Introduction
Subcutaneous panniculitis-like T-cell lymphoma (SPTCL) is a rare subtype of primary cutaneous T-cell lymphoma defined by neoplastic α/β T-cell infiltration of the subcutaneous fat, mimicking an inflammatory panniculitis.1, 2, 3 SPTCL makes up only 1% of primary cutaneous lymphomas.4 Given the paucity of cases reported in the literature, little is known about the precise epidemiology, risk factors, and causative factors in the development of SPTCL. We describe a young woman who developed SPTCL at the injection site of a recent COVID-19 vaccination.
Case report
A previously healthy 28-year-old woman received the primary dose of the recombinant replication-incompetent adenovirus type 26 (Ad26) viral vector–based COVID-19 vaccine (Janssen Pharmaceuticals) in her upper arm. A few days later, she developed a nodule at the injection site. Over the next 3 months, the nodule progressively enlarged to become a plaque with associated pain, warmth, and paresthesias. She also developed cervical and left axillary lymphadenopathy, intermittent fevers, fatigue, and unintentional weight loss. Presumptive treatment for cellulitis with several courses of oral antibiotics was ineffective.
Physical examination revealed a large erythematous indurated plaque on the upper arm and cervical and axillary lymphadenopathy (Fig 1). The patient presented with fever (38 °C), mild anemia, leukopenia, and neutropenia. Punch biopsies revealed a lobular panniculitis with a subcutaneous infiltrate of atypical lymphocytes rimming adipocytes with fibrinoid necrosis and large histiocytes containing apoptotic debris (Fig 2). The atypical lymphocytes were CD3+, CD7+, CD8+, perforin+, T-cell receptor (TCR)–beta+, TCR-delta-, CD4-, CD56-, EBER-, ISH-, and CD30- with an elevated proliferation index highlighted by Ki-67. Following extraction of genomic DNA, the TCR gamma gene was amplified using a multiplex polymerase chain reaction master mix according to Trainor et al.5 Polymerase chain reaction products were resolved by capillary electrophoresis. Molecular genetic analysis for TCR-gamma gene rearrangement revealed a polyclonal distribution, although with 2 suspicious peaks: one at 187 bp of master mix I and the second at 220 bp of master mix I. According to the World Health Organization–European Organization for Research and Treatment of Cancer classification, the clinical and histopathologic findings were consistent with SPTCL.3 Primary cutaneous γ/δ T-cell lymphoma, a more aggressive variant distinguished from SPTCL, was excluded by the lack of CD56 positivity and the absence of ulcerating plaques and tumors.3 Secondary hemophagocytic lymphohistiocytosis (HLH) was excluded by HLH-2004 criteria.6
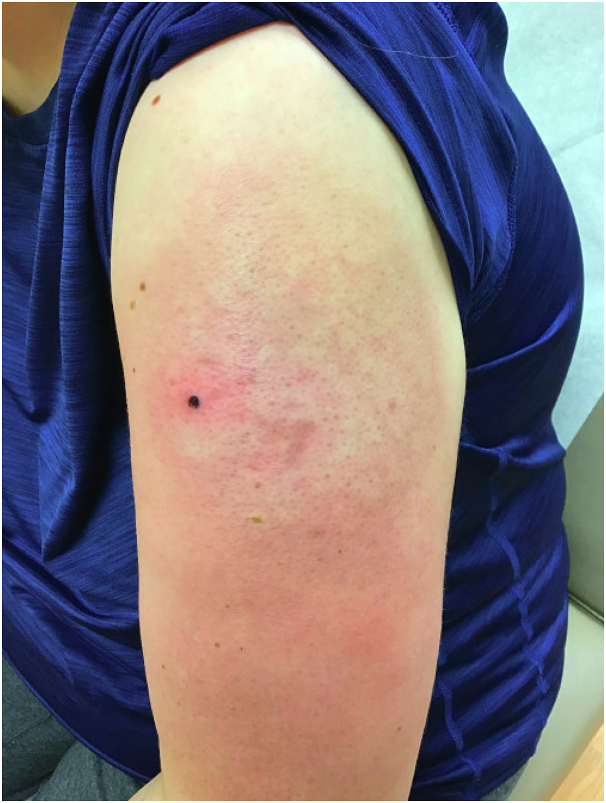
Fig 1.
Erythematous, indurated plaque on the upper arm.
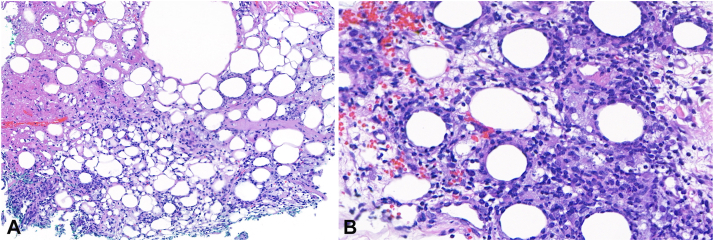
Fig 2.
Lobular panniculitis with fibrinoid necrosis (A: H&E, 150× magnification), atypical medium-sized lymphocytes rimming adipocytes, and histiocytes containing apoptotic debris (B: H&E, 400× magnification).
The patient developed tender cervical, submandibular, right axillary, and right popliteal lymphadenopathy with persistence of the subcutaneous plaque and new subcutaneous nodules on both arms. Positron emission tomography–computed tomography (PET-CT) scan showed multifocal subcutaneous lesions in the right forearm (standardized uptake value [SUV] max: 10.34), left arm and axilla (SUV max: 10.26), jaw (SUV max: 11.9), right supraclavicular region (SUV max: 8.88), back (SUV max: 3.72), pelvis (SUV max: 6.7), right calf (SUV max: 5.27), and left knee (SUV max: 3.66). Patchy fluorodeoxyglucose uptake was also seen in the tibias. The spleen was enlarged to 13.7 cm with increased fluorodeoxyglucose uptake relative to the liver.
Treatment was initiated with cyclosporine 5 mg/kg/day. Since the patient only showed modest improvement after 2 weeks, prednisone 50 mg daily was added. Three weeks after combination therapy, the anemia, leukopenia, and neutropenia resolved. By 6 weeks, the patient’s fatigue, the subcutaneous nodules, and the lymphadenopathy had nearly resolved. The original plaque at the site of vaccination resolved with prominent atrophy (Fig 3). PET-CT scan at 3-month follow-up showed improvement or resolution of all widespread subcutaneous lesions, no new lesions, and decreased splenomegaly. The prednisone was tapered over 4 months, and she has completed 8 months of a 12-month course of cyclosporine therapy.
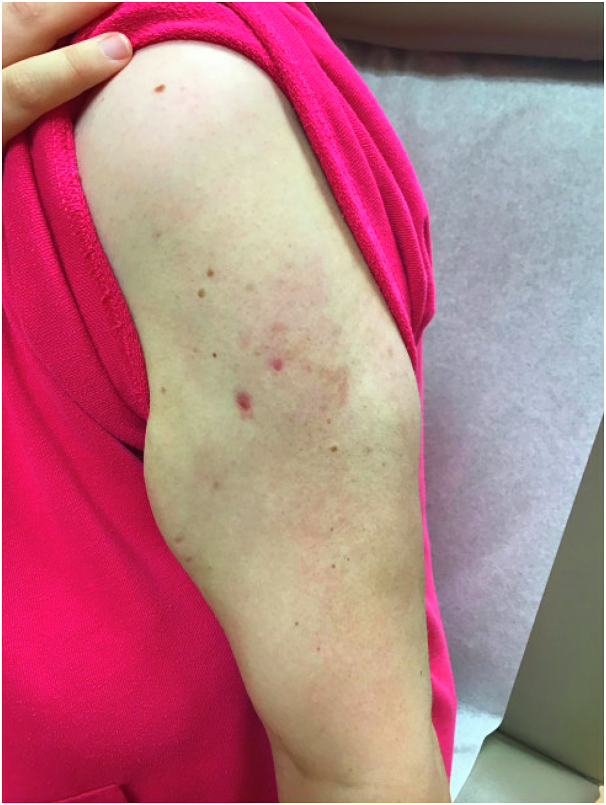
Fig 3.
Resolution with atrophy following 6 weeks of combined treatment with cyclosporine and prednisone.
Discussion
Due to the rarity of SPTCL, there are few data to characterize the demographic risk factors, comorbid medical conditions, and inciting factors associated with the disease. The median age of presentation is 36 years, females are twice as likely to be affected as males, and up to 20% of patients have a co-existing autoimmune disease.1 In this manuscript, we present a case of SPTCL that developed following the Ad26 viral vector–based COVID-19 vaccine (Janssen Pharmaceuticals). Although causation cannot be established in this single case, this case supports the understanding that certain immunologic triggers, such as a modified adenovirus vaccine, may contribute to the development or exacerbation of SPTCL. Aberrant immune function or lymphocyte hyperstimulation may also be responsible. For example, an association has been established between SPTCL and systemic lupus erythematosus, Sjögren’s syndrome, type 1 diabetes mellitus, and juvenile idiopathic arthritis.1
CD56-negative, α/β SPTCL typically follows an indolent course, rarely with involvement of lymphatic structures. In this case, PET-CT scan showed diffuse subcutaneous, lymph node, and splenic enhancement, representing relatively aggressive disease for this diagnosis. However, consistent with SPTCL and in contrast to primary cutaneous γ/δ T-cell lymphoma, her disease responded promptly to conservative treatment.
No standardized treatment protocol currently exists for SPTCL. Historically, polychemotherapy was used for treatment. However, following the 2008 World Health Organization–European Organization for Research and Treatment of Cancer update excluding the more aggressive γ/δ phenotype,3 systemic corticosteroids were shown to be an effective first-line therapy for SPTCL without HLH.7 Further evidence has demonstrated higher rates of complete remission and lower rates of progression with immunosuppressant therapies in SPTCL.8
Success has also been reported with bexarotene9 and romidepsin monotherapy.10 In the case presented, cyclosporine and prednisone therapy resulted in significant improvement with resolution of induration and erythema, systemic symptoms, and subcutaneous nodules. This case adds to accumulating data favoring the use of immunosuppressive agents as first-line treatment for SPTCL rather than chemotherapy.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
Consent for the publication of all patient photographs and medical information was provided by the authors at the time of article submission to the journal stating that all patients gave consent for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
References
- 1.Willemze R., Jansen P.M., Cerroni L., et al. Subcutaneous panniculitis-like T-cell lymphoma: definition, classification, and prognostic factors: an EORTC Cutaneous Lymphoma Group Study of 83 cases. Blood. 2008;111(2):838–845. doi: 10.1182/blood-2007-04-087288. [DOI] [PubMed] [Google Scholar]
- 2.Parveen Z., Thompson K. Subcutaneous panniculitis-like T-cell lymphoma: redefinition of diagnostic criteria in the recent World Health Organization–European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas. Arch Path Lab. 2009;133(2):303–308. doi: 10.5858/133.2.303. [DOI] [PubMed] [Google Scholar]
- 3.Willemze R., Cerroni L., Kempf W., et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133(16):1703–1714. doi: 10.1182/blood-2018-11-881268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abouyabis A.N., Shenoy P.J., Lechowicz M.J., Flowers C.R. Incidence and outcomes of the peripheral T-cell lymphoma subtypes in the United States. Leuk Lymphoma. 2008;49(11):2099–2107. doi: 10.1080/10428190802455867. [DOI] [PubMed] [Google Scholar]
- 5.Trainor K.J., Brisco M.J., WanNeoh J.H., Grist S., Morley A.A. Gene rearrangement in B- and T-lymphoproliferative disease detected by the polymerase chain Reaction. Blood. 1991;78:192–196. [PubMed] [Google Scholar]
- 6.Henter J.I., Horne A., Aricó M., et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124–131. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 7.Guenova E., Schanz S., Hoetzenecker W., et al. Systemic corticosteroids for subcutaneous panniculitis-like T-cell lymphoma. Br J Dermatol. 2014;171(4):891–894. doi: 10.1111/bjd.13053. [DOI] [PubMed] [Google Scholar]
- 8.Michonneau D., Petrella T., Ortonne N., et al. Subcutaneous panniculitis-like-T-cell lymphoma: immunosuppressive drugs induce better response than polychemotherapy. Acta Derm Venereol. 2017;97(3):358–364. doi: 10.2340/00015555-2543. [DOI] [PubMed] [Google Scholar]
- 9.Mehta N., Wayne A.S., Kim Y.H., et al. Bexarotene is active against subcutaneous panniculitis-like T-cell lymphoma in adult and pediatric populations. Clin Lymphoma Myeloma Leuk. 2012;12(1):20–25. doi: 10.1016/j.clml.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jothishankar B., Espinosa M.L., Zain J., Parekh V., Di Raimondo C., Abdulla F. Complete response to romidepsin as monotherapy in treatment-resistant subcutaneous panniculitis-like T-cell lymphoma. JAAD Case Rep. 2020;6(12):1245–1247. doi: 10.1016/j.jdcr.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]