Abstract
Objectives
To assess whether COVID-19 could be a concurrent factor in the genesis and/or worsening of stroke and to provide data on COVID-19 –associated stroke patients during the first pandemic wave and comparative data on COVID-19 negative stroke patients in the same period.
Materials and Methods
This is a retrospective, observational, case-control, single centre study, carried out in a General Hospital in northern Italy. Sixty-three consecutive stroke patients were included, COVID-19-associated stroke was classified as cases and non COVID-19-associated stroke as controls.
Results
A total of 19/63 (28.8%) had a COVID-19-associated stroke, 11 /63 (17.5%) were haemorrhagic and 52/63 (82.5%) ischaemic. COVID-19-associated strokes were more severe (p-value 0.019) and had a higher risk of severe disability and/or death (OR 3.79, CI 95%: 1.21-11.93, p-value 0.19). The COVID-19-associated stroke patients with onset during hospitalization for COVID-19 had a more severe stroke than patients with COVID-19 onset during hospitalization for stroke (p-value 0.019).
Conclusion
Although no relationship was observed between the stroke aetiology and COVID-19, intriguingly, COVID-associated stroke turned out to be more severe and disabling. Hopefully, further studies will provide more data and help in the management of this emerging population.
Keywords: COVID-19, SARS-CoV-2, COVID-19-associated stroke, Case-control study, Large vessel occlusion
Introduction
It was in December of 2019 that an unexplained interstitial pneumonia was first observed in Wuhan, China. In January 2020, this led to the recognition of the novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) as the etiological agent of the corona virus disease 2019 (COVID-19) and The World Health Organisation (WHO) declared the COVD-19 infection outbreak a pandemic, on March 11th, 2020.1
Since then, numerous studies have been carried out, aimed at describing the clinical characteristics, neuroimaging findings and outcomes of COVID-19-associated stroke.
International literature links COVID-19-associated stroke to a more severe course, which frequently involves the large vessels rather than the smaller ones.2, 3, 4, 5, 6, 7, 8 Several case-series and small observational studies have described other clinical characteristics, i.e., young COVID-19 positive patients with carotid artery dissection (CAD), central nervous system vasculitis like phenotype, etc.2 , 3 , 6
Severe COVID-19 and Acute Respiratory Distress Syndrome (ARDS) share some risk factors with stroke, i.e., being elderly, having hypertension, cardiovascular diseases, neoplasms, etc.9 Therefore, it would be reasonable to presume that, in a selected at risk population, there might well be a higher stroke incidence. However, surprisingly, there was a drop in the number of admissions to casualty departments for stroke during the COVID-19 pandemic.10 , 11 This intriguing observation may be attributed to numerous factors, including the fear of being infected when going to a hospital structure and a tendency to diagnose COVID-19 infection as the primary cause of seeking help.10 , 11 , 12
Although the pathogenesis of the COVID-19 has not yet been fully clarified, inflammatory cytokine storms and viral evasion of cellular immune response have been observed to play a pivotal role in disease progression and severity.13 , 14 It also seems that hyperinflammation promotes endothelial cell activation and dysfunction, leading to a prothrombotic state. Indeed, COVID-19 induced coagulopathy (CIC) has been reported to be a contributing factor to morbidity and mortality.15 , 16 A systematic review supported this hypothesis by describing a low-grade intravascular clotting activation, which was more evident in severe disease.17 Conversely, some haemorrhagic complications, such as autoimmune thrombocytopenic purpura, have also been reported.18 Although some observational studies do compare COVID-19-associated stroke with historical stroke cohorts,19 , 5 case-control studies are scanty.
Although a relationship between stroke and COVID-19 severity has been described,20 , 21 where patients with a more severe Covid-19 were reported to have a more severe stroke, whether a Covid-19-associated stroke is more severe than a non Covid-19 associated stroke is still a question of debate. However, Covid-19 patients subsequently affected by stroke differ from patients that had COVID-19 onset during hospitalisation for stroke, as they may lack factors that influence stroke severity at onset (e.g., cytokine storm, endothelial damage, severe COVID-19).
This study was carried out to provide data on COVID-19–associated stroke patients during the first pandemic waves and comparative data on COVID-19 negative stroke patients, in the same period; Patients hospitalised for stroke who subsequently acquired COVID-19 were also compared to patients hospitalized for COVID-19, who were subsequently affected by stroke.
Materials and methods
Study design and patients
This study was carried-out in the largest General Hospital in Piacenza, one of the epicentres heavily burdened by the first SARS-CoV-2 waves. All the consecutive stroke patients admitted to the Neurology Unit of the “Guglielmo da Saliceto” Hospital, from February 21st to April 30th, 2020, have been assessed retrospectively. Patients with COVID-19-associated stoke were classified as cases and patients with stroke alone as controls. The study was approved by the local ethics committee, who waived informed consent due to its retrospective nature.
Data collection
All the data were saved in an electronic clinical database specifically customized for stroke patients, named, “Galileo” (trademark of Dedalus Italia, S.P.A.). It contains the National Institutes of Health Stroke Scale (NIHSS), the modified Rankin Scale (mRS) and the “Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification”.
All the neurologists responsible for the collection of data were certificated for NIHSS and mRS use and skilled in the TOAST classification. The data reported in this study are available from the corresponding author upon reasonable request. The neurologists identified the study population by searching the International Disease Classification-10 (ICD-10) discharge diagnosis for stroke in the institutional database. The demographics, NIHSS, mRS at admission and discharge, stroke aetiology and diagnostic procedures were extracted anonymously. The strokes were then classified according to the TOAST criteria.22 Any undetermined strokes were further divided into Undetermined and Embolic Stroke of Undetermined Source (ESUS),23 according to our institutional stroke cause classification in the electronic medical records and stroke diagnosis was in line with the WHO definition.24
A diagnosis of COVID-19 was made after microbiological confirmation, according to the WHO recommendations. If there was still a high clinical suspicion of infection, even after two negative nasopharyngeal swabs, bronchoalveolar lavage (BAL) sampling was performed. All the stroke patients admitted to the casualty department had a nasopharyngeal swab, followed by a high-resolution lung computed tomography (CT) scan if the swab was positive for SARS-CoV-2. The swab was repeated every 72 hours throughout hospitalization in previously negative patients.
For the purpose of this study, COVID-19 onset was considered to be when the first symptoms appeared, e.g., a temperature, cough and/or dyspnea. All stroke patients had a brain CT scan at onset, cardiac and carotid sonography, ECG and routine laboratory tests. Dual-phase 16 slice CT angiography at admission,25 , 26 brain magnetic resonance imaging (MRI) or CT scan after 48 hours from symptom onset and/or a 24-hour Holter ECG, were performed at the neurologist's discretion.
Analyses and outcome measures
A total of 63 consecutive stroke patients were admitted to our department from 21st February to 30th April, 2020, 19/63 (28.8%) had a concomitant diagnosis of stroke and COVID-19.
Cases and controls were compared for demographic and baseline clinical characteristics.
Two analyses were performed:
-
-
a case-control analysis: COVID-19-associated stroke was compared to non-COVID-19-associated stroke as to demographics, stroke aetiologies, NIHSS, mRS, recombinant tissue Plasminogen Activator (rtPA) treatment, mortality and laboratory tests.
-
-
a COVID-19-associated stroke analysis: stroke course in patients hospitalized for COVID-19 was compared to that of patients hospitalized for stroke who became SARS-CoV-2 positive during hospitalization, as to demographics, NIHSS, mRS, mortality, laboratory tests and interstitial pneumonia were assessed at lung CT scan.
Statistical analyses
Categorial data were described by frequencies, continuous variables were tested for normal distribution by the Kolgomorov-Smirnoff test and described by average and standard deviations (SD) or median and interquartile range (IQR), depending on their distribution. The data were analyzed by the Student's t-test assuming equal or unequal variances (determined by Levene's Test), the Mann-Whitney U Test, Fisher's exact test and binary logistic regression when appropriate; a p-value of less than 0.05 was considered significant. Statistical analysis was performed by SPSS 28.0.0.0 (IBM Corp. IBM SPSS Statistics for Mac, Armonk, NY: IBM Corp.).
Results
A total of 11/63 (17.5%) patients had haemorrhagic stroke and 52/63 (82.5%) ischaemic stroke. The demographic data are reported in Table 1 , whilst the ischaemic stroke etiologies are reported in Table 2 .
Table 1.
Demographics.
| Frequency | Average | Range | Standard deviation | |
|---|---|---|---|---|
| Age | 74.3 | 29-92 | 11.61 | |
| Gender | 49.2% females | |||
| 50.8% males | ||||
| Diabetes | 12.70% | |||
| Hypertension | 76.20% | |||
| Coronary Heart disease | 9.50% | |||
| Atrial fibrillation | 27% | |||
| Obesity | 12.70% | |||
| Chronic renal failure | 11.10% | |||
| rt-PA | 23.10% | |||
| mRs 0-2 | 83.90% | |||
| mRs 0-1 | 85.50% |
Table 2.
Stroke etiologies the whole population.
| Stroke aetiology | Frequency |
|---|---|
| Large vessel disease | 17.3% |
| Cardioembolism | 32.7% |
| Small vessel disease | 9.6% |
| Other (dissection, vasculitis, etc.) | 1.9% |
| Undertemined | 23.1% |
| ESUS (percentage within undetermined) | 15.4% |
COVID-19-aassociated stroke patients versus non COVID-19-associated stroke
The average age of our study group was 76.05 +/- 8.8 for cases and 73.2 +/- 12.61 for controls (p-value n.s.), there were 47.4% of females and 52.6% of males in cases and 52.3% of females and 47.7% of males in controls (p-value n.s.). There was no statistically significant difference between cases and controls as to diabetes, hypertension, coronary artery disease, atrial fibrillation, chronic renal failure, obesity, or neoplasm (p-values n.s.). Nor was there a statistically significant difference between cases and controls as to haemorrhagic stroke frequency (10.5% and 20.5% respectively, p-value n.s.), rtPA treatment (15.5% and 20.5% respectively, p-value n.s.), in-hospital mortality (15.8% and 6.8% respectively, p-value n.s.) or stroke etiologies, such as large vessel disease (21.1% and 11.4% respectively, p-value n.s.). Cases had a higher median NIHSS (9, IQR 4-15 versus 4, IQR 2-8, p-value 0.019), risk of death or serious disability (mRS 4-5) (OR 3.79, CI 95%: 1.21-11.93, p-value 0.19), median LDH value (314, IQR 203-504 versus 232, IQR 194-271, p-value < 0.001), median D-dimer level (6600, IQR 1448.8-27068 versus 839-5, IQR 324.5-1235.8, p-value 0.001). A multivariate logistic regression analysis confirmed an association only for the LDH level (p-value 0.003).
Stroke in COVID-19 patients
A total of 15/19 (78.9%) cases had stroke onset during hospitalisation for COVID-19, whereas stroke preceded COVID-19 diagnosis in 4/19 (21.1%). The average time lapse between COVID-19 diagnosis and stroke was 6.05 days (SD 10.17) in the whole population. It was 8.33 days (SD 10.31) in COVID-19-associated stroke with onset prior to stroke and -2.5 days (SD 1) in the group with stroke onset before COVID-19 diagnosis.
COVID-19-associated stroke with onset during and before COVID-19 differed as to average age (78.2 +/- 8.5 vs 68 +/- 4.8 years respectively, p-value 0.036), LDH (412.53 +/- 166.72 vs 245.75 +/- 66.35 respectively, p-value 0.009), NIHSS (11.4 +/- 6.62 vs 3.75 +/- 3.1, p-value 0.041) and male gender (OR 1.8, CI 95% 1.003-3.229, p-value 0.033). The multivariate analysis confirmed associations for male gender (p-value 0.018), age (p-value 0.035) and NIHSS (p-value 0.039).
Interstitial pneumonia was evidenced in 13/19 (68.4%) patients, with a trend towards a higher frequency in males (X2 p-value 0.057). Only two cases required non-invasive mechanical ventilation in the Intensive Care Unit (ICU) and had elevated NIHSS scores.17 , 21
Discussion
The relationship between stroke and COVID-19 is a complex question and still a matter of debate. Though rare, stroke is a potentially life-threatening complication of COVID-19, affecting approximately 1 to 3% of hospitalised cases and 6% of ICU cases.27, 28, 29, 30 Stroke and COVID-19 share several common risk factors, e.g., obesity, hypertension and diabetes. It has been reported that there was a 7.6-fold increase in the odds of having a stroke with a COVID-19 infection, compared to influenza.31 Moreover, another study reported that COVID-19 was the strongest predictor of in-hospital stroke onset. All of which led to the hypothesis that COVID-19 infection may well be an independent risk factor for stroke.32
In literature, COVID-associated stroke had a higher rate of large vessel occlusion, a lower rate of small vessel disease and lacunar stroke,5 atypical stroke presentation,33 multi-territory involvement, stroke in territory of distribution of uncommonly affected vessels and a worse outcome.34 A proinflammatory-prothrombotic state, CIC and endothelial injury, associated with SARS-CoV-2 infection, also supports the hypothesis of COVID-19- associated stroke.13, 14, 15, 16, 17
A reduction in stroke access to causality departments was described during the first wave of the Pandemic,10 probably due to the fear of contracting COVID-19, meaning that those with minor strokes tended to avoid hospitals, compared to those with large artery disease.12 Therefore, historical cohort-controlled studies could be biased due the pandemic, e.g. historical cohorts may have a higher prevalence of minor stroke and most likely, a case-control study design would minimize such a bias.
In contrast with other studies, we observed no reduction in small-vessel disease, even if there was an excess of large-artery disease in cases (20.5%), compared to controls (10.4%). However, this difference did not reach statistical significance as the study was underpowered for this analysis (the study power to detect such a difference, given a 0.05 level of significance, was 21.7%, meaning that if there was a significant association, the present study had only a 21.7% probability of finding it), therefore, this association cannot be excluded.
Although this datum was not confirmed by the multivariable analysis, there was an association between stroke severity at onset and COVID-19. The risk of death and/or serious disability (mRS 4-5) was higher in cases (OR 3.79, CI 95%: 1.21-11.93, p-value 0.19) than in controls. Three deaths occurred in the case group: one was a 92 year-old woman with a severe stroke (admission NIHSS 14) and a mild COVID-19 (without acute respiratory failure), the other two patients were 74 and 85 year-old men who had severe stroke (admission NIHSS 20 and 15 respectively) and severe COVID-19 (pneumonia involving 60% of the lungs, as evidenced by the admission CT scan); these two patients died of acute respiratory failure.
Furthermore, in cases, the association between stroke severity and stroke onset during hospitalization for COVID-19 was statistically significant in the multivariable analysis: stroke with onset during hospitalization for SARS-CoV-2 infection seems to lead to a particularly severe disability. Our study supports the hypothesis that COVID-19 could be an independent risk factor for stroke,32 especially in patients who have a cerebrovascular accident during hospitalization for severe COVID-19. A previous study of ours supported an association between stroke severity and interstitial pneumonia extension,20 in line with the hypothesis of an association between stroke severity and COVID-19 severity (Brain CT, CTA and lung CT scan of a case with large vessel occlusion, severe stroke and severe COVID-19 interstitial pneumonia are represented in Fig. 1 ).
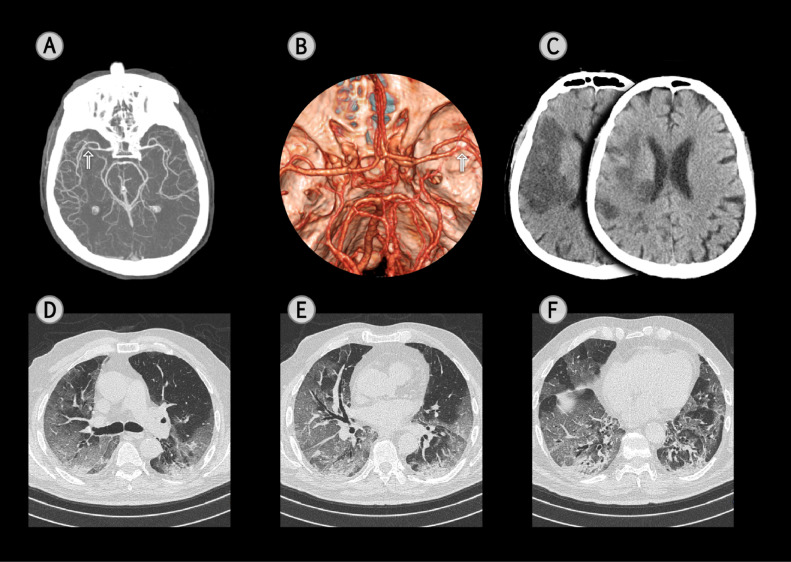
Fig. 1.
A Computed Tomography Angiography (CTA) of a 65-year-old male, COVID-19-associated stroke patient, with left hemiparesis and neglect (NIHSS 18) after having had a temperature, cough and dyspnea for 7 days. The arterial phase of the CTA evidences a steno-occlusion of right middle cerebral artery (arrow) (A: maximum intensity projection; B: volume rendering technique with top view). A head Computed Tomography scan (CT), performed 24 hrs. later, documented an extensive ischaemic lesion in the right temporal and frontal lobes (C). A chest CT scan, on the 2nd day after admission, for respiratory symptoms, evidenced a bilateral reticular pattern, superimposed on a background of ground-glass opacities (GGO) (D-F) and bibasilar subpleural consolidations (F). Fibrous stripes and multiple small vascular enlargement were also observed (E and F). Well aerated lung with a visual score of 40%.
Noteworthy is the fact that the same percentage of cases and controls was treated with rtPA, thanks to the set-up of dedicated pathways for Covid-19 patients in the casualty department.34
To the best of our knowledge there are five case-control studies investigating COVID-19-associated stroke versus stroke21 , 35, 36, 37, 38 and a single case-control on COVID-19-associated stroke versus COVID-1939 (Table 3 ). All the studies that investigated mortality reported a higher mortality rate in COVID-19-associated stroke versus non COVID-19-associated stroke and versus COVID-19 patients without stroke.
Table 3.
COVID-19-associated stroke case-control studies.
| Study | Case-control definition | NIHSS (cases vs controls) | LVO (cases vs controls) | Mortality (cases vs controls) | mRS on discharge (cases vs controls) | Stroke etiology subtype (TOAST) |
|---|---|---|---|---|---|---|
| Perry et al, JNNP 2021 | COVID-19-associated stroke vs stroke | 8 vs 5, p < 0.002 | n.s. | 19.8% vs 6.9%, p < 0.0001 | 4 vs 3, p < 0.0001 | n.s. |
| Kihira et al, AJR 2020 | COVID-19-associated stroke vs stroke | n.s. | 31.7% vs 15.3%, p 0.001 | - | - | - |
| Topcuoglu et al, JSCVD 2021 | COVID-19-associated stroke vs stroke | 12 vs 9, p 0.034 | - | 54% vs 8%, p < 0.0001 | n.s. | n.s. |
| Yaghi et al, Stroke 2020 | COVID-19-associated stroke vs stroke | 19 vs 8, p 0.007 | n.s. | 63% vs 9%, p < 0.001 | - | Cryptogenetic stroke 65.6% vs 30.4%, p 0.003 |
| Garcia-Lamberecht | COVID-19-associated stroke vs stroke | - | - | OR 1.77, 95% CI 1.37-2.3 | - | - |
| Khorvash et al., J Res Med Sci | COVID-19-associated stroke vs COVID-19 | - | - | 52% vs 18%, p 0.001 | - | - |
NIHSS National Institute of Health Stroke Scale, LVO Large Vessel Occlusion, mRS modified Rankin Scale, TOAST
Three case-control studies21 , 35 , 36 assessed COVID-19-associated stroke versus non COVID-19-associated stroke. Two reported an association between stroke severity and COVID-19-associated stroke35 , 36 and one an association with multiple large vessel disease.35 Another study reported an excess of Large Vessel Occlusion in COVID-19-associated stroke37 and only one study reported an association between cryptogenetic stroke and COVID-19-associated stroke.
We are aware that our study does have some limitations, the main being the small sample size. Indeed, we report a non-significant excess of large vessel disease, even if the statistical power to reach significance was only 21.7%. Therefore, this association cannot be excluded, nor can a higher mortality in patients with concomitant stroke and COVID-19. However, the strength of the present study is the case-control design, which is not affected by “non-contemporaneous control bias” (i.e., secular changes in definitions, exposures, diagnoses, diseases, and treatment may render non-contemporaneous controls non-comparable).40
The pandemic is a dynamic phenomenon and mortality depends on many factors that vary according to COVID-19 prevalence, health-care organization, etc,41 , 42 therefore, estimating the mortality rate in the course of pandemic is no easy task. However, several methods can be used to analyze mortality during a pandemic: “excess death” esteems the increase of mortality compared to a given period in the past. At time of writing excess death decreased by up to 2.5% during the subsequent pre-Delta, Delta and Omicron waves and there was no excess mortality in the working-age population.43 Although we are still experiencing pandemic waves, excess mortality seems to involve only populations at risk for severe COVID-19.43 The present study reported that COVID-19-associated strokes were particularly severe when onset was during hospitalization for COVID-19. This observation should support the indication to treat people at risk for severe COVID-19 evolution early so as to reduce COVID-19 complications and associated stroke.
Conclusions
As the concurrence of COVID-19 and stroke is very challenging, a dedicated COVID-19 pathway might make the management of COVID-associated stroke quicker and safer. This case-control study evidenced an association between stroke severity and COVID-19, with a high frequency of large vessel disease in COVID-19, in line with previous historical-control studies and the hypothesis that severe COVID-19 could be an independent stroke risk factor. Hopefully, further large multicentre case-control studies will help to better clarify the association between stroke and COVID-19.
Funding
None.
Disclosures
None of the authors have anything to disclose.
Acknowledgments
The authors thank Barbara Wade for her linguistic advice.
References
- 1.https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020
- 2.Oxley T J, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 2020;382:e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morassi M., Bagatto D., Cobelli M., et al. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020;267:2185–2192. doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyrouti R., Adams M.E., Benjamin L., et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. 2020;91:889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahjouei S, Tsivgoulis G, Farahmand G, et al. SARS-CoV-2 and stroke characteristics. a report from the multinational COVID-19 stroke study group. Stroke. 2021;53:e117–e130. doi: 10.1161/STROKEAHA.120.032927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogrig A, Gigli GL, Bnà C, Morassi M. Stroke in patients with COVID-19: clinical and neuroimaging characteristics. Neuroscience Letters. 2021;743 doi: 10.1016/j.neulet.2020.135564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. 2020;382:2268–2270. doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W, Ni Z, Hu Y, et al. For the China Medical Treatment Expert Group for Covid-19*. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morelli N, Rota E, Terracciano C, et al. The baffling case of ischemic stroke disappearance from the casualty department in the COVID-19 Era. Eur Neurol. 2020;14:1–3. doi: 10.1159/000507666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hugh S M, Brainin M. COVID-19 and stroke—a global world stroke organization perspective. Int J Stroke. 2020;15:361–364. doi: 10.1177/1747493020923472. [DOI] [PubMed] [Google Scholar]
- 12.Bersano A, Panton I.L. Stroke care in Italy at the time of COVID-19 pandemic: a lesson to learn. J Neurol. 2021;268:2307–2313. doi: 10.1007/s00415-020-10200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta PM, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michael Henry B, Vikse J, Benoit S, et al. Hyperinflammation and derangement of renin-angiotensin-aldosterone system in COVID-19: a novel hypothesis for clinically suspected hypercoagulopathy and microvascular immunothrombosis. Clin Chim Acta. 2020 doi: 10.1016/j.cca.2020.04.027. S0009-8981:30183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Violi F, Pastori D, Cangemi R, et al. Hypercoagulation and antithrombotic treatment in coronavirus 2019: a new challenge. Thromb Haemost. 2020;120:949–956. doi: 10.1055/s-0040-1710317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zulfiqar AA, Hasler P, Andres E. Immune thrombocytopenic Purpura in a patient with Covid-19. N Engl J Med. 2020;382:e43. doi: 10.1056/NEJMc2010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz JM, Libman RB, Wang JJ, et al. Cerebrovascular complications of COVID-19. Stroke. 2020;51:e227–e231. doi: 10.1161/STROKEAHA.120.031265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Immovilli P, Terracciano C, Zaino D, et al. Stroke in COVID-19 patients—a case series from Italy. Int J stroke. 2020;15:701–702. doi: 10.1177/1747493020938294. [DOI] [PubMed] [Google Scholar]
- 21.Perry R J, Smith C J, Roffe C, et al. Characteristics and outcomes of COVID-19 associated stroke: a UK multicentre case-control study. J Neurol Neurosurg Psychiatry. 2021;92:242–248. doi: 10.1136/jnnp-2020-324927. [DOI] [PubMed] [Google Scholar]
- 22.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke definitions for use in a multicenter clinical trial. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 23.Palline Hart RG, Catanese L, Perera KS, et al. Embolic stroke of undetermined source. a systematic review and clinical update. Stroke. 2017;48:867–872. doi: 10.1161/STROKEAHA.116.016414. [DOI] [PubMed] [Google Scholar]
- 24.WHO MONICA Project Investigators The World Health Organization MONICA Project (Monitoring trends and determinants in cardiovascular disease) J Clin Epidemiol. 1998;41:105–114. doi: 10.1016/0895-4356(88)90084-4. [DOI] [PubMed] [Google Scholar]
- 25.Morelli N, Rota E, Colombi D, et al. Goliath and the ant: whole-brain CT perfusion against 16-slice CT angiography in stroke imaging. J Neuroradiol. 2019;46:398–400. doi: 10.1016/j.neurad.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Morelli N, Rota E, Immovilli P, et al. Dual-phase 16 slice CT angiography in stroke imaging: a poor man's multiphase study? Acta Neurol Belg. 2019;119:187–192. doi: 10.1007/s13760-018-1019-4. [DOI] [PubMed] [Google Scholar]
- 27.Ellul M A, Benjamin L, Singh B, et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Wang M, Zhou Y, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5:279–284. doi: 10.2139/ssrn.3550025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan Y-K, Goh C, Leow A S T, et al. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J. Thromb. Thrombolysis. 2020;50:587–595. doi: 10.1007/s11239-020-02228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegler J E, Cardona P, Arenillas J F, et al. Cerebrovascular events and outcomes in hospitalized patients with COVID-19: the SVIN COVID-19 multinational registry. Int. J. Stroke. 2021;16:437–447. doi: 10.1177/1747493020959216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merkler A E, Parikh N S, Mir S, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77:1366–1372. doi: 10.1001/jamaneurol.2020.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz JM, Libman RB, Wang JJ. Cerebrovascular complications of COVID-19. Stroke. 2020;51:e227–e231. doi: 10.1161/STROKEAHA.120.031265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernandez-Fernandez F, Valencia H S, Barbella-Aponte R A, et al. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain. 2020;143:3089–3103. doi: 10.1093/brain/awaa239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khosravani H, Rajendram P, Notario L, et al. Protected code stroke: hyperacute stroke management during the coronavirus disease 2019 (COVID-19) pandemic. Stroke. 2020;51:1891–1895. doi: 10.1161/STROKEAHA.120.029838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Topcouglu M A, Pektezel M Y, Oge D D, et al. Stroke mechanism in COVID-19 infection: a prospective case- control study. J Stroke Cerebrovasc Dis. 2021;30 doi: 10.1016/j.jstrokecerebrovasdis.2021.105919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García-Lamberechts E J, Mirò O, Fragiel M, et al. A case-control analysis of stroke in COVID-19 patients: results of unusual manifestations of COVID-19–study 11. Acad Emerg Med. 2021;28:1236–1250. doi: 10.1111/acem.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kihira S, Schefflein J, Mahmoudi K, et al. Case-control Study. AJR Am J Roeentgenol. 2021;216:150–156. doi: 10.2214/AJR.20.23847. [DOI] [PubMed] [Google Scholar]
- 38.Yaghi S, Ishida K, Torres J, et al. SARS-CoV-2 and Stroke in a New York Healthcare System. Stroke. 2020;51:2002–2011. doi: 10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khorvash F, Najafi MA, Kheradmand M, et al. New-onset acute ischemic stroke following COVID-19: a case-control study. J Res Med Sci. 2022;27:31. doi: 10.4103/jrms.jrms_255_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sackett DL. Bias in analytic research. J Chronic Dis. 1979;32:51–63. doi: 10.1016/0021-9681(79)90012-2. [DOI] [PubMed] [Google Scholar]
- 41.Immovilli P, Morelli N, Rota E, Guidetti D. COVID-19 mortality and health-care resources: organization. Med Intensiva. 2021;45(6):383–384. doi: 10.1016/j.medin.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Immovilli P, Morelli N, Antonucci E, Radaelli G, Barbera M, Guidetti D. COVID-19 mortality and ICU admission: the Italian experience. Crit Care. 2020;24(1):228. doi: 10.1186/s13054-020-02957-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alicandro G, Remuzzi G, Centanni S, Gerli A, La Vecchia C. No excess mortality among working-age Italians during the Omicron wave of Covid-19. Med Lav. 2022;113(3) doi: 10.23749/mdl.v113i3.13092. [DOI] [PMC free article] [PubMed] [Google Scholar]