Abstract
Background and Aims
Whereas the use of nicotine vaping products (NVPs) is widespread, their impact on smoking prevalence is controversial. This study considered the potential impact of NVPs on smoking prevalence in England.
Design
Indirect simulation model. The England SimSmoke model is validated through 2012, before NVP use became more widely used by smokers. Because information on NVP-related transitions is limited, an indirect method is used; the difference in observed smoking prevalence (reflecting NVPs) is compared with a 2012–2019 counterfactual No-NVP scenario (without NVPs) to estimate the impact of NVPs on smoking and smoking-attributable deaths.
Setting
England, 2000–2019.
Participants
Nationally representative sample of population.
Measurements
England’s population, mortality rates and smoking prevalence estimates from three national surveys and tobacco control policies.
Findings
Between 2000 and 2012, SimSmoke projected a decline in age 18+ smoking prevalence of 23.5% in men and 27.0% in women. These projections, as well as those by specific age groups, were generally consistent with findings from the three national surveys. Comparing 2012–2019 relative reduction in age 18+ prevvalence from the Annual Population Survey (males, 27.5%) with the model-predicted No-NVP reduction (males, 7.3%), the implied NVP-attributable relative reduction in adult smoking prevalence was 20.2% (95% CI, 18.8%–22.0%) for males and 20.4% (18.7%–22.2%) for females. The NVP-attributable reduction was 27.2% (22.8%–31.6%) for males and 31.7% (27.4%–36.5%) for females ages 18–24 and 18.6% (15.2%–21.8%) for males and 15.0% (11.1%–18.8%) for females ages 25–34, with similar reductions for ages 35+. The implied reduction in smoking prevalence between 2012 and 2019 equates to 165660 (132453–199501) averted deaths by 2052. Other surveys yielded smaller, but relatively consistent results.
Conclusions
An indirect method of simulation modelling indicates that substantial reductions in smoking prevalence occurred in England from 2012–2019 coinciding with the growth in nicotine vaping product use.
Keywords: E-cigarettes, England, public health, simulation model, smoking, vaping
INTRODUCTION
Nicotine vaping products (NVPs) represent a new generation of nicotine delivery products, which have become progressively more efficient in delivering nicotine to the user [1–3]. NVPs first came onto the market in 2009, but only gained popularity in 2012 when third generation vaping devices became available [4,5]. Although NVP use (‘vaping’) has been found to help smokers to stop cigarette use (‘smoking’) [6–10], their impact on public health is more complex [11]. Vaping improves public health when used by those who would have otherwise initiated smoking or who would not have otherwise quit smoking and harms public health when used as a gateway to smoking or as a substitute for smoking cessation among those who would have stopped completely [11]. The population level impact of NVPs on smoking remains unknown.
Simulation modelling provides a virtual laboratory in which disparate sources of data can be combined to examine the effects of policies over time in complex social systems [12,13]. The SimSmoke tobacco control simulation model has been applied and validated for a wide range of countries [14–25] and was previously adapted to Great Britain [24]. Great Britain SimSmoke was used to estimate that increased cigarette taxes, strong smoke-free legislation, comprehensive cessation treatment, restricted tobacco marketing, limited youth access and strong health warnings implemented between 1998 and 2009 reduced smoking prevalence by 23% and would avert 168000 smoking-attributable deaths by 2040.
This study extends the previous Great Britain model to more recent years and considers the potential impact of NVPs on smoking prevalence and smoking-attributable deaths. Because tobacco control policies are more uniform in England, and because the Smoking Toolkit Study data for England enabled us to better validate the model and measure policy inputs, we focus on England rather than Great Britain. England also has been one of the more active tobacco control nations but has less restrictive policies toward NVPs than most other nations [26,27]. As such, England provides a useful case study of the potential effects of NVPs in a country with strong tobacco control policies [28].
The ability to model the impact of NVPs in England as well as for other countries depends on picking apart a complex set of interactions [11]. Short-term transitions involving NVPs must be distinguished from long-term transitions and smoking transitions that would have occurred in the absence of NVPs must be distinguished from those resulting from NVP use [11]. In addition, these transitions may vary over time, because of the uncertain impact of innovations in NVPs (i.e. their disruptive nature) and changing NVP regulations. Consequently, the ability to explicitly model the direct and indirect impact of NVPs on smoking prevalence is limited.
Rather than explicitly attempting to model NVP transitions, we have developed a novel, indirect method that does not involve explicitly modelling NVP use. To gauge the impact of NVPs on smoking, we first validated England SimSmoke over the period 2000–2012. The 2012 cut-off was chosen, because last 30-day use of NVPs increased from 3% of smokers and recent ex-smokers in 2011 to ~7% in 2012 and increased to ~18% in 2013 with the advent of third generation devices, and that level was maintained through 2019 [29]. The validated model is then used to project the smoking prevalence during the post-2012 period. Because the model does not incorporate NVPs, the post-2012 prediction of smoking rates serves as the No-NVP ‘counterfactual’ (i.e. projected smoking prevalence in the absence of NVPs). The impact of NVPs is then estimated by comparing observed post-2012 survey trends in smoking prevalence with model predictions, because the surveys reflect any impact of vaping on smoking trends. Thereby, we indirectly infer the impact of NVPs on smoking prevalence and resulting smoking-attributable deaths.
METHODS
Model overview
SimSmoke includes separate components for population, smoking, smoking-attributable death and tobacco control policies [13,30,31]. A discrete, first-order Markov process is used to project the population through deaths and immigration and to project smoking via initiation, cessation and relapse. Tobacco control policies reduce initiation and increase cessation.
A brief description of the model is presented below and in more depth in the Supplementary Data.
Population and smoking
Projected and actual population estimates by age and gender were obtained from the United Kingdom Office of National Statistics (ONS) [32]. We incorporated net immigration [32] and deaths [33], distinguished by smoking status as described below.
Data from the 2000 ONS Opinions and Lifestyle Survey (OPN) [34] were used to distinguish current, former and never smokers in 2000. Current smokers were defined as individuals who ‘smoke cigarettes at all nowadays’. Former smokers were defined as individuals who do not currently smoke but smoked regularly in the past. Never smokers have never regularly smoked cigarettes.
SimSmoke uses smoking prevalence rates in the initial model year to estimate initiation rates net of quitting, measured as the difference between smoking prevalence at a given age and the previous age. Because smoking rates increased until age 25 for males and age 19 for females, net initiation was applied through those ages. Cessation is incorporated from the last age of net initiation. Data on past-year quit rates by age groups were obtained from the 2000 OPN, measured as those who quit less or equal to one year, calculated as quit ≤1 year/(current smokers + quit ≤1 year). Because data on relapse were not available for England, age and gender-specific relapse rates by years since quit were based on the rates for United States (US) smokers [35–37].
Smoking-attributable deaths are based on the excess death risks of current and former smokers compared with never smokers. Relative risks of current and former smokers [38,39] are used to distinguish their corresponding deaths rates. The number of current smokers at each age is multiplied by the excess mortality risks (current smoker death rate minus never smoker death rate) and then summed over ages to obtain smoking attributable deaths for current smokers. The same procedure is applied to former smokers and summed with current smokers to obtain totals.
Policy effects
Policy effect sizes are in terms of percentage changes in prevalence, initiation and cessation rates as shown in Table 1 and described further in the Supplementary Data. They are generally applied to smoking prevalence in the year when the policy is implemented and applied to initiation and cessation rates in future years if the policy is sustained [34]. The effect of each newly implemented policy depends on the change from its previous level and is tracked from 2000–2019. Policy levels are based on MPOWER reports [40–44] and other policy reports [28,45]. The SimSmoke projections for males ages 16+ and policies are shown in Fig. 1.
Table 1.
Tobacco control policies, specifications and effect sizes applied in England SimSmoke.
| Policy | Description | Policy effect size |
|---|---|---|
| Cigarette excise taxes | ||
| Cigarette price/tax | The effect of taxes is directly incorporated through the average price after tax; the price elasticity is used to convert the price changes (%) into effect sizes | Elasticities |
| −0.4 for ages 14–17 | ||
| −0.3 for ages 18–24 | ||
| −0.2 for ages 25–34 | ||
| −0.1 for ages 35–64 | ||
| −0.2 for ages 65+ | ||
| Smoke-free air laws | ||
| Worksite smoking ban | Ban in all indoor worksites, with strong enforcement of laws (reduced by 1/3 if allowed in ventilated areas and by 2/3 if allowed in common areas) | −6% prevalence and initiation, +6% cessation |
| Restaurant smoking ban | Ban in all indoor restaurants (scaled for lower coverage), with strong enforcement of laws | −2% prevalence and initiation, +2% cessation |
| Pubs and bars smoking ban | Ban in all indoor in pubs and bars (scaled for lower coverage), with strong enforcement of laws | −1% prevalence and initiation, +1% cessation |
| Other place bans | Ban in 3 out of 4 government buildings (scaled for lower coverage), retail stores, public transportation and elevators, with strong enforcement of laws | −1% prevalence and initiation, +1% cessation |
| Enforcement and publicity | Government agency enforces the laws and publicity via tobacco control campaigns | Effects reduced 50% absent publicity and enforcement |
| Media campaigns | ||
| High level media campaign | Campaign publicized heavily with state and local programs with strong funding (>$0.50 USD) | −6.5% prevalence and initiation, +6.5% cessation |
| Medium level media campaign | Campaign publicized with funding of at least $0.10 USD per capita | −3.25% prevalence and initiation, +3.25% cessation |
| Low level media campaign | Campaign publicized only sporadically with minimal funding (< $0.10 USD per capita) | −1.63% prevalence and initiation, +1.63% cessation |
| Marketing restrictions | ||
| Comprehensive marketing ban | Ban on all forms of direct advertising including point of sale and indirect marketing | −5% prevalence, −8% initiation, +4% cessation |
| Moderate marketing ban | Ban on broadcast media, newspapers and billboards marketing and at least some indirect marketing (sponsorship, branding, giveaways) | −3% prevalence, −4% initiation, +2% cessation |
| Minimal marketing ban | Ban on broadcast media advertising | −1% prevalence and −1% initiation only |
| Enforcement | Government agency enforces the laws | Effects reduced 50% absent enforcement |
| Health warnings | ||
| Additional impact of plain packaging with strong health warnings | The outside of the package is drab, with brand and variant names appearing once on the front, top and bottom surfaces and no inserts | −2% prevalence, −2% initiation, +2% cessation |
| High health warnings | Labels are large, bold and graphic, and cover at least 50% of pack | −4% prevalence, −6% initiation, +10% cessation |
| Moderate health warnings | Laws cover at least 30% of package, not bold or graphic | −2% prevalence, −2% initiation, +4% cessation |
| Low health warnings | Laws cover <30% of package, not bold or graphic | −1% prevalence, −1% initiation, +2% cessation |
| Cessation treatment policies | ||
| Availability of pharmacotherapies | Legality of nicotine replacement therapy and/or bupropion and varenicline | −1% prevalence, +4% cessation |
| Cessation treatment financial coverage | Payments to cover pharmacotherapy and behavioural cessation treatment with high publicity (effect size reduced by 12.5% with moderate publicity and 18.75% with low publicity) | −2.25% prevalence, +8% cessation |
| Quit line | Three quit line types: passive, proactive and active with follow-up (effect size reduced by 1/3 if quit line is proactive, reduced by 2/3 if quit line passive) | −1% prevalence, +6% cessation |
| Brief interventions | Advice by health care provider to quit and methods provided | −1% prevalence, +6% cessation |
| All cessation policies combined | Complete availability and reimbursement of pharmaco- and behavioural treatments, quit lines and brief interventions | −5.68% prevalence, +29.4% cessation |
| Youth access policies | ||
| Strong enforcement and well publicized | Compliance checks conducted 4 times per year per outlet, penalties are potent and enforced with heavy publicity | −16% initiation and prevalence for ages 16–17 and −24% for ages 10–15 |
| Moderate enforcement with some publicity | Compliance checks conducted regularly, penalties are potent and publicity and merchant training are included | −8% initiation and prevalence ages 16–17 and −12% for ages 10–15 |
| Low enforcement | Compliance checks are conducted sporadically, penalties are weak | −2% initiation and prevalence ages 16–17 and −3% ages 10–15 |
Note: Unless otherwise indicated, the effects are in terms of the reduction in prevalence during the first year, the reduction in initiation and increase in quit rates during future years that the policy is in effect.
Figure 1.
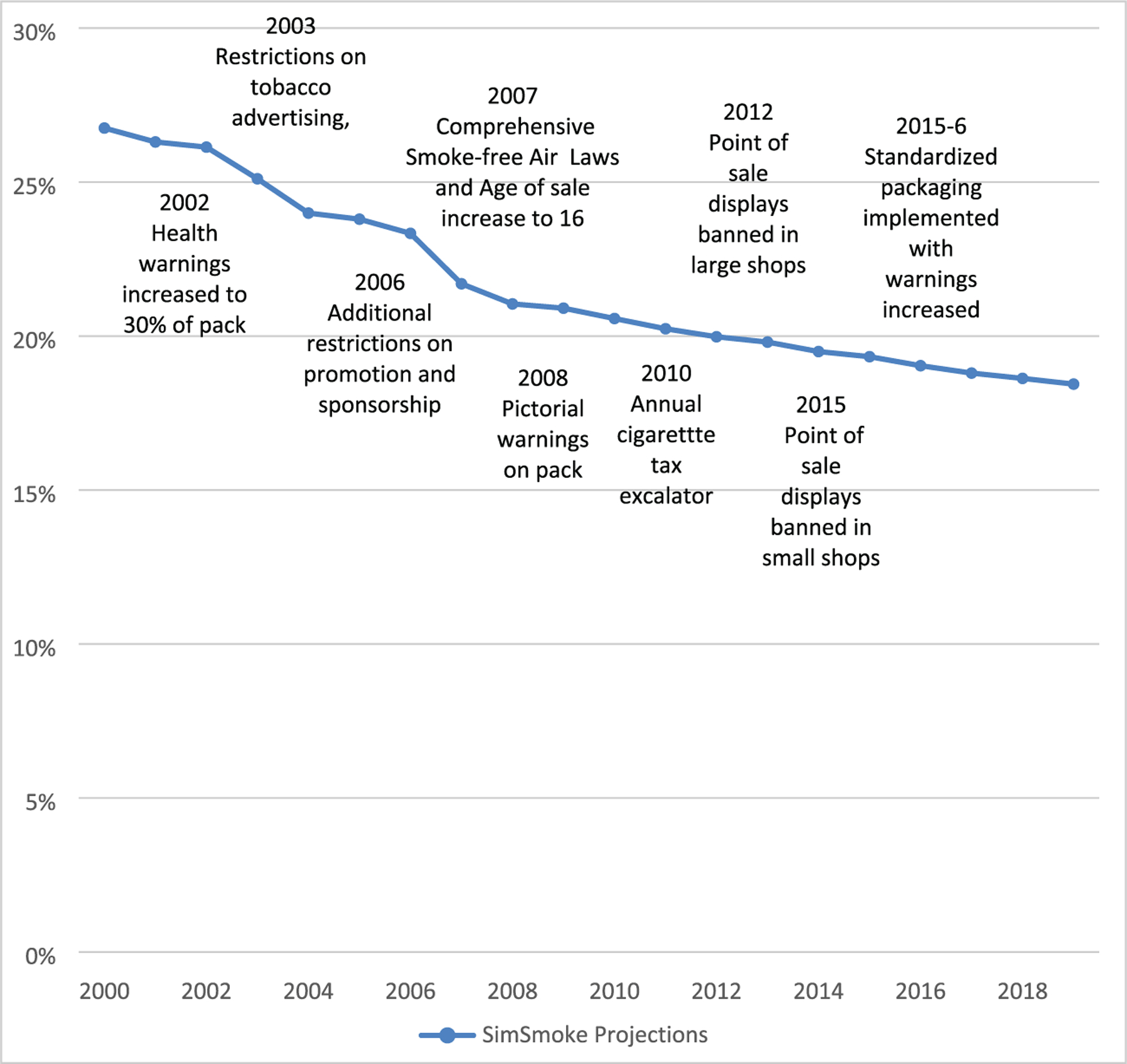
England SimSmoke projections and policies, males and females, ages 16+, 2000–2019.
Changes in cigarette price are translated into changes in smoking prevalence through an equation dependent on price elasticities that vary by age [46]. Cigarette prices and the consumer price index (CPI) for all household items for England for all years were obtained from ONS [47]. Because studies indicate substantial cost minimizing strategies (i.e. substituting to lower price brands) [48–51], we estimated prices actually paid by dividing average weekly smoking expenditures by consumption to obtain average spending per cigarette pack, using 2007–2019 data from the Smoking Toolkit Study (STS) [6,52,53]. Cigarette prices were adjusted by the CPI.
The smoke-free air laws include restriction on: (i) worksites; (ii) restaurants; (iii) pubs and bars; and (iv) other public places and incorporates enforcement. From weak initial laws in 2000–2006, comprehensive smoke-free legislation covering workplaces and almost all enclosed public places were enacted in England in 2007 [4], with enforcement set at the highest level.
Media campaigns were set at a low level in 2000, then increased to a moderate level in 2004 and maintained at that level, with more campaigns and media expenditures averaging ~7 million pounds since 2007 in most years [54].
Marketing restrictions include both direct (advertising) and indirect (sponsorship and branding). In 2000, England had restrictions on broadcast advertising considered a minimal marketing ban. In February 2003, the Tobacco Advertising and Promotion Act 2002 came into effect, making it illegal to advertise tobacco products on billboards, newspapers and magazines; it was considered a moderate ban. With restrictions on sponsorship and branding, the policy level was increased to a 50% moderate and 50% comprehensive marketing ban in 2006. With the display of tobacco products in large shops banned in 2012, the levels were increased to 20% moderate and 80% comprehensive marketing ban in 2012. This ban was extended to smaller shops in 2015 [45], considered 10% moderate and 90% comprehensive marketing ban (some forms of sponsorship were still permitted). Based on MPOWER reports, enforcement is set at the highest level.
Health warnings in England were considered at a 100% low level in 2000 (small text only), increasing to a 100% moderate level in 2004–2007 with warnings increased to 30% of the pack, and graphic warnings became mandatory in 2008 covering over 40% of the front and back; this was considered 10% moderate and 90% high level. In 2016, packs were required to have 65% of their principal display areas; this was considered 100% high level. Plain packaging was first introduced in May 2016 and implemented by May 2017.
Cessation treatment policies include pharmacotherapy (PT) availability, financial coverage of treatments, quit lines and health care provider brief intervention. Nicotine replacement therapy became available at general stores or pharmacies without prescription since 2001. Bupropion became available in 2000 and varenicline in 2006, both by prescription. Based on MPOWER reports, we set both behavioural and pharmacotherapy coverage at 25% (partial) in 2000, increasing to fully covered in 2003. The quit line is considered passive in 2000 and active with follow-up since 2001. Health care provider involvement, which includes asking about smoking, advising to quit and recommending effective cessation treatments, increased from 50% in 2000 to 75% in 2001, with the increased focus on cessation services.
Youth access policy includes enforcement and publicity. The minimum purchase age for tobacco was raised from 16 to 18 in England in 2007. Youth access is set to no policy until 2008 and then maintained at a low enforcement level from 2009 onward with publicity.
Calibration, validation and the impact of NVPs
SimSmoke estimates two primary outcomes: smoking prevalence and smoking-attributable deaths, both distinguished by age and gender. These outcomes are projected for the 2000–2019 observation period and for future years 2020 through 2052.
England SimSmoke was first calibrated by comparing the projected prevalence against reported smoking prevalence from OPN surveys through 2004, and then validated over the period through 2012. We compared the relative difference in smoking prevalence from 2000 to 2012 (e.g. (prevalence 2012 — prevalence 2000)/prevalence 2000), because 2000 levels vary across surveys. We also considered whether the 2012 model projections are within the 2012 survey 95% CI where available. The comparisons are made by gender for those ages 18+ and for age groups available for each survey.
Once validated, England SimSmoke was used to project the No-NVP counterfactual for 2012–2019 as described earlier. In developing the implied impact of NVPs, we compare the smoking prevalence from England SimSmoke (that does not incorporate NVP impact) to survey estimates. Because 2012 prevalence levels vary across surveys, we compare relative differences from 2012–2019 [i.e. (prevalence 2019 — prevalence 2012)/prevalence 2012]. To assess uncertainty, we apply the upper and lower bounds of the CI of the 2019 smoking prevalence from each survey. We, then, calculate the implied impact of NVPs by subtracting the relative change in smoking prevalence from 2012–2019 from SimSmoke to those from England surveys. Once obtaining the NVP-attributable reduction in smoking prevalence, we incorporated the individual effects of each survey back into SimSmoke as yearly adjustments in 2013–2019 to smoking prevalence (the NVP-adjusted SimSmoke) to estimate future outcomes by gender and age. The difference in smoking-attributable deaths between the NVP-adjusted SimSmoke and the No-NVP counterfactual is then used to estimate the implied impact of NVPs from 2012–2052. The analysis was not pre-registered, and the results should be considered exploratory.
We used three national surveys to validate England SimSmoke and estimate the impact of NVPs on smoking prevalence: 2000–2019 Opinions and Lifestyle Survey (OPN) [34], 2006–2019 Smoking Toolkit Study (STS) [55] and 2010–2019 Annual Population Survey (APS) [56]. We focus on the APS and STS survey to estimate the impact of NVPs on smoking-attributable deaths, because of the greater consistency of results across age groups and the larger sample size compared to OPN. In the Supplementary Data, we also applied the Health Survey for England.
RESULTS
Validation of smoking prevalence prediction from 2000–2012
The England SimSmoke gender- and age-specific projection and estimates for smoking prevalence from the three surveys are presented in Table 2. SimSmoke predicted a relative reduction in smoking prevalence for adult males (females) age 16+ of 23.7% (27.2%) from 2000–2012. Over this same time period, OPN showed similar relative reductions of 23.1% (28.4%). The STS was only available since 2007. Comparing over the years 2007–2012, STS showed greater relative reductions for males than SimSmoke from 2007–2012 (−8.4% vs. −4.7%), but the 2012 SimSmoke projection for males age 16+ were within the 2012 STS 95% CI. The 2012 SimSmoke male projection was also with the APS CI. Although the age 16 + 2012 projections for females were nearly identical to the STS and OPN estimates, the 18+ projections were outside the APS 95% CI for females.
Table 2.
Validation of England SimSmoke current smoking prevalence (%) predictions against three national surveysa by age and gender, 2000–2012.
| Age | Source | 2000 | 2007 | 2012 | Percent change 2000–2007 (%) |
Percent change 2007–2012 (%) |
Percent change 2000–2012 (%) |
|
|---|---|---|---|---|---|---|---|---|
| Males | ||||||||
| 16+ | SimSmoke | 28.6 | 23.5 | 21.8 | −17.9 | −4.7 | −23.7 | |
| OPN | 28.6 | 22.0 | 22.0 | −23.1 | −8.2 | −23.1 | ||
| STS | 25.6 | 22.1 | −8.4 | |||||
| 95% CI | (21.3, 22.9) | |||||||
| 18+ | SimSmoke | 28.8 | 23.7 | 22.0 | −17.6 | −4.7 | −23.5 | |
| APS | 21.8 | |||||||
| 95% CI | (21.4, 22.1) | |||||||
| 16–24 | SimSmoke | 33.1 | 26.7 | 24.8 | −19.1 | −4.4 | −24.9 | |
| OPN | 33.6 | 28.2 | 22.7 | −16.1 | −17.7 | −32.4 | ||
| STS | 31.8 | 24.3 | −14.3 | |||||
| 95% CI | (22.3, 26.3) | |||||||
| 18–24 | SimSmoke | 35.7 | 29.2 | 27.0 | −18.4 | −4.6 | −24.4 | |
| APS | 27.4 | |||||||
| 95% CI | (26.2, 28.6) | |||||||
| 25–34 | SimSmoke | 38.1 | 30.2 | 28.5 | −20.8 | −3.8 | −25.3 | |
| APS age | 28.8 | |||||||
| 95% CI | (27.8, 29.8) | |||||||
| 35–44 | SimSmoke | 32.0 | 27.0 | 26.3 | −15.5 | −2.6 | −17.8 | |
| APS age | 25.8 | |||||||
| 95% CI | (24.9, 26.6) | |||||||
| 25–44 | SimSmoke | 35.1 | 29.1 | 27.4 | −17.2 | −3.7 | −21.9 | |
| STS | 30.4 | 29.0 | −2.4 | |||||
| 95% CI | (27.5, 30.5) | |||||||
| 35–59 | SimSmoke | 29.4 | 25.4 | 23.6 | −13.7 | −4.5 | −19.7 | |
| OPN | 29.5 | 23.6 | 23.4 | −20.0 | −5.6 | −20.8 | ||
| 45–64 | SimSmoke | 26.4 | 22.7 | 21.5 | −13.8 | −3.8 | −18.3 | |
| STS | 24.9 | 20.7 | −11.2 | |||||
| 95% CI | (19.3, 22.1) | |||||||
| 45–54 | SimSmoke | 28.2 | 22.4 | 22.1 | −20.5 | −1.2 | −21.5 | |
| APS | 22.4 | |||||||
| 95% CI | (21.6, 23.1) | 95% CI | ||||||
| 55–64 | SimSmoke | 24.1 | 21.3 | 20.8 | −11.6 | −2.2 | −13.5 | |
| APS | 18.7 | |||||||
| 95% CI | (18.0, 19.5) | |||||||
| 45–64 | SimSmoke | 26.4 | 22.7 | 21.5 | −13.8 | −3.8 | −18.3 | |
| STS | 24.9 | 20.7 | −11.2 | |||||
| 95% CI | (19.3, 22.1) | |||||||
| 60+ | SimSmoke | 16.6 | 13.8 | 13.0 | −17.2 | −3.1 | −21.8 | |
| OPN | 15.9 | 12.3 | 13.0 | −22.6 | 1.6 | −18.2 | ||
| 65+ | SimSmoke | 14.5 | 10.7 | 10.3 | −26.4 | −4.0 | −29.2 | |
| STS | 10.9 | 9.3 | −7.3 | |||||
| 95% CI | (7.9, 10.6) | |||||||
| APS | 10.5 | |||||||
| 95% CI | (10.0, 11.0) | |||||||
| Females | ||||||||
| 16+ | SimSmoke | 24.9 | 19.9 | 18.1 | −20.1 | −5.8 | −27.2 | |
| OPN | 25.0 | 19.2 | 17.9 | −23.2 | −0.5 | −28.4 | ||
| STS | 22.8 | 18.1 | −15.0 | |||||
| 95% CI | (17.3, 18.8) | |||||||
| 18+ | SimSmoke | 24.9 | 19.9 | 18.2 | −19.9 | −5.8 | −27.0 | |
| APS | 17.0 | |||||||
| 95% CI | (16.7, 17.3) | |||||||
| 16–24 | SimSmoke | 31.5 | 25.6 | 23.6 | −18.6 | −4.8 | −25.0 | |
| OPN | 32.2 | 26.2 | 21.7 | −18.6 | −7.3 | −32.6 | ||
| STS | 33.8 | 22.2 | −19.8 | |||||
| 95% CI | (20.2, 24.3) | |||||||
| 18–24 | SimSmoke | 33.1 | 27.4 | 25.2 | −17.3 | −4.9 | −23.8 | |
| APS | 23.0 | |||||||
| 95% CI | (22.0, 24.1) | |||||||
| 25–34 | SimSmoke | 31.8 | 25.9 | 24.6 | −18.5 | −3.6 | −22.7 | |
| APS | 20.7 | |||||||
| 95% CI | (19.9, 21.5) | |||||||
| 35–44 | SimSmoke | 28.2 | 23.5 | 21.5 | −16.6 | −5.4 | −24.0 | |
| APS | 19.0 | |||||||
| 95% CI | (18.3, 19.7) | |||||||
| 25–44 | SimSmoke | 30.0 | 24.6 | 23.0 | −18.1 | −4.4 | −23.5 | |
| STS | 26.1 | 20.6 | −15.7 | |||||
| 95% CI | (19.2, 21.9) | |||||||
| 35–59 | SimSmoke | 26.1 | 21.5 | 19.7 | −17.7 | −5.3 | −24.5 | |
| OPN | 26.4 | 21.2 | 19.4 | −20.0 | −4.5 | −26.6 | ||
| 45–54 | SimSmoke | 24.9 | 20.5 | 19.1 | −17.4 | −4.7 | −23.1 | |
| APS | 18.7 | |||||||
| 95% CI | (18.0, 19.3) | |||||||
| 55–64 | SimSmoke | 22.3 | 17.2 | 15.7 | −22.9 | −6.5 | −29.7 | |
| APS | 16.4 | |||||||
| 95% CI | (15.8, 17.1) | |||||||
| 45–64 | SimSmoke | 23.7 | 18.9 | 17.6 | −20.2 | −5.2 | −25.9 | |
| STS | 21.8 | 18.3 | −10.8 | |||||
| 95% CI | (17.0, 19.6) | |||||||
| 60+ | SimSmoke | 15.8 | 11.7 | 10.2 | −26.4 | −8.1 | −35.5 | |
| OPN | 15.2 | 11.3 | 11.7 | −25.7 | 12.4 | −23.0 | ||
| 65+ | SimSmoke | 14.3 | 10.2 | 8.9 | −28.8 | −9.0 | −37.8 | |
| STS | 11.3 | 10.9 | −12.1 | |||||
| 95% CI | (9.6, 12.2) | |||||||
| APS | 9.0 | |||||||
| 95% CI | (8.5, 9.4) | |||||||
OPN = Opinion and Lifestyle Survey conducted by UK Office of National Statistics, which defines smokers as those who smoke at all nowadays; STS = Smoking Toolkit Study conducted by the British Market Research Bureau, which defines smokers as those who smoke cigarettes every day or not every day; APS = Annual Population Survey conducted by UK Office of National Statistics, which defines smokers as those who smoke at all nowadays.
Data from surveys are compared to England SimSmoke by matching age groups. For unmatched age groups (e.g. age 16+ and age 18+), the SimSmoke projections for two age groups are provided.
SimSmoke also generally performed well in predicting smoking prevalence for most age groups. For males, all 2012 projections were within or close (within 0.1%) of the 2012 CI, except males age groups 55-64 and 65+ using the APS. For females, the projections were within STS CI except for ages 25–44 and 65+, and for the APS age groups below age 45.
Impact of NVPs on smoking prevalence relative to a No-NVP counterfactual
To estimate the potential impact of NVPs, we compared the relative reductions in smoking prevalence over the period 2012–2019 from SimSmoke to survey estimates. The results with 95% CI shown in parentheses are presented in Table 3.
Table 3.
Smoking prevalence (%) predictions from unadjusted England SimSmoke model compared to three national surveys, by age group and gender, 2012–2019.
| Age | Sourcea | 2012 | 2019 | Relative reduction in 2012–2019 (%) | Difference from SimSmoke 2012–2019 (%) |
|---|---|---|---|---|---|
| Males | |||||
| 16+ | SimSmoke | 21.8 | 20.2 | 7.2 | |
| OPN | 22.0 | 17.3 | 21.4 | 14.2 | |
| 95% CI | (15.2, 19.5) | (11.4, 30.9) | (4.2, 23.7) | ||
| STS | 22.1 | 16.7 | 24.4 | 17.2 | |
| 95% CI | (21.3, 22.9) | (16.0, 17.4) | (21.1, 27.7) | (14.0, 20.5) | |
| 18+ | SimSmoke | 22.0 | 20.4 | 7.3 | |
| APS | 21.8 | 15.8 | 27.5 | 20.2 | |
| 95% CI | (21.4, 22.1) | (15.4, 16.1) | (26.1, 29.4) | (18.8, 22.0) | |
| 16–24 | SimSmoke | 24.8 | 23.1 | 6.8 | |
| OPN | 22.7 | 22.1 | 2.6 | −4.1 | |
| 95% CI | (12.9, 31.3) | (−37.9, 43.2) | (−44.7, 36.4) | ||
| STS | 24.3 | 19.9 | 18.0 | 11.2 | |
| 95% CI | (22.3, 26.3) | (17.9, 22.0) | (9.5, 26.5) | (2.7, 19.7) | |
| 18–24 | SimSmoke | 27.0 | 25.1 | 7.1 | |
| APS | 27.4 | 18.0 | 34.3 | 27.2 | |
| 95% CI | (26.2, 28.6) | (16.8, 19.2) | (29.9, 38.7) | (22.8, 31.6) | |
| 25–34 | SimSmoke | 28.5 | 26.6 | 6.7 | |
| OPN | 31.6 | 24.7 | 21.8 | 15.1 | |
| 95% C | (16.6, 32.8) | (−3.8, 47.5) | (−10.5, 40.8) | ||
| I APS | 28.8 | 21.5 | 25.3 | 18.6 | |
| 95% CI | (27.8, 29.8) | (20.6, 22.5) | (21.9, 28.5) | (15.2, 21.8) | |
| 35–44 | SimSmoke | 26.3 | 24.6 | 6.6 | |
| APS | 25.8 | 18.2 | 29.5 | 22.9 | |
| 95% CI | (24.9, 26.6) | (17.3, 19.0) | (26.4, 32.9) | (19.8, 26.4) | |
| 25–44 | SimSmoke | 27.4 | 25.7 | 6.3 | |
| STS | 29.0 | 21.3 | 26.5 | 20.2 | |
| 95% CI | (27.5, 30.5) | (19.9, 22.7) | (21.7, 31.3) | (15.4, 25.0) | |
| 35–49 | SimSmoke | 25.3 | 24.0 | 5.1 | |
| OPN | 24.3 | 17.2 | 29.2 | 24.1 | |
| 95% CI | (12.6, 21.7) | (10.7, 48.1) | (5.6, 43.1) | ||
| 35–59 | SimSmoke | 23.6 | 22.3 | 5.5 | |
| OPN | 23.4 | 18.2 | 22.2 | 16.7 | |
| 45–54 | SimSmoke | 22.1 | 22.0 | 0.7 | |
| APS | 22.4 | 17.3 | 22.8 | 22.0 | |
| 95% CI | (21.6, 23.1) | (16.5, 18.1) | (19.2, 26.3) | (18.4, 25.6) | |
| 55–64 | SimSmoke | 20.8 | 18.8 | 9.4 | |
| APS | 18.7 | 14.1 | 24.6 | 15.2 | |
| 95% CI | (18.0, 19.5) | (13.4, 14.8) | (20.9, 28.3) | (11.4, 18.9) | |
| 45–64 | SimSmoke | 21.5 | 20.5 | 4.8 | |
| STS | 20.7 | 16.0 | 22.8 | 18.0 | |
| 95% CI | (19.3, 22.1) | (14.7, 17.3) | (16.7, 28.9) | (11.9, 24.1) | |
| 50–59 | SimSmoke | 20.7 | 20.0 | 3.5 | |
| OPN | 21.9 | 19.7 | 10.0 | 6.6 | |
| 95% CI | (14.6, 24.9) | (−13.7, 33.3) | (−17.2, 29.9) | ||
| 60+ | SimSmoke | 13.0 | 11.8 | 9.2 | |
| OPN | 13.0 | 9.5 | 26.9 | 17.7 | |
| 95% CI | (7.5, 11.4) | (12.3, 42.3) | (3.1, 33.1) | ||
| 65+ | SimSmoke | 10.3 | 9.6 | 6.4 | |
| STS | 9.3 | 8.3 | 10.8 | 4.4 | |
| 95% CI | (7.9, 10.6) | (7.1, 9.4) | (−1.9, 23.5) | (−8.3, 17.1) | |
| APS | 10.5 | 8.4 | 20.0 | 13.6 | |
| 95% CI | (10.0, 11.0) | (7.9, 8.9) | (15.2, 24.8) | (8.8, 18.3) | |
| Females | |||||
| 16+ | SimSmoke | 18.1 | 16.6 | 8.3 | |
| OPN | 17.9 | 13.8 | 22.9 | 14.6 | |
| 95% CI | (11.9, 15.7) | (12.3, 33.5) | (4.0, 25.2) | ||
| STS | 18.1 | 14.2 | 21.1 | 12.8 | |
| 95% CI | (17.3, 18.8) | (13.6, 14.9) | (17.4, 24.8) | (9.1, 16.5) | |
| 18+ | SimSmoke | 18.2 | 16.6 | 8.4 | |
| APS | 17.0 | 12.1 | 28.8 | 20.4 | |
| 95% CI | (16.7, 17.3) | (11.8, 12.4) | (27.1, 30.6) | (18.7, 22.2) | |
| 16–24 | SimSmoke | 23.6 | 22.0 | 7.0 | |
| OPN | 21.7 | 16.2 | 25.3 | 18.4 | |
| 95% CI | (8.4, 24.1) | (−11.1, 61.3) | (−18.0, 54.3) | ||
| STS | 22.2 | 15.5 | 30.5 | 23.5 | |
| 95% CI | (20.2, 24.3) | (13.5, 17.4) | (21.8, 39.2) | (14.8, 32.3) | |
| 18–24 | SimSmoke | 25.2 | 23.4 | 7.4 | |
| APS | 23.0 | 14.0 | 39.1 | 31.7 | |
| 95% CI | (22.0, 24.1) | (12.9, 15.0) | (34.8, 43.9) | (27.4, 36.5) | |
| 25–34 | SimSmoke | 24.6 | 22.9 | 6.8 | |
| OPN | 21.8 | 21.2 | 2.8 | −4.0 | |
| 95% CI | (14.4, 28.0) | (−28.4, 33.9) | (−35.2, 27.2) | ||
| APS | 20.7 | 16.2 | 21.7 | 15.0 | |
| 95% CI | (19.9, 21.5) | (15.4, 17.0) | (17.9, 25.6) | (11.1, 18.8) | |
| 35–44 | SimSmoke | 21.5 | 19.9 | 7.2 | |
| APS | 19.0 | 12.7 | 33.2 | 26.0 | |
| 95% CI | (18.3, 19.7) | (12.0, 13.3) | (30.0, 36.8) | (22.8, 29.7) | |
| 25–44 | SimSmoke | 23.0 | 21.5 | 6.3 | |
| STS | 20.6 | 18.2 | 5.4 | ||
| 95% CI | (19.2, 21.9) | (16.9, 19.5) | (5.3, 18.0) | (−1.0, 11.7) | |
| 35–49 | SimSmoke | 20.8 | 19.4 | 6.9 | |
| OPN | 20.6 | 13.8 | 33.0 | 26.1 | |
| 95% CI | (9.2, 18.4) | (10.7, 55.3) | (3.8, 48.5) | ||
| 45–54 | SimSmoke | 19.1 | 18.0 | 5.8 | |
| APS | 18.7 | 13.9 | 25.7 | 19.8 | |
| 95% CI | (18.0, 19.3) | (13.2, 14.5) | (22.5, 29.4) | (16.6, 23.6) | |
| 55–64 | SimSmoke | 15.7 | 14.7 | 6.1 | |
| APS | 16.4 | 12.7 | 22.6 | 16.5 | |
| 95% CI | (15.8, 17.1) | (12.0, 13.3) | (18.9, 26.8) | (12.8, 20.8) | |
| 45–64 | SimSmoke | 17.6 | 16.5 | 6.4 | |
| STS | 18.3 | 14.7 | 19.8 | 13.4 | |
| 95% CI | (17.0, 19.6) | (13.5, 15.9) | (13.1, 26.4) | (6.8, 20.0) | |
| 50–59 | SimSmoke | 17.7 | 16.6 | 6.5 | |
| OPN | 17.4 | 14.0 | 19.5 | 13.1 | |
| 95% CI | (9.9, 18.1) | (−4.0, 43.1) | (−10.5, 36.6) | ||
| 60+ | SimSmoke | 10.2 | 9.2 | 10.4 | |
| OPN | 11.7 | 8.6 | 26.5 | 16.1 | |
| 95% CI | (7.0, 10.1) | (13.7, 40.2) | (3.3, 29.8) | ||
| 65+ | SimSmoke | 8.9 | 7.9 | 11.7 | |
| STS | 10.9 | 7.8 | 28.6 | 16.9 | |
| 95% CI | (9.6, 12.2) | (6.7, 8.8) | (19.0, 38.2) | (7.3, 26.5) | |
| APS | 9.0 | 6.6 | 26.7 | 15.0 | |
| 95% CI | (8.5, 9.4) | (6.2, 7.0) | (22.2, 31.1) | (10.6, 19.4) | |
OPN = Opinion and Lifestyle Survey conducted by UK Office of National Statistics (ONS), which defines smokers as those who smoke at all nowadays; STS = Smoking Toolkit Study conducted by the British Market Research Bureau, which defines smokers as those who smoke cigarettes (including hand-rolled) every day or not every day; APS = Annual Population Survey conducted by UK Office of National Statistics, which defines smokers as those who smoke at all nowadays.
APS age 25–44, 45–64 and ONS 35–59 age are combined from smaller age groups (35–34, 35–44, 45–54, 55–64, 35–49, and 50–59), and no 95% CI is available for them.
For ages 18+, APS showed relative reductions in smoking prevalence of 27.5% (95% CI = 26.1%–29.4%) for males and 28.8% (27.1%–30.6%) for females, which implies NVP-attributable reductions of 20.2% (18.8%–22.0%) and 20.4% (18.4%–22.2%) compared to SimSmoke. For ages 16+, OPN indicated NVP-attributable reductions of 14.2% (4.2%–23.7%) for males and 14.6% (4.0%–25.2%) for females compared to 17.2% (14.0%–20.5%) for males and 12.8% (9.1%–16.5%) for females using STS. The 2019 No-NVP adult projections were also outside the 95% CI of all surveys.
The No-NVP projections were outside the 95% CI for all age groups using the APS and STS, except for females age 65+. We also considered NVP-attributable differences in smoking prevalence across age groups. For ages 18–24, APS NVP-attributable reductions in smoking prevalence were 27.2% (22.8%–31.6%) for males and 31.7% (27.4%–36.5%) for females, compared to 11.2% (2.7%–19.7%) for males and 3.5% (14.8%–32.3%) for females from STS ages 16–24. For ages 25–44, STS implied NVP-attributable reductions of 20.2% (15.4%–25.0%) for males and 5.4% (−1.0%–11.7%) for females from STS, whereas APS implied NVP-attributable reductions of 18.6% (15.2%–21.8%) and 15.0% (11.1%–18.8%) for females ages 25–34 and of 22.9% (19.8%–26.4%) for males and 26.0% (22.8%–29.7%) for females ages 35–44. For ages 45–64, STS implied NVP-attributable reductions of 18.0% (11.9%–24.1%) for males and 13.4% (6.8%–20.0%) for females, whereas APS implied NVP-attributable reductions of 22.0% (18.4%–25.6%) for males and 19.8% (16.6%–23.6%) for females ages 45–54 and of 15.2% (11.4%–18.9%) for males and 16.5% (12.8%–20.8%) for females ages 55–64. For ages 65+, APS yielded a reduction of 13.6% (8.8%–18.3%) for males and 15.0% (10.6%–19.4%) for females, compared to 4.4% (−8.3%–17.1%) for males and 16.9% (7.3%–26.5%) for females from STS. OPN reported wider CI for comparable age categories.
Impact of NVP use during 2012–2019 on 2012–2052 smoking-attributable deaths
The number of smoking-attributable deaths in the No-NVP scenario and the NVP-adjusted scenarios and the implied impact of vaping on smoking-attributable deaths are shown in Table 4. In 2019, SimSmoke with the APS adjustment for NVPs projected 26315 (25769–26852) male and 15909 (15547–16252) female smoking-attributable deaths compared to 28675 for males and 17331 for females in the No-NVP (unadjusted) scenario, resulting in 2360 (1813–2906) male and 1423 (1079–1784) female deaths averted. Cumulatively, over the period 2012–2052, the APS-adjusted model estimated 107238 (86501–127621) male and 58422 (45952–71879) female deaths averted. Using the STS-adjusted NVP impact for 2012–2052, SimSmoke estimated 87102 (49671–125353) male and 41516 (17143–66326) female adding to a total of 128 617 (66814–191679) deaths averted.
Table 4.
Projected smoking-attributable deaths and deaths averted, unadjusted England SimSmoke compared to STS and APS NVP-adjusted England SimSmoke, by gender, 2012–2052.
| Gender | Measurea | Adjustment | 2012 | 2019 | 2032 | 2052 | Total 2012–2019 | Total 2012–2052 |
|---|---|---|---|---|---|---|---|---|
| Male | Smoking-attributable deaths | Unadjustedb | 27659 | 28675 | 27968 | 23111 | 226162 | 1103472 |
| STS-adjusted | 27659 | 27048 | 25385 | 20757 | 219734 | 1016370 | ||
| 95% CI | – | (25838, 28243) | (24287, 26460) | (19950, 21549) | (214522, 224731) | (978119, 1053801) | ||
| APS-adjusted | 27659 | 26315 | 24841 | 20275 | 216540 | 996235 | ||
| 95% CI | – | (25769, 26862) | (24251, 25438) | (19790, 20778) | (214191, 218863) | (975851, 1016971) | ||
| Deaths averteda | STS-adjusted | – | 1627 | 2582 | 2354 | 6429 | 87102 | |
| 95% CI | – | (432, 2837) | (1508, 3681) | (1561, 3161) | (1431, 11640) | (49671, 125353) | ||
| APS-adjusted | – | 2360 | 3127 | 2836 | 9622 | 107238 | ||
| 95% CI | – | (1813, 2906) | (2530, 3716) | (2332, 3320) | (7299, 11971) | (86501, 127621) | ||
| Female | Smoking-attributable deaths | Unadjustedb | 18136 | 17331 | 16665 | 14738 | 141957 | 674943 |
| STS-adjusted | 18136 | 16047 | 15458 | 13843 | 136532 | 633428 | ||
| 95% CI | – | (15318, 16768) | (14741, 16163) | (13280, 14398) | (133323, 139609) | (608618, 657800) | ||
| APS-adjusted | 18136 | 15909 | 15001 | 13180 | 136049 | 616521 | ||
| 95% CI | – | (15547, 16252) | 14613, 15359) | (12849, 13485) | (134481, 137525) | (603064, 628991) | ||
| Deaths averteda | STS-adjusted | – | 1284 | 1207 | 895 | 5425 | 41516 | |
| 95% CI | – | (563, 2013) | (503, 1924) | (339, 1457) | (2348, 8633) | (17143, 66326) | ||
| APS-adjusted | – | 1423 | 1665 | 1558 | 5907 | 58422 | ||
| 95% CI | – | (1079, 1784) | (1306, 2052) | (1252, 1888) | (4432, 7475) | (45952, 71879) | ||
| Both genders | Smoking-attributable deaths | Unadjusted | 45795 | 46006 | 44633 | 37848 | 368119 | 1778416 |
| STS-adjusted | 45795 | 43095 | 40843 | 34599 | 356265 | 1649798 | ||
| 95% CI | – | (41156, 45011) | (39028, 42622) | (33230, 35948) | (347845, 364340) | (1586737, 1711601) | ||
| APS-adjusted | 45795 | 42223 | 39841 | 33455 | 352589 | 1612756 | ||
| 95% CI | – | (41316, 43115) | (38865, 40797) | (32640, 34264) | (348672, 356388) | (1578915, 1645962) | ||
| Deaths averteda | STS-adjusted | – | 2911 | 3790 | 3249 | 11853 | 128617 | |
| 95% CI | – | (995, 4851) | (2011, 5605) | (1901, 4619) | (3779, 20274) | (66814, 191679) | ||
| APS-adjusted | – | 3783 | 4792 | 4394 | 15530 | 165660 | ||
| 95% CI | – | (2891, 4690) | (3836, 5768) | (3585, 5209) | (11731, 19447) | (132453, 199501) |
Deaths averted is calculated as the difference between the smoking-attributable deaths in the APS and STS NVP-adjusted model and the No-NVP model. The lower (upper) bound of those estimates is determined by the upper (lower) bound of the smoking-attributed deaths of the APS and STS NVP-adjusted projection, i.e. the more smoking-attributable deaths in the NVP-adjusted projection (corresponding to the lower bound in the prevalence estimates), the fewer lives saved.
’Unadjusted’ refers to the SimSmoke model without NVP adjustment in 2012–2019 and APS and STS adjustments refer NVP-adjusted projections using the 2012–2019 relative reductions based on comparison of the SimSmoke model projections with the APS and STS surveys. 95% CI refers to the implementation of APS and STS adjustments using the annual relative difference in 2012–2019 derived from the lower and upper bound of the 95% CI from survey in 2019. STS = Smoking Toolkit Study; OPN = Opinion and Lifestyle Survey; APS = Annual Population Survey.
DISCUSSION
Because of the inherent uncertainty in ascertaining transitions to and from smoking involving NVP use, we have developed a novel, indirect method for gauging the impact of NVPs. England SimSmoke validated well through the year 2012, just before NVP use became more widespread. By comparing the projected trends in smoking from 2012–2019 (the No-NVP counterfactual) to actual trends from three different surveys, we indirectly inferred the potential effects of NVPs on cigarette use. Based on this methodology, we estimated an implied NVP-attributable reduction in adult (18+) smoking prevalence of ~20.2% for males and 20.4% for females using the APS, the largest survey. The implied reductions were larger for the 18–24 age group, but otherwise relatively consistent across age groups.
The results indicate that NVPs played an important role in reducing smoking prevalence in England in 2012–2019. Other studies have found significant impacts of NVPs on smoking cessation [52,57–63] and initiation [64] in England. Based on a time-series analysis with a 34.3% quit attempt rate of which 35.2% used e-cigarettes with a 6% increase in quit success rate and 5.4% increase in overall quit rate, Beard et al. [52] estimated between 0.7% (34.3% × 35.2% × 6%) and 1% (18.5% smokers × 5.4% quit rate per smoker) of smokers additionally quit as a consequence of e-cigarette use in 2017, similar to an earlier estimate [59]. The Beard et al. [52] rates are lower than our annual estimated annual reduction of ~3% from APS and 2% from STS, but are based on data for 2017 before the relatively larger smoking reduction observed in 2018 and 2019 and do not incorporate any impact of NVP use on initiation, long-term relapse and quitting by other smokers. For example, contact with individuals using NVPs was found to increase the likelihood of smoker quit attempts and quit success [65].
We also estimated the impact of the 2012–2019 NVP-attributable reduction in smoking prevalence on smoking-attributable deaths. Based on the APS estimates, we projected 166000 fewer smoking-attributable deaths from 2012–2052. Although some of the reduction in smoking-attributable deaths will be offset by NVP-attributable deaths, the mortality risks of exclusive NVP use are expected to be substantially less than for smokers [66–68]. In addition, our estimates are only for the reduction in smoking prevalence inferred for NVP use during the years 2012–2019. Additional smoking-attributable deaths would be averted from any NVP-induced reduction in smoking after 2019, although those who had previously switched to NVPs or quit all use may also relapse back to smoking.
Our results are subject to limitations. The results depend on the assumptions built into the model and the data used. The impact of NVPs is inferred based on the England SimSmoke projection of smoking prevalence in the absence of NVP but controlling for the impact of new and previously implemented tobacco control policies. This method assumes that vaping was the only factor not modelled that would have substantially influenced smoking prevalence trends. However, the inferred impact of NVPs may also be because of other factors not incorporated into the model, such as changes in the effectiveness of policies, cigarette companies’ reactions to policies or changes in attitude toward risks as reflected by changes in alcohol consumption [69,70]. Nevertheless, England SimSmoke was validated for years 2000–2012 and generally performed well. In addition, SimSmoke [14–25] has generally been well-validated for other countries that have implemented a wide range of policies.
The results also depend on a particular set of policy effect sizes that define the magnitude and time pattern of policy impacts. Over the post-2012 period (when NVPs became more prominent), the only substantial changes in tobacco control policy were cigarette prices, marketing restrictions and packaging, which were modelled. Using upper and lower bounds for policy effects (±50% of the policy effect, except ±25% for taxes) based on a literature review [71], we applied these bounds to policy changes in SimSmoke projections over the time period 2012–2019. The relative reduction in the adult smoking prevalence was 7.3% (6.5%–8.1%) for males and 8.4% (7.5%–9.2%) for females. Therefore, the uncertainty regarding policy changes for 2012–2019 was found to contribute to only an 0.8% absolute variation in the male and female SimSmoke projections for 2019, therefore having a relatively minor influence on the projected net impact of NVPs. For example, based on the APS, the implied NVP impact for males is 20.2%, therefore implying 4% (0.8%/20.2%) of the variation. The effects were greatest for price, which alone contributed to 0.6% of the 0.8% deviation.
We also considered different measures of policy levels. When we used an ONS cigarette price index (based on retail prices) instead of STS prices (based on prices paid) during 2007–2019, the inferred impact of NVPs on smoking prevalence was reduced in absolute terms by 4.1% (approximately half of the 7.3%–8.4% relative reduction because of policies) in 2012–2019. In addition, two studies [72,73] indicate that media campaigns were substantially reduced in 2010 (to what would be considered a low level). When we denoted a low instead of a moderate level media campaign in 2010–2019, the inferred impact of NVPs increased in absolute terms by 0.5% by 2019, therefore implying a larger impact of NVPs on smoking.
Although we attribute a relatively small impact of the recent decline smoking prevalence to policies, strong cigarette-oriented policies in England both before and after 2012 may have played a major role by enhancing the impact of NVPs. In examining trends in smoking prevalence relative to a scenario where policies are maintained at 2000 levels, England SimSmoke projects that smoking prevalence had been reduced by ~29% between 2000 and 2019 because of policies. Conducting the same analysis for the period 2012–2019, only 36% (2.7%/ %) of male and 33% (2.7%/8.4%) of the female relative reduction in smoking prevalence was attributable to policies. However, the effect of past and newly implemented cigarette-oriented policies may have been enhanced by the availability of NVPs, because smokers had a potentially viable alternative to cigarettes. For example, NVPs have been found to be a substitute for cigarettes in demand studies [74–77], and NVPs have been used in England as an alternative by those having failed with traditional cessation treatments [6–9,78,79]. Therefore, by providing a viable substitute for smoking, part of the impact of NVPs may be the indirect impact of making past and newly implemented cigarette-oriented policies more effective.
Another limitation is that the NVP-attributable impacts depend of the accuracy of estimates from the surveys. Because the estimates of prevalence for a given year vary considerably among the surveys and in comparison to SimSmoke projections, we focused on relative reductions in smoking prevalence (i.e. relative to initial prevalence levels) from SimSmoke and from surveys. However, the relative reductions also varied substantially from survey to survey, therefore providing an indication of the uncertainty in our results. We also conducted a sensitivity analysis based on the 95% CI of the 2019 surveys estimates to further indicate the uncertainty in our estimates. We note that these estimates imply greater uncertainty for specific age groups, especially those at younger ages. Further, our validation in some cases depended on the year chosen for some surveys. When we examined the sensitivity of results to the initial and final year chosen for examining NVP-attributable impacts (not discussed above), we obtained similar results using the years 2011, 2012 and 2013 as the initial projection years, but results were more sensitive to the choice of the final projection year. For example, the STS male (female) smoking prevalence showed a relative reduction of 24% (21%) for 2012–2019 compared to a reduction of 15% (13%) for 2012–2018 reduction.
In conclusion, England provides a valuable case study because it already had strong tobacco control policies directed at smoking. Yet, our analysis indicates substantial reductions in smoking prevalence associated with NVP use observed for both genders and across all age groups. Whereas our model does not distinguish the role of NVP-oriented from cigarette-oriented policies, the impact of NVPs may have been greater because of the strong cigarette-oriented policies working in tandem with relatively extensive, but proportionate NVP policies. Further research using models that explicitly incorporate NVP use and the resulting transitions to and from cigarette use and studies evaluating the impact of cigarette-oriented vis-a-vis NVP-oriented policies can shed further light on the public health impact of NVPs. However, as new models that include NVPs transitions are developed, it will be important to compare the results from the different models to develop a better understanding of the impact of NVPs.
Supplementary Material
Table S1 Tobacco control policies, specifications and effect sizes applied in England SimSmoke.
Table S2 Validation of England SimSmoke current smoking prevalence predictions against national surveys, by age and gender, 2000–2012.
Table S3a Smoking prevalence and smoking attributable deaths projected by the unadjusted SimSmoke model under individual policy scenarios by gender, 2000–2040.
Table S3b Smoking prevalence projected by the No-NVP SimSmoke model under multiple policy scenarios by gender in 2012–2019.
Table S4a Sensitivity analysis of smoking prevalence (%) at age 18+ by gender under multiple policy effects sizes in 2012–2019.
Table S4b Sensitivity analysis of smoking prevalence (%) at age 18+ by gender under multiple policy effects sizes only in the marginal change of the policies in 2012–2019.
Table S4c Sensitivity analysis of smoking prevalence (%) at age 18+ by gender under different policy specifications for price and media campaigns.
Table S5 Smoking prevalence predictions from unadjusted SimSmoke model compared to national surveys, by age group and gender, 2012–2018/19.
Table S6 Projected smoking prevalence (%), No-NVP SimSmoke versus NVP SimSmoke, age 16+ by gender, 2012–2052.
Table S7 Projected smoking-attributable deaths and lives saved, unadjusted England SimSmoke compared to STS NVP-adjusted and APS NVP-adjusted England SimSmoke, by gender, 2012–2052.
Acknowledgements
We would like to thank Emma Beard from providing data from the STS and Robert West for comments on earlier work. D.T.L., L.M.S., Y.L. and Y.Z. received funding through P01 grant (P01CA200512) from the Division of Cancer Prevention, National Cancer Institute, US National Institutes of Health. A.M. also received funding through P01 grant (P01CA200512) and is a National Institute for Health Research (NIHR) Senior Investigator. The views expressed in this article are those of the authors and not necessarily those of the NIHR, or the Department of Health and Social Care. The STS is currently primarily funded by Cancer Research UK (C1417/A14135). J.B. and A.M. are members of SPECTRUM, a UK Prevention Research Partnership Consortium (MR/S037519/1). UKPRP is an initiative funded by the UK Research and Innovation Councils, the Department of Health and Social Care (England) and the UK devolved administrations, and leading health research charities.
Footnotes
Declaration of Interest
JB has received unrestricted research funding to study smoking cessation from companies who manufacture smoking cessation medications. JB declares no financial links with tobacco companies or e-cigarette manufacturers or their representatives. Other declare no conflict of interest.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.Farsalinos KE, Spyrou A, Tsimopoulou K, Stefopoulos C, Romagna G, Voudris V Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Sci Rep 2014; 4: 4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagener TL, Floyd EL, Stepanov I, Driskill LM, Frank SG, Meier E, et al. Have combustible cigarettes met their match? The nicotine delivery profiles and harmful constituent exposures of second-generation and third-generation electronic cigarette users. Tob Control 2017; 26: e23–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glasser AM, Collins L, Pearson JL, Abudayyeh H, Niaura RS, Abrams DB, et al. Overview of electronic nicotine delivery systems: a systematic review. Am J Prev Med 2017; 52: e33–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy DT, Yuan Z, Li Y, Mays D, Sanchez-Romero LM An examination of the variation in estimates of E-cigarette prevalence among U.S. adults. Int J Environ Res Public Health 2019Aug 30; 16: pii: E3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marynak KL, Gammon DG, King BA, et al. National and state trends in sales of cigarettes and E-cigarettes, U.S., 2011-2015. Am J Prev Med 2017; 3: 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beard E, West R, Michie S, Brown J Association between electronic cigarette use and changes in quit attempts, success of quit attempts, use of smoking cessation pharmacotherapy, and use of stop smoking services in England: time series analysis of population trends. BMJ 2016; 354: i4645. [DOI] [PubMed] [Google Scholar]
- 7.Caraballo RS, Shafer PR, Patel D, Davis KC, McAfee TA Quit methods used by US adult cigarette smokers, 2014-2016. Prev Chronic Dis 2017; 14: E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy DT, Yuan Z, Luo Y, Abrams DB The relationship of E-cigarette use to cigarette quit attempts and cessation: in-sights from a large, nationally representative U.S. survey. Nicotine Tob Res 2018; 20: 931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel D, Davis KC, Cox S, Bradfield B, King BA, Shafer P, et al. Reasons for current E-cigarette use among U.S. adults. Prev Med 2016; 93: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, et al. A randomized trial of E-cigarettes versus nicotine-replacement therapy. N Engl J Med 2019; 380: 629–37. [DOI] [PubMed] [Google Scholar]
- 11.Levy DT, Cummings KM, Villanti AC, Niaura R, Abrams DB, Fong GT, et al. A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction 2017; 112: 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Homer JB, Hirsch GB System dynamics modelling for public health: background and opportunities. Am J Public Health 2006; 96: 452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy DT, Bauer JE, Lee HR Simulation modelling and tobacco control: creating more robust public health policies. Am J Public Health 2006; 96: 494–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currie LM, Blackman K, Clancy L, Levy DT The effect of tobacco control policies on smoking prevalence and smoking-attributable deaths in Ireland using the IrelandSS simulation model. Tob Control 2013; 22: e25–e32. [DOI] [PubMed] [Google Scholar]
- 15.Levy D, de Almeida LM, Szklo A The Brazil SimSmoke policy simulation model: the effect of strong tobacco control policies on smoking prevalence and smoking-attributable deaths in a middle income nation. PLoS Med 2012; 9: e1001336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levy D, Gallus S, Blackman K, Carreras G, la Vecchia C, Gorini G Italy SimSmoke: the effect of tobacco control policies on smoking prevalence and smoking attributable deaths in Italy. BMC Public Health 2012; 12: 709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levy D, Rodriguez-Buno RL, Hu TW, Moran AE The potential effects of tobacco control in China: projections from the China SimSmoke simulation model. BMJ 2014; 348: g1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levy D, Benjakul S, Ross H, Ritthiphakdee B The role of tobacco control policies in reducing smoking and deaths in a middle income nation: results from the Thailand SimSmoke simulation model. Tob Control 2008; 17: 53–9. [DOI] [PubMed] [Google Scholar]
- 19.Levy DT, Blackman K, Currie LM, Mons U Germany SimSmoke: the effect of tobacco control policies on future smoking prevalence and smoking-attributable deaths in Germany. Nicotine Tob Res 2013; 15: 465–73. [DOI] [PubMed] [Google Scholar]
- 20.Levy D, Cho S, Kim Y-M, Park S, Suh M-K, Kam S An evaluation of the impact of tobacco control policies in Korea using the SimSmoke model: the unknown success story. Am J Public Health 2010; 100: 1267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynales-Shigematsu LM, Fleischer NL, Thrasher JF, Zhang Y, Meza R, Cummings KM, et al. Effects of tobacco control policies on smoking prevalence and tobacco-attributable deaths in Mexico: the SimSmoke model. Rev Panam Salud Publica 2015; 38: 316–25. [PubMed] [Google Scholar]
- 22.Maslennikova GY, Oganov RG, Boytsov SA, et al. Russia SimSmoke: the long-term effects of tobacco control policies on smoking prevalence and smoking-attributable deaths in Russia. Tob Control 2013. [DOI] [PMC free article] [PubMed]
- 23.Nagelhout GE, Levy DT, Blackman K, Currie L, Clancy L, Willemsen MC The effect of tobacco control policies on smoking prevalence and smoking-attributable deaths. Findings from the Netherlands SimSmoke tobacco control policy simulation model. Addiction 2012; 107: 407–16. [DOI] [PubMed] [Google Scholar]
- 24.Levy DT, Currie L, Clancy L Tobacco control policy in the UK: blueprint for the rest of Europe. Eur J Public Health 2013; 23: 201–6. [DOI] [PubMed] [Google Scholar]
- 25.Levy DT, Meza R, Zhang Y, Holford TR, Gauging the Effect of U.S. Tobacco Control Policies From Through 2014 using SimSmoke. Am J Prev Med 2016 1965; 50: 535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.House of Commons Science and Technology Committee. Ecigarettes, Seventh Report of Session 2017–19 2018. Available at: https://publications.parliament.uk/pa/cm201719/cmselect/cmsctech/505/505.pdf (accessed March 29 2020).
- 27.McNeill A, Brose L, Calder R, Bauld L, Robson D Vaping in England: an evidence update including mental health and pregnancy, March 2020: a report commissioned by Public Health England London: Public Health England; March 2020. [Google Scholar]
- 28.Joossens L, Feliu A, Fernandez E The Tobacco Control Scale 2019 in Europe 2020. Available at: http://www.tobaccocontrolscale.org/TCS2019.pdf.
- 29.Cancer Research UK’s Health Behaviour Research Centre UCL, University of London, Smoking Toolkit Study 2020. Available at: http://www.smokinginengland.info/latest-statis-tics/ (accessed 27 February 2020).
- 30.Levy DT, Nikolayev N, Mumford EA Recent trends in smoking and the role of public policies: results from the SimSmoke tobacco control policy simulation model. Addiction 2005; 10: 1526–37. [DOI] [PubMed] [Google Scholar]
- 31.Levy DT, Nikolayev N, Mumford EA The healthy people 2010 smoking prevalence and tobacco control objectives: results from the SimSmoke tobacco control policy simulation model. Cancer Causes Control 2005; 16: 359–71. [DOI] [PubMed] [Google Scholar]
- 32.United Kingdom National Office of Statistics. Population Statistics 2019. Available at: www.ons.gov.uk/peoplepopulation-andcommunity/populationandmigration/populationestimates/methodologies/methodologyguideformid2012tomid2016uk-populationestimatesenglandandwalesmarch2018#home-armed-forces (accessed 23 December 2019).
- 33.United Kingdom National Office of Statistics. Mortality Statistics 2019. Available at: ons.gov.uk/peoplepopulation-andcommunity/birthsdeathsandmarriages/deaths/datasets/deathregistrationssummarytablesenglandandwalesdeathsby-singleyearofagetables (accessed 23 December 2019).
- 34.United Kingdom National Office of Statistics. OPN-ONS Smoking Statistics 2020. Available at: www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/datasets/adultsmokinghabitsinengland (accessed 20 February 2020).
- 35.Gilpin EA, Pierce JP, Farkas AJ Duration of smoking abstinence and success in quitting. J Natl Cancer Inst 1997; 89: 572–6. [DOI] [PubMed] [Google Scholar]
- 36.Hughes JR, Keely J, Naud S Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004; 99: 29–38. [DOI] [PubMed] [Google Scholar]
- 37.Hughes JR, Peters EN, Naud S Relapse to smoking after 1 year of abstinence: a meta-analysis. Addict Behav 2008; 33: 1516–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doll R, Peto R, Boreham J, Sutherland I Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004; 328: 1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pirie K, Peto R, Reeves GK, Green J, Beral V Million women study C. the 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet 2013; 381: 133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.World Health Organization. WHO Report on the Global Tobacco Epidemic The MPOWER package. Gen 2008; 2008. [Google Scholar]
- 41.World Health Organization. WHO Report on the Global Tobacco Epidemic The MPOWER package. Gen 2011; 2012. [Google Scholar]
- 42.World Health Organization. WHO discussion paper: a comprehensive global monitoring framework and voluntary global targets for the prevention and control of NCDs. Comprehensive global monitoring framework including indicators and a set of voluntary global targets for the prevention and control of NCDs 2013. Available at: http://www.who.int/nmh/events/2012/consultation_april_2012/en/ (accessed 2 January 2014).
- 43.World Health Organization. WHO report on the global tobacco epidemic, 2015: Raising taxes on tobacco 2015. Available at: http://www.who.int/tobacco/global_report/2015/report/en/ (accessed 12 March 2016).
- 44.World Health Organization. WHO report on the global tobacco epidemic 2017 Geneva: WHO; 2017. [Google Scholar]
- 45.Barber S Tobacco control policy overview. In: House of Commons, editor. House of Commons Library. London; 2017. [Google Scholar]
- 46.Levy DT, Cummings KM, Hyland A Increasing taxes as a strategy to reduce cigarette use and deaths: results of a simulation model. Prev Med 2000; 31: 279–86. [DOI] [PubMed] [Google Scholar]
- 47.United Kingdom National Office of Statistics O-O. Price statistics 2020. Available at: https://www.ons.gov.uk/economy/inflationandpriceindices/timeseries/dobn/mm23# and https://www.ons.gov.uk/economy/inflationandpriceindices/timeseries/l522/mm23 (accessed 2 February 2020).
- 48.Branston JR, McNeill A, Gilmore AB, Hiscock R, Partos TR Keeping smoking affordable in higher tax environments via smoking thinner roll-your-own cigarettes: findings from the international tobacco control four country survey 2006-15. Drug Alcohol Depend 2018; 193: 110–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Partos TR, Branston JR, Hiscock R, Gilmore AB, McNeill Individualised tobacco affordability in the UK 2002-2014: findings from the international tobacco control policy evaluation project. Tob Control 2019; 28: s9–s19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Partos TR, Gilmore AB, Hitchman SC, Hiscock R, Branston JR, McNeill A Availability and use of cheap tobacco in the United Kingdom 2002-2014: findings from the international tobacco control project. Nicotine Tob Res 2018; 20: 714–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuipers MA, Partos T, McNeill A, et al. Smokers’ strategies across social grades to minimise the cost of smoking in a period with annual tax increases: evidence from a national survey in England. BMJ Open 2019; 9: e026320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beard E, West R, Michie S, Brown J Association of prevalence of electronic cigarette use with smoking cessation and cigarette consumption in England: a time-series analysis between 2007 and 2017. Addiction 2019. [DOI] [PMC free article] [PubMed]
- 53.Jackson SE, Beard E, Kujawski B, Sunyer E, Michie S, Shahab L, et al. Comparison of trends in self-reported cigarette consumption and sales in England, 2011 to 2018. JAMA Netw Open 2019; 2: e1910161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuipers MAG, Beard E, West R, Brown J Associations between tobacco control mass media campaign expenditure and smoking prevalence and quitting in England: a time series analysis. Tob Control 2018; 27: 455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fidler JA, Shahab L, West O, Jarvis MJ, McEwen A, Stapleton JA, et al. ‘The smoking toolkit study’: a national study of smoking and smoking cessation in England. BMC Public Health 2011; 11: 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.United Kingdom OoNS. Smoking Habits in the UK, Annual Population Survey 2019. Available at: www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/datasets/smokinghabitsintheuk-anditsconstituentcountries (accessed 4 December 2019).
- 57.Brose LS, Hitchman SC, Brown J, West R, McNeill A Is the use of electronic cigarettes whereas smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction 2015; 110: 1160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown J, Beard E, Kotz D, Michie S, West R Real-world effectiveness of e-cigarettes when used to aid smoking cessation: a cross-sectional population study. Addiction 2014; 109: 1431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beard E, Brown J, Michie S, West R Is prevalence of e-cigarette and nicotine replacement therapy use among smokers associated with average cigarette consumption in England? A time-series analysis. BMJ Open 2018; 8: e016046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beard E, Jackson SE, West R, Kuipers MAG, Brown J Population-level predictors of changes in success rates of smoking quit attempts in England: a time series analysis. Addiction 2020; 115: 315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Beard E, Jackson SE, West R, Kuipers MAG, Brown J Trends in attempts to quit smoking in England since 2007: a time series analysis of a range of population-level influences. Nicotine Tob Res 2019; epub. [DOI] [PMC free article] [PubMed]
- 62.Beard E, West R, Michie S, Brown J Association of prevalence of electronic cigarette use with smoking cessation and cigarette consumption in England: a time-series analysis between 2006 and 2017. Addiction 2020; 115: 961–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.West R, Shahab L, Brown J Estimating the population impact of e-cigarettes on smoking cessation in England. Addiction 2016; 111: 1118–9. [DOI] [PubMed] [Google Scholar]
- 64.Shahab L, Beard E, Brown J Association of initial e-cigarette and other tobacco product use with subsequent cigarette smoking in adolescents: a cross-sectional, matched control study. Tob Control 2020; tobaccocontrol-2019-055283. [DOI] [PMC free article] [PubMed]
- 65.Jackson SE, Beard E, Michie S, Shahab L, Raupach T, West R, et al. Are smokers who are regularly exposed to e-cigarette use by others more or less motivated to stop or to make a quit attempt? A cross-sectional and longitudinal survey. BMC Med 2018; 16: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nutt DJ, Phillips LD, Balfour D, Curran HV, Dockrell M, Foulds J, et al. E-cigarettes are less harmful than smoking. Lancet 2016; 387: 1160–2. [DOI] [PubMed] [Google Scholar]
- 67.National Academy of Sciences EaM Public Health Consequences of E-cigarettes Washington, DC: The National Acadamies Press; 2018. [Google Scholar]
- 68.McNeill A, Brose L, Calder R, Bauld L, Robson D Evidence review of e-cigarettes and heated tobacco products 2018 London: A report commissioned by Public Health England; 2018. [Google Scholar]
- 69.Beard E, West R, Michie S, Brown J Association between smoking and alcohol-related behaviours: a time-series analysis of population trends in England. Addiction 2017; 112: 1832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jackson SE, Beard E, Michie S, West R, Brown J Is the use of e-cigarettes for smoking cessation associated with alcohol consumption? A population-level survey of successful quitters in England. Addict Behav 2020; 101: 106138. [DOI] [PubMed] [Google Scholar]
- 71.Levy DT, Tam J, Kuo C, Fong GT, Chaloupka F The impact of implementing tobacco control policies: the 2017 tobacco control policy scorecard. J Public Health Manag Pract 2018; 24: 448–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hopkinson NS, Millett C, Glantz S, Arnott D, McNeill A UK government should fund stop smoking media campaigns not give tax breaks to films with smoking imagery. Addiction 2016; 111: 2066–7. [DOI] [PubMed] [Google Scholar]
- 73.Langley T, Szatkowski L, Lewis S, McNeill A, Gilmore AB, Salway R, et al. The freeze on mass media campaigns in England: a natural experiment of the impact of tobacco control campaigns on quitting behaviour. Addiction 2014; 109: 995–1002. [DOI] [PubMed] [Google Scholar]
- 74.Huang J, Gwarnicki C, Xu X, Caraballo RS, Wada R, Chaloupka FJ A comprehensive examination of own- and cross-price elasticities of tobacco and nicotine replacement products in the U.S. Prev Med 2018; 117: 107–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pesko MF, Huang J, Johnston LD, Chaloupka FJ E-cigarette price sensitivity among middle- and high-school students: evidence from monitoring the future. Addiction 2018; 113: 896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zheng Y, Zhen C, Dench D, Nonnemaker JMUS demand for tobacco products in a system framework. Health Econ 2016. [DOI] [PubMed]
- 77.Huang J, Tauras J, Chaloupka FJ The impact of price and tobacco control policies on the demand for electronic nicotine delivery systems. Tob Control 2014; 23: iii41–iii47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beard E, Brose LS, Brown J, West R, McEwen A How are the English stop smoking services responding to growth in use of electronic cigarettes? Patient Educ Couns 2014; 94: 276–81. [DOI] [PubMed] [Google Scholar]
- 79.Beard E, Brown J, McNeill A, Michie S, West R Has growth in electronic cigarette use by smokers been responsible for the decline in use of licensed nicotine products? Findings from repeated cross-sectional surveys. Thorax 2015; 70: 974–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Tobacco control policies, specifications and effect sizes applied in England SimSmoke.
Table S2 Validation of England SimSmoke current smoking prevalence predictions against national surveys, by age and gender, 2000–2012.
Table S3a Smoking prevalence and smoking attributable deaths projected by the unadjusted SimSmoke model under individual policy scenarios by gender, 2000–2040.
Table S3b Smoking prevalence projected by the No-NVP SimSmoke model under multiple policy scenarios by gender in 2012–2019.
Table S4a Sensitivity analysis of smoking prevalence (%) at age 18+ by gender under multiple policy effects sizes in 2012–2019.
Table S4b Sensitivity analysis of smoking prevalence (%) at age 18+ by gender under multiple policy effects sizes only in the marginal change of the policies in 2012–2019.
Table S4c Sensitivity analysis of smoking prevalence (%) at age 18+ by gender under different policy specifications for price and media campaigns.
Table S5 Smoking prevalence predictions from unadjusted SimSmoke model compared to national surveys, by age group and gender, 2012–2018/19.
Table S6 Projected smoking prevalence (%), No-NVP SimSmoke versus NVP SimSmoke, age 16+ by gender, 2012–2052.
Table S7 Projected smoking-attributable deaths and lives saved, unadjusted England SimSmoke compared to STS NVP-adjusted and APS NVP-adjusted England SimSmoke, by gender, 2012–2052.


