Visual Abstract
Keywords: monosodium glutamate, PSMA, PET/CT, xerostomia, salivary glands;
Abstract
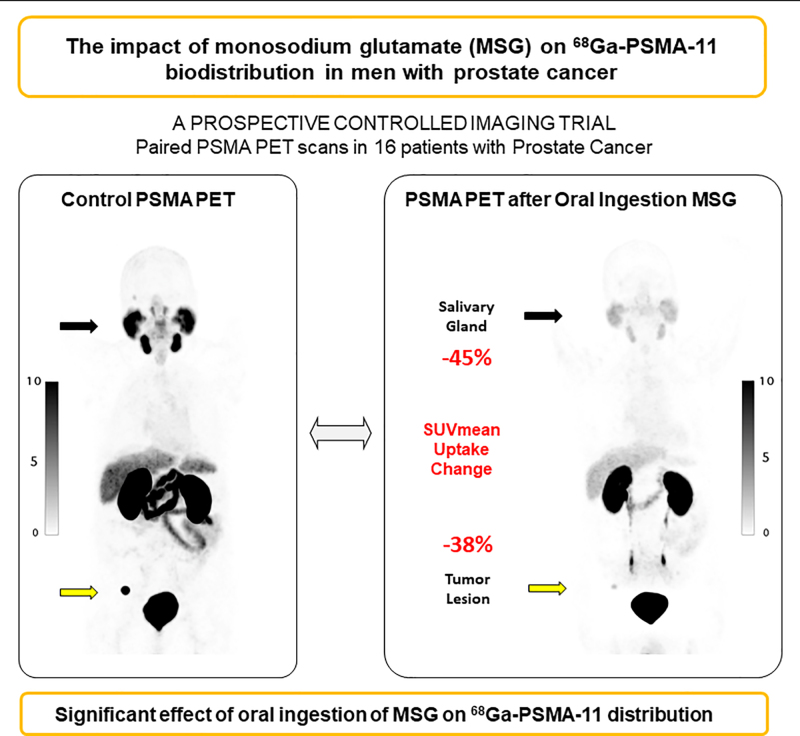
The prostate-specific membrane antigen (PSMA) has been targeted for PET imaging and radioligand therapy (RLT) in patients with prostate cancer. Xerostomia is a common side effect of RLT because of the high salivary gland uptake of PSMA radioligands. Here, we aimed to determine the impact of monosodium glutamate (MSG) administration on PSMA-radioligand biodistribution within healthy organs and tumor lesions by using 68Ga-PSMA-11 PET imaging. Methods: Sixteen men with prostate cancer were randomized (1:1) into oral ingestion and oral topical application (“swishing”) arms. Each subject underwent 2 68Ga-PSMA-11 PET/CT scans within 14 d under baseline and MSG conditions. The salivary glands and whole-body tumor lesions were segmented using qPSMA software. We quantified tracer uptake via SUVmean and SUVmax and compared parameters within each patient. Results: For the oral ingestion arm, salivary gland SUVmean and SUVmax decreased on average from the control scan to the MSG scan by 45% ± 15% (P = 0.004) and 53% ± 11% (P < 0.001), respectively. Tumor lesion SUVmean and SUVmax also decreased by 38% (interquartile range, −67% to −33%) and −52% (interquartile range, −70% to −49%), respectively (P = 0.018). Swishing had no significant effect on 68Ga-PSMA-11 accumulation in normal organs or tumor lesions. Conclusion: Oral ingestion but not topical application of MSG reduced 68Ga-PSMA-11 uptake in salivary glands. Tumor uptake also declined; therefore, the clinical application of MSG is unlikely to be useful in the framework of RLT.
Prostate-specific membrane antigen (PSMA) is a transmembrane glycoprotein highly overexpressed by prostate cancer (PCa) cells (1). In recent years, PSMA has become an attractive target for both diagnosis and treatment of PCa (2). After their introduction for whole-body imaging with PET/CT, small-molecule PSMA ligands with a DOTA chelator, such as PSMA I&T or PSMA-617, were labeled with β-emitting (177Lu) or α-emitting (225Ac) isotopes for therapeutic purposes. PSMA-targeted radioligand therapy (RLT) with 177Lu demonstrated significant reductions in serum prostate-specific antigen (PSA) levels in phase 2 trials of metastatic castration-resistant PCa (3) and is currently being investigated in a phase 3 trial (VISION: NCT03511664). PSMA RLT with 225Ac, an α-emitter with a high energy deposition, may have enhanced therapeutic efficacy but has a less favorable toxicity profile (4, 5). The most concerning side effects include xerostomia, long-term nephrotoxicity, and myelosuppression (6–8). In particular, 225Ac-PSMA is associated with grade 2 or higher xerostomia, which often led to treatment cessation despite an initially favorable PSA response (4, 5, 9, 10). After the preliminary effects of 225Ac-PSMA on serum PSA levels, multiple efforts have failed to apply protective measures against salivary gland and kidney toxicity (11–14).
The salivary gland binding and uptake mechanism of PSMA radioligands remain unclear. There appears to be limited target expression by the salivary glands (low or intermediate immunohistochemistry PSMA staining intensity; patchy and focal expression, limited in extent [5% of salivary gland tissue]), whereas radioligand uptake is very high (15). In contrast, PSMA-targeted radioantibodies, such as 111In-J591 and 177Lu-J591, do not accumulate in the salivary glands or accumulate only at low levels (16). The high accumulation of the PSMA radioligands in the salivary glands may thus represent an off-target effect (i.e., related not to the PSMA target expression but to the radioligand molecules).
PSMA (also known as glutamate carboxypeptidase II) is targeted by small molecules via interaction of the glutamate moiety of the radioligands (among other features) with its enzymatic region, which has high glutamate affinity (17–19). Therefore, it was hypothesized that the administration of monosodium glutamate (MSG), a well-known food additive, could act as a competitor by blocking the binding of the PSMA-targeting radioligands. In a preclinical model, MSG reduced 68Ga-PSMA-11 salivary gland and renal uptake, whereas tumor accumulation was unaffected, in LNCap-bearing mice (20). Moreover, MSG stimulates salivary flow as shown in a controlled study, with up to a 1 mL/min mean salivary flow compared with 0.25 mL/min at baseline (21). We also hypothesized that MSG could be used as an oral salivary flow stimulant to remove accumulated radioligands from the salivary glands.
68Ga-PSMA-11 PET imaging is a rapid, noninvasive, and safe technique that provides reliable estimates of the biodistribution of therapeutic PSMA ligands.
In this imaging-controlled study on men with PCa, we determined the impact of MSG administration on PSMA-radioligand biodistribution in normal organs and tumors by using 68Ga-PSMA-11 PET/CT with and without MSG administration. We tested 2 administration methods: “swishing” (i.e., oral topical, to increase the salivary flow) and oral ingestion (for competitive binding).
MATERIALS AND METHODS
Study Design and Patient Population
This was a prospective single-center, open-label, randomized, controlled imaging study conducted at UCLA using 16 paired PSMA PET/CT studies with (MSG scan) and without (control scan) MSG administration, with less than 14 d between the 2 scans. The study was investigator-initiated, self-funded, conducted under an investigational new drug application (application 130649), approved by the local institutional review board (approval 18-001776), and registered on clinicaltrial.gov (NCT04282824).
Patients with histopathologically proven PCa who volunteered to undergo 2 PSMA PET/CT scans within 14 d and without any treatment change between the 2 scans were eligible. Patients with prior salivary gland surgery or radiation therapy, a history of salivary gland disease, severe uncontrolled hypertension, or a known allergic responses to MSG or who were unable to comply with the study procedures were excluded (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org). We obtained oral and written informed consent from all patients.
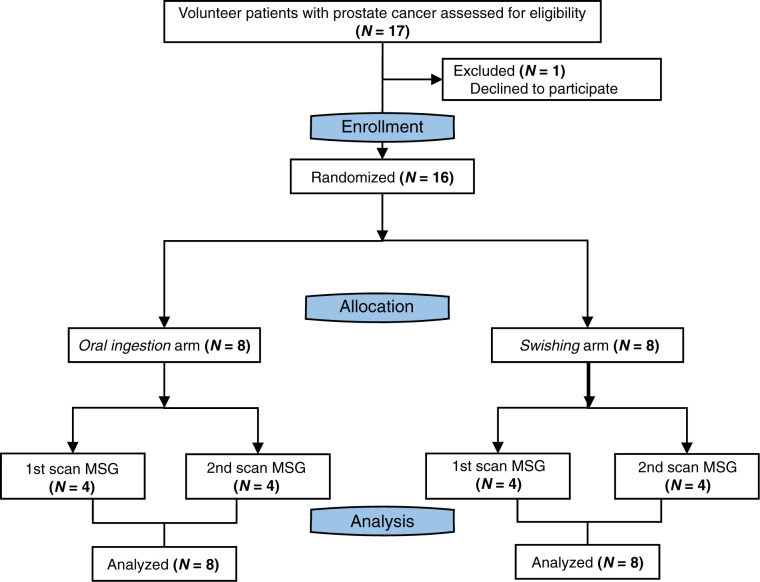
To preclude the potential confounding factor of stimulus effect, patients were initially randomized into 2 arms based on the type of MSG administration: oral ingestion (n = 8) and swishing (n = 8). A second randomization process subdivided the patients into receiving the control or MSG scan first. Figure 1 depicts the study flowchart.
FIGURE 1.
Study flowchart.
Procedures
MSG Administration
We obtained food-grade MSG as a sealed salt powder (Ajinomoto). The U.S. Food and Drug Administration has designated MSG to be generally recognized as safe. Patients randomized to oral ingestion received a 150 mg/kg dose of food-grade MSG dissolved in 300 mL of drinking-grade water 30 min before 68Ga-PSMA-11 injection. Patients randomized to swishing received 0.5 M MSG, which they swished within the mouth for 30 s before removing the solution without swallowing. The swishing procedure was repeated at 0, 30, and 45 min after 68Ga-PSMA-11 injection.
Image Acquisition
68Ga-PSMA-11 (Glu-NH-CO-NH-Lys-(Ahx)-[68Ga(HBED-CC)]) was used as the PSMA ligand and was obtained from the Biomedical Cyclotron Facility at UCLA. 68Ga-PSMA-11 PET/CT imaging was performed according to international guidelines (22). The target injected activity dose was 185 MBq (allowed range, 111–259 MBq). The target uptake period was 60 min (allowed range, 50–100 min). We applied oral but no intravenous CT contrast medium for the control and MSG scans. We acquired images using a 64-detector PET/CT scanner (2007 Biograph 64 Truepoint or 2010 Biograph mCT 64; Siemens). The same scanner was used for both visits. A diagnostic CT scan (200–240 mAs, 120 kV) with a 5-mm slice thickness was obtained. PET images were acquired in 3-dimensional mode from mid thigh to vertex (whole-body scan) with a time of 2–4 min per bed position using a weight-based protocol (22). All PET images were reconstructed using corrections for attenuation, dead time, random events, and scatter. PET images were reconstructed with an iterative algorithm (ordered-subset expectation maximization) in an axial 168 × 168 matrix on the Biograph 64 Truepoint (2-dimensional, 2 iterations, 8 subsets, 5.0-mm gaussian filter) and in a 200 × 200 matrix on the Biograph mCT 64 (3-dimensional, 2 iterations, 24 subsets, 5.0-mm gaussian filter).
Image Analyses
Board-certified nuclear medicine physicians and radiologists used a PSMA PET/CT–based TNM staging system (PROMISE) to generate clinical reports of the control scans by consensus (23).
Two nuclear medicine physicians, who did not know the study condition (control vs. MSG administration and type of MSG application), used qPSMA software to interpret the research MSG and control PSMA PET/CT scans by consensus (24). They segmented all detected tumor lesions and normal organs manually. Normal organs included the lacrimal glands, parotid glands, submandibular glands, liver, spleen, kidneys, and urinary bladder. Output parameters included SUVmean and SUVmax for both tumor lesions and normal organs.
Measurements of Salivary Radioactivity
To assess the effect of MSG on radioligand excretion, we collected saliva from all patients at 5 time points after 68Ga-PSMA-11 injection at 0 min (range, 0–7 min), 10 min (range, 9–17 min), 30 min (range, 28–39 min), 45 min (range, 44–54 min), and 100 min (range, 88–126 min). We transferred saliva collected in disposable medication cups to disposable borosilicate test tubes. Samples were weighed and radioactivity was measured in a γ-well counter (Capintec CAPRAC-t; Mirion Technologies). Background was measured before each patient injection. We assayed 68Ga decay within a range of 10–1,200 keV and recorded the time of radioactivity collection and measurement to adjust for tracer decay. We corrected tracer uptake in saliva for background and radioactive decay.
Safety
We monitored safety before/after injection of the radiotracer, before/after the MSG administration, and before/after the scan procedure. We recorded blood pressure and heart rate before injection of 68Ga-PSMA-11 and directly after completion of the scan. We communicated with all patients within 72 h after the scan and asked whether they had any untoward side effects or symptoms. Adverse events were documented and evaluated according to the Common Terminology Criteria for Adverse Events, version 5.0.
Outcomes
The primary objective of this trial was to compare the degree of 68Ga-PSMA-11 uptake in the salivary glands with and without MSG administration. A 2-fold reduction after MSG administration was a priori defined as a successful reduction in salivary gland PSMA uptake (25). The secondary objectives were to determine the impact of MSG administration on 68Ga-PSMA-11 uptake in normal organs and tumor lesions, to measure whether MSG stimulates 68Ga-PSMA-11 excretion in the saliva, and to assess the safety of oral MSG ingestion and salivary flow stimulation at the proposed doses.
Statistical Analyses
Radiation doses to the salivary glands from 1 cycle of 225Ac-PSMA or 177Lu-PSMA were estimated at 17 and 10 Gy, respectively (9, 10, 26, 27). The commonly applied safe upper limit for external-beam salivary gland radiation therapy is 32 Gy, which can be reached after 2 cycles of 225Ac-PSMA (28). On the basis of these numbers, we aimed to achieve a 2-fold reduction in the 68Ga-PSMA-11 accumulation in the salivary glands after MSG administration. The primary endpoint measure was the mean difference in SUVmax and SUVmean in all assessable salivary glands with and without the administration of MSG interventions. Patients were randomized (1:1) using a computer-generated randomization list. The randomization plan used a permuted block design with 2 blocks of n = 8 (arms A and B, Supplemental Table 2).
We report descriptive values as mean ± SD or median and interquartile range (if data were not normally distributed according to the Shapiro–Wilk test). Because individual patients served as their own control, paired t tests were performed. Differences between paired data that were not normally distributed were determined using the Wilcoxon signed-rank test. The independent t test was used to compare the means between unrelated groups. In each analysis, a P value of less than 0.05 was considered statistically significant. We conducted all analyses using SPSS Statistics, version 26.0 (IBM Corp.), and R Studio, version 3.6.1 (R Foundation for Statistical Computing).
RESULTS
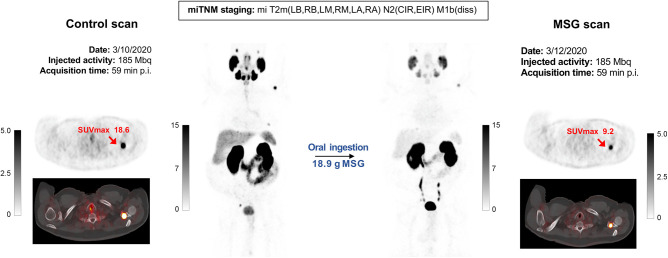
A summary image of each of the 16 patients, with all measurements, is provided in the supplemental materials (Supplemental Fig. 1). One patient example (patient 012) is displayed in Figure 2.
FIGURE 2.
Set of images of 73-y-old patient after radiation therapy (initial PSA, 16 ng/mL; biopsy Gleason score, 8; pT2c) and concurrent androgen hormone treatment, currently presenting for rising PSA value (6.27 ng/mL). After enrollment, patient was randomized to oral ingestion arm and received 18.9 g of MSG before second 68Ga-PSMA-11 injection. PSMA PET/CT images revealed multifocal prostate involvement, common iliac right and external iliac right pelvic lymph nodes, and multiple bone lesions. Maximum-intensity-projection images show overall decline in 68Ga-PSMA-11 accumulation within normal organs as well as tumor lesions on MSG scan relative to control scan. Axial view images display relevant case example of bone lesion with significant PSMA decrease after MSG administration (SUVmax from 18.6 to 9.2). p.i. = after injection.
Patient Population
Between December 20, 2019, and April 4, 2020, 17 patients were screened to identify 16 patients who met the eligibility criteria (Fig. 1). One patient declined to participate. The demographics and clinical characteristics of the study population are presented in Table 1. Two of 16 (16%) patients underwent the PSMA PET/CT scan for initial staging of PCa, 7 of 16 (42%) for localization of biochemical recurrence, and 7 of 16 (42%) for restaging of metastatic disease.
TABLE 1.
Patient Characteristics
| Characteristic | Data |
|---|---|
| Age (y) | 72 (56–81) |
| Time since diagnosis of PCa (y) | 7 (0.6–21) |
| PSA at diagnosis (ng/mL) | 36 (2.5–308) |
| Gleason score at diagnosis* | |
| <8 | 7 (44%) |
| ≥8 | 8 (50%) |
| T stage at diagnosis* | |
| T1 | 1 (6%) |
| T2 | 11 (66%) |
| T3 | 3 (18%) |
| M status at diagnosis† | |
| M0 | 15 (94%) |
| M1 | 1 (6%) |
| Primary treatment‡ | |
| Prostatectomy ± lymphadenectomy | 7 (49%) |
| Local radiotherapy | 6 (42%) |
| Systemic treatment | 1 (7%) |
| Salvage treatment | |
| None | 9 (56%) |
| Radiotherapy | 3 (19%) |
| Systemic treatment | 4 (25%) |
| Indication for scan | |
| Primary staging | 2 (12%) |
| Biochemical recurrence | 7 (42%) |
| Metastatic restaging | 7 (42%) |
| PSA at time of PSMA (ng/mL) | 6.2 (0.2–53.7) |
Data missing for 1 patient.
M1 was defined as metastatic disease (distant metastases).
Data missing for 2 patients.
Qualitative data are number and percentage (total n = 16); continuous data are median and range.
PSMA PET/CT Images
In the oral ingestion arm, the mean injected activity was 184 ± 1 and 183 ± 2 MBq for the MSG and control scans, respectively (P = 0.18). Image acquisition commenced at 61 ± 8 and 61 ± 7 min, respectively, after tracer injection (P = 0.87).
In the swishing arm, the mean injected activity was 184 ± 1 and 184 ± 1 MBq for the MSG and control scans, respectively (P = 0.40). Image acquisition commenced at 67 ± 15 and 66 ± 14 min, respectively, after tracer injection (P = 0.87).
Table 2 summarizes the scan findings and PSMA PET–based staging. Three patients had no visible PCa lesions (1 in the oral ingestion arm and 2 in the swishing arm). There was no change in stage between the control and MSG scans.
TABLE 2.
PSMA PET Findings
| Swishing | Oral ingestion | |||
|---|---|---|---|---|
| Study arm | Control | MSG | Control | MSG |
| 68Ga-PSMA-11 PET/CT+ | ||||
| Prostate/prostate bed (T+) | 4 (50%) | 4 (50%) | 5 (63%) | 5 (63%) |
| Pelvic LN (N1) | 1 (13%) | 1 (13%) | 2 (25%) | 2 (25%) |
| Extrapelvic LN (M1a) | 1 (13%) | 1 (13%) | 1 (13%) | 1 (13%) |
| Bone (M1b) | 2 (25%) | 1 (13%) | 3 (38%) | 3 (38%) |
| Visceral (M1c) | 0 | 0 | 0 | 0 |
| 68Ga-PSMA-11 TNM pattern | ||||
| PSMA T0 N0 M0 | 2 (25%) | 2 (25%) | 1 (13%) | 1 (13%) |
| PSMA T+ N0 M0 | 3 (38%) | 3 (38%) | 2 (25%) | 2 (25%) |
| PSMA T0 N1 M0 | 0 | 0 | 0 | 0 |
| PSMA T+ N1 M0 | 1 (13%) | 1 (13%) | 1 (13%) | 1 (13%) |
| PSMA T+ N0 M1 | 0 | 0 | 1 (13%) | 1 (13%) |
| PSMA T0 N0 M1 | 2 (25%) | 2 (25%) | 2 (25%) | 2 (25%) |
| PSMA T0 N1 M1 | 0 | 0 | 0 | 0 |
| PSMA T+ N1 M1 | 0 | 0 | 1 (13%) | 1 (13%) |
Data are number and percentage.
68Ga-PSMA-11 Uptake in Normal Organs
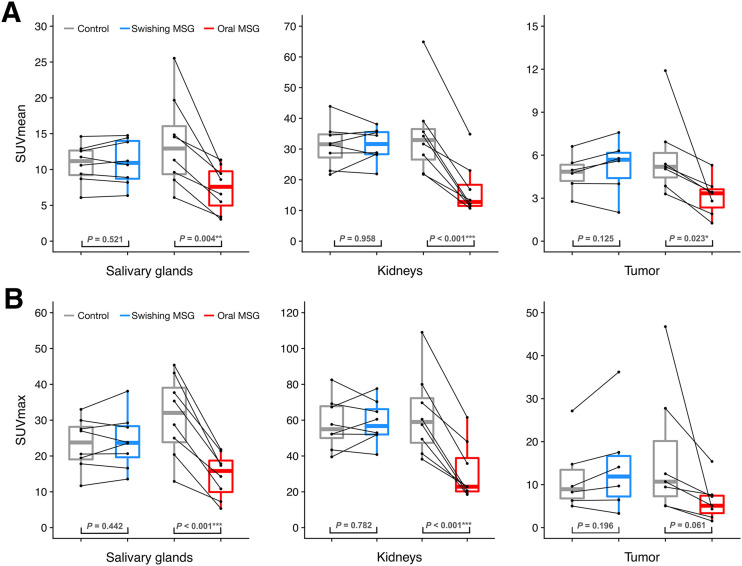
In the oral ingestion arm, MSG administration was associated with a significant decrease in 68Ga-PSMA-11 accumulation in all normal organs (P < 0.05) and a large increase in bladder activity (mean difference, +372% SUVmean and +593% SUVmax; Table 3). 68Ga-PSMA-11 uptake decreased by more than 50% in the salivary glands (mean difference, −46% SUVmean and −53% SUVmax), with a more prominent effect on the submandibular glands (mean difference, −59% SUVmean and −63% SUVmax) than on the parotid glands (mean difference, −33% SUVmean and −34% SUVmax).
TABLE 3.
Comparison of 68Ga-PSMA11 Uptake in Normal Organs in Control and MSG Scans
| SUVmean | SUVmax | |||||||
|---|---|---|---|---|---|---|---|---|
| Site | Control | MSG | Change | P | Control | MSG | Change | P |
| Oral ingestion (n = 8) | ||||||||
| Lacrimal glands | 5.5 ± 2.3 | 2.6 ± 0.9 | −50.9% ± 6.5% | 0.001 | 9.9 ± 4.7 | 4.3 ± 1.6 | −55.5% ± 6.1% | 0.002 |
| Parotid glands | 10.9 ± 4.1 | 6.9 ± 3.3 | −32.9% ± 22.6% | 0.002 | 19.0 ± 6.6 | 13.2 ± 6.2 | −34.1% ± 21.7% | 0.020 |
| Submandibular glands | 15.9 ± 5.7 | 6.6 ± 2.5 | −58.9% ± 8.9% | <0.001 | 29.0 ± 10.5 | 10.4 ± 3.7 | −63.2% ± 9.9% | <0.001 |
| Salivary glands | 12.1 ± 4.6 | 6.8 ± 3.1 | −45.5% ± 14.6% | 0.004 | 29.0 ± 10.5 | 13.5 ± 5.9 | −53.4% ± 11.1% | <0.001 |
| Liver | 4.6 ± 0.5 | 2.1 ± 0.3 | −54.2% ± 5.3% | <0.001 | 10.7 ± 1.3 | 5.5 ± 0.4 | −50.1% ± 8.3% | <0.001 |
| Spleen | 8.1 ± 2.0 | 3.0 ± 0.9 | −62.8% ± 6.9% | <0.001 | 13.7 ± 3.5 | 5.3 ± 1.5 | −61.5% ± 8.6% | <0.001 |
| Kidney | 34.5 ± 14.8 | 17.4 ± 8.8 | −51.5% ± 13.7% | <0.001 | 63.6 ± 25.0 | 32.7 ± 16.6 | −51.3% ± 13.3% | <0.001 |
| Urinary bladder | 16.0 ± 8.7 | 69.1 ± 34.3 | +371.6% ± 300.2% | 0.004 | 33.7 ± 23.9 | 184.2 ± 97.5 | +593.2% ± 659.5% | 0.003 |
| Total tumor lesions | 6.6 ± 3.5 | 3.5 ± 2.1 | −45.0% ± 19.0% | 0.023 | 21.5 ± 19.8 | 8.2 ± 8.7 | −55.7% ± 22.0% | 0.061 |
| Swishing (n = 8) | ||||||||
| Lacrimal glands | 6.5 ± 1.6 | 6.0 ± 2.5 | +4.9% ± 22.0% | 0.625 | 12.3 ± 3.2 | 11.1 ± 4.6 | −5.2% ± 25.0% | 0.556 |
| Parotid glands | 11.7 ± 2.2 | 12.0 ± 2.7 | +0.1% ± 8.4% | 0.917 | 22.2 ± 6.1 | 22.5 ± 6.0 | +1.9% ± 10.4% | 0.792 |
| Submandibular glands | 12.8 ± 3.5 | 13.4 ± 3.6 | +5.6% ± 6.8% | 0.061 | 21.3 ± 7.0 | 22.6 ± 7.3 | +7.7% ± 11.6% | 0.188 |
| Salivary glands | 11.5 ± 2.3 | 11.9 ± 2.9 | +1.6% ± 7.8% | 0.521 | 25.8 ± 6.0 | 26.6 ± 7.2 | +4.3% ± 12.3% | 0.442 |
| Liver | 4.1 ± 0.8 | 4.3 ± 0.9 | +5.7% ± 17.3% | 0.317 | 9.8 ± 1.3 | 10.4 ± 3.0 | +5.3% ± 15.7% | 0.321 |
| Spleen | 6.1 ± 2.0 | 6.3 ± 1.7 | +6.2% ± 18.6% | 0.458 | 10.8 ± 3.9 | 10.1 ± 2.6 | −0.3% ± 15.2% | 0.622 |
| Kidney | 30.1 ± 8.0 | 30.1 ± 5.8 | + 1.9% ± 13.9% | 0.958 | 57.1 ± 15.9 | 57.6 ± 13.6 | +3.4% ± 15.8% | 0.782 |
| Urinary bladder | 20.2 ± 13.5 | 24.2 ± 8.3 | +36.5% ± 41.4% | 0.357 | 50.6 ± 42.4 | 56.4 ± 20.2 | +52.0% ± 70.0% | 0.651 |
| Total tumor lesions | 4.9 ± 1.1 | 5.4 ± 1.6 | 7.8% ± 13.8% | 0.125 | 12.5 ± 7.7 | 15.0 ± 11.3 | 12.6% ± 27.8% | 0.196 |
Data are average ± SD.
In the swishing arm, no statistically significant difference in 68Ga-PSMA-11 accumulation measured by either SUVmean and SUVmax was observed in normal organs after MSG administration (P > 0.05) (Table 3).
68Ga-PSMA-11 Uptake in Tumor Lesions
In the oral ingestion arm, MSG administration was associated with a significant decline in 68Ga-PSMA-11 accumulation in tumor lesions (median difference, −38% SUVmean and −52% SUVmax; Table 4). One pelvic bone lesion showed a dramatic decrease in SUVmax (from 46.8 to 4.3) after MSG administration (case MSG05 in the supplemental materials).
TABLE 4.
Comparison of SUVmean and SUVmax Derived from Control and MSG Scans
| Parameter | Control | MSG | Change (%) | P |
|---|---|---|---|---|
| Oral ingestion (n = 7) | ||||
| SUVmean | 5.4 (3.9, 11.4) | 3.3 (1.9, 3.8) | −37.8 (−67.3, −32.5) | 0.018 |
| SUVmax | 10.7 (6.5, 46.8) | 5.1 (2.6, 9.7) | −52.3 (−70.0, −48.5) | 0.018 |
| Swishing (n = 6) | ||||
| SUVmean | 4.9 (4.2, 5.5) | 5.7 (4.1, 6.3) | 13.8 (−4.0, 15.4) | 0.116 |
| SUVmax | 9.0 (7.8, 14.8) | 11.9 (6.7, 17.5) | 17.9 (−14.0, 33.3) | 0.173 |
Data are median and interquartile range for total tumor lesions.
In the swishing arm, no significant difference in tumor accumulation of 68Ga-PSMA-11 as measured by SUVmean and SUVmax was observed between the 2 PET scans (P = 0.11 and P = 0.17, respectively) (Table 4).
A comparison of pooled SUVmean and SUVmax between the control and MSG studies for each arm is depicted in Figure 3.
FIGURE 3.
SUVmean (A) and SUVmax (B) of salivary glands, kidneys, and tumor lesions in control and MSG studies in oral ingestion and swishing arms.
Saliva Radioactivity Measurements
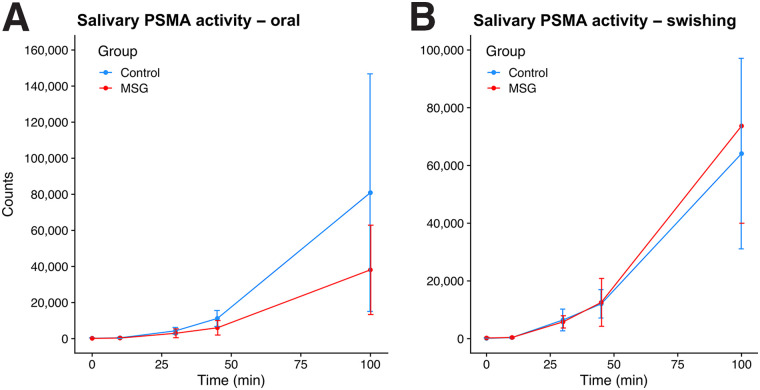
Salivary radioactivity increased over time, demonstrating 68Ga-PSMA-11 salivary excretion. The median activity counts after tracer injection for both arms are provided in Supplemental Table 3. Figure 4 shows the median saliva counts over time.
FIGURE 4.
Median changes in 68Ga-PSMA11 activity in saliva between control and MSG groups at 0, 10, 30, 45, and 100 min after tracer injection for oral ingestion arm (A) and swishing arm (B).
In the oral ingestion arm, a significant decrease in salivary activity counts was observed at 45 and 100 min, with median reductions of −42% and −53%, respectively. In the swishing arm, no significant difference in salivary activity was observed at any time point (P > 0.05).
Adverse Events
Grade 1 nausea after administration was recorded in 1 (6%) of 16 patients after oral ingestion of MSG. Five non–study-related events were recorded (diarrhea [n = 2 in each arm] and abdominal discomfort [n = 1 in the swishing arm]; Supplemental Table 4).
DISCUSSION
This prospective randomized imaging study revealed that oral ingestion of MSG, a food additive, is associated with a significant decrease in 68Ga-PSMA-11 accumulation within normal organs and tumor lesions, whereas topical oral application of MSG has no impact on 68Ga-PSMA-11 biodistribution. The primary endpoint of at least a 50% decrease in 68Ga-PSMA-11 accumulation in the salivary glands was met when expressed as change in SUVmax (53%). However, oral administration of MSG also significantly diminished 68Ga-PSMA-11 uptake in tumor lesions (52% and 39% decline in SUVmax and SUVmean, respectively) and all other organs. A 3-fold increase in 68Ga-PSMA-11 signal in the urinary bladder highlighted rapidly increased urinary excretion after oral MSG administration. Previous work found a repeatability coefficient of 33%–38% for SUV measurements in PSMA PET/CT, indicating that the reduction in tumor uptake in our patients (>45%) is related to MSG administration (29). The application of MSG to reduce salivary gland toxicity (xerostomia) induced by PSMA RLT is therefore unlikely to be a successful clinical strategy.
Various direct attempts to reduce the salivary gland toxicity of 225Ac-PSMA have been reported: salivary gland duct dilation and clearance via sialendoscopy (30), vasoconstriction of parotid gland blood vessels through external cooling with ice packs (11, 13), and local injection of botulinum toxin A to suppress saliva formation metabolically (31). Indirect attempts to alter the biochemical mechanism of off-target binding by competition included PSMA inhibitors such as 2-(phosphonomethyl) pentanedioic acid or serum glutamate–elevating approaches (20, 32–35). Dosimetry data showed that coadministration of oral polyglutamate administration may reduce salivary gland ligand uptake. However, the impact on tumor uptake has not yet been determined (36).
Application of MSG in murine models reduced salivary PSMA radioligand uptake in a dose-dependent matter without affecting tumor uptake (20). In contrast, oral MSG administration in humans led to significant decreases in tumor uptake. Consistent with our findings, a significant decrease in 18F-DCFPyL accumulation in normal organs and tumor lesions after oral administration of MSG was also observed by others (32). Harsini et al. (32) applied a fix dose of 12.7 g, whereas our patients received a 150 mg/kg dose of MSG, which led to higher average dose of 15.1 g. This difference might explain the higher impact of MSG on tracer biodistribution observed in our study, a finding that suggests a dose-dependent effect of MSG. Although the MSG dosages were 10-fold higher than the MSG concentration in a normal meal (37), intake of food containing glutamate (e.g., MSG, umami, tomatoes, cheese, and mushrooms) may impact the biodistribution of PSMA radioligands, and a potential impact on diagnostic or therapy efficacy cannot be formally excluded. Further studies investigating the impact of food containing glutamate on imaging-and-therapy PSMA radioligands may be warranted.
68Ga-PSMA-11 is excreted in the saliva, as shown by our measurements. Oral ingestion of MSG led to diminished salivary excretion of 68Ga-PSMA-11. This finding suggests that its off-target accumulation in the salivary glands interacts with saliva formation, potentially impacting ductal cell transporters within the glands (15, 38). Alternatively, the macromolecular composition of saliva itself may be interacting with PSMA and glutamate, trapping or binding to the molecules and causing an accumulation in the salivary glands within saliva.
Our study had limitations. First, both the dosing and the timing of MSG administration were chosen empirically on the basis of studies largely concerned with safe dosing of MSG rather than application as a blood glutamate–modulating tool (39). Second, although not evaluated in this study, tumor burden may play a role in the efficacy of MSG’s impact on radioligand distribution. Although PSMA RLT is currently offered in heavily metastasized patients with late-stage metastatic castration-resistant PCa, our patients were mainly in earlier disease stages that have a low tumor burden. Nevertheless, considering the tumor sink effect, we expect a higher impact of MSG administration on tumor uptake in patients with a high tumor burden (40).
CONCLUSION
Oral administration of MSG successfully decreased 68Ga-PSMA-11 uptake in normal organs, including the salivary glands and kidneys, in human subjects but also reduced tumor uptake significantly. This result suggests that MSG strategies reducing the salivary gland toxicity of PSMA RLT will negatively impact tumor PSMA uptake. Thus, clinical applicability is unlikely. Future investigations evaluating different doses and timings of MSG administration are warranted, considering the possibility that a lower dose may show differential preference for tumor or normal tissue.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: What is the impact of MSG administration on 68Ga-PSMA11 biodistribution?
PERTINENT FINDINGS: This prospective single-center, randomized imaging study, which included 16 men with PCa, met its primary endpoint, defined as a 50% reduction in 68Ga-PSMA11 accumulation in the salivary glands when MSG was administered orally (−53.4% SUVmax, P < 0.001). However, the radiotracer reduction in normal organs was accompanied by a significant reduction within tumor lesions (−55.7% SUVmax, P = 0.061).
IMPLICATIONS FOR PATIENT CARE: MSG is capable of modulating 68Ga-PSMA-11 biodistribution, including tumor uptake, which limits its clinical application in the setting of PSMA RLT.
REFERENCES
- 1. Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 2. Eiber M, Fendler WP, Rowe SP, et al. Prostate-specific membrane antigen ligands for imaging and therapy. J Nucl Med. 2017;58(suppl):67S–76S. [DOI] [PubMed] [Google Scholar]
- 3. Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–833. [DOI] [PubMed] [Google Scholar]
- 4. Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med. 2018;59:795–802. [DOI] [PubMed] [Google Scholar]
- 5. Sathekge M, Bruchertseifer F, Knoesen O, et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2019;46:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okamoto S, Thieme A, Allmann J, et al. Radiation dosimetry for 177Lu-PSMA I&T in metastatic castration-resistant prostate cancer: absorbed dose in normal organs and tumor lesions. J Nucl Med. 2017;58:445–450. [DOI] [PubMed] [Google Scholar]
- 7. Fendler WP, Reinhardt S, Ilhan H, et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget. 2017;8:3581–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baum RP, Kulkarni HR, Schuchardt C, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016;57:1006–1013. [DOI] [PubMed] [Google Scholar]
- 9. Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: dosimetry estimate and empiric dose finding. J Nucl Med. 2017;58:1624–1631. [DOI] [PubMed] [Google Scholar]
- 10. Kratochwil C, Bruchertseifer F, Giesel FL, et al. 225Ac-PSMA-617 for PSMA-targeted alpha-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med. 2016;57:1941–1944. [DOI] [PubMed] [Google Scholar]
- 11. van Kalmthout LWM, Lam M, de Keizer B, et al. Impact of external cooling with icepacks on 68Ga-PSMA uptake in salivary glands. EJNMMI Res. 2018;8:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Langbein T, Chausse G, Baum RP. Salivary gland toxicity of PSMA radioligand therapy: relevance and preventive strategies. J Nucl Med. 2018;59:1172–1173. [DOI] [PubMed] [Google Scholar]
- 13. Yilmaz B, Nisli S, Ergul N, Gursu RU, Acikgoz O, Cermik TF. Effect of external cooling on 177Lu-PSMA uptake by the parotid glands. J Nucl Med. 2019;60:1388–1393. [DOI] [PubMed] [Google Scholar]
- 14. Bander NH, Milowsky MI, Nanus DM, Kostakoglu L, Vallabhajosula S, Goldsmith SJ. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J Clin Oncol. 2005;23:4591–4601. [DOI] [PubMed] [Google Scholar]
- 15. Rupp NJ, Umbricht CA, Pizzuto DA, et al. First clinicopathologic evidence of a non-PSMA-related uptake mechanism for 68Ga-PSMA-11 in salivary glands. J Nucl Med. 2019;60:1270–1276. [DOI] [PubMed] [Google Scholar]
- 16. Tagawa ST, Vallabhajosula S, Christos PJ, et al. Phase 1/2 study of fractionated dose lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Cancer. 2019;125:2561–2569. [DOI] [PubMed] [Google Scholar]
- 17. Wang H, Byun Y, Barinka C, et al. Bioisosterism of urea-based GCPII inhibitors: synthesis and structure-activity relationship studies. Bioorg Med Chem Lett. 2010;20:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eder M, Schafer M, Bauder-Wust U, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23:688–697. [DOI] [PubMed] [Google Scholar]
- 19. Navrátil M, Ptacek J, Sacha P, et al. Structural and biochemical characterization of the folyl-poly-gamma-l-glutamate hydrolyzing activity of human glutamate carboxypeptidase II. FEBS J. 2014;281:3228–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rousseau E, Lau J, Kuo HT, et al. Monosodium glutamate reduces 68Ga-PSMA-11 uptake in salivary glands and kidneys in a preclinical prostate cancer model. J Nucl Med. 2018;59:1865–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hodson NA, Linden RW. The effect of monosodium glutamate on parotid salivary flow in comparison to the response to representatives of the other four basic tastes. Physiol Behav. 2006;89:711–717. [DOI] [PubMed] [Google Scholar]
- 22. Fendler WP, Eiber M, Beheshti M, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–1024. [DOI] [PubMed] [Google Scholar]
- 23. Eiber M, Herrmann K, Calais J, et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59:469–478. [DOI] [PubMed] [Google Scholar]
- 24. Gafita A, Bieth M, Krönke M, et al. qPSMA: semiautomatic software for whole-body tumor burden assessment in prostate cancer using 68Ga-PSMA11 PET/CT. J Nucl Med. 2019;60:1–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kratochwil C, Fendler WP, Eiber M, et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur J Nucl Med Mol Imaging. 2019;46:2536–2544. [DOI] [PubMed] [Google Scholar]
- 26. Delker A, Fendler WP, Kratochwil C, et al. Dosimetry for 177Lu-DKFZ-PSMA-617: a new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:42–51. [DOI] [PubMed] [Google Scholar]
- 27. Yadav MP, Ballal S, Tripathi M, et al. Post-therapeutic dosimetry of 177Lu-DKFZ-PSMA-617 in the treatment of patients with metastatic castration-resistant prostate cancer. Nucl Med Commun. 2017;38:91–98. [DOI] [PubMed] [Google Scholar]
- 28. Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. [DOI] [PubMed] [Google Scholar]
- 29. Pollard JH, Raman C, Zakharia Y, et al. Quantitative test-retest measurement of 68Ga-PSMA-HBED-CC in tumor and normal tissue. J Nucl Med. 2020;61:1145–1152. [DOI] [PubMed] [Google Scholar]
- 30. Rathke H, Kratochwil C, Hohenberger R, et al. Initial clinical experience performing sialendoscopy for salivary gland protection in patients undergoing 225Ac-PSMA-617 RLT. Eur J Nucl Med Mol Imaging. 2019;46:139–147. [DOI] [PubMed] [Google Scholar]
- 31. Baum RP, Langbein T, Singh A, et al. Injection of botulinum toxin for preventing salivary gland toxicity after PSMA radioligand therapy: an empirical proof of a promising concept. Nucl Med Mol Imaging. 2018;52:80–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harsini S, Saprunoff H, Alden T, Mohammadi B, Wilson D, Bénard F. The effects of monosodium glutamate on PSMA radiotracer uptake in men with recurrent prostate cancer: a prospective, randomized, double-blind, placebo-controlled intraindividual imaging study. J Nucl Med. 2021;62:81–87. [DOI] [PubMed] [Google Scholar]
- 33. Paganelli G, Sarnelli A, Severi S, et al. Dosimetry and safety of 177Lu PSMA-617 along with polyglutamate parotid gland protector: preliminary results in metastatic castration-resistant prostate cancer patients. Eur J Nucl Med Mol Imaging. 2020;47:3008–3017. [DOI] [PubMed] [Google Scholar]
- 34. Majer P, Jancarik A, Krecmerova M, et al. Discovery of orally available prodrugs of the glutamate carboxypeptidase II (GCPII) inhibitor 2-phosphonomethylpentanedioic acid (2-PMPA). J Med Chem. 2016;59:2810–2819. [DOI] [PubMed] [Google Scholar]
- 35. Kratochwil C, Giesel FL, Leotta K, et al. PMPA for nephroprotection in PSMA-targeted radionuclide therapy of prostate cancer. J Nucl Med. 2015;56:293–298. [DOI] [PubMed] [Google Scholar]
- 36. Sarnelli A, Belli ML, Di Iorio V, et al. Dosimetry of 177Lu-PSMA-617 after mannitol infusion and glutamate tablet administration: preliminary results of EUDRACT/RSO 2016-002732-32 IRST protocol. Molecules. 2019;24:621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He K, Du S, Xun P, et al. Consumption of monosodium glutamate in relation to incidence of overweight in Chinese adults: China Health and Nutrition Survey (CHNS). Am J Clin Nutr. 2011;93:1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sunavala-Dossabhoy G. Radioactive iodine: an unappreciated threat to salivary gland function. Oral Dis. 2018;24:198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fernstrom JD, Cameron JL, Fernstrom MH, McConaha C, Weltzin TE, Kaye WH. Short-term neuroendocrine effects of a large oral dose of monosodium glutamate in fasting male subjects. J Clin Endocrinol Metab. 1996;81:184–191. [DOI] [PubMed] [Google Scholar]
- 40. Gaertner FC, Halabi K, Ahmadzadehfar H, et al. Uptake of PSMA-ligands in normal tissues is dependent on tumor load in patients with prostate cancer. Oncotarget. 2017;8:55094–55103. [DOI] [PMC free article] [PubMed] [Google Scholar]