Abstract
From the lipid fraction of a freeze-dried cell mass of a strain of the Mycobacterium avium-Mycobacterium intracellulare complex, a new glycolipid was isolated and was characterized as 5-mycoloyl-α-arabinofuranosyl (1→1′)-glycerol, mainly on the basis of nuclear magnetic resonance spectroscopy studies.
Mycobacterial cell envelopes are known to contain a range of characteristic antigenic glycolipids. From the envelope of strains of the Mycobacterium avium-Mycobacterium intracellulare complex, glycopeptidolipids (1–4) and 5-mycoloyl-β- arabinofuranosyl (1→2)-5-mycoloyl-α-arabinofuranosyl (1→1′)-glycerol (Gl-ai) (26) have been obtained. The glycopeptidolipids, formerly collectively termed “C-mycosides,” are reportedly serotype-specific antigens characterizing the M. avium-M. intracellulare complex and used for typing of the species. Use of combinations of the glycopeptidolipids from several different serotypes for serodiagnosis of the diseases caused by this species has also been reported (20, 24). Gl-ai, on the other hand, was considered to be a species-specific antigen, as discussed previously, because it was detected in all of the M. avium-M. intracellulare complex and Mycobacterium scrofulaceum strains tested (14, 26). Gl-ai has been shown to be a useful single antigen for serodiagnosis of this family of pathogens to give reliable information about the clinical stage of M. avium complex (MAC) disease patients (14). Later, Gl-ai was also detected in M. kansasii (28), but the content in M. kansasii seemed to be too low to interfere with the use of this glycolipid antigen in serodiagnosis of the diseases caused by strains of the M. avium-M. intracellulare complex.
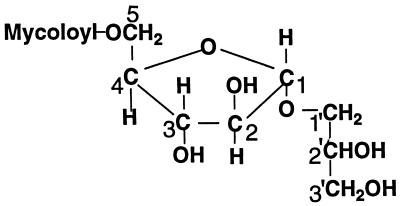
In the present study, we isolated from cells of strain KK-41-24 of the M. avium-M. intracellulare complex (obtained from the Type Culture Collection of the Research Institute of Tuberculosis, Kiyose, Tokyo [clinical isolate]), which had been shake cultured at 37°C for 3 to 4 weeks and freeze-dried, a new minor glycolipid, Gl-ai-II, and characterized it as 5-mycoloyl-α-arabinofuranosyl (1→1′)-glycerol, mainly by the nuclear magnetic resonance (NMR) spectroscopy studies of the glycolipid and its acetate (Fig. 1). Whether the arabinose was a d- or l-arabinose was not examined. However, since the arabinofuranoses in the majority of the cell envelope glycolipids are all reportedly of the d form, the arabinose in the structure of Gl-ai-II in Fig. 1 is shown in the d form.
FIG. 1.
Structure of Gl-ai-II.
The crude glycolipid fraction prepared from the freeze-dried cell mass, as described previously (26), was placed on a silica gel column (Fuji-Davidson’s microbead silica gel 4B, 200/350 mesh). After being washed with hexane-CHCl3 (1:1 [vol/vol]) and CHCl3, the column was eluted with CHCl3-methanol (MeOH) (94:6 [vol/vol]). The earlier eluates contained mainly Gl-ai, and the later eluates contained other more polar glycolipids, as shown by thin-layer chromatography (TLC) on Merck 5547 plates {development with CHCl3-MeOH (92:8 [vol/vol]) or toluene-acetone (65:35 [vol/vol])}. Detection was by heating after the samples had been sprayed with a phosphomolybdic acid reagent (5% [wt/vol] in ethanol [EtOH]) or a 1-naphthol reagent {3% [wt/vol] in H2SO4-H2O-EtOH (65:40:400 [vol/vol])}. The later eluates were combined and subjected to repeated preparative TLC on Merck 5744 plates {development with CHCl3-MeOH (92:8 [vol/vol])}. Detection under UV light after the samples had been sprayed with a rhodamine 6G solution (0.01% [wt/vol] in EtOH) led to the isolation of pure Gl-ai-II. The yield was about 0.02% (wt/wt) of the freeze-dried cell mass (about 10% of the yield of the major glycolipid, Gl-ai).
The alkaline and acid hydrolysis of Gl-ai-II performed as described previously (26) revealed that it consisted of arabinose, glycerol, and mycolic acid. Matrix-assisted laser desorption ionization–time-of-flight/mass spectrometry (MALDI-TOF/MS) of Gl-ai-II acetate on a PerSeptive Biosystems Voyager RS (reflector mode, with dihydroxybenzoic acid as the matrix) showed that the Gl-ai-II molecule consisted of one glycerol, one arabinose and one mycoloyl residue with an average molecular weight of about 1,250.
Since Gl-ai-II had no acetyl group, as revealed by the 1H-NMR spectrum, and since better resolution of 1H-NMR signals was obtained by acetylation of the hydroxyl groups, the glycolipid was acetylated in pyridine-Ac2O (1:1 [vol/vol]) at room temperature overnight, and detailed NMR spectral analysis of its peracetate was performed with a Bruker model DRX 500 spectrometer (500 MHz for 1H-NMR and 125 MHz for 13C-NMR) with CDCl3.
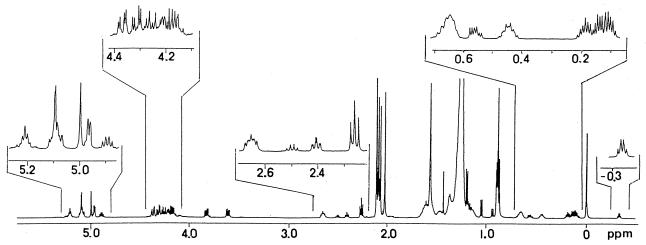
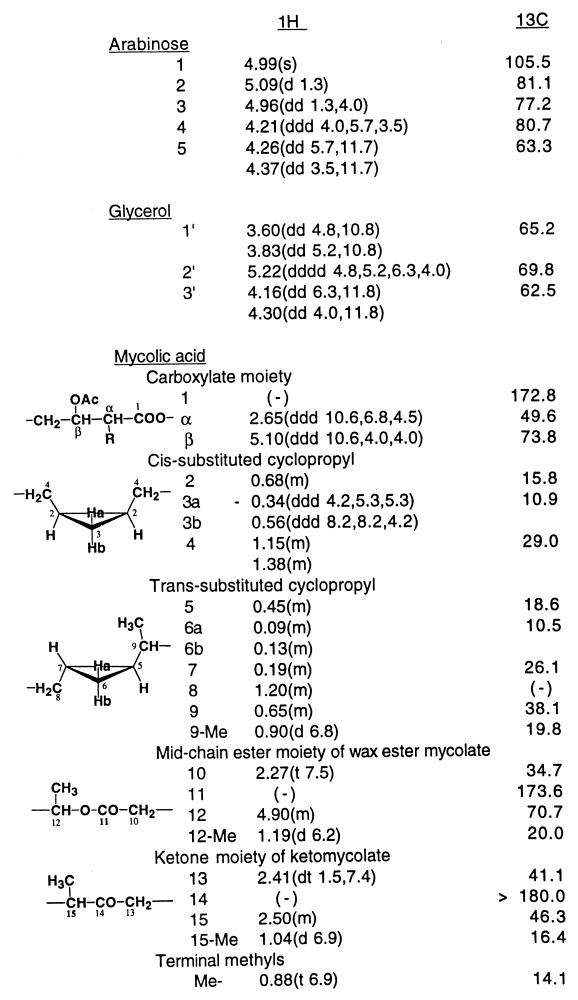
The 1H-NMR spectrum of Gl-ai-II pentaacetate, with the proton NMR signals of acetoxymethyls at 2.01, 2.06, 2.08, and 2.10 (2 methyls) (Fig. 2), was generally similar to that of Gl-ai acetate. The only major difference observed was that Gl-ai-II had proton signals due to one pentose unit and one mycolic acid residue, as expected from its mass spectral data, whereas Gl-ai had proton signals corresponding to two pentose units and two mycolic acid residues. Assignment of the proton and carbon signals in the 1H- and 13C-NMR spectra of Gl-ai-II acetate was made on the basis of 1H-1H- and 13C-1H-NMR COSY analyses. Table 1 gives the chemical shifts of the proton and carbon signals caused by the arabinose, glycerol, and representative groups of mycolates of Gl-ai-II acetate.
FIG. 2.
Proton NMR spectrum of Gl-ai-II acetate in CDCl3.
TABLE 1.
Chemical shifts (in parts per million) of 1H- and 13C-NMR signals of Gl-ai-II acetate in CDCl3a
Data in parentheses are coupling constants in hertz. s, singlet; d, doublet; m, multiplet; (−), nonexistent or not identified.
The 13C-1H-NMR COSY heteronuclear multiple-bond coherence spectrum revealed the linkages between the three components. Thus, the signal from the end carboxylic acid carbonyl carbon of mycolic acid at 172.8 ppm was correlated with the signals from the arabinose-5 protons at 4.26 and 4.37 ppm, the mycolic acid α-proton at 2.65 ppm, and the mycolic acid β-proton at 5.10 ppm, revealing that the mycolic acid formed an ester linkage with the arabinose-5 hydroxyl group.
The signal of the arabinose-1 carbon at 105.5 ppm was correlated with the signals of glycerol-1′ protons at 3.60 and 3.83 ppm, the arabinose-2 proton at 5.09 ppm, and the arabinose-3 proton at 4.96 ppm, showing that the arabinose formed a glycosidic linkage with glycerol-1′. The signal of glycerol-1′ carbon at 65.2 ppm was correlated with the signals of the arabinose-1 anomeric proton at 4.99 ppm, the glycerol-2′ proton at 5.22 ppm, and the glycerol-3′ protons at 4.16 and 4.30 ppm. The glycerol-2′ carbon signal at 69.8 ppm was correlated with the signals of the glycerol-1′ protons at 3.60 and 3.83 ppm, the glycerol-3′ protons at 4.16 and 4.30 ppm, and the methyl protons of the acetoxy group at 2.08 ppm, and the glycerol-3′ carbon signal at 62.5 ppm was correlated with the signals of the glycerol-1′ protons at 3.60 and 3.83 ppm, the glycerol-2′ proton at 5.22 ppm and the methyl protons of the acetoxy group at 2.06 ppm, showing that the glycerol-2′ and -3′ hydroxyls were free in original Gl-ai-II and confirming that the hydroxyl of glycerol-1′ was linked to arabinose-1.
Regarding the conformation of the glycosidic linkage of arabinose-1, the singlet signal of the anomeric proton of arabinose in the 1H-NMR spectrum suggested that it was an α form (23). Accordingly, Gl-ai-II was characterized as 5-mycoloyl-α-arabinofuranosyl (1→1′)-glycerol.
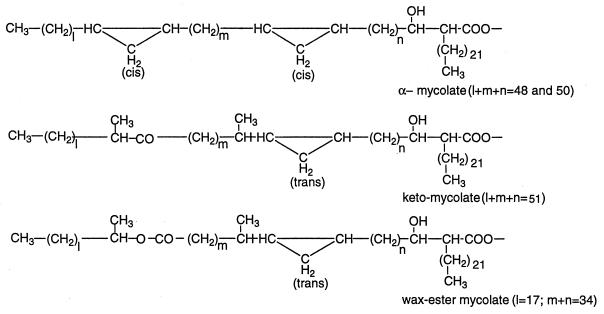
Regarding the mycolic acid residues, this species is known to contain three types of mycolic acids: α-, keto-, and wax ester mycolic acids (Fig. 3) (22). Alkaline hydrolysis of Gl-ai-II and subsequent methylation of the diethyl ether-soluble fraction of the hydrolysate with CH2N2, followed by separation by preparative TLC with hexane-diethyl ether (9:1 [vol/vol], three times), as described previously (26), gave an alcohol and α-, keto-, and ω-carboxymycolic acid methyl esters.
FIG. 3.
Structures of mycolates of the M. avium-M. intracellulare complex. Values in parentheses represent the respective major components.
MALDI-TOF/MS of the methylmycolates (Fig. 3) revealed that the major α-mycolic acid was C80 and C82 acids (l+m+n = 48 and 50, of about the same signal height) with some C78 and C84 homologues, that the major keto-mycolic acid was a C85 acid (l+m+n = 51) with some C83 and C87 homologues, and that the major ω-carboxymycolic acid was a C65 diacid (l+m = 34) with some C64 and C67 homologues. The major α-mycolic acid of this species is reported to be C80 acid with smaller amounts of its larger and smaller homologues (18, 19). The chain length of the α-alkyl group of these three mycolic acids was 22, as reported previously (18). Gas chromatography-mass spectrometry of the trimethylsilyl derivative of the alcohol showed that it was an almost pure C20 alcohol (l = 17) with traces of C22 and C18 alcohols.
The α-mycolic acid methyl ester was shown to have two cis-disubstituted cyclopropyl groups by the characteristic NMR signal of the highly shielded cis-disubstituted cyclopropyl proton at −0.34 ppm (2H), although it did not negate the possibility of the presence of a trans-disubstituted cyclopropyl group. The proton NMR spectra of the keto- and ω-carboxymycolates showed that their major disubstituted cyclopropyl groups were both trans. However, a high magnification of the NMR spectra in the higher magnetic field showed small peaks of the characteristic proton signal at −0.34 ppm, suggesting that the ketomycolate might contain about 1% and the ω-carboxymycolate might contain 5 to 10% of the cis-disubstituted cyclopropyl group.
Regarding the composition of the mycolates of Gl-ai-II, the ratios of the areas of proton signals characteristic of each type of mycolate provided a rough estimation of the composition of mycolates in Gl-ai-II. Thus, the areas of the signals at 2.65 (mycolic acid α-proton), 2.50, and 2.41 ppm (methine and methylene protons, respectively, adjacent to the ketone carbonyl of ketomycolate) and 2.27 ppm (methylene protons next to the mid-chain ester carbonyl of the wax ester mycolate) gave a molecular ratio between the total mycolate, ketomycolate, and wax ester mycolate of 3.45:0.92:1.84, and, accordingly, a molecular ratio between the α-, keto-, and wax ester mycolates in Gl-ai-II of 1:1.3:2.7. Different preparations of Gl-ai-II from different cultures of this strain gave about the same result. An analogous assay of the mycolic acid composition of the major glycolipid, Gl-ai, gave a ratio of 1:0.5:0.95 (26).
The antigenicity of Gl-ai-II was not tested: because its content was very low, its value in clinical diagnosis is not important, even if it was antigenic.
Gl-ai-II was detected as a minor glycolipid in all 12 of the strains tested in the previous work (26) (strains from the Type Culture Collection of the Research Institute of Tuberculosis, Tokyo, all forming the usual small smooth opaque colonies on egg slants), when hexane extracts of freeze-dried cell mass of these strains were subjected to TLC and the plates were sprayed with the 1-naphthol reagent.
Gl-ai-II, 5-mycoloyl-α-arabinofuranosyl (1→1′)-glycerol, constitutes part of the major glycolipid of the species Gl-ai, 5-mycoloyl-β-arabinofuranosyl (1→2)-5-mycoloyl-α-arabino-furanosyl (1→1′)-glycerol, whose glycosyl moiety, in turn, constitutes the end portion of the terminal mycolylated pentaarabinosyl motif of the mycobacterial cell wall arabinogalactan. As discussed previously, however, although Gl-ai and the terminal pentaarabinosyl unit share the same di-(mycoloyl-arabinosyl) structure, Gl-ai is not likely a degradation product of the cell wall arabinogalactan, since only the sera of active MAC patients gave very high anti-Gl-ai antibody titers, and scarcely any tuberculosis patients’ sera immunoreacted with Gl-ai (14). Gl-ai-II, in which the end 5-mycoloyl-β-arabinofuranosyl residue of Gl-ai is missing, is probably not a degradation product of the mycolylated pentaarabinosyl motif, either.
Major mycobacterial glycolipid antigens are often accompanied by several of their homologues, which normally differ in the saccharide moiety. For example, the glycosyl phenolphthiocerol dimycocerosates of M. kansasii include one major (10, 11) and six minor (13, 25, 28) homologues, those of Mycobacterium leprae contain one major (15, 16) and three minor (8, 12, 17) homologues, and those of Mycobacterium tuberculosis contain two major (5, 9, 27) and three minor (6, 7) homologues. Those coexisting minor homologues are generally regarded as possible intermediates in the biosynthesis of the respective major glycolipids of the species (6, 13). Accordingly, and since the yield was always about 10% of the major glycolipid Gl-ai, in young cultures (2 weeks) and in older cultures (3 and 4 weeks), Gl-ai-II might be considered analogously as an intermediate of the biosynthesis of Gl-ai.
Mycolylation of the terminal mycolylated pentaarabinofuranosyl motif of the cell wall arabinogalactan is reportedly an “all or none” event (21). It may be that the fully mycolylated pentaarabinofuranosyl motif is formed somewhere and then is linked to the major skeleton. In that light, those 5-mycolylated arabinofuranosyl compounds having the glycosidic linkages of the same nature as the pentaarabinosyl motif might somehow be related to the biosynthetic route of the cell wall arabinogalactan.
REFERENCES
- 1.Besra G S, Brennan P J. The glycolipids of mycobacteria. In: Fenselau C, editor. Mass spectrometry for the characterization of microorganisms. Washington, D.C: American Chemical Society; 1994. pp. 203–232. [Google Scholar]
- 2.Brennan P J, Goren M B. Structural studies on the type-specific antigens and lipids of the Mycobacterium avium/Mycobacterium intracellulare/Mycobacterium scrofulaceum serocomplex. J Biol Chem. 1979;254:4205–4211. [PubMed] [Google Scholar]
- 3.Brennan P J. Structures of the typing antigens of atypical mycobacteria: a brief review of present knowledge. Mycobacterium intracellulare serotype 9. Rev Infect Dis. 1981;3:905–913. doi: 10.1093/clinids/3.5.905. [DOI] [PubMed] [Google Scholar]
- 4.Brennan P J, Mayer H, Aspinall G O, Nam Shin J E. Structures of glycopeptidolipid antigens from serovars in the Mycobacterium avium/Mycobacterium intracellulare/Mycobacterium scrofulaceum serocomplex. Eur J Biochem. 1981;115:7–15. doi: 10.1111/j.1432-1033.1981.tb06190.x. [DOI] [PubMed] [Google Scholar]
- 5.Daffé M, Lacave C, Laneélle M A, Laneélle G. Structure of the major triglycosyl phenolphthiocerol of Mycobacterium tuberculosis. Eur J Biochem. 1987;167:155–160. doi: 10.1111/j.1432-1033.1987.tb13317.x. [DOI] [PubMed] [Google Scholar]
- 6.Daffé M, Laneélle M A, Lacave C, Laneélle G. Monoglycosyldiacylphenol-phthiocerol of Mycobacterium tuberculosis and Mycobacterium bovis. Biochim Biophys Acta. 1988;958:443–449. doi: 10.1016/0005-2760(88)90230-5. [DOI] [PubMed] [Google Scholar]
- 7.Daffé M. Further specific triglycosyl phenol phthiocerol diester from Mycobacterium tuberculosis. Biochim Biophys Acta. 1989;1002:256–270. doi: 10.1016/0005-2760(89)90295-6. [DOI] [PubMed] [Google Scholar]
- 8.Daffé M, Laneélle M A. Diglycosyl phenol phthiocerol diester of Mycobacterium leprae. Biochim Biophys Acta. 1989;1002:333–337. doi: 10.1016/0005-2760(89)90347-0. [DOI] [PubMed] [Google Scholar]
- 9.Daffé M, Servin P. Scalar, dipolar-correlated and J-resolved 2D NMR spectroscopy of the specific phenolic mycoside of Mycobacterium tuberculosis. Eur J Biochem. 1989;185:157–162. doi: 10.1111/j.1432-1033.1989.tb15097.x. [DOI] [PubMed] [Google Scholar]
- 10.Fournié J J, Rivierè M, Puzo G. Structural elucidation of the major phenolic glycolipid from Mycobacterium kansasii. J Biol Chem. 1987;262:3174–3179. [PubMed] [Google Scholar]
- 11.Fournié J J, Rivierè M, Papa F, Puzo G. Structural elucidation of major phenolic glycolipid from Mycobacterium kansasii. II. Presence of a novel dideoxyhexose. J Biol Chem. 1987;262:3180–3184. [PubMed] [Google Scholar]
- 12.Fujiwara T, Hunter S W, Cho S-N, Aspinell G O, Brennan P J. Chemical synthesis and serology of disaccharides and trisaccharides of phenolic glycolipid antigens from the leprosy bacillus and preparation of a disaccharide protein conjugate for serodiagnosis of leprosy. Infect Immun. 1984;43:245–252. doi: 10.1128/iai.43.1.245-252.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilleron M, Venisse A, Rivierè M, Servin P, Puzo G. Carbohydrate epitope structural elucidation by 1H-NMR spectroscopy of a new Mycobacterium kansasii phenolic glycolipid antigen. Eur J Biochem. 1990;193:449–475. doi: 10.1111/j.1432-1033.1990.tb19359.x. [DOI] [PubMed] [Google Scholar]
- 14.Honda I, Kawajiri K, Watanabe M, Toida I, Kawamata K, Minnikin D E. Evaluation of the use of 5-mycoloyl-β-arabinofuranosyl-(1→2)-5-mycoloyl-α-arabinofuranosyl-(1→1′)-glycerol in serodiagnosis of Mycobacterium avium-intracellulare complex infection. Res Microbiol. 1993;144:229–235. doi: 10.1016/0923-2508(93)90048-7. [DOI] [PubMed] [Google Scholar]
- 15.Hunter S W, Brennan P J. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J Bacteriol. 1981;147:728–735. doi: 10.1128/jb.147.3.728-735.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter S W, Fujiwara T, Brennan P J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J Biol Chem. 1982;257:15072–15078. [PubMed] [Google Scholar]
- 17.Hunter S W, Brennan P J. Further specific extracellular phenolic glycolipid antigens and a related diacylphthiocerol from Mycobacterium leprae. J Biol Chem. 1983;258:7556–7562. [PubMed] [Google Scholar]
- 18.Kaneda K, Naito S, Imaizumi S, Yano I, Mizuno S, Tomiyasu I, Baba T, Kusunose E, Kusunose M. Determination of molecular species composition of C80 or longer-chain α-mycolic acids in Mycobacterium spp. by gas chromatography-mass spectrometry and mass chromatography. J Clin Microbiol. 1986;24:1060–1070. doi: 10.1128/jcm.24.6.1060-1070.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaneda K, Imaizumi S, Mizuno S, Baba T, Tsukamura M, Yano I. Structures and molecular species composition of three homologous series of α-mycolic acids from Mycobacterium spp. J Gen Microbiol. 1988;134:2213–2229. doi: 10.1099/00221287-134-8-2213. [DOI] [PubMed] [Google Scholar]
- 20.Lee B-Y, Chatterjee D, Bozic C M, Brennan P J, Cohn D L, Bales J D, Harrison S M, Andron L A, Orme I M. Prevalence of serum antibody to the type-specific glycolipid antigens of Mycobacterium avium in human immunodeficiency virus-positive and -negative individuals. J Clin Microbiol. 1991;29:1026–1029. doi: 10.1128/jcm.29.5.1026-1029.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNeil M, Daffé M, Brennan P J. Location of the mycolyl ester substituents in the cell walls of mycobacteria. J Biol Chem. 1991;266:13217–13223. [PubMed] [Google Scholar]
- 22.Minnikin D E, Minnikin S M, Parlett J H, Goodfellow M, Magnusson M. Mycolic acid patterns of some species of mycobacterium. Arch Microbiol. 1984;139:225–231. doi: 10.1007/BF00402005. [DOI] [PubMed] [Google Scholar]
- 23.Mizutani K, Kasai R, Nakamura R, Tanaka O. NMR spectral study of α-and β-l-arabinofuranosides. Carbohydr Res. 1989;185:27–38. [Google Scholar]
- 24.Orme I M, Chatterjee D. Antibody level to the type-specific glycopeptidolipid antigens of Mycobacterium avium in homosexual men: possible implications (8th Forum in Microbiology) Res Microbiol. 1992;143:381–385. doi: 10.1016/0923-2508(92)90050-x. [DOI] [PubMed] [Google Scholar]
- 25.Rivierè M, Fournié J J, Puzo G. A novel mannose containing phenolic glycolipid from Mycobacterium kansasii. J Biol Chem. 1987;262:14879–14884. [PubMed] [Google Scholar]
- 26.Watanabe M, Kudoh S, Yamada Y, Iguchi K, Minnikin D E. A new glycolipid from Mycobacterium avium-Mycobacterium intracellulare complex. Biochim Biophys Acta. 1992;1165:53–60. doi: 10.1016/0005-2760(92)90075-7. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe M, Yamada Y, Iguchi K, Minnikin D E. Structural elucidation of new phenolic glycolipids from Mycobacterium tuberculosis. Biochim Biophys Acta. 1994;1210:174–180. doi: 10.1016/0005-2760(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe M, Aoyagi Y, Ohta A, Minnikin D E. Structures of phenolic glycolipids from Mycobacterium kansasii. Eur J Biochem. 1997;248:93–98. doi: 10.1111/j.1432-1033.1997.00093.x. [DOI] [PubMed] [Google Scholar]