Abstract
Purpose:
Dual blockade of Bruton’s tyrosine kinase with ibrutinib and selinexor has potential to deepen responses for patients with chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL).
Experimental Design:
In this phase I study (clinicaltrials.gov:NCT02303392), adult patients with CLL/NHL, relapsed/refractory to ≥1 prior therapy were enrolled. Patients received weekly oral selinexor and daily oral ibrutinib in 28-day cycles until progression or intolerance. Primary objective was to determine maximum tolerated dose (MTD).
Results:
Included patients had CLL (n=16) or NHL (n=18; 9 Richter’s transformation, 6 diffuse large B-cell lymphoma, and 3 mantle cell lymphoma). Median prior therapies was 4 (range=1–14) and 59% previously received ibrutinib. The established MTD was 40mg of selinexor (Days 1, 8, 15) and 420mg daily ibrutinib. Common non-hematologic adverse events were fatigue (56%), nausea (53%), anorexia (41%), and diarrhea (41%) and were mostly low grade. Overall response rate was 32%. An additional 47% achieved stable disease (SD), some prolonged (up to 36 months). Median progression-free survival for patients with CLL and NHL was 8.9 [(95%CI:3.9–16.1] and 2.7 (95%CI:0.7–5.4) months, respectively. For CLL patients who did not receive prior ibrutinib, only 20% (1/5) progressed. Estimated 2-year overall survival was 73.7% (95%CI:44.1–89.2%) and 27.8% (95%CI:10.1–48.9%) for CLL and NHL patients, respectively.
Conclusion:
The selinexor and ibrutinib combination has demonstrated tolerability in patients with relapsed/refractory CLL/NHL. Responses were durable. Notable responses were seen in CLL patients with minimal prior therapy. Future study of this combination will focus on efforts to deepen remissions in patients with CLL receiving ibrutinib therapy.
Introduction
For patients with chronic lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), and refractory diffuse large B-cell lymphoma (DLBCL), cure is very unlikely. Chronic activation of the B-cell receptor signaling pathway is critical for the survival of CLL, DLBCL, and MCL cells1–3. Bruton’s tyrosine kinase (BTK) is a key component of the B-cell receptor signaling pathway. Treatment with ibrutinib, an oral BTK inhibitor, demonstrated efficacy and tolerability and has been approved for the treatment of CLL, MCL, and other B-cell malignancies4–6. Ibrutinib must be given continuously to retain response and achievement of complete remission (CR) is rare4–6. Resistance to ibrutinib therapy is common and leads to poor clinical outcomes7,8. There is significant clinical need to develop therapeutic strategies to induce deeper remissions. Rationally designed combination strategies are one approach to this problem.
Selinexor is a selective oral inhibitor of exportin-1 (XPO1)-mediated nuclear transport. XPO1 is an essential shuttle protein that identifies cargo molecules including ribonucleoprotiens (RNPs) and tumor suppressor proteins (TSPs), facilitating their transport from the nucleus into the cytoplasm thereby regulating the sub-cellular localization of these cargos and influencing their activity9. XPO1 inhibition induces apoptosis and suppresses downstream effectors of B-cell activation, proliferation and migration in CLL cells10. XPO1 inhibition down-modulates BTK, and synergizes with ibrutinib in CLL cells cultured ex vivo, and in mouse models of acquired ibrutinib resistance. Selinexor’s ability to overcome ibrutinib-mediated resistance was attributed to the drug’s ability to target multiple B-cell receptor signaling nodes in a manner independent of BTK kinase activity, and thereby, preserving the ability to block adaptive signaling responses in ibrutinib-resistant subclones11. In a phase I trial of relapsed/refractory (RR) lymphoid malignancies selinexor was tolerable and resulted in an overall response rate (ORR) of 31%12. The maximum tolerated dose (MTD) was 60mg twice weekly 12. ORR was 28% in a phase II study of 127 RR DLBCL patients13. Median duration of response (DOR) was 9.3 months; 23 months in patients with CR13. Selinexor was approved for DLBCL patients.
With a goal of inducing deep remissions with synergistic dual blockade of BTK with ibrutinib and selinexor, we designed a phase 1 trial for RR CLL and NHL patients. We hypothesized that selinexor could be combined with continuous ibrutinib at a safe and tolerable dose. Herein, we report the results of this study.
Methods and Materials
Patients/Treatment
After obtaining written informed consent, patients with CLL, DLBCL, Richter’s Transformation (RT), or MCL were enrolled at Ohio State University and University of Utah. Eligible patients were ≥18 years, had ≥1prior therapy and ECOG performance status 0–1. CLL patients met criteria for therapy based on 2008 International Working Group for CLL (iwCLL) criteria. Patients received selinexor (per dose level) orally 1–2 times weekly (3 weeks of 4-week cycle) and daily oral ibrutinib (420mg) starting Cycle 1 Day 8. To minimize nausea and weight-loss previously associated with selinexor, dexamethasone (12mg or equivalent) and ondansetron (8mg or equivalent) were administered with each dose of selinexor. Dexamethasone could be tapered and discontinued per discretion of treating physician. Treatment cycles were repeated until disease progression, intolerance, death or discontinuation of trial participation. The study was conducted in accordance with the Declaration of Helsinki and approved by local Institutional Review Boards.
Pharmacodynamic/Pharmacokinetic Analysis
Pharmacodynamic/pharmacokinetic analyses were performed as described in the Supplementary Data.
Statistical Methods
The primary objective was to determine the MTD of the combination. Dose-limiting toxicity (DLT) was defined and evaluated as detailed in the Supplementary data. Dose-finding was guided by a Bayesian continual reassessment method using a two-parameter logistic model and a target DLT rate of 30%14. MTD was defined as the highest dose with model-estimated DLT rate <30% when the first of 10–12 patients were treated at a dose level or 36 patients were evaluated for DLT, at which 10 CLL and 10 NHL patients were to be treated in expansion cohorts. Adverse events (AEs) were graded according to Common Terminology Criteria of Adverse Events (CTCAE) version 4 and were summarized for all patients who received treatment.
Response was assessed by iwCLL 2008 for CLL patients or the 2007 International Working Group Response Criteria for NHL patients. Additional statistical methods are included in the Supplemental Data.
Data Availability Statement
The data generated in this study are available within the article and its Supplementary Data files.
Results
Patient Characteristics
Thirty-four patients were enrolled from June 2015 through July 2019. Included patients had diagnoses of CLL (n=16) or NHL (n=18; 9 RT, 6 DLBCL, and 3 MCL). Median age was 65 years (range=50–80). Median number of prior therapies was 4 (range=1–14), and 20 (59%) patients had previously received ibrutinib, including 11 (69%) of 16 patients with CLL. Baseline characteristics are detailed in Table 1.
Table 1.
Baseline Patient Characteristics
| CLL (n=16) | DLBCL* (n=6) | MCL (n=3) | Richter’s (n=9) | All (n=34) | |
|---|---|---|---|---|---|
|
| |||||
| Median age, years (range) | 61 (50–75) | 73.5 (52–80) | 70 (66–74) | 62 (59–78) | 65 (50–80) |
|
| |||||
| Male sex, no. (%) | 11 (69) | 4 (67) | 3 (100) | 5 (56) | 23 (68) |
|
| |||||
| Caucasian race, no. (%) | 16 (100) | 6 (100) | 3 (100) | 9 (100) | 34 (100) |
|
| |||||
| Median number of prior therapies, no. (range) | 3.5 (1–8) | 3.5 (1–8) | 4 (1–11) | 6 (3–14) | 4 (1–14) |
|
| |||||
| Number of prior therapies, no. (%) | |||||
| 1 | 3 (19) | 1 (17) | 1 (33) | 0 (0) | 5 (15) |
| 2 | 2 (13) | 1 (17) | 0 (0) | 0 (0) | 3 (9) |
| 3–8 | 11 (69) | 4 (67) | 1 (33) | 5 (56) | 21 (62) |
| 10–14 | 0 (0) | 0 (0) | 1 (33) | 4 (44) | 5 (15) |
|
| |||||
| Prior ibrutinib therapy, no. (%) | 11 (69) | 0 (0) | 1 (33) | 8 (89) | 20 (59) |
|
| |||||
| Prior acalabrutinib therapy, no. (%) | 1 (6) | 0 (0) | 0 (0) | 0 (0) | 1 (3) |
|
| |||||
| Prior venetoclax therapy, no. (%) | 1 (6) | 0 (0) | 1 (33) | 3 (33) | 5 (15) |
|
| |||||
| Prior idelalisib therapy, no. (%) | 2 (13) | 1 (17) | 0 (0) | 2 (22) | 5 (15) |
|
| |||||
| Prior autologous stem cell transplant, no. (%) | 1 (6) | 2 (33) | 1 (33) | 1 (11) | 5 (15) |
|
| |||||
| Prior allogeneic stem cell transplant, no. (%) | 1 (6) | 0 (0) | 0 (0) | 1 (11) | 2 (6) |
|
| |||||
| Del(11q) | 7 (44) | NA | NA | NA | NA |
|
| |||||
| Del(17p) | 4 (25) | NA | NA | NA | NA |
|
| |||||
| Unmutated IgVH | 16 (100) | NA | NA | NA | NA |
|
| |||||
| Complex karyotype | 13 (81) | NA | NA | NA | NA |
|
| |||||
| BTK Mutation | 10** | NA | NA | NA | NA |
1 GCB, 2 non GCB subtype, 3 not evaluated
9 C481S, 1 C481R/C481S, 6 not evaluated or unknown.
BTK=Bruton’s tyrosine kinase, CLL=chronic lymphocytic leukemia, DLBCL=diffuse large B-cell lymphoma, GCB=germinal center B-cell, MCL=mantle cell lymphoma
Safety
MTD is 40mg of selinexor weekly (D1, 8, 15 of 28-day cycle) and 420mg daily ibrutinib (dose level 1; DL1). After the first 2 evaluable patients treated at DL1 did not have DLT, a second cohort of 2 patients was treated at DL2 with 30mg of selinexor twice weekly and 420mg daily ibrutinib. While the patients did not have grade ≥3 fatigue, both patients noted that fatigue was limiting their daily activities. While the grade of the toxicity did not officially meet DLT criteria, they were considered to have experienced DLT since they were not able to continue on the planned dose of the selinexor. As such, DL2 and all higher dose levels were deemed intolerable.
At data cutoff, three patients remain on therapy. Of the 31 patients who discontinued therapy, median duration of therapy was 3.2 months (range=0.2–37 months). Most common reasons for therapy discontinuation were progressive disease (PD) (65%) and toxicity (23%). AEs leading to discontinuation were nausea and vomiting (n=2), pneumonitis (n=2), mouth sores, palpitations, and fatigue and abdominal cramping (n=1 each). Hematologic AEs of any grade were common with 20 (59%), 19 (56%), and 13 (38%) patients experiencing thrombocytopenia, anemia, and neutropenia, respectively. Grade ≥3 hematologic AEs were uncommon with 8 (24%), 6 (18%), and 4 (12%) patients experiencing thrombocytopenia, anemia, and neutropenia, respectively. Most common non-hematologic AEs of any grade were fatigue (56%), nausea (53%), anorexia (41%), and diarrhea (41%). Thirty-five nausea events occurred in 18 patients during treatment, 40%, 14%, 6%, 14%, 6%, 3%, and 6% in cycles 1, 2, 3, 4, 5–7, 16, and 22, respectively. Grade ≥3 non-hematologic AEs were uncommon with 6 patients (18%) experiencing hypertension and 5 patients (15%) experiencing asymptomatic hyponatremia (Table 2). A complete list of AEs can be found in Supplementary Table 2. AEs of interest are detailed in Table 2. There were 4 patient deaths while on study; 2 related to disease progression and 2 related to infection. The range of time from last recorded dose of study treatment to death was 7–81 days.
Table 2.
Any ≥ Grade 3 AEs That Occurred in ≥ 1 Patient and Non-Hematologic AEs of Interest
| Event of Interest | ≥ Grade 3 | Any Grade |
|---|---|---|
| Hematologic AEs, no. (%) | ||
| Thrombocytopenia | 8 (24) | 20 (59) |
| Anemia | 6 (18) | 19 (56) |
| Neutropenia | 4 (12) | 13 (38) |
| Lymphocyte count increased | 2 (6) | 10 (29) |
| Lymphopenia | 4 (12) | 9 (27) |
| Non-hematologic AEs, no. (%) | ||
| Fatigue | 0 (0) | 19 (56) |
| Nausea | 1 (3) | 18 (53) |
| Anorexia | 0 (0) | 14 (41) |
| Diarrhea | 2 (6) | 14 (41) |
| Hypertension | 6 (18) | 12 (35) |
| Joint/Bone Pain | 1 (3) | 12 (35) |
| Hyponatremia | 5 (15) | 10 (29) |
| Pneumonia | 3 (9) | 9 (27) |
| Hyperglycemia | 2 (6) | 9 (27) |
| Weight Loss | 0 (0) | 9 (27) |
| Fall | 2 (6) | 5 (15) |
| Bleeding* | 0 (0) | 4 (12) |
| Cataract | 2 (6) | 4 (12) |
| Cognitive Disturbance | 2 (6) | 4 (12) |
| Hypophosphatemia | 2 (6) | 4 (12) |
| Arthralgia | 0 (0) | 3 (9) |
| Atrial Fibrillation | 0 (0) | 3 (9) |
| Death | 3 (9) | 3 (9) |
| Hypoxia | 2 (6) | 2 (6) |
Grade 1/2 epistaxis (n=2), hematuria (n=1), menorrhagia (n=1)
Response to Therapy
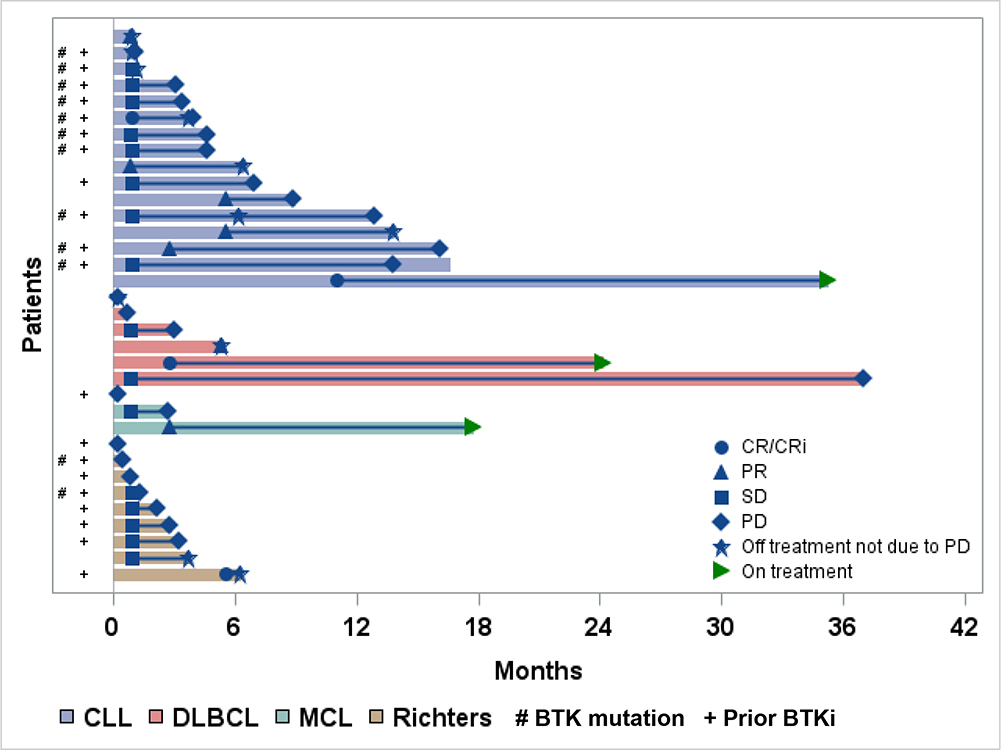
Of all patients (n = 34), the ORR was 32%. ORR for CLL, DLBCL, MCL, and RT were 44%, 33%, 33%, and 11%, respectively. An additional 16 (47%) patients maintained stable disease (SD; Supplementary Figure 5). Figure 1 depicts the DOR. Of the 16 patients with SD, 15 had >6 months of follow-up, and 20% (3/15) had maintained SD for >6 months. In the CLL cohort, 94% (15/16) achieved at least SD as the best response. The patient who achieved CR with no detectable residual disease received only one prior therapy, and discontinued selinexor after maintaining CR for almost 24 months. The patient who achieved CRi had previously received ibrutinib with a known BTK resistance mutation and a complex karyotype. This patient discontinued both agents secondary to presumed ibrutinib-related cardiac dysrhythmia toxicity while in CRi after a duration of remission of 3 months. Of 9 evaluable CLL patients with known BTK mutations, 2 responded to therapy (1 CRi, 1 PR) and 7 had SD. Median range of DOR or SD in this group was 3.7 (range=0.2–13.4) months. Of the 6 DLBCL patients, 1 achieved CR. The patient with CR had received one prior therapy and benefited from a prolonged 22-month DOR. One of two DLBCL patients with SD maintained SD for 36 months. Of 9 RT patients, 1 achieved CR and 5 achieved SD. The RT patient who achieved CR had 3 prior therapies, including ibrutinib. This patient discontinued therapy at one month after achieving CR to receive allogeneic hematopoietic stem cell transplantation. In the 5 RT patients who achieved SD, the median time from first achieving SD to progression or death was 1.3 months (range=0.4–4.5). In the 11 patients on study who achieved at least PR, only 3 had progressed or died, and the median DOR was not reached (NR) (95%CI:3.0 months-NR).
Figure 1. Treatment Duration and Best Response for Individual Patients By Disease Subtype.
Swimmers plot. The length of the bar indicates the time on treatment. CLL=chronic lymphocytic leukemia, CR=complete response, CRi=complete response with incomplete count recovery, DLBCL=diffuse large B-cell lymphoma, MCL=mantle cell lymphoma, PD=progressive disease, PR=partial response, SD=stable disease
Survival
Median progression-free survival (PFS) for CLL and NHL patients were 8.9 (95%CI:3.9–16.1) and 2.7 (95%CI:0.7–5.4) months, respectively (Figure 2A). Median PFS for CLL patients who received prior ibrutinib or did not was 4.6 months (95%CI:3.1–12.9) and not reached (95%CI:8.9-NR), respectively. For CLL patients who received ≥3 prior therapies, 91% (10/11) had PD. All but 1 of these patients had previously received ibrutinib. Only 1 of 5 patients (20%) who received 1–2 prior therapies progressed, and he had previously received ibrutinib. With median follow-up of 33.3 (range=12.9–45.3) and 24.2 (range=17.7–50.3) months among survivors, median OS for CLL and NHL patients was 58.9 (95%CI:19.6–58.9) and 5.1 (95%CI:3.0–14.8) months, respectively. Estimated 2-year OS was 73.7% (95%CI:44.1–89.2%) and 27.8% (95%CI:10.1–48.9%) for CLL and NHL patients, respectively (Figure 2B).
Figure 2. Progression-Free (A) and Overall Survival (B) by Disease Subtype.
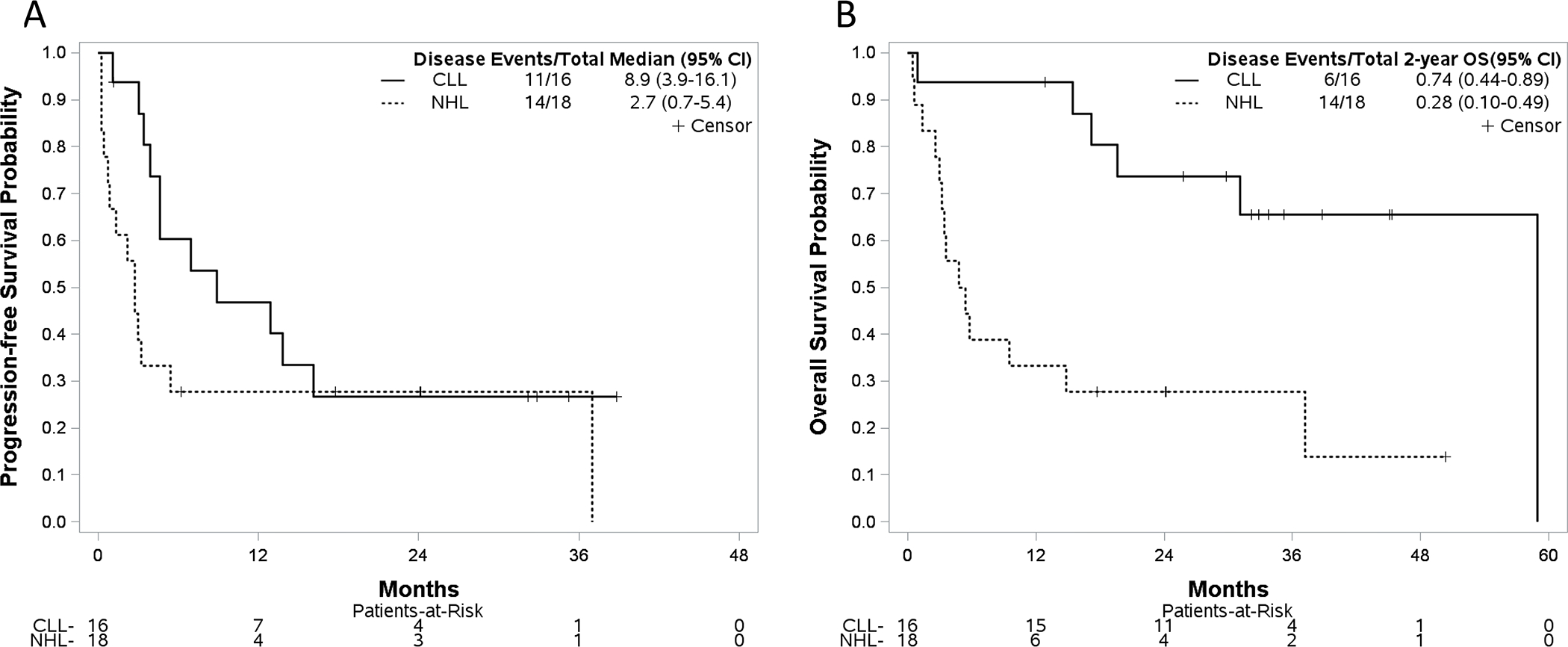
(A) Progression-free survival for patients with chronic lymphocytic leukemia [median 8.9 (95%CI: 3.9–16.1) months] and non-Hodgkin lymphoma [median 2.7 (95%CI: 0.7–5.4) months]. (B) Estimated 2-year overall survival was 73.7% (95%CI:44.1–89.2%) and 27.8% (95%CI:10.1–48.9%) for CLL and NHL patients, respectively.
Pharmacodynamic and Pharmacokinetic Analyses
A detailed summary of the pharmacodynamic and pharmacokinetic analyses can be found in the Supplementary Data. Notably, in the one RT patient who achieved CR, at least 2-fold nuclear accumulation was observed at the C1D1-post time point for c-Myc, FOXO3a, IκBα, and BCL2 proteins proteins (Supplementary Figure 2). At the C1D2, a reduction in Myc mRNA expression was observed in patients with better clinical response, whereas a significant increase in Myc mRNA was variably observed in patients with poor response.
The pharmacokinetic data suggests there is no major drug interaction between selinexor and ibrutinib.
Discussion
In this study, the combination of ibrutinib and selinexor was tolerable and responses were observed in this heavily pretreated population of CLL and NHL patients. Efficacy may be greater in earlier lines of therapy. Further investigation of this combination is needed to optimize response rates and durability.
When compared to studies of single-agent ibrutinib or selinexor in patients with RR lymphoid malignancies, the combination resulted in slightly more toxicity than what was seen with single-agent ibrutinib15,16. The most common grade 1/2 AEs demonstrated in this phase 1 combination study were fatigue (56%), nausea (50%), anorexia (41%), and diarrhea (35%). These toxicities were similar to what was seen with single-agent selinexor with 49%, 65%, 56%, and 32% and experiencing grade 1/2 fatigue, nausea, anorexia, and diarrhea, respectively12. In a phase II study of patients with relapsed DLBCL treated with single-agent selinexor similar rates of grade 1/2 fatigue (36%), nausea (52%), anorexia (33%), and diarrhea (32%) were observed.13 As expected, the same toxicities were slightly more than what was seen with single-agent ibrutinib with 37%, 41%, 42%, and 30% of patients experiencing grade 1/2 nausea, fatigue, diarrhea and anorexia, respectively15. The comparable toxicity seen in this combination study is likely related to the lower and less frequent dosing of selinexor than what was used in the single-agent phase 1 selinexor study (60mg in 2 weekly doses) and the aggressive prophylactic measures implemented on this study to minimize nausea and weight-loss associated with the drug. Dexamethasone and ondansetron were administered with each dose of selinexor. The incidence of nausea events decreased after cycle 1, and most patients were able to taper or discontinue the dexamethasone over time indicating that tolerance of nausea improved over time for the patients on this combination study. Of note, there are now data to support that the second-generation BTK inhibitors acalabrutinib and zanubrutinib cause less toxicity than ibrutinib17,18. Additionally, third generation BTK inhibitors such as pirtobrutinib and ARQ-531 have very favorable toxicity profiles19,20. A combination of one of these newer agents with selinexor could potentially lead to less toxicity than demonstrated in this study.
The ORR derived from the combination for patients with DLBCL was 33%, which was similar to the ORR demonstrated in patients with DLBCL treated with single-agent selinexor in the phase II study (28%).13 As there were only 6 patients with DLBCL on our study, limited data precludes determination of whether cell of origin affects response rates. Notably, in the two DLBCL patients who achieved SD, one maintained SD status for 36 months and the other for 2 months. Maintaining stable disease control with this regimen could allow patients to be bridged to another therapy, such as chimeric antigen receptor T-cell therapy.
Similarly, patients with RT typically have aggressive disease and five of nine were able to achieve SD with this combination therapy. The median duration of SD was 1.3 months (range=0.4–4.5), which could prolong survival long enough to allow for these patients to be bridged to another therapy or clinical trial. Notably, the one patient with RT who achieved CR demonstrated a robust pharmacodynamic response following initiation of selinexor treatment, where inhibiting XPO1-mediated nuclear export resulted in significant nuclear accumulation of proteins including p53, FOXO3a, MYC, and BCL2. This patient was then able to transition to more definitive therapy with an allogeneic hematopoietic stem cell transplantation. Interestingly, while variably observed in this limited sample set, a significant increase in Myc mRNA expression following selinexor treatment was observed in patients with poor clinical response, whereas a decrease in Myc expression was predominantly observed in patients with better response. Aberrant Myc expression has been linked to CLL progression and RT, warranting further evaluation of Myc as a prognostic indicator following ibrutinib/selinexor combination therapy.
The responses demonstrated by the combination was notable in two CLL populations: those with known BTK mutations and those with minimal prior therapy. Two of 10 CLL patients with known BTK mutations had response to therapy and 7 had SD. This indicates that the dual inhibition of BTK may be able to overcome ibrutinib resistance. All 3 CLL patients who received only one line of prior therapy responded to this combination; 1 achieved CR with no detectable residual disease, the other 2 had PR. The patient with CR discontinued selinexor after achieving response and has a current DOR of almost 24 months. This response is notable as ibrutinib alone rarely leads to CR and even more rarely leads to remission with no residual disease. This indicates that early dual inhibition of BTK with this combination may deepen responses seen with ibrutinib. As such, a planned expansion cohort aims to study this combination in CLL patients with only one prior line of therapy.
In summary, this study combining selinexor (40mg weekly) and ibrutinib (420mg daily) induced responses and demonstrated tolerability in patients with RR B-cell malignancies. The combination held similar toxicities to what has been demonstrated in studies with single-agent selinexor. Patients with DLBCL and RT maintained disease control with the regimen that may allow for bridging to other therapies. Responses were seen in patients with CLL who had minimal prior therapy. Future study of this regimen will focus on efforts to deepen remissions in patients with CLL receiving ibrutinib therapy.
Supplementary Material
Statement of Translational Relevance:
Dual blockade of Bruton’s tyrosine kinase with ibrutinib and selinexor has the potential to deepen responses for patients with chronic lymphocytic leukemia (CLL) and non-Hodgkin lymphoma (NHL). In this phase I study, adult patients with relapsed/refractory CLL (n=16) or NHL (n=18) were enrolled. The established MTD was 40mg of selinexor (Days 1, 8, 15) and 420mg daily ibrutinib. Common non-hematologic adverse events were mostly low grade. Overall response rate was 32%. An additional 47% of patients achieved stable disease (SD), some prolonged (up to 36 months). Median progression-free survival for patients with CLL and NHL was 8.9 and 2.7 months, respectively. The selinexor and ibrutinib combination induced responses and was tolerated in patients with relapsed/refractory CLL/NHL. Notable responses were seen in CLL patients with minimal prior therapy. Future study of this combination will focus on efforts to deepen remissions in patients with CLL receiving ibrutinib therapy.
Acknowledgements
The authors would like to thank the following funding sources for research conducted resulting in the preparation of this manuscript:
NIH/NCI grant K23 CA212271: D. Stephens
NIH/NCI grant NCATS TL1 TR002735: J. Walker
NIH/NCI R35 CA197734: J. Byrd
NIH/NCI R01 CA192928: R. Lapalombella, J. Woyach, and J. Byrd
Leukemia and Lymphoma Society Scholar in Clinical Research grant CDP 2331-20: K. A. Rogers
Images were generated using instruments and services at the Campus Microscopy and Imaging Facility (CMIF) at The Ohio State University. This facility is supported in part by NCI grant P30 CA016058-45.
Footnotes
Conflicts of Interest: DS has received research funding from Acerta Pharma, Gilead Sciences, Karyopharm Therapeutics, Mingsight, Arqule, Novartis, Verastem, Juno Therapeutics. She has received consulting fees from Pharmacyclics/Janssen, Karyopharm Therapeutics, Beigene, Innate, AstraZeneca, Abbvie, CSL Behring, Celegene, TG Therapeutics, and Innate Pharma. BH has received research funding from Roche, Miragen, Celgene and CRISPR Therapeutics. HS has received research funding from Epizyme, Seattle Genetics and Beigene. KR has received research funding from Genentech, AbbVie, Janssen, and Novartis, has consulted for Acerta Pharma, Genentech, AbbVie, Pharmacyclics, AstraZeneca, and Innate Pharma, and received travel funding from AstraZeneca. SB has received consulting fees from AstraZeneca and Beigene. She has received honoraria from AstraZeneca, OncLive, and Aptitude Health. SJ has received research funding from Kite, Novartis, Juno/BMS, and Caribou Biosciences. She has received consulting fees from Kite, Novartis, Juno/BMS, CRISPR Therapeutics, and Takeda. RL is on the medical advisory board for Vincera Pharmaceutical, Inc. JCB has received research funding from AstraZeneca, Gilead, Karyopharm, Celgene, Pharmacyclics/Abvie, Kartos, Telios, and Newave. He has consulted for Jannsen, Pharmacyclics, AstraZeneca, Novartis, Trillium, and Syndax. He is a founder and major stock owner in Vincera Pharmaceutical, Inc. JAW has received research funding from Karyopharm, Pharmacyclics, Janssen, and Schrodinger. She has received consulting fees from Abbvie, AstraZeneca, Beigene, Pharmacyclics, Janssen, Genentech, and Loxo. YH, AR, JSW, DC, CC, QF, SB, RV, HL have no relevant conflicts of interest to disclose.
Clinical Trial Registration Number: www.clinicaltrials.gov NCT02303392
References
- 1.Davis RE, Ngo VN, Lenz G, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463(7277):88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baran-Marszak F, Boukhiar M, Harel S, et al. Constitutive and B-cell receptor-induced activation of STAT3 are important signaling pathways targeted by bortezomib in leukemic mantle cell lymphoma. Haematologica. 2010;95(11):1865–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ringshausen I, Schneller F, Bogner C, et al. Constitutively activated phosphatidylinositol-3 kinase (PI-3K) is involved in the defect of apoptosis in B-CLL: association with protein kinase Cdelta. Blood. 2002;100(10):3741–3748. [DOI] [PubMed] [Google Scholar]
- 4.Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. The New England journal of medicine. 2013;369(1):32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. The New England journal of medicine. 2013;369(6):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nature medicine. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of Ibrutinib Therapy Discontinuation and Outcomes in Patients With Chronic Lymphocytic Leukemia. JAMA oncology. 2015;1(1):80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheah CY, Chihara D, Romaguera JE, et al. Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Annals of Oncology : Official Journal of the European Society for Medical Oncology / ESMO. 2015. [DOI] [PubMed] [Google Scholar]
- 9.Arnaoutov A, Azuma Y, Ribbeck K, et al. Crm1 is a mitotic effector of Ran-GTP in somatic cells. Nature cell biology. 2005;7(6):626–632. [DOI] [PubMed] [Google Scholar]
- 10.Lapalombella R, Sun Q, Williams K, et al. Selective inhibitors of nuclear export show that CRM1/XPO1 is a target in chronic lymphocytic leukemia. Blood. 2012;120(23):4621–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hing ZA, Mantel R, Beckwith KA, et al. Selinexor is effective in acquired resistance to ibrutinib and synergizes with ibrutinib in chronic lymphocytic leukemia. Blood. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuruvilla J, Savona M, Baz R, et al. Selective inhibition of nuclear export with selinexor in patients with non-Hodgkin lymphoma. Blood. 2017;129(24):3175–3183. [DOI] [PubMed] [Google Scholar]
- 13.Kalakonda N, Maerevoet M, Cavallo F, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. The Lancet Haematology. 2020;7(7):e511–e522. [DOI] [PubMed] [Google Scholar]
- 14.Piantadosi S, Fisher JD, Grossman S. Practical implementation of a modified continual reassessment method for dose-finding trials. Cancer Chemother Pharmacol. 1998;41(6):429–436. [DOI] [PubMed] [Google Scholar]
- 15.Advani RH, Buggy JJ, Sharman JP, et al. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) has significant activity in patients with relapsed/refractory B-cell malignancies. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31(1):88–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuruvilla J, Gutierrez M, Shah BD, et al. Preliminary Evidence Of Anti Tumor Activity Of Selinexor (KPT-330) In a Phase I Trial Ofa First-In-Class Oral Selective Inhibitor Of Nuclear Export (SINE) In Patients (pts) With Relapsed / Refractory Non Hodgkin’s Lymphoma (NHL) and Chronic Lymphocytic L…. Blood. 2013;122(21):90–90. [Google Scholar]
- 17.Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J Clin Oncol. 2021:Jco2101210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hillmen P, Brown JR, Eichhorst BF, et al. ALPINE: zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Future Oncology. 2020;16(10):517–523. [DOI] [PubMed] [Google Scholar]
- 19.Mato AR, Shah NN, Jurczak W, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021;397(10277):892–901. [DOI] [PubMed] [Google Scholar]
- 20.Woyach J, Stephens DM, Flinn IW, et al. Final Results of Phase 1, Dose Escalation Study Evaluating ARQ 531 in Patients with Relapsed or Refractory B-Cell Lymphoid Malignancies. Blood. 2019;134(Supplement_1):4298–4298. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available within the article and its Supplementary Data files.