Abstract
Introduction
Pressure injury (PI) impacts the quality of life, and socioeconomic and psychological well-being negatively in persons with Spinal Cord Injury (SCI). Autologous Platelet Rich Plasma (PRP) and Platelet Rich Fibrin (PRF) showed promising roles in wound healing. PRF is considered a second-generation PRP, contains more growth factors and is more biocompatible than PRP. It possesses an additional favourable impact on wound healing due to its three-dimensional fibrin architecture, and antimicrobial property. There are no studies on PRF membrane use for PI healing in SCI.
Case presentation
A 25-year-old male with operated traumatic T10 American Spinal Injury Association Impairment Scale grade A paraplegia with neurogenic bowel, and bladder and a stage II PI over the left greater trochanter, was admitted for inpatient rehabilitation. The chronic non-healing PI which did not show any improvement following normal saline (0.9%) dressing for the past 3 months, was treated with autologous PRF membrane weekly for four weeks. The PI healed completely and no adverse events were noted. Weekly total scores of the Spinal Cord Impairment Pressure Ulcer Monitoring Tool and Pressure Ulcer Scale for Healing were 6, 6, 5, 2, 0 and 12, 10, 10, 3, and 0 respectively.
Discussion
To the best of our knowledge, this is the first case report on the healing of PI in SCI with the use of PRF. This novel biomaterial is a safe and effective promising agent for PI management in SCI. But further randomized trials are needed to establish stronger evidence regarding feasibility and effectiveness.
Subject terms: Spinal cord diseases, Megakaryocytes
Introduction
Pressure injury (PI) is one of the most serious secondary complications following spinal cord injury (SCI). The global magnitude of PIs among the population with SCI is around 32% [1]. The altered physiological status of SCI along with its numerous consequences like immobility, sensory-motor impairments, incontinence of bowel-bladder, etc constitute the major risk factors for PIs [2]. The high cost of management of PI and its complication makes it an economic burden for this population [3]. Moreover, it also affects the psychosocial well-being and quality of life negatively [4, 5].
Among all the available treatment options for PIs, nothing is superior to the other and in the chronic phase of SCI, PIs become difficult to manage. Platelet-rich plasma (PRP) has been studied for the treatment of PIs in SCI previously [6–8]. Platelet-rich fibrin (PRF) is considered a second-generation PRP which possesses potential characteristics for wound healing [9–12]. It is found to be safe and promising in ‘hard-to-heal’ skin ulcers [13]. Though PRF has been tried with promising results in dental procedures, maxillofacial surgeries and in a few cases of neuropathic ulcers due to leprosy, it has never been tried in PIs in people with SCI. In this case, we used PRF as a dressing material in a chronic non-healing PI in an individual with SCI, and in a short duration complete healing of the PI was observed.
Case presentation
A 25-year-old, unmarried, right-handed male with operated traumatic T10 American Spinal Injury Association (ASIA) Impairment Scale (AIS) grade A paraplegia, presented to our rehabilitation unit 16-month post-injury. He had neurogenic bladder and bowel and had developed a pressure injury (stage II and without signs of infection according to NPUAP classification) over the left greater trochanter 3 months back and it had not improved following dressing with normal saline (0.9%) daily. He was admitted for inpatient rehabilitation and weekly dressing with PRF was planned for PI. The patient was hemodynamically stable and had no fever, anaemia, thrombocytopenia, history of malignancy, coagulation disorders, cardiac disorders, and was not on any non-steroidal anti-inflammatory drug (NSAIDs), steroid medication, fibrinolytic therapy, or anticoagulant.
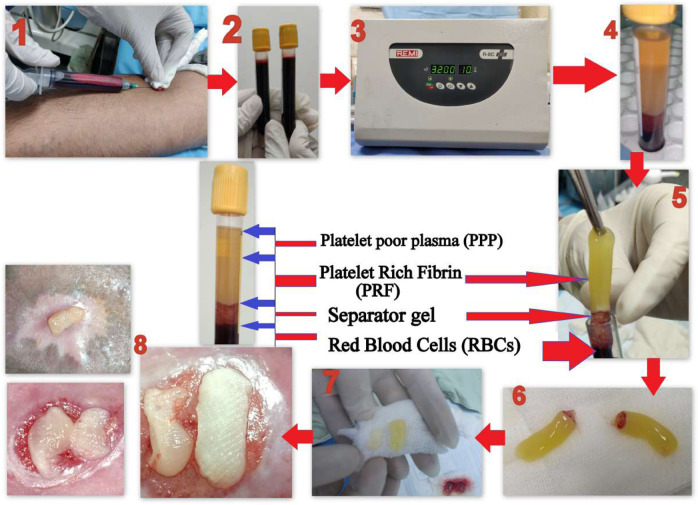
For preparing PRF 10 ml of venous blood was collected under all aseptic precautions from his antecubital vein into a vial (with no anticoagulant) and was immediately (within 2 min) centrifuged at 3200 revolutions per minute (rpm) for 10 min (min) using REMI (R-8C Plus) centrifuge machine. This yielded a natural fibrin matrix gel which was compressed between two sterile gauze pieces into a thin membrane rich in platelets. This was placed directly over the ulcer base, was covered with a sterile dressing, and was left in situ for 7 days. This was repeated weekly for a total of 4 weeks. Details of the step-by-step procedure have been depicted in the schematic illustration [Fig. 1].
Fig. 1. Step-by-step procedure of PRF preparation and dressing.
1 Drawing of venous blood from patient. 2 Collection of blood into anticoagulant free vial (clotted vial). 3 Centrifugation of sample (3200 rpm 10 min). 4 PFR formation in the vial. 5 Obtaining PRF by sterile forceps. 6 Separated PRF from separator gel. 7 Compressing by gauge to make PRF membrane. 8 Putting PRF membrane over ulcer base for dressing.
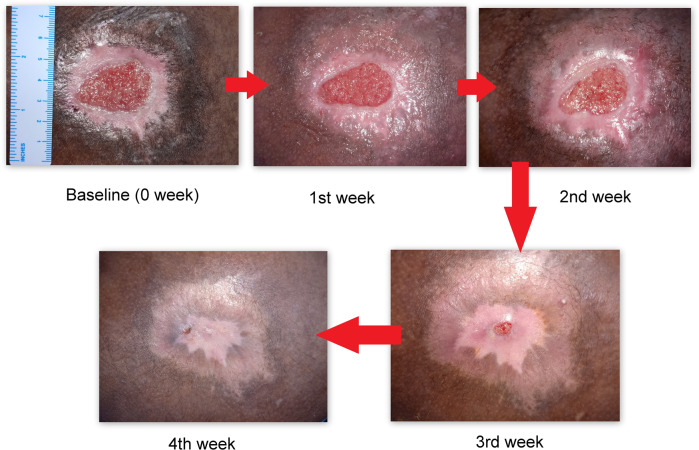
A baseline assessment of PI was done and images were taken before the starting of PRF dressing and these assessments were repeated weekly. The first reassessment done 7 days after the initiation of PRF dressing showed the formation of significant granulation tissue at the base of PI. This trend was observed during the subsequent weekly reassessments and complete healing of PI happened by the 4th week of PRF dressing.
At baseline, the PI was oval in shape and had a pink-red floor with a surrounding area of hypopigmentation, followed by hyperpigmentation. At the end of the first week, there was a significant increase in pink-red granulation tissue, a reduction in the size of the wound and the area of surrounding hyperpigmentation. A further decrease in size was noted during the following reassessments and at the end of the third-week maximum healing was observed, areas of pigment changes disappeared completely except for a small area of hypopigmentation, and at the end of 4th week, PI had healed completely [Figs. 2 and 3].
Fig. 2.
Weekly pressure injury images following weekly PRF membrane dressing.
Fig. 3.
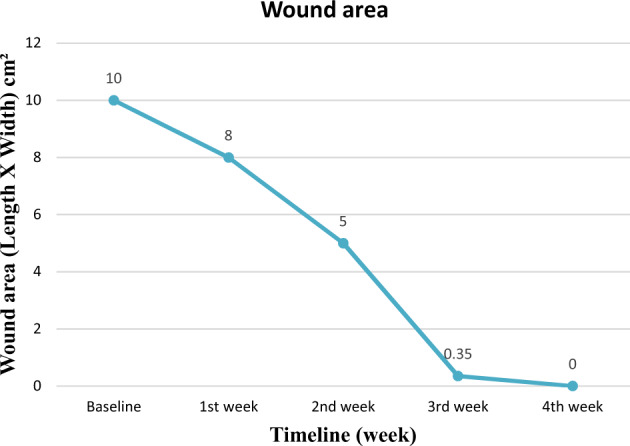
Graph showing wound area (length width) changes with time.
As the initial stage was II, NPUAP staging could not be used for follow-up [14], we used more detailed scales to monitor the healing process such as Spinal Cord Impairment Pressure Ulcer Monitoring Tool (SCI-PUMT) [15] and Pressure Ulcer Scale for Healing (PUSH tool 3.0) [16]. Furthermore, weekly pictorial documentation was also carried out.
SCI-PUMT was 6 at baseline, 6 at the end of the first week, 5 at the end of the second week, 2 at the end of the 3rd week, and completely healed (complete epithelisation) at the end of the 4th week [Fig. 4]. PUSH total score was 12 at baseline, 10 at the end first week and second week and 3 at the end of the third week and zero (healed) at the end of the fourth week [Fig. 5]. Though PUSH total score was the same in the first and second week, there was a reduction in length X width from 8 cm2 to 5 cm2.
Fig. 4.
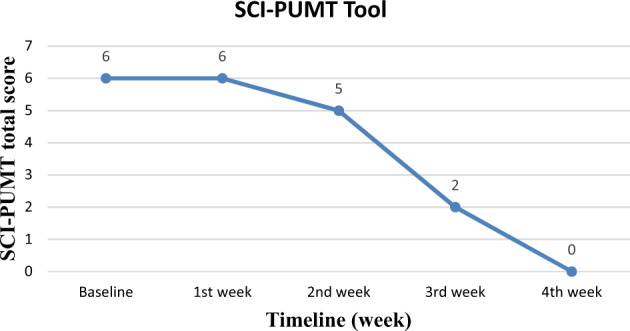
Graph showing changes in SCI-PUMT total score with time.
Fig. 5.
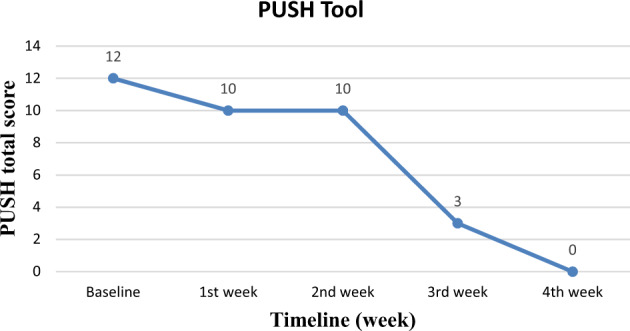
Graph showing changes in PUSH tool total score with time.
He was taught posture-positioning with pressure relief techniques and recommended nutritional care (protein: approximately 1.5 g/kg of body weight/day, calories: 30 Kcal/kg of body weight/day) was also followed [17]. His routine blood investigations (complete hemogram, liver function tests, kidney function tests) were within normal range.
To continue the follow-up during the Covid-19 pandemic and lockdown period, he received telerehabilitation services and on later dates, he also took a physical visit to the rehabilitation outpatient department. There is currently no recurrence of PI at the concerned site. There were no immediate adverse reactions noted and also till now no untoward side effects have been observed.
Discussion
To the best of our knowledge, this is the first case report of healing of PI following PRF dressing in people with SCI. Though few studies have documented the effectiveness of PRP in the healing of PIs in persons with SCI [6, 18], there are no studies on PRF for pressure injuries in SCI. Multiple in-vitro [19–21], and in-vivo studies on animal [22], and human subjects [23–27] on the use of PRF in wound healing and chronic ulcers favour its use.
Autologous PRP (first generation) contains a 6–8-fold increased amount of growth factors than whole blood. Eventually, it has been popular in the treatment of wound healing but with time it was also noticed that the use of anticoagulants may cause a delay in wound healing. Second-generation PRP then came into the picture where anticoagulants are not utilized for its preparation. It was named PRF which additionally contains white blood cells. It was first prepared by Choukroun et al. [28]. Multiple human studies used a centrifugation speed of 3000-3500 rpm for 10–15 min [29], but in our case, a well-formed PRF was prepared after 10 min of centrifugation at 3200 rpm. In the three-dimensional scaffold of PRF, apart from platelets, macrophages also play a key role in wound healing by the secretion of growth factors such as transforming growth factor-beta, platelet-derived growth factors (PDGF), and vascular endothelial growth factor [30, 31] and the neutrophils remove debris and microbes thus preventing infections at the wound site. Furthermore, mesenchymal stem cells (MSCs) in the fibrin meshwork of PRF also have a profound role in the healing and regeneration [32]. Thus, PRF assists in tissue regeneration, angiogenesis, and infection prevention leading to a collaborative impact on wound healing.
A Cochrane review suggested that PRP might be useful in the healing of diabetic foot ulcers but the quality of evidence was low [33]. In a study done on three patients of SCI in which a sustained-release PRP preparation was used for the treatment of chronic pressure injuries, an accelerated rate of wound healing was observed [18]. In a prospective case series of patients with SCI, histopathological evidence of ulcer healing was noted following the use of PRP [19]. Moreover, a recent systematic review based on in-vitro studies has also concluded in favour of PRF in terms of cellular proliferation, migration, adhesion, differentiation, and reduced inflammation leading to wound healing [34]. Despite multiple studies on tissue regeneration and repair with PRF conclusive evidence is currently lacking for wound healing [29].
An in-vivo study by Tunali et. al where PRF was prepared with 3500 rpm for 15 min, showed new connective tissue formation in a rabbit model of wound healing within 30 days [22]. In our human model (SCI) of wound healing, we used 3200 rpm for 10 min and healing took place within 30 days but new connective tissue formation was evident even as early as 7 days. Though a few previous studies on PRF showed contrasting results [23, 35], it was found to be a safe and effective option in treating recalcitrant ulcers [24]. One study on the antimicrobial property of PRF revealed that it induces the expression of hBD-2, an antimicrobial agent that helps in healing chronic wounds [36]. In chronic PIs in SCI, Staphylococcus aureus is the most prevalent (35%) bacteria causing wound infection [37], and PRF is known to possess antimicrobial properties against biofilm-producing Staphylococcus aureus [38]. This may imply the safety of PRF use in SCI in terms of infection. Another in-vitro study revealed that the PRF membrane showed gradual and increased release of growth factors up to the 19th day and then consecutive slow but constant release till the 23rd day [21]. Such release is influenced by the fibrin meshwork [39, 40]. In our case also maximum healing was observed at the end of 3rd week.
PRF is more biocompatible than PRP as additives are not required for the preparation of PRF and here the natural property of autologous blood is involved in fibrin formation. When the rate of release of growth factors is compared, it is early and immediate in the case of PRP whereas PRF shows extended release partly due to its composition which prevents early proteolysis of growth factors [41] and also due to their higher concentration [42].
In stage II PI, non-operative wound care is usually the treatment of choice (moist dressing most of the time) [43], and other methods like debridement and antibacterial ointments are not indicated. Modalities like negative pressure wound therapy, autologous PDGFs, surgical management and adjunctive therapies such as electrical stimulation, hyperbaric oxygen etc. are also available. In non-healing chronic ulcers, the natural healing process is deranged leading to an abnormally long duration of the inflammatory phase of healing. Reducing the inflammatory phase, in particular, can help in accelerating the healing process. In such a context, PRF is a good option as it has anti-inflammatory potential.
Advantages we found with the use of PRF are that it requires changing wound dressing less frequently, thus reducing the risk for wound infection and improving patient compliance. In addition, it does not involve any biochemical handling of blood. The only disadvantage is that it may not be feasible in home settings. One might encounter practical challenges while using PRF dressing such as patients having large or multiple PIs, in which cases PRF dressing might have to be done either in multiple sittings on the same day or on different days, the reason being large PIs require large PRF membrane which in turn needs large amount patient’s blood for preparation. Considering all factors, PRF would be a good option for both outpatients as well as inpatient rehabilitation.
Though there are studies on the use of PRF in ulcers of different grades due to different aetiologies (diabetic foot ulcers, venous ulcers, etc) there are no studies on PI in SCI [24–26]. Theoretically, it might be possible to use PRF in PIs of stages III & IV, practically it might be difficult as mentioned earlier due to needing a PRF membrane of larger size for dressing in a single sitting. Further studies are required to comment on this aspect in particular.
We would also like to emphasize that, while using PRF in persons with SCI, the general contraindications followed in the PRP preparation for regenerative therapy (thrombocytopenia, anticoagulation therapy, systemic steroid use, sepsis etc.) should be followed [44]. In addition, as the role of growth factors in oncogenesis is already known [45], and the fact that rapid growth of granulation tissue is observed with PRF we would like to recommend against the use of PRF in PIs where locally suspicious abnormal growth is seen or in cases of history of any malignancy.
PRF is safe and may be an effective option in PI management in SCI. Since it is autologous, the risk of transmission of infection, immunological reactions and cost of PI management are minimal. Further research on feasibility, effectiveness, cost-effectiveness and safety in the long-term period in people with SCI should be evaluated in randomized trials, and also comparative study with normal saline (0.9%) dressing would be needed. A future histopathological study and growth factor estimation studies might throw light on the microscopic and molecular basis of the effects of such biomaterial in PIs in persons with SCI.
Supplementary Information
Data availability
All the authors confirm that the data supporting the findings of this study are available within the article. Further details, if needed, can be elucidated from the corresponding author on a reasonable scientific request.
Competing interests
These authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41394-022-00540-8.
References
- 1.Shiferaw WS, Akalu TY, Mulugeta H, Aynalem YA. The global burden of pressure ulcers among patients with spinal cord injury: A systematic review and meta-analysis. BMC Musculoskelet Disord. 2020;21:334.. doi: 10.1186/s12891-020-03369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Byrne DW, Salzberg CA. Major risk factors for pressure ulcers in the spinal cord disabled: A literature review. Spinal Cord. 1996;34:255–63. doi: 10.1038/sc.1996.46. [DOI] [PubMed] [Google Scholar]
- 3.Chan BC, Nanwa N, Mittmann N, Bryant D, Coyte PC, Houghton PE, et al. The average cost of pressure ulcer management in a community dwelling spinal cord injury population. Int Wound J. 2013;10:431–40. doi: 10.1111/j.1742-481X.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lala D, Dumont FS, Leblond J, Houghton PE, Noreau L. Impact of pressure ulcers on individuals living with a spinal cord injury. Arch Phys Med Rehabil. 2014;95:2312–9. doi: 10.1016/j.apmr.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Adriaansen JJE, Ruijs LEM, van Koppenhagen CF, van Asbeck FWA, Snoek GJ, van Kuppevelt D, et al. Conditions and quality of life in persons living with spinal cord injury for at least ten years. J Rehabil Med. 2016;48:853–60. doi: 10.2340/16501977-2166. [DOI] [PubMed] [Google Scholar]
- 6.Singh R, Rohilla RK, Dhayal RK, Sen R, Sehgal PK. Role of local application of autologous platelet-rich plasma in the management of pressure ulcers in spinal cord injury patients. Spinal Cord. 2014;52:809–16. doi: 10.1038/sc.2014.144. [DOI] [PubMed] [Google Scholar]
- 7.Rappl LM. Effect of platelet rich plasma gel in a physiologically relevant platelet concentration on wounds in persons with spinal cord injury. Int Wound J. 2011;8:187–95. doi: 10.1111/j.1742-481X.2011.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scevola S, Nicoletti G, Brenta F, Isernia P, Maestri M, Faga A, et al. Allogenic platelet gel in the treatment of pressure sores: a pilot study. Int Wound J. 2010;7:184–90. doi: 10.1111/j.1742-481X.2010.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, Dohan SL, et al. Platelet-rich fibrin (PRF): A secondgeneration platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radio Endod. 2006;101:e56. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A secondgeneration platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radio Endod. 2006;101:e37. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): a secondgeneration platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radio Endod. 2006;101:e45. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): a secondgeneration platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radio Endod. 2006;101:e51. doi: 10.1016/j.tripleo.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Wan Y, Lin Y, Jiang H. Platelet-rich fibrin and concentrated growth factors as novel platelet concentrates for chronic hard-to-heal skin ulcers: A systematic review and Meta-analysis of randomized controlled trials. J Dermatolog Treat. 2020:1–9. 10.1080/09546634.2020.1773386. [DOI] [PubMed]
- 14.NPUAP Position Statement on Staging – 2017 Clarifications. 2017. https://cdn.ymaws.com/npuap.site-ym.com/resource/resmgr/position_statements/npuap-position-statement-on-.pdf. Accessed on 11 August, 2021.
- 15.Thomason SS, Luther SL, Powell-Cope GM, Harrow JJ, Palacios P. Validity and reliability of a pressure ulcer monitoring tool for persons with spinal cord impairment. J Spinal Cord Med. 2014;37:317–27. doi: 10.1179/2045772313Y.0000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stotts NA, Rodeheaver GT, Thomas DR, Frantz RA, Bartolucci AA, Sussman C, et al. An instrument to measure healing in pressure ulcers: Development and validation of the pressure ulcer scale for healing (PUSH) J Gerontol A Biol Sci Med Sci. 2001;56:M795–M799. doi: 10.1093/gerona/56.12.M795. [DOI] [PubMed] [Google Scholar]
- 17.Dorner B, Posthauer ME, Thomas D. The role of nutrition in pressure ulcer prevention and treatment: National Pressure Ulcer Advisory Panel white paper. Adv Ski Wound Care. 2009;22:212–21. doi: 10.1097/01.ASW.0000350838.11854.0a. [DOI] [PubMed] [Google Scholar]
- 18.Sell SA, Ericksen JJ, Reis TW, Droste LR, Bhuiyan MB, Gater DR, et al. A case report on the use of sustained release platelet-rich plasma for the treatment of chronic pressure ulcers. J Spinal Cord Med. 2011;34:122–7. doi: 10.1179/107902610X12923394765616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundquist R, Dziegiel MH, Agren MS. Bioactivity and stability of endogenous fibrogenic factors in plateletrich fibrin. Wound Repair Regen. 2008;16:356. doi: 10.1111/j.1524-475X.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- 20.Lundquist R, Holmstrøm K, Clausen C, Jørgensen B, Karlsmark T. Characteristics of an autologous leukocyte and platelet-rich fibrin patch intended for the treatment of recalcitrant wounds. Wound Repair Regen. 2013;21:66. doi: 10.1111/j.1524-475X.2012.00870.x. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee A, Debnath K. Comparative evaluation of growth factors from platelet concentrates: An in vitro study. J Indian Soc Periodontol. 2019;23:322–8. doi: 10.4103/jisp.jisp_678_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tunalı M, Özdemir H, Küçükodacı Z, Akman S, Fıratlı E. In vivo evaluation of titanium-prepared platelet-rich fibrin (T-PRF): A new platelet concentrate. Br J Oral Maxillofac Surg. 2013;51:438. doi: 10.1016/j.bjoms.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Steenvoorde P, van Doorn LP, Naves C, Oskam J. Use of autologous platelet-rich fibrin on hard-to-heal wounds. J Wound Care. 2008;17:60. doi: 10.12968/jowc.2008.17.2.28179. [DOI] [PubMed] [Google Scholar]
- 24.Jørgensen B, Karlsmark T, Vogensen H, Haase L, Lundquist R. A pilot study to evaluate the safety and clinical performance of leucopatch, an autologous, additive-free, platelet-rich fibrin for the treatment of recalcitrant chronic wounds. Int J Low Extrem Wounds. 2011;10:218. doi: 10.1177/1534734611426755. [DOI] [PubMed] [Google Scholar]
- 25.Crisci A, Marotta G, Licito A, Serra E, Benincasa G, Crisci M, et al. Use of Leukocyte Platelet (L-PRF) Rich Fibrin in Diabetic Foot Ulcer with Osteomyelitis (Three Clinical Cases Report) Diseases. 2018;6:30.. doi: 10.3390/diseases6020030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagaraju U, Sundar PK, Agarwal P, Raju BP, Kumar M. Autologous Platelet-rich Fibrin Matrix in Non-healing Trophic Ulcers in Patients with Hansen’s Disease. J Cutan Aesthet Surg. 2017;10:3–7. doi: 10.4103/JCAS.JCAS_17_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anandan V, Jameela WA, Saraswathy P, Sarankumar S. Platelet rich plasma: Efficacy in treating trophic ulcers in leprosy. J Clin Diagn Res. 2016;10:WC06–WC09. doi: 10.7860/JCDR/2016/21899.8758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choukroun J, Adda F, Schoefflfflffler C, Vervelle A. Une opportunite’ en paro-implantologie: Le PRF. Implantodontie. 2001;42:55–62. [Google Scholar]
- 29.Miron RJ, Fujioka-Kobayashi M, Bishara M, Zhang Y, Hernandez M, Choukroun J. Platelet-rich fibrin and soft tissue wound healing: A systematic review. Tissue Eng Part B Rev. 2017;23:83–99. doi: 10.1089/ten.teb.2016.0233. [DOI] [PubMed] [Google Scholar]
- 30.Adamson R. Role of macrophages in normal wound healing: An overview. J Wound Care. 2009;18:349. doi: 10.12968/jowc.2009.18.8.43636. [DOI] [PubMed] [Google Scholar]
- 31.Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB. Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. J Periodontol. 2010;81:546–55. doi: 10.1902/jop.2009.090531. [DOI] [PubMed] [Google Scholar]
- 32.Bensaïd W, Triffitt JT, Blanchat C, Oudina K, Sedel L, Petite HA, et al. biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24:2497–502. doi: 10.1016/S0142-9612(02)00618-X. [DOI] [PubMed] [Google Scholar]
- 33.Martinez-Zapata MJ, Martí-Carvajal AJ, Solà I, Expósito JA, Bolíbar I, Rodríguez L, et al. Autologous platelet-rich plasma for treating chronic wounds. Cochrane Database Syst Rev. 2016;CD006899; 10.1002/14651858.CD006899.pub3. [DOI] [PMC free article] [PubMed]
- 34.Strauss FJ, Nasirzade J, Kargarpoor Z, Stähli A, Gruber R. Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: A systematic review of in vitro studies. Clin Oral Investig. 2020;24:569–84. doi: 10.1007/s00784-019-03156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danielsen P, Jorgensen B, Jorgensen LN, Agren MS, Karlsmark T. Effect of topical autologous plateletrich fibrin versus no intervention on epithelialization of donor sites and meshed split-thickness skin autografts: A randomized clinical trial. Plast Reconstr Surg. 2008;122:1431. doi: 10.1097/PRS.0b013e318188202c. [DOI] [PubMed] [Google Scholar]
- 36.Bayer A, Lammel J, Rademacher F, Groß J, Siggelkow M, Lippross S, et al. Platelet-released growth factors induce the antimicrobial peptide human beta-defensin-2 in primary keratinocytes. Exp Dermatol. 2016;25:460. doi: 10.1111/exd.12966. [DOI] [PubMed] [Google Scholar]
- 37.Dana AN, Bauman WA. Bacteriology of pressure ulcers in individuals with spinal cord injury: What we know and what we should know. J Spinal Cord Med. 2015;38:147–60. doi: 10.1179/2045772314Y.0000000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jasmine S, A T, Janarthanan K, Krishnamoorthy R, Alshatwi AA. Antimicrobial and antibiofilm potential of injectable platelet rich fibrin-a second-generation platelet concentrate-against biofilm producing oral staphylococcus isolates. Saudi J Biol Sci. 2020;27:41–46. doi: 10.1016/j.sjbs.2019.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dohan Ehrenfest DM, Bielecki T, Jimbo R, Barbé G, Del Corso M, Inchingolo F, et al. Do the fibrin architecture and leukocyte content influence the growth factor release of platelet concentrates? An evidence-based answer comparing a pure platelet-rich plasma (P-PRP) gel and a leukocyte- and platelet-rich fibrin (L-PRF) Curr Pharm Biotechnol. 2012;13:1145–52. doi: 10.2174/138920112800624382. [DOI] [PubMed] [Google Scholar]
- 40.Bai MY, Wang CW, Wang JY, Lin MF, Chan WP. Three-dimensional structure and cytokine distribution of platelet-rich fibrin. Clin (Sao Paulo) 2017;72:116–24. doi: 10.6061/clinics/2017(02)09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lundquist R, Dziegiel MH, Agren MS. Bioactivity and stability of endogenous fibrogenic factors in platelet-rich fibrin. Wound Repair Regen. 2008;16:356–63. doi: 10.1111/j.1524-475X.2007.00344.x. [DOI] [PubMed] [Google Scholar]
- 42.Masuki H, Okudera T, Watanebe T, Suzuki M, Nishiyama K, Okudera H, et al. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (APRF), and concentrated growth factors (CGF) Int J Implant Dent. 2016;2:1–6. doi: 10.1186/s40729-016-0052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kruger EA, Pires M, Ngann Y, Sterling M, Rubayi S. Comprehensive management of pressure ulcers in spinal cord injury: current concepts and future trends. J Spinal Cord Med. 2013;36:572–85. doi: 10.1179/2045772313Y.0000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jain NK, Gulati M. Platelet-rich plasma: A healing virtuoso. Blood Res. 2016;51:3–5. doi: 10.5045/br.2016.51.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heinemann V, Jehn U. Growth factors. A new dimension in understanding oncogenesis. Klin Wochenschr. 1985;63:740–6. doi: 10.1007/BF01733825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the authors confirm that the data supporting the findings of this study are available within the article. Further details, if needed, can be elucidated from the corresponding author on a reasonable scientific request.