Before a new tracer can be used in clinical research, it is customary to perform dosimetry scans in animals and humans to assess whether the radiation exposure is acceptable. The main parameter to assess the radiation exposure is the effective dose, which is expressed in sieverts and defined as the tissue-weighted sum of the equivalent doses in the different organs. According to the U.S. Food and Drug Administration, new radiotracers require an investigational-new-drug application. Although there are no formal dose limitations for investigational new drugs, most institutions limit the yearly effective dose from research scans to 50 mSv. European countries apply a limit of 10 mSv for minor-to-intermediate risk levels, based on the medical exposures directive (97/43/Euratom) established by the European Commission. Sometimes, the dose to individual organs is needed as well, especially for tracers administered under the conditions specified in the Radioactive Drug Research Committee regulations.
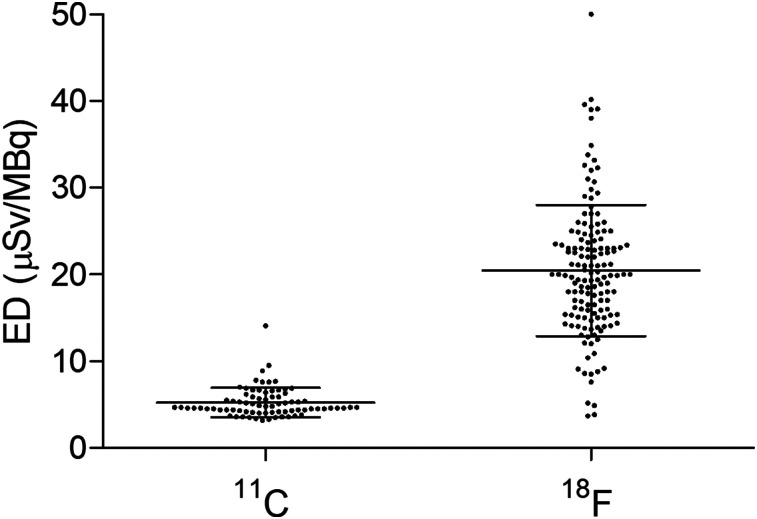
New PET tracers are commonly labeled with either 11C or 18F. However, these 2 isotopes are different from a dosimetric standpoint, because the average effective dose from 11C tracers (5.2 ± 1.7 μSv/MBq; n = 77) (Supplemental Table 1; supplemental materials are available at http://jnm.snmjournals.org) is about one fourth the average effective dose from 18F tracers (20.5 ± 7.6 μSv/MBq; n = 144) (Supplemental Table 2). In addition, 11C doses have a smaller variability than 18F doses: the dose range is 3.2–14.1 μSv/MBq for 11C (a 4-fold difference) and 3.7–50 μSv/MBq for 18F (a ratio of 13.5).
We argue that performing 11C dosimetry scans is antithetical to 2 widely accepted principles that govern medical ethics committees, namely to reduce animal experimentation and to avoid unnecessary radiation exposure to the general public.
Instead, 11C dosimetry scans for new tracers should be abandoned in both animals and humans and replaced by a standard average dose of 5 μSv/MBq. This would not compromise the safety of healthy volunteers and patients and would not significantly reduce the accuracy of dose estimation because, first, dose calculations in animals, even primates, have little predictive value for humans and, second, the results obtained from human dosimetry, in terms of both effective dose and organ dose, are dependent mostly on how the dose is calculated.
As Figure 1 clearly shows, 11C dosimetry estimations are remarkably consistent, with only 1 outlying value: the dose of 14.1 μSv/MBq for the serotonin 1A receptor tracer 11C-WAY-100635 (1), which stands at about 7 SDs from the average of the other 11C tracers. Arguably, extreme dose values may be explained by methodologic issues rather than by biodistribution. The dosimetry of 11C-WAY-100635 in rats was estimated at 4.1 μSv/MBq (MRC Cyclotron Unit of Hammersmith Hospital, unpublished data). In addition, tracers for the same target, and labeled with the same isotope, should not be radically different from a biologic and biophysical point of view: the effective dose of 11C-CUMI-101, also a tracer for serotonin 1A receptors, is only 5.3 μSv/MBq (2). In any case, even if the dose of 14.1 μSv/MBq for 11C-WAY-100635 was correct, it would still be about 1 SD below the average dose for 18F tracers. Notably, the 18F group also has 1 major outlier: the dose from 18F-tetrafluoroborate was estimated at the very high value of 50 μSv/MBq in healthy volunteers (average between the male dose at 36 μSv/MBq and the female dose at 64 μSv/MBq) in 1 study (3), but the estimated value in another study was 32.6 μSv/MBq (4) despite the dose having been calculated in thyroid cancer patients, and no significant differences between male and female doses were reported.
FIGURE 1.
Scatterplots showing estimation of human effective dose (ED) for 11C tracers (n = 77) and for 18F tracers (n = 144). Dose of 11C tracers is about one fourth that of 18F tracers, and its variability lower.
Even without considering extreme outliers, variations around the mean values are largely due to how the dose is calculated. For instance, the choice of using a dynamic bladder model and its voiding time may significantly affect the final dose. The doses for different voiding times are not systematically reported, but, for example, a faster voiding schedule would reduce the effective dose of 11C-flumazenil by 13% (5) and that of 18F-CP-18 by 61% (6).
Comparing the dose obtained by 2 different teams for the same tracer is a useful natural experiment to evaluate the weight of dose calculation approaches. In the literature, there are 21 tracers for which human dosimetry has been estimated more than once by 2 different teams. In 18 of these, the effective dose was reported for both tracers. The average relative difference among these 18 tracers was 42% (Supplemental Table 3). Only for 3 tracers did the 2 teams find a dose difference smaller than 10%.
The dose to the target organ is estimated even more variably than the effective dose. For organs that can void their content, the dose is largely dependent on the voiding parameters simulated in the study. To take the tracers above described, a faster bladder voiding reduced the dose to the bladder by 33% for 11C-flumazenil (5) and by 74% for 18F-CP-18 (6). Similarly, the dose to the gallbladder, the target organ for 18F-fluortriopride, was reduced by 71% by a fatty meal (7). Among the 21 tracers with at least 2 dosimetry evaluations by different teams, for only 11 tracers did the 2 teams identify the same target organ, with an average relative difference in dose of 165% (and a median of 72%) (Supplemental Table 3).
In summary, given their narrow variability around the mean value of 5 μSv/MBq, the dosimetry estimates reported in 11C papers could be as different as the dose found for another 11C tracer, had a different team performed the analysis or a different methodology to calculate the dose been used.
Among the animals used to extrapolate the human dose, monkeys are a better model than rodents, because they are more closely related to humans. Monkeys, however, are not widely available, are expensive, and require sophisticated medical monitoring.
We verified the agreement in terms of effective dose (Supplemental Table 4) and target organ doses (Supplemental Table 5) of 16 11C tracers and 21 18F tracers for which dosimetry from human and nonhuman primates was available. For both groups of tracers, monkey scans unpredictably under- or overestimated the human effective dose, with a mean absolute percentage difference of 31%. Of these 37 tracers, the target organ was reported for both species in 32. Of these 32 pairs of studies, in only 11 was the target organ the same in both monkeys and humans, and the monkey dose poorly predicted the human dose (mean difference of 42 absolute percentage points). To highlight the impact of methodology on the outcome of dosimetry studies, the same team (or part of the same team) performed the calculations for both species in 9 of the 11 tracers for which the target organ was the same, but the same team was responsible for 13 of 21 studies for which the target organ was different.
Finally, we wish to make clear that we advocate abandoning dosimetry scans only for 11C ligands, not for isotopes with a longer half-life. The dosimetry (in humans) for 18F tracers should be maintained, because they deliver a higher dose and have a higher variability (Fig. 1). With the aim of reducing unnecessary exposure to the general public, we nevertheless suggest use of either the first-in-humans protocol implemented at the National Institutes of Health (8), which recommends dosimetry scans only for those tracers that prove to be successful, or the approach used at the Amsterdam University Medical Center, where only a single low-dose (74 MBq) 18F whole-body scan is performed before proof-of-concept studies, to rule out abnormal tracer distributions. The dosimetry of more irradiating positron emitters, such as 89Zr—whose dose is about 2 orders of magnitude higher than that of 11C (9,10)—should be calculated for each tracer before it can be used.
DISCLOSURE
This work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health (project ZIAMH002852). The views expressed in this article do not necessarily represent the views of the National Institutes of Health, the Department of Health and Human Services, or the United States Government. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Parsey RV, Belanger MJ, Sullivan GM, et al. Biodistribution and radiation dosimetry of 11C-WAY100,635 in humans. J Nucl Med. 2005;46:614–619. [PubMed] [Google Scholar]
- 2.Hines CS, Liow J-S, Zanotti-Fregonara P, et al. Human biodistribution and dosimetry of 11C-CUMI-101, an agonist radioligand for serotonin-1A receptors in brain. PLoS One. 2011;6:e25309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang H, Schmit NR, Koenen AR, et al. Safety, pharmacokinetics, metabolism and radiation dosimetry of 18F-tetrafluoroborate (18F-TFB) in healthy human subjects. EJNMMI Res. 2017;7:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Doherty J, Jauregui-Osoro M, Brothwood T, et al. 18F-tetrafluoroborate, a PET probe for imaging sodium/iodide symporter expression: whole-body biodistribution, safety, and radiation dosimetry in thyroid cancer patients. J Nucl Med. 2017;58:1666–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laymon CM, Narendran R, Mason NS, et al. Human biodistribution and dosimetry of the PET radioligand [11C]flumazenil (FMZ). Mol Imaging Biol. 2012;14:115–122. [DOI] [PubMed] [Google Scholar]
- 6.Doss M, Kolb HC, Walsh JC, et al. Biodistribution and radiation dosimetry of 18F-CP-18, a potential apoptosis imaging agent, as determined from PET/CT scans in healthy volunteers. J Nucl Med. 2013;54:2087–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doot RK, Dubroff JG, Scheuermann JS, et al. Validation of gallbladder absorbed radiation dose reduction simulation: human dosimetry of [18F]fluortriopride. EJNMMI Phys. 2018;5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zanotti-Fregonara P, Lammertsma AA, Innis RB. Suggested pathway to assess radiation safety of 18F-labeled PET tracers for first-in-human studies. Eur J Nucl Med Mol Imaging. 2013;40:1781–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Donoghue JA, Lewis JS, Pandit-Taskar N, et al. Pharmacokinetics, biodistribution, and radiation dosimetry for 89Zr-trastuzumab in patients with esophagogastric cancer. J Nucl Med. 2018;59:161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pandit-Taskar N, O’Donoghue JA, Ruan S, et al. First-in-human imaging with 89Zr-Df-IAB2M Anti-PSMA minibody in patients with metastatic prostate cancer: pharmacokinetics, biodistribution, dosimetry, and lesion uptake. J Nucl Med. 2016;57:1858–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]