Abstract
Prostate-specific membrane antigen (PSMA) ligand PET induces management changes in patients with prostate cancer. We aim to better characterize the impact of 68Ga-PSMA-11 PET (68Ga-PSMA PET) on management of recurrent prostate cancer in a large prospective cohort. Methods: We report management changes after 68Ga-PSMA PET, a secondary endpoint of a prospective multicenter trial in men with biochemical recurrence of prostate cancer. Pre-PET (Q1), post-PET (Q2), and posttreatment (Q3) questionnaires were sent to referring physicians recording site of recurrence and intended (Q1 to Q2 change) and implemented (Q3) therapeutic and diagnostic management. Results: Q1 and Q2 response was collected for 382 of 635 patients (60%, intended cohort), and Q1, Q2, and Q3 response was collected for 206 patients (32%, implemented cohort). An intended management change occurred in 260 of 382 (68%) patients. The intended change was considered major in 176 of 382 (46%) patients. Major changes occurred most often for patients with prostate-specific antigen of 0.5 to less than 2.0 ng/mL (81/147, 55%). By analysis of stage groups, management change was consistent with PET disease location, that is, a majority of major changes toward active surveillance (47%) for unknown disease site (103/382, 27%), toward local or focal therapy (56%) for locoregional disease (126/382, 33%), and toward systemic therapy (69% M1a; 43% M1b/c) for metastatic disease (153/382, 40%). According to Q3 responses, the intended management was implemented in 160 of 206 (78%) patients. In total, 150 intended diagnostic tests, mostly CT (n = 43, 29%) and bone scans or 18F-NaF PET (n = 52, 35%), were prevented by 68Ga-PSMA PET; 73 tests, mostly biopsies (n = 44, 60%) as requested by the study protocol, were triggered. Conclusion: According to referring physicians, sites of recurrence were clarified by 68Ga-PSMA PET, and disease localization translated into management changes in more than half of patients with biochemical recurrence of prostate cancer.
Keywords: BCR, change in management, impact, molecular imaging, PET, prostate cancer
PET using 68Ga-labeled ligands of the prostate-specific membrane antigen (PSMA) stages prostate cancer with high accuracy (1,2). Among other factors, disease location and extent critically guide management of recurrent prostate cancer (3,4).
The impact of 68Ga-PSMA-11 PET (68Ga-PSMA PET) on the management of biochemically recurrent prostate cancer has been assessed in several retrospective studies or smaller prospective cohorts (5–8). A recent metaanalysis investigating the impact of 68Ga-PSMA PET on management at primary staging or biochemical recurrence reported management changes in approximately half of patients but found considerable heterogeneity among trials depending on prostate-specific antigen (PSA) level, PET positivity, and type-of-change definition (9). Overall, common management pathways and their association with PSA or 68Ga-PSMA PET stage have not been characterized in a large prospective patient cohort yet.
Our recent prospective multicenter trial confirmed high detection rates, positive predictive value, and interreader reproducibility along with favorable safety for 68Ga-PSMA PET in 635 patients with biochemically recurrent prostate cancer (10–12). Here, we assess the impact of 68Ga-PSMA PET on the diagnostic and therapeutic management of biochemically recurrent prostate cancer, a secondary endpoint of this trial. To identify management pathways, the intended and implemented management change was determined for different stage groups, defined by 68Ga-PSMA PET.
MATERIALS AND METHODS
Study Design
Patients were recruited at the University of California, Los Angeles (UCLA) (NCT02940262), and the University of California, San Francisco (UCSF) (NCT03353740) (10). In brief, patients with histopathologically confirmed prostate adenocarcinoma and biochemical recurrence were eligible. Biochemical recurrence was defined as a PSA of 0.2 ng/mL or higher measured more than 6 wk after prostatectomy or a PSA rise of 2 ng/mL or higher above nadir after radiation therapy.
68Ga-PSMA PET
The imaging procedure was reported previously (10). In brief, all patients underwent 68Ga-PSMA PET/CT or PET/MRI in accordance with present imaging guidelines (13). Images were interpreted by local clinical reading and, for the study report, additionally by 3 masked readers using an image-based TNM staging system (PROMISE) following regions for recurrence: prostate, prostate bed, and seminal vesicle remnants (Tr), pelvic lymph nodes (N1) (internal iliac, obturator, external iliac, perirectal, presacral, common iliac, and other), extrapelvic lymph nodes (M1a) (retroperitoneal, inguinal, chest, and other), bone (M1b), and visceral organs (M1c) (14).
Management
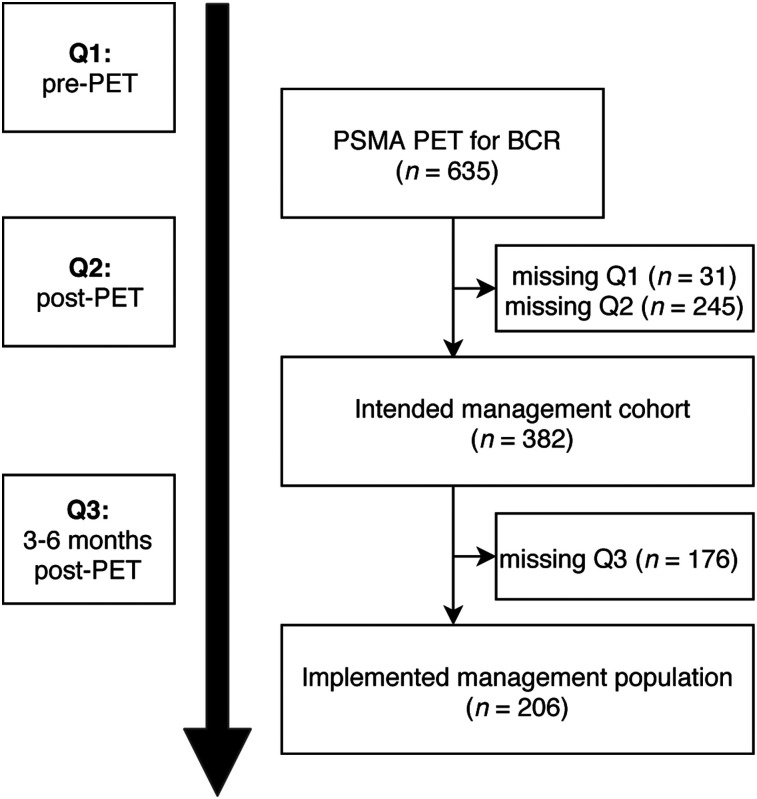
Figure 1 illustrates patient flow and physician surveys. To assess change in intended management after 68Ga-PSMA PET, referring physicians received a pre-PET questionnaire (Q1, Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org) on scheduling of the patient and a post-PET questionnaire (Q2, Supplemental Fig. 2) along with the written 68Ga-PSMA PET report and a digital video disc with PET/CT or PET/MR images. In Q1, referrers were asked to indicate their pre-PET site of recurrence, which diagnostic tests they would order, and their currently intended management if 68Ga-PSMA PET were not available. In Q2, referrers were asked again to indicate post-PET site of recurrence and their intended management based on the current clinical work-up, including 68Ga-PSMA PET/CT or PET/MRI. Additionally, they were asked whether 68Ga-PSMA PET enabled them to avoid or triggered any test or procedure. As part of follow-up, referring physicians received a 3- to 6-mo follow-up questionnaire (Q3, Supplemental Fig. 3) asking whether the intended management noted on Q2 was implemented.
FIGURE 1.
Patient flow and study design.
Intermodality changes were considered major changes, with the exception of adjuvant androgen deprivation therapy added to or removed from local therapy, which was considered a minor change. Furthermore, we considered a switch of systemic treatment (i.e., modality abiraterone/enzalutamide to chemotherapy) as a major change. Otherwise, intramodality changes were regarded as minor changes. A detailed description of change categories can be found in Supplemental Table 1.
This study was approved by local institutional review boards at the UCSF and the UCLA, and written informed consent was obtained from all patients. Trial data were collected in a central REDCap database. Descriptive statistics were used to analyze and present data. All analyses were performed using R statistics (R, version 3.4.0).
RESULTS
Baseline Characteristics
Of the 635 (60%) patients, 382 had complete Q1 and Q2 surveys (intended management cohort). Complete Q1, Q2, and Q3 surveys were available for 206 patients (32%, implemented management cohort).
Baseline characteristics of the intended management cohort are summarized in Table 1. Before 68Ga-PSMA PET, referring physicians responded that the location of disease was unknown in 262 of 382 patients (68%); 64 of 382 (17%) patients had locoregional disease, and 56 of 382 (15%) patients had metastatic disease.
TABLE 1.
Characteristics of Intended (n = 382) and Implemented Management Cohorts (n = 206)
| Characteristic | Median | Range | Intended (n) | Implemented (n) |
| Age (y) | 70.1 | 43.8–95.3 | ||
| Ethnicity/race | ||||
| White American | 333 (87%) | 177 (86%) | ||
| Black or African American | 8 (2%) | 2 (1%) | ||
| Asian American | 10 (3%) | 4 (2%) | ||
| Other | 14 (4%) | 7 (3%) | ||
| Missing data | 17 (5%) | 15 (7%) | ||
| Initial therapy | ||||
| Prostatectomy only | 166 (44%) | 86 (42%) | ||
| Radiotherapy only | 101 (26%) | 50 (24%) | ||
| Prostatectomy and salvage radiotherapy | 115 (30%) | 70 (34%) | ||
| Other prior therapy | ||||
| Local salvage therapy | 56 (15%) | 19 (9%) | ||
| Androgen deprivation therapy | 145 (38%) | 80 (39%) | ||
| Abiraterone/enzalutamide | 11 (3%) | 4 (2%) | ||
| Chemotherapy | 12 (3%) | 3 (1%) | ||
| Bone-targeted treatment | 4 (1%) | 1 (0%) | ||
| Other | 24 (6%) | 3 (1%) | ||
| Gleason score | ||||
| <8 | 237 (62%) | 125 (61%) | ||
| ≥8 | 112 (29%) | 62 (30%) | ||
| Missing data | 33 (9%) | 19 (9%) | ||
| PSA | ||||
| Intended cohort | 1.86 | 0.05–425 | ||
| Implemented cohort | 1.75 | 0.2–425 | ||
| PSA doubling time* | 6.30 | 0.43–5,018 | ||
| <6 mo | 150 (39%) | 83 (40%) | ||
| ≥6 mo | 160 (42%) | 94 (46%) | ||
| Missing data | 72 (19%) | 19 (9%) | ||
| Prior staging examination within 6 mo of 68Ga-PSMA PET | ||||
| Negative for prostate cancer | 101 (26%) | 56 (27%) | ||
| Positive for prostate cancer | 46 (12%) | 24 (12%) | ||
| Equivocal | 25 (7%) | 14 (7%) | ||
| None | 210 (55%) | 112 (54%) |
In accordance with Pound et al. (22).
Site of Recurrence and Intended Management Change
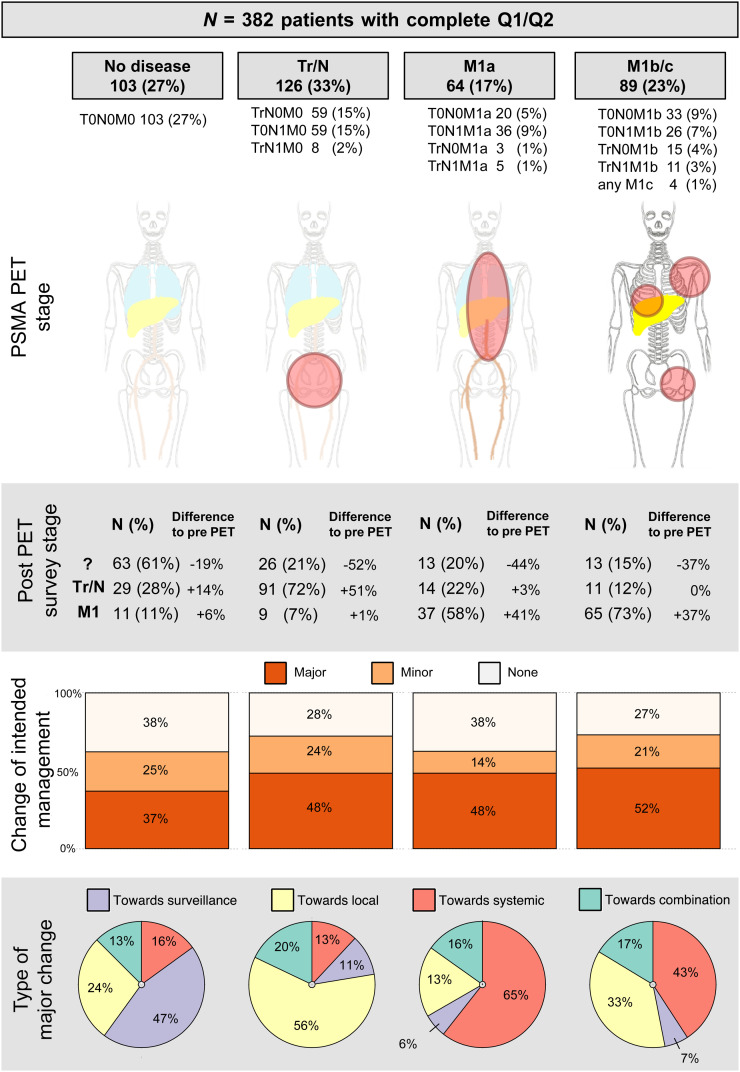
Figure 2 illustrates survey-based site of recurrence and intended management changes (Q1/Q2) stratified by 68Ga-PSMA PET disease stage groups.
FIGURE 2.
Summary of intended management change after 68Ga-PSMA PET. ? = unknown stage.
In the subgroup with no lesion localization by 68Ga-PSMA PET (103/382, 27%), referring physicians reported an unknown disease location for 63 of 103 (61%; −19% change from baseline) patients according to the Q2 survey. Major change was recorded for 38 of 103 (37%) patients, with the largest subgroup (18/38, 47%) changing to intended active surveillance.
In the subgroup with locoregional disease by 68Ga-PSMA PET (126/382, 33%), referring physicians reported suspicion of locoregional disease in 91 of 126 (72%; +51% change from baseline) patients according to the Q2 survey. A major change was recorded in 61 of 126 (48%) patients, with the largest subgroup being intended for local treatment options (34/61, 56%).
In the subgroup with extrapelvic nodal metastatic disease (M1a) according to 68Ga-PSMA PET (64/382, 17%), referring physicians reported suspicion of metastatic disease in 37 of 64 (58%; +41% change from baseline) patients after PET. A major change was recorded in 31 of 64 (48%) patients, with the largest group shifting toward systemic therapy (20/31, 65%) after PET.
In the subgroup with osseous (n = 85, M1b) or visceral metastatic (n = 4, M1c) disease by 68Ga-PSMA PET, referring physicians reported suspicion of metastatic disease in 65 of 89 (73%; +37% change from baseline) patients after PET. A major change in intended management occurred in 46 of 89 (52%) patients, with the largest groups being intended for either focal (15/46, 33%) or systemic (20/46, 43%) therapy after PET.
The rate of major change was different for the predefined PSA ranges: 39% for less than 0.5 ng/mL (n = 85), 58% for 0.5 to less than 1.0 ng/mL (n = 57), 53% for 1.0 to less than 2.0 ng/mL (n = 90), 45% for 2.0 to less than 5.0 ng/mL (n = 96), and 35% for 5.0 ng/mL or more (n = 54) as demonstrated in Supplemental Figure 4.
The rate of major change was different among patients with previous prostatectomy, radiotherapy, or both (Table 2). The highest proportion of management changes was observed in patients having had both (57%). The intended management change (Q1/Q2) was not considerably different among patients currently undergoing versus not undergoing androgen deprivation therapy.
TABLE 2.
Change in Intended Management Stratified by Previous Therapy and Hormone Status (n = 382)
| Previous therapy |
Hormone status |
||||
| Change category | Prostatectomy (n = 166) | Prostatectomy + radiotherapy (n = 115) | Radiotherapy (n = 101) | No current ADT (n = 328) | Current ADT (n = 54) |
| Major change (n = 176) | 63 (38%) | 66 (57%) | 47 (46%) | 150 (46%) | 26 (48%) |
| Minor change (n = 84) | 46 (28%) | 11 (10%) | 27 (27%) | 75 (23%) | 9 (17%) |
| No change (n = 122) | 57 (34%) | 38 (33%) | 25 (27%) | 103 (31%) | 19 (35%) |
ADT = androgen deprivation therapy
Triggered or Prevented Diagnostic Tests
Table 3 lists diagnostic tests planned before and prevented or triggered after 68Ga-PSMA PET according to the referring physicians. Before 68Ga-PSMA PET, referring physicians intended to perform 443 tests on 382 patients. According to Q2, 150 tests were prevented. One test was prevented in 45 of 382 patients (12%), and multiple tests were prevented in 48 of 382 patients (13%). Mostly bone scans or 18F-NaF PET (52/150 tests, 35%) and CT scans (43/150 tests, 29%) were prevented by 68Ga-PSMA PET. After 68Ga-PSMA PET, 73 diagnostic tests were triggered in 70 patients. One test was triggered in 67 of 382 patients (18%), and 2 tests were triggered in 3 of 382 patients (1%). Biopsies to confirm 68Ga-PSMA PET–positive sites of disease (44/73 tests, 60%) were triggered most often.
TABLE 3.
Diagnostic Tests Triggered or Prevented After 68Ga-PSMA PET
| Test type | MRI | CT | PET | 18F-NaF or bone scan | Biopsy | Other | Total |
| Tests planned before 68Ga-PSMA PET (Q1) | 56 (13%) | 77 (17%) | 145 (33%) | 144 (33%) | 8 (2%) | 13 (3%) | 443 |
| Tests prevented by 68Ga-PSMA PET (Q2) | 16 (11%) | 43 (29%) | 17 (11%) | 52 (35%) | 18 (12%) | 4 (3%) | 150 |
| Tests triggered after 68Ga-PSMA PET (Q2) | 8 (11%) | 7 (10%) | 2 (3%) | 5 (7%) | 44 (60%) | 7 (10%) | 73 |
Implemented Management
Management implementation rates are given in Table 4. According to Q3 responses, the intended management was implemented in 160 (78%) patients. The management was implemented in 98 of 135 (72%) of patients with an intended change. Continuation of the pre-PET management was implemented in 62 of 70 (89%) patients. The implementation rate was consistent and ranged from 66% to 78% for the various management-change pathways (Table 5).
TABLE 4.
Management Implementation (n = 206)
| Management change | Implemented | Not implemented |
| Change intended (n = 136) | 98 (72%) | 38 (28%) |
| No change intended (n = 70) | 62 (89%) | 8 (11%) |
TABLE 5.
Management Implementation Details (n = 206)
| Change category | Implemented | Not implemented |
| Major change to combination (n = 16) | 11 (69%) | 5 (31%) |
| Major change to local (n = 34) | 26 (76%) | 8 (24%) |
| Major change to surveillance (n = 17) | 11 (65%) | 6 (35%) |
| Major change to systemic (n = 29) | 19 (66%) | 10 (34%) |
| Minor change (n = 40) | 31 (78%) | 9 (22%) |
| No change (n = 70) | 62 (89%) | 8 (11%) |
DISCUSSION
For clinical impact, diagnostic tests need to translate into relevant changes in management. Analyses of the National Oncologic PET Registry (NOPR) demonstrated a change in management in 37% of cancer patients after 18F-FDG PET and resulted in 18F-FDG PET reimbursement for a wide range of indications in the United States (15). However, a subanalysis of the NOPR study revealed a somewhat lower rate of change in management for prostate cancer than for other entities, possibly because of low 18F-FDG uptake and limited lesion detection (16). Since NOPR completion, several novel radiotracers have been introduced for prostate cancer imaging. Of these, radiolabeled PSMA ligands have been studied extensively since their introduction. Recently, a high positive predictive value, detection rate, and interreader agreement were reported for 68Ga-PSMA PET in a prospective multicenter trial (10). Here, we present a NOPR-like survey-based impact on management data, a secondary endpoint of this prospective study.
68Ga-PSMA PET resulted in a change in management in more than half of patients undergoing 68Ga-PSMA PET for localization of biochemically recurrent prostate cancer. Referring physicians frequently accepted the reported site of disease according to Q2 surveys. Subsequent management pathways were consistent with 68Ga-PSMA PET disease locations; that is, local treatment was considered more often for local disease (54/126 patients, 44%), whereas systemic disease was associated more often with an intended change toward systemic or combination approaches (106/153 patients, 69%). Our findings demonstrate that the accuracy of 68Ga-PSMA PET translates into a change in disease stage and management consistent with PET-positive sites of recurrent prostate cancer.
After 68Ga-PSMA PET, the proportion of patients with unknown sites of disease declined from about two thirds to one third according to the referring clinicians. The 68Ga-PSMA PET disease location was frequently accepted by referring physicians. Individual management pathways are diverse (Supplemental Fig. 5). However, changes demonstrate detectable patterns: patients without detectable disease by 68Ga-PSMA PET more often experienced intended major deescalation toward active surveillance (47%), whereas patients with locoregional disease had an intended major transition toward focal therapy (56%). In cases of extrapelvic nodal disease (M1a), clinicians tended toward a major change to systemic therapy (65%). In patients with bone metastasis (M1b) or visceral metastasis (M1c), major systemic or local treatment changes were most common (43% and 33%, respectively).
Accurate localization of disease is a critical early step in the management of patients with biochemical recurrence of prostate cancer. Focal and salvage therapies need accurate target delineation. On the other hand, the presence of distant metastases may trigger additional or alternative systemic therapy (3). Therefore, the updated European Association of Urology guidelines recommend 68Ga-PSMA PET in biochemical recurrence after radical prostatectomy if the results will influence subsequent treatment decisions (3). In this study, major changes occurred most often in patients with a PSA of 0.5 to less than 2.0 ng/mL. However, an impact on subsequent treatment decisions occurred also in patients with undetectable or extensive disease. We further demonstrate that detectable management pathways follow guideline recommendations: focal or salvage therapy is offered for local disease, and systemic treatment is recommended in cases of metastatic spread (3). Whether 68Ga-PSMA PET–induced management changes translate into survival benefits remains unknown. Prospective studies with long-term follow-up are required to answer this question. With this intent, trials investigating 68Ga-PSMA PET–guided therapy are currently under way (17,18).
A previous study reported management changes based on surveys and chart review in an initial UCLA cohort (n = 101) of the presented study (5). Systematic chart review confirms that intended management changes frequently differ from implemented changes based on subsequent diagnostic tests, tumor board decisions, or patient preference (5). However, even when considering subsequent modification, the overall proportion of patients experiencing a major implemented management change remains high (5). In our expanded cohort (n = 382), survey-based implemented management differed from intended management in 22% of patients overall; discrepancy was somewhat higher in patients with an intended management change (38%). The proportion of management change was similar in the biochemical failure cohort of a recent Australian multicenter study finding altered management in 62% of patients (6). Similarly, Müller et al. found a 60% management change in a retrospective cohort of recurrent prostate cancer and, of note, a high response rate to subsequent focal therapy (19). Overall, the impact on management was higher than reported in a recent metaanalysis of 1,163 patients at primary diagnosis and biochemical recurrence, with a change in management occurring in 54% of patients (95%CI, 47%–60%) (9). In this study, we report in more detail how management pathways are associated with PET stage, indicating that referring physicians have high confidence in 68Ga-PSMA PET findings.
One hundred fifty diagnostic tests were prevented by 68Ga-PSMA PET, according to the survey response. Most of these were CT scans (43 cases) or bone scans or 18F-NaF PET/CT (52 cases). The decision to omit these diagnostic tests is in line with several studies that demonstrated superior accuracy for 68Ga-PSMA PET when compared with one or a combination of the prevented diagnostic instruments for prostate cancer localization (8,12,20,21). Specifically, 68Ga-PSMA PET demonstrated superior detection sensitivity when compared head-to-head with bone scanning or the recently approved 18F-fluciclovine, especially at a PSA of 2 ng/mL or less (20,21). Although more diagnostic tests were prevented than triggered, the addition of 68Ga-PSMA PET increases the total diagnostic work-up. On the other hand, at the time of enrollment, referring physicians had little experience with 68Ga-PSMA PET, and some of the diagnostic tests, including biopsies (44/382, 12%), were encouraged by the study protocol for lesion validation. Histopathologic validation resulted in a positive predictive value of 84% for 68Ga-PSMA PET both on a per-patient basis and on a per-region basis (10). As availability improves, by an increasing number of clinical trials or an anticipated approval of PSMA ligand PET, additional tests, especially potentially burdensome biopsies, may be ordered less frequently in the future clinical setting.
Although this study benefits from a large cohort, missing questionnaires are a limitation to our study. More specifically, Q1 and Q2 were completed for 60% of patients, and all 3 questionnaires were available for only 32% of patients. A greater likelihood that proponents of new imaging technologies would reply may have introduced a responder bias. Furthermore, information on implemented management was not confirmed by file review, and a potential discrepancy between intended and finally implemented management, reported previously (5), was not resolved. The low Q3 rate may be due to the late request to respond, that is, 3–6 mo after 68Ga-PSMA PET—disconnected from the PET report and outside typical clinical timelines. Also, a more frequent Q3 response for closely monitored or high-risk patients might have led to an overestimation of the management implementation rate. On the other hand, similar patient characteristics between the intended and implemented management cohorts (Table 1) indicate no relevant selection bias. Only a small proportion of patients were African-American. This underrepresentation may have led to a selection bias, and findings might not be entirely applicable to this ethnic group.
CONCLUSION
68Ga-PSMA PET findings were accepted by referring physicians and induced management changes in more than half of patients with biochemically recurrent prostate cancer. Management pathways aligned with PET disease location: focal or salvage therapy for local disease; systemic treatment for distant metastases. Future randomized trials aim to evaluate the impact of management changes on oncologic outcomes.
DISCLOSURE
Wolfgang Fendler received academic financial support from the German Research Foundation (Deutsche Forschungsgemeinschaft [DFG], FE1573/1-1/807122 and FE1573/3-1/659216), Mercator Research Center Ruhr (MERCUR, An-2019-0001), IFORES, Doktor Robert Pfleger-Stiftung, and Wiedenfeld-Stiftung/Stiftung Krebsforschung Duisburg; is a consultant for Ipsen, Endocyte, and BTG; and received personal fees from RadioMedix, outside the submitted work. Thomas Hope was supported by the Prostate Cancer Foundation (2017 Jonathan Kovler Young Investigator Award) and the National Institutes of Health (grant R01CA212148); is a consultant for Curium and Ipsen; and receives grant support from Philips. Jeremie Calais received grants from the Fondation ARC pour la recherche sur le cancer (SAE20160604150) and Philippe Foundation Inc. (New York). Johannes Czernin received grants from the U.S. Department of Energy (DESC0012353), Prostate Cancer Foundation (2017 Challenge Award, 17CHAL02), and Johnson Comprehensive Cancer Center (NIH-NCI Cancer Center support grant P30 CA016042); is a founder and board member and holds equity in Sofie Biosciences and Trethera Therapeutics; serves on the medical advisory board of Actinium; and is a member of the VISION trial steering committee, a clinical trial sponsored by Endocyte. Matthias Eiber is a consultant for ABX and Blue Earth Diagnostics. Robert Flavell receives grant support from Fukushima SiC. Matthew Rettig is a speaker and advisory board member for Janssen and Bayer, receives research funding from Novartis, and consults for Amgen and Ambrx. Ken Herrmann receives personal fees from Bayer, SIRTEX, Adacap, Curium, Endocyte, BTG, IPSEN, Siemens Healthineers, GE Healthcare, Amgen, Novartis, and ymabs; other fees from Sofie Biosciences; nonfinancial support from ABX; and grants from BTG, outside the submitted work. Intellectual property patented by the University of California is licensed to Sofie Biosciences and Trethera Therapeutics. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Does 68Ga-PSMA PET impact the management of men with biochemically recurrent prostate cancer?
PERTINENT FINDINGS: We demonstrated that 68Ga-PSMA PET findings were frequently accepted by referring physicians and induced management changes in 260 of 382 (68%) patients with biochemically recurrent prostate cancer. Furthermore, management pathways aligned with PET disease location: local therapy was chosen more often for local disease; a change toward systemic treatment was seen more often for distant metastases.
IMPLICATIONS FOR PATIENT CARE: 68Ga-PSMA PET accuracy translates into a change in management for patients with recurrent prostate cancer. The potential benefit of 68Ga-PSMA PET–guided management now needs to be assessed in prospective trials with oncologic outcome.
Acknowledgments
We thank the patients who volunteered to participate in this trial and the investigators and staff who cared for them. We thank the 68Ga-PSMA PET masked expert readers: Okamoto Shozo, MD, Louise Emmett, MD, Helle D. Zacho, MD, Harun Ilhan, MD, Axel Wetter, MD, Christoph Rischpler, MD, Heiko Schoder, MD, and Irene A. Burger, MD. We thank Jens Eickhoff from the University of Wisconsin–Madison and Carina Demel, PhD, from the Max Planck Institute for Biophysical Chemistry, Göttingen, Germany, for assistance with the statistical analysis. We thank Martin Allen-Auerbach, MD, and Nicholas Nickols, MD, PhD, from UCLA, as well as Ashley Mishoe, PhD, and Rahul Aggarwal, MD, from UCSF for their contribution and support throughout the course of this study.
REFERENCES
- 1.Perera M, Papa N, Roberts M, et al. Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions—a systematic review and meta-analysis. Eur Urol. 2020;77:403–417. [DOI] [PubMed] [Google Scholar]
- 2.von Eyben FE, Picchio M, von Eyben R, Rhee H, Bauman G. 68Ga-labeled prostate-specific membrane antigen ligand positron emission tomography/computed tomography for prostate cancer: a systematic review and meta-analysis. Eur Urol Focus. 2018;4:686–693. [DOI] [PubMed] [Google Scholar]
- 3.Cornford P, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–642. [DOI] [PubMed] [Google Scholar]
- 4.Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17:479–505. [DOI] [PubMed] [Google Scholar]
- 5.Calais J, Fendler WP, Eiber M, et al. Impact of 68Ga-PSMA-11 PET/CT on the management of prostate cancer patients with biochemical recurrence. J Nucl Med. 2018;59:434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roach PJ, Francis R, Emmett L, et al. The impact of 68Ga-PSMA PET/CT on management intent in prostate cancer: results of an Australian prospective multicenter study. J Nucl Med. 2018;59:82–88. [DOI] [PubMed] [Google Scholar]
- 7.Hope TA, Aggarwal R, Chee B, et al. Impact of Ga-68 PSMA-11 PET on management in patients with biochemically recurrent prostate cancer. J Nucl Med. 2017;58:1956–1961. [DOI] [PubMed] [Google Scholar]
- 8.Morigi JJ, Stricker PD, van Leeuwen PJ, et al. Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med. 2015;56:1185–1190. [DOI] [PubMed] [Google Scholar]
- 9.Han S, Woo S, Kim YJ, Suh CH. Impact of 68Ga-PSMA PET on the management of patients with prostate cancer: a systematic review and meta-analysis. Eur Urol. 2018;74:179–190. [DOI] [PubMed] [Google Scholar]
- 10.Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afshar-Oromieh A, Holland-Letz T, Giesel FL, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–674. [DOI] [PubMed] [Google Scholar]
- 13.Fendler WP, Eiber M, Beheshti M, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–1024. [DOI] [PubMed] [Google Scholar]
- 14.Eiber M, Herrmann K, Calais J, et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59:469–478. [DOI] [PubMed] [Google Scholar]
- 15.Hillner BE, Siegel BA, Liu D, et al. Impact of positron emission tomography/computed tomography and positron emission tomography (PET) alone on expected management of patients with cancer: initial results from the National Oncologic PET Registry. J Clin Oncol. 2008;26:2155–2161. [DOI] [PubMed] [Google Scholar]
- 16.Hillner BE, Siegel BA, Shields AF, et al. Relationship between cancer type and impact of PET and PET/CT on intended management: findings of the National Oncologic PET Registry. J Nucl Med. 2008;49:1928–1935. [DOI] [PubMed] [Google Scholar]
- 17.Calais J, Czernin J, Fendler WP, Elashoff D, Nickols NG. Randomized prospective phase III trial of 68Ga-PSMA-11 PET/CT molecular imaging for prostate cancer salvage radiotherapy planning [PSMA-SRT]. BMC Cancer. 2019;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofman MS, Murphy DG, Williams SG, et al. A prospective randomized multicentre study of the impact of gallium-68 prostate-specific membrane antigen (PSMA) PET/CT imaging for staging high-risk prostate cancer prior to curative-intent surgery or radiotherapy (proPSMA study): clinical trial protocol. BJU Int. 2018;122:783–793. [DOI] [PubMed] [Google Scholar]
- 19.Müller J, Ferraro DA, Muehlematter UJ, et al. Clinical impact of 68Ga-PSMA-11 PET on patient management and outcome, including all patients referred for an increase in PSA level during the first year after its clinical introduction. Eur J Nucl Med Mol Imaging. 2019;46:889–900. [DOI] [PubMed] [Google Scholar]
- 20.Calais J, Ceci F, Eiber M, et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019;20:1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pyka T, Okamoto S, Dahlbender M, et al. Comparison of bone scintigraphy and 68Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:2114–2121. [DOI] [PubMed] [Google Scholar]
- 22.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. [DOI] [PubMed] [Google Scholar]