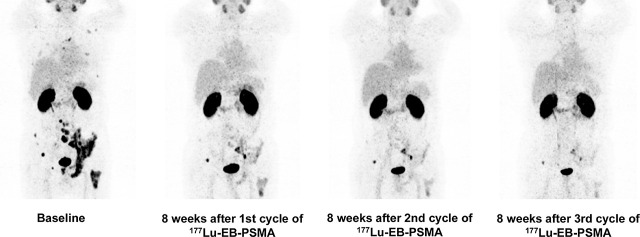
Visual Abstract
Keywords: radioligand therapy, 177Lu, Evans blue, prostate-specific membrane antigen (PSMA), metastatic castration-resistant prostate cancer (mCRPC)
Abstract
This study was designed to assess the safety and therapeutic response to 177Lu-labeled Evans blue–modified prostate-specific membrane antigen (PSMA) 617 (EB-PSMA-617) treatment with escalating doses in patients with metastatic castration-resistant prostate cancer. Methods: With institutional review board approval and informed consent, patients were randomly divided into 3 groups: group A (n = 10) was treated with a 1.18 ± 0.09 GBq dose of 177Lu-EB-PSMA. Group B (n = 10) was treated with a 2.12 ± 0.19 GBq dose of 177Lu-EB-PSMA. Group C (n = 8) was treated with a 3.52 ± 0.58 GBq dose of 177Lu-EB-PSMA. Eligible patients received up to 3 cycles of 177Lu-EB-PSMA therapy, at 8-wk intervals. Results: Because of disease progression or bone marrow suppression, 4 of 10, 5 of 10, and 5 of 8 patients completed 3 cycles of therapy as planned in groups A, B, and C, respectively. The prostate-specific antigen response was correlated with treatment dose, and the prostate-specific antigen disease control rates were higher in groups B (70%) and C (75%) than in group A (10%) (P = 0.007), but no correlation between groups B and C was found. 68Ga-PSMA PET/CT showed a response in all treatment groups; however, there was no significant difference among the 3 groups. A hematologic toxicity study found that platelets decreased more in groups B and C than in group A and that grade 4 thrombocytopenia occurred in 2 (25.0%) patients in group C. No serious nephritic or hepatic side effects were observed. Conclusion: This study demonstrated that a 2.12-GBq dose of 177Lu-EB-PSMA seems to be safe and adequate in tumor treatment. Further investigations with an increased number of patients are warranted.
Prostate cancer is one of the most common types of cancer worldwide and the second most frequent cause of cancer deaths for adult men (1). The cause of death in most patients who die from prostate cancer is metastatic castration-resistant prostate cancer (mCRPC) (2,3). Radioligand therapy targeting prostate-specific membrane antigen (PSMA), which is overexpressed in most cases of prostate cancer and is even further increased in metastatic and castration-resistant carcinomas, has been demonstrated to be effective and safe in men with mCRPC (4–15). The European Association of Nuclear Medicine procedure guidelines for radionuclide therapy with 177Lu-labeled PSMA ligands were issued in 2019 (16). So far, the 2 most common radiotherapeutic agents have been 177Lu-PSMA-617 and 177Lu-PSMA I&T. Because they are small molecules that are cleared relatively quickly from the circulation, the radiotherapy requires a high therapy activity and frequent administrations.
To increase tumor uptake and improve radiotherapeutic efficacy, Evans blue–modified PSMA-617 (EB-PSMA-617), which binds to both serum albumin and PSMA, was synthesized and conjugated to DOTA chelator and labeled with 177Lu. Preclinical studies showed that EB-PSMA-617 had significantly higher accumulation in PSMA-positive tumors and highly effective radiotherapeutic efficacy (17). Then, we performed the first-in-humans study of 177Lu-EB-PSMA-617 to evaluate its safety, dosimetry, and therapeutic response and found that 177Lu-EB-PSMA-617 had 2.15- to 5.68-fold higher tumor accumulation than 177Lu-PSMA-617 and that all patients tolerated the treatment well at a low dose (18).
This translational study was designed to assess the safety and response to 177Lu-EB-PSMA-617 treatment with escalating doses and multiple administrations in patients with mCRPC, to provide guidance about optimal doses.
MATERIALS AND METHODS
Patients
This study was registered at clinicaltrials.gov (NCT03780075) and approved by the Institutional Review Board of Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, and Peking Union Medical College, and all subjects gave written informed consent. In total, 28 patients with histologically confirmed prostate cancer were recruited between April 2018 and June 2019 in this prospective study. The inclusion criteria were progressive mCRPC, increasing blood prostate-specific antigen (PSA) levels, and PSMA expression of distant metastases as determined by 68Ga-PSMA-617 PET/CT within 1 wk before treatment. Patients were not eligible if they had clinically significant impaired bone marrow, liver, or kidney function with a hemoglobin level of less than 9.0 g/dL, a white blood cell count of less than 2.5 × 109/L, a platelet count of less than 75 × 109/L, a serum creatinine level of more than 150 μmol/L, a total bilirubin level of more than 60 μmol/L, and a serum albumin level of more than 3.0 g/dL. Patients with an Eastern Cooperative Oncology Group performance status score of more than 2 were also excluded from the study.
Patients stratified to receive 177Lu-EB-PSMA were randomly divided into 3 groups. Group A (10 patients, 70 ± 9 y old) was treated with a 1.18 ± 0.09 GBq dose (31.79 ± 2.44 mCi), group B (10 patients, 70 ± 6 y old) was treated with a 2.12 ± 0.19 GBq dose (57.39 ± 5.29 mCi), and group C (8 patients, 68 ± 4 y old) was treated with a 3.52 ± 0.58 GBq dose (95.12 ± 15.56 mCi).
Treatment Regimen and Follow-up
Preparation of EB-PSMA-617 and 177Lu labeling were performed as described previously (18). Patients received intravenous hydration (2,000 mL of 0.9% NaCl) starting 30 min before 177Lu administration. The radiopharmaceutical diluted in 100 mL of normal saline was coadministered slowly in an intravenous infusion for over 15–25 min. To minimize dry mouth syndrome, patients received ice packs over the parotid and submandibular glands at 30 min before administration of the radiopharmaceutical. Eligible patients in each group received up to 3 cycles of 177Lu-EB-PSMA therapy at 8-wk intervals.
Blood tests, including hematologic status, liver function, and renal function, were performed before and every 2 wk after each cycle of treatment for 8 wk. The serum PSA response was documented monthly until 8 wk after the last treatment cycle. Adverse events were categorized using the Common Toxicity Criteria for Adverse Events, version 5.0.
Response Assessment
PSA Response
According to the recommendations of Prostate Cancer Working Group 3 (19), biochemical response was classified as the following: partial response (PR) if there was a PSA decrease of at least 50%, progressive disease (PD) if there was a PSA increase of at least 25%, and stable disease if there was a PSA increase of less than 25% or a PSA decrease of less than 50%.
68Ga-PSMA PET/CT Response
All patients underwent 68Ga-PSMA-617 whole-body PET/CT 8 wk after each cycle of treatment. The molecular response was classified according to adapted PERCIST 1.0 (20). Complete response was complete resolution of 68Ga-PSMA-617 uptake in the target lesions. PR was defined as more than a 30% decrease in the SUVmax uptake of the target lesions from the baseline scan, and PD was defined as more than a 30% increase in the SUVmax of the target lesions from the baseline scan. Neither complete response, PR, nor PD was considered stable disease if there was less than a 30% decrease or less than a 30% increase in the target lesion. Changes in SUV between pre- and posttherapeutic PET were calculated.
Data Analysis and Statistics
Calculations were performed using SPSS (IBM SPSS Statistics for Windows, version 21.0). P values of less than 0.05 were accepted as statistically significant. All quantitative data were expressed as the mean ± SD, and differences among groups were compared with 1-way ANOVA analysis or Student t tests. The χ2 test was used to compare treatment response rates.
RESULTS
Patients
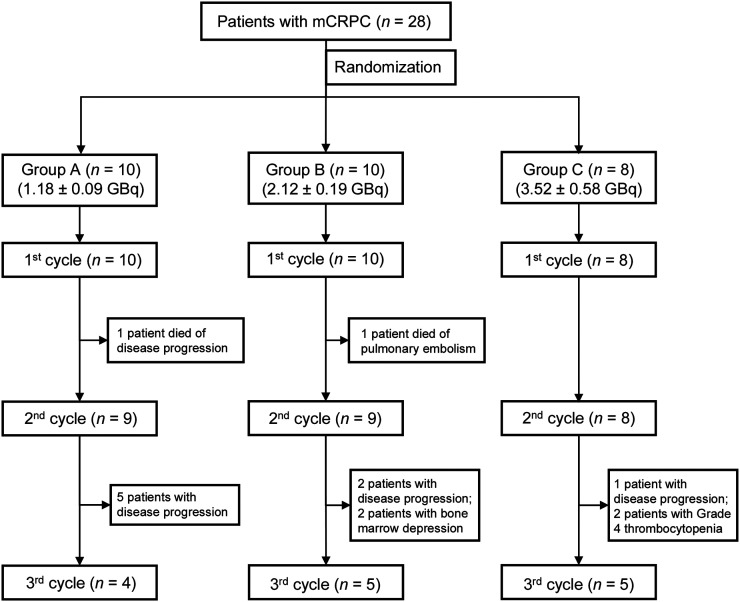
Because of disease progression, bone marrow suppression, or death, all 10 patients in group A received cycle 1 of 177Lu-EB-PSMA, and 9 (90%) and 4 (40%) patients received cycles 2 and 3, respectively. All 10 patients in group B received cycle 1 of 177Lu-EB-PSMA, and 9 (90%) and 5 (50%) patients received cycles 2 and 3, respectively. In addition, all 8 patients in group C received cycle 1 of 177Lu-EB-PSMA, and 8 (100%) and 5 (63%) patients received cycles 2 and 3, respectively. The patients’ basic characteristics and flowchart are listed in Supplemental Table 1 (supplemental materials are available at http://jnm.snmjournals.org) and Figure 1, respectively.
FIGURE 1.
Patient flowchart of 3 randomized groups.
Safety Evaluation
Administration of 177Lu-EB-PSMA-617 was well tolerated, with no immediate adverse effects recorded during injection and no treatment-related deaths. One death occurred in group A because of disease progression, and 1 death occurred in group B because of pulmonary embolism. No deaths occurred in group C for at least 2 mo after the last cycle of treatment.
In group A, grade 1 xerostomia was observed in 3 (30.0%) patients, and grade 1 nausea was observed in 2 (20.0%) patients. In group B, 5 (50%) patients experienced grade 1 xerostomia, and 3 (30%) patients experienced grade 1 nausea. In group C, grade 1–2 xerostomia occurred in 4 (50.0%) patients and grade 1 nausea occurred in 2 (25.0%) patients.
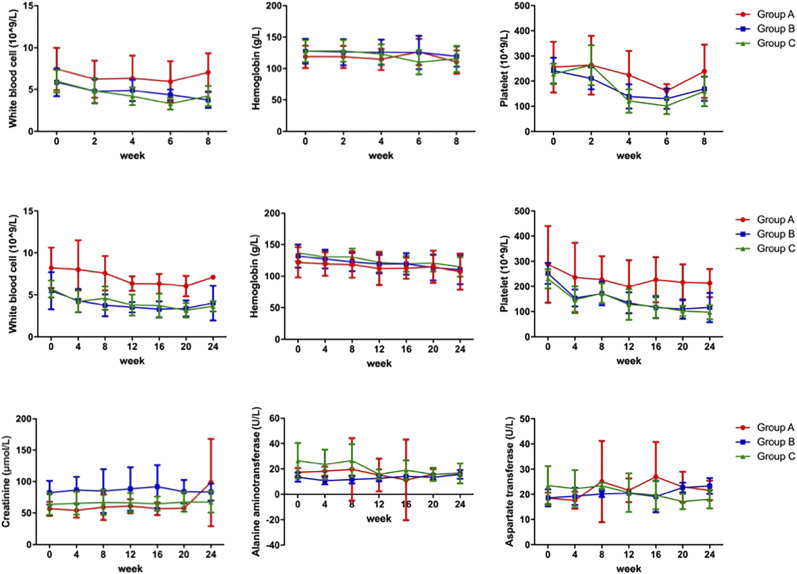
Hematologic parameters within 8 wk after the first cycle of treatment were collected for all patients in each group. The mean counts for white blood cells, hemoglobin, and platelets over 8 wk are shown in Figure 2. White blood cells reached a nadir at weeks 6–8, and platelets at week 6, in all groups. No differences in hemoglobin were observed before and after the first cycle of treatment in any group. For patients who completed 3 cycles, the white blood cell, hemoglobin, and platelet counts over the complete 24-wk period are shown in Figure 2. Platelets dropped the most, followed by white blood cells. A slight decrease in hemoglobin count was observed. In addition, the decrease in platelets was greater in groups B and C than that in group A. However, the mean platelet counts were still in the reference range. Details are shown in Supplemental Table 2.
FIGURE 2.
Hematologic toxicity, nephrotoxicity, and hepatotoxicity. The first row showed change in white blood cells, hemoglobin, and platelets over 8 wk after first cycle for all patients, and the middle row showed change in hematologic parameters over 24 wk for patients receiving 3 cycles of 177Lu-EB-PSMA therapy for 3 groups. The last row showed mean creatinine, alanine aminotransferase, and aspartate transferase over 24 wk for patients receiving 3 cycles of therapy.
All patients who had received at least 1 cycle of 177Lu-EB-PSMA were included in safety analyses. In group A, grade 3 anemia occurred in 4 (40.0%) patients (2 patients had grade 2 anemia at baseline). In group B, grade 3 anemia, leukopenia, and thrombocytopenia occurred in 2 (20.0%), 1 (10.0%), and 1(10.0%) patients, respectively. In group C, grade 3–4 anemia, leukopenia, and thrombocytopenia occurred in 3 (37.5%), 1 (12.5%), and 3 (37.5%) patients, respectively (1 patient with thalassemia showed both grade 4 anemia and thrombocytopenia). Most patients with grade 3–4 hematologic side effects had prior chemotherapy and diffuse bone metastasis. Details are shown in Supplemental Table 3.
No nephrotoxicity or hepatotoxicity (grade 3 or 4) occurred within the 2-mo period of observation after the last cycle of therapy in any patients. For the patients who completed 3 cycles, the mean creatinine, alanine aminotransferase, and aspartate transferase over 24 wk are shown in Figure 2.
Treatment Efficacy
Improvement in Clinical Symptoms
Except for 2 patients who died, the Eastern Cooperative Oncology Group scores of the other patients remained stable during therapy. In group A, 3 patients (30%) had partial remission of pain, 4 (40%) reported no changes, and 3 (30%) had worsening bone pain. Three patients (30%) in group B had partial remission of pain, 5 (50%) reported no changes, and 2 (20%) had worsening bone pain. In group C, 1 patient (13%) had complete resolution of pain, 1 (13%) reported partial remission, and 6 (85%) showed no changes.
PSA Response
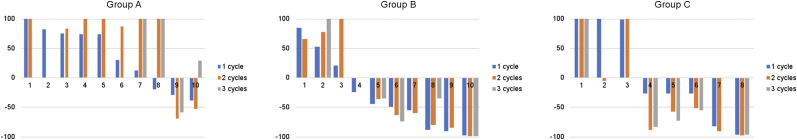
After the first treatment cycle, a decline in the PSA level was observed in 3 (30.0%), 7 (70.0%), and 5 (62.5%) patients in groups A, B, and C, respectively. A decline in the PSA level of greater than 50% (PR) occurred in 0 (0%), 4 (40.0%), and 2 (25.0%) of patients in groups A, B, and C, respectively. Compared with the baseline PSA value, 2 (22.2%), 6 (66.6%), and 6 (75.0%) patients in groups A, B, and C, respectively, showed a PSA decline 2 mo after the second cycle, with 2 (22.2%), 5 (55.5%), and 5 (62.5%), respectively, showing a PSA decline of more than 50%. At 2 mo after the third treatment cycle, 1 (25.0%), 4 (80.0%), and 4 (80.0%) patients in groups A, B, and C, respectively, showed a PSA decline, with 1 (25.0%), 2 (40.0%), and 4 (80.0%), respectively, showing a PSA decline of more than 50%. The waterfall plots in Figure 3 represent the PSA response at 8 wk after each cycle of treatment.
FIGURE 3.
Waterfall graphs of PSA response as compared with baseline level after each cycle of treatment for 3 groups. PSA increase of 100% was cropped for simplification.
In total, PSA response before and 8 wk after the last cycle of treatment in all enrolled patients who had received at least 1 cycle of therapy was analyzed, and we found that 1 (10.0%) patient had PR and 9 (90%) patients had PD in group A. In addition, 4 (40.0%), 3 (30%), and 3 (30%) patients showed PR, stable disease, and PD, respectively, in group B, and 5 (62.5%), 1 (12.5%), and 2 (25.0%) patients showed PR, stable disease, and PD, respectively, in group C. The disease control rates (PR + stable disease) were higher in groups B and C than in group A (group A vs. group B vs. group C: 10% vs. 70% vs.75%, respectively [P = 0.007]; group A vs. group B: 10% vs. 70%, respectively [P = 0.02]; group A vs. group C: 10% vs. 75%, respectively [P = 0.012]; group B vs. group C: 70% vs. 75%, respectively [P = 1.00] on χ2 testing). Table 1 further details the response data after each cycle of therapy for the 3 groups according to PSA-level measurements.
TABLE 1.
PSA Response After Each Cycle of Therapy for 3 Groups
| Group A |
Group B |
Group C |
||||||||||
| PSA response | First | Second | Third | All* | First | Second | Third | All* | First | Second | Third | All* |
| PR | 0/10 (0.0%) | 2/9 (22.2%) | 1/4 (25.0%) | 1/10 (10.0%) | 4/10 (40.0%) | 5/9 (55.5%) | 2/5 (40.0%) | 4/10 (40.0%) | 2/8 (25.0%) | 5/8 (62.5%) | 4/5 (80.0%) | 5/8 (62.5%) |
| Stable disease | 4/10 (40.0%) | 0/9 (0.0%) | 0/4 (0.0%) | 0/10 (0.0%) | 4/10 (40.0%) | 1/9 (11.1%) | 2/5 (40.0%) | 3/10 (30.0%) | 3/8 (37.5%) | 1/8 (12.5%) | 0/5 (0.0%) | 1/8 (12.5%) |
| PD | 6/10 (60.0%) | 7/9 (77.7%) | 3/4 (75.0%) | 9/10 (90.0%) | 2/10 (20.0%) | 3/9 (33.3%) | 1/5 (20.0%) | 3/10 (30.0%) | 3/8 (37.5%) | 2/8 (25.0%) | 1/5 (20.0%) | 2/8 (25.0%) |
PSA response before and 8 wk after last cycle of treatment in all enrolled patients who had received at least 1 cycle of therapy.
There was a significant correlation between patient response to the first cycle and the third cycle compared with baseline PSA (P = 0.027). In total, 14 patients accepted 3 cycles of treatment in all groups. Among them, 11 patients showed a PSA decline after the first cycle, and 9 (81.8%) also showed a PSA decline 2 mo after the third cycle, when compared with their baseline PSA value. Three patients who did not show any PSA decline after the first cycle also showed no response after the third cycle.
68Ga-PSMA PET/CT Response
After the first treatment cycle, according to the adapted PERCIST 1.0, 6 (60%), 3 (30%), and 1 (10%) patients in group A showed PR, stable disease, and PD, respectively; 5 (50%), 4 (40%), and 1 (10%) patients had PR, stable disease, and PD, respectively, in group B. PR, stable disease, and PD were demonstrated for 4 (50.0%), 2 (25.0%), and 2 (25%) patients, respectively, in group C. When the bone metastases with comparable baseline SUVmax from 10.0 to 40.0 were selected from the 3 groups for comparison, there was no significant difference demonstrated by changes in SUV from 68Ga-PSMA PET/CT before and after the treatment (−36.00% ± 23.35% [n = 36] vs. −38.63% ± 42.57% [n = 27] vs. −33.83% ± 39.63% [n = 19], respectively; P = 0.895).
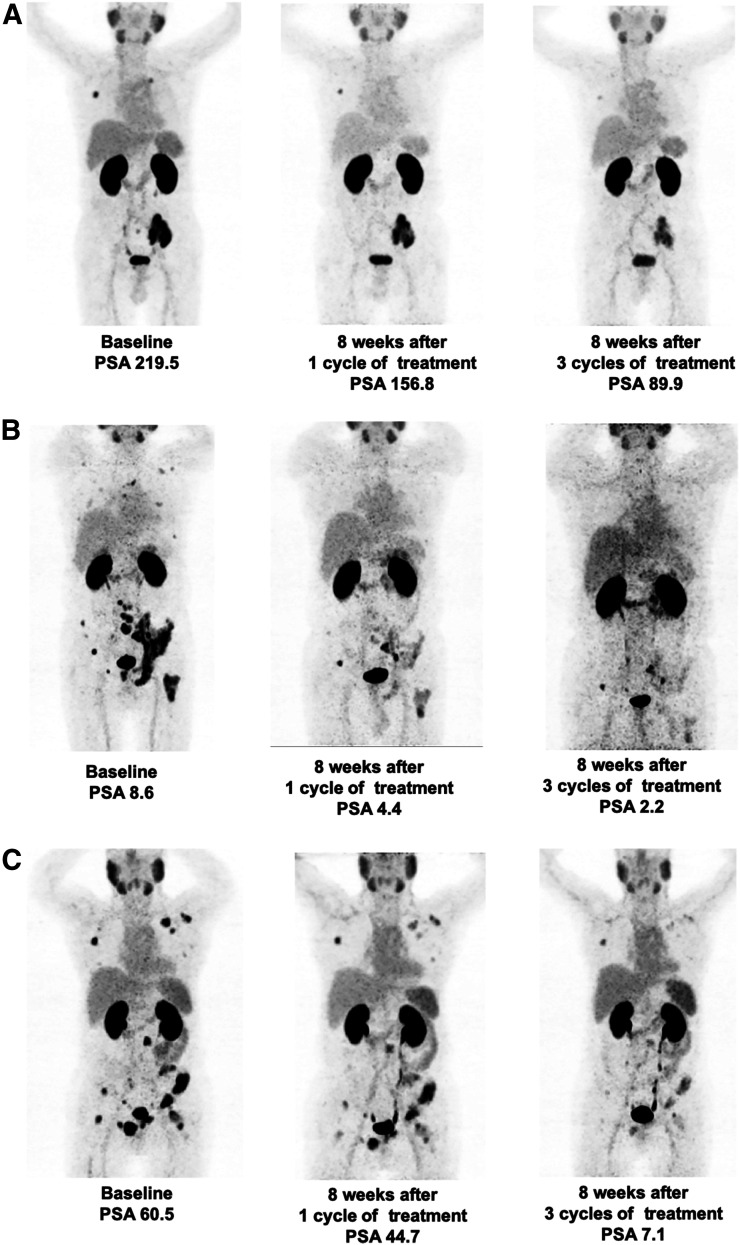
An objective radiologic response at 8 wk after the second cycle of treatment was demonstrated for 4 of 9 patients in group A, 5 of 9 patients in group B, and 5 of 8 patients in group C. The response of PR was demonstrated for 3 (33.3%), 3 (33.3%), and 3 (37.5%) patients in groups A, B, and C, respectively. After the third cycle of therapy, 2 of 4, 5 of 5, and 4 of 5 patients repeated 68Ga-PSMA PET/CT in groups A, B, and C, respectively. In the end, 1 (25.0%), 3 (60.0%), and 2 (40%) patients showed PR in groups A, B, and C, respectively. There was no major difference in 68Ga-PSMA PET/CT response after each cycle of treatment among the 3 groups. Details are shown in Table 2. Representative 68Ga-PSMA PET/CT images in 3 groups after each cycle of therapy are shown in Figure 4.
TABLE 2.
68Ga-PSMA PET/CT Response After Each Cycle of Therapy for 3 Groups
| Group A |
Group B |
Group C |
|||||||
| 68Ga-PSMA PET/CT response | First | Second | Third | First | Second | Third | First | Second | Third |
| PR | 6/10 (60.0%) | 3/9 (33.3%) | 1/4 (25.0%) | 5/10 (50.0%) | 3/9 (33.3%) | 3/5 (60.0%) | 4/8 (50.0%) | 3/8 (37.5%) | 2/5 (40.0%) |
| Stable disease | 3/10 (30.0%) | 0/9 (0.0%) | 0/4 (0.0%) | 4/10 (40.0%) | 2/9 (22.2%) | 2/5 (40.0%) | 2/8 (25.0%) | 1/8 (12.5%) | 2/5 (40.0%) |
| PD | 1/10 (10.0%) | 1/9 (11.1%) | 1/4 (25.0%) | 1/10 (10.0%) | 0/9 (0.0%) | 0/5 (0.0%) | 2/8 (25.0%) | 1/8 (12.5%) | 0/5 (0.0%) |
| Not performed | 0/10 (0.0%) | 5/9 (55.5%) | 2/4 (50.0%) | 0/10 (0.0%) | 4/9 (44.4%) | 0/5 (0.0%) | 0/8 (0.0%) | 3/8 (37.5%) | 1/5 (20.0%) |
FIGURE 4.
Representative patients for 68Ga-PSMA PET/CT and PSA response evaluation in group A (A), group B (B), and group C (C).
DISCUSSION
The present study showed that PSA response and hematologic toxicity were related to treatment activity. Patients in groups B and C had a better PSA response than those in group A. Platelets also decreased more in groups B and C than in group A; however, in most patients, the absolute counts were still in the reference range.
Recent studies showed that 177Lu-PSMA-617 and 177Lu-PSMA I&T radionuclide therapy is a safe and effective approach for mCRPC patients. A PSA decrease of at least 50% was seen in 25%–40% of patients from the first cycle of 177Lu-PSMA-617 with the average doses of 5.9–6.1 GBq (21–23). This study showed that a decline of greater than 50% in PSA level after the first cycle occurred in 0%, 40%, and 25.0% of patients in groups A, B, and C, respectively. In a recent phase II trial of 177Lu-PSMA-617 radioligand therapy, 57% of patients showed a PSA decline of more than 50% for all patients in the analysis (24). In our study, a decline of greater than 50% (PR) in the PSA level occurred in 10%, 40.0%, and 62.5% of patients in groups A, B, and C, respectively. The PSA response rate in groups B and C seems to be similar to that of 5.9–8.7 GBq of 177Lu-PSMA-617 therapy. In addition, the disease control rates (PR + stable disease) were higher in groups B (70%) and C (75%) than in group A (10%), but there was no significant difference between groups B and C, probably because of the limited number of patients.
Yordanova et al. found that 43% of patients showed PR with 68Ga-PSMA PET/CT response according to adapted PERCIST after 1 cycle of 177Lu-PSMA-617 treatment (23). In our study, 6 (60%), 5 (50%), and 4 (40%) patients showed PR in groups A, B, and C, respectively, after the first cycle treatment. However, there was no difference in response rate among the 3 groups, again likely because of the limited number of patients. Moreover, when we chose comparable bone metastases with baseline SUVmax of 10.0–40.0 from 3 groups, there were still no significant differences demonstrated by changes in SUV. The imaging-based response did not correlate well with the PSA response in some patients, in accordance with the findings of a study showing that the therapy effects on SUV from 68Ga-PSMA-PET/CT are mostly independent of PSA response (25). A preclinical study found that PSMA uptake in the tumor was directly associated with the number of tumor cells and that decreased PSMA uptake after therapy was not due to treatment-induced changes but rather reliably reflects the number of living tumor cells (26). In addition, the changes in 68Ga-PSMA PET/CT reflect the changes in PSMA receptor expression and do not represent changes in the tumor as a whole, because some tumors also express other receptors, such as somatostatin receptors. These findings might also explain the discordance between 68Ga-PSMA PET/CT response and PSA changes in our study.
Hematologic toxicity is the most commonly reported adverse side effect related to 177Lu-PSMA therapy, especially for the patients with a heavy burden of bone metastases and borderline marrow function. Because of albumin binding, 177Lu-EB-PSMA-617 also had a significantly higher effective dose in red bone marrow than did 177Lu-PSMA-617, as also in kidneys and liver. On the basis of a dose limit of 2 Gy to red marrow and the dosimetry result in our previous study, patients can be injected with as much as 34 GBq of 177Lu-EB-PSMA. In the current study, no serious nephrotoxicity or hepatotoxicity was observed. After the first cycle of treatment, only grade 3 anemia occurred in 1 patient who had thalassemia but was not diagnosed before the treatment in group C; this rate is in accordance with a German multicenter study that showed 10% grade 3–4 anemia in patients with 177Lu-PSMA-617 therapy (7). A recent phase II trial reported that the most common toxic effects possibly were grade 3 neutropenia in 7% of patients, grade 3 anemia in 23%, and grade 3 or 4 thrombocytopenia in 27% (24). In our study, grade 3 anemia occurred in 40.0% of patients in group A, and grade 3 anemia occurred in 20.0%, leukopenia in 10.0%, and thrombocytopenia 10.0% in group B. In group C, grade 3–4 anemia, leukopenia, and thrombocytopenia occurred in 37.5%, 12.5%, and 37.5% patients, respectively. As the dose escalated, platelets decreased more in groups B and C than in group A, but the absolute counts were still in the reference range for most patients. To weigh the advantages and disadvantages, patients in group B had a better treatment response and acceptable hematologic side effects, and 177Lu-EB-PSMA treatment should be carefully performed with 2.12-GBq doses and monitored more closely, especially for patients with diffuse red marrow infiltration.
177Lu-EB-PSMA allowed the radioactivity to remain in the target, prolonged tumor retention, and maximized the therapeutic effect with lower doses. A relatively low dose (2.12 GBq) of 177Lu-EB-PSMA can achieve a therapeutic effect similar to that of 5.9–8.7 GBq of 177Lu-PSMA-617, improve the use of 177Lu, and make delivery to vulnerable normal organs fall within the acceptable level. At the same time, lower doses of 177Lu can lower the cost and reduce radiation exposure to medical workers and the public. This new compound has great potential to be used in patients with mCRPC.
There were several limitations in our study. The most prominent was the small number of patients in each group. Second, our study lacked long-term observations of side effects and survival analysis; such observations will be implemented in our future studies. Third, because we did not set 177Lu-PSMA-617 therapy as the control group, the data in our study could be compared only with the literature. Further studies with more patients subjected to 177Lu-EB-PSMA therapy and with 177Lu-PSMA-617 therapy as the control group are warranted, but patients with diffuse bone marrow involvement should be vetted carefully.
CONCLUSION
This study demonstrated that PSA response and hematologic toxicity were related to treatment activity. Grade 4 thrombocytopenia observed in the 3.52-GBq dose group might imply that the potential for further dose escalations is limited, and a 2.12-GBq dose of 177Lu-EB-PSMA—with relatively high efficacy and acceptable side effects—seems to be optimal from the trade-off.
DISCLOSURE
This work was supported by the National Natural Science Foundation of China (project 81871392), the Chinese Academy of Medical Science Major Collaborative Innovation Project (2019-I2M-1-001), and the Intramural Research Program of the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is 177Lu-EB-PSMA therapy with escalating doses and multiple administrations safe and effective in patients with mCRPC, and what is the optimal dose?
PERTINENT FINDINGS: In this clinical study, 28 patients with mCRPC were randomly divided into 3 dose groups for 177Lu-EB-PSMA treatment. The PSA response was correlated with treatment dose, with PSA disease control rates being higher in the 2.12-GBq group (70%) and 3.52-GBq group (75%) than in the 1.18-GBq group (10%) (P = 0.007).
IMPLICATIONS FOR PATIENT CARE: A 2.12-GBq dose of 177Lu-EB-PSMA seems to be the best choice in balancing safety with adequacy in tumor treatment.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract. 2011;65:1180–1192. [DOI] [PubMed] [Google Scholar]
- 3.Hotte SJ, Saad F. Current management of castrate-resistant prostate cancer. Curr Oncol. 2010;17(suppl 2):S72–S79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav MP, Ballal S, Tripathi M, et al. 177Lu-DKFZ-PSMA-617 therapy in metastatic castration resistant prostate cancer: safety, efficacy, and quality of life assessment. Eur J Nucl Med Mol Imaging. 2017;44:81–91. [DOI] [PubMed] [Google Scholar]
- 5.Rahbar K, Schmidt M, Heinzel A, et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: a multicenter retrospective analysis. J Nucl Med. 2016;57:1334–1338. [DOI] [PubMed] [Google Scholar]
- 6.Rahbar K, Bogeman M, Yordanova A, et al. Delayed response after repeated 177Lu-PSMA-617 radioligand therapy in patients with metastatic castration resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:243–246. [DOI] [PubMed] [Google Scholar]
- 7.Rahbar K, Ahmadzadehfar H, Kratochwil C, et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med. 2017;58:85–90. [DOI] [PubMed] [Google Scholar]
- 8.Kulkarni HR, Singh A, Schuchardt C, et al. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: the Bad Berka experience since 2013. J Nucl Med. 2016;57(suppl 3):97S–104S. [DOI] [PubMed] [Google Scholar]
- 9.Ahmadzadehfar H, Wegen S, Yordanova A, et al. Overall survival and response pattern of castration-resistant metastatic prostate cancer to multiple cycles of radioligand therapy using [177Lu]Lu-PSMA-617. Eur J Nucl Med Mol Imaging. 2017;44:1448–1454. [DOI] [PubMed] [Google Scholar]
- 10.Baum RP, Kulkarni HR, Schuchardt C, et al. 177Lu-labeled prostate-specific membrane antigen radioligand therapy of metastatic castration-resistant prostate cancer: safety and efficacy. J Nucl Med. 2016;57:1006–1013. [DOI] [PubMed] [Google Scholar]
- 11.Heck MM, Tauber R, Schwaiger S, et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with 177Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur Urol. 2019;75:920–926. [DOI] [PubMed] [Google Scholar]
- 12.Ahmadzadehfar H, Rahbar K, Kurpig S, et al. Early side effects and first results of radioligand therapy with 177Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res. 2015;5:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kratochwil C, Giesel FL, Stefanova M, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J Nucl Med. 2016;57:1170–1176. [DOI] [PubMed] [Google Scholar]
- 14.Rahbar K, Bode A, Weckesser M, et al. Radioligand therapy with 177Lu-PSMA-617 as a novel therapeutic option in patients with metastatic castration resistant prostate cancer. Clin Nucl Med. 2016;41:522–528. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh A, Heston WD. Tumor target prostate specific membrane antigen (PSMA) and its regulation in prostate cancer. J Cell Biochem. 2004;91:528–539. [DOI] [PubMed] [Google Scholar]
- 16.Kratochwil C, Fendler WP, Eiber M, et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur J Nucl Med Mol Imaging. 2019;46:2536–2544. [DOI] [PubMed] [Google Scholar]
- 17.Wang Z, Tian R, Niu G, et al. Single low-dose injection of Evans blue modified PSMA-617 radioligand therapy eliminates prostate-specific membrane antigen positive tumors. Bioconjug Chem. 2018;29:3213–3221. [DOI] [PubMed] [Google Scholar]
- 18.Zang J, Fan X, Wang H, et al. First-in-human study of 177Lu-EB-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scher HI, Morris MJ, Stadler WM, et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J Clin Oncol. 2016;34:1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(suppl 1):122S–150S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rathke H, Giesel FL, Flechsig P, et al. Repeated 177Lu-labeled PSMA-617 radioligand therapy using treatment activities of up to 9.3 GBq. J Nucl Med. 2018;59:459–465. [DOI] [PubMed] [Google Scholar]
- 22.Bräuer A, Grubert LS, Roll W, et al. 177Lu-PSMA-617 radioligand therapy and outcome in patients with metastasized castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging. 2017;44:1663–1670. [DOI] [PubMed] [Google Scholar]
- 23.Yordanova A, Linden P, Hauser S, et al. Outcome and safety of rechallenge [177Lu]Lu-PSMA-617 in patients with metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2019;46:1073–1080. [DOI] [PubMed] [Google Scholar]
- 24.Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–833. [DOI] [PubMed] [Google Scholar]
- 25.Taeger P, Hammes J, Hohberg M, et al. Discrete evaluation of multi-cycle Lu-177-PSMA-617-therapy effects on bone versus lymph node metastases in patients with metastasized castration-resistant prostate cancer (mCRPC) [abstract]. J Nucl Med. 2018;59(suppl 1):523.28775202 [Google Scholar]
- 26.Hillier SM, Kern AM, Maresca KP, et al. 123I-MIP-1072, a small-molecule inhibitor of prostate-specific membrane antigen, is effective at monitoring tumor response to taxane therapy. J Nucl Med. 2011;52:1087–1093. [DOI] [PubMed] [Google Scholar]