Abstract
A new series of 1-(6-amino-9H-purin-9-yl)-1-deoxy-N-ethyl-β-d-ribofuranuronamide-bearing N-arylureas or N-arylcarboxamido groups at the purine 6 position and N-arylureas combined with halogens or alkynyl chains at the 2 position have been synthesized and tested for affinity at A1 and A2A adenosine receptors in rat brain membranes and at cloned rat A3 receptors expressed in CHO cells. The derivatives contained the 5′ substituent found in the potent, nonselective agonist 1-(6-amino-9H-purin-9-yl)-1-deoxy-N-ethyl-β-d-ribofuranuronamide (NECA). While the carboxamido derivatives (9–13) showed affinity for A1 receptors, the urea derivatives (30–45) showed different degrees of affinity and selectivity for the A3 adenosine receptor subtype. In particular the derivative bearing a p-sulfonamidophenyl-urea at the 6 position, 31 showed a high affinity (Ki = 9 nM) and selectivity for the A3 receptors compared to that of the reference compound 1-[6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-d-ribofuranuronamide (IB-MECA). Furthermore, the importance of the stereochemistry in the interaction of these ligands at the rat A3 adenosine receptors has been evaluated by introducing a chiral chain at the 6 position. The introduction of halogens or alkynyl chains at the purine 2 position of selected ureas did not give the expected enhancement of potency at A2A and/or A3 receptors but rather showed a dramatic reduction of A2A affinity, resulting in compounds with good A2A/A3 selectivity. For example, the 2-(3-hydroxy-3-phenyl-1-propyn-1-yl)-6-(4-methoxyphenylurea) derivative 61 showed the capability to bind simultaneously to A1 and A3 receptor subtypes, excluding the A2A receptor. Compound 31 was shown to be an agonist, 9-fold more potent than NECA, at A3 receptors in rat RBL-2H3 mast cell membranes through stimulation of binding of [35S]GTP-γ-S.
Introduction
Four subtypes of adenosine receptors (A1, A2A, A2B, and A3) have been cloned1–4 and characterized pharmacologically with the use of potent agonists and antagonists. The A1 and A2 receptor subtypes are linked to inhibition and stimulation, respectively, of adenylyl cyclase.5–7 A3 receptors are coupled both to the stimulation of phospholipase C, as shown in rat RBL-2H3 mast cells8 and in rat brain slices,9 and to the inhibition of adenylyl cyclase.4
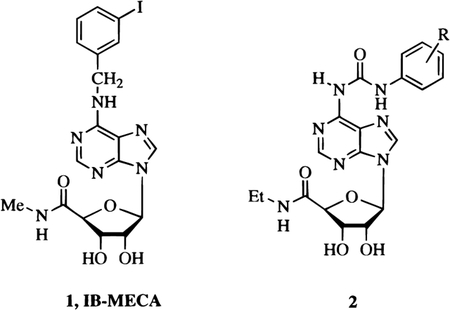
Many selective agents have been developed for the A110–15 and A2A16–19 receptor subtypes. Some of these seem promising as potential therapeutic agents in the treatment of Parkinson’s disease,20 cognitive deficits,21 schizophrenia,22 epilepsy23a and renal failure.23b Selective and/or high affinity agonists and antagonists for the A2B receptor have not yet been reported. Since the cloning of the A3 receptor from a rat brain cDNA library,4 recent efforts have been made in order to develop selective agonist ligands24 as well as some selective antagonists.25–28 The selective agonists, 1-[6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-d-ribofuranuronamide (IB-MECA) 1, shows a Ki value of 1.1 ± 0.3 nM at rat A3 receptors and a 50-fold selectivity vs either A1 or A2A receptors.29 The related agonist,[125I]1-[6-[[(4-amino-3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-d-ribofuranuronamide ([125I]AB-MECA),30 has become a useful radioligand for the screening of new derivatives interacting with cloned A3 receptors.
The A3 receptor mediates many processes such as inflammation,31 hypotension,32 and mast cell degranulation.33 Also, the A3 receptor apparently has a role in the central nervous system. IB-MECA 1 induces behavioral depression34 and, upon chronic administration, protects against cerebral ischemia.35 Furthermore, at high concentrations A3 selective agonists were also found to induce apoptosis in HL-60 human leukemia cells.36
From the point of view of structure–activity relationships, the ribose moiety of adenosine has to be maintained unchanged, with the exception of the 5′ position, at which alkyluronamide groups are well tolerated.37 Furthermore, some substitutions at the purine 6 and/or 2 positions modulates affinity and selectivity of adenosine agonists for the different receptor subtypes.24 Alkyl or aryl derivatives at the 6 position of the purine nucleus are A1 selective, and many derivatives at the 2 position bearing C, N, or O substituents are A2A selective. 6-Benzyl analogues also containing the 5′-uronamide modification, such as 1, have been found to be potent and selective A3 agonists.38 We have recently reported a series of adenosine 5′-uronamide derivatives, substituted at the purine 6 position, of general formula 2, which have shown high affinity at adenosine A3 receptors. In particular, NECA derivatives containing at the 6-positon the 3-chloro-, 2-chloro-, or 4-methoxyphenyl-carbamoyl moieties have shown affinity for A3 adenosine receptors with 4–7 nM Ki values. However, while the selectivity with respect to A2A receptors was high (A2A/A3 from 96 to 510), the discrimination between A1 and A3 receptors was very low (A1/A3 from 4- to 10-fold).39
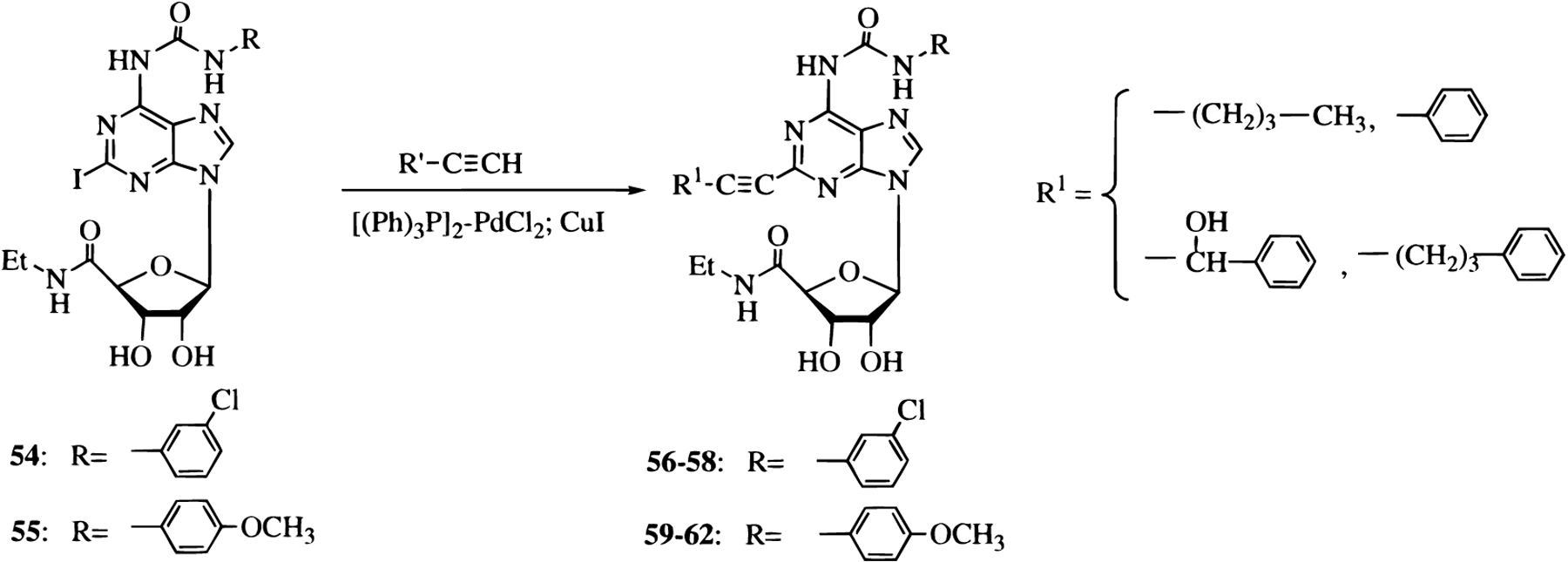
A new series of carboxamido and urea derivatives at the 6 position of NECA (9–13, 30–45, and 52–62) have been prepared to further explore the structural basis for the selectivity for A3 vs A1 receptors. In particular, we were interested in evaluating the influence of the length of the acyl chain at the adenine N position on the receptor affinity. We prepared some amide derivatives of NECA (9–13), which are structurally related to our previously reported series of urea derivatives.39 Also, some new urea derivatives at purine 6 position of NECA (30–45) were synthesized to evaluate the physicochemical parameters involved in the interaction with A3 adenosine receptors. Furthermore, we introduced a chiral center in compounds 33 and 34 to evaluate the stereoselectivity of binding of the substituent at 6 position.
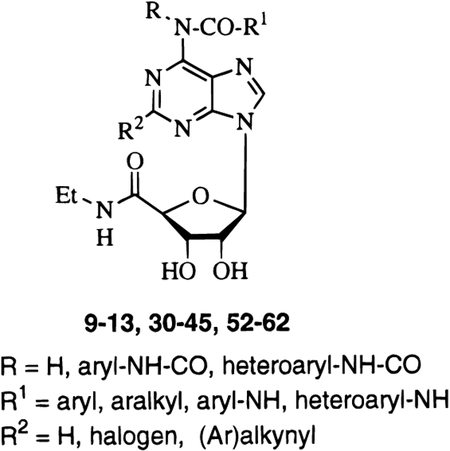
On the basis of the observation that 2-(1-hexyn-1-yl)-1-(6-amino-9H-purin-9-yl)-1-deoxy-N-ethyl-β-d-ribofuranuronamide (HENECA), a potent A2A adenosine agonist,16,40 also showed high affinity at rat A3 receptors,41 we combined ureido moieties at the purine 6 position, that were favorable in A3 receptor binding, with the substitution at the 2 position. Using results from a previous work,39 we introduced the A3 receptor affinity enhancing 4-methoxyphenyl-and 3-chlorophenyl-urea groups at the purine 6 position, while substituting the 2 position with halogens (Cl, I) (52–55) and alkynyl groups (56–62).
Results and Discussion
Chemistry.
The preparation of N-acyl derivatives of NECA, compounds 9–13, was performed following the general synthetic strategy depicted in Scheme 1.
Scheme 1.
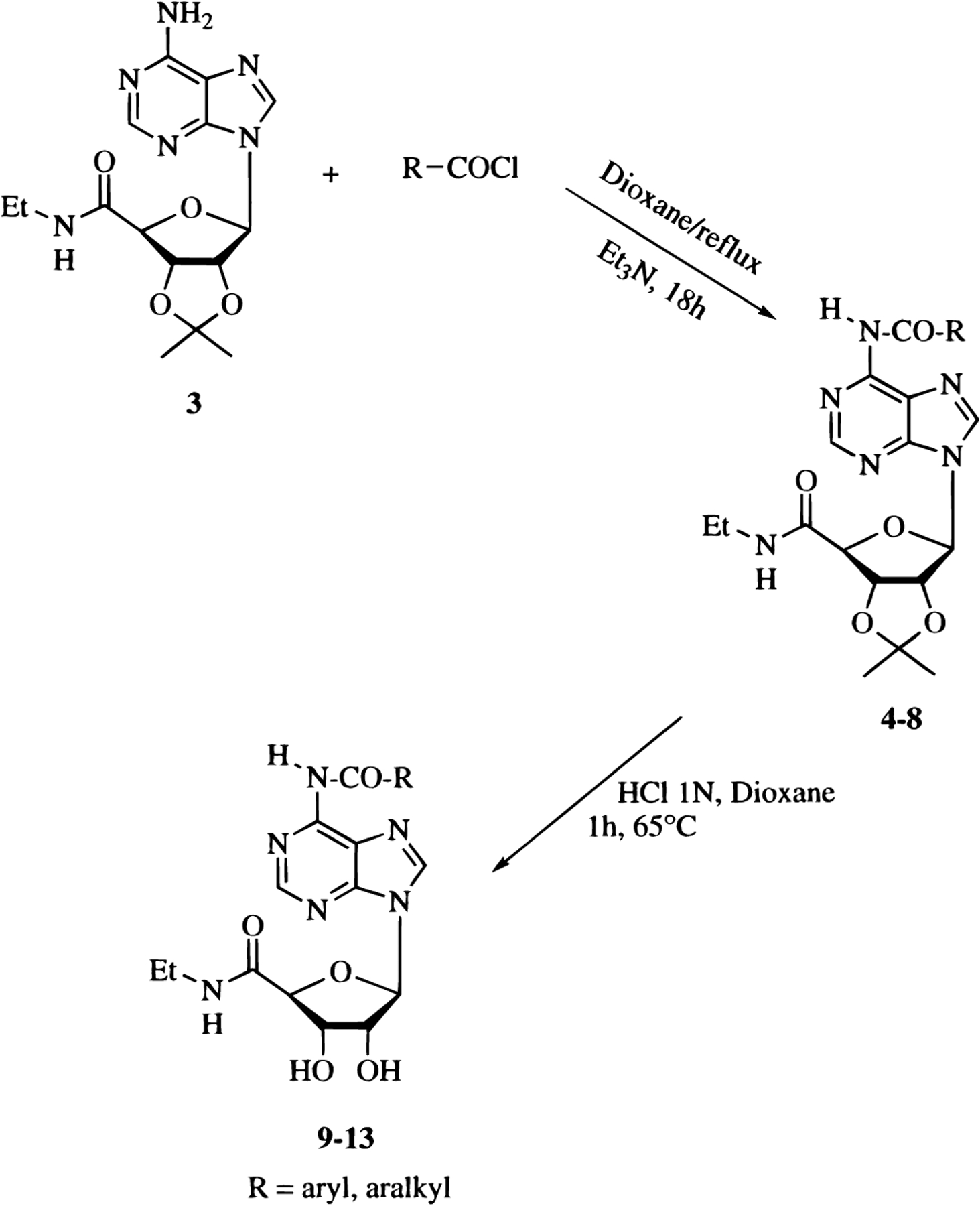
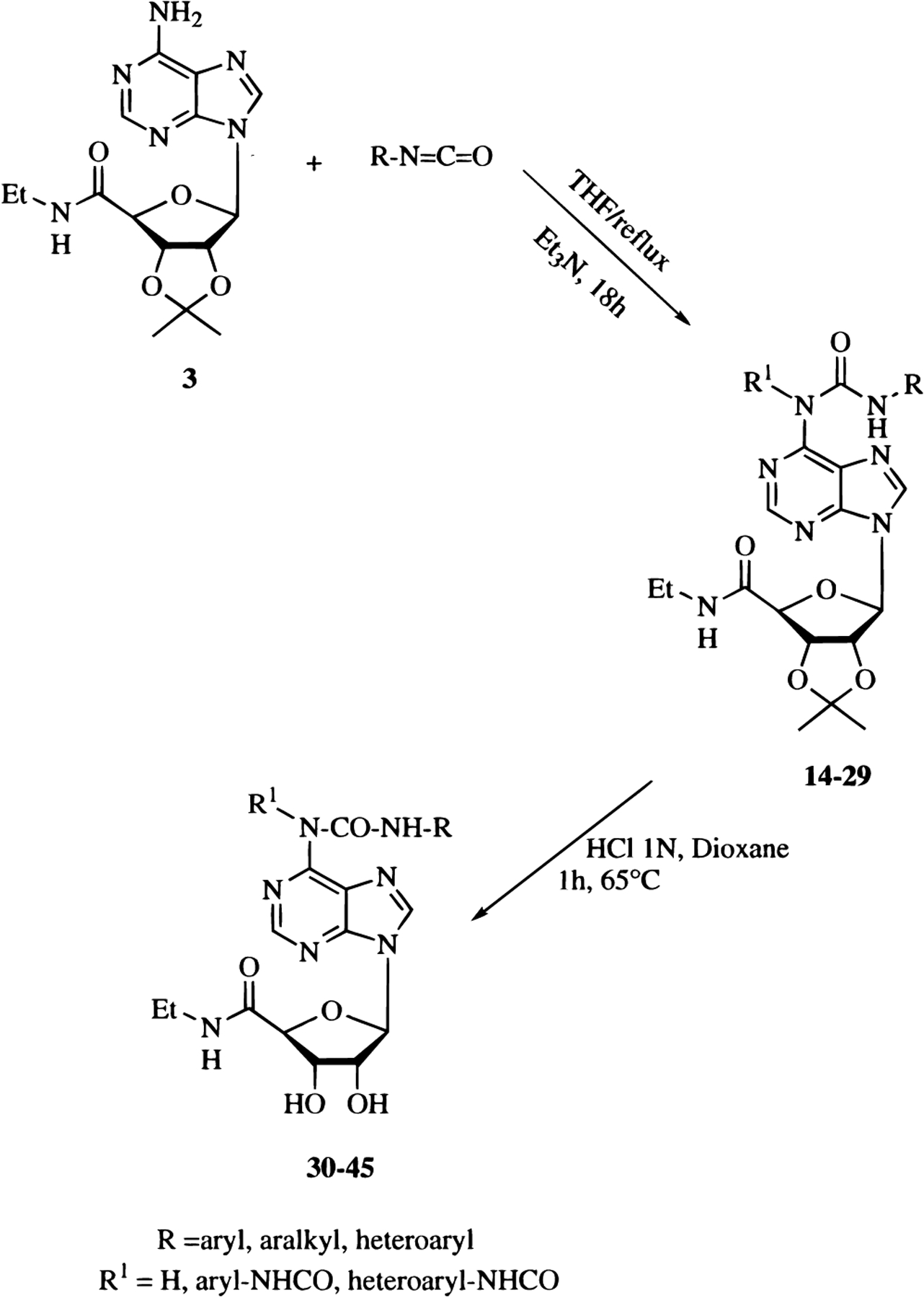
The amino group at the 6 position of NECA is not very reactive; therefore, it was necessary to protect the hydroxyl groups of the ribose moiety during selective acylation. Reaction of 2′,3′-O-isopropylidene-protected NECA (3) with the appropriate acyl chloride in the presence of a catalytic amount of triethylamine at reflux afforded the adducts 4–8 in a good yield, which were in turn converted into the desired compounds (9–13) by deprotection in aqueous 1 N HCl and dioxane at 65 °C. In a similar manner, derivatives 30–45 were prepared by nucleophilic reaction of the appropriate isocyanate with 3 and then by deprotection of the ribosyl moiety (Scheme 2).
Scheme 2.
When not commercially available, the isocyanate was prepared by reacting the corresponding substituted anilines using trichloromethylchloroformate, as described previously.42
Using 4-nitrophenyl isocyanate or 5-chloropyridin-2-yl isocyanate under the conditions mentioned above, the bis adducts 44 and 45 were the only products isolated from the reaction mixture.
The 2-chloro- and the 2-iodo-6-arylcarbamoyl derivatives of NECA 52–55 were synthesized from 2′,3′-O-isopropylidene-2-chloro-NECA (46)43 and 2′,3′-O-isopropylidene-2-iodo-NECA (47),44 respectively (Scheme 3).
Scheme 3.
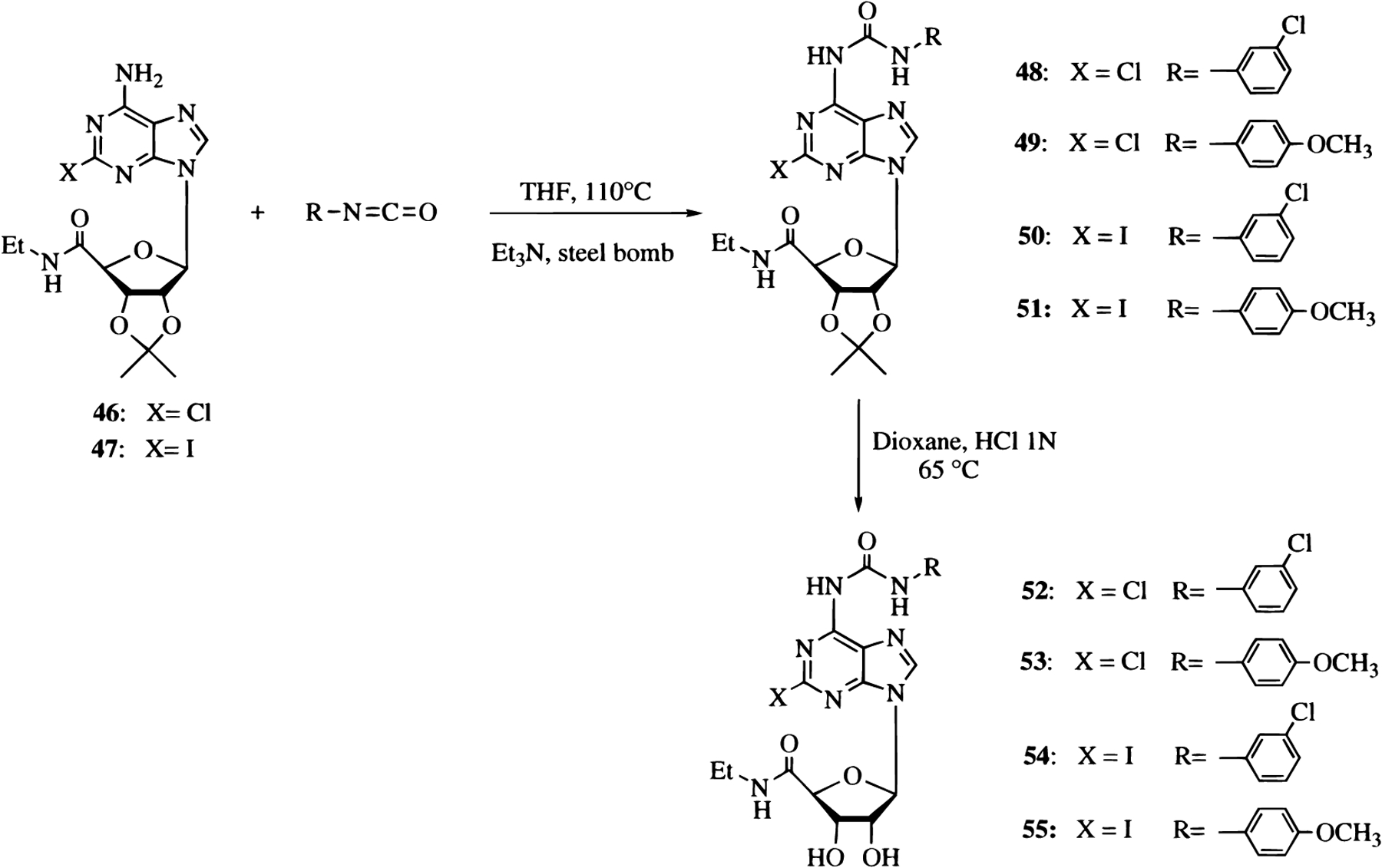
The reaction of 46 or 47 in dry THF with the appropriate isocyanate to afford the protected nucleosides 48–51 was carried out in a steel bomb at 110 °C for 24 h. The nucleosides were deprotected in 1 N HCl and dioxane at 65 °C to give the corresponding compounds 52–55. The synthesis of the (ar)alkynyl derivatives 56–62 was carried out by a modification of the palladium-catalyzed cross-coupling reaction.40
The 2-iodo derivatives 54 and 55 in dry acetonitrile, DMF, and triethylamine reacted with cuprous iodide, bis(triphenylphosphine)palladium dichloride, and the appropriate terminal alkyne, at room temperature for several hours under an atmosphere of N2, to produce the alkynyl derivatives 56–58 and 59–62, respectively (Scheme 4).
Scheme 4.
Biological Activity.
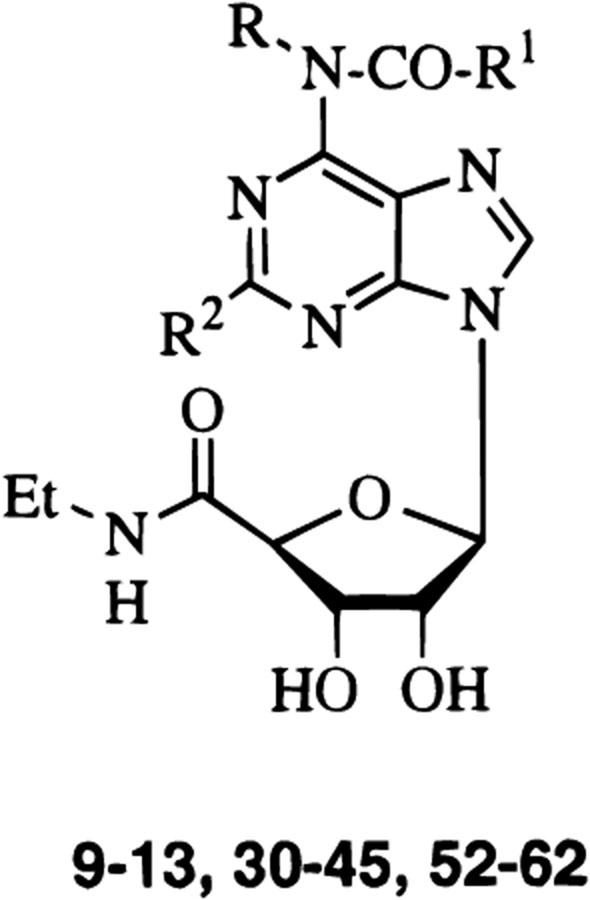
All the synthesized compounds (9–13, 30–45, and 52–62) were tested in radioligand binding assays for affinity at the rat brain A1, A2A, and A3 receptors, and the results are summarized in Table 1.45
Table 1.
Affinities of Synthesized Derivatives (9–13, 30–45, and 52–62) in Radioligand Binding Assays at Rat Brain A1, A2A, and A3 Adenosine Receptors
| ||||||||
|---|---|---|---|---|---|---|---|---|
| compd | R | R1 | R2 | K1 (nM) or % inhibition | A1/A3 | A2A/A3 | ||
| Ki(A1)a | Ki(A2A)b | Ki(A3)c | ||||||
| IB-MECA, 1 | 54 ± 5 | 56 ± 8 | 1.1 ± 0.3 | 49 | 51 | |||
| C1-IB-MECA | 820 ± 570 | 470 ± 365 | 0.33 ± 0.08 | 2500 | 1400 | |||
| NECAd | 6.3 | 10.3 | 113 | 0.071 | 0.091 | |||
| 9 | H | 4-biphenyl | H | 5.92 ± 1.04 | 3580 ± 850 | 979 ± 22 | 0.0060 | 3.7 |
| 10 | H | 2,4-Cl-Ph-CH2 | H | 16.0 ± 3.3 | 26.6 ± 8.8 | 167 ± 7 | 0.095 | 0.16 |
| 11 | H | 4-CH3O-Ph | H | 86.4 ± 14.2 | 237 ± 46 | 837 ± 142 | 0.10 | 0.28 |
| 12 | H | 2-Cl-Ph | H | 1170 ± 220 | 1960 ± 300 | 754 ± 132 | 1.6 | 2.6 |
| 13 | H | Ph | H | 252 ± 52 | 670 ± 211 | 824 ± 126 | 0.30 | 0.81 |
| 30 | H | PhCH2NH | H | 171 ± 15 | 1793 ± 230 | 38.3 ± 6.4 | 4.5 | 47 |
| 31 | H | 4-SO2NH2-PhNH | H | 453 ± 141 | 1180 ± 360 | 9.73 ± 0.75 | 47 | 120 |
| 32 | H | 4-CH3CO-PhNH | H | 72.7 ± 11.8 | 1050 ± 270 | 20.9 ± 7.51 | 3.5 | 50 |
| 33 | H | (R)-α-phenylethyl-NH | H | 433 ± 171 | 279 ± 171 | 16.3 ± 3.7 | 27 | 17 |
| 34 | H | (S)-α-phenylethyl-NH | H | 537 ± 33 | 2970 ± 930 | 319 ±134 | 1.7 | 9.3 |
| 35 | H | 5-Me-isoxazol-3-yl-NH | H | 146 ± 39 | 884 ± 232 | 532 ± 240 | 0.27 | 1.7 |
| 36 | H | l,3,4-thiadiazol-2-yl-NH | H | 208 ± 42 | 917 ± 254 | 5550 ± 2800 | 0.037 | 0.16 |
| 37 | H | 4-n-C3H7O-PhNH | H | 247 ± 43 | 255 ± 85 | 107 ± 34 | 2.3 | 2.4 |
| 38 | H | Ph-CH2CH2NH | H | 129 ± 44 | 2650 ± 640 | 149 ± 26 | 0.86 | 18 |
| 39 | H | 3,4-MeO-Ph-CH2CH2NH | H | 1770 ± 490 | 1570 ± 220 | 411 ± 178 | 4.3 | 3.8 |
| 40 | H | fur-2-yl-CH2NH | H | 419 ± 22 | 1860 ± 370 | 713 ± 91 | 0.58 | 2.6 |
| 41 | H | 4-(pyridin-2-yl-NHSO2)PhNH | H | 292 ± 76 | 740 ± 283 | 54.1 ± 10.8 | 5.4 | 14 |
| 42 | H | 4-(5-Me-isoxazol-3-yl-NHSO2)PhNH | H | 901 ± 62 | 977 ± 425 | 155 ± 5 | 5.8 | 6.3 |
| 43 | H | 4-(pyrimidin-2-yl-NHSO2)PhNH | H | 725 ± 141 | 16.8 ± 3.1 | 405 ± 12 | 1.7 | 0.041 |
| 44 | 4-NO2-Ph-NH-CO | 4-NO2-Ph-NH | H | 89.1 ± 8.8 | 2530 ± 200 | 168 ± 42 | 0.53 | 15 |
| 45 | 5-Cl-pyridin-2-yl-NH-CO | 5-Cl-pyridin-2-yl-NH | H | 761 ± 187 | 799 ±191 | 5700 ± 100 | 0.13 | 0.14 |
| 52 | H | 3-Cl-Ph-NH | Cl | 90.0 ± 23.7 | 557 ± 112 | 77.6 ± 25 | 1.2 | 4.3 |
| 53 | H | 4-MeO-Ph-NH | Cl | 22.0 ± 5.5 | 59.0 ± 5.3 | 17.1 ± 8.6 | 1.2 | 3.5 |
| 54 | H | 3-Cl-Ph-NH | I | 103 ± 23 | 10 600 ± 3700 | 315 ± 63 | 0.33 | 34 |
| 55 | H | 4-MeO-Ph-NH | I | 29.4 ± 12.1 | 260 | 251 ± 95 | 0.12 | 1.0 |
| 56 | H | 3-Cl-Ph-NH | nC4H9–C≡C | 2040 ± 540 | 7130 ± 2570 | 581 ± 82 | 3.5 | 12 |
| 57 | H | 3-Cl-Ph-NH | Ph–C≡C | 1330 ± 490 | 48700 ± 1400 | 611 ± 100 | 2.1 | 80 |
| 58 | H | 3-Cl-Ph-NH | PhCH(OH)–C≡C | 814 ± 113 | 12 ± 3% (10−4) | 696 ±149 | 1.2 | >140 |
| 59 | H | 4-MeO-Ph-NH | nC4H9–C≡C | 428 ± 124 | 2330 ± 480 | 211±104 | 2.0 | 11 |
| 60 | H | 4-MeO-Ph-NH | Ph–C≡C | 4790 ± 760 | 29 ± 3% (10−4) | 154 ± 46 | 31 | >4000 |
| 61 | H | 4-MeO-Ph-NH | PhCH(OH)–C≡C | 75.1 ± 20.6 | 12 ± 6% (10−4) | 324 ± 65 | 0.23 | >300 |
| 62 | H | 4-MeO-Ph-NH | Ph(CH2)3–C≡C | 14400 ± 4000 | 1670 ± 420 | 89.5 ± 19 | 160 | 19 |
Displacement of specific [3H]R–PIA binding (A1) in rat brain membranes, expressed as Ki ± SEM in nM (n = 3–6).
Displacement of specific [3H]CGS 21680 binding (A2A) in rat striatal membranes expressed as Ki ± SEM in nM (n = 3–6).
Displacement of specific [125I]AB-MECA binding at rat A3 receptors expressed in CHO cells, expressed as Ki ± SEM in nM (n = 3–6).
Values at A1 and A2A receptors are taken from Bruns et al.45 Ki values at A1 receptors are vs specific binding of [3H]-N6-cyclohexyladenosine ([3H]-CHA). Ki values at A2A receptors are vs specific binding of [3H]-NECA in the presence of 50 nM CPA in rat striatal membranes.
In our previous studies, we demonstrated that the 6-substituted adenosine uronamides are full agonists in inhibiting adenylate cyclase via rat A3 receptors.39
From the binding assays, it appeared that the presence of an amide vs urea functionality at the 6 position (9–13) was generally detrimental in terms of affinity at rat A3 receptors, whereas several of these derivatives (11 and 13) showed roughly 1 order of magnitude increase in affinity for adenosine A2A receptor. Affinity at A1 receptors was highly variable, ranging from 5.9 nM (biphenyl derivative, 9) to 1.2 μM. For example, the o-chlorobenzoyl derivative (12) was 100-fold less potent at A3 receptors and was 10-fold less potent at A1 receptors than the corresponding urea derivative.39 The p-methoxybenzoyl derivative (11) was 127-fold less potent at A3 receptors than the corresponding p-methoxyphenyl urea derivative.39 These results suggested that a small modification in the chain at the 6 position could modulate the affinity for A1 or A3 receptors. In the series of urea-derivatives (30–45), a substituted-phenyl (31, 32, and 37) or substituted-benzyl (30, 33, and 34) group led to higher affinity and selectivity at A3 adenosine receptors. In fact, derivatives 31, 32, and 37 showed a high affinity (9.7–107 nM) at A3 receptors with varying degrees of A1/A3 selectivity. In particular, compound 31 was less active than IB-MECA (9.7 nM vs 1.1 nM) but showed selectivity for A3 vs either A1 or A2A receptors comparable to the reference compound. In addition, it seemed necessary to have a free amino group on the sulfonamido moiety, since its substitution with some heteroaryl groups (e.g., compounds 41–43) led to derivatives with modest affinity at rat A3 adenosine receptors and low selectivity with respect to A1 and A2A receptors.
The data demonstrate the importance of a phenylurea moiety at the purine 6 position for an interaction with the A3 adenosine receptor subtype.46 When the phenyl ring was replaced with various heterocycles (35, 36, and 40) both affinity and selectivity for A3 receptors decreased.
Moreover, the disubstitution at the 6 position led to derivatives (44–45) with diminished activity, in particular at A2A and A3 receptors. This could be explained by the absence of one hydrogen atom at N6, which could act as a hydrogen bond donor, already noted as a requirement for binding at the A1 and A2A receptor subtypes.47
The benzylic series (30, 33, and 34) showed a SAR pattern comparable to the phenylic series. In fact, the unsubstituted compound 30 showed affinity and selectivity at A3 receptors very similar to those of the previously reported phenyl derivative.39 Interestingly, comparing the two diastereomers 33 and 34 in which substituents at the purine 6 position are of opposite configuration, the R-isomer (33) was more potent than the S-isomer (34) at the rat A2A and A3 adenosine receptor subtypes. The binding stereoselectivity of the N6 substituent confirms the importance of the stereochemistry at this subregion of the adenosine receptor binding site, similar to that already observed for the A1 agonists R-PIA and S-PIA.48 A similar observation has been made for the C2 subregion of the A2A subtype, both with geometric isomers of 2-alkenyl-NECA49 and with diastereomers of 2-alkynyl derivatives of NECA [R- and S-PHPNECA].50
The data also demonstrate the importance of chain length at the 6 position and suggest that phenyl or benzyl chains are the preferred substituents for the interaction with the A3 adenosine receptor subtype. Longer chains, with respect to the phenylic or benzylic moieties, seemed not to improve the affinity at rat A3 receptors. Thus, compounds, 38 and 39, bearing 2-phenylethyl and 3,4-dimethoxy-2-phenylethyl groups at the purine 6 position, respectively, showed submicromolar affinity but low selectivity at rat A3 receptors.
These results and our previous work39 seem to indicate that the lipophilicity of the para substituents of a phenyl ring plays a significant role in A3 affinity. A quantitative correlation was not found, but compounds 31 (π SO2NH2 = −1.82), 32 (π COCH3 = −0.55), and the previously reported 6-phenyl urea derivative substituted with a methoxy group at the para position (π OCH3 = −0.02), bearing the least lipophilic substituents, show some of the most favorable Ki values within the series.51
Species differences, which are pronounced for antagonists but not for agonists, in binding of ligands to A3 receptors have been noted. The most interesting derivative in the present study (31) showed affinity at human A3 adenosine receptors (56.1 ± 9.1 nM) comparable to that at rat A3 receptors, but there was 6-fold more potency at rat receptors. This finding confirms, as already observed, significant differences between rat and human A3 adenosine receptor subtypes.28
A functional assay indicated that compound 31 acted as a full agonist at rat A3 receptors. The assay consisted of agonist-induced stimulation of binding of a guanine nucleotide to membranes of rat RBL-2H3 mast cells, which contain a high density of A3 receptors.30 Figure 1 shows that the potent A3 agonists 1-[6-[[(4-amino-3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-d-ribofuranuronamide (I-AB-MECA) and 2-chloro-1-[6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-d-ribofuranuronamide (Cl-IB-MECA) increased binding of [35S]GTP-γ-S in a dose-dependent manner and with 9-fold greater potency than NECA, as expected for A3 receptors.
Figure 1.
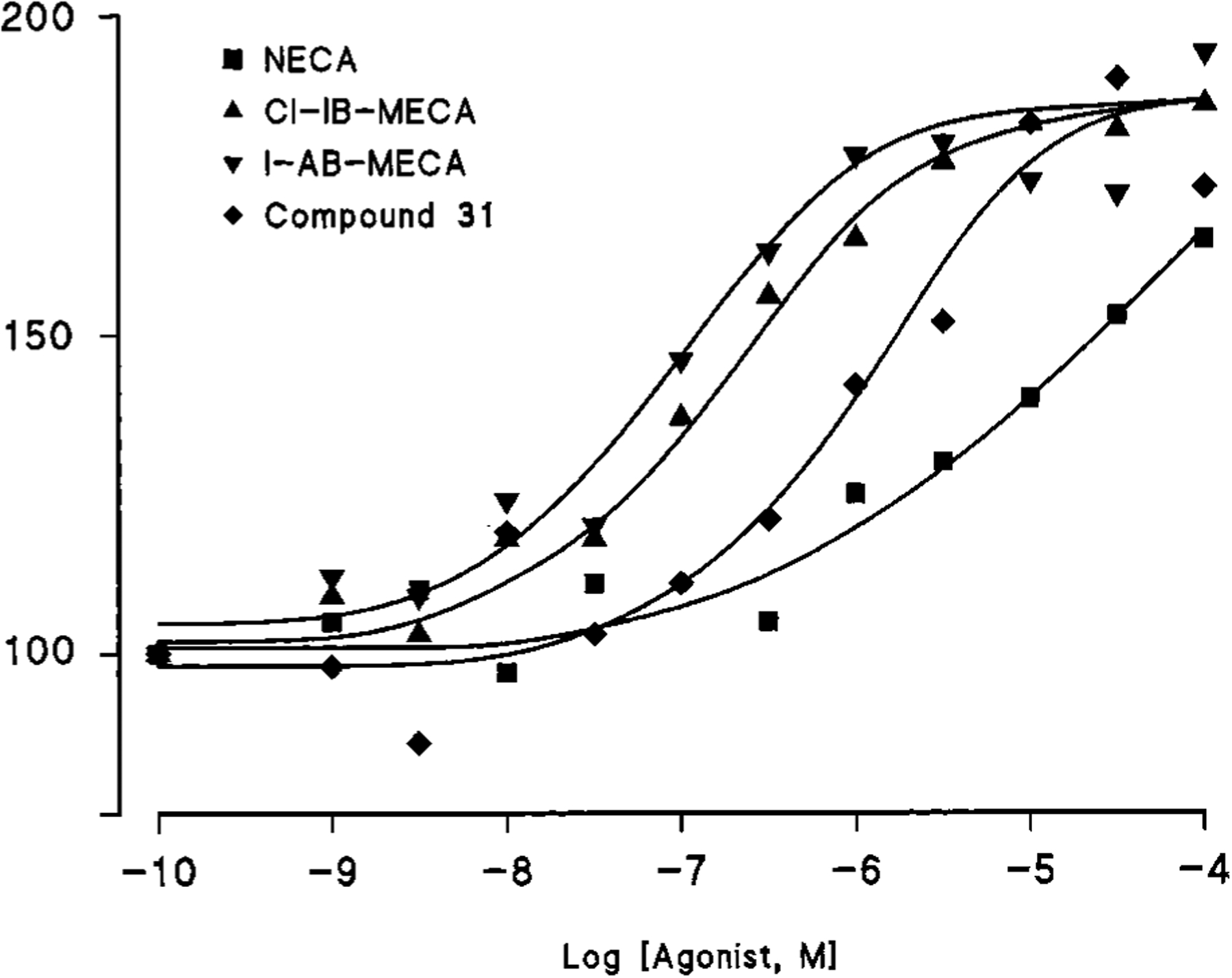
Effect of the adenosine agonists NECA, compound 31, Cl-IB-MECA, and I-AB-MECA on the binding of [35S]GTP-γ-S to membranes of rat RBL-2H3 mast cells. EC50 values were NECA (10.6 μM), compound 31 (1.15 μM), Cl-IB-MECA (174 nM), and I-AB-MECA (86.8 nM).
A correlation between binding affinity and potency in this functional assay has been demonstrated.52
Compound 31 was as efficacious as these two potent agonists but approximately 1 order of magnitude less potent, with an EC50 value of 1.15 μM.
The introduction of halogens at the 2 position of selected urea derivatives at the purine 6 position led to compounds 52–55, which show an A3 affinity ranging from 17 nM (53) to 315 nM (54). For example, the presence of a chlorine atom in the 2 position reduced the affinity and selectivity with respect to the corresponding 2-unsubstituted urea derivatives39 (52, Ki at A3 = 77.6 nM vs 4.4 nM; 53, Ki at A3 = 17 nM vs 6.6 nM), unlike data for various other 6-substituted A153 and A329 receptor agonists. Similarly, the presence of an iodine atom further decreased affinity and selectivity (54, Ki at A3 = 315 nM; 55, Ki at A3 = 251 nM).
The introduction of an alkynyl chains at the 2 position of these 5′-carboxamide adenosine derivatives was expected to enhance the potency at A3 receptors, consistent with the high affinity demonstrated by HENECA (Ki at A3 = 25.6 nM).41 Surprisingly, the 2-alkynyl-substituted N6-urea 5′-carboxamide derivatives (56–62), in fact, showed a dramatic reduction of A2A affinity, indicating the interdependence of substitution at the purine 2 and 6 positions. The triple substitution resulted in compounds with extremely high A3 vs A2A receptor selectivity, 60 > 61 > 58 > 57 (in order of selectivity). Thus, the most potent compounds in this group, showing high selectivity versus the A2A subtype and which also showed a comparable affinity for A1 and A3 receptors, the 2-(3-hydroxy-3-phenyl-1-propyn-1-yl) derivative 61, could be an interesting candidate for the simultaneous stimulation of both A1 and A3 subtypes. This could be interesting since an interaction between these two subtypes has been demonstrated in the rat hippocampus, in the presynaptic control of release of glutamic acid.54 Since both the A1 and A3 receptors are coupled to the inhibition of adenylate cyclase, it may prove useful to have such pharmacological probes of mixed A1 and A3 selectivity such that they would exert a dual, simultaneous action on the same second messenger system. Conversely, compound 62 showed very high selectivity for the A3 versus the A1 subtype.
Conclusions
The present study provides useful information concerning the structural requirements necessary for recognition by the A3 adenosine receptor. It appears that a phenyl- or benzylcarbamoyl moiety at the purine 6 position seems favorable for A3 adenosine receptor affinity and selectivity, while an arylcarboxamido group enhances A1 affinity and selectivity. In particular, in the urea series, compound 31 was shown to be less potent but with approximately the same degree of A3 selectivity vs that of either A1 or A2A with respect to the reference compound IB-MECA.
Furthermore, a simultaneous substitution at the 6 and 2 positions does not improve affinity and selectivity for A3. However, the 2-(3-hydroxy-3-phenyl-1-propyn-1-yl) derivative 61 showed the capability to bind simultaneously to A1 and A3 receptor subtypes, excluding the A2A receptor.
Experimental Section
Chemistry.
Reactions were routinely monitored by thin-layer chromatography (TLC) on silica gel (precoated F254 Merck plates), and products were visualized with iodine or aqueous potassium permanganate. Infrared spectra (IR) were measured on a Perkin-Elmer 257 instrument. 1H NMR spectra were determined in CDCl3 or DMSO-d6 solutions with a Bruker AC 200 spectrometer, peak positions are given in parts per million (δ) downfield from tetramethylsilane as internal standard, and J values are given in Hz. Light petroleum ether refers to the fractions boiling at 40–60 °C. Melting points were determined on a Buchi-Tottoli instrument and are uncorrected. Chromatography was performed with Merck 60–200 mesh silica gel. All products reported showed IR and 1H NMR spectra in agreement with the assigned structures. Organic solutions were dried over anhydrous magnesium sulfate. Elemental analyses were performed by the microanalytical laboratory of Dipartimento di Chimica, University of Ferrara, and were within ±0.4% of the theoretical values for C, H, and N.
General Procedure for the Preparation of 1-Deoxy-1-[6-[[(substituted)carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (4–8).
2′,3′-O-Isopropylidene-NECA 3 (0.43 mmol) was dissolved in freshly distilled dioxane (4 mL), and the appropriate acyl chloride (1.3 equiv) and a catalytic amount of triethylamine (2–3 drops) were added. The mixture was refluxed under argon for 15 h. The solvent was then removed under reduced pressure, and the residue was purified by flash chromatography (CH2Cl2–EtOAc 20%) to afford the desired compound 4–8.
1-Deoxy-1-[6-[[(4-biphenyl)carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (4):
yield 80%; pale yellow foam; IR (neat) cm−1 3445, 1720, 1640, 1575; 1H NMR (CDCl3) δ 0.84 (t, 3H, J = 7), 1.39 (s, 3H), 1.62 (s, 3H), 3.02–3.11 (m, 2H), 4.71 (d, 1H, J = 2), 5.39–5.46 (m, 2H), 6.19 (d, 1H, J = 2), 6.66 (t, 1H, J = 2), 7.40–7.74 (m, 7H), 8.09–8.16 (m, 3H), 8.74 (s, 1H), 9.44 (bs, 1H). Anal. (C28H28N6O5) C, H, N.
1-Deoxy-1-[6-[[(2,4-dichlorobenzyl)carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (5):
yield 67%; white foam; IR (neat) cm−1 3440, 1730, 1625, 1565, 1210; 1H NMR (CDCl3) δ 0.82 (t, 3H, J = 7), 1.17 (s, 3H), 1.26 (s, 3H), 2.99–3.13 (m, 2H), 4.32 (s, 2H), 4.72 (d, 1H, J = 1.8), 5.38–5.48 (m, 2H), 6.16 (d, 1H, J = 1.8), 6.56 (t, 1H, J = 2), 7.20–7.32 (m, 3H), 7.41 (s, 1H), 8.20 (s, 1H), 8.67 (s, 1H). Anal. (C23H24Cl2N7O5) C, H, N.
1-Deoxy-1-[6-[[(4-methoxyphenyl)carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (6):
yield 85%; white solid, mp 105–107 °C (CH2Cl2–Et2O); IR (KBr) cm−1 3445, 1715, 1640, 1555, 1210; 1H NMR (CDCl3) δ 0.85 (t, 3H, J = 7), 1.40 (s, 3H), 1.63 (s, 3H), 3.03–3.12 (m, 2H), 3.90 (s, 3H), 4.72 (d, 1H, J = 2), 5.41–5.49 (m, 2H), 6.16 (d, 1H, J = 2), 6.61 (t, 1H, J = 2), 7.01 (d, 2H, J = 9), 8.02 (s, 2H, J = 9), 8.10 (s, 1H), 8.74 (s, 1H), 9.09 (bs, 1H). Anal. (C23H26N6O6) C, H, N.
1-Deoxy-1-[6-[[(2-chlorophenyl)carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (7):
yield 72%; white foam; IR (neat) cm−1 3435, 1720, 1620, 1550, 1230; 1H NMR (CDCl3) δ 0.83 (t, 3H, J = 7), 1.39 (s, 3H), 1.62 (s, 3H), 3.00–3.07 (m, 2H), 4.71 (d, 1H, J = 2), 5.41–5.44 (m, 2H), 6.18 (d, 1H, J = 2), 6.67 (t, 1H, J = 2), 7.30–7.44 (m, 3H), 7.71–7.76 (m, 1H), 8.16 (s, 1H), 8.69 (s, 1H), 10.24 (bs, 1H). Anal. (C22H23 Cl N7O5) C, H, N.
1-Deoxy-1-[6-[[(phenyl)carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (8):
yield 90%; pale yellow oil; IR (neat) cm−1 3425, 1730, 1640, 1555, 1240; 1H N:MR (CDCl3) δ 0.79 (t, 3H, J = 7), 1.39 (s, 3H), 1.61 (s, 3H), 2.91–3.03 (m, 2H), 4.71 (d, 1H, J = 2), 5.45–5.52 (m, 2H), 6.18 (d, 1H, J = 2), 6.40 (t, 1H, J = 2), 7.27–7.53 (m, 3H), 7.83–7.87 (m, 2H), 8.19 (s, 1H), 8.63 (s, 1H), 9.18 (bs, 1H). Anal. (C22H24N6O5) C, H, N.
General Procedure for the Preparation of 1-Deoxy-1-[6-[[[(substituted)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (14–29).
2′,3′-O-Isopropylidene-NECA 3 (0.43 mmol) was dissolved in freshly distilled THF (4 mL), and then the appropriate isocyanate (1.3 equiv) and a catalytic amount of triethylamine (two drops) were added. The mixture was refluxed under argon for 18 h. Next the solvent was removed under reduced pressure, and the residue was purified by flash chromatography (CH2Cl2–EtOAc 20%) to afford the desired compound 14–29.
1-Deoxy-1-[6-[[[(benzyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (14):
yield 82%; white solid, mp 109–111 °C (CH2Cl2–Et2O); IR (KBr) cm−1 3440, 1740, 1610, 1550, 1210; 1H NMR (CDCl3) δ 0.76 (t, 3H, J = 7), 1.21 (s, 3H), 1.63 (s, 3H), 2.96–3.03 (m, 2H), 4.64 (d, 2H, J = 6), 4.71 (d, 1H, J = 1.8), 5.44–5.48 (m, 2H), 6.16 (d, 1H, J = 2), 6.52 (t, 1H, J = 2), 7.26–7.38 (m, 5H), 8.14 (s, 1H), 8.45 (s, 1H), 8.46 (s, 1H), 9.79 (bs, 1H). Anal. (C26H31N7O5) C, H, N.
1-Deoxy-1-[6-[[[(4-sulfonamidophenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (15):
yield 60%; white solid, mp 207 °C (CH2Cl2–Et2O); IR (KBr) cm−1 3450–3250, 1730, 1610, 1565, 1360, 1230; 1H NMR (CDCl3) δ 0.57 (t, 3H, J = 7), 1.35 (s, 3H), 1.54 (s, 3H), 2.96–3.03 (m, 2H), 4.60 (d, 1H, J = 2), 5.47–5.50 (m, 2H), 6.47 (d, 1H, J = 2), 6.60 (t, 1H, J = 2), 7.26 (d, 2H, J = 9), 7.74 (d, 2H, J = 9), 8.60 (s, 1H), 8.64 (s, 1H), 9.22 (s, 1H), 10.47 (bs, 1H), 12.05 (s, 1H). Anal. (C22H26N8O7S) C, H, N.
1-Deoxy-1-[6-[[[(4-acetylphenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (16):
yield 77%; white solid, mp 204 °C (CH2Cl2–Et2O); IR (KBr) cm−1 3430, 1740, 1730, 1630, 1545, 1240; 1H NMR (CDCl3) δ 0.83 (t, 3H, J = 7), 1.42 (s, 3H), 1.64 (s, 3H), 2.61 (s, 3H), 2.99–3.08 (m, 2H), 4.74 (d, 1H, J = 2), 5.47–5.55 (m, 2H), 6.21 (d, 1H, J = 2), 6.47 (t, 1H, J = 2), 7.74 (d, 2H, J = 9), 7.98 (d, 2H, J = 9), 8.21 (s, 1H), 8.63 (s, 1H), 8.67 (bs, 1H), 12.01 (s, 1H). Anal. (C21H27N7O6) C, H, N.
1-Deoxy-1-[6-[[[((R)-1-phenylethyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (17):
yield 65%; white solid, mp 110–111 °C (CH2Cl2–Et2O); IR (KBr) cm−1 3430, 1730, 1620, 1555, 1230; 1H NMR (CDCl3) δ 0.68 (t, 3H, J = 7), 1.41 (s, 3H), 1.63 (s, 3H), 1.64 (d, 3H, J = 7), 2.87–2.96 (m, 2H), 4.71 (d, 1H, J = 1.8), 5.16 (m, 1H), 5.48–5.51 (m, 2H), 6.16 (d, 1H, J = 2), 6.52 (bs, 1H), 7.25–7.40 (m, 5H), 8.15 (s, 1H), 8.51 (s, 1H), 9.84 (bs, 1H), 10.94 (s, 1H). Anal. (C23H29N7O5) C, H, N.
1-Deoxy-1-[6-[[[((S)-1-phenylethyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (18):
yield 82%; white solid, mp 107–108 °C (CH2Cl2–Et2O); IR (KBr) cm−1 3445, 1725, 1615, 1560, 1230; 1H NMR (CDCl3) δ 0.85 (t, 3H, J = 7), 1.40 (s, 3H), 1.63 (s, 3H), 1.64 (d, 3H, J = 7), 3.00–3.09 (m, 2H), 4.72 (d, 1H, J = 1.8), 5.17 (m, 1H), 5.39–5.50 (m, 2H), 6.14 (d, 1H, J = 2), 6.57 (bs, 1H), 7.26–7.45 (m, 5H), 8.12 (s, 1H), 8.51 (s, 1H), 9.81 (bs, 1H), 10.87 (s, 1H). Anal. (C23H29N7O5) C, H, N.
1-Deoxy-1-[6-[[[(5-methyl-isoxazol-3-yl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (19):
yield 83%; pale yellow solid, mp 120 °C (CH2Cl2–Et2O); IR (KBr) cm−1 3450, 1720, 1620, 1550, 1215; 1H NMR (CDCl3) δ 0.82 (t, 3H, J = 7), 1.20 (s 3H), 1.41 (s, 3H), 2.44 (s, 3H), 3.00–3.07 (m, 2H), 4.74 (d, 1H, J = 2), 5.46–5.50 (m, 2H), 6.21 (d, 1H, J = 2), 6.55 (t, 1H, J = 2), 6.70 (s, 1H), 8.37 (s, 1H), 8.63 (s, 1H), 9.39 (bs, 1H), 12.34 (bs, 1H). Anal. (C20H24N8O6) C, H, N.
1-Deoxy-1-[6-[[[(1,3,4-thiadiazol-2-yl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (20):
yield 70%; yellow solid, mp 148 °C (CH2Cl2–Et2O); IR (KBr) cm−1 3430, 1730, 1615, 1560, 1240; 1H NMR (CDCl3) δ 0.86 (t, 3H, J = 7), 1.44 (s 3H), 1.67 (s, 3H), 3.07–3.14 (m, 2H), 4.78 (d, 1H, J = 1.8), 5.47–5.51 (m, 2H), 6.24 (d, 1H, J = 1.8), 6.52 (t, 1H, J = 2), 8.36 (s, 1H), 8.75 (s, 1H), 8.91 (bs, 1H), 9.40 (bs, 1H), 11.72 (bs, 1H). Anal. (C18H21N9O5S) C, H, N.
1-Deoxy-1-[6-[[[(4-n-propyloxy-phenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (21):
yield 70%; pale yellow foam; IR (neat) cm−1 3440, 1725, 1620, 1555, 1220; 1H NMR (CDCl3) δ 0.71 (t, 3H, J = 7), 0.90 (t, 3H, J = 7), 1.40 (s 3H), 1.62 (s, 3H), 1.81–2.03 (m, 2H), 2.95–3.01 (m, 2H), 3.90 (t, 2H, J = 7), 4.71 (d, 1H, J = 1.8), 5.46–5.51 (m, 2H), 6.19 (d, 1H, J = 1.8), 6.51 (t, 1H, J = 2), 6.89 (d, 2H, J = 9), 7.48 (d, 2H, J = 9), 8.25 (s, 1H), 8.56 (s, 1H), 8.80 (bs, 1H), 11.48 (s, 1H). Anal. (C22H25N7O5) C, H, N.
1-Deoxy-1-[6-[[[(2-phenylethyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (22):
yield 60%; pale yellow solid, mp 88–90 °C (EtOAc–light petroleum); IR (KBr) cm−1 3440, 1730, 1615, 1550, 1225; 1H NMR (CDCl3) δ 0.70 (t, 3H, J = 7), 1.40 (s 3H), 1.62 (s, 3H), 2.94–3.23 (m, 4H), 3.67 (t, 2H, J = 6.8), 4.71 (d, 1H, J = 1.8), 5.46–5.51 (m, 2H), 6.18 (d, 1H, J = 1.8), 6.49 (t, 1H, J = 6), 7.24–7.32 (m, 5H), 8.25 (s, 1H), 8.30 (s, 1H), 9.48 (bs, 1H), 11.48 (s, 1H). Anal. (C24H29N7O5) C, H, N.
1-Deoxy-1-[6-[[[(3,4-dimethoxy-2-phenylethyl)amino]-carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (23):
yield 55%; yellow solid, 85–87 °C mp (EtOAc–light petroleum); IR (KBr) cm−1 3440, 1724, 1623, 1554, 1225; 1H NMR (CDCl3) δ 0.73 (t, 3H, J = 7), 1.23 (t, 2H, J = 8), 1.41 (s 3H), 1.62 (s, 3H), 2.95–3.07 (m, 2H), 3.85 (s, 3H), 3.87 (s, 3H), 4.71 (d, 1H, J = 1.8), 5.47–5.51 (m, 2H), 6.17 (d, 1H, J = 1.8), 6.51 (t, 1H, J = 2), 6.60 (s, 1H), 6.62 (s, 2H), 8.23 (s, 1H), 8.36 (s, 1H), 8.71 (s, 1H), 9.46 (bs, 1H). Anal. (C26H33N7O7) C, H, N.
1-Deoxy-1-[6-[[[(fur-2-yl-methyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (24):
yield 58%; yellow solid mp 90–93 °C (EtOAc–light petroleum); IR (KBr) cm−1 3445, 1727, 1620, 1550, 1222; 1H NMR (CDCl3) δ 0.72 (t, 3H, J = 7), 1.40 (s, 3H), 1.62 (s, 3H), 2.84–2.99 (m, 2H), 4.6 (d, 2H, J = 6), 4.71 (d, 1H, J = 1.8), 5.45–5.55 (m, 2H), 6.19 (d, 1H, J = 1.8), 6.30–6.36 (m, 2H), 6.60 (s, 1H), 6.5 (t, 1H, J = 6), 7.38 (d, 1H, J = 2), 8.32 (s, 1H), 8.49 (s, 1H), 9.00 (s, 1H), 9.84 (bs, 1H). Anal. (C21H25N7O6) C, H, N.
1-Deoxy-1-[6-[[[(4-(pyridin-2-yl-aminosulfonyl)phenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (25):
yield 48%; yellow solid mp 168–170 °C (EtOAc–light petroleum); IR (KBr) cm−1 3450, 1730, 1625, 1550, 1350, 1220; 1H NMR (CDCl3) δ 0.71 (t, 3H, J = 7), 1.41 (s, 3H), 1.60 (s, 3H), 2.80–2.95 (m, 2H), 4.73 (d, 1H, J = 1.8), 5.40–5.60 (m, 2H), 6.19 (d, 1H, J = 1.8), 6.65 (t, 1H, J = 6), 7.15–7.25 (m, 1H), 7.45 (d, 2H, J = 9), 7.55–7.75 (m, 3H), 7.92 (d, 2H, J = 9), 8.27 (s, 1H), 8.50 (s, 1H), 8.99 (s, 1H), 9.80 (bs, 1H), 11.02 (bs, 1H). Anal. (C27H29N9O7S) C, H, N.
1-Deoxy-1-[6-[[[(4-(5-methyl-isoxazol-3-yl-aminosulfonyl)phenyl)amino] carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuron-amide (26):
yield 56%; yellow solid mp 157–159 °C (EtOAc–light petroleum); IR (KBr) cm−1 3455, 1725, 1630, 1557, 1355, 1224; 1H NMR (DMSO-d6) δ 0.79 (t, 3H, J = 7), 1.40 (s, 3H), 1.62 (s, 3H), 2.30 (s, 3H), 2.91–3.04 (m, 2H), 4.68 (d, 1H, J = 1.8), 5.40–5.48 (m, 2H), 6.11 (d, 1H, J = 1.8), 6.39 (s, 1H), 7.06 (t, 1H, J = 6), 7.63 (d, 2H, J = 9), 7.85 (d, 2H, J = 9), 8.44 (s, 1H), 8.59 (s, 1H), 9.07 (s, 1H), 9.62 (bs, 1H), 12.11 (bs, 1H). Anal. (C26H29N9O8S) C, H, N.
1-Deoxy-1-[6-[[[(4-(pyrimidin-2-yl-aminosulfonyl)-phenyl)amino] carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (27):
yield 53%; yellow solid mp 157–159 °C (EtOAc–light petroleum); IR (KBr) cm−1 3450, 1722, 1633, 1552, 1350, 1220; 1H NMR (DMSO-d6) δ 0.57 (t, 3H, J = 7), 1.35 (s, 3H), 1.54 (s, 3H), 2.71–2.80 (m, 2H), 4.59 (d, 1H, J = 1.8), 5.40–5.46 (m, 2H), 6.46 (d, 1H, J = 1.8), 7.03 (t, 1H, J = 6), 7.56 (m, 1H), 7.60 (d, 2H, J = 9), 7.85 (d, 2H, J = 9), 8.48 (s, 1H), 8.51 (s, 1H), 8.60 (d, 2H, J = 7), 10.45 (bs, 1H), 11.71 (bs, 1H), 12.04 (s, 1H). Anal. (C26H28N10O7S) C, H, N.
1-Deoxy-1-[6,6-[[[bis-(4-nitrophenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (28):
yield 65%; yellow solid, mp 261 °C (CH2Cl2–Et2O); IR (KBr) cm−1 3430, 1730, 1620, 1555, 1350, 1210; 1H NMR (DMSO-d6) δ 0.56 (t, 3H, J = 7), 1.34 (s, 3H), 1.54 (s, 3H), 2.67–2.83 (m, 2H), 4.61 (d, 1H, J = 1.8), 5.47–5.50 (m, 2H), 6.47 (d, 1H, J = 1.8), 7.61 (t, 1H, J = 2), 7.71 (d, 2H, J = 9), 7.91 (d, 2H, J = 9), 8.22 (d, 2H, J = 9), 8.24 (s, 1H), 8.64 (d, 2H, J = 9), 9.72 (s, 1H), 10.63 (bs, 1H), 12.24 (s, 1H). Anal. (C29H28N10O10) C, H, N.
1-Deoxy-1-[6,6-[[[bis-(5-chloro-pyridin-2-yl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (29):
yield 60%; yellow foam; IR (neat) cm–1 3440, 1710, 1630, 1540, 1220; 1H NMR (DMSO-d6) δ 0.58 (t, 3H, J = 7), 1.35 (s 3H), 1.54 (s, 3H), 2.71–2.85 (m, 2H), 4.60 (d, 1H, J = 2), 5.47–5.51 (m, 2H), 6.47 (d, 1H, J = 2), 7.52 (t, 1H, J = 2), 7.79–7.92 (m, 4H), 8.34–8.38 (m, 2H), 8.60 (s, 1H), 8.65 (s, 1H), 10.66 (bs, 1H), 12.22 (bs, 1H). Anal. (C27H26Cl2N8O6) C, H, N.
General Procedure for the Preparation of 1-Deoxy-1-[6-[[(substituted)carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (9–13) and 1-Deoxy-1-[6-[[[(substituted)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (30–45).
A solution of the isopropylidene derivative, 4–8 or 14–29, (0.084 mmol) in aqueous 1 N HCl (5 mL) and dioxane (5 mL) was stirred at 65 °C for 1 h. The solvent was then removed under reduced pressure, and the residue was crystallized from ethanol to afford the desired compound, 9–13 or 30–45.
1-Deoxy-1-[6-[[(4-biphenyl)carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (9):
yield 65%; white solid, mp 221 °C (EtOH); IR (KBr) cm−1 3500–3100, 1720, 1615, 1550; 1H NMR (DMSO-d6) δ 1.07 (t, 3H, J = 7), 3.17–3.24 (m, 2H), 4.23–4.25 (m, 1H), 4.36 (d, 1H, J = 2), 4.71–4.73 (m, 1H), 5.70 (d, 1H, J = 8), 5.79 (d, 1H, J = 4), 6.13 (d, 1H, J = 8), 7.43–7.53 (m, 3H), 7.78–7.89 (m, 4H), 8.16 (d, 2H, J = 11), 8.46 (t, 1H, J = 4), 8.80 (s, 1H), 8.82 (s, 1H), 11.34 (bs, 1H). Anal. (C25H24N6O5) C, H, N.
1-Deoxy-1-[6-[[(2,4-dichlorobenzyl)carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (10):
yield 55%; off white solid, mp 132–134 °C (EtOH); IR (KBr) cm−1 3450–3050, 1730, 1635, 1545, 1230; 1H NMR (DMSO-d6) δ 1.08 (t, 3H, J = 7), 3.18–3.25 (m, 2H), 3.34 (s, 2H), 4.12–4.15 (m, 1H), 4.30 (s, 1H), 4.49–4.62 (m, 2H), 5.57 (d, 1H, J = 8), 5.77 (d, 1H, J = 4), 5.95 (d, 1H, J = 8), 7.44–7.51 (m, 3H), 8.19 (s, 1H), 8.39 (s, 1H), 8.95 (bs, 1H). Anal. (C20H20Cl2N6O5) C, H, N.
1-Deoxy-1-[6-[[(4-methoxyphenyl)carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (11):
yield 80%; white solid, mp 167–169 °C (EtOH); IR (KBr) cm−1 3550–3150, 1710, 1640, 1530, 1270; 1H NMR (DMSO-d6) δ 1.07 (t, 3H, J = 7), 3.15–3.23 (m, 2H), 3.86 (s, 3H), 4.20–4.24 (m, 1H), 4.35 (d, 1H, J = 2), 4.70–4.75 (m, 1H), 5.69 (d, 1H, J = 7), 5.77 (d, 1H, J = 4), 6.11 (d, 1H, J = 7), 7.08 (d, 2H, J = 9), 8.04 (d, 2H, J = 9), 8.44 (bs, 1H), 8.77 (s, 1H), 8.78 (s, 1H), 11.12 (s, 1H). Anal. (C20H22N6O6) C, H, N.
1-Deoxy-1-[6-[[(2-chlorophenyl)carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (12):
yield 78%; white solid, mp 179–180 °C (EtOH); IR (KBr) cm−1 3510–3050, 1720, 1660, 1510, 1250; 1H NMR (DMSO-d6) δ 1.06 (t, 3H, J = 7), 3.15–3.22 (m, 2H), 4.22 (bs, 1H), 4.34 (s, 1H), 4.68–4.70 (m, 1H), 5.66 (d, 1H, J = 7), 5.76 (d, 1H, J = 4), 6.10 (d, 1H, J = 7), 7.43–7.61 (m, 4H), 8.44 (bs, 1H), 8.67 (s, 1H), 8.82 (bs, 1H), 11.53 (bs, 1H). Anal. (C19H19N6O5) C, H, N.
1-Deoxy-1-[6-[[(phenyl)carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (13):
yield 67%; white solid, mp 157–159 °C (EtOH); IR (KBr) cm−1 3500–3000, 1715, 1630, 1535, 1260; 1H NMR (DMSO-d6) δ 1.03 (t, 3H, J = 7), 3.16–3.19 (m, 2H), 3.74 (bs, 2H), 4.22 (bs, 1H), 4.36 (s, 1H), 4.51–4.63 (m, 1H), 6.13 (d, 1H, J = 6.8), 7.48–7.59 (m, 3H), 8.04–8.11 (m, 2H), 8.52 (bs, 1H), 8.79 (s, 1H), 8.83 (s, 1H), 11.22 (s, 1H). Anal. (C19H20N6O5) C, H, N.
1-Deoxy-1-[6-[[[(benzyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (30):
yield 70%;white solid, mp 184–186 °C (EtOH); IR (KBr) cm−1 3500–3100, 1675, 1620, 1565, 1520, 1310; 1H NMR (DMSO-d6) δ 1.06 (t, 3H, J = 7), 3.10–3.20 (m, 2H), 4.05 (bs, 2H), 4.17–4.20 (m, 1H), 4.36 (s, 1H), 4.47–4.50 (m, 2H), 4.55–4.65 (m, 1H), 6.05–6.10 (m, 1H), 7.17–7.40 (m, 5H), 8.30–8.40 (m, 1H), 8.62 (s, 1H), 8.86 (s, 1H), 9.50–9.60 (m, 1H), 10.30 (bs, 1H). Anal. (C20H24N7O5) C, H, N.
1-Deoxy-1-[6-[[[(4-sulfonamidophenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (31):
yield 63%; white solid, mp 183–185 °C (EtOH); IR (KBr) cm−1 3550–3050, 1715, 1630, 1545, 1370, 1250; 1H NMR (DMSO-d6) δ 1.07 (t, 3H, J = 7), 3.16–3.26 (m, 2H), 4.21 (bs, 1H), 4.36 (s, 1H), 4.63–4.69 (m, 1H), 5.62 (d, 1H, J = 6.8), 5.78 (d, 1H, J = 4), 6.11 (d, 1H, J = 6.8), 7.31 (bs, 2H), 7.81 (s, 4H), 8.49 (bs, 1H), 8.75 (s, 1H), 8.89 (s, 1H), 9.69 (bs, 1H), 11.92 (bs, 1H). Anal. (C19H22N8O7S) C, H, N.
1-Deoxy-1-[6-[[[(4-acetylphenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (32):
yield 83%; white solid, mp 187 °C (EtOH); IR (KBr) cm−1 3550–3100, 1740, 1720, 1630, 1525, 1215; 1H NMR (DMSO-d6) δ 1.08 (t, 3H, J = 7), 2.55 (s, 3H), 3.16–3.23 (m, 2H), 4.18–4.20 (m, 1H), 4.21 (d, 1H, J = 2), 4.45 (bs, 2H), 4.64–4.69 (m, 1H), 6.11 (d, 1H, J = 7), 7.77 (d, 2H, J = 9), 7.98 (d, 2H, J = 9), 8.49 (bs, 1H), 8.76 (s, 1H), 8.88 (s, 1H), 10.62 (bs, 1H), 11.94 (bs, 1H). Anal. (C21H23N7O6) C, H, N.
1-Deoxy-1-[6-[[[((R)-1-phenylethyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (33):
yield 65%; white solid, mp 153–155 °C (EtOH); IR (KBr) cm−1 3550–3100, 1720, 1635, 1535, 1240; 1H NMR (DMSO-d6) δ 1.05 (t, 3H, J = 7), 1.51 (d, 3H, J = 8), 3.14–3.21 (m, 2H), 3.35–3.41 (m, 1H), 4.20 (s, 1H), 4.36 (s, 1H), 4.93–5.00 (m, 3H), 6.09 (d, 1H, J = 6.8), 7.25–7.39 (m, 5H), 8.48 (bs, 1H), 8.65 (s, 1H), 8.89 (s, 1H), 9.51 (bs, 1H), 10.38 (bs, 1H). Anal. (C21H25N7O5) C, H, N.
1-Deoxy-1-[6-[[[((S)-1-phenylethyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (34):
yield 72%; white solid, mp 151 °C (EtOH); IR (KBr) cm−1 3550–3100, 1725, 1630, 1525, 1240; 1H NMR (DMSO-d6) δ 1.06 (t, 3H, J = 7), 1.49 (d, 3H, J = 8), 3.15–3.21 (m, 2H), 3.28–3.39 (m, 1H), 4.00 (bs, 2H), 4.35 (s, 1H), 4.61–4.66 (m, 1H), 4.94–5.01 (m, 1H), 6.07 (d, 1H, J = 6.8), 7.24–7.39 (m, 5H), 8.48 (bs, 1H), 8.64 (s, 1H), 8.83 (s, 1H), 9.57 (bs, 1H), 10.12 (bs, 1H). Anal. (C21H25N7O5) C, H, N.
1-Deoxy-1-[6-[[[(5-methyl-isoxazol-3-yl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (35):
yield 58%; white solid, mp 197 °C (EtOH); IR (KBr) cm−1 3550–3050, 1715, 1620, 1535, 1240; 1H NMR (DMSO-d6) δ 1.06 (t, 3H, J = 7), 2.40 (s 3H), 3.12–3.25 (m, 2H), 4.21–4.23 (m, 1H), 4.36 (d, 1H, J = 2), 4.64–4.70 (m, 1H), 5.68 (m, 2H), 6.09 (d, 1H, J = 6.8), 6.67 (s, 1H), 8.46 (t, 1H, J = 6), 8.74 (s, 1H), 8.84 (s, 1H), 10.75 (bs, 1H), 12.19 (bs, 1H). Anal. (C17H20N8O6) C, H, N.
1-Deoxy-1-[6-[[[(1,3,4-thiadiazol-2-yl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (36):
yield 65%; white solid, mp 197 °C (EtOH); IR (KBr) cm−1 3450–300, 1720, 1635, 1515, 1240; 1H NMR (DMSO-d6) δ 1.06 (t, 3H, J = 7), 3.15–3.22 (m, 2H), 3.72 (bs, 2H), 4.21–4.24 (m, 1H), 4.36 (d, 1H, J = 2), 4.65–4.70 (m, 1H), 6.12 (d, 1H, J = 6.8), 8.45 (t, 1H, J = 7), 8.80 (s, 1H), 8.89 (s, 1H), 9.19 (s, 1H), 10.35 (bs, 1H), 11.38 (bs, 1H). Anal. (C15H17N4O5S) C, H, N.
1-Deoxy-1-[6-[[[(4-n-propyloxy-phenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (37):
yield 66%; white solid, mp 270 °C (EtOH); IR (KBr) cm−1 3450–3050, 1720, 1630, 1535, 1240; 1H NMR (DMSO-d6) δ 0.95 (t, 3H, J = 7), 1.09 (t, 3H, J = 7), 1.65–1.75 (m, 2H), 3.06–3.23 (m, 2H), 3.67 (bs, 2H), 3.89 (t, 2H, J = 7), 4.18–4.20 (m, 1H), 4.33 (s, 1H), 4.51–4.61 (m, 1H), 6.05 (d, 1H, J = 6.8), 6.91 (d, 2H, J = 9), 7.52 (d, 2H, J = 9), 7.92 (t, 1H, J = 7), 8.22 (s, 1H), 8.52 (s, 1H), 9.65 (bs, 1H), 11.52 (bs, 1H). Anal. (C22H27N7O6) C, H, N.
1-Deoxy-1-[6-[[[(2-phenylethyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (38):
yield 82%; light brown solid, mp 166 °C (EtOH); IR (KBr) cm−1 3450–3000, 1725, 1610, 1560, 1210; 1H NMR (DMSO-d6) δ 1.04 (t, 3H, J = 7), 2.84–2.95 (m, 2H), 3.01–3.23 (m, 2H), 4.15–4.20 (m, 1H), 4.47 (s, 1H), 4.54–4.79 (m, 3H), 5.39–5.48 (m, 2H), 6.21 (d, 1H, J = 1.8), 6.53 (t, 1H, J = 6), 7.31–7.44 (m, 5H), 8.32 (s, 1H), 8.41 (s, 1H), 9.55 (bs, 1H), 11.18 (s, 1H). Anal. (C21H25N7O5) C, H, N.
1-Deoxy-1-[6-[[[(3,4-dimethoxy-2-phenylethyl)amino]-carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (39):
yield 68%; light brown solid, mp 120–122 °C (EtOH); IR (KBr) cm−1 3540–3060, 1734, 1633, 1500, 1215; 1H NMR (DMSO-d6) δ 1.11 (t, 3H, J = 7), 1.23 (t, 2H, J = 8), 2.88–3.01 (m, 2H), 3.78 (s, 3H), 3.81 (s, 3H), 4.18–4.23 (m, 1H), 4.42 (s, 1H), 4.54–4.59 (m, 1H), 4.87–5.02 (bs, 2H), 5.47–5.51 (m, 2H), 6.15 (d, 1H, J = 1.8), 6.59 (t, 1H, J = 2), 6.65 (s, 1H), 6.71 (s, 2H), 8.31 (s, 1H), 8.47 (s, 1H), 9.05 (s, 1H), 10.46 (bs, 1H). Anal. (C23H29N7O7) C, H, N.
1-Deoxy-1-[6-[[[(fur-2-yl-methyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (40):
yield 79%; brown solid mp 189–191 °C (EtOH); IR (KBr) cm−1 3500–3087, 1731, 1630, 1550, 1335, 1221; 1H NMR (DMSO-d6) δ 1.06 (t, 3H, J = 7), 2.94–3.05 (m, 2H), 4.20–4.24 (m, 1H), 4.38 (s, 1H), 4.56 (s, 2H, J = 6), 4.61–4.67 (m, 1H), 4.96–5.04 (bs, 2H), 6.17 (d, 1H, J = 1.8), 6.35–6.46 (m, 1H), 6.62 (s, 1H), 6.56 (t, 1H, J = 6), 7.48 (d, 1H, J = 2), 8.38 (s, 1H), 8.54 (s, 1H), 9.08 (s, 1H), 9.94 (bs, 1H). Anal. (C18H21N7O6) C, H, N.
1-Deoxy-1-[6-[[[(4-(pyridin-2-yl-aminosulfonyl)phenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (41):
yield 88%; brown solid mp 163 °C (EtOH); IR (KBr) cm−1 3500–3050, 1734, 1633, 1552, 1345, 1213; 1H NMR (DMSO-d6) δ 1.05 (t, 3H, J = 7), 2.99–3.18 (m, 2H), 4.23–4.26 (m, 1H), 4.42 (s, 1H), 4.54–4.62 (m, 1H), 4.91–5.15 (bs, 2H), 6.16 (d, 1H, J = 1.8), 6.71 (t, 1H, J = 6), 7.18–7.31 (m, 1H), 7.57 (d, 2H, J = 9), 7.63–7.71 (m, 3H), 7.95 (d, 2H, J = 9), 8.37 (s, 1H), 8.78 (s, 1H), 9.05 (s, 1H), 10.15 (bs, 1H), 12.45 (bs, 1H). Anal. (C24H25N9O7S) C, H, N.
1-Deoxy-1-[6-[[[(4-(5-methyl-isoxazol-3-yl-aminosulfonyl)phenyl)amino] carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (42):
yield 77%; pale yellow solid mp 184 °C (EtOH); IR (KBr) cm−1 3550–3150, 1730, 1638, 1550, 1350, 1210; 1H NMR (DMSO-d6) δ 1.08 (t, 3H, J = 7), 2.30 (s, 3H), 3.17–3.20 (m, 2H), 4.22–4.23 (m, 1H), 4.36 (s, 1H), 4.40–4.95 (m, 3H), 6.11 (d, 1H, J = 1.8), 6.15 (s, 1H), 6.68 (t, 1H, J = 6), 7.37 (d, 2H, J = 9), 7.84 (d, 2H, J = 9), 8.42 (bs, 1H), 8.73 (s, 1H), 8.85 (s, 1H), 9.63 (bs, 1H), 11.37 (bs, 1H), 12.02 (s, 1H). Anal. (C23H25N9O8S) C, H, N.
Deoxy-1-[6-[[[(4-(pyrimidin-2-yl-aminosulfonyl)-phenyl)amino] carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (43):
yield 80%; brown solid mp 184 °C (EtOH); IR (KBr) cm−1 3550–3100, 1720, 1630, 1555, 1355, 1200; 1H NMR (DMSO-d6) δ 1.08 (t, 3H, J = 7), 3.17–3.23 (m, 2H), 4.22–4.25 (m, 1H), 4.37 (s, 1H), 4.64–4.70 (m, 1H), 4.90–5.12 (bs, 2H), 6.11 (d, 1H, J = 1.8), 6.60 (t, 1H, J = 6), 7.03–7.08 (m, 1H), 7.81 (d, 2H, J = 9), 7.98 (d, 2H, J = 9), 8.51 (d, 2H, J = 6), 8.74 (s, 1H), 8.89 (s, 1H), 9.55 (bs, 1H), 11.19 (bs, 1H), 11.90 (s, 1H). Anal. (C23H24N10O7S) C, H, N.
1-Deoxy-1-[6,6-[[[bis-(4-nitrophenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (44):
yield 52%; white solid, mp 213 °C (EtOH); IR (KBr) cm−1 3500–3050, 1720, 1630, 1545, 1340, 1220; 1H NMR (DMSO-d6) δ 1.07 (t, 3H, J = 7), 3.12–3.23 (m, 2H), 4.21–4.23 (m, 1H), 4.35 (d, 1H, J = 2), 4.64–4.69 (m, 1H), 5.69 (bs, 2H), 6.09 (d, 1H, J = 6.8), 7.68 (d, 2H, J = 9), 7.91 (d, 2H, J = 9), 8.18–8.29 (m, 4H), 8.83 (s, 1H), 9.69 (s, 1H), 10.68 (bs, 1H), 12.26 (bs, 1H). Anal. (C26H29N10O10) C, H, N.
1-Deoxy-1-[6,6-[[[bis-(5-chloro-pyridin-2-yl)amino]-carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (45):
yield 68%; white solid, mp 220 °C (EtOH); IR (KBr) cm−1 3550–3100, 1725, 1630, 1525, 1250; 1H NMR (DMSO-d6) δ 1.06 (t, 3H, J = 7), 3.15–3.22 (m, 2H), 4.22–4.24 (m, 1H), 4.36 (d, 2H, J = 2), 4.64–4.69 (m, 1H), 5.95 (bs, 2H), 6.10 (d, 1H, J = 6.8), 7.80–8.06 (m, 6H), 8.39 (t, 1H, J = 7), 8.76 (s, 1H), 8.93 (s, 1H), 10.39 (bs, 1H), 12.11 (bs, 1H). Anal. (C24H22Cl2N8O6) C, H, N.
General Procedure for the Synthesis of 2-Chloro- and 2-Iodo-1-deoxy-1-[6-[[[(substituted)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (48–51).
The appropriate commercially available isocyanate (2.1 mmol) was added to a solution of 2′,3′-O-isopropylidene-2-chloro-NECA (46)43 or to 2′,3′-O-isopropylidene-2-iodo-NECA (47)44 (0.42 mmol) and a catalytic amount of triethylamine in THF (10 mL), and the mixture was heated at 110 °C in a steel bomb for 24 h. After the mixture was concentrated in vacuo, the residue was flash chromatographed on a silica gel column eluting with CHCl3–MeOH (99:1) to give the desired protected nucleosides 48–51.
2-Chloro-1-deoxy-1-[6-[[[(3-chlorophenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (48):
yield 33%; white solid, mp 98–100 °C dec (CH3CN–EtOH); IR (KBr) cm−1 3400–3050, 1685, 1650, 1520, 1235; 1H NMR (DMSO-d6) δ 0.66 (t, 3H, J = 7.2 Hz), 1.37 (s, 3H), 1.56 (s, 3H), 2.74–2.92 (br m, 2H), 4.61 (s, 1H), 5.42 (s, 2H), 6.42 (s, 1H), 7.17 (d, 1H, J = 7.0 Hz), 7.32–7.42 (m, 2H),7.60–7.70 (m, 1H), 7.79 (s, 1H), 8.59 (s, 1H), 10.64 (s, 1H), 10.79 (s, 1H). Anal. (C22H23Cl2N7O5) C, H, N.
2-Chloro-1-deoxy-1-[6-[[[(4-methoxyphenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (49):
yield 35%; vitreous solid; IR (KBr) cm−1 3450–3050, 1680, 1650, 1510, 1230; 1H NMR (DMSO-d6) δ 0.67 (t, 3H, J = 7.0 Hz), 1.38 (s, 3H), 1.56 (s, 3H), 2.76–2.94 (m, 2H), 3.76 (s, 3H), 4.61 (s, 1H), 5.42 (s, 2H), 6.41 (s, 1H), 6.93–7.03 (m, 2H), 7.41–7.53 (m, 2H), 7.65–7.75 (m, 1H), 8.61 (s, 1H), 10.43 (s, 1H), 11.11 (s, 1H). Anal. (C23H26ClN7O6) C, H, N.
2-Iodo-1-deoxy-1-[6-[[[(3-chlorophenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (50):
yield 62%; white solid, mp 127–130 °C (CH3CN–EtOH); IR (KBr) cm−1 3450–3050, 1690, 1650, 1520, 1240; 1H NMR (DMSO-d6) δ 0.66 (t, 3H, J = 7.2 Hz), 1.37 (s, 3H), 1.56 (s, 3H), 2.74–2.92 (br m, 2H), 4.61(s, 1H), 5.42 (s, 2H), 6.42 (s, 1H), 7.17 (d, 1H, J = 7.0 Hz), 7.32–7.42 (m, 2H), 7.56–7.66 (m, 1H), 7.79 (s, 1H), 8.50 (s, 1H), 10.64 (s, 1H), 11.33 (s, 1H). Anal. (C22H23ClIN7O5) C, H, N.
2-Iodo-1-deoxy-1-[6-[[[(4-methoxyphenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-2,3-O-(1-methylethylidene)-β-d-ribofuranuronamide (51):
yield 70%; white solid, mp 127–130 °C (CH3CN–EtOH); IR (KBr) cm−1 3450–3025, 1680, 1660, 1510, 1230; 1H NMR (DMSO-d6) δ 0.67 (t, 3H, J = 7.0 Hz), 1.38 (s, 3H), 1.56 (s, 3H), 2.76–2.94 (m, 2H), 3.76 (s, 3H), 4.61 (s, 1H), 5.42 (s, 2H), 6.41 (s, 1H), 6.93–7.03 (m, 2H), 7.41–7.53 (m, 2H), 7.54–7.65 (m, 1H), 8.49 (s, 1H), 10.43 (s, 1H), 11.11 (s, 1H). Anal. (C23H26IN7O6) C, H, N.
General Procedure for the Synthesis of 2-Chloro- and 2-Iodo-1-deoxy-1-[6-[[[(substituted)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (52–55).
To a solution of 2′,3′-O-isopropylidene-2-chloro-6-ureas (48–49) or 2′,3′–O-isopropylidene-2-iodo-6-ureas (50–51) (0.96 mmol) in dioxane (50 mL) was added 1 N HCl (50 mL), and the mixture was heated at 65 °C for 1 h. After the mixture was concentrated in vacuo, the residue was flash chromatographed on a silica gel column eluting with CHCl3–MeOH (95:5) to give the desired compounds 52–55.
2-Chloro-1-deoxy-1-[6-[[[(3-chlorophenyl)amino]-carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (52):
yield 66%; white solid, mp 150–153 °C dec (EtOH); IR (KBr) cm−1 3500–3000, 1690, 1640, 1530, 1240; 1H NMR (DMSO-d6) δ 1.07 (t, 3H, J = 7.2 Hz), 3.13–3.31 (m, 2H), 4.25 (s, 1H,), 4.38 (d,1H, J = 2.1 Hz), 4.67 (pq, 1H), 6.05 (d, 1H, J = 6.7 Hz), 7.17 (d, 1H, J = 5.2 Hz), 7.35–7.44 (m, 2H), 8.82 (s, 1H), 8.26–8.33 (m, 1H), 8.87 (s, 1H), 10.80 (s, 1H), 11.33 (s, 1H). Anal. (C19H19Cl2N7O5) C, H, N.
2-Chloro-1-deoxy-1-[6-[[[(4-methoxyphenyl)amino]-carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuron-amide (53):
yield 67%; white solid, mp 158–160 °C dec (EtOH); IR (KBr) cm−1 3400–3050, 1680, 1660, 1520, 1240; 1H NMR (DMSO-d6) δ 1.02–1.14 (m, 3H), 3.12–3.30 (m, 2H), 3.75 (s, 3H), 4.20–4.30 (m, 1H), 4.37 (s,1H), 4.61–4.72 (m, 1H), 6.04 (d, 1H, J = 6.8 Hz), 6.97 (d, 2H, J = 8.9 Hz), 7.47 (d, 2H, J = 8.9 Hz), 8.22–8.35 (m, 1H), 8.85 (s, 1H), 10.51 (s, 1H), 10.62 (s, 1H). Anal. (C20H22ClN7O6) C, H, N.
2-Iodo-1-deoxy-1-[6-[[[(3-chlorophenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (54):
yield 85%; white solid, mp 202–204 °C dec (EtOH); IR (KBr) cm−1 3500–3000, 1700, 1640, 1530, 1240; 1H NMR (DMSO-d6) δ 1.07 (t, 3H, J = 7.2 Hz), 3.13–3.31 (m, 2H), 4.25 (s, 1H), 4.38 (d, 1H, J = 2.1 Hz), 4.67 (pq, 1H), 6.05 (d, 1H, J = 6.7 Hz), 7.17 (d, 1H, J = 5.2 Hz), 7.35–7.44 (m, 2H), 8.82 (s, 1H), 8.13–8.23 (m, 1H), 8.79 (s, 1H), 10.66 (s, 1H), 11.33 (s, 1H). Anal. (C19H19ClIN7O5) C, H, N.
2-Iodo-1-deoxy-1-[6-[[[(4-methoxyphenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuron-amide (55):
yield 89%; white solid, mp 214–218 °C dec (EtOH); IR (KBr) cm−1 3375–3050, 1680, 1650, 1510, 1240; 1H NMR (DMSO-d6) δ 1.01–1.12 (m, 3H), 3.12–3.32 (m, 2H), 3.76 (s, 3H), 4.20–4.28 (m, 1H), 4.36 (s,1H), 4.60–4.73 (m, 1H), 6.04 (d, 1H, J = 6.6 Hz), 6.93–7.04 (m, 2H), 7.44–7.56 (m, 2H), 8.11–8.21 (m, 1H), 8.76 (s, 1H), 10.45 (s, 1H), 11.09 (s, 1H). Anal. (C20H22IN7O6) C, H, N.
General Procedure for the Synthesis of 2-(Ar)alkynyl-1-deoxy-1-[6-[[[(substituted)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (56–62).
Cuprous iodide (0.12 mg), bis(triphenylphosphine)palladium dichloride (1.7 mg) and the appropriate terminal alkyne (0.6 mmol) were added to a solution of 2-iodo-6-ureas (54 or 55) (0.12 mmol) in a mixture of dry acetonitrile (4 mL), DMF (4 mL), and triethylamine (0.3 mL), and the mixture was stirred at room temperature for 16 h in an atmosphere of N2. The solvent was removed under vacuum, and the residue was chromatographed on silica gel column, eluting with a suitable mixture of solvents (see below) to give the desired alkynyl derivatives 56–62.
2-(1-Hexyn-1-yl)-1-deoxy-1-[6-[[[(3-chlorophenyl)amino]-carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (56):
chromatography solvent, CHCl3–MeOH (92:8); yield 75%; white solid, mp 196–198 °C dec (EtOH); IR (KBr) cm−1 3500–3150, 2235, 1680, 1660, 1545, 1260; 1H NMR (DMSO-d6) δ 0.91–1.0 (m, 3H), 1.02–1.13 (m, 3H), 1.40–1.71 (m, 4H), 2.52–2.60 (m, 2H), 3.19–3.34 (m, 2H), 4.18–4.26 (m, 1H), 4.37 (d, 1H, J = 2.2 Hz), 4.60–4.70 (m, 1H), 6.07 (d, 1H, J = 7.1 Hz), 7.11–7.22 (m, 1H), 7.41 (d, 2H, J = 5.1 Hz), 7.74 (s, 1H), 8.40–8.50 (m, 1H), 8.84 (s, 1H), 10.56 (s, 1H), 11.80 (s, 1H). Anal. (C25H28ClN7O5) C, H, N.
2-(Phenylethynyl)-1-deoxy-1-[6-[[[(3-chlorophenyl)-amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (57):
chromatography solvent, CHCl3–MeOH (92:8); yield 59%; white solid, mp 210–214 °C dec (EtOH); IR (KBr) cm−1 3450–3050, 2210, 1680, 1670, 1550, 1270; 1H NMR (DMSO-d6) δ 1.02–1.13 (m, 3H), 3.17–3.34 (m, 2H), 4.20–4.29 (m, 1H), 4.39 (s, 1H), 4.63–4.74 (m, 1H), 6.13 (d, 1H, J = 7.0 Hz), 7.17 (d, 1H, J = 7.2 Hz), 7.36–7.59 (m, 5H), 7.69–7.79 (m, 3H), 8.35–8.44 (m, 1H), 8.92 (s, 1H), 10.61 (s, 1H), 11.65 (s, 1H). Anal. (C27H24ClN7O5) C, H, N.
2-(3-Hydroxy-3-phenyl-1-propyn-1-yl)-1-deoxy-1-[6-[[[(3-chlorophenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (58):
chromatography solvent, CHCl3-cC6H12–CH3CN–MeOH (73:10:5:12); yield 43%; yellow solid, mp 214–216 °C dec (EtOH); IR (KBr) cm−1 3450–3050, 2325, 1700, 1640, 1540, 1250; 1H NMR (DMSO-d6) δ 0.96–1.14 (m, 3H), 3.04–3.30 (m, 2H), 4.30–4.45 (m, 2H), 4.78–4.90 (m, 1H), 6.19 (d, 1H, J = 6.6 Hz), 7.12–8.34 (m, 11H), 8.94 (s, 1H), 10.51 (s, 1H), 11.47 (s, 1H). Anal. (C28H26ClN7O6) C, H, N.
2-(1-Hexyn-1-yl)-1-deoxy-1-[6-[[[(4-methoxyphenyl)-amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (59):
chromatography solvent, CHCl3–C6H6–MeOH (81:10:9); yield 71%; white solid, mp 175–178 °C dec (EtOH); IR (KBr) cm−1 3500–3000, 2240, 1660, 1600, 1520, 1240; 1H NMR (DMSO-d6) δ 0.91–1.02 (m, 3H), 1.04–1.17 (m, 3H), 1.43–1.70 (m, 4H), 2.50–2.61 (m, 2H), 3.18–3.32 (m, 2H), 3.77 (s, 3H), 4.18–4.28 (m, 1H), 4.37 (d, 1H, J = 1.7 Hz), 4.66 (pq, 1H), 6.07 (d, 1H, J = 7.3 Hz), 6.96 (d, 2H, J = 8.9 Hz), 7.48 (d, 2H, J = 9.1 Hz), 8.40–8.51 (m, 1H), 8.82 (s, 1H), 10.31 (s, 1H), 11.49 (s, 1H). Anal. (C26H31N7O6) C, H, N.
2-(Phenylethynyl)-1-deoxy-1-[6-[[[(4-methoxyphenyl)-amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (60):
chromatography solvent, CHCl3–C6H6–MeOH (80:10:10); yield 60%; white solid, mp 204–206 °C dec (EtOH); IR (KBr) cm−1 3400–3050, 2205, 1675, 1660, 1510, 1240; 1H NMR (DMSO-d6) δ 0.98–1.14 (m, 3H), 3.15–3.34 (m, 2H), 3.77 (s, 3H), 4.20–4.30 (m, 1H), 4.39 (s, 1H), 4.65–4.75 (m, 1H), 6.12 (d, 1H, J = 6.9 Hz), 6.99 (d, 2H, J = 8.8 Hz), 7.44–7.62 (m, 5H), 7.64–7.78 (m, 2H), 8.34–8.47 (m, 1H), 8.90 (s, 1H), 10.36 (s, 1H), 11.29 (s, 1H). Anal. (C28H27N7O6) C, H, N.
2-(3-Hydroxy-3-phenyl-1-propyn-1-yl)-1-deoxy-1-[6-[[[(4-methoxyphenyl)amino]carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (61):
chromatography solvent, CHCl3–C6H6–MeOH (80:10:10); yield 40%; yellow solid, mp 218–220 °C dec (EtOH); IR (NaCl) cm−1 3450–3050, 2300, 1680, 1650, 1510, 1240; 1H NMR (DMSO-d6) δ 0.90–1.10 (m, 3H), 3.13–3.23 (m, 2H), 3.77 (s, 3H), 4.30–4.46 (m, 2H), 4.76–4.89 (m, 1H), 6.19 (d, 1H, J = 6.7 Hz), 6.88–7.02 (m, 2H), 7.43–7.83 (m, 5H), 8.03–8.30 (m, 4H), 8.92 (s, 1H), 10.30 (s, 1H), 11.32 (s, 1H). Anal. (C29H29N7O7) C, H, N.
2-(5-Phenyl-1-pentyn-1-yl)-1-deoxy-1-[6-[[[(4-methoxyphenyl)amino] carbonyl]amino]-9H-purin-9-yl]-N-ethyl-β-d-ribofuranuronamide (62):
chromatography solvent, CHCl3–MeOH (94:6); yield 77%; white solid, mp 210–212 °C dec (EtOH); IR (NaCl) cm−1 3450–3050, 2240, 1680, 1660, 1510, 1240; 1H NMR (DMSO-d6) δ 1.02–1.16 (m, 3H), 1.86–2.04 (m, 2H), 2.49–2.64 (m, 2H), 2.73–2.88 (m, 2H), 3.16–3.32 (m, 2H), 3.71 (s, 3H), 4.18–4.29 (m, 1H), 4.37 (s, 1H), 4.66 (pq, 1H), 6.07 (d, 1H, J = 7.0 Hz), 6.84 (d, 2H, J = 8.1 Hz), 7.17–7.38 (m, 5H), 7.41–7.54 (m, 2H), 8.39–8.52 (m, 1H), 8.83 (s, 1H), 10.32 (s, 1H), 11.50 (s, 1H). Anal. (C31H33N7O6) C, H, N.
Methods for Receptor Binding Assays.
Procedures for preparation of rat brain membranes and CHO cell membranes were reported previously.30,38,55 For binding experiments, membrane homogenates were frozen and stored at −20 °C for ≤2 months. Adenosine deaminase (ADA) was from Boehringer Mannheim (Indianapolis, IN). [3H]R-PIA was from Amersham (Arlington Heights, IL), and [3H]CGS 21680 was from DuPont NEN (Boston, MA). [125I]-AB-MECA was prepared as described by Olah et al.30
Binding of [125I]-AB-MECA to CHO cells stably transfected with the rat A3 receptor clone or to HEK-293 cells stably expressing the human A3 receptor was performed essentially as described.28,30,38,55 Assays were performed in 50 mM Tris/10 mM MgCl2/1 mM EDTA buffer (adjusted to pH 8.26 at 5 °C) in glass tubes containing 100 μL of the membrane suspension, 50 μL of [125I]-AB-MECA (final concentration 0.3 nM), and 50 μL of inhibitor. Inhibitors were routinely dissolved in DMSO. Concentrations of DMSO in incubations never exceeded 1%, at which concentration [125I]-AB-MECA binding was not affected. Incubations were carried out in duplicate for 1 h at 37 °C and were terminated by rapid filtration over Whatman GF/B filters, using a Brandell cell harvester (Brandell, Gaithersburg, MD). Tubes were washed three times with 3 mL of buffer. Radioactivity was determined in a Beckman gamma 5500B γ-counter. Nonspecific binding was determined in the presence of 200 μM NECA. Ki values were calculated according to Cheng–Prusoff56 assuming a Kd for [125I]-AB-MECA of 1.48 nM.
Binding of [3H]R-PIA to A1 receptors from rat cortical membranes and of [3H]CGS 21680 to A2A receptors from rat striatal membranes was performed as described previously.57Adenosine deaminase (2 units/mL) was present during the preparation of brain membranes. Additional deaminase was not added during incubation with the radioligand.
Binding of [35S]GTP-γ-S.
The binding of [35S]GTP-γ-S (Amersham, Chicago, IL, specific activity 1275 Ci/mmol) was carried out using rat RBL-2H3 mast cell membranes by the general method of Lorenzen et al.58 Membranes (approximately 5 μg) were suspended in a buffer containing 50 mM Tris (pH 7.4), 3 units/mL adenosine deaminase, 1 mM EDTA, 1 mM DTT, 10 μM GDP, 100 mM NaCl, and 10 mM MgCl2. [35S]GTP-γ-S was added to a final concentration of 0.1 nM in a total volume of 125 μL, and the mixture was incubated for 2 h at 30 °C. Nonspecific binding was determined in the presence of 10 μM GTP-γ-S (Sigma, St. Louis, MO). Incubation of the reaction mixture was terminated by filtration over GF/B glass filter using a Brandell cell harvester and washed twice with the same buffer.
Acknowledgment.
This work was partially supported by Consiglio Nazionale delle Ricerche (CNR), Ministero della Ricerca Scientifica e Tecnologica (MURST, grant 40 and 60%).
Abbreviations:
- [125I]AB-MECA
[125I]1-[6-[[(4-amino-3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-d-ribofuranuronamide
- HENECA
that 2-(1-hexyn-1-yl)-1-(6-amino-9H-purin-9-yl)-1-deoxy-N-ethyl-β-d-ribofuranuronamide
- CHO
cells, Chinese hamster ovary cells
- CGS 21680
2-[4-[(2-carboxyethyl)phenyl]ethyl-amino]1-(6-amino-9H-purin-9-yl)-1-deoxy-N-ethyl-β-d-ribofuranuronamide
- Cl-IB-MECA
2-Chloro-1-[6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-d-ribofuranuronamide
- R-PIA
1-[6-[[(phenylisopropyl]amino]-9H-purin-9-yl]-1-deoxy-β-d-ribofuranose
- DMSO
dimethysulfoxide
- EDTA
ethylenediaminotetraacetate, I-AB-MECA, 1-[6-[[(4-amino-3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-d-ribofuranuronamide
- IB-MECA
1-[6-[[(3-iodophenyl)methyl]amino]-9H-purin-9-yl]-1-deoxy-N-methyl-β-d-ribofuranuronamide
- Ki
equilibrium inhibition constant
- NECA
1-(6-amino-9H-purin-9-yl)-1-deoxy-N-ethyl-β-d-ribofuranuronamide
- PHPNECA
2-(3-hydroxy-3-phenyl-1-propyn-1-yl)-1-(6-amino-9H-purin-9-yl)-1-deoxy-N-ethyl-β-d-ribofuranuronamide
- THF
tetrahydrofuran
- Tris
tris(hydroxymethyl)aminomethane
References
- (1).(a) Libert F; Schiffmann SN; Lefort A; Parmentier M; Gerard C; Dumont JE; Vanderhaeghen JJ; Vassart G The orphan receptor cDNA RDC7 encodes an A1 adenosine receptor. EMBO J. 1991, 10, 1677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Fredholm BB; Abbracchio MP; Burnstock G; Daly JW; Harden TK; Jacobson KA: Williams M Nomenclature and classification of purinoceptors. Pharmacol. Rev 1994, 46, 143–156. [PMC free article] [PubMed] [Google Scholar]
- (2).Maenhaut C; Sande JV; Libert F; Abramowicz M; Parmentier M; Vanderhaeghen JJ; Dumont JE; Vassart G; Schiffmann S RDC8 codes for an adenosine A2 receptor with physiological constitutive activity. Biochem. Biophys. Res. Commun 1990, 173, 1169–1178. [DOI] [PubMed] [Google Scholar]
- (3).Stehle JH; Rivkees SA; Lee JJ; Weaver DR; Deeds JD; Reppert SM Molecular cloning and expression of the cDNA for a novel A2-adenosine receptor subtype. Mol. Endocrinol 1992, 6, 384–393. [DOI] [PubMed] [Google Scholar]
- (4).Zhou QY; Li CY; Olah ME; Johnson RA; Stiles GL; Civelli O Molecular cloning and characterization of an adenosine receptor-the A3 adenosine receptor. Proc. Natl. Acad. Sci. U.S.A 1992, 89, 7432–7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Londos C; Cooper DM; Wolff J Subclasses of external adenosine receptors. Proc. Natl. Acad. Sci. U.S.A 1980, 77, 2551–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Hamprecht B; Van Calker D Nomenclature of adenosine receptors. Trends Pharmacol. Sci 1985, 6, 153–154. [Google Scholar]
- (7).Ukena D; Olsson RA; Daly JW Definition of subclasses of adenosine receptors associated with adenylate cyclase: interaction of adenosine analogs with inhibitory A1 receptors and stimulatory A2 receptors. Can. J. Physiol. Pharmacol 1987, 65, 365–376. [DOI] [PubMed] [Google Scholar]
- (8).Ramkumar V; Stiles GL; Beaven MA; Ali H The A3AR is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J. Biol. Chem 1993, 268, 6871–6890. [PubMed] [Google Scholar]
- (9).Abbracchio MP; Brambilla R; Ceruti S; Kim HO; von Lubitz DKJE; Jacobson KA; Cattabeni F G-protein-dependent activation of phospholipase C by adenosine A3 receptors in rat brain. Mol. Pharmacol 1995, 48, 1038–1045. [PubMed] [Google Scholar]
- (10).(a) Bruns RF; Daly JW; Snyder SH Adenosine receptors in brain membranes: binding of N-cyclohexyl[3H] adenosine and 1,3-diethyl-8[3H] phenylxanthine. Proc. Natl. Acad. Sci. U.S.A 1980, 77, 5547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jacobson KA; van Rhee AM Development of selective purinoceptor agonists and antagonists. In Purinergic Approaches Experimental Therapy; Jacobson KA, Jarvis MF, Eds; Wiley-Liss Publisher: New York, 1997; pp 101–128. [Google Scholar]
- (11).Olsson RA; Kusachi S; Thompson SD; Ukena D; Padgett W; Daly JW N6-substituted N-alkyladenosine-5′-uronamides: bifunctional ligands having recognition groups for A1 and A2 adenosine receptors. J. Med. Chem 1986, 29, 1683–1689. [DOI] [PubMed] [Google Scholar]
- (12).Jacobson KA; Nikodijevic O; Ji XD; Berkich DA; Eveleth D; Dean RL; Hiramatsu K; Kassel NF; van Galen PJM; Lee KS; Bartus RT; Daly JW; Lanoue KF; Maillard M Synthesis and biological activity of N6-(p-sulfophenyl) alkyl and N6-sulfoalkyl derivatives of adenosine. Water soluble and peripherally selective adenosine agonists. J. Med. Chem 1992, 35, 4143–4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Shamim MT; Ukena D; Padgett WL; Hong O; Daly JW 8-Aryl- and 8-cycloalkyl-1,3-dipropylxanthines: further potent and selective antagonists for A1-adenosine receptors. J. Med. Chem 1988, 31, 613–617. [DOI] [PubMed] [Google Scholar]
- (14).Linden J; Patel A; Earl CQ; Craig RH; Daluge SM [125I]-labelled 8-phenylxanthine derivatives: antagonist radio-ligands for adenosine A1 receptors. J. Med. Chem 1988, 31, 745–751. [DOI] [PubMed] [Google Scholar]
- (15).Jacobson KA; de la Cruz R; Schulick R; Kiriasis KL; Padgett W; Pfleiderer W; Kirk KL; Neumeyer JL; Daly JW 8-substituted xanthines as antagonists at A1 and A2 adenosine receptors. Biochem. Pharmacol 1988, 37, 3653–3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Cristalli G; Eleuteri A; Vittori S; Volpini R; Lohse MJ; Klotz KN 2-alkynyl derivatives of adenosine and adenosine-5′-N-ethyluronamide as selective agonists at A2 adenosine receptors. J. Med. Chem 1992, 35, 2363–2368. [DOI] [PubMed] [Google Scholar]
- (17).Jarvis MF; Schulz R; Hutchison AJ; Do UH; Sills MA; Williams M [3H]CGS 21680, a selective A2 adenosine receptor agonist directly labels A2 receptors in rat brain. J. Pharmacol. Exp. Ther 1989, 251, 888–893. [PubMed] [Google Scholar]
- (18).Shimada J; Suzuki K; Nonaka H; Ishii A; Ichikawa S (E)-1,3-dialkyl-7-methyl-8-(3,4,5-trimethoxystyryl)xanthines: potent and selective adenosine A2 antagonists. J. Med. Chem 1992, 35, 2342–2345. [DOI] [PubMed] [Google Scholar]
- (19).(a) Jacobson KA; Gallo-Rodriguez C; Melman N; Fischer B; Maillard M; van Bergen A; van Galen PJM; Karton Y Structure–activity relationship of 8-styrylxanthines as A2-selective adenosine antagonists. J. Med. Chem 1993, 36, 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Baraldi PG; Manfredini S; Simoni D; Zappaterra L; Zocchi C; Dionisotti S; Ongini E Synthesis and activity of new pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c] pyrimidine and 1,2,3-triazolo[4,5-e]1,2,4-triazolo[1,5-c]pyrimidine displaying potent and selective activity as A2A adenosine receptor antagonist. Bioorg. Med. Chem. Lett 1994, 4, 2539–2544. [Google Scholar]; (c) Baraldi PG; Cacciari B; Spalluto G; Borioni A; Viziano M; Dionisotti S; Ongini E Current developments of A2A adenosine receptor antagonists. Cur. Med. Chem 1995, 2, 707–722. [Google Scholar]
- (20).Richardson PJ; Kase H; Jenner PG Trends Pharmacol. Sci. 1997, 18, 338–344. [DOI] [PubMed] [Google Scholar]
- (21).Schingnitz G; Küfner-Mühl U; Ensinger H; Lehr E; Kuhn FJ Selective A1-antagonists for treatment of cognitive deficits. Nucleosides Nucleotides. 1991, 10, 1067–1076. [Google Scholar]
- (22).(a) Bridges AJ; Moos WH; Szotek DL; Trivedi BK; Bristol JA; Heffner TG; Bruns RF; Downs DA N6-(2,2-diphenylethyl)adenosine, a novel adenosine receptor agonist with antipsychotic-like activity. J. Med. Chem 1987, 30, 1709–1711. [DOI] [PubMed] [Google Scholar]; (b) Ferré S; O’Connor WT; Snaprud P; Ungerstedt U; Fuxe K Antagonistic interactions between adenosine A2A receptors and dopamine D2 receptors in the ventral striopallidal system-implications for the treatment of schizophrenia. Neuroscience. 1994, 63, 765–773. [DOI] [PubMed] [Google Scholar]
- (23).(a) Knutsen LJS; Murray TF Adenosine and ATP in epilepsy. In Purinergic Approaches Experimental Therapy Jacobson KA, Jarvis MF, Eds; Wiley-Liss Publisher: New York, 1997; pp 423–447. [Google Scholar]; (b) Jacobson KA; Trivedi BK; Churchill PC; Williams M Novel therapeutics acting via purine receptors. Biochem. Pharmacol 1991, 41, 1399–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Jacobson KA; Kim HO; Siddiqi SM; Olah ME; Stiles G; von Lubitz DKJ A3 adenosine receptors: design of selective ligands and therapeutic prospects. Drugs Future. 1995, 20, 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Ji XD; Melman N; Jacobson KA Interactions of flavonoids and other phytochemicals with adenosine receptors. J. Med. Chem 1996, 39, 781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Karton Y; Jiang J-I; Ji XD; Melman N; Olah ME; Stiles GL; Jacobson KA Synthesis and biological activities of flavonoid derivatives as A3 adenosine receptor antagonists. J. Med. Chem 1996, 39, 2293–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).van Rhee AM; Jiang J-I; Melman N; Olah ME; Stiles GL; Jacobson KA Intercaction of 1,4-dihydropyridine and pyridine derivative with adenosine receptors: selectivity for A3 receptors. J. Med. Chem 1996, 39, 2980–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Kim Y-C, Ji XD; Jacobson KA Derivatives of the triazoloquinazoline adenosine antagonist (CGS15943) are selective for the human A3 receptor subtype. J. Med. Chem 1996, 39, 4142–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Kim HO; Ji XD; Siddiqi SM; Olah ME; Stiles GL; Jacobson KA 2-substitution of N6-benzyladenosine-5′-uronamides enhances selectivity for A3 adenosine receptors. J. Med. Chem 1994, 37, 3614–3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Olah ME; Gallo-Rodriguez C; Jacobson KA; Stiles GL [125I]AB-MECA, a high affinity radioligand for the rat A3 adenosine receptor. Mol. Pharmacol 1994, 45, 978–982. [PMC free article] [PubMed] [Google Scholar]
- (31).Linden J Cloned adenosine A3 receptors-pharmacological properties, species, differences and receptor functions. Trends Pharmacol. Sci 1994, 15, 298–306. [DOI] [PubMed] [Google Scholar]
- (32).Hannon JP; Pfannkuche HJ; Fozard JR A role for mast cells in adenosine A3 receptor-mediated hypothension in the rat. Br. J. Pharmacol 1995, 115, 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Fozard JR; Pfannkuche HJ; Schuurman HJ Mast cell degranulation following adenosine A3 receptor activation in rats. Eur. J. Pharmacol 1996, 298, 293–297. [DOI] [PubMed] [Google Scholar]
- (34).Jacobson KA; Nikodijevic O; Shi D; Gallo-Rodriguez C; Olah ME; Stiles GL; Daly JW A role for central A3-adenosine receptors: Mediation of behavioral depressant effects. FEBS Lett. 1993, 336, 57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).von Lubitz DKJE; Lin RCS; Popik P; Carter MF; Jacobson KA Adenosine A3 receptor stimulation and cerebral ischemia. Eur. J. Pharmacol 1994, 263, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Kohno Y; Sei Y; Koshiba M; Kim HO; Jacobson KA Induction of apoptosis in HL-60 human promyelocytic leukemia cells by selective adenosine A3 receptor agonists. Biochem. Biophys. Res. Commun 1996, 219, 904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Jacobson KA; van Galen PJM; Williams M Perspective Adenosine Receptors: pharmacology, structure–activity relationship and therapeutic potential. J. Med. Chem 1992, 35, 407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Gallo-Rodriguez C; Ji XD; Melman N; Siegman BD; Sanders LH; Orlina J; Pu QL; Olah ME; van Galen PJM; Stiles GL; Jacobson KA Structure–activity relationship of N6-benzyladenosine-5′-uronamides as A3-selective adenosine agonists. J. Med. Chem 1994, 37, 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Baraldi PG; Cacciari B; Spalluto G; Ji XD; Olah ME; Stiles G; Dionisotti S; Zocchi C; Ongini E; Jacobson KA Novel N6-(substituted-phenylcarbamoyl)adenosine-5′-uronamides as potent agonists for A3 adenosine receptors. J. Med. Chem 1996, 39, 802–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Cristalli G; Volpini R; Vittori S; Camaioni E; Monopoli A; Conti A; Dionisotti S; Zocchi C; Ongini E 2-Alkynyl derivatives of adenosine-5′-N-ethyluronamide: selective A2 adenosine receptor agonists with potent inhibitory activity on platelet aggregation. J. Med. Chem 1994, 37, 1720–1726. [DOI] [PubMed] [Google Scholar]
- (41).Siddiqui SM; Jacobson KA; Esker JL; Olah ME; Ji X; Melman N; Tiwari KN; Secrist JA III; Schneller SW; Cristalli G; Stiles GL; Johnson CR; IJzerman AP Search for new purine- and ribose-modified adenosine analogues as selective agonists and antagonists at adenosine receptors. J. Med. Chem 1995, 38, 1174–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Kurita K; Iwakura Y Trichloromethyl-Chloroformate as a phosgene equivalent: 3-isocyanatopropanoylchloride. Organic Synthesis; Wiley & Sons: New York, 1988; Collect. Vol VI, pp 715–718. [Google Scholar]
- (43).Hutchison AJ; Williams M; de Jesus R; Yokoyama R; Oei HH; Ghai GR; Webb RL; Zoganas HC; Stone GA; Jarvis MF 2-(Arylalkylamino)adenosin-5′-uronamides: a new class of highly selective adenosine A2 receptor ligands. J. Med. Chem 1990, 33, 1919–1924. [DOI] [PubMed] [Google Scholar]
- (44).Cristalli G; Volpini R; Vittori S; Camaioni E; Monopoli A; Conti A; Dionisotti S; Zocchi C; Ongini E 2-Aralkynyl and 2-heteroalkynyl derivatives of adenosine-5′-N-ethyluronamide as selective A2a adenosine receptor agonists. J. Med. Chem 1995, 38, 1462–1472. [DOI] [PubMed] [Google Scholar]
- (45).Bruns RF; Lu GH; Pugsley TA Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat striatal membranes. Mol. Pharmacol 1986, 29, 331–346. [PubMed] [Google Scholar]
- (46).Siddiqi SM; Pearlstein RA; Sanders LH; Jacobson KA Comparative molecular field analysis of selective A3 adenosine receptor agonists. Bioorg. Med. Chem 1995, 3, 1331–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).van Galen PJM; Stiles GL; Michaels G; Jacobson KA Adenosine A1 and A2 receptors: structure-function relationships. Med. Res. Rev 1992, 12, 423–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Müller CE; Scior T Adenosine receptors and their modulators. Pharmaceutica Acta Helvitiae. 1993, 68, 77–111. [DOI] [PubMed] [Google Scholar]
- (49).Vittori S; Camaioni E; Di Francesco E; Volpini R; Monopoli A; Dionisotti S; Ongini E; Cristalli G 2-Alkenyl and 2-alkyl derivatives of adenosine and adenosine-5′-N-ethyluronamide: different affinity and selectivity of E- and Z-diastereomers at A2A adenosine receptors. J. Med. Chem 1996, 39, 4211–4217. [DOI] [PubMed] [Google Scholar]
- (50).Camaioni E; Di Francesco E; Vittori S; Volpini R; Cristalli G Adenosine receptor agonists: synthesis and biological evaluation of the diastereoisomers of 2-(3-hydroxy-3-phenyl-1-propyn-1-yl)NECA. J. Med. Chem 1997, 5, 2267–2275. [DOI] [PubMed] [Google Scholar]
- (51).Bodwen K Electronic effects in drugs. In Comprehensive Medicinal Chemistry, 1st ed.; Hansch C, Sammes PG, Taylor JB, Eds.; Pergamon Press: Elmsford, NY, 1990; Vol. 4, pp 205–239. [Google Scholar]; (b) Leo AJ Methods of calculating partition coefficients. In Comprehensive Medicinal Chemistry, 1st ed.; Hansch C, Sammes PG, Taylor JB, Eds.; Pergamon Press: Elmsford, NY, 1990; Vol. 4, pp 295–319. [Google Scholar]
- (52).Park KS; Hoffmann C; Kim HO; Padgett WL; Daly JW; Brambilla R; Motta C; Abbracchio MP; Jacobson KA Activation and desensitization of rat A3-adenosine receptors by selective adenosine derivatives and xanthine-7-ribosides. Drug Dev. Res, submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Lohse MJ; Klotz K-N; Schwabe U; Cristalli G; Vittori S; Grifantini M 2-Chloro- N6-cyclopentyladenosine: a highly selective agonist at A1 adenosine receptors. Naunyn–Schmiedeberg’s Arch. Pharmacol 1988, 337, 687–689. [DOI] [PubMed] [Google Scholar]
- (54).Macek TA; Conn PJ PKC Inhibition of Group II mGlu Autoreceptor Function at Medial and Lateral Performant. Path. Soc. Neurosci 1997, Abstr. 685.17, 23, pp 1754. [Google Scholar]
- (55).van Galen PJM; van Bergen AH; Gallo-Rodriguez C; Melman N; Olah ME; IJzerman AP; Stiles GL; Jacobson KA A binding site model and structure-activity relationship for the rat A3 adenosine receptor. Mol. Pharmacol 1994, 45, 1101–1111. [PMC free article] [PubMed] [Google Scholar]
- (56).Cheng YC; Prusoff HR Relationships between the inhibition constant (Ki) and the concentration of inhibitor which causes 50% inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol 1973, 22, 3099–3108. [DOI] [PubMed] [Google Scholar]
- (57).van Bergen A; van Galen PJM; Stiles GL; Jacobson KA A3 receptors: Structure activity relationship and molecular modeling. ACS 206th National Meeting, Chicago, IL, August, 1993, Abstract MEDI217. [Google Scholar]
- (58).Lorenzen A; Guerra L; Vogt H; Schwabe U Interaction of full and partial agonists of the A1 adenosine receptor with receptor/G protein complexes in rat brain membranes. Mol. Pharmacol 1996, 49, 915–926. [PubMed] [Google Scholar]


