Abstract
Background and Purpose:
To evaluate the performance of multiparametric MR images in differentiation of different regions of the gross tumor area and for assessment of glioma grade.
Methods:
Forty-six glioma subjects (18 grade II, 11 grade III, 17 grade IV) underwent a comprehensive MR and spectroscopic imaging procedure. Maps were generated by subtraction of T1-weighted images from contrast-enhanced T1-weighted images (ΔT1 map) and T1-weighted images from T2-weighted images (ΔT2 map). Regions of interest (ROI) were positioned in normal appearing white matter (NAWM), enhancing tumor, hyperintense T2, necrotic region, immediate and distal peritumoral regions (IPR and DPR). Relative signal contrast was estimated as difference between mean intensities in ROIs and NAWM. Classification using support vector machines was applied to all image series to determine the efficacy of regional contrast measures for differentiation of low and high-grade lesions and grade III and IV lesions.
Results:
ΔT1 and ΔT2 maps offered higher contrast as compared to other parametric maps in differentiating enhancing tumor and edematous regions, respectively, and provided the highest classification accuracy for differentiating low and high-grade tumors, of 91% and 90.4%. Choline/N-acetylaspartate maps provided significant contrast for delineating IPR and DPR. For differentiating high-grade gliomas, ΔT2 and ΔT1 maps provided a mean accuracy of 90.9% and 88.2%, which was lower than that obtained using Cerebral Blood Volume (93.7%) and Choline/Creatine (93.3%) maps.
Conclusion:
This study showed that subtraction maps provided significant contrast in differentiating several regions of the gross tumor area and are of benefit for accurate tumor grading.
Keywords: MRI, subtraction, contrast, glioma, brain tumor, MRSI
INTRODUCTION
For detection of gliomas, a post-processing method based on the difference between two images of differing contrasts, or subtraction images, has been presented as a way to improve image contrast and benefit tumor detection, quantification, and evaluation of progression.1 A combination of longer T1 and T2 relaxation times in gliomas and hyper-intensities seen in contrast-enhanced (CE) images that are correlated to tumor grade, therefore, presents an opportunity to improve visualization of gliomas, and potentially to improve grading, by creating subtraction maps derived by subtracting non-enhanced T1-weighted images from CE T1-weighted images; and subtracting non-enhanced T1-weighted images from T2-weighted images.
Previous studies have demonstrated the use of images derived from subtraction of non-enhanced T1-weighted images from CE T1-weighted images to provide better visualization and quantification of gliomas treated with bevacizumab.2,3 The utility of T1 subtraction maps in patients with new or recurrent glioblastoma multiforme (GBM), showed a 25% reduction in T1 CE tumor volume compared to baseline.4 Kim et al. showed increased contrast-to-noise ratio as compared to traditional imaging techniques using subtraction maps generated from the subtraction of T2-weighted images from T1-weighted images in gliomas and cortical dysplasia.5
Several studies have evaluated multiparametric MRI methods for tissue classification and grading of glioma;6,7 however, these did not consider the use of T1 or T2 subtraction images. This study’s primary aim was to assess if subtraction images generated using CE T1-weighted, non-enhanced T1-weighted and T2-weighted images offered better contrast in differentiating tumor and edema from NAWM as compared to traditionally acquired anatomic, diffusion-weighted imaging (DWI), perfusion-weighted imaging (PWI) and MR spectroscopy imaging (MRSI) sequences. A secondary aim was to evaluate if image metrics obtained from subtraction images were correlated to tumor grade and whether these metrics can be used for classification of gliomas.
METHODS
Participants
A retrospective data analysis was carried out for 46 subjects with untreated gliomas histologically verified with World Health Organization classifications of grade II (n = 18), III (n = 11), and IV (n = 17). Subjects varied in age from 14-69 years comprising of 36 males and 10 females. Out of the 56 subjects included, 8 subjects were excluded due to lack of CE T1-weighted images, and 2 subjects were excluded due to poor image registration results. All subjects had gross total resection and were treatment naive before imaging receiving no steroids. Informed consent was acquired from each subject and the protocol was approved by the institution's human subjects' research review board.
MRI Image Acquisition
Subjects underwent MR imaging at 3T (Signa HDxt; GE Healthcare, Wisconsin) comprising of pre- and post-CE T1-weighted MR, DWI, CE perfusion measurement (DCE-MRI), T2-weighted MR imaging, and whole-brain MRSI. Details of the imaging methods are previously reported7 and hence are described only in brief. T1-weighted imaging was carried out using a 3D IR-prepared Fast spoiled gradient (SPGR) sequence at 1x1x1 mm; TR/TE/TI=3.01/8.02/400 ms; FA, 13°; image matrix, 256x256. T2-weighted imaging was acquired using TE/TR of 101.8/3500 ms; image matrix, 256x256x46; and pixel size, 1.02x1.02x3 mm. DWI data were acquired in 12 directions in addition to the reference measurement with b=0 s/mm2 and parametric maps for apparent diffusion coefficient (ADC), and fractional anisotropy (FA) were estimated. DCE-MRI was performed using a 3D SPGR sequence with Gd-DTPA-BMA (Omniscan; GE Healthcare, Piscataway, NJ) administered at the beginning of the fourth time point at 5 mL/s and a dose of 0.2 mmol/kg body weight. Quantitative analysis of the concentration-time curve was performed to calculate cerebral blood volume (CBV) maps corrected for contrast agent leakage effect due to the disrupted BBB.8 Whole-brain MRSI was acquired using echo-planar acquisition with voxel volume of 1.5 ml; TR/TE/TI=1710/70/198 ms; and FOV of 280x280x180 mm3. MRSI data were processed using the MIDAS package,9 for automated spectral analysis for N-acetylaspartate (NAA), creatine and phosphocreatine (Cr), and glycerophosphocholine, phosphocholine, and free choline (Cho). All images and parametric maps were registered to pre-contrast the T1-weighted image for a subject using rigid registration.10
Generation of Subtraction Maps following Intensity Normalization
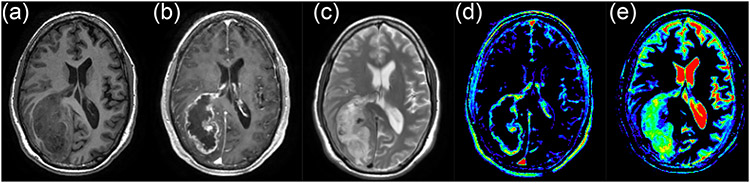
Two variants of subtraction maps were generated, namely, T1-weighted subtraction (ΔT1) maps generated by subtraction of pre-contrast T1-weighted images from CE T1-weighted images, and T2-weighted subtraction (ΔT2) maps derived by subtraction of T2-weighted image from pre-contrast T1-weighted image. Before image subtraction, the CE T1-weighted and T2- weighted images were both registered to the pre-contrast T1-weighted image using a 3D multi-modality medical image alignment algorithm10 and Gaussian normalization2,11 was used to normalize the intensity of all images before subtraction. Figure 1 shows an example of structural MR images and generated subtraction images for a subject with a grade IV glioma.
Figure 1:
Structural MR images and derived subtraction maps for a grade IV glioma subject, showing the pre-contrast T1-weighted image (a), Contrast Enhaced T1-weighted image (b), T2-weighted image (c), T1-weighted subtraction map (d), and T2-weighted subtraction map (e).
Quantitative Analysis
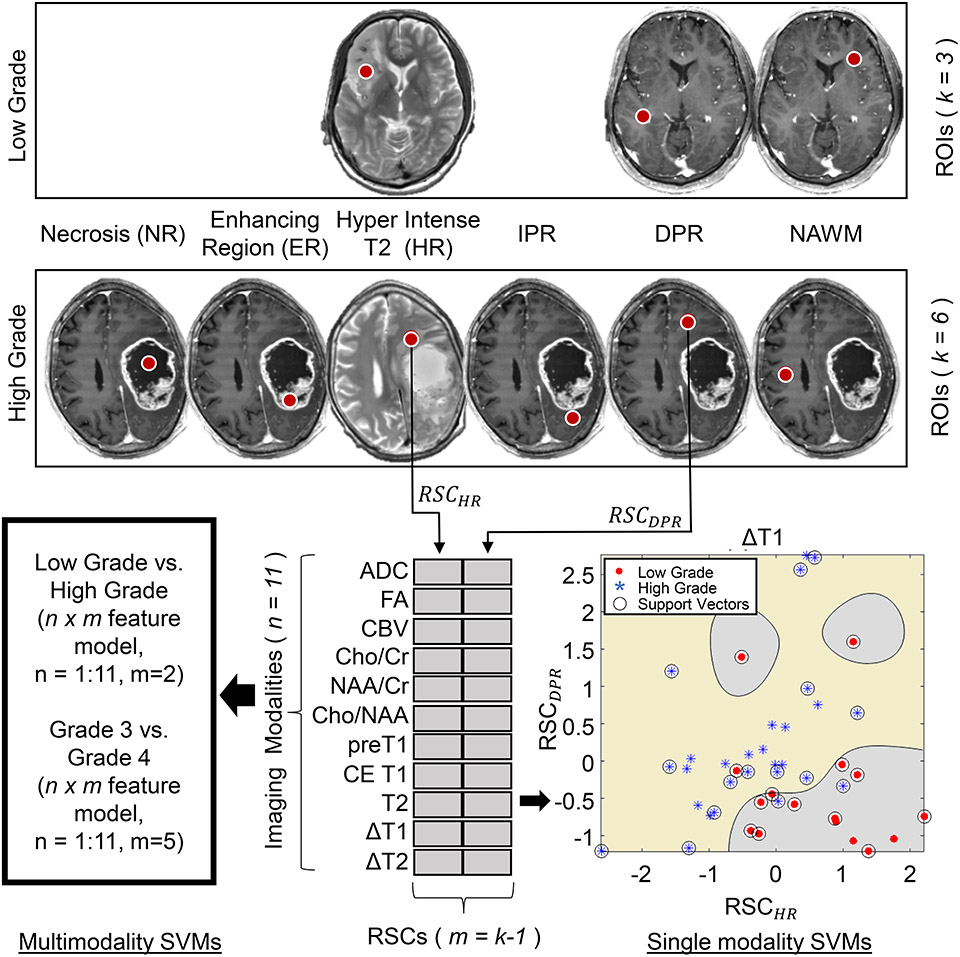
To estimate the image contrast of each imaging modality, the mean image value within six regions of interest (ROI) was calculated. ROIs were manually drawn for each subject based on features seen in the T1 and T2-weighted images as shown in Figure 2, and were located in (1) NAWM in the contralateral hemisphere to the gross tumor, (2) enhancing tumor region (ER) as seen on CE T1-weighted imaging, (3) hyperintense regions (HR) on T2 images located in the gross tumor volume but not in the enhancing or necrotic regions, (4) necrotic region (NR), (5) immediate peritumoral region (IPR) defined as a 1 cm wide band around the enhancing region, and (6) distal peritumoral region (DPR) defined as a band approximately 2 −3 cm away from the enhancing region on the ipsilateral side of the tumor. To improve the power of the comparison three ROIs were sampled and averaged for each region.
Figure 2:
Outline of the Support Vector Machine (SVM) classification methodology showing selection of regions of interests (ROIs) in Enhancing Region on CE-T1 (ER), Hyperintense T2 Region (HR), Immediate Peritumoral Region (IPR), Distal Peritumoral Region (DPR), Necrotic Region (NR), and normal appearing white matter (NAWM) in low and high-grade gliomas. Metrics are generated from 11 images/maps and single and multi-modality (combination) SVMs are executed for classification.
Abbreviations: RSC, Relative signal contrast; m, number of RSCs used in the classification model; n, number of modalities ADC, Apparent Diffusion Coefficeint; CBV, Cerebral Blood Volume; Cho, Choline; NAA, N-acetylaspartate; Cr, Creatine; FA, fractional anisotropy; CE, Contrast Enhanced; DWI, diffusion-weighted imaging; MRS, MR spectroscopy.
ROIs drawn on CE T1-weighted images and T2-weighted images were transferred to co-aligned ADC, FA, CBV, MRSI (Cho/Cr, Cho/NAA and NAA/Cr) and subtraction maps (ΔT1 and ΔT2). Relative signal contrast (RSC) was estimated as the difference in the mean intensities between the ROI under consideration and contralateral NAWM, i.e.
| [1] |
where, μROI is the mean signal intensity within an ROI and μNAWM, the mean signal intensity within the contralateral NAWM, is considered the mean background value.
Five contrasts were evaluated for differentiating ER (RSCER), HR (RSCHR), NR (RSCNR), IPR (RSCIPR), and DPR (RSCDPR) from NAWM for each of the MR maps.
Statistical Analysis
One-way analysis of variance (ANOVA) was carried out separately for each tissue contrast to ascertain which image offered the best contrast for visualization of ER, HR, NR, IPR, and DPR from surrounding NAWM. Post-hoc analysis was carried out using Tukey’s test for pair-wise comparisons. RSCs calculated for each tissue ROI were compared across tumor grades separately and after pooling subjects of all grades together. Additionally, a multinomial logistic regression analysis was carried out to estimate associations between RSCs and tumor grade. Statistical significance for each test was determined by a two-tailed False Discovery Rate (FDR) procedure12 adjusted probability of less than 0.05.
Classification Studies
To determine which image/map provided the highest accuracy for classification of tumor grade support vector machines (SVM) were used with the histologically determined grades treated as the gold standard. Figure 2 shows the outline of the SVM classification methodology.
To classify low-grade tumors from high-grade tumors, RSCs from HR and DPR were chosen as features for SVM. The choice of these RSCs was driven by the knowledge that most low-grade tumors did not show an enhancing or necrotic region, and SVM analysis was limited to use only those measures. The discriminative power of each image and parameter map was then determined. Classification models were created for each image/map with RSCHR and RSCDPR calculated from each image type, acting as a feature set to train the SVM. The resultant 2-dimensional (2D) SVM created for each image/map was assessed to calculate the accuracy, sensitivity, and specificity in each case with leave-one-out cross-validation employed for cross-validation. An example SVM created for the ΔT1 map is shown in Figure 2 with low and high-grade tumors shown in red and blue, respectively.
Finally, multimodality SVMs were created to test the potential of using a combination of more than one image/map towards the classification process. Six combination models were evaluated that comprised of: (1) SVM constructed using the two best-performing modalities in terms of accuracy obtained from the single modality experiments (4D model); (2) SVM constructed using the best three modalities (6D model); (3) SVM constructed using all the available modalities (20D model); (4) SVM constructed using DWI ADC and FA maps (4D model); (5) SVM constructed using all available MRS maps (6D model); and (6) SVM constructed using the two studied subtraction images (4D model). For each combination model, the complexity is m*n, where m is the number of RSCs used in the classification model and n is the number of modalities combined to form the classifier.
To differentiate between grade III and IV tumors, a similar analysis, using all the five available contrasts were carried out. High-grade tumors not possessing either enhancing or necrotic regions (n = 9/28, 32%) were excluded from the classification process. To account for the n = 9/28 (32%) high grade tumors not possessing either enhancing or necrotic regions, a ‘reduced’ model that incorporated only RSCHR and RSCDPR, similar to the classification of low-grade and high-grade tumors, was implemented.
SVM classification was performed using MATLAB’s Statistics and Machine Learning Toolbox. The SVM uses a sequential minimal optimization scheme to implement an L1 soft-margin SVM classifier with a Gaussian Radial Basis Function kernel with an empirical scaling factor of 3 to map the original features via the selected kernel function to construct a maximum margin classifier in high-dimensional feature space.13 Each classifier was executed 100 times with a randomized initialization and the mean and standard deviation of the performance metrics are reported. Leave-one-out cross-validation was used for assessing the variability of the data.
RESULTS
Tissue Relative Signal Contrasts
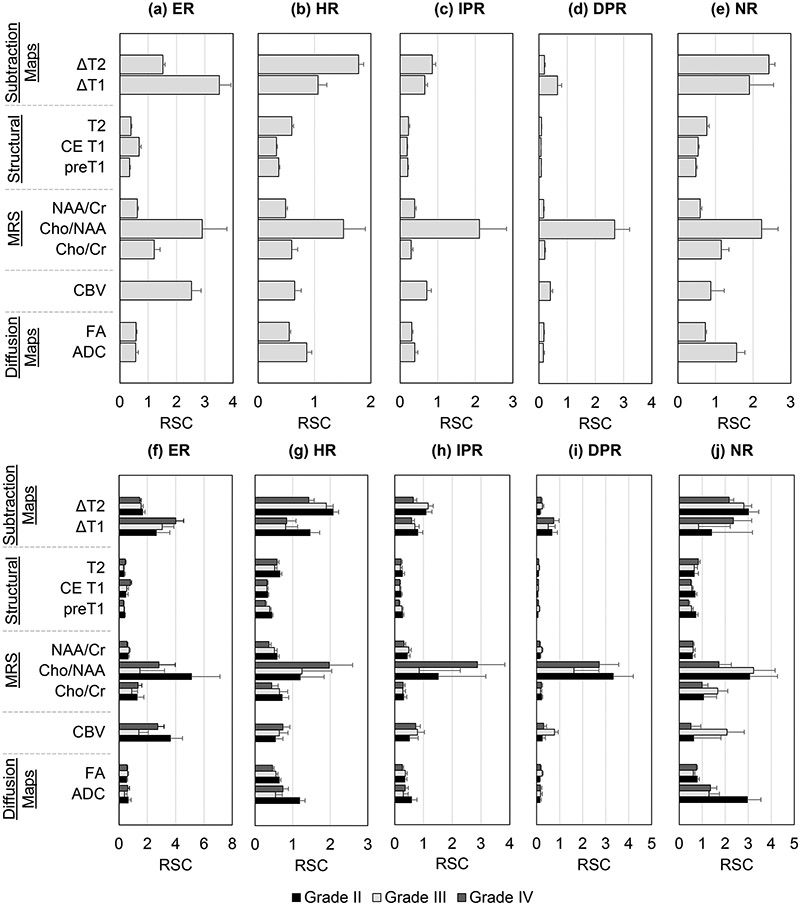
In Figure 3 are shown the mean RSCs for all image types and each of the five contrasts, with the top row (a-e) showing results for all tumors, whereas the bottom row (f-j) show results separated by tumor grade.
Figure 3:
Relative signal contrast (RSC) calculated for Enhancing Region on Contrast Enhanced T1 (ER), Hyperintense T2 Region (HR), Immediate Peritumoral Region (IPR), Distal Peritumoral Region (DPR) and Necrotic Region (NR) with respect to normal-appearing white matter. Top row (a-e) shows the contrasts calculated across subjects not accounting for grade, whereas bottom row (f-j) shows the contrasts calculated separately for grade II, III, and IV tumors.
Abbreviations: ADC, Apparent Diffusion Coefficeint; CBV, Cerebral Blood Volume; Cho, Choline; NAA, N-acetylaspartate; Cr, Creatine; FA, fractional anisotropy; CE, Contrast Enhanced; MRS, MR spectroscopy.
ER: ΔT1 and Cho/NAA offered the highest contrast for differentiation of ER from contralateral NAWM (p < 0.001), with no difference in pair-wise comparisons (p > 0.05). CBV, Cho/Cr and ΔT2 maps also provided contrast for differentiating ER from NAWM (p > 0.05), but Tukey's pair-wise comparisons indicated no differences between these. Moreover, ΔT1 RSC was seen to increase with tumor grade (Figure 3f); however, regression showed no significant correlation of ΔT1 RSC with tumor grade after multiple comparison correction (p = 0.27, FDR q = 0.55).
HR: For differentiation of edema from NAWM, it was found that ΔT2 and Cho/NAA offered the highest contrast (p < 0.001) with no difference in Tukey’s pair-wise comparisons. ΔT2 maps showed that RSCHR reduces with tumor grade, whereas for Cho/NAA maps an increase in RSCHR was seen with tumor grade. Pair-wise comparisons revealed that ΔT2 RSC in the HR was approximately similar between the different DWI measures namely ADC, and FA (p > 0.05). Regression of ΔT1 (q < 0.05) and ΔT2 (q < 0.05) RSC with tumor grade was found to be significant after FDR correction, with decreasing mean RSC values with tumor grade.
IPR and DPR: For differentiation of IPR and DPR from NAWM, Cho/NAA maps provided highest contrast (p < 0.001). ΔT2 and ΔT1 had the second-highest contrast; however, failed to reach significance for pair-wise analysis. The mean RSC values were smaller than for differentiation of other tissue regions, which can be attributed to the fact that IPR and, especially, DPR are very similar in appearance to the NAWM on most parametric maps.
NR: Tukey’s pair-wise comparisons show that ΔT2, ΔT1, and Cho/NAA maps offer the best differentiation between necrotic regions and NAWM, with no significant difference among them (p > 0.05). Correlation of RSCNR observed in ΔT2, ΔT1, and Cho/NAA to tumor grade failed to reach significance after multiple comparison correction (q > 0.05).
Classification Results
Table 1 shows the mean accuracies obtained for differentiation between low and high-grade lesions. The single modality classifiers show that the best accuracy was obtained using ΔT1 maps (91.0 ± 1.4%), with similar performance obtained for ΔT2 (90.4 ± 2.8%), ADC (90.1 ± 2.3%) and CBV (88.4 ± 1.8%). Within spectroscopy images, Cho/NAA maps provided greatest classification accuracy (86.3 ± 1.8%), followed by Cho/Cr (85.6 ± 2.5%) and NAA/Cr (85.3 ± 1.7%). Structural images (T2, T1, and CE T1) provided the least classification accuracy. Multimodal SVMs created using a combination of the best two single modality classifiers, namely subtraction maps ΔT1 and ΔT2, provided an improved average accuracy of 97.2 ± 1.3%. A classifier constructed using all the available images/maps provided an accuracy of 87.3 ± 1.4%. Limiting the analysis to a particular modality resulted in DWI based classifiers providing an accuracy of 84.5 ± 2.0%, whereas MRS based maps provided a combined accuracy of 91.3 ± 1.3%, which was higher than that provided by either MRSI map individually.
Table 1:
Classification accuracy obtained for classification of low-grade (II) and high-grade (III and IV) gliomas.
| Modality | Complexity Dimensions |
Accuracy (%) | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|
| Single Modality Models | ΔT1 | 2 | 91.0 ± 1.4 | 87.4 ± 3.6 | 93.1 ± 0.9 |
| ΔT2 | 2 | 90.4 ± 2.8 | 79.6 ± 7.3 | 96.5 ± 2.0 | |
| ADC | 2 | 90.1 ± 2.3 | 73.4 ± 6.3 | 100 ± 0 | |
| CBV | 2 | 88.4 ± 1.7 | 80.0 ± 4.7 | 92.8 ± 0.8 | |
| Cho/NAA | 2 | 86.3 ± 1.8 | 73.2 ± 5.4 | 99.6 ± 1.4 | |
| Cho/Cr | 2 | 85.6 ± 2.5 | 61.3 ± 6.8 | 100 ± 0 | |
| NAA/Cr | 2 | 85.3 ± 1.7 | 59.4 ± 4.6 | 100 ± 0 | |
| FA | 2 | 84.8 ± 1.7 | 58.3 ± 4.8 | 100 ± 0 | |
| T2 | 2 | 82.4 ± 1.7 | 62.5 ± 4.6 | 93.7 ± 1.8 | |
| T1 | 2 | 82.3 ± 2.0 | 61.5 ± 6.7 | 94.2 ± 3.4 | |
| CE T1 | 2 | 82.1 ± 2.0 | 49.3 ± 6.3 | 99.8 ± 1.0 | |
| Combination Models | Top 2 (ΔT1, ΔT2) | 4 | 97.2 ± 1.3 | 92.8 ± 3.7 | 99.7 ± 1.0 |
| Top 3 (ΔT1, ΔT2, ADC) | 6 | 92.0 ± 1.6 | 78.8 ± 4.3 | 99.7 ± 0.9 | |
| All | 22 | 87.3 ± 1.4 | 63.8 ± 3.9 | 99.5 ± 1.3 | |
| DWI (ADC, FA) | 4 | 84.5 ± 2.0 | 57.4 ± 5.5 | 100 ± 0 | |
| MRS (Cho/NAA, Cho/Cr, NAA/Cr) | 6 | 91.3 ± 2.7 | 78.2 ± 7.1 | 99.0 ± 2.0 | |
| SUBTRACTION (ΔT1, ΔT2) | 4 | 97.2 ± 1.3 | 92.8 ± 3.7 | 99.7 ± 1.0 |
All the data represents mean ± standard deviation unless otherwise indicated. Abbreviations: ADC, Apparent Diffusion Coefficeint; CBV, Cerebral Blood Volume; Cho, Choline; NAA, N-acetylaspartate; Cr, Creatine; FA, fractional anisotropy; CE, Contrast Enhanced; DWI, diffusion-weighted imaging; MRS, MR spectroscopy
In Table 2 are shown results for distinguishing between grade III and IV lesions using RSCs derived from all the studied ROIs. Classification results showed that CBV provided the best accuracy (93.7 ± 2.0%), followed by Cho/Cr maps (93.3 ± 5.0%), ΔT2 (90.9 ± 2.7%), and ΔT1 (88.2 ± 4.0%). Similar to the results seen for classification of low and high-grade lesions, structural images (T2, CE T1, and T1) provided the least accuracy. Combination SVMs using the two modalities that provided the best accuracy in single modalities analysis (CBV and Cho/Cr) do not offer significantly different results as obtained from either of them individually. A 55D model constructed using all the available RSCs and image/maps resulted in reduced accuracy of 91.8 ± 2.8% compared to single modality models generated from either CBV, Cho/Cr, or ΔT2 maps. Combination models comprised of DWI, MRS, and subtraction maps did not improve the accuracy provided by CBV alone. Reduced single modality SVMs implemented using ΔT1, ΔT2 and CBV maps showed a greater than 10% reduction in accuracy in segregation of grade III and IV lesions demonstrating the added value offered by RSCs obtained from the ER, NR and IPR regions.
Table 2:
Classification accuracy obtained for classification of grade III and grade IV gliomas.
| Modality | Complexity Dimensions | Accuracy (%) |
Sensitivity (%) |
Specificity (%) |
|
|---|---|---|---|---|---|
| Single Modality Models | CBV | 5 | 93.7 ± 1.9 | 71.5 ± 8.7 | 100 ± 0 |
| Cho/Cr | 5 | 93.3 ± 5.0 | 69.8 ± 22.6 | 100 ± 0 | |
| ΔT2 | 5 | 90.9 ± 2.7 | 59.0 ± 12.1 | 100 ± 0 | |
| ΔT1 | 5 | 88.2 ± 4.0 | 46.8 ± 18.0 | 100 ± 0 | |
| Cho/NAA | 5 | 88.0 ± 3.9 | 37.0 ± 17.6 | 100 ± 0 | |
| NAA/Cr | 5 | 86.8 ± 2.9 | 40.8 ± 13.1 | 100 ± 0 | |
| FA | 5 | 86.5 ± 4.1 | 39.3 ± 18.6 | 100 ± 0 | |
| ADC | 5 | 83.8 ± 3.1 | 35.0 ± 12.3 | 100 ± 0 | |
| T2 | 5 | 81.4 ± 3.5 | 16.5 ± 15.6 | 100 ± 0 | |
| CE T1 | 5 | 80.6 ± 3.3 | 12.5 ± 14.9 | 100 ± 0 | |
| T1 | 5 | 80.4 ± 3.0 | 12.0 ± 13.5 | 100 ± 0 | |
| Combination Models | Top 2 (CBV, Cho/Cr) | 10 | 93.5 ± 2.1 | 70.8 ± 9.4 | 100 ± 0 |
| Top 3 (CBV, Cho/Cr, ΔT2) | 15 | 92.1 ± 2.9 | 64.5 ± 12.9 | 100 ± 0 | |
| All | 55 | 91.8 ± 2.8 | 63.0 ± 12.6 | 100 ± 0 | |
| DWI (ADC, FA) | 10 | 88.9 ± 4.7 | 55.5 ± 18.7 | 100 ± 0 | |
| MRS (Cho/Cr, Cho/NAA, NAA/Cr) | 15 | 85.8 ± 3.3 | 43.0 ± 13.3 | 100 ± 0 | |
| SUBTRACTION (ΔT1, ΔT2) | 10 | 89.4 ± 4.0 | 52.3 ± 18.2 | 100 ± 0 |
All the data represents mean ± standard deviation unless otherwise indicated. Abbreviations: ADC, Apparent Diffusion Coefficeint; CBV, Cerebral Blood Volume; Cho, Choline; NAA, N-acetylaspartate; Cr, Creatine; FA, fractional anisotropy; CE, Contrast Enhanced; DWI, diffusion-weighted imaging; MRS, MR spectroscopy
DISCUSSION
The main finding of this study was that subtraction maps, ΔT2 and ΔT1, provided better contrast in identifying regions of the gross tumor area than other parametric images/maps generated using DWI, PWI or MRSI, except for the Cho/NAA map, which provides considerable contrast in identifying IPR and DPR, and subtraction maps provided the best diagnostic classification between low and high-grade gliomas, whereas CBV provided the highest discriminative power towards segregation of grade III and IV lesions. Classification studies showed that combinational models can improve the classification accuracy in discriminating low and high-grade gliomas, but not for differentiating grade III and IV gliomas.
Tissue relative signal contrasts
This study supports previous observations of improved efficacy of T1-weighted subtraction images.2,3,5 In this study, ΔT1 maps provided a 5-fold increase in contrast between enhancing tumor regions and NAWM as compared to conventional post-contrast T1 images, and a 17% and 27% improvement over the Cho/NAA and CBV maps, respectively, which are among the highest contrasts provided by the other imaging modalities included in this study. For differentiation of enhancing tumor regions from vasogenic edema, ΔT1 maps provided a 64.7% improvement in contrast over conventional CE T1 images and a 42.3% improvement over CBV maps.
T2-weighted subtraction images were studied by Connor et al. who found improved estimation of tumor volume in low-grade gliomas as compared to conventional imaging.14 Here, ΔT2 images provided a 66.5% improvement in contrast in identifying edematous regions from NAWM as compared to conventional T2 images. Moreover, ΔT2 images offered 29.9% increase in contrast in isolating enhancing tumor regions from surrounding edema as compared to conventional CE T1 images and an approximately 15-fold increase as compared to conventional T2 images.
Correlations to tumor grade
This study found no significant association of the RSCER derived from CBV with tumor grade, in contrast to previous reports of higher maximum tumor CBV for high-grade gliomas as compared to low-grade gliomas.6,15 This finding may be attributed to the way RSC was calculated in the study, where the ratio of the mean CBV within the ROI to NAWM was used, as compared to the maximum CBV within the tumor. Other factors may be the inclusion of oligodendrogliomas in the subject selection, which has been shown to influence CBV values.7
The RSC for NAA/Cr showed significant correlation to tumor grade only for the T2 hyperintense region of the tumor, but not in the contrast-enhancing tumor. Cho/Cr and Cho/NAA maps also showed no significant correlation to tumor grade. Several MRS studies have indicated that glioma is characterized by increased Cho, with values increasing with tumor grade, combined with reduced NAA and the presence of lactate and lipid signals.16,17 Moreover, tumor Cho levels have been shown to be correlated to the cellular density of the tumor18 and the degree of tumor infiltration.19 The discrepancies in the findings may reflect the large variation in Cho/Cr ratios that have been reported and a significant overlap in gliomas of different grades.16,17
Results showed a strong correlation between RSC derived from the HR and tumor grade for ΔT2 images. A combined effect of longer T1 and T2 relaxation times in higher grade lesions20,21 may contribute to increased contrast for ΔT2 images as well as the significant correlation observed with grade for the RSC of ΔT2 images in the edematous regions.
Tumor grading
Several studies have investigated single and multiparametric imaging measures for lesion classification;7,22 however, the utility of subtraction maps towards this grading has not been explored. This study found that subtraction maps provided the highest accuracy in differentiation between low and high-grade lesions. Generally, addition of more parametric images to a classification model improves classification accuracy. However, as seen in Table 1, the addition of ADC to an existing SVM composed of subtraction maps results in reduction of accuracy by 5.2%, and a full combination model provided a classification accuracy of 87.3%. The reduced accuracy may be attributed to the diminished contrast seen across grades for ADC, or structural images as seen in Figure 3.
Few studies have examined differentiation between grade III and IV tumors.11,23-25 This distinction is of interest due to their different prognosis and treatment.26 This report provided accuracies of 93.7% using CBV, and 93.3% using Cho/Cr, respectively (see Table 2). ΔT1 and ΔT2 provided significant differentiation of grade III and IV tumors, which may be due to an improved ability to isolate the different tumor regions due to the increased contrast. Additionally, this study used five regional measurements as features in developing classification models, in contrast to most studies that have used the mean or maximum intensity in the tumor. This could also contribute to the increased classification accuracy seen with spectroscopy models as these models include inferences from varied regions of the gross tumor volume, which may show changes in spectroscopic measures in the absence of changes in the structural images.17
Limitations
Firstly, the study evaluated 2D ROIs, similar to that done in other studies;11,23 however, the lack of 3D ROIs may introduce a selection bias and not provide a complete representation of the tumor regions, although we note in this regard that multiple representative ROIs were used for each tissue region. Secondly, ROIs drawn in the HR or IPR may not represent true vasogenic edema or peripheral tissue respectively but may sample infiltrating tumor regions that cannot be differentiated on T1- or T2-weighted images. Moreover, IPR regions chosen based on a 1 cm distance from the ER may have significant overlap with HR regions due to their similar visualization on T1- or T2-weighted images and impact the contrast estimates.The study bases the classification of the subjects based on WHO 2007 scores and the lack of the updated WHO 2016 grading or c-IMPACT pathologic grading may be a limitation of this study. Finally, the lack of 1p19q codeletion and IDH mutation status is a shortcoming of the paper since some imaging markers have shown to be affected by altered genetic status.27
Acknowledgements and Disclosure:
This work was supported by National Institute of Health (NIH) [grants R01CA172210 and R01EB016064], and Indo-US Science & Technology Forum [award #20-2009]. The authors have no financial conflicts-of-interests to report.
References
- 1.Ellingson BM, Bendszus M, Sorensen AG, Pope WB. Emerging techniques and technologies in brain tumor imaging. Neuro Oncol 2014;16(Suppl 7):12–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellingson BM, Kim HJ, Woodworth DC, et al. Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology 2014;271:200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Downs RK, Bashir MH, Ng CK, Heidenreich JO. Quantitative contrast ratio comparison between T1 (TSE at 1.5T, FLAIR at 3T), magnetization prepared rapid gradient echo and subtraction imaging at 1.5T and 3T. Quant Imaging Med Surg 2013;3:141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardy A, Prins R, Kong X, et al. Enhancing tumor response using quantitative volumetry from Tl subtraction maps in malignant glioma treated with dendritic cell vaccination. Neuro Oncol 2014;16. [Google Scholar]
- 5.Kim HG, Heo YC, Cho JH. Study on a method to improve T1 image contrast by the subtraction technique for 3.0 T brain examination. Clin Imag 2014;38:91–5. [DOI] [PubMed] [Google Scholar]
- 6.Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 2003;24:1989–98. [PMC free article] [PubMed] [Google Scholar]
- 7.Roy B, Gupta RK, Maudsley AA, et al. Utility of multiparametric 3T MRI for glioma characterization. Neuroradiology 2013;55:603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh A, Haris M, Rathore D, et al. Quantification of physiological and hemodynamic indices using T(1) dynamic contrast-enhanced MRI in intracranial mass lesions. J Magn Reson Imaging 2007;26:871–80. [DOI] [PubMed] [Google Scholar]
- 9.Maudsley AA, Domenig C, Govind V, et al. Mapping of brain metabolite distributions by volumetric proton MR spectroscopic imaging (MRSI). Magn Reson Med 2009;61:548–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Studholme C, Hill DLG, Hawkes DJ. An overlap invariant entropy measure of 3D medical image alignment. Pattern Recogn 1999;32:71–86. [Google Scholar]
- 11.Ellingson BM, Zaw T, Cloughesy TF, et al. Comparison between intensity normalization techniques for dynamic susceptibility contrast (DSC)-MRI estimates of cerebral blood volume (CBV) in human gliomas. J Magn Reson Imaging 2012;35:1472–7. [DOI] [PubMed] [Google Scholar]
- 12.Benjamini Y, Hochberg Y. Controlling the false discovery rate - A practical and powerful approach to multiple testing. J Roy Stat Soc B Met 1995;57:289–300. [Google Scholar]
- 13.Zhou Q, Goryawala M, Cabrerizo M, et al. An optimal decisional space for the classification of Alzheimer's disease and mild cognitive impairment. IEEE Trans Biomed Eng 2014;61:2245–53. [DOI] [PubMed] [Google Scholar]
- 14.Connor SE, Gunny R, Hampton T, O'Gorman R. Magnetic resonance image registration and subtraction in the assessment of minor changes in low grade glioma volume. Eur Radiol 2004;14:2061–6. [DOI] [PubMed] [Google Scholar]
- 15.Sugahara T, Korogi Y, Kochi M, et al. Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am J Roentgenol 1998;171:1479–86. [DOI] [PubMed] [Google Scholar]
- 16.Murphy M, Loosemore A, Clifton AG, et al. The contribution of proton magnetic resonance spectroscopy (1HMRS) to clinical brain tumour diagnosis. Br J Neurosurg 2002;16:329–34. [DOI] [PubMed] [Google Scholar]
- 17.Maudsley AA, Roy B, Gupta RK, et al. Association of metabolite concentrations and water diffusivity in normal appearing brain tissue with glioma grade. J Neuroimaging 2014;24:585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller BL, Chang L, Booth R, et al. In vivo 1H MRS choline: correlation with in vitro chemistry/histology. Life Sci 1996;58:1929–35. [DOI] [PubMed] [Google Scholar]
- 19.Croteau D, Scarpace L, Hearshen D, et al. Correlation between magnetic resonance spectroscopy imaging and image-guided biopsies: Semiquantitative and qualitative histopathological analyses of patients with untreated glioma. Neurosurgery 2001;49:823–9. [DOI] [PubMed] [Google Scholar]
- 20.Bodsch W, Rommel T, Ophoff BG, Menzel J. Factors responsible for the retention of fluid in human-tumor edema and the effect of dexamethasone. J Neurosurg 1987;67:250–7. [DOI] [PubMed] [Google Scholar]
- 21.Oh J, Cha S, Aiken AH, et al. Quantitative apparent diffusion coefficients and T2 relaxation times in characterizing contrast enhancing brain tumors and regions of peritumoral edema. J Magn Reson Imaging 2005;21:701–8. [DOI] [PubMed] [Google Scholar]
- 22.Fink JR, Carr RB, Matsusue E, et al. Comparison of 3 Tesla proton MR spectroscopy, MR perfusion and MR diffusion for distinguishing glioma recurrence from posttreatment effects. J Magn Reson Imaging 2012;35:56–63. [DOI] [PubMed] [Google Scholar]
- 23.Caulo M, Panara V, Tortora D, et al. Data-driven grading of brain gliomas: a multiparametric MR imaging study. Radiology 2014;272:494–503. [DOI] [PubMed] [Google Scholar]
- 24.Fudaba H, Shimomura T, Abe T, et al. Comparison of multiple parameters obtained on 3T pulsed arterial spin-labeling, diffusion tensor imaging, and MRS and the Ki-67 labeling index in evaluating glioma grading. AJNR Am J Neuroradiol 2014;35:2091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weber MA, Zoubaa S, Schlieter M, et al. Diagnostic performance of spectroscopic and perfusion MRI for distinction of brain tumors. Neurology 2006;66:1899–906. [DOI] [PubMed] [Google Scholar]
- 26.Stupp R, Tonn JC, Brada M, Pentheroudakis G, Group EGW. High-grade malignant glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21(Suppl 5):190–3. [DOI] [PubMed] [Google Scholar]
- 27.Goryawala M, Saraf-Lavi E, Nagornaya N, et al. The association between whole-brain MR spectroscopy and IDH mutation status in gliomas. J Neuroimaging 2020;30:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]