Abstract
C─H functionalization reactions are playing an increasing role in the preparation and modification of complex organic molecules, including pharmaceuticals, agrochemicals, and polymer precursors. Radical C─H functionalization reactions, initiated by hydrogen-atom transfer (HAT) and proceeding via open-shell radical intermediates, have been expanding rapidly in recent years. These methods introduce strategic opportunities to functionalize C(sp3)─H bonds. Examples include synthetically useful advances in radical-chain reactivity and biomimetic radical-rebound reactions. A growing number of reactions, however, proceed via "radical relay" whereby HAT generates a diffusible radical that is functionalized by a separate reagent or catalyst. The latter methods provide the basis for versatile C─H cross-coupling methods with diverse partners. In the present review, highlights of recent radical-chain and radical-rebound methods provide context for a survey of emerging radical-relay methods, which greatly expand the scope and utility of intermolecular C(sp3)─H functionalization and cross coupling.
Graphical Abstract

Radical reaction pathways have evolved sufficiently that C(sp3)─H bonds may now be viewed as strategic "reagents" for bond formation in synthetic organic chemistry. This Review highlights the diverse approaches for C(sp3)–H functionalization and cross-coupling reactions, emphasizing radical-relay reactions.
Introduction
Efficient synthesis of organic molecules is crucial to drug discovery and development, materials synthesis, and many other domains. C─H functionalization methods provide a means to streamline synthetic routes by avoiding substrate preactivation, accessing target molecules in fewer steps, and providing efficient strategies to diversify existing chemical structures, including modification of their physicochemical properties and three-dimensional structures. Multiple mechanistic pathways are available for C(sp3)─H functionalization, including those initiated by organometallic C─H activation1-4; atom-transfer methods5-7, such as carbene and nitrene C─H insertion; and those initiated by hydrogen-atom transfer (HAT) to generate radical intermediates4,8-10. The synthetic utility of radical C─H functionalization reactions was historically viewed with skepticism11, owing to the challenges in controlling reaction selectivity; however, recent advances have changed this perception. Radical pathways are featured in a growing number of synthetically useful methods that enable site-selective functionalization of C(sp3)─H bonds. C─H functionalization reactions that feature a one-for-one replacement of a hydrogen atom with another functional group, such as a halogen, pseudohalogen, or oxygen atom, have been complemented by C─H cross-coupling reactions that permit efficient access to dozens, if not hundreds, of derivatives via reaction of the C─H substrate with broad classes of reaction partners, such as aryl halides, boronic acids, alcohols, and amine derivatives. The latter reactions represent an important class of reactions for pharmaceutical and agrochemical discovery efforts due to their ability to enable rapid elaboration of simple building blocks, core structures of moderate complexity, and late-stage structures and bioactive compounds.
Multiple mechanisms are available for radical C─H functionalization reactions. Radical-chain mechanisms (FIG. 1Aa) are involved in a number of large-scale industrial processes, including chlorination of methane and autoxidation of hydrocarbons, such as cyclohexane and cumene12,13. These reactions feature three general steps: initiation, propagation, and termination. The initiation step generates new radical species, typically by thermal or photochemical cleavage of a weak bond in dihalogens (e.g., Cl2 and Br2), peroxides, or specialized reagents, such as such as AIBN (2,2'-azobis(2-methylpropionitrile). Propagation steps account for net transformation of the starting material into the reaction product(s). These steps regenerate the radical carrier without net consumption of radical species. The chain process ends in termination steps that consume radicals, such as the direct coupling of two radicals. Recent advances have highlighted the utility of radical-chain reactions for selective functionalization of more complex molecules, benefiting from the development of new reagents that lead to improved site selectivity in the HAT propagation step14-17.
Fig. 1 ∣. Mechanisms and components of radical-chain, radical-rebound, and radical-relay reactions.
Aa ∣ General mechanism of radical-chain reaction. Ab ∣ Representative initiators/reagents for radical-chain reactions. Ba ∣ General catalytic cycle of radical-rebound reactions. Bb ∣ Representative iron and manganese catalysts for radical-rebound reactions. Ca ∣ General mechanism of radical-relay reactions involving radical trapping reagents. Cb ∣ General mechanism of catalytic radical-relay reactions. Cc ∣ Representative oxidants and HAT reagents for radical-relay reactions. Cd ∣ Representative radical trapping reagents. Ce ∣ Representative catalytic radical-relay coupling partners. [O] = O2/2e−, H2O2, or other O-atom transfer reagent. NFSI, N-fluorobenzenesulfonimide; Selectfluor I (R = CH2Cl), 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate); Selectfluor II (R = CH3), 1-fluoro-4-methyl-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate); TMP, 5,10,15,20-tetramesitylporphyrin; TPFPP, 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin; PDP, N,N′-bis(2-pyridylmethyl)-2,2′-bipyrrolidine; HAT, hydrogen atom transfer; ET, electron transfer; TBPB, tert-butyl peroxybenzoate; DTBP, di-tert-butyl peroxide; NHPI, N-hydroxyphthalimide; B2cat2, bis(catecholato)diboron.
The radical-rebound mechanism (FIG. 1Ba) is closely associated with iron-containing heme enzymes such as cytochrome P450 (CYP) and related non-heme iron enzymes in biology18. This collection of biological and synthetic reactions involves high-valent metal-oxo species generated via reaction of a reduced metal complex with oxygen-atom donors, including O2, H2O2, alkyl or acyl hydroperoxides, and PhI=O. HAT from a C(sp3)─H bond to the reactive metal-oxo is followed by rapid recombination (i.e., rebound) of the resulting carbon-centered radical with the incipient metal-hydroxide to afford the oxygenated product. Variations of this reaction pathway can lead to other products if the organic radical recombines with a different ligand within the metal coordination sphere. For example, C─H halogenation products can arise from HAT by a metal oxo, followed by reaction of the organic radical with a halide ligand rather than the hydroxide ligand. Analogous C─H amination reactivity is possible with metal-nitrene intermediates derived from the reaction of a reduced metal complex with nitrogen-atom donors, including organic azides19 and PhI=NR reagents20. Recent advances include the discovery of new catalysts that promote radical-rebound reactions with complex molecules.
Radical-relay reactions (FIG. 1C) complement radical-chain and radical-rebound reactions and significantly expand the scope of accessible synthetic transformations. An HAT step generates a diffusible organic radical, which is then functionalized by a second reagent or catalyst. In some cases, the radical undergoes direct coupling with a substrate partner or radical trapping reagent (FIG. 1Ca). In other cases, the radical undergoes metal-catalyzed coupling, reacting by one of several possible mechanisms (FIG. 1Cb). It can undergo radical-polar crossover, in which electron-transfer (ET) generates a carbocation that reacts readily with diverse nucleophiles. Alternatively, the carbon-centered radical can react by one of two possible pathways with a transition-metal catalyst containing NiII, CuII, or another metal ion: (a) direct addition of the radical to a coordinated ligand on the transition-metal complex, or (b) radical addition to the metal center and subsequent reductive elimination with the coordinated coupling partner. Each of these pathways support C─H cross-coupling reactions that generate new carbon-carbon and carbon-heteroatom bonds via reaction with diverse reaction partners. Recent advances have introduced new methods to access HAT reagents from chemical oxidants and photoactive reagents, in addition to new catalyst systems that support diverse product formation.
The intermolecular C(sp3)─H functionalization and cross-coupling methods represented by these reaction classes provide valuable strategies to diversify organic molecules ranging from simple building blocks to complex molecules21-23. Intramolecular reactions, such as Hoffmann-Loeffler-Freitag and related reactions24-26, feature closely related mechanisms but are outside the synthetic scope of this review. The content herein begins with a survey of new radical-chain and radical-rebound reactions, followed by a presentation of radical-relay reactions that use C─H bonds as latent nucleophiles in carbon─carbon and carbon–heteroatom bond-forming reactions. Cross-coupling methods include those that feature direct C─H cross coupling, in addition to those that proceed via stepwise C─H functionalization/diversification sequences. Mechanistic features of these reactions are presented, emphasizing those with implications for the synthetic scope of the reactions.
Radical-chain and radical-rebound reactions
Radical chain.
Conventional radical-chain reactions involving Cl2 and Br2 often lack the selectivity required to support synthetically useful, site-selective C─H functionalization of complex molecules. Over the past decade, however, a number of N─X (X = F, Cl) reagents have been shown to promote radical-chain C─H fluorination and chlorination with improved yields and selectivity (FIG. 2Aa). N-Fluorobenzenesulfonimide (NSFI) and Selectfluor reagents are featured in a number of C(sp3)─H fluorination methods, and they have been paired with various initiators, including azobisisobutyronitrile (AIBN)27, BEt3/O228, and copper(I)29 (FIG. 2Ab, condition 1). Reactions using Fe(acac)2/Selectfluor30 (acac = acetylacetonate) and CuOAc/NFSI31 support selective fluorination of benzylic C─H bonds and the reactivity is consistent with a radical-chain pathway. A multicomponent Cu(bis-imine)/Selectfluor/N-hydroxyphthalimide (NHPI) catalyst system with KB(C6F5)4 as an anionic phase-transfer catalyst supports fluorination of stronger aliphatic C─H bonds32. N─F reagents have also been paired with photocatalysts, including diarylketone33 and tetrabutylammonium decatungstate (TBADT)27,34. These reagents are capable of promoting HAT from C─H substrates (FIG. 2Ab, condition 2 and 3), and the resulting carbon-centered radicals can react with fluorinating reagents to afford the desired C─F bonds. It is possible that HAT could proceed via both radical-chain and photocatalyst-promoted steps in these reactions. Fluorination of benzylic C(sp3)─H bonds has been achieved using 1,2,4,5-tetracyanobenzene, although this photocatalyst is proposed to initiate an electron transfer pathway35. Collectively, these methods provide effective routes to monofluorinated benzylic and aliphatic C(sp3)─H products. The reactions show good selectivity for benzylic C─H bonds in the photocatalyzed (e.g., diaryl ketone, TBADT) and metal-initiated radical chains. Difluorination can be achieved under more forcing condition and by employing additional Selectfluor and diaryl ketone as photocatalyst33.
Fig. 2 ∣. Late-stage C(sp3)─H functionalization reactions via radical chain and radical rebound.
Aa ∣ General mechanism for radical-chain reaction with a N─X reagent. Ab ∣ Fluorination reactions of C(sp3)─H bonds. Ac ∣ Site-selective aliphatic C─H halogenation and xanthylation reactions with N─X reagents. Ad ∣ Benzylic C(sp3)─H hydroxylation reaction. B ∣ Iron-catalyzed C─H oxidation reaction. Ca ∣ Manganese-non-heme catalyzed chiral desymmetrization of cyclohexane. Cb ∣ Manganese-catalyzed benzylic amination reaction. Cc∣ Manganese-porphyrin or -salen catalyzed C─H functionalization reactions. HFIP, hexafluoroisopropanol; PDP, N,N′-bis(2-pyridylmethyl)-2,2′-bipyrrolidine; TIPSmcp, N,N′-dimethyl N,N′-bis(2-(5-triisopropylsilylpyridyl)methyl)-1,2-trans-diaminocyclohexane; Pc, phthalocyanine; TMP, 5,10,15,20-tetramesitylporphyrin; TMS, trimethylsilyl.
Complementary reagents have been developed to support selective radical-chain chlorination. A sterically encumbered N-chloroamide reagent supports chlorination of aliphatic C(sp3)─H substrates under visible-light irradiation, taking advantage of difference in electronics and sterics of various C─H bonds to achieve improved selectivity relative to conventional chlorination methods (FIG. 2Ac, X = Cl)14. Steric modulation of aryl ring of the N-chloroamide reagent, by shifting two trifluoromethyl groups from meta to ortho positions with respect to the carbonyl group, alters regioselectivity in the chlorination of aliphatic C─H bonds36. A sterically hindered, 2,2,6,6-tetramethylpiperidinium N─Cl reagent (cf. FIG. 1Ab) is an effective chlorinating reagent and chain carrier for photoinitiated C─H chlorination37. The electrophilicity and steric hindrance of the aminium radical confers excellent selectivity in reactions with less sterically hindered methylene C─H sites and with weaker tertiary C(sp3)─H bonds. Radical-chain chlorination of primary or secondary benzylic C─H bonds with N-chlorosuccinimide (NCS) has been initiated by reductive activation of NCS with an acridinium-based photocatalyst to generate a nitrogen-centered radical38. In addition to N–Cl reagents, tert-butyl hypochlorite (tBuOCl) has been used with a phenanthroline (phen)-ligated silver catalyst to chlorinate C(sp3)─H bonds39. The reaction has been proposed to proceed via silver-mediated C─Cl bond formation; however, a radical-chain pathway could also account for the observed reactivity. In the latter pathway, a carbon-centered radical generated by HAT could react via chain propagation with tBuOCl.
Radical-chain reactions have been developed for selective conversion of C(sp3)─H bonds into C─Br, C─S, and C─O bonds. Variants of the N-chloroamide reagent in FIG 2Ac in which N─Cl is replaced with N─Br or N─xanthyl (xanthyl = −S(C=S)OEt) supports C─H bromination15 and xanthylation16, respectively. Xanthylated products are versatile intermediates in two-step C─H functionalization/diversification reactions in which the xanthyl group may be displaced in a second step by other groups, including allyl, vinyl, CF3, D, NR2, N3, OH, and SR (FIG. 2Ac)16,40. Bis(methanesulfonyl) peroxide supports radical-chain C─H oxygenation of benzylic C(sp3)─H bonds in the presence of a copper(I) initiator, and the resulting benzyl mesylates undergo facile displacement by water in the presence of hexafluoroisopropanol (HFIP) to afford benzyl alcohols (FIG. 2Ad).17
Collectively, these synthetic methods and reagents highlight the emergence of numerous synthetically useful radical-chain reactions. Sterically and electronically tailored N─X and O─X reagents (X = heteroatom substituent) have begun to overcome historical limitations of radical-chain reactions involving simple Cl2, Br2, and related reagents. Moreover, recent applications of radical-chain reactivity have demonstrated the ability to employ C─H substrates as the limiting reagent and exhibit good site selectivity and compatibility with functional groups that are sensitive to polar reagents.
Radical rebound.
Radical-rebound C(sp3)─H functionalization takes inspiration from heme and non-heme iron oxygenase enzymes18,41. Early studies of model complexes for non-heme iron enzymes investigated reactions with hydrogen peroxide as the oxidant and provided clear evidence for generation of high-valent iron-oxo species42-46. For example, stereospecificity observed in the hydroxylation of alkanes supports rapid rebound of the organic radical with an intermediate Fe─OH species generated via HAT (cf. FIG 1Ba)46-47,48. These and related studies established a foundation for more recent synthetic applications of this reactivity using similar catalyst systems. Nitrogen-ligated iron catalysts have been developed for stereo- and site-selective oxidation of aliphatic C─H bonds in molecules containing multiple secondary and tertiary C─H bonds49. The C─H substrate is used as the limiting reagent in these reactions, distinguishing these reactions from the earlier studies, which used excess hydrocarbon substrate with limiting oxidant. Electronic, steric, and stereoelectronic factors contribute to site selectivity,50 with reactivity favored at electron-rich and less hindered sites, in addition to sites that relieve strain in the HAT step51,52. These catalyst systems have been implemented for hydroxylation of structurally complex molecules49,52-54, and modification of the ligand structure allows for enhanced and/or altered site-selectivity (FIG. 2B). Manganese catalysts analogous to the Fe-based catalyst systems have also been employed in synthetically important radical-rebound oxygenation reactions55-57. Enantioselective oxidation of methylene C─H bonds has been achieved by using a chiral tetradentate nitrogen ligand to support enantioselective HAT (FIG. 2Ca)58. Carboxylic acid additives contribute significantly to the reaction outcome by promoting O─O bond cleavage of the hydroperoxide intermediate to afford the reactive metal-oxo species and by replacing a metal-bound hydroxide with a carboxylate ligand that influences both site-selectivity and enantioselectivity in C─H oxidation reactions49,51-54,58-61.
Various manganese-based catalysts show that the radical-rebound pathway can provide the basis for aliphatic C(sp3)─H functionalization beyond oxygenation (FIG. 2Cb). Mn-porphyrin and Mn-salen complexes support chemoselective halogenation (fluorination, chlorination)62-65 and pseudohalogenation, with azide66and isocyanate67 sources. Most of these reactions employ iodosylbenzene (PhIO) as the oxidant. The radical-rebound mechanism is not limited to oxygen-centered HAT species. A Mn-phthalocyanine catalyst supports sulfonamidation of benzylic C(sp3)─H bonds using a trichloroethoxysulfonamide-derived hypervalent iodine reagent (PhI=NTces)20 (FIG 2Cb). The reaction is highly selective for benzylic sites in the presence of other aliphatic C─H bonds and exhibits good functional group tolerance. These efforts showcase synthetically useful applications of the radical-rebound mechanism to achieve site-selective carbon-heteroatom bond forming reactions.
Radical-relay reactions involving direct coupling with a substrate partner or radical trapping reagent
The development of radical-relay reactions in recent years has significantly expanded the scope of C(sp3)─H functionalization. The term "radical relay" is used here to describe non-chain radical C─H functionalization reactions in which the HAT species does not incorporate the group or reagent that undergoes coupling with the intermediate organic radical (cf. FIG. 1). The first class of radical-relay reactions, summarized in this section, feature direct addition of an organic radical to a trapping reagent or coupling partner. The HAT species used to generate the organic radical may be generated one of several different pathways, including redox processes that lead to generation an oxyl radical (e.g., via oxidation of an O─H bond or reductive cleavage of an N─O bond); photochemical excitation of decatungstate anion or aryl ketones; photon-induced ligand-to-metal charge transfer of a transitional metal reagent, resulting in homolytic cleavage of a M─OR or M─Cl bond; reductive activation of N─F reagents, such as NFSI or Selectfluor reagents; among others.
Radical-relay C─H oxygenation reactions may be achieved by combining an HAT reagent with O2 or another oxygen-atom source as the radical trap4,68,69. These methods often exhibit improved selectivity relative to conventional autoxidation methods. A prominent example of this concept features the use of N-hydroxyphthalimide (NHPI), which generates the HAT species phthalimido-N-oxyl (PINO) in the presence of O2 and a cobalt catalyst (FIG. 3Aa)70,71. PINO-mediate HAT from weak C(sp3)─H bonds generates a carbon-centered radical that reacts rapidly with O2, ultimately affording ketones, carboxylic acids, or other oxygenation products. This reactivity was initially demonstrated with simple hydrocarbons70, but it has recently been applied to oxygenation of heterobenzylic C─H bonds in pharmaceutically relevant building blocks71. The scope of alkylated heterocycles was extended by the development of an iron, rather than cobalt, cocatalyst72. The oxygenation of methylarenes under Co/NHPI/O2 conditions typically generates benzoic acid derivatives; however, the use of an HFIP solvent system enables selective formation of benzyaldehydes. Hydrogen bonding from HFIP is proposed to polarize the carbonyl group of the aldehyde to prevent further oxidation to benzoic acid73. Decatungstate (DT) photocatalysts serve as an effective HAT reagent upon near-ultraviolet irradiation, and they support reactivity at C(sp3)─H bonds stronger that those typically reactive with PINO. The excited-state DT species is an electrophilic HAT reagent, and protonated amines undergo reaction at C─H bonds remote from the electron-withdrawing ammonium group, forming ketones in the presence of H2O2 (FIG. 3Ab)74. TBADT has been used as an HAT photocatalyst to support oxygenation of (hetero)benzylic and aliphatic C─H bonds in the presence of O2 under batch75 and continuous flow76 conditions.
Fig. 3 ∣. Radical relay involving radical addition to trapping reagents.
Aa ∣ Oxygenation with Co/NHPI cocatalysts. Ab ∣ Oxygenation with decatungstate photocatalysts. Ba/b ∣ PINOylation methods. Ca ∣ Azidation/cyanation via radical trapping with sulfonyl–X reagents (X = N3, CN). Cb ∣ Thiol coupling via radical trapping with sulfonylthiolates. Da ∣ Amination of heterobenzylic C(sp3)─H bonds with DEAD. Db ∣ Amination initiated by LMCT with CeIV reagents. Ea ∣ Giese-type alkylation with decatungstate photocatalysts. Eb ∣ Polarity reversal HAT/Giese-type alkylation. Fa ∣ Minisci reaction with decatungstate photocatalyst. Fb ∣ Photochemical Minisci-type pyridylation. G ∣ Photochemical borylation. NHPI, N-hydroxyphthalimide; DEAD, diethyl azodicarboxylate; LMCT, ligand-to-metal charge transfer; TFA, trifluoroacetic acid; (TRIPS)2, bis(2,4,6-triisopropylphenyl) disulfide; PFBI–OH, hydroxyl perfluorobenziodoxole; HFIP, hexafluoroisopropanol; B2cat2, bis(catecholato)diboron; ClB(cat), B-chlorocatecholborane; DT, decatungstate.
PINO is a meta-stable radical, and, in addition to serve as an HAT reagent, it can trap carbon-centered radicals to afford PINOylated products (FIG. 3B). This reactivity has been achieved by combining catalytic copper(I) chloride, (diacetoxyiodo)benzene (PhI(OAc)2), and NHPI with the C─H substrate (FIG. 3Ba)77. Similar reactivity has been achieved with a reaction system comprising catalytic copper(II) acetate, Selectfluor, and NHPI (FIG. 3Bb)78. The mechanism of these reactions is not well understood, but reactive HAT species could be generated by oxidation of NHPI or reductive activation of the stoichiometric oxidant, and the Cu catalyst could contribute to each of these processes. Ultimately, the PINOylation product may be formed by coupling of the carbon-centered radical derived from HAT with PINO.
Sulfonyl–X reagents (X = N3, CN, SR, SCF3, SePh, Cl, CH=CHSO2Ph, and C≡Ar) react with carbon-centered radicals to afford C─X bonds, and they are employed in a number of radical-relay C(sp3)─H functionalization reactions. Various methods may be used to generate the radical via HAT from an C(sp3)─H bond. Azidation of aliphatic C─H bonds can proceed under metal-free conditions, using potassium persulfate as the HAT precursor with an arylsulfonyl azide trapping reagent (FIG. 3Ca)79. Photoexcited decatungstate has been used to promote HAT in the presence of tosyl cyanide to support C─H cyanation (FIG. 3Ca, bottom)80. DT-mediated HAT has also been used other radical traps, including sulfur dioxide81, (trifluoromethylthio)phthalimide82, and other sulfonyl–X reagents (e.g., methanesulfonyl alkynes83) to achieve C─H functionalization. Reductive activation of the N─F reagent N-(tert-butyl)-N-fluoro-3,5-bis(trifluoromethyl)benzene-sulfonamide by copper(I) has been used to generate a nitrogen-centered radical capable of promoting HAT in the presence of PhSO2–X reagents (X = SCF3, SePh, C≡CPh, CN, N3, Cl, CH=CHSO2Ph). This reactivity was most extensively demonstrated for thiolation of C(sp3)─H bonds (FIG. 3Cb)84. This chemistry is closely related to Cu/NFSI-based C─H functionalization methods elaborated below, with the distinction that the carbon-centered radical intermediate directly adds to the sulfonyl–X reagent rather than undergoing Cu-catalyzed functionalization (see FIG. 1Ca/b and content below for additional context).
Dialkyl azodicarboxylate reagents react as radical traps to support amination of C(sp3)─H bonds. A copper(II)/organic HAT catalyst system using diethyl azodicarboxylate (DEAD) has been used to achieve site selective amination of heterobenzylic C(sp3)─H bonds (FIG. 3Da)85. Copper(II) acts as a Lewis acid catalyst to enhance the reactivity of heterobenzylic positions, leading to high selectivity in presence of weaker benzylic C─H bonds. Photoinduced ligand-to-metal charge transfer (LMCT) in cerium(IV)–X reagents has been use to generate reactive heteroatom-based radicals that promote HAT from C(sp3)─H bonds. The initial reports proposed formation of an oxygen-centered radical via LMCT within a cerium(IV)-alkoxide complex86,87; however, a subsequent study implicated the formation of chlorine radical via LMCT involving a CeIV─Cl fragment88. In both proposed mechanisms, the heteratom radical promotes HAT, and the resulting carbon-centered radical adds to the dialkyl azodicarboxylate reagent to form a C─N bond. This steps leads to formation of an adjacent nitrogen-centered radical that is proposed to be reduced by cerium(III) and undergo protonation to afford the final product (FIG. 3Db)87. The N─N bond is subjected to hydrogenolysis over Raney Ni to afford the primary amine derivative. DT photocatalysis has also been used to support HAT from C─H bonds in the presence of dialkyl azodicarboxylate to afford the C─N coupling products89,90. A photoreactor with high intensity LED light has been used to achieve this reactivity under flow conditions91.
Electron-deficient alkenes can serve as radical traps that provide the basis for C(sp3)─H alkylation. The addition to carbon-centered radicals to alkenes ("Giese reactions"92-94) generates a new carbon-centered radical that can react with a hydrogen-atom donor (e.g., a thiol or other reagnet) or undergo one-electron reduction and protonation to afford the alkylate product. Light-promoted LMCT from copper(II)95, cerium(IV)86,87, and iron(III)96 reagents and DT photocatalysts97-100 have been used to generate reactive species that support HAT from the C─H substrate. A DT-catalyzed Giese-type radical addition reaction was implemented in continuous flow to forge new C(sp3)─C(sp3) bonds between light alkanes, including methane, ethane, propane, and isobutane, and electron-deficient alkenes (FIG. 3Ea)101. HAT reagents typically consist of electrophilic radicals that favor reaction at electron-rich C─H bonds (e.g., 3° aliphatic and benzylic C─H bonds); however, polarity-reversed HAT102 from acidic C─H bonds adjacent to electron-withdrawing groups was enabled by using an amine-borane catalyst under photochemical conditions (FIG. 3Eb)103. The organic radical subsequently adds to an unactivated alkene followed by abstracting a hydrogen atom from a thiol-based H-atom donor to afford the C(sp3)─C(sp3) coupling product.
Electron-deficient arenes and heterocycles, such as pyridinium groups, also react readily with nucleophilic radicals ("Minisci reactions"104,105). This reactivity may be used to support (hetero)arylation of C(sp3)─H bonds by generating carbon-centered radicals via HAT. Representative HAT reagents that have been used in Minisci-type coupling reactions include oxygen-and nitrogen-centered radicals derived from peroxides/alcohols and amides106-109, DT photocatalysts110-113, azide radical114, and hypervalent iodine114. For example, a ruthenium photoredox catalyst can undergo SET with hydroxyl perfluorobenziodoxole (PFBI–OH) to generate an oxygen-centered radical114. HAT generates a carbon-centered radical that adds to a protonated N-heteroarene, and subsequent loss of an electron and proton from the adduct affords the Minisci coupling product (FIG. 3Fa). In another example, anthraquinone proved superior to TBADT and benzoquinone as a photocatalyst/HAT reagent in the direct coupling of alkanes with N-aminopyridinium salts115. The radical adds to the electron-deficient heterocycle, similar to Minisci reactions, with C4 selectivity (FIG. 3Fb). The reaction employs 5 equiv of alkane, aldehyde, and other coupling partners (e.g., P─H, Si-H substrates).
A primary-selective C─H borylation is initiated by photoinduced electron transfer upon irradiating N-(trifluoroethoxy)phthalimide in the presence of bis(catecholato)diboron (B2cat2), 10 equiv of the C─H substrate, and 20 mol% B-chlorocatecholborane [ClB(cat)] (FIG. 3G)116. Mesolytic cleavage of the N─O bond affords a trifluoroethoxy radical that is proposed to react with ClB(cat) to generate the reactive HAT species. The lack of a photoredox catalyst supports radical, rather than radical-polar crossover, reactivity. Carbon-boron bond formation arises from reaction of the intermediate organic radical with B2cat2. High primary C─H selectivity, even in the presence of weaker secondary tertiary C─H bonds, is rationalized by the involvement of a chlorine radical–boron ‘ate’ complex that selectively cleaves sterically unhindered C─H bonds.
Kharasch-Sosnovsky reactions: Early examples of catalytic radical relay
The Kharasch-Sosnovsky (K-S) allylic oxidation reaction, first reported in 1958, represents a seminal precedent for catalytic radical-relay reactivity (FIG. 1Cb)117,118. The original transformation used copper(I) bromide and tert-butyl peroxybenzoate (TBPB) to achieve allylic oxidation of cyclohexene, 1-hexene, and 1-octene, affording the allylic benzoate derivatives (FIG. 4a). The proposed mechanism involves CuI-mediated activation of the peroxide, allylic HAT by tBuO• and coupling of the allylic radical with CuII-benzoate (FIG. 4b)119. Asymmetric C─O coupling, which was initially achieved by using a catalytic amount of L-proline as a chiral ligand (40% yield, 30% ee)120, implicates inner-sphere coupling of the C─O bond via radical addition to CuII and C─O reductive elimination (FIG. 4c). Improved enantioselectivity was achieved by using chiral bis- or trisoxazoline ligands (FIG. 4e)121_124. Most K-S reactions use excess C─H substrate relative to the peroxide reagent. Low temperatures (e.g., −20 °C) are needed to enhance enantioselectivity, but these conditions often result in multiple-day reaction times. K-S oxidation methods have been used in synthetic chemistry, for example, to access triterpene natural products (FIG. 4d)125,126. These reactions and later oxidation of the sesquiterpene valencene127,128 are among the few examples of K-S reactions that use the C─H substrate as the limiting reagent (FIG. 4j). Adaptation of K-S-type reactivity has led to oxidation of aliphatic C─H bonds. For example, di-tert-butyl peroxide (DTBP) has been used as an oxidant in combination with nitrogen-ligated Cu complexes to achieve tert-butyletherification of cyclohexane129 and dehydrogenative coupling of carboxylic acids with cyclohexane130. These reactions use a large excess of the C─H substrate (≥ 10 equiv).
Fig. 4 ∣. Timeline of representative Kharasch-Sosnovsky (K-S)-type reactions.
a ∣ Original Kharasch-Sosnovsky reaction. b ∣ Kharasch-Sosnovsky reaction mechanism. c ∣ First asymmetric allylic oxidation. d ∣ Synthesis of triterpenes. e ∣ Examples of asymmetric allylic oxidation. f ∣ Cross-coupling reaction of benzylic C─H bonds and 1,3-dicarbonyl compounds. g ∣ An alternative HAT reagent. h ∣ C─N bond formation reaction. i ∣ Mechanistic insights for C─N bond formation. j ∣ Allylic oxidation with limiting C─H substrate. k ∣ Cross coupling of benzylic C─H bonds and arylboronic esters. l ∣ Cross coupling of C(sp3)─H and C(sp2)─H bonds. TBS, tert-butyldimethylsilyl; BPhen, bathophenanthroline; N~N, β-diketiminate ligand: 2,4-bis-(2,6-dichlorophenylimino)pentyl; Ad, 1-adamantyl; Phen, phenanthroline; phd, 1,10-phenanthroline-5,6-dione.
K-S-type reactions may be used to achieve C─N bond formation, for example, with amide, sulfonamide, and imide coupling partners (FIG. 4g-i)118,131-136. The reactions commonly use tert-butyl acylperoxides (i.e., derivatives of TBPB) or DTBP as the oxidant. For example, TBPB derivatives with electron-withdrawing groups on the benzoyl group (3-Cl, 3-CF3) achieve C─N coupling between primary and secondary sulfonamides and simple methylarenes (25 equiv relative to the nitrogen nucleophile) (FIG. 4h)133. A well-defined diketiminate-ligated Cu complex was used in combination with DTBP to support amination of a small number of aliphatic C─H substrates (indane, ethylbenzene, cyclohexane) with 1-adamantylamine and other aliphatic amines (FIG. 4g)134. The amine ligand undergoes exchange with a [Cu]─OtBu intermediate to form a copper(II)-amido complex, and mechanistic studies indicate that this species can promote direct HAT from the C─H substrate. This reactivity differs from most K-S-type reactions, which feature HAT by an alkoxyl radical. Amination of cyclic and acyclic aliphatic C─H bonds proceeds with various amides, sulfonamides, and phthalimide using a 4,7-dimethoxyphenantronline/CuI catalyst with DTBP as the oxidant (FIG. 4i)135. A variant of the conventional K-S mechanism was proposed, in which a CuI-amidate activates the peroxide to generate the tBuO•, which promotes HAT from the C─H substrate135. The resulting alkyl radical reacts with a CuII-amidate species to afford the C─N bond. A photochemical copper(II)-catalyzed method initiates O─O bond homolysis of DTBP at room temperature, supporting C─N coupling of alkanes with various nitrogen nucleophiles under mild conditions136. As with the other K-S-type reactions involving aliphatic substrates, a large excess of the C─H substrate (≥ 10 equiv) is used in these reactions.
K-S reactivity may be used to support C─C coupling reactions with various carbon nucleophiles, including 1,3-dicarbonyl compounds, arylboronic esters, and fluorinated arenes. In the reaction of benzylic C─H substrates with TBPB as the oxidant, C─C coupling with 1,3-dicarbonyl compounds proceeds via sequential formation of a benzylic benzoate, followed by nucleophilic displacement of the benzoate (FIG. 4f)137. Redox-neutral alkyenylation of C─H bonds adjacent to oxygen in THF (and related cyclic ether solvents) exhibits feature reminiscent of K-S reactions, employing terminal alkynes as coupling partners together with tBuOOH and catalytic CuICl under blue LED irradiation138. Arylation of benzylic C─H bonds with arylboronic esters proceeds with DTBP as the oxidant (FIG. 4k)139. The reaction is proposed to involve transmetalation between the arylboronic ester and a CuII–OtBu intermediate to afford an arylcopper(II) intermediate, which undergoes coupling with a benzylic radical generated by HAT from tBuO•. This reaction affords a variety of diarylmethane and diarylalkane products. β-Diketiminate copper catalysts support direct C─H/C─H coupling of polyfluoroarenes and allylic, benzylic, and aliphatic C─H substrates using DTBP as the oxidant (FIG. 4l)140. Each of these reactions employs the C(sp3)─H substrate in excess (e.g., 10 equiv) relative to the coupling partner; however, they have synthetic appeal beyond the related C─O and C─N coupling reactions because the more valuable coupling partner (alkyne, arylboronic acid, fluoroarene) is used as the limiting reagent. Thus, inexpensive cyclic ethers and alkylarenes may be used to support alkylation of valuable coupling partners.
Copper/NFSI-catalyzed radical-relay reactions
The need for excess C─H substrate restricts the synthetic utility of K-S and related oxidative coupling reactions; however, this limitation has been overcome by using NFSI, rather than peroxide-based oxidants, in combination with copper catalysts. These methods show very high selectivity for benzylic C(sp3)─H bonds relative to other positions (e.g., 3° C─H bonds), and they are among the most versatile methods for site-selective C─H functionalization and cross-coupling, leading to the formation of new C─X (X = halide, psueudohalide), C─N, C─O, C─S, and C─C bonds (FIG. 5).
Fig. 5 ∣. Summary of Cu/NFSI-catalyzed radical-relay functionalization, functionalization/diversification, and cross-coupling reactions of benzylic C(sp3)─H bonds.
a ∣ Sulfonimidation. b ∣ Enantioselective cyanation. c ∣ Trifluoromethylation. d ∣ Azidation reaction. e ∣ Amination methods with amides and ammonia surrogates. f ∣ Thiocyanation. g ∣ Isocyanation/amine coupling sequence. h ∣ Fluorination/diversification. i ∣ Chlorination/diversification. j ∣ Cross coupling of benzylic C─H bonds and alcohols. k ∣ Cross coupling of benzylic C─H bonds and azoles with N-site selectivity. l ∣ Enantioselective cross coupling of benzylic C─H bonds and arylboronic acids. m ∣ Enantioselective alkynylation. NFSI, N-fluorobenzenesulfonimide; Selectfluor II, 1-fluoro-4-methyl-1,4-diazoniabicylco[2.2.2]octanebis(tetrafluoroborate); 1-Np, 1-naphthalene; TMS, trimethylsilyl; F-TEDA-PF6, 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(hexafluorophosphate); TBACl, tetrabutylammonium chloride; TMSOTf, trimethylsilyl triflate; TFA, trifluoroacetyl-protected amine.
Cu/NFSI-catalyzed C(sp3)─H functionalization and functionalization/diversification methods.
The first Cu/NFSI benzylic C─H functionalization method led to sulfonimidation of benzylic C─H bonds, using NFSI as the HAT reagent and as the coupling partner (FIG. 5a)141. This method, which is compatible with primary and secondary benzylic C─H bonds, highlighted the use of the benzylic C─H substrates as the limiting reagent. The first demonstration of Cu/NFSI reactivity for oxidative coupling of C─H substrates and a separate nucleophilic reaction partner featured cyanation of benzylic C─H bonds with TMSCN (TMS = trimethylsilyl) (FIG. 5b)142. These reactions employ a chiral bisoxazoline ligand and achieve high enantioselectivity (many with >90% ee), good functional group compatibility, and very high site selectivity for less hindered secondary benzylic C─H bonds. Density functional theory (DFT) calculations suggest enantioselective C─C bond formation arises from reversible radical addition to a chiral CuII–CN species followed by selectivity determining reductive elimination (FIG. 6a, inner sphere radical coupling).
Fig. 6 ∣. Catalytic cycle and radical functionalization mechanisms for Cu/NFSI-catalyzed radical-relay reactions.
a ∣ Inner sphere radical coupling via reductive elimination. b ∣ Radical-polar crossover pathway. c ∣ Radical addition to Cu-bound ligand. NFSI, N-fluorobenzenesulfonimide.
Benzylic C─H trifluoromethylation was demonstrated using a copper(I) source, NFSI or Selectfluor II as the oxidant, and (bpy)Zn(CF3)2 as the trifluoromethyl nucleophile (FIG. 5c)143. NFSI is generally the most effective oxidant for primary benzylic substrates, but Selectfluor II, which generates a more reactive nitrogen-centered radical, exhibits improved reactivity with electron-deficient substrates. Improved outcomes were observed with secondary benzylic substrates when using Zn(OTf)2 and Zn(OAc)2 additives, an effect attributed to more efficient transmetalation of the trifluoromethyl anion to copper(II) and higher concentration of CuII–CF3 species.
Cu/NFSI-catalyzed C─H azidation is achieved by using trimethylsilyl azide (TMSN3) as the nucleophilic coupling partner (FIG. 5d)144. The reaction exhibits very high site-selectivity for secondary benzylic C─H bonds over tertiary aliphatic and benzylic C─H bonds. This selectivity, which exceeds that observed with other radical azidation methods66, 145, 146 is rationalized by the steric and electronic properties of the sulfonimidyl N-centered radical that promotes HAT. Azide is an effective ammonia surrogate, in addition to serving as a precursor to various heterocycles (FIG. 5d). Complementary Cu/NFSI methods have been developed for coupling with other ammonia surrogates, including carbamates147, sulfonamides, and primary carboxamides (FIG. 5e)148. These and several other Cu/NFSI-catalyzed oxidative coupling reactions afford racemic products, even when using chiral bisoxazoline ligands similar to those in the cyanation reaction144. Experimental and computational data indicate the azidation reaction proceeds via a radical-polar crossover mechanism in which the benzylic radical is oxidized to a cation prior to reaction with an azide nucleophile. The different stereochemical outcomes of these reactions is likely correlated with the influence of different nucleophiles as ligands that modulate the redox properties of the CuII species, altering the fate of the radical intermediate.
TMSNCS and TMSNCO are additional effective pseudohalide coupling partners, affording benzylic thiocyanate and isocyanate derivatives (FIG. 5f and 5g)149,150. Inter- and intramolecular competition studies in the thiocyanation reaction indicate that HAT from benzylic C─H bonds favors 3° > 2° > 1° positions, differing from the azidation reaction, which favors 2° sites. These observations suggest the identity of the HAT reagent may not be identical in all reactions. Both thiocyanate and isocyanate product classes represent versatile precursors to other valuable products. The benzylic thiocyanate products may be converted in two- or three-step sequences, without isolation of the intermediates, into other products, including isothiocyanates, thioureas, trifluoromethylthio, difluoromethylthio, 5-S-benzbromarone-linked tetrazole, and disulfide derivatives. This concept was expanded for benzylic isocyanates through the development of a benzylic isocyanation/amine coupling sequence suitable for implementation in a high-throughput format, enabling efficient access to large numbers of benzylic ureas with drug-like physiochemical properties150.
The C─H functionalization/diversification strategy illustrated for azides, thiocyanates, and isocyanates in FIGS. 5d, 5f, and 5g, is even more versatile with C─H halogenation methods. C─H fluorination was demonstrated by variation of the original Cu/NFSI conditions, which led to C─H sulfonimidation (FIG. 5h)31,151. The use of a Brønsted base (Li2CO3) and MeB(OH)2 as an in situ reductant for the copper catalyst (i.e., a "redox buffer"; see mechanistic discussion below) account for the switch in selectivity31 via initiation of a CuI-promoted radical-chain pathway involving NFSI (see Radical Chain section above for further discussion and additional references). From a synthetic perspective, benzyl fluorides are not inert like many other alkyl fluorides but are quite labile. This feature may be exploited to support nucleophilic displacement of the fluoride, promoted by the presence of a hydrogen-bond donor, such as hexafluoroisopropanol (HFIP), or a Lewis acid, such as boron trifluoride diethyl etherate (BF3•Et2O)152-155. The resulting fluorination/substitution sequence enables the replacement of benzylic C─H bonds with a wide range of oxygen-, nitrogen-, and carbon-based groups151. Similar concepts have been demonstrated with a Cu/NFSI-catalyzed chlorination method (FIG. 5i), in which chlorination/substitution access products that can be difficult to access under the acidic conditions required for fluoride substitution156. For example, reactions of phenols with benzyl fluorides promoted by HFIP leads to C─C bond formation, with phenol reacting at the ortho position, while reactions with benzyl chlorides under basic conditions leads to C─O bond formation, with phenol reacting at the oxygen atom. These two-step functionalization/diversification methods, initiated by fluorination and chlorination, enable net C─H functionalization with oxidatively sensitive nucleophiles that would not be compatible with a one-step oxidative coupling method using NFSI as the oxidant. This reactivity complements an independent Cu-catalyzed chlorination approach using a dichloramine-T as the oxidant that selectively functionalizes C(sp3)─H bonds in ketones, enones, and alkylbenzenes157,158. This study also demonstrated nucleophilic displacement of secondary benzyl chlorides with N-Bocpiperazine, demonstrating another example functionalization/diversification.
Variants of NFSI have been developed in which one phenylsulfonyl group in NFSI is replaced with a tert-alkyl group and the other phenysulfonyl is replaced an electron-deficient arylsulfonyl (e.g., 4-CF3- or 3,5-(CF3)2-phenyl). These reagents have been used to achieve complementary radical-relay C─H functionalization, including regio- and enantioselective cyanation of allylic C(sp3)─H bonds with TMSCN (with limiting C─H substrate)159, and thiolation of C─H bonds with S-aryl benzenethiosulfonates (3 equiv C─H substrate) (see FIG. 3Cb)84. In the former study, DFT calculations implicate a CuII-bound nitrogen-centered radical as the reactive HAT species, and this species was proposed to account for modulation of site selectivity. This proposal aligns with the observations above that the specific HAT species could be modified under different conditions. Selectfluor is a related N─F reagent that generates an amine radical-cation, which is a stronger HAT reagent than the N-centered radical derived from NFSI. In presence of copper catalysts, Selectfluor enables HAT with aliphatic C─H substrates, used in excess (3 equiv to solvent level) relative to the coupling partner160. Esterification was achieved with various carboxylic acids as coupling partners in presence of pentanenitrile as additive160. The Ritter-type amidation product derive from pentanenitrile was obtained when triflic acid was attempted as the coupling partner (i.e., in an effort to use triflate as the nucleophile).
Cu/NFSI-catalyzed C(sp3)─H cross coupling.
Cross-coupling methods that combine reagents from diverse pools of substrates are especially important methods in synthetic chemistry, and several examples of C─H cross coupling have been achieved by using Cu/NFSI catalysis. The coupling of benzylic C─H substrates and alcohols enable access to a diverse scope of benzyl ethers (FIG. 5j)161. Medicinally relevant benzylic C─H substrates undergo cross coupled with sterically and electronically diverse aliphatic alcohols. Mechanistic studies indicate that this reactivity proceeds by a radical-polar crossover mechanism (FIG. 6b). In addition, successful reactivity required inclusion of a dialkyl phosphite additive as a sacrificial reductant to regenerate the reduced catalyst (see mechanistic discussion below). Azoles have also been used as reaction partners in Cu/NFSI-catalyzed benzylic C─H cross-coupling reactions. Pyrazoles exhibit controlled regioselectivity, with different N-site coupling observed when the reaction was conducted in the presence of a tetrabutylammonium halide (TBAX) or silyl triflate additive (FIG. 5k)162. The kinetically favored N2 site selectivity with TBACl as the additive contrasts the N1 site selectivity more commonly observed, for example, in substitution reactions with pyrazolide reagents163, 164. Various azoles and other nitrogen heterocycles are effective coupling partners.
Cu/NFSI-catalyzed methods enable carbon-carbon bond formation via benzylic C─H cross-coupling with aryl and alkynyl nucleophiles. While K-S-type oxidative coupling of benzylic substrates and aryl boronic acids with DTBP as the oxidant uses excess C─H substrate139, the use of NFSI as the oxidant enables reactions to proceed with limiting C─H substrate165. The latter reactions are effective with electron-deficient arylboronic acids and proceed at ambient temperature, rather than the high temperatures (90 °C) required for K-S-type reaction. Use of a chiral bisoxazoline ligand supports enantioselective arylation of benzylic C─H bonds of alkylnaphthalenes and related derivatives (FIG. 5l)166. Enantioselective coupling of benzylic C─H bonds and alkynyl silane derivatives is also possible, again using chiral bisoxazoline ligands (FIG. 5m)167. A modified N─F reagent, in which the two phenyl groups of NSFI were replaced with a 4-fluorophenyl and a 1-naphthyl substituent aryl, led to improvements in the yield and enantioselectivity. The alkylarene is used as the limiting reagent in combination with simple alkynyl trimethoxylsilane derivatives.
Mechanistic features of Cu/NFSI-catalyzed C─H functionalization and cross-coupling methods.
The catalytic cycle for these Cu/NFSI reactions is believed to follow a mechanism similar to that proposed for K-S reactions (FIG. 6)142. Reductive activation of NFSI by copper(I) initiates the reaction, generating a CuII–F intermediate and a nitrogen-centered radical (•NSI). The latter species promotes HAT from the C─H bond to generate a diffusible benzylic radical. Studies of a closely related allylic cyanation reaction, which employed modified N─F reagents, provided evidence for an adduct between CuII and the N-centered radical as the HAT reagent159. Modification of the nitrogen-centered radical in this manner could account for some variations in site selectivity that have been observed in different Cu/NFSI described above.
Functionalization of the organic radical can proceed by several different pathways. In some reactions, the nucleophilic coupling partner (e.g., TMSCN) exchanges with fluoride to generate a CuII–Nuc species. Subsequent addition of the benzylic radical to the CuII center affords an CuIII(benzyl)(Nuc) intermediate that can undergo reductive elimination to afford the desired coupling product via an inner-sphere mechanism (FIG. 6a). This pathway rationalizes the enantioselective outcomes of certain coupling reactions, including benzylic cyanation, arylation, and alkynylation. Computational studies of the cyanation reaction suggest radical addition to CuII is reversible and stereoselectivity is determined in the reductive elimination step142. Alternatively, radical functionalization can proceed via a radical-polar crossover pathway (FIG. 6b, radical-polar crossover), involving outer-sphere electron transfer from the radical to CuII. The resulting benzyl cation is then trapped by a nucleophile to afford the desired product. This pathway is supported by racemic product formation in many of the reactions in FIG. 5, such as etherification161, which has also been analyzed by DFT computational studies. DFT analysis of the azidation reaction indicated that radical addition the distal nitrogen of a coordinated azide ligand (FIG. 6c) is energetically similar to the radical-polar crossover mechanism144. As noted above, the electronic/donor properties of different nucleophiles are expected to influence the CuII/I redox potential and influence whether a benzyl radical adds to CuII to generate a CuIII intermediate, introducing the possibility of enantioselective bond formation, or it undergoes outer-sphere electron transfer, resulting in racemic product formation.
The •NSI species generated by reductive activation of NFSI by CuI can oxidize a second equivalent of CuI to generate a CuII–NSI species, rather than promoting HAT from the C─H substrate (FIG. 6, bottom cycle). This undesired side reaction leads to unproductive consumption of the NFSI oxidant and CuI species, stalling the reaction. This problem was first identified in the study of benzylic etherification161, and it was overcome by identifying dialkylphosphite as a mild reductant that slowly reduces CuII to CuI during the reaction, without undergoing direct reaction with NFSI. This "redox buffer" concept has been employed in a number of Cu/NFSI radical-relay reactions, including etherification161, isocyanation150, fluorination31, chlorination156, and azole coupling162. Reagents that have been used as redox buffers include dialkylphosphites150,156,161,162, MeB(OH)2, and B2pin231,151. Not all Cu/NFSI radical-relay reactions require a redox buffer. If the coupling partner, such as TMSCN or ArB(OH)2 in the cyanation and arylation reactions, can undergo background reduction of CuII, for example, via homocoupling to generate cyanogen or biaryls, then no redox buffer is required.
Radical-relay oxidative coupling reactions with catalysts other than copper
Various metals other than copper have been used to support synthetically useful oxidative coupling reactions that proceed via radical intermediates (FIG. 7). Iron(II) chloride and DTBP support the cross coupling of benzylic C─H bonds and 1,3-dicarbonyl compounds168 and imidazoles169 (FIG. 7, Fe), and iron(III) chloride and DTBP was used to achieve coupling of 2-benzylbenzoxazoles and anilines170. Iron(II) acetate with nitrogen donor ligands catalyzes azidation of various C(sp3)─H bonds with a hypervalent azidoiodine(III) reagent145, often favoring tertiary C─H functionalization. This reactivity was applied to late-stage functionalization of various complex molecules171. Cobalt(II) bromide catalysts support cross-coupling of benzylic C─H bonds with amides and sulfonamides and with DTBP as oxidant (FIG. 7, Co)172. Two equivalents of the C─H substrate were used in these reactions. A radical-polar crossover pathway involving a benzylic cation was proposed.
Fig. 7 ∣. Radical-relay C─H functionalization and cross-coupling reactions with catalysts other than copper.
DTBP, di-tert-butyl peroxide; dppb, 1,4-bis(diphenylphosphino)butane; Ln, ligands; DCP, dicumyl peroxide; phd, 1,10-phenanthroline-5,6-dione.
Nickel catalysts have been paired with peroxide oxidants to support oxidative alkynylation, arylation, and methylation of C(sp3)─H bonds. A Ni/Cu/Ag-cocatalyst system promotes oxidative C(sp3)─H alkynylation of unactivated alkanes (as a cosolvent), using terminal alkynes as the limiting reagent (FIG. 7, Ni)173. Ni/triphenylphosphine catalyst systems promote arylation of aliphatic C(sp3)─H bonds with arylboronic acids coupling partners as the limiting reagent174,175. Effective C─H substrates include tetrahydrofuran (THF), 1,4-dioxane, and cyclohexane, which are used as the solvent in the reactions. Methylation of C(sp3)─H bonds with limiting C─H substrates is enabled by photosensitization of peroxides and nickel-mediated radical coupling (FIG. 7, Ni)176. Tertiary alkoxyl radicals generated from O─O homolysis of peroxides undergo HAT with C─H substrates and self-decomposition β-methyl scission to produce methyl radicals. Various reaction parameters, including temperature, concentration, solvent, and structures of peroxides, may be tuned to balance the relative rates of HAT and methyl-radical formation. Protonation of C─H substrates containing basic amines deactivates C(sp3)─H sites adjacent to nitrogen to enable methylation at the benzylic positions. This methylation method creates opportunities to explore “magic methyl” effects on medicinally relevant building blocks and drug molecules177.
Silver catalysts have also been used in radical reaction reactions (FIG. 7, Ag)178. A silver-promoted oxidative benzylic C─H trifluoromethoxylation with C─H substrate as the limiting reagent uses potassium persulfate as oxidant179. The reaction favors functionalization at secondary > primary > tertiary benzylic C─H site and can be modified to introduce a fluorine atom and a trifluoromethoxy group at the same site.
Photochemical radical-relay reactions
Photochemical and visible-light photoredox methods provide a versatile strategy to generate organic radicals from a C(sp3)─H substrate and promote radical-relay coupling with diverse functional groups180-186. The reactions are initiated by abstraction of a hydrogen atom from the C─H substrate. The HAT reagent is generated by photocatalyst-mediated activation of an oxidant, such as a hypervalent iodine reagent, N-acyloxyphthalimide, or persulfate, or by direct photoactivation of a reagent, such as decatungstate or benzophenone (FIG. 8)187. The introduction of functional groups, including hydroxyl, ammonia surrogates, trifluoromethyl, alkyl, and aryl groups, can take place by various mechanisms, similar to the methods outlined above (cf. FIG. 1Cb and FIG. 6). Radical-polar crossover reactions, which generate the corresponding cation or anion (FIG. 8a, SET), are used to support subsequent coupling reactions with nucleophiles and electrophiles, respectively. Transition-metal reagents and cocatalysts can promote coupling via radical-polar crossover or inner-sphere coupling pathways (FIG. 8a, Cu, Ni). Iodine has been used to trap and support further functionalization of the alkyl radicals (FIG. 8a, I). Examples of these photochemical radical-relay reactions will be surveyed below, divided into C(sp3)─H functionalization (one-for-one replacement of a C─H bond with another functional group) and cross-coupling reactions (reaction of a C─H bond with a group of similar reagents).
Fig. 8 ∣. Photoredox C(sp3)─H functionalization/cross coupling via radical relay.
a ∣ Four pathways for photoredox-promoted C─H functionalization/cross coupling. b ∣ The photoredox toolbox includes HAT reagents, photocatalysts, and metal-/iodine-based catalysts utilized for representative methodologies. HAT, hydrogen atom transfer; SET, single electron transfer; BI─OH, hydroxyl benziodoxole, PFBI─OH, hydroxyl perfluorobenziodoxole; PIDA, phenyliodonium diacetate; TBADT, tetra-n-butylammonium decatungstate; TBPB, tert-butyl peroxybenzoate; 4CzIPN, 2,4,5,6-tetra(carbazole-9-yl)isophthalonitrile; [Acr+Mes], 9-mesityl-1,3,6,8-tetramethoxy-10-phenylacridin-10-ium tetrafluoroborate; bpy, 2,2'-bipyridine; PhI(pBBA)2, bis(4-bromobenzoyloxy)iodobenzene; p-F-ppy, 5-fluoro-2-(2-pyridinyl-κN)phenyl-κC; dFppy, 2-(2,4-difluorophenyl)pyridine; dtbbpy, 4,4'-di-tert-butyl-2,2’-bipyridyl; 5,5'-dmbpy, 5,5'-dimethyl-2,2'-bipyridyl.
Photoredox C(sp3)─H functionalization reactions.
Photoredox-promoted radical-relay methods have been used to support functionalization of C(sp3)─H bonds with halogens, pseudohalogens, acetate, ammonia surrogates, and a trifluoromethyl group. Nucleophilic fluorination of C(sp3)─H bonds has been achieved in two complementary studies by using Ir-based photoredox catalysts. In one case, single electron transfer (SET) from the excited state photocatalyst promotes reductive activation of N-acetoxyphthalimide188. N─O cleavage and decarboxylation of the acetoxyl radical generates a methyl radical that promotes HAT from a benzylic or allylic C─H bond. The stabilized carbon-centered radical then undergoes SET oxidation by the IrIV photocatalyst to generate a carbocation that can react with Et3N•HF. A related study employed a peroxide-based oxidant (TBPB) with an Ir photoredox catalyst, resulting in generation of tert-butoxyl radical, which promotes HAT from secondary/tertiary benzylic C─H bonds189. A similar radical-polar crossover step, involving SET oxidation of the radical by IrIV, generates a carbocation that reacts with Et3N•HF. Both studies prioritized fluorination; however, other nucleophiles were shown to trap the carbocation under the reaction conditions, including chloride, azide, alcohols, thiols, and 1,3,5-trimethoxybenzene (FIG. 9a, SET).
Fig. 9 ∣. Different methods for radical-relay C(sp3)─H functionalization and cross coupling using carbon-centered radicals access via photoredox methods.
a ∣ Photoredox-promoted C─H functionalization/cross-coupling reactions involving single electron transfer. b ∣ Photoredox-promoted C─H functionalization/cross-coupling reactions involving copper catalysts. c ∣ Photoredox-promoted C─H functionalization/cross-coupling reactions involving nickel catalysts. d ∣ Photoredox-promoted C─H functionalization/cross-coupling reactions involving iodine-based catalysts . SET, single electron transfer; PhI(pBBA)2, bis(4-bromobenzoyloxy)iodobenzene; BI–OH, hydroxyl benziodoxole, PFBI–OH, hydroxyl perfluorobenziodoxole; TFA, trifluoroacetic acid, TBADT, tetra-n-butylammonium decatungstate; NaDT, sodium decatungstate; Ketone-1, 4-methoxy-4'-trifluoromethylbenzophenone; Ketone-2, 4,4'-dichlorobenzophenone, BRSM, based on recovered starting material.
C(sp3)─H oxygenation and amidation has been achieved by pairing photoredox catalysts with hypervalent iodine oxidants. [Ru(bpy)3]Cl2 mediates photochemical reduction of benziodoxole (BI─OH) and its perfluorinated analog (PFBI─OH) to generate species that promote HAT from tertiary aliphatic and benzylic C(sp3)─H bonds. The RuIII state of the photocatalyst then promotes SET oxidation of the organic radical to generate a carbocation, which can react with H2O to afford the hydroxylation product190. Inclusion of CH3CN or PhCN in the reaction mixtures accesses the corresponding acetamide or benzamide products via a Ritter-type mechanism (FIG. 9a, SET)191,192. Acetoxylation of primary and secondary benzylic C─H bonds proceeds with and acridinium-based photocatalyst and phenyliodonium diacetate (PIDA) as the oxidant and source of acetate193. Photoredox-based reductive activation of PIDA is proposed to generate a methyl radical, which undergoes HAT with the C─H substrate. The oxidized photocatalyst can then oxidize the organic radical to a carbocation intermediate, and subsequent reaction of acetate with the carbocation affords the acetoxylated product.
An alternative approach for functionalization of organic radicals involves SET reduction to carbanions, followed by reaction with different electrophiles (FIG. 9a, SET)194,195. This approach has been used to convert alkylarenes to aryl acetic acids and related products via C─H coupling with CO2194 and to homobenzylic secondary and tertiary alcohols via C─H coupling with aldehydes and ketones195. These reactions use carbazole- or diarylamine-substituted dicyanoarene photocatalysts. The excited-state photocatalyst oxidizes the thiolate of iPr3SiSH to generate a thiyl radical, which promotes HAT from the activated C─H bond. The reduced photocatalyst then promotes SET reduction of the carbon-centered radical to afford a carbanion that can react with an electrophile (CO2 or a ketone) to form a C─C bond.
Light-assisted iodine-catalyzed intramolecular C(sp3)─H amination been the focus of considerable development25 Intermolecular amination of C(sp3)─H bonds has been achieved using bis(4-bromobenzoyloxy)iodobenzene as oxidant and trifluoromethanesulfonamide (H2NTf) as an HAT reagent and ammonia surrogate (FIG. 9d, I)196. In situ reaction of H2NTf and the hypervalent iodine reagent generates N-iodo triflamide (I–NHTf), which can undergo light-induced homolysis of the N─I bond to afford a sulfonamidyl radical. This step is followed by HAT from the C(sp3)─H substrate, carbon─iodine bond formation, and nucleophilic substitution of the iodide to form the C─N product.
Installation of trifluoromethyl group has also been achieved via a photoredox radical-relay approach with cooperative copper catalysis (FIG. 9, Cu). The CF3 group was introduced to the benzylic sites by irradiating a reaction mixture of ammonium persulfate and a bench-stable bpyCu(CF3)3 complex197 in mixed organic/aqueous media (acetone/H2O 1:1) (FIG. 9b, Cu)198. This strategy was also applied to trifluoromethylation of unactivated C(sp3)─H bonds199. HAT from C─H substrates could be promoted by sulfate radical anion or trifluoromethyl-based radical, via light-induced homolysis of persulfate or bpyCu(CF3)3, respectively. The organic radical then reacts with an equivalent of the Cu─CF3 species to afford a trifluoromethylated product. C(sp3)─H trifluoromethylation has also been achieved using bpyCu(CF3)3 and oxone200. In this case, the mechanism is proposed to involve HAT from the C─H substrate by a trifluoromethyl radical, oxidation of the carbon-centered radical to a carbocation by oxone, and reaction of an anionic trifluoromethyl source with the carbocation. In yet another approach, sodium decatungstate has been used in combination with a copper catalyst (CuCl2) and a trifluoromethyl hypervalent iodine-based oxidant to convert C(sp3)─H bonds to the corresponding C(sp3)─CF3 products (FIG. 9b, Cu)201. The photoexcited decatungstate promotes HAT to form a carbon-centered radical, and a CuII─CF3 species, generated in situ from CuCl2 and the CF3 source, is proposed to react with the organic radical to afford the trifluoromethylated products.
Photoredox C(sp3)─H cross coupling.
Photoredox methods enable cross coupling of benzylic and aliphatic C─H substrates with diverse aryl or alkyl halide reaction partners. Three general strategies have been employed to support HAT in these reactions: (a) combining a photocatalyst with a tertiary amine to generate an amine radical cation species, (b) use of a reagent that is capable to promoting HAT from its photoexcited state, and (c) photochemical activation of a nickel halide to generate a halogen radical HAT species.
The first example of this reactivity used 3-acetoxyquinuclidine, an Ir-based photocatalyst, and nickel(II) bromide to support cross coupling of activated C(sp3)─H bonds, such as those adjacent to nitrogen in pyrrolidines, with aryl bromides/chlorides (FIG.9c, Ni)202. SET oxidation of 3-acetoxyquinuclidine by the excited-state IrIII photocatalyst generates a quinuclidine radical cation capable of promoting HAT from the C(sp3)─H bond. This radical generation process is paired with a Ni-based radical functionalization cycle. The nickel co-catalyst reacts with the organic halide species to afford an organonickel(II) species, which promotes C─C coupling by reaction with the radical derived from the C─H substrate. Cross coupling of activated C(sp3)─H and alkyl halides employing quinuclidine, an Ir-based photoredox catalyst, and nickel(II) bromide has been achieved via similar mechanistic pathway203. Quinuclidine also promotes HAT from aldehyde204 and the α-C─H bonds of alcohols205 to enable direct arylation and alkylation via Ni-catalyzed cross coupling. Nitrogen-centered radicals from sulfonamides have been shown to abstract hydrogen atoms from C─H bonds adjacent to heteroatoms, generating radicals that undergo Ni-catalyzed cross coupling with alkyl halides to afford alkylated products206. Photoexcited 4CzIPN (1,2,3,5-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene) is proposed to oxidize sulfonamide anion to the nitrogen-centered radical, which is responsible for HAT.
TBADT serves as a combined photocatalyst/HAT reagent and supports arylation of strong aliphatic C─H bonds with aryl bromides207. Disproportionation of two singly reduced decatungstate species regenerates the active HAT photocatalyst and the doubly reduced decatungstate species, which can reduce the nickel cocatalyst as needed for activation of the aryl bromide coupling partner. Benzophenone derivatives are similar to TBADT in their ability to promote HAT from their photoexcited triplet state208,209. This feature has been exploited in combination with nickel catalysis for cross coupling of activated C(sp3)─H bonds with aryl and alkyl halides (FIG. 9c, Ni)210. 4,4'-Dichlorobenzophenone (FIG. 8b, Ketone-2) has been used as a photocatalyst to achieve arylation of primary benzylic C(sp3)─H bonds211. Aryl halides, including chlorides, bromides, and iodides, can be utilized as coupling partners. Benzaldehyde has also been used as a photocatalyst for arylation and alkylation of activated C(sp3)─H bonds (FIG. 9c, Ni)212,213.
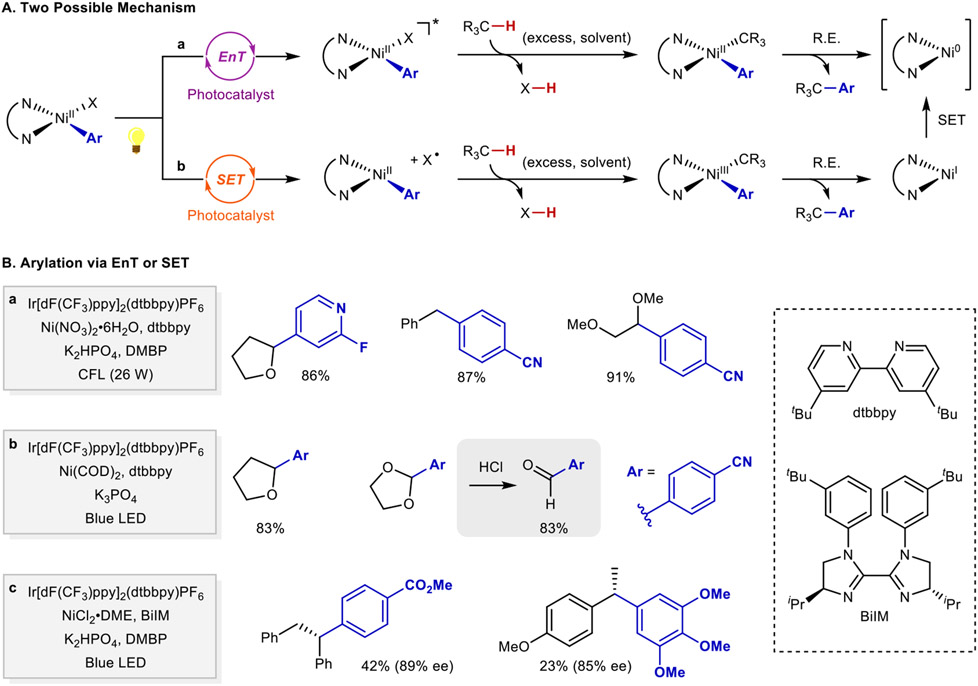
Arylation of activated C(sp3)─H bonds has been achieved by combining an Ir-based photoredox catalyst and Ni cocatalyst, in the absence of an HAT reagent (FIG. 10Ba). In the coupling of aryl bromides with THF and other solvents with activated C─H bonds, the excited-state photocatalyst IrIII* is proposed to undergo triplet-triplet energy transfer with a ArNiII–Br species to generate a triplet NiII* species. (FIG. 10Aa). HAT from the C(sp3)─H bond may involve a bromine radical or proceed via a concerted four-membered transition state involving the Ni─Br bond. The resulting carbon-centered radical can react at Ni to afford the C(sp3)─C(sp2) bond 214. A complementary study, involving the coupling of aryl chlorides with THF and related solvents (FIG. 10Bb) 215, proposes that the IrIII photocatalyst serves as a SET reagent. Oxidation of an ArNiII–Cl intermediate by the excited state photocatalyst IrIII* generates a NiIII species. Subsequent loss of a chlorine atom leads to HAT from the activated C(sp3)─H bond of the solvent to afford an organic radical that can undergo coupling with the arylnickel(II) species (FIG. 10Ab). The synthetic utility of the latter reaction was demonstrated in a separate report in the cross coupling of (hetero)aryl chlorides with 1,3-dioxolane as the solvent216. The resulting cross-coupled products are readily converted to (hetero)aryl aldehydes following a mild acidic workup at room temperature. An enantioselective benzylic C─H arylation has been achieved with an Ir-based photocatalyst, nickel(II), and a chiral bis-imidazoline ligand (FIG. 10Bc)217. Aryl bromide coupling partners generate ArNiII–Br, which is proposed to generate bromine radical as the HAT reagent upon irradiation in the presence of a photocatalyst. An enantioselective benzylic C(sp3)─H alkenylation reaction has also been achieved with alkenyl bromides as the coupling partners in presence of an Ir-photoredox catalyst, nickel(II), and a chiral bis-imidazoline-based ligand218. Complementary nickel/photoredox dual catalysis enables acylation219 and arylation220 reactions of α-amino C(sp3)─H bonds. In these cases, N-arylpyrrolidine substrates are proposed to generate stabilized α-amino radicals via a more conventional process involving SET oxidation of the substrate.
Fig. 10 ∣. Nickel-catalyzed arylation of C(sp3)─H bonds of inexpensive (co)solvents enabled by in situ generation of HAT reagents.
Aa ∣ Energy transfer pathway for arylation of C(sp3)─H bonds. Ab ∣ Single electron transfer pathway for arylation of C(sp3)─H bonds. Ba ∣ Examples of arylated products involving energy transfer. Bb ∣ Examples of arylated product involving single electron transfer. Bc ∣ Enantioselective arylation of benzylic C(sp3)─H bonds with a chiral bis-imidazoline ligand. EnT, energy transfer; SET, single electron transfer; R.E., reductive elimination; dF(CF3)ppy, 3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl]phenyl; dtbbpy, 4,4'-di-tert-butyl-2,2'-dipyridyl; BiIM, biimidazoline; DMBP, 4,4'-dimethoxybenzophenone.
Electrochemical radical-relay reactions
Electrochemical methods provide additional strategies to achieve C(sp3)─H functionalization via radical relay221,222. N-hydroxyphthalimide (NHPI) was first demonstrated as an electrochemical mediator for C(sp3─H) oxygenation in the 1980s223-225. NHPI undergoes proton-coupled oxidation to phthalimide N-oxyl (PINO) as the electrode, and PINO- mediated HAT then generates an organic radical that can react with O2 or other oxidants, resulting in C─H functionalization (FIG. 11Aa)226. These methods complement transition-metal/NHPI cocatalyst systems for C─H oxygenation with O268,71,72. Modified NHPI derivatives can lead to improved performance, evident from the use of tetrachloro-N-hydroxyphthalimide (Cl4NHPI) as the HAT mediator in electrochemical oxidation of allylic C(sp3)─H bonds (FIG. 11Ab) 227 , and an electrochemical NHPI/O2 system proved effective for C─H oxygenation of alkyl-substituted heterocycles that exhibit poor reactivity with a chemical Co/NHPI/O2 catalyst system (FIG. 11Ac)71. Flow conditions have been developed for the latter process228.
Fig. 11 ∣. Radical-relay reactions involving electrochemistry.
Aa ∣ General mechanism for NHPI-mediated electrochemical oxygenation reaction of C(sp3)─H bonds. Ab ∣ Oxygenation of allylic C─H bonds. Ac ∣ Oxygenation of heterobenzylic C(sp3)─H bonds. Ad ∣ Quinuclidine-mediated oxygenation of C(sp3)─H bonds. Ae ∣ N-alkyl ammonium ylides as a new class of HAT-mediators. Af ∣ NHPI-mediated iodination followed by pyridination of alkylarenes. Ba ∣ Oxygenation reactions involving metal-oxo complexes. Bb ∣ Oxygenation of secondary benzylic C(sp3)─H bonds using Fe-TAML complex. Bc ∣ Hydroxylation of C(sp3)─H bonds in amine derivatives using a ruthenium-based catalyst. C ∣ Azidation of C(sp3)─H bonds using MnIII catalyst. D ∣ Photoelectrochemical azidation reaction of C(sp3)─H bonds. NHPI, N-hydroxyphthalimide; Cl4NHPI, tetrachloro-N-hydroxyphthalimide; RVC, reticulated vitreous carbon; TBAP, tetrabutylammonium perchlorate; TAML, tetraamido macrocylic ligand; dtbpy, 4,4'-di-tert-butyl-2,2'-dipyridyl; DDQ, 2,3-dichloro-5,6-dicyano-p-benzoquinone; 1,10-Phen, 1,10-phenanthroline; TFA, trifluoroacetic acid.
Different mediators provide a means to expand reactivity beyond activated C─H bonds. Electrochemical oxidation of quinuclidine generates an amine radical cation that promotes HAT and oxygenation of diverse C(sp3)─H bonds under aerobic conditions (FIG. 11Ad)229. N-alkyl ammonium ylides have been developed as a new class of electrochemical mediators for C(sp3)─H oxygenation through the support of computational method (FIG. 11Ae)230. These reagents show modified chemo- and site-selectivity relative to previous chemical and electrochemical methods for C(sp3)─H oxygenation.
NHPI has been paired with iodine, rather than O2, to enable the iodination of methyl arenes231. The resulting benzyl iodide may be employed in subsequent SN2-base derivatization reactions to access new C─C, C─O, and C─N bonds or undergo in situ substitution, for example, by pyridine (FIG. 11Af). This mediated HAT strategy allows electrochemical C─H functionalization to proceed at electrode potentials 0.7–1.3 V lower than those required by conventional SET-initiated electrochemical benzylic C─H functionalization methods232,233.
Metal-oxo complexes capable of promoting C─H oxygenation have been generated via electrochemical proton-coupled oxidation of metal-aquo complexes (FIG. 11Ba). Electrochemical oxidation of [(TAML)FeIII(OH)2]Na (TAML = tetraamido macrocyclic ligand) generates FeIV and FeV oxo species. The FeV-oxo is more reactive and oxidizes secondary benzylic C─H bonds to ketones using water as oxygen-atom source (FIG. 11Bb)234. Ruthenium-based oxo species derived from cis-bis(4,4'-di-tert-butylpyridine)ruthenium complex effects electrochemical hydroxylation of C(sp3)─H bonds (FIG. 11Bc)235. Basic amines are tolerated, owing to the use of acidic reaction conditions that protect the amines as ammonium salts.
Two complementary electrochemical methods have been developed for C(sp3)─H azidation. Manganese(III) porphyrin or salen complexes and sodium azide promote azidation of C(sp3)─H bonds (FIG. 11C)236. The reaction is proposed to proceed via oxidation of MnIII to a MnIV-diazide species, which promotes HAT from the substrate to generate an organic radical that affords an azide product via coupling with another MnIV-diazide complex. An electrophotochemical azidation protocol uses MnF2 in combination with a ketone-based photocatalyst [e.g. 9-fluorenone, 2,3-dichloro-5,6-dicyano-p-benzoquinone (DDQ), bis(4-methoxyphenyl)methanone] and sodium azide (FIG. 11D)237. The excited-state photocatalyst promotes HAT, and an electrochemically generated MnIII-azide is proposed to react with the carbon-centered radical.
Conclusion
Radical reaction pathways offer some of the most versatile methods available for C(sp3)─H functionalization and cross-coupling, accessing synthetic scope and utility that is unmatched by other reaction pathways. Major advances have been made in each of the different radical reaction classes, including radical chain, radical rebound, and radical relay. Many of these reactions are able to use the C─H substrate as the limiting reagent, addressing one of the long-standing limitations of this field and supporting application to late-stage functionalization. Radical-relay methods are particularly versatile. These reactions not only enable installation of a specific functional groups, including -CN, ─CF3, ─N3, ─OH, ─SCN, ─F, ─Cl, ─NCO, but also provide the basis for sequential functionalization/diversification methods and direct cross-coupling methods that leverage substrates derived from large pools of reaction partners, such as alcohols, amines/amides, arylboronic acids, and aryl halides. The latter feature greatly expands the scope of accessible products relative those obtained from typical C─H "functionalization" methods and has profound implications for drug discovery and medicinal chemistry where access to diverse structures is crucial to the success of the endeavors.
Acknowledgements
This work was supported by funding from the NIH (R35 GM134929).
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.He J, Wasa M, Chan KSL, Shao Q & Yu J-Q Palladium-catalyzed transformations of alkyl C─H bonds. Chem. Rev 117, 8754–8786 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J & Baudoin O Functionalization of organic molecules by transition-metal-catalyzed C(sp3)─H activation. Chem. Eur. J 16, 2654–2672 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Saint-Denis TG, Zhu R-Y, Chen G, Wu Q-F & Yu J-Q Enantioselective C(sp3)─H bond activation by chiral transition metal catalysts. Science 359, eaao4798 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A, Kumar J, Rahaman A, Singh AK & Bhadra S Functionalization of C(sp3)─H bonds adjacent to heterocycles catalyzed by earth abundant transition metals. Tetrahedron 98, 132415 (2021). [Google Scholar]
- 5.Davies HML & Manning JR Catalytic C─H functionalization by metal carbenoid and nitrenoid insertion. Nature 451, 417–424 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle MP, Duffy R, Ratnikov M & Zhou L Catalytic carbene insertion into C─H bonds. Chem. Rev 110, 704–724 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Roizen J, Harvey ME & Du Bois J Metal-catalyzed nitrogen-atom transfer methods for the oxidant of aliphatic C─H bonds. Acc. Chem. Res 45, 911–922 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi H et al. Recent advances in radical C─H activation/radical cross-coupling. Chem. Rev 117, 9016–9085 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Zhang C, Li Z-L, Gu Q-S & Liu X-Y Catalytic enantioselective C(sp3)─H functionalization involving radical intermediates. Nat. Commun 12, 475 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Studer A & Curran DP Catalysis of radical reactions: A radical chemistry perspective. Angew. Chem. Int. Ed 55, 58–102 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Labinger JA & Bercaw JE Understanding and exploiting C─H bond activation. Nature 417, 507–514 (2002). [DOI] [PubMed] [Google Scholar]
- 12.Sheldon RA & Kochi JK in Metal-catalyzed oxidations of organic compounds: Mechanistic principles and synthetic methodology including biochemical processes (Academic Press, Inc., New York, 1981). [Google Scholar]
- 13.Hermans I, Spier ES, Neuenschwander U, Turrà N & Baiker A Selective oxidation catalysis: Opportunities and challenges. Top. Catal 52, 1162–1174 (2009). [Google Scholar]
- 14.Quinn RK et al. Site-selective aliphatic C─H chlorination using N-chloroamides enables a synthesis of chlorolissoclimide. J. Am. Chem. Soc 138, 696–702 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt VA, Quinn RK, Brusoe AT & Alexanian EJ Site-selective aliphatic C─H bromination using N-bromoamides and visible light. J. Am. Chem. Soc 136, 14389–14392 (2014). [DOI] [PubMed] [Google Scholar]
- 16.Czaplyski WL, Na CG & Alexanian EJ C─H Xanthylation: A synthetic platform for alkane functionalization. J. Am. Chem. Soc 138, 13854–13857 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanwar L, Börgel J & Ritter T Synthesis of benzylic alcohols by C─H oxidation. J. Am. Chem. Soc 141, 17983–17988 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang X & Groves JT Beyond ferryl-mediated hydroxylation: 40 years of rebound mechanism and C─H activation. J. Biol. Inorg. Chem 22, 185–207 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang X & Groves JT Taming azide radicals for catalytic C─H azidation. ACS Catal. 6, 751–759 (2016). [Google Scholar]
- 20.Clark JR, Feng K, Sookezian A & White MC Manganese-catalysed benzylic C(sp3)─H amination for late-stage functionalization. Nature Chem. 10, 583–591 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lovering F, Bikker J & Humblet C Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem 52, 6752–6756 (2009). [DOI] [PubMed] [Google Scholar]
- 22.Blakemore DC et al. Organic synthesis provides opportunities to transform drug discovery. Nature Chem. 10, 383–394 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Guillemard L, Kaplaneris N, Ackermann L & Johansson MJ Late-stage C─H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem 5, 522–545(2021). [DOI] [PubMed] [Google Scholar]
- 24.Wolff ME Cyclization of N-halogenated amines (the Hofmann-Löffler reaction). Chem. Rev 63, 55–64 (1963). [Google Scholar]
- 25.Statement LM, Nakafuku KM & Nagib DA Remote C─H functionalization via selective hydrogen atom transfer. Synthesis 50, 1569–1586 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarkar S Cheung KPS & Gevorgyan V C─H functionalization reactions enabled by hydrogen atom transfer to carbon-centered radicals. Chem. Sci 11, 12974–12993 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nodwell MB et al. Direct photocatalytic fluorination of benzylic C─H bonds with N-fluorobenzenesulfonimide. Chem. Commun 51, 11783–11786 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Pitts CR, Ling B, Woltornist R, Liu R & Lectka T Triethylborane-initiated radical chain fluorination: A synthetic method derived from mechanistic insight. J. Org. Chem 79, 8895–8899 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Pitts CR et al. Direct, catalytic monofluorination of sp3 C─H bonds: A radical-based mechanism with ionic selectivity. J. Am. Chem. Soc 136, 9780–9791 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Bloom S et al. Iron(II)-catalyzed benzylic fluorination. Org. Lett 15, 1722–1724 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Buss JA, Vasilopoulos A, Golden DL & Stahl SS Copper-catalyzed functionalization of benzylic C─H bonds with N-fluorobenzenesulfonimide: Switch from C─N to C─F bond formation promoted by a redox buffer and Brønsted base. Org. Lett 22, 5749–5752 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bloom S et al. A polycomponent metal-catalyzed aliphatic, allylic, and benzylic fluorination. Angew. Chem. Int. Ed 51, 10580–10583 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Xia J-B, Zhu C & Chen C Visible light-promoted metal-free C─H activation: Diarylketone-catalyzed selective benzylic mono- and difluorination. J. Am. Chem. Soc 135, 17494–17500 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halperin SD, Fan H, Chang S, Martin RE & Britton R A convenient photocatalytic fluorination of unactivated C─H bonds. Angew. Chem. Int. Ed 53, 4690–4693 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Bloom S, McCann M & Lectka T Photocatalyzed benzylic fluorination: Shedding “light” on the involvement of electron transfer. Org. Lett 16, 6338–6341 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Carestia AM, Ravelli D & Alexanian EJ Reagent-dictated site selectivity in intermolecular aliphatic C─H functionalizations using nitrogen-centered radicals. Chem. Sci 9, 5360–5365 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MacMillan AJ et al. Practical and selective sp3 C─H bond chlorination via aminium radicals. Angew. Chem. Int. Ed 60, 7132–7139 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiang M et al. Visible light-catalyzed benzylic C─H bond chlorination by a combination of organic dye (Acr+-Mes) and N-chlorosuccinimide. J. Org. Chem 85, 9080–9087 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Ozawa J & Kanai M Silver-catalyzed C(sp3)─H chlorination. Org. Lett 19, 1430–1433 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Quiclet-Sire B & Zard SZ The xanthate route to amines, anilines, and other nitrogen compounds. A brief account. Synlett. 27, 680–701 (2016). [Google Scholar]
- 41.Huang X & Groves JT Oxygen activation and radical transformations in heme proteins and metallophorphyrins. Chem. Rev 118, 2491–2553 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leising RA, Norman RE & Que L Jr. Alkane functionalization by non-porphyrin iron complexes: Mechanistic insights. Inorg. Chem 29, 2553–2555 (1990). [Google Scholar]
- 43.Chen K, Costas M & Que L Jr. Spin state tuning of non-heme iron-catalyzed hydrocarbon oxidations: Participation of FeIII–OOH and FeV=O intermediates. J. Chem. Soc., Dalton Trans 672–679 (2002). [Google Scholar]
- 44.Kim J, Kim C, Harrison RG, Wilkinson EC & Que L Jr. Fe(TPA)-catalyzed alkane hydroxylation can be a metal-based oxidation. J. Mol. Catal. A: Chem 117, 83–89 (1997). [Google Scholar]
- 45.Chen K & Que L Jr. Stereospecific alkane hydroxylation by non-heme iron catalysts: Mechanistic evidence for an FeV=O active species. J. Am. Chem. Soc 123, 6327–6337 (2001). [DOI] [PubMed] [Google Scholar]
- 46.Costas M, Tipton AK, Chen K, Jo D-H & Que L Jr. Modeling Rieske dioxygenases: The first example of iron-catalyzed asymmetric cis-dihydroxylation of olefins. J. Am. Chem. Soc 123, 6722–6723 (2001). [DOI] [PubMed] [Google Scholar]
- 47.Kim C, Chen K, Kim J & Que L Jr. Stereospecific alkane hydroxylation with H2O2 catalyzed by an iron(II)–tris(2-pyridylmethyl)amine complex. J. Am. Chem. Soc 119, 5964–5965 (1997). [Google Scholar]
- 48.Costas M & Que L Jr. Ligand topology tuning of iron-catalyzed hydrocarbon oxidations. Angew. Chem. Int. Ed 41, 2179–2181 (2002). [PubMed] [Google Scholar]
- 49.Chen MS & White MC A predictably selective aliphatic C─H oxidation reaction for complex molecule synthesis. Science. 318, 783–787 (2007). [DOI] [PubMed] [Google Scholar]
- 50.St John P, Guan Y, Kim Y, Kim S, Paton RS Prediction of homolytic bond dissociation enthalpies for organic molecules at near chemical accuracy with sub-second computational cost. Nat. Commun 11, 2328, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen MS & White MC Combined effects on selectivity in Fe-catalyzed methylene oxidation. Science. 327, 566–571 (2010). [DOI] [PubMed] [Google Scholar]
- 52.Gormisky PE & White MC Catalyst-controlled aliphatic C─H oxidations with a predictive model for site-selectivity. J. Am. Chem. Soc 135, 14052–14055 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Howell JM, Feng K, Clark JR, Trzepkowski LJ & White MC Remote oxidation of aliphatic C─H bonds in nitrogen-containing molecules. J. Am. Chem. Soc 137, 14590–14593 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Osberger TJ, Rogness DC, Kohrt JT, Stepan AF & White MC Oxidative diversification of amino acids and peptides by small-molecule iron catalysis. Nature 537, 214–219 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dantignana V et al. Chemoselective aliphatic C─H bond oxidation enabled by polarity reversal. ACS Cent. Sci 3, 1350–1358 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borrell M, Gil-Caballero S, Bietti M & Costas M Site-selective and product chemoselective aliphatic C─H bond hydroxylation of polyhydroxylated substrates. ACS. Catal 10, 4702–4709 (2020). [Google Scholar]
- 57.Zhao J, Nanjo T, de Luca EC & White MC Chemoselective methylene oxidation in aromatic molecules. Nature Chem. 11, 213–221 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Milan M, Bietti M & Costas M Highly enantioselective oxidation of nonactivated aliphatic C─H bonds with hydrogen peroxide catalyzed by manganese complexes. ACS. Cent. Sci 3, 196–204 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.White MC, Doyle AG & Jacobsen EN A synthetically useful, self-assembling MMO mimic system for catalytic alkene epoxidation with aqueous H2O2. J. Am. Chem. Soc 123, 7194–7195 (2001). [DOI] [PubMed] [Google Scholar]
- 60.Oloo WN & Que L Jr. Bioinspired nonheme iron catalysts for C─H and C=C bond oxidation: Insights into the nature of the metal-based oxidants. Acc. Chem. Res 48, 2612–2621 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Kal S, Xu S & Que L Jr. Bio-inspired nonheme iron oxidation catalysis: Involvement of oxoiron(V)oxidants in cleaving strong C─H bonds. Angew. Chem. Int. Ed 59, 7332–7349 (2020). [DOI] [PubMed] [Google Scholar]
- 62.Liu W & Groves JT Manganese catalyzed C─H halogenation. Acc. Chem Res 48, 1727–1735 (2015). [DOI] [PubMed] [Google Scholar]
- 63.Liu W et al. Oxidative aliphatic C─H fluorination with fluoride ion catalyzed by a manganese porphyrin. Science. 337, 1322–1325 (2012). [DOI] [PubMed] [Google Scholar]
- 64.Li G, Dilger AK, Cheng PT, Ewing WR and Groves JT Selective C─H halogenation with a highly fluorinated manganese porphyrin. Angew. Chem. Int. Ed 57, 1251–1255 (2018). [DOI] [PubMed] [Google Scholar]
- 65.Liu W et al. Site-selective 18F fluorination of unactivated C─H bonds mediated by a manganese porphyrin. Chem. Sci 9, 1168–1172 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang X, Bergsten TM & Groves JT Manganese-catalyzed late-stage aliphatic C─H azidation. J. Am. Chem. Soc 137, 5300–5303 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Huang X et al. Alkyl isocyanates via manganese-catalyzed C─H activation for the preparation of substituted ureas. J. Am. Chem. Soc 139, 15407–15413 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Ishii Y, Sakaguchi S & Iwahama T Innovation of hydrocarbon oxidation with molecular oxygen and related reactions. Adv. Synth. Catal 343, 393–427 (2001). [Google Scholar]
- 69.Sterckx H, Morel B & Maes BUW Catalytic aerobic oxidation of C(sp3)─H bonds. Angew. Chem. Int. Ed 58, 7946–7970 (2019). [DOI] [PubMed] [Google Scholar]
- 70.Ishii Y; Iwahama T; Sakaguchi S; Nakayama K & Nishiyama Y Alkane oxidation with molecular oxygen using a new efficient catalytic system: N-hydroxyphthalimide (NHPI) combined with Co(acac)n (n = 2 or 3). J. Org. Chem 61, 4520–4526 (1996). [DOI] [PubMed] [Google Scholar]
- 71.Hruszkewycz DP, Miles KC, Thiel OR & Stahl SS Co/NHPI-mediated oxygenation of benzylic C─H bonds in pharmaceutically relevant molecules. Chem. Sci 8, 1282–1287 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cooper JC, Luo C, Kameyama R & Van Humbeck JF Combined iron/hydroxytriazole dual catalytic system for site selective oxidation adjacent to azaheterocycles. J. Am. Chem. Soc 140, 1243–1246 (2018). [DOI] [PubMed] [Google Scholar]
- 73.Gaster E, Kozuch S & Pappo D Selective aerobic oxidation of methylarenes to benzaldehydes catalyzed by N-hydroxyphthalimide and cobalt(II) acetate in hexafluoropropan-2-ol. Angew. Chem. Int. Ed 56, 5912–5915 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Schultz DM et al. Oxyfunctionalization of the remote C─H bonds of aliphatic amines by decatungstate photocatalysis. Angew. Chem. Int. Ed 56, 15274–15278 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Wu W et al. (nBu4N)W10O32-catalyzed selective oxygenation of cyclohexane by molecular oxygen under visible light irradiation. Appl. Catal. B: Environ 164, 113–119 (2015). [Google Scholar]
- 76.Laudadio G et al. Selective C(sp3)─H aerobic oxidation enabled by decatungstate photocatalysis in flow. Angew. Chem. Int. Ed 57, 4078–4082 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee JM, Park J, Cho SH & Chang S Cu-facilitated C─O bond formation using N-hydroxyphthalimide: Efficient and selective functionalization of benzyl and allylic C─H bonds. J. Am. Chem. Soc 130, 7824–7825 (2008). [DOI] [PubMed] [Google Scholar]
- 78.Guo Z, Jin C, Zhou J & Su W Copper(II)-catalyzed cross dehydrogenative coupling reaction of N-hydroxyphthalimide with alkanes and ethers via unactivated C(sp3)─H activation at room temperature. RSC. Adv 6, 79016–79019 (2016). [Google Scholar]
- 79.Zhang X, Yang H & Tang P Transition-metal-free oxidative aliphatic C─H azidation. Org. Lett 17, 5828–5831 (2015). [DOI] [PubMed] [Google Scholar]
- 80.Kim K, Lee S & Hong SH Direct C(sp3)─H cyanation enabled by highly active decatungstate photocatalyst. Org. Lett 23, 5501–5505 (2021). [DOI] [PubMed] [Google Scholar]
- 81.Sarver PJ, Bissonnette NB & MacMillan DWC Decatungstate-catalyzed C(sp3)─H sulfinylation: Rapid access to diverse organosulfur functionality. J. Am. Chem. Soc 143, 9737–9743 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schirmer TE, Rolka AB, Karl TA, Holzhausen F & König B Photocatalytic C─H trifluoromethylthiolation by the decatungstate anion. Org. Lett 23, 5729–5733 (2021). [DOI] [PubMed] [Google Scholar]
- 83.Capaldo L & Ravelli D Decatungstate as direct hydrogen atom transfer photocatalyst for SOMOphilic alkynylation. Org. Lett 23, 2243–2247 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mao R; Bera S; Turla AC & Hu X Copper-catalyzed intermolecular functionalization of unactivated C(sp3)─H bonds and aliphatic carboxylic acids. J. Am. Chem. Soc 143, 14667–14675 (2021). [DOI] [PubMed] [Google Scholar]
- 85.Bentley KW, Dummit KA & Van Humbeck JF A highly site-selective radical sp3 C─H amination of azaheterocycles. Chem. Sci 9, 6440–6445 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu A, Guo J-J, Pan H & Zuo Z Selective functionalization of methane, ethane, and higher alkanes by cerium photocatalysis. Science 361, 668–672 (2018). [DOI] [PubMed] [Google Scholar]
- 87.An Q et al. Cerium-catalyzed C─H functionalizations of alkanes utilizing alcohols as hydrogen atom transfer agents. J. Am. Chem. Soc 142, 6216–6226 (2020). [DOI] [PubMed] [Google Scholar]
- 88.Yang Q et al. Photocatalytic C─H activation and the subtle role of chlorine radical complexation in reactivity. Science 372, 847–852 (2021). [DOI] [PubMed] [Google Scholar]
- 89.Ryu I et al. Efficient C─H/C─N and C─H/C─CO─N conversion via decatungstate-photoinduced alkylation of diisopropyl azodicarboxylate. Org. Lett 15, 2554–2557 (2013). [DOI] [PubMed] [Google Scholar]
- 90.Bonassi F, Ravelli D, Protti S & Fagnoni M Decatungstate photocatalyzed acylations and alkylations in flow via hydrogen atom transfer. Adv. Synth. Catal 357, 3687–3695 (2015). [Google Scholar]
- 91.Wan T et al. Accelerated and scalable C(sp3)─H amination via decatungstate photocatalysis using a flow photoreactor equipped with high-intensity LEDs. ACS Cent. Sci Accepted Article (2021) 10.1021/acscentsci.1c01109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Giese B Formation of CC bonds by addition of free radicals to alkenes. Angew. Chem. Int. Ed. Engl 22, 753–764 (1983). [Google Scholar]
- 93.Crespi S & Fagnoni M Generation of alkyl radicals: From the tyranny of tin to the photon democracy. Chem. Rev 120, 9790–9833 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kanegusuku ALG & Roizen JL Recent advances in photoredox-mediated radical conjugate addition reactions: An expanding toolkit for the Giese reaction. Angew. Chem. Int. Ed 60, 2–36 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Treacy SM & Rovis T Copper-catalyzed C(sp3)─H bond alkylation via photoinduced ligand-to-metal charge transfer. J. Am. Chem. Soc 143, 2729–2735 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang YC, Treacy SM & Rovis T Iron-catalyzed photoinduced LMCT: A 1° C─H abstraction enables skeletal rearrangements and C(sp3)─H alkylation. ACS. Catal 11, 7442–7449 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Angioni S et al. Tetrabutylammonium decatungstate (chemo)selective photocatalyzed, radical C─H functionalization in amides. Adv. Synth. Catal 350, 2209–2214 (2008). [Google Scholar]
- 98.Yamada K et al. Photocatalyzed site-selective C─H to C─C conversion of aliphatic nitriles. Org. Lett 17, 1292–1295 (2015). [DOI] [PubMed] [Google Scholar]
- 99.Fukuyama T et al. Photocatalyzed site-selective C(sp3)─H functionalization of alkylpyridines at non-benzylic positions. Org. Lett 19, 6436–6439 (2017). [DOI] [PubMed] [Google Scholar]
- 100.Fukuyama T, Nishikawa T & Ryu I Site-selective C(sp3)─H functionalization of fluorinated alkanes driven by polar effects using a tungstate photocatalyst. Eur. J. Org. Chem 1424–1428 (2020). [Google Scholar]
- 101.Laudadio G et al. C(sp3)─H functionalizations of light hydrocarbon using decatungstate photocatalysis in flow. Science 369, 92–96 (2020). [DOI] [PubMed] [Google Scholar]
- 102.Roberts BP Polarity-reversal catalysis of hydrogen-atom abstraction reactions: Concepts and applications in organic chemistry. Chem. Soc. Rev 28, 25–35 (1999). [Google Scholar]
- 103.Lei G, Xu M, Chang R, Funes-Ardoiz I & Ye J Hydroalkylation of unactivated olefins via visible-light-driven dual hydrogen atom transfer catalysis. J. Am. Chem. Soc 143, 11251–11261 (2021). [DOI] [PubMed] [Google Scholar]
- 104.Minisci F, Bernardi R, Bertini F, Galli R, Perchinummo M Nucleophilic character of alkyl radicals—VI: A new convenient selective alkylation of heteroaromatic bases. Tetrahedron 27, 3575–3579 (1971). [Google Scholar]
- 105.Proctor RSJ & Phipps RJ Recent advances in Minisci-type reactions. Angew. Chem. Int. Ed 58, 13666–13699 (2019). [DOI] [PubMed] [Google Scholar]
- 106.Leonov D & Elad D Ultraviolet- and γ-ray-induced reactions of nucleic acid constituents. Reactions of purines with ethers and dioxolane. J. Org. Chem 39, 1470–1473 (1974). [DOI] [PubMed] [Google Scholar]
- 107.Deng G, Ueda K, Yanagisawa S, Itami K & Li C-J Coupling of nitrogen heteroaromatics and alkanes without transition metals: A new oxidative cross-coupling at C─H/C─H bonds. Chem. Eur. J 15, 333–337 (2009). [DOI] [PubMed] [Google Scholar]
- 108.Xia R, Niu H-Y, Qu G-R & Guo H-M CuI controlled C─C and C─N bond formation of heteroaromatics through C(sp3)─H activation. Org. Lett 14, 5546–5549 (2012). [DOI] [PubMed] [Google Scholar]
- 109.Shao X, Wu X, Wu S & Zhu C Metal-free radical-mediated C(sp3)─H heteroarylation of alkanes. Org. Lett 22, 7450–7454 (2020). [DOI] [PubMed] [Google Scholar]
- 110.Tzirakis MD, Lykakis IN & Orfanopoulos M Decatungstate as an efficient photocatalyst in organic chemistry. Chem. Soc. Rev 38, 2609–2621 (2009). [DOI] [PubMed] [Google Scholar]
- 111.Quattrini MC et al. Versatile cross-dehydrogenative coupling of heteroaromatics and hydrogen donors via decatungstate photocatalysis. Chem. Commun 53, 2335–2338 (2017). [DOI] [PubMed] [Google Scholar]
- 112.De Waele V, Poizat O, Fagnoni M, Bagno A & Ravelli D Unraveling the key features of the reactive state of decatungstate anion in hydrogen atom transfer (HAT) photocatalysis. ACS Catal. 6, 7174–7182 (2016). [Google Scholar]
- 113.Ravelli D, Fagnoni M, Fukuyama T, Nishikawa T & Ryu I Site-selective C─H functionalization by decatungstate anion photocatalysis: Synergistic control by polar and steric effects expands the reaction scope. ACS Catal. 8, 701–703 (2018). [Google Scholar]
- 114.Li G-X, Hu X, He G & Chen G Photoredox-mediated Minisci-type alkylation of N-heteroarenes with alkanes with high methylene selectivity. ACS Catal. 8, 11847–11853 (2018). [Google Scholar]
- 115.Lee W, Jung S, Kim M & Hong S Site-selective direct C─H pyridylation of unactivated alkanes by triplet excited anthraquinone. J. Am. Chem. Soc 143, 3003–3012 (2021). [DOI] [PubMed] [Google Scholar]
- 116.Shu C, Noble A & Aggarwal VK Metal-free photoinduced C(sp3)─H borylation of alkanes. Nature 586, 714–719 (2020). [DOI] [PubMed] [Google Scholar]
- 117.Kharasch MS & Sosnovsky G The reactions of t-butyl perbenzoate and olefins – a stereospecific reaction. J. Am. Chem. Soc 80, 756 (1958). [Google Scholar]
- 118.Kharasch M & Fono A Radical substitution reactions. J. Org. Chem 23, 325–326 (1958). [Google Scholar]
- 119.Kochi JK Copper salt-catalyzed reaction of butenes with peresters. J. Am. Chem. Soc 84, 774–784 (1962). [Google Scholar]
- 120.Muzart J Enantioselective copper-catalyzed allylic acetoxylation of cyclohexene. J. Mol. Catal 64, 381–384 (1991). [Google Scholar]
- 121.Andrus MB, Argade AB, Chen X & Pamment MG The asymmetric Kharasch reaction. Catalytic enantioselective allylic acyloxylation of olefins with chiral copper(I) complexes and tert-butyl perbenzoate. Tetrahedron Lett. 36, 2945–2948 (1995). [Google Scholar]
- 122.Gokhale AS, Minidis ABE & Pfaltz A Enantioselective allylic oxidation catalyzed by chiral bisoxazoline-copper complexes. Tetrahedron Lett. 36, 1831–1834 (1995). [Google Scholar]
- 123.Kawasaki K, Tsumura S & Katsuki T Enantioselective allylic oxidation using biomimetic tris(oxazolines)-copper(II) complex. Synlett. 1245–1246 (1995). [Google Scholar]
- 124.Andrus MB & Zhou Z Highly enantioselective copper–bisoxazoline-catalyzed allylic oxidation of cyclic olefins with tert-butyl p-nitroperbenzoate. J. Am. Chem. Soc 124, 8806–8807 (2002). [DOI] [PubMed] [Google Scholar]
- 125.Corey EJ & Lee J Enantioselective total synthesis of oleanolic acid, erythrodiol, β-amyrin, and other pentacyclic triterpenes from a common intermediate. J. Am. Chem. Soc 115, 8873–8874 (1993). [Google Scholar]
- 126.Neukirch H et al. Improved anti-inflammatory activity of three new terpenoids derived, by systematic chemical modifications, from the abundant triterpenes of the flowery plant calendula officinalis. Chem. Biodiversity 2, 657–671 (2005). [DOI] [PubMed] [Google Scholar]
- 127.García-Cabeza AL et al. Allylic oxidation of alkenes catalyzed by a copper–aluminum mixed oxide. Org. Lett 16, 1598–1601 (2014). [DOI] [PubMed] [Google Scholar]
- 128.García-Cabeza AL et al. Optimization by response surface methodology (RSM) of the Kharasch–Sosnovsky oxidation of valencene. Org. Process Res. Dev 19, 1662–1666 (2015). [Google Scholar]
- 129.Gephart RT et al. Reaction of CuI with dialkyl peroxides: CuII-alkoxides, alkoxy radicals, and catalytic C─H etherification. J. Am. Chem. Soc 134, 17350–17353 (2012). [DOI] [PubMed] [Google Scholar]
- 130.Tran BL, Driess M & Hartwig JF Copper-catalyzed oxidative dehydrogenative carboxylation of unactivated alkanes to allylic esters via alkenes. J. Am. Chem. Soc 136, 17292–17301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kohmura Y, Kawasaki K & Katsuki T Benzylic and allylic amination. Synlett. 12, 1456–1458 (1997). [Google Scholar]
- 132.Pelletier G & Powell DA Copper-catalyzed amidation of allylic and benzylic C─H bonds. Org. Lett 8, 6031–6034 (2006). [DOI] [PubMed] [Google Scholar]
- 133.Powell DA & Fan H Copper-catalyzed amination of primary benzylic C─H bonds with primary and secondary sulfonamides. J. Org. Chem 75, 2726–2729 (2010). [DOI] [PubMed] [Google Scholar]
- 134.Wiese S et al. Catalytic C─H amination with unactivated amines through copper(II) amides. Angew. Chem. Int. Ed 49, 8850–8855 (2010). [DOI] [PubMed] [Google Scholar]
- 135.Tran BL, Li B, Driess M & Hartwig JF Copper-catalyzed intermolecular amidation and imidation of unactivated alkanes. J. Am. Chem. Soc 136, 2555–2563 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zheng Y-W, Narobe R, Donabauer K, Yabukov S, & König B Copper(II)-photocatalyzed N─H alkylation with alkanes. ACS. Catal 10, 8582–8589 (2020). [Google Scholar]
- 137.Borduas N & Powell DA Copper-catalyzed oxidative coupling of benzylic C─H bonds with 1,3-dicarbonyl compounds. J. Org. Chem 73, 7822–7825 (2008). [DOI] [PubMed] [Google Scholar]
- 138.Song Z-Q et al. Photoredox oxo-C(sp3)─H bond functionalization via in situ Cu(I)-acetylide catalysis. Org. Lett 22, 832–836 (2020). [DOI] [PubMed] [Google Scholar]
- 139.Vasilopoulos A, Zultanski SL & Stahl SS Feedstocks to pharmacophores: Cu-catalyzed oxidative arylation of inexpensive alkylarenes enabling direct access to diarylalkanes. J. Am. Chem. Soc 139, 7705–7708 (2017). [DOI] [PubMed] [Google Scholar]
- 140.Xie W, Heo J, Kim D & Chang S Copper-catalyzed direct C─H alkylation of polyfluoroarenes by using hydrocarbons as an alkylating source. J. Am. Chem. Soc 142, 7487–7496 (2020). [DOI] [PubMed] [Google Scholar]
- 141.Ni Z et al. Highly regioselective copper-catalyzed benzylic C─H amination by N-fluorobenzenesulfonimide. Angew. Chem. Int. Ed 51, 1244–1247 (2012). [DOI] [PubMed] [Google Scholar]
- 142.Zhang W et al. Enantioselective cyanation of benzylic C─H bonds via copper-catalyzed radical relay. Science, 353, 1014–1018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xiao H et al. Copper-catalyzed late-stage benzylic C(sp3)─H trifluoromethylation. Chem. 5, 940–949 (2019). [Google Scholar]
- 144.Suh S-E et al. Site-selective copper-catalyzed azidation of benzylic C─H bonds. J. Am. Chem. Soc 142, 11388–11393 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sharma A & Hartwig JF Metal-catalyzed azidation of tertiary C─H bonds suitable for late-stage functionalization. Nature 517, 600–604 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Margrey KA, Czaplyski WL, Nicewicz DA & Alexanian EJ A general strategy for aliphatic C─H functionalization enabled by organic photoredox catalysis. J. Am. Chem. Soc 140, 4213–4217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu S et al. Copper-catalyzed oxidative benzylic C(sp3)─H amination: Direct synthesis of benzylic carbamates. Chem. Commun 56, 13013–13016 (2020). [DOI] [PubMed] [Google Scholar]
- 148.Wang A, DeOliveira CC & Emmert M Non-directed, copper catalyzed benzylic C─H amination avoiding substrate excess. ChemRxiv. Preprint at 10.26434/chemrxiv.8792243.v2 (2019). [DOI] [Google Scholar]
- 149.Jiang C, Chen P & Liu G Copper-catalyzed benzylic C─H bond thiocyanation: Enabling late-stage diversifications. CCS Chem. 2, 1884–1893 (2020). [Google Scholar]
- 150.Suh S-E, Nkulu LE, Lin S, Krska SW & Stahl SS Benzylic C─H isocyanation/amine coupling sequence enabling high-throughput synthesis of pharmaceutically relevant ureas. Chem. Sci 12, 10380–10387 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vasilopoulos A, Golden DL, Buss JA & Stahl SS Copper-catalyzed C─H fluorination/functionalization sequence enabling benzylic C─H cross coupling with diverse nucleophiles. Org. Lett 22, 5753–5757 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Champagne PA et al. Enabling nucleophilic substitution reactions of activated alkyl fluorides through hydrogen bonding. Org. Lett 15, 2210–2213 (2013). [DOI] [PubMed] [Google Scholar]
- 153.Champagne PA, Benhassine Y, Desroches J & Paquin J-P Friedel–Crafts reaction of benzyl fluorides: Selective activation of C─F bonds as enabled by hydrogen bonding. Angew. Chem. Int. Ed 53, 13835–13839 (2014). [DOI] [PubMed] [Google Scholar]
- 154.Hemelaere R, Champagne PA, Desroches J & Paquin J-F Faster initiation in the Friedel–Crafts reaction of benzyl fluorides using trifluoroacetic acid as activator. J. Fluorine Chem 190, 1–6 (2016). [Google Scholar]
- 155.Hamel J-D & Paquin J-F Activation of C─F bonds α to C─C multiple bonds. Chem. Commun 54, 10224–10239 (2018). [DOI] [PubMed] [Google Scholar]
- 156.Lopez MA, Buss JA & Stahl SS Site-selective chlorination of benzylic C─H bonds by Cu-catalysis. Org. Lett 24, (2022), in press, DOI: 10.1021/acs.orglett.1c04038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jin J et al. Copper(I)-catalysed site-selective C(sp3)─H bond chlorination of ketones, (E)-enones and alkylbenzenes by dichloramine-T. Nat. Commun 12, 4065 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fawcett A, Keller MJ, Herrera Z & Hartwig JF Site selective chlorination of C(sp3)─H bonds suitable for late-stage functionalization. Angew. Chem. Int. Ed 60, 8276–8283 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li J et al. Site-specific allylic C─H bond functionalization with a copper-bound N-centered radical. Nature 574, 516–521 (2019). [DOI] [PubMed] [Google Scholar]
- 160.Zhou J, Jin C, Li X & Su W Copper-catalyzed oxidative esterification of unactivated C(sp3)─H bonds with carboxylic acids via cross dehydrogenative coupling. RCS Adv. 5, 7232–7236 (2015). [Google Scholar]
- 161.Hu H et al. Copper-catalyzed benzylic C─H coupling with alcohols via radical relay enabled by redox buffering. Nat. Catal 3, 358–367 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen S-J, Golden DL, Krska SW & Stahl SS Copper-catalyzed cross-coupling of benzylic C─H bonds and azoles with controlled N-site selectivity. J. Am. Chem. Soc 143, 14438–14444 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ivanova AE et al. Ambident polyfluoroalkyl-substituted pyrazoles in the methylation reactions. J. Fluorine Chem 195, 47–56 (2017). [Google Scholar]
- 164.Huang A et al. Regioselective synthesis, NMR, and crystallographic analysis of N1-substituted pyrazoles. J. Org. Chem 82, 8864–8872 (2017). [DOI] [PubMed] [Google Scholar]
- 165.Zhang W, Chen P & Liu G Copper-catalyzed arylation of benzylic C─H bonds with alkylarenes as the limiting reagents. J. Am. Chem. Soc 139, 7709–7712 (2017). [DOI] [PubMed] [Google Scholar]
- 166.Zhang W, Wu L, Chen P & Liu G Enantioselective arylation of benzylic C─H bonds by copper-catalyzed radical relay. Angew. Chem. Int. Ed 58, 6425–6429 (2019). [DOI] [PubMed] [Google Scholar]
- 167.Fu L, Zhang Z, Chen P, Lin Z & Liu G Enantioselective copper-catalyzed alkynylation of benzylic C─H bonds via radical relay. J. Am. Chem. Soc 142, 12493–12500 (2020). [DOI] [PubMed] [Google Scholar]
- 168.Li Z, Cao L & Li C-J FeCl2-catalyzed selective C─C bond formation by oxidative activation of a benzylic C─H bond. Angew. Chem. Int. Ed 46, 6505–6507 (2007). [DOI] [PubMed] [Google Scholar]
- 169.Xia Q, Chen W & Qiu H Direct C─N coupling of imidazoles and benzylic compounds via iron-catalyzed oxidative activation of C─H bonds. J. Org. Chem 76, 7577–7582 (2011). [DOI] [PubMed] [Google Scholar]
- 170.Kumar J, Suresh E & Bhadra S Catalytic direct α-amination of arylacetic acid synthons with anilines. J. Org. Chem 85, 13363–13374 (2020). [DOI] [PubMed] [Google Scholar]
- 171.Karimov RR, Sharma A & Hartwig JF Late Stage Azidation of Complex Molecules. ACS Cent. Sci 2, 715–724 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ye Y-H, Zhang J, Wang G, Chen S-Y & Yu X-Q Cobalt-catalyzed benzylic C─H amination via dehydrogenative-coupling reaction. Tetrahedron 67, 4649–4654 (2011). [Google Scholar]
- 173.Tang S, Wang P, Li H & Lei A Multimetallic catalysed radical oxidative C(sp3)─H /C(sp)─H cross-coupling between unactivated alkanes and terminal alkynes. Nat. Commun 7, 11676 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu D, Liu C, Li H & Lei A Direct functionalization of tetrahydrofuran and 1,4-dioxane: Nickel-catalyzed oxidative C(sp3)─H arylation. Angew. Chem. Int. Ed 52, 4453–4456 (2013). [DOI] [PubMed] [Google Scholar]
- 175.Liu D et al. Nickel-catalyzed oxidative radical cross-coupling: An effective strategy for inert Csp3─H functionalization. Org. Lett 17, 998–1001 (2015). [DOI] [PubMed] [Google Scholar]
- 176.Vasilopoulos A, Krska SW & Stahl SS C(sp3)─H methylation enabled by peroxide photosensitization and Ni-mediated radical coupling. Science 372, 398–403 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schönherr H & Cernak T Profound methyl effects in drug discovery and a call for new C─H methylation reactions. Angew. Chem. Int. Ed 52, 12256–12267 (2013). [DOI] [PubMed] [Google Scholar]
- 178.Xu P, Guo S, Wang L & Tang P Silver-catalyzed oxidative activation of benzylic C─H bonds for the synthesis of difluoromethylated arenes. Angew. Chem. Int. Ed 126, 6065–6068 (2014). [DOI] [PubMed] [Google Scholar]
- 179.Yang H et al. Silver-promoted oxidative benzylic C─H trifluoromethoxylation. Angew. Chem. Int. Ed 57, 13266013270 (2018). [DOI] [PubMed] [Google Scholar]
- 180.Skubi KL, Blum TR & Yoon TP Dual catalysis strategies in photochemical synthesis. Chem. Soc. Rev 116, 10035–10074 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Twilton J et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem 1, 0052 (2017). [Google Scholar]
- 182.Shaw MH, Twilton J & MacMillan DWC Photoredox catalysis in organic chemistry. J. Org. Chem 81, 6898–6926 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Levin MD, Kim S & Toste FD Photoredox catalysis unlocks single-electron elementary steps in transition metal catalyzed cross-coupling. ACS Cent. Sci 2, 293–301 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Matsui JK, Lang SB, Heitz DR & Molander GA Photoredox-mediated routes to radicals: The value of catalytic radical generation in synthetic methods development. ACS Catal. 7, 2563–2575 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.McAtee RC, McClain EJ & Stephenson CRJ Illuminating Photoredox Catalysis. Trends Chem. 1, 111–125 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chan AY et al. Metallaphotoredox: The merger of photoredox and transition metal catalysis. Chem. Rev Accepted Article (2021) 10.1021/acs.chemrev.1c00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Capaldo L; Ravelli D & Fagnoni M Direct photocatalyzed hydrogen atom transfer (HAT) for aliphatic C─H bonds elaboration. Chem. Rev Accepted Article (2021) 10.1021/acs.chemrev.1c00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Leibler IN-M, Tekle-Smith MA & Doyle A A general strategy for C(sp3)─H functionalization with nucleophiles using methyl radical as a hydrogen atom abstractor. Nat. Commun 12, 6950 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zhang Y et al. A photoredox-catalyzed approach for formal hydride abstraction to enable a general Csp3─H fluorination with HF. Chem. Catalysis 2, 292–308 (2022). [Google Scholar]
- 190.Li G-X et al. A unified photoredox-catalysis strategy for C(sp3)─H hydroxylation and amidation using hypervalent iodine. Chem. Sci 8, 7180–7185 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Michaudel Q, Thevenet D & Baran PS Intermolecular Ritter-type C─H amination of unactivated sp3 carbons. J. Am. Chem. Soc 134, 2547–2550 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kiyokawa K, Takemoto K & Minakata S Ritter-type amination of C─H bonds at tertiary carbon centers using iodic acid as an oxidant. Chem. Commun 52, 13082–13085 (2016). [DOI] [PubMed] [Google Scholar]
- 193.Maeda B, Sakakibara Y, Murakami K & Itami K Photoredox-catalyzed benzylic esterification via radical-polar crossover. Org. Lett 23, 5113–5117 (2021). [DOI] [PubMed] [Google Scholar]
- 194.Meng Q-Y, Schirmer TE, Berger AL, Donabauer K & König B Photocarboxylation of benzylic C─H bonds. J. Am. Chem. Soc 141, 11393–11397 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Berger AL, Donabauer K & König B Photocatalytic carbanion generation from C─H bonds – reductant free Barbier/Grignard-type reactions. Chem. Sci 10, 10991–10996 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bosnidou AE & Muñiz K Intermolecular radical C(sp3)─H amination under iodine catalysis. Angew. Chem. Int. Ed 58, 7485–7489 (2019). [DOI] [PubMed] [Google Scholar]
- 197.Romine AM et al. Easy access to the copper(III) anion [Cu(CF3)4]−. Angew. Chem. Int. Ed 54, 2745–2749 (2015). [DOI] [PubMed] [Google Scholar]
- 198.Guo S, AbuSalim DI & Cook SP Aqueous benzylic C─H trifluoromethylation for late-stage functionalization. J. Am. Chem. Soc 140, 12378–12382 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.He J, Nguyen TN, Guo S & Cook SP Csp3─H trifluoromethylation of unactivated aliphatic systems. Org. Lett 23, 702–705 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Choi G, Lee GS, Park B, Kim D & Hong SH Direct C(sp3)─H trifluoromethylation of unactivated alkanes enabled by multifunctional trifluoromethyl copper complexes. Angew. Chem. Int. Ed 60, 5467–5474 (2021). [DOI] [PubMed] [Google Scholar]
- 201.Sarver PJ et al. The merger of decatungstate and copper catalysis to enable aliphatic C(sp3)─H trifluoromethylation. Nat. Chem 12, 459–467 (2020). [DOI] [PubMed] [Google Scholar]
- 202.Shaw MH, Shurtleff VW, Terrett JA, Cuthbertson JD & MacMillan DWC Native functionality in triple catalytic cross-coupling: sp3 C─H bonds as latent nucleophiles. Science 352, 1304–1308 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Le C, Liang Y, Evans RW, Li X & MacMillan DWC Selective sp3 C─H alkylation via polarity-match-based cross-coupling. Nature 547, 79–83 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhang X & MacMillan DWC Direct aldehyde C─H arylation and alkylation via the combination of nickel, hydrogen atom transfer, and photoredox catalysis. J. Am. Chem. Soc 139, 11353–11356 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Twilton J et al. Selective hydrogen atom abstraction through induced bond polarization: Direct α-arylation of alcohols through photoredox, HAT, and nickel catalysis. Angew. Chem. Int. Ed 57, 5369–5373 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ma Z-Y et al. Sulfonamide as photoinduced hydrogen-atom transfer catalyst for regioselective alkylation of C(sp3)─H bonds adjacent to heteroatoms. Org. Lett 23, 474–479 (2021). [DOI] [PubMed] [Google Scholar]
- 207.Perry IB et al. Direct arylation of strong aliphatic C─H bonds. Nature 560, 70–75 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Berger M, Goldblatt IL & Steel C Photochemistry of benzaldehyde. J. Am. Chem. Soc 95, 1717–1725 (1973). [Google Scholar]
- 209.Dórman G, Nakamura H Pulsipher A & Prestwich GD The life of pi star: Exploring the exciting and forbidden worlds of the benzophenone photophore. Chem. Rev 116, 15284–15398 (2016). [DOI] [PubMed] [Google Scholar]
- 210.Shen Y, Gu Y & Martin R sp3 C─H arylation and alkylation enabled by the synergy of triplet excited ketones and nickel catalysts. J. Am. Chem. Soc 140, 12200–12209 (2018). [DOI] [PubMed] [Google Scholar]
- 211.Dewanji A, Krach PE & Rueping M The dual role of benzophenone in visible-light/nickel photoredox-catalyzed C─H arylations: Hydrogen-atom transfer and energy transfer. Angew. Chem. Int. Ed 58, 3566–3570 (2019). [DOI] [PubMed] [Google Scholar]
- 212.Zhang L et al. The combination of benzaldehyde and nickel-catalyzed photoredox C(sp3)─H alkylation/arylation. Angew. Chem. Int. Ed 58, 1823–1827 (2019). [DOI] [PubMed] [Google Scholar]
- 213.Si X, Zhang L & Hashmi SK Benzaldehyde- and nickel-catalyzed photoredox C(sp3)─H alkylation/arylation with amides and thioethers. Org. Lett 21, 6329–6332 (2019). [DOI] [PubMed] [Google Scholar]
- 214.Heitz DR, Tellis JC & Molander GA Photochemical nickel-catalyzed C─H arylation: Synthetic scope and mechanistic investigations. J. Am. Chem. Soc 138, 12715–12718 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Shields BJ & Doyle AG Direct C(sp3)─H cross-coupling enabled by catalytic generation of chlorine radicals. J. Am. Chem. Soc 138, 12719–12722 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Nielsen MK et al. Mild, redox-neutral formylation of aryl chlorides through the photocatalytic generation of chlorine radicals. Angew. Chem. Int. Ed 56, 7191–7194 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Chang X, Lu H & Lu Z Enantioselective benzylic C─H arylation via photoredox and nickel dual catalysis. Nat. Commun 10, 3549 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cheng X; Li T; Liu Y & Lu Z Stereo- and enantioselective benzylic C─H alkenylation via photoredox/nickel dual catalysis. ACS Catal. 11, 11059–11065 (2021). [Google Scholar]
- 219.Joe CL & Doyle AG Direct acylation of C(sp3)─H bonds enabled by nickel and photoredox catalysis. Angew. Chem. Int. Ed 55, 4040–4043 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ahneman DT & Doyle AG C─H functionalization of amines with aryl halides by nickel-photoredox catalysis. Chem Sci. 7, 7002–7006 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Novaes LF et al. Electrocatalysis as an enabling technology for organic synthesis. Chem. Soc. Rev 50, 7941–8002 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Chen N & Xu H-C Electrochemical generation of nitrogen-centered radicals for organic synthesis. Green Synth. Catal 2, 165–178 (2021). [Google Scholar]
- 223.Masui M, Hara S, Ueshima T, Kawagushi T & Ozaki S Anodic oxidation of compounds having benzylic or allylic carbon and α-carbon to hetero atom using N-hydroxyphthalimide as a mediator. Chem Pharm. Bull 31, 4209–4211 (1983). [Google Scholar]
- 224.Masui M, Hosomi K, Tsuchida K & Ozaki S Electrochemical oxidation of olefins using N-hydroxyphthalimide as a mediator. Chem. Pharm. Bull 33, 4798–4802 (1985). [Google Scholar]
- 225.Masui M, Hara S & Ozaki S Anodic oxidation of amides and lactams using N-hydroxyphthalimide as a mediator. Chem. Pharm. Bull 34, 975–979 (1986). [Google Scholar]
- 226.Nutting JE, Rafiee MR & Stahl SS Tetramethylpiperidine N-oxyl (TEMPO), phthalimide N-oxyl (PINO), and related N-Oxyl species: Electrochemical properties and their use in electrocatalytic reactions. Chem. Rev 118, 4834–4885 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Horn EJ et al. Scalable and sustainable electrochemical allylic C─H oxidation. Nature 533, 77–81 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mo Y & Jensen KF Continuous N-hydroxyphthalimide (NHPI)-mediated electrochemical aerobic oxidation of benzylic C─H bonds. Chem. Eur. J 24, 10260–10265 (2018). [DOI] [PubMed] [Google Scholar]
- 229.Kawamata Y et al. Scalable, electrochemical oxidation of unactivated C─H bonds. J. Am. Chem. Soc 139, 7448–7451 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Saito M et al. N-ammonium ylide mediators for electrochemical C─H oxidation. J. Am. Chem. Soc 143, 7859–7867 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rafiee M, Wang F, Hruszkewycz DP & Stahl SS N-hydroxyphthalimide-mediated electrochemical iodination of methylarenes and comparison to electron-transfer-initiated C─H functionalization. J. Am. Chem. Soc 140, 22–25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hayashi R, Shimizu A & Yoshida J The stabilized cation pool method: Metal- and oxidant- free benzylic C─H/aromatic C─H cross-coupling. J. Am. Chem. Soc 138, 8400–8403 (2016). [DOI] [PubMed] [Google Scholar]
- 233.Zhu Y et al. A promising electro-oxidation of methyl-substituted aromatic compounds to aldehydes in aqueous imidazole ionic liquid solutions. J. Electroanal. Chem 751, 105–110 (2015). [Google Scholar]
- 234.Das A; Nutting JE & Stahl SS Electrochemical C─H oxygenation and alcohol dehydrogenation involving Fe-oxo species using water as the oxygen source. Chem. Sci 10, 7542–7548 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Robinson SG, Mack JBC, Alektiar SN, Du Bois J & Sigman MS Electrochemical ruthenium-catalyzed C─H hydroxylation of amine derivatives in aqueous acid. Org. Lett 22, 7060–7063 (2020). [DOI] [PubMed] [Google Scholar]
- 236.Meyer TH, Samanta RC, Del Vecchio A & Ackermann L Mangana(III/IV)electro-catalyzed C(sp3)─H azidation. Chem. Sci 12, 2890–2897 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Niu L et al. Manganese-catalyzed oxidative azidation of C(sp3)─H bonds under electrophotocatalytic conditions. J. Am. Chem. Soc 142, 17693–17702 (2020). [DOI] [PubMed] [Google Scholar]