Abstract
The Klebsiella pneumoniae mdcR gene, which encodes a LysR-type regulator, was overexpressed in Escherichia coli. Purified MdcR was found to bind specifically to the control region of either the malonate decarboxylase (mdc) genes or mdcR. We have also demonstrated that MdcR is an activator of the expression of the mdc genes, whereas it represses the transcription of the putative control region of mdcR, PmdcR, indicating a negative autoregulatory control.
Many bacterial species can utilize malonate as a carbon source. In these bacteria, the dissimilation of C3 dicarboxylic acid proceeds via a decarboxylation process with acetate and CO2 as the end products (7). The malonate decarboxylase (MDC) in some of the bacteria has been purified and characterized and was shown to be a multisubunit complex (9, 12, 19, 21). Genes encoding MDC as well as those encoding other accessory proteins required for malonate catabolism have recently been isolated from Klebsiella pneumoniae (10, 17), Acinetobacter calcoaceticus (14), and Malonomonas rubra (3).
By using a screening of the malonate-inducible MDC activity in Escherichia coli, we have isolated a recombinant plasmid, namely pHP817 (Fig. 1A), which contains the entire mdcABCDEFGHR gene cluster of K. pneumoniae CG43-17 (6). Two lines of evidence have indicated that a controlling system that is required for the regulation of malonate utilization is included in pHP817. First, the MDC activity in E. coli JM109(pHP817) can be significantly induced with malonate and was found to be comparable with that of K. pneumoniae CG43-17. Second, in the results from an in vivo 35S-labeling assay, the proteins synthesized upon malonate induction in E. coli JM109(pHP817) were identical to those found in K. pneumoniae CG43-17. Both the malonate-induced MDC activity and protein synthesis were not observed in the E. coli strain harboring a pHP817 derivative with a truncated mdcR. Nevertheless, the defect could be restored by providing the recombinant E. coli strain with an mdcR-expressing plasmid. The data together with the sequence analysis of mdcR (GenBank accession no. U14004), which shows extensive amino acid similarity with the members of the LysR family (18), strongly support the hypothesis that mdcR is the regulatory gene for controlling the expression of mdc genes.
FIG. 1.
(A) Organization of the mdc gene cluster. The transcriptional directions of the genes are indicated by arrows. The restriction map of the DNA segment contained in pHP817 is also shown. B, BamHI; RV, EcoRV; Sc, SacI; Sal, SalI. (B) Nucleotide sequences of the putative control regions Pmdc and PmdcR. Nucleotide sequences containing Pmdc and PmdcR for EMSA are shown. The start sites of the mdc and mdcR transcripts are shown (∗). The possible Shine-Dalgarno sequences are in bold and are underlined. The primers used in the study and the dyad symmetry in front of the mdcR coding region are also indicated.
Determination of the respective control regions of mdcR and the mdcABCDEFGH genes.
The regulatory gene of the LysR family is usually transcribed divergently and shares the same promoter regions with its target operon (18). However, the mdcR gene is located downstream and is transcribed convergently with the mdc gene cluster (Fig. 1A). The gene organization suggests that the regulatory mechanism of MdcR may be different from the other LysR-type regulators. To address this question, it is necessary to identify the promoters of mdc genes and mdcR. Our previous analysis of several deletion derivatives of pHP817 and their MDC activities upon malonate induction has allowed us to determine roughly the 5′ termini of the mdc genes and mdcR (Fig. 1B). To locate the promoter region more precisely, primer extension experiments were performed with the RNA templates isolated from K. pneumoniae CG43-17, which was grown in an M9 minimal medium supplemented with 40 mM sodium malonate. Two synthetic oligonucleotides, MDC28 and MDC3 (Fig. 1B), were used in the reactions for the identification of the transcriptional starts of the mdc genes and the mdcR gene, respectively. The assay mixture contained 10 pmol of the synthetic primer; 20 μg of total cellular RNA; 0.2 mM concentrations (each) of dATP, dTTP, and dGTP; 10 μCi of [α-32P]dCTP (∼3,000 Ci/mmol; Amersham, Little Chalfont, Buckinghamshire, United Kingdom); 5 U of RNasin; and 5 U of Moloney murine leukemia virus reverse transcriptase (Life Technologies, Gaithersburg, Md.). The reaction was performed at 37°C for 20 min and continued for another 10 min after the addition of excess dCTP. The primer extension product was analyzed on a sequencing gel side by side with the sequence ladders generated with the same primer by using pHP817 as the template. As shown in Fig. 2A, the transcription start site of the mdc genes was mapped to an A residue located 29 bp upstream of the mdcA start codon. The transcription start of mdcR (Fig. 2B) was also mapped to an A residue which is 11 bp downstream of a perfect dyad symmetrical sequence (Fig. 1B). Upstream of the two transcription starts, we found no consensus sequences of −10 and −35 as in a typical E. coli ς70 promoter. Since many members of the LysR family exert their roles as repressors, the 5′ part of the coding sequence of the MdcR target genes that is likely to contain the operator for MdcR was included as the putative control region for subsequent studies. Thus, two primer pairs, N2-C1 and RN1-RC2 (Fig. 1B), were synthesized for PCR to amplify the putative control regions of mdc genes and mdcR, Pmdc and PmdcR.
FIG. 2.
(A) Primer extension analysis of K. pneumoniae mdc genes. The transcription start site was mapped by extension of the primer MDC28, with a reverse transcriptase, and the product was analyzed on a 6% polyacrylamide-8M urea sequencing gel. The dideoxy sequencing ladder was generated by using the same primer. Lanes T, A, C, and G show the sequencing reaction products. Lane P shows the primer extension product, which is marked (∗). (B) Localization of the transcription start site of the K. pneumoniae mdcR gene. Lanes T, A, C, and G show the sequencing ladder generated with the same primer (MDC3) as that used for the primer extension reaction. Lanes 1 to 3 represent 10-fold increasing amounts of the primer extension product, and the start site (S) is indicated.
Heterologous expression of mdcR in E. coli and purification of the recombinant protein.
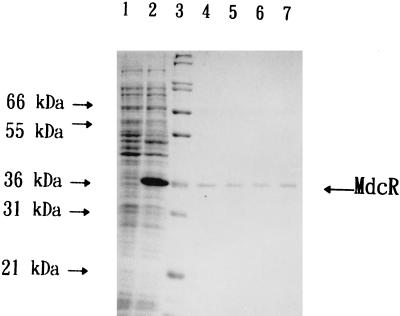
In order to facilitate the MdcR purification, the coding sequence of mdcR was amplified by PCR and the reaction product was digested with restriction enzymes EcoRI and NcoI prior to ligation into the pET30c vector (Novagen). The resulting plasmid, pHPm23, contains mdcR in frame fused with a hexahistidine sequence (HisTag) which allows the fusion protein to be purified by affinity chromatography through the HisBind resin (Novagen). E. coli NovaBlue(DE3) (Novagen) harboring pHPm23 was grown in Luria-Bertani medium at 37°C with vigorous shaking until an optical density at 600 nm of 0.3 had been reached. Isopropyl-β-d-thiogalactopyranoside (IPTG) was then added to a final concentration of 1 mM, and the incubation was continued for 4 h. The cells were collected, resuspended in 4 volumes of 1× binding buffer (5 mM imidazole, 500 mM NaCl, 20 mM Tris-HCl, pH 7.9), and disrupted by a sonicator (Ultrasonic Processor model XL; Heat Systems, Farmingdale, N.Y.). The cell extracts were clarified by centrifugation and applied to the affinity column packed with the HisBind resin. The column was washed to remove unbound proteins, and the MdcR was eluted under conditions recommended by Novagen. The eluted fractions were resolved on a sodium dodecyl sulfate-polyacrylamide gel, and the proteins were visualized by means of Coomassie brilliant blue R-250 staining. As shown in Fig. 3, a protein of approximately 36 kDa could be observed only from the cells harboring pHPm23 upon IPTG induction. The size of the protein is in good agreement with that predicted for the mdcR gene product plus the HisTag.
FIG. 3.
Expression and purification of recombinant MdcR. Whole-cell protein profiles and the purified fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Lanes 1 and 2 contain total proteins isolated from E. coli NovaBlue(DE3)(pHPm23). The whole-cell protein in lane 2 was obtained from IPTG-induced cells. Lane 3 shows the molecular size markers. Lanes 4 to 7 contain MdcR purified through HisBind resin. The sizes of the molecular mass markers are shown on the left. The position of purified MdcR is indicated on the right.
Specific binding of Pmdc by MdcR.
The electrophoresis mobility shift assay (EMSA) was used to investigate the binding of MdcR to the control region of the mdc genes (Pmdc). The DNA fragment containing Pmdc was obtained by PCR amplification, and the reaction product was purified from agarose gels and labeled with [γ-32P]ATP. The conditions for EMSA were as described previously (4) with slight modifications. The end-labeled DNA was incubated with increasing amounts of the purified MdcR protein in a buffer containing 20 mM Tris-HCl (pH 8.0), 5 mM MgCl2, 100 mM KCl, 7.5% glycerol, and 0.5 mM dithiothreitol at 25°C for 10 min. The reaction mixtures were then resolved on a 5% polyacrylamide gel by means of electrophoresis at 15°C with a constant current of 15 mA. The gels were dried, and the signals were visualized by autoradiography. As demonstrated in Fig. 4A, MdcR is capable of binding to the 0.21-kb Pmdc-containing DNA. A second type of binding complex (Fig. 4A) appeared as the concentrations of MdcR increased from 18 to 60 ng, suggesting the formation of oligomeric binding complexes.
FIG. 4.
(A) EMSA of purified MdcR and Pmdc. The approximately 0.21-kb PCR product amplified with the primer pair N2 and C1 was end labeled with [γ-32P]ATP and included in each reaction mixture with different concentrations of the purified MdcR protein. Lane 1 contains only the labeled DNA. Lanes 2 to 8 contain the labeled DNA with increasing amounts of purified MdcR (6, 12, 18, 60, 120, 180, and 240 ng, respectively). (B) Binding of MdcR to PmdcR. Lane 1 is labeled PmdcR. Lanes 2 to 5 show the binding mixture of the labeled DNA with 60, 120, 180, and 240 ng of purified MdcR, respectively. (C) Specific binding between MdcR and PmdcR. Lane 1 contains only labeled PmdcR. Lane 2 contains the binding mixture of labeled PmdcR and 120 ng of purified MdcR. Lanes 3 to 6 contain the binding mixtures of labeled DNA with 120 ng of purified MdcR and the following: unlabeled PmdcR DNA as a specific competitor (lane 3), pUC18 DNA as a nonspecific competitor (lane 4), an unlabeled DNA fragment from nucleotide position −83 to +83 of PmdcR (lane 5), and DHO1 oligonucleotide (lane 6). (D) Effects of malonate on the formation of the binding complex of MdcR and Pmdc. Lane 1 contains the labeled, free DNA. Lanes 2 to 4 are the complexes of MdcR with labeled PmdcR. Sodium malonate was added to the reaction mixture at concentrations of 20 mM (lane 3), 2 mM (lane 4), and 0.2 mM (lane 5). The arrow indicates the newly formed binding complex after the addition of the malonate.
Specific binding of PmdcR by MdcR.
Again, EMSA was performed to study the binding of MdcR to the control region of mdcR (PmdcR). The primer set RN1-RC2 (Fig. 1B) was used to amplify the DNA fragment containing PmdcR. Unlabeled PmdcR DNA and a synthetic oligonucleotide, DHO1 (5′-AAAAGGGAAGACCATGGTCTTCCCTTTT-3′), corresponding to the dyad symmetrical sequence contained in PmdcR were used as specific competitors in the binding reaction, whereas unlabeled pUC18 was the nonspecific competitor. In this case, only one binding complex was observed (Fig. 4B), indicating that the interactions between MdcR and Pmdc and between MdcR and PmdcR are somewhat different. In addition, MdcR appeared to have a higher affinity for Pmdc, since an approximately 20-fold higher concentration of MdcR is required for the formation of the binding complex of MdcR and PmdcR. The addition of unlabeled pUC18 DNA in the reaction mixture had no effect on the formation of the binding complex. On the other hand, we found that unlabeled PmdcR DNA at the same concentration and the synthetic oligomer DHO1 effectively compete the binding of the probe by MdcR. These data indicated that the binding between MdcR and PmdcR is rather specific. Interestingly, a DNA fragment (from nucleotide position −83 to +83 of PmdcR) in which the dyad symmetrical sequence was not included was still capable of competing the binding of MdcR to the labeled DNA. Like the other LysR-type regulators, MdcR is capable of binding to the specific DNA in the absence of malonate. Nevertheless, adding malonate to the reaction mixture appeared to affect the oligomerization of the MdcR and Pmdc binding complex as shown in Fig. 4D.
Regulation of mdc and mdcR promoters by MdcR.
A number of LysR-type regulatory proteins have been found to autoregulate the expression of their own genes negatively (18). Whether mdcR controls its own expression was investigated. Plasmid pUCD1752, a derivative of pUCD607 (20), containing bacterial luxAB was used as the reporter system (kindly provided by S.-T. Liu, Department of Microbiology and Immunology, Chang-Gung University, Taiwan). The upstream region of mdcR, from nucleotide positions −216 to +1 (Fig. 1B), was amplified by PCR, and the products were subcloned into the HindIII-KpnI site of pUCD1752 to make a transcriptional fusion with the luxAB genes. The DNA fragment containing PmdcR-luxAB was then subcloned into the HindIII-BamHI site of pACYC184 (5), a plasmid carrying a replication origin from p15A that allows the vector to replicate compatibly with the ColE1-derived plasmids. The resulting plasmid, pHPm52, was cotransformed with pHPm23 into E. coli NovaBlue(DE3) (Novagen), and the luciferase activity was determined. Briefly, the bacteria for luciferase assay were grown in Luria-Bertani medium to late log phase, decyl aldehyde was then added to the culture at a final concentration of 20 μl/ml, and the luminescence was measured with an Autolumat model LB953. The luciferase activity was expressed in relative luciferase units as described previously (12). As shown in Table 1, the expression of PmdcR-luxAB was reduced significantly if IPTG was added to induce MdcR synthesis. In contrast, the addition of malonate to the culture medium did not have any effect. The data indicated that the mdcR control region is negatively regulated by MdcR, independent of the presence of malonate.
TABLE 1.
Determination of the role of MdcR in the control of Pmdc and PmdcR with the luciferase activity assay
| E. coli NovaBlue(DE3) with plasmid | Luciferase activitya with:
|
|||
|---|---|---|---|---|
| Nothing | IPTG | Na malonate | IPTG + Na malonate | |
| pHPm52 | 58,518 | 42,030 | 45,817 | 68,311 |
| pHPm52 + pHPm23 | 65,800 | 1,209 | 72,927 | 1,333 |
| pHPm56 | 769 | 1,104 | 1,108 | 1,068 |
| pHPm56 + pHPm23 | 1,735 | 2,676 | 71,157 | 64,500 |
Data are averages from two independent experiments; variation between duplicates was less than 15%. Luciferase activity is rendered in relative luciferase units with the optical density at 600 nm. IPTG was added at a concentration of 0.2 mM, and sodium malonate was added at a concentration of 20 mM.
Similarly, the way that MdcR regulates the expression of mdc genes was also investigated by using luxAB as a reporter. The plasmid carrying Pmdc-luxAB in pACYC184, namely pHPm56, was transformed into E. coli NovaBlue(DE3)(pHPm23), and the luciferase activity of the transformant was measured. A significant increase in the luciferase activity upon malonate induction was observed in the bacteria (Table 1), which supported the previous notion that MdcR functions as an activator for Pmdc and that malonate is a coinducer. Finally, the luciferase activity of the bacteria carrying only pHPm52 was found to be comparable with that of the bacteria carrying pHPm56 and pHPm23 in an activated condition, suggesting that PmdcR is a relatively strong promoter.
Concluding remarks.
One interesting feature about the organization of the mdc gene cluster is that there is an 11-bp overlap between the 3′ coding sequences of mdcR and the putative mdcH gene. It has been demonstrated that when two genes are being transcribed convergently, the alteration in superhelicity resulting from the transcription of one gene would certainly affect the transcription of the other (23). Whether a similar interaction occurs between mdcH and mdcR remains to be investigated. Nevertheless, such a gene organization is likely to provide an additional type of regulation for mdc gene expression.
For a coordinate regulation by MdcR, we expected to find some features common to the two control regions, Pmdc and PmdcR. Indeed, several copies of a T-N11-A sequence, the consensus binding motif for the LysR-type regulators (1, 2, 8, 15), are present in both Pmdc and PmdcR (Fig. 1B). The perfect 15-bp dyad symmetry that is commonly found in promoters of the LysR-type regulators encoding genes is present in PmdcR but not in Pmdc. It has been shown that the binding to the control region by the LysR-type regulator is likely at several sites but is specific (14, 16, 22). The EMSA results shown in Fig. 4 also demonstrated that MdcR is capable of specifically binding to either Pmdc or PmdcR. Since PmdcR is a relatively strong promoter, it is likely that the cells retain a certain level of MdcR from time to time. Thus, a small amount of MdcR preferentially binds to the T-N11-A sequences in Pmdc and activates the expression of mdc genes when the coinducer malonate is present. In the absence of malonate, the accumulated MdcR then moves to bind PmdcR, which contains the dyad symmetry, to prevent its gene from being transcribed.
Like other members of the LysR-type transcriptional regulators, MdcR is a positive regulatory protein for its target promoter. However, it is intriguing that the overexpression of mdcR did not further increase the expression level of luciferase activity. One likely explanation is that overexpressed MdcR competes with the binding of the limited amount of malonate, which renders only a small percentage of MdcR in the activated form. This possibility remains to be verified. Nevertheless, the autoregulation of mdcR by MdcR resembles that for the other LysR-like proteins, which exert negative autoregulatory effects.
Acknowledgments
This work was supported by grants from the National Science Council of the Republic of China (NSC 86-2316-B182-007 to H.-L.P and NSC 86-2314-B182-080 to H.-Y.C).
REFERENCES
- 1.Belitsky B R, Janssen P J, Sonenshein A L. Sites required for GltC-dependent regulation of Bacillus subtilis glutamate synthase expression. J Bacteriol. 1995;177:5686–5695. doi: 10.1128/jb.177.19.5686-5695.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belitsky B R, Sonenshein A L. Mutations in GltC that increase Bacillus subtilis gltA expression. J Bacteriol. 1995;177:5696–5700. doi: 10.1128/jb.177.19.5696-5700.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg M, Hilbi H, Dimroth P. Sequence of a gene cluster from Malonomonas rubra encoding components of the malonate decarboxylase Na+ pump and evidence for their function. Eur J Biochem. 1997;245:103–115. doi: 10.1111/j.1432-1033.1997.00103.x. [DOI] [PubMed] [Google Scholar]
- 4.Carety J. Gel retardation. Methods Enzymol. 1991;208:103–117. doi: 10.1016/0076-6879(91)08010-f. [DOI] [PubMed] [Google Scholar]
- 5.Chang A C Y, Cohen S N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the p15A cryptic miniplasmid. J Bacteriol. 1978;134:1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang H Y, Lee J H, Deng W L, Fu T Z, Peng H L. Virulence and outer membrane properties of a galU mutant of Klebsiella pneumoniae CG43. Microb Pathog. 1996;20:255–261. doi: 10.1006/mpat.1996.0024. [DOI] [PubMed] [Google Scholar]
- 7.Dimroth P, Hilbi H. Enzymatic and genetic basis for bacterial growth on malonate. Mol Microbiol. 1997;25:3–10. doi: 10.1046/j.1365-2958.1997.4611824.x. [DOI] [PubMed] [Google Scholar]
- 8.Goehtals K, VanMontagu M, Holsters M. Conserved motifs in a divergent nod box of Azorhizobium caulinodans ORS571 reveal a common structure in promoters regulated by LysR-type proteins. Proc Natl Acad Sci USA. 1992;89:1646–1650. doi: 10.1073/pnas.89.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilbi H, Dehning I, Schink B, Dimroth P. Malonate decarboxylase of Malonomonas rubra, a novel type of biotin-containing acetyl enzyme. Eur J Biochem. 1992;207:117–123. doi: 10.1111/j.1432-1033.1992.tb17028.x. [DOI] [PubMed] [Google Scholar]
- 10.Hoenke S, Schmid M, Dimroth P. Sequence of a gene cluster from Klebsiella pneumoniae encoding malonate decarboxylase and expression of the enzyme in Escherichia coli. Eur J Biochem. 1997;246:530–538. doi: 10.1111/j.1432-1033.1997.00530.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y S, Byun H S. Purification and properties of a novel type of malonate decarboxylase from Acinetobacter calcoaceticus. J Biol Chem. 1994;269:29636–29641. [PubMed] [Google Scholar]
- 12.Kondo T, Strayer C A, Kulkarni R D, Taylor W, Ishiura M, Golden S S, Johnson C H. Circadian rhythms in prokaryotes: luciferase as a reporter of circadian gene expression in cyanobacteria. Proc Natl Acad Sci USA. 1993;90:5672–5676. doi: 10.1073/pnas.90.12.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koo J H, Jung S B, Byun H S, Kim Y S. Cloning and sequencing of gene encoding malonate decarboxylase in Acinetobacter calcoaceticus. Biochim Biophys Acta. 1997;1354:49–54. doi: 10.1016/s0167-4781(97)00134-6. [DOI] [PubMed] [Google Scholar]
- 14.Kullik I, Steven J, Toledano M B, Storz G. Mutational analysis of the redox-sensitive transcriptional regulator OxyR: regions important for DNA binding and multimerization. J Bacteriol. 1995;177:1285–1291. doi: 10.1128/jb.177.5.1285-1291.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsek M R, Ye W R, Pun P, Chakrabarty A M. Critical nucleotides in the interaction of a LysR-type regulator with its target promoter region. J Biol Chem. 1994;269:11279–11284. [PubMed] [Google Scholar]
- 16.Parsek M R, Kivisaar M, Chakrabarty A M. Differential DNA bending introduced by the Pseudomonas putida LysR-type regulator, CatR, at the plasmid-borne pheBA and chromosomal catBC promoters. Mol Microbiol. 1995;15:819–828. doi: 10.1111/j.1365-2958.1995.tb02352.x. [DOI] [PubMed] [Google Scholar]
- 17.Peng H L, Chang H Y. A novel regulatory protein which transactivates malonate utilization operon in Klebsiella pneumoniae. Presented at the 16th International Congress of Biochemistry and Molecular Biology, New Dehli, India. 1994. [Google Scholar]
- 18.Schell M A. Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol. 1993;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- 19.Schmid M, Berg M, Domroth P. Malonate decarboxylase of Klebsiella pneumoniae catalyses the turnover of acetyl and malonyl thioester residues on a coenzyme-A-like prosthetic group. Eur J Biochem. 1996;237:221–228. doi: 10.1111/j.1432-1033.1996.0221n.x. [DOI] [PubMed] [Google Scholar]
- 20.Shaw J J, Kado C I. Development of a Vibrio bioluminescence gene-set to monitor phytopathogenic bacteria during the ongoing disease process in a non-disruptive manner. Bio/Technology. 1986;4:560–564. [Google Scholar]
- 21.Takamura Y, Kitayama Y. Purification and some properties of malonate decarboxylase from Pseudomonas ovalis: an oligomeric enzyme with bifunctional properties. Biochem Int. 1981;3:483–491. [Google Scholar]
- 22.Wang L, Winans S C. The sixty nucleotides OccR operator contains a subsite essential and sufficient for OccR binding and a second subsite required for ligand-responsive DNA bending. J Mol Biol. 1995;253:691–702. doi: 10.1006/jmbi.1995.0583. [DOI] [PubMed] [Google Scholar]
- 23.Wu H Y, Tan J, Fang M. Long-range interaction between two promoters: activation of the leu-500 promoter by a distant upstream promoter. Cell. 1995;82:445–451. doi: 10.1016/0092-8674(95)90433-6. [DOI] [PubMed] [Google Scholar]