Abstract
Purpose
Accumulating evidence has reported that microRNAs (miRNAs) play a critical role in the mechanism of keloid formation, and recent research found that miR-23b-3p was upregulated in keloid fibroblasts (KFs). Herein, we explored the potential effect of miR-23b-3p on fibroblasts in keloid.
Materials and Methods
Clinical tissues, primary KFs and KEL FIB cells were used to detect the expression of miR-23b-3p by performing qRT-PCR. Gene knockdown was carried out to evaluate the molecular and biological changes of primary KFs and KEL FIB cells by conducting CCK-8 assay, flow cytometry and Western blot. The online databases and luciferase reporter assay were utilized to screen and identify the potential target of miR-23b-3p.
Results
Upregulation of miR-23b-3p was detected in keloid tissues, primary KFs and KF cell line KEL FIB cells, and inhibition of miR-23b-3p promoted apoptosis and suppressed proliferation and the expression of collagen I, collagen III and fibronectin of primary KFs and KEL FIB cells. Further investigation revealed that TNFAIP3, the ubiquitin-editing enzyme A20, was the direct target of miR-23b-3p, and inhibition of miR-23b-3p promoted the expression of A20 in primary KFs and KEL FIB cells. The in vitro assays indicated that A20 suppression inhibited apoptosis and facilitated proliferation and the expression of collagen I, collagen III and fibronectin of miR-23b-3p inhibitor-transfected primary KFs and KEL FIB cells. Finally, we found that miR-23b-3p inhibitor reduced the expression of receptor interacting serine/threonine protein kinase 1 (RIPK1), which was partially reversed by A20 inhibition.
Conclusion
These findings suggested that inhibition of miR-23b-3p/A20/RIPK1 axis induced apoptosis, limited proliferation and decreased extracellular matrix of KFs, providing a potential therapeutic target for treatment of keloid.
Keywords: keloid fibroblasts, proliferation, apoptosis, extracellular matrix, MiR-23b-3p
Introduction
Keloid is defined as a fibro-proliferative disease pathologically characterized by the excessive growth of fibroblasts and deposition of extracellular matrix in dermis after a series of insults (eg, wound, infection and inflammation).1,2 It has been recognized that keloid fibroblasts (KFs) share some biological similarities to tumor cells, such as uncontrolled proliferative capacity and increased invasive ability.3 Although surgical excision, steroid injection, radiation and laser therapy have been applied to keloid treatment, the efficacy remains unsatisfied because of the relatively high recurrence rate.4–6 Therefore, unveiling the underlying mechanism is a prerequisite for the development of alternative treatment for keloid.
Non-coding RNAs, a group of molecules involved in development and disease, have no ability to encode any functional proteins directly, but they could specifically modulate the expression of some messenger RNAs (mRNAs) to function in cells, among which microRNAs (miRNAs) are well studied.7 It has been well documented that miRNAs bind to the 3’-untranslated regions (3’-UTRs) of the targeted mRNAs to repress the translation process, which results in the downregulation of the corresponding proteins.8 Moreover, accumulating evidence has stated that miRNAs are implicated in the regulation of biological phenotypes of fibroblasts to participate in the pathogenesis of keloid.9 Liu et al reported that the miR-21 was upregulated in keloid tissues, and inhibition of miR-21 accelerated apoptosis of KFs by relieving the suppression to FasL, a proapoptotic molecule.10 An in vitro and in vivo study suggested that decreased expression of miR-21 could be regarded as a molecular biomarker that reflected the effectiveness of treatment of Galla Chinensis for keloid.11 In addition, some miRNAs are downregulated in keloid. Xu et al found that the expression of miR-194-5p was significantly decreased in KEL FIB cells, and the malignant phenotypes of KEL FIB cells were abated by exogenous upregulation of miR-194-5p.12 Therefore, the differential expression of miRNAs might play the opposite role in biological function of KFs and keloid.
Recently, Li and colleagues detected the miRNA expression profile in KFs, and screened some upregulated miRNAs, among which miR-23b-3p ranked first.13 A previous review article showed that miR-23b-3p was dysregulated in different tumors to modulate tumor biology.14 However, the potential role of miR-23b-3p in the biological changes of KFs remains unknown. Herein, we performed in vitro assays to evaluate the effects of miR-23b-3p on proliferation, apoptosis and the expression of extracellular matrix-related molecules in KFs through cell transfection, and to explore the target of miR-23b-3p to clarify the underlying mechanism via bioinformatics and biological experiments.
Materials and Methods
Tissue Preparation
A total of 30 patients (16 male patients and 14 female patients) with keloid were collected from Department of Oral and Maxillofacial Surgery and Plastic Surgery, the Affiliated Hospital of Jiangxi University of Chinese Medicine. The mean value of the age of male patients was 27.56 years, and the mean value of the age of male patients was 25.64 years. The keloid tissues were surgically removed from earlobe or chest of the patients, who did not receive any other therapies before. The tissues used in the control group was adjacent to keloid and its appearance presented the appearance of the normal skin. The informed consent form was signed by each participant. The research was approved by the Ethics Committee of the Affiliated Hospital of Jiangxi University of Chinese Medicine, and the procedures complied with the Declaration of Helsinki.
Isolation of Primary KFs
The primary KFs were obtained from the fresh tissues, of which the epidermis and subcutaneous adipose tissues were removed. Next, the tissues were cut, and then put into Trypsin-EDTA Solution (Beyotime, China) at 4 °C overnight. After washing and centrifugation, Collagenase Type I (Thermo Fisher Scientific, USA) was added, and the tissues were incubated at 37 °C for 2 h, followed by washing and centrifugation. DMEM (Thermo Fisher Scientific, USA) containing 10% fetal bovine serum (Thermo Fisher Scientific, USA) was used to resuspend. The cell suspension was placed in the cell incubator for 10 min, and the supernatant was transferred to the cell-culture dish to obtain the primary KFs.
Cell Culture
The KF cell line KEL FIB cells were purchased from ATCC (Manassas, USA). The primary KFs and KEL FIB cells were both cultured in DMEM (Thermo Fisher Scientific, USA) containing 10% fetal bovine serum (Thermo Fisher Scientific, USA) in the cell incubator containing 5% CO2 37 °C. Cell passage was performed every 3 d.
Cell Transfection
The primary KFs and KEL FIB cells were seeded into 96-well plates or 6-well plates with a number of 8000 cells/well or 2.5 × 105 cells/well, respectively. miR-23b-3p inhibitor and/or small interfering RNA (siRNA) against A20 (si-A20), both of which were purchased from GenePharma (China), were diluted with serum-free DMEM to treat the cells in the presence of Lipofectamine 3000 (Thermo Fisher Scientific, USA) according to the manufacturer’s guidelines. After 8 h, the serum-free DMEM was replaced by DMEM containing 10% fetal bovine serum to incubate the cells for 48 h.
RNA Extraction and Detection
After cell transfection or TGF-β1 (5 ng/mL, Merck, Germany) treatment, the cells were collected and the total RNA was extracted with RNeasy Mini Kit (QIAGEN, Germany). After quantification, the RNA was utilized to obtain cDNA by using PrimeScript RT Reagent Kit (Takara, Japan). Next, SYBR Premix Ex Taq II Kit (Takara, Japan) was used to conduct quantitative real time-polymerase chain reaction (qRT-PCR), and 2−ΔΔCT method was carried out to evaluate the expression of the indicated genes (Table 1), and the primers of the above were purchased from Sangon (China).
Table 1.
The Sequences of Primers
| Gene | Sequence (5’-3’) |
|---|---|
| miR-23b-3p | F: TCACGGTCCAGTTTTCCCAG |
| R: GGAAGGAGGCAAATCCAGCT | |
| U6 | F: CTCGCTTCGGCAGCACAT |
| R: AACGCTTCACGAATTTGCGT | |
| TNFAIP3 (A20) | F: TGGCCAATGGTTCACCTACC |
| R: TTCACCCGTCCTGTTTGGAT | |
| COL1A1 (collagen I) | F: GGCAGTTCTTGGTCTCGTCA |
| R: AGGGGCGGGAAGTGAAAAAT | |
| COL3A1 (collagen III) | F: CCTGAAGCTGATGGGGTCAA |
| R: AATGCTGACTGCTTTATGACCT | |
| FN1 (fibronectin) | F: GAGCATGTGGCTTGGCACTA |
| R: TCAAACATGAATTGCACTCACCT | |
| RIPK1 | F: CCGCCAGCAAGATGAGGAAT |
| R: CCACGTCCATCACGGGATTA | |
| GAPDH | F: AAGGGCCCTGACAACTCTTTT |
| R: CTGGTGGTCCAGGGGTCTTA |
Evaluation of Cell Viability
Cell Counting Kit-8 (CCK8, Beyotime, China) was utilized to measure the cell viability according to the manufacturer’s guidelines. After cell transfection, the primary KFs and KEL FIB cells were seeded in a 96-well plate at a density of 7000 cells per well and cultured for indicated time. Next, the cells in each well were incubated with a mixed solution containing 200 μL fresh medium and 20 μL reagents at 37 °C for 1.5 h. The value of optical density (OD) at 450 nm was detected by Microplate Reader (Bio-Rad, USA).
Flow Cytometry
The Annexin V-PE/7-AAD Apoptosis Detection Kit (Solarbio, China) was utilized to monitor apoptosis of primary KFs and KEL FIB cells with indicated treatment according to the instruction. The cells were collected in specific tubes and 5 μL of Annexin V-PE was added for incubation for 5 min, followed by incubation with 10 μL of 7-AAD for 15 min at room temperature. Apoptotic cells were detected and analyzed by CytoFLEX LX (Beckman Coulter, USA).
Detection of Protein Expression
RIPA lysis buffer (Beyotime, China) and protease inhibitor PMSF (Beyotime, China) were used to lyse the cells, and protein concentration was evaluated by utilizing Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, USA). The protein samples were added into SDS-PAGE gels (Beyotime, China) in the electrophoresis apparatus for indicated period, followed by being transferred to Immobilon-FL PVDF (Merck, Germany). Next, PVDF was incubated with 5% non-fat milk for 90 min at room temperature, followed by been incubated with primary antibodies (A20 Rabbit mAb, Cell Signaling Technology, USA; Rabbit monoclonal to Collagen I, Abcam, USA; Rabbit monoclonal to Collagen III, Abcam, USA; Rabbit monoclonal to Fibronectin, Abcam, USA; RIP Rabbit mAb, Cell Signaling Technology, USA; GAPDH Mouse Monoclonal Antibody, Beyotime, China) overnight at 4 °C. After incubation with secondary antibodies (Beyotime, China), PVDF was detected with BeyoECL Plus reagent (Beyotime, China).
Luciferase Reporter Assay
The cells were seeded into a 96-well plate with a density of 8000 cells per well. The pmirGLO vector carrying wild type (WT) or mutant (Mut) TNFAIP3, combined with miR-23b-3p mimic or negative control (NC) mimic, was co-transfected into KEL FIB cells by using Lipofectamine 3000 (Thermo Fisher Scientific, USA). After 48 h, the cells were tested with Bio-GloTM Luciferase Assay System (Promega, USA).
Statistical Analysis
The data were processed with GraphPad Prism 8.0 Software (GraphPad, USA) and depicted as mean ± standard deviation (SD). Each assay was performed three times independently. Non-parametric t tests and two-way ANOVA were used to analyze the data, and P < 0.05 was regarded as statistical difference.
Results
MiR-23b-3p Was Overexpressed in Keloid
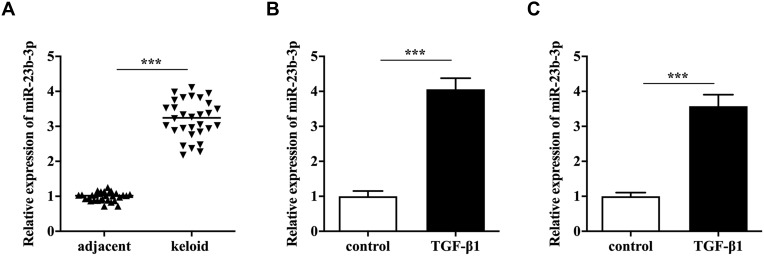
First, qRT-PCR analysis showed that keloid tissues expressed a significantly higher level of miR-23b-3p compared to the adjacent tissues (Figure 1A). Next, primary KFs were treated with TGF-β1, and we found TGF-β1 induced upregulation of miR-23b-3p (Figure 1B). In parallel, miR-23b-3p was overexpressed in TGF-β1-treated KEL FIB cells (Figure 1C). These results suggested that miR-23b-3p might be involved in the pathogenesis of keloid.
Figure 1.
MiR-23b-3p was overexpressed in keloid. The relative expression of miR-23b-3p in keloid tissues (A), TGF-β1-treated primary KFs cells (B) and TGF-β1-treated KEL FIB cells (C). ***P<0.001.
Anti-miR-23b-3p Suppressed Proliferation and Promoted Apoptosis of KFs
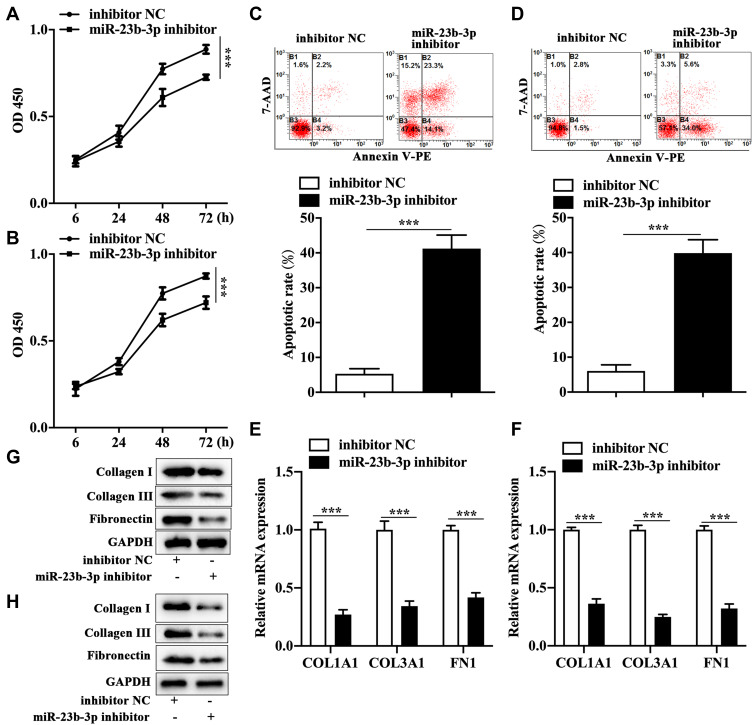
To explore the exact role of miR-23b-3p in keloid, we performed cell transfection in both primary KFs and KEL FIB cells with miR-23b-3p inhibitor. The CCK-8 assay showed that miR-23b-3p inhibitor restrained proliferation of primary KFs (Figure 2A) and KEL FIB cells (Figure 2B). Correspondingly, miR-23b-3p inhibitor enhanced apoptotic rate of primary KFs (Figure 2C) and KEL FIB cells (Figure 2D).
Figure 2.
Anti-miR-23b-3p suppressed proliferation and expression of extracellular matrix-related molecules and promoted apoptosis of KFs. Changes of cell viability of miR-23b-3p inhibitor-transfected primary KFs cells (A) and KEL FIB cells (B). Apoptosis of miR-23b-3p inhibitor-transfected primary KFs cells (C) and KEL FIB cells (D). The mRNA expression of collagen I, collagen III and fibronectin in miR-23b-3p inhibitor-transfected primary KFs cells (E) and miR-23b-3p inhibitor-transfected KEL FIB cells (F). The protein level of collagen I, collagen III and fibronectin in miR-23b-3p inhibitor-transfected primary KFs cells (G) and miR-23b-3p inhibitor-transfected KEL FIB cells (H). ***P<0.001.
Abbreviation: inhibitor NC, negative control inhibitor.
Anti-miR-23b-3p Inhibited Extracellular Matrix-Related Molecules in KFs
Next, we examined the expression of key components of the extracellular matrix. The qRT-PCR analysis revealed that mRNA level of collagen I, collagen III and fibronectin was prohibited in primary KFs (Figure 2E) and KEL FIB cells (Figure 2F) with miR-23b-3p inhibitor transfection. In parallel, transfection with miR-23b-3p inhibitor decreased the protein expression of collagen I, collagen III and fibronectin in primary KFs (Figure 2G) and KEL FIB cells (Figure 2H).
A20 Was the Direct Target of miR-23b-3p
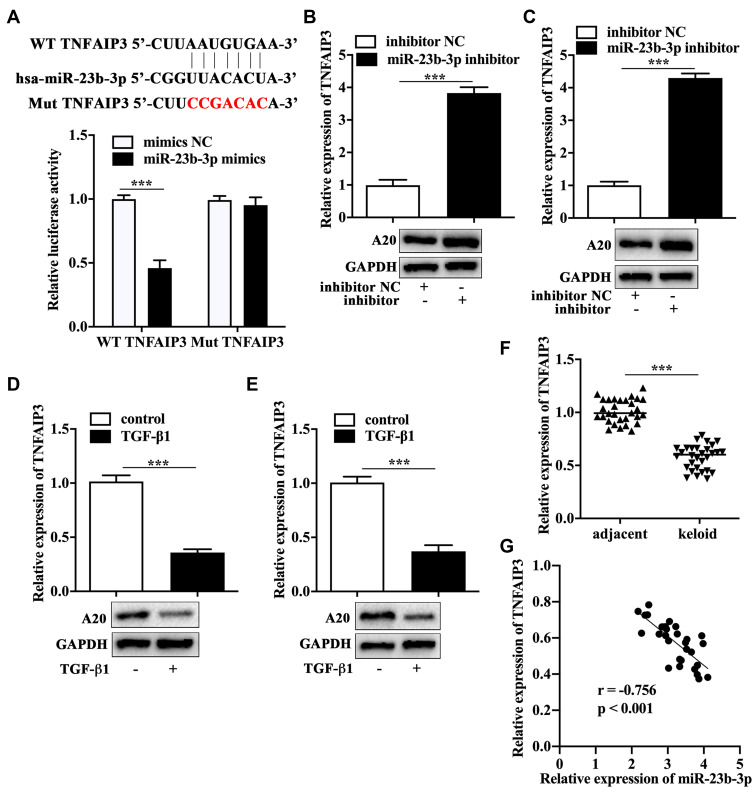
It has been acknowledged that miRNAs function in cell biology by regulating the expression of functional molecules. ENCORI database (https://starbase.sysu.edu.cn/agoClipRNA.php?source=mRNAandflag=miRNAandclade=mammalandgenome=humanandassembly=hg19andmiRNA=hsa-miR-23b-3pandclipNum=anddeNum=andpanNum=andproNum=andprogram=andtarget= - modal) and TargetScanHuman database (https://starbase.sysu.edu.cn/agoClipRNA.php?source=mRNAandflag=targetandclade=mammalandgenome=humanandassembly=hg19andmiRNA=hsa-miR-23b-3pandclipNum=1anddeNum=0andpanNum=0andproNum=1andprogram=andtarget=TNFAIP3) were utilized to screen the potential target of miR-23b-3p, and we found TNFAIP3, encoding A20 protein, was one of the key downstream molecules of miR-23b-3p. The luciferase reporter assay showed that the cells co-transfected with miR-23b-3p mimics and wild type TNFAIP3 displayed a significantly lower luciferase activity compared with that co-transfected with mimics NC and wild type TNFAIP3 (Figure 3A), which indicated that TNFAIP3 was the direct target of miR-23b-3p. We found that miR-23b-3p inhibitor-transfected primary KFs (Figure 3B) and KEL FIB cells (Figure 3C) expressed a higher level of A20 as assessed by qRT-PCR and Western blot analysis. In addition, TGF-β1 treatment reduced the mRNA and protein expression of A20 in primary KFs (Figure 3D) and KEL FIB cells (Figure 3E). We also found that the mRNA expression of A20 was dramatically lower in keloid tissues compared to the adjacent tissues (Figure 3F), which was negatively correlated to the expression of miR-23b-3p in keloid tissues (Figure 3G).
Figure 3.
A20 was the direct target of miR-23b-3p. (A) Schematic graph of binding sites between miR-23b-3p and TNFAIP3, and the evaluation of binding ability by dual luciferase reporter assay. The expression of A20 in miR-23b-3p inhibitor-transfected primary KFs (B), miR-23b-3p inhibitor-transfected KEL FIB cells (C), TGF-β1-treated primary KFs (D) and TGF-β1-treated KEL FIB cells (E). (F) The mRNA expression of A20 in keloid tissues. (G) The correlation between the expression of A20 and miR-23b-3p. ***P<0.001.
Abbreviation: mimics NC, negative control mimics.
A20 Knockdown Antagonized the Effects of miR-23b-3p Inhibitor on the Biological Phenotypes of KFs
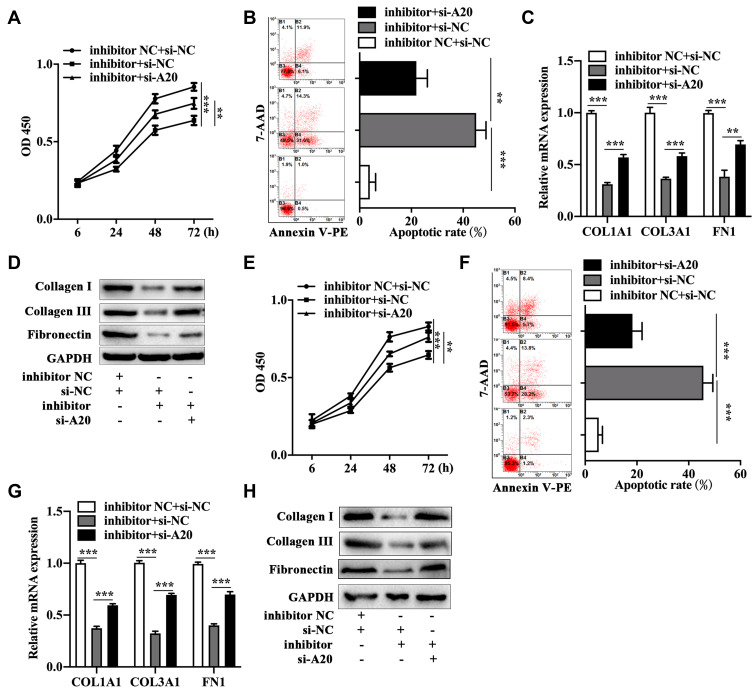
To illuminate the potential effects of A20 on miR-23b-3p inhibitor-mediated biological change of KFs, we conducted the following assays. A20 siRNA and miR-23b-3p inhibitor co-transfected primary KFs presented an increased proliferative ability (Figure 4A) and a decreased apoptotic rate (Figure 4B) compared to the negative control siRNA and miR-23b-3p inhibitor co-transfected primary KFs. Meanwhile, the mRNA (Figure 4C) and protein (Figure 4D) expression of collagen I, collagen III and fibronectin were higher in A20 siRNA and miR-23b-3p inhibitor co-transfected primary KFs compared to that in negative control siRNA and miR-23b-3p inhibitor co-transfected primary KFs. The similar findings were found in the A20 siRNA and miR-23b-3p inhibitor co-transfected KEL FIB cells as was determined by CCK-8 assay (Figure 4E), flow cytometry (Figure 4F), qRT-PCR (Figure 4G) and Western blot (Figure 4H). The above results suggested that A20 inhibition played an opposite role in miR-23b-3p inhibitor-mediated biological changes of KFs.
Figure 4.
A20 knockdown antagonized the effects of miR-23b-3p inhibitor on the biological phenotypes of KFs. Measurement of cell viability (A), apoptosis (B), mRNA level of collagen I, collagen III and fibronectin (C) and protein expression of collagen I, collagen III and fibronectin (D) in primary KFs with co-transfection. Evaluation of cell viability (E), apoptosis (F), mRNA level of collagen I, collagen III and fibronectin (G) and protein expression of collagen I, collagen III and fibronectin (H) in KEL FIB cells with co-transfection. **P<0.01, ***P<0.001.
Abbreviations: inhibitor NC, negative control inhibitor; si-NC, negative control siRNA; inhibitor, miR-23b-3p inhibitor; si-A20, siRNA against TNFAIP3.
RIPK1 Was Regulated by miR-23b-3p/A20 Axis
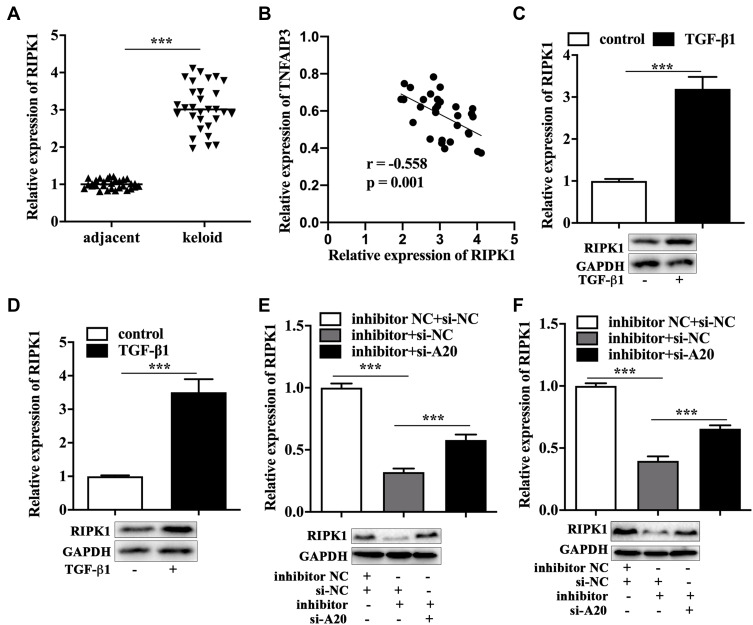
A20 could induce K48-ubiquitylation and degradation of RIPK1, a key molecule of cell survival.15 The qRT-PCR analysis showed a higher level of RIPK1 in keloid tissues compared to that in adjacent tissues (Figure 5A), and the correlation between the expression of A20 and RIPK1 in keloid tissues was negative (Figure 5B). In addition, TGF-β1 treatment facilitated the mRNA and protein expression of RIPK1 in primary KFs (Figure 5C) and KEL FIB cells (Figure 5D). We also found that miR-23b-3p inhibitor induced downregulation of RIPK1, while A20 siRNA partially reversed the decreased expression of RIPK1 in miR-23b-3p inhibitor-transfected primary KFs (Figure 5E) and KEL FIB cells (Figure 5F) by performing qRT-PCR analysis and Western blot.
Figure 5.
RIPK1 was regulated by miR-23b-3p/A20 axis. (A) The mRNA expression of RIPK1 in keloid tissues. (B) The correlation between the expression of A20 and RIPK1. The mRNA and protein expression of RIPK1 in TGF-β1-treated primary KFs (C) and TGF-β1-treated KEL FIB cells (D). The mRNA and protein expression of RIPK1 in primary KFs with co-transfection (E) and KEL FIB cells with co-transfection (F). ***P<0.001.
Abbreviations: inhibitor NC, negative control inhibitor; si-NC, negative control siRNA; inhibitor, miR-23b-3p inhibitor; si-A20, siRNA against TNFAIP3.
Discussion
It comes to light that KFs are the predominant effector cells in keloid. The overactivation of KFs function, the unbalance of proliferation and apoptosis of KFs, and the increased secretion of extracellular matrix are all the key factors that facilitate the keloid formation, which are also the hallmarks of keloid.3,16,17 Currently, multiple approaches are employed to the clinical treatment for keloid, but the high recurrence rate prompts investigators to unveil the underlying mechanisms so as to improve the clinical efficacy and outcomes. Theoretically, overcoming the excessive proliferation of KFs and the increased deposition of extracellular matrix might be a promising strategic management for keloid treatment.
Increasing evidence has demonstrated that miRNAs are involved in the modulation of biological function of fibroblast, which further contributes to pathogenesis of keloid.9 In the present study, we verified the upregulation of miR-23b-3p existed in keloid tissues, primary KFs and KF cell line KEL FIB cells, which was in line with the previous bioinformatics analysis.13 Currently, studies about miR-23b-3p mostly concentrate on tumors. A recent research found that miR-23b-3p was overexpressed in patients with non-small cell lung cancer, which was a biomarker of tumor progression and also could be regarded as a poor prognosis biomarker.18 In patients with hepatocellular carcinoma, the upregulation of miR-23b-3p predicted a poor overall survival rate.19 Functional study demonstrated that inhibition of the upregulated miR-23b-3p in osteosarcoma cells suppressed cell proliferation and tumor growth.20 Besides, miR-23b-3p plays a critical role in non-neoplastic diseases. In patients with idiopathic pulmonary fibrosis, lung fibroblasts generated increased amount of miR-23b-3p to induce reactive oxygen species production to damage lung epithelial cells, which aggregated idiopathic pulmonary fibrosis, and the elevated miR-23b-3p was a biomarker that reflected the severity of idiopathic pulmonary fibrosis.21 Yang and colleagues demonstrated that both atrial appendage tissues of patients with atrial fibrillation and the corresponding cell model derived from human atrial fibroblasts expressed a higher level of miR-23b-3p, which promoted the expression of collagen I, collagen III and α-smooth muscle actin.22 In our research, the in vitro assays revealed that proliferation slowed down and apoptotic cells increased after downregulation of miR-23b-3p in primary KFs and KEL FIB cells. In addition, we also found that knockdown of miR-23b-3p dramatically decreased the expression of collagen I, collagen III and fibronectin, key components of extracellular matrix. The above findings suggest that miR-23b-3p is a key regulator in fibroblasts and keloid.
To probe the downstream mechanism, we screened and identified a potential target of miR-23b-3p by using online databases and dual luciferase reporter assay, namely TNFAIP3 which encodes A20. As a crucial ubiquitin-editing protein, A20 protects cell function by inhibiting apoptosis and inflammation.23–25 Literally, A20 represses recruitment of RIPK1 to death domain of tumor necrosis factor receptor 1, which blocks cleavage of caspase cascade to restrain apoptotic process.26 In nasopharyngeal carcinoma cells, A20 overexpression reduced cell proliferation and increased cell apoptosis, whereas A20 knockdown exerted the opposite role.27 A previous study found that the decreased expression of A20 was engaged in tumor necrosis factor-induced apoptosis of dermal fibroblasts.28 In addition, TGF-β facilitated downregulation of A20 in fibroblasts, and A20 overexpression cut down the production of extracellular matrix in Toll-like receptor 4-treated fibroblasts.29 Our research showed that A20 was downregulated in Keloid tissues and KFs, and TGF-β1 inhibited A20 expression in vitro. We also found that A20 knockdown antagonized the pro-apoptotic and anti-proliferative roles of miR-23b-3p inhibition, and miR-23b-3p inhibition-induced decreased expression of collagen I, collagen III and fibronectin was reversed by A20 silencing. Moreover, keloid tissues and KFs expressed a higher level of RIPK1, which might involve the downregulation of A20 in keloid tissues and KFs, as A20 could degrade RIPK1.15 A recent investigation manifested that RIPK1 was overexpressed in human hypertrophic scar fibroblasts, and inhibition of RIPK1 by using its inhibitor Necrostatin-1 partially limited proliferation and migration of the hypertrophic scar fibroblasts.30 An in vitro and in vivo exploration from Mou and colleagues also indicated that Necrostatin-1, inhibitor of RIPK1, weakened proliferative ability of pulmonary fibroblasts, suppressed the expression of collagen I, collagen IV, α-smooth muscle actin and fibronectin, decreased collagen deposition and ameliorated fibrosis of lung tissues.31 Combined with our research, these findings suggested that RIPK1 might promote proliferation of KFs and increase extracellular matrix in keloid.
Conclusion
Taken together, our study made clear that upregulation of miR-23b-3p was detectable in clinical samples and fibroblasts of keloid, and silencing miR-23b-3p reduced proliferation and extracellular matrix and increased apoptosis of KFs. Furthermore, miR-23b-3p positively modulated the expression of RIPK1 by targeting A20. These findings highlighted a pivotal role of miR-23b-3p/A20/RIPK1 axis in the biological function of keloid fibroblasts, which provided an alternative mechanism to deepen the pathogenesis of keloid. Notably, further in vivo experiments are needed to expound the exact role of miR-23b-3p/A20/RIPK1 axis in keloid.
Acknowledgments
We were grateful for the kind help of Prof. Huicai Wen and Prof. Yisen Shao. We also appreciated the participation of the participants in this work.
Data Sharing Statement
All the data in the research are available from the corresponding author on reasonable request.
Ethics Statement
This study was conducted with the signing of the informed consent form and complied with the Declaration of Helsinki Principle. This work was approved by the Ethics Committee of the Affiliated Hospital of Jiangxi University of Chinese Medicine.
Disclosure
The authors declare no conflict of interests.
References
- 1.Macarak EJ, Wermuth PJ, Rosenbloom J, Uitto J. Keloid disorder: fibroblast differentiation and gene expression profile in fibrotic skin diseases. Exp Dermatol. 2021;30(1):132–145. doi: 10.1111/exd.14243 [DOI] [PubMed] [Google Scholar]
- 2.Wang ZC, Zhao WY, Cao Y, et al. The Roles of Inflammation in Keloid and Hypertrophic Scars. Front Immunol. 2020;11:603187. doi: 10.3389/fimmu.2020.603187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan S, Khumalo N, Bayat A. Understanding Keloid Pathobiology From a Quasi-Neoplastic Perspective: less of a Scar and More of a Chronic Inflammatory Disease With Cancer-Like Tendencies. Front Immunol. 2019;10:1810. doi: 10.3389/fimmu.2019.01810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekstein SF, Wyles SP, Moran SL, Meves A. Keloids: a review of therapeutic management. Int J Dermatol. 2020;60(6):661–671. doi: 10.1111/ijd.15159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold MH, Nestor MS, Berman B, Goldberg D. Assessing keloid recurrence following surgical excision and radiation. Burns Trauma. 2020;8:tkaa031. doi: 10.1093/burnst/tkaa031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Limmer EE, Glass DA. A Review of Current Keloid Management: mainstay Monotherapies and Emerging Approaches. Dermatol Ther (Heidelb). 2020;10(5):931–948. doi: 10.1007/s13555-020-00427-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodall GJ, Wickramasinghe VO. RNA in cancer. Nat Rev Cancer. 2021;21(1):22–36. doi: 10.1038/s41568-020-00306-0 [DOI] [PubMed] [Google Scholar]
- 8.Chipman LB, Pasquinelli AE. miRNA Targeting: growing beyond the Seed. Trends Genet. 2019;35(3):215–222. doi: 10.1016/j.tig.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stevenson AW, Deng Z, Allahham A, Prele CM, Wood FM, Fear MW. The epigenetics of keloids. Exp Dermatol. 2021;30(8):1099–1114. doi: 10.1111/exd.14414 [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Ren L, Liu W, Xiao Z. MiR-21 regulates the apoptosis of keloid fibroblasts by caspase-8 and the mitochondria-mediated apoptotic signaling pathway via targeting FasL. Biochem Cell Biol. 2018;96(5):548–555. doi: 10.1139/bcb-2017-0306 [DOI] [PubMed] [Google Scholar]
- 11.Tang Z, Ding J, Zhai X, Jing M, Guan Z, Li Y. MicroRNA-21 may be involved in the therapeutic effects of Galla chinensis ointment on keloid. J Int Med Res. 2020;48(3):300060520909602. doi: 10.1177/0300060520909602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Q, Jiang S. miR-194-5p serves a suppressive role in human keloid fibroblasts via targeting NR2F2. Mol Med Rep. 2021;23:1. [DOI] [PubMed] [Google Scholar]
- 13.Li C, Bai Y, Liu H, et al. Comparative study of microRNA profiling in keloid fibroblast and annotation of differential expressed microRNAs. Acta Biochim Biophys Sin (Shanghai). 2013;45(8):692–699. doi: 10.1093/abbs/gmt057 [DOI] [PubMed] [Google Scholar]
- 14.Grossi I, Salvi A, Baiocchi G, Portolani N, De Petro G. Functional Role of microRNA-23b-3p in Cancer Biology. Microrna. 2018;7(3):156–166. doi: 10.2174/2211536607666180629155025 [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, He X, Huang N, Yu J, Shao B. A20: a master regulator of arthritis. Arthritis Res Ther. 2020;22(1):220. doi: 10.1186/s13075-020-02281-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lv W, Ren Y, Hou K, et al. Epigenetic modification mechanisms involved in keloid: current status and prospect. Clin Epigenetics. 2020;12(1):183. doi: 10.1186/s13148-020-00981-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen W, Zhang Z, Ma J, Lu D, Lyu L. The Ubiquitin Proteasome System and Skin Fibrosis. Mol Diagn Ther. 2021;25(1):29–40. doi: 10.1007/s40291-020-00509-z [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Xue H, Zhu Z, Gao J, Zhao M, Ma Z. Expression of serum exosomal miR-23b-3p in non-small cell lung cancer and its diagnostic efficacy. Oncol Lett. 2020;20(4):30. doi: 10.3892/ol.2020.11891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayashi M, Yamada S, Kurimoto K, et al. miR-23b-3p Plays an Oncogenic Role in Hepatocellular Carcinoma. Ann Surg Oncol. 2020;1:847. [DOI] [PubMed] [Google Scholar]
- 20.Zhu R, Li X, Ma Y. miR-23b-3p suppressing PGC1alpha promotes proliferation through reprogramming metabolism in osteosarcoma. Cell Death Dis. 2019;10(6):381. doi: 10.1038/s41419-019-1614-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kadota T, Yoshioka Y, Fujita Y, et al. Extracellular Vesicles from Fibroblasts Induce Epithelial-Cell Senescence in Pulmonary Fibrosis. Am J Respir Cell Mol Biol. 2020;63(5):623–636. doi: 10.1165/rcmb.2020-0002OC [DOI] [PubMed] [Google Scholar]
- 22.Yang Z, Xiao Z, Guo H, et al. Novel role of the clustered miR-23b-3p and miR-27b-3p in enhanced expression of fibrosis-associated genes by targeting TGFBR3 in atrial fibroblasts. J Cell Mol Med. 2019;23(5):3246–3256. doi: 10.1111/jcmm.14211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li KZ, Liao ZY, Li YX, et al. A20 rescues hepatocytes from apoptosis through the NF-kappaB signaling pathway in rats with acute liver failure. Biosci Rep. 2019;39:1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Prasad AS, Bao B. Molecular Mechanisms of Zinc as a Pro-Antioxidant Mediator: clinical Therapeutic Implications. Antioxidants. 2019;8(6):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Priem D, Devos M, Druwe S, et al. A20 protects cells from TNF-induced apoptosis through linear ubiquitin-dependent and -independent mechanisms. Cell Death Dis. 2019;10(10):692. doi: 10.1038/s41419-019-1937-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kattah MG, Shao L, Rosli YY, et al. A20 and ABIN-1 synergistically preserve intestinal epithelial cell survival. J Exp Med. 2018;215(7):1839–1852. doi: 10.1084/jem.20180198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng Z, Qu JQ, Yi HM, et al. MiR-125b regulates proliferation and apoptosis of nasopharyngeal carcinoma by targeting A20/NF-kappaB signaling pathway. Cell Death Dis. 2017;8(6):e2855. doi: 10.1038/cddis.2017.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seol JY, Mihich E, Berleth ES, Apoptosis Protection TNF. Fraction (TAPF) prevents apoptosis induced by TNF, but not by Fas or TRAIL, via NF-kappaB-induced increase in cFLIP. Cytokine. 2015;75(2):321–329. doi: 10.1016/j.cyto.2015.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharyya S, Wang W, Graham LV, Varga J. A20 suppresses canonical Smad-dependent fibroblast activation: novel function for an endogenous inflammatory modulator. Arthritis Res Ther. 2016;18(1):216. doi: 10.1186/s13075-016-1118-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin PT, Xue XD, Zhao ZD, Lu JY, Xie PL. Necrostatin-1, RIP1/RIP3 inhibitor, relieves transforming growth factor beta-induced wound-healing process in formation of hypertrophic scars. J Cosmet Dermatol. 2020;20:2612–2618. doi: 10.1111/jocd.13860 [DOI] [PubMed] [Google Scholar]
- 31.Mou F, Mou C. Necrostatin-1 Alleviates Bleomycin-Induced Pulmonary Fibrosis and Extracellular Matrix Expression in Interstitial Pulmonary Fibrosis. Med Sci Monit. 2020;26:e919739. doi: 10.12659/MSM.919739 [DOI] [PMC free article] [PubMed] [Google Scholar]