Abstract
The genus Colletotrichum includes important plant pathogens, endophytes, saprobes and human pathogens. Even though the polyphasic approach has facilitated Colletotrichum species identification, knowledge of the overall species diversity and host distribution is largely incomplete. To address this, we examined 952 Colletotrichum strains isolated from plants representing 322 species from 248 genera, or air and soil samples, from 87 locations in China, as well as 56 strains from Saudi Arabia, Thailand, Turkey, and the UK. Based on morphological characteristics and multi-locus phylogenetic analyses, the strains were assigned to 107 species, including 30 new species described in this paper and 18 new records for China. The currently most comprehensive backbone tree of Colletotrichum, comprising 16 species complexes (including a newly introduced C. bambusicola species complex) and 15 singleton species, is provided. Based on these analyses, 280 species with available molecular data are accepted in this genus, of which 139 have been reported in China, accounting for 49.6 % of the species. Colletotrichum siamense, C. karsti, C. fructicola, C. truncatum, C. fioriniae, and C. gloeosporioides were the most commonly detected species in China, as well as the species with the broadest host range. By contrast, 76 species were currently found to be associated with a single plant species or genus in China. To date, 33 Colletotrichum species have been exclusively reported as endophytes. Furthermore, we generated and assembled whole-genome sequences of the 30 new and a further 18 known species. The most comprehensive genome tree comprising 94 Colletotrichum species based on 1 893 single-copy orthologous genes was hence generated, with all nodes, except four, supported by 100 % bootstrap values. Collectively, this study represents the most comprehensive investigation of Colletotrichum diversity and host occurrence to date, and greatly enhances our understanding of the diversity and phylogenetic relationships in this genus.
Taxonomic novelties: New species: Colletotrichum arecacearum F. Liu, Z.Y. Ma & L. Cai, Colletotrichum bicoloratum F. Liu, W.P. Wu & L. Cai, Colletotrichum bromeliacearum F. Liu & L. Cai, Colletotrichum buxi F. Liu, W.P. Wu & L. Cai, Colletotrichum chamaedoreae F. Liu, W.P. Wu & L. Cai, Colletotrichum crousii F. Liu, Z.Y. Ma & L. Cai, Colletotrichum danxiashanense F. Liu, W.P. Wu & L. Cai, Colletotrichum diversisporum F. Liu, W.P. Wu & L. Cai, Colletotrichum diversum F. Liu & L. Cai, Colletotrichum dolichoconidiophori F. Liu, W.P. Wu & L. Cai, Colletotrichum iris F. Liu & L. Cai, Colletotrichum monsterae F. Liu, W.P. Wu & L. Cai, Colletotrichum multiseptatum F. Liu, W.P. Wu & L. Cai, Colletotrichum nageiae F. Liu, W.P. Wu & L. Cai, Colletotrichum obovoides F. Liu & L. Cai, Colletotrichum parabambusicola F. Liu, W.P. Wu & L. Cai, Colletotrichum paraendophytum F. Liu, W.P. Wu & L. Cai, Colletotrichum reniforme F. Liu, Z.Y. Ma & L. Cai, Colletotrichum schimae F. Liu, W.P. Wu & L. Cai, Colletotrichum shivasii F. Liu & L. Cai, Colletotrichum sinuatum F. Liu, W.P. Wu & L. Cai, Colletotrichum subacidae F. Liu, Z.Y. Ma & L. Cai, Colletotrichum subsalicis F. Liu & L. Cai, Colletotrichum subvariabile F. Liu, W.P. Wu & L. Cai, Colletotrichum syngoniicola F. Liu, Z.Y. Ma & L. Cai, Colletotrichum telosmae F. Liu, W.P. Wu & L. Cai, Colletotrichum tibetense F. Liu & L. Cai, Colletotrichum variabile F. Liu, W.P. Wu & L. Cai, Colletotrichum zhaoqingense F. Liu & L. Cai, Colletotrichum zhejiangense F. Liu, W.P. Wu & L. Cai.
Citation: Liu F, Ma ZY, Hou LW, Diao YZ, Wu WP, Damm U, Song S, Cai L (2022). Updating species diversity of Colletotrichum, with a phylogenomic overview. Studies in Mycology 101: 1–56. doi: 10.3114/sim.2022.101.01
Keywords: Backbone tree, Fungal systematics, Multi-locus phylogeny, New taxa, Phylogenomics, Plant pathogen, Taxonomy
INTRODUCTION
Colletotrichum is the only genus of the Glomerellaceae (Glomerellales, Sordariomycetes, Ascomycota), and is regarded as one of the 10 most important genera of plant pathogenic fungi in the world (Dean et al. 2012). A few species are opportunistic human pathogens, including C. dematium, C. gigasporum, C. gloeosporioides and C. truncatum that can cause keratitis and subcutaneous infections (Guarro et al. 1998, Damm et al. 2009, Shiraishi et al. 2011, Shivaprakash et al. 2011, Liu et al. 2014, Buchta et al. 2019). In rare cases, Colletotrichum species have been reported to infect animals, e.g. C. fioriniae (as C. acutatum var. fioriniae), infecting a scale insect, and C. acutatum (s. lat.), infecting a sea turtle (Manire et al. 2002, Marcelino et al. 2008). Colletotrichum also includes plant endophytes, and saprobes from a wide range of substrates, such as the soil, water, and air (Liu et al. 2014).
Accurate species identification is important for understanding biodiversity, host-parasite interaction, and evolutionary history, and for monitoring and controlling plant pathogens, and developing quarantine measures. Previous host- and morphology-oriented systematics of Colletotrichum is, however, not regarded as natural, does not reflect the phylogenetic relationships, and has largely impeded meaningful investigation of species diversity in this genus. To establish a stable and natural classification system, the use of molecular data in combination with morphological, geographical, and ecological data has increasingly been employed (Cai et al. 2009, Cannon et al. 2012, Marin-Felix et al. 2017, Jayawardena et al. 2020).
In the genus Colletotrichum, a species complex (also called an ‘aggregate’) is defined as a group of species that form a monophyletic clade and exhibit shared characteristics (e.g. similar conidial morphology) (Cannon et al. 2012). The current classification system of Colletotrichum comprises 15 species complexes, i.e. the C. acutatum, C. agaves, C. boninense, C. caudatum, C. dematium, C. destructivum, C. dracaenophilum, C. gigasporum, C. gloeosporioides, C. graminicola, C. magnum, C. orbiculare, C. orchidearum, C. spaethianum, and C. truncatum species complexes, as well as a number of singletons (Marin-Felix et al. 2017, Damm et al. 2019, Jayawardena et al. 2020, Bhunjun et al. 2021). The C. caudatum species complex forms an inner clade of the C. graminicola species complex, and was annotated as C. caudatum sub-aggregate in the phylogenetic tree, but referred to as a species complex by Crouch (2014). Subsequently, many researchers refer to this group as a C. caudatum species complex (e.g. Marin-Felix et al. 2017, Jayawardena et al. 2020). Recently, Bhunjun et al. (2021) recommended treating the two species complexes as one, i.e. the C. graminicola-caudatum species complex.
ITS is a useful DNA barcode for assigning Colletotrichum species to species complexes (Cannon et al. 2012), but different loci are being employed to resolve the different species complexes. For example, six loci (act, chs-1, gapdh, his3, ITS, and tub2) have been used for the C. acutatum, C. dematium, C. destructivum, C. dracaenophilum, C. magnum, C. orchidearum, C. spaethianum and C. truncatum species complexes (Damm et al. 2009, 2012a, 2014, 2019), with an additional locus each for the C. boninense (cal) and C. orbiculare (gs) species complexes (Damm et al. 2012b, 2013), and three additional loci (ApMat, cal, and gs) for the C. gloeosporioides species complex (Weir et al. 2012, Liu et al. 2015). Meanwhile, act, chs-1, gapdh, ITS, and tub2 have been used for the C. gigasporum species complex (Liu et al. 2014), and apn2, ITS, Mat1/Apn2, and sod2 have been used for the C. caudatum and C. graminicola species complexes (Crouch et al. 2009a, Crouch 2014). Furthermore, the combined use of ApMat and gs in phylogenetic analysis is very useful for species delimitation in the C. gloeosporioides species complex (Liu et al. 2015).
Based on phylogenetic analyses of multiple loci, the backbone tree of Colletotrichum has been constructed and is frequently updated by the addition of newly described species. The tree includes 119 species in Cannon et al. (2012); 189 species in Jayawardena et al. (2016) and Marin-Felix et al. (2017); 247 species in Jayawardena et al. (2020); and 248 species in Bhunjun et al. (2021). The continuous discovery of new species indicates very high species diversity in this genus. In China, most Colletotrichum taxa are reported in the form of single or few species, or species associated with a certain host (e.g. Tao et al. 2013, Liu et al. 2015, Zhang et al. 2020), and there is a lack of systematic and biodiversity investigation of these fungi.
In the current study, we aimed to: 1) resolve the systematic placement of 1 008 Colletotrichum strains collected, mostly in China, since 1993; 2) characterise newly discovered species based on all available data; 3) supplement missing sequences of the act, chs-1, gapdh, his3, and tub2 genes of some known species, and build an integrated dataset for Colletotrichum; 4) construct a robust and reliable phylogeny of Colletotrichum including all species with type-derived sequences; and 5) improve knowledge on the diversity and host occurrence of Colletotrichum species in China. In addition, all Colletotrichum species with an available genome sequence were used for the construction of a whole-genome species tree to help resolve species boundaries and define species complexes.
MATERIALS AND METHODS
Isolates
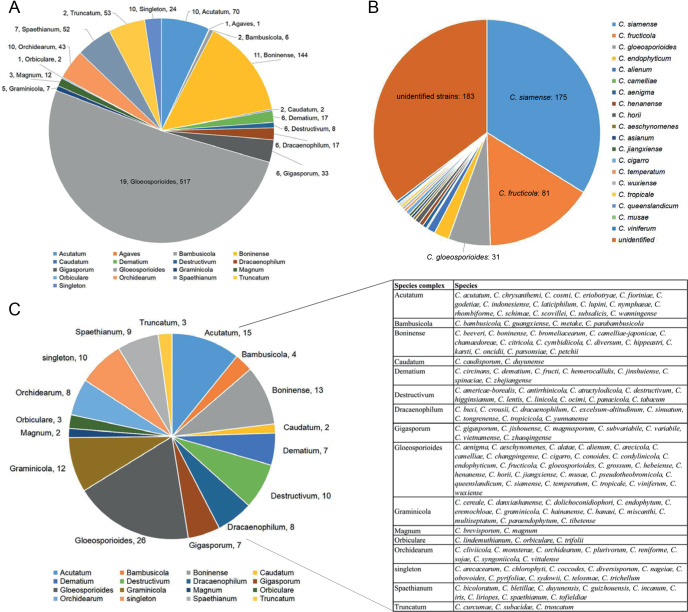
In the current study, 1 008 Colletotrichum isolates, associated with at least 322 host plant species in 248 genera, from the LC Culture Collection (a personal culture collection of Lei Cai, housed in the Institute of Microbiology, Chinese Academy of Sciences) and the Novozymes Culture Collection were analysed. Of these, 952 were collected at 87 locations in China and 56 at 26 locations in Saudi Arabia, Thailand, Turkey, and the UK. Representative cultures of the new species described herein were deposited in the China General Microbiological Culture Collection (CGMCC). Type specimens were deposited in the Mycological Herbarium, Institute of Microbiology, Chinese Academy of Sciences, Beijing, China (HMAS).
Morphology
The isolates were cultivated on potato dextrose agar (PDA; DifcoTM, Becton, Dickinson and Company, Sparks, MD, USA) and synthetic nutrient-poor agar (SNA; Nirenberg 1976) supplemented with double-autoclaved pine needles placed on the agar surface. The cultures were incubated at room temperature (25 °C) under a 12 h day/night regime. After 7 d, fungal growth rates were measured and the colony characteristics were noted. Colony colours were rated using the colour charts of Rayner (1970). Morphological observations of reproductive structures were performed using a Nikon AZ100 dissecting microscope (DM) and a Nikon Eclipse 80i compound microscope with differential interference contrast (DIC) illumination, both equipped with a Nikon DS-Ri2 high-definition colour digital camera. Slides were prepared using lactic acid. Measurements and descriptions of microscopic structures were preferentially made from cultures grown on SNA. If sterile on SNA, structures produced on PDA, oatmeal agar (OA), malt extract agar (MEA) (Crous et al. 2019), or pine needles were described. Hyphal appressoria were induced using a slide culture technique (Cai et al. 2009) or observed directly on the reverse side of colonies grown on SNA. At least 30 measurements were made for each structure, and the mean value, standard deviation, and minimum–maximum values are given, with the extreme measurements in parentheses. Descriptions and illustrations of taxonomic novelties were deposited in MycoBank (www.MycoBank.org; Crous et al. 2004).
Molecular analyses using barcoding sequences
Total genomic DNA was extracted from fresh mycelia of each isolate using a modified CTAB protocol (Guo et al. 2000). All primers used in the current study are listed in Table 1. PCR amplification was performed as described by Crouch et al. (2009a) and Liu et al. (2016). PCR amplicons were purified and sequenced by the SinoGenoMax Company (Beijing, China). The forward and reverse reads were paired, and consensus sequences calculated in MEGA v. 7.0.21 (Kumar et al. 2016).
Table 1.
Primers used in this study, with originating loci, sequences and references.
| Locus | Product name | Primer name | Direction | Sequence (5’–3’) | Reference |
|---|---|---|---|---|---|
| act | Actin | ACT-512F | Forward | ATGTGCAAGGCCGGTTTCGC | Carbone & Kohn (1999) |
| ACT-783R | Reverse | TACGAGTCCTTCTGGCCCAT | Carbone & Kohn (1999) | ||
| apn2 | Mat1 and the adjacent DNA lyase gene | Apn1W1F | Forward | ATGGAGCACAAAAACGAACA | Crouch et al. (2009b) |
| Apn1W1R | Reverse | GCGGAGCAGAGGATGTAGTC | Crouch et al. (2009b) | ||
| cal | Calmodulin | CL1C | Forward | GAATTCAAGGAGGCCTTCTC | Weir et al. (2012) |
| CL2C | Reverse | CTTCTGCATCATGAGCTGGAC | Weir et al. (2012) | ||
| chs-1 | Chitin synthase | CHS-79F | Forward | TGGGGCAAGGATGCTTGGAAGAAG | Carbone & Kohn (1999) |
| CHS-345R | Reverse | TGGAAGAACCATCTGTGAGAGTTG | Carbone & Kohn (1999) | ||
| ApMat | Apn2-Mat1-2 intergenic spacer and partial mating type Mat1-2 gene | AMF1 | Forward | CCAGAAATACACCGAACTTGC | Silva et al. (2012) |
| AMR1 | Reverse | TCATTCTACGTATGTGCCCG | Silva et al. (2012) | ||
| gapdh | Glyceraldehyde-3-phosphate dehydrogenase | GDF1 | Forward | GCCGTCAACGACCCCTTCATTGA | Guerber et al. (2003) |
| GDR1 | Reverse | GGGTGGAGTCGTACTTGAGCATGT | Guerber et al. (2003) | ||
| gs | Glutamine synthetase | GSF1 | Forward | ATGGCCGAGTACATCTGG | Guerber et al. (2003) |
| GSR1 | Reverse | GAACCGTCGAAGTTCCAC | Guerber et al. (2003) | ||
| GSLF2 | Forward | TACACGAGSAAAAGGATACGC | Liu et al. (2016) | ||
| GSLR1 | Reverse | AGRCGCACATTGTCAGTATCG | Liu et al. (2016) | ||
| his3 | Histone3 | CYLH3F | Forward | AGGTCCACTGGTGGCAAG | Crous et al. (2004) |
| CYLH3R | Reverse | AGCTGGATGTCCTTGGACTG | Crous et al. (2004) | ||
| ITS | Internal transcribed spacer | ITS1 | Forward | TCCGTAGGTGAACCTGCGG | White et al. (1990) |
| ITS4 | Reverse | TCCTCCGCTTATTGATATGC | White et al. (1990) | ||
| Mat1/Apn2 | The 3′ end of apn2 and the 5′ end of the mating type gene Mat1-2 | Mat1M72F | Forward | ACGGCAAACGGCTCAGGGAGTG | Crouch et al. (2009b) |
| Mat1M72R | Reverse | AATGCCGAGTCCCACGAGGTTCG | Crouch et al. (2009b) | ||
| sod2 | Manganese-superoxide dismutase | SOD625F | Forward | GCCCACAGTACATATTGCCTAAGC | Crouch et al. (2006) |
| SOD625R | Reverse | TCATCCCGGGAGCCAGAAAACCT | Crouch et al. (2006) | ||
| tub2 | β-tubulin 2 | T1 | Forward | AACATGCGTGAGATTGTAAGT | O’Donnell & Cigelnik (1997) |
| Bt2b | Reverse | ACCCTCAGTGTAGTGACCCTTGGC | Glass & Donaldson (1995) |
Primarily, ApMat, gapdh, gs, ITS, or tub2, which are good discriminative loci in different species complexes in Colletotrichum (Damm et al. 2012a, b, 2013, 2014, 2019, Liu et al. 2015, Jayawardena et al. 2016), were selected for PCR amplification and sequencing. All efforts were made to assign isolates to species complexes and to identify them to species level, by BLASTn searches of the NCBI GenBank or by phylogenetic analyses using single locus sequences.
For isolates of species that could not be determined based on the above analyses, an ITS tree was first used for inferring delimitation to the species complex level, and further multi-locus phylogenetic analyses and phenotypic characterisation were then performed for species delimitation. Regarding the multi-locus analyses, a concatenated sequence dataset of act, chs-1, gapdh, his3, ITS, and tub2, including all Colletotrichum species for which molecular data are available, was used to construct the overview phylogeny for the genus (Cannon et al. 2012, Damm et al. 2012a, b, 2013, 2014, 2019, Weir et al. 2012, Liu et al. 2014, Jayawardena et al. 2016, Marin-Felix et al. 2017). Two additional sequence datasets were used for the C. caudatum species complex (apn2, ITS, Mat1/Apn2, and sod2) and C. graminicola species complex (act, chs-1, ITS, sod2, and tub2) (Crouch et al. 2009a, Cannon et al. 2012, Crouch 2014). All novel sequences obtained in the current study were submitted to the NCBI GenBank (www.ncbi.nlm.nih.gov; Tables S1–S3).
Sequence alignments of the individual loci were prepared using MAFFT v. 7 (http://mafft.cbrc.jp/alignment/server/index.html) and manually edited in MEGA v. 7.0.21. Maximum-likelihood (ML) and Bayesian analysis (BA) were used for phylogenetic inferences of the ITS alignment and concatenated alignments. MrModelTest v. 2.2 (Nylander 2004) was used to determine the optimal nucleotide substitution model for each locus. The individual gene trees were assessed for clade conflicts between the individual phylogenies.
Maximum-likelihood and BA were implemented using the CIPRES Science Gateway portal (https://www.phylo.org/; Miller et al. 2012) using RAxML-HPC BlackBox v. 8.2.10 (Stamatakis 2014) and MrBayes v. 3.2.6 (Huelsenbeck & Ronquist 2001, Ronquist & Huelsenbeck 2003), respectively. For ML analyses, GTR+GAMMA substitution model with 1 000 bootstrap iterations was set. Bayesian analyses were computed with four simultaneous Markov Chain Monte Carlo chains, 200 M generations, and a sampling frequency of 1 000 generations for the first dataset and 100 generations for the other two datasets, ending the run automatically when standard deviation of split frequencies fell below 0.01. The burn-in fraction was set to 0.25, after which the 50 % majority rule consensus trees and posterior probability (PP) values were calculated. The resulting trees were plotted using FigTree v. 1.4.2 (http://tree.bio.ed.ac.uk/software/figtree).
Whole-genome sequencing
Phylogenomic analysis was performed to better define the species complex boundaries for Colletotrichum and help to understand the fungal evolution in this genus. Whole-genome sequences were generated for ex-type strains of new species described in the current study and for 18 known species that were available. Reference genomes were retrieved from NCBI (Table S4). All isolates were purified using a single-spore isolation method (Zhang et al. 2013). Hypha of 4-d-old colonies growing on PDA were transferred to 50 mL of potato dextrose broth (PDB) and cultivated for 3–6 d at 25 °C at 150 rpm. Fresh mycelia were filtered through four layers of sterile gauze and were then stored at −80 °C. DNA libraries were prepared using DNeasy Plant Mini kit (Qiagen). The libraries were sequenced as 150 bp pair-end reads using Illumina NovaSeq 6000 platform. Genome assemblies were deposited in the National Microbiology Data Center (NMDC) under BioProject NMDC10017886.
Genome assembly and gene annotation
Read quality was assessed by using FastQC v. 0.11.8 (Andrews & Babraham 2010). Clean reads were assembled with SPAdes v. 3.12.0 (Bankevich et al. 2012), using the ‘careful’ mode and various kmers (21, 33, 55, 77, 99). Scaffolds shorter than 200 bp were removed from subsequent analyses. Genome assembly quality was assessed using QUAST v. 5.0.2 (Alexey et al. 2013). Genome completeness was assessed using genome mode in BUSCOs v. 2.0.1 (Mathieu et al. 2019), with Sordaromyceta_odb9 gene set.
Gene prediction for the 48 newly sequenced and 46 previously published genomes of Colletotrichum (Table S4) were done using the Funannotate pipeline v. 1.7.0 (Palmer 2016). Repetitive elements were initially soft-masked using default parameters. Next, the prediction step of funannotate pipeline (funannotate predict) was implemented using --busco_db Sordariomycetes, --busco_seed_species Verticillium longporum1 and default parameters. Predicted proteins were firstly annotated using eggNOG-mapper v. 2 (Huerta-Cepas et al. 2017) and then compared via BlastP (e-value ≤ 1e-10) against Fungal Cytochrome P450 (Moktali et al. 2012) and Transporters Classification Database (Saier et al. 2016). Carbohydrate-active enzymes annotation was predicted using dbCAN v. 2.0.6 (Yin et al. 2012) with DIAMOND, Hotpep and HMMER as default settings, and the genes found by at least two tools were regarded as candidates. The SignalP v. 5.5 (Armenteros et al. 2019), TMHMM v. 2.0 (Krogh et al. 2001), TargetP v. 2.0 (Emanuelsson et al. 2000), EffectorP v. 1.0 (Sperschneider et al. 2016) were incorporated to predict effectors.
Phylogenomic tree construction
The phylogenetic relationships between Colletotrichum members were inferred based on orthologs of all (94) assembled genomes (Table S4), using Verticillium dahliae as the outgroup. Predicted proteins were clustered into orthologous groups by using Orthofinder v. 2.3.3 (Emms & Kelly 2019). Amino acid sequences of 1 893 single-copy orthologs were aligned using MAFFT v. 7.407 (Katoh & Standley 2013) with default settings. Conserved sites in the alignment were extracted using Gblocks v. 0.91b (Castresana 2000) and then concatenated. A JTT substitution model of the concatenated alignment resulting from analysis in ProtTest v. 3.4.2 (Darriba et al. 2011) was used for phylogenomic tree construction using RAxML v. 8.2.12 (Stamatakis 2014) with 1 000 bootstrap iterations.
RESULTS
Single-locus analysis
Based on BLASTn search and single-locus phylogenetic analyses using ApMat, gapdh, gs, or tub2, we attempted to assign the 1 008 strains analysed (Table S5) to species level. For the undetermined strains, a single ITS phylogenetic analysis was performed to allocate them into species complexes. The ITS alignment contained 618 characters, including alignment gaps. The ML search revealed a best tree with an InL of −8314.217491. The BA was run for 20 025 000 generations, and a 50 % consensus tree and posterior probabilities were calculated from 30 040 trees from two analysis runs. The analysed strains were thus separated into 14 species complexes and six singleton clades. Four taxa (C. bambusicola, C. hsienjenchang, C. metake, and C. parabambusicola sp. nov.), characterised by straight conidia, formed a main clade, which was denoted as a new species complex (Fig. S1). The C. graminicola species complex was polyphyletic in the ITS tree. Colletotrichum riograndense, which had previously been considered as belonging to the C. spaethianum species complex, was however phylogenetically basal to the C. bambusicola and C. spaethianum species complexes. Furthermore, the topologies of the single ITS tree and multi-locus tree were compared to determine whether the grouping of species within species complexes was congruent.
Multi-locus phylogeny
Overview phylogeny of Colletotrichum based on six loci
The combined act, chs-1, gapdh, his3, ITS, and tub2 sequence alignment that was used for overview phylogeny construction contained 250 currently accepted Colletotrichum species and Monilochaetes infuscans (CBS 869.96) as the outgroup. The final alignment contained 2 659 characters (act: 322; chs-1: 251; gapdh: 441; his3: 417; ITS: 606; tub2: 622) including the alignment gaps, and 1 944 characters were unique site patterns. The ML search revealed a best tree with an InL of −74738.627777. MrModelTest recommended Dirichlet base frequencies for all data partitions of the BA. The GTR+I+G model was suggested for act, gapdh, and ITS, and the HKY+I+G model for chs-1, his3, and tub2. The BA was run for 39 980 000 generations, and a 50 % consensus tree and posterior probabilities were calculated from 59 972 trees from two runs.
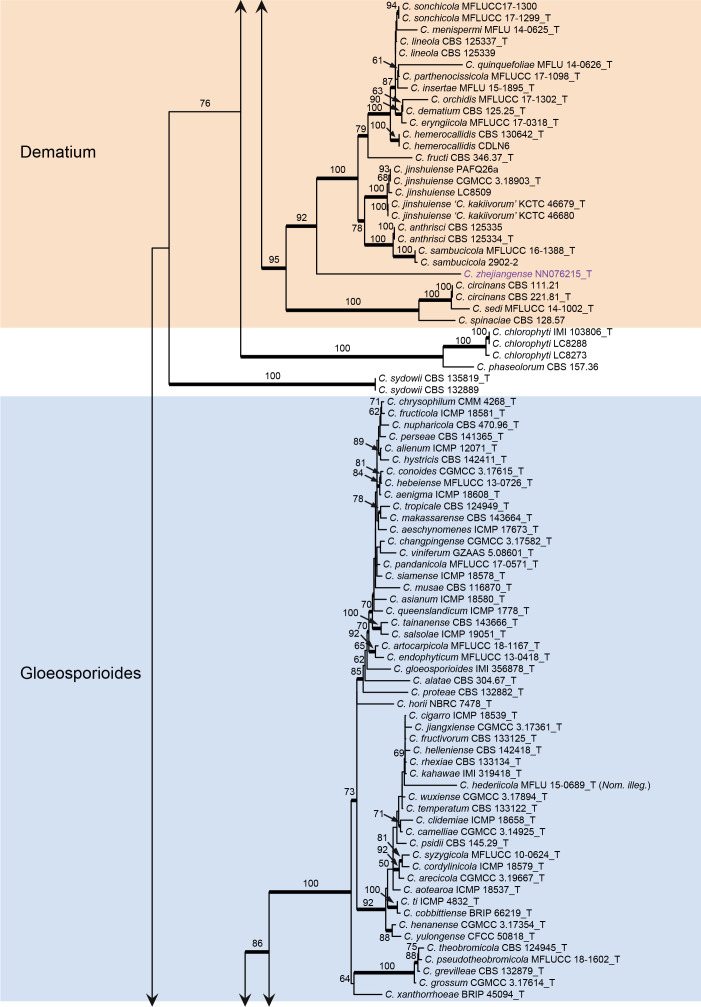
The topologies of the six-locus phylogenetic trees generated by ML and BA were congruent and consistent with the species complex delimitation reported previously (Cannon et al. 2012, Marin-Felix et al. 2017, Bhunjun et al. 2021), but differed from the ITS tree constructed in the current study in the grouping of C. guangxiense and C. riograndense on species complex level. In the six-locus tree, C. guangxiense and four additional taxa (C. bambusicola, C. hsienjenchang, C. metake, and C. parabambusicola sp. nov.) formed a new species complex (Fig. 1). By contrast, C. guangxiense was basal to the C. caudatum, C. destructivum and C. graminicola species complexes in the ITS tree (Fig. S1). Colletotrichum riograndense, belonging to the C. spaethianum species complex in the six-locus phylogeny (Fig. 1), was in the ITS tree basal to a clade formed by the C. bambusicola, C. caudatum, C. destructivum, C. graminicola, and C. spaethianum species complexes (Fig. S1).
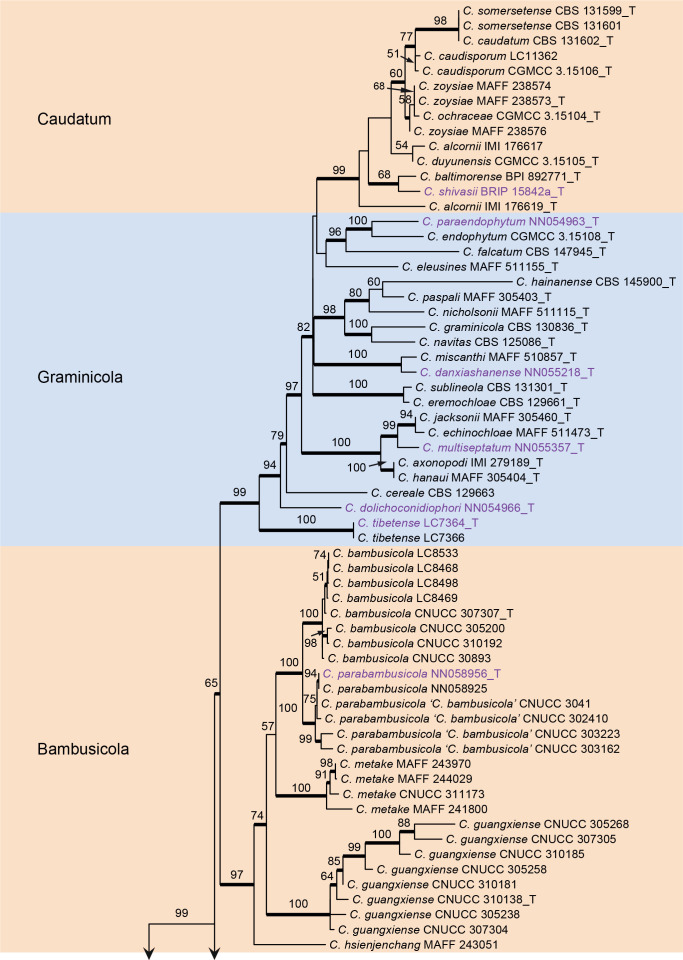
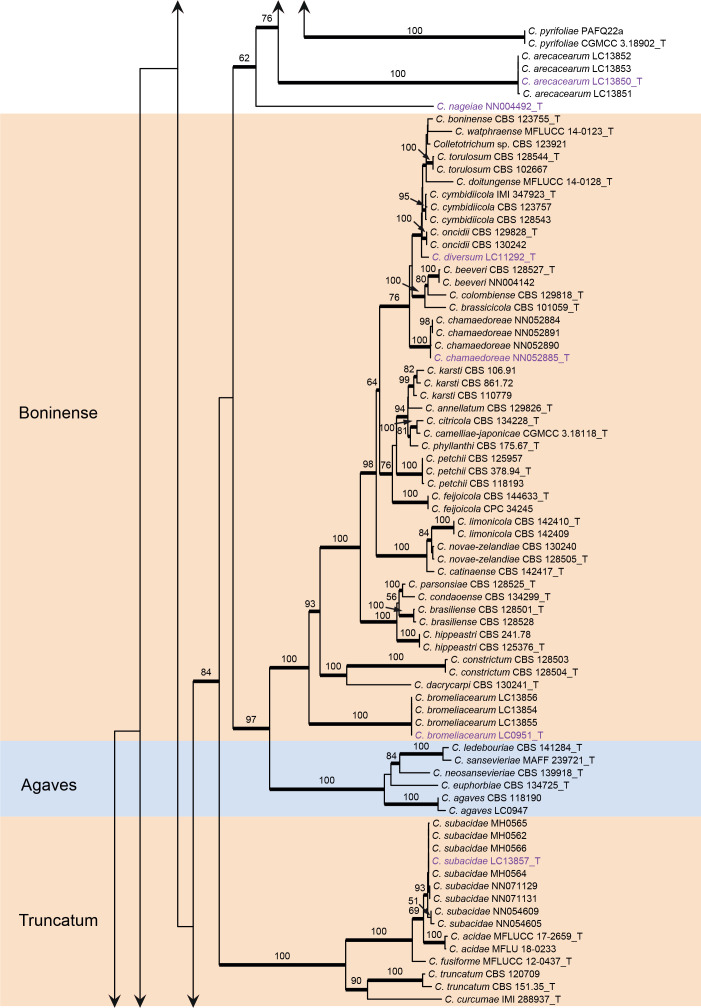
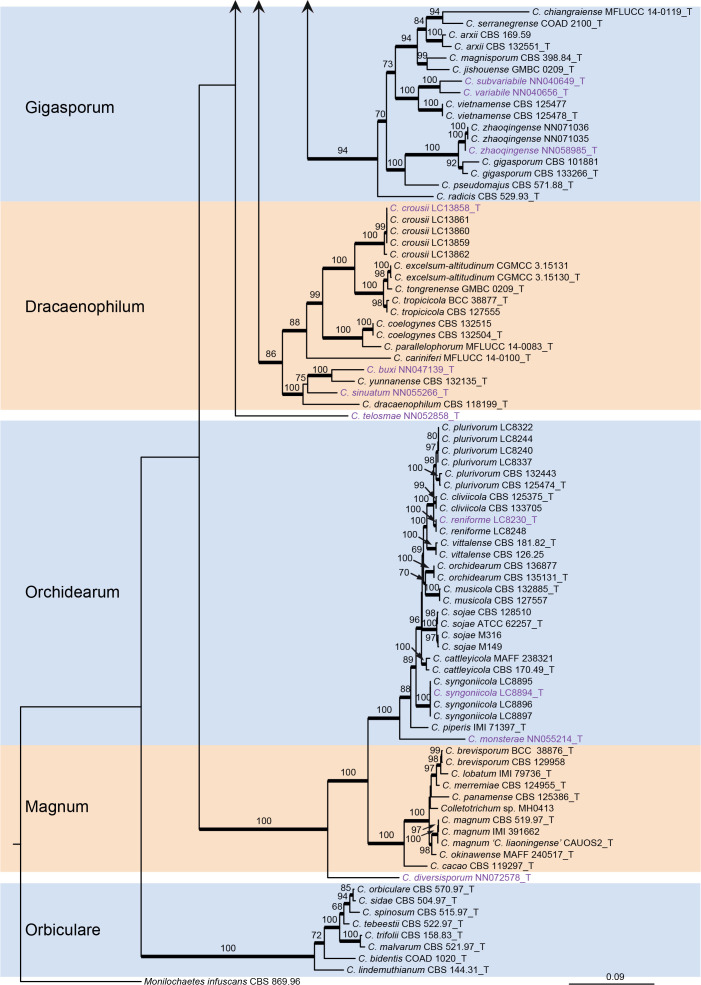
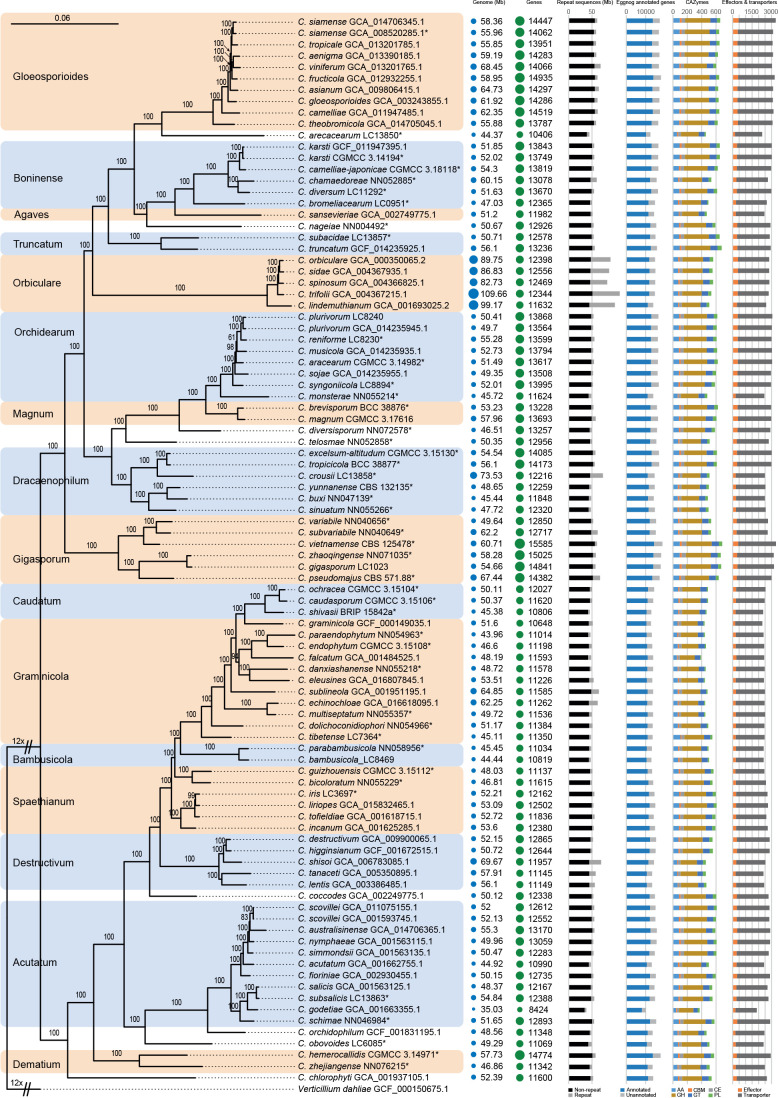
Fig. 1.
Phylogenetic tree of Colletotrichum calculated with a maximum likelihood analysis of the combined act, chs-1, gapdh, his3, ITS, and tub2 sequence alignment. Bayesian posterior probabilities (PP > 0.90) are emphasised by thickened branches, maximum likelihood bootstrap support values (> 50 %) are shown at the nodes. Species complexes are indicated with coloured boxes, with their names listed at the left. Ex-type strains are indicated with “T” in the end of the taxa labels. Latin names and ex-type strain numbers of the new species described in the current study are shown in purple font.
The C. caudatum species complex formed a subclade of the C. graminicola complex and some taxa in the two species complexes were indistinguishable in the six-locus tree (Fig. 1), e.g. C. caudatum and C. somersetense, C. ochraceae and C. zoysiae, C. axonopodi and C. hanaui.
The phylogenies of the C. caudatum and C. graminicola species complexes
As two different sets of loci were previously used for the phylogenetic analyses of C. caudatum and C. graminicola species complexes, separate analyses were performed herein.
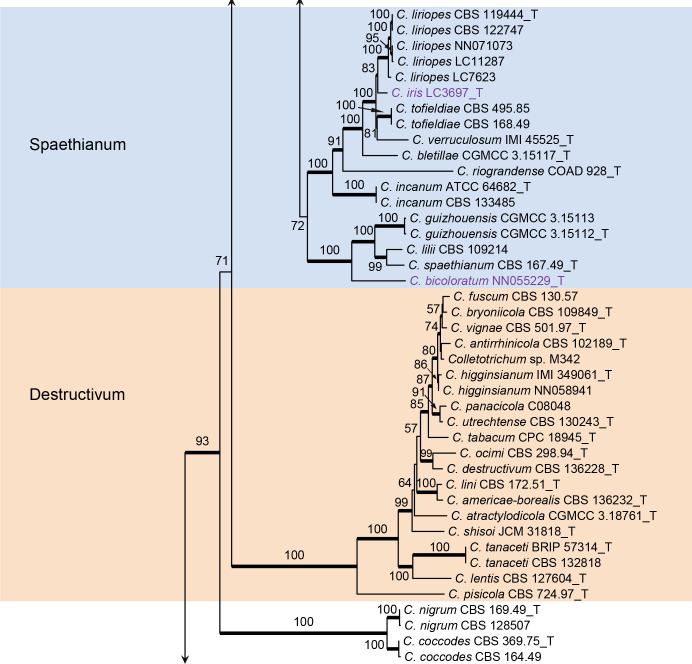
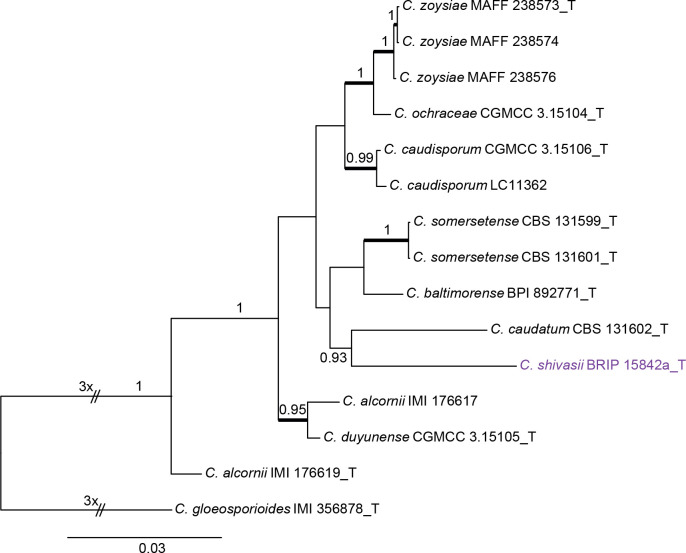
Colletotrichum caudatum species complex (Fig. 2): The dataset consisted of 14 strains, with C. gloeosporioides (IMI 356878) as the outgroup. The final sequence alignment contained 2 949 characters (apn2: 805; ITS: 439; Mat1/Apn2: 1253; sod2: 452) including alignment gaps, and 301 characters were unique site patterns. The ML search revealed a best tree with an InL of −6 746.046698. The BA was run for 260 000 generations, and a 50 % consensus tree and posterior probabilities were calculated from 5 202 trees from two runs. The topologies of phylogenetic trees generated by ML and BA were congruent. In the four-locus phylogenetic tree of C. caudatum species complex (Fig. 2), strain BRIP 15842a formed a sister clade to the ex-type of C. baltimorense on a long branch, distinct from the other clades, representing a novel species. The two strains of C. alcornii (IMI 176617 and IMI 176619) were separated into two distant clades, sharing 97 % (419/431) ITS sequence similarity, and may represent different species.
Fig. 2.
Phylogenetic tree of the C. caudatum species complex resulting from a Bayesian analysis of the combined apn2, ITS, Mat1/Apn2, and sod2 sequence alignment. Maximum likelihood bootstrap support values (> 70 %) are emphasised by thickened branches, bayesian posterior probabilities (PP > 0.90) are shown at the nodes. The scale bar represents the expected number of changes per site. Ex-type strains are indicated with “T” in the end of the taxa labels. Latin names and ex-type strain numbers of the new species described in the current study are shown in purple font.
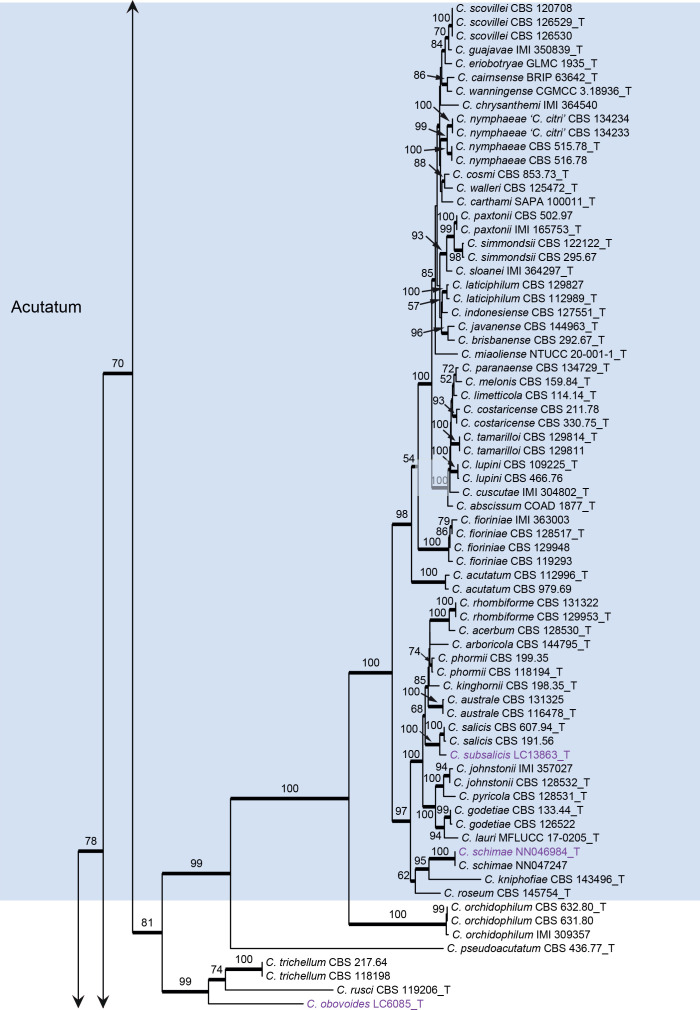
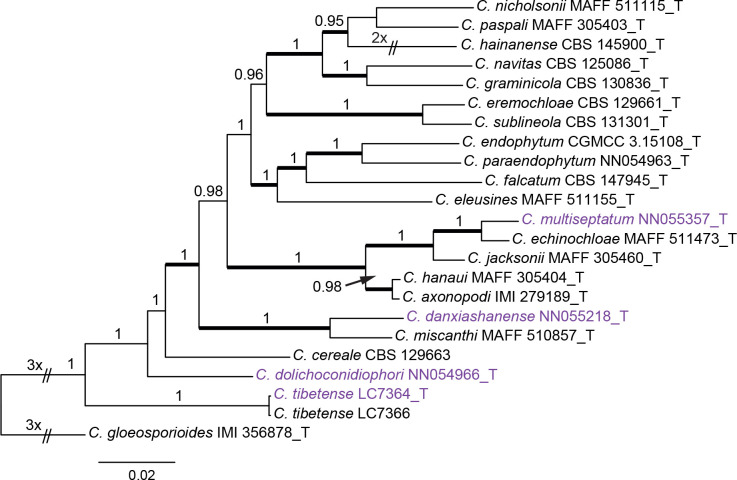
Colletotrichum graminicola species complex (Fig. 3): The dataset consisted of 22 strains, with C. gloeosporioides (IMI 356878) as the outgroup. The final alignment contained 2 117 characters (act: 255; chs-1: 278; ITS: 507; sod2: 564; tub2: 513) including alignment gaps, and 725 characters were unique site patterns. The ML search revealed a best tree with an InL of −10629.345037. The BA was run for 1 000 generations, and a 50 % consensus tree and posterior probabilities were calculated from 202 trees from two runs. The topologies of the phylogenetic trees generated by ML and BA were congruent. In the five-locus phylogenetic tree of the C. graminicola species complex (Fig. 3), the analysed strains formed five distinct clades on long branches.
Fig. 3.
Phylogenetic tree of the C. graminicola species complex resulting from a Bayesian analysis of the combined act, chs-1, ITS, sod2, and tub2 sequence alignment. Maximum likelihood bootstrap support values (> 70 %) are emphasised by thickened branches, bayesian posterior probabilities (PP > 0.90) are shown at the nodes. The scale bar represents the expected number of changes per site. Ex-type strains are indicated with “T” in the end of the taxa labels. Latin names and ex-type strain numbers of the new species described in the current study are shown in purple font.
Whole-genome data and phylogenomic assessment
The Colletotrichum genomes varied from 35.03 Mbp to 109.66 Mbp in size and encoded from 8 424 to 14 841 protein-coding genes (Fig. 4, Table S4), and neither correlated with the phylogenetic position (Fig. 4) nor lifestyle of the species (Table S4), except for the genome sizes of the C. orbiculare species complex (> 82 Mbp) that were generally larger than those of other species (Fig. 4). However, disproportionate to the large genome, the C. orbiculare species complex encodes relatively smaller number of genes, CAZymes and transporters than a few other species complexes, e.g. C. gigasporum, C. gloeosporioides, and C. orchidearum species complexes. On the contrary, C. orbiculare species complex possesses the highest proportion of repeat content than other species in this genus (Fig. 4), which may contribute to its large genome (Haridas et al. 2020). Moreover, compared to other members in Colletotrichum, species associated with gramineous plants in the C. bambusicola, C. caudatum, and C. graminicola species complexes are characterised by small genome size and small number of genes, CAZymes and transporters.
Fig. 4.
Maximum likelihood phylogenomic tree generated from a concatenated alignment of sets of orthologous protein sequences. A total of 1 893 single-copy orthologs were retained. The tree was estimated based on the JTT substitution model. Ex-type strains are indicated with “*” in the end of the taxa labels. Genome and assembly features are shown at the right side of the phylogenetic tree. From left to right: Genomes (Mb): genome size in Mb; Genes: number of predicted genes; Repeat sequences (Mb): genome sizes showing proportion of repeat elements in these genomes; Eggnog annotated genes: number of predicted protein coding genes with and without functional annotation based on eggnog database; CAZymes: gene number with the distribution of CAZyme classes, auxiliary activities (AA), carbohydrate binding molecules (CBM), carbohydrate esterases (CE), glycoside hydrolases (GH), glycosyl transferases (GT), polysaccharide lyases (PL); Effectors & transporters: number of predicted effectors and transporters.
Using 94 Colletotrichum species covering 16 species complexes and one outgroup, a high confidence whole-genome-based phylogenetic tree was generated (Fig. 4). The Gblocks filtered alignment of 1 893 single-copy orthologs consisted of 655 956 characters, including alignment gaps. All nodes, except for four (two in the C. orchidearum species complex, and the other two in the C. graminicola and C. spaethianum species complexes respectively), received 100 % bootstrap support (Fig. 4). The species tree supported the taxonomic status of all species newly described in the current study (Fig. 4). The strain with the assembly accession GCA_001593745.1 was labeled as C. acutatum at the time the genome was published; it was however revealed to be C. scovillei in the species tree (Fig. 4).
Taxonomy
Five taxa (C. bambusicola, C. guangxiense, C. hsienjenchang, C. metake, and C. parabambusicola sp. nov.) characterised by straight conidia formed a well-supported clade in the six-locus tree (Fig. 1), representing a new species complex, herein called the C. bambusicola species complex. Based on the molecular analyses, morphological examination, and habitat and geographical comparisons, 30 new species are introduced herein.
Colletotrichum arecacearum F. Liu, Z.Y. Ma & L. Cai, sp. nov. MycoBank MB 841370. Fig. 5.
Fig. 5.

Colletotrichum arecacearum (ex-type culture LC13850). A. Disease symptom on the host plant. B, C. Front and reverse colony on PDA (7 d). D. Colony surface on OA (7 d), with immersed, olivaceous conidiomata. E–L. Conidiogenous cells and conidia. M. Conidia. Scale bars = 10 μm.
Etymology: Named after its host plant family, Arecaceae.
Description: Colonies on PDA 19–22 mm diam in 7 d, flat with entire edge, saffron in the centre, white at the margin, aerial mycelium sparse, reverse creamy white. Vegetative hyphae hyaline, smooth-walled, septate, branched. Sporulating on OA, conidiomata scattered or confluent, immersed, olivaceous. Conidiophores usually reduced to conidiogenous cells. Conidiogenous cells hyaline, smooth-walled, ampulliform, rarely subcylindrical, straight or curved, collarette distinct, periclinal thickening sometimes visible, 8.5–15 × 4.5–7.5 μm. Conidia hyaline, aseptate, smooth-walled, guttulate, cylindrical, occasionally slightly curved, with one end round and one end subacute, 12.5–18.5 × 4–5.5 μm (av. ± SD = 15.5 ± 1.3 × 4.7 ± 0.3 μm), L/W ratio = 3.3. Appressoria and setae not observed.
Typus: China, Guangxi, Guangxi Botanical Garden of Medicinal Plants, on leaves of an unidentified species in Arecaceae, Jun. 2017, Z.Y. Ma & L.W. Hou (holotype HMAS 350634, ex-type culture CGMCC 3.20509 = LC13850 = MH0003).
Additional materials examined: China, Guangxi, Guangxi Botanical Garden of Medicinal Plants, on leaves of an unidentified species in Arecaceae, Jun. 2017, Z.Y. Ma & L.W. Hou, living cultures LC13851 (= MH0003-1), LC13852 (= MH0003-2), LC13853 (= MH0003-3).
Notes: Colletotrichum arecacearum, C. pyrifoliae, and C. nageiae sp. nov. clustered basal to the broadly known C. gloeosporioides species complex (Fig. 1). These three species produce cylindrical conidia, similar to those produced by the C. gloeosporioides complex (Weir et al. 2012), but are temporarily considered as singleton species because the ingroup taxa of the C. gloeosporioides complex are much more tightly related to each other than to them. Colletotrichum arecacearum is distinct from other species in this genus at each locus sequenced in the current study, and morphologically differs from the most closely related species C. pyrifoliae in producing shorter and wider conidiogenous cells (8.5–15 × 4.5–7.5 μm vs. 15–32 × 3–5 μm), and slightly shorter and thinner conidia (12.5–18.5 × 4–5.5 μm vs. 14–23 × 5.5–7 μm), and differs from C. nageiae sp. nov. in producing wider conidiogenous cells (8.5–15 × 4.5–7.5 μm vs. 10–20 × 2.5–4.5 μm) and longer conidia (12.5–18.5 × 4–5.5 μm vs. 9–13.5 × 4.5–6 μm).
Colletotrichum bambusicola C.L. Hou & Q.T. Wang, Mycologia 113: 452. 2021. Fig. 6.
Fig. 6.

Colletotrichum bambusicola (C–E, H, J–N from LC8469, F–G, I, O from LC8468, P–T from LC8533). A, B. Disease symptoms on the host plants. C. Front and reverse colony on PDA (7 d). D. Acervulus. E–G. Conidiophores, conidiogenous cells and conidia. H, I. Setae. J–M. Appressoria. N, O. Conidia. P, Q. Ascomata. R. Ascomata wall. S, T. Asci and ascospores. Scale bars =10 μm. Scale bar of M applies to J–M.
Description: Colonies on PDA 52–56 mm diam in 7 d, flat with undulate edge, white to pale grey, aerial mycelium dense, reverse pale grey with mouse grey to black halo, white at the edge. Sexual morph developed on SNA. Ascomata ovoid to obpyriform, medium to dark brown, 180–195 × 95–110 μm, glabrous, ostiolate, neck pale brown, outer layer composed of angular cells, medium brown, 8–18.5 μm diam. Asci cylindrical to obclavate, hyaline, 58–72 × 7–10 μm, 8-spored. Ascospores uni- to bi-seriate, hyaline, smooth-walled, aseptate, allantoid with rounded ends, rarely straight or very slightly curved, 14–19 × 4–6 μm (av. ± SD = 16 ± 1.1 × 5 ± 0.5 μm), L/W ratio=3.2.
On SNA, conidiomata acervular, scattered, semi-immersed, conidiophores and setae formed on a cushion of roundish, hyaline to pale brown cells. Setae pale to dark brown, smooth-walled, straight or flexuous, 3–4-septate, 60–74 μm long, basal cell cylindrical, 4–4.5 μm diam, tip more or less acute. Conidiophores hyaline to pale brown, smooth-walled, septate, branched. Conidiogenous cells hyaline, smooth-walled, cylindrical, occasionally ampulliform, 21–33 × 2.5–3 μm. Conidia hyaline, aseptate, smooth-walled, cylindrical, straight, occasionally slightly curved, both ends rounded, or one end rounded and one end ± acute, 12–15.5 × 3.5–6 μm (av. ± SD = 14.0 ± 1.0 × 4.3 ± 0.7 μm), L/W ratio = 3.2. Appressoria single or gregarious, olivaceous, irregular outline with crenate or lobed margin, or clavate, 8.5–17.5 × 5–12 μm (av. ± SD = 12.3 ± 2.3 × 8.2 ± 1.7 μm).
Typus: China, Guangxi Zhuang Autonomous region, on seeds of Phyllostachys edulis, Sep. 2016, C.L. Hou & Q.T. Wang (holotype CAF80001, ex-type culture CFCC 54250 = ACCC 39709 = CNUCC 307307).
Additional materials examined: China, Fujian, Fuzhou, Wuyi Mountain, on Petasites hybridus, Aug. 2016, Z.Y. Ma, WYS14, living culture LC8469 (= M0288); ibid. living cultures LC8468 (= M0287); Fujian, Fuzhou, Wuyi Mountain, on P. hybridus, Aug. 2016, Z.Y. Ma, WYS40, living culture LC8498 (= M0322); Fujian, Fuzhou, Wuyi Mountain, on Patrinia villosa, Aug. 2016, Z.Y. Ma, WYS66, living culture LC8533 (= M0362).
Notes: Here, we describe the sexual morph of C. bambusicola, which was lacking in the original description based on collections from bamboo in Wang et al. (2021). This fungus was treated in a broader sense although two subclades were recognised. However, after broader sampling for the phylogenetic analysis and detailed morphological comparisons, we concluded that a new species should be proposed to distinguish the two clades (Fig. 1). Colletotrichum bambusicola differs in 3 bp in act, 2 bp in gapdh, 24 in his3, 1 bp in ITS, 11 bp in tub2 from C. parabambusicola sp. nov. Morphologically, C. bambusicola differs from C. parabambusicola sp. nov. in that it produces longer asci (58–72 × 7–10 μm vs. 47–55 × 6.5–7.5 μm) and ascospores (14–19 × 4–6 μm vs. 10–14.5 × 3–5.5 μm), and a larger conidium L/W ratio (3.2 vs. 2.6).
Colletotrichum bicoloratum F. Liu, W.P. Wu & L. Cai, sp. nov. MycoBank MB 841371. Fig. 7.
Fig. 7.
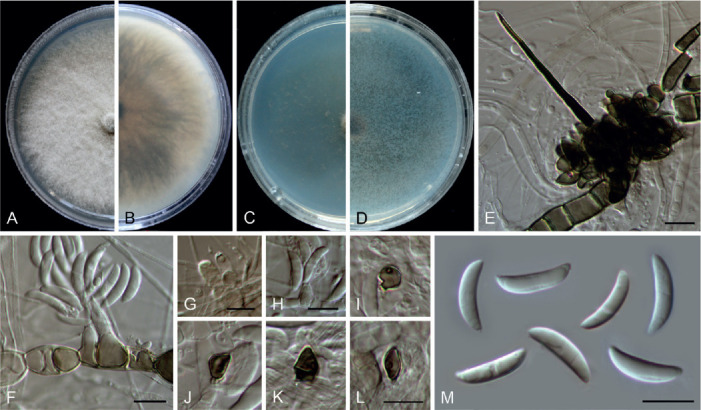
Colletotrichum bicoloratum (ex-type culture NN055229). A, B. Front and reverse colony on PDA (7 d). C, D. Front and reverse colony on SNA (7 d). E. Seta and conidiophores. F–H. Conidiogenous cells and conidia. I–L. Appressoria. M. Conidia. Scale bars = 10 μm.
Etymology: Named to reflect the bicoloured conidiogenous cells.
Description: Colonies on PDA 52 mm diam in 7 d, flat with entire edge, white, aerial mycelium dense, reverse greyish sepia to fuscous black. On MEA, setae rarely observed, dark brown, smooth-walled, straight, 3–4-septate, up to 51 μm long, basal cell cylindrical, 3.5 μm diam, tip acute. Conidiophores formed directly on hyphae, usually reduced into conidiogenous cells. Conidiogenous cells hyaline or dark brown, smooth-walled, ampulliform, 6–10.5 × 4–6 μm (av. ± SD = 8.8 ± 1.4 × 4.8 ± 0.6 μm). Conidia hyaline, aseptate, smooth-walled, curved, tapering towards apex and base, base usually obtuse and broader than the apex, 12–16 × 3–4 μm (av. ± SD = 14 ± 0.9 × 3.6 ± 0.3 μm), L/W ratio = 3.9. Appressoria single, medium to dark brown, usually ellipsoidal to subcircular, or irregularly shaped, rarely 2-celled, 5–9 × 4–6.5 μm.
Typus: China, Guangdong Province, Guangzhou, Yuexiu Park, on dead leaves of Ophiopogon japonicus, 29 Dec. 2012, W.P. Wu (holotype HMAS 350648, ex-type culture CGMCC 3.20510 = LC13882 = NN055229).
Notes: Colletotrichum bicoloratum is phylogenetically closely related to C. guizhouensis, C. lilii and C. spaethianum in the C. spaethianum species complex, but differs from C. guizhouensis and C. lilii in producing shorter and wider conidiogenous cells (6–10.5 × 4–6 μm vs. 12–25 × 2–2.5 μm in C. guizhouensis, 7–20 × 2–3.5 μm in C. lilii) and smaller appressoria (5–9× 4–6.5 μm vs. 6–14.5 × 5–11 μm in C. guizhouensis, 7.5–28.5 × 4.5–14 μm in C. lilii), and differs from C. spaethianum in producing wider conidiogenous cells (6–10.5 × 4–6 μm vs. 6–16 × 3–4 μm) and shorter conidia (12–16 × 3–4 μm vs. 13.5–29 × 3–4.5 μm) (Damm et al. 2009). Colletotrichum bicoloratum can be identified to species level by analysing any of the locus used in the current study.
Like most species in the C. spaethianum species complex, the associated host of C. bicoloratum, Ophiopogon japonicus, is also a petaloid monocotyledon plant. To the best of our knowledge, this is the first report of a Colletotrichum species associated with this plant host in China (Farr & Rossman 2021).
Colletotrichum bromeliacearum F. Liu & L. Cai, sp. nov. MycoBank MB 841372. Fig. 8.
Fig. 8.
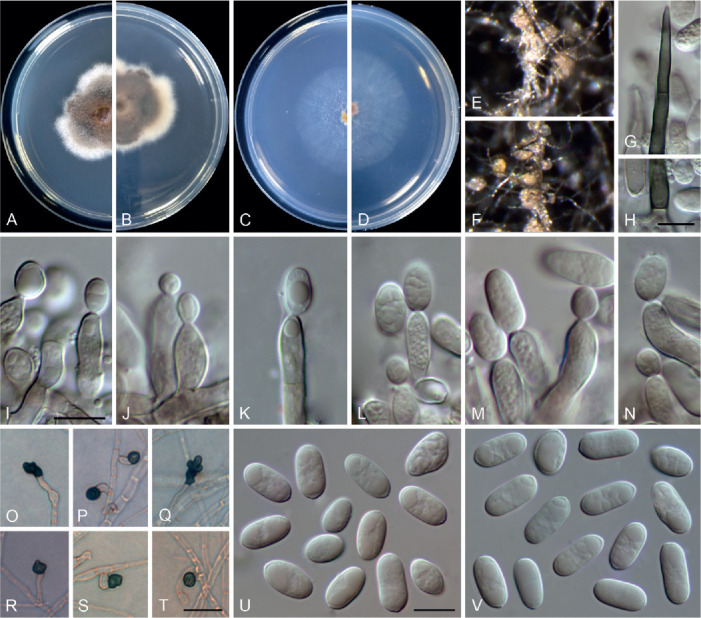
Colletotrichum bromeliacearum (ex-type culture LC0951). A, B. Front and reverse colony on PDA (9 d). C, D. Front and reverse colony on SNA (9 d). E, F. Conidiomata on PDA. G. Tip of a seta. H. Base of a seta. I–N. Conidiogenous cells and conidia. O–T. Appressoria. U, V. Conidia. Scale bars: H, I, U = 10 μm; T = 20 μm. Scale bar of I applies to I–N; T applies to O–T; U applies to U, V.
Etymology: Named after the host plant family, Bromeliaceae.
Description: Colonies on PDA 40–47 mm diam in 7 d, flat with undulate edge, cinnamon in the centre, white towards the margin, aerial mycelia sparse, reverse brown vinaceous, dark brick, white towards margin. On PDA, conidiomata acervular, gregarious, pale luteous. Setae olivaceous grey to olivaceous black, smooth-walled, 2–3-septate, 21–73 μm long, basal cell cylindrical or cylindric-conical, 3–5 μm diam, tip more or less acute. Conidiophores formed from a cushion of roundish and pale brown cells, solitary or branched, septate, hyaline to pale brown. Conidiogenous cells hyaline or pale brown, smooth-walled, cylindrical, ovoid, 9–20 × 3.5–7 μm. Conidia hyaline, aseptate, smooth-walled, cylindrical with both ends round, 8.5–16 × 5–7.5 μm (av. ± SD = 12 ± 1.8 × 6.2 ± 0.7 μm), L/W ratio =1.9. Appressoria single, mostly globose to subglobose, rarely subcylindrical or irregular outline, with undulate edge, 5–8(–15) × 4.5–8 μm (av. ± SD = 6.7 ± 0.9 × 6 ± 0.9 μm).
Typus: China, Yunnan, on a bromeliad plant (Bromeliaceae), 2010, F. Liu (holotype HMAS 350626, ex-type culture CGMCC 3.20527 = LC0951).
Additional materials examined: China, Yunnan, on a bromeliad plant (Bromeliaceae), 2010, F. Liu, living cultures LC13854, LC13855, LC13856.
Notes: Four strains of C. bromeliacearum formed a distinct clade in the C. boninense species complex (Fig. 1). This species is distinct from other species in this genus at each locus sequenced in the current study. Hitherto, five Colletotrichum species were known from Bromeliaceae, i.e. C. ananas, C. brevisporum, C. truncatum, C. gloeosporioides, and C. setosum (Farr & Rossman 2021). Colletotrichum bromeliacearum is easily distinguished from C. brevisporum, C. truncatum, and C. gloeosporioides based on molecular and morphological characters (different species complexes), and from C. ananas [Nom. inval., Art. 39.1 (Shenzhen)] and C. setosum in producing different shapes of conidia (cylindrical and straight vs. curved) (Garud 1968), as well as shorter and wider conidia (8.5–16 × 5–7.5 μm vs. 15–17 × 4–5 μm) (Patterson 1900), respectively.
Colletotrichum buxi F. Liu, W.P. Wu & L. Cai, sp. nov. MycoBank MB 841373. Fig. 9.
Fig. 9.
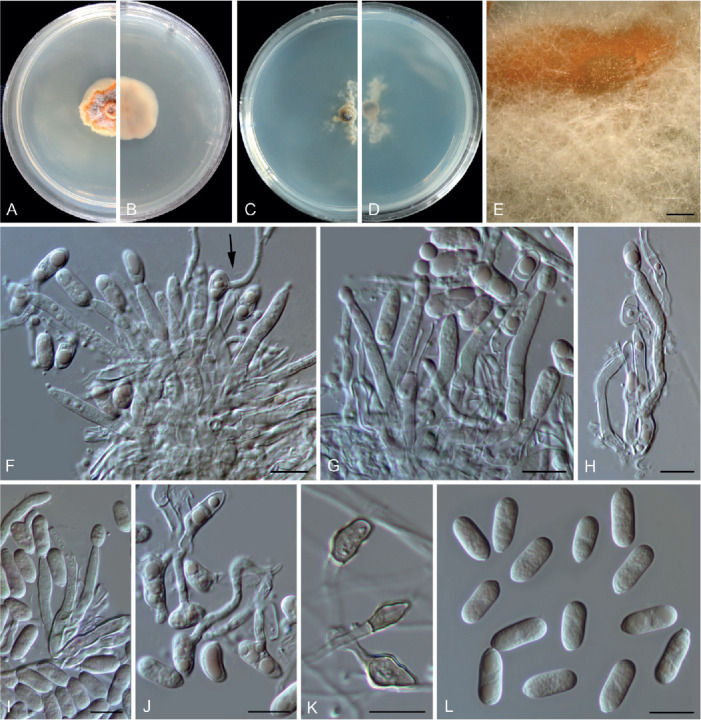
Colletotrichum buxi (ex-type culture NN047139). A, B. Front and reverse colony on PDA (7 d). C, D. Front and reverse colony on SNA (7 d). E. Conidial masses on PDA. F–I. Conidiophores, conidiogenous cells and conidia (arrow in F points to a germinating conidium). J. Germinating conidia. K. Appressoria. L. Conidia. Scale bars: E = 300 μm; F–L = 10 μm.
Etymology: Named after the host plant genus, Buxus.
Description: Colonies on PDA growing very slowly, reaching 8–11 mm diam after 7 d, flat with undulate edge, saffron to orange, conidial masses abundant, orange, aerial mycelium sparse, reverse salmon. Conidiomata not developed, abundant conidial masses (Fig. 9 E) formed on the surface of PDA, covered by aerial mycelium, orange, confluent. Conidiophores formed directly on hyphae, hyaline, septate, branched, 32–74 μm in length. Conidiogenous cells hyaline, smooth-walled, cylindrical to subcylindrical, variable in size, 9.5–26 × 2–4.5 μm (av. ± SD =18.6 ± 4.1 × 3.4 ± 0.7 μm). Conidia hyaline, mostly aseptate, sometimes 1-septate and germinating with thin and flexuous germ tubes developing from the middle or apex of the conidia (Fig. 9 F, J), smooth-walled, cylindrical, both ends round, or one end obtuse and another end round, 9.5–14.5 × 4–6 μm (av. ± SD =12.2 ± 1.2 × 5.1 ± 0.4 μm), L/W ratio =2.4. Appressoria mostly clavate, sometimes subcylindrical or with an irregular outline and with a crenate or lobed margin, 5–12 × 3.5–6 μm (av. ± SD = 7.5 ± 1.8 × 4.4 ± 0.7 μm). Setae not observed.
Typus: China, Yunnan Province, Kunming, Kunming Botanical Garden, on healthy leaves of Buxus sp., 10 May 2002, W.P. Wu (holotype HMAS 350642, ex-type culture CGMCC 3.20511 = LC13873 = NN047139).
Additional material examined: China, Yunnan Province, Kunming, Kunming Botanical Garden, on Buxus sinica var. parvifolia, 20 Dec. 1993, W.P. Wu, living culture LC14551 (= NN004149).
Notes: Colletotrichum buxi resides within the C. dracaenophilum species complex in the multi-locus phylogenetic tree (Fig. 1). It shares low sequence similarity with the phylogenetically related species C. yunnanense at act (93.8 %), chs-1 (98.8 %), gapdh (89.5 %), his3 (94.9 %), tub2 (97.3 %), and ITS (97.4 %), and differs in producing longer conidiophores (32–74 μm vs. 10–30 μm) and conidiogenous cells (9.5–26 μm vs. 6–12 μm), and shorter conidia (9.5–14.5 μm vs. 14–21 μm) (Liu et al. 2007).
Colletotrichum chamaedoreae F. Liu, W.P. Wu & L. Cai, sp. nov. MycoBank MB 841374. Fig. 10.
Fig. 10.
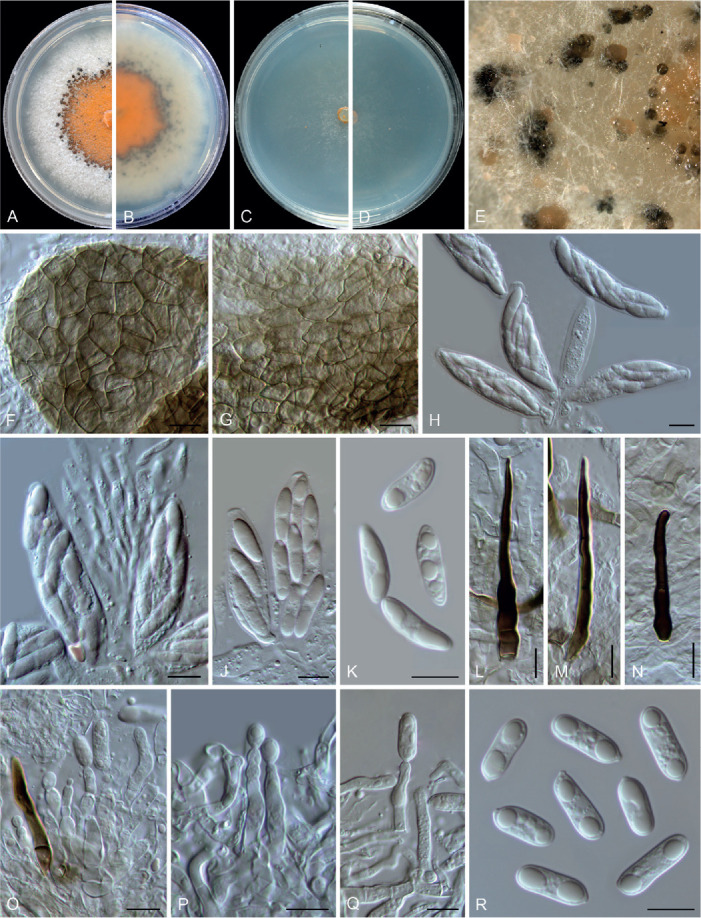
Colletotrichum chamaedoreae (ex-type culture NN052885). A, B. Front and reverse colony on PDA (7 d). C, D. Front and reverse colony on SNA (7 d). E. Conidiomata and ascomata on SNA. F, G. Ascomata wall. H–J. Asci, ascospores and paraphyses. K. Ascospores. L–N. Seta. O–Q. Conidiophores, conidiogenous cells and conidia. R. Conidia. Scale bars = 10 μm.
Etymology: Named after the host plant genus, Chamaedorea.
Description: Colonies on PDA 42–48 mm diam in 7 d, orange in the centre due to the formation of abundant conidial masses, white at the margin, reverse orange in the centre and white towards the margin. Vegetative hyphae hyaline, smooth-walled, septate, branched, 2.5–4.5 μm diam. On PDA, ascomata globose, subglobose or with an irregular shape, solitary or gregarious, brown to black, sub-immersed or immersed, ostiolate, outer wall composed of pale brown to dark brown angular cells, 6.5–19.5 × 3.5–12 μm diam (av. ± SD = 12.8 ± 3.1 × 7.3 ± 2.2 μm). Interascal tissue composed of hyaline, thin-walled, septate paraphyses, 2.5–5.5 μm diam. Asci obclavate or clavate, hyaline, 41–65 × 12–16 μm, 8-spored. Ascospores uniseriately or irregularly arranged, hyaline, smooth-walled, aseptate, fusoid or subcylindrical with gently tapering rounded ends, straight or slightly curved, 14.5–21.5 × 4.5–6.5 μm (av. ± SD = 17.7 ± 1.8 × 5.3 ± 0.5 μm), L/W ratio = 3.3.
Conidial masses amber to buff, protruded from the dark brown to black conidiomata. Setae medium brown to dark brown, smooth-walled, 1–7-septate, 36–95 μm long, basal cells cylindrical, sometimes inflated in the middle, 4–8 μm diam, the tip acute or rounded. Conidiophores 0–1-septate, usually reduced to conidiogenous cells. Conidiogenous cells hyaline, cylindrical to subcylindrical, smooth-walled, straight or slight curved, sometimes extending to form new conidiogenous loci, 11–27 × 3.5–9.5 μm. Conidia hyaline, aseptate, smooth-walled, guttulate, often with two big and a number of small guttules, cylindrical, the apex round, the base with a prominent truncate scar, straight, 13.5–19 × 4.5–6.5 μm (av. ± SD = 15.7 ± 1.4 × 5.6 ± 0.3 μm), L/W ratio = 2.8. Appressoria not observed.
Typus: China, Yunnan Province, Jinghong, Xishuangbanna Botanical Garden, on healthy leaves of Chamaedorea erumpens, 19 Mar. 2010, W.P. Wu (holotype HMAS 350639, ex-type culture CGMCC 3.20512 = LC13868 = NN052885).
Additional materials examined: China, Yunnan Province, Jinghong, Xishuangbanna Botanical Garden, on healthy leaves of Chamaedorea erumpens, 19 Mar. 2010, W.P. Wu, living cultures LC13867 (= NN052884), LC13869 (= NN052890), LC13870 (= NN052891).
Notes: Colletotrichum chamaedoreae belongs to the C. boninense species complex (Fig. 1). Conidiogenous cells that extend to form new conidiogenous loci have previously been observed in species of the C. boninense species complex, e.g. in C. annellatum, C. constrictum, C. cymbidiicola, C. novae-zelandiae, and C. oncidii. However, their conidia are shorter and the conidium L/W ratio is lower than those of C. chamaedoreae (Damm et al. 2012b). In addition, C. chamaedoreae is distinct from other species in this genus at each locus sequenced in the current study, and morphologically differs from the most closely related species C. brassicicola in that it produces shorter asci (41–65 × 12–16 μm vs. 65–105 × 12–13.5 μm), and longer and wider conidiogenous cells (11–27 × 3.5–9.5 μm vs. 7–14 × 4–5.5 μm) (Damm et al. 2012b).
Colletotrichum chiangraiense X.Y. Ma et al., MycoKeys 43: 34. 2018.
Notes: Colletotrichum chiangraiense was shown to be a member of the C. boninense species complex in the original description (Ma et al. 2018), but resided within the C. gigasporum species complex in our multi-locus tree, forming an unusually long branch (Fig. 1). BLASTn search of the NCBI GenBank using ITS and act sequences of C. chiangraiense (ex-type MFLUCC 14-0119) yielded closest matches with species in the C. gigasporum species complex, while using tub2 yielded closest matches with species in the C. boninense species complex. Therefore, it is very likely that the sequences provided in Ma et al. (2018) were misplaced or with sequencing errors. The taxonomic status of C. chiangraiense requires confirmation by re-examination and re-sequencing of the type.
Colletotrichum crousii F. Liu, Z.Y. Ma & L. Cai, sp. nov. MycoBank MB 841375. Fig. 11.
Fig. 11.

Colletotrichum crousii (ex-type culture LC13858). A–D. Disease symptoms on Rhaphidophora spp. E, F. Front and reverse colony on PDA (7 d). G, H. Front and reverse colony on SNA (7 d). I. Conidioma on SNA. J–L, N, O. Conidiophores, conidiogenous cells and conidia. M, P. Conidia. Scale bars = 10 μm. Scale bar of J applies to J–L, N, O.
Etymology: Named in honour of the mycologist Pedro Crous, one of the major contributors to recent improvements in Colletotrichum systematics.
Description: Colonies on PDA 44–46 mm diam in 7 d, flat with undulate edge, rosy buff, covered by cottony and white aerial mycelium, reverse dark vinaceous buff, white towards margin. On PDA, conidiomata not observed, conidiophores formed directly from hyphae, conidial masses pale vinaceous buff, confluent. Conidiophores hyaline, solitary, sometimes branched at the base, up to 35 μm, usually reduced to conidiogenous cells. Conidiogenous cells hyaline, aseptate, smooth-walled, cylindrical to ampulliform, 9–22 × 4.5–6 μm. Conidia hyaline, aseptate, smooth-walled, cylindrical, both ends obtuse, or apex obtuse and bottom gradually narrowed with an prominent truncate scar, 12.5–18.5 × 6.5–8 μm (av. ± SD =16 ± 1.6 × 7 ± 1.6 μm), L/W ratio =2.2. Appressoria and setae not observed.
Typus: China, Guangxi, Chongzuo, Guangxi Nonggang National Nature Reserve, on leaf of Rhaphidophora sp., Jun. 2017, Z.Y. Ma & L.W. Hou, NG56 (holotype HMAS 350636, ex-type culture CGMCC 3.20513 = LC13858 = MH0588).
Additional materials examined: China, Guangxi, Chongzuo, Guangxi Nonggang National Nature Reserve, on leaf of Rhaphidophora sp., Jun. 2017, Z.Y. Ma & L.W. Hou, NG56 (Fig. 11A, B), living cultures LC13859 (= MH0589), LC13860 (= MH0592), LC13861 (= MH0727); on leaf of Rhaphidophora sp., Jun. 2017, Z.Y. Ma & L.W. Hou, NG58 (Fig. 11C, D), living culture LC13862 (= MH0759).
Notes: Colletotrichum crousii is phylogenetically related to C. excelsum-altitudinum, C. tongrenense, and C. tropicicola in the C. dracaenophilum species complex, but differs in that it produces wider conidia (6.5–8 μm vs. 5–7 μm in C. excelsum-altitudinum and C. tongrenense, 4.5–5.5 μm in C. tropicicola) with a lower L/W ratio (2.2 vs. generally 2.4–3.3) (Tao et al. 2013, Damm et al. 2019, Zhou et al. 2019), and larger conidiogenous cells (9–22 × 4.5–6 μm vs. 2–11 × 1–2 μm in C. tongrenense) (Zhou et al. 2019). Moreover, C. crousii is distinct from other species in this genus at each locus sequenced in the current study.
Colletotrichum danxiashanense F. Liu, W.P. Wu & L. Cai, sp. nov. MycoBank MB 841376. Fig. 12.
Fig. 12.
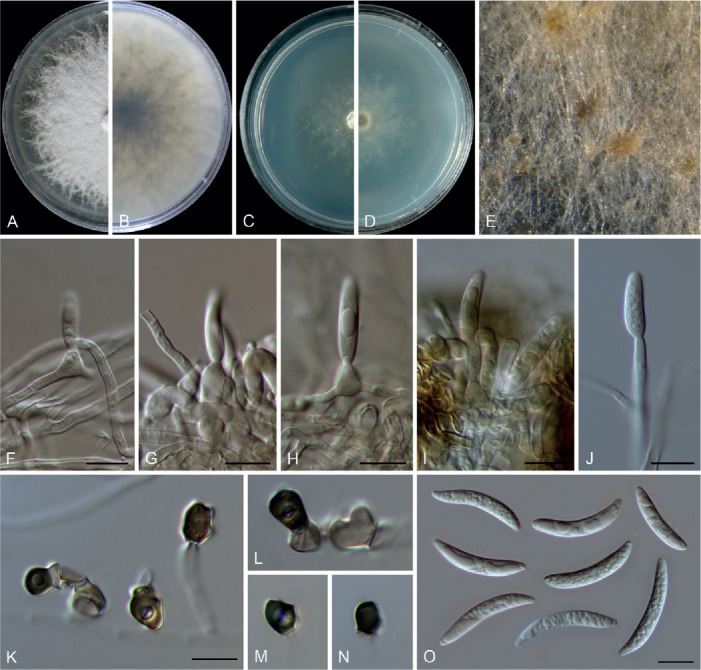
Colletotrichum danxiashanense (ex-type culture NN055218). A, B. Front and reverse colony on PDA (7 d). C, D. Front and reverse colony on SNA (7 d). E. Conidial masses on PDA. F–J. Conidiogenous cells and conidia. K–N. Appressoria. O. Conidia. Scale bars = 10 μm. Scale bar of K applies to K–N.
Etymology: Named after the location where the fungus was collected, Danxia Mountain.
Description: Colonies on PDA 52 mm diam in 7 d, flat with rhizoids edge, surface covered by floccose white, aerial mycelium, reverse pale luteous to brown. Vegetative hyphae hyaline or pale brown, smooth-walled, septate, branched. On SNA, conidiomata not developed, conidiophores formed directly on hyphae, terminally or laterally. Setae not observed. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline, rarely pale brown, smooth-walled, ampulliform to cylindrical, 6–12 × 2.5–5.5 μm, periclinal thickening not observed. Conidia hyaline, aseptate, smooth-walled, guttulate, falcate with ± acute or obtuse ends, 18.5–29.5 × 3–4.5 (av. ± SD ± 24.4 ± 3.1 × 3.8 ± 0.4), L/W ratio = 6.4. Appressoria single, medium brown to dark brown, subcircular, subcylindrical, or irregularly shaped, 6–10 × 6–7 μm (av. ± SD = 8.3 ± 1.4 × 6.4 ± 0.4 μm).
Typus: China, Guangdong Province, Shaoguan, Danxia Mountain, on dead leaves of probable Miscanthus sp., 25 Dec. 2012, W.P. Wu (holotype HMAS 350650, ex-type culture CGMCC 3.20514 = LC13885 = NN055218).
Notes: Colletotrichum danxiashanense belongs to the C. graminicola species complex and forms a sister clade to C. miscanthi (Fig. 3). Both species are morphologically similar and associated with the same host genus, Miscanthus, but with clearly different act (98.3 % identity, with 4 bp differences), chs-1 (99.6 % identity, with 1 bp difference), ITS (98.9 % identity, with 5 bp differences), sod2 (90.5 % identity, with 35 bp differences), and tub2 (97.9 % identity, with 10 bp differences) sequences.
Colletotrichum diversisporum F. Liu, W.P. Wu & L. Cai, sp. nov. MycoBank MB 841377. Fig. 13.
Fig. 13.
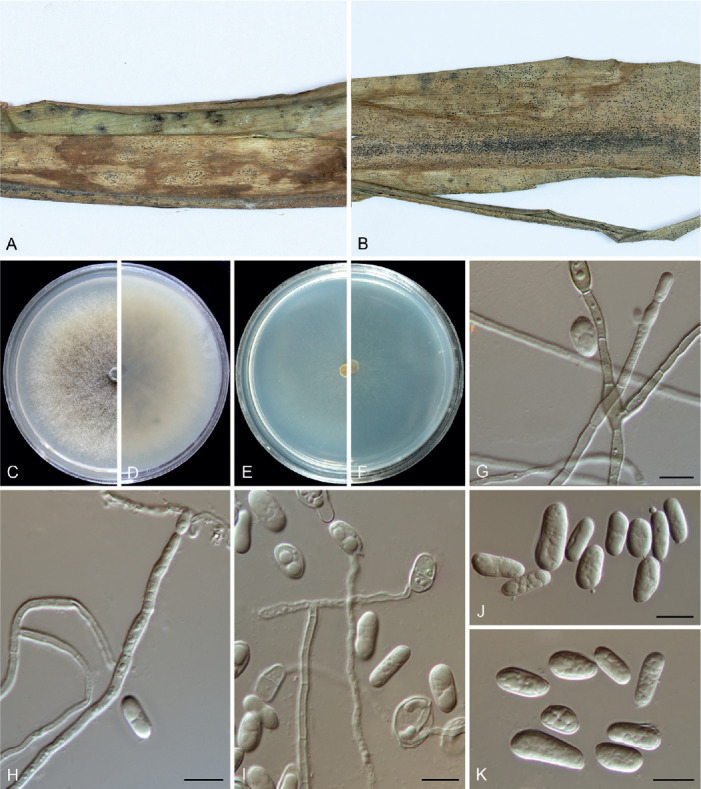
Colletotrichum diversisporum (ex-type culture NN072578). A, B. Disease symptoms on host plant. C, D. Front and reverse colony on PDA (7 d). E, F. Front and reverse colony on SNA (7 d). G, H. Conidiophores, conidiogenous cells and conidia. I. Germinating conidia. J, K. Conidia. Scale bars = 10 μm.
Etymology: Refers to the diverse shapes of conidia.
Description: Colonies on PDA 49 mm diam in 7 d, flat with entire edge, smoke to mouse grey in the centre, white towards the margin, aerial mycelium more or less sparse, reverse smoke grey. Vegetative hyphae hyaline, smooth-walled, septate, branched. On PDA, conidiomata not developed, conidiophores formed directly on hyphae. Conidiophores hyaline or very pale brown, septate, branched. Conidiogenous cells hyaline, smooth-walled, cylindrical, 14–33 × 2.5–3.5 μm. Conidia hyaline, aseptate, smooth-walled, guttulate, variable in shape and size, cylindrical, ellipsoidal or ovoid, sometimes constricted in the centre or near the base, 9.5–16.5 × 3.5–8 μm (av. ± SD ± 11.5 ± 1.5 × 5.2 ± 1.1 μm), L/W ratio = 2.2. Sometimes conidia becoming 1-septate and germinating after 10 d, germ tubes flexuous and thinner than hyphae (Fig. 13 G). Appressoria and setae not observed.
Typus: China, Guangdong Province, Guangzhou, South China Botanical Garden, on dead leaves of Dracaena angustifolia, 28 Feb. 2016, W.P. Wu (holotype HMAS 350655, ex-type culture CGMCC 3.20515 = LC13890 = NN072578).
Notes: Colletotrichum diversisporum is basal to the C. magnum and C. orchidearum species complexes (Fig. 1). It is characterised by a production of conidia of variable shapes and sizes, and can be easily differentiated from other species by analysing any of the loci sequenced in the current study.
Colletotrichum diversum F. Liu & L. Cai, sp. nov. MycoBank MB 841378. Fig. 14.
Fig. 14.
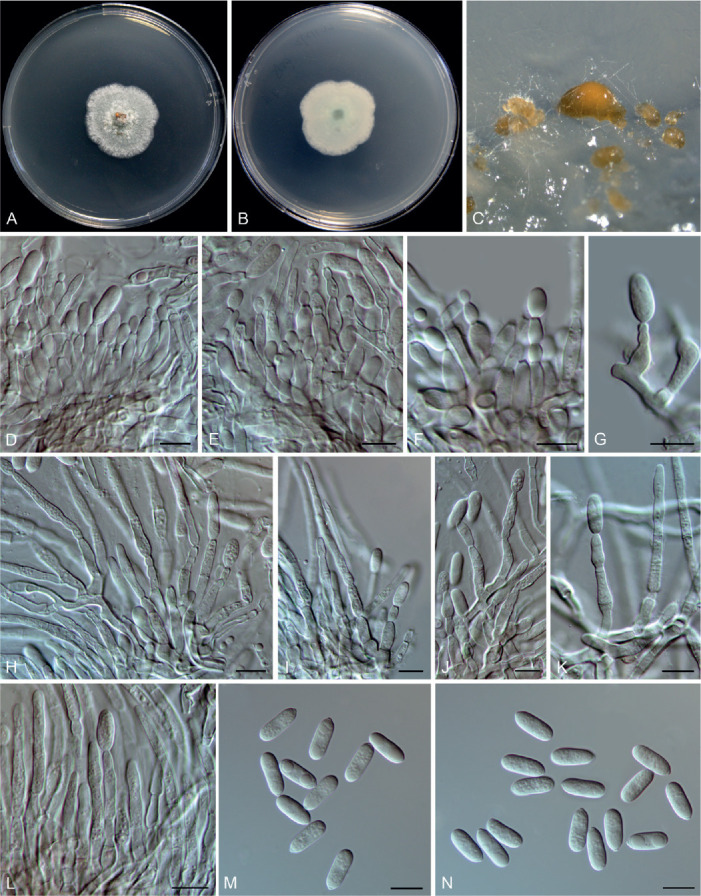
Colletotrichum diversum (ex-type culture LC11292). A, B. Front and reverse colony on PDA (6 d). C. Conidiomata and conidial masses. D–G. Type I of conidiophores and conidia. H–L. Type II of conidiophores and conidia. M, N. Conidia. Scale bars = 10 μm.
Etymology: Named to reflect the formation of two different types of conidiophores.
Description: Colonies on PDA 36–39 mm diam in 7 d, flat with undulate edge, pale glaucous grey, aerial mycelium sparse, surface partly covered with orange conidial masses, reverse white. Vegetative hyphae hyaline, smooth-walled, septate, branched. On SNA, conidiomata acervular, scattered or gregarious, conidiophores formed on a cushion of subglobose pale brown cells (type I, Fig. 14 D–F) or directly from vegetative hyphae (type II, Fig. 14 H–L). The first type of conidiophores hyaline, branched, up to 45 μm, sometimes reduced to conidiogenous cells; conidiogenous cells hyaline, ampulliform to obclavate, sometimes cylindrical, often extending to form new conidiogenous loci, 11.5–17.5(–22.5) × 3–5.5 μm (av. ± SD = 14.6 ± 2.4 × 4.3 ± 0.6 μm), periclinal thickening distinct. The second type of conidiophores hyaline, septate, branched, up to 80 μm; conidiogenous cells hyaline, smooth-walled, cylindrical or subcylindrical, sometimes irregularly inflated, variable in length, (7–)12–32 × 3–4.5 μm (av. ± SD = 20.9 ± 6.2 × 3.7 ± 0.6 μm), usually extending to form new conidiogenous loci, periclinal thickening distinct. Conidia hyaline, aseptate, smooth-walled, guttulate, straight, cylindrical with one end round and one end slightly acute, 12–15.5 × 4.5–5.5 μm (av. ± SD = 14.1 ± 0.8 × 5.2 ± 0.3), L/W ratio = 2.7. Appressoria and setae not observed.
Typus: China, Yunnan Province, Honghe Hani and Yi Autonomous Prefecture, Mengzi county, Nanhu park, on Philodendron selloum, 12 May 2016, Q. Chen (holotype HMAS 350633, ex-type culture CGMCC 3.20516 = LC11292 = CQ775).
Notes: Colletotrichum diversum is basal to the phylogenetic clade that comprises C. boninense, C. cymbidiicola, C. doitungense C. oncidii, C. torulosum and C. watphraense in the C. boninense species complex (Fig. 1), and shares low sequence similarity with the most closely related species C. oncidii at act (97.9 %) and gapdh (96.9 %) and 99 % similarities at his3, ITS and tub2. Morphologically, the two species differ with respect to the formation of conidiophores and conidiogenous cells. On SNA, C. diversum forms conidiophores either on a cushion of subglobose pale brown cells or directly from vegetative hyphae, while C. oncidii only forms conidiophores directly from hyphae. In addition, C. diversum produces ampulliform to obclavate or (sub-)cylindrical conidiogenous cells, while only cylindrical cells have been observed for C. oncidii (Damm et al. 2012b).
To date, Colletotrichum species known to be associated with Philodendron selloum are C. orchidearum (Hou et al. 2016, Damm et al. 2019), and C. philodendri which lacks type-derived sequence data (Alfieri et al. 1984). Furthermore, C. diversum morphologically differs from C. philodendri with respect to the shape (cylindrical with obtuse ends vs. round at one end and acute at the other end) and width (4.5–5.5 μm vs. 3.5–4 μm) of the conidia. Geographically, C. philodendri was reported in the Americas (Brazil and the US) (Hennings 1905, Alfieri et al. 1984), while, to date, C. diversum is only known in China.
Colletotrichum dolichoconidiophori F. Liu, W.P. Wu & L. Cai, sp. nov. MycoBank MB 841379. Fig. 15.
Fig. 15.
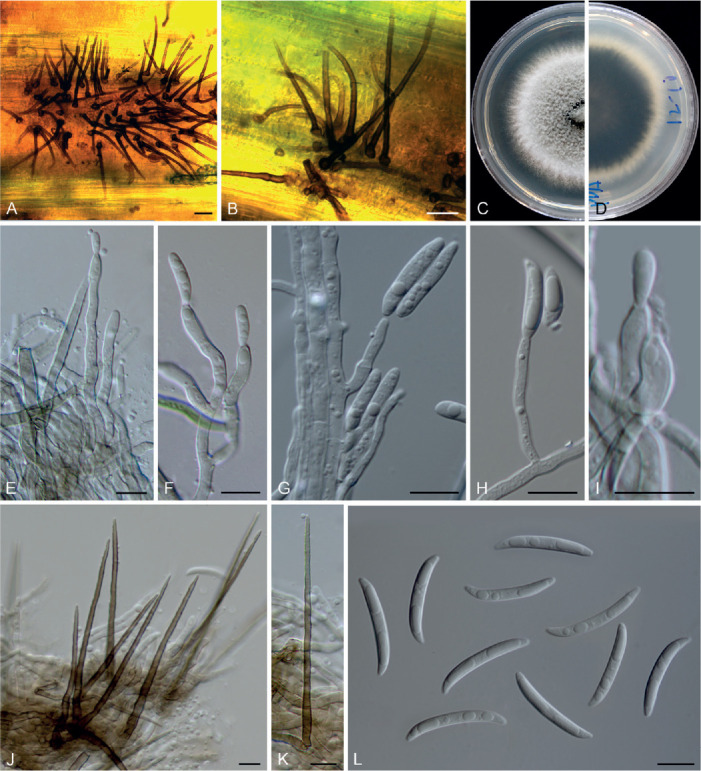
Colletotrichum dolichoconidiophori (ex-type culture NN054966). A, B. Conidiomata with setae on the host plant. C, D. Front and reverse colony on PDA (7 d). E–I. Conidiophores, conidiogenous cells and conidia. J, K. Setae. L. Conidia. Scale bars: A, B = 20 μm; E–L = 10 μm.
Etymology: Named to reflect the formation of long conidiophores.
Description: Colonies on PDA 40–41 mm diam in 7 d, flat with fimbriate edge, pale grey in the centre, white toward the margin, aerial mycelium floccose, reverse fuscous black, white at margin. Vegetative hyphae hyaline to pale brown, smooth-walled, septate, branched. Conidiomata not developed. On PDA, conidiophores formed directly on hyphae, hyaline to very pale brown, smooth-walled, cylindrical, up to 250 μm. Conidiogenous cells cylindrical to subcylindrical, rarely ampulliform, hyaline, 6–23 × 2–4 μm. Setae abundant, brown, smooth- and thick-walled, 0–3-septate, 41–110 μm long, basal cell cylindrical, inflated, flask shaped or globose, 3.5–8 μm diam, tip ± acute. Conidia hyaline, aseptate, smooth-walled, falcate, central part of the conidium almost straight with parallel walls, gradually tapering towards a slightly rounded to ± acute apex and a truncate base, with a similar radian, 19–27.5 × 2.5–3.5 μm (av. ± SD ± 24.9 ± 1.92 × 3.1 ± 0.29 μm), L/W ratio = 8. Appressoria not observed.
Typus: China, Beijing, Huairou, Beigoucun, on an unidentified grass, 10 Sep. 2012, W.P. Wu (holotype HMAS 350654, ex-type culture CGMCC 3.20517 = LC13889 = NN054966).
Notes: Colletotrichum dolichoconidiophori belongs to the C. graminicola species complex (Fig. 1). It shares very low sequence similarity with all currently accepted species in the genus, even with the most closely related species C. cereale CBS 129663 (Figs 1, 3, act: 90.7 %; chs-1: 96 %; ITS: 96.7 %; sod2: 91.8 %; tub2: 92.2 %). Morphologically, C. dolichoconidiophori differs from C. cereale in that it produces longer conidiogenous cells (6–23 × 2–4 μm vs. 2–6 × 1–2 μm).
Colletotrichum guangxiense C.L. Hou & Q.T. Wang, Mycologia 113: 454. 2021.
Notes: Intraspecific branches in the C. guangxiense clade suggest a high degree of genetic variation in this species, mainly within the act and tub2 sequences examined in the current study.
Colletotrichum iris F. Liu & L. Cai, sp. nov. MycoBank MB 841383. Fig. 16.
Fig. 16.
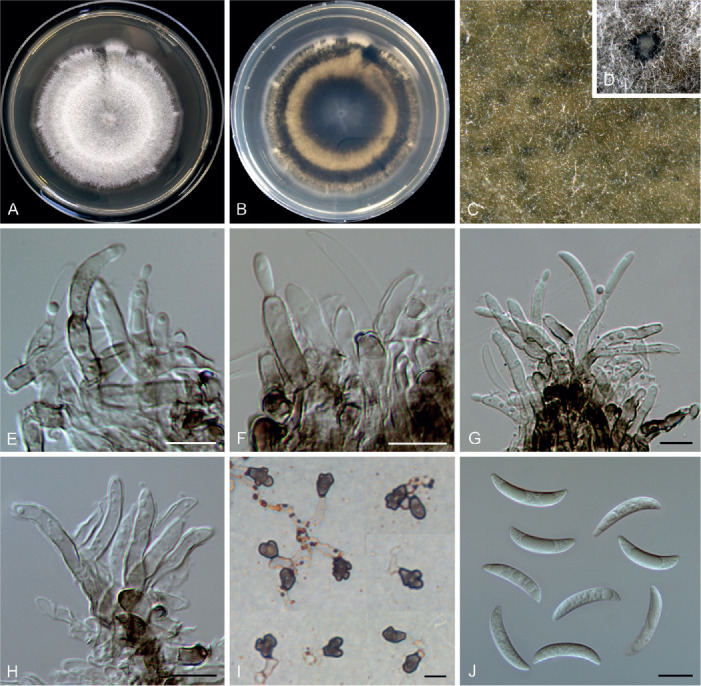
Colletotrichum iris (ex-type culture LC3697). A, B. Front and reverse colony on PDA (7 d). C, D. Immersed conidiomata. E–H. Conidiophores, conidiogenous cells and conidia. I. Appressoria. J. Conidia. Scale bars = 10 μm.
Etymology: Named after the host plant genus, Iris.
Description: Colonies on PDA 33–34 mm diam in 7 d, flat, aerial mycelium dense, white, reverse pale luteous, centre part fuscous black, and with a fuscous black halo towards margin. On PDA, conidiomata scattered or confluent, dark brown to black, immersed. Setae not observed. Conidiophores hyaline to pale brown, branched, septate, up to 65 μm. Conidiogenous cells hyaline or pale brown, smooth-walled, cylindrical, occasionally ampulliform, straight or flexuous, 12.5–22.5 × 3–4.5 μm. Conidia hyaline, aseptate, smooth-walled, curved, apex ± acute, base usually broader and truncate, 18–28 × 2–4 μm (av. ± SD = 22 ± 2.6 × 3.4 ± 0.5 μm), L/W ratio = 6.5. Appressoria single or in small groups, medium brown, mostly irregular shaped, occasionally ellipsoidal to subcircular, 5–16 × 3–10 μm (av. ± SD = 9.6 ± 2.6 × 6.7 ± 1.8 μm).
Typus: China, Jiangxi, Lushan Botanical Garden, on Iris japonica, 4 Sep. 2013, N. Zhou (holotype HMAS 350628, ex-type culture CGMCC 3.20518 = LC3697).
Notes: Colletotrichum iris is closely related to C. liriopes in the C. spaethianum species complex (Fig. 1), but differs genetically in 2 bp of the act nucleotide sequence, 1 bp of chs-1, 13 bp of gapdh, 12 bp of his3, 1 bp of ITS and 3 bp of tub2. Morphologically, C. iris differs from C. liriopes in that it produces thick-walled, pale brown conidiogenous cells and a higher conidium L/W ratio (6.5 vs.5.0) (Damm et al. 2009). Like most other species in the C. spaethianum complex (Damm et al. 2009), C. iris was isolated from the petaloid monocotyledon plant, Iris japonica. Six other Iris-associated species in the genus are C. circinans, C. coccodes, C. dematium, C. gloeosporioides, C. siamense, and C. tofieldiae (Farr & Rossman 2021), which are phylogenetically distinct from C. iris. Moreover, C. iris morphologically differs from another Iris-associated species C. liliacearum (DNA sequences are unavailable) in producing longer conidia (18–28 × 2–4 μm μm vs.12–17 × 2.5–3.5) (Ferraris 1902).
Colletotrichum jasminigenum Wikee et al., Fungal Diversity 46: 174. 2011.
Notes: Colletotrichum jasminigenum was shown to be a member of the C. truncatum species complex (Hyde et al. 2014). However, BLASTn search of the NCBI GenBank using gapdh and act sequences of C. jasminigenum (ex-holotype MFLUCC 10-0273) yielded the closest matches with species of the C. gloeosporioides species complex, while using ITS and tub2 sequences indicated that it belongs to the C. truncatum species complex. Therefore, it is likely that the sequences of MFLUCC 10-0273 provided in Wikee et al. (2011) were mixed up with those of other species or contained major sequencing errors. To clarify the taxonomic status of C. jasminigenum, type specimen and culture inquiries were submitted to the culture collections (CGMCC and MFLUCC) mentioned in the original publication (Wikee et al. 2011). Unfortunately, the ex-type could not be located in CGMCC. We suspected that it might not have been preserved in the culture collection, as the strain number was not mentioned in Wikee et al. (2011). Furthermore, specimen delivery from MFLUCC was not allowed because of the current quarantine measures for international mail. Therefore, the taxonomic status of C. jasminigenum requires further examination and re-sequencing of the type.
Colletotrichum jinshuiense M. Fu & G.P. Wang, Persoonia 42: 21. 2018.
Synonym: Colletotrichum kakiivorum H.Y. Jung & S.Y. Lee [as ‘kakivorum’], Mycol. Prog. 17: 1118. 2018.
Typus: China, Hubei Province, Wuhan, on leaves of Pyrus pyrifolia cv. Jinshui, 1 Aug. 2016, M. Fu (holotype HMAS 247824, ex-type culture CGMCC 3.18903).
Additional material examined: China, Fujian Province, Fuzhou, Wuyi Mountain, on Dioscorea zingiberensis, Aug. 2016, L. Cai & Z.Y. Ma, living culture LC8509 (= M0333).
Notes: The ex-type strains of C. jinshuiense (Fu et al. 2019, published online 24 Jul. 2018) and C. kakiivorum (Lee & Jung 2018, published online: 30 Jul. 2018) cluster together in a well-supported clade within the C. dematium species complex (Fig. 1), and their act, chs-1, gapdh, his3, ITS, and tub2 sequences share high similarity (99.8 %), with only two base-pair differences. Morphologically, C. jinshuiense and C. kakiivorum produce conidiogenous cells, conidia, and appressoria with essentially similar shapes and dimensions. Hence, C. kakiivorum is synonymised with the previously published C. jinshuiense.
Colletotrichum liriopes Damm et al., Fungal Diversity 39: 71. 2009. Fig. 17.
Fig. 17.
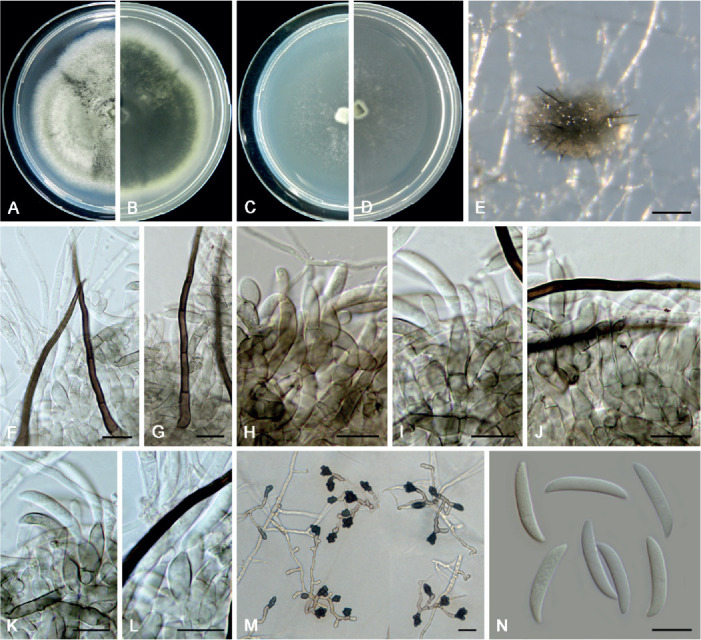
Colletotrichum liriopes (LC11287). A, B. Front and reverse colony on PDA (7 d). C, D. Front and reverse colony on SNA (7 d). E. Conidioma. F, G. Setae. H–L. Conidiophores, conidiogenous cells and conidia. M. Appressoria. N. Conidia. Scale bars: E = 80 μm; F–N = 10 μm.
Typus: Mexico, APHIS interception Houston 057263, on Liriope muscari, 29 Nov. 2000, M.J. Segall (holotype CBS H-20364, ex-type culture CBS 119444).
Additional materials examined: China, Shan’xi, Xi An, Huaqing Hot Spring, on dead leaves of Osmanthus fragrans, 1 Jul. 2015, W.P. Wu, living culture NN071073; Tibet, Bomi county, Suotong village, on Poaceae, 13 Jun. 2015, F. Liu, living culture LC7623; Yunnan Province, Honghe Hani and Yi Autonomous Prefecture, Mengzi county, DaTun Sea, on Liriope spicata, 12 May 2016, Q. Chen, living culture LC11287.
Notes: Colletotrichum liriopes is phylogenetically related to C. iris in the C. spaethanium species complex. This species is pathogenic to Eria coronaria (Yang et al. 2011), Liriope muscari (Damm et al. 2009), L. spicata (this study), and Rohdea japonica (Kwon & Kim 2013), endophytic in Bletilla ochracea and Peione bulbocodioides (Yang et al. 2011), and saprophytic on dead stalk of Hemerocallis fulva (Yang et al. 2011) and dead leaves of Osmanthus fragrans (this study).
Colletotrichum magnum (Jenkins & Winstead) Rossman & W.C. Allen, IMA Fungus 7: 4. 2016.
Basionym: Glomerella magna Jenkins & Winstead, Phytopathology 54: 453. 1964.
Synonym: Colletotrichum liaoningense Y.Z. Diao, et al., Persoonia 38: 34. 2017.
Typus: USA, on Citrullus lanatus, unknown collection date, R. Rodriguez, ex-epitype culture CBS 519.97.
Additional material examined: China, Liaoning Province, Xingcheng city, on chili fruits (Capsicum annuum var. conoides), Oct. 2012, Y.Z. Diao, ex-type culture of C. liaoningense CGMCC 3.17616 (= CAUOS2 = LC6228).
Notes: Since the sequence data and phylogenetic assessment of C. liaoningense are questionable (Damm et al. 2019), we re-sequenced six loci of its ex-type culture CAUOS2, twice, in the present study. Comparison with sequences deposited in the NCBI GenBank by Diao et al. (2017) revealed that the sequences of four loci were consistent except for 1–3 base-pair differences [99 % sequence similarity shared at cal (433/435), chs-1 (294/297), gapdh (257/258), and tub2 (679/680)]; however, the sequences of ITS and act differed at many positions [96 % similarity on ITS (478/500) and act (252/263)]. Therefore, the original sequences of CAUOS2 are incorrect, and the sequences newly generated in the current study have been deposited in GenBank (Table S1) and used for six-locus phylogenetic analyses (Fig. 1).
Colletotrichum liaoningense is phylogenetically indistinguishable from C. magnum (Fig. 1), and their act, chs-1, his3, ITS, and tub2 sequences are 100 % identical, while the gapdh sequences are 96 % (250/260) identical. Consequently, C. liaoningense is synonymised with the older name C. magnum.
Colletotrichum metake Sacc., Annls Mycol. 6: 557. 1908.
Notes: Colletotrichum metake was originally described on a dead culm of bamboo (Arundinaria japoniea) in Italy (Costa, Vittorio, Treviso) and characterised by oblong conidia (22 × 5.5–6 μm) (Saccardo 1908), but without type designation. In the current study, four bamboo-associated strains of C. metake (Sato et al. 2012, Wang et al. 2021) clustered in a well-supported clade (Fig. 1). Three of these strains were from Pleioblastus simonii in Japan and one from Chimonobambusa quadrangularis in China. However, none of these strains is suitable for type designation because of the inconsistency with the original collection site and host of the species (Hyde & Zhang 2008).
Colletotrichum monsterae F. Liu, W.P. Wu & L. Cai, sp. nov. MycoBank MB 841384. Fig. 18.
Fig. 18.

Colletotrichum monsterae (ex-type culture NN055214). A, B. Front and reverse colony on PDA (7 d). C, D. Front and reverse colony on SNA (7 d). E. Conidiomata (black, submerged) and conidial masses (buff, superficial). F–I. Conidiophores bearing conidia. J–O. Appressoria. P. Conidia formed on SNA. Q. Conidia formed on PDA. Scale bars = 10 μm.
Etymology: Named after the host plant genus, Monstera.
Description: Colonies on PDA 52 mm diam in 7 d, flat with entire edge, aerial mycelium white and floccose, reverse saffron. Vegetative hyphae hyaline to pale brown, smooth-walled, septate, branched. Conidiomata black, submerged, conidial masses buff, abundantly formed on the surface of the agar medium or directly on hyphyae. Setae not observed. Conidiophores brown, base dark brown, mostly gradually becoming paler towards the tip, 1–3-septate, solitary or branched at the bottom, 46–102 μm long. Conidiogenous cells pale to medium brown, smooth-walled, cylindrical, 15–51.5 × 3.5–6 μm (av. ± SD ± 33 ± 11.1 × 4.7 ± 0.8). Conidia hyaline, aseptate, smooth-walled, guttulate, cylindrical to subcylindrical, rarely obvoid, straight, sometimes slightly curved, with obtuse ends or gradually tapering towards the base, on SNA 13–22.5 × 4.5–6 μm (av. ± SD ± 18.2 ± 2.1 × 5 ± 0.4), L/W ratio = 3.6, on PDA 16.5–24.5 × 3.5–5 (av. ± SD ± 20.6 ± 1.9 × 4.4 ± 0.3), L/W ratio = 4.7. Appressoria single, medium to dark brown, mostly irregularly shaped, rarely cylindrical, with undulate to lobate margins, 8.5–19 × 4.5–11.5 μm.
Typus: China, Guangdong Province, Guangzhou, Zhaoqing, Seven Star Cave (Qixingyan), on diseased leaf of Monstera deliciosa, 24 Dec. 2012, W.P. Wu (holotype HMAS 350640, ex-type culture CGMCC 3.20519 = LC13871 = NN055214).
Notes: Although represented by a single strain, C. monsterae is phylogenetically distinct from all currently accepted species of Colletotrichum at each locus sequenced in the current study and is basal to other species in the C. orchidearum species complex (Fig. 1). Morphologically it is different from most species in the genus, including the closest related species, C. piperis, and the two other Monstera deliciosa-associated species, C. gloeosporioides (French 1989) and C. orchidearum (Damm et al. 2019), by producing long brown conidiophores reminiscent of setae.
Colletotrichum multiseptatum F. Liu, W.P. Wu & L. Cai, sp. nov. MycoBank MB 841385. Fig. 19.
Fig. 19.
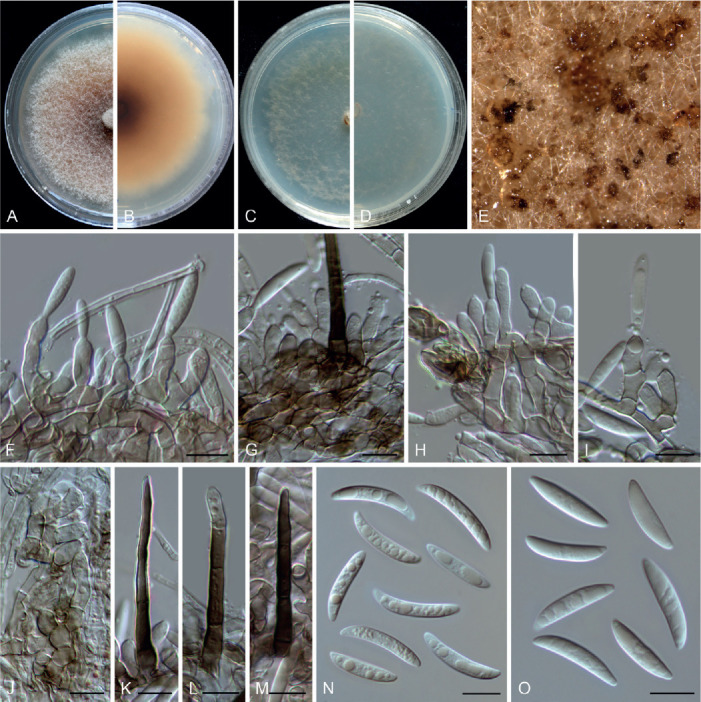
Colletotrichum multiseptatum (ex-type culture NN055357). A, B. Front and reverse colony on PDA (7 d). C, D. Front and reverse colony on SNA (7 d). E. Conidial masses on PDA. F–I. Conidiophores, conidiogenous cells and conidia on PDA. J. Conidiogenous cells on SNA. K–M. Setae on PDA. N. Conidia on SNA. O. Conidia on PDA. Scale bars = 10 μm.
Etymology: Named to reflect the multiple septa of its seta.
Description: Colonies on PDA 49 mm diam in 7 d, flat with entire edge, saffron to peach, aerial mycelium unconsolidated, reverse peach to umber. Vegetative hyphae hyaline to pale brown, smooth-walled, septate, branched. Conidiomata not developed, abundant conidial masses formed on the surface of medium, salmon, brown to black, solitary or gregarious. Setae medium brown to dark brown, smooth-walled, 50–62 μm long, 2–4-septate, base cylindrical, sometimes slightly inflated, 4.5–6 μm diam, tip ± acute or rounded. Conidiophores formed directly from hyphae, hyaline to pale brown, 1–3-septate, usually branched at the bottom and reduced to conidiogenous cells. Conidiogenous cells hyaline or pale brown, smooth-walled, ampulliform, bullet-shaped, or subcylindrical, variable in length, 8.5–21.5 × 3–5.5 μm (av. ± SD = 13.1 ± 3 × 4 ± 0.5 μm), collarette observed. Conidia hyaline, aseptate, smooth-walled, guttulate, curved, or slightly curved, gradually tapering towards the round apex and truncate base, sometimes less curved towards the base, on PDA 16.5–26 × 3–4.5 μm (av. ± SD = 19.8 ± 2.3 × 3.7 ± 0.3 μm), L/W ratio = 5.4, on SNA 20–25.5 × 3.5–4.5 μm (av. ± SD = 21.8 ± 1.5 × 3.9 ± 0.2 μm), L/W ratio = 5.6. Only one appressorium observed, irregular shaped, dark brown, 10 × 5.5 μm.
Typus: China, Guangdong Province, Shaoguan, Danxia Mountain, on dead culm of an unidentified grass, 25 Dec. 2012, W.P. Wu (holotype HMAS 350651, ex-type culture CGMCC 3.20520 = LC13886 = NN055357).
Notes: Colletotrichum multiseptatum, belonging to the C. graminicola species complex, is phylogenetically related to C. echinochloae (Fig. 3), but shares low sequence similarity at sod2 (97 %) and even lower whole-genome sequence similarity (95 %) with that species. Morphologically, C. multiseptatum differs from C. echinochloae in that it produces shorter setae [50–62 μm vs.79.8–145.5(–186.3) μm] and exhibits a higher L/W ratio of the conidia (5.4–5.6 vs.3.5–5) (Moriwaki & Tsukiboshi 2009). Furthermore, the conidia of C. echinochloae are more curved and both ends are more acute than those of C. multiseptatum.
Colletotrichum nageiae F. Liu, W.P. Wu & L. Cai, sp. nov. MycoBank MB 841386. Fig. 20.
Fig. 20.
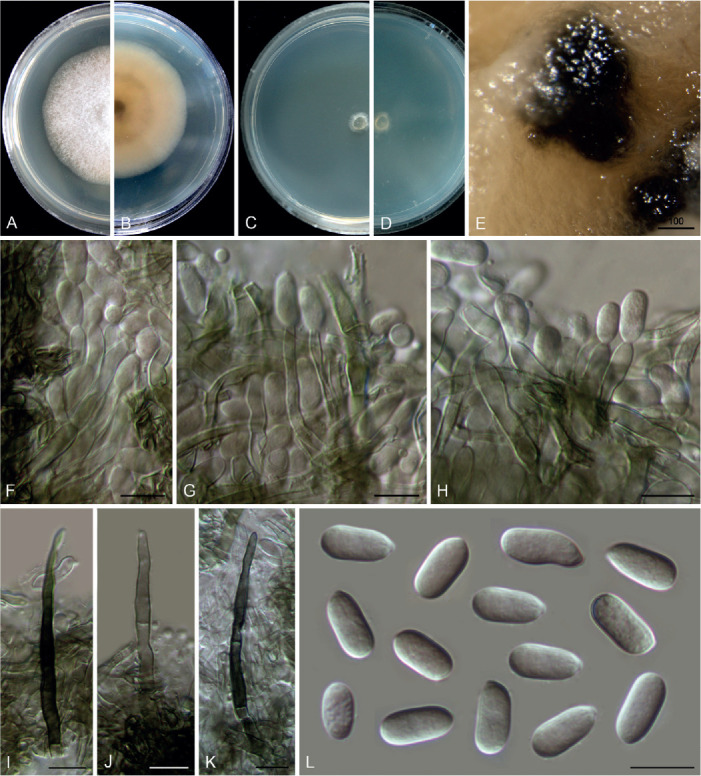
Colletotrichum nageiae (ex-type culture NN004492). A, B. Front and reverse colony on PDA (7 d). C, D. Front and reverse colony on SNA (7 d). E. Conidioma. F–H. Conidiogenous cells and conidia. I–K. Setae. L. Conidia. Scale bars: E = 100 μm; F–L = 10 μm.
Etymology: Named after the host plant genus, Nageia.
Description: Colonies on PDA growing slowly, reaching 32 mm diam after 7 d, flat with entire edge, white, aerial mycelium sparse, reverse pale buff. On SNA, vegetative hyphae hyaline to brown, smooth-walled, septate, branched, 1.5–2 μm diam. Conidiomata dark brown to black, beneath the media. Setae pale brown to brown, straight to flexuous, smooth-walled, 2–3-septate, 57–62 μm long, basal cells cylindrical, 3.5–4.5 μm diam, the tip rounded. Conidiophores septate, branched, conidiogenous cells hyaline to pale brown, cylindrical, smooth-walled, straight, 10–20 × 2.5–4.5 μm. Conidia hyaline, aseptate, smooth-walled, cylindrical, straight, 9–13.5 × 4.5–6 μm (av. ± SD = 11.5 ± 0.9 × 5.3 ± 0.4 μm), L/W ratio = 2.2, the apex obtuse, the bottom gradually narrowed with a prominent truncate scar. Appressoria not observed.
Typus: China, Yunnan Province, Kunming, Kunming Botanical Garden, on healthy leaves of Nageia nagi, 20 Dec. 1993, W.P. Wu (holotype HMAS 350638, ex-type culture CGMCC 3.20521 = LC13866 = NN004492).
Notes: Although the conidial morphology of C. nageiae resembles that of species in the C. boninense species complex, it is a singleton species that clusters basal to C. gloeosporioides species complex and is phylogenetically distinct from all currently accepted Colletotrichum species (Fig. 1). Furthermore, BLASTn search revealed very low sequence similarity with the sequences of other species deposited in the NCBI GenBank, based on the comparisons of act (up to 86 % identity shared with species in the C. gloeosporioides and C. gigasporum species complexes), cal (up to 80 % identity shared with species in the C. gloeosporioides and C. gigasporum species complexes), chs-1 (up to 93 % identity shared with species in the C. boninense species complex), gapdh (up to 87 % identity shared with species in the C. boninense species complex), gs (up to 78 % identity shared with species in the C. boninense species complex), his3 (up to 91 % identity shared with species in the C. gloeosporioides and C. gigasporum species complexes), and tub2 (up to 83 % identity shared with species in the C. boninense species complex).
Colletotrichum obovoides F. Liu & L. Cai, sp. nov. MycoBank MB 841387. Fig. 21.
Fig. 21.
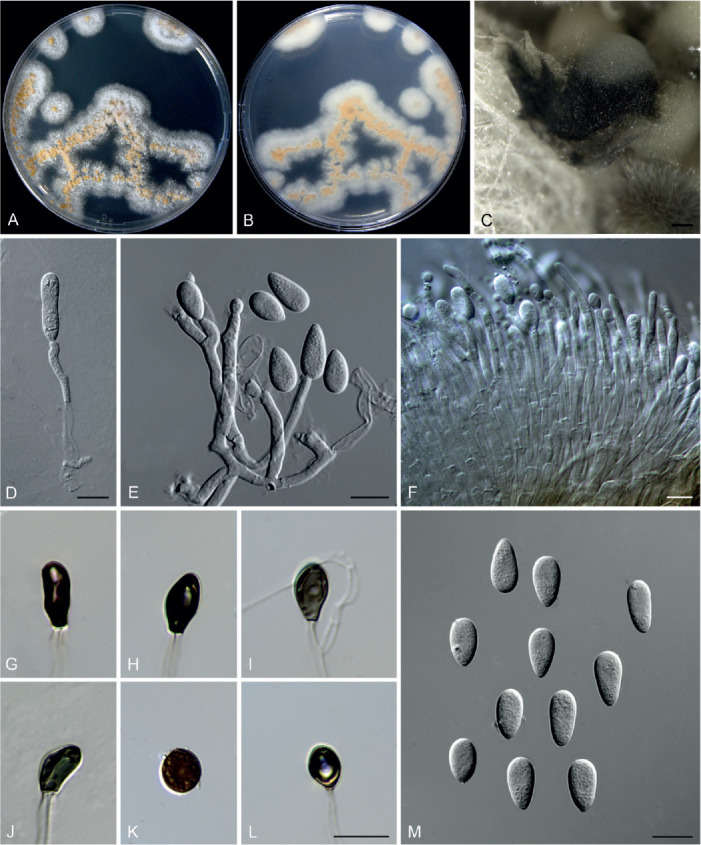
Colletotrichum obovoides (ex-type culture LC6085). A, B. Front and reverse colony on PDA (6 d). C. Conidioma. D–F. Conidiophores, conidiogenous cells and conidia. G–L. Appressoria. M. Conidia. Scale bars: C = 100 μm; D, E, L, M = 10 μm; F = 20 μm. Scale bar of L applies to G–L.
Etymology: Named to reflect the obovoid shape of conidia.
Description: Colonies on PDA 28–33 mm diam in 7 d, flat with fimbriate edge, white, abundant pale luteous and confluent conidial masses formed on the surface of medium, reverse white, or buff because of the production of conidial masses. Vegetative hyphae hyaline, smooth-walled, septate, branched. On SNA, conidiomata acervular, superficial or semi-immersed, black, protruding with white or buff conidial masses. Setae not observed. Conidiophores hyaline, septate, branched, formed directly from hyphae or on a cushion of pale brown cells, up to 70 μm. Conidiogenous cells hyaline, smooth-walled, cylindrical, rarely ampulliform, 5–24 × 2.5–4 μm. Conidia hyaline, aseptate, smooth-walled, obovoid, sometimes clavate, 10.5–15.5 × 5.5–7.5 μm (av. ± SD ± 12.7 ± 1.1 × 6.5 ± 0.4), L/W ratio = 1.9. Appressoria solitary, brown to dark brown, aseptate, smooth-walled, subglobose, bullet-shaped, clavate, or navicular, 7.5–13 × 4.5–9.5 μm (av. ± SD = 10.2 ± 1.7 × 6.2 ± 1.2 μm).
Typus: China, Tibet, Bomi county, Suotong village, on leaf of an unidentified plant, 13 Jun. 2015, Q. Chen, ST10 (holotype HMAS 350629, ex-type culture CGMCC 3.20522 = LC6085 = JJR053).
Notes: Colletotrichum obovoides is a singleton species, not belonging to any species complex (Fig. 1), and differs genetically from the most closely related species C. rusci in 24 bp of the act nucleotide sequence, 18 bp of chs-1, 41 bp of his3, 40 bp of ITS, and 53 bp of tub2. Morphologically, C. obovoides distinctly differs from the two phylogenetically related species, C. trichellum (conidia fusoid, straight or slightly curved, 15–28 × 3–5 μm, Duke 1928) and C. rusci (conidia subfusoid or cymbiform, curved, 16–23 × 4–5 μm, Damm et al. 2009), in that it produces obovoid or clavate, and smaller conidia (10.5–15.5 × 5.5–7.5 μm).
Colletotrichum orchidis Jayaward. et al., Mycosphere 11: 595. 2020.
Notes: Phylogenetically, C. orchidis belongs to the C. dematium species complex (Fig. 1). However, BLASTn search of the NCBI GenBank using sequences of different loci of C. orchidis yielded controversial results. The closest matches identified by BLASTn search using gapdh, chs-1, act, and tub2 sequences of the ex-type MFLUCC 17-1302 (Jayawardena et al. 2020) are species that belong to the C. dematium species complex. However, based on the ITS sequence similarity, the closest matches are C. panacicola, C. destructivum, and C. utrechtense, which belong to the C. destructivum species complex, and C. trifolii, which belongs to the C. orbiculare species complex. This inconsistence might explain the unusually long branch of C. orchidis in the six-locus tree (Fig. 1), and requires re-examination of the ex-type.
Colletotrichum parabambusicola F. Liu, W.P. Wu & L. Cai, sp. nov. MycoBank MB 841388. Fig. 22.
Fig. 22.
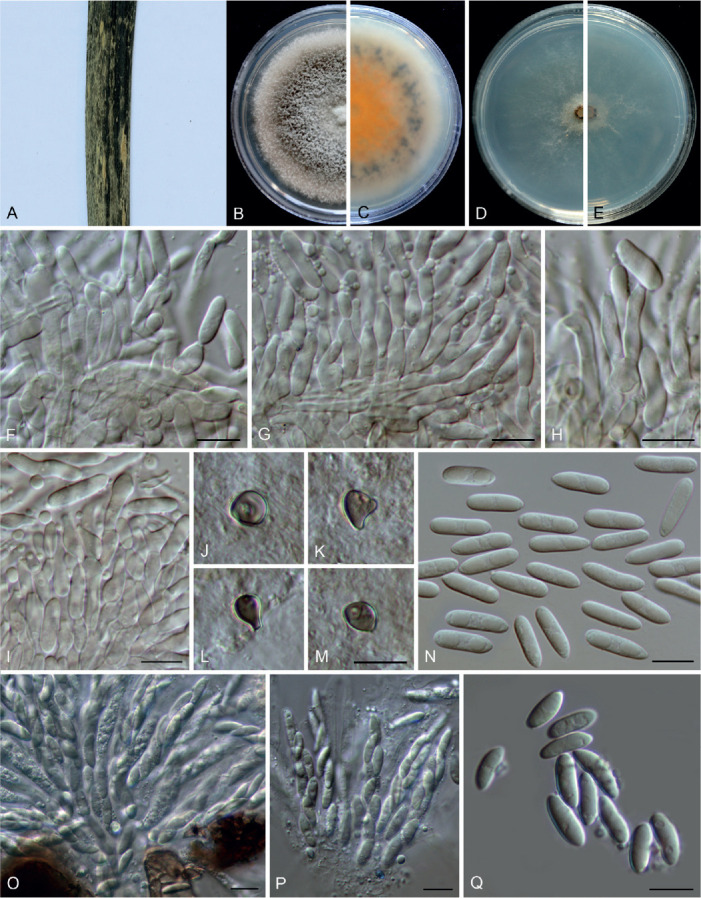
Colletotrichum parabambusicola (ex-type culture NN058956). A. Disease symptom on host plant. B, C. Front and reverse colony on PDA (7 d). D, E. Front and reverse colony on SNA (7 d). F–I. Conidiophores, conidiogenous cells and conidia. J–M. Appressoria. N. Conidia. O, P. Asci. Q. Ascospores. Scale bars: F–I, M–Q = 10 μm. Scale bar of M applies to J–M.
Etymology: Named after its close phylogenetic relationship with C. bambusicola.
Description: Colonies on PDA 48–52 mm diam in 7 d, flat with crenate edge, pale grey to mouse grey, aerial mycelium dense and floccose, reverse saffron. Sexual morph formed on PDA. Ascomata medium to dark brown, outer wall composed of dark brown angular or rarely subglobose cells. Interascal tissue composed of paraphyses, thin-walled, hyaline, septate, 1–3 μm diam. Asci cylindrical, hyaline, 47–55 × 6.5–7.5 μm, 8-spored. Ascospores arranged uniseriately or irregularly, hyaline, smooth-walled, aseptate, allantoid or cylindrical to ellipsoidal, 10–14.5 × 3–5.5 μm, (av. ± SD = 12 ± 1.1 × 4 ± 0.6 μm), L/W ratio=3. Conidiomata subimmersed, globose, black, solitary or gregarious, conidial masses buff. Setae not observed. Conidiophores formed directly on hyphae, hyaline, septate, branched, 21–48 μm long. Conidiogenous cells hyaline, smooth-walled, ampulliform, subcylindrical, 8–21.5 × 3–5 μm (av. ± SD = 12.9 ± 3.5 × 3.8 ± 0.5 μm). Conidia hyaline, aseptate, smooth-walled, guttulate, variable in shape, ellipsoidal, cylindrical, ovoid, ossiform, obclavate, with obtuse ends, 9.5–20.5 × 4–8 (av. ± SD ± 13.9 ± 2.7 × 5.3 ± 1), L/W ratio = 2.6. Appressoria single, brown, subcircular, globose to subglobose, clavate, pyriform, 6.5–15 × 4.5–6.5 μm.
Typus: China, Shanghai Botanical Garden, on dead culm of bamboo, 22 May 2015, W.P. Wu (holotype HMAS 350649, ex-type culture CGMCC 3.20523 = NN058956 = LC13884).
Additional material examined: China, Shanghai Botanical Garden, on dead culm of bamboo, 22 May 2015, W.P. Wu, living culture LC13883 (= NN058925).
Notes: Colletotrichum parabambusicola shares low sequence similarity with the phylogenetically related species C. bambusicola at act (97.7 %), gapdh (92.1 %), his3 (94.2 %), ITS (99.8 %), and tub2 (97.3 %). Morphologically, it differs from C. bambusicola in producing smaller asci (47–55 × 6.5–7.5 μm vs.58–72 × 7–10 μm) and ascospores (10–14.5 × 3–5.5 μm vs.14–19 × 4–6 μm) and exhibiting a lower conidium L/W ratio (2.6 vs.3.2).
Colletotrichum paraendophytum F. Liu, W.P. Wu & L. Cai, sp. nov. MycoBank MB 841389. Fig. 23.
Fig. 23.
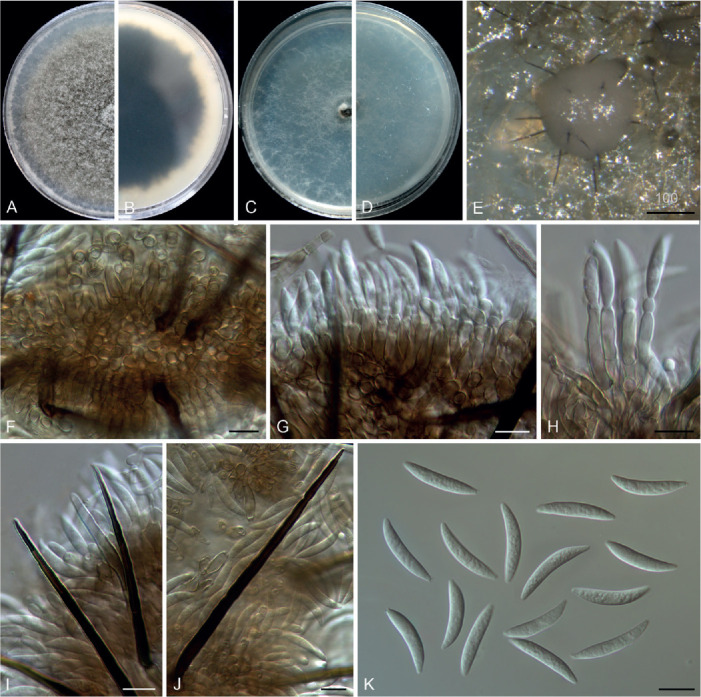
Colletotrichum paraendophytum (ex-type culture NN054963). A, B. Front and reverse colony on PDA (7 d). C, D. Front and reverse colony on SNA (7 d). E. Conidial mass with setae. F–H. Conidiophores, conidiogenous cells and conidia. I, J. Setae. K. Conidia. Scale bars: F–K = 10 μm.
Etymology: Named to reflect its close phylogenetic relationship with C. endophytum.
Description: Colonies on PDA 52 mm diam in 7 d, flat with entire edge, mouse grey, aerial mycelium fluffy, reverse fuscous black with a white margin. Vegetative hyphae hyaline to pale brown, smooth-walled, septate, branched. On SNA, conidiomata scattered, semi-immersed. Setae dark brown, smooth-walled, 1–2-septate, 38–160 μm long, basal cell cylindrical, sometimes paler than the other cells, 3–6 μm diam, tip acute or round. Conidiophores either septate, branched, formed from a cushion of roundish brown cells, or reduced to conidiogenous cells. Conidiogenous cells hyaline to pale brown, smooth-walled, cylindrical to ampulliform, straight, 9.5–15 × 3–5 μm, periclinal thickening distinct, sometimes extending to form a new conidiogenous locus. Conidia hyaline, aseptate, guttulate, smooth-walled, slightly curved, gradually tapering towards a rounded apex and a usually truncate base, with a similar radian, 18.5–23 × 3–4.5 (av. ± SD = 21 ± 0.9 × 3.8 ± 0.3 μm), L/W ratio = 5.5. Appressoria not observed.
Typus: China, Beijing, Huairou, Beigoucun, on an unidentified grass, 10 Sep. 2012, W.P. Wu (holotype HMAS 350653, ex-type culture CGMCC 3.20524 = LC13888 = NN054963).
Notes: Colletotrichum paraendophytum is phylogenetically closely related to C. endophytum in the C. graminicola species complex (Figs 1, 3), but differs in that it produces differently shaped conidia (slightly curved vs.pronouncedly curved) and with a different conidium L/W ratio (5.5 vs.4.8). In addition, C. paraendophytum produces semi-immersed brown conidiomata, which are absent in C. endophytum (Tao et al. 2013). Furthermore, the two species share low sequence similarity at act (95.2 %), chs-1 (98.2 %), ITS (97.9 %), sod2 (92.8 %), and tub2 (92.1 %).
Colletotrichum plurivorum Damm et al., Stud. Mycol. 92: 31. 2018. Fig. 24.
Fig. 24.

Colletotrichum plurivorum (D–E, H–J. LC8337; F–G, K–S. LC8240). A, B. Disease symptoms on Paederia foetida. C. Disease symptom on Vigna unguiculata. D–G. Front and reverse colonies on PDA (6 d). H, I. Ascomata. J. Conidiomata and conidial mass. K, L. Setae. M, N. Asci. O. Ascospores. P–R. Conidiophores, conidiogenous cells and conidia. S. Conidia. Scale bar = 10 μm.
Description: Colonies on PDA 35–51 mm diam in 7 d, flat with undulate edge. Vegetative hyphae hyaline to medium brown, smooth-walled, septate, branched. On PDA, ascomata globose, subglobose or with an irregular shape, solitary or gregarious, black, 130–300 μm diam, formed on the surface of the medium and usually covered by aerial mycelium, ostiolate, outer wall composed of greenish grey angular cells, 2–6 μm diam. Interascal tissue composed of thin-walled, hyaline, septate paraphyses. Asci cylindrical, obclavate to clavate, 33–54.5 × 7.5–13 μm, 8-spored. Ascospores hyaline, smooth-walled, aseptate, fusoid, clavate, with rounded ends, straight or slightly curved, 15–22.5(–25.5) × 4.5–6 μm, (av. ± SD = 18.5 ± 2.7 × 5.3 ± 0.5 μm), L/W ratio = 3.5. Conidiomata acervular, solitary or confluent, superficial or semi-immersed, protruding pale luteous conidial masses. Setae pale brown to dark brown, usually smooth-walled at the base and verruculose at the top, 1–3-septate, 54–82 μm long, basal cells cylindrical, 3.5–6 μm diam, the tip ± acute. Conidiophores solitary or branched, septate, usually reduced to conidiogenous cells. Conidiogenous cells hyaline, cylindrical, ovoid to obclavate, smooth-walled, 11–31 × 2.5–5 μm. Conidia hyaline, aseptate, smooth-walled, guttulate, cylindrical with obtuse ends, straight, 9–13.5 × 4.5–5.5 μm (av. ± SD = 11.3 ± 1.1 × 5 ± 0.3 μm), L/W ratio = 2.3. Appressoria not observed.
Typus: Vietnam, Da Lat-Lam Dong, from anthracnose on leaf of Coffea sp., collection date unknown, P. Nguyen & E. Liljeroth (holotype CBS H-21496, ex-type culture CBS 125474).
Additional materials examined: China, Fujian, Fuzhou, Wuyi Mountain, on Paederia foetida, Aug. 2016, Z.Y. Ma & L. Cai, WYS8, living cultures LC8240 = (M51), LC8244 (= M55), LC8322 (= M136); on Vigna unguiculata, Aug. 2016, Z.Y. Ma & L. Cai, FJWYS01, living culture LC8337 (= M151).
Notes: Colletotrichum plurivorum exhibits a large host range and high degree of genetic variation (Damm et al. 2019). This is the first report of C. plurivorum on Paederia foetida and Vigna unguiculata.
Colletotrichum quinquefoliae Jayaward. et al., Fungal Diversity 78: 83. 2016.
Notes: Colletotrichum quinquefoliae was described as a distinct species in the C. dematium species complex (Li et al. 2016) and forms an unusually long branch in the multi-locus phylogenetic tree (Fig. 1). Based on the BLASTn searches, ITS, gapdh, and tub2 sequences of its type MFLU 14-0626 (Li et al. 2016) share high similarity with those of species in the C. dematium species complex. However, the closest matches using the act sequence (KU236389.1) is C. siamense (> 99 % identity), belonging to the C. gloeosporioides species complex, which very likely reflects a confusion with another specimen and may explain the strangely long branch of C. quinquefoliae. The type of C. quinquefoliae and its derived sequences are thus in need of reexamination.
Colletotrichum reniforme F. Liu, Z.Y. Ma & L. Cai, sp. nov. MycoBank MB 841390. Fig. 25.
Fig. 25.

Colletotrichum reniforme (ex-type culture LC8230). A. Disease symptom on Smilax cocculoides. B. Front and reverse colony on PDA (6 d). C, D. Conidiomata and conidial masses on pine needle and SNA, respectively. E–H. Appressoria. I. Conidiophores. J. Conidia. K–O. Setae. P, Q. Ascomata on SNA and pine needle, respectively. R, S. Asci. T. Ascospores. Scale bars: P, Q = 200 μm; R = 20 μm; H–K, M, O, S, T = 10 μm. Scale bar of H applied to E–H.
Etymology: Named to reflect the reniform shape of ascospores.
Description: Colonies on PDA 60–64 mm diam in 7 d, flat with entire edge, pale grey with white edge, floccose aerial mycelium, reverse olivaceous grey to iron grey with off-white edge. Vegetative hyphae hyaline, smooth-walled, septate, branched. Sexual morph formed on SNA. Ascomata solitary or gregarious, superficial to semi-immersed, globose or irregular, black, outer wall composed of angular cells, medium brown. Interascal tissue composed of paraphyses, thin-walled, hyaline, septate. Asci cymbiform to subcylindrical, hyaline, 33.5–49.5 × 6.5–11.5 μm, 8-spored. Ascospores uniseriately arranged, hyaline, smooth-walled, aseptate, allantoid to reniform with rounded ends, 13.5–16 × 4–5 μm (av. ± SD = 14.8 ± 0.7 × 4.5 ± 0.3 μm), L/W ratio = 3.2. On SNA amended with pine needle, conidiomata acervular, scattered or gregarious, semi-immersed. Setae straight or flexuous, smooth-walled, sometimes verrucous in the upper half, basal cell medium brown, gradually becoming dark brown towards the tip, 1–2-septate, 65–89 μm long, basal cell cylindrical, 3.5–4.5 μm diam, the tip ± acute. Conidiophores hyaline to pale brown, septate, branched, 25–35 μm, usually reduced to conidiogenous cells. Conidiogenous cells hyaline, smooth-walled, cylindrical, subcylindrical to ampulliform, 5.5–11.5(–14) × 2.5–5 μm. Conidia hyaline, aseptate, smooth-walled, guttulate, straight, cylindrical, both ends obtuse, 11.5–16 × 4–5.5 μm (av. ± SD = 13.6 ± 0.9 × 4.7 ± 0.3), L/W ratio = 2.9. Appressoria single, medium to dark brown, various in shape, subcircular, ovoid, navicular, ellipsoidal or irregular in outline, 9–14.5 × 4.5–7.5 μm (av. ± SD = 11.6 ± 1.4 × 5.5 ± 0.7 μm).
Typus: China, Fujian, Fuzhou, Wuyi Mountain, on Smilax cocculoides, Aug. 2016, Z.Y. Ma & L. Cai, WYS3 (holotype HMAS 350631, ex-type culture CGMCC 3.20525 = LC8230 = M41).
Additional material examined: China, Fujian, Fuzhou, Wuyi Mountain, on Paederia foetida, Aug. 2016, Z.Y. Ma & L. Cai, WYS8, living culture LC8248 (= M59).
Notes: Colletotrichum reniforme is closely related to C. cliviicola in the C. orchidearum species complex (Fig. 1), but these two species are easily differentiated on the basis of morphological characteristics. The conidiophores of C. reniforme are inconspicuous on SNA and pine needles, and difficult to distinguish from the pale brown acervulus cells, while those of C. cliviicola are easily recognised and usually elongating up to 70 or 80 μm in 3 wk (Damm et al. 2019). The conidiogenous cells of C. reniforme are shorter and thinner than those of C. cliviicola [5.5–11.5(–14) × 2.5–5 μm vs.7.5–23 × 4.5–7.5 μm]. Furthermore, microcyclic conidiation is observed in C. cliviicola, but not in C. reniforme. According to Damm et al. (2019) and Yang et al. (2009), the appressoria of C. cliviicola usually have undulate to lobate margins, while those of C. reniforme are flat.
Colletotrichum schimae F. Liu, W.P. Wu & L. Cai, sp. nov. MycoBank MB 841391. Fig. 26.
Fig. 26.
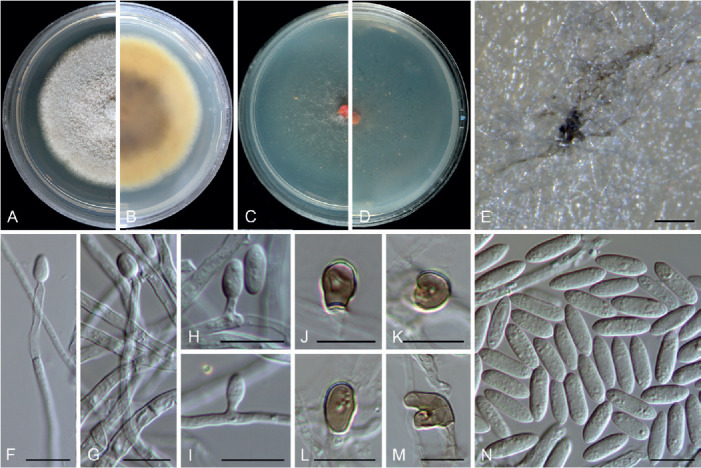
Colletotrichum schimae (ex-type culture NN046984). A, B. Front and reverse colony on PDA (7 d). C, D. Front and reverse colony on SNA (7 d). E. Conidial mass on SNA. F–I. Conidiophores, conidiogenous cells and conidia. J–M. Appressoria. N. Conidia. Scale bars = 10 μm.
Etymology: Named after the host plant genus, Schima.
Description: Colonies on PDA 33–38 mm diam in 7 d, flat with entire or undulate edge, white, aerial mycelium dense, reverse umber in the centre, pale luteous towards the margin. Conidiomata and setae not observed. On SNA, conidiophores formed directly from aerial mycelium, hyaline, aseptate or septate. Conidiogenous cells hyaline, cylindrical, formed terminally or laterally on hyphae, variable in size, up to 45 μm long. Conidia hyaline, smooth-walled, guttulate, cylindrical to fusoid, with ± acute ends, 8.5–14 × 2.5–4 μm (av. ± SD = 11.6 ± 1.1 × 3.5 ± 0.3 μm), L/W ratio = 3.3. Appressoria pale brown, solitary, ellipsoidal, subglobose, bullet or irregular shape, 5–12.5 × 3.5–7 μm.
Typus: China, Hunan Province, Chenzhou, Yizhang County, Mangshan, on healthy leaves of Schima sp., 12 Apr. 2002, W.P. Wu (holotype HMAS 350647, ex-type culture CGMCC 3.20526 = LC13880 = NN046984).
Additional material examined: China, Hunan Province, Chenzhou, Yizhang County, Mangshan, on healthy leaves of Schima sp., 12 Apr. 2002, W.P. Wu, living culture LC13881 (= NN047247).
Notes: Colletotrichum schimae is phylogenetically related to C. kniphofiae in the C. acutatum species complex, and shares low sequence similarity at act (92 %), chs-1 (96.2 %), gapdh (89.8 %), ITS (98.4 %), and tub2 (94.9 %). Moreover, C. schimae is distinct from all other species in this genus at each locus sequenced in the current study. Morphologically, C. schimae differs from C. kniphofiae in that it produces smaller-sized conidia (8.5–14 × 2.5–4 μm vs.17–37 × 5–7 μm) (Crous et al. 2018).
Colletotrichum shivasii F. Liu & L. Cai, sp. nov. MycoBank MB 841392. Fig. 27.
Fig. 27.
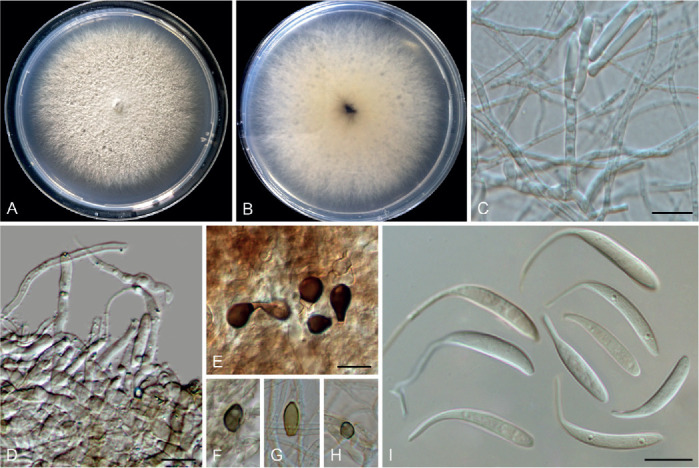
Colletotrichum shivasii (ex-type culture BRIP 15842a). A, B. Front and reverse colony on PDA (7 d). C, D. Conidiophores and conidiogenous cells. E–H. Appressoria. I. Conidia. Scale bars = 10 μm.
Etymology: Named in honour of the mycologist Roger Shivas, who provided the ex-type culture of this species for the study.
Description: Colonies on PDA 75–80 mm diam in 7 d, flat with fimbriate edge, aerial mycelium white, reverse white at first, becoming orange to black at the centre with time. On PDA, conidiomata not observed. Conidiophores formed directly from aerial mycelium, hyaline to very pale grey, septate, up to 37 μm. Setae not observed. Conidiogenous cells cylindrical, smooth-walled, 12–16 μm in length. Conidia hyaline, smooth-walled, apex reduced into a filiform appendage (10–)12.5–23 μm, conidia body slightly curved or straight, 27.5–47.5 μm long (including appendages), 2.5–4 μm wide (av ± SD = 39.4 ± 5.2 × 3.6 ± 0.5 μm), L/W ratio = 10.9. Appressoria pale to medium brown, pyriform to subcircular, 6–11.5 × 4.5–7.5 μm (av. ± SD = 7.8 ± 1.5 × 6 ± 1.1 μm).
Typus: Australia, Queensland, Tablelands, Wongabel Road, Atherton, on leaf of Themeda triandra, Feb. 2004, probably J.L. Alcorn (holotype HMAS 350627, ex-type culture LC1400 = BRIP 15842a).
Notes: Colletotrichum shivasii belongs to the C. caudatum species complex. Species in this complex comprise widespread fungal pathogens of warm-season grasses, and are easily differentiated from other species in the genus by producing caudate conidia (Crouch 2014). Colletotrichum shivasii, associated with the perennial tussock-forming grass Themeda triandra in Australia, is morphologically similar to the closely related species C. caudatum, but harbours nucleotide polymorphisms at ITS (96.9 % similarity), sod2 (92.9 % similarity), and Mat/Apn2 (82.1 % similarity).
Colletotrichum sinuatum F. Liu, W.P. Wu & L. Cai, sp. nov. MycoBank MB 841393. Fig. 28.
Fig. 28.
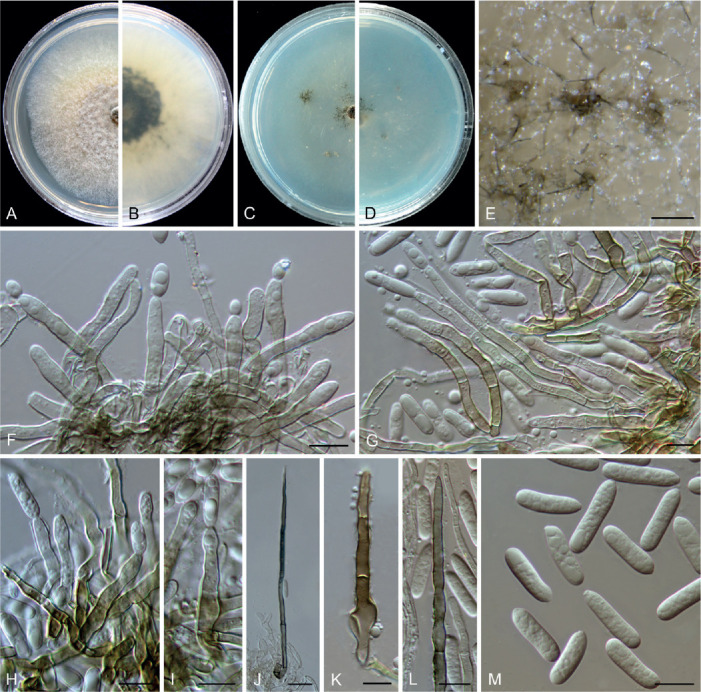
Colletotrichum sinuatum (ex-type culture NN055266). A, B. Front and reverse colony on PDA (7 d). C, D. Front and reverse colony on SNA (7 d). E. Conidial masses and setae on SNA. F–I. Conidiophores, conidiogenous cells and conidia. J–L. Setae. M. Conidia. Scale bars: E = 100 μm; J = 20 μm; F–I, K–M = 10 μm.
Etymology: Named to reflect the sinuate setae and conidiophores.
Description: Colonies on PDA 42 mm diam in 7 d, flat with entire edge, white to pale saffron, aerial mycelium sparse, reverse pale saffron with a black centre. Conidiomata not formed, conidial masses and setae abundantly formed on the surface of medium. Setae medium to dark brown, smooth-walled or verruculose, mostly sinuate, 2–5-septate, 67–175 μm long, basal cells cylindrical, sometimes inflated, 4–9.5 μm diam, the tip acute or rounded. Conidiophores formed directly from hyphae, hyaline to brown, branched, 1–4-septate, 32–122 μm in length. Conidiogenous cells hyaline to pale brown, smooth-walled, cylindrical, rarely subulate, variable in length, on SNA 11.5–34 × 2.5–5 μm (av. ± SD = 21.9 ± 5.4 × 3.8 ± 0.6 μm), on PDA 15–37 × 3–5 μm (av. ± SD = 24.8 ± 6.5 × 4 ± 0.5 μm). Conidia hyaline, aseptate, smooth-walled, cylindrical, apex obtuse, tapering at base to a truncate hilum, mostly straight, rarely slightly curved, on SNA 14.5–19 × 3.5–5 μm (av. ± SD = 16.9 ± 1.2 × 4.4 ± 0.4 μm), L/W ratio = 3.8, on PDA 14.5–21 × 4.5–5.5 μm (av. ± SD = 17.8 ± 1.5 × 4.8 ± 0.3 μm), L/W ratio = 3.7. Appressoria not observed.
Typus: China, Guangdong Province, Guangzhou, Yuexiu Park, on dead leaves of Ophiopogon japonicus, 29 Dec. 2012, W.P. Wu (holotype HMAS 350643, ex-type culture CGMCC 3.20528 = LC13874 = NN055266).
Notes: Colletotrichum sinuatum belongs to the C. dracaenophilum species complex. It shares low sequence similarity with the most closely related species C. yunnanense at act (91.6 %), chs-1 (97.6 %), gapdh (87.4 %), his3 (92.4 %), ITS (96.5 %), and tub2 (96.3 %). Moreover, C. schimae is distinct from all other species in this genus at each locus sequenced in the current study. This species morphologically differs from the phylogenetically related species C. buxi sp. nov., C. dracaenophilum, and C. yunnanense in that it produces distinctly longer conidiophores and conidiogenous cells. Two other O. japonicus-associated species, C. truncatum and C. falcatum (Raabe et al. 1981, Miller 1992), are easily distinguished from C. sinuatum in that they produce curved conidia.
Colletotrichum subacidae F. Liu, Z.Y. Ma & L. Cai, sp. nov. MycoBank MB 841394. Fig. 29.
Fig. 29.

Colletotrichum subacidae (ex-type culture LC13857). A. Disease symptom on host plant. B, C. Front and reverse colony on PDA (6 d). D. Conidioma on PDA. E, F. Setae. G–K. Conidiophores, conidiogenous cells and conidia. L–O. Appressoria. P, Q. Conidia. Scale bars: F, M = 20 μm; G, P = 10 μm. Scale bar of G applies to G–K; M applies to L–O; P applies to P–Q.
Etymology: Named to reflect its close phylogenetic relationship with C. acidae.
Description: Colonies on PDA 43–51 mm diam in 7 d, flat with undulate edge, glaucous grey to smoke grey in the centre, white at the margin, aerial mycelium sparse, reverse greenish grey in the centre, white at the margin. On PDA, conidiomata acervular, gregarious, semi-immersed. Setae dark brown, 1–3-septate, 80–165 μm long, base cylindrical, smooth-walled, 5–9 μm diam, tip acute to obtuse. Conidiophores hyaline to pale brown, smooth-walled, septate, solitary or branched, formed from a cushion of roundish brown cells. Conidiogenous cells hyaline, smooth-walled, cylindrical, 9–20 × 2–4.5 μm. Conidia hyaline, aseptate, smooth-walled, slightly curved, central part of conidium almost straight with parallel walls, apex more or less acute, base usually truncate, 21–30 × 2.5–4 μm (av. ± SD = 26 ± 2.3 × 3 ± 0.3 μm), L/W ratio = 8.5. Appressoria brown, usually in groups, mostly oblong to subcylindrical, rarely irregular in outline, 8–25 × 5–8 μm (av. ± SD = 15.5 ± 4.4 × 6.5 ± 1.0 μm), L/W ratio = 2.3.
Typus: China, Guangxi, Chongzuo, Guangxi Nonggang National Nature Reserve, on Tetrastigma obovatum, Jun. 2017, Z.Y. Ma & L.W. Hou (holotype HMAS 350635, ex-type culture CGMCC 3.20529 = LC13857 = LH01).
Additional materials examined: China, Beijing, Xibeiwang, Beijing Medical Botanical Garden, on a diseased stem of Asparagus officinalis, 9 Sep. 2012, W.P. Wu, living culture NN054605; on a diseased leaf of Hosta sp., 9 Sep. 2012, W.P. Wu, living culture NN054609; Guangxi, Chongzuo, Guangxi Nonggang National Nature Reserve, on Tetrastigma obovatum, Jun. 2017, Z.Y. Ma & L.W. Hou, living cultures LC15821, LC15822, LC15823; Hubei Province, Wuhan Botanical Garden, on a dead leaf petiole of Ailanthus altissima, 2 Aug. 2015, W.P. Wu, living cultures NN071129, NN071131.
Notes: Colletotrichum subacidae is phylogenetically allied with C. acidae (Fig. 1), but these two species share low sequence similarity at gapdh (93.8 %), act (94.3 %), and tub2 (97.6 %). Morphologically, C. subacidae differs from C. acidae with respect to the shape and size of appressoria (oblong to subcylindrical, 8–25 × 5–8 μm vs.round, oval or irregular, 11–23 × 9–18 μm) and the size of conidiogenous cells (9–20 × 2–4.5 μm vs.1–2 × 2–3.5 μm) (Samarakoon et al. 2018). Moreover, the conidia of C. acidae are more strongly curved than those of C. subacidae.
Colletotrichum subsalicis F. Liu & L. Cai, sp. nov. MycoBank MB 841395. Fig. 30.
Fig. 30.
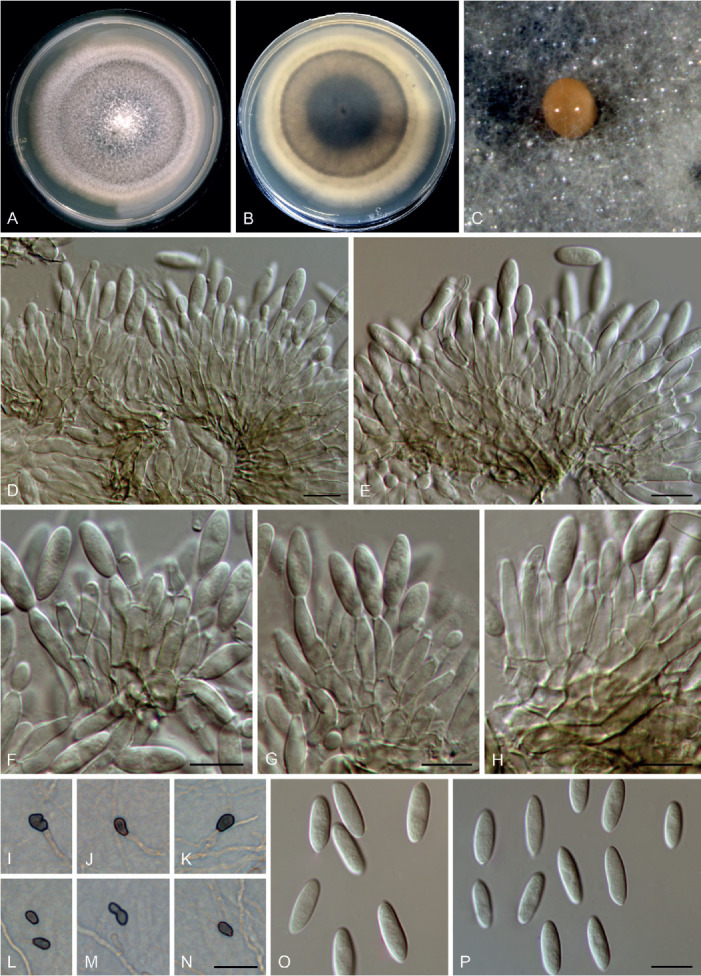
Colletotrichum subsalicis (ex-type culture LC13863). A, B. Front and reverse colony on PDA (13 d). C. Conidioma on PDA. D–H. Conidiophores, conidiogenous cells and conidia. I–N. Appressoria. O, P. Conidia. Scale bars: D–H, P = 10 μm; N = 20 μm. Scale bar of N applies to I–N; P applies to O, P.
Etymology: Named to reflect its close phylogenetic relationship with C. salicis.
Description: Colonies on PDA 39–40 mm diam in 7 d, flat with entire edge, pale mouse grey in the centre, white at the margin, aerial mycelium sparse, reverse brown vinaceous, mouse grey and white towards the margin. On PDA, conidiomata acervular, scattered, semi-immersed, protruding saffron conidial masses. Setae not observed. Conidiophores formed from a cushion of pale brown angular cells, septate, branched, 17–41 μm. Conidiogenous cells hyaline to pale brown, smooth-walled, mostly cylindrical to ampulliform, rarely ovoid, 11–20 × 2–5 μm, periclinal thickening visible. Conidia hyaline, aseptate, smooth-walled, fusoid, 13–16 × 3.5–5 μm (av. ± SD = 14 ± 0.9 × 4.5 ± 0.9 μm), L/W ratio = 3.2. Appressoria single, olivaceous, smooth-walled, mostly clavate with a truncate base, sometimes irregular, 6–13 × 2–6 μm (av. ± SD = 8.5 ± 1.7 × 4 ± 1.7 μm), L/W ration = 2.1.
Typus: China, Beijing, Baihuashan National Nature Reserve Forest Station, Populus alba, 19 Sep. 2018, Q. Chen (holotype HMAS 350637, ex-type culture CGMCC 3.20530 = LC13863 = CQ1168).
Notes: Colletotrichum subsalicis forms a sister clade to C. salicis in the C. acutatum species complex (Fig. 1), but morphologically differs from the latter in conidium shape (fusoid vs.cylindrical to clavate) and appressorium size (6–13 × 2–6 μm, av. ± SD = 8.5 × 4 μm, L/W ratio = 2.1 vs. 6–19.5 × 5–9.5 μm, av. ± SD = 11.5 × 7.6 μm, L/W ratio = 1.5) (Damm et al. 2012a). Furthermore, the sexual morph of C. salicis is more commonly observed in culture than the asexual morph (Damm et al. 2012a), but is absent in C. subsalicis. Colletotrichum subsalicis and C. salicis share 98.8 % genomic similarity.
Colletotrichum subvariabile F. Liu, W.P. Wu & L. Cai, sp. nov. MycoBank MB 841396. Fig. 31.
Fig. 31.
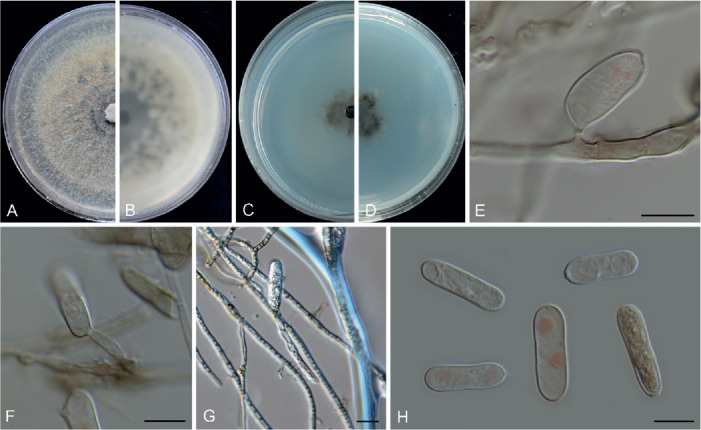
Colletotrichum subvariabile (ex-type culture NN040649). A, B. Front and reverse colony on PDA (7 d). C, D. Front and reverse colony on SNA (7 d). E–G. Conidiophores, conidiogenous cells and conidia. H. Conidia. Scale bars = 10 μm.
Etymology: Named to reflect its close phylogenetic relationship with C. variabile.
Description: Colonies on PDA 48–49 mm diam in 7 d, flat with entire edge, pale salmon, covered by white and pale greenish grey aerial mycelium, reverse pale salmon, variegated with pale greenish grey spots. On PDA, conidiomata subimmersed, globose, black, solitary or gregarious, in which conidiophores hardly observed. Conidiophores, formed directly on hyphae, usually reduced to conidiogenous cells, terminally or laterally. Conidiogenous cells rarely observed, hyaline to pale brown, cylindrical, or ampulliform, 2–12 μm in length. Conidia hyaline with salmon guttules, smooth-walled, cylindrical with obtuse apex and truncate base, 18–27 × 5.5–8 μm, av. ± SD = 22.1 ± 2.5 × 6.7 ± 0.8 μm, L/W ratio = 3.3. Setae and appressoria not observed.
Typus: China, Yunnan Province, Kunming, Kunming Botanical Garden, on healthy leaves of an unknown plant (endophyte), 20 Dec. 1993, W.P. Wu (holotype HMAS 350645, ex-type culture CGMCC 3.20531 = LC13876 = NN040649).
Notes: Colletotrichum subvariabile, belonging to the C. gigasporum species complex (Fig. 1), shares low sequence similarity with the phylogenetically related species C. variabile sp. nov. at act (95.2 %), chs-1 (97.2 %), gapdh (94.2 %), his3 (95 %), tub2 (97.9 %), and ITS (97.8 %). Morphologically, C. subvariabile differs from C. variabile in that it produces shorter conidiogenous cells (2–12 μm vs.9–34 μm) and has a lower conidium L/W ratio (3.3 vs.3.8).
Colletotrichum syngoniicola F. Liu, Z.Y. Ma & L. Cai, sp. nov. MycoBank MB 841397. Fig. 32.
Fig. 32.

Colletotrichum syngoniicola (ex-type culture LC8894). A. Disease symptom on Syngonium sp. B. Front and reverse colony on PDA (6 d). C, D. Conidiomata and conidial masses on pine needle and SNA, respectively. E–I. Conidiophores, conidiogenous cells and conidia. J–M. Appressoria. N. Conidia. Scale bars = 10 μm.
Etymology: Named after the host plant genus, Syngonium.
Description: Colonies on PDA 56–58 mm diam in 7 d, flat with entire edge, smoke grey to greenish grey with white edge, aerial mycelium sparse and short, reverse greenish grey to olivaceous black, off-white at the margin. On SNA, vegetative hyphae hyaline or brown, smooth-walled, septate, branched. Conidiomata not developed. Conidiophores formed directly from hyphae, conidial masses buff to honey. Setae not observed. Conidiophores hyaline to brown, septate, branched, up to 130 μm long, basal cells more or less thick-walled. Conidiogenous cells hyaline, rarely pale brown, smooth-walled, cylindrical or slightly tapering towards the apex, 8–19(–24) × 3.5–5.5 μm. Conidia hyaline, aseptate, smooth-walled, guttulate, cylindrical with obtuse ends, sometimes the base tapering to a truncate hilum, (9–)11–18.5(–23.5) × 3.5–5.5 (av. ± SD = 14.9 ± 1.2 × 4.1 ± 0.4), L/W ratio = 3.6. Appressoria single or gregarious, medium to dark brown, terminally at the tip of the hyphae, mostly irregularly shaped, with undulate to lobate margins, sometimes elliptical with an entire margin, 7.5–18 × 3.5–9 μm (av. ± SD = 10.1 ± 2.7 ×5.8 ± 1.7 μm).
Typus: China, Guangdong, Shenzhen, from a leaf spot of Syngonium sp., Nov. 2016, Y.Z. Diao, SZ36 (holotype HMAS 350632, ex-type culture CGMCC 3.20532 = LC8894 = M0745).
Additional materials examined: China, Guangdong, Shenzhen, from leaf spots of Syngonium sp., Nov. 2016, Y.Z. Diao, SZ36, living cultures LC8895 (= M0746), LC8896 (= M0747), LC8897 (= M0748).
Notes: Colletotrichum syngoniicola belongs to the C. orchidearum species complex (Fig. 1), and shares low sequence similarity with the phylogenetically related species C. piperis at act (95.3 %), chs-1 (98.4 %), gapdh (93.2 %), his3 (96.5 %), tub2 (97.5 %), and ITS (99 %). Morphologically, C. syngoniicola differs from C. piperis in that it produces relatively darker conidiophores (Damm et al. 2019). Moreover, although C. syngoniicola is characterised by the unusual conidiophores that are long-branched and brown, resembling those of C. monsterae sp. nov., the two species share low sequence similarity at act (89.3 %), chs-1 (92.8 %), gapdh (87.7 %), his3 (91.9 %), ITS (97.6 %), and tub2 (93.5 %).
Colletotrichum telosmae F. Liu, W.P. Wu & L. Cai, sp. nov. MycoBank MB 841398. Fig. 33.
Fig. 33.
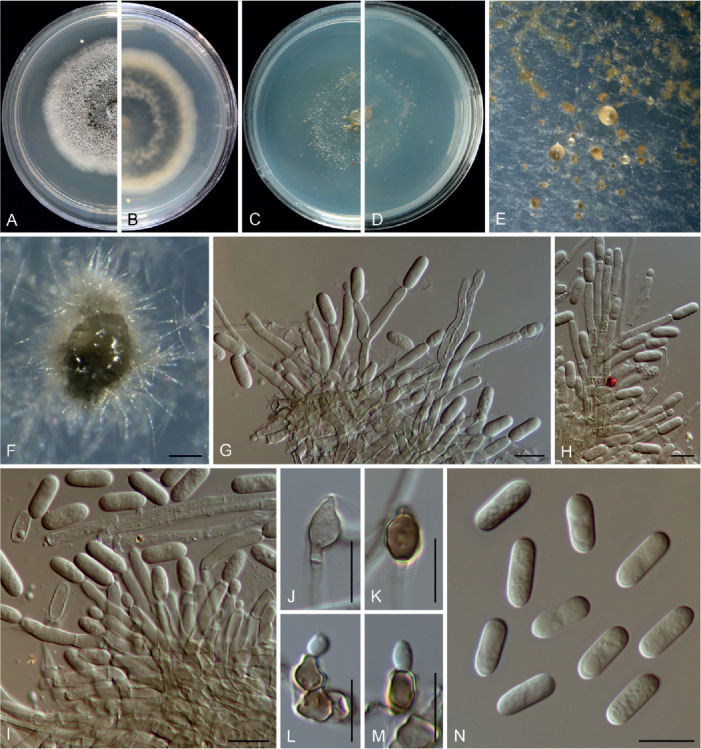
Colletotrichum telosmae (ex-type culture NN052858). A, B. Front and reverse colony on PDA (7 d). C, D. Front and reverse colony on SNA (7 d). E, F. Conidial masses on SNA. G–I. Conidiophores, conidiogenous cells and conidia. J–M. Appressoria. N. Conidia. Scale bars: F = 100 μm; G–N = 10 μm.
Etymology: Named after the host plant genus, Telosma.
Description: Colonies on PDA growing slowly, 28–29 mm diam in 7 d, raised with concave edge, grey, reverse iron grey with pale amber margin, a pale amber ring more towards the centre of the colony corresponds to the production of conidial masses on the surface. Vegetative hyphae hyaline to pale brown, smooth-walled, septate, branched. On SNA, conidiomata not developed, conidiophores formed directly on hyphae, conidial masses buff to brown. Setae not observed. Conidiophores hyaline to pale brown, 1–4-septate, branched, up to 80 μm long. Conidiogenous cells hyaline, smooth-walled, cylindrical, 8–24 × 2–4 μm (av. ± SD ± 18.3 ± 3.5 × 3 ± 0.3 μm), collarette visible. Conidia hyaline, aseptate, smooth-walled, guttulate, cylindrical with obtuse ends, 10.5–11.5 × 3.5–5 μm (av. ± SD ± 11 ± 0.4 × 4 ± 0.2 μm), L/W ratio = 2.8. Appressoria ellipsoidal, subcircular, medium brown to dark brown, 5.5–11.5 × 3.5–7 μm (av. ± SD ± 7.2 ± 1.6 × 5 ± 1 μm), with conidia-like cells formed from the appressoria, 6.5–7.5 × 5–5.5 μm.
Typus: China, on healthy leaves of Telosma cordarum, 24 Mar. 2010, W.P. Wu (holotype HMAS 350641, ex-type culture CGMCC 3.20533 = LC13872 = NN052858).
Notes: The endophytic C. telosmae, phylogenetically related to the C. dracaenophilum species complex (Fig. 1), is a singleton species and can be distinguished from all currently accepted species of Colletotrichum at each locus sequenced in the current study. Its cylindrical conidia resemble species in many complexes, especially the C. gloeosporioides and C. dracaenophilum complexes. However, the conidia-like cells that form on the C. telosmae appressoria (Fig. 33L–M) have not been previously observed in this genus. This is the first report of a Colletotrichum species on Telosma cordarum.
Colletotrichum tibetense F. Liu & L. Cai, sp. nov. MycoBank MB 841399. Fig. 34.
Fig. 34.
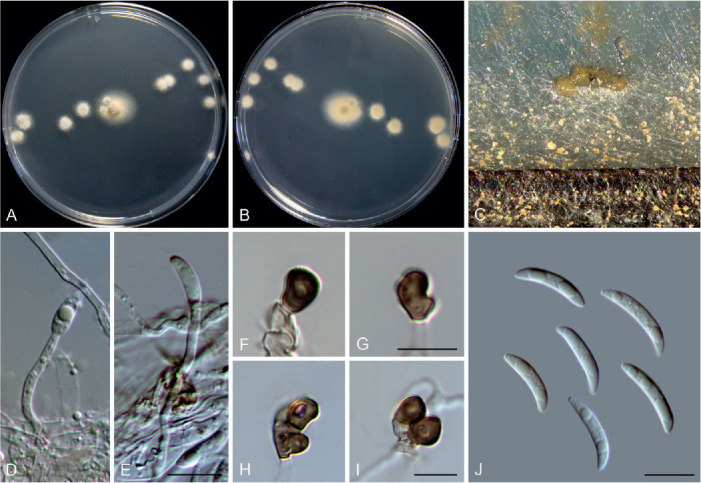
Colletotrichum tibetense (ex-type culture LC7364). A, B. Front and reverse colony on PDA (6 d). C. Conidial masses on pine needle and SNA. D, E. Conidiogenous cells and conidia. F–I. Appressoria. J. Conidia. Scale bars = 10 μm. Scale bar of E applies to D, E; G applies to F, G; I applies to H, I.
Etymology: Named after the location where the fungus was collected, Tibet.
Description: Colonies on PDA growing slowly, 21–24 mm diam in 7 d, flat with fimbriate edge, straw, aerial mycelium sparse, reverse straw. Vegetative hyphae hyaline, smooth-walled, septate, branched. Sporulating on SNA and pine needle, conidiomata not developed, conidiophores formed directly from hyphae and hardly observed, conidial masses abundant, pale luteous to buff, scattered or confluent. Setae not observed. Conidiophores hyaline, aseptate, unbranched, reduced to conidiogenous cells. Conidiogenous cells hyaline, smooth-walled, cylindrical, straight or flexuous, 19.5–32.5 × 2 μm. Conidia hyaline, aseptate, guttulate, smooth-walled, curved, central part of conidium almost straight with parallel walls, gradually tapering towards a ± acute or slightly rounded apex and a usually truncate base, with a similar radian, 11.5–18.5 × 2.5–4 μm (av. ± SD = 16.4 ± 1.7 × 3.1 ± 0.3 μm), L/W ratio = 5.3. Appressoria discrete or gregarious, brown, smooth-walled, subglobose, obovoid, clavate with truncate base, or sometimes irregularly shaped, lobed, 6–9.5 × 3.5–7 μm (av. ± SD = 8.1 ± 0.9 × 5.3 ± 0.8 μm).
Typus: China, Tibet, Bomi county, Suotong village, on an unidentified species of Poaceae, 13 Jun. 2015, F. Liu, BM12 (holotype HMAS 350630, ex-type culture CGMCC 3.20534 = LC7364 = LJM48).
Additional material examined: China, Tibet, Bomi county, Suotong village, on Poaceae, 13 Jun. 2015, F. Liu, BM12, living culture LC7366.
Notes: Colletotrichum tibetense belongs to the C. graminicola species complex (Fig. 1) and is characterised by slow growth rate on PDA (21–24 mm diam in 7 d) and abundant sporulation. It morphologically differs from the closely related species C. dolichoconidiophori (Figs 1, 3) in producing longer conidiogenous cells (19.5–32.5 × 2 μm vs.6–23 × 2–4 μm), shorter conidia (11.5–18.5 × 2.5–4 μm vs.19–27.5 × 2.5–3.5 μm), and with a lower conidium L/W ratio (5.3 vs.8). The closest matches revealed by BLASTn search using the act, chs-1, sod2, tub2, and ITS sequences of the ex-type strain LC7364 were C. cereale (92.7 %), C. hanaui (95 %), C. sublineola (86.3 %), C. navitas (89.3 %), and C. tofieldiae (99.8 %), respectively.
Colletotrichum variabile F. Liu, W.P. Wu & L. Cai, sp. nov. MycoBank MB 841400. Fig. 35.
Fig. 35.
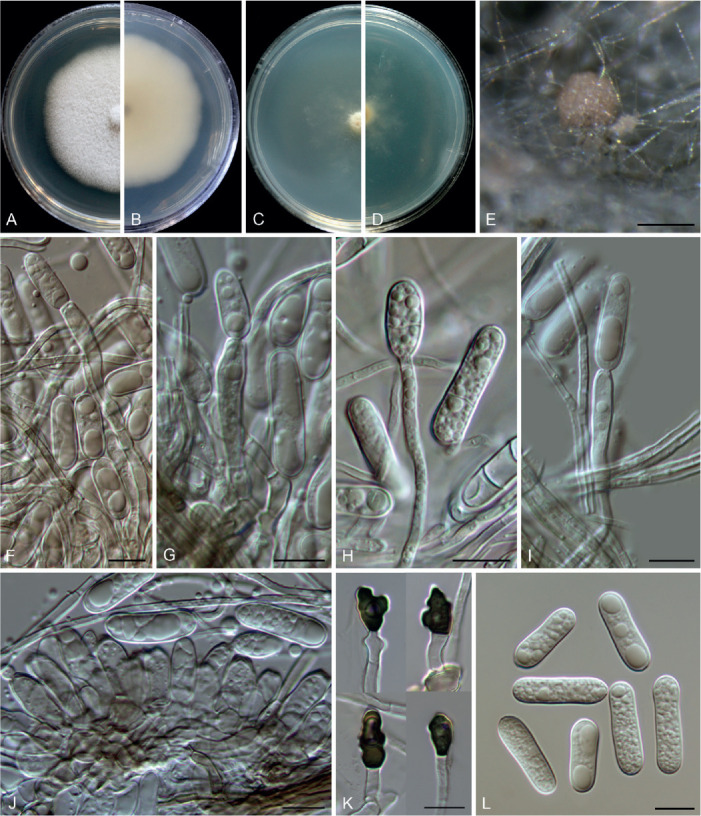
Colletotrichum variabile (ex-type culture NN040656). A, B. Front and reverse colony on PDA (7 d). C, D. Front and reverse colony on SNA (7 d). E. Conidial mass. F–J. Conidiophores, conidiogenous cells and conidia. K. Appressoria. L. Conidia. Scale bars = 10 μm.
Etymology: Named to reflect the variable length of conidiogenous cells.
Description: Colonies on PDA 42 mm diam in 7 d, flat with entire edge, white to pale saffron, aerial mycelium sparse, reverse pale saffron with a black region in the centre. On PDA, conidiomata and setae not observed. Conidial masses salmon, formed among the aerial mycelium. Conidiophores formed directly from the aerial mycelium, hyaline, branched, 1–5-septate. Conidiogenous cells cylindrical to subcylindrical, rarely ovoid, hyaline, 9–34 × 4–5.5 μm. Conidia hyaline, smooth-walled, guttulate, cylindrical with obtuse ends, sometimes slightly narrowed at the centre, becoming 1–3-septate with age, (16–)19–27.5 × 4.5–7 μm, av. ± SD = 22.5 ± 2.2 × 6.0 ± 0.6 μm, L/W ratio = 3.8. Appressoria irregular outline with crenate or lobed margin, clavate, brown, 7.5–9 × 4.5–5.5 μm.
Typus: China, Yunnan Province, Kunming, Kunming Botanical Garden, from healthy leaves of an unknown plant, 20 Dec. 1993, W.P. Wu (holotype HMAS 350644, ex-type culture CGMCC 3.20535 = LC13875 = NN040656).
Notes: Colletotrichum variabile belongs to the C. gigasporum species complex. It shares low sequence similarity with the phylogenetically related species C. subvariabile sp. nov. at act (95.2 %), chs-1 (97.2 %), gapdh (94.2 %), his3 (95 %), tub2 (97.9 %), and ITS (97.8 %), and differs from that species in that it produces longer conidiogenous cells (9–34 μm vs.2–12 μm). In addition, C. variabile differs from other species in the C. gigasporum species complex in the mode of conidiophore and conidium formation (directly on the aerial mycelia vs.on a cushion of cells).
Colletotrichum zhaoqingense F. Liu & L. Cai, sp. nov. MycoBank MB 841401. Fig. 36.
Fig. 36.
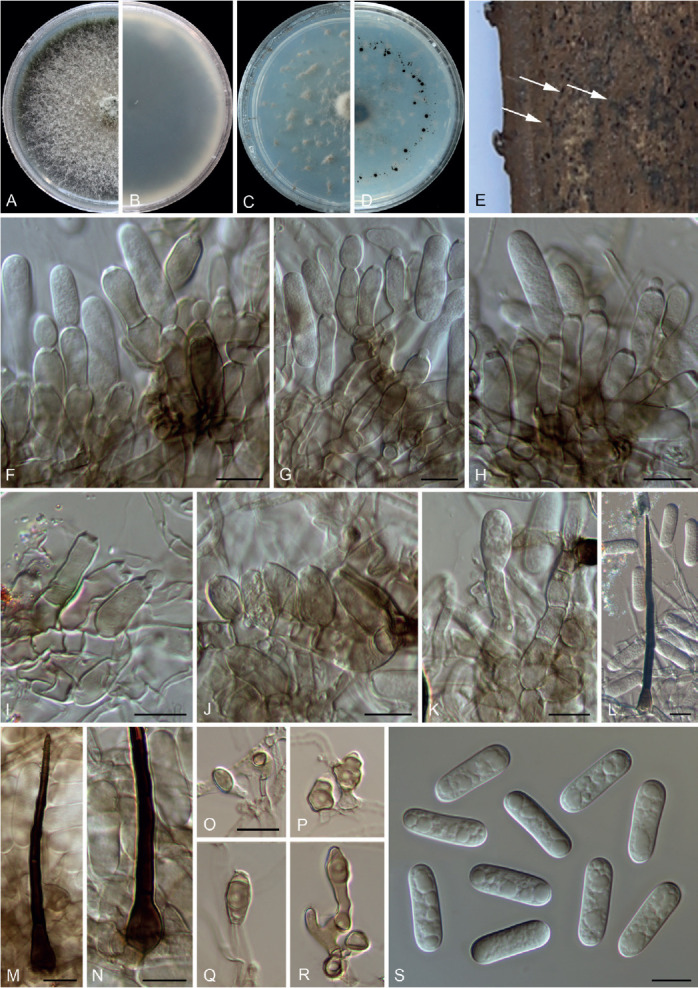
Colletotrichum zhaoqingense (A–D, I–L, N–S: ex-type culture NN058985, E–H, M: NN071035). A, B. Front and reverse colony on PDA (7 d). C, D. Front and reverse colony on SNA (7 d). E. Conidiomata (black) on dead petiole of palm. F–K. Conidiophores, conidiogenous cells and conidia. L–N. Setae. O–R. Appressoria. S. Conidia. Scale bars: F–O, S = 10 μm. Scale bar of O applies to O–R.
Etymology: Named after the location at which the fungus was collected, Zhaoqing.
Description: Colonies on PDA 52 mm diam in 7 d, flat with entire edge, medium to dark mouse grey, aerial mycelium floccose, reverse dark mouse grey. On SNA, conidiomata acervular, scattered, semi-immersed to immersed, protruding hyaline or salmon conidial masses, surrounded by dark brown setae. Setae 1–4-septate, 66–112 μm long, basal cells cylindrical, sometimes conical, smooth-walled, 5–6.5 μm diam, tip acute. Conidiophores formed from a cushion of roundish to angular pale brown cells, or formed directly on aerial mycelium, solitary or branched, septate, hyaline to pale brown, 0–4-septate. Conidiogenous cells cylindrical, rarely ovoid, hyaline to pale brown, 10–22 × 4.5–8 μm. Conidia hyaline, smooth-walled, guttulate, cylindrical with obtuse ends, 20–24 × 5.5–7 μm, av. ± SD = 21.3 ± 0.9 × 6.3 ± 0.6 μm, L/W ratio = 3.4. Appressoria variable in shape, globose, subglobose, ovoid, or irregular outline with a crenate or lobed margin, pale brown, 5.5–18 × 3.5–6.5 μm.
Typus: China, Zhejiang Province, Hangzhou Botanical Garden, on dead petiole of an unidentified palm (Arecaceae), 12 Jun. 2015, W.P. Wu (holotype HMAS 350646, ex-type culture CGMCC 3.20536 = LC13878 = NN071035).
Additional materials examined: China, Guangdong Province, Zhaoqing, Seven Star Cave (Qixingyan), on Musa sp., 24 Dec. 2012, W.P. Wu, living culture NN055284; Guangzhou, on Carica papaya, 10 Dec. 2013, W.P. Wu, living culture NN057644; Zhejiang Province, Hangzhou Botanical Garden, on dead petiole of palm (Arecaceae), 12 Jun. 2015, W.P. Wu, living cultures LC13877 (= NN058985), LC13879 (= NN071036).
Notes: Colletotrichum zhaoqingense forms a sister clade to C. gigasporum (Fig. 1), but differs morphologically from the latter in producing smaller conidia (20–24 × 5.5–7 μm vs.22–32 × 6–9 μm) (Liu et al. 2014). Furthermore, on the molecular level, these two species share low sequence similarity at chs-1 (98 %), gapdh (97.3 %), and ITS (93.2 %).
Colletotrichum zhejiangense F. Liu, W.P. Wu & L. Cai, sp. nov. MycoBank MB 841402. Fig. 37.
Fig. 37.
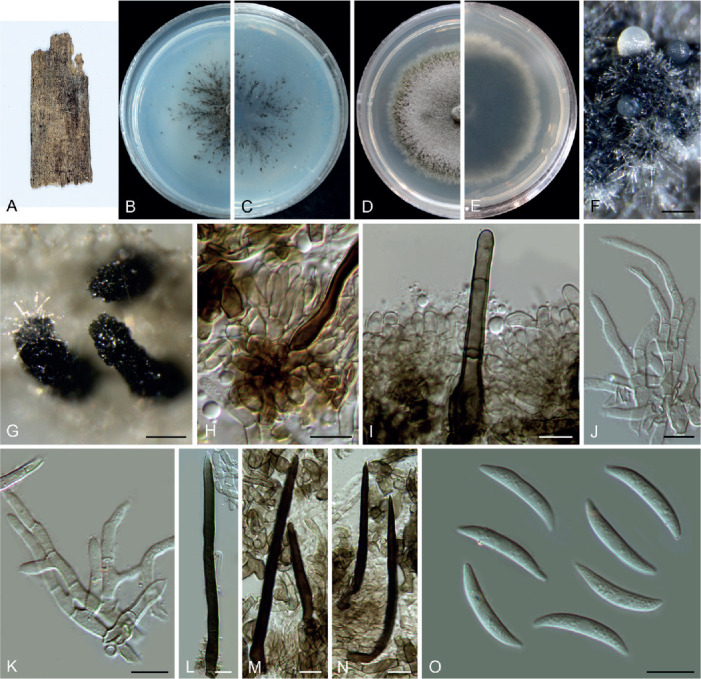
Colletotrichum zhejiangense (ex-type culture NN076215). A. Symptom on the dead leaf of an unidentified tree. B, C. Front and reverse colony on SNA (7 d). D, E. Front and reverse colony on PDA (7 d). F, G. Conidiomata. H, I. Conidiophores, conidiogenous cells and setae. J, K. Conidiophore-like hyphae. L–N. Setae. O. Conidia. Scale bars: F, G = 100 μm; H–O = 10 μm.
Etymology: Named after the location at which the fungus was collected, Zhejiang Province.
Description: Colonies on PDA 34–35 mm diam in 7 d, flat with crenate edge, grey to purple slate, aerial mycelium dense, reverse violet slate with grey margin. Conidiomata black, columnar, straight, conidial masses hyaline. Conidiophores formed from a cushion of elliptical or angular and medium brown cells, branched, septate, hyaline to pale brown. Setae brown to olivaceous black, smooth-walled, 1–3-septate, 45–139 μm long, basal cells cylindrical to slightly conical, 4–8 μm diam, tip acute or round. Conidiogenous cells smooth-walled, cylindrical, 5.5–11.5 × 2.5–4.5 μm (av. ± SD = 8.8 ± 1.9 × 3.5 ± 0.5 μm). Conidia hyaline, aseptate, smooth-walled, curved, central part almost straight with parallel walls, gradually tapering towards the ends with a similar radian, 20.5–24.5 × 3–4 μm (av. ± SD = 22 ± 0.9 × 3.3 ± 0.2 μm), L/W ratio = 6.7. Appressoria not observed.
Typus: China, Zhejiang Province, Chun’an County, Qiandao Lake, on dead leaves of an unidentified tree, 18 Oct. 2018, W.P. Wu (holotype HMAS 350652, ex-type culture CGMCC 3.20537 = LC13887 = NN076215).
Notes: Colletotrichum zhejiangense belongs to the C. dematium species complex (Figs 1, S1), and is characterised by typical curved conidia with parallel walls in the middle, as is also observed for other species in this group. BLASTn search of C. zhejiangense sequences in the NCBI GenBank revealed very low sequence similarity with other species; the closest matches of the act, chs-1, gapdh, ITS and tub2 sequences were C. fructi (89.1 % identity), C. insertae (94.4 %), C. lineola (68.9 %), C. fructi (98.6 %) and C. dematium (89.2 %), respectively.
Species diversity of Colletotrichum in China
In the current study, based on BLASTn searches and phylogenetic analyses of single-, multi-locus and whole-genome sequences, 1 008 strains were assigned to 107 species, belonging to 16 species complexes and 10 singletons (Fig. 38), of which 97 were isolated in China (Tables S5, S6). The majority of analysed strains belong to the C. gloeosporioides species complex (Fig. 38). However, because of the unavailability of ApMat and gs sequences for some taxa in the C. gloeosporioides species complex, species identification in this group was difficult or unfeasible. Hence, only tentative identification was provided for 183 strains in the current study. Among all the identified species, C. siamense was the most common taxon, followed by C. karsti, C. fructicola, C. truncatum, C. fioriniae, and C. gloeosporioides (Fig. 39).
Fig. 38.
Statistics of Colletotrichum strains and species in this study. A. Proportion of all 1 008 strains analysed in this study (left: number of species, right: number of strains). B. Proportion of the 517 strains (right: number of strains) of species in the C. gloeosporioides species complex analysed in this study. C. Proportion and summary of species within each species complex distributed in China based on this and previous studies (right: number of species).
Fig. 39.
Statistics of Colletotrichum species in this study. A. The most common ten species identified in this study. B. The ten species with the widest host range in China based on this study.
Furthermore, we summarised the host-association data for Colletotrichum species from China, that were retrieved from 224 peer-reviewed papers published in 2009 or later (Table S6), and in which the species had been identified employing a modern classification approach. As of 1 Apr. 2021, 139 species belonging to 15 species complexes and 10 singleton species have been reported in China, including the 30 new species and 18 new records reported in the current study (Table S6, Fig. 38). The top six most common species listed in the preceding paragraph are also the species with the widest host range in China (Fig. 39). On the other hand, 76 species have to date been reported from a single plant species or genus (Table S6).
DISCUSSION
In the current study, we generated 67 type-derived sequences for known Colletotrichum species that were omitted or have been erroneously sequenced in various previous publications. This helped to clarify the existing taxonomic confusion (e.g. C. liaoningense and C. ochraceae), and also greatly contributed to the reconstruction of a robust backbone tree for Colletotrichum. Nevertheless, the identity of a few ambiguous species is still pending clarification, as we were unable to obtain their type or type-derived DNA, e.g. in the case of C. chiangraiense, C. jasminigenum, and C. quinquefoliae. Several strains that had been isolated from different bamboo hosts form a distinct clade in the multi-locus phylogeny (Fig. 1), which we refer to as the C. bambusicola species complex. The strains previously identified as C. metake and C. hsienjenchang (Sato et al. 2012) do not include type material, and hence their identity has not yet been clarified. Furthermore, the C. metake strains neither originate from the type location (Italy) nor from the host that C. metake was originally described on (Arundinaria japonica, Saccardo 1908), and might even represent more than one species. The two species will be examined in detail in a subsequent study (U. Damm, unpub. data). Overall, together with the 30 species newly described in the current study, 265 of the 280 Colletotrichum species were herein assigned to 16 species complexes, with the remaining 15 species regarded as singletons (Fig. 1, Tables S1–S3).
Among the 16 species complexes, the C. acutatum, C. boninense, and C. gloeosporioides species complexes contain more species than the other species complexes. Coincidentally, most strains analysed in the current study belong to these three species complexes (Fig. 38), with more than half (518/1 008) belonging to the C. gloeosporioides species complex. Although the combined use of ApMat and gs is very effective in resolving species in the C. gloeosporioides species complex (Liu et al. 2015), we did not employ the two loci in the multi-locus analyses herein, as they had been only rarely sequenced from species in other species complexes. To date, 51 species for which DNA sequence data are available have been accepted in the C. gloeosporioides species complex (Fig. 1); however, the species boundaries are still not well defined for several species. For example, the sequences of the six loci analysed and even whole-genome sequences of C. kahawae s. str. and its closest relatives (C. cigarro, C. fructivorum, C. hedericola, C. helleniense, C. kahawae, C. rhexiae and C. jiangxiense), C. conoides and C. hebeiense, C. wuxiense and C. temperatum, and C. cobbitiiense and C. ti exhibit very few nucleotide differences (Liu et al., unpub. data). Hence, resolution of the taxonomy of the C. gloeosporioides species complex requires a further in-depth study, preferably involving whole-genome data.
Based on the findings of the current study, data retrieved from the USDA fungal database (Farr & Rossman 2021), and data from previous studies (Table S6), we observed that Colletotrichum species vary widely with respect to host specificity and host range, although determination of the host range is somewhat biased as pathogenic fungi have received much more attention to date than endophytes and saprobes. Species with both broad and narrow host range are found in most species complexes (Crouch et al. 2014, Table S6), especially in the C. acutatum, C. boninense and C. gloeosporioides species complexes. In contrast, species in the C. caudatum and C. graminicola species complexes are exclusively associated with monocots, and mostly restricted to single species or members of the Poaceae (Crouch et al. 2009a, 2014). Comparative genomic analyses indicate that host specificity may be related to gene family contractions (Baroncelli et al. 2016), loss of functional genes (Gan et al. 2016, Stajich 2017), and maintenance of a targeted arsenal of virulence factors (O’Connell et al. 2012). In-depth comparative genomic and transcriptomic analyses, as well as verification experiments of functional genes, are required to elucidate the molecular mechanisms of host specialisation and expansion.
Many Colletotrichum species have been isolated from healthy plant tissue and are referred to as endophytes, of which some are also known to be plant pathogenic. However, this does not necessarily imply that all endophytes can switch to a necrotrophic lifestyle (Cannon et al. 2012), and distinguishing between the two life strategies is difficult. According to Bhunjun et al. (2021), 16 out of the 40 endophytic Colletotrichum species can cause disease symptoms in host plants. Our large-scale survey revealed only three additional necrotrophs among the previously known endophytic species (C. caudisporum, C. duyunensis, and C. metake) (Table S7). By contrast, six newly described species (C. buxi, C. chamaedoreae, C. nageiae, C. schimae, C. telosmae, and C. variabile) have been isolated exclusively as endophytes to date. This largely implies that some of the 33 Colletotrichum species that are currently reported as endophytes only (Table S7) might live as beneficial organisms in the host plant and may not cause plant disease.
Although morphological characters are insufficient to distinguish Colletotrichum species, they are considered as important taxonomic characters for the identification of species to species complexes (Cannon et al. 2012). For example, conidia of most species in the C. acutatum species complex have acute ends or at least one acute end (Damm et al. 2012a); conidia of most species in the C. gigasporum species complex are notably larger than those formed by other species complexes (Liu et al. 2014); and typical conidia of C. boninense species complex are cylindrical with a prominent basal scar (Damm et al. 2012b). However, many species in various species complexes (e.g. the C. dracaenophilum, C. magnum, and C. orchidearum species complexes), as well as singleton species, form cylindrical conidia with round ends, which is regarded as a typical feature of the C. gloeosporioides complex (Damm et al. 2019). A schematic overview of the typical conidium and ascospore features of each Colletotrichum species complex is provided in Fig. 40. In general, species of the C. acutatum, C. bambusicola, C. boninense, C. dracaenophilum, C. gigasporum, C. gloeosporioides, C. magnum, C. orbiculare, and C. orchidearum species complexes produce straight conidia, while species in the C. caudatum, C. dematium, C. graminicola, C. spaethianum, and C. truncatum species complexes produce curved conidia (Damm et al. 2012a, b, 2013, 2014, 2019, 2020, Weir et al. 2012, Yang et al. 2012, Crouch 2014, Liu et al. 2014, 2015, Jayawardena et al. 2016, Liu 2016, Fu et al. 2019, Zhang et al. 2020). Of note, species complexes with curved conidia are scattered throughout the phylogenetic tree, which indicates that curved spores may have evolved more than once within the genus.
Fig. 40.

An illustration of the diversity of conidia and ascospores in different species complexes of Colletotrichum. A, B. Acutatum (A. Conidia of C. schimae, NN046984; B. Ascospores of C. salicis, CBS 607.94). C. Agaves (Conidia of C. agaves, CBS 118190). D, E. Bambusicola (D. Conidia of C. bambusicola, LC8468; E. Ascus and ascospores of C. bambusicola, LC8533). F, G. Boninense (F. Conidia of C. chamaedoreae, NN052885; G. Ascus and ascospores of C. chamaedoreae, NN052885). H. Caudatum (Conidia of C. shivasii, BRIP 15842a). I. Dematium (Conidia of C. zhejiangense, NN076215). J. Destructivum (Conidia of Colletotrichum sp., LC8517). K. Dracaenophilum (Conidia of C. buxi, NN047139). L, M. Gigasporum (L. Conidia of C. magnisporum, CBS 398.84; M. Ascospores of C. pseudomajus, CBS 571.88). N, O. Gloeosporioides (N. Conidia of C. gloeosporioides, CGMCC 3.17360; O. Ascospores of C. alienum, CBS 115183). P. Graminicola (Conidia of C. multiseptatum, NN055357). Q. Magnum (Conidia of C. magnum, CGMCC 3.17616). R. Orbiculare (Conidia of C. orbiculare, CBS 570.97). S,T. Orchidearum (S. Conidia of C. orchidearum, CBS 135131; T. Ascospores of C. reniforme, LC8230). U. Spaethianum (Conidia of C. iris, LC3697). V. Truncatum (Conidia of C. subacidae, LC13857). Scale bars = 10 μm.
The ITS and multi-locus trees constructed in the current study are consistent with previous reports that the species complexes in Colletotrichum are monophyletic except for the C. graminicola species complex (Cannon et al. 2012, Marin-Felix et al. 2017, Bhunjun et al. 2021). In the multi-locus phylogenetic tree of the current study (Fig. 1), the C. graminicola species complex is inclusive of the C. caudatum species complex residing at the top of the clade. However, morphologically, the C. caudatum species complex is easily differentiated from the C. graminicola species complex by the formation of a filiform appendage at the apex of the curved conidia (Crouch 2014, Fig. 40). Initially, we suspected that this incongruence might be a consequence of the largely incomplete sequence dataset for this group. Specifically, the his3/gapdh sequences of most species in the C. graminicola species complex, and those of gapdh/chs-1/his3/act/tub2 of most species in the C. caudatum species complex are unavailable, and treated as missing data in the alignments (e.g. Marin-Felix et al. 2017, Bhunjun et al. 2021, and the current study). However, the subsequent species tree based on 1 893 single-copy orthologous genes (Fig. 4) revealed the same topology of this group as that in the multi-locus tree (Fig. 1). It is therefore most likely that species in the C. caudatum species complex are descendants of a common ancestor of the C. graminicola species complex, from which evolved a filiform appendage at the conidial apex. The two species complexes probably should be regarded as one species complex (the C. graminicola-caudatum species complex) as proposed by Bhunjun et al. (2021), or as C. caudatum sub-aggregate in the C. graminicola species complex, as proposed by Crouch (2014).
Although ITS is generally useful for assigning Colletotrichum species to species complexes, the allocation of a few individual species within the C. bambusicola, C. caudatum, C. destructivum, C. graminicola, and C. spaethianum species complexes somewhat contradicts that achieved by using multiple loci. For example, C. riograndense, a member of the C. spaethianum species complex according to the six-locus phylogeny (Fig. 1), is basal to the C. bambusicola and C. spaethianum species complexes in the ITS tree (Fig. S1). By contrast, the affiliation of species to species complexes is much more congruent between the six-locus tree and the whole-genome tree (Fig. 4), except for the C. spaethianum species complex, which is divided into two subclades in the genome tree. These contradictions among the single- and six-locus gene trees, and the genome tree might result from incomplete lineage sorting, horizontal gene transfer, and hybridisation or recombination through speciation events (Degnan & Rosenberg 2009).
By 3 June 2021, 207 whole genomes of 69 Colletotrichum species (Table S4) have been deposited in the NCBI and JGI databases, representing 24.6 % of the currently accepted Colletotrichum species. Whole-genome sequences of additional 48 species, including 30 new and 18 known species, were generated and assembled in the current study, increasing the number of genome-sequenced species to 116 (41.4 % of the species, Table S4). To better define the species complex boundaries and reveal the evolutionary relationship of Colletotrichum, we generated a whole-genome-based phylogenetic tree in this study. In accordance with the six-locus tree (Fig. 1), most species complexes formed well-supported clades in the species tree, except for the C. spaethianum species complex, which did not form a monophyletic clade. As expected, the C. boninense and C. gloeosporioides species complexes with a large number of species and a wide range of hosts had generally larger number of genes and CAZymes (Fig. 4). This is consistent with the conclusion of Baroncelli et al. (2016) that host range is associated with gene family expansion and contraction in Colletotrichum. However, the numbers of genes and CAZymes greatly varied among species in the C. acutatum species complex, another species rich group with broad host range. For example, C. godetiae, associated with at least 18 genera of host plants (Farr & Rossman 2021), possesses the smallest genome size and number of genes, CAZymes and transporters of this genus (Fig. 4). Considering the importance of the genus Colletotrichum, we recommend genome sequencing of all species, especially those plant and human pathogens. This effort will not only pave the way toward a fully resolved Colletotrichum tree of life, but also provide essential data revealing their evolution and adaptation mechanisms, and improve the understanding of the genetic basis of various biological features and metabolic potential of these fungi.
Acknowledgments
We thank Yuanying Su, Wei Sun, Qian Chen, Zhifeng Zhang, Dianming Hu, Parinn Noireung, Dimuthu S. Manamgoda, Dhanushka Udayanga, Junmin Liang and Roger Shivas for providing assistance in sample sharing and culture collection. We also acknowledge Pedro W. Crous for his support on the taxonomy of phytopathogenic fungi and Clement K.M. Tsui for his comments and suggestions on this manuscript. Hui Li, Haoning Qiu and Wanying Kang are thanked for their help on generating partial sequences. This study was financially supported by the National Natural Science Foundation of China (31725001 & 31770009), the Youth Innovation Promotion Association of Chinese Academy of Sciences (2021085), and the National Science and Technology Fundamental Resources Investigation Program of China (2021FY100900). Yongzhao Diao thanks the National Natural Science Foundation of China (31700436) for financial support.
DECLARATION ON CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Supplementary Material: https://studiesinmycology.org/
Phylogenetic tree of Colletotrichum resulting from the RAxML analysis of the ITS sequence alignment. Bootstrap support values (1 000 replicates, GTR-GAMMA model) > 50 % are shown at the nodes. The scale bar represents the expected number of changes per site. Species complexes are indicated with coloured boxes, their names are listed at the left. Ex-type strains are indicated with “T” in the end of the taxa labels.
DNA barcodes of all accepted Colletotrichum spp. except for the ones in the C. graminicola and C. caudatum species complexes.
DNA barcodes of the accepted Colletotrichum spp. in the C. caudatum species complex.
DNA barcodes of the accepted Colletotrichum spp. in the C. graminicola species complex.
Colletotrichum species for which whole-genome sequences are available, retrieved from NCBI and JGI, or generated in the current study.
All strains identified in the current study.
The substrate/host information for Colletotrichum species analysed in the current study, and host information of species reported in China retrieved from literature.
Colletotrichum species reported as endophytes in previous publications and in the current study.
REFERENCES
- Alexey G, Vladislav S, Nikolay V, et al. (2013). QUAST: quality assessment tool for genome assemblies. Bioinformatics 29: 1072–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri SA, Jr, Langdon KR, Wehlburg C, et al. (1984). Index of Plant Diseases in Florida (revised). Florida Department of Agriculture & Consumer Services, Division of Plant Industry, Bulletin 11: 1–389. [Google Scholar]
- Andrews S, Babraham B. (2010). FastQC: a quality control tool for high throughput sequence data. <http://www.bioinformatics.babraham.ac.uk/projects/fastqc>. [Google Scholar]
- Armenteros JJA., Tsirigos KD, Sonderby CK, et al. (2019). SignalP 5.0 improves signal peptide predictions using deep neural networks. Nature Biotechnology 37: 420–423. [DOI] [PubMed] [Google Scholar]
- Bankevich A, Nurk S, Antipov D, et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. Journal of Computational Biology 19: 455–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroncelli R, Amby DB, Zapparata A, et al. (2016). Gene family expansions and contractions are associated with host range in plant pathogens of the genus Colletotrichum. BMC Genomics 17: 555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhunjun CS, Phukhamsakda C, Jayawardena R, et al. (2021). Investigating species boundaries in Colletotrichum. Fungal Diversity 107: 107–127. [Google Scholar]
- Buchta V, Nekolová J, Jirásková N, et al. (2019). Fungal keratitis caused by Colletotrichum dematium: case study and review. Mycopathologia 184: 441–453. [DOI] [PubMed] [Google Scholar]
- Cai L, Hyde KD, Taylor PWJ, et al. (2009). A polyphasic approach for studying Colletotrichum. Fungal Diversity 39: 183–204. [Google Scholar]
- Cannon PF, Damm U, Johnston PR, et al. (2012). Colletotrichum current status and future directions. Studies in Mycology 73: 181–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone I, Kohn LM. (1999). A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91: 553–556. [Google Scholar]
- Castresana J. (2000). Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Molecular Biology and Evolution 17: 540–552. [DOI] [PubMed] [Google Scholar]
- Crouch JA, Clarke BB, White JFJ, et al. (2009a). Systematic analysis of the falcate-spored graminicolous Colletotrichum and a description of six new species from warm-season grasses. Mycologia 101: 717–732. [DOI] [PubMed] [Google Scholar]
- Crouch JA, Tredway LP, Clarke BB, et al. (2009b). Phylogenetic and population genetic divergence correspond with habitat for the pathogen Colletotrichum cereale and allied taxa across diverse grass communities. Molecular Ecology 18: 123–135. [DOI] [PubMed] [Google Scholar]
- Crouch JA. (2014). Colletotrichum caudatum s.l. is a species complex. IMA Fungus 5: 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch J, O’Connell R, Gan P, et al. (2014). The genomics of Colletotrichum. In: Genomics of plant-associated fungi: monocot pathogens (Dean RA, Lichens-Park A, Kole C, eds). Springer-Verlag Berlin Heidelberg, Germany: 69–102. [Google Scholar]
- Crous PW, Gams W, Stalpers JA, et al. (2004). MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22. [Google Scholar]
- Crous PW, Schumacher RK, Wingfield MJ, et al. (2018). New and interesting fungi. 1. Fungal Systematics and Evolution 1: 169–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, et al. (eds) (2019). Fungal Biodiversity, 2nd ed. Westerdijk Fungal Biodiversity Institute, Utrecht, Netherlands: [Westerdijk Laboratory Manual Series 1]. [Google Scholar]
- Damm U, Cannon PF, Woudenberg JHC, et al. (2012a). The Colletotrichum acutatum species complex. Studies in Mycology 73: 37–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Cannon PF, Woudenberg JHC, et al. (2012b). The Colletotrichum boninense species complex. Studies in Mycology 73: 1–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Cannon PF, Liu F, et al. (2013). The Colletotrichum orbiculare species complex: Important pathogens of field crops and weeds. Fungal Diversity 61: 29–59. [Google Scholar]
- Damm U, O’Connell RJ, Groenewald JZ, et al. (2014). The Colletotrichum destructivum species complex – hemibiotrophic pathogens of forage and field crops. Studies in Mycology 79: 49–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Sato T, Alizadeh A, et al. (2019). The Colletotrichum dracaenophilum, C. magnum and C. orchidearum species complexes. Studies in Mycology 92: 1–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm U, Sun YC, Huang CJ. (2020). Colletotrichum eriobotryae sp. nov. and C. nymphaeae, the anthracnose pathogens of loquat fruit in central Taiwan, and their sensitivity to azoxystrobin. Mycological Progress 19: 367–380. [Google Scholar]
- Damm U, Woudenberg JHC, Cannon PF, et al. (2009). Colletotrichum species with curved conidia from herbaceous hosts. Fungal Diversity 39: 45–87. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, et al. (2011). ProtTest 3: fast selection of best-fit models of protein evolution. Bioinformatics 27: 1164–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R, van Kan JA, Pretorius ZA, et al. (2012). The Top 10 fungal pathogens in molecular plant pathology. Molecular Plant Pathology 13: 414–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan JH, Rosenberg NA. (2009). Gene tree discordance, phylogenetic inference and the multispecies coalescent. Trends in Ecology & Evolution 24: 332–340. [DOI] [PubMed] [Google Scholar]
- Diao YZ, Zhang C, Liu F, et al. (2017). Colletotrichum species causing anthracnose disease of chili in China. Persoonia 38: 20–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke MM. (1928). The genera Vermicularia Fr. and Colletotrichum Cda. Transactions of the British Mycological Society 13: 156–184. [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, et al. (2000). Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. Journal of Molecular Biology 300: 1005–1016. [DOI] [PubMed] [Google Scholar]
- Emms DM, Kelly S. (2019). OrthoFinder: phylogenetic orthology inference for comparative genomics. Genome Biology 20: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr DF, Rossman AY. (2021). Fungal Databases, U.S. National Fungus Collections, ARS, USDA. <https://nt.ars-grin.gov/fungaldatabases/>. Accessed on 16 March 2021. [Google Scholar]
- Ferraris T. (1902). Materiali per una flora micologica del Piemonte. Miceti raccolti nei dintorni di Crescentino. Seconda contribuzione. Malpighia 16: 1–46. [Google Scholar]
- Fu M, Crous PW, Bai Q, et al. (2019). Colletotrichum species associated with anthracnose of Pyrus spp. in China. Persoonia 42: 1–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan P, Narusaka M, Kumakura N, et al. (2016). Genus-wide comparative genome analyses of Colletotrichum species reveal specific gene family losses and gains during adaptation to specific infection lifestyles. Genome Biology and Evolution 8: 1467–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garud AB. (1968). An anthracnose disease of pineapple in India. Plant Disease Reporter 52: 436–437. [Google Scholar]
- Glass NL, Donaldson GC. (1995). Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology 61: 1323–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarro J, Svidzinski TE, Zaror L, et al. (1998). Subcutaneous hyalohyphomycosis caused by Colletotrichum gloeosporioides. Journal of Clinical Microbiology 36: 3060–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo LD, Hyde KD, Liew ECY. (2000). Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytologist 147: 617–630. [DOI] [PubMed] [Google Scholar]
- Guerber JC, Liu B, Correll JC, et al. (2003). Characterization of diversity in Colletotrichum acutatum sensu lato by sequence analysis of two gene introns, mtDNA and intron RFLPs, and mating compatibility. Mycologia 95: 872–895. [PubMed] [Google Scholar]
- Haridas S, Albert R, Binder M, et al. (2020). 101 Dothideomycetes genomes: A test case for predicting lifestyles and emergence of pathogens. Studies in Mycology 96: 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings P. (1905). Fungi Amazonici IV. a cl. Ernesto Ule collecti (Appendix). Hedwigia 44: 57–71. [Google Scholar]
- Hou LW, Liu F, Duan WJ, et al. (2016). Colletotrichum aracearum and C. camelliae-japonicae, two holomorphic new species from China and Japan. Mycosphere 7: 1111–1123. [Google Scholar]
- Huelsenbeck JP, Ronquist F. (2001). MrBayes: Bayesian inference of phylogeny. Bioinformatics 17: 754–755. [DOI] [PubMed] [Google Scholar]
- Huerta-Cepas J, Forslund K, Coelho LP, et al. (2017). Fast genome-wide functional annotation through orthology assignment by eggNOG-Mapper. Molecular Biology and Evolution 34: 2115–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde KD, Nilsson RH, Alias SA. et al. (2014). One stop shop: backbones trees for important pytopathogenic genera: I. Fungal Diversity 67: 21–125. [Google Scholar]
- Hyde KD, Zhang Y. (2008). Epitypification: should we epitypify? Journal of Zhejiang University Science B 9: 842–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayawardena RS, Hyde KD, Damm U, et al. (2016). Notes on currently accepted species of Colletotrichum. Mycosphere 7: 1192–1260. [Google Scholar]
- Jayawardena RS, Hyde KD, Chen YJ, et al. (2020). One stop shop IV: taxonomic update with molecular phylogeny for important phytopathogenic genera: 76–100. Fungal Diversity 103: 87–218. [Google Scholar]
- Katoh K, Standley DM (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, et al. (2001). Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. Journal of Molecular Biology 305: 567–580. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JH, Kim J. (2013). First report of anthracnose on Rohdea japonica caused by Colletotrichum liriopes in Korea. Plant Disease 97: 559. [DOI] [PubMed] [Google Scholar]
- Lee SY, Jung HY. (2018). Colletotrichum kakivorum sp. nov., a new leaf spot pathogen of persimmon in Korea. Mycological Progress 17: 1113–1121. [Google Scholar]
- Li GJ, Hyde KD, Zhao RL. et al. (2016). Fungal diversity notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 78: 1–237. [Google Scholar]
- Liu F. (2016). Taxonomy and biodiversity of Colletotrichum Ph.D. dissertation. University Utrecht, The Netherlands. [Google Scholar]
- Liu F, Cai L, Crous PW, et al. (2014). The Colletotrichum gigasporum species complex. Persoonia 33: 83–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Wang M, Damm U, et al. (2016). Species boundaries in plant pathogenic fungi: a Colletotrichum case study. BMC Evolutionary Biology 16: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Weir BS, Damm U, et al. (2015). Unravelling Colletotrichum species associated with Camellia: employing ApMat and GS loci to resolve species in the C. gloeosporioides complex. Persoonia 35: 63–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XJ, Xie X, Duan J. (2007). Colletotrichum yunnanense sp. nov., a new endophytic species from Buxus sp. Mycotaxon 100: 137–144 [Google Scholar]
- Ma X, Nontachaiyapoom S, Jayawardena RS (2018). Endophytic Colletotrichum species from Dendrobium spp. in China and Northern Thailand. MycoKeys 43: 23–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manire CA, Rhinehart HL, Sutton DA, et al. (2002). Disseminated mycotic infection caused by Colletotrichum acutatum in a Kemp’s ridley sea turtle (Lepidochelys kempi). Journal of Clinical Microbiology 40: 4273–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelino J, Giordano R, Gouli S et al. (2008). Colletotrichum acutatum var. fioriniae (teleomorph: Glomerella acutata var. fioriniae var. nov.) infection of a scale insect. Mycologia 100: 353–374. [DOI] [PubMed] [Google Scholar]
- Marin-Felix Y, Groenewald JZ, Cai L. et al. (2017). Genera of phytopathogenic fungi: GOPHY 1. Studies in Mycology 86: 99–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu S, Mosè M, Evgeny MZ. (2019). BUSCO: assessing genome assembly and annotation completeness. Methods in Molecular Biology 1962: 227–245. [DOI] [PubMed] [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2012). The CIPRES science gateway: enabling high-impact science for phylogenetics researchers with limited resources. In: Proceedings of the 1st conference of the extreme science and engineering discovery environment: Bridging from the extreme to the campus and beyond. Association for Computing Machinery, USA: 1–8. [Google Scholar]
- Miller JW. (1992). Bureau of Plant Pathology. Triology Technical Report Of Division, Plant Industry, Florida 31: 5–8. [Google Scholar]
- Moktali V, Park J, Fedorova-Abrams ND, et al. (2012). Systematic and searchable classification of cytochrome P450 proteins encoded by fungal and oomycete genomes. BMC Genomics 13: 525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki J, Tsukiboshi T. (2009). Colletotrichum echinochloae, a new species on Japanese barnyard millet (Echinochloa utilis). Mycoscience 50: 273–280. [Google Scholar]
- Nirenberg HI. (1976). Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem 169: 1–117. [Google Scholar]
- Nylander JAA. (2004). MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University, Sweden. [Google Scholar]
- O’Donnell K, Cigelnik E (1997). Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116. [DOI] [PubMed] [Google Scholar]
- O’Connell RJ, Thon MR, Hacquard S. et al. (2012). Life-style transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nature Genetics 44: 1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer JM. (2016). Funannotate: pipeline for genome annotation. <https://funannotate.readthedocs.io/en/latest/index.html>. [Google Scholar]
- Patterson W. (1900). New species of fungi. Bulletin of the Torrey Botanical Club 27: 282–286. [Google Scholar]
- Raabe RD, Conners IL, Martinez AP. (1981). Checklist of plant diseases in Hawaii. College of Tropical Agriculture and Human Resources, University of Hawaii, USA. [Google Scholar]
- Rayner RW. (1970). A Mycological Colour Chart. Commonwealth Mycological Institute, Kew, UK. [Google Scholar]
- Ronquist F, Huelsenbeck JP. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574. [DOI] [PubMed] [Google Scholar]
- Samarakoon MC, Persoh D, Hyde KD, et al. (2018). Colletotrichum acidae sp. nov. from northern Thailand and a new record of C. dematium on Iris sp. Mycosphere 9: 583–597. [Google Scholar]
- Saccardo PA. (1908). Notae mycologicae. Series X. Annales Mycologici 6: 553–569. [Google Scholar]
- Saier MH, Reddy VS, Tsu BV, et al. (2016). The Transporter Classification Database (TCDB): recent advances. Nucleic Acids Research 44: D372–D379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Moriwaki J, Uzuhashi S, et al. (2012). Molecular phylogenetic analyses and morphological re-examination of strains belonging to three rare Colletotrichum species in Japan. Microbiology and Culture Collections 28: 121–134. [Google Scholar]
- Shiraishi A, Araki-Sasaki A, Mitani A, et al. (2011). Clinical characteristics of keratitis due to Colletotrichum gloeosporioides. Journal of Ocular Pharmacology and Therapeutics 27: 487–491. [DOI] [PubMed] [Google Scholar]
- Shivaprakash MR, Appannanavar SB, Dhaliwal M, et al. (2011). Colletotrichum truncatum: an unusual pathogen causing mycotic keratitis and endophthalmitis. Journal of Clinical Microbiology 49: 2894–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva DN, Talhinhas P, Várzea V, et al. (2012). Application of the Apn2/MAT locus to improve the systematics of the Colletotrichum gloeosporioides complex: an example from coffee (Coffea spp.) hosts. Mycologia 104: 396–409. [DOI] [PubMed] [Google Scholar]
- Sperschneider J, Gardiner DM, Dodds PN, et al. (2016). EffectorP: predicting fungal effector proteins from secretomes using machine learning. New Phytologist 210: 743–761. [DOI] [PubMed] [Google Scholar]
- Stajich JE. (2017). Fungal genomes and insights into the evolution of the kingdom. Microbiol Spectrum 5: FUNK-0055-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao G, Liu ZY, Liu F, et al. (2013). Endophytic Colletotrichum species from Bletilla ochracea (Orchidaceae), with descriptions of seven new species. Fungal Diversity 61: 139–164. [Google Scholar]
- Wang Q, Liu F, Hou CL, et al. (2021). Species of Colletotrichum on bamboos from China. Mycologia 113: 450–458. [DOI] [PubMed] [Google Scholar]
- Weir BS, Johnston PR, Damm U. (2012). The Colletotrichum gloeosporioides species complex. Studies in Mycology 73: 115–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns TD, Lee S, et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications (White TJ, Sninsky JJ, Gelfand DH, et al., eds). Academic Press, San Diego, USA: 315–322. [Google Scholar]
- Wikee S, Cai L, Pairin N, et al. (2011). Colletotrichum species from Jasmine (Jasminum sambac). Fungal Diversity 46: 171–182. [Google Scholar]
- Yang Y, Liu Z, Cai L, et al. (2012). New species and notes of Colletotrichum on daylilies (Hemerocallis spp.). Tropical Plant Pathology 37: 165–174. [Google Scholar]
- Yang YL, Liu ZY, Cai L, et al. (2009). Colletotrichum anthracnose of Amaryllidaceae. Fungal Diversity 39: 123–146. [Google Scholar]
- Yang Y, Cai L, Liu Z, et al. (2011). Colletotrichum species on Orchidaceae in southwest China. Cryptogamie, Mycologie 32: 229–253. [Google Scholar]
- Yin Y, Mao X, Yang J, et al. (2012). dbCAN: a web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Research 40: W445–W451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Su YY, Cai L. (2013). An optimized protocol of single spore isolation for fungi. Cryptogamie, Mycologie 34: 349–356. [Google Scholar]
- Zhang W, Damm U, Crous PW, et al. (2020). Anthracnose disease of carpetgrass (Axonopus compressus) caused by Colletotrichum hainanense sp. nov. Plant Disease 104: 1744–1750. [DOI] [PubMed] [Google Scholar]
- Zhou S, Qiao L, Jayawardena RS, et al. (2019). Two new endophytic Colletotrichum species from Nothapodytes pittosporoides in China. MycoKeys 49: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic tree of Colletotrichum resulting from the RAxML analysis of the ITS sequence alignment. Bootstrap support values (1 000 replicates, GTR-GAMMA model) > 50 % are shown at the nodes. The scale bar represents the expected number of changes per site. Species complexes are indicated with coloured boxes, their names are listed at the left. Ex-type strains are indicated with “T” in the end of the taxa labels.
DNA barcodes of all accepted Colletotrichum spp. except for the ones in the C. graminicola and C. caudatum species complexes.
DNA barcodes of the accepted Colletotrichum spp. in the C. caudatum species complex.
DNA barcodes of the accepted Colletotrichum spp. in the C. graminicola species complex.
Colletotrichum species for which whole-genome sequences are available, retrieved from NCBI and JGI, or generated in the current study.
All strains identified in the current study.
The substrate/host information for Colletotrichum species analysed in the current study, and host information of species reported in China retrieved from literature.
Colletotrichum species reported as endophytes in previous publications and in the current study.