Abstract
Chaetomiaceae comprises phenotypically diverse species, which impact biotechnology, the indoor environment and human health. Recent studies showed that most of the traditionally defined genera in Chaetomiaceae are highly polyphyletic. Many of these morphology-based genera, such as Chaetomium, Thielavia and Humicola, have been redefined using multigene phylogenetic analysis combined with morphology; however, a comprehensive taxonomic overview of the family is lacking. In addition, the phylogenetic relationship of thermophilic Chaetomiaceae species with non-thermophilic taxa in the family is largely unclear due to limited taxon sampling in previous studies. In this study, we provide an up-to-date overview on the taxonomy and phylogeny of genera and species belonging to Chaetomiaceae, including an extensive taxon sampling of thermophiles. A multigene phylogenetic analysis based on the ITS (internal transcribed spacers 1 and 2 including the 5.8S nrDNA), LSU (D1/D2 domains of the 28S nrDNA), rpb2 (partial RNA polymerase II second largest subunit gene) and tub2 (β-tubulin gene) sequences was performed on 345 strains representing Chaetomiaceae and 58 strains of other families in Sordariales. Divergence times based on the multi-gene phylogeny were estimated as aid to determine the genera in the family. Genera were delimited following the criteria that a genus must be a statistically well-supported monophyletic clade in both the multigene phylogeny and molecular dating analysis, fall within a divergence time of over 27 million years ago, and be supported by ecological preference or phenotypic traits. Based on the results of the phylogeny and molecular dating analyses, combined with morphological characters and temperature-growth characteristics, 50 genera and 275 species are accepted in Chaetomiaceae. Among them, six new genera, six new species, 45 new combinations and three new names are proposed. The results demonstrate that the thermophilic species fall into seven genera (Melanocarpus, Mycothermus, Remersonia, Thermocarpiscus gen. nov., Thermochaetoides gen. nov., Thermothelomyces and Thermothielavioides). These genera cluster in six separate lineages, suggesting that thermophiles independently evolved at least six times within the family. A list of accepted genera and species in Chaetomiaceae, together with information on their MycoBank numbers, living ex-type strains and GenBank accession numbers to ITS, LSU, rpb2 and tub2 sequences is provided. Furthermore, we provide suggestions how to describe and identify Chaetomiaceae species.
Taxonomic novelties: new genera: Parvomelanocarpus X.Wei Wang & Houbraken, Pseudohumicola X.Wei Wang, P.J. Han, F.Y. Bai & Houbraken, Tengochaeta X.Wei Wang & Houbraken, Thermocarpiscus X.Wei Wang & Houbraken, Thermochaetoides X.Wei Wang & Houbraken, Xanthiomyces X.Wei Wang & Houbraken; New species: Botryotrichum geniculatum X.Wei Wang, P.J. Han & F.Y. Bai, Chaetomium subaffine Sergejeva ex X.Wei Wang & Houbraken, Humicola hirsuta X.Wei Wang, P.J. Han & F.Y. Bai, Subramaniula latifusispora X.Wei Wang, P.J. Han & F.Y. Bai, Tengochaeta nigropilosa X.Wei Wang & Houbraken, Trichocladium tomentosum X.Wei Wang, P.J. Han & F.Y. Bai; New combinations: Achaetomiella gracilis (Udagawa) Houbraken, X.Wei Wang, P.J. Han & F.Y. Bai, Allocanariomyces americanus (Cañete-Gibas et al.) Cañete-Gibas, Wiederhold, X.Wei Wang & Houbraken, Amesia dreyfussii (Arx) X.Wei Wang & Houbraken, Amesia raii (G. Malhotra & Mukerji) X.Wei Wang & Houbraken, Arcopilus macrostiolatus (Stchigel et al.) X.Wei Wang & Houbraken, Arcopilus megasporus (Sörgel ex Seth) X.Wei Wang & Houbraken, Arcopilus purpurascens (Udagawa & Y. Sugiy.) X.Wei Wang & Houbraken, Arxotrichum deceptivum (Malloch & Benny) X.Wei Wang & Houbraken, Arxotrichum gangligerum (L.M. Ames) X.Wei Wang & Houbraken, Arxotrichum officinarum (M. Raza & L. Cai) X.Wei Wang & Houbraken, Arxotrichum piluliferoides (Udagawa & Y. Horie) X.Wei Wang & Houbraken, Arxotrichum repens (Guarro & Figueras) X.Wei Wang & Houbraken, Arxotrichum sinense (K.T. Chen) X.Wei Wang & Houbraken, Botryotrichum inquinatum (Udagawa & S. Ueda) X.Wei Wang & Houbraken, Botryotrichum retardatum (A. Carter & R.S. Khan) X.Wei Wang & Houbraken, Botryotrichum trichorobustum (Seth) X.Wei Wang & Houbraken, Botryotrichum vitellinum (A. Carter) X.Wei Wang & Houbraken, Collariella anguipilia (L.M. Ames) X.Wei Wang & Houbraken, Collariella hexagonospora (A. Carter & Malloch) X.Wei Wang & Houbraken, Collariella pachypodioides (L.M. Ames) X.Wei Wang & Houbraken, Ovatospora amygdalispora (Udagawa & T. Muroi) X.Wei Wang & Houbraken, Ovatospora angularis (Yu Zhang & L. Cai) X.Wei Wang & Houbraken, Parachaetomium biporatum (Cano & Guarro) X.Wei Wang & Houbraken, Parachaetomium hispanicum (Guarro & Arx) X.Wei Wang & Houbraken, Parachaetomium inaequale (Pidopl. et al.) X.Wei Wang & Houbraken, Parachaetomium longiciliatum (Yu Zhang & L. Cai) X.Wei Wang & Houbraken, Parachaetomium mareoticum (Besada & Yusef) X.Wei Wang & Houbraken, Parachaetomium muelleri (Arx) X.Wei Wang & Houbraken, Parachaetomium multispirale (A. Carter et al.) X.Wei Wang & Houbraken, Parachaetomium perlucidum (Sergejeva) X.Wei Wang & Houbraken, Parachaetomium subspirilliferum (Sergejeva) X.Wei Wang & Houbraken, Parathielavia coactilis (Nicot) X.Wei Wang & Houbraken, Parvomelanocarpus tardus (X.Wei Wang & Samson) X.Wei Wang & Houbraken, Parvomelanocarpus thermophilus (Abdullah & Al-Bader) X.Wei Wang & Houbraken, Pseudohumicola atrobrunnea (X.Wei Wang et al.) X.Wei Wang, P.J. Han, F.Y. Bai & Houbraken, Pseudohumicola pulvericola (X.Wei Wang et al.) X.Wei Wang, P.J. Han, F.Y. Bai & Houbraken, Pseudohumicola semispiralis (Udagawa & Cain) X.Wei Wang, P.J. Han, F.Y. Bai & Houbraken, Pseudohumicola subspiralis (Chivers) X.Wei Wang, P.J. Han, F.Y. Bai & Houbraken, Staphylotrichum koreanum (Hyang B. Lee & T.T.T. Nguyen) X.Wei Wang & Houbraken, Staphylotrichum limonisporum (Z.F. Zhang & L. Cai) X.Wei Wang & Houbraken, Subramaniula lateralis (Yu Zhang & L. Cai) X.Wei Wang & Houbraken, Thermocarpiscus australiensis (Tansey & M.A. Jack) X.Wei Wang & Houbraken, Thermochaetoides dissita (Cooney & R. Emers.) X.Wei Wang & Houbraken, Thermochaetoides thermophila (La Touche) X.Wei Wang & Houbraken, Xanthiomyces spinosus (Chivers) X.Wei Wang & Houbraken; New names: Chaetomium neoglobosporum X.Wei Wang & Houbraken, Thermothelomyces fergusii X.Wei Wang & Houbraken, Thermothelomyces myriococcoides X.Wei Wang & Houbraken; Lecto- and / or epi-typifications (basionyms): Botryoderma rostratum Papendorf & H.P. Upadhyay, Botryotrichum piluliferum Sacc. & Marchal, Chaetomium carinthiacum Sörgel, Thielavia heterothallica Klopotek.
Citation: Wang XW, Han PJ, Bai FY, Luo A, Bensch K, Meijer M, Kraak B, Han DY, Sun BD, Crous PW, Houbraken J (2022). Taxonomy, phylogeny and identification of Chaetomiaceae with emphasis on thermophilic species. Studies in Mycology 101: 121–243. doi: 10.3114/sim.2022.101.03.
Keywords: Generic divergence times, Identification, Multi-gene phylogeny, New taxa, Taxonomic novelties, Thermophilic species
INTRODUCTION
Species of the family Chaetomiaceae exhibit high phenotypical and ecological diversity and are medically and economically important. Well-known taxa of the family include the indoor contaminant Chaetomium globosum, the mycetoma-causing agent Madurella mycetomatis and the enzyme producer Thermothelomyces thermophilus (= Myceliophthora thermophila) (Ahmed et al. 2002, van den Brink et al. 2012, Samson et al. 2019). Chaetomiaceae have a worldwide distribution. The majority are saprobes and occur in soil, dung, air, seed, compost, rotting plant materials and indoor environments (Cooney & Emerson 1964, Tiscornia et al. 2009, Betancourt et al. 2013, Wang et al. 2016a).
Species in Chaetomiaceae gained attention in biotechnology because they are producers of industrial-relevant enzymes (Berka et al. 2011, Harreither et al. 2011, Glass et al. 2013, Vivi et al. 2019), with the thermophilic species being used for the production of plant-biomass degrading thermostable enzymes (Margaritis et al. 1986, Haki & Rakshit 2003, Viikari et al. 2007, Berka et al. 2011, van den Brink et al. 2012, Yang et al. 2014a, Singh 2016). Other potential applications of Chaetomiaceae are their use as biological control organisms of plant diseases, bioorganic fertilisers (Lang et al. 2012, Hu et al. 2013, Yang et al. 2014b, Zhang et al. 2014b, Larran et al. 2016) or growth promoters of Agaricus bisporus mycelium (Straatsma et al. 1994). Chaetomiaceae are able to produce various bioactive secondary metabolites that display a wide range of cytotoxic, anticancer, antioxidant, antibacterial or antimalarial activities (Kharwar 2011, Gond et al. 2012, Gutierrez et al. 2012, Zhang et al. 2012, Selim et al. 2014, Wang et al. 2017, Gao et al. 2019, Yadav et al. 2019)
In contrast to the positive aspects mentioned above, some Chaetomiaceae species are negatively associated with human health. For example, Madurella species are agents of human subcutaneous mycoses, causing human mycetoma in arid areas of northeastern Africa (Ahmed et al. 2002). The presence of medically important species in Chaetomiaceae is not restricted to Madurella, and infective species are distributed across the family (e.g., Canariomyces subthermophilus, Chaetomium globosum, Humicola atrobrunnea and Subramaniula anamorphosa) (Ahmed et al. 2016, Wang et al. 2019b). Most human infections by Chaetomiaceae species are caused by traumatic inoculations into otherwise healthy humans, and rarely occur as deep infections in severely immunocompromised hosts (Abbott et al. 1995, Guppy et al. 1998, Ahmed et al. 2002, Barron et al. 2003, Al-Aidaroos et al. 2007, Hubka et al. 2011). Furthermore, the aflatoxin precursor and mycotoxin sterigmatocystin can be produced by several species within the family (Rank et al. 2011). In addition, Chaetomiaceae also occur in the indoor environment, causing disfigurement of surfaces and contribute to the development of rhinitis and asthma due to the production of mycotoxins, microbial volatile organic compounds and fungal particles (ascospores, hyphal fragments) (Wang et al. 2016b).
In 1817, Gustav Kunze introduced Chaetomium with Ch. globosum as type (Kunze & Schmidt 1817). The family Chaetomiaceae was established in 1885 to accommodate fungi that produce non-stromatic ascomata with a membranaceous ascomatal wall, fasciculate and evanescent asci and single-celled, smooth, pigmented ascospores (Winter 1885, Ames 1963, Hawksworth 1971). In the long taxonomic history of Chaetomiaceae, the most important change was made by von Arx et al. (1986). Rather than focusing on the variable ascomatal hairs, he laid emphasis on asci and ascospores characters, the presence of germ pores on ascospores and the structure of the ascomatal wall to delimit species. However, there were limited changes to the generic concept. For example, Chaetomium remained for species producing ostiolate ascomata covered by relatively well-developed hairs, Achaetomium for species having ostiolate ascomata covered by hypha-like ascomatal hairs, Chaetomidium for taxa producing non-ostiolate ascomata with pseudoparenchymatous wall covered by well-developed ascomatal hairs, and Thielavia for those having non-ostiolate, glabrous or tomentose ascomata with a wall of textura epidermoidea (von Arx et al. 1986, 1988, Abdel-Azeem 2020). The only change at the generic level was splitting several genera from previously existing genera, such as separating Corynascus (for species producing ascospores with two apical germ pores and a chrysosporium-like conidial morph) and Corynascella (for species producing ascospores with two apical germ pores but lacking a chrysosporium-like conidial morph) from Thielavia (von Arx 1973b, 1975a), and separating Subramaniula from Achaetomium for species producing urniform and nearly glabrous ascomata with a translucent wall and a wide ostiole surrounded by a hyaline collar (von Arx 1985). The morphologically-defined Chaetomium became a large genus with more than 400 proposed species epithets and approximately 270 accepted species (Abdel-Azeem 2020).
Traditional taxonomic studies of Chaetomiaceae mainly focused on sexually reproducing species (Zopf 1881, Ames 1963, von Arx et al. 1986, 1988). However, phylogenetic studies showed that different asexual morphs are present in the family, and these can be, for example, acremonium-, humicola-, staphylotrichum- or trichocladium-like (Wang et al. 2019a). Recent taxonomic studies based on molecular phylogenetic analyses also recognised the polyphyly of many morphologically-defined genera, including Chaetomium, Chaetomidium and Thielavia (Greif et al. 2009, van den Brink et al. 2015, Wang et al. 2016b). A modern classification system of Chaetomiaceae that includes monophyletic lineages and that is consistent with the current single name nomenclature system has been established. In total, 26 genera are recently proposed: Allobotryotrichum (Raza et al. 2019), Allocanariomyces (Mehrabi et al. 2020), Amesia and Arcopilus (Wang et al. 2016b), Arxotrichum (Crous et al. 2018), Batnamyces (Noumeur et al. 2020), Brachychaeta, Carteria, Chrysanthotrichum and Chrysocorona (Wang et al. 2019b), Collariella (Wang et al. 2016b), Condenascus (Wang et al. 2019b), Crassicarpon (Marin-Felix et al. 2015), Dichotomopilus (Wang et al. 2016b), Floropilus, Hyalosphaerella and Microthielavia (Wang et al. 2019b), Mycothemus (Natvig et al. 2015, Wang et al. 2019a), Ovatospora (Wang et al. 2016b), Parachaetomium (Mehrabi et al. 2020), Parathielavia (Wang et al. 2019b), Pseudocanariomyces (Ryan et al. 2021), Pseudothielavia and Stolonocarpus (Wang et al. 2019b), Thermothelomyces (Marin-Felix et al. 2015), Thermothielavioides (Wang et al. 2019b). Many existing genera have also been re-defined, including Acrophialophora, Botryotrichum, Canariomyces, Chaetomium, Humicola, Staphylotrichum, Subramaniula, Thielavia, and Trichocladium (Wang et al. 2016b, 2019a, b). Although this series of recent studies have elucidated the phylogenetic relationships of Chaetomiaceae (Wang et al. 2016a, b, 2019a, b), a comprehensive taxonomic overview is still lacking, which may hamper correct species identification, resulting in incorrect classification at species and generic levels (Raza et al. 2019).
Thermophilic fungi were defined as those with a maximum growth temperature above 50 °C and a minimum growth temperature at 20 °C or even higher (Cooney & Emerson 1964), or with a faster growth rate at 45 °C than at 34 °C (Morgenstern et al. 2012). They are of great importance as a potential source of thermostable enzymes in industry and as a production platform for biotechnology at elevated temperatures (van den Brink et al. 2012). Morgenstern et al. (2012) reported the presence of 23 thermophilic species in Kingdom Fungi and demonstrated their polyphyly: 13 species (of which three proved to be conspecific, Wang et al. 2019a) fell into the Chaetomiaceae (Sordariales), six belonged to the Eurotiales and one to the Onygenales in Ascomycota, and three in the Mucoromycota. This clearly shows that Chaetomiaceae harbours the most thermophilic species in Kingdom Fungi. Various studies adopted inconsistent names for some of the thermophilic Chaetomiaceae species, mainly caused by confusing taxonomy based on morphology. Natvig et al. (2015) introduced the name Mycothermus thermophilus for a fungus, which historically was named Scytalidium thermophilum or Torula thermophila. Subsequently, Wang et al. (2019b) synonymised Humicola insolens and Humicola grisea var. thermoides with Mycothermus thermophilus. During the phylogenetic re-evaluation of the genus Thielavia, Thermothielavioides terrestris was introduced to accommodate the thermophilic species “Thielavia terrestris” which produces thielavia-like ascomata, but is phylogenetically distant from the type species of Thielavia (Wang et al. 2019b). Marin-Felix et al. (2015) segregated Myceliophthora sensu van den Brink et al. (2012) into four genera: Myceliophthora, the resurrected genus Corynascus, and two newly-proposed thermophilic genera Crassicarpon and Thermothelomyces. In their analysis, however, only four other Chaetomiaceae species were included as a reference, and their phylogenetic relationships with other genera in the family remain unclear. Despite these studies, the classification and relationships of some other thermophilic species in the family is still poorly addressed. Chaetomium thermophilum, for example, is one of the few thermophilic fungal species with the optimum growth temperature at 45–50 °C and maximum up to 60 °C, reaching the upper limit of growth for Eukarya (Millner 1977, Morgenstern et al. 2012, de Oliveira et al. 2015). There has been evidence that Ch. thermophilum is distantly related to Chaetomium sensu stricto (van den Brink et al. 2012, Wang et al. 2016a, b, Zhang et al. 2017b); however, no taxonomic update has been made for this species.
Molecular-clock dating analysis proved helpful to delimit taxa at different taxonomic levels. The molecular evolutionary clock concept or the molecular clock hypothesis was already proposed in the 1960s, postulating a constant evolutionary rate at the molecular level (Zuckerkandl & Pauling 1965). Molecular-clock dating analysis greatly advanced over the past decades and with the availability of DNA sequence data and suitable fossil calibrations, it has been widely used to estimate timescales for different life forms on earth or in studying the macroevolutionary process (Bourguignon et al. 2014, Zanne et al. 2014, dos Reis et al. 2015, Chen et al. 2019, Ho 2020). In mycology, molecular-clock dating has been employed to infer macroevolutionary patterns of speciation and extinction of mushroom-forming fungi (Agaricomycetes) (Varga et al. 2019), and to infer the origin and diversification of genera and fungi in certain specific environments over time (Wang et al. 2018, Zhang et al. 2018, Steenwyk et al. 2019, Wang et al. 2019c, Zhu et al. 2019). It has also been used as additional evidence for classification arrangements at different taxonomic levels. Hyde et al. (2017) proposed a series of evolutionary periods that could be used as a guide to determine the various higher ranks in Kingdom Fungi: phyla >550 million years ago (Mya), subphyla 400–550 Mya; classes 300–400 Mya; subclasses 250–300 Mya, orders 150–250 Mya and families 50–150 Mya. They furthermore proposed that classification schemes and ranking of taxa should, where possible, incorporate a polyphasic approach including phylogeny, phenotype, and estimate of divergence times. Molecular dating analyses have been applied in various taxonomic studies. For example, to standardise taxonomic ranks of Basidiomycota, a universal criterion was proposed in which taxa must be monophyletic and statistically well-supported in molecular dating analyses (Zhao et al. 2017). In order to stabilise ranks in Basidiomycota, He et al. (2019) subsequently estimated the divergence times within this phylum (to family level). Examples in Ascomycota include those of Píchová et al. (2018), who used molecular dating to support their proposal of an infrageneric classification in Claviceps and Guterres et al. (2018), who used multilocus phylogenetic analyses followed by divergence time estimation to demonstrate a natural placement of Apiosphaeria guaranitica (the causal agent of brown crust disease of bignoniaceous plants) within Diaporthaceae (Diaporthales) rather than in Phyllachoraceae (Phyllachorales). In the present study, molecular dating analysis was used as an addition to the commonly used phylogenetic analyses for revealing phylogenetic relationships of genera in Chaetomiaceae.
Lists of accepted species are compiled to assist users of the taxonomy in basic and applied research fields to obtain the correct species names. These lists have been prepared for various genera, such as Aspergillus, Cladosporium, Fusarium, Penicillium and Trichoderma, and sometimes also include data on reference sequences, (ex-)type information and MycoBank numbers (Samson et al. 2014, Visagie et al. 2014, Yilmaz et al. 2014, Bissett et al. 2015, Marin-Felix et al. 2017, Crous et al. 2021). Historically, overviews of accepted Chaetomiaceae species were provided in monographs dealing with specific genera, but these monographs are outdated (Arx et al. 1986, 1988, Abdel-Azeem 2020). Though our recent studies have updated the taxonomy of Chaetomiaceae and most of the generic descriptions have been emended (Wang et al. 2016a, b, 2019a, b), a comprehensive modern classification of the Chaetomiaceae providing a better insight into the evolutionary relationships among the species and genera is lacking. The first aim of this study is to determine the phylogenetic relationships of taxa within the Chaetomiaceae, including thermophilic taxa and previously described species and genera that have not yet been treated in phylogenetic studies of the family before. Secondly, we suggest methods for identifying and describing Chaetomiaceae species using molecular markers and morphology, and thirdly, we propose a list of accepted species and genera in Chaetomiaceae with their MycoBank numbers, type information and GenBank numbers to reference sequences.
SUGGESTED METHODS TO DESCRIBE AND IDENTIFY CHAETOMIACEAE
During our studies on the taxonomy of Chaetomiaceae (Wang et al. 2014, 2016a, b, 2019a, b), we gained experience in describing and identifying strains belonging to this family. In this section we intend to share our accumulated knowledge.
Markers for identification and phylogenetic analysis
Amplification and sequencing
An overview of primers used for amplification and sequencing of ITS, LSU, rpb2 and tub2 is given in Table 1. The primer combination V9G and LS266 is preferred for ITS amplification and sequencing, and the combination ITS5/ITS4 can be used as an alternative. The ITS barcode and a part of LSU region (D1/D2) can also be amplified in one reaction with the primers ITS5 and NL4; however, in that case sequencing should be preferably performed with the additional internal primers, e.g., LR0R and LS266 or ITS4. The primer combination rpb2-5F2/rpb2-7CR is recommended for amplification and sequencing of a part of the rpb2 gene, and the reverse primer rpb2AM-7R is suggested as alternative. Successful amplification is usually obtained with an annealing temperature of 55 °C in combination with 35 cycles. The PCR enhancer dimethyl sulfoxide (DMSO, 5 %) is added to the PCR master mix for obtaining ITS, LSU and tub2 amplicons and bovine serum albumin (BSA, 0.05 %) is added to increase the success rate of the rpb2 PCR reaction.
Table 1.
Primers used for amplification and sequencing of Chaetomiaceae strains.
| Locus | Primer | Direction | Preferred/alternative | Primer sequence (5’-3’) | Reference |
|---|---|---|---|---|---|
| Internal Transcribed Spacer (ITS) | V9G | Forward | Preferred | TTA CGT CCC TGC CCT TTG TA | de Hoog & Gerrits van den Ende (1998) |
| LS266 | Reverse | Preferred | GCA TTC CCA AAC AAC TCG ACT C | Masclaux et al. (1995) | |
| ITS5 | Forward | Alternative | GGA AGT AAA AGT CGT AAC AAG G | White et al. (1990) | |
| ITS4 | Reverse | Alternative | TCC TCC GCT TAT TGA TAT GC | White et al. (1990) | |
| 28S large subunit (LSU) nrDNA | LR0R | Forward | Preferred | ACC CGC TGA ACT TAA GC | Vilgalys & Sun (1994) |
| LR5 | Reverse | Preferred | TCC TGA GGG AAA CTT CG | Vilgalys & Hester (1990) | |
| ITS+LSU, combined | ITS5 | Forward | Preferred | GGA AGT AAA AGT CGT AAC AAG G | White et al. (1990) |
| NL4 | Reverse | Preferred | GGT CCG TGT TTC AAG ACG | O’Donnell (1993) | |
| β-tubulin (tub2) | T1 | Forward | Preferred | AAC ATG CGT GAG ATT GTA AGT | O’Donnell & Cigelnik (1997) |
| TUB4Rd | Reverse | Preferred | CCR GAY TGR CCR AAR ACR AAG TTG TC | Woudenberg et al. (2009) | |
| RNA polymerase II second largest subunit (rpb2) | rpb2-5F2 | Forward | Preferred | GGG GWG AYC AGA AGA AGG C | Sung et al. (2007) |
| rpb2-7CR | Reverse | Preferred | CCC ATR GCT TGY TTR CCC AT | Liu et al. (1999) | |
| rpb2AM-7R | Reverse | Alternative | GAA TRT TGG CCA TGG TRT CCA T | Miller & Huhndorf (2005) |
DNA-based identification
Identification of Chaetomiaceae strains using morphological characters is challenging and suffers from phenotypic plasticity and genetic variability (Tekpinar & Kalmer 2019). Strains can lose their typical morphology when preserved over time, or do not or poorly sporulate on the agar media recommended for identification (e.g., Batnamyces, Madurella) (Wang et al. 2019a, Noumeur et al. 2020). Comparative sequence-based methods are the current standard for strain identification. The ITS region is the accepted DNA barcode for fungi (Schoch et al. 2012). In common with some other ascomycete genera and families, this marker is unreliable for identification because different species can share the same ITS sequence (Wang et al. 2016b, 2019a). A good genetic identification marker should have enough variability to allow species identification and an extensive reference sequence dataset should be available for comparison. Of the markers commonly used in Chaetomiaceae (LSU, ITS, rpb2 and tub2), the latter two are suitable for strain identification. However, we recommend the use of tub2 and as secondary identification marker because this gene has a better species resolution (Wang et al. 2016b) and is easier to amplify than rpb2 (pers. obs.).
Some entries in GenBank might not reflect the new taxonomic concepts and/or sequences in GenBank might be deposited under an incorrect name (Nilsson et al. 2006); both negatively affecting the identification result. It is recommended to check whether the taxonomy of the identification result is correct, e.g., by using the list of accepted species supplied in this article. In case of doubt, we recommend constructing a phylogram using the tub2 reference sequences provided in the list of accepted species here and using the phylograms in this article (Fig. 7, Supplementary Fig. S3) as a guide. Alternatively, a local BLAST database can be assembled using verified tub2 sequences.
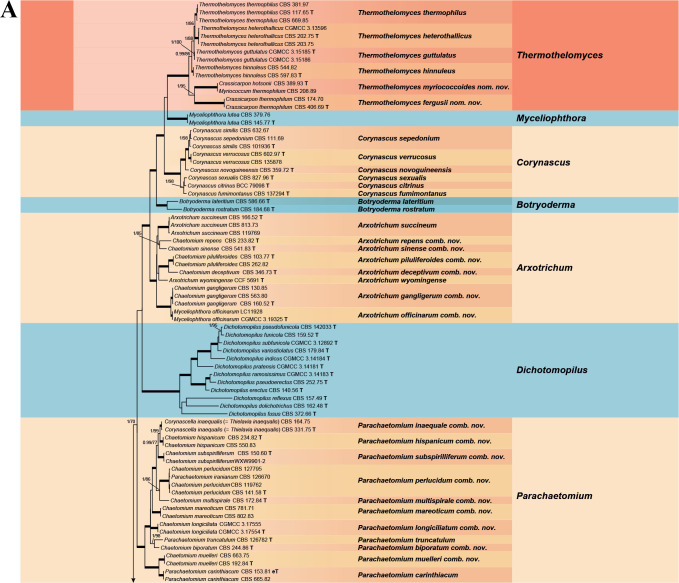
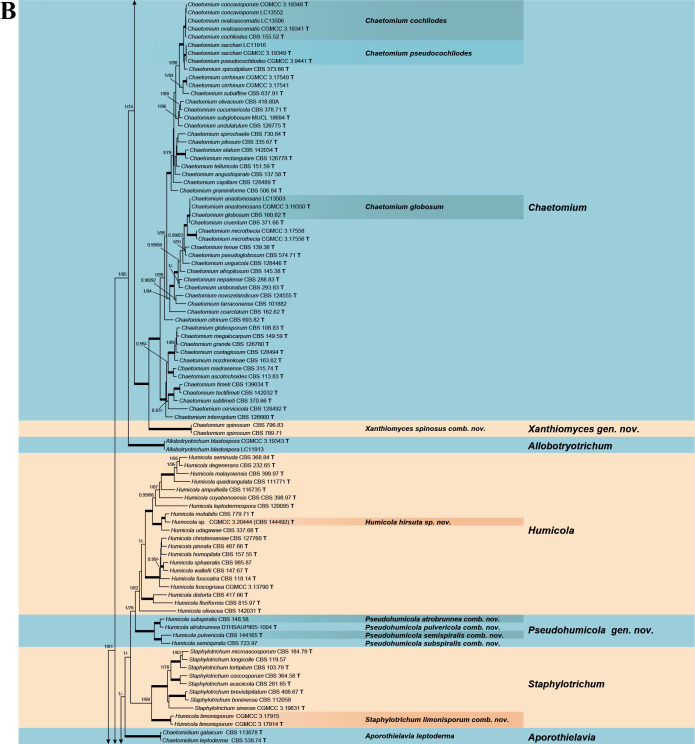
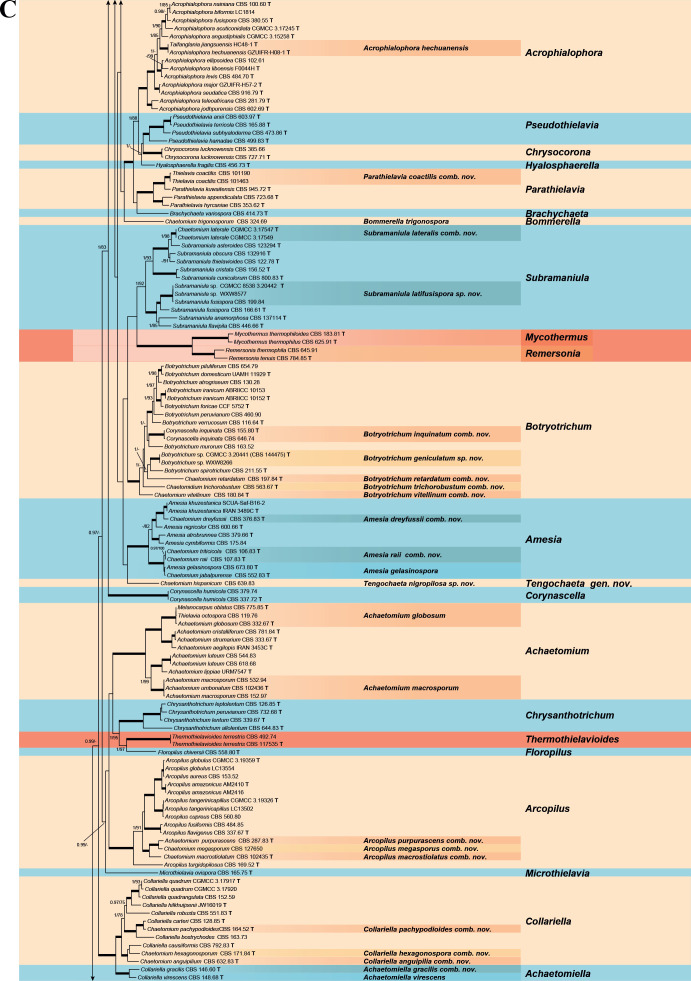
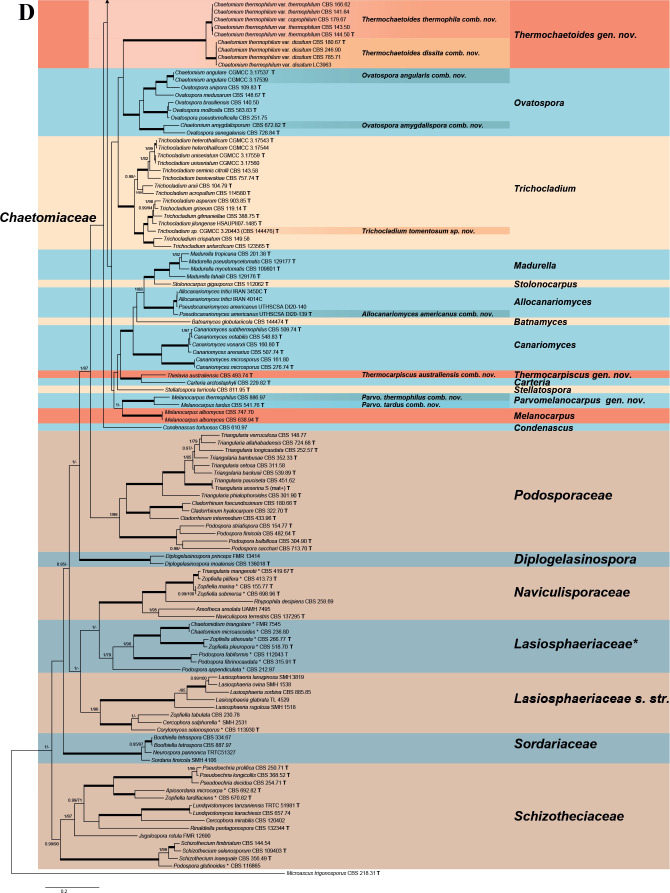
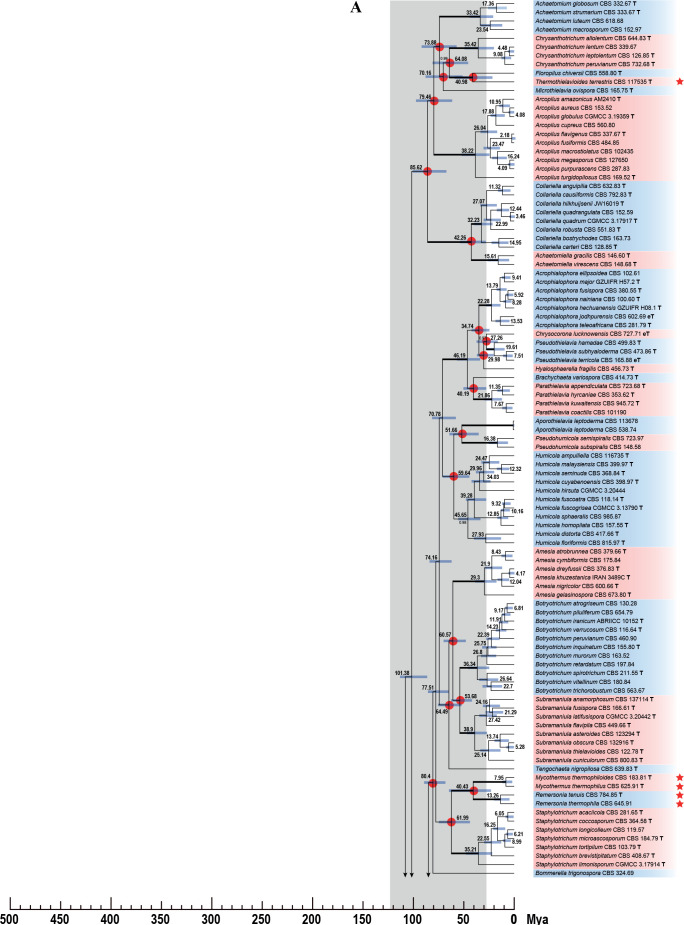
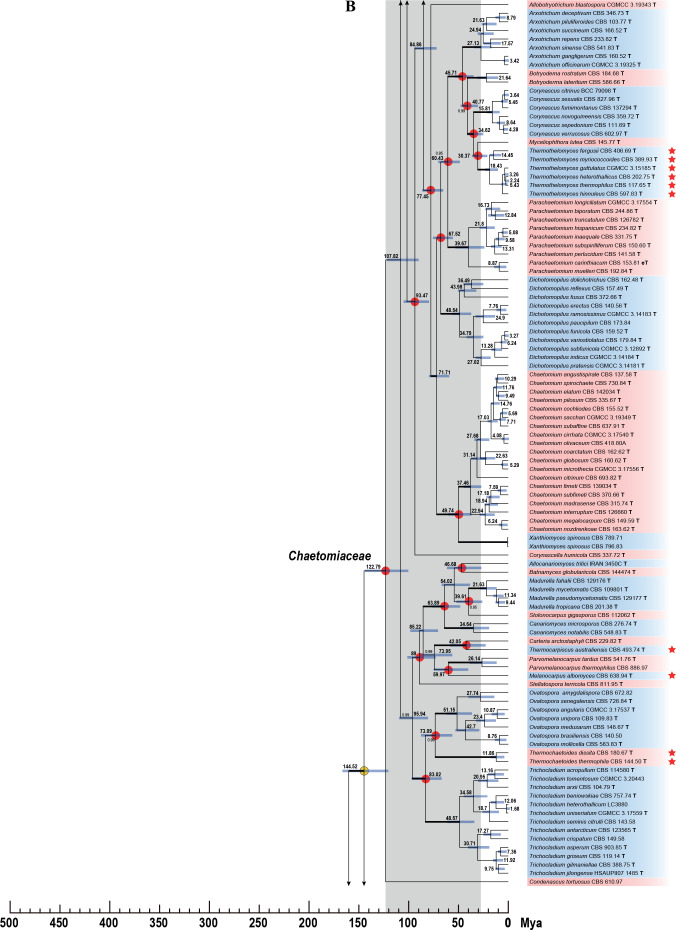
Fig. 7.
Phylogenetic tree resulting from ML analysis of the concatenated partial rpb2, tub2, ITS and LSU gene region alignment, with the confidence values indicated at the notes: the posterior probabilities from the Bayesian analysis before the slash, bootstrap proportions from the ML analysis after the slash. The “-” indicates lacking statistical support (<70 % for bootstrap proportions from ML analysis; <0.95 for posterior probabilities from Bayesian analysis). The branches with full statistical support (PP = 1.0; ML-BS = 100 %) are highlighted by thickened branches. Genus/potential new species or combination clades are discriminated with boxes in different colours and clades containing thermophilic species are highlighted with an orange background. Ex-type strains are marked with “T” after the culture number. “eT” represents the ex-epitype designated in this study. *Taxa with names of genus/family not necessarily reflecting molecular phylogenetic relationships. The scale bar shows the expected number of changes per site. The tree is rooted with Microascus trigonosporus in the Microascales.
Phylogenetic analysis
The tub2 gene is recommended for routine identification of species, but analysis of a combined dataset of ITS, LSU, rpb2 and tub2 sequences is suggested for phylogenetic analysis. β-tubulin is difficult to align, especially when the dataset includes multiple genera, and this also applies, to a lesser extent, to the ITS dataset. The LSU and rpb2 sequence datasets have the advantage that they are easier to align above species level. For the description of new Chaetomiaceae species, we recommend generating at least ITS, LSU, rpb2 and tub2 sequences of the ex-type strain. The relationships of the new species will be confidently determined using this 4-gene approach, and it will enable us to recognise new species more easily.
Morphological characters
Nowadays, Chaetomiaceae taxonomy often relies more heavily on molecular phylogenetic data than on morphological characters. Morphological observations, however, are essential for describing new taxa in the family, understanding the generic and species concepts and achieving insight into the biology of the species. Before the single name nomenclature era, the production of non-stromatic perithecia covered with hairs was a hallmark for Chaetomiaceae. The majority of species in the family reproduces sexually in a homothallic manner and lacks an asexual morph; however, some species produce sexual and asexual morphs in one culture (e.g., many species in Humicola, several Chaetomium species, Corynascella humicola, Corynascus species). Other species or genera are only known by their asexual morph (e.g., all species of Allobotryotrichum, Botryoderma, Mycothermus and Remersonia, most species in Acrophialophora and some species in Botryotrichum, Humicola, Staphylotrichum and Trichocladium). Here, we provide recommendations for obtaining morphological data in order to properly identify and describe Chaetomiaceae species.
Cultivation of Chaetomiaceae strains
Media
Colony characteristics vary on different media. Oatmeal agar (OA; composition and preparation, see Samson et al. 2019) is recommended as standard medium for Chaetomiaceae and morphological descriptions are mainly based on cultures grown on this medium. Ascomata are key structures for sexually reproducing species. Von Arx et al. (1986) recommended cornmeal agar (CMA); however, our experience is that the development of sexual structures is better on OA. Potato carrot agar (PCA; composition and preparation, see Samson et al. 2019) is recommended as an alternative medium for species that poorly develop ascomata on OA (e.g., Arxotrichum repens), but in contrast to OA, the cultures on PCA often fail to produce coloured exudates. Furthermore, aerial mycelium development is poor on PCA and this hampers preparation of slides for the observation of those asexual morphs which are formed on mycelium. Malt extract agar (MEA, Oxoid) and potato dextrose agar (PDA) are recommended media for extrolite profiling (Wang et al. 2016b, Samson et al. 2019). However, these media are not suitable for studying the morphology because the formation of a sexual morph is generally poorly induced. Some strains/species easily lose their ability to sporulate sexually. Covering the OA and/or PCA medium with a sterile cellophane membrane before inoculation might help to induce the development of ascomata when adding sterile filter paper fails (Wang et al. 2019b).
Inoculation
Inoculations are made from freshly prepared ascospore or conidium suspensions in a solution containing 2.0 g/L agar and 0.5 g/L Tween 80. We recommend using a micropipette for inoculation of the agar media with the spore suspension. The agar plates are inoculated in a three-point pattern with 1–2 μL per spot. For strains that do not or poorly sporulate, we recommend using agar plugs as inoculum. Agar plugs are cut with a cork borer along the edges of fresh colonies. Inoculating media with spore suspensions preserved at −20 °C or −80 °C is not recommended for measuring growth rates because of possible growth delay.
Incubation
Inoculated agar medium plates are incubated reverse side up in the dark at 25 °C. Exceptions are for thermotolerant or thermophilic species where an incubation temperature of 37 °C and/or 45 °C is recommended. Plates should not be wrapped with Parafilm, because this restricts air exchange and often inhibits growth and sporulation. Incubation times for measuring colony diameters are standardised at 7 d with the exception for some thermophilic species that grow fast at 45 °C; for those species the incubation time is shortened to 3 d. Asci are studied in young cultures of generally less than 2 wk old, while ascomata and ascospores are examined from cultures with fully developed ascomata, usually present after 3 wk or more.
Macromorphology
The macromorphology of a Chaetomiaceae on an agar medium provides the first impression of a species. Colony characters used for characterising species include colony diameters, degree of sporulation, colour of mycelium and colony reverse, and the presence or absence and texture of aerial mycelium, the presence or absence and distribution of ascomata and asexual morphs, soluble pigments and exudates.
Micromorphology
Microscopy
A dissecting microscope is used to observe the developmental stage of the ascomata in culture. The ostiolate ascomata are studied for the presence of ascomatal hairs, ascospore masses on the ascomata and their colour in reflected light. The top of the ascomata can be observed by placing the agar plate under the dissecting microscope, and the side view by cutting out a block of agar with well-developed ascomata and tipping it onto one side.
Slide preparation
Up to five slides are needed to study the morphology of a holomorphic Chaetomiaceae species: 1) ascomata together with ascomatal hairs, 2) asci, 3) ascospores, 4) the ascomatal wall and 5) the asexual morph. Historically, ascomycete taxonomists used water as mounting medium for the observation and measurement of ascospores (von Arx et al. 1986). Considering its rapid desiccation and the difficulty of observing germ pore(s) of ascospores properly, we suggest to use lactic acid (80 %) instead of water. We made a tentative study to get insight in the effect of these two mounting fluids on the ascospore size. Ascospore size data of 15 strains derived from previous studies were compared (Supplementary Table S1). Seven strains seemed slightly (0.5–2 μm) smaller in size (both in length and width) in lactic acid than in water, the length of two strains was slightly less in lactic acid than in water (no difference in width), one strain had a similar size in lactic acid and water, two strains were slightly narrower in width or in lateral width (for its bilaterally flattened ascospores) with no difference in length, two strains produces ascospores of similar length, but had a broader width and one strain was slightly shorter in length and broader in width. This tentative comparison shows that the ascospores of Chaetomiaceae are slightly smaller in lactic acid than in water, and observing germ pores is more easy in lactic acid. A more detailed study would be needed to confirm these data. Shear’s solution (Samson et al. 2019) is a good alternative for lactic acid (and water), especially as a mounting medium for asci. In our experience, both lactic acid and Shear’s solution are very suitable for photomicrography.
Ascomata and ascomatal hairs (Figs 1, 2)
Fig. 1.

Ascoma diversity in Chaetomiaceae under stereomicroscope (A–H) and light microscope (I–Z). A, B. Amesia nigricolor CBS 291.83. C, D. Staphylotrichum longicolle CBS 119.57. E, F. Brachychaeta variospora CBS 414.73. G. Canariomyces vonarxii CBS 160.80. H. Hyalosphaerella fragilis CBS 456.73. I. Chaetomium subaffine CBS 637.91. J. Arcopilus cupreus CBS 560.80. K. Collariella bostrychodes DTO 324-H6. L. Arxotrichum repens CBS 233.82. M. Humicola seminuda CBS 368.84. N. Collariella carteri CBS 128.85. O. Botryotrichum murorum DTO 324-G9. P. Dichotomopilus pratensis CBS 860.68. Q. Trichocladium acropullum CBS 114580. R. Chaetomium umbonatum CBS 293.83. S. Staphylotrichum longicolle CBS 119.57. T. Humicola hirsuta CBS 144492. U. Parachaetomium muelleri CBS 192.84. V. Chaetomium subfimeti CBS 370.66. W. Chrysanthotrichum peruvianum CBS 732.68. X. Botryotrichum geniculatum CBS 144475. Y. Trichocladium arxii CBS 104.79. Z. Hyalosphaerella fragilis CBS 456.73. Scale bars: I–L, O, P, R, U, X, Y, = 100 μm; M, Q, S, T = 50 μm ; N, Z = 20 μm ; V = 500 μm.
Fig. 2.

Structure diversity of ascomatal wall in Chaetomiaceae. A. Pseudothielavia arxii CBS 603.97. B. Chaetomium globosum MUCL 39526. C. Aporothielavia leptoderma CBS 538.74. D. Humicola seminuda CBS 368.84. E. Trichocladium arxii CBS 104.79. Scale bars = 10 μm.
A fine needle can be used to transfer ascomata into the lactic acid mounting medium. Ascomata are picked up one by one under a dissecting microscope to avoid being damaged. After the preparation is covered with a coverslip, the slide is gently heated on a hotplate or above a low flame on a lab gas burner to remove air bubbles and ascospore masses trapped inside terminal ascomatal hairs. After this procedure, the whole structure of the terminal ascomatal hairs including their lower parts can be observed. Ascomata of Chaetomiaceae (Fig. 1) are non-stromatic perithecia (e.g., Fig. 1A–F, I–U) or cleistothecia (e.g., Fig. 1G–H, V–Z) and are usually produced superficially on the agar surface and occasionally immersed in the medium (e.g., Fig. 1H). The ascomata can be glabrous (e.g., Fig. 1G, H, Z) or covered by highly diverse hairs (e.g., Fig. 1A–F, I–Y). The ascomatal hairs can be erect [e.g., Fig. 1M, N, S, T, W (partial), Y], flexuous [e.g., Fig. 1I, W (partial)], undulate [e.g., Fig. 1L, O, U (long)], coiled (e.g., Fig. 1K, Q), arcuate [e.g., Fig. 1J, U (short)], apically circinate or coiled [e.g., Fig. 1J, O, W (partial)], branched (e.g., Fig. 1P), hypha-like [e.g., Fig. 1R, V (short), X], or consisting of two different types (e.g., Fig. 1U, V). The ascomatal walls (peridium) can be membranaceous, composed of textura epidermoidea (Fig. 2A), intricata (Fig. 2B) or angularis (Fig. 2D) in surface view, or cephalothecoid (composed of radially elongated cells and often surrounded by lines of dehiscence in surface view) in a few species (Fig. 2C, E).
Asci (Fig. 3)
Fig. 3.
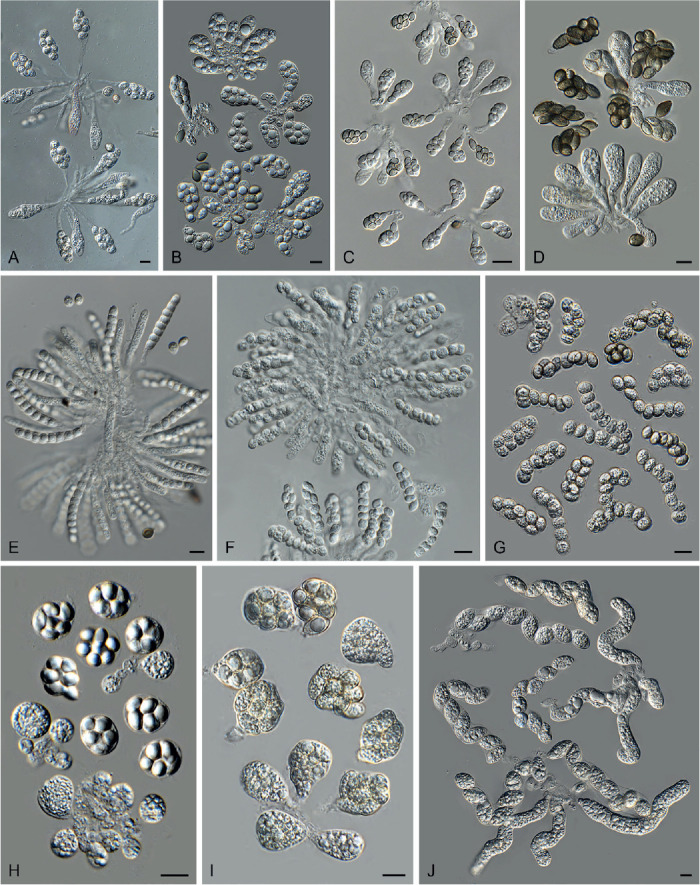
Ascus diversity in Chaetomiaceae. A. Chaetomium globosum DTO 333-E3. B. Hyalosphaerella fragilis CBS 456.73. C. Thermothielavioides terrestris CBS 492.74. D. Parathielavia appendiculata CBS 723.68. E. Ovatospora pseudomollicella CBS 251.75. F. Humicola ampulliella CBS 116735. G. Trichocladium antarcticum CBS 123565. H. Canariomyces microsporus CBS 161.80. I. Corynascus fumimontanus CBS 137294. J. Condenascus tortuosus CBS 610.97. Scale bars = 10 μm.
Examining asci in Chaetomiaceae is a challenge because they are commonly evanescent and disappear before maturation of the ascospores. Because of this, we usually observe hyaline ascospores in an ascus (Fig. 3). In some studies, the ascospores of Chaetomium globosum were even wrongly assumed to be conidia (Luo et al. 2019). For observing asci, careful attention should be paid to the formation of ascomata. It is very important to prepare slides from young ascomata at the early stage of the culture, normally within 2 wk. When the cultures are incubated longer, the majority of ascomata are mature and it becomes difficult to observe asci, even when you pick up young ascomata at the edge of the colony. For species that produce non-ostiolate ascomata, hyaline young ascomata are usually a good choice for ascus observation. Several young ascomata are transferred in a drop of Shear’s solution on a microscope slide. After the preparation is covered with a coverslip, the blunt end of a needle can be used to gently squash the ascomata. After tapping several times, fasciculate and dissociated asci can be found beside the cracked ascomata. In some species/strains, persistent asci that retain until ascospores mature can be observed (Fig. 3D). The shape of the asci can be fusiform (Fig. 3A), clavate (Fig. 3B–D), cylindrical (Fig. 3E–F), ovoid to subglobose (Fig. 3H–I) or twisted (Fig. 3J). The asci contain eight (rarely four) ascospores that are uniseriate [e.g., Fig. 3E, F (partial), G (partial), J (partial)], biseriate [e.g., Fig. 3A, D (partial), F (partial), G (partial)] or irregularly-arranged [e.g., Fig. 3B, D (partial), H, I].
Ascospores (Fig. 4)
Fig. 4.

Ascospore diversity in Chaetomiaceae. A. Collariella bostrychodes DTO 324-H6. B. Dichotomopilus variostiolatus CBS 179.84. C. Collariella quandrangulata CBS 152.59. D. Humicola ampulliella CBS 116735. E. Parathielavia kuwaitensis CBS 353.62. F. Ovatospora brasiliensis CBS 140.50. G. Bommerella trigonospora CBS 324.69. H. Arcopilus cupreus CBS 560.80. I. Canariomyces microsporus CBS 161.80. J. Arxotrichum piluliferoides CBS 103.77. K. Stellatospora terricola CBS 811.95. L. Parachaetomium subspirilliferum CBS 150.60. M. Staphylotrichum longicolle CBS 119.57. N. Pseudothielavia arxii CBS 603.97. O. Parachaetomium inaequale CBS 331.75. P. Dichotomopilus fusus CBS 114.83. Q. Chaetomium globosum DTO 319-B2. R. Chaetomium umbonatum CBS 293.83. S. Chaetomium citrinum CBS 693.82. T. Chaetomium ascotrichoides CBS 110.83. U. Chaetomium megalocarpum CBS 149.59. V. Chaetomium globosporum CBS 108.83. W. Pseudothielavia hamadae CBS 499.83. X. Stolonocarpus gigasporus CBS 112062. Y. Pseudothielavia terricola CBS 165.88. Z. Corynascus sepedonium CBS 111.69. AA. Brachychaeta variospora CBS 414.73. AB. Pseudothielavia subhyaloderma CBS 473.86. AC. Aporothielavia leptoderma CBS 538.74. AD. Parachaetomium perlucidum CBS 141.58. AE. Chrysanthotrichum peruvianum CBS 732.68. AF. Arxotrichum repens CBS 233.82. AG. Xanthiomyces spinosus CBS 789.71. AH. Parachaetomium biporatum CBS 244.86. AI. Corynascella humicola CBS 337.72. AJ. Amesia dreyfussii CBS 376.83. AK. Corynascus sexualis CBS 827.96. AL. Botryotrichum geniculatum CBS 144475. AM. Thermochaetoides thermophila CBS 179.67. AN. Humicola quadrangulata CBS 111771. AO. Parathielavia hyrcaniae CBS 353.62. AP. Condenascus tortuosus CBS 610.97. AQ. Chaetomium nozdrenkoae CBS 163.62. Scale bars = 10 μm, applies to all.
The species that produce ostiolate ascomata usually have their mature ascospores extruded in a sticky mass or cirrhus at the top of the ascoma. In many species, these ascospores are wrapped in numerous ascomatal hairs. It is easy to pick up ascospores from the top of these ascomata for slide preparation. To study ascospores produced in non-ostiolate ascomata, the ascomata must be squashed on a slide to release the ascospores. As mentioned above, water, Shear’s solution and lactic acid can be used as mounting media. Water has the disadvantage of rapid desiccation and exposure to the air might make ascospores become dehydrated, which may make them shrunken or concave. In our experience, germ pores are not easily observed in such ascospores (e.g. in Aporothielavia leptoderma (Fig. 11, see notes below) and Arxotrichum piluliferoides (= Chaetomium piluliferoides; Fig. 14, see notes below)). Heating the ascospore slide gently above a low flame on a lab gas burner or on a hotplate will not only allow ascospores to restore their normal shape, but also helps to visualise the germ pore on the spores. The position and number of germ pores can be observed on rolling ascospores in the (heated) lactic acid. When ascospores are immobile, photos can be taken of ascospores with germ pore(s). The ascospores of Chaetomiaceae are aseptate, pigmented, smooth and vary in shape and size, with one (in most species), two (e.g., Fig. 4O, Z, AA, AI, AQ) or rarely more [e.g., Fig. 4U, AQ (partial)] germ pores. The position of the germ pore is apical in most species, or subapical to oblique (e.g., Fig. 4E, AD, AO, AP) or lateral [e.g., Fig. 4N, U, AQ (partial)]. The ascospore measurements should include the extreme values given in parentheses and, in between, the 95 % confidence interval of 30 individual measurements. For the measurements of bilaterally flattened ascospores, the size was reported as “length” × “width in front view” × “width in lateral view”.
Fig. 11.
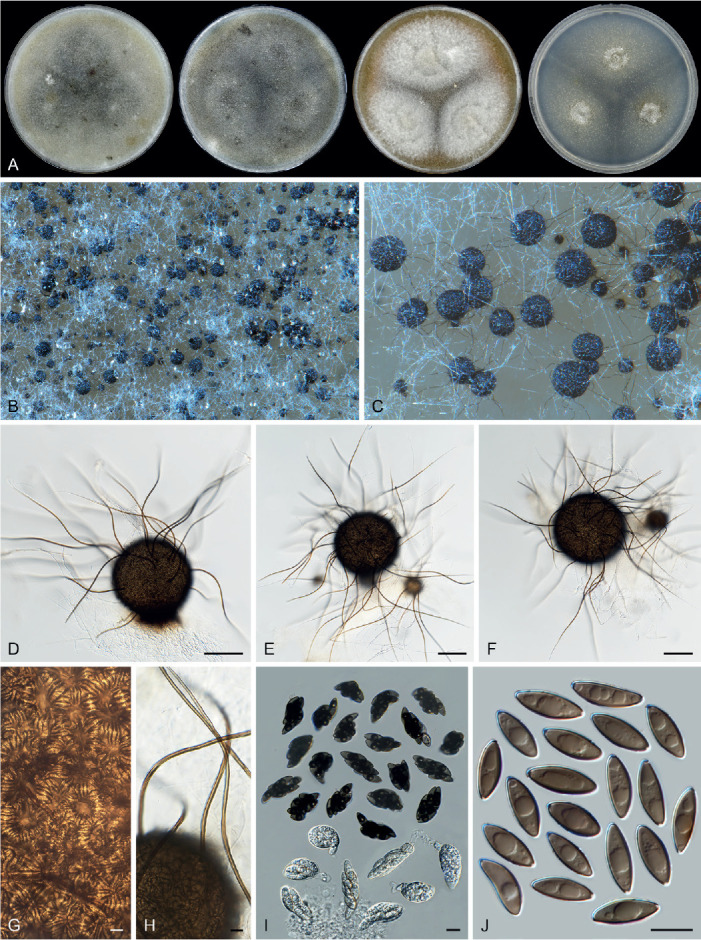
Sexual morph of Aporothielavia leptoderma (CBS 538.74, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Ascomatal hairs. I. Asci. J. Ascospores. Scale bars: D–F = 100 μm; G–J = 10 μm.
Fig. 14.
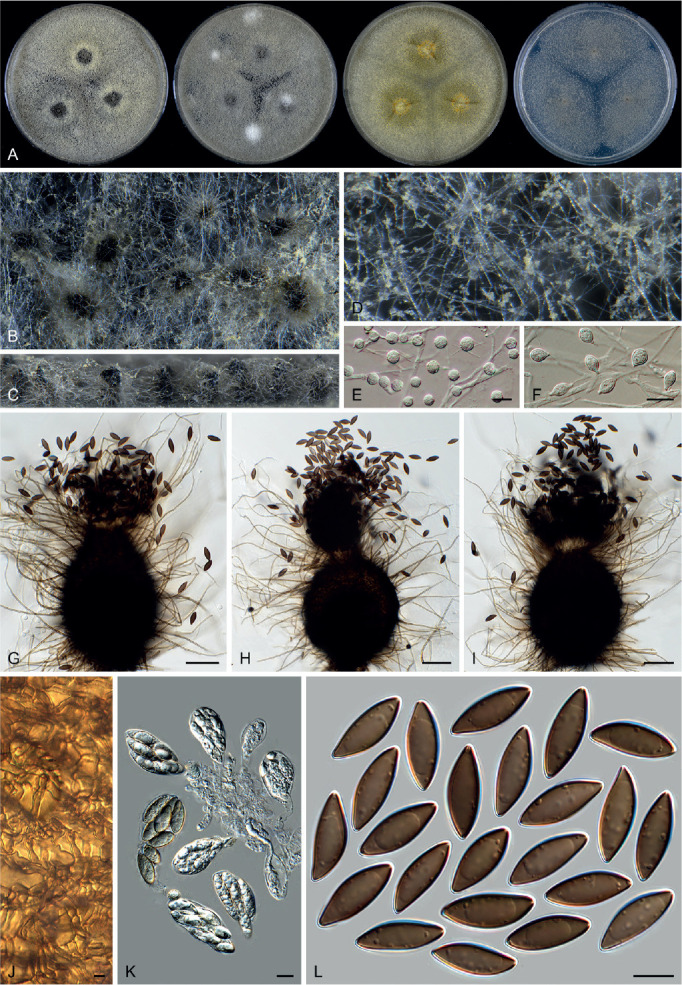
Arxotrichum piluliferoides (CBS 103.77, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B. Part of the colony showing mature ascomata on OA, top view. C. Mature ascomata on OA, side view. D. Conidia on aerial hyphae. E, F. Conidia and hyphae. G–I. Ascomata mounted in lactic acid. J. Structure of ascomatal wall in surface view. K. Asci. L. Ascospores. Scale bars: E, F, J–L = 10 μm; G–I = 50 μm.
Asexual morph (Figs 5, 6)
Fig. 5.
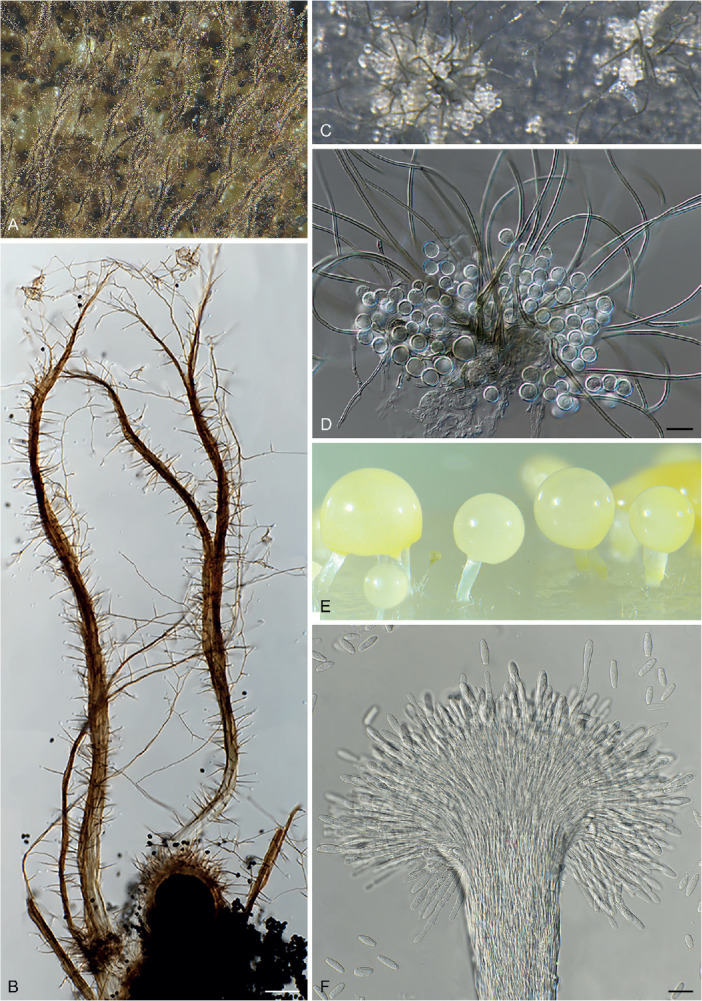
Diversity of asexual structures growing up into the air above the medium in Chaetomiaceae. A–B. Corynascella humicola CBS 337.72. C–D. Botryotrichum piluliferum DTO 254-B8. E–F. Remersonia thermophila CBS 645.91. Scale bars: B = 100 μm; D, F = 20 μm.
Fig. 6.
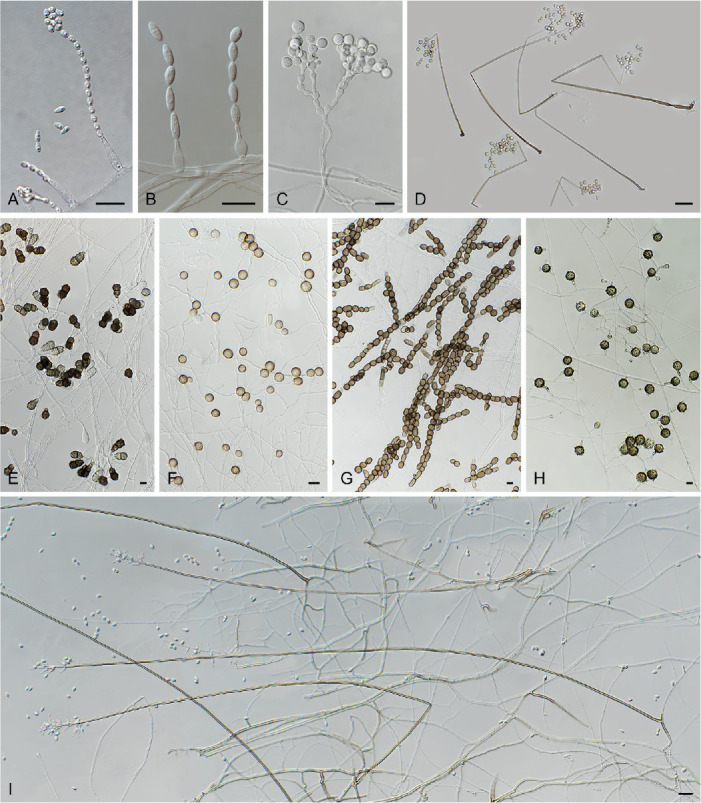
Asexual diversity in Chaetomiaceae observed by means of inclined coverslip method. A. Chaetomium elatum DTO 319-B3. B. Acrophialophora ellipsoidea CBS 102.61. C. Trichocladium beniowskiae CBS 757.74. D. Staphylotrichum coccosporum CBS 364.58. E. Trichocladium asperum CBS 903.85. F. Humicola fuscoatra CBS 118.14. G. Mycothermus thermophilus CBS 625.91. H. Botryotrichum verrucosum CBS 116.64. I. Acrophialophora nainiana CBS 100.60. Scale bars: A, B, E–H = 10 μm; C, D, I = 20 μm.
In general, the asexual morph of Chaetomiaceae is produced either on the substrate or in the aerial mycelium. The asexual structures of Corynascella humicola, Botryotrichum spp. (except for Botry. verrucosum) and Remersonia spp. grow up into the air above the medium (Fig. 5), and it is easy to prepare microscopic slides of these structures in lactic acid (80 %). The asexual morphs developed at mycelium (Fig. 6), especially those produced by species that also produce abundant well-developed ascomata, can easily be missed and cultures should therefore be carefully examined. A slide culture method (Riddell 1950, modified) is recommended. An agar block is cut out of a culture, placed on a sterile glass microscope slide, and a sterile coverslip is subsequently put on the top of the block. After 1–2 wk inoculation in a damp chamber, the coverslip is carefully removed from the block and used for the preparation of a microscope slide. The material that has grown around the block onto the microscope slide is used to make another slide (after removal of the agar block). The inclined coverslip method (Kawato & Shinobu 1959, revised in Nugent et al. 2006) can be used as an alternative. A sterile coverslip is inserted into the OA agar medium at a 45° angle, and the target strain is subsequently inoculated at one side of the inserted coverslip to allow the fungus to creep onto it. After 7–10 d, when the mycelium has covered about a third of the coverslip, the coverslip is carefully taken out of the OA medium. After cleaning the other side of the coverslip with tissue paper dipped in alcohol, it is used to make a slide for observation. Lactic acid (80 %) is used as mounting medium. Air bubbles inside the slide can be removed by gently heating the slide above a low flame. Diverse asexual morphs are associated with Chaetomiaceae (e.g., acremonium-, humicola-, staphylotrichum- or trichocladium-like, Wang et al. 2019a) and we refer to Seifert et al. (2011) for more details on these structures.
MATERIALS AND METHODS
Strains
In addition to previously studied strains (Wang et al. 2016a, b, 2019a, b), 106 strains were obtained from the CBS culture collection (CBS) housed at the Westerdijk Fungal Biodiversity Institute (WI), Utrecht, the Netherlands. Six isolates were obtained from the personal collection of Xue-Wei Wang (WXW) housed at the State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences, which represent four potential new species. Details on these strains are provided in Table 2.
Table 2.
Details of strains sequenced and studied in the present study.
| Current name | Culture accession number | Previous identification (if different) | Origin |
GenBank accession numbers1
|
|||
|---|---|---|---|---|---|---|---|
| ITS | LSU | tub2 | rpb2 | ||||
| Achaetomium strumarium | CBS 781.84 = ATCC 58164 | Achaetomium cristalliferum | Arid saline soil; New Valley Department, near Kharga-Beris, Egypt; type of Achaetomium cristalliferum | MH861836 | – | MZ343033 | MZ342994 |
| Achaetomium globosum | CBS 775.85 | Melanocarpus oblatus | Unknown substrate; Niger; type of Melanocarpus oblatus | MZ334727 | MZ343031 | MZ342992 | |
| CBS 119.76 = IMI 291729 | Thielavia octospora | Dead branch; Pabbi Hills, Pakistan | MZ334731 | MZ351416 | MZ343009 | MZ342970 | |
| Achaetomium macrosporum | CBS 102436 = FMR 6778 = IMI 381871 | Achaetomium umbonatum | Garden soil; Kanpur, India; type of Achaetomium umbonatum | MZ334718 | AJ312099 | MZ343007 | MZ342966 |
| Amesia dreyfussii | CBS 376.83 = MUCL 40177 | Chaetomium dreyfussii | Dung of hare; Israel; isotype of Chaetomium dreyfussii | MH861613 | MH873331 | MZ343023 | MZ342985 |
| Amesia gelasinospora | CBS 552.83 = IMI 157256 | Chaetomium jabalpurense | Unknown substrate and location | – | – | MZ343026 | MZ342987 |
| Amesia raii | CBS 107.83 | Chaetomium raii | Wood of Mangifera indica; India; type of Chaetomium raii | – | – | – | MZ342968 |
| CBS 106.83 = IMI 232292 | Chaetomium triticicola | Stored wheat grain; New Delhi, India; type of Chaetomium triticicola | – | – | MZ342967 | ||
| Aporothielavia leptoderma | CBS 538.74 = IMI 054770 | Chaetomidium leptoderma, Thielavia leptoderma | Soil; Surrey, England; isotype of Thielavia leptoderma | NR_164219 | NG_067253 | MZ343025 | MZ342986 |
| CBS 113678 = FMR 8192 | Chaetomidium leptoderma | Black spot on granite rock sample; Serra de Xurés, Galicia, Spain; type of Chaetomidium galaicum | – | MZ351417 | MZ343008 | MZ342969 | |
| Arcopilus aureus | CGMCC 3.19359 | Arcopilus globulus | Root of Saccharum officinarum, Guangxi, China | MN215741 | MN215579 | MZ343038 | MN255422 |
| Arcopilus cupreus | CGMCC 3.19326 | Arcopilus turgidopilosus | Root of Saccharum officinarum, Guangzhou, China | MN215743 | MN215581 | MN329904 | MZ342999 |
| Arcopilus macrostiolatum | CBS 102435 = FMR 6780 = IMI 381870 = MUCL 43147 | Chaetomium macrostiolatum | Forest soil; Enugu state, Isi-uzo, Nigeria; type of Chaetomium macrostiolatum | MZ334722 | MZ351418 | MZ343006 | MZ342965 |
| Arcopilus megasporum | CBS 127650 | Chaetomium megasporum | Agricultural soil; Minnesota, near East Bethel, USA | – | – | MZ343010 | MZ342971 |
| Arcopilus purpurascens | CBS 287.83 | Chaetomium purpurascens | Soil; Gandaki, Nepal; type of Achaetomium purpurascens | – | – | MZ343021 | MZ342982 |
| Arxotrichum deceptivum comb. nov. | CBS 346.73 | Chaetomium deceptivum | Dung of pack rat; California, USA; isotype of Chaetomium deceptivum | MK919276 | MK919276 | MK919390 | MK919332 |
| Arxotrichum gangligerum comb. nov. | CBS 160.52 = ATCC 11206 | Chaetomium gangligerum | Wood sample; Virginia, USA; type of Chaetomium gangligerum | MK919277 | MK919277 | MK919391 | MK919333 |
| CBS 130.85 | Chaetomium gangligerum | Dung of rabbit; Ontario, Canada | MK919278 | MK919278 | MK919392 | MK919334 | |
| CBS 563.80 | Chaetomium gangligerum | Dung of rabbit; Ontario, Canada | MK919279 | MK919279 | MK919393 | MK919335 | |
| Arxotrichum piluliferoides comb. nov. | CBS 103.77 = IFM 4531 = IMI 210880 | Chaetomium piluliferoides | Grassland soil; Sugadaira, Naguna Prefecture, Japan; isotype of Chaetomium piluliferoides | MK919280 | MK919280 | MK919394 | MK919336 |
| CBS 262.82 | Chaetomium piluliferoides | Dung; Tarragona, Spain | MK919281 | MK919281 | MK919395 | MK919337 | |
| Arxotrichum repens comb. nov. | CBS 233.82 | Chaetomium repens | Soil; Tarragona, Spain; isotype of Chaetomium repens | MK919282 | MK919282 | MK919396 | MK919338 |
| Arxotrichum sinense comb. nov. | CBS 541.83 | Chaetomium sinense | Soil; China | MK919283 | MK919283 | MK919397 | MK919339 |
| Arxotrichum succineum | CBS 166.52 = ATCC 11216 = MUCL 18704 | Chaetomium succineum | Abies magnifica var. shastensis; California, USA; type of Chaetomium succineum | MK919284 | MK919284 | MK919398 | MK919340 |
| CBS 813.73 = DAOM 24174 = IMI 044210 | Abies magnifica var. shastensis; California, USA | MK919285 | MK919285 | MK919399 | MK919341 | ||
| CBS 119769 | Chaetomium succineum | Soil; Xinjiang, China | MK919286 | MK919286 | MK919400 | MK919342 | |
| Bommerella trigonospora | CBS 324.69 | Soil; Tokyo, Japan | MZ351419 | MZ343022 | MZ342984 | ||
| Botryoderma lateritium | CBS 586.66 = ATCC 18926 = IMI 158956 = MUCL 8790 | Soil mixed with leaf litter; Transvaal, South Africa | MK919287 | MK919287 | MK919401 | MK919343 | |
| Botryoderma rostratum | CBS 184.68 = ATCC 18927 = IMI 158957 | Sandy soil; Maranhâo, Brazil; type of Botryoderma rostratum | MK919288 | MK919288 | MK919402 | MK919344 | |
| Botryotrichum geniculatum sp. nov | CBS 144475 = WXW8287 | Soil; Xinjiang, China | MZ334719 | MZ351422 | MZ343011 | MZ342972 | |
| WXW8266 | Soil; Xinjiang, China | MZ334720 | MZ351423 | MZ343039 | MZ343000 | ||
| Botryotrichum inquinatum comb. nov. | CBS 155.80 | Corynascella inquinata | Sewage sludge; Nagasaki Pref., Japan; type of Corynascella inquinata | MK919289 | MK919289 | MK919403 | MK919345 |
| CBS 646.74 | Thielavia hyalocarpa | Desert soil; Egypt | MK919290 | MK919290 | MK919404 | MK919346 | |
| Botryotrichum retardatum | CBS 197.84 | Chaetomium retardatum | Dung of herbivore; Lake Amboseli, Kenya | – | – | MZ343019 | MZ342980 |
| Botryotrichum trichorobustum | CBS 563.67 | Chaetomidium trichorobustum | Dung of rabbit; near Hamburg, Germany | – | MZ351420 | MZ343027 | MZ342988 |
| Botryotrichum vitellinum | CBS 180.84 | Chaetomium vitellinum | Soil of field; Turkey | MZ334725 | MZ351421 | MZ343018 | MZ342979 |
| Chaetomium nepalense | CBS 288.83 | Soil; Godawari; Nepal | MH861591 | MH873316 | MZ342983 | ||
| Chaetomium tarraconense | CBS 101882 = FMR 6638 = IMI 380425 = MUCL 43149 | Soil; Tarragona, Spain; type of Chaetomium tarraconensis | – | – | MZ343005 | MZ342964 | |
| Collariella anguipilia | CBS 632.83 | Chaetomium anguipilium | Dung of rabbit; New Mexico, USA; type of Chaetomium anguipilium | MZ334721 | MZ351424 | MZ343028 | MZ342989 |
| Collariella hexagonospora | CBS 171.84 = FMR 7235 | Chaetomium hexagonosporum | Dung of pack rat; Nevada, USA; type of Chaetomium hexagonosporum | MH861717 | MZ343016 | MZ342977 | |
| Collariella pachypodioides | CBS 164.52 = ATCC 11213 = IMI 012266 = IMI 287299 = MUCL 9586 | Chaetomium pachypodioides | Vegetable detritus; Tennessee, USA; type of Chaetomium pachypodioides | MH856980 | MH868500 | MZ343014 | MZ342975 |
| Corynascella humicola | CBS 337.72 | Soil; North Carolina, Piedmont, USA; type of Corynascella humicola | KX976656 | KX976751 | KX976998 | MK942091 | |
| CBS 379.74 | Soil; North Carolina, Piedmont, USA; type of Corynascella humicola | KX976657 | KX976752 | KX976999 | MK942092 | ||
| Corynascus fumimontanus | CBS 137294 = FMR 12372 | Forest soil; Tennessee, USA; type of Corynascus fumimontanus | LK932694 | MK919291 | MK919405 | MK919347 | |
| Corynascus novoguineensis | CBS 359.72 | Myceliophthora novoguineensis | Soil; New Britain, Rabaul, Papua New Guinea; type of Thielavia novoguineensis | HQ871762 | MK919292 | MK919406 | MK919348 |
| Corynascus sepedonium | CBS 111.69 = IMI 136625 | Myceliophthora sepedonium | Soil; Allahabad, India; type of Thielavia sepedonium var. minor | HQ871751 | KX976777 | KX977027 | MK919349 |
| CBS 632.67 | Corynascus similis | Soil; Uzbekistan; type of Thielavia lutescens | HQ871759 | MK919293 | MK919407 | MK919350 | |
| CBS 101936 = FMR 5693 | Corynascus similis | Soil; Ajmed, India | MK919294 | MK919294 | MK919408 | MK919351 | |
| Corynascus sexualis | CBS 827.96 = FMR 5691 | Soil; Jaipur, India | AJ224202 | MK919295 | MK919409 | MK919352 | |
| Corynascus verrucosus | CBS 602.97 = IMI 378522 = FMR 5904 | Soil; Quilmes, Argentina | AJ224203 | MK919296 | MK919410 | MK919353 | |
| CBS 135878 = FMR 12783 | Forest soil; Tennessee, USA | MK919297 | MK919297 | MK919411 | MK919354 | ||
| Humicola hirsuta sp. nov. | CBS 144492 = WXW 9028 | Soil; Sanxi, China | MZ334726 | MZ351425 | MZ343013 | MZ342974 | |
| Melanocarpillus thermophilus | CBS 886.97 = FMR 6190 (representative) | Thielavia minuta var. thermophila/Melanocarpus thermophilus | Soil; Agra, India | KM655350 | MH874288 | MZ343037 | xKM655434 |
| CBS 379.76 = ATCC 14741 = IMI 086454 | Usar soil; Lucknow, Uttar Pradesh, India; type of Sporotrichum carthusioviride | MK919302 | MK919302 | MK919416 | MK919359 | ||
| Ovatospora amygdalispora | CBS 672.82 = IMI 291735 | Chaetomium amygdalisporum | Soil; Japan; type of Chaetomium amygdalisporum | – | – | MZ343030 | MZ342991 |
| Parachaetomium biporatum comb. nov. | CBS 244.86 = FMR 854 = IMI 330348 | Chaetomium biporatum | Soil; Valencia, Spain; type of Chaetomium biporatum | MK919303 | MK919303 | MK919417 | MK919360 |
| Parachaetomium carinthiacum | CBS 153.81 | Chaetomium carinthiacum | Unknown substrate; Meylan, France | MK919298 | MK919298 | MK919412 | MK919355 |
| CBS 665.82 | Chaetomium carinthiacum | Thymus sp.; Japan | MK919299 | MK919299 | MK919413 | MK919356 | |
| Parachaetomium hispanicum comb. nov. | CBS 234.82 | Chaetomium hispanicum | Dung; Tarragona, Spain; type of Chaetomium hispanicum | MK919304 | MK919304 | MK919418 | MK919361 |
| CBS 550.83 = FMR 502 | Chaetomium hispanicum | Soil; Reus, Spain | MK919305 | MK919305 | MK919419 | MK919362 | |
| Parachaetomium inaequalis comb. nov. | CBS 331.75 = IMI 196527 | Corynascella inaequalis | Soil of oak forest; Kirovograd, Ukraine; type of Thielavia inaequalis | MK919306 | MK919306 | MK919420 | MK919363 |
| CBS 164.75 | Corynascella inaequalis | Soil; Kirovograd, Ukraine | MK919307 | MK919307 | MK919421 | MK919364 | |
| Parachaetomium mareoticum comb. nov. | CBS 802.83 | Chaetomium mareoticum | Dung; Moledet, Israel | MZ334723 | MZ351426 | MZ343036 | MZ342997 |
| CBS 781.71 | Dung of gazelle; Israel | MZ343032 | MZ342993 | ||||
| Dimorphopilus muelleri comb. nov. | CBS 192.84 | Chaetomium muelleri | Decayed twig; Lahore, Pakistan; type of Chaetomium muelleri | MK919300 | MK919300 | MK919414 | MK919357 |
| CBS 663.75 | Chaetomium muelleri | Unknown substrate; Bornova-Izmir, Turkey | MK919301 | MK919301 | MK919415 | MK919358 | |
| Parachaetomium multispirale comb. nov. | CBS 172.84 = TRTC 66609 | Chaetomium multispirale | Dung of herbivore; Mt. Kenya, Kenya; type of Chaetomium multispirale | MH861718 | – | MZ343017 | MZ342978 |
| Parachaetomium perlucidum comb. nov. | CBS 141.58 = IMI 074954 = MUCL 18693 = MUCL 39399 | Chaetomium perlucidum | Dead herbaceous stem; Kiev, Ukraine; type of Chaetomium perlucidum | MK919308 | MK919308 | MK919422 | MK919365 |
| CBS 119762 = AS 3.9405 | Chaetomium raii | Soil; Xinjiang, China | MK919309 | MK919309 | MK919423 | MK919366 | |
| CBS 126670 | Chaetomium iranianum | Leaf of Hordeum vulgare; East Azerbaijan Prov., Iran; type of Chaetomium iranianum | MK919310 | MK919310 | MK919424 | MK919367 | |
| CBS 127795 | Chaetomium perlucidum | Soil; Wyoming, USA | MK919311 | MK919311 | MK919425 | MK919368 | |
| Parachaetomium subspirilliferum comb. nov. | CBS 150.60 = ATCC 14534 = IMI 081771 = MUCL 18698 | Chaetomium subspirilliferum | Soil; Kulundinskaya steppe, Altai, Russia; type of Chaetomium subspirilliferum | MK919312 | MK919312 | MK919426 | MK919369 |
| WXW 9901-2 | Chaetomium subspirilliferum | Soil; Xinjiang, China | MK919313 | MK919313 | MK919427 | MK919370 | |
| Parathielavia coactilis comb. nov. | CBS 101190 (representative) | Thielavia coactilis | Bark of lower branches of Atraphaxis replicata; Mangyschlak Peninsula, near Mt. Kunabai, Kazakhstan | – | – | MZ343003 | MZ342962 |
| CBS 101463 | Dead leaves of Carpinus betulus; Ile de France, France | – | – | MZ343004 | MZ342963 | ||
| Subramaniula fusispora | CBS 166.61 | Soil, red-brown earth; Adelaide, South Australia, Australia; type of Chaetomium fusisporum | MH858011 | MH869571 | MZ343015 | MZ342976 | |
| Subramaniula lateralis comb. nov. | CGMCC 3.17547 | Chaetomium laterale | Leymus chinensis, Inner Mongolia, China | KP336789 | KP336838 | KP336887 | MZ342998 |
| Subramaniula latifusispora sp. nov. | CGMCC 20442 = WXW 8538 | Sheep dung; Xinjiang, China | MZ334728 | MZ351428 | MZ343040 | MZ343001 | |
| WXW 8577 | Fallen spruce fruit; Xinjiang, China | MZ334729 | MZ351427 | MZ343041 | MZ343002 | ||
| Tengochaeta nigropilosa gen. et sp. nov. | CBS 639.83 | Soil from Pinus forest; Tenerife, Spain | MZ334730 | MZ343029 | MZ342990 | ||
| Thermocarpusella australiensis gen. et comb. nov. | CBS 493.74 = ATCC 28236 = DAOM 145919 | Thielavia australiensis | Nesting material of incubator bird; Pulletop Nature Reserve near Griffith, New South Wales, Australia; type of Thielavia australiensis | KM655339 | KM655378 | MZ343024 | KM655419 |
| Thermochaetoides dissita gen. et comb. nov. | CBS 180.67 = ATCC 16452 = IMI 126332 | Chaetomium thermophilum var. dissitum | Straw of Typha; California, USA; type of Chaetomium thermophilum var. dissitum | MK919319 | MK919319 | MK919433 | MK919375 |
| CBS 246.90 | Chaetomium thermophilum var. dissitum | Dung of pig with sawdust; Netherlands | MK919320 | MK919320 | MK919434 | MK919376 | |
| CBS 785.71 | Chaetomium thermophilum var. dissitum | Dung of gazelle; Israel | MK919321 | MK919321 | MK919435 | MK919377 | |
| Thermochaetoides thermophila comb. nov. | CBS 144.50 | Chaetomium thermophilum var. thermophilum | Decaying wheat straw; Leeds, UK; type of Chaetomium thermophilum var. thermophilum | MK919314 | MK919314 | MK919428 | KM655436 |
| CBS 143.50 | Chaetomium thermophilum var. thermophilum | Decaying wheat straw; Leeds, UK | MK919315 | MK919315 | MK919429 | MK919371 | |
| CBS 166.62 | Chaetomium thermophilum var. thermophilum | Mushroom compost; Netherlands | MK919316 | MK919316 | MK919430 | MK919372 | |
| CBS 141.64 | Chaetomium thermophilum var. thermophilum | Mushroom compost; Zürich, Switzerland | MK919317 | MK919317 | MK919431 | MK919373 | |
| CBS 179.67 = ATCC 16451= IMI 126331 | Chaetomium thermophilum var. coprophilum | Horse dung; California, USA; type of Chaetomium thermophilum var. coprophilum | MK919318 | MK919318 | MK919432 | MK919374 | |
| Thermothelomyces fergusii nom. nov. | CBS 406.69 = ATCC 22067 | Crassicarpon thermophilum | Mushroom compost, Pennsylvania, USA; type of Thielavia thermophila | HQ871794 | KX976776 | KX977024 | MK919378 |
| CBS 174.70 = IMI 145136 | Myceliophthora fergusii | Wheat straw compost; Cambridge, England | MK919322 | MK919322 | MK919436 | MK919379 | |
| Thermothelomyces guttulatus | CGMCC 3.15185 | Soil; Hunan, China | KC352943 | MK919323 | MK919437 | MK919380 | |
| CGMCC 3.15186 | Soil; Hunan, China | KC352944 | MK919324 | MK919438 | MK919381 | ||
| Thermothelomyces heterothallicus | CBS 203.75 | Soil; Indiana, USA; authentic strain of Thielavia heterothallica | HQ871772 | MK919325 | MK919439 | MK919382 | |
| CGMCC 3.13596 | Soil; USA | MK919326 | MK919326 | MK919440 | MK919383 | ||
| Thermothelomyces hinnuleus | CBS 597.83 | Cultivated soil; Japan; type of Myceliophthora hinnulea | HQ871791 | MK919327 | MK919441 | MK919384 | |
| CBS 544.82 | Soil; Christchurch, New Zealand | MK919328 | MK919328 | MK919442 | MK919385 | ||
| Thermothelomyces myriococcoides nom. nov. | CBS 389.93 = ATCC 22112 = CBS 736.70 | Myriococcum thermophilum | Surface of heated compost; Switzerland; type of Papulaspora thermophila | MK919329 | MK919329 | MK919443 | MK919386 |
| CBS 208.89 | Myriococcum thermophilum | Self-heating horse manure; Netherlands | MK919330 | MK919330 | MK919444 | KM655394 | |
| Thermothelomyces thermophilus | CBS 117.65 | Dry pasture soil; England; isotype of Sporotrichum thermophilum | HQ871764 | MK919331 | MK919445 | MK919387 | |
| CBS 669.85 | Mutant of CBS 866.85; USA | HQ871767 | KX976778 | KX977028 | MK919388 | ||
| CBS 381.97 | Man; unknown location | HQ871766 | KX976779 | KX977029 | MK919389 | ||
| Trichocladium tomentosum sp. nov. | CBS 144476 = WXW 8615 | Soil; Qinghai, China | MZ334732 | MZ351431 | MZ343012 | MZ342973 | |
| Xanthiomyces spinosum gen. et comb. nov. (representative) | CBS 789.71 | Chaetomium spinosum | Culture of algae; Zürich, Switzerland | MH860357 | MZ351429 | MZ343034 | MZ342995 |
| CBS 796.83 | Straw; Bloney, Switzerland | MZ334724 | MZ351430 | MZ343035 | MZ342996 | ||
| CBS 236.80 | Chaetomium microascoides | Soil; Spain | MH861259 | MH873028 | MZ343020 | MZ342981 | |
1 Sequences generated in this study are indicated in bold.
Morphology
The methods and media used for the morphological analysis are described in the “suggested methods” section above. Mature ascomata (top or side view) or a part of the colony were photographed using a Nikon SMZ25 stereo microscope. Focused images were obtained by z-stacking using the software Nikon NIS-Element D. Microscopic photographs were taken using a Zeiss AxioImager.2A microscope with a Nikon DS-Ri2 camera, or sometimes using Nikon Eclipse 80i microscope with a Nikon Digital Sight DS-Fi1 camera, both equipped with differential interference contrast (DIC) illumination. Ascospores with clear germ pore(s) were selected from the originally taken photos to get composite images using the “Healing Brush Tool” of Adobe Photoshop.
DNA isolation, sequencing and phylogenetic analyses
Genomic DNA was extracted from fungal mycelium grown on oatmeal agar (OA, Samson et al. 2019) using the DNeasy® UltraClean® Microbial Kit (Qiagen, Germany) following the manufacturer’s instructions. The internal transcribed spacer 1 and 2 including the intervening 5.8S nrDNA (ITS), the D1/D2 domains of the 28S nrDNA (LSU), partial RNA polymerase II second largest subunit gene (rpb2) and partial β-tubulin gene (tub2) were selected for phylogenetic inference. The PCR conditions, primers used for PCR amplification and sequencing were the same as those described by Wang et al. (2019a). Each amplicon was sequenced in both directions using the same set of primers. A consensus sequence for each locus was assembled in MEGA v. 6 (Tamura et al. 2013).
Novel sequences generated in this study were deposited in GenBank (http://www.ncbi.nlm.nih.gov, Table 2) and these datasets were merged with reference sequences obtained from previous studies (Wang et al. 2016a, b, 2019a, b) or retrieved from GenBank (see list of accepted species below and Supplementary Table S2). Alignments and treefiles are available in Figshare: https://Figshare.com/s/d251b9512f9d77522ef7. Phylogenetic analyses were based on Bayesian inference (BI) and Maximum Likelihood (ML) as described previously (Wang et al. 2019b). For BI, the best evolutionary model for each locus was determined using MrModeltest v. 2.0 (Nylander 2004). Obtained trees were viewed in FigTree v. 1.1.2 (Rambaut 2009) and subsequently visually prepared and edited in Adobe® Illustrator® CS6.
Divergence time estimation within Chaetomiaceae
Divergence time analysis was introduced to evaluate the phylogenetically-delimited genera in Chaetomiaceae. Five calibration points were selected (Samarakoon et al. 2019) (Table 3). Bayesian molecular-clock dating analysis was carried out using BEAST v. 2.6.3 (Bouckaert et al. 2019) with the concatenated rpb2, tub2, ITS and LSU sequence dataset including all genera and representative species of Chaetomiaceae as well as reference taxa. The reference sequences were retrieved from GenBank and listed in Supplementary Table S2. The introns in the protein coding genes and ITS1, ITS2 fragments in ITS locus were excluded to avoid an uncertain or dubious estimate.
Table 3.
Overview of selected calibration points (Samarakoon et al. 2019) in this study.
| No. | Crown calibrating point | Fossil taxa | Minimum age (Ma) |
|---|---|---|---|
| 1 | Pezizomycotina | Paleopyrenomycites devonicus | 410 |
| 2 | Diaporthales | Spataporthe taylorii | 136 |
| 3 | Ophiocordyceps | Paleoophiocordyceps coccophagus | 99 |
| 4 | Colletotrichum | Protocolletotrichum deccanensis | 61 |
| 5 | Aspergillus | Aspergillus collembolorum | 35 |
The GTR substitution model was assigned for each gene with a gamma distribution accounting for rate variation among sites. An uncorrelated lognormal relaxed-clock model was applied to the four genes together with a uniform (10–6,1) hyperprior for the mean rate. Following a recent work on divergence time calibrations for ancient lineages of Ascomycota based on reliable fossil data (Samarakoon et al. 2019), the following five calibrations were used with priors: a uniform (35,55) distribution for Aspergillus, a uniform (61.6,72.3) distribution for Colletotrichum, a uniform (136,188) distribution for Diaporthales, a uniform (98.17,99.41) distribution for Ophiocordyceps, and an offset-exponential distribution with a mean 10 million years ago (Mya) and an offset 410 Mya for Pezizomycotina. Using the Yule process (Yule 1925) with a gamma (0.001,1000) distribution for the speciation rate, we performed two independent runs of Markov chain Monte Carlo (MCMC) sampling, with samples drawn every 10 000 steps over 100 million steps, discarding the first 25 %. After the convergence was checked based on the combined samples, the maximum-clade-credibility tree was identified among posteriors using TreeAnnotator v. 2.6.0.
RESULTS
Phylogeny
Phylogenetic analyses were performed on the individual LSU, ITS, rpb2 and tub2 datasets and a combined dataset of all four loci. The LSU and ITS phylograms were poorly supported. Compared to the phylogram based on the combined dataset (Fig. 7, discussed below), 27 generic clades were supported (ML-BS > 80 %; PP = 1.00) or formed monotypic lineages in the ITS phylogram; the other recognised generic clades did not receive robust support or did not form monophyletic lineages (Supplementary Fig. S1). The LSU failed to resolve most of the recognised species and genera (data not shown). In the tub2 phylogeny, 45 of the 47 generic clades recognised in the combined phylogram were supported (ML-BS > 78 %; PP > 0.97) or formed monotypic lineages. Humicola was not statistical supported and Melanocarpus albomyces was distant from the other Melanocarpus species (Supplementary Fig. S2). In the rpb2 phylogeny, the Remersonia/Mycothermus clade nested on a long branch inside Staphylotrichum (Supplementary Fig. S3). The position of the Remersonia and Mycothermus together is questionable (rpb2) or lacks support (LSU, ITS, tub2, combined) in the single gene and the combined phylograms (Fig. 7C, Supplementary Figs S1–S3). Furthermore, the Chaetomium, Humicola and Melanocarpus clades did not receive statistical support in the rpb2 phylogram (Supplementary Fig. S3). No topological conflicts were observed when the 70 % bootstrap reciprocal tree topologies based on the single datasets were compared (Supplementary Figs S1–S3). Therefore, all four loci were combined to reveal the generic relationships in the family following the argument of Cunningham (1997) that combining incongruent partitions could increase phylogenetic accuracy.
The concatenated dataset of LSU, ITS, rpb2 and tub2 contains sequences of 404 strains and includes representatives of all genera and most accepted species of Chaetomiaceae. Exceptions are species that are only known by their ITS and/or LSU sequence(s), e.g., several Acrophialophora species and Humicola koreana, as well as Chaetomium iranicum, Collariella capillicompacta, Trichocladium amorphum and Trichocladium nigrospermum. Furthermore, representative species belonging to Lasiosphaeriaceae sensu lato, Podosporaceae and Sordariaceae were included, and Microascus trigonosporus CBS 218.31 (Microascales) was selected as the outgroup. The alignment contained 3 622 characters (including gaps) and was composed of four partitions: 883 characters for rpb2, 1 354 characters for tub2, 798 characters for ITS and 587 characters for the D1/D2 regions of LSU. Of these, 1 352 characters were constant, 1 980 were parsimony-informative, and 290 were parsimony-uninformative. For the Bayesian inference, GTR+I+G was the most optimal model for all four partitions. The result of the phylogenetic analysis is shown in Fig. 7. Forty-seven monophyletic lineages are recognised in the Chaetomiaceae, each corresponding to a previously defined genus or a potential new genus, which were all highly supported (ML-BS ≥ 92 %; PP = 1.00). The only exceptions were the Melanocarpus lineage (ML-BS < 70 %; PP = 1.00, Fig. 7D) and Humicola lineage (ML-BS = 78 %; PP = 1.00, Fig. 7B). The fifteen thermophilic species grouped into seven genus-level clades (Fig. 7, highlighted in orange blocks). The Thermothelomyces clade (ML-BS = 100 %; PP = 1.00) was confirmed as closely related to the four non-thermophilic genera Arxotrichum, Botryoderma, Corynascus and Myceliophthora (ML-BS = 100 %; PP = 1.00, Fig. 7A). Mycothermus and Remersonia were confirmed as sister genera (ML-BS = 100 %; PP = 1.00, Fig. 7C). Thermothielavioides was closely related to the non-thermophilic genus Floropilus (ML-BS = 97 %; PP = 1.00), and these two genera clustered close to but separate from the non-thermophilic genus Chrysanthotrichum (ML-BS = 95 %; PP = 1.00, Fig. 7C). The Chaetomium thermophilum clade (ML-BS = 100 %; PP = 1.00, Fig. 7D) consists of two species clades, with no statistically-supported close relatives. The Thielavia australiensis clade is sister to the genus Carteria (ML-BS = 100 %; PP = 1.00, Fig. 7D).
To delimit species boundaries using the gene concordance phylogenetic species concept (GCPSR), the phylogenies based on ITS (if data available), tub2 and rpb2 sequences are compared (see Supplementary Figs S1–S3) and discussed in the notes of the relevant species in the taxonomy section below. The LSU phylogeny failed to resolve most of the recognised species and genera (data not shown) and is therefore not discussed.
Divergence time estimation (Figs 8, 9)
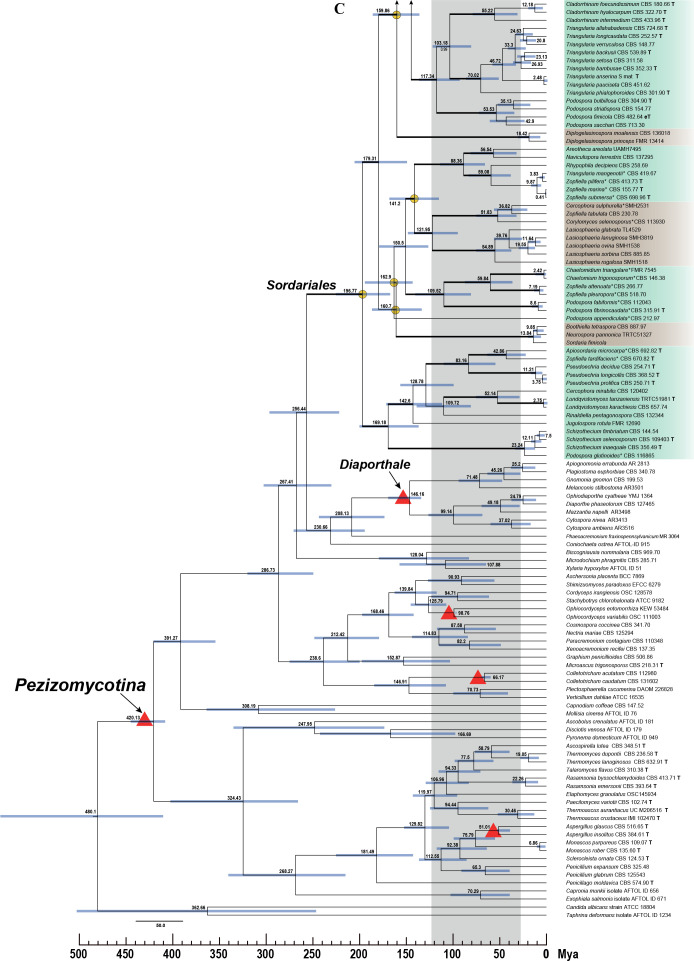
Fig. 8.
Maximum clade credibility tree of Sordariales based on rpb2, tub2, ITS and LSU sequences. Blue bars around each internode correspond to 95 % divergence time confidence intervals for each branch. For reference, the time scale is shown right below the phylogenetic tree. Different genera are depicted using different-coloured blocks. Dating estimates were calibrated using five constraints marked by red triangles. Mean divergence times of genera in Chaetomiaceae are marked in red dots and those of families in Sordariales marked in yellow dots. The robust confidence values (posterior probabilities ≥ 0.95) for genera or higher clades of Sordariales indicated at the notes and the branches with full statistical support (PP = 1.0) are highlighted by thickened branches. The red stars at the right of species names highlight the thermophilic species.
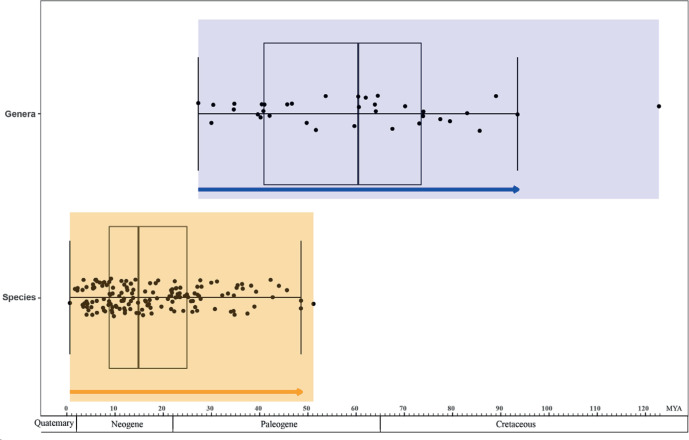
Fig. 9.
Comparison of divergence times between species (yellow block) and genera (blue block) in Chaetomiaceae.
Three hundred and eighteen taxa were selected for dating analysis, containing 204 representative species of Chaetomiaceae, 16 Podosporaceae species, 38 Lasiosphaeriaceae sensu lato species and three Sordariaceae species (all in the Sordariales), together with 55 Pezizomycotina species that included five calibrating points (Table 3, Fig. 8). Taphrina deformans and Candida albicans were used as outgroups. Divergence time of genera (blue) and species (yellow) in Chaetomiaceae are shown in Fig. 9. The Turkey`s test reveals two outliers: 51.15 Mya in the species boxplot and 122.79 Mya in the genus boxplot. After removing these two outliers, the divergence times of the species range from 0.64 Mya to 48.57 Mya and those of the genera range from 27.26 Mya to 93.47 Mya. The molecular dating analysis indicated that all the previously defined genera in the Chaetomiaceae diverged from about 27 Mya (Chrysocorona) to 123 Mya (Condenascus) and that these generic clades are all fully supported (PP = 1.0, highlighted by thickened branches in Fig. 8), with the Humicola and Melanocarpus clades being the only exception. The previously phylogenetically-defined Humicola lineage appeared to be polyphyletic, with two subclades estimated to diverge from each other about 60 Mya, with one of them closer to Aporothielavia, having diverged from the latter about 52 Mya. Two subclades within the Melanocarpus lineage (PP < 0.9) diverged from each other about 60 Mya. The other thermophilic lineages diverged from their non-thermophilic neighbours at least 30 Mya.
TAXONOMY
Six genera, Parvomelanocarpus, Pseudohumicola, Tengochaeta, Thermocarpiscus, Thermochaetoides and Xanthiomyces, are newly proposed based on molecular dating and multi-gene phylogenetic analyses (Figs 7, 8) in combination with (shared) morphological characters and/or ecological features. The genus Botryoderma is confidentially positioned in the Chaetomiaceae and Achaetomiella, Aporothielavia and Bommerella are resurrected and redefined or redescribed. The generic concepts of Collariella and Humicola are emended because of the introduction of Achaetomiella and Pseudohumicola. Allocanariomyces, Amesia, Arcopilus, Arxotrichum, Botryotrichum, Chaetomium, Ovatospora, Parachaetomium, Parathielavia, Staphylotrichum, Subramaniula and Thermothelomyces are expanded with new combinations and/or new species. Among them, four genera (Arxotrichum, Botryotrichum, Parachaetomium and Staphylotrichum) are redefined. In the current concept, Corynascella and Melanocarpus are restricted to their type species. Six new species belonging to six different genera (Botryotrichum geniculatum, Chaetomium subaffine, Humicola hirsuta, Subramaniula latifusispora, Tengochaeta nigropilosa and Trichocladium tomentosum) are introduced. The delimitation of Corynascus and Myceliophthora by Marin-Felix et al. (2015) is confirmed. In total, 50 genera and 275 species are accepted in the Chaetomiaceae, while “Chaetomium microascoides” and “Chaetomidium triangulare” proved to be members of Lasiosphaeriaceae sensu lato, distant from the Chaetomiaceae.
Arxotrichum, Botryoderma, Corynascus, Myceliophthora, Parachaetomium and Thermothelomyces are studied here in more detail. These genera include thermophilic species or species phylogenetically related to them. Together with Dichotomopilus (Wang et al. 2016b), these seven genera share a common ancestor (ML-BS = 70 %; PP = 1.00, Fig. 7A). New combinations are mainly based on the results of the phylogenetic analyses (Fig. 7, Supplementary Figs S1–S3). A number of new species combinations is fully illustrated and described, as examples for a genus.
Achaetomiella Arx, The genera of fungi sporulating in pure culture: 247. 1970. Fig. 10.
Fig. 10.
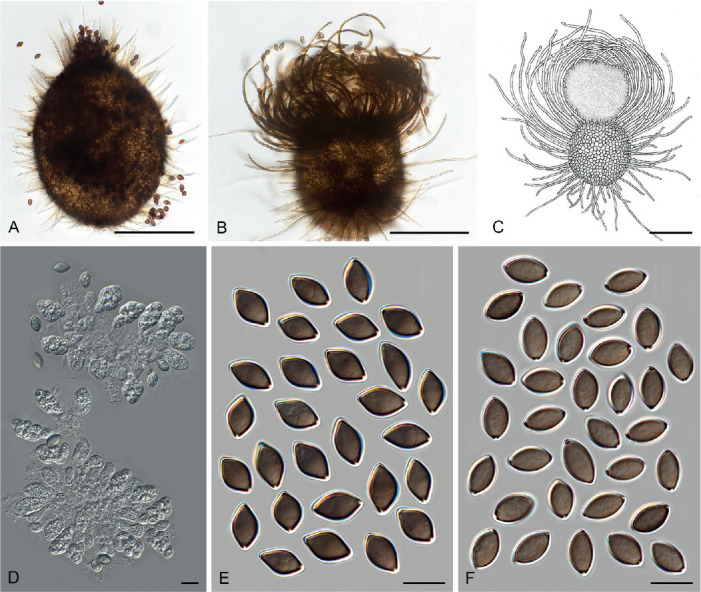
Morphology of Achaetomiella. Ascomata (A–C): A. Ach. virescens (CBS 148.68T). B. Ach. gracilis (CBS 249.75). C. Line drawing of Ach. gracilis (CGMCC 3.3782). D. Asci of Ach. gracilis (CBS 249.75). Ascospores (E, F): E. Ach. virescens (CBS 148.68 T). F. Ach. gracilis (CBS 249.75). Scale bars: A–C = 100 μm; D–F =10 μm.
Micromorphology: Ascomata superficial, ostiolate, subglobose or ovate, with brown walls of textura angularis in surface view. Terminal hairs straight, flexuous, undulate or arcuate. Lateral hairs straight, flexuous. Asci fasciculate, clavate, fusiform or obovate, with eight irregularly-arranged ascospores, evanescent. Ascospores brown when mature, ellipsoidal or fusiform, with an apical or sometimes slightly sub-apical germ pore, usually more than 9 μm in length. Asexual morph not observed.
Type species: Achaetomiella virescens Arx
Notes: Achaetomiella, typified by Ach. virescens, was introduced by von Arx (1970) as an intermediate between Achaetomium and Chaetomium. This genus was characterised by the production of simple ascomatal hairs that are evenly distributed over the ascoma. Udagawa (1980) transferred Ach. virescens to Chaetomium and this was accepted by Cannon (1986) and von Arx et al. (1986). Based on a multigene phylogenetic analysis (Wang et al. 2016b), Ach. virescens was transferred to Collariella. At that time, two morphologically distinct groups were observed within the genus. Molecular dating analysis indicated that these two groups diverged from each other as early as about 42 Mya (Fig. 8A). Group I includes the type species of Collariella and is redefined here as Collariella sensu stricto (Fig. 10), and Achaetomiella is resurrected to accommodate taxa belonging to group II (Fig. 22). Collariella and Achaetomiella are sister genera (Fig. 7C, Supplementary Figs S2, S3) and the generic concept of Collariella is redefined below. Collariella is characterised by 1) ascomata that usually have a darkened collar around the ostiolar pore, 2) broadly limoniform to quadrangular, bilaterally flattened ascospores with an apical germ pore, 3) ascospores length usually less than 7.5 μm, with Col. hexagonospora (9–10.5 μm long) being the only exception (see also notes of Collariella sensu stricto below). In contrast, Achaetomiella species lack a darkened collar around the ostiolar pore of ascomata, and their ascospores can be ellipsoidal or fusiform, but never limoniform or quadrangular, and never bilaterally flattened. Two species are accepted in this genus, Achaetomiella gracilis and Achaetomiella virescens. Ascomatal hairs cannot be used as a diagnostic characteristic for Achaetomiella.
Fig. 22.
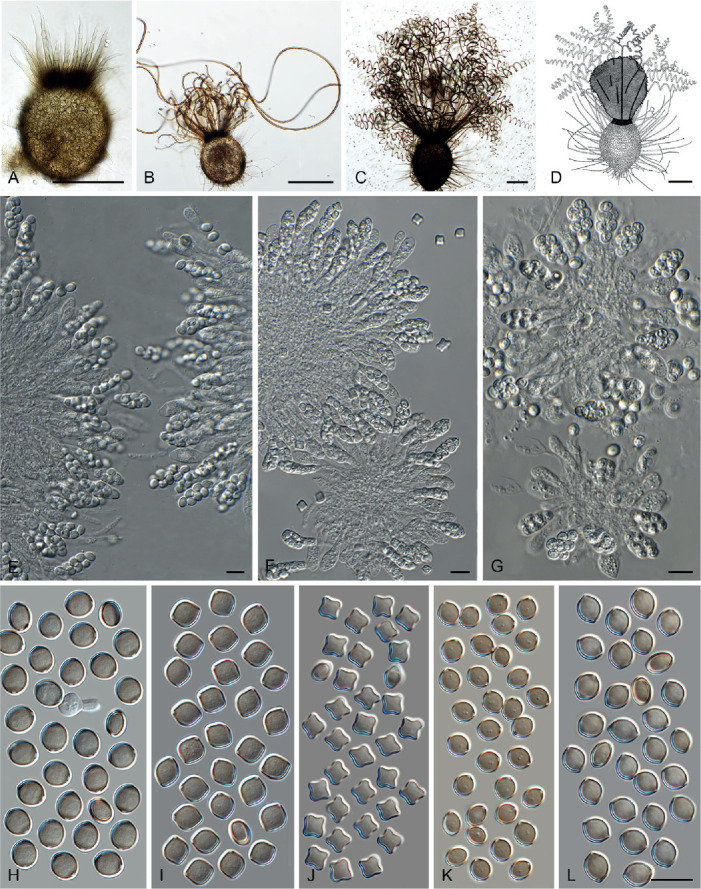
Morphology of Collariella. Ascomata (A–D): A. Collariella intricata (CBS 128.85T). B. Col. causiiformis (CBS 792.83T). C. Col. bostrychodes (DTO 326-H6). D. Line drawing of Col. bostrychodes (CGMCC 3.1054). Asci (E–G): E. Col. bostrychodes (DTO 326-H6). F. Col. quandrangulata (CBS 152.59). G. Col. causiiformis (CBS 792.83T). Ascospores (H–L): H. Col. bostrychodes (DTO 326-H6). I. Col. robusta (CBS 551.83T). J. Col. quandrangulata (CBS 152.59). K. Col. causiiformis (CBS 792.83T). L. Col. intricata (CBS 128.85T). Scale bars: A–D = 100 μm; E–G = 10 μm; L = 10 μm, also applied to H–K.
Achaetomiella gracilis (Udagawa) Houbraken, X.Wei Wang, P.J. Han & F.Y. Bai, comb. nov. MycoBank MB 840195. Fig. 10B–D, F.
Basionym: Chaetomium gracile Udagawa, J. Gen. Appl. Microbiol. 6: 235. 1960.
Synonym: Collariella gracilis (Udagawa) X.Wei Wang & Samson, Stud. Mycol. 84: 185. 2016.
Notes: This species can be distinguished from Ach. virescens by numerous arcuate ascomatal hairs surrounding the truncated apical ostiole (Fig. 10B, C), while the ascomata of Ach. virescens are tapered at the apex and covered by sparse, straight and short hairs (Fig. 10A).
Achaetomiella virescens Arx, The genera of fungi sporulating in pure culture: 247. 1970. Fig. 10A, E.
Synonyms: Chaetomium virescens (Arx) Udagawa, Trans. Mycol. Soc. Japan 21: 34. 1980.
Collariella virescens (Arx) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016.
Notes: According to the description of von Arx et al. (1986), this species produces ascospores with two apical germ pores, but the figure in their monograph (Plate 91D) shows ascospores with only one apical germ pore. Our observations confirmed that ascospores have one apical germ pore (Fig. 10E).
Allocanariomyces Mehrabi et al., Mycol. Prog. 19: 1417. 2020.
Synonym: Pseudocanariomyces Cañete-Gibas et al., Mycopathologia 186: 443. 2021.
Micromorphology and illustrations: See Mehrabi et al. (2020). Species producing both a sexual and asexual morph.
Type species: Allocanariomyces tritici Mehrabi, Asgari & Zare
Notes: Allocanariomyces was first proposed for a seed endophyte of Triticum boeoticum. This genus is morphologically similar to Canariomyces in its asexual (solitary and appressonium-like conidia laterally produced from hyphae) and sexual morph (producing non-ostiolate ascomata and ellipsoidal-fusiform ascospores with a subapical or apical germ pore), but is phylogenetically distinct (Mehrabi et al. 2020). Later, Ryan et al. (2021) proposed Pseudocanariomyces to accommodate strains isolated from a prosthetic hip infection of a 65-yr-old white woman and a human ear respectively. Our phylogenetic analysis showed that Pseudocanariomyces is a synonym of Allocanariomyces (Fig. 7D).
Allocanariomyces americanus (Cañete-Gibas et al.) Cañete-Gibas, Wiederhold, X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840154.
Basionym: Pseudocanariomyces americanus Cañete-Gibas et al., Mycopathologia 186: 443. 2021.
Notes: Two strains identified as Pseudocanariomyces americanus were reported in Ryan et al. (2021). The ex-type strain UTHSCSA DI20-139 (= CBS 147185), isolated from a prosthetic hip infection of a patient, represents a species belonging to Allocanariomyces and this combination is proposed here. The other strain (UTHSCSA DI20-140 = CBS 147186), isolated from a human ear, is phylogenetically different and is re-identified here as Allocanariomyces tritici (Fig. 7D). Allocanariomyces americanus produces smaller ascomata than Allocan. tritici (15–90 × 20–92.5 μm vs 100–130 μm diam), but has ascospores (12.5–25 × 8.75–15 μm vs 13–22.8 × 9–16 μm) and conidia (5–7.75 × 2.5–5 μm vs 3–9 × 3–4.5 μm) similar to those of Allocan. tritici in shapes and sizes (Mehrabi et al. 2020, Ryan et al. 2021).
Amesia X.Wei Wang et al., Stud. Mycol. 84: 156. 2016.
Micromorphology and illustrations: See Wang et al. (2016b; p. 156–163). Species producing only a sexual morph.
Type species: Amesia atrobrunnea (L.M. Ames) X.Wei Wang & Samson
Notes: The genus Amesia was proposed for four species that originally were described in Chaetomium (Wang et al. 2016b). These four species produce ostiolate ascomata, but the morphological diversity in ascomatal hairs and ascospores among species in the genus proved to be large. The ascomatal hairs of Amesia species can be straight, flexuous, undulate or spirally coiled, and ascopores can be fusiform, elongated fusiform, ovate, elongated ovate or pyriform, with an apical or sub-apical germ pore. Two more chaetomium-like species proved to be members of this genus based on our phylogenetic analysis (Fig. 7C). Both morphologically fit in the definition of Amesia (von Arx et al. 1986, Wang et al. 2016b).
Amesia dreyfussii (Arx) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840132.
Basionym: Chaetomium dreyfussii Arx, Beih. Nova Hedwigia 84: 6. 1986.
Notes: This species can be distinguished from other Amesia species by the production of seta-like terminal hairs surrounding the apical ostioles and elongated fusiform or pyriform ascospores with an apical germ pore at the relatively broad end (Fig. 4-AJ). Von Arx et al. (1986) incorrectly described the ascospores of this species with an apical germ pore at the most attenuated end; however, this does not match with what is shown in their supplied illustration (Plate 22D).
Amesia raii (G. Malhotra & Mukerji) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840137.
Basionym: Chaetomium raii G. Malhotra & Mukerji, Rev. Mycol. (Paris) 40: 182. 1976.
Notes: Amesia raii produces ascomata covered by undulate to spirally coiled terminal hairs and fusiform or elongated ovate ascospores with a subapical germ pore. As indicated by von Arx et al. (1986), this species is morphologically quite like Para. perlucidum (Fig. 37, see below), but phylogenetically distant (Fig. 7A, C). Amesia gelasinospora is phylogenetically related to Am. raii (Fig. 7C, Supplementary Fig. S3; ITS and tub2 sequences of Am. raii are not available) and produces ascospores with a subapical germ pore. The former species can be distinguished by the production of numerous and more regularly coiled ascomatal hairs, and by the shape of its ascospores that are ovate or broadly fusiform (Wang et al. 2016b).
Fig. 37.

Parachaetomium perlucidum (CBS 141.58, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 18 d incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D. Mature ascomata on OA, side view. E, F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: E, F = 100 μm; G–J = 10 μm.
Aporothielavia Malloch & Cain, Mycologia 65: 1074. 1973.
Micromorphology: Ascomata superficial, non-ostiolate, spherical, pilose. Ascomatal wall brown, consisting of cephalothecoid plates in surface view. Ascomatal hairs brown, slightly undulate, tapering towards the tips, smooth, sometimes absent. Asci pyriform to fusiform with eight irregularly-arranged ascospores. Ascospores olivaceous when mature, fusiform, with an apical or slightly subapical germ pore. Asexual morph produced as intercalary or terminal chlamydospores, solitary or catenulate, ellipsoidal to globose, brown, 1-celled, smooth-walled, lacking germ pores. Containing only one species with both asexual and sexual morphs.
Type species: Aporothielavia leptoderma (C. Booth) Malloch & Cain
Notes: Booth (1961) originally described the type species of Aporothielavia in Thielavia. In the original description it was mentioned that this species produces fusiform ascospores with an indistinct germ pore. However, Malloch & Cain (1973) re-examined the ex-type culture and noted that their strain produced ascospores without germ pores. Based on these observations, they introduced Aporothielavia to accommodate this species. Later, Greif & Currah (2007) transferred Apor. leptoderma to Chaetomidium because they observed an apical germ pore and noticed the species’ morphological similarity to Chaetomidium arxii (= Trichocladium arxii) (both have non-ostiolate ascomata with long hairs). Our examination confirmed the presence of a germ pore in the ascospores (Fig. 11J), but phylogenetic analysis showed that this species forms a unique clade in the family (Fig. 7B). The monotypic genus Aporothielavia is therefore resurrected and redefined.
Aporothielavia leptoderma (C. Booth) Malloch & Cain, Mycologia 65: 1074. 1973. Fig. 11.
Basionym: Thielavia leptoderma C. Booth [as ‘leptodermus’], Mycol. Pap. 83: 3. 1961.
Synonyms: Chaetomidium leptoderma (C. Booth) Greif & Currah, Mycol. Res. 111: 74. 2007.
Chaetomidium gallecicum Stchigel & Guarro [as ‘galaicum’], Stud. Mycol. 50: 217. 2004.
Micromorphology: Ascomata superficial or covered by aerial mycelium, solitary to loosely aggregated, non-ostiolate, leaden black when mature in reflected light due to the dark ascomatal wall, spherical, pilose, (100–)155–475 μm diam. Ascomatal wall brown, consisting of cephalothecoid plates which are composed of radially elongated cells in surface view. Ascomatal hairs brown, slightly undulate, tapering towards the tips, smooth, (2.5–)3–5 μm diam near the base, sometimes absent. Asci pyriform to fusiform, spore-bearing part 20–43 × 12–18 μm, with stalks 7–15 μm long, with eight irregularly-arranged ascospores, sometimes persistent till ascospores mature. Ascospores olivaceous when mature, fusiform, often inequilateral, (14–)14.5–16.5(–18) × (5–) 5.5–6.5(–7.5) μm, with an apical or slightly subapical germ pore. Asexual morph (fide Malloch & Cain 1973), formed as intercalary or terminal chlamydospores, solitary or catenulate, ellipsoidal to globose, brown, 1-celled, smooth-walled, 4–14 μm diam, lacking germ pores.
Culture characteristics: Colonies on OA with an entire edge, 51–57 mm diam in 7 d at 25 °C, with white aerial mycelium, without coloured exudates; reverse pale mouse grey. Colonies on CMA similar to those on OA, 45–51 mm diam in 7 d at 25 °C. Colonies on MEA with an entire edge, 52–58 mm diam in 7 d at 25 °C, with white aerial mycelium, texture floccose, obverse white; reverse ochreous to fulvous. Colonies on PCA with an entire edge, 45–51 mm diam in 7 d at 25 °C, with sparse aerial mycelium, obverse uncoloured, without coloured exudates, reverse uncoloured.
Material examined: UK, England, Surrey, Chobham, isolated from soil, 1953, G.W.F. Sewell (ex-type culture of Thielavia leptoderma, CBS 538.74 = IMI 054770). Spain, Galicia, Orense, Serra de Xurés, isolated from black spot on granite rock sample, 10 Nov. 2001, V. Jato & A.M. Stchigel (CBS 113678 = FMR 8192, ex-type of Chaetomidium gallecicum).
Notes: Based on the phylogenetic analyses, Chaetomidium gallecicum is a synonym of Apor. leptoderma (Fig. 7B, Supplementary Figs S2, S3; ITS sequences of the two strains are not available). Aporothielavia leptoderma can be easily recognised by the cephalothecoid ascomatal wall of non-ostiolate ascomata with long hairs (sometimes missing), pyriform to fusiform asci and elongated fusiform ascospores with an apical or slightly subapical germ pore.
Arcopilus X.Wei Wang et al., Stud. Mycol. 84: 159. 2016.
Micromorphology and illustrations: See Wang et al. (2016b; p.159, 165). Containing species with only sexual morph.
Type species: Arcopilus aureus (Chivers) X.Wei Wang & Samson
Notes: The genus Arcopilus was proposed based on phylogenetic analysis (Wang et al. 2016b) and species belonging to this genus produce arcuate ascomatal hairs, colourful colonies (due to its ascomata and exudates) and diverse ascospores that are more or less inequilateral with one or two apical germ pores. Three more chaetomium-like species proved to be members of this genus based on our phylogenetic analysis (Fig. 7C). Each of them morphologically fits in the definition of Arcopilus (Wang et al. 2016b).
Arcopilus macrostiolatus (Stchigel et al.) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840138.
Basionym: Chaetomium macrostiolatum Stchigel et al., Mycologia 94: 121. 2002.
Notes: Arcopilus macrostiolatus produces terminal hairs arcuate and recurved at the apex, fitting the general morphology of the genus. This species is phylogenetically most closely related to Ar. megasporus and Ar. purpurascens (Fig. 7C). Arcopilus macrostiolatus can be distinguished from these and other species in the genus by its limoniform, umbonate, bilaterally flattened ascospores that have an apical germ pore (Rodríguez et al. 2002). Arcopilus turgidopilosus also produces limoniform and bilaterally flattened ascospores, but can be distinguished by production of biapiculate or less umbonate ascospores with two apical germ pores (von Arx et al. 1986, Wang et al. 2016b).
Arcopilus megasporus (Sörgel ex Seth) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840139.
Basionym: Chaetomium megasporum Sörgel ex Seth, Beih. Nova Hedwigia 37: 82. 1972.
Notes: Arcopilus megasporus produces fusiform or navicular ascospores with two germ pores at the ends and red exudates on OA and/or CMA, fitting the overall morphology of the genus (von Arx et al. 1986). It can be distinguished from the other species in the genus by its ascomata covered by sparse, hypha-like flexuous hairs.
Arcopilus purpurascens (Udagawa & Y. Sugiy.) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840140.
Basionym: Achaetomium purpurascens Udagawa & Y. Sugiy., Rep. Cryptogam. Stud. Nepal: 13. 1982.
Synonym: Chaetomium purpurascens (Udagawa & Y. Sugiy.) Arx, Proc. Indian Acad. Sci., Pl. Sci. 94: 344. 1985.
Notes: Arcopilus purpurascens is morphologically similar to Ar. megasporus and was treated as a synonym of the latter by von Arx et al. (1986). The four-locus phylogeny as well as the tub2 and rpb2 phylograms show Ar. purpurascens is a distinct species, most closely related to Ar. megasporus (Fig. 7C, Supplementary Figs S2, S3).
Arxotrichum A. Nováková & M. Kolařik, Persoonia 40: 259. 2018.
Micromorphology: Containing asexual species, sexual species and species with both asexual and sexual morphs. Ascomata superficial, occasionally sub-immersed in the medium, ostiolate, ovoid, in some species possessing a short tapering beak fading towards the tip with a pale brown to subhyaline apex. Ascomatal wall brown, composed of irregular or angular cells. Ascomatal hairs pale to pale brown, finely verrucose, verrucose or punctulate, septate, in some species without differentiation between terminal and lateral ones, hypha-like, straight or flexuous, tapering and fading towards the tips; in other species terminal hairs spirally coiled, loosely coiled, undulate or flexuous, usually erect or flexuous at lower part, lateral hairs flexuous, shorter than terminal ones. Asci fasciculate, fusiform or clavate, stalked, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, ellipsoidal with attenuated or rounded ends, or fusiform, sometimes reniform or navicular, not bilaterally flattened, with an apical, subapical, oblique or lateral germ pore, or with two apical or slightly subapical germ pores, each at one end. Asexual morph present in four species. Conidiophores ramified, unbranched or reduced to conidiogenous cells. Conidiogenous cells developing at the ends of branches of conidiophores, or lateral or intercalary directly from the hyphae, monoblastic. Conidia 1-celled, smooth, verruculose or rugose, hyaline or pinkish coloured.
Type species: Arxotrichum wyomingense A. Nováková & M. Kolařik
Notes: Arxotrichum was recently proposed to accommodate an asexual species, Arx. wyomingense (the type species), and the sexual species Chaetomium succineum which was renamed Arx. succineum (Crous et al. 2018). The type species produces poorly differentiated conidiophores that resemble the micronematous ones of a Staphylotrichum species. Based on our four-gene phylogenetic analysis (Fig. 7A), six additional chaetomium-like species are transferred into the genus Arxotrichum. Several species in the genus, e.g., Arx. gangligerum, Arx. officinarum and Arx. piluliferoides, produce both sexual and asexual morphs. They link the asexual species Arx. wyomingense to the strictly sexually reproducing species in the genus.
Arxotrichum deceptivum (Malloch & Benny) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 830917. Fig. 12.
Fig. 12.
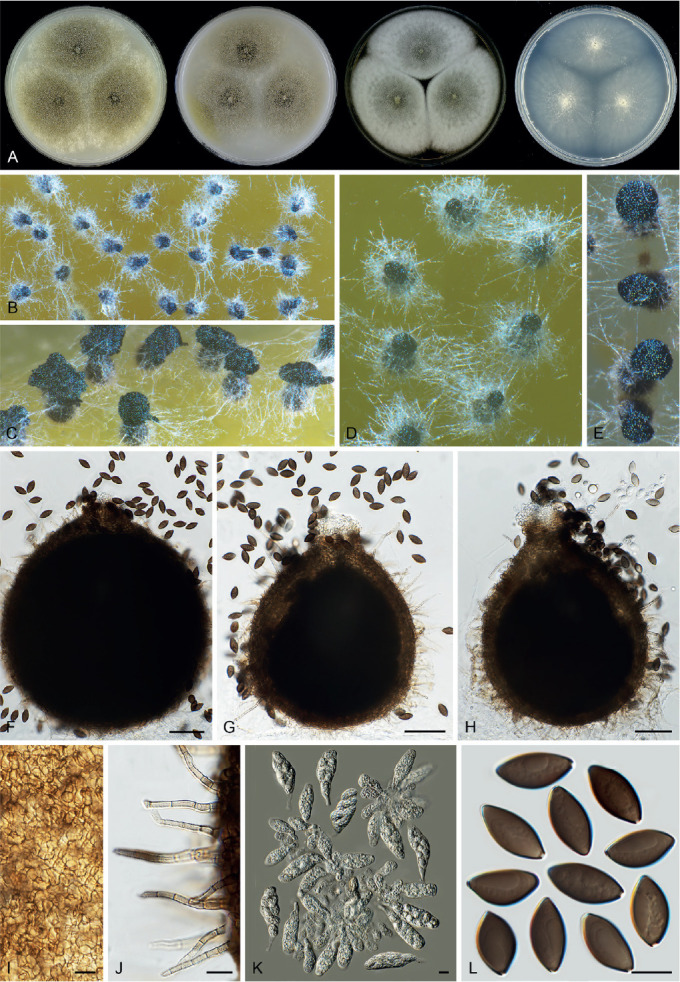
Arxotrichum deceptivum (CBS 346.73, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 2 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, side view. D, E. Mature ascomata on OA, top view. F–H. Ascomata mounted in lactic acid. I. Structure of ascomatal wall in surface view. J. Terminal ascomatal hairs. K. Asci. L. Ascospores. Scale bars: F–H = 50 μm; I–L = 10 μm.
Basionym: Chaetomium deceptivum Malloch & Benny, Mycologia 65: 648. 1973.
Micromorphology: Ascomata superficial, sometimes sub-immersed in the medium, leaden black to amber due to ascomata and masses of ascospores in reflected light, ostiolate, ovoid, with a short papillate beak fading towards the tip, usually with a hyaline apex, 190–335 μm high, 150–300 μm diam. Ascomatal wall brown, composed of irregular or angular cells. Ascomatal hairs pale brown, short, straight or flexuous, tapering and fading towards the tips, finely verrucose, septate, 2.5–3.5 μm diam near the base, usually less than 70 μm long. Asci fusiform, sometimes clavate, spore-bearing part 37–55 × 15–20.5 μm, with stalks being 7–16.5 μm long, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, fusiform or ellipsoidal with both ends attenuated, often inequilateral, occasionally navicular, (14.5–)16.5–19.5(–21.5) × (8–)8.5–9.5(–10) μm, with an apical or subapical germ pore. Asexual morph unknown.
Culture characteristics: On OA with an entire edge, 39–45 mm diam in 7 d at 25 °C, with sparse aerial mycelium, obverse olivaceous buff due to ascomata; reverse hazel. On CMA similar to those on OA. On MEA with an entire edge, 44–50 mm diam in 7 d at 25 °C, texture floccose, obverse white to smoke grey due to aerial mycelium mixed with ascomata, reverse ochreous, or mouse grey in the central part. On PCA with an entire edge, 42–48 mm diam in 7 d at 25 °C, without sparse aerial mycelium, obverse pale olivaceous grey, without coloured exudates; reverse uncoloured.
Material examined: USA, California, Riverside County, Lake Hemet, isolated from dung of pack rat, 10 Nov. 1968, coll. R.K. Benjamin, isol. C.L. Benny (culture ex-type CBS 346.73 = RSA 1993).
Notes: Arxotrichum deceptivum can easily be distinguished from the other known species in the genus by its short, hypha-like ascomatal hairs. The ascomata produced by Arx. deceptivum are reminiscent of those of Achaetomiella virescens and an Achaetomium species. Von Arx et al. (1986) suggested that Arx. deceptivum was related to Chaetomium murorum (= Botryotrichum murorum, Wang et al. 2016b) based on the similarities of their ascospores. These two species are phylogenetically distant from each other (Fig. 7A, C, Supplementary Figs S1–S3). Morphologically, Arx. deceptivum can be distinguished from Botryot. murorum by its larger (16.5–19.5 × 8.5–9.5 μm vs 12.5–15 × 7.5–8.5 μm) and often inequilateral ascospores, and the production of undeveloped ascomatal hairs.
Arxotrichum gangligerum (L.M. Ames) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 830918. Fig. 13.
Fig. 13.
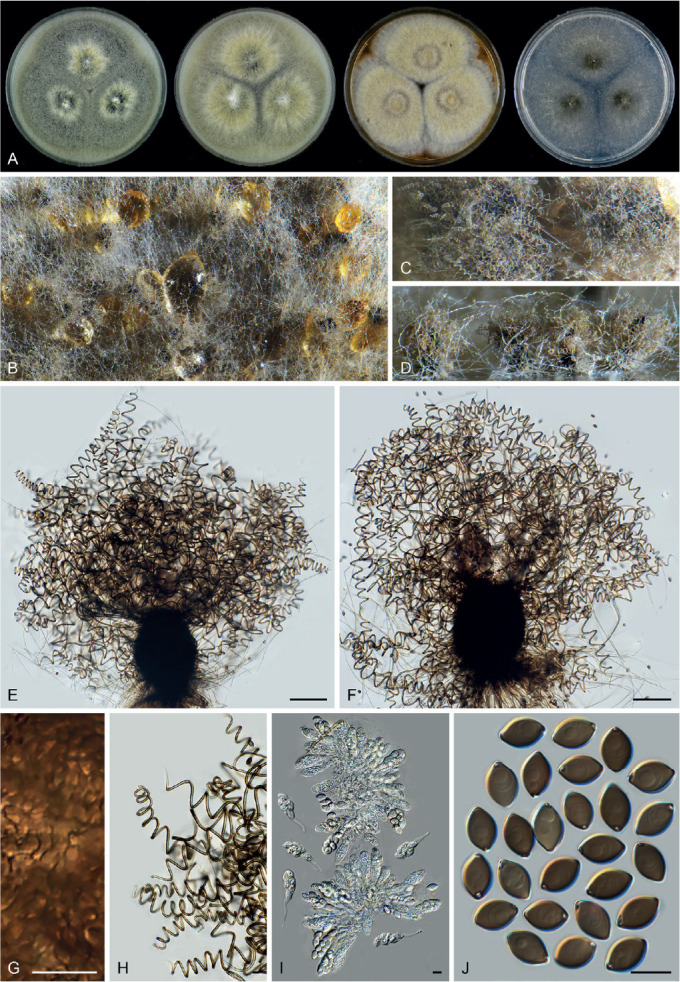
Sexual morph of Arxotrichum gangligerum (CBS 160.52, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 2 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D. Mature ascomata on OA, side view. E, F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: E, F = 100 μm; G–J = 10 μm.
Basionym: Chaetomium gangligerum L.M. Ames, Mycologia 41: 640. 1950.
Micromorphology: Ascomata superficial, vinaceous buff due to ascomatal hairs in reflected light, ostiolate, ovoid or ellipsoidal, 140–230 μm high, 130–200 μm diam. Ascomatal wall brown, composed of textura epidermoidea in surface view. Ascomatal hairs numerous, brown, spirally coiled, finely verrucose, septate, erect or flexuous at lower part, 2–3.5 μm diam near the base. Lateral hairs flexuous. Asci fusiform or clavate, spore-bearing part 31.5–45.5 × 12–17.5 μm, with stalks being 14–36.5 μm long, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, ellipsoidal with both ends attenuated, (9.5–)10.5–12.5(–13.5) × (6.5–)7–8.5 μm, with a subapical or oblique germ pore. Asexual morph (fide von Arx et al. 1986): Conidia spherical or ellipsoidal, mostly formed intercalary, singly or catenate, occasionally clustered, smooth or verrucose, hyaline or pale brown, 5–8 μm diam.
Culture characteristics: On OA with an entire edge, 46–52 mm diam in 7 d at 25 °C, obverse primrose or pale mouse grey to grey olivaceous due to ascomata mixed with aerial mycelium; reverse mouse grey. On CMA with an entire edge, 49–55 mm diam in 7 d at 25 °C, with a thicker layer of aerial mycelium, obverse olivaceous buff to greenish olivaceous, reverse greenish olivaceous. On MEA with an entire edge, 45–51 mm diam in 7 d at 25 °C, texture floccose, obverse smoke grey to olivaceous buff, reverse fuscous black. On PCA with an entire edge, 49–55 mm diam in 7 d at 25 °C, with sparse aerial mycelium, obverse smoke grey, or grey olivaceous in the central part, without coloured exudates; reverse uncoloured, or olivaceous grey in the central part.
Material examined: Canada, Ontario, Haliburton Co., Dorset, isolated from dung of rabbit, Oct. 1979, A. Carter (CBS 563.80 = CBS 130.85 = TRTC 48537). USA, Virginia, Fort Belvoir, isolated from wood sample under test conditions in Tropical Testing Chamber, date unknown, L.M. Ames (culture ex-type CBS 160.52 = ATCC 11206).
Notes: Arxotrichum gangligerum is characterised by ascospores with a subapical or oblique germ pore, and by the production of numerous spirally coiled ascomatal hairs that are relatively long and often intertwined with aerial mycelium. Arxotrichum sinense produces similar ascospores (with an oblique to lateral germ pore), but can be distinguished from Arx. gangligerum by its pyriform or ovoid asci, larger ascospores (13.5–15 × 8.5–9.5 μm vs 10.5–13 × 7–8.5 μm), and flexuous or undulate rather than spirally coiled ascomatal hairs. The asexual morph was not observed in our study and more work is required to compare the conidia of this species with those of Arx. piluliferoides and Arx. wyomingense.
Arxotrichum officinarum (M. Raza & L. Cai) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840142.
Basionym: Myceliophthora officinarum M. Raza & L. Cai, Fungal Diversity 99: 89. 2019.
Micromorphology: See Raza et al. (2019; p. 87, 89).
Notes: Raza et al. (2019) classified this species in Myceliophthora based on their phylogenetic analysis. However, no representatives of Arxotrichum were included in their phylogenetic analysis. In our phylogenetic analyses (Fig. 7A, Supplementary Figs S1–S3), Arx. officinarum forms a sister lineage to Arx. gangligerum. This species is morphologically similar to Arx. gangligerum with both sexual and asexual morphs, but can be distinguished by thicker ascomatal hairs (2.5–4 μm vs 2–3.5 μm diam near the base) and larger conidia (7.0–10.5 × 6–10.5 μm vs 5–8 μm diam). The original description reported that the ascospores of Arx. officinarum have an “apical germ slit”, and the ex-type strain needs to be re-examined to confirm this observation.
Arxotrichum piluliferoides (Udagawa & Y. Horie) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 830920. Fig. 14.
Basionym: Chaetomium piluliferoides Udagawa & Y. Horie, Trans. Mycol. Soc. Japan 16: 337. 1975.
Micromorphology: Ascomata superficial, olivaceous grey in reflected light due to ascomatal hairs, ovoid to obpyriform or subglobose, often with a short beak, 140–245 μm high, 115–195 μm diam. Ascomatal wall brown, composed of irregular or angular cells. Ascomatal hairs flexuous, punctulate or verrucose, brown, septate, 1.5–3 μm diam near the base. Asci pyriform to clavate, spore-bearing part 31–58 × 18–24 μm, with stalks being 9–10 μm long, containing eight irregularly-arranged or biseriate ascospores, evanescent, sometimes persistent until ascospores mature. Ascospores olivaceous brown when mature, fusiform, often inequilateral, (19–)21–24(–26) × 7.5–9 (–9.5) μm, with one or two apical or slightly subapical germ pores. Conidiophores absent. Conidiogenous cells reduced to a hyphal cell, monoblastic, laterally or terminally producing conidia. Conidia arising laterally from aerial hyphae, or from short branches of hyphae, sometimes intercalary, hyaline, globose to subglobose, sometimes ovate to fusiform, hyaline, verrucose, 4.5–9 μm diam.
Culture characteristics: On OA with an entire edge, 51–57 mm diam in 7 d at 25 °C, texture floccose, obverse olivaceous buff to pale luteous due to conidia on mycelium mixed with ascomata; reverse olivaceous grey. On CMA similar to those on OA, 49–55 mm diam in 7 d at 25 °C. On MEA with an entire edge, 47–53 mm diam in 7 d at 25 °C, texture thick floccose, obverse buff to pale luteous; reverse ochreous to umber. On PCA with an entire edge, 46–52 mm diam in 7 d at 25 °C, without aerial mycelium, obverse smoke grey due to ascomata, without coloured exudates, reverse smoke grey or dark brick.
Material examined: Japan, Sugadaira, Naguna Prefecture, isolated from grassland soil, 17 Oct. 1972, J.Y. Horie (culture ex-type CBS 103.77 = IFM 4531 = IMI 210880 = NHL 2738). Spain, Tarragona, isolated from dung, date unknown, J. Guarro (CBS 262.82).
Notes: Arxotrichum piluliferoides is closely related to Arx. deceptivum (Fig. 7A, Supplementary Figs S1–S3). Both species produce hypha-like ascomatal hairs with no differentiation between the terminal and lateral ones. Arxotrichum piluliferoides can be distinguished from Arx. deceptivum by olivaceous grey ascomatal hairs and elongated fusiform ascospores. Conidia of this species can easily be observed in the aerial mycelium (Fig. 14B–F). Von Arx et al. (1986) described the ascospores of this species “with paler ends, but without sharply delimited germ pores”. In our study we used lactic acid as mounting fluid and two apical germ pores (Fig. 14L) could be observed in the ascospores.
Arxotrichum repens (Guarro & Figueras) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 830921. Fig. 15.
Fig. 15.
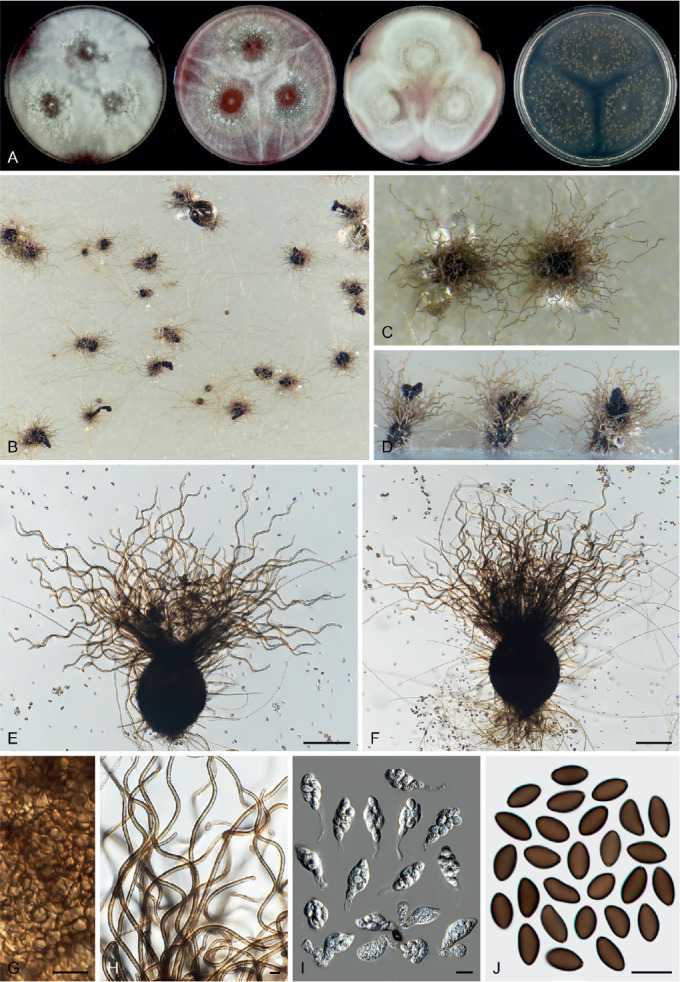
Arxotrichum repens (CBS 233.82, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony on PCA. C. Mature ascomata on PCA, top view. D. Mature ascomata on PCA, side view. E, F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: E, F = 100 μm; G–J = 10 μm.
Basionym: Chaetomium repens Guarro & Figueras, Beih. Nova Hedwigia 84: 6. 1986.
Micromorphology: Ascomata superficial, buff to greyish sepia due to ascomatal hairs in reflected light, ovoid or subglobose, ostiolate, 160–230 μm high, 130–220 μm diam. Ascomatal wall brown, composed of irregular or angular cells. Terminal hairs brown, regularly undulate to slightly coiled, verrucose, septate, erect or flexuous in the lower parts, 2.5–4.5 μm diam near the base. Lateral hairs flexuous. Asci fusiform, sometimes clavate or pyriform, spore-bearing part 16.5–28.5 × 11–16 μm, with stalks being 4–20.5 μm long, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, ellipsoidal or reniform, rounded or slightly attenuated at both ends, often inequilateral, (7.5–)8–10(–10.5) × (4.5–)5–6 μm, with an inconspicuous apical germ pore. Asexual morph unknown.
Culture characteristics: On OA with an entire edge, 47–53 mm diam in 7 d at 25 °C, texture floccose, obverse white to smoke grey due to aerial mycelium, non-sporulating; reverse bay due to coloured exudates diffusing into the medium. On CMA similar to those on OA. On MEA with an entire edge, 47–53 mm diam in 7 d at 25 °C, texture floccose, obverse white with margins rosy or rosy vinaceous, reverse rust. On PCA with an entire edge, 50–56 mm diam in 7 d at 25 °C, without aerial mycelium, obverse buff due to ascomata, without coloured exudates; reverse uncoloured or buff.
Material examined: Spain, Tarragona, isolated from soil in Montblanc, date unknown, J. Guarro (culture ex-type CBS 233.82 = FFBA 310).
Notes: Arxotrichum repens is phylogenetically most closely related to Arx. sinense (Fig. 7A). These two species could be differentiated based in their tub2 and rpb2 sequences. In contrast, the ITS phylogeny fails to separate these two species (Supplementary Figs S1–S3). Arxotrichum repens can be distinguished from Arx. sinense by its regularly undulate to slightly coiled ascomatal hairs, often fusiform and smaller asci (16.5–28.5 × 11–16 μm vs 22–35 × 17.5–23 μm in spore-bearing part), and ellipsoidal or reniform and smaller ascospores (8–10 × 5–6 μm vs 13.5–15 × 8.5–9.5 μm) with an inconspicuous apical germ pore. This species is only known from the ex-type strain. Our cultures on OA and CMA remained sterile after prolonged incubation, but ascomata were obtained on PCA. Arxotrichum repens is mainly characterised by its ellipsoidal or reniform ascospores with rounded ends. In contrast, the majority of Chaetomiaceae species produce ascospores with at least one attenuated end. The only exceptions are those that produce spherical and bilaterally flattened ascospores, such as Chaetomium globosporum, Ch. grande and Ch. megalocarpum (von Arx et al. 1986, 1988, Wang et al. 2016a, b, 2019a, b).
Arxotrichum sinense (K.T. Chen) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 830922. Fig. 16.
Fig. 16.
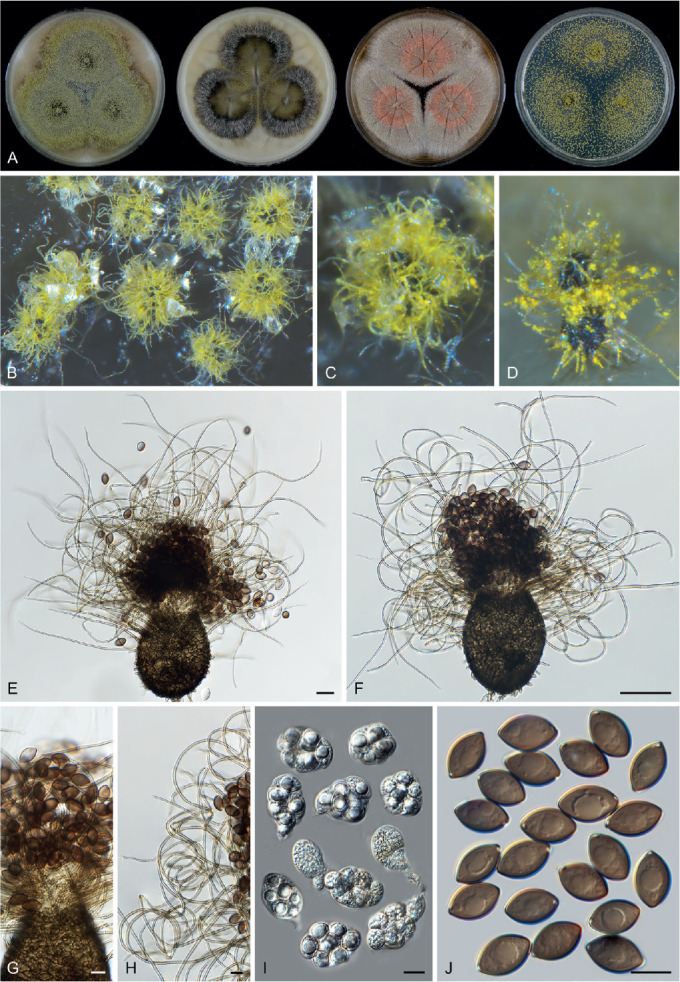
Arxotrichum sinense (CBS 541.83, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D. Mature ascomata on OA, side view. E, F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: E = 20 μm; F = 50 μm; G–J = 10 μm.
Basionym: Chaetomium sinense K.T. Chen, Acta Microbiol. Sin. 13: 125. 1973.
Micromorphology: Ascomata superficial, sulphur-yellow to pure yellow due to ascomatal hairs in reflected light, ovoid or subglobose, ostiolate, often with a short papillate beak, 100–145 μm high, 82–120 μm diam. Ascomatal wall brown, with apical beak paler, composed of irregular or angular cells. Terminal hairs brown, flexuous or undulate, sometimes recurved or slightly circinate at the apex, tapering towards the tips, finely verrucose, septate, erect or flexuous in the lower parts, 2–3.5 μm diam near the base. Lateral hairs flexuous. Asci pyriform or ovoid, spore-bearing part 22–35 × 17.5–23 μm, with short stalks being 3.5–7 μm long, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, ellipsoidal with both ends attenuated, (13–)13.5–15(–15.5) × (7.5–)8.5–9.5(–10) μm, with an oblique to lateral germ pore. Asexual morph unknown.
Culture characteristics: On OA with an entire edge, 47–53 mm diam in 7 d at 25 °C, without aerial mycelium, obverse olivaceous buff to greenish olivaceous due to ascomata, with margins hazel due to coloured exudates diffusing into the medium; reverse buff, or fuscous black in the central part. On CMA with an entire edge, 47–53 mm diam in 7 d at 25 °C, poorly sporulating, obverse olivaceous due to coloured exudates diffusing into the medium, with a ring of aerial mycelium around the centre; reverse mouse grey. On MEA with an entire edge, 47–53 mm diam in 7 d at 25 °C, obverse smoke grey or slightly peach due to aerial mycelium, reverse sienna or umber. On PCA with an entire edge, 45–51 mm diam in 7 d at 25 °C, without aerial mycelium, obverse sulphur-yellow to amber due to ascomata, without coloured exudates; reverse smoke grey.
Material examined: China, isolated from soil, date unknown, J.D. Chen (culture ex-type CBS 541.83 = FFBA 388).
Notes: Chaetomium sinense was synonymised with Ch. gangligerum by von Arx et al. (1986). These two species are phylogenetically not closely related (Fig. 7A, Supplementary Figs S1–S3). Our morphological examination showed that Arx. sinense (= Ch. sinense) produces similar shaped ascospores; however, they are larger than those of Arx. gangligerum (= Ch. gangligerum) (13.5–15 × 8.5–9.5 μm vs 10.5–13 ×7–8.5 μm). Furthermore, the terminal hairs of Arx. sinense are never spirally coiled, different from those of Arx. gangligerum. For more details, see notes of Arx. gangligerum.
Arxotrichum succineum (L.M. Ames) A. Nováková & M. Kolařik, Persoonia 40: 259. 2018. Fig. 17.
Fig. 17.
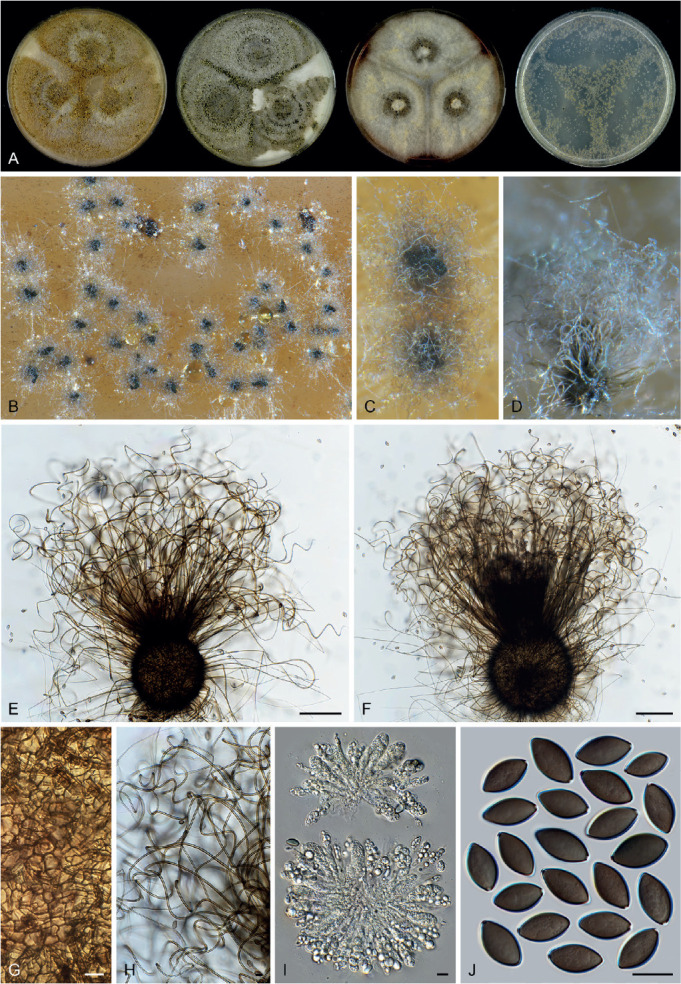
Arxotrichum succineum (CBS 166.52, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D. Mature ascomata on OA, side view. E, F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: E, F = 100 μm; G–J = 10 μm.
Basionym: Chaetomium succineum L.M. Ames, Mycologia 41: 645. 1949.
Micromorphology: Ascomata superficial, buff to greenish olivaceous due to ascomatal hairs in reflected light, subglobose to ovoid, ostiolate, 110–280 μm high, 90–255 μm diam. Ascomatal wall brown, composed of irregular or angular cells. Terminal hairs brown, flexuous, undulate or irregularly loosely coiled in the upper part, finely verrucose, septate, erect or flexuous at lower part, 2–4 μm diam near the base. Lateral hairs flexuous. Asci clavate or fusiform, spore-bearing part 30.5–38.5 × 13–17 μm, with stalks being 11–20 μm long, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous when mature, ellipsoidal or fusiform with both ends attenuated, sometimes inequilateral, (11.5–)12.5–14(–15) × (6–)6.5–7.5(–8.5) μm, with an apical germ pore. Asexual morph unknown.
Culture characteristics: On OA with an entire edge, 39–45 mm diam in 7 d at 25 °C, obverse greenish olivaceous to hazel due to ascomata mixed with aerial mycelium and conidia; reverse cinnamon. On CMA similar to those on OA, obverse grey olivaceous; reverse honey or hazel. On MEA with an entire edge, 40–46 mm diam in 7 d at 25 °C, texture floccose, obverse smoke grey or slightly buff to pale luteous due to conidia on aerial mycelium, reverse rust. On PCA with an entire edge, 43–49 mm diam in 7 d at 25 °C, without aerial mycelium, obverse olivaceous buff due to ascomata, without coloured exudates; reverse uncoloured.
Material examined: China, Xinjiang, Altai, isolated from soil, 2003, X.W. Wang (CBS 119769 = CGMCC 3.9426). USA, California, Mount Shasta, isolated from Abies magnifica var. shastensis, date unknown, G.W. Martin (culture ex-type CBS 166.52 = ATCC 11216 = MUCL 18704); (CBS 813.73 = DAOM 24174 = IMI 044210 = QM 1044).
Notes: Arxotrichum succineum can be distinguished from the other known species in the genus by its thin, flexuous, undulate or irregularly coiled ascomatal hairs and fusiform ascospores having an apical germ pore. When Arxotrichum was introduced, this was the only known sexually reproducing species in the genus (Crous et al. 2018). The description of the sexual morph of Arxotrichum was therefore based on the description of Ch. succineum as reported by von Arx et al. (1986). In the present study, we re-describe six additional species in Arxotrichum and then redefine the genus.
Arxotrichum wyomingense A. Nováková & M. Kolařik, Persoonia 40: 259. 2018.
Micromorphology: See Nováková and Kolařik (Crous et al. 2018): On MEA. Conidiophores septate, 250–400 μm long, stipe with basal part yellowish brown, smooth to finely rough-walled, 3 μm wide, upper part colourless, smooth, 2.5 μm wide, ramified, branches racemose. Conidiogenous cells borne at the ends of branches, hyaline. Conidia solitary, aseptate, 5(–7) μm diam, hyaline to pinkish coloured, subglobose, rough-walled to rugose, flattened from side view with distinct spiral (bands) and visible scars. Sexual morph not observed.
Notes: Although Arx. wyomingense was designated as the type species of Arxotrichum, this is the only species in the genus that only produces an asexual morph (Crous et al. 2018). The conidiophores of this species are ramified with racemose branches and the aseptate conidia are produced solitary, are flattened from side view and have rough to rugose walls with distinct spiral (bands) and visible scars. Three other species in the genus (Arx. gangligerum, Arx. officinarum and Arx. piluliferoides) also produce solitary and aseptate conidia. However, these species do not produce conidiophores and the conidia develop lateral or intercalary directly from the hyphae. We did not study the ex-type of this species. It needs to be noted that the description of Arx. wyomingense was based on MEA, and this medium is often unsuitable for Chaetomiaceae species to develop ascomata. It is therefore necessary to check whether this species is able to produce a sexual morph on more suitable media such as OA or PCA.
Bommerella Marchal, Bull. Soc. Roy. Bot. Belgique 24: 164. 1885.
Micromorphology (from von Arx et al. 1986): Ascomata ostiolate, with a short conical beak. Ascomatal wall composed of elongate cells arranged in petaloid patterns (cephalothecoid). Ascomatal hairs seta-like, straight, septate, smooth or verrucose, tapering towards the tips. Asci fasciculate, clavate or fusiform, stalked, containing eight ascospores, evanescent. Ascospores triangular in front view, ellipsoidal in side view, brown when mature, with an apical germ pore. Conidia formed in basipetal chains on percurrently elongating conidiogenous cells, pyriform, truncate at base, punctulate, hyaline. Containing species with both asexual and sexual morphs.
Type species: Bommerella trigonospora Marchal
Notes: This is a monotypic genus. Bommerella trigonospora is characterised by its ascospores which are triangular in front view and elliosoidal in side view (Fig. 4G), and by the presence of conidia which are pyriform, hyaline, and formed in basipetal chains (von Arx et al. 1986). Chivers (1915) combined this species in Chaetomium (Ch. trigonosporum) and this was followed by others (Ames 1963, von Arx et al. 1986). Our phylogenetic analyses showed that this species forms a single lineage with no known close relatives (Fig. 7C), thus the generic name is resurrected. Another chaetomium-like species that produces trianglar ascospores is “Chaetomium microascoides” which can be distinguished by the absence of conidia and terminal ascomatal hairs around ostiolates. Phylogenetic analysis indicated that “Chaetomium microascoides” belongs to Lasiosphaeriaceae sensu lato, distant from Chaetomiaceae (Fig. 7D).
Botryoderma Papendorf & H.P. Upadhyay, Trans. Brit. Mycol. Soc. 52: 257. 1969.
Micromorphology: Fertile hyphae hyaline, 1–4 μm wide. Conidiophores reduced. Conidiogenous cells arising laterally or terminally from fertile hyphae, densely clustered with some sterile hyphal branches, hyaline, subglobose, ellipsoidal or ovoid, monoblastic or polyblastic. Conidia produced singly, rhexolytic when seceding, single-celled, hyaline, smooth, ellipsoidal or obovoid, with a rounded apex or an apical spine-like beak and a narrow basal or oblique secession scar where a membranous frill is often attached. Sterile hyphal branches arising laterally or terminally from fertile hyphae and forming dense clusters together with conidiogenous cells, erect, seta-like, flexuous or recurved. Sexual morph not observed.
Type species: Botryoderma lateritium Papendorf & H.P. Upadhyay
Notes: Since the genus Botryoderma was established (Papendorf & Upadhyay 1969), it remained as “incertae sedis” in the Pezizomycotina, Ascomycota (Kirk et al. 2008, www.mycobank.org, www.indexfungorum.org). In the present study, phylogenetic analysis clearly located Botryoderma in the Chaetomiaceae, Sordariales. This genus forms a lineage closely related to the genera Arxotrichum, Corynascus, Myceliophthora and Thermothelomyces (Fig. 7A). Botryoderma lateritium and Botryod. rostratum can easily be recognised by their conidiogenous cells and sterile hyphal branches arising laterally or terminally from fertile hyphae in dense clusters. The other two species described in this genus, Botryod. gigasporum (Kapoor & Lal 1982) and Botryod. nigrum (Lopez et al. 1995) also produce single-celled conidia attached with remains of ruptured conidiogenous cell due to rhexolytic secession, but differ in lacking sterile hyphal branches within the clusters of conidiogenous cells. Furthermore, Botryod. gigasporum produces conspicuous conidiophores (Kapoor & Lal 1982) and Botryod. nigrum pigmented to black conidia (Lopez et al. 1995). It remains necessary to confirm the classification of the latter two species in Botryoderma.
Botryoderma lateritium Papendorf & H.P. Upadhyay, Trans. Brit. Mycol. Soc. 52: 258. 1969. Fig. 18.
Fig. 18.
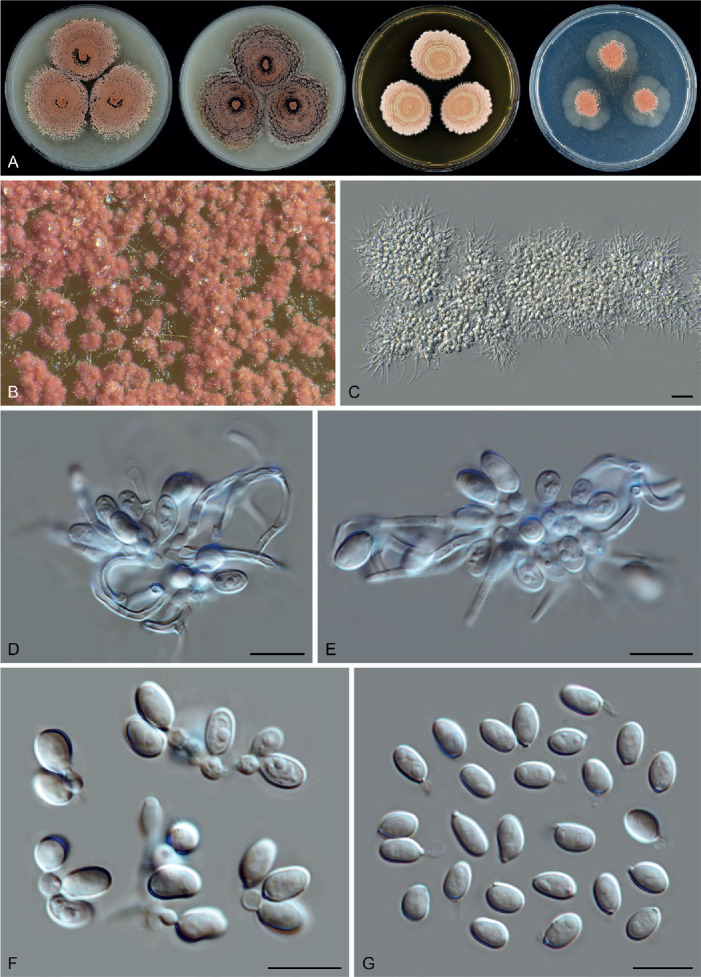
Botryoderma lateritium (CBS 586.66, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony on OA. C. Clusters of conidiogenous cells with conidia and sterile hyphal branches. D, E. Part of the clusters of conidiogenous cells with conidia and sterile hyphal branches. F. Conidiogenous cells and conidia. G. Conidia. Scale bars: C = 20 μm; D–G = 10 μm.
Micromorphology: Conidiophores usually reduced. Conidiogenous cells arising laterally or terminally from fertile hyphae, densely clustered with some sterile hyphal branches, hyaline, subglobose, ellipsoidal or ovoid, polyblastic or monoblastic, 2.5–4 × 2–3 μm. Conidia single-celled, hyaline, smooth, ellipsoidal or obovoid, (4.5–)5.5–8(–9) × 3–5 μm, rhexolytic when seceding, usually with a rounded apex and a truncate base or a narrow secession scar often attached with ruptured remain of conidiogenous cell when detached. Sterile filaments arising laterally or terminally from fertile hyphae and forming dense clusters together with conidiogenous cells, erect, flexuous or recurved, 1.5–2.5 μm diam near the bases, up to 40 μm long. Sexual morph not observed.
Culture characteristics: On OA with an entire edge, 12–18 mm diam after 7 d at 25 °C, without aerial mycelium, obverse peach to brick due to the clusters of conidiogenous cells and conidia; reverse cinnamon. On CMA with a crenate edge, 12–18 mm diam after 7 d at 25 °C, without aerial mycelium, obverse olivaceous to brown vinaceous due to coloured exudates diffusing into the medium, with several peach to brick and crenate concentric rings due to the formation of clusters of conidiogenous cells and conidia; reverse dark mouse grey. On MEA with a crenate edge, 13–19 mm diam after 7 d at 25 °C, obverse saffron in the central part, with several salmon, buff or rosy buff concentric rings; reverse fawn to hazel. On PCA translucent, with an entire or lobate edge, 5–11 mm diam after 7 d at 25 °C, without aerial mycelium, obverse peach to brick, reverse saffron.
Material examined: South Africa, Transvaal, Potchefstroom, isolated from soil mixed with leaf litter of Acacia karroo, Jan.–Feb. 1964, M.C. Papendorf (culture ex-type CBS 586.66 = ATCC 18926 = IMI 158956 = MUCL 8790 = PRE 44223).
Notes: Botryoderma lateritium, the type species of the genus, produces conidia that usually have a rounded apex and a truncate base or a narrow secession scar. Two subsequently described species, Botryod. gigasporum and Botryod. nigrum have similar conidia. In comparison with Botryod. lateritium, Botryod. gigasporum (Kapoor & Lal 1982) produces larger conidia (11.5–30 × 10–22 μm vs 5.5–8 × 3–5 μm), while Botryod. nigrum (Lopez et al. 1995) produces smaller and brown to black conidia (3–6 × 2.5–3.5 μm vs 5.5–8 × 3–5 μm). Furthermore, Botryod. gigasporum and Botryod. nigrum lack sterile hyphal branches in the clusters of the conidiogenous cells.
Botryoderma rostratum Papendorf & H.P. Upadhyay, Trans. Brit. Mycol. Soc. 52: 260. 1969. Fig. 19.
Fig. 19.
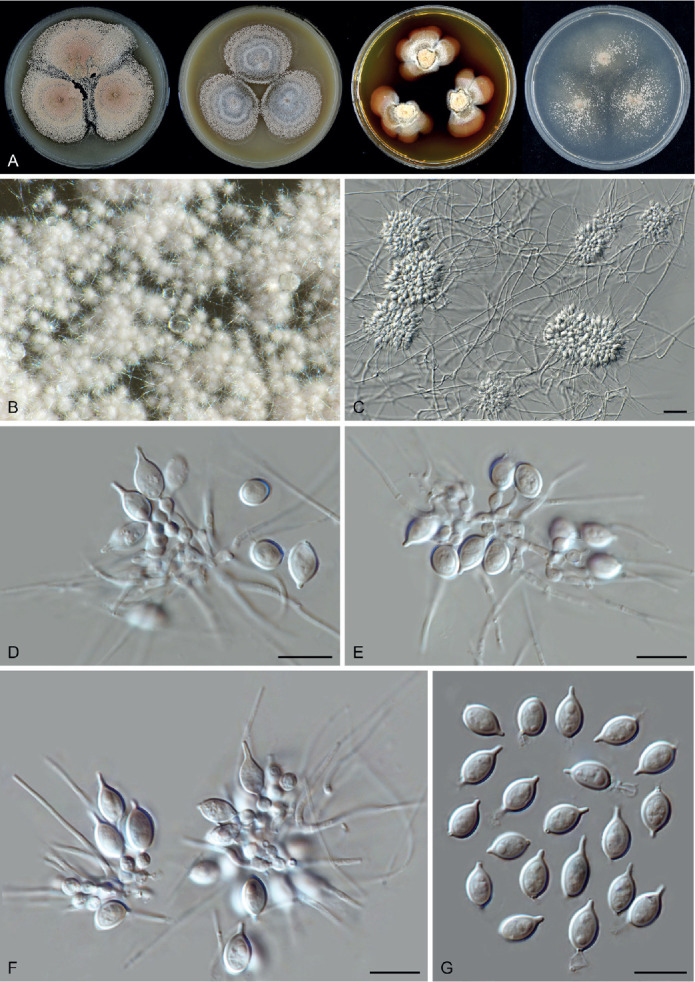
Botryoderma rostratum (CBS 184.68, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony on OA. C. Hyphae, clusters of conidiogenous cells with conidia and sterile hyphal branches arising from hyphae. D–F. Part of the clusters of conidiogenous cells with conidia and sterile hyphal branches arising from hyphae. G. Conidia. Scale bars: C = 20 μm; D–G = 10 μm.
Micromorphology: Conidiophores reduced. Conidiogenous cells arising laterally from fertile hyphae, densely clustered with seta, hyaline, subglobose, ellipsoidal or ovoid, monoblastic or polyblastic, 2.5–4 × 2–3 μm. Conidia single-celled, hyaline, smooth, ellipsoidal or obovoid, usually with an apical spine-like beak which is 1–3.5 μm long, rhexolytic when seceding, usually with a truncate base or a secession scar often attached with ruptured remain of conidiogenous cell when detached, (7.5–)8–10(–11.5) × 4–6 μm (excluding beak). Sterile seta arising laterally or terminally from fertile hyphae and forming dense clusters together with conidiogenous cells, sometimes branched, erect or recurved, 1–2 μm diam near the bases, up to 80 μm long. Sexual morph not observed.
Culture characteristics: On OA with an entire edge, 23–29 mm diam after 7 d at 25 °C, with sparse aerial mycelium, obverse vinaceous buff due to clusters of conidiogenous cells and conidia; reverse iron grey. On CMA like those on OA, with clusters of conidiogenous cells and conidia mainly in the central part and forming several concentric rings. On MEA with a lobate edge, 6–12 mm diam after 7 d at 25 °C, obverse white to buff or saffron, with a sienna margin, olivaceous around the colonies due to coloured exudates diffusing into the medium; reverse sienna. On PCA with an entire or slightly crenate edge, 16–22 mm diam after 7 d at 25 °C, without aerial mycelium, obverse white to rosy buff, reverse buff.
Material examined: Lectotype designated here: CBS H-24915, MBT 10004828. Brazil, Prov. Maranhâo, isolated from sandy soil, 1964, M.C. Papendorf (culture ex-lectotype CBS 184.68 = ATCC 18927 = IMI 158957).
Notes: Papendorf & Upadhyay (1969) described Botryoderma rostratum and Botryod. lateritium in the same article, but only designated a holotype of Botryod. lateritium. For Botryod. rostratum, the protologue simply mentioned that “Cultures of the type have been deposited in the Mycotheca, University of Recife, Brazil; the Centraalbureau voor Schimmelcultures, Baarn, Netherlands, and the Cryptogamic Herbarium, University of Potchefstroom.” Consequently, we prepared a herbarium specimen from the type culture CBS 184.68 as lectotype of this species. Both species described by Papendorf & Upadhyay (1969) produce conidiogenous cells and sterile hyphal branches in dense clusters. Botryoderma rostratum can easily be distinguished from Botryod. lateritium, Botryod. gigasporum and Botryod. nigrum, by its conidia having an apical spine-like beak. For more morphological comparisons, see notes of Botryod. lateritium.
Botryotrichum Sacc. & Marchal, Bull. Soc. Roy. Bot. Belgique 24: 66. 1885.
Micromorphology: Containing asexual species, sexual species and species with both asexual and sexual morphs. Ascomata superficial, or sub-immersed to immersed in the medium, ostiolate or non-ostiolate, subglobose to ovoid, covered by well-developed ascomatal hairs, or in some species glabrous or with sparse and hypha-like hairs. Ascomatal wall brown, non-translucent to semi-translucent, or in some species subhyaline to olivaceous grey and translucent, composed of textura epidermoidea, intricata or angularis in surface view. Ascomatal hairs, if present, flexuous or undulate and often circinate at the apex, or spirally coiled, or sparse and hyphal-like. Asci fasciculate, clavate, fusiform, ovoid or irregular, stalked, containing eight ascospores, evanescent. Ascospores olivaceous brown when mature, smooth, ellipsoidal with attenuated ends, or ellipsoidal-fusiform, not bilaterally flattened, with one or two apical germ pores. Conidiophores solitary or clustered with a tuft of sterile setae, hyaline to slightly pigmented, sympodially branched to produce several conidiogenous cells, or unbranched, sometimes reduced to conidiogenous cells. Conidiogenous cells hyaline, subhyaline to pale brown, cylindrical or slightly swollen, monoblastic or sympodially polyblastic. Conidia 1-celled, hyaline or pigmented, globose to subglobose, smooth to warted, solitary or rarely formed in chains of a few spores.
Type species: Botryotrichum piluliferum Sacc. & Marchal
Notes: As described above, Botryotrichum encompasses a high morphological diversity (Wang et al. 2016b, 2019a). Based on previous phylogenetic analyses, ten species have been included in this genus. Among the seven asexually reproducing species, Botryot. atrogriseum, Botryot. peruvianum and the type species Botryot. piluliferum produce monoblastic or sympodially polyblastic conidiogenous cells on sympodially or simply branched conidiophores that often cluster with sterile setae (Wang et al. 2016b). Botryotrichum domesticum (Schultes et al. 2019), Botryot. foricae (Crous et al. 2019) and Botryot. iranicum (Alidadi et al. 2020) produce similar conidiogenous cells, but usually lack sterile setae clustering with conidiophores or conidiogenous cells. Botryotrichum verrucosum produces solitary and often unbranched conidiophores (Wang et al. 2019a). Although the type species Botryot. piluliferum was originally described as an asexual species (Marchal 1885, Saccardo 1886), its sexual morph was later discovered (Daniels 1961). The sexual morph of Botryot. piluliferum is morphologically similar to that of Botryot. murorum and both produce ostiolate ascomata covered by unbranched ascomatal hairs with circinate tips and ellipsoidal ascospores (Daniels 1961, von Arx et al. 1986). Botryotrichum murorum has no asexual morph. Botryotrichum spirotrichum can be distinguished from the other species in the genus by its non-ostiolate ascomata which are usually ellipsoidal to doliiform and have two (or three) tufts of spirally coiled hairs at the two opposite ends. Based on phylogenetic data (Fig. 7), four new combinations in Botryotrichum are proposed below and one new species is described. The morphological diversity of Botryotrichum expands with the addition of this new species and four chaetomium- or corynascella-like species. Two of those species are (re)described and illustrated here: Botryot. geniculatum with a chaetomidium-like sexual morph and Botryot. inquinatum with a corynascella-like sexual morph. More details are given below.
Botryotrichum geniculatum X.Wei Wang, P.J. Han & F.Y. Bai, sp. nov. MycoBank MB 840127. Fig. 20.
Fig. 20.
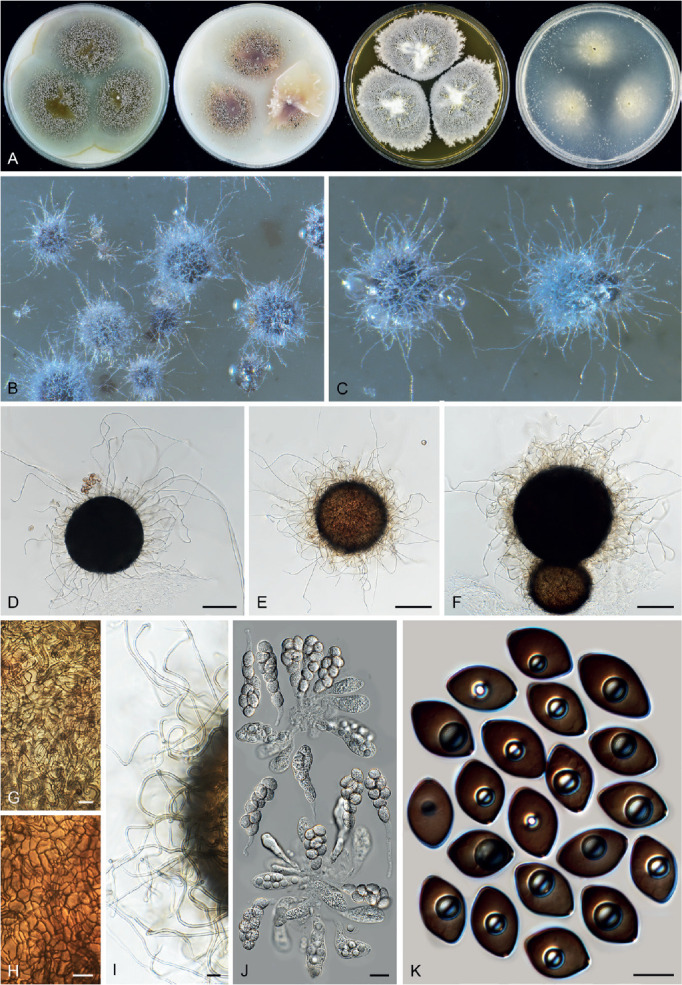
Botryotrichum geniculatum (CGMCC 3.20441, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B, C. Mature ascomata on OA, top view. D–F. Ascomata mounted in lactic acid. G, H. Structure of ascomatal wall in surface view. I. Ascomatal hairs. J. Asci. K. Ascospores. Scale bars: D–F = 100 μm; G–I, K = 10 μm; J = 20 μm.
Etymology: The name refers to its ascomatal hairs, which are geniculate in the lower parts.
Micromorphology: Ascomata superficial, occasionally immersed, solitary to several clustered, non-ostiolate, pale olivaceous grey in reflected light due to ascomatal hairs, spherical, 150–310 μm diam. Ascomatal wall dark brown, composed of textura epidermoidea or intricata when young, and then textura angularis when mature in surface view. Ascomatal hairs covering the whole ascomata, hypha-like, smooth or finely verrucose, flexuous or slightly undulate in the upper part, tapering and fading to hyaline towards the tips, geniculate in the lower part, brown at the base, 2–3.5 μm near the base, varying in length, some up to 400 μm long, occasionally up to 1 300 μm long. Asci clavate, spore-bearing part 48–72 × 20–26 μm, with stalks 12–35(–59) μm long, containing eight irregularly-arranged or biseriate ascospores, evanescent. Ascospores 1-celled, smooth, dark brown when mature, elongated limoniform to broadly fusiform, sometimes inequilateral, (16.5–)17–20(–30) × (11–)11.5–13(–14) μm, with an apical germ pore. Asexual morph unknown.
Culture characteristics: On OA with an entire edge, 18–24 mm diam in 7 d at 25 °C, aerial mycelium absent; obverse pale olivaceous grey due to the masses of ascomata, with soluble pigment greenish glaucous to greenish olivaceous; reverse smoke grey to greenish olivaceous due to exudates diffusing into the medium. On CMA similar to those on OA, 17–23 mm diam in 7 d at 25 °C, with soluble pigment pale vinaceous grey to vinaceous grey. On MEA with an fimbriate edge, 11–17 mm diam in 7 d at 25 °C, obverse pale mouse grey due to thick aerial mycelium, texture floccose, reverse cinnamon. On PCA with an entire edge, 10–16 mm diam in 7 d at 25 °C, obverse white, aerial mycelium absent, without coloured exudates, reverse uncoloured.
Material examined: China, Burjin County, Altay Prefecture, Xinjiang, isolated from soil under herb, Aug. 2004 , X.W. Wang (holotype HMAS 350293, isotype CBS H-23629, culture ex-type CGMCC 3.20441 = CBS 144475 = WXW 8287); Yili Prefecture, Xinjiang, near Sayram Lake, isolated from soil under Trollius chinensis, Aug. 2004, X.W. Wang (culture WXW 8266).
Notes: This species is characterised by its non-ostiolate ascomata covered by hypha-like ascomatal hairs, which are geniculate in their lower part and by its large limoniform to broadly fusiform ascospores (17–20 × 11.5–13 μm) with an apical germ pore. Three other Botryotrichum species produce non-ostiolate ascomata: Botry. inquinatum also has large ascospores (17–19 × 12.5–14.5 μm), but can be distinguished by two apical or subapical germ pores on each ascospore and by glabrous ascomata (see below); Botry. spirotrichum can be distinguished by smaller ascospores (5.5–8.0 × 4.5–6.5 μm) and by ascomata covered by coiled hairs (von Arx et al. 1988); Botry. trichorobustum is different in ascomata covered by flexuous or slightly undulate hairs, partly recurved or circinate at the apex, and in slightly smaller ascospores (10.5–18.5 × 10.5–13.5 μm) (Seth 1968, see below).
Botryotrichum inquinatum (Udagawa & S. Ueda) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 830923. Fig. 21.
Fig. 21.
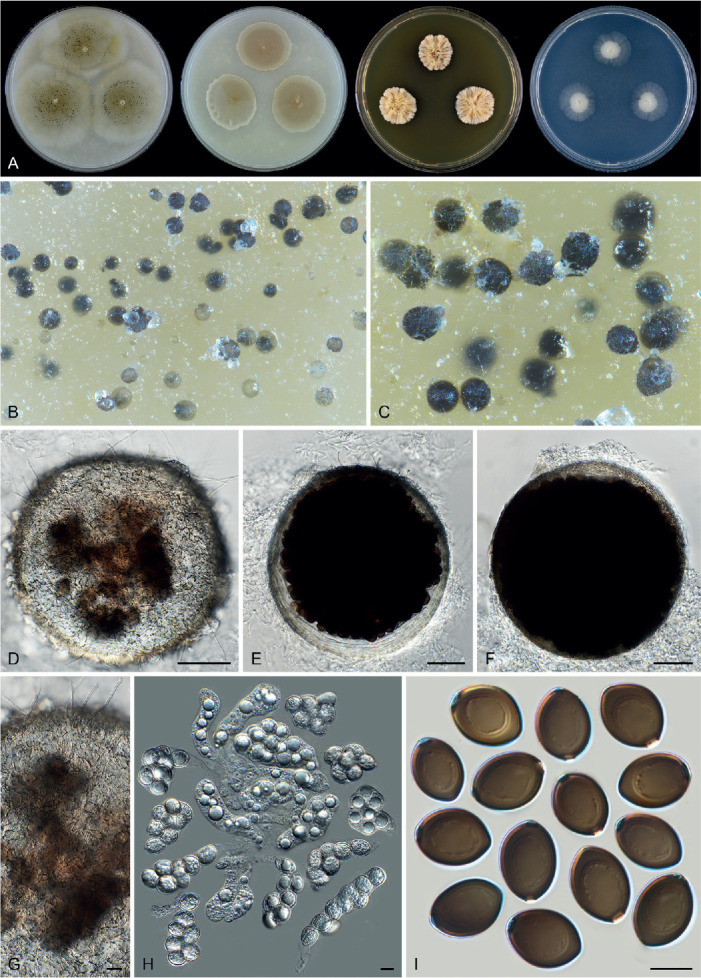
Botryotrichum inquinatum (CBS 155.80, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B, C. Mature ascomata on OA, top view. D–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Asci. I. Ascospores. Scale bars: D–F = 50 μm; G–I = 10 μm.
Basionym: Corynascella inquinata Udagawa & S. Ueda, Mycotaxon 8: 292. 1979.
Micromorphology: Ascomata superficial, subimmersed to immersed in the medium, solitary or aggregated, non-ostiolate, mouse grey to dark mouse grey when mature in reflected light, subglobose, glabrous, or with sparse, hypha-like hairs, (120–)175–330 μm diam. Ascomatal wall subhyaline to olivaceous grey, translucent, composed of textura epidermoidea in surface view. Asci pyriform, clavate, ovoid or irregular, spore-bearing part 42–86 × 21–31 μm, with stalks being 17–36.5 μm long, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, ellipsoidal, often biapiculate, (16–)17–19(–20.5) × (11.5–)12.5–14.5 μm, with two apical or subapical germ pores. Asexual morph unknown.
Culture characteristics: On OA with an entire or crenate edge, 10–16 mm diam in 7 d at 25 °C, without aerial mycelium, obverse olivaceous buff due to coloured exudates diffusing into the medium, producing mouse grey ascomata; reverse buff to olivaceous buff. On CMA a crenate edge, 7–13 mm diam in 7 d at 25 °C, without aerial mycelium, obverse buff to olivaceous buff; reverse olivaceous buff. On MEA with a lobate edge, 5–11 mm diam in 7 d at 25 °C, without aerial mycelium, obverse buff to rosy buff, wrinkled; reverse cinnamon. On PCA with a crenate edge, 3–9 mm diam in 7 d at 25 °C, without aerial mycelium, obverse grey white, with thin and translucent margins, without coloured exudates, reverse uncoloured.
Material examined: Egypt, isolated from desert soil, date unknown, J. Mouchacca (CBS 646.74). Japan, Nagasaki Pref., Isahaya-shi, isolated from sewage sludge, 22 Mar. 1978, S. Ueda (culture ex-type CBS 155.80 = ATCC 18927 = NHL 2841).
Notes: Botryotrichum inquinatum was originally placed in Corynascella based on its production of non-ostiolate ascomata and ascospores with two germ pores (Udagawa & Ueda 1979). Our phylogenetic analysis indicates that this species belongs to Botryotrichum, distantly related to the type species of Corynascella, C. humicola (Fig. 7C). This species is distinguished from the other species in the genus by its translucent, nearly glabrous non-ostiolate ascomata and its ascospores with two apical or subapical germ pores.
Botryotrichum retardatum (A. Carter & R.S. Khan) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840141.
Basionym: Chaetomium retardatum A. Carter & R.S. Khan, Canad. J. Bot. 60: 1255. 1982.
Notes: The position of this species in Botryotrichum (Fig. 7C) is supported in the combined and the tub2 and rpb2 phylogenies (Fig. 7C, Supplementary Figs S2, S3, ITS sequence not available). Botryotrichum retardatum can be distinguished from other species by the production of ostiolate ascomata covered by sparse hairs and ellipsoidal to fusiform ascospores with two subapical germ pores (von Arx et al. 1986).
Botryotrichum trichorobustum (Seth) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840143.
Basionym: Chaetomidium trichorobustum Seth, Nova Hedwigia 16: 430. 1968.
Notes: The phylogenetic relationship of Botryo. trichorobustum with other species in the genus is unresolved and it seems to take a basal position in the genus, though bootstrap support is lacking. Botryotrichum trichorobustum can be distinguished from other species in the genus by the production of non-ostiolate ascomata covered by smooth and thick (9–14 μm near the base) ascomatal hairs, partly with recurved or circinate apex. The species produces limoniform to broadly fusiform ascospores. No germ pores were observed and reported in the original description of the species (Seth 1968).
Botryotrichum vitellinum (A. Carter) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840144.
Basionym: Chaetomium vitellinum A. Carter, Mycologia 75: 531. 1983.
Notes: Phylogenetically, Botryo. vitellinum is sister to all other species in the genus (Fig. 7C). It can be distinguished from other species in the genus by the production of ostiolate ascomata covered by sparse, delicate hairs and ovate to fusiform ascospores with an apical germ pore (von Arx et al. 1986).
Chaetomium Kunze, Mykol. Hefte 1: 15. 1817.
Micromorphology and illustrations: See Wang et al. (2016a; p.167). Containing sexual species and species with both asexual and sexual morphs.
Type species: Chaetomium globosum Kunze
Notes: Chaetomium sensu stricto is characterised by the production of globose, ellipsoid to ovate or obovate ascomata, most often ostiolate and non-ostiolate in a few species. The ascomatal wall is usually composed of textura intricata or epidermoidea in surface view, or of textura angularis in a few species; the ascomatal hairs are hypha-like, flexuous, undulate, coiled to simply branched or dichotomously branched, mostly with verrucose surface and smooth in a few species. The asci of Chaetomium species are clavate or fusiform with eight biseriate or irregularly arranged ascospores. The ascospores are limoniform to globose (irregular in a few species), bilaterally flattened and usually more than 7 μm in length. An asexual morph is produced in some species and is, if present, acremonium-like (Wang et al. 2016a, b). We accept 43 species in Chaetomium. It is the largest genus in the family (Fig. 7B).
Chaetomium neoglobosporum X.Wei Wang & Houbraken, nom. nov. MycoBank MB 841112.
Replaced synonym: Chaetomium globosporum Rikhy & Mukerji, Kavaka 1: 38. 1974, non Chaetomium globisporum Lodha. 1964.
Etymology: The species name refers to “globosporum”, the epithet of the replaced synonym.
Micromorphology and illustrations: See Wang et al. (2016a; p. 101–102).
Notes: The epithets of Ch. globosporum and Ch. globisporum are similar, have the same meaning and can be easily confused. Because of this, Ch. globosporum is regarded as an illegitimate later homonym of Ch. globisporum (Art. 53.1), and we therefore propose the replacement name “neoglobosporum” for the former one. No (ex-)type material of the latter one “Ch. globisporum” was included in our study, so the taxonomic position of the latter species remains unknown.
Chaetomium subaffine Sergejeva ex X.Wei Wang & Houbraken, sp. nov. MycoBank MB 842311.
Synonym: Chaetomium subaffine Sergejeva, Bot. Mater. Otd. Sporov. Rast. Bot. Inst. Komarova Akad. Nauk S.S.S.R. 14: 148. 1961. (nom. inval., Art. 39.1).
Etymology: The epithet “subaffine” was used in the description of Sergejeva (1961), referring to Chaetomium affine Corda sensu Bainier.
Diagnosis: Phylogenetic inference (Fig. 7B) indicates that Ch. subaffine is most closely related to Ch. cirrhatum, Ch. cochliodes, Ch. pseudocochliodes and Ch. spiculipilium. Chaetomium subaffine can be distinguished from them by the abundant white mycelia covering the ascomata, mostly straight to flexuous ascomatal hairs, and the production of an acremonium-like asexual morph. The ascospores of Ch. subaffine are also larger (11.5–13.5 × 8.5–10 × 6.5–7.5 μm) than those of Ch. cochliodes (9–10 × 7.5–8.5 × 5–6 μm), Ch. pseudocochliodes (9.5–11 × 7.5–8.5 × 5.5–6.5 μm) and Ch. spiculipilium (10–13 × 7.5–9 × 5.5–6.5 μm) (Wang et al. 2016a, Zhang et al. 2017).
Description and illustration: See Wang et al. (2016a; p.121–122).
Material examined: Russia, isolated from seed and dead stem of cereal, date unknown, K.S. Sergejeva (holotype CBS H-24916, culture ex-type CBS 637.91 = ATCC 14531 = IMI 090489 = MUCL 18695 = VKM F-1945).
Notes: Bainier’s concept (1910) of Ch. affine differs from the original description of Corda (1840) and therefore Sergejeva (1961) introduced Ch. subaffine for Bainier’s species. Chaetomium subaffine Sergejeva lacks a Latin diagnosis and is therefore invalidly described. Sergejeva (1961) refers to Bainier (1910), but this publication also lacks a Latin description (only in French). In addition, the reference to Bainier’s illustration is insufficient. To validate this species, an English diagnosis is given above, with the name of the original author maintained.
Collariella X.Wei Wang, Samson & Crous, Stud. Mycol. 84: 177. 2016. Fig. 22.
Micromorphology (emended description): Ascomata superficial, ostiolate, ovate, obovate, ampulliform or cylindrical with brown walls of textura angularis in surface view. Apices of ascomata truncated, usually with a darkened collar around the ostiolar pore. Terminal hairs highly diverse, straight, flexuous, undulate or spirally coiled or presenting two different types. Lateral hairs straight, flexuous. Asci fasciculate, fusiform or clavate, stalked, with eight biseriate or irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown at maturity, broadly limoniform to quadrangular, bilaterally flattened, with an apical germ pore, usually less than 7.5 μm in length, occasionally up to 10.5 μm long. Asexual morph not observed.
Type species: Collariella bostrychodes (Zopf) X.Wei Wang & Samson
Notes: Collariella was originally delimited based on phylogenetic data and included two subclades (Wang et al. 2016b). Species in each subclade share certain morphological characters, yet the two subclades are distinctively different in the morphology of their ascomata and ascospores (Figs 10, 22). The molecular dating analysis performed here (Fig. 8A) indicated that these two subclades diverged from each other about 42 Mya, before the later time limit (about 27 Mya, Fig. 8A, 9) of the other accepted genera in the family. This result supports segregating them as two genera. Thus, Collariella sensu stricto is restricted to subclade 1 (Wang et al. 2016b) and Achaetomiella is revived above to accommodate species belonging to the other subclade.
Phylogenetic analyses showed that three additional chaetomium-like species belong to Collariella (Fig. 7C, Supplementary Figs S1, S2). The morphology of these species fits in the modified definition of Collariella as given above.
Collariella anguipilia (L.M. Ames) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840145.
Basionym: Chaetomium anguipilium L.M. Ames, A monograph of the Chaetomiaceae: 12. 1963.
Notes: Collariella anguipilia is phylogenetically closely related to Col. causiiformis and Col. hexagonospora (Fig. 7C, Supplementary Figs S1–S3). Morphologically, Col. anguipilia is similar to Col. causiiformis. Both have two types of ascomatal hairs, partly shorter and partly longer, and possess ascospores of similar shape and size. Collariella anguipilia can be distinguished from Col. causiiformis by its shorter type of ascomatal hairs, which are undulate and unbranched (Ames 1963, von Arx et al. 1986). Collariella hexagonospora can be distinguished by coiled ascomatal hairs and larger ascospores.
Collariella hexagonospora (A. Carter & Malloch) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840146.
Basionym: Chaetomium hexagonosporum A. Carter & Malloch, Canad. J. Bot. 60: 1249. 1982.
Notes: Collariella hexagonospora has the largest ascospores (9–10.5 μm long) in the genus (von Arx et al. 1986). Ascospores of the other known Collariella species are usually less than 7.5 μm long. The presence of a darkened collar around the ostiolar pore was not reported in the original description, though von Arx et al. (1986) mentioned that its ascomata were darker than those of other species (von Arx et al. 1986). More work is required to verify whether this species also produces a darkened collar around the ostiolar pore, as do the other species in the genus.
Collariella pachypodioides (L.M. Ames) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840147.
Basionym: Chaetomium pachypodioides L.M. Ames, Mycologia 37: 145. 1945.
Notes: This species was once treated as a synonym of Col. bostrychodes (von Arx et al. 1986). Our phylogenetic analysis indicated that it is distinct (Supplementary Figs S1–S3), and closer to Col. carteri than to Col. bostrychodes (Fig. 7C). Collariella carteri can be distinguished from Col. pachypodioides by short and seta-like terminal ascomatal hairs (Wang et al. 2016b), while the latter only produces spirally coiled terminal hairs. Collariella pachypodioides can be distinguished from Col. bostrychodes by its ascomatal shape (elongated ovate vs globose or subglobose) (Greathouse & Ames 1945, Wang et al. 2016b). Collariella capillicompacta is recently described as being closely related to Col. carteri and is phenotypically similar as Col. pachypodioides (Aghyl et al. 2021).
Corynascella Arx & Hodges, Stud. Mycol. 8: 23. 1975.
Micromorphology: Ascomata superficial, immersed or sub-immersed into the medium, solitary or aggregated, non-ostiolate at the beginning, later ostiolate especially on CMA, with masses of released ascospores on the top, subglobose or ovoid, usually covered by sparse aerial hyphae on which conidia are produced. Ascomatal wall brown, composed of textura epidermoidea in surface view. Ascomatal hairs straight or flexuous, brown, fading towards the tips, septate, smooth. Asci fasciculate, clavate, stalked, containing eight biseriate or irregularly-arranged ascospores, evanescent. Ascospores brown when mature, ellipsoidal, oblate, ovoid or doliiform, usually irregular and inequilateral, with two apical germ pores. Synnemata composed of compact groups of parallel or sometimes intricate hyphae, brown, often split in two or more branches in the upper part and tapering and fading towards the tips. Conidiophores arising laterally from synnemata or from aerial hyphae, erect or flexuous, brown, septate, unbranched or simply branched near the base. Conidiogenous cells hyaline to buff, cylindrical or clavate, apically surrounded by masses of conidia, polyblastic, with conidiogenous scars after conidial secession. Conidia formed in slimy heads, single-celled, hyaline, smooth, ellipsoidal, obovate or clavate with a truncate base. Containing species with both asexual and sexual morphs.
Type species: Corynascella humicola Arx & Hodges
Notes: In his morphological treatment of Thielavia, von Arx (1973a, 1975b) classified species producing ascospores with two germ pores in Corynascus (see below in detail) and Corynascella, and species with a single germ pore in Thielavia. Corynascella was distinguished from Corynascus by lacking a myceliophthora-like asexual morph (von Arx 1973b, 1975a). In the protologue of the type species Corynascella humicola, von Arx (1975a) simply mentioned that occasionally some blastoconidia were formed in the aerial mycelium or on tips of hyphal branches. Up to now, four species have been described in this genus, but except for the type species, none of the other species produce an asexual morph (von Arx 1975b, Udagawa & Ueda 1979, von Arx et al. 1988, Guarro et al. 1997). In the present study, three of the four species were examined. Our phylogenetic analysis demonstrated that these three Corynascella species belong to three different genera (Fig. 7A, C). Corynascella is restricted only to its type species. Corynascella inaequalis is transferred below to Parachaetomium as Parach. inaequale and Corynascella inquinata is transferred to Botryotrichum as Botryot. inquinatum. The unique structure of the asexual morph in the type species was re-described and illustrated (Fig. 24). No asexual morph is reported in Corynascella arabica (Guarro et al. 1997) and the phylogenetic position of this species remains to be resolved.
Fig. 24.
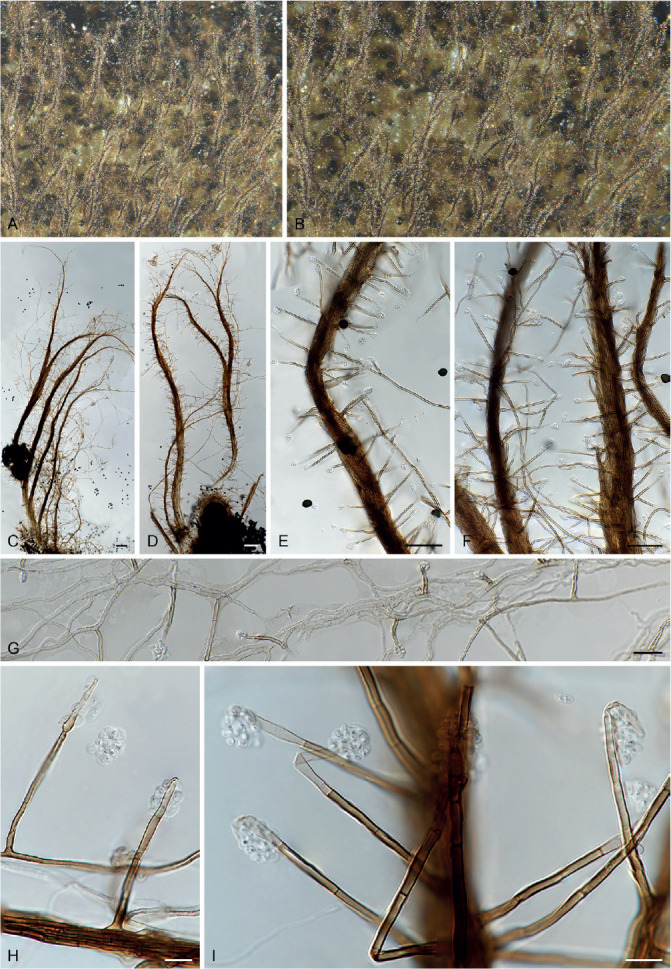
Asexual morph of Corynascella humicola (CBS 337.72, ex-type culture). A, B. Aerial part of the colony, showing synnemata bearing conidiophores on sides and apex, on CMA. C–D. Synnemata bearing conidiophores on sides and apex, mounted in lactic acid. E, F. Part of synnemata and solitary fertile hyphae, from which conidiophores arise. G. Hyphae from which solitary conidiophores arise. H, I. Conidiophores, hyaline conidiogenous cells and masses of conidia. Scale bars: C, D = 100 μm; E, F = 50 μm; G = 20 μm; H, I = 10 μm.
Corynascella humicola Arx & Hodges, Stud. Mycol. 8: 23. 1975. Fig. 23, 24.
Fig. 23.
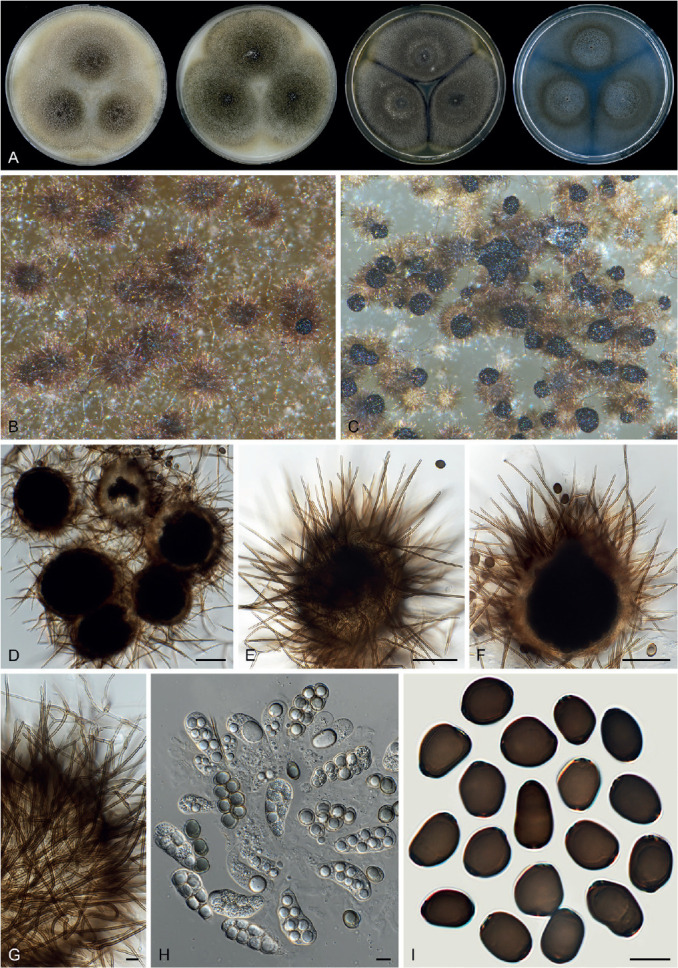
Sexual morph of Corynascella humicola (CBS 337.72, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony, showing ascomata on OA, top view; most non-ostiolate. C. Part of the colony, showing mature and ostiolate ascomata with masses of ascospores on the top, top view on OA. D–F. Ascomata mounted in lactic acid. G. Ascomatal hairs. H. Asci. I. Ascospores. Scale bars: D–F = 50 μm; G–I = 10 μm.
Micromorphology: Ascomata on OA and CMA usually superficial, sometimes immersed or sub-immersed into the medium, solitary or aggregated, non-ostiolate at the beginning, later ostiolate especially on CMA, with masses of released ascospores on the top, cinnamon due to ascomatal hairs, or fuscous black when mature due to exposed ascospores in reflected light, subglobose or ovoid, 110–170 μm high, 95–140 μm diam, usually covered by sparse aerial hyphae on which conidia are produced. Ascomatal wall brown, composed of textura epidermoidea in surface view. Ascomatal hairs straight or flexuous, brown, fading towards the tips, septate, smooth, 1–3 μm diam near the base, up to 105 μm long. Asci clavate, spore-bearing part 38–52 × 16–20 μm, with stalks being 7–20.5 μm long, containing eight biseriate or irregularly-arranged ascospores, evanescent. Ascospores dark brown when mature, irregularly ellipsoidal, oblate, ovoid or doliiform, usually irregular and inequilateral, (11–)12.5–14.5(–16.5) × (9–)9.5–11(–12.5) μm, with two apical germ pores. Synnemata usually produced in the central part of colonies on OA or forming a circle ring around the centre on CMA, composed of compact groups of parallel or sometimes intricate hyphae, brown, 10–100 μm diam near the bases, often split in two or more branches in the upper part and tapering and fading towards the tips, up to 2.5 mm long. Conidiophores arising laterally from synnemata or from aerial hyphae, erect or flexuous, brown, septate, unbranched or simply branched near the base, 2–4 μm diam near the base, 20–80 μm long. Conidiogenous cells hyaline to buff, cylindrical or clavate, surrounded by masses of conidia, 9–16 × 2–2.5 μm, polyblastic, with conidiogenous scars after conidial secession (Fig. 24H). Conidia formed compactly in slimy heads on top of the conidiogenous cells, single-celled, hyaline, smooth, ellipsoidal, obovate or clavate with a truncate base, 2.5–8 × 2–4.5 μm, schizolytic when seceding.
Culture characteristics: On OA with an entire edge, 30–36 mm diam in 7 d at 25 °C, with sparse olivaceous aerial hyphae on which simple conidiophores and conidia are produced; reverse uncoloured. On CMA with an entire edge, 30–36 mm diam in 7 d at 25 °C, olivaceous black due to masses of ascospores on top of ascomata, with a greyish sepia and floccose circle ring composed of synnemata around the central part; reverse buff. On MEA with an entire edge, about 29–35 mm diam in 7 d at 25 °C, with thick olivaceous floccose aerial hyphae covering the aggregated ascomata, obverse fuscous black; reverse buff to olivaceous black. On PCA with a lobate or crenate edge, about 27–33 mm diam in 7 d at 25 °C, translucent, obverse pale mouse grey to dark mouse grey due to simple conidiophores and ascomata; reverse olivaceous to olivaceous black due to the immersed hyphae, immersed ascomata and coloured exudates diffusing into the medium.
Material examined: USA, North Carolina, Piedmont, isolated from soil, 1971, C.S. Hodges (culture ex-type CBS 337.72); North Carolina, Piedmont, isolated from soil, 1971, C.S. Hodges (CBS 379.74).
Notes: The asexual morph of Corynascella humicola was carefully examined for the first time in the present study (Fig. 24). The same asexual morph was observed in the cultures CBS 337.72T and CBS 379.74. The synnemata seem to be better developed on CMA than on OA, MEA and PCA. Von Arx (1975a) observed solitary conidiophores in the aerial mycelium and conidia measuring 4–8 × 1.5–3 μm, similar to those arising directly from the aerial hyphae in our observations. The conidia are usually aggregated compactly in slimy heads, and it is not easy to find discrete conidia free from the aggregations. The ascomata of Corynascella humicola were always described as being non-ostiolate; however, we observed ostioles on the mature ascomata where masses of released ascospores are clearly present (Fig. 23C).
Corynascus Arx, Proc. Kon. Ned. Akad. Wetensch., C 76: 295. 1973.
Micromorphology: Ascomata superficial to immersed, often covered by aerial hyphae together with conidial structures, non-ostiolate, small (usually less than 115 μm diam), glabrous, globose or subglobose. Ascomatal wall brown, composed of textura epidermoidea in surface view. Asci obovoid, pyriform or clavate, usually with short or indistinct stalks, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, single-celled, smooth, fusiform to limoniform, with two apical, subapical or oblique germ pores. Conidiophores usually reduced to conidiogenous cells that arise laterally or terminally from hyphae. Conidiogenous cells hyaline, denticle-like or inflated and ampulliform, fusiform, clavate or obovoid, sometimes reduced to a hyphal cell, monoblastic or polyblastic synchronous. Conidia schizolytic when seceding, hyaline, single-celled, verrucose, or smooth in a few species, globose, subglobose, ellipsoidal or obovoid, sometimes with a narrow and truncate base. Containing species with both asexual and sexual morphs.
Type species: Corynascus sepedonium (C.W. Emmons) Arx
Notes: Corynascus was defined to produce homothallic ascomata together with a myceliophthora-like (historically called chrysosporium-like, see “Myceliophthora” below) asexual morph and this genus was therefore considered a sexual morph of Myceliophthora (von Arx 1973b, van Oorschot 1980, von Arx et al. 1988, Stchigel et al. 2000). Several recent studies dealt with the generic delimitation of Myceliophthora sensu lato. Van den Brink et al. (2012) studied the phylogeny of 48 strains representing five Myceliophthora and three Corynascus species, but only a limited number of other Chaetomiaceae species was included in their analysis. Even though their study indicated segregation of homothallic Corynascus from the other species, they nevertheless suggested a broad generic concept for Myceliophthora, in which all Corynascus species were included. Zhang et al. (2014a) followed the taxonomy of van den Brink et al. (2012), but Marin-Felix et al. (2015) segregated Myceliophthora sensu van den Brink et al. (2012) into four genera: the two non-thermophilic genera Corynascus and Myceliophthora and the two thermophilic genera Crassicarpon and Thermothelomyces. Phylogenetically, Corynascus is sister to Myceliophthora and Thermothelomyces (Fig. 7A). Our molecular dating analysis (Fig. 8B) indicated that Myceliophthora sensu van den Brink et al. (2012) has a mean stem age of about 41 Mya. The Corynascus clade within this Myceliophthora sensu van den Brink et al. clade diverged from the others about 35 Mya. Based on literature (von Arx 1975a, Stchigel et al. 2000, van den Brink et al. 2015, Marin-Felix et al. 2015), Corynascus species are mesophilic or thermotolerant, and no species are thermophilic. All described Corynascus species are homothallic, produce ascomata and a myceliophothora-like morph in culture. The combination of these characters sets this genus apart from the other genera in Chaetomiaceae.
Corynascus fumimontanus Y. Marín et al., Mycologia 107: 628. 2015. Fig. 25.
Fig. 25.

Corynascus fumimontanus (CBS 137294, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B–D. Part of the colony on OA, showing ascomata mixed with hyphae and conidia on OA, top view. E–G. Hyphae, conidiogenous cells and conidia. H, I. Ascomata mounted in lactic acid. J. Structure of ascomatal wall in surface view. K. Asci. L. Ascospores. Scale bars: E–G, J–L = 10 μm; H = 20 μm; I = 50 μm.
Micromorphology: See Marín-Felix et al. (2015).
Notes: Based on our measurements, Corynascus fumimontanus produces larger ascomata (up to 165 μm diam) than other Corynascus species, which are usually less than 120 μm diam. Its conidia arise laterally or terminally from hyphae rather than from well-differentiated conidiogenous cells. Marín-Felix et al. (2015) emphasised the morphological characters of this species such as verrucose ascomatal wall cells, mostly irregularly shaped ascospores and sessile conidia. Our examination showed that similar ascomatal wall cells are also found in Corynascus novoguineensis, Coryn. sexualis and Coryn. verrucosus, and ascospores of the accepted species of that genus are more or less inequilateral as well. Corynascus sepedonium produces a similar asexual morph, but has smaller ascomata (25–45 μm vs 65–165 μm), broader and shorter ascospores (12–14.5 × 7.5–9 μm vs 13–15.5 × 7.5–8.5 μm) and smooth-walled conidia (see below).
Corynascus novoguineensis (Udagawa & Y. Horie) Arx, Proc. Kon. Ned. Akad. Wetensch., C 76: 295. 1973. Fig. 26.
Fig. 26.
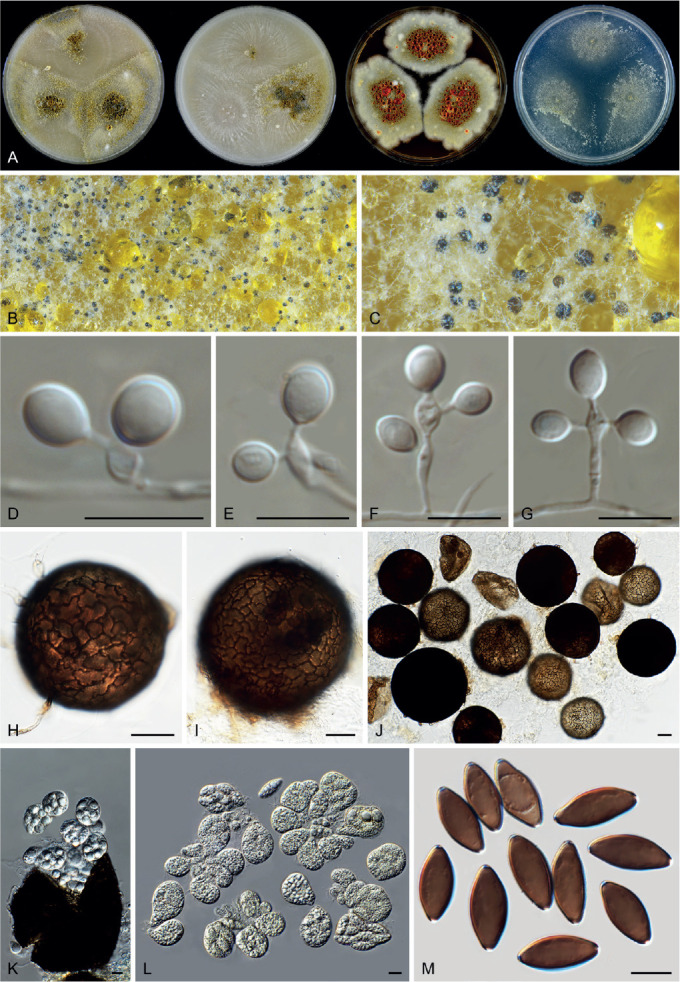
Corynascus novoguineensis (CBS 359.72, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B, C. Part of the colony on OA, showing ascomata mixed with hyphae and conidia on OA, top view. D–G. Hyphae, conidiogenous cells and conidia. H–J. Ascomata mounted in lactic acid. K. Asci coming from a broken ascoma. L. Asci. M. Ascospores. Scale bars: D–G, K–M = 10 μm; H–J = 20 μm.
Basionym: Thielavia novoguineensis Udagawa & Y. Horie, Bull. Natl. Sci. Mus. Tokyo 15: 191. 1972.
Synonym: Myceliophthora novoguineensis (Udagawa & Y. Horie) van den Brink & Samson, Fungal Diversity 52: 206. 2011 [2012], nom. inval., Art. 41.5.
Micromorphology: Ascomata superficial, often covered by aerial mycelium together with conidial structures, solitary or aggregated, non-ostiolate, honey to leaden black, glabrous, globose or subglobose, 50–115 μm diam. Ascomatal wall brown, composed of textura epidermoidea in surface view. Asci obovoid or pyriform, 24–43 × 20–28 μm, with short stalks being 2–9 μm long, containing eight irregularly-arranged ascospores, evanescent. Ascospores dark brown when mature, fusiform or ellipsoidal with attenuated ends, often inequilateral, (16.5–)18.5–21(–22) × (7.5–)8–9(–9.5) μm, with two apical germ pores. Conidiophores hypha-like, usually reduced to conidiogenous cells. Conidiogenous cells produced terminally on the short branches of hyphae or arising laterally from hyphae, hyaline, ampulliform, fusiform or obovoid due to swollen conidiogenous part, usually synchronously polyblastic, 4–10.5 × 1.5–4 μm. Conidia produced on the pedicels arising from the conidiogenous cells, hyaline, smooth, subglobose, ellipsoidal, obovoid, with a narrow and truncate base, (5–)5.5–7.5(–9) × (4.5–) 5.5 –7.5(–8.5) μm.
Culture characteristics: On OA with an entire edge, 41–47 mm diam in 7 d at 25 °C, obverse pale luteous or honey due to the formation of conidia on aerial mycelium together with liquid drops of coloured exudates on the surface of the mycelium; reverse luteous. On CMA similar to those on OA, 39–45 mm diam in 7 d at 25 °C. On MEA with a crenate or lobate edge, 32–38 mm diam in 7 d at 25 °C, texture floccose, obverse olivaceous buff due to aerial mycelium, with orange liquid drops of coloured exudates near the central part; reverse sienna. On PCA with an entire or slightly crenate edge, 36–42 mm diam in 7 d at 25 °C, with sparse aerial mycelium, obverse olivaceous buff; reverse uncoloured.
Material examined: Papua New Guinea, New Britain, Rabaul, isolated from soil, 27 Dec. 1969, S. Udagawa (culture ex-type CBS 359.72 = NHL 22501).
Notes: Corynascus novoguineensis can easily be distinguished from the other species in the genus by the production of smooth-walled conidia that often synchronously arise from swollen and polyblastic conidiogenous cells. This species also produces large ascospores (18.5–21 × 8–9 μm), while the ascospores of other Corynascus species are usually less than 16 μm in length.
Corynascus sepedonium (C.W. Emmons) Arx, Proc. Kon. Ned. Akad. Wetensch., C 76: 292. 1973. Fig. 27.
Fig. 27.
Corynascus sepedonium (CBS 111.69, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 2 wk incubation. B, C. Part of the colony on OA, showing ascomata mixed with hyphae and conidia on OA, top view. D, E. Hyphae, conidiogenous cells and conidia. F. Conidia. G, H. Mature ascomata mixed with hyphae and conidia. I. Asci coming from a broken ascoma. J. Mature ascospores and a broken ascoma. K. Ascospores. Scale bars: D–F, I–K = 10 μm; G, H = 20 μm.
Basionym: Thielavia sepedonium C.W. Emmons, Bull. Torrey Bot. Club 59: 417. 1932.
Synonyms: Myceliophthora sepedonium (C.W. Emmons) van den Brink & Samson, Fungal Diversity 52: 206. 2011 [2012], nom. inval., Art. 41.5.
Thielavia lutescens Kamyschko, Novosti Sist. Nizsh. Rast. 2: 116. 1965.
Corynascus similis Stchigel et al., Mycol. Res. 104: 881. 2000.
Myceliophthora similis (Stchigel et al.) van den Brink & Samson, Fungal Diversity 52: 206. 2011 [2012], nom. inval., Art. 41.5.
Micromorphology: Ascomata superficial, often covered by aerial mycelium together with conidial structures, solitary or aggregated, non-ostiolate, leaden black in reflected light, glabrous, globose or subglobose, 25–45 μm diam. Ascomatal wall brown, composed of angular or irregular cells in surface view. Asci clavate or pyriform, 24–28 × 13–18 μm, with indistinct stalks, containing eight irregularly-arranged ascospores, quickly evanescent. Ascospores olivaceous brown when mature, ellipsoidal with attenuated ends to fusiform, sometimes slightly inequilateral, (11.5–)12–14.5(–16) × (7–)7.5–9(–10.5) μm, with two apical germ pores. Conidiophores reduced. Conidiogenous cells arising laterally from hyphae, hyaline, ampulliform or denticle-like, monoblastic, 1.5–6 × 1–3 μm. Conidia hyaline, verrucose, globose or subglobose, (6–)7–9(10.5) μm diam, occasionally ovoid, 9.5–13 × 8–8.5 μm.
Culture characteristics: On OA with an entire edge, 31–37 mm diam in 7 d at 25 °C, obverse buff or olivaceous buff due to aerial mycelium and conidia; reverse cinnamon. On CMA with an entire edge, 28–34 mm diam in 7 d at 25 °C, obverse grey white with cinnamon margins; reverse buff to honey. On MEA with an entire or slightly crenate edge, 28–34 mm diam in 7 d at 25 °C, texture floccose, obverse buff with white margins; reverse apricot. On PCA with an entire edge, 27–33 mm diam in 7 d at 25 °C, with sparse aerial mycelium, obverse pale smoke grey; reverse uncoloured.
Material examined: India, Allahabad, isolated from soil, 1968, B.S. Mehrotra (CBS 111.69 = IMI 136625, ex-type of Coryn. sepedonium var. minor); Ajmed, isolated from soil, 2 Nov. 1995, J. Guarro (CBS 101936 = FMR 5693, ex-type of Coryn. similis). Uzbekistan, isolated from soil, date unknown, O.P. Kamyschko (CBS 632.67 = VKM F-1142, ex-type of Thielavia lutescens).
Notes: CBS 340.33 was isolated by the original author C.W. Emmons and was considered as ex-type of Coryn. sepedonium (originally Thielavia sepedonium) by von Arx (1975a). Von Arx (1975a) treated Coryn. sepedonium var. minor and Thielavia lutescens as synonyms of Coryn. sepedonium. In his study, von Arx also reported that Coryn. sepedonium had a large variation in the size and shape of ascospores and conidia. His treatment was supported by multigene phylogenetic analysis (van den Brink et al. 2012). CBS 111.69, the ex-type of Coryn. sepedonium var. minor, was often incorrectly assumed to be the ex-type of Coryn. sepedonium (van den Brink et al. 2012, Marin-Felix et al. 2015). According to von Arx (1975a), the type strain of Coryn. sepedonium (CBS 340.33) produced similar sized conidia (8–12 μm), but larger ascospores than those of CBS 111.69 [15–19 × 8–10 μm vs (11.5–)12–14.5(–16) × (7–)7.5–9(–10.5) μm]. Our measurements of ascomata of CBS 111.69 (25–45 μm diam) were also much smaller than those in the previous description given by von Arx (50–120 μm diam, 1975a) or by Malloch & Cain (20–150 μm diam, 1973). Its asci are evanescent and difficult to observe and measure. Our phylogenetic analysis (Fig. 7A) confirmed that the ex-type culture of Coryn. similis clustered with the strains of Coryn. sepedonium, and we therefore follow Marín-Felix et al. (2015) and treat Coryn. similis as a synonym of Coryn. sepedonium. In the original description, however, Coryn. similis was described to produce ascospores with two subapical or oblique germ pores. We did not see such ascospores and cannot confirm this observation.
Corynascus sexualis Stchigel et al., Mycol. Res. 104: 880. 2000. Fig. 28.
Fig. 28.
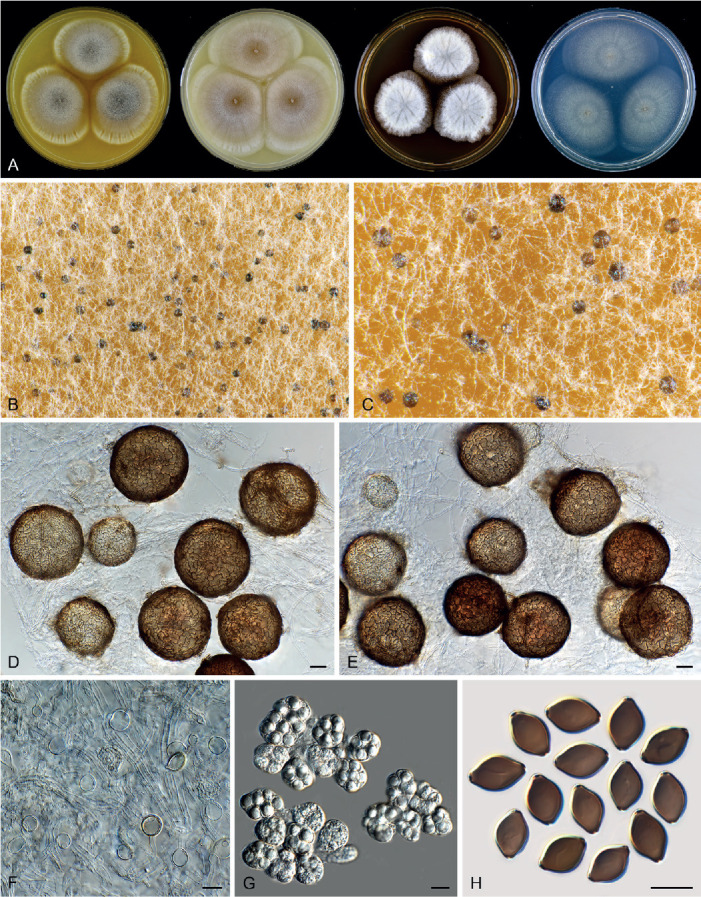
Corynascus sexualis (CBS 827.96, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B, C. Part of the colony on OA, showing ascomata mixed with hyphae and conidia on OA, top view. D, E. Ascomata, hyphae and conidia mounted in lactic acid. F. Hyphae and conidia. G. Asci. H. Ascospores. Scale bars: D, E = 20 μm; F–H = 10 μm.
Synonym: Myceliophthora sexualis (Stchigel et al.) van den Brink & Samson, Fungal Diversity 52: 206. 2011 [2012], nom. inval., Art. 41.5.
Micromorphology: Ascomata superficial to immersed in the medium, often covered by aerial mycelium, solitary or aggregated, non-ostiolate, fawn to olivaceous in reflected light, glabrous, globose or subglobose, 60–115 μm diam. Ascomatal wall brown, composed of textura epidermoidea in surface view. Asci obovoid or pyriform, 18–25 × 14–18 μm, with short stalks being 3–7 μm long, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, limoniform or broad fusiform, umbonate at both ends, sometimes slightly inequilateral, 11.5–13.5(–14.5) × 8–9 μm, with two apical germ pores. Conidiophores reduced. Conidiogenous cells reduced to a hyphal cell, inconspicuous, monoblastic. Conidia inconspicuous, arising laterally or terminally from hyphae, subhyaline, thin-walled, smooth, subglobose, ellipsoidal, obovoid, 6–11 × 5–10.5 μm.
Culture characteristics: On OA with an entire or slightly lobate edge, 24–30 mm diam in 7 d at 25 °C, obverse olivaceous buff or smoke grey due to ascomata mixed with aerial mycelium, pale luteous to luteous around the colonies due to coloured exudates diffusing into the mycelium; reverse pale luteous to sienna. On CMA with an entire edge, 26–32 mm diam in 7 d at 25 °C, less producing ascomata, obverse buff to rosy buff, without coloured exudates; reverse pale luteous to luteous. On MEA with a slightly lobate edge, 16–22 mm diam in 7 d at 25 °C, texture floccose, obverse white due to aerial mycelium; reverse luteous to orange. On PCA translucent, with an entire or slightly crenate edge, 22–28 mm diam in 7 d at 25 °C, without or with sparse aerial mycelium; reverse uncoloured.
Material examined: India, Jaipur, isolated from soil, Oct. 1995, J. Guarro (culture ex-type CBS 827.96 = FMR 5691 = IMI 378520).
Notes: The conidiophores and conidiogenous cells of Coryn. sexualis are extremely reduced, and its conidia are sparsely produced. This might explain why the original description of this species lacks the description of an asexual morph. Phylogenetic analysis showed that Coryn. sexualis is closely related to Coryn. citrinus and Coryn. fumimontanus (Fig. 7A). These species can be identified using tub2 and rpb2 sequencing; the ITS sequence fails to distinguish the three species (Supplementary Figs S1–S3). Corynascus sexualis is distinct in sparsely producing conidia, and can also be distinguished from Coryn. citrinus by its larger ascospores (11.5–13.5 × 8–9 μm vs 9–12 × 6–8 μm) and from Coryn. fumimontanus by shorter and broader ascospores (11.5–13.5 × 8–9 μm vs 13–15.5 × 7.5–8.5 μm).
Corynascus verrucosus Stchigel et al., Mycol. Res. 104: 884. 2000. Fig. 29.
Fig. 29.
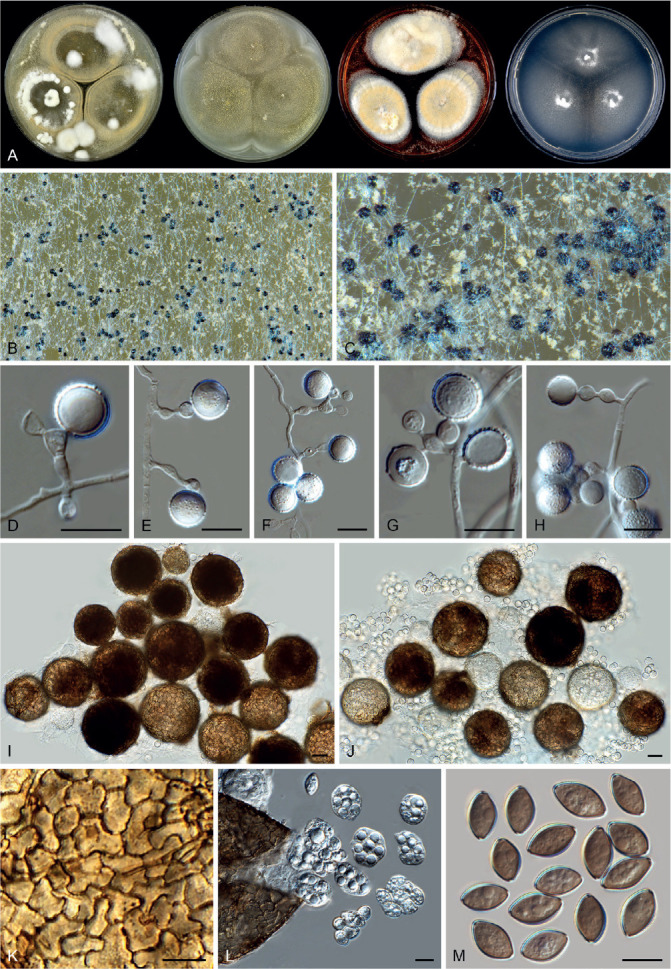
Corynascus verrucosus (CBS 602.97, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 4 wk incubation. B, C. Part of the colony on OA, showing ascomata mixed with hyphae and conidia on OA, top view. D–H. Hyphae, conidiogenous cells and conidia. I, J. Ascomata and conidia mounted in lactic acid. K. Structure of ascomatal wall in surface view. L. Asci coming from a broken ascoma. M. Ascospores. Scale bars: D–H, K–M = 10 μm; I, J = 20 μm.
Synonym: Myceliophthora verrucosa (Stchigel et al.) van den Brink & Samson, Fungal Diversity 52: 206. 2011 [2012], nom. inval., Art. 41.5.
Micromorphology: Ascomata superficial, often covered by aerial mycelium together with conidial structures, solitary or aggregated, non-ostiolate, leaden black in reflected light, glabrous, globose or subglobose, 50–85 μm diam. Ascomatal wall brown, composed of textura epidermoidea in surface view. Asci obovoid or pyriform, 20–28 × 14–22 μm, with short or indistinct stalks being 0–5 μm long, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, ellipsoidal with attenuated ends or fusiform, often inequilateral, (9–)11–13(–13.5) × (6–)7–8 μm, with two apical or slightly subapical germ pores. Conidiophores hypha-like or reduced to conidiogenous cells. Conidiogenous cells arising laterally from hyphae, hyaline, often sympodially polyblastic or proliferating, swollen to ampulliform, doliiform or clavate, 5–15 × 1.5–4 μm. Conidia produced on pedicels arising from the conidiogenous cells, hyaline, verrucose, globose or subglobose, (6–)7.5–9.5(–11) μm diam.
Culture characteristics: On OA with an entire edge, 37–43 mm diam in 7 d at 25 °C, obverse olivaceous buff due to the formation of conidia on aerial mycelium, often partly with white floccose aerial mycelium; reverse ochreous. On CMA similar to those on OA, 37–43 mm diam in 7 d at 25 °C, obverse buff. On MEA with an entire or slightly crenate edge, 27–33 mm diam in 7 d at 25 °C, texture floccose, obverse white to buff; reverse apricot to chestnut. On PCA translucent, with an entire edge, 23–29 mm diam in 7 d at 25 °C, without aerial mycelium, obverse buff; reverse buff.
Material examined: Argentina, Quilmes, Buenos Aires Province, isolated from soil, Aug. 1996, A.M. Stchigel (culture ex-type CBS 602.97 = FMR 5904 = IMI 378522). USA, Tennessee, Great Smokey Mountain National Park, isolated from forest soil, 10 Aug. 2008, A. Miller, M. Calduch & A. Stchigel (CBS 135878 = FMR 12783).
Notes: Phylogenetic analysis shows that Coryn. verrucosus is sister to Coryn. sepedonium (Fig. 7A). Partial tub2 and rpb2 sequencing can be used for identification but ITS fails to distinguish the two species (Supplementary Figs S1–S3). This species can be distinguished from Coryn. sepedonium by larger, better developed conidiogenous cells (2–15 × 1.5–4 μm vs 1.5–6 × 1–3 μm) which are polyblastic or proliferating and often swollen. This species was originally described to produce ascospores with a larger length range (11–18 μm, Stchigel et al. 2000) than what we measured (11–13 μm).
Humicola Traaen, Nytt Mag. Naturvidensk. 52: 31. 1914.
Micromorphology (emended description): Asexual morphs producing aleurioconidia-like conidia, humicola-like, an acremonium-like morph co-occurring in several species. Humicola-like morph: conidiogenous cells reduced to a hyphal cell, intercalary or lateral, monoblastic; conidiophores absent. Aleurioconidia-like conidia arising laterally, intercalary or terminally, 1-celled, solitary or rarely in chains of a few spores, globose, subglobose, oblate, occasionally obovoid, pyriform or irregular-shaped, light olivaceous, olivaceous, brown or dark brown, smooth, in persisted state on hyphae or rhexolytic when seceding, germ pores rare. Acremonium-like morph: Phialides lateral or occasionally terminal, hyaline. Conidia in basipetal chains, hyaline, aseptate, smooth, obovoid, usually with a truncated base and a rounded apex. Ascomata absent or present, when present superficial, or covered by aerial hyphae, ostiolate. Ascomatal wall brown, composed of textura angularis in surface view. Terminal hairs seta-like, flexuous, undulate, or arcuate with apices incurved. Asci clavate, with eight biseriate or irregularly-arranged ascospores, evanescent before ascospores become mature. Ascospores limoniform to quadrangular, bilaterally flattened, with an apical germ pore. Containing asexual species and species with both asexual and sexual morphs.
Type species: Humicola fuscoatra Traaen
Notes: In our phylogenetic analyses, we noticed that our previously defined Humicola (MP-BS < 50 %, ML-BS = 78 %; PP = 1.0, Wang et al. 2019a) seemed unstable. With the addition of rpb2 sequences of CBS 113678 and CBS 538.74 to our analysis, representing Aporothielavia, Humicola splits into two clades, with one of them clustering with Aporothielavia (ML-BS < 70 %; PP = 1.0, Supplementary Fig. S3). This result suggested that not all the species in the Humicola clade share a common recent ancestor. Molecular dating analysis reinforced our suspicion that a small clade splits from the other Humicola species and is here named Aporothielavia (Fig. 8A). Molecular dating analysis was based on a dataset in which ITS1, ITS2 and introns in protein coding genes were excluded. The topology of the resulting tree is expected to be more stable than the normal phylogenetic tree. Therefore, we segregate Humicola sensu Wang et al. (2019a) into two genera. Our molecular dating estimation showed that the two “Humicola” clades diverged from each other as early as about 60 Mya, supporting their segregation. Humicola sensu stricto is modified as shown above, and a new genus (Pseudohumicola) is proposed for the other clade (see below for more details). Morphologically, both genera produce similar asexual morphs, but the ascomata in Pseudohumicola (if produced) usually have coiled terminal hairs, while such hairs are rare in sexual Humicola s. str. species.
Humicola hirsuta X.Wei Wang, P.J. Han & F.Y. Bai, sp. nov. MycoBank MB 840128. Fig. 30.
Fig. 30.

Humicola hirsuta (CGMCC 3.20444, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony showing mature ascomata on OA, top view. C. Mature ascomata on OA, side view. D, E. Ascomata mounted in lactic acid. F. Structure of ascomatal wall in surface view. G. Terminal ascomatal hair. H. Asci. I. Ascospores. J. Hyphae and conidia. Scale bars: D = 100 μm; E = 50 μm; F, G = 20 μm; H–J = 10 μm.
Etymology: The name refers to its terminal ascomatal hairs, which are relatively long and erect.
Micromorphology: Ascomata superficial, ostiolate, leaden black with honey hairs in reflected light, elongated obpyriform, obclavate or ampulliform below, apically attenuated to an elongated conical or short cylindrical neck, 190–290 μm high, 70–130 μm diam at the widest part. Ascomatal wall brown, composed of angular and irregular cells, or elongate to cylindrical cells in the neck part in surface view. Terminal hairs around ostiole relatively short, seta-like and delicate, smooth, tapering and fading to hyaline towards the tips, 1.5–4 μm diam near the base, usually surrounded by numerous long, thick and seta-like hairs which are 3.5–5.5 μm diam near the base, closely septate. Lateral hairs similar to thick terminal ones, tapering and fading towards the tips. Asci clavate, spore-bearing part 23–31 × 9–12 μm, with stalks about 12–25 μm long, containing eight biseriate or irregularly-arranged ascospores, evanescent. Ascospores olivaceous or olivaceous brown when mature, limoniform, biapiculate or slightly umbonate at both ends, bilaterally flattened, (7–)7.5–9(–10.5) × (5.5–)6–7.5(–8) × 4.5–5.5 μm, with an apical germ pore. Conidia usually subglobose, arising laterally or terminally from the hyaline aerial hyphae, solitary, sometimes two cells in chains or several in clusters, cinnamon to fawn, 6–10.5 μm diam.
Culture characteristics: Colonies on OA 33–39 mm diam after 7 d at 25 °C; edge entire or slightly crenate; obverse showing leaden black mature ascomata mixed with young ascomata covered by pale luteous to amber ascomata hairs, and sparse white aerial hypha; soluble pigment absent; reverse uncoloured. Colonies on CMA similar to those on OA, 31–37 mm diam after 7 d at 25 °C. Colonies on MEA 29–35 mm diam after 7 d at 25 °C; edge entire, obverse showing a thin layer of white aerial mycelium mixed with sparse ascomata; reverse saffron to ochreous. Colonies on PCA 27–33 mm diam after 7 d at 25 °C, edge entire; translucent; aerial hyphae absent; soluble pigment absent; reverse uncoloured.
Material examined: China, Qinling Mountains in Shaanxi Province, isolated from soil, Jun. 2004, X.W. Wang (holotype HMAS 350292, isotype CBS H-23638, culture ex-type CGMCC 3.20444 = CBS 144492 = WXW 9028).
Notes: Humicola hirsuta is phylogenetically most closely related to H. mutabilis (Fig. 7B) and this relationship is also concordant among the single gene phylograms (Supplementary Figs S1–S3). Humicola hirsuta produces uniformly-shaped ascomata and long, honey (in reflected light) terminal hairs around the beak, while H. mutabilis produces ascomata that are variable in shape, with buff to amber hairs in reflected light. Humicola hirsuta is morphologically similar to H. ampulliella, but differs in ascus and ascospore shape. Humicola ampulliella produces narrowly clavate to cylindrical asci and prominently umbonate ascospores, while clavate asci and biapiculate or slightly umbonate ascospores are present in H. hirsuta.
Melanocarpus Arx, Stud. Mycol. 8: 17. 1975.
Micromorphology (fide von Arx et al. 1988, Guarro et al. 1996): Colonies expanding rapidly. Ascomata superficial, non-ostiolate, spherical, smooth, black. Ascomatal wall dark brown, composed of textura angularis in surface view. Asci fasciculate, obovate or clavate, stalked, containing eight ascospores, evanescent. Ascospores bilaterally flattened, globose to broadly ovate in face view and elliptical in side view, dark brown, with an apical germ pore. Conidia usually catenate, cylindrical, fusiform or clavate with a truncate base, hyaline. Thermophilic. Containing species with both asexual and sexual morphs.
Type species: Melanocarpus albomyces (Cooney & R. Emers.) Arx
Notes: Melanocarpus was first introduced for Myriococcum albomyces Cooney & R. Emers., a thermophilic species producing non-ostiolate ascomata with a pseudoparenchymatous wall, ovoid-oblate ascospores with an apical germ pore, and hyaline, cylindrical, fusiform or clavate, usually catenate conidia (von Arx 1975a). Later, four more species (Mel. coprophilus, Mel. oblatus, Mel. tardus and Mel. thermophilus) were described or transferred to the genus, all with similar ascomata and ascospores (Guarro et al. 1996, Wang et al. 2016b).
Our present analysis indicates that the morphologically defined Melanocarpus is polyphyletic. Melanocarpus oblatus is a synonym of Achaetomium globosum (Fig. 7C, Supplementary Figs S1–S3). In the phylogram based on the combined dataset, Mel. tardus and Mel. thermophilus cluster together as a sister clade to Mel. albomyces (Fig. 7D). A similar clustering is observed in the rpb2 phylogram (Supplementary Fig. S3), but the tub2 and ITS phylogenies place Mel. albomyces distantly from the two other species (Supplementary Figs S1, S2). These three species have different growth rates and temperature growth profiles (Fig. 39): Mel. albomyces grows fast and has a higher growth rate at 45 °C than at 37 °C (thermophilic), while Mel. tardus and Mel. thermophilus grow very slowly and show optimal growth at 37 °C (thermotolerant). Molecular dating analysis shows that Mel. albomyces diverged from the two other species quite early (about 59.97 Mya, Fig. 8B). Therefore, we restrict Melanocarpus to its thermophilic type species and at the same time, the new genus Parvomelanocarpus is proposed for Mel. tardus and Mel. thermophilus (see below for more details).
Fig. 39.
Comparison of growth temperature between Melanocarpus and Parvomelanocarpus. Left to right: 5-d-old colonies on OA 25 °C, OA 37 °C, OA 45 °C, PDA 25 °C, PDA 37 °C, PDA 45 °C; top to bottom: Melanocarpus albomyces CBS 638.94, Parvomelanocarpus tardus CBS 541.76, Parvomelanocarpus thermophilus CBS 886.97.
No material of Mel. coprophilus was included in our study. According to the original description (Guarro et al. 1996), this species is mesophilic and does not produce an asexual morph. It is therefore unlikely that this species belongs to Melanocarpus.
Myceliophthora Costantin, Compt. Rend. Hebd. Séances Acad. Sci. D 114: 849. 1892.
Micromorphology: Conidiophores absent. Conidiogenous cells reduced to a hyphal cell, or originating laterally or terminally from hyphae, swollen, subglobose, fusiform, clavate or ampulliform, monoblastic or synchronously polyblastic with one or more conidia developing from one conidiogenous cell. Conidia solitary or in short acropetal chains, single-celled, smooth, hyaline, ovoid or subglobose, apically rounded, often with a narrow and truncate base, rhexolytic when seceding. Sexual morph not observed. Thermotolerant.
Type species: Myceliophthora lutea Costantin
Notes: For many years, thermophilic species that produce single-celled blastoconidia with narrow bases attached directly to hyphae or conidiogenous cells were placed in Myceliophthora (van Oorschot 1977, Berka et al. 2011, van den Brink et al. 2012, Zhang et al. 2014a). The taxonomy of this genus has long been tumultuous.The type species, Myceliophthora lutea was first described as a pathogen in mushroom cultivation (Costantin 1892). Apinis (1962) described Sporotrichum thermophilum, a thermophilic species that produces a conidial morph similar to that of My. lutea. In the same year, Carmichael (1962) transferred My. lutea into his broad genus Chrysosporium as Chry. luteum. Von Arx (1973a) re-described Sporotrichum as a basidiomycete genus because clamp connections were observed on the septa of the hyphae of the type species, Sporotrichum aureum, and suggested moving Chry. luteum from Chrysosporium back to Myceliophthora. Other “sporotrichum-like” fungi were classified in Chrysosporium, which produce conidia with a broad base (being separated from the conidiogenous cell by a cross wall) and lack clamp connections, such as Sp. thermophilum (von Arx 1973a). Later, another thermophilic species, Chrysosporium fergusii was described (von Klopotek 1974). Van Oorschot (1977) formally reintroduced Myceliophthora for species producing blastoconidia with a narrow base and lacking intercalary arthroconidia. Three species mentioned above were accepted in Myceliophthora: the type species My. lutea, My. thermophila (= Sporotrichum thermophilum) and My. fergusii (= Chrysosporium fergusii). Marín-Felix et al. (2015) suggested to restrict Myceliophthora only to its type species My. lutea on the basis of their multigene phylogenetic analysis. Our phylogenetic analyses (Fig. 7A) confirmed the treatment of Marín-Felix et al. (2015). Myceliophthora lutea grows faster at 37 °C than at 45 °C, indicating that it is thermotolerant rather than thermophilic. Our molecular dating analysis indicated that Myceliophthora diverged from its thermophilic relatives about 30 Mya, before the later time limit (about 27 Mya, Fig. 8, 9) of the other accepted genera in the family.
Myceliophthora lutea Costantin, Compt. Rend. Hebd. Séances Acad. Sci., Sér. D 114: 850.1892. Fig. 31.
Fig. 31.
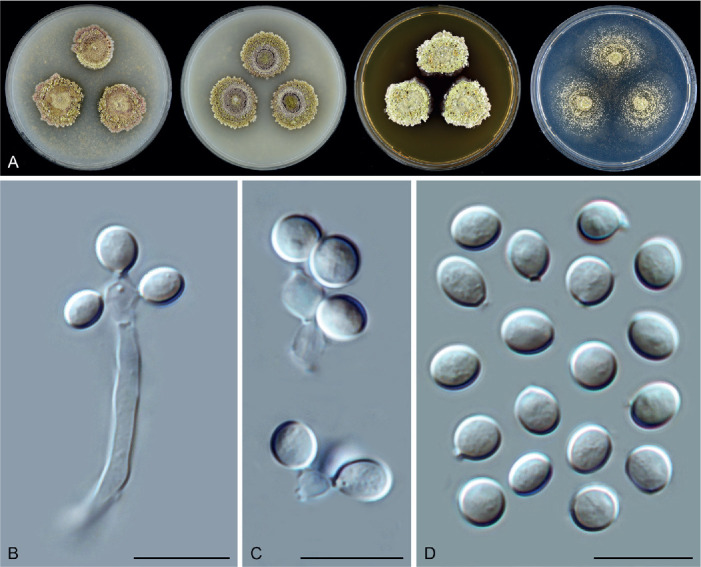
Myceliophthora lutea (CBS 145.77, ex-neotype culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B, C. Conidiogenous cells and conidia. D. Conidia. Scale bars = 10 μm.
Synonyms: Scopulariopsis lutea (Costantin) Tubaki, Nagaoa 5: 29. 1955.
Chrysosporium luteum (Costantin) J.W. Carmich., Canad. J. Bot. 40: 1158. 1962.
Sporotrichum carthusioviride J.N. Rai & Mukerji, Mycopathol. Mycol. Appl. 18: 122. 1962.
Micromorphology: Conidiophores absent. Conidiogenous cells often reduced to a hyphal cell, or originating laterally or terminally from hyphae, swollen, subglobose, fusiform, clavate or ampulliform, synchronously polyblastic or monoblastic, 1–4 conidia developing from one conidiogenous cell, 3–5 × 2.5–3.5 μm. Conidia solitary or in short chains, single-celled, smooth, hyaline, ovoid or subglobose, rhexolytic when seceding, with a truncate base, (3.5–)4.5–5.5(–6) × (3–)3.5–4.5(–5) μm diam. Sexual morph unknown.
Culture characteristics: On OA with a crenate edge, 11–17 mm diam in 7 d at 25 °C, texture cottony, obverse olivaceous buff or hazel due to conidia mixed with aerial mycelium, isabelline around the colonies due to coloured exudates diffusing in to the medium; reverse olivaceous. On CMA similar to those on OA, obverse olivaceous buff or greenish olivaceous. On MEA with a crenate edge, 11–17 mm diam in 7d at 25 °C, texture cottony, obverse olivaceous buff; reverse umber. On PCA with an entire edge, 12–18 mm diam in 7 d at 25 °C, obverse olivaceous buff due to the formation of conidia on aerial mycelium, without coloured exudates; reverse olivaceous buff.
Material examined: India, Uttar Pradesh, Lucknow, isolated from usar soil, date unknown, Rai & Mukerji (CBS 379.76, ex-type culture of Sporotrichum carthusioviride). UK, Newmarket, isolated from hay, 1974, M.T. Archer (culture ex-neotype CBS 145.77 = IMI 182034).
Notes: Myceliophthora lutea has been isolated from mushroom beds, soil, hay, Hordeum vulgare, air in pig sty, and dust in a stable (van Oorschot 1977). This species produces a similar asexual morph as Thermothelomyces species, but can be distinguished by its restricted growth on the agar media (Fig. 31A) and by its thermotolerant rather than thermophilic nature. Because a type specimen was not designated by the original author with no illustration in the original publication, van Oorschot (1977) designated CBS 145.77 as neotype for this species.
Ovatospora X.Wei Wang et al., Stud. Mycol. 84: 207. 2016.
Micromorphology and illustrations: See Wang et al. (2016b; p. 207, 214–216). Containing species with only the sexual morph.
Type species: Ovatospora brasiliensis (Batista & Pontual) X.Wei Wang et al.
Notes: The genus Ovatospora is mainly characterised by its ascospore shape and the arrangement of these ascospores in the asci (Wang et al. 2016b). The ascospores of Ovatospora are broadly ovate, bilaterally flattened, rounded at one end, with an apical or subapical germ pore at another attenuate or apiculate end. The eight ascospores are usually uniseriate in cylindrical asci, in a few species biseriate or irregularly-arranged in clavate asci. Species in the genus produce ostiolate ascomata with walls of textura angularis in surface view, and usually covered by coiled terminal hairs, sometimes with coiled branches, which were originally placed in Chaetomium. Two more chaetomium-like species proved to be members of this genus based on our phylogenetic analysis (Fig. 7D). Morphologically, each taxon fits the definition of Ovatospora (Udagawa & Muroi 1981, Wang et al. 2016b, Zhang et al. 2017).
Ovatospora amygdalispora (Udagawa & T. Muroi) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840155.
Basionym: Chaetomium amygdalisporum Udagawa & T. Muroi, Trans. Mycol. Soc. Japan 22: 13. 1981.
Notes: This species is combined in Ovatospora based on our phylogenetic analysis of the ex-type culture (Fig. 7D). It is closely related to O. senegalensis (Fig. 7D), but the latter produces smaller ascospores with a subapical or oblique germ pore (9–11 × 7–8 × 6–7 μm vs 13–18 × 10–14 × 9–12 μm). Apparently, O. amygdalispora is the species with the largest ascospores in the genus. Von Arx et al. (1986) treated this species as a synonym of Ch. uniapiculatum, but there is no type material of the latter species available to confirm this treatment using DNA sequence data.
Ovatospora angularis (Yu Zhang & L. Cai) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840156.
Basionym: Chaetomium angulare Yu Zhang & L. Cai, Fungal Biol. 121: 28. 2016.
Notes: Ovatospora angularis produces broadly ovate and bilaterally flattened ascospores with an apical germ pore, uniseriate in cylindrical asci (Zhang et al. 2017), morphologically fitting the definition of Ovatospora. Species previously recognised in Ovatospora produce coiled terminal hairs covering their ostiolate ascomata. Ovatospora angularis is the only known Ovatospora species producing flexuous or slightly undulate ascomatal hairs with no differentiation between terminal and lateral hairs. It is phylogenetically closely related to O. unipora (Fig. 7D), but the latter produces clavate asci and larger ascospores (9–11 × 8–10 × 5–7 μm vs 6.5–8.5 × 5.5–7.5 × 5–6 μm) in addition to their difference in ascomatal hairs.
Parachaetomium Mehrabi et al., Mycol. Prog. 19: 1422. 2020.
Micromorphology (emended description): Ascomata superficial, sometimes immersed in the medium, ostiolate, non-ostiolate in one species (Parach. inaequale), globose, subglobose to ovate. Ascomatal wall brown, composed of irregular or angular cells. Ascomatal hairs highly diverse, some verrucose, undulate to loosely coiled, or irregularly coiled, erect or flexuous in the lower part, with lateral hairs flexuous; or finger-like (short) to hypha-like, unbranched, straight or flexuous, finely verrucose, covering the whole ascoma or without differentiation between terminal and lateral ones; sometimes with two distinct types of hairs (called type I and type II): type I numerous, shorter and thinner, often arcuate, apically circinate, undulate or irregularly coiled, verrucose, brown, tapering and fading towards the tips; type II only a few, longer and thicker, undulate or loosely coiled, verrucose, brown, tapering towards the tips, sometimes recurved or circinate at the apex. Asci fasciculate, fusiform, clavate or pyriform, stalked, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous or olivaceous brown when mature, elongated ellipsoidal or fusiform, with an apical, subapical or oblique germ pore, or with two apical germ pores, each at one end. Asexual morph not observed.
Type species: Parachaetomium perlucidum (Sergejeva) X.Wei Wang & Houbraken
Notes: Parachaetomium was introduced to accommodate three chaetomium-like species with Parach. iranianum as the type species (Mehrabi et al. 2020). Our phylogenetic analysis revealed that Parach. iranianum resides in a clade along with the older species Ch. perlucidum, here combined in Parachaetomium as Parach. perlucidum (Fig. 7A, Supplementary Figs S1–S3). The species resemble each other morphologically (von Arx et al. 1986, Mehrabi et al. 2020) and we therefore consider Parach. iranianum a synonym of Parach. perlucidum. Nine more species are transferred to this genus, resulting in a total of eleven accepted species (Fig. 7A). All species only produce a sexual morph. A high morphological diversity is present (for more details, see below). Ten species produce ostiolate ascomata and were previously classified in Chaetomium. Parachaetomium inaequale produces non-ostiolate ascomata and was previously classified in Corynascella.
Parachaetomium biporatum (Cano & Guarro) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 830926. Fig. 32.
Fig. 32.
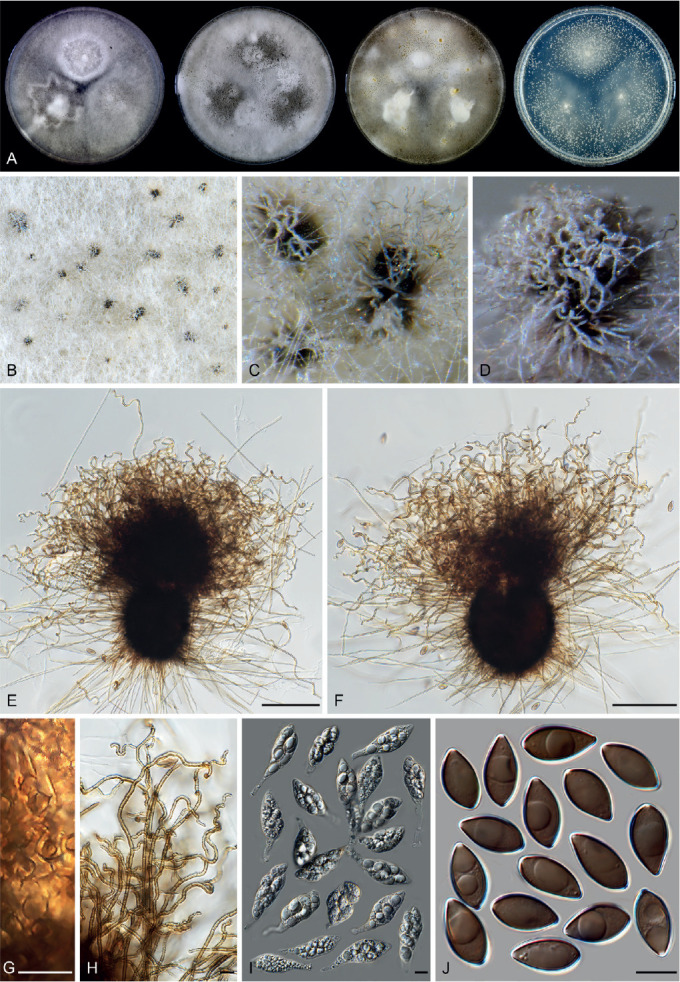
Parachaetomium biporatum (CBS 244.86, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 5 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D. Mature ascomata on OA, side view. E, F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: E, F = 100 μm; G–J = 10 μm.
Basionym: Chaetomium biporatum Cano & Guarro, Nova Hedwigia 44: 543. 1987.
Micromorphology: Ascomata superficial, solitary, usually covered by white aerial mycelium, leaden black due to masses of ascospores, with smoke grey ascomatal hairs in reflected light, subglobose, ostiolate, 95–165 μm high, 95–155 μm diam. Ascomatal wall brown, composed of textura epidermoidea in surface view. Terminal hairs brown, septate, verrucose, irregularly undulate to irregularly coiled, often with undulate to irregularly coiled branches, erect or flexuous at lower part, 1.5–3 μm diam near the base. Lateral hairs flexuous. Asci fusiform, spore-bearing part 34–51 × 16–24 μm, with stalks being 10–36.5 μm long, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, elongated ovoid or fusiform, often inequilateral, (13.5–)15.5–18(–19.5) × (7.5–)8–9(–10) μm, with two apical germ pores. Asexual morph unknown.
Culture characteristics: On OA with an entire edge, 34–40 mm diam in 7 d at 25 °C, texture floccose, obverse white due to aerial mycelium; reverse buff. On CMA similar to those on OA, 31–37 mm diam in 7 d at 25 °C; reverse buff to ochreous. On MEA with an entire edge, 30–36 mm diam in 7 d at 25 °C, obverse white or grey olivaceous due to ascomata mixed with aerial mycelium, reverse ochreous to olivaceous grey. On PCA with an entire edge, 30–36 mm diam in 7 d at 25 °C, without aerial mycelium, producing pale olivaceous grey ascomata, without coloured exudates; reverse uncoloured.
Material examined: Spain, Valencia, Betera, isolated from soil, Aug. 1985, J. Guarro (culture ex-type CBS 244.86 = FMR 854 = IMI 330348).
Notes: Parachaetomium biporatum can be distinguished from the other known species in the genus by the production of elongated ovoid or fusiform ascospores with two apical germ pores (Fig. 32J) and by the occurrence of numerous irregularly undulate to coiled terminal hairs with undulate to irregularly coiled branches (Fig. 32H).
Parachaetomium carinthiacum (Sörgel) Mehrabi et al., Mycol. Prog. 19: 1422. 2020. Fig. 33.
Fig. 33.
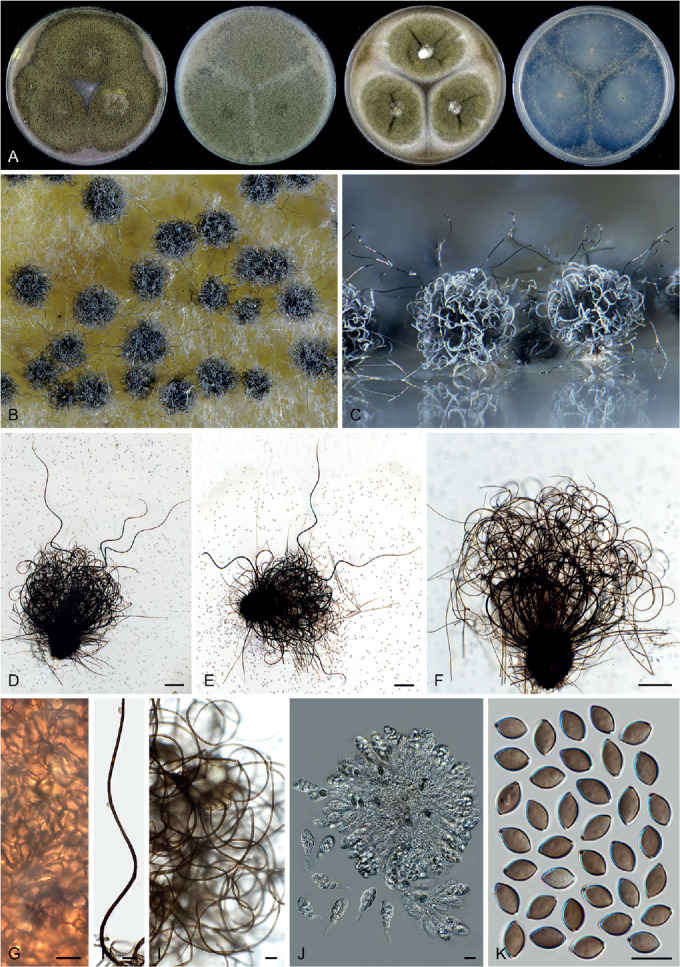
Parachaetomium carinthiacum (CBS 153.81, ex-epitype culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 18 d incubation. B. Part of the colony showing mature ascomata on OA, top view. C. Mature ascomata on OA, side view. D–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Part of a long terminal ascomatal hair. I. Short terminal ascomatal hairs. J. Asci. K. Ascospores. Scale bars: D–F = 100 μm; H, I = 20 μm; G, J–K = 10 μm.
Basionym: Chaetomium carinthiacum Sörgel, Arch. Mikrobiol. 40: 393. 1961.
Micromorphology: Ascomata superficial, mouse grey in reflected light due to ascomatal hairs, globose or ovoid, ostiolate, 140–190 μm high, 125–165 μm diam. Ascomatal wall brown, composed of angular or irregular cells in surface view. Terminal hairs of two types: type I numerous, shorter, often erect or arcuate in the lower part, irregularly undulate to loosely and irregularly coiled in the upper part, verrucose, brown, septate, tapering and fading towards the tips, 2–4 μm diam near the base; type II only a few, longer, undulate, verrucose, brown, septate, tapering towards the tips, sometimes recurved or circinate at the apex, 4–6 μm diam near the base. Lateral hairs straight or flexuous. Asci fusiform or clavate, spore-bearing part 20–31.5 × 10.5–13 μm, with stalks being 6–16.5 μm long, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous when mature, ellipsoidal-fusiform, attenuated at both ends, sometimes often slightly inequilateral, (7.5–)8–9(–10) × 5–6 μm, with an apical or subapical to oblique germ pore. Asexual morph unknown.
Culture characteristics: On OA with an entire edge, 39–45 mm diam in 7 d at 25 °C, without aerial mycelium, grey olivaceous due to ascomata; reverse olivaceous grey. On CMA similar to those on OA, obverse greenish olivaceous; reverse olivaceous buff to honey. On MEA with an entire edge, 42–48 mm diam in 7 d at 25 °C, obverse greenish olivaceous with white margins due to aerial mycelium; reverse cinnamon to umber. On PCA with an entire edge, 37–43 mm diam in 7 d at 25 °C, without aerial mycelium and coloured exudates; reverse uncoloured.
Material examined: Lectotype designated here: Abb. 7 a, b. in Sörgel, Arch. Mikrobiol. 40: 392, 1961 (based on the ex-type culture from a dead leaf collected in Germany), MBT 10002835. France, Meylan, date and substrate unknown, Laboratoire de Biologie Végétale Cryptogamie Meylan (CBS H-10007, epitype of Chaetomium carinthiacum designated here, MBT 10002836, culture ex-epitype CBS 153.81). Japan, isolated from Thymus sp., date unknown, S. Udagawa (CBS 665.82 = NHL 2884).
Notes: Parachaetomium carinthiacum is characterised by the production of two types of terminal ascomatal hairs. Another species in the genus, Parach. muelleri, also has two types of terminal ascomatal hairs. The two species are sister taxa (Fig. 7A). They are indistinguishable in ITS phylogeny (Supplementary Fig. S1), but can be identified using tub2 and rpb2 sequencing. Parachaetomium carinthiacum differs from Parach. muelleri by numerous short type I hairs, which are often erect or arcuate in the lower part, and irregularly undulate to loosely and irregularly coiled in the upper part, while type I hairs of Parach. muelleri are arcuate, but relatively sparse, some may be apically recurved, but not undulate or coiled. Type II terminal hairs of Parach. carinthiacum are flexuous or undulate, but never coiled like those of Parach. muelleri.
Parachaetomium hispanicum (Guarro & Arx) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 830927. Fig. 34.
Fig. 34.
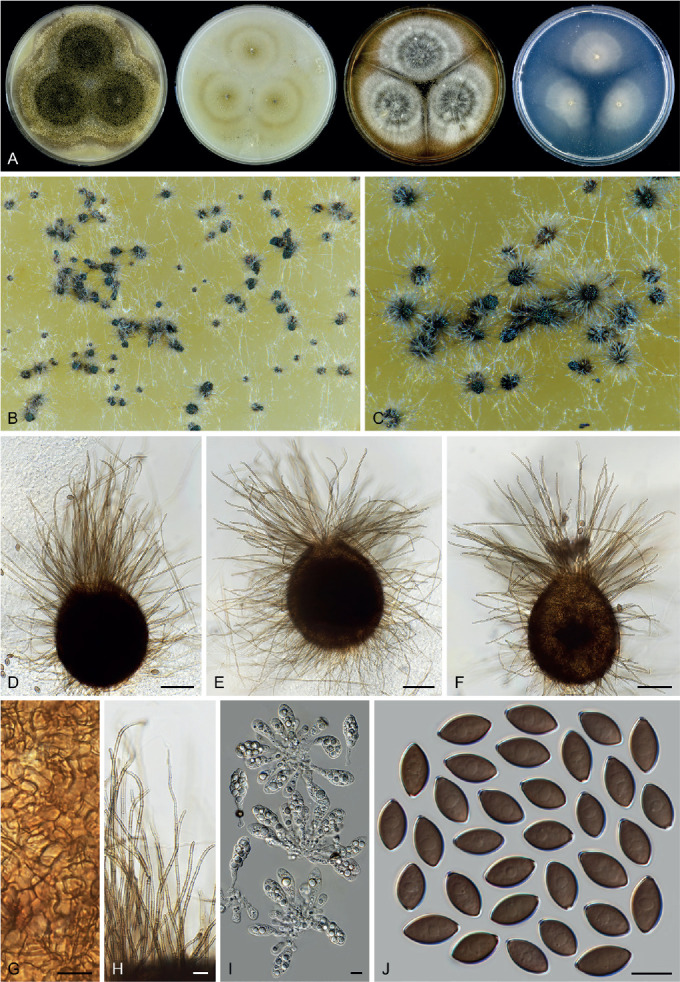
Parachaetomium hispanicum (CBS 550.83). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B, C. Part of the colony, showing ascomata on OA, top view. D–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: D–F = 50 μm; G–J = 10 μm.
Basionym: Chaetomium hispanicum Guarro & Arx, Beih. Nova Hedwigia 84: 6. 1986.
Micromorphology: Ascomata superficial, solitary, grey olivaceous due to ascomatal hairs and masses of ascospores in reflected light, ovoid, ostiolate, 110–220 μm high, 100–190 μm diam. Ascomatal wall brown, composed of angular or irregular cells. Terminal hairs hypha-like, pale brown, septate, finely verrucose, straight or flexuous, sometimes apically recurved, unbranched, 1.5–3 μm diam near the base. Lateral hairs similar to terminal ones. Asci clavate, spore-bearing part 30–35 × 13.5–16 μm, with stalks being 9–20 μm long, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, ellipsoidal, attenuated at both ends, often slightly inequilateral, 12–14(–15) × 7–8 μm, with an apical germ pore. Asexual morph unknown.
Culture characteristics: On OA with an entire edge, 32–38 mm diam in 7 d at 25 °C, without aerial mycelium, obverse grey olivaceous to isabelline due to ascomata; reverse isabelline. On CMA with an entire edge, 30–36 mm diam in 7 d at 25 °C, without aerial mycelium, poorly sporulating, obverse olivaceous buff due to coloured exudates diffusing into the medium; reverse olivaceous buff. On MEA with an entire edge, 28–34 mm diam in 7 d at 25 °C, texture floccose, obverse pale smoke grey due to ascomata mixed with aerial mycelium, reverse ochreous or dark brick. On PCA with an entire edge, 25–34 mm diam in 7 d at 25 °C, without aerial mycelium, without coloured exudates; reverse uncoloured.
Material examined: Spain, Tarragona, isolated from dung, date unknown, J. Guarro (culture ex-type CBS 234.82 = FFBA 313); Reus, isolated from soil, date unknown, J. Guarro (CBS 550.83 = FMR 502).
Notes: The ex-type culture of this species is degenerated as sterile and the description above is based on the culture CBS 550.83. Parachaetomium hispanicum is characterised by the production of hypha-like and unbranched ascomatal hairs, with no differentiation between the terminal and lateral ones.
Parachaetomium inaequale (Pidopl. et al.) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 830928. Fig. 35.
Fig. 35.
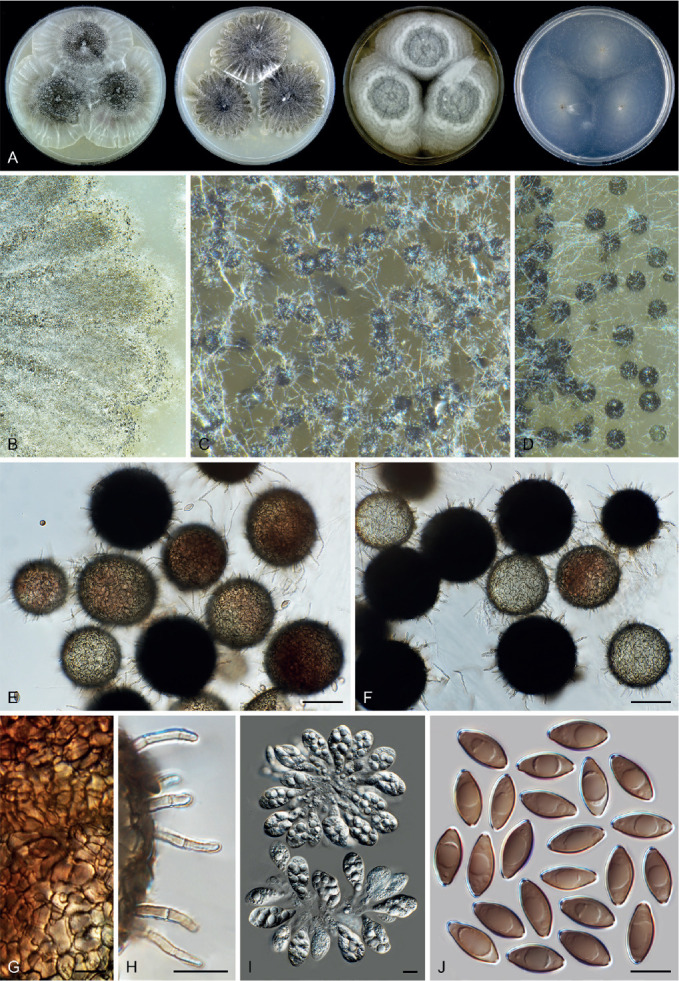
Parachaetomium inaequale (CBS 331.75, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 18 d incubation. B. Part of the colony on CMA. C. Mature ascomata on OA, top view. D. Mature ascomata on CMA, top view. E, F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: E, F = 50 μm; G–J = 10 μm.
Basionym: Thielavia inaequalis Pidopl. et al., Mikrobiol. Zhurn. 35: 723. 1973.
Synonym: Corynascella inaequalis (Pidopl. et al.) Arx, Kavaka 3: 34. 1976.
Micromorphology: Ascomata superficial or immersed in the medium, solitary or aggregated, non-ostiolate, fuscous black when mature in reflected light, spherical to oblate, pilose, 65–110 μm diam. Ascomatal wall brown, composed of textura epidermoidea in surface view. Ascomatal hairs short, finger-like, straight or flexuous, finely verrucose, septate, 1.5–2 μm diam near the base, less than 30 μm long. Asci clavate or pyriform, spore-bearing part 25.5–33.5 × 15–17 μm, with stalks being 5–13 μm long, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, elongated ellipsoidal or fusiform, often inequilateral, (12–)13.5–15.5(–16.5) × (5.5–)6.5–7.5(–8.5) μm, with two apical germ pores. Asexual morph unknown.
Culture characteristics: On OA with a crenate edge, 27–33 mm diam in 7 d at 25 °C, with a thin layer of white aerial mycelium, obverse mouse grey due to ascomata; reverse buff. On CMA similar to those on OA, 28–34 mm diam in 7 d at 25 °C, forming masses of ascomata radially straited with lobate margins. On MEA with an entire or slightly crenate edge, 26–32 mm diam in 7 d at 25 °C, with white aerial mycelium, obverse pale mouse grey in the central part, wrinkled, with several white concentric and crenated rings; reverse ochreous. On PCA with an entire edge, 29–35 mm diam in 7 d at 25 °C, without aerial mycelium, without coloured exudates, reverse uncoloured.
Material examined: Ukraine, Kirovograd, isolated from soil in oak forest, May 1968, collector unknown (culture ex-type CBS 331.75 = IMI 196527 = VKM F-1922); Kirovograd District, Ashen plantation, isolated from soil, 1 Jun. 1968, T.S. Kirilenko (CBS 164.75 = VKM F-1565).
Notes: Parachaetomium inaequale is the only species in the genus with non-ostiolate ascomata. This species was originally described in Thielavia, and later combined in Corynascella because of the production of ascospores with two apical germ pores (von Arx 1975b). Phylogenetic analysis indicates that Parach. inaequale is a sister species of Parach. hispanicum, distantly related to the type species of Corynascella (Fig. 7A). Parachaetomium inaequale differs morphologically from the type species of Corynascella (Figs 23, 24) by lacking an asexual morph and having elongated, ellipsoidal or fusiform ascospores, rather than irregularly ellipsoidal, oblate, ovoid or doliiform and usually irregular and inequilateral ascospores produced by the latter.
Parachaetomium muelleri (Arx) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 830925. Fig. 36.
Fig. 36.
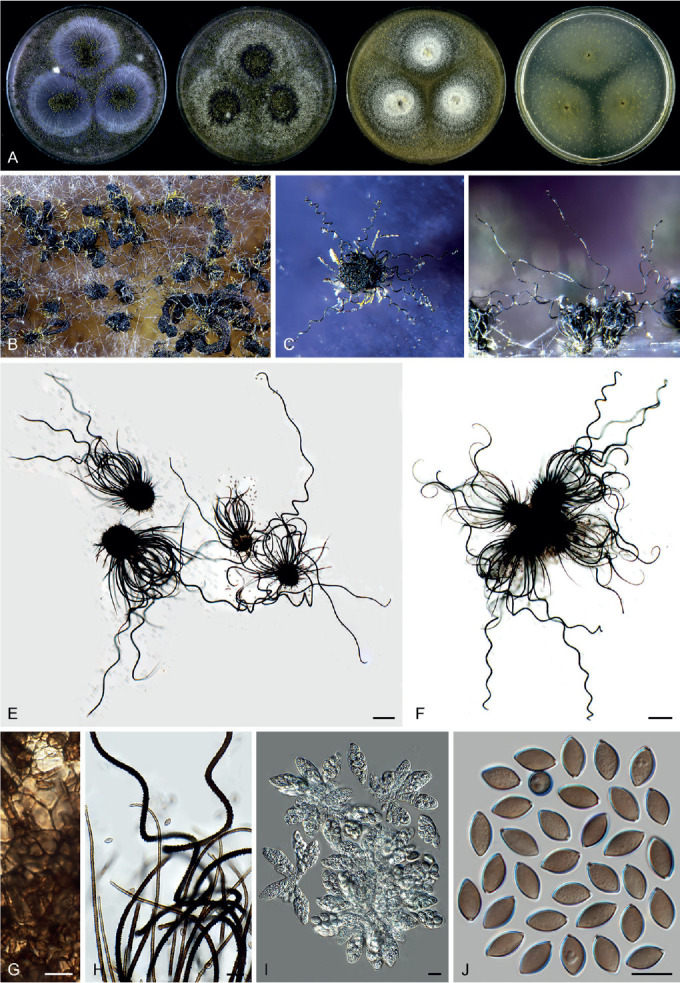
Parachaetomium muelleri (CBS 192.84, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D. Mature ascomata on OA, side view. E, F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: E, F = 100 μm; G–J = 10 μm.
Basionym: Chaetomium muelleri Arx, Beih. Nova Hedwigia 84: 6. 1986.
Micromorphology: Ascomata superficial, olivaceous grey in reflected light due to ascomatal hairs and masses of ascospores, subglobose, ostiolate, 100–200 μm high, 90–195 μm diam. Ascomatal wall brown, composed of angular or elongate cells in surface view. Terminal hairs in two types: type I shorter, arcuate, some apically recurved, verrucose, brown, septate, tapering and fading towards the tips, 3.5–6 μm diam near the base; type II longer, undulate or loosely coiled, verrucose, dark brown, tapering towards the tips, sometimes recurved or circinate at the apex, 5.5–7 μm diam near the base. Lateral hairs short, straight or flexuous. Asci fusiform, occasionally clavate, spore-bearing part 23–36.5 × 11.5–15 μm, with stalks being 6–15 μm long, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous when mature, ellipsoidal-fusiform, attenuated at both ends, sometimes slightly inequilateral, (9–)9.5–10.5(–11) × 5.5–6.5 μm, with an apical or slightly subapical germ pore. Asexual morph unknown.
Culture characteristics: On OA with an entire edge, 49–55 mm diam in 7 d at 25 °C, without aerial mycelium, obverse lavender to violet due to coloured exudates diffusing into the medium; reverse olivaceous grey. On CMA with an entire edge, 50–56 mm diam in 7 d at 25 °C, with white aerial mycelium, obverse citrine due to ascomata; reverse violet slate. On MEA with an entire edge, 52–58 mm diam in 7 d at 25 °C, with aerial mycelium, obverse buff to isabelline; reverse cinnamon. On PCA with an entire edge, 52–58 mm diam in 7 d at 25 °C, without aerial mycelium, without coloured exudates, obverse and reverse olivaceous buff.
Material examined: Pakistan, Lahore, isolated from decayed twig, 1976, S. Ahmed (culture ex-type CBS 192.84). Turkey, Bornova-Izmir, date and substrate unknown, M. Esentepe (CBS 663.75).
Notes: Parachaetomium muelleri can be easily recognised by the production of lavender to violet exudates on OA. It differs from its sister species, Parach. carinthiacum, by sparser, thicker (3.5–6 μm vs 2–4 μm diam near the base) and shorter type I terminal hairs, which are arcuate, some apically recurved, and by thicker terminal hairs of type II (5.5–7 μm diam vs 4–6 μm near the base), which are undulate to loosely coiled. Parachaetomium muelleri also produces larger ascospores than Parach. carinthiacum (9.5–10.5 × 5.5–6.5 μm vs 8–9 × 5–6 μm).
Parachaetomium perlucidum (Sergejeva) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 830930. Fig. 37.
Basionym: Chaetomium perlucidum Sergejeva, Bot. Mater. Otd. Sporov. Rast. Bot. Inst. Komarova Akad. Nauk S.S.S.R. 11: 108. 1956.
Synonyms: Chaetomium iranianum Asgari & Zare, Mycologia 103: 877. 2011.
Parachaetomium iranianum (Asgari & Zare) Mehrabi et al., Mycol. Prog. 19: 1422. 2020.
Micromorphology: Ascomata superficial, smoke grey due to ascomatal hairs in reflected light, subglobose to ovate, ostiolate, 95–230 μm high, 85–200 μm diam. Ascomatal wall brown, composed of irregular or angular cells. Terminal hairs in reflected light orange or luteous near the base, fading to pale smoke grey towards the tips, brown when mounting, septate, verrucose, loosely coiled, erect or flexuous in the lower part, 2–3.5 μm diam near the base. Lateral hairs flexuous. Asci fusiform, spore-bearing part 28–38 × 12–14 μm, with stalks being 8–18 μm long, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous when mature, fusiform or elongated ovoid, (11–)12–13.5(–14.5) × (5.5–)6–6.5(–7.5) μm, with a subapical or oblique germ pore. Asexual morph unknown.
Culture characteristics: On OA with an entire edge, 38–44 mm diam in 7 d at 25 °C, obverse smoke grey due to ascomatal hairs, without aerial mycelium; reverse honey. On CMA similar to those on OA, 35–41 mm diam in 7 d at 25 °C, with a thin layer of white aerial mycelium; reverse buff or ochreous. On MEA with an entire or slightly crenate edge, 37–43 mm diam in 7 d at 25 °C, obverse white and floccose due to aerial mycelium, reverse ochreous or fulvous. On PCA with an entire edge, 37–43 mm diam in 7 d at 25 °C, with sparse aerial mycelium, without coloured exudates; reverse uncoloured.
Material examined: China, Xinjiang, Changji, isolated from soil, 2013, X.W. Wang (culture CBS 119762). Iran, East Azerbaijan Prov., Sarab, isolated from leaf of Hordeum vulgare, 22 May 2005, B. Asgari (CBS 126670 = IRAN 861C, ex-type of Chaetomium iranianum). Ukraine, Kiev, isolated from dead herbaceous stem, date unknown, K.S. Sergejeva (culture ex-type CBS 141.58 = IMI 074954 = MUCL 18693 = MUCL 39399 = VKM F-1950). USA, Wyoming, isolated from soil, 1976, M. Christensen (CBS 127795 = RMF H 140).
Notes: Parachaetomium perlucidum is characterised by having ascospores with a subapical or oblique germ pore, fusiform asci and loosely coiled terminal hairs. As noted above, Parach. iranianum is treated as a synonym of this species based on morphological similarities and phylogenetic analysis.
Parachaetomium subspirilliferum (Sergejeva) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 830931. Fig. 38.
Fig. 38.
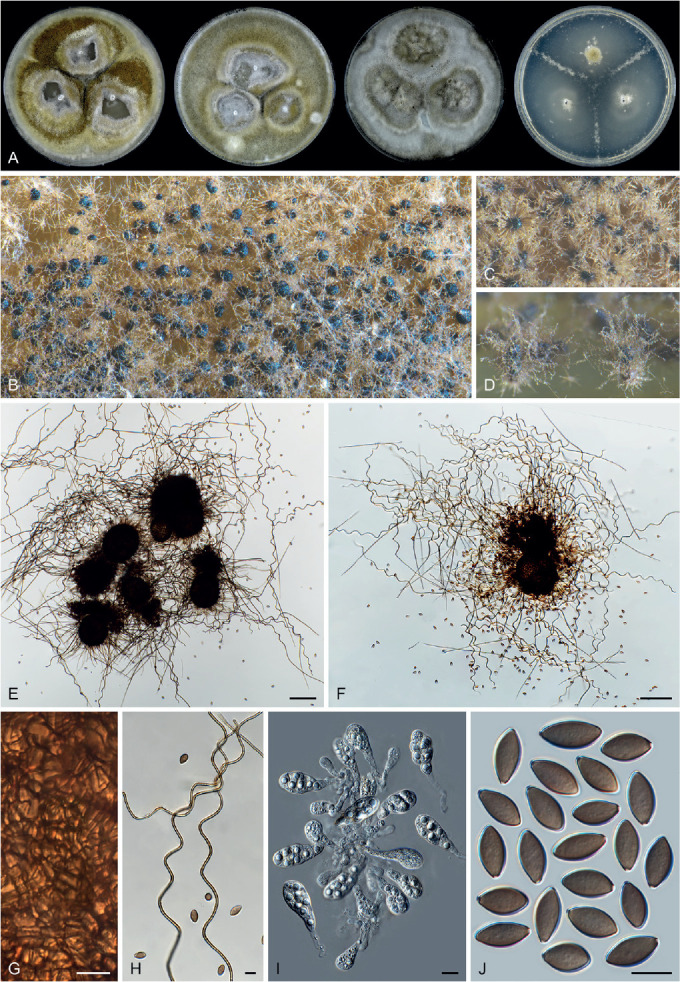
Parachaetomium subspirilliferum (CBS 150.60, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D. Mature ascomata on OA, side view. E, F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: E, F = 100 μm; G–J = 10 μm.
Basionym: Chaetomium subspirilliferum Sergejeva, Bot. Mater. Otd. Sporov. Rast. Bot. Inst. Komarova Akad. Nauk S.S.S.R. 13: 174. 1960.
Micromorphology: Ascomata superficial, greenish olivaceous due to ascomatal hairs in reflected light, spherical to ovate, ostiolate, 95–150 μm high, 80–130 μm diam. Ascomatal wall brown, composed of angular or elongate cells. Terminal hairs brown, septate, finely verrucose, undulate to loosely coiled, erect or flexuous at lower part, 1.5–3 μm diam near the base. Lateral hairs flexuous. Asci clavate, spore-bearing part 25.5–36 × 12–14.5 μm, with stalks being 11–21 μm long, containing eight irregularly-arranged ascospores, evanescent quickly in one week. Ascospores olivaceous brown when mature, elongated ellipsoidal, elongated ovoid, attenuated at both ends, or fusiform, often slightly inequilateral, (10.5–)12–13.5(–14) × (6–)6.5–7.5 μm, with an apical or occasionally slightly subapical germ pore. Asexual morph unknown.
Culture characteristics: On OA with an entire edge, 34–40 mm diam in 7 d at 25 °C, obverse grey olivaceous to isabelline due to ascomatal hairs, with sparse white aerial mycelium; reverse hazel. On CMA similar to those on OA. On MEA with an entire edge, 28–34 mm diam in 7 d at 25 °C, obverse white or greenish olivaceous due to ascomata mixed with aerial mycelium, reverse ochreous or fulvous. On PCA with an entire edge, 34–40 mm diam in 7 d at 25 °C, without aerial mycelium, sparsely producing ascomata, without coloured exudates; reverse uncoloured.
Material examined: China, Xingjiang, Altai, isolated from soil, 2003, X.W. Wang (WXW 9901-2). Russia, Altai, Kulundinskaya steppe, isolated from soil, date unknown, D.T. Degtyareva & M.V. Nosdrenko (culture ex-type CBS 150.60 = ATCC 14534 = IMI 081771 = MUCL 18698 = VKM F-1943).
Notes: Parachaetomium subspirilliferum produces undulate to loosely coiled terminal hairs, similar to Parach. perlucidum. This species can be differentiated from the latter species by its ascospores, which have an apical (occasionally slightly subapical, but never oblique) germ pore, clavate rather than fusiform asci and slightly thinner terminal hairs (1.5–3 μm diam vs 2–3.5 μm diam near the base).
Three more chaetomium-like species are combined in Parachaetomium based on phylogenetic data (Fig. 7A, Supplementary Figs S2, S3). These species produce fusiform ascospores and coiled terminal hairs covering ostiolate ascomata, similar to Parach. perlucidum and Parach. subspirilliferum:
Parachaetomium longiciliatum (Yu Zhang & L. Cai) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840157.
Basionym: Chaetomium longiciliatum [as ‘longiciliata’] Yu Zhang & L. Cai, Fungal Biol. 121: 31. 2016.
Note: Parachaetomium longiciliatum is characterised by producing shorter ascospores (9–12.5 × 5.5–8 μm) than Parach. perlucidum (12–13.5 × 6–6.5 μm) and Parach. subspirilliferum (12–13.5 × 6.5–7.5 μm), and having an apical or slightly subapical germ pore (Zhang et al. 2017).
Parachaetomium mareoticum (Besada & Yusef) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840158.
Basionym: Chaetomium mareoticum Besada & Yusef, Trans. Brit. Mycol. Soc. 52: 502. 1969.
Notes: Parachaetomium mareoticum produces rather thin terminal hairs (2–3 μm diam near the base), similar to Parach. perlucidum and Parach. subspirilliferum. This species can be distinguished by its large ascospores (15–18 × 7–8.5 μm) with two apical germ pores (von Arx et al. 1986). Apparently, Parach. mareoticum produces the largest ascospores in the genus.
Parachaetomium multispirale (A. Carter et al.) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840159.
Basionym: Chaetomium multispirale A. Carter et al., Canad. J. Bot. 60: 1256. 1982.
Notes: Parachaetomium multispirale produces relatively thick terminal hairs (2.5–4 μm diam near the base), similar to Parach. perlucidum, but can be distinguished by its smaller ascospores (7–10 × 5–6 μm vs 11.5–13.5 × 6–7 μm) with an apical germ pore (Carter & Khan 1982, von Arx et al. 1986), rather than a subapical or oblique germ pore like the latter. Parachaetomium multispirale produces more regularly coiled terminal hairs (Carter & Khan 1982), in contrast to the loosely coiled hairs of Parach. perlucidum.
Parathielavia X.Wei Wang & Houbraken, Stud. Mycol. 93: 208. 2019.
Micromorphology and illustrations: See Wang et al. (2019b; p. 208, 210–212).
Type species: Parathielavia hyrcaniae (Nicot) X.Wei Wang & Houbraken
Notes: Parathielavia is characterised by producing non-ostiolate, pilose or glabrous ascomata, with a brown and semi-translucent wall and having ascospores with a subapical germ pore (Wang et al. 2019b). Thielavia coactilis, previously not studied when introducing Parathielavia (Wang et al. 2019b), proved to belong to this genus based on our phylogenetic analysis (Fig. 7C). The morphology of this species fits in the current circumscription of the genus. The combination is introduced below.
Parathielavia coactilis (Nicot) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840160.
Basionym: Thielavia coactilis Nicot, Compt. Rend. Hebd. Séances Acad. Sci., Sér. D 253: 304. 1961.
Notes: Parathielavia coactilis was published together with Parath. hyrcaniae (= Thielavia hyrcaniae), and can be distinguished by glabrous ascomata and smaller ascospores (6–11 × 5–7 μm vs 11–13 × 6–7 μm) (von Arx 1975a, Wang et al. 2019b). No type material of this species is available. Our sequence data are from two recent isolates deposited in the CBS culture collection and these are phylogenetically most closely related to the type of Parath. kuwaitensis.
Parvomelanocarpus X.Wei Wang & Houbraken, gen. nov. MycoBank MB 840124.
Etymology: The name refers to its smaller ascospores and slower growth than those of the genus Melanocarpus.
Micromorphology: Ascomata superficial, or embedded in aerial mycelium, discrete to aggregated, non-ostiolate, spherical, glabrous or covered by finger-like ascomatal hairs, black when mature in reflected light due to the dark ascomatal wall. Ascomatal wall brown, composed of textura angularis, epidermoidea or intricata in surface view. Asci ovate to broadly ovate, containing eight irregularly-arranged ascospores, evanescent. Ascospores 1-celled, smooth, olivaceous brown or brown when mature, ovate to broadly ovate, bilaterally flattened, with an apical germ pore, usually shorter than 10 μm. Asexual morph unknown.
Culture characteristics: Colonies on agar media growing slowly, less than 20 mm diam in 5 d at optimal temperature (about 37 °C). Thermotolerant.
Type species: Parvomelanocarpus tardus (X.Wei Wang & Samson) X.Wei Wang & Houbraken
Notes: The proposal of this new genus is supported by its differences from Melanocarpus albomyces in morphology, reproduction, temperature adaptation (Fig. 39) and by their divergence time (Fig. 8B). Parvomelanocarpus species are thermotolerant, while Melanocarpus species are thermophilic. Furthermore, no asexual morph is observed in Parvomelanocarpus species, they grow very slow on agar media and produce smaller ascospores. The genus diverged from Melanocarpus about 60 Mya (Fig. 8B). For more details, see notes of Melanocarpus.
Parvomelanocarpus tardus (X.Wei Wang & Samson) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840152.
Basionym: Melanocarpus tardus X.Wei Wang & Samson, Stud. Mycol. 84: 205. 2016.
Micromorphology and illustrations: See Wang et al. (2016b; p. 205, 213).
Note: Parvomelanocarpus tardus is characterised by the production of non-ostiolate, glabrous ascomata; the ascospores of this species are ovate to broadly ovate, bilaterally flattened, 7–8(–8.5) × (6–) 6.5–7.5 × 5–6 μm, having an apical germ pore at the attenuated end.
Parvomelanocarpus thermophilus (Abdullah & Al-Bader) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840167. Fig. 40.
Fig. 40.

Parvomelanocarpus thermophilus (CBS 886.97). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony. C. Mature ascomata on OA, top view. D, F. Mature ascomata mounted in lactic acid. E. Young ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Ascomatal hairs. I. Asci. J. Ascospores. Scale bars: D = 100 μm; E, F = 50 μm; G–J = 10 μm.
Basionym: Thielavia minuta var. thermophila Abdullah & Al-Bader, Basrah J. Agric. Sci. 5: 116. 1992.
Synonym: Melanocarpus thermophilus (Abdullah & Al-Bader) Guarro et al., Mycol. Res. 100: 75. 1996.
Micromorphology: Ascomata superficial, discrete or aggregated to form a ring around the central point, non-ostiolate, dark slate blue in reflected light, covered by hairs, globose or subglobe, 60–160 μm diam. Ascomatal wall brown, ochreous or fulvous when young, dark brown when mature, textura epidermoidea or intricata in surface view. Ascomatal hairs brown, finger-like, often geniculate, finely verrucose, septate, 2–4 μm diam near the base, usually less than 20 μm long. Asci fasciculate, ovate to broadly ovate, spore-bearing portion 13.5–20 × 10.5–14.5 μm, with short or indistinct stalks being 0–5 μm long, containing eight irregularly-arranged ascospores, quickly evanescent. Ascospores dark brown when mature, broadly ovate, slightly bilaterally flattened, (6–)7–9(–9.5) × (6–)6.5–8(–8.5) × 6–7 μm, with an apical germ pore at the attenuated end. Asexual morph unknown.
Culture characteristics: On OA with a crenated edge, 8–14 mm diam in 5 d at 37 °C, obverse mouse grey due to the formation of ascomata, with sparse white aerial mycelium, without coloured exudates; reverse uncoloured. On CMA similar to those on OA. On MEA with an entire or slightly lobate edge, 14–20 mm diam in 5 d at 37 °C, obverse pale mouse grey due to ascomata mixed with aerial mycelium, reverse saffron. On PCA with a crenated edge, 6–12 mm diam in 5 d at 37 °C, with sparse aerial mycelium, without coloured exudates; reverse uncoloured.
Material examined: India, Agra, isolated from soil, 3 Nov. 1995, A.M. Stchigel (CBS 886.97 = FMR 6190).
Notes: Parvomelanocarpus thermophilus can be distinguished from Par. tardus by its ascomata, which are covered by finger-like ascomatal hairs, while those of Par. tardus are usually glabrous (Wang et al. 2016b). The ascospores of Par. thermophilus are slightly larger than those of Par. tardus (7–9 × 6.5–8 × 6–7 μm vs 7–8 × 6.5–7.5 × 5–6 μm).
Pseudohumicola X.Wei Wang, P.J. Han, F.Y. Bai & Houbraken, gen. nov. MycoBank MB 840123.
Etymology: The name refers to the morphologically related genus Humicola.
Micromorphology: Containing asexual species and species with both asexual and sexual morphs. Asexual morphs humicola-like and producing aleurioconidia-like conidia and/or acremonium-like. Conidiogenous cells of humicola-like morph reduced to a hyphal cell, intercalary or lateral, monoblastic. Aleurioconidia-like conidia arising laterally, intercalary or terminally, 1-celled, solitary or rarely in chains or in clusters of a few spores, globose, subglobose, oblate, occasionally obovoid, pyriform or clavate, light olivaceous, olivaceous, brown or dark brown, smooth or not, in persisted state on hyphae or rhexolytic when seceding, germ pores or thinning area of wall present. Acremonium-like phialides lateral or occasionally terminal, hyaline. Acremonium-like conidia in basipetal chains, hyaline, aseptate, smooth, obovoid, usually with a truncated base and a rounded apex. Ascomata absent or present, when present superficial, or covered by aerial hyphae, ostiolate. Terminal hairs straight, flexuous, undulate or coiled in the upper part. Asci clavate, containing eight biseriate or irregularly-arranged ascospores, evanescent. Ascospores limoniform, bilaterally flattened, with an apical germ pore.
Type species: Pseudohumicola subspiralis (Chivers) X.Wei Wang, P.J. Han, F.Y. Bai & Houbraken
Notes: Humicola sensu Wang et al. (2019a) receives moderate statistic support in our phylogenetic analysis (ML-BS = 78 %; PP = 1.0, Fig. 7B). In contrast, our molecular dating analysis shows that a group of species (Humicola atrobrunnea, H. pulvericola, H. semispiralis, H. subspiralis) closely clusters to Aporothielavia, separating them from the other Humicola species. The new genus Pseudohumicola is therefore proposed here for these species. The ascomata (if produced) in Pseudohumicola usually have coiled terminal hairs, while such ascomatal hairs are rare in the sexual species of Humicola s. str. In addition, germ pores or a thin area on the aleurioconidia-like conidia is more often observed in Pseudohumicola than in Humicola species. For more details, see notes on Humicola above.
Pseudohumicola atrobrunnea (X.Wei Wang et al.) X.Wei Wang, P.J. Han, F.Y. Bai & Houbraken, comb. nov. MycoBank MB 840148.
Basionym: Humicola atrobrunnea X.Wei Wang et al., Stud. Mycol. 93: 76. 2018.
Micromorphology and illustrations: See Wang et al. (2019a; p. 76, 78).
Notes: This species only produces an asexual morph with aleurioconidia-like conidia. The smooth, dark brown and thick-walled conidia are produced solitary, sometimes in a chain of 2–3 conidia or in a cluster. A germ pore can be observed on some conidia.
Pseudohumicola pulvericola (X.Wei Wang et al.) X.Wei Wang, P.J. Han, F.Y. Bai & Houbraken, comb. nov. MycoBank MB 840149.
Basionym: Humicola pulvericola X.Wei Wang et al., Stud. Mycol. 93: 96. 2018.
Micromorphology and illustrations: See Wang et al. (2019a; p. 96, 98).
Notes: Pseudohumicola pulvericola only produces an asexual morph with both aleurioconidia-like conidia and an acremonium-like morph. The slightly verrucose, dark brown and thick-walled aleurioconidia-like conidia are mostly produced solitary and occasionally in chains of two. A germ pore or thinning area of wall can sometimes be observed on the conidia. Acremonium-like conidiophores can be present in the aerial mycelium.
Pseudohumicola semispiralis (Udagawa & Cain) X.Wei Wang, P.J. Han, F.Y. Bai & Houbraken, comb. nov. MycoBank MB 840150.
Basionym: Chaetomium semispirale Udagawa & Cain, Canad. J. Bot. 47: 1947. 1969.
Synonym: Humicola semispiralis (Udagawa & Cain) X.Wei Wang & Houbraken, Stud. Mycol. 93: 100. 2018.
Micromorphology and illustrations: See Wang et al. (2019a; p. 100, 102).
Notes: Pseudohumicola semispiralis produces a sexual morph and an asexual morph with aleurioconidia-like conidia. The terminal ascomatal hairs are partly straight to flexuous, partly spirally coiled in the upper part. The conidia are smooth, subhyaline or olivaceous. A germ pore or thinning area of wall can be observed on some conidia.
Pseudohumicola subspiralis (Chivers) X.Wei Wang, P.J. Han, F.Y. Bai & Houbraken, comb. nov. MycoBank MB 840151.
Basionym: Chaetomium subspirale Chivers, Proc. Amer. Acad. Arts 48: 84. 1912.
Synonym: Humicola subspiralis (Chivers) X.Wei Wang & Houbraken, Stud. Mycol. 93: 104. 2018.
Micromorphology and illustrations: See Wang et al. (2019a; p. 104, 105).
Notes: Pseudohumicola subspiralis produces both a sexual and asexual morph. Von Arx et al. (1986) observed aleurioconidia-like conidia and noted that such thick-walled conidia could occasionally be absent. In our study, only acremonium-like phialides were observed in the ex-type culture. Apparently, this species can potentially produce both aleurioconidia-like conidia and an acremonium-like morph. The ascomatal hairs of this species are undulate to spirally coiled in the upper parts, without differentiation between the terminal and lateral ones.
Staphylotrichum J.A. Mey. & Nicot, Bull. Trimestriel Soc. Mycol. France 72: 322. 1957.
Micromorphology (emended description): Species producing only an asexual morph or both an asexual and sexual morph. Asexual morphs usually of two types. Type one macronematous. Conidiophores arising from an intercalary, thick-walled, pigmented foot cell, usually pigmented and thick-walled in the lower part, tapering and fading towards the tips, apically branched. Conidiogenous cells terminally on the top branches of conidiophores, cylindrical or denticle-like, monoblastic or sympodial polyblastic. Type two micronematous. Conidiophores absent. Conidiogenous cells arising directly from hyphae, cylindrical or denticle-like, monoblastic, rarely sympodial polyblastic. Conidia solitary, single-celled, smooth or slightly verrucose, hyaline to pale brown, usually globose, subglobose or obovoid, rhexolytic when seceding. Sexual morph absent or present. If present, of two types: Ascomata of type I with a conspicuous neck, superficial or covered by aerial hyphae, ostiolate, elongated obpyriform, obclavate or ampulliform below, usually apically attenuated to a cylindrical, thread-like neck which is composed of fused basal part of the terminal hairs, with terminal hairs seta-like or whip-like, smooth, fused in the lower part to form a channel through which a column of ascospores emerges from the ascomata. Ascomata of type II without a conspicuous neck, superficial, ostiolate, ovate to subglobose, with terminal hairs spirally or loosely coiled in the upper parts. Asci clavate to fusiform, with eight irregularly-arranged ascospores, evanescent. Ascospores broad limoniform to nearly globose, often somewhat biapiculate, bilaterally flattened, with an apical germ pore.
Type species: Staphylotrichum coccosporum J.A. Mey. & Nicot.
Notes: Asexual species usually produce both macro- and micronematous asexual morphs, while sexual species only produce a micronematous asexual morph. With the addition of Staph. limonisporum, the concept of Staphylotrichum needs to be emended (see above) to include species producing ascomata lacking a long neck. All known species produce similar conidia, asci and ascospores. The ascospores of Staphylotrichum are limoniform to broad limoniform, bilaterally flattened, with an apical germ pore. The conidia of Staphylotrichum species are easily confused with those of Humicola species. Staphylotrichum species produce cylindrical or denticle-like conidiogenous cells, while Humicola species produce conidia that arise laterally, intercalary or terminally from hyphae without differentiated conidiophores or conidiogenous cells (Fig. 6F). Two more species are transferred to this genus.
Staphylotrichum koreanum (Hyang B. Lee & T.T.T. Nguyen) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840161.
Basionym: Humicola koreana Hyang B. Lee & T.T.T. Nguyen, Fungal Diversity 78: 97. 2016.
Notes: Based on the illustration in the original description, H. koreana produces typical micronematous conidia on denticle-like to cylindrical conidiogenous cells (Li et al. 2016), similar to other Staphylotrichum species. ITS and LSU sequences indicate that H. koreana belongs to Staphylotrichum (Supplementary Fig. S4). No rpb2 and tub2 sequences are available for this species and it is therefore not included in our multigene phylogenetic tree.
Staphylotrichum limonisporum (Z.F. Zhang & L. Cai) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840162.
Basionym: Humicola limonispora [as ‘limonisporum’] Z.F. Zhang & L. Cai, Persoonia 39: 15. 2017.
Notes: Staphylotrichum limonisporum was originally described as a Humicola species (Zhang et al. 2017a). This was mainly due to the limited taxon sampling in their phylogenetic analysis. The phylogenetic position in Staphylotrichum is supported by multigene phylogeny (Fig. 7B), as well as the tub2 and rpb2 phylogenies (Supplementary Figs S2, S3). Staphylotrichum limonisporum is the only known Staphylotrichum species that produces ascomata without a conspicuous neck. Molecular dating indicated that this species diverged from the other Staphylotrichum species about 35 Mya. Considering that Staph. limonisporum produces similar ascospores and conidia as those of typical Staphylotrichum species, it is transferred to Staphylotrichum above.
Subramaniula Arx, Proc. Indian Acad. Sci., Pl. Sci. 94: 344. 1985.
Micromorphology: Containing sexual species and asexual species. Sexual species: Ascomata superficial to immersed in the medium, ostiolate, urniform, subglobose or ovoid. Ascomatal wall brown, usually composed of textura angularis in surface view. Ascomatal hairs absent or present and highly diverse, if present, hypha-like, flexuous or undulate, sometimes coiled in the upper part, or apically irregularly-curved and branched repeatedly to form a network in some species. Asci fasciculate, clavate, fusiform or obovate, stalked, containing eight ascospores, evanescent. Ascospores olivaceous brown or dark brown when mature, smooth, fusiform or ellipsoidal-fusiform, sometimes inaequilateral or irregular, not bilaterally flattened, with an apical, subapical or lateral germ pore. Asexual morph unknown. Asexual species: Somatic hyphae hyaline, sometimes becoming pigmented with age, or forming chlamydospore- or microsclerotium-like structures. Chlamydospores formed in chains, pigmented, thick-walled. Microsclerotium-like structures composed of brown or dark brown, thick-walled, subglobose or irregular cells. Conidiophores phialidic, terminally or intercalary from hyphae, hyaline, cylindrical, obclavate or reduced to conidiogenous cells. Conidia smooth-walled, hyaline, unicellular, obovoidal or ellipsoidal in slimy heads or in basipetal chains. Sexual morph unknown.
Type species: Subramaniula thielavioides (Arx et al.) Arx
Notes: Subramaniula species exhibit a highly diverse morphology. Three asexually-reproducing species (Sub. anamorphosa, Sub. asteroides and Sub. obscura) are opportunistic human pathogens that were once misclassified as Papulaspora spp. (Ahmed et al. 2016). These asexual species intermingle among the sexual species in the phylogenetic tree (Fig. 7C), although their sexual morphs are unknown. One new species is described here and an additional chaetomium-like species is transferred to this genus. In total, seven sexual species are now included in the genus with no asexual morph observed. Subramaniula thielavioides, the type species, produces glabrous ascomata, while the other species have ascomata covered by different ascomatal hairs.
Subramaniula latifusispora X.Wei Wang, P.J. Han & F.Y. Bai, sp. nov. MycoBank MB 840129. Fig. 41.
Fig. 41.
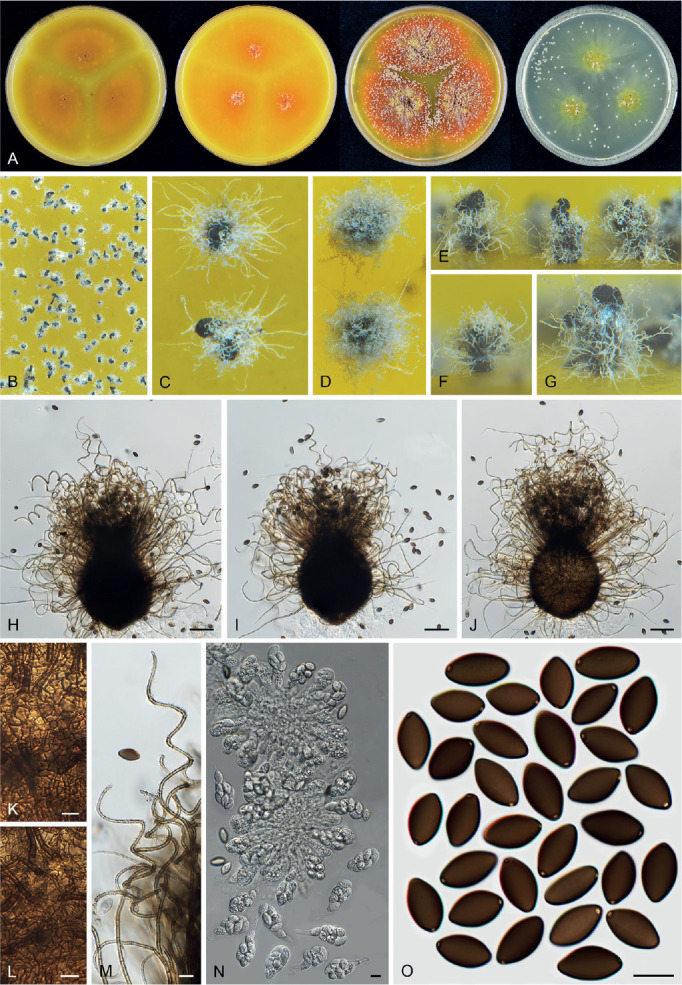
Subramaniula latifusispora (CGMCC 3.20442, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony. C, D. Mature ascomata on OA, top view. E–G. Mature ascomata on OA, side view. H–J. Ascomata mounted in lactic acid. K, L. Structure of ascomatal wall in surface view. M. Terminal ascomatal hairs. N. Asci. O. Ascospores. Scale bars: H–J = 50 μm; K–O = 10 μm.
Etymology: The name refers to the ascospores, which are broader than those of its closest relative Subramaniula fusispora.
Micromorphology: Ascomata superficial, ostiolate, olivaceous buff due to ascomatal hairs in reflected light, then becoming greenish black due to ascospores aggregating on the top, ovate or subglobose, 140–220 μm high, 125–190 μm diam. Ascomatal wall brown, of textura angularis in surface view. Terminal hairs brown, septate, fading towards the tips, flexuous in the lower part, 2–3 μm diam near the base, coiled or undulate in the upper part. Lateral hairs flexuous, undulate or slightly coiled. Asci fasciculate, fusiform or clavate, spore-bearing part 32–45 × 16–21 μm, with stalks 10–20.5 μm long, containing eight biseriate or irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, fusiform, (11–)13–15.5(–17.5) × 7.5–9(–11) μm, with a subapical or lateral germ pore. Asexual morph unknown.
Culture characteristics: On OA with an entire edge, 28–38 mm diam in 7 d at 25 °C, without aerial hyphae, obverse luteous or ochreous to orange due to exudates diffusing into the medium, often forming ascomata sparsely; reverse luteous to ochreous or orange. On CMA similar to those on OA, 23–33 mm diam after 7 d at 25 °C. On MEA with an entire edge, 25–35 mm diam in 7 d at 25 °C, without aerial hyphae, obverse orange with a luteous edge due to exudates diffusing into the medium, forming ascomata; reverse orange with a luteous edge. On PCA with an entire edge, 26–33 mm diam in 7 d at 25 °C, without aerial hyphae, sparsely forming ascomata, obverse amber in the centre due to exudates diffusing into the medium; reverse amber in the centre due to exudates diffusing into the medium.
Material examined: Canada, Banff, isolated from dung of marmot, 10 Sep. 1977, D.W. Malloch (culture CBS 199.84). China, Gongliu Forest Farm in Yili, Xinjiang, isolated from dung of sheep, Aug. 2004, X.W. Wang (holotype HMAS 350267, culture ex-type CGMCC 3.20442 = WXW 8538); near Sayram Lake in Yili, Xinjiang, isolated from fallen spruce fruit, Aug. 2004, X.W. Wang (WXW 8577).
Notes: Subramaniula latifusispora is phylogenetically related, but separate, to Sub. fusispora (Fig. 7C). Morphologically, Sub. latifusispora can be distinguished from Sub. fusispora by broader ascospores (13–15.5 × 7.5–9 μm vs 12.5–14.5 × 6.5–7 μm) and luteous or ochreous to orange exudates on OA.
Subramaniula lateralis (Yu Zhang & L. Cai) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840164.
Basionym: Chaetomium laterale Yu Zhang & L. Cai, Fungal Biol. 121: 30. 2017.
Notes: Subramaniula lateralis is characterised by hypha-like ascomatal hairs that are flexuous or slightly undulate, and by fusiform ascospores with a subapical germ pore (Zhang et al. 2017a). It is closely related to the asexual species Sub. asteroides (Fig. 7C). Subramaniula flavipila (= Chaetomium irregulare) also produces hypha-like ascomatal hairs, but can be distinguished by ascospores with an apical germ pore.
Tengochaeta X.Wei Wang & Houbraken, gen. nov. MycoBank MB 830915.
Etymology: Named after S.C. Teng (1902–1975), honouring his pioneering study on Chinese Chaetomiaceae.
Micromorphology: Ascomata superficial, often covered by aerial mycelium, ostiolate, ellipsoidal or subglobose, sometimes forming two ostioles from one ascoma. Ascomatal wall brown, composed of angular or irregular cells. Terminal hairs brown, septate, verrucose, flexuous to undulate, unbranched. Lateral hairs similar to terminal ones. Asci pyriform or broadly clavate, evanescent. Ascospores olivaceous brown when mature, ellipsoidal to fusiform, attenuated at both ends, often slightly inequilateral, with an apical germ pore. Asexual morph unknown.
Type species: Tengochaeta nigropilosa X.Wei Wang & Houbraken
Tengochaeta nigropilosa X.Wei Wang & Houbraken, sp. nov. MycoBank MB 840130. Fig. 42.
Fig. 42.
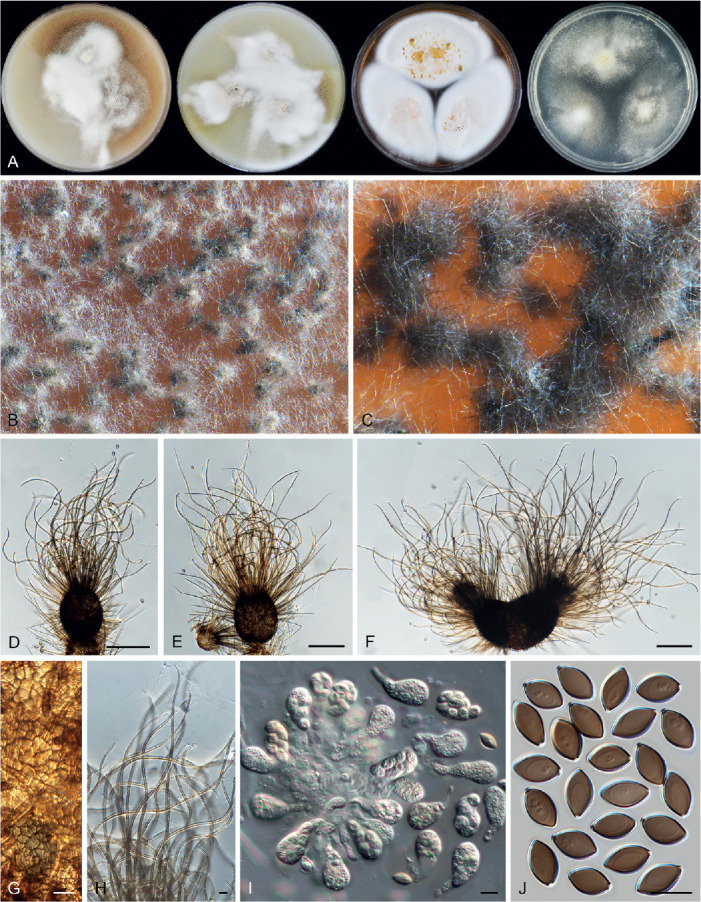
Tengochaeta nigropilosa (CBS 639.83, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony. C. Mature ascomata on OA, top view. D–F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: D–F = 100 μm; G–J = 10 μm.
Etymology: The name refers to the ascomatal hairs of the species that look like dark hairs fully covering the ascoma.
Micromorphology: Ascomata superficial, often covered by white aerial mycelium, solitary or clustered, grey olivaceous due to ascomatal hairs in reflected light, ellipsoidal or subglobose, ostiolate, sometimes forming two ostioles from one ascoma, 105–195 μm high, 85–140 μm diam, or 130–280 μm diam when with two ostioles. Ascomatal wall brown, composed of angular or irregular cells. Terminal hairs brown, septate, verrucose, flexuous to undulate, unbranched, 2–3 μm diam near the base. Lateral hairs similar to terminal ones, but shorter. Asci pyriform or broadly clavate, spore-bearing part 25–30 × 13–16.5 μm, with stalks being 8–13 μm long, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous brown when mature, ellipsoidal to fusiform, attenuated at both ends, often slightly inequilateral, (9.5–)10.5–12(–12.5) × (5.5–)6–7 μm, with an apical germ pore. Asexual morph unknown.
Culture characteristics: On OA with an entire edge, 27–33 mm diam in 7 d at 25 °C, obverse ochreous to umber due to coloured exudates diffusing into the medium, or forming thick aerial mycelium, texture floccose; reverse apricot. On CMA with an entire edge, 25–31 mm diam in 7 d at 25 °C, obverse white due to thick aerial mycelium, texture floccose, often pale luteous at the edge due to coloured exudates diffusing into the medium; reverse pale luteous to orange. On MEA with an entire edge, 32–38 mm diam in 7 d at 25 °C, texture floccose, obverse white due to thick aerial mycelium, reverse apricot to scarlet. On PCA with an entire edge, 25–31 mm diam in 7 d at 25 °C, obverse white due to aerial mycelium, without coloured exudates; reverse uncoloured or pale luteous in the centre.
Material examined: Spain, Tenerife, Aguamansa, isolated from soil in Pinus forests, date unknown, M. Dreyfuss (holotype CBS H-24774, culture ex-type CBS 639.83).
Notes: Based on morphology, von Arx et al. (1986) identified CBS 639.83 as Ch. hispanicum (= Par. hispanicum). Our phylogenetic analysis shows that CBS 639.83 is distinct from Parachaetomium (Fig. 7A) and forms a separate lineage, with no statistically supported close relatives (Fig. 7C). Tengochaeta nigropilosa and Par. hispanicum have different morphologies. Tengochaeta nigropilosa can be distinguished from Par. hispanicum (Fig. 34) by producing darker ascomatal hairs which are flexuous to undulate and often covered by aerial mycelium, pyriform or broadly clavate asci, while Par. hispanicum produces straight or flexuous, hypha-like ascomatal hairs and clavate asci. Tengochaeta nigropilosa also produces smaller ascospores than those of Par. hispanicum (10.5–12 × 6–7 μm vs 12–14 × 7–8 μm).
Thermocarpiscus X.Wei Wang & Houbraken, gen. nov. MycoBank MB 840163.
Etymology: The name refers to the thermophilic nature of the genus and the production of small cleistothecia.
Micromorphology (fide Tansey & Jack 1975): Ascomata superficial, spherical, glabrous, usually less than 100 μm diam, black when mature in reflected light due to the dark ascomatal wall. Ascomatal wall brown, composed of textura epidermoidea in surface view. Asci broadly ovate to subglobose, containing eight irregularly-arranged ascospores. Ascospores 1-celled, smooth, olivaceous brown when mature, ovate, with an apical germ pore. Conidiophores absent. Conidiogenous cells arising laterally or terminally from hyphae, or reduced to a hyphal cell, monoblastic. Conidia 1-celled, solitary, hyaline, smooth, usually ovoid. Containing one species with both sexual and asexual morph.Thermophilic.
Type species: Thermocarpiscus australiensis (Tansey & M.A. Jack) X.Wei Wang & Houbraken
Notes: This is a monotypic genus. The ex-type culture of the type species CBS 493.74 is in poor condition and is no longer producing ascomata, and we failed to study the phenotypic characteristics of this species. According to the original description, the species had a growth minimum temperature of 20 °C and they observed that the fungus grew at above 50 °C.
Thermocarpiscus australiensis (Tansey & M.A. Jack) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840165.
Basionym: Thielavia australiensis Tansey & M.A. Jack, Canad. J. Bot. 53: 81. 1975.
Micromorphology and illustrations: See Tansey & Jack (1975).
Thermochaetoides X.Wei Wang & Houbraken, gen. nov. MycoBank MB 830916.
Etymology: The name refers to the thermophilic habit and its morphological similarity to Chaetomium.
Micromorphology: Ascomata superficial, subglobose or ovoid, ostiolate. Ascomatal wall brown, composed of irregular or angular cells. Ascomatal hairs brown, flexuous, usually tortuous or geniculate, unequally thickened and being frequently and irregularly constricted along their length, dichotomously or irregularly branched, verrucose, septate. Asci fasciculate, cylindrical, occasionally elongated clavate, stalked, containing eight uniseriate (occasionally biseriate) ascospores, evanescent. Ascospores olivaceous when mature, single-celled, smooth, globose, subglobose or broad ovoid, bilaterally flattened, with a distinctly protuberant apical germ pore. Asexual morph unknown.
Type species: Thermochaetoides thermophila (La Touche) X.Wei Wang & Houbraken
Notes: Our phylogenetic analysis (Fig. 7D) shows that this genus forms an isolated lineage with no known close relatives in the Chaetomiaceae. After the description of Chaetomium thermophilum (La Touche 1950), Cooney & Emerson (1964) proposed two new varieties based on cultural characteristics. In our phylogenetic analyses, these varieties clearly group into two lineages (Fig. 7D, Supplementary Figs S1–S3). We here propose two new combinations, Thermoc. dissita and Thermoc. thermophila (see below).
Thermochaetoides dissita (Cooney & R. Emers.) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 830932. Fig. 43.
Fig. 43.
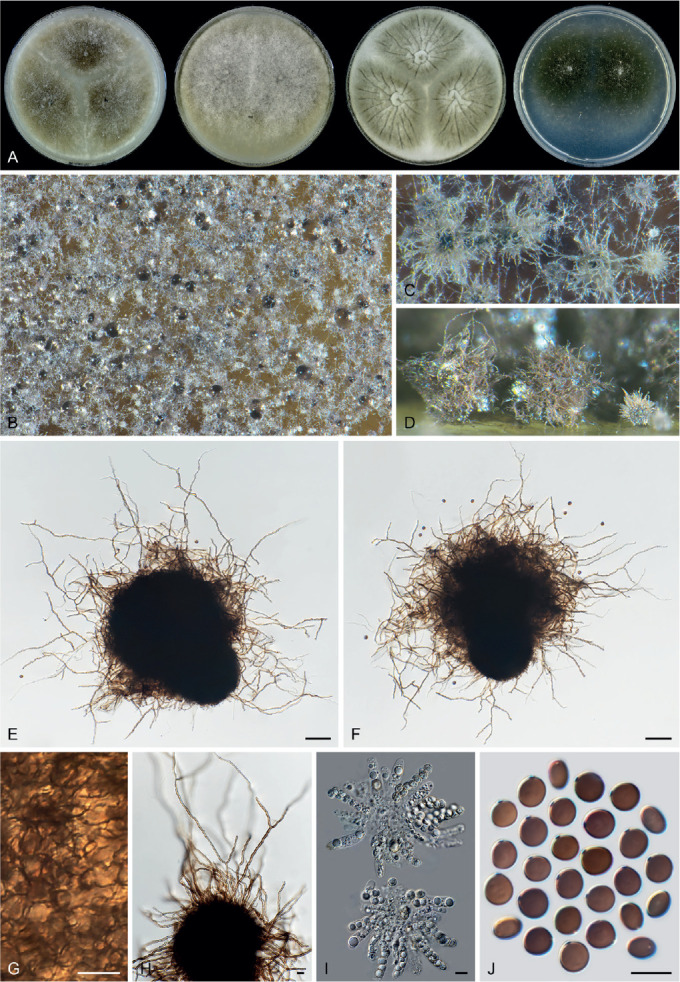
Thermochaetoides dissita (CBS 180.67, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 d incubation at 45 °C. B. Part of the colony. C. Mature ascomata on OA, top view. D. Mature ascomata on OA, side view. E, F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: E, F = 50 μm; G–J = 10 μm.
Basionym: Chaetomium thermophilum var. dissitum Cooney & R. Emers., Thermophilic Fungi: 68. 1964.
Micromorphology: Ascomata superficial, pale olivaceous grey due to ascomatal hairs in reflected light, subglobose or ovoid, ostiolate, 100–200 μm high, 90–155 μm diam. Ascomatal wall brown, composed of irregular or angular cells. Terminal hairs brown, geniculate and flexuous, dichotomously or irregularly branched, verrucose, septate, 2–4 μm diam near the base. Lateral hairs similar but sparse and shorter. Asci cylindrical, sometimes elongated clavate or elongated obclavate, spore-bearing part 32–52 × 7.5–11 μm, with stalks being 4–8 μm long, containing eight uniseriate, occasionally biseriate ascospores, evanescent. Ascospores olivaceous when mature, globose, subglobose or ovoid, bilaterally flattened, (6.5–)7–8(–9) × (6–)6.5–7.5(–9) × (4.5–)5–5.5(–6) μm, with a distinctly protuberant apical germ pore. Asexual morph unknown.
Culture characteristics: Optimum growth temperature 45–55 °C. On OA with an entire or slightly crenate edge, over 70 mm diam in 3 d at 45 °C, obverse pale smoke grey to grey olivaceous; reverse smoke grey. On CMA similar to those on OA. On MEA with an entire edge, over 70 mm diam in 3 d at 45 °C, obverse olivaceous buff or smoke grey, radially or irregularly striated due to wrinkling; reverse dark mouse grey. On PCA with an entire or slightly crenate edge, over 70 mm diam in 3 d at 45 °C, obverse mouse grey to dark mouse grey; reverse mouse grey.
Material examined: Israel, isolated from dung of gazelle, date unknown, E. Müller (CBS 785.71). Netherlands, isolated from dung of pig with sawdust, date unknown, G. Straatsma & Proefstation voor de Champignoncultuur (CBS 246.90). USA, California, Alameda Co., isolated from straw of Typha used as nesting material by the common coot or mud hen, 1949, D.G. Cooney & R. Emerson (culture ex-type CBS 180.67 = ATCC 16452 = DSM 1494 = IMI 126332).
Notes: Thermochaetoides dissita can be distinguished from Thermoc. thermophila by smaller ascospores (7–8 × 6.5–7.5 × 5–5.5 μm vs 8–9.5 × 8–9 × 5.5–6.5 μm) and the absence of crenate concentric rings in colonies grown on OA and CMA. Colonies of this species grow faster at 45 °C (over 70 mm diam vs < 60 mm diam on MEA after 3 d) and ascomata often develop discretely over the colony. Furthermore, Thermoc. thermophila has numerous ascomatal hairs covering the whole ascoma without differentiation of terminal and lateral hairs, while distinct forms of terminal and lateral hairs can be found in Thermoc. dissita.
Thermochaetoides thermophila (La Touche) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 830933. Fig. 44, 45.
Fig. 44.
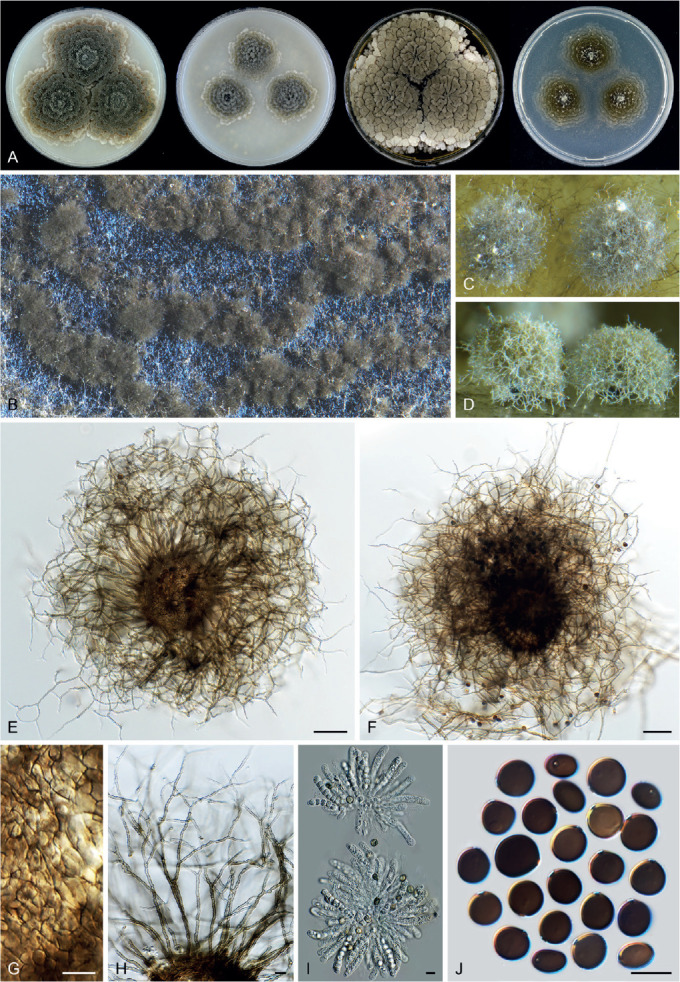
Thermochaetoides thermophila (CBS 144.50, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 d incubation at 45 °C. B. Part of the colony. C. Mature ascomata on OA, top view. D. Mature ascomata on OA, side view. E, F. Ascomata mounted in lactic acid. G. Structure of ascomatal wall in surface view. H. Terminal ascomatal hairs. I. Asci. J. Ascospores. Scale bars: E, F = 50 μm; G–J = 10 μm.
Fig. 45.
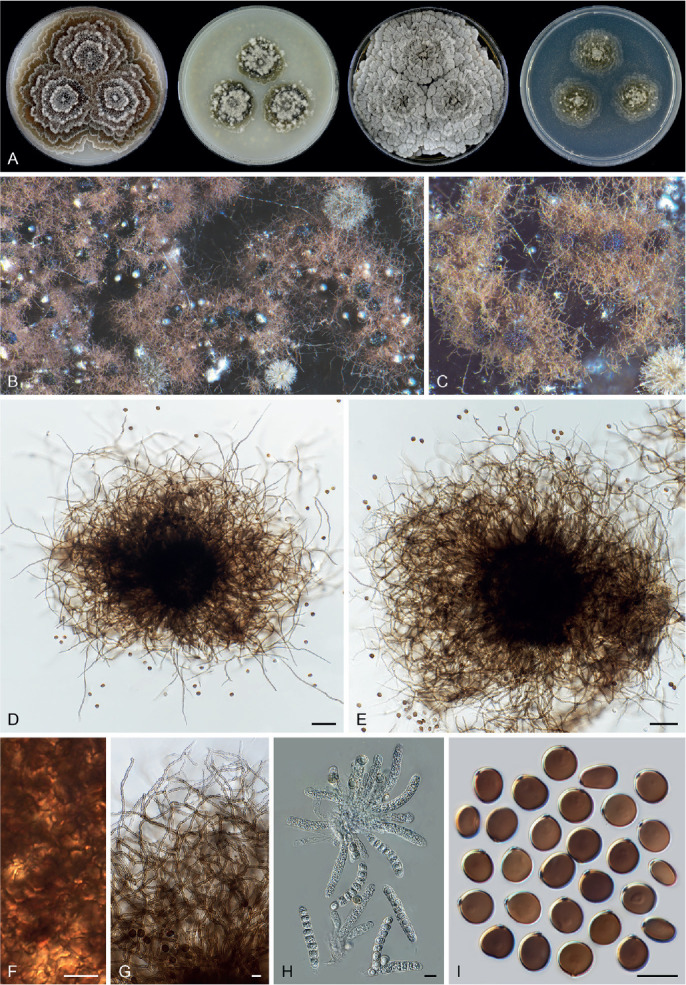
Thermochaetoides thermophila (CBS 179.67). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 d incubation at 45 °C. B, C. Part of the colony, showing ascomata on OA, top view. D, E. Ascomata mounted in lactic acid. F. Structure of ascomatal wall in surface view. G. Terminal ascomatal hairs. H. Asci. I. Ascospores. Scale bars: D, E = 50 μm; F–I = 10 μm.
Basionym: Chaetomium thermophilum La Touche (as ‘thermophile’), Trans. Brit. Mycol. Soc. 33: 95. 1950.
Synonyms: Chaetomium thermophilum var. thermophilum La Touche, Trans. Brit. Mycol. Soc. 33: 95. 1950.
Chaetomium thermophilum var. coprophilum Cooney & R. Emers., Thermophilic Fungi: 68. 1964.
Micromorphology: Ascomata superficial, olivaceous grey or greyish sepia due to ascomatal hairs in reflected light, subglobose, ostiolate, (80–)105–160 μm diam. Ascomatal wall brown, composed of irregular or angular cells. Ascomatal hairs numerous, covering the whole ascoma with no differentiation between terminal hairs and lateral hairs, brown, geniculate and flexuous, dichotomously or irregularly branched, verrucose, septate, 2–4 μm diam near the base. Asci cylindrical, spore-bearing part 46–58 × 8–11.5 μm, with stalks being 6–17 μm long, containing eight uniseriate ascospores, evanescent. Ascospores olivaceous when mature, globose, subglobose or ovoid, bilaterally flattened, 8–9.5(–10.5) × (7.5–)8–9(–9.5) × 5.5–6.5 μm, with a distinctly protuberant germ pore. Asexual morph unknown.
Culture characteristics: Optimum growth temperature 45–55 °C. On OA with a crenate-lobate edge, 44–73 mm diam in 3 d at 45 °C, obverse smoke grey to mouse grey or greyish sepia, with several crenate concentric rings due to the formation of ascomata; reverse olivaceous or fawn. On CMA with a crenate-lobate edge, 25–55 mm diam in 3 d at 45 °C, obverse smoke grey or olivaceous buff to greenish olivaceous, sometimes with crenate concentric rings; reverse pale mouse grey. On MEA with a crenate-lobate edge, 53–over 70 mm diam in 3 d at 45 °C, obverse pale mouse grey or olivaceous grey, radially or irregularly striated to form several concentric rings; reverse iron grey. On PCA with a lobate edge, 26–62 mm diam in 7 d at 45 °C, obverse smoke grey to grey olivaceous or mouse grey to olivaceous grey, with several lobate concentric rings; reverse pale olivaceous grey to olivaceous grey or hazel.
Material examined: Netherlands, isolated from mushroom compost, date unknown, H.C. Bels-Koning (CBS 166.62). Switzerland, isolated from mushroom compost, date unknown, E. Müller (CBS 141.64). UK, Leeds, isolated from decaying wheat straw, 1949, C.J. La Touche (culture ex-type CBS 144.50 = DAOM 24625 = DSM 1495 = IMI 039719; CBS 143.50). USA, California, Alameda Co., isolated from horse dung, 1950, D.G. Cooney & R. Emerson (CBS 179.67, ex-type culture of Chaetomium thermophilum var. coprophilum).
Notes: The variety Ch. thermophilum var. coprophilum (Fig. 45) is morphologically undistinguishable from the ex-type culture of Ch. thermophilum (Fig. 44). Our phylogenetic analysis confirmed that these two taxa are conspecific (Fig. 7D, Supplementary Figs S1–S3). Thermochaetoides thermophila can easily be distinguished from Thermoc. dissita, as noted above.
Thermothelomyces Y. Marín et al., Mycologia 107: 630. 2015.
Micromorphology: Asexual morph: Conidiophores hypha-like, sometimes simply branched, or reduced to conidiogenous cells. Conidiogenous cells reduced to a hyphal cell, or terminally or occasionally laterally from conidiophores, solitary, sometimes verticillate, or in short unbranched or branched chain, sometimes swollen, subglobose, fusiform, clavate or ampulliform, monoblastic or synchronously polyblastic with two or more conidia developing from one conidiogenous cell. Conidia solitary or in short chains, single-celled, mostly hyaline and smooth, in a few species pigmented and verrucose, ovoid, ellipsoidal, pyriform or subglobose, often apically rounded, in a few species apically attenuated, with a narrow and truncate base, rhexolytic when seceding. In one species (Thermoth. myriococcoides), only sterile microsclerotia-like structures were formed, mainly in aerial mycelium, subglobose, ellipsoidal to irregular, composed of angular or irregular cells, surrounded by several layers of outer cells; conidial morph not observed. Sexual morph (fide Fergus & Sinden 1969, von Klopotek 1976) not observed in the culture of an individual strain, but in two heterothallic species (Thermoth. fergusii and Thermoth. heterothallicus), different and compatible strains growing together induce the formation of ascomata. Ascomata, if present, superficial, sub-immersed to immersed, non-ostiolate, globose, glabrous, black when mature. Asci globose, ovoid, clavate or ellipsoidal, containing four or eight ascospores, evanescent. Ascospores brown when mature, unicellular, ellipsoidal or ovoid, with one or two apical germ pores. The genus contains asexual species and heterothallic species with asexual and sexual morphs when both mating types present. Thermophilic.
Type species: Thermothelomyces thermophilus (Apinis) Y. Marín et al.
Notes: Marín-Felix et al. (2015) segregated the thermophilic species of Myceliophthora sensu van den Brink et al. (2012) into two genera: Crassicarpon (with hyaline, smooth-walled conidia) and Thermothelomyces (with brown, ornamented conidia). The present study does not accept the invalidly described genus Crassicarpon (Art. F.5.1). Three of the four species in Thermothelomyces sensu Marín-Felix et al. (2015) are observed to produce hyaline and smooth conidia (Figs 46, 47, 48), which means that “Crassicarpon” and Thermothelomyces species have, besides their thermophilicity, also overlapping morphological characters. In addition, molecular dating analysis shows that divergence between “Crassicarpon” and Thermothelomyces sensu Marín-Felix et al. (2015) happened more recently (at about 18 Mya) than the later time limit (at about 27 Mya, Fig. 8B, 9) of the other accepted genera in the family. We therefore merge “Crassicarpon” in Thermothelomyces sensu Marín-Felix et al. (2015) and redefine this genus. Thermothelomyces diverged from the closest relative Myceliophthora about 30 Mya (Fig. 8B).
Fig. 46.
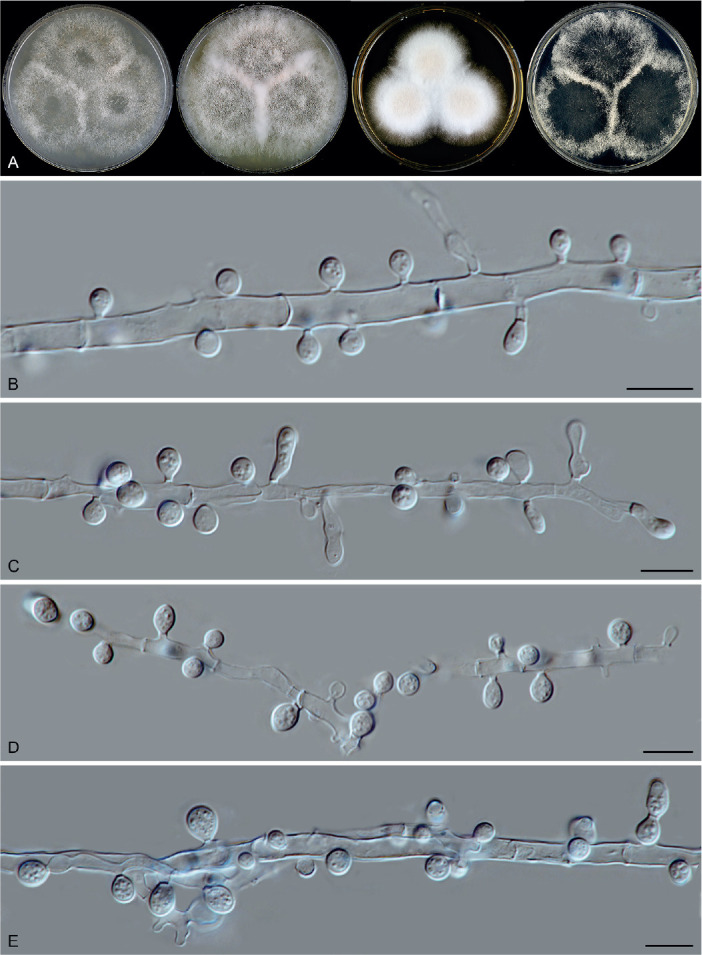
Thermothelomyces fergusii (CBS 406.69, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 7 d incubation at 37 °C. B–E. Hyphae, conidiogenous cells and conidia. Scale bars = 10 μm.
Fig. 47.
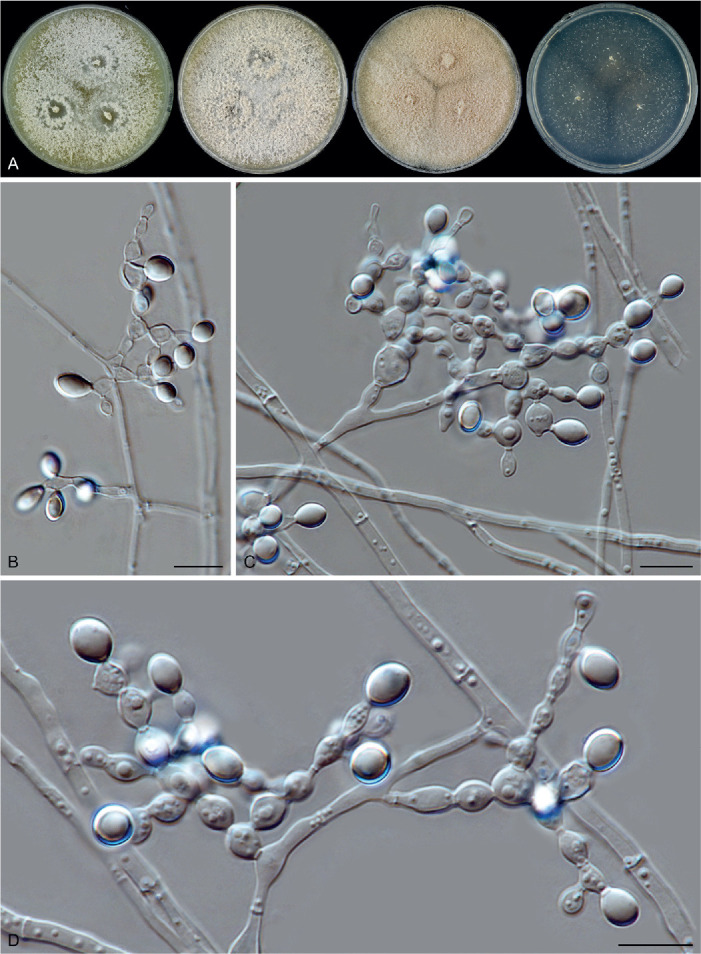
Thermothelomyces guttulatus (CGMCC 3.15185, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 d incubation at 45 °C. B–D. Hyphae, conidiogenous cells and conidia. Scale bars = 10 μm.
Fig. 48.
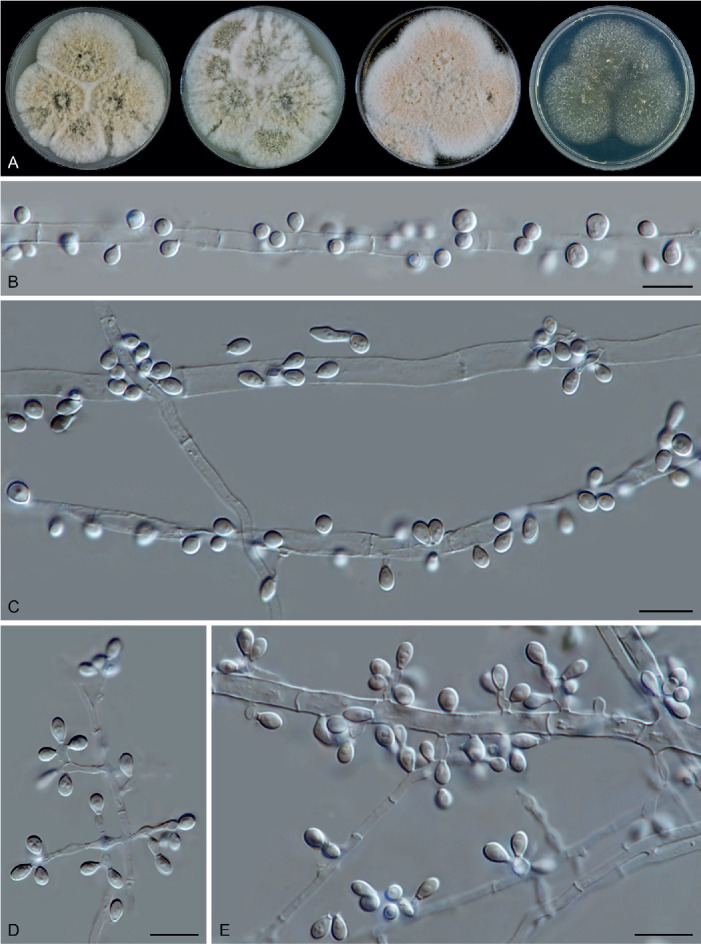
Thermothelomyces heterothallicus (CBS 202.75, ex-epitype culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 d incubation at 45 °C. B–E. Hyphae, conidiogenous cells and conidia. Scale bars = 10 μm.
Thermothelomyces fergusii X.Wei Wang & Houbraken, nom. nov. MycoBank MB 830934. Fig. 46.
Replaced synonym: Thielavia thermophila Fergus & Sinden, Canad. J. Bot. 47: 1635. 1969, non Thermothelomyces thermophilus (Apinis) Y. Marín et al., Mycologia 107: 630. 2015.
Synonyms: Chrysosporium fergusii Klopotek, Arch. Mikrobiol. 98: 366. 1974. (conidial morph).
Corynascus thermophilus (Fergus & Sinden) Klopotek, Arch. Mikrobiol. 98: 366. 1974.
Myceliophthora fergusii (Klopotek) Oorschot, Persoonia 9: 406. 1977.
Crassicarpon thermophilum (Fergus & Sinden) Y. Marín et al., Mycologia 107: 630. 2015, nom. inval., Art. 35.1.
Micromorphology: Conidiophores absent. Conidiogenous cells reduced to an intercalary or terminal hyphal cell or as a denticle laterally arising from a hyphal cell, monoblastic, or occasionally polyblastic. Conidia single-celled, solitary, occasionally in chains of two, smooth, hyaline, subglobose, ovoid, ellipsoidal, pyriform or clavate, usually apically rounded, with a narrow and truncate base, (4–)5–7(–9) × 4–6(–7.5) μm. Sexual morph heterothallic formed by crossing between compatible strains (fide Fergus & Sinden 1969): Ascomata superficial to immersed, non-ostiolate, globose, glabrous, black when mature, 190–260 μm diam. Asci globose or ovoid, 30–37 × 40–52 μm, without distinct stalks, containing four irregularly-arranged ascospores, quickly evanescent. Ascospores brown when mature, unicellular, ellipsoidal, 23–32 × 17–23 μm, with two apical germ pores.
Culture characteristics: On OA with an entire edge, over 70 mm diam in 3 d at 45 °C, texture floccose, obverse white due to aerial mycelium, without coloured exudates; reverse uncoloured. On CMA similar to those on OA. On MEA with an entire edge, over 70 mm diam in 3 d at 45 °C, with a thick layer of aerial mycelium, obverse white; reverse uncoloured. On PCA translucent, with an entire edge, over 70 mm diam in 3 d at 45 °C, with sparse aerial mycelium mainly at the margins, without coloured exudates; reverse uncoloured.
Material examined: UK, Cambridge, isolated from wheat straw compost, date unknown, H.J. Hudson (CBS 174.70 = IMI 145136). USA, Pennsylvania, isolated from mushroom compost, date unknown, C.L. Fergus (culture ex-type CBS 406.69 = ATCC 22067 = R46w2).
Notes: Fergus & Sinden (1969) induced ascomata after growing compatible strains (CBS 406.69 and CBS 405.69) together, and they described this holomorph as Thielavia thermophila. Subsequently, von Klopotek (1974) transferred Thielavia thermophila to Corynascus and redescribed its conidial morph as Chrysosporium fergusii (based on “strain S22”, isolated from Germany designated as ex-type). He also determined the mating types of the following strains: CBS 405.69 and S22 (+); CBS 406.69 and CBS 174.70 (-). The asexual species was later transferred into Myceliophthora as My. fergusii by van Oorschot (1977). Based on a three-locus phylogenetic analysis, Marín-Felix et al. (2015) combined this species in the invalidly-proposed Crassicarpon (published without identifier, Art. F.5.1), a genus related to Thermothelomyces. Crassicarpon is not accepted here (see notes of Thermothelomyces above). In the present study, we only examined the individual strains and did not attempt mating experiments to induce ascomata.
Thermothelomyces guttulatus (Yu Zhang & L. Cai) Y. Marín et al., Mycologia 107: 630. 2015. Fig. 47.
Basionym: Myceliophthora guttulata Yu Zhang & L. Cai, Mycol. Prog. 13: 168. 2013.
Micromorphology: Conidiophores hypha-like, or simply branched, hyaline, sometimes reduced. Conidiogenous cells often several formed in unbranched or branched chains, swollen, subglobose, ellipsoidal, doliiform, ampulliform or fusiform, monoblastic, or polyblastic with two or more conidia formed from one cell, 3.5–6.5(–8) × 2–5.5 μm. Conidia solitary or in short chains, single-celled, smooth, hyaline, subglobose, ellipsoidal or ovoid, apically rounded or slightly attenuated, with a narrow and truncate base, (4–)4.5–7.5(–10) × (3–)3.5–5(–5.5) μm diam. Sexual morph not observed.
Culture characteristics: On OA with an entire edge, over 70 mm diam in 3 d at 45 °C, texture cottony, obverse white due to the formation of conidia on aerial mycelium, greyish yellow-green around the colonies due to coloured exudates diffusing into the medium; reverse buff. On CMA similar to those on OA, over 70 mm diam in 3 d at 45 °C, reverse cinnamon due to coloured exudates diffusing into the medium. On MEA with an entire edge, over 70 mm diam in 3 d at 45 °C, texture thick cottony, obverse white to pale smoke grey; reverse umber. On PCA translucent, with an entire edge, over 70 mm diam in 3 d at 45 °C, with sparse aerial mycelium, without coloured exudates; reverse uncoloured.
Material examined: China, Hunan Province, Yizhang county, Mangshan National Forest Park, isolated from soil, 10 Sep. 2000, W.-P. Wu (culture ex-type CGMCC 3.15185; culture CGMCC 3.15186).
Notes: No sexual morph was observed and we speculate that this species is heterothallic. Mating type analysis in combination with growth experiments might induce ascomata formation. Thermothelomyces guttulatus can be distinguished from other species by its swollen conidiogenous cells.
Thermothelomyces heterothallicus (Klopotek) Y. Marín et al., Mycologia 107: 630. 2015. Fig. 48.
Basionym: Thielavia heterothallica Klopotek, Arch. Mikrobiol. 107: 223. 1976.
Synonyms: Corynascus heterothallicus (Klopotek) Arx, Persoonia 12: 174. 1984.
Myceliophthora heterothallica (Klopotek) van den Brink & Samson, Fungal Diversity 52: 206. 2012, nom. inval., Art. 41.5.
Micromorphology: Conidiophores absent. Conidiogenous cells reduced to intercalary hyphal cells, or differentiated laterally from hyphae, sometimes two or more in short simple or branched chains, slightly swollen, fusiform, clavate or ampulliform, monoblastic, or polyblastic with two or more conidia developed from one cell, 2–7 × 1–4 μm. Conidia solitary or in short chains, single-celled, smooth, hyaline, ovoid, ellipsoidal, pyriform, usually apically rounded, with a narrow and truncate base, (3–)3.5–5(–6.5) × (2–)2.5–3 μm. Sexual morph heterothallic formed by crossing between compatible strains (fide von Klopotek 1976): Ascomata sub-immersed to immersed, non-ostiolate, globose, brown or black when mature, 70–180 μm diam. Ascomatal wall composed of textura epidermoidea. Asci clavate or ellipsoidal, 25–35 × 10–15 μm, with stalks, containing eight ascospores, evanescent. Ascospores brown when mature, unicellular, ellipsoidal or ovoid, 7.5–11 × 4.5–7 μm, with one apical germ pore.
Culture characteristics: On OA with an entire edge, 42–55 mm diam in 3 d at 45 °C, with a thick layer of aerial mycelium, texture cottony, obverse white to buff due to the formation of conidia on aerial mycelium; reverse pale luteous. On CMA similar to those on OA, 46–54 mm diam in 3 d at 45 °C, obverse white. On MEA similar to those on OA, 51–58 mm diam in 3 d at 45 °C, obverse white to rosy buff; reverse ochreous. On PCA translucent, with an entire edge, 36–50 mm diam in 3 d at 45 °C, with sparse aerial mycelium, without coloured exudates; reverse uncoloured.
Material examined: Germany, Giessen, isolated from garden soil, date unknown, A. von Klopotek (CBS 202.75), (holotype CBS H-18810, dried culture of crossing of CBS 202.75 and CBS 203.75, designated by von Klopotek (1976), epitype CBS H-24878, designated here, MBT 10004825; culture ex-epitype CBS 202.75) USA, Indiana, Bloomington, isolated from soil, 8 Aug. 1974, M.R. Tansey (CBS 203.75); isolated from soil, date and collector unknown (CGMCC 3.13596 = ACCC 30346 = IFFI 2441).
Notes: A holotype specimen was prepared by von Klopotek (1976) consisting of a dried culture of a crossing with CBS 202.75 and CBS 203.75. This specimen, which was later labeled as CBS H-18810 contains many ascomata. Although this specimen demonstrates the sexual component of the life cycle, it is necessary to designate an epitype from one of the strains in order to present the phylogenetic relationship of this species with other ones. Here, we designate CBS H-24878, derived from CBS 202.75, as the epitype. Thermothelomyces heterothallicus is phylogenetically closely related to Thermoth. thermophilus, but can be distinguished by its simpler conidiogenous structures and the production of conidia with a rounded apex. Within the genus, Thermot. heterothallicus is phylogenetically more distant from Thermoth. fergusii (Fig. 7A). Nevertheless, the conidial morphs of the two species are quite similar. However, Thermot. heterothallicus produces smaller and narrower conidia (3.5–5 × 2.5–3 μm vs 5–7 × 4–6 μm). Both species are hetherothallic. The sexual morph of Thermoth. heterothallicus distinctively differs from that of Thermoth. fergusii. The former species produces clavate or ellipsoidal asci containing eight smaller ascospores (7.5–11 × 4.5–7 μm) with an apical germ pore, while the latter species has globose or ovoid asci containing four larger ascospores (23–32 × 17–23 μm) with two apical germ pores (Fergus & Sinden 1969, von Klopotek 1976). In the present study, we only examined the individual strains and did not undertake mating experiments to induce ascomata for study.
Thermothelomyces hinnuleus (Awao & Udagawa) Y. Marín et al., Mycologia 107: 630. 2015. Fig. 49.
Fig. 49.
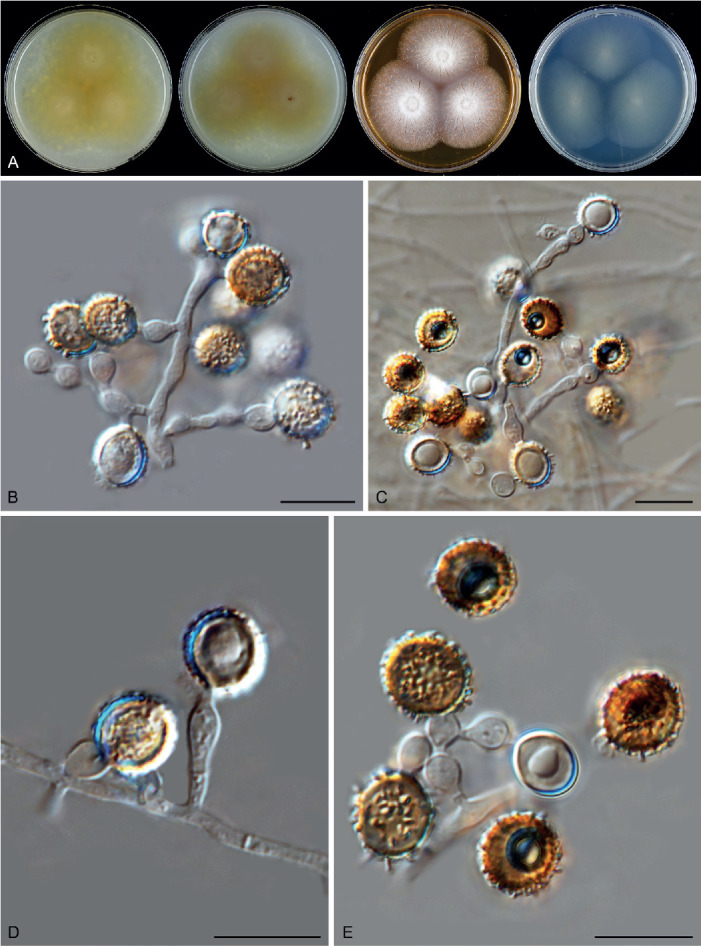
Thermothelomyces hinnuleus (CBS 597.83, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 7 d incubation at 37 °C. B–E. Hyphae, conidiogenous cells and conidia. Scale bars = 10 μm.
Basionym: Myceliophthora hinnulea Awao & Udagawa, Mycotaxon 16: 436. 1983.
Micromorphology: Conidiophores hypha-like or reduced. Conidiogenous cells lateral or terminal, solitary or several in simple or branched chains, swollen, pyriform, clavate or ampulliform, usually monoblastic, 4–7 × 2.5–3.5 μm. Conidia solitary or in short chains, single-celled, verrucose, orange, umber or fulvous, subglobose, (7–)7.5–9 (–10.5) × (6–)6.5–8(–8.5) μm diam. Sexual morph not observed.
Culture characteristics: On OA with an entire edge, 50–56 mm diam in 3 d at 45 °C, with sparse aerial mycelium, obverse pale luteous; reverse pale luteous. On CMA similar to those on OA. On MEA with an entire edge, 48–54 mm diam in 3 d at 45 °C, obverse white due to aerial mycelium, forming radiating furrows; reverse ochreous to orange. On PCA translucent, with an entire edge, 48–54 mm diam in 3 d at 45 °C, without coloured exudates; reverse uncoloured.
Material examined: Japan, Shizuoka Pref., Tagatagun, Niriayama-machi, isolated from cultivated soil, 24 Feb. 1973, T. Awao (culture ex-type CBS 597.83 = AJ 6773 = ATCC 52474 = NHL 2909). New Zealand, Christchurch, isolated from soil, date unknown, A.L.J. Cole (CBS 544.82).
Notes: Thermothelomyces hinnuleus is the only species of the genus that produces verrucose, pigmented conidia. Phylogenetic analysis shows that it is closely related to Thermoth. guttulatus, Thermoth. heterothallicus and Thermoth. thermophilus (Fig. 7A). No sexual morph has been observed or described for this species.
Thermothelomyces myriococcoides X.Wei Wang & Houbraken, nom. nov. MycoBank MB 830935. Fig. 50.
Fig. 50.
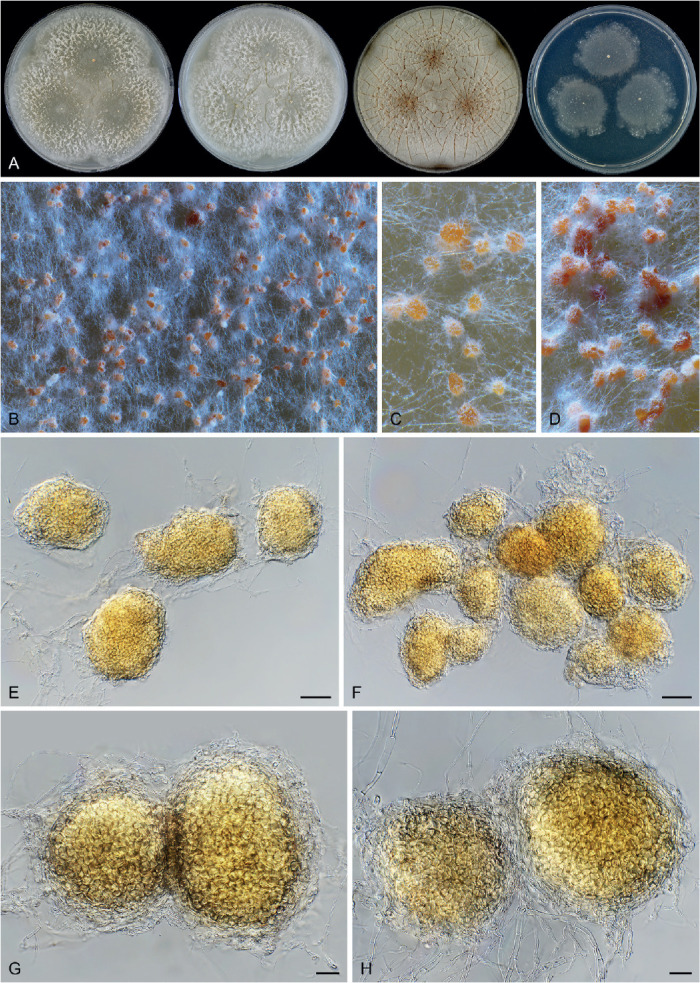
Thermothelomyces myriococcoides (CBS 389.93, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3d incubation at 45 °C. B–D. Part of the colony, showing microsclerotium-like structures on aerial mycelium. E–H. Hyphae and microsclerotium-like structures. Scale bars: E, F = 50 μm; G, H = 20 μm.
Replaced synonym: Papulaspora thermophila Fergus, Mycologia 632: 426. 1971, non Thermothelomyces thermophilus (Apinis) Y. Marín et al., Mycologia 107: 630. 2015.
Synonyms: Myriococcum thermophilum (Fergus) Aa, Verh. Kon. Ned. Akad. Wetensch., Afd. Natuurk. 61(4): 60. 1973.
Crassicarpon hotsonii (Fergus) Koukol, Pl. Syst. Evol. 302: 967. 2016, nom. inval., Art. 35.1.
Etymology: The epithet refers to the genus Myriococcum, to which the species is morphologically similar.
Micromorphology: Microsclerotium-like structures originating mainly from aerial mycelium, subglobose, ellipsoidal to irregular, pale luteous to apricot in reflected light, pale luteous to luteous when mounted in lactic acid, 85–230 × 75–180 μm, composed of angular or irregular cells, surrounded by the outer 2–5 layers consisting of pale or hyaline, elongate cells. Other reproductive structures not observed. Thermophilic.
Culture characteristics: On OA with a crenate edge, 59–65 mm diam in 3 d at 45 °C, texture cottony, with white aerial mycelium, obverse grey white to buff due to microsclerotium-like structures mixed with aerial mycelium, without coloured exudates; reverse uncoloured. On CMA similar to those on OA. On MEA with a crenate edge, over 70 mm diam in 3d at 45 °C, obverse vinaceous buff, wrinkled to form radiating furrows, reverse ochreous. On PCA with a crenate or lobate edge, 36–48 mm diam in 3 d at 25 °C, with sparse aerial mycelium, without coloured exudates; reverse uncoloured.
Material examined: Netherlands, Limburg, isolated from self-heating horse manure, 30 Mar. 1987, G. Straatsma (CBS 208.89). Switzerland, Gossau-Zürich, isolated from surface of heated compost, 1969, C.L. Fergus (culture ex-type CBS 389.93 = CBS 736.70 = ATCC 22112).
Notes: Neither a conidial nor a sexual (ascomata) morph has been observed in Thermoth. myriococcoides. This species is thermophilic and produces numerous microsclerotium-like structures in the aerial mycelium. Thermothelomyces myriococcoides was originally described in Papulaspora (Fergus 1971), and later transferred to Myriococcum by van der Aa (1973). Myriococcum, typified by Myriococcum praecox, was described by Fries (1823) as a sterile fungus producing sclerotia. As a consequence, several unrelated species were classified in this genus, including the thermophilic species Myriococcum thermophilum. Koukol (2016) showed that the genus is highly polyphyletic and the generic type belongs to the family Stephanosporaceae (Agaricomycetes). Furthermore, Myriococcum thermophilum was transferred to the invalidly proposed genus Crassicarpon as Crass. hotsonii. Based on our phylogenetic analysis, this species belongs to Thermothelomyces.
Thermothelomyces thermophilus (Apinis) Y. Marín et al., Mycologia 107: 630. 2015. Fig. 51.
Fig. 51.
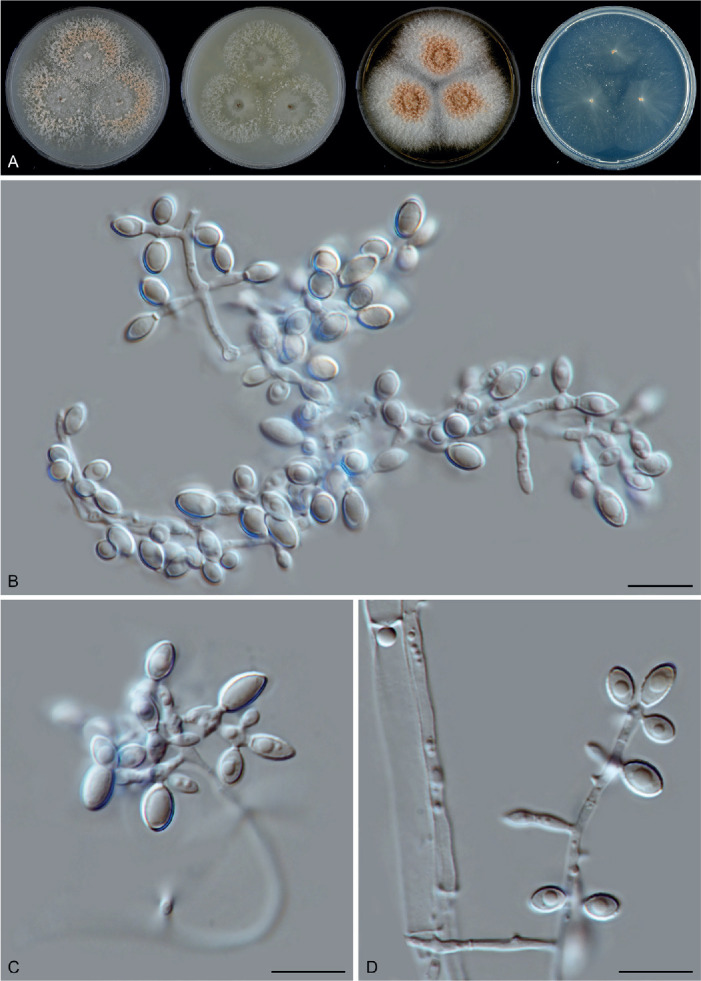
Thermothelomyces thermophilus (CBS 117.65, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 d incubation at 37 °C. B–D. Hyphae, conidiogenous cells and conidia. Scale bars = 10 μm.
Basionym: Sporotrichum thermophilum [‘thermophile’] Apinis, Nova Hedwigia 5: 74. 1962.
Synonyms: Chrysosporium thermophilum (Apinis) Klopotek, Arch. Mikrobiol. 98: 366. 1974.
Myceliophthora thermophila (Apinis) Oorschot, Persoonia 9: 403. 1977.
Micromorphology: Conidiophores hyaline, hypha-like. Conidiogenous cells verticillate, lateral or terminal, occasionally intercalary, slightly swollen, fusiform, clavate or ampulliform, usually polyblastic with two or more conidia developed from one cell, 4–7 × 1.5–3 μm. Conidia solitary or in short chains, single-celled, smooth, hyaline to buff, ovoid, ellipsoidal, pyriform, often apically attenuated, with a narrow and truncate base, (3.5–)4–5.5(–6) × (2.5–)3–3.5(–4) μm diam. Sexual morph not observed.
Culture characteristics: On OA with an entire edge, 38–44 mm diam in 3 d at 45 °C, texture cottony, obverse grey white to rosy buff due to the formation of conidia on aerial mycelium, without coloured exudates; reverse uncoloured. On CMA similar to those on OA, 44–50 mm diam in 3 d at 45 °C, obverse grey white. On MEA with an entire edge, 59–65 mm diam in 3 d at 45 °C, texture thick cottony, obverse grey white to rosy buff due to the formation of conidia on aerial mycelium; reverse ochreous. On PCA translucent, with an entire edge, 40–46 mm diam in 3 d at 45 °C, with sparse aerial mycelium, without coloured exudates; reverse uncoloured.
Material examined: UK, Attenborough, isolated from dry pasture soil, Jul. 1951, A.E. Apinis (culture ex-type CBS 117.65 = BDUN 274). Unknown, isolated from man, biopsy of nasal cavity, HIV pos. patient affected by sinusitis with infiltration of the orbit & exophthalmos, date unknown, L. Polonelli (CBS 381.97). USA, Ajinomoto Co., substrate and date unknown (CBS 669.85, mutant of CBS 866.85 producing cellulase).
Notes: Von Klopotek (1976) treated Thielavia heterothallica (= Myceliophthora heterothallica = Thermothelomyces heterothallicus) as the sexual morph of this species, followed by van Oorschot (1977). Previous studies showed that these two species are closely related, but distinct (van den Brink et al. 2015, Marín-Felix et al. 2015). Our phylogenetic analysis confirmed their separation (Fig. 7A, Supplementary Figs S1–S3). Morphologically, Thermoth. thermophilus and Thermoth. heterothallicus can be differentiated by their conidiophore structure. The former species produces conidiophores with verticillate, lateral or terminal conidiogenous cells and its conidia are often apically attenuated, while the conidiogenous cells of Thermoth. heterothallicus are often reduced or arise laterally from hyphae, developing apically rounded conidia.
Trichocladium Harz, Bull. Soc. Imp. Naturalistes Moscou 44: 125. 1871.
Micromorphology: Containing asexual species, sexual species and species with both asexual and sexual morphs. Conidiophores originating laterally or terminally from hyphae, simple or branched, sometimes reduced. Conidiogenous cells monoblastic or polyblastic, occasionally reduced to a hyphal cell, in one species (Tri. beniowskiae) swollen and in simple or branched chains. Conidia solitary or in chains of a few spores, 1-celled, didymo-, phragmo- or muriform, globose, subglobose, oblate, ellipsoid, obovoid, pyriform or quadrangular, olivaceous, brown or dark brown, or hyaline in Tri. beniowskiae, smooth to verrucose, rhexolytic when seceding, often with germ pore(s). Acremonium-like phialides present in a few species, lateral or occasionally terminal, hyaline. Acremonium-like conidia in basipetal chains or in a false slimy head, hyaline, aseptate, smooth, obovoid, usually with a truncated base and a rounded apex. In one species (Tri. amorphum) only intercalary arthroconidia, chlamydospores or microsclerotia produced. Ascomata, if present, superficial or immersed in the thick mycelium, ostiolate or non-ostiolate. Asci cylindrical with eight (four) uniseriate ascospores, or clavate to fusiform with eight biseriate ascospores, evanescent. Ascospores typically broadly ovate, bilaterally flattened, sometimes ellipsoidal and non-flattened, with an apical germ pore.
Type species: Trichocladium asperum Harz
Notes: Trichocladium is morphologically highly diverse in both its asexual and sexual morphs (Wang et al. 2019a). Among the sexual species, ascomata are ostiolate in five species, and non-ostiolate in two species (Tri. antarcticum and Tri. arxii). Here we describe a third species producing non-ostiolate ascomata.
Trichocladium tomentosum X.Wei Wang, P.J. Han & F.Y. Bai, sp. nov. MycoBank MB 840131. Fig. 52.
Fig. 52.
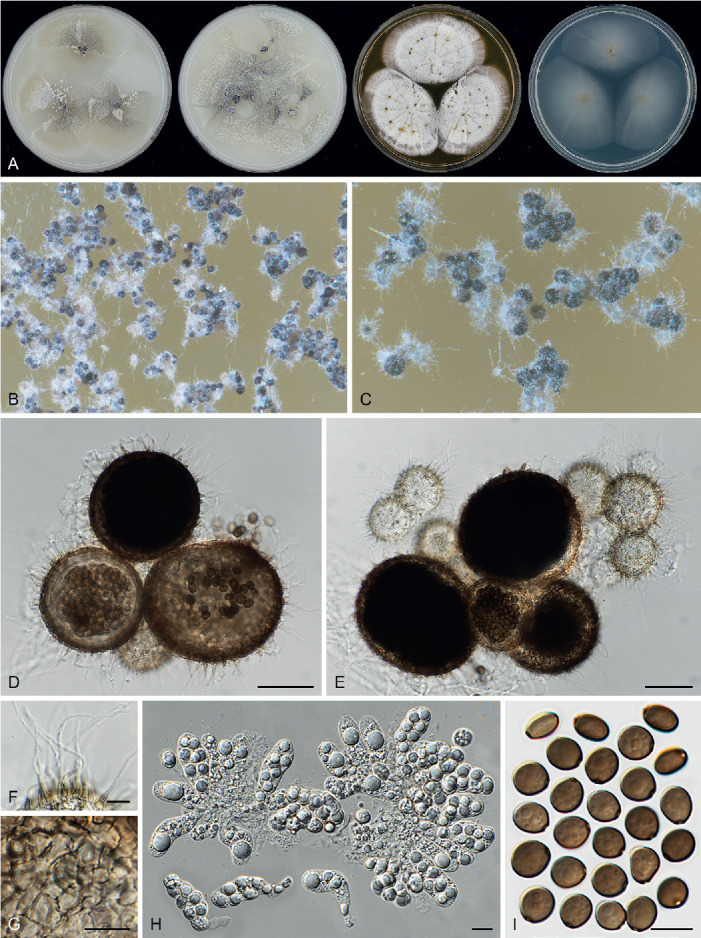
Trichocladium tomentosum (CGMCC 3.20443, ex-type culture). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony on OA. C. Mature ascomata on OA, top view. D, E. Ascomata mounted in lactic acid. F. Ascomatal hairs. G. Structure of ascomatal wall in surface view. H. Asci. I. Ascospores. Scale bars: D, E = 50 μm; F–I = 10 μm.
Etymology: The name refers to its downy ascomatal hairs.
Micromorphology: Ascomata superficial, solitary to aggregated, non-ostiolate, leaden black when mature in reflected light due to the dark ascomatal wall covered with short ascomatal hairs, spherical, 50–150 μm diam. Ascomatal wall brown, semi-translucent, composed of textura epidermoidea in surface view. Ascomatal hairs covering the whole ascomata, hypha-like, smooth or finely verrucose, brown at the base, septate, tapering and fading to hyaline in the upper part, with basal cells swelling, 2.5–4.5 μm wide. Asci clavate, spore-bearing part 28.5–35 × 12–15 μm, with stalks 7–15 μm long, containing eight irregularly-arranged ascospores, evanescent. Ascospores 1-celled, smooth, olivaceous when mature, smooth, broadly ovoid, bilaterally flattened, (6.5–)8–9.5(–10) × (7–)7.5–8.5(–9.5) × (5–)5.5–6.5(–7) μm, with an apical germ pore at the attenuated end. Asexual morph unknown.
Culture characteristics: On OA with an entire edge, 15–21 mm diam in 7 d at 25 °C, with sparse white aerial mycelium, obverse white or pale olivaceous grey due to masses of ascomata, without coloured exudates, or greyish sepia in the centre of the old culture due to exudates diffusing into the medium; reverse buff to fawn. On CMA similar to those on OA, 17–23 mm diam in 7 d at 25 °C, usually without coloured exudates. On MEA with an entire or slightly undulate edge, 18–24 mm diam in 7 d at 25 °C, with thick white aerial mycelium, texture floccose, obverse white, reverse ochraceous to umber. On PCA with an entire edge, 17–23 mm diam in 7 d at 25 °C, without aerial mycelium, obverse uncoloured, without coloured exudates, reverse uncoloured.
Material examined: China, Qinghai, isolated from soil near Qinghai Lake, Jul. 2003, X.W. Wang (holotype HMAS 350294, isotype CBS H-23643, culture ex-type CGMCC 3.20443 = CBS 144476 = WXW 8615).
Notes: Trichocladium tomentosum is phylogenetically closely related to several asexually reproducing species, such as Tri. asperum, Tri. gilmaniellae, Tri. griseum and Tri. jilongense (Fig. 7D). No asexual morph was found in our single Tri. tomentosum strain. The closest sexually reproducing species are Tri. crispatum with ostiolate ascomata and Tri. antarcticum with non-ostiolate ascomata (Wang et al. 2019a). The new species produces non-ostiolate ascomata and broadly ovoid, bilaterally flattened ascospores with an apical germ pore, similar to those of Tri. antarcticum, but can be differentiated by the production of hypha-like ascomatal hairs and clavate asci (rather than glabrous ascomata and cylindrical to elongated clavate asci as in Tri. antarcticum) and by the lack of an acremonium-like asexual morph.
Xanthiomyces X.Wei Wang & Houbraken, gen. nov. MycoBank MB 840125.
Etymology: The name refers to its ascomata, which look like the fruit of the plant genus Xanthium.
Micromorphology: Ascomata superficial, ellipsoidal, subglobose or ovoid, ostiolate. Ascomatal wall dark brown, composed of angular or irregular cells in surface view. Ascomatal hairs erect, seta-like, verrucose. Asci fasciculate, elongated fusiform or clavate, stalked, containing eight irregularly-arranged ascospores, evanescent. Ascospores olivaceous grey when mature, ovate to elongated ovate, with an apical germ pore. Asexual morph unknown.
Type species: Xanthiomyces spinosus (Chivers) X.Wei Wang & Houbraken
Notes: “Chaetomium spinosum” forms a sister clade to Chaetomium sensu stricto (Fig. 7B). This species produces ovate ascospores that are usually less than 7 μm in length (Fig. 53K), in contrast to the limoniform to globose ascospores of Chaetomium, which are longer than 7 μm (Fig. 4Q–V). The molecular dating analysis indicates that this species diverged from Chaetomium about 50 Mya, significantly earlier than the later time limit of the other accepted genera in the family (about 27 Mya, Fig. 8B, 9). Based on the morphological difference and the divergence date, the new genus Xanthiomyces is proposed here to accommodate Chaetomium spinosum.
Fig. 53.

Xanthiomyces spinosus (CBS 789.71). A. Colonies from left to right on OA, CMA, MEA and PCA after 3 wk incubation. B. Part of the colony on OA. C, D. Mature ascomata on OA. E–G. Ascomata mounted in lactic acid. H. Structure of ascomatal wall in surface view. I. Terminal ascomatal hairs. J. Asci. K. Ascospores. Scale bars: E–G = 100 μm; H–K = 10 μm.
Xanthiomyces spinosus (Chivers) X.Wei Wang & Houbraken, comb. nov. MycoBank MB 840166. Fig. 53.
Basionym: Chaetomium spinosum Chivers, Proc. Amer. Acad. Arts 48: 86. 1912.
Micromorphology: Ascomata superficial, ostiolate, yellow in reflected light due to ascomatal hairs, ellipsoidal, suglobose or ovoid, 150–230 μm high, 120–180 μm diam. Ascomatal wall dark brown, composed of angular or irregular cells in surface view. Terminal hairs dark brown, often covered by yellow crystals, seta-like, verrucose, tapering, 4–7 μm near the base. Lateral hairs similar to, but shorter than terminal ones. Asci elongated fusiform or clavate, with spore-bearing part 15–27 × 7–9 μm, with stalks 6–11 μm long, containing eight irregularly-arranged or biseriate ascospores, evanescent. Ascospores 1-celled, olivaceous grey when mature, smooth, ovate to elongated ovate, slightly bilaterally flattened, with an apical germ pore, (5–)5.5–6.5(–7) × (3–)3.5–4(–4.5) × 3–4 μm, with an apical germ pore at the attenuated end. Asexual morph unknown.
Culture characteristics: On OA with an entire edge, 53–59 mm diam in 7 d at 25 °C, obverse olivaceous buff or white, sometimes pale luteous due to aerial mycelium, or primrose due to the formation of ascomata; reverse buff to pale luteous, or saffron due to exudates diffusing into the medium. On CMA similar to those on OA, 54–60 mm diam in 7 d at 25 °C; reverse honey. On MEA with an entire edge, over 70 mm diam in 7 d at 25 °C, obverse olivaceous buff, primrose or straw due to aerial mycelium, reverse umber. On PCA with an entire edge, 34–42 mm diam in 7 d at 25 °C, obverse white, aerial mycelium sparse, without coloured exudates; reverse uncoloured.
Material examined: Switzerland, Zürich, isolated from culture of algae, date unknown, E. Müller (representative strain CBS 789.71 = ETH 7700); Bloney, isolated from straw, date unknown, M. Dreyfuss (culture CBS 796.83).
Notes: Xanthiomyces spinosus is morphologically reminiscent of Dichotomopilus species which are characterised by their seta-like to dichotomously or irregularly branched ascomatal hairs, and small (usually less than 7.5 μm long) and narrowly ovate, ovate or broad ovate ascospores with an apical or slightly sub-apical germ pore (Fig. 4B, Wang et al. 2016b). Phylogenetically, this species is distantly related to Dichotomopilus (Fig. 7A, B).
List of genera and species in Chaetomiaceae
Since its introduction, over 400 species were described in genera of Chaetomiaceae. In the list provided below, we accept only the species that we could classify in the family using DNA sequence data (species shown in bold font). Species originally described in genera of Chaetomiaceae, but excluded or reclassified by other authors, or which have not been re-examined recently, are not included in our list. A few excluded species that we have examined, or were sequenced by other authors, are included in a brief overview at the end of this list. Synonyms of the accepted species are provided (in italics, non-bold font) only when these are known and confirmed by sequence data. Most of these data originate from previous studies (Wang et al. 2016a, b, 2019 a, b). The list includes 275 accepted species, distributed across 50 genera. For each species, we provide the full reference including MycoBank number, provide information (if known) of the holotype and ex-type strain/specimen and GenBank numbers of reference sequences. In addition, we indicate if the species is able to produce a sexual and/or an asexual morph.
List of accepted genera in Chaetomiaceae
Achaetomiella Arx, The genera of fungi sporulating in pure culture: 247. 1970. [MB 37]. — Type: Achaetomiella virescens. Reproduction: sexual.
Achaetomium J.N. Rai et al., Canad. J. Bot. 42: 693. 1964. [MB 38]. — Type: Achaetomium globosum. Reproduction: sexual.
Acrophialophora Edward, Mycologia 51: 784. 1961. [MB 7037]. — Type: Acrophialophora nainiana. Reproduction: asexual or sexual.
Allobotryotrichum M. Raza & L. Cai, Fungal Diversity 99: 74. 2019. [MB 556672]. — Type: Allobotryotrichum blastosporum. Reproduction: asexual.
Allocanariomyces Mehrabi et al., Mycol. Prog. 19: 1417. 2020. [MB 835853]. — Type: Allocanariomyces tritici. Reproduction: sexual & asexual.
Amesia X.Wei Wang et al., Stud. Mycol. 84: 156. 2016. [MB 818829]. — Type: Amesia atrobrunnea. Reproduction: sexual.
Aporothielavia Malloch & Cain, Mycologia 65: 1074. 1973. [MB 283]. — Type: Aporothielavia leptoderma. Reproduction: sexual.
Arcopilus X.Wei Wang et al., Stud. Mycol. 84: 159. 2016. [MB 818835]. — Type: Arcopilus aureus. Reproduction: sexual.
Arxotrichum A. Nováková & M. Kolařík, Persoonia 40: 259. 2018. [MB 824080]. — Type: Arxotrichum wyomingense. Reproduction: sexual & asexual.
Batnamyces Noumeur, Mycol. Prog. 19: 593. 2020. [MB 832844]. — Type: Batnamyces globulariicola. Reproduction: asexual.
Bommerella Marchal, Bull. Soc. Roy. Bot. Belgique 24: 164. 1885. [MB 622]. — Type: Bommerella trigonospora. Reproduction: sexual.
Botryoderma Papendorf & H.P. Upadhyay, Trans. Brit. Mycol. Soc. 52: 257. 1969. [MB 7419]. — Type: Botryoderma lateritium. Reproduction: asexual.
Botryotrichum Sacc. & Marchal, Bull. Soc. Roy. Bot. Belgique 24: 66. 1885. [MB 7431]. — Type: Botryotrichum piluliferum. Reproduction: asexual & sexual.
Brachychaeta X.Wei Wang & Houbraken, Stud. Mycol. 93: 186. 2019. [MB 829842]. — Type: Brachychaeta variospora. Reproduction: sexual.
Canariomyces Arx, Persoonia 12: 185. 1984. [MB 25789]. — Type: Canariomyces notabilis. Reproduction: sexual & asexual.
Carteria X.Wei Wang & Houbraken, Stud. Mycol. 93: 194. 2019. [MB 829850]. — Type: Carteria arctostaphyli. Reproduction: sexual.
Chaetomium Kunze, Mykol. Hefte 1: 15. 1817. [MB 953]. — Type: Chaetomium globosum. Reproduction: sexual & asexual.
Chrysanthotrichum X.Wei Wang & Houbraken, Stud. Mycol. 93: 194. 2019. [MB 829852]. — Type: Chrysanthotrichum lentum. Reproduction: sexual.
Chrysocorona X.Wei Wang & Houbraken, Stud. Mycol. 93: 201. 2019. [MB 829858]. — Type: Chrysocorona lucknowensis. Reproduction: sexual.
Collariella X.Wei Wang et al., Stud. Mycol. 84: 177. 2016. [MB 818839]. — Type: Collariella bostrychodes. Reproduction: sexual.
Condenascus X.Wei Wang & Houbraken, Stud. Mycol. 93: 203. 2019. [MB 829860]. — Type: Condenascus tortuosus. Reproduction: sexual.
Corynascella Arx & Hodges, Stud. Mycol. 8: 23. 1975. [MB 1256]. — Type: Corynascella humicola. Reproduction: sexual & asexual.
Corynascus Arx, Proc. Kon. Ned. Akad. Wetensch., Sect. C 76: 295. 1973. [MB 1257]. — Type: Corynascus sepedonium. Reproduction: sexual & asexual.
Dichotomopilus X.Wei Wang et al., Stud. Mycol. 84: 185. 2016. [MB 818840]. — Type: Dichotomopilus indicus. Reproduction: sexual.
Floropilus X.Wei Wang & Houbraken, Stud. Mycol. 93: 203. 2019. [MB 829862]. — Type: Floropilus chiversii. Reproduction: sexual.
Humicola Traaen, Nytt Mag. Naturvidensk. 52: 31. 1914. [MB 8566]. — Type: Humicola fuscoatra. Reproduction: sexual & asexual.
Hyalosphaerella X.Wei Wang & Houbraken, Stud. Mycol. 93: 205. 2019. [MB 829864]. — Type: Hyalosphaerella fragilis. Reproduction: sexual.
Madurella Brumpt, Compt.-Rend. Séances Mém. Soc. Biol. 58: 999. 1905. [MB 8824]. — Type: Madurella mycetomatis. Reproduction: asexual/sterile.
Melanocarpus Arx, Stud. Mycol. 8: 17. 1975. [MB 3063]. — Type: Melanocarpus albomyces. Reproduction: sexual & asexual.
Microthielavia X.Wei Wang & Houbraken, Stud. Mycol. 93: 208. 2019. [MB 829866]. — Type: Microthielavia ovispora. Reproduction: sexual.
Myceliophthora Costantin, Compt. Rend. Hebd. Séances Acad. Sci. 114: 849. 1892. [MB 9013]. — Type: Myceliophthora lutea. Reproduction: asexual.
Mycothermus D.O. Natvig et al., Mycologia 107: 321. 2015. [824453]. — Type: Mycothermus thermophilus. Reproduction: asexual.
Ovatospora X.Wei Wang et al., Stud. Mycol. 84: 207. 2016. [MB 818850]. — Type: Ovatospora brasiliensis. Reproduction: sexual.
Parachaetomium Mehrabi et al., Mycol. Prog. 19: 1422. 2020. [MB 835855]. — Type: Parachaetomium perlucidum. Reproduction: sexual.
Parathielavia X.Wei Wang & Houbraken, Stud. Mycol. 93: 208. 2019. [MB 829868]. — Type: Parathielavia hyrcaniae. Reproduction: sexual & asexual.
Parvomelanocarpus X.Wei Wang et al., this study. [MB 840124]. — Type: Parvomelanocarpus tardus. Reproduction: sexual.
Pseudohumicola X.Wei Wang et al., this study. [MB 840123]. — Type: Pseudohumicola subspiralis. Reproduction: sexual & asexual.
Pseudothielavia X.Wei Wang & Houbraken, Stud. Mycol. 93: 213. 2019. [MB 829872]. — Type: Pseudothielavia terricola. Reproduction: sexual.
Remersonia Samson & Seifert, Canad. J. Bot. 75: 1160. 1997. [MB 27809]. — Type: Remersonia thermophila. Reproduction: asexual.
Staphylotrichum J. Mey. & Nicot, Bull. Trimestriel Soc. Bot. France 72: 322. 1957. [MB 10065]. — Type: Staphylotrichum coccosporum. Reproduction: sexual & asexual.
Stellatospora Tad. Ito & Nakagiri, Mycoscience 35: 413. 1994. [MB 27456]. — Type: Stellatospora terricola. Reproduction: sexual.
Stolonocarpus X.Wei Wang & Houbraken, Stud. Mycol. 93: 221. 2019. [MB 829877]. — Type: Stolonocarpus gigasporus. Reproduction: sexual.
Subramaniula Arx, Proc. Indian Acad. Sci., Pl. Sci. 94: 344. 1985. [MB 25699]. — Type: Subramaniula thielavioides. Reproduction: sexual & asexual.
Tengochaeta X.Wei Wang & Houbraken, this study. [MB 830915]. — Type: Tengochaeta nigropilosa. Reproduction: sexual.
Thermocarpiscus X.Wei Wang & Houbraken, this study. [MB 840163]. — Type: Thermocarpiscus australiensis. Reproduction: sexual & asexual.
Thermochaetoides X.Wei Wang & Houbraken, this study. [MB 830916]. — Type: Thermochaetoides thermophila. Reproduction: sexual.
Thermothelomyces Y. Marín et al., Mycologia 107: 630. 2015. [MB 809489]. — Type: Thermothelomyces thermophilus. Reproduction: asexual & sexual (heterothallic).
Thermothielavioides X.Wei Wang & Houbraken, Stud. Mycol. 93: 223. 2019. [MB 829879]. — Type: Thermothielavioides terrestris. Reproduction: sexual & asexual.
Trichocladium Harz, Bull. Soc. Imp. Naturalistes Moscou 44: 125. 1871. [MB 10278]. — Type: Trichocladium asperum. Reproduction: sexual & asexual.
Xanthiomyces X.Wei Wang & Houbraken, this study. [MB 840125]. — Type: Xanthiomyces spinosus. Reproduction: sexual.
List of accepted species and their synonyms
Achaetomiella
Achaetomiella gracilis (Udagawa) Houbraken et al., this study. [MB 840195]. Basionym: Chaetomium gracile. — Type: NHL 2251. Ex-type: CBS 146.60 = ATCC 16153 = IFO 6568 = IMI 084227 = NHL 2251. Reproduction: sexual. ITS barcode: KX976648 (alternative markers: LSU = KX976743; tub2 = KX976990; rpb2 = KX976842).
Achaetomiella virescens Arx, The genera of fungi sporulating in pure culture: 247. 1970. [MB 308086]. — Type: CBS 148.68. Ex-type: CBS 148.68 = IMI 136212 = IMI 159035. Reproduction: sexual. ITS barcode: KX976654 (alternative markers: LSU = KX976749; tub2 = KX976996; rpb2 = KX976848).
Achaetomium
Achaetomium aegilopis Mehrabi et al., Mycol. Prog. 19: 1422. 2020. [MB 835859]. — Type: IRAN 17712F. Ex-type: IRAN 3453C. Reproduction: sexual. ITS barcode: MT568841 (alternative markers: LSU = MT568844; tub2 = MT568852; rpb2 = n/a).
Achaetomium cristalliferum Faurel & Locq.-Lin., Cryptog. Mycol. 1: 235. 1980. [MB 113114]. — Type: PC 3252. Ex-type: CBS 781.84. Reproduction: sexual. ITS barcode: MH861836 (alternative markers: LSU = n/a; tub2 = MZ343033; rpb2 = MZ342994). Note: Based on the phylogenetic analysis (Fig. 7), we consider Achaetomium cristalliferum a synonym of Achaetomium strumarium.
Achaetomium globosum J.N. Rai & J.P. Tewari, Canad. J. Bot. 42: 693. 1964. [MB 325764]. — Type: IMI 82626. Ex-type: CBS 332.67 = IMI 082626 = IMI 082626ii = IMI 136483 = NRRL A-10899. Reproduction: sexual. ITS barcode: KX976570 (alternative markers: LSU = KX976695; tub2 = KX976911; rpb2 = KX976793).
Achaetomium hamadae Udagawa, Trans. Mycol. Soc. Japan 23: 287. 1982. [MB 124435]; basionym of Pseudothielavia hamadae.
Achaetomium irregulare (Sörgel ex W. Gams) K. Rodr. et al., Stud. Mycol. 50: 81. 2004. [MB 500020]. Note: The basionym of Achaetomium irregulare, Chaetomium irregulare, was replaced by a new name in Subramaniula (as flavipila).
Achaetomium lippiae M.G. Viana et al., Persoonia 39: 283. 2017. [MB 820711]. — Type: URM 90067. Ex-type: URM 7547. Reproduction: sexual. ITS barcode: KY855413 (alternative markers: LSU = KY855414; tub2 = KY855412; rpb2 = n/a).
Achaetomium luteum J.N. Rai & J.P. Tewari, Canad. J. Bot. 42: 694. 1964. [MB 325765]. — Type: IMI 96678. Ex-type: IMI 96678; Representative strain: CBS 618.68 = ATCC 18524 = IMI 141563. Reproduction: sexual. ITS barcode: KX976571 (alternative markers: LSU = KX976696; tub2 = KX976912; rpb2 = KX976794).
Achaetomium macrosporum J.N. Rai et al., Indian Phytopathol. 23: 54. 1970. [MB 308089]. — Type: IMI 132137. Representative strain: CBS 152.97. Reproduction: sexual. ITS barcode: KX976573 (alternative markers: LSU = KX976698; tub2 = KX976914; rpb2 = KX976796).
Achaetomium nepalense Udagawa & Y. Sugiy., Rep. Cryptog. Stud. Nepal: 11. 1982 [MB 115709]; basionym of Chaetomium nepalense.
Achaetomium purpurascens Udagawa & Y. Sugiy., Rep. Cryptog. Stud. Nepal: 13. 1982. [MB 115710]; basionym of Arcopilus purpurascens.
Achaetomium strumarium J.N. Rai et al., Canad. J. Bot. 42: 694. 1964. [MB 325766]. — Type: IMI 82624. Ex-type: CBS 333.67 = ATCC 58165 = IMI 082624 = IMI 082624ii = MI 136213 = NRRL A-10898. Reproduction: sexual. ITS barcode: AY681204 (alternative markers: LSU = AY681170; tub2 = AY681238; rpb2 = KC503254).
Achaetomium thielavioides Arx et al., Persoonia 10: 144. 1978. [MB 308092]; basionym of Subramaniula thielavioides.
Achaetomium umbonatum K. Rodr. et al., Stud. Mycol. 50: 78. 2004. [MB 500019]. — Type: IMI 38289. Ex-type: CBS 102436 = FMR 6778 = IMI 381871. Reproduction: sexual. ITS barcode: MZ334718 (alternative markers: LSU = AJ312099; tub2 = MZ343007; rpb2 = MZ342966). Note: Based on the phylogenetic analysis (Fig. 7), we consider Ach. umbonatum a synonym of Achaetomium macrosporum.
Acremonium (Hypocreales, Sordariomycetes)
Acremonium nigrospermum Schwein., Trans. Amer. Philos. Soc., n.s. 4: 283. 1832. [MB 248467]; basionym of Trichocladium nigrospermum.
Acrophialophora
Acrophialophora acuticonidiata Yu Zhang & L. Cai, Mycologia 107: 771. 2015. [MB 807461]. — Type: HMAS 245076. Ex-type: CGMCC 3.17245. Reproduction: asexual. ITS barcode: KJ026975 (alternative markers: LSU = n/a; tub2 = KJ147441; rpb2 = n/a).
Acrophialophora angustiphialis Yu Zhang & L. Cai, Mycologia 107: 772. 2015. [MB 807078]. — Type: HMAS 244840. Ex-type: CGMCC 3.15258. Reproduction: asexual. ITS barcode: KJ026972 (alternative markers: LSU = n/a; tub2 = KJ147438; rpb2 = n/a).
Acrophialophora biformis (Z.Q. Liang et al.) Yu Zhang & L. Cai, Mycologia 107: 772. 2015. [MB 810301]. Basionym: Paecilomyces biformis. — Type: GZDXIFR-H28. Ex-type: GZDXIFR-H28-1. Reproduction: asexual. ITS barcode: DQ191963 (alternative markers: LSU = n/a; tub2 = n/a; rpb2 = n/a).
Acrophialophora cinerea (Z.Q. Liang et al.) Yu Zhang & L. Cai, Mycologia 107: 772. 2015. [MB 810302]. Basionym: Paecilomyces cinereus. — Type: GZDXIFR-H57-1. Ex-type: GZDXIFR-H57-1. Reproduction: asexual. ITS barcode: DQ243694 (alternative markers: LSU = n/a; tub2 = n/a; rpb2 = n/a).
Acrophialophora curticatenata (Z.Q. Liang & Y.F. Han) Yu Zhang & L. Cai, Mycologia 107: 772. 2015. [MB 810303]. Basionym: Paecilomyces curticatenatus. — Type: GZDXIFR-H-125-2. Ex-type: GZUIFR-H125-2. Reproduction: asexual. ITS barcode: EU004811 (alternative markers: LSU = n/a; tub2 = n/a; rpb2 = n/a).
Acrophialophora ellipsoidea Yu Zhang & L. Cai, Mycologia 107: 772. 2015. [MB 807077]. — Type: HMAS 244841. Ex-type: CGMCC 3.15256. Reproduction: asexual. ITS barcode: MK926786 (alternative markers: LSU = MK926786; tub2 = MK926886; rpb2 = MK876748).
Acrophialophora furcata (Z.Q. Liang et al.) Yu Zhang & L. Cai, Mycologia 107: 775. 2015. [MB 810304]. Basionym: Paecilomyces furcatus. — Type: GZDXIFR-H104-1. Ex-type: GZDXIFR-H104-1. Reproduction: asexual. ITS barcode: DQ243695 (alternative markers: LSU = n/a; tub2 = n/a; rpb2 = n/a).
Acrophialophora fusispora (S.B. Saksena) Samson, Acta Bot. Neerl. 19: 805. 1970. [MB 308237]. Basionym: Paecilomyces fusisporus. — Type: n/a. Ex-type: CBS 380.55 = ATCC 22556 = IMI 057442 = UAMH 10771. Reproduction: asexual. ITS barcode: MK926788 (alternative markers: LSU = MK926788; tub2 = MK926888; rpb2 = MK876750).
Acrophialophora hechuanensis (Z.Q. Liang et al.) Yu Zhang & L. Cai, Mycologia 107: 775. 2015. [MB 810305]. Basionym: Taifanglania hechuanensis. — Type: GZUIFR H08-1. Ex-type: GZUIFR-H08-1. Reproduction: asexual. ITS barcode: MK926789 (alternative markers: LSU = MK926789; tub2 = MK926889; rpb2 = MK876751).
Acrophialophora jiangsuensis (Y.F. Han & Z.Q. Liang) Yu Zhang & L. Cai, Mycologia 107: 775. 2015. [MB 810306]. Basionym: Taifanglania jiangsuensis. — Type: GZUIFR HC48.1. Ex-type: GZUIFR HC48.1. Reproduction: asexual. ITS barcode: KF719171 (alternative markers: LSU = n/a; tub2 = KP143112; rpb2 = n/a). Note: Based on the comparison of the available ITS and tub2 sequences (Fig. 7), we consider this species a synonym of Acrophialophora hechuanensis.
Acrophialophora jodhpurensis (Lodha) X.Wei Wang & Houbraken, Stud. Mycol. 93: 179. 2019. [MB 829844]. Basionym: Chaetomium jodhpurense. — Type: fig. 8 in Lodha, J. Indian Bot. Soc. 43: 132, 1964 (lectotype). CBS H-10019 (epitype). Ex-epitype: CBS 602.69. Reproduction: sexual. ITS barcode: MK926790 (alternative markers: LSU = MK926790; tub2 = MK926890; rpb2 = MK876752).
Acrophialophora levis Samson & T. Mahmood, Acta Bot. Neerl. 19: 807. 1970. [MB 308238]. — Type: CBS 484.70. Ex-type: CBS 484.70 = ATCC 22557 = UAMH 10773. Reproduction: asexual. ITS barcode: KP233038 (alternative markers: LSU = KM995840; tub2 = KP233044; rpb2 = n/a).
Acrophialophora liboensis Y.W. Zhang et al., Phytotaxa 302: 270. 2016. [MB 818669]. — Type: GZUIFR-F0044. Ex-type: CGMCC 3.18309. Reproduction: asexual. ITS barcode: KP192127 (alternative markers: LSU = n/a; tub2 = KP999978; rpb2 = n/a).
Acrophialophora major (Z.Q. Liang et al.) Yu Zhang & L. Cai, Mycologia 107: 775. 2015. [MB 810307]. Basionym: Paecilomyces inflatus var. major. — Type: GZDX-IFR H-57-2. Ex-type: GZUIFR-H57-2. Reproduction: asexual. ITS barcode: MK926792 (alternative markers: LSU = MK926792; tub2 = MK926892; rpb2 = MK876754).
Acrophialophora nainiana Edward, Mycologia 51: 784. 1961. [MB 325807]. — Type: IMI, anon. s.n.; MPPD, anon. s.n.; WVA, anon. s.n.; Department of Biology, Allahabad Agricultural Institute, anon. s.n. Ex-type: CBS 100.60. Reproduction: asexual. ITS barcode: MK926793 (alternative markers: LSU = MK926793; tub2 = MK926893; rpb2 = MK876755).
Acrophialophora seudatica (Subrahm.) Sand.-Den. et al., J. Clin. Microbiol. 53: 1552. 2015. [MB 811225]. Basionym: Ampullifera seudatica. — Type: CBS 916.79. Ex-type: CBS 916.79 = ATCC 36866. Reproduction: asexual. ITS barcode: LN736030 (alternative markers: LSU = LN736031; tub2 = LN736032; rpb2 = n/a). Note: Based on ITS, LSU and tub2 sequence data (Fig. 7), we consider this species a synonym of Acrophialophora major.
Acrophialophora teleoafricana X.Wei Wang & Houbraken, Stud. Mycol. 93: 185. 2019. [MB 829843]. — Type: CBS H-23631. Ex-type: CBS 281.79. Reproduction: sexual. ITS barcode: MK926795 (alternative markers: LSU = MK926795; tub2 = MK926895; rpb2 = MK876757).
Allobotryotrichum
Allobotryotrichum blastosporum [as ‘blastospora’] M. Raza & L. Cai, Fungal Diversity 99: 74. 2019. [MB 636270]. — Type: HMAS 248065. Ex-type: CGMCC 3.19343 = LC11912. Reproduction: asexual. ITS barcode: MN215716 (alternative markers: LSU = MN215554; tub2 = MN329887; rpb2 = MN255397).
Allocanariomyces
Allocanariomyces americanus (Cañete-Gibas et al.) Cañete-Gibas et al., this study. [MB 840154]. Basionym: Pseudocanariomyces americanus. — Type: CBS H-24761. Ex-type: CBS 147185 = UTHSCSA DI20-139. Reproduction: sexual & asexual. ITS barcode: MT902181 (alternative markers: LSU = MT902391; tub2 = MT904876; rpb2 = MT904877).
Allocanariomyces tritici Mehrabi et al., Mycol. Prog. 19: 1420. 2020. [MB 835854]. — Type: IRAN 17711F. Ex-type: IRAN 3450C. Reproduction: sexual & asexual. ITS barcode: MT568839 (alternative markers: LSU = MT568842; tub2 = MT568850; rpb2 = MT568845).
Amesia
Amesia atrobrunnea (L.M. Ames) X.Wei Wang & Samson, Stud. Mycol. 84: 158. 2016. [MB 818832]. Basionym: Chaetomium atrobrunneum. — Type: BPI 1100755. Ex-type: CBS 379.66. Reproduction: sexual. ITS barcode: JX280771 (alternative markers: LSU = JX280666; tub2 = KX976916; rpb2 = KX976798).
Amesia cymbiformis (Lodha) X.Wei Wang & Samson, Stud. Mycol. 84: 158. 2016. [MB 818833]. Basionym: Chaetomium cymbiforme. — Type: n/a. Representative strain: CBS 175.84. Reproduction: sexual. ITS barcode: KX976576 (alternative markers: LSU = KX976701; tub2 = KX976918; rpb2 = KX976800).
Amesia dreyfussii (Arx) X.Wei Wang & Houbraken, this study. [MB 840132]. Basionym: Chaetomium dreyfussii. — Type: CBS H-6864. Ex-type: CBS 376.83 = MUCL 40177. Reproduction: sexual. ITS barcode: MH861613 (alternative markers: LSU = MH873331; tub2 = MZ343023; rpb2 = MZ342985).
Amesia gelasinospora (Aue & E. Müll.) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 818854]. Basionym: Chaetomium gelasinosporum. — Type: ETH. Ex-type: CBS 673.80. Reproduction: sexual. ITS barcode: KX976580 (alternative markers: LSU = KX976705; tub2 = KX976922; rpb2 = KX976804).
Amesia khuzestanica Mehrabi-Koushki et al., Mycol. Prog. 19: 939. 2020. [MB 832229]. — Type: IRAN 17597F. Ex-type: IRAN 3489C = SCUA-Saf-B16. Reproduction: sexual. ITS barcode: MT551117 (alternative markers: LSU = n/a; tub2 = MN275701; rpb2 = MN275706).
Amesia nigricolor (L.M. Ames) X.Wei Wang & Samson, Stud. Mycol. 84: 159. 2016. [MB 818834]. Basionym: Chaetomium nigricolor. — Type: BPI. Ex-type: CBS 600.66 = ATCC 11211 = DSM 3703 = IMI 250971. Reproduction: sexual. ITS barcode: KX976578 (alternative markers: LSU = KX976703; tub2 = KX976920; rpb2 = KX976802). Notes: Several specimens in BPI are labeled as type according to Mycoportal: BPI 1101431, BPI 1101434, BPI 580553, BPI 580554, BPI 580555, BPI 580556, BPI 580557, BPI 580558, BPI 580559. A database search and/or examination of these specimens is needed to determine which of them is the holotype and which are isotypes, or if a lectotypification is necessary.
Amesia raii (G. Malhotra & Mukerji) X.Wei Wang & Houbraken, this study. [MB 840137]. Basionym: Chaetomium raii. — Type: DUH KG 326. Ex-type: CBS 107.83 = ITCC 1944. Reproduction: sexual. ITS barcode: n/a (alternative markers: LSU = n/a; tub2 = n/a; rpb2 = MZ342968).
Aporothielavia
Aporothielavia leptoderma (C. Booth) Malloch & Cain, Mycologia 65: 1074. 1973. [MB 308869]. Basionym: Thielavia leptoderma [as ‘leptodermus’]. — Type: IMI 54770. Ex-type: CBS 538.74. Reproduction: sexual. ITS barcode: NR_164219 (alternative markers: LSU = NG_067253; tub2 = MZ343025; rpb2 = MZ342986).
Arcopilus
Arcopilus amazonicus T.F. Sousa & G.F. Silva, Phytotaxa 456: 150. 2020. [MB 835577]. — Type: INPA2410. Ex-type: INPA2410. Reproduction: sexual. ITS barcode: MH777083 (alternative markers: LSU = MH780043; tub2 = MH784466; rpb2 = MH784457).
Arcopilus aureus (Chivers) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 818855]. Basionym: Chaetomium aureum. — Type: BPI 1100494. Representative strain: CBS 153.52. Reproduction: sexual. ITS barcode: KX976582 (alternative markers: LSU = KX976707; tub2 = KX976924; rpb2 = KX976806). Notes: BPI 1100494 is tentatively indicated as holotype. Thaxter is indicated as the collector of this specimen, which is in agreement with the protologue. However, specimen BPI 1100497 rather than BPI 1100494 is labeled as type. No collector information is given for BPI 1100497.
Arcopilus cupreus (L.M. Ames) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 818856]. Basionym: Chaetomium cupreum. — Type: BPI 580275. Representative strain: CBS 560.80. Reproduction: sexual. ITS barcode: KX976584 (alternative markers: LSU = KX976709; tub2 = KX976926; rpb2 = KX976808).
Arcopilus eremanthi [as ‘eremanthusum’] D.G. Tavares et al., Arch. Microbiol. 204: 156, 5. 2022. [MB 843294]. — Type: VIC 47,499. Ex-type: CML 3766 = A2C54. Reproduction: sexual. ITS barcode: MN539886 (alternative markers: LSU = MN539910, tub2 = n/a, rpb2 = MN551186).
Arcopilus flavigenus (Van Warmelo) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 818858]. Basionym: Chaetomium flavigenum. — Type: PRE 43080. Ex-type: CBS 337.67. Reproduction: sexual. ITS barcode: KX976587 (alternative markers: LSU = KX976712; tub2 = KX976929; rpb2 = KX976811).
Arcopilus fusiformis (Chivers) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 818857]. Basionym: Chaetomium fusiforme. — Type: n/a. Representative strain: CBS 484.85. Reproduction: sexual. ITS barcode: KX976585 (alternative markers: LSU = KX976710; tub2 = KX976927; rpb2 = KX976809).
Arcopilus globulus M. Raza & L. Cai, Fungal Diversity 99: 75. 2019. [MB 556674]. — Type: HMAS 248066. Ex-type: CGMCC 3.19359 = LC11930. Reproduction: sexual. ITS barcode: MN215741 (alternative markers: LSU = MN215579; tub2 = MZ343038; rpb2 = MN255422). Note: Based on sequence data, we consider this species a synonym of Arcopilus aureus.
Arcopilus macrostiolatus (Stchigel et al.) X.Wei Wang & Houbraken, this study. [MB 840138]. Basionym: Chaetomium macrostiolatum [as ‘macrostiolum’]. — Type: IMI 382896. Ex-type: CBS 102435. Reproduction: sexual. ITS barcode: MZ334722 (alternative markers: LSU = MZ351418; tub2 = MZ343006; rpb2 = MZ342965).
Arcopilus megasporus (Sörgel ex Seth) X.Wei Wang & Houbraken, this study. [MB 840139]. Basionym: Chaetomium megasporum. — Type: IMA 73514. Representative strain: CBS 127650. Reproduction: sexual. ITS barcode: n/a (alternative markers: LSU = n/a; tub2 = MZ343010; rpb2 = MZ342971).
Arcopilus navicularis Kubátová et al., Persoonia 46: 417. 2021. [MB 839209]. — Type: PRM 954081. Ex-type: CCF 3252 = CBS 147158. Reproduction: sexual. ITS barcode: MW798185 (alternative markers: LSU = MW798181; tub2 = MW816125; rpb2 = MW816124).
Arcopilus purpurascens (Udagawa & Y. Sugiy.) X.Wei Wang & Houbraken, this study. [MB 840140]. Basionym: Achaetomium purpurascens. — Type: NHL 2896. Ex-type: CBS 287.83. Reproduction: sexual. ITS barcode: n/a (alternative markers: LSU = n/a; tub2 = MZ343021; rpb2 = MZ342982).
Arcopilus tangerinicapillus M. Raza & L. Cai, Fungal Diversity 99: 78. 2019. [MB 556675]. — Type: HMAS 248067. Ex-type: CGMCC 3.19326 = LC11936. Reproduction: sexual. ITS barcode: MN215743 (alternative markers: LSU = MN215581; tub2 = MN329904; rpb2 = MZ342999). Note: Based on sequence data, we consider Ar. tangerinicapillus a synonym of Arcopilus cupreus.
Arcopilus turgidopilosus (L.M. Ames) X.Wei Wang & Samson, Stud. Mycol. 84: 159. 2016. [MB 818836]. Basionym: Chaetomium turgidopilosum. — Type: ISC-F-0123588. Ex-type: CBS 169.52. Reproduction: sexual. ITS barcode: KX976588 (alternative markers: LSU = KX976713; tub2 = KX976930; rpb2 = KX976812).
Arxotrichum
Arxotrichum deceptivum (Malloch & Benny) X.Wei Wang & Houbraken, this study. [MB 830917]. Basionym: Chaetomium deceptivum. — Type: TRTC 46369. Ex-type: CBS 346.73. Reproduction: sexual. ITS barcode: MK919276 (alternative markers: LSU = MK919276; tub2 = MK919390; rpb2 = MK919332).
Arxotrichum gangligerum (L.M. Ames) X.Wei Wang & Houbraken, this study. [MB 830918]. Basionym: Chaetomium gangligerum. — Type: BPI. Ex-type: CBS 160.52 = ATCC 11206. Reproduction: sexual. ITS barcode: MK919277 (alternative markers: LSU = MK919277; tub2 = MK919391; rpb2 = MK919333). Notes: Two specimens in BPI are labeled as type according to Mycoportal: BPI 1100713 and BPI 580418. A database search and/or examination of these specimens is needed to determine which is the holotype and which the isotype, or if a lectotypification is necessary.
Arxotrichum officinarum (M. Raza & L. Cai) X.Wei Wang & Houbraken, this study. [MB 840142]. Basionym: Myceliophthora officinarum. — Type: HMAS 248073. Ex-type: CGMCC 3.19325. Reproduction: sexual & asexual. ITS barcode: MN215767 (alternative markers: LSU = MN215605; tub2 = MN337032; rpb2 = MN255448).
Arxotrichum piluliferoides (Udagawa & Y. Horie) X.Wei Wang & Houbraken, this study. [MB 830920]. Basionym: Chaetomium piluliferoides. — Type: NHL 2738. Ex-type: CBS 103.77 = IFM 4531 = IMI 210880 = NHL 2738. Reproduction: sexual & asexual. ITS barcode: MK919280 (alternative markers: LSU = MK919280; tub2 = MK919394; rpb2 = MK919336).
Arxotrichum repens (Guarro & Figueras) X.Wei Wang & Houbraken, this study. [MB 830921]. Basionym: Chaetomium repens. — Type: CBS H-6890. Ex-type: CBS 233.82 = FFBA 310. Reproduction: sexual. ITS barcode: MK919282 (alternative markers: LSU = MK919282; tub2 = MK919396; rpb2 = MK919338).
Arxotrichum sinense (K.T. Chen) X.Wei Wang & Houbraken, this study. [MB 830922]. Basionym: Chaetomium sinense. — Type: CBS H-10001 (isotype). Ex-type: CBS 541.83 = FFBA 388. Reproduction: sexual. ITS barcode: MK919283 (alternative markers: LSU = MK919283; tub2 = MK919397; rpb2 = MK919339).
Arxotrichum succineum (L.M. Ames) A. Nováková & M. Kolařík, Persoonia 40: 259. 2018. [MB 824082]. Basionym: Chaetomium succineum. — Type: ISC-F-0123587?. Ex-type: CBS 166.52 = ATCC 11216 = MUCL 18704. Reproduction: sexual. ITS barcode: MK919284 (alternative markers: LSU = MK919284; tub2 = MK919398; rpb2 = MK919340).
Arxotrichum wyomingense A. Nováková & M. Kolařík, Persoonia 40: 259. 2018. [MB 824081]. — Type: PRM 945788. Ex-type: CCF 5691. Reproduction: asexual. ITS barcode: LT968153 (alternative markers: LSU = LT968143; tub2 = LT971393; rpb2 = n/a).
Batnamyces
Batnamyces globulariicola Noumeur, Mycol. Prog. 19: 593. 2020. [MB 832845]. — Type: CBS H-23624. Ex-type: CBS 144474. Reproduction: asexual. ITS barcode: MT075917 (alternative markers: LSU = MT075917; tub2 = MT075919; rpb2 = MT075918).
Beniowskia
Beniowskia macrospora M.D. Mehrotra, Sydowia 17: 149. 1964 [MB 326968]; replaced synonym of Trichocladium beniowskiae.
Bommerella
Bommerella trigonospora Marchal, Bull. Soc. Roy. Bot. Belgique. 24: 164. 1885. [MB 221255]. — Type: BR5020093738364. Representative strain: CBS 324.69. Reproduction: sexual. ITS barcode: n/a (alternative markers: LSU = MZ351419; tub2 = MZ343022; rpb2 = MZ342984).
Botryoderma
Botryoderma lateritium Papendorf & H.P. Upadhyay, Trans. Brit. Mycol. Soc. 52: 258. 1969. [MB 327102]. — Type: PRE 44223. Ex-type: CBS 586.66 = ATCC 18926 = IMI 158956 = MUCL 8790. Reproduction: asexual. ITS barcode: MK919287 (alternative markers: LSU = MK919287; tub2 = MK919401; rpb2 = MK919343).
Botryoderma rostratum Papendorf & H.P. Upadhyay, Trans. Brit. Mycol. Soc. 52: 260. 1969. [MB 327103]. — Type: CBS H-24915 (lectotype). Ex-lectotype: CBS 184.68 = ATCC 18927 = IMI 158957. Reproduction: asexual. ITS barcode: MK919288 (alternative markers: LSU = MK919288; tub2 = MK919402; rpb2 = MK919344).
Botryotrichum
Botryotrichum atrogriseum J.F.H. Beyma, Verh. Kon. Ned. Akad. Wetensch., Afd. Natuurk. 26: 14. 1928. [MB 257744]. — Type: CBS 130.28. Ex-type: CBS 130.28 = IMI 092902 = MUCL 1110. Reproduction: asexual. ITS barcode: KX976589 (alternative markers: LSU = KX976714; tub2 = KX976931; rpb2 = KX976813).
Botryotrichum domesticum D.W. Li & N.P. Schultes, Botany 97: 314. 2019. [MB 828185]. — Type: NHES L1707. Ex-type: UAMH 11929. Reproduction: asexual. ITS barcode: MH899168 (alternative markers: LSU = MH899169; tub2 = MH899172; rpb2 = MH899171).
Botryotrichum foricae Jurjević & Hubka, Persoonia 42: 389. 2019. [MB 830668]. — Type: BPI 910933. Ex-type: CCF 5752 = EMSL 2683. Reproduction: asexual. ITS barcode: LR584032 (alternative markers: LSU = LR584033; tub2 = LR584034; rpb2 = n/a).
Botryotrichum geniculatum X.Wei Wang et al., this study. [MB 840127]. — Type: HMAS 350293 (holotype); CBS H-23629 (isotype). Ex-type: CGMCC 3.20441 = CBS 144475 = WXW 8287. Reproduction: sexual. ITS barcode: MZ334719 (alternative markers: LSU = MZ351422; tub2 = MZ343011; rpb2 = MZ342972).
Botryotrichum inquinatum (Udagawa & S. Ueda) X.Wei Wang & Houbraken, this study. [MB 830923]. Basionym: Corynascella inquinata. — Type: NHL 2841. Ex-type: CBS 155.80 = NHL 2841. Reproduction: sexual. ITS barcode: MK919289 (alternative markers: LSU = MK919289; tub2 = MK919403; rpb2 = MK919345).
Botryotrichum iranicum A. Alidadi, Mycol. Prog. 19: 1578. 2020. [MB 831975]. — Type: ABRIICC 106H. Ex-type: ABRIICC 10152. Reproduction: asexual. ITS barcode: MN134583 (alternative markers: LSU = n/a; tub2 = MN128435; rpb2 = MN128437).
Botryotrichum murorum (Corda) X.Wei Wang & Samson, Stud. Mycol. 84: 164. 2016. [MB 818837]. Basionym: Chaetomium murorum. — Type: PRM. Representative strain: CBS 163.52 = ATCC 11210 = MUCL 40179. Reproduction: sexual. ITS barcode: KX976591 (alternative markers: LSU = KX976716; tub2 = KX976933; rpb2 = KX976815). Note: No ex-type strains are available and epitypification of the species based on a suitable specimen needs to be performed.
Botryotrichum peruvianum Matsush., Icones Microfungorum a Matsushima lectorum: 17. 1975. [MB 309895]. — Type: MFC 2649. Representative strain: CBS 460.90 = FMR 3674. Reproduction: asexual. ITS barcode: KX976595 (alternative markers: LSU = KX976720; tub2 = KX976937; rpb2 = KX976819).
Botryotrichum piluliferum Sacc. & Marchal, Bull. Soc. Roy. Bot. Belgique 24: 66. 1885. [MB 221757]. — Type: Pl. II, figs 5–8, in Marchal, Bull. Soc. Roy. Bot. Belgique 24: 68, 1885 (lectotype designated here, MBT 10005041; CBS H-24868 [dried culture] – epitype designated here, MBT 10005042). Ex-epitype: CBS 654.79. Reproduction: asexual and sexual. ITS barcode: KX976597 (alternative markers: LSU = KX976722; tub2 = KX976939; rpb2 = KX976821). Note: No holotype specimen could be located (e.g., in BR) and therefore the illustration in the protologue is designated here as lectotype.
Botryotrichum retardatum (A. Carter & R.S. Khan) X.Wei Wang & Houbraken, this study. [MB 840141]. Basionym: Chaetomium retardatum. — Type: TRTC 66.1778b. Ex-type: CBS 197.84. Reproduction: sexual. ITS barcode: n/a (alternative markers: LSU = n/a; tub2 = MZ343019; rpb2 = MZ342980).
Botryotrichum spirotrichum (R.K. Benj.) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 818860]. Basionym: Magnusia spirotricha. — Type: RSABG 116. Ex-type: CBS 211.55 = ATCC 12128 = IMI 060034. Reproduction: sexual. ITS barcode: KX976601 (alternative markers: LSU = KX976726; tub2 = KX976943; rpb2 = KX976825).
Botryotrichum trichorobustum (Seth) X.Wei Wang & Houbraken, this study. [MB 840143]. Basionym: Chaetomidium trichorobustum. — Type: CBS H-6840. Ex-type: CBS 563.67 = ATCC 18247 = IMI 130230. Reproduction: sexual. ITS barcode: n/a (alternative markers: LSU = MZ351420; tub2 = MZ343027; rpb2 = MZ342988).
Botryotrichum verrucosum (Pugh et al.) X.Wei Wang & Houbraken, Stud. Mycol. 93: 72. 2018. [MB 824410]. Basionym: Thermomyces verrucosus. — Type: IMI 96466. Ex-type: CBS 116.64 = ATCC 22222 = IMI 096466 = IMI 096466ii = MUCL 30565. Reproduction: asexual. ITS barcode: LT993567 (alternative markers: LSU = LT993567; tub2 = LT993648; rpb2 = LT993486).
Botryotrichum vitellinum (A. Carter) X.Wei Wang & Houbraken, this study. [MB 840144]. Basionym: Chaetomium vitellinum. — Type: TRTC 48873. Ex-type: CBS 180.84 = IMI 283627 = TRTC 48873. Reproduction: sexual. ITS barcode: MZ334725 (alternative markers: LSU = MZ351421; tub2 = MZ343018; rpb2 = MZ342979).
Brachychaeta
Brachychaeta variospora (Udagawa & Y. Horie) X.Wei Wang & Houbraken, Stud. Mycol. 93: 186. 2019. [MB 829845]. Basionym: Chaetomium variosporum. — Type: NHL 22698. Ex-type: CBS 414.73 = IMI 172986 = NHL 2698. Reproduction: sexual. ITS barcode: MK926797 (alternative markers: LSU = MK926797; tub2 = MK926897; rpb2 = MK876759).
Canariomyces
Canariomyces arenarius (Mouch.) X.Wei Wang & Houbraken, Stud. Mycol. 93: 189. 2019. [MB 829846]. Basionym: Thielavia arenaria. — Types: PC (holotype); CBS H-7846 (isotype); CBS H-7847 (isotype). Ex-type: CBS 507.74. Reproduction: sexual & asexual. ITS barcode: MK926798 (alternative markers: LSU = MK926798; tub2 = MK926898; rpb2 = KM655438).
Canariomyces microsporus (Mouch.) X.Wei Wang & Houbraken, Stud. Mycol. 93: 190. 2019. [MB 829847]. Basionym: Thielavia microspora. — Type: PC. Ex-type: CBS 276.74. Reproduction: sexual & asexual. ITS barcode: MK926799 (alternative markers: LSU = MK926799; tub2 = MK926899; rpb2 = MK876760).
Canariomyces notabilis Arx, Persoonia 12: 185. 1984. [MB 107785]. — Type: CBS 548.83. Ex-type: CBS 548.83. Reproduction: sexual & asexual. ITS barcode: MK926802 (alternative markers: LSU = MK926802; tub2 = MK926902; rpb2 = MK876763).
Canariomyces subthermophilus (Mouch.) X.Wei Wang & Houbraken, Stud. Mycol. 93: 190. 2019. [MB 829848]. Basionym: Thielavia subthermophila. — Type: PC. Ex-type: CBS 509.74. Reproduction: sexual & asexual. ITS barcode: MK926804 (alternative markers: LSU = MK926804; tub2 = MK926904; rpb2 = MK876764).
Canariomyces vonarxii X.Wei Wang & Houbraken, Stud. Mycol. 93: 190. 2019. [MB 829849]. — Type: CBS H-18817. Ex-type: CBS 160.80 = NHL 2831. Reproduction: sexual & asexual. ITS barcode: MK926805 (alternative markers: LSU = MK926805; tub2 = MK926905; rpb2 = MK876765).
Carteria
Carteria arctostaphyli X.Wei Wang & Houbraken, Stud. Mycol. 93: 194. 2019. [MB 829851]. — Type: CBS H-23640. Ex-type: CBS 229.82. Reproduction: sexual. ITS barcode: MK926807 (alternative markers: LSU = MK926807; tub2 = MK926907; rpb2 = MK876767).
Chaetomidium (synonym of Chaetomium)
Chaetomidium arxii Benny, Mycologia 80: 832. 1980. [MB 112727]; basionym of Trichocladium arxii.
Chaetomidium fimeti (Fuckel) Sacc., Syll. Fung. 1: 39. 1882. [MB 174464]; synonym of Chaetomium fimeti.
Chaetomidium fragile Natarajan, Proc. Indian Acad. Sci., B 37: 124. 1972 [1971]. [MB 310876]; basionym of Hyalosphaerella fragilis.
Chaetomidium gallecicum [as ‘galaicum’] Stchigel & Guarro, Stud. Mycol. 50: 217. 2004. [MB 368919]; synonym of Aporothielavia leptoderma.
Chaetomidium leptoderma (C. Booth) Greif & Currah, Mycol. Res. 111: 74. 2007. [MB 510052]; synonym of Aporothielavia leptoderma.
Chaetomidium peruvianum Goch., Mycologia 60: 1118. 1968. [MB 328015]; basionym of Chrysanthotrichum peruvianum.
Chaetomidium pilosum (C. Booth & Shipton) Arx, Stud. Mycol. 8: 16. 1975. [MB 310879]; synonym of Chaetomium pilosum.
Chaetomidium spirotrichum (R.K. Benj.) Malloch & Cain, Mycologia 65: 1069. 1973. [MB 310882]; synonym of Botryotrichum spirotrichum.
Chaetomidium subfimeti Seth, Trans. Brit. Mycol. Soc. 50: 46. 1967. [MB 328016]; basionym of Chaetomium subfimeti.
Chaetomidium thermophilum (Fergus & Sinden) Lodha, Taxonomy of fungi (Proc. Int. Symp. Madras, 1973) 1: 248. 1978. [MB 310883]; synonym of Thermothelomyces fergusii.
Chaetomidium trichorobustum Seth, Nova Hedwigia 16: 430. 1968. [MB 328017]; basionym of Botryotrichum trichorobustum.
Chaetomium
Chaetomium acropullum X.Wei Wang, Nova Hedwigia 80: 414. 2005. [MB 336138]; basionym of Trichocladium acropullum.
Chaetomium afropilosum X.Wei Wang et al., Persoonia 36: 91. 2015 [2016]. [MB 812942]. — Type: CBS H-22192. Ex-type: CBS 145.38 = DAOM 19448. Reproduction: sexual. ITS barcode: KT214574 (alternative markers: LSU = KT214605; tub2 = KT214751; rpb2 = KT214675).
Chaetomium amberpetense P. Rama Rao & Ram Reddy, Mycopathol. Mycol. Appl. 24: 114. 1964. [MB 328021]; synonym of Amesia nigricolor.
Chaetomium amesii Sergejeva, Novosti Sist. Nizsh. Rast. 2: 112. 1965. [MB 328022]; synonym of Humicola homopilata.
Chaetomium ampulliellum X.Wei Wang, Nova Hedwigia 81: 248. 2005. [MB 356099]; basionym of Humicola ampulliella.
Chaetomium amygdalisporum Udagawa & T. Muroi, Trans. Mycol. Soc. Japan 22: 13. 1981. [MB 111238]; basionym of Ovatospora amygdalispora.
Chaetomium anamorphosum S.A. Ahmed et al., Fungal Diversity 76: 18. 2015. [MB 810426]; basionym of Subramaniula anamorphosa.
Chaetomium anastomosans M. Raza & L. Cai, Fungal Diversity 99: 78. 2019. [MB 556676]. — Type: HMAS 248069. Ex-type: CGMCC 3.19350 = LC11926. Reproduction: sexual. ITS barcode: MN215745 (alternative markers: LSU = MN215583; tub2 = MN337028; rpb2 = MN255426). Note: This species is a synonym of Chaetomium globosum.
Chaetomium anguipilium L.M. Ames, A monograph of the Chaetomiaceae: 12. 1963. [MB 328023]; basionym of Collariella anguipilia.
Chaetomium angulare Yu Zhang & L. Cai, Fungal Biol. 121: 28. 2016 [2017]. [MB 811149]; basionym of Ovatospora angularis.
Chaetomium angustispirale Sergejeva, Not. Syst. Sect. Crypt. Inst. Bot. Acad. Sci. USSR 11: 115. 1956. [MB 294684]. — Type: –. Ex-type: CBS 137.58 = IMI 074952 = VKM F-1942. Reproduction: sexual & asexual. ITS barcode: JN209862 (alternative markers: LSU = JN209862; tub2 = JN256141; rpb2 = KF001824).
Chaetomium ascotrichoides Calviello, Revista Mus. Argent. Ci. Nat., Bernardino Rivadavia Inst. Nac. Invest. Ci. Nat., Bot. 3: 372. 1972. [MB 310890]. — Type: n/a. Ex-type: CBS 113.83 = IMI 182725. Reproduction: sexual. ITS barcode: KC109752 (alternative markers: LSU = KC109752; tub2 = KC109770; rpb2 = KF001832).
Chaetomium atrobrunneum L.M. Ames, Mycologia 41: 641. 1949. [MB 294685]; basionym of Amesia atrobrunnea.
Chaetomium aureum Chivers, Proc. Amer. Acad. Arts Sci. 48: 86. 1912. [MB 161470]; basionym of Arcopilus aureus.
Chaetomium biporatum Cano & Guarro, Nova Hedwigia 44: 543. 1987. [MB 130544]; basionym of Parachaetomium biporatum.
Chaetomium bostrychodes Zopf, Verh. Bot. Vereins Provinz Brandenburg 19: 173. 1877. [MB 161575]; basionym of Collariella bostrychodes.
Chaetomium brasiliense Bat. & Pontual, Bol. Agric. Pernambuco 15: 70. 1948. [MB 294688]; basionym of Ovatospora brasiliensis.
Chaetomium camelliae Jayaward. et al., Mycosphere 12: 471. 2021. [MB 558001]. — Type: JZBH3340001. Ex-type: JZB3340001. Reproduction: sexual. ITS barcode: MT535751 (alternative markers: LSU = MT535749, tub2 = MT535533, rpb2 = MT535537).
Chaetomium cancroideum Tschudy, Amer. J. Bot. 24: 472. 1938. [MB 120075]; synonym of Dichotomopilus funicola.
Chaetomium capillare X.Wei Wang et al., Persoonia 36: 92. 2015. [2016]. [MB 812975]. — Type: CBS H-22187. Ex-type: CBS 128489 = UTHSC 03-1339. Reproduction: sterile. ITS barcode: KT214583 (alternative markers: LSU = KT214614; tub2 = KT214760; rpb2 = KT214686).
Chaetomium carinthiacum Sörgel, Arch. Mikrobiol. 40: 393. 1961. [MB 328032]; basionym of Parachaetomium carinthiacum.
Chaetomium causiiforme L.M. Ames, Mycologia 41: 637. 1949. [MB 118825]; basionym of Collariella causiiformis.
Chaetomium cervicicola X.Wei Wang et al., Persoonia 36: 93. 2015 [2016]. [MB 812976]. — Type: CBS H-22188. Ex-type: CBS 128492 = UTHSC 07-3593. Reproduction: sterile. ITS barcode: KT214558 (alternative markers: LSU = KT214592; tub2 = KT214735; rpb2 = KT214662).
Chaetomium chiversii (J.C. Cooke) A. Carter, Nova Hedwigia 84: 19. 1986. [MB 104874]; synonym of Floropilus chiversii.
Chaetomium cirrhatum [as ‘cirrhata’] Yu Zhang & L. Cai, Fungal Biol. 121: 28. 2016 [2017]. [MB 840133]. — Type: HMAS 245784. Ex-type: CGMCC 3.17540. Reproduction: sexual. ITS barcode: KP336792 (alternative markers: LSU = KP336841; tub2 = KP336890; rpb2 = KT149508).
Chaetomium citrinum Udagawa & T. Muroi, Trans. Mycol. Soc. Japan 22: 15. 1981. [MB 111239]. — Type: NHL 2873. Ex-type: CBS 693.82 = NHL 2873. Reproduction: sexual. ITS barcode: KT214587 (alternative markers: LSU = KT214617; tub2 = KT214764; rpb2 = KT214691).
Chaetomium coarctatum Sergejeva, Bot. Mater. Otd. Sporov. Rast. Bot. Inst. Komarova Akad. Nauk S.S.S.R. 14: 146. 1961. [MB 328033]. — Type: –. Ex-type: CBS 162.62 = ATCC 14530 = IMI 090491 = MUCL 18697 = VKM F-1946. Reproduction: sexual. ITS barcode: JN209863 (alternative markers: LSU = JN209863; tub2 = JN256142; rpb2 = KF001802).
Chaetomium cochliodes Palliser, North American Flora 3: 61. 1910. [MB 257241]. — Type: NY01050409; HMAS 244354 (epitype). Ex-epitype: CBS 155.52. Reproduction: sexual. ITS barcode: KC109754 (alternative markers: LSU = KC109754; tub2 = KC109772; rpb2 = KF001811).
Chaetomium concavisporum M. Raza & L. Cai, Fungal Diversity 99: 80. 2019. [MB 556677]. — Type: HMAS 248070. Ex-type: CGMCC 3.19348 = LC11924. Reproduction: sexual. ITS barcode: MN215747 (alternative markers: LSU = MN215585; tub2 = MN329916; rpb2 = MN255428); synonym of Chaetomium cochliodes.
Chaetomium contagiosum X.Wei Wang et al., Persoonia 36: 98. 2015 [2016]. [MB 812977]. — Type: CBS H-22189. Ex-type: CBS 128494 = UTHSC 10-726. Reproduction: sterile. ITS barcode: KT214555 (alternative markers: LSU = KT214589; tub2 = KT214732; rpb2 = KT214659).
Chaetomium crispatum (Fuckel) Fuckel, Jahrb. Nassauischen Vereins Naturk. 23-24: 90. 1870. [MB 156792]; synonym of Trichocladium crispatum.
Chaetomium cristatum L.M. Ames, Mycologia 41: 639. 1950. [MB 294690]; basionym of Subramaniula cristata.
Chaetomium cruentum L.M. Ames, A monograph of the Chaetomiaceae: 20. 1963. [MB 120272]. — Type: n/a, see Notes, CBS H-6860 (isotype). Ex-type: CBS 371.66. Reproduction: sexual. ITS barcode: JN209871 (alternative markers: LSU = JN209871; tub2 = JN256148; rpb2 = KF001795). Notes: This species is phylogenetically identical to Chaetomium globosum, but is morphologically distinct (Wang et al. 2016a). We tentatively accept this species in Chaetomium. Several specimens are labeled as type according to Mycoportal: BPI 1101395, NY01050414 and NY01050415. A database search and/or examination of these specimens is needed to determine which of them is the holotype and which are isotypes, or if a lectotypification is necessary.
Chaetomium cucumericola X.Wei Wang et al., Persoonia 36: 98. 2015 [2016]. [MB 812978]. — Type: CBS H-22190. Ex-type: CBS 378.71. Reproduction: sterile. ITS barcode: KT214579 (alternative markers: LSU = KT214610; tub2 = KT214756; rpb2 = KT214680).
Chaetomium cuniculorum Fuckel, Fungi Rhen. Exs. Suppl. Fasc. 5: no. 1961. 1867. [MB 171246]; basionym of Subramaniula cuniculorum.
Chaetomium cupreum L.M. Ames, Mycologia 41: 642. 1949. [MB 294691]; basionym of Arcopilus cupreus.
Chaetomium cuyabenoense [as ‘cuyabenoensis’] Decock & Hennebert, Mycol. Res. 101: 309. 1997. [MB 628862]; basionym of Humicola cuyabenoensis.
Chaetomium cymbiforme Lodha, J. Indian Bot. Soc. 43: 129. 1964. [MB 328037]; basionym of Amesia cymbiformis.
Chaetomium deceptivum Malloch & Benny, Mycologia 65: 648. 1973. [MB 310899]; basionym of Arxotrichum deceptivum.
Chaetomium distortum L.M. Ames, A monograph of the Chaetomiaceae: 21. 1963. [MB 328039]; basionym of Humicola distorta.
Chaetomium dolichotrichum L.M. Ames, Mycologia 37: 145. 1945. [MB 285133]; basionym of Dichotomopilus dolichotrichus.
Chaetomium dreyfussii Arx, Beih. Nova Hedwigia 84: 6. 1986. [MB 104875]; basionym of Amesia dreyfussii.
Chaetomium elatum Kunze, Deutschl. Schwämme, Achte Lieferung: 3, no. 184. 1818. [MB 172050]. — Type: CBS H-22851 (neotype). Ex-neotype: CBS 142034. Reproduction: sexual & asexual. ITS barcode: KX976612 (alternative markers: LSU = KX976733; tub2 = KX976954; rpb2 = KX976832).
Chaetomium erectum Skolko & J.W. Groves, Canad. J. Res. 26: 277. 1948. [MB 285134]; basionym of Dichotomopilus erectus.
Chaetomium fimeti Fuckel, Jahrb. Nassauischen Vereins Naturk. 15: 64. 1860. [MB 160431]. — Type: G00127165 (holotype), CBS H-22198 (epitype). Ex-epitype: DSM 62108 = CBS 139034. Reproduction: sexual. ITS barcode: KT214559 (alternative markers: LSU = KT214593; tub2 = KT214736; rpb2 = KT214663).
Chaetomium flavigenum Van Warmelo, Mycologia 58: 847. 1966. [MB 328042]; basionym of Arcopilus flavigenus.
Chaetomium floriforme Gené & Guarro, Mycol. Res. 100: 1005. 1996. [MB 415630]; basionym of Humicola floriformis.
Chaetomium funicola Cooke, Grevillea 1: 176. 1873. [MB 172830]; basionym of Dichotomopilus funicola.
Chaetomium fusiforme Chivers, Proc. Amer. Acad. Arts 48: 87. 1912. [MB 172131]; basionym of Arcopilus fusiformis.
Chaetomium fusisporum G. Sm., Trans. Brit. Mycol. Soc. 44: 46. 1961. [MB 328044]; basionym of Subramaniula fusispora.
Chaetomium fusum L.M. Ames, A monograph of the Chaetomiaceae Ser. 2: 25. 1963. [MB 328045]; basionym of Dichotomopilus fusus.
Chaetomium gangligerum L.M. Ames, Mycologia 41: 640. 1950. [MB 294695]; basionym of Arxotrichum gangligerum.
Chaetomium gelasinosporum Aue & E. Müll., Ber. Deutsch. Bot. Ges. 77: 193. 1967. [MB 328046]; basionym of Amesia gelasinospora.
Chaetomium globosporum Rikhy & Mukerji, Kavaka: 38. 1974. [MB 310902]; replaced synonym of Chaetomium neoglobosporum.
Chaetomium globosum Kunze, Mykol. Hefte 1: 16. 1817. [MB 172545]. — Type: CBS H-22185 (neotype). Ex-neotype: CBS 160.62. Reproduction: sexual. ITS barcode: KT214565 (alternative markers: LSU = KT214596; tub2 = KT214742; rpb2 = KT214666).
Chaetomium globosum var. flavoviride E.K. Novák, Ann. Univ. Sci. Budapest. Rolando Eötvös, Sect. Biol. 8: 207. 1966. [MB 349175]; synonym of Chaetomium globosum.
Chaetomium globosum var. griseum E.K. Novák, Ann. Univ. Sci. Budapest. Rolando Eötvös, Sect. Biol. 8: 207. 1966. [MB 353346]; synonym of Chaetomium globosum.
Chaetomium gracile Udagawa, J. Gen. Appl. Microbiol. Tokyo 6: 235. 1960. [MB 328048]; basionym of Achaetomiella gracilis.
Chaetomium graminiforme X.Wei Wang et al., Persoonia 36: 106. 2015 [2016]. [MB 812979]. — Type: CBS H-22193. Ex-type: CBS 506.84 = TRTC 47862. Reproduction: sexual. ITS barcode: KT214584 (alternative markers: LSU = KT214615; tub2 = KT214761; rpb2 = KT214687).
Chaetomium grande Asgari & Zare, Mycologia 103: 874. 2011. [MB 519105]. — Type: IRAN 14608F. Ex-type: IRAN 1064C = CBS 126780. Reproduction: sexual. ITS barcode: HM365253 (alternative markers: LSU = HM365253; tub2 = HM365273; rpb2 = KT214657).
Chaetomium hamadae (Udagawa) Arx, Proc. Indian Acad. Sci., Pl. Sci. 94: 343. 1985. [MB 105136]; synonym of Pseudothielavia hamadae.
Chaetomium heterothallicum Yu Zhang & L. Cai, Fungal Biol. 121: 29. 2016 [2017]. [MB 811151]; basionym of Trichocladium heterothallicum.
Chaetomium hexagonosporum A. Carter & Malloch, Canad. J. Bot. 60: 1249. 1982. [MB 109668]; basionym of Collariella hexagonospora.
Chaetomium hispanicum Guarro & Arx, Beih. Nova Hedwigia 84: 6. 1986. [MB 104876]; basionym of Parachaetomium hispanicum.
Chaetomium homopilatum Omvik, Mycologia 47: 749. 1955. [MB 294697]; basionym of Humicola homopilata.
Chaetomium indicum Corda, Icon. Fung. 4: 38, tab. 8, fig. 104. 1840. [MB 150904]; basionym of Dichotomopilus indicus.
Chaetomium interruptum Asgari & Zare, Mycologia 103: 874. 2011. [MB 519104]. — Type: IRAN 14607F. Ex-type: IRAN 1278C = CBS 126660. Reproduction: sexual. ITS barcode: HM365246 (alternative markers: LSU = HM365246; tub2 = KT214741; rpb2 = KT214665).
Chaetomium iranianum Asgari & Zare, Mycologia 103: 877. 2011. [MB 519106]. — Type: IRAN 14609F. Ex-type: IRAN 861C = CBS 126670. Reproduction: sexual. ITS barcode: HM365257 (alternative markers: LSU = HM365257; tub2 = HM365297; rpb2 = MT568848). Note: This species was transferred to the genus Parachaetomium (as P. iranianum); however, the current name is Parachaetomium perlucidum (this study).
Chaetomium iranicum M. Mehrabi-Koushki et al., Sydowia 73: 24. 2020. [MB 835248]. — Type: IRAN 17599F. Ex-type: IRAN 3379C = SCUA-Agh-26. Reproduction: sexual. ITS barcode: n/a (alternative markers: LSU = n/a; tub2 = MN520421; rpb2 = MT273944).
Chaetomium irregulare Sörgel ex W. Gams, Nova Hedwigia 12: 386. 1966. [MB 328055]; replaced synonym of Subramaniula flavipila.
Chaetomium jabalpurense D.P. Tiwari et al., Curr. Sci. 46: 578. 1977. [MB 310905]. — Type: IMI 157256. Ex-type: CBS 552.83 = IMI 157256. Reproduction: sexual. ITS barcode: n/a (alternative markers: LSU = n/a; tub2 = MZ343026; rpb2 = MZ342987). Note: Based on the phylogenetic analysis (Fig. 7), we consider Chaetomium jabalpurense a synonym of Amesia gelasinospora.
Chaetomium jatrophae Rohit Sharma, Mycotaxon 124: 120. 2013. [MB 563940]. — Type: AMH 9558. Ex-type: CBS 13426 = MCC 1025. Reproduction: sexual. ITS barcode: JQ246354 (alternative markers: LSU = HE981193; tub2 = HE981190; rpb2 = n/a). Notes: The tub2 sequence deposited on GenBank is 99.3 % similar to that of CBS 673.80, the ex-type of Amesia gelasinospora. We therefore consider this species a synonym of Amesia gelasinospora.
Chaetomium jodhpurense Lodha, J. Indian Bot. Soc. 43: 132. 1964. [MB 328056]; basionym of Acrophialophora jodhpurensis.
Chaetomium laterale Yu Zhang & L. Cai, Fungal Biol. 121: 30. 2016. [MB 811152]; basionym of Subramaniula lateralis.
Chaetomium lentum Van Warmelo, Mycologia 58: 850. 1967. [MB 328057]; basionym of Chrysanthotrichum lentum.
Chaetomium longiciliatum [as ‘longiciliata’] Yu Zhang & L. Cai, Fungal Biol. 121: 31. 2016 [2017]. [MB 840134]; basionym of Parachaetomium longiciliatum.
Chaetomium longicolle [as ‘longicolleum’] Krzemien. & Badura, Acta Soc. Bot. Poloniae 23: 748. 1954. [MB 491886]; basionym of Staphylotrichum longicolle [as ‘longicolleum’].
Chaetomium longirostre (Farrow) L.M. Ames, A monograph of the Chaetomiaceae: 29. 1963. [MB 282966]; synonym of Staphylotrichum longicolle.
Chaetomium lucknowense J.N. Rai & J.P. Tewari, Canad. J. Bot. 40: 1379. 1963. [MB 328059]; basionym of Chrysocorona lucknowensis.
Chaetomium luteum (J.N. Rai & J.P. Tewari) P.F. Cannon, Trans. Brit. Mycol. Soc. 87: 60. 1986. [MB 103140]; synonym of Achaetomium luteum.
Chaetomium macrostiolatum [as ‘macrostiolum’] Stchigel et al., Mycologia 94: 121. 2002. [MB 484629]; basionym of Arcopilus macrostiolatus.
Chaetomium madrasense Natarajan, Proc. Indian Acad. Sci., Sect. B 74: 255. 1971. [MB 310909]. — Type: CBS H-6877. Ex-type: CBS 315.74. Reproduction: sexual. ITS barcode: KC109751 (alternative markers: LSU = KC109751; tub2 = KC109769; rpb2 = KF001831).
Chaetomium malaysiense (D. Hawksw.) Arx, Beih. Nova Hedwigia 84: 38. 1986. [MB 104877]; synonym of Humicola malaysiensis.
Chaetomium mareoticum Besada & Yusef, Trans. Brit. Mycol. Soc. 52: 502. 1969. [MB 310911]; basionym of Parachaetomium mareoticum.
Chaetomium medusarum J.A. Mey. & Lanneau, Bull. Trimestriel Soc. Bot. France 83: 318. 1967. [MB 328061]; basionym of Ovatospora medusarum.
Chaetomium megalocarpum Bainier, Bull. Trimestriel Soc. Bot. France 25: 202. 1910. [MB 165525]. — Type: Pl. XVI, figs 1–4 in Bainier, Bull. Trimestriel Soc. Bot. France 25: 202, 1910 (lectotype); CBS H-22186 (epitype). Ex-epitype: MUCL 9589 = CBS 149.59. Reproduction: sexual. ITS barcode: KC109744 (alternative markers: LSU = KC109744; tub2 = KC109762; rpb2 = KF001828).
Chaetomium megasporum Sörgel ex Seth, Beih. Nova Hedwigia 37: 82. 1972. [MB 310912]; basionym of Arcopilus megasporus.
Chaetomium microthecium [as ‘microthecia’] Yu Zhang & L. Cai, Fungal Biol. 121: 32. 2016 [2017]. [MB 840135]. — Type: HMAS 245781. Ex-type: CGMCC 3.17556. Reproduction: sexual. ITS barcode: KP336785 (alternative markers: LSU = KP336834; tub2 = KP336883; rpb2 = KT149505).
Chaetomium mollicellum L.M. Ames, A monograph of the Chaetomiaceae: 30. 1963. [MB 328063]; basionym of Ovatospora mollicella.
Chaetomium mollipilium L.M. Ames, Mycologia 42: 644. 1950. [MB 294702]; synonym of Chaetomium globosum.
Chaetomium muelleri Arx, Beih. Nova Hedwigia 84: 6. 1986. [MB 104878]; basionym of Parachaetomium muelleri.
Chaetomium multispirale A. Carter et al., Canad. J. Bot. 60: 1256. 1982. [MB 109669]; basionym of Parachaetomium multispirale.
Chaetomium murorum Corda, Icon. Fung. 1: 24, tab. 7, fig. 293B. 1837. [MB 165260]; basionym of Botryotrichum murorum.
Chaetomium neoglobosporum X.Wei Wang & Houbraken, [MB 841112]. Replaced synonym: Chaetomium globosporum. — Type: IMI 166876. Ex-type: CBS 108.83 = ITCC 1835. Reproduction: sexual. ITS barcode: KC109750 (alternative markers: LSU = KC109750; tub2 = KC109768; rpb2 = KF001825).
Chaetomium nepalense (Udagawa & Y. Sugiy.) Arx, Proc. Indian Acad. Sci., Sect. B 94: 344. 1985. [MB 105137]. Basionym: Achaetomium nepalense. — Type: NHL 2895. Ex-type: CBS 288.83 = IMI 288623 = NHL 2895. Reproduction: sexual. ITS barcode: MH861591 (alternative markers: LSU = MH873316; tub2 = n/a; rpb2 = MZ342983).
Chaetomium nigricolor L.M. Ames, Mycologia 42: 645. 1950. [MB 294703]; basionym of Amesia nigricolor.
Chaetomium novozelandicum X.Wei Wang et al., Persoonia 36: 110. (2015) [2016]. [MB 812980]. — Type: AEB 1071 (holotype); CBS H-22191 (isotype). Ex-type: CBS 124555. Reproduction: sterile. ITS barcode: KT214576 (alternative markers: LSU = KT214607; tub2 = KT214753; rpb2 = KT214677).
Chaetomium nozdrenkoae Sergejeva, Bot. Mater. Otd. Sporov. Rast. Bot. Inst. Komarova Akad. Nauk S.S.S.R. 14: 140. 1961. [MB 328064]. — Type: –. Ex-type: CBS 163.62 = ATCC 14528 = IMI 090490 = IMI 090490 = MUCL 18703 = VKM F-1953. Reproduction: sexual. ITS barcode: KT214556 (alternative markers: LSU = KT214590; tub2 = KT214733; rpb2 = KT214660).
Chaetomium olivaceum Cooke & Ellis, Grevillea 6: 96. 1878. [MB 164649]. — Type: n/a. Representative strain: CBS 418.80A. Reproduction: sexual. ITS barcode: JN209914 (alternative markers: LSU = JN209914; tub2 = JN256184; rpb2 = KF001806). Notes: Two collections of Ellis are cited in the protologue and an illustration is available. Chaetomium olivaceum remains to be lecto- and epitypified based on suitable specimens.
Chaetomium ovatoascomatis M. Raza & L. Cai, Fungal Diversity 99: 82. 2019. [MB 556679]. — Type: HMAS 248072. Ex-type: CGMCC 3.19341 = LC13510. Reproduction: sexual. ITS barcode: MN215753 (alternative markers: LSU = MN215591; tub2 = MN329920; rpb2 = MN255434); synonym of Chaetomium cochliodes.
Chaetomium pachypodioides L.M. Ames, Mycologia 37: 145. 1945. [MB 285136]; basionym of Collariella pachypodioides.
Chaetomium perlucidum Sergejeva, Not. Syst. Sect. Crypt. Inst. Bot. Acad. Sci. USSR 11: 108. 1956. [MB 294704]; basionym of Parachaetomium perlucidum.
Chaetomium pilosum (C. Booth & Shipton) X.Wei Wang & Crous, Persoonia 36: 112. 2015 [2016]. [MB 812981]. Basionym: Thielavia pilosa. — Type: IMI 113231. Ex-type: CBS 335.67 = IMI 113231 = VKM F-1851. Reproduction: sexual. ITS barcode: KT214586 (alternative markers: LSU = FJ666356; tub2 = KT214763; rpb2 = FJ666387).
Chaetomium piluliferoides Udagawa & Y. Horie, Trans. Mycol. Soc. Japan: 337. 1975. [MB 310914]; basionym of Arxotrichum piluliferoides.
Chaetomium piluliferum J. Daniels, Trans. Brit. Mycol. Soc. 44: 84. 1961. [MB 328069]; synonym of Botryotrichum piluliferum.
Chaetomium pinnatum L.M. Ames, A monograph of the Chaetomiaceae: 33. 1963. [MB 328070]; basionym of Humicola pinnata.
Chaetomium pratense X.Wei Wang, Mycol. Prog. 13: 723. 2014. [MB 563348]; basionym of Dichotomopilus pratensis.
Chaetomium pseudocochliodes X.Wei Wang et al., Persoonia 36: 113. 2015 [2016]. [MB 812982]. — Type: CBS H-22197. Ex-type: CGMCC 3.9441. Reproduction: sexual. ITS barcode: JN209925 (alternative markers: LSU = JN209925; tub2 = JN256195; rpb2 = KF001816).
Chaetomium pseudoglobosum X.Wei Wang et al., Persoonia 36: 115. 2015 [2016]. [MB 812983]. — Type: CBS H-10083. Ex-type: CBS 574.71. Reproduction: sexual. ITS barcode: KT214573 (alternative markers: LSU = KT214604; tub2 = KT214750; rpb2 = KT214674).
Chaetomium quadrangulatum Chivers, Proc. Amer. Acad. Arts Sci. 48: 85. 1912. [MB 173351]; basionym of Collariella quadrangulata.
Chaetomium raii G. Malhotra & Mukerji, Rev. Mycol., (Paris) 40(2): 182. 1976. [MB 283388]; basionym of Amesia raii.
Chaetomium ramipilosum Schaumann, Arch. Mikrobiol. 91: 98. 1973. [MB 310916]; synonym of Chaetomium elatum.
Chaetomium ramosissimum X.Wei Wang & L. Cai, Mycol. Prog. 13: 725. 2014. [MB 801734]; basionym of Dichotomopilus ramosissimus.
Chaetomium rectangulare Asgari & Zare, Mycologia 103: 872. 2011. [MB 519103]. — Type: IRAN 14606F. Ex-type: IRAN 1641C = CBS 126778. Reproduction: sexual & asexual. ITS barcode: HM365239 (alternative markers: LSU = HM365239; tub2 = HM365285; rpb2 = KT214688).
Chaetomium reflexum Skolko & J.W. Groves, Canad. J. Res. 26: 279. 1948. [MB 285137]; basionym of Dichotomopilus reflexus.
Chaetomium repens Guarro & Figueras, Beih. Nova Hedwigia 84: 6. 1986. [MB 104870]; basionym of Arxotrichum repens.
Chaetomium retardatum A. Carter & R.S. Khan, Canad. J. Bot. 60: 1255. 1982. [MB 109670]; basionym of Botryotrichum retardatum.
Chaetomium robustum L.M. Ames, A monograph of the Chaetomiaceae: 35. 1963. [MB 328075]; basionym of Collariella robusta.
Chaetomium sacchari M. Raza & L. Cai, Fungal Diversity 99: 88. 2019. [MB 556680]. — Type: HMAS 248071. Ex-type: CGMCC 3.19349 = LC11918. Reproduction: sexual. ITS barcode: MN215759 (alternative markers: LSU = MN215597; tub2 = MN329926; rpb2 = MN255440); synonym of Chaetomium cryptocochliodes.
Chaetomium seminis-citrulli [as ‘semen-citrulli’] Sergejeva, Not. Syst. Sect. Crypt. Inst. Bot. Acad. Sci. USSR 11: 113. 1956. [MB 537941]; basionym of Trichocladium seminis-citrulli.
Chaetomium seminudum L.M. Ames, Mycologia 41: 642. 1949. [MB 294709]; basionym of Humicola seminuda.
Chaetomium semispirale Udagawa & Cain, Canad. J. Bot. 47: 1947. 1969. [MB 310918]; basionym of Pseudohumicola semispiralis.
Chaetomium senegalense L.M. Ames, A monograph of the Chaetomiaceae: 36. 1963. [MB 328077]; basionym of Ovatospora senegalensis.
Chaetomium serpentinum L.M. Ames ex A. Carter, Canad. J. Bot. 61: 2605. 1983. [MB 106675]; synonym of Amesia cymbiformis.
Chaetomium sinense K.T. Chen, Acta Microbiol. Sin. 13(2): 125. 1973. [MB 310920]; basionym of Arxotrichum sinense.
Chaetomium sphaerale Chivers, Proc. Amer. Acad. Arts Sci. 48: 84. 1912. [MB 158635]; basionym of Humicola sphaeralis.
Chaetomium spiculipilium L.M. Ames, A monograph of the Chaetomiaceae: 37. 1963. [MB 328078]. — Type: BPI 1100708 (holotype); CBS H-6893 (isotype). Ex-type: CBS 373.66. Reproduction: sexual. ITS barcode: KC109756 (alternative markers: LSU = KC109756; tub2 = KC109774; rpb2 = KF001809).
Chaetomium spinosum Chivers, Proc. Amer. Acad. Arts 48: 86. 1912. [MB 152061]; basionym of Xanthiomyces spinosus.
Chaetomium spirochaete Palliser, North American Flora 3: 61. 1910. [MB 167661]. — Type: NY01050443 (holotype); HMAS 244438 (epitype). Ex-epitype: CBS 730.84 = IMI 287303 = QM 6702. Reproduction: sexual. ITS barcode: JN209921 (alternative markers: LSU = JN209921; tub2 = JN256191; rpb2 = KF001819).
Chaetomium strumarium (J.N. Rai et al.) P.F. Cannon, Trans. Brit. Mycol. Soc. 87: 64. 1986. [MB 103141]; synonym of Achaetomium strumarium.
Chaetomium subaffine Sergejeva ex X.Wei Wang & Houbraken, this study. [MB 842311]. — Type: CBS H-24916. Ex-type: CBS 637.91 = ATCC 14531 = IMI 90489 = VKM F-1945. Reproduction: sexual & asexual. ITS barcode: JN209929 (alternative markers: LSU = JN209929; tub2 = JN256199; rpb2 = KF001817).
Chaetomium subfimeti (Seth) X.Wei Wang & Crous, Persoonia 36: 121. 2015 [2016]. [MB 812984]. Basionym: Chaetomidium subfimeti. — Type: IMI 116692 (holotype); CBS H-6839 (isotype). Ex-type: CBS 370.66 = ATCC 18209 = IMI 116692 = LCP 82.3317. Reproduction: sexual. ITS barcode: KT214562 (alternative markers: LSU = FJ666354; tub2 = KT214739; rpb2 = FJ666385).
Chaetomium subfunicola X.Wei Wang & L. Cai, Mycol. Prog. 13: 723. 2014. [MB 801733]; basionym of Dichotomopilus subfunicola.
Chaetomium subglobosum Sergejeva, Bot. Mater. Otd. Sporov. Rast. Bot. Inst. Komarova Akad. Nauk S.S.S.R. 13: 172. 1960. [MB 328081]. — Type: –. Ex-type: CBS 149.60 = ATCC 14533 = IMI 081770 = MUCL 18694 = VKM F-1951. Reproduction: sexual. ITS barcode: JN209930 (alternative markers: LSU = JN209930; tub2 = JN256200; rpb2 = KF001808).
Chaetomium subspirale Chivers, Proc. Amer. Acad. Arts Sci. 48: 84. 1912. [MB 167796]; basionym of Pseudohumicola subspiralis.
Chaetomium subspirilliferum Sergejeva, Bot. Mater. Otd. Sporov. Rast. Bot. Inst. Komarova Akad. Nauk S.S.S.R. 13: 174. 1960. [MB 328082]; basionym of Parachaetomium subspirilliferum.
Chaetomium subterraneum Swift & Povah, Mycologia 21: 210. 1929. [MB 168290]; synonym of Chaetomium globosum.
Chaetomium succineum L.M. Ames, Mycologia 41: 645. 1949. [MB 294710]; basionym of Arxotrichum succineum.
Chaetomium tarraconense [as ‘tarraconensis’] Stchigel et al., Mycologia 94: 125. 2002. [MB 541276]. — Type: IMI 382893. Ex-type: CBS 101882 = FMR 6638 = IMI 380425 = MUCL 43149. Reproduction: sexual. ITS barcode: n/a (alternative markers: LSU = n/a; tub2 = MZ343005; rpb2 = MZ342964).
Chaetomium tectifimeti X.Wei Wang & Samson, Stud. Mycol. 84: 177. 2016. [MB 818838]. — Type: CBS H-22844. Ex-type: CBS 142032. Reproduction: sexual. ITS barcode: KX976640 (alternative markers: LSU = KX976737; tub2 = KX976982; rpb2 = KX976836).
Chaetomium telluricola X.Wei Wang et al., Persoonia 36: 124. 2015 [2016]. [MB 812985]. — Type: CBS H-676. Ex-type: CBS 151.59 = IMI 032543. Reproduction: sexual. ITS barcode: KT214582 (alternative markers: LSU = KT214613; tub2 = KT214759; rpb2 = KT214685).
Chaetomium tenue X.Wei Wang et al., Persoonia 36: 125. 2015 [2016]. [MB 812986]. — Type: CBS H-22195. Ex-type: CBS 139.38. Reproduction: sexual. ITS barcode: KT214568 (alternative markers: LSU = KT214599; tub2 = KT214745; rpb2 = KT214669).
Chaetomium thermophilum La Touche, Trans. Brit. Mycol. Soc. 33: 95. 1950. [MB 344053]; basionym of Thermochaetoides thermophila.
Chaetomium thermophilum var. dissitum Cooney & R. Emers., Thermophilic Fungi: 68. 1964. [MB 353347]; basionym of Thermochaetoides dissita.
Chaetomium trigonosporum (Marchal & É.J. Marchal) Chivers, Mem. J. Torrey Bot. Soc. 14: 166. 1915. [MB 162780]; synonym of Bommerella trigonospora.
Chaetomium trilaterale var. chiversii J.C. Cooke, Mycologia 65: 1218. 1973. [MB 347891]; basionym of Floropilus chiversii.
Chaetomium triticicola Lal & J.N. Kapoor, Indian Phytopathol. 30: 136. 1978. [MB 310928]. — Type: n/a. Ex-type: CBS 106.83 = IMI 232292 = ITCC 2038. Reproduction: sexual. ITS barcode: n/a (alternative markers: LSU = n/a; tub2 = n/a; rpb2 = MZ342967). Note: This species shares identical rpb2 sequences with Amesia raii and is tentatively considered a synonym of this species.
Chaetomium truncatulum Asgari & Zare, Mycologia 103: 877. 2011. [MB 519107]; basionym of Parachaetomium truncatulum.
Chaetomium turgidopilosum L.M. Ames, Mycologia 41: 639. 1949. [MB 294711]; basionym of Arcopilus turgidopilosus.
Chaetomium udagawae Sergejeva ex Udagawa, Trans. Mycol. Soc. Japan 20: 476. 1979. [MB 118491]; basionym of Humicola udagawae.
Chaetomium umbonatum D. Brewer, Proc. & Trans. Nova Scotian Inst. Sci. 27(2): 59. 1974. [MB 310929]. — Type: IMI 138895 (holotype); CBS H-6904 (isotype). Ex-type: CBS 293.83 = ATCC 28768 = IMI 138895. Reproduction: sexual. ITS barcode: KT214575 (alternative markers: LSU = KT214606; tub2 = KT214752; rpb2 = KT214676).
Chaetomium undulatulum Asgari & Zare, Mycologia 103: 870. 2011. [MB 519102]. — Type: IRAN 14605F. Ex-type: CBS 126775 = IRAN 857C. Reproduction: sexual. ITS barcode: HM365251 (alternative markers: LSU = HM365251; tub2 = HM365279; rpb2 = KT214682).
Chaetomium unguicola X.Wei Wang et al., Persoonia 36: 128. 2015 [2016]. [MB 812987]. — Type: CBS H-22196. Ex-type: CBS 128446 = UTHSC 07-2213. Reproduction: sexual. ITS barcode: KT214567 (alternative markers: LSU = KT214598; tub2 = KT214744; rpb2 = KT214668).
Chaetomium uniporum Aue & E. Müll., Ber. Schweiz. Bot. Ges. 77: 189. 1967. [MB 328088]; basionym of Ovatospora unipora.
Chaetomium uniseriatum Yu Zhang & L. Cai, Fungal Biol. 121: 33. 2016. [MB 811156]; basionym of Trichocladium uniseriatum.
Chaetomium variosporum Udagawa & Y. Horie, Rep. Tottori Mycol. Inst. 10: 430. 1973. [MB 310931]; basionym of Brachychaeta variospora.
Chaetomium variostiolatum A. Carter, Canad. J. Bot. 61: 2603. 1983. [MB 106676]; basionym of Dichotomopilus variostiolatus.
Chaetomium venezuelense L.M. Ames, A monograph of the Chaetomiaceae: 42. 1963. [MB 328089]; synonym of Chrysocorona lucknowensis.
Chaetomium virescens (Arx) Udagawa, Trans. Mycol. Soc. Japan 21: 34. 1980. [MB 121660]; synonym of Collariella virescens.
Chaetomium virgicephalum [as ‘virgecephalum’] L.M. Ames, A monograph of the Chaetomiaceae: 42. 1963. [MB 121663]; synonym of Chaetomium elatum.
Chaetomium vitellinum A. Carter, Mycologia 75: 531. 1983. [MB 108760]; basionym of Botryotrichum vitellinum.
Chaetomium wallefii J.A. Mey. & Lanneau, Bull. Trimestriel Soc. Bot. France 83: 320. 1967. [MB 328093]; basionym of Humicola wallefii.
Chrysanthotrichum
Chrysanthotrichum allolentum X.Wei Wang & Houbraken, Stud. Mycol. 93: 196. 2019. [MB 829853]. — Type: CBS H-23634. Ex-type: CBS 644.83. Reproduction: sexual. ITS barcode: MK926808 (alternative markers: LSU = MK926808; tub2 = MK926908; rpb2 = MK876768).
Chrysanthotrichum lentum (Van Warmelo) X.Wei Wang & Houbraken, Stud. Mycol. 93: 196. 2019. [MB 829855]. Basionym: Chaetomium lentum. — Type: PRE 43084. Ex-type: CBS 339.67 = IMI 128308. Reproduction: sexual. ITS barcode: MK926809 (alternative markers: LSU = MK926809; tub2 = MK926909; rpb2 = MK876769).
Chrysanthotrichum leptolentum X.Wei Wang & Houbraken, Stud. Mycol. 93: 196. 2019. [MB 829856]. — Type: CBS H-23633. Ex-type: CBS 126.85. Reproduction: sexual. ITS barcode: MK926810 (alternative markers: LSU = MK926810; tub2 = MK926910; rpb2 = MK876770).
Chrysanthotrichum peruvianum (Goch.) X.Wei Wang & Houbraken, Stud. Mycol. 93: 201. 2019. [MB 829857]. Basionym: Chaetomidium peruvianum. — Type: NY, Gochenaur 68-11. Ex-type: CBS 732.68 = ATCC 18511 = IMI 135024. Reproduction: sexual. ITS barcode: MK926812 (alternative markers: LSU = MK926812; tub2 = MK926912; rpb2 = MK876772).
Chrysocorona
Chrysocorona lucknowensis (J.N. Rai & J.P. Tewari) X.Wei Wang & Houbraken, Stud. Mycol. 93: 201. 2019. [MB 829859]. Basionym: Chaetomium lucknowense. — Type: figs 16–28 in Rai & Tewari, Canad. J. Bot. 40: 1380, 1962 (lectotype); CBS H-10081 (epitype). Ex-epitype: CBS 727.71 = LUP-22. Reproduction: sexual. ITS barcode: MK926813 (alternative markers: LSU = MK926813; tub2 = MK926913; rpb2 = MK876773).
Chrysosporium (Onygenales, Eurotiomycetes)
Chrysosporium fergusii Klopotek, Arch. Mikrobiol. 98: 366. 1974. [MB 311104]; synonym of Thermothelomyces fergusii.
Coccospora
Coccospora agricola Goddard, Bot. Gaz. 56: 264. 1913. [MB 206263]; synonym of Botryotrichum piluliferum.
Collariella
Collariella anguipilia (L.M. Ames) X.Wei Wang & Houbraken, this study. [MB 840145]. Basionym: Chaetomium anguipilium. — Type: BPI 1101397. Ex-type: CBS 632.83. Reproduction: sexual. ITS barcode: MZ334721 (alternative markers: LSU = MZ351424; tub2 = MZ343028; rpb2 = MZ342989).
Collariella bostrychodes (Zopf) X.Wei Wang & Samson, Stud. Mycol. 84: 179. 2016. [MB 818862]. Basionym: Chaetomium bostrychodes. — Type: n/a. Representative strain: CBS 163.73 = ATCC 24468 = IMI 171508 = TRTC 661727b. Reproduction: sexual. ITS barcode: KX976641 (alternative markers: LSU = KX976738; tub2 = KX976983; rpb2 = KX976837).
Collariella capillicompacta M. Mehrabi-Koushki et al., Sydowia 73: 27. 2020. [MB 832919]. — Type: IRAN 17599F. Ex-type: IRAN 3496C = SCUA-Agh-20H. Reproduction: sexual. ITS barcode: n/a (alternative markers: LSU = n/a; tub2 = MN520423; rpb2 = MN520427).
Collariella carteri X.Wei Wang et al., Stud. Mycol. 84: 179. 2016. [MB 818863]. — Type: CBS H-22845. Ex-type: CBS 128.85 = TRTC 50691. Reproduction: sexual. ITS barcode: KX976647 (alternative markers: LSU = KX976742; tub2 = KX976989; rpb2 = KX976841).
Collariella causiiformis (L.M. Ames) X.Wei Wang & Samson, Stud. Mycol. 84: 179. 2016. [MB 818864]. Basionym: Chaetomium causiiforme. — Type: BPI. Ex-type: CBS 792.83 = ATCC 11198 = CBS 139.56 = IFO 9139. Reproduction: sexual. ITS barcode: KX976646 (alternative markers: LSU = KX976741; tub2 = KX976988; rpb2 = KX976840). Notes: Several specimens are labeled as type according to Mycoportal: BPI 845270, BPI 1100724 and BPI 1101427. A database search and/or examination of these specimens is needed to determine which of them is the holotype and which are isotypes, or if a lectotypification is necessary.
Collariella gracilis (Udagawa) X.Wei Wang & Samson, Stud. Mycol. 84: 185. 2016. [MB 818865]; synonym of Achaetomiella gracilis.
Collariella hexagonospora (A. Carter & Malloch) X.Wei Wang & Houbraken, this study. [MB 840146]. Basionym: Chaetomium hexagonosporum. — Type: TRTC 48872. Ex-type: CBS 171.84 = TRTC 48872 = FMR 7235. Reproduction: sexual. ITS barcode: MH861717 (alternative markers: LSU = n/a; tub2 = MZ343016; rpb2 = MZ342977).
Collariella hilkhuijsenii X.Wei Wang, Persoonia 39: 463. 2017. [MB 823460]. — Type: CBS H-23232. Ex-type: CBS 143305. Reproduction: sexual. ITS barcode: MG432011 (alternative markers: LSU = MG432012; tub2 = MF716586; rpb2 = MF716587).
Collariella pachypodioides (L.M. Ames) X.Wei Wang & Houbraken, this study. [MB 840147]. Basionym: Chaetomium pachypodioides. — Type: FH, Ames 1044.3 (holotype); CBS H-6883 (isotype). Ex-type: CBS 164.52 = ATCC 11213 = IFO 9109 = IMI 012266 = IMI 287299 = MUCL 9586. Reproduction: sexual. ITS barcode: MH856980 (alternative markers: LSU = MH868500; tub2 = MZ343014; rpb2 = MZ342975).
Collariella quadrangulata (Chivers) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 818861]. Basionym: Chaetomium quadrangulatum. — Type: Chivers No. 29, CUP. Representative strain: CBS 152.59. Reproduction: sexual. ITS barcode: KX976651 (alternative markers: LSU = KX976746; tub2 = KX976993; rpb2 = KX976845).
Collariella quadrum Z.F. Zhang et al., Persoonia 39: 14. 2017. [MB 818249]. — Type: HMAS 246923. Ex-type: CGMCC 3.17917. Reproduction: sexual. ITS barcode: KU746675 (alternative markers: LSU = KU746721; tub2 = KU746767; rpb2 = KY575870).
Collariella robusta (L.M. Ames) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 818872]. Basionym: Chaetomium robustum. — Type: BPI 1101399. Ex-type: CBS 551.83. Reproduction: sexual. ITS barcode: KX976652 (alternative markers: LSU = KX976747; tub2 = KX976994; rpb2 = KX976846).
Collariella virescens (Arx) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 819488]; synonym of Achaetomiella virescens.
Condenascus
Condenascus tortuosus (Udagawa & Y. Sugiy.) X.Wei Wang & Houbraken, Stud. Mycol. 93: 203. 2019. [MB 829861]. Basionym: Thielavia tortuosa. — Type: NHL 2890. Ex-type: CBS 691.82 (contaminated); CBS 610.97 (representative strain) = FMR 5780. Reproduction: sexual. ITS barcode: MK926817 (alternative markers: LSU = MK926817; tub2 = MK926917; rpb2 = MK876777).
Coniothyrium (Pleosporales, Dothideomycetes)
Coniothyrium terricola J.C. Gilman & E.V. Abbott, Iowa St. Coll. J. Sci. 1: 267. 1927. [MB 255077]; basionym of Pseudothielavia terricola.
Corynascella
Corynascella humicola Arx & Hodges, Stud. Mycol. 8: 23. 1975. [MB 312209]. — Type: CBS H-6963. Ex-type: CBS 337.72. Reproduction: sexual & asexual. ITS barcode: KX976656 (alternative markers: LSU = KX976751; tub2 = KX976998; rpb2 = MK942091).
Corynascella inaequalis (Pidopl. et al.) Arx, Kavaka 3: 34. 1975. [MB 312210]; synonym of Parachaetomium inaequale.
Corynascella inquinata Udagawa & S. Ueda, Mycotaxon 8: 292. 1979. [MB 312211]; basionym of Botryotrichum inquinatum.
Corynascus
Corynascus citrinus A. Giraldo & Crous, Persoonia 36: 449. 2016. [MB 816971]. — Type: BCC 79098 (metabolically inactive). Ex-type: BCC 79098. Reproduction: sexual & asexual. ITS barcode: KX262667 (alternative markers: LSU = KX228351; tub2 = n/a; rpb2 = KX262668).
Corynascus fumimontanus Y. Marín et al., Mycologia 107: 628. 2015. [MB 809486]. — Type: CBS H-21594. Ex-type: CBS 137294 = FMR 12372. Reproduction: sexual & asexual. ITS barcode: MK919291 (alternative markers: LSU = MK919291; tub2 = MK919405; rpb2 = MK919347).
Corynascus heterothallicus (Klopotek) Arx, Persoonia 12: 174. 1984, nom. inval., Art. 41.4. [MB 107879]; synonym of Thermothelomyces heterothallicus [as ‘heterothallica’].
Corynascus novoguineensis (Udagawa & Y. Horie) Arx, Proc. Kon. Ned. Akad. Wetensch., Sect. C 76: 292. 1973. [MB 312212]. Basionym: Thielavia novoguineensis. — Type: NHL 22501. Ex-type: CBS 359.72 = NHL 22501. Reproduction: sexual & asexual. ITS barcode: MK919292 (alternative markers: LSU = MK919292; tub2 = MK919406; rpb2 = MK919348).
Corynascus sepedonium (C.W. Emmons) Arx, Proc. Kon. Ned. Akad. Wetensch., Sect. C 76: 292. 1973. [MB 312213]. Basionym: Thielavia sepedonium. — Type: n/a. Ex-type: CBS 340.33; representative strain: CBS 111.69 = IMI 136625. Reproduction: sexual & asexual. ITS barcode: HQ871751 (alternative markers: LSU = KX976777; tub2 = KX977027; rpb2 = MK919349).
Corynascus sexualis Stchigel et al., Mycol. Res. 104: 880. 2000. [MB 467480]. — Type: IMI 378520 (holotype); FMR 5691 (isotype). Ex-type: CBS 827.96 = FMR 5691. Reproduction: sexual & asexual. ITS barcode: MK919295 (alternative markers: LSU = MK919295; tub2 = MK919409; rpb2 = MK919352).
Corynascus similis Stchigel et al., Mycol. Res. 104: 881. 2000. [MB 467481]; synonym of Corynascus sepedonium.
Corynascus thermophilus (Fergus & Sinden) Klopotek, Arch. Mikrobiol. 98: 366. 1974. [MB 312215]; synonym of Thermothelomyces fergusii.
Corynascus verrucosus Stchigel et al., Mycol. Res. 104: 884. 2000. [MB 467482]. — Type: IMI 378522 (holotype); FMR 5904 (isotype). Ex-type: CBS 602.97 = FMR 5904. Reproduction: sexual & asexual. ITS barcode: MK919296 (alternative markers: LSU = MK919296; tub2 = MK919410; rpb2 = MK919353).
Crassicarpon nom. inval., Art. F.5.1; synonym of Thermothelomyces.
Crassicarpon hotsonii Koukol, Pl. Syst. Evol. 302: 967. 2016. nom. inval., Art. 35.1. (Shenzhen). [MB 816112]; synonym of Thermothelomyces myriococcoides.
Crassicarpon thermophilum (Fergus & Sinden) Y. Marín et al., Mycologia 107: 629. 2015. nom. inval., Art. 35.1. (Shenzhen). [MB 809488]; synonym of Thermothelomyces fergusii.
Dichotomopilus
Dichotomopilus dolichotrichus (L.M. Ames) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 818866]. Basionym: Chaetomium dolichotrichum. — Type: FH, Ames 1044.7. Ex-type: CBS 162.48 = ATCC 11203 = IMI 012264 = MUCL 9598. Reproduction: sexual. ITS barcode: HM449049 (alternative markers: LSU = HM449063; tub2 = JF772462; rpb2 = KX976852).
Dichotomopilus erectus (Skolko & J.W. Groves) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 818867]. Basionym: Chaetomium erectum. — Type: DAOM 14205. Ex-type: CBS 140.56 = DAOM 14205 = IMI 032249. Reproduction: sexual. ITS barcode: HM449044 (alternative markers: LSU = HM449058; tub2 = JF772458; rpb2 = KX976854).
Dichotomopilus finlandicus O. Kedves et al., Pathogens 10, 1133: 9. 2021. [MB 840621]. — Type: SZMC 26529. Ex-type: SZMC 26529 (metabolically inactive). Reproduction: sexual. ITS barcode: MW541926 (alternative markers: LSU = n/a, tub2 =MZ665529, rpb2 = MZ665531).
Dichotomopilus funicola (Cooke) X.Wei Wang & Samson, Stud. Mycol. 84: 189. 2016. [MB 818841]. Basionym: Chaetomium funicola. — Type: K(M) 189267 (holotype), HMAS 244231 (epitype). Ex-epitype: CBS 159.52. Reproduction: sexual. ITS barcode: GU563369 (alternative markers: LSU = GU563354; tub2 = JF772461; rpb2 = KX976856).
Dichotomopilus fusus (L.M. Ames) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 818868]. Basionym: Chaetomium fusum. — Type: BPI 579938. Ex-type: CBS 372.66. Reproduction: sexual. ITS barcode: KX976660 (alternative markers: LSU = KX976754; tub2 = KX977002; rpb2 = KX976859).
Dichotomopilus indicus (Corda) X.Wei Wang & Samson, Stud. Mycol. 84: 189. 2016. [MB 818842]. Basionym: Chaetomium indicum. — Type: PRM 155406 (holotype), HMAS 244232 (epitype). Ex-epitype: CGMCC 3.14184. Reproduction: sexual. ITS barcode: GU563367 (alternative markers: LSU = GU563360; tub2 = JF772453; rpb2 = KX976861).
Dichotomopilus pratensis (X.Wei Wang & L. Cai) X.Wei Wang & Samson, Stud. Mycol. 84: 191. 2016. [MB 818843]. Basionym: Chaetomium pratense. — Type: HMAS 242921. Ex-type: CBS 133396 = CGMCC 3.14181. Reproduction: sexual. ITS barcode: GU563372 (alternative markers: LSU = GU563357; tub2 = JF772450; rpb2 = KX976866).
Dichotomopilus pseudoerectus X.Wei Wang & Samson, Stud. Mycol. 84: 191. 2016. [MB 818844]. — Type: CBS H-22846. Ex-type: CBS 252.75. Reproduction: sexual. ITS barcode: KX976667 (alternative markers: LSU = KX976761; tub2 = KX977009; rpb2 = KX976869).
Dichotomopilus pseudofunicola X.Wei Wang & Samson, Stud. Mycol. 84: 195. 2016. [MB 818845]. — Type: CBS H-22847. Ex-type: CBS 142033. Reproduction: sexual. ITS barcode: KX976668 (alternative markers: LSU = KX976762; tub2 = KX977010; rpb2 = KX976870).
Dichotomopilus ramosissimus (X.Wei Wang & L. Cai) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 818869]. Basionym: Chaetomium ramosissimum. — Type: HMAS 244195. Ex-type: CGMCC 3.14183. Reproduction: sexual. ITS barcode: GU563371 (alternative markers: LSU = GU563361; tub2 = JF772452; rpb2 = KX976871).
Dichotomopilus reflexus (Skolko & J.W. Groves) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 818870]. Basionym: Chaetomium reflexum. — Type: DAOM 14201. Ex-type: CBS 157.49 = DAOM 14201 = IMI 032252 = MUCL 18700. Reproduction: sexual. ITS barcode: HM449051 (alternative markers: LSU = HM449055; tub2 = JF772460; rpb2 = KX976873).
Dichotomopilus subfunicola (X.Wei Wang & L. Cai) X.Wei Wang & Samson, Stud. Mycol. 84: 195. 2016. [MB 818846]. Basionym: Chaetomium subfunicola. — Type: HMAS 244194. Ex-type: CGMCC 3.12892. Reproduction: sexual. ITS barcode: JX867125 (alternative markers: LSU = JX867125; tub2 = JX867122; rpb2 = KX976875).
Dichotomopilus variostiolatus (A. Carter) X.Wei Wang & Samson, Stud. Mycol. 84: 203. 2016. [MB 818847]. Basionym: Chaetomium variostiolatum. — Type: TRTC QM 36d. Ex-type: CBS 179.84. Reproduction: sexual. ITS barcode: KX976672 (alternative markers: LSU = KX976766; tub2 = KX977014; rpb2 = KX976879).
Farrowia synonym of Staphylotrichum.
Farrowia cuyabenoensis (Decock & Hennebert) D. Hawksw., Systema Ascomycetum 16 (1-2): 52. 1998. [MB 442618]; synonym of Humicola cuyabenoensis.
Farrowia longicollis [as ‘longicollea’] (Krzemien. & Badura) D. Hawksw., Persoonia 8: 174. 1975. [MB 314068]; synonym of Staphylotrichum longicolle.
Farrowia malaysiensis D. Hawksw., Persoonia 8: 178. 1975. [MB 314069]; basionym of Humicola malaysiensis.
Farrowia seminuda (L.M. Ames) D. Hawksw., Persoonia 8: 181. 1975. [MB 314070]; synonym of Humicola seminuda.
Floropilus
Floropilus chiversii (J.C. Cooke) X.Wei Wang & Houbraken, Stud. Mycol. 93: 205. 2019. [MB 829863]. Basionym: Chaetomium trilaterale var. chiversii. — Type: CBS H-10077 (neotype). Ex-neotype: CBS 558.80 = IMI 250966 = MUCL 40052 = TRTC 48533. Reproduction: sexual. ITS barcode: MK926818 (alternative markers: LSU = MK926818; tub2 = MK926918; rpb2 = MK876778).
Gilmaniella
Gilmaniella macrospora Moustafa, Persoonia 8: 332. 1975. [MB 314495]. Replaced synonym of Trichocladium gilmaniellae.
Humicola
Humicola ampulliella (X.Wei Wang) X.Wei Wang & Houbraken, Stud. Mycol. 93: 76. 2018. [MB 824419]. Basionym: Chaetomium ampulliellum. — Type: HMAS 86813. Ex-type: CBS 116735 = CGMCC 3.6696. Reproduction: sexual & asexual. ITS barcode: LT993568 (alternative markers: LSU = LT993568; tub2 = LT993649; rpb2 = LT993487).
Humicola atrobrunnea X.Wei Wang et al., Stud. Mycol. 93: 76. 2018. [MB 824420]; basionym of Pseudohumicola atrobrunnea.
Humicola christenseniae [as ‘christensenii’] X.Wei Wang & Houbraken, Stud. Mycol. 93: 76. 2018. [MB 827854]. — Type: CBS H-23482. Ex-type: CBS 127760 = RMF 9051. Reproduction: sexual & asexual. ITS barcode: LT993571 (alternative markers: LSU = LT993571; tub2 = LT993652; rpb2 = LT993490).
Humicola cuyabenoensis (Decock & Hennebert) X.Wei Wang & Houbraken, Stud. Mycol. 93: 80. 2018. [MB 824423]. Basionym: Chaetomium cuyabenoense. — Type: MUCL 38838. Ex-type: CBS 398.97 = MUCL 38838. Reproduction: sexual & asexual. ITS barcode: LT993573 (alternative markers: LSU = LT993573; tub2 = LT993654; rpb2 = LT993492).
Humicola degenerans X.Wei Wang & Houbraken, Stud. Mycol. 93: 80. 2018. [MB 824424]. — Type: CBS H-23483. Ex-type: CBS 232.65 = IMI 109880. Reproduction: sexual & asexual. ITS barcode: LT993574 (alternative markers: LSU = LT993574; tub2 = LT993655; rpb2 = LT993493).
Humicola distorta (L.M. Ames) X.Wei Wang & Houbraken, Stud. Mycol. 93: 80. 2018. [MB 824427]. Basionym: Chaetomium distortum. — Type: BPI 579118 (holotype); NY01050417 (isotype). Ex-type: CBS 417.66. Reproduction: sexual & asexual. ITS barcode: LT993577 (alternative markers: LSU = LT993577; tub2 = LT993658; rpb2 = LT993496).
Humicola floriformis (Gené & Guarro) X.Wei Wang & Houbraken, Stud. Mycol. 93: 85. 2018. [MB 824429]. Basionym: Chaetomium floriforme. — Type: IMI 368520. Ex-type: CBS 815.97 = MUCL 40181. Reproduction: sexual (easy to lose) and asexual. ITS barcode: LT993578 (alternative markers: LSU = LT993578; tub2 = LT993659; rpb2 = LT993497).
Humicola fuscoatra Traaen, Nytt Mag. Naturvidensk. 52: 33. 1914. [MB 188714]. — Type: n/a. Ex-type: CBS 118.14 = ATCC 22721 = MUCL 8010. Reproduction: asexual. ITS barcode: LT993579 (alternative markers: LSU = LT993579; tub2 = LT993660; rpb2 = LT993498).
Humicola fuscogrisea Y.L. Jiang & T.Y. Zhang, Mycosystema 28: 649. 2009. [MB 513355]. — Type: HSAUP II 04 6083. Ex-type: CGMCC 3.13790. Reproduction: asexual. ITS barcode: LT993581 (alternative markers: LSU = LT993581; tub2 = LT993662; rpb2 = LT993500).
Humicola grisea Traaen, Nytt Mag. Naturvidensk. 52: 34. 1914. [MB 148670]; basionym of Trichocladium griseum.
Humicola grisea var. thermoidea Cooney & R. Emers., Thermophilic Fungi: 74. 1964. [MB 349549]; synonym of Mycothermus thermophilus.
Humicola hirsuta X.Wei Wang et al., this study. [MB 840128]. — Type: HMAS 350292 (holotype); CBS H-23638 (isotype). Ex-type: CBS 144492 = CGMCC 3.20444 = WXW 9028. Reproduction: sexual & asexual. ITS barcode: MZ334726 (alternative markers: LSU = MZ351425; tub2 = MZ343013; rpb2 = MZ342974).
Humicola homopilata (Omvik) X.Wei Wang & Houbraken, Stud. Mycol. 93: 89. 2018. [MB 824432]. Basionym: Chaetomium homopilatum. — Type: CBS 157.55. Ex-type: CBS 157.55 = IMI 182125 = MUCL 40178. Reproduction: sexual & asexual. ITS barcode: LT993582 (alternative markers: LSU = LT993582; tub2 = LT993663; rpb2 = LT993501).
Humicola insolens Cooney & R. Emers., Thermophilic Fungi: 79. 1964. [MB 332024]; synonym of Mycothermus thermophilus.
Humicola jilongensis Y.M. Wu & T.Y. Zhang, Mycotaxon 121: 148. 2012. [MB 563887]; basionym of Trichocladium jilongense.
Humicola koreana Hyang B. Lee & T.T.T. Nguyen, Fungal Diversity 78: 97. 2016. [MB 814402]; basionym of Staphylotrichum koreanum.
Humicola leptodermospora X.Wei Wang & Houbraken, Stud. Mycol. 93: 89. 2018. [MB 824435]. — Type: CBS H-23484. Ex-type: CBS 120095 = FMR 9050. Reproduction: sexual (easy to lose) and asexual. ITS barcode: LT993584 (alternative markers: LSU = LT993584; tub2 = LT993665; rpb2 = LT993503).
Humicola limonispora [as ‘limonisporum’] Z.F. Zhang & L. Cai, Persoonia 39: 15. 2017. [MB 840136]; basionym of Staphylotrichum limonisporum.
Humicola malaysiensis (D. Hawksw.) X.Wei Wang & Houbraken, Stud. Mycol. 93: 89. 2018. [MB 824437]. Basionym: Farrowia malaysiensis. — Type: IMI 183184. Ex-type: CBS 399.97 = IMI 183184 = MUCL 39402. Reproduction: sexual & asexual. ITS barcode: LT993586 (alternative markers: LSU = LT993586; tub2 = LT993667; rpb2 = LT993505).
Humicola mutabilis X.Wei Wang & Houbraken, Stud. Mycol. 93: 93. 2018. [MB 824438]. — Type: CBS H-23485. Ex-type: CBS 779.71. Reproduction: sexual & asexual. ITS barcode: LT993588 (alternative markers: LSU = LT993588; tub2 = LT993669; rpb2 = LT993507).
Humicola olivacea X.Wei Wang & Samson, Stud. Mycol. 84: 203. 2016. [MB 818848]. — Type: CBS H-22848. Ex-type: CBS 142031. Reproduction: asexual. ITS barcode: LT993589 (alternative markers: LSU = LT993589; tub2 = LT993670; rpb2 = LT993508).
Humicola pinnata (L.M. Ames) X.Wei Wang & Houbraken, Stud. Mycol. 93: 96. 2018. [MB 824440]. Basionym: Chaetomium pinnatum. — Type: BPI 580625. Ex-type: CBS 467.66. Reproduction: sexual & asexual. ITS barcode: LT993590 (alternative markers: LSU = LT993590; tub2 = LT993671; rpb2 = LT993509).
Humicola pulvericola X.Wei Wang et al., Stud. Mycol. 93: 96. 2018. [MB 824444]; basionym of Pseudohumicola pulvericola.
Humicola quadrangulata X.Wei Wang & Houbraken, Stud. Mycol. 93: 96. 2018. [MB 825446]. — Type: CBS H-23487. Ex-type: CBS 111771. Reproduction: sexual & asexual. ITS barcode: LT993593 (alternative markers: LSU = LT993593; tub2 = LT993674; rpb2 = LT993512).
Humicola seminuda (L.M. Ames) X.Wei Wang & Houbraken, Stud. Mycol. 93: 100. 2018. [MB 824447]. Basionym: Chaetomium seminudum. — Type: figs 23–29 in Ames, Mycologia 41: 643, 1949 (lectotype); CBS H-23488 (epitype). Ex-epitype: CBS 368.84. Reproduction: sexual & asexual. ITS barcode: LT993594 (alternative markers: LSU = LT993594; tub2 = LT993675; rpb2 = LT993513).
Humicola semispiralis (Udagawa & Cain) X.Wei Wang & Houbraken, Stud. Mycol. 93: 100. 2018. [MB 824448]; synonym of Pseudohumicola semispiralis (based on Chaetomium semispirale).
Humicola sphaeralis (Chivers) X.Wei Wang & Houbraken, Stud. Mycol. 93: 100. 2018. [MB 824449]. Basionym: Chaetomium sphaerale. — Type: NY01050440. Ex-type: CBS 985.87. Reproduction: sexual & asexual. ITS barcode: LT993598 (alternative markers: LSU = LT993598; tub2 = LT993679; rpb2 = LT993517).
Humicola subspiralis (Chivers) X.Wei Wang & Houbraken, Stud. Mycol. 93: 104. 2018. [MB 824450]; synonym of Pseudohumicola subspiralis.
Humicola udagawae (Sergejeva ex Udagawa) X.Wei Wang & Houbraken, Stud. Mycol. 93: 104. 2018. [MB 824451]. Basionym: Chaetomium udagawae. — Type: NHL 2259. Ex-type: CBS 337.68 = NHL 2259. Reproduction: sexual & asexual. ITS barcode: LT993601 (alternative markers: LSU = LT993601; tub2 = LT993682; rpb2 = LT993520).
Humicola wallefii (J.A. Mey. & Lanneau) X.Wei Wang & Houbraken, Stud. Mycol. 93: 107. 2018. [MB 824452]. Basionym: Chaetomium wallefii. — Type: n/a. Ex-type: CBS 147.67 = IMI 126039. Reproduction: sexual (easy to lose) and asexual. ITS barcode: LT993602 (alternative markers: LSU = LT993602; tub2 = LT993683; rpb2 = LT993521).
Hyalosphaerella
Hyalosphaerella fragilis (Natarajan) X.Wei Wang & Houbraken, Stud. Mycol. 93: 205. 2019. [MB 829865]. Basionym: Chaetomidium fragile. — Type: n/a. Ex-type: CBS 456.73 = IMI 169641. Reproduction: sexual. ITS barcode: KX976693 (alternative markers: LSU = KX976791; tub2 = KX977042; rpb2 = MK876779).
Madurella
Madurella fahalii de Hoog et al., J. Clin. Microbiol. 50: 991. 2012. [MB 560128]. — Type: CBS H-20690. Ex-type: CBS 129176. Reproduction: asexual/sterile. ITS barcode: MK926819 (alternative markers: LSU = MK926819; tub2 = MK926919; rpb2 = MK876780).
Madurella mycetomatis (Laveran) Brumpt, Compt.-Rend. Séances Mém. Soc. Biol. 58: 997. 1905. [MB 535193]. Basionym: Streptothrix mycetomatis. — Type: CBS 109801 (neotype, de Hoog et al. 2004). Ex-neotype: CBS 109801. Reproduction: asexual/sterile. ITS barcode: MK926820 (alternative markers: LSU = MK926820; tub2 = MK926920; rpb2 = MK876781).
Madurella pseudomycetomatis Yan et al. ex de Hoog et al., J. Clin. Microbiol. 50: 991. 2012. [MB 509682]. — Type: CBS H-20691. Ex-type: CBS 129177. Reproduction: asexual/sterile. ITS barcode: MK926821 (alternative markers: LSU = MK926821; tub2 = MK926921; rpb2 = MK876782).
Madurella tropicana de Hoog et al., J. Clin. Microbiol. 50: 993. 2012. [MB 800571]. — Type: CBS H-20692. Ex-type: CBS 201.38. Reproduction: asexual/sterile. ITS barcode: MK926824 (alternative markers: LSU = MK926824; tub2 = MK926924; rpb2 = MK876785).
Magnusia (Microascales, Sordariomycetes)
Magnusia spirotricha R.K. Benj., Aliso 3: 199. 1955. [MB 300049]; basionym of Botryotrichum spirotrichum.
Melanocarpus
Melanocarpus albomyces (Cooney & R. Emers.) Arx, Stud. Mycol. 8: 17. 1975. [MB 317449]. Basionym: Myriococcum albomyces. — Type: UPS F-646091. Ex-type: CBS 638.94 = ATCC 16460 = CBS 177.67 = IMI 126326. Reproduction: sexual & asexual. ITS barcode: KX976679 (alternative markers: LSU = KX976773; tub2 = KX977021; rpb2 = KX976886). Note: A specimen of Cooney and Emersons material is deposited in Uppsala University, Museum of Evolution (UPS:BOT) under UPS F-646091.
Melanocarpus oblatus Guarro & Aa, Persoonia 13: 270. 1987. [MB 132107]. — Type: CBS 775.85. Ex-type: CBS 775.85. Reproduction: sexual. ITS barcode: MZ334727 (alternative markers: LSU = n/a; tub2 = MZ343031; rpb2 = MZ342992). Note: This species is a synonym of Achaetomium globosum.
Melanocarpus tardus X.Wei Wang & Samson, Stud. Mycol. 84: 205. 2016. [MB 818849]; basionym of Parvomelanocarpus tardus.
Melanocarpus thermophilus (Abdullah & Al-Bader) Guarro et al., Mycol. Res. 100: 75. 1996. [MB 413444]; synonym of Parvomelanocarpus thermophilus.
Microthielavia
Microthielavia ovispora (Pidopl. et al.) X.Wei Wang & Houbraken, Stud. Mycol. 93: 208. 2019. [MB 829867]. Basionym: Thielavia ovispora. — Type: Instituto Microbiol. et Virusol. Acad. Sci., Ucrainae (Kiovia) sub N 52128. Ex-type: CBS 165.75 = IMI 196525 = VKM F-1596. Reproduction: sexual. ITS barcode: MK926826 (alternative markers: LSU = MK926826; tub2 = MK926926; rpb2 = MK876787).
Myceliophthora
Myceliophthora fergusii (Klopotek) Oorschot, Persoonia 9: 406. 1977. [MB 317954]; synonym of Thermothelomyces fergusii.
Myceliophthora guttulata Yu Zhang & L. Cai, Mycol. Prog. 13: 168. 2013. [MB 80233]; basionym of Thermothelomyces guttulatus [as ‘guttulata’].
Myceliophthora heterothallica (Klopotek) van den Brink & Samson, Fungal Diversity 52: 206. 2011 [2012], nom. inval., Art. 41.5. [MB 519538]; synonym of Th. heterothallicus.
Myceliophthora hinnulea Awao & Udagawa, Mycotaxon 16: 436. 1983. [MB 109090]; basionym of Thermothelomyces hinnuleus [as ‘hinnulea’].
Myceliophthora lutea Costantin, Compt. Rend. Hebd. Séances Acad. Sci. 114: 850. 1892. [MB 232833]. — Type: CBS 145.77 (neotype). Ex-neotype: CBS 145.77 = IMI 182034. Reproduction: asexual. ITS barcode: HQ871775(alternative markers: LSU = KM655351; tub2 = KX977026; rpb2 = KM655395).
Myceliophthora novoguineensis (Udagawa & Y. Horie) van den Brink & Samson, Fungal Diversity 52: 206. 2011 [2012], nom. inval., Art. 41.5. [MB 561526]; synonym of Corynascus novoguineensis.
Myceliophthora officinarum M. Raza & L. Cai, Fungal Diversity 99: 89. 2019. [MB 556681]; basionym of Arxotrichum officinarum.
Myceliophthora sepedonium (C.W. Emmons) van den Brink & Samson, Fungal Diversity 52: 206. 2011 [2012], nom. inval., Art. 41.5. [MB 561525]; synonym of Corynascus sepedonium.
Myceliophthora sexualis (Stchigel et al.) van den Brink & Samson, Fungal Diversity 52: 206. 2011 [2012], nom. inval., Art. 41.5. [MB 561527]; synonym of Corynascus sexualis.
Myceliophthora similis (Stchigel et al.) van den Brink & Samson, Fungal Diversity 52: 206. 2011 [2012], nom. inval., Art. 41.5. [MB 561528]; synonym of Corynascus similis.
Myceliophthora verrucosa (Stchigel et al.) van den Brink & Samson, Fungal Diversity 52: 206. 2011 [2012], nom. inval., Art. 41.5. [MB 561529]; synonym of Corynascus verrucosus.
Mycothermus
Mycothermus thermophiloides X.Wei Wang & Houbraken, Stud. Mycol. 93: 107. 2018. [MB 824455]. — Type: CBS H-23489. Ex-type: CBS 183.81. Reproduction: asexual. ITS barcode: LT993603 (alternative markers: LSU = LT993603; tub2 = LT993684; rpb2 = LT993522).
Mycothermus thermophilus (Cooney & R. Emers.) X.Wei Wang et al., Stud. Mycol. 93: 107. 2018. [MB 824454]. Basionym: Torula thermophila. — Type: UC 1206525. Ex-type: CBS 625.91 = ATCC 16463. Reproduction: asexual. ITS barcode: LT993604 (alternative markers: LSU = LT993604; tub2 = LT993685; rpb2 = LT993523).
Myriococcum (Atheliales, Agaricomycetes)
Myriococcum albomyces Cooney & R. Emers., Thermophilic Fungi: 60. 1964. [MB 335011]; basionym of Melanocarpus albomyces.
Myriococcum thermophilum (Fergus) Aa, Verh. Kon. Ned. Akad. Wetensch., Afd. Natuurk., Sect. 2 61(4): 60. 1973. [MB 318413]; synonym of Thermothelomyces myriococcoides.
Ovatospora
Ovatospora amygdalispora (Udagawa & T. Muroi) X.Wei Wang & Houbraken, this study. [MB 840155]. Basionym: Chaetomium amygdalisporum. — Type: NHL 2874. Ex-type: CBS 672.82 = IMI 291735 = NHL 2874. Reproduction: sexual. ITS barcode: n/a (alternative markers: LSU = n/a; tub2 = MZ343030; rpb2 = MZ342991).
Ovatospora angularis (Yu Zhang & L. Cai) X.Wei Wang & Houbraken, this study. [MB 840156]. Basionym: Chaetomium angulare. — Type: HMAS 245780. Ex-type: CGMCC 3.17537. Reproduction: sexual. ITS barcode: KP336763 (alternative markers: LSU = KP336812; tub2 = KP336861; rpb2 = KT149486).
Ovatospora brasiliensis (Bat. & Pontual) X.Wei Wang & Samson, Stud. Mycol. 84: 207. 2016. [MB 818851]. Basionym: Chaetomium brasiliense. — Type: n/a. Representative strain: CBS 140.50 = IMI 031638 = MUCL 9590. Reproduction: sexual. ITS barcode: KX976683 (alternative markers: LSU = KX976781; tub2 = KX977031; rpb2 = KX976896).
Ovatospora medusarum (J.A. Mey. & Lanneau) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 818871]. Basionym: Chaetomium medusarum. — Type: n/a. Ex-type: CBS 148.67 = IMI 126040 = IMI 126040ii = MUCL 10171. Reproduction: sexual. ITS barcode: KX976684 (alternative markers: LSU = KX976782; tub2 = KX977032; rpb2 = KX976897).
Ovatospora mollicella (L.M. Ames) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 818873]. Basionym: Chaetomium mollicellum. — Type: BPI. Ex-type: CBS 583.83. Reproduction: sexual. ITS barcode: KX976685 (alternative markers: LSU = KX976783; tub2 = KX977033; rpb2 = KX976898). Notes: Ames’ type of Ch. mollicellum is probably maintained in BPI. Specimen BPI 580521 is not labeled as type, but could well be used for the species description. More work is needed to eludicate the status of this specimen.
Ovatospora pseudomollicella X.Wei Wang & Samson, Stud. Mycol. 84: 207. 2016. [MB 818852]. — Type: CBS H-22850. Ex-type: CBS 251.75. Reproduction: sexual. ITS barcode: KX976686 (alternative markers: LSU = KX976784; tub2 = KX977034; rpb2 = KX976899).
Ovatospora senegalensis (L.M. Ames) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 818874]. Basionym: Chaetomium senegalense. — Type: BPI. Ex-type: CBS 728.84. Reproduction: sexual. ITS barcode: KX976687 (alternative markers: LSU = KX976785; tub2 = KX977035; rpb2 = KX976900). Notes: Three specimens are labeled as type: BPI 1100707, BPI 1101433 (collected in 1974, so should not be the type material) and BPI 580647. More work is needed to eludicate the status of these specimens.
Ovatospora unipora (Aue & E. Müll.) X.Wei Wang & Samson, Stud. Mycol. 84: 217. 2016. [MB 818875]. Basionym: Chaetomium uniporum. — Type: ETH 7503. Ex-type: CBS 109.83. Reproduction: sexual. ITS barcode: KX976689 (alternative markers: LSU = KX976787; tub2 = KX977037; rpb2 = KX976902).
Paecilomyces (Eurotiales, Eurotiomycetes)
Paecilomyces biformis Z.Q. Liang et al., Fungal Diversity 27: 97. 2007. [MB 510977]; basionym of Acrophialophora biformis.
Paecilomyces cinereus Z.Q. Liang et al., Mycotaxon 97: 16. 2006. [MB 501355]; basionym of Acrophialophora cinerea.
Paecilomyces curticatenatus Z.Q. Liang & Y.F. Han, Mycosystema 26: 14. 2007. [MB 510908]; basionym of Acrophialophora curticatenata.
Paecilomyces furcatus Z.Q. Liang et al., Mycotaxon 97: 16. 2006. [MB 501356]; basionym of Acrophialophora furcata.
Paecilomyces fusisporus S.B. Saksena, J. Indian Bot. Soc. 32: 186. 1953. [MB 302189]; basionym of Acrophialophora fusispora.
Paecilomyces inflatus var. major Z.Q. Liang et al., J. Fungal Res. 2: 43. 2004. [MB 509628]; basionym of Acrophialophora major.
Papulaspora (Melanosporales, Sordariomycetes)
Papulaspora thermophila Fergus, Mycologia 63: 426. 1971. [MB 319160]; synonym of Thermothelomyces myriococcoides.
Parachaetomium
Parachaetomium biporatum (Cano & Guarro) X.Wei Wang & Houbraken, this study. [MB 830926]. Basionym: Chaetomium biporatum. — Type: FMR 854. Ex-type: CBS 244.86 = FMR 854 = IMI 330348. Reproduction: sexual. ITS barcode: MK919303 (alternative markers: LSU = MK919303; tub2 = MK919417; rpb2 = MK919360).
Parachaetomium carinthiacum (Sörgel) Mehrabi et al., Mycol. Prog. 19: 1422. 2020. [MB 835858]. Basionym: Chaetomium carinthiacum. — Type: Abb. 7a and b in Sörgel, Arch. Mikrobiol. 40: 392, 1961 (lectotype), CBS H-10007 (epitype). Ex-epitype: CBS 153.81. Reproduction: sexual. ITS barcode: HM365265 (alternative markers: LSU = HM365265; tub2 = HM365299; rpb2 = MT568847).
Parachaetomium hispanicum (Guarro & Arx) X.Wei Wang & Houbraken, this study. [MB 830927]. Basionym: Chaetomium hispanicum. — Type: CBS 234.82. Ex-type: CBS 234.82. Reproduction: sexual. ITS barcode: MK919304 (alternative markers: LSU = MK919304; tub2 = MK919418; rpb2 = MK919361).
Parachaetomium inaequale (Pidopl. et al., X.Wei Wang & Houbraken, this study. [MB 830928]. Basionym: Thielavia inaequalis. — Type: Instituto Microbiol. et Virusol. Acad. Sci., Ucrainae (Kiovia) sub N 55042. Ex-type: CBS 331.75 = IMI 196527 = VKM F-1922. Reproduction: sexual. ITS barcode: MK919306 (alternative markers: LSU = MK919306; tub2 = MK919420; rpb2 = MK919363).
Parachaetomium iranianum (Asgari & Zare) Mehrabi et al., Mycol. Prog. 19: 1422. 2020. [MB 835856]. Basionym: Chaetomium iranianum. — Type: IRAN 14609F. Ex-type: IRAN 861C = CBS 126670. Reproduction: sexual. ITS barcode: HM365257 (alternative markers: LSU = HM365257; tub2 = HM365297; rpb2 = MT568848); synonym of Parachaetomium perlucidum.
Parachaetomium longiciliatum (Yu Zhang & L. Cai) X.Wei Wang & Houbraken, this study. [MB 840157]. Basionym: Chaetomium longiciliatum [as ‘longiciliata’]. — Type: HMAS 245782. Ex-type: CGMCC 3.17554. Reproduction: sexual. ITS barcode: KP336774 (alternative markers: LSU = KP336823; tub2 = KP336872; rpb2 = KT149497).
Parachaetomium mareoticum (Besada & Yusef) X.Wei Wang & Houbraken, this study. [MB 840158]. Basionym: Chaetomium mareoticum. — Type: IMI 78435. Representative strain: CBS 802.83. Reproduction: sexual. ITS barcode: MZ334723 (alternative markers: LSU = MZ351426; tub2 = MZ343036; rpb2 = MZ342997).
Parachaetomium muelleri (Arx) X.Wei Wang & Houbraken, this study. [MB 830925]. Basionym: Chaetomium muelleri. — Type: CBS H-6879. Ex-type: CBS 192.84. Reproduction: sexual. ITS barcode: MK919300 (alternative markers: LSU = MK919300; tub2 = MK919414; rpb2 = MK919357).
Parachaetomium multispirale (A. Carter et al.) X.Wei Wang & Houbraken, this study. [MB 840159]. Basionym: Chaetomium multispirale. — Type: TRTC 66.609f. Ex-type: CBS 172.84 = TRTC 66609. Reproduction: sexual. ITS barcode: MH861718 (alternative markers: LSU = n/a; tub2 = MZ343017; rpb2 = MZ342978).
Parachaetomium perlucidum (Sergejeva) X.Wei Wang & Houbraken, this study. [MB 830930]. Basionym: Chaetomium perlucidum. — Type: –; CBS H-6885 (isotype). Ex-type: CBS 141.58 = IMI 074954 = MUCL 18693 = MUCL 39399 = VKM F-1950. Reproduction: sexual. ITS barcode: MK919308 (alternative markers: LSU = MK919308; tub2 = MK919422; rpb2 = MK919365).
Parachaetomium subspirilliferum (Sergejeva) X.Wei Wang & Houbraken, this study. [MB 830931]. Basionym: Chaetomium subspirilliferum. — Type: CBS H-6894; CBS H-6895 (isotype). Ex-type: CBS 150.60 = ATCC 14534 = IMI 081771 = MUCL 18698 = VKM F-1943. Reproduction: sexual. ITS barcode: MK919312 (alternative markers: LSU = MK919312; tub2 = MK919426; rpb2 = MK919369).
Parachaetomium truncatulum (Asgari & Zare) Mehrabi et al., Mycol. Prog. 19: 1422. 2020. [MB 835857]. Basionym: Chaetomium truncatulum. — Type: IRAN 14610F. Ex-type: CBS 126782 = IRAN 918C. Reproduction: sexual. ITS barcode: HM365263 (alternative markers: LSU = HM365263; tub2 = HM365298; rpb2 = MT568849).
Parathielavia
Parathielavia appendiculata (M.P. Srivast. et al.) X.Wei Wang & Houbraken, Stud. Mycol. 93: 210. 2019. [MB 829869]. Basionym: Thielavia appendiculata. — Type: IMI 104944. Ex-type: CBS 723.68 = IMI 104944. Reproduction: sexual. ITS barcode: MK926827 (alternative markers: LSU = MK926827; tub2 = MK926927; rpb2 = MK876788).
Parathielavia coactilis (Nicot) X.Wei Wang & Houbraken, this study. [MB 840160]. Basionym: Thielavia coactilis. — Type: PC 1644. Representative strain: CBS 101190 = TRTC 52103. Reproduction: sexual. ITS barcode: n/a (alternative markers: LSU = n/a; tub2 = MZ343003; rpb2 = MZ342962).
Parathielavia hyrcaniae (Nicot) X.Wei Wang & Houbraken, Stud. Mycol. 93: 210. 2019. [MB 829870]. Basionym: Thielavia hyrcaniae. — Type: PC 1645. Ex-type: CBS 353.62 = IFO 8807 = LCP 1645. Reproduction: sexual. ITS barcode: KM655329(alternative markers: LSU = KM655368; tub2 = KX977043; rpb2 = KM655401).
Parathielavia kuwaitensis (Moustafa) X.Wei Wang & Houbraken, Stud. Mycol. 93: 210. 2019. [MB 829871]. Basionym: Thielavia kuwaitensis. — Type: CBS H-7848. Ex-type: CBS 945.72. Reproduction: sexual & asexual. ITS barcode: KM655332 (alternative markers: LSU = KM655371; tub2 = KX977044; rpb2 = KM655404).
Parvomelanocarpus
Parvomelanocarpus tardus (X.Wei Wang & Samson) X.Wei Wang & Houbraken, this study. [MB 840152]. Basionym: Melanocarpus tardus. — Type: CBS H-22849. Ex-type: CBS 541.76. Reproduction: sexual. ITS barcode: KX976681 (alternative markers: LSU = KX976775; tub2 = KX977023; rpb2 = KX976888).
Parvomelanocarpus thermophilus (Abdullah & Al-Bader) X.Wei Wang & Houbraken, this study. [MB 840167]. Basionym: Thielavia minuta var. thermophila. — Type: BSR 1006. Representative strain: CBS 886.97 = FMR 6190. Reproduction: sexual. ITS barcode: KM655350 (alternative markers: LSU = MH874288; tub2 = MZ343037; rpb2 = KM655434).
Pseudocanariomyces (synonym of Allocanariomyces; this study).
Pseudocanariomyces americanus Cañete-Gibas et al., Mycopathologia 186: 443. 2021. [MB 839083]; basionym of Allocanariomyces americanus.
Pseudohumicola
Pseudohumicola atrobrunnea (X.Wei Wang et al.) X.Wei Wang et al., this study. [MB 840148]. Basionym: Humicola atrobrunnea. — Type: CBS H-23481. Ex-type: HSAUP II 05-1004 = CBS 114167. Reproduction: asexual. ITS barcode: LT993570 (alternative markers: LSU = LT993570; tub2 = LT993651; rpb2 = LT993489).
Pseudohumicola pulvericola (X.Wei Wang et al.) X.Wei Wang et al., this study. [MB 840149]. Basionym: Humicola pulvericola. — Type: CBS H-23486. Ex-type: CBS 144165. Reproduction: asexual. ITS barcode: LT993591 (alternative markers: LSU = LT993591; tub2 = LT993672; rpb2 = LT993510).
Pseudohumicola semispiralis (Udagawa & Cain) X.Wei Wang et al., this study. [MB 840150]. Basionym: Chaetomium semispirale. — Type: TRTC 30103. Ex-type: CBS 723.97 = IMI 250972 = MUCL 40089. Reproduction: sexual & asexual. ITS barcode: LT993597 (alternative markers: LSU = LT993597; tub2 = LT993678; rpb2 = LT993516).
Pseudohumicola subspiralis (Chivers) X.Wei Wang et al., this study. [MB 840151]. Basionym: Chaetomium subspirale. — Type: NY01050446. Representative strain: CBS 148.58 = IMI 075855. Reproduction: sexual & asexual. ITS barcode: LT993599 (alternative markers: LSU = LT993599; tub2 = LT993680; rpb2 = LT993518).
Pseudothielavia
Pseudothielavia arxii (Stchigel & Guarro) X.Wei Wang & Houbraken, Stud. Mycol. 93: 213. 2019. [MB 829873]. Basionym: Thielavia arxii. — Type: IMI 374725. Ex-type: CBS 603.97 = FMR 5875. Reproduction: sexual. ITS barcode: MK926830 (alternative markers: LSU = MK926830; tub2 = MK926930; rpb2 = MK876791). Note: This species is phylogenetically close to Pseudothielavia terricola, though phenotypically distinct.
Pseudothielavia hamadae (Udagawa) X.Wei Wang & Houbraken, Stud. Mycol. 93: 213. 2019. [MB 829874]. Basionym: Achaetomium hamadae. — Type: NHL 2910. Ex-type: CBS 499.83 = IMI 288714ii = NHL 2910. Reproduction: sexual. ITS barcode: MK926832 (alternative markers: LSU = MK926832; tub2 = MK926932; rpb2 = MK876793).
Pseudothielavia subhyaloderma X.Wei Wang & Houbraken, Stud. Mycol. 93: 217. 2019. [MB 829875]. — Type: CBS H-6866. Ex-type: CBS 473.86 = TRTC 36863. Reproduction: sexual. ITS barcode: MK926833 (alternative markers: LSU = MK926833; tub2 = MK926933; rpb2 = MK876794).
Pseudothielavia terricola (J.C. Gilman & E.V. Abbott) X.Wei Wang & Houbraken, Stud. Mycol. 93: 217. 2019. [MB 829876]. Basionym: Coniothyrium terricola. — Type: fig. 17 in Gilman & Abbott, Iowa St. Coll. J. Sci. 1(3): 267, 1927 (lectotype); CBS H-24049 (epitype). Ex-epitype: CBS 165.88 = TRTC 50997. Reproduction: sexual. ITS barcode: KX976694 (alternative markers: LSU = KX976792; tub2 = KX977045; rpb2 = MK876795).
Remersonia
Remersonia tenuis X.Wei Wang et al., Stud. Mycol. 93: 111. 2018. [MB 824456]. — Type: CBS H-18610. Ex-type: CBS 784.85 = IMI 295313. Reproduction: asexual. ITS barcode: LT993609 (alternative markers: LSU = LT993609; tub2 = LT993690; rpb2 = LT993528).
Remersonia thermophila (Fergus) Seifert & Samson, Canad. J. Bot. 75: 1160. 1997. [MB 437277]. Basionym: Stilbella thermophila. — Type: PAC. Ex-type: ATCC 22073; Representative strain: CBS 645.91. Reproduction: asexual. ITS barcode: JF412016 (alternative markers: LSU = n/a; tub2 = LT993692; rpb2 = KF958020).
Scytalidium (Helotiales, Leotiomycetes)
Scytalidium thermophilum (Cooney & R. Emers.) Austwick, New Zealand J. Agric. Res. 19: 29. 1976. [MB 123497]; synonym of Mycothermus thermophilus.
Sphaeria
Sphaeria crispata Fuckel, Fungi Rhen. Exs., Suppl. Fasc. 6: no 2022. 1867. [MB 165726]; basionym of Trichocladium crispatum.
Sporotrichum (Agaricomycotina, Basidiomycota)
Sporotrichum thermophilum Apinis, Nova Hedwigia 5: 74. 1963. [MB 344529]; basionym of Thermothelomyces thermophilus [as ‘thermophila’].
Staphylotrichum
Staphylotrichum acaciicola X.Wei Wang & Houbraken, Stud. Mycol. 93: 113. 2018. [MB 824457]. — Type: CBS H-23490. Ex-type: CBS 281.65. Reproduction: asexual. ITS barcode: LT993613 (alternative markers: LSU = LT993613; tub2 = LT993694; rpb2 = LT993532).
Staphylotrichum boninense Nonaka et al., Mycoscience 53: 315. 2012. [MB 561191]. — Type: TNS-F-41734. Ex-type: JCM 17908; Representative strain: CBS 112059. Reproduction: asexual. ITS barcode: LT993616 (alternative markers: LSU = LT993616; tub2 = LT993697; rpb2 = LT993535).
Staphylotrichum brevistipitatum X.Wei Wang & Houbraken, Stud. Mycol. 93: 118. 2018. [MB 824458]. — Type: CBS H-18521. Ex-type: CBS 408.67. Reproduction: asexual. ITS barcode: LT993619 (alternative markers: LSU = LT993619; tub2 = LT993700; rpb2 = LT993538).
Staphylotrichum coccosporum J.A. Mey. & Nicot, Bull. Trimestriel Soc. Bot. France 72: 323. 1957. [MB 306413]. — Type: n/a. Ex-type: CBS 364.58 = CBS 293.55 = IMI 57899. Reproduction: asexual. ITS barcode: LT993620 (alternative markers: LSU = LT993620; tub2 = LT993701; rpb2 = LT993539).
Staphylotrichum koreanum (Hyang B. Lee & T.T.T. Nguyen) X.Wei Wang & Houbraken, this study. [MB 840161]. Basionym: Humicola koreana. — Type: EML-UD33-1. Ex-type: JMRC:SF:012183. Reproduction: asexual. ITS barcode: KU058192 (alternative markers: LSU = KU058190; tub2 = n/a; rpb2 = n/a).
Staphylotrichum limonisporum (Z.F. Zhang & L. Cai) X.Wei Wang & Houbraken, this study. [MB 840162]. Basionym: Humicola limonispora. — Type: HMAS 246922. Ex-type: CGMCC 3.17914. Reproduction: sexual & asexual. ITS barcode: KU746672 (alternative markers: LSU = KU746718; tub2 = KU746764; rpb2 = KY575867).
Staphylotrichum longicolle [as ‘longicolleum’] (Krzemien. & Badura) X.Wei Wang & Houbraken, Stud. Mycol. 93: 122. 2018. [MB 827915]. Basionym: Chaetomium longicolle [as ‘longicolleum’]. — Type: n/a. Representative strain: CBS 119.57. Reproduction: sexual. ITS barcode: LT993621 (alternative markers: LSU = LT993621; tub2 = LT993702; rpb2 = LT993540).
Staphylotrichum microascosporum X.Wei Wang & Houbraken, Stud. Mycol. 93: 122. 2018. [MB 824460]. — Type: CBS H-12643. Ex-type: CBS 184.79. Reproduction: sexual. ITS barcode: LT993624 (alternative markers: LSU = LT993624; tub2 = LT993705; rpb2 = LT993543).
Staphylotrichum sinense M. Qiao et al., Int. J. Syst. Evol. Microbiol. 71 (3, no. 004747): 2. 2021. [MB 832671]. — Type: YMFT 1.05760. Ex-type: YMF 1.05760 = CGMCC3.19631. Reproduction: asexual. ITS barcode: MN271027 (alternative markers: LSU = MN271026; tub2 = MN340040; rpb2 = MN233643).
Staphylotrichum tortipilum X.Wei Wang & Houbraken, Stud. Mycol. 93: 126. 2018. [MB 824461]. — Type: CBS H-12642. Ex-type: CBS 103.79. Reproduction: sexual. ITS barcode: LT993625 (alternative markers: LSU = LT993625; tub2 = LT993706; rpb2 = LT993544).
Stellatospora
Stellatospora terricola Tad. Ito & Nakagiri, Mycoscience 35: 413. 1994. [MB 414193]. — Type: IFO H-12166. Ex-type: CBS 811.95 = IFO 32597. Reproduction: sexual. ITS barcode: MK926835 (alternative markers: LSU = MK926835; tub2 = MK926935; rpb2 = MK876797).
Stilbella (Hypocreales, Sordariomycetes)
Stilbella thermophila Fergus, Mycologia 56: 277. 1964. [MB 339742]; basionym of Remersonia thermophila.
Stolonocarpus
Stolonocarpus gigasporus (Mustafa & Abdel-Azeem) X.Wei Wang & Houbraken, Stud. Mycol. 93: 221. 2019. [MB 829878]. Basionym: Thielavia gigaspora. — Type: IMI 39131. Ex-type: CBS 112062 = IMI 39131. Reproduction: sexual. ITS barcode: MK926836 (alternative markers: LSU = MK926836; tub2 = MK926936; rpb2 = MK876798).
Streptothrix
Streptothrix mycetomatis Laveran, Bull. Acad. Méd. Paris, ser. 3, 47: 776. 1902. [MB 492359]; basionym of Madurella mycetomatis.
Subramaniula
Subramaniula anamorphosa (S.A. Ahmed et al.) X.Wei Wang & Samson, Stud. Mycol. 84: 220. 2016. [MB 818876]. Basionym: Chaetomium anamorphosum. — Type: CBS H-21973. Ex-type: CBS 137114. Reproduction: asexual. ITS barcode: KP862598 (alternative markers: LSU = KP970641; tub2 = KP900704; rpb2 = KP900667).
Subramaniula asteroides S.A. Ahmed et al., Fungal Diversity 76: 20. 2015. [MB 810427]. — Type: CBS H-21971. Ex-type: CBS 123294. Reproduction: asexual. ITS barcode: HQ906667 (alternative markers: LSU = JX280731; tub2 = KP900703; rpb2 = KP900666).
Subramaniula cristata (L.M. Ames) X.Wei Wang & Samson, Stud. Mycol. 84: 212. 2016. [MB 818853]. Basionym: Chaetomium cristatum. — Type: ISC-F-0123561. Ex-type: CBS 156.52 = ATCC 11201 = DSM 3702. Reproduction: sexual. ITS barcode: KX976690 (alternative markers: LSU = KX976788; tub2 = KX977038; rpb2 = KX976903).
Subramaniula cuniculorum (Fuckel) X.Wei Wang & Samson, Stud. Mycol. 84: 220. 2016. [MB 818877]. Basionym: Chaetomium cuniculorum. — Type: Fuckel, Fungi Rhen. 1961, e.g., HAL, S-F267436. Representative strain: CBS 800.83. Reproduction: sexual. ITS barcode: KX976692 (alternative markers: LSU = KX976790; tub2 = KX977040; rpb2 = KX976905).
Subramaniula flavipila X.Wei Wang & Samson, Stud. Mycol. 84: 220. 2016. [MB 818878]. Replaced synonym: Chaetomium irregulare. — Type: B 505 (holotype); CBS H-6876 (isotype). Ex-type: CBS 446.66 = IMI 153340. Reproduction: sexual. ITS barcode: KP862600 (alternative markers: LSU = KP970647; tub2 = KP900706; rpb2 = KP900669).
Subramaniula fusispora (G. Sm.) X.Wei Wang & Samson, Stud. Mycol. 84: 220. 2016. [MB 818879]. Basionym: Chaetomium fusisporum. — Type: LSHTM BB382. Ex-type: CBS 166.61 = IMI 086560. Reproduction: sexual. ITS barcode: MH858011 (alternative markers: LSU = MH869571; tub2 = MZ343015; rpb2 = MZ342976).
Subramaniula lateralis (Yu Zhang & L. Cai) X.Wei Wang & Houbraken, this study. [MB 840164]. Basionym: Chaetomium laterale. — Type: HMAS 245785. Ex-type: CGMCC 3.17547. Reproduction: sexual. ITS barcode: KP336789 (alternative markers: LSU = KP336838; tub2 = KP336887; rpb2 = MZ342998).
Subramaniula latifusispora X.Wei Wang et al., this study. [MB 840129]. — Type: HMAS 350267. Ex-type: CGMCC 3.20442 = WXW 8538. Reproduction: sexual. ITS barcode: MZ334728 (alternative markers: LSU = MZ351428; tub2 = MZ343040; rpb2 = MZ343001).
Subramaniula obscura S.A. Ahmed et al., Fungal Diversity 76: 21. 2015. [MB 810428]. — Type: CBS H-21972. Ex-type: CBS 132916. Reproduction: asexual. ITS barcode: KP862595 (alternative markers: LSU = KP970653; tub2 = KP900700; rpb2 = KP900662).
Subramaniula thielavioides (Arx et al.) Arx, Proc. Indian Acad. Sci. Sect. B 94: 344. 1985. [MB 105812]. Basionym: Achaetomium thielavioides. — Type: CBS H-6628. Ex-type: CBS 122.78 = IMI 288625. Reproduction: sexual. ITS barcode: KP862597 (alternative markers: LSU = KP970654; tub2 = KP900708; rpb2 = KP900670).
Taifanglania (synonym of Acrophialophora)
Taifanglania biformis (Z.Q. Liang et al.) Z.Q. Liang et al., Fungal Diversity 34: 74. 2009. [MB 512815]; synonym of Acrophialophora biformis.
Taifanglania hechuanensis Z.Q. Liang et al., Fungal Diversity 34: 72. 2009. [MB 512804]; basionym of Acrophialophora hechuanensis.
Taifanglania jiangsuensis Y.F. Han & Z.Q. Liang, Mycotaxon 112: 328. 2010. [MB 516504]; basionym of Acrophialophora jiangsuensis.
Tengochaeta
Tengochaeta nigropilosa X.Wei Wang & Houbraken, this study. [MB 840130]. — Type: CBS H-24774. Ex-type: CBS 639.83. Reproduction: sexual. ITS barcode: MZ334730 (alternative markers: LSU = n/a; tub2 = MZ343029; rpb2 = MZ342990).
Thermocarpiscus
Thermocarpiscus australiensis (Tansey & M.A. Jack) X.Wei Wang & Houbraken, this study. [MB 840165]. Basionym: Thielavia australiensis. — Type: DAOM, microscope slide no. 3/19/74-8. Ex-type: CBS 493.74 = ATCC 28236 = DAOM 145919. Reproduction: sexual & asexual. ITS barcode: KM655339 (alternative markers: LSU = KM655378; tub2 = MZ343024; rpb2 = KM655419).
Thermochaetoides
Thermochaetoides dissita (Cooney & R. Emers.) X.Wei Wang & Houbraken, this study. [MB 830932]. Basionym: Chaetomium thermophilum var. dissitum. — Type: UC 1206513. Ex-type: CBS 180.67 = ATCC 16452 = DSM 1494 = IMI 126332. Reproduction: sexual. ITS barcode: MK919319 (alternative markers: LSU = MK919319; tub2 = MK919433; rpb2 = MK919375).
Thermochaetoides thermophila (La Touche) X.Wei Wang & Houbraken, this study. [MB 830933]. Basionym: Chaetomium thermophilum. — Type: IMI, anon. MRA112. Ex-type: CBS 144.50 = DAOM 24625 = DSM 1495 = IMI 039719. Reproduction: sexual. ITS barcode: MK919314 (alternative markers: LSU = MK919314; tub2 = MK919428; rpb2 = KM655436).
Thermomyces (Eurotiales, Eurotiomycetes)
Thermomyces verrucosus Pugh et al., Trans. Brit. Mycol. Soc. 47: 116. 1964. [MB 340048]; basionym of Botryotrichum verrucosum.
Thermothelomyces
Thermothelomyces fergusii X.Wei Wang & Houbraken, this study. [MB 830934]. Replaced synonym: Thielavia thermophila. — Type: PAC, Fergus & Sinden R46w1×R46w2. Ex-type: CBS 406.69 = ATCC 22067. Reproduction: asexual & sexual (heterothallic). ITS barcode: HQ871794 (alternative markers: LSU =KX976776; tub2 = KX977024; rpb2 = MK919378).
Thermothelomyces guttulatus [as ‘guttulata’] (Y. Zhang & L. Cai) Y. Marín et al., Mycologia 107: 630. 2015. [MB 823051]. Basionym: Myceliophthora guttulata. — Type: HMAS 244238. Ex-type: CGMCC 3.15185. Reproduction: asexual. ITS barcode: MK919323 (alternative markers: LSU = MK919323; tub2 = MK919437; rpb2 = MK919380).
Thermothelomyces heterothallicus [as ‘heterothallica’] (Klopotek) Y. Marín et al., Mycologia 107: 630. 2015. [MB 823052]. Basionym: Thielavia heterothallica. — Type: CBS H-18810 (holotype), CBS H-24878 (epitype). Ex-epitype: CBS 202.75. Reproduction: asexual & sexual (heterothallic). ITS barcode: HQ871771 (alternative markers: LSU = KM655354; tub2 = KX977025; rpb2 = KM655391).
Thermothelomyces hinnuleus [as ‘hinnulea’] (Awao & Udagawa) Y. Marín et al., Mycologia 107: 630. 2015. [MB 823053]. Basionym: Myceliophthora hinnulea. — Type: NHL AJ-6773. Ex-type: CBS 597.83 = ATCC 52474 = NHL 2909. Reproduction: asexual. ITS barcode: MK919327 (alternative markers: LSU = MK919327; tub2 = MK919441; rpb2 = MK919384).
Thermothelomyces myriococcoides (Fergus) X.Wei Wang & Houbraken, this study. [MB 830935]. Replaced synonym: Papulaspora thermophila. — Type: BPI 844852. Ex-type: CBS 389.93 = ATCC 22112. Reproduction: asexual. ITS barcode: MK919329 (alternative markers: LSU = MK919329; tub2 = MK919443; rpb2 = MK919386).
Thermothelomyces thermophilus [as ‘thermophila’] (Apinis) Y. Marín et al., Mycologia 107: 630. 2015. [MB 823054]. Basionym: Sporotrichum thermophilum. — Type: BDUN 274 (holotype); CBS H-7380, CBS H-7381 (isotypes). Ex-type: CBS 117.65 = BDUN 274. Reproduction: asexual. ITS barcode: MK919331 (alternative markers: LSU = MK919331; tub2 = MK919445; rpb2 = MK919387).
Thermothielavioides
Thermothielavioides terrestris (Apinis) X.Wei Wang & Houbraken, Stud. Mycol. 93: 223. 2019. [MB 829880]. Basionym: Allescheria terrestris. — Type: BDUN 278. Ex-type: CBS 117535 = CBS 355.66 = BDUN 278 = UAMH 3988. Reproduction: sexual & asexual. ITS barcode: MK926837 (alternative markers: LSU = MK926837; tub2 = MK926937; rpb2 = MK876799).
Thielavia (Melanosporales, Sordariomycetes)
Thielavia antarctica Stchigel & Guarro, Mycologia 95: 1225. 2004. [MB 489459]; basionym of Trichocladium antarcticum.
Thielavia appendiculata M.P. Srivast. et al., Mycopathol. Mycol. Appl. 30: 205. 1966. [MB 340050]; basionym of Parathielavia appendiculata.
Thielavia arenaria Mouch., Bull. Trimestriel Soc. Mycol. France 89: 308. 1973. [MB 324545]; basionym of Canariomyces arenarius.
Thielavia arxii Stchigel & Guarro, Mycol. Res. 106: 979. 2002. [MB 483974]; basionym of Pseudothielavia arxii.
Thielavia australiensis Tansey & M.A. Jack, Canad. J. Bot. 53: 81. 1975. [MB 324546]; basionym of Thermocarpiscus australiensis.
Thielavia coactilis Nicot, Compt. Rend. Hebd. Séances Acad. Sci. Paris 253: 304. 1961. [MB 340051]; basionym of Parathielavia coactilis.
Thielavia fimeti (Fuckel) Malloch & Cain, Mycologia 65: 1064. 1973. [MB 324554]; synonym of Chaetomium fimeti.
Thielavia fragilis (Natarajan) Arx, Stud. Mycol. 8: 8. 1975. [MB 324555]; synonym of Hyalosphaerella fragilis.
Thielavia gigaspora Moustafa & Abdel-Azeem, Microbiol. Res. 163: 442. 2008. [MB 487453]; basionym of Stolonocarpus gigasporus.
Thielavia heterothallica Klopotek, Arch. Microbiol. 107: 223. 1976. [MB 324556]; basionym of Thermothelomyces heterothallicus [as ‘heterothallica’].
Thielavia hyrcaniae Nicot, Compt. Rend. Hebd. Séances Acad. Sci., Sér. D 253: 304. 1961. [MB 340053]; basionym of Parathielavia hyrcaniae.
Thielavia inaequalis Pidopl. et al., Mikrobiol. Zhurn. 35(6): 723. 1973. [MB 324558]; basionym of Parachaetomium inaequale.
Thielavia kirilenkoae Beliakova, Mikol. Fitopatol. 8(2): 73. 1974. [MB 324559]; synonym of Microthielavia ovispora.
Thielavia kuwaitensis Moustafa, Trans. Brit. Mycol. Soc. 66: 336. 1976. [MB 324560]; basionym of Parathielavia kuwaitensis.
Thielavia leptoderma C. Booth [as ‘leptodermus’], Mycol. Pap. 83: 3. 1961. [MB 340054]; basionym of Aporothielavia leptoderma.
Thielavia microspora Mouch., Bull. Trimestriel Soc. Mycol. France 89: 300. 1973. [MB 324563]; basionym of Canariomyces microsporus.
Thielavia novoguineensis Udagawa & Y. Horie, Bull. Nat. Sci. Mus. Tokyo 15: 191. 1972. [MB 324566]; basionym of Corynascus novoguineensis.
Thielavia octospora (Natarajan) Arx, Stud. Mycol. 8: 6. 1975. [MB 283722]. Basionym: Thielaviella octospora. — Type: MUBL 2250. Representative strain: CBS 119.76. ITS barcode: MZ334731 (alternative markers: LSU = MZ351416; tub2 = MZ343009; rpb2 = MZ342970). Note: Based on the phylogenetic analysis (Fig. 7), we consider Thielavia octospora a synonym of Achaetomium globosum.
Thielavia ovispora Pidopl. et al., Mikrobiol. Zhurn. 35(6): 724. 1973. [MB 324568]; basionym of Microthielavia ovispora.
Thielavia peruviana (Goch.) Malloch & Cain, Mycologia 65: 1067. 1973. [MB 324571]; synonym of Chrysanthotrichum peruvianum.
Thielavia pilosa C. Booth & Shipton, Trans. Brit. Mycol. Soc. 49: 665. 1966. [MB 340058]; basionym of Chaetomium pilosum.
Thielavia sepedonium C.W. Emmons, Bull. Torrey Bot. Club 59: 417. 1932. [MB 277883]; basionym of Corynascus sepedonium.
Thielavia spirotricha (R.K. Benj.) Malloch & Cain, Mycologia 65: 1069. 1973. [MB 324575]; synonym of Botryotrichum spirotrichum.
Thielavia subfimeti (Seth) Malloch & Cain, Mycologia 65: 1070. 1973. [MB 324576]; synonym of Chaetomium subfimeti.
Thielavia subthermophila Mouch., Bull. Trimestriel Soc. Mycol. France 89: 297. 1973. [MB 324577]; basionym of Canariomyces subthermophilus.
Thielavia terrestris (Apinis) Malloch & Cain, Canad. J. Bot. 50: 66. 1972. [MB 324578]; synonym of Thermothielavioides terrestris.
Thielavia terricola (J.C. Gilman & E.V. Abbott) C.W. Emmons, Bull. Torrey Bot. Club 57: 124. 1930. [MB 255078]; synonym of Pseudothielavia terricola.
Thielavia tetraspora (Lodhi & Mirza) Arx, The genera of fungi sporulating in pure culture: 115. 1974. [MB 283723]; synonym of Boothiella tetraspora.
Thielavia thermophila Fergus & Sinden, Canad. J. Bot. 47: 1635. 1969. [MB 340061]. Replaced synonym of Thermothelomyces fergusii.
Thielavia tortuosa Udagawa & Y. Sugiy., Trans. Mycol. Soc. Japan 22: 197. 1981. [MB 111966]; basionym of Condenascus tortuosus.
Thielavia minuta var. thermophila Abdullah & Al-Bader, Basrah J. Agric. Sci. 5: 116. 1992. [MB 444607]; basionym of Parvomelanocarpus thermophilus.
Torula (Pleosporales, Dothideomycetes)
Torula thermophila Cooney & R. Emers., Thermophilic Fungi: 92. 1964. [MB 340149]; basionym of Mycothermus thermophilus.
Trichocladium
Trichocladium acropullum (X.Wei Wang) X.Wei Wang & Houbraken, Stud. Mycol. 93: 126. 2018. [MB 824462]. Basionym: Chaetomium acropullum. — Type: HMAS 86808. Ex-type: CBS 114580. Reproduction: sexual & asexual. ITS barcode: LT993626 (alternative markers: LSU = LT993626; tub2 = LT993707; rpb2 = LT993545).
Trichocladium amorphum X.Wei Wang & Houbraken, Stud. Mycol. 93: 130. 2018. [MB 824463]. — Type: CBS H-23491. Ex-type: CBS 127763. Reproduction: asexual. ITS barcode: LT993628 (alternative markers: LSU = LT993628; tub2 = LT993709; rpb2 = LT993547).
Trichocladium antarcticum (Stchigel & Guarro) X.Wei Wang & Houbraken, Stud. Mycol. 93: 130. 2018. [MB 824464]. Basionym: Thielavia antarctica. — Type: IMI 389346. Ex-type: CBS 123565 = FMR 7920. Reproduction: sexual & asexual. ITS barcode: LT993629 (alternative markers: LSU = LT993629; tub2 = LT993710; rpb2 = LT993548).
Trichocladium arxii (Benny) X.Wei Wang & Houbraken, Stud. Mycol. 93: 130. 2018. [MB 824465]. Basionym: Chaetomidium arxii. — Type: FLAS-F52103. Ex-type: CBS 104.79. Reproduction: sexual. ITS barcode: LT993631 (alternative markers: LSU = LT993631; tub2 = LT993712; rpb2 = LT993550).
Trichocladium asperum Harz, Bull. Soc. Imp. Naturalistes Moscou 44: 125. 1871. [MB 171452]. — Type: Tab. II fig. 1 in Harz, Bull. Soc. Imp. Naturalistes Moscow 44, 1871 (lectotype), CBS H-23060 (epitype, designated here; MBT 10002832). Ex-epitype: CBS 903.85. Reproduction: asexual. ITS barcode: LT993632 (alternative markers: LSU = LT993632; tub2 = LT993713; rpb2 = LT993551).
Trichocladium beniowskiae X.Wei Wang & Houbraken, Stud. Mycol. 93: 134. 2018. [MB 824466]. Replaced synonym: Beniowskia macrospora. — Type: IMI 99625. Ex-type: CBS 757.74 = IMI 099625. Reproduction: asexual. ITS barcode: LT993635 (alternative markers: LSU = LT993635; tub2 = LT993716; rpb2 = LT993554).
Trichocladium crispatum (Fuckel) X.Wei Wang & Houbraken, Stud. Mycol. 93: 137. 2018. [MB 824467]. Basionym: Sphaeria crispata. — Type: G00127921 (holotype), CBS H-23492 (epitype). Ex-epitype: CBS 149.58. Reproduction: sexual. ITS barcode: LT993636 (alternative markers: LSU = LT993636; tub2 = LT993717; rpb2 = LT993555).
Trichocladium gilmaniellae X.Wei Wang & Houbraken, Stud. Mycol. 93: 137. 2018. [MB 824468]. Replaced synonym: Gilmaniella macrospora. — Type: CBS 388.75. Ex-type: CBS 388.75. Reproduction: asexual. ITS barcode: LT993638 (alternative markers: LSU = LT993638; tub2 = LT993719; rpb2 = LT993557).
Trichocladium griseum (Traaen) X.Wei Wang & Houbraken, Stud. Mycol. 93: 141. 2018. [MB 824469]. Basionym: Humicola grisea. — Type: CBS H-23493 (neotype). Ex-neotype: CBS 119.14 = ATCC 22724 = IMI 075664 = MUCL 8008. Reproduction: asexual. ITS barcode: LT993639 (alternative markers: LSU = LT993639; tub2 = LT993720; rpb2 = LT993558).
Trichocladium heterothallicum (Yu Zhang & L. Cai) X.Wei Wang & Houbraken, Stud. Mycol. 93: 141. 2018. [MB 824470]. Basionym: Chaetomium heterothallicum. — Type: HMAS 245783. Ex-type: CGMCC 3.17543 = LC3796. Reproduction: sexual & asexual. ITS barcode: KP336755 (alternative markers: LSU = KP336804; tub2 = KP336853; rpb2 = n/a).
Trichocladium jilongense (Y.M. Wu & T.Y. Zhang) X.Wei Wang & Houbraken, Stud. Mycol. 93: 141. 2018. [MB 824471]. Basionym: Humicola jilongensis. — Type: HSAUP II 07 1485. Ex-type: HSAUP II 07 1485. Reproduction: asexual. ITS barcode: LT993642 (alternative markers: LSU = LT993642; tub2 = LT993723; rpb2 = LT993561).
Trichocladium nigrospermum (Schwein.) X.Wei Wang & Houbraken, Stud. Mycol. 93: 141. 2018. [MB 824472]. Basionym: Acremonium nigrospermum. — Type: PH. Representative strain: CBS 103.36. Reproduction: asexual. ITS barcode: LT993644 (alternative markers: LSU = LT993644; tub2 = LT993725; rpb2 = LT993563).
Trichocladium seminis-citrulli (Sergejeva) X.Wei Wang & Houbraken, Stud. Mycol. 93: 145. 2018. [MB 824473]. Basionym: Chaetomium seminis-citrulli. — Type: –; CBS H-6892 (isotype). Ex-type: CBS 143.58 = IMI 074953 = VKM F-1952. Reproduction: sexual & asexual. ITS barcode: LT993645 (alternative markers: LSU = LT993645; tub2 = LT993726; rpb2 = LT993564).
Trichocladium tomentosum X.Wei Wang et al., this study. [MB 840131]. — Type: HMAS 350294 (holotype); CBS H-23643 (isotype). Ex-type: CGMCC 3.20443 = CBS 144476 = WXW 8615. Reproduction: sexual. ITS barcode: MZ334732 (alternative markers: LSU = MZ351431; tub2 = MZ343012; rpb2 = MZ342973).
Trichocladium uniseriatum (Yu Zhang & L. Cai) X.Wei Wang & Houbraken, Stud. Mycol. 93: 145. 2018. [MB 824475]. Basionym: Chaetomium uniseriatum. — Type: HMAS 245787. Ex-type: CGMCC 3.17559 = LC3756. Reproduction: sexual & asexual. ITS barcode: KP336751 (alternative markers: LSU = KP336800; tub2 = KP336849; rpb2 = KT149475).
Xanthiomyces
Xanthiomyces spinosus (Chivers) X.Wei Wang & Houbraken, this study. [MB 840166]. Basionym: Chaetomium spinosum. — Type: CUP, Chivers No. 7. Representative strain: CBS 789.71. Reproduction: sexual. ITS barcode: MH860357 (alternative markers: LSU = MZ351429; tub2 = MZ343034; rpb2 = MZ342995).
Doubtful and excluded species
Achaetomium thermophilum M. Basu, Curr. Sci. 51: 524. 1982. [MB 109578]. — Type: n/a. Ex-type: CBS 250.85 = CBS 152.97. Reproduction: sexual. ITS barcode: JX280859 (alternative markers: LSU = JX280740; tub2 = n/a; rpb2 = n/a). Notes: The two representative cultures of Achaetomium thermophilum in the CBS collection differ: one resembles Chaetomium vitellinum and the other one Achaetomium macrosporum. The taxonomic position of this species is doubtful and needs further study.
Chaetomidium triangulare Stchigel & Guarro, Stud. Mycol. 50: 218. 2004. [MB 500062]. — Type: IMI 392313. Ex-type: CBS 113677 = FMR 7545. Reproduction: sexual. ITS barcode: (alternative markers: LSU = FJ666362; tub2 = n/a; rpb2 = FJ666393). Note: Chaetomidium triangulare and Chaetomium microascoides cluster together in “clade 6, Lasiosphaeriaceae” outside the Chaetomiaceae (Fig. 7).
Chaetomium microascoides Guarro, Nova Hedwigia 41: 445. 1985. [MB 103923]. — Type: n/a. Ex-type: CBS 236.80 = CBS 540.83. Reproduction: sexual. ITS barcode: MH861259 (alternative markers: LSU = MH873028; tub2 = MZ343020; rpb2 = MZ342981). Note: Chaetomium microascoides and Chaetomidium triangulare cluster together in “clade 6, Lasiosphaeriaceae” outside the Chaetomiaceae (Fig. 7).
Chaetomium olivicolor K. Rodr. et al., Mycologia 94: 123. 2002. [MB 484630]. — Type: IMI 382895. Ex-type: CBS 102434 = FMR 6779 = IMI 381869 = MUCL 43148. Reproduction: sexual. ITS barcode: KM655318 (alternative markers: LSU = KM655357; tub2 = n/a; rpb2 = KM655428). Note: Chaetomium olivicolor is phylogenetically related to Achaetomiella and more research is needed to determine the position of this species.
Chaetomium siamense Pornsuriya & Soytong, Mycotaxon 115: 21. 2011. [MB 514033]. — Type: TMACC001. Ex-type: CP-2009. Reproduction: sexual. ITS barcode: AB506801 (alternative markers: LSU = n/a; tub2 = n/a; rpb2 = n/a). Notes: A BLAST search with the ITS sequence deposited on GenBank showed a 97.2 % homology with CBS 337.67, the ex-type of Ar. flavigenus. More research is needed to confidentially combine this species in Arcopilus.
Chaetomium tetrasporum S. Hughes, Trans. Brit. Mycol. Soc. 29: 72. 1946. [MB 285138]. — Notes: No ex-type or authentic representative culture was available to determine the position of this species. A strain maintained in the CBS culture collection (CBS 351.77) as Chaetomium tetrasporum is sterile.
Humicola siamensis Chatmala & E.B.G. Jones, Nova Hedwigia 83: 226. 2006. [MB 522318]. — Type: BCC 9511. Ex-type: BCC 9511. Reproduction: sexual & asexual. ITS barcode: n/a (alternative markers: LSU = DQ237875; tub2 = n/a; rpb2 = n/a). Note: A BLAST search with the LSU sequence present in GenBank shows that this species doesn’t belong to the Chaetomiaceae, and probably belongs to Halosphaeriaceae (Microascales).
Taifanglania parvispora Y. Wang et al., Mycosystema 34: 347. 2015. [MB 805936]. — Type: GZUIFR-E21402H. Ex-type: GZUIFR-E21402H. Reproduction: asexual. ITS barcode: KF719170 (alternative markers: LSU = n/a; tub2 = n/a; rpb2 = n/a). Notes: The morphology of Tai. parvispora is that of a typical Acrophialophora species; however, comparison of the ITS sequence indicated a relationship with species in Subramaniula. More data are needed to confidentially determine its generic position.
DISCUSSION
Fifty genera are recognised in Chaetomiaceae, of which six are newly proposed in this study. Multi-gene phylogenetic analysis resolved most of the genera as monophyletic lineages with robust support. However, in some cases we are faced with the choice to define genera in a broader or narrower sense. In combination with ecological, morphological and phylogenetic data, we used divergence times as an additional criterion for evaluating genera that are difficult to delimit. The results indicate that all the well-defined genera in the Chaetomiaceae diverged earlier than 27 Mya (Figs 8, 9). Chrysocorona, Pseudothielavia and Hyalosphaerella seem to have diverged most recently from each other at about 27 and 30 Mya, respectively. Even though they cluster together, no statistical evidence supports the close relationships of these three genera in our combined phylogenetic analysis (Fig. 7C). Furthermore, they do have striking morphological differences and we therefore accept them as separate genera. The criteria used here to delimit genera led to the introduction of the new genera Parvomelanocarpus, Pseudohumicola and Xanthiomyces, the reintroduction of Achaetomiella, the synonymy of “Crassicarpon” with Thermothelomyces, and the acceptance of Parachaetomium delimited by Mehrabi et al. (2020). Divergence times also helped us to confirm several genera which were delimitated in our previous studies. For example, the two sexually-reproducing chaetomium-like species Acro. jodhpurensis and Acro. teleoafricana were classified in the traditionally asexual genus Acrophialophora based on their close phylogenetic relationship. The molecular dating analysis showed that the two sexual species diverged about 22 Mya, more recent than the later time limit of the other accepted genera in the family (about 27 Mya, Fig. 8, 9), supporting them within the genus. The phylogenetically-defined Trichocladium is also supported by molecular dating analysis. The species in this genus possess a highly diverse morphology in their asexual and sexual morphs. In total, the mean stem ages of the genera in the family range from 27 to 122 Mya (Figs 8, 9).
Most of the morphologically-defined traditional genera in Chaetomiaceae have proven to be poly- or paraphyletic with a few exceptions like Achaetomium, Corynascus and Stellatospora (von Arx 1973, von Arx et al. 1988, Ito & Nakagiri 1994, Wang et al. 2016a, b, 2019a, b). Species with a chaetomium-like morph (the traditionally morphology-based concept of Chaetomium) are distributed in 23 genera, namely Achaetomiella, Acrophialophora, Amesia, Arcopilus, Arxotrichum, Botryotrichum, Brachychaeta, Chaetomium sensu stricto, Chrysanthotrichum, Chrysocorona, Collariella, Dichotomopilus, Floropilus, Humicola, Ovatospora, Parachaetomium, Pseudohumicola, Staphylotrichum, Subramaniula, Tengochaeta, Thermochaetoides, Trichocladium, Xanthiomyces (Wang et al. 2016a, Crous et al. 2018, Wang et al. 2019a, b, Mehrabi et al. 2020, this study). The thielavia-like species in Chaetomiaceae (species once placed in Thielavia) are distributed in 11 genera: Carteria, Chrysanthotrichum, Condenascus, Hyalosphaerella, Microthielavia, Parathielavia, Pseudothielavia, Stolonocarpus, Thermocarpiscus, Trichocladium and Thermothielavioides (Wang et al. 2019a, b, this study). Three species, which produce non-ostiolate ascomata containing ascospores with two apical germ pores, were previously classified in Corynascella and current insight shows that these belong to three different genera: Corynascella sensu stricto (Coryl. humicola; Fig. 23, 24), Botryotrichum (Bot. inquinatum; Fig. 21) and Parachaemium (Parach. inaequale = Coryl. inaequalis = Thielavia inaequalis; Fig. 35). Species with a chaetomidium-like morph are distributed over four lineages: Aporothielavia (Ap. leptoderma = Chd. leptoderma; Fig. 11), Botryotrichum (Bot. trichorobustum = Chd. trichorobustum), Chaetomium (Ch. fimeti = Chd. fimeti, Ch. pilosum = Chd. pilosum, Ch. subfimeti = Chd. subfimeti, Ch. tectifimeti, Wang et al. 2016a, b) and Lasiosphaeriaceae sensu lato outside Chaetomiaceae (“Chaetomidium triangulare”).
After this phylogenetic revision, most of the genera in the family consist of species which share morphological features. Examples are Collariella and Dichotomopilus. Collariella can be easily recognised by the production of 1) broadly limoniform to quadrangular and bilaterally flattened ascospores with an apical germ pore which are usually less than 7.5 μm long, and 2) a darkened collar around the ostiolar pore of ascomata. Dichotomopilus is characterised by seta-like to dichotomously or irregularly branched ascomatal hairs and by narrowly ovate to broad ovate and slightly bilaterally-flattened small ascospores, which are usually less than 7.5 μm long, and have an apical or slightly sub-apical germ pore. On the other hand, some genera, such as Botryotrichum and Trichocladium are morphologically highly diverse. Species in these genera can reproduce asexually with different asexual structures or sexually with ostiolate or non-ostiolate ascomata.
At species level, 275 species are accepted in Chaetomiaceae based on the results of the phylogenetic analyses (Fig. 7, Supplementary Figs S1, S2). Few species were not included in the multigene analysis because these were very recently described (Arcopilus eremanthi, Chaetomium camelliae, Dichotomopilus finlandicus) or only ITS and/or LSU sequences were available (e.g., several Acrophialophora species, Staphylotrichum koreanum = Humicola koreana) and these failed to be combined in the analysis. Most of the species have been recently (re)described and photo plates of these species are available (Asgar & Zare 2011, Marin-Felix et al. 2015, Wang et al. 2016a, b, 2019a, b, Crous et al. 2016, 2017, 2018, 2019, Zhang et al. 2017a, b, Raza et al. 2019, Schultes et al. 2019, Alidadi et al. 2020, Mehrabi et al. 2020, Safi et al. 2020, Sousa et al. 2020, Qiao et al. 2021, Ryan et al. 2021, this study), making identification easier. In addition, “Chaetomium microascoides” (ex-type culture CBS 236.80) and “Chaetomidium triangulare” (ex-type culture CBS 113677) proved to be members of Lasiosphaeriaceae sensu lato, outside the Chaetomiaceae. Further study is needed to determine their exact position at both genus and family level. A few species in Chaetomiaceae were found to have a distinct morphology, while no differences in their molecular barcodes (ITS, LSU, rpb2 and tub2) were present (e.g., Ch. globosum and Ch. cruentum; figs 14, 16 in Wang et al. 2016a). These species are morphologically distinct, but phylogenetically undistinguishable (Fig. 7B, Supplementary Figs S1–S3). We decided to tentatively accept these species and genome studies may give more insight into the evolution of these taxa.
Morgenstern et al. (2012) demonstrated that thermophilic species are polyphyletic in kingdom Fungi, and that they are present in Sordariales, Eurotiales and Onygenales in Ascomycota and in Mucoromycota. The study of van den Brink et al. (2015) showed that thermophilic species in Chaetomiaceae are also polyphyletic. The present study resolved 15 thermophilic Chaetomiaceae species in seven genera: Melanocarpus, Mycothermus, Remersonia, Thermocarpusella, Thermochaetoides, Thermothelomyces and Thermothielavioides. It is clear that thermophiles independently evolved at least six times within Chaetomiaceae. Mycothermus and Remersonia are sister genera with a mean stem age of about 40 Mya. The other five thermophilic genera do not have thermophilic sister genera and clearly diverged from mesophilic genera, probably by adaptation to elevated temperatures. Each of them is estimated to diverge from their close non-thermophilic relatives over 30 Mya.
Some genera have optimum and maximum growth temperatures over a relatively large temperature range. One example is the genus Trichocladium. Trichocladium antarcticum was reported to have optimal growth temperature at about 15 °C (Stchigel et al. 2003), and the optimal growth temperature of Tri. acropullum is between 25–28 °C (maximum growth temperature at 32 °C) (Wang & Zheng 2005). In contrast, Tri. seminis-citrulli has an optimal growth temperature of about 30 °C and a maximum growth temperature at 40 °C (Millner 1977). Another example is Collariella, in which Col. quadrangulata has an optimal growth temperature at about 25 °C and maximum growth temperature at 37–38 °C, while Col. causiiformis and Col. gracilis have an optimal growth temperature around 35–40 °C and maximum growth temperature between 45 and 50 °C (Millner 1977). This suggests that species with psychrophilic, mesophilic and even thermotolerant habits in a certain genus have diverged rather recently.
A few species which can grow at 37 °C have been reported as causal agents of systemic and deep infections in humans, such as Achaetomium strumarium (= Chaetomium atrobrunneum, Abbott et al. 1995), Amesia atrobrunnea (= Chaetomium atrobrunneum, Abbott et al. 1995) and Parachaetomium perlucidum (= Chaetomium perlucidum, Barron et al. 2003). To our knowledge, no thermophilic species in Chaetomiaceae have been reported to infect humans although some of them can also grow well at 37 °C. More work is required to determine the cardinal growth temperature of each Chaetomiaceae species, and to clarify the correlations between cardinal growth temperatures, infection potential in humans and the ability to produce useful enzymes for industry.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (NSFC, Project No. 32070014). The authors are grateful to the CBS culture collection at the Westerdijk Fungal Biodiversity Institute, the curator Gerard Verkleij and the technical staff Yda Vlug, Trix Merkx, Diana Vos and Arien van Iperen for their kind help in providing strains from the culture collection, preparing cultures and in the deposit of fungarium material. The authors acknowledge prof. dr Keith Seifert for his kind help in the study of asexual morphs of Chaetomiaceae. The first author would like to thank Dr Ewald Groenewald for his valuable comments on this study.
DECLARATION ON CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Supplementary Material: https://studiesinmycology.org/
Phylogenetic tree resulting from ML analysis of the ITS gene region alignment. The confidence values are indicated at the nodes in the same way as in Fig. 7. Genus clades are discriminated with boxes in different colours and clades containing thermophilic species are highlighted with an orange background. The scale bar shows the expected number of changes per site. The tree is rooted with Pseudoechria longicollis and Schizotheciaceae, in the Schizotheciaceae.
Phylogenetic tree resulting from ML analysis of the partial tub2 gene region alignment. The confidence values are indicated at the nodes in the same way as in Fig. 7. Genus clades are discriminated with boxes in different colours and clades containing thermophilic species are highlighted with an orange background. The scale bar shows the expected number of changes per site. The tree is rooted with Pseudoechria longicollis and Schizotheciaceae, in the Schizotheciaceae, Sordariales.
Phylogenetic tree resulting from ML analysis of the partial rpb2 gene region alignment. The confidence values are indicated at the nodes in the same way as in Fig. 7. Genus clades are discriminated with boxes in different colours and clades containing thermophilic species are highlighted with an orange background. The scale bar shows the expected number of changes per site. The tree is rooted with Pseudoechria longicollis and Pseudoechria prolifica, in the Schizotheciaceae.
Phylogenetic location of “Humicola koreana” based on the separate analyses of partial gene sequences of ITS (A) and LSU (B). The confidence values are indicated at the nodes in the same way as in Fig. 7. The scale bar shows the expected number of changes per site. The tree is rooted with Achaetomium strumarium.
Comparison ascospore sizes measured in lactic acid (present study) and water (literature) as mounting medium.
Overview species and strains used in phylogenetic analysis.
REFERENCES
- Abbott SP, Sigler L, McAleer R, et al. (1995). Fatal cerebral mycoses caused by the ascomycete Chaetomium strumarium. Journal of Clinical Microbiology 33: 2692–2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Azeem AM. (2020). Recent developments on genus Chaetomium. Springer International Publishing, Cham. [Google Scholar]
- Aghyl H, Mehdi Mehrabi-Koushki M, Esfandiari M. (2021). Chaetomium iranicum and Collariella capillicompacta spp. nov. and notes to new hosts of Amesia species in Iran. Sydowia 73: 21–30. [Google Scholar]
- Ahmed A, Adelmann D, Fahal A, et al. (2002). Environmental occurrence of Madurella mycetomatis, the major agent of human eumycetoma in Sudan. Journal of Clinical Microbiology 40: 1031–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SA, Khan Z, Wang XW, et al. (2016). Chaetomium-like fungi causing opportunistic infections in humans: a possible role for extremotolerance. Fungal Diversity 76: 11–26. [Google Scholar]
- Al-Aidaroos A, Bin-Hussain I, Solh HE, et al. (2007). Invasive Chaetomium infection in two immunocompromised pediatric patients. The Pediatric Infectious Disease Journal 26: 456–458. [DOI] [PubMed] [Google Scholar]
- Alidadi A, Vala SA, Jouzani GS. (2020). Botryotrichum iranicum sp. nov. and Trematosphaeria magenta sp. nov. as two new species from Iran. Mycological Progress 19: 1575–1586. [Google Scholar]
- Ames LM. (1963). A Monograph of the Chaetomiaceae. Research and Development Service of U. S. Army. [Google Scholar]
- Apinis AE. (1962). Occurrence of thermophilous microfungi in certain alluvial soils near Nottingham. Nova Hedwigia. 5: 57–78. [Google Scholar]
- Asgari B, Zare R. (2011). The genus Chaetomium in Iran, a phylogenetic study including six new species. Mycologia 103: 863–882. [DOI] [PubMed] [Google Scholar]
- Bainier G. 1910. Monographie des Chaetomidium et des Chaetomium. Bulletin de la Société Mycologique de France 25: 191–237. [Google Scholar]
- Barron MA, Sutton DA, Veve R, et al. (2003). Invasive mycotic infections caused by Chaetomium perlucidum, a new agent of cerebral phaeohyphomycosis. Journal of Clinical Microbiology 41: 5302–5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berka RM, Grigoriev IV, Otillar R, et al. (2011). Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris. Nature Biotechnology 29: 922–927. [DOI] [PubMed] [Google Scholar]
- Betancourt O, Zaror L, Senn C. (2013). Isolation of filamentous fungi from haircoat cats without skin lesions in temuco, Chile. Revista Cientifica de la Facultad de Ciencias Veterinarias de la Universidad del Zulia 23: 380–387. [Google Scholar]
- Bissett J, Gams W, Jaklitsch W, et al. (2015). Accepted Trichoderma names in the year 2015. IMA Fungus 6: 263–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth C. (1961). Studies of Pyrenomycetes. VI Thielavia. With notes on some allied genera. Mycological Papers 83: 1–15. [Google Scholar]
- Bouckaert R, Vaughan TG, Barido-Sottani J, et al. (2019). BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLOS Computational Biology 15: e1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon T, Lo N, Cameron SL, et al. (2014). The Evolutionary History of Termites as Inferred from 66 Mitochondrial Genomes. Molecular Biology and Evolution 32: 406–421. [DOI] [PubMed] [Google Scholar]
- Cannon PF. (1986). A revision of Achaetomium, Achaetomiella, and Subramaniula, and some silimar species of Chaetomium. Transactions of the British Mycological Society 87: 45–76. [Google Scholar]
- Carmichael JW. (1962). Chrysosporium and some other aleuriosporic hyphomycetes. Canadian Journal of Botany 40: 1137–1173. [Google Scholar]
- Carter A, Khan RS. (1982). New and interesting Chaetomium species from East Africa. Canadian Journal of Botany 60: 1253–1262. [Google Scholar]
- Chen L, Qiu Q, Jiang Y, et al. (2019). Large-scale ruminant genome sequencing provides insights into their evolution and distinct traits. Science 364: eaav6202. [DOI] [PubMed] [Google Scholar]
- Chivers AH. (1915). A monograph of the genera Chaetomium and Ascotricha. Memoirs of the Torrey Botanical Club 14: 155–240. [Google Scholar]
- Cooney DG, Emerson R. (1964). Thermophilic Fungi: an account of their biology, activities and classification. W.H. Freeman and Co., San Francisco, London [Google Scholar]
- Corda ACJ. (1840). Icones fungorum hucusque cognitorum. 4: 1–53. [Google Scholar]
- Costantin J. (1892). Sur quelques maladies du blanc de Champignon. Comptes Rendues des Séances Hebdomadaires de l’Académie des Sciences Paris 114: 849–851. [Google Scholar]
- Crous PW, Carnegie AJ, Wingfield MJ, et al. (2019). Fungal Planet description sheets: 868–950 . Persoonia 42: 291–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Lombard L, Sandoval-Denis M, et al. (2021). Fusarium: more than a node or a foot-shaped basal cell. Studies in Mycology 98: 100116: 1–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. (2017). Fungal Planet description sheets: 625–715. Persoonia 39: 270–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. (2018). Fungal Planet description sheets: 716–784. Persoonia 40: 240–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Richardson DM, et al. (2016). Fungal Planet description sheets: 400–468. Persoonia 36: 316–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CW. (1997). Can three incongruency tests predict when data should be combined? Molecular Biology and Evolution 14: 733–740. [DOI] [PubMed] [Google Scholar]
- Daniels J. (1961). Chaetomium piluliferum sp. nov., the perfect state of Botryotrichum piluliferum. Transactions of the British Mycological Society 44: 79–86. [Google Scholar]
- de Hoog GS, Adelmann D, Ahmed AOA, et al. (2004). Phylogeny and typification of Madurella mycetomatis, with a comparison of other agents of eumycetoma. Mycoses. 47: 121–130. [DOI] [PubMed] [Google Scholar]
- de Hoog GS, Gerrits van den Ende AHG. (1998). Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycosis 41: 183–189. [DOI] [PubMed] [Google Scholar]
- de Oliveira TB, Gomes E, Rodrigues A. (2015). Thermophilic fungi in the new age of fungal taxonomy. Extremophiles 19: 31–37. [DOI] [PubMed] [Google Scholar]
- dos Reis M, Donoghue PCJ, Yang ZH. (2015). Bayesian molecular clock dating of species divergences in the genomics era. Nature Reviews Genetics 17: 71-80. [DOI] [PubMed] [Google Scholar]
- Fergus CL, Sinden JW. (1969). A new thermophilic fungus from mushroom compost: Thielavia thermophila spec. nov. Canadian Journal of Botany. 47: 1635–1637. [Google Scholar]
- Fergus CL. (1971). The temperature relationships and thermal resistance of a new thermophilic Papulaspora from mushroom compost. Mycologia 63: 426–431. [Google Scholar]
- Fries EM. (1823). Systema mycologicum, vol 2. Officina Berlingiana, Lundae. [Google Scholar]
- Gao WX, He Y, Li FL, et al. (2019). Antibacterial activity against drug-resistant microbial pathogens of cytochalasan alkaloids from the arthropod-associated fungus Chaetomium globosum TW1-1. Bioorganic Chemistry 83: 98–104. [DOI] [PubMed] [Google Scholar]
- Glass NL, Schmoll M, Cate JHD, et al. (2013). Plant cell wall deconstruction by ascomycete fungi. Annual Review of Microbiology 67: 477–98. [DOI] [PubMed] [Google Scholar]
- Gond SK, Mishra A, Sharma VK, et al. (2012). Diversity and antimicrobial activity of endophytic fungi isolated from Nyctanthes arbor-tristis, a well-known medicinal plant of India. Mycoscience 53: 113–121. [Google Scholar]
- Greathouse GA, Ames LM. (1945). Fabric deterioration by thirteen described and three new species of Chaetomium. Mycologia 37: 138–155. [Google Scholar]
- Greif MD, Currah RS. (2007). Development and dehiscence of the cephalothecoid peridium in Aporothielavia leptoderma shows it belongs in Chaetomidium. Mycological Research 111: 70–77. [DOI] [PubMed] [Google Scholar]
- Greif MD, Stchigel AM, Huhndorf SM. (2009). A re-evaluation of genus Chaetomidium based on molecular and morphological characters. Mycologia 101: 554–564. [DOI] [PubMed] [Google Scholar]
- Guarro J, Abdullah SK, Al-Bader SM, et al. (1996). The genus Melanocarpus. Mycological Research 100: 75–78. [Google Scholar]
- Guarro J, Al-Saadoon AH, Gene J, et al. (1997). Two new cleistothecial Ascomycetes from Iraq. Mycologia 89: 955–961. [Google Scholar]
- Guppy KH, Thomas C, Thomas K, et al. (1998). Cerebral fungal infections in the immunocompromised host: a literature review and a new pathogen – Chaetomium atrobrunneum: case report. Neurosurgery 43: 1463–1469. [DOI] [PubMed] [Google Scholar]
- Guterres DC, Galvão-Elias S, de Souza BCP, et al. (2018). Taxonomy, phylogeny, and divergence time estimation for Apiosphaeria guaranitica, a Neotropical parasite on bignoniaceous hosts. Mycologia 110: 526–545. [DOI] [PubMed] [Google Scholar]
- Gutierrez RMP, Gonzalez AMN, Ramirez AM. (2012). Compounds derived from endophytes: a review of phytochemistry and pharmacology. Current Medicinal Chemistry 19: 2992–3030. [DOI] [PubMed] [Google Scholar]
- Haki GD, Rakshit SK. (2003). Developments in industrially important thermostable enzymes: a review. Bioresource Technology 89: 17–34. [DOI] [PubMed] [Google Scholar]
- Harreither W, Sygmund C, Augustin M, et al. (2011). Catalytic properties and classification of cellobiose dehydrogenases from ascomycetes. Applied and Environmental Microbiology 77: 1804–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth DL. (1971). A revision of the genus Ascotricha Berk. Mycological Papers 126: 1–28. [Google Scholar]
- He MQ, Zhao RL, Hyde KD, et al. (2019). Notes, outline and divergence times of Basidiomycota. Fungal Diversity 99: 105–367. [Google Scholar]
- Ho SYW. (2020). The Molecular Evolutionary Clock: Theory and Practice. Springer, Cham, Switzerland. [Google Scholar]
- Hubka V, Mencl K, Skorepova M, et al. (2011). Phaeohyphomycosis and onychomycosis due to Chaetomium spp., including the first report of Chaetomium brasiliense infection. Medical Mycology 49: 724–733. [DOI] [PubMed] [Google Scholar]
- Hu Y, Zhang WP, Zhang P, et al. (2013). Nematicidal activity of chaetoglobosin A produced by Chaetomium globosum NK102 against Meloidogyne incognita. Journal of Agricultural and Food Chemistry 61: 41–46. [DOI] [PubMed] [Google Scholar]
- Hyde KD, Maharachchikumbura SSN, Hongsanan S, et al. (2017). The ranking of fungi: a tribute to David L. Hawksworth on his 70th birthday. Fungal Diversity 84: 1–23. [Google Scholar]
- Ito T, Nakagiri A. (1994). Stellatospora, a new genus of the Sordariaceae. Mycoscience 35: 413–415. [Google Scholar]
- Kapoor JN, Lal SP. (1982). A new species of Botryoderma from India. Indian Phytopathology 35: 732–733. [Google Scholar]
- Kawato M, Shinobu R. (1959). On Streptomyces herbaricolor, nov. sp., supplement: a simple technique for the microscopic observation. Memoirs of Osaka University of the Liberal Arts and Education. Series B. Natural Sciences 8: 114–119. [Google Scholar]
- Kharwar RN, Mishra A, Gond SK, et al. (2011). Anticancer compounds derived from fungal endophytes: their importance and future challenges. Natural Product Reports 28: 1208–1228. [DOI] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, et al. (2008). Ainsworth & Bisby’s Dictionary of the Fungi, 10th ed. CABI, UK. [Google Scholar]
- Koukol O. (2016). Myriococcum revisited: a revision of an overlooked fungal genus. Plant Systematics and Evolution 302: 957–969. [Google Scholar]
- Kunze G, Schmidt JK. (1817). Mykologische Hefte 1: 1–109. Leipzig, Germany. [Google Scholar]
- Lang J, Hu J, Ran W, et al. (2012). Control of cotton Verticillium wilt and fungal diversity of rhizosphere soils by bio-organic fertilizer. Biology and Fertility of Soils 48: 191–203. [Google Scholar]
- Larran S, Simón MR, Moreno MV, et al. (2016). Endophytes from wheat as biocontrol agents against tan spot disease. Biological Control 92: 17–23. [Google Scholar]
- La Touche CJ. (1950). On a thermophile species of Chaetomium. Transactions of the British Mycological Society 33: 95–104. [Google Scholar]
- Li GJ, Hyde HD, Zhao RL, et al. (2016). Fungal diversity notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal diversity 78: 1–237. [Google Scholar]
- Liu YJJ, Whelen S, Benjamin DH. (1999). Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808 [DOI] [PubMed] [Google Scholar]
- Lopez SE, Nelson EE, Thies WG (1995). A new species of Botryoderma and other hyphomycetes isolated from roots of fumigated and nonfumigated Douglas-fir stumps in Oregon. Mycotaxon 55: 269–274. [Google Scholar]
- Luo ZL, Hyde KD, Liu JK, et al. (2019). Freshwater Sordariomycetes. Fungal Diversity 99: 451–660. [Google Scholar]
- Malloch D, Cain RF. (1973). The genus Thielavia. Mycologia 65: 1055–1077. [Google Scholar]
- Marchal E. (1885). Champignons coprophiles de Belgique. Bulletin de la Société Royale de Botanique de Belgique 24: 57–77. [Google Scholar]
- Margaritis A, Merchant RFJ, Yaguchi M. (1986). Thermostable cellulases from thermophilic microorganisms. Critical Reviews in Biotechnology 4: 327–367. [Google Scholar]
- Marín-Felix Y, Groenewald JZ, Cai L, et al. (2017). Genera of phytopathogenic fungi: GOPHY1. Studies in Mycology 86: 99–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Felix Y, Stchigel AM, Miller AN, et al. (2015). A re-evaluation of the genus Myceliophthora (Sordariales, Ascomycota): its segregation into four genera and description of Corynascus fumimontanus sp. nov. Mycologia 107: 619–632. [DOI] [PubMed] [Google Scholar]
- Masclaux F, Guého E, de Hoog GS, et al. (1995). Phylogenetic relationships of human-pathogenic Cladosporium (Xylohypha) species inferred from partial LS rRNA sequences. Journal of Medical and Veterinary Mycology 33: 327–338. [DOI] [PubMed] [Google Scholar]
- Mehrabi M, Asgari B, Zare R. (2020). Description of Allocanariomyces and Parachaetomium, two new genera, and Achaetomium aegilopis sp. nov. in the Chaetomiaceae. Mycological Progress 19: 1415–1427. [Google Scholar]
- Miller AN, Huhndorf SM. (2005). Multi-gene phylogenies indicate ascomal wall morphology is a better predictor of phylogenetic relationships than ascospore morphology in the Sordariales (Ascomycota, Fungi). Molecular Phylogenetics and Evolution 35: 60–75. [DOI] [PubMed] [Google Scholar]
- Millner PD. (1977). Radial growth responses to temperature by 58 Chaetomium species, and some taxonomic relationships. Mycologia 69: 492–502. [Google Scholar]
- Morgenstern I, Powlowski J, Ishmael N, et al. (2012). A molecular phylogeny of thermophilic fungi. Fungal Biology 116: 489–502. [DOI] [PubMed] [Google Scholar]
- Natvig DO, Taylor JW, Tsang A, et al. (2015). Mycothermus thermophilus gen. et comb. nov., a new home for the itinerant thermophile Scytalidium thermophilum (Torula thermophila). Mycologia 107: 319–327. [DOI] [PubMed] [Google Scholar]
- Nilsson RH, Ryberg M, Kristiansson E, et al. (2006). Taxonomic reliability of DNA sequences in public sequence databases: a fungal perspective. PLoS ONE 1: e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noumeur SR, Teponno RB, Helaly SE (2020). Diketopiperazines from Batnamyces globulariicola, gen. & sp. nov. (Chaetomiaceae), a fungus associated with roots of the medicinal plant Globularia alypum in Algeria. Mycological Progress 19: 589–603. [Google Scholar]
- Nugent LK, Sangvichen E, Sihanonth P, et al. (2006). A revised method for the observation of conidiogenous structures in fungi. Mycologist 20: 111–114. [Google Scholar]
- Nylander JAA. (2004). MrModeltest v. 2. Programme distributed by the author. Evolutionary Biology Centre, Uppsala University. [Google Scholar]
- O’Donnell K. (1993). Fusarium and its near relatives. In: The Fungal Holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics (Reynolds D, Taylor JW, eds). CAB International, Wallingford: 225–233. [Google Scholar]
- O’Donnell K, Cigelnik E. (1997). Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution 7: 103–116. [DOI] [PubMed] [Google Scholar]
- Papendorf MC, Upadhyay HP. (1969). Botryoderma lateritium and B. rostratum gen. et spp. nov. from soil in South Africa and Brazil. Transactions of the British Mycological Society 52: 257–265. [Google Scholar]
- Píchová K, Pažoutová S, Martin Kostovčík M, et al. (2018). Evolutionary history of ergot with a new infrageneric classification (Hypocreales: Clavicipitaceae: Claviceps). Molecular Phylogenetics and Evolution 123: 73–87. [DOI] [PubMed] [Google Scholar]
- Qiao M, Zhang Z, Yang LY, et al. (2021). Staphylotrichum sinense sp. nov., a new hyphomycete (Chaetomiaceae) from China. International Journal of Systematic and Evolutionary Microbiology 71: 1–8. [DOI] [PubMed] [Google Scholar]
- Rambaut A. (2009). FigTree v. 1.3.1. Computer program and documentation distributed by the author at http://tree.bio.ed.ac.uk/software/. [Google Scholar]
- Rank C, Nielsen KF, Larsen TO, et al. (2011). Distribution of sterigmatocystin in filamentous fungi. Fungal Biology 115: 406–420. [DOI] [PubMed] [Google Scholar]
- Raza M, Zhang ZF, Hyde KD, et al. (2019). Culturable plant pathogenic fungi associated with sugarcane in southern China. Fungal Diversity 99: 1–104. [Google Scholar]
- Riddell RW. (1950). Permanent stained mycological preparations obtained by slide culture. Mycologia 42: 265–270. [Google Scholar]
- Rodríguez K, Stchigel A, Guarro J. (2002). Three new species of Chaetomium from soil. Mycologia 94: 116–126. [DOI] [PubMed] [Google Scholar]
- Ryan K, Cañete-Gibas C, Sanders C, et al. (2021). Pseudocanariomyces americanus, gen. nov., sp. nov., a new thielavia-like species in the Chaetomiaceae: identification and management of a prosthetic hip infection. Mycopathologia 186: 441–447. [DOI] [PubMed] [Google Scholar]
- Saccardo PA. (1886). Sylloge Fungorum 4: 1–807. [Google Scholar]
- Safi A, Mehrabi-Koushki M, Farokhinejad R. (2020). Amesia khuzestanica and Curvularia iranica spp. nov. from Iran. Mycological Progress 19: 935–945. [Google Scholar]
- Samarakoon MC, Hyde KD, Hongsanan S, et al. (2019). Divergence time calibrations for ancient lineages of Ascomycota classification based on a modern review of estimations. Fungal Diversity 96: 285–346. [Google Scholar]
- Samson RA, Houbraken J, Thrane U, et al. (2019). Food and Indoor Fungi. 2nd Ed. Westerdijk Laboratory Manual series 2. Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands. [Google Scholar]
- Samson RA, Visagie CM, Houbraken J, et al. (2014). Phylogeny, identification and nomenclature of the genus Aspergillus. Studies in Mycology 78: 141–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S, et al. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proceedings of the National Academy of Sciences of the United States of America 109: 6241–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultes NP, Strzalkowski N, Li DW, et al. (2019). Botryotrichum domesticum sp. nov., a new hyphomycete from an indoor environment. Botany 97: 311–319. [Google Scholar]
- Seifert K, Morgan-Jones G, Gams W, et al. (2011). The genera of Hyphomycetes. CBS Biodiversity series 9. Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands. [Google Scholar]
- Selim KA, El-Beih AA, Abdel-Rahman TM, et al. (2014). Biological evaluation of endophytic fungus, Chaetomium globosum J N711454, as potential candidate for improving drug discovery. Cell Biochemistry and Biophysics 68: 67–82. [DOI] [PubMed] [Google Scholar]
- Sergejeva KS. (1961). Species novae generis Chaetomium III. Notulae Systematicae e Sectione Cryptogamica Instituti Botanici Komarovii Academiae Scientiarum U.R.S.S. 14: 139–150. [Google Scholar]
- Seth HK. (1968). Chaetomidium trichorobustum sp. nov. from Germany. Nova Hedwigia 16: 429–432. [Google Scholar]
- Singh B. (2016). Myceliophthora thermophila syn. Sporotrichum thermophile: a thermophilic mould of biotechnological potential. Critical Reviews in Biotechnology 36: 59–69. [DOI] [PubMed] [Google Scholar]
- Sousa TF, Santos AO, Silva FMA, et al. (2020). Arcopilus amazonicus (Chaetomiaceae), a new fungal species from the Amazon rainforest native plant Paullinia cupana. Phytotaxa 456: 145–156. [Google Scholar]
- Stchigel AM, Guarro J, Cormack WM. (2003). Apiosordaria antarctica and Thielavia antarctica, two new ascomycetes from Antarctica. Mycologia 95: 1218–1226. [DOI] [PubMed] [Google Scholar]
- Stchigel AM, Sagués M, Cano J, et al. (2000). Three new thermotolerant species of Corynascus from soil, with a key to the known species. Mycological Research 104: 879–887. [Google Scholar]
- Steenwyk JL, Shen XX, Lind AL, et al. (2019). A robust phylogenomic time tree for biotechnologically and medically important fungi in the genera Aspergillus and Penicillium. mBio 10: e00925-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straatsma G, Samson RA, Olijnsma TW, et al. (1994). Ecology of thermophilic fungi in mushroom compost, with emphasis on Scytalidium thermophilum and growth stimulation of Agaricus bisporus mycelium. Applied and Environmental Microbiology 60: 454–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung GH, Sung JM, Hywel-Jones NL, et al. (2007). A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localized incongruence using a combinational bootstrap approach. Molecular Phylogenetics and Evolution 44: 1204–1223. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, et al. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution 30: 2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey MR, Jack MA. (1975). Thielavia australiensis sp. nov., a new thermophilic fungus from incubator-bird (mallee fowl) nesting material. Canadian Journal of Botany 53: 81–83. [Google Scholar]
- Tekpinar AD, Kalmer A. (2019). Utility of various molecular markers in fungal identification and phylogeny. Nova Hedwigia 109: 87–224. [Google Scholar]
- Tiscornia S, Seguí C, Bettucci L. (2009). Composition and characterization of fungal communities from different composted materials. Cryptogamie, Mycologie 30: 363–376. [Google Scholar]
- Udagawa SI, Ueda S. (1979). Corynascella inquinata, a new cleistothecial ascomycete from sewage sludge. Mycotaxon 8: 292–296. [Google Scholar]
- Udagawa S. (1980). New or noteworthy ascomycetes from Southeast Asian soil. I. Transactions of the Mycological Society of Japan 21: 17–34. [Google Scholar]
- Udagawa S, Muroi T. (1981). Notes on some Japanese Ascomycetes XVI. Transactions of the Mycological Society of Japan 22: 11–26. [Google Scholar]
- van den Brink J, Facun K, de Vries M, et al. (2015). Thermophilic growth and enzymatic thermostability are polyphyletic traits within Chaetomiaceae. Fungal Biology 119: 1255–1266. [DOI] [PubMed] [Google Scholar]
- van den Brink J, Samson RA, Hagen F, et al. (2012). Phylogeny of the industrial relevant, thermophilic genera Myceliophthora and Corynascus. Fungal Diversity 52: 197–207. [Google Scholar]
- van der Aa HA. (1973). Progress report. Verhandelingen der Koninklijke Nederlandsche Akademie van Wetenschappen Afdeeling Natuurkunde, Sectie 2 61: 60–61. [Google Scholar]
- van Oorschot CAN. (1977). The genus Myceliophthora. Persoonia 9: 401–408. [Google Scholar]
- van Oorschot CAN. (1980). A revision of Chrysosporium and allied genera. Studies in Mycology 20: 1–89. [Google Scholar]
- Varga T, Krizsán K, Földi C, et al. (2019). Megaphylogeny resolves global patterns of mushroom evolution. Nature Ecology & evolution 3: 668–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viikari L, Alapuranen M, Puranen T, et al. (2007). Thermostable enzymes in lignocellulose hydrolysis. Advances in Biochemical Engineering Biotechnology 108: 121–145. [DOI] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R, Sun BL. (1994). Ancient and recent patterns of geographic speciation in the oyster mushroom Pleurotus revealed by phylogenetic analysis of ribosomal DNA sequences. Proceedings of the National Academy of Sciences of the United States of America 91: 4599–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visagie CM, Houbraken J, Frisvad JC, et al. (2014). Identification and nomenclature of the genus Penicillium. Studies in Mycology 78: 343–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivi VK, Martins-Franchetti SM, Attili-Angelis D. (2019). Biodegradation of PCL and PVC: Chaetomium globosum (ATCC 16021) activity. Folia Microbiologica 64:1–7. [DOI] [PubMed] [Google Scholar]
- von Arx JA. (1970). The Genera of Fungi Sporulating in Pure Culture. Lehre: J. Cramer. [Google Scholar]
- von Arx JA. (1973a). Further observations on Sporotrichum and some similar fungi. Persoonia 7: 127–130. [Google Scholar]
- von Arx JA. (1973b). Ostiolate and nonostiolate pyrenomycetes. Proceedings Proceedings, Koninklijke Nederlandse Akademie van Wetenschappen, Series C, Biological and Medical Sciences 76: 289–296. [Google Scholar]
- von Arx JA. (1975a). On Thielavia and some similar genera of ascomycetes. Studies in Mycology 8: 1–32. [Google Scholar]
- von Arx JA. (1975b). On Thielavia angulata and some recently described Thielavia species. Kavaka 3: 33–36. [Google Scholar]
- von Arx JA. (1985). On Achaetomium and a new genus Subramaniula. Proceedings of the Indian Academy of Science (Plant Science) 94: 341–345. [Google Scholar]
- von Arx JA, Figueras MJ, Guarro J (1988). Sordariaceous ascomycetes without ascospore ejaculation. Beihefte zur Nova Hedwigia 94: 1–104. [Google Scholar]
- von Arx JA, Guarro J, Figueras MJ. (1986). The Ascomycete genus Chaetomium. Beihefte zur Nova Hedwigia 84: 1–162. [Google Scholar]
- von Klopotek A. (1974). Revision der thermophilen Sporotrichum-Arten: Chrysosporium thermophilum (Apinis) comb. nov. Und Chrysosporium fergusii spec. nov. = status conidialis von Corynascus thermophilus (Fergus und Sinden) comb. nov. Archiv für Mikrobiologie 98: 365–369. [DOI] [PubMed] [Google Scholar]
- von Klopotek A. (1976). Thielavia heterothallica spec. nov., die perfekte Form von Chrysosporium thermophilum. Archives of Microbiology 107: 223–224. [DOI] [PubMed] [Google Scholar]
- Wang FQ, Jiang J, Hu S, et al. (2017). Secondary metabolites from endophytic fungus Chaetomium sp. induce colon cancer cell apoptotic death. Fitoterapia 121: 86–93. [DOI] [PubMed] [Google Scholar]
- Wang XH, Halling RE, Hofstetter V, et al. (2018). Phylogeny, biogeography and taxonomic reassessment of Multifurca (Russulaceae, Russulales) using three-locus data. PLoS ONE 13: e0205840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XW, Bai FY, Bensch K, et al. (2019b). Phylogenetic re-evaluation of Thielavia with the introduction of a new family Podosporaceae. Studies in Mycology 93: 155–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XW, Houbraken J, Groenewald JZ, et al. (2016b). Diversity and taxonomy of Chaetomium and chaetomium-like fungi from indoor environments. Studies in Mycology 84: 145–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XW, Lombard L, Groenewald JZ, et al. (2016a). Phylogenetic reassessment of the Chaetomium globosum species complex. Persoonia 36: 83–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XW, Wang XL, Fu-Jiang Liu FJ, et al. (2014). Phylogenetic assessment of Chaetomium indicum and allied species, with the introduction of three new species and epitypification of C. funicola and C. indicum. Mycological Progress 13: 719–732. [Google Scholar]
- Wang XW, Yang FY, Meijer M, et al. (2019a). Redefining Humicola sensu stricto and related genera in the Chaetomiaceae. Studies in Mycology 93: 65–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XW, Zheng RY. (2005). Chaetomium acropullum sp. nov. (Chaetomiaceae, Ascomycota), a new psychrotolerant mesophilic species from China. Nova Hedwigia 80: 413–418. [Google Scholar]
- Wang Y, Youssef NH, Couger MB, et al. (2019c). Molecular dating of the emergence of anaerobic rumen fungi and the impact of laterally acquired genes. mSystems 4: e00247-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns TD, Lee S, et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: A Guide to Methods and Applications (Innis MA, Gelfand DH, eds). Academic Press, London: 315–322. [Google Scholar]
- Winter G. (1885). Ascomyceten: Gymnoasceen und Pyrenomyceten. Dr. L. Rabenhorst’s Kryptogamen Flora von Deutschland, Oesterreich und der Schweiz. Zweite Auflage 1(2): i–viii: 1–928. [Google Scholar]
- Woudenberg JH, Aveskamp MM, de Gruyter J, et al. (2009). Multiple Didymella teleomorphs are linked to the Phoma clematidina morphotype. Persoonia 22: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav M, Singh A, Balan V, et al. (2019). Biological treatment of lignocellulosic biomass by Chaetomium globosporum: process derivation and improved biogas production. International Journal of Biological Macromolecules 128: 176–183. [DOI] [PubMed] [Google Scholar]
- Yang CH, Lin MJ, Su HJ, et al. (2014a). Multiple resistance-activating substances produced by Humicola phialophoroides isolated from soil for control of Phytophthora blight of pepper. Botanical Studies 55: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Shi P, Huang H, et al. (2014b). Two xylose-tolerant GH43 bifunctional β-xylosidase/α-arabinosidases and one GH11 xylanase from Humicola insolens and their synergy in the degradation of xylan. Food Chemistry 148: 381–387. [DOI] [PubMed] [Google Scholar]
- Yilmaz N, Visagie CM, Houbraken J, et al. (2014). Polyphasic taxonomy of the genus Talaromyces. Studies in Mycology 78: 175–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule GU. (1925). A Mathematical theory of evolution, based on the conclusions of Dr. J. C. Willis, F.R.S. Philosophical Transactions of the Royal Society B 213: 21–87. [Google Scholar]
- Zanne AE, Tank DC, Cornwell WK, et al. (2014). Three keys to the radiation of angiosperms into freezing environments. Nature 506: 89–92. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Li HQ, Zong SC, et al. (2012). Chemical and bioactive diversities of the genus Chaetomium secondary metabolites. Mini-Reviews in Medicinal Chemistry 12: 127–148. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wu W, Cai L. (2017b). Polyphasic characterisation of Chaetomium species from soil and compost revealed high number of undescribed species. Fungal Biology 121: 21–43. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wu WP, Dian MH, et al. (2014a). A new thermophilic species of Myceliophthora from China. Mycological Progress 13: 165–170. [Google Scholar]
- Zhang QH, Zhang J, Yang L, et al. (2014b). Diversity and biocontrol potential of endophytic fungi in Brassica napus. Biological Control 72: 98–108. [Google Scholar]
- Zhang ZF, Liu F, Zhou X, et al. (2017a). Culturable mycobiota from Karst caves in China, with descriptions of 20 new species. Persoonia 39: 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZF, Zhao P, Cai L. (2018). Origin of cave fungi. Frontiers in Microbiology 9: 1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao RL, Li GJ, Sánchez-Ramírez S, et al. (2017). A six-gene phylogenetic overview of Basidiomycota and allied phyla with estimated divergence times of higher taxa and a phyloproteomics perspective. Fungal Diversity 84: 43–74. [Google Scholar]
- Zhu L, Song J, Zhou JL, et al. (2019) Species diversity, phylogeny, divergence time, and biogeography of the genus Sanghuangporus (Basidiomycota). Frontiers in Microbiology 10: 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zopf W. (1881). Zur Entwicklungsgeschichte der Ascomyceten: Chaetomium. Nova Acta Academiae Caesareae Leopoldino-Carolinae Germanicae Naturae Curiosorum 42: 199–292. [Google Scholar]
- Zuckerkandl E, Pauling L. (1965). Evolutionary divergence and convergence in proteins. In: Evolving genes and proteins (Bryson V, Vogel HJ, eds). Academic Press INC, USA: 97–166. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic tree resulting from ML analysis of the ITS gene region alignment. The confidence values are indicated at the nodes in the same way as in Fig. 7. Genus clades are discriminated with boxes in different colours and clades containing thermophilic species are highlighted with an orange background. The scale bar shows the expected number of changes per site. The tree is rooted with Pseudoechria longicollis and Schizotheciaceae, in the Schizotheciaceae.
Phylogenetic tree resulting from ML analysis of the partial tub2 gene region alignment. The confidence values are indicated at the nodes in the same way as in Fig. 7. Genus clades are discriminated with boxes in different colours and clades containing thermophilic species are highlighted with an orange background. The scale bar shows the expected number of changes per site. The tree is rooted with Pseudoechria longicollis and Schizotheciaceae, in the Schizotheciaceae, Sordariales.
Phylogenetic tree resulting from ML analysis of the partial rpb2 gene region alignment. The confidence values are indicated at the nodes in the same way as in Fig. 7. Genus clades are discriminated with boxes in different colours and clades containing thermophilic species are highlighted with an orange background. The scale bar shows the expected number of changes per site. The tree is rooted with Pseudoechria longicollis and Pseudoechria prolifica, in the Schizotheciaceae.
Phylogenetic location of “Humicola koreana” based on the separate analyses of partial gene sequences of ITS (A) and LSU (B). The confidence values are indicated at the nodes in the same way as in Fig. 7. The scale bar shows the expected number of changes per site. The tree is rooted with Achaetomium strumarium.
Comparison ascospore sizes measured in lactic acid (present study) and water (literature) as mounting medium.
Overview species and strains used in phylogenetic analysis.