Objectives:
This is a descriptive study to characterize rates of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in pediatric solid organ transplant (SOT) recipients during the early days of the pandemic. We hypothesized that asymptomatic infection may represent a large proportion of SARS-CoV-2 infection in pediatric SOT recipients.
Methods:
We queried Organ Transplant Tracking Record (OTTR) for all pediatric SOT recipients followed at our center and reviewed medical records to identify patients tested for SARS-CoV-2 between March 15, 2020 and June 30, 2021. Patients were tested by polymerase chain reaction (PCR): prior to planned procedures or because of symptoms; OR: tested by measurement of IgG to spike protein with their routine labs q 2-monthly. A positive PCR was called acute infection. A positive IgG with negative PCR was called convalescence. For immunologic studies, blood was obtained when the PCR or IgG was positive. Statistical comparisons were made between (1) acute infection versus convalescence; (2) acute infection versus SOT recipients without infection (called healthy controls); (3) liver transplant (LT) versus small bowel (SB)/multivisceral transplant (MVT); (4) positive versus negative test result.
Results:
Of 257 LT recipients, 99 were tested: 6 were PCR positive, 13 were antibody positive. Of 150 SB/MVT recipients, 55 were tested: 4 were PCR positive, 6 were antibody positive. Of 8 simultaneous liver, kidney transplant recipients, 3 were tested: 1 was PCR positive. Symptoms when present were mostly mild. Patients with a positive test result were younger (6.3 vs 10.0 years; P = 0.017). We observed a rapid decline in viral load within 96 hours without a change in immunosuppression. Antibody lasted >8 months beyond the time it was monitored. Acute infection was associated with increased CD4 and CD8 TEM cell frequency (P = 0.04, P = 0.03, respectively), decreased interferon (IFN)-γ production from T-cells (2.8% vs 11.3%; P = 0.006), and decreased CD8 TEMRA frequency (4.56% vs 11.70%; P = 0.006).
Conclusions:
Early in the pandemic, COVID-19 disease was mostly mild in pediatric SOT recipients with no rejection, patient death, or graft loss observed.
Keywords: COVID-19 disease, solid organ transplantation
What Is Known
Children represent a small but increasing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) burden.
Once infected, the risk of severe disease and death lower compared to adults.
What Is New
Effector memory responses observed in pediatric solid organ transplant recipients following SARS-CoV-2 infection.
Despite the above observation, no rejection or graft loss observed following SARS-CoV-2 infection in pediatric solid organ transplant recipients.
Despite receiving immunosuppressive therapy, pediatric solid organ transplant recipients demonstrated an effective immune response with viral clearance.
Immunosuppression minimization likely not needed following SARS-CoV-2 infection.
Children represent 1%–2% of the total severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) burden (1). Outside of the multisystem inflammatory syndrome in children associated with COVID-19 disease, (MIS-C), critical illness is rare, with most children being asymptomatic, or having mild symptoms.
Among the many unknowns is the immune response to infection in pediatric solid organ transplant (SOT) recipients. These patients rarely have co-morbidities known to increase risk of severe disease, and thus prevalence of severe disease would presumably be rare.
This descriptive study characterizes the rate of SARS-CoV-2 infection in pediatric SOT recipients and assesses immunologic response to infection. We hypothesized that asymptomatic infection may represent a large proportion of SARS-CoV-2 infection in pediatric SOT recipients. Demographic and immunologic comparisons were made between (1) symptomatic versus asymptomatic patients; (2) acute infection versus convalescence; (3) isolated liver transplant (LT) versus small bowel (SB)/multivisceral transplant (MVT) recipients; (4) SARS-CoV-2–positive versus SARS-CoV-2–negative patients.
METHODS
Patients
In March 2020, our pediatric SOT center instituted testing for SARS-CoV-2 IgG antibody to spike protein in all recipients. Testing was done monthly (for isolated small bowel/multivisceral transplant recipients) or every 2 months (for isolated liver or simultaneous liver/kidney transplant recipients). Additionally, patients with symptoms of SARS-CoV-2 infection or pre-procedure (ileoscopy, endoscopy, colonoscopy, liver biopsy) were tested by polymerase chain reaction (PCR). For this descriptive study, the Organ Transplant Tracking Record database, Organ Transplant Tracking Record (OTTR), was queried for all pediatric SOT recipients followed at our center. Medical records were reviewed to identify patients who had at least 1 test for SARS-CoV-2 between March 15, 2020 (when the above transplant center policy was instituted) and June 30, 2021 (Fig. 1). For immunologic studies, patients were recruited as soon as a positive test was identified. Specifically, blood was obtained within 1, 4, and 7 days of a positive SARS-CoV-2 PCR or SARS-CoV-2 IgG in 4, 4, and 3 patients, respectively. Positive SARS-CoV-2 IgG antibody to spike protein with a negative PCR was defined as convalescent. Acute SARS-CoV-2 infection was defined as a positive SARS-CoV-2 PCR on a nasopharyngeal sample or bronchoalveolar sample. Healthy controls comprised pediatric SOT recipient blood samples that predated October 2019. Thus, the blood samples were from patients who had no exposure to SARS-CoV-2. Information on clinical symptoms, blood count, presence of co-morbidities associated with severe disease (eg, obesity, diabetes, hypertension), immunosuppression and drug levels at diagnosis was obtained from the medical record. Demographics and clinical characteristics are summarized in Tables 1–3 and Tables 2 and 4, Supplemental Digital Content, http://links.lww.com/MPG/C866.
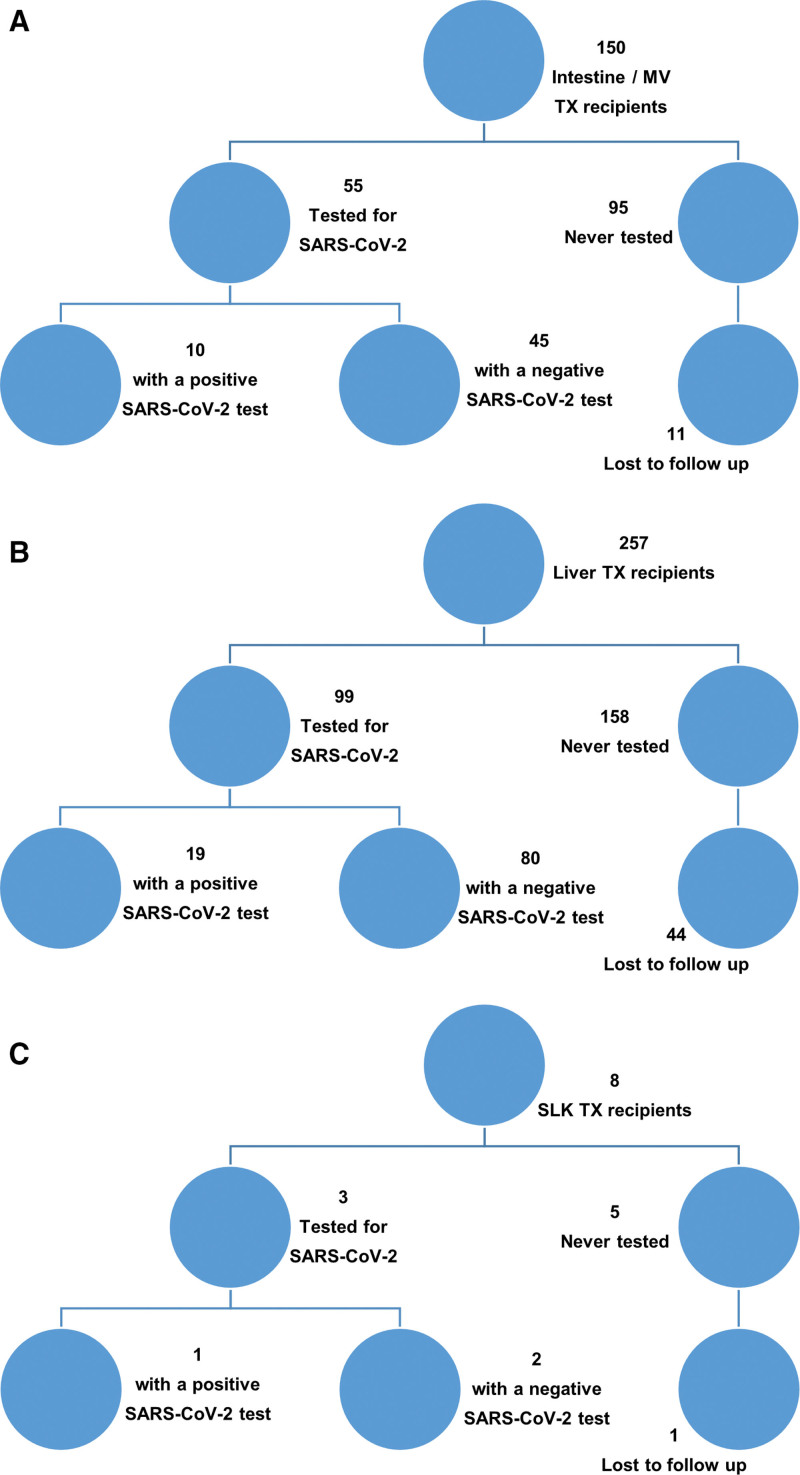
FIGURE 1.
Flow diagram showing participant recruitment. (A) Small bowel/multivisceral transplanted patient cohort. (B) Isolated liver transplanted patient cohort. (C) Simultaneous liver/kidney transplanted patient cohort. MV = multivisceral; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2; SLK = simultaneous liver-kidney; TX = transplant.
TABLE 1.
Demographic data stratified by COVID-19 test positive vs COVID-19 test negative
| Variable | COVID-19 test negative (n = 127) | COVID-19 test positive*† (n = 30) | P value |
|---|---|---|---|
| Age, y | |||
| Median (25%–75% IQR) | 10.02 (5.66–14.57) | 6.30 (4.33–10.08) | 0.017 |
| Duration from TX at testing, y | |||
| Median (25%–75% IQR) | 5.28 (1.82–9.45) | 4.36 (1.73–6.06) | 0.33 |
| Tacrolimus level, ng/mL | |||
| Median (25%–75% IQR) | 5.20 (3.70–7.90) | 4.50 (3.00–7.40) | 0.61 |
| Gender | |||
| Female | 66 (52) | 10 (33) | 0.10 |
| Male | 61 (48) | 20 (67) | |
| Transplant type | |||
| LT | 80 (63) | 19 (63) | 0.66 |
| MVT/SB | 45 (35) | 10 (33) | |
| SLK | 2 (2) | 1 (3) | |
| No. of IS drugs‡ | |||
| 1 | 38 (30) | 8 (27) | 0.90 |
| 2 | 67 (53) | 17 (57) | |
| ≥3 | 21 (17) | 5 (17) | |
The italicized P value is statistically significant. IS = immunosuppression; LT = liver transplant; MVT = multivisceral transplant; SB = small bowel; SLK = simultaneous liver-kidney.
Listed patient excluded.
SARS-CoV-2 polymerase chain reaction and IgG-positive patients.
1 patient off all IS.
TABLE 3.
Clinical characteristics of SARS-CoV-2 IgG–positive convalescent patients
| Patient ID | Gender | TX type | Duration from TX at detection, y | Age at detection, y | Previous documented non-SARS-CoV-2 coronavirus infection | Symptomatic or asymptomatic |
|---|---|---|---|---|---|---|
| 013 | F | MVT | 5.81 | 10.1 | HKU1 | Symptomatic |
| 014 | M | SB | 8.26 | 9.99 | No | Symptomatic |
| 015 | F | LT | 1.12 | 4.26 | No | Asymptomatic |
| 016 | M | LT | 5.12 | 6.20 | No | Asymptomatic |
| 017 | F | LT | 5.04 | 5.65 | No | Asymptomatic |
| 018 | M | LT | 14.32 | 15.59 | No | Asymptomatic |
| 019 | F | LT | 4.94 | 6.33 | No | Asymptomatic |
| 020 | M | MVT | 4.30 | 5.04 | No | Asymptomatic |
| 021 | M | LT | 2.25 | 3.87 | No | Symptomatic |
| 022 | M | LT | 14.01 | 14.09 | No | Asymptomatic |
| 023 | M | LT | 0.10 | 0.99 | No | Symptomatic |
| 024 | M | SB | 0.55 | 1.80 | No | Asymptomatic |
| 025 | F | LT | 9.61 | 10.03 | No | Asymptomatic |
| 026 | F | LT | 4.29 | 4.90 | No | Asymptomatic |
| 027 | M | LT | 4.31 | 18.78 | No | Asymptomatic |
| 028 | M | LT | 5.10 | 7.33 | No | Asymptomatic |
| 029 | M | LT | 10.24 | 12.45 | No | Asymptomatic |
| 030 | M | MVT | 6.15 | 8.16 | No | Asymptomatic |
| 031 | M | SB | 11.27 | 12.43 | No | Symptomatic |
LT = liver transplant; MVT = multivisceral transplant; PCR = polymerase chain reaction; SB = small bowel.
Complete details of the methods of antibody testing, immunophenotyping including activation cocktail used, quantitative reverse transcriptase (RT)-PCR, and multiplex immunohistochemistry are described in Table 1, Supplemental Digital Content, http://links.lww.com/MPG/C866.
Statistics
Clinical characteristics of patients with acute infection and convalescent patients were descriptively summarized. The primary endpoint is seropositivity rate, defined as the rate of either SARS-CoV-2–PCR positive or SARS-CoV-2 IgG positive. Fisher’s exact 95% confidence interval was used to assess seropositivity rate for overall or by each group. Duration of seropositivity was examined using Kaplan-Meier method. Wilcoxon rank sum test was used to compare absolute lymphocyte count (ALC) and tacrolimus levels between acute infection and convalescent groups. Analysis of variance (ANOVA) was used to compare CD3, CD20, CD4, and CD8 cell frequencies among acute infection, convalescent, and healthy control groups. For exploratory analyses, principal component analysis (PCA) was conducted to examine grouped activities of 7 flow cytometry parameters (CD4 naive, CD8 naive, CD4 memory, CD8 memory, activated CD4 cells, CD4 IFN-γ, CD8 IFN-γ) among acute infection, convalescent, and healthy control groups. Volcano plots report log2fold changes and unadjusted P values based on Wilcoxon rank sum test for 40 flow cytometry parameters in acute infection relative to healthy controls, and in convalescence relative to healthy controls. Two-sided P < 0.05 is considered statistically significant and no multiplicity correction was applied for exploratory analyses.
This study was approved by Georgetown University IRB (Study Number: 2017-0365).
RESULTS
Demographics
Of 257 recipients of an isolated LT, 99 were tested, 6 were PCR positive, and 13 were antibody positive. Of 150 isolated SB/MVT recipients, 55 were tested, 4 were PCR positive, and 6 were antibody positive. Of 8 simultaneous liver, kidney transplant recipients, 3 were tested, 1 patient was PCR positive. One listed patient was PCR positive while awaiting MVT (Fig. 1A–C; Table 1). Patients with a positive test were significantly younger (6.3 vs 10.0 years; P = 0.017; Table 1). No patient was vaccinated. This suggests in the period March 2020 to June 2021, a minority of pediatric SOT recipients in the DC, Maryland, Virginia (DMV) region had been exposed to SARS-CoV-2.
Clinical Outcomes
Twelve patients (11 post-transplant; 1 listed) were PCR positive (Table 2). Patients with acute infection trended closer to transplant (P = 0.09; Table 2, Supplemental Digital Content, http://links.lww.com/MPG/C866). Four patients had contact with infected adult household member. Two patients had biopsy-proven acute rejection (BPAR) 6 months and 3½ years preceding infection, respectively. Six patients received corticosteroids; 3, sirolimus; and 2, mycophenolate; 6 months preceding infection. Additional immunosuppression at diagnosis included corticosteroids in 6 patients, mycophenolate in 1 patient, and sirolimus in 4 patients.
TABLE 2.
Clinical characteristics of SARS-CoV-2 PCR–positive patients with acute infection
| Patient ID | Gender | TX type | Duration from TX at diagnosis, y | Age at diagnosis, y | Viral load, cp/mL | Ct value | Previous documented non-SARS-CoV-2 corona virus infection | Symptomatic or asymptomatic |
|---|---|---|---|---|---|---|---|---|
| 001 | F | LT | 0.45 | 6.3 | nd | n/a | No | Symptomatic |
| 002 | M | SLK | 1.18 | 19.3 | nd | n/a | No | Asymptomatic |
| 003 | M | MVT | 5.74 | 6.4 | nd | n/a | OC43 | Asymptomatic |
| 004 | M | LT | 0.68 | 1.6 | Day 12: 212 | Day 12: 38.24 | HKU1 | Symptomatic |
| 005 | F | LT | 3.12 | 6.7 | nd | n/a | No | Symptomatic |
| 006 | F | LT | 1.62 | 2.3 | Day 0: 26,415 | Day 0: 17.23 | No | Asymptomatic |
| Day 4: 567 | Day 4: 33.46 | |||||||
| 007 | M | MVT | 2.07 | 3.5 | Day 0: 102,785,648 | Day 0: 15.12 | No | Symptomatic |
| Day 1: 60,879,327 | Day 1: 16.14 | |||||||
| Day 19: 41,278 | Day 19: 27.6 | |||||||
| 008 | M | LT | 4.41 | 6.1 | nd | n/a | No | Symptomatic |
| 009 | M | MVT | 13.86 | 14.4 | nd | n/a | No | Symptomatic |
| 010 | F | MVT | 1.54 | 2.5 | Day 0: 50,676,225 Day 1: nd |
Day 0: 16.63 Day 1: 19.1 |
No | Symptomatic |
| 011 | M | LT | 3.40 | 4.6 | nd | n/a | No | Symptomatic |
| 012* | F | n/a | n/a | 1.1 | Day 0: 567 Day 16: 312 |
Day 0: 33.46 Day 16: 35.56 |
No | Symptomatic |
LT = liver transplant; MVT = multivisceral transplant; n/a = not applicable; nd = not done; PCR = polymerase chain reaction; SLK = simultaneous liver-kidney.
Listed patient.
Of 7 patients admitted, duration of admission ranged between 24 and 72 hours in initial 12 months of the pandemic; between April and June 2021, admission duration exceeded 72 hours. Only 1 patient required supplemental oxygen. One patient underwent bronchoscopy (PCR on bronchoalveolar lavage fluid was positive). That patient received dexamethasone and remdesivir. One patient had elevated liver enzymes, underwent a liver biopsy that showed mild non-specific lymphocytic portal inflammation with absence of viral copies in the liver on qPCR. ALC and tacrolimus trough level shown in Figures 1 and 2, Supplemental Digital Content, http://links.lww.com/MPG/C866. Viral load rapidly declined over the course of the illness even in the absence of immunosuppression changes (Table 2). Six of the 12 PCR-positive patients went on to develop antibody as at manuscript preparation.
Nineteen patients had positive SARS-CoV-2 IgG antibody (Table 3). None of the 19 IgG antibody-positive patients had a previously documented PCR positive test. ALC and tacrolimus level at IgG antibody detection shown in Figures 1 and 2, Supplemental Digital Content, http://links.lww.com/MPG/C866. Four patients had BPAR 2 months, 2 years, and 4 years preceding antibody detection. One patient received infliximab 6 months prior to antibody detection. Fourteen patients were on additional immunosuppression, namely, prednisolone (n = 7), mycophenolate (n = 5), sirolimus (n = 3), ruxolitinib (n = 1) at antibody detection.
Two patients were admitted: the first was hospitalized for management of poor glucose control and was found to have a skin rash, which led to antibody being checked. Skin biopsy of the rash was thought to be consistent with eczema. The duration of hospitalization was 5 days, mostly for social reasons. The second patient was admitted for fever in a patient with a central line. Blood culture was negative. The patient developed multiorgan failure resulting in a prolonged pediatric intensive care unit (PICU) stay and had positive antibody though a preceding positive PCR had never been documented and it is unclear the relationship, if any, between the positive antibody and multiorgan failure. The patient recovered and was discharged.
Overall, no major changes were made to immunosuppression in response to a positive PCR or positive IgG antibody. There were no cases of rejection, graft loss, or mortality.
Altogether, seropositivity rate was 19/99; 19% [isolated LT: 95% confidence interval (CI) 0.11–0.27]; 10/55; 18% (SB/MVT: 95% CI 0.09–0.30), with a median duration of seropositivity >250 days (Figure 3, Supplemental Digital Content, http://links.lww.com/MPG/C866). No patient had re-infection during the study period.
The above suggests SARS-CoV-2 infection was not associated with increased morbidity in our patient cohort. Moreover, being on immunosuppression and a preceding history of rejection did not result in poorer outcomes.
We found no significant differences between symptomatic and asymptomatic patients with respect to immunosuppression, co-morbidities, ALC, age at infection, and duration from transplant (Tables 3 and 4, Supplemental Digital Content, http://links.lww.com/MPG/C866). The median (range) ALC in symptomatic and asymptomatic patients shown in Figure 1, Supplemental Digital Content, http://links.lww.com/MPG/C866. Mean white blood cell count, absolute neutrophil and monocyte count at diagnosis was significantly higher in isolated LT versus SB/MVT patients [white blood cell (WBC) 8.7 k/µL vs 5.6 k/µL; absolute neutrophil count (ANC) 4.6 k/µL vs 2.5 k/µL; absolute monocyte count (AMC) 0.6 k/µL vs 0.2 k/µL; P = 0.02; P = 0.015; P = 0.008, respectively].
In summary, lymphopenia was not a hallmark of SARS-CoV-2 infection in our patient cohort, neither did the presence of co-morbidities result in poor outcomes. Similarly, none of the isolated LT recipients who had higher AMC had severe disease.
Immune Response to SARS-CoV-2 Infection in Pediatric SOT Recipients
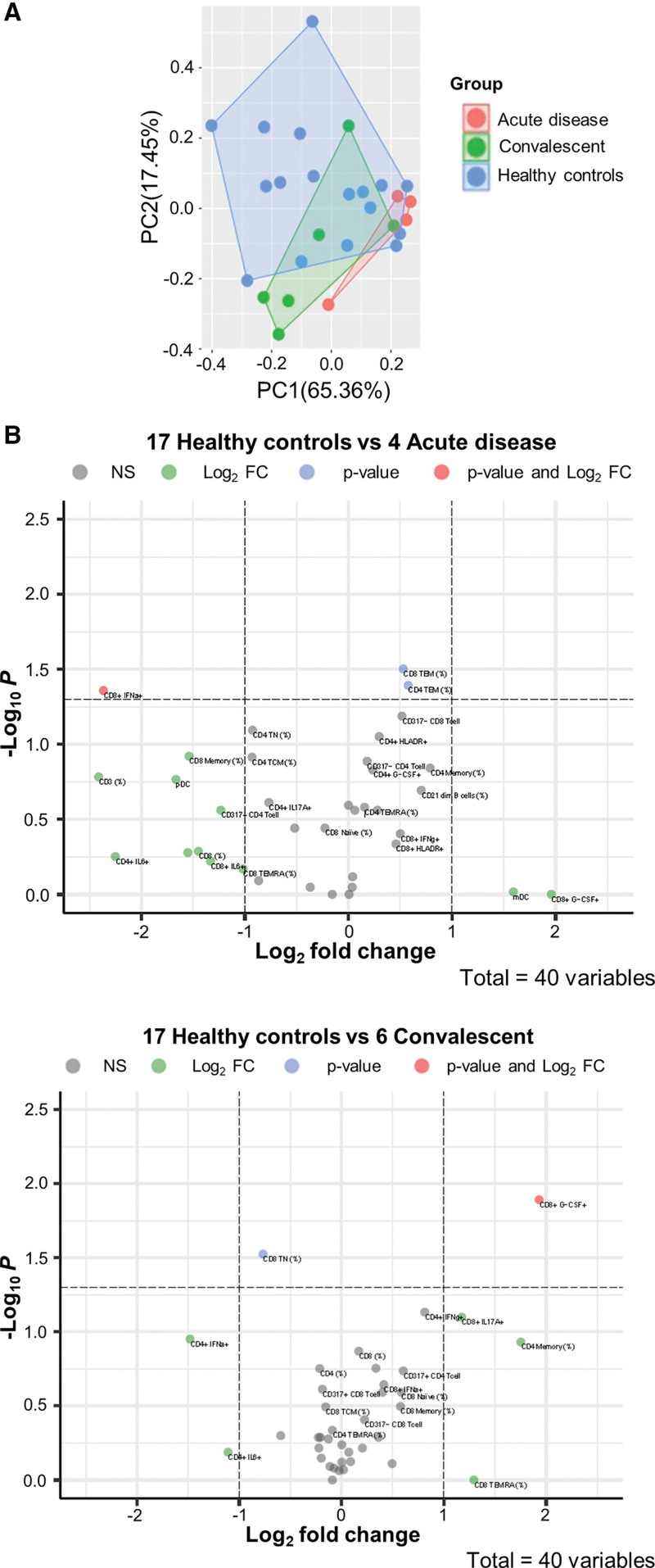
Frequencies of CD3, CD4, CD8 T-cells, and CD20 B-cells were not significantly different between patients with acute infection, convalescent patients, and healthy pediatric SOT patients with no exposure to/infection with SARS-CoV-2 (Figure 4, Supplemental Digital Content, http://links.lww.com/MPG/C866), neither were they significantly different between symptomatic and asymptomatic patients (data not shown). Number of immunosuppressive drugs, tacrolimus level, age, gender, duration from transplant, and race was not significantly different between patients with a positive test versus healthy pediatric SOT patients (Table 5, Supplemental Digital Content, http://links.lww.com/MPG/C866). To test the hypothesis that children with infection would display an over-arching immune signature, we undertook PCA for all patients with acute infection, convalescent patients, and controls with no exposure or infection for whom full datasets was available as is required for PCA. In seeking a signature of active infection, we began by considering only 7 flow cytometry parameters which segregated patients with acute infection from control and convalescent (seropositive) patients; there was some overlap between convalescent and control patients (Fig. 2A).
FIGURE 2.
Immunophenotyping showing distinct features of the immune system in COVID-19 disease. (A) Principal component analysis shows that patients with acute infection segregate away from healthy control and convalescent patients; there was some overlap between convalescent and healthy control patients. Color denotes disease status (red: acute disease n = 4; green: convalescent n = 6; blue: healthy controls n = 17). (B) Volcano plot shows that when acute infection was compared with healthy controls there is a (0.57 log2fold increase in CD4 TEM and 0.53 log2fold increase in CD8 TEM cell frequency; P = 0.04, P = 0.03, respectively). Concomitantly, CD8 IFN-α cells were depleted (−2.4 log2fold decrease; P = 0.04). When convalescent was compared with healthy controls, there was enrichment in CD8 GCSF cells (1.9 log2fold increase; P = 0.013). Concomitantly, CD8 TN cells were depleted (−0.8 log2fold decrease; P = 0.03). IFN = interferon.
T lymphocytes are pivotal in tackling viral infections as cytotoxic CD8+ T lymphocytes kill infected cells and CD4+ T cells provide the signals to optimize effective and durable adaptive immune responses. The absence of reduction of T lymphocytes, particularly absence of reduction of CD8+ T cells probably reflects the fact that most patients in our study had mild disease as patients with severe disease are reported to have contraction of the CD8+ lymphocyte compartment.
To identify statistically significant discriminators between SARS-CoV-2 positive and controls, we analyzed effect sizes for all flow cytometry data. This depicted a SARS-CoV-2 immunophenotype enriched in CD4 TEM, and CD8 TEM when acute infection was compared with healthy controls (0.57 log2fold increase in CD4 TEM and 0.53 log2fold increase in CD8 TEM cell frequency; P = 0.04, P = 0.03, respectively). Concomitantly, CD8 interferon (IFN)-α cells were depleted (−2.4 log2fold decrease; P = 0.04; Fig. 2B and Table 6, Supplemental Digital Content, http://links.lww.com/MPG/C866). The convalescent versus healthy controls comparison depicted a SARS-CoV-2 immunophenotype enriched in CD8 GCSF cells (1.9 log2fold increase; P = 0.013). Concomitantly, CD8 TN cells were depleted (−0.8 log2fold decrease; P = 0.03; Fig. 2B and Table 6, Supplemental Digital Content, http://links.lww.com/MPG/C866).
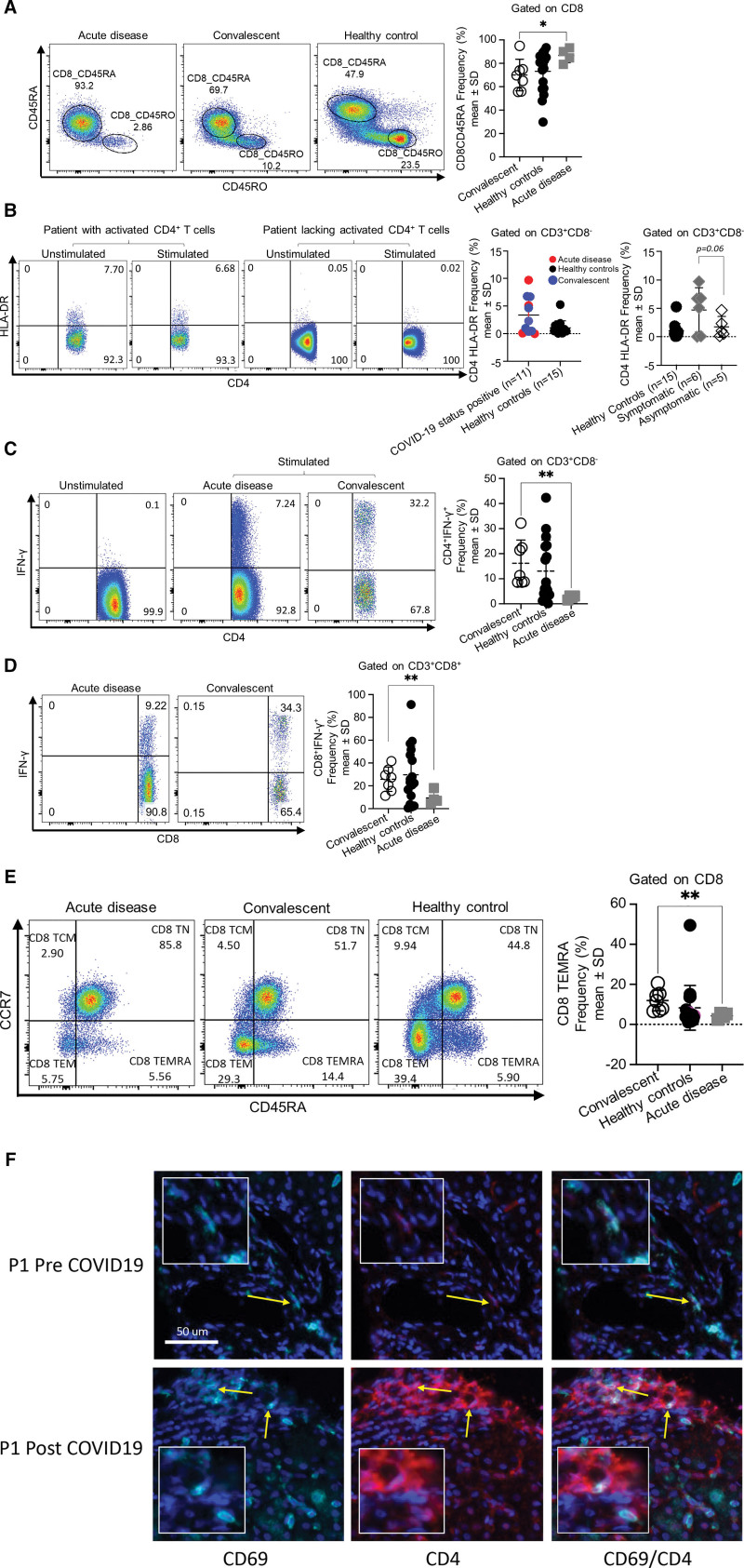
We next sought to characterize the SARS-CoV-2 infection T-cell signature. Patients with acute infection had an overall higher frequency of CD8CD45RA naive cells compared to convalescent patients (Fig. 3A; P = 0.04) and similarly demonstrated a trend toward higher CD4CD45RA naive cells compared to convalescent patients (Figure 5, Supplemental Digital Content, http://links.lww.com/MPG/C866; P = 0.07). Patients with acute infection and convalescent patients segregated into 2 groups: activated CD4+ T-cells, and limiting-response CD4+ T-cells (Fig. 3B). Majority of patients with activated CD4+ T-cells were symptomatic (Fig. 3B). Of the 5 patients in our cohort who had activated CD4+ T-cells, 3 continue to be antibody positive 9, 10, and 6 months, respectively, since the first antibody positive result noted; 2 are presently antibody negative but notably were antibody positive for 6 and 3 months, respectively. Four of 5 had short symptom duration, with only 1 patient having a prolonged hospital course.
FIGURE 3.
Patients with COVID-19 disease display low TEMRA and IFN-γ frequencies, with variable activation of T cells. (A) Increase frequency of CD8+ naive cells during acute infection. Representative flow cytometry plot and summary graph (acute infection, n = 4, convalescent, n = 7, healthy controls, n = 17). (B) Patients with COVID-19 disease segregate into 2 groups: 1 group with activated CD4+ T cells, and the second group with limited CD4+ T cell responses. Representative flow cytometry plot and summary graph (COVID-19–positive status n = 11, healthy controls n = 15; symptomatic n = 6, asymptomatic n = 5). (C and D) Low IFN-γ production from T cells during acute infection. Representative flow cytometry plot and summary graph (acute infection n = 4, convalescent n = 7, healthy controls n = 17). (E) Low CD8+ TEMRA frequency during acute infection. Representative flow cytometry plot and summary graph (acute infection n = 4, convalescent n = 7, healthy controls n = 17). Plots denote mean and standard deviation. *P < 0.05 **P < 0.01. (F) Immunohistochemistry of liver showing infrequent CD4 TRM cells (second row) within the portal tract of convalescent patient, and absence of same in liver biopsy of same patient pre-dating onset of COVID-19 pandemic (first row). TRM defined as CD4+CD69+. Lt -> Rt: CD69 magenta; CD4 red; CD4CD69 co-expressing cells. Larger square magnification ×20. IFN = interferon.
CD4+ and CD8+ T-cells of patients with acute infection produced significantly less IFN-γ compared to CD4+ and CD8+ T-cells of convalescent patients (Fig. 3C, D). Additionally, CD8 TEMRA frequency was significantly lower in patients with acute infection compared to CD8 TEMRA in convalescent patients (Fig. 3E). Gating strategy (Figure 6, Supplemental Digital Content, http://links.lww.com/MPG/C866).
The above findings suggest an ongoing CD8 T cell response to infection and may explain the milder clinical outcomes in our patient cohort as immunocompetent adults with severe disease show a reduced frequency of CD8 TEM cells (2). Moreover, the lower IFN-γ production by T cells may drive a less inflammatory environment that promotes milder COVID-19 outcomes.
To understand whether local changes in the immune landscape of the transplanted allograft contribute to the decreased susceptibility to infection in children, we profiled how the immune cell composition, specifically TRM CD8+ and CD4+ T-cell subset, in the transplanted graft changed pre- and post-infection. We found infrequent CD4 T TRM in liver biopsies performed at the time of PCR positivity or seropositivity compared to biopsies from the same patients that pre-date SARS-CoV-2–positive status (Fig. 3F).
DISCUSSION
This is the largest cohort of pediatric SOT recipients that includes seropositivity data and characterizes T-cell responses during acute infection and recovery. We had no mortality and documented a rapid decline in viral load within the first 96 hours in most instances (Table 2) without changing immunosuppression management, suggesting pediatric SOT recipients mount an effective immune response that clears virus. The literature on viral load and mortality prediction is contradictory (3–5). We posited a protective role of past non–SARS-CoV-2 infection, however a minority of patients had a history of non–SARS-CoV-2 infection suggesting this did not account for the good outcomes in our patients. We observed different patterns of lymphocyte responses in acute infection compared to the recovery phase, specifically higher frequency of CD8CD45RA naive cells in the former (Fig. 3A), and CD4+, CD8+ T-cells of the latter exhibiting a TH1 response associated with an increased frequency of CD8 TEMRA (Fig. 3C–E). Like the report by Mathew et al (6), some patients displayed robust CD4 T-cell activation (Fig. 3B). Activated T-cells can migrate to the site of infection and facilitate viral clearance, suggesting an immunoprotective potential (7), moreover, polyfunctional T-cells that express more than 1 cytokine or effector molecule have been described as a hallmark of protective immunity in viral infections (8,9). Specifically, IFN-γ and tumor necrosis factor (TNF)-α co-producing CD4+ and CD8+ T-cells have been shown to indicate effector/memory phenotype and long-term protection in SARS-CoV infection (10,11). Though we did not observe differences in production of effector cytokines such as interleukin (IL)-6, granzyme B, between patients with acute infection versus convalescent and symptomatic versus asymptomatic patients (data not shown), the differences in IFN-γ production were striking and significant. Moreover, the convalescent patients had cleared virus and were PCR negative suggesting that there were differences in T-cell responses in patients who had cleared the virus versus those who did not. The exact time of infection in our cohort (as in most other reports) is not known. We observed that increases of IFN-γ did not result in or contribute toward a hyperinflammatory response. In contrast to adults with severe COVID-19 disease who have marked depletion of CD4 TEM and CD8 TEM cells (12), our patients had a log2 fold increase in CD4TEM and CD8TEM frequency which is likely a reflection of their mild, symptomatic disease and the absence of T-cell cytopenia. Depletion in CD8 TEMRA cells, naive (TN), and central memory (TCM) CD8+ cells is reported in adults with severe COVID-19 disease and attributed in part to ongoing differentiation into other states (12). CD8 TEMRA frequency was lower in our patients with acute infection versus convalescent patients and was not accompanied by depletions in the other CD8 subsets (TN, TCM, TEM) suggesting the absence of ongoing differentiation into other states, and probably a finding associated with mild disease. In contrast to adults (13–15), our patients had normal or elevated ALC (Figure 1, Supplemental Digital Content, http://links.lww.com/MPG/C866), with no significant differences in CD3, CD4, CD8, and CD20 frequencies compared to healthy pediatric SOT patients (Figure 4, Supplemental Digital Content, http://links.lww.com/MPG/C866). This may have contributed to better clinical outcomes. Given that mild, symptomatic disease was more prevalent in our cohort with zero mortality, our results may reflect expected findings in patients with uneventful recovery. Poor COVID-19 disease outcomes have been reported in patients with inflammatory co-morbidities and the common link has been hypothesized to be inflammasome activation (16). Though most of our patients lacked co-morbidities (Table 3, Supplemental Digital Content, http://links.lww.com/MPG/C866), severe disease was absent in the few with co-morbidities. We did not examine for the presence of inflammasome activation in our cohort but acknowledge the need to investigate for this as it may explain some of the differences observed in our pediatric cohort compared to the adult cohort (16).
A feature of our cohort was antibody durability (Figure 3, Supplemental Digital Content, http://links.lww.com/MPG/C866). Chen et al (17) describe a subset of adult patients who showed sustained antibody levels, shorter symptom duration, and increases in frequencies of previously activated CD4+ cells suggesting a distinct immunophenotype connecting symptomatic disease resolution kinetics and antibody duration dynamics. While our numbers are small, interrogating our patients for presence of somatic hypermutation in virus-specific memory B-cell antibody genes like the patients described by Chen et al would be prudent (17). We did not follow the T-cell responses and are unable to comment on the stability of CD4 T-cell activation and durability of T cell responses but acknowledge further work is required to define mechanisms that underlie our observations and evaluate the durability of protective immune responses elicited by infection with SARS-CoV-2.
Finally, our demonstration of TRM CD4+ T-cells in the liver biopsies of 2 (1 acutely-infected and 1 convalescent) patients (but not in liver biopsies of same patients done in 2019) was surprising given the fact that pathogen specific TRM cells are a frontline defense that exert an immediate response following virus re-exposure (18). While we acknowledge we do not know for certain that these were SARS-CoV-2 virus-specific TRM CD4+T-cells, we cannot ignore previous biopsies from the same patients showed no TRM T cells, and only biopsies done during acute SARS-CoV-2 infection and during convalescence demonstrated this finding. Deposition of influenza virus-specific TRM along the airways is a key for establishing long-term heterosubtypic immunity to influenza (19), and the loss of influenza-specific TRM renders the host susceptible to re-infection (19–21). More work needs to be done to understand the role, if any, TRM play in maintaining immunity to SARS-CoV-2 infection.
There are several limitations to our study. First, our patient numbers are small. It is therefore important that our observations are confirmed in future studies with a larger patient number. Our observations highlight our avoidance of “knee jerk” changes to immunosuppression in response to infection, that may have resulted in a different graft outcome. Second, stimulation was done with phorbol myristate acetate (PMA)/ionomycin which bypasses the T-cell membrane receptor complex and not SARS-CoV-2 peptide. While we acknowledge our immunological observations are not definitively in response to SARS-CoV-2 infection, our results will form the basis for future studies in this patient population. Third, this was a cross-sectional study that did not follow the dynamics of T-cell responses, which is planned in future studies, and will elucidate understanding of immune response to SARS-CoV-2 infection in pediatric SOT recipients. Fourth, it is possible the true incidence of SARS-CoV-2 infection is underestimated in our cohort given concerns about the ability of this patient cohort to effectively generate antibody responses to infection; Moreover, not all patients were tested at the time of manuscript submission so the true incidence of SARS-CoV-2 infection may have been underestimated. Finally, our study did not evaluate specific cellular immune response to SARS-CoV-2. This will need to be done in the future.
CONCLUSIONS
In our small single center report, we observed that SARS-CoV-2 infection in pediatric SOT recipients is generally mild and associated with rapid viral clearance. The seropositivity rate is 19% in isolated LT, and 18% in SB/MVT recipients, with a median duration exceeding 8 months. We had zero mortality and zero graft loss, and infection did not result in a generalized immune activation that could lead to rejection.
Supplementary Material
Footnotes
The data that support the findings of this study are available from the corresponding author upon request.
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.jpgn.org).
REFERENCES
- 1.Buonsenso D, Sali M, Pata D, et al. Children and COVID-19: microbiological and immunological insights. Pediatr Pulmonol 2020;55:2547–55. [DOI] [PubMed] [Google Scholar]
- 2.Notarbartolo S, Ranzani V, Bandera A, et al. Integrated longitudinal immunophenotypic, transcriptional and repertoire analyses delineate immune responses in COVID-19 patients. Sci Immunol 2021;6:eabg5021. [DOI] [PubMed] [Google Scholar]
- 3.Pujadas E, Chaudhry F, McBride R, et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med 2020;8:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh KA, Jordan K, Clyne B, et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect 2020;81:357–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelleni MT. SARS CoV-2 viral load might not be the right predictor of COVID-19 mortality. J Infect 2021;82:e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathew D, Giles JR, Baxter AE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020;369:eabc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 2020;183:158–168.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duvall MG, Precopio ML, Ambrozak DA, et al. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol 2008;38:350–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006;107:4781–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol 2004;78:5535–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Channappanavar R, Fett C, Zhao J, et al. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J Virol 2014;88:11034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laing AG, Lorenc A, Del Molino Del Barrio I, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med 2020;26:1623–1635 [DOI] [PubMed] [Google Scholar]
- 13.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine 2020;55:102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen R, Sang L, Jiang M, et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J Allergy Clin Immunol 2020;146:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroemer A, Khan K, Plassmeyer M, et al. Inflammasome activation and pyroptosis in lymphopenic liver patients with COVID-19. J Hepatol 2020;73:1258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Zuiani A, Fischinger S, et al. Quick COVID-19 healers sustain anti-SARS-CoV-2 antibody production. Cell 2020;183:1496–1507.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen TH, McAuley JL, Kim Y, et al. Influenza, but not SARS-CoV-2, infection induces a rapid interferon response that wanes with age and diminished tissue-resident memory CD8(+) T cells. Clin Transl Immunol 2021;10:e1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu T, Hu Y, Lee YT, et al. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol 2014;95:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakim LM, Smith J, Caminschi I, et al. Antibody-targeted vaccination to lung dendritic cells generates tissue-resident memory CD8 T cells that are highly protective against influenza virus infection. Mucosal Immunol 2015;8:1060–71. [DOI] [PubMed] [Google Scholar]
- 21.Pizzolla A, Nguyen THO, Smith JM, et al. Resident memory CD8(+) T cells in the upper respiratory tract prevent pulmonary influenza virus infection. Sci Immunol 2017;2:eaam6970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.