Abstract
Hypocretin/Orexin (HCRT) is a neuropeptide that is associated with both stress and reward systems in humans and rodents. The different contributions of signaling at hypocretin-receptor 1 (HCRT-R1) and hypocretin-receptor 2 (HCRT-R2) to compulsive alcohol drinking are not yet fully understood. Thus, the current studies used pharmacological and viral-mediated targeting of HCRT to determine participation in compulsive alcohol drinking and measured HCRT-receptor mRNA expression in the extended amygdala of both alcohol-dependent and non-dependent male rats. Rats were made dependent through chronic intermittent exposure to alcohol vapor and were tested for the acute effect of HCRT-R1-selective (SB-408124; SB-R1), HCRT-R2-selective (NBI-80713; NB-R2), or dual HCRT-R1/2 (NBI-87571; NB-R1/2) antagonism on alcohol intake. NB-R2 and NB-R1/2 antagonists each dose-dependently decreased overall alcohol drinking in alcohol-dependent rats, whereas, SB-R1 decreased alcohol drinking in both alcohol-dependent and non-dependent rats at the highest dose (30 mg/kg). SB-R1, NB-R2, and NB-R1/2 treatment did not significantly affect water drinking in either alcohol-dependent or non-dependent rats. Additional PCR analyses revealed a significant decrease in Hcrtr1 mRNA expression within the central amygdala (CeA) of dependent rats under acute withdrawal conditions compared to nondependent rats. Lastly, a shRNA-encoding adeno-associated viral vector with retrograde function was used to knockdown HCRT in CeA-projecting neurons from the lateral hypothalamus (LH). LH-CeA HCRT knockdown significantly attenuated alcohol self-administration in alcohol-dependent rats. These observations suggest that HCRT signaling in the CeA is necessary for alcohol-seeking behavior during dependence. Together, these data highlight a role for both HCRT-R1 and -R2 in dependent alcohol-seeking behavior.
Keywords: Hypocretin/orexin, alcohol, central amygdala, retro-adeno-associated viral vector, alcohol-dependence, self-administration
1. Introduction
Alcohol use disorder (AUD), is a multifaceted psychiatric disorder characterized by compulsive alcohol seeking, loss of control over intake, and the emergence of a negative emotional state, including dysphoria, anxiety, depression, that occurs during alcohol withdrawal. During alcohol withdrawal, brain stress systems are dysregulated in both humans [1–4] and rodents [5–8], resulting in an increased sensitivity to stress. Exposure to alcohol vapor in rodents is an animal model of dependence that closely mimics somatic and motivational signs of AUD resembling those of the human condition [9,10]. It is hypothesized that following prolonged alcohol dependence, negative reinforcement mechanisms predominate, in which brain stress systems are recruited and the drug is taken to alleviate negative affective states associated with drug withdrawal (for review, [11]).
Hypocretin/orexin (HCRT) is a neuropeptide believed to be dysregulated in alcohol dependence (for review, [12–14]). HCRT neuropeptides have been associated with both stress and drug seeking behaviors [13–15]. The two HCRT neuropeptides, HCRT-1 and HCRT-2, are synthesized within a restricted region of the dorsal hypothalamus, including the lateral hypothalamus proper, adjacent perifornical area, and dorsomedial hypothalamus [16,17]. HCRT neurons project widely throughout the brain [18–21] and target two G-protein-coupled receptors, HCRT receptor 1 and receptor 2 (HCRT-R1 and -R2, respectively) [17]. HCRT projections include reciprocal connections to the extended amygdala and other subcortical regions that are implicated in negative reinforcement [19,20]. These brain regions, including the central amygdala (CeA) and nucleus accumbens shell (NAs), are suspected to contribute towards enhanced motivation underlying compulsive-like intake during drug and alcohol dependence [11]. HCRT-R1 exhibits a higher affinity for HCRT-1, whereas HCRT-R2 has relatively equal affinities for HCRT-1 and −2 [17]. Antagonists targeting HCRT-Rs are either selective for a single hypocretin-receptor subtype (e.g., HCRT-R1: SB-334867, SB-408124, ACT-539313; HCRT-R2: JNJ-10394049, NBI-80713) or non-selective for dual hypocretin-receptors (e.g., almorexant, suvorexant, lemborexant, etc.), all with varying degrees of affinity and specificity for respective HCRT-R subtypes [22,23]. HCRT neurotransmission has been shown to mediate the reinforcement of drug-seeking behavior for all major drug classes, including psychostimulants [24–33], nicotine [34–38], opioids [39–41], and alcohol [42–51]. Relevant to alcohol, studies indicate a role for both HCRT-R1 and HCRT-R2 signaling in self-administration of alcohol in alcohol-preferring rats [52–55]. Additionally, antagonism of HCRT-R1 can reduce alcohol intake in mice made alcohol-dependent by chronic intermittent alcohol vapor [56]. Treatment with the dual HCRT-R1/2 antagonist, TCS1102, prevented stress-induced reinstatement in rats made dependent by chronic intermittent exposure to alcohol vapor [57]. However, little is known about the role of HCRT neurotransmission at HCRT-R1 and -R2, individually or in combination, on the compulsive-like responding in animal models of alcohol dependence.
Here, we tested the hypothesis that altered HCRT signaling at both HCRT-R1 and -R2, mediate compulsive-like alcohol intake associated with repeated withdrawal periods in an alcohol-dependent state. Thus, we examined the effects of varying HCRT-receptor-specific antagonists administered systemically on responding for alcohol in non-dependent rats and rats made dependent on alcohol by exposure to chronic intermittent ethanol vapor (CIEV). Additionally, Hcrtr1 and Hcrtr2 gene expression was examined in reward/stress-related brain regions of the CeA and NAs of dependent rats during withdrawal from alcohol and in non-dependent controls. Finally, a novel shRNA-encoding adeno-associated viral vector (AAV) with retrograde function was microinjected into the CeA to knockdown HCRT projections from the lateral hypothalamus (LH) to the CeA in alcohol-dependent rats. This retrograde AAV Hcrt silencing was expected to decrease alcohol self-administration in alcohol-dependent rats due to the decrease in HCRT presence in the LH-CeA pathway.
2. Material and Methods
2.1. Animals
Adult male Wistar rats (N = 80; Charles River, Raleigh, NC), weighing between 225–275 grams at the beginning of the experiments, were housed in groups of 2–3 per cage in a temperature-controlled (22°C) vivarium on a 12/12-hour light/dark cycle (lights on at 18:00) with ad libitum access to food and water. The animals were allowed to acclimate to the animal facility for at least 7 days before training. All procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute (behavioral pharmacology experiments), the National Institute on Drug Abuse (AAV experiments), and at East Tennessee State University (AAV timeline experiments).
2.2. Experiment 1: Behavioral pharmacology of HCRT-R-specific antagonism
2.2.1. Alcohol and water self-administration
Alcohol and water self-administration sessions were conducted simultaneously in standard operant conditioning chambers (Med Associates, St. Albans, VT, USA). Briefly, for the training of alcohol and water lever pressing, rats were first given free-choice access to alcohol (10% w/v) and water for 1 day in their home cages, and then were given one overnight session in the operant chamber with access to food (ad libitum) and one lever that delivered alcohol on a fixed ratio (FR1) schedule of reinforcement where one lever press resulted in 0.1 ml alcohol delivery. In the following days, the rats were transitioned to 30 min sessions with two levers available (alcohol or water; FR1; 0.1 ml liquid/press) until stable levels of 10% (w/v) alcohol intake were reached with sessions occurring 4–5 days per week. Upon stable levels of responding for alcohol, rats were split into two groups (alcohol-dependent [CIEV-exposed; n = 21] and non-dependent [air-exposed; n = 21]) and operant behavior was maintained in self-administration sessions 2 times per week thereafter.
2.2.2. Alcohol-dependence induction via CIEV exposure
Following operant self-administration training, half of the rats were made dependent on alcohol via daily exposure to CIEV as previously described [7,58,59]. Cycles of alcohol intoxication and withdrawal (i.e., alcohol 14h ON/10h OFF) occurred daily for a minimum of 2 and up to 4 weeks, after which blood alcohol concentration (BAC) levels during vapor exposure ranged between 150–250 mg/dL. Following dependence induction, alcohol-dependent rats were maintained at BACs of 150–250 mg/dL with daily CIEV. Behavioral testing of operant alcohol self-administration occurred during the 10-hour period without alcohol vapor, 6–8 hours into withdrawal when brain and blood alcohol levels are negligible [60]. In this model, rats exhibit somatic withdrawal signs and negative emotional symptoms reflected by anxiety-like responses and elevated brain reward thresholds [59,61–67]. Rats exposed to ambient air were used as non-dependent controls. All behavioral testing occurred 2 times per week in 30-minute sessions for both alcohol-dependent and non-dependent rats. Alcohol-dependent rats were returned to CIEV chambers following operant testing.
2.2.3. Pharmacological testing
SB-408124 (SB-R1; AdooQ Bioscience, Irvine, CA, USA), NBI-80713 and NBI-87571 (NB-R2 and NB-R1/2, respectively; Neurocrine Biosciences, Inc., San Diego, CA, USA) were dissolved in 5% dimethyl sulfoxide and 5% Cremophor EL in sterile water. NBI-87571 ((3S)-2-[2-(3,4-dimethoxyphenoxy)ethyl]-N-[(1R)-1-phenylethyl]-1,2,3,4-tetrahydroisoquinoline-3-carboxamide, MW=460.57 g/mol) is a high-affinity dual HCRT-R1/2 antagonist (Rat HCRT-R1 Ki= 3 nM; Rat HCRT-R2 Ki=10.4 nM).
All drugs were injected intraperitoneally in a volume of 3 ml/kg 20 minutes prior to behavioral testing. Doses were chosen based on previously published studies and limited pilot studies [40,68,69]. For alcohol self-administration, the animals received all doses of one antagonist type (SB-R1: 0, 3, 10, 30 mg/kg; NB-R2: 0, 7.5, 15, 30 mg/kg; or NB-R1/2: 0, 3, 10, 30 mg/kg) in a within-subject Latin-Square design. A regular FR1 alcohol self-administration session without antagonist treatment was performed between testing days.
2.3. Experiment 2: HCRT-R mRNA expression levels in the extended amygdala of alcohol-dependent rats
2.3.1. Reverse transcription and quantitative PCR
A separate cohort of rats were trained for operant alcohol self-administration, as described above. Rats were then made alcohol-dependent via CIEV (as described above; n = 9) or non-dependent (air-exposed controls; n =9) and allowed 12 operant self-administration sessions (over 3 weeks) prior to euthanasia. Brains from alcohol-dependent and non-dependent rats were collected and snap-frozen with isopentane for measurements of Hcrtr1 and Hcrtr2 mRNA levels during acute alcohol withdrawal (approximately 24 hours after the vapor was turned off). Importantly, 6–48 hours after the end of exposure to CIEV, rats exhibit both somatic and motivational signs of withdrawal, and the motivational signs can still be observed 3–5 weeks into alcohol abstinence [58,59,63,64,66]. Thus, the time point of 24 hours for brain collection should be well-within the period of withdrawal. Furthermore, this time point was chosen to target more stable dysregulations of gene expression and to avoid potentially transient effects caused by early withdrawal. Brains were sliced on a cryostat, and bilateral tissue punches were collected from NAs (500 μm thickness, 0.5 mm diameter) and the CeA (500 μm thickness, 1.0 mm diameter). RNA was extracted and purified from the brain punches using the PureLink RNA Mini Kit (Ambion, Austin, TX, USA) following the manufacturer’s instructions. cDNA was reverse transcribed from total RNA using the Superscript III First Strand Synthesis System (Invitrogen, Carlsbad, CA, USA). Gene expression levels were determined by quantitative polymerase chain reaction (qPCR) using a TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA, USA). Reactions were carried out as described previously [7] and cDNA concentrations of Hcrtr1 and Hcrtr2 were calculated according to the relative quantification (delta-delta Ct) method, corrected for differences in PCR efficiency, normalized to glyceraldehyde-3-phosphate dehydrogenase (Gapdh). Primers used were as follows: TaqMan qPCR utilized commercially available Hcrtr1 (Rn00565032_m1), Hcrtr2 (Rn00565155_m1) and Gapdh (Rn99999916_s1) primer/probe sets (Applied Biosystems Inc., Foster City, CA), with PCR conditions according to the manufacturer’s protocol.
2.4. Experiment 3: Retrograde AAV knockdown of HCRT projections to CeA
2.4.1. AAV constructs
Recombinant short hairpin RNA (shRNA) encoding AAV vectors were produced using an AAV helper-free system (Stratagene, France; as described in [70]). All AAV vectors were produced by the Genetic Engineering and Viral Vector Core at the National Institute on Drug Abuse Intramural Research Program. In these vectors, the shRNA sequence is expressed under the control of the mouse ubiquitin 6 (mU6) promoter, while the enhanced green fluorescent protein (GFP) tag is expressed under the control of the cytomegalovirus (CMV) promoter to label transduced cells. The shRNA sequence targeting the Hcrt transcript (-HCRT; 5’-GTCTTCTATCCCTGTCCTAGT-3’) was selected using the BLOCK-iT RNAi Designer algorithm (ThermoFisher). A scrambled sequence (-SCR; 5’-GCTTACTTTCGGCTCTCTACT-3’) was used as negative control. Loop sequence was 5’-AGTCGACA-3’ for both. The shRNA-HCRT and -SCR constructs were packaged into a retrogradely transported AAV2 serotype (AAVretro; titer of 7.4×1011 GU/mL). Specificity for these particular HCRT shRNA plasmid constructs [26, 71] and AAVretro-HCRTshRNA function [71] have been previously described by our group and others, indicating no direct off-target effects of the shRNA-AAV on neurons anatomically common to the dorsal hypothalamus that co-exist with HCRT, including prodynorphin- and melanin concentrating hormone-producing neurons.
2.4.2. AAVretro microinjections
Rats were anesthetized with isoflurane (1–3%) and mounted in a stereotaxic frame (Kopf Instruments, Tujiunga, CA, USA). A stainless steel 30-gauge injector was used to microinject either the AAVretro-HCRT or -SCR vectors contra- and unilaterally (time course experiment) or bilaterally (alcohol behavioral experiment) into the CeA (AP −2.2 mm; ML ± 4.5 mm from bregma, and DV −8.3 mm from dura). Micronjections (0.5 μL/site) were made using a micro-infusion pump (Kd Scientific LEGATO 130 pump, Holliston, MA, USA) with a flow rate of 0.15 μL/min over 3.3 minutes. Injectors remained in place for 10 minutes to assure adequate diffusion of solution and prevent backflow along the injector track.
2.4.3. Immunohistochemistry and HCRT cell quantification
In AAVretro studies, rats were euthanized, perfused with normal saline followed by 4% formaldehyde at 2, 4, or 6 weeks (time course experiment) or approximately 8 weeks (alcohol behavioral experiment) following AAVretro microinjection. The brains were collected, post-fixed overnight in 4% formaldehyde, and then transferred to a sucrose solution (20–30%) the following day. Brains were cryosectioned (40 μm) and stored in 0.01M phosphate buffered saline (PBS) with 0/1% sodium azide at 4°C. For HCRT immunolabeling, sections were rinsed with 0.3% Triton X-100 in 0.01 M PBS, then quenched with 0.75% hydrogen peroxide and blocked with 10% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA). Sections were then rinsed and incubated for 24 hours at room temperature with rabbit anti-rat prepro-HCRT antibody (AB3096, 1:1000; diluted in 0.1 M PBS with 10% Triton X-100; EMD Millipore, Billerica, MA, USA). Tissue was then rinsed with 0.01 M PBS, incubated with biotinylated goat anti-rabbit secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) for 90 minutes, rinsed with PBS-Tx, incubated in avidin-biotin complex (Elite ABC-HRP kit, Vector Laboratories) for 90 minutes, rinsed with 0.01 M PBS and then visualized with Vector SG substrate (Vector Laboratories) to yield a blue–gray precipitate. After staining, sections were mounted on microscope slides, dehydrated, cleared (Histoclear; Fisher Scientific, Itasca, IL, USA) and coverslipped.
Immunostained sections were analyzed on an AmScope Fluorescence/Light Microscope equipped with AmScopeAmLite software and a 20MP C-mount microscope camera (MU2003-BI-RU1, AmScope, Irvine, CA, USA). GFP fluorescence was used to detect the brain area within the CeA transduced by the viral vector and was confirmed for correct placement along with visualization of the most ventral point of the needle track using brightfield. Quantification of HCRT-positive cells containing a distinct soma with blue precipitate. For the time course experiment, counting was performed on two coronal sections (anterior-posterior level ranging from −2.6 to −2.9 mm from Bregma) using manual counting at a magnification of 20x by an observer blind to the experimental condition. HCRT-positive cell counts were derived from a capture window that encompassed the entire HCRT-neuronal field dorsoventrally and mediolaterally. Counts were totaled across two sections for each hemisphere. For the alcohol behavioral experiment, counting was performed on one coronal section (anterior-posterior level −2.8 mm from Bregma) using manual counting at a magnification of 20x by an observer blind to the experimental condition. HCRT-positive cell counts were derived from a capture window that encompassed the entire HCRT-neuronal field dorsoventrally and mediolaterally. Counts were totaled across both hemispheres.
2.5. Statistical analysis
All data are expressed as means and standard errors of the mean (+SEM). Self-administration data were analyzed using a repeated-measures two-way analysis of variance (ANOVA), with group (non-dependent and dependent) as the between-subjects factor and dose (SB-R1 and NB-R1/2: 0, 3, 10, and 30 mg/kg; NB-R2: 0, 7.5, 15, and 30 mg/kg) as the within-subjects factor, with post hoc comparisons performed using a Holm-Sidak multiple-comparison test when appropriate. Dose-response curves for each antagonist on alcohol and water self-administration were analyzed using a repeated-measures one-way ANOVA on alcohol-dependent and non-dependent groups separately, with post hoc comparisons performed using a Dunnett’s multiple comparison test when appropriate. A two-way mixed-factor ANOVA was used to assess the efficacy of the 30 mg/kg dose for each antagonist on alcohol and water self-administration in alcohol-dependent and non-dependent rats with group (non-dependent and dependent) as the between-subjects factor and HCRT-R antagonist (SB-R1, NB-R2, and NB-R1/2) as the within-subjects factor. When appropriate, post hoc comparisons were performed using a Holm-Sidak multiple-comparisons test. For quantitative PCR analyses, data are expressed as relative mean mRNA expression fold change (2^ΔΔCT) and were analyzed using a non-parametric Mann-Whitney U test for each region and genotype tested. AAVretro data were analyzed using a repeated-measures two-way ANOVA, with AAVretro-treatment group (AAVretro-HCRT and -SCR) as the between-subjects factor and time (BL, +3, +4, and +5 weeks; or +2, +4 and +6 weeks for time course experiment) as the within-subjects factor. When appropriate, post hoc comparisons were performed using a Dunnett’s (behavioral testing) or Holm-Sidak (time course) multiple-comparison test. P values less than 0.05 were considered statistically significant for all tests.
3. Results
3.1. Experiment 1: Behavioral pharmacology of HCRT-R-specific antagonism
3.1.1. Effects of HCRT-R1 antagonism on alcohol and water self-administration
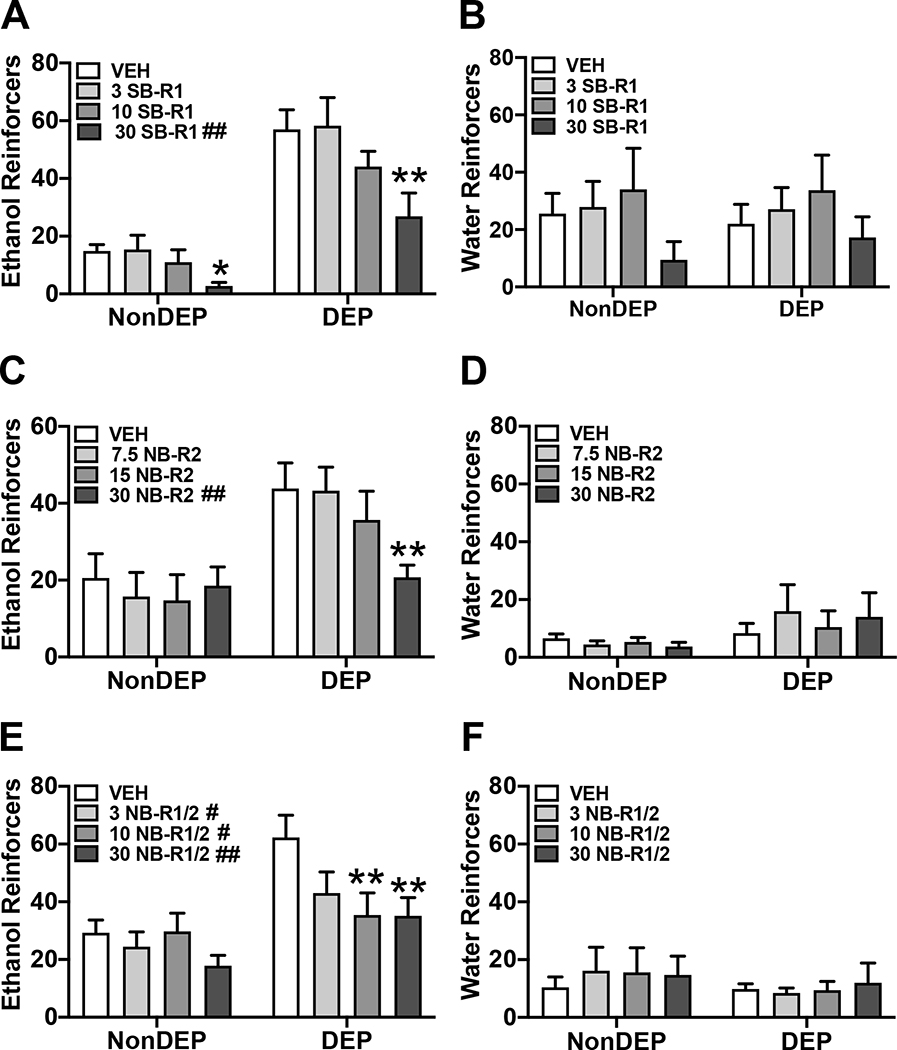
SB-R1 (0, 3, 10, or 30 mg/kg) was used to assess the effects of HCRT-R1 antagonism on alcohol self-administration in alcohol-dependent (n=7) and non-dependent (n=7) rats. Water self-administration was assessed concurrently during the session. There was a main effect of dose and group on alcohol drinking in rats (Dose: F(3,36)= 10.02, p< 0.01; Group: F(1,12)= 32.34, p< 0.01; Dose × group: F(3,36)= 1.86, p= 0.15). Post hoc analyses for the main effect of SB-R1 dose on alcohol drinking showed the 30 mg/kg, but not 3 or 10 mg/kg, dose significantly reduced alcohol drinking compared to 0 mg/kg vehicle controls. The 30 mg/kg dose of SB-R1 also significantly reduced alcohol drinking compared to the 3 and 10 mg/kg doses. The results of the repeated-measures one-way ANOVA revealed that treatment with SB-R1 antagonist significantly reduced alcohol drinking in both non-dependent rats (Figure 1A; F(3,18)= 3.37, p< 0.05) and dependent rats (Figure 1A; F(3,18)= 6.77, p< 0.01). Post hoc analyses showed the 30mg/kg dose significantly decreased alcohol drinking in non-dependent (p< 0.05) and dependent rats (p< 0.01) compared to vehicle controls. For water drinking, there was a main effect of dose (Dose: F(3,36)= 3.53, p< 0.05; Group: F(1,12)= 0.01, p= 0.94; Dose × group: F(3,36)= 0.29, p= 0.83). Post hoc analyses for the main effect of dose on water drinking showed only the 30 mg/kg dose significantly reduced alcohol drinking compared to the 10 mg/kg dose of SB-R1. Water drinking in non-dependent rats (Figure 1B; F(3,18)= 1.98, p= 0.15) and dependent rats (Figure 1B; F(3,18)= 1.78, p= 0.19) was not significantly reduced compared to vehicle controls. Treatment with SB-R1 significantly reduced alcohol drinking in non-dependent and dependent rats, but did not significantly reduce water drinking in either group.
Figure 1.
HCRT-R-subtype antagonists have differential effects on alcohol self-administration. A, C, and E) Bars represent mean number (+SEM) of ethanol reinforcers per dose for SB-R1 (A), NB-R2 (C), or NB-R1/2 (E) treatment in both non-dependent (NonDEP) and alcohol-dependent (DEP) rats. A) SB-R1 (30 mg/kg) significantly decreased the number of ethanol reinforcers in both NonDEP and DEP rats. C) NB-R2 (30 mg/kg) dose significantly decreased the number of ethanol reinforcers in DEP rats. E) NB-R1/2 (10 and 30 mg/kg) significantly decreased the number of ethanol reinforcers in DEP rats. B, D, and F) Bars represent mean number (+SEM) of water reinforcers per dose for SB-R1 (B), NB-R2 (D), or NB-R1/2 (F) treatment in both NonDEP and DEP rats. SB-R1 (B), NB-R2 (D), and NB-R1/2 (F) did not significantly change the number of water reinforcers in either NonDEP or DEP rats versus respective vehicle. #p < 0.05 and ##p< 0.01 versus vehicle (analyzed using two-way ANOVA); *p<0.05 and **p<0.01 versus respective vehicle (analyzed using one-way ANOVA).
3.1.2. Effects of HCRT-R2 antagonism on alcohol and water self-administration
NB-R2 (0, 7.5, 15, or 30 mg/kg) was used to assess the effects of HCRT-R2 antagonism on alcohol self-administration in a separate cohort of alcohol-dependent (n=7) and non-dependent (n=7) rats. Water self-administration was assessed concurrently during the session. There was a main effect of dose and group, and interaction of dose × group on alcohol drinking (Figure 1C; Dose: F(3,36)= 5.25, p< 0.01; Group: F(1,12)= 6.04, p< 0.05; Dose × group: F(3,36)= 5.50, p< 0.01). Post hoc analyses for the interaction of NB-R2 dose × group on alcohol drinking showed the 30 mg/kg, but not 7.5 or 15 mg/kg, dose significantly reduced alcohol drinking compared to 0 mg/kg vehicle controls in dependent rats only. The 30 mg/kg dose of NB-R2 also significantly reduced alcohol drinking compared to the 7.5 and 15 mg/kg doses in dependent rats. There was no effect of NB-R2 on alcohol drinking in non-dependent rats. The results of the repeated-measures one-way ANOVA revealed that treatment with NB-R2 antagonist significantly reduced alcohol drinking in dependent rats (Figure 1C; F(3,18)= 7.28, p< 0.01), but not in non-dependent rats (Figure 1C; F(3,18)= 1.03, p= 0.40). Post hoc analyses showed the 30mg/kg dose significantly decreased alcohol drinking in dependent rats (p< 0.01) compared to vehicle control. For water drinking, there was no main effect of dose or group on water drinking (Figure 1D; Dose: F(3,36)= 0.47, p= 0.70; Group: F(1,12)= 1.25, p= 0.29; Dose × group: F(3,36)= 1.64, p= 0.20). Water drinking in non-dependent rats (Figure 1D; F(3,18)= 0.91, p= 0.45) and dependent rats (Figure 1D; F(3,18)= 1.08, p= 0.38) was not significantly reduced compared to vehicle controls. Thus, the effects of NB-R2 were specific for alcohol drinking in dependent rats.
3.1.3. Effects of dual HCRT-R1/2 antagonism on alcohol and water self-administration
NB-R1/2 (0, 3, 10, 30 mg/kg) was used to assess the effects of HCRT-R1/2 antagonism on alcohol self-administration in separate cohort of alcohol-dependent (n=7) and non-dependent (n=7) rats. Water self-administration was assessed concurrently during the session. There was a main effect of dose and group on alcohol drinking (Dose: F(3,36)= 5.85, p< 0.01; Group: F(1,12)= 7.92, p< 0.05; Dose × group: F(3,36)= 2.80, p= 0.05). Post hoc analyses for the main effect of NB-R1/2 dose on alcohol drinking showed the 10 and 30 mg/kg, but not 3 mg/kg, dose significantly reduced alcohol drinking compared to 0 mg/kg vehicle controls. The results of the repeated-measures one-way ANOVA revealed that treatment with NB-R1/2 antagonist significantly reduced alcohol drinking in dependent rats (Figure 1E; F(3,18)= 5.00, p< 0.05), but not in non-dependent rats (Figure 1E; F(3,18)= 2.5, p= 0.09). Post hoc analyses showed the 10 (p< 0.01) and 30 (p< 0.01) mg/kg doses significantly reduced alcohol drinking in dependent rats compared to vehicle control. For water drinking, there was no main effect of dose or group on water drinking (Dose: F(3,36)= 0.44, p= 0.73; Group: F(1,12)= 0.36, p= 0.561; Dose × group: F(3,36)= 0.61, p= 0.61).Water drinking in non-dependent rats (Figure 1F; F(3,18)= 0.83, p= 0.49) and dependent rats (Figure 1F; F(3,18)= 0.25, p= 0.86) was not significantly changed compared to vehicle control. Thus, the effects of NB-R1/2 were specific for alcohol drinking in dependent rats.
3.1.4. Comparative effects of HCRT-R antagonism
To directly compare efficacy of HCRT-R antagonism on alcohol and water self-administration at an equal, maximally effective dose (30 mg/kg) across compounds, a mixed-factor two-way ANOVA was performed on the percentage change from vehicle (0 mg/kg dose) scores. There was a main effect of HCRT-R antagonist type and group × HCRT-R antagonist interaction on the percent change of alcohol drinking from vehicle (Figure 2A; Antagonist type: F(2,36)= 7.01, p< 0.01; Group: F(1,36)= 2.62, p= 0.11; Antagonist type × group: F(2,36)= 4.75, p< 0.05). Post hoc analyses show that the percent change of alcohol drinking from vehicle is significantly different in non-dependent rats treated with SB-R1 compared to NB-R2 (p< 0.01) or NB-R1/2 (p< 0.05). Also, there is a significant difference in the percent change of alcohol drinking from vehicle of non-dependent rats treated with NB-R2 compared to NB-R1/2 (p< 0.05). Thus, the HCRT-R antagonists have varying degrees of efficacy in non-dependent controls. In dependent rats, the HCRT-R antagonists did not exhibit significantly different effects on the percent change of alcohol drinking from vehicle. Thus, HCRT-R antagonists have similar maximal efficacy in attenuating alcohol self-administration by approximately 50% relative to vehicle treatment in dependent rats. In water self-administration, there was no main effect of HCRT-R antagonist type or group (Figure 1E; Antagonist type: F(2,36)= 1.12, p= 0.34; Group: F(1,36)= 0.13, p= 0.72; Antagonist type × group: F(2,36)= 0.26, p< 0.78).
Figure 2.
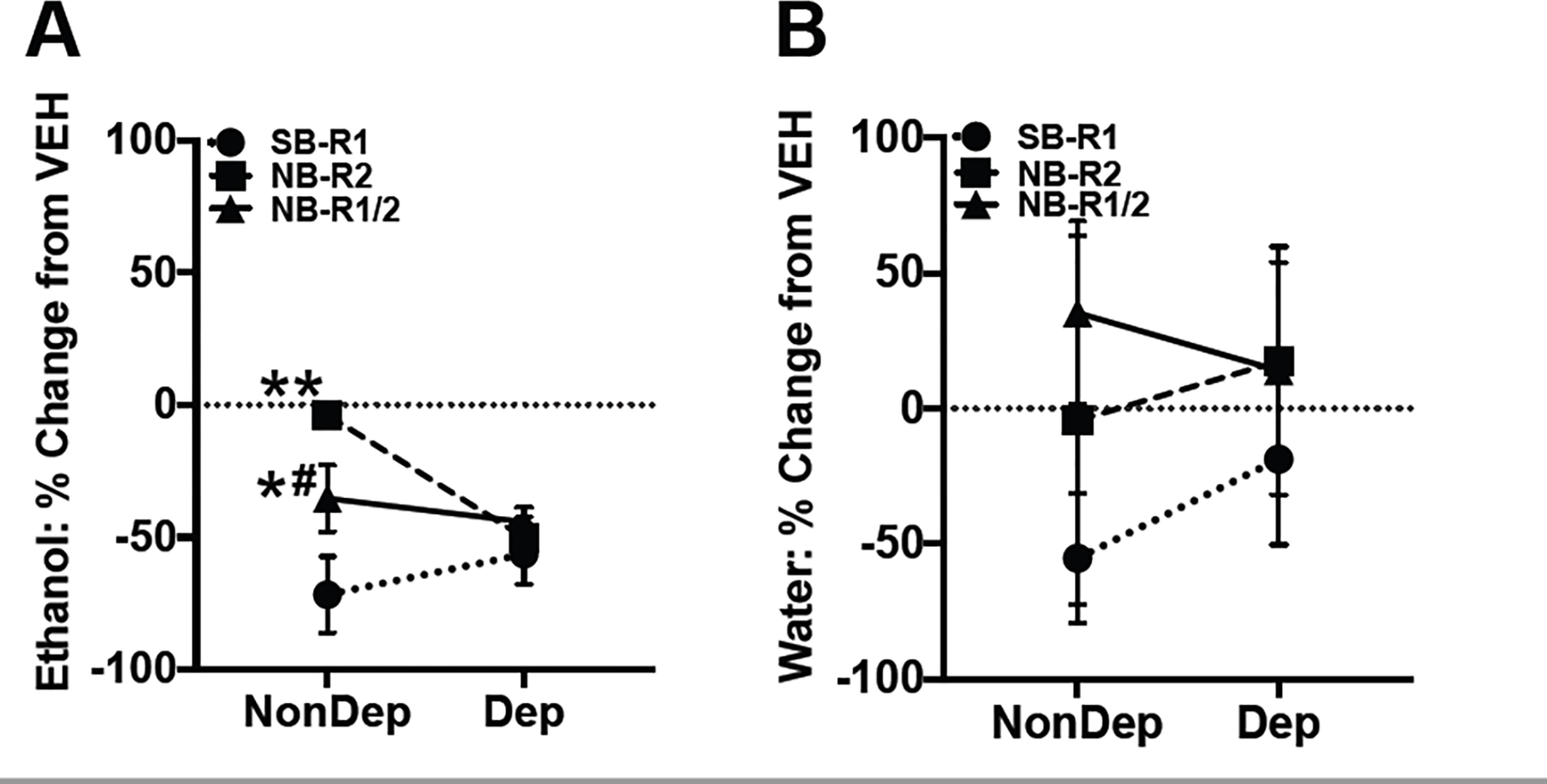
Comparative effects of HCRT-R antagonism efficacy on alcohol and water self-administration. A) Markers represent the percent change in mean number (±SEM) of ethanol reinforcers from vehicle (VEH; 0 mg/kg) treatment per HCRT-R-subtype antagonist for SB-R1 (circle), NB-R2 (square), or NB-R1/2 (triangle) treatment (30 mg/kg) in both non-dependent (NonDEP) and alcohol-dependent (DEP) rats. A) In NonDEP rats, SB-R1 treatment was significantly more effective at attenuating alcohol self-administration than NB-R2 and NB-R1/2 treatment, and NB-R1/2 treatment was more effective than NB-R2 treatment. In DEP rats, all HCRT-R antagonists similarly reduced alcohol self-administration. B) Markers represent the percent change in mean number (±SEM) of water reinforcers from vehicle (VEH; 0 mg/kg) for SB-R1 (circle), NB-R2 (square), or NB-R1/2 (triangle) treatment (30 mg/kg) in both NonDEP and DEP rats. There was no significant difference in water self-administration among rats treated with either SB-R1, NB-R2, or NB-R1/2 in NonDEP or DEP rats. *p < 0.05 and **p < 0.01 versus SB-R1. #p < 0.05 versus NB-R2.
3.2. Experiment 2: Extended amygdalar HCRT-R mRNA in alcohol-dependent rats
3.2.1. RT-PCR: HCRT-R mRNA expression in the CeA and NAs
Hcrtr1 and Hcrtr2 mRNA expression in two stress/reward-related brains regions of alcohol-dependent and non-dependent rats (n=5–9 per group/brain region) were investigated using reverse transcription and quantitative PCR. Alcohol-dependent rats were euthanized 24 hours into alcohol withdrawal. Non-dependent control rats were exposed to ambient air instead of alcohol vapor, but also had exposure to alcohol self-administration (as described; Section 2.2.1). Hcrtr1 mRNA expression was significantly decreased in the CeA (Figure 3A; Non-dependent Md=1.00, Dependent Md=0.27; U=3, p< 0.05) but not in the NAs (Figure 3B; Non-dependent Md=1.14, Dependent Md=1.43; U=16, p= 0.82) of alcohol-dependent rats compared to non-dependent rats. There was no significant difference in Hcrtr2 mRNA expression in the CeA (Figure 3A; Non-dependent Md=0.97, Dependent Md=1.32; U=27, p= 0.42) or NAs (Figure 3B; Non-dependent Md=1.15, Dependent Md=1.10; U=31.5, p= 0.69) between dependent and non-dependent rats. These data suggest a downregulation of HCRT-R1 in the CeA during withdrawal from alcohol in dependent rats.
Figure 3.
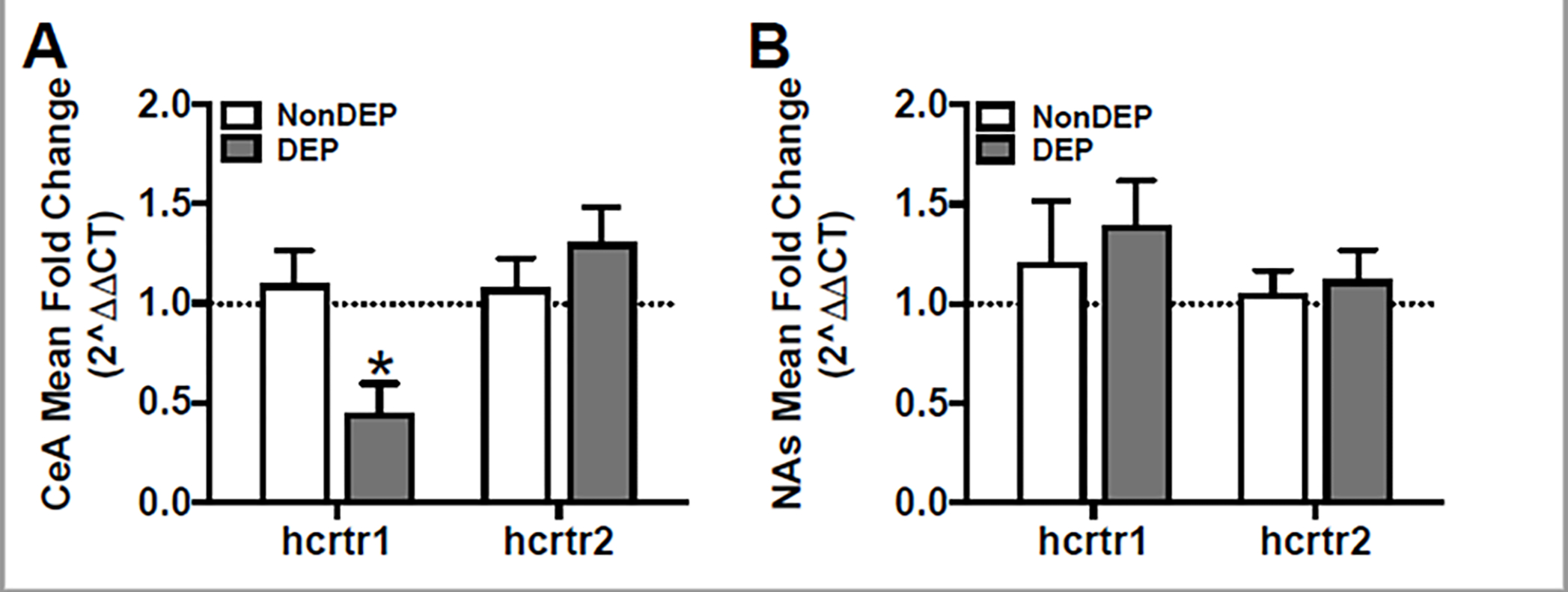
Decreased hcrtr1 mRNA expression in the CeA of alcohol-dependent rats. A-B) Relative mRNA expression of each gene is expressed as mRNA expression mean (+SEM) fold change (2^ΔΔCT) in the CeA (A) and NAs (B) of alcohol non-dependent (NonDep) and dependent (Dep) rats. A) mRNA expression of hcrtr1, but not hcrtr2, was significantly decreased in the CeA of Dep (gray bars) compared to NonDep (white bars) rats. B) Neither hcrtr1 nor hcrtr2 mRNA expression in the NAs were significantly different between Dep and NonDep rats. *p< 0.05 versus respective NonDEP control.
3.3. Experiment 3: AAVretro knockdown of HCRT projections to CeA
3.3.1. Effects of AAVretro-HCRT microinjections into CeA on alcohol intake in dependent rats
First, to characterize the time-course of HCRT knockdown of the AAVretro-HCRT vector, a separate, alcohol-naïve cohort of rats (N=12) was unilaterally microinjected with each AAVretro-HCRT and -SCR into contralateral hemispheres and HCRT-positive cells were counted in each hemisphere after 2-, 4-, or 6-weeks (n= 4/time point) following microinjection. One rat from the 4-week group was dropped due to tissue-handling damage. Microinjection of AAVretro-HCRT yielded a significant knockdown of Hcrt in dorsal hypothalamic HCRT-producing neurons compared to the AAVretro-SCR-treated control hemisphere (Figure 4C; AAVretro group: F(1,8)= 22.12, p< 0.01; Time: F(2,8)= 0.03, p= 0.97; AAVretro group × time: F(2,8)= 0.64, p= 0.55). AAVretro-HCRT hemispheres showed an approximate 14%, 23%, and 24% retrograde knockdown of HCRT-positive neurons compared to the AAVretro-SCR treated control at 2-, 4-, and 6-weeks post-microinjection, respectively. Post hoc analyses showed a significant decrease of HCRT-positive neurons in the AAVretro-HCRT-treated hemisphere compared to the AAVretro-SCR-treated control hemisphere at 4- (p< 0.05) and 6-weeks (p< 0.5) following AAVretro microinjection. To confirm the importance of HCRT-R1 signaling in the CeA in alcohol drinking, we examined the knockdown of the HCRT LH-CeA pathway in alcohol-dependent rats. CIEV exposure was used to generate alcohol-dependent rats that were trained to self-administer alcohol (as described 2.2.1). The baseline number of alcohol reinforcers were recorded prior to bilateral microinjection with either AAVretro-HCRT or -SCR (n= 5 and 3, respectively) into the CeA (Figure 4E). Post-mortem analyses of the tissue showed an approximate 34% retrograde knockdown of HCRT-positive cells in the LH of AAVretro-HCRT-treated rats versus AAVretro-SCR-treated rats (Figure 4D; t(6)=3.90, p<0.01). AAVretro-HCRT knockdown significantly attenuated alcohol self-administration in dependent rats versus AAVretro-SCR-treated rats (Figure 4F; AAVretro group: F(1,6)= 3.49, p=0.11; Time: F(3,18)= 2.63, p=0.08; Group × Time: F(3,18)= 3.33, p< 0.05). Post hoc analysis revealed a significant decrease in alcohol self-administration in AAVretro-HCRT-treated rats at each timepoint compared to baseline self-administration, and no significant effect in AAVretro-SCR-treated rats. These data suggest that the LH-CeA HCRT-signaling pathway is critical to alcohol self-administration in dependent rats.
Figure 4.
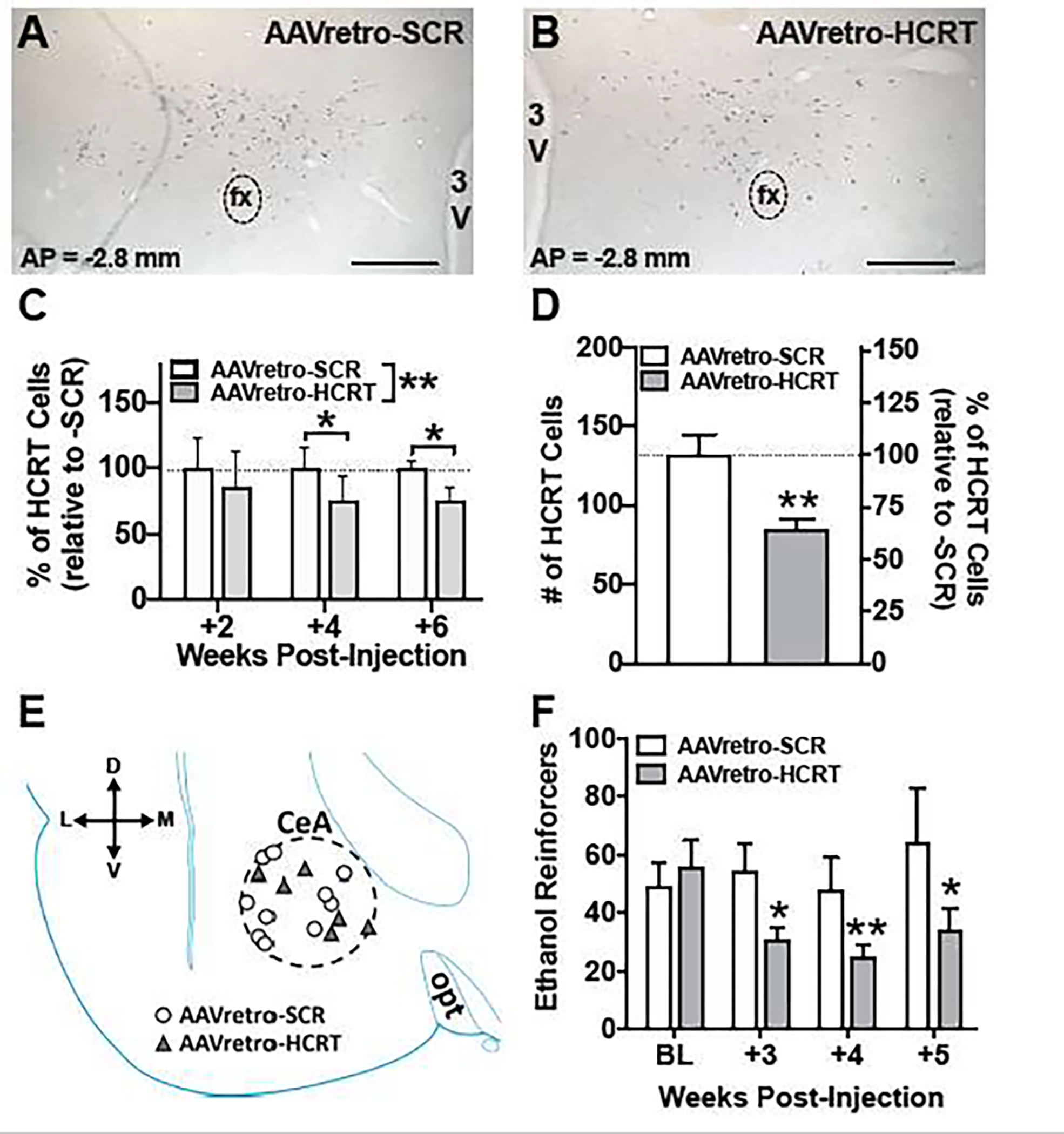
AAVretro-HCRT microinjection into the CeA of alcohol-dependent rats reduces alcohol self-administration. A-B) Representative coronal sections stained with anti-preproHCRT antibody (blue/gray precipitate) to indicate HCRT-positive cells, following either AAVretro-SCR (A; scramble control) or AAVretro-HCRT (B; hcrt-silenced) microinjection. C) Bars represent mean percentage (+SEM) of HCRT-positive cells relative to control AAVretro-SCR treated rats at 2-, 4-, and 6-weeks following AAVretro injection in alcohol naïve rats. D) Bars represent mean number (+SEM; left) and percentage (+SEM; right; relative to control) of HCRT-positive cells in the hypothalamus for AAVretro-SCR (control) or -HCRT treated alcohol-dependent rats. AAVretro-HCRT significantly decreased number of HCRT-positive cells. E) Schematic of AAVretro-SCR (white circles) or -HCRT (gray triangles) microinjection site locations in the CeA (dashed circle) in alcohol-dependent rats; both hemispheres are superimposed onto a diagram of the left hemisphere. F) Bars represent mean number (+SEM) of self-administered ethanol reinforcers in AAVretro-SCR or -HCRT treated alcohol-dependent rats. AAVretro-HCRT, but not AAVretro-SCR, resulted in a significant attenuation of ethanol reinforcers at 3-, 4-, and 5-weeks post-AAVretro-microinjection versus baseline (BL) responding. *p<0.05 and **p<0.01 versus respective control. Horizontal scale bar (A-B) = 500μm. 3V, third ventricle; CeA, central amygdala; D, dorsal; fx, fornix; L, lateral; M, medial; opt, optic tract; V, ventral.
4. Discussion
The different contributions of HCRT-R1 and HCRT-R2 signaling in alcohol drinking are not yet fully understood. We hypothesized that altered brain stress systems, including contributions from HCRT signaling at HCRT-R1 and -R2, mediate highly-motivated alcohol intake associated with an alcohol-dependent state. Here, we investigated the extent to which selective and dual HCRT-R antagonism affects alcohol drinking in alcohol-dependent and non-dependent rats. Systemic HCRT-R1 antagonism (SB-R1), but not HCRT-R2 (NB-R2) nor -R1/2 (NB-R1/2), attenuated alcohol intake in non-dependent rats. In contrast to the effects in non-dependent rats, all three antagonists significantly reduced alcohol self-administration in dependent rats under acute withdrawal conditions, with the HCRT dual-receptor antagonist NB-R1/2 having the most selective effect on alcohol intake. To further investigate site-specific actions of HCRT-R neurotransmission, additional studies were performed in withdrawal/stress-related regions of the CeA and NAs to determine potential HCRT neuroadaptations during acute alcohol withdrawal. PCR analysis showed that hcrtr1 mRNA was significantly decreased in the CeA of dependent rats during acute withdrawal compared to non-dependent rats. Therefore, the contribution of HCRT signaling in the CeA of alcohol-dependent rats was investigated by using an AAV with retrograde function to knockdown hcrt expression in projections from the LH to the CeA. HCRT knockdown in the LH-CeA neurocircuitry significantly attenuated alcohol-seeking behavior following AAVretro-HCRT microinjection in alcohol-dependent rats compared to AAVretro-SCR controls. Combined, these results suggest a functional role for HCRT-R neurotransmission in alcohol drinking in dependent rats, and that HCRT projections from the LH to the CeA are integral to highly-motivated alcohol self-administration behavior observed in alcohol dependence.
4.1. Pharmacological blockade of HCRT signaling in alcohol-dependent rats
Previous research implicating the HCRT system in drug- and alcohol-seeking behavior largely focuses on signaling at HCRT-R1, with far fewer studies investigating HCRT-R2 signaling. Generally, HCRT-R1 is thought to be more involved in mediating drug-seeking behavior because of its association with motivation and reward, whereas, HCRT-R2 is associated with arousal and is therefore more prevalent in sleep research. More recently, there has been renewed focus by researchers on targeting dual-HCRT-Rs with the introduction of FDA-approved drugs like suvorexant and lemborexant for the treatment of insomnia. In alcohol research, the use of dual HCRT-R antagonists has also become more prevalent. Dual HCRT-R antagonism has been shown to attenuate alcohol responding in self-administration (fixed and progressive ratio schedules of reinforcement), stress-induced reinstatement, and in binge drinking models [43,45,57]. In the current study, Experiment 1 examined the effects of various HCRT-R-specific antagonists in rats under conditions of acute withdrawal during alcohol dependence and in non-dependent rats.
Treatment with the SB-R1 antagonist dose-dependently reduced alcohol self-administration in both alcohol-dependent and non-dependent rats at the highest dose (30 mg/kg). However, treatment with either NB-R2 or NB-R1/2 selectively showed reduced alcohol self-administration in dependent rats. This may suggest that signaling at HCRT-R2 is particularly sensitive under conditions of highly motivated-behavior like that of dependent alcohol-seeking. Previous research has shown a non-specific attenuation of operant, consummatory behavior of natural rewards are modulated, in part, by HCRT-R1 signaling [72–77]. In this way, the effect of SB-R1 on alcohol self-administration in both non-dependent and dependent rats may be a reduction of overall consummatory behavior and not necessarily specific to alcohol drinking. An additional or alternative explanation for the attenuating effect of the SB-R1 antagonist on alcohol self-administration is the potential sedative-like side-effect of the antagonist decreasing locomotor activity, especially at high doses. This has been shown elsewhere, particularly under conditions of low-motivated behavior during the active phase of the light-dark cycle [78]. However, there was no effect of the SB-R1 antagonist compared to vehicle-treated controls on water self-administration, suggesting that general operant and locomotor activity were unaffected.
In regard to HCRT-R2 antagonism, NB-R2 and NB-R1/2 had no effect on alcohol self-administration in non-dependent rats, but did dose-dependently reduce alcohol self-administration in alcohol-dependent rats. Although HCRT-R2 signaling is highly implicated in arousal and locomotor-producing activity [79–81], it is unlikely that effects on alcohol self-administration observed here are due to reduced arousal/activity as there was no effect on water self-administration. In alcohol-dependent rats, all three antagonists (SB-R1, NB-R2, and NB-R1/2) significantly reduced responding for alcohol during acute withdrawal. Importantly, the treatment also had no effect on water self-administration in these rats. In dependent rats, any potential confounding effects of decreased operant activity or decreased arousal level associated with HCRT-R antagonism are likely mitigated with highly-salient, goal-directed alcohol-seeking behavior. Consistent with this hypothesis, we and others have shown HCRT-R antagonists to have little to no effect on general activity in animals that exhibit strong addiction-like phenotypes with enhanced motivation, even at high doses [40,43,55,57,82].
Finally, in order to directly compared efficacy of HCRT-R antagonism on alcohol self-administration in alcohol-dependent and non-dependent rats, we specifically analyzed the most effective dose that was equal across all antagonists, 30 mg/kg. The results displayed in Figure 2 show that the HCRT-R antagonism have differential effects on alcohol self-administration in alcohol-dependent rats compared to non-dependent rats, with more variability in efficacy across HCRT-R antagonists in the non-dependent rats. Furthermore, it appears the HCRT-R antagonists have a greater overall effect on attenuation of alcohol self-administration in alcohol-dependent rats compared to non-dependent rats. Further preclinical studies are warranted to investigate the efficacy of such HCRT-R drugs on additional models of alcohol seeking and taking, and for other classes of drugs of abuse.
4.2. Contribution and neuroadaptation of the HCRT system in the extended amygdala
The pharmacological blockade of HCRT receptors in the first experiment utilized a systemic approach to demonstrate the differential effects of selective and dual HCRT-R-antagonism on alcohol drinking, particularly in alcohol-dependent rats in acute withdrawal. Additional experiments were conducted in order to understand the role HCRT signaling plays in the extended amygdala, including the CeA and NAs, distinct brain regions associated with stress- and alcohol-withdrawal related neurocircuitry [11].
Experiment 2 investigated the degree to which Hcrtr1 and Hcrtr2 mRNA levels are altered in alcohol-dependent animals at the time of acute withdrawal compared to non-dependent alcohol-drinking rats. PCR analysis showed that Hcrtr1 mRNA was downregulated in the CeA in alcohol-dependent rats compared to non-dependent rats. Interestingly, HCRT has been shown to regulate, in part, the hypothalamic-pituitary-adrenal (HPA) response to stress [83–85]. A similar downregulation has been observed with other HPA axis modulators in response to alcohol dependence-related stress, namely corticosterone, corticotropin-releasing factor and glucocorticoids [7,8,86], in which the HPA axis becomes hyporesponsive. In the same way, the decrease in Hcrtr1 mRNA observed may be a negative feedback mechanism due to a preceding increase in exogenous HCRT peptide in the CeA recruited in an alcohol-dependent state. Consistent with this hypothesis, mRNA of the HCRT peptide precursor, prepro-HCRT, has been shown to be upregulated in rats following chronic alcohol consumption [50,54], thus suggesting an increase in HCRT signaling. However, Hcrtr2 mRNA levels in the CeA were not significantly changed in alcohol-dependent rats, so there may be differential effects of chronic alcohol on mRNA HCRT-R subtype within regions of the CeA. The possible changes to HCRT-R1 and -R2 numbers, sensitivity, and function during acute alcohol withdrawal are important topics for future studies. There were also no significant changes in either Hcrtr1 or Hcrtr2 mRNA levels in the Nas of alcohol-dependent rats. Other studies have shown mixed results concerning the effect alcohol exposure on HCRT-R mRNA expression. For example, rats euthanized 30 minutes following chronic intermittent-drinking exhibited no change in Hcrtr1 or Hcrtr2 mRNA in the posterior paraventricular thalamus [50], whereas CIEV-exposed rats euthanized at 8 hours of abstinence had significantly higher Hcrtr1 and Hcrtr2 mRNA in the same region compared to alcohol-naïve and non-dependent controls [57]. Thus, length of alcohol exposure and specific time of tissue collection following alcohol exposure should be considered in the interpretation of mRNA data as levels are likely subject to state- and time-dependent dynamics. The current studies used a period of 24 hours following CIEV for brain collection, a timepoint that likely targets more stable gene expression while animals are still in acute withdrawal [58,59,63,64,66], and avoids possible transient effects to expression occurring early in withdrawal. However, it may also be that this timepoint did not capture dynamic changes to Hcrtr2 mRNA that may otherwise have relevant implications for understanding the role of HCRT-R2 in dependent alcohol-seeking. Alternatively, or additionally, combined with the behavioral pharmacology studies, the lack of effect on Hcrtr2 expression may also suggest that HCRT-R2 neurotransmission may modulate, at least in part, dependent alcohol-seeking in a brain region outside of the two areas considered herein. Nonetheless, the current findings demonstrate altered Hcrtr1 mRNA expression in the CeA of alcohol-dependent rats, implicating the extended amygdala in dependence-related neurocircuitry under conditions of acute withdrawal.
Experiment 3 sought to test whether HCRT projections to the CeA are specifically involved with alcohol drinking in dependent rats. An shRNA AAV with retrograde function was used for site-specific knockdown of HCRT projections from the LH to the CeA. Alcohol self-administration was significantly decreased at 3-, 4-, and 5-weeks following AAVretro-HCRT microinjections into the CeA of rats made dependent on alcohol via CIEV exposure. There was no effect on alcohol self-administration in AAVretro-SCR-treated (control AAV) alcohol-dependent rats. AAVretro-HCRT microinjections resulted in a 34% knockdown of HCRT-positive neurons compared to AAVretro-SCR alcohol-dependent controls, as measured at 8-weeks following AAVretro microinjections. Likewise, alternative use of this same AAVretro-HCRT vector by another group showed a similar HCRT-knockdown efficacy (35%) in cocaine self-administering rats microinjected with AAVretro-HCRT into the ventral tegmental area [71]. In the time course experiment conducted in alcohol-naïve rats herein, a similar, albeit lesser knockdown of HCRT was observed at 4- and 6-weeks following AAVretro-HCRT microinjection (23% and 24% knockdown, respectively). While it is possible that the extent of knockdown at the time of behavioral testing in alcohol-dependent rats in the current studies was different from observed end-of-study values (and possibly more like that of the time course subjects), the behavioral data suggest that the extent of knockdown at the time of testing was sufficient to affect behavior. Finally, it is also possible that the AAVretro construct uptake did not exclusively occur in terminal HCRT-CeA neuronal synaptic fibers, but also into non-terminal HCRT fibers of passage, although the retrograde vector used here (AAV2-retro) has not been stated to infect axons of passage [87]. These studies by our group and others also confirm specificity of this exact shHcrt construct, despite possible compensatory adaptations of prodynorphin (via vesicular co-release with HCRT) and/or neighboring melanin-concentrating hormone-containing neuron activity resulting from prolonged HCRT-projection knockdown following AAV transfection [26, 71]. Thus, it is possible that these concomitant peptide adaptations may have contributed to the reduced alcohol intake in AAVretro-HCRT treated rats, but this would need to be directly tested in future studies.
These AAVretro-HCRT results suggest that HCRT signaling in the CeA is necessary for alcohol drinking during dependence. These findings extend a growing literature implicating CeA in alcohol dependence-related neurocircuitry [5,88–90]. Neuronal ensemble recruitment in the CeA is associated with alcohol dependence, and our research suggests that HCRT signaling is an important aspect of this recruitment [91,92].
In conclusion, the current studies provide evidence that both selective and dual-HCRT-R antagonismsignificantly attenuates alcohol self-administration in alcohol-dependent rats without affecting water self-administration. Further, that alcohol exposure dynamically regulates HCRT-R expression in the CeA and that LH-CeA HCRT projections are necessary for alcohol drinking during dependence. Together, these findings support a role HCRT-R1 and -R2 modulation of dependent alcohol-seeking behavior and suggest HCRT-R antagonists as a target for understanding and potentially treating AUD.
Acknowledgements
This research was financially supported by grants DA036355 (BES) and DA042110 (BES) from the National Institute on Drug Abuse, and P60-AA006420 (OG), AA026081 (OG), AA028982 (VRC, PPS), and AA021667 (PPS) from the National Institute on Alcohol Abuse and Alcoholism. A portion of this work was also supported by the Intramural Research Program of the National Institute on Drug Abuse. The authors thank Neurocrine Biosciences, Inc. for providing us NBI-87571 and the Genetic Engineering and Viral Vector Core at the National Institute on Drug Abuse Intramural Research Program for providing us with viral vectors.
Footnotes
Funding and Disclosures
The authors declare no conflict of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Adinoff B, Iranmanesh A, Veldhuis J, Fisher L, Disturbances of the Stress Response, Alcohol Health Res. World. 22 (1998) 67–72. [PMC free article] [PubMed] [Google Scholar]
- [2].Errico AL, King AC, Lovallo WR, Parsons OA, Cortisol Dysregulation and Cognitive Impairment in Abstinent Male Alcoholics, Alcohol. Clin. Exp. Res. 26 (2002) 1198–1204. 10.1111/j.1530-0277.2002.tb02656.x. [DOI] [PubMed] [Google Scholar]
- [3].Lovallo WR, Dickensheets SL, Myers DA, Thomas TL, Nixon SJ, Blunted Stress Cortisol Response in Abstinent Alcoholic and Polysubstance-Abusing Men, Alcohol. Clin. Exp. Res. 24 (2000) 651–658. 10.1111/j.1530-0277.2000.tb02036.x. [DOI] [PubMed] [Google Scholar]
- [4].Sinha R, Fuse T, Aubin L-R, O’Malley SS, Psychological stress, drug-related cues and cocaine craving, Psychopharmacology (Berl.). 152 (2000) 140–148. 10.1007/s002130000499. [DOI] [PubMed] [Google Scholar]
- [5].Funk CK, O’Dell LE, Crawford EF, Koob GF, Corticotropin-Releasing Factor within the Central Nucleus of the Amygdala Mediates Enhanced Ethanol Self-Administration in Withdrawn, Ethanol-Dependent Rats, J. Neurosci. 26 (2006) 11324–11332. 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Richardson HN, Zhao Y, Fekete ÉM, Funk CK, Wirsching P, Janda KD, Zorrilla EP, Koob GF, MPZP: A novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist, Pharmacol. Biochem. Behav. 88 (2008) 497–510. 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF, Corticosteroid-Dependent Plasticity Mediates Compulsive Alcohol Drinking in Rats, J. Neurosci. 32 (2012) 7563–7571. 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zorrilla EP, Valdez GR, Weiss F, Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats, Psychopharmacology (Berl.). 158 (2001) 374–381. 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
- [9].Vendruscolo LF, Roberts AJ, Operant alcohol self-administration in dependent rats: Focus on the vapor model, Alcohol. 48 (2014) 277–286. 10.1016/j.alcohol.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Meinhardt MW, Sommer WH, Postdependent state in rats as a model for medication development in alcoholism, Addict. Biol 20 (2015) 1–21. 10.1111/adb.12187. [DOI] [PubMed] [Google Scholar]
- [11].Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW, George O, Addiction as a stress surfeit disorder, Neuropharmacology. 76 (2014) 370–382. 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang C, Wang Q, Ji B, Pan Y, Xu C, Cheng B, Bai B, Chen J, The Orexin/Receptor System: Molecular Mechanism and Therapeutic Potential for Neurological Diseases, Front. Mol. Neurosci 11 (2018) 220. 10.3389/fnmol.2018.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].James MH, Mahler SV, Moorman DE, Aston-Jones G, A Decade of Orexin/Hypocretin and Addiction: Where Are We Now?, in: Lawrence AJ, de Lecea L (Eds.), Behav. Neurosci. OrexinHypocretin, Springer International Publishing, Cham, 2017: pp. 247–281. 10.1007/7854_2016_57. [DOI] [Google Scholar]
- [14].Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G, Chapter 7 - Multiple roles for orexin/hypocretin in addiction, in: Shekhar A (Ed.), Prog. Brain Res, Elsevier, 2012: pp. 79–121. 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A, Chapter 9 - Orexin, stress, and anxiety/panic states, in: Shekhar A (Ed.), Prog. Brain Res, Elsevier, 2012: pp. 133–161. 10.1016/B978-0-444-59489-1.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].de Lecea L, Kilduff TS, Peyron C, Gao X-B, Foye PE, Danielson PE, Fukuhara C, Battenberg ELF, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG, The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity, Proc. Natl. Acad. Sci. 95 (1998) 322–327. 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu W-S, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M, Orexins and Orexin Receptors: A Family of Hypothalamic Neuropeptides and G Protein-Coupled Receptors that Regulate Feeding Behavior, Cell. 92 (1998) 573–585. 10.1016/S0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- [18].Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K, Distribution of orexin neurons in the adult rat brain1Published on the World Wide Web on 17 March 1999.1, Brain Res. 827 (1999) 243–260. 10.1016/S0006-8993(99)01336-0. [DOI] [PubMed] [Google Scholar]
- [19].Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS, Neurons Containing Hypocretin (Orexin) Project to Multiple Neuronal Systems, J. Neurosci. 18 (1998) 9996–10015. 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Baldo BA, Daniel RA, Berridge CW, Kelley AE, Overlapping distributions of orexin/hypocretin- and dopamine-β-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress, J. Comp. Neurol. 464 (2003) 220–237. 10.1002/cne.10783. [DOI] [PubMed] [Google Scholar]
- [21].Schmitt O, Usunoff KG, Lazarov NE, Itzev DE, Eipert P, Rolfs A, Wree A, Orexinergic innervation of the extended amygdala and basal ganglia in the rat, Brain Struct. Funct. 217 (2012) 233–256. 10.1007/s00429-011-0343-8. [DOI] [PubMed] [Google Scholar]
- [22].Scammell TE, Winrow CJ, Orexin Receptors: Pharmacology and Therapeutic Opportunities, Annu. Rev. Pharmacol. Toxicol. 51 (2011) 243–266. 10.1146/annurev-pharmtox-010510-100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ruoff C, Cao M, Guilleminault C, Hypocretin Antagonists in Insomnia Treatment and Beyond, Curr. Pharm. Des 17 (2011) 1476–1482. 10.2174/138161211796197089. [DOI] [PubMed] [Google Scholar]
- [24].Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L, Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior, Proc. Natl. Acad. Sci. 102 (2005) 19168–19173. 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Steiner N, Rossetti C, Sakurai T, Yanagisawa M, de Lecea L, Magistretti PJ, Halfon O, Boutrel B, Hypocretin/orexin deficiency decreases cocaine abuse liability, Neuropharmacology. 133 (2018) 395–403. 10.1016/j.neuropharm.2018.02.010. [DOI] [PubMed] [Google Scholar]
- [26].Schmeichel BE, Matzeu A, Koebel P, Vendruscolo LF, Sidhu H, Shahryari R, Kieffer BL, Koob GF, Martin-Fardon R, Contet C, Knockdown of hypocretin attenuates extended access of cocaine self-administration in rats, Neuropsychopharmacology. 43 (2018) 2373–2382. 10.1038/s41386-018-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DCS, Jones SR, The hypocretin–orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system, Eur. J. Neurosci. 31 (2010) 336–348. 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Khosrowabadi E, Karimi-Haghighi S, Jamali S, Haghparast A, Differential Roles of Intra-accumbal Orexin Receptors in Acquisition and Expression of Methamphetamine-Induced Conditioned Place Preference in the Rats, Neurochem. Res. 45 (2020) 2230–2241. 10.1007/s11064-020-03084-1. [DOI] [PubMed] [Google Scholar]
- [29].Matzeu A, Kerr TM, Weiss F, Martin-Fardon R, Orexin-A/Hypocretin-1 Mediates Cocaine-Seeking Behavior in the Posterior Paraventricular Nucleus of the Thalamus via Orexin/Hypocretin Receptor-2, J. Pharmacol. Exp. Ther. 359 (2016) 273–279. 10.1124/jpet.116.235945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Rusyniak DE, Zaretsky DV, Zaretskaia MV, Durant PJ, DiMicco JA, The orexin-1 receptor antagonist SB-334867 decreases sympathetic responses to a moderate dose of methamphetamine and stress, Physiol. Behav. 107 (2012) 743–750. 10.1016/j.physbeh.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Martin-Fardon R, Weiss F, Blockade of hypocretin receptor-1 preferentially prevents cocaine seeking: comparison with natural reward seeking, Neuroreport. 25 (2014) 485–488. 10.1097/WNR.0000000000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gentile TA, Simmons SJ, Watson MN, Connelly KL, Brailoiu E, Zhang Y, Muschamp JW, Effects of Suvorexant, a Dual Orexin/Hypocretin Receptor Antagonist, on Impulsive Behavior Associated with Cocaine, Neuropsychopharmacology. 43 (2018) 1001–1009. 10.1038/npp.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Prince CD, Rau AR, Yorgason JT, España RA, Hypocretin/Orexin Regulation of Dopamine Signaling and Cocaine Self-Administration Is Mediated Predominantly by Hypocretin Receptor 1, ACS Chem. Neurosci 6 (2015) 138–146. 10.1021/cn500246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ, Insular hypocretin transmission regulates nicotine reward, Proc. Natl. Acad. Sci. 105 (2008) 19480–19485. 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].LeSage MG, Perry JL, Kotz CM, Shelley D, Corrigall WA, Nicotine self-administration in the rat: effects of hypocretin antagonists and changes in hypocretin mRNA, Psychopharmacology (Berl.). 209 (2010) 203–212. 10.1007/s00213-010-1792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nishizawa D, Kasai S, Hasegawa J, Sato N, Yamada H, Tanioka F, Nagashima M, Katoh R, Satoh Y, Tagami M, Ujike H, Ozaki N, Inada T, Iwata N, Sora I, Iyo M, Yamada M, Kondo N, Won M-J, Naruse N, Uehara-Aoyama K, Itokawa M, Ohi K, Hashimoto R, Tanisawa K, Arai T, Mori S, Sawabe M, Naka-Mieno M, Yamada Y, Yamada M, Sato N, Muramatsu M, Tanaka M, Irukayama-Tomobe Y, Saito YC, Sakurai T, Hayashida M, Sugimura H, Ikeda K, Associations between the orexin (hypocretin) receptor 2 gene polymorphism Val308Ile and nicotine dependence in genome-wide and subsequent association studies, Mol. Brain 8 (2015) 50. 10.1186/s13041-015-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Plaza-Zabala A, Flores Á, Martín-García E, Saravia R, Maldonado R, Berrendero F, A Role for Hypocretin/Orexin Receptor-1 in Cue-Induced Reinstatement of Nicotine-Seeking Behavior, Neuropsychopharmacology. 38 (2013) 1724–1736. 10.1038/npp.2013.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Plaza-Zabala A, Martín-García E, de Lecea L, Maldonado R, Berrendero F, Hypocretins Regulate the Anxiogenic-Like Effects of Nicotine and Induce Reinstatement of Nicotine-Seeking Behavior, J. Neurosci. 30 (2010) 2300–2310. 10.1523/JNEUROSCI.5724-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].O’Connor SL, Fragale JE, James MH, Aston-Jones G, The dual orexin/hypocretin receptor antagonist suvorexant reduces addiction-like behaviors for the opioid fentanyl, BioRxiv. (2020) 2020.04.25.061887. 10.1101/2020.04.25.061887. [DOI] [Google Scholar]
- [40].Schmeichel BE, Barbier E, Misra KK, Contet C, Schlosburg JE, Grigoriadis D, Williams JP, Karlsson C, Pitcairn C, Heilig M, Koob GF, Vendruscolo LF, Hypocretin Receptor 2 Antagonism Dose-Dependently Reduces Escalated Heroin Self-Administration in Rats, Neuropsychopharmacology. 40 (2015) 1123–1129. 10.1038/npp.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Smith RJ, Aston-Jones G, Orexin / hypocretin 1 receptor antagonist reduces heroin self-administration and cue-induced heroin seeking, Eur. J. Neurosci. 35 (2012) 798–804. 10.1111/j.1460-9568.2012.08013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Morganstern I, Chang G-Q, Barson J, Ye Z, Karatayev O, Leibowitz SF, Differential effects of acute and chronic ethanol exposure on orexin expression in the perifornical lateral hypothalamus, Alcohol. Clin. Exp. Res. 34 (2010) 886–896. 10.1111/j.1530-0277.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Anderson RI, Becker HC, Adams BL, Jesudason CD, Rorick-Kehn LM, Orexin-1 and orexin-2 receptor antagonists reduce ethanol self-administration in high-drinking rodent models, Front. Neurosci. 0 (2014). 10.3389/fnins.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Moorman DE, James MH, Kilroy EA, Aston-Jones G, Orexin/hypocretin-1 receptor antagonism reduces ethanol self-administration and reinstatement selectively in highly-motivated rats, Brain Res. 1654 (2017) 34–42. 10.1016/j.brainres.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Srinivasan S, Simms JA, Nielsen CK, Lieske SP, Bito-Onon JJ, Yi H, Hopf FW, Bonci A, Bartlett SE, The Dual Orexin/Hypocretin Receptor Antagonist, Almorexant, in the Ventral Tegmental Area Attenuates Ethanol Self-Administration, PLOS ONE. 7 (2012) e44726. 10.1371/journal.pone.0044726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cannella N, Economidou D, Kallupi M, Stopponi S, Heilig M, Massi M, Ciccocioppo R, Persistent Increase of Alcohol-Seeking Evoked by Neuropeptide S: an Effect Mediated by the Hypothalamic Hypocretin System, Neuropsychopharmacology. 34 (2009) 2125–2134. 10.1038/npp.2009.37. [DOI] [PubMed] [Google Scholar]
- [47].Olney JJ, Navarro M, Thiele TE, Binge-Like Consumption of Ethanol and Other Salient Reinforcers is Blocked by Orexin-1 Receptor Inhibition and Leads to a Reduction of Hypothalamic Orexin Immunoreactivity, Alcohol. Clin. Exp. Res. 39 (2015) 21–29. 10.1111/acer.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dhaher R, Hauser SR, Getachew B, Bell RL, McBride WJ, McKinzie DL, Rodd ZA, The Orexin-1 Receptor Antagonist SB-334867 Reduces Alcohol Relapse Drinking, but not Alcohol-Seeking, in Alcohol-Preferring (P) Rats, J. Addict. Med 4 (2010) 153–159. 10.1097/ADM.0b013e3181bd893f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kwok C, Lei K, Pedrozo V, Anderson L, Ghotra S, Walsh M, Li L, Yu J, Hopf FW, Differential importance of nucleus accumbens Ox1Rs and AMPARs for female and male mouse binge alcohol drinking, Sci. Rep. 11 (2021) 231. 10.1038/s41598-020-79935-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Barson JR, Ho HT, Leibowitz SF, Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2: aPVT is involved in drinking, Addict. Biol 20 (2015) 469–481. 10.1111/adb.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lei K, Wegner SA, Yu J-H, Hopf FW, Orexin-1 receptor blockade suppresses compulsive-like alcohol drinking in mice, Neuropharmacology. 110 (2016) 431–437. 10.1016/j.neuropharm.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Brown RM, Khoo SY-S, Lawrence AJ, Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats, Int. J. Neuropsychopharmacol 16 (2013) 2067–2079. 10.1017/S1461145713000333. [DOI] [PubMed] [Google Scholar]
- [53].Jupp B, Krivdic B, Krstew E, Lawrence AJ, The orexin1 receptor antagonist SB-334867 dissociates the motivational properties of alcohol and sucrose in rats, Brain Res. 1391 (2011) 54–59. 10.1016/j.brainres.2011.03.045. [DOI] [PubMed] [Google Scholar]
- [54].Lawrence AJ, Cowen MS, Yang H-J, Chen F, Oldfield B, The orexin system regulates alcohol-seeking in rats, Br. J. Pharmacol. 148 (2006) 752–759. 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Shoblock JR, Welty N, Aluisio L, Fraser I, Motley ST, Morton K, Palmer J, Bonaventure P, Carruthers NI, Lovenberg TW, Boggs J, Galici R, Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement, Psychopharmacology (Berl.). 215 (2011) 191–203. 10.1007/s00213-010-2127-x. [DOI] [PubMed] [Google Scholar]
- [56].Lopez MF, Moorman DE, Aston-Jones G, Becker HC, The highly selective orexin/hypocretin 1 receptor antagonist GSK1059865 potently reduces ethanol drinking in ethanol dependent mice, Brain Res. 1636 (2016) 74–80. 10.1016/j.brainres.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Matzeu A, Martin-Fardon R, Blockade of Orexin Receptors in the Posterior Paraventricular Nucleus of the Thalamus Prevents Stress-Induced Reinstatement of Reward-Seeking Behavior in Rats With a History of Ethanol Dependence, Front. Integr. Neurosci 0 (2020). 10.3389/fnint.2020.599710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Vapor Inhalation of Alcohol in Rats - Gilpin - 2008 - Current Protocols in Neuroscience - Wiley Online Library, (n.d.). 10.1002/0471142301.ns0929s44 (accessed August 6, 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].O’Dell LE, Roberts AJ, Smith RT, Koob GF, Enhanced Alcohol Self-Administration after Intermittent Versus Continuous Alcohol Vapor Exposure, Alcohol. Clin. Exp. Res. 28 (2004) 1676–1682. 10.1097/01.ALC.0000145781.11923.4E. [DOI] [PubMed] [Google Scholar]
- [60].Gilpin NW, Smith AD, Cole M, Weiss F, Koob GF, Richardson HN, Operant Behavior and Alcohol Levels in Blood and Brain of Alcohol-Dependent Rats, Alcohol. Clin. Exp. Res. 33 (2009) 2113–2123. 10.1111/j.1530-0277.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Schulteis G, Markou A, Cole M, Koob GF, Decreased brain reward produced by ethanol withdrawal, Proc. Natl. Acad. Sci. 92 (1995) 5880–5884. 10.1073/pnas.92.13.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF, Excessive Ethanol Drinking Following a History of Dependence: Animal Model of Allostasis, Neuropsychopharmacology. 22 (2000) 581–594. 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- [63].Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF, Increased Ethanol Self-Administration and Anxiety-Like Behavior During Acute Ethanol Withdrawal and Protracted Abstinence: Regulation by Corticotropin-Releasing Factor, Alcohol. Clin. Exp. Res. 26 (2002) 1494–1501. 10.1111/j.1530-0277.2002.tb02448.x. [DOI] [PubMed] [Google Scholar]
- [64].Rimondini R, Sommer W, Heilig M, A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication., J. Stud. Alcohol 64 (2003) 445–449. 10.15288/jsa.2003.64.445. [DOI] [PubMed] [Google Scholar]
- [65].Zhao Y, Weiss F, Zorrilla EP, Remission and Resurgence of Anxiety-Like Behavior Across Protracted Withdrawal Stages in Ethanol-Dependent Rats, Alcohol. Clin. Exp. Res. 31 (2007) 1505–1515. 10.1111/j.1530-0277.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- [66].Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA, Upregulation of Voluntary Alcohol Intake, Behavioral Sensitivity to Stress, and Amygdala Crhr1 Expression Following a History of Dependence, Biol. Psychiatry. 63 (2008) 139–145. 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- [67].Harte-Hargrove LC, Dow-Edwards DL, Withdrawal from THC during adolescence: Sex differences in locomotor activity and anxiety, Behav. Brain Res. 231 (2012) 48–59. 10.1016/j.bbr.2012.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Dugovic C, Shelton JE, Aluisio LE, Fraser IC, Jiang X, Sutton SW, Bonaventure P, Yun S, Li X, Lord B, Dvorak CA, Carruthers NI, Lovenberg TW, Blockade of Orexin-1 Receptors Attenuates Orexin-2 Receptor Antagonism-Induced Sleep Promotion in the Rat, J. Pharmacol. Exp. Ther. 330 (2009) 142–151. 10.1124/jpet.109.152009. [DOI] [PubMed] [Google Scholar]
- [69].Bonaventure P, Yun S, Johnson PL, Shekhar A, Fitz SD, Shireman B, Lebold TP, Nepomuceno D, Lord B, Wennerholm M, Shelton J, Carruthers N, Lovenberg TW, Dugovic C, A selective orexin-1 receptor antagonist attenuates stress induced hyperarousal without hypnotic effects, J. Pharmacol. Exp. Ther. (2015). 10.1124/jpet.114.220392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Darcq E, Koebel P, Boca CD, Pannetier S, Kirstetter A-S, Garnier J-M, Hanauer A, Befort K, Kieffer BL, RSK2 signaling in brain habenula contributes to place aversion learning, Learn. Mem 18 (2011) 574–578. 10.1101/lm.2221011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Pantazis CB, James MH, O’Connor S, Shin N, Aston-Jones G, Orexin-1 receptor signaling in ventral tegmental area mediates cue-driven demand for cocaine, Neuropsychopharmacology. (2021) 1–11. 10.1038/s41386-021-01173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Haynes AC, Jackson B, Chapman H, Tadayyon M, Johns A, Porter RA, Arch JRS, A selective orexin-1 receptor antagonist reduces food consumption in male and female rats, Regul. Pept. 96 (2000) 45–51. 10.1016/S0167-0115(00)00199-3. [DOI] [PubMed] [Google Scholar]
- [73].Parise EM, Lilly N, Kay K, Dossat AM, Seth R, Overton JM, Williams DL, Evidence for the role of hindbrain orexin-1 receptors in the control of meal size, Am. J. Physiol.-Regul. Integr. Comp. Physiol 301 (2011) R1692–R1699. 10.1152/ajpregu.00044.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Ishii Y, Blundell JE, Halford JCG, Upton N, Porter R, Johns A, Rodgers RJ, Satiety enhancement by selective orexin-1 receptor antagonist SB-334867: influence of test context and profile comparison with CCK-8S, Behav. Brain Res. 160 (2005) 11–24. 10.1016/j.bbr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- [75].Zheng H, Patterson LM, Berthoud H-R, Orexin Signaling in the Ventral Tegmental Area Is Required for High-Fat Appetite Induced by Opioid Stimulation of the Nucleus Accumbens, J. Neurosci. 27 (2007) 11075–11082. 10.1523/JNEUROSCI.3542-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Choi DL, Davis JF, Fitzgerald ME, Benoit SC, The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats, Neuroscience. 167 (2010) 11–20. 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- [77].Alcaraz-Iborra M, Carvajal F, Lerma-Cabrera JM, Valor LM, Cubero I, Binge-like consumption of caloric and non-caloric palatable substances in ad libitum-fed C57BL/6J mice: Pharmacological and molecular evidence of orexin involvement, Behav. Brain Res. 272 (2014) 93–99. 10.1016/j.bbr.2014.06.049. [DOI] [PubMed] [Google Scholar]
- [78].Morairty SR, Revel FG, Malherbe P, Moreau J-L, Valladao D, Wettstein JG, Kilduff TS, Borroni E, Dual Hypocretin Receptor Antagonism Is More Effective for Sleep Promotion than Antagonism of Either Receptor Alone, PLOS ONE. 7 (2012) e39131. 10.1371/journal.pone.0039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jacobson LH, Chen S, Mir S, Hoyer D, Orexin OX2 Receptor Antagonists as Sleep Aids, in: Lawrence AJ, de Lecea L (Eds.), Behav. Neurosci. OrexinHypocretin, Springer International Publishing, Cham, 2017: pp. 105–136. 10.1007/7854_2016_47. [DOI] [PubMed] [Google Scholar]
- [80].De Boer P, Drevets WC, Rofael H, van der Ark P, Kent JM, Kezic I, Parapatics S, Dorffner G, van Gerven J, Beneš H, Keicher C, Jahn H, Seiden DJ, Luthringer R, A randomized Phase 2 study to evaluate the orexin-2 receptor antagonist seltorexant in individuals with insomnia without psychiatric comorbidity, J. Psychopharmacol. (Oxf.) 32 (2018) 668–677. 10.1177/0269881118773745. [DOI] [PubMed] [Google Scholar]
- [81].Mochizuki T, Arrigoni E, Marcus JN, Clark EL, Yamamoto M, Honer M, Borroni E, Lowell BB, Elmquist JK, Scammell TE, Orexin receptor 2 expression in the posterior hypothalamus rescues sleepiness in narcoleptic mice, Proc. Natl. Acad. Sci. 108 (2011) 4471–4476. 10.1073/pnas.1012456108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Schmeichel BE, Herman MA, Roberto M, Koob GF, Hypocretin Neurotransmission Within the Central Amygdala Mediates Escalated Cocaine Self-administration and Stress-Induced Reinstatement in Rats, Biol. Psychiatry. 81 (2017) 606–615. 10.1016/j.biopsych.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Kuru M, Ueta Y, Serino R, Nakazato M, Yamamoto Y, Shibuya I, Yamashita H, Centrally administered orexin/hypocretin activates HPA axis in rats, Neuroreport. 11 (2000) 1977–1980. 10.1097/00001756-200006260-00034. [DOI] [PubMed] [Google Scholar]
- [84].Spinazzi R, Andreis PG, Rossi GP, Nussdorfer GG, Orexins in the Regulation of the Hypothalamic-Pituitary-Adrenal Axis, Pharmacol. Rev 58 (2006) 46–57. 10.1124/pr.58.1.4. [DOI] [PubMed] [Google Scholar]
- [85].Grafe LA, Eacret D, Luz S, Gotter AL, Renger JJ, Winrow CJ, Bhatnagar S, Orexin 2 receptor regulation of the hypothalamic-pituitary-adrenal (HPA) response to acute and repeated stress, Neuroscience. 348 (2017) 313–323. 10.1016/j.neuroscience.2017.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Richardson HN, Lee SY, O’Dell LE, Koob GF, Rivier CL, Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state, Eur. J. Neurosci. 28 (2008) 1641–1653. 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Tervo DGR, Hwang B-Y, Viswanathan S, Gaj T, Lavzin M, Ritola KD, Lindo S, Michael S, Kuleshova E, Ojala D, Huang C-C, Gerfen CR, Schiller J, Dudman JT, Hantman AW, Looger LL, Schaffer DV, Karpova AY, A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons, Neuron. 92 (2016) 372–382. 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, Caviness VS, Hodge SM, Tang L, Albaugh M, Ziegler DA, Davis OC, Kissling C, Schumann G, Breiter HC, Heinz A, Amygdala Volume Associated With Alcohol Abuse Relapse and Craving, Am. J. Psychiatry 165 (2008) 1179–1184. 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- [89].Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH, Corticotropin Releasing Factor–Induced Amygdala Gamma-Aminobutyric Acid Release Plays a Key Role in Alcohol Dependence, Biol. Psychiatry. 67 (2010) 831–839. 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Gilpin NW, Misra K, Herman MA, Cruz MT, Koob GF, Roberto M, Neuropeptide Y Opposes Alcohol Effects on Gamma-Aminobutyric Acid Release in Amygdala and Blocks the Transition to Alcohol Dependence, Biol. Psychiatry. 69 (2011) 1091–1099. 10.1016/j.biopsych.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].de Guglielmo G, Crawford E, Kim S, Vendruscolo LF, Hope BT, Brennan M, Cole M, Koob GF, George O, Recruitment of a Neuronal Ensemble in the Central Nucleus of the Amygdala Is Required for Alcohol Dependence, J. Neurosci. 36 (2016) 9446–9453. 10.1523/JNEUROSCI.1395-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Koob GF, Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking, Proc. Natl. Acad. Sci. 109 (2012) 18156–18161. 10.1073/pnas.1116523109. [DOI] [PMC free article] [PubMed] [Google Scholar]