Abstract
Dental personnel are ranked among the highest risk occupations for exposure to SARS-CoV-2 due to their close proximity to the patient’s mouth and many aerosol generating procedures encountered in dental practice. One method to reduce aerosols in dental settings is the use of intraoral evacuation systems. Intraoral evacuation systems are placed directly into a patient’s mouth and maintain a dry field during procedures by capturing liquid and aerosols. Although multiple intraoral dental evacuation systems are commercially available, the efficacy of these systems is not well understood. The objectives of this study were to evaluate the efficacy of four dental evacuation systems at mitigating aerosol exposures during simulated ultrasonic scaling and crown preparation procedures. We conducted real-time respirable (PM4) and thoracic (PM10) aerosol sampling during ultrasonic scaling and crown preparation procedures while using four commercially available evacuation systems: a high-volume evacuator (HVE) and three alternative intraoral systems (A, B, C). Four trials were conducted for each system. Respirable and thoracic mass concentrations were measured during procedures at three locations including (1) near the breathing zone (BZ) of the dentist, (2) edge of the dental operatory room approximately 0.9 m away from the mannequin mouth, and (3) hallway supply cabinet located approximately 1.5 m away from the mannequin mouth. Respirable and thoracic mass concentrations measured during each procedure were compared with background concentrations measured in each respective location. Use of System A or HVE reduced thoracic (System A) and respirable (HVE) mass concentrations near the dentist’s BZ to median background concentrations most often during the ultrasonic scaling procedure. During the crown preparation, use of System B or HVE reduced thoracic (System B) and respirable (HVE or System B) near the dentist’s BZ to median background concentrations most often. Although some differences in efficacy were noted during each procedure and aerosol size fraction, the difference in median mass concentrations among evacuation systems was minimal, ranging from 0.01 to 1.48 μg/m3 across both procedures and aerosol size fractions.
Keywords: COVID-19, exposure mitigation, respirable, thoracic
Introduction
Recent spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), has resulted in a global pandemic, with some occupations identified as having a greater risk for exposure to SARS-CoV-2 than others (Zhang 2021). Healthcare professions have the highest predicted risk for contracting diseases such as COVID-19 and dental professions specifically, including dental hygienists, oral and maxillofacial surgeons, dental assistants, and general dentists, were ranked as the top four individual occupations at highest risk of exposure (Zhang 2021). Dental personnel appear to be at increased risk for exposure to SARS-CoV-2 due to the unique nature of dentistry including close proximity of the provider to the patient’s mouth combined with many routinely performed aerosol-generating procedures (Ather et al. 2020; Cirillo 2020).
Dental personnel routinely perform procedures that produce aerosols. Observed aerosol sizes vary widely by procedure type, with aerosols across a large size range (submicron sized aerosols (<1 micrometers (μm)) upward to 300 μm droplets) occurring during procedures such as ultrasonic scaling and submicronsized aerosols occurring during procedures such as grinding or drilling teeth (Sotiriou et al. 2008; Van Landuyt et al. 2014; Liu et al. 2019; Mirbod Parisa et al. 2021; Pierre-Bez et al. 2021). Additionally, a wide range of aerosol sizes (0.8–500 μm) are generated during speech and breathing with most aerosols falling in the submicron size range (Duguid 1946; Loudon and Roberts 1967; Papineni and Rosenthal 1997; Edwards et al. 2004; Fabian et al. 2008; Morawska et al. 2009; Xie et al. 2009; Gupta et al. 2010; Asadi et al. 2019; Stadnytskyi et al. 2020). Previous studies reported bimodal size-distributions of SARS-CoV-2 aerosols generated by patients with COVID-19, with peaks observed in size ranges from 0.25 to 1 μm and >2.5 μm; sizes with settling times of approximately hours to days (Liu et al. 2020). Three recent studies found SARS-CoV-2 in particles less than 5 μm, with two of the three studies finding SARS-CoV-2 in particles less than 1 μm and these respirable sized particles showed evidence of infectivity in cell culture (Chia et al. 2020; Liu et al. 2020; Santarpia et al. 2020). Small aerosols generated during dental procedures or during speech and breathing are problematic given their long settling times and subsequent increased likelihood of inhalation by dental personnel or other patients. Further, these small aerosols have the potential to travel and contaminate distant sites in a clinic when unmitigated (Holliday et al. 2021).
Additionally, dental personnel perform tasks that can generate mineral and acrylic dusts associated with occupational lung diseases such as pneumoconiosis and pulmonary fibrosis (Carles et al. 1978; De et al. 1986; Choudat 1994; Ergün et al. 2014). Thoracic aerosols (50% cut-point (d50) < 10 μm; also referred to as particulate matter (PM) < 10 μm or PM10) and respirable aerosols (d50 < 4 μm, or PM4) are exposures of particular concern in the development of occupational lung disease because (1) thoracic aerosol (PM10) can reach airways beyond the larynx and (2) respirable aerosol (PM4) is capable of reaching the unciliated lower airways including the gas-exchange region of the lungs (Cherrie and Aitken 1999; Brown et al. 2013). Further, a recent cluster of idiopathic pulmonary fibrosis (IPF) observed among dental personnel highlighted how source control is needed to mitigate aerosol exposures in dental settings beyond the immediate needs of the current pandemic (Nett et al. 2018). A defining feature of IPF is the disease is an interstitial pneumonia without a known cause; however, a recent study by Abramson et al. identified occupational exposure to respirable dust (PM4) as a risk factor for IPF (Abramson et al. 2020).
Both the increased risk for exposure to SARS-CoV-2, as well as the recent cluster of IPF observed among dental personnel, indicate a need to review aerosol exposure mitigation strategies within dental settings. The most effective means of mitigating aerosol exposure during dental procedures is to capture aerosol at the source (i.e., the patient’s mouth), using source controls such as high-volume evacuation (HVE) and other alternative dental evacuation systems. Although many dental evacuation systems are currently marketed to dental personnel, few have been thoroughly evaluated for efficacy in aerosol exposure mitigation. To the authors’ knowledge, only one previous study evaluated HVE and alternative intraoral dental evacuation systems’ ability to mitigate total aerosol (e.g., spatter consisting of droplets up to 50 μm) and fine particulate matter (PM2.5) exposure in dental settings (Comisi et al. 2021). Additionally, previous studies of HVE or other dental evacuation systems collected aerosol measurements in only one location in the operatory (Nulty et al. 2020; Comisi et al. 2021). Our study described here, evaluated multiple dental evacuation systems’ efficacy at mitigating PM10 and PM4 aerosol in the (1) breathing zone (BZ) of a dentist, (2) operatory more broadly, and (3) adjacent corridor during simulated dental procedures in a dental clinic.
Methods
Simulated dental procedures were performed in a clinic with five chairs in semi-separated operatories (Figure 1). Each dental operatory bay was approximately 3.7 m wide by 3.7 m deep and separated by floor to ceiling walls laterally on either side of the chair. Supply air was provided by three supply air vents located in three of the five operatories (Figure 1). Return air was exhausted from the semi-open operatories and adjacent spaces though six return air ducts located in the adjacent hallways and was filtered through air filters with a Minimum Efficiency Reporting Value (MERV) rating of 8 (MERV 8). All dental procedures were performed by a licensed practicing dentist. The clinic was not occupied with patients during the days on which experiments were conducted. PM10 and PM4 mass concentrations were measured during two dental procedures using a mannequin with simulated saliva flow and four dental evacuation systems. The order of experiments was randomized, and each procedure was repeated four times for each evacuation system for a total of 32 experiments.
Figure 1.
Clinic operatory layout with air sampling and ventilation locations.
Mannequin and dental procedures
A mannequin with an anatomically correct mouth (ModuPRO One dentoform Model MP-R320 Acadental, Inc., Overland Park, KS), movable silicone tongue, and flexible silicone mouth lining was attached to a metal skull mannequin head to simulate a patient. The mannequin was attached to a dental chair headrest in the usual supine patient position with the maxillary arch perpendicular to the floor. Salivary flow was simulated using flexible Tygon tubing with four equidistance holes perforated in the tube at locations approximating salivary gland locations, similar to the setup described by Holliday et al. (2021) and Coulthard et al. (2020). Phosphate buffered saline (PBS pH 7.2, Thermo Fisher Scientific, Waltham, MA, USA) was introduced into the mouth of the mannequin using 4 mm inner diameter tubing connected to a syringe pump (Harvard Apparatus, Holliston, MA) operating at 1.5 mL/min, simulating high normal stimulated salivary flow (Holliday et al. 2021). Two dental procedures were simulated: ultrasonic scaling and crown preparation. Ultrasonic scaling was completed along the left side on the lower-left quadrant of the patient’s mouth for 10 min. Crown preparations were completed on tooth number 21. Details on the handpieces and settings used by the dentist are in the Supplemental Material.
Dental evacuation systems
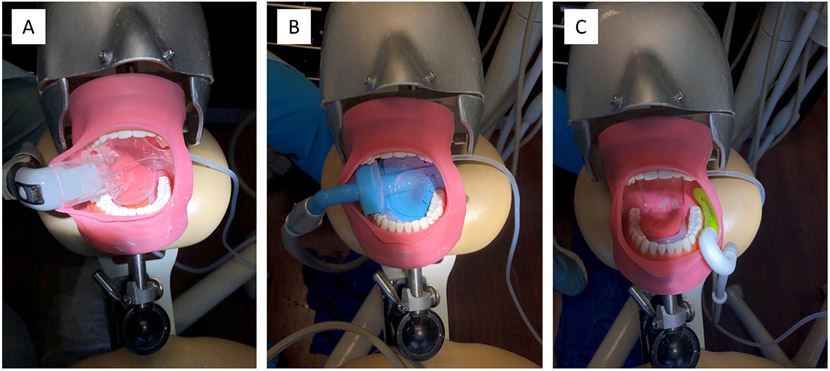
HVE and three alternative dental evacuation systems were evaluated. The HVE system is a suction device commonly used during more extensive dental procedures. Typically, HVE systems operate at > 10.6 standard cubic feet of air per minute (SCFM) and are fitted with a semi-flexible disposable polyvinylchloride (PVC) tube as the mouthpiece that is placed in the mouth as close to the aerosol-generating procedure as possible. Three other alternative dental evacuation systems were evaluated and included Systems A (Figure 2a), B (Figure 2b), and C (Figure 2c). Additional information on the design and operation of these systems can be found in the Supplementary Material. Suction flow rate was measured prior to each experiment using a 0–20 SCFM flowmeter (Easy-Read High-Pressure Flowmeter for Air, McMaster-Carr, Elmhurst, IL) connected to the vacuum ports, before removing the flowmeter and connecting the evacuation system.
Figure 2.
Mannequin setup with System A (panel A), System B (panel B), and System C (panel C) systems.
Aerosol measurements
Aerosol mass and number concentrations were measured using personal DataRAMs (pDRs) (pDR-1500, Thermo Scientific, Franklin, MA) and an Aerodynamic Particle Sizer (APS, TSI, Inc., Maplewood, MN), respectively. Two pDRs per sampling location were equipped with a red cyclone and operated at 1.19 liters per minute (Lpm) and 2.65 Lpm to obtain PM10 (thoracic) and PM4 (respirable) size cut-points and mass concentrations, respectively. Mass concentrations were measured with pDRs at three locations including (1) near the BZ of the dentist (same height as BZ and within 0.45 m of BZ), (2) edge of the dental operatory room approximately 0.9 m away from the mannequin mouth, and (3) supply cabinet located approximately 1.5 m away from the mannequin mouth. The pDR recorded measurements every 10 sec. The APS was positioned near the dentist’s BZ (within 0.45 m of BZ and co-located with the BZ pDRs) to measure number concentration, count median diameters (CMDs) and size distribution (0.5–20 μm) of aerosols generated during each procedure. All instruments were started and stopped with the start and completion of each procedure. APS measurements were also logged at 10-sec intervals. Background aerosol concentration measurements were collected with pDRs and the APS at each respective sampling location for 20 minutes each day before any procedures were performed. All instruments were zero calibrated at the beginning of each sampling day.
Statistical analyses
All analyses were performed in R (version 1.4.1106, RStudio, PBC) and JMP (version 15, SAS Institute Inc., Cary, NC). Because air measurements were not normally distributed, median mass concentrations of PM4 or PM10 aerosol concentrations were used for comparing background aerosol concentrations with aerosol concentrations measured while performing each procedure with each evacuation system in each location. The percentage frequency of PM4 or PM10 aerosol measurements reduced to concentrations below median background concentrations were calculated for each evacuation system (Table 1). Percentage frequency was calculated by dividing the sum of the number of PM4 or PM10 measurements below the median background concentration by the sum of the total number of respirable or thoracic measurements multiplied by 100%.
Table 1.
Count median diameter (CMD), geometric mean (GM), and geometric standard deviation (GSD) of aerosols measured near the dentist’s breathing zone during scaling and crown preparation procedures with HVE, System A, System B, and System C.
| Procedure | Dental evacuation system |
CMD (μm) | GM (μm) | GSD |
|---|---|---|---|---|
| Background, Day 1 | None | 0.65 | 0.69 | 1.30 |
| Background, Day 2 | None | 0.63 | 0.67 | 1.28 |
| Scaling | HVE | 0.64 | 0.68 | 1.27 |
| System A | 0.64 | 0.68 | 1.28 | |
| System B | 0.64 | 0.69 | 1.28 | |
| System C | 0.64 | 0.69 | 1.26 | |
| Crown Preparation | HVE | 0.64 | 0.69 | 1.29 |
| System A | 0.64 | 0.69 | 1.32 | |
| System B | 0.64 | 0.69 | 1.30 | |
| System C | 0.64 | 0.69 | 1.34 |
Each 10-sec aerosol measurement data point was background corrected by subtracting the average background concentration in each respective location on each day of testing, prior to running statistical analyses for comparisons between aerosol measurements collected while using each respective evacuation system and background concentrations. Background measurements were also background corrected using the average background concentration to ensure background measurements were normalized when comparing to aerosol measurements collected while using each respective evacuation system. Pairwise comparisons using Wilcoxon rank sum tests with a Bonferroni correction were conducted with pooled observations (across trials) to compare evacuation systems with background concentrations and identify when group medians were significantly elevated above background concentrations.
Results
Median background concentrations of PM4 and PM10 aerosol at each respective location can be seen in Supplementary Table 1. Suction flow rates, measured prior to each procedure, ranged from 13.5 to 19.5 SCFM, with average flow rates measured as 17.8, 17.7, 17.7, and 17.7 SCFM for HVE, system A, system B, and system C, respectively.
CMDs measured in the dentist’s BZ during ultrasonic scaling and the crown preparation procedure were 0.64 μm and did not vary significantly by evacuation system or procedure (Table 1). Background CMDs ranged from 0.63 to 0.65 μm.
PM10 and PM4 aerosol mass concentrations near the dentist’s BZ during ultrasonic scaling
No significant elevations of PM10 or PM4 aerosol mass concentrations above background concentrations were observed near the dentist’s BZ for any of the evacuation systems tested during the ultrasonic scaling procedure (Figure 3). System A reduced PM10 aerosol concentrations near the dentist’s BZ to median background concentrations most often during the ultrasonic scaling procedure (53.5% of scaling procedure; Table 2). HVE and System B reduced PM4 aerosol concentrations to background concentrations most often during the ultrasonic scaling procedure (HVE = 70.0%; System B = 60.5%; Table 2). Differences between median PM10 and PM4 mass concentrations between systems were small with differences in median mass concentrations ranging from 0.45–1.48 micrograms per cubic meter of air (μg/m3) for PM10 and 0.24–0.85 μg/m3 for PM4 aerosol.
Figure 3.
Box-whisker plots of background corrected mass concentrations in the dentist’s breathing zone during the scaling (top) and crown preparation (bottom) procedures. Significant increase above background concentrations is indicated by * (p = <0.05).
Table 2.
Percentage frequency* of how often evacuation systems reduced respirable or thoracic mass concentrations below median background concentrations.
| Procedure | Aerosol size fraction (Cutpoint) |
Dental evacuation system |
Near breathing zone (%) |
Edge of operatory (%) |
Hallway outside operatory (%) |
|---|---|---|---|---|---|
| Scaling | Thoracic (10 μm) | HVE† | 32.2 | 21.8 | 33.5‡ |
| System A | 53.5 | 56.2 | 26.2‡ | ||
| System B | 16.0 | 48.1 | 19.5‡ | ||
| System C | 18.5 | 21.3‡ | 0.01‡ | ||
| Respirable (4 μm) | HVE† | 70.0 | 68.8 | 43.8 | |
| System A | 50.2 | 57.3 | 45.9 | ||
| System B | 60.5 | 83.5 | 50.4‡ | ||
| System C | 44.9 | 74.5 | 19.0‡ | ||
| Crown Prep | Thoracic (10 μm) | HVE† | 14.2 | 39.8 | 21.2‡ |
| System A | 16.5 | 30.0‡ | 13.4‡ | ||
| System B | 23.6 | 45.3 | 13.0‡ | ||
| System C | 10.3‡ | 34.7‡ | 13.4‡ | ||
| Respirable (4 μm) | HVE† | 43.7 | 54.1 | 36.9‡ | |
| System A | 14.2‡ | 60.4 | 17.7‡ | ||
| System B | 47.2 | 64.1 | 40.8 | ||
| System C | 20.2‡ | 75.9 | 31.4‡ |
Percentage frequency of aerosol measurements reduced to concentrations below median background concentrations were calculated by dividing the sum of the number of aerosol measurements below the median background concentration during the procedure, by the sum of the total number of aerosol measurements during the procedure, multiplied by 100%.
HVE indicates high volume evacuation.
indicates significant increase above background concentrations (p = <0.05).
PM10 and PM4 aerosol mass concentrations in the dentist’s BZ during crown preparation
Significant elevation of PM10 mass concentrations above background concentrations were observed near the dentist’s BZ when using System C (p < 0.0001) during the crown preparation procedure (Figure 3). HVE, System A, and System B reduced PM10 aerosol concentrations to median background concentrations most often during the crown preparation procedure (HVE = 14.2%; System A = 16.5%; System B = 23.6%; Table 2). Differences in PM10 median mass concentrations among evacuation systems were small and ranged from 0.26–1.13 μg/m3.
Significant elevation of PM4 mass concentrations above background concentrations were observed near the dentist’s BZ when using System A (p = 0.006) or System C (p < 0.0001) during the crown preparation procedure (Figure 3). HVE and System B reduced PM4 aerosol concentrations to median background concentrations most often during the crown preparation procedure (HVE = 43.7%; System B = 47.2%; Table 2). Differences in PM4 median mass concentrations among evacuation systems were small and ranged from 0.01–0.39 μg/m3.
PM10 and PM4 aerosol mass concentrations at the edge of dental operatory during ultrasonic scaling
Significant elevation of PM10 mass concentrations above background concentrations were observed at the edge of the dental operatory when using System C (p = 0.0002) during the ultrasonic scaling procedure (Figure 4). System A and System B reduced PM10 aerosol concentrations to median background concentrations most often during the ultrasonic scaling procedure (System A = 56.2%; System B = 48.1%; Table 2). Differences in median PM10 mass concentrations were small and ranged from 0.05–0.35 μg/m3.
Figure 4.
Box-whisker plots of background corrected mass concentrations at the edge the of operatory during the scaling (top) and crown preparation (bottom) procedures. Significant increase above background concentrations is indicated by * (p = <0.05).
No significant elevations of PM4 mass concentrations above background concentrations were observed at the edge of the dental operatory for any of the evacuation systems tested during the ultrasonic scaling procedure (Figure 4). All systems tested reduced PM4 aerosol concentrations to median background concentrations for more than 50% of the ultrasonic scaling procedure, with System B reducing concentrations most often (System B = 83.5%; Table 2). Differences in median PM4 mass concentrations were small and ranged from 0.21–1.19 μg/m3.
PM10 and PM4 aerosol mass concentrations at the edge of dental operatory during crown preparation
Significant elevation of PM10 mass concentrations above background concentrations were observed at the edge of the dental operatory when using System A (p = 0.001) or System C (p = 0.0001) during the crown preparation procedure (Figure 4). HVE and System B reduced PM10 aerosol concentrations to median background concentrations most often during the crown preparation procedure (HVE = 39.2%; System B = 45.3%; Table 2). Differences in median PM10 aerosol mass concentrations were small and ranged from 0.03–0.35 μg/m3.
No significant elevations of PM4 mass concentrations above background concentrations were observed at the edge of the dental operatory for any of the evacuation systems tested during the crown preparation procedure (Figure 4). All systems similarly reduced PM4 (range: 54.1–75.9%) aerosol concentrations to median background concentrations at the edge of the operatory during the crown preparation, though some differences were noted (Table 2, Figure 4). Differences in median PM4 aerosol mass concentrations were small and ranged from 0.02–0.45 μg/m3.
PM10 and PM4 aerosol mass concentrations in the hallway outside of open operatory room during ultrasonic scaling
Significant elevation of PM10 mass concentration above background concentrations were observed in the hallway outside the operatory when using any of the four systems during the ultrasonic scaling procedure (p < 0.0001) (Figure 5). HVE and System A reduced PM10 mass concentrations in the hallway outside the operatory to median background concentrations most often during the ultrasonic scaling procedure (HVE = 33.5%; System A = 26.2%; Table 2). Differences in PM10 median mass concentrations among the evacuation systems were small and ranged from 0.12–0.82 μg/m3.
Figure 5.
Box-whisker plots of background corrected mass concentrations in the hallway outside the operatory during the scaling (top) and crown preparation (bottom) procedures. Significant increase above background concentrations is indicated by * (p = <0.05).
Significant elevation of PM4 mass concentrations above background concentrations were observed in the hallway outside the operatory when using System B (p = 0.049) or System C (p < 0.0001) during the ultrasonic scaling procedure (Figure 5). System B, System A, and HVE reduced PM4 mass concentrations to median background concentrations most often (System B = 50.4%; System A = 45.9%; HVE = 43.8%; Table 2). Differences in PM4 median mass concentrations among evacuation systems were small and ranged from 0.02–0.27 μg/m3.
PM10 and PM4 aerosol mass concentrations in the hallway outside of open operatory room during crown preparation
Significant elevation of PM10 mass concentration above background concentrations were observed in the hallway outside the operatory when using any of the four systems during the crown preparation procedure (p < 0.0001) (Figure 5). HVE reduced PM10 mass concentrations to median background concentrations most often (HVE = 21.2%; Table 2). Differences between systems were small and ranged from 0.11–0.23 μg/m3.
Significant elevation of PM4 mass concentration above background concentrations were observed in the hallway outside the operatory when using HVE (p = 0.0004), System A (P < 0.0001), and System C (p < 0.0001) during the crown preparation procedure (Figure 5). System B reduced PM4 mass concentrations below median background concentrations most often (System B = 40.8%; Table 2). Differences in PM4 median mass concentrations were small and ranged from 0.10–0.27 μg/m3.
Discussion
The ongoing COVID-19 pandemic combined with dental professions identified as among the highest risk occupations for COVID-19 highlights a need to mitigate risks associated with SARS-CoV-2 transmission in dental clinics (Zhang 2021). To minimize risk for exposure to SARS-CoV-2, guidance documents published by the Centers for Disease Control and Prevention (CDC 2020) and other national associations (ASHRAE 2021) advised dental professions to implement engineering, administrative, and personal protective equipment (PPE) controls such as considering postponing elective procedures and non-urgent outpatient visits, optimizing ventilation systems, implementing screening protocols for signs and symptoms of COVID-19 among patients and providers, performing targeted SARS-CoV-2 testing of asymptomatic patients, implementing environmental cleaning and disinfection procedures after each patient, and implementing universal masking and use of PPE. However, some of these controls might not sufficiently mitigate risk, as evidenced by recent studies that observed SARS-CoV-2 in the saliva of asymptomatic persons indicating that even the most detailed screening procedures for patients could miss potentially infectious patients (Huang et al. 2021). Further, testing all asymptomatic patients is likely not feasible for most dental practices. Source controls such as the dental evacuation systems evaluated in this study are advantageous because they can capture potentially harmful aerosols directly at the mouth of the patient before the aerosol can enter ambient air and subsequently be inhaled by nearby dental personnel, patients, or others, immediately or hours later.
Source control methods are dependent on the proximity of the capture device to the source, efficiency of the evacuation inlet, and air flow rates of the system. However, the efficiency of evacuation systems can vary widely by design and manufacturer, and the effectiveness of these systems has not been extensively evaluated in previous studies. Our results expand upon the findings of previous studies by evaluating concentrations of respirable (PM4) aerosols, an aerosol size of concern for both COVID-19 and occupational lung diseases, yet previously not evaluated, during commonly encountered dental procedures. Respirable (PM4) aerosol is of particular concern due to increased (1) ability to remain suspended in the air for hours to days, (2) likelihood of escaping past personal protective equipment like face shields, and (3) ability to reach and deposit in the unciliated lower airways including the gas-exchange region of the lungs (Cherrie and Aitken 1999; Hinds 2012; Brown et al. 2013; Konda et al. 2020; Ou et al. 2020; Pei et al. 2020; Ou et al. 2021). We observed median particle sizes in the submicron range for all procedures and evacuation systems which is consistent with previous studies of aerosols generated during crown preparations or ultrasonic scaling (Sotiriou et al. 2008; Balanta-Melo et al. 2020; Ehtezazi et al. 2021). Our study and previous studies’ observations of submicron aerosol generated during routine dental procedures with high-speed handpieces or ultrasonic scalers further emphasizes the need for effective aerosol source controls to minimize dental personnel’s risks for exposure to potentially harmful dental aerosols hours or days later.
A recent study by Nulty et al. evaluated the effectiveness of HVE at reducing PM2.5 and PM10 during five different restorative procedures, including ultrasonic scaling and tooth drilling, and observed that PM2.5 and PM10 were reduced to background levels when HVE was used during dental procedures (Nulty et al. 2020) which is consistent with the findings we report here. Similarly, Ou et al. observed the use of HVE significantly reduced emission of large aerosols ranging in size from 30 μm to 150 μm during ultrasonic scaling, although efficiency decreased with increasing aerosol size (Ou et al. 2021). We observed the most consistent reductions of PM10 mass concentrations near the BZ of the dentist when HVE or System A during an ultrasonic procedure. Similarly, HVE, System B, or System A resulted in the most consistent reductions of PM4 mass concentrations near the BZ of the dentist during the ultrasonic scaling procedure.
Another study by Comisi et al. evaluated the effectiveness of different aerosol exposure mitigation systems, including three of the four systems tested in our study here (HVE, System A, and System C), at reducing PM2.5 and spatter during a simulated crown preparation (Comisi et al. 2021). We note that some of our results and methods differed from those reported by Comisi et al. First, we also evaluated System B and observed it to have the most consistent PM4 and PM10 aerosol reductions near the BZ of the dentist during a crown preparation among all systems tested in our study. Among systems evaluated in both studies, Comisi et al. reported System A reduced PM2.5 to background concentrations near the BZ of a dentist during a simulated crown preparation. In our study here, System A reduced PM10, but not PM4, to background concentrations. Additionally, Comisi et al. observed HVE or System C did not reduce PM2.5 to background levels (Comisi et al. 2021). In contrast, we observed that HVE reduced PM10 and PM4 concentrations near the BZ of the dentist during a crown preparation to background concentrations. Although both our study and the study by Comisi et al. evaluated aerosol generation during a simulated crown procedure, some differences were noted in methodology which could contribute to differing results. First, we did not measure the same aerosol size fractions; Comisi et al. measured PM2.5 whereas we measured PM4 and PM10 aerosol. Also, the sampling duration differed between studies; Comisi et al. collected aerosol measurements during 2 minutes of a crown preparation, whereas we collected aerosol measurements for the entire duration of the crown preparation. Additionally, Comisi et al. performed a crown preparation on tooth number 30, whereas we performed a crown preparation on tooth number 21. Last, Comisi et al. do not report the suction flow rate of the evacuation systems tested, and differences in operational flow rates could potentially contribute to differences between their results and our results reported here.
System C resulted in a larger range of mass concentrations observed during either procedure and was associated with less consistent reduction of PM10 and PM4 aerosol, similar to findings reported by Comisi et al. (2021). In general, System C reduced PM10 and PM4 mass concentrations to background concentrations least often during either procedure and resulted in significantly elevated PM10 and PM4 mass concentrations near the BZ of the dentist during the crown preparation. Unlike the other alternative intraoral evacuation systems tested, System C is designed to be placed in the patient’s mouth between the teeth and cheek. Its smaller surface area and placement off to the side, as opposed to across the mouth like Systems A and B, could have contributed to its inability to effectively reduce both aerosol size fractions to background concentrations near the BZ of the dentist during the crown preparation, and its overall least consistent reductions in PM10 and PM4 aerosol among all systems evaluated. We note that although differences were noted when comparing all four systems evaluated in our study here, the difference in median mass concentrations among evacuation systems was minimal, ranging from 0.01–1.48 μg/m3.
To the best of our knowledge, no previous study has measured PM4 and PM10 aerosol concentrations in multiple locations both in and outside of a dental operatory during dental procedures when utilizing dental evacuation systems. Overall, we observed lower concentrations of both aerosol sizes in the region behind the head of the mannequin at the edge of the operatory as compared to the dentist’s BZ. The scaling and crown prep tools typically projected aerosols laterally or forward from the patient’s mouth, potentially creating a region of lower concentrations directly behind the patient and at the edge of the dental operatory where sampling equipment was located. We also observed higher concentrations of both aerosol sizes in the hallway outside the operatory room. Although the clinic was closed to patients on the days of our experiments, staff assisting with the aerosol sampling (n = 3) used the hallway just outside the operatory to move from one side of the operatory to the other when starting, stopping, or monitoring the aerosol sampling equipment during procedures. Increased foot traffic in the hallway outside the operatory from staff assisting with aerosol sampling could have contributed to increased aerosol concentrations as well. Additionally, we noted that there were no supply air vents in the operatory used for the procedures (Figure 1). The closest air vents were two return air (exhaust) vents located in the hallway outside the semi-open operatory, located approximately 2.9m and 5m from the mannequin head (Figure 1). Although the direction of air currents was not directly assessed in our study, the presence of exhaust vents in the hallway outside the semi-open operatory likely created negative pressure in the hallway outside the semi-open operatory when the high-volume suction was not in use between procedures. Aerosols generated during the procedure and not captured by the evacuation system could have been pulled toward the exhaust vents in the hallway outside the open operatory and could have contributed to an accumulation of PM4 and PM10 aerosol in the hallway outside the operatory. These findings further emphasize the importance of (1) source control to capture and remove small aerosol before it can accumulate within a clinic and (2) optimized ventilation systems designed to move air from clean-to-less-clean areas such that air is moved from hallways to the rear of operatories, thereby minimizing both exposures of personnel in the operatory and the potential for accumulation of aerosol outside of operatories.
Intraoral dental evacuation systems are advantageous because they are placed directly in the patient’s mouth and do not require positioning of an external device or alterations to dental instruments that might impede the dentist’s ability to perform the procedure. One disadvantage of HVE, either when used solely or in conjunction with an additional evacuation system, is that HVE often requires the use of one of the dental personnel’s hands or an additional dental assistant devoted to holding the HVE in place during four-handed procedures. Further, when used in conjunction with an additional evacuation system, the additional suction line can cause the suction flow rate to vary if the same vacuum pump is utilized, as is often the case in individual operatories or open bay operatories. An advantage of the intraoral evacuation systems evaluated in our study here is that they can be placed directly into a patient’s mouth and allow the dental personnel to freely use both hands while performing dental procedures, thus minimizing the number of personnel potentially exposed.
The vacuum flow rate will directly affect evacuation systems’ efficiency and can vary due to several factors such as increased resistance due to build-up of residues within the vacuum system or increased pressure drops from simultaneous use of other vacuum systems in adjacent dental operatories that rely on the same vacuum pump. In Europe, a minimum of 10.6 SCFM for intraoral suction devices has been used for decades to ensure the optimal performance of suction devices (Koch 2020). We measured flow rates well above 10.6 SCFM, and these flow rates were relatively consistent among procedures. However, no other dental operatories were in use at the time of our study. Simultaneous use of multiple suction systems that utilize the same vacuum pump could contribute to variability in flow rates observed in clinic settings. Thus, field studies evaluating evacuation systems in clinics should measure and report the vacuum flow rates. Clinics that use HVE or alternative dental evacuation systems such as those described here should routinely test the flow rate of their vacuum system lines in each dental operatory to ensure optimal performance.
There are several limitations of this study. First, we conducted simulated dental procedures with a mannequin and simulated dental procedures do not account for real-world variability in mouth and tongue anatomy and size, which could affect the efficiency of the systems tested. Similarly, because we collected measurements during simulated dental procedures, we were not able to identify aerosols that were patient-derived (e.g., saliva, blood, mucins, or plaque) vs. procedure-based (e.g., water from the handpiece). Additionally, we only simulated two commonly encountered dental procedures and four commercially available intraoral evacuation systems. We are currently planning additional studies of the effectiveness of additional dental evacuation systems at mitigating PM4 and PM10 aerosol during clinical practice with patients during multiple types of routine procedures to expand upon the results presented here. We note that designing a study to directly quantify SARS-CoV-2 infection risk for dental personnel is difficult because the infectious dose of SARS-CoV-2 needed to transmit infection has not been established (CDC 2021). The infectious dose for a respiratory virus can depend on multiple factors such as viral load of the patient; amount of virus shedding; aerosol size, number, and mass concentration; temperature; humidity; and immune status of the exposed individual (Leung 2021; Samet et al. 2021). Despite this knowledge gap, studies can be designed to assess theoretical exposure risk by assessing patient-derived bioaerosols present during dental procedures. In our future work, we aim to evaluate patient-derived bioaerosols and procedure-based aerosols to assess the effectiveness of dental evacuation systems at mitigating risk for exposure to potentially infectious patient-derived aerosol during clinical practice.
We chose to focus on PM4 and PM10 aerosol because (1) their small size and ability to remain suspended for hours to days is a potential hazard to dental personnel and patients if not adequately controlled, (2) aerosols in this size range (a) have been documented to contain SARS-CoV-2 virus capable of infecting cells in culture and (b) are more likely to escape past PPE such as face shields, (3) both size fractions can potentially contribute to occupational lung disease, and (4) no previous studies evaluated PM4 and PM10 aerosol generated during dental procedures while using source controls such as dental evacuation systems. However, larger aerosols in the inhalable size range can also contribute to potential respiratory virus infection risk. Additional studies are needed to evaluate the efficiency of source controls for inhalable aerosol exposure mitigation during dental procedures.
Further, because our study did not include aims to characterize airflow patterns in the dental clinic or conduct hazard mapping, we did not collect air measurements of all supply air and return ducts to model air currents in the facility. Therefore, although we observed elevations in PM4 and PM10 aerosol in the hallway outside the operatory, we were not able to identify potential dead zones or areas where PM4 and PM10 aerosol might accumulate due to decreased air flow. In future work, we plan to evaluate the effect of airflow patterns on spatial distributions of aerosols throughout a clinic during and after dental procedures with and without intraoral evacuation systems. Such studies would increase an understanding of airflow effects on the spatial distribution of aerosols, especially PM4 and PM10 aerosols that can remain suspended for hours or days.
The source controls evaluated in our study can be utilized alongside other engineering controls recommended by ASHRAE and CDC, such as utilizing well-ventilated individual operatories when possible and working with a ventilation professional to optimize air movement in a clean-to-less-clean flow direction, including placing supply-air vents that deliver clean air into corridors with return-air vents that remove contaminated air from the rear of dental operatories (ASHRAE 2021). Source controls like the dental evacuation systems evaluated here are of even greater importance for dental facilities with open floor plans which could have difficulty retrofitting their open floor design to have (1) semi-individual operatories by adding physical barriers and (2) ventilation designed with a clean-to-less-clean flow direction from corridors to patient operatories.
Conclusions
Source controls such as the dental evacuation systems evaluated in our study can help minimize dental personnel’s exposure to harmful aerosols generated during dental procedures, including infectious bioaerosols, and other hazardous aerosols such as metals or silica. Source controls capture aerosol at the mouth of the patient and minimize its wider distribution within the dental operatory or dental clinic where it could be potentially inhaled by other personnel or bystanders. Our results contribute to the limited understanding of the effectiveness of dental evacuation systems at mitigating PM4 and PM10 aerosol exposure and can help dental personnel make informed decisions as to which engineering controls might best suit the needs of their practice or clinic. Additional studies are warranted to evaluate the effectiveness of these systems at mitigating patient-derived bioaerosols in clinical practice during multiple types of routine procedures. Future studies are also needed to evaluate the effect of airflow patterns on distributions of aerosols throughout a clinic during and after dental procedures with and without intraoral evacuation systems to understand their effect on the distribution of aerosols.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Kenneth Mead, Ethan Fechter-Leggett, and Caroline P. Groth for reviewing the manuscript.
Funding
This work was supported by NIOSH research funds.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention. Mention of any company or product does not constitute endorsement by the U.S. Government, National Institute for Occupational Safety and Health, or Centers for Disease Control and Prevention.
Supplemental data for this article is available online at https://doi.org/10.1080/15459624.2022.2053140. AIHA and ACGIH members may also access supplementary material at http://oeh.tandfonline.com.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Abramson MJ, Murambadoro T, Alif SM, Benke GP, Dharmage SC, Glaspole I, Hopkins P, Hoy RF, Klebe S, Moodley Y, et al. 2020. Occupational and environmental risk factors for idiopathic pulmonary fibrosis in Australia: case–control study. Thorax. 75(10):864–869. [DOI] [PubMed] [Google Scholar]
- Asadi S, Wexler AS, Cappa CD, Barreda S, Bouvier NM, Ristenpart WD. 2019. Aerosol emission and superemission during human speech increase with voice loudness. Sci Rep. 9(1):2348. doi: 10.1038/s41598-019-38808-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHRAE. 2021. Coronavirus (COVID-19) response resources: dental facilities. Peachtree Corners (GA): ASHRAE; [accessed 2021]. https://www.ashrae.org/technical-resources/dental-facilities. [Google Scholar]
- Ather A, Patel B, Ruparel NB, Diogenes A, Hargreaves KM. 2020. Coronavirus disease 19 (COVID-19): implications for clinical dental care. J Endod. 46(5):584–595. doi: 10.1016/j.joen.2020.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balanta-Melo J, Gutiérrez A, Sinisterra G, Díaz-Posso M, Gallego D, Villavicencio J, Contreras A. 2020. Rubber dam isolation and high-volume suction reduce ultrafine dental aerosol particles: an experiment in a simulated patient. Appl Sci. 10(18):6345. doi: 10.3390/app10186345 [DOI] [Google Scholar]
- Brown JS, Gordon T, Price O, Asgharian B. 2013. Thoracic and respirable particle definitions for human health risk assessment. Part Fibre Toxicol. 10(1):12. doi: 10.1186/1743-8977-10-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carles P, Fabre J, Pujol M, Duprez A, Bollinelli R. 1978. [Complex pneumoconiosis in dental laboratory technicians]. Poumon et Le Coeur. 34(3):189–192. [PubMed] [Google Scholar]
- CDC. 2020. Interim infection prevention and control guidance for dental settings during the coronavirus disease 2019 (COVID-19) pandemic. U.S. Centers for Disease Control and Prevention; [accessed 2021 Dec 1]. https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html. [Google Scholar]
- CDC. 2021. Scientific brief: SARS-CoV-2 transmission. U.S. Centers for Disease Control and Prevention; [updated 5/7/2021; accessed 2022 Feb 3]. https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/sars-cov-2-transmission.html. [Google Scholar]
- Cherrie JW, Aitken RJ. 1999. Measurement of human exposure to biologically relevant fractions of inhaled aerosols. Occup Environ Med. 56(11):747–752. doi: 10.1136/oem.56.11.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia PY, Coleman KK, Tan YK, Ong SWX, Gum M, Lau SK, Lim XF, Lim AS, Sutjipto S, Lee PH, et al. 2020. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat Commun. 11(1):2800. doi: 10.1038/s41467-020-16670-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudat D. 1994. Occupational lung diseases among dental technicians. Tuber Lung Dis. 75(2):99–104. doi: 10.1016/0962-8479(94)90037-X [DOI] [PubMed] [Google Scholar]
- Cirillo N. 2020. COVID-19 outbreak: succinct advice for dentists and oral healthcare professionals. Clin Oral Investig. 24(7):2529–2535. doi: 10.1007/s00784-020-03323-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comisi JC, Ravenel TD, Kelly A, Teich ST, Renne W. 2021. Aerosol and spatter mitigation in dentistry: analysis of the effectiveness of 13 setups. J Esthet Restor Dent. 33(3):466–479. doi: 10.1111/jerd.12717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulthard P, Thomson P, Dave M, Coulthard FP, Seoudi N, Hill M. 2020. The COVID-19 pandemic and dentistry: the clinical, legal and economic consequences—part 2: consequences of withholding dental care. Br Dent J. 229(12):801–805. doi: 10.1038/s41415-020-2406-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vuyst P, Vande Weyer R, De Coster A, Marchandise FX, Dumortier P, Ketelbant P, Jedwab J, Yernault JC. 1986. Dental technician’s pneumoconiosis. a report of two cases [Case Reports Research Support, Non-U.S. Gov’t]. Am Rev Respir Dis. 133(2):316–320. [DOI] [PubMed] [Google Scholar]
- Duguid JP. 1946. The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. J Hyg (Lond). 44(6):471–479. doi: 10.1017/s0022172400019288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DA, Man JC, Brand P, Katstra JP, Sommerer K, Stone HA, Nardell E, Scheuch G. 2004. Inhaling to mitigate exhaled bioaerosols. Proc Natl Acad Sci USA. 101(50):17383–17388. doi: 10.1073/pnas.0408159101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehtezazi T, Evans DG, Jenkinson ID, Evans PA, Vadgama VJ, Vadgama J, Jarad F, Grey N, Chilcott RP. 2021. SARS-CoV-2: characterisation and mitigation of risks associated with aerosol generating procedures in dental practices. Br Dent J. doi: 10.1038/s41415-020-2504-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ergün D, Ergün R, Ozdemir C, Oziş Tür N, Yilmaz Hç, Akkurt I. 2014. Pneumoconiosis and respiratory problems in dental laboratory technicians: analysis of 893 dental technicians. Int J Occup Med Environ Health. 27(5):785–796. doi: 10.2478/s13382-014-0301-9 [DOI] [PubMed] [Google Scholar]
- Fabian P, McDevitt JJ, DeHaan WH, Fung RO, Cowling BJ, Chan KH, Leung GM, Milton DK. 2008. Influenza virus in human exhaled breath: an observational study. PLoS One. 3(7):e2691. doi: 10.1371/journal.pone.0002691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta JK, Lin CH, Chen Q. 2010. Characterizing exhaled airflow from breathing and talking. Indoor Air. 20(1):31–39. doi: 10.1111/j.1600-0668.2009.00623.x [DOI] [PubMed] [Google Scholar]
- Hinds WC. 2012. Aerosol technology: properties, behavior, and measurement of airborne particles. Hoboken (NJ): John Wiley & Sons. [Google Scholar]
- Holliday R, Allison J, Currie C, Edwards D, Bowes C, Pickering K, Reay S, Durham J, Lumb J, Rostami N, et al. 2021. Evaluating contaminated dental aerosol and splatter in an open plan clinic environment: implications for the COVID-19 pandemic. J Dent. 105:103565. doi: 10.1016/j.jdent.2020.103565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang N, Pérez P, Kato T, Mikami Y, Okuda K, Gilmore RC, Conde CD, Gasmi B, Stein S, Beach M, et al. 2021. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 27(5):892–903. doi: 10.1038/s41591-021-01296-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M. 2020. Aerosol reduction by means of an intraoral spray mist suction—first findings from an experimental pilot study. Studie DÜRR DENTAL SE, Höpfigheimer Str. 17, 74321 Bietigheim-Bissingen; [accessed 2021 Dec 15]. https://www.airtechniques.com/wp-content/uploads/AT-White-paper.pdf. [Google Scholar]
- Konda A, Prakash A, Moss GA, Schmoldt M, Grant GD, Guha S. 2020. Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano. 14(5):6339–6347. doi: 10.1021/acsnano.0c03252 [DOI] [PubMed] [Google Scholar]
- Leung NHL. 2021. Transmissibility and transmission of respiratory viruses. Nat Rev Microbiol. 19(8):528–545. doi: 10.1038/s41579-021-00535-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M-H, Chen C-T, Chuang L-C, Lin W-M, Wan G-H. 2019. Removal efficiency of central vacuum system and protective masks to suspended particles from dental treatment. PLoS One. 14(11):e0225644. doi: 10.1371/journal.pone.0225644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, Sun L, Duan Y, Cai J, Westerdahl D, et al. 2020. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 582(7813):557–560. doi: 10.1038/s41586-020-2271-3 [DOI] [PubMed] [Google Scholar]
- Loudon RG, Roberts RM. 1967. Droplet expulsion from the respiratory tract. Am Rev Respir Dis. 95(3):435–442. doi: 10.1164/arrd.1967.95.3.435 [DOI] [PubMed] [Google Scholar]
- Mirbod Parisa HEA, Bagheri M, Higham J. 2021. Aerosol formation due to a dental procedure: insights leading to the transmission of diseases to the environment. J R Soc Interface. 18:20200967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawska L, Johnson G, Ristovski Z, Hargreaves M, Mengersen K, Corbett S, Chao CYH, Li Y, Katoshevski D. 2009. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J Aerosol Sci. 40(3):256–269. doi: 10.1016/j.jaerosci.2008.11.002 [DOI] [Google Scholar]
- Nett RJ, Cummings KJ, Cannon B, Cox-Ganser J, Nathan SD. 2018. Dental personnel treated for idiopathic pulmonary fibrosis at a tertiary care center—Virginia, 2000-2015. MMWR Morb Mortal Wkly Rep. 67(9):270–273. doi: 10.15585/mmwr.mm6709a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nulty A, Lefkaditis C, Zachrisson P, Van Tonder Q, Yar R. 2020. A clinical study measuring dental aerosols with and without a high-volume extraction device. Br Dent J. doi: 10.1038/s41415-020-2274-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Q, Pei C, Kim SC, Abell E, Pui DY. 2020. Evaluation of decontamination methods for commercial and alternative respirator and mask materials—view from filtration aspect. J Aerosol Sci. 150:105609. doi: 10.1016/j.jaerosci.2020.105609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Q, Placucci RG, Danielson J, Anderson G, Olin P, Jardine P, Madden J, Yuan Q, Grafe TH, Shao S, et al. 2021. Characterization and mitigation of aerosols and splatters from ultrasonic scalers. medRxiv.2021.2002.2026.21252487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papineni RS, Rosenthal FS. 1997. The size distribution of droplets in the exhaled breath of healthy human subjects. J Aerosol Med. 10(2):105–116. doi: 10.1089/jam.1997.10.105 [DOI] [PubMed] [Google Scholar]
- Pei C, Ou Q, Kim SC, Chen S-C, Pui DY. 2020. Alternative face masks made of common materials for general public: fractional filtration efficiency and breathability perspective. Aerosol Air Qual Res. 20(12):2581–2591. doi: 10.4209/aaqr.2020.07.0423 [DOI] [Google Scholar]
- Pierre-Bez AC, Agostini-Walesch GM, Bradford Smith P, Hong Q, Hancock DS, Davis M, Marcelli-Munk G, Mitchell JC. 2021. Ultrasonic scaling in COVID-era dentistry: a quantitative assessment of aerosol spread during simulated and clinical ultrasonic scaling procedures. Int J Dent Hyg. 19(4):474–480. doi: 10.1111/idh.12548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM, Burke TA, Lakdawala SS, Lowe JJ, Marr LC, Prather KA, Shelton-Davenport M, Volckens J. 2021. SARS-CoV-2 indoor air transmission is a threat that can be addressed with science. Proc Natl Acad Sci USA. 118(45):e2116155118. doi: 10.1073/pnas.2116155118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarpia JL, Rivera DN, Herrera VL, Morwitzer MJ, Creager HM, Santarpia GW, Crown KK, Brett-Major DM, Schnaubelt ER, Broadhurst MJ, et al. 2020. Aerosol and surface contamination of SARS-CoV-2 observed in quarantine and isolation care. Sci Rep. 10(1):12732. doi: 10.1038/s41598-020-69286-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriou M, Ferguson SF, Davey M, Wolfson JM, Demokritou P, Lawrence J, Sax SN, Koutrakis P. 2008. Measurement of particle concentrations in a dental office. Environ Monit Assess. 137(1-3):351–361. doi: 10.1007/s10661-007-9770-7 [DOI] [PubMed] [Google Scholar]
- Stadnytskyi V, Bax CE, Bax A, Anfinrud P. 2020. The airborne lifetime of small speech droplets and their potential importance in SARS-CoV-2 transmission. Proc Natl Acad Sci USA. 117(22):11875–11877. doi: 10.1073/pnas.2006874117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Landuyt K, Hellack B, Van Meerbeek B, Peumans M, Hoet P, Wiemann M, Kuhlbusch T, Asbach C. 2014. Nanoparticle release from dental composites. Acta Biomater. 10(1):365–374. doi: 10.1016/j.actbio.2013.09.044 [DOI] [PubMed] [Google Scholar]
- Xie X, Li Y, Sun H, Liu L. 2009. Exhaled droplets due to talking and coughing. J R Soc Interface. 6(Suppl 6):S703–S714. doi: 10.1098/rsif.2009.0388.focus [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M 2021. Estimation of differential occupational risk of COVID-19 by comparing risk factors with case data by occupational group. Am J Ind Med. 64(1):39–47. doi: 10.1002/ajim.23199 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.