Introduction
NMDA-type glutamate receptors (NMDARs) play vital roles in nervous system function, contributing to a broad range of physiological functions spanning the basics of excitatory neurotransmission to the complexities of synaptic plasticity, learning and memory. The ability of a single receptor type to support this diverse array of functions is tied to the structural and functional properties of the receptor and its complex and dynamic regulation (Hansen et al., 2018; Stroebel and Paoletti, 2021). NMDARs are tetrameric receptors composed of two obligatory GluN1 subunits and two GluN2 or GluN3 subunits. Upon binding of the agonist glutamate to the GluN2 subunit and the co-agonist glycine or d-serine to the GluN1 or GluN3 subunits, NMDARs undergo a conformational change that opens a non-selective cation channel, resulting in net influx of cations and depolarization of the neuron. NMDAR GluN1 subunits exhibit extensive alternative splicing and GluN2 and GluN3 subunits are encoded by different genes with different developmental and spatial expression patterns, resulting in a broad array of NMDAR compositions with distinct ion channel properties (Iacobucci and Popescu, 2017; Perez-Otano et al., 2016; Petit-Pedrol and Groc, 2021; Rajani et al., 2020). Furthermore, the intracellular C-terminal tails have been shown to be modified by kinases, phosphatases, ions, and second messengers, and can interact with a broad variety of scaffolding proteins and intracellular signaling molecules (Lau and Zukin, 2007; Lussier et al., 2015; Tovar and Westbrook, 2017; Warnet et al., 2021). Notably, alterations in NMDAR function have been associated with brain disorders that result in cognitive deficits, including Alzheimer’s disease and schizophrenia (Burnashev and Szepetowski, 2015; Ogden et al., 2017; Vieira et al., 2021).
Historically, the fundamental role of NMDARs in synaptic function and plasticity has been attributed to their specialized properties of ion flux, such as high permeability to calcium ions, slow kinetics, voltage-dependent block by magnesium ions, and physiological modulation by a wide variety of intracellular and extracellular signaling molecules. More recently, a growing number of studies support that NMDARs also signal in an ion flux-independent manner. Here, we review these non-ionotropic NMDAR signaling mechanisms that have been reported to contribute to a broad array of neuronal functions and dysfunctions including synaptic transmission, long-term depression (LTD), dendritic spine structural plasticity, cell death and survival, and neurological disorders.
Non-ionotropic NMDAR signaling in neuronal physiology
NMDAR endocytosis and trafficking
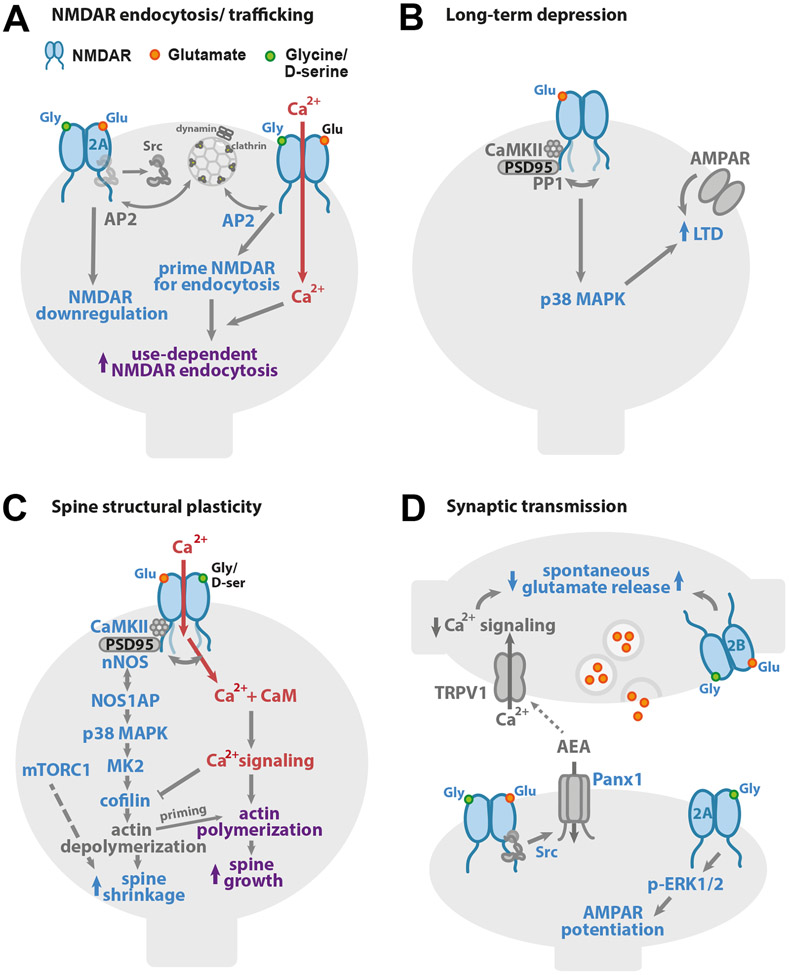
Among the earliest studies reporting non-ionotropic NMDAR signaling were those showing that agonist binding to the NMDAR influenced its surface expression and trafficking, independent of ion flux (Fig. 1A). In an initial study, Vissel and colleagues demonstrated that glutamate binding to the NMDAR enhanced tyrosine dephosphorylation of the GluN2A subunit, independent of ion flux through the NMDAR (Vissel et al., 2001). Tyrosine dephosphorylation led to a reduction of the number of functional NMDARs, an effect that was blocked by expression of a dominant-negative subunit of the clathrin-adaptor protein, AP2. This NMDAR downregulation was shown to depend on the binding of both glutamate and co-agonist.
Figure 1. Non-ionotropic NMDAR signaling in neuronal physiology.
(A) Binding of one or both co-agonists drives NMDAR downregulation. On the left, binding of NMDAR agonist and co-agonist drives dephosphorylation of GluN1 Y837 and GluN2A Y842, even when NMDAR ion flux is inhibited, leading to downregulation of the number of functional NMDARs. On the right, high concentrations of glycine (>10 μM) increase the interaction with AP2 and prime the NMDAR for dynamin-dependent endocytosis. (B) Glutamate binding to the NMDAR is sufficient to drive long-term depression (LTD) of synaptic strength, independent of ion flux through the NMDAR. p38 MAPK activation is required for LTD induction. Agonist binding to the NMDAR initiates a conformational change which drives the GluN1 C-tails apart and alters its interactions with CaMKII and PP1. (C) Ion flux-independent NMDAR signaling contributes to bidirectional spine structural plasticity. Glutamate binding with little or no ion flux through the NMDAR drives dendritic spine shrinkage via a signaling pathway that includes the interaction of nNOS and NOS1AP, and the activities of nNOS, p38 MAPK, CaMKII, MK2, and cofilin. Furthermore, the activity of mTORC1 supports a requirement for new protein synthesis. Notably, when this non-ionotropic NMDAR signaling pathway is activated in combination with strong calcium influx, it instead drives dendritic spine growth. (D) Non-ionotropic NMDAR signaling plays a role in the regulation of neurotransmitter release and synaptic strength. On the left, ligand binding to postsynaptic NMDARs activates Pannexin-1 channels through Src. Opening of Pannexin-1 drives clearance of AEA, a ligand for presynaptic TRPV1, from the synaptic cleft. With decreased AEA, TRPV1 closes, reducing calcium available for spontaneous release of glutamate. On the right bottom, in a second mechanism, co-agonist binding to GluN2A-containing postsynaptic NMDARs activates ERK1/2 and increases synaptic strength. On the right top, activation of presynaptic NMDARs by agonist, or both agonist and co-agonist, enhances spontaneous release of glutamate in an ion flux-independent manner.
In another related series of studies, co-agonist binding alone has been demonstrated to prime the NMDAR for clathrin-mediated, dynamin-dependent endocytosis (Nong et al., 2003). In this example, occupancy of the NMDAR co-agonist site by glycine or d-serine at high concentrations primes the NMDAR for endocytosis, which depends on AP2. This signaling occurs independent of the identity of the GluN2 subunit, and a single amino acid on the GluN1 subunit (A714) has been identified as critical for this glycine priming of NMDARs (Han et al., 2013). Furthermore, priming depends on specific splice variants of the obligatory GluN1 subunit, as only GluN1 isoforms that lack the N1 cassette allow for co-agonist priming of the NMDAR for endocytosis (Li et al., 2021). Because hippocampal pyramidal neurons, but not interneurons, naturally express GluN1 isoforms lacking the N1 cassette, co-agonist priming of the NMDAR for endocytosis can be observed in the former but not in the latter.
Additional studies have focused on influences of agonist and co-agonist binding on NMDAR trafficking and lateral mobility, independent of ion flux. In one such study (Barria and Malinow, 2002), the authors show that binding of agonist and co-agonist drives the activity-dependent synaptic insertion of GluN2A-containing NMDARs in CA1 pyramidal neuron dendrites in organotypic cultured hippocampal slices, independent of synaptic NMDAR current. Notably, spontaneous activity in the presence of tetrodotoxin (TTX) was sufficient to drive insertion of GluN2A-containing NMDARs, which was inhibited by competitive antagonists of either the NMDAR agonist or co-agonist sites. In contrast, synaptic insertion of GluN2B occurred even when agonist or co-agonist binding was inhibited. In another study tracking NMDAR subunits in cultured hippocampal neurons using antibody-conjugated quantum dots, d-serine was shown to decrease the lateral mobility of GluN2B-, but not GluN2A-containing NMDARs even when ion flow through the NMDAR was blocked by AP5 (Ferreira et al., 2017; Papouin et al., 2012). Furthermore, glycine was shown to decrease the lateral mobility of GluN2A-, but not GluN2B-, containing NMDARs independent of NMDAR currents (Ferreira et al., 2017; Papouin et al., 2012). Thus, the relative local abundance of glutamate, glycine and d-serine can act to tune NMDAR trafficking and synaptic insertion, and thus NMDAR signaling, independent of ion flux.
Synaptic plasticity
Over the past decade, there has been increased focus on the role of non-ionotropic NMDAR signaling in synaptic plasticity. Although earlier evidence had emerged that NMDAR-dependent long-term depression (NMDAR-LTD) of synaptic strength could occur independently of ion flux through the receptor (Mayford et al., 1995), recent reports have highlighted and further defined this role for ion flux-independent NMDAR signaling (Carter and Jahr, 2016; Dore and Malinow, 2020; Nabavi et al., 2013; Stein et al., 2015; Stein et al., 2020; Wong and Gray, 2018) (Fig. 1B). Using NMDAR antagonists that block ion flux through the NMDAR but still allow for glutamate binding such as co-agonist blocker 7-chlorokynurenic acid (7-CK) or channel pore blocker MK-801, NMDAR-LTD was successfully induced even when ion flow through the NMDAR was blocked. In contrast, NMDAR antagonists that inhibit glutamate binding, such as AP5 or CPP, blocked LTD induction. Thus, glutamate binding to the NMDAR, not ion flux through the NMDAR, is required for LTD. Notably, high frequency stimulation protocols that are typically used to induce long-term potentiation (LTP) of synaptic strength, instead drive LTD when ion flow through the NMDAR is inhibited by blocking the pore or co-agonist binding (Nabavi et al., 2013; Stein et al., 2020), further supporting that glutamate binding to the NMDAR is sufficient to drive LTD.
There have been several insights into the ion flux-independent molecular signaling mechanisms that drive LTD in response to glutamate binding to the NMDAR. Downstream of glutamate binding, p38 mitogen-activation protein kinase (p38 MAPK) was shown to attain its active phosphorylated state during LTD induction by NMDA treatment in the presence of the NMDAR pore blocker MK-801 (Nabavi et al., 2013), demonstrating NMDA-dependent activation of intracellular signaling independent of ion flux through the NMDAR. In a subsequent study, pharmacological inhibition confirmed that p38 MAPK is required for non-ionotropic NMDAR-LTD (Stein et al., 2020). Even though NMDAR-mediated calcium influx is not required, successful NMDAR-LTD induction requires a basal level of intracellular calcium, which is proposed to maintain synaptic transmission through calcineurin-mediated restriction of AMPAR levels at the synapse (Nabavi et al., 2013). Notably, despite the differences between GluN2A and GluN2B in their developmental expression patterns, synaptic location, protein modifications, and interactions at the C-tail (Paoletti et al., 2013), a recent study has shown that non-ionotropic NMDAR-LTD is not specifically associated with either the GluN2A or GluN2B subunit (Wong and Gray, 2018).
Despite the accumulating evidence in support of ion flux-independent NMDAR signaling driving LTD, there is still controversy. Several studies report contrasting results, finding instead that the use-dependent pore inhibitor MK-801 inhibits LTD (Babiec et al., 2014; Coultrap et al., 2014; Sanderson et al., 2016). Calcium leak through the NMDARs appears unlikely as calcium influx in the presence of co-agonist blockers such as 7-CK is not detectable (Stein et al., 2015) and alternate sources of calcium such as voltage-gated calcium channels (VGCCs) and downstream signaling from Group I metabotropic glutamate receptors (Group I mGluRs) do not play a role (Nabavi et al., 2013; Stein et al., 2020). It has been suggested that a contributing variable may be age-dependence in the time window for non-ionotropic NMDAR signaling, in that higher expression of PSD-95 in older animals could block non-ionotropic NMDAR signaling by obstructing the conformational change of the GluN1 C-tails (Dore and Malinow, 2020). Due to the large number of variables in experimental conditions, including method of tissue preparation, specifics of the LTD induction protocols, concentration and timing of washout of MK-801, and recipes for extracellular and intracellular solutions including concentrations of co-agonist and ion channel inhibitors, the reason for discrepancy between studies remains unclear.
In addition to synaptic weakening driven by NMDAR agonist binding, NMDAR co-agonist binding has been reported to drive an increase in synaptic strength through postsynaptic modifications that are independent of ion flow (Fig. 1D). Particularly, glycine binding to GluN2A containing NMDAR complexes increases AMPAR responses through activation of extracellular signal-regulated protein kinase (ERK1/2) in an ion flux-independent manner (Li et al., 2016b). Interestingly, ERK1/2 activation had earlier been reported downstream of agonist-induced non-ionotropic NMDAR signaling in conjunction with co-activation of metabotropic glutamate receptor 5 (mGluR5) (Yang et al., 2004).
Bidirectional spine structural plasticity
A large body of work has established that the decreased synaptic transmission strength that is observed following induction of LTD is accompanied by changes in synaptic structure, including a decrease in dendritic spine volume and spine loss (Stein and Zito, 2019). Notably, in parallel with the observations for LTD, several recent studies reported that NMDAR pore blockers or inhibitors of NMDAR co-agonist binding do not block NMDAR-LTD associated dendritic spine shrinkage, which is blocked by inhibiting NMDAR agonist binding (Stein et al., 2015; Stein et al., 2020; Thomazeau et al., 2020) (Fig. 1C). These studies show that non-ionotropic NMDAR signaling is sufficient to drive dendritic spine shrinkage and loss, independent of ion flux through the NMDAR.
The molecular signaling pathway that drives spine shrinkage downstream of non-ionotropic NMDAR signaling has begun to be elucidated. In addition to a requirement for p38 MAPK (Stein et al., 2015), both neuronal nitric oxide synthase (nNOS), which is closely associated with the NMDAR through its interaction with PSD-95 (Li et al., 2013), and the interaction between the adaptor protein NOS1AP and nNOS (Zhu et al., 2014) are critical for non-ionotropic NMDAR-mediated spine shrinkage (Stein et al., 2020). Downstream targets of p38 MAPK, the kinase MK2 and the actin-depolymerizing protein cofilin were also demonstrated to be essential (Stein et al., 2020). Furthermore, CaMKII, which has recently been identified to play a role in LTD (Coultrap et al., 2014), was also observed to be required for spine shrinkage mediated by non-ionotropic NMDAR signaling, although it is unclear whether the role of CaMKII is upstream near the NMDAR (Aow et al., 2015) or downstream at the actin cytoskeleton, as shown for CaMKIIβ (Kim et al., 2015; Sanabria et al., 2009). Additionally, mammalian target of rapamycin complex 1 (mTORC1), which is important for protein synthesis, has been shown to be required for spine shrinkage mediated by non-ionotropic NMDAR signaling (Thomazeau et al., 2020). Despite significant progress, much remains to be elucidated concerning how these molecular signaling mechanisms link to each other and how they are initiated by conformational movement of the NMDAR.
As glutamate binding to the NMDAR is sufficient to activate non-ionotropic NMDAR signaling, it is likely that the same signaling pathways are also activated during activity-induced dendritic spine growth. Indeed, a recent report shows that many components of the non-ionotropic NMDAR signaling pathway required for LTD-associated spine shrinkage are also required for LTP-associated spine growth: pharmacological inhibition of nNOS, nNOS-NOS1AP interactions, p38 MAPK, or MK2 all blocked LTP-induced spine growth (Stein et al., 2021). Furthermore, simultaneous influx of calcium, regardless of its source, is sufficient to drive spine growth in conjunction with non-ionotropic NMDAR signaling. In contrast, there is no evidence yet that non-ionotropic NMDAR signaling plays a role in synaptic strengthening. Despite the co-occurrence of LTP and spine growth, the pathway for the two processes appears to diverge upstream of p38 MAPK, as inhibition of p38 MAPK blocks LTP-associated spine growth but not LTP (Stein et al., 2021). Altogether, these data fit a model (Stein et al., 2021) in which non-ionotropic NMDAR signaling, initiated by glutamate binding during synaptic plasticity, destabilizes the actin cytoskeleton, leading to spine shrinkage when associated with a weak stimulus as in the case of LTD, or to actin branching and spine growth (Bosch et al., 2014) when in the presence of a strong stimulus and the robust calcium influx as seen with LTP.
Spontaneous glutamate release
In addition to postsynaptic signaling, it has been reported that spontaneous release of glutamate is regulated by non-ionotropic NMDAR signaling, both in Cajal-Retzius cells synapsing onto layer 5 (L5) pyramidal cells of the visual cortex (Abrahamsson et al., 2017) and in hippocampal CA1 neurons (Bialecki et al., 2020; Weilinger et al., 2016) (Fig. 1D). In L5 cells of the visual cortex, the authors observed enhancement of spontaneous neurotransmitter release via ion flux-independent signaling of presynaptic NMDARs (Abrahamsson et al., 2017). In contrast, in the hippocampus, non-ionotropic NMDAR signaling was shown to lead to decreased spontaneous neurotransmitter release (Bialecki et al., 2020; Weilinger et al., 2016).
The signaling pathway regulating neurotransmitter release in the hippocampus via non-ionotropic NMDAR signaling was further defined. The authors demonstrated that simultaneous glutamate and co-agonist binding activates both sarcoma (Src) kinase and the ion channel Pannexin-1 (Panx1), which form a complex with the NMDAR (Bialecki et al., 2020; Weilinger et al., 2016). The opening of Pannexin-1 controls the extracellular levels of anandamide (AEA), an endogenous agonist for the presynaptically located transient receptor potential vanilloid 1 (TRPV1) channel, which regulates the spontaneous release of glutamate (Bialecki et al., 2020). The authors propose that this type of trans-synaptic signaling is a homeostatic mechanism in which decreased spontaneous release of glutamate drives reduced non-ionotropic NMDAR signaling at the postsynaptic terminal, which ultimately feeds back to increase the frequency of spontaneous release via presynaptic TRPV1 channels (Fig. 1D). In addition, binding of the co-agonist glycine to GluN2A-containing receptors has been observed to increase the frequency of miniature excitatory postsynaptic currents (mEPSCs) through ion flux-independent NMDAR signaling (Li et al., 2016b).
Activation of non-ionotropic NMDA receptor signaling
To assess conformational movements of the NMDAR during non-ionotropic NMDAR signaling, several studies have used FRET to monitor movements of the C terminus of the NMDAR. These studies reported that NMDA or glutamate binding to the NMDAR drives GluN1 C-tails to move away from each other, independent of NMDAR ion flux (Aow et al., 2015; Dore et al., 2015; Ferreira et al., 2017). Moreover, antibody-mediated restriction of this movement inhibited ion flux-independent NMDAR-LTD (Aow et al., 2015; Dore et al., 2015). The agonist-binding driven conformational change also caused the GluN1 C-tail to move away from both the protein phosphatase PP1 and the protein kinase CaMKII (Aow et al., 2015), both of which have demonstrated roles in mediating LTD (Coultrap et al., 2014; Lee et al., 2009). Interestingly, the movement between the GluN1 C-tail and CaMKII is driven by PP1 activity and delayed by several minutes. Notably, the same movement of GluN1 C-tails occurs whether in the presence of NMDA or glutamate alone or along with co-agonist (Dore et al., 2015; Ferreira et al., 2017).
As co-agonist binding induces non-ionotropic NMDAR signaling that is distinct from that caused by agonist binding, it is to be expected that it also causes distinct conformational changes of the NMDAR. Indeed, while glutamate binding to the NMDAR causes the GluN1 C-tails to move further apart, d-serine binding to the NMDAR has been observed to cause GluN1 C-tails to move closer to one another (Ferreira et al., 2017). Surprisingly, binding of the co-agonist glycine does not cause any detectable movement of GluN1 C-tails (Ferreira et al., 2017), although the lack of measurable response could be due to isometric rotation of the C-tails that cannot be picked up by FRET or, alternatively, signaling could originate from movement of the GluN2 C-tail, which has not yet been investigated. Recently, agonist and co-agonist have been shown also to induce different movement of the amino-terminal domains (ATD) of the NMDAR (Vyklicky et al., 2021). Glutamate binding alone to GluN2 subunit induces two step conformational change of the ATD that results in ATD dimer separation but not pore opening (Vyklicky et al., 2021). Glycine binding alone to GluN1 subunit does not induce any conformational changes of the ATD, although it is not possible to rule out isometric rotation not observable with FRET (Vyklicky et al., 2021).
Non-ionotropic NMDAR signaling in non-neuronal physiology
Non-ionotropic NMDAR signaling is not limited to neurons. For example, endothelial cells that make up the blood-brain barrier (BBB) also have been reported to exhibit ion flux-independent NMDAR signaling. A first example occurs via the action of tissue plasminogen activator (tPA). Earlier work had established that tPA acts to potentiate NMDAR-mediated calcium influx through binding to the N-terminal domain of GluN1 and increasing both the surface dynamics and clustering of NMDARs (Lesept et al., 2016; Nicole et al., 2001). Notably, when tPA binds to the NMDAR in the presence of either NMDAR agonist or co-agonist, a signaling cascade is initiated in which Rho-associated coiled-coil containing protein kinase (ROCK) phosphorylates myosin light chain, driving cytoskeletal modifications that shrink the endothelial cells and increase BBB permeability, independent of ion flux through the NMDAR (Mehra et al., 2020). Furthermore, brain endothelial NMDARs have been reported to have agonist-binding dependent, non-ionotropic signaling. In this second example, NMDA binding induces increase in intracellular calcium levels through the opening of endoplasmic reticulum and lysosomal calcium stores (Negri et al., 2021). This ion flux-independent opening of intracellular calcium stores is mediated by group 1 mGluRs, although the exact mechanism or link between NMDARs and mGluRs in this signaling pathway is unknown.
Finally, astrocytes have been reported to express NMDARs and exhibit ion-flux independent NMDAR signaling (Montes de Oca Balderas and Aguilera, 2015; Montes de Oca Balderas et al., 2020). In these studies, the authors report that NMDAR signaling, even when the pore is blocked by MK-801, leads to calcium release from the endoplasmic reticulum in a process that involves tyrosine kinase. This mechanism was shown to be activated by H+, suggesting that acidic conditions could elicit a similar non-ionotropic NMDAR signaling in other cell types.
Non-ionotropic NMDAR signaling in neuronal dysfunction and disease
Excitotoxicity
In addition to the essential role of the NMDAR for healthy neuronal physiology, excessive activation of the NMDAR, as observed in situations such as brain ischemia, has been shown to lead to excessive calcium influx and ultimately to cell death (Aarts et al., 2002; Rossi et al., 2000). Although intracellular calcium levels are clearly a critical component in driving cell death, recent studies have demonstrated that ion flux-independent NMDAR signaling is also sufficient to initiate signaling pathways associated with excitotoxicity (Fig. 2A).
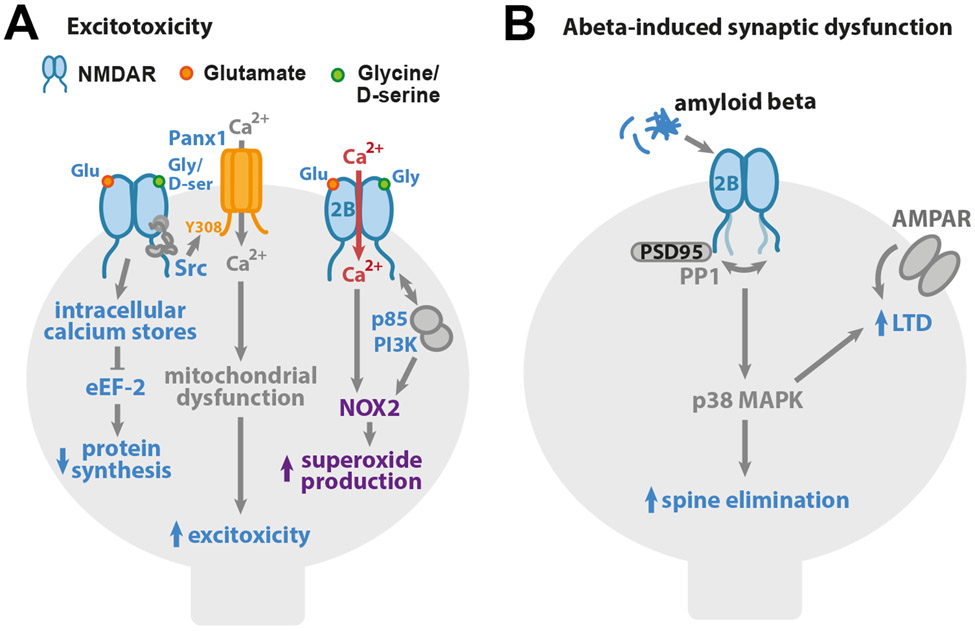
Figure 2. Non-ionotropic NMDAR signaling in neuronal dysfunction and disease.
(A) Non-ionotropic NMDAR signaling contributes to excitotoxicity. Ligand binding to the NMDAR drives opening of intracellular calcium stores and inhibition of eEF-2 and protein synthesis. Glutamate and co-agonist binding to the NMDAR leads to Src kinase activation and the opening of Pannexin-1 channels and Ca2+ influx, which drives the mitochondrial dysfunction, contributing to excitotoxicity. Ligand binding to GluN2B-containing NMDARs increases binding of p85 to GluN2B C-tail, removing the PI3K regulatory domain so PI3K can activate NOX2 and superoxide production that results in excitotoxicity. (B) Synaptic dysfunction associated with Alzheimer’s disease can be mediated through non-ionotropic NMDAR signaling, β-amyloid acting via GluN2B subunit-containing NMDAR complexes results in activation of p38 MAPK and drives LTD and dendritic spine elimination.
Excitotoxic conditions of brain ischemia include the inhibition of protein synthesis, which can be mediated through ion flux-independent NMDAR signaling (Gauchy et al., 2002). In this study, the authors show that application of NMDAR agonist drives the opening of intracellular calcium stores, leading to phosphorylation of eukaryotic Elongation Factor 2 (eEF-2) and, subsequently, slowing protein synthesis. Notably, protein synthesis was inhibited by NMDA application even in the absence of extracellular Ca2+ and in the presence of Mg2+, supporting that ion flux-independent NMDAR signaling was sufficient. Removal of d-serine or competitive inhibition of the NMDAR co-agonist binding site blocked the NMDA-induced inhibition of protein synthesis, demonstrating a requirement for both agonist and co-agonist binding.
Another study demonstrated that both agonist and co-agonist binding to the NMDAR triggers a non-ionotropic NMDAR signaling pathway that results in mitochondrial dysfunction and, ultimately, cell death (Weilinger et al., 2016). Using electrophysiological recordings and biochemical approaches, the authors showed that non-ionotropic NMDAR signaling drives activation and recruitment of both Src kinase and Pannexin-1 to the NMDAR to create a signaling complex. Phosphorylation of Pannexin-1 by Src kinase allows for its activation and the calcium influx that results in mitochondrial dysfunction. As calcium influx through Pannexin-1, but not through NMDAR, is required for cell death, this non-ionotropic NMDAR pathway for excitotoxicity depends on the localized calcium signaling of Pannexin-1. As Src kinase is known to bind to NMDAR indirectly through NADH dehydrogenase 2, conformational change of the NMDAR may be sufficient to bring Src kinase in proximity of Y308 on the C-tail of Pannexin-1 for activation that is essential for cell death (Fig. 2A).
Beyond calcium dysregulation, non-ionotropic NMDAR signaling has also been identified as a vital component of NADPH oxidase-2 (NOX2)-mediated superoxide production (Minnella et al., 2018). Although reactive oxygen species are a normal byproduct and necessary for cellular homeostasis, overproduction of superoxides leads to a variety of undesirable effects such as altered protein folding and mitochondrial dysfunction that will ultimately lead to cell death (Forrester et al., 2018; Wang and Swanson, 2020). Triggered by glutamate binding alone, the p85 regulatory subunit of phosphoinositide 3-kinase (PI3K) interacts with the GluN2B C-tail (Minnella et al., 2018). The activated PI3K is required for NOX2 activity, but calcium influx from any source is also required for superoxide production that eventually leads to cell death (Fig. 2A).
Neuroprotection
Interestingly, in addition to promoting excitotoxicity when bound to the receptor alongside with agonist, co-agonist binding alone to NMDARs has been demonstrated to promote cell survival (Chen et al., 2017; Hu et al., 2016). This mechanism of the NMDAR is ion-flux independent, as glycine treatment was observed to attenuate ischemic injury through increased phosphorylation and activation of the cell survival-promoting kinase Akt, even in the presence of the channel pore blocker MK-801. Notably, this mechanism required the presence of GluN2A subunits (Hu et al., 2016). As the mechanism of cell survival through activated Akt involves modification of various substrates such as the class O of forkhead box transcription factors (FOXO) and the B-cell lymphoma 2 (Bcl-2) family members, it warrants investigation if these targets of Akt can also be modified in an ion flux-independent manner. It is possible that non-ionotropic NMDAR signaling induced by co-agonist binding could be neuroprotective both by activating signaling for promoting cell survival and by driving endocytosis of NMDARs and thus preventing calcium dysregulation and superoxide production.
Alzheimer’s disease
Excessive production of β-amyloid is thought to contribute to the etiology of Alzheimer’s disease by disrupting synaptic and circuit function (Hardy and Selkoe, 2002; Jackson et al., 2019). Notably, non-ionotropic NMDAR signaling has been implicated in β-amyloid induced synaptic deficits (Fig. 2B). Initial studies using inhibitors of co-agonist binding or pore blockers showed that β-amyloid treatment induces NMDAR-dependent synaptic depression independent of NMDAR-mediated ion flux (Kessels et al., 2013; Tamburri et al., 2013). In contrast, NMDAR antagonists that block glutamate binding resulted in robust inhibition of β-amyloid-induced synaptic deficits. β-amyloid-induced synaptic depression depended on the presence of the GluN2B subunit (Kessels et al., 2013; Tamburri et al., 2013). Notably, activation of p38 MAPK was shown also to be required for β-amyloid induced dendritic spine elimination (Birnbaum et al., 2015).
As non-ionotropic NMDAR-mediated LTD and β-amyloid-induced synaptic depression both share a requirement for glutamate binding to the NMDAR and both lead to activation of p38 MAPK, it appears likely that the signaling cascade mediating non-ionotropic NMDAR-LTD contributes to the synaptic dysfunction associated with Alzheimer’s disease. Indeed, β-amyloid was found to cause a similar conformational change of the GluN1 C-tails as that observed with agonist binding to the NMDAR in the absence of co-agonist binding (Dore et al., 2021). Furthermore, overexpression of PSD-95 is sufficient to block agonist- and β-amyloid-induced NMDAR conformational changes, non-ionotropic NMDAR-LTD, and β-amyloid-induced synaptic depression (Dore et al., 2021; Dore and Malinow, 2020). Finally, large spines, which are more enriched for PSD-95 (De Roo et al., 2008; Lambert et al., 2017), appear less susceptible to β-amyloid-induced synaptic depression, and pharmacological block of PSD-95 depalmitoylation is sufficient to reverse β-amyloid-induced synaptic depression (Dore et al., 2021). These findings have led to the proposal that enhancement of PSD-95 levels could serve as a potential therapeutic route for addressing synaptic dysfunction associated with Alzheimer’s disease (Dore et al., 2021).
Schizophrenia
In addition to potentially mediating synaptic changes associated with Alzheimer’s disease, there is cause to believe that non-ionotropic NMDAR signaling may also contribute to neuronal dysfunction associated with schizophrenia. NMDAR hypofunction has long been proposed to contribute to the etiology of schizophrenia through driving changes in synaptic and circuit function (Nakazawa and Sapkota, 2020). The NMDAR hypofunction model of schizophrenia is supported by findings that schizophrenia is associated with decreased levels of d-serine in CSF (Bendikov et al., 2007; Hashimoto et al., 2005) and that NMDAR antagonists such as PCP and ketamine produce schizophrenia-like symptoms in healthy individuals (Javitt and Zukin, 1991; Krystal et al., 1994; Newcomer et al., 1999). Furthermore, decreasing d-serine levels by genetic deletion of enzyme serine racemase (SRKO) causes schizophrenia-like neuroanatomical changes and behavioral deficits in mice (Balu et al., 2013; Basu et al., 2009; Puhl et al., 2015).
An intriguing hypothesis (Park et al., 2021) asserts that the decreased d-serine levels associated with schizophrenia create an enhanced opportunity for glutamate binding to the NMDAR in the absence of co-agonist, leading to the initiation of non-ionotropic NMDAR signaling, disrupting spine growth and stabilization (Hill and Zito, 2013), and instead biasing toward synaptic weakening (Nabavi et al., 2013) and spine shrinkage and loss (Stein et al., 2015). Notably, a recent preprint has reported that SRKO mice exhibit a bias for activity-dependent destabilization of dendritic spines (Park et al., 2021), whereby stronger stimuli that normally lead to long-term spine growth in WT animals instead lead to no change or shrinkage in SRKO animals.
Summary
Although the role of NMDARs in synaptic plasticity, cell survival, cell death, and neurological disorders had been long considered to be mediated by ion flux, we have highlighted here the accumulating reports that NMDARs also signal in an ion flux-independent manner (Table 1). Indeed, these studies provide substantial evidence that ligand binding to the NMDAR is sufficient to mediate many physiological functions of the NMDAR in the absence of ion flux. Notably, over the past 25 years, many studies have brought to light similar ion flux-independent functions of other ionotropic receptors and ion channels (Valbuena and Lerma, 2016), such as kainate receptors (Rodriguez-Moreno and Lerma, 1998), AMPARs (Bai et al., 2002; Hayashi et al., 1999; Wang et al., 1997), and voltage-gated calcium channels (Li et al., 2016a; Servili et al., 2019). Further studies focused on ion flux-independent signaling modes of the NMDAR will greatly expand our understanding of their physiological functions and should also provide additional opportunities for the development of therapeutics for neurological disorders resulting from NMDAR dysfunction.
Table 1.
Examples of ion flux-independent NMDAR signaling
| Phenomenon | Agonist | Co-agonist | Agonist + Co-agonist |
Other | References |
|---|---|---|---|---|---|
| NEURONAL PHYSIOLOGY | |||||
| NMDAR endocytosis/ trafficking | x | Vissel 2001, Barria 2002 | |||
| x | Nong 2003, Papouin 2012, Han 2013, Ferreira 2017, Li 2021 | ||||
| Synaptic plasticity | x | Nabavi 2013, Aow 2015, Stein 2015, Carter 2016, Wong 2018, Dore 2020, Stein 2020 | |||
| Spine structural plasticity | x | Stein 2015, Stein 2020, Thomazeau 2020, Stein 2021 | |||
| Synaptic transmission | x | Li 2016b | |||
| x | ** | Abrahamsson 2017 | |||
| x | Bialecki 2020 | ||||
| Synapse to nucleus gene expression | x | DHPG | Yang 2004 | ||
| NON-NEURONAL PHYSIOLOGY | |||||
| Astrocytic ER Ca2+ release | x | Montes de Oca Balderas 2015, Montes de Oca Balderas 2020 | |||
| BBB permeability | x | x | tPA | Mehra 2020 | |
| NEURONAL DYSFUNCTION & DISEASE | |||||
| Excitotoxicity | |||||
| Inhibition of protein synthesis | x | Gauchy 2002 | |||
| Mitochondrial dysfunction | x | Weilinger 2016 | |||
| Superoxide production | x | Minnella 2018 | |||
| Cerebral ischemia/Traumatic brain injury | |||||
| Neuroprotection | x | Hu 2016, Chen 2017 | |||
| Alzheimer's disease | |||||
| Synaptic weakening | β-amyloid | Kessels 2013, Tamburri 2013, Dore 2021 | |||
| Spine structural plasticity | β-amyloid | Birnbaum 2015 | |||
| Schizophrenia | |||||
| Spine structural plasticity | x | Park 2021 | |||
not tested
NMDA receptors (NMDARs) can signal in an ion flux-independent manner
Binding of agonist and/or co-agonist initiates non-ionotropic NMDAR signaling
Non-ionotropic NMDAR signaling regulates synaptic transmission and plasticity
Agonist and co-agonist induce different conformational movements of the NMDAR
Non-ionotropic NMDAR signaling is implicated in Alzheimer’s disease and schizophrenia
Acknowledgements:
This work was supported by the NIH (R01 NS062736) and an ARCS Scholar Award (D.K.P.). We thank J. Wong and M. Anisimova for critical reading of the manuscript.
Footnotes
Declaration of interests: The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, and Tymianski M (2002). Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science 298, 846–850. [DOI] [PubMed] [Google Scholar]
- Abrahamsson T, Chou CYC, Li SY, Mancino A, Costa RP, Brock JA, Nuro E, Buchanan KA, Elgar D, Blackman AV, et al. (2017). Differential Regulation of Evoked and Spontaneous Release by Presynaptic NMDA Receptors. Neuron 96, 839–855 e835. [DOI] [PubMed] [Google Scholar]
- Aow J, Dore K, and Malinow R (2015). Conformational signaling required for synaptic plasticity by the NMDA receptor complex. Proc Natl Acad Sci U S A 112, 14711–14716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiec WE, Guglietta R, Jami SA, Morishita W, Malenka RC, and O'Dell TJ (2014). Ionotropic NMDA receptor signaling is required for the induction of long-term depression in the mouse hippocampal CA1 region. J Neurosci 34, 5285–5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Muller RU, and Roder JC (2002). Non-ionotropic cross-talk between AMPA and NMDA receptors in rodent hippocampal neurones. J Physiol 543, 23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Li Y, Puhl MD, Benneyworth MA, Basu AC, Takagi S, Bolshakov VY, and Coyle JT (2013). Multiple risk pathways for schizophrenia converge in serine racemase knockout mice, a mouse model of NMDA receptor hypofunction. Proc Natl Acad Sci U S A 110, E2400–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, and Malinow R (2002). Subunit-specific NMDA receptor trafficking to synapses. Neuron 35, 345–353. [DOI] [PubMed] [Google Scholar]
- Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, Jiang ZI, Benneyworth MA, Froimowitz MP, Lange N, et al. (2009). Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol Psychiatry 14, 719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendikov I, Nadri C, Amar S, Panizzutti R, De Miranda J, Wolosker H, and Agam G (2007). A CSF and postmortem brain study of D-serine metabolic parameters in schizophrenia. Schizophr Res 90, 41–51. [DOI] [PubMed] [Google Scholar]
- Bialecki J, Werner A, Weilinger NL, Tucker CM, Vecchiarelli HA, Egana J, Mendizabal-Zubiaga J, Grandes P, Hill MN, and Thompson RJ (2020). Suppression of Presynaptic Glutamate Release by Postsynaptic Metabotropic NMDA Receptor Signalling to Pannexin-1. J Neurosci 40, 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum JH, Bali J, Rajendran L, Nitsch RM, and Tackenberg C (2015). Calcium flux-independent NMDA receptor activity is required for Abeta oligomer-induced synaptic loss. Cell Death Dis 6, e1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Castro J, Saneyoshi T, Matsuno H, Sur M, and Hayashi Y (2014). Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron 82, 444–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N, and Szepetowski P (2015). NMDA receptor subunit mutations in neurodevelopmental disorders. Curr Opin Pharmacol 20, 73–82. [DOI] [PubMed] [Google Scholar]
- Carter BC, and Jahr CE (2016). Postsynaptic, not presynaptic NMDA receptors are required for spike-timing-dependent LTD induction. Nat Neurosci 19, 1218–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hu R, Liao H, Zhang Y, Lei R, Zhang Z, Zhuang Y, Wan Y, Jin P, Feng H, et al. (2017). A non-ionotropic activity of NMDA receptors contributes to glycine-induced neuroprotection in cerebral ischemia-reperfusion injury. Sci Rep 7, 3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coultrap SJ, Freund RK, O'Leary H, Sanderson JL, Roche KW, Dell'Acqua ML, and Bayer KU (2014). Autonomous CaMKII mediates both LTP and LTD using a mechanism for differential substrate site selection. Cell Rep 6, 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roo M, Klauser P, Mendez P, Poglia L, and Muller D (2008). Activity-dependent PSD formation and stabilization of newly formed spines in hippocampal slice cultures. Cereb Cortex 18, 151–161. [DOI] [PubMed] [Google Scholar]
- Dore K, Aow J, and Malinow R (2015). Agonist binding to the NMDA receptor drives movement of its cytoplasmic domain without ion flow. Proc Natl Acad Sci U S A 112, 14705–14710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore K, Carrico Z, Alfonso S, Marino M, Koymans K, Kessels HW, and Malinow R (2021). PSD-95 protects synapses from beta-amyloid. Cell Rep 35, 109194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore K, and Malinow R (2020). Elevated PSD-95 Blocks Ion-flux Independent LTD: A Potential New Role for PSD-95 in Synaptic Plasticity. Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira JS, Papouin T, Ladepeche L, Yao A, Langlais VC, Bouchet D, Dulong J, Mothet JP, Sacchi S, Pollegioni L, et al. (2017). Co-agonists differentially tune GluN2B-NMDA receptor trafficking at hippocampal synapses. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, and Griendling KK (2018). Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ Res 122, 877–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauchy C, Nairn AC, Glowinski J, and Premont J (2002). N-Methyl-D-aspartate receptor activation inhibits protein synthesis in cortical neurons independently of its ionic permeability properties. Neuroscience 114, 859–867. [DOI] [PubMed] [Google Scholar]
- Han L, Campanucci VA, Cooke J, and Salter MW (2013). Identification of a single amino acid in GluN1 that is critical for glycine-primed internalization of NMDA receptors. Mol Brain 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Yi F, Perszyk RE, Furukawa H, Wollmuth LP, Gibb AJ, and Traynelis SF (2018). Structure, function, and allosteric modulation of NMDA receptors. J Gen Physiol 150, 1081–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, and Selkoe DJ (2002). The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Shimizu E, Komatsu N, Watanabe H, Shinoda N, Nakazato M, Kumakiri C, Okada S, Takei N, and Iyo M (2005). No changes in serum epidermal growth factor levels in patients with schizophrenia. Psychiatry Res 135, 257–260. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Umemori H, Mishina M, and Yamamoto T (1999). The AMPA receptor interacts with and signals through the protein tyrosine kinase Lyn. Nature 397, 72–76. [DOI] [PubMed] [Google Scholar]
- Hill TC, and Zito K (2013). LTP-induced long-term stabilization of individual nascent dendritic spines. J Neurosci 33, 678–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R, Chen J, Lujan B, Lei R, Zhang M, Wang Z, Liao M, Li Z, Wan Y, Liu F, et al. (2016). Glycine triggers a non-ionotropic activity of GluN2A-containing NMDA receptors to confer neuroprotection. Sci Rep 6, 34459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobucci GJ, and Popescu GK (2017). NMDA receptors: linking physiological output to biophysical operation. Nat Rev Neurosci 18, 236–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J, Jambrina E, Li J, Marston H, Menzies F, Phillips K, and Gilmour G (2019). Targeting the Synapse in Alzheimer's Disease. Front Neurosci 13, 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, and Zukin SR (1991). Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148, 1301–1308. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Nabavi S, and Malinow R (2013). Metabotropic NMDA receptor function is required for beta-amyloid-induced synaptic depression. Proc Natl Acad Sci U S A 110, 4033–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Lakhanpal G, Lu HE, Khan M, Suzuki A, Hayashi MK, Narayanan R, Luyben TT, Matsuda T, Nagai T, et al. (2015). A Temporary Gating of Actin Remodeling during Synaptic Plasticity Consists of the Interplay between the Kinase and Structural Functions of CaMKII. Neuron 87, 813–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr., and Charney DS (1994). Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 51, 199–214. [DOI] [PubMed] [Google Scholar]
- Lambert JT, Hill TC, Park DK, Culp JH, and Zito K (2017). Protracted and asynchronous accumulation of PSD95-family MAGUKs during maturation of nascent dendritic spines. Dev Neurobiol 77, 1161–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, and Zukin RS (2007). NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci 8, 413–426. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Escobedo-Lozoya Y, Szatmari EM, and Yasuda R (2009). Activation of CaMKII in single dendritic spines during long-term potentiation. Nature 458, 299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesept F, Chevilley A, Jezequel J, Ladepeche L, Macrez R, Aimable M, Lenoir S, Bertrand T, Rubrecht L, Galea P, et al. (2016). Tissue-type plasminogen activator controls neuronal death by raising surface dynamics of extrasynaptic NMDA receptors. Cell Death Dis 7, e2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Tadross MR, and Tsien RW (2016a). Sequential ionic and conformational signaling by calcium channels drives neuronal gene expression. Science 351, 863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Rajani V, Han L, Chung D, Cooke JE, Sengar AS, and Salter MW (2021). Alternative splicing of GluN1 gates glycine site-dependent nonionotropic signaling by NMDAR receptors. Proc Natl Acad Sci U S A 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LJ, Hu R, Lujan B, Chen J, Zhang JJ, Nakano Y, Cui TY, Liao MX, Chen JC, Man HY, et al. (2016b). Glycine Potentiates AMPA Receptor Function through Metabotropic Activation of GluN2A-Containing NMDA Receptors. Front Mol Neurosci 9, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LL, Ginet V, Liu X, Vergun O, Tuittila M, Mathieu M, Bonny C, Puyal J, Truttmann AC, and Courtney MJ (2013). The nNOS-p38MAPK pathway is mediated by NOS1AP during neuronal death. J Neurosci 33, 8185–8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier MP, Sanz-Clemente A, and Roche KW (2015). Dynamic Regulation of N-Methyl-d-aspartate (NMDA) and alpha-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptors by Posttranslational Modifications. J Biol Chem 290, 28596–28603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Wang J, Kandel ER, and O'Dell TJ (1995). CaMKII regulates the frequency-response function of hippocampal synapses for the production of both LTD and LTP. Cell 81, 891–904. [DOI] [PubMed] [Google Scholar]
- Mehra A, Guerit S, Macrez R, Gosselet F, Sevin E, Lebas H, Maubert E, De Vries HE, Bardou I, Vivien D, et al. (2020). Nonionotropic Action of Endothelial NMDA Receptors on Blood-Brain Barrier Permeability via Rho/ROCK-Mediated Phosphorylation of Myosin. J Neurosci 40, 1778–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnella AM, Zhao JX, Jiang X, Jakobsen E, Lu F, Wu L, El-Benna J, Gray JA, and Swanson RA (2018). Excitotoxic superoxide production and neuronal death require both ionotropic and non-ionotropic NMDA receptor signaling. Sci Rep 8, 17522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes de Oca Balderas P, and Aguilera P (2015). A Metabotropic-Like Flux-Independent NMDA Receptor Regulates Ca2+ Exit from Endoplasmic Reticulum and Mitochondrial Membrane Potential in Cultured Astrocytes. PLoS One 10, e0126314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes de Oca Balderas P, Matus Nunez M, Picones A, and Hernandez-Cruz A (2020). NMDAR in cultured astrocytes: Flux-independent pH sensor and flux-dependent regulator of mitochondria and plasma membrane-mitochondria bridging. FASEB J 34, 16622–16644. [DOI] [PubMed] [Google Scholar]
- Nabavi S, Kessels HW, Alfonso S, Aow J, Fox R, and Malinow R (2013). Metabotropic NMDA receptor function is required for NMDA receptor-dependent long-term depression. Proc Natl Acad Sci U S A 110, 4027–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, and Sapkota K (2020). The origin of NMDA receptor hypofunction in schizophrenia. Pharmacol Ther 205, 107426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negri S, Faris P, Maniezzi C, Pellavio G, Spaiardi P, Botta L, Laforenza U, Biella G, and Moccia DF (2021). NMDA receptors elicit flux-independent intracellular Ca(2+) signals via metabotropic glutamate receptors and flux-dependent nitric oxide release in human brain microvascular endothelial cells. Cell Calcium 99, 102454. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, Craft S, and Olney JW (1999). Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology 20, 106–118. [DOI] [PubMed] [Google Scholar]
- Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, and Buisson A (2001). The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med 7, 59–64. [DOI] [PubMed] [Google Scholar]
- Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, and Salter MW (2003). Glycine binding primes NMDA receptor internalization. Nature 422, 302–307. [DOI] [PubMed] [Google Scholar]
- Ogden KK, Chen W, Swanger SA, McDaniel MJ, Fan LZ, Hu C, Tankovic A, Kusumoto H, Kosobucki GJ, Schulien AJ, et al. (2017). Molecular Mechanism of Disease-Associated Mutations in the Pre-M1 Helix of NMDA Receptors and Potential Rescue Pharmacology. PLoS Genet 13, e1006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, and Zhou Q (2013). NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14, 383–400. [DOI] [PubMed] [Google Scholar]
- Papouin T, Ladepeche L, Ruel J, Sacchi S, Labasque M, Hanini M, Groc L, Pollegioni L, Mothet JP, and Oliet SH (2012). Synaptic and extrasynaptic NMDA receptors are gated by different endogenous coagonists. Cell 150, 633–646. [DOI] [PubMed] [Google Scholar]
- Park DK, Petshow S, Anisimova M, Barragan EV, Gray JA, Stein IS, and Zito K (2021). Reduced d-serine levels drive enhanced non-ionotropic NMDA receptor signaling and destabilization of dendritic spines in a mouse model for studying schizophrenia. bioRxiv 2021.03.04.434016; doi: 10.1101/2021.03.04.434016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Otano I, Larsen RS, and Wesseling JF (2016). Emerging roles of GluN3-containing NMDA receptors in the CNS. Nat Rev Neurosci 17, 623–635. [DOI] [PubMed] [Google Scholar]
- Petit-Pedrol M, and Groc L (2021). Regulation of membrane NMDA receptors by dynamics and protein interactions. J Cell Biol 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puhl MD, Mintzopoulos D, Jensen JE, Gillis TE, Konopaske GT, Kaufman MJ, and Coyle JT (2015). In vivo magnetic resonance studies reveal neuroanatomical and neurochemical abnormalities in the serine racemase knockout mouse model of schizophrenia. Neurobiol Dis 73, 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajani V, Sengar AS, and Salter MW (2020). Tripartite signalling by NMDA receptors. Mol Brain 13, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, and Lerma J (1998). Kainate receptor modulation of GABA release involves a metabotropic function. Neuron 20, 1211–1218. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, and Attwell D (2000). Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature 403, 316–321. [DOI] [PubMed] [Google Scholar]
- Sanabria H, Swulius MT, Kolodziej SJ, Liu J, and Waxham MN (2009). {beta}CaMKII regulates actin assembly and structure. J Biol Chem 284, 9770–9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JL, Gorski JA, and Dell'Acqua ML (2016). NMDA Receptor-Dependent LTD Requires Transient Synaptic Incorporation of Ca(2)(+)-Permeable AMPARs Mediated by AKAP150-Anchored PKA and Calcineurin. Neuron 89, 1000–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servili E, Trus M, and Atlas D (2019). Ion occupancy of the channel pore is critical for triggering excitation-transcription (ET) coupling. Cell Calcium 84, 102102. [DOI] [PubMed] [Google Scholar]
- Stein IS, Gray JA, and Zito K (2015). Non-Ionotropic NMDA Receptor Signaling Drives Activity-Induced Dendritic Spine Shrinkage. J Neurosci 35, 12303–12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein IS, Park DK, Claiborne N, and Zito K (2021). Non-ionotropic NMDA receptor signaling gates bidirectional structural plasticity of dendritic spines. Cell Rep 34, 108664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein IS, Park DK, Flores JC, Jahncke JN, and Zito K (2020). Molecular Mechanisms of Non-ionotropic NMDA Receptor Signaling in Dendritic Spine Shrinkage. J Neurosci 40, 3741–3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein IS, and Zito K (2019). Dendritic Spine Elimination: Molecular Mechanisms and Implications. Neuroscientist 25, 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroebel D, and Paoletti P (2021). Architecture and function of NMDA receptors: an evolutionary perspective. J Physiol 599, 2615–2638. [DOI] [PubMed] [Google Scholar]
- Tamburri A, Dudilot A, Licea S, Bourgeois C, and Boehm J (2013). NMDA-receptor activation but not ion flux is required for amyloid-beta induced synaptic depression. PLoS One 8, e65350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomazeau A, Bosch M, Essayan-Perez S, Barnes SA, De Jesus-Cortes H, and Bear MF (2020). Dissociation of functional and structural plasticity of dendritic spines during NMDAR and mGluR-dependent long-term synaptic depression in wild-type and fragile X model mice. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, and Westbrook GL (2017). Modulating synaptic NMDA receptors. Neuropharmacology 112, 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valbuena S, and Lerma J (2016). Non-canonical Signaling, the Hidden Life of Ligand-Gated Ion Channels. Neuron 92, 316–329. [DOI] [PubMed] [Google Scholar]
- Vieira MM, Jeong J, and Roche KW (2021). The role of NMDA receptor and neuroligin rare variants in synaptic dysfunction underlying neurodevelopmental disorders. Curr Opin Neurobiol 69, 93–104. [DOI] [PubMed] [Google Scholar]
- Vissel B, Krupp JJ, Heinemann SF, and Westbrook GL (2001). A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat Neurosci 4, 587–596. [DOI] [PubMed] [Google Scholar]
- Vyklicky V, Stanley C, Habrian C, and Isacoff EY (2021). Conformational rearrangement of the NMDA receptor amino-terminal domain during activation and allosteric modulation. Nat Commun 12, 2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, and Swanson RA (2020). Superoxide and Non-ionotropic Signaling in Neuronal Excitotoxicity. Front Neurosci 4, 861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Small DL, Stanimirovic DB, Morley P, and Durkin JP (1997). AMPA receptor-mediated regulation of a Gi-protein in cortical neurons. Nature 389, 502–504. [DOI] [PubMed] [Google Scholar]
- Warnet XL, Bakke Krog H, Sevillano-Quispe OG, Poulsen H, and Kjaergaard M (2021). The C-terminal domains of the NMDA receptor: How intrinsically disordered tails affect signalling, plasticity and disease. Eur J Neurosci 54, 6713–6739. [DOI] [PubMed] [Google Scholar]
- Weilinger NL, Lohman AW, Rakai BD, Ma EM, Bialecki J, Maslieieva V, Rilea T, Bandet MV, Ikuta NT, Scott L, et al. (2016). Metabotropic NMDA receptor signaling couples Src family kinases to pannexin-1 during excitotoxicity. Nat Neurosci 19, 432–442. [DOI] [PubMed] [Google Scholar]
- Wong JM, and Gray JA (2018). Long-Term Depression Is Independent of GluN2 Subunit Composition. J Neurosci 38, 4462–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Mao L, Tang Q, Samdani S, Liu Z, and Wang JQ (2004). A novel Ca2+-independent signaling pathway to extracellular signal-regulated protein kinase by coactivation of NMDA receptors and metabotropic glutamate receptor 5 in neurons. J Neurosci 24, 10846–10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LJ, Li TY, Luo CX, Jiang N, Chang L, Lin YH, Zhou HH, Chen C, Zhang Y, Lu W, et al. (2014). CAPON-nNOS coupling can serve as a target for developing new anxiolytics. Nat Med 20, 1050–1054. [DOI] [PubMed] [Google Scholar]