Abstract
Puf5, a Puf-family RNA-binding protein, binds to 3´ untranslated region of target mRNAs and negatively regulates their expression in Saccharomyces cerevisiae. The puf5Δ mutant shows pleiotropic phenotypes including a weakened cell wall, a temperature-sensitive growth, and a shorter lifespan. To further analyze a role of Puf5 in cell growth, we searched for a multicopy suppressor of the temperature-sensitive growth of the puf5Δ mutant in this study. We found that overexpression of CLB2 encoding B-type cyclin suppressed the temperature-sensitive growth of the puf5Δ mutant. The puf5Δ clb2Δ double mutant displayed a severe growth defect, suggesting that Puf5 positively regulates the expression of a redundant factor with Clb2 in cell cycle progression. We found that expression of CLB1 encoding a redundant B-type cyclin was decreased in the puf5Δ mutant, and that this decrease of the CLB1 expression contributed to the growth defect of the puf5Δ clb2Δ double mutant. Since Puf5 is a negative regulator of the gene expression, we hypothesized that Puf5 negatively regulates the expression of a factor that represses CLB1 expression. We found such a repressor, Ixr1, which is an HMGB (High Mobility Group box B) protein. Deletion of IXR1 restored the decreased expression of CLB1 caused by the puf5Δ mutation and suppressed the growth defect of the puf5Δ clb2Δ double mutant. The expression of IXR1 was negatively regulated by Puf5 in an IXR1 3´ UTR-dependent manner. Our results suggest that IXR1 mRNA is a physiologically important target of Puf5, and that Puf5 and Ixr1 contribute to the cell cycle progression through the regulation of the cell cycle-specific expression of CLB1.
Author summary
Cell cycle progression is strictly regulated by cyclin-dependent kinases (CDKs). The activity of CDK is determined by the expression of cell cycle-specific cyclins. In this paper, we demonstrate a novel regulatory mechanism for expression of a cyclin, CLB1, in budding yeast provoked by the RNA-binding protein Puf5 and the HMGB protein Ixr1. The RNA-binding protein Puf5 binds to the 3’ untranslated region of IXR1 mRNA, inducing sequential negative regulation of the Ixr1 protein levels. The Ixr1 protein negatively regulates CLB1 expression via the CLB1 promoter. This discovery of new regulators of cyclins indicates that the control of cyclin gene expression may be elaborately regulated in response to environmental conditions.
Introduction
RNA-binding proteins play an important role in controlling the translation, turnover, and localization of mRNAs. RNA-binding proteins affect the fate of target mRNAs through binding to certain sequences found in the 5´ untranslated region (UTR), the coding region, or the 3´ UTR [1,2,3,4]. Among the three regions, the 3´ UTR has been reported to be the most common region where the sequences are found [4]. One well-known family of RNA-binding proteins is PUF (Pumilio and FBF) family. PUF family proteins are evolutionary conserved in many eukaryotes. In Saccharomyces cerevisiae, 6 Puf proteins have been reported [3,5]. Among them, we have focused on Puf5, also known as Mpt5. Puf5 binds to 3´ UTR of target mRNAs and recruits the Ccr4-Not complex to promote RNA degradation [6,7]. Puf5 has been reported to bind to more than 1,000 RNAs and regulate the expression of genes functioning in broad biological processes, including cell wall integrity (CWI) or cell cycle progression [8,9]. Among the binding targets, two physiologically important targets have been reported: one is HO mRNA encoding an endonuclease involved in mating-type switching [10], and the other is LRG1 mRNA encoding a GTPase-activating protein (GAP) for Rho1 GTPase involved in regulating CWI [11–13]. Puf5 negatively regulates LRG1 expression together with Ccr4 and controls the CWI pathway [11–15]. The puf5Δ mutant shows pleiotropic phenotypes including an abnormal mating-type switching in daughter cells [10], a weakened cell wall, a temperature-sensitive growth [11,14,15], and a shorter lifespan [5,14]. These pleiotropic effects indicate the multiple functions of Puf5 in cell growth, but the target mRNAs of Puf5 involved in growth control, other than LRG1 mRNA, have not been fully understood.
Cell cycle progression is strictly regulated by a periodic change in the activity of cyclin-dependent kinase (Cdk). In S. cerevisiae, Cdc28 functions as a sole Cdk [16]. Activated Cdc28 phosphorylates various substrates necessary for the cell cycle progression, and this periodical regulation of the phosphorylation status of the proteins ensures a proper transition of the cell cycle. The main regulators of the periodicity of the Cdk activity are cyclin proteins. Cyclins are indispensable for Cdk activity, and the periodical change in the cyclin abundance controls the activity of Cdk [16]. In S. cerevisiae, there are two major groups of cyclins, G1 cyclins and B-type cyclins. G1 cyclins including Cln1, Cln2, and Cln3 promote the G1/S transition. B-type cyclins consist of six cyclins, Clb1, Clb2, Clb3, Clb4, Clb5, and Clb6. Clb5/Clb6 accumulate in the late G1 phase and contribute to initiating the S phase. Clb3/Clb4 are abundant in the S and G2 phases and promote spindle formation. The abundance of Clb1/Clb2 is increased in the G2 and M phases, and they play an important role in the transition from the G2 phase to the M phase. Clb2 performs as the major G2/M cyclin and is the most important of the six cyclin B proteins in mitosis [16]. On the other hand, the abundance of Clb2 is low in meiosis, and Clb1 acts as a major G2/M cyclin [17]. In regulating the cyclin abundance, both transcriptional control and protein degradation play crucial roles. B-type cyclin transcripts accumulate at the proper timing. As for G2/M cyclins, their transcription is regulated by cell cycle-dependent activity of the Mcm1-Fkh2-Ndd1 activator complex [18,19]. Even though the importance of transcriptional activators has been reported, the contribution of transcriptional repressors to the regulation of the CLB1/2 expression remains unclear.
In this study, we further analyzed the role of Puf5 in cell growth. We found that overexpression of CLB2 suppressed the temperature-sensitive phenotype of the puf5Δ mutant, and that the puf5Δ clb2Δ double mutant showed a severe growth defect. In addition, the expression of CLB1 encoding a redundant B-type cyclin with Clb2 was decreased in the puf5Δ mutant, and overexpression of CLB1 recovered the growth defect of the puf5Δ clb2Δ mutant. We also found that deletion of IXR1 encoding a transcriptional repressor suppressed the growth defect of the puf5Δ clb2Δ double mutant, and that the ixr1Δ mutation restored the reduced CLB1 expression caused by the puf5Δ mutation. The expression of IXR1 was negatively regulated by Puf5. Our data suggest that IXR1 mRNA is a new physiologically important target of Puf5, and that the IXR1 regulation engendered by Puf5 leads to the positive regulation of CLB1 expression and assures the proper cell cycle progression.
Materials and methods
Strains and media
To manipulate DNA, the Escherichia coli, DH5α strain, was used. The Saccharomyces cerevisiae, W303 strain, was used as a yeast strain. The strains used in this study are described in S1 Table. Standard procedures were followed for yeast manipulations [20]. The medium used in this study includes YPD medium (2% Glucose, 2% bactopeptone, and 1% yeast extract) and synthetic complete medium (SC) [20]. For the strains needed to reduce osmolarity stress, 10% sorbitol was added. SC medium lacking amino acids (e.g., SC-Ura medium was SC medium lacking uracil) were used to select transformants.
Plasmids
Plasmids used in this study are described in S2 Table. For the construction of YEplac195-PUF5 plasmid, the fragment containing the PUF5 gene together with upstream and downstream regions was amplified by PCR using genomic DNA as a template. The fragment was inserted between SalI and EcoRI sites of YEplac195 plasmid. YEplac195-CLB2 and YEplac195-CLB1 plasmids were constructed in a similar manner. For the construction of YCplac33-IXR1 plasmid, the fragment containing the IXR1 gene together with upstream and downstream regions was amplified by PCR and inserted between SalI and EcoRI sites of YCplac33 plasmid.
YCplac33-CLB1-HA was constructed as follows. The fragment containing CLB1 promoter-CLB1 ORF and the fragment containing CLB1 3´ UTR were amplified by PCR from genomic DNA, and the fragment encoding HA was amplified by PCR using the pFA6a-HA-kanMX6 plasmid as a template [21]. The recognition sequence of EcoRI was added to the top of the CLB1 promoter-CLB1 ORF fragment, and the sequence of SalI was added to the end of the CLB1 3´ UTR fragment. To the other ends of the CLB1 promoter-CLB1 ORF fragment and the CLB1 3´ UTR fragment, the upper sequence and downstream sequence of HA fragment were added, respectively. Then, the fragments were inserted between SalI and EcoRI sites of YCplac33 plasmid. YCplac33-IXR1-HA plasmid was constructed in a similar manner. YCplac33-IXR1-HA-ADH1 3´ UTR plasmid was constructed by inserting the fragment of IXR1 promoter-IXR1 ORF amplified from genomic DNA and the fragment of HA-ADH1 3´ UTR amplified from the pFA6a-HA-kanMX6 plasmid between SalI and EcoRI sites of YCplac33 plasmid.
YCplac33-CLB1 promoter -GFP-ADH1 3´ UTR and YCplac33-CLB2 promoter -GFP-ADH1 3´ UTR were constructed as follows. The fragments corresponding to CLB1/CLB2 promoter were amplified by PCR from genomic DNA, and the GFP-ADH1 3´ UTR fragment was amplified by PCR from the pFA6a-GFP(S65T)-kanMX6 plasmid. Then, the fragments were inserted between EcoRI and SalI site of YCplac33 plasmid. YCplac33-MCM2 promoter -GFP-IXR1 3´ UTR were constructed using MCM2 promoter, GFP, and IXR1 3´ UTR fragments amplified by PCR from genomic DNA, the pFA6a-GFP(S65T)-kanMX6 plasmid, and genomic DNA, respectively.
The pCgLEU2, pCgHIS3, and pCgTRP1 plasmids, which were pUC19 carrying the Candida glabrata LEU2, HIS3, and TRP1 genes, respectively, were used to delete genes [22]. The pKl-URA3 plasmid, pUC19 carrying the Kluyveromyces lactis URA3 were also used for gene deletion.
Gene deletion
Deletions of PUF5, CLB2, CLB1, IXR1, BAR1, and LRG1 were constructed by PCR-based gene-deletion method [22–24]. Primer sets used in this study were listed in S3 Table. The fragments amplified by PCR were transformed into the wild-type strain and transformants were selected on the SC medium lacking the corresponding amino acids.
Screening for multicopy suppressor of the temperature-sensitive phenotype of the puf5Δ mutant
The genomic DNA library in the YEp13 plasmid [25] was transformed into the puf5Δ mutant strain harboring pRS316-3xFLAG-LRG1 and plated on SC-Ura-Leu plates containing 10% sorbitol at room temperature for 4 days. pRS316-3xFLAG-LRG1 was transformed for the purpose of obtaining both downstream factors of Puf5 and other regulators of Lrg1. Then, the plates were replicated onto YPD plates and incubated at 37°C for 3 days. Twenty-six transformants that formed colonies at 37°C were identified. The corresponding plasmids were isolated from the transformants, and those that conferred the ability to proliferate at 37°C to the puf5Δ strain were identified by retransformation. Four of twenty-six plasmids gave the ability to proliferate at 37°C. Sequencing of the insert DNAs of the four recovered plasmids revealed that two contained the SSD1 gene, one contained the CLB2 gene, and one contained the ZDS1 gene. Regional analysis of the suppression ability confirmed that the SSD1, CLB2, and ZDS1 genes were responsible for ensuring the growth of the puf5Δ strain.
Western blot analysis
Yeast cells were pre-cultured overnight in a liquid medium, then diluted to OD600 = 0.5 (optical density measured at a wavelength of 600 nm), and cultured for 4 hours. Then, cells were collected by centrifuge and treated with sodium hydroxide for protein extraction [26]. Protein samples were loaded onto an SDS-PAGE gel for protein electrophoresis and then transferred to a PDVF membrane (Millipore). Then, the membrane was reacted with the primary antibody at 4°C overnight and reacted with the secondary antibody for 1 hour at room temperature. The anti-HA monoclonal antibody HA11, the anti-Pgk1 monoclonal antibody 22C5D8 (Invitrogen), the anti-GFP monoclonal antibody (Roche), the anti-FLAG monoclonal antibody M2 (Sigma), and the anti-c-Myc monoclonal antibody 9E10 (Santa Cruz) were used as the primary antibodies. The anti-mouse IRDye 800CW secondary antibodies and IRDye 680RD secondary antibodies (LI-COR) were used as the secondary antibodies. The Pgk1 protein was used as a loading control. ODYSSEY CLx (LI-COR) was used to visualize and quantify the Clb1-HA protein, Ixr1-HA protein, GFP protein, Puf5-Myc protein, and Pgk1 protein. The fold change of the Clb1-HA, Ixr1-HA protein, GFP protein, and Puf5-Myc protein levels, normalized with the intensity of Pgk1, was calculated and statistically analyzed using Excel (Microsoft).
RNA isolation, quantitative real-time PCR (qRT-PCR), and microarray analysis
Yeast cells were pre-cultured overnight in a liquid medium, then diluted to OD600 = 0.5 (optical density measured at a wavelength of 600 nm), and cultured for 4 hours. Then, cells were collected by centrifuge. RNAs were extracted from the collected cells using ISOGEN reagent (Nippon Gene). From the extract, genomic DNAs were removed using RNeasy Mini kit (Qiagen), and reverse transcription was performed using the Prime Script RT reagent Kit (Takara). The cDNA levels were quantified by qRT-PCR using QuantStudio 5 (Thermo Fisher Scientific) with SYBR Premix Ex Taq (Takara). The primers used for the qRT-PCR were listed in S4 Table. The fold change of the mRNAs was calculated using SCR1 or ACT1 as internal control genes and statistically analyzed using Excel (Microsoft).
The microarray analysis was performed by the KURABO Bio-Medical Department using the Affymetrix GeneChip Yeast Genome 2.0 Array (Affymetrix, Santa Clara, California, USA). Microarray data sets are available at the Gene Expression Omnibus at http://www.ncbi.nlm.nih.gov/geo (GEO accession number GSE124908)
Cell cycle synchronization by α-factor block and release
In the pheromone-induced cell cycle synchronization procedure, we used the MATa bar1Δ strains to prevent degradation of α-factor and followed the procedure previously reported [27]. Yeast cells were pre-cultured overnight in a YPD medium at 28°C, then transferred into a fresh YPD medium, and cultured for 4 hours. When culturing strains harboring the YCplac33-IXR1-HA-IXR1 3´UTR plasmid, yeast cells were pre-cultured overnight in an SC-Ura medium at 28°C, and then cells were collected by centrifuge, suspended in a small amount of YPD medium, and cultured at 28°C for 2 hours. Sequentially, cells were transferred into a fresh YPD medium and cultured for 4 hours. We confirmed that more than 95% of cells carried the YCplac33-IXR1-HA-IXR1 3´ UTR plasmid in YPD culture. After the 4-hour culture, α-factor was added into the medium, and incubation was continued for 2 hours. Then, after collecting the 0-minute sample, cells were washed with a fresh YPD medium by centrifuge, transferred into a fresh YPD medium, and incubated at 28°C. Samples were collected by centrifuge every 10 minutes from the time at release.
RNA immunoprecipitation (RIP) analysis
To examine the interaction between Puf5 and IXR1 mRNA, ixr1Δ PUF5-3xFLAG-ADH1 3´ UTR strains harboring YEplac195-IXR1 with/without Puf5-binding element were used. Untagged ixr1Δ PUF5 strains harboring the same plasmids were used as a negative control. Yeast cells were pre-cultured overnight in an SC-Ura medium, then diluted to OD600 = 0.5 (optical density measured at a wavelength of 600 nm), and cultured for 4 hours. Then, cells were collected.
Cells were dissolved into the extraction buffer (0.3M XT buffer (0.05 M Hepes, 0.3 M KCl, and 0.004 M MgCl2), protease inhibitors (APMSF and aprotinin), a reducing agent dithiothreitol, a detergent NP40, and RNasin (Promega)) and extracts were obtained by the beads crushing. The supernatant from the crude extracts was labeled as SUP samples. From the supernatant, Puf5-3xFLAG protein was immunoprecipitated with the anti-FLAG M2 affinity agarose gel (Sigma). After washing with extraction buffer with 10% glycerol, we obtained the immunoprecipitants labeled as beads samples. Protein and RNA were extracted from each sample, and the enrichment of IXR1 mRNA was examined by qRT-PCR analysis.
Statistical analysis
Graph data is presented as means ± standard error (SE) of biological samples. Statistical analyses were performed with Microsoft Office Excel using Tukey’s test, and differences were regarded as significant when they were either **P < 0.01 or *P < 0.05.
Results
Overexpression of CLB2 suppressed the temperature-sensitive phenotype of the puf5Δ mutant
Deletion of PUF5 leads to a weakened cell wall, temperature-sensitive growth, and a short life span [11,14,15]. In order to further analyze the function of Puf5 in cell growth, we screened a genomic library for a gene whose overexpression suppresses the temperature-sensitive growth of the puf5Δ mutant. We identified CLB2 encoding a B-type cyclin [16,17] and ZDS1 involved in polarized growth [28] as a multicopy suppressor (for details of the screening, see Materials and methods). We also identified SSD1, the gene encoding a translational repressor contributing to polarized growth and CWI [14,29], in this screening, but we did not study the SSD1 gene further here, since the W303 stains carry a defective SSD1 allele. Overexpression of PUF5 and ZDS1 recovered the growth defect of the puf5Δ mutant at 35°C and 37°C (Fig 1A). However, multicopy CLB2 suppressed the phenotype at 35°C but not enough at 37°C (Fig 1A). Therefore, CLB2 seems to weakly suppress the phenotype of the puf5Δ mutant. Among three genes, we focused on the CLB2.
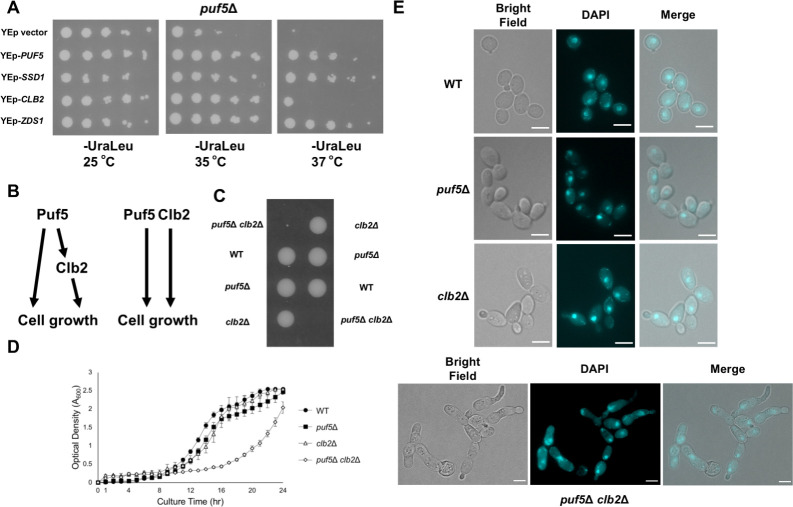
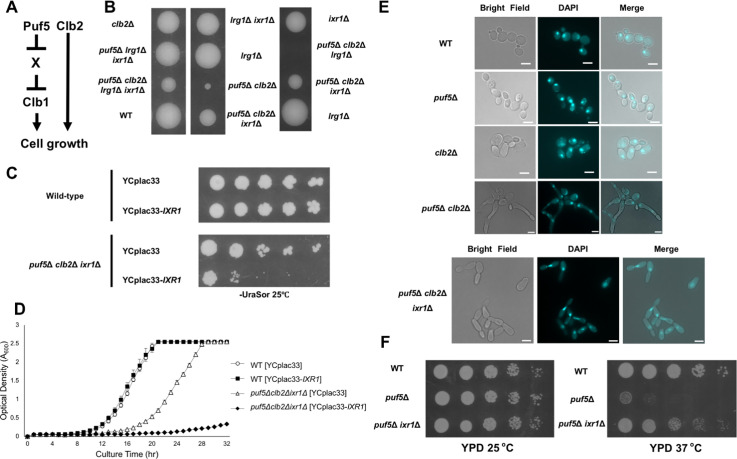
Fig 1. Overexpression of CLB2 repressed the temperature-sensitive phenotype of the puf5Δ mutant.
(A) The multi-copy suppressors of the temperature-sensitive phenotype of the puf5Δ mutant. The puf5Δ mutants harboring YEp13, YEp13-PUF5, YEp13-SSD1, YEp13-CLB2, or YEp13-ZDS1 in addition to the plasmids pRS316-3xFLAG-LRG1 were cultured at 25°C, serially diluted, and spotted onto an SC-Ura-Leu plate, and incubated for 4 days at 25°C, 35°C, or 37°C. (B) Models showing how Puf5 and Clb2 function in cell growth. One is that Clb2 is a downstream target of Puf5, and the other is that Puf5 and Clb2 function in a parallel manner. (C) The tetrad analysis of the strains that are heterozygous for the alleles of PUF5 and CLB2. The cells were sporulated, dissected on a YPD plate, and cultured at 30°C for 3 days. (D) The growth curve of wild-type (black circle), the puf5Δ mutant (black square), the clb2Δ mutant (white triangle), and the puf5Δ clb2Δ mutant (white rhombus) at 25°C. The strains were pre-cultured in a YPD medium containing 10% sorbitol overnight at 25°C, and then transferred into a fresh YPD-Sorbitol medium and cultured at 25°C for 1 day. The data shows the mean± SE (n = 3) of the optical density. (E) Morphology of wild-type, the puf5Δ mutant, the clb2Δ mutant, and the puf5Δ clb2Δ double mutant cells. Bright-field (left), DAPI staining (middle), and the overlayed (right) were shown. The scale bar represents 5 μm.
The puf5Δ clb2Δ double mutant shows severe growth defect
Based on the result that overexpression of CLB2 suppressed the temperature-sensitive phenotype of the puf5Δ mutant (Fig 1A), we considered two possibilities described in Fig 1B: the first is that CLB2 functions downstream of Puf5 in the growth control, and the second is that CLB2 has a redundant role with PUF5 in the growth control. To clarify these possibilities, we genetically analyzed the interaction between PUF5 and CLB2. We generated the puf5Δ clb2Δ double mutant and examined the cell growth. While the puf5Δ and the clb2Δ single mutants grew similarly to wild-type at 30°C, the puf5Δ clb2Δ double mutant showed dramatical poor growth or lethality (Fig 1C). We also confirmed the severe growth defect of the puf5Δ clb2Δ double mutant by the overtime culture in a liquid medium. Consistently, the puf5Δ clb2Δ double mutant grew more slowly than wild-type, the puf5Δ mutant, or the clb2Δ mutant (Fig 1D).
The clb2Δ mutant has been reported to show an abnormally elongated shape and G2/M delay [30]. Regarding that, we examined whether the puf5Δ mutation accelerates this phenotype by observing the morphology of the puf5Δ clb2Δ double mutant. The clb2Δ mutant cells showed an elongated shape (Fig 1E, clb2Δ) as reported previously [30]. Corresponding to a previous report that the puf5Δ mutant shows a highly polarized shape [31], the puf5Δ mutant was enlarged compared to wild-type cells (Fig 1E, WT, puf5Δ). The puf5Δ clb2Δ double mutant was severely elongated (Fig 1E, puf5Δ clb2Δ), and the elongation was severer than that in the clb2Δ mutant. Nuclear staining patterns of the puf5Δ clb2Δ double mutant showed partial defects in chromosome segregation (Fig 1E, puf5Δ clb2Δ DAPI). Taken together, these results suggest that Clb2 functions in parallel with Puf5 in cell growth rather than acts as a downstream factor of Puf5.
CLB1 expression was diminished in the puf5Δ mutant
Since Puf5 is an RNA-binding protein that binds to 3´ UTR of target mRNA [3,5], it is unlikely that Puf5 directly functions as a redundant factor with Clb2. Rather, it is more likely that Puf5 regulates expression of a target gene that has a redundant role with Clb2 in cell cycle progression, and that expression of the target gene is decreased in the puf5Δ mutant. In order to find a target, we conducted microarray analysis using wild-type and the puf5Δ mutant and screened the genes that exhibited a certain decrease in expression (log2 fold change ≦ -0.5) upon PUF5 deletion. In the puf5Δ mutant, mRNA levels of 59 genes were decreased (log2 fold change ≦ -0.5). Among 59 genes, expression of CLB1 and CLB6 encoding different B-type cyclins were decreased to approximately 50% and 60%, respectively (Table 1). Expression of other cyclin genes, CLB2, CLB3, CLB4, CLB5, CLN1, CLN2, and CLN3 did not show a significant change in the puf5Δ mutant (Table 1). The whole data of this microarray analysis is available in Valderrama et al. [32].
Table 1. The extract data of the expression of CLB and CLN from previous microarray data.
The microarray data of each gene in wild-type and the puf5Δ mutant strains are presented. The whole data is presented in (Valderrama et al., 2021) [32]. The log2 fold change of the expression of each gene in the puf5Δ mutant normalized to that in wild-type are shown within parenthesis.
| GENE | wild-type | puf5Δ |
|---|---|---|
| CLB1 | 694.6 (1) |
411.8 (-0.75) |
| CLB2 | 569.4 (1) |
585.5 (0.04) |
| CLB3 | 637.7 (1) |
507.6 (-0.33) |
| CLB4 | 347.7 (1) |
291.1 (-0.26) |
| CLB5 | 557.7 (1) |
502.4 (-0.15) |
| CLB6 | 270.5 (1) |
172.1 (-0.65) |
| CLN1 | 701.1 (1) |
661 (-0.08) |
| CLN2 | 620.9 (1) |
667.7 (0.10) |
| CLN3 | 1251.1 (1) |
1096.5 (-0.19) |
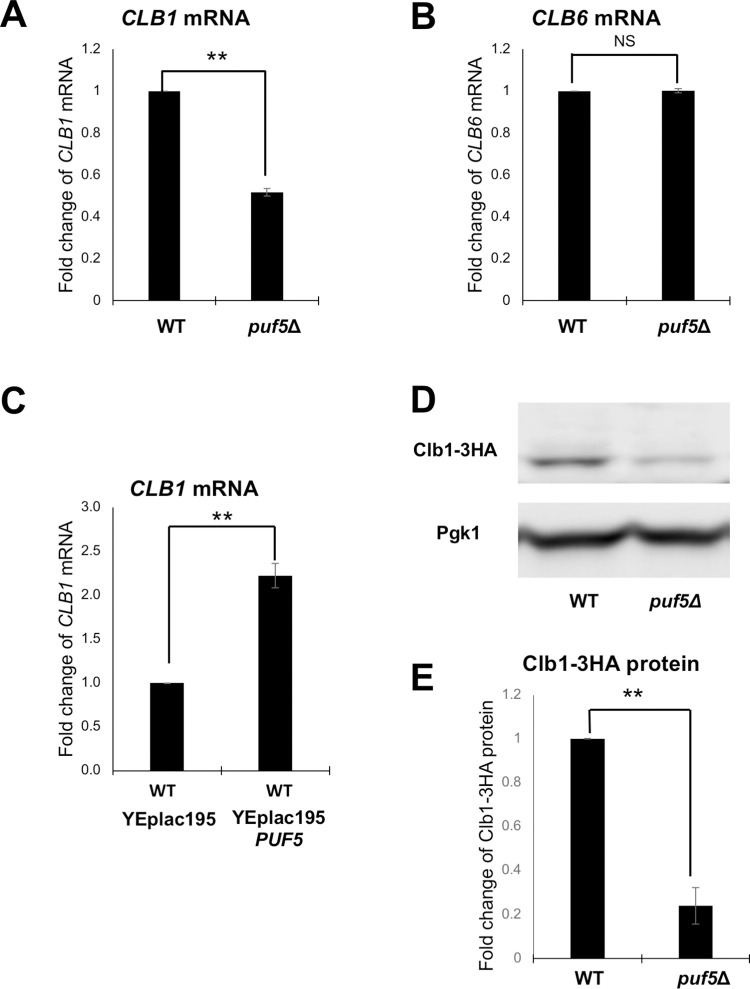
To confirm the microarray results, we examined the CLB1 and CLB6 mRNA levels in wild-type and the puf5Δ mutant by qRT-PCR. The CLB1 mRNA level in the puf5Δ mutant was decreased to 52% of that in wild-type with a statistically significant difference (Fig 2A). The CLB6 mRNA levels were not statistically significantly different between the puf5Δ mutant and wild-type (Fig 2B). Therefore, we further analyzed the regulation of the CLB1 expression by Puf5. While deletion of PUF5 caused the decrease in the CLB1 mRNA level, overexpression of PUF5 in wild-type cells increased the CLB1 mRNA level (Fig 2C). We also examined Clb1 protein levels in wild-type and the puf5Δ mutant. Clb1-HA protein level in the puf5Δ mutant was statistically significantly decreased to 24% of that in wild-type (Fig 2D and 2E). These results suggest that Puf5 positively regulates CLB1 expression.
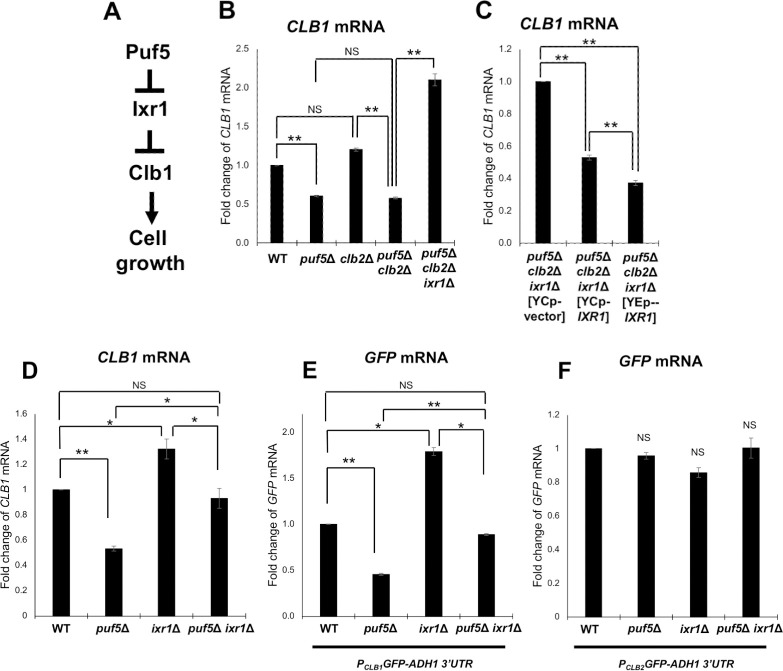
Fig 2. Puf5 positively regulates CLB1 expression.
(A, B) The mRNA levels of CLB1 (A) and CLB6 (B) in wild-type and the puf5Δ mutant. The cells were cultured in a YPD medium containing 10% sorbitol at 25°C until the log phase. The CLB mRNA levels were quantified by qRT-PCR analysis, and the relative mRNA levels were calculated using the SCR1 reference gene. The data shows the mean ± SE (n = 3) of the fold change of CLB1 mRNA (A) and CLB6 mRNA (B) relative to the mRNA level in wild-type. *P < 0.05, **P < 0.01 as determined by Tukey’s test. (C) The mRNA levels of CLB1 in the wild-type cell overexpressing PUF5. The wild-type strains harboring plasmids YEplac195 or YEplac195-PUF5 were cultured in an SC-Ura medium at 28°C until the exponential phase. The CLB1 mRNA levels were quantified by qRT-PCR analysis, and the relative mRNA levels were calculated using the SCR1 reference gene. The data shows the mean ± SE (n = 3) of the fold change of CLB1 mRNA relative to the mRNA level in wild-type harboring the YEplac195 plasmid. *P < 0.05, **P < 0.01 as determined by Tukey’s test. (D, E) The Clb1 protein levels in wild-type and the puf5Δ mutant, and the quantitative analysis data of Clb1-HA protein level. The strains harboring the YCplac33-CLB1-HA-CLB1 3´ UTR plasmid were cultured in an SC-Ura medium at 28°C. The extracts were immunoblotted with anti-HA antibody or anti-Pgk1 antibody. Clb1-HA protein level was quantified and normalized with the Pgk1 protein level. The data shows the mean ± SE (n = 3) of the fold change of Clb1-HA protein relative to the protein level in wild-type (E). *P < 0.05, **P < 0.01 as determined by Tukey’s test.
The decrease of the CLB1 expression in the puf5Δ mutant contributes to the growth defect of the puf5Δ clb2Δ double mutant
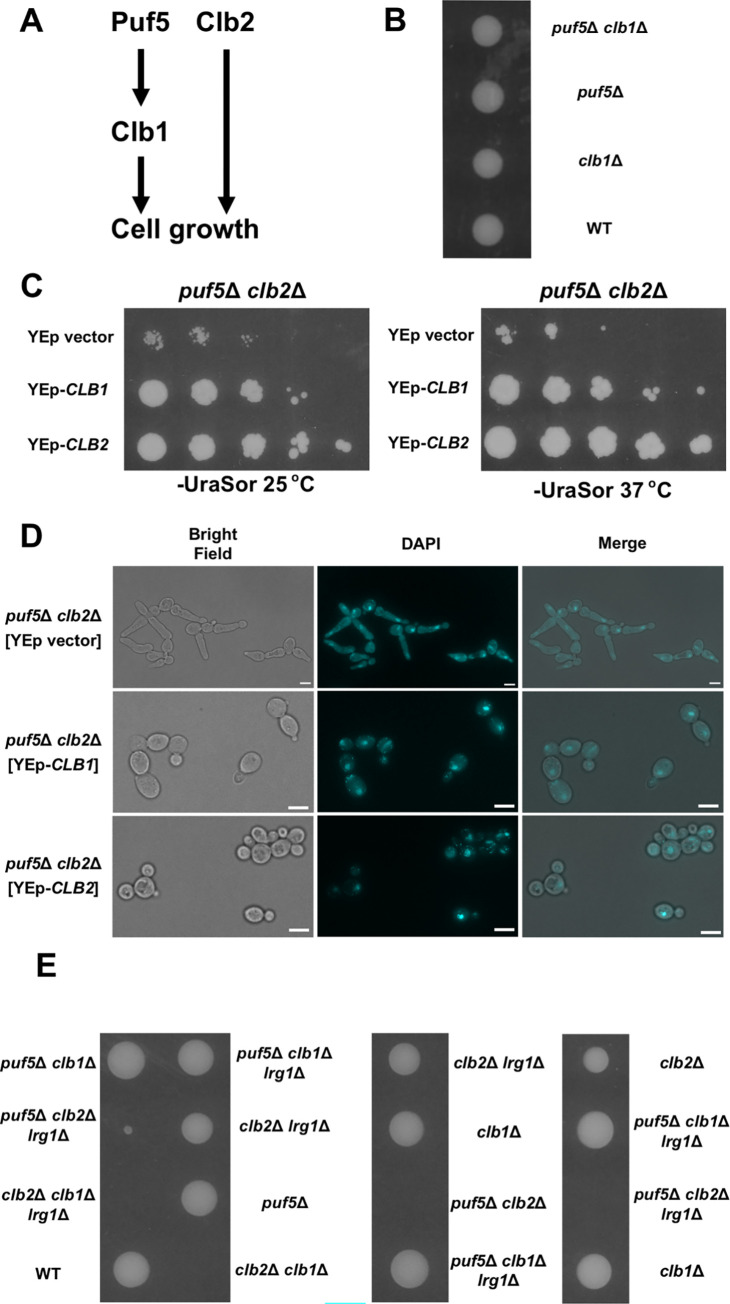
Above data suggested that Puf5 positively regulates CLB1 expression, a paralog of CLB2, encoding a G2/M cyclin (Fig 3A). To confirm that CLB1 acts downstream of Puf5, we deleted PUF5 and CLB1 in a diploid cell and performed tetrad analysis. Each single mutant, the puf5Δ mutant or the clb1Δ mutant, grew as well as wild-type (Fig 3B puf5Δ, clb1Δ, WT). The growth of the puf5Δ clb1Δ double mutant did not apparently differ from that of single mutants. These data corroborate the model that CLB1 is a downstream factor of Puf5. Regarding that Puf5 positively regulates CLB1 expression, we asked if this control influences cell growth together with Clb2 (Fig 3A). To make it clear, we exogenously overexpressed CLB1 and CLB2 in the puf5Δ clb2Δ double mutant. Overexpression of either CLB1 or CLB2 suppressed the growth defect of the puf5Δ clb2Δ double mutant, although the growth recovery by CLB1 overexpression was weaker than that by CLB2 overexpression (Fig 3C). We also examined whether overexpression of CLB1 or CLB2 suppresses the elongated phenotype of the puf5Δ clb2Δ double mutant. Consistent with the observation shown in Fig 1E, the puf5Δ clb2Δ double mutant harboring YEp vector showed severely elongated morphology (Fig 3D, puf5Δ clb2Δ [YEp vector]). When CLB1 or CLB2 was overexpressed, the elongated phenotype was suppressed (Fig 3D, puf5Δ clb2Δ [YEp-CLB1], puf5Δ clb2Δ [YEp-CLB2]). However, the cell sizes were still larger in the CLB1-overexpressed strain than in the CLB2-overexpressed one. This may be reflected in the partial restoration of the growth defect of the puf5Δ clb2Δ double mutant by overexpression of CLB1 (Fig 3C). These results supported the model that the positive regulation of CLB1 expression by Puf5 plays a key role in cell growth when Clb2 is absent. To further confirm this model, we deleted PUF5, CLB2, CLB1, and LRG1 in diploid cell and performed tetrad analysis. Since we and other groups have previously reported that LRG1 deletion suppresses the high temperature-sensitive growth of the puf5Δ mutant [11,14,15], we considered a possibility that Lrg1 is involved in the growth defect of the puf5Δ clb2Δ double mutant. Consistent with the results shown in Fig 3B, the clb1Δ single and the puf5Δ clb1Δ double mutant grew as well as wild-type (Fig 3E clb1Δ, puf5Δ clb1Δ, WT). The clb2Δ single mutant showed slower growth than wild-type, but the difference was not remarkable (Fig 3E clb2Δ, WT). As also shown in Fig 1C, the puf5Δ clb2Δ double mutant showed a severe growth defect or lethality (Fig 3E puf5Δ clb2Δ). The clb2Δ clb1Δ double mutant was also lethal (Fig 3E clb2Δ clb1Δ) as previously reported [33]. Deletion of LRG1, another target of Puf5, did not affect the phenotype of the puf5Δ clb2Δ double mutant and the clb2Δ clb1Δ double mutant (Fig 3E puf5Δ clb2Δ lrg1Δ, clb2Δ clb1Δ lrg1Δ). These results suggest that each of Clb2 and Clb1 complements their function each other to restore the decrease in the amount of G2/M cyclin. Deletion of PUF5 showed a severe growth defect only in combination with CLB2 deletion and had no effect in combination with CLB1 deletion, and this growth defect was independent of LRG1, another target of Puf5. Thus, the positive regulation of CLB1 expression by Puf5 was physiologically important when CLB2 expression was lost.
Fig 3. The decrease of CLB1 expression in the puf5Δ mutant contributes to the growth defect of the puf5Δ clb2Δ double mutant.
(A) A model of the Puf5 function in cell growth showing the hypothesis that Puf5 controls cell growth through the positive regulation of CLB1, a paralog of CLB2. (B) The tetrad analysis of the strains that are heterozygous for the alleles of PUF5 and CLB1. The cells were sporulated, dissected on a YPD plate, and cultured at 30°C for 3 days. (C) The effects of overexpression of CLB1 and CLB2 are presented. The puf5Δ clb2Δ double mutant strains harboring plasmids YEplac195, YEplac195-CLB1, or YEplac195-CLB2 were cultured in an SC-Ura medium containing 10% sorbitol at 25°C until the exponential phase. Cells were serially diluted, spotted onto an SC-Ura plate containing 10% sorbitol, and incubated for 4 days at 25°C or 37°C. (D) Morphology of the puf5Δ clb2Δ double mutant strains harboring YEp vector, YEp195-CLB1, or YEp195-CLB2. Bright-field (left), DAPI staining (middle), and overlayed (right) were shown. The scale bar represents 5μm. (E) The tetrad analysis of the strains that are heterozygous for the alleles of PUF5, CLB2, CLB1, and LRG1. The cells were sporulated, dissected on a YPD plate, and cultured at 30°C for 3 days.
Puf5 regulates CLB1 expression through the CLB1 promoter
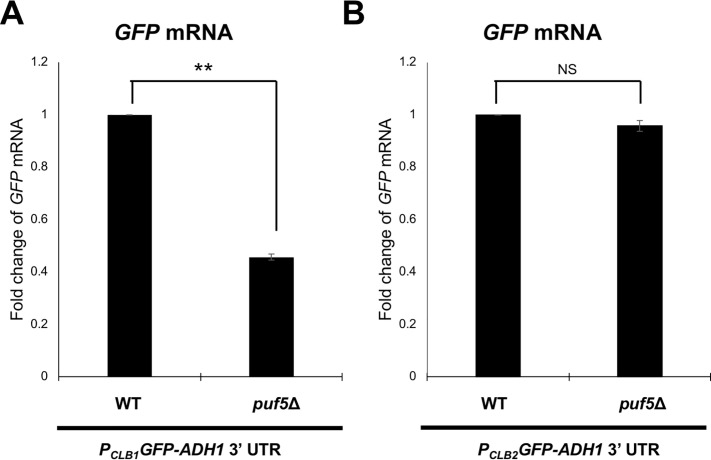
Since Puf5 regulates CLB1 expression positively rather than negatively, we investigated whether Puf5 regulates CLB1 expression at the transcriptional level. For this purpose, we constructed a reporter plasmid, the CLB1 promoter-GFP-ADH1 3´ UTR plasmid that harbors the GFP driven by the CLB1 promoter. The ADH1 3´ UTR was used in this reporter plasmid, since the expression of ADH1 was not affected by PUF5 deletion (S1A Fig). We examined the expression of GFP mRNA driven by the CLB1 promoter in wild-type and the puf5Δ mutant by qRT-PCR. As a result, the GFP mRNA level in the puf5Δ mutant was decreased to 46% of that in wild-type with a statistically significant difference (Fig 4A). This data corresponds to the decrease of endogenous CLB1 mRNA level in the puf5Δ mutant (Fig 2A). To confirm that the decrease of GFP mRNA level in the puf5Δ mutant is dependent on the CLB1 promoter, we also constructed another reporter plasmid, the CLB2 promoter-GFP-ADH1 3´ UTR plasmid, and examined the GFP mRNA levels in wild-type and the puf5Δ mutant. We used the CLB2 promoter in consideration of the data that CLB2 mRNA is expressed to the same extent between wild-type and the puf5Δ mutant (S1B Fig). The expression of GFP mRNA driven by the CLB2 promoter did not show a statistically significant difference between wild-type and the puf5Δ mutant (Fig 4B). These data suggest that Puf5 positively regulates CLB1 expression via the CLB1 promoter at the transcriptional level.
Fig 4. Puf5 positively regulates CLB1 transcription through the CLB1 promoter.
(A, B) The mRNA levels of GFP in wild-type and the puf5Δ mutant. The strains harboring the YCplac33-CLB1 promoter-GFP-ADH1 3´ UTR plasmid (A) or YCplac33-CLB2 promoter-GFP-ADH1 3´ UTR plasmid (B) were cultured in an SC-Ura medium at 28°C until the exponential phase. The GFP mRNA levels were quantified by qRT-PCR analysis, and the relative mRNA levels were calculated using the SCR1 reference gene. The data shows the mean ± SE (n = 3) of the fold change of GFP mRNA relative to the mRNA level in wild-type. *P < 0.05, **P < 0.01 as determined by Tukey’s test.
The HMGB protein Ixr1 functions downstream of Puf5 and contributes to the growth defect of the puf5Δ clb2Δ mutant
For the mechanism of how Puf5 positively regulates CLB1 transcription, we hypothesized that Puf5 negatively regulates expression of a transcriptional repressor of CLB1 (Fig 5A), regarding that Puf5 is thought to be a negative regulator of gene expression [10–12,15].
Fig 5. Deletion of IXR1 recovered the growth defect of the puf5Δclb2Δ double mutant.
(A) A model showing how Puf5 functions in cell growth through the CLB1 regulation. The hypothesis is presented that Puf5 negatively regulates the expression of X, and X negatively regulates CLB1 expression. (B) The tetrad analysis of the strains that are heterozygous for the alleles of PUF5, CLB2, IXR1, and LRG1. The cells were sporulated, dissected on a YPD plate, and cultured at 30°C for 3 days. (C) The spot assay of wild-type and the puf5Δ clb2Δ ixr1Δ triple mutant harboring plasmids YCplac33 or YCplac33-IXR1. The strains were cultured in an SC-Ura medium containing 10% sorbitol at 25°C until the exponential phase and collected. Cells were serially diluted, spotted onto an SC-Ura plate containing 10% sorbitol, and incubated for 4 days at 25°C. (D) The growth curve of wild-type and the puf5Δ clb2Δ ixr1Δ triple mutant harboring plasmids YCplac33 or YCplac33-IXR1 at 25°C. The strains were pre-cultured in an SC-Ura medium overnight at 25°C, then transferred into a fresh SC-Ura medium, and cultured at 25°C for 1.5 days. The data shows the mean± SE (n = 3) of the optical density. The white circle markers show wild-type [YCplac33], the black square markers show wild-type [YCplac33-IXR1], the white triangle markers show the puf5Δ clb2Δ ixr1Δ [YCplac33], and the black rhombus markers show the puf5Δ clb2Δ ixr1Δ [YCplac33-IXR1]. (E) Morphology of wild-type, the puf5Δ mutant, the clb2Δ mutant, the puf5Δ clb2Δ double mutant, and the puf5Δ clb2Δ ixr1Δ triple mutant strains. Bright field (left), DAPI staining (middle), and overlayed (right) were shown. The scale bar represents 5 μm. (F) The spot assay of wild-type, the puf5Δ mutant, and the puf5Δ ixr1Δ double mutant. The strains were cultured in a YPD medium at 25°C until the exponential phase. Cells were serially diluted, spotted onto YPD plate and incubated for 1 day at 25°C or 37°C.
We searched the Saccharomyces Genome Database (SGD https://www.yeastgenome.org/) and found 16 genes encoding a regulator of CLB1. We examined the mRNA levels of these 16 genes in wild-type and the puf5Δ mutant by qRT-PCR, and found that the expression of IXR1, FKH1, FKH2, HFI1, HIR1, and STE12 genes was increased (fold change > 1.5) in the puf5Δ compared to wild-type (S5 Table). IXR1 encodes an HMGB (High Mobility Group box B) protein. [34,35]. Fkh1/ Fkh2 are members of the winged-helix/forkhead (FOX) transcription factor gene family and regulate the transcription of CLB1 and CLB2 in the G2/M phase [18,36]. Hfi1 is a component of the SAGA complex [37], Hir1 is a corepressor involved in the transcriptional regulation of histone gene [38], and Ste12 is a MAPK cascade-activated transcription factor taking a part in the pheromone response and pseudohyphal growth [39]. We deleted each gene in addition to PUF5, CLB2, and LRG1 genes in diploid cells and performed tetrad analysis to examine whether the deletion recovered the severe growth defect of the puf5Δ clb2Δ double mutant. LRG1 was additionally deleted in this tetrad analysis for the purpose of ensuring that the regulator of CLB1 expression acts independently of LRG1. Consequently, we found that deletion of IXR1 recovered the growth defect of puf5Δ clb2Δ double mutant (Fig 5B puf5Δ clb2Δ, puf5Δ clb2Δ ixr1Δ). This recovery was also observed in the lrg1Δ background (Fig 5B puf5Δ clb2Δ ixr1Δ, puf5Δ clb2Δ lrg1Δ ixr1Δ). None of the deletions of other regulatory genes recovered the growth defect of the puf5Δ clb2Δ double mutant (S2A–S2E Fig). Fkh1 and Fkh2 have been reported to regulate the expression of CLB1 and CLB2 [18,36], but loss of FKH1 or FKH2 also did not impair the growth of puf5Δ mutant or puf5Δ clb2Δ double mutant (S2A and S2B Fig). Therefore, we focused on the IXR1 gene. IXR1 encodes a transcriptional repressor with the HMG domain and has been found as a factor binding to cisplatin-DNA adducts [34,35,40,41]. Ixr1 represses the expression of hypoxic genes during normoxia and regulates hypoxic response [42]. While the ixr1Δ mutation restored the growth defect of the puf5Δ clb2Δ double mutant, this restoration was not observed at 37°C (S3 Fig puf5Δ clb2Δ, puf5Δ clb2Δ ixr1Δ). Since the lrg1Δ mutation retrieved the growth defect of the puf5Δ clb2Δ ixr1Δ triple mutant (S3 Fig puf5Δ clb2Δ ixr1Δ, puf5Δ clb2Δ lrg1Δ ixr1Δ), the ixr1Δ mutation and the lrg1Δ mutation seem to affect cell growth independently. To confirm the influence of IXR1 on the growth defect of the puf5Δ clb2Δ double mutant, we introduced YCplac33-IXR1 in the puf5Δ clb2Δ ixr1Δ triple mutant. The expression of IXR1 suppressed the growth of the puf5Δ clb2Δ ixr1Δ triple mutant (Fig 5C). This result was confirmed by the overtime culture in a liquid medium (Fig 5D). We also examined whether IXR1 deletion restored not only the growth defect of the puf5Δ clb2Δ double mutant but also its elongated morphology. Consistent to the results shown in Fig 1E, the clb2Δ mutant cells showed an elongated shape compared to wild-type cells, and the puf5Δ mutation accelerated the elongation (Fig 5E WT, clb2Δ, puf5Δ clb2Δ). The puf5Δ clb2Δ ixr1Δ triple mutant cells were still elongated, but the degree of the elongation was much smaller than in the puf5Δ clb2Δ double mutant (Fig 5E puf5Δ clb2Δ ixr1Δ, puf5Δ clb2Δ). These results suggest that Ixr1 functions downstream of Puf5 and contributes to the growth defect of the puf5Δ clb2Δ double mutant.
To further investigate whether Ixr1 acts downstream of Puf5, not Clb2, we examined whether the ixr1Δ mutation suppresses the temperature-sensitive phenotype of the puf5Δ CLB2+ mutant. The puf5Δ mutant grew poorly at 37°C compared to wild-type (Fig 5F). Although the effect was not enough to completely suppress the temperature-sensitiveness, the ixr1Δ mutation recovered the growth defect of the puf5Δ mutant at 37°C (Fig 5F). This data supports the model that Ixr1 functions downstream of Puf5.
Ixr1 negatively regulates the CLB1 transcription
We next examined whether Ixr1 negatively regulates CLB1 expression (Fig 6A). First, we examined whether the ixr1Δ mutation restored the decreased expression of CLB1 in the puf5Δ clb2Δ double mutant. The CLB1 mRNA levels was decreased in the puf5Δ mutant and the puf5Δ clb2Δ double mutant with a statistically significant difference, and this decreased expression of CLB1 mRNA was restored in the puf5Δ clb2Δ ixr1Δ triple mutant (Fig 6B). When we examined the CLB1 mRNA levels in the puf5Δ clb2Δ ixr1Δ triple mutant harboring plasmids YCp vector, YCp-IXR1, or YEp-IXR1, the CLB1 mRNA levels in the puf5Δ clb2Δ ixr1Δ triple mutant harboring YCp-IXR1 and YEp-IXR1 was significantly decreased to 53% and 37%, respectively, of that in the triple mutant with YCp vector (Fig 6C). These results suggest that IXR1 deletion restored the decreased expression of CLB1 caused by the puf5Δ mutation and simultaneously recovered the severe growth defect of the puf5Δ clb2Δ double mutant as shown in Fig 5B–5E.
Fig 6. Ixr1 is a downstream factor of Puf5 and negatively regulates CLB1 expression.
(A) A model showing how Puf5 functions in cell growth through the CLB1 regulation mediated by Ixr1. The hypothesis is presented that Puf5 negatively regulates the expression of the Ixr1 repressor, and Ixr1negatively regulates the CLB1 transcription. (B)The CLB1 mRNA levels in wild-type, the puf5Δ mutant, the clb2Δ mutant, the puf5Δ clb2Δ double mutant, and the puf5Δ clb2Δ ixr1Δ triple mutant. The cells were cultured in a YPD medium containing 10% sorbitol at 28°C until the exponential phase. The CLB1 mRNA levels were quantified by qRT-PCR analysis, and the relative mRNA levels were calculated using the SCR1 reference gene. The data shows the mean ± SE (n = 5) of the fold change of CLB1 mRNA relative to the mRNA level in wild-type. *P < 0.05, **P < 0.01 as determined by Tukey’s test. (C) The CLB1 mRNA levels in the puf5Δ clb2Δ ixr1Δ mutant harboring plasmids YCplac33, YCplac33-IXR1, or YEplac195-IXR1. The strains were cultured in an SC-Ura medium at 25°C until the exponential phase. The CLB1 mRNA levels were quantified by qRT-PCR analysis, and the relative mRNA levels were calculated using the SCR1 reference gene. The data shows the mean ± SE (n = 5) of the fold change of CLB1 mRNA relative to the mRNA level in the strain harboring the YCplac33 plasmid. *P < 0.05, **P < 0.01 as determined by Tukey’s test. (D) The CLB1 mRNA levels in wild-type, the puf5Δ mutant, the ixr1Δ mutant, and the puf5Δ ixr1Δ double mutant. The cells were cultured in a YPD medium at 28°C until the exponential phase. The CLB1 mRNA levels were quantified by qRT-PCR analysis, and the relative mRNA levels were calculated using the SCR1 reference gene. The data shows the mean ± SE (n = 5) of the fold change of CLB1 mRNA relative to the mRNA level in wild-type. *P < 0.05, **P < 0.01 as determined by Tukey’s test. (E, F) The GFP mRNA levels in wild-type, the puf5Δ mutant, the ixr1Δ mutant, and the puf5Δ ixr1Δ double mutant. The strains harboring the YCplac33-CLB1 promoter-GFP-ADH1 3´ UTR plasmid (E) or YCplac33-CLB2 promoter-GFP-ADH1 3´ UTR plasmid (F) were cultured in an SC-Ura medium at 28°C until the exponential phase. The GFP mRNA levels were quantified by qRT-PCR analysis, and the relative mRNA levels were calculated using the SCR1 reference gene. The data shows the mean ± SE (n = 3) of the fold change of GFP mRNA relative to the mRNA level in wild-type. *P < 0.05, **P < 0.01 as determined by Tukey’s test.
Next, we examined the endogenous CLB1 mRNA level in the ixr1Δ mutant. Consistent with the results in Fig 2A, the CLB1 mRNA level in the puf5Δ mutant was decreased to 53% of that in wild-type (Fig 6D). In the ixr1Δ mutant, the CLB1 mRNA level was approximately 1.4-times as much as that in wild-type (Fig 6D). We also investigated the expression of the reporter plasmid, CLB1 promoter-GFP-ADH1 3´ UTR, in the ixr1Δ mutant to clarify whether Ixr1 negatively regulates CLB1 expression through the CLB1 promoter. Since the expression of ADH1 mRNA was not influenced by deletion of PUF5 or IXR1 (S1A Fig), we utilized the ADH1 3’ UTR. The expression of GFP mRNA driven by the CLB1 promoter was 1.8-times increased in the ixr1Δ mutant than that in wild-type with a statistically significant difference (Fig 6E). To confirm that Ixr1 specifically regulates the CLB1 promoter, we also examined the GFP mRNA level driven by the CLB2 promoter in wild-type, the puf5Δ mutant, the ixr1Δ mutant, and the puf5Δ ixr1Δ double mutant, since the CLB2 mRNA level was not affected by IXR1 deletion (S1B Fig). As a result, the expression of GFP mRNA driven by the CLB2 promoter did not show a statistically significant difference among the four strains examined (Fig 6F). These results suggest that Ixr1 negatively regulates CLB1 transcription. When we compare the expression of both endogenous CLB1 and GFP reporter between the ixr1Δ mutant and the puf5Δ ixr1Δ double mutant, the puf5Δ mutation decreased the endogenous CLB1 and GFP mRNA levels even in the absence of Ixr1 (Fig 6D and 6E). These results imply that Puf5 regulates CLB1 expression in both Ixr1-dependent and -independent manners.
Since the above data suggested that Ixr1 negatively regulates CLB1 transcription, we questioned whether this regulation results from the direct binding of Ixr1 to the CLB1 promoter. However, since the interaction between Ixr1 and CLB1 promoter has not been reported in a previous genome-wide chromatin immunoprecipitation (ChIP) analysis [43], it is unlikely that Ixr1 acts as a repressor that directly binds to the CLB1 promoter. It is known that CLB1 expression is induced by the activated Mcm1-Fkh2-Ndd1 complex [18,19]. Fkh1, a paralog of Fkh2, also regulates the transcription of CLB1 and CLB2 in the G2/M phase partially complementarily with Fkh2. Therefore, to consider a possibility that Ixr1 functions dependently on Fkh1 and Fkh2, we examined a genetic interaction between IXR1 and FKH1/FKH2. We constructed the diploid strain heterozygous for IXR1, FKH1, and FKH2 alleles and performed tetrad analysis. The fkh1Δ and fkh2Δ single mutants grew as well as wild-type (S4 Fig fkh2Δ, fkh1Δ, WT). In contrast, the fkh1Δ fkh2Δ double mutant showed a growth defect as previously reported [44]. This growth defect was not restored by IXR1 deletion (S4 Fig fkh1Δ fkh2Δ, ixr1Δfkh1Δ fkh2Δ). These data implied the possibility that Fkh1 and Fkh2 may act downstream rather than parallel with Ixr1. Ixr1 may function via regulating expression of the transcriptional activators of CLB1. However, this result alone does not completely rule out the possibility that Fkh1 and Fkh2 regulate CLB1 expression in parallel with Ixr1.
Puf5 negatively regulates IXR1 through the IXR1 3´ UTR
Ixr1 mediates the regulation of the CLB1 expression by Puf5. As additional evidence, a previous genome-wide analysis reported that Puf5 binds to the 3´ UTR of IXR1 mRNA [8]. Therefore, we investigated whether Puf5 regulates IXR1 expression as a model shown in Fig 7A. The IXR1 mRNA level in the puf5Δ mutant was 1.2-times as much as that in wild-type (Fig 7B). This difference was small, but reproducible. We also examined Ixr1 protein level. The level of Ixr1 protein tagged with HA in the puf5Δ mutant was 1.7-times as much as that in wild-type (Fig 7C and 7D). Since Puf5 has been reported to bind to the 3´ UTR of IXR1 mRNA [8], we examined the effect of the IXR1 3´ UTR using the IXR1-HA-IXR1 3´ UTR and IXR1-HA-ADH1 3´ UTR constructs. The increase in Ixr1 protein level by PUF5 deletion was significant in the construct harboring the IXR1 3´ UTR but not in the construct harboring the ADH1 3´ UTR (Fig 7C and 7D). These results implied that Puf5 negatively regulates IXR1 expression via the IXR1 3´ UTR. In the IXR1 3´ UTR, we asked which region is responsible for the regulation by Puf5. Previous HITS-CLIP analysis has revealed that Puf5 binds to the element 5´-UGUAACAUUA in the 3´ UTR of IXR1 mRNA [8]. We constructed YCplac33-MCM2 promoter-GFP-IXR1 3´ UTR Δ UGUAACAUUA in which the Puf5-binding element is deleted (Fig 7E) and analyzed the effect of the deletion on GFP protein level. The deletion increased the GFP protein level 2.5-fold (Fig 7F and 7G), suggesting that Puf5-binding to the element negatively regulates expression of IXR1 mRNA.
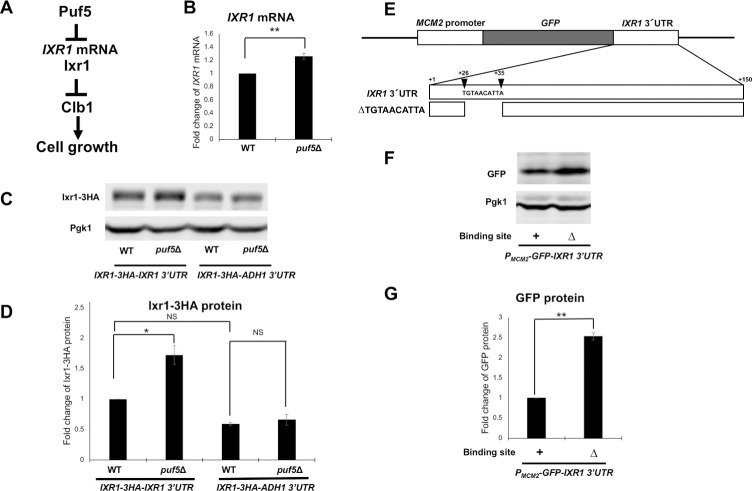
Fig 7. Puf5 negatively regulates IXR1 expression through the IXR1 3’ UTR.
(A) A model showing how Puf5 functions in cell growth through the CLB1 regulation, which is mediated by Ixr1. The hypothesis is presented that Puf5 negatively regulates the IXR1 expression through the IXR1 mRNA, and that Ixr1negatively regulates the CLB1 transcription. (B) The IXR1 mRNA levels in wild-type and the puf5Δ mutant. The cells were cultured in a YPD medium containing 10% sorbitol at 25°C until the exponential phase. The IXR1 mRNA levels were quantified by qRT-PCR analysis, and the relative mRNA levels were calculated using the SCR1 reference gene. The data shows the mean ± SE (n = 3) of the fold change of IXR1 mRNA relative to the mRNA level in wild-type. *P < 0.05, **P < 0.01 as determined by Tukey’s test. (C, D) The Ixr1 protein levels in wild-type and the puf5Δ mutant and the quantitative analysis data of Ixr1-HA protein level. The strains harboring the YCplac33-IXR1-HA-IXR1 3´ UTR plasmid or the YCplac33-IXR1-HA-ADH1 3´ UTR plasmid were cultured in an SC-Ura medium at 28°C. The extracts were immunoblotted with anti-HA antibody or anti-Pgk1 antibody. Ixr1-HA protein level was quantified and normalized with the Pgk1 protein level. The data shows the mean ± SE (n = 3) of the fold change of Ixr1-HA protein relative to the protein level in wild-type harboring the YCplac33-IXR1-HA-IXR1 3´ UTR plasmid (D). *P < 0.05, **P < 0.01 as determined by Tukey’s test. (E) Scheme of the YCplac33-IXR1-HA-IXR1 3´ UTR plasmid. ΔTGTAACATTA harbors the deletion of the sequence encoding the Puf5-binding site of IXR1 mRNA, 5´-TGTAACATTA. Described numbers correspond to the number of bases from the stop codon of IXR1. (F, G) The GFP protein levels and the quantitative analysis data of GFP protein level in wild-type strain compared by the presence of the Puf5-binding site deletion. The strains harboring the YCplac33-MCM2 promoter-GFP-IXR1 3´ UTR plasmid with or without the Puf5-binding site were cultured in an SC-Ura medium at 28°C. The extracts were immunoblotted with anti-GFP antibody or anti-Pgk1 antibody. GFP protein level was quantified and normalized with the Pgk1 protein level. The data shows the mean ± SE (n = 3) of the fold change of GFP protein relative to the protein level in the strains harboring the YCplac33-MCM2 promoter-GFP-IXR1 3´ UTR plasmid with the Puf5-binding site (G). *P < 0.05, **P < 0.01 as determined by Tukey’s test.
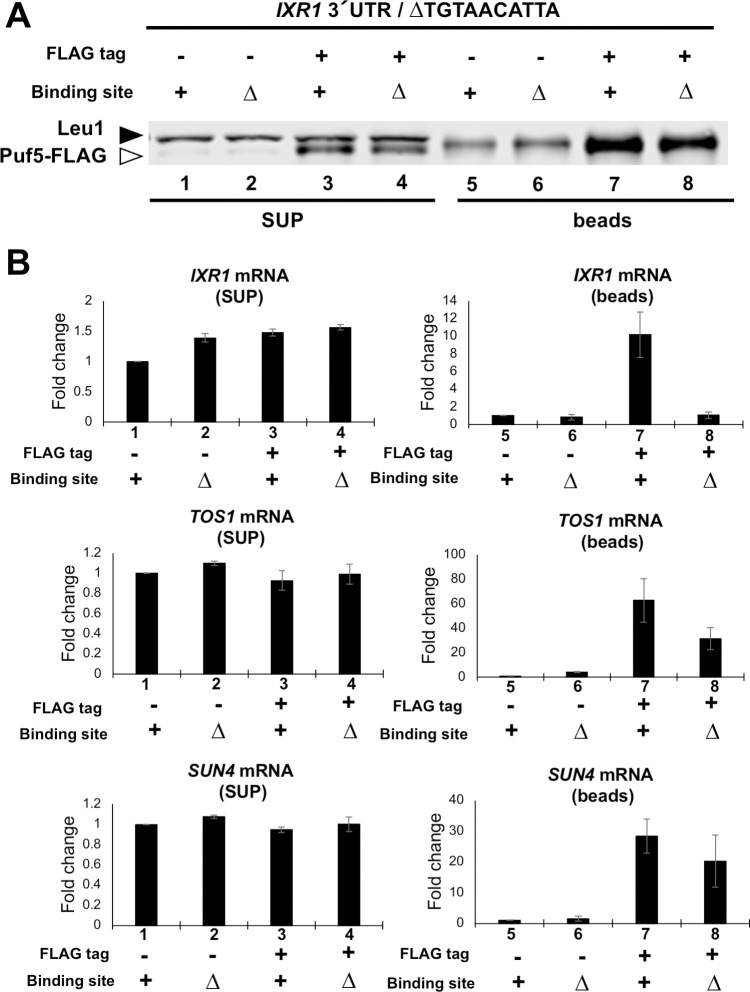
To further analyze the interaction between Puf5 and IXR1 mRNA through the 3’ UTR, we performed RNA immunoprecipitation (RIP). Puf5-FLAG protein was immunoprecipitated with anti-FLAG antibody, mRNAs were purified from the immunoprecipitants, and the enrichment of IXR1 mRNA was analyzed (for details, see Materials and methods). Strains harboring untagged-Puf5 were used as a negative control. Western blot analysis was performed to confirm that Puf5-FLAG protein was contained in the supernatant and immunoprecipitants. While the bands of Leu1 protein recognized by the anti-FLAG M2 antibody were detected simultaneously, the bands of Puf5-FLAG protein were detected in the supernatant (Fig 8A). Although the bands of the Leu1 and Puf5-FLAG proteins was not separated in the immunoprecipitants, the signal intensity of the overlapping bands were clearly increased in the Puf5-FLAG strains compared to the untagged strain, indicating that the Puf5-FLAG protein was successfully immunoprecipitated (Fig 8E). Consistently, SUN4 and TOS1 mRNAs, which are reported to be strongly immunoprecipitated with Puf5 in the previous HITS-CLIP analysis [8], were highly enriched in the Puf5-FLAG immunoprecipitants compared to the untagged immunoprecipitants (Fig 8B). Similarly, IXR1 mRNA was significantly enriched in the Puf5-FLAG immunoprecipitants compared to the untagged immunoprecipitants (Fig 8B). We also investigated the contribution of Puf5-binding element to the enrichment of IXR1 mRNA. The enrichment of IXR1 mRNA was dependent on the Puf5-binding element (Fig 8B). In contrast, SUN4 mRNA and TOS1 mRNA enriched independently of the binding element in the IXR1 3´ UTR (Fig 8B). These data confirmed that Puf5 specifically binds to the motif 5´-UGUAACAUUA in the IXR1 3´ UTR.
Fig 8. Puf5 binds to 3´ UTR of IXR1 mRNA.
(A,B) RIP analysis data clarifying the binding between Puf5 protein and IXR1 mRNA. The extract was obtained from ixr1Δ PUF5-FLAG-ADH1 3´ UTR strain harboring the YEplac195-IXR1 plasmid, and Puf5-FLAG protein was immunoprecipitated with anti-FLAG antibody. The ixr1Δ untagged-PUF5 strain was used as a negative control. Sample 1–4 contain the supernatant, and sample 5–8 contain the immunoprecipitants. Sample 1 and 5, untagged strain with Puf5-binding element; sample 2 and 6, untagged strain without Puf5-binding element; sample 3 and 7, FLAG-tagged strain with Puf5-binding element; sample 4 and 8, FLAG-tagged strain without Puf5-binding element. (A) Puf5-FLAG protein level in supernatant and immunoprecipitants. The white arrowhead corresponds to Puf5-FLAG protein, and the black to Leu1 protein. (B) IXR1 mRNA levels in the supernatant and the immunoprecipitants. TOS1 mRNA levels and SUN4 mRNA levels were presented as positive controls. The mRNA levels were quantified by qRT-PCR analysis, and the fold change was calculated relative to the mRNA level in the untagged strain with Puf5-binding element (sample 1 for the supernatant, and sample 5 for the immunoprecipitants).
Puf5 and Ixr1 contribute to the cell-cycle-dependent expression of CLB1
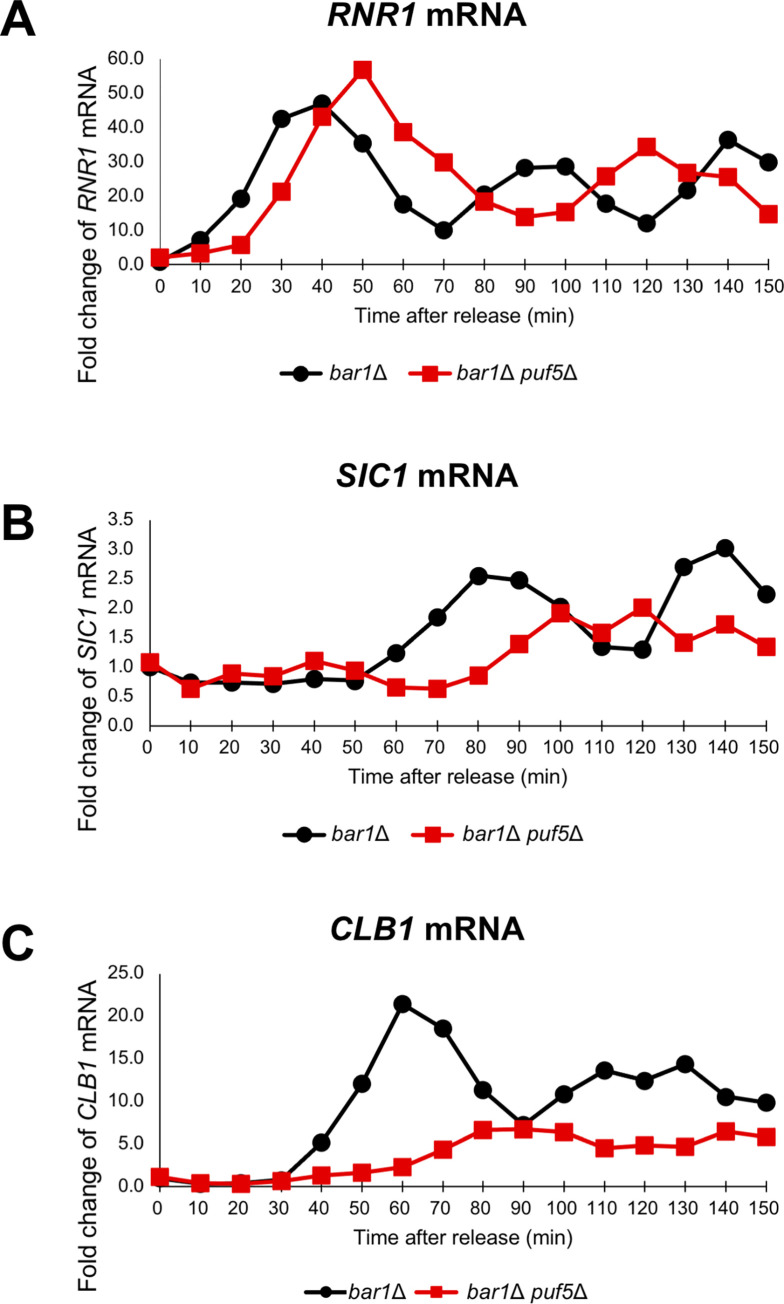
The above data suggest that Puf5 regulates CLB1 expression through regulating IXR1 expression. However, when examining an asynchronous culture, the decrease of the CLB1 expression in the puf5Δ mutant, approximately 50% decrease, was not prominent (Figs 2A, 6B and 6D). Regarding that the expression of CLBs dramatically transits depending on the cell cycle [16], the decrease of the CLB1 expression in the puf5Δ mutant may be observed at a higher extent in a synchronous culture. Therefore, we synchronized the cell cycle by pheromone-induced G1 arrest and examined the effects of Puf5 and Ixr1 on the cell cycle-specific expression of CLB1 mRNA. For this purpose, we utilized the MATa bar1Δ and MATa bar1Δ puf5Δ cells, in which α-factor protease Bar1 was absent [27]. The MATa bar1Δ and MATa bar1Δ puf5Δ cells were arrested in the G1 phase with α-factor and synchronized by releasing, and RNAs were extracted from the samples collected from 0 minutes (just before releasing) to 150 minutes (for details, see Materials and methods). We examined the expression of RNR1 and SIC1 in cell cycle-synchronized strains as an S phase and a late M phase marker, respectively. In the bar1Δ cell, the RNR1 mRNA level peaked at 40 min first and at 90–100 min second (Fig 9A bar1Δ), and the SIC1 mRNA level did at 80 min first and at 140 min second (Fig 9B bar1Δ). In the bar1Δ puf5Δ mutant, the RNR1 mRNA level reached the summit at 50 min first and at 120 min second (Fig 9A bar1Δ puf5Δ), and the SIC1 mRNA level did at 100-120 min (Fig 9B bar1Δ puf5Δ). These data show that the cell cycle was completed within the time tested in both strains, although the cell cycle was delayed for 10–40 min in the bar1Δ puf5Δ mutant. The expression of CLB1 mRNA was induced from 40 min, peaked at 60 min, and decreased immediately after the peak in the bar1Δ cell (Fig 9C bar1Δ). As expected, the CLB1 mRNA level was dramatically decreased in the bar1Δ puf5Δ mutant: the expression of CLB1 was slightly induced at 80-90 min, but the extent of the induction was much smaller in the bar1Δ puf5Δ mutant than that in the bar1Δ cell (Fig 9C bar1Δ, bar1Δ puf5Δ). These results suggest that Puf5 ensures the utmost expression of CLB1 during the cell cycle through positive regulation of CLB1 expression.
Fig 9. The cell cycle-regulated expression of CLB1 was diminished in the puf5Δ mutant.
(A-C) The cell cycle-dependent mRNA levels of CLB1 in the synchronized bar1Δ cell (black circle) and bar1Δ puf5Δ mutant (red square). The cell cycle was arrested in the G1 phase by α-factor, and, after release, cells were collected from 0 min (just before releasing) to 150 min. The levels of RNR1 mRNA (A), an S phase marker, SIC1 mRNA (B), a late M phase marker, and CLB1 mRNA (C) were quantified by qRT-PCR analysis, and the relative mRNA levels were calculated using the SCR1 reference gene. The vertical axis shows the fold change of mRNA relative to the mRNA level in the bar1Δ 0 min sample, and the horizontal axis shows the time after release.
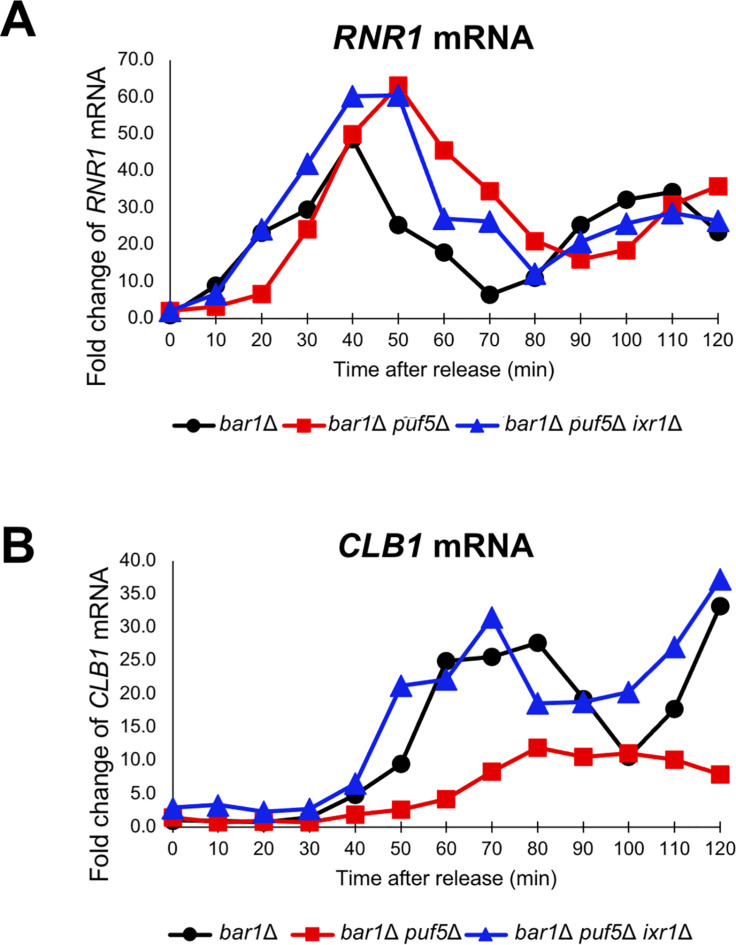
Regarding that Ixr1 mediates the regulation of CLB1 expression by Puf5, we examined whether the ixr1Δ mutation restored the decreased expression of CLB1 in the synchronous puf5Δ mutant. The CLB1 mRNA levels in the synchronous bar1Δ cell and bar1Δ puf5Δ ixr1Δ mutant was compared to that in bar1Δ puf5Δ mutant shown in Fig 9C. The expression of RNR1 mRNA, an S-phase marker, showed similar patterns in the three strains and peaked at 40–50 min (Fig 10A bar1Δ, bar1Δ puf5Δ, bar1Δ puf5Δ ixr1Δ). This result suggests that the cell cycle progresses normally in all three strains. As shown in Fig 9C, the cell cycle-specific expression of CLB1 mRNA was remarkably diminished in the bar1Δ puf5Δ mutant compared to that in the bar1Δ cell (Fig 10B bar1Δ, bar1Δ puf5Δ), and the diminished expression was restored in the bar1Δ puf5Δ ixr1Δ mutant (Fig 10B bar1Δ puf5Δ ixr1Δ). These results indicate that Ixr1 functions downstream of Puf5 and mediates the positive control of the cell cycle-specific CLB1 expression. To confirm the involvement of Ixr1 in cell cycle progression, we also examined the CLB1 mRNA level in the cell cycle-synchronized bar1Δ ixr1Δ mutant. The peak of RNR1 mRNA level was observed at 40 min in the bar1Δ cell and 50 min in the bar1Δ puf5Δ mutant as shown in Fig 9A and 10A (Fig 11A bar1Δ, bar1Δ puf5Δ). In the bar1Δ ixr1Δ mutant, the RNR1 mRNA level went highest at 20 min first, approximately 20 minutes earlier than in the bar1Δ cell, and at 80 min second (Fig 11A bar1Δ ixr1Δ). The extent of the peak expression was similar to that in the bar1Δ cell (Fig 11A bar1Δ, bar1Δ ixr1Δ). Although the expression of RNR1 was induced earlier than in bar1Δ cell, these data suggest that the cell cycle progresses regularly in the ixr1Δ background. As shown in Fig 10B, the CLB1 mRNA level was highest around 60–80 min in the bar1Δ cell, and the peak was remarkably lessened in the bar1Δ puf5Δ mutant (Fig 11B bar1Δ, bar1Δ puf5Δ). Comparing the bar1Δ cell and the bar1Δ ixr1Δ mutant, the expression of CLB1 was induced at an earlier stage, and the extent of the peak expression was significantly higher in the bar1Δ ixr1Δ mutant than in the bar1Δ cell (Fig 11B bar1Δ, bar1Δ ixr1Δ). These data suggest that Ixr1 negatively regulates cell cycle-depending induction of CLB1 expression. Puf5, a negative regulator of IXR1 expression, seems to control this Ixr1-mediated repression and indirectly control the maximum level of the cell cycle-dependent CLB1 expression, which probably leads to the control of the proper cell cycle progression.
Fig 10. IXR1 deletion restored the decreased expression of CLB1 caused by PUF5 deletion.
(A, B) The cell cycle-dependent mRNA levels of CLB1 (B) in the cell cycle synchronized bar1Δ cell (black circle), bar1Δ puf5Δ mutant (red square), and bar1Δ puf5Δ ixr1Δ mutant (blue triangle). The cell cycle was arrested in the G1 phase by α-factor, and, after release, cells were collected from 0 min (just before releasing) to 120 min. The levels of RNR1 mRNA (A), an S phase marker, and CLB1 mRNA (B) were quantified by qRT-PCR analysis, and the relative mRNA levels were calculated using the SCR1 reference gene. The vertical axis shows the fold change of mRNA relative to the mRNA level in the bar1Δ 0 min sample, and the horizontal axis shows the time after release.
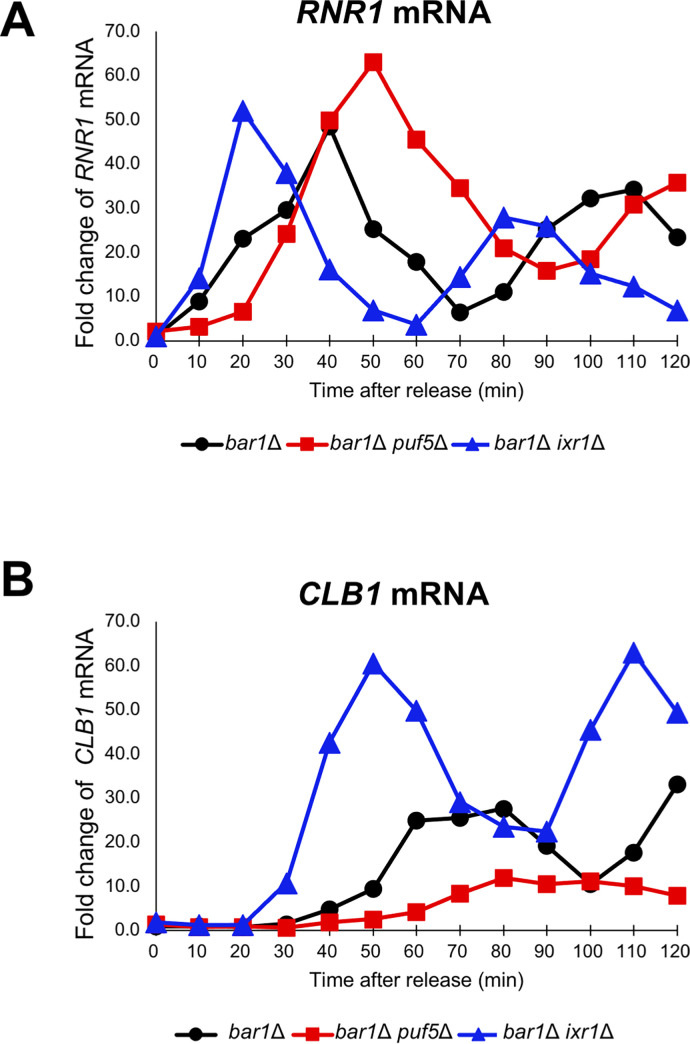
Fig 11. Ixr1 negatively regulates the cell cycle-specific expression of CLB1.
(A, B) The cell cycle-dependent mRNA levels of CLB1 (B) in the synchronized bar1Δ cell (black circle), bar1Δ puf5Δ mutant (red square), and bar1Δ ixr1Δ mutant (blue triangle). The cell cycle was arrested in the G1 phase by α-factor, and, after release, cells were collected from 0 min (just before releasing) to 120 min. The levels of RNR1 mRNA (A), an S phase marker, and CLB1 mRNA (B) were quantified by qRT-PCR analysis, and the relative mRNA levels were calculated using the SCR1 reference gene. The vertical axis shows the fold change of mRNA relative to the mRNA level in the bar1Δ 0 min sample, and the horizontal axis shows the time after release.
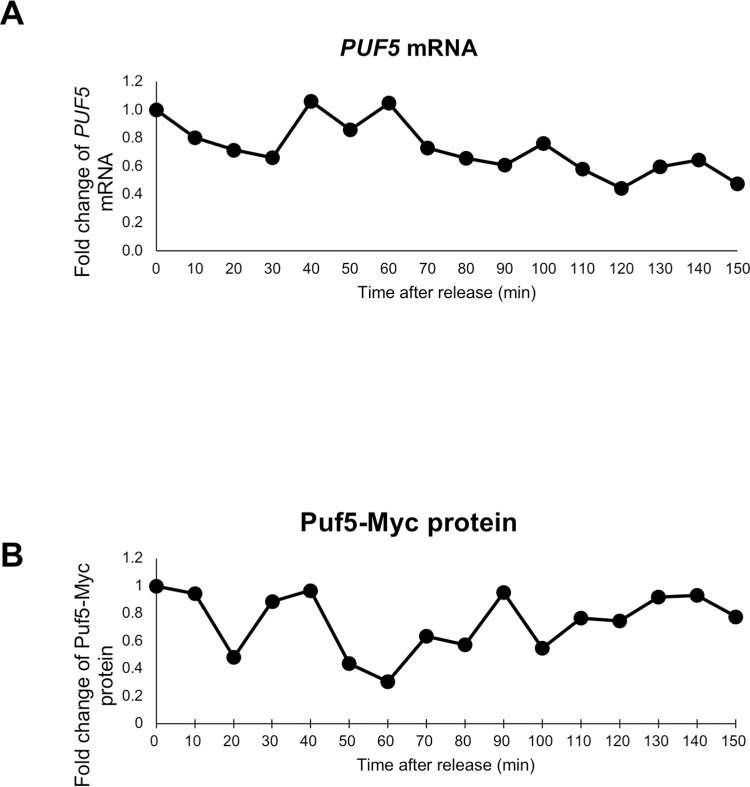
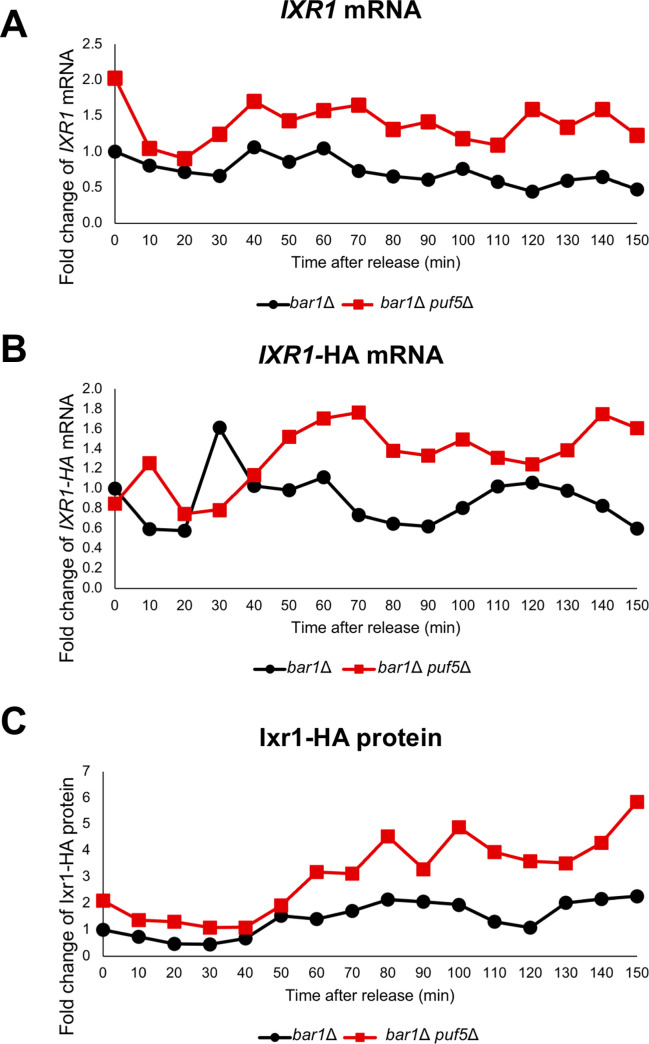
Puf5 negatively regulates IXR1 expression throughout the cell cycle
Regarding that Puf5 and Ixr1 seem to regulate the cell cycle progression, we questioned whether their expression rhythmically transits during the cell cycle. We first examined PUF5 expression in the cell cycle. PUF5 mRNA levels in synchronized bar1Δ cells did not change significantly throughout the cell cycle (Fig 12A). Consistent with the mRNA level, Puf5-Myc protein levels did not change significantly throughout the cell cycle examined using the bar1Δ PUF5-Myc strain (Fig 12B). Next, we analyzed the transition of IXR1 expression in a synchronous culture. IXR1 mRNA levels in both the bar1Δ cell and the bar1Δ puf5Δ mutant did not change significantly throughout the cell cycle (Fig 13A bar1Δ, bar1Δ puf5Δ). Even though the periodic expression of IXR1 mRNA was not observed, the IXR1 mRNA level was higher in the bar1Δ puf5Δ mutant than that in the bar1Δ cell over the cell cycle (Fig 13A bar1Δ, bar1Δ puf5Δ). The Ixr1 protein level was quantified by exploiting the YCplac33-IXR1-HA-IXR1 3´ UTR plasmid in the bar1Δ cell and the bar1Δ puf5Δ mutant. Since the plasmids were used, we first examined the IXR1-HA mRNA level; the IXR1-HA mRNA level in the bar1Δ cell did not change significantly during the cell cycle, consistently with the endogenous IXR1 mRNA level shown in Fig 13A (Fig 13B bar1Δ). In the bar1Δ puf5Δ mutant, the IXR1-HA mRNA level was increased and went higher following the cell cycle progression than that in the bar1Δ cell (Fig 13B bar1Δ puf5Δ, bar1Δ). Similar to the IXR1-HA mRNA level, the Ixr1 protein level in the bar1Δ cell did not change significantly during the cell cycle (Fig 13C bar1Δ). In the bar1Δ puf5Δ mutant, the Ixr1 protein level was increased following the cell cycle progression than that in the bar1Δ cell (Fig 13C bar1Δ puf5Δ, bar1Δ). These results suggest that expression of Puf5 and Ixr1 does not change significantly during the cell cycle, but Puf5 negatively regulates IXR1 expression throughout the cell cycle.
Fig 12. PUF5 expression is invariable during the cell cycle.
(A) The cell cycle-dependent mRNA levels of PUF5 in the synchronized bar1Δ cell, the same sample used in Fig 9. The cell cycle was arrested in the G1 phase by α-factor, and, after release, cells were collected from 0 min (just before releasing) to 150 min. The levels of PUF5 mRNA were quantified by qRT-PCR analysis, and the relative mRNA levels were calculated using the SCR1 reference gene. The vertical axis shows the fold change of mRNA relative to the mRNA level in the bar1Δ 0 min sample, and the horizontal axis shows the time after release. (B) The cell cycle-dependent Puf5 protein level in the synchronized bar1Δ cell integrated PUF5-13Myc-ADH1 3´ UTR gene. The cell cycle was arrested in the G1 phase by α-factor, and, after release, cells were collected from 0 min (just before releasing) to 150 min. The extracts were immunoblotted with anti-Myc antibody or anti-Pgk1 antibody. The blot image is presented in S5A Fig. Puf5-Myc protein level was quantified and normalized with the Pgk1 protein level. The vertical axis shows the fold change of protein relative to the protein level in the bar1Δ 0 min sample, and the horizontal axis shows the time after release.
Fig 13. IXR1 expression is invariable during the cell cycle.
(A) The IXR1 mRNA levels in the synchronized bar1Δ cell (black circle) and bar1Δ puf5Δ mutant (red square), the same sample used in Fig 9. The cell cycle was arrested in the G1 phase by α-factor, and, after release, cells were collected from 0 min (just before releasing) to 150 min. The IXR1 mRNA level was quantified by qRT-PCR analysis, and the relative mRNA levels were calculated using the SCR1 reference gene. The mRNA level in the bar1Δ 0 min sample was set as a reference. The vertical axis shows the fold change of IXR1 mRNA relative to the mRNA level in the bar1Δ 0 min sample, and the horizontal axis shows the time after release. (B) The IXR1-HA mRNA levels in the synchronized bar1Δ cell (black circle) and bar1Δ puf5Δ mutant (red square) harboring the YCplac33-IXR1-HA-IXR1 3´ UTR plasmid. The cell cycle was arrested in the G1 phase by α-factor, and, after release, cells were collected from 0 min (just before releasing) to 150 min. The IXR1-HA mRNA level was quantified by qRT-PCR analysis, and the relative mRNA levels were calculated using the SCR1 reference gene. The mRNA level in the bar1Δ 0 min sample was set as a reference. The vertical axis shows the fold change of IXR1-HA mRNA relative to the mRNA level in the bar1Δ 0 min sample, and the horizontal axis shows the time after release. (C) The Ixr1-HA protein level in the synchronized bar1Δ cell (black circle) and bar1Δ puf5Δ mutant (red square) harboring the YCplac33-IXR1-HA-IXR1 3´ UTR plasmid. The cell cycle was arrested in the G1 phase by α-factor, and, after release, cells were collected from 0 min (just before releasing) to 150 min. The extracts were immunoblotted with anti-HA antibody or anti-Pgk1 antibody. The blot image is presented in S5B–S5D Fig. Ixr1-HA protein level was quantified and normalized with the Pgk1 protein level. The Ixr1-HA protein level in the bar1Δ 0 min sample was set as a reference. The vertical axis shows the fold change of protein relative to the protein level in the bar1Δ 0 min sample, and the horizontal axis shows the time after release.
Discussion
Puf5 contributes to cell growth through the positive regulation of the CLB1 expression, which is mediated by Ixr1
Puf5 has been reported to play multiple roles including mating-type switching, maintaining CWI, and extending life span [3,5,8–15]. Here, we identified CLB2 as a multicopy suppressor of the temperature-sensitive phenotype of the puf5Δ mutant. To reveal the machinery for the suppression by CLB2, we further analyzed the Puf5 function in cell growth using the genetic approaches. We found that Puf5 positively regulates the CLB1 transcription, and that this regulation has a phenotypic effect in the absence of Clb2. For the mechanism of how Puf5 regulates the CLB1 transcription, we hypothesized that Puf5 negatively regulates the expression of a factor that negatively regulates the transcription of CLB1, since Puf5 has been reported to negatively regulate the expression of target mRNAs via their 3´ UTRs [6,7,10–13]. We identified such a factor Ixr1. Preceding genome-wide RIP analysis data have revealed the interaction of Puf5 with cyclin mRNAs, CLB1, CLB2, CLB3, and CLN1 mRNA [8,9]. Among them, only CLB1 expression was affected by puf5Δ mutation (Table 1). Regardless of the protein-RNA interaction, Puf5 indirectly regulates CLB1 expression: Puf5 negatively regulates expression of IXR1 encoding a transcriptional repressor with the HMG domain [34,35]. Previous microarray analysis has reported that CLB1 expression is increased in the ixr1Δ mutant [42], but we first clarified that Ixr1 negatively regulates CLB1 transcription. Together with our data that the ixr1Δ mutation recovered the growth defect of puf5Δ clb2Δ double mutant, Ixr1 negatively regulates the CLB1 transcription with physiological importance. However, it remains unclear whether Ixr1 represses the CLB1 transcription directly or indirectly, by binding to the CLB1 promoter or by controlling other regulatory factors of CLB1. Previous genome-wide ChIP analysis data did not report the binding of Ixr1 to the CLB1 promoter [43]. Ixr1 has been reported to contribute to the transcriptional regulation of ribosomal genes [43]. Moreover, the association of Ixr1 with Ssn8 and Tdh3, general transcriptional regulators, was also reported [45]. Taken together, Ixr1 may function as a general repressor. For another possibility, Ixr1 may regulate the expression of the transcriptional activators of CLB1. The CLB1 expression is induced by the activated Mcm1-Fkh2-Ndd1 complex [18,19,36,46]. Our result suggests that Fkh1/Fkh2 may function downstream of Ixr1 (S4 Fig), but this result does not completely rule out the possibility that Fkh1/Fkh2 functions parallel to Ixr1. Further analysis is needed to reveal the detailed machinery of the Ixr1 function.
IXR1 mRNA is a physiologically important target of Puf5
Puf5 negatively regulates IXR1 expression, which leads to positive regulation of CLB1 expression. This machinery of regulating IXR1 expression depends on the Puf5-binding element in the IXR1 3´ UTR (Fig 7F and 7G). Furthermore, the interaction between Puf5 protein and IXR1 mRNA through the IXR1 3’ UTR was confirmed (Fig 8). Taking these data into account, IXR1 mRNA is a physiologically important target of Puf5. Considering the mechanism of how Puf5 regulates IXR1 expression, the increase of IXR1 expression in the puf5Δ mutant was observed more significantly in the protein level (approximately 1.7-fold) than in the mRNA level (1.2-fold) (Fig 7). Therefore, the negative regulation of IXR1 mRNA by Puf5 may be caused by the translational control rather than by the mRNA stability control. Previously, it has been reported that Puf5 functions in the translational repression through binding to 3´ UTR of target mRNAs in vitro [47]. Regarding that, Puf5 may repress the translation of IXR1 mRNA depending on the IXR1 3´ UTR.
Puf5 regulates the expression of physiological targets, IXR1 and LRG1, independently
The first observation that CLB2 is a multicopy suppressor of the puf5Δ mutant led us to the finding of the Puf5 function in the CLB1 regulation. However, since multicopy CLB2 suppressed the phenotype at 35°C but not enough at 37°C (Fig 1A), CLB2 seems to weakly suppress the phenotype. It appears that other factors also contribute to the defect of the puf5Δ mutant.
We and other groups have previously reported that Puf5 negatively regulates the expression of LRG1 encoding a Rho1 GAP involved in maintaining CWI [11–13,15]. This negative regulation involves CCR4 and POP2, and the puf5Δ mutant, the ccr4Δ mutant, and the pop2Δ mutant show the cell lysis phenotype at the elevated temperature [11–13,15]. The lrg1Δ mutation partially suppresses the cell lysis phenotype, while LRG1 overexpression exacerbates the cell lysis phenotype. In the regulation of the CLB1 expression, Puf5 seems to function independently of controlling CWI through the LRG1 regulation: the growth defect of the puf5Δ clb2Δ double mutant was not recovered by the lrg1Δ mutation (Fig 3E). In addition, IXR1 deletion restored the growth of the puf5Δ clb2Δ double mutant even in the lrg1Δ background (Fig 5B). While IXR1 deletion suppressed the temperature-sensitive phenotype of the puf5Δ mutant (Fig 5F), IXR1 deletion did not retrieve the temperature sensitiveness of the puf5Δ clb2Δ double mutant (S3 Fig). The lrg1Δ mutation recovered the growth defect of the puf5Δ clb2Δ ixr1Δ triple mutant at the high temperature (S3 Fig). This maybe because the two physiologically important targets, IXR1 and LRG1, independently act on cell growth. The Ixr1-mediated decrease of CLB1 expression in the puf5Δ mutant partially causes the temperature-sensitive phenotype, and this phenotype is severely accelerated when the clb2Δ mutation is combined. Since the lrg1Δ mutation that partially suppresses the cell lysis phenotype affects cell growth independently of the Ixr1-mdiated CLB1 regulation, the mutation is able to recover the growth defect of the puf5Δ clb2Δ double mutant at the elevated temperature.
The cell cycle-specific expression of CLB1 is regulated by not only positive regulators but also negative regulators
Expression of cyclin genes dramatically transits depending on the cell cycle [16]. Here we found that Puf5 and its physiological target Ixr1 are involved in controlling the cell cycle-specific expression of CLB1, which leads to the control of the proper cell cycle progression. When examined in a synchronous culture, the cell cycle was delayed for approximately 10–20 min in the puf5Δ background (Fig 9) and started to progress at an earlier stage in the ixr1Δ background (Fig 11). We think of two hypotheses for the observation. (a) Puf5 and Ixr1 are involved in pheromone recovery, since we synchronized the cell cycle using pheromone-induced cell cycle arrest. (b) Puf5 and Ixr1 generally regulate the cell cycle progression. Further analyses are needed for the detailed mechanism. Previous study has revealed that Ixr1 positively regulates the expression of RNR1 [48]. However, from our data, the RNR1 expression was induced earlier in the ixr1Δ mutant, and the ixr1Δ mutation did not affect the extent of the peak expression. These data are not consistent with the previous report [48]. The difference of the yeast strains may result in this inconsistency.
Puf5 and its target Ixr1 regulate the CLB1 expression specifically in G2/M phase. Therefore, there should be the regulatory machinery of the cell cycle-depending activity of Puf5 and Ixr1. However, the Puf5 and Ixr1 mRNA levels were invariable through the cell cycle (Figs 12 and 13). Similarly, our western blot data examining Puf5 or Ixr1 protein levels during the cell cycle showed plural bands of each protein (S5A–S5D Fig). Therefore, cell cycle-regulated modification, such as phosphorylation, of Puf5 and Ixr1 protein may determine their phase-specific activity. Further analyses are required to explore the mechanism of how the activities of Puf5 and Ixr1 are rhythmically regulated and how the rhythmicity influences the CLB1 expression.
The cell cycle-dependent transcription of CLB1/CLB2 has been reported to be regulated by the periodic activity of the Mcm1-Fkh2-Ndd1 activator complex [18,19,36,46]. Even though the importance of the transcriptional activators has been reported, little has been reported about the contribution of a transcriptional repressor to the regulation of the G2/M phase transcription. Our data first revealed that Ixr1 transcriptionally represses the cell cycle-specific expression of CLB1, although the detailed mechanism of the repression is unclear. Here we identified Puf5 and Ixr1 as cell cycle regulators. Ixr1 represses the G2/M phase-specific expression of CLB1, and Puf5 positively regulates the expression through the negative regulation of the IXR1 expression. This machinery ensures the appropriate expression of CLB1 and sequentially contributes to the proper cell cycle progression. Regarding how this regulation administers to the cell cycle regulation, we consider that this regulation is involved in the G2/M phase checkpoint. In S. cerevisiae, DNA damage and DNA replication failure provoke the G2/M phase checkpoint [49]. IXR1 gene has been reported to interact with RAD9 gene encoding an adaptor protein involved in DNA damage checkpoint [50]. Moreover, the ixr1Δ mutation improves cell growth when replication stress is induced [51]. Ixr1 indirectly positively regulates the dNTP pool [48], and the decreased concentration of dNTP accommodates cells to the replication stressed condition [51]. Taking together, Ixr1 may be a downstream factor of the DNA damage or DNA replication checkpoint. If this hypothesis is true, the activity of Ixr1 controlled by the checkpoint alters the amount of CLB1 transcripts, leading to the appropriate cell division. In humans, HMGB proteins also play a role in DNA repair, and increased expression of HMGB1, one type of HMGB protein, is associated with tumor progression or metastasis [52], suggesting that HMGB proteins are closely related to maintaining ordered cell proliferation.
Insights into RNA regulatory network
In the regulation of cell cycle progression, the importance of the post-transcriptional regulation is less investigated. Previously, RNA-binding protein Whi3 has been reported to interact with CLN3 mRNA [53,54], and, more recently, we have shown that Ccr4, a catalytic subunit of Ccr4-Not complex, has been found to destabilize CLB6 mRNA [55]. However, it has been unclear whether RNA-binding proteins contribute to the regulation of the expression of B-type cyclins. In this study, we found that Puf5-binding to the 3´ UTR of IXR1 mRNA leads to the negative regulation of IXR1 expression, which results in the positive regulation of CLB1 expression and control of the cell cycle progression. These findings indicate the significance of the RNA regulation upon cell cycle control. In other species, such as mouse, Xenopus, and zebrafish, PUF family protein Pum1 directly binds to cyclin B1 mRNA and negatively regulates the translation of cyclinB1 in oocytes [56–59]. Contrarily, in S. cerevisiae, Puf5 indirectly regulates the expression of CLB1, a cyclin B gene, via the regulation of the expression of IXR1. This difference shows a variety of the function of PUF family proteins acquired during evolution. Puf5 functions in tolerating various stress, such as replication stress, weakened cell wall, and high temperature, and positively regulates life span [11–15]. Such pleiotropically functioning Puf5 controls the appropriate CLB1 expression level at the G2/M phase or upon DNA damage, suggesting that Puf5 controls the cell cycle progression in a proper state for the circumstances. This regulation may enhance the fitness of cells and prolong the life span.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(A) The mRNA levels of ADH1 in wild-type, the puf5Δ mutant, the ixr1Δ mutant, and the puf5Δ ixr1Δ double mutant. The cells were cultured in a YPD medium at 28°C until the exponential phase. The ADH1 mRNA levels were quantified by qRT-PCR analysis, and the relative mRNA levels were calculated using the ACT1 reference gene. The data shows the mean ± SE (n = 3) of the fold change of ADH1 mRNA relative to the mRNA level in wild-type. *P < 0.05, **P < 0.01 as determined by Tukey’s test. (B) The mRNA levels of CLB2 in wild-type, the puf5Δ mutant, the ixr1Δ mutant, and the puf5Δ ixr1Δ double mutant. The cells were cultured in a YPD medium at 28°C until the exponential phase. The CLB2 mRNA levels were quantified by qRT-PCR analysis, and the relative mRNA levels were calculated using the SCR1 reference gene. The data shows the mean ± SE (n = 3) of the fold change of CLB2 mRNA relative to the mRNA level in wild-type. *P < 0.05, **P < 0.01 as determined by Tukey’s test.
(TIFF)
(A) The tetrad analysis of the strains that are heterozygous for the alleles of PUF5, CLB2, FKH1, and LRG1 (A), PUF5, CLB2, FKH2, and LRG1 (B), PUF5, CLB2, HFI1, and LRG1 (C), PUF5, CLB2, and HIR1 (D), PUF5, CLB2, STE12, and LRG1 (E). The cells were sporulated, dissected on a YPD plate containing 10% sorbitol, and cultured at 30°C for 3 days.
(TIFF)
The effect of the ixr1Δ mutation and the lrg1Δ mutation on cell growth at 37°C. The sets of the strains obtained from the tetrad analysis shown in Fig 5B were picked on a YPD plate containing 10% sorbitol and incubated at 25°C for 1 day. Then, the plate was replicated to a YPD plate and incubated at 37°C for 1 day.
(TIFF)
The tetrad analysis of the strains that are heterozygous for the alleles of IXR1, FKH1, and FKH2. The cells were sporulated, dissected on a YPD plate containing 10% sorbitol, and cultured at 30°C for 3 days.
(TIFF)
(A) The Puf5 protein level in the synchronized bar1Δ cell integrated PUF5-Myc-ADH1 3´ UTR gene. No tag sample was loaded as a negative control. The arrowheads show two different bands of Puf5-Myc protein observed. Band 1 and band 2 were quantified and normalized with the Pgk1 protein level. Fold change is presented in Fig 12B. (B, C) The Ixr1 protein level in the synchronized bar1Δ cell (B) and bar1Δ puf5Δ mutant (C) harboring the YCplac33-IXR1-HA-IXR1 3´ UTR plasmid. No tag sample was loaded as a negative control. The arrowheads show three different bands of Ixr1-HA protein observed. Band 1 and band 2 were quantified and normalized with the Pgk1 protein level. Fold change is presented in Fig 13C. (D, E) The comparison of the Ixr1 protein level (D) and quantitative analysis data of Ixr1-HA protein (E) between the synchronized bar1Δ cell and bar1Δ puf5Δ mutant harboring the YCplac33-IXR1-HA-IXR1 3´ UTR plasmid. 100 min samples of each strain were loaded. The arrowheads show three different bands of Ixr1-HA protein observed. Band 1 and band 2 were quantified and normalized with the Pgk1 protein level. The data shows the fold change of Ixr1-HA protein relative to the protein level in the bar1Δ cell (E).
(TIFF)
Acknowledgments
We thank all the members of the Molecular Cell Biology Laboratory for valuable discussions.
Data Availability
Microarray data sets are available at the Gene Expression Omnibus at http://www.ncbi.nlm.nih.gov/geo (GEO accession number GSE124908).
Funding Statement
This research was supported by JSPS KAKENHI Grant Number 18K06053 and 22K06074 (to KI). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Parker R. RNA degradation in Saccharomyces cerevisae. Genetics. 2012; 191: 671–702. doi: 10.1534/genetics.111.137265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchell SF, Parker R. Principles and properties of eukaryotic mRNPs. Mol Cell. 2014; 54: 547–558. doi: 10.1016/j.molcel.2014.04.033 [DOI] [PubMed] [Google Scholar]
- 3.Wang M, Ogé L, Perez-Garcia MD, Hamama L, Sakr S. The PUF Protein Family: Overview on PUF RNA Targets, Biological Functions, and Post Transcriptional Regulation. Int J Mol Sci. 2018; 19: 410. doi: 10.3390/ijms19020410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogan DJ, Riordan DP, Gerber AP, Herschlag D, Brown PO. Diverse RNA-binding proteins interact with functionally related sets of RNAs, suggesting an extensive regulatory system. PLoS Biol. 2008; 6: e255. doi: 10.1371/journal.pbio.0060255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wickens M, Bernstein DS, Kimble J, Parker R. RNA. Trends Genet. 2002; 18: 150–157. doi: 10.1016/s0168-9525(01)02616-6 [DOI] [PubMed] [Google Scholar]
- 6.Goldstrohm AC, Hook BA, Seay DJ, Wickens M. PUF proteins bind Pop2p to regulate messenger RNAs. Nat Struct Mol Biol. 2006; 13: 533–539. doi: 10.1038/nsmb1100 [DOI] [PubMed] [Google Scholar]
- 7.Goldstrohm AC, Seay DJ, Hook BA, Wickens M. PUF protein-mediated deadenylation is catalyzed by Ccr4p. J Biol Chem. 2007; 282: 109–114. doi: 10.1074/jbc.M609413200 [DOI] [PubMed] [Google Scholar]
- 8.Miller JE, Zhang L, Jiang H, Li Y, Pugh BF, Reese JC. Genome-Wide Mapping of Decay Factor-mRNA Interactions in Yeast Identifies Nutrient-Responsive Transcripts as Targets of the Deadenylase Ccr4. G3 (Bethesda). 2018; 8: 315–330. doi: 10.1534/g3.117.300415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapointe CP, Preston MA, Wilinski D, Saunders HAJ, Campbell ZT, Wickens M. Architecture and dynamics of overlapped RNA regulatory networks. RNA. 2017; 23: 1636–1647. doi: 10.1261/rna.062687.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tadauchi T, Matsumoto K, Herskowitz I, Irie K. Post-transcriptional regulation through the HO 3’-UTR by Mpt5, a yeast homolog of Pumilio and FBF. EMBO J. 2001; 20: 552–561. doi: 10.1093/emboj/20.3.552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viet NTM, Duy DL, Saito K, Irie K, Suda Y, Mizuno T, Irie K. Regulation of LRG1 expression by RNA-binding protein Puf5 in the budding yeast cell wall integrity pathway. Genes Cells, 2018; 23: 988–997. doi: 10.1111/gtc.12646 [DOI] [PubMed] [Google Scholar]
- 12.Duy DL, Suda Y, Irie K. Cytoplasmic deadenylase Ccr4 is required for translational repression of LRG1 mRNA in the stationary phase. PLoS One. 2017; 12: e0172476. doi: 10.1371/journal.pone.0172476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito W, Li X, Irie K, Mizuno T, Irie K. RNA-binding protein Khd1 and Ccr4 deadenylase play overlapping roles in the cell wall integrity pathway in Saccharomyces cerevisiae. Eukaryot Cell. 2011; 10: 1340–1347. doi: 10.1128/EC.05181-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaeberlein M, Guarente L. Saccharomyces cerevisiae MPT5 and SSD1 function in parallel pathways to promote cell wall integrity. Genetics. 2002; 160: 83–95. doi: 10.1093/genetics/160.1.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart MS, Krause SA, McGhie J, Gray JV. Mpt5p, a stress tolerance- and lifespan-promoting PUF protein in Saccharomyces cerevisiae, acts upstream of the cell wall integrity pathway. Eukaryot Cell. 2007; 6: 262–270. doi: 10.1128/EC.00188-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendenhall MD, Hodge AE. Regulation of Cdc28 cyclin-dependent protein kinase activity during the cell cycle of the yeast Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1998; 62: 1191–1243. doi: 10.1128/MMBR.62.4.1191-1243.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Futcher B. Cyclins in meiosis: lost in translation. Dev Cell. 2008; 14: 644–645. doi: 10.1016/j.devcel.2008.04.009 [DOI] [PubMed] [Google Scholar]
- 18.Breeden LL. Cyclin transcription: Timing is everything. Curr Biol. 2000; 10: R586–R588. doi: 10.1016/s0960-9822(00)00634-5 [DOI] [PubMed] [Google Scholar]
- 19.Reynolds D, Shi BJ, McLean C, Katsis F, Kemp B, Dalton S. Recruitment of Thr 319-phosphorylated Ndd1p to the FHA domain of Fkh2p requires Clbkinase activity: a mechanism for CLB cluster gene activation. Genes Dev. 2003; 17: 1789–1802. doi: 10.1101/gad.1074103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams A, Gottschling DE, Kaiser CA, Stearn T. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 1997. [Google Scholar]
- 21.Longtine MS, Mckenzie A III, Demarini DJ, Shah NG, Wach A, Brachat A, et al. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998; 14: 953–961. doi: [DOI] [PubMed] [Google Scholar]
- 22.Sakumoto N, Mukai Y, Uchida K, Kouchi T, Kuwajima J, Nakagawa Y, et al. A series of protein phosphatase gene disruptants in Saccharomyces cerevisiae. Yeast. 1999; 15: 1669–1679. doi: [DOI] [PubMed] [Google Scholar]
- 23.Baudin A, Ozier-Kalogeropoulos O, Denouel A, Lacroute F, Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993; 21: 3329–3330. doi: 10.1093/nar/21.14.3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider BL, Steiner B, Seufert W, Futcher AB. pMPY-ZAP: a reusable polymerase chain reaction-directed gene disruption cassette for Saccharomyces cerevisiae. Yeast. 1996; 12:129–134. doi: [DOI] [PubMed] [Google Scholar]
- 25.Sekiya-Kawasaki M, Abe M, Saka A, et al. Dissection of upstream regulatory components of the Rho1p effector, 1,3-beta-glucan synthase, in Saccharomyces cerevisiae. Genetics. 2002; 162: 663–676. doi: 10.1093/genetics/162.2.663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kushnirov VV. Rapid and reliable protein extraction from yeast. Yeast. 2000; 16: 857–860. doi: [DOI] [PubMed] [Google Scholar]
- 27.Futcher B. Cell cycle synchronization. Methods Cell Sci. 1999; 21: 79–86. doi: 10.1023/a:1009872403440 [DOI] [PubMed] [Google Scholar]
- 28.Yasutis K, Vignali M, Ryder M, et al. Zds2p regulates Swe1p-dependent polarized cell growth in Saccharomyces cerevisiae via a novel Cdc55p interaction domain. Mol Biol Cell. 2010; 21: 4373–4386. doi: 10.1091/mbc.E10-04-0326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen JM, Wanless AG, Seidel CW, Weiss EL. Cbk1 regulation of the RNA-binding protein Ssd1 integrates cell fate with translational control. Curr Biol. 2009; 19: 2114–2120. doi: 10.1016/j.cub.2009.10.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richardson H, Lew DJ, Henze M, Sugimoto K, Reed SI. Cyclin-B homologs in Saccharomyces cerevisiae function in S phase and in G2. Genes Dev. 1992; 6: 2021–2034. doi: 10.1101/gad.6.11.2021 [DOI] [PubMed] [Google Scholar]
- 31.Bourens M, Panozzo C, Nowacka A, Imbeaud S, Mucchielli MH, Herbert CJ. Mutations in the Saccharomyces cerevisiae kinase Cbk1p lead to a fertility defect that can be suppressed by the absence of Brr1p or Mpt5p (Puf5p), proteins involved in RNA metabolism. Genetics. 2009; 183: 161–173. doi: 10.1534/genetics.109.105130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valderrama AL, Fujii S, Duy DL, Irie K, Mizuno T, Suda Y, Irie K. Pbp1 mediates the aberrant expression of genes involved in growth defect of ccr4Δ and pop2Δ mutants in yeast Saccharomyces cerevisiae. Genes Cells. 2021; 26: 381–398. doi: 10.1111/gtc.12846 [DOI] [PubMed] [Google Scholar]
- 33.Surana U, Robitsch H, Price C, Schuster T, Fitch I, Futcher AB, Nasmyth K. The role of CDC28 and cyclins during mitosis in the budding yeast S. cerevisiae. Cell. 1991; 65:145–161. doi: 10.1016/0092-8674(91)90416-v [DOI] [PubMed] [Google Scholar]
- 34.Lambert JR, Bilanchone VW, Cumsky MG. The ORD1 gene encodes a transcription factor involved in oxygen regulation and is identical to IXR1, a gene that confers cisplatin sensitivity to Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1994; 91: 7345–7349. doi: 10.1073/pnas.91.15.7345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vizoso-Vázquez A, Lamas-Maceiras M, Fernández-Leiro R, Rico-Díaz A, Becerra M, Cerdán ME. Dual function of Ixr1 in transcriptional regulation and recognition of cisplatin-DNA adducts is caused by differential binding through its two HMG-boxes. Biochim Biophys Acta Gene Regul Mech. 2017; 1860: 256–269. doi: 10.1016/j.bbagrm.2016.11.005 [DOI] [PubMed] [Google Scholar]
- 36.Jorgensen P, Tyers M. The fork’ed path to mitosis. Genome Biol. 2000; 1: REVIEWS1022. doi: 10.1186/gb-2000-1-3-reviews1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horiuchi J, Silverman N, Piña B, Marcus GA, Guarente L. ADA1, a novel component of the ADA/GCN5 complex, has broader effects than GCN5, ADA2, or ADA3. Mol Cell Biol. 1997;17: 3220–3228. doi: 10.1128/MCB.17.6.3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherwood PW, Tsang SV, Osley MA. Characterization of HIR1 and HIR2, two genes required for regulation of histone gene transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1993; 13: 28–38. doi: 10.1128/mcb.13.1.28-38.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tedford K, Kim S, Sa D, Stevens K, Tyers M. Regulation of the mating pheromone and invasive growth responses in yeast by two MAP kinase substrates. Curr Biol. 1997; 7: 228–238. doi: 10.1016/s0960-9822(06)00118-7 [DOI] [PubMed] [Google Scholar]
- 40.Brown SJ, Kellett PJ, Lippard SJ. Ixr1, a yeast protein that binds to platinated DNA and confers sensitivity to cisplatin. Science. 1993; 261: 603–605. doi: 10.1126/science.8342024 [DOI] [PubMed] [Google Scholar]
- 41.Chow CS, Whitehead JP, Lippard SJ. HMG domain proteins induce sharp bends in cisplatin-modified DNA. Biochemistry. 1994; 33: 15124–15130. doi: 10.1021/bi00254a023 [DOI] [PubMed] [Google Scholar]
- 42.Vizoso-Vázquez A, Lamas-Maceiras M, Becerra M, González-Siso MI, Rodríguez-Belmonte E, Cerdán ME. Ixr1p and the control of the Saccharomyces cerevisiae hypoxic response. Appl Microbiol Biotechnol. 2012; 94: 173–184. doi: 10.1007/s00253-011-3785-2 [DOI] [PubMed] [Google Scholar]
- 43.Vizoso-Vázquez Á, Lamas-Maceiras M, González-Siso MI, Cerdán ME. Ixr1 Regulates Ribosomal Gene Transcription and Yeast Response to Cisplatin. Sci Rep. 2018; 8: 3090. doi: 10.1038/s41598-018-21439-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pic A, Lim FL, Ross SJ, Veal EA, Johnson AL, Sultan MR, et al. The forkhead protein Fkh2 is a component of the yeast cell cycle transcription factor SFF. EMBO J. 2000; 19: 3750–3761. doi: 10.1093/emboj/19.14.3750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barreiro-Alonso A, Lamas-Maceiras M, Cerdán EM, Vizoso-Vázquez Á. The HMGB protein Ixr1 interacts with Ssn8 and Tdh3 involved in transcriptional regulation. FEMS Yeast Res. 2018; 18: foy013. doi: 10.1093/femsyr/foy013 [DOI] [PubMed] [Google Scholar]
- 46.Darieva Z, Pic-Taylor A, Boros J, Spanos A, Geymonat M, Reece RJ, et al. Cell cycle-regulated transcription through the FHA domain of Fkh2p and the coactivator Ndd1p. Curr Biol. 2003; 13: 1740–1745. doi: 10.1016/j.cub.2003.08.053 [DOI] [PubMed] [Google Scholar]
- 47.Chritton JJ, Wickens M. Translational repression by PUF proteins in vitro. RNA. 2010; 16: 1217–1225. doi: 10.1261/rna.2070110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsaponina O, Barsoum E, Aström SU, Chabes A. Ixr1 is required for the expression of the ribonucleotide reductase Rnr1 and maintenance of dNTP pools. PLoS Genet. 2011; 7: e1002061. doi: 10.1371/journal.pgen.1002061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murray A. Cell cycle checkpoints. Curr Opin Cell Biol. 1994; 6: 872–876. doi: 10.1016/0955-0674(94)90059-0 [DOI] [PubMed] [Google Scholar]
- 50.Murakami-Sekimata A, Huang D, Piening BD, Bangur C, Paulovich AG. The Saccharomyces cerevisiae RAD9, RAD17 and RAD24 genes are required for suppression of mutagenic post-replicative repair during chronic DNA damage. DNA Repair (Amst). 2010; 9: 824–834. doi: 10.1016/j.dnarep.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fumasoni M, Murray AW. The evolutionary plasticity of chromosome metabolism allows adaptation to constitutive DNA replication stress. Elife. 2020; 9: e51963. doi: 10.7554/eLife.51963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005; 5: 331–342. doi: 10.1038/nri1594 [DOI] [PubMed] [Google Scholar]
- 53.Garí E, Volpe T, Wang H, Gallego C, Futcher B, Aldea M. Whi3 binds the mRNA of the G1 cyclin CLN3 to modulate cell fate in budding yeast. Genes Dev. 2001; 15: 2803–2808. doi: 10.1101/gad.203501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai Y, Futcher B. Effects of the yeast RNA-binding protein Whi3 on the half-life and abundance of CLN3 mRNA and other targets. PLoS One. 2013; 8: e84630. doi: 10.1371/journal.pone.0084630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Revilleza JEC, Sato M, Irie K, Suda Y, Mizuno T, Irie K. Regulation of CLB6 expression by the cytoplasmic deadenylase Ccr4 through its coding and 3’ UTR regions. PLoS One. 2022; 17: e0268283. doi: 10.1371/journal.pone.0268283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takei N, Takada Y, Kawamura S, Sato K, Saitoh A, Bormann J, et al. Changes in subcellular structures and states of pumilio 1 regulate the translation of target Mad2 and cyclin B1 mRNAs. J Cell Sci. 2020; 133: jcs249128. doi: 10.1242/jcs.249128 [DOI] [PubMed] [Google Scholar]
- 57.Piqué M, López JM, Foissac S, Guigó R, Méndez R. A Combinatorial Code for CPE-Mediated Translational Control. Cell. 2008: 132; 434–448. doi: 10.1016/j.cell.2007.12.038 [DOI] [PubMed] [Google Scholar]
- 58.Ota R, Kotani T, Yamashita M. Biochemical characterization of Pumilio1 and Pumilio2 in Xenopus oocytes. J Biol Chem. 2011; 286: 2853–2863. doi: 10.1074/jbc.M110.155523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kotani T, Yasuda K, Ota R, Yamashita M. Cyclin B1 mRNA translation is temporally controlled through formation and disassembly of RNA granules. J Cell Biol. 2013; 202: 1041–1055. doi: 10.1083/jcb.201302139 [DOI] [PMC free article] [PubMed] [Google Scholar]