Abstract
Sudden unexpected death in epilepsy (SUDEP) is the leading cause of epilepsy-related death. SUDEP typically happens during the night following a seizure. Many aspects of mammalian physiology are regulated by circadian rhythms in ways that might make seizures that occur during the night more dangerous. Using two mouse models of seizure-induced death we demonstrate that time-of-day and circadian rhythms alter vulnerability to seizure-induced death. We exposed normally entrained DBA/1 mice to a potentially seizure-inducing acoustic stimulus at different times of day and compared the characteristics and outcomes of the seizures. Time-of-day did not alter the likelihood of a seizure, but it did alter the likelihood of seizure-induced death. To determine whether circadian rhythms alter vulnerability to seizure-induced death we induced maximal electroshock seizures in free-running C57BL/6J mice at different circadian time points while measuring breathing via whole body plethysmography. Circadian phase did not affect seizure severity, but it did alter postictal respiratory outcomes and the likelihood of seizure-induced death. Contrary to our expectations, in entrained and free-running mice, vulnerability to seizure-induced death was greatest during the night and subjective night, respectively. These findings suggest that circadian rhythmicity may be responsible for the increased nighttime prevalence of SUDEP and that the underlying mechanism is phase conserved between nocturnal and diurnal mammals. All seizures in this investigation were induced during wakefulness indicating that the effect of time point on vulnerability to seizure-induced death was not the result of sleep. Understanding why SUDEP happens more during the night may inform future preventative countermeasures.
Keywords: circadian rhythms, seizures, mortality, SUDEP
Introduction
The epilepsies are a heterogenous array of diseases characterized by spontaneous seizures (Fisher et al., 2014). The leading cause of premature death in epilepsy patients is sudden unexpected death in epilepsy (SUDEP; Thurman et al., 2014). SUDEP results in more years of potential life lost than any other neurological condition with the exception of stroke (Thurman et al., 2014). There are currently no means by which SUDEP can be reliably predicted or prevented (Massey et al., 2014). Convergent lines of evidence from epilepsy patients and animal models suggest that SUDEP is the result of some amalgamation of seizure-induced respiratory, cardiac, autonomic, and electrocerebral dysfunction (Ryvlin et al., 2013; Massey et al., 2014). Unfortunately, why some seizures result in death when so many others do not remains unclear.
It has been consistently observed that SUDEP happens more during the night. Meta-analyses of definite, probable, and possible SUDEP report that a majority of SUDEP cases occur during the night, presumably during sleep (Ali et al., 2017). More selective studies of only definite, autopsy-confirmed SUDEP cases also suggest that SUDEP occurs more frequently at night (Lamberts et al., 2012). Patients with nocturnal seizures have been identified as being at an increased risk of SUDEP (Lamberts et al., 2012; Harden et al., 2017). Most cases of SUDEP occur while the patient is alone making it difficult to determine the exact time of death (Sveinsson et al., 2018). Potentially the most accurate data on the timing of SUDEP comes from cases in which the patient was under observation in an epilepsy monitoring unit at the time of the fatal seizure. A case series of SUDEP occurring in epilepsy monitoring units reported that 14 of 16 deaths occurred during the night (Ryvlin et al., 2013). Additionally, death is more common during the night in rodent models of epilepsy with spontaneous seizure-induced death (Moore et al., 2014; Teran et al., 2019). Why SUDEP happens more during the night is unknown (Purnell et al., 2018).
Circadian rhythms influence the temporal distribution of seizures in both human epilepsy patients and animal models (Van Luijtelaar and Coenen, 1988; Quigg, 2000). Daily patterns in seizure frequency vary depending on the location of the patients seizure focus (Durazzo et al., 2008; Spencer et al., 2016). The use of long term intercranial monitoring has provided additional evidence suggesting that circadian rhythms alter the distribution of seizures (Duckrow and Tcheng, 2007; Baud et al., 2018). Additionally, acute seizures can elicit circadian phase shifts and chronic models of epilepsy display circadian disruption (Quigg et al., 2001; Kalume et al., 2015). Whether the effect of circadian rhythms on seizure frequency or the deleterious effect of seizures on circadian rhythms is meaningful to SUDEP etiology is unclear
Circadian rhythms alter aspects of physiology in ways that might influence SUDEP vulnerability. Cardiac dysfunction has been implicated in SUDEP and a prolonged QT interval has been proposed as a biomarker of SUDEP vulnerability (Tavernor et al., 1996). QT interval is altered by circadian rhythms and tends to be longer during the night (Ishida et al., 1997). In cases of SUDEP in which simultaneous EKG and breathing data are available the primary cause of death appears to be respiratory arrest (Ryvlin et al., 2013). Breathing, at baseline and in response to challenges such as increased CO2, is altered by circadian rhythms (Stephenson et al., 2000; Purnell and Buchanan, 2020). Seizures disrupt autonomic tone, often causing rapid swings in sympathetic/parasympathetic balance and potentially facilitating SUDEP (Poh et al., 2012). Autonomic tone is subject to circadian regulation as indicated by changes in heart rate variability (Huikuri et al., 1994).
The goal of this study was to test the hypothesis that circadian rhythms alter vulnerability to seizure-induced death. Using two models of seizure-induced death we demonstrate empirically that vulnerability to seizure-induced death is altered by time-of-day and circadian phase. In this study, seizures were induced during wakefulness, indicating that the effect of time point on vulnerability to seizure-induced death was not the result of differing sleep states of seizure origin. These findings suggest that circadian rhythms may contribute to the increased nighttime incidence of SUDEP.
Materials and Methods
Ethical Approval
All procedures and protocols used in this study were approved by the University of Iowa Institutional Animal Care and Use Committee (IACUC) in accordance with the international guidelines set by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Care was taken to use the minimum number of animals possible and to minimize their pain and distress. The methods used in this study and the manner in which the results are reported are in compliance with the principles and standards outlined by the Journal of Physiology (Grundy, 2015).
Animals.
DBA/1 mice used in this study were bred on-site in a University of Iowa Office of Animal Resources vivarium. DBA/1 mice were housed under normal 12:12 LD conditions (lights on at 0600, lights off at 1800). DBA/1 animals were weaned at postnatal day 21 and experimented on between postnatal day 23–30. The C57BL/6J mice used in this study were acquired from Jackson Labs at 7–8 weeks old (000664; Bar Harbor, ME). C57BL/6J mice were initially housed under normal 12:12 LD conditions followed by constant darkness as detailed in the ‘wheel-running behavior’ methods subsection. All animals (DBA/1 and C57BL/6J) were singly housed and had ad libitum access to food (NIH-31 Mouse Diet) and water. Animals used in this study that did not experience seizure-induced death were euthanized with CO2 exposure. Briefly, 100% CO2 was passed into the animal’s home cage (2 L/min). CO2 exposure was sustained until at least one minute after the animal stopped breathing. Death was confirmed with cervical dislocation.
Experimental design.
Experiment 1. Determine whether time-of-day alters vulnerability to seizure-induced death.
DBA/1 mice were exposed to a potentially seizure-inducing acoustic stimulus during wakefulness at one of eight times of day (ZT 0, 2, 6, 10, 12, 14, 18, and 22; n = 15 per time point). Animals were monitored by video during seizure trials. The following variables were quantified for each seizure trail: (1) the occurrence of wild running seizure activity, (2) mortality, (3) latency to onset of wild running seizure activity, (4) latency to seizure-induced death, (5) number of wild running episodes, (6) duration of wild running seizure activity, and (7) time spent in wild running seizure activity or undergoing seizure-induced death. Our hypothesis was that the likelihood of seizure-induced death is altered by time-of-day with greatest vulnerability during the day (ZT 2, 6, and 10). Male and female mice were included in this experiment. Further methodological details for this experiment are provided in the ‘DBA/1 audiogenic seizures’ methods subsection.
Experiment 2. Determine whether circadian phase alters vulnerability to seizure-induced death.
Maximal electroshock (MES) seizures were induced in C57BL/6J mice kept in free-running conditions during wakefulness at one of six circadian phases (CT 2, 6, 10, 14, 18, and 22; n = 9 per time point). Animals were monitored by video and whole body plethysmography during seizure trials. The following variables were quantified for each seizure trail: (1) mortality, (2) motor seizure severity, (3) first apnea duration, (4) total apnea duration, (5) largest postictal breath volume, and (6) postictal respiratory rate, tidal volume, and ventilation. Plethysmography data was analyzed for the 300 s following seizure induction. Respiratory parameters are plotted as change from baseline (60 s prior to the seizure). Our hypothesis was that seizure-induced respiratory changes and the likelihood of seizure-induced death are altered by circadian phase with greatest mortality during the subjective day (CT 2, 6, and 10). Only male mice were included in this experiment. Further methodological details for this experiment are provided in the ‘maximal electroshock seizures’, ‘wheel-running behavior’, and ‘whole body plethysmography’ methods subsections.
The model of seizure-induced death was switched from DBA/1 audiogenic seizures in the time-of-day experiments to MES in C57BL/6J mice for the circadian free-running experiments. Vulnerability of DBA/1 mice to seizure-induced death decreases with age (Faingold and Randall, 2013). Animals are typically allowed to free-run for ≥ 14 days in constant conditions prior to experimentation, during this time the animals vulnerability to seizure-induced death might decrease to a degree which would adversely impact the experimental design. Furthermore, young DBA/1 mice tend to be small (Martin et al., 2020). It was doubtful that 23–30 day old DBA/1 mice would be able to reliably operate running a running wheel.
Statistical analyses.
Circadian rhythms in non-dichotomous variables (e.g. apnea duration, latency to wild running) were assessed by the cosinor method (Refinetti et al., 2007) using GraphPad Prism (Version 7; GraphPad Software Inc., La Jolla, CA). Briefly, a cosine function which best fit the time series data was generated using the least squares method. If the 95% confidence bands of the fitted cosine function did not overlap the midline estimating statistic of rhythm (MESOR), then a statistically significant oscillation could be inferred with a p < 0.05 (Refinetti et al., 2007). Circadian rhythms in dichotomous variables (e.g. wild running, mortality) were assessed using a Hodges-Ajne test of circular non-uniformity (Zar, 1999). The Hodges-Ajne test was employed in these cases instead of cosinor analysis due to its compatibility with dichotomous variables. Furthermore, the Hodges-Ajne test works well with non-unimodal distributions which was suspected in this case in light of previous evidence (Moore et al., 2014; Teran et al., 2019). All data scoring and analysis was conducted blind to experimental condition.
Whenever possible, data is plotted as mean with standard deviation. All ‘n’ values indicate the number of animals used in that group. All time-of-day or circadian phase data is plotted as a traditional time series graph and as a rose plot. While it is easier to compare across different time points with a tradition time series graph it can mislead the viewer due to the physical separation between plotted points not corresponding to their distance in time. For example, on a traditional time series graph ZT 0 appears to be much closer to ZT 2 than ZT 22 despite the fact that they are temporally equidistant. Wheel-running behavior.
Mice were singly housed so that wheel-running behavior could be monitored for each animal individually. The home cage of each animal (Maximiser #9; Thoren Caging Systems, Hazelton, PA) contained a running wheel (CC01A02EXERNC; Thoren Caging Systems). A magnet (5 mm dia.; 0.75 mm thick; neodymium; K & J Magnetics, Plumsteadville, PA) was mounted to the spoke of the running wheel. With each revolution of the wheel the magnet closed the circuit of a reed switch (MITI-3v1-6-12.5; Littelfuse, Schiphol, The Netherlands). Wheel revolutions, indicated by closure of the circuit, were relayed to a multiplexer (ACTI-556B; Actimetrics, Wilmette, IL) and recorded on a desktop computer running ClockLab Data Collection software (Version 3, Actimetrics).
The animals wheel-running behavior was plotted using well established methods (Jud et al., 2005). Briefly, wheel-running data for each 24 hour period was plotted as a histogram. These histograms were concatenated vertically into a column. Wheel-running activity was double-plotted to facilitate visual identification of the rhythm (each 24 hour segment of data is plotted twice, once in each vertical column).
Home cages were placed in custom-made, fan-ventilated, light-impermeable boxes (1.5 m × 48 cm × 45 cm). The inside of these boxes was outfitted with programable lights, these lights were initially set to 12:12 LD (lights on at 0600, lights off at 1800). To prevent novelty-induced phase shifts, running wheels were locked during the first 24 hours (Reebs and Mrosovsky, 1989). Animals were allowed to acclimate to 12:12 LD conditions for ≥ 14 days. By convention, the beginning of the light and dark phases were designated zeitgeber time (ZT) 0 and 12, respectively (Jud et al., 2005). When the animal’s wheel-running activity had adequately entrained to LD conditions, the animals were released into free-running conditions of constant darkness (DD). By convention, the beginning of the active phase was designated circadian time (CT) 12 (Jud et al., 2005). The mice free-ran for ≥ 14 days prior to experimentation to confirm a free-running phenotype with stable periodicity.
Whole body plethysmography.
Before MES seizure trials, animals were removed from their home cage and placed in a cast acrylic plethysmography chamber (350 cm3). The plethysmography chamber was contained in a custom-made, light-impermeable container (40 cm × 30 cm × 30 cm). If the animal was in darkness prior to the trial, the transfer from the home cage to the recording chamber was performed under dim red light (635–700 nm, < 4 lux). Compressed air (21% O2 / 79% N2; Praxair Inc., Cedar Rapids, IA) was passed through the chamber at 0.40–0.45 L/min. Two flow meters (32907-67; Cole-Parmer; Vernon Hills, IL) and a vacuum regulator (V-800-4988, Safe House) and an infrared camera (R2; Foscam, Shenzhen, China) was mounted inside the chamber. The animals behavior was recorded via video for the duration of the trial. The broadband acoustic stimulus was provided by the bell for 60 s during a period of wakefulness as assessed by real-time video examination. Seizure trials resulted in30; Airtrol Components Inc., New Berlin, WI) were used to maintain the balance of air flowing through the chamber. An ultra-low volume pressure transducer (DC002NDR5; Honeywell International Inc., Morris Plains, NJ) was connected to the chamber to record the pressure changes produced by the animal’s breaths. The signal from the pressure transducer was amplified (100×), band-pass filtered (0.3–30 Hz), digitized (1,000 samples/s; NI USB-6008; National Instruments, Austin, TX), and then recorded with custom MATLAB software on a desktop computer. Prior to experimentation, mice were implanted with a subcutaneous temperature telemeter (IPTT-300; Bio Medic Data Systems Inc., Seaford, DE). Under inhaled isoflurane anesthesia (0.5–5% in O2) a small patch of fur near the animals scruff was shaved and the skin coated in betadine. Using aseptic technique, a 12 gauge stainless steel needle was inserted under the animals skin and a plunger was used to push the temperature telemeter through the shaft of the needle and under the skin. The puncture from the needle was closed using non-absorbable sutures. Animals were allowed to recover for at least 1 week prior to experimentation.
After being placed in the plethysmography chamber a rodent mechanical ventilator (MiniVent 845; Hugo Sachs Elektronik, Grünstraße, Germany) was used to deliver 200 s of calibration air pulses (300 μL, 180/min) into the recording chamber. Respiratory frequency (fR), tidal volume (VT), and minute ventilation (VE) were determined from artifact free segments of breathing in which the animal was not sniffing or moving around the chamber. An apnea was defined as an interval between breaths of one second or more. Baseline and postictal epochs were defined as the time 60 s before and 300 s after seizure induction, respectively. VT was calculated using a barometric flow-through method (Drorbaugh and Fenn, 1955) of incorporating breath amplitude, barometric pressure (www.wunderground.com), flow rate, mouse temperature, air temperature, and the amplitude of calibration air pulses. A digital thermometer was used to assess the air temperature of the room (401014; ExTech Instruments, Nashua, NH). Room temperature ranged from 20 to 22 °C. Mouse body temperature was recorded with a subcutaneous telemeter (IPTT-300; Bio Medic Data Systems Inc.). Assessment of periictal breathing using this flow-through method of rodent whole body plethysmography has been described previously by our lab (Buchanan and Richerson, 2010).
DBA/1 audiogenic seizures.
DBA/1 mice are vulnerable to audiogenic seizures which sometimes result in fatal respiratory arrest (Faingold et al., 2010). During seizure trials, mice were placed in a custom-made, light-impermeable, sound-attenuating chamber (33.5 cm × 30.5 cm × 30.5 cm). A 93 dB bell (model 49-4988, Safe House) and an infrared camera (R2; Foscam, Shenzhen, China) was mounted inside the chamber. The animals behavior was recorded via video for the duration of the trial. The broadband acoustic stimulus was provided by the bell for 60 s during a period of wakefulness as assessed by real-time video examination. Seizure trials resulted in one of three outcomes: the animal did not experience a seizure, the animal experienced a non-fatal seizure, or the animal experienced a fatal seizure (Fig. 1A). During seizure trials during the dark phase (ZT 12 14, 18, and 22) the animal was transferred from its home cage to the experimental chamber under dim red light (635–700 nm, < 4 lux).
Figure 1. Time-of-day alters the occurrence of seizure-induced death, but not wild running in DBA/1 mice.
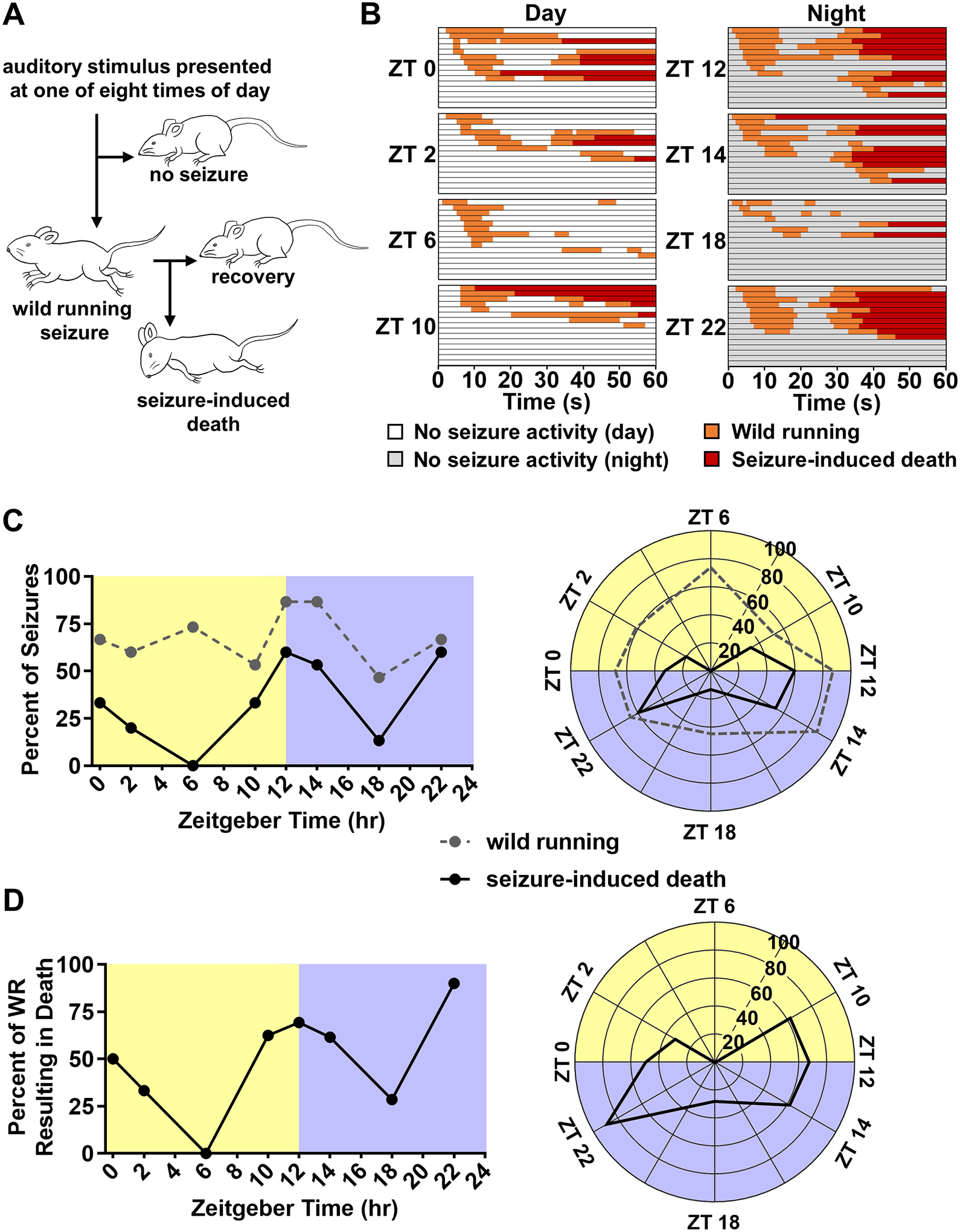
(A) Diagrammatic representation of experiment 1. DBA/1 mice were exposed to a high intensity, broadband, acoustic stimulus for 60 s at one of eight times of day (ZT 0, 2, 6, 10, 12, 14, 18, and 22; n = 15 per time point). A subset of animals exhibited seizures characterized by wild running. A subset of animals which experienced seizures then underwent seizure-induced death characterized by tonic hindlimb extension while others recovered. (B) Timelines illustrating the progression of seizure trials in DBA/1 mice. Each horizontal line corresponds to an individual trial. Each horizontal section depicts seizure trials conducted at a specific zeitgeber time (ZT, n = 15 per time point). Segments of time are color coded in relation the animals behavior: no seizure activity, white (time points in light) or gray (time points in darkness); wild running, orange; seizure-induced death, red. Within each ZT section trials are arranged in order of wild running onset. (C) Line graph (left) and rose plot (right) depicting percent of total trials resulting in wild running (dashed gray line) or seizure-induced death (solid black line) at different times of day in animals entrained to normal light-dark conditions (A, n = 15 per time point). (D) Line graph (left) and rose plot (right) depicting percent of trials which resulted in a seizure that ended in seizure-induced death (B, n = 7–13 per time point).
Maximal electroshock seizures.
C57BL6J mice were housed in wheel-running cages in LD and then DD as described in the ‘wheel-running behavior’ methods subsection. Prior to the seizure trial animals were acclimated to the experimental apparatus for at least one hour on two separate days. At the time of the seizure trial, the animal was transferred from its home cage to the plethysmography chamber within a light-impermeable container under dim red light. An infrared video camera inside of the light-impermeable container recorded the animal’s behavior within the transparent plethysmography chamber (R2, Foscam). Video and baseline breathing data was recorded for 30 minutes prior to the seizure trial. After baseline data collection the animal received a single electroshock stimulation (50 mA, 0.2 s, 60 Hz sine wave; Rodent Shocker; Harvard Apparatus, Cambridge, MA) via ear-clip electrodes wrapped with saline moistened gauze (7236K51; McMaster Carr Supply Co., Elmhurst, IL). Seizures were induced during wakefulness as assessed by live video. A subset of mice undergoing seizures induced with these parameters experience seizure-induced death (Buchanan et al., 2014; Li and Buchanan, 2019). Non-fatal MES seizures are characterized by a long initial apnea followed by a series of smaller apneas. Whole body plethysmography allowed for the quantification of these apneas. The extension-to-flexion ratio (E/F ratio) was used to assess seizure severity. E/F ratio is computed by dividing the time the hindlimbs were flexed (extended < 90° in relation to the torso) by the time that the hindlimbs were extended (projecting beyond 90° in relation to the torso). Higher E/F ratios are indicative of more widespread propagation of epileptiform activity (Anderson et al., 1986).
Results
The likelihood of seizure-induced death in DBA/1 mice depends on time-of day.
DBA/1 mice are vulnerable to audiogenic seizures which sometimes result in fatal respiratory arrest (Faingold et al., 2010). The likelihood that a seizure results in respiratory arrest increases with the number of seizures that a DBA/1 mouse has experienced (Faingold et al., 2010). The seizure-induced respiratory arrest seen in DBA/1 mice is frequently used to study the potential mechanisms of SUDEP (Faingold et al., 2011; Faingold et al., 2014; Zhang et al., 2018). Typically, DBA/1 mice are subjected to a process called ‘priming’ in the days following weaning. During the priming process the animals are exposed to the audiogenic stimulus daily until that stimulus consistently elicits seizure-induced respiratory arrest. The mice are then quickly revived by mechanical ventilation so that they can be used in subsequent priming sessions or experimental trials (Faingold et al., 2010; Zhang et al., 2018).
The mice used in this study were not primed, instead the animals were exposed to the acoustic stimulus only once and the result was recorded. Of interest in this experiment was whether the time-of-day in which a seizure was induced would alter the probability that the seizure resulted in death. Priming causes DBA/1 mice to experience seizure-induced death following acoustic stimulation almost invariably. If the animals in this study had been primed it is likely that there would have been a confounding ‘ceiling effect’ in which all mice experienced seizure-induced death regardless of time-of-day.
To determine the effect of time-of-day on vulnerability to seizure-induced death, separate groups of normally entrained DBA/1 mice were exposed to a potentially seizure-inducing acoustic stimulus at different time points. Time-of-day did not alter the distribution of wild running seizure activity as assessed by a Hodges-Ajne test (p = 0.09, n = 15 per time point; Fig. 1B–C). Probability of wild running ranged from a nadir of 46.7% (ZT 18) to 86.7% (ZT 12 and 14). Across all time points, the probability of wild running was 67.5%. During the day (ZT 0, 2, 6 and 10) the audiogenic stimulus resulted in wild running in 63.3% of trials. Conversely, during the night (ZT 12, 14, 18 and 22) the audiogenic stimulus resulted in wild running in 71.7% of trials.
Time-of-day altered the distribution of seizure-induced death as assessed by a Hodges-Ajne test (p < 0.01, n = 15 per time point; Fig. 1B–C). Probability of seizure-induced death ranged from a nadir of 0% (ZT 6) to 60% (ZT 12 and 22). Across all time points, the probability of seizure-induced death was 34.2%, this value is consistent with prior reports of seizure-induced death rates upon initial exposure to the acoustic stimulus (Faingold et al., 2010). During the day (ZT 0, 2, 6 and 10) the audiogenic stimulus resulted in seizure-induced death in 21.7% of trials. Conversely, during the night (ZT 12, 14, 18 and 22) the audiogenic stimulus resulted in seizure-induced death in 46.7% of trials.
In DBA/1 mice, wild running seizure activity is generally considered to be a prerequisite to tonic hindlimb extension and seizure-induced death. As such, it is conceivable that an alteration in the distribution of wild running could contribute to an alteration in the distribution of seizure-induced death without time-of-day exerting any direct influence on whether a wild running seizure evolved into seizure-induced death. This does not appear to be the case as there was statistically significant non-uniformity in seizure-induced death, but not wild running. Nevertheless, it may be useful to consider the percentage of trials which resulted death while excluding trials in which there was no wild running seizure activity (Fig. 1D).
Cosinor analysis did not reveal a statistically significant oscillation in any of the following variables: Latency to wild running (p = 0.34, MESOR = 13.84, R2 = 0.04, n = 7–13 per time point; Fig. 2A). Latency to seizure-induced death (p = 0.98, MESOR = 38.37, R2 < 0.01, n = 2–9 per time point; Fig. 2B). Number of wild running episodes per trial (p = 0.16, MESOR = 1.04, R2 = 0.04, n = 15 per time point; Fig. 2C). Wild running duration (p = 0.91, MESOR = 9.63, R2 < 0.01, n = 15 per time point; Fig. 2D). Total time spent wild running and in respiratory arrest (p = 0.48, MESOR = 17.02, R2 = 0.02, n = 15 per time point; Fig. 3).
Figure 2. Time-of-day does not affect latency to wild running, latency to seizure-induced death, number of wild running bouts, or wild running duration.
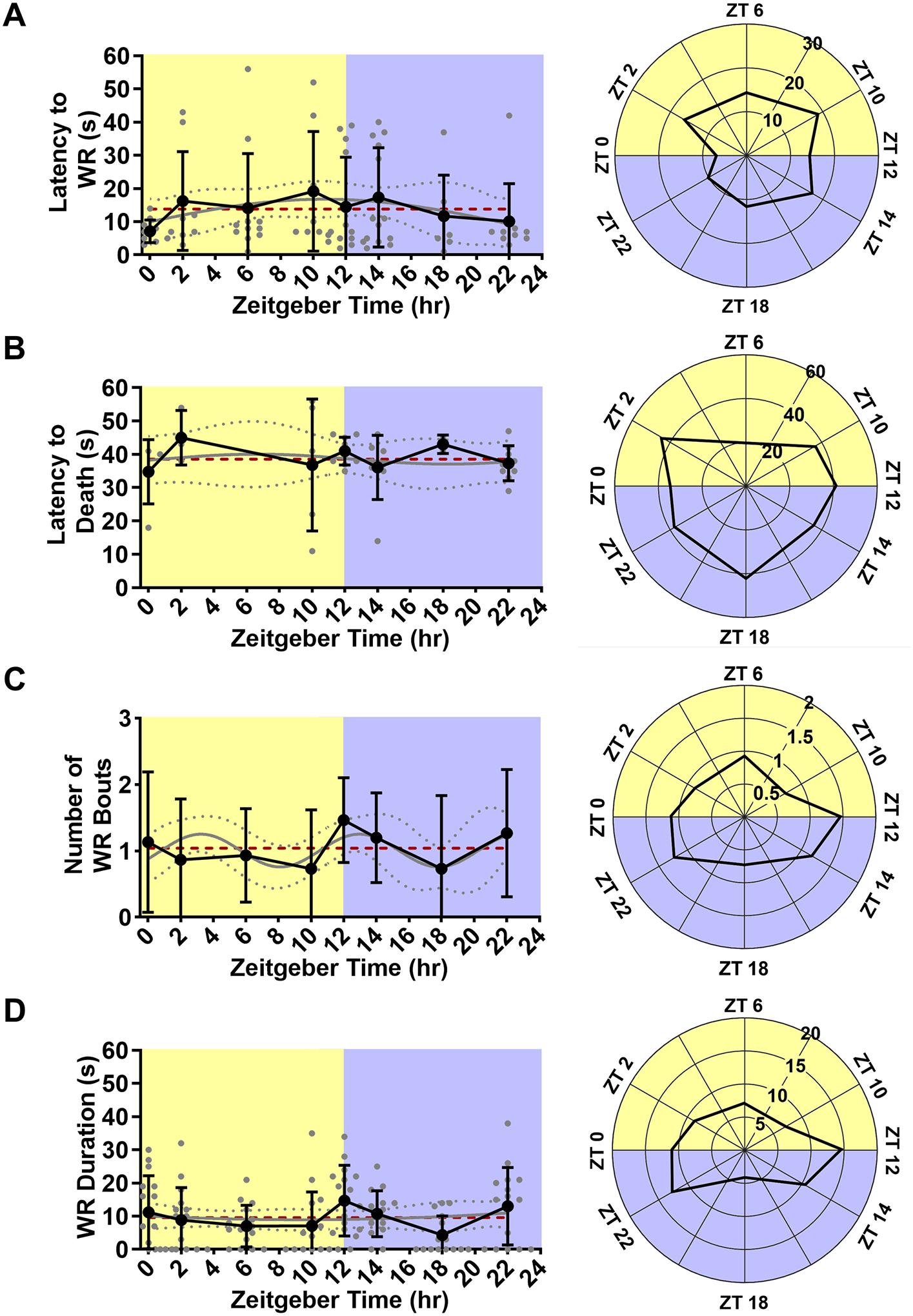
Line graph (left) and rose plot (right) depicting latency to wild running (A, n = 7–13 per time point). Line graph (left) and rose plot (right) depicting latency to seizure-induced death (B, n = 2–9 per time point). Line graph (left) and rose plot (right) depicting number of wild running episodes (C, n = 15 per time point). Line graph (left) and rose plot (right) depicting wild running duration (D, n = 15 per time point). All data is plotted as mean with standard deviation (black line), with individual values for continuous variables (grey circles). The cosinor line of best fit is depicted as a gray curve with 95% confidence intervals (dotted gray curves). The midline estimating statistic of rhythm (MESOR) is illustrated with a dashed red line.
Figure 3. Time-of-day does not affect time spent wild running and in respiratory arrest.
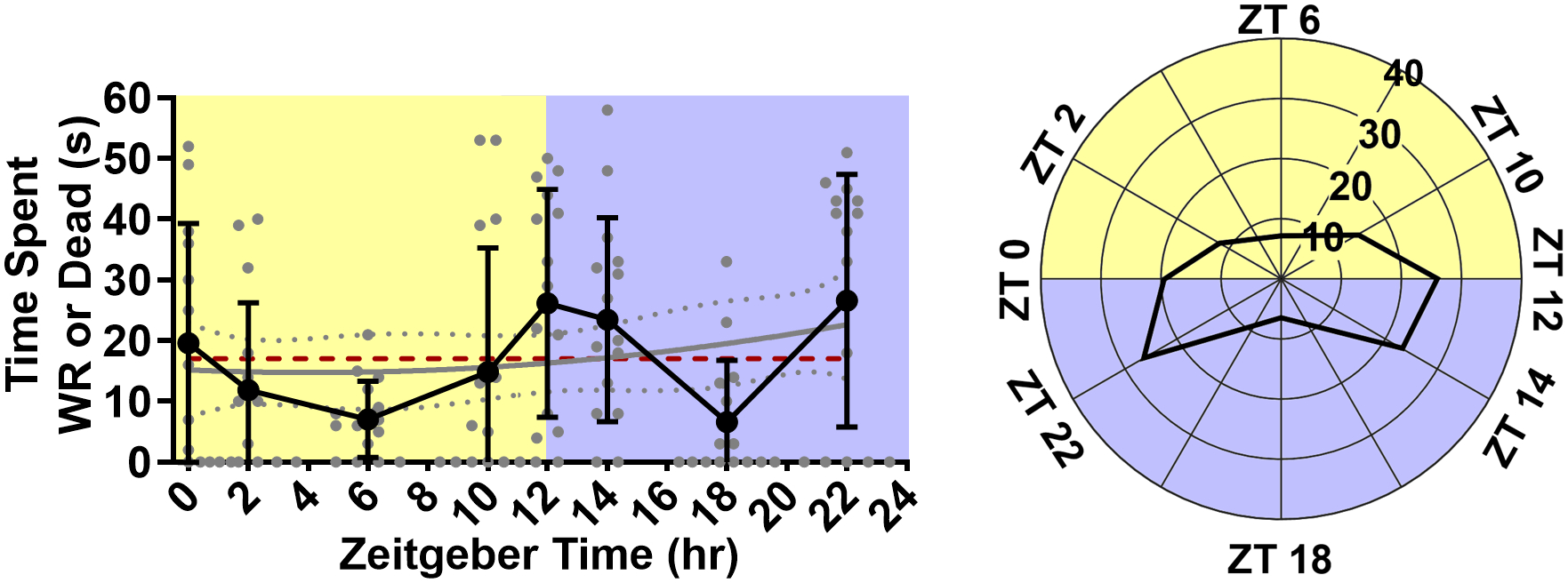
Line graph (left) and rose plot (right) depicting time spent wild running and in respiratory arrest. plotted as mean with standard deviation (black line), with individual values (grey circles, n = 15 per time point). The cosinor line of best fit is depicted as a gray curve with 95% confidence intervals (dotted gray curves). The midline estimating statistic of rhythm (MESOR) is illustrated with a dashed red line.
Postictal breathing and the likelihood of seizure-induced death following MES seizures depends on circadian phase.
To determine the effect of circadian phase on vulnerability to seizure-induced death, MES seizures were induced in separate groups of free-running C57Bl/6J mice at different circadian time points (Fig. 4). Seizure-induced death following MES seizures was significantly non-uniformly distributed across circadian time points as assessed by a Hodges-Ajne test (p < 0.01, n = 9 per time point; Fig. 4E, 5A). Probability of seizure-induced death ranged from a nadir of 33.3% (ZT 6 and 10) to 77.7% (ZT 2 and 22). Across all time points, the probability of seizure-induced death was 59.3%, this value is consistent with prior reports of seizure-induced death rates following a 50 mA MES stimulation (Buchanan et al., 2014). During the subjective day (CT 2, 6, and 10) the MES stimulus resulted in seizure-induced death in 48.1% of trials. Conversely, during the subjective night (CT 14, 18, and 22) the MES stimulus resulted in seizure-induced death in 70.4% of trials.
Figure 4. Maximal electroshock (MES) seizures result in seizure-induced respiratory arrest and death.
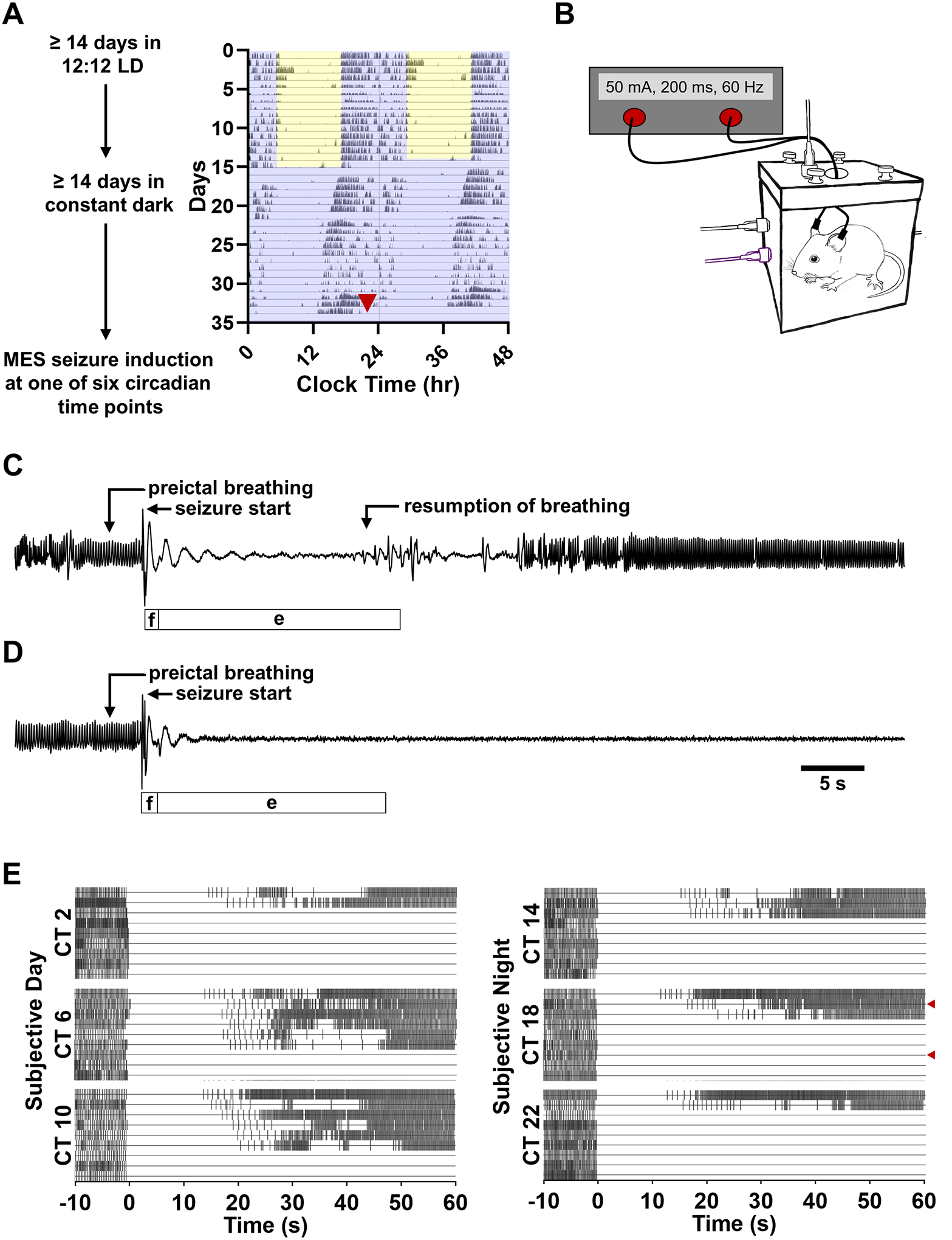
(A) Diagrammatic representation of experiment 2. C57BL/6J mice were normally entrained to 12:12 LD conditions for ≥ 14 days before being released into constant darkness. Mice free-ran for ≥ 14 days before a MES seizure trial in a plethysmography chamber at one of six circadian time points. Wheel running data was used to track the free-running rhythmicity of each animal so that seizures trials could be appropriately timed. Red arrowhead indicates the time at which the animal was removed from its home cage for the seizure trial. (B) Schematic of the experimental apparatus used to induce MES seizures concomitant to whole body plethysmography. Plethysmography traces depicting 10 s of preictal breathing followed by the respiratory sequelae of a non-fatal (C) and fatal (D) MES seizure. Labels above each trace highlight notable respiratory features ascertained from the plethysmography trace. Bars below each trace depict the duration of the flexion and extension components of the motor seizure ascertained from video, labeled ‘f’ and ‘e’ respectively. (E) Raster plots depicting the timing of breaths before and after MES seizure induction. Each horizontal line corresponds to an individual trial. Each horizontal section depicts seizure trials conducted at a specific circadian time (CT, n = 9 per time point). Each vertical hash-mark corresponds to a breath as detected by whole body plethysmography. Within each CT section trials are arranged in order of first postictal breath. Red arrowheads indicate the non-fatal and fatal seizure trials depicted in panels C-D.
Figure 5. Circadian phase alters the occurrence of seizure-induced death, but does not affect seizure severity following MES seizures.
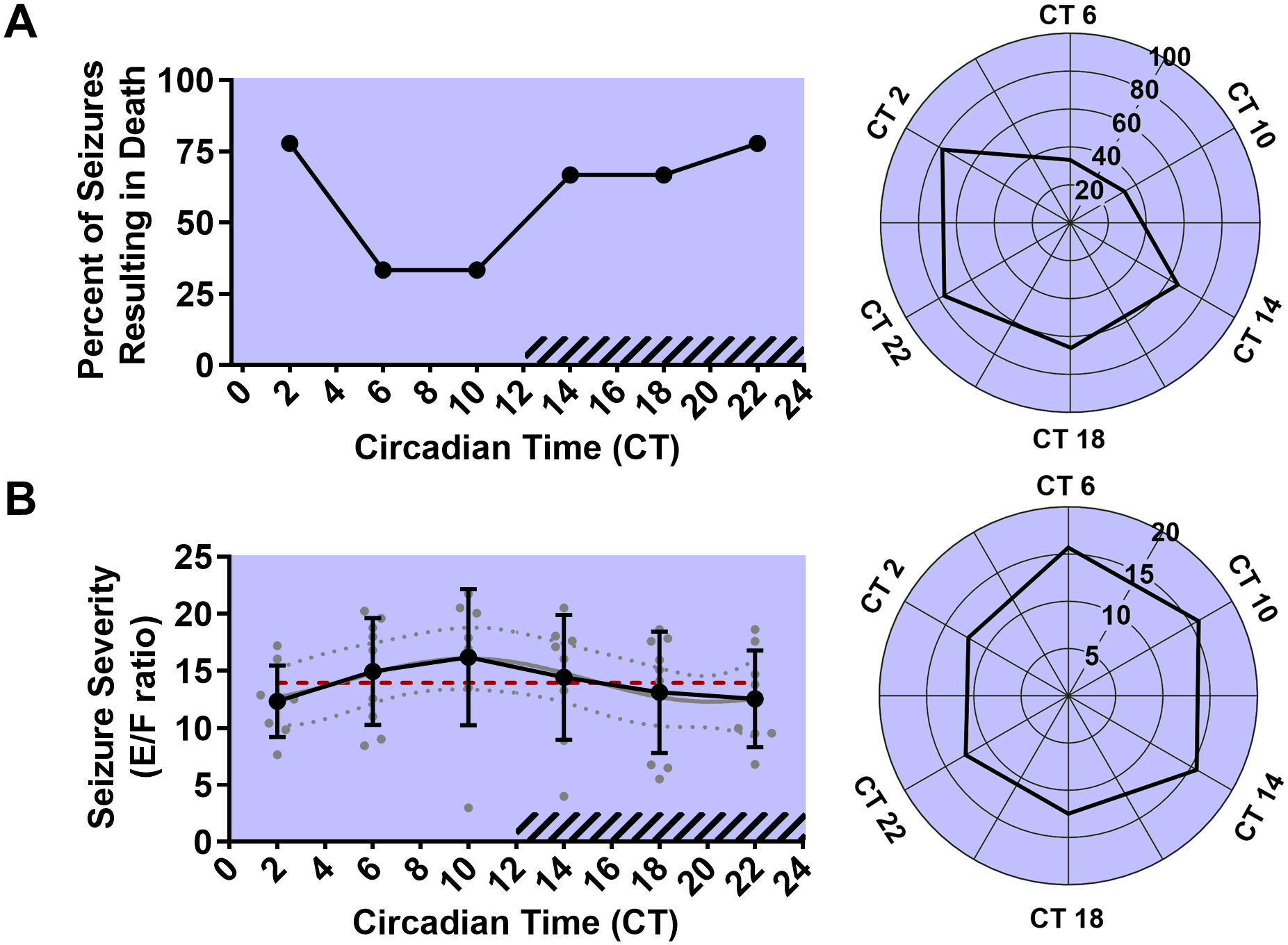
Line graph (left) and rose plot (right) depicting percent of total trials resulting in seizure-induced death (solid black line) at different circadian phases in animals kept in free-running conditions (A, n = 9 per time point). Line graph (left) and rose plot (right) depicting seizure severity as measured by extension/flexion ratio plotted as mean with standard deviation (black line), with individual values for continuous variables (grey circles). The cosinor line of best fit is depicted as a gray curve with 95% confidence intervals (dotted gray curves). The midline estimating statistic of rhythm (MESOR) is illustrated with a dashed red line. Time points during the subjective night are indicated with diagonal hash-marks.
Cosinor analysis did not reveal a statistically significant oscillation in seizure severity in all seizure trials (p = 0.29, MESOR = 13.93, R2 = 0.09, n = 9 per time point; Fig. 5B). There was also no statistically significant oscillation when the data was separated into non-fatal seizure trials (p = 0.83, MESOR = 16.06, R2 = 0.06, n = 2–6 per time point) or fatal seizure trials (p = 0.58, MESOR = 12.63, R2 = 0.07, n = 3–7 per time point). Mean E/F ratios ranged from 12.35 (CT 2) to 16.20 (CT 10). Across all time points, the average E/F ratio was 13.94, this value is consistent with prior reports of E/F ratios following 50 mA MES stimulation in this strain (Kruse et al., 2019).
MES seizures are followed by a long initial apnea followed by death or the spontaneous resumption of breathing initially characterized by large breaths mixed with smaller apneas. We examined the duration of the initial apnea and compared it across time points as an assay of postictal respiratory disruption. There was no statistically significant oscillation in first apnea duration (p = 0.27, MESOR = 16.18, R2 = 0.19, n = 2–6 per time point; Fig. 6A) or total apnea duration (p = 0.51, MESOR = 28.09, R2 = 0.12, n = 2–6 per time point; Fig. 6B) as assessed by the cosinor method. It was not possible to include a precise value associated with apnea duration in the case of animals which experienced seizure-induced death as their apneas were fatal and, as such, effectively infinite in duration; however, entirely excluding animals which died from the apnea analysis is problematic for two reasons: First, because the proportion of animals that died was variable across the different time points the sample size becomes substantially inconsistent. Second, the animals that died of respiratory failure in essence had longer apneas than any animal that survived and their omission is likely to skew the data from time points with high mortality. To address these issues, we repeated our analysis with fatal trials coded as having had a first apnea duration or total apnea duration equal to highest value from any surviving mouse (20.7 s and 40.4 s respectively). Cosinor analysis on the resulting data did not reveal a statistically significant oscillation in first apnea duration (p = 0.79, MESOR = 19.02, R2 = 0.02, n = 9 per time point; Fig. 6C) or total apnea duration (p = 0.77, MESOR = 35.59, R2 = 0.02, n = 9 per time point; Fig. 6D).
Figure 6. Circadian phase does not affect first apnea duration or total apnea duration following MES seizures.
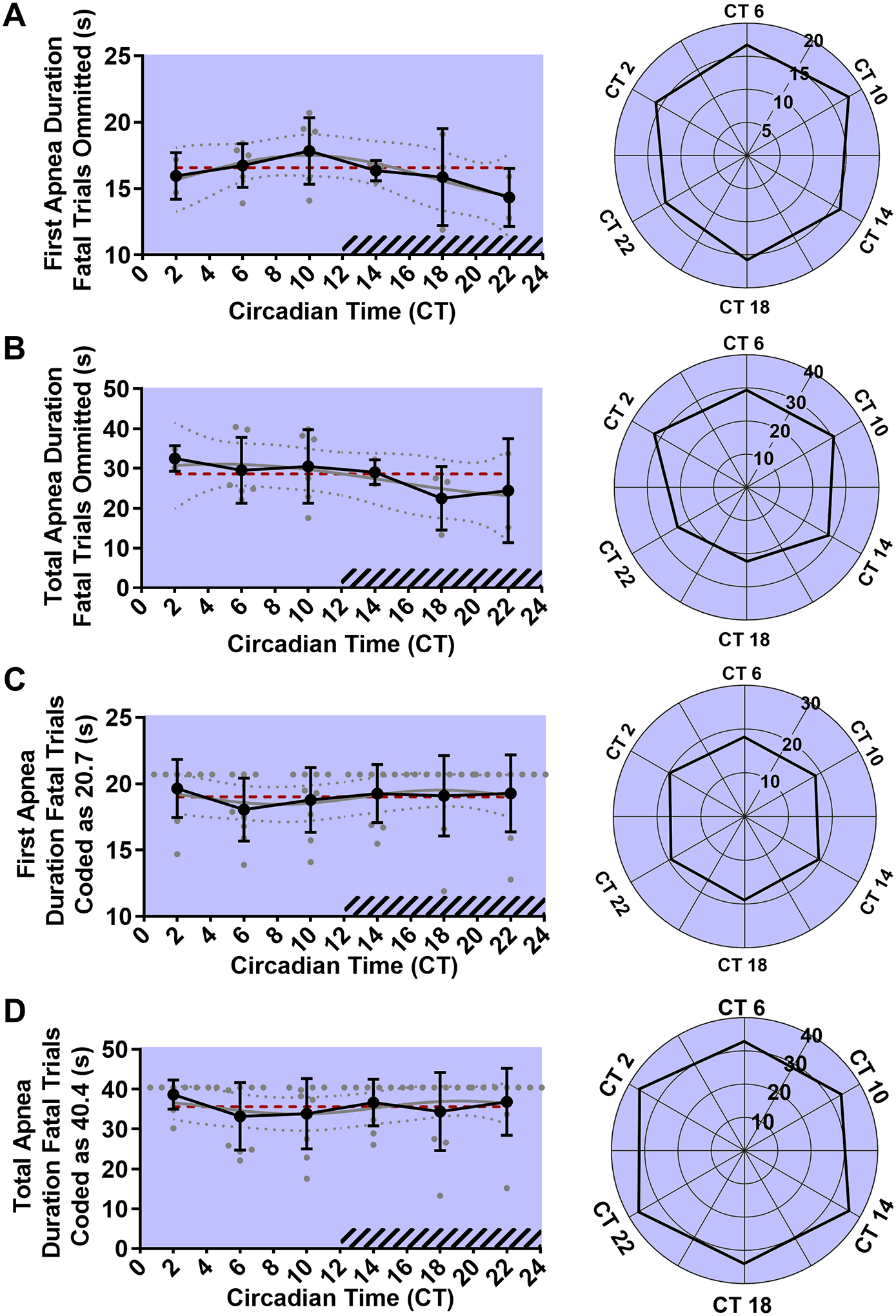
Line graph (left) and rose plot (right) depicting first apnea duration of surviving animals (A, n = 2–6 per time point). Line graph (left) and rose plot (right) depicting total apnea duration of surviving animals (B, n = 2–6 per time point). Line graph (left) and rose plot (right) depicting first apnea duration of all animals. Fatal seizures have been coded as having a first apnea duration equal to largest of surviving animals of (20.7 s, C, n = 9 per time point). Line graph (left) and rose plot (right) depicting total apnea duration of all animals. Fatal seizures have been coded as having a total apnea duration equal to largest of surviving animals of (40.4 s, D, n = 9 per time point). Values are plotted as mean with standard deviation (black line), with individual values for continuous variables (grey circles). The cosinor line of best fit is depicted as a gray curve with 95% confidence intervals (dotted gray curves). The midline estimating statistic of rhythm (MESOR) is illustrated with a dashed red line. Time points during the subjective night are indicated with diagonal hash-marks.
Whole body plethysmography was used to quantify breathing during MES seizure trials and compared across time points to determine if circadian phase affected seizure-induced respiratory disruption. Cosinor analysis revealed statistically significant oscillations in baseline to postictal changes in fR (p < 0.01, MESOR = −177.5, R2 = 0.21, n = 9 per time point; Fig. 7A), VT (p = 0.04, MESOR = −9.56, R2 = 0.16, n = 9 per time point; Fig. 7B), and VE (p < 0.01, MESOR = −2.66, R2 = 0.23, n = 9 per time point; Fig. 7C). Postictal reduction of fR ranged from −4.48 (CT 22) to −1.23 bpm (CT 10). Averaged across all time points, fR was lower during the postictal period by −177.5 bpm. Postictal reduction of VT ranged from −13.66 (CT 2) to −4.26 uL/g (CT 10). Averaged across all time points, VT was lower during the postictal period by −9.56 uL/g. Postictal suppression of VE ranged from −3.90 (CT 22) to −0.42 mL/min/g (CT 10). Averaged across all time points, VE was lower during the postictal period by −2.66 mL/min/g. Cosinor analysis revealed a statistically significant oscillation in largest postictal breath volume (p = 0.04, MESOR = 0.52, R2 = 0.15, n = 9 per time point; Fig. 7D). Max VT ranged from 0.25 (CT 22) to 0.93 mL (CT 6). Averaged across all time points, max VT was 0.52 mL.
Figure 7. Circadian phase alters postictal breathing following MES seizures.
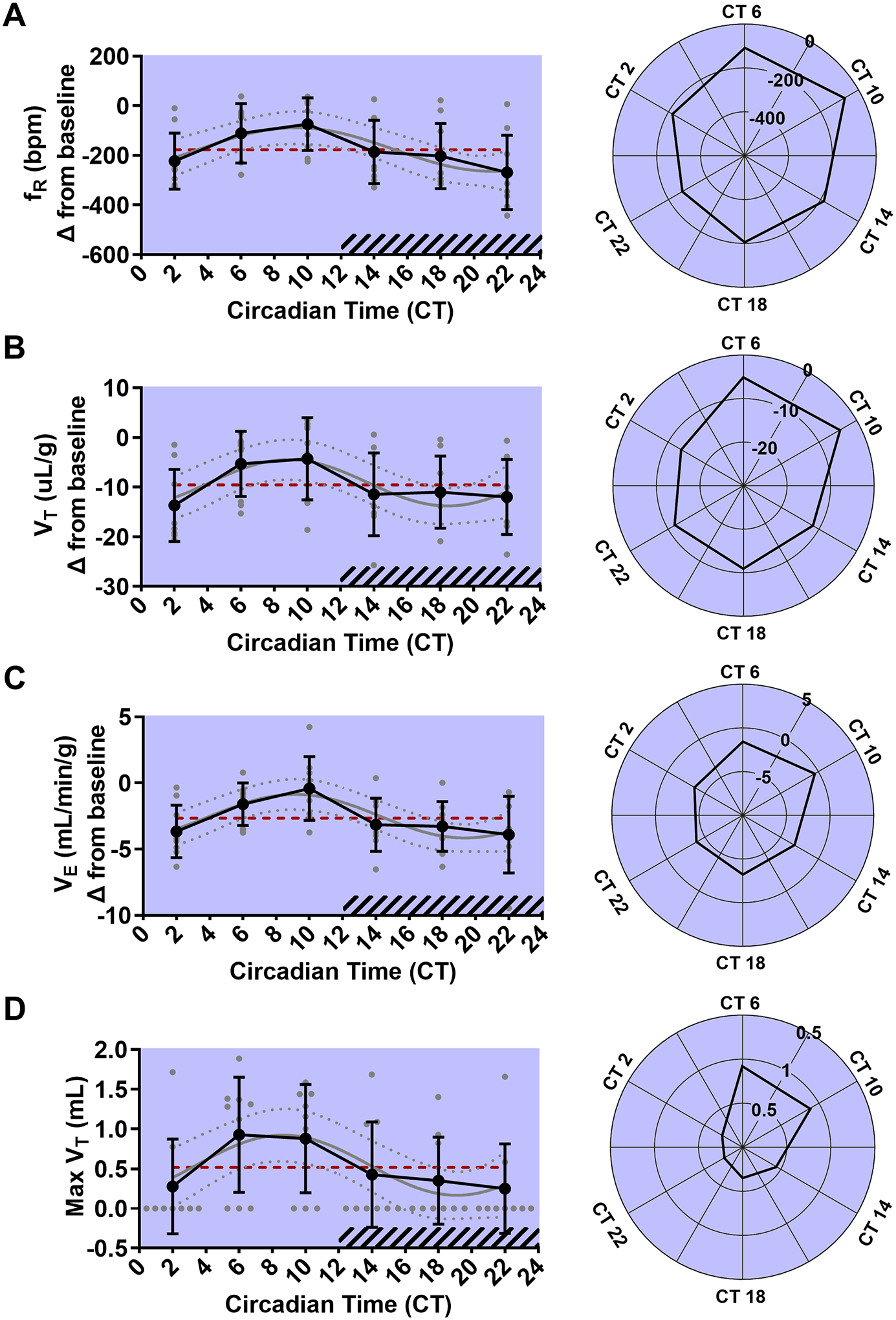
Line graphs (left) and rose plots (right) depicting postictal changes in respiratory frequency (fR) in breaths per minute (bpm), tidal volume (VT), and ventilation (VE) at different circadian time points following MES seizures (A-C, n = 9 per time point). Line graphs (left) and rose plots (right) depicting the largest postictal breath. Values are plotted as mean with standard deviation (black line), with individual values for continuous variables (grey circles). The cosinor line of best fit is depicted as a gray curve with 95% confidence intervals (dotted gray curves). The midline estimating statistic of rhythm (MESOR) is illustrated with a dashed red line. Time points during the subjective night are indicated with diagonal hash-marks.
Discussion
SUDEP is the leading cause of epilepsy-related death. Mortality in SUDEP often happens during the night following a seizure. Why SUDEP happens more during the night is unclear. Here we found that time-of-day in normally entrained mice and circadian phase in free-running mice alter vulnerability to seizure-induced death with greatest vulnerability during the night and subjective night, respectively. In this study, all seizures were induced during wakefulness allowing us to differentiate the effect of time-of-day and circadian phase on vulnerability to death from that of sleep.
To determine whether time-of-day alters vulnerability to seizure-induced death, separate groups of normally entrained DBA/1 mice were exposed to a potentially seizure-inducing acoustic stimulus during wakefulness at different time points. Though there was no difference in seizure susceptibility, the likelihood of seizure-induced death was altered by time-of-day. The bimodal distribution of mortality seen in the DBA/1 animals in the study resembles observations made in mouse models of spontaneous seizure-induced death. Transgenic Kcna1−/− mice, which lack voltage-gated Kv1.1 shaker-like channels, experience spontaneous seizures and seizure-induced death (Glasscock et al., 2010). Mortality in Kv1.1 null mice happens more during the night (Moore et al., 2014). Dravet syndrome is a genetic infantile onset disorder characterized by high SUDEP rates (Dravet, 1978; Genton et al., 2011). Transgenic Scn1aR1407X/+ mice, which have a loss-of-function mutation in the voltage-gated sodium channel Nav1.1, recapitulate many of the characteristics of Dravet Syndrome such as spontaneous seizures and seizure-induced death (Claes et al., 2001). Mortality in Scn1aR1407X/+ mice happens more during the night (Teran et al., 2019). The distribution of seizures in Scn1aR1407X/+ mice generally resembles that of seizure-induced death indicating that the increase in seizure-induced death during the night might be the result of an increase in seizure frequency (Teran et al., 2019). Neither of these studies was able to determine the vigilance state of the animals prior to the fatal seizure; therefore, it is difficult to differentiate the effect of vigilance state from that of time-of-day (Moore et al., 2014; Teran et al., 2019). In contrast, a vigilance state independent effect of time-of-day on mortality has been previously documented using MES (Silverberg et al., 2010). Mice were restrained at different times of day and subjected to MES during wakefulness via subcutaneous needle electrodes above the skull. Vulnerability to seizure-induce death was observed to depend on time-of-day with a nadir in mortality during the day at approximately ZT 10 (4pm in a 7am-6pm LD cycle; Silverberg et al., 2010). That the time of the seizure could be controlled in the models employed in this study allowed us to parse the effects of time-of-day and circadian phase from those of vigilance state.
Previous reports indicate that seizure-induced death occurs infrequently in unprimed DBA/1 mice which have had no prior exposure to the acoustic stimulus (Faingold and Randall, 2013). Our results indicate that the perception of how vulnerable unprimed DBA/1 mice are to seizure-induced death has likely been influenced by the time at which experiments are typically conducted. Stimulations during the day rarely resulted in death as would be expected from the existing literature (Faingold and Randall, 2013); however, stimulations during the night frequently resulted in death. Thus, punctilious control over the time of experimentation is needed in studies using DBA/1 mice to avoid a potential confound.
All prior investigations examining the effect of time-of-day on vulnerability to SUDEP, as well as our DBA/1 experiment, were conducted in a normal light-dark cycle. To determine whether circadian rhythms affect vulnerability to seizure-induced death, we performed our MES study in free-running conditions of constant darkness. The effect of time point on mortality and postictal breathing in free-running conditions suggests that circadian rhythms alter vulnerability to seizure-induced death independent of day/night differences in lighting conditions. Additionally, all seizures in this study were induced during wakefulness suggesting that the effect of time point we observed was not the result of differing vigilance states of seizure origin. Importantly, this simply controls for effects of sleep-wake state, but does not eliminate a role for sleep-wake state in seizure-induced death.
Conversely, circadian phase did not affect seizure severity. This result may indicate that vulnerability to seizure-induced death is not directly related to motor seizure severity. This may seem paradoxical; however, previous investigations have demonstrated that mortality and seizure severity are not necessarily correlated in the MES model (Buchanan et al., 2014; Kruse et al., 2019). Circadian rhythms did not alter apnea related parameters in animals that survived. Together, this data may suggest that the effect of circadian rhythms on vulnerability to seizure-induced death is mediated by capacity to reinitiate breathing as opposed to seizure severity or postictal breathing per se. The pattern of postictal breathing seen in non-fatal seizures (protracted apnea followed by intermittent breaths of increasing size and then restoration of eupneic breathing) is similar to that observed in episodic anoxia models of autoresuscitation (Krause et al., 2016).
Most circadian rhythms are inverted or ‘flipped’ between diurnal and nocturnal mammals (Smale et al., 2003). For example, in diurnal mammals core body temperature is higher during the day, but in nocturnal mammals temperature is higher during the night (Sutherland and Galbraith, 1906; Bolles and Duncan, 1969). This is true of aspects of physiology which might be relevant to seizure-induced death such as respiratory function (Buchanan, 2013; Purnell and Buchanan, 2020). The results of this investigation as well as prior observations in rodent models of spontaneous seizure-induced death indicate that vulnerability to seizure-induced death is phase-conserved between nocturnal and diurnal mammals. In a prior investigation, we induced MES seizures in normally entrained mice at two time points, the early part of the day (ZT 2–6) and the early part of the night (ZT 14–18). We found that seizures induced during the day were associated with a greater degree of postictal respiratory and electrographic disruption, but we observed no effect of time-of-day on mortality (Purnell et al., 2017). The expanded number of time points in the current investigation tell a different story. The source of the discrepancy between the findings of the current investigation and our prior experiment is readily apparent when the time points from our prior investigation in LD are compared to the analogous time points in DD from the current investigation. Previously we observed no significant difference in mortality rates between ZT 2 and ZT 14, in the current investigation mortality rates at CT 2 and CT 14 are remarkably similar (Purnell et al., 2017). Only by incorporating additional time points was the oscillation in vulnerability to seizure-induced death revealed. This finding highlights the importance of carefully controlling the time of experimentation in studies which are not using time-of-day as an independent variable and using numerous time points in studies which are.
The fact that both nocturnal and diurnal mammals appear to be more vulnerable to seizure-induced death during the night has significant implications for the underlying mechanism responsible for day/night differences in SUDEP occurrence. In mammals, the suprachiasmatic nucleus (SCN) operates as a central circadian pacemaker (Moore and Eichler, 1972; Stephan and Zucker, 1972). Circadian oscillations in clock gene expression and neuronal activity in the SCN are similar in nocturnal and diurnal mammals (Caldelas et al., 2003; Peirson et al., 2006; Silver and Lesauter, 2008); however, the daily patterns of physiology and behavior are very different between these organisms (Silver and Lesauter, 2008). A substantial portion of projections from the SCN go to the subparaventricular zone (Nunez et al., 1999; Schwartz et al., 2004). Unlike the SCN, the neuronal activity patterns of the subparaventricular zone are different in nocturnal and diurnal organisms (Nunez et al., 1999; Schwartz et al., 2004). As a result, it is theorized that the subparaventricular zone is responsible for inverting the SCN signal between nocturnal and diurnal organisms (Nunez et al., 1999; Schwartz et al., 2004). The lack of temporal inversion in vulnerability to seizure-induced death between nocturnal and diurnal organisms suggests that the circuit responsible for circadian variations in vulnerability to seizure-induced death involves the projections of the SCN to brain areas other than the subparaventricular zone.
Circadian variations in a number of processes including serotonin signaling, SCN activity, and melatonin release are conserved between nocturnal and diurnal species and thus could be driving the effect of circadian phase on vulnerability to seizure-induced death (Ralph et al., 1971; Kubota et al., 1981; Sato and Kawamura, 1984; Rao et al., 1994; Mateos et al., 2009; Martin-Fairey et al., 2015). Taking serotonin as an example, convergent lines of evidence suggest that serotonergic neurotransmission is protective against seizure-induced death (Tupal and Faingold, 2006; Buchanan et al., 2014; Petrucci et al., 2019). Serotonergic function is impaired by seizure activity (Zhan et al., 2016). Serotonin concentrations oscillate with a similar circadian pattern in nocturnal rodents and diurnal humans with peaks during the day and troughs at night (Purnell et al., 2018).
Because rodents sleep primarily during the day and appear to be more vulnerable to seizure-induced death during sleep it seems counterintuitive that they would experience more seizure-induced death during the night; however, it is important to note that sleep in rodents is highly fragmentary and mice typically have numerous bouts of sleep during the night. No study documenting spontaneous seizure-induced death in rodents has been able to determine the vigilance state of seizure origin (Moore et al., 2014; Teran et al., 2019). It may be that the fatal seizures which occurred during the night in these studies were emerging from the intermittent bouts of sleep that occur during the animals active phase.
Evidence from human SUDEP and animal models of seizure-induced death suggest that seizures which emerge from sleep might be more dangerous (Ryvlin et al., 2013; Hajek and Buchanan, 2016). All experiments in this study were conducted while the animal was awake. Thus, we were able to demonstrate that, during wakefulness, circadian phase alters vulnerability to seizure-induced death. Whether circadian phase alters vulnerability to seizure-induced death for seizures emerging from sleep remains to be determined. Furthermore, if circadian phase does in fact alter vulnerability to seizure-induced death during sleep it may, or may not, share the same temporal pattern of vulnerability to seizure-induced death that has been documented during wakefulness.
It may be that vulnerability to seizure-induced death at any given time is influenced by the compounding effects of vigilance state and circadian rhythms. A similar phenomenon has been documented in the case of respiration (Stephenson et al., 2001). The effects of sleep and circadian rhythms on breathing are additive (Stephenson et al., 2001). For example, if an organism breathes more slowly during the night due to circadian factors and breathes more slowly during non-REM sleep one would expect the organism to breathe more slowly when sleeping during the night than when sleeping during the day or awake during the night. Further experimentation in which seizures are induced in different vigilance states at different circadian phases will be necessary to determine whether sleep and circadian rhythms have additive effects on vulnerability to seizure-induced death.
Conceptualizing the likelihood of a seizure occurring and the likelihood of a seizure resulting in SUDEP as independent constructs may be critical to understanding the timing of SUDEP. Most if not all cases of SUDEP occur following a seizure (Ryvlin et al., 2013; Lhatoo et al., 2016). As such, a seizure is likely to be a gating factor to the occurrence of SUDEP. While the ability of the scientific community to predict seizures is imperfect it is clear that the timing of seizures is not random (Bou Assi et al., 2017). It has long been appreciated that circadian rhythms alter the temporal distribution of seizures (Quigg, 2000; Baud et al., 2018). In light of this investigation, other experimental evidence using animal models (Silverberg et al., 2010; Moore et al., 2014; Teran et al., 2019), and clinical data (Lamberts et al., 2012; Ryvlin et al., 2013), it also seems probable that circadian rhythms alter the likelihood that a seizure results in death; however, the underlying mechanisms responsible for circadian differences in seizure threshold and vulnerability to seizure-induced death might be different. For example, a patient might have the overwhelming majority of their seizures in the morning, but any given seizure might be more likely to cause SUDEP during the night. This patient would be more likely to die of SUDEP during the morning due to the preponderance of seizures during that time, despite the fact that, in abstract, the patient’s vulnerability to seizure-induced death would be greater during the night.
This study is not without limitations. Only male animals were used in the circadian MES experiment. Data from human patients suggests that there might be sex differences in vulnerability to SUDEP (Sveinsson et al., 2017). In future experiments examining both sexes at different circadian time points might yield interesting results. For logistical reasons detailed in the methods section the model of seizure-induced death had to be switched between the entrained time-of-day experiment and the free-running circadian experiment; therefore, data collected in these two sets of experiments are not directly comparable.
The primary findings of this investigation are: Time-of-day alters vulnerability to audiogenic seizure-induced death in normally entrained DBA/1 mice. Circadian phase alters MES postictal breathing and vulnerability to seizure-induced death in free-running C57BL/6J mice. In both models used in this study, seizure-induced death was higher during the active phase (the night or subjective night). Together, these results suggest that endogenous circadian rhythms, independent of vigilance state, might contribute to day/night differences in the occurrence of SUDEP. Future investigations should focus on evaluating potential underlying mechanisms for circadian differences in vulnerability to seizure-induced death. Additionally, by only inducing seizures in wakefulness the effect of time-of-day/circadian phase were isolated from that of vigilance state; however, examining how time-of-day/circadian phase might interact with vigilance state to potentiate SUDEP might be instructive in future experiments.
Supplementary Material
Key Points.
Sudden unexpected death in epilepsy (SUDEP) is the leading cause of premature death in patients with refractory epilepsy.
SUDEP typically happens during the night, but the reason for this is unclear.
We found that in normally entrained mice time-of-day alters vulnerability to seizure-induced death.
We found that in free-running mice circadian phase alters vulnerability to seizure-induced death.
These findings suggest that circadian rhythmicity may be responsible for the increased nighttime prevalence of SUDEP
Funding
This work was supported by a Post-Comprehensive Exam Research Fellowship from the University of Iowa Graduate College, and NIH/NINDS T32NS007421 and F31NS106819 to B.S.P., and NIH/NINDS R01NS095842 and the Beth L. Tross Epilepsy Professorship to G.F.B.
Footnotes
Competing interests.
The authors declare that they have no competing interests.
Data Availability Statement
The data that support the findings of the present study are available from the corresponding author upon reasonable request
References
- Ali A, Wu S, Issa NP, Rose S, Towle VL, Warnke P, Tao JX (2017) Association of sleep with sudden unexpected death in epilepsy. Epilepsy Behav 76:1–6. [DOI] [PubMed] [Google Scholar]
- Anderson RE, Howard RA, Woodbury DM (1986) Correlation between effects of acute acetazolamide administration to mice on electroshock seizure threshold and maximal electroshock seizure pattern, and on carbonic anhydrase activity in subcellular fractions of brain. Epilepsia 27:504–509. [DOI] [PubMed] [Google Scholar]
- Baud MO, Kleen JK, Mirro EA, Andrechak JC, King-Stephens D, Chang EF, Rao VR (2018) Multi-day rhythms modulate seizure risk in epilepsy. Nat Commun 9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolles RC, Duncan PM (1969) Daily course of activity and subcutaneous body temperature in hungry and thirsty rats. Physiology & Behavior 4:87–89. [Google Scholar]
- Bou Assi E, Nguyen DK, Rihana S, Sawan M (2017) Towards accurate prediction of epileptic seizures: A review. Biomedical Signal Processing and Control 34:144–157. [Google Scholar]
- Buchanan GF (2013) Timing, sleep, and respiration in health and disease. Prog Mol Biol Transl Sci 119:191–219. [DOI] [PubMed] [Google Scholar]
- Buchanan GF, Richerson GB (2010) Central serotonin neurons are required for arousal to co2. Proc Natl Acad Sci U S A 107:16354–16359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan GF, Murray NM, Hajek MA, Richerson GB (2014) Serotonin neurones have anti-convulsant effects and reduce seizure-induced mortality. J Physiol 592:4395–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldelas I, Poirel VJ, Sicard B, Pvet P, Challet E (2003) Circadian profile and photic regulation of clock genes in the suprachiasmatic nucleus of a diurnal mammal arvicanthis ansorgei. Neuroscience 116:583–591. [DOI] [PubMed] [Google Scholar]
- Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P (2001) De novo mutations in the sodium-channel gene scn1a cause severe myoclonic epilepsy of infancy. Am J Hum Genet 68:1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravet C (1978) Les épilepsies graves de l’enfant. VIE MED 59: 543–548. [Google Scholar]
- Duckrow RB, Tcheng TK (2007) Daily variation in an intracranial eeg feature in humans detected by a responsive neurostimulator system. Epilepsia 48:1614–1620. [DOI] [PubMed] [Google Scholar]
- Durazzo TS, Spencer SS, Duckrow RB, Novotny EJ, Spencer DD, Zaveri HP (2008) Temporal distributions of seizure occurrence from various epileptogenic regions. Neurology 70:1265–1271. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Randall M (2013) Effects of age, sex, and sertraline administration on seizure-induced respiratory arrest in the dba/1 mouse model of sudden unexpected death in epilepsy (sudep). Epilepsy Behav 28:78–82. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Randall M, Tupal S (2010) Dba/1 mice exhibit chronic susceptibility to audiogenic seizures followed by sudden death associated with respiratory arrest. Epilepsy Behav 17:436–440. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Randall M, Mhaskar Y, Uteshev VV (2011) Differences in serotonin receptor expression in the brainstem may explain the differential ability of a serotonin agonist to block seizure-induced sudden death in dba/2 vs. Dba/1 mice. Brain Res 1418:104–110. [DOI] [PubMed] [Google Scholar]
- Faingold CL, Kommajosyula SP, Long X, Plath K, Randall M (2014) Serotonin and sudden death: Differential effects of serotonergic drugs on seizure-induced respiratory arrest in dba/1 mice. Epilepsy Behav 37:198–203. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, Engel J Jr., Forsgren L, French JA, Glynn M, Hesdorffer DC, Lee BI, Mathern GW, Moshe SL, Perucca E, Scheffer IE, Tomson T, Watanabe M, Wiebe S (2014) Ilae official report: A practical clinical definition of epilepsy. Epilepsia 55:475–482. [DOI] [PubMed] [Google Scholar]
- Genton P, Velizarova R, Dravet C (2011) Dravet syndrome: The long-term outcome. Epilepsia 52 Suppl 2:44–49. [DOI] [PubMed] [Google Scholar]
- Glasscock E, Yoo JW, Chen TT, Klassen TL, Noebels JL (2010) Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. J Neurosci 30:5167–5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015) Principles and standards for reporting animal experiments in the journal of physiology and experimental physiology. J Physiol 593:2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek MA, Buchanan GF (2016) Influence of vigilance state on physiological consequences of seizures and seizure-induced death in mice. J Neurophysiol 115:2286–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden C, Tomson T, Gloss D, Buchhalter J, Cross JH, Donner E, French JA, Gil-Nagel A, Hesdorffer DC, Smithson WH, Spitz MC, Walczak TS, Sander JW, Ryvlin P (2017) Practice guideline summary: Sudden unexpected death in epilepsy incidence rates and risk factors: Report of the guideline development, dissemination, and implementation subcommittee of the american academy of neurology and the american epilepsy society. Neurology 88:1674–1680. [DOI] [PubMed] [Google Scholar]
- Huikuri HV, Niemela MJ, Ojala S, Rantala A, Ikaheimo MJ, Airaksinen KE (1994) Circadian rhythms of frequency domain measures of heart rate variability in healthy subjects and patients with coronary artery disease. Effects of arousal and upright posture. Circulation 90:121–126. [DOI] [PubMed] [Google Scholar]
- Ishida S, Nakagawa M, Fujino T, Yonemochi H, Saikawa T, Ito M (1997) Circadian variation of qt interval dispersion: Correlation with heart rate variability. J Electrocardiol 30:205–210. [DOI] [PubMed] [Google Scholar]
- Jud C, Schmutz I, Hampp G, Oster H, Albrecht U (2005) A guideline for analyzing circadian wheel-running behavior in rodents under different lighting conditions. Biol Proced Online 7:101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalume F, Oakley JC, Westenbroek RE, Gile J, de la Iglesia HO, Scheuer T, Catterall WA (2015) Sleep impairment and reduced interneuron excitability in a mouse model of dravet syndrome. Neurobiol Dis 77:141–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause A, Nowak Z, Srbu R, Bell HJ (2016) Respiratory autoresuscitation following severe acute hypoxemia in anesthetized adult rats. Respir Physiol Neurobiol 232:43–53. [DOI] [PubMed] [Google Scholar]
- Kruse SW, Dayton KG, Purnell BS, Rosner JI, Buchanan GF (2019) Effect of monoamine reuptake inhibition and alpha1 blockade on respiratory arrest and death following electroshock-induced seizures in mice. Epilepsia 60:495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamberts RJ, Thijs RD, Laffan A, Langan Y, Sander JW (2012) Sudden unexpected death in epilepsy: People with nocturnal seizures may be at highest risk. Epilepsia 53:253–257. [DOI] [PubMed] [Google Scholar]
- Lhatoo SD, Nei M, Raghavan M, Sperling M, Zonjy B, Lacuey N, Devinsky O (2016) Nonseizure sudep: Sudden unexpected death in epilepsy without preceding epileptic seizures. Epilepsia 57:1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Buchanan GF (2019) Scurrying to understand sudden expected death in epilepsy: Insights from animal models. Epilepsy Curr 19:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Dieuset G, Pawluski JL, Costet N, Biraben A (2020) Audiogenic seizure as a model of sudden death in epilepsy: A comparative study between four inbred mouse strains from early life to adulthood. Epilepsia 61:342–349. [DOI] [PubMed] [Google Scholar]
- Massey CA, Sowers LP, Dlouhy BJ, Richerson GB (2014) Mechanisms of sudden unexpected death in epilepsy: The pathway to prevention. Nat Rev Neurol 10:271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty DJ, Harper RM (1976) Dorsal raphe neurons: Depression of firing during sleep in cats. Brain Research 101:569–575. [DOI] [PubMed] [Google Scholar]
- Moore BM, Jerry Jou C, Tatalovic M, Kaufman ES, Kline DD, Kunze DL (2014) The kv1.1 null mouse, a model of sudden unexpected death in epilepsy (sudep). Epilepsia 55:1808–1816. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB (1972) Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42:201–206. [DOI] [PubMed] [Google Scholar]
- Nunez AA, Bult A, McElhinny TL, Smale L (1999) Daily rhythms of fos expression in hypothalamic targets of the suprachiasmatic nucleus in diurnal and nocturnal rodents. J Biol Rhythms 14:300–306. [DOI] [PubMed] [Google Scholar]
- Peirson SN, Butler JN, Duffield GE, Takher S, Sharma P, Foster RG (2006) Comparison of clock gene expression in scn, retina, heart, and liver of mice. Biochem Biophys Res Commun 351:800–807. [DOI] [PubMed] [Google Scholar]
- Petrucci AN, Joyal KG, Purnell BS, Buchanan GF (2019) Serotonin and sudden unexpected death in epilepsy. Exp Neurol 325:113145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh MZ, Loddenkemper T, Reinsberger C, Swenson NC, Goyal S, Madsen JR, Picard RW (2012) Autonomic changes with seizures correlate with postictal eeg suppression. Neurology 78:1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell BS, Buchanan GF (2020) Free-running circadian breathing rhythms are eliminated by suprachiasmatic nucleus lesion. J Appl Physiol (1985) 129:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell BS, Hajek MA, Buchanan GF (2017) Time-of-day influences on respiratory sequelae following maximal electroshock-induced seizures in mice. J Neurophysiol 118:2592–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell BS, Thijs RD, Buchanan GF (2018) Dead in the night: Sleep-wake and time-of-day influences on sudden unexpected death in epilepsy. Front Neurol 9:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigg M (2000) Circadian rhythms: Interactions with seizures and epilepsy. Epilepsy Res 42:43–55. [DOI] [PubMed] [Google Scholar]
- Quigg M, Straume M, Smith T, Menaker M, Bertram EH (2001) Seizures induce phase shifts of rat circadian rhythms. Brain Research 913:165–169. [DOI] [PubMed] [Google Scholar]
- Rao ML, Gross G, Strebel B, Halaris A, Huber G, Braunig P, Marler M (1994) Circadian rhythm of tryptophan, serotonin, melatonin, and pituitary hormones in schizophrenia. Biol Psychiatry 35:151–163. [DOI] [PubMed] [Google Scholar]
- Reebs SG, Mrosovsky N (1989) Large phase-shifts of circadian rhythms caused by induced running in a re-entrainment paradigm: The role of pulse duration and light. J Comp Physiol A 165:819–825. [DOI] [PubMed] [Google Scholar]
- Refinetti R, Lissen GC, Halberg F (2007) Procedures for numerical analysis of circadian rhythms. Biol Rhythm Res 38:275–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, Boon P, Crespel A, Dworetzky BA, Hogenhaven H, Lerche H, Maillard L, Malter MP, Marchal C, Murthy JM, Nitsche M, Pataraia E, Rabben T, Rheims S, Sadzot B, Schulze-Bonhage A, Seyal M, So EL, Spitz M, Szucs A, Tan M, Tao JX, Tomson T (2013) Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (mortemus): A retrospective study. Lancet Neurol 12:966–977. [DOI] [PubMed] [Google Scholar]
- Schwartz MD, Nunez AA, Smale L (2004) Differences in the suprachiasmatic nucleus and lower subparaventricular zone of diurnal and nocturnal rodents. Neuroscience 127:13–23. [DOI] [PubMed] [Google Scholar]
- Silver R, Lesauter J (2008) Circadian and homeostatic factors in arousal. Ann N Y Acad Sci 1129:263–274. [DOI] [PubMed] [Google Scholar]
- Silverberg J, Ginsburg D, Orman R, Amassian V, Durkin HG, Stewart M (2010) Lymphocyte infiltration of neocortex and hippocampus after a single brief seizure in mice. Brain Behav Immun 24:263–272. [DOI] [PubMed] [Google Scholar]
- Smale L, Lee T, Nunez AA (2003) Mammalian diurnality: Some facts and gaps. J Biol Rhythms 18:356–366. [DOI] [PubMed] [Google Scholar]
- Spencer DC, Sun FT, Brown SN, Jobst BC, Fountain NB, Wong VS, Mirro EA, Quigg M (2016) Circadian and ultradian patterns of epileptiform discharges differ by seizure-onset location during long-term ambulatory intracranial monitoring. Epilepsia 57:1495–1502. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I (1972) Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A 69:1583–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson, Mohan RM, Duffin J, Jarsky TM (2000) Circadian rhythms in the chemoreflex control of breathing. Am J Physiol Regul Integr Comp Physiol 278:R282–286. [DOI] [PubMed] [Google Scholar]
- Stephenson, Liao KS, Hamrahi H, Horner RL (2001) Circadian rhythms and sleep have additive effects on respiration in the rat. The Journal of Physiology 536:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland S, Galbraith J (1906) Iv.—observations on the normal temperature of the monkey and its diurnal variation, and on the effect of changes in the daily routine on this variation. Transactions of the Royal Society of Edinburgh 45:65–104. [Google Scholar]
- Sveinsson O, Andersson T, Carlsson S, Tomson T (2017) The incidence of sudep: A nationwide population-based cohort study. Neurology 89:170–177. [DOI] [PubMed] [Google Scholar]
- Sveinsson O, Andersson T, Carlsson S, Tomson T (2018) Circumstances of sudep: A nationwide population-based case series. Epilepsia 59:1074–1082. [DOI] [PubMed] [Google Scholar]
- Tavernor SJ, Brown SW, Tavernor RME, Gifford C (1996) Electrocardiograph qt lengthening associated with epileptiform eeg discharges—a role in sudden unexplained death in epilepsy? Seizure 5:79–83. [DOI] [PubMed] [Google Scholar]
- Teran FA, Kim Y, Crotts MS, Bravo E, Emaus KJ, Richerson GB (2019) Time of day and a ketogenic diet influence susceptibility to sudep in scn1a (r1407x/+) mice. Front Neurol 10:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman DJ, Hesdorffer DC, French JA (2014) Sudden unexpected death in epilepsy: Assessing the public health burden. Epilepsia 55:1479–1485. [DOI] [PubMed] [Google Scholar]
- Trulson ME, Jacobs BL (1979) Raphe unit activity in freely moving cats: Correlation with level of behavioral arousal. Brain Research 163:135–150. [DOI] [PubMed] [Google Scholar]
- Tupal S, Faingold CL (2006) Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in dba/2 mice. Epilepsia 47:21–26. [DOI] [PubMed] [Google Scholar]
- Van Luijtelaar ELJM, Coenen AML (1988) Circadian rhythmicity in absence epilepsy in rats. Epilepsy Research 2:331–336. [DOI] [PubMed] [Google Scholar]
- Zar J (1999) Biostatistical analysis, 4th Edition Edition. [Google Scholar]
- Zhan Q, Buchanan GF, Motelow JE, Andrews J, Vitkovskiy P, Chen WC, Serout F, Gummadavelli A, Kundishora A, Furman M, Li W, Bo X, Richerson GB, Blumenfeld H (2016) Impaired serotonergic brainstem function during and after seizures. J Neurosci 36:2711–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhao H, Zeng C, Van Dort C, Faingold CL, Taylor NE, Solt K, Feng HJ (2018) Optogenetic activation of 5-ht neurons in the dorsal raphe suppresses seizure-induced respiratory arrest and produces anticonvulsant effect in the dba/1 mouse sudep model. Neurobiol Dis 110:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of the present study are available from the corresponding author upon reasonable request


