Abstract
Desflurane, isoflurane and sevoflurane, three halogenated ethers, are commonly used inhaled anesthetics, both in the operating room and in the intensive care unit (ICU). The potency and dosage of these drugs is expressed by the MAC value (minimum alveolar concentration). Their interaction with hemoglobin and its affinity for oxygen, best described by the oxygen dissociation curve (ODC), has already been investigated, with conflicting results. Altered by many factors, the ODC can be shifted to the left or to the right, therefore increasing or decreasing hemoglobin oxygen (Hb-O2) affinity. In venous blood samples of 22 healthy participants (11 female, 11 male) ODC were measured with a high-throughput method in vitro. Blood samples were either exposed to control or to three different concentrations of desflurane, isoflurane or sevoflurane prior to and during measurements (low, medium and high corresponding to MAC 0.5, MAC 1.0 and MAC 2.0). With increasing concentrations from control to medium, desflurane and isoflurane significantly decreased Hb-O2 affinity by shifting the ODC to the right (p = 0.016 and p < 0.001) but sevoflurane showed no effects. When further increasing concentrations from medium to high, all three inhaled anesthetics shifted the ODC back to the left (p < 0.001). Comparing only controls to high concentrations, a significant increase in Hb-O2 affinity for desflurane (p = 0.005) and sevoflurane (p < 0.001) was detected. Our study shows a varying effect at different doses of inhaled anesthetics on Hb-O2 affinity. While the underlying mechanisms remain unclear, these results show an effect which needs to be further investigated to determine if patients undergoing anesthesia may potentially benefit or get disadvantage from this slightly increased (e.g. impaired pulmonary oxygen uptake), or decreased Hb-O2 affinity (e.g. arterial vascular disease).
Trial registration: This study is registered with clinicaltrials.gov (NCT04612270).
Subject terms: Physiology, Medical research, Molecular medicine
Introduction
In mammals, the essential role of transporting oxygen(O2) from the lungs to the tissues is provided by the molecule hemoglobin (Hb)1. The interaction between hemoglobin and oxygen is represented by the oxygen dissociation curve (ODC), which was first reported in the early 1900s by Bohr, who demonstrated a reduced hemoglobin oxygen (Hb-O2) affinity in acidic conditions and described the sigmoid shape of the curve2. The ODC is best described by two main parameters: the p50, representing the partial pressure of O2 at 50% Hb-O2 saturation, and the Hill Coefficient (HC), representing the maximum steepness of the curve in the logarithmic Hill plot3. Many agents and conditions have the capability to modify the interaction between hemoglobin and O2, indicated by a change in the ODC. In general, a shift to the right (increase in p50) indicates a decrease, whereas a shift to the left indicates an increase in Hb-O2 affinity1.
Inhaled anesthetics play a central role in modern anesthesia. Desflurane, isoflurane and sevoflurane—all halogenated ethers—are the most commonly used inhaled anesthetics in modern operation theaters and are often used in combination with intravenous anesthetics and analgesics for anesthesia maintenance4. In intensive care medicine inhaled anesthetics are used for sedation, particularly in the context of distinct tolerance or tachyphylaxis to intravenous sedatives, severe bronchospasms and epilepsy refractory to regular treatment or as anesthetic preconditioning5.
In the clinical setting the potency and dosage of inhaled anesthetics are often described by the Minimum Alveolar Concentration (MAC), which expresses the concentration at sea level where 50% of the volunteers did not show purposeful movement after a surgical stimulus6. The MAC value is mainly influenced by age, but also by temperature, barometric pressure and blood sodium levels6. For 18–30 year-olds, MAC 1 values for desflurane, isoflurane and sevoflurane are 7.25%, 1.3% and 2.4%7, respectively.
The choice between these drugs depends on several factors, amongst them the interaction with the cardiovascular system. While desflurane and sevoflurane show the strongest negative inotropic effects, desflurane and isoflurane can cause an increase in heart rate. With regard to the respiratory system, all three anesthetics cause a bronchodilation, while desflurane and isoflurane can trigger laryngeal spasms and are therefore considered unfeasible for induction of anesthesia8.
The interaction of inhaled anesthetics with Hb-O2 affinity was already investigated in the ‘70s and ‘80s of the twentieth century, where the studies conducted by Smith et al. were able to show that halothane, enflurane and nitrous oxide shift the ODC to the right, thereby decreasing Hb-O2 affinity9. On the other hand, Lanza et al. reported no change in Hb-O2 affinity for halothane and enflurane, but confirmed the abovementioned effects for nitrous oxide10. Furthermore, Kambam et al. also showed a right shift of the ODC not only for nitrous oxide but also for isoflurane11,12, while for sevoflurane no effects were found13. Overall, the effects of various inhaled anesthetics on the ODC and the influence of differing dosages is not well established and for some anesthetics even contradictory. Therefore, the influence on Hb-O2 affinity cannot be taken into consideration by clinicians up to date.
In this study, we aimed to investigate the effect at different dosage of the commonly used inhaled anesthetics desflurane, isoflurane and sevoflurane on Hb-O2 affinity in whole blood samples using a new in vitro method for ODC determination.
Materials and methods
Venous blood samples were drawn with a minimum period of stasis from 11 female and 11 male healthy volunteers aged between 18 and 40 years. All subjects were nonsmokers, not pregnant or breastfeeding, had no known hemoglobinopathy, or recent history of illness, trauma, recent surgery, blood loss or multi-day trips to high altitude (> 3000 m). Immediately after blood sampling, the heparinized samples were stored on ice, while blood gas analysis (ABL 800 flex, Radiometer) was performed from aliquots. Another aliquot was separated and stored at − 80 °C for the quantification of 2,3-bisphosphoglycerate (2,3-BPG) and adenosine triphosphate (ATP) concentrations with a validated liquid chromatography–tandem mass spectrometry method described before14.
The in-vitro ODC measurements were performed with a high-throughput method for the recording of ODCs15. In the four channel ODC plate, blood samples and an internal hemoglobin standard solution15 were exposed to desflurane, isoflurane, and sevoflurane side by side with a control, exposed to anesthetic-free standard gas mixes only15. In three runs, samples were exposed to low, medium and high doses of inhaled anesthetics, corresponding to a MAC of 0.5, 1.0 and 2.0 when referring to the population present in this study (18–40 years, female and male; Table 1)7. Gas concentrations were measured at the end of the gas flow-through system with a multi-gas module and an intensive care monitor (Mindray Benevision with Multigas Module, Mindray Bio-Medical Electronics Co., Ltd, China).
Table 1.
Concentrations of inhaled anesthetics based on MAC values adapted from Thiel & Roewer’s “Anästhesiologische Pharmakotherapie” used in this study7.
| MAC 0.5 (%) | MAC 1.0 (%) | MAC 2.0 (%) | |
|---|---|---|---|
| Desflurane | 3.75 | 7.5 | 15 |
| Isoflurane | 0.75 | 1.5 | 3 |
| Sevoflurane | 1.25 | 2.5 | 5 |
MAC minimal alveolar concentration.
Inhaled anesthetics were vaporized and volumetrically added to the oxygen containing and oxygen free gas mixes used for the in vitro method. Prior to the study, stability of inhaled anesthetics in the gas sampling bags was confirmed by preparation of a defined gas mixture and repeated measurements over 24 h using the above-mentioned gas monitor. Additionally, interference of the inhaled anesthetics with absorption measurements was excluded in advance. Triplicate measurements were performed for every concentration, substance and blood sample. The thermostatic experimental setup was set to 37 °C, all gas mixtures contained 40 mmHg PCO2.
Excel (Microsoft 2016) was used for curve fittings, P50 and HC calculations. Statistical analysis was performed using R (v4.0.2, R Core Team, www.R-project.org) and RStudio (v1.2.5001, RStudio Inc., Boston, MA, USA). Due to non-normal distribution of the small sample size, non-parametric tests were applied. ANOVA type statistics modified for non-parametric longitudinal data (R package: nparLD) was utilized to analyze the effects of different inhaled concentrations of anesthetics with regard to controls16. A p < 0.05 was considered significant. Data are presented as median and interquartile range (IQR; first and third quartile).
Ethical approval
This study was approved by the ethics committee of the Medical University of Innsbruck (vote nr. 1265/2020) and is registered with clinicaltrials.gov (NCT04612270, 02/11/2020). The authors confirm that all experiments were performed in accordance with relevant guidelines and regulations. Written informed consent was given by all subjects.
Results
A total of 22 subjects (11 female; 11 male) were included in the study. General demographics and baseline characteristics are presented in Table 2. Particularly hemoglobin concentration and hematocrit were within normal ranges for this study population. The pCO2 values in our venous whole blood samples were slightly elevated and pH on the lower end of the normal range. Baseline values used as control for p50 and HC were 27.9 (26.3–29.4) mmHg and 2.9 (2.7–3.0), respectively (Table 2). ODCs of all subjects for the three anesthetics at low, medium and high concentration are shown in Supplementary Material.
Table 2.
Baseline characteristics of the study population (median and interquartile range, IQR).
| Median | IQR (Q1–Q3) | |
|---|---|---|
| Age (y) | 29.0 | 28.0–30.0 |
| Hemoglobin (g/dl) | 14.4 | 13.4–15.0 |
| Hematocrit (%) | 44.1 | 41.3–46.0 |
| pCO2 (mmHg) | 44.3 | 40.4–52.9 |
| pH | 7.36 | 7.33–7.38 |
| P50 (mmHg) | 27.9 | 26.3–29.4 |
| Hill coefficient | 2.9 | 2.7–3.0 |
| 2,3 BPG (µmol/gHb) | 19.9 | 18.6–22.4 |
| ATP (µmol/gHb) | 5.0 | 4.7–5.5 |
pCO2 carbon dioxide partial pressure in blood, p50 oxygen partial pressure at 50% saturation, 2,3 BPG 2,3-bisphospholycerate, ATP adenosine triphosphate.
Sex specific analysis revealed no difference between sexes therefore results of the combined data set is provided.
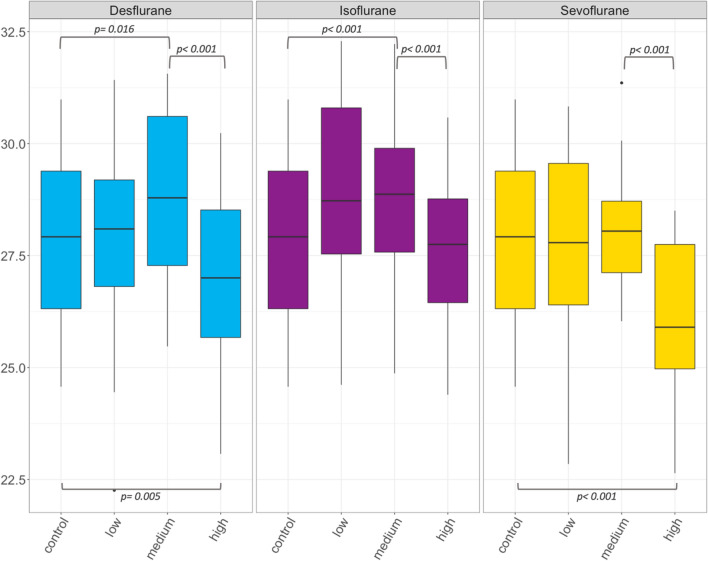
Overall, a significant, unspecific effect of the examined volatile anesthetics on p50 and the HC was found (p < 0.001; Table 3; Figs. 1, 2). In detail, with increasing anesthetic gas concentrations from control over low to medium concentrations, a significant increase in p50 was shown for desflurane (p = 0.016) and isoflurane (p < 0.001) but not for sevoflurane (p = 0.613; Table 3; Fig. 1). Subsequently, desflurane, isoflurane and sevoflurane showed a significant decrease in p50 when further increasing the concentration from medium to high concentrations (p < 0.001; Fig. 1). Comparing controls to high concentrations only, a significant decrease in p50 was shown for desflurane (p = 0.005) and sevoflurane (p < 0.001), but not for isoflurane (p = 0.542).
Table 3.
P50 and HC (median and interquartile range, IQR) of controls and three inhaled anesthetics at low, medium and high concentration.
| Desflurane median (IQR) | Isoflurane median (IQR) | Sevoflurane median (IQR) | ||||
|---|---|---|---|---|---|---|
| P50 (mmHg) | HC | P50 (mmHg) | HC | P50 (mmHg) | HC | |
| Control | 27.9 (26.3–29.4) | 2.9 (2.7–3.0) | 27.9 (26.3–29.4) | 2.9 (2.7–3.0) | 27.9 (26.3–29.4) | 2.9 (2.7–3.0) |
| Low concentration | 28.1 (26.8–29.2) | 3.0 (2.9–3.1) | 28.7 (27.5–30.8) | 3.0 (2.9–3.3) | 27.8 (26.4–29.6) | 2.9 (2.7–2.9) |
| Medium concentration | 28.8 (27.3–30.6) | 3.2 (3.0–3.3) | 28.9 (27.6–29.9) | 3.3 (3.1–3.4) | 28.1 (27.1–28-7) | 3.1 (2.8–3.2) |
| High concentration | 27.0 (25.7–28.5) | 2.9 (2.7–3.1) | 27.8 (26.5–28.8) | 3.1 (2.9–3.2) | 25.9 (25.0–27.8) | 2.7 (2.5–2.9) |
P50 oxygen partial pressure at 50% saturation, HC the Hill coefficient.
Figure 1.
Box plots of p50 (in mmHg) for desflurane, isoflurane and sevoflurane at low (corresponding to MAC 0.5), medium (MAC 1.0) and high (MAC 2.0) concentration. Significant differences are indicated by brackets and p values are reported. P50 is oxygen partial pressure at 50% saturation.
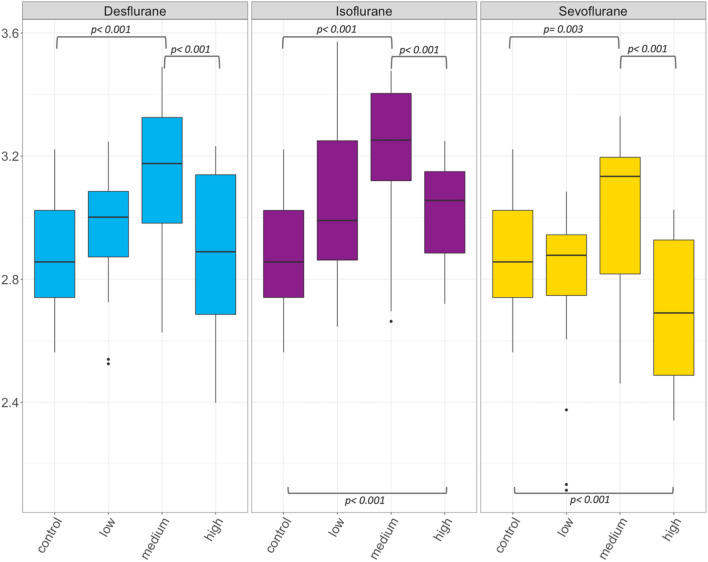
Figure 2.
Box plots of HC for desflurane, isoflurane and sevoflurane at low (corresponding to MAC 0.5), medium (MAC 1.0) and high (MAC 2.0) concentration. Significant differences are indicated by brackets and p values are reported. HC is Hill coefficient.
With regard to the HC, desflurane (p < 0.001), isoflurane (p < 0.001) and sevoflurane (p = 0.003) led to a significant increase while progressing from control over low to medium concentrations (Fig. 2). Further increasing gas concentrations from medium to high significantly decreased the HC again for all volatiles (p < 0.001). Compared to controls only, the HC in high concentrations did not differ for desflurane (p = 0.437), increased for isoflurane (p < 0.001) and decreased for sevoflurane (p < 0.001).
Discussion
In this study, significant effects of desflurane, isoflurane and sevoflurane on p50, HC and hence Hb-O2 affinity in human whole blood samples are shown. With varying effects at different doses, p50 and HC increased with desflurane and isoflurane from controls to medium concentrations but subsequently decreased substantially when further increasing the applied concentrations to a high level. Sevoflurane however, showed no effect on p50 up to medium concentrations followed by a significant decrease when applying high concentrations. Compared to controls only, the total effect of high concentrations was (1) a significant decrease of p50 for desflurane and sevoflurane but non-significant differences for isoflurane and (2) no difference in the HC for desflurane, an increase of the HC for isoflurane and a decrease of the HC for sevoflurane. The described effects translate into an initial right shift of the ODC and therefore a decrease in Hb-O2 affinity for desflurane and isoflurane up to medium concentrations, followed by a subsequent increase again when further increasing the dose. Although unaffected by low to medium concentrations, sevoflurane was able to increase Hb-O2 affinity the most by decreasing p50 when applying high concentrations.
These findings are partially in line with the results of Kambam et al.11,12, who showed an ODC shift to the right for isoflurane and nitrous oxide. Furthermore, in their studies, no influence of sevoflurane on Hb-O2 affinity was detected. Noteworthy, the applied concentrations approximately corresponded to our low to medium concentrations where also no effects on Hb-O2 affinity was shown13. In contrast to our findings, Wade et al. reported that isoflurane at a concentration of 2%, corresponding to a MAC between 1 and 2, increased the oxygen affinity of sickle hemoglobin, shifting the ODC to the left17. Of course, sickle cell hemoglobin per se exhibits decreased oxygen affinity potentially explaining discrepancies when comparing results to healthy adults.
The underlying mechanisms leading to the reported modification of Hb-O2 affinity by inhaled anesthetics are unclear. An interaction with several receptors, modifying the electrolyte balance and, as a consequence, intracellular pH in red blood cells seems possible: Altikat et al. showed that the activity of glucose-6-phosphate dehydrogenase (G6PD) in red blood cells was inhibited by sevoflurane and isoflurane, but not by halothane. Thus, the production of NAPDH was reduced, hence exposing the red blood cell to reactive oxygen species18. Fomitcheva et al. showed that isoflurane in clinically relevant concentrations inhibits the activity of plasma membrane Ca2+-transporting adenosine triphosphatase (PMCA), thus increasing intracellular Ca2+ concentrations19. This increase was linked to a right shift of the ODC and a lower affinity for oxygen. As a potential cause, red blood cell shrinkage, resulting in an increase in 2,3-DPG levels was ruled out as the right shift of the ODC was also observed in red blood cells where shrinkage was prevented. It was hypothesized that the effects are based on an intracellular pH change due to interactions of increased free Ca2+-concentrations with the protonation of hemoglobin20,21. Further increasing the concentration to a MAC of approximately 3 was also shown to inhibit several types of ATPase like the Na+-K+-ATPase or the Mg2+-ATPase19. This may partially explain the underlying mechanisms behind the opponent effects on Hb-O2 affinity when progressing the dose to rather high concentrations, as observed in our study. Moreover, possible genetic influences on drug effects at an individual level, in terms of pharmacogenetics, must be taken into account22,23.
Although the demonstrated shift in p50 might seem minor, clinical relevance cannot be excluded and needs to be further evaluated. Whether a shift of the ODC to the right or to the left may be beneficial is still under debate and certainly depends on the individual patient and his underlying disease24. Changes in pulmonary O2 absorption capacity may be counteracted by numerous factors at the peripheral tissue level. A left shift might be capable to increase the oxygen absorption in the lungs, but at the same time it may reduce oxygen delivery at the peripheral cellular level. On the other side, Woodson et al. showed that in rats, a left shifted ODC can raise the coronary and brain blood flow without increasing the workload of the heart—similar to anemia25. However, at the tissue level hypercapnia, acid metabolites, and even local hyperthermia might easily mitigate these effects. A right shift of the ODC on the other hand, can increase the amount of O2 released in the tissues, although a reduced absorption of oxygen in the lungs must be considered. In patients suffering from an oxygenation limitation, for example COVID-19 pneumonia or ARDS, a left shift of the ODC might be beneficial in improving the O2 uptake in the lungs26. On the other hand, in heart failure, peripheral vascular disease and other conditions where tissue oxygenation is at risk, a right shift of the ODC might mitigate tissue hypoxia.
An anesthetist must consider several parameters when choosing the optimal anesthetic for the given patient. Cardiovascular risk profile, obstructive pulmonary disease, type of surgery and required depth of anesthesia, just to name a few. Currently, inhaled anesthetics are standard care in general anesthesia7. Despite many common properties, each inhaled anesthetic has its own characteristics: desflurane seems to maintain cardiopulmonary function at best, sevoflurane outperforms in terms of adverse effects like airway irritation, and isoflurane shows a high blood-gas partition coefficient, thus minimalized consumption and faster induction and awakening time4,8.
The shown decrease in Hb-O2 affinity by desflurane and isoflurane in low to medium concentrations, increase in Hb-O2 affinity by desflurane and sevoflurane in high concentrations as well as absent influence on Hb-O2 affinity by sevoflurane in low to medium concentrations, needs to be further investigated since it is still unclear if the effects shown in this experimental setting may translate into clinically relevant effects.
Limitations
Due to the in vitro design of our study, further studies are certainly necessary to confirm our result by investigating the impact of all three inhaled anesthetics in vivo. In addition, underlying pathophysiological mechanisms require further investigation.
Conclusions
In low to medium concentrations in vitro, the use of isoflurane or desflurane is accompanied by a decrease in Hb-O2 affinity, where sevoflurane seems to have no effects. At high concentrations in vitro, desflurane and sevoflurane increase Hb-O2 affinity.
Supplementary Information
Acknowledgements
We thank Martin Streicher, Mario Hirsch, Matthias Triendl and Karl Soraperra for the valuable technical support in the experimental setup.
Author contributions
Conceptualization, S.W., N.M., T.H., M.R., H.G., C.R. and M.S.; data curation, S.W.; formal analysis, C.R.; investigation, S.W., N.M., T.H., M.R., C.R. and M.S.; 2,3-BPG and ATP determination and validation, H.O. and D.P.; methodology, S.W., N.M., T.H.; project administration, S.W., T.H. and M.S.; supervision, T.H., M.S. and C.R.; validation, S.W. and C.R.; writing—original draft, M.R., S.W. and C.R.; writing—review and editing, S.W., N.M., T.H., M.R., D.P., H.O., H.G., C.R. and M.S.. All authors have read and agreed to the published version of the manuscript.
Funding
The authors thank the Department of Innovation, Research, University and Museums of the Autonomous Province of Bozen/Bolzano for covering the Open Access publication costs.
Data availability
The raw data supporting the conclusions of this article will be made available by the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-17789-6.
References
- 1.Mairbäurl H, Weber RE. Oxygen transport by hemoglobin. Compr. Physiol. 2012;2(2):1463–1489. doi: 10.1002/cphy.c080113. [DOI] [PubMed] [Google Scholar]
- 2.Bohr C, Hasselbalch K, Krogh A. Ueber einen in biologischer Beziehung wichtigen Einfluss, den die Kohlensäurespannung des Blutes auf dessen Sauerstoffbindung übt 1. Skand Arch. Für Physiol. 1904;16(2):402–412. doi: 10.1111/j.1748-1716.1904.tb01382.x. [DOI] [Google Scholar]
- 3.Imai K. Allosteric Effects in Haemoglobin. Cambridge University Press; 1982. [Google Scholar]
- 4.Miller AL, Theodore D, Widrich J. Inhalational Anesthetic. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. http://www.ncbi.nlm.nih.gov/books/NBK554540/. Accessed Nov 8 2021. [PubMed]
- 5.Jerath A, Parotto M, Wasowicz M, Ferguson ND. Volatile anesthetics. Is a new player emerging in critical care sedation? Am. J. Respir. Crit. Care Med. 2016;193(11):1202–1212. doi: 10.1164/rccm.201512-2435CP. [DOI] [PubMed] [Google Scholar]
- 6.Quasha AL, Eger EI, Tinker JH. Determination and applications of MAC. Anesthesiology. 1980;53(4):315–334. doi: 10.1097/00000542-198010000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Thiel H, Roewer N. Anästhesiologische Pharmakotherapie: Von den Grundlagen der Pharmakologie zur Medikamentenpraxis. Thieme; 2014. p. 491. [Google Scholar]
- 8.Deile M, Damm M, Heller AR. Inhaled anesthetics. Anaesthesist. 2013;62(6):493–504. doi: 10.1007/s00101-013-2175-9. [DOI] [PubMed] [Google Scholar]
- 9.Smith TC, Colton ET, Behar MG. Does anesthesia alter hemoglobin dissociation? Anesthesiology. 1970;32(1):5–10. doi: 10.1097/00000542-197001000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Lanza V, Mercadante S, Pignataro A. Effects of halothane, enflurane, and nitrous oxide on oxyhemoglobin affinity. Anesthesiology. 1988;68(4):591–594. doi: 10.1097/00000542-198804000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Kambam JR. Effect of isoflurane on P50 and Po2 measurements. Anesth. Rev. 1987;14:40–42. [Google Scholar]
- 12.Kambam JR, Holaday DA. Effect of nitrous oxide on the oxyhemoglobin dissociation curve and PO2 measurements. Anesthesiology. 1987;66(2):208–209. doi: 10.1097/00000542-198702000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Kambam JR, Horton BF, Parris WC, Holaday DA. Effect of sevoflurane on P50 and on measurement of oxygen tension. J. Clin. Monit. 1988;4(4):261–263. doi: 10.1007/BF01617323. [DOI] [PubMed] [Google Scholar]
- 14.Woyke S, Mair N, Haller T, Ronzani M, Plunser D, Oberacher H, et al. The impact of nebulized epoprostenol and iloprost on hemoglobin oxygen affinity: An ex vivo experiment. Am. J. Physiol. Lung Cell Mol. Physiol. 2022;322(6):L898–903. doi: 10.1152/ajplung.00084.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woyke S, Ströhle M, Brugger H, Strapazzon G, Gatterer H, Mair N, et al. High-throughput determination of oxygen dissociation curves in a microplate reader—a novel, quantitative approach. Physiol. Rep. 2021;9(16):e14995. doi: 10.14814/phy2.14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noguchi, K., Gel, Y. R., Brunner, E., & Konietschke, F. nparLD: An R Software Package for the Nonparametric Analysis of Longitudinal Data in Factorial Experiments. J Stat Softw [Internet]. 2012;50(12). http://www.jstatsoft.org/v50/i12/. Accessed 18 Feb 2022.
- 17.Wade M, Joshi R, Nazir A, Head CA, Meiler SE. Isoflurane reduces hypoxia-induced RBC sickling and increases oxygen affinity of sickle hemoglobin. Blood. 1995;86(10):3676–3684. doi: 10.1182/blood.V86.10.3676.bloodjournal86103676. [DOI] [Google Scholar]
- 18.Altikat S, Ciftçi M, Büyükokuroğlu ME. In vitro effects of some anesthetic drugs on enzymatic activity of human red blood cell glucose 6-phosphate dehydrogenase. Pol. J. Pharmacol. 2002;54(1):67–71. [PubMed] [Google Scholar]
- 19.Fomitcheva I, Kosk-Kosicka D. Volatile anesthetics selectively inhibit the Ca(2+)-transporting ATPase in neuronal and erythrocyte plasma membranes. Anesthesiology. 1996;84(5):1189–1195. doi: 10.1097/00000542-199605000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Bogdanova A, Makhro A, Wang J, Lipp P, Kaestner L. Calcium in red blood cells—a perilous balance. Int. J. Mol. Sci. 2013;14(5):9848–9872. doi: 10.3390/ijms14059848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makhro A, Hänggi P, Goede JS, Wang J, Brüggemann A, Gassmann M, et al. N-Methyl-d-aspartate receptors in human erythroid precursor cells and in circulating red blood cells contribute to the intracellular calcium regulation. Am. J. Physiol. Cell Physiol. 2013;305(11):C1123–1138. doi: 10.1152/ajpcell.00031.2013. [DOI] [PubMed] [Google Scholar]
- 22.Nair AS. Pharmacogenomics of inhalational anesthetic agents. Med. Gas Res. 2019;9(1):52–53. doi: 10.4103/2045-9912.254641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Searle R, Hopkins PM. Pharmacogenomic variability and anaesthesia. Br. J. Anaesth. 2009;103(1):14–25. doi: 10.1093/bja/aep130. [DOI] [PubMed] [Google Scholar]
- 24.Dempsey JA. With haemoglobin as with politics–should we shift right or left? J Physiol. 2020;598(8):1419–1420. doi: 10.1113/JP279555. [DOI] [PubMed] [Google Scholar]
- 25.Woodson RD, Auerbach S. Effect of increased oxygen affinity and anemia on cardiac output and its distribution. J. Appl. Physiol. 1982;53(5):1299–1306. doi: 10.1152/jappl.1982.53.5.1299. [DOI] [PubMed] [Google Scholar]
- 26.Woyke S, Rauch S, Ströhle M, Gatterer H. Modulation of Hb-O2 affinity to improve hypoxemia in COVID-19 patients. Clin. Nutr. 2021;40(1):38–39. doi: 10.1016/j.clnu.2020.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the corresponding author upon reasonable request.