Abstract
Primates, represented by 521 species, are distributed across 91 countries primarily in the Neotropic, Afrotropic, and Indo-Malayan realms. Primates inhabit a wide range of habitats and play critical roles in sustaining healthy ecosystems that benefit human and nonhuman communities. Approximately 68% of primate species are threatened with extinction because of global pressures to convert their habitats for agricultural production and the extraction of natural resources. Here, we review the scientific literature and conduct a spatial analysis to assess the significance of Indigenous Peoples’ lands in safeguarding primate biodiversity. We found that Indigenous Peoples’ lands account for 30% of the primate range, and 71% of primate species inhabit these lands. As their range on these lands increases, primate species are less likely to be classified as threatened or have declining populations. Safeguarding Indigenous Peoples’ lands, languages, and cultures represents our greatest chance to prevent the extinction of the world’s primates.
Supporting Indigenous Peoples’ land rights is an effective solution to protect the world’s primates from extinction.
INTRODUCTION
A growing human population and globally expanding economic activities exert unsustainable demands on nature, resulting in widespread deforestation, biodiversity loss, erosion of ecosystem services, and accelerating climate change (1). Consequently, about 1 million animal and plant species are threatened with extinction (2). This imperiled biodiversity includes the world’s nonhuman primates (primates from here on), our closest living biological relatives. Primates (prosimians, tarsiers, monkeys, and apes) are the third most speciose mammalian radiation (521 extant species; only Rodentia and Chiroptera have more species), are an essential component of forest biodiversity, and play important roles in the livelihoods, cultures, and belief systems of many societies worldwide. Primates are present in 91 countries in the Neotropic (178 species), Afrotropic (mainland Africa 107 species; Madagascar 107 species), and Indo-Malayan (130 species) realms (Fig. 1 and tables S1 to S6) (3). Across these biogeographic regions, primates inhabit a wide range of tropical, subtropical, and temperate forests, as well as woodlands and savannas. Overall, 97% of primate species (n = 508) exploit ranges that include forested environments (3). Across these biomes, primates play a critical role in supporting community-wide ecological functions, processes, and services (e.g., seed dispersal, pollination, carbon sequestration, and predator-prey relationships) that sustain healthy ecosystems benefiting local human communities (4, 5). Disturbingly, ~68% of primate species for which data are available are in danger of extinction (listed as Vulnerable, Endangered, and Critically Endangered), while 93% have declining populations (3). In the past 5 years, the number of primate species considered threatened has increased by 8%, and primates currently represent the most vulnerable large taxonomically diverse mammal group [in comparison, Rodentia has 17% of species threatened and Chiroptera, 21%; (3)]. Moreover, given their relatively slow life history, long interbirth interval, and that most species give birth to a single infant, in many forest communities, primates are considered “indicator” or “sentinel” species, warning of the deleterious effects of hunting and habitat conversion on biodiversity and ecosystems health (6–8).
Fig. 1. Selected primate species in the Neotropics, Afrotropics, and Indo-Malayan realm whose distributional ranges intersect Indigenous Peoples’ lands.
Shown also is their IUCN conservation status (CR, Critically Endangered; VU, Vulnerable; EN, Endangered; LC, Least Concern). (A) Neotropics: 1, Northern muriqui (Brachyteles hypoxanthus), CR (photo credit: Priscila Pereira); 2, Colombian night monkey (Aotus lemurinus), VU (photo credit: Juan Felipe León León); 3, Black-capped capuchin (Sapajus apella), LC (photo credit: Joan de la Malla); 4, Munduruku marmoset (Mico munduruku), VU (photo credit: Marlyson Costa. (B) Afrotropics: 1, Western gorilla (Gorilla gorilla), CR (photo credit: Rhett Butler); 2, Grivet monkey (Cholorocebus aethiops), LC (photo credit: Sarie Van Belle); 3, Western chimpanzee (Pan troglodytes), CR (photo credit: K.H.); 4, Spectacled Lesser Galago (Galago matschiei), LC (photo credit: Yvonne A. de Jong and Thomas M. Butynski). (C) Indo-Malay: 1, Sumatran orangutan (Pongo abelii), CR (photo credit: Perry van Duijnhoven); 2, White-headed langur (Trachypithecus poliocephalus), EN (photo credit Paul A. Garber); 3, Philippine Slow Loris (Nycticebus menagensis), VU (photo credit: Hery Sudarno); 4, Skywalker gibbon (Hoolock tianxing), EN (photo credit: Fan Peng-Fei). See tables S3 to S5 for spatial metrics for each of these species.
A key factor placing primate populations at risk is high deforestation rates (~11 million ha/year between 2001 and 2018 across the primate range) to satisfy the unsustainable demands of industrial societies for food and nonfood commodities (9, 10). Tropical deforestation accounts for 20 to 25% of total human-generated greenhouse gas emissions worldwide and the overwhelming majority of emissions in many primate habitat countries (11). A global assessment of forest loss in the tropics indicates that between 2000 and 2010, the proportion of forest edge increased from 27 to 31% of the total forested area, resulting in a marked reduction in habitat connectivity and an expansion of fragmented landscapes (12). Additional threats to primate populations are infrastructure development, urban expansion, climate change, human and domestic animal–borne infectious diseases, unsustainable subsistence hunting, the illegal trade of wild meat, body parts, and live individuals, and the dispossession (i.e., loss of residence, political, and economic control) of Indigenous Peoples from their traditional homelands (3, 4, 9, 13–16).
Indigenous Peoples represent a large proportion of the world’s contemporary cultural diversity, including ~3000 languages and systems of beliefs, knowledge, and relationships concerning humans and the rest of the natural world (17, 18). They account for 6% (~370 million to 476 million people) of the world’s population and live in 90 countries, principally in the tropics and subtropics [(19, 20); Supplementary Text for the definition of Indigeneity]. Indigenous Peoples manage some 38 million km2 of land and have been pivotal in safeguarding global biodiversity and mitigating climate change (21–24) by contributing to global carbon sequestration through collective ownership of forested lands. Some 24% of global carbon stored above ground in the world’s tropical forests [~54.5 million metric tons of carbon (MtC)] is estimated to be managed by Indigenous Peoples and local communities (19, 25). Across seven Amazonian countries, Indigenous Peoples’ lands store more than 50% (41,991 MtC) of the region’s carbon (26), illustrating the importance of Indigenous stewardship for sustainable forest management and global climate stability (19, 27).
Indigenous Peoples have a long and collective ancestral relationship to their lands and natural resources. This relationship is grounded in their beliefs, practices, systems of knowledge, and social norms, which in turn are dependent on their physical, cultural, and spiritual well-being and the resilience of the ecosystems in which they live (28–30). Given that a substantial proportion of the world’s biodiversity inhabits lands managed by Indigenous Peoples, there is a growing recognition among researchers and conservationists that Indigenous perspectives, knowledge systems, and histories hold globally important conservation lessons (24, 31, 32). Many Indigenous leaders, scholars, and knowledge holders have been making this case for decades (if not longer) and raising awareness of the biocultural value of their lands (33, 34). For example, in many cases, Indigenous histories of land use, occupation, and management have resulted in “landscape domestication” (35), with the species composition of standing forests being altered to benefit human needs with minimal disruption to community-wide ecological functions and the region’s conservation value (36). Biodiversity decline is significantly lower on Indigenous Peoples’ lands than in other areas across the globe (2, 37). On the basis of the Human Footprint Index (which evaluates measures of human population density, roads, rail, and electrical power–generating infrastructures, agricultural and pasture lands, and the built environment associated with cities and towns), 45.2% of Indigenous Peoples’ lands are characterized as having a low human impact on the environment (23).
Indigenous sociocultural identities are intricately interwoven with the plant, fungus, and animal species found on Indigenous Peoples’ lands (29). In this regard, the close evolutionary relationship between humans and nonhuman primates, along with their long histories of coexistence in many parts of the world, has resulted in a vast body of traditional Indigenous knowledge of primate ecology and behavior, including rich representations of primates in local cultural and spiritual practices (38–41). Globally, ~36% of lands (11.6 million km2) with high environmental value (i.e., not strongly affected by human activities) and classified as Intact Forest Landscapes (42) are managed by Indigenous Peoples (22). The human population density on Indigenous Peoples’ lands has seldom exceeded 1 to 2 individuals/km2 [mean world population density in 2018 was ~59/km2; (43)]. We note that, in the case of Indigenous communities, modeling indicates that it is less their overall population density and more the distribution of the population across the landscape that affects primate densities (44). For example, in areas of Amazonia, where nearby Indigenous communities hunt primates across common areas of overlap using guns (see discussion of source-sink dynamics below), large-bodied primate species experience population decline. In contrast, when Indigenous communities rely on more traditional hunting technologies and space settlements across the landscape, large-bodied primate populations can persist at carrying capacity, even during periods of Indigenous population growth (44). Moreover, Indigenous communities traditionally have relied on multifaceted resource-based subsistence economies that focus on hunting and gathering, horticulture, and herding. In many cases, Indigenous Peoples tend to shift their patterns of land use seasonally or yearly and, in doing so, rarely exhaust or permanently undermine their natural resource base (45, 46).
Despite our understanding of the overall importance of Indigenous Peoples for conserving biodiversity, there is a lack of information on their role in safeguarding the world’s primate communities. For example, a recent study found that 60% of 4460 mammalian species had at least 10% of their range on Indigenous Peoples’ lands and that some 1000 species had over 50% of their range on Indigenous Peoples’ lands (23). However, the data were not evaluated by taxonomic group and, therefore, do not indicate the importance of Indigenous Peoples’ lands for primate conservation. Similarly, a global analysis examining the impact of the human footprint on over 5000 species of terrestrial vertebrates, including 1277 mammals, did not address the effects of anthropogenic change on primate survivorship (47). Given the impending extinction crisis faced by the over 500 primate species, and the role that primates play as indicators of ecosystem health (4), we combine information from the scientific literature on Indigenous and non-Indigenous land use with a spatial analysis of primate distributions to assess the role of Indigenous Peoples worldwide in protecting primate populations. Specifically, we examined four aspects associated with Indigenous Peoples well-being and primate survivorship: (i) Indigenous Peoples’ knowledge systems, cultures, and subsistence activities; (ii) the geographical overlap between primate range distributions and Indigenous Peoples’ lands, protected areas, and other lands (we define other lands as neither Indigenous Peoples’ lands nor protected areas) in the Neotropics, mainland Africa, Madagascar, and the Indo-Malayan realm; (iii) the effects of the human footprint on or near Indigenous Peoples’ lands and primate conservation; and (iv) the threat of land dispossession on both Indigenous Peoples’ livelihoods and primate survivorship. In addition, we test the hypothesis that Indigenous Peoples’ lands in the Neotropics, mainland Africa, and the Indo-Malay realm contain significantly more primate species than expected by chance compared to equally sized areas randomly located across each realm.
We recognize that data on primate species biomass, population density, genetic variability, and precise estimates of areas of suitable habitat would offer the strongest evidence for a causative relationship between Indigenous land management and primate population health (48). Such information, however, does not exist for most primate species. Therefore, we have relied on large and standardized datasets, including the International Union for Conservation of Nature (IUCN) shapefiles. In the case of primates, these distribution maps are based on expert knowledge of verified species locations. In this regard, they represent an improvement from other spatial metrics used in the IUCN Red List to assess species extinction risk such as the extent of occurrence (i.e., EOO, a minimum convex polygon that includes all recorded locations of the species range) and the area of occupancy [AOO, the area actually occupied by a species; see the IUCN Standards and Petitions Committee 2022 (49) and Supplementary Text for a discussion of the strengths and limitations of using different spatial estimates to assess a species distribution]. In the present study, we combined spatial analyses using the IUCN dataset with information on the number and occurrence of IUCN threatened (Vulnerable, Endangered, and Critically Endangered) primate species on Indigenous Peoples’ lands, protected areas [from the World Database for Protected Areas, (50)], and on other lands, as an indication of the value of these areas in promoting primate persistence.
The definition of Indigeneity adopted here is consistent with other recent studies of Indigenous Peoples (21, 23) and aligns with that found in the International Labor Organization Indigenous and Tribal Peoples Convention 1989 (No. 169) Article 1 [(51); Supplementary Text]. This definition does not include communities of people that manage resources in ways similar to others who are formally recognized as Indigenous Peoples but do not identify or are not identified as Indigenous. In doing so, we note that local communities in Madagascar, a country of exceptional significance with over 100 species of primates, do not meet the definition of Indigenous Peoples by the International Labor Organization Indigenous and Tribal Peoples Convention 1989 (No. 169) Article 1 (Supplementary Text). Madagascar has many local communities that maintain intergenerational connections to place and nature through their livelihoods, cultural identities, worldviews, institutions, and ecological knowledge (52). Although many of these communities share certain characteristics with Indigenous Peoples (e.g., long histories of place-based living, subsistence economies, and distinct cultural practices), they do not self-identify as Indigenous. The academic literature refers to them as non-Indigenous local communities [e.g., (53, 54)]. Consequently, we treat Madagascar separately from all other primate regions in most of our analyses (see below).
INDIGENOUS PEOPLES’ TRADITIONAL KNOWLEDGE SYSTEMS AND PRACTICES AND PRIMATE CONSERVATION
Sustainable land use
Most Indigenous communities have developed land-use systems that promote three notable features vital for sustainability: (i) high levels of biodiversity, (ii) socioecological resilience, and (iii) stable stewardship over long periods. The formation of landscape mosaics under Indigenous management is critical in maintaining and promoting biodiversity (17, 22, 24, 55–57). Indigenous resource use strategies result from the intergenerational transmission of knowledge, are often communicated through oral histories, and encompass traditional systems of species and landscape classification, sustainable resource use, and symbolic ritual and religious practices. These systems have enabled Indigenous societies to persist for millennia in a wide range of environments, often through coexistence with local biodiversity, including primates (32, 58, 59). Most traditional knowledge is linguistically exclusive such that each Indigenous language encapsulates and represents unique information concerning plants, animals, landscapes, and their sustainable management (18, 60–62).
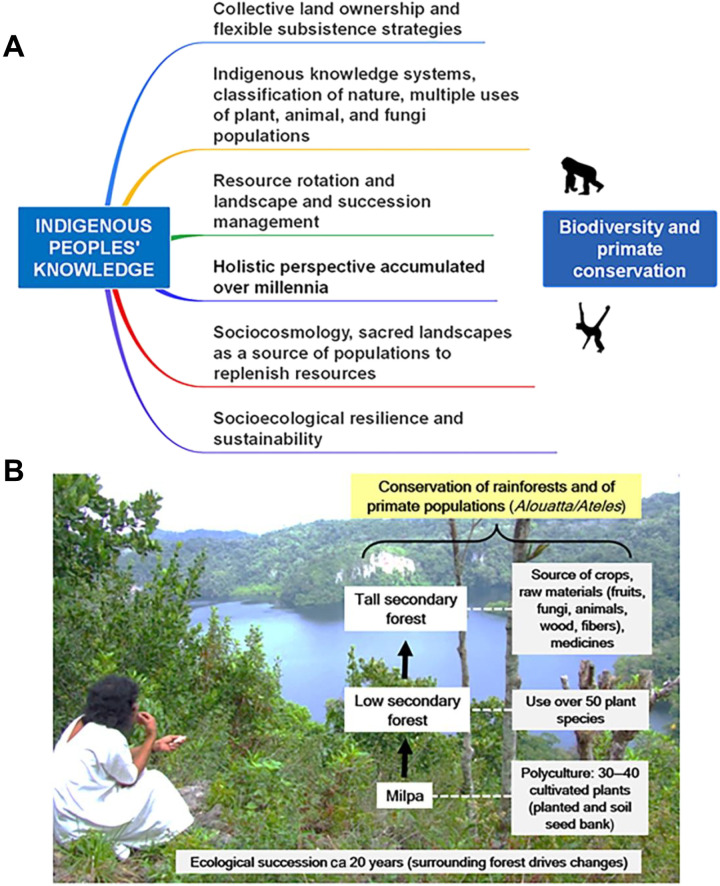
Many Indigenous Peoples practice low impact and resilient land use, which often includes patterns of spatial and temporal resource rotation and landscape management, incorporated within sociocosmologies that value and promote biodiversity (Fig. 2) (17, 29, 45, 46, 55, 63–65). An assessment of vertebrate biodiversity in Brazil, Canada, and Australia found that Indigenous Peoples’ lands and protected areas were more species rich than a random set of other lands of similar size. Within each country, species richness was highest on Indigenous Peoples’ lands and lowest on other lands (31). Moreover, a geospatial analysis of the nine countries that comprise the Amazon basin found that from 2000 to 2015, 8% of deforestation occurred on Indigenous Peoples’ lands, 7% in protected areas, and 83% on other lands (29, 66–69). In Brazil, more than half of all Indigenous Peoples’ lands (n = 587 of the 690 Indigenous territories recognized by the national government) retain 90% of their natural vegetation. Within a 10-km buffer of Indigenous Peoples’ lands, natural vegetation cover has been reduced to 52% (70). Several geospatial analyses across the Amazon basin have shown that Indigenous Peoples’ lands play a fundamental role in buffering against deforestation and forest degradation (29, 66–69, 71). In addition, data collected over a 34-year period found that natural vegetation conversion in Brazil was lower in protected areas and areas governed by Indigenous Peoples than on other lands (72). These current trends are reinforced by archaeological data on human alteration of Amazonian tropical forests over the past 5000 years. Over many millennia, Indigenous Peoples were primary actors, knowledge holders, managers, stewards, stakeholders, and decision makers over their lands, and coexisted with and helped sustain large expanses of comparatively unmodified forests (32, 73). As the species richness of primates usually increases with the landscape-scale cover of old-growth forests (74–76), primates benefit from minimally disturbed forests and areas of low human population density, conditions often present on Indigenous Peoples’ lands (77).
Fig. 2. Indigenous Peoples’ sustainable use of natural resources.
(A) Summary of critical concepts in Indigenous Peoples’ sustainable use of natural resources favoring biodiversity and primate conservation. Indigenous Peoples’ knowledge systems are transmitted orally from generation to generation (61). (B) Example of ecological engineering by Lancandon Indigenous Peoples of southern Mexico involving the sustainable use of natural resources. “Milpa” is a crop-growing system used throughout Mesoamerica. Lacandon Indigenous Peoples’ flow diagram based on information in (247). Photo by A.E. taken with Lacandon Indigenous Peoples’ informed consent.
Primate hunting by Indigenous Peoples
Traditional foods are those that Indigenous Peoples consume locally and are embedded in systems of ontology, cultural knowledge, and beliefs. These foods are procured through farming, herding, or the harvesting of plants, animals, and fungi (78–81). Within Indigenous communities, hunting lore, behavioral proscriptions, and food taboos often emphasize environmental balances, and reciprocal exchanges between humans, prey species, and other nonhuman beings. These, at least in principle, constrain excessive hunting and can facilitate sustainable ecosystem relationships (63, 82, 83). Moreover, although overhunting and climate change were likely factors leading to the extinction of several species of large-bodied mammals during the late Pleistocene and early Quaternary in North America, South America, and Madagascar, it is likely that unsustainable predator-prey relationships developed when naïve prey encountered human hunters for the first time (84, 85). This contrasts with the situation with many Indigenous Peoples today, who now have long histories in their homelands. In Amazonia, for example, many Indigenous Peoples consider primates to be fundamentally human in their origins and underlying essence (41). Even when used as a source of meat, they commonly occupy a unique role in mythology, culture, and pet-keeping practices (41, 86). Among the Awá-Guajá people of Brazil, for example, the fundamental humanity of howler monkeys is evident both in the way they are hunted (which involves reciprocal “singing” between humans and howlers) and in adopting orphaned howler monkey juveniles as pets in their settlements, where these primates and other wild animal pets often “surpass the number of human beings” (86). In some cases, food taboos involving primates appear to be rooted not in conservation considerations, but rather as a strategy to avoid zoonotic diseases or avoidance of foul-tasting meat (52, 86).
Primate hunting has been critical to the food sovereignty of Indigenous Peoples for thousands of years (87–91). In the Neotropics, primates are among the three most numerically dominant orders of prey mammals hunted by the Waorani (92, 93), the Waiwai (6, 94, 95), the Waimiri (96), the Kayapó (97), the Matis (98), the Shuar (99), and the Matsigenka (100). The Aguaruna target yellow-tailed woolly monkeys (Lagothrix flavicauda) for their festivals, in part, to make headdresses and for meat (101). The Canelos Kichwa target woolly monkeys (Lagothrix spp.) during their hunting hista festival, which “lasts for four days and is preceded by weeks of preparation, during which long hunting trips to provide meat for the celebrations play a central role” (102). In central African forests, the most consumed terrestrial mammals by all hunters are ungulates (40 to 80% of offtake biomass), rodents (10 to 30%), and primates (4 to 30%) (103–106). Patterns of primate hunting by Indigenous Peoples in Asia are less well studied than in Amazonia and Africa (107–109). Generally, pigs, ungulates, and primates are the most frequently harvested taxa across the region (45, 110, 111), with primates more highly preferred by Indigenous groups such as the Iban, Jahai, and Mentawai in Southeast Asia and the Mishmi, Meyor, and Nyishi of South Asia (87, 92, 93). Among Indigenous communities in Asia, primate hunting preferences vary considerably in response to religious taboos, the prevalence of crop raiding by primate species, and the local use of primate body parts for traditional medicines (112). In particular, primates such as gibbons, langurs, and orangutans are frequently hunted for food, for traditional medicine, or as pets (113–115). Historically, most Indigenous Peoples hunted for subsistence purposes, but externally imposed political/economic conditions, land dispossession, and high demand for primate (and other wildlife) parts by consumers in countries such as China have increased commercial hunting, potentially altering the sociocultural contexts and constraints for primate harvesting (116). For example, bushmeat hunting of primates for sale in local markets has become more common in some areas, particularly in central Africa (104, 117). In Asia, the internet has created a new and expanding illegal market for primates as pets (118, 119).
Not all primate hunting is sustainable. However, the greatest threats to primates globally are not from Indigenous communities but from unsustainable non-Indigenous hunting, deforestation, and industrial agriculture (e.g., palm oil), in addition to transport and hydroelectric infrastructure development. These actions are supported by national governments, agribusinesses, and international financial institutions (9, 14). Primates, particularly larger-bodied species, are vulnerable to overhunting due to their relatively low reproductive rates and extended period of juvenile development (90, 120, 121). Thus, at some sites where Indigenous hunting occurs, densities of large- and medium-bodied taxa have been reduced by 20 to 60% (121–125). Indigenous hunting has directly led to local extinctions of primate species such as orangutans at several sites in Borneo (114). Similarly, central Amazonian Indigenous hunters may have led to the extirpation of primates like black spider monkeys (Ateles paniscus) in the Solimões-Rio Negro river interfluvium before European conquest (82). More recently, engagement with the global economy has meant that some Indigenous and other traditional peoples with access to commercially valuable faunal resources have become engaged in regional or even international markets for wild animal meat, hides, or parts, with potentially devastating effects on tropical biodiversity (126, 127).
The widespread adoption of firearms by Indigenous and other hunters worldwide is a major technological transformation that can lead to the local extinctions of large-bodied primates and other vulnerable prey (97, 128), a factor often overlooked in anthropological studies of Indigenous hunting ideology (82). Numerous authors have argued that changes in Indigenous hunting techniques (from blowguns and bows and arrows to firearms) increased assimilation into the cash economy, and increases in human population size have pushed even Indigenous hunting to unsustainable levels (44, 129–132). However, other assessments indicate that technology (i.e., guns versus bow and arrow) and the spatial overlap of hunting zones between adjacent settlements—but not human population growth per se—are the primary factors in primate population decline in Amazonia. At one site, the introduction of firearms so severely depleted large-bodied primate populations that hunting efficiency (measured in kilogram harvested per hour) dropped to pre-firearm levels within only 7 to 15 years (7). Therefore, without the appropriate cultural safeguards, any short-lived increase in human welfare brought about by firearms is counterbalanced over the long term by a marked increase in the rarity of primate species (82), a decrease in ecosystem services, and a disruption of the long-term well-being and livelihoods of Indigenous communities (133).
However, many Indigenous Peoples practice tacit or explicit social and ecological controls that serve to reduce their impact on harvest-sensitive species ( Fig. 2) (134). Although primates represent an essential source of wild meat for many Indigenous Peoples, low human population densities on Indigenous Peoples lands, management practices that favor the maintenance of standing forests, less efficient traditional weaponry, food taboos, and other cultural norms have facilitated the long-term survival of primates and other vulnerable wildlife on Indigenous Peoples’ lands (32, 113, 134–136). The Matsigenka people of Manu National Park, Peru, for example, refrain from hunting monkeys during the dry season, preferring to wait until the rainy period when species “fatten” on ripe fruits, thus giving vulnerable taxa a seasonal reprieve (82, 137). Indigenous Peoples of the upper Xingu River practice some of the most extensive food taboos of any peoples of Amazonia, including a near-total avoidance of consuming large primate species (82). In the western Amazon, the Cocama (Kukama) people of the Samiria River adapt to seasonal declines of wild meat populations by markedly reducing the hunting of primates and other large mammals and expanding their fishing activities. This allows mammal populations to recover, facilitating more sustainable management of natural resources (Fig. 2) (134, 138).
Tzeltal and Mestizo hunters in the Lacandon Forest, Chiapas, Mexico rarely hunt primates, as they claim that “monkeys look like small people” (139). The Baka people of Cameroon consider the gorilla and chimpanzee to be special animals related to humans through reincarnation (140). In the ontologies of several Indigenous forager peoples of the Congo basin, primates often cross interspecies boundaries between humans and nonhumans [e.g., (141–143)]. Not surprisingly, many of these Indigenous populations hunt fewer primates than nontraditional populations of the Congo: In a multisite comparison of hunting between Indigenous and non-Indigenous communities throughout central Africa, Indigenous hunters harvested primates (4% of offtake) much less frequently than non-Indigenous hunters (16%) (Table 1) and sold a considerably smaller percentage of their harvest (35%) than did non-Indigenous communities (65%) (103).
Table 1. Global primate range.
Estimated area in square kilometers (millions) of the primate distributional range, IPLs, PAs, and OLs. IPL∩PA indicates the area common to both IPLs and PAs. “IPLs alone and PAs alone” refers to the extent of each area type that does not overlap the other, that is, their exclusive area. Although according to the International Labor Organization Indigenous and Tribal Peoples Convention 1989 (No. 169, Article 1) (51), Madagascar has no Indigenous Peoples, it is included here for comparison with the other regions in terms of the extent of primate ranges and protected areas.
| Total primate range | IPLs total | PAs total | OLs | IPL∩PA | IPLs alone | PAs alone | No. of PAs | No. of PAs in IPLs | |
| Neotropics | 14.4 | 3.0 | 5.2 | 6.3 | 2.2 | 0.8 | 2.9 | 5416 | 1734 |
| Mainland Africa | 20.0 | 6.6 | 4.4 | 9.1 | 1.5 | 5.1 | 2.9 | 6229 | 1837 |
| Madagascar | 0.46 | – | 0.07 | – | – | – | – | 171 | – |
| Indo-Malayan | 11.2 | 4.4 | 1.0 | 6.0 | 0.5 | 3.7 | 0.5 | 4942 | 1070 |
| Global | 46.0 | 14.0 | 10.6 | 21.4 | 4.2 | 9.6 | 6.3 | 16,758 | 4641 |
Indigenous hunting is mostly consistent with a central place foraging model such that hunters create local “sinks” within a 10- to 15-km radius around settlements (depending on the spatial spread of hunters) that are replenished as animals disperse from unhunted “source” areas, which sometimes include sacred landscapes, outside the sink radius (6, 44, 94, 144, 145). These source-sink dynamics can contribute to sustainable hunting over long periods with limited large-scale faunal depletion (Supplementary Text and fig. S1) (6, 24, 29, 80, 83, 93, 135–138, 146–149). Moreover, if the human population density of colonists bordering Indigenous Peoples lands is relatively low, even vulnerable primate species and ecosystems can persist by repopulating from distant, nonhunted zones (44, 133). As long as Indigenous Peoples’ lands remain under Indigenous Peoples’ sovereignty, this natural mechanism of species recovery can be enhanced by community-based management of subsistence hunting in areas where the forest cover is largely intact (44, 82, 150).
GEOGRAPHICAL OVERLAP OF PRIMATE DISTRIBUTIONS ON INDIGENOUS PEOPLES’ LANDS, PROTECTED AREAS, AND OTHER LANDS
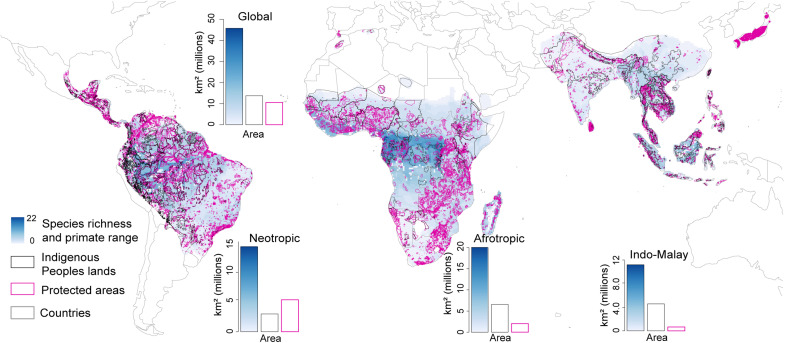
Given the role of Indigenous Peoples in environmental stewardship, we conducted a spatial analysis of primate species distributions, primate species’ diversity, and primate species conservation status on Indigenous Peoples’ lands, protected areas, and other lands. The results indicate that the global primate range encompasses ~46 million km2. Mainland Africa has the largest area (40%) of the global primate range followed by the Neotropics (30%), the Indo-Malayan (24%) realm, and Madagascar (1%) (Table 1 and Fig. 3). Indigenous Peoples’ lands account for 30% of the primate range, protected areas 23%, and other lands 47% (Fig. 3 and table S1). In the Indo-Malay realm, Indigenous Peoples’ lands account for ~36% of the primate range, in mainland Africa ~33%, and in the Neotropics ~21%. In contrast, protected areas account for ~35% of the primate range in the Neotropics, ~22% in mainland Africa, and only ~9% in the Indo-Malay realm. Across these regions, other lands account for between 44 and 55% of the primate distribution (Figs. 3 and 4A, Table 1, and table S1). Overall, Indigenous Peoples’ lands overlap the ranges of 71% of the world’s primate species.
Fig. 3. Global geographic overlap among primate distributions, Indigenous Peoples’ lands, and protected areas.
The primate species range data source was the IUCN Red List 2021. The number of primate species per realm is indicated to the immediate right or left of that region. We defined species richness as the overlap of the polygons describing the geographic distributions of all primate species onto a cell grid of 0.5° resolution in latitude and longitude. Numbers by each region refer to the number of primate species present. The source of data on Indigenous Peoples’ lands is from (21). Protected areas from Protected Planet (50). For this spatial analysis, we did not separate protected areas into their different categories; we considered categories I to VI as a group. The resolution may be inexact because boundaries between Indigenous and other lands are often contested. Given Madagascar’s unique situation regarding no Indigenous Peoples, we estimated that its primate range encompasses an area of ~0.469 million km2 or 80% of Madagascar’s land area (ca 587,041 km2); protected areas account for 17% of the primate range and other lands 83% (Table 1 and fig. S5).
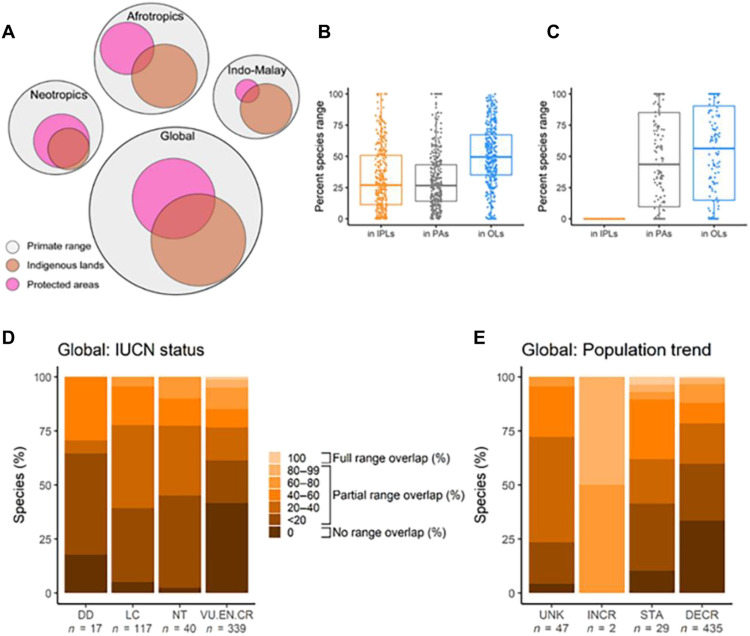
Fig. 4. Spatial distribution of Indigenous Peoples’ lands and the conservation status of primate species that inhabit these lands.
(A) Euler plots of the intersection between Indigenous Peoples’ lands (IPLs) and protected areas (PAs) across the distributional range of primates globally and in each realm. Circles and areas of intersection in the global and ecoregion plots are proportional to the area of the natural range of primates (global, ~46 million km2; Neotropics, ~14 million km2; mainland Africa, ~20 million km2; Indo-Malayan realm, ~12 million km2; Table 1). (B) Boxplots showing the global percent of primate species (n = 362) whose ranges intersect with IPLs, PAs, and other lands (OLs). Other lands may include non-Indigenous community lands and protected areas not included in the World Database on Protected Areas (21, 50). (C) Global percent of primate species whose range does not intersect Indigenous Peoples’ lands (n = 151; Neotropics, 5 species; mainland Africa, 20 species; Madagascar, 107 species; and Indo-Malayan realm, 19 species). Also shown for these species is the percent of their range that intersects PAs and OLs (table S5). Boxplots for the Neotropics, mainland Africa, and Indo-Malayan region are presented in fig. S2. Original data are in tables S2 to S4. (D) Percent of primate species whose range overlaps with Indigenous Peoples’ lands’ and their IUCN conservation category. (E) Percent of primate species whose range overlaps with Indigenous Peoples’ lands and their IUCN population trend. IUCN conservation categories: DD, Data Deficient; LC, Least Concerned; NT, Near Threatened; VU, Vulnerable; EN, Endangered; CR, Critically Endangered; UK, Unknown; INCR, Increasing; STA, Stable; DECR, Decreasing. Source of IUCN data IUCN Red List (https://iucnredlist.org/). Bar graphs include the species from Madagascar since their ranges do not overlap Indigenous Peoples’ lands. See tables S2 to S6 for data on primate species in each primate range region.
We tested the hypothesis that Indigenous Peoples’ lands in the Neotropics, mainland Africa, and the Indo-Malay realm each contain significantly more primate species than expected by chance compared to equally sized random locations across each ecoregion. To accomplish this, we created polygons the same size as those of Indigenous Peoples’ lands and placed them randomly across a given primate ecoregion. Then, we compared the expected species richness of these randomly located polygons with the actual primate species richness on Indigenous Peoples’ lands. The procedure was repeated 100 times per primate ecoregion to determine the probability of the null hypothesis, namely, that Indigenous Peoples’ lands contain the same number of primates species as any other equally sized area, including protected areas and other lands in that region. We found evidence of significantly greater primate species richness on Indigenous Peoples’ lands than in protected areas and other lands in the Neotropics (observed richness = 170 species; expected richness = 160 species; standardized mean difference θ = 1.873; P < 0.01) and in the Indo-Malayan region (observed richness = 106; expected richness = 81; θ = 3.32; P = 0.00). In the Neotropics, 41% (n = 70) of primates species have 25 to 75% of their distribution on Indigenous Peoples’ lands (only 30% of these species are threatened; table S2). In the Indo-Malayan realm, 85% (n = 88) have 25 to 100% of their range on Indigenous Peoples’ lands (92% of these species are threatened; table S4).
A different pattern of species richness characterized mainland Africa. In Africa, primate species richness on Indigenous Peoples’ lands was not greater than expected (observed richness = 86 species; expected richness = 89; θ = −0.278; P = 1.00). The results for Africa can be explained by the fact that protected areas in Africa are extremely primate species rich. Despite representing a smaller percentage of the land area than Indigenous Peoples’ lands, protected areas in mainland Africa contain 195 primate species, whereas Indigenous Peoples’ lands contain only 87 primate species (table S3). We found that 45% (n = 38) of these 87 species have between 25 and 82% of their total distribution on Indigenous Peoples’ lands (50% are threatened; table S3).
According to the Convention of Biological Diversity, many Indigenous Peoples’ lands can be considered as “other effective area-based conservation measures” (OECMs). OECMs are defined by the Convention of Biological Diversity as “a geographically defined area other than a Protected Area, which is governed and managed in ways that achieve positive and sustained long-term outcomes for the in situ conservation of biodiversity, with associated ecosystem functions and services and where applicable, cultural, spiritual, socioeconomic, and other locally relevant values” (151, 152). Our spatial analyses revealed that alongside nationally protected areas, Indigenous Peoples’ lands considerably add to the natural habitat occupied by primate populations (Fig. 4A and tables S1 to S4). In addition, we found that the percentage of primate species classified by the IUCN as Critically Endangered is significantly lower for primate species whose ranges overlap Indigenous Peoples’ lands than for those whose range does not overlap Indigenous Peoples’ lands (Neotropics, 40 versus 8%; mainland Africa, 35 versus 5%; Indo-Malayan region, 32 versus 17%) (Fig. 4, D and E and table S5). It is clear that national governments must engage with Indigenous leadership to create mutually agreed-upon policies that support Indigenous Peoples’ land tenure and management practices, enhance local and national primate conservation, and promote healthy ecosystems and the well-being of Indigenous people.
Globally, the ranges of 362 primate species (71% of the world’s primate species) intersect Indigenous Peoples’ lands (Fig. 4, A and B, and tables S2 to S4). Of these, 48% are from the Neotropics, 28% are from the Indo-Malayan realm, and 24% are from mainland Africa. These species also have part of their ranges intersecting protected areas and other lands (tables S2 to S4 and fig. S3). We found no primate species whose range is only on Indigenous Peoples’ lands. The ranges of the remaining 151 primate species (29%) do not overlap with any Indigenous Peoples’ lands (Fig. 4C and table S5). Of these, 71% are from Madagascar, 13% are from mainland Africa, 13% are from the Indo-Malayan region, and 3% are from the Neotropics (table S5). While 93% of the 151 primate species whose ranges do not overlap with Indigenous Peoples’ lands are classified as threatened (Vulnerable, Endangered, Critically Endangered) by the IUCN, only 55% of the 362 primate species whose ranges intersect Indigenous Peoples’ lands are threatened. Similar patterns are evident for each primate region (Fig. 4D and tables S2 to S4).
We note that these results are partially driven by the heavy representation of lemurs among threatened primates and the fact that there are no areas classified as Indigenous Peoples’ lands in Madagascar (table S6). However, even when the lemurs of Madagascar are removed from the analysis, 88% (38 of 43 species for which data are available) of primates species in Africa, the Neotropics, and the Indo-Malay realm, whose ranges do not overlap Indigenous Peoples’ lands, are threatened with extinction (table S5), compared to 55% of primate species whose ranges overlap Indigenous Peoples’ lands. Moreover, the number of primate species classified as threatened by the IUCN decreases from 20 to 5% as the extent of that species range on Indigenous Peoples’ lands reaches 75% (Fig. 4D). A similar pattern is evident regarding the number of primate species classified by the IUCN as having decreasing populations. As the extent of their range on Indigenous Peoples’ lands increases, fewer primate species are classified as having declining populations (reduction from 26 to <3%; Fig. 4E). Analogous trends are evident for each primate region (fig. S4). Although the evidence presented here is correlative, these data offer strong support for the contention that Indigenous Peoples’ lands provide essential safeguards for primate species population persistence.
Madagascar represents an unusual situation for primates. Madagascar maintains 107 endemic species of lemurs (20.5% of all primate species), and some 90% of its original forest has been lost. Approximately 96% of Malagasy primate species are listed by the IUCN as threatened, and 100% have declining populations (table S6). Data contained in table S6 indicate that there are 22 Malagasy primate species that have 100% of their range on protected lands. Each of these 22 species is threatened (10 are Critically Endangered, 11 are Endangered, and 1 is Vulnerable). There are no differences in the conservation status of Malagasy primates living in protected reserves or on other lands. In addition, although many ethnic groups in Madagascar practice community forest management (CFM), a study by Rasolofoson et al. (153) found that deforestation on CFM lands was not significantly lower than on non-CFM lands (0.02% less deforestation on CFM lands). Therefore, regardless of whether Malagasy primates live in protected areas or on other lands, including those managed by non-Indigenous ethnic communities, they are threatened with extinction. It appears that historical factors associated with colonization have played a major role in deforestation, land conversion, and lemur decline across Madagascar. For example, between 1895 and 1925, three-fourths of the island’s primary forests were cleared (154). From 1950 to 1985, deforestation rates in the eastern rainforest of Madagascar averaged 1.5% per year (155). Some 61 lemur species inhabit the eastern rainforest (3), and their ultimate survival is dependent on protecting Madagascar’s remaining natural forests.
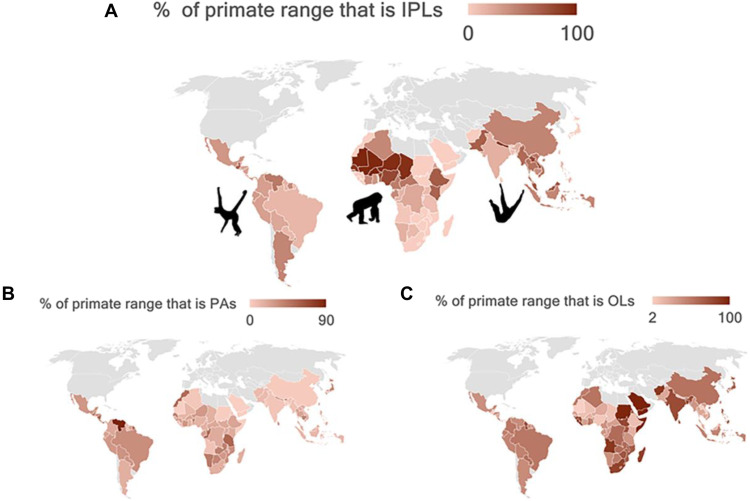
Worldwide, the distribution of Indigenous Peoples’ lands varies greatly across primate range countries, something necessary to consider in designing effective policies to promote primate conservation (Fig. 5 and table S1). In the Neotropics, 6 of 20 primate range countries (Brazil, Venezuela, Colombia, Peru, Bolivia, and Mexico) account for ~86% of the primate distribution on Indigenous Peoples’ lands (ca. 2.8 million km2) (Fig. 5 and table S1). In mainland Africa (45 countries), Mali, Ethiopia, Nigeria, Chad, Niger, and Democratic Republic of the Congo (DRC) make up ~62% of the primate range on Indigenous Peoples’ lands (6.5 million km2) (table S2). In the Indo-Malayan region (23 countries), China, India, Indonesia, Pakistan, Myanmar, and Thailand account for ~81% of the primate range on Indigenous Peoples’ lands (4.3 million km2) (Fig. 5 and table S1). Although the most effective conservation solutions require input and consensus at the country, regional, and local levels, strengthening Indigenous land rights will have particular benefits for primate species and populations in these countries.
Fig. 5. Percent of primate ranges across all primate range countries that overlap with IPLs, PAs, and OLs.
(A) IPLs, (B) PAs, and (C) OLs (table S1). PAs refer to IUCN categories I to VI. Country profiles of IPLs and PAs in fig. S2. Original data in table S1. Primate silhouettes from www.phylopic.org.
HABITAT CONVERSION, INDIGENOUS PEOPLES’ LANDS, AND PRIMATE CONSERVATION
In many tropical regions, large-scale land conversion of native vegetation for industrial, agricultural, and natural resource extraction by multinational corporations, and with the support of national governments, has resulted in severe habitat loss and degradation. These are major threats to primate populations (4, 9). These same factors imperil Indigenous Peoples’ lands, languages, cultures, systems of knowledge, and livelihoods (62, 156). Given projected increases in human population growth, infrastructure development, mega-dam construction, and the unsustainable demands of consumer nations, ecosystem health across several primate species–rich regions, including the Atlantic Forest of Brazil, the Guinean Forests of West Africa, and most of South and Southeast Asia, is expected to continue to decline (3, 14, 157). To better understand the potential severity of critical anthropogenic threats to primates, Indigenous Peoples, and the environment, we used the most recent version of the Human Footprint (HF) database (157) to compare the degree of human pressure within Indigenous Peoples’ lands relative to adjacent areas. The HF provides a quantitative measure of the accumulated effects of the expansion of urban areas, croplands, pasture lands, human population density, nighttime lighting, roads, railways, and navigable waterways on the environment. Although the HF does not consider all factors that directly contribute to habitat degradation and primate population decline (i.e., fires, hunting, forest fragmentation, and capture for the pet trade), ecosystems characterized by a higher human footprint tend to be more fragmented, less species rich, and less resilient to additional habitat loss (158). The HF ranges from a score of 1 (minimal pressure) to a score of 50 (maximum pressure).
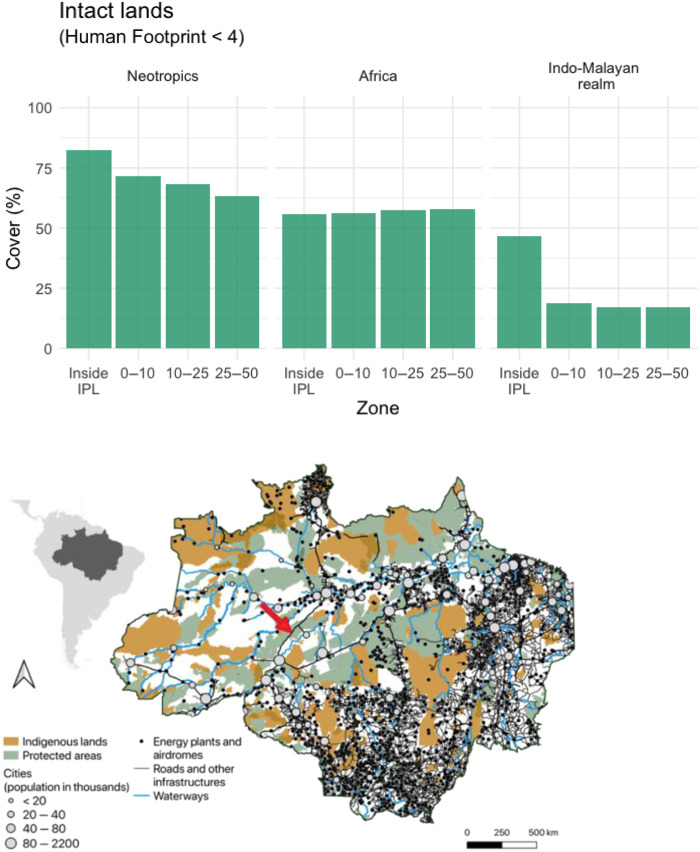
Following previous research (159, 160), we defined “intact land” as those areas with a Human Footprint value of <4. This threshold serves as a boundary beyond which anthropogenic activities significantly change the habitat from largely natural to highly modified (159). We extracted information on the HF from 330,000 randomly selected locations inside Indigenous Peoples’ lands, within 10 km of Indigenous borders, and at distances of 10 to 25 km and 25 to 50 km from the border of Indigenous Peoples’ lands. Although the selection of these distances is arbitrary, we feel that they offer a reasonable measure of the impact of habitat conversion on lands immediately adjacent to and at greater distances to Indigenous Peoples’ lands. In our analysis, the number of random locations was proportional to the area contained in each of these four zones (56% of HF scores were located inside Indigenous Peoples’ lands, 12% within a 10-km buffer of Indigenous Peoples’ lands, 14% within a 25-km buffer, and 19% within a 25- to 50-km buffer of Indigenous Peoples’ lands). We used binomial generalized linear models (GLMs), with intact land as the dependent variable and “zone” as a single predictor. Comparisons were performed for each primate region separately (Neotropics, Afrotropics, and Indo-Malayan realm).
We found that in the Neotropic and Indo-Malayan realms, the likelihood of having intact landscapes (HF value <4) within Indigenous Peoples’ lands was significantly greater than for all adjacent zones located up to 50 km from the borders of Indigenous Peoples’ lands (table S7). This suggests that it is not simply isolation/distance from developed areas that is driving these patterns. In contrast, the proportion of intact land did not differ inside and outside of Indigenous Peoples’ lands in Africa (Fig. 6, top). In the Neotropics, the proportion of intact lands was found to decrease with increasing distances from Indigenous Peoples’ lands, whereas in the Indo-Malayan realm the proportion of intact lands was relatively low and equal at distances from 1 to 50 km of the borders of Indigenous Peoples’ lands (Fig. 6A, top, and table S7). The fact that the proportion of intact land was lower in areas immediately outside (<10 km) of Indigenous Peoples’ lands in the Neotropic and Indo-Malayan realms reinforces the need to guarantee land rights to Indigenous Peoples to ensure that their territories are protected from further colonization and deforestation (161).
Fig. 6. The Human Footprint, infrastructure density, Indigenous Peoples’ lands, and primate geographic distributions.
(Top) Frequency of intact lands, i.e., areas with a Human Footprint <4, within IPLs and on lands immediately adjacent to IPLs: up to 10 km from IPL borders, 10 to 25 km from IPL borders, and 25 to 50 km from IPL borders, across the Neotropic, African, and Indo-Malayan realms. (Bottom) Infrastructure distribution across the Legal Amazon of Brazil (dark gray area in the inset). Infrastructure data were obtained from MapBiomas (https://mapbiomas.org/en) and OpenStreetMap (https://openstreetmap.org/#map=4/38.01/-95.84). Together with state and federal roads, we also show energy infrastructures, mining areas, waterways (used for people and cargo transportation), main cities (size proportional to population), energy plants, and aerodromes. BR-319 is highlighted by the red arrow. IPLs and PAs are from TerraBrasilis (URL: terrabrasilis.dpi.inpe.br; accessed in May 2021). The road data in OpenStreetMap are ~83% complete. Therefore, our results may underestimate the extent of roads in the Brazilian Amazon. See fig. S6, Supplementary Text, and (248) for procedures.
The situation in Africa (relatively equal proportion of intact habitat inside and within 50 km of Indigenous Peoples’ lands) may be best explained by a combination of factors. For example, across parts of the Sahel and East Africa (fig. S7), a large proportion of people who identify as Indigenous are itinerant pastoralists (African Commission on Human and Peoples’ Rights 2006). Much of their land overlaps or intersects with altered agricultural landscapes occupied by non-Indigenous sedentary farmers and therefore has a high human footprint (21). In contrast, in Africa’s Congo basin, which maintains the largest area of tropical forest outside of Amazonia (162), the human footprint inside and immediately outside of the boundaries of Indigenous Peoples’ lands has remained relatively small (fig. S7). Thus, historical differences in patterns of Indigenous transhumance and subsistence, along with regional differences in anthropogenically driven habitat conversion, serve to distinguish the conservation challenges faced by Indigenous Peoples and primates across the Neotropics, Africa, and the Indo-Malayan realm.
A critical driver of habitat change is linear infrastructure development. Well-planned transportation infrastructures, such as roads and railways, can help improve a country’s local and national economy, improve access to essential services and markets, and increase global economic integration (163, 164). Yet, these infrastructure projects facilitate the movement of people into remote areas, often resulting in resource extraction, and highly fragmented and affected landscapes (15, 162). Current projections estimate that, by 2050, an additional 2 million km of roads will be built in primate range regions, with an average increase in road length of 16% in South America, 41% in Africa, and 25% in Asia (165). Many of these new infrastructure and transport connectivity projects are part of China’s one trillion USD Belt and Road Initiative (BRI) (166). Officially launched in 2013, the BRI is primarily intended to increase trade among China, much of Asia, Europe, and Africa. Similar infrastructure initiatives targeting low- and middle-income primate habitat countries, such as the G7’s ‘Build Back Better World’ (167), the European Union’s “Global Gateway” (168), and the Integration of Regional Infrastructure in South America (169), are designed to add or upgrade large numbers of roads, railways, mega-dams, powerlines, deepwater ports, and other infrastructures.
These massive infrastructure investments will create itinerant or permanent settlements of colonists, loggers, miners, poachers, agricultural workers, and others, legally or illegally exploiting natural resources in these newly opened landscapes (170–172). This process will likely further disrupt natural ecosystems on the borders of and within Indigenous Peoples’ lands through water, air, and soil pollution, logging, habitat fragmentation, bush-meat hunting, the live primate trade, and the spread of zoonotic diseases and invasive species. This will create permanent barriers to primate dispersal and gene flow (173–178). A recent study identified 32 primate species that are considered highly vulnerable to the environmental impacts of infrastructure development, and whose distributional ranges already include a high density of transportation and energy infrastructures (14, 15). These include the Critically Endangered Tapanuli orangutan (Pongo tapanuliensis) found in Sumatra, Indonesia. This species has lost 95% of its historical range since 1940. The remaining total population consists of less than 800 individuals, inhabiting a small, fragmented forest area (179). There is heightened concern worldwide that the proposed Batang Toru hydropower dam project will cause the extinction of the last remaining population of this ape species (172). Similarly, the western chimpanzee (Pan troglodytes verus) is negatively affected by roads across 95.7% of its present geographical distribution, with the road-effect zone (i.e., the distance up to which the presence of major roads reduces the density of a species) estimated as 17.2 km (95% confidence interval, 15.8 to 18.6 km), which is three times greater than that of minor roads (180). Likewise, several primate species endemic to the Atlantic Forest of Brazil (which has lost some 70% of its original habitat) (181), including the Endangered golden lion tamarin (Leontopithecus rosalia), the Critically Endangered southern muriqui (Brachyteles arachnoides), and the Endangered blonde capuchin (Sapajus flavius), are threatened with extinction due to deforestation and infrastructure development (173). Moreover, major hydroelectric projects in tropical river basin systems such as the Amazon, Congo, and the Mekong, and the expansion of large-scale industrial agriculture and road networks by multinational corporations have resulted in the widespread displacement of Indigenous Peoples, leaving all remaining biodiversity and forest habitats vulnerable to invasion and overexploitation (9, 182, 183).
The potential benefits of roads and other infrastructures to Indigenous Peoples have seldom been quantified (184). However, an analysis of the expected environmental, social, and economic impacts of 75 road projects (ca. 12,000 km of new roads) planned for the Amazon region found that each project would negatively affect both the environment and the Indigenous Peoples living there (185). Road projects such as the “Capitán Augusto Rivadeneira–Reperado” in Ecuador and the “Mitú–Monforth” and the “Puerto Leguizamo–La Tagua” in Colombia would cut across Indigenous territories, opening the region to recurrent exploitation and deforestation by poachers, loggers, miners, agribusiness, and cattle ranchers (185, 186). Similarly, recent changes in government policy in the Brazilian Legal Amazon, an area covering ca. 115 million ha and home to 424 Indigenous territories (186) and some 95 primate species (roughly 75% of all primate species in Brazil, the world’s primate-richest country with 131 primate species), threaten to destabilize this critical ecosystem (3). The northern half of the Brazilian Legal Amazon is relatively free of infrastructure surrounding Indigenous Peoples’ lands; however, this is not the case on the southern rim, which is characterized by an arc of deforestation dominated by high-density infrastructures such as roads, pipelines, and powerlines (Fig. 6, bottom) on the boundary of Indigenous Peoples’ lands and protected areas (29, 77). In addition, a recent study found that in regions such as the Amazon, an increase in road density is expected to result in increased Indigenous economic dependency and the expansion of non-Indigenous government control over Indigenous communities. This will limit the ability of Indigenous Peoples’ to fully engage in their traditional lifestyle and cultural practices, resulting in the endangerment and loss of numerous Indigenous languages (187).
Even in those cases in which infrastructure projects remain outside of Indigenous Peoples’ lands, they facilitate access to the core areas of these lands and therefore promote extractive activities, land dispossession, and the spread of disease (188). One example is the paving of BR-319, a highway cutting through Brazil’s Amazon (Fig. 6, bottom) between Porto Velho, an area already heavily deforested, and Manaus, a city of over 2 million people. Once completed, this highway, together with accompanying side roads, is expected to increase deforestation within 150 km of the road by over 1200% by the year 2100. This will severely fragment and isolate forested areas on 63 Indigenous Peoples’ lands that are home to at least 18,000 Indigenous People (189). Assuming a road-effect zone of 150 km (188), at least 25 primate species will also likely be affected. Most notably, more than 66% of the distributional ranges of Pissinatti’s bald-faced saki (Pithecia pissinattii) and Nash’s titi (Plecturocebus stephennashi) will be negatively affected. The current conservation status of both species remains largely unknown (IUCN category Data Deficient).
Given the accumulated loss and fragmentation of natural habitat, biodiversity, and Indigenous sovereignty caused by an expanding human footprint across the global primate range (14, 190), immediate government actions and legislation are needed to mitigate these outside pressures on Indigenous Peoples’ lands and end land dispossession of Indigenous Peoples. Unfortunately, in primate range nations like Brazil, national legislation strengthening the ability of ranchers and business owners to assert legal claims over public lands, including Indigenous Peoples’ lands, while limiting the ability of Indigenous Peoples to delay or halt development, including infrastructure projects, mining concessions, and the expansion of industrial agriculture on their lands, is now law (70).
LAND DISPOSSESSION, INDIGENOUS PEOPLES, AND PRIMATE CONSERVATION
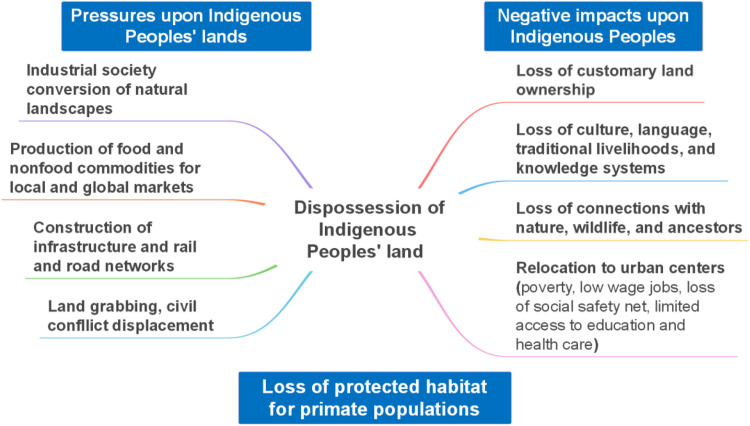
Land dispossession leads to the breakdown of Indigenous communities and their cultural, technological, and traditional practices. It erodes the languages and knowledge systems that have contributed to maintaining biodiversity for millennia (62). Dispossession also results in the loss of symbolic connections with nature and ancestors (Fig. 7) (32, 185, 188–191). This has occurred regardless of the United Nations Declaration on the Rights of Indigenous Peoples (UNDRIP), which recognizes that (i) “Indigenous peoples have the right to the lands, territories, and resources which they have traditionally owned, occupied or otherwise used or acquired” (192), (ii) “Indigenous Peoples have the right to own, use, develop and control the lands, territories, and resources that they possess because of traditional ownership or other traditional occupation or use, as well as those which they have otherwise acquired” [(185), article 26-2], and (iii) “States shall give legal recognition and protection to these lands, territories, and resources. And that such recognition shall be conducted with due respect to the customs, traditions and land tenure systems of the Indigenous peoples concerned” [(185), article 26-3]. Moreover, article 10 of the Declaration states, “Indigenous peoples shall not be forcibly removed from their lands or territories. No relocation shall take place without the free, prior and informed consent of the Indigenous peoples concerned and after agreement on just and fair compensation and, where possible, with the option of return” [(193); see also (194)].
Fig. 7. Major global socioeconomic pressures on IPLs.
(Left) Primary factors negatively affect the ecological integrity and ownership of IPLs resulting in land dispossession. (Right) Key consequences of land dispossession on Indigenous Peoples’ knowledge systems, culture, symbolic life, and well-being.
Notwithstanding their specialized systems of knowledge in managing their lands, Indigenous Peoples have rarely been given a voice or allowed to contribute to national or international decision-making. This has accelerated Indigenous land dispossession by industry, government, ranchers, and agribusinesses (Fig. 7) (20, 195–200). In 2013, the United Nations reported that the tenure of 90% of the land in rural parts of the Afrotropics was undocumented and, therefore, susceptible to confiscation or other types of dispossession (201). Loss of land and access to forest resources, including medicinal plants and wild foods, has irreversibly altered the lives of, for example, Indonesia’s Indigenous Peoples, who have depended for thousands of years on these traditional resources (202, 203). The Orang Asli communities of Malaysia are permitted to reside in particular natural areas but cannot own these lands, and authorities can force them to abandon an area without compensation (204). In Sarawak, Malaysia, construction of the Bakun and Murum dams, the two largest dams in the country, required the relocation of 10,000 and 3400 Indigenous Peoples, respectively, the vast majority of which were removed involuntarily (205). This has devastating consequences on Indigenous Peoples’ sense of place, further weakening their cultures and identities. In addition, dam construction resulted in the flooding of almost 95,000 ha of biodiverse forest, home to threatened primate species such as the Bornean gibbon (Hylobates muelleri), the proboscis monkey (Nasalis larvatus), the Bornean banded langur (Presbytis chrysomelas), the silvery lutung (Trachypithecus cristatus), and the Bornean orangutan (Pongo pygmaeus) (205).
Highly biodiverse Indigenous Peoples’ lands also attract many external conservation agents, both governmental and nongovernmental, who also often fail to respect Indigenous rights (206, 207). The gazettement of conservation areas is one of the primary causes of land dispossession of Indigenous Peoples’ territories worldwide (208, 209). As part of an ongoing legacy of colonial intervention (210), some mainstream conservation efforts often consider local and Indigenous peoples as a counter to environmental conservation or discourage or hinder Indigenous communities from initiating conservation programs (211–214). These kinds of interventions perpetuate tensions and hostilities between local communities and conservationists. International programs and organizations aimed at safeguarding natural environments that fail to recognize the essential roles of Indigenous communities in perpetuating natural environments contribute to the marginalization of Indigenous Peoples, land appropriation, forced displacements, and refugee crises (215, 216). Conservation organizations must avoid an anti-Indigenous Peoples discourse and actions, such as lobbying and state interventions that may inadvertently contribute to human rights violations by pressuring governments into asserting sovereignty over Indigenous Peoples’ lands to achieve their conservation goals (208).
Schemes such as REDD+ and other types of payments for ecosystem services can also result in environmental injustice toward Indigenous Peoples (217, 218). Indigenous Peoples generally have limited political and socioeconomic power when competing stakeholders each assert legal authority to manage forested lands. The outcome is that Indigenous Peoples’ legitimate claims are often ignored (219). Actions by colonists, local governments, exploitative businesses, and illegal syndicates have resulted in numerous human rights violations against Indigenous communities, acts that are often underreported (216, 220, 221). Violent clashes, forced evictions, and even systematic killings have been documented as Indigenous leaders organize to secure their land rights (222).
The establishment of protected areas, which in some cases includes the construction of roads and facilities to support tourism, has also led to the dispossession of Indigenous Peoples’ lands. For example, Maasai pastoralists in Kenya were removed from their homeland to make way for the Amboseli National Park (223). In Tanzania, the British colonial government forcibly removed Indigenous pastoral Maasai communities from the first formal National Park, Serengeti National Park, gazetted in 1959, with no compensation (224). After Tanzanian independence, Indigenous Peoples were displaced during the establishment of Mkomazi National Park in the late 1980s (225) and from Ruaha National Park in 2006 (226).
Between 1960 and 1970, 580 Bambuti/Batwa families (approximately 3000 to 6000 individuals) were forced out of the Kahuzi-Biega forest in DRC to establish the Kahuzi-Biega National Park (19, 226). In Botswana and Namibia, the Kwe Peoples were stripped of their traditional lands to establish game reserves and national parks, which generate large sums of money from tourism but provide limited jobs for Indigenous Peoples (227). In these and many other cases, the community land rights of Indigenous Peoples were violated (228). Even in those instances in which the government has granted land rights, as is the case of the Yanomami of Brazil, significant parts of their territory have been invaded by thousands of illegal miners who use toxic chemicals such as mercury, resulting in extensive environmental damage, water and soil pollution, and significant risks to Yanomami and wildlife health (229, 230). Other major pressures upon Indigenous Peoples’ lands are large-scale infrastructure projects and armed civil conflict that force Indigenous People to leave their traditional lands (15, 231–233) and relocate to urban centers, with the expected decline of traditional livelihoods.
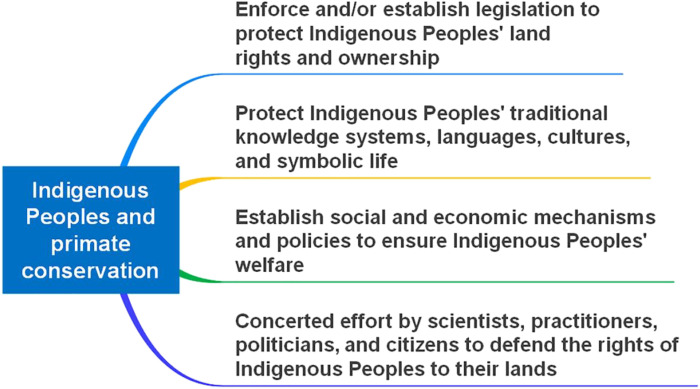
Dispossessed lands that are developed for infrastructure, resource extraction, agriculture, and industry become highly fragmented landscapes that divide once continuous primate populations by creating barriers to migration and gene flow, thus increasing the likelihood of small effective population size and extirpation (14, 179, 234). Halting Indigenous land dispossession, returning land to dispossessed Indigenous Peoples, and respecting and safeguarding their sovereignty represent critical priorities for Indigenous Peoples that are also central to protecting primate biodiversity (11). While primates play critical roles in ecosystem dynamics and sustainability, they are also fundamental to local and regional Indigenous Peoples’ traditional systems of knowledge, culture, beliefs, and mythology. Numerous primate species have millennia-old sympatric relationships with humans. For many Indigenous societies, primates are integrated into their historical tales as sacred cultural figures and persons (39). As primates become locally extirpated or rare due to the unsustainable demands of industrial societies, intricate ecological, social, and cultural relationships that have developed between humans and nonhuman primates over thousands of years are put at risk. While Indigenous Peoples may not frame their stewardship practices with an explicit focus on what conservationists would term “environmental preservation” (29, 56), they often manage their lands in ways that are compatible with or actively support nature conservation (24, 67, 82). Several generations of Indigenous leaders, scholars, thinkers, and philosophers have significantly contributed to raising global awareness about the ecological values of their territories [e.g., 235–238]. Consequently, the implementation of effective land tenure policies by local and national governments and international organizations that safeguard and guarantee, in perpetuity, Indigenous Peoples’ land rights will ensure their cultural, economic, environmental, and social well-being. This will protect primate populations and plant and animal biodiversity present on these lands (Fig. 8) (55, 66, 67, 175, 182, 194, 230).
Fig. 8. Key global societal actions needed to support Indigenous People.
These actions will enable Indigenous Peoples to continue their land stewardship and culture in ways that will benefit primate conservation. In general, these actions apply to all primate range nations. Ultimately, it will be Indigenous people who decide whether their engagement with their lands will allow them to continue their traditional ways of life—the actions indicated above will make it possible for them to have that option.
Last, we acknowledge certain limitations in this study. Our results provide strong correlational evidence that primate species living on Indigenous Peoples’ lands are less threatened than primate species living on other lands. However, measures of population size, species biomass, and genetic diversity are needed to better understand the conservation value of Indigenous Peoples’ lands to individual primate species and the set of measures that could be implemented to protect those taxa that remain most vulnerable. In addition, the IUCN shape files (3) may result in underestimating or overestimating species ranges and the true area of occupancy (239). Future studies will need to prioritize measures, such as the remaining area of suitable habitat (AOH), the extent of forest fragmentation, and fragment size, in assessing primate population persistence (48, 240).
KEY CHALLENGES AHEAD
As we try to mitigate the existential challenges of the Anthropocene—climate change, large-scale environmental modification, and mass extinctions—there is a relatively direct and highly effective way to sequester carbon, restore natural landscapes, and safeguard primate biodiversity. That way is for national governments, international organizations, and global citizens to support Indigenous Peoples in their efforts to continue stewardship of their lands, culture, oral traditions, and treaties already in place (56, 62, 241). Sustainable primate conservation solutions depend on acknowledging the needs and strengthening the rights of Indigenous Peoples (Fig. 8). Conservation organizations and national governments need to work against the negative impacts of ongoing environmental destruction on Indigenous Peoples’ well-being and translate conservation-oriented scientific information into policies that support Indigenous Peoples’ sustainable land management practices (24, 62, 123). We also need to raise worldwide scientific and public appreciation of the critical roles played by Indigenous Peoples in biodiversity conservation. Given the diverse histories of colonialism, inequity, racism, genocide, and political oppression, many contemporary Indigenous Peoples still have no sovereignty over their ancestral lands (56, 242).
We can only achieve successful implementation of national and international conservation and sustainable development goals by recognizing and strengthening Indigenous rights to their traditional lands and resources (19, 195–197, 241, 243, 244). We also need to explore ways of providing Indigenous Peoples with opportunities to enhance primate and biodiversity conservation by establishing links between their lands and other lands held communally or traditionally, and protected areas not included in the IUCN categories I to VI. Although primates fare better on Indigenous Peoples’ lands than on other lands not officially protected by law, 55% of primate species that overlap with Indigenous territories are nevertheless threatened. While this is considerably better than the 93% of primate species listed as threatened on other lands, limiting non-Indigenous resource extraction on Indigenous Peoples’ lands must become an international conservation priority. This priority must include strengthening Indigenous land rights, with support from scientifically based and culturally appropriate strategies for reducing human impacts on primate populations.
Madagascar is the second primate-richest country in the world (based on number of species). However, given that Madagascar does not have any recognized Indigenous groups, alternate approaches to primate conservation must be considered. These include community-based forest management programs, which have expanded substantially in recent decades in response to the limitations of state-run reserves in achieving effective conservation outcomes and in addressing the needs of the rural poor (153, 245). Unfortunately, the success of many of these community-based conservation programs has been limited. This is due, in large part, to the fact that conservation restrictions associated with community-based forest management have reduced local annual income by 27 to 84%, with these losses borne disproportionately by the poorest community members (245). Financial compensation to households for lost income can be a viable and cost-effective tool in incentivizing forest management and lemur conservation. This will require a sustained, decades-long, national and international commitment to provide the necessary funding, education, and oversight (245).
CONCLUDING REMARKS
Given that the ranges of 71% of primate species intersect Indigenous Peoples’ lands, we will only avert the mass extinction of primates if we respect and support biocultural diversity and the efforts of Indigenous Peoples to maintain their languages, and cultural and symbolic ties to their lands and waters. Indigenous Peoples must be supported in their efforts to shield their lands from the unsustainable demands of multinational corporations, consumer nations, and national governments that favor short-term economic benefits over human rights, biodiversity, and environmental health. The enforced loss of connection between Indigenous Peoples and their lands worldwide has resulted in the overexploitation of natural resources and the erosion of unique sociocultural connections between people and nature (33, 34, 73, 235–238, 246). The challenges faced by Indigenous Peoples in conserving their lands, traditional knowledge, cultures, and natural resources require that primate conservationists actively and constructively engage with Indigenous Peoples, politicians, the business community, and global citizens (Fig. 8). Indigenous Peoples should be respected for their systems of knowledge and considered by the global conservation community as holders of essential information, land rights, and partners in the quest to safeguard biodiversity. This will conserve local, regional, and global ecosystems, and offers our best chance to prevent the extinction of the world’s primates, our closest living biological relatives.
Acknowledgments
A.E. thanks the Tarahumara and the Lacandon Indigenous Peoples of Mexico for triggering his interest in developing this writing project. A.E. thanks his colleague coauthors for their sustained interest and original contributions to this evaluation. P.A.G. wishes to thank Chrissie, Sara, Jenni, and Dax for continuing to inspire him to help save the world’s primates from extinction. C.S. would like to thank P. Suse, C. Yukuma, and E. Marawanaru, who contributed important data to this manuscript, and all the Waiwai of Masakenari for their research collaboration. We acknowledge the importance of the world’s Indigenous Peoples as critical custodians of nature.
Funding: F.A. was funded by Fundação para a Ciência e Tecnologia (FCT, CEECIND/03265/2017). The authors declare no other sources of funding to support the writing of this review paper. This is principally a review manuscript and therefore did not require IRB and/or IACUC approval.
Author contributions: A.E. and P.A.G. conceived and designed the review and contributed to all sections. S.T.G. provided the spatial layers used in the global analysis of Indigenous Peoples’ lands within primate range regions and provided many useful comments on the manuscript. S.G. and R.D. conducted the spatial analysis of the global distribution of primate species found on Indigenous Peoples’ lands, protected areas, and other lands. F.A. did the global spatial analysis of infrastructure and primate distributions. A.F.-L., A.F., and G.H.S. developed several segments touching upon Indigenous Peoples’ knowledge systems. C.S. developed the section on hunting. All authors contributed data, discussed data analyses, and commented on earlier versions of the manuscript.
Competing interests: The authors declare that they have no competing interests. No institutional review board or institutional animal and welfare committee approval was needed for this study.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials (tables S1 to S7 and figs. S1 to S7).
Supplementary Materials
This PDF file includes:
Supplementary Text
Figs. S1 to S7
Tables S1 to S7
References
REFERENCES AND NOTES
- 1.Díaz S., Settele J., Brondízio E. S., Ngo H. T., Agard J., Arneth A., Balvanera P., Brauman K. A., Butchart S. H. M., Chan K. M. A., Garibaldi L. A., Ichii K., Liu J., Subramanian S. M., Midgley G. F., Miloslavich P., Molnár Z., Obura D., Pfaff A., Polasky S., Purvis A., Razzaque J., Reyers B., Chowdhury R. R., Shin Y.-J., Visseren-Hamakers I., Willis K. J., Zayas C. N., Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 366, eaax3100 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES Secretariat, 2019).
- 3.IUCN, IUCN Redlist of Threatened Species (2022); https://iucnredlist.org/.
- 4.Estrada A., Garber P. A., Rylands A. B., Roos C., Fernandez-Duque E., Di Fiore A., Nekaris K. A., Nijman V., Heymann E. W., Lambert J. E., Rovero F., Barelli C., Setchell J. M., Gillespie T. R., Mittermeier R. A., Arregoitia L. V., de Guinea M., Gouveia S., Dobrovolski R., Shanee S., Shanee N., Boyle S. A., Fuentes A., MacKinnon K. C., Amato K. R., Meyer A. L. S., Wich S., Sussman R. W., Pan R., Kone I., Li B., Impending extinction crisis of the world’s primates: Why primates matter. Sci. Adv. 3, e1600946 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodie J. F., Williams S., Garner B., The decline of mammal functional and evolutionary diversity worldwide. Proc. Natl. Acad. Sci. 118, e1921849118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shaffer C. A., Yukuma C., Marawanaru E., Suse P., Assessing the sustainability of Waiwai subsistence hunting in Guyana by comparison of static indices and spatially explicit, biodemographic models. Anim. Conserv. 21, 148–158 (2018). [Google Scholar]
- 7.Levi T., Shepard G. H., Ohl-Schacherer J., Wilmers C. C., Peres C. A., Yu D. W., Spatial tools for modeling the sustainability of subsistence hunting in tropical forests. Ecol. Appl. 21, 1802–1818 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Cronin D. T., Sesink Clee P. R., Mitchell M. W., Bocuma Meñe D., Fernández D., Riaco C., Fero Meñe M., Esara Echube J. M., Hearn G. W., Gonder M. K., Conservation strategies for understanding and combating the primate bushmeat trade on Bioko Island, Equatorial Guinea. Am. J. Primatol. 79, 22663 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Estrada A., Garber P. A., Chaudhary A., Expanding global commodities trade and consumption place the world’s primates at risk of extinction. PeerJ 7, e7068 (2019). [Google Scholar]
- 10.Estrada A., Garber P. A., Chaudhary A., Current and future trends in socio-economic, demographic and governance factors affecting global primate conservation. PeerJ 8, e9816 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urbancic N., How granting Indigenous peoples’ land titles contributes to forest conservation in Latin America. Birkbeck Law Rev. 7, 26–57 (2020). [Google Scholar]
- 12.Fischer R., Taubert F., Müller M. S., Groeneveld J., Lehmann S., Wiegand T., Huth A., Accelerated forest fragmentation leads to critical increase in tropical forest edge area. Sci. Adv. 7, eabg7012 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.C. L. Nunn, T. R. Gillespie, in An Introduction to Primate Conservation, S. A. Wich, A. J. Marshall, Eds. (Oxford Univ. Press, 2016), pp. 157–174; https://oxford.universitypressscholarship.com/view/10.1093/acprof:oso/9780198703389.001.0001/acprof-9780198703389-chapter-10.
- 14.Ascensão F., Yogui D. R., Alves M. H., Alves A. C., Abra F., Desbiez A. L. J., Preventing wildlife roadkill can offset mitigation investments in short-medium term. Biol. Conserv. 253, 108902 (2021). [Google Scholar]
- 15.Ascensão F., D’Amico M., Barrientos R., No planet for Apes? Assessing global priority areas and species affected by linear infrastructures. Int. J. Primatol. 43, 57–73 (2021). [Google Scholar]
- 16.Boonratana R., Asian primates in fragments: Understanding causes and consequences of fragmentation, and predicting primate population viability. Am. J. Primatol. 82, e23082 (2020). [DOI] [PubMed] [Google Scholar]
- 17.V. M. Toledo, in Encyclopedia of Biodiversity (Elsevier, ed. 2, 2013), pp. 269–278; https://researchgate.net/profile/Victor-Toledo-2/publication/255585922_Indigenous_Peoples_and_Biodiversity/links/5a1d6cb0a6fdcc0af326d9e5/Indigenous-Peoples-and-Biodiversity.pdf.
- 18.UNESCO, UNESCO Atlas of the World’s Languages in Danger (2020); https://unesdoc.unesco.org/ark:/48223/pf0000380132.
- 19.IWGIA, The Indigenous World 2020 (Eks-Skolen Trykkeri, 2020); http://iwgia.org/images/yearbook/2020/IWGIA_The_Indigenous_World_2020.pdf.
- 20.World Bank, Indigenous Peoples (2021); https://worldbank.org/en/topic/indigenouspeoples.
- 21.Garnett S. T., Burgess N. D., Fa J. E., Fernández-Llamazares Á., Molnár Z., Robinson C. J., Watson J. E. M. M., Zander K. K., Austin B., Brondizio E. S., Collier N. F., Duncan T., Ellis E., Geyle H., Jackson M. V., Jonas H., Malmer P., McGowan B., Sivongxay A., Leiper I., A spatial overview of the global importance of Indigenous lands for conservation. Nat. Sustain. 1, 369–374 (2018). [Google Scholar]
- 22.Fa J. E., Watson J. E. M., Leiper I., Potapov P., Evans T. D., Burgess N. D., Molnár Z., Fernández-Llamazares Á., Duncan T., Wang S., Austin B. J., Jonas H., Robinson C. J., Malmer P., Zander K. K., Jackson M. V., Ellis E., Brondizio E. S., Garnett S. T., Importance of Indigenous Peoples’ lands for the conservation of intact forest landscapes. Front. Ecol. Environ. 18, 135–140 (2020). [Google Scholar]
- 23.O’Bryan C. J., Garnett S. T., Fa J. E., Leiper I., Rehbein J. A., Fernández-Llamazares Á., Jackson M. V., Jonas H. D., Brondizio E. S., Burgess N. D., Robinson C. J., Zander K. K., Molnár Z., Venter O., Watson J. E. M., The importance of Indigenous Peoples’ lands for the conservation of terrestrial mammals. Conserv. Biol. 35, 1002–1008 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fletcher M.-S., Hamilton R., Dressler W., Palmer L., Indigenous knowledge and the shackles of wilderness. Proc. Natl. Acad. Sci. 118, e2022218118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D. Mamo, K. Wessendorf, in The Indigenous World 2020, D. Mamo, Ed. (Eks-Skolen Trykkeri, 2020), pp. 6–23. [Google Scholar]
- 26.Walker W. S., Gorelik S. R., Baccini A., Aragon-Osejo J. L., Josse C., Meyer C., Macedo M. N., Augusto C., Rios S., Katan T., de Souza A. A., Cuellar S., Llanos A., Zager I., Mirabal G. D., Solvik K. K., Farina M. K., Moutinho P., Schwartzman S., The role of forest conversion, degradation, and disturbance in the carbon dynamics of Amazon indigenous territories and protected areas. Proc. Natl. Acad. Sci. 117, 3015–3025 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.N. Harris, D. Gibbs, Forests Absorb Twice As Much Carbon As They Emit Each Year (World Resources Institute, 2021); https://wri.org/insights/forests-absorb-twice-much-carbon-they-emit-each-year.
- 28.Ellis E. C., Gauthier N., Klein Goldewijk K., Bliege Bird R., Boivin N., Díaz S., Fuller D. Q., Gill J. L., Kaplan J. O., Kingston N., Locke H., McMichael C. N. H., Ranco D., Rick T. C., Shaw M. R., Stephens L., Svenning J.-C., Watson J. E. M., People have shaped most of terrestrial nature for at least 12,000 years. Proc. Natl. Acad. Sci. 118, e2023483118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernández-Llamazares Á., Virtanen P. K., Game masters and Amazonian Indigenous views on sustainability. Curr. Opin. Environ. Sustain. 43, 21–27 (2020). [Google Scholar]
- 30.R. E. Kasperson, K. Dow, E. R. M. Archer, D. Cáceres, T. E. Downing, T. Elmqvist, S. Eriksen, C. Folke, G. Han, K. Iyengar, C. Vogel, K. A. Wilson, G. Ziervogel, in Ecosystems and Human Well-being: Current State and Trends, R. Norgaard, D. Rapport, Eds. (Island Press, 2005), vol. 56, pp. 143–164. [Google Scholar]
- 31.Schuster R., Germain R. R., Bennett J. R., Reo N. J., Arcese P., Vertebrate biodiversity on indigenous-managed lands in Australia, Brazil, and Canada equals that in protected areas. Environ. Sci. Policy 101, 1–6 (2019). [Google Scholar]
- 32.Piperno D. R., McMichael C. H., Pitman N. C. A., Guevara Andino J. E., Paredes M. R., Heijink B. M., Torres-Montenegro L. A., A 5,000-year vegetation and fire history for tierra firme forests in the Medio Putumayo-Algodón watersheds, northeastern Peru. Proc. Natl. Acad. Sci. 118, e2022213118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salmón E., Kincentric ecology: Indigenous perceptions of the human-nature relationship. Ecol. Appl. 10, 1327–1332 (2000). [Google Scholar]
- 34.Redvers N., Celidwen Y., Schultz C., Horn O., Githaiga C., Vera M., Perdrisat M., Mad Plume L., Kobei D., Kain M. C., Poelina A., Rojas J. N., Blondin B., The determinants of planetary health: An Indigenous consensus perspective. Lancet Planet. Heal. 6, e156–e163 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Franco-Moraes J., Baniwa A. F. M. B., Costa F. R. C., Lima H. P., Clement C. R., Shepard G. H., Historical landscape domestication in ancestral forests with nutrient-poor soils in northwestern Amazonia. For. Ecol. Manage. 446, 317–330 (2019). [Google Scholar]
- 36.Odonne G., van den Bel M., Burst M., Brunaux O., Bruno M., Dambrine E., Davy D., Desprez M., Engel J., Ferry B., Freycon V., Grenand P., Jérémie S., Mestre M., Molino J. F., Petronelli P., Sabatier D., Hérault B., Long-term influence of early human occupations on current forests of the Guiana Shield. Ecology 100, e02806 (2019). [DOI] [PubMed] [Google Scholar]
- 37.Grantham H. S., Duncan A., Evans T. D., Jones K. R., Beyer H. L., Schuster R., Walston J., Ray J. C., Robinson J. G., Callow M., Clements T., Costa H. M., DeGemmis A., Elsen P. R., Ervin J., Franco P., Goldman E., Goetz S., Hansen A., Hofsvang E., Jantz P., Jupiter S., Kang A., Langhammer P., Laurance W. F., Lieberman S., Linkie M., Malhi Y., Maxwell S., Mendez M., Mittermeier R., Murray N. J., Possingham H., Radachowsky J., Saatchi S., Samper C., Silverman J., Shapiro A., Strassburg B., Stevens T., Stokes E., Taylor R., Tear T., Tizard R., Venter O., Visconti P., Wang S., Watson J. E. M., Anthropogenic modification of forests means only 40% of remaining forests have high ecosystem integrity. Nat. Commun. 11, 5978 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voss R. S., Fleck D. W., Mammalian diversity and matses ethnomammalogy in amazonian peru part 1: Primates. Bull. Am. Mus. Nat. Hist. 351, 1–81 (2011). [Google Scholar]
- 39.Fuentes A., Ethnoprimatology and the anthropology of the human-primate interface. Ann. Rev. Anthropol. 41, 101–117 (2012). [Google Scholar]
- 40.Parathian H. E., McLennan M. R., Hill C. M., Frazão-Moreira A., Hockings K. J., Breaking through disciplinary barriers: Human–wildlife interactions and multispecies ethnography. Int. J. Primatol. 39, 749–775 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.J. P. Boubli, B. Urbani, H. Caballero-Arias, G. H. Shepard, M. Lizarralde, in Neotropical Ethnoprimatology. Ethnobiology, B. Urbani, M. Lizarralde, Eds. (Springer, 2020), pp. 199–224; 10.1007/978-3-030-27504-4_9. [DOI]
- 42.Potapov P., Hansen M. C., Laestadius L., Turubanova S., Yaroshenko A., Thies C., Smith W., Zhuravleva I., Komarova A., Minnemeyer S., Esipova E., The last frontiers of wilderness: Tracking loss of intact forest landscapes from 2000 to 2013. Sci. Adv. 3, e1600821 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Bank, Population Density (2020); https://data.worldbank.org/indicator/EN.POP.DNST.
- 44.Levi T., Shepard G. H., Ohl-Schacherer J., Peres C. A., Yu D. W., Modelling the long-term sustainability of indigenous hunting in Manu National Park, Peru: Landscape-scale management implications for Amazonia. J. Appl. Ecol. 46, 804–814 (2009). [Google Scholar]
- 45.E. L. Bennett, J. G. Robinson, “Hunting of wildlife in tropical forests: Implications for biodiversity and forest peoples” (Biodiversity Series, 2000); https://openknowledge.worldbank.org/bitstream/handle/10986/18297/multi0page.pdf?sequence=1&isAllowed=y.
- 46.Albert B., Le Tourneau F., Ethnogeography and resource use among the Yanomami. Curr. Anthropol. 48, 584–592 (2007). [Google Scholar]
- 47.Allan J. R., Watson J. E. M., Di Marco M., O’Bryan C. J., Possingham H. P., Atkinson S. C., Venter O., Hotspots of human impact on threatened terrestrial vertebrates. PLOS Biol. 17, e3000158 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brooks T. M., Pimm S. L., Akçakaya H. R., Buchanan G. M., Butchart S. H. M., Foden W., Hilton-Taylor C., Hoffmann M., Jenkins C. N., Joppa L., Li B. V., Menon V., Ocampo-Peñuela N., Rondinini C., Measuring terrestrial area of habitat (AOH) and its utility for the IUCN Red list. Trends Ecol. Evol. 34, 977–986 (2019). [DOI] [PubMed] [Google Scholar]
- 49.IUCN Standards and Petitions Committee, Guidelines for Using the IUCN Red List Categories and Criteria (2022); https://iucnredlist.org/resources/redlistguidelines.
- 50.UNEP-WCMC and IUCN, Protected Planet: The World Database on Protected Areas (WDPA) (2020); https://protectedplanet.net/.
- 51.ILO, in Convention Concerning Indigenous and Tribal Peoples in Independent Countries (ILO, 1989); https://ilo.org/dyn/normlex/en/f?p=NORMLEXPUB:12100:0::NO::P12100_INSTRUMENT_ID:312314.
- 52.Golden C. D., Comaroff J., The human health and conservation relevance of food taboos in northeastern Madagascar. Ecol. Soc. 20, art42 (2015). [Google Scholar]
- 53.Tengö M., Johansson K., Rakotondrasoa F., Lundberg J., Andriamaherilala J. A., Rakotoarisoa J. A., Elmqvist T., Taboos and forest governance: Informal protection of hot spot dry forest in southern Madagascar. Ambio 36, 683–691 (2007). [DOI] [PubMed] [Google Scholar]
- 54.Rocha R., Fernández-Llamazares Á., López-Baucells A., Andriamitandrina S. F. M., Andriatafika Z. E., Temba E. M., Torrent L., Burgas D., Cabeza M., Human-bat interactions in rural Southwestern Madagascar through a biocultural lens. J. Ethnobiol. 41, 53–69 (2021). [Google Scholar]
- 55.Toledo V. M., Ortiz-Espejel B. F., Cortés L., Moguel P., de J. Ordoñez M., The multiple use of tropical forests by indigenous peoples in Mexico: A case of adaptive management. Conserv. Ecol. 7, art9 (2003). [Google Scholar]
- 56.Reyes-García V., Fernández-Llamazares Á., Aumeeruddy-Thomas Y., Benyei P., Bussmann R. W., Diamond S. K., García-del-Amo D., Guadilla-Sáez S., Hanazaki N., Kosoy N., Lavides M., Luz A. C., McElwee P., Meretsky V. J., Newberry T., Molnár Z., Ruiz-Mallén I., Salpeteur M., Wyndham F. S., Zorondo-Rodriguez F., Brondizio E. S., Recognizing Indigenous peoples’ and local communities’ rights and agency in the post-2020 biodiversity agenda. Ambio 51, 84–92 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Armstrong C. G., Miller J. E. D., McAlvay A. C., Ritchie P. M., Lepofsky D., Historical indigenous land-use explains plant functional trait diversity. Ecol. Soc. 26, art6 (2021). [Google Scholar]
- 58.Reyes-García V., Benyei P., Indigenous knowledge for conservation. Nat. Sustain. 2, 657–658 (2019). [Google Scholar]
- 59.Ford J. D., King N., Galappaththi E. K., Pearce T., McDowell G., Harper S. L., The resilience of indigenous peoples to environmental change. One Earth 2, 532–543 (2020). [Google Scholar]
- 60.Hill R., Adem Ç., Alangui W. V., Molnár Z., Aumeeruddy-Thomas Y., Bridgewater P., Tengö M., Thaman R., Yao C. Y. A., Berkes F., Carino J., da Cunha M. C., Diaw M. C., Díaz S., Figueroa V. E., Fisher J., Hardison P., Ichikawa K., Kariuki P., Karki M., Lyver P. O., Malmer P., Masardule O., Yeboah A. A. O., Pacheco D., Pataridze T., Perez E., Roué M.-M., Roba H., Rubis J., Saito O., Xue D., Working with Indigenous, local and scientific knowledge in assessments of nature and nature’s linkages with people. Curr. Opin. Environ. Sustain. 43, 8–20 (2020). [Google Scholar]
- 61.Cámara-Leret R., Bascompte J., Language extinction triggers the loss of unique medicinal knowledge. Proc. Natl. Acad. Sci. 118, e2103683118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fernández-Llamazares Á., Lepofsky D., Lertzman K., Armstrong C. G., Brondizio E. S., Gavin M. C., Lyver P. O., Nicholas G. P., Pascua P., Reo N. J., Reyes-García V., Turner N. J., Yletyinen J., Anderson E. N., Balée W., Cariño J., David-Chavez D. M., Dunn C. P., Garnett S. C., Greening (La’goot) S., Jackson (NiniwumSelapem) S., Kuhnlein H., Molnár Z., Odonne G., Retter G.-B., Ripple W. J., Sáfián L., Bahraman A. S., Torrents-Ticó M., Vaughan M. B., Scientists’ warning to humanity on threats to indigenous and local knowledge systems. J. Ethnobiol. 41, 144–169 (2021). [Google Scholar]
- 63.G. H. Shepard, in Primates Face to Face (Cambridge Univ. Press, 2002), pp. 101–136; https://cambridge.org/core/product/identifier/CBO9780511542404A017/type/book_part.
- 64.Allison E. H., Cho V., River conservation by an Indigenous community. Nature 588, 589–590 (2020). [DOI] [PubMed] [Google Scholar]
- 65.Koning A. A., Perales K. M., Fluet-Chouinard E., McIntyre P. B., A network of grassroots reserves protects tropical river fish diversity. Nature 588, 631–635 (2020). [DOI] [PubMed] [Google Scholar]
- 66.Vuohelainen A. J., Coad L., Marthews T. R., Malhi Y., Killeen T. J., The effectiveness of contrasting protected areas in preventing deforestation in Madre de Dios, Peru. Environ. Manage. 50, 645–663 (2012). [DOI] [PubMed] [Google Scholar]
- 67.Blackman A., Corral L., Lima E. S., Asner G. P., Titling indigenous communities protects forests in the Peruvian Amazon. Proc. Natl. Acad. Sci. 114, 4123–4128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Environmental Defense Fund, Amazonian Indigenous Peoples Territories and Their Forests Related to Climate Change: Analyses and Policy Options (2017); https://edf.org/sites/default/files/indigenous-territories-barrier-to-deforestation.pdf.
- 69.Fernández-Llamazares Á., Garteizgogeascoa M., Basu N., Brondizio E. S., Cabeza M., Martínez-Alier J., McElwee P., Reyes-García V., A state-of-the-art review of indigenous peoples and environmental pollution. Integr. Environ. Assess. Manag. 16, 324–341 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Begotti R. A., Peres C. A., Rapidly escalating threats to the biodiversity and ethnocultural capital of Brazilian Indigenous Lands. Land Use Policy 96, 104694 (2020). [Google Scholar]
- 71.Nepstad D., Schwartzman S., Bamberger B., Santilli M., Ray D., Schlesinger P., Lefebvre P., Alencar A., Prinz E., Fiske G., Rolla A., Inhibition of Amazon deforestation and fire by parks and indigenous lands. Conserv. Biol. 20, 65–73 (2006). [DOI] [PubMed] [Google Scholar]
- 72.Gonçalves-Souza D., Vilela B., Phalan B., Dobrovolski R., The role of protected areas in maintaining natural vegetation in Brazil. Sci. Adv. 7, eabh29932 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.D. Kopenawa, B. Albert, The Falling Sky: Words of a Yanomami Shaman (Belknap Press, 2013). [Google Scholar]
- 74.Gibson L., Lee T. M., Koh L. P., Brook B. W., Gardner T. A., Barlow J., Peres C. A., Bradshaw C. J. A., Laurance W. F., Lovejoy T. E., Sodhi N. S., Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 478, 378–381 (2011). [DOI] [PubMed] [Google Scholar]
- 75.Bernard H., Bili R., Matsuda I., Hanya G., Wearn O. R., Wong A., Ahmad A. H., Species richness and distribution of primates in disturbed and converted forest landscapes in Northern Borneo. Trop. Conserv. Sci. 9, 194008291668010 (2016). [Google Scholar]
- 76.de Almeida-Rocha J. M., Peres C. A., Oliveira L. C., Primate responses to anthropogenic habitat disturbance: A pantropical meta-analysis. Biol. Conserv. 215, 30–38 (2017). [Google Scholar]
- 77.Fernández-Llamazares Á., Terraube J., Gavin M. C., Pyhälä A., Siani S. M. O., Cabeza M., Brondizio E. S., Reframing the wilderness concept can bolster collaborative conservation. Trends Ecol. Evol. 35, 750–753 (2020). [DOI] [PubMed] [Google Scholar]
- 78.Berkes F., Colding J., Folke C., Rediscovery of traditional ecological knowledge as adaptive management. Ecol. Appl. 10, 1251–1262 (2000). [Google Scholar]
- 79.F. Berkes, Sacred Ecology (Taylor & Francis, 2008). [Google Scholar]
- 80.Arora D., Shepard G. H., Mushrooms and economic botany. Econ. Bot. 62, 207–212 (2008). [Google Scholar]
- 81.H. V. Kuhnlein, B. Erasmus, D. Spigelski, Indigenous Peoples’ Food Systems: The Many Dimensions of Culture, Diversity and Environment for Nutrition and Health (FAO, 2009).
- 82.Shepard G. H. Jr., Levi T., Neves E. G., Peres C. A., Yu D. W., Hunting in ancient and modern Amazonia: Rethinking sustainability. Am. Anthropol. 114, 652–667 (2012). [Google Scholar]
- 83.Pezzuti J. C. B., Lima J. P., da Silva D. F., Begossi A., Uses and taboos of turtles and tortoises along rio Negro, Amazon Basin. J. Ethnobiol. 30, 153–168 (2010). [Google Scholar]
- 84.Diniz-Filho J. A. F., Macroecological analyses support an overkill scenario for late Pleistocene extinctions. Brazilian J. Biol. 64, 407–414 (2004). [DOI] [PubMed] [Google Scholar]
- 85.Wolfe A. L., Broughton J. M., A foraging theory perspective on the associational critique of North American Pleistocene overkill. J. Archaeol. Sci. 119, 105162 (2020). [Google Scholar]
- 86.Cormier L., A preliminary review of neotropical primates in the subsistence and symbolism of indigenous lowland South American peoples. Ecol. Environ. Anthropol. 2, 14–32 (2006). [Google Scholar]
- 87.Cartelle C., Hartwig W. C., A new extinct primate among the Pleistocene megafauna of Bahia. Brazil. Proc. Natl. Acad. Sci. 93, 6405–6409 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Balée W., Honorato de Oliveira V., dos Santos R., Amaral M., Rocha B., Guerrero N., Schwartzman S., Torres M., Pezzuti J., Ancient transformation, current conservation: Traditional forest management on the Iriri River, Brazilian Amazonia. Hum. Ecol. 48, 1–15 (2020). [Google Scholar]
- 89.Jerozolimski A., Peres C. A., Bringing home the biggest bacon: A cross-site analysis of the structure of hunter-kill profiles in Neotropical forests. Biol. Conserv. 111, 415–425 (2003). [Google Scholar]
- 90.De Thoisy B., Lacoste V., Germain A., Muñoz-Jordán J., Colón C., Mauffrey J.-F., Delaval M., Catzeflis F., Kazanji M., Matheus S., Dussart P., Morvan J., Setién A. A., Deparis X., Lavergne A., Dengue infection in neotropical forest mammals. Vector Borne Zoonotic Dis. 9, 157–170 (2009). [DOI] [PubMed] [Google Scholar]
- 91.Borgerson C., Fisher B. L., Razafindrapaoly B. N., Rasolofoniaina B. J. R., Randriamanetsy J. M., Razafindrapaoly B. L., Rajaona D., Herrera P., Van Itterbeeck J., Martinez K. M., Aardema M. L., A nutrient-rich traditional insect for improving food security and reducing biodiversity loss in Madagascar and sub-Saharan Africa. Conserv. Sci. Pract. 3, e480 (2021). [Google Scholar]
- 92.Franzen M., Evaluating the sustainability of hunting: A comparison of harvest profiles across three Huaorani communities. Environ. Conserv. 33, 36–45 (2006). [Google Scholar]
- 93.S. Papworth, in Ethnoprimatology, M. T. Waller, Ed. (Springer International Publishing, 2016), pp. 95–115. [Google Scholar]
- 94.Shaffer C. A., Milstein M. S., Yukuma C., Marawanaru E., Suse P., Sustainability and comanagement of subsistence hunting in an indigenous reserve in Guyana. Conserv. Biol. 31, 1119–1131 (2017). [DOI] [PubMed] [Google Scholar]
- 95.C. Shaffer, E. Marawanaru, C. Yukuma, in Ethnoprimatology: A Practical Guide to Research at the Human-Nonhuman Interface, K. M. Dore, E. P. Riley, A. Fuentes, Eds. (Cambridge Univ. Press, 2016), pp. 232–250. [Google Scholar]
- 96.De Souza-Mazurek R. R., Pedrinho T., Feliciano X., Hilário W., Gerôncio S., Marcelo E., Subsistence hunting among the Waimiri Atroari Indians in central Amazonia, Brazil. Biodivers. Conserv. 9, 579–596 (2000). [Google Scholar]
- 97.Peres C. A., Nascimento H. S., Impact of game hunting by the Kayapó of south-eastern Amazonia: Implications for wildlife conservation in tropical forest indigenous reserves. Biodivers. Conserv. 15, 2627–2653 (2006). [Google Scholar]
- 98.P. Erikson, in Beyond the Visible and the Material: The Amerindianization of Society in the Work of Peter Rivière, L. Rival, N. Whitehead, Eds. (Oxford Univ. Press, 2001), pp. 101–121. [Google Scholar]
- 99.Zapata-Ríos G., Urgilés C., Suárez E., Mammal hunting by the Shuar of the Ecuadorian Amazon: Is it sustainable? Oryx 43, 375 (2009). [Google Scholar]
- 100.Ohl-Schacherer J., Shepard G. H., Kaplan H., Peres C. A., Levi T., Yu D. W., The sustainability of subsistence hunting by matsigenka native communities in Manu National Park, Peru. Conserv. Biol. 21, 1174–1185 (2007). [DOI] [PubMed] [Google Scholar]
- 101.Shanee N., Shanee S., Maldonado A. M., Conservation assessment and planning for the yellow tailed woolly monkey (Oreonax flavicauda) in Peru. Wildl. Biol. Pract. 3, 73–82 (2007). [Google Scholar]
- 102.Sirén A., Machoa J., Fish, wildlife, and human nutrition in tropical forests: A fat gap? Interciencia 33, 186–193 (2008). [Google Scholar]
- 103.Fa J. E., Olivero J., Farfán M. A., Lewis J., Yasuoka H., Noss A., Hattori S., Hirai M., Kamgaing T. O. W., Carpaneto G., Germi F., Márquez A. L., Duarte J., Duda R., Gallois S., Riddell M., Nasi R., Differences between pygmy and non-pygmy hunting in Congo Basin forests. PLOS ONE 11, e0161703 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nasi R., Taber A., Van Vliet N., Empty forests, empty stomachs? Bushmeat and livelihoods in the Congo and Amazon Basins. Int. For. Rev. 13, 355–368 (2011). [Google Scholar]
- 105.Abernethy K. A., Coad L., Taylor G., Lee M. E., Maisels F., Extent and ecological consequences of hunting in Central African rainforests in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 368, 20120303 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Petrozzi F., Amori G., Franco D., Gaubert P., Pacini N., Eniang E. A., Akani G. C., Politano E., Luiselli L., Ecology of the bushmeat trade in west and central Africa. Trop. Ecol. 57, 545–557 (2016). [Google Scholar]
- 107.Corlett R. T., The impact of hunting on the mammalian fauna of tropical asian forests. Biotropica 39, 292–303 (2007). [Google Scholar]
- 108.T. M. Lee, A. Sigouin, M. Pinedo-Vasquez, R. Nasi, “The harvest of wildlife for bushmeat and traditional medicine in East, South and Southeast Asia” (Occasional Paper 115, 2014).
- 109.Nielsen M. R., Meilby H., Smith-Hall C., Pouliot M., Treue T., The importance of wild meat in the global south. Ecol. Econ. 146, 696–705 (2018). [Google Scholar]
- 110.H. Nooren, G. Claridge, Wildlife Trade in Laos: The End of the Game (IUCN-The World Conservation Union, 2001). [Google Scholar]
- 111.Rao M., Myint T., Zaw T., Htun S., Hunting patterns in tropical forests adjoining the Hkakaborazi National Park, north Myanmar. Oryx 39, 292–300 (2005). [Google Scholar]
- 112.Nijman V., Decline of the endemic Hose’s langur Presbytis hosei in Kayan Mentarang National Park, East Borneo. Oryx 39, 223–226 (2005). [Google Scholar]
- 113.Loke V. P. W., Lim T., Campos-Arceiz A., Hunting practices of the Jahai indigenous community in northern peninsular Malaysia. Glob. Ecol. Conserv. 21, e00815 (2020). [Google Scholar]
- 114.Marshall A. J., Nardiyono, Engström L. M., Pamungkas B., Palapa J., Meijaard E., Stanley S. A., The blowgun is mightier than the chainsaw in determining population density of Bornean orangutans (Pongo pygmaeus morio) in the forests of East Kalimantan. Biol. Conserv. 129, 566–578 (2006). [Google Scholar]
- 115.Nijman V., Nekaris K., Traditions, taboos and trade in slow lorises in Sundanese communities in southern Java, Indonesia. Endanger. Species Res. 25, 79–88 (2014). [Google Scholar]
- 116.Wilkie D. S., Bennett E. L., Peres C. A., Cunningham A. A., The empty forest revisited. Ann. N. Y. Acad. Sci. 1223, 120–128 (2011). [DOI] [PubMed] [Google Scholar]
- 117.L. Coad, J. E. Fa, K. Abernethy, N. Van Vliet, C. Santamaria, D. Wilkie, H. R. El Bizri, D. J. Ingram, D.-M. Cawthorn, R. Nasi, Toward A Sustainable, Participatory and Inclusive Wild Meat Sector (CIFOR, 2019); 10.1111/j.1749-6632.2010.05908.x. [DOI]
- 118.Siriwat P., Nekaris K. A. I., Nijman V., The role of the anthropogenic Allee effect in the exotic pet trade on Facebook in Thailand. J. Nat. Conserv. 51, 125726 (2019). [Google Scholar]
- 119.Norconk M. A., Atsalis S., Tully G., Santillán A. M., Waters S., Knott C. D., Ross S. R., Shanee S., Stiles D., Reducing the primate pet trade: Actions for primatologists. Am. J. Primatol. 82, 1–9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.K. H. Redford, J. G. Robinson, in Latin America Mammology: History, Biodiversity, and Conservation, M. A. Mares, D. J. Schmidly, Eds. (University of Oklahoma Press, 1991), pp. 227–237. [Google Scholar]
- 121.Peres C. A., Effects of subsistence hunting on vertebrate community structure in Amazonian forests. Conserv. Bio. 14, 240–253 (2000). [Google Scholar]
- 122.Freese C. H., Heltne P. G., Napoleon C. R., Whitesides G., Patterns and determinants of monkey densities in Peru and Bolivia, with notes on distributions. Int. J. Primatol. 3, 53–90 (1982). [Google Scholar]
- 123.Peres C. A., Indigenous reserves and nature conservation in Amazonian forests. Conserv. Biol. 8, 586–588 (1994). [Google Scholar]
- 124.de Thoisy B., Renoux F., Julliot C., Hunting in northern French Guiana and its impact on primate communities. Oryx 39, 149–157 (2005). [Google Scholar]
- 125.Ripple W. J., Abernethy K., Betts M. G., Chapron G., Dirzo R., Galetti M., Levi T., Lindsey P. A., Macdonald D. W., Machovina B., Newsome T. M., Peres C. A., Wallach A. D., Wolf C., Young H., Bushmeat hunting and extinction risk to the world’s mammals. R. Soc. Open Sci. 3, 160498 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Espinosa S., Branch L. C., Cueva R., Road development and the geography of hunting by an Amazonian Indigenous Group: Consequences for wildlife conservation. PLOS ONE 9, e114916 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van Vliet N., Quiceno-Mesa M. P., Cruz-Antia D., de Aquino L. J. N., Moreno J., Nasi R., The uncovered volumes of bushmeat commercialized in the Amazonian trifrontier between Colombia, Peru and Brazil. Ethnobiol. Conserv. 3, 1–11 (2014). [Google Scholar]
- 128.Peres C. A., Palacios E., Basin-wide effects of game harvest on vertebrate population densities in Amazonian forests: Implications for animal-mediated seed dispersal. Biotropica 39, 304–315 (2007). [Google Scholar]
- 129.Alvard M. S., Robinson J. G., Redford K. H., Kaplan H., The sustainability of subsistence hunting in the neotropics La Sustentabilidad de la Caza de Subsistencia en el Neotropico. Conserv. Biol. 11, 977–982 (1997). [Google Scholar]
- 130.Fa J. E., García Yuste J. E., Commercial bushmeat hunting in the Monte Mitra forests, Equatorial Guinea: Extent and impact. Anim. Biodivers. Conserv. 24, 31–52 (2001). [Google Scholar]
- 131.Luz A. C., Paneque-Gálvez J., Guèze M., Pino J., Macía M. J., Orta-Martínez M., Reyes-García V., Continuity and change in hunting behaviour among contemporary indigenous peoples. Biol. Conserv. 209, 17–26 (2017). [Google Scholar]
- 132.Suarez E., Zapata-Ríos G., Managing subsistence hunting in the changing landscape of Neotropical rain forests. Biotropica 51, 282–287 (2019). [Google Scholar]
- 133.Peres C. A., Emilio T., Schietti J., Desmoulière S. J. M., Levi T., Dispersal limitation induces long-term biomass collapse in overhunted Amazonian forests. Proc. Natl. Acad. Sci. 113, 892–897 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bodmer R., Mayor P., Antunez M., Fang T., Chota K., Yuyarima T. A., Flores S., Cosgrove B., López N., Pizuri O., Puertas P., Wild meat species, climate change, and indigenous Amazonians. J. Ethnobiol. 40, 218–233 (2020). [Google Scholar]
- 135.Avila Martin E., Ros Brull G., Funk S. M., Luiselli L., Okale R., Fa J. E., Wild meat hunting and use by sedentarised Baka Pygmies in southeastern Cameroon. PeerJ 8, e9906 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.W. R. Townsend, R. B. Wallace, K. Lara-Delgado, G. Miranda-Chumacero, in Neotropical Ethnoprimatology. Ethnobiology, B. Urbani, M. Lizarralde, Eds. (Springer, 2020), pp. 343–362; 10.1007/978-3-030-27504-4_17. [DOI]
- 137.da Silva M. N. F., Shepard G. H., Yu D. W., Conservation implications of primate hunting practices among the Matsigenka of Manu National Park. Neotrop. Primates 13, 31 (2005). [Google Scholar]
- 138.Luz A., Guèze M., Paneque-Gálvez J., Pino J., Macía M., Orta-Martínez M., Reyes-García V., How does cultural change affect indigenous peoples’ hunting activity? An empirical study among the tsimane’ in the Bolivian Amazon. Conserv. Soc. 13, 382 (2015). [Google Scholar]
- 139.Naranjo E. J., Guerra M. M., Bodmer R. E., Bolaños J. E., Subsistence hunting by three ethnic groups of the lacandon forest, Mexico. J. Ethnobiol. 24, 233–253 (2004). [Google Scholar]
- 140.Duda R., Gallois S., Reyes-Garcia V., Hunting techniques, wildlife offtake and market integration. A perspective from individual variations among the baka (Cameroon). Afr. Study Monogr. 38, 97–118 (2017). [Google Scholar]
- 141.Köhler A., Of apes and men: Baka and Bantu attitudes to wildlife and the making of eco-goodies and baddies. Conserv. Soc. 3, 407–435 (2005). [Google Scholar]
- 142.Kohler F., Brondizio E. S., Considering the needs of indigenous and local populations in conservation programs. Conserv. Biol. 31, 245–251 (2017). [DOI] [PubMed] [Google Scholar]
- 143.Oishi T., Human-gorilla and gorilla-human: Dynamics of human-animal boundaries and interethnic relationships in the central African rainforest1. Rev. Primatol. , (2013). [Google Scholar]
- 144.C. Shaffer, E. Marawanaru, C. Yukuma, in Ethnoprimatology: A Practical Guide to Research at the Human-Nonhuman Interface, K. M. Dore, E. P. Riley, A. Fuentes, Eds. (Cambridge Univ. Press, 2017), pp. 232–250. [Google Scholar]
- 145.C. A. Shaffer, M. S. Milstein, P. Suse, E. Marawanaru, C. Yukuma, in Primate Research and Conservation in the Anthropocene, A. M. Behie, J. A. Teichrobe, N. Malone, Eds. (Cambridge Univ. Press, 2019), pp. 74–98. [Google Scholar]
- 146.Antunes A. P., Fewster R. M., Venticinque E. M., Peres C. A., Levi T., Rohe F., Shepard G. H., Empty forest or empty rivers? A century of commercial hunting in Amazonia. Sci. Adv. 2, e1600936 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Read J. M., Fragoso J. M. V., Silvius K. M., Luzar J., Overman H., Cummings A., Giery S. T., de Oliveira L. F., Space, place, and hunting patterns among indigenous peoples of the Guyanese Rupununi Region. J. Latin Am. Geogr. 9, 213–243 (2010). [Google Scholar]
- 148.Riu-Bosoms C., Vidal T., Duane A., Fernandez-Llamazares Onrubia A., Gueze M., Luz A. C., Paneque-Gálvez J., Macia M. J., Reyes-Garcia V., Exploring indigenous landscape classification across different dimensions: A case study from the Bolivian Amazon. Landsc. Res. 40, 318–337 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Virtanen P. K., Ancestors’ times and protection of Amazonian Indigenous biocultural heritage. Altern. Int. J. Indig. Peoples 15, 330–339 (2019). [Google Scholar]
- 150.de Mattos Vieira M. R., von Muhlen E., Shepard G., Participatory monitoring and management of subsistence hunting in the Piagaçu-Purus Reserve, Brazil. Conserv. Soc. 13, 254 (2015). [Google Scholar]
- 151.IUCN-WCPA Task Force on OECMs, Recognising and Reporting Other Effective Area-Based Conservation Measures (IUCN, 2019). [Google Scholar]
- 152.CBD, in Conference of the Parties to the Convention on Biological Diversity, Fourteeth Meeting, Agenda Item 24 CBD/COP/DEC/14/8 (CBD, 2018), p. 19; https://cbd.int/doc/decisions/cop-14/cop-14-dec-08-en.pdf.
- 153.Rasolofoson R. A., Ferraro P. J., Jenkins C. N., Jones J. P. G., Effectiveness of community forest management at reducing deforestation in Madagascar. Biol. Conserv. 184, 271–277 (2015). [Google Scholar]
- 154.Jarosz L., Defining and explaining tropical deforestation: Shifting cultivation and population growth in colonial Madagascar (1896-1940). Econ. Geogr 69, 366–379 (1993). [PubMed] [Google Scholar]
- 155.Sussman R. W., Green G. M., Sussman L. K., Satellite imagery, human ecology, anthropology, and deforestation in Madagascar. Hum. Ecol. 22, 333–354 (1994). [Google Scholar]
- 156.IPBES, Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES Secretariat, 2019).
- 157.Venter O., Sanderson E. W., Magrach A., Allan J. R., Beher J., Jones K. R., Possingham H. P., Laurance W. F., Wood P., Fekete B. M., Levy M. A., Watson J. E. M., Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 7, 12558 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Correa Ayram C. A., Mendoza M. E., Etter A., Pérez Salicrup D. R., Anthropogenic impact on habitat connectivity: A multidimensional human footprint index evaluated in a highly biodiverse landscape of Mexico. Ecol. Indic. 72, 895–909 (2017). [Google Scholar]
- 159.Watson J. E. M., Jones K. R., Fuller R. A., Di Marco M., Segan D. B., Butchart S. H. M., Allan J. R., McDonald-Madden E., Venter O., Persistent disparities between recent rates of habitat conversion and protection and implications for future global conservation targets. Conserv. Lett. 9, 413–421 (2016). [Google Scholar]
- 160.Beyer H. L., Venter O., Grantham H. S., Watson J. E. M., Substantial losses in ecoregion intactness highlight urgency of globally coordinated action. Conserv. Lett. 13, 1–9 (2020). [Google Scholar]
- 161.Baragwanath K., Bayi E., Collective property rights reduce deforestation in the Brazilian Amazon. Proc. Natl. Acad. Sci. 117, 20495–20502 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.WWF, Congo Basin (2022); https://worldwildlife.org/places/congo-basin.
- 163.Asher S., Novosad P., Rural roads and local economic development. Am. Econ. Rev. 110, 797–823 (2018). [Google Scholar]
- 164.Ibisch P. L., Hoffmann M. T., Kreft S., Pe’er G., Kati V., Biber-Freudenberger L., DellaSala D. A., Vale M. M., Hobson P. R., Selva N., A global map of roadless areas and their conservation status. Science 354, 1423–1427 (2016). [DOI] [PubMed] [Google Scholar]
- 165.Meijer J. R., Huijbregts M. A. J., Schotten K. C. G. J., Schipper A. M., Global patterns of current and future road infrastructure. Environ. Res. Lett. 13, 64006 (2018). [Google Scholar]
- 166.OECD, in OECD Business and Finance Outlook 2018 (OECD Publishing, 2018), pp. 1–44. [Google Scholar]
- 167.White House, FACT SHEET: President Biden and G7 Leaders Launch Build Back Better World (B3W) Partnership (2021); https://whitehouse.gov/briefing-room/statements-releases/2021/06/12/fact-sheet-president-biden-and-g7-leaders-launch-build-back-better-world-b3w-partnership/.
- 168.European Commission, Global Gateway: Up to € 300 billion for the European Union’s strategy to boost sustainable links around the world, press release (2021), p. 3.
- 169.Kis Madrid C., Hickey G. M., Bouchard M. A., Strategic environmental assessment effectiveness and the initiative for the integration of regional infrastructure in South America (IIRSA): A multiple case review. J. Environ. Assess. Policy Manag. 13, 515–540 (2011). [Google Scholar]
- 170.Hughes A. C., Understanding and minimizing environmental impacts of the Belt and Road Initiative. Conserv. Biol. 33, 883–894 (2019). [DOI] [PubMed] [Google Scholar]
- 171.Hughes A. C., Lechner A. M., Chitov A., Horstmann A., Hinsley A., Tritto A., Chariton A., Li B. V., Ganapin D., Simonov E., Morton K., Toktomushev K., Foggin M., Tan-Mullins M., Orr M. C., Griffiths R., Nash R., Perkin S., Glémet R., Kim M., Yu D. W., Horizon scan of the Belt and Road initiative. Trends Ecol. Evol. 35, 583–593 (2020). [DOI] [PubMed] [Google Scholar]
- 172.Sloan S., Alamgir M., Campbell M. J., Setyawati T., Laurance W. F., Development corridors and remnant-forest conservation in Sumatra, Indonesia. Trop. Conserv. Sci. 12, 194008291988950 (2019). [Google Scholar]
- 173.Ascensão F., Niebuhr B. B., Moraes A. M., Alexandre B. R., Assis J. C., Alves-Eigenheer M. A., Ribeiro J. W., Morais M. M., Martins A. F., Oliveira A., Moraes E., Ramos J. H., Lorini M. L., Ferraz L. P., Culot L., Dietz J. M., Ruiz-Miranda C. R., Ribeiro M. C., End of the line for the golden lion tamarin? A single road threatens 30 years of conservation efforts. Conserv. Sci. Pract. 1, e89 (2019). [Google Scholar]
- 174.Grandia L., Road Mapping: Megaprojects and land grabs in the northern Guatemalan lowlands. Dev. Change 44, 233–259 (2013). [Google Scholar]
- 175.Edwards D. P., Sloan S., Weng L., Dirks P., Sayer J., Laurance W. F., Mining and the African environment. Conserv. Lett. 7, 302–311 (2014). [Google Scholar]
- 176.Barber C. P., Cochrane M. A., Souza C. M., Laurance W. F., Roads, deforestation, and the mitigating effect of protected areas in the Amazon. Biol. Conserv. 177, 203–209 (2014). [Google Scholar]
- 177.R. Stavenhagen, Indigenous Peoples in Comparative Perspective—Problems and Policies (HDOCPA-2004-14, Human Development Report Office (HDRO), United Nations Development Programme (UNDP), 2004).
- 178.Sales L. P., Ribeiro B. R., Pires M. M., Chapman C. A., Loyola R., Recalculating route: Dispersal constraints will drive the redistribution of Amazon primates in the Anthropocene. Ecography 42, 1789–1801 (2019). [Google Scholar]
- 179.Meijaard E., Ni’matullah S., Dennis R., Sherman J., Onrizal, Wich S. A., The historical range and drivers of decline of the Tapanuli orangutan. PLOS ONE 16, e0238087 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Andrasi B., Jaeger J. A. G., Heinicke S., Metcalfe K., Hockings K. J., Quantifying the road-effect zone for a critically endangered primate. Conserv. Lett. 14, e12839 (2021). [Google Scholar]
- 181.Rezende C. L., Scarano F. R., Assad E. D., Joly C. A., Metzger J. P., Strassburg B. B. N., Tabarelli M., Fonseca G. A., Mittermeier R. A., From hotspot to hopespot: An opportunity for the Brazilian Atlantic Forest. Perspect. Ecol. Conserv. 16, 208–214 (2018). [Google Scholar]
- 182.Lees A. C., Peres C. A., Fearnside P. M., Schneider M., Zuanon J. A. S., Hydropower and the future of Amazonian biodiversity. Biodivers. Conserv. 25, 451–466 (2016). [Google Scholar]
- 183.Jones I. L., Bull J. W., Major dams and the challenge of achieving “No Net Loss” of biodiversity in the tropics. Sustain. Dev. 28, 435–443 (2020). [Google Scholar]
- 184.Clements G. R., Aziz S. A., Bulan R., Giam X., Bentrupperbaumer J., Goosem M., Laurance S., Laurance W. F., Not everyone wants roads: Assessing Indigenous People’s support for roads in a globally important tiger conservation landscape. Hum. Ecol. 46, 909–915 (2018). [Google Scholar]
- 185.Vilela T., Malky Harb A., Bruner A., Laísa da Silva Arruda V., Ribeiro V., Alencar A. A. C., Grandez A. J. E., Rojas A., Laina A., Botero R., A better Amazon road network for people and the environment. Proc. Natl. Acad. Sci. 117, 7095–7102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.ISA, Table of Indigenous peoples, in Povos Indígenas no Brasil (2021); https://pib.socioambiental.org/en/Table_of_Indigenous_Peoples.
- 187.Bromham L., Dinnage R., Skirgård H., Ritchie A., Cardillo M., Meakins F., Greenhill S., Hua X., Global predictors of language endangerment and the future of linguistic diversity. Nat. Ecol. Evol. 6, 163–173 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ferrante L., Gomes M., Fearnside P. M., Amazonian indigenous peoples are threatened by Brazil’s Highway BR-319. Land Use Policy 94, 104548 (2020). [Google Scholar]
- 189.Ferrante L., Fearnside P. M., The Amazon’s road to deforestation. Science 369, 634 (2020). [DOI] [PubMed] [Google Scholar]
- 190.Engert J. E., Ishida F. Y., Laurance W. F., Rerouting a major Indonesian mining road to spare nature and reduce development costs. Conserv. Sci. Pract. 3, e521 (2021). [Google Scholar]
- 191.United Nations, United Nations Declaration on the Rights of Indigenous Peoples (United Nations, 2007); https://un.org/development/desa/indigenouspeoples/declaration-on-the-rights-of-indigenous-peoples.html.
- 192.UN, in 61/295. United Nations Declaration on the Rights of Indigenous Peoples (United Nations, 2007), pp. 1–29; https://un.org/development/desa/indigenouspeoples/wp-content/uploads/sites/19/2018/11/UNDRIP_E_web.pdf.
- 193.UN, in General Assembly GA/SHC/3891 (United Nations, 2007); https://un.org/press/en/2007/gashc3891.doc.htm.
- 194.J. C. Tresierra, Rights of Indigenous Groups over Natural Resources in Tropical Forests (IADB, 2020); https://publications.iadb.org/en/rights-indigenous-groups-over-natural-resources-tropical-forests.
- 195.G. Freire, M. Schartz Orellana, S. D. Zumaeta Aurazo, D. C. Costa, J. M. Ludvall, L. R. Viveros Mendoza, M. C. Lucchetti, L. L. Moreno Herrera, L. D. C. Souza, Indigenous Latin America in the Twenty-First Century: The First Decade (Washington D.C., 2015); http://documents.worldbank.org/curated/en/145891467991974540/pdf/Indigenous-Latin-America-in-the-twenty-first-century-the-first-decade.pdf.
- 196.UNDESA, Education (United Nations Department of Economic and Social Affairs – Indigenous Peoples, 2020); https://un.org/development/desa/indigenouspeoples/mandated-areas1/education.html.
- 197.UNDESA, Indigenous Peoples & the COVID-19 Pandemic: Considerations (New York, NY, 2020); https://un.org/development/desa/indigenouspeoples/wp-content/uploads/sites/19/2020/04/COVID19_IP_considerations.pdf.
- 198.UN, The State of World’s Indigenous Peoples: Education (United Nations, 2017); https://un.org/development/desa/indigenouspeoples/wp-content/uploads/sites/19/2017/12/State-of-Worlds-Indigenous-Peoples_III_WEB2018.pdf.
- 199.UNDESA, COVID-19 and Indigenous Peoples (United Nations Department of Economic and Social Affairs, 2020); https://un.org/development/desa/indigenouspeoples/covid-19.html.
- 200.Jurkevics A., Land grabbing and the perplexities of territorial sovereignty. Polit. Theory 50, 32–58 (2021). [Google Scholar]
- 201.F. Pearce, Common Ground: Securing Land Rights and Safeguarding the Earth (International Land Coalition, 2016). [Google Scholar]
- 202.Human Rights Watch, ‘When We Lost the Forest, We Lost Everything’: Oil Palm Plantations and Rights Violations in Indonesia (Human Rights Watch, 2019); https://hrw.org/sites/default/files/report_pdf/indonesia0919_web.pdf.
- 203.J. Siringoringo, V. Mambor, in The Indigenous World 2020 (Eks-Skolen Trykkeri, 2020), pp. 250–266. [Google Scholar]
- 204.Nagata S., Dallos C., The Orang Asli of West Malaysia: An update. Moussons 4, 97–112 (2001). [Google Scholar]
- 205.J. A. Schertow, Indigenous Peoples of Sarawak Fighting Malaysian Plan to Build 12 Mega-Dams (Deep Green Resistance News Service, 2019); https://dgrnewsservice.org/civilization/colonialism/indigenous-peoples-of-sarawak-fighting-malaysian-plan-to-build-12-mega-dams/.
- 206.Ramutsindela M., Sinthumule I., Property and difference in nature conservation. Geogr. Rev. 107, 415–432 (2017). [Google Scholar]
- 207.Witter R., Satterfield T., Rhino poaching and the “slow violence” of conservation-related resettlement in Mozambique’s Limpopo National Park. Geoforum 101, 275–284 (2019). [Google Scholar]
- 208.E. I. Laltaika, K. M. Askew, Modes of Dispossession of Indigenous Lands and Territories in Africa (United Nations, 2018); https://un.org/development/desa/indigenouspeoples/wp-content/uploads/sites/19/2018/01/Laltaika-and-Askew_UN-paper_rev3.pdf.
- 209.Anaya F. C., Espírito-Santo M. M., Protected areas and territorial exclusion of traditional communities: Analyzing the social impacts of environmental compensation strategies in Brazil. Ecol. Soc. 23, art8 (2018). [Google Scholar]
- 210.Domínguez L., Luoma C., Decolonising conservation policy: How colonial land and conservation ideologies persist and perpetuate Indigenous injustices at the expense of the environment. Landarzt 9, 65 (2020). [Google Scholar]
- 211.Bocarejo D., Ojeda D., Violence and conservation: Beyond unintended consequences and unfortunate coincidences. Geoforum 69, 176–183 (2016). [Google Scholar]
- 212.Lunstrum E., Ybarra M., Deploying difference: Security threat narratives and state displacement from protected areas. Conserv. Soc. 16, 114–124 (2018). [Google Scholar]
- 213.Shanee N., Reclaim Conservation: Conservation discourse and initiatives of the rondas campesinas, North-Eastern Peru. Conserv. Soc. 17, 270–282 (2020). [Google Scholar]
- 214.Shanee S., Shanee N., Lock W., Espejo-Uribe M. J., The development and growth of non-governmental conservation in Peru: Privately and communally protected areas. Hum. Ecol. 48, 681–693 (2020). [Google Scholar]
- 215.Dehm J., Authorizing appropriation?: Law in contested forested spaces. Eur. J. Int. Law 28, 1379–1396 (2017). [Google Scholar]
- 216.Kurian A. L., Ecotourism and conservation refugees: The Indian. Ecol. Environ. Conserv. 27, 308–314 (2021). [Google Scholar]
- 217.Milne S., Mahanty S., Value and bureaucratic violence in the green economy. Geoforum 98, 133–143 (2019). [Google Scholar]
- 218.Aguilar-Støen M., Better safe than sorry? Indigenous Peoples, carbon cowboys and the governance of REDD in the Amazon. Forum Dev. Stud. 44, 91–108 (2017). [Google Scholar]
- 219.Hoang C., Satyal P., Corbera E., ‘This is my garden’: Justice claims and struggles over forests in Vietnam’s REDD+. Clim. Policy. 19, S23–S35 (2019). [Google Scholar]
- 220.Sylvander N., ‘Territorial cleansing’ for whom? Indigenous rights, conservation, and state territorialization in the Bosawas Biosphere Reserve, Nicaragua. Geoforum. 121, 23–32 (2021). [Google Scholar]
- 221.J. Mbaria, M. Ogada, The Big Conservation Lie: The Untold Story of Wildlife Conservation in Kenya (Lens & Pens Publishing, 2016). [Google Scholar]
- 222.Weldemichel T. G., Othering Pastoralists, state violence, and the remaking of boundaries in Tanzania’s militarised wildlife conservation sector. Antipode 52, 1496–1518 (2020). [Google Scholar]
- 223.Western D., Tyrrell P., Brehony P., Russell S., Western G., Kamanga J., Conservation from the inside-out: Winning space and a place for wildlife in working landscapes. People Nat. 2, 279–291 (2020). [Google Scholar]
- 224.Sirima A., Backman K. F., Communities’ displacement from national park and tourism development in the Usangu Plains, Tanzania. Curr. Issues Tour. 16, 719–735 (2013). [Google Scholar]
- 225.Brockington D., Community conservation, inequality and injustice: Myths of power in protected area management. Conserv. Soc. 2, 411–432 (2004). [Google Scholar]
- 226.K. Zephyrin, Rwanda: The Situation of the Batwa Forest Dwellers and Conservation of the Volcanoes National Park and Nyungwe Natural Forest (Peoples Programme, Anderen, Netherlands, 2001); https://forestpeoples.org/sites/fpp/files/publication/2010/10/rwandaeng.pdf.
- 227.A. K. Barume, Heading Towards Extinction? Indigenous Rights in Africa: The Case of the TWA of the Kahuzi-Biega National Park, Democratic Republic of Congo (International Work Group for Indigenous Affairs, 2000).
- 228.V. Couillard, J. Gilbert, J. Kenrick, C. Kidd, Land Rights and the Forest Peoples of Africa: Historical, Legal and Anthropological Perspectives (Moreton-in-Marsh, 2009); http://forestpeoples.org/sites/fpp/files/publication/2010/05/overviewlandrightsstudy09eng.pdf.
- 229.Plummer J., The Yanomami: Illegal mining, law, and indigenous rights in the Brazilian Amazon. Georg. Int. Environ. Law Rev. 27, 479–496 (2015). [Google Scholar]
- 230.C. Torres, Illegal Gold Mining Causing Mercury Contamination In Indigenous Groups (Mongabay, 2016); https://news.mongabay.com/2016/07/illegal-gold-mining-causing-mercury-contamination-in-indigenous-groups/.
- 231.ONIC, The National Indigenous Organization of Colombia (2020); https://onic.org.co/.
- 232.UNHCR, ‘To lose our land is to lose ourself’: Indigenous people and forced displacement in Colombia (2020); https://acnur.org/fileadmin/Documentos/RefugiadosAmericas/Colombia/EN/Indigenous_people_and_forced_displacement_in_Colombia.pdf?view.
- 233.Hitchcock R. K., The impacts of conservation and militarization on indigenous peoples. Hum. Nat. 30, 217–241 (2019). [DOI] [PubMed] [Google Scholar]
- 234.Sales L., Ribeiro B. R., Chapman C. A., Loyola R., Multiple dimensions of climate change on the distribution of Amazon primates. Perspect. Ecol. Conserv. 18, 83–90 (2020). [Google Scholar]
- 235.G. Cajete, Native Science: Natural Laws of Interdependence (Clear Light Publishers, 2000). [Google Scholar]
- 236.C. Carroll, Roots of Our Renewal (University of Minnesota Press, 2015); http://jstor.org/stable/10.5749/j.ctt15hvz24.
- 237.J. Mohawke, in Thinking in Indian: A John Mohawk Reader, J. Barreiro, Ed. (Fulcrum Publishing, 2010), pp. 274–277. [Google Scholar]
- 238.J. Hernandez, Fresh Banana Leaves: Healing Indigenous Landscapes Through Indigenous Science (North Atlantic Books, 2022). [Google Scholar]
- 239.Venter O., Fuller R. A., Segan D. B., Carwardine J., Brooks T., Butchart S. H. M., Di Marco M., Iwamura T., Joseph L., O’Grady D., Possingham H. P., Rondinini C., Smith R. J., Venter M., Watson J. E. M., Targeting global protected area expansion for imperiled biodiversity. PLOS Biol. 12, e1001891 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yanqing G., Baoping R., Qiang D., Jun Z., Garber P. A., Jiang Z., Habitat estimates reveal that there are fewer than 400 Guizhou snub-nosed monkeys, Rhinopithecus brelichi, remaining in the wild. Glob. Ecol. Conserv. 24, e01181 (2020). [Google Scholar]
- 241.IUCN, in IUCN World Conservation Congress - Marseille (2021).
- 242.Urzedo D., Chatterjee P., The colonial reproduction of deforestation in the Brazilian Amazon: Violence against Indigenous peoples for land development. J. Genocide Res. 23, 302–324 (2021). [Google Scholar]
- 243.UNDESA, “State of the World’s Indigenous Peoples Rights to Lands, Territories and Resources” (New York, 2021); https://un.org/development/desa/indigenouspeoples/wp-content/uploads/sites/19/2021/03/State-of-Worlds-Indigenous-Peoples-Vol-V-Final.pdf.
- 244.R. K. Dhir, U. Cattaneo, M. V. Ormaza Cabrera, H. Coronado, M. Oelz, Implementing the ILO Indigenous and Tribal Peoples Convention No. 169: Towards an Inclusive, Sustainable and Just Future (International Labour Organization, 2019; https://ilo.org/wcmsp5/groups/public/---dgreports/---dcomm/---publ/documents/publication/wcms_735607.pdf).
- 245.Poudyal M., Jones J. P. G., Rakotonarivo O. S., Hockley N., Gibbons J. M., Mandimbiniaina R., Rasoamanana A., Andrianantenaina N. S., Ramamonjisoa B. S., Who bears the cost of forest conservation? PeerJ 6, e5106 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.E. Richard Atleo, Principles of Tsawalk: An Indigenous Approach to Global Crisis (UBC Press, 2012). [Google Scholar]
- 247.Falkowski T. B., Diemont S. A. W., Chankin A., Douterlungne D., Lacandon Maya traditional ecological knowledge and rainforest restoration: Soil fertility beneath six agroforestry system trees. Ecol. Eng. 92, 210–217 (2016). [Google Scholar]
- 248.Padgham M., Lovelace R., Salmon M., Rudis B., Osmdata. J. Open Source Softw. 2, 305 (2017). [Google Scholar]
- 249.Hansford J., Wright P. C., Rasoamiaramanana A., Pérez V. R., Godfrey L. R., Errickson D., Thompson T., Turvey S. T., Early Holocene human presence in Madagascar evidenced by exploitation of avian megafauna. Sci. Adv. 4, eaat6925 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Serva M., The Settlement of Madagascar: What dialects and languages can tell us. PLOS ONE 7, e30666 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Serva M., Pasquini M., Dialects of Madagascar. PLOS ONE 15, e0240170 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Hurles M. E., Sykes B. C., Jobling M. A., Forster P., The dual origin of the Malagasy in Island Southeast Asia and East Africa: Evidence from maternal and paternal lineages. Am. J. Hum. Genet. 76, 894–901 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Li H., Sinha A., Anquetil André A., Spötl C., Vonhof H. B., Meunier A., Kathayat G., Duan P., Voarintsoa N. R. G., Ning Y., Biswas J., Hu P., Li X., Sha L., Zhao J., Edwards R. L., Cheng H., A multimillennial climatic context for the megafaunal extinctions in Madagascar and Mascarene Islands. Sci. Adv. 6, eabb2459 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Pierron D., Heiske M., Razafindrazaka H., Rakoto I., Rabetokotany N., Ravololomanga B., Rakotozafy L. M. A., Rakotomalala M. M., Razafiarivony M., Rasoarifetra B., Raharijesy M. A., Razafindralambo L., Ramilisonina, Fanony F., Lejamble S., Thomas O., Abdallah A. M., Rocher C., Arachiche A., Tonaso L., Pereda-loth V., Schiavinato S., Brucato N., Ricaut F.-X., Kusuma P., Sudoyo H., Ni S., Boland A., Deleuze J.-F., Beaujard P., Grange P., Adelaar S., Stoneking M., Rakotoarisoa J.-A., Radimilahy C., Letellier T., Genomic landscape of human diversity across Madagascar. Proc. Natl. Acad. Sci. 114, E6498–E6506 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.World Bank, The World Bank in Madagascar (2021); https://worldbank.org/en/country/madagascar/.
- 256.Olson D. M., Dinerstein E., Wikramanayake E. D., Burgess N. D., Powell G. V. N., Underwood E. C., D’Amico J. A., Itoua I., Strand H. E., Morrison J. C., Loucks C. J., Allnutt T. F., Ricketts T. H., Kura Y., Lamoreux J. F., Wettengel W. W., Hedao P., Kassem K. R., Terrestrial ecoregions of the world: A new map of life on Earth. Bioscience 51, 933–938 (2001). [Google Scholar]
- 257.R Core Development Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2021).
- 258.T. Keitt, R. Bivand, E. Pebesma, B. Rowlingson, rgdal: Bindings for the geospatial data abstraction library (2010).
- 259.Hijmans R., van Etten J., Raster: Geographic data analysis and modeling. R Packag. Version 517, 2–12 (2014). [Google Scholar]
- 260.H. Wickham, ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag, 2016). [Google Scholar]
- 261.Rondinini C., Di Marco M., Chiozza F., Santulli G., Baisero D., Visconti P., Hoffmann M., Schipper J., Stuart S. N., Tognelli M. F., Amori G., Falcucci A., Maiorano L., Boitani L., Global habitat suitability models of terrestrial mammals. Philos. Trans. R. Soc. B Biol. Sci. 366, 2633–2641 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ficetola G. F., Rondinini C., Bonardi A., Katariya V., Padoa-Schioppa E., Angulo A., An evaluation of the robustness of global amphibian range maps. J. Biogeogr. 41, 211–221 (2014). [Google Scholar]
- 263.Hoffmann M., Hilton-Taylor C., Angulo A., Böhm M., Brooks T. M., Butchart S. H. M., Carpenter K. E., Chanson J., Collen B., Cox N. A., Darwall W. R. T., Dulvy N. K., Harrison L. R., Katariya V., Pollock C. M., Quader S., Richman N. I., Rodrigues A. S. L., Tognelli M. F., Vié J.-C., Aguiar J. M., Allen D. J., Allen G. R., Amori G., Ananjeva N. B., Andreone F., Andrew P., Ortiz A. L. A., Baillie J. E. M., Baldi R., Bell B. D., Biju S. D., Bird J. P., Black-Decima P., Blanc J. J., Bolaños F., Bolivar-G W., Burfield I. J., Burton J. A., Capper D. R., Castro F., Catullo G., Cavanagh R. D., Channing A., Chao N. L., Chenery A. M., Chiozza F., Clausnitzer V., Collar N. J., Collett L. C., Collette B. B., Fernandez C. F. C., Craig M. T., Crosby M. J., Cumberlidge N., Cuttelod A., Derocher A. E., Diesmos A. C., Donaldson J. S., Duckworth J. W., Dutson G., Dutta S. K., Emslie R. H., Farjon A., Fowler S., Freyhof J., Garshelis D. L., Gerlach J., Gower D. J., Grant T. D., Hammerson G. A., Harris R. B., Heaney L. R., Hedges S. B., Hero J.-M., Hughes B., Hussain S. A., Icochea M J., Inger R. F., Ishii N., Iskandar D. T., Jenkins R. K. B., Kaneko Y., Kottelat M., Kovacs K. M., Kuzmin S. L., La Marca E., Lamoreux J. F., Lau M. W. N., Lavilla E. O., Leus K., Lewison R. L., Lichtenstein G., Livingstone S. R., Lukoschek V., Mallon D. P., McGowan P. J. K., McIvor A., Moehlman P. D., Molur S., Alonso A. M., Musick J. A., Nowell K., Nussbaum R. A., Olech W., Orlov N. L., Papenfuss T. J., Parra-Olea G., Perrin W. F., Polidoro B. A., Pourkazemi M., Racey P. A., Ragle J. S., Ram M., Rathbun G., Reynolds R. P., Rhodin A. G. J., Richards S. J., Rodríguez L. O., Ron S. R., Rondinini C., Rylands A. B., de Mitcheson Y. S., Sanciangco J. C., Sanders K. L., Santos-Barrera G., Schipper J., Self-Sullivan C., Shi Y., Shoemaker A., Short F. T., Sillero-Zubiri C., Silvano D. L., Smith K. G., Smith A. T., Snoeks J., Stattersfield A. J., Symes A. J., Taber A. B., Talukdar B. K., Temple H. J., Timmins R., Tobias J. A., Tsytsulina K., Tweddle D., Ubeda C., Valenti S. V., van Dijk P. P., Veiga L. M., Veloso A., Wege D. C., Wilkinson M., Williamson E. A., Xie F., Young B. E., Akçakaya H. R., Bennun L., Blackburn T. M., Boitani L., Dublin H. T., da Fonseca G. A. B., Gascon C., Lacher T. E., Mace G. M., Mainka S. A., McNeely J. A., Mittermeier R. A., Reid G. M., Rodriguez J. P., Rosenberg A. A., Samways M. J., Smart J., Stein B. A., Stuart S. N., The impact of conservation on the status of the world’s vertebrates. Science 330, 1503–1509 (2010). [DOI] [PubMed] [Google Scholar]
- 264.Strassburg B. B. N., Rodrigues A. S. L., Gusti M., Balmford A., Fritz S., Obersteiner M., Kerry Turner R., Brooks T. M., Impacts of incentives to reduce emissions from deforestation on global species extinctions. Nat. Clim. Chang. 2, 350–355 (2012). [Google Scholar]
- 265.Jenkins C. N., Van Houtan K. S., Pimm S. L., Sexton J. O., US protected lands mismatch biodiversity priorities. Proc. Natl. Acad. Sci. 112, 5081–5086 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Davidson L. N. K., Dulvy N. K., Global marine protected areas to prevent extinctions. Nat. Ecol. Evol. 1, 0040 (2017). [DOI] [PubMed] [Google Scholar]
- 267.Le Saout S., Hoffmann M., Shi Y., Hughes A., Bernard C., Brooks T. M., Bertzky B., Butchart S. H. M., Stuart S. N., Badman T., Rodrigues A. S. L., Protected areas and effective biodiversity conservation. Science 342, 803–805 (2013). [DOI] [PubMed] [Google Scholar]
- 268.González-Maya J. F., Víquez-R L. R., Belant J. L., Ceballos G., Effectiveness of protected areas for representing species and populations of terrestrial mammals in Costa Rica. PLOS ONE 10, e0124480 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Klein C. J., Brown C. J., Halpern B. S., Segan D. B., McGowan J., Beger M., Watson J. E. M., Shortfalls in the global protected area network at representing marine biodiversity. Sci. Rep. 5, 17539 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Shanee S., Shanee N., Monteferri B., Allgas N., Alarcon Pardo A., Horwich R. H., Protected area coverage of threatened vertebrates and ecoregions in Peru: Comparison of communal, private, and state reserves. J. Environ. Manage. 202, 12–20 (2017). [DOI] [PubMed] [Google Scholar]
- 271.Fernández-Llamazares Á., López-Baucells A., Velazco P. M., Gyawali A., Rocha R., Terraube J., Cabeza M., The importance of Indigenous Territories for conserving bat diversity across the Amazon biome. Perspect. Ecol. Conserv. 19, 10–20 (2021). [Google Scholar]
- 272.Montesino Pouzols F., Toivonen T., Di Minin E., Kukkala A. S., Kullberg P., Kuusterä J., Lehtomäki J., Tenkanen H., Verburg P. H., Moilanen A., Global protected area expansion is compromised by projected land-use and parochialism. Nature 516, 383–386 (2014). [DOI] [PubMed] [Google Scholar]
- 273.J. Larsson, eulerr: Area-proportional Euler and Venn diagrams with ellipses (2020; https://cran.r-project.org/package=eulerr).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Text
Figs. S1 to S7
Tables S1 to S7
References