Abstract
Aims
Genetic testing in relatives of hypertrophic cardiomyopathy (HCM) patients leads to early identification of pathogenic DNA variant carriers (G+), before the onset of left ventricular hypertrophy. Routine phenotyping consists of electrocardiography (ECG) and transthoracic echocardiography (TTE). Cardiovascular magnetic resonance (CMR) has become valuable in the work-up of HCM. In this study, we investigated the value of CMR in phenotyping of G+ family members.
Methods and results
This study included 91 G+ subjects who underwent ECG, TTE and CMR, with a maximal wall thickness (MWT) <15 mm on TTE. The relative performance of TTE and CMR regarding wall thickness measurements and HCM diagnoses was assessed. HCM was defined as MWT of ≥13 mm. Logistic regression was performed to assess whether ECG and TTE parameters can predict CMR results. Most subjects (75%) had an MWT <13 mm on TTE, of which 23 (34%) were diagnosed with HCM based on CMR. MWT differences (range 1–10 mm) were often caused by an anterobasal hook-shaped thickening of the myocardium not visible on TTE. Two of 23 (9%) subjects with HCM on TTE were reclassified as no HCM on CMR. Normal ECG and TTE results almost excluded reclassifications by CMR. The prevalence of other HCM-related abnormalities on CMR was low.
Conclusion
CMR reclassified 27% of subjects. Subjects with normal ECG/TTE results were reclassified in a low number of cases, justifying screening with ECG and TTE in G+ relatives. In subjects with abnormal ECGs and/or poor TTE image quality, CMR is indicated.
Keywords: hypertrophic cardiomyopathy, echocardiography, cardiovascular magnetic resonance, genetics, screening
Graphical Abstract
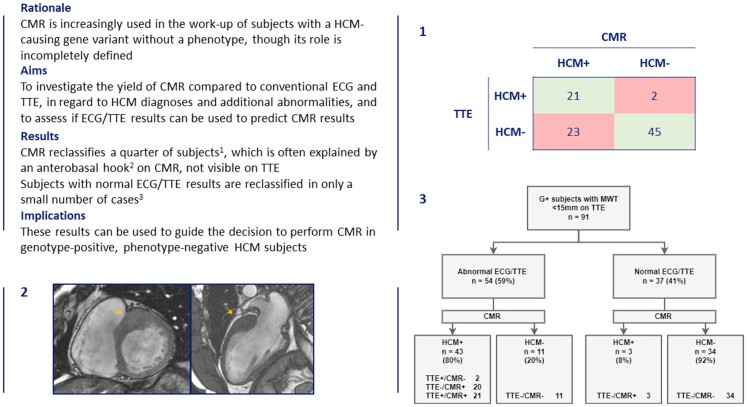
Graphical Abstract.
Overview of current study as a structured abstract in top left, demonstrating the use of cardiac magnetic resonance imaging (CMR) on top of electrocardiography (ECG) and transthoracic echocardiography (TTE) in subjects evaluated for hypertrophic cardiomyopathy (HCM) through family screening. Presence or absence of HCM as judged by TTE and CMR imaging is cross-tabulated (1). More than a quarter of subjects are reclassified when using CMR, as shown by the red boxes. Reclassifications mostly occurred in subjects without HCM on TTE (23/68, 34%), and only rarely in those with HCM on TTE (2/23, 9%). In 10 subjects reclassified as HCM on CMR, a prominent anterobasal hook was found on CMR, a structure not visible on TTE (2, arrows). Results from ECG and TTE can be used to predict diagnoses on HCM (3), as subjects with normal ECG/TTE results (defined as the absence of left ventricular hypertrophy on ECG or TTE, and the lack of left ventricular outflow tract obstruction on TTE), were reclassified in only a small number of cases. These findings highlight the potential role of CMR in family screening for HCM, and show how ECG and TTE can be used to assess whether or not it is useful to perform CMR studies in this subject group. G+ = genotype positive.
See the editorial comment for this article ‘Cardiovascular magnetic resonance for screening in hypertrophic cardiomyopathy: the new family plan’, by Ethan J. Rowin and Martin S. Maron, https://doi.org/10.1093/ehjci/jeac126.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common hereditary cardiac condition, with a pathogenic sarcomere gene variant found in up to 60% of cases.1 Genetic counselling and testing of HCM patients facilitate cascade screening of relatives. Most genotype-positive (G+) relatives are phenotype-negative (Ph−) at initial evaluation [maximal wall thickness (MWT) <13 mm], and are followed regularly to assess development of HCM.2 Classically, phenotyping consists of electrocardiography (ECG) and transthoracic echocardiography (TTE), although cardiovascular magnetic resonance (CMR) is increasingly used.1,3 Advantages of CMR include accurate measurement of wall thickness in all segments, the detection of late gadolinium enhancement (LGE) and myocardial crypts.1 CMR is recommended in case of insufficient echocardiographic windows.1 The additive value of CMR imaging in G+ subjects with no or borderline HCM is less clear, as is the role of the ECG and TTE in guiding the decision to perform CMR. In this study, we investigated the incremental value of CMR in diagnosing HCM and assessed which subjects are most likely to be impacted by CMR imaging in addition to the routine assessment by ECG and TTE.
Methods
Study population
For this single-centre retrospective observational study, we screened all G+ subjects who underwent ECG, TTE, and CMR and had an MWT <15 mm on TTE. Subjects with TTE and CMR studies performed up to one year apart from each other were eligible for inclusion. Those with definite HCM (MWT ≥15 mm), or with a high suspicion thereof (i.e. apical hypertrophy but with inadequate tissue delineation to allow accurate measurements), were excluded as were those with pathogenic variants associated with HCM phenocopies (e.g. amyloidosis). The diagnosis of HCM is based on an MWT ≥15 mm, or ≥13 mm in those with first-degree relatives with HCM or in those with a genetic substrate, not solely explained by loading conditions, in accordance with the guidelines.1,4 The latter cut-off is based on the concept that subtle hypertrophy is a sign of disease expression in the context of familial HCM. This study conforms to the principles of the Declaration of Helsinki. All subjects gave informed consent for inclusion in the registry. According to the local institutional review board, this study did not meet the requirements of a study subject to the Medical Research Involving Human Subjects Act.
Genetic analysis and family screening
Genetic counselling and testing is routinely offered to HCM patients visiting our cardiogenetic outpatient clinic. Subjects are considered G+ in case of a likely pathogenic or pathogenic variant (class 4 or 5), in accordance with the American College of Medical Genetics and Genomics recommendations.5 Cascade genetic screening is offered to relatives targeting the variant found in the proband. G+ relatives are then referred for cardiac screening. The cardiogenetic testing procedure has been previously described.6
ECG analysis
Standard 12-lead ECG analysis (10 mm = 1 mV, 25 mm/s) was performed in supine position and during quiet respiration. Left ventricular hypertrophy (LVH) was evaluated using Sokolow–Lyon criteria (S V1 + R V5 or V6 ≥ 35 mm or R aVL >11 mm), Romhilt–Estes score, and Cornell voltage criteria (S V3 + R aVL >28 mm in men, >20 mm in women), based on their superior performance in previous research.7,8 Pathological Q waves were (i) any Q wave >0.02 s or QS in V2-V3, (ii) Q wave ≥0.03 s and ≥1 mm deep or QS in I, II, aVL, aVF, or V4-V6 in ≥2 contiguous leads, or (iii) R wave >0.04 s in V1-V2 and R/S > 1 with a concordant positive T wave in absence of conduction defects.9 T wave inversion (TWI) was defined as an inversion ≥3 mm in ≥2 contiguous leads.
TTE and CMR analysis
TTE and CMR studies were separately analyzed by two researchers (R.H., N.v.d.V.), who were blinded to the results of the other modalities’ data. To assess intra- and inter-reader reproducibility, a random sample of 30 TTE and CMR studies was analyzed 12 months later by the same researchers and by two others (TTE: A.S., CMR: A.H.). All patients underwent TTE using a standardized guideline-based protocol.10,11 TTEs were considered normal in the absence of LVH and left ventricular outflow tract (LVOT) obstruction. LVH was defined as MWT >10 mm for men and >9 mm for women.11 LVOT obstruction was defined as a gradient ≥30 mmHg at rest or during valsalva. The anterior mitral valve leaflet (AMVL) was measured in the apical three-chamber (CH) view, from the tip of the leaflet to the insertion of the non-coronary aortic valve leaflet.12 Left ventricular (LV) systolic and diastolic function were analyzed according to the guidelines.10,11 LV diastolic function was defined as normal, abnormal relaxation, pseudonormal, or restrictive filling, based on Doppler mitral inflow patterns including early (E) and late (A) LV filling velocities, deceleration time, tissue Doppler imaging-derived septal early diastolic velocities (e′) and left atrial dimensions. Image quality was graded optimal, fair, suboptimal or poor based on the visibility of all segments, endocardial border delineation and axis alignment.
All subjects seen at our cardiogenetic outpatient clinic are invited to undergo CMR scanning. Those who undergo CMR scanning represent approximately 90% of the contemporary outpatient population. The remaining 10% either have contra-indications (claustrophobia, metal objects) or refuse CMR for other reasons. CMR examinations were performed on different clinical 1.5 T (n = 81) and 3 T (n = 10) systems (GE Healthcare, Milwaukee, WI, USA) with dedicated cardiac/anterior array coils, ECG gating and breath-hold techniques. The imaging protocol consisted of retrospectively ECG-gated steady-state free precession (SSFP) cine imaging and two-dimensional LGE images.
SSFP images were obtained during breath-hold in a contiguous short-axis (SA) view, with coverage from base to apex and in standard long-axis views (2-, 3-, and 4CH). Functional and volumetric measurements were determined by manually drawing epi- and endocardial contours in end-systole and -diastole in the SA view, excluding papillary muscles and trabeculations from the myocardium. Morphological features such as crypts were assessed on all available SSFP cine images and AMVL length was measured in the 3CH view (from the leaflet to its insertion in the posterior aortic wall, mid- or end-diastole).13 Typical SSFP cine imaging scan parameters were: slice thickness 6–8 mm, interslice gap 2–4 mm, TR/TE 3.2–4.5/1.4–2.0 ms, flip angle 45–85°, ASSET 2, field of view 280–380 × 250–340 mm, acquired matrix 192–280 × 160–256, and 30 phases per cardiac cycle.
We registered the presence of ‘anterobasal hooks’, hook-shaped configurations of the anterobasal segment, defined as an isolated hypertrophic protuberance with a wall thickness ratio ≥2 compared with the adjacent myocardium, visible on the long-axis 2CH view and measured at end-diastole.14
LGE imaging was performed at least 15 min after intravenous administration of a gadolinium-based contrast agent (0.2 mmol/kg; Gadovist, Bayer, Mijdrecht, The Netherlands), using a breath-held two-dimensional segmented inversion-recovery gradient-echo pulse sequence. LGE images were obtained in SA and long-axis views. If necessary, preset inversion time was adjusted to null normal myocardium for LGE imaging. Typical LGE scan parameters were: slice thickness 6–8 mm, interslice gap 2–4 mm, TR/TE 4.5–7.01/1.2–3.3 ms, flip angle 15–25°, ASSET 1.5, field of view 280–400 × 250–400 mm and acquired matrix 200–256 × 160–200. Isolated hinge point fibrosis was registered separately from mid-myocardial replacement fibrosis contained in the LV myocardium.
Statistical analysis
Values were expressed as mean ± standard deviation, median [25–75th percentile] or number (%). Continuous data were assessed for normality using Q–Q plots and the Shapiro–Wilk test, and analyzed using the Student’s t-test or Mann–Whitney U test. Categorical data were compared using Pearson’s χ² test or Fisher’s exact test. Paired data were compared using the paired t-test and McNemar’s test. Agreement between TTE and CMR as well as intra- and inter-reader reproducibility was visualized using Bland–Altman plots and reported using the intraclass correlation coefficient (ICC). The cohort was split into groups and analyzed based on presence or absence of HCM on TTE and CMR. Logistic regression was used to assess the predictive ability of ECG and TTE parameters for reclassifications on CMR. Variable selection was based on the Akaike Information Criterion. Multicollinearity was assessed by calculating variance inflation factors, with a cut-off of 5 indicating high multicollinearity. Subjects were stratified according to the presence of ECG/TTE abnormalities to identify subgroups with low and high odds of reclassifications and CMR-related abnormalities. We explored which independent predictors could refine these results by assessing their predictive ability through receiver operator characteristic curves. Testing was two-tailed and P values <0.05 were considered statistically significant. All analyses were performed using SPSS version 25 (IBM Corp., Armonk, New York) and R version 3.6.1 (https://cran.r-project.org/).
Results
Study population
After excluding two subjects lacking a formal diagnosis of HCM on TTE but highly suspected thereof (LVH on CT, abnormalities in the apex suggestive of apical HCM), we included 91 subjects (40% male, 46 ± 13 years). Most (n = 68, 75%) had no HCM on TTE (TTE−), of which 23 (34%) were diagnosed with HCM following CMR (CMR+). Of 23 (25%) subjects diagnosed with HCM on TTE (TTE+), 2 (9%) were reclassified as no HCM on CMR (CMR−). The number of HCM diagnoses was significantly different between modalities (P < 0.001).
Table 1 illustrates ECG and imaging characteristics of the total cohort and TTE− subgroups, stratified by the presence of HCM on CMR. Most ECGs were unremarkable, with TWI and LVH by Sokolow-Lyon criteria being particularly rare. There was some degree of systolic and diastolic impairment, although this was mild in almost all cases. There were four subjects with LVOT obstruction, with peak LVOT gradient values between 32 and 58 mmHg. Myocardial crypts and mid-myocardial LGE were found in 39 (43%) and 13 (14%) subjects. TTE−/CMR+ subjects were, on average, 7 years older than the TTE−/CMR− group. They were less likely to have neutral septal contours (70 vs. 96%, P < 0.01), had a lower septal e′ velocity and a higher E/e′ ratio accordingly (8.0 ± 3.1 vs. 9.8 ± 2.5 cm/s; 9.8 ± 3.5 vs. 7.8 ± 2.2, P < 0.05).
Table 1.
Characteristics for total group and TTE subjects, stratified by HCM on CMR
| Variables | All subjectsa (n = 91) | TTE−/CMR− (n = 45) | TTE−/CMR+ (n = 23) | P-value |
|---|---|---|---|---|
| Age, years | 46 ± 13 | 42 ± 13 | 49 ± 13 | 0.03 |
| Male | 36 (40%) | 13 (29%) | 8 (35%) | 0.62 |
| Genotype | 0.78 | |||
| MYBPC3 | 69 (76%) | 33 (73%) | 18 (78%) | |
| MYH7 | 12 (13%) | 6 (13%) | 3 (13%) | |
| MYL2 | 8 (9%) | 4 (9%) | 2 (9%) | |
| Other | 2 (2%) | 2 (4%) | 0 (0%) | |
| Negative inotropes | 3 (3%) | 1 (2%) | 0 (0%) | 1.00 |
| ECG | ||||
| QRS duration, ms | 95 ± 11 | 92 ± 11 | 97 ± 13 | 0.13 |
| QTc duration, ms | 399 ± 21 | 394 ± 21 | 409 ± 19 | <0.01 |
| Pathological Q wave | 8 (9%) | 2 (4%) | 2 (9%) | 0.48 |
| T-wave inversion | 3 (3%) | 2 (4%) | 0 (0%) | 0.55 |
| Sokolow–Lyon criteria | 3 2(3%) | 2 (4%) | 0 (0%) | 0.55 |
| Cornell voltage criteria | 11 (11%) | 4 (9%) | 2 (9%) | 1.00 |
| Romhilt–Estes score | 0.17 | |||
| <4 | 86 (95%) | 44 (98%) | 20 (87%) | |
| 4 | 4 (4%) | 1 (2%) | 2 (9%) | |
| ≥5 | 1 (1%) | 0 (0%) | 1 (4%) | |
| Echocardiography | ||||
| Suboptimal/poor image quality | 32 (35%) | 14 (31%) | 9 (39%) | 0.51 |
| Interventricular septal wall thickness, mm | 10 [8–12] | 9 [8–10] | 11 [10–11] | <0.001 |
| Septal curvature | <0.01 | |||
| Neutral | 64 (70%) | 43 (96%) | 16 (70%) | |
| Reverse | 24 (26%) | 2 (4%) | 5 (22%) | |
| Sigmoid | 3 (3%) | 0 (0%) | 2 (9%) | |
| Posterior wall thickness, mm | 8 [7–10] | 8 [7–9] | 8 [8–9] | 0.21 |
| MWT, mm | 11 [9–13] | 9 [8–10] | 11 [10–12] | <0.001 |
| MWT location | 0.12 | |||
| Septum | 71 (78%) | 28 (62%) | 20 (87%) | |
| Posterior | 7 (8%) | 7 (16%) | 0 (0%) | |
| Inferolateral | 1 (1%) | 1 (2%) | 0 (0%) | |
| Concentric | 12 (13%) | 9 (20%) | 3 (13%) | |
| Left atrial diameter, mm | 36 ± 5 | 35 ± 5 | 36 ± 5 | 0.33 |
| AMVL length, mm | 27 ± 4 | 26 ± 3 | 27 ± 4 | 0.36 |
| Systolic anterior motion of the mitral valve | 3 (3%) | 0 (0%) | 1 (4%) | 0.34 |
| Complete | 2 (2%) | 0 (0%) | 1 (4%) | |
| Partial | 1 (1%) | 0 (0%) | 0 (0%) | |
| Without LVOT obstruction | 1 (1%) | 0 (0%) | 0 (0%) | - |
| Chordal systolic anterior motion | 2 (2%) | 0 (0%) | 0 (0%) | - |
| LVOT gradient, mmHg | 5 [4–7] | 4 [4–5] | 6 [5–12] | 0.02 |
| Rest valsalva | 7 [5–9] | 6 [4–7] | 8 [4–10] | 0.12 |
| LVOT obstruction | 4 (4%) | 0 (0%) | 1 (4%) | 0.34 |
| Impaired systolic function | 9 (10%) | 6 (13%) | 2 (9%) | 0.71 |
| Mitral inflow E wave, cm/s | 73 ± 16 | 76 ± 17 | 71 ± 17 | 0.28 |
| Mitral inflow A wave, cm/s | 61 ± 18 | 55 ± 16 | 68 ± 22 | <0.01 |
| E/A ratio | 1.3 ± 0.6 | 1.5 ± 0.6 | 1.2 ± 0.5 | 0.03 |
| Septal e′, cm/s | 8.8 ± 2.8 | 9.8 ± 2.5 | 8.0 ± 3.1 | 0.01 |
| E/e′ ratio | 8.9 ± 2.8 | 7.8 ± 2.2 | 9.8 ± 3.5 | <0.01 |
| Diastolic function | 0.04 | |||
| Normal | 63 (69%) | 38 (84%) | 13 (57%) | |
| Abnormal relaxation | 19 (21%) | 4 (9%) | 7 (30%) | |
| Pseudonormal filling | 9 (10%) | 3 (7%) | 3 (13%) | |
| LV mass, g/m² | 76 ± 18 | 68 ± 16 | 79 ± 14 | <0.01 |
| CMR | ||||
| LV mass, g/m² | 49 ± 9 | 46 ± 8 | 49 ± 10 | 0.08 |
| Interventricular septal wall thickness, mm | 10 [8–11] | 8 [8–9] | 11 [10–12] | <0.001 |
| Septal curvature | 0.02 | |||
| Neutral | 69 (76%) | 43 (93%) | 18 (78%) | |
| Reverse | 13 (14%) | 3 (7%) | 1 (4%) | |
| Sigmoid | 9 (10%) | 0 (0%) | 4 (17%) | |
| Posterior wall thickness, mm | 7 [6–8] | 7 [6–8] | 7 [6–8] | 0.33 |
| MWT, mm | 12 [10–14] | 10 [10–11] | 14 [13–15] | <0.001 |
| MWT location | 0.40 | |||
| Anteroseptal basal | 65 (71%) | 30 (67%) | 19 (83%) | |
| Anteroseptal mid | 2 (2%) | 2 (4%) | 0 (0%) | |
| Inferoseptal basal | 14 (15%) | 8 (18%) | 3 (13%) | |
| Inferoseptal mid | 5 (6%) | 1 (2%) | 1 (4%) | |
| Anterolateral basal | 5 (6%) | 4 (9%) | 0 (0%) | |
| Myocardial crypts | 39 (43%) | 19 (42%) | 12 (52%) | 0.44 |
| Multiple | 12 (13%) | 5 (11%) | 4 (18%) | 0.47 |
| Anterobasal hook | 26 (29%) | 10 (22%) | 10 (44%) | 0.07 |
| AMVL length, mm | 23 ± 3 | 23 ± 3 | 23 ± 4 | 0.95 |
| Myocardial fibrosisb | 13 (14%) | 0 (0%) | 3 (14%) | 0.04 |
| Hinge point fibrosis | 8 (13%) | 2 (5%) | 6 (27%) | 0.02 |
Data expressed as mean ± standard deviation, median [25–75th percentile] or number (%). AMVL, anterior mitral valve leaflet. LVOT, left ventricular outflow tract. MWT, maximal wall thickness.
Includes TTE+/CMR+ and TTE+/CMR− groups.
Excludes isolated hinge point fibrosis.
Measurement discrepancies
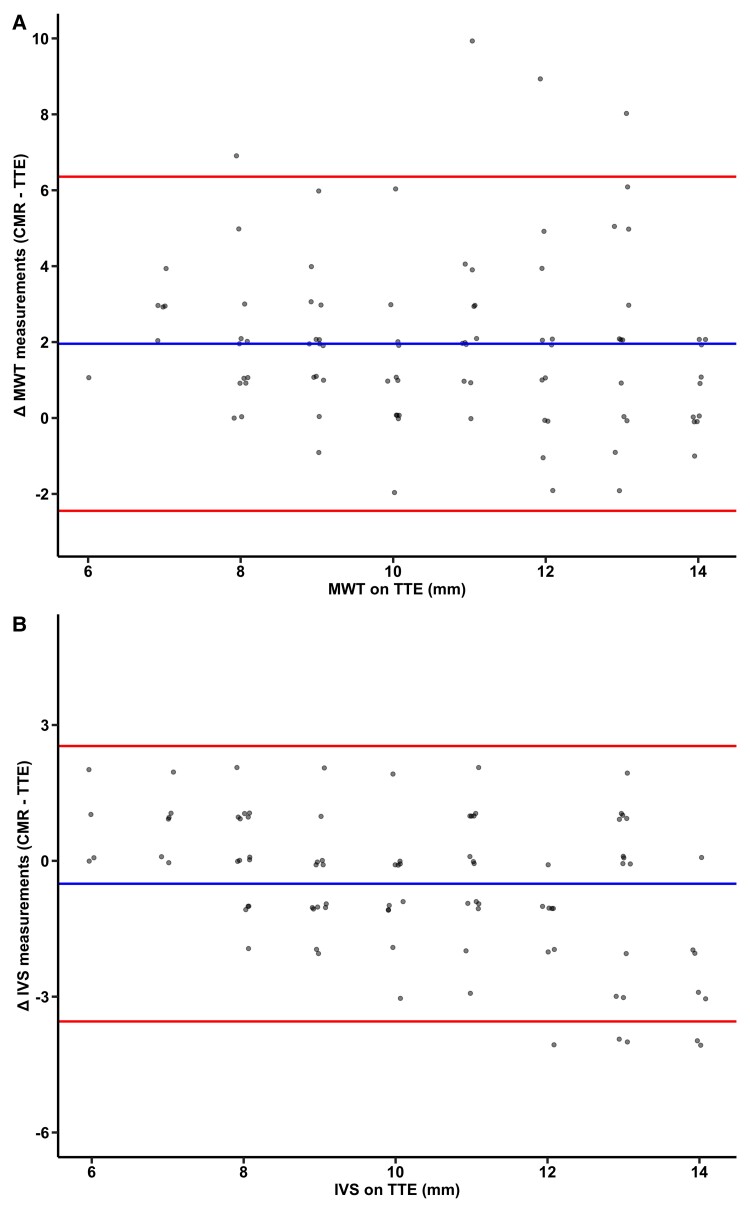
Overall, MWT was significantly higher on CMR (12.6 ± 3.0 vs. 10.6 ± 2.2 mm, P < 0.001), whereas interventricular septum (IVS) thickness was lower (9.8 ± 2.1 vs. 10.3 ± 2.3 mm, P < 0.001). Mean differences were 2.0 ± 2.2 mm and −0.5 ± 1.6 mm, respectively, corresponding with poor and good agreement (ICC MWT: 0.49 [0.07–0.72]; IVS: 0.73 [0.60–0.82], Figure 1). Discrepancies in MWT were present along the full spectrum of measurements, whereas differences in IVS values were more pronounced with increasing IVS thickness on TTE. TTE and CMR agreed on MWT location in 60 (66%) cases, although the difference in MWT was equal in subjects with and without discrepant MWT locations (1.9 ± 2.4 vs. 2.0 ± 2.2 mm, P = 0.90).
Figure 1.
Bland–Altman plots visualizing discrepancies between CMR and TTE. Differences between CMR and TTE for MWT (A) and IVS (B) measurements (y axis) are displayed along the full range of wall thickness values on TTE (x axis). Blue inner line: mean difference, red outer lines: limits of agreement (mean ± 2 SDs).
In 32 (35%) subjects with suboptimal and poor TTE image quality, mean MWT difference was similar to those with satisfactory image quality (2.0 ± 2.5 vs. 1.9 ± 2.1, P = 0.82), with a similar reclassification rate (31 vs. 25%, P = 0.55).
Mean intra- and inter-reader differences for measurements repeated in 30 random subjects were 0.0 ± 0.7 mm and 0.5 ± 1.3 mm for TTE and 0.2 ± 1.4 mm and 0.5 ± 0.8 mm for CMR (see Supplementary data online, Figure S1). The implications of these differences for HCM diagnoses and subsequent reclassifications are shown in Supplementary data online, Table S1. Over all combinations, on average 21% of subjects were reclassified using CMR. Intra-reader reproducibility for TTE and CMR MWT measurements was 0.94 [0.89–0.97] and 0.97 [0.95–0.99], respectively. Inter-reader reproducibility was 0.78 [0.62–0.89] and 0.92 [0.86–0.96], respectively, indicating good and excellent inter- and intra-reader reproducibility.
Reclassifications of HCM diagnoses
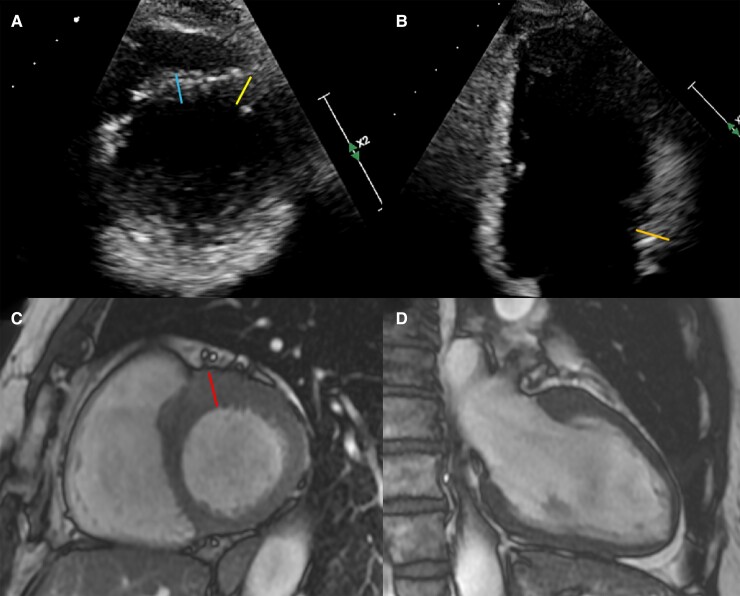
A total of 25 (27%) reclassifications occurred, predominantly in TTE- subjects (n = 23, 34%). Table 1 compares ECG and imaging characteristics between the groups. In the TTE−/CMR+ group the difference between MWT on CMR and TTE was almost 4 mm (range 1–10 mm), and was 5 mm or more in 7 (30%) subjects. Figure 2 illustrates a representative TTE−/CMR+ subject. The MWT location was the septum in all cases, in 10 (43%) cases being caused by a hook-shaped protuberance of the anterobasal segment clearly visible on CMR, but not on TTE (see Supplementary data online, Figure S2). TTE− subjects with this hook had a higher median MWT on CMR and MWT difference than those without (13 [11–15] vs. 11 [10–13] mm and 2.5 [2–4] vs. 1.0 [0–2], P < 0.05 for both). This characteristic finding was not seen on TTE. In the TTE- group, modest LVH (MWT 10–13 mm) was found in 24 subjects. Other HCM-related features (defined here as LVOT obstruction or septal e′ velocities <7 cm/s) were found in 11 subjects, 9 (82%) of which were reclassified. In the 13 subjects without these features, 8 (62%) were reclassified.
Figure 2.
Representative example of a subject reclassified as HCM on CMR. A and B illustrate parasternal SA (basal level) and apical two chamber images on TTE, with corresponding orientations on CMR on C and D. MWT on TTE was 11 mm (A, right yellow line, B, orange line), blue line (A, left line) measures 10 mm. MWT on CMR (red line, panel C) was measured at 16 mm.
There were two TTE+/CMR− subjects, both with poor acoustic windows. The MWT location in these subjects was the septum on both modalities, and the MWT difference was 1 and 2 mm. The ECG showed left axis deviation and intraventricular conduction delay in one subject. Multiple myocardial crypts were visible on CMR in both subjects, there was no LGE.
Anterobasal hooks
A sub-analysis of subjects with and without hooks was performed to determine whether this finding was associated with a specific phenotypic profile. There were 26 subjects with hooks, most common in the TTE−/CMR+ group (n = 10, 43%), followed by the TTE+/CMR+ and TTE−/CMR− groups (n = 6, 29%; n = 10, 22%). Those with hooks were older, more often female, had an increased MWT and lower indexed LV mass (see Supplementary data online, Table S2). Diastolic function was more often impaired and they had more LVOT obstruction.
Yield of CMR based on ECG and TTE
Logistic regression analysis was performed assessing which variables were predictive of reclassifications, restricting the analysis to TTE− subjects, because the factors underlying reclassifications in this group potentially differ from those in TTE+ subjects. After multivariable adjustment, significant predictors included MWT/posterior wall thickness ratio (1.93 [1.19–3.14], P < 0.01) and indexed LV mass (1.01 [1.00–1.02], P = 0.03), as shown in Table 2.
Table 2.
Results of logistic regression analyses for the prediction of HCM on CMR in subjects without HCM on TTE.
| Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| Odds ratio | P-value | Odds ratio | P-value | |
| Age (per year) | 1.05 [1.01–1.10] | 0.03 | ||
| Corrected QT interval (per ms) | 1.03 [1.01–1.07] | 0.01 | 1.00 [1.00–1.01] | 0.43 |
| IVS/PWT ratio | 31.7 [2.99–542.7] | <0.01 | ||
| MWT/PWT ratio | 30.0 [2.58–547.3] | 0.01 | 1.93 [1.19–3.14] | <0.01 |
| Mitral inflow A wave (per cm/s) | 1.04 [1.01–1.07] | 0.02 | ||
| Septal e′ (per cm/s) | 0.78 [0.62–0.94] | 0.01 | 1.02 [0.96–1.08] | 0.47 |
| E/A ratio | 0.26 [0.07–0.81] | 0.03 | ||
| E/e′ ratio | 1.33 [1.08–1.70] | 0.01 | 1.05 [1.00–1.11] | 0.06 |
| Impaired diastolic functiona | 4.18 [1.34–13.79] | 0.02 | ||
| LV mass, indexed (per g/m2) | 1.05 [1.01–1.09] | 0.01 | 1.01 [1.00–1.02] | 0.03 |
| Reverse curve morphology | 5.97 [1.17–44.48] | 0.04 | ||
Only variables with significant results in univariable analysis are displayed. Variable selection was done in a stepwise manner based on the Akaike Information Criterion.
Includes all grades of diastolic dysfunction.
PWT, posterior wall thickness.
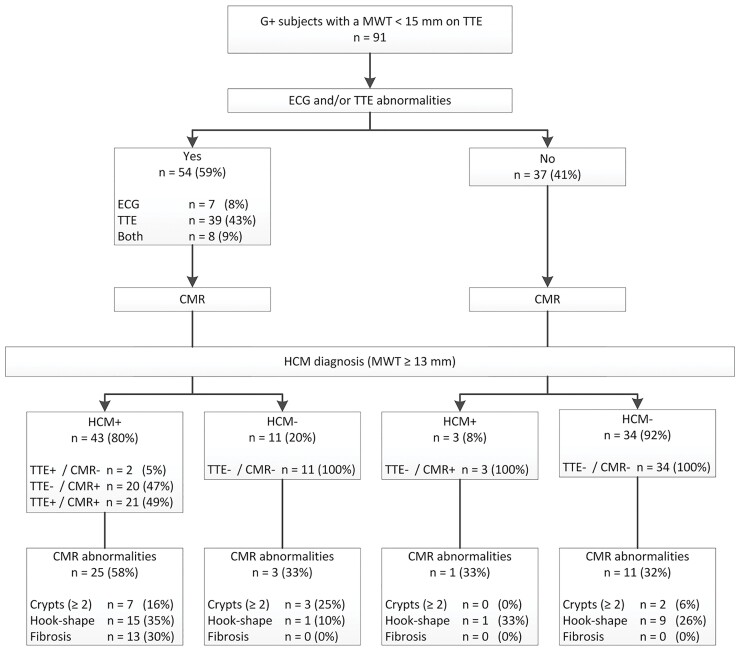
There were 61 subjects with ECG/TTE abnormalities, defined as LVH on ECG (Romhilt–Estes, Cornell, Sokolow–Lyon criteria) and/or LVH (MWT >10 mm for men and >9 mm for women) or LVOT obstruction on TTE. Of these, 13 (21%) subjects did not have HCM on any modality. ECG/TTE abnormalities were less specific for HCM diagnoses (on CMR) when adding other abnormalities (pathological Q waves or TWI). Specificity improved after excluding the Sokolow–Lyon criteria from the ECG definition and when applying a universal cut-off for LVH of >10 mm for both men and women, shown in Figure 3. Of the 37 subjects without ECG/TTE abnormalities, 3 (8%) were reclassified as CMR+, all of which had an MWT on CMR of 13 mm. The majority with ECG/TTE abnormalities were CMR+ (n = 41, 76%), in 20 (49%) cases undiscovered on TTE. CMR revealed more HCM-related abnormalities in those with ECG/TTE abnormalities, although this was not statistically significant (P = 0.07). Myocardial fibrosis was found only in subjects with ECG/TTE abnormalities, and only in those with HCM on CMR. Multiple myocardial crypts were rare (see Figure 3).
Figure 3.
Yield of CMR imaging following ECG and TTE. ECGs were abnormal in case of positive Cornell criteria or Romhilt–Estes score ≥4, TTEs were abnormal in case of LVH (MWT >10 mm) or LVOT obstruction. CMR reclassifies 8% of those without ECG/TTE abnormalities. CMR reveals additional abnormalities in all groups, but mostly in those with HCM.
To further stratify remaining subjects, we attempted to incorporate other ECG- and TTE-based predictors of CMR+ status based on logistic regression results. However, in 31 TTE− subjects with ECG/TTE abnormalities, no variables had significant predictive utility as determined by their areas under the curve.
Discussion
This study explored the incremental value of CMR on top of clinical evaluation and phenotyping using ECG and TTE in a cohort of pathogenic sarcomere gene variant carriers with no or limited LVH. Twenty-seven percent of subjects were reclassified by CMR. The number of reclassifications in subjects without ECG/TTE abnormalities was low, indicating that ECG and TTE can be used to guide the decision to perform CMR.
Being able to predict the yield of CMR based on conventional ECG and TTE results is clinically useful, guiding decision-making in the work-up of a larger growing population of ‘phenotype-negative’ sarcomere variant carriers, resulting in a more time- and cost-effective approach for these subjects. Only three (8%) reclassifications occurred in subjects with normal ECG/TTE results, and the additional yield of other CMR-specific abnormalities was low. These findings suggest that ECG and TTE can be used to determine whether or not to perform CMR. For a better understanding of the phenotypic spectrum of HCM and for research purposes, the use of CMR in these individuals will remain valuable for high-volume HCM centres with specific scientific interest.
IVS measurements on TTE and CMR agreed relatively well, whereas MWT measurements differed more profoundly, which was not explained by discrepancies in MWT locations nor by image quality. In several cases this is explained by the superior performance of CMR in the three-dimensional appraisal of the LV, with improved visualization of specific LV segments (i.e. the anterolateral wall), in other cases with concordant MWT locations CMR measurements benefited from a more accurate assessment of the myocardial wall.
Unsurprisingly, reclassifications occurred mostly in those with borderline LVH. In seven (32%) TTE−/CMR+ subjects, the difference in MWT was >5 mm, indicating that substantial LVH can be missed by TTE. In several subjects without LVH on TTE, LVH on ECG predicted HCM on CMR, indicating the importance of the ECG for these subjects, beyond the appraisal of features suggesting specific diseases (e.g. apical HCM, HCM phenocopies). The relatively large number of reclassifications illustrate the value of CMR in the work-up of subjects evaluated for the presence of HCM. Routine use of CMR should be considered in centres with sufficient CMR facilities, given the management implications for reclassified subjects. An additional advantage of CMR is the ability to more reliably compare measurements longitudinally in the same subject, which is particularly relevant for those who are re-evaluated for HCM later in life.
The two TTE+/CMR− cases had poor acoustic windows, therefore already representing a group for which subsequent CMR scanning is indicated. Importantly, myocardial thickness measurements are not always interchangeable irrespective of image quality or MWT location, as seen in previous reports in HCM patients or sarcomere variant carriers.3,15,16 As the MWT difference was mild, we believe that this is indicative of the inherent differences between the two modalities, which by itself should not spur physicians to retract prior given diagnoses. We stress that the practice of dichotomizing MWT to diagnose HCM, while clinically useful and necessary, foregoes the fact that HCM develops along a continuum, and the possible discrepancies between modalities represent a specific issue within this paradigm, perhaps calling for modality-specific reference values. This is further complicated by the fact that normal values on CMR differ between segments and sex,17 with a mean regional wall thickness difference of 1 mm between men and women. The clinical relevance of these differences is demonstrated in a large study (n = 4972) performed in the Multi-Ethnic Study of Atherosclerosis cohort, in which unexplained LVH (≥15 mm in ≥2 LV segments on CMR) was found in 61 men and 6 women, corresponding to a prevalence of 2.6% of men and 0.2% of women. It would be interesting to see the prevalence after application of sex-specific cut-offs, as this could also offer an explanation to the predominance of men observed in HCM cohorts.6
CMR allows for the detection of several abnormalities considered part of the phenotypic spectrum of HCM. Multiple myocardial crypts in particular have been found to be strongly associated with the presence of sarcomere gene variants.18 Its value in those with proven pathogenic variants is unclear. To our knowledge, there are no studies demonstrating whether multiple myocardial crypts or other CMR-derived parameters predict progression to HCM. In HCM, presence of (extensive) LGE is a modifier of sudden cardiac death risk beyond traditional risk factors. The small number of TTE−/CMR+ subjects with LGE could indicate missed diagnoses in those at risk of life-threatening arrhythmias. However, the impact of non-severe LGE in subjects without other compelling risk factors is likely very limited. As the occurrence of replacement fibrosis (not confined to the hinge points) was low, both in the overall group and in reclassified subjects, we believe these abnormalities do not justify performance of CMR by itself, and it can be considered to forego CMR in this population if there are no other indications to do so.
An anterobasal hook was present in 26 (29%) subjects, a pattern akin to the shape of a rounded hook because of its relative hypertrophy compared to the adjacent myocardium, which was not found on TTE images (see Supplementary data online, Figure S2). This pattern of isolated hypertrophy was previously described by Maron et al.,14 and lacked any specific associations with hypertrophy or curve morphology. The initial hypertrophic response is presumed to occur in the basal septum due to increased wall stress in that area, owing to a larger radius and the influence of non-basal LV contraction and right ventricular pressure.19 We hypothesize that this hook either represents the area of initial hypertrophy or increases in size in parallel to the basal septum, with the resultant curve morphology dependent on genetic, cellular or hemodynamic differences.20,21 The 4 (4%) subjects with LVOT obstruction all had mild LVH on TTE (MWT 12–14 mm) and more pronounced LVH on CMR (MWT 15–21 mm) caused by the anterobasal hook. The occurrence of LVOT obstruction depends on morphological abnormalities, and we suspect that hearts with this particular shape are prone to LVOT obstruction.21
Limitations
The inherent limitations of retrospective studies apply here. A larger sample size would allow for more robust analyses. Subjects in which MWT could not be accurately measured but who were suspected to have HCM were excluded (n = 2). Contrast echocardiography is not routinely performed at our centre for wall thickness measurement. Its use may improve MWT measurements on TTE particularly in those with poor images, impacting comparisons between TTE and CMR. Our results would likely change if we would incorporate, for example, subjects suspected of apical HCM with concomitant TWI on ECG. Finally, our results are potentially over- or underestimated, as they are primarily based on single TTE and CMR measurements. However, absolute intra- and inter-reader differences were small and reclassifications were common even after considering other measurement combinations. Also, our results show that reclassifications are not confined to those with borderline LVH and that differences between modalities can be profound, indicating that differences between TTE and CMR are not confined to the error margins of wall thickness measurements.
Conclusion
In conclusion, CMR reclassified a quarter of all subjects. Only 8% of subjects without LVH on ECG and TTE are reclassified using CMR, and they have a low prevalence of LGE and myocardial crypts, indicating that it can be justified to not perform CMR in these subjects. Conversely, in subjects with abnormal ECGs and/or poor TTE image quality, CMR is indicated.
Supplementary data online, Figure S1 demonstrates intra- and inter-reader differences for TTE and CMR MWT measurements. Supplementary data online, Figure S2 gives examples of anterobasal hooks on CMR. Supplementary data online, Table S1 illustrates agreement between TTE and CMR for each pair of observers. Supplementary data online, Table S2 gives baseline, ECG and imaging data stratified by the presence of an anterobasal hook.
Supplementary Material
Contributor Information
Roy Huurman, Department of Cardiology, Room Rg-419, Thoraxcenter, Erasmus MC, University Medical Center Rotterdam, Dr. Molewaterplein 40, Rotterdam 3015 GD, The Netherlands.
Nikki van der Velde, Department of Cardiology, Room Rg-419, Thoraxcenter, Erasmus MC, University Medical Center Rotterdam, Dr. Molewaterplein 40, Rotterdam 3015 GD, The Netherlands; Department of Radiology and Nuclear Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam 3015 GD, The Netherlands.
Arend F L Schinkel, Department of Cardiology, Room Rg-419, Thoraxcenter, Erasmus MC, University Medical Center Rotterdam, Dr. Molewaterplein 40, Rotterdam 3015 GD, The Netherlands.
H Carlijne Hassing, Department of Cardiology, Room Rg-419, Thoraxcenter, Erasmus MC, University Medical Center Rotterdam, Dr. Molewaterplein 40, Rotterdam 3015 GD, The Netherlands; Department of Radiology and Nuclear Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam 3015 GD, The Netherlands.
Ricardo P J Budde, Department of Cardiology, Room Rg-419, Thoraxcenter, Erasmus MC, University Medical Center Rotterdam, Dr. Molewaterplein 40, Rotterdam 3015 GD, The Netherlands; Department of Radiology and Nuclear Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam 3015 GD, The Netherlands.
Marjon A van Slegtenhorst, Department of Clinical Genetics, Erasmus MC, University Medical Center Rotterdam, Rotterdam 3015 GD, The Netherlands.
Judith M A Verhagen, Department of Clinical Genetics, Erasmus MC, University Medical Center Rotterdam, Rotterdam 3015 GD, The Netherlands.
Alexander Hirsch, Department of Cardiology, Room Rg-419, Thoraxcenter, Erasmus MC, University Medical Center Rotterdam, Dr. Molewaterplein 40, Rotterdam 3015 GD, The Netherlands; Department of Radiology and Nuclear Medicine, Erasmus MC, University Medical Center Rotterdam, Rotterdam 3015 GD, The Netherlands.
Michelle Michels, Department of Cardiology, Room Rg-419, Thoraxcenter, Erasmus MC, University Medical Center Rotterdam, Dr. Molewaterplein 40, Rotterdam 3015 GD, The Netherlands.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Availability
Data is available upon reasonable request.
References
- 1. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 2. van Velzen HG, Schinkel AFL, Baart SJ, Oldenburg RA, Frohn-Mulder IME, van Slegtenhorst MA, et al. Outcomes of contemporary family screening in hypertrophic cardiomyopathy. Circ Genom Precis Med 2018;11:e001896. [DOI] [PubMed] [Google Scholar]
- 3. Valente AM, Lakdawala NK, Powell AJ, Evans SP, Cirino AL, Orav EJ, et al. Comparison of echocardiographic and cardiac magnetic resonance imaging in hypertrophic cardiomyopathy sarcomere mutation carriers without left ventricular hypertrophy. Circ Cardiovasc Genet 2013;6:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cardim N, Galderisi M, Edvardsen T, Plein S, Popescu BA, D'Andrea A, et al. Role of multimodality cardiac imaging in the management of patients with hypertrophic cardiomyopathy: an expert consensus of the European Association of Cardiovascular Imaging Endorsed by the Saudi Heart Association. Eur Heart J Cardiovasc Imaging 2015;16:280. [DOI] [PubMed] [Google Scholar]
- 5. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Velzen HG, Schinkel AFL, Baart SJ, Huurman R, van Slegtenhorst MA, Kardys I, et al. Effect of gender and genetic mutations on outcomes in patients with hypertrophic cardiomyopathy. Am J Cardiol 2018;122:1947–1954. [DOI] [PubMed] [Google Scholar]
- 7. Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009;53:992–1002. [DOI] [PubMed] [Google Scholar]
- 8. Delcrè SD, Di Donna P, Leuzzi S, Miceli S, Bisi M, Scaglione M, et al. Relationship of ECG findings to phenotypic expression in patients with hypertrophic cardiomyopathy: a cardiac magnetic resonance study. Int J Cardiol 2013;167:1038–1045. [DOI] [PubMed] [Google Scholar]
- 9. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J 2019;40:237–269. [DOI] [PubMed] [Google Scholar]
- 10. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–1360. [DOI] [PubMed] [Google Scholar]
- 11. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 12. Alhaj EK, Bette K, Cantales D, Uretsky S, Chaudry FA, Sherrid MV. Symptomatic exercise-induced left ventricular outflow tract obstruction without left ventricular hypertrophy. J Am Soc Echocardiogr 2013;5:556–565. [DOI] [PubMed] [Google Scholar]
- 13. Maron MS, Olivotto I, Harrigan C, Appelbaum E, Gibson CM, Lesser JR, et al. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation 2011;124:40–47. [DOI] [PubMed] [Google Scholar]
- 14. Maron MS, Maron BJ, Harrigan C, Buros J, Gibson CM, Olivotto I, et al. Hypertrophic cardiomyopathy phenotype revisited after 50 years with cardiovascular magnetic resonance. J Am Coll Cardiol 2009;54:220–228. [DOI] [PubMed] [Google Scholar]
- 15. Hindieh W, Weissler-Snir A, Hammer H, Adler A, Rakowski H, Chan RH. Discrepant measurements of maximal left ventricular wall thickness between cardiac magnetic resonance imaging and echocardiography in patients with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging 2017;10:e006309. [DOI] [PubMed] [Google Scholar]
- 16. Bois JP, Geske JB, Foley TA, Ommen SR, Pellikka PA. Comparison of maximal wall thickness in hypertrophic cardiomyopathy differs between magnetic resonance imaging and transthoracic echocardiography. Am J Cardiol 2017;119:643–650. [DOI] [PubMed] [Google Scholar]
- 17. Kawel N, Turkbey EB, Carr JJ, Eng J, Gomes AS, Hundley WG, et al. Normal left ventricular myocardial thickness for middle-aged and older subjects with steady-state free precession cardiac magnetic resonance: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging 2012;5:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brouwer WP, Germans T, Head MC, van der Velden J, Heymans MW, Christiaans I, et al. Multiple myocardial crypts on modified long-axis view are a specific finding in pre-hypertrophic HCM mutation carriers. Eur Heart J Cardiovasc Imaging 2012;13:292–297. [DOI] [PubMed] [Google Scholar]
- 19. Kelshiker MA, Mayet J, Unsworth B, Okonko DO. Basal septal hypertrophy. Curr Cardiol Rev 2013;9:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Binder J, Ommen SR, Gersh BJ, Van Driest SL, Tajik AJ, Nishimura RA, et al. Echocardiography-guided genetic testing in hypertrophic cardiomyopathy: septal morphological features predict the presence of myofilament mutations. Mayo Clin Proc 2006;81:459–467. [DOI] [PubMed] [Google Scholar]
- 21. Kwon DH, Smedira NG, Popovic ZB, Lytle BW, Setser RM, Thamilarasan M, et al. Steep left ventricle to aortic root angle and hypertrophic obstructive cardiomyopathy: study of a novel association using three-dimensional multimodality imaging. Heart 2009;95:1784–1791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available upon reasonable request.