Abstract
Aims
We evaluated the associations of left atrial (LA) structure and function with prevalent and incident cardiovascular disease (CVD), independent of left ventricular (LV) metrics, in 25 896 UK Biobank participants.
Methods and results
We estimated the association of cardiovascular magnetic resonance (CMR) metrics [LA maximum volume (LAV), LA ejection fraction (LAEF), LV mass : LV end-diastolic volume ratio (LVM : LVEDV), global longitudinal strain, and LV global function index (LVGFI)] with vascular risk factors (hypertension, diabetes, high cholesterol, and smoking), prevalent and incident CVDs [atrial fibrillation (AF), stroke, ischaemic heart disease (IHD), myocardial infarction], all-cause mortality, and CVD mortality. We created uncorrelated CMR variables using orthogonal principal component analysis rotation. All five CMR metrics were simultaneously entered into multivariable regression models adjusted for sex, age, ethnicity, deprivation, education, body size, and physical activity. Lower LAEF was associated with diabetes, smoking, and all the prevalent and incident CVDs. Diabetes, smoking, and high cholesterol were associated with smaller LAV. Hypertension, IHD, AF (incident and prevalent), incident stroke, and CVD mortality were associated with larger LAV. LV and LA metrics were both independently informative in associations with prevalent disease, however LAEF showed the most consistent associations with incident CVDs. Lower LVGFI was associated with greater all-cause and CVD mortality. In secondary analyses, compared with LVGFI, LV ejection fraction showed similar but less consistent disease associations.
Conclusion
LA structure and function measures (LAEF and LAV) demonstrate significant associations with key prevalent and incident cardiovascular outcomes, independent of LV metrics. These measures have potential clinical utility for disease discrimination and outcome prediction.
Keywords: lef, t atrium, left ventricle, cardiovascular magnetic resonance, vascular risk factors, atrial fibrillation, stroke, ischaemic heart disease, cardiovascular outcomes, mortality
Graphical Abstract
Graphical Abstract.
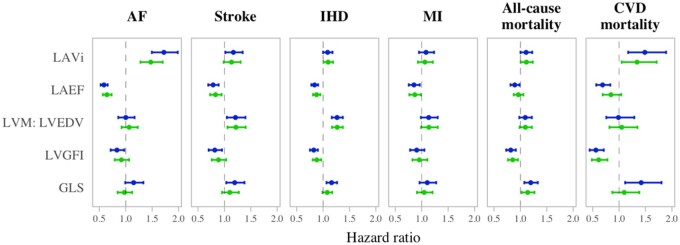
Association of CMR metrics with incident cardiovascular disease considered separately (blue) and in models mutually adjusted for all the CMR metrics (green). The points indicate point estimate for the hazard ratio and the intervals indicate the corresponding 95% confidence intervals from Cox hazard proportional models. Confounders are age, sex, ethnicity, deprivation, education, body mass index, hypertension, high cholesterol, diabetes, physical activity, and smoking. The blue results are from models with individual adjustment for CMR metrics (Supplementary data online, Table S9). The green results are from models mutually adjusting for all the CMR metrics (Table 3). AF, atrial fibrillation; CMR, cardiovascular magnetic resonance; CVD, cardiovascular disease; LVGFI, left ventricular global function index; GLS, global longitudinal strain; i, indicates indexation to body surface area; IHD, ischaemic heart disease; LAEF, left atrial ejection fraction; LAV, maximum left atrial volume; LVEDV, left ventricular end-diastolic volume; LVM, left ventricular mass; MI, myocardial infarction.
Introduction
The left atrium (LA) is highly sensitive to subtle left ventricular (LV) haemodynamic changes.1,2 Alterations in LA structure and function may precede detectable LV dysfunction and, as such, have potential utility for earlier and more accurate disease discrimination than LV metrics.1–3 In particular, LA size and function are altered in response to elevated LV filling pressures, an early feature of diastolic dysfunction and a key component of heart failure with preserved ejection fraction (HFpEF).1,3 Furthermore, clinically important arrhythmias, such as atrial fibrillation (AF), primarily result in atrial (rather than ventricular) remodelling. Thus, atrial metrics may provide better indicators for the presence and occurrence of these conditions and provide incremental predictive value for key related health outcomes, such as stroke.4
The association of echocardiography derived measures of LA structure and function with incident and prevalent cardiovascular diseases (CVDs) has been repeatedly demonstrated.5–9 However, whilst the incremental value of LA over LV metrics seems biologically plausible, formal demonstration of this requires further study. Furthermore, although echocardiography is a valuable first line modality in clinical settings, cardiovascular magnetic resonance (CMR) is the reference standard for cardiac chamber quantification providing highly reproducible metrics calculated with fewer geometric assumptions than in echocardiography. Existing CMR studies of the utility of LA metrics are mostly based on small select samples of clinical cohorts,10–12 with a paucity of data from larger population-based samples.
The UK Biobank is a very large population-based cohort study including detailed participant characterization, linked longitudinally tracked health outcome data, and detailed standardized CMR. Thus, we evaluated, in 25 896 UK Biobank participants, clinical associations of LA structure and function independent of LV metrics. We estimated associations of CMR derived LA and LV metrics with vascular risk factors (VRFs), prevalent CVD, incident CVD, and mortality outcomes. We considered a wide range of demographic and clinical confounders and, critically, we assessed the independent value of LA metrics over measures of LV structure and function.
Methods
Setting and study participants
The UK Biobank includes over 500 000 participants from across the UK. Individuals aged 40–69 years old were identified using National Health Service (NHS) registers and recruited between 2006 and 2010 through postal invitations.13 Baseline assessment comprised detailed characterization of participant demographic, lifestyle, environmental, and medical factors, as well as a series of physical measures and blood sampling. Individuals who could not complete baseline assessment due to discomfort or ill health were not recruited. The UK Biobank protocol is publicly available.14 Linkages have been established with key routine health data including hospital episode statistics (HES) and death registers, with health outcomes documented according to standardized International Classification of Diseases (ICD) codes. This linked information is continually updated allowing reliable longitudinal tracking of incident events for all participants. Furthermore, the UK Biobank has produced adjudicated algorithmically defined incident health outcome data for key illnesses, such as myocardial infarction (MI) and stroke.15 The UK Biobank imaging study, launched in 2015, aims to scan a random 100 000 subset of the original participants and includes, amongst other things, detailed CMR imaging.16
CMR image acquisition
The UK Biobank imaging study is performed using standardized pre-defined operating procedures, equipment, and staff training. CMR imaging was with 1.5 T scanners (MAGNETOM Aera, Syngo Platform VD13A, Siemens Healthcare, Erlangen, Germany), the acquisition protocol is published elsewhere.17 In brief, cardiac function assessment comprised three long axis cines and a complete short axis stack covering the left and right ventricles acquired at one slice per breath hold using balanced steady-state free precession sequences.
CMR image analysis
CMR indices were derived using a fully automated quality-controlled image analysis pipeline previously developed and validated in a large subset of the UK Biobank.18,19 CMR metrics were available for the first 26, 891 UK Biobank CMR studies, of these both LA and LV data were available for 25 896 participants, which we include in the study (Supplementary data online, Figure S1). We considered the following CMR measures: LA maximum volume (LAV), LA ejection fraction (LAEF, calculated as: LA maximum volume−LA minimum volume/LA maximum volume), LV mass : LV end-diastolic volume ratio (LVM : LVEDV), and global longitudinal strain (GLS). We considered LV global function index (LVGFI) as an additional measure of LV function. Previous reports have identified LVGFI as a strong predictor of heart failure and CVD events with incremental utility over LV ejection fraction (LVEF).20,21 As per previous descriptions,20,21 we defined LVGFI (%) as LV stroke volume/LV global volume × 100, where LV global volume was calculated as the sum of the LV mean cavity volume [(LV end-diastolic volume + LV end-systolic volume)/2] and myocardium volume (LV mass/density). Density of LV was specified as 1.05 g/mL. A higher LVGFI reflects better LV function. As LVEF is a more clinically established metric, we also considered associations with LVEF and compared its performance to LVGFI.
Defining participant characteristics
Sex and ethnicity were taken as self-reported at baseline. Ethnicity was converted into a binary variable of White and Black Asian and minority ethnic (BAME) groups. Socioeconomic deprivation was recorded at the baseline UK Biobank assessment as the Townsend index, a measure of deprivation relative to national averages.22 Age was calculated at the time of imaging. Body mass index (BMI) was calculated from height and weight recorded at imaging. Educational level and smoking status were taken from self-report. Physical activity level was expressed as a continuous value of metabolic equivalent (MET) minutes/week, calculated by weighting different types of activity (walking, moderate, or vigorous) by its energy requirements using values derived from the International Physical Activity Questionnaire (IPAQ) study.23
Ascertainment of vascular risk factors, cardiovascular disease, and mortality outcomes
We considered the following VRFs: hypertension, diabetes, high cholesterol, and smoking; and the following CVDs (incident and prevalent): AF, stroke, ischaemic heart disease (IHD), and MI. Mortality outcomes were ascertained from death register data. We considered all-cause and CVD mortality; the latter was defined as primary cause of death recorded as any CVD (ICD10 Chapter IX I00-I99). Incident CVDs and mortality outcomes were considered as those occurring after CMR imaging. The average follow-up time available for HES and mortality data was 4.2 ± 1.2 (range: 2.5–6.9) years.
For ascertainment of prevalent VRFs and CVDs, we referred to baseline verbal interview, documentation of relevant HES codes, or record in UK Biobank algorithmically defined health outcomes (for MI and stroke). For diabetes and high cholesterol, we also referred to biochemistry data (glycosylated haemoglobin >48 mmol/mol and total cholesterol >7 mmol/L, respectively). The approach to ascertainment of VRFs and CVDs along with a full list of ICD codes used is presented in Supplementary data online, Table S1.
Statistical analysis
Statistical analysis was performed using R version 4.0.324 and RStudio Version 1.3.1093.25 We included all UK Biobank participants with quality-controlled CMR data available.
We performed orthogonal principal component analysis (PCA) rotation of the five CMR metrics (LAVi, LAEF, LVM : LVEDV, GLS, and LVGFI), creating uncorrelated CMR variables whilst retaining >90% of their individual variance, as described in previous work.26–28 Thus, we removed significant interdependencies between the CMR metrics and were able to include the rotated CMR variables together as exposures in the same model. For inclusion to the PCA, LAV, and LAEF contained 0.03% missing values and GLS contained 3.7% missing values that were imputed with the mean. For comparison of LVGFI and LVEF, we created a separate set of PCA rotated CMR metrics replacing LVGFI with LVEF. The PCA loadings are presented in Supplementary data online, Tables S2 and S3. We estimated the independent association of the PCA rotated CMR metrics with VRFs (hypertension, diabetes, high cholesterol, and smoking) and prevalent CVDs (AF, stroke, IHD, and MI) in multivariable logistic regression models, simultaneously modelling all five CMR metrics and adjusting for confounders (age, sex, ethnicity, deprivation, education, physical activity, and BMI). We used Cox proportional hazards regression for incident CVDs and mortality outcomes (with covariate adjustment as before). In associations with incident CVDs, we excluded participants who had already had the same outcome prior to CMR.
We also present associations with individually entered raw CMR metrics. For associations with prevalent diseases, we used multivariable linear regression, considering individual raw CMR metrics as the model outcome, and VRFs and prevalent CVDs as exposure variables. For incident outcomes, we used Cox proportional hazards regression with raw CMR metrics entered individually as exposure variables. We adjusted for confounders as before. In all models, LAV was log-transformed to remove skew. We corrected for multiple comparisons using a false discovery rate of 0.05 across exposure variables.
Results
Population characteristics
We studied 25 896 participants for whom CMR data were available (Supplementary data online, Figure S1). The cohort had an average age of 62.9 (±7.5) years old; 52% (n = 13 488) were women (Table 1). The proportion of participants with hypertension, diabetes, high cholesterol, and smoking was 32.7%, 5.7%, 34.5%, and 3.7%, respectively. There was, overall, less socio-economic deprivation than UK national averages. The proportion of participants with prevalent AF, stroke, IHD, and MI at time of CMR was 1.5%, 1.9%, 6.0%, and 2.4%, respectively (Table 1).
Table 1.
Participant characteristics
| Whole sample | Men | Women | |
|---|---|---|---|
| (n = 25 896) | (n = 12 408) | (n = 13 488) | |
| Age at imaging (years) | 62.9 (±7.5) | 63.6 (±7.6) | 62.2 (±7.4) |
| Townsend deprivation index | −2.7 (−3.9, −0.7) | −2.7 (−4.0, −0.7) | −2.6 (−3.9, −0.7) |
| Education | |||
| Left school ≤14 years without qualifications | 67 (0.3%) | 38 (0.3%) | 29 (0.2%) |
| Left school ≥ 15 years without qualifications | 1844 (7.1%) | 855 (6.9%) | 989 (7.3%) |
| Secondary school qualification | 3485 (13.5%) | 1313 (10.6%) | 2172 (16.1%) |
| A levels/AS levels or equivalent | 1465 (5.7%) | 653 (5.3%) | 812 (6.0%) |
| Other professional qualification | 7258 (28.0%) | 3666 (29.5%) | 3592 (26.6%) |
| Higher education (e.g. university) degree | 11 511 (44.5%) | 5755 (46.4%) | 5756 (42.7%) |
| Missing | 266 (1.0%) | 128 (1.0%) | 138 (1.0%) |
| BMI (kg/m2) | 25.9 (23.5, 28.9) | 26.5 (24.3, 29.1) | 25.3 (22.8, 28.6) |
| Physical activity (summed MET-min/week) | 1899 (896, 3573) | 1971 (958, 3666) | 1838 (834, 3514) |
| Smoker (current) | 960 (3.7%) | 540 (4.4%) | 420 (3.1%) |
| Hypertension | 8471 (32.7%) | 4914 (39.6%) | 3557 (26.4%) |
| High cholesterol | 8947 (34.5%) | 5117 (41.2%) | 3830 (28.4%) |
| Diabetes | 1485 (5.7%) | 924 (7.4%) | 561 (4.2%) |
| Prevalent cardiovascular disease | |||
| Atrial fibrillation | 386 (1.5%) | 273 (2.2%) | 113 (0.8%) |
| Stroke | 503 (1.9%) | 320 (2.6%) | 183 (1.4%) |
| IHD | 1560 (6.0%) | 1092 (8.8%) | 468 (3.5%) |
| MI | 633 (2.4%) | 502 (4.0%) | 131 (1.0%) |
| Incident CVD and mortality outcomes | |||
| Atrial fibrillation | 180 (0.7%) | 127 (1.0%) | 53 (0.4%) |
| Stroke | 178 (0.7%) | 114 (0.9%) | 64 (0.5%) |
| IHD | 530 (2.0%) | 347 (2.8%) | 183 (1.4%) |
| MI | 197 (0.8%) | 140 (1.1%) | 57 (0.4%) |
| All-cause mortality | 331 (1.3%) | 220 (1.8%) | 111 (0.8%) |
| CVD mortality | 58 (0.2%) | 44 (0.4%) | 14 (0.1%) |
| Any of AF, stroke, IHD, MI, or CVD death | 880 (3.4%) | 583 (4.7%) | 297 (2.2%) |
| CMR metrics | |||
| LAV (mL) | 70.0 (57.0, 85.3) | 75.9 (61.1, 92.6) | 65.9 (54.5, 78.7) |
| LAVi (mL/m2) | 38.0 (31.5, 45.4) | 38.0 (30.9, 46.0) | 38.1 (31.9, 45.0) |
| LAEF (%) | 61.3 (±9.1) | 60.6 (±9.6) | 61.9 (±8.5) |
| LVM : LVEDV (g/mL) | 0.57 (0.52, 0.63) | 0.60 (0.56, 0.66) | 0.54 (0.50, 0.59) |
| LVSVi (mL/m2) | 47.1 (±8.4) | 48.8 (±9.1) | 45.6 (±7.4) |
| LVEF (%) | 59.6 (±6.1) | 57.8 (±6.1) | 61.1 (±5.5) |
| LVGFI (%) | 47.7 (±6.8) | 44.8 (±6.2) | 50.4 (±6.2) |
| GLS (%) | −18.5 (±2.7) | −17.8 (±2.6) | −19.1 (±2.7) |
Counts variables are presented as number (percentage), continuous variables as mean (standard deviation) or median (inter-quartile range) based on skew.
BMI, body mass index; CMR, cardiovascular magnetic resonance; CVD, cardiovascular disease; GLS, global longitudinal strain; i, indexation to body surface area; IHD, ischaemic heart disease; LAEF, left atrial ejection fraction; LAV, maximum left atrial volume; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVM, left ventricular mass; LVGFI, left ventricular global function index; MET, metabolic equivalent; MI, myocardial infarction.
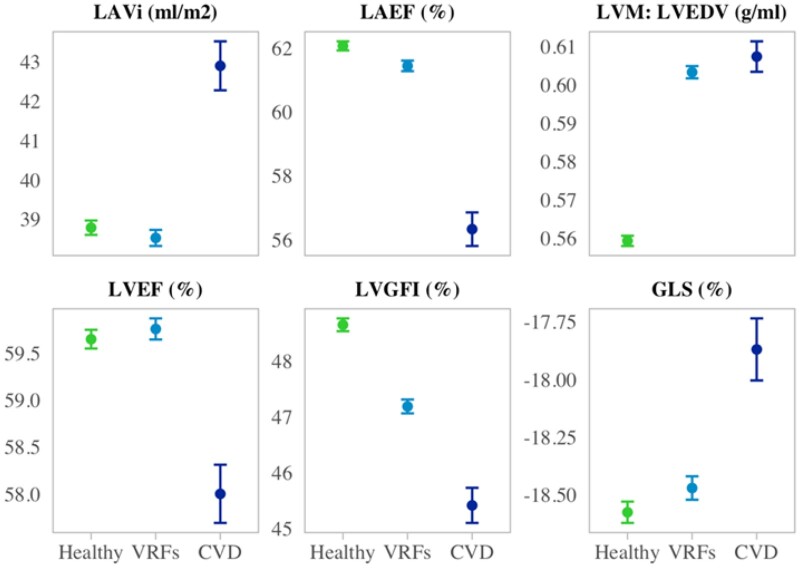
LA size and function were comparable in men and women after adjustment for body size (Table 1). Compared with women, men had, on average, more concentric LV remodelling patterns (higher LVM : LVEDV) and poorer LV function by GLS, LVGFI, and LVEF (Table 1). We additionally examined CMR metrics in subsets of participants (i)without VRFs or CVD (healthy), (ii)with VRFs, but without CVD, and (iii)with CVD (Figure 1 and Supplementary data online, Table S4). There was a stepwise decline in LV (by LVGFI and GLS) and LA function (by LAEF) from the healthy subset to those with VRFs and to those with CVD (Figure 1). Average LVEF was higher in the participants with VRFs compared with the healthy subset and lower than both subsets in those with CVD. Individuals with VRFs had smaller LAVi than healthy participants; those with CVD had the largest LAVi. The VRF and CVDs subset had higher LVM : LVEDV than the healthy cohort (Figure 1 and Supplementary data online, Table S4).
Figure 1.
CMR metric means, and 95% confidence interval of the mean stratified by disease status. Within the ‘Healthy’, ‘VRFs’, and ‘CVD’ subsets, we include participants without prevalent CVD or VRFs, with VRFs but without prevalent CVDs, and with prevalent CVDs, respectively. CMR, cardiovascular magnetic resonance; CVD, cardiovascular disease; LVGFI, left ventricular global function index; GLS, global longitudinal strain; i, indexation to body surface area; LAEF, left atrial ejection fraction; LAV, maximum left atrial volume; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; left ventricular mass; MI, myocardial infarction.
Over the average follow-up time of 4.2 ± 1.2 years, we observed incidence of 180 (0.7%) AF, 178 (0.7%) stroke, 530 (2.0%) IHD, and 197 (0.8%) MI events. There were 331 deaths during the available follow-up period; of these, 58 were attributed to CVD. In total, 880 (3.4%) participants had at least one incident event, of these 34% (n = 297) were women (Supplementary data online, Table S5). Participants who experienced an incident event had higher burden of VRFs than the whole cohort, with hypertension, diabetes, high cholesterol, and smoking documented in 50.5%, 9.9%, 46.7%, and 3.9%, respectively (Supplementary data online, Table S5).
Association of CMR metrics with vascular risk factors
In fully adjusted logistic regression models, including all the PCA rotated CMR metrics, we observed association of all the VRFs with poorer LA function (lower LAEF), with statistically significant relationships observed with diabetes and smoking (Table 2). Diabetes, high cholesterol, and smoking were associated with smaller LA sizes (lower LAV), whilst hypertension was associated with larger LA size (Table 2). There was significant association of all the VRFs with concentric LV remodelling patterns (higher LVM : LVEDV). Hypertension, diabetes, and smoking were associated with significantly poorer LV function by LVGFI and GLS (Table 2). In mutually adjusted models with LVEF instead of LVGFI, diabetes was associated with significantly lower LVEF; associations of LVEF with other VRFs were not statistically significant (Supplementary data online, Table S6). There was a similar pattern of associations in models using raw CMR metrics entered individually (Supplementary data online, Table S7).
Table 2.
Associations of mutually adjusted CMR metrics with vascular risk factors and prevalent cardiovascular disease in multivariable logistic regression models with full confounder adjustment
| Vascular risk factors | Prevalent cardiovascular disease | |||||||
|---|---|---|---|---|---|---|---|---|
| CMR metric | Hypertension | Diabetes | High cholesterol | Smoking (current) | AF | Stroke | IHD | MI |
| LAVi (mL/m2) | 1.24* | 0.87* | 0.96* | 0.88* | 1.30* | 0.96 | 1.14* | 1.09 |
| (1.21–1.28) | (0.83–0.92) | [0.94, 0.99] | [0.83, 0.94] | [1.18, 1.44] | [0.88, 1.05] | [1.08, 1.20] | [1.01, 1.18] | |
| 7.59 × 10−46 | 1.31 × 10−6 | 0.0129 | 1.65 × 10−4 | 4.15 × 10−7 | 0.3661 | 2.73 × 10−6 | 0.0377 | |
| LAEF (%) | 0.99 | 0.94* | 0.99 | 0.93* | 0.40* | 0.88* | 0.82* | 0.82* |
| (0.96–1.02) | (0.89–0.98) | [0.96, 1.02] | [0.87, 0.99] | [0.36, 0.43] | [0.82, 0.96] | [0.78, 0.86] | [0.76, 0.88] | |
| 0.6064 | 0.0110 | 0.3950 | 0.0266 | 6.15 × 10−91 | 0.0027 | 1.36 × 10−14 | 3.82 × 10−8 | |
| LVM : LVEDV | 1.43* | 1.20* | 1.10* | 1.29* | 0.80* | 1.04 | 0.85* | 0.75* |
| (1.38–1.48) | (1.14–1.27) | [1.07, 1.14] | [1.21, 1.38] | [0.71, 0.90] | [0.95, 1.14] | [0.81, 0.91] | [0.68, 0.82] | |
| 2.40 × 10−99 | 1.10 × 10−11 | 6.28 × 10−9 | 1.05 × 10−13 | 2.16 × 10−4 | 0.4062 | 1.19 × 10−7 | 1.52 × 10−10 | |
| LVGFI (%) | 0.93* | 0.87* | 1.00 | 0.88* | 1.23* | 0.95 | 0.88* | 0.71* |
| (0.90–0.96) | (0.82–0.92) | [0.96, 1.03] | [0.82, 0.94] | [1.11, 1.37] | [0.87, 1.05] | [0.84, 0.94] | [0.65, 0.77] | |
| 1.07 × 10−5 | 1.89 × 10−6 | 0.7519 | 2.43 × 10−4 | 8.50 × 10−5 | 0.3310 | 2.63 × 10−5 | 7.96 × 10−16 | |
| GLS (%) | 1.03* | 1.15* | 0.97 | 1.12* | 1.11 | 1.04 | 1.01 | 1.07 |
| (1.00–1.07) | (1.09–1.21) | [0.94, 1.00] | [1.05, 1.20] | [1.01, 1.22] | [0.96, 1.14] | [0.95, 1.06] | [0.99, 1.16] | |
| 0.0251 | 9.34 × 10−7 | 0.0382 | 9.47 × 10−4 | 0.0387 | 0.3420 | 0.8414 | 0.0836 | |
Results are odds ratios, 95% confidence intervals, and P-values. Models are logistic regression models with disease of interest entered as the response (outcome) variable. For the vascular risk factor models, covariates include mutually entered PCA rotated CMR metrics (LAV, LAEF, LVM/LVEDV, GLS, and LVGLFI), age, sex, ethnicity, deprivation, education, body mass index, physical activity, and all the VRFs (except the one set as the model outcome). For the prevalent cardiovascular disease models covariates include mutually entered PCA rotated CMR metrics (LAV, LAEF, LVM/LVEDV, GLS, and LVGLFI), age, sex, ethnicity, deprivation, education, body mass index, physical activity, hypertension, high cholesterol, diabetes, and smoking.
AF, atrial fibrillation; CMR, cardiovascular magnetic resonance; LVGFI, left ventricular global function index; GLS, global longitudinal strain; i, indexation to body surface area; IHD, ischaemic heart disease; LAEF, left atrial ejection fraction; LAV, maximum left atrial volume; LVEDV, left ventricular end-diastolic volume; LVM, left ventricular mass; MI, myocardial infarction; PCA, principal component analysis.
Statistically significant P-values with a false discovery rate of 0.05, giving an approximate threshold of 0.025.
Association of CMR metrics with prevalent cardiovascular disease
In fully adjusted logistic regression models, including all the PCA rotated CMR metrics, all the prevalent CVDs were associated with significantly lower LAEF (Table 2). AF and IHD were associated with significantly larger LA sizes (Table 2). As expected, these relationships appeared most dominant for AF (Table 2). AF, IHD, and MI were associated with more eccentric LV remodelling pattern (lower LVM : LVEDV). IHD and MI were associated with poorer LV function by LVGFI (Table 2). The same pattern of associations was observed with LVEF in mutually adjusted models with LVEF instead of LVGFI (Supplementary data online, Table S6). These relationships were broadly similar in models using individual raw CMR metrics; in these models, MI and AF were additionally associated with significantly poorer GLS, LVGFI, and LVEF, but these relationships were attenuated in the mutually adjusted models (Supplementary data online, Table S7).
Association of CMR metrics with incident cardiovascular disease
In fully adjusted Cox regression models, with mutual inclusion of all the PCA rotated CMR metrics, poorer LA function (lower LAEF) was associated with significantly higher risk of incidence of all the CVDs considered, specifically AF, stroke, IHD, and MI (Table 3 and Graphical Abstract). Larger LA size was associated with significantly higher risk of incident AF. More concentric LV remodelling patterns (higher LVM : LVEDV) were associated with significantly increased risk of incident stroke and incident IHD (Table 3). Lower LVGFI was associated with significantly higher risk of incident IHD (Table 3). In mutually adjusted models with LVEF instead of LVGFI, there was no significant association between LVEF and any of the incident CVDs (Supplementary data online, Table S8). In equivalent Cox regression models with raw individually entered CMR metrics, the associations with LA metrics were largely unchanged (Supplementary data online, Table S9). In these models, AF, stroke, and IHD were associated with significantly lower LVGFI, stroke, and IHD were associated with significantly poorer GLS, and AF was associated with lower LVEF (Supplementary data online, Table S9); these relationships (with exception of IHD and LVGFI) were attenuated in models mutually adjusting for all the CMR metrics (Table 3).
Table 3.
Associations of mutually adjusted CMR metrics with incident cardiovascular disease and mortality outcomes in Cox proportional hazard models with full confounder adjustment
| CMR metric | AF | Stroke | IHD | MI | All-cause mortality | CVD mortality |
|---|---|---|---|---|---|---|
| LAVi (mL/m2) | 1.47* | 1.13 | 1.10 | 1.06 | 1.11 | 1.34* |
| (1.28–1.70) | (0.98–1.31) | (1.01–1.19) | (0.93–1.21) | (1.00–1.23) | (1.05–1.71) | |
| 8.46 × 10−8 | 0.0807 | 0.0302 | 0.4002 | 0.0407 | 0.0185 | |
| LAEF (%) | 0.64* | 0.83* | 0.88* | 0.87* | 0.96 | 0.85 |
| (0.56–0.73) | (0.73–0.95) | (0.81–0.95) | (0.76–0.99) | (0.87–1.06) | (0.69–1.04) | |
| 2.50 × 10−11 | 0.0060 | 9.95 × 10−4 | 0.0294 | 0.4029 | 0.1119 | |
| LVM : LVEDV | 1.06 | 1.22* | 1.27* | 1.14 | 1.09 | 1.05 |
| (0.92–1.23) | (1.06–1.40) | (1.17–1.37) | (0.99–1.30) | (0.98–1.22) | (0.82–1.35) | |
| 0.4036 | 0.0065 | 1.27x10−8 | 0.0732 | 0.0959 | 0.7039 | |
| LVGFI (%) | 0.92 | 0.89 | 0.88* | 0.95 | 0.85* | 0.61* |
| (0.79–1.06) | (0.76–1.03) | (0.80–0.96) | (0.82–1.11) | (0.76–0.95) | (0.48–0.78) | |
| 0.2521 | 0.1266 | 0.0063 | 0.5372 | 0.0050 | 5.95 × 10−5 | |
| GLS (%) | 0.97 | 1.10 | 1.08 | 1.05 | 1.14* | 1.10 |
| (0.85–1.12) | (0.95–1.27) | (0.99–1.17) | (0.91–1.20) | (1.02–1.27) | (0.87–1.38) | |
| 0.7171 | 0.1880 | 0.0888 | 0.5254 | 0.0170 | 0.4314 |
Results are hazard ratios, 95% confidence intervals, and P-values. Covariates are LAV, LAEF, LVM/LVEDV, GLS, GLFI, age, sex, ethnicity, deprivation, education, body mass index, hypertension, high cholesterol, diabetes, physical activity, and smoking. The CMR variables are principal component analysis rotated variables.
AF, atrial fibrillation; CMR, cardiovascular magnetic resonance; CVD, cardiovascular disease; LVGFI, left ventricular global function index; GLS, global longitudinal strain; i, indicates indexation to body surface area; IHD, ischaemic heart disease; LAEF, left atrial ejection fraction; LAV, maximum left atrial volume; LVEDV, left ventricular end-diastolic volume; LVM, left ventricular mass; MI, myocardial infarction.
Statistically significant P-values with a false discovery rate of 0.05, giving an approximate threshold of 0.028.
Association of CMR metrics with mortality outcomes
In fully adjusted Cox regression models, including all the PCA rotated CMR metrics, larger LAVi was associated with significantly greater hazard of CVD mortality. Poorer GLS was associated with significantly higher risk of all-cause mortality. Lower LVGFI was associated with significantly higher risk of both all-cause and CVD mortality (Table 3). In mutually adjusted models with LVEF instead of LVGFI, LVEF was also associated with significantly lower risk of all-cause and CVD mortality, but with slightly smaller effect sizes than LVGFI (Supplementary data online, Table S8 and Table 3).
Discussion
Summary of findings
In this study of 25 896 UK Biobank participants, we demonstrate associations of CMR derived LA structure and function metrics with VRFs, prevalent CVDs, incident CVDs, and mortality outcomes, independent of LV measures and a wide range of clinical confounders.
Lower LAEF emerged as a consistent and independent indicator of VRFs (diabetes and smoking) and prevalent and incident CVDs (AF, stroke, IHD, and MI). Diabetes, high cholesterol, and smoking were associated with smaller LAV. Hypertension and IHD were associated with larger LAV, perhaps reflecting more advanced diastolic dysfunction in these conditions. Both prevalent and incident AF were associated with larger LA sizes. More concentric LV remodelling patterns were associated with VRFs and incident CVDs, whilst prevalent CVDs were associated with more eccentric LV remodelling. These observations likely reflect differential dominance of LV pressure and volume overload in the transition from risk factor to disease, with volume overload becoming dominant after disease occurrence. LVGFI, GLS, and LVEF provided good indications of VRFs and prevalent CVDs, with LVGFI showing the most consistent results. LVGFI and LVEF were independent predictors of all-cause and CVD mortality, with larger effect sizes observed with LVGFI. Higher LAVi was independently associated with significantly higher CVD mortality.
Both the LV and LA metrics were independently informative in associations with risk factors and prevalent disease. In associations with incident outcomes many of the LV associations were attenuated, whilst LAEF associations with all incident CVDs remained robust independent of LV metrics and other confounders. Larger LAVi appeared a strong independent predictor for CVD mortality. These observations demonstrate the independent utility of LA structure and function metrics, particularly for prediction of incident outcomes, which likely reflects pre-clinical LA remodelling before establishment of LV alterations.
Comparison with existing research
We observed strong and significant associations of lower LAEF and larger LAV with both prevalent and incident AF. Consistently, Bertelsen et al.29 also demonstrate significant association of larger CMR-derived LA volumes and poorer LA function with greater risk of AF detected on an implantable loop recorder in 203 participants with stroke risk factors but without pre-existing AF.29 These LA alterations likely reflect underlying atrial remodelling, which predisposes to (and can also occur as a result of) AF. In a study of 1148 MESA (Multi-Ethnic Study of Atherosclerosis) participants, Heckbert et al.30 demonstrate association of lower total LAEF and larger LAV with greater burden of premature atrial contractions on ambulatory electrocardiographic monitoring; such arrhythmias may be precursors of AF and indicative of atrial fibrosis. Indeed, in a study of 111 patients without a prior history of atrial arrhythmia, Quail et al.11 demonstrate association of LA late gadolinium enhancement (a marker of atrial fibrosis) with incident atrial arrhythmias.
We observed association of poorer LAEF with both prevalent and incident stroke independent of other CMR metrics. Larger LAV was associated with significantly greater risk of incident stroke in individual models, but not in models including other CMR metrics. In a study of 169 patients with AF referred for catheter ablation, Inoue et al.12 similarly demonstrate the association of poorer LA function (LAEF) with prior stroke or transient ischaemic attack. Habibi et al.31 also report significant association of lower LAEF, but not LA size, with incident ischaemic stroke in 4261 MESA participants. We additionally demonstrate significant associations of lower LAEF with incident IHD and incident MI, independent of other CMR metrics. In a study of 536 diabetic MESA participants without clinical CVD, Markman et al.32 also demonstrate the association of poorer LA function with incident CVD (defined as composite of MI, resuscitated cardiac arrest, angina, stroke, heart failure, or AF). Our findings add to the literature by demonstrating specific independent association of larger LAVi and higher CVD mortality risk.
We observed association of diabetes with smaller LAV and lower LAEF, along with more concentric LV remodelling and poorer LV function metrics. Two studies of small diabetic cohorts have also demonstrated lower LAEF in diabetics compared with controls but have not demonstrated any significant difference in LA size.10,33 Similar to our observations, studies using the first release of the UK Biobank CMR data have demonstrated association of diabetes with smaller atrial volumes.34,35 Conversely, some echocardiography studies have demonstrated association of diabetes with larger LA sizes. For example, Armstrong et al.9 demonstrate association of larger LA diameter with prevalent diabetes in 2903 CARDIA study participants. LA alterations evolve with disease progression, with LA dilatation reflecting persistently elevated LV filling pressures and advancement of diastolic (and systolic) LV dysfunction.3 Thus, the duration of exposure to and control of the diabetes, as well as the overall risk factor profile of participants likely influence associations with LA size. As the UK Biobank comprises a relatively healthy cohort, our observations reflect milder disease. Indeed, we observed significant association of larger LAV with pre-existing IHD, a condition associated with more advanced LV impairment. This is further supported by the observed association of larger LAV with greater CVD mortality risk. In our study, and in existing literature, LAEF appears as a reliable and consistent indicator of diabetes and other key morbidities.
We observed the association of more concentric LV remodelling patterns with VRFs and incident CVDs, whilst prevalent CVDs were associated with more eccentric LV remodelling patterns. These observations likely reflect predominance of pressure overload in the presence of VRFs and prior to disease occurrence, but dominance of volume overload after development of clinical CVD. Our analysis also demonstrates consistent and significant association of lower LVGFI with VRFs, prevalent CVDs, incident IHD, and higher risk of all-cause and CVD mortality. In separate analyses comparing LVGFI to LVEF, the latter showed similar but less consistent associations. Our findings add strength to existing studies which have proposed the high utility of this LVGFI as a measure of LV function.20,21
Clinical implications
In this study of 25 896 UK Biobank participants, we describe independent clinical associations of CMR derived measures of LA structure and function (LAV and LAEF). These metrics, particularly LAEF, show robust associations with key cardiovascular outcomes independent of LV measures. Thus, there is potential utility for these metrics as components of clinical risk prediction algorithms. In the next stages towards development of such clinical models, there is need for evaluation of clinical relationships in other cohorts and settings. Any proposed clinical risk stratification models will require careful validation and evaluation of model performance prior to use in clinical practice.
Strengths and limitations
The highly detailed participant characterization and standardized CMR data in the UK Biobank permitted evaluation of associations of CMR phenotypes with key VRFs and CVDs in a very large cohort, whilst considering a wide range of confounders. The linked reliably recorded health outcome data also permitted assessment of associations with incident CVDs. The duration of follow up was relatively short and the proportion of participants with incident events was small (n = 880/25 896, 3.4%), and even fewer when considering subgroups of participants (Supplementary data online, Table S10). Given that this limits our power to detect statistically significant associations with incident events, the observed significant relationships between LA metrics and incident CVDs are all the more notable. Identification of incident outcomes using HES is ideal for conditions such as acute MI and stroke, which almost always require hospitalization. However, this approach is not optimal for endpoints that do not always require hospital admission, such as diastolic heart failure or mitral valve disease, which we were unable to consider in the analysis. There were few CVD mortality events, which means that analysis with this outcome is likely underpowered to appreciate the full picture of CMR associations (particularly small and moderate effect sizes). As events accrue in the UK Biobank, more adequately powered analyses may be conducted with possibility of evaluating associations with more granular disease-specific mortality outcomes. Finally, due to the observational nature of the study, we cannot exclude residual confounding or reverse causation; however, the primary aim of the present study is description of associations rather than causal inference.
Conclusions
LA structure and function measures (LAEF and LAV) demonstrate significant associations with key prevalent and incident cardiovascular outcomes, independent of LV metrics. These measures have potential clinical utility for disease discrimination and outcome prediction.
Supplementary data
Supplementary data are available at European Heart Journal - Cardiovascular Imaging online.
Supplementary Material
Acknowledgements
This study was conducted using the UK Biobank resource under access application 2964. We would like to thank all the participants, staff involved with planning, collection and analysis, including core lab analysis of the CMR imaging data.
Contributor Information
Zahra Raisi-Estabragh, William Harvey Research Institute, NIHR Barts Biomedical Research Centre, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK; Barts Heart Centre, St Bartholomew’s Hospital, Barts Health NHS Trust, London EC1A 7BE, UK.
Celeste McCracken, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, National Institute for Health Research Oxford Biomedical Research Centre, Oxford University Hospitals NHS Foundation Trust, Oxford OX3 9DU, UK.
Dorina Condurache, London North West University Healthcare NHS Trust, Harrow HA1 3UJ, UK.
Nay Aung, William Harvey Research Institute, NIHR Barts Biomedical Research Centre, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK; Barts Heart Centre, St Bartholomew’s Hospital, Barts Health NHS Trust, London EC1A 7BE, UK.
Jose D Vargas, William Harvey Research Institute, NIHR Barts Biomedical Research Centre, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK; MedStar Georgetown University Hospital, Washington, DC 20007, USA.
Hafiz Naderi, William Harvey Research Institute, NIHR Barts Biomedical Research Centre, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK; Barts Heart Centre, St Bartholomew’s Hospital, Barts Health NHS Trust, London EC1A 7BE, UK.
Patricia B Munroe, William Harvey Research Institute, NIHR Barts Biomedical Research Centre, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK.
Stefan Neubauer, Division of Cardiovascular Medicine, Radcliffe Department of Medicine, University of Oxford, National Institute for Health Research Oxford Biomedical Research Centre, Oxford University Hospitals NHS Foundation Trust, Oxford OX3 9DU, UK.
Nicholas C Harvey, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, UK; NIHR Southampton Biomedical Research Centre, University of Southampton, University Hospital Southampton NHS Foundation Trust, Southampton, UK.
Steffen E Petersen, William Harvey Research Institute, NIHR Barts Biomedical Research Centre, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK; Barts Heart Centre, St Bartholomew’s Hospital, Barts Health NHS Trust, London EC1A 7BE, UK; Health Data Research UK, London, UK; Alan Turing Institute, London, UK.
Funding
Z.R.-E. was supported by a British Heart Foundation Clinical Research Training Fellowship (FS/17/81/33318). S.E.P. acknowledges support from the ‘SmartHeart’ EPSRC programme grant (www.nihr.ac.uk; EP/P001009/1) and also from the CAP-AI programme, London’s first AI enabling programme focused on stimulating growth in the capital’s AI Sector. CAP-AI is led by Capital Enterprise in partnership with Barts Health NHS Trust and Digital Catapult and is funded by the European Regional Development Fund and Barts Charity. S.E.P. has also received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 825903 (euCanSHare project). S.E.P. and S.N. acknowledge the British Heart Foundation for funding the manual analysis to create a cardiovascular magnetic resonance imaging reference standard for the UK Biobank imaging resource in 5000 CMR scans (www.bhf.org.uk; PG/14/89/31194). S.N. and C.M. were supported by the Oxford NIHR Biomedical Research Centre and S.N. by Oxford NIHR Biomedical Research Centre and the Oxford British Heart Foundation Centre of Research Excellence. N.C.H acknowledges support from the UK Medical Research Council (MRC #405050259 and #MC_UU_12011/1), NIHR Southampton Biomedical Research Centre, University of Southampton, and University Hospital Southampton. N.A. recognizes the National Institute for Health Research (NIHR) Integrated Academic Training programme which supports his Academic Clinical Lectureship posts. H.N. was supported by a British Heart Foundation Clinical Research Training Fellowship (FS/20/22/34640). This project was enabled through access to the MRC eMedLab Medical Bioinformatics infrastructure, supported by the Medical Research Council (www.mrc.ac.uk; MR/L016311/1). The funders provided support in the form of salaries for authors as detailed above but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Ethics
This study complies with the Declaration of Helsinki; the work was covered by the ethical approval for UK Biobank studies from the NHS National Research Ethics Service on 17 June 2011 (Ref 11/NW/0382) and extended on 18 June 2021 (Ref 21/NW/0157) with written informed consent obtained from all participants.
Data Availability
The data underlying this article were provided by the UK Biobank under access application 2964. UK Biobank will make the data available to bona fide researchers for all types of health-related research that is in the public interest, without preferential or exclusive access for any persons. All researchers will be subject to the same application process and approval criteria as specified by UK Biobank. For more details on the access procedure, see the UK Biobank website: http://www.ukbiobank.ac.uk/register-apply/.
References
- 1. Stefanadis C, Dernellis J, Toutouzas P.. A clinical appraisal of left atrial function. Eur Heart J 2001;22:22–36. [DOI] [PubMed] [Google Scholar]
- 2. Blume GG, McLeod CJ, Barnes ME, Seward JB, Pellikka PA, Bastiansen PMet al. Left atrial function: physiology, assessment, and clinical implications. Eur J Echocardiogr 2011;12:421–30. [DOI] [PubMed] [Google Scholar]
- 3. Kurt M, Wang J, Torre-Amione G, Nagueh SF.. Left atrial function in diastolic heart failure. Circ Cardiovas Imaging 2009;2:10–5. [DOI] [PubMed] [Google Scholar]
- 4. Gupta DK, Shah AM, Giugliano RP, Ruff CT, Antman EM, Grip LT, et al. ; for the Effective aNticoaGulation with factor xA next GEneration in AF-Thrombolysis In Myocardial Infarction 48 (ENGAGE AF-TIMI 48) Echocardiographic Study Investigators . Left atrial structure and function in atrial fibrillation: ENGAGE AF-TIMI 48. Eur Heart J 2014;35:1457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ou Q, Chen Y, Yu S, Guo X, Zhao H, Sun Y.. Prevalence of left atrial enlargement and its risk factors in general Chinese population. BMC Cardiovasc Disord 2016;16:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D.. Left atrial size and the risk of stroke and death: the Framingham Heart Study. Circulation 1995;92:835–41. [DOI] [PubMed] [Google Scholar]
- 7. Proietti M, Raparelli V, Basili S, Olshansky B, Lip GYH.. Relation of female sex to left atrial diameter and cardiovascular death in atrial fibrillation: the AFFIRM Trial. Int J Cardiol 2016;207:258–63. [DOI] [PubMed] [Google Scholar]
- 8. Inciardi RM, Giugliano RP, Claggett B, Gupta DK, Chandra A, Ruff CT, et al. ; ENGAGE AF-TIMI 48 Investigators . Left atrial structure and function and the risk of death or heart failure in atrial fibrillation. Eur J Heart Fail 2019;21:1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Armstrong AC, Gidding SS, Colangelo LA, Kishi S, Liu K, Sidney Set al. Association of early adult modifiable cardiovascular risk factors with left atrial size over a 20-year follow-up period: the CARDIA study. BMJ Open 2014;4:e004001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shang Y, Zhang X, Leng W, Lei X, Chen L, Liang Zet al. Left atrium passive ejection fraction is the most sensitive index of type 2 diabetes mellitus-related cardiac changes. Int J Cardiovasc Imaging 2018;34:141–51. [DOI] [PubMed] [Google Scholar]
- 11. Quail M, Grunseich K, Baldassarre LA, Mojibian H, Marieb MA, Cornfeld Det al. Prognostic and functional implications of left atrial late gadolinium enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2019;21:2. doi: 10.1186/s12968-018-0514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Inoue YY, Alissa A, Khurram IM, Fukumoto K, Habibi M, Venkatesh BAet al. Quantitative tissue-tracking cardiac magnetic resonance (CMR) of left atrial deformation and the risk of stroke in patients with atrial fibrillation. J Am Heart Assoc 2015;4:e001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Allen NE, Sudlow C, Peakman T, Collins R, UK Biobank . UK Biobank data: come and get it. Sci Transl Med 2014;6:224ed4. [DOI] [PubMed] [Google Scholar]
- 14. UK Biobank: protocol for a large-scale prospective epidemiological resource , 2007. https://www.ukbiobank.ac.uk/wp-content/uploads/2011/11/UK-Biobank-Protocol.pdf (13 December 2020, date last accessed).
- 15. Schnier C, Sudlow Biobank CU . Algorithmically-defined health outcomes (Chief Scientist), with input from members of the UK Biobank Follow-up and Outcomes Adjudication Group, 2017. https://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/alg_outcome_main.pdf (27 March 2020, date last accessed).
- 16. Raisi-Estabragh Z, Harvey NC, Neubauer S, Petersen SE.. Cardiovascular magnetic resonance imaging in the UK Biobank: a major international health research resource. Eur Heart J Cardiovasc Imaging 2021;22:251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Petersen SE, Matthews PM, Francis JM, Robson MD, Zemrak F, Boubertakh Ret al. UK Biobank’s cardiovascular magnetic resonance protocol. J Cardiovasc Magn Reson 2016;18:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bai W, Sinclair M, Tarroni G, Oktay O, Rajchl M, Vaillant Get al. Automated cardiovascular magnetic resonance image analysis with fully convolutional networks. J Cardiovasc Magn Reson 2018;20:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petersen SE, Aung N, Sanghvi MM, Zemrak F, Fung K, Paiva JMet al. Reference ranges for cardiac structure and function using cardiovascular magnetic resonance (CMR) in Caucasians from the UK Biobank population cohort. J Cardiovasc Magn Reson 2017;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nwabuo CC, Moreira HT, Vasconcellos HD, Mewton N, Opdahl A, Ogunyankin KOet al. Left ventricular global function index predicts incident heart failure and cardiovascular disease in young adults: the coronary artery risk development in young adults (CARDIA) study. Eur Heart J Cardiovasc Imaging 2019;20:533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mewton N, Opdahl A, Choi E-Y, Almeida ALC, Kawel N, Wu COet al. Left ventricular global function index by magnetic resonance imaging–a novel marker for assessment of cardiac performance for the prediction of cardiovascular events: the multi-ethnic study of atherosclerosis. Hypertens 2013;61:770–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Townsend P, Phillimore P, Beattie A.. Health and deprivation: inequality and the north. Nurs Stand 1988;2:34. [DOI] [PubMed] [Google Scholar]
- 23. Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BEet al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003;35:1381–95. [DOI] [PubMed] [Google Scholar]
- 24. R Core Team . 2019. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 25. RStudio: Integrated Development for R. Boston, MA: RStudio, Inc. https://rstudio.com/ (18 October 2020, date last accessed).
- 26. Wiley JF, Carrington MJ.. A metabolic syndrome severity score: a tool to quantify cardio-metabolic risk factors. Prev Med 2016;88:189–95. [DOI] [PubMed] [Google Scholar]
- 27. Kochunov P, Charlesworth J, Winkler A, Hong LE, Nichols TE, Curran JEet al. Transcriptomics of cortical gray matter thickness decline during normal aging. Neuroimage 2013;82:273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré Get al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013;36:27–46. [Google Scholar]
- 29. Bertelsen L, Diederichsen SZ, Haugan KJ, Brandes A, Graff C, Krieger Det al. Left atrial volume and function assessed by cardiac magnetic resonance imaging are markers of subclinical atrial fibrillation as detected by continuous monitoring. Europace 2020;22:724–31. [DOI] [PubMed] [Google Scholar]
- 30. Heckbert SR, Jensen PN, Austin TR, Chen LY, Post WS, Venkatesh BAet al. Associations of left atrial function and structure with supraventricular ectopy: the multi-ethnic study of atherosclerosis. J Am Heart Assoc 2021;10:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Habibi M, Zareian M, Ambale Venkatesh B, Samiei S, Imai M, Wu Cet al. Left atrial mechanical function and incident ischemic cerebrovascular events independent of AF: insights from the MESA Study. JACC Cardiovasc Imaging 2019;12:2417–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Markman TM, Habibi M, Venkatesh BA, Zareian M, Wu C, Heckbert SRet al. Association of left atrial structure and function and incident cardiovascular disease in patients with diabetes mellitus: results from multi-ethnic study of atherosclerosis (MESA). Eur Heart J Cardiovasc Imaging 2017;18:1138–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Graça B, Ferreira MJ, Donato P, Gomes L, Castelo-Branco M, Caseiro-Alves F.. Left atrial dysfunction in type 2 diabetes mellitus: insights from cardiac MRI. Eur Radiol 2014;24:2669–2676. [DOI] [PubMed] [Google Scholar]
- 34. Petersen SE, Sanghvi MM, Aung N, Cooper JA, Paiva JM, Zemrak Fet al. The impact of cardiovascular risk factors on cardiac structure and function: insights from the UK Biobank imaging enhancement study. PLoS One 2017;12:e0185114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jensen MT, Fung K, Aung N, Sanghvi MM, Chadalavada S, Paiva JMet al. Changes in cardiac morphology and function in individuals with diabetes mellitus. Circ Cardiovasc Imaging 2019;12:e009476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by the UK Biobank under access application 2964. UK Biobank will make the data available to bona fide researchers for all types of health-related research that is in the public interest, without preferential or exclusive access for any persons. All researchers will be subject to the same application process and approval criteria as specified by UK Biobank. For more details on the access procedure, see the UK Biobank website: http://www.ukbiobank.ac.uk/register-apply/.