Abstract
The COVID-19 pandemic has disrupted systems of care for infectious diseases—including tuberculosis—and has exposed pervasive inequities that have long marred efforts to combat these diseases. The resulting health disparities often intersect at the individual and community levels in ways that heighten vulnerability to tuberculosis. Effective responses to tuberculosis (and other infectious diseases) must respond to these realities. Unfortunately, current tuberculosis programmes are generally not designed from the perspectives of affected individuals and fail to address structural determinants of health disparities. We describe a person-centred, equity-oriented response that would identify and focus on communities affected by disparities, tailor interventions to the mechanisms by which disparities worsen tuberculosis, and address upstream determinants of those disparities. We detail four key elements of the approach (data collection, programme design, implementation, and sustainability). We then illustrate how organisations at multiple levels might partner and adapt current practices to incorporate these elements. Such an approach could generate more substantial, sustainable, and equitable reductions in tuberculosis burden at the community level, highlighting the urgency of restructuring post-COVID-19 health systems in a more person-centred, equity-oriented way.
Introduction
The COVID-19 pandemic has crippled existing systems for diagnosis, prevention, and treatment of tuberculosis and other infectious diseases in high-burden countries.1, 2, 3 By some accounts, COVID-19 has set back progress against tuberculosis by 5 years or more.2 In 2020, it is estimated that tuberculosis mortality rose for the first time in over a decade.4 Although it will be several years before we understand the pandemic's full effect on tuberculosis and other diseases, we cannot wait to begin rebuilding and strengthening health systems for those diseases.5, 6 It is therefore timely to consider how innovative systems to control and eliminate infectious diseases in a post-COVID-19 world might best be constructed.
The COVID-19 pandemic has laid bare that most modern societies are characterised by deep disparities rooted in socioeconomic opportunity, health-care access, political and legal power, and demographics.7 These disparities have led to socioeconomic, racial, and ethnic inequities in COVID-19-related outcomes.8 Disparities of a similar nature have long been identified as the fuel that feeds tuberculosis epidemics.9, 10, 11 Unfortunately, as illustrated by the COVID-19 response, reliance on existing health systems generally results in a deepening of disparities.12, 13
Need for a new approach
Tuberculosis programmes have historically been organised in a top-down and disease-specific (ie, vertical) way. This structure is well suited for adopting biomedical advances that lend themselves to one-size-fits-all policies14, 15—including, for tuberculosis, more sensitive diagnostic tests,16 shorter-course preventive therapy,17 and novel drugs for treatment.18 However, even these policies are often implemented slowly and sporadically. More recently, some attention has been paid to social interventions such as cash transfers19 and other social protection strategies.20 Although a welcome advance, social interventions for tuberculosis have been even less widely and consistently implemented than biomedical interventions; those that exist are often delivered in a broad, undifferentiated way and narrowly centred around reducing catastrophic costs.
Although undeniably important, top-down approaches reach a limit of feasible effect, after which the social and historical factors that underlie health disparities often shape disease burden.21 Furthermore, top-down approaches do not generally consider the complex interactions of human experiences that factor into one's risk of developing tuberculosis disease and seeking care. For example, marginalised communities are more likely to face barriers to care because of stigma, poverty, language or cultural differences, or distrust.22 This resulting disparity in access to care is unlikely to be addressed by making diagnostic assays or drugs cheaper, superior, or more readily available—nor by providing government-issued poverty grants. Health systems also need to focus on building trust, meeting community needs, understanding the reasons for health disparities, improving disease awareness, and partnering to achieve mutual priorities.23
To bend the curve of tuberculosis (and other infectious diseases), health systems must be enhanced to respond to the unique needs of individuals affected by health disparities. As a step in this process, we sought to develop a conceptual framework for a person-centred, equity-oriented approach to tuberculosis and to illustrate how such an approach can result in sustainable reductions in tuberculosis burden when traditional systems might not. We anticipate that these principles will also apply to other diseases that disproportionally affect disadvantaged populations.
Conceptual overview
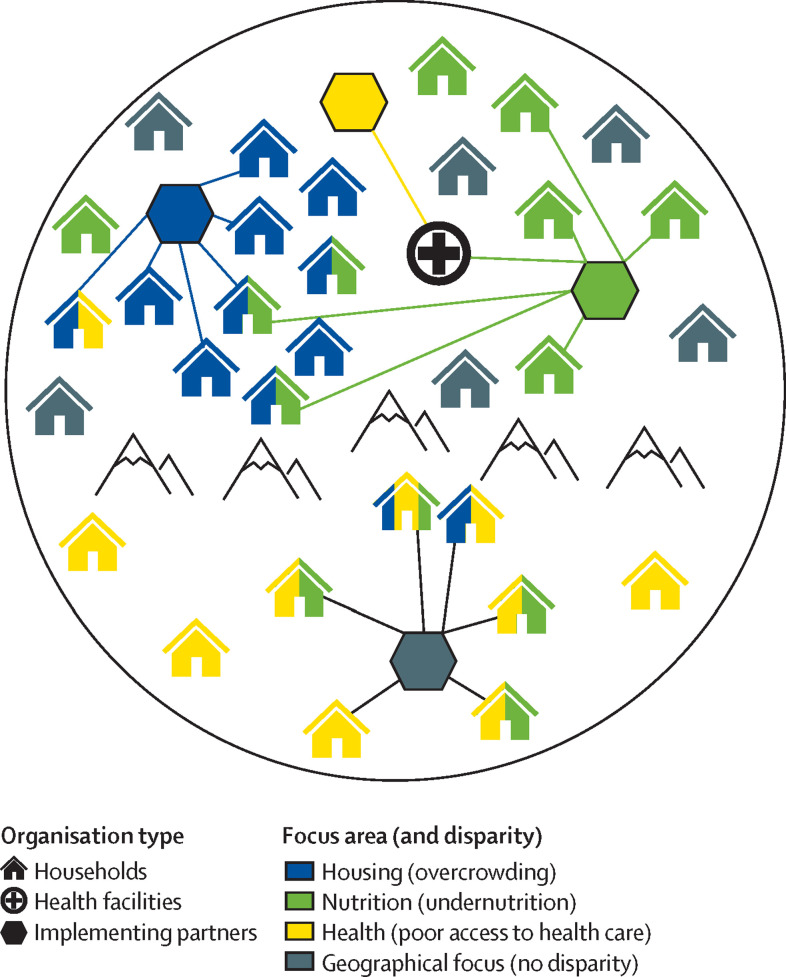
Our conceptual framework envisions tuberculosis vulnerability through the lens of individual experiences (person-centred) that are affected by structural determinants (equity-oriented). Figure 1 illustrates how such structural determinants can combine to increase individual tuberculosis risk. In this figure, health disparities are conceptualised as corresponding to mechanisms of vulnerability to tuberculosis. For example, disparities in food security might lead to higher rates of tuberculosis disease progression in individuals without access to adequate nutrition, whereas disparities in access to health care might cause lower rates of diagnosis and treatment.24 Figure 2 depicts how these disparities often overlap, resulting in heightened tuberculosis risk in affected communities. Importantly, these disparities are not always geographically segregated, and the people most aware of the structural determinants affecting different communities tend to be local implementing partners (eg, members of community-based non-governmental organisations [NGOs] and civil society), community leaders, and health workers—not national-level officials (figure 3 ).
Figure 1.
The contribution of multiple disparities to increased TB risk in individuals
An individual's risk of having TB reflects their combined vulnerability to infection with Mycobacterium tuberculosis, progression to active disease after infection, and inadequate or delayed diagnosis and treatment if active TB develops. Specific structural disparities might act on one of these mechanisms more than others. TB burden can therefore be most effectively reduced by identifying the people most affected by given disparities, and then acting both upstream to reduce the vulnerabilities created by those disparities (dashed arrows) and downstream to mitigate the effects of those vulnerabilities on TB burden (solid arrows). TB=tuberculosis.
Figure 2.
Overlapping disparities increase tuberculosis risk in households and communities
Multiple disparities act together to elevate tuberculosis risk in households, which themselves coalesce into communities. Members of households affected by multiple disparities (shown in the centre of this figure) often face barriers to diagnosis, treatment, and prevention that can only be overcome using a person-centred approach. This overlap of disparities also occurs on the level of communities (rather than just individual households). By collecting data on how multiple disparities combine to affect individuals, households, and communities, one can appropriately target interventions to maximise their effect.
Figure 3.
Disparity-affected communities and organisations contributing to a person-centred, equity-oriented approach to tuberculosis
The figure illustrates a hypothetical district (or other subnational locality) in a high tuberculosis burden setting. Households are coloured according to three selected disparities that increase their vulnerability to tuberculosis; households not affected by these disparities are shown in grey. Disparities tend to be clustered geographically, but not exclusively. Organisations that could partner with the local tuberculosis programme to implement an equity-oriented, person-centred response include health facilities, local government and community groups, and implementing partners, such as non-governmental organisations or civil society organisations. These partners often focus on specific topical domains (indicated via colours: nutrition, housing, or health) or geographical locations (grey: the organisation in the isolated community below the mountain range). Lines indicate that some implementing partners work within communities (such as the blue and grey), others primarily through health facilities (yellow), and some with both (green).
The components of a person-centred, equity-oriented approach to tuberculosis therefore include identifying the disparities causing vulnerability to tuberculosis at the local level, clarifying the mechanisms by which those disparities increase vulnerability, and addressing the barriers corresponding to these disparities and mechanisms. We propose that such an approach should focus on four key elements: data collection, programme design, implementation, and sustainability (table 1 ). This proposed approach would merge a locally relevant evidence base, a comprehensive set of interventions (both tuberculosis-specific and upstream), and a focus on support to individuals and sustainability. The same prevention, diagnostic, and treatment interventions used in top-down systems are still necessary, but their delivery would be structured from the perspective of affected individuals (including a respect for human rights, especially among stigmatised populations29), and the allocation of these tools would be tailored to the mechanisms by which disparities increase tuberculosis risk in each community. Furthermore, addressing additional upstream barriers would be considered essential.
Table 1.
Elements of a person-centred, equity-oriented approach to ending tuberculosis
| Person-centred | Equity-oriented | Example | |
|---|---|---|---|
| Data collection | Engage affected communities to understand sources of individual and community vulnerability to tuberculosis | Identify communities most affected by existing disparities | Identification of communities experiencing high tuberculosis burden due to the effect of food insecurity25 |
| Design | Multifaceted, scalable approaches address the multiple barriers faced by individual people | Disparity-matched approaches address the specific vulnerabilities caused by key disparities | Preventive therapy facilitated by streamlining the flow of clinic visits and providing nutritional supplements26 |
| Implementation | Interventions enable patients to complete the full cascades of diagnosis, treatment, and prevention | Interventions target underlying mechanisms by which disparities increase vulnerability to tuberculosis | Implementation of treatment for tuberculosis infection with context-specific supporting interventions27 |
| Sustainability | Efforts keep people connected to care and build trust in communities | Efforts address upstream determinants so vulnerabilities do not rebound | Efforts to build trust and reduce stigma associated with tuberculosis infection in identified communities28 |
At a local level, some components of this approach might be coordinated by district or regional tuberculosis programmes, which would house relevant expertise on tuberculosis diagnosis, treatment, and prevention. However, the success of such an approach depends crucially on collaboration between stakeholders at different levels and keeping affected individuals and communities at the centre (figure 4 ). Table 2 describes the roles that various stakeholders—including local community leaders and implementing partners, organisations at multiple levels of the health-care system, external funders and policy makers, and civil society—could play to advance a person-centred, equity-oriented approach. In promoting such an approach, closer communication and partnership is a cross-cutting theme.30 In the following sections, we describe how stakeholders at all levels might partner to address each of the four key elements in table 1, thereby resulting in a more person-centred, equity-oriented—and more effective—tuberculosis response.
Figure 4.
National, regional, local, and community level partnerships to advance a person-centred, equity-oriented approach to tuberculosis
The figure shows how various organisations and stakeholders could collaborate to contribute towards a person-centred, equity-oriented approach to tuberculosis. The upper set of partners represents the tuberculosis programme, the lower right represents funders and NGOs, and the lower left represents non-tuberculosis-specific governmental agencies. This approach is centred at the local level—including affected communities, local health-care workers, community leaders, and local implementing partners—to ensure that priorities and approaches are locally relevant. Partners at higher levels (regional=middle ring, national and international=outer ring) are nonetheless essential for garnering political will, ensuring financial sustainability, and maintaining accountability. Specific organisational roles and opportunities for engagement are described in more detail in table 2. CSOs=civil society organisations. NGOs=non-governmental organisations.
Table 2.
Organisational roles and engagement opportunities to advance a person-centred, equity-oriented approach to ending tuberculosis
| Current roles | Engagement opportunities | |
|---|---|---|
| Community leaders | Understand community priorities; administer local programmes; advocate for communities | Formal connections with tuberculosis programmes (eg, community advisory boards) to represent community priorities and build trust; community activities (eg, information campaigns) to link constituents to tuberculosis services |
| Implementing partners (eg, non-governmental and civil society organisations) | Identify disparity-affected populations; implement interventions; coordinate with local clinics to support health programmes; provide expertise on key topics; advocate for communities | Funding partnerships with tuberculosis programmes; collaborative efforts to share expertise and set priorities; collection and sharing of data (eg, on social determinants); communication of health priorities to affected populations |
| Local health-care workers | Diagnose, treat, and prevent tuberculosis; collect data for local and national tuberculosis programmes; link tuberculosis programmes with other health programmes (eg, HIV and diabetes) | Formal engagement with tuberculosis programmes and implementing partners (eg, clinical advisory boards); collaboration with non-clinical partners (eg, cross-referrals) as part of targeted interventions; expanded local data on health disparities affecting patients |
| District tuberculosis programmes | Ensure quality of tuberculosis care; implement and disseminate guidelines; set regional priorities for tuberculosis; collect data to report to national tuberculosis programme | Formal partnerships with local implementing partners and community leaders; structured activities for community engagement; incorporation of local data into reports and priority-setting |
| National tuberculosis programmes | Set national priorities for tuberculosis; fund tuberculosis programmes; advocacy at the national level for efforts to end tuberculosis; report data to other national and international organisations | Boards of district-level programmes and community representatives; flexible funding mechanisms for locally guided interventions; guidelines and accountability mechanisms that allow local priorities to be incorporated |
| Ministries of health | Address sources of ill health and health disparities; advance universal health coverage and other social supports to improve health | Champions and guidelines for integrated care; novel joint funding mechanisms; political will to advance patient-centred, equity-oriented approaches |
| District and national government (outside Ministry of Health) | Provide programmes (eg, education and housing) with potential health effects; provide funding for such programmes; collect data on relevant programmes and sectors | Partnerships to implement comprehensive tuberculosis interventions (eg, case finding in schools or slum areas); multisectoral data collection and programming efforts |
| External funding agencies | Provide financial support to improve health | Representation of patients and communities in funding opportunities; accountability structures to ensure local relevance, patient centredness, and focus on equity; funding for partnerships across disease areas and sectors |
Data collection
Without local data on tuberculosis vulnerability and the influence of social determinants, it is impossible to design tuberculosis responses that are person-centred, equity-oriented, and evidence-based. However, vulnerable and marginalised communities are often those for which we have the least data. Targeted data collection efforts can be embedded within routine systems and combined with data that those systems are already collecting. District tuberculosis programmes could partner with community-based organisations to investigate the relative prevalence and severity of known determinants of tuberculosis risk. For example, in communities thought to have a high burden of undernutrition (which is linked to increased tuberculosis progression risk31), targeted surveys or routine health records could be used to estimate the distribution of BMI in the population. For people affected by incarceration (which is linked to increased tuberculosis transmission risk32), prison release records could be systematically abstracted to estimate the frequency and duration of incarceration among residents of different neighbourhoods. Tuberculosis officials could provide expertise as to the mechanism of risk and strength of association with tuberculosis for each determinant (eg, based on published literature), and local organisations could provide input on the determinants thought to be most important in their constituent communities.
In collecting and using local data for decision making, an inherent tension exists between feasibility and utility. For example, routine tuberculosis notifications might be easy to collect and evaluate, but high notification rates in a community could reflect higher tuberculosis burden or better access to care.33, 34 More detailed data on access to care (eg, estimated symptom duration) could be informative, but would require more intensive effort to collect. Similarly, to inform interventions that are highly tailored geographically, data on a hyper-local level are needed—but collection of such data might be infeasible.29 Ultimately, these tensions can only be effectively resolved through partnership between vertical programmes (that offer expertise and funding) and local partners (who are more familiar with specific communities). In some cases, the value of implementing highly targeted, evidence-based interventions will justify the cost and logistical challenge of collecting better data. In other cases, use of routinely available data—coupled with expert opinion—might be the only affordable option.
Intervention design
To be effective, interventions must be not only person-centred and equity-oriented, but also feasible. Borrowing from the literature on disaster preparedness,35, 36 vulnerability mapping could be a useful first step in designing interventions. Vulnerability maps show geographical areas where social vulnerabilities cluster. For example, social vulnerability indices37 have been used to map vulnerability for interventions from climate change mitigation38 to COVID-19 vaccines.39 Multidimensional, disease-specific social vulnerability indices—for example, incorporating specific components of social vulnerability that are relevant to tuberculosis risk and link to specific responses—are an important area for future research.
Implementation of vulnerability indices and maps should also recognise that vulnerabilities—and corresponding interventions—might not always be geographically distributed. Furthermore, some implementing partners might serve specific populations, not just specific geographies (figure 3). For example, men whose work schedules restrict their ability to access health care (who are more likely to have undiagnosed tuberculosis40) might be better served by occupational programmes than location-specific programmes. Using social vulnerability indices to identify key populations and partner with the local organisations that serve them would be an important advance.
Teams of tuberculosis experts and local partners could use data on tuberculosis risk, incorporated into vulnerability maps or indices, to design interventions that address the most important determinants of tuberculosis risk at the local level. Programmes could use these data to tailor the mix of interventions to the mechanisms by which these disparities yield higher tuberculosis burdens. For example, people with poor access to care might benefit most from active case finding and treatment support, whereas preventive treatment might be better focused on people affected by vulnerabilities that increase the risk of disease progression (eg, undernutrition or HIV). Matching vulnerabilities to mechanisms of action can enable resource-intensive interventions to be implemented more efficiently, affordably, and cost-effectively.
As an illustrative example, consider an NGO that provides nutrition-focused services to people with undernutrition (ie, low BMI). If undernourishment were to double the risk of progression from tuberculosis infection to disease,24, 41 then implementing tuberculosis preventive therapy (TPT) in this population (eg, in partnership with this NGO) could be nearly twice as efficient as an untargeted approach. By tailoring the mechanism of intervention action (TPT prevents disease progression) to the mechanism of disparity (undernutrition increases risk of disease progression), this programme's effect could be optimised. Targeting an intervention that is not tailored to the mechanism of the disparity would have less effect.
In designing such an intervention, additional considerations would need to be addressed—including cost-effectiveness, affordability, ethical and human rights implications, and spillover effects on other health and non-health outcomes. But if interventions are not designed to address both the barriers faced by affected individuals (person-centred) and the underlying determinants of health disparities (equity-oriented), they are unlikely to have substantive effects on tuberculosis burden.
Intervention implementation
Following appropriate data collection and design, interventions must also be implemented in a person-centred way that meets the unique needs of the people to whom the interventions are targeted and supports them in completing the full cascade of care. Without careful attention to all steps in the care cascade, interventions with proven efficacy will not reduce the burden of disease.42, 43, 44, 45 Furthermore, unless the social, economic, and geographical barriers to care faced by vulnerable populations are considered, broad implementation could exacerbate existing disparities.46 As an example from the COVID-19 pandemic, adverse consequences of lockdown policies have disproportionately affected vulnerable populations.47 Collaborating with those who already work closely with affected communities (including NGOs, civil society, community leaders, and other influential and trusted community champions) and obtaining input from communities themselves can help programmes craft interventions that are more person-centred.48, 49, 50
Effective interventions must also be implemented in a way that is responsive to perceived needs in the community—such needs often reflect underlying vulnerabilities. For example, communities facing high levels of stigma (eg, racial and ethnic minorities) might have poor access to care,51 but in a way that differs from that of communities experiencing financial or geographical barriers. Problem-focused interventions to improve access to tuberculosis care—such as contact investigation using uniformed health-care workers52 or streamlining of traditional clinic services53 to reduce the number of visits—might be very effective for communities with financial barriers to health-care access, but are unlikely to reach stigmatised populations who do not trust the traditional health-care system.54 Interventions must therefore be implemented in a way that begins by considering local patient-level barriers, rather than simply seeking to solve a problem (eg, poor access to care) as perceived by decision makers at a national level.
Sustainability
Finally, to be sustainable, interventions should also directly address upstream determinants such as stigma, socioeconomic factors (which can contribute to phenomena such as undernutrition and crowding), lack of trust in the health-care system, and health literacy. Addressing upstream determinants can help ensure that impact is sustained over time and is also likely to yield effects that extend beyond tuberculosis.55 For example, preventing undernutrition might not only sustainably reduce the burden of tuberculosis, but it could also reduce vulnerability to other infectious diseases56 and lead to improvements in child growth and development.57 Similarly, cash transfers and other social protection programmes targeting impoverished households can reduce poverty, yielding widespread benefits beyond tuberculosis.58 The COVID-19 pandemic has demonstrated the need to synergise disease-specific programmes and programmes that target broader social determinants of health. Urgent attention to this issue is needed in disease areas other than COVID-19 so that this momentum and awareness is not lost.
Historically, sustainability has often been interpreted as a need to reduce costs. Here, we envision sustainability as lasting impact: approaches that fail to support individuals throughout the entire cascade of care or address upstream vulnerabilities contributing to tuberculosis risk are unlikely to be effective in the long term. However, it is important to build an understanding among governments, donors, and other funding agencies that a person-centred, equity-oriented approach to tuberculosis will require additional resources up front. Thus, an important priority is to develop an explicit business case for that investment, to show its long-term value and potential spillover effects. A person-centred, equity-oriented approach might be resource intensive and costly, especially when implemented for the first time, but it could also generate savings in the long term, bolster human rights, and yield effects beyond tuberculosis-related outcomes.
Challenges
Despite potential gains, availability of funding will be a major challenge to implementing a person-centred, equity-oriented approach. Most funding for tuberculosis still originates at the national level, and there is still a need for a robust national tuberculosis programme with the expertise and infrastructure to diagnose, treat, and prevent tuberculosis at all levels of the health-care system. However, additional resources—such as interventions to support patients and address upstream determinants of disparities—will be required to carry out elements of this approach. These resources are often not present in existing top-down programmes.
Resource constraints not only affect the selection and implementation of interventions, but also data collection. A balance must be struck between collecting data on a geographical and population scale that is sufficiently granular to inform targeted interventions, but sufficiently focused to remain affordable. Local partners can lend expertise in this regard, sharing existing data streams and describing which data are likely to be helpful for informing decision making, and which are likely to be superfluous. In light of the COVID-19 pandemic, the need for locally relevant data has never been more apparent, and a window to increase investment in public health data streams might still be open.
Additionally, a person-centred, equity-oriented approach requires that many activities be carried out by, or in partnership with, implementing partners with close knowledge of local drivers of vulnerability. Building partnerships between district tuberculosis programmes and the many other stakeholders involved in the proposed approach will take time and effort, and additional capacity might be required among district tuberculosis programmes to coordinate this approach in their localities. Current vertical funding might need to be made more flexible to allow for such collaborative arrangements; consideration of spillover effects beyond tuberculosis has the potential to open up diverse funding streams.59
Implementing a person-centred, equity-oriented approach to tuberculosis will ultimately take time and require substantial resources. However, this approach also has the potential to use limited resources more efficiently and for greater impact. Consideration of other diseases might allow even greater opportunities for synergy, as some interventions (eg, household visits and engagement in care) could improve health outcomes across disease areas, in a person-centred way and with relatively small additional cost.
Conclusion
In summary, the COVID-19 pandemic, which devastated health systems around the world, adds urgency to the need to recast our approach to tuberculosis. As we respond to the challenges created by COVID-19, we should consider restructuring current top-down approaches. Specifically, we should prioritise person-centred, equity-oriented approaches that identify communities that experience heightened vulnerability to tuberculosis, support those communities with interventions tailored to their specific challenges, consider the perspective of individual community members facing multifaceted barriers, and address the underlying social determinants responsible for entrenching those vulnerabilities. Collaborative efforts among diverse stakeholders will be crucial for designing and implementing these solutions; in developing these relationships and adopting this change in perspective, there is no time to lose.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
DWD was funded by a Catalyst Award from the Johns Hopkins University Office of Research. KR was funded by the National Institutes of Health (F32HL158019). The funders had no role in the study's design; collection, analysis, or interpretation of data; the writing of the report; or the decision to submit for publication.
Contributors
DWD, LC, KR, and TR conceptualised the study and contributed to the initial drafting of the manuscript. TR led the initial drafting of the sections corresponding to the four key elements and of the section on challenges, LC led the conceptualisation and application of elements related to sustainability and person-centredness, and KR performed the primary literature review. TR, LC, and KR all contributed to the development of tables and figures. AK, ST, and DWD provided overarching guidance on the study, including the framing of the results and implications. SZ-M, RA, EAK, and SS contributed to subsequent drafts of the study and provided recommendations on the analysis and conceptual approach. All authors accept responsibility to submit for publication.
References
- 1.McQuaid CF, Vassall A, Cohen T, Fiekert K, White RG. The impact of COVID-19 on TB: a review of the data. Int J Tuberc Lung Dis. 2021;25:436–446. doi: 10.5588/ijtld.21.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cilloni L, Fu H, Vesga JF, et al. The potential impact of the COVID-19 pandemic on the tuberculosis epidemic a modelling analysis. EClinicalMedicine. 2020;28 doi: 10.1016/j.eclinm.2020.100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan MS, Rego S, Rajal JB, et al. Mitigating the impact of COVID-19 on tuberculosis and HIV services: a cross-sectional survey of 669 health professionals in 64 low and middle-income countries. PLoS One. 2021;16 doi: 10.1371/journal.pone.0244936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Global tuberculosis report. 2021. https://www.who.int/teams/global-tuberculosis-programme/tb-reports
- 5.Pai M, Kasaeva T, Swaminathan S. COVID-19's devastating effect on tuberculosis care—a path to recovery. N Engl J Med. 2022;386:1490–1493. doi: 10.1056/NEJMp2118145. [DOI] [PubMed] [Google Scholar]
- 6.Adepoju P. Tuberculosis and HIV responses threatened by COVID-19. Lancet HIV. 2020;7:e319–e320. doi: 10.1016/S2352-3018(20)30109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson G, Frank JW, Naylor CD, Wodchis W, Feng P. Using socioeconomics to counter health disparities arising from the COVID-19 pandemic. BMJ. 2020;369 doi: 10.1136/bmj.m2149. [DOI] [PubMed] [Google Scholar]
- 8.Magesh S, John D, Li WT, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.34147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lönnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M. Drivers of tuberculosis epidemics: the role of risk factors and social determinants. Soc Sci Med. 2009;68:2240–2246. doi: 10.1016/j.socscimed.2009.03.041. [DOI] [PubMed] [Google Scholar]
- 10.Harling G, Ehrlich R, Myer L. The social epidemiology of tuberculosis in South Africa: a multilevel analysis. Soc Sci Med. 2008;66:492–505. doi: 10.1016/j.socscimed.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 11.Oxlade O, Murray M. Tuberculosis and poverty: why are the poor at greater risk in India? PLoS One. 2012;7 doi: 10.1371/journal.pone.0047533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrams EM, Szefler SJ. COVID-19 and the impact of social determinants of health. Lancet Respir Med. 2020;8:659–661. doi: 10.1016/S2213-2600(20)30234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burstrom B, Tao W. Social determinants of health and inequalities in COVID-19. Eur J Public Health. 2020;30:617. doi: 10.1093/eurpub/ckaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vesga JF, Hallett TB, Reid MJA, et al. Assessing tuberculosis control priorities in high-burden settings: a modelling approach. Lancet Glob Health. 2019;7:e585–e595. doi: 10.1016/S2214-109X(19)30037-3. [DOI] [PubMed] [Google Scholar]
- 15.Ross JM, Badje A, Rangaka MX, et al. Isoniazid preventive therapy plus antiretroviral therapy for the prevention of tuberculosis: a systematic review and meta-analysis of individual participant data. Lancet HIV. 2021;8:e8–15. doi: 10.1016/S2352-3018(20)30299-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walzl G, McNerney R, du Plessis N, et al. Tuberculosis: advances and challenges in development of new diagnostics and biomarkers. Lancet Infect Dis. 2018;18:e199–e210. doi: 10.1016/S1473-3099(18)30111-7. [DOI] [PubMed] [Google Scholar]
- 17.Faust L, Ruhwald M, Schumacher S, Pai M. How are high burden countries implementing policies and tools for latent tuberculosis infection? A survey of current practices and barriers. Health Sci Rep. 2020;3:e158. doi: 10.1002/hsr2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pontali E, Sotgiu G, Tiberi S, et al. Combined treatment of drug-resistant tuberculosis with bedaquiline and delamanid: a systematic review. Eur Respir J. 2018;52 doi: 10.1183/13993003.00934-2018. [DOI] [PubMed] [Google Scholar]
- 19.Reis-Santos B, Shete P, Bertolde A, et al. Tuberculosis in Brazil and cash transfer programs: a longitudinal database study of the effect of cash transfer on cure rates. PLoS One. 2019;14 doi: 10.1371/journal.pone.0212617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrade KVF, Nery JS, Souza RA, Pereira SM. Effects of social protection on tuberculosis treatment outcomes in low or middle-income and in high-burden countries: systematic review and meta-analysis. Cad Saude Publica. 2018;34 doi: 10.1590/0102-311X00153116. [DOI] [PubMed] [Google Scholar]
- 21.Fee E, Krieger N. Understanding AIDS: historical interpretations and the limits of biomedical individualism. Am J Public Health. 1993;83 doi: 10.2105/ajph.83.10.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:1–9. doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhillon RS, Kelly JD. Community trust and the Ebola endgame. N Engl J Med. 2015;373:787–789. doi: 10.1056/NEJMp1508413. [DOI] [PubMed] [Google Scholar]
- 24.Sinha P, Lönnroth K, Bhargava A, et al. Food for thought: addressing undernutrition to end tuberculosis. Lancet Infect Dis. 2021;21:e318–e325. doi: 10.1016/S1473-3099(20)30792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chileshe M. Tuberculosis, HIV, food insecurity, and poverty in rural Zambia: an ethnographic account of the Southern province. 2008. https://open.uct.ac.za/handle/11427/10192
- 26.Kadota JL, Musinguzi A, Nabunje J, et al. Protocol for the 3HP options trial: a hybrid type 3 implementation-effectiveness randomized trial of delivery strategies for short-course tuberculosis preventive therapy among people living with HIV in Uganda. Implement Sci. 2020;15:65. doi: 10.1186/s13012-020-01025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16:1269–1278. doi: 10.1016/S1473-3099(16)30216-X. [DOI] [PubMed] [Google Scholar]
- 28.van der Westhuizen HM, Nathavitharana RR, Pillay C, Schoeman I, Ehrlich R. The high-quality health system ‘revolution': re-imagining tuberculosis infection prevention and control. J Clin Tuberc Other Mycobact Dis. 2019;17 doi: 10.1016/j.jctube.2019.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stop TB. Data for action for tuberculosis key, vulnerable and underserved populations. 2017. https://stoptb.org/assets/documents/communities/Data%20for%20Action%20for%20Tuberculosis%20Key,%20Vulnerable%20and%20Underserved%20Populations%20Sept%202017.pdf
- 30.WHO Multisectoral Accountability Framework for TB (MAF-TB) Baseline Assessment Checklist for country use in pursuing a national MAF-TB. 2019. https://www.who.int/publications/m/item/who-multisectoral-accountability-framework-for-tb-(maf-tb)-baseline-assessment-checklist-for-country-use-in-pursuing-a-national-maf-tb
- 31.Semba RD, Darnton-Hill I, De Pee S. Addressing tuberculosis in the context of malnutrition and HIV coinfection. Food Nutr Bull. 2010;31:S345–S364. [PubMed] [Google Scholar]
- 32.Cords O, Martinez L, Warren JL, et al. Incidence and prevalence of tuberculosis in incarcerated populations: a systematic review and meta-analysis. Lancet Public Health. 2021;6:e300. doi: 10.1016/S2468-2667(21)00025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chitwood MH, Pelissari DM, Drummond Marques da Silva G, et al. Bayesian evidence synthesis to estimate subnational TB incidence: an application in Brazil. Epidemics. 2021;35 doi: 10.1016/j.epidem.2021.100443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allorant A, Biswas S, Ahmed S, et al. Finding gaps in routine TB surveillance activities in Bangladesh. Int J Tuberc Lung Dis. 2022;26:356–362. doi: 10.5588/ijtld.21.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrow BH. Identifying and mapping community vulnerability. Disasters. 1999;23:1–18. doi: 10.1111/1467-7717.00102. [DOI] [PubMed] [Google Scholar]
- 36.Bankoff G, Frerks G, Hilhorst D. Taylor & Francis; Abingdon: 2004. Mapping vulnerability: disasters, development and people. [Google Scholar]
- 37.Flanagan BE, Hallisey EJ, Adams E, Lavery A. Measuring community vulnerability to natural and anthropogenic hazards: the Centers for Disease Control and Prevention's Social Vulnerability Index. J Environ Health. 2018;80:34. [PMC free article] [PubMed] [Google Scholar]
- 38.Rabby YW, Hossain MB, Hasan MU. Social vulnerability in the coastal region of Bangladesh: an investigation of social vulnerability index and scalar change effects. Int J Disaster Risk Reduct. 2019;41 [Google Scholar]
- 39.Hughes MM, Wang A, Grossman MK, et al. County-level COVID-19 vaccination coverage and social vulnerability—United States, December 14, 2020–March 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:431–436. doi: 10.15585/mmwr.mm7012e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chikovore J, Pai M, Horton KC, et al. Missing men with tuberculosis: the need to address structural influences and implement targeted and multidimensional interventions. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2019-002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lönnroth K, Williams BG, Cegielski P, Dye C. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol. 2010;39:149–155. doi: 10.1093/ije/dyp308. [DOI] [PubMed] [Google Scholar]
- 42.Subbaraman R, Nathavitharana RR, Satyanarayana S, et al. The tuberculosis cascade of care in India's public sector: a systematic review and meta-analysis. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bastos ML, Melnychuk L, Campbell JR, Oxlade O, Menzies D. The latent tuberculosis cascade-of-care among people living with HIV: a systematic review and meta-analysis. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun AY, Denkinger CM, Dowdy DW. The impact of novel tests for tuberculosis depends on the diagnostic cascade. Eur Respir J. 2014;44 doi: 10.1183/09031936.00111014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016;16:1269–1278. doi: 10.1016/S1473-3099(16)30216-X. [DOI] [PubMed] [Google Scholar]
- 46.Andrews JR, Basu S, Dowdy DW, Murray MB. The epidemiological advantage of preferential targeting of tuberculosis control to the poor. Int J Tuberc Lung Dis. 2015;19:375. doi: 10.5588/ijtld.14.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glover RE, van Schalkwyk MCI, Akl EA, et al. A framework for identifying and mitigating the equity harms of COVID-19 policy interventions. J Clin Epidemiol. 2020;128:35. doi: 10.1016/j.jclinepi.2020.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mullan Z. Reaching the unreached and expecting the unexpected. Lancet Glob Health. 2014;2:e182. doi: 10.1016/S2214-109X(14)70214-1. [DOI] [PubMed] [Google Scholar]
- 49.Pai M, Yadav P, Anupindi R. Tuberculosis control needs a complete and patient-centric solution. Lancet Glob Health. 2014;2:e189–e190. doi: 10.1016/S2214-109X(14)70198-6. [DOI] [PubMed] [Google Scholar]
- 50.Reid MJA, Goosby E. Patient-centered tuberculosis programs are necessary to end the epidemic. J Infect Dis. 2017;216(suppl 7):S673–S674. doi: 10.1093/infdis/jix373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Craig GM, Daftary A, Engel N, O'Driscoll S, Ioannaki A. Tuberculosis stigma as a social determinant of health: a systematic mapping review of research in low incidence countries. Int J Infect Dis. 2017;56:90–100. doi: 10.1016/j.ijid.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 52.Kerrigan D, West N, Tudor C, et al. Improving active case finding for tuberculosis in South Africa: informing innovative implementation approaches in the context of the Kharitode trial through formative research. Health Res Policy Syst. 2017;15:42. doi: 10.1186/s12961-017-0206-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shete PB, Nalugwa T, Farr K, et al. Feasibility of a streamlined tuberculosis diagnosis and treatment initiation strategy. Int J Tuberc Lung Dis. 2017;21:746–752. doi: 10.5588/ijtld.16.0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Armstrong-Hough M, Ggita J, Turimumahoro P, et al. “Something so hard”: a mixed-methods study of home sputum collection for tuberculosis contact investigation in Uganda. Int J Tuberc Lung Dis. 2018;22 doi: 10.5588/ijtld.18.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thornton RLJ, Glover CM, Cené CW, Glik DC, Henderson JA, Williams DR. Evaluating strategies for reducing health disparities by addressing the social determinants of health. Health Aff (Millwood) 2016;35 doi: 10.1377/hlthaff.2015.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schaible UE, Kaufmann SHE. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4:e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Black RE, Victora CG, Walker SP, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 58.Bastagli F, Hagen-Zanker J, Harman L, Barca V, Sturge G, Schmidt T. The impact of cash transfers: a review of the evidence from low- and middle-income countries. J Soc Policy. 2019;48:569–594. [Google Scholar]
- 59.McGuire F, Vijayasingham L, Vassall A, et al. Financing intersectoral action for health: a systematic review of co-financing models. Global Health. 2019;15:1–18. doi: 10.1186/s12992-019-0513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]