Abstract
Background
Emerging SARS-CoV-2 variants and evidence of waning vaccine efficacy present substantial obstacles towards controlling the COVID-19 pandemic. Booster doses of SARS-CoV-2 vaccines might address these concerns by amplifying and broadening the immune responses seen with initial vaccination regimens. We aimed to assess the immunogenicity and safety of a homologous booster dose of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373).
Methods
This secondary analysis of a phase 2, randomised study assessed a single booster dose of a SARS-CoV-2 recombinant spike protein vaccine with Matrix-M adjuvant (NVX-CoV2373) in healthy adults aged 18–84 years, recruited from 17 clinical centres in the USA and Australia. Eligible participants had a BMI of 17–35 kg/m2 and, for women, were heterosexually inactive or using contraception. Participants who had a history of SARS-CoV or SARS-CoV-2, confirmed diagnosis of COVID-19, serious chronic medical conditions, or were pregnant or breastfeeding were excluded. Approximately 6 months following their primary two-dose vaccination series (administered day 0 and day 21), participants who received placebo for their primary vaccination series received a placebo booster (group A) and participants who received NVX-CoV2373 for their primary vaccination series (group B) were randomly assigned (1:1) again, via centralised interactive response technology system, to receive either placebo (group B1) or a single booster dose of NVX-CoV2373 (5 μg SARS-CoV-2 rS with 50 μg Matrix-M adjuvant; group B2) via intramuscular injection; randomisation was stratified by age and study site. Vaccinations were administered by designated site personnel who were masked to treatment assignment, and participants and other site staff were also masked. Administration personnel also assessed the outcome. The primary endpoints are safety (unsolicited adverse events) and reactogenicity (solicited local and systemic) events and immunogenicity (serum IgG antibody concentrations for the SARS-CoV-2 rS protein antigen) assessed 14 days after the primary vaccination series (day 35) and 28 days following booster (day 217). Safety was analysed in all participants in groups A, B1, and B2, according to the treatment received; immunogenicity was analysed in the per-protocol population (ie, participants in groups A, B1, and B2) who received all assigned doses and who did not test SARS-CoV-2-positive or received an authorised vaccine, analysed according to treatment assignment). This trial is registered with ClinicalTrials.gov, NCT04368988.
Findings
1610 participants were screened from Aug 24, 2020, to Sept 25, 2020. 1282 participants were enrolled, of whom 173 were assigned again to placebo (group A), 106 were re-randomised to NVX-CoV2373–placebo (group B1), and 104 were re-randomised to NVX-CoV2373–NVX-CoV2373 (group B2); after accounting for exclusions and incorrect administration, 172 participants in group A, 102 in group B1, and 105 in group B2 were analysed for safety. Following the active booster, the proportion of participants with available data reporting local (80 [82%] of 97 participants had any adverse event; 13 [13%] had a grade ≥3 event) and systemic (75 [77%] of 98 participants had any adverse event; 15 [15%] had a grade ≥3 event) reactions was higher than after primary vaccination (175 [70%] of 250 participants had any local adverse event, 13 [5%] had a grade ≥3 event; 132 [53%] of 250 had any systemic adverse event, 14 [6%] had a grade ≥3 event). Local and systemic events were transient in nature (median duration 1·0–2·5 days). In the per-protocol immunogenicity population at day 217 (167 participants in group A, 101 participants in group B1, 101 participants in group B2), IgG geometric mean titres (GMT) had increased by 4·7-fold and MN50 GMT by 4·1-fold for the ancestral SARS-CoV-2 strain compared with the day 35 titres.
Interpretation
Administration of a booster dose of NVX-CoV2373 resulted in an incremental increase in reactogenicity. For both the prototype strain and all variants evaluated, immune responses following the booster were similar to or higher than those associated with high levels of efficacy in phase 3 studies of the vaccine. These data support the use of NVX-CoV2373 in booster programmes.
Funding
Novavax and the Coalition for Epidemic Preparedness Innovations.
Introduction
The COVID-19 pandemic began shortly after the emergence of the novel SARS-CoV-2 in Wuhan, China, in December, 2019. Since then, SARS-CoV-2 transmission has continued, resulting in millions of deaths globally. Multiple vaccines produced using various technologies have been developed to confer immunity against the ancestral SARS-CoV-2 spike (S) protein (eg, mRNA-1273 [Moderna], BNT162b2 [Pfizer–BioNTech], AD26.COV2.S [Johnson & Johnson], and ChAdOx1 nCoV-19 [AstraZeneca]), with approximately 11 billion doses administered to date.1 Although clinical studies and real-world evidence have shown that these vaccines are highly effective in reducing severe disease and death due to COVID-19, there are also signs that their protection for these clinical endpoints might be waning since vaccination campaigns began in December, 2020. Additionally, several SARS-CoV-2 variants have emerged with changes in the S protein, enabling them to partially avoid neutralisation in people who are vaccinated. Five such variants have been classified by WHO as variants of concern: alpha (B.1.1.7), beta (B.1.351), gamma (P.1), delta (B.1.617.2), and omicron (B.1.1.529, including sublineages). The development of variant-specific vaccines and the use of homologous vaccine booster doses are being investigated as potential avenues to bolster protection against SARS-CoV-2 and its variants.
Novavax has developed a SARS-CoV-2 recombinant S protein nanoparticle vaccine (SARS-CoV-2 rS), comprising the full-length, prefusion trimers of the ancestral SARS-CoV-2 S glycoprotein, co-formulated with a saponin-based adjuvant, Matrix-M (NVX-CoV2373), for the ancestral strain of SARS-CoV-2, which might be effective for new variants. Favourable vaccine efficacy for NVX-CoV2373 against mild, moderate, or severe COVID-19 was established in two phase 3, randomised, placebo-controlled clinical trials of healthy and medically stable adult participants using a two-dose series (two doses administered 3 weeks apart).2, 3 One trial of 15 139 participants in the UK reported a vaccine efficacy against symptomatic infection of 89·7% (95% CI 80·2–94·6) for all participants: 86·3% (71·3–93·5) for the alpha variant and 96·4% (73·8–99·5) for the ancestral strain.2 The second trial consisted of 29 582 participants in the USA and Mexico, and the resultant vaccine efficacy against symptomatic infection was 90·4% (95% CI 82·9–94·6) for all participants: 100·0% (80·8–100·0) for variants that were not classified as of concern or interest, 93·2% (83·9–97·1) for variants of concern and variants of interest, and 93·6% (81·7–97·8) for participants with the alpha variant of SARS-CoV-2.3
As part of an ongoing phase 2, randomised, placebo-controlled clinical trial of NVX-CoV2373, a single booster dose was administered to healthy adult participants approximately 6 months following their primary two-dose vaccination series.4 We aimed to compare the immunogenicity and safety data during the 28 days following the booster dose with the data from the primary vaccination series in the same participant population.
Methods
Study design
We administered a single booster dose of NVX-CoV2373 to participants as part of an ongoing phase 2, randomised, observer-blinded, placebo-controlled study of NVX-CoV2373 in 17 clinical sites across Australia and the USA. The trial protocol was approved by the Alfred Hospital Ethics Committee (Melbourne, VIC, Australia) and Advarra Central Institutional Review Board (Columbia, MD, USA) and was performed in accordance with the International Conference on Harmonisation, Good Clinical Practice guidelines. The protocol is available in the appendix (pp 10–166).
Research in context.
Evidence before this study
We searched PubMed for research articles from March 1, 2020, to May 31, 2022, using the search terms “SARS-CoV-2”, “immunogenicity”, “booster”, and “protein vaccine”, limiting the results to clinical trial data. We did not apply any language restrictions. There are few (n=2) published immunogenicity studies on SARS-CoV-2 protein vaccines. Clinical studies and real-world evidence have shown that vaccines that induce immunity against SARS-CoV-2 spike (S) protein are highly effective in reducing severe disease and death due to COVID-19. However, studies indicate that vaccine protection might wane over time, necessitating additional booster doses. A SARS-CoV-2 recombinant S protein nanoparticle vaccine coformulated with a saponin-based adjuvant Matrix-M (NVX-CoV2373) has been shown to have approximately 90% efficacy in two phase 3 studies.
Added value of this study
This study reports on the first available safety and immunogenicity data for a booster dose of NVX-CoV2373 administered approximately 6 months following the primary two-dose vaccination. Results indicate an incremental increase in reactogenicity and enhanced immune responses after booster dosing. For the prototype strain and all SARS-CoV-2 variants evaluated, immune responses following the booster were similar to or higher than those associated with high levels of efficacy in phase 3 studies of the vaccine.
Implications of all the available evidence
Maintaining robust immunity might be important for continued protection against SARS-CoV-2, particularly as infection levels remain high as new variants circulate. These data support the use of NVX-CoV2373 in booster programmes.
Participants
Healthy male and female participants aged 18–84 years were recruited from the clinical sites for enrolment in this study. Participants were eligible if they had a BMI of 17–35 kg/m2, were able to provide informed consent before enrolment, and (for female participants) agreed to be heterosexually inactive or use approved forms of contraception to prevent pregnancy.
Participants with a previous history of severe acute respiratory syndrome, confirmed diagnosis of COVID-19, serious chronic medical condition (eg, diabetes, congestive heart failure, autoimmune conditions, or malignancy), or who were being assessed for an undiagnosed illness that might lead to a new diagnosis were excluded from the study. Pregnant or breastfeeding women were also excluded. Full details of eligibility criteria are presented on ClinicalTrials.gov (NCT04368988) and in the study protocol (appendix pp 44–48).
Participants were recruited via advertisements at study sites; advertisements were approved by the investigational review boards. Written informed consent was obtained from all participants before their enrolment. Participants in the study were notified of the availability of COVID-19 vaccines shortly after the vaccines were first authorised and were reconsented at that time.
Randomisation and masking
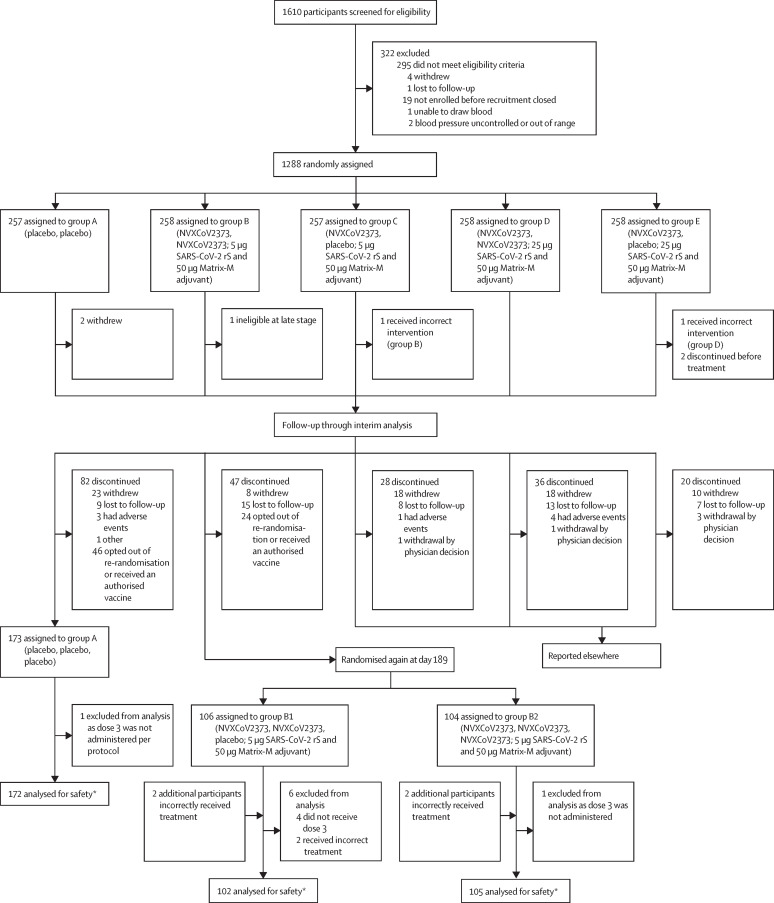
The randomisation schemes used in this trial were executed using a centralised interactive response technology system according to pre-generated randomisation schedules. Two-factor, two-level stratification was used (ie, stratified by ages 18–59 years or 60–84 years; stratified by study site). At the start of the trial, participants were randomly assigned to one of five initial treatment groups (1:1:1:1:1) as previously described by Formica and colleagues (figure 1 ).4 Of the five treatment groups, one was a placebo control (group A) and two were active vaccine groups that were considered for additional vaccination with a booster (group B and group C). As group C did not receive a second dose in the primary vaccination series, this group will not be reported herein, because this Article focuses on the primary vaccination dose regimen that is authorised globally. Group D (ie, two primary vaccination doses of 25 μg SARS-CoV-2 rS and 50 μg Matrix-M adjuvant with no booster) and group E (ie, one primary vaccination dose of 25 μg SARS-CoV-2 rS and 50 μg Matrix-M adjuvant with no booster) are not reported herein as they do not represent the dose that is authorised globally.
Figure 1.
Consort diagram for study 2019nCoV-101 (part 2): booster dosing of group B participants
Group A received two doses of placebo. Group B received two doses of NVX-CoV2373 (5 μg SARS-CoV-2 rS with 50 μg Matrix-M adjuvant). Group C received one dose of NVX-CoV2373 (5 μg SARS-CoV-2 rS with 50 μg Matrix-M adjuvant) and one dose of placebo. Group D received two doses of NVX-CoV2373 (25 μg SARS-CoV-2 rS with 50 μg Matrix-M adjuvant). Group E received one dose of NVX-CoV2373 (25 μg SARS-CoV-2 rS with 50 μg Matrix-M adjuvant) and one dose of placebo. *Patients who received three doses in the corresponding group. Patients were analysed according to treatment received.
After approximately 6 months, consenting participants from groups B and C who had been randomly assigned to receive a primary vaccination series of either two doses of NVX-CoV2373 (5 μg SARS-CoV-2 rS and 50 μg Matrix-M adjuvant) on day 0 and day 21 (group B) or one dose of NVX-CoV2373 (5 μg SARS-CoV-2 rS and 50 μg Matrix-M adjuvant) on day 0 and placebo on day 21 (group C) could continue in the study and receive a booster dose. Participants were randomly assigned again (1:1) to receive either a single booster dose of NVX-CoV2373 at the same dose (groups B2, hereafter known as NVX-CoV2373–NVX-CoV2373, and C2) or placebo (groups B1, hereafter known as NVX-CoV2373–placebo, and C1) at day 189 (appendix p 4). Participants could decline to be randomly assigned to a new group again for any reason (eg, they had already received an emergency authorised vaccine), but participants who declined were included in the safety analysis for the placebo group and the NVX-CoV2373–placebo group. After the local authorisation of a vaccine for SARS-CoV-2, participants were notified that they might be eligible to receive the vaccine. At that point, participants could be unmasked to whether they received placebo and informed that they might benefit from receiving the newly authorised vaccine (appendix p 5).
Randomisation was conducted by a central interactive response technology system that was able to maintain masked and unmasked roles. A masked team at the contract research organisation, PPD (Wilmington, NC, USA), generated the sequence, and allocation was performed by the interactive response technology system. Sites were responsible for enrolling participants and were masked to treatment allocation.
Participants who received two doses of NVX-CoV2373 (5 μg SARS-CoV-2 rS and 50 μg Matrix-M adjuvant) for their primary vaccination series and who subsequently received a single booster dose of NVX-CoV2373 (NVX-CoV2373–NVX-CoV2373 group) are the focus of this Article as they were vaccinated with the NVX-CoV2373 dose and regimen that are approved for use and commercial distribution. Participants who received two doses of placebo for their primary vaccination series and a single booster dose of placebo are included for safety comparisons (hereafter known as the placebo group). Results for group C will be reported elsewhere. All other treatment groups are continuing to be followed up for safety events only and results will be reported elsewhere.
All vaccinations were prepared on an outpatient basis by unmasked designated site personnel to maintain masking of observers. Participants and other site staff, including personnel who administered and observed outcomes, were masked to individual treatment assignment. To maintain masking, placebo vaccination via an intramuscular route was used, and personnel who were not masked managed the vaccine logistics and preparation so as to maintain masking for all other site personnel and the participants. A blue translucent tape was applied to vials by staff who were not masked to make the placebo and active booster identical in looks.
Procedures
All active booster vaccinations were administered approximately 6 months after primary vaccination schedule at the same dose as the primary vaccination series (5 μg SARS-CoV-2 rS with 50 μg Matrix-M adjuvant) via a single intramuscular injection. All booster vaccinations, active or placebo (0·9% saline), were administered at an injection volume of 0·5 mL.
Participants used an electronic diary to record reactogenicity on the day of vaccination and for an additional 6 days thereafter (ie, 7 days in total). Adverse events were graded according to the US Food and Drug Administration toxicity grading scales, as previously described.5 Blood samples for immunogenicity analysis were collected immediately before and 28 days after receipt of the booster at the sites that participants were recruited from, with safety follow-up also performed throughout the 28 days.
Measures of immune response included assays for serum IgG antibodies, neutralising antibody activity (ie, microneutralisation assay at an inhibitory concentration >50% [MN50]), and ACE2 receptor binding inhibition. Serum IgG antibody concentrations specific for the SARS-CoV-2 rS protein antigen were detected with a qualified IgG ELISA (Novavax Clinical Immunology, Gaithersburg, MD, USA) for the ancestral strain or validated IgG ELISA (Novavax Clinical Immunology, Gaithersburg, MD, USA) for the beta variant. Neutralising antibodies that were specific for SARS-CoV-2 were measured with a qualified wild Victoria strain or validated wild beta variant MN50 assay (360biolabs, Melbourne, VIC, Australia) for the ancestral strain and beta variant. Serum IgG and MN50 assay data were collected for both the ancestral and beta variant SARS-CoV-2 strains. Fit-for-purpose functional ACE2 receptor binding inhibition and anti-recombinant spike (rS) IgG activity assays were both used to analyse responses against the ancestral, beta, alpha, delta, and omicron variant strains of SARS-CoV-2. An additional microneutralisation assay (performed at the University of Maryland School of Medicine, Baltimore, MD, USA) was performed to evaluate microneutralisation at an inhibitory concentration of more than 99% (MN99). Methods were similar to those previously reported, except that VeroE6 cells overexpressing TMPRSS2 were used instead of VeroE6 cells.5
Outcomes
The primary endpoints of the phase 2 trial were comparisons of treatment regimens for the following safety and immunogenicity outcomes. The primary safety outcomes were the proportion of patients with solicited adverse events for 7 days following each primary vaccination and unsolicited adverse events up until day 35.4 The primary immunogenicity outcomes were serum IgG antibody concentrations specific for the SARS-CoV-2 rS protein antigen detected by ELISA using GMT or seroconversion rate (ie, the proportion of participants with >4-fold increases in antibody titres compared with baseline) for the two-dose regimens by dose at day 35 and have previously been reported.4
In this follow-up analysis of the phase 2 data, reported here, the key safety outcomes included participant-reported reactogenicity events for 7 days following the booster and unsolicited adverse events occurring until 28 days after booster. Solicited local and systemic reactogenicity were documented separately from one another. Data were also collected on whether an adverse event was serious, was related to vaccination, was related to COVID-19, was a potentially immune-mediated medical condition, or led to discontinuation or an unscheduled visit to a health-care practitioner (ie, medically attended AE). Key immunogenicity outcomes included serum IgG antibody concentrations for the SARS-CoV-2 rS protein antigen detected by ELISA using GMT at days 189 (immediately before administration of booster), 217 (28 days after booster), and 357 (data for day 357 will be reported separately) for treatment group B for boosting assessment with either placebo or active boost, and neutralising antibody activity at days 35, 217, and 357 (data for day 357 will be reported separately). Additional endpoints included assays to measure immune response (including ACE2 receptor binding inhibition) and immune responses to SARS-CoV-2 variants. A complete list of all prespecified endpoints can be found in the appendix (pp 95–98).
Participants who received three doses of NVX-CoV2373 (5 μg SARS-CoV-2 rS and 50 μg Matrix-M adjuvant) are the focus of this Article as they were vaccinated using the NVX-CoV2373 dose and regimen that is approved for use and commercial distribution. As such, safety and immunogenicity data are not reported here for groups C, D, and E; these data will be reported separately.
Statistical analysis
The sample size for the trial was based on clinical and practical considerations, not on a formal statistical power calculation. No multiplicity adjustments were made for this early phase study in which multiple treatment groups and endpoints were evaluated. With 150 participants in each treatment group, there was a greater than 99·9% probability of observing at least one participant with an adverse event if the true incidence of the adverse event was 5%, and a 77·9% probability if the true incidence of the adverse event was 1%. Sample size was calculated for the primary immunogenicity outcome at day 35 and was not re-estimated at second randomisation for the booster dose.
Analyses included data from participants in groups A and B obtained during and after their primary vaccination series (ie, days 0, 21, 35, 105, and 189) for comparison with data collected from the NX-CoV2373–placebo group and the NVX-CoV2373–NVX-CoV2373 group 28 days following receipt of the booster dose (day 217). Results were analysed in all participants (18–84 years) and by participant age group: age 18–59 years and age 60–84 years.
The safety analysis included all participants who received placebo for the two-dose primary vaccination series and as a booster (placebo group) and all participants who received the NVX-CoV2373 two-dose primary vaccination series and a subsequent single booster injection of NVX-CoV2373 (NVX-CoV2373–NVX-CoV2373) or placebo (NVX-CoV2373–placebo). Participants who declined randomisation at day 180 were included in the placebo group or the NVX-CoV2373–placebo group, and therefore not all participants in the safety analysis set received three doses of the placebo or NVX-CoV2373. Participants in the safety analysis set are counted according to the treatment received to accommodate for treatment errors. Safety data are presented as proportion of participants with solicited local and systemic adverse events analysed for 7 days after each vaccination and unsolicited adverse events during the 28 days following the booster. Safety data were not analysed for statistical significance.
The per-protocol analysis set was used for immunogenicity analysis and includes participants who received placebo for the two-dose primary vaccination series and as a booster (placebo group) and all participants who received the NVX-CoV2373 two-dose primary vaccination series (group B) and a subsequent single booster injection of NVX-CoV2373 (NVX-CoV2373–NVX-CoV2373). To be included in the per-protocol analysis set, participants must have received the primary vaccination series both on day 0 and day 21 (SARS-CoV-2 rS or placebo) for all analyses at day 35, and received the primary vaccination series both on day 0 and day 21 and the booster dose on day 189 for all analyses at day 217. All participants in the per-protocol analysis set were analysed according to the randomised treatment assignment. Any participant who was SARS-CoV-2-positive by qualitative PCR testing from screening and before the immunogenicity assessment for a particular timepoint was excluded from the per-protocol analysis set for that timepoint onwards. Participants who received an authorised vaccine were excluded from further immunogenicity analysis after receipt. Safety oversight for the study was provided by an independent safety monitoring committee. We used SAS (version 9.4) for statistical analysis.
This study is registered on ClinicalTrials.gov, NCT04368988.
Role of the funding source
The trial was designed by Novavax, with funding support from the Coalition for Epidemic Preparedness Innovations. Several employees of Novavax fulfilled authorship criteria and participated in the data analysis, interpretation, and writing of this report.
Results
1610 participants were screened from Aug 24, 2020, to Sept 25, 2020, and 1283 participants were enrolled and and received their assigned intervention. Of the 258 participants who were allocated to receive two doses of NVX-CoV2373 (group B), 210 completed the two-dose primary vaccination series and were then re-randomised to the NVX-CoV2373–placebo group (n=106) and the NVX-CoV2373–NVX-CoV2373 group (n=104; figure 1).
Demographics and baseline characteristics were generally balanced between the NVX-CoV2373–NVX-CoV2373 and NVX-CoV2373–placebo booster groups (table 1 ), except for a higher proportion of female participants in the NVX-CoV2373–placebo group than in the NVX-CoV2373–NVX-CoV2373 group. Across the placebo, NVX-CoV2373–placebo, and NVX-CoV2373–NVX-CoV2373 groups, the median age was 57·2 years (range 18–83 years), and 172 (45%) of 379 of participants were aged 60–84 years. Most participants were White (330 [87%]) and not Hispanic or Latino (362 [96%]). Baseline SARS-CoV-2 serostatus was predominantly negative (372 [98%] participants).
Table 1.
Demographics and baseline characteristics for the placebo, NVX-CoV2373–placebo, and NVX-CoV2373–NVX-CoV2373 groups in the safety analysis set who received three doses
| Placebo group (n=172) | NVX-CoV2373–placebo group (n=102) | NVX-CoV2373–NVX-CoV2373 group (n=105) | |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 51·9 (17·2) | 52·0 (17·0) | 51·7 (17·1) |
| Median (range) | 56·0 (18–83) | 57·5 (19–80) | 58·0 (19–82) |
| Age group | |||
| 18–59 years | 95 (55%) | 55 (54%) | 57 (54%) |
| 60–84 years | 77 (45%) | 47 (46%) | 48 (46%) |
| Sex | |||
| Male | 100 (58%) | 43 (42%) | 58 (55%) |
| Female | 72 (42%) | 59 (58%) | 47 (45%) |
| Race | |||
| White | 151 (88%) | 86 (84%) | 93 (89%) |
| Black or African American | 2 (1%) | 3 (3%) | 3 (3%) |
| Asian | 15 (9%) | 10 (10%) | 7 (7%) |
| American Indian or Alaska Native | 2 (1%) | 1 (1%) | 1 (1%) |
| Multiple | 2 (1%) | 1 (1%) | 1 (1%) |
| Missing | 0 | 1 (1%) | 0 |
| Ethnicity | |||
| Hispanic or Latino | 11 (6%) | 3 (3%) | 1 (1%) |
| Not Hispanic or Latino | 161 (94%) | 97 (95%) | 104 (99%) |
| Unknown | 0 | 2 (2%) | 0 |
| Baseline BMI, kg/m2 | |||
| Mean (SD) | 27·29 (4·207) | 26·69 (4·060) | 27·43 (4·040) |
| Median (IQR) | 27·40 (24·30–30·70) | 26·50 (24·10–29·50) | 27·10 (25·10–30·70) |
| Baseline SARS-CoV-2 status | |||
| Negative | 169 (98%) | 101 (99%) | 102 (97%) |
| Positive | 2 (1%) | 1 (1%) | 3 (3%) |
| Indeterminate | 1 (1%) | 0 | 0 |
Data are n (%), unless otherwise stated.
After emergency use authorisation of other SARS-CoV-2 vaccines, participants could be unmasked (appendix p 5). As a result, at least 119 (47%) of 254 participants in the placebo group, 34 (22%) of 153 in the NVX-CoV2373–placebo group, and six (6%) of 105 in the NVX-CoV2373–NVX-CoV2373 group who received up to three doses of their allocated intervention had receipt of an authorised vaccine recorded in the study database by May 31, 2022. Participants who received an authorised vaccine were excluded from the immunogenicity analysis.
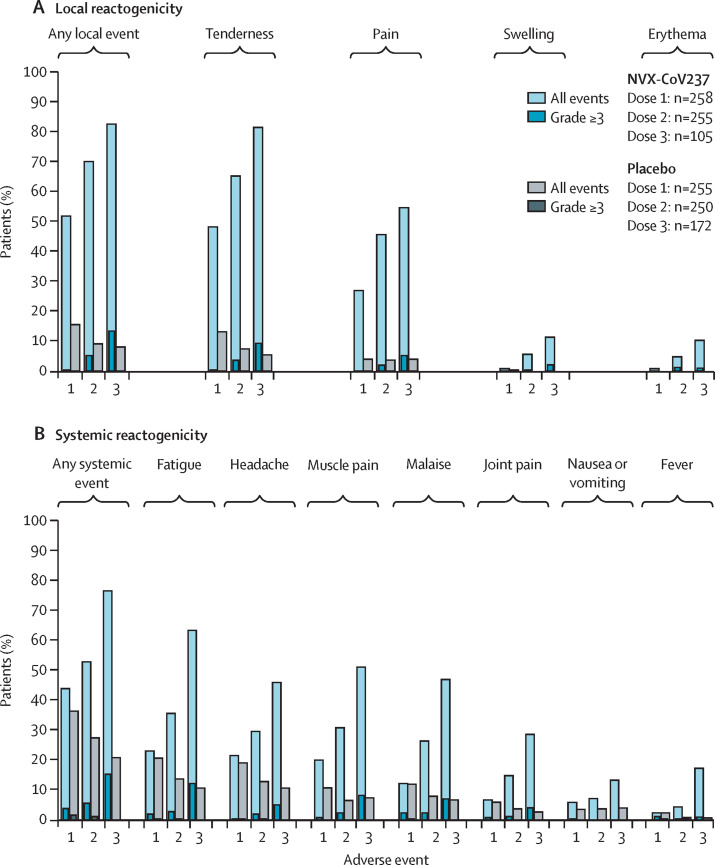
Safety reporting of solicited local and systemic reactogenicity events showed an increasing incidence after each of the three doses of NVX-CoV2373 (group B and the NVX-CoV2373–NVX-CoV2373 group; figure 2 ). By contrast, the incidence of solicited local and systemic reactogenicity events stayed the same or decreased with each dose of placebo.
Figure 2.
Local and systemic vaccine reactogenicity in NVX-CoV2373 and placebo groups by dose
Participants who received placebo were from group A. Participants who received NVX-CoV2373 were from group B (dose 1 and dose 2) and group B2 (dose 3). Proportion of participants with solicited local (A) and systemic (B) adverse events by symptom, vaccination dose, vaccine type, and maximum US Food and Drug Administration toxicity grade.
Among the participants included in the safety population, not all participants filled out their safety diary at each timepoint; therefore, the number of participants who had data available in their diary is given throughout this section. Following the NVX-CoV2373 booster, 80 (82%) of 97 participants with data available (of the 105 participants who were analysed) had local reactions (ie, tenderness, pain, swelling, and erythema) of any grade and 13 (13%) participants had local reactions of at least grade 3, whereas 175 (70%) of 250 participants with data available (of the 255 participants who were analysed) had reactions of any grade and 13 (5%) participants had events of at least grade 3 following the primary vaccination series (figure 2A). Grade 4 local reactions were rare, with two events (ie, pain and tenderness) reported by one participant after the booster compared with no participants following the primary vaccination series. Following the booster, local reactions were short lived, with a median duration of 2·0 days (IQR 1·0–3·0 for pain and swelling, 2·0–4·0 for tenderness) for all events except erythema (2·5 days [2·0–4·0]). Local reactions were also short lived following the primary vaccination series, with median durations of 2·0 days (IQR 1·0–2·0) for pain, 2·0 days (1·0–3·0) for tenderness, 2·0 days (1·0–2·0) for swelling, and 1·0 day (1·0–1·0) for erythema.
Systemic reactions showed a similar pattern with an incidence rate for any event (ie, fatigue, headache, muscle pain, malaise, joint pain, nausea or vomiting, or fever of 77% (75 of 98 participants with data available) for any grade (15% [15] grade ≥3) after booster dosing compared with 53% (132 of 250 participants with data available) for any grade (6% [14] grade ≥3) following the primary vaccination series (figure 2B). Grade 4 systemic reactions were rare, with three events reported by one participant after the booster (ie, headache, malaise, and muscle pain) compared with no participants following the primary vaccination series. Following the booster, systemic reactions were transient in nature with a median duration of 1·0 day (IQR 1·0–2·0) for all events except muscle pain, which had a duration of 2·0 days (1·0–2·0 days). All systemic reactions were also short lived following the primary vaccination series, with a median duration of 1·0 day (IQR 1·0–3·0) for all events.
Local and systemic reactogenicity events were less frequent and less severe in older adults (ie, aged 60–84 years) than in younger adults (ie, aged 18–59 years) following either the primary vaccination series or booster dose. In the younger cohort (n=57), 45 (85%) of 53 participants with data available had postbooster local reactions of any grade (ten [19%] participants had reactions of grade ≥3) and 45 (85%) participants had systemic reactions of any grade (14 [26%] participants had reactions of ≥grade 3). In the older cohort (n=48), 35 (80%) of 44 participants with data available were postbooster local reactions of any grade (three [7%] reported reactions of grade ≥3) and 30 (68%) participants reported systemic reactions of any grade (one [2%] reported reactions of grade ≥3).
Unsolicited adverse events were summarised across the participants who received an active booster (NVX-CoV2373–NVX-CoV2373 group), participants who received a placebo booster (NVX-CoV2373–placebo), and participants receiving three doses of placebo throughout the study. Participants who received less than three doses in the placebo group and the NVX-CoV2373–placebo group were also included (table 2 ). Participants who initially received active vaccine for their primary vaccination series had a higher incidence of treatment-emergent unsolicited adverse events than did those who received only placebo. A similar trend was seen for unsolicited severe adverse events.
Table 2.
Overall summary of unsolicited adverse events for placebo, NVX-CoV2373–placebo, and NVX-CoV2373–NVX-CoV2373 groups in the safety analysis set
| Placebo group | NVX-CoV2373–placebo group | NVX-CoV2373–NVX-CoV2373 group | ||
|---|---|---|---|---|
| Participants who received up to three doses* | ||||
| Any unsolicited TEAE | 92 (36%) of 254 | 65 (42%) of 153 | 48 (46%) of 105 | |
| Treatment-related | 9 (4%) of 254 | 5 (3%) of 153 | 5 (5%) of 105 | |
| Severe | 6 (2%) of 254 | 6 (4%) of 153 | 6 (6%) of 105 | |
| Leading to vaccination discontinuation | 6 (2%) of 254 | 0 | 1 (1%) of 105 | |
| Leading to study discontinuation | 3 (1%) of 254 | 0 | 0 | |
| Any serious TEAEs | 4 (2%) of 254 | 5 (3%) of 153 | 6 (6%) of 105 | |
| Treatment-related | 0 | 0 | 0 | |
| Treatment-emergent MAAEs | 59 (23%) of 254 | 40 (26%) of 153 | 32 (30%) of 105 | |
| Treatment-related | 3 (1%) of 254 | 0 | 2 (2%) of 105 | |
| PIMMC | 1 (<1%) of 254 | 0 | 1 (1%) of 105 | |
| Treatment-related | 0 | 0 | 0 | |
| AESIs relevant to COVID-19 | 0 | 0 | 0 | |
| Participants who received three doses | ||||
| Any unsolicited TEAE | 20 (12%) of 172 | 14 (14%) of 102 | 13 (12%) of 105 | |
| Treatment-related | 1 (1%) of 172 | 1 (1%) of 102 | 4 (4%) of 105 | |
| Severe | 0 | 2 (2%) of 102 | 0 | |
| Leading to vaccination discontinuation | 0 | 0 | 0 | |
| Leading to study discontinuation | 0 | 0 | 0 | |
| Any serious TEAEs | 1 (1%) of 172 | 2 (2%) of 102 | 1 (1%) of 105 | |
| Treatment-related | 0 | 0 | 0 | |
| Treatment-emergent MAAEs | 13 (8%) of 172 | 9 (9%) of 102 | 6 (6%) of 105 | |
| Treatment-related | 1 (1%) of 172 | 0 | 1 (1%) of 105 | |
| PIMMC | 0 | 0 | 0 | |
| Treatment-related | 0 | 0 | 0 | |
| AESIs relevant to COVID-19 | 0 | 0 | 0 | |
Data are n (%). Participants in the safety analysis set are counted according to the treatment received to accommodate for treatment errors. All events were reported any time from day 0 to day 217, with the exception of events during the 28 days after booster. AESI=adverse event of special interest. MAAE=medically attended adverse event. PIMMC=potentially immune-mediated medical condition. TEAE=treatment-emergent adverse event.
Participants who declined to be rerandomised were assigned to the placebo group and the NVX-CoV2373–placebo group, and therefore some participants included in these data did not receive three doses.
Overall, medically attended adverse events occurred with a slightly higher frequency in participants who received an active booster than in the placebo group, with related events reported in few participants (table 2). Events considered to be potentially immune-mediated medical conditions were rare across the study, with one participant in the NVX-CoV2373–NVX-CoV2373 group and placebo group reporting a single event each; both events were assessed as not related to study treatment. No participant reported an adverse event related to COVID-19.
The overall per-protocol immunogenicity analysis included 167 participants who received placebo, 101 participants who received NVX-CoV2373–placebo, and 101 participants who received NVX-CoV2373–NVX-CoV2373; however, the numbers for each timepoint vary because of protocol deviations and withdrawal from the study, including after receipt of an authorised vaccine (table 3 ). As expected, declines in NVX-CoV2373 IgG and MN50 geometric mean titres (GMTs) were observed following the primary vaccination series (day 35) until day 189. 28 days following the booster (day 217), IgG and MN50 titres increased robustly compared with both the prebooster titres and the day 35 titres produced by the primary series (figure 3 , table 3).
Table 3.
Serum IgG GMTs and neutralising antibody activity after primary and booster vaccination for the ancestral and beta variant SARS-CoV-2 strains by study day and age group for participants receiving NVX-CoV2373–NVX-CoV2373
|
Ancestral strain |
Beta variant |
||||
|---|---|---|---|---|---|
| Day 35 | Day 189 | Day 217 | Day 189 | Day 217 | |
| Serum IgG GMT | |||||
| All participants | 43 905 (37 500–51 403; n=242) | 6064 (4625–7952; n=85) | 204 367 (164 543–253 828; n=74) | 4317 (3261–5715; n=85) | 175 190 (139 895–219 391; n=74) |
| 18–59 years | 65 255 (55 747–76 385; n=128) | 8102 (6041–10 866; n=47) | 270 224 (214 304–340 736; n=41) | 6310 (4642–8578; n=47) | 226 103 (176 090–290 321; n=41) |
| 60–84 years | 28 137 (21 617–36 623; n=114) | 4238 (2631–6826; n=38) | 144 440 (99 617–209 431; n=33) | 2700 (1682–4333; n=38) | 127 601 (86 809–187 561; n=33) |
| MN50GMT | |||||
| All participants | 1470 (1008–2145; n=50) | 63 (49–81; n=84) | 6023 (4542–7988; n=64) | 13 (11–15; n=84) | 661 (493–886; n=65) |
| 18–59 years | 2281 (1414–3678; n=24) | 80 (56–114; n=47) | 8568 (6646–11 046; n=35) | 14 (11–18; n=47) | 871 (656–1156; n=36) |
| 60–84 years | 981 (560–1717; n=26) | 47 (33–65; n=37) | 3936 (2341–6620; n=29) | 12 (10–15; n=37) | 469 (270–816; n=29) |
Data are ELISA units (95% CI). The number of participants at each timepoint vary because of protocol deviations and withdrawal from the study. Not all participants in the per-protocol immunogenicity population had adequate samples for immunogenicity analyses, so some data are missing in this table and some participants had only one assay performed. The ancestral strain IgG assay method is qualified, and the beta variant IgG assay method is validated. GMT=geometric mean titre. MN50=microneutralisation assay at an inhibitory concentration of more than 50%.
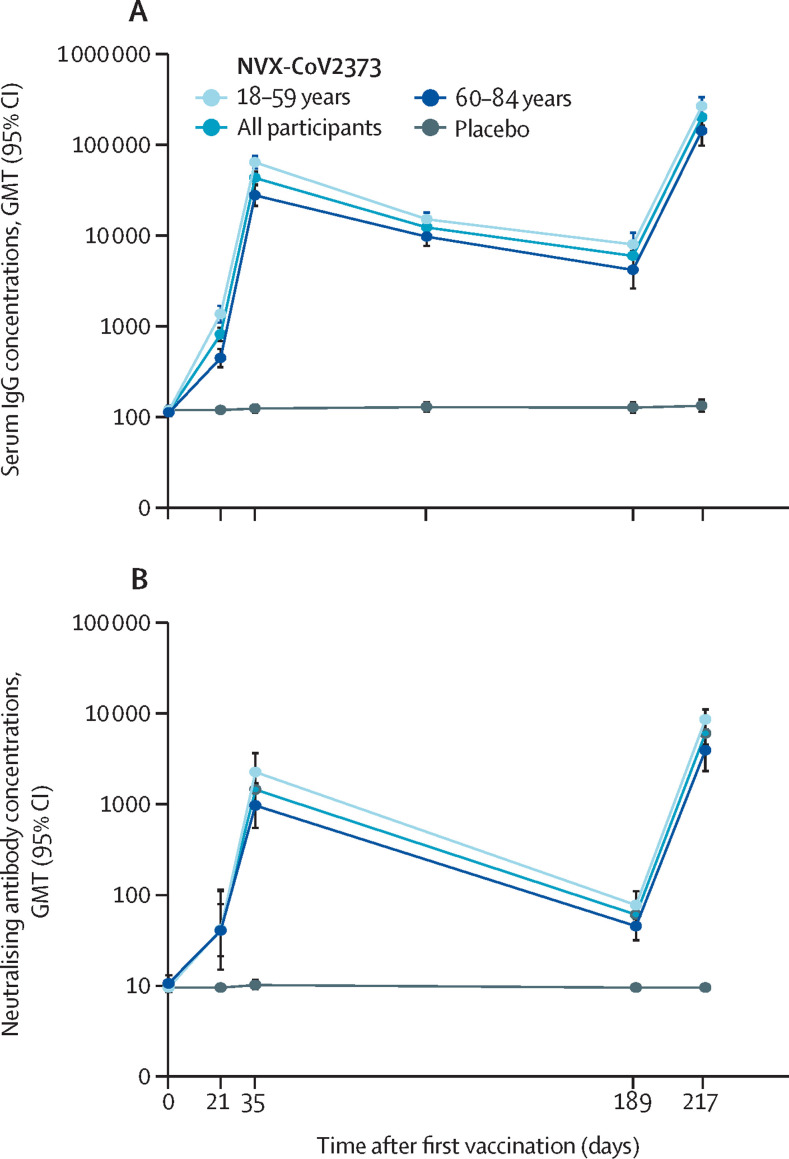
Figure 3.
Serum IgG titres and neutralising antibody activity for the ancestral SARS-CoV-2 strain by study day (log scale)
(A) Geometric mean anti-spike IgG ELISA unit responses to recombinant SARS-CoV-2 protein antigens at baseline (day 0), 3 weeks after first vaccination (day 21), 2 weeks after second vaccination (day 35), 12 weeks after second vaccination (day 105), on the day of third vaccination (day 189), and 4 weeks after third vaccination (day 217). Data are from a qualified ancestral strain IgG assay. (B) Microneutralisation antibodies showing response at an inhibitory concentration of more than 50% to recombinant SARS-CoV-2 protein antigens at baseline (day 0), 3 weeks after first vaccination (day 21), 2 weeks after second vaccination (day 35), 12 weeks after second vaccination (day 105), on the day of third vaccination (day 189), and 4 weeks after third vaccination (day 217). Values represent the GMTs and error bars represent the 95% CIs. Data are from a qualified ancestral strain microneutralisation assay. Data are shown overall and by age group in the NVX-CoV2373–NVX-CoV2373 group, and overall in the group that received three doses of placebo. GMT=geometric mean titre.
For the ancestral SARS-CoV-2 strain, serum IgG GMTs increased from day 189 (ie, prebooster) to day 217, reflecting a postbooster increase of approximately 33·7-fold (table 3). These titres were approximately 4·7-fold higher than the primary vaccination series at day 35. Fold increases after boosting were higher in older adults (ie, aged 60–84 years; 5·1-fold) than in younger adults (ie, aged 18–59 years; 4·1-fold). The ratio of serum IgG GMTs in older adults to serum IgG GMTs in younger adults was 2·32 at day 35 and 1·87 at day 217, and the neutralising antibody response ratio was 2·32 at day 35 and 2·17 at day 217. Similarly, MN50 assay GMTs specific to the ancestral SARS-CoV-2 strain increased by approximately 4·1-fold between day 35 and day 217, with a 4·0-fold increase in older adults and a 3·8-fold increase in younger adults. MN50 assay GMTs increased approximately 95·6-fold between day 189 and day 217.
For the beta variant, IgG GMTs increased from day 189 (ie, prebooster) to day 217, reflecting a postbooster increase of approximately 40·6-fold (table 3). These titres were 4·0-fold higher than those observed at day 35 for the ancestral strain. Beta variant MN50 assay data showed a similar fold increase in titres from prebooster (day 189) to post-booster (day 217) of approximately 50·8-fold, although titres were lower than those seen for the ancestral strain at day 35 (table 3).
As expected, participants who received a primary vaccination series of NVX-CoV2373 and booster dose with placebo (NVX-CoV2373–placebo group) had a slight decrease in IgG GMTs and MN50 assay GMTs from day 189 to day 217 for both the ancestral strain and the beta variant (appendix p 6).
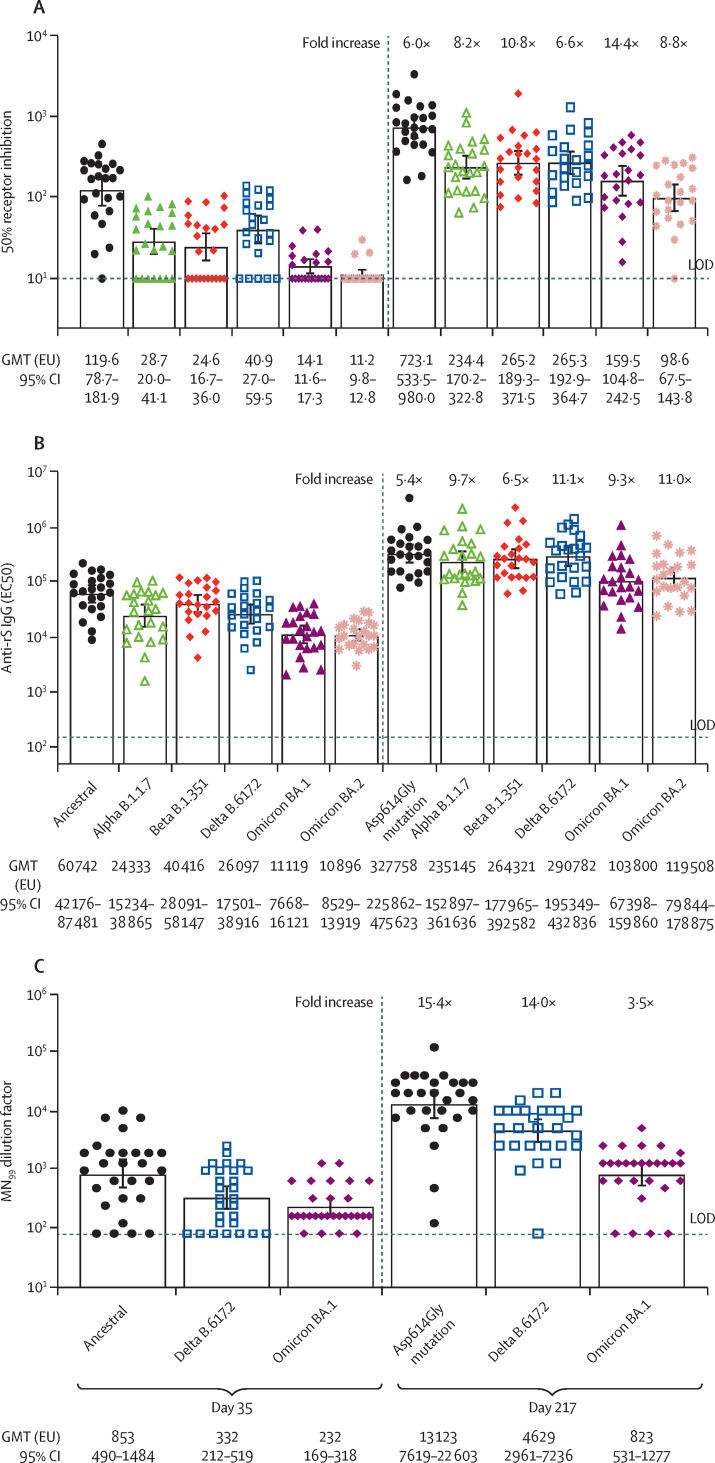
Three fit-for-purpose assays were developed to assess immune responses against additional SARS-CoV-2 variants by use of participant serum samples from the NVX-CoV2373–NVX-CoV2373 group (figure 4 ; appendix pp 7–9). A functional ACE2 receptor binding inhibition assay was used to compare activity against the ancestral strain and the alpha, beta, delta, and omicron (BA.1 and BA.2) variants of SARS-CoV-2 (figure 4A; appendix p 7). 54·4-fold (ancestral), 21·9-fold (alpha), 24·5-fold (beta), 24·4-fold (delta), and 20·0-fold (BA.1) increases in ACE2 inhibition titres were observed from day 189 (immediately prebooster; BA.2 analysis could not be completed) to day 217, and 6·0-fold (ancestral), 8·2-fold (alpha), 10·8-fold (beta), 6·6-fold (delta), 14·4-fold (BA.1) and 8·8-fold (BA.2) increases from day 35 to day 217. A second assay comparing anti-rS IgG activity across the same strains of SARS-CoV-2 identified that 61·1-fold (ancestral), 85·8-fold (alpha), 65·0-fold (beta), 92·5-fold (delta), and 73·4-fold (omicron BA.1) higher titres were observed from day 189 to day 217 and 5·4-fold (ancestral), 9·7-fold (alpha), 6·5-fold (beta), 11·1-fold (delta), 9·3-fold (BA.1), and 11·0-fold (BA.2) higher titres were observed from day 35 to day 217 (figure 4B; appendix p 8). A third assay comparing wild-type neutralisation titres (MN99) showed 15·4-fold (ancestral), 14·0-fold (delta), and 3·5-fold (omicron BA.1) higher titres after the booster (figure 4C; appendix p 9).
Figure 4.
ACE2 receptor binding inhibition, anti-rS IgG, and neutralisation titres for SARS-CoV-2 variants after primary vaccination at day 35 and after booster at day 217
(A) ACE2 receptor binding assay results showing 50% inhibition at day 35 and after booster at day 217 for the ancestral and variants including alpha, beta, delta, and omicron (BA.1 and BA.2). (B) Geometric mean anti-spike IgG ELISA unit responses to recombinant SARS-CoV-2 protein antigens after primary vaccination (day 35) and after booster (day 217) for ancestral and variants including alpha, beta, delta, and omicron (BA.1 and BA.2). (C) Geometric mean microneutralisation antibodies showing response at an inhibitory concentration higher than 99% to recombinant SARS-CoV-2 protein antigens after primary vaccination (day 35) and after booster (day 217) for ancestral and variants including delta and omicron (BA.1). Bars represent the GMT and error bars represent 95% CIs. Fold increase from day 35 to day 217 is indicated above the day 217 graphs. Assay methodology is fit for purpose. Anti-rS IgG=anti-recombinant spike IgG antibody. EC50=50% effective concentration. EU=ELISA units. GMT=geometric mean titres. LOD=limit of detection. MN99=microneutralisation at inhibitory concentration higher than 99%.
Discussion
In this Article, we describe the first available safety and immunogenicity data for a booster dose of NVX-CoV2373 in the context of an ongoing phase 2, randomised, observer-masked, placebo-controlled study. Administration of a single booster dose of the vaccine approximately 6 months following the primary two-dose series resulted in an incremental increase in reactogenicity events along with significantly enhanced immunogenicity.
Before boosting at day 189, anti-SARS-CoV-2 antibody titres in immunised participants were notably lower than in samples taken after the primary vaccination series at day 35 (NVX-CoV2373 group IgG GMTs decreased from 43 905 ELISA units [EU] to 6064 EU and MN50 GMTs decreased from 1470 EU to 63 EU). Correlating falling antibody titres in individuals immunised or previously exposed to SARS-CoV-2 with waning immunity can be complex, although there is established evidence that the presence of neutralising antibodies is strongly indicative of protection against symptomatic COVID-19. Further, vaccines that have been shown to generate high neutralising antibody titres (eg, NVX-CoV2373, mRNA-1273, BNT162b2, and Sputnik V) have shown higher vaccine efficacy in clinical trials than those associated with lower titres (eg, ChAdOx1 nCoV-19, Ad26.COV2.S, and CoronaVac).6, 7
Maintaining robust immunity might be especially important in the presence of SARS-CoV-2 variants that can cause breakthrough infections. Other mechanisms for maintaining long-term immunity among multiple variants could involve mixing vaccines with heterologous boosters or using vaccine technologies that result in broadly cross-reacting immunity, as observed with an adjuvanted recombinant nanoparticle influenza vaccine.6, 8
In our study, antibody responses to the booster were assessed for the ancestral vaccine strain and SARS-CoV-2 variants, including alpha, beta, delta, and omicron (BA.1 and BA.2). For the ancestral strain, IgG titres at day 217 were approximately 33·7-fold higher than the prebooster day 189 titres, whereas neutralising antibody titres increased approximately 95·6-fold after the booster. Both IgG and microneutralisation titres after the booster were more than 4-fold higher than after the primary two-dose series at day 35, which is notable because the day 35 titres corresponded to high levels of clinical efficacy in both a phase 3 study in the UK (89·7%) and a phase 3 study in the USA and Mexico (90·4%).2, 3
When data for the ancestral strain were broken down by age group, serum IgG and neutralising antibody concentrations were similar between age groups, although antibody responses in older adults (ie, aged 60–84 years) were slightly lower than those in younger adults (ie, aged 18–59 years).
For the beta variant, 40–50-fold increases in IgG and microneutralisation antibody titres were seen following the booster, and IgG titres were approximately 4·0-fold higher than those seen for the ancestral strain after the primary vaccination series. Unlike IgG, MN50 GMTs for the beta variant were lower following the booster than those for the ancestral strain following the primary vaccination series (GMT 661 EU vs 1470 EU), in alignment with the known decreased neutralising responses for this variant. Although the worldwide prevalence of the beta variant has decreased to less than 1% from a high of 8% in April, 2021, antibody responses to this variant are of interest due to the Glu484Lys mutation found in this variant. Glu484Lys is responsible for marked decreases in neutralisation titres for vaccines, monoclonal antibodies, and convalescent serum samples, and the mutation exists in the gamma, P.2, and the mu variants of SARS-CoV-2.9, 10
For the delta and omicron variants of SARS-CoV-2, 24·4-fold (delta) and 20·0-fold (omicron [BA.1]) increases in functional ACE2 receptor binding inhibition titres were identified when comparing the postbooster titres from day 217 to day 189 titres.9 Anti-rS IgG activity compared at these same timepoints showed 92·5-fold (delta) and 73·4-fold (omicron [BA.1]) higher titres associated with the booster. Microneutralisation (MN99) titres increased, although to a lesser degree, at 14·0-fold (delta) and 3·5-fold (omicron [BA.1]). When comparing postbooster titres (day 217) to titres after the primary vaccination series (day 35), the fold increase for omicron BA.2 data were similar to those for BA.1.
These findings of significantly increased antibody titres following boosting are important as they come during a time when SARS-CoV-2 vaccine booster doses are being widely considered or implemented by many countries to counteract the waning antibody titres and decreased effectiveness of approved vaccines.6 The continued high levels of SARS-CoV-2 circulation combined with increasing immunological pressure could also give rise to new escape variants; increasing individuals' titres to these variants through use of a booster dose might extend protection in previously vaccinated individuals and combat the spread of SARS-CoV-2 variants.
The incidence of both local and systemic reactogenicity was higher following the 6-month booster dose than following previous doses, reflecting the increased immunogenicity seen with the third dose. However, the incidence of grade 3 or worse events was low, with only fatigue (12 [12%] of 98 participants) being reported by greater than 10% of participants. In total, five grade 4 (ie, potentially life threatening) solicited local and systemic adverse events were reported. All five of these events (ie, pain, tenderness, headache, malaise, and muscle pain) were reported by the same participant in the active booster group concurrently with an adverse event of drug hypersensitivity related to the vaccine. The drug hypersensitivity event was assessed as mild in severity. The participant did not seek any medical attention for this event, and all the participant's symptoms resolved over a period of 6 days.
Although the relative magnitude of the reactogenicity rates are difficult to compare across studies, the proportion of participants who had a reactogenicity event after a third dose of NVX-CoV2373 appears to be similar to that after a booster dose of the BNT162b2 and mRNA-1273 vaccines and somewhat elevated compared with the ChAdOx1 nCoV-19 vaccine.11, 12, 13 Reactogenicity after vaccination might be an indicator of humoral response as suggested by others;14, 15 however, we did not assess for a link between reactogenicity and immunogenicity in our study. Although the incidence of unsolicited adverse events was higher in vaccine recipients (48 [46%] of 105 participants) than in placebo recipients (92 [36%] of 254 participants), all events were classified as mild or moderate in severity. Medically attended adverse events, potentially immune-mediated medical conditions, and serious adverse events occurred infrequently following the booster dose and were balanced between vaccine and placebo groups.
Our study was subject to some limitations. As these results are from an ongoing phase 2 study with a small sample size, clinical efficacy of the booster dose was not evaluated. Additionally, between-strain comparisons of antibody data should be made with caution, as correlations with clinical efficacy might vary for each variant of COVID-19. Because of the rapidly changing environment of SARS-CoV-2 variants, microneutralisation titres were not available for the omicron BA.2 variant; however, we were able to assess responses to this variant using a functional ACE2 receptor inhibition assay. Additionally, although evidence suggests an important role for memory B cells, T cells, and other immune mechanisms in long-term immunogenicity against SARS-CoV-2,16 our study focused on serum antibody responses, which have been shown to correlate with protection.7, 17 Future studies assessing the effect of NVX-CoV2373 on other mediators of immunity are warranted.
Overall, a single booster dose of NVX-CoV2373 administered approximately 6 months after the primary series induced substantial increases in humoral antibodies for both the prototype strain and all evaluated variants that were similar to or higher than those associated with high levels of efficacy in phase 3 studies of the vaccine. These findings, together with the acceptable safety profile, support use of the vaccine in booster programmes.
Data sharing
The trial protocol was a part of the peer-review process and will be included with the published manuscript. Data will be shared at the required timelines to https://clinicaltrials.gov/ct2/show/NCT04368988. Additional requests will be considered on publication of the manuscript by request to the corresponding author.
Declaration of interests
RMM, SP, BW, AM, GA, HM, MR, JSP, MZ, SC-C, BZ, GC, AR, SM, GS, NP, and GMG are Novavax employees and as such receive a salary for their work. NF is a consultant contractor providing clinical development support. AM provided medical writing support. MBF, HLH, LB, and JL are supported for these studies through funding by Novavax. MBF is also on the scientific advisory board of Aikido Pharma. The Frieman Laboratory has received unrelated funding support in sponsored research agreements from AstraZeneca Pharmaceuticals, Regeneron, Pfizer, Emergent Biosolutions, and Aikido Pharma.
Acknowledgments
Acknowledgments
We thank the study participants who volunteered for this study. This study was funded by Novavax and the Coalition for Epidemic Preparedness Innovations. Graphics and editorial assistance for this manuscript were provided by Kelly Cameron (Ashfield MedComms, USA, an Inizio company), supported by Novavax.
Contributors
RMM, MBF, BW, AR, SP, GA, GMG, NF, JSP, and MZ were involved in the study design. HLH, LB, JL, BW, BZ, NP, SCC, SM, HM, and SP were involved in data collection. AR, MBF, and GC performed the statistical analyses. RMM, MBF, BW, GS, NP, GMG, NF, JSP, and MZ were involved in data interpretation. MR was involved in project administration and supervision. RMM and MBF verified the data. RMM, AM, and MBF wrote the first draft. All authors reviewed, commented on, and approved this manuscript before submission. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Contributor Information
Novavax 2019nCoV101 Study Group:
Mark Adams, Mark Arya, Eugene Athan, Ira Berger, Paul Bradley, Toby Briskin, Richard Glover II, Paul Griffin, Joshua Kim, Scott Kitchener, Terry Klein, Amber Leah, Indika Leelasena, Charlotte Lemech, Jason Lickliter, Mary Beth Manning, Fiona Napier-Flood, Paul Nugent, Susan Thackwray, and Mark Turner
Supplementary Material
References
- 1.Our World in Data Coronavirus (COVID-19) vaccinations. https://ourworldindata.org/covid-vaccinations
- 2.Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. N Engl J Med. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunkle LM, Kotloff KL, Gay CL, et al. Efficacy and safety of NVX-CoV2373 in adults in the United States and Mexico. N Engl J Med. 2022;386:531–543. doi: 10.1056/NEJMoa2116185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Formica N, Mallory R, Albert G, et al. Different dose regimens of a SARS-CoV-2 recombinant spike protein vaccine (NVX-CoV2373) in younger and older adults: a phase 2 randomized placebo-controlled trial. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keech C, Albert G, Cho I, et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamady A, Lee J, Loboda ZA. Waning antibody responses in COVID-19: what can we learn from the analysis of other coronaviruses? Infection. 2022;50:11–25. doi: 10.1007/s15010-021-01664-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 8.Shinde V, Cai R, Plested J, et al. Induction of cross-reactive hemagglutination inhibiting antibody and polyfunctional CD4+ T-cell responses by a recombinant matrix-M-adjuvanted hemagglutinin nanoparticle influenza vaccine. Clin Infect Dis. 2021;73:e4278–e4287. doi: 10.1093/cid/ciaa1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nextstrain Genomic epidemiology of SARS-CoV-2 with subsampling focused globally over the past 6 months. https://nextstrain.org/ncov/gisaid/global
- 10.Harvey WT, Carabelli AM, Jackson B, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaxman A, Marchevsky NG, Jenkin D, et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: a substudy of two randomised controlled trials (COV001 and COV002) Lancet. 2021;398:981–990. doi: 10.1016/S0140-6736(21)01699-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi A, Koch M, Wu K, et al. Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis. Nat Med. 2021;27:2025–2031. doi: 10.1038/s41591-021-01527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration Application for licensure of a booster dose for COMINARTY (COVID-19 vaccine, mRNA).BNT162b2: evaluation of a booster dose (third dose) Sept 17, 2021. https://www.fda.gov/media/152176/download
- 14.Lim SY, Kim JY, Park S, et al. Correlation between reactogenicity and immunogenicity after the ChAdOx1 nCoV-19 and BNT162b2 mRNA vaccination. Immune Netw. 2021;21:e41. doi: 10.4110/in.2021.21.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bauernfeind S, Salzberger B, Hitzenbichler F, et al. Association between reactogenicity and immunogenicity after vaccination with BNT162b2. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Earle KA, Ambrosino DM, Fiore-Gartland A, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine. 2021;39:4423–4428. doi: 10.1016/j.vaccine.2021.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The trial protocol was a part of the peer-review process and will be included with the published manuscript. Data will be shared at the required timelines to https://clinicaltrials.gov/ct2/show/NCT04368988. Additional requests will be considered on publication of the manuscript by request to the corresponding author.