Abstract
Purpose
This systematic review endeavors to find an effective treatment protocol for subacute thyroiditis (SAT) to minimize side effects, recurrence and long-term hypothyroidism.
Materials and Methods
We analyzed available original studies on treatment protocols for SAT. A thorough literature search was performed on the following online databases PubMed, Cochrane library nd Google Scholar using appropriate keywords for choosing relevant articles. Two reviewers assessed the methodological quality of selected articles independently using a critical appraisal instrument. The results were analyzed and synthesized qualitatively using the level of evidence method.
Results
The literature search retrieved a total of 460 publications after abstract screening; out of which 36 articles met the inclusion criteria. After full text screening, 23 articles were further excluded as they were focusing on aspects of SAT other than management, the remaining 15 articles were investigated for both reliability and validity. Thirteen studies provided low-quality evidence, and two randomized control trials (RCT) provided a high quality of evidence. Steroid therapy was found to be the most effective for moderate to severe SAT and provided relief from acute symptoms but was found to not be a risk factor for recurrence. Low initial doses of steroid (15 mg) were preferred over high initial dosage (30–40 mg). Furthermore, a look into the mode of steroid delivery (RCT) revealed that intrathyroidal steroid therapy can potentially become a safer and faster mode of therapy. The duration of tapering was found to be of significance as a short tapering period was linked with greater recurrence rates.
Conclusion
Low initial doses of steroid along with an extended tapering period may help lower recurrence rates; also, intrathyroidal steroid injections are potentially a better alternative to oral prednisone (PSN) with regard to safety and speed of action. However, the evidence is of moderate quality and further investigation is required.
Keywords: subacute thyroiditis, de Quervain thyroiditis, painful thyroiditis, steroid, NSAIDS, recurrence
Video abstract

Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Subacute thyroiditis (SAT) (also called de Quervain thyroiditis or granulomatous thyroiditis) is a self-limited, inflammatory thyroid disorder of a possible viral origin associated commonly with pain in the thyroid and various systemic symptoms.1
SAT is a common cause of thyrotoxicosis and is the most common painful thyroid disease. Clinical signs associated with SAT include: a frequent history of recent viral illness; enlarged and firm thyroid gland; tenderness; thyrotoxicosis; referred pain to the parietal regions, occipital regions and the ears, jaw, or the throat; raised erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels; and decreased radioactive Iodine (I) uptake. SAT may also present with mild anemia, diffuse heterogeneous ultrasonography (USG) changes, and occasionally decreased color Doppler blood flow signals.3 The recent pandemic involving the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) has shown to be a strong etiological factor in the development of subacute thyroiditis, with some studies showing close to 18.8% of all SAT cases were due to SARS-CoV2.4
SARS-CoV2 induced SAT is generally found to have slightly higher severity with regards to clinical presentation and lab findings compared to non-SARS-Cov2 patients with SAT in predisposed individuals.5
No definitive curative treatment for SAT exists, but there are effective treatments that will ameliorate symptoms and allow the disease to run its course as an asymptomatic condition. Corticosteroids have proved to be extremely valuable in the treatment of most SAT patients. They have a rapid clinical response, with many cases reporting total relief of symptoms within 24 hours. However, the most effective treatment protocol for SAT is not well defined. In this systematic review we have gone through all the relevant original articles on the management of SAT published between January 2000 and May 2022 in order to determine the most effective treatment protocol for SAT.
Materials and Methods
Search Strategy
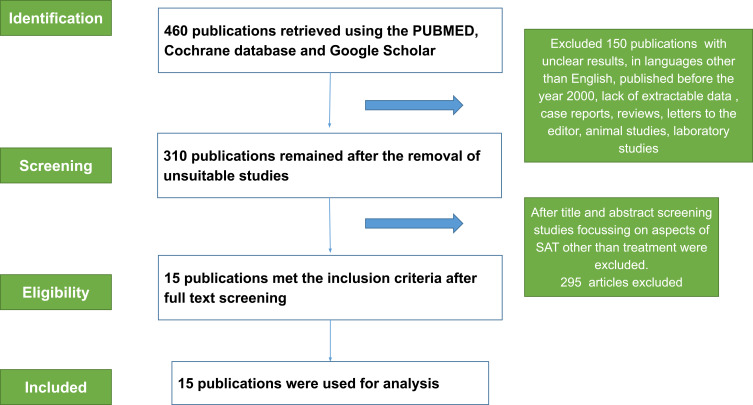
To investigate a treatment protocol for SAT a systematic literature search was performed. The latest edition of the PRISMA statement was adhered to, and both the checklist and flow diagram (Figure 1) are listed.6 The Google search engine was used to search relevant articles in PubMed, PubMed Central, Cochrane database and Google Scholar using the following keywords: “SAT”, “thyroiditis”, “De Quervain thyroiditis”, “painful thyroiditis”, “Prednisone (PSN)”, “NSAIDS”, “Prednisolone” (PSL), “recurrence”, “hypothyroidism” combined with “And” and “In”. The search language was English, and the search time was from the year 2000 to May 2022. Once the search was completed, the articles were manually screened with the following criteria: the articles must be in English, must be from within the past 22 years, a full text version of the articles must be available and only human studies were included.
Figure 1.
PRISMA diagram.
Notes: PRISMA figure adapted from Moher D, Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.
Abbreviation: SAT, subacute thyroiditis.
Inclusion and Exclusion Criteria
Original clinical studies/trials published in the English language, studies with human subjects, studies using appropriate statistical tools for data analysis, recent studies published between 2000 and 2022, studies reporting the effectiveness of various treatment protocols for SAT along with changes in laboratory parameters like TSH, ESR, CRP, T4 and T3 before treatment and after follow up along with clear demonstration of recurrence rates and long term hypothyroidism rates post therapy were included in our systematic review. Studies with unclear results, in languages other than English, published before the year 2000, which lacked extractable data regarding treatment protocols for SAT, case reports, systematic reviews, literature reviews, letters to the editor, animal studies, laboratory studies, articles without SAT/subacute granulomatous thyroiditis/de Quervain thyroiditis in the title name, articles that focused on other aspects of SAT unrelated to the management of SAT, and studies with inaccessible full text versions were excluded. Descriptive data was extracted; these included physical examination, laboratory tests, radio-imaging and cytological confirmation, dosage and outcome of therapy along with recurrence rate and evidence of long-term hypothyroidism.
Data Extraction
Two reviewers I. Ray and B. D’Souza independently reviewed titles and extracts. Any titles or extracts that were not clearly relevant to the purpose of this study were discarded. In cases of uncertainty of eligibility of a study title or abstract the full text was reviewed. Articles that were considered relevant were included and reviewed after initial screening under primary search strategy. Any disagreements were resolved through discussion with a third assessor. The included articles also had their references manually searched and those references that fit the inclusion criteria were also included under secondary search strategy, however; this was only done once and subsequent additions did not have their reference lists searched. The data was extracted and recorded on a table in Microsoft Excel: the first author, year of publication, origin/country, and aim of the study, study design, study sample and valid information for evaluating the reliability and validity of the treatment protocol for SAT, recurrence rate and long-term hypothyroidism were analyzed.
Quality Assessment
The methodological quality of each selected article was assessed using the criteria proposed by Brink et al.7 The Brink criteria includes 13 items scored “Yes”, “No” or “n/a” (Table 1). Two reviewers used the instrument to assess the methodological quality of each article independently and any disagreement was resolved through discussion. A score of ≥60% was considered as high quality (Table 2).
Table 1.
Methodological Quality of Selected Studies
| Criteria | Hepsen S16 | Fatourechi V18 | Duan L9 | Mizukoshi T13 | Sato J12 | Zhao N15 | Arao T14 | Sencar ME17 | Li F19 | Kubota S11 | Saklamaz A20 | Benbassat CA21 | Forkert IO10 | Martinez DS23 | Bahadir ÇT22 | Quality of Study Score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | If human subjects were used, did the author give a detailed description of the sample of subjects used to perform the index test? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| 2 | Did the authors clarify the qualification, or competence of the raters who performed the index test? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| 3 | Was the reference standard explained? | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| 4 | If inter rater ability was tested, were raters blinded to the findings of other raters? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| 5 | If intra rater reliability was tested, were raters blinded to their own prior findings of the test under evaluation? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| 6 | Was the order of examination varied? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| 7 | If human subjects were used, was the time period between the reference standard and the index test short enough to be reasonably sure that the target condition did not change between the two tests? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| 8 | Was the stability (or theoretical stability) of the variable being measured taken into account when determining the suitability of the time interval between repeated measures? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| 9 | Was the reference standard independent of the index test? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| 10 | Was the execution of the (index) test described in sufficient detail to permit replication of the test? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| 11 | Was the execution of the reference standard described in sufficient detail to permit its replication? | Yes | No | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| 12 | Were withdrawals from the study explained? | NA | No | Yes | No | No | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
| 13 | Were the statistical methods appropriate for the purpose of the study? | NA | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Moderate |
Table 2.
Level of Evidence
| Level of evidence | Number of studies with the corresponding level of evidence |
|---|---|
| Strong | Consistent findings among ≥3 high-quality studies |
| Moderate | Consistent findings among ≥1 high-quality studies and ≥1 low-quality studies |
| Limited | Findings from ≥1 low-quality studies or only 1 study irrespective of study quality |
| Conflicting | Inconsistent findings among ≥2 studies irrespective of study quality |
| No evidence | No studies found |
Data Analysis
A table was made in MS Word including the 15 studies with columns indicating the study, its aim and model, diagnostic criteria, therapy, outcome followed by results and conclusions and was assessed qualitatively through the levels of evidence approach adapted from Tulder et al.8
Results
Articles Selection
The flow diagram (Figure 1) based on the PRISMA guidelines presents the process of reviewing the articles followed by the authors.
Methodological Quality of Selected Articles
As shown in Table 1, level of evidence for all 13 criteria, according to the quality assessment model suggested by Brink et al,6 was of moderate quality as findings were consistent amongst 2 high-quality studies (randomized controlled trial - RCT) and 13 low-quality studies (retrospective and prospective cohorts) which was according to the level of evidence approach adapted by Tulder et al8 as depicted in Table 2.
Characteristics of Selected Articles
The 15 articles selected were published from 2000 to 2022, from China (3 studies), Japan (4 studies), USA (2 studies), Israel (1 study), Ukraine (1 study) and Turkey (4 studies) out of which 11 articles were retrospective and 4 articles were prospective. 2 out of the 4 prospective studies were RCTs, one compared the efficacy of a short term 2 week combination treatment for SAT vs a traditional 6 week treatment with PSN, the other RCT compared the efficiency of intrathyroidal steroid injection to oral PSN in SAT patients.
The following are the conclusions from the 15 studies analyzed in this systematic review:-
Duan et al reported that the short term treatment of SAT with PSN in combination with NSAIDS for 2 weeks had a lower frequency of side effects compared to long term treatment for moderate to severe SAT.9 Intrathyroidal steroid therapy had a shorter duration of disease, with faster results and no side effects when compared with oral, PSN, albeit with further studies required.10 Low initial doses of PSL like 15 mg/day may help prevent long term steroid related side effects compared to traditional doses of 30–40 mg/day as per Kubota et al11 and Sato et al.12 An extended tapering period of PSN therapy to 5 mg/day has been shown to reduce recurrence by Mizukoshi et al and Arao et al,13,14 SAT leads to a lower thyroid gland volume in the study by Zhao et al.15 With regards to treatment response and hypothyroidism rate there is essentially no stark difference between low dose steroid therapy (16 mg methylprednisolone) and high dose steroid therapy (48 mg methylprednisolone), suggesting low dose steroid therapy sufficient for the treatment of SAT.16 The presence of anti TPO antibodies and ibuprofen treatment were found to be independent risk factors for permanent hypothyroidism by Sencar et al.17 Fatourechi et al found steroid therapy more effective in providing relief from acute symptoms with no effect on recurrence or long term hypothyroidism.18 Newer adjuvant therapeutic modalities Prunella vulgaris (PV) need to be explored.19 There seems to be no causal relationship between the development of permanent hypothyroidism and the different treatment options used in SAT.20 No difference in outcome is observed between the treated group and untreated group of SAT. Although corticosteroids shorten the duration of the disease but with no difference in duration of hyperthyroidism, highest TSH level and peak FT4 levels were observed when compared to NSAID.21
Choice of treatment modality has shown to be an independent risk factor in the recurrence of SAT, as patients with steroids had a higher rate of recurrence of the disease when compared to those under NSAID treatment. Patients who have had mild thyrotoxicosis also had a higher rate of recurrences of SAT.22 Sodium ipodate and sodium iopanoate therapy for SAT with hyperthyroidism has shown effectiveness in controlling hyperthyroidism symptoms, with reduced recurrence of SAT which occurs often when the steroid is tapered.23
The following were the limitations of the 15 studies analyzed in this systematic review: The small sample size was a limitation reported by authors of all the 15 studies. Lack of multicenter clinical trials, mild cases of SAT not seeking medical care leading to underreported case numbers and not accounting for the side effects of other drugs being given alongside the target therapy were some other cited limitations.9,18 The retrospective nature of studies leads to the probability of higher rates of recurrence and permanent hypothyroidism in patients treated with steroids was due to the more initial damage to thyroid follicles compared to moderate cases not given steroids.18 Intrathyroidal steroid therapy needs further evaluation with different population groups and different body masses.10 The absence of any large scale studies examining colchicine and Prunella vulgaris in SAT.19,24
Reliability and Validity
Table 1 presents the accumulated level of evidence for efficacy treatment protocols of SAT and incidence of recurrence and long term hypothyroidism. A moderate level of evidence was found for the effective treatment of SAT and the incidence of long term hypothyroidism. The strength of the level of evidence was in the RCTs which were of high quality due to high reliability and validity and the weaknesses were when the level of evidence was of low quality due to the low reliability and validity of the other 2 prospective and 11 retrospective cohorts according to the quality appraisal model by Brink et al6 and limited number of articles available.
Discussion
Subacute granulomatous thyroiditis (SAT) or de Quervain thyroiditis is the most common cause of painful thyroiditis. The disease is known to have a viral origin.25 The pathognomic feature is the destruction of the thyroid follicular structure due to an influx of acute inflammatory cells and histiocytes producing pseudo giant cells. In the early disease, the serums T4 and T3 are both elevated. There is a rise in plasma thyroglobulin and ESR.26 The 24 hour iodine or technetium-99 uptake of the thyroid gland is low due to the destruction of parenchyma. A hypoechoic, irregular lesion in the thyroid lobe is seen on thyroid USG.15 In many patients three distinct phases of the disease are seen. The hyperthyroid phase of SAT continues till the gland gets depleted of preformed colloid. Lack of biosynthesis of new thyroid hormone leads to a transient hypothyroid phase. As recovery occurs, the iodine uptake of the thyroid gland begins to rise gradually, eventually restoring the thyroid function to normal with permanent hypothyroidism in some cases.26
Brancatella et al conducted a retrospective, cross-sectional, observational study from January 2016 to December 2020 where 312 subjects were studied in Italy to study the effect of COVID-19 on the annual timing and severity of SAT cases. In the second and fourth quarters of 2020, higher levels of free thyroxine (FT4), C-reactive protein (CRP), and thyroglobulin (Tg) were observed in SAT cases compared with the same quarter of the years 2016–2019 where most of the SAT cases were observed in the third quarter of the year only. This study shows that the advent of COVID-19 increased the annual occurrence and also altered the timing and frequency of the annual peaks of SAT cases when compared to SAT occurrence in a pre COVID era.5
The primary aim of treatment of SAT is to decrease pain effectively along with minimal side effects, followed by a reduction in frequency of relapse and permanent hypothyroidism.
NSAIDS have shown success in patients with milder or moderate forms of SAT. PSN or a similar analogue in the class of corticosteroids, are considered effective in more severe cases of SAT due to their immunosuppressant properties.26 The American Thyroid Association recommends 40 mg/day of PSN for the treatment of moderate subacute thyroiditis, leading to improvement of the initial symptoms within 72 hours.3,7 Due to the adrenal suppression by use of exogenous high dose corticosteroid, the dosage is then gradually tapered over a period of 6 weeks followed by its complete cessation.3 The Chinese guidelines recommend initiation with 20–40 mg/day PSN for 1–2 weeks and then slow reduction of dose according to symptoms, signs and levels of ESR. The usual course lasts for 6–8 weeks but 20% of patients require therapy for longer than 8 weeks due to the relapse of symptoms.11 SAT shows an unpredictable clinical course not being affected by clinical features and treatment regimen. Intrathyroidal steroid has shown superiority over oral steroids with regards to efficacy and safety and limiting the disease duration, but further studies on a wide range of populations are needed to prove their validity.10 No definite treatment protocol is yet available for SAT and treatment regimens followed vary substantially among various published articles.2
Clinical and Research Implications
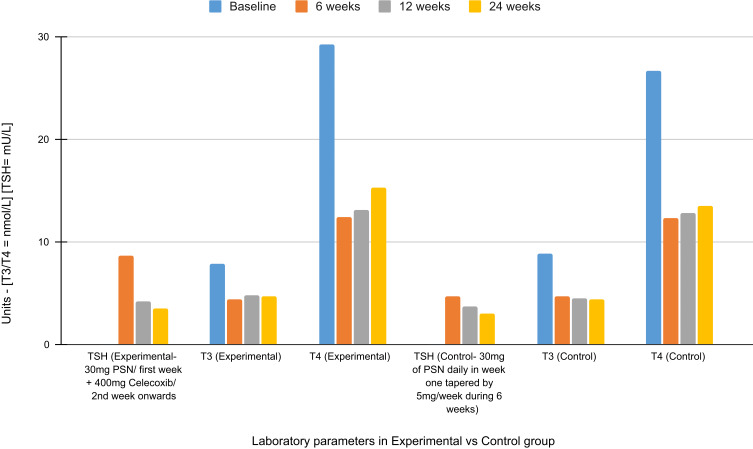
In this Systematic Review we have thoroughly gone through all relevant published articles on the treatment of SAT and tried to determine an efficient treatment protocol for the same Table 3. An RCT conducted by Duan et al, compared the safety and efficacy of short-term vs 6 week PSN therapy for treating moderate-to-severe symptoms of SAT due to the severe side effects associated with long term steroid therapy. The experimental group was treated with 30 mg PSN for 1 week and for the 2nd week onwards they received 400 mg celecoxib for the next 7 days before discontinuing celecoxib treatment. The control group received 30 mg of PSN daily during week one tapered by 5 mg/week during the 6 week treatment. The primary endpoint or efficacy and recurrence rates were not statistically different between the two groups. The secondary endpoints. namely differences between levels of various laboratory parameters in the two groups (Figure 2), were also not significantly different. They concluded the study by stating that short term glucocorticoid therapy might be an effective and safe alternative treatment for SAT.9
Table 3.
Table Depicting the Study Model, Therapy for Subacute Thyroidits, Therapy Outcome and Results of the Fifteen Articles Analyzed in This Systematic Review
| S. No | Study | Aim of study | Study model | Therapy | Outcome of therapy | Post therapy R [R] and prognosis | Results and conclusions |
|---|---|---|---|---|---|---|---|
| 1 | Sencar ME, PUBL 2019, ANKARA, (TURKEY).17 | Differentiate b/w therapeutic effects of steroids and NSAIDs in SAT. | Retrospective cohort study, Jan 2014 and Sept 2018. Sample size was 217. |
1) Ibuprofen (NSAID) Pt No.: 126 Dose: 1800 mg in 3 doses Duration: 14 d 2) Methyl PSL (Steroid) Pt No.: 91 Dose: 48 mg in 2 doses. Tapering: 8 mg for 1st 4 wks and 4 mg for last wk Duration: 6 wks |
Mean Rx duration for NSAID shorter than PSL as nonresponsive NSAID grp were shifted to steroids | NSAID: R in 7 Pts. PH in 9 Pts. Methyl PSL: R in 21 Pts. PH in 6 pts. |
NSAIDs: less than 50% efficacy in pts. With ↑ ESR & ↑ CRP. PSL therapy Important in SAT pts. With Anti-TPO, ↑ ESR &↑ CRP to induce R & prevent PH. The total PH rate was 12.4%. This was seen to be linked to Anti-TPO Ab & Ibuprofen Rx. (p:0.03 and p:0.04) |
| 2 | Fatourechi V, PUBL 2003, OLMSTEAD COUNTY, MINNESOTA (USA)18 | Determine the incidence of transient hypothyroidism and PH post Rx of SAT with NSAIDs and PSL alone or in combination along with the incidence of R. | Retrospective cohort study sample size: 94 [1960 to 1997] | 1) NSAIDs alone: Pt no: 39 Mean duration: 35d 2) NSAIDs alone or with PSL: Pt No. 57 Dose: 40 mg PSL (mean dose) for 7 d tapering for 34 d (median) 3) PSL alone: Pt No.: 15 Dose: 40 mg PSL for 7 d tapering for 34 d 4) PSL alone or with other Rx Pt No.: 34 Dose: 40 mg PSL for 7 d followed by tapering for 34d 5) Acetaminophen Pt No.: 21 Duration:35 d 6) Thyroidectomy: 1 Pt. |
NA | 1) No difference in transient hypothyroidism during the early phase b/w PSL vs NSAID vs BOTH grp. 1) Early R 10% of pts. 2) Early hypothyroidism.: 34% 3) Late R 4% of pts. 4) Late hypothyroidism: 15% of pts. 5) Nodular goitre was seen in 5% of pts. 6) 5% a/w autoimmune disease 7) 11% of pts a/w non-thy cancers. |
There is a ↑ incidence of transient hypothyroidism in the 1st year post Rx (34%) The PSL Rx grp had a ↑ rate of hypoth. on long-term follow-up (P < 0.05). |
| 3 | Duan L, PUBL. 2020 CHONGQING, CHINA9 | Short term vs 6 wk PSN in the Rx of SAT: Randomised Control Trial (RCT). | RCT. Prospective single blind RCT Duration 24 wks Pts AUG 2013 to DEC 2014, Sample size: 50. |
Randomly assigned to short-term (EXP grp) or 6-wk (CONTROL grp) 1) EXP GRP WK 1: 20 mg and 10mg PSN in morn and noon daily. WK 2: 400 mg celecoxib on d 1 and 200 mg twice daily for the next 6d CONTROL GRP: WK 1: 20mg and 10 mg PSN in morn and noon daily. WK 2: Taper by 5mg/wk from 2nd wk until withdrawal in 6th wk. All pts. given PPIS |
Efficacy defined as no pain in thy, no clinical Sy, normalization of ESR and CRP. R: development of goitre with Tenderness, fever, and ↑ of markers during PSN tapering or at Rx end Primary end points: no sig difference in two grps in rates of efficacy and R. |
R: - EXP Grp 3 pts had R during Rx and 4 after Rx. CONTROL Grp: 2 pts R During RX and 4 pts After RX. MANAGEMENT OF R: 10 mg PSN in morn and 10 mg in noon. Incidence of overt and SUBCLINICAL HYPOTHY: At diagnosis 86% During follow up 61% in both grps. |
The Study demonstrated that both grps had similar rates of effects. The short-term Rx had fewer s/e eg Markers of bone turnover and BP. Short-term Rx with a may be an optional strategy for moderate-to-severe SAT. |
| 4 | Sato J, PUBL 2016, TOKYO, JAPAN12 | Diff. b/w Rx effects of PSL and NSAIDs in SAT. | Retrospective study b/w 2008–2014. Sample size: 42 |
25 pts with PSL. dose of PSL was 15 mg 17 pts NSAIDs. loxoprofen at 180 mg/d |
Time for normalisation of thy function: PSL GRP Mean – 25 d NSAIDs Mean 32 d Time for disappearance of initial sy PSL grp. Mean: 7 d NSAIDs grp Mean 21 d |
R within two months of stopping drug PSL grp 3 pts. NSAIDs grp 1 pt No difference in the R rate b/w the two grps (p = 0.635). |
PSL not only shortens the duration of resolution of sy but also reduces the time to normalisation of thy function |
| 5 | Kubota S, PUBL: 2013, KOBE, JAPAN.11 | 1) Determine if Initial Rx with 15 mg PSN daily is sufficient for most SAT pts. | Prospective Cohort Study SAMPLNG b/w Feb 2005 - Jan 2008. Final sample size: 219. |
Initial dose: 15 mg/d of PSL. Tapering: 5 mg every 2 wks Duration: variable. Follow up every 2w, |
SHORT MED. GRP.113 Recovered within 6wks. LONG MED. GRP: Recovered in 12wks or more. 61pts in 7–8 wks Longest RX: 40 wks: 7pts needed increased PSL dose: |
The Rx protocol had 15 mg/d of PSL as the initial dose for SAT, with tapering by 5mg every 2 wks, and was effective and safe for Japanese pts. However, 20% of pts. with SAT needed longer than 8 wks to recover from the inflammation. | |
| 6 | LI F PUBL CHINA, 201819 | To retrospectively analyse the effects and safety of PV extract and low-dose PSL in SAT | Retrospective cohort study Sept 2013 -May 2016 sample size: 87 |
The control grp= PSL 20 mg/dy as initial dose tapering 5mg every 3 wks The experimental grp. = 10 mg/dy of PSL+ 1.4 g/dy PV tapering of 5mg every 3 wks |
GRP. 1 – PSL ONLY R Rate (6months): 4 (9.5%) Incidence of transient hypothyroidism: 2 (4.8%) GRP. 2 – PSL WITH PV R Rate (6months): 2 (4.4%) Incidence of transient hypothy: 1 (2.2%) |
The results have shown that the new combination of PSL and PV alleviated fever, pain, thy swelling more efficiently and had a lower R rate. This could be a result of the immunosuppressive and antiviral effect of PV. |
|
| 7 | ARAO T, PUBL. 2015, JAPAN14 | To determine the PSL Dosing Regimen for Rx of SAT | Retrospective cohort study 26 pts. Jan 2004 - July 2013 |
The non-R grp and The R Grp both received PSN doses b/w 15 mg/dy to 30 mg/dy Analysis of the 2 grp showed no differences in the initial PSL dose. |
R was diag. in 4 out of 26 pts. With an estimated R rate of 15.3% |
4 pts. experienced R at The time when the PSL dose was 5 mg/dy. The No. of dys required tapering the PSL dose to 5 mg/dy was sigly shorter in the R grp |
The SAT R rate was 15.3%. There was no sig difference in the initial PSL dose b/w the non-R and R grps. However, for the primary endpoint, sig differences were found b/w the two grps in time required for tapering PSL to 5 mg/dy (non-R: 44.3 ± 15.3 dys, R: 19.0 ± 11.9 dys, |
| 8 | Mizukoshi T 2001, PUBL, JAPAN13 | To confirm the R rate of PSL and to compare the findings b/w R and non-R grps. | Retrospective cohort study b/w Jan 1997 to Dec 1998 Sample Size - 36 |
PSL was administered at 30 mg or 25 mg daily, and tap. by 5 mg per wk, for 5 or 6 wks | Most pts. Became euthyroid by the second wk after the initial presentation of thyrotoxicosis. | Among 36 pts. 8 recurred (22%) | The R rate of SAT with treated PSL is about 20%. A modified protocol should be further investigated to decrease the R of SAT |
| 9 | ZHAO N, PUBL. 2020, SHENYANG (CHINA)15 | To explore the early indicators of hypothyroidism and the final changes in thy Volume in SAT pts. | Prospective cohort study Sample size: 61 |
NSAIDS: 46 pts. PSL: 6 pts, NSAID± PSL: 7 No therapy: 2 |
1 out of 13 pts. On PSL Rx had hypothyroidism, 19 of48 pts. who did not receive PSL had hypothyroidism |
Pts. With clinical hypothy. And subclinical hypothy. after the acute SAT received LT4 therapy (dose 12.5 or 25 µg/dy) |
In conclusion, during the 2 years of follow-up, the gland volume of SAT pts. especially with hypothy, were smaller than those of healthy controls after the acute stage of the disease. |
| 10 | Saklamaz A. 2017, PUBL, TURKEY20 | To compare the treatment options on the development of permanent hypothyroidism in SAT patients. | Retrospective cohort study from medical records of sat between 2010 to 2015 Sample size:81 |
Patients classified into treatment groups: NSAID (n = 33) Steroid (n = 29) NSAID+steroid (n = 19) and 1 year outcome noted |
All patients had tsh level return back to baseline. No permanent hypothy. Seen in either of the 3 treatment groups. | Treatment drug option did not affect the permanent hypothyroidism one year after in our SAT patients. | |
| 11 | Benbassat CA 2007 PUBL ISRAEL21 | To identify predictive factors of clinical outcome of SAT | Retrospective case series between 1999 to 2005. Sample size 56 pts. | 10 pts.received no treatment, 43 pts.received treatment {NSAID (n = 25), steroids) n = 18)} 3 pts. were missing | Hypothy. Phase documented in 31 pts. Mean duration of disease was 77 days. Shorter duration of disease in pts. Given glucocorticoids when compared with NSAIDS. But short-term outcome between treated and untreated remains the same. |
6 pts. Remained permanently hypothy. Peak FT4 level was positively correlated with highest TSH level and disease duration. |
SAT follows and unpredictable clinical course hardly affected by clinical features or Rx |
| 12 | Forkert IO. 2021, PUBL Ukraine10 | To compare the efficiency of intrathyroidal steroid injection with respect to oral steroid intake in SAT patients | RCT Between 2019–2021 Sample size 32 patients |
32 pts. Randomly divided into 2 equal groups of 16. 1st grp received 2 intrathyroid steroid injection,2nd grp got oral PSL | Pts in grp 1 showed faster USG result compared to grp 2, a ↑ ESR, CRP drop at corresponding weeks measured.no side effects were noted in grp 1. 2nd grp experienced side effects like weight gain (n = 6), glucose intolerance (n = 5), HTN (n = 4), irregular menses (n = 2) | All 32 patients recovered fully and no incidence of PH or R | Intrathyroid steroid therapy is safer faster and better tolerated by pt.s when compared with oral PSL. |
| 13 | Hepsen S 2021 PUBL Turkey16 | To evaluate difference between low dose and high dose steroid treatment | Retrospective observation study for 1 year, a total of 91 patients included | 44 patients received 16 mg methylprednisolone (low dose) 47 patients received 48 mg methylprednisolone dose (high dose) |
Recovery achieved in all patients Treatment duration needed to be extended for 1 (2.1%) and 6 (13.6%) of the patients in the 48-mg and 16-mg Methylprednisolone groups, respectively.48-mg Methylprednisolone group had a higher SAT recurrence rate than the 16-mg group. |
Permanent Hypothyroidism developed in 5 (10.6%) of patients in the 48-mg MPS and 3 (6.8%) in the 16-mg MPS group | Low dose steroid therapy is sufficient to achieve complete therapy. No difference is observed with respect to treatment response and hypothyroidism rates between high and low dose steroid groups. High dose steroid group have a higher recurrence rate of SAT. |
| 14 | Bahadir ÇT 2022 PUBL Turkey22 | Aimed to evaluate the factors affecting recurrence of SAT especially with regards to treatment | Retrospective study, includes 137 patients from jan 2008 to jan 2020 | 72 patients received steroid therapy while 65 patients had received NSAIDS According to patient records |
Risk of recurrence was higher in those with steroid therapy (p = 0.015) | Sixty-five (47.4%) patients were treated with NSAIDs, and 72 (52.6%) patients were treated with steroids. Of 137 patients, 12 (8.8%) had recurrence and the remaining 125 (91.2%) patients had | The risk of SAT recurrence was higher with steroid therapy than with NSAIDs. Patients who had mild thyrotoxicosis need a longer duration of treatment as they had higher recurrence rates |
| 15 | Martinez DS, 2003 PUBL USA23 | To evaluate the effects of using sodium ipodate and sodium iopanoate as treatment for SAT | Retrospective study with 10 patients with SAT | 10 SAT patients received sodium ipodate and sodium iopanoate | Hyperthyroidism symptoms controlled and improved without any evidence of relapse of SAT after withdrawal of cholecystography agents. | No side effects of treatment with oral cholecystography agents. | sodium ipodate and sodium iopanoate are safe and effective in treatment of hyperthyroidism symptoms in SAT and prevent relapse of SAT |
Abbreviations: SAT, subacute thyroiditis; b/w, between; No., number; PUBL, published; NSAIDS, non-steroidal anti-inflammatory drugs; pts, patients; pt, patient; ESR, erythrocyte sedimentation rate; CRP, C reactive protein; Thy, thyroid; USG, ultrasonography; Sy, symptoms; wks, weeks; wk, week; grp, group; grps, groups; PSL, prednisolone; PSN, prednisone; Rx, treatment; d, days; R, recurrence; PH, post therapy hypothyroidism; Anti TPO, anti thyroid peroxidase; Ab, antibody; I, iodine; Jan, January; Sept, September; Phy, physical; Lab, laboratory; FT4, free thyroxine; FT3, free triiodothyronine; TSH, thyroid stimulating hormone; hypoth., hypothyroidism; a/w, associated with; ↑, high; ↓, low; PSN, prednisone; EXP, experimental; morn, morning; BP, blood pressure; NA, not applicable; PV, Prunella vulgaris; sig, significant; effect, effectiveness; s/e, side effects; HTN, hypertension; MED, medication.
Figure 2.
Short term vs 6-week treatment for subacute thyroiditis. Figure 2 demonstrates the progressive change in laboratory parameters in patients with SAT over a period of 6 weeks, 12 weeks and 24 weeks from the start of therapy in experimental group receiving a short-term steroid therapy [30 mg PSN for 1 week and 400 mg celecoxib for 7 days (from week 2)] vs a control group receiving 30 mg of PSN daily during week one tapered by 5 mg/week during the 6 week treatment in an RCT conducted by Duan and colleagues. Data from Duan et al.9
Abbreviations: SAT, subacute thyroiditis; Rx, therapy; TSH, thyroid stimulating hormone; T3, triiodothyronine; T4, thyroxine; PSN, prednisone; RCT, randomized controlled trial; SAT, subacute thyroiditis.
Forkert et al conducted an RCT study, the aim of which was to compare the efficiency of intrathyroidal steroid therapy to oral PSN therapy in SAT patients. This included 32 SAT patients with ineffective non-steroidal anti-inflammatory drug (NSAID) response divided into two groups. The 1st group received 2 intrathyroidal steroid injections while the 2nd group received only oral steroid (PSN). Ultrasound was used to evaluate treatment results along with ESR and CRP at different weeks of the treatment. Patients in the first group showed a faster response, shorter duration of disease and no side effects when compared to second group which showed side effects such as weight gain, glucose intolerance, HTN, and irregular menses. However, owing to the small sample size of only 32, no correction for body weight was performed and further research needs to be done to explore this treatment modality.10 A study by Sencar et al tried to evaluate steroid and NSAID treatments in SAT in relation to persistent hypothyroidism and recurrence. They observed that many non-responsive patients in the NSAID group had to be shifted to steroids hence the mean duration therapy was shorter for NSAIDs. Steroids were not found to be a risk factor for recurrence. TPO antibodies and ibuprofen treatment were found to be independent risk factors for hypothyroidism.17 Mizukoshi et al speculated that recurrence usually occurs when PSL dose is being lowered and 10 mg daily PSL is the break point for recurrence. Hence they suggest that extending the duration of 10 mg/day PSL therapy will decrease the recurrence rate.13 A similar study by Arao et al shows that a relatively long tapering period for PSL to 5 mg/day is crucial to reduce SAT recurrence.14
In a study done by Fatourechi et al it was seen that steroid therapy works well in mitigating symptoms but does not prevent hypothyroidism.18
Kubota et al aimed to determine a suitable dose of PSL for treating SAT among Japanese patients. Instead of a high initial doses of PSL (25–40 mg), like that used traditionally, Kubota et al used a 15 mg initial dose.11,13,18 Results showed that 15 mg/day of PSL followed by tapering by 5 mg every 2 weeks was an effective therapy for Japanese patients which may vary according to ethnic groups and weight of the patients.11
Junko Sato et al conducted a study to compare NSAIDS and PSL and used a low initial dose of PSL (15 mg/day) to treat SAT. The time taken for normalization of thyroid dysfunction was similar in the PSL and NSAID group but the time taken for the disappearance of initial symptoms was significantly shorter in the PSL group.12
There are some studies which have tried to explore new alternative treatment modalities or adjuvant therapies for SAT. Colchicine plays an important role in anti-inflammatory action as it inhibits phagocytosis in neutrophils.27 Martinez conducted a study using two oral cholecystography agents (sodium ipodate and sodium iopanoate) and observed a reduction in hyperthyroidism symptoms and recurrence rate of SAT, even in patients who previously relapsed from steroid treatment.23 Li et al conducted a study using PV (Prunella vulgaris) along with PSL for SAT and observed that PV reduced the PSL dose needed to treat SAT thereby reducing steroid related side effects.19 A retrospective study conducted by Bahadir which included a total of 137 patients, 72 of which had received steroids while the rest had received NSAIDs, concluded a significantly increased recurrence rate of SAT in the steroid treatment group and also in those who have mild thyrotoxicosis.22 Benbassat conducted a study to identify the predictive factors on the clinical outcome of 56 patients with SAT, of which 10 were given no treatment, 43 received treatment (NSAID in 25) (glucocorticoid in 18), while data for 3 patients was missing. The study revealed no difference in outcome between the treatment and non-treatment groups.21 Hepsen conducted a retrospective observational study comprising of 91 patients divided into a low dose steroid (16 mg PSN) group (n = 44) and a high dose steroid (48 mg PSN) group (n = 47). The study concluded that the low dose steroid therapy was sufficient to achieve complete recovery and there is no significant difference between the two groups with respect to hypothyroidism rates and treatment response. A higher steroid dose was associated with significant recurrent rates of SAT.16 The retrospective study conducted by Saklamaz compared the treatment options for SAT with permanent hypothyroidism by dividing 81 patients into steroid group, NSAID group and combined group for one year and concluded that drug treatment had no role in the development of post SAT permanent hypothyroidism.20
Strengths and Limitations
The strengths of the present review are its systematic nature, the use of studies that included different effective treatment modalities for SAT, the inclusion of 2 RCTs; all 15 studies included were human studies and were recently published from 2000–2022. However, as in all systematic reviews, the results are dependent on the studies included.
The inclusion of only two RCTs with high-level evidence and the small sample size of the other 13 low-evidence studies can be cited as the first limitation of this review. Moreover, of the two RCTs one study in particular related to intrathyroidal steroid injection therapy had many limitations with regards to sample size, population groups, lack of adjustment of dose with body mass to surface area and in the overall quality of the study protocol. Lastly, most of the original articles included were conducted in Asian countries like Japan and China and the proper correction for body weight was not performed. Hence, these results cannot be directly extrapolated to people of other races like Caucasian and African origin races.10
Conclusion
Steroid therapy for SAT was observed to be more effective for mitigating acute symptoms compared to NSAIDS. Short-term 2-week steroid therapy in combination with celecoxib can be an effective treatment modality for SAT compared to traditional long-term steroid therapy with various side effects. An initial low dose of PSN can be an effective treatment in mild to moderate cases and only severe patients and patients who had relapsed should be given higher steroid doses. Furthermore, a study proposed giving patients intrathyroidal steroid injections; however this theory needs further assessment through studies with a larger sample size. We conclude by saying that more large scale, double blinded multicenter trials are needed to explore short-term steroid- NSAID combination therapy for SAT along with use of colchicine and PV in SAT therapy. Moreover, since most of the current studies were done on Chinese or Japanese individuals with smaller body masses, we need to conduct further studies on the management of SAT on Caucasian and African origin individuals so that a proper correction for body weight can be performed giving us a broader view of management of SAT taking into account all related factors and data.
Funding Statement
There is no funding to report.
Disclosure
The authors report no conflict of interest in work.
References
- 1.Ross DS, Burch HB, Cooper DS, et al. 2016 American thyroid association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis [published correction appears in Thyroid. 2017 Nov;27(11):1462]. Thyroid. 2016;26(10):1343–1421. doi: 10.1089/thy.2016.0229 [DOI] [PubMed] [Google Scholar]
- 2.Stasiak M, Lewiński A. New aspects in the pathogenesis and management of subacute thyroiditis. Rev Endocr Metab Disord. 2021;22(4):1027–1039. doi: 10.1007/s11154-021-09648-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross DS. Syndromes of thyrotoxicosis with low radioactive iodine uptake. Endocrinol Metab Clin North Am. 1998;27(1):169–185. doi: 10.1016/s0889-8529(05)70305-4 [DOI] [PubMed] [Google Scholar]
- 4.Bahçecioğlu AB, Karahan ZC, Aydoğan BI, Kalkan IA, Azap A, Erdoğan MF. Subacute thyroiditis during the COVID-19 pandemic: a prospective study. J Endocrinol Invest. 2022;45(4):865–874. doi: 10.1007/s40618-021-01718-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brancatella A, Viola N, Rutigliano G, Sgrò D, Santini F, Latrofa F. Subacute thyroiditis during the SARS-CoV-2 pandemic. J Endocr Soc. 2021;5(10):bvab130. doi: 10.1210/jendso/bvab130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brink Y, Louw QA. Clinical instruments: reliability and validity critical appraisal. J Eval Clin Pract. 2012;18(6):1126–1132. doi: 10.1111/j.1365-2753.2011.01707.x [DOI] [PubMed] [Google Scholar]
- 8.van Tulder M, Furlan A, Bombardier C, Bouter L; Editorial Board of the Cochrane Collaboration Back Review Group. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine. 2003;28(12):1290–1299. doi: 10.1097/01.BRS.0000065484.95996.AF [DOI] [PubMed] [Google Scholar]
- 9.Duan L, Feng X, Zhang R, et al. Short-term versus 6-week prednisone in the treatment of subacute thyroiditis: a randomized controlled trial. Endocr Pract. 2020;26(8):900–908. doi: 10.4158/EP-2020-0096 [DOI] [PubMed] [Google Scholar]
- 10.Forkert IO, Melekhovets OK, Kalynychenko DO, Melekhovets YV, Kovalenko EL. Painful subacute thyroiditis treatment approach. Wiad Lek. 2021;74(8):1921–1924. doi: 10.36740/WLek202108125 [DOI] [PubMed] [Google Scholar]
- 11.Kubota S, Nishihara E, Kudo T, Ito M, Amino N, Miyauchi A. Initial treatment with 15 mg of prednisolone daily is sufficient for most patients with subacute thyroiditis in Japan. Thyroid. 2013;23(3):269–272. doi: 10.1089/thy.2012.0459 [DOI] [PubMed] [Google Scholar]
- 12.Sato J, Uchida T, Komiya K, et al. Comparison of the therapeutic effects of prednisolone and nonsteroidal anti-inflammatory drugs in patients with subacute thyroiditis. Endocrine. 2017;55(1):209–214. doi: 10.1007/s12020-016-1122-3 [DOI] [PubMed] [Google Scholar]
- 13.Mizukoshi T, Noguchi S, Murakami T, Futata T, Yamashita H. Evaluation of recurrence in 36 subacute thyroiditis patients managed with prednisolone. Intern Med. 2001;40(4):292–295. doi: 10.2169/internalmedicine.40.292 [DOI] [PubMed] [Google Scholar]
- 14.Arao T, Okada Y, Torimoto K, et al. Prednisolone dosing regimen for treatment of subacute thyroiditis. J UOEH. 2015;37(2):103–110. doi: 10.7888/juoeh.37.103 [DOI] [PubMed] [Google Scholar]
- 15.Zhao N, Wang S, Cui XJ, et al. Two-years prospective follow-up study of subacute thyroiditis. Front Endocrinol (Lausanne). 2020;11:47. doi: 10.3389/fendo.2020.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hepsen S, Akhanli P, Sencar ME, et al. The evaluation of low- and high-dose steroid treatments in subacute thyroiditis: a retrospective observational study. Endocr Pract. 2021;27(6):594–600. doi: 10.1016/j.eprac.2020.11.009 [DOI] [PubMed] [Google Scholar]
- 17.Sencar ME, Calapkulu M, Sakiz D, et al. An evaluation of the results of the steroid and non-steroidal anti-inflammatory drug treatments in subacute thyroiditis in relation to persistent hypothyroidism and recurrence. Sci Rep. 2019;9(1):16899. doi: 10.1038/s41598-019-53475-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fatourechi V, Aniszewski JP, Fatourechi GZ, Atkinson EJ, Jacobsen SJ. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab. 2003;88(5):2100–2105. doi: 10.1210/jc.2002-021799 [DOI] [PubMed] [Google Scholar]
- 19.Li F, Wu Y, Chen L, Hu L, Liu X. Initial treatment combined with Prunella vulgaris reduced prednisolone consumption for patients with subacute thyroiditis. Ann Transl Med. 2019;7(3):45. doi: 10.21037/atm.2019.01.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saklamaz A. Is there a drug effect on the development of permanent hypothyroidism in subacute thyroiditis? Acta Endocrinol. 2017;13(1):119–123. doi: 10.4183/aeb.2017.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benbassat CA, Olchovsky D, Tsvetov G, Shimon I. Subacute thyroiditis: clinical characteristics and treatment outcome in fifty-six consecutive patients diagnosed between 1999 and 2005. J Endocrinol Invest. 2007;30(8):631–635. doi: 10.1007/BF03347442 [DOI] [PubMed] [Google Scholar]
- 22.ÇT B, Yilmaz M, Kiliçkan E. Factors affecting recurrence in subacute granulomatous thyroiditis [published online ahead of print, 2022 May 12]. Arch Endocrinol Metab. 2022;2359–3997000000473. doi: 10.20945/2359-3997000000473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez DS, Chopra IJ. Use of oral cholecystography agents in the treatment of hyperthyroidism of subacute thyroiditis. Panminerva Med. 2003;45(1):53–57. [PubMed] [Google Scholar]
- 24.Tian Z, Su Y, Zhang M, Zhang X, Guan Q. Successful management of recurrent subacute thyroiditis by adding colchicine to glucocorticoid treatment: a case series study [published correction appears in Horm Metab Res. 2020 May 19]. Horm Metab Res. 2020;52(10):712–717. doi: 10.1055/a-1148-2260 [DOI] [PubMed] [Google Scholar]
- 25.Yasuji I. Subacute thyroiditis in a patient with juvenile idiopathic arthritis undergoing etanercept treatment: a case report and review of the literature. Mod Rheumatol. 2013;23(2):397–400. doi: 10.1007/s10165-012-0670-5 [DOI] [PubMed] [Google Scholar]
- 26.Volpé R. The management of subacute (DeQuervain’s) thyroiditis. Thyroid. 1993;3(3):253–255. doi: 10.1089/thy.1993.3.253 [DOI] [PubMed] [Google Scholar]
- 27.Antonopoulos AS, Papanikolaou E, Vogiatzi G, Oikonomou E, Tousoulis D. Anti-inflammatory agents in peripheral arterial disease. Curr Opin Pharmacol. 2018;39:1–8. doi: 10.1016/j.coph.2017.11.001 [DOI] [PubMed] [Google Scholar]