Abstract
Purpose of the review:
To summarize the state-of-the-art literature on the epidemiology, disease progression and mediators of heart failure (HF), tachyarrhythmias, and sudden cardiac death in people living with HIV (PLWH) to inform prevention strategies.
Recent findings:
Recent studies corroborate the role of HIV as a risk enhancer for HF and arrhythmias, which persists despite adjustment for cardiovascular risk factors and unhealthy behaviors. Immune activation and inflammation contribute to the risk. HF occurs more frequently at younger ages, and among women and ethnic minorities living with HIV, highlighting disparities. Prospective outcome studies remain sparse in PLWH limiting prevention approaches. However, subclinical cardiac and electrophysiologic remodeling and dysfunction detected by noninvasive testing are powerful disease surrogates that inform our mechanistic understanding of HIV-associated cardiovascular disease and offer opportunities for early diagnosis.
Summary:
Aggressive control of HIV viremia and cardiac risk factors and abstinence from unhealthy behaviors remain treatment pillars to prevent HF and arrhythmic complications. The excess risk among PLWH warrants heightened vigilance for HF and arrhythmic symptomatology and earlier testing as subclinical abnormalities are common. Future research needs include identifying novel therapeutic targets to prevent HF and arrhythmias and testing of interventions in diverse groups of PLWH.
Keywords: HIV infection, heart failure, arrhythmia, sudden cardiac death, antiretroviral therapy
Introduction
Access to anti-retroviral therapy (ART) has transformed HIV epidemiology. People living with HIV (PLWH) enjoy extended longevity but are vulnerable to non-AIDS-related chronic morbidities such as cardiovascular disease (CVD) that deter healthy aging. Numerous factors potentially contribute to prevalent and incident CVD among PLWH. Even with durable HIV viral suppression, immune dysregulation and heightened inflammation persist as do viral reservoirs and viral replication which are cardiotoxic. ART is associated with potential side effects including dyslipidemia, insulin resistance, lipodystrophy and pro-arrhythmogenicity. The burden of traditional cardiovascular risk factors increases as PLWH age. Behavioral factors such as smoking, substance use and unhealthy diet and exercise patterns, which are more common among PLWH, further heighten risk. Finally, disparities in health care access and utilization may also play roles.
Here, we aim to summarize the state-of-the-art knowledge in epidemiology, disease progression and prognosis and potential mediators of non-atherosclerotic CVD in PLWH, focusing on heart failure (HF), atrial and ventricular arrhythmia, and sudden cardiac death (SCD) outcomes. Such an understanding can inform prevention strategies, management and subsequent research directions. Prevention of atherosclerotic CVD has been reviewed recently and is not covered here [1*,2*].
EPIDEMIOLOGY OF HEART FAILURE IN PLWH (Table 1)
Table 1:
Prevalence, incidence and risk factors associated with HF, atrial arrhythmias and ventricular arrhythmias/sudden cardiac death in PLWH
| HF | Atrial arrhythmias | Ventricular arrhythmias / sudden cardiac death | |
|---|---|---|---|
| Prevalence | 6.5–7.2% | 2–3% | -- |
| Incidence | 0.23–0.9 per 100 person-years to 2.1% per year | 18.2 per 1000 person-years | 232 per 100,000 person-years to 3.5–13% per year |
| Age | Younger age of onset | Older age | Younger |
| Sex | Women | --- | Men |
| Race/ethnicity | Blacks, Asian-Pacific Islanders | Caucasian race | Caucasian race |
| Traditional cardiac risk factors | Increased burden | Increased burden | Increased burden |
| HIV viral load ≥500 copies/ml | HFrEF only | -- | Increased risk |
| Nadir CD4 <200 cells/ml | Increased risk | Increased risk | Increased risk |
| Protease inhibitors | Increased risk | -- | -- |
| Substance use | Cocaine increases risk | -- | Cocaine increases risk Opiates increase risk |
Early and durable ART has decreased the frequency of HIV-associated dilated cardiomyopathies characterized by severe left ventricular systolic dysfunction, especially in developed countries. However, numerous studies report an excess burden of HF, due to both reduced (HFrEF) and preserved ejection fraction (HFpEF) [3–12]. In an analysis of a national database of electronic health care records, a HF diagnosis occurred in 7.2% of PLWH and 4.4% of controls (relative risk 1.66 [1.60–1.72], p<0.0001) [3]. In the Veteran Aging Cohort Study (VACS), a multicenter longitudinal study, veterans living with HIV had a 61% increased risk of incident HFrEF and a 21% increased risk of incident HFpEF compared with uninfected veterans after multivariable adjustment [4]. When physician-adjudicated HF was used instead of administrative coding in another study, the excess incident risk associated with HIV was even higher (relative risk 2.10, 95% confidence interval, 1.38–3.21 versus uninfected controls) [5]. Both prevalent and incident HFrEF and HFpEF are associated with lower nadir CD4+ T-cell count (CD4 count) <200 cells/mm3 [3,5,6], while HIV viral load ≥500 copies/mL is associated only with HFrEF [4]. Another analysis of national health insurance data reported lower incident HF risk with increasing ART duration [9]. Incident and prevalent HF risks among PLWH are higher at younger ages [3,4,6,7*,13*], among women [3,7*] [6], blacks [14], and Asian-Pacific Islanders [7*], and independent of coronary artery disease [3,4,7*]. PLWH with HF have an increased burden of cardiovascular risk factors and substance use compared to controls [14].
Once diagnosed with HF, PLWH have worse outcomes including higher rates of all-cause mortality and recurrent HF and all-cause hospital admission [14–16]. Outcomes are particularly worse among PLWH with low CD4 count or unsuppressed HIV viral load [14,15]. However, among HFrEF patients, for those PLWH with undetectable viral load and CD4 count ≥200 cells/mm3, outcome is similar versus uninfected patients [15]. Cocaine use and coronary artery disease are associated with increased risk for 30 day HF readmission [15] Use of protease inhibitors, compared to other ART, is associated with increased 30 day HF readmission rate [17]. Recipients of protease inhibitors have higher rates of hyperlipidemia, diabetes mellitus, and coronary artery disease and lower left ventricular ejection fraction (LVEF), further exacerbating adverse outcome [17]. Among PLWH hospitalized with HF, persistently reduced LVEF with lack of LVEF recovery at follow-up is more common than seen in other chronic inflammatory diseases [18].
SUBCLINICAL DISEASE MEASURES AND RISK OF HF IN PLWH (Table 2)
Table 2:
Subclinical manifestations of increased risk for HF, atrial arrhythmias and ventricular arrhythmias/sudden cardiac death in PLWH
| HF | Atrial arrhythmias | Ventricular arrhythmias / sudden cardiac death |
|---|---|---|
Echocardiography:
|
Echocardiography:
|
Echocardiography:
|
There are few prospective longitudinal studies of and no interventional studies of HF prevention in PLWH. However, much can be learned from imaging-based investigations of subclinical structural heart disease associated with HIV. Changes in cardiac structure and function precede symptomatic HF (pre-HF Stage B) in the general population [19]. Echocardiography and cardiovascular magnetic resonance imaging (CMR) identify imaging features associated with increased risk for symptomatic HF. Reduced LVEF is much less frequent than in the pre-ART era with only a 2.4% prevalence reported in a recent large echocardiography study of men living with HIV enrolled in the Multicenter AIDS Cohort Study (MACS) which was statistically similar to the 1.9% prevalence among uninfected comparators [20*]. In uninfected individuals with preserved LVEF, LV global longitudinal strain (GLS) reflects contractility, detects subclinical impairment in deformation and is incrementally prognostic for HF outcomes [21]. In a retrospective cohort of PLWH, abnormal GLS was associated with lower CD4 count [22]. A Ugandan study of PLWH with high prevalence of hypertension also reported reduced GLS compared to uninfected controls [23]. CMR studies similarly note reduced LV GLS and circumferential strain among PLWH versus controls [24–27].
A number of studies have identified abnormal imaging metrics associated with diastolic impairment among PLWH [10,20,23,28,29]. These include increased LV mass/hypertrophy and diastolic dysfunction using various criteria including the 2009 [30] and 2016 American Society of Echocardiography guidelines [31] and those proposed by the Characterization of Heart Function on Antiretroviral Therapy (CHART) study [28]. Prevalent diastolic dysfunction ranges from 22–37% among cohorts of middle-aged PLWH [10,20,29]. However, among some studies of PLWH in African nations, diastolic dysfunction is much less prevalent (1–7%) [32,33] and similar by HIV serostatus [32,33], perhaps due to younger participants, few traditional cardiac risk factors and yet unidentified differences in environmental factors. Nonetheless, LV mass was higher among these younger African PLWH compared to uninfected controls which may predispose to future diastolic dysfunction [32,33]. Risk factors for LV hypertrophy include CD4 count ≤200 cells/mm3, lack of viral suppression [29], and later initiation of ART [33] while lower CD4 count [29] and more severe HIV disease [33] correlate with diastolic dysfunction.
Biomarker studies provide additional insight into potential mechanisms of cardiac dysfunction in HIV. An earlier study correlated levels of suppression of tumorgenicity-2 (ST2) with diastolic dysfunction [34]. More recently, diastolic dysfunction was associated with higher levels of N-terminal pro-B-type natriuretic peptide (NT-pro-BNP), troponin-I, and carboxyl-terminal telopeptide of collagen type I with borderline correlation with interleukin-6 [28] suggesting roles for cardiac stress, injury, collagen turnover and inflammation. Among a group of HIV seropositive and seronegative Ugandans, NT-proBNP, growth differentiation factor-15 (GDF-15), ST2, and cystatin C were associated with LV mass while NT-proBNP and GDF-15 were associated with diastolic dysfunction [35*]. The relationship between the serum biomarkers and LV mass was stronger for men than women, highlighting sex as well as potential geographic and/or racial/ethnic differences [35*].
CMR also provides insight into HIV-related myocardial involvement by detecting focal, macroscopic scar via late gadolinium enhancement (LGE), diffuse fibrosis via T1 mapping techniques, and myocardial steatosis by spectroscopy. Several studies have reported increased prevalences of focal LGE among PLWH [24–26,36] often in a nonischemic pattern involving the midwall and subepicardium [24,25,37*]. PLWH have increased extracellular volume fraction (ECV) or native T1 times reflecting diffuse fibrosis compared to uninfected referents [25–27,37*,38**]. Such patterns are adverse prognostic markers in HIV seronegative cohorts [39]. Elevated native T1 times among PLWH were recently associated with adverse cardiovascular outcomes including HF [38**]. Studies have also reported higher intramyocardial lipid levels in PLWH [25,36,40] which, in combination with higher amounts of fibrosis, are associated with strain abnormalities [36]. While dysfunction of immune and metabolic fat pathways likely exacerbate cardiac stress and injury and increased collagen turnover, our understanding of the specific pathways by which HIV increases burden of myocardial scar, fibrosis, and fat remains incomplete. A recent report correlating levels of transmethylamine-N-oxide (TMAO), a dietary gut metabolite, with ECV among PLWH suggests a possible role for gut permeability and microbial translocation contributing to immune activation that warrants further investigation [41**].
Few studies have examined the time course of imaging characteristics among PLWH and are restricted to echocardiography. A retrospective analysis of clinically-acquired echocardiograms from PLWH with LVEF>40% observed higher LV mass and lower LV end-diastolic volume over 5 years independent of CD4 count or HIV viral suppression and no change in LV systolic volume, LV GLS or E/e’ ratio [42*]. A smaller cohort study of women with and at risk for HIV enrolled in the Women’s Interagency HIV Study (WIHS) performed repeat echocardiograms 12 years following baseline [43*]. Incident LV dysfunction occurred in 13.8%, most of which (79.2%) comprised diastolic dysfunction and neither rate of new systolic nor diastolic dysfunction differed by HIV serostatus [43*]. However, coinfection with hepatitis C correlated with a three-fold increased risk for incident LV dysfunction in women living with HIV [43*]. Larger validation studies are needed.
EPIDEMIOLOGY OF ATRIAL ARRHYTHMIAS IN PLWH (Table 1)
The literature is mixed regarding the association between HIV and atrial arrhythmias [44–47] due to differences in rhythm validation (hospital diagnosis coding versus physician adjudication) and cohort selection. Prevalent atrial arrhythmia is estimated at 2–3% [45,46] with an incidence rate for PLWH of 18.2 (versus 8.9 per 1,000 person-years for controls) [47]. Most studies report associations between older age, white race, diabetes, hypertension, coronary artery disease, HF, and/or chronic obstructive pulmonary disease and incident atrial arrhythmias among PLWH, common to those in the general population [45,48,49]. Additionally, nadir CD4 <200 cells/mm3 [45], lack of ART or the prescription of multiple ART regimens [48], perhaps a marker of treatment failure and/or duration of HIV, also correlate with increased risk for incident arrhythmia.
SUBCLINICAL DISEASE MEASURES AND RISK OF ATRIAL ARRHYTHMIAS IN PLWH (Table 2)
Abnormal atrial structure and function predispose to subsequent atrial fibrillation in people without HIV [50]. While subclinical changes in atrial size and function are described among PLWH [20*,37*], the progression to clinical arrhythmias and differences in trajectory or risk predictors from that of uninfected individuals are unknown.
EPIDEMIOLOGY OF VENTRICULAR ARRHYTHMIAS AND SUDDEN CARDIAC DEATH IN PLWH (Table 1)
Published studies suggest an increased risk for SCD among PLWH, estimated at 232 per 100,000 person-years to 3.5–13% per year, depending on the definition of SCD used and the cohort – e.g. public HIV clinic [51], longitudinal cohort study [52**], administrative hospital database [53*] versus hospitalized HF patients [54]. Low CD4 counts and higher HIV viral loads correlate with increased SCD risk [52,54]. Rates of SCD among PLWH with CD4 count ≥200 cell/mm3 and undetectable viral load are similar to those of controls [54]. Other risk factors for SCD in PLWH generally mirror those of uninfected referents, including coronary artery disease, low LVEF, worse functional class, as well as cocaine use, lower prescription of beta blockers, and longer QRS and QT interval durations [52,54]. In a study of PLWH hospitalized for HF with low rates of viral suppression (51%) and clinically-indicated implantable cardioverter defibrillators, appropriate and inappropriate discharges occurred more frequently compared to uninfected comparators and were associated with greater risk for HF admission and cardiovascular mortality [55]. Among PLWH, cocaine use and coronary artery disease as well as longer QRS duration and worse functional class were associated with increased odds of a device discharge [55].
A recent study compared results of autopsy and toxicology and histology testing in out-of-hospital arrest victims with and without HIV [56**]. Occult drug overdose was the leading cause of sudden death among PLWH, exceeding that in referents (34% vs 13%). Arrhythmic SCD comprised 47% of all sudden deaths with a trend toward higher incidence rate among PLWH versus controls (25.0 versus 13.3 arrhythmic deaths per 100,000 person-years; incidence rate ratio 1.87, 95% confidence interval 0.93–3.78). PLWH with arrhythmic SCD were predominantly men (94%), Caucasian (77%), and ~9 years younger than HIV seronegative cardiac arrest victims. The prevalences of cardiovascular risk factors and disease, including myocardial infarction and HF, were similar by HIV serostatus. The burden of total myocardial fibrosis, and specifically interstitial (but not replacement) fibrosis, was higher in PLWH. These findings highlight the lower rates of arrhythmic SCD when using an autopsy-based definition; the frequency of occult drug overdose as a cause of presumed cardiac arrest among PLWH; and the association between myocardial fibrosis burden and ventricular arrhythmic outcomes not unique to HIV. The study also confirms the increased prevalence of non-ischemic scar among PLWH reported in the CMR studies discussed in the HF section above.
SUBCLINICAL DISEASE MEASURES AND RISK OF VENTRICULAR ARRHYTHMIAS AND SUDDEN CARDIAC DEATH IN PLWH (Table 2)
Abnormal myocardial structure and function, including reduced LVEF and myocardial scar, not only correlate with increased risk for HF, as reviewed above, but also predispose to ventricular arrhythmias and SCD [57]. Additional factors that increase arrhythmic risk include electrophysiologic and autonomic dysfunction. PLWH may be susceptible to prolonged ventricular repolarization, as measured by the electrocardiographic QT interval, due to direct HIV viral effects on cardiac ion channels [58], side effects of ART [59], drug-drug interactions between ART and other medications known to increase QT interval duration [60], and substances such as opioids [61]. Prevalent QT prolongation ranges from 3–23% [62–66*]. Several studies report longer absolute corrected QT and QT subcomponent intervals among PLWH versus controls, both in developed and low income countries [64,67–69*]. Risk factors for prolonged QT in PLWH include male sex [66*], Asian race [66*], high HIV viral load [63,65,66*], lower CD4 count [63,65], ART use [69*], and increased inflammatory biomarker levels [64,68]. The data correlating specific ART classes and QT prolongation are conflicting but support increased risk with older generation protease inhibitors, efavirenz, and rilpivirine [65,70]. Few studies have examined longitudinal changes in QT intervals. One recent study reported a 1.6% incidence of new QT prolongation within 2 years among virally suppressed PLWH [71*]. Relatively high prevalences of early repolarization abnormalities (12.4%) and electrocardiographic LV hypertrophy (8.3%) were also recently reported in the multinational Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) Study [66*]. While studies in the general population suggest the association between electrocardiographic early repolarization [72] and LV hypertrophy with SCD [73], long term clinical follow-up is required to determine clinical significance in PLWH.
Complementary to static measurements, QT variability (QTV) quantifies spontaneous, temporal fluctuation in the QT interval, reflects electrical instability and correlates with increased risk for SCD in HIV seronegative cohorts [74]. Heart rate variability (HRV), a measure of autonomic function, is also an informative marker of arrhythmic risk in the general population. HIV may result in sympathetic dominance that can potentially alter both HRV and repolarization dynamics, predisposing to ventricular arrhythmias [75,76]. This hypothesis was tested in a recent study of MACS participants which used an ambulatory electrocardiography patch monitor to examine differences in QTV index (QTVI) by HIV serostatus and is defined as the ratio between QTV and HRV [77]. A higher QTVI (prognostically worse) was seen among men living with HIV compared to uninfected referents, even after adjusting for traditional cardiovascular risk factors. The association between higher QTVI and HIV seropositivity was stronger among men with HIV and detectable viral loads. In examining the QTV and HRV subcomponents of QTVI, HIV seropositivity was associated with lower (prognostically worse) HRV and higher (prognostically worse) QTV, supporting HIV-associated autonomic dysfunction and repolarization abnormalities as mechanisms for proarrhythmia. Higher inflammatory biomarker levels correlated with higher QTVI and partly attenuated the HIV association, supporting a role for inflammation-mediated changes in electrophysiology and autonomic function.
Other subclinical markers of ventricular arrhythmic risk studied in PLWH include ventricular ectopy (VE) and non-sustained ventricular tachycardia (NSVT), using administrative coding [78] and ambulatory electrocardiographic patch monitoring [79]. Burden of VE/NSVT appears similar by HIV serostatus [78,79] though among men with HIV, higher viral load [78], lower CD4 count [78], and higher QTVI correlate with more frequent VE/NSVT [77].
The Figure shows a conceptual diagram of the risk factors, injury mechanisms and interplay between adverse cardiac and electrophysiologic remodeling that contribute to ventricular arrhythmias/SCD.
Figure 1.
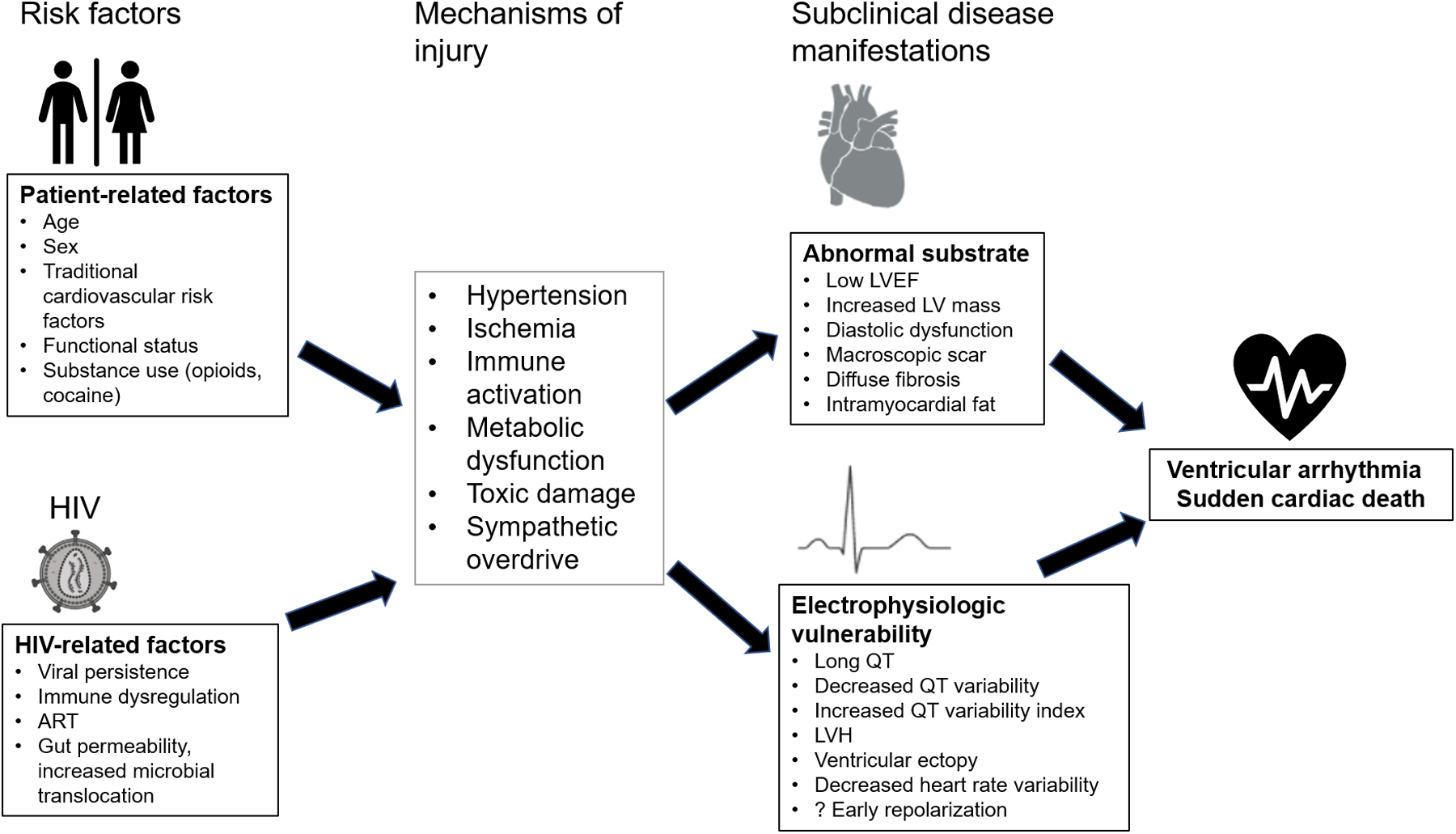
Conceptual diagram of mechanisms of ventricular arrhythmia/SCD in PLWH. The risk factors, injury mechanisms and interplay between adverse cardiac and electrophysiologic remodeling that contribute to ventricular arrhythmias/SCD are shown in this Figure (created with Biorender.com).
Conclusions
The recent literature corroborates HIV infection as a risk enhancer for HF and arrhythmias. While PLWH often have an increased burden of traditional cardiovascular risk factors and unhealthy behaviors, increased risk for these outcomes persists despite multivariable adjustment, reinforcing other HIV-specific mechanisms. HF endpoints in particular occur at younger ages, and with higher frequency among women and ethnic minorities living with HIV, highlighting disparities. The evidence supports a role for immune activation as a mediator, though exact pathways remain unclear, limiting intervention options. The associated metabolic sequelae of ART regimens also contribute but management of the individual patient is restricted to knowledge of general class effects.
There remains a lack of HIV-specific strategies and guidelines directing prevention, diagnostic and treatment approaches stemming from limitations of current outcomes studies. Few include independently adjudicated endpoints or individual HF, tachyarrhythmic and SCD events. Current estimates of the excess risk attributable to HIV suffer from imperfect comparison groups. Many observational studies include HIV seronegative referents matched only on a few demographic parameters but otherwise lack the risk factors known to be enriched in PLWH. Few include large numbers of women and diverse races/ethnicities. However, subclinical cardiac and electrophysiologic remodeling and dysfunction detected by noninvasive testing remain powerful surrogates of disease that inform our mechanistic understanding of HIV-related CVD, help identify potential preventive and therapeutic targets and offer opportunities for early diagnosis.
Durable and aggressive control of HIV viremia and cardiac risk factors as well as abstinence from unhealthy substances and behaviors remain primary treatment goals to prevent cardiovascular complications. The enhanced risk among PLWH warrants heightened vigilance for potential HF and arrhythmic symptomatology and merits earlier testing and diagnosis. Future research needs include the identification of therapeutic targets specifically for prevention of HF and arrhythmias and testing of interventions in diverse groups of PLWH.
Key points.
Enrichment for traditional cardiac risk factors as well as unhealthy behaviors and substance use contributes to the excess risk associated with HIV for HF and arrhythmic outcomes, but does not fully explain the relationships.
Higher HIV viral load, lower CD4 count, and younger age are associated with more frequent HF and ventricular arrhythmia endpoints among PLWH while women and ethnic minorities with HIV are at higher risk for HF endpoints.
Among PLWH, the subclinical phenotype associated with increased risk for HF includes preserved LV ejection fraction but reduced global longitudinal strain, increased LV mass, larger left atrial size, diastolic dysfunction, and larger amounts of myocardial interstitial fibrosis.
Electrocardiographic abnormalities are common among PLWH and prolonged QT duration, lower heart rate variability, higher QT variability and QT variability index support increased risk for ventricular arrhythmias and sudden cardiac death.
Inflammation and immune activation mediate subclinical changes in cardiac structural and functional and electrophysiologic properties in PLWH compared to HIV seronegative comparators.
Financial support and sponsorship:
KCW and WSP were principal investigators of the “Identifying Risk Factors for Subclinical Myocardial Disease in HIV Infection” study (National Institutes of Health, NIH R01HL126552) and are co-investigators on the Multicenter AIDS Cohort Study (MACS) / Women’s Interagency HIV Study (WIHS) Combined Cohort Study (CCS) (NIH U01HL146201).
Footnotes
Conflicts of interest: none
Disclosures: none
References
- 1.Durstenfeld MS, Hsue PY. Mechanisms and primary prevention of atherosclerotic cardiovascular disease among people living with HIV. Curr Opin HIV AIDS 2021; 16:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This is a recent comprehensive review of mechanisms and primary prevention of atherosclerotic cardiovascular disease beyond the scope of what is covered here.
- 2.Achhra AC, Lyass A, Borowsky L, et al. Assessing Cardiovascular Risk in People Living with HIV: Current Tools and Limitations. Curr HIV/AIDS Rep 2021; 18:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This review covers the current status of the application of risk prediction tools to PLWH, primarily with regard to atherosclerotic outcomes and highlights limitations with equations developed in the general population.
- 3.Al-Kindi SG, ElAmm C, Ginwalla M, et al. Heart failure in patients with human immunodeficiency virus infection: Epidemiology and management disparities. Int J Cardiol 2016; 218:43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freiberg MS, Chang CH, Skanderson M, et al. Association Between HIV Infection and the Risk of Heart Failure With Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results From the Veterans Aging Cohort Study. JAMA Cardiol 2017; 2:536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feinstein MJ, Steverson AB, Ning H, et al. Adjudicated Heart Failure in HIV-Infected and Uninfected Men and Women. J Am Heart Assoc 2018; 7:e009985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Gao Y, Zhou Y, et al. Human Immunodeficiency Virus Infection and Incident Heart Failure: A Meta-Analysis of Prospective Studies. J Acquir Immune Defic Syndr 2021; 87:741–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Go AS, Reynolds K, Avula HR, et al. Human Immunodeficiency Virus Infection and Variation in Heart Failure Risk by Age, Sex, and Ethnicity: The HIV HEART Study. Mayo Clin Proc 2022; 97:465–479. [DOI] [PMC free article] [PubMed] [Google Scholar]; *HIV HEART is a retrospective, age/gender/race-matched, case-control cohort study of patients enrolled in Kaiser-Permanente health care delivery systems. PLWH had a higher adjusted incident HF rate that was independent of acute coronary syndrome and more pronounced among women and younger patients, corrobating other studies and as well as identifying higher risk among Asian-Pacific Islanders. Strengths include the large cohort size and ethnic diversity.
- 8.Alonso A, Barnes AE, Guest JL, et al. HIV Infection and Incidence of Cardiovascular Diseases: An Analysis of a Large Healthcare Database. J Am Heart Assoc 2019; 8:e012241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yen YF, Ko MC, Yen MY, et al. Human Immunodeficiency Virus Increases the Risk of Incident Heart Failure. J Acquir Immune Defic Syndr 2019; 80:255–263. [DOI] [PubMed] [Google Scholar]
- 10.Erqou S, Lodebo BT, Masri A, et al. Cardiac Dysfunction Among People Living With HIV: A Systematic Review and Meta-Analysis. JACC Heart Fail 2019; 7:98–108. [DOI] [PubMed] [Google Scholar]
- 11.Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation 2019; 140:e98–e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prasada S, Rivera A, Nishtala A, et al. Differential Associations of Chronic Inflammatory Diseases With Incident Heart Failure. JACC Heart Fail 2020; 8:489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erqou S, Jiang L, Choudhary G, et al. Age at Diagnosis of Heart Failure in United States Veterans With and Without HIV Infection. J Am Heart Assoc 2021; 10:e018983. [DOI] [PMC free article] [PubMed] [Google Scholar]; *In this retrospective study of predominantly male, US veterans, characteristics of hospitalized HF patients living with and without HIV were compared using Veterans Health Administration healthcare data. PLWH were diagnosed with HF at a median age that was 5 years younger compared to HIV seronegative referents, supporting the concept of earlier physiologic aging among PLWH.
- 14.Erqou S, Jiang L, Choudhary G, et al. Heart Failure Outcomes and Associated Factors Among Veterans With Human Immunodeficiency Virus Infection. JACC Heart Fail 2020; 8:501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvi RM, Afshar M, Neilan AM, et al. Heart failure and adverse heart failure outcomes among persons living with HIV in a US tertiary medical center. Am Heart J 2019; 210:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brouch D, Tashtish N, Di Felice C, et al. Human Immunodeficiency Virus Infection and Risk of Heart Failure Rehospitalizations. Am J Cardiol 2019; 124:1232–1238. [DOI] [PubMed] [Google Scholar]
- 17.Alvi RM, Neilan AM, Tariq N, et al. Protease Inhibitors and Cardiovascular Outcomes in Patients With HIV and Heart Failure. J Am Coll Cardiol 2018; 72:518–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivera AS, Sinha A, Ahmad FS, et al. Long-Term Trajectories of Left Ventricular Ejection Fraction in Patients With Chronic Inflammatory Diseases and Heart Failure: An Analysis of Electronic Health Records. Circ Heart Fail 2021; 14:e008478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozkurt B, Coats AJ, Tsutsui H, et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail 2021. [Google Scholar]
- 20.Doria de Vasconcellos H, Post WS, Ervin AM, et al. Associations Between HIV Serostatus and Cardiac Structure and Function Evaluated by 2-Dimensional Echocardiography in the Multicenter AIDS Cohort Study. J Am Heart Assoc 2021; 10:e019709. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This is the largest contemporary echocardiographic study of men with HIV and HIV seronegative comparators enriched for similar traditional cardiovascular risk factors and risk factors for HIV acquisition. There was an independent association between HIV seropositivity and greater LV mass, left atrial and right ventricular sizes, lower right ventricular function and diastolic abnormalities but not LV ejection fraction. These abnormalities may portend increased risk for heart failure with preserved ejection fraction among men with HIV, even if virally suppressed.
- 21.Potter E, Marwick TH. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC Cardiovasc Imaging 2018; 11:260–274. [DOI] [PubMed] [Google Scholar]
- 22.Alenezi F, Bloomfield GS, Okeke NL, et al. Global Longitudinal Strain and Immune Status in Patients Living With Human Immunodeficiency Virus. Am J Cardiol 2019; 124:966–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buggey J, Yun L, Hung CL, et al. HIV and pericardial fat are associated with abnormal cardiac structure and function among Ugandans. Heart 2020; 106:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luetkens JA, Doerner J, Schwarze-Zander C, et al. Cardiac Magnetic Resonance Reveals Signs of Subclinical Myocardial Inflammation in Asymptomatic HIV-Infected Patients. Circ Cardiovasc Imaging 2016; 9:e004091. [DOI] [PubMed] [Google Scholar]
- 25.Holloway CJ, Ntusi N, Suttie J, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation 2013; 128:814–822. [DOI] [PubMed] [Google Scholar]
- 26.Ntusi N, O’Dwyer E, Dorrell L, et al. HIV-1-Related Cardiovascular Disease Is Associated With Chronic Inflammation, Frequent Pericardial Effusions, and Probable Myocardial Edema. Circ Cardiovasc Imaging 2016; 9:e004430. [DOI] [PubMed] [Google Scholar]
- 27.Yan C, Li R, Guo X, et al. Cardiac Involvement in Human Immunodeficiency Virus Infected Patients: An Observational Cardiac Magnetic Resonance Study. Front Cardiovasc Med 2021; 8:756162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butler J, Greene SJ, Shah SH, et al. Diastolic Dysfunction in Patients With Human Immunodeficiency Virus Receiving Antiretroviral Therapy: Results From the CHART Study. J Card Fail 2019; 26:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okeke NL, Alenezi F, Bloomfield GS, et al. Determinants of Left Ventricular Hypertrophy and Diastolic Dysfunction in an HIV Clinical Cohort. J Card Fail 2018; 24:496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 2009; 22:107–133. [DOI] [PubMed] [Google Scholar]
- 31.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016; 29:277–314. [DOI] [PubMed] [Google Scholar]
- 32.Woldu B, Temu TM, Kirui N, et al. Diastolic dysfunction in people with HIV without known cardiovascular risk factors in Western Kenya. Open Heart 2022; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mahtab S, Lawrenson J, Jamieson-Luff N, et al. Echocardiographic Findings in a Cohort of Perinatally HIV-Infected Adolescents Compared with Uninfected Peers from the Cape Town Adolescent Antiretroviral Cohort. J Am Soc Echocardiogr 2020; 33:604–611. [DOI] [PubMed] [Google Scholar]
- 34.Secemsky EA, Scherzer R, Nitta E, et al. Novel Biomarkers of Cardiac Stress, Cardiovascular Dysfunction, and Outcomes in HIV-Infected Individuals. JACC Heart Fail 2015; 3:591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kipke J, Margevicius S, Kityo C, et al. Sex, HIV Status, and Measures of Cardiac Stress and Fibrosis in Uganda. J Am Heart Assoc 2021; 10:e018767. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study compared serum biomarker and echocardiographic measurements in 100 PLWH on ART and 100 HIV seronegative Ugandans with at least one cardiovascular risk factor. The study highlighted sex-based differences in serum biomarker levels of cardiac fibrosis and stress among PLWH that may differ from that seen in developed countries and warrants further investigation.
- 36.Thiara DK, Liu CY, Raman F, et al. Abnormal Myocardial Function Is Related to Myocardial Steatosis and Diffuse Myocardial Fibrosis in HIV-Infected Adults. J Infect Dis 2015; 212:1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu KC, Haberlen SA, Plankey MW, et al. Human immunodeficiency viral infection and differences in interstitial ventricular fibrosis and left atrial size. Eur Heart J Cardiovasc Imaging 2021; 22:888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This is the largest CMR study that included predominantly virologically suppressed men with HIV and HIV seronegative referents with common risk factors for HIV acquisition, thus allowing for robust adjustment of covariates to better isolate HIV effects. HIV seropositivity was associated with larger amounts of diffuse myocardial fibrosis as well as larger left atrial size which represent markers of increased risk for HF and arrhythmias.
- 38.de Leuw P, Arendt CT, Haberl AE, et al. Myocardial Fibrosis and Inflammation by CMR Predict Cardiovascular Outcome in People Living With HIV. JACC Cardiovasc Imaging 2021; 14:1548–1557. [DOI] [PubMed] [Google Scholar]; **In this outcome study of 156 PLWH followed prospectively after CMR acquisition, higher T1 times (a marker of myocardial fibrosis) and higher LV mass were independently associated with increased risk for major adverse cardiovascular events including HF.
- 39.Shanbhag SM, Greve AM, Aspelund T, et al. Prevalence and prognosis of ischaemic and non-ischaemic myocardial fibrosis in older adults. Eur Heart J 2019; 40:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neilan TG, Nguyen KL, Zaha VG, et al. Myocardial Steatosis Among Antiretroviral Therapy-Treated People With Human Immunodeficiency Virus Participating in the REPRIEVE Trial. J Infect Dis 2020; 222:S63–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colaco NA, Wang TS, Ma Y, et al. Transmethylamine-N-Oxide Is Associated With Diffuse Cardiac Fibrosis in People Living With HIV. J Am Heart Assoc 2021; 10:e020499. [DOI] [PMC free article] [PubMed] [Google Scholar]; **In this study of PLWH with and without diastolic dysfunction enrolled in the CHART-HIV study, levels of TMAO correlated with greater diffuse fibrosis, assessed by serum biomarkers and CMR, increased LV filling pressure measured by echocardiography, and levels of monocyte activation marker, sCD14. The findings suggest a biologic mechanism linking HIV-associated gut permeability and the resultant microbial translocation with immune activation and myocardial fibrosis.
- 42.Bloomfield GS, Alenezi F, Chiswell K, et al. Progression of cardiac structure and function in people with human immunodeficiency virus. Echocardiography 2022; 39:268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This is the 2nd published study to examine serial echocardiograms in PLWH. Among 150 PLWH with LVEF≥40% who underwent clinically-acquired echocardiograms, there was an increase in LV mass index and decrease in indexed LV end-diastolic volume over a median of 5 years independent of CD4 count or HIV viral suppression status and no change in LV systolic volume, LV GLS or E/e’ ratio.
- 43.Shitole SG, Lazar JM, Hanna DB, et al. HIV, hepatitis C virus and risk of new-onset left ventricular dysfunction in women. AIDS 2021; 35:1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This is 1 of 2 studies that examined longitudinal trajectories of echocardiographic indices. It included 219 women with HIV and 92 women at risk for HIV enrolled in the Women’s Interagency HIV Study (WIHS). Incidence rates for neither systolic nor diastolic dysfunction differed by HIV serostatus. Co-infection with hepatitis C was found to increase the risk for incident LV dysfunction by three-fold in women living with HIV.
- 44.Hsu JC, Li Y, Marcus GM, et al. Atrial fibrillation and atrial flutter in human immunodeficiency virus-infected persons: incidence, risk factors, and association with markers of HIV disease severity. J Am Coll Cardiol 2013; 61:2288–2295. [DOI] [PubMed] [Google Scholar]
- 45.Sanders JM, Steverson AB, Pawlowski AE, et al. Atrial arrhythmia prevalence and characteristics for human immunodeficiency virus-infected persons and matched uninfected controls. PLoS One 2018; 13:e0194754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osuji N, Haberlen SA, Ashikaga H, et al. Association between human immunodeficiency virus serostatus and the prevalence of atrial fibrillation. Medicine (Baltimore) 2021; 100:e26663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sardana M, Hsue PY, Tseng ZH, et al. Human Immunodeficiency Virus Infection and Incident Atrial Fibrillation. J Am Coll Cardiol 2019; 74:1512–1514. [DOI] [PubMed] [Google Scholar]
- 48.Nance RM, Delaney JAC, Floyd JS, et al. Risk factors for atrial fibrillation in a multi-center US clinical cohort of people with HIV infection. AIDS 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jung H, Yang PS, Jang E, et al. Prevalence and Associated Stroke Risk of Human Immunodeficiency Virus-Infected Patients With Atrial Fibrillation- A Nationwide Cohort Study. Circ J 2019; 83:2547–2554. [DOI] [PubMed] [Google Scholar]
- 50.Habibi M, Samiei S, Ambale Venkatesh B, et al. Cardiac Magnetic Resonance-Measured Left Atrial Volume and Function and Incident Atrial Fibrillation: Results From MESA (Multi-Ethnic Study of Atherosclerosis). Circ Cardiovasc Imaging 2016; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tseng ZH, Secemsky EA, Dowdy D, et al. Sudden cardiac death in patients with human immunodeficiency virus infection. J Am Coll Cardiol 2012; 59:1891–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freiberg MS, Duncan MS, Alcorn C, et al. HIV Infection and the Risk of World Health Organization-Defined Sudden Cardiac Death. J Am Heart Assoc 2021; 10:e021268. [DOI] [PMC free article] [PubMed] [Google Scholar]; **In the predominantly male, prospective, longitudinal Veterans Aging Cohort Study, HIV seropositivity was independently associated with increased risk for sudden cardiac death defined by World Health Criteria and was more pronounced among those with CD4 count<200 cells/mm3 or HIV viral load>500 copies/ml. The comparison group comprised veterans matched by age, sex, race/ethnicity, and clinical site.
- 53.Sardana M, Nah G, Hsue PY, et al. Human Immunodeficiency Virus Infection and Out-of-Hospital Cardiac Arrest. Am J Cardiol 2022; 163:124–129. [DOI] [PubMed] [Google Scholar]; *This study used an administrative database of California state residents receiving medical care in a California emergency department, ambulatory surgery, or inpatient hospital unit to compare rates and risk factors for out-of-hospital arrest by HIV serostatus. Risk was 2.5-fold higher among PLWH and disproportionately affected younger and female patients as well as those with hypertension, HF and chronic kidney disease.
- 54.Alvi RM, Neilan AM, Tariq N, et al. The Risk for Sudden Cardiac Death Among Patients Living With Heart Failure and Human Immunodeficiency Virus. JACC Heart Fail 2019; 7:759–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alvi RM, Neilan AM, Tariq N, et al. Incidence, Predictors, and Outcomes of Implantable Cardioverter-Defibrillator Discharge Among People Living With HIV. J Am Heart Assoc 2018; 7:e009857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tseng ZH, Moffatt E, Kim A, et al. Sudden Cardiac Death and Myocardial Fibrosis, Determined by Autopsy, in Persons with HIV. N Engl J Med 2021; 384:2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This is a unique postmortem study that compared results of autopsy, toxicology and histology testing in out-of-hospital arrest victims with and without HIV occurring in San Franciso County, California. This study highlighted the relationship between higher amounts of interstitial fibrosis on histopathology among PLWH with sudden death compared to HIV seronegative referents.
- 57.Deyell MW, Krahn AD, Goldberger JJ. Sudden cardiac death risk stratification. Circ Res 2015; 116:1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Es-Salah-Lamoureux Z, Jouni M, Malak OA, et al. HIV-Tat induces a decrease in IKr and IKsvia reduction in phosphatidylinositol-(4,5)-bisphosphate availability. J Mol Cell Cardiol 2016; 99:1–13. [DOI] [PubMed] [Google Scholar]
- 59.Anson BD, Weaver JG, Ackerman MJ, et al. Blockade of HERG channels by HIV protease inhibitors. Lancet 2005; 365:682–686. [DOI] [PubMed] [Google Scholar]
- 60.Manga P, McCutcheon K, Tsabedze N, et al. HIV and Nonischemic Heart Disease. J Am Coll Cardiol 2017; 69:83–91. [DOI] [PubMed] [Google Scholar]
- 61.Wedam EF, Haigney MC. The Impact of Opioids on Cardiac Electrophysiology. Curr Cardiol Rev 2016; 12:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reinsch N, Arendt M, Geisel MH, et al. Prolongation of the QTc interval in HIV-infected individuals compared to the general population. Infection 2017; 45:659–667. [DOI] [PubMed] [Google Scholar]
- 63.Gili S, Mancone M, Ballocca F, et al. Prevalence and predictors of long corrected QT interval in HIV-positive patients: a multicenter study. J Cardiovasc Med (Hagerstown) 2017; 18:539–544. [DOI] [PubMed] [Google Scholar]
- 64.Wu KC, Zhang L, Haberlen SA, et al. Predictors of electrocardiographic QT interval prolongation in men with HIV. Heart 2019; 105:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chastain DB, Veve MP, Wagner JL. Abnormal QTc syndrome in HIV-infected patients: a systematic review of prevalence and risk factors. Antivir Ther 2019; 24:459–465. [DOI] [PubMed] [Google Scholar]
- 66.Bloomfield GS, Weir IR, Ribaudo HJ, et al. Prevalence and Correlates of Electrocardiographic Abnormalities in Adults With HIV: Insights From the Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE). J Acquir Immune Defic Syndr 2022; 89:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]; *In 7720 PLWH enrolled in the multinational, multiethnic Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE), baseline ECGs showed relatively high prevalences of early repolarization abnormalities (12.4%), left ventricular hypertrophy (8.3%) and prolonged QT duration (8%). Longitudinal trajectory of these ECGs findings, association with incident outcomes, and effect of primary prevention with statins will be examined over the course of the study and will provide unique information.
- 67.Knudsen AD, Kofoed KF, Gelpi M, et al. Prevalence and risk factors of prolonged QT interval and electrocardiographic abnormalities in persons living with HIV. AIDS 2019; 33:2205–2210. [DOI] [PubMed] [Google Scholar]
- 68.Wu KC, Bhondoekhan F, Haberlen SA, et al. Associations between QT interval subcomponents, HIV serostatus, and inflammation. Ann Noninvasive Electrocardiol 2020; 25:e12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roozen GVT, Meel R, Peper J, et al. Electrocardiographic and echocardiographic abnormalities in urban African people living with HIV in South Africa. PLoS One 2021; 16:e0244742. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This cross-sectional analysis of electrocardiographic and echocardiographic indices included relatively young, well-treated PLWH from urban South Africa and HIV seronegative community controls. There were no associations between HIV serostatus and echocardiographic findings, in contrast to other studies. ART use was associated with longer QTc, similar to other studies. The findings highlight potential geographic and/or racial/ethnic differences in cardiovascular risk among PLWH.
- 70.Hosseini Z, Mollazadeh R, Dehghan-Manshadi SA, et al. Association between exposure to Efavirenz and substrates of dysrhythmia in HIV-infected young adults. Clin Cardiol 2021; 44:1448–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knudsen AD, Graff C, Nielsen JB, et al. De novo electrocardiographic abnormalities in persons living with HIV. Sci Rep 2021; 11:20750. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study examined longitudinal changes in electrocardiographic indices over 2 years among participants in the Copenhagen comorbidity in HIV infection (COCOMO) study. Prevalent and incident QT prolongation were rare in this Danish cohort with relatively few cardiovascular risk factors.
- 72.Liu LJ, Tang N, Bi WT, et al. Association Between Temporal Changes in Early Repolarization Pattern With Long-Term Cardiovascular Outcome: A Population-Based Cohort Study. J Am Heart Assoc 2022; 11:e022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Porthan K, Kentta T, Niiranen TJ, et al. ECG left ventricular hypertrophy as a risk predictor of sudden cardiac death. Int J Cardiol 2019; 276:125–129. [DOI] [PubMed] [Google Scholar]
- 74.Baumert M, Porta A, Vos MA, et al. QT interval variability in body surface ECG: measurement, physiological basis, and clinical value: position statement and consensus guidance endorsed by the European Heart Rhythm Association jointly with the ESC Working Group on Cardiac Cellular Electrophysiology. Europace 2016; 18:925–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McIntosh RC, Lobo JD, Hurwitz BE. Current assessment of heart rate variability and QTc interval length in HIV/AIDS. Curr Opin HIV AIDS 2017; 12:528–533. [DOI] [PubMed] [Google Scholar]
- 76.McIntosh RC. A meta-analysis of HIV and heart rate variability in the era of antiretroviral therapy. Clin Auton Res 2016; 26:287–294. [DOI] [PubMed] [Google Scholar]
- 77.Heravi AS, Etzkorn LH, Urbanek JK, et al. HIV Infection Is Associated With Variability in Ventricular Repolarization: The Multicenter AIDS Cohort Study (MACS). Circulation 2020; 141:176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meyer A, Dandamudi S, Achenbach C, et al. Ventricular Ectopy and Arrhythmia Characteristics for Persons Living with HIV and Uninfected Controls. J Int Assoc Provid AIDS Care 2019; 18:2325958219852123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Feinstein MJ, Haberlen SA, Ashikaga H, et al. Ventricular ectopy and arrhythmia by HIV serostatus, viremia, and CD4+ cell count. AIDS 2021; 35:846–849. [DOI] [PMC free article] [PubMed] [Google Scholar]


