Abstract
Purpose:
To determine whether SD-101, a Toll-like receptor 9 agonist, potentiates the antitumor activity of anti-PD-1 antibodies in patients with anti-PD-1/PD-L1 naïve, recurrent/metastatic head and neck squamous cell carcinoma (HNSCC).
Patients and Methods:
Patients with PD-1 Ab-naïve HNSCC received either 2 mg SD-101 injected in one to four lesions or 8 mg SD-101 injected into a single lesion weekly × 4 doses then every 3 weeks × 7 doses. Pembrolizumab was administered at 200 mg every 3 weeks.
Results:
A total of 28 patients received 2 mg and 23 received 8 mg per injection, respectively. A total of 76% of patients had received prior systemic therapy. Combined positive score was ≥1 to < 20 in 35 patients (70%) and ≥ 20 in 15 patients (30%) of 50 patients with available data. There were 12 patients with grade ≥3 treatment-related adverse events (24%), and no treatment-related deaths. The objective response rate was 24% including 2 complete and 10 partial responses. The median duration of response was 7.0 [95% confidence interval (CI): 2.1–11.1] months. The response rate was higher in human papillomavirus–positive (HPV+) patients (44%, N = 16). Responses were not associated with PD-L1 expression levels or IFNγ-related gene expression at baseline. Responses were observed both in injected (32%) and in noninjected lesions (29%). Progression-free and overall survival at 9 months were 19.0% (95% CI: 9.1–31.7) and 64.7% (95% CI: 45.3–78.7), respectively.
Conclusions:
SD-101 combined with pembrolizumab induced objective responses, especially in HPV+ tumors, which were frequently associated with increased intratumoral inflammation and effector immune cell activity.
Translational Relevance.
The absence of inflammatory gene expression and effector T cells in the tumor environment predict poor response to anti-PD-1 antibodies in patient with advanced solid tumors including head and neck squamous cell carcinoma (HNSCC). Toll-like receptor agonists including SD-101 induce inflammatory antitumor immune responses and they have demonstrated clinical synergy with anti-PD-1 antibodies in PD-1 Ab-naïve and PD-1 Ab-refractory melanoma. In the current study, we demonstrate that SD-101 synergizes with pembrolizumab in patients with PD-1 Ab-naïve HNSCC, increasing cellular immunity and inflammatory gene expression, demonstrating that SD-101 is active across tumor types. This led to objective tumor responses, even in patients whose tumors had low IFNγ-related gene expression at baseline. Furthermore, there was a high response rate in human papillomavirus–positive tumors suggesting that the combination may be particularly promising in this virally-induced HNSCC subtype.
Introduction
Prior to the advent of immune checkpoint inhibitors, particularly anti-PD-1 antibodies (Abs), recurrent and metastatic squamous cell carcinoma (HNSCC) was associated with poor prognosis. First-line therapy with platinum, 5-fluorouracil, and cetuximab was the standard of care, but yielded a median overall survival (OS) of approximately 10 months with more than 80% of patients experiencing grade 3 or higher treatment-associated adverse events (AE). Single-agent chemotherapy was the standard of care in the second line, but response rates were poor and the median OS in patients requiring second-line therapy was less than 7 months (1, 2).
More recently, first- and second-line immunotherapy with anti-PD-1 Abs has been shown to improve survival in patients with recurrent/metastatic (R/M) HNSCC. In a randomized, open-label, phase III study, patients with progressive disease (PD) on platinum-containing treatment were randomly assigned to receive either pembrolizumab or the investigator's choice of standard doses of methotrexate, docetaxel, or cetuximab. Pembrolizumab yielded a superior median OS of 8.4 months [95% confidence interval (CI), 6.4–9.4] compared with 6.9 months (5.9–8.0) observed in patients receiving chemotherapy (HR, 0.80; 0.65–0.98; nominal P = 0.0161; ref. 1). Moreover, fewer patients treated with pembrolizumab than with standard-of-care chemotherapy had grade 3 or worse treatment-related AEs (TRAE). Similarly, the anti-PD-1 Ab nivolumab improved survival in patients with platinum-refractory R/M HNSCC in a separate phase III study (3). KEYNOTE-048 demonstrated that pembrolizumab improves survival in patients with first-line R/M HNSCC who had not previously received systemic therapy for metastatic or recurrent disease (4). On the basis of these findings, pembrolizumab was approved for use in combination with platinum and fluorouracil for all patients and as a single agent for patients whose tumors express PD-L1 [combined positive score (CPS) ≥1] as determined by an FDA-approved test.
Despite significant progress in HNSCC treatment with the arrival of checkpoint inhibitors, OS is still limited to about a year in the vast majority of patients and there remains a high unmet need for synergistic combinations that could increase long-term remission rates. Several factors have been associated with increased response rates to anti-PD-1 Abs in HNSCC including tumor mutation burden, human papillomavirus (HPV) antigen expression, intratumoral inflammation, the relative abundance of effector T cells in the tumor microenvironment, and, most commonly, PD-L1 protein expression by IHC. Many of the factors associated with response to anti-PD-1 Abs can be enhanced through application of exogenous pro-inflammatory agents, and there are several injectable agents in clinical development including the Toll-like-receptor (TLR) 7/8 agonist MEDI9197 (5), the TLR8 agonist motolimod (6), the TLR9 agonist tilsotolimod (7), the STING agonist MK1454 (8), and an electroporated plasmid encoding IL12, tavokinogene telseplasmid (8). However, it has yet to be established whether these agents can potentiate the activity of anti-PD-1 Abs in HNSCC.
SD-101 is a synthetic class-C CpG-oligodeoxynucleotide TLR9 agonist, which stimulates human plasmacytoid dendritic cells to release IFNα and mature into efficient antigen-presenting cells, enhancing both innate and adaptive immune responses. Preclinical studies in multiple mouse tumor models, including HNSCC, have demonstrated that intratumoral injection of SD-101, combined with a systemic anti-PD-1 Ab, can lead to complete and durable immune-mediated rejection of nearly all injected tumors and a large fraction of noninjected tumors in mice with multiple tumors (9, 10). Clinically, intratumoral SD-101 has been shown to stimulate significant antitumor immunity when combined with local radiation in patients with non–Hodgkin lymphoma (11) and with anti-PD-1 Abs in patients with advanced melanoma (12).
HNSCC is a tumor that is often easy to visualize and access in common tumor locations including the oral cavity, oropharynx, and visible or palpable metastases to cervical lymph nodes. Thus, HNSCC represents an ideal malignancy for study with intratumoral agents. Here, we report the results from a phase II study of SD-101 and pembrolizumab in patients with PD-1 Ab-naïve HNSCC with and without prior exposure to cytotoxic chemotherapy in the R/M setting. Two SD-101 dose levels were assessed: 8 mg injected into one lesion and 2 mg per lesion injected in up to four lesions.
Patients and Methods
This was a phase II study assessing the efficacy of the combination of SD-101 and pembrolizumab in advanced/metastatic HNSCC. Patients were stratified on the basis of level of PD-L1 expression and HPV status (positive or negative).
Two dose levels of intratumoral SD-101, 2 mg per injection for up to four injections and 8 mg in one single injection, were tested.
Inclusion criteria
Eligible patients were 18 years or older; with Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; adequate bone marrow, liver, and renal function; and histologically or cytologically confirmed R/M anti-PD-1/PD-L1–naïve HNSCC that could not be treated with curative intent. At least two measurable (RECIST v1.1) sites of disease were required (13), including at least one accessible, measurable lesion that was amenable to multiple intratumoral injections. Investigators were advised to select lesions for injection that were of sufficient size so that biopsies of these lesions would not significantly impact tumor response assessments, but exceptions to biopsy requirements were permitted when biopsies were judged to be medically inappropriate or technically infeasible. Tumors situated in previously irradiated areas or in areas subjected to other locoregional therapy were not considered to be measurable unless they had demonstrated growth following local therapy. Patients with any prior combination therapy involving agents given by intratumoral injection targeting the innate immune system such as oncolytic viral or microbial therapy (e.g., talimogene laherparepvec), TLR agonists, STING, or RIG-1 and an anti-PD-1/L1 inhibitor were excluded from the study.
Patients were ineligible if they had known immunodeficiency, active hepatitis B, hepatitis C, human immunodeficiency virus infection, autoimmune disease requiring systemic treatment, or a history of pneumonitis requiring steroids. Patients could not be pregnant or breastfeeding, or expecting to conceive or father children within the projected duration of the trial through 120 days after the last dose of study treatment.
Written, informed consent was obtained from all patients prior to study participation under the supervision of the Institutional Review Boards at participating institutions. All study procedures were performed in accordance with Good Clinical Practice guidelines and the Code of Ethics of the World Medical Association as outlined in the Declaration of Helsinki.
Study design
This was a phase II single-arm study, performed in patients with R/M HNSCC. Patients received either an 8 mg dose of SD-101 injected into a single lesion or a 2 mg per lesion dose was injected into each of one to four lesions to explore the potential impact of per-lesion dose exposure and the impact of exposure to SD-101 in multiple lesions that might vary in antigen repertoire. All patients were scheduled to receive two courses of SD-101 separated by 9 weeks. Each course was comprised of four weekly doses followed by up to seven additional doses administered every 3 weeks on schedule with pembrolizumab. Pembrolizumab was administered intravenously at a fixed dose of 200 mg on day 1 and then every 3 weeks until confirmed disease progression, unacceptable adverse reaction, or until 35 doses were completed. SD-101 was administered before pembrolizumab on days when both drugs were scheduled to be administered. The protocol allowed patients with unconfirmed PD who were clinically stable to continue on study until confirmed PD [per immune-related RECIST (irRECIST; ref. 14)] per investigator discretion and with Dynavax Medical Monitor approval. Pembrolizumab could be continued as a single agent if SD-101 was discontinued because of AEs per investigator decision.
Objectives
The primary objective of this study was to determine the systemic overall response rate (ORR) for the combination of SD-101 and pembrolizumab. Secondary objectives included response rate in injected (local response) and uninjected lesions (distant response), safety and tolerability of SD-101 in combination with pembrolizumab, and additional clinical parameters including time to response, duration of response (DOR), and progression-free survival (PFS). Exploratory objectives included identifying changes in correlative biomarkers including tumor-infiltrating lymphocytes and PD-L1 expression at baseline and after SD-101 and pembrolizumab treatment.
Safety assessment
The safety of the combination of pembrolizumab and SD-101 was assessed on the basis of AE reporting, vital signs, and physical examination, electrocardiograms, and laboratory tests. AEs were graded using the NCI Common Terminology Criteria for Adverse Events Version 4.03 (https://www.eortc.be/services/doc/ctc/ctcae_4.03_2010-06-14_quickreference_5x7.pdf). All safety data were analyzed descriptively and were based on the all-treated population.
Efficacy assessment
Efficacy endpoints included the ORR, DOR, disease control rate (DCR), PFS, and OS. Disease evaluation was performed at week 10 and then every 9 weeks thereafter. For the calculation of PFS, initial PD per RECIST v1.1 had to be confirmed per irRECIST (14), and a follow-up scan obtained at least 4 weeks later that also met the criteria of PD per RECIST v1.1 was required. The ORR was defined as the proportion of patients who achieved a best objective response of either complete response (CR) or partial response (PR). The DCR is the proportion of patients who had CR, PR, or stable disease for at least 9 weeks. The time to response was defined as the time from the first dose of SD-101 until the onset of CR or PR. The DOR was defined as the period of time from the date of initial confirmed PR or CR until the date of PD or death, whichever was earlier. The PFS was defined as the period of time from baseline to confirmed PD or death. OS was defined as the period of time from baseline to death.
Assessment of response and progression for determining study efficacy endpoints were based on RECIST v1.1. Treatment continuation decisions were based on the irRECIST modification of RECIST v 1.1 (14, 15). Target lesions were chosen independently from the choice of lesions for injection. Injected lesions that were also chosen as target lesions were included in sum of diameters measurements. Injected lesions that were not chosen as RECIST targets contributed to response assessments as nontargets. Written informed consent had to be obtained from a patient prior to receiving study treatment after confirmed disease progression. Patients treated beyond progression were followed for survival until the end of study.
At any time during the study, if pembrolizumab was discontinued, then SD-101 also had to be discontinued. Subjects who discontinued both SD-101 and pembrolizumab were followed for assessment of response and progression until confirmed PD, and then followed for survival. Subjects who started new anticancer therapy were followed for survival.
Study objectives, sample size, and statistical analysis
Recruitment of approximately 60 patients with unresectable or metastatic disease, who had no previous exposure to anti-PD-1 Abs was anticipated. The safety population included all enrolled patients who received at least one dose of both pembrolizumab and SD-101. The intent-to-treat efficacy population was comprised of all patients who received at least one dose of either pembrolizumab or SD-101, regardless of whether they had a postbaseline radiographic assessment.
The primary objective of the study was to assess the tumor response both locally and systemically. The primary endpoint was ORR. Assessments of treatment responses in injected and noninjected lesions were included as part of the primary objective. Secondary objectives included assessing the safety and tolerability of the treatment combination, assessing the time frame of tumor responses (including time to response and DOR) and assessing PFS. Exploratory objectives included assessing changes in the tumor and in the peripheral blood associated with treatment.
The reported results were taken from an expansion cohort for patients with PD-1 Ab-naïve HNSCC. Prior to the initiation of the study, the plan was to enroll approximately 25 patients with a null hypothesis that the response rate is < 20% was be tested against a one-sided alternative at a significance level of 0.05. The design provided greater than 80% power to detect a true response rate > 40%. There were no adjustments for multiplicity. Ultimately, separate cohorts of PD-1 Ab-naïve patients were evaluated at different SD-101 doses and the cohorts were pooled for post hoc analysis.
PD-L1 and HPV expression assessment
PD-L1 expression was assessed using PD-L1 IHC 22C3 PharmDx assay and reported as CPS = number of PD-L1+ cells (tumor cells, lymphocytes, macrophages) divided by total number of tumor cells × 100.
Tumor p16 status was identified through verification of all pathology reports. Tumors were categorized as p16 positive (p16+) if the pathology report referred to widespread, diffuse, or strong staining for p16, described staining in 70% or more of tumor cells, or indicated that the tumor was positive for p16 without other qualifiers. Tumors were categorized as p16 negative (p16−) if the p16 staining was noted as “negative,” less than 70%, or staining was only focally positive.
Immune expression profiling in tumors
Tissue from baseline and on-therapy intratumorally injected lesions was collected via punch or core-needle biopsy during screening and 1 week after the fourth SD-101 dose, placed into RNA stabilizing agent (RNAlater, Qiagen), and frozen. RNA was isolated and analyzed with the nCounter PanCancer Immune Profiling Panel (NanoString Technologies, Inc.) to evaluate the immunophenotype of the tumor microenvironment. NanoString data were analyzed using the nSolver Analysis Software. Statistical analyses were performed using Prism software v5 (GraphPad Software). A two-tailed paired t test was used to compare pre-treatment and on-treatment samples.
Results
A total of 51 patients were enrolled in this phase II study. Twenty-eight patients were treated with 2 mg per lesion with a maximum total dose of 8 mg (four lesions) and 23 patients were treated with 8 mg SD-101 injected into a single tumor on each intratumoral treatment date. One patient was taken off study before starting any treatment.
Demographics and baseline disease characteristics of the patients treated on study are displayed in Table 1. A majority of patients had oral or oropharyngeal primary tumors. Most of the patients had either distant metastases or distant metastases plus local recurrence (92.6% in the 2 mg group and 91.3% in the 8 mg group), and 52% of patients in the 2 mg group and 61% in the 8 mg group, respectively, had PD-L1–positive tumors. Thirty-six percent and 26% of patients were HPV positive (HPV+), respectively. Twelve patients had prior exposure to cetuximab including 3 patients in the 2 mg cohort and 9 patients in the 8 mg cohort. A majority of patients had had surgery and radiotherapy, and 76.5% had received one previous line of systemic therapy.
Table 1.
Patient demographics and baseline disease characteristics.
| 2 mg per injection | 8 mg one injection | Total | ||
|---|---|---|---|---|
| Patients' characteristics - n (%) unless noted | (N = 28) | (N = 23) | (N = 51) | |
| Age – Median (range) | 63 (38–93) | 65 (43–91) | 64.5 (38–93) | |
| Sex | Male | (67.9) | (91.3) | (78.4) |
| Female | (32.1) | (8.7) | (21.6) | |
| ECOG PS | 0 | (17.9) | (26.1) | (21.5) |
| 1 | (82.1) | (73.9) | (78.5) | |
| Primary tumor location | Hypopharyngeal | 2 (7.1) | — | 2 (3.9) |
| Nasopharyngeal | — | 3 (13.0) | 3 (5.9) | |
| Oral | 13 (46.4) | 13 (56.5) | 26 (51.0) | |
| Oropharyngeal | 9 (32.1) | 2 (8.7) | 11 (21.6) | |
| Laryngeal | 3 (10.7) | 4 (17.4) | 7 (13.7) | |
| Unknown | 0 | 1 (4.3) | 1 (2.0) | |
| Staging at enrollment | Local | 2 (7.1) | 2 (8.7) | 4 (7.8) |
| Metastatic | 16 (57.1) | 14 (60.9) | 30 (58.8) | |
| Local + Metastatic | 10 (35.7) | 7 (30.4) | 17 (33.3) | |
| Organ involvement | Liver | 2 (7.1) | 2 (8.7) | 4 (7.8) |
| Lung | 9 (32.1) | 6 (26.1) | 15 (29.4) | |
| Bone | 2 (7.4) | 2 (8.7) | 4 (7.8) | |
| Skin/subcutaneous | 3 (10.7) | 8 (34.8) | 11 (21.6) | |
| Tissue | 16 (57.1) | 11 (47.8) | 27 (52.9) | |
| Lymph nodes | 13 (46.4) | 16 (69.6) | 29 (56.9) | |
| Other organs | ||||
| Prior locoregional therapy | Prior radiotherapy | 21 (75.0) | 20 (87.0) | 41 (80.3) |
| Prior surgery | 24 (85.7) | 22 (95.7) | 46 (90.2) | |
| Prior lines of systemic therapy | 0 | 9 | 3 | 12 |
| 1 | 15 | 11 | 26 | |
| 2 | 3 | 6 | 9 | |
| ≥3 | 1 | 3 | 4 | |
| Number of target lesions | 1 | 11 (39.3) | 6 (26.1) | 17 (33.3) |
| 2 | 10 (35.7) | 5 (21.7) | 15 (29.4) | |
| 3 | 4 (14.3) | 8 (34.8) | 12 (23.5) | |
| 4 | 1 (3.6) | 2 (8.7) | 3 (5.9) | |
| 5 | 2 (7.1) | 2 (8.7) | 4 (7.8) | |
| PD-L1 status | <1 | — | — | — |
| ≥1 to <20 | 16 (59.3) | 19 (82.6) | 35 (70.0) | |
| ≥20 | 11 (40.7) | 4 (17.4) | 15 (30) | |
| HPV status | Negative | 11 (39.3) | 6 (26.1) | 17 (33.3) |
| Positive | 10 (35.7) | 6 (26.1) | 16 (31.4) | |
| Unknown | 7 (25.0) | 11 (47.8) | 18 (35.2) | |
Safety
Of patients who were evaluable for toxicity, 92.6% of patients in the 2 mg cohort and 100% of patients in the 8 mg cohort had at least one treatment-emergent AE (TEAE; Table 2). Grade 3 and higher AEs included fatigue, (24%) myalgia (24%), vomiting, constipation, increased gamma-glutamyl transferase (GGT), injection site erythema, and pyrexia. Eleven percent and 22% experienced predefined immune-related AEs of interest in the 2 and 8 mg group, respectively, including 6 patients with thyroid dysfunction and 1 patient each with pneumonitis and colitis. Four treatment-related serious AEs were observed including 1 patient each with atrial fibrillation, systemic inflammatory response syndrome, infusion reaction, and dehydration. There were no treatment-related deaths on study.
Table 2.
TEAEs and all grade 3 or 4 TRAEs observed in at least 5% of patients.
| 2 mg (N = 27) | 8 mg (N = 23) | |||
|---|---|---|---|---|
| Event | Any grade TEAE | Grade 3–4 TRAE | Any grade TEAE | Grade 3–4 TRAE |
| Any | 92.6 | 14.8 | 100 | 34.8 |
| ALT Increased | 7.4 | — | 8.7 | — |
| Anemia | 14.8 | — | 21.7 | — |
| Anxiety | — | 13 | — | |
| Chills | 11.1 | — | 43.5 | — |
| Constipation | 14.8 | — | 17.4 | — |
| Cough | 11.1 | — | 8.7 | — |
| Decreased appetite | 11.1 | — | 13 | — |
| Dehydration | — | 13 | 4.3 | |
| Diarrhea | — | 30.4 | — | |
| Dizziness | 18.5 | — | 4.3 | — |
| Dysphagia | 18.5 | — | 26.1 | — |
| Fatigue | 55.6 | — | 73.9 | 17.4 |
| Headache | 11.1 | — | 43.5 | 4.3 |
| Hyponatremia | 11.1 | 4.3 | 8.7 | — |
| Injection site erythema | 3.7 | — | 17.4 | — |
| Injection site pain | — | 26.1 | 8.7 | |
| Injection site swelling | 7.4 | — | 13 | — |
| Insomnia | 11.1 | — | 8.7 | — |
| Malaise | — | 52.2 | — | |
| Myalgia | 3.7 | — | 39.1 | 8.7 |
| Nausea | 29.6 | — | 34.8 | — |
| Pneumonia | 14.8 | — | 4.3 | — |
| Pruritus | 7.4 | — | 17.4 | — |
| Pyrexia | 22.2 | — | 26.1 | — |
| Tumor hemorrhage | 18.5 | — | — | — |
| Tumor pain | 14.8 | — | 8.7 | — |
| Vomiting | 18.5 | — | 13 | — |
Note: No grade 5 treatment-related adverse events were observed.
Efficacy
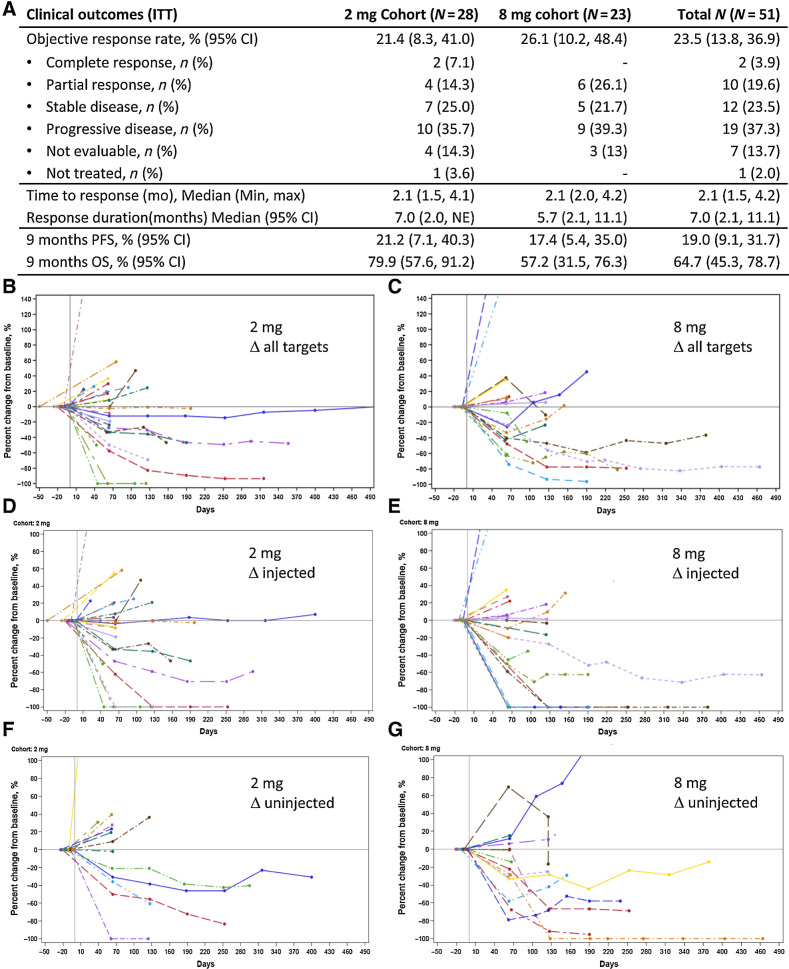
The objective responses rate was 21% in the 2 mg group and 26% in the 8 mg dose group, for a global response rate of 24% in the total population (Fig. 1A). Two CRs (7.1%) were observed in the 2 mg group and the overall DCR was 47%. The time to response of 2.1 months was consistent between the two groups.
Figure 1.
A, Clinical outcomes in the intent-to-treat population. B–G, The percent change in lesions over time. B, 2 mg cohort, all targets. C, 8 mg cohort, all targets. D, 2 mg cohort, injected. E, 8 mg cohort, injected. F, 2 mg cohort, uninjected. G, 8 mg cohort, uninjected.
With a median follow-up of 3.1 months for the 2 mg group and 5.6 months for the 8 mg group, the median DOR was 7.0 months in the 2 mg group and 5.7 months in the 8 mg group (Fig. 1A; Supplementary Fig. S1). The median PFS for the 2 mg and 8 mg cohorts were 2.5 months (2.0–4.2) and 2.3 months (2.1–4.1), respectively. The landmark PFS rates at 9 months, chosen based on available follow-up data as a surrogate for long-term benefit, were 21.2% (95% CI: 7.1–40.3) in the 2 mg group and 17.4% (95% CI: 5.4–35.0) in the 8 mg group. The median OS for the 2 mg and 8 mg cohorts were not reached (9.0–not reached) and 9.0 months (5.6–11.2), respectively. The OS rate at 9 months was 79.9% (95% CI: 57.6–91.2) and 57.2% (95% CI: 31.5–76.3), respectively.
The ORR varied according to the disease stage, with a 27% ORR in purely metastatic disease, 24% in metastatic and local relapse, and 0% in local relapse only (Supplementary Table S1). The nominal ORR was higher in patients with oropharyngeal (36%) and laryngeal (50%) primaries, and lower in patients with oral (12%) primaries. Very few patients with nasopharyngeal (N = 3) or hypopharyngeal (N = 2) carcinoma were treated on study and there were no objective responses in these patients. There were no objective responses in subjects with liver metastases while 38% of subjects with other metastatic visceral locations including the lung had objective responses.
The ORR also varied on the basis of this site of the injected lesion, with 8 responses in 22 patients with nodal lesions injected (36%) and response rates tended to be lower at most other sites, although formal comparison is limited by the sample sizes in these subgroups (Supplementary Table S2). Responses were also uncommon following injection into previously irradiated sites, including in the 4 patients with local recurrence only after irradiation. One of the patients with locally recurrent disease had paired biopsies that showed an increase in immune markers on treatment.
In 50 patients with available PD-L1 results, there were no PD-L1 CPS <1 tumors. In tumors that expressed PD-L1 CPS ≥1 to <20 (n = 35) and ≥20 (n = 15), the objective response rate was 22.9% and 26.7%, respectively (Supplementary Table S1). Response rates appeared to be higher in HPV+ tumors (44%) compared with HPV-negative tumors (12%). As in previous reports in patients receiving PD-1 Ab monotherapy, there were few responses in patients with prior exposure to the anti-EGFR mAb cetuximab (ORR with prior cetuximab 8.3% vs. 35.5% in patients without prior exposure; ref. 3).
Similar rates of tumor regression occurred in injected and noninjected lesions (Fig. 1B–G; Supplementary Fig. S2).
Tumor biopsies
Paired samples were available from 21 of the 51 patients. The principal reasons for the absence of paired data were technical failure of a sample, failure to collect biopsies both pre- and on-treatment, patient refusal for on-treatment samples, and failure to collect on-treatment samples due to withdrawal of consent or rapid progression. Most patients with objective responses (8/12; 67%) and PD (12/19; 63%) had evaluable paired biopsies. Patients with stable disease as the best response (5/12; 42%) and those with clinical progression (0/7; 0%) were underrepresented.
Immune expression profiling in tumors
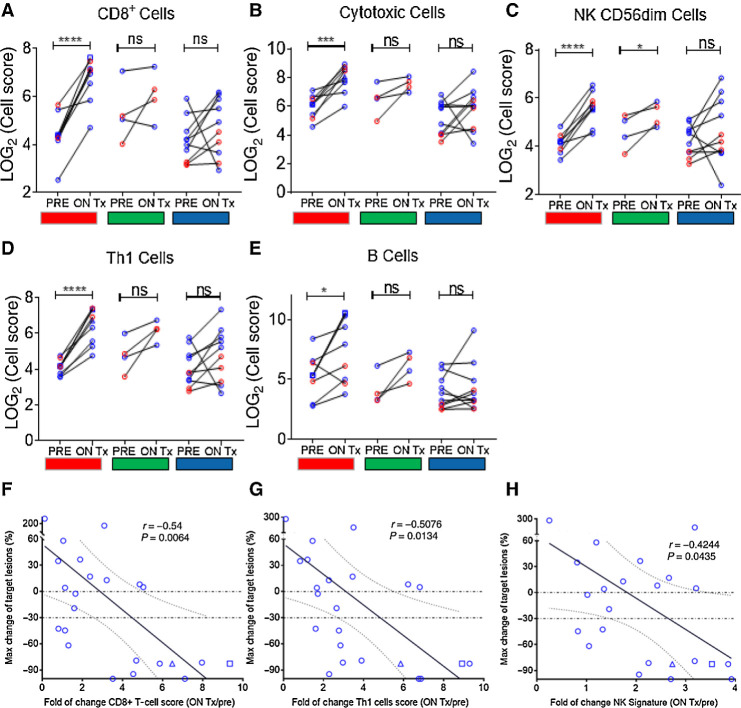
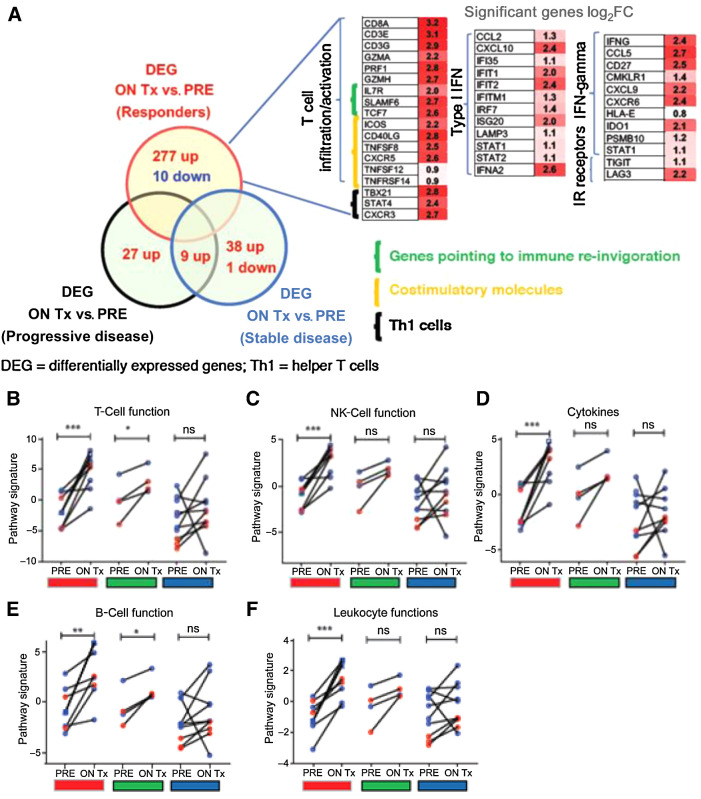
NanoString transcriptomic analysis of baseline and on-therapy biopsies from SD-101 injected lesions showed consistent increases in the expression of genes representing a variety of immune cell types, including CD8+ T cells, cytotoxic T cells, activated natural killer (NKCD56dim) cells, Th1 cells, and B cells in patients treated with SD-101 in combination with pembrolizumab. Changes were seen primarily in responding patients (Fig. 2A–E). Changes in CD8+, Th1, and natural killer (NK)-cell scores were correlated with changes in tumor burden (Fig. 2F–H). Changes in transcript associated with immune cell function were also significantly upregulated following SD-101 treatment (Fig. 3A–E). These changes were significantly more pronounced in patients experiencing a clinical response (PR or CR) when compared with patients with PD. One patient who underwent additional biopsies 3 weeks after the sixth SD-101 injection and 3 weeks after the 10th SD-101 injection, had consistent upregulation of immune cell signatures, possibly suggesting the establishment of a self-perpetuating cycle of immune cell activation (Figs. 2 and 3).
Figure 2.
A–E, Transcriptomic analysis of baseline and on-therapy biopsies from SD-101 injected lesions showed consistent increases in the expression of genes representing a variety of immune cell types, including CD8+ T cells, cytotoxic T cells, activated NK (NKCD56dim) cells, Th1 cells, and B cells in patients treated with SD-101 in combination with pembrolizumab. Changes were seen primarily in responding patients (red). F–H, Changes in CD8+, Th1, and NK cell scores were correlated with changes in tumor burden. All patients had one biopsy at baseline and second biopsy 1 week following the fourth SD-101 treatment. One patient received two additional biopsies at day 106 (3 weeks after the sixth SD-101 injection, blue square) and day 190 (3 weeks after the 10th SD-101 injection, blue triangle). Fold of changes in lymphocyte infiltration within SD-101–treated lesions was correlated with the maximum percentage change in target lesions from baseline using Pearson correlation coefficient.
Figure 3.
Treatment with SD-101 and pembrolizumab induces increased transcripts associated with A. T-cell function (B), NK-cell function (C), pro-inflammatory cytokines (D), B-cell function (E), and leukocyte function (F). These increases were observed primarily in responding patients (red) although there were also significant increases in the expression of transcripts associated with B- and T-cell function in patients with stable disease (green) as the best response. Data shown as open red circles (2 mg cohort) and blue circles (8 mg cohort). All patients included in the analysis had one biopsy at baseline and second biopsy 1 week following the fourth SD-101 treatment. One patient received two additional biopsies at day 106 (3 weeks after the sixth SD-101 injection, blue square) and day 190 (3 weeks after the 10th SD-101 injection, blue triangle). F, Broad changes in gene expression were observed primarily in responding patients including increased transcripts associated with T-cell infiltration and activation, type I IFN, IFNγ, and IR receptors. A–E, All statistical analyses were performed using Prism software v5 (GraphPad Software). A two-tailed paired t test was done between matched on-treatment (ON Tx) and baseline (PRE) biopsies. F, Analysis of changes in gene expression was performed using nSolver software (Nanostring). Differentially expressed genes (DEG; cutoff:LOG2FC ± 0.6, P = 0.05) in between matched on-treatment (ON Tx) and baseline (PRE) biopsies. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; and ****, P ≤ 0.0001.
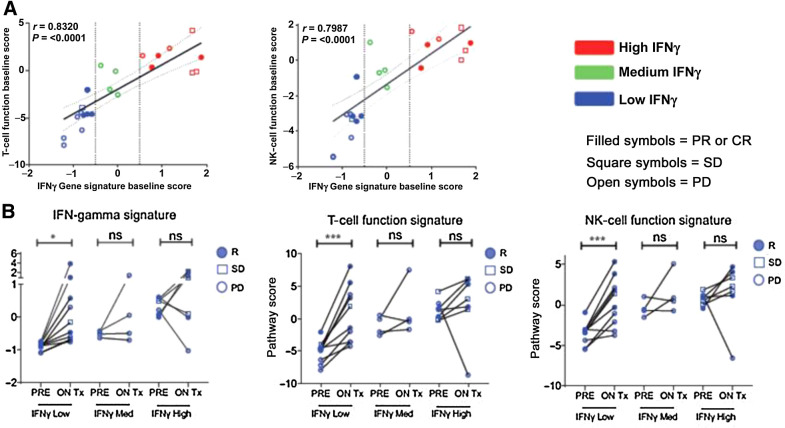
More broadly, changes in gene expression were observed in responding patients beyond those associated with T-cell infiltration and activation, and these included increases in transcripts associated with type I IFN, IFNγ, and IR receptors (Fig. 3F). Furthermore, baseline inflammatory gene expression was not a prerequisite for response. Patients whose tumors exhibited a low IFNγ signatures score at baseline (<−0.05) also had low T-cell and NK-cell function signature scores, consistent with immunologically cold tumors, but even these patients responded to the combination of SD-101 and pembrolizumab, demonstrating immunomodulation of relevant gene signature scores associated with clinical response (Fig. 4).
Figure 4.
Immune-related changes by baseline immune status in HNSCC tumor biopsies. A, Patients whose tumors exhibited a low IFNγ signature score at baseline (blue; score < −0.05) had low T-cell and NK-cell function signature scores, consistent with immunologically cold tumors. B, Patients whose tumors showed low IFNγ at baseline responded to SD-101 and demonstrated immunomodulation of relevant gene signature scores and clinical response. All statistical analyses were performed using Prism software v5 (GraphPad Software). A, Correlation was assessed using Pearson correlation coefficient. B, A two-tailed paired t test was done between matched on-treatment (ON Tx) and baseline (PRE) biopsies. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; and ****, P ≤ 0.0001.
Discussion
The combination of SD-101 and pembrolizumab induced objective responses in 24% of patients, the vast majority of whom had received one or more prior lines of cytotoxic chemotherapy. Treatment was well tolerated, with grade 3 or higher AEs only observed in 24% of patients. Although this response rate did not exceed the threshold for clinical significance established a priori at either dose level, with the added context of our translational data, our data suggest the addition of SD-101 to pembrolizumab may lead to biologically meaningful changes in the tumor microenvironment. In particular, treatment increased inflammatory gene expression and cellular immune responses, both factors that have been associated with improved outcomes in patient treated with PD-1 Abs. Although prior studies have demonstrated that the addition of SD-101 to pembrolizumab can induce objective immune responses in patients with PD-1 Ab-refractory melanoma, the relative contribution of SD-101 in the current study to improved clinical outcomes is more difficult to isolate. Cross-trial comparisons are complicated by differences in study populations, but this 24% response rate compares favorably with the response rates observed in patients with PD-L1–positive tumors receiving pembrolizumab (ORR = 17.0) or nivolumab (ORR = 17.3%) monotherapy in the second line (1, 3). Similar patients in the postchemotherapy setting (with > 1% PD-L1 expression) treated with PD-1 Ab monotherapy have demonstrated a median OS of 8.7 months, which was exceeded in our population, although this could be influenced by patient selection and other uncontrolled differences between studies. Collectively, these results suggest that intratumoral therapy with SD-101 in combination with pembrolizumab is active in HNSCC, a noncutaneous solid tumor often with lesions that are accessible to intratumoral therapy.
It is also notable that the response rates were particularly high in patients with HPV-associated tumors. Similar findings were observed in a study of the PD-L 1 Ab durvalumab administered as monotherapy in patients with PD-L1 expression levels higher than 25%, in which the response rate in HPV-associated HNSCC was also higher than seen in virus-negative tumors (29.4% vs. 10.9%, respectively; ref. 16). Post hoc analyses of HNSCC patients treated with PD-1 monotherapy suggest that the tumor mutation burden is correlated with the rate of response to anti-PD-1 Abs in patients with non-virally associated malignancies, but not in patients with HPV- or Epstein-Barr Virus (EBV)-associated disease. This suggests that viral proteins, for example, the E6/E7 protein produced by HPV, may be inherently antigenic (17, 18). In contrast to the tumor mutation burden, higher levels of intratumoral inflammation were associated with response in both virally associated and non-virally associated HNSCC. Thus, it is possible that increases in intratumoral inflammation caused by SD-101 combined with the antigenic E6/E7 proteins in HPV-associated tumors provided optimal conditions for response to pembrolizumab. One caveat to this argument is that all patients included in the current study had PD-L1 CPS scores greater than 1 and objective response rates were similar in patients across baseline CPS scores. We also acknowledge that the limited sample size (N = 16) in the HPV+ subgroup and the inclusion of 4 patients with p16+ tumors originating outside the oropharynx (3 larynx, 1 oral cavity) could impact interpretations of these data.
Our biomarker data suggested, however, that intratumoral inflammation cannot be reduced to a simple binary function of “positive” or “negative” in our PD-L1–positive population. Treatment with SD-101 in combination with pembrolizumab induced many of the cellular changes associated with increased intratumoral inflammation, particularly in responding patients. These changes included increases in CD8+ cells overall, and cytotoxic cells, TH-1–positive cells, and B cells. In addition, there were signs that treatment modulated innate immunity yielded an increase in intratumoral NK cells in patients benefiting from therapy, including those with stable disease. Many of these changes were positively correlated with a decrease in target lesion diameter. In addition, paired biopsy analysis demonstrated an increase in T-cell, NK-cell, and B-cell functionality in patients benefiting from treatment. Thus, the combination of SD-101 and pembrolizumab yielded favorable changes in the tumor immune microenvironment that were associated with clinical responses supporting the role of inflammatory agents even in tumors that are nominally PD-L1 positive.
The potential role of SD-101 in potentiating responses to pembrolizumab in HNSCC is consistent with existing data in other advanced solid tumors. In a phase IB study, SD-101 in combination with pembrolizumab induced objective responses in 8 of 9 patients with treatment-naïve melanoma and objective responses were also seen in patients with documented PD-1 Ab-refractory disease (12). SD-101 has also induced systemic responses in combination with low-dose radiation in patients with untreated indolent lymphoma (11), and TLR agonists alone have also induced responses in treated and untreated lesions in mycosis fungoides (19). Most notably, the TLR8 agonist motolimod failed to improve the median PFS in patients with HNSCC overall when added to chemotherapy (20), but there was a significant improvement in a preplanned analysis of patients with HPV+ disease, mirroring our findings in patients treated with SD-101 and pembrolizumab.
In summary, the combination of SD-101 and pembrolizumab induced objective responses in patients with R/M HNSCC and translational data suggest substantial improvements in cellular immune responses, particularly in patients who benefit from treatment. This combination warrants further testing in a randomized phase III clinical trial, particularly in patients with HPV+ tumors.
Supplementary Material
Acknowledgments
This study was funded by Dynavax Technologies, Inc.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
E.E.W. Cohen reports personal fees from MSD outside the submitted work. D.J. Wong reports grants from Dynavax during the conduct of the study. D.J. Wong also reports grants from Merck Sharp and Dohme, Bristol Myers Squibb, Checkmate Pharmaceuticals, Pfizer, TopAlliance, FSTAR, Iovance, LOXO Therapeutics, Elevar Therapeutics, and Enzychem; grants and personal fees from Regeneron; and personal fees from Sanofi outside the submitted work. T. Day reports other support from Dynavax during the conduct of the study. M. Milhem reports other support from Syneos, Exicure, Novartis, Immunocore, BioNTech, Amgen, Array, and Trieza outside the submitted work. M. Jameson reports other support from Dynavax Technologies during the conduct of the study, as well as other support from Bayer, Beigene, Roche, Bristol Meyers Squibb, GlaxoSmithKline, Gilead, Huya, Sanofi, Merck, Millenium, and Pfizer outside the submitted work. O. Guntinas-Lichius reports grants from MED-EL and other support from Merz Pharma outside the submitted work. E. Whitman reports personal fees from Merck, Bristol Myers Squibb, Regeneron, and Sanofi Genzyme outside the submitted work. M. Chisamore reports other support from Merck & Co., Inc outside the submitted work. R. Janssen reports other support from Dynavax Technologies Corporation during the conduct of the study, as well as other support from Dynavax Technologies Corporation outside the submitted work. E. Gamelin reports personal fees from Dynavax during the conduct of the study, as well as personal fees from Dynavax outside the submitted work. A.P. Algazi reports other support from Dynavax and Merck during the conduct of the study. A.P. Algazi also reports personal fees and non-financial support from OncoSec; non-financial support from Valitor; personal fees from Regeneron and Array; and other support from BMS, AstraZeneca, Genentech, Tessa, Idera, Checkmate, Sensei, Acerta, Incyte, ISA, Loxo, and Pfizer outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
E.E.W. Cohen: Conceptualization, supervision, writing–original draft, project administration, writing–review and editing. L. Nabell: Supervision, investigation, project administration, writing–review and editing. D.J. Wong: Supervision, investigation, writing–review and editing. T. Day: Supervision, investigation, writing–review and editing. G.A. Daniels: Supervision, investigation, project administration, writing–review and editing. M. Milhem: Supervision, investigation, project administration, writing–review and editing. S. Deva: Supervision, investigation, project administration, writing–review and editing. M. Jameson: Supervision, investigation, project administration, writing–review and editing. O. Guntinas-Lichius: Supervision, investigation, project administration, writing–review and editing. M. Almubarak: Supervision, investigation, project administration, writing–review and editing. M. Strother: Supervision, investigation, project administration, writing–review and editing. E. Whitman: Supervision, investigation, project administration, writing–review and editing. M. Chisamore: Conceptualization, writing–review and editing. C. Obiozor: Data curation, formal analysis, writing–review and editing. T. Bagulho: Data curation, formal analysis, writing–review and editing. J. Gomez-Romo: Data curation, formal analysis, writing–review and editing. C. Guiducci: Conceptualization, resources, data curation, funding acquisition, writing–original draft, writing–review and editing. R. Janssen: Conceptualization, resources, supervision, funding acquisition, investigation, methodology, writing–original draft, writing–review and editing. E. Gamelin: Conceptualization, resources, formal analysis, supervision, funding acquisition, methodology, writing–original draft, writing–review and editing. A.P. Algazi: Data curation, formal analysis, supervision, validation, investigation, writing–original draft, writing–review and editing.
References
- 1. Cohen EEW, Soulières D, Le Tourneau C, Dinis J, Licitra L, Ahn M-J, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 2019;393:156–67. [DOI] [PubMed] [Google Scholar]
- 2. Lala M, Chirovsky D, Cheng JD, Mayawala K. Clinical outcomes with therapies for previously treated recurrent/metastatic head-and-neck squamous cell carcinoma (R/M HNSCC): a systematic literature review. Oral Oncol 2018;84:108–20. [DOI] [PubMed] [Google Scholar]
- 3. Ferris RL, Blumenschein G, Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burtness B, Harrington KJ, Greil R, Soulières D, Tahara M, de Castro G, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 2019;394:1915–28. [DOI] [PubMed] [Google Scholar]
- 5. Siu L, Brody J, Gupta S, Marabelle A, Jimeno A, Munster P, et al. Safety and clinical activity of intratumoral MEDI9197 alone and in combination with durvalumab and/or palliative radiation therapy in patients with advanced solid tumors. J Immunother Cancer 2020;8:e001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Northfelt DW, Ramanathan RK, Cohen PA, Von Hoff DD, Weiss GJ, Dietsch GN, et al. A phase I dose-finding study of the novel Toll-like receptor 8 agonist VTX-2337 in adult subjects with advanced solid tumors or lymphoma. Clin Cancer Res 2014;20:3683–91. [DOI] [PubMed] [Google Scholar]
- 7. Butler MO, Robert C, Negrier S, In GK, Walker JWT, Krajsova I, et al. ILLUMINATE 301: a randomized phase 3 study of tilsotolimod in combination with ipilimumab compared with ipilimumab alone in patients with advanced melanoma following progression on or after anti-PD-1 therapy. Available from: https://meetinglibrary.asco.org/record/178033/abstract.
- 8. Merck Sharp & Dohme Corp. Phase 1 open-label, multicenter study of MK-1454 administered by intratumoral injection as monotherapy and in combination with pembrolizumab for patients with advanced/metastatic solid tumors or lymphomas [Internet]. clinicaltrials.gov; 2020 Dec. Report No.: NCT03010176. Available from: https://clinicaltrials.gov/ct2/show/NCT03010176.
- 9. Wang S, Campos J, Gallotta M, Gong M, Crain C, Naik E, et al. Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells. Proc Natl Acad Sci U S A 2016;113:E7240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sato-Kaneko F, Yao S, Ahmadi A, Zhang SS, Hosoya T, Kaneda MM, et al. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight 2017;2:e93397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Frank MJ, Reagan PM, Bartlett NL, Gordon LI, Friedberg JW, Czerwinski DK, et al. In situ vaccination with a TLR9 agonist and local low-dose radiation induces systemic responses in untreated indolent lymphoma. Cancer Discov 2018;8:1258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ribas A, Medina T, Kummar S, Amin A, Kalbasi A, Drabick JJ, et al. SD-101 in combination with pembrolizumab in advanced melanoma: results of a phase Ib, multicenter study. Cancer Discov 2018;8:1250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 14. Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer 2018;88:38–47. [DOI] [PubMed] [Google Scholar]
- 15. Le Lay J, Jarraya H, Lebellec L, Penel N. irRECIST and iRECIST: the devil is in the details. Ann Oncol 2017;28:1676–8. [DOI] [PubMed] [Google Scholar]
- 16. Zandberg DP, Algazi AP, Jimeno A, Good JS, Fayette J, Bouganim N, et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: results from a single-arm, phase II study in patients with ≥25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur J Cancer 2019;107:142–52. [DOI] [PubMed] [Google Scholar]
- 17. Haddad RI, Seiwert TY, Chow LQM, Gupta S, Weiss J, Gluck I, et al. Genomic determinants of response to pembrolizumab in head and neck squamous cell carcinoma (HNSCC). J Clin Oncol 2017;35:6009. [Google Scholar]
- 18. Wang J, Sun H, Zeng Q, Guo X-J, Wang H, Liu H-H, et al. HPV-positive status associated with inflamed immune microenvironment and improved response to anti-PD-1 therapy in head and neck squamous cell carcinoma. Sci Rep 2019;9:13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim YH, Gratzinger D, Harrison C, Brody JD, Czerwinski DK, Ai WZ, et al. In situ vaccination against mycosis fungoides by intratumoral injection of a TLR9 agonist combined with radiation: a phase 1/2 study. Blood 2012;119:355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferris RL, Saba NF, Gitlitz BJ, Haddad R, Sukari A, Neupane P, et al. Effect of adding motolimod to standard combination chemotherapy and cetuximab treatment of patients with squamous cell carcinoma of the head and neck: The Active8 Randomized Clinical Trial. JAMA Oncol 2018;4:1583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.