Abstract
Purpose:
To evaluate efficacy and safety of venetoclax + azacitidine among treatment-naïve patients with IDH1/2-mutant (mut) acute myeloid leukemia (AML).
Patients and Methods:
Data were pooled from patients enrolled in a phase III study (NCT02993523) that compared patients treated with venetoclax + azacitidine or placebo + azacitidine and a prior phase Ib study (NCT02203773) where patients were treated with venetoclax + azacitidine. Enrolled patients were ineligible for intensive therapy due to age ≥75 years and/or comorbidities. Patients on venetoclax + azacitidine received venetoclax 400 mg orally (days 1–28) and azacitidine (75 mg/m2; days 1–7/28-day cycle).
Results:
In the biomarker-evaluable population, IDH1/2mut was detected in 81 (26%) and 28 (22%) patients in the venetoclax + azacitidine and azacitidine groups. Composite complete remission [CRc, complete remission (CR)+CR with incomplete hematologic recovery (CRi)] rates (venetoclax + azacitidine/azacitidine) among patients with IDH1/2mut were 79%/11%, median duration of remission (mDoR) was 29.5/9.5 months, and median overall survival (mOS) was 24.5/6.2 months. CRc rates among patients with IDH1/2 wild-type (WT) were 63%/31%, mDoR 17.5/10.3 months, and mOS 12.3/10.1 months. In patients with IDH1mut, CRc rates (venetoclax + azacitidine/azacitidine) were 66.7%/9.1% and mOS 15.2/2.2 months. In patients with IDH2mut, CRc rates were 86.0%/11.1% and mOS not reached (NR)/13.0 months. Patients with IDH1/2 WT AML treated with venetoclax + azacitidine with poor-risk cytogenetics had inferior outcomes compared with patients with IDH1/2mut, who had superior outcomes regardless of cytogenetic risk (mOS, IDH1/2mut: intermediate-risk, 24.5 months; poor-risk, NR; IDH1/2 WT: intermediate, 19.2 and poor, 7.4 months). There were no unexpected toxicities in the venetoclax + azacitidine group.
Conclusions:
Patients with IDH1/2mut who received venetoclax + azacitidine had high response rates, durable remissions, and significant OS; cytogenetic risk did not mitigate the favorable outcomes seen from this regimen for IDH1/2mut.
Translational Relevance.
Approximately 20% of patients with acute myeloid leukemia (AML) have isocitrate dehydrogenase (IDH) mutations that result in tumorigenesis due to alterations in cellular metabolism. The preliminary analysis of the ongoing randomized phase III trial (NCT02993523) reported that patients treated with venetoclax and azacitidine had higher remission rates and more prolonged overall survival (OS) than patients treated with placebo and azacitidine. We further evaluated the efficacy and safety of the combination and reported that the remission rates and OS among patients with IDH1/2 mutations were superior as compared with azacitidine alone, and patients with IDH2 mutations had numerically better outcomes than patients with IDH1 mutations. Additional analysis demonstrated that cytogenetic risk by National Comprehensive Cancer Network was not prognostic of OS in patients with IDH1/2 mutations when treated with the combination, whereas among IDH1/2 wild-type, cytogenetic risk was prognostic of OS. These findings warrant validation in future analyses.
Introduction
Acute myeloid leukemia (AML) is a biologically and clinically heterogeneous malignancy (1, 2) in which outcomes to therapy may vary by commonly occurring cytogenetic and molecular features (3). Recurrent mutations in isocitrate dehydrogenase (IDH) enzymes have been identified to occur in approximately 20% of patients with AML, with 7%–14% and 8%–19% of patients possessing the IDH1- and IDH2-mutant subtypes, respectively (4). The IDH enzymes catalyze the conversion of isocitrate to α-ketoglutarate, a critical intermediate in cellular metabolism. Mutations in IDH1/2 produce high levels of the oncometabolite 2-hydroxyglutarate (2-HG; ref. 5). Elevated 2HG levels can lead to multiple downstream effects related to inhibition of α-ketoglutarate-mediated reactions, resulting in a hypermethylated phenotype, impaired cellular differentiation, and ultimately, the promotion of tumorigenesis (1, 6–8). For IDH1, the heterozygous mutations are predominantly located at codon 132 (IDH1R132), and for IDH2, IDH2R140 are the more common alterations than IDH2R172 (9, 10). The prognostic impact of IDH1R132, IDH2R140, and IDH2R172 mutations have not been fully elucidated and likely depend on the presence or absence of other prognostic cooccurring mutations (6, 10, 11).
For treatment-naïve patients with AML and IDH1/2 mutations who are ineligible for induction chemotherapy, there is no standard therapy. Ivosidenib and enasidenib are oral small-molecule inhibitors that are approved for the targeted inhibition of mutant IDH1 and IDH2 in relapsed/refractory AML, respectively. For treatment-naïve and chemotherapy-ineligible IDH1-mutated AML patients, ivosidenib as monotherapy is also approved by FDA (12). The safety and efficacy of both molecules are currently being evaluated in combination with azacitidine (NCT02677922) and have been demonstrated to be safe with encouraging efficacy (13–15).
Venetoclax is a potent BCL-2 inhibitor. Preclinical investigations of venetoclax showed that cells with IDH1/2 mutations were especially susceptible to venetoclax therapy (7). IDH mutations predicted higher rates of response to BCL-2 inhibition through the production of 2-HG and inhibition of cytochrome c oxidase (COX) activity making AML cells dependent on BCL-2 for survival, as COX inhibition increases activation of the BAX/BAK complex leading to apoptosis upon BCL-2 inhibition (7).
In a previous phase 1b study, treatment with venetoclax and a hypomethylating agent (azacitidine or decitabine) resulted in a favorable composite complete remission [CRc, defined by complete remission (CR) plus CR with incomplete hematologic recovery (CRi)]. CR rate was 71% in treatment-naive ineligible patients with AML and IDH1/2 mutation (16). The results from the randomized phase III study demonstrated significantly higher CRc rates (75.4% vs. 10.7%) and longer median overall survival [not reached (NR) vs. 6.2 months] for the venetoclax and azacitidine group as compared with azacitidine alone (17). Herein, we further detail the efficacy and safety of venetoclax and azacitidine among treatment-naïve patients with AML and co-morbidities, and/or age ≥ 75 years, ineligible for intensive treatment and harboring IDH1/2 mutations. We also explore the prognostic impact of cytogenetic risk and molecular genetics on treatment.
Patients and Methods
Patients and treatment
This pooled analysis included patients from the phase III study (NCT02993523, VIALE-A) and a non-randomized, single-arm phase Ib study (NCT02203773). The full study designs and eligibility criteria have been previously reported (16, 17). Patients assessed in this pooled analysis were scheduled to receive either 400-mg venetoclax by mouth daily on days 1–28 and 75 mg/m2 azacitidine intravenously or subcutaneously on days 1–7 every 28-day cycle, or azacitidine monotherapy. Individuals enrolled in both trials had a confirmed diagnosis of AML by the World Health Organization criteria and must have been ineligible for standard induction chemotherapy due to age ≥75 years or the presence of co-morbidities.
Both studies were approved by the local ethics committees and were conducted in accordance with the International Conference on Harmonization, Good Clinical Practice guidelines, and the Declaration of Helsinki. All patients provided written informed consent.
Assessment of outcomes
Response assessments were performed at screening, end of cycle 1, and every three cycles thereafter and were evaluated per modified International Working Group (IWG) response criteria for AML (18). Duration of CRc was defined as the number of days from the date of first response (CR or CRi) per the modified IWG criteria for AML to the earliest evidence of confirmed morphologic relapse, confirmed disease progression, or death due to disease progression. OS was defined as the time from randomization to the date of death from any cause. Adverse events were graded according to the NCI Common Terminology Criteria for Adverse Events Version 4.0 (19). Baseline cytogenetic risk was determined locally and evaluated using the National Comprehensive Cancer Network (NCCN) criteria (Supplementary Table S1; ref. 17).
Assessment of molecular data
DNA was isolated from bone marrow aspirates collected from patients prior to the first dose of the study drug and analyzed centrally. Different assays were used to detect IDH1 and IDH2 mutations across the two studies. The phase Ib study utilized the MyAML panel (Invivoscribe), while patient samples from the Phase 3 study were analyzed using the MyAML panel and/or a RealTime IDH1 or IDH2 assays (Abbott). Concordance between the RealTime IDH1 or IDH2 assays and MyAML assays for IDH1/2 mutation was evaluated using positive percent agreement and negative percent agreement instead of sensitivity and specificity as both assays have the potential for false-negative tests. The limit of detection was 2% and 2.5% for the CDx assay and MyAML assay, respectively. Patients with missing specimens or inconclusive results were excluded from the analysis. FLT3 mutations were evaluated by the MyAML assay for the phase Ib study and the Leukostrat FLT3 CDx assay and/or MyAML for the phase III study. In addition, the MyAML assay was also used to detect the presence of other mutations, including NPM1 and TP53 in both the phase Ib and phase III studies.
Statistical analysis
Demographics were summarized by descriptive statistics. Remission rates were summarized in counts and proportions. OS and DoR were evaluated by the Kaplan–Meier methodology. The HRs and 95% confidence intervals (CI) between treatment groups were estimated using the Cox proportional-hazards model.
Data sharing statement
This clinical trial data can be requested by any qualified researchers who engage in rigorous, independent scientific research and will be provided following the review and approval of a research proposal and Statistical Analysis Plan (SAP) and execution of a Data Sharing Agreement (DSA). Data requests can be submitted at any time, and the data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing/data-and-information-sharing-with-qualified-researchers.html.
Results
Patient disposition and baseline characteristics
The clinical data cut-off dates were January 4, 2020, for the phase III study and July 19, 2019, for the phase Ib study. The pooled analysis included 353 patients in the venetoclax and azacitidine group (phase III, n = 286; phase Ib, n = 67) and 145 patients in the azacitidine group.
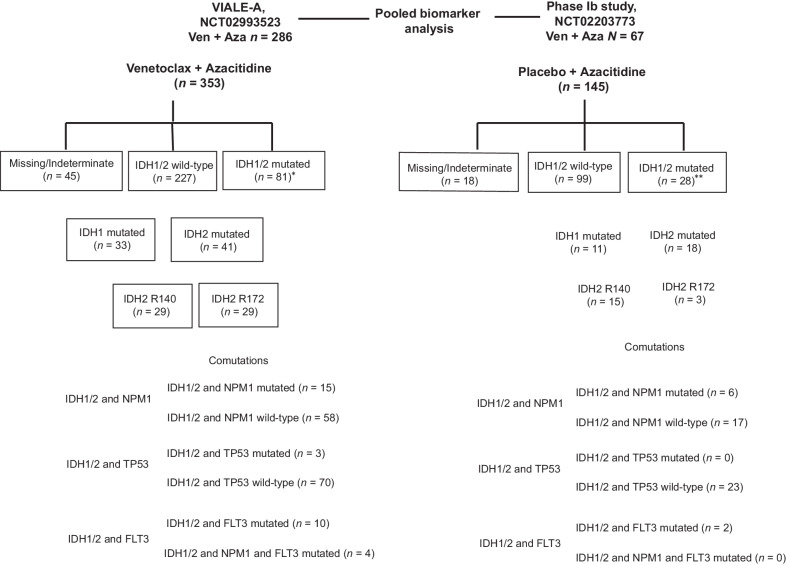
The biomarker evaluable population included 308/353 (87%) venetoclax and azacitidine patients and 127/145 (88%) azacitidine patients. IDH1/2 mutations were detected in 81 of 308 (26%) in the venetoclax and azacitidine group and 28 of 127 (22%) in the azacitidine group. Two patients in the venetoclax and azacitidine group and one patient in the azacitidine group had both IDH1 and IDH2 mutations, respectively. An overview of the study design and the molecular categorization of patients is shown in Fig. 1.
Figure 1.
Study design and molecular classification. *Two patients had both IDH1/2 mutations. **One patient had both IDH1/2 mutations. Ven, venetoclax; Aza, azacitidine.
Baseline characteristics of patients with IDH1/2 mutations are shown in Table 1. Compared with IDH1/2-mutated patients, IDH1/2 WT had a higher incidence of poor-risk cytogenetics [venetoclax and azacitidine group (IDH1/2 mutated vs. IDH1/2 WT): 23.5% vs. 42.7%; azacitidine group: 32.1% vs. 42.4%], and a higher incidence of blast percentage <30 [venetoclax and azacitidine group (IDH1/2 mutated vs. IDH1/2 WT): 17.3% vs. 36.1%; azacitidine group: 21.4% vs. 30.3%].
Table 1.
Baseline characteristics of patients.
| Venetoclax + Azacitidine | Azacitidine | |||
|---|---|---|---|---|
| IDH1/2 mutated | IDH1/2 wild-type | IDH 1/2 mutated | IDH 1/2 wild-type | |
| n = 81a | n = 227 | n = 28b | n = 99 | |
| Median age, years (min, max) | 76 (64, 90) | 76 (49, 91) | 77.5 (62, 90) | 76 (60, 87) |
| <65, n (%) | 1 (1.2) | 8 (3.5) | 1 (3.6) | 3 (3.0) |
| 65–<75 | 27 (33.3) | 77 (33.9) | 10 (35.7) | 32 (32.3) |
| ≥75 years | 53 (65.4) | 142 (62.6) | 17 (60.7) | 64 (64.6) |
| Sex, n (%) | ||||
| Female | 34 (42.0) | 92 (40.5) | 11 (39.3) | 40 (40.4) |
| Male | 47 (58.0) | 135 (59.5) | 17 (60.7) | 59 (59.6) |
| AML type, n (%) | ||||
| De novo | 60 (74.1) | 164 (72.2) | 24 (85.7) | 69 (69.7) |
| Secondary | 21 (25.9) | 63 (27.8) | 4 (14.3) | 30 (30.3) |
| AML with myelodysplasia-related changes, n (%) | 19 (23.5) | 83 (36.6) | 7 (25.0) | 41 (41.4) |
| ECOG performance status, n (%) | ||||
| 0–1 | 46 (56.8) | 133 (58.6) | 19 (67.9) | 60 (60.6) |
| 2–3 | 35 (43.2) | 94 (41.4) | 9 (32.1) | 39 (39.4) |
| Cytogenetic risk category, n (%) | ||||
| Intermediate | 62 (76.5) | 130 (57.3) | 19 (67.9) | 57 (57.6) |
| Poor | 19 (23.5) | 97 (42.7) | 9 (32.1) | 42 (42.4) |
| Bone marrow blast count, n (%) | ||||
| <30% | 14 (17.3) | 82 (36.1) | 6 (21.4) | 30 (30.3) |
| ≥30–<50% | 20 (24.7) | 51 (22.5) | 5 (17.9) | 24 (24.2) |
| ≥50% | 47 (58.0) | 94 (41.4) | 17 (60.7) | 45 (45.5) |
Abbreviations: AML-MRC, AML with myelodysplasia-related changes; ECOG, Eastern Cooperative Oncology Group.
aTwo patients had both IDH1 and IDH2 mutations.
bOne patient had both IDH1 and IDH2 mutations.
Remission rates
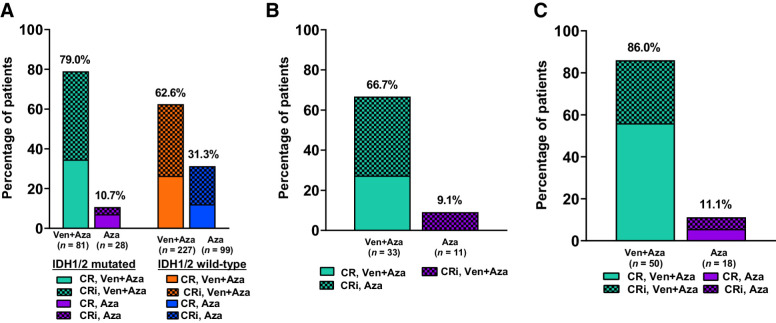
Among patients with IDH1/2 mutation (venetoclax and azacitidine vs. azacitidine), the median number of treatment cycles administered was 8.0 (range: 1.0 ― 37.0) versus 2.5 (1.0 ― 18.0). Patients with IDH1/2 mutations and treated with venetoclax and azacitidine had higher CRc rates than patients with IDH1/2 mutations and treated with azacitidine [79.0% (n = 64) vs. 10.7% (n = 3); Fig. 2A], the median time to CR or CRi response was 1.1 (range: 0.7–8.8) versus 3.4 (2.1 - 7.1) months, and the median DoR was 29.5 [95% CI, 16.7–not estimable (NE)] versus 9.5 (3.5–NE) months. In the IDH1/2 WT (venetoclax and azacitidine group vs. azacitidine group), the CRc rates were 62.6% (n = 142) versus 31.3% (n = 31); the median time to first response for CR or CRi was 1.3 (range: 0.8–9.9) versus 2.9 (0.8–13.2) months, and the median DoR was 17.5 (95% CI, 10.6–23.5) versus 10.3 (5.0–13.8) months.
Figure 2.
A, Remission rates in patients with IDH1/2 mutations and IDH1/2 wild-type by treatment groups. B, Remission rates in patients with IDH1 mutations in the venetoclax and azacitidine group. C, Remission rates in patients with IDH2 mutations in the venetoclax and azacitidine group.
In patients with IDH1, the CRc rate (venetoclax and azacitidine group vs. azacitidine group) was 66.7% (n = 22) versus 9.1% (n = 1; Fig. 2B) and the median time to first response for CR or CRi was 1.2 (range: 0.8–8.1) versus 3.4 (3.4–3.4) months. The median DoR in the IDH1-mutated venetoclax and azacitidine group was 21.9 (95% CI: 7.8–NE) months. In patients with IDH2 mutations, the CRc rate for venetoclax and azacitidine was 86% (n = 43) versus 11.1% (n = 2) for azacitidine (Fig. 2C) and median time to CR or CRi was 1.1 (range 0.7–8.8) versus 4.6 (2.1–7.1) months. The median DoR in the venetoclax and azacitidine group was not reached (95% CI, 16.7–NE), while the median DoR was 9.5 (3.5–NE) months for azacitidine. The estimated 12-month median DoR for patients with IDH2 mutation who received venetoclax and azacitidine was 71.1% (95% CI, 53.8%–82.9%).
The outcomes in patients harboring IDH2R140 and IDH2R172 mutations were similar and are shown in Supplementary Table S2. The remission rates of patients who achieved CR and CR with partial hematologic remission (CRh) are shown in Supplementary Table S3.
Overall survival
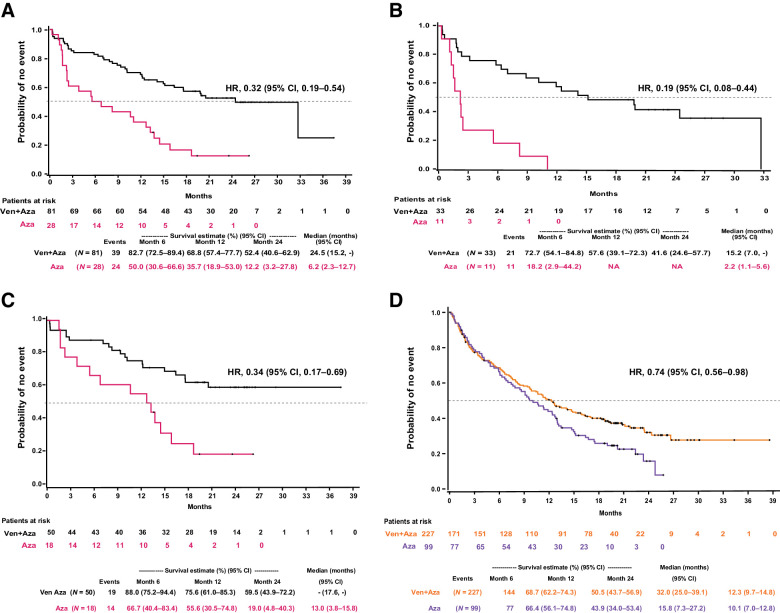
In patients with IDH1/2 mutations treated with venetoclax and azacitidine versus azacitidine, the median OS was 24.5 months (95% CI, 15.2–NE) versus 6.2 months (95% CI, 2.3–12.7); HR: 0.32 (95% CI, 0.19–0.54; Fig. 3A). In patients with IDH1 mutations, the median OS in the venetoclax and azacitidine vs. azacitidine group was 15.2 months (95% CI, 7.0–NE) versus 2.2 months (95% CI, 1.1, 5.6), HR: 0.19 (0.08–0.44), respectively (Fig. 3B). The median OS in patients with IDH2 mutations (venetoclax and azacitidine vs. azacitidine) was NR (95% CI, 17.6–NE) versus 13.0 (95% CI: 3.8, 15.8) months (HR, 0.34; 95% CI, 0.17–0.69), respectively (Fig. 3C). In patients with IDH1/2 WT, the median OS was 12.3 months (95% CI, 9.7–14.8) in patients treated with venetoclax and azacitidine and 10.1 (7.0–12.8) months, HR: 0.74 (95% CI, 0.56–0.98) in patients treated with azacitidine (Fig. 3D).
Figure 3.
Kaplan–Meier curves for OS (A) patients with IDH1/2 mutation treated with venetoclax (Ven) and azacitidine (Aza) versus azacitidine groups, patients with IDH1 mutation by treatment groups (B); patients with IDH2 mutated by treatment groups (C); and patients with IDH1/2 wild-type by treatment groups (D).
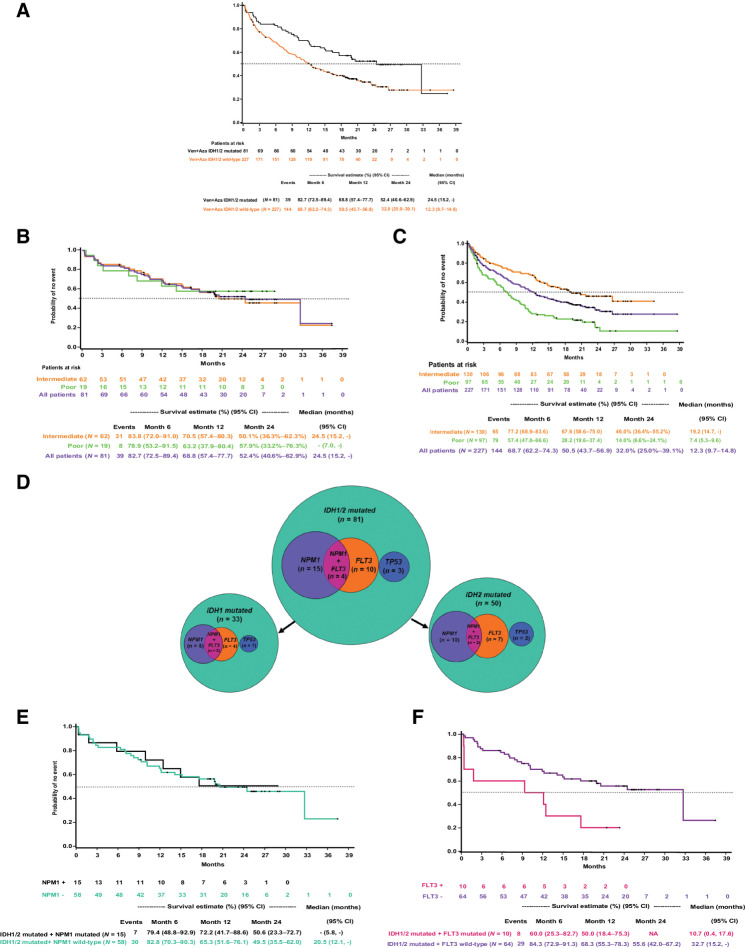
Among patients treated with venetoclax and azacitidine, those with IDH1/2 mutations have longer OS than IDH1/2 WT (Fig. 4A). Further analysis revealed that NCCN cytogenetic risk is prognostic in patients with IDH1/2 WT but does not impact OS in patients with IDH1/2 mutation. In IDH1/2 mutated, patients with intermediate and poor-risk had comparable OS (intermediate-risk, 24.5 months; poor-risk, NR; estimated 12-month median OS for intermediate-risk, 70.5%; poor-risk, 63.2%; Fig. 4B). Whereas in IDH1/2 WT, the median OS was inferior among patients with poor cytogenetic risk (7.4 months) than those with intermediate-risk (19.2 months; Fig. 4C). These findings show that venetoclax and azacitidine provide similar OS benefits to patients with intermediate cytogenetic risk regardless of IDH1/2 mutation, while in patients with poor cytogenetic risk, IDH 1/2 mutation is associated with differential outcomes.
Figure 4.
Kaplan–Meier curves for overall survival (A) patients with IDH1/2 mutated and IDH1/2 wild-type and treated with venetoclax and azacitidine; patients with IDH1/2 mutation treated with venetoclax and azacitidine and stratified by NCCN risk categories for AML (B); patients with IDH1/2 wild-type treated with venetoclax and azacitidine and stratified by NCCN cytogenetic risk categories (B); Venn diagrams showing co-mutations of NPM1, FLT3, and TP3 with IDH1/2 in patients treated with venetoclax and azacitidine (C); patients with IDH1/2 and NPM1-mutant or wild-type in the venetoclax and azacitidine group (D); patients with IDH1/2- and FLT3-mutant or wild-type in the venetoclax and azacitidine group (E).
In the IDH1/2-mutated cohort treated with venetoclax and azacitidine, only a small subset of patients exhibited a comutation with NPM1 (n = 19/81, 23%) or FLT3 (n = 14/81, 17%; Fig. 4D). Among patients with IDH1/2 mutated AML, the median OS was similar in patients with versus without NPM1 comutation (Fig. 4E). However, median OS was inferior in the presence of FLT3 comutation versus no FLT3 comutation, as shown in Fig. 4F. The median OS of patients who had IDH1/2 with a co-mutation in NPM1 or FLT3 and treated with azacitidine are shown in Supplementary Table S4. Three patients treated with venetoclax and azacitidine had IDH1/2 and TP53 co-mutation, and the 12-month estimate of OS was 33.3% (95% CI, 0.9%–77.4%).
Safety
All patients in the safety population experienced at least one adverse event, regardless of the treatment group (Table 2). Higher rates of grade ≥3 hematologic AEs were observed in the venetoclax and azacitidine group compared to the azacitidine group, regardless of mutation status, as was previously reported (17). In the overall population of the pooled analysis, 79.3% of patients in the venetoclax and azacitidine group experienced grade ≥3 hematologic AEs compared with 66.9% of patients in the azacitidine group. In the venetoclax and azacitidine group, grade ≥3 pneumonia was reported in 27.2% of patients with IDH1/2 mutations versus 22.7% in the overall population of the pooled analysis.
Table 2.
Treatment-emergent adverse events.
| Venetoclax + Azacitidine | Azacitidine | |||
|---|---|---|---|---|
| IDH1/2 mutated | Overall population | IDH1/2 mutated | Overall population | |
| Adverse eventa, n (%) | n = 81 | n = 353 | n = 28 | n = 145 |
| AE of any grade | 81 (100) | 349 (98.9) | 28 (100) | 143 (98.6) |
| AE grade ≥3 | 79 (97.5) | 343 (97.2) | 26 (92.9) | 138 (95.2) |
| Hematologic AE | 67 (82.7) | 280 (79.3) | 19 (67.9) | 97 (66.9) |
| Anemia | 24 (29.6) | 94 (26.6) | 7 (25.0) | 29 (20.0) |
| Febrile neutropenia | 34 (42.0) | 143 (40.5) | 7 (25.0) | 27 (18.6) |
| Neutropenia | 28 (34.6) | 131 (37.1) | 7 (25.0) | 41 (28.3) |
| Thrombocytopenia | 37 (45.7) | 141 (39.9) | 10 (35.7) | 55 (37.9) |
| Infections | 48 (59.3) | 215 (60.9) | 15 (53.6) | 73 (50.3) |
| Pneumonia | 22 (27.2) | 80 (22.7) | 9 (32.1) | 36 (24.8) |
| Serious AEs | 66 (81.5) | 288 (81.6) | 23 (82.1) | 105 (72.4) |
| Febrile neutropenia | 24 (29.6) | 102 (28.9) | 4 (14.3) | 15 (10.3) |
| Pneumonia | 17 (21.0) | 66 (18.7) | 8 (28.6) | 32 (22.1) |
| Anemia | 6 (7.4) | 15 (4.2) | 1 (3.6) | 6 (4.1) |
| Sepsis | 5 (6.2) | 17 (4.8) | 4 (14.3) | 12 (8.3) |
| Neutropenia | 4 (4.9) | 13 (3.7) | 1 (3.6) | 3 (2.1) |
| Atrial fibrillation | 3 (3.7) | 13 (3.7) | 0 | 2 (1.4) |
Abbreviation: AE, adverse event.
aIncludes all patients who received at least one dose of study treatment.
Serious AEs occurred in 81.5% of IDH1/2 mutant in the venetoclax and azacitidine group and 82.1% in the azacitidine group. In both the venetoclax and azacitidine and azacitidine groups, febrile neutropenia (29.6% vs.14.3%) and pneumonia (21.0% vs. 28.6%) were the most common serious AEs among patients with IDH1/2 mutant AML. No incidences of tumor lysis syndrome were reported among patients with IDH1/2 mutations in either treatment group.
Among patients with IDH1/2 mutations, 6 of 81 (7.4%) in the venetoclax and azacitidine group and 3 of 28 (10.7%) in the azacitidine group received concomitant hydroxyurea therapy.
Discussion
For older patients with AML who are ineligible for intensive chemotherapy, treatment with venetoclax and azacitidine is now recommended as the new standard of care by the NCCN clinical practice guidelines for AML regardless of mutation status (20). Among all patients with IDH1/2-mutated AML, treatment with venetoclax and azacitidine led to a higher remission rate (68%) and a 68% reduction in the risk of death compared with treatment with azacitidine alone.
In this analysis, patients with IDH1/2-mutated AML were confirmed to benefit from the combination of venetoclax and azacitidine, with an overall CRc rate of 79% and OS of 24.5 months. Specifically, among the IDH1-mutated subgroup (n = 33) receiving the combination, the CRc rate was 67%, and the CR rate was 27%, with a median OS of 15 months. This compares favorably to the outcomes with ivosidenib monotherapy for newly diagnosed AML, with CRc 42% and OS of 12.6 months (12). Ivosidenib combinations are also under investigation in this population; in a phase Ib trial of azacitidine and ivosidenib, CRc rate was 69.6% with CR rate 60% (13); a phase III placebo-controlled study of azacitidine ± ivosidenib is ongoing for patients with IDH1 mutations. Patients with IDH2-mutated AML appear to derive particular benefit with venetoclax and azacitidine, with a CRc rate of 86%, a CR rate of 56%, and a median OS that was not reached. Results from an ongoing randomized phase II study of the targeted IDH2 inhibitor enasidenib in combination with azacitidine (vs. azacitidine alone) reported promising efficacy as well, with the combination leading to improved responses including 63% CRc rate and 54% CR, although OS benefit with the azacitidine and enasidenib combination (vs. azacitidine alone) was not confirmed (21). Future studies may explore alternate combinations using venetoclax, with or without azacitidine, and a targeted IDH1/2 inhibitor to maximize efficacy and minimize the toxicity of treating patients with IDH1/2-mutated AML. Currently, an early clinical trial evaluating ivosidenib with venetoclax with or without azacitidine (NCT03471260) among treatment-naïve patients reported that the combination therapy with or without azacitidine had an acceptable safety profile and efficacy (22).
In an additional analysis, we found that in the presence of IDH1/2 mutations, the NCCN cytogenetic classification system of intermediate and poor-risk cytogenetics was not prognostic of OS. However, in the absence of an IDH1/2 mutation, this classification system remained relevant and predictive of OS; intermediate-risk had superior outcomes while the patients with poor-risk had inferior outcomes. Further evaluation may describe the underlying biology of these findings.
The presence of a comutation of IDH1/2 with FLT3 resulted in inferior OS, while the presence of a comutation with NPM1 did not impact survival. FLT3-ITD mutations have been reported to confer rapid escape from venetoclax as a single agent and associate with earlier relapse and outgrowth of FLT3-mutant clones in HMA or LDAC and venetoclax combination, likely through upregulation of alternative BCL-2 family proteins like MCL-1, not targeted by venetoclax (23). In such patients, the addition of FLT3 inhibitor to HMA and venetoclax can be exploited. Further research is also warranted to understand whether additional prognostic factors, such as the cooccurrence of a TP53 mutation, may impact the efficacy of venetoclax and azacitidine.
The safety and tolerability of venetoclax and azacitidine among patients were similar irrespective of IDH1/2 mutation status. The toxicities were predominantly hematological and consistent with the safety data of the overall study population (16, 17). No significant increase in neutropenia-related AEs was identified, and no events of IDH differentiation syndrome were reported. All toxicities were effectively managed by the standard of care.
There were a few limitations of the study. First, data were pooled from a phase III and a phase Ib trial to increase the number of patients with IDH1/2 mutations for this analysis; however, due to the 2:1 randomization of VIALE-A and single-arm design of the phase Ib trial, the number of patients in the azacitidine group was limited and interpretation of key findings between the two treatments were not possible. In addition, patients included in this study may not truly represent all IDH1/2-mutated patients in real-world clinical practice; hence, the descriptive results of treatment effects in the groups warrant caution and validation in future studies. Likewise, the small sample sizes in the molecular subgroups further limited detailed comparisons between the IDH-mutant isoforms and various cooccurring mutations.
The current data demonstrate the efficacy of venetoclax and azacitidine in patients with IDH1/2-mutated AML that warrant further investigation, including extending this treatment to younger “fit” patients with IDH1/2 mutations, and/or evaluating time-limited therapy for responding patients with this genomic profile who achieve measurable residual disease negativity. Future analyses can also establish the role and potential benefit of sequencing or combining additional effective IDH1/2-directed therapies such as targeted inhibitors of IDH1/2.
Authors' Disclosures
D.A. Pollyea reports grants and personal fees from AbbVie during the conduct of the study as well as grants and personal fees from AbbVie; personal fees from Syros, Bristol Myers Squibb, Celgene, Glycomimetics, Genentech, Novartis, Syndax, Kiadis, Takeda, Foghorn, Aprea, Astellas, Gilead, Jazz, Beigene, Bergen Bio, and Amgen; grants from Teva; and grants and personal fees from Karyopharm outside the submitted work. C.D. DiNardo reports other support from Notable Labs; personal fees from Novartis, Takeda, Aprea, and GSK; grants and personal fees from Agios/Servier, Celgene/BMS, AbbVie/Genentech, ImmuneOnc, Foghorn, and Cleave outside the submitted work. M.L. Arellano reports personal fees from Gilead Sciences, personal fees from SYNDAX Pharmaceutical, Inc, and other support from Cephalon Oncology outside the submitted work. A. Pigneux reports grants from AbbVie, Agios, Astellas, Pfizer, Jazz, and Novartis outside the submitted work. W. Fiedler reports personal fees and non-financial support from AbbVie; grants, personal fees, and non-financial support from Amgen and Pfizer; and personal fees from Jazz Pharmaceuticals, Celgene, Morphosys, Ariad/Incyte, Daiichi Sankyo, and Servier outside the submitted work; in addition, W. Fiedler has a patent for Amgen issued; and support for medical writing: Amgen, Pfizer, AbbVie. M. Konopleva reports other support from AbbVie during the conduct of the study as well as grants and other support from AbbVie, Genentech, F. Hoffman La-Roche, and Stemline Therapeutics; grants from Eli Lilly, Cellectis, Calithera, Ablynx, Agios, Ascentage, Astra Zeneca, Rafael Pharmaceutical, and Sanofi; grants and other support from Forty Seven; other support from Janssen; grants from Immunomet; and other support from Reata Pharmaceutical outside the submitted work. D.A. Rizzieri reports grants from AbbVie during the conduct of the study as well as personal fees from AbbVie outside the submitted work. R.M. Lemoli reports personal fees from Jazz Pharma and AbbVie; other support from Sanofi; and personal fees from Celgene outside the submitted work. M. Dail reports other support from Genentech/Roche during the conduct of the study as well as other support from Genentech/Roche outside the submitted work. Y. Duan reports other support from AbbVie, Inc during the conduct of the study as well as other support from AbbVie, Inc outside the submitted work. B. Chyla reports other support from AbbVie during the conduct of the study as well as other support from AbbVie outside the submitted work. J. Potluri is an employee of AbbVie. C.L. Miller reports other support from AbbVie, Inc outside the submitted work. No disclosures were reported by the other authors.
Supplementary Material
Acknowledgments
The authors wish to thank the patients and their families, the study coordinators, and support staff. The authors would also like to acknowledge all investigators of studies M14–358 and M15–656.
D.A. Pollyea is supported by the Leukemia and Lymphoma Scholar in Clinical Research Award and the Robert H. Allen MD Chair in Hematology Research. Medical writing support was provided by Rachel M. Richardson, PharmD, MS, and Dalia Majumdar, Ph.D., employees of AbbVie.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This article is featured in Highlights of This Issue, p. 2717
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Contributions
D.A. Pollyea: Supervision, writing–review and editing. C.D. DiNardo: Supervision, writing–review and editing. M.L. Arellano: Supervision, writing–review and editing. A. Pigneux: Supervision, writing–review and editing. W. Fiedler: Supervision, writing–review and editing. M. Konopleva: Supervision, writing–review and editing. D.A. Rizzieri: Supervision, writing–review and editing. B.D. Smith: Supervision, writing–review and editing. A. Shinagawa: Supervision, writing–review and editing. R.M. Lemoli: Supervision, writing–review and editing. M. Dail: Supervision, writing–review and editing. Y. Duan: Data curation, supervision, methodology, writing–review and editing. B. Chyla: Data curation, methodology, writing–review and editing. J. Potluri: Conceptualization, data curation, supervision, methodology, writing–review and editing. C.L. Miller: Conceptualization, supervision, methodology, writing–review and editing. H.M. Kantarjian: Supervision, writing–review and editing.
References
- 1. Marando L, Huntly BJP. Molecular landscape of acute myeloid leukemia: prognostic and therapeutic implications. Curr Oncol Rep 2020;22:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papaemmanuil E, Dohner H, Campbell PJ. Genomic classification in acute myeloid leukemia. N Engl J Med 2016;375:900–1. [DOI] [PubMed] [Google Scholar]
- 3. Lagunas-Rangel FA, Chávez-Valencia V, Gómez-Guijosa MÁ, Cortes-Penagos C. Acute myeloid leukemia-genetic alterations and their clinical prognosis. Int J Hematol Oncol Stem Cell Res 2017;11:328–39. [PMC free article] [PubMed] [Google Scholar]
- 4. Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med 2015;373:1136–52. [DOI] [PubMed] [Google Scholar]
- 5. Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 2010;17:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DiNardo CD, Ravandi F, Agresta S, Konopleva M, Takahashi K, Kadia T, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol 2015;90:732–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chan SM, Thomas D, Corces-Zimmerman MR, Xavy S, Rastogi S, Hong WJ, et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat Med 2015;21:178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Montalban-Bravo G, DiNardo CD. The role of IDH mutations in acute myeloid leukemia. Future Oncol 2018;14:979–93. [DOI] [PubMed] [Google Scholar]
- 9. Marcucci G, Maharry K, Wu Y-Z, Radmacher MD, Mrózek K, Margeson D, et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol 2010;28:2348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thol F, Damm F, Wagner K, Göhring G, Schlegelberger B, Hoelzer D, et al. Prognostic impact of IDH2 mutations in cytogenetically normal acute myeloid leukemia. Blood 2010;116:614–6. [DOI] [PubMed] [Google Scholar]
- 11. Duchmann M, Micol J-B, Duployez N, Raffoux E, Thomas X, Marolleau J-P, et al. Prognostic significance of concurrent gene mutations in intensively treated patients with IDH-mutated AML: an ALFA study. Blood 2021;137:2827–37. [DOI] [PubMed] [Google Scholar]
- 12. Roboz GJ, DiNardo CD, Stein EM, de Botton S, Mims AS, Prince GT, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood 2020;135:463–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DiNardo CD, Stein AS, Stein EM, Fathi AT, Frankfurt O, Schuh AC, et al. Mutant isocitrate dehydrogenase 1 inhibitor ivosidenib in combination with azacitidine for newly diagnosed acute myeloid leukemia. J Clin Oncol 2021;39:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dinardo CD, Stein AS, Stein EM, Fathi AT, Schuh AC, Fernández PM, et al. Mutant IDH (mIDH) inhibitors, ivosidenib or enasidenib, with azacitidine (AZA) in patients with acute myeloid leukemia (AML). J Clin Oncol 2018;36:7042. [Google Scholar]
- 15. Pollyea DA, Tallman MS, de Botton S, Kantarjian HM, Collins R, Stein AS, et al. Enasidenib, an inhibitor of mutant IDH2 proteins, induces durable remissions in older patients with newly diagnosed acute myeloid leukemia. Leukemia 2019;33:2575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019;133:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med 2020;383:617–29. [DOI] [PubMed] [Google Scholar]
- 18. Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 2003;21:4642–9. [DOI] [PubMed] [Google Scholar]
- 19. US Department of Health and Human Services. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.03. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 20. National Comprehensive Cancer Network. NCCN Guidelines for patients with Acute Myeloid Leukemia, Version 3.2021; 2021.
- 21. DiNardo CD, Schuh AC, Stein EM, Montesinos P, Wei AH, de Botton S, et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant-IDH2 acute myeloid leukaemia (AG221-AML-005): a single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol 2021;22:1597–608. [DOI] [PubMed] [Google Scholar]
- 22. Lachowiez CA, Borthakur G, Loghavi S, Zeng Z, Kadia TM, Masarova L, et al. A phase Ib/II study of ivosidenib with venetoclax ± azacitidine in IDH1-mutated myeloid malignancies. J Clin Oncol 2021;39:7012-. [Google Scholar]
- 23. DiNardo CD, Tiong IS, Quaglieri A, MacRaild S, Loghavi S, Brown FC, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood 2020;135:791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.