Abstract
Purpose:
Transcriptomic profiling was performed for microsatellite instability-high (MSI-H)/mismatch repair-deficient (dMMR) gastrointestinal tumors to determine the predictors of response to PD-1 blockade.
Experimental Design:
Thirty-six patients with MSI-H/dMMR gastrointestinal tumors, including gastric cancer, colorectal cancer, cholangiocarcinoma, small intestine cancer, and pancreatic cancer, being treated with PD-1 blockade were analyzed. We conducted the transcriptomic analysis of gastrointestinal tumors using RNA sequencing data, including the consensus molecular subtypes (CMS) of colorectal cancer.
Results:
Gene set enrichment analysis (GSEA) demonstrated that non-responders had upregulations of epithelial–mesenchymal transition, angiogenesis, hypoxia, mTORC1, TNF-α, KRAS, Wnt/β-catenin, TGF-β, and various metabolism-related signaling pathways. Meanwhile, the IFNγ pathway was enriched in responders. On the basis of the leading-edge analysis of GSEA, VEGF-A was significantly correlated with enriched pathways in non-responders. Patients with high VEGF-A expression, compared with those with low expression, had significantly shorter progression-free survival [PFS; median 4.8 months vs. not reached (NR), P = 0.032] and overall survival (median 11.1 months vs. NR, P = 0.045). Among 13 patients with colorectal cancer evaluable for CMS classification, the objective response rate was 100%, 0%, 0%, and 16.7% in CMS1, CMS2, CMS3, and CMS4, respectively. Patients with CMS1 had significantly longer PFS (NR vs. 4.8 months, P = 0.017) than those with CMS2, CMS3, or CMS4.
Conclusions:
Several transcriptomic features, including CMS classification and related genes, were associated with response to PD-1 blockade in MSI-H/dMMR gastrointestinal tumors. These findings can help develop predictive biomarkers or combination immunotherapies.
Translational Relevance.
Approximately half of microsatellite instability-high (MSI-H)/mismatch repair-deficient (dMMR) tumors do not respond to immune checkpoint inhibitors (ICI), highlighting the importance of identifying predictive biomarkers or molecular profiling correlated with resistance to these agents. We performed transcriptomic profiling of MSI-H/dMMR gastrointestinal tumors treated with PD-1 blockade, including the consensus molecular subtypes (CMS) of colorectal cancer. Our study revealed that non-responders had the enrichment of epithelial–mesenchymal transition, angiogenesis, hypoxia, mTORC1, KRAS, Wnt/β-catenin, TGFβ, and various metabolism-related signaling pathways, which have been associated with an immunosuppressive tumor microenvironment. Meanwhile, the IFNγ pathway was upregulated in responders. High expression of VEGF-A might predict a negative response to ICIs in this population. Moreover, CMS classification possibly correlated with clinical outcomes of PD-1 blockade in MSI-H/dMMR patients with colorectal cancer. These transcriptomic features could help in the development of predictive biomarkers or combination immunotherapies in the future.
Introduction
Microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) tumors exhibit elevated mutation rates (including frameshifts or missense mutations) and hypermethylation (including hypermethylation at the MLH1 promoter), resulting in the enhanced expression of neoantigens and high infiltration of CD8+ T cells into the tumor microenvironment (TME; refs. 1, 2). Tumors with these distinct molecular and immunological characteristics may respond well to immune checkpoint inhibitors (ICI; ref. 3). In several trials, the FDA has approved anti–PD-1 antibodies (e.g., pembrolizumab and dostarlimab) for patients with treatment-resistant MSI-H/dMMR solid tumors (4, 5). Currently, MSI-H/dMMR is one of the most consistent predictive biomarkers for ICIs. However, approximately 30% of MSI-H/dMMR tumors had during the first months of therapy progressive disease with anti–PD-1 therapy, and thus it is crucial to identify the predictive biomarkers or molecular profiling correlated with resistance to these agents (6–8). In our recent study of 45 MSI-H/dMMR gastrointestinal tumors, we found that tumor mutational burden (TMB)-low tumors and PTEN mutations, especially in the phosphatase domain with immunosuppressive microenvironments, might be associated with decreased responsiveness to PD-1 blockade (9). Other gene alterations, such as STK11, FBXW7, JAK1, B2M, and HLA mutations, were also observed in non-responders (9).
Aside from mutational status, gene signatures detected via RNA sequencing, especially those induced by IFNγ, may also be potential biomarkers for predicting clinical benefit to anti–PD-1 or anti–PD-L1 therapies in microsatellite-stable (MSS)/MMR-proficient (pMMR) solid tumors (10). A preclinical study reported that the Wnt/β-catenin pathway induced intrinsic resistance to ICIs in melanoma (11). However, there is a paucity of information regarding the transcriptomic landscape in MSI-H/dMMR gastrointestinal tumors treated with anti–PD-1 therapy (12). Thus, uncovering the molecular determinants of responsiveness to ICI therapy can lead to the development of novel biomarkers or combination therapeutic modalities to overcome resistance to these agents in MSI-H/dMMR gastrointestinal tumors.
This study aimed to elucidate predictors of responsiveness to ICI therapy in MSI-H/dMMR gastrointestinal tumors. We performed transcriptomic profiling of MSI-H/dMMR gastrointestinal tumors treated with PD-1 blockade, including the consensus molecular subtypes (CMS) of colorectal cancer.
Materials and Methods
Patients
We performed transcriptomic analysis to uncover the signatures associated with the PD-1 blockade response in patients with advanced MSI-H/dMMR gastrointestinal tumors at National Cancer Center Hospital East (Kashiwa, Chiba, Japan). The inclusion criteria were as follows: (i) an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 to 1; (ii) histologically proven, unresectable, locally advanced, or metastatic gastrointestinal tumor that is refractory or intolerant to at least 1 chemotherapy regimen; (iii) MSI-H or dMMR status verified via local PCR or IHC as described below; (iv) adequate bone marrow, hepatic, and renal function as indicated by medical records; (v) received an anti–PD-1 inhibitor either alone (i.e., pembrolizumab or nivolumab) or as combination therapy (i.e., pembrolizumab plus napabucasin; ref. 13) from July 2015 to April 2021; and (vi) had adequate tumor samples for RNA sequencing. All patients provided written informed consent for the biomarker analysis of formalin-fixed paraffin-embedded (FFPE) tissue specimens from archival tissue samples. The study was approved by the Institutional Review Board of the National Cancer Center Hospital East (Kashiwa, Chiba, Japan) and was carried out in accordance with the guidelines for biomedical research specified in the Declaration of Helsinki.
MSI and MMR status
MSI status was analyzed using a Promega MSI analysis system (Promega), which used 5 mononucleotide markers for the detection of MSI: BAT-25, BAT-26, NR-21, NR-24, and MONO-27 (14). Tumors were classified as MSI-H if instability was noted in at least two markers. On the other hand, MMR status was assessed via IHC using the following monoclonal antibodies: anti-mutL homolog 1 (MLH1, ES05), anti-mutS homolog 2 (MSH2, FE11), anti-postmeiotic segregation increased 2 (PMS2, EP51), and anti-mutS homolog 6 (MSH6, EP49; Agilent Technologies). Tumors that lacked MLH1, MSH2, PMS2, or MSH6 expression were considered dMMR.
Whole-exome sequencing
Mutational status, including TMB, was assessed via whole-exome sequencing as we previously reported (9). TMB was defined as the total number of nonsynonymous mutations, including indels, mutations per megabase (muts/Mb) in whole-exome sequencing and TMB-high was defined as ≥ 10 muts/Mb.
RNA sequencing
Total RNA was extracted from FFPE specimens using the RNeasy FFPE Kit (QIAGEN). Ribosomal RNA was depleted from the total RNA with an NEBNext rRNA Depletion Kit (New England Biolabs). Sequencing libraries for RNA-seq were prepared with a NEBNext Ultra RNA Library Prep Kit (New England Biolabs); these prepared RNA-seq libraries underwent 150 bp, paired-end NGS sequencing. To perform a gene set enrichment analysis (GSEA), the gene set variation analysis (GSVA) in R package and the GSEA tool (https://www.gsea-msigdb.org/gsea/index.jsp) were used to calculate the signaling pathway variation score and normalized enrichment score (NES), respectively (15, 16). In GSEA, gene sets with both nominal P value and FDR q value of <0.05 were considered as significantly enriched pathways. These analyses were performed according to the hallmark gene sets, which were downloaded from the Molecular Signatures Database (MSigDB). After GSEA, leading-edge analysis was performed to determine genes with a high impact on each pathway; the genes that overlapped across significant pathways were further identified (15, 16). A unique CMS in patients with colorectal cancer was also assessed as previously described (17).
Multiplex immunofluorescence IHC and PD-L1 expression
The protein expression levels of CD3, CD4, CD8, and cytokeratin in FFPE samples were assessed using multiplex fluorescence IHC with each monoclonal antibody. The details of multiplex immunofluorescence are available in the Supplementary Methods.
A trained pathologist (T. Kuwata) who was blinded to the diagnoses and/or other identifying information assessed the PD-L1 combined positive score (CPS) using PD-L1 IHC 22C3 pharmDx (Dako). CPS was defined as the ratio of the number of PD-L1–positive cells (including tumor cells, lymphocytes, and macrophages) to the total number of tumor cells multiplied by 100.
Outcomes and statistical analysis
Progression-free survival (PFS) was defined as the time from the initiation of PD-1 blockade to disease progression or death from any cause. Overall survival (OS) was defined as the time from the initiation of PD-1 blockade to death from any cause. Tumor response was assessed in patients with measurable lesions using the RECIST version 1.1. Overall response rate (ORR) was defined as the proportion of patients whose best overall response was a complete response (CR) or partial response (PR). Disease control rate (DCR) was defined as the proportion of patients who achieved a best overall response of a CR, PR, or stable disease (SD) lasting more than 6 weeks from the start of study treatment.
Quantitative data are expressed as median and interquartile range (IQR) and the cutoff values were set to the median. The Wilcoxon rank sum test was used to compare continuous variables, whereas Fisher's exact test was used to compare categorical variables. Survival curves were estimated using the Kaplan–Meier method, and differences between groups were tested using the log-rank test. Hazard ratios (HR) were estimated using the Cox proportional hazards model. PFS and OS were analyzed using univariate and multivariate Cox regression analyses. The backward selection method was used to select factors retained in the multivariate analysis (P < 0.1). All P values of <0.05 were considered statistically significant. All statistical analyses were performed using the statistical program R version 4.1.0 (The R Foundation for Statistical Computing).
Data availability
Raw RNA sequencing data used in the present study were uploaded to the Sequence Read Archive of DNA DataBank of Japan (DRA: https://www.ddbj.nig.ac.jp/dra/index.html) under accession No. DRA013565. All other data are available from the corresponding author upon reasonable request. Those requests will be reviewed by a study steering committee to verify whether the request is subject to any intellectual property or confidentiality obligations.
Results
Patient overview
To examine transcriptomic profiling associated with the response to PD-1 blockade, we identified patients with gastrointestinal tumors and evaluated their characteristics and responses to PD-1 blockade. A total of 36 patients with available RNA sequencing data met the inclusion criteria and had the following cancers: gastric cancer (n = 18), colorectal cancer (n = 13), cholangiocarcinoma (n = 2), small intestine cancer (n = 2), and pancreatic cancer (n = 1). The patient characteristics are summarized in Table 1. In addition, the details of MSI/MMR status with TMB are summarized in Supplementary Table S1. Of the total 36 patients, 9 were analyzed by both Promega MSI analysis and MMR IHC test, and the remaining 27 were analyzed by either test [Promega MSI analysis (n = 15) or MMR IHC (n = 12); Supplementary Table S1]. Tumor specimens were collected from primary tumor samples before PD-1 blockade.
Table 1.
Patient characteristics.
| Total (n = 36) | |
|---|---|
| Age, y | |
| Median (range) | 67 (30–84) |
| ≥65 | 20 (55.6) |
| Sex, n (%) | |
| Male | 20 (55.6) |
| Female | 16 (44.4) |
| Previous treatment regimens, n (%) | |
| 1 | 19 (52.8) |
| ≥2 | 17(47.2) |
| Primary cancer, n (%) | |
| Gastric | 18 (50.0) |
| Colorectal | 13 (36.1) |
| Small intestine | 2 (5.6) |
| Cholangiocarcinoma | 2 (5.6) |
| Pancreatic | 1 (2.8) |
| ECOG PS, n (%) | |
| 0 | 23 (63.9) |
| 1 | 13 (36.1) |
| Surgery on primary tumor, n (%) | |
| No | 14 (38.9) |
| Yes | 22 (61.1) |
| Metastatic sites, n (%) | |
| Liver | 5 (13.9) |
| Lung | 5 (13.9) |
| Peritoneal | 16 (44.4) |
| Lymph node | 29 (80.6) |
| Number of metastatic organs, n (%) | |
| 1 | 19 (52.8) |
| ≥2 | 17 (47.2) |
| PD-L1 CPS, n (%) | |
| CPS < 1 | 7 (19.4) |
| 1 ≤ CPS < 10 | 5 (13.9) |
| CPS ≥ 10 | 23 (63.9) |
| Missing | 1 (5.3) |
| TMB, mutations/Mb (range) | 38.7 (3.6–93.0) |
| Treatment, n (%) | |
| Nivolumab | 6 (16.7) |
| Pembrolizumab | 24 (66.7) |
| Pembrolizumab with napabucasin | 6 (16.7) |
Abbreviations: CPS, combined positive score; ECOG, Eastern Cooperative Oncology Group; PS, Performance status; TMB, tumor mutational burden.
The maximum percentage change in tumor size from baseline is shown in Fig. 1. All patients had measurable lesions. The overall population had an ORR and DCR of 52.7% (19/36) and 83.3% (30/36), respectively. The median follow-up at the time of the analysis was 28.2 months. In the overall population, the median PFS was 24.5 months [95% confidence interval (CI), 4.4 to not reached (NR)], whereas the median OS was 29.5 months (95% CI, 15.2–NR), with 16 mortalities (44.4%; Supplementary Fig. S1).
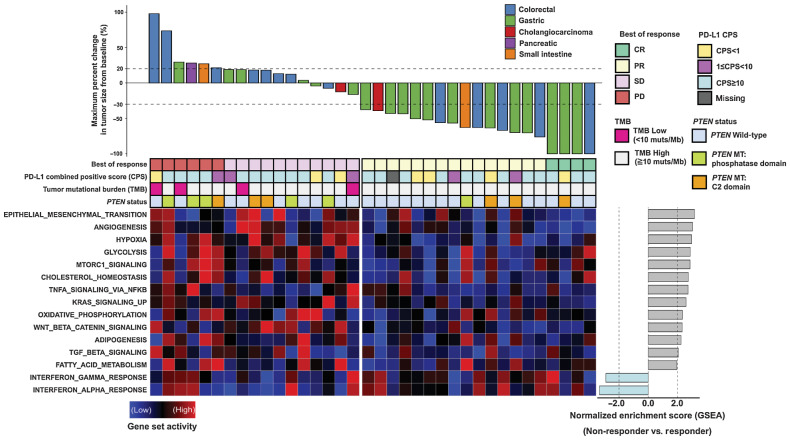
Figure 1.
Waterfall plot of the maximum percentage change in tumor size from baseline measured using the RECIST version 1.1 and gene set activity of signaling pathways between responders and non-responders. Top, percentages of maximum tumor volume changes during PD-1 blockade measured using the RECIST 1.1 criteria. The bottom dotted line represents a tumor reduction of 30%, as per RECIST 1.1, representing partial response (PR) or complete response (CR). The top dotted line represents a tumor increase of more than 20%, representing disease progression (PD). Middle, summary of best of response, combined positive score (CPS), tumor mutational burden (TMB), and PTEN mutation status. Each column represents an individual patient with available data. Bottom left, a heat map showing the relative expression levels of associated gene sets between samples calculated using the gene set variation analysis (GSVA). Bottom right, a bar plot representing normalized enrichment score between responders and non-responders for each gene set calculated using the gene set enrichment analysis (GSEA).
Transcriptome features associated with the response to PD-1 blockade
The gene set activity in enriched pathways is illustrated using a heat map among samples calculated using GSVA, with a bar plot representing NES between non-responders [SD or progressive disease (PD)] and responders (CR or PR) generated using GSEA (Fig. 1). On GSEA, non-responders had upregulations of epithelial–mesenchymal transition (EMT), angiogenesis, hypoxia, mTORC1, TNFα, KRAS, Wnt/β-catenin, TGFβ, and various metabolism-related signaling pathways. On the other hand, the IFNγ and α pathways were upregulated in responders.
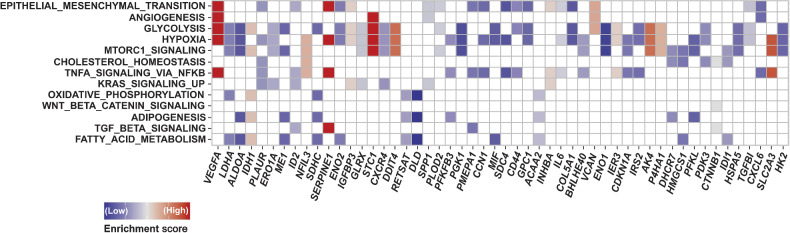
Next, we performed leading-edge analysis of the GSEA to identify the individual genes that were significantly related to enriched pathways in non-responders. Specifically, the top 5 genes were VEGF-A, LDHA, ALDOA, IDH1, and PLAUR (Fig. 2). Of these, patients with high VEGF-A mRNA expression, compared with those with low expression, had significantly shorter PFS (median, 4.8 months; 95% CI, 3.0–30.7 vs. NR, 95% CI, 8.4–NR; HR, 2.79; 95% CI, 1.10–7.09; P = 0.032) and OS (median, 11.1 months; 95% CI, 5.3–NR vs. NR, 95% CI, 15.2–NR; HR, 2.97; 95% CI, 1.02–8.62; P = 0.045; Fig. 3). On the other hand, the expression of the other genes was not associated with PFS and OS (data not shown). Multivariate analyses showed that high expression of VEGF-A mRNA was independently associated with shorter PFS (HR, 3.23; 95% CI, 1.18–8.87; P = 0.023) and OS (HR, 3.61; 95% CI, 1.06–12.29; P = 0.040; Supplementary Tables S2 and S3). Among other VEGF-related genes, the expression of PIGF and PDGF-A were significantly higher in non-responders than in responders. Furthermore, the expression of VEGFR-1 and PDGF-B tended to be higher in non-responders than in responders (Supplementary Fig. S2).
Figure 2.
The leading-edge analysis of the gene set enrichment analysis (GSEA). The heatmap created by the leading-edge analysis of the GSEA indicates the relative expression level of each gene associated with 3 or more pathways. The top 5 genes related to significantly enriched pathways in non-responders to PD-1 blockade were VEGF-A, LDHA, ALDOA, IDH1, and PLAUR.
Figure 3.
Kaplan–Meier plots of progression-free survival (PFS; A) and overall survival (OS; B) according to the expression of VEGF-A mRNA. Patients with high VEGF-A mRNA expression, compared with those with low expression, had significantly lower PFS [median: 4.8 months; (95% CI, 3.0–30.7) vs. not reached, NR (95% CI, 8.4–NR); log-rank P = 0.025] and OS [median, 11.1 months; (95% CI, 5.3–NR) vs. NR (95% CI, 15.2–NR); log-rank P = 0.035].
CMS classification of colorectal cancer and response to PD-1 blockade
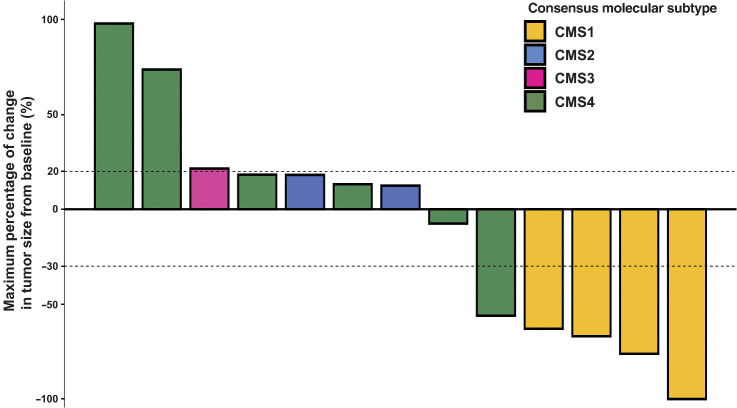
Among 13 patients with colorectal cancer evaluable for CMS classification, CMS1, CMS2, CMS3, and CMS4 were detected in 4, 2, 1, and 6 cases, respectively. Among the 4 cases of CMS1, 1 had CR, while the remaining 3 had PR. Both cases of CMS2 had SD. The single case of CMS3 had PD. Among the 6 cases of CMS4, 1 had PR, 3 had SD, and the remaining 2 patients had PD. Consequently, the ORR was 100% (4/4), 0% (0/2), 0% (0/1), and 16.7% (1/6) for CMS1, CMS2, CMS3, and CMS4, respectively (Fig. 4). Patients with CMS1 had a significantly longer PFS (median, NR; 95% CI, NR–NR vs. 4.8 months; 95% CI, 1.1–30.7; P = 0.017) and numerically longer OS (median, NR; 95% CI, NR–NR vs. 31.0 months; 95% CI, 2.6–NR; P = 0.105) than those with CMS2, CMS3, or CMS4 (Fig. 5).
Figure 4.
Waterfall plot of the maximum percentage change in tumor size from baseline according to the consensus molecular subtype (CMS) of colorectal cancer (CRC) as measured using the RECIST version 1.1. Among 13 patients with CRC evaluable for CMS classification, CMS1, CMS2, CMS3, and CMS4 were detected in 4, 2, 1, and 6 cases, respectively. The ORR was 100% (4/4), 0% (0/2), 0% (0/1), and 16.7% (1/6) for patients with CMS1, CMS2, CMS3, and CMS4, respectively.
Figure 5.
Kaplan–Meier plots of progression-free survival (PFS; A) and overall survival (OS; B) according to the expression of consensus molecular subtype (CMS). Patients with CMS1 showed significantly longer PFS [Not reached, NR (95% CI, NR–NR) vs. 4.8 months (95% CI, 1.1–30.7); P = 0.017] and numerically longer OS [Not reached (95% CI, NR–NR) vs. 31.0 months (95% CI, 2.6–NR); P = 0.105] than those with other types of CMS.
TME according to the expression of VEGF-A mRNA
The TME was analyzed via multiplex fluorescence IHC (data shown as median; IQR). Tumors with high VEGF-A mRNA expression, compared with those with low expression, had significantly lower levels of intratumoral CD8+ T cells [146.8/mm2 (95% CI, 63.7–336.2) vs. 269.1/mm2 (95% CI, 193.1–621.2); P = 0.041], as well as numerically lower levels of intratumoral CD3+ [352.6/mm2 (95% CI, 258.8–471.8) vs. 414.7/mm2 (95% CI, 348.9–873.4); P = 0.133] and CD4+ T cells [52.1/mm2 (95% CI, 27.1–100.1) vs. 96.1/mm2 (95% CI, 16.3–169.2); P = 0.405; Supplementary Fig. S3).
Discussion
We performed transcriptomic profiling, including CMS of colorectal cancer, for patients with MSI-H/dMMR gastrointestinal tumors treated with PD-1 blockade to elucidate the determinants of responsiveness to ICI therapy. To the best of our knowledge, this is the first report to comprehensively describe the transcriptomic landscape of tumors (specifically related to PD-1 blockade) among patients with MSI-H/dMMR gastrointestinal tumors.
First, our study revealed that non-responders exhibited enrichment of the EMT, angiogenesis, hypoxia, mTORC1, KRAS, Wnt/β-catenin, TGF-β, and various metabolism-related signaling pathways, all of which have been associated with an immunosuppressive TME (11, 18–25). On the other hand, the IFNγ pathway was upregulated in responders, which was in line with previous studies showing that an IFNγ-related mRNA profile correlated with favorable clinical outcomes with ICIs in several malignancies (26).
In a recent study, Kwon and colleagues (12) reported that non-responders had upregulations in the Wnt/β-catenin pathway in a phase II trial of pembrolizumab in cases of advanced MSI-H gastric cancer. Similarly, the non-responders to anti–PD-1 therapy in our cohort also demonstrated an enriched Wnt/β-catenin pathway. Recently, a selective inhibitor of the interaction between β-catenin and CREB-binding protein demonstrated synergistic antitumor activity in combination with PD-1 blockade via modulation of the Wnt/β-catenin pathway and alteration of the tumor immune microenvironments in vivo (27, 28). Thus, ICI resistance in MSI-H/dMMR gastrointestinal tumors can potentially be overcome by targeting the Wnt/β-catenin pathway.
In our study, non-responders to PD-1 blockade had upregulations in the pathways of angiogenesis. Moreover, on the basis of the leading-edge analysis of GSEA, tumors with high VEGF-A expression were associated with poorer clinical outcomes after PD-1 blockade therapy compared with those with low VEGF-A expression. These results were consistent with previous reports demonstrating that VEGF-A levels were elevated among non-responders to ICI therapy in melanoma and other cancers (29, 30). VEGF has been reported to reduce the infiltration of CD8+ T cells through decreasing the expression of vascular cell adhesion molecule-1 and increasing the expression of Fas ligand (31, 32). In line with this, we observed significantly fewer CD8+ T cells in tumors with high VEGF-A expression in our study. Recently, a phase III trial of atezolizumab (anti–PD-L1 antibody) plus bevacizumab (anti–VEGF-A antibody) showed promising results for hepatocellular carcinoma, leading to its FDA approval (33). Furthermore, on the basis of the results of phase III trials, the FDA has approved anti–PD-1/PD-L1 antibodies plus tyrosine kinase inhibitors of VEGF receptor in renal cell carcinoma or endometrial carcinoma (34, 35). Given that the levels of VEGF-A and other VEGF related genes (i.e., PIGF, PDGF-A/ B, and VEGFR-1) increased in non-responders in our cohort, therapy with anti-VEGF antibodies or tyrosine kinase inhibitors of VEGF receptor might overcome resistance to ICIs in MSI-H/dMMR gastrointestinal tumors.
In addition, we evaluated CMS and their clinical outcomes in MSI-H/dMMR colorectal cancer. As expected, patients with CMS1, characterized by inflamed tumors with infiltrating activated lymphocytes and high levels of immune checkpoint molecules (36), exhibited a greater tumor response (ORR, 100%) and longer survival outcomes compared with the other types of CMS. Notably, among 6 patients with CMS4, only 1 patient showed an objective response, whereas 2 showed apparent tumor enlargement. CMS4 is characterized by immune-excluded tumors, wherein the immune system is engaged, but factors in its microenvironment (e.g., angiogenesis or TGFβ) prevent activity (37, 38). Thus, dual inhibition of PD-1 and angiogenesis or TGFβ might be a promising strategy for MSI-H/dMMR colorectal cancer with CMS4, warranting further investigations in future clinical trials.
The major limitations of this study were its small sample size and retrospective, single-center design. Therefore, this was a hypothesis-generating study. Further preclinical studies and larger cohort studies are needed to confirm our findings. However, given that MSI-H/dMMR is a rare subtype in patients with gastrointestinal tumors (39), our study provides new insight regarding the development of predictive biomarkers or combination therapies for PD-1/PD-L1 blockade in this population. Also, this research included different gastrointestinal cancer type even if they all shared MSI/dMMR status. The difference of primary tumor location was possibly related with that of RNA expression level (40). We should analyze transcriptomic profiling of each cancer type in a future study with a large cohort of patients, though the RNA expressions of VEGF related gene, which were significantly enriched in non-responders compared with responders, were not significantly different between primary tumor locations in our cohort (data not shown). Finally, the proportion of CMS1 in our study was lower than in a previous report probably due to its small sample size (36). Furthermore, although Marisa and colleagues (41) recently reported that several CMS may coexist within a same colorectal cancer, the heterogeneity was not assessed in our study, which should be investigated in future studies.
In conclusion, several signaling pathways were associated with the response to PD-1 therapies in patients with MSI-H/dMMR gastrointestinal tumors. Of note, high VEGF-A expression might be a predictor of negative response to these agents in this population. Moreover, CMS classification possibly correlated with the clinical outcomes of PD-1 blockade in MSI-H/dMMR patients with colorectal cancer. These transcriptomic features could help in the future development of predictive biomarkers or combination immunotherapies.
Supplementary Material
Acknowledgments
The authors would like to thank all patients and their families for participating. The authors also would like to thank Enago (www.enago.jp) for the English language review. This study was supported by research funding from the National Cancer Center Hospital East and grants from the Japan Agency for Medical Research and Development (AMED; JP21cm0106502) and the Japan Society for the Promotion of Science (JSPS; KAKENHI, 16K07143 and 21H02772; to M. Kawazu).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This article is featured in Highlights of This Issue, p. 1991
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
K. Chida reports personal fees from Bayer outside the submitted work. A. Kawazoe reports personal fees from Daiichi Sankyo, Lilly, Ono, Taiho, Bristol Myers Squibb, Merck Seronobiopharma, MSD, Bayer, Sumitomo Dainippon, and AstraZeneca outside the submitted work. Y. Nakamura reports grants from Taiho Pharmaceutical, Chugai Pharmaceutical, Seagen, Guardant Health, Genomedia, Daiichi Sankyo, and Roche Diagnostics outside the submitted work. Y. Kuboki reports grants and personal fees from Taiho, ONO, Takeda, Boehringer Ingelheim, and Amgen, as well as grants from Bayer, Sanofi, AbbVie, AstraZeneca, Incyte, Chugai, GSK, Genmab, Astellas, and Daiichi-Sankyo outside the submitted work. D. Kotani reports personal fees from Takeda, Chugai, Bristol Myers Squibb, Eli Lilly, and Merckbiopharma; grants and personal fees from Ono and MSD; and grants from Novartis, IQVIA, Syneos Health, CMIC Shift Zero, and Janssen outside the submitted work. T. Kojima reports grants and personal fees from Ono Pharmaceutical and MSD; grants from Amgen Astellas BioPharma, Taiho Pharmaceutical, Shionogi, Chugai Pharmaceutical, and BeiGene Ltd.; and personal fees from Oncolys BioPharma, Astellas Pharma, BMS, and Merk outside the submitted work. H. Bando reports personal fees from Eli Lilly Japan, Taiho Pharmaceutical, and Ono Pharmaceutical, as well as grants from Ono Pharmaceutical outside the submitted work. S. Mishima reports personal fees from Merck Seronobiopharma, as well as grants from Roche Diagnostics outside the submitted work. T. Kuwata reports grants from Roche Diagnostics Japan, as well as personal fees from AstraZeneca, MSD, Bristol Myers Squibb Japan, and Ono Pharmaceutical outside the submitted work. J. Watanabe reports personal fees from Johnson and Johnson, Medtronic, and Eli Lilly, as well as grants from Medtronic, AMCO, and TERUMO outside the submitted work. H. Mano reports grants from Ono Pharmaceutical during the conduct of the study, as well as grants from Daiichi Sankyo, PFDeNA, Sysmex, and Konica–Minolta outside the submitted work. M. Ikeda reports personal fees from ASLAN, NIHON SERVIER, NanoCarrier, Novartis, Bayer, Eli Lilly, Chugai, Shire, Taiho, Sumitomo Dainippon, Teijin Pharma, MSD, Mylan, Astellas, EA Pharma, Gilead, and Otsuka, as well as other support from Yakult, Ono, AstraZeneca, J-Pharma, ASLAN, Merck Serono, NIHON SERVIER, Bristol Myers Squibb, NanoCarrier, Novartis, Bayer, Eli Lilly, MSD, Chugai, Pfizer, Takeda, and Chiome Bioscience outside the submitted work. K. Shitara reports grants from Astellas Pharma, Ono Pharmaceutical, Novartis, Chugai Pharma, Medi-Science, Eisai, and AbbVie Inc.; personal fees from Eli Lilly and Company, Bristol Myers Squibb, Takeda Pharmaceuticals, GlaxoSmithKline, Boehringer Ingelheim, Janssen, and Pfizer Inc.; and grants and personal fees from Daiichi Sankyo, Taiho Pharmaceutical, Merck Pharmaceutical, and Amgen outside the submitted work. I. Endo reports grants from Taiho Pharmaceutical, Ono Pharmaceutical, Chugai Pharmaceutical, Takeda Pharmaceutical, Asahi Kasei Pharmaceutical, Eisai Pharmaceutical, and Eli Lilly Japan outside the submitted work. T. Yoshino reports grants and personal fees from Chugai; personal fees from Takeda, Eli Lily, and Merck Biopharma; and grants from GlaxoSmithKline, Novartis, Sanofi, Ono, Daiichi Sankyo, Parexel, and Sumitomo Dainippon outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
K. Chida: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, investigation, visualization, writing–original draft, project administration, writing–review and editing. A. Kawazoe: Conceptualization, resources, data curation, formal analysis, supervision, funding acquisition, investigation, visualization, writing–original draft, project administration, writing–review and editing. T. Suzuki: Formal analysis, supervision, investigation, visualization, writing–review and editing. M. Kawazu: Methodology, writing–review and editing. T. Ueno: Data curation, writing–review and editing. K. Takenouchi: Formal analysis, visualization, writing–review and editing. Y. Nakamura: Writing–review and editing. Y. Kuboki: Writing–review and editing. D. Kotani: Writing–review and editing. T. Kojima: Writing–review and editing. H. Bando: Writing–review and editing. S. Mishima: Writing–review and editing. T. Kuwata: Writing–review and editing. N. Sakamoto: Writing–review and editing. J. Watanabe: Writing–review and editing. H. Mano: Writing–review and editing. M. Ikeda: Writing–review and editing. K. Shitara: Supervision, writing–review and editing. I. Endo: Writing–review and editing. T. Nakatsura: Writing–review and editing. T. Yoshino: Funding acquisition, writing–review and editing.
References
- 1. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med 2017;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li K, Luo H, Huang L, Luo H, Zhu X. Microsatellite instability: a review of what the oncologist should know. Cancer Cell Int 2020;20:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chao J, Fuchs CS, Shitara K, Tabernero J, Muro K, Cutsem EV, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability–high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol 2021;7:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Casak SJ, Marcus L, Fashoyin-Aje L, Mushti SL, Cheng J, Shen YL, et al. FDA approval summary: pembrolizumab for the first-line treatment of patients with MSI-H/dMMR advanced unresectable or metastatic colorectal carcinoma. Clin Cancer Res 2021;27:4680–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Markham A. Dostarlimab: first approval. Drugs. 2021;81:1213–9. [DOI] [PubMed] [Google Scholar]
- 6. Marabelle A, Le DT, Ascierto PA, Giacomo AMD, Jesus-Acosta AD, Delord J-P, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2020;38:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le DT, Kim TW, Cutsem EV, Geva R, Jäger D, Hara H, et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol 2019;38:11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. André T, Shiu K-K, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-instability–high advanced colorectal cancer. New Engl J Med 2020;383:2207–18. [DOI] [PubMed] [Google Scholar]
- 9. Chida K, Kawazoe A, Kawazu M, Suzuki T, Nakamura Y, Nakatsura T, et al. A low tumor mutational burden and PTEN mutations are predictors of a negative response to PD-1 blockade in MSI-H/dMMR gastrointestinal tumors. Clin Cancer Res 2021;27:3714–24. [DOI] [PubMed] [Google Scholar]
- 10. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016;17:e542–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015;523:231–5. [DOI] [PubMed] [Google Scholar]
- 12. Kwon M, An M, Klempner SJ, Lee H, Kim K-M, Sa JK, et al. Determinants of response and intrinsic resistance to PD-1 blockade in microsatellite instability–high gastric cancer. Cancer Discov 2021;11:2168–85. [DOI] [PubMed] [Google Scholar]
- 13. Kawazoe A, Kuboki Y, Shinozaki E, Hara H, Nishina T, Komatsu Y, et al. Multicenter phase I/II trial of napabucasin and pembrolizumab in patients with metastatic colorectal cancer (EPOC1503/SCOOP Trial). Clin Cancer Res 2020;26:5887–94. [DOI] [PubMed] [Google Scholar]
- 14. Bacher JW, Flanagan LA, Smalley RL, Nassif NA, Burgart LJ, Halberg RB, et al. Development of a fluorescent multiplex assay for detection of MSI-high tumors. Dis Markers 2004;20:237–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hänzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinf 2013;14:7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005;102:15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guinney J, Dienstmann R, Wang X, de RA, Schlicker A, Soneson C, et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015;21:1350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang G, Xu D, Zhang Z, Li X, Shi J, Sun J, et al. The pan-cancer landscape of crosstalk between epithelial–mesenchymal transition and immune evasion relevant to prognosis and immunotherapy response. NPJ Precis Oncol 2021;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zeng D, Li M, Zhou R, Zhang J, Sun H, Shi M, et al. Tumor microenvironment characterization in gastric cancer identifies prognostic and immunotherapeutically relevant gene signatures. Cancer Immunol Res 2019;7:737–50. [DOI] [PubMed] [Google Scholar]
- 20. Conciatori F, Bazzichetto C, Falcone I, Pilotto S, Bria E, Cognetti F, et al. Role of mTOR signaling in tumor microenvironment: an overview. Int J Mol Sci 2018;19:2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ischenko I, D'Amico S, Rao M, Li J, Hayman MJ, Powers S, et al. KRAS drives immune evasion in a genetic model of pancreatic cancer. Nat Commun 2021;12:1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Takeuchi Y, Tanegashima T, Sato E, Irie T, Sai A, Itahashi K, et al. Highly immunogenic cancer cells require activation of the WNT pathway for immunological escape. Sci Immunol 2021;6:eabc6424. [DOI] [PubMed] [Google Scholar]
- 23. Derynck R, Turley SJ, Akhurst RJ. TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol 2021;18:9–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu F, Cheng Y, Wu L, Zhang W, Zheng W, Wang Q, et al. Emerging landscapes of tumor immunity and metabolism. Frontiers Oncol 2020;10:575037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol 2009;9:609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-γ–related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest 2017;127:2930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamada K, Hori Y, Inoue S, Yamamoto Y, Iso K, Kamiyama H, et al. E7386, a selective inhibitor of the interaction between β-catenin and CBP, exerts antitumor activity in tumor models with activated canonical Wnt signaling. Cancer Res 2021;81:1052–62. [DOI] [PubMed] [Google Scholar]
- 28. Zhang H, Bi Y, Wei Y, Liu J, Kuerban K, Ye L. Blocking Wnt/β-catenin signal amplifies anti-PD-1 therapeutic efficacy by inhibiting tumor growth, migration, and promoting immune infiltration in glioblastomas. Mol Cancer Ther 2021;20:1305–15. [DOI] [PubMed] [Google Scholar]
- 29. Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, et al. Genomic and transcriptomic features of response to anti–PD-1 therapy in metastatic melanoma. Cell 2016;165:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen P-L, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov 2016;6:827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell 2019;176:1248–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bourhis M, Palle J, Galy-Fauroux I, Terme M. Direct and indirect modulation of T cells by VEGF-A counteracted by anti-angiogenic treatment. Front Immunol 2021;12:616837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Casak SJ, Donoghue M, Fashoyin-Aje L, Jiang X, Rodriguez L, Shen Y-L, et al. FDA approval summary: atezolizumab plus bevacizumab for the treatment of patients with advanced unresectable or metastatic hepatocellular carcinoma. Clin Cancer Res 2021;27:1836–41. [DOI] [PubMed] [Google Scholar]
- 34. Motzer R, Choueiri TK. Lenvatinib plus pembrolizumab for renal cell carcinoma. New Engl J Med 2021;385:287. [DOI] [PubMed] [Google Scholar]
- 35. Arora S, Balasubramaniam S, Zhang W, Zhang L, Sridhara R, Spillman D, et al. FDA approval summary: pembrolizumab plus lenvatinib for endometrial carcinoma, a collaborative international review under project Orbis. Clin Cancer Res 2020;26:5062–7. [DOI] [PubMed] [Google Scholar]
- 36. Sveen A, Johannessen B, Tengs T, Danielsen SA, Eilertsen IA, Lind GE, et al. Multilevel genomics of colorectal cancers with microsatellite instability—clinical impact of JAK1 mutations and consensus molecular subtype 1. Genome Med 2017;9:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Soldevilla B, Carretero-Puche C, Gomez-Lopez G, Al-Shahrour F, Riesco MC, Gil-Calderon B, et al. The correlation between immune subtypes and consensus molecular subtypes in colorectal cancer identifies novel tumour microenvironment profiles, with prognostic and therapeutic implications. Eur J Cancer 2019;123:118–29. [DOI] [PubMed] [Google Scholar]
- 38. Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer 2017;17:79–92. [DOI] [PubMed] [Google Scholar]
- 39. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Uhlen M, Zhang C, Lee S, Sjöstedt E, Fagerberg L, Bidkhori G, et al. A pathology atlas of the human cancer transcriptome. Science 2017;357:eaan2507. [DOI] [PubMed] [Google Scholar]
- 41. Marisa L, Blum Y, Taieb J, Ayadi M, Pilati C, Malicot KL, et al. Intratumor CMS heterogeneity impacts patient prognosis in localized colorectal cancer. Clin Cancer Res 2021;27:4768–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw RNA sequencing data used in the present study were uploaded to the Sequence Read Archive of DNA DataBank of Japan (DRA: https://www.ddbj.nig.ac.jp/dra/index.html) under accession No. DRA013565. All other data are available from the corresponding author upon reasonable request. Those requests will be reviewed by a study steering committee to verify whether the request is subject to any intellectual property or confidentiality obligations.



![Figure 3. Kaplan–Meier plots of progression-free survival (PFS; A) and overall survival (OS; B) according to the expression of VEGF-A mRNA. Patients with high VEGF-A mRNA expression, compared with those with low expression, had significantly lower PFS [median: 4.8 months; (95% CI, 3.0–30.7) vs. not reached, NR (95% CI, 8.4–NR), log-rank P = 0.025] and OS [median, 11.1 months; (95% CI, 5.3–NR) vs. NR (95% CI, 15.2 to NR); log-rank P = 0.035].](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/6ba9/9365358/94058166cc59/2110fig3.jpg)
![Figure 5. Kaplan–Meier plots of progression-free survival (PFS; A) and overall survival (OS; B) according to the expression of consensus molecular subtype (CMS). Patients with CMS1 showed significantly longer PFS [Not reached, NR (95% CI, NR–NR) vs. 4.8 months (95% CI, 1.1–30.7); P = 0.017] and numerically longer OS [Not reached (95% CI, NR–NR) vs. 31.0 months (95% CI, 2.6–NR); P = 0.105] than those with other types of CMS.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/6ba9/9365358/b2f51e58be4f/2110fig5.jpg)