Abstract
Purpose:
Testing safety of Delta24-RGD (DNX-2401), an oncolytic adenovirus, locally delivered by convection enhanced delivery (CED) in tumor and surrounding brain of patients with recurrent glioblastoma.
Patients and Methods:
Dose-escalation phase I study with 3+3 cohorts, dosing 107 to 1 × 1011 viral particles (vp) in 20 patients. Besides clinical parameters, adverse events, and radiologic findings, blood, cerebrospinal fluid (CSF), brain interstitial fluid, and excreta were sampled over time and analyzed for presence of immune response, viral replication, distribution, and shedding.
Results:
Of 20 enrolled patients, 19 received the oncolytic adenovirus Delta24-RGD, which was found to be safe and feasible. Four patients demonstrated tumor response on MRI, one with complete regression and still alive after 8 years. Most serious adverse events were attributed to increased intracranial pressure caused by either an inflammatory reaction responding to steroid treatment or viral meningitis being transient and self-limiting. Often viral DNA concentrations in CSF increased over time, peaking after 2 to 4 weeks and remaining up to 3 months. Concomitantly Th1- and Th2-associated cytokine levels and numbers of CD3+ T and natural killer cells increased. Posttreatment tumor specimens revealed increased numbers of macrophages and CD4+ and CD8+ T cells. No evidence of viral shedding in excreta was observed.
Conclusions:
CED of Delta24-RGD not only in the tumor but also in surrounding brain is safe, induces a local inflammatory reaction, and shows promising clinical responses.
Translational Relevance.
In this phase I trial, we demonstrate the safety of prolonged infusion by convection enhanced delivery of the oncolytic adenovirus Delta24-RGD, in recurrent glioblastoma (GBM) and peritumoral regions. Despite the potential effects of immunotherapeutic approaches in GBM, including immune checkpoint blockade and therapeutic vaccines, limitations affecting treatment efficacy are apparent. Our study results show promising clinical responses and indications for an antitumor immune response, providing a base for future testing of (combinatorial) Delta24-RGD treatment in GBM. This trial was the first to assess the local and locoregional responses upon infusion of an oncolytic virus into the tumor and surrounding brain by sequential sampling of brain interstitial fluid and cerebrospinal fluid (CSF). Cytokine and chemokine analysis in CSF suggested that IFNγ and TNFα levels may represent a potential biomarker for response in future oncolytic viral trials. Biomarker assays may ultimately aid in the identification of responding patients and improve the response rate to oncolytic viral therapy.
Introduction
Despite aggressive multimodal therapy the prognosis of patients with glioblastoma (GBM) remains dismal, with a median survival of 12 to 15 months and almost always a fatal outcome (1). The need for novel treatments of GBM has led to the development and evaluation of several virus-based therapeutic strategies, among which oncolytic viruses (2).
In this clinical study we tested Delta24-RGD (DNX-2401) in patients with recurrent GBM. This oncolytic adenovirus harbors a 24 base pair deletion in the viral E1A genomic region, which renders the adenovirus unable to replicate in normal cells, but capable of replicating in cells with disrupted Rb pathway (3, 4). The p16/Rb pathway is inactivated in more than 90% of gliomas, making this pathway an excellent target for designing tumor-specific replication-competent viruses (5–9). In addition, a RGD-4C peptide is inserted in the fiber knob, altering the primary attachment from the Coxsackie Adenovirus Receptor (CAR) to αvβ5 and αvβ3 integrin receptors, improving the infection efficiency of glioma cells (10, 11). The oncolytic potency of Delta24-RGD has been confirmed in several preclinical studies (12–15).
Apart from direct oncolysis, studies in syngeneic mouse glioma models demonstrated that local Delta24-RGD treatment induced T-cell-mediated antitumor responses and established a protective immune memory (16, 17). This immune-mediated antitumor activity was abolished upon co-treatment with the immune suppressive agent dexamethasone or by depletion of CD4+ or CD8+ T cells, resulting in complete loss of survival benefit (16, 18). More recently, we showed that local Delta24-RGD therapy promotes a prolonged shift in tumor macrophage phenotype from the protumoral M2 toward pro-inflammatory M1 in human GBM, contributing to a tumor-detrimental microenvironment (19). Combination treatment with the immune checkpoint inhibitor anti–PD-1 improved the efficacy of Delta24-RGD treatment in two syngeneic mouse models for GBM (20). Together, these studies underscore the important role of the immune system in Delta24-RGD therapy of GBM.
A recently published phase I clinical trial with a “window of opportunity” component testing local injection of Delta24-RGD into the tumor of patients with recurrent GBM showed evidence of viral replication in tumor cells and activation of antitumor immune responses in posttreatment specimens. These biological effects translated into complete and durable antitumor responses in several patients. No toxic side effects were observed and 15% of the antitumor responses were long-lasting, suggesting that activation of the immune system is an important mechanism of this agent (21).
In view of the recognized heterogeneity and the diffuse spread of GBM, we applied convection enhanced delivery (CED) for local administration. With this method of prolonged micro-infusion, a large volume of distribution can be achieved (22). Herewith, different subclonal tumor populations may be infected by the virus, as well as infiltrating tumor cells in the surrounding brain.
In this clinical study, we enrolled 20 patients with recurrent GBM in a dose escalation set-up, administering Delta24-RGD by CED to larger areas of tumor and tumor-infiltrated brain with the purpose of evaluating the safety and tolerability of Delta24-RGD. A unique aspect of the study was that serial blood and cerebrospinal fluid (CSF) samples were obtained before, during, and for prolonged periods after virus infusion, which enabled analysis of reactive changes in blood and CSF composition. Microdialysis of tumor tissue enabled measurement of interstitial, extracellular fluid composition before and during virus infusion. Urine, saliva, and fecal sampling enabled us to assess potential viral shedding after virus administration to the brain.
Patients and Methods
Clinical trial
We conducted a standard dose-escalation phase I trial, followed by a small dose expansion cohort (see “treatment plan” and “dose cohort” below), evaluating administration of Delta24-RGD by CED to tumor and surrounding brain. This trial was conducted in accordance with the Declaration of Helsinki and approved by the Dutch regulatory authorities, that is, the Central Committee on Research Involving Human Subjects, the Ministry of Health and the Ministry of Infrastructure and Environment. All participants provided written informed consent. Patient safety was monitored by an independent Data Safety Monitoring Board (DSMB).
Inclusion and exclusion criteria
Eligible patients had a recurrence of a histologically proven GBM after surgery and/or chemo- and/or radiotherapy, confirmed on MRI scan within 3 weeks prior to enrollment. All patients were between 18 and 70 years old; had a Karnofsky performance status rating ≥70%; had recovered from the toxic effects of prior therapy; had adequate hepatic, renal, and bone marrow function, defined as absolute neutrophil count (ANC) ≥1.5 × 109 L−1, platelet count of ≥100 × 109 L−1, ALT (SGPT), AST (SGOT), and alkaline phosphatase ≤2 times ULN, total bilirubin < 26 μmol/L, creatinine <1.5 times ULN, and urea (BUN) <1.5 times ULN. Recurring tumors had to be restricted to one hemisphere, without signs of subependymal spreading, be accessible for surgery or biopsy, be unifocal without a midline shift >0.5 cm and without radiologic signs of uncal herniation. Before start of virus infusion, histologic analysis of the resected or biopsied tumor had to support the diagnosis of tumor recurrence. Patients were excluded if they had an active uncontrolled infection; had an upper pulmonary infection and/or flu-like symptoms or the presence of adenovirus in pretreatment throat-swab or serum sample as determined by PCR; had evidence of bleeding diathesis or use of anticoagulants that could not be safely interrupted; had systemic diseases or other unstable conditions associated with increased anesthetic/surgical risks; were immune-compromised or known to have HIV; were pregnant or lactating; or had another primary malignancy than GBM.
Study drug
Delta24-RGD is a conditionally replication-competent adenovirus, containing a 24 base pair deletion (bases 923–946) in the E1A gene and an integrin-binding motif (RGD-4C) insertion in the H1 loop of the fiber. The production of the virus was commissioned and supplied by the Biological Resources Branch (BRB), Development Therapeutics Program (DTP) of the Division of Cancer Treatment & Diagnostics (DCTD) at the National Cancer Institute-Frederick (NIH), and manufactured by The National Vector Production Laboratory at Baylor college of Medicine in Houston.
The drug was supplied in a sterile, pyrogen-free solution at a concentration as specified on the Certificate of Analysis diluted in 20 mmol/L Tris, 25 mmol/L NaCl, 2.5% (w/v) glycerol as stabilizer and pH 8.0. Delta24-RGD was provided in sterile single use vials, containing 0.25 mL fill volume each, with a titer of 2 × 1011 vp/mL (6 × 109 pfu/mL). Just prior to administration, the virus was dissolved in 0.9% NaCl and 0.2% human serum albumin and divided into syringes of which the number corresponded with the number of CED-infusion catheters to be used. Virus stability and compatibility with the catheter-tubing system was assessed in advance.
Treatment plan
Patients not amenable for surgical resection underwent needle biopsy, placement of CED catheters, a ventricular catheter with subcutaneous reservoir for CSF sampling and a microdialysis probe in a single surgical procedure. Four temporary CED catheters were inserted, of which two in the enhancing tumor core and two in the tumor-infiltrated surrounding brain. The temporary microdialysis probe (cut-off 100,000 Da; M Dialysis AB), enabling continuous sampling of brain interstitial fluid (BIF), was inserted in the tumorcore. Patients with an indication for tumor debulking first underwent resection in a separate procedure at least 1 week before study treatment.
Before virus infusion, the position of catheters was evaluated by CT scanning. Convective properties and function of catheters were assessed by 4 to 6 hours test infusion (flow rate of 0.2 to 0.3 mL/h) of a gadolinium-based contrast agent followed by MR scanning. Catheters not showing convective spread of gadolinium were not used for virus infusion.
Virus was infused over a time period of 44 to 66.7 hours with a rate of 0.2 to 0.3 mL/h, depending on number of catheters used. Hereafter CED catheters and the microdialysis probe were removed. Patients remained in isolated care during virus infusion and until daily testing for virus shedding was negative for minimally 3 consecutive days after ending of Delta24-RGD infusion, provided that surgical wounds were without signs of CSF leakage after removal of the catheters and dialysis probe.
Dose cohorts
Patients were subsequently assigned into one of six Delta24-RGD incremental dose cohorts, ranging from 1 × 107 vp to 1 × 1011 vp. If no dose-limiting toxicities (DLT) occurred in the first three patients, subsequent patients were entered in the next dose level (Supplementary Fig. S1). In the event of a DLT, three additional patients were enrolled at the same dose level. DLT was defined as any Delta24-RGD related ≥grade 3 organ system toxicity, or grade 4 hematology or grade 4 nonorgan system metabolic/laboratory toxicity. The MTD was defined as one dose level below the dose causing DLT in ≥33.3% of the patients with a minimum of 6 treated patients treated at that dose level. An extension cohort of 6 patients was planned to be treated at the MTD.
Endpoints
The primary endpoint of this study was to determine the MTD. Safety was further assessed by evaluation of adverse events (AE), laboratory examinations (biochemistry, hematology, CSF sampling), dissemination of virus in body fluids analyzed by PCR, vital signs, physical examination, KPS score, and neurologic examination. The secondary endpoints of this study were PFS, overall survival (OS), and tumor response on MRI.
Safety assessment
During hospitalization patients had daily physical and neurologic examinations, registration of vital signs, AEs, serum, CSF, and urine analysis, saliva, blood, urine, and feces samples for virus culturing and use of concomitant medication. The same assessments were performed at every study visit after hospital discharge until 4 weeks after completion of the Delta24-RGD infusion. The severity (Grade) of each AE was assigned according to the NCI Common Terminology Criteria (CTC) for Adverse Events, version 3.0.
Response assessment
Radiographic tumor assessment on T1 contrast enhancing, FLAIR, and T2 images was performed by MRI scan every 12 weeks. Efficacy was studied by determination of PFS and OS.
Collection of blood, cerebrospinal fluid, and BIF
Blood and CSF were collected 24 and 6 hours before start of virus infusion respectively (indicated as pre-infusion time points) and 6, 24, 48, 72, and 96 hours, and 1, 2, 4, and 12 weeks there after (indicated as post-infusion time points). Samples were centrifuged within 1 hour to remove erythrocytes from CSF or to collect serum or plasma from blood and aliquoted and stored at −80°. BIF was collected at 6 hours before and 2, 6, 22, and 44 hours after start of virus infusion and frozen at −80° immediately.
Analytical plan of the phase I trial
Sample size was variable and dependent on the number of patients needed to find the MTD with a dose-limiting toxicity level of <33.3% and a maximum of 6 patients treated at any dose level. AEs were coded using the CTCAE v3.0 and MedDRA v 10.0 AE dictionaries and listed by severity and by relatedness with the study treatment. Laboratory data were analyzed with shift tables and summaries of change from baseline to maximum posttreatment value.
Laboratory analyses
IHC of posttreatment tissues
Tumor sections were made from the formalin-fixed paraffin-embedded tissue of patient 7 (postmortem, 3 months after treatment) and 11 (resection 6 weeks after virus treatment). Antigens were retrieved at 97°C for 16 minutes (CD8) or 64 minutes (CD68, CD16, CD4) using ULTRA Cell Conditioning (Ventana Medical Systems Inc.). Sections were stained with the following antibodies: anti-hexon (Millipore), anti-CD68/k (Clone M0814; Dako Agilent Pathology Solutions), anti-CD16 (Clone 2H7, Neomarkers Inc.), anti-CD4 (Ventana Medical Systems Inc.), and anti-CD8 (clone Sp57, Ventana Medical Systems Inc.). Sections were analyzed after signal amplification and counterstaining with Hematoxylin II and Bluing Reagent (All Ventana Medical Systems Inc.).
Detection of Delta24-RGD viral genomes
From CSF and excreta samples (urine, throat, and anal swabs) nucleic acids were extracted for qPCR analysis of the adenoviral RGD-modified fiber gene using the High Pure Viral Nucleic Acid Extraction Kit of the Magnapure LC (Roche Molecular Systems). Amplification was performed using the 2× Taqman Universal Mastermix (Life Technologies) and primers add24-RGDfwd (5′-acactaaacggtacacaggaaacag-3′) and add24-RGDrev (5′-gccagaccagtcccatgaaa-3′) and FAM-BHQ1 labeled probe add24-RGDprobe (5′-ccgcggagactgtttctgccca-3′). Real-time PCR amplification was read on a Lightcycler 480 (Roche Molecular Systems). In parallel, a calibration curve of a Delta24-RGD stock with a known viral particle titer was run. The minimal detection level of RGD-specific adenoviral genomes was 20 vp/mL.
Cytokine and chemokine measurements
For cytokine and chemokine analysis, the CSF and BIF samples were thawed on ice and 25 μL of each sample (in duplicate for CSF) was used to detect IFNγ, IL1β, IL2, IL4, IL5, IL6, IL8, IL10, IL12p70, IL13, TNFα, IP-10, MCP-1, MCP-4, MIP-1β, TARC, and Eotaxin using the Meso Scale Discovery Human Cytokine Ultra-Sensitive Multiplex Assay (MSD) according to the manufacturer's instructions. Plates were run on the SECTOR Imager Instrument (MSD) and results were analyzed using the Workbench analysis software (MSD).
Blood, CSF, and tissue flow cytometry analysis
Blood and CSF samples were processed for 6-color flow cytometry analysis as described by de Graaf and colleagues (23). The absolute numbers and percentages of leukocytes (lymphocytes, granulocytes, and monocytes), lymphocytes [T (CD4 and CD8), natural killer (NK), NKT and B cells], T cells (naïve, central memory, effector memory, and regulatory), and dendritic cell (DC) subsets (myeloid and plasmacytoid) were assessed. Immediately upon staining, list mode data were acquired on a 6-color FACS Canto flow cytometer (BD Biosciences). Analysis was performed using FCS Express software (De Novo Software). Resection tissue (patient 16) obtained 26 months posttreatment was processed and analyzed by flow cytometry as described previously using the following antibodies CD3, CD4, CD8, CD14, CD19, CD27, CD45, and CD56 (19).
Serum and cerebrospinal fluid adenovirus antibody titers
Serum and cerebrospinal fluid immunoglobulin antibodies against adenovirus were determined quantitatively by adenovirus IgG and adenovirus IgM ELISA kits (Creative Diagnostics) according to manufacturer's instructions. For both IgG and IgM assays, samples obtained before virus treatment and at 4 weeks after were thawed and duplicate serial dilutions up to 1:2,000 were prepared followed by testing against the provided standard curves of the kits. The absorbance was read on a VERSAmax tunable microplate reader (Molecular Devices). Results are expressed in arbitrary units (U/mL).
Statistical analysis
Statistical analysis was performed using the Prism Graphpad Software (Graphpad Software Inc.). Differences were considered statistically significant when P < 0.05. Time to progression and survival was summarized by Kaplan–Meier methods.
Results
Patient characteristics and virus treatment
In total, 20 patients were enrolled, 15 in the dose escalation cohort, 5 in the dose expansion cohort (Supplementary Fig. S1). Patients had a median age of 53.5 years (range 29–69 years) and Karnofsky Performance Score (KPS) between 70 and 100 (median 90; Table 1). Most patients suffered from a second or third tumor recurrence (n = 17; 85%) after prior treatments of resection at initial diagnosis (n = 19; 95%), re-resection after recurrence (n = 5; 20%), radiation (n = 20; 100%), concomitant and adjuvant temozolomide (n = 19; 95%), second-line lomustine (n = 15; 75%), and second-line bevacizumab (n = 6; 30%). Tumors were determined to be IDH1 wild-type (n = 15; 75%), mutant (n = 1; 5%), or unknown (n = 4; 20%). Nineteen of 20 patients completed Delta24-RGD virus infusion according to protocol; 5 of 20 patients (25%) had tumor debulking/resection prior to virus treatment. All patients received four CED catheters, except one in whom a small venous superficial cortical hemorrhage prohibited entry and placement of one catheter. Of 79 catheters placed in 20 patients, 70 were used for virus infusion. In 5 patients one of four catheters was excluded for infusion due to mispositioning of the catheter tip in the lateral ventricle (1) and contrast leakage to CSF spaces at test infusion (4). One patient showed lowering of KPS below 70 after the surgical procedure for needle biopsy and catheter placement, resulting in failure to meet the inclusion criteria for virus infusion.
Table 1.
Patient characteristics: sex (F, female, M, male), age (in years), KPS, prior treatments before enrollment in this trial (RT, radiotherapy; TMZ, temozolomide), received viral dose (vp), if patients underwent resection prior to treatment, and isocitrate dehydrogenase (IDH) status.
| Prior treatment | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Sex | Age | KPS | Standard therapy | Second-line | Third-line | Dose (vp) | Resection | IDH status |
| 1 | F | 42 | 90 | Resection, RT/TMZ, aTMZ | Bevacizumab + lomustine | 107 | Yes | Wild-type | |
| 2 | F | 29 | 90 | Resection, RT/TMZ, aTMZ | Bevacizumab + lomustine | 107 | No | n/a | |
| 3 | M | 54 | 80 | Resection, RT | Temsirolimus | Bevacizumab + lomustine | 107 | No | Wild-type |
| 4 | F | 46 | 90 | Resection, RT/TMZ, aTMZ | Lomustine | 108 | No | Wild-type | |
| 5 | M | 53 | 100 | Resection, RT/TMZ, aTMZ | Bevacizumab + lomustine | 108 | No | Wild-type | |
| 6 | F | 60 | 90 | Resection, RT, TMZ | 108 | Yes | Wild-type | ||
| 7 | M | 67 | 80 | Resection, RT/TMZ | Dasatinib + lomustine | 109 | No | Wild-type | |
| 8 | M | 64 | 90 | Resection, RT/TMZ, aTMZ | Lomustine | 109 | No | n/a | |
| 9 | M | 60 | 90 | Resection, RT/TMZ | DC therapy | Lomustine | 109 | No | n/a |
| 10 | F | 54 | 90 | Biopsy, RT, TMZ | Lomustine | 1010 | No | Wild-type | |
| 11 | F | 48 | 80 | Resection, RT/TMZ | Re-resection, aTMZ | 1010 | No | Wild-type | |
| 12 | M | 52 | 90 | Resection, RT/TMZ, aTMZ | 1010 | Yes | Wild-type | ||
| 13 | M | 45 | 90 | Resection, RT/TMZ, aTMZ | Re-resection, lomustine | 3 × 1010 | No | Wild-type | |
| 14 | M | 37 | 90 | Resection, re-resection, TMZ, RT | Lomustine | Not infused | No | n/a | |
| 15 | F | 40 | 100 | Resection, RT/TMZ, aTMZ | Lomustine | 3 × 1010 | No | Mutant | |
| 16 | M | 55 | 100 | Resection, RT/TMZ, aTMZ | 1010 | No | Wild-type | ||
| 17 | F | 66 | 90 | Resection, RT/TMZ, aTMZ | Bevacizumab + lomustine | Re-resection | 1010 | Yes | Wild-type |
| 18 | M | 69 | 90 | Resection, RT/TMZ, | Re-resection, aTMZ | Re-resection | 1010 | Yes | Wild-type |
| 19 | M | 38 | 80 | Resection, RT/TMZ, aTMZ | Bevacizumab + lomustine | 1010 | No | Wild-type | |
| 20 | M | 56 | 80 | Resection, RT/TMZ, aTMZ | Re-resection, lomustine | 1010 | No | Wild-type | |
Toxicities
A summary of AEs is shown in Table 2. In 14 patients, 17 serious AEs (SAE) occurred, of which 8 were unrelated to the study treatment, including one fatal SAE (gastrointestinal perforation). The remaining nine SAEs that were possibly related are described in detail below (Table 3).
Table 2.
All reported adverse events arranged by body systems and toxicity grades.
| Toxicity grade | Total | ||||||
|---|---|---|---|---|---|---|---|
| Body system | 1 | 2 | 3 | 4 | 5 | Missing data | |
| Blood/bone marrow | 95 | 5 | 3 | 103 | |||
| Cardiac general | 5 | 1 | 6 | ||||
| Coagulation | 15 | 15 | |||||
| Constitutional symptoms | 21 | 9 | 3 | 34 | |||
| Dermatology/skin | 6 | 1 | 2 | 9 | |||
| Endocrine | 2 | 1 | 3 | ||||
| Gastrointestinal | 20 | 18 | 2 | 1 (perforation) | 42 | ||
| Hemorrhage/bleeding | 8 | 1 | 9 | ||||
| Infection | 4 | 7 | 1 | 12 | |||
| Lymphatics | 4 | 1 | 5 | ||||
| Metabolic/laboratory | 138 | 19 | 7 | 1 | 165 | ||
| Musculoskeletal/soft tissue | 6 | 5 | 7 | 1 | 19 | ||
| Neurology | 27 | 26 | 14 | 3 | 70 | ||
| Ocular/visual | 2 | 3 | 5 | ||||
| Pain | 22 | 15 | 3 | 40 | |||
| Pulmonary/upper respiratory | 10 | 4 | 2 | 16 | |||
| Renal/genitourinary | 3 | 2 | 2 | 7 | |||
| Syndromes | 1 | 1 | |||||
| Total | 389 | 117 | 48 | 5 | 1 | 1 | 561 |
Table 3.
All reported SAEs per patient, viral dose, and relation to treatment.
| Patient | Dose (vp) | SAE | Grade | Relation to treatment |
|---|---|---|---|---|
| 5 | 108 | Neck pain (in combination with fever) | 2 | Unrelated |
| 6 | 108 | Hydrocephalus | 4 | Unrelated |
| 7 | 109 | Urinary tract infection | 2 | Unrelated |
| 7 | 109 | Neurological deterioration | 3 | Unrelated |
| 7 | 109 | Diverticulitis resulting in sigmoid perforation and sepsis | 5 | Unrelated |
| 9 | 109 | Confusion | 4 | Related to Delta24-RGD infusion |
| 10 | 1010 | Seizure (generalized) | 3 | Related to Delta24-RGD infusion |
| 11 | 1010 | Neurological deterioration | 2 | Unrelated |
| 12 | 1010 | Chronic subdural hematoma | 2 | Unrelated |
| 13 | 3 × 1010 | Increased intracranial pressure | 3 | Related to Delta24-RGD infusion |
| 14 | Not infused | Neurological deterioration | 3 | Related to catheter surgery |
| 15 | 3 × 1010 | Meningitis with hydrocephalus | 3 | Related to Delta24-RGD infusion |
| 16 | 1010 | Wound dehiscence at the site of the Ommaya | 3 | Related to other study procedure |
| 17 | 1010 | Increased intracranial pressure | 3 | Related to Delta24-RGD infusion |
| 17 | 1010 | Wound dehiscence at the site of the Ommaya | 3 | Related to other study procedure |
| 19 | 1010 | Depressed level of consciousness | 4 | Unrelated |
| 20 | 1010 | Seizure | 3 | Related to Delta24-RGD infusion |
Patient 9 (dose level 3, 1 × 109 vp) was readmitted with a CTC grade 4 confusion and signs of delirium 4 weeks after virus infusion. Although this event was possibly related to virus treatment, he recovered after haloperidol and antibiotic treatment because of a suspected urinary tract infection.
Patient 10 (dose level 4, 1 × 1010 vp) was hospitalized 2 weeks after virus treatment because of a first generalized seizure, CTC grade 3. Seizures resolved after starting anti-epileptic medication and correction of a hyponatremia and hypopotassemia. The event was possibly related to virus infusion, but more likely related to the GBM itself in combination with hyponatremia.
Patient 13 (dose level 5, 3 ×1010 vp) was readmitted 4 weeks after virus treatment with symptoms of increased intracranial pressure and increased peritumoral edema on CT scan, CTC grade 3. Administration of dexamethasone provided rapid improvement.
Patient 14 experienced neurologic deterioration after surgery for needle biopsy and catheter placement. His preoperative KPS score of 70 dropped to 50 resulting in failure to meet the inclusion criteria. This grade 3 SAE was related to study related surgery and not to the virus itself.
Patient 15, treated at dose level 5 (3 × 1010 vp), was readmitted with mild fever, nausea, and vomiting, CTC grade 3, hydrocephalus, and CSF leakage from the surgical head wound 2 weeks after virus infusion. After bacterial meningitis was ruled out, the patient was diagnosed with viral meningitis and subsequent mild hydrocephalus, which was supported by the finding of a high Delta24-RGD titer in CSF. Spontaneous recovery occurred after 2 weeks.
Patient 16 experienced a wound dehiscence, CTC grade 3, related to other study procedure.
Patient 17 (dose level 4, 1 × 1010 vp) was readmitted 2 weeks after virus infusion with an increase of preexisting mild hemiparesis, headache, nausea, and vomiting, CTC grade 3. A CT scan showed increased edema around the tumor and mid-line shift, after dexamethasone and mannitol therapy symptoms disappeared. This patient also experienced a wound dehiscence, CTC grade 3.
Patient 20 (dose level 4, 1 × 1010 vp) was readmitted 1 week after virus treatment with dysphasia and mild fever, CTC grade 3, both disappeared spontaneously. A CT scan did not reveal new findings and an EEG showed possible postictal changes. Because of previous epilepsy-related speech disorders, it was concluded that this event was caused by an epileptic seizure, possibly related to virus infusion.
Four patients developed dysregulation of the CSF circulation. This resulted in re-closure of a CSF-leaking head wound (patient 15), placement of a CSF shunt (patients 6 and 12), and head bandage for a subcutaneous CSF collection with ventricular enlargement on CT scan (patient 10). Two of these patients and an additional other patient (patient 17) had signs of viral meningitis consisting of headache, neck pain, and nausea, occurring between week 2 and 4, which were all self-limiting. Although a clear relation between occurrence of these symptoms, CSF circulation disturbances, and viral CSF titers is lacking, virus delivery through CED catheters in the tumor and the surrounding brain may have led to spreading of viral particles, not only in tissue but also in the CSF, causing symptoms of viral meningitis.
MTD as a result from toxicity was not reached in this study. Because of CSF leakage from a head wound 2 weeks after treatment at dose level 5 (3 × 1010 vp) in patient 15, dose escalation was evaluated by the Dutch environmental safety authority and DSMB upon which it was recommended to stop dose escalation, not because of toxicity but to avoid a possible risk of virus shedding. Therefore, patients in the dose expansion cohort were subsequently treated at dose level 4 (1 × 1010 vp).
Survival
Median PFS was 82 days (range 29–287 days), with a median OS of 129 days (range 68 days to more than 7 years). Six of 19 patients had an OS of more than 6 months, of which 2 (15 and 16) achieved long-term survival of 7.5 and 2.5 years, respectively. Patient 15 had a complete response, is still alive and well, without signs of tumor recurrence and without any other treatments (Table 4).
Table 4.
Survival (in days) of the trial patients and reported MRI responses.
| Patient | Dose (vp) | Response type | Survival in days |
|---|---|---|---|
| 1 | 107 | 169 | |
| 2 | 107 | Survival | 280 |
| 3 | 107 | Survival | 225 |
| 4 | 108 | 86 | |
| 5 | 108 | 98 | |
| 6 | 108 | 129 | |
| 7 | 109 | MRI | 96 |
| 8 | 109 | 94 | |
| 9 | 109 | 96 | |
| 10 | 1010 | Survival | 211 |
| 11 | 1010 | MRI | 106 |
| 12 | 1010 | 131 | |
| 13 | 3 × 1010 | 95 | |
| 14 | Not infused | ||
| 15 | 3 × 1010 | MRI + survival | 2,792a |
| 16 | 1010 | MRI + survival | 976 |
| 17 | 1010 | 82 | |
| 18 | 1010 | 146 | |
| 19 | 1010 | 68 | |
| 20 | 1010 | Survival | 199 |
aPatient currently alive 7.5 years after initial Delta24-RGD treatment.
MRI response
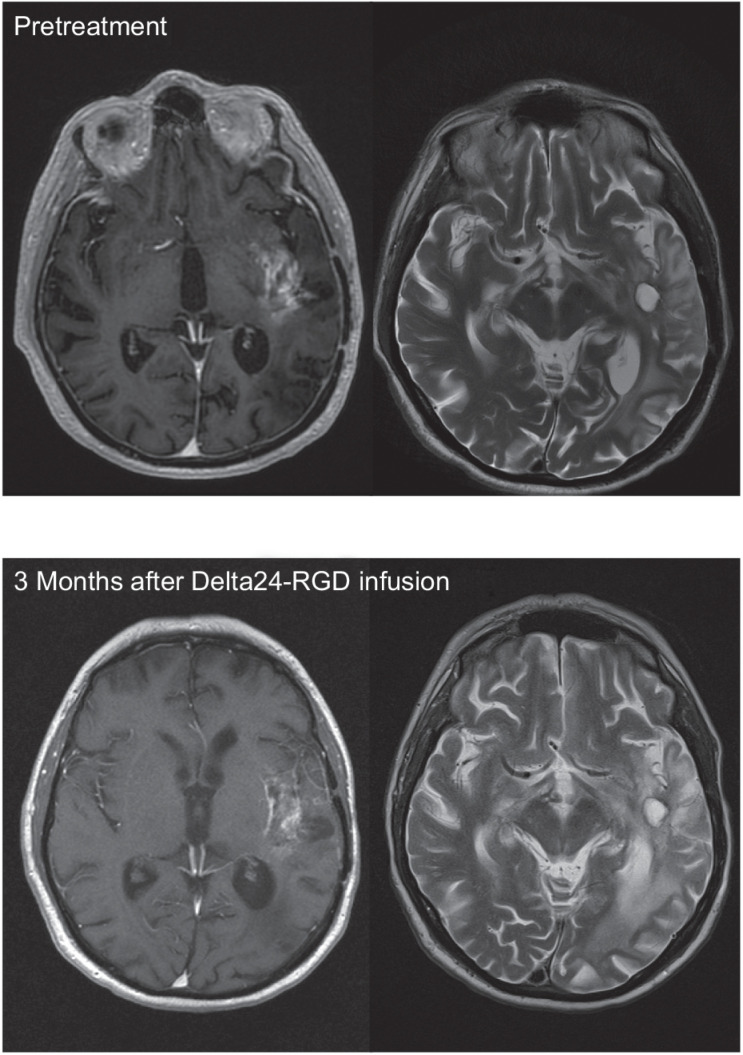
Using three-dimensional volume measurements on T1 contrast enhancing as well as FLAIR and T2 images, we could identify 4 patients with a radiographic response. Patient 7 (dose 1 × 109 vp) had at 3 months unchanged tumor size on T2, an increase of FLAIR signal interpreted as edema and a decrease of contrast enhancement on T1 (Fig. 1). Patient 11 (dose 1 × 1010 vp) showed tumor lysis around the track of a CED-infusion catheter (Fig. 2). Two patients with long-term survival (dose 1 × 1010 vp and 3 × 1010 vp) showed tumor regression. Patient 15 had increased enhancement at the initial follow-up MRI scan (Fig. 3), which was later interpreted as pseudoprogression, because after more than a year the tumor gradually disappeared over the course of another year, without having had any other treatment (Table 4).
Figure 1.
Illustrative case patient 7, a 67-year-old male with a second recurrence of a GBM, diagnosed 13 months earlier. Virus infusion of 1 × 109 vp was uncomplicated, but patient was readmitted 3 months later due to neurological deterioration. MR showed a decrease of the contrast-enhancing part of the tumor but an increase of the signal intensity in the peritumoral area on the T2- and FLAIR-images, interpreted as increased edema and stable tumor (left side: T1 after gadolinium; right side: T2 images). During hospitalization, patient deteriorated rapidly, dying with clinical signs of sepsis. At autopsy a diverticulitis with sigmoid perforation was found. Brain autopsy revealed a relatively small tumor in relation to the MR images, containing immune cell infiltrates (Fig. 5A), necrotic areas, infarctions, and absence of vascular proliferation.
Figure 2.
Illustrative case patient 11, a 48-year-old female, who was treated 21 months after GBM diagnosis for a second recurrence at dose level 4 with 1 × 1010 vp. After 2 weeks, MRI showed a large area with cavity formation suggestive of tumor lysis in the target area of one of the intratumoral catheters used for virus administration [A, T1 after gadolinium prior to treatment and 2 weeks after treatment; B, postoperative neuronavigation screenshot demonstrating the relation of the CED catheter (trajectory in blue) and the necrotic area]. Six weeks after virus infusion her preexisting paresis of the left leg deteriorated due to increasing mass effect of the treated tumor for which she underwent subtotal re-resection. After initial improvement, she deteriorated again 6 weeks later, at this point she declined further diagnostic tests and treatment. She died 106 days after virus infusion. PCR analysis of CSF samples demonstrated increasing virus titers 3 months post-infusion (Fig. 5). The resected tumor material contained besides tumor cells also viral proteins and immune cell infiltrates (Fig. 4B). Our routine tumor cell culture protocol of this tumor failed due to virus-induced lysis of tumor cells. Unfortunately, we were unable to discriminate between true tumorprogression or an inflammatory reaction known as pseudoprogression.
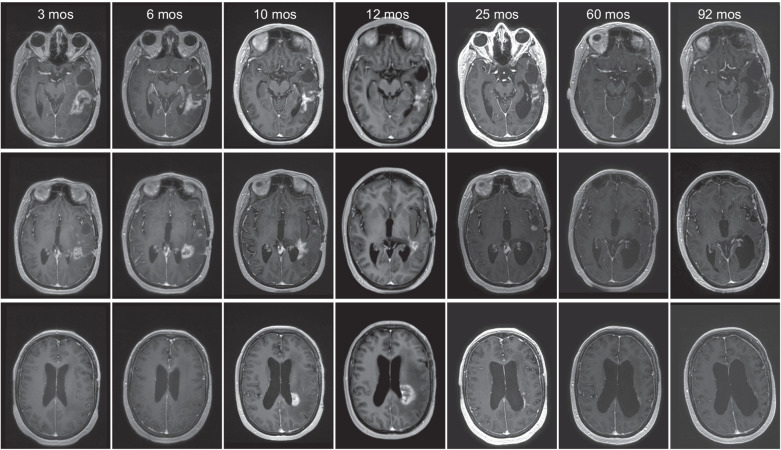
Figure 3.
Illustrative case patient 15, a 40-year-old female who received 3 × 1010 vp for her second GBM recurrence, 13 months after initial diagnosis of an IDH mutant GBM. T1 after gadolinium images posttreatment demonstrated tumor regression of the virus-infused tumor site (top) after initial pseudoprogression, followed by a slow prolonged regression of the distant tumor sites (middle and bottom). One year after virus infusion patient neurologically deteriorated despite high-dose steroid treatment. Ultimately, she was bedridden with palliative care and all medication, including steroids, was withdrawn. After this she made a remarkable recovery, regaining a KPS of 70. Patient is still alive and in stable clinical condition 8 years after Delta24-RGD treatment without having had any other treatments.
Shedding data
In all but 1 patients daily PCR analyses of urine, throat-swab, and anal-swab samples were negative for Delta24-RGD during 3 consecutive days after ending of virus infusion. From patient 19 (dose level 4), one of the in triplicate tested anal-swab samples was positive for Delta24-RGD on the first day after completing virus infusion. Although laboratory contamination could not be ruled out, isolated care was prolonged for an extra day according to protocol after which further PCR tests remained negative (results not shown).
Prolonged presence of viral DNA in CSF
The kinetics of Delta24-RGD replication after CED administration were assessed by PCR analyses of viral DNA on sequential CSF samples in 18 of 19 patients (Fig. 4). At 24 to 48 hours after start of infusion, CSF viral DNA titers were low or undetectable in 15 patients and high in patients 8, 12, and 13. Hereafter, most patients show a substantial increase of CSF viral titers with a peak at 2 weeks post-infusion. In patients 1, 3, 7, and 16, titers remained low or undetectable up to 4 weeks after viral infusion. Interestingly, in three of the six available 12-week CSF samples, viral DNA was still present. No correlation could be found between administered viral dose, the level of or fold increase of virus particles in CSF over time and tumor response. However, in 4 of 7 patients surviving more than 6 months, the amount of viral DNA increased over time up to 4 weeks after treatment (patients 2, 10, 15, and 20), with the best responding patient 15 having a steep increase.
Figure 4.
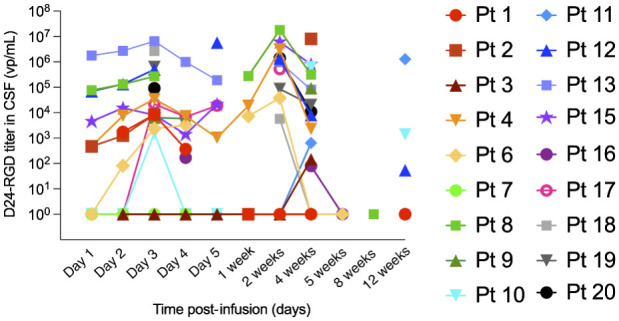
Prolonged presence of Delta24-RGD DNA in cerebrospinal fluid of treated patients. Samples collected at indicated time points from permanent ventricular catheter were analyzed by a Delta24-RGD specific PCR for the presence of viral copy numbers (expressed as viral particles per mL CSF).
Presence of intratumoral adenoviral proteins
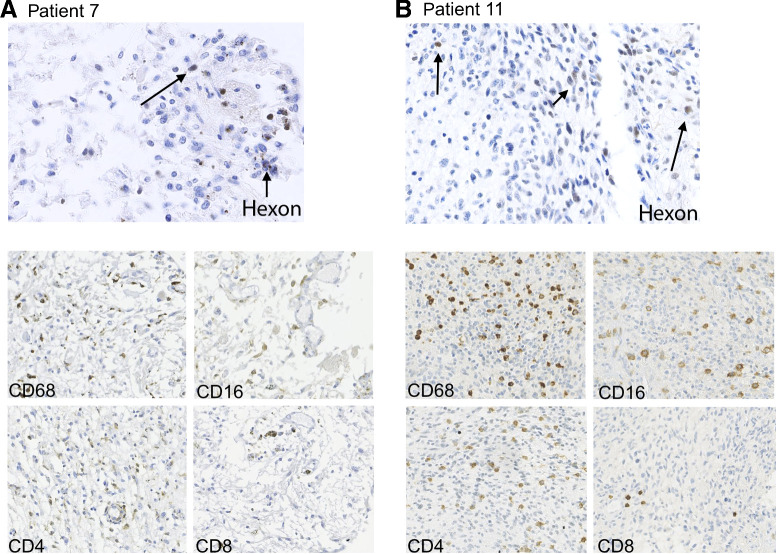
In the post-mortem brain of patient 7, at 3 months and the re-resected tumor of patient 11, at 6 weeks after virus infusion hexon-positive cells were present. In patient 7, some scattered hexon-positive cells were observed (Fig. 5A, top image) whereas in patient 11 abundant hexon-positive cells were present in tumor around the cavity that had emerged after the virus infusion (Fig. 5B, top image), suggestive for viral replication. Furthermore, we routinely establish tumor cell cultures with a success rate of over 75%, however, from this virus-treated tumor specimen of patient 11 we were not able to establish a viable cell culture and clear signs of virus-induced cytopathic effects were observed (results not shown). These observations are suggestive of prolonged intratumoral presence and/or activity of the virus.
Figure 5.
IHC analysis of post-mortem tumor material of patient 7 (A, 3 months after virus administration, left) and re-resection material of patient 11 (B, 4 weeks after virus administration, right) for the adenoviral protein hexon (top images), showing scattered hexon-positive cells and for the presence of immune cells (bottom images); CD68+ cells (macrophage marker), CD16+ (monocyte marker), and CD4+ lymphocytes are quite abundant in both samples. CD8+ lymphocytes are less frequent in both samples.
Intratumoral induction of proinflammatory cytokines and chemokines
In 11 patients, including patient 14 who underwent catheter placement but did not receive virus, BIF samples were acquired by microdialysis during the period of virus infusion. Directly after the surgical procedure in which CED catheters were placed and before viral infusion, levels of acute-phase cytokines, including IL6 and TNFα (Supplementary Fig. S2A) were increased in almost all patients. These increases are probably the result of the surgical procedure itself as most cytokines show a steady decrease during the following 44 hours, with the exception of macrophage inflammatory protein-1β (MIP-1β, Supplementary Fig. S2B). Indeed, patient 14 (black line), who did not receive virus but had follow-up CSF analyses showed similar cytokine kinetics. The cytokines IL2, IL4, IL5, and IL12 (p70) were not detectable in the BIF samples (results not shown).
Elevated levels of cytokines and chemokines in CSF after viral infusion
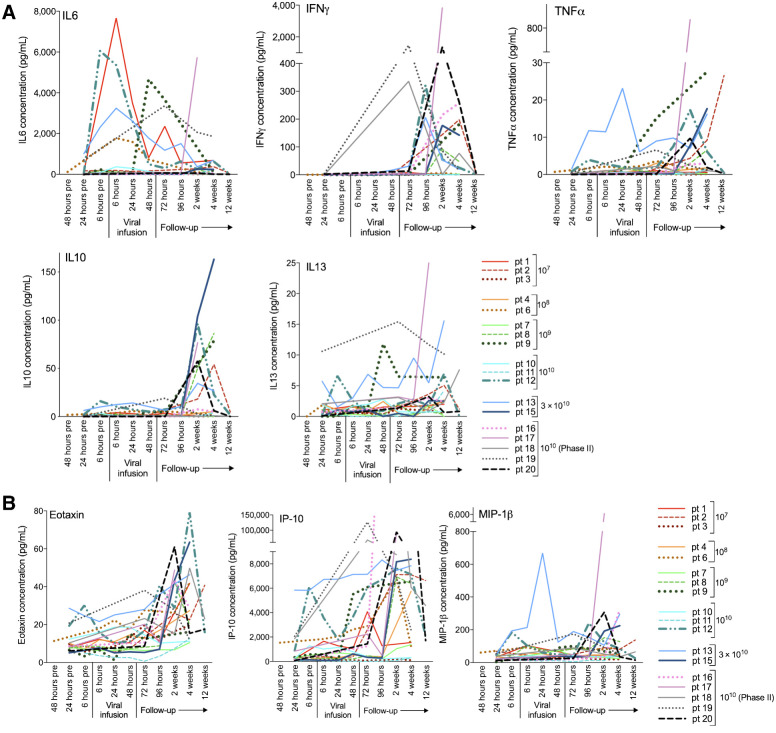
The levels of Th1-associated cytokines IFNγ and TNFα increased in most patients after virus administration, peaking between 2 and 4 weeks. IL6 levels showed large inter-patient variability. Of the Th2-associated cytokines, IL10 and IL13 were detectable in the CSF samples with initial low levels, rising after 96 hours (Fig. 6A). The cytokines IL4, IL5, and IL12 (p70) were not detectable (results not shown).
Figure 6.
CSF levels of the cytokines IL6, IFNγ, TNFα, IL10, and IL13 (A) and the chemokines Eotaxin, IP-10, and MIP-1β (B) at indicated time points prior to, during, and after Delta24-RGD infusion. Concentrations are expressed in pg/mL CSF.
Chemokines were quite abundantly present in the CSF in response to virus. In particular, Eotaxin, IP-10, and MIP-1β (Fig. 6B) showed a steep increase after virus infusion. The remaining chemokines that could be detected, IL8, MCP-1, and MCP4, showed a more variable response pattern (Supplementary Fig. S3).
Together these analyses are indicative of an inflammatory response to the virus infusion in the majority of the patients.
Phenotyping of locoregional inflammatory cells
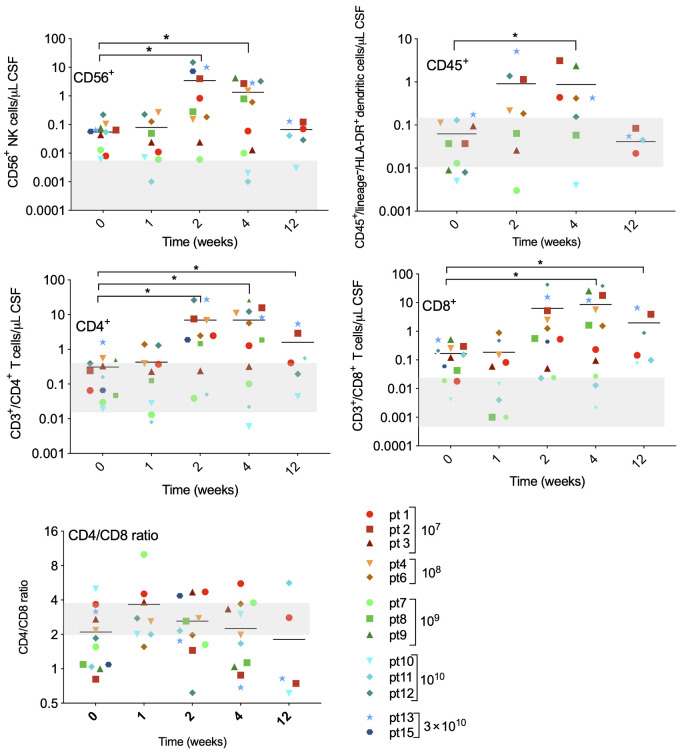
CSF samples of patients 1 to 15 were analyzed for the presence of immune cells. As shown in Fig. 7, marked changes were seen after virus infusion. Significant increases of CD56+ NK cells and DCs (CD45+/lineage−/HLA-DR+) were seen 2 and 4 weeks after treatment. Both CD4+ and CD8+ T-cell subsets rose up to 100-fold compared with pre-infusion values and were well above normal range (gray beam in the graphs). Peak numbers were measured on days 14 and 28 after virus infusion. Interestingly, the ratio of CD4+:CD8+ cells decreased most remarkably in patients with longest OS (for which CSF samples could be obtained at day 85), indicating a relative abundance of CD8+ (cytotoxic) T cells in these patients.
Figure 7.
Immune cells were isolated from cerebrospinal fluid at indicated timepoints after Delta24-RGD infusion and analyzed by flow cytometry with the following markers: CD45+CD3−CD56+ (NK cells), DCs (CD45+/lineage−/HLA-DR+), CD45+CD3+/CD4+ (CD4+ T-lymphocytes), and CD45+CD3+/CD8+ (CD8+ T-lymphocytes). Immune cell subsets are presented as absolute numbers per μL CSF. Gray-shaded areas indicate the normal range for each cell type.
IHC staining revealed the presence of abundant numbers of macrophages (CD68+ and CD16+ cells), CD4+ T cells and to a lesser extent CD8+ cells in tumors that were exposed to virus of patients 11 and 16 (Fig. 5). Flow cytometry of a tumor sample obtained 26 months after virus treatment (patient 16) revealed increased percentages of macrophages, CD4+ and CD8+ lymphocytes within the intratumoral leukocyte population compared with these numbers in four control GBM samples, suggestive for a prolonged local immune activation (Supplementary Fig. S4).
Systemic immune response
No changes in serum levels of cytokines and chemokines, nor in immune cell subsets of 12 patients could be detected (Supplementary Fig. S5). Numbers of immune cell were below the normal range, probably due to prolonged effects of prior chemotherapy as well as the reported systemic immune suppressive effects of the tumor itself (24, 25).
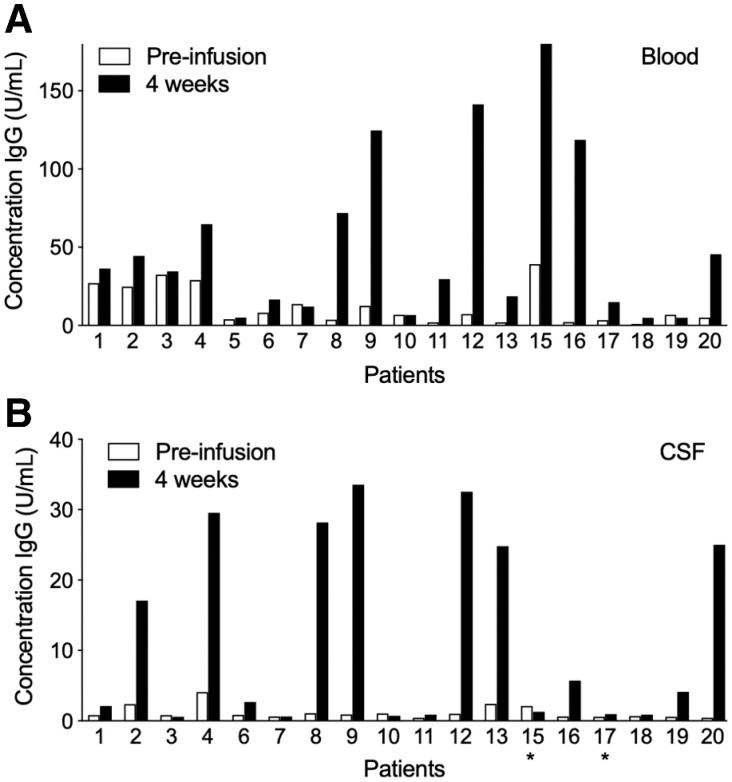
Serum adenovirus-specific IgG and IgM levels prior to and 4 weeks after virus infusion revealed that most patients had low levels of pre-existing antibodies to adenovirus (IgG in Fig. 8A). Interestingly, long-term surviving patient 15 had the highest level of preexisting antibodies. In approximately half of the patients, a considerable rise in antibody titers was observed at 4 weeks after virus infusion, with again patient 15 showing the highest levels followed by patients 12 and long-term surviving patient 16. Similar results were obtained for anti-adenovirus IgM levels (not shown) and for IgG levels in the cerebrospinal fluid of treated patients (Fig. 8B). These results suggest that pre-existing anti-adenovirus antibodies do not preclude a therapeutic response to virus treatment.
Figure 8.
Serum-specific anti-adenovirus IgG antibodies were determined in all patients prior to (white bars) and 4 weeks after (black bars) Delta24-RGD treatment (A). Levels are expressed in units per mL serum. Anti-adenovirus IgG antibodies found in the CSF of patients prior to (white bars) and 4 weeks after (black bars) Delta24-RGD treatment are shown in B. Stars indicate usages of a 2-week sample instead of 4 weeks post-infusion.
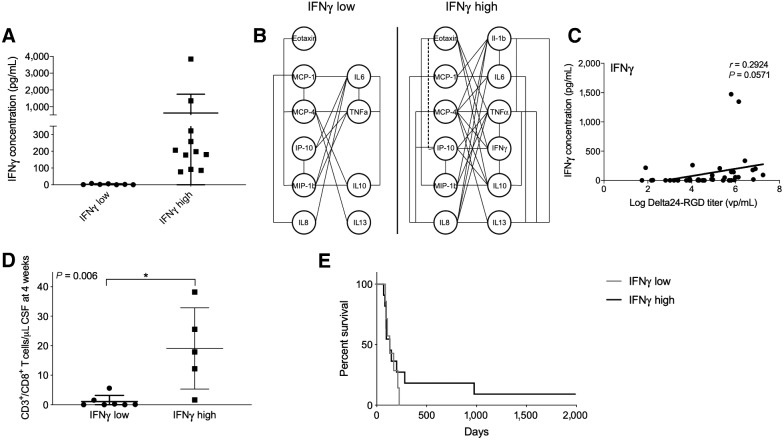
Inflammatory response correlated with increased survival
A subset of patients showed a substantial increase in CSF IFNγ levels 4 weeks after viral infusion, whereas in the other subset no increase was detected. These subgroups were defined as IFNγ-high (>50 pg/mL) and IFNγ-low <10 pg/mL; Fig. 9A). To further investigate this phenomenon, reciprocal correlations between cytokine and chemokine production in these two groups were calculated. In patients with a high IFNγ concentration, significant positive correlations between almost all cytokines and chemokines were detected, whereas in patients with low IFNγ concentration only few correlations were found (Fig. 9B). Although levels of IFNγ were not significantly correlated with the CSF Delta-24RGD levels (P = 0.057), in the 2 patients with the highest viral titers, IFNγ levels were increased compared with the other patients (Fig. 9C). Furthermore, CD8+ T cells were more abundant in the CSF in the IFNγ-high group at 4 weeks after viral infusion (Fig. 9D) and a trend toward a prolonged survival compared with patients with a IFNγ-low profile was observed (Fig. 9E).
Figure 9.
The degree of inflammatory response is correlated with survival. In a subset of patients, termed IFNγ-high, CSF IFNγ levels rose above 50 pg/mL at 2 to 4 weeks after treatment (A). In these patients, almost all measured cytokines and chemokines are significantly correlated and increased. Significant correlated cytokines/chemokines are connected with a straight line (P < 0.05) or with a dotted line (P < 0.001; B). The levels of IFNγ are not significantly correlated with levels of Delta24-RGD (C). The IFNγ-high group also revealed higher CD8+ T cells numbers in CSF compared with IFNγ-low group (D). Kaplan–Meier survival curve of treated patients separated into IFNγ-high and IFNγ-low groups. Both long-term surviving patients (nos. 15 and 16) belong to the IFNγ-high group (E, black line).
Of note, the acute-phase cytokine TNFα significantly correlated with the CSF levels of Delta-24RGD (Supplementary Fig. S6); however only 3 patients had a higher titer of TNFα at 4 weeks after viral infusion. Of these 3 patients, one showed a prolonged survival.
Discussion
In this study we have shown for the first time that prolonged infusion by CED technique of the oncolytic adenovirus Delta24-RGD, in recurrent GBM as well as in the surrounding brain, is safe. Study-related SAEs that occurred caused symptoms mostly related to increased intracranial pressure caused by inflammation-related edema or viral meningitis, however these signs and symptoms were all temporary. Two of 19 treated patients showed long-term survival, of whom one is still alive, free of tumor 8 years without any other treatment. In CSF and tumor of specific patients, including the long-term surviving patient, virus remained present over a long time period, up to 12 weeks, the number of immune cells and levels of modulatory cytokines and chemokines were increased, suggesting that an antitumor immune response can be elicited.
Several trials have shown that local delivery of replicating oncolytic viruses into malignant glioma is feasible and safe (26–30), and recent studies demonstrated impressive durable responses in a significant proportion of patients (21, 31, 32). Lang and colleagues tested Delta24-RGD in 37 patients with recurrent GBM (21). Five of 25 patients (20%) receiving a single intratumoral injection survived more than 3 years. Furthermore, analysis of resected treated tumor revealed presence of intratumoral CD8+ and T-bet+ cells 2 weeks after Delta24-RGD treatment, suggesting an immunogenic antitumor response. Treatment of 61 patients with recurrent GBM with the polio-virus derived PVS-RIPO achieved a similar therapeutic effect of 21% long-term survivors (31). Treatment of 45 patients with the retrovirus derived TOCA-511 virus in a phase II trial led to an OS of 13.6 months (32). Although the phase III study with TOCA-511 was negative (33), we do believe that oncolytic viral therapy holds strong therapeutic potential, based on the relatively large number of responders in early-phase trials.
In the current trial, we observed viral meningitis as treatment related toxicity in 3 patients, which is most likely related to the mode of delivery using CED. At the time of this study, dedicated CED catheters designed for backflow reduction, were not available yet. The use of these suboptimal CED catheters in this trial may have contributed to shedding of virus into the CSF, causing viral meningitis. Although we cannot outweigh the benefits or risks of use of CED for delivering adenovirus to tumor and surrounding brain, the finding that virus remains present in the CSF for a long period of time, is new. This may be caused by continuous production of viral progeny in the infected tumor cells, the viral spill in the CSF due to inadequate CED catheters or a combination.
In this dose-escalating study with six dose levels, the MTD was not reached. A wound healing problem occurred in a patient treated at dose level 5 (3 × 1010 vp), with subsequent CSF leakage, 2 weeks after hospital discharge. Due to the risk of viral shedding as observed by the national environmental safety authority, the DSMB recommended to continue the study at dose level 4. Risk of environmental shedding of the virus through excreta was found to be nearly nil.
Although median OS is within the expected range for patients with recurrent GBM, 2 of 19 treated patients showed long-term survival, with one complete responder being still alive. Although these results may not seem impressive, it must be recognized that in this trial patients were heavily pretreated and in far stage of disease. The majority of patients in this study, 14 of 19, were treated at second or even third recurrence. Furthermore, during the conduct of this trial, it could not be discerned whether symptoms as headache and nausea were caused by viral meningitis, tumor growth, or an inflammatory reaction causing edema. MR and CT imaging were not helpful in discriminating between these different etiologies of increased intracranial pressure. These symptoms were treated with steroids, in retrospect this treatment may have hampered the desired antitumor immune response. A striking observation was that long-term surviving patient 15, whose neurologic deterioration that was accompanied by signs of increased intracranial pressure was initially treated with high-dose dexamethasone, improved dramatically after withdrawal of steroids. These support the notion that steroid use should be avoided in oncolytic viral therapies.
This clinical trial was also the first to assess the local and loco-regional responses upon infusion of an oncolytic virus into the tumor and surrounding brain by sequential sampling of BIF and CSF. PCR analysis in 12 of 17 evaluable CSF samples showed presence of viral DNA at 4 weeks after treatment, in 5 patients these levels were increased compared with measurements at earlier time points. At 12 weeks, 4 patients still had viral DNA present in CSF, of which 2 were increased compared with the 4-week measurement. These findings are in support of the anticipated biological effect of virus replication in tumor cells, which may continue for a longer period of time than previously anticipated. Not only reactive immune cell activation but also preexisting antiviral antibodies may limit the efficacy of virus-based therapies due to rapid elimination of the virus or viral vector (34, 35). However, therapy-enhancing effects of preexisting antibodies have also been reported (36, 37). In the Delta24-RGD-treated patients, the levels of preexisting anti-adenovirus antibodies did not correlate with level and duration of viral DNA in the CSF. Interestingly, the best responding patient had the highest pre- and posttreatment antibody titers.
Also, the Th1-associated cytokines IFNγ and TNFα, rose in CSF of a large subset of patients and peaked between 2 and 4 weeks after treatment. This peak coincided with significant increases in CD4+, CD8+ T-cell subsets and CD56+ NK cells. These findings were in line with the analysis of post-mortem tumor tissue of patient 7 and re-resected tumor of patient 11 showing large numbers of immune cell infiltrates. Re-resected tumor obtained 26 months after treatment from patient 16, revealed the long-term nature of this local inflammatory response to viral treatment, which was also accompanied by a macrophage phenotypic shift from a more tumor-supportive M2 phenotype toward a more tumor detrimental M1 phenotype as we described previously (19). Importantly, presence of high levels of IFNγ in the CSF was associated with high numbers of CD8+ T cells in the CSF. In this small patient series, segregation of the overall survival graph into patients with high versus low CSF IFNγ levels, suggests a survival benefit of high IFNγ levels. CSF IFNγ and TNFα levels may therefore represent a potential biomarker for response in future oncolytic virus trials.
BIF analysis showed high levels of acute-phase cytokines and chemokines already prior to virus infusion, presumably resulting from tissue damage during surgery for biopsy and catheter placement. Such effects have been reported previously and were described to resolve within approximately 6 hours (38). However, a more prolonged increase in cytokines and in particular chemokines was noted in a subset of our patients, often returning to baseline values after 44 hours. This may indicate that these increases measured beyond 6 hours are related to the virus infusion. Because we were unable to sample BIF after the time point of 44 hours prolonged kinetics could not be assessed.
Further research is required to validate whether local cytokine levels represent biomarkers for response and whether such responses can be modelled ex vivo. Such biomarker assays may ultimately aid in the identification of responding patients and improve the response rate to oncolytic viral therapy.
Supplementary Material
Acknowledgments
This project has been supported through the NCI-RAID Program of the Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, NCI, and by grants of the Dutch Cancer Society (KWF); the Netherlands Organization for Health Research and Development (ZonMw) Program Translational Gene Therapy Research; and the Erasmus MC Mrace program. We thank R. Verdijk, M.D., Ph.D., for evaluating selected pathology samples; W. van den Bossche, M.D., for the flow cytometric analysis of the re-resection tumor sample; Lisette Vogelezang and Eftichia Stavrakaki for technical assistance; and the members of the Data Safety Monitoring Board (P. Sillevis Smitt, R. Hoeben, J. Heijmans, C. Bangma, F.F. Lang). We also wish to express our gratitude and respect to the late Charles A. Conrad for his achievements in the field of oncolytic viral therapy and valuable contributions to this study.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
E.H.P. van Putten reports grants from NCI, Dutch Cancer Society (KWF), Dutch Organization for Health Research and Development (ZonMw), and Erasmus MC Mrace during the conduct of the study. A. Kleijn reports grants from NCI-RAID, Dutch Cancer Society, the Netherlands Health Research and Development (ZonMW), and ErasmusMC Mrace Program during the conduct of the study. V.W. van Beusechem reports grants from ZonMw during the conduct of the study; other support from ORCA Therapeutics BV outside the submitted work. J. Fueyo reports other support from DNATrix, Inc. during the conduct of the study; also has a patent licensed to DNAtrix. F.F. Lang reports other support from DNAtrix, Inc. during the conduct of the study; other support from Insightec; personal fees from Theracle, PolyPid, and Nektar Therapeutics outside the submitted work; also has a patent for US 2015/0306160 A1 pending. C.E. Teunissen reports grants from KWF Dutch Cancer Society during the conduct of the study; other support from ADx Neurosciences and Quanterix; grants from Axon NeuroSciences, Biogen, Boehringer, Brainstorm Therapeutics, EIP farma, Esai, Janssen prevention center, Roche, Toyama, and Vivoryon outside the submitted work. W. Gerritsen reports grants from Dutch Goverment (ZONMW) during the conduct of the study. M.L.M. Lamfers reports grants from Dutch government ZonMW and Dutch Cancer Society KWF during the conduct of the study; other support from DNAtrix outside the submitted work. C.M.F. Dirven reports grants from Dutch Government ZONMW and Dutch Cancer Society (KWF) during the conduct of the study; other support from DNAtrix outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
E.H.P. van Putten: Resources, formal analysis, investigation, visualization, writing–original draft, project administration, writing–review and editing. A. Kleijn: Resources, formal analysis, investigation, visualization, writing–original draft, project administration, writing–review and editing. V.W. van Beusechem: Conceptualization, formal analysis, funding acquisition, writing–review and editing. D. Noske: Conceptualization, resources, supervision. C.H.J. Lamers: Resources, data curation, formal analysis. A.L. de Goede: Conceptualization, supervision. S. Idema: Resources, investigation. D. Hoefnagel: Resources, data curation, investigation, project administration. J.J. Kloezeman: Resources, investigation. J. Fueyo: Conceptualization, methodology. F.F. Lang: Conceptualization, methodology. C.E. Teunissen: Conceptualization, resources, formal analysis, methodology. R.M. Vernhout: Resources, data curation, software, formal analysis, project administration. C. Bakker: Data curation, software, supervision, methodology, project administration. W. Gerritsen: Conceptualization, supervision, methodology. D.T. Curiel: Conceptualization, funding acquisition, methodology. A. Vulto: Conceptualization, supervision, funding acquisition, methodology. M.L.M. Lamfers: Conceptualization, resources, formal analysis, supervision, funding acquisition, visualization, methodology, writing–original draft, project administration, writing–review and editing. C.M.F. Dirven: Conceptualization, resources, formal analysis, supervision, funding acquisition, visualization, methodology, writing–original draft, project administration, writing–review and editing.
References
- 1. Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncology 2009;10:459–66. [DOI] [PubMed] [Google Scholar]
- 2. Stavrakaki E, Dirven CMF, Lamfers MLM. Personalizing oncolytic virotherapy for glioblastoma: in search of biomarkers for response. Cancers 2021;13:614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene 2000;19:2–12. [DOI] [PubMed] [Google Scholar]
- 4. Jiang H, Gomez-Manzano C, Lang F, Alemany R, Fueyo J. Oncolytic adenovirus: preclinical and clinical studies in patients with human malignant gliomas. Curr Gene Ther 2009;9:422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Henson JW, Schnitker BL, Correa KM, von Deimling A, Fassbender F, Xu H-J, et al. The retinoblastoma gene is involved in malignant progression of astrocytomas. Ann Neurol 1994;36:714–21. [DOI] [PubMed] [Google Scholar]
- 6. Jen J, Harper JW, Bigner SH, Bigner DD, Papadopoulos N, Markowitz S, et al. Deletion of p16 and p15 genes in brain tumors. Cancer Res 1994;54:6353–8. [PubMed] [Google Scholar]
- 7. Costello JF, Berger MS, Huang HS, Cavenee WK. Silencing of p16/CDKN2 expression in human gliomas by methylation and chromatin condensation. Cancer Res 1996;56:2405–10. [PubMed] [Google Scholar]
- 8. Kyritsis AP, Zhang B, Zhang W, Xiao M, Takeshima H, Bondy ML, et al. Mutations of the p16 gene in gliomas. Oncogene 1996;12:63–67. [PubMed] [Google Scholar]
- 9. Schmidt EE, Ichimura K, Reifenberger G, Collins VP. CDKN2 (p16/MTS1) gene deletion or CDK4 amplification occurs in the majority of glioblastomas. Cancer Res 1994;54:6321–4. [PubMed] [Google Scholar]
- 10. Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol 1998;72:9706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamfers MLM, Grill J, Dirven CM, Van Beusechem VW, Geoerger B, Van Den Berg J, et al. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res 2002;62:5736–42. [PubMed] [Google Scholar]
- 12. Alonso MM, Gomez-Manzano C, Bekele BN, Yung WKA, Fueyo J. Adenovirus-based strategies overcome temozolomide resistance by silencing the O6-methylguanine-DNA methyltransferase promoter. Cancer Res 2007;67:11499–504. [DOI] [PubMed] [Google Scholar]
- 13. Alonso MM, Jiang H, Yokoyama T, Xu J, Bekele NB, Lang FF, et al. Delta-24-RGD in combination with RAD001 induces enhanced anti-glioma effect via autophagic cell death. Mol Ther 2008;16:487–93. [DOI] [PubMed] [Google Scholar]
- 14. Lamfers MLM, Idema S, Bosscher L, Heukelom S, Moeniralm S, van der Meulen-Muileman IH, et al. Differential effects of combined Ad5- delta 24RGD and radiation therapy in in vitro versus in vivo models of malignant glioma. Clin Cancer Res 2007;13:7451–8. [DOI] [PubMed] [Google Scholar]
- 15. Suzuki K, Fueyo J, Krasnykh V, Reynolds PN, Curiel DT, Alemany R. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin Cancer Res 2001;7:120–6. [PubMed] [Google Scholar]
- 16. Kleijn A, Kloezeman J, Treffers-Westerlaken E, Fulci G, Leenstra S, Dirven C, et al. The in vivo therapeutic efficacy of the oncolytic adenovirus Delta24-RGD is mediated by tumor-specific immunity. PLoS ONE 2014;9:e97495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang H, Clise-Dwyer K, Ruisaard KE, Fan X, Tian W, Gumin J, et al. Delta-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. PLoS ONE 2014;9:e97407–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kleijn A, van den Bossche W, Haefner ES, Belcaid Z, Burghoorn-Maas C, Kloezeman JJ, et al. The sequence of Delta24-RGD and TMZ administration in malignant glioma affects the role of CD8+T cell anti-tumor activity. Mol Ther Oncolytics 2017;5:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van den Bossche WBL, Kleijn A, Teunissen CE, Voerman JSA, Teodosio C, Noske DP, et al. Oncolytic virotherapy in glioblastoma patients induces a tumor macrophage phenotypic shift leading to an altered glioblastoma microenvironment. Neuro-Oncology 2018;20:1494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Belcaid Z, Berrevoets C, Choi J, van Beelen E, Stavrakaki E, Pierson T, et al. Low-dose oncolytic adenovirus therapy overcomes tumor-induced immune suppression and sensitizes intracranial gliomas to anti-PD-1 therapy. Neuro-Oncology Advances 2020;2:vdaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lang FF, Conrad C, Gomez-Manzano C, Yung WKA, Sawaya R, Weinberg JS, et al. Phase I study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. Journal of Clinical Oncology 2018;36:1419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA 1994;91:2076–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Graaf MT, Smitt PAES, Luitwieler RL, van Velzen C, van den Broek PDM, Kraan J, et al. Central memory CD4+ T cells dominate the normal cerebrospinal fluid. Cytometry B Clin Cytom 2011;80:43–50. [DOI] [PubMed] [Google Scholar]
- 24. Jackson C, Ruzevick J, Phallen J, Belcaid Z, Lim M. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Dev Immunol 2011;2011:732413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gustafson MP, Lin Y, New KC, Bulur PA, O'Neill BP, Gastineau DA, et al. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro-Oncology 2010;12:631–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, Hunter WD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther 2000;7:867–74. [DOI] [PubMed] [Google Scholar]
- 27. Forsyth P, Roldán G, George D, Wallace C, Palmer CA, Morris D, et al. A phase I trial of intratumoral administration of reovirus in patients with histologically confirmed recurrent malignant gliomas. Mol Ther 2008;16:627–32. [DOI] [PubMed] [Google Scholar]
- 28. Kicielinski KP, Chiocca EA, Yu JS, Gill GM, Coffey M, Markert JM. Phase 1 clinical trial of intratumoral reovirus infusion for the treatment of recurrent malignant gliomas in adults. Mol Ther 2014;22:1056–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther 2004;10:958–66. [DOI] [PubMed] [Google Scholar]
- 30. Geletneky K, Hajda J, Angelova AL, Leuchs B, Capper D, Bartsch AJ, et al. Oncolytic H-1 parvovirus shows safety and signs of immunogenic activity in a first phase I/IIa glioblastoma trial. Mol Ther 2017;25:2620–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Desjardins A, Gromeier M, Herndon JE, Beaubier N, Bolognesi DP, Friedman AH, et al. Recurrent glioblastoma treated with recombinant poliovirus. N Engl J Med 2018;379:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cloughesy TF, Landolfi J, Vogelbaum MA, Ostertag D, Elder JB, Bloomfield S, et al. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro-Oncology 2018;20:1383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cloughesy TF, Petrecca K, Walbert T, Butowski N, Salacz M, Perry J, et al. Effect of vocimagene amiretrorepvec in combination with flucytosine vs standard of care on survival following tumor resection in patients with recurrent high-grade glioma: a randomized clinical trial. JAMA Oncol 2020;6:1939–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsai V. Impact of human neutralizing antibodies on antitumor efficacy of an oncolytic adenovirus in a murine model. Clin Cancer Res 2004;10:7199–206. [DOI] [PubMed] [Google Scholar]
- 35. Chen Y, Yu D-C, Charlton D, Henderson DR. Pre-existent adenovirus antibody inhibits systemic toxicity and antitumor activity of CN706 in the nude mouse LNCaP xenograft model: Implications and proposals for human therapy. Hum Gene Ther 2000;11:1553–67. [DOI] [PubMed] [Google Scholar]
- 36. Ricca JM, Oseledchyk A, Walther T, Liu C, Mangarin L, Merghoub T, et al. Pre-existing immunity to oncolytic virus potentiates its immunotherapeutic efficacy. Mol Ther 2018;26:1008–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Berkeley RA, Steele LP, Mulder AA, van den Wollenberg DJM, Kottke TJ, Thompson J, et al. Antibody-neutralized reovirus is effective in oncolytic virotherapy. Cancer Immunol Res 2018;6:1161–73. [DOI] [PubMed] [Google Scholar]
- 38. Marcus HJ, Carpenter KLH, Price SJ, Hutchinson PJ. In vivo assessment of high-grade glioma biochemistry using microdialysis: a study of energy-related molecules, growth factors and cytokines. J Neurooncol 2010;97:11–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



![Figure 2. Illustrative case patient 11, a 48-year-old female, who was treated 21 months after GBM diagnosis for a second recurrence at dose level 4 with 1 × 1010 vp. After 2 weeks, MRI showed a large area with cavity formation suggestive of tumor lysis in the target area of one of the intratumoral catheters used for virus administration [A, T1 after gadolinium prior to treatment and 2 weeks after treatment; B, postoperative neuronavigation screenshot demonstrating the relation of the CED catheter (trajectory in blue) and the necrotic area]. Six weeks after virus infusion her preexisting paresis of the left leg deteriorated due to increasing mass effect of the treated tumor for which she underwent subtotal re-resection. After initial improvement, she deteriorated again 6 weeks later, at this point she declined further diagnostic tests and treatment. She died 106 days after virus infusion. PCR analysis of CSF samples demonstrated increasing virus titers 3 months post-infusion (Fig. 5). The resected tumor material contained besides tumor cells also viral proteins and immune cell infiltrates (Fig. 4B). Our routine tumor cell culture protocol of this tumor failed due to virus-induced lysis of tumor cells. Unfortunately, we were unable to discriminate between true tumorprogression or an inflammatory reaction known as pseudoprogression.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/29be/9365362/fb33bb0120fa/1572fig2.jpg)