Abstract
Purpose:
Entrectinib potently inhibits tropomyosin receptor kinases (TRKAs)/B/C and ROS1, and previously induced deep [objective response rate (ORR) 57.4%] and durable [median duration of response (DoR) 10.4 months] responses in adults with NTRK fusion-positive solid tumors from three phase I/II trials. This article expands prior reports with additional patients and longer follow-up.
Patients and Methods:
Patients with locally advanced/metastatic NTRK fusion-positive solid tumors and ≥12 months' follow-up were included. Primary endpoints were ORR and DoR by blinded independent central review (BICR); secondary endpoints included progression-free survival (PFS), intracranial efficacy, and safety. The safety-evaluable populations included all patients who had received ≥1 entrectinib dose.
Results:
At clinical cut-off (August 31, 2020), the efficacy-evaluable population comprised 121 adults with 14 tumor types and ≥30 histologies. Median follow-up was 25.8 months; 61.2% of patients had a complete (n = 19) or partial response (n = 55). Median DoR was 20.0 months [95% confidence interval (CI), 13.0–38.2]; median PFS was 13.8 months (95% CI, 10.1–19.9). In 11 patients with BICR-assessed measurable central nervous system (CNS) disease, intracranial ORR was 63.6% (95% CI, 30.8–89.1) and median intracranial DoR was 22.1 (95% CI, 7.4–not estimable) months. The safety profile of entrectinib in adults and pediatric patients was aligned with previous reports. Most treatment-related adverse events (TRAEs) were grade 1/2 and manageable/reversible with dose modifications. TRAE-related discontinuations occurred in 8.3% of patients.
Conclusions:
With additional clinical experience, entrectinib continues to demonstrate durable systemic and intracranial responses and can address the unmet need of a CNS-active treatment in patients with NTRK fusion-positive solid tumors.
Translational Relevance.
In previous reports of an integrated analysis of three phase I/II trials, entrectinib yielded deep and durable overall and intracranial responses in 54 patients with NTRK fusion-positive solid tumors, including rare tumor types. We report updated results from this analysis with a longer follow-up and a larger patient cohort (n = 121). At clinical cut-off (August 31, 2020), the overall and intracranial efficacy of entrectinib were confirmed with response rate 61.2%, median duration of response 20.0 months, median progression-free survival 13.8 months, and intracranial response rate 63.6% (in 11 patients with measurable central nervous system disease by blinded independent review). In line with previous reports, entrectinib had a manageable safety profile, with most treatment-related adverse events grade 1/2 and reversible. These results confirm data that supported the United States (2019) and European (2020) approvals and inform physicians of the latest advances in the field of tumor-agnostic therapies, improving patient access to treatments tailored for their condition.
Introduction
Gene fusions of the neurotrophic tyrosine receptor kinase gene [NTRK1/2/3; coding for tropomyosin receptor kinases (TRK)A/B/C] lead to the expression of constitutively active chimeric TRK proteins that are potential oncogenic drivers across a large range of tumor types (1, 2). NTRK gene fusions occur at a low frequency (<1%) in common solid tumors but can be found in more than 90% of secretory breast carcinoma, mammary analog secretory carcinoma (MASC), and rare pediatric tumors (3, 4).
NTRK fusion-positive solid tumors can be treated using targeted therapies, such as larotrectinib and entrectinib, the first two TRK inhibitors approved in the United States (5). Entrectinib is a potent inhibitor of TRK, ROS1, and ALK and was specifically designed to penetrate and remain in the central nervous system (CNS; refs. 6, 7). In 2019 and 2020, entrectinib received United States and European Union approval/marketing authorizations for the treatment of patients ≥12 years old with NTRK fusion-positive solid tumors and adults with ROS1 fusion-positive non–small cell lung cancer (NSCLC). We have previously reported that entrectinib induced durable and clinically meaningful responses in 54 adults with advanced/metastatic NTRK fusion-positive solid tumors enrolled in three phase I/II clinical trials (ALKA-372-001; STARTRK-1; STARTRK-2; ref. 8). In that integrated analysis (data cut-off May 31, 2018), entrectinib yielded a 57.4% objective response rate (ORR), a 10.4-month median duration of response (DoR), and an 11.2-month median progression-free survival (PFS). Importantly, intracranial responses were also shown in six of 11 patients with baseline CNS metastases, per blinded independent central review (BICR). These preliminary results demonstrated the systemic and CNS activity of entrectinib across multiple tumor types. Entrectinib was also well tolerated, with a manageable safety profile.
We present updated efficacy and safety data of this integrated analysis with a larger number of patients and longer follow-up.
Patients and Methods
Study design and patients
This analysis included patients aged ≥18 years in one of two phase I studies (ALKA-372-001 or STARTRK-1) or a phase II global basket study (STARTRK-2), across more than 150 sites in 16 countries. Patients enrolled before July 31, 2019, were included in the efficacy analysis to ensure they had ≥12 months' follow-up from their first on-study scan (≥13 months after enrollment) at the time of the clinical cut-off date (August 31, 2020). Study designs of the three ongoing trials included in this integrated analysis have been described previously (8, 9).
Briefly, all included patients had a solid tumor that harbored a fusion in NTRK1, NTRK2, or NTRK3 confirmed by molecular profiling in tissue samples (e.g., next-generation sequencing or PCR; Appendix), measurable disease at baseline as assessed by the investigator (RECIST version 1.1), Eastern Cooperative Oncology Group performance status ≤2, and no prior treatment with a TRK inhibitor.
All studies were conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines; all patients provided written informed consent. Protocols were approved by the relevant institutional review boards and/or ethics committees.
Treatments and assessments
Patients received entrectinib orally until documented radiographic progression, unacceptable toxicity, or withdrawal of consent (treatment postprogression was allowed at the investigator's discretion if the patient derived clinical benefit). The intended entrectinib dose for all patients was 600 mg/day; three out of the 121 efficacy-evaluable patients received doses more than 600 mg daily within the phase I dose-escalation studies.
Tumor screenings (including brain scans) were performed at baseline ≤30 days before the first administration of entrectinib. Subsequent tumor assessments were scheduled at the end of cycle 1 (4 weeks), every 8 weeks thereafter, and at the end of treatment if not done in the previous 4 weeks or whenever progression was suspected. Patients with baseline CNS metastases per investigator assessment (RECIST v1.1) had brain scans performed at every tumor assessment. All imaging scans were submitted for BICR. CNS follow-up of patients without baseline CNS metastases was performed as clinically indicated based on symptomatic progression or routine CNS scans where customary. Objective tumor response was confirmed radiographically ≥4 weeks after the first evidence of complete response (CR) or partial response (PR).
Safety assessments were performed through clinical laboratory tests, physical examinations, and monitoring of adverse events (AEs) at each patient visit. AEs were coded using the Medical Dictionary for Regulatory Activities (version 21.0 or higher) and graded using the NCI Common Terminology Criteria for Adverse Events (version 4.03).
Outcomes
Primary endpoints were ORR, defined as the proportion of patients with confirmed CR or PR as best overall response, and DoR (measured from the date of first objective response (OR) to first documentation of radiographic disease progression or death due to any cause, whichever occurred first), per BICR. For patients without disease progression or death, DoR was censored at the last tumor assessment.
Secondary endpoints included PFS by BICR (defined as the time from the first dose of entrectinib to first documentation of radiographic disease progression or death due to any cause at data cutoff time); overall survival (OS; time from the first dose of entrectinib to the date of death due to any cause); safety and tolerability. For patients with CNS metastases at baseline, further secondary endpoints evaluated entrectinib efficacy specifically in the brain and included BICR-assessed intracranial ORR, PFS, and DoR. Radiographic CNS metastases progression was defined as an occurrence of a new CNS lesion or progression in pre-existing CNS lesions per RECIST v1.1. Per RECIST v1.1, nonmeasurable CNS disease could only be categorized as CR, non-CR/nonprogressive disease (PD), or PD.
Statistical analyses
The efficacy-evaluable population comprised TRK inhibitor-naïve patients with extracranial NTRK fusion-positive solid tumors who received ≥1 dose of entrectinib and had measurable disease at baseline. The overall safety-evaluable population included all patients who had received ≥1 dose of entrectinib while enrolled in ALKA-372-001, STARTRK-1, STARTRK-2, or STARTRK-NG. STARTRK-NG (NCT02650401) is an ongoing pediatric phase I/II study of entrectinib in patients aged ≤22 years (10, 11). The NTRK fusion-positive safety subpopulation comprised all safety-evaluable adult patients with NTRK fusion-positive solid tumors.
Patient demographic and safety data were summarized descriptively. For BICR-assessed ORs, the number, proportion, and corresponding two-sided Clopper–Pearson exact 95% confidence intervals (CIs) were summarized. All medians and event-free probabilities for time-to-event endpoints (DoR, PFS, and OS) were estimated via the Kaplan–Meier method. SAS (version 9.3 or higher) was used for all statistical analyses.
Data availability statement
The data were generated and analyzed under the auspices of Roche, which is a member of the Vivli Center for global clinical research data (https://vivli.org/ourmember/roche/). Roche will share/allow access to individual patient-level data from the clinical trials via Vivli, providing certain criteria are met. Please see the criteria and exceptions on the Roche member section of the Vivli homepage at https://vivli.org/ourmember/roche/. Please see also the Roche Global Data Sharing Policy (https://www.roche.com/dam/jcr:1c46aa73-cea0-4b9b-8eaa-e9a788ed021b/roche_global_policy_on_sharing_of_clinical_study_informationV2.1%20April2020%20(1).pdf) for more details. To request access to individual patient-level data from the clinical trials, first locate the clinical trial in Vivli (https://search.vivli.org/ requires sign up and log in) using the trial registration number (given above), then click the “Request Study” button and follow the instructions. In the event that you cannot see a specific study in the Roche list, an Enquiry Form can be submitted to confirm the availability of the specific study.
To request access to related clinical study documents (eg: protocols, clinical study reports, safety reports), please use Roche's Clinical study documents request form: https://www.roche.com/research_and_development/who_we_are_how_we_work/research_and_clinical_trials/our_commitment_to_data_sharing/clinical_study_documents_request_form.htm.
Results
Patients
At the clinical cut-off date, the NTRK efficacy-evaluable population comprised 121 patients with advanced or metastatic solid tumors who had received ≥1 dose of entrectinib with ≥12 months of follow-up from first planned tumor assessment (ALKA-372-001: n = 1; STARTRK-1: n = 2; STARTRK-2: n = 118; Appendix Fig. A1).
Patient demographics and baseline characteristics of the efficacy-evaluable population are presented in Table 1. Twenty-six (21.5%) patients had baseline CNS metastases by investigator assessment; this was confirmed by BICR for 19 patients (15.7%). Patients presented with 14 different tumor types with ≥30 distinct histologies (Appendix Table A1). The most common tumor types were sarcoma [26 patients (21.5%)], MASC [24 patients (19.8%)], and NSCLC [22 patients (18.2%)]. Forty-nine patients (40.5%) had received ≥2 prior lines of therapy for metastatic disease; 37 patients (30.6%) had not received any prior therapy for metastatic disease.
Table 1.
Patient demographics and baseline characteristics in patients with NTRK fusion-positive solid tumors.
| Characteristic | NTRK efficacy-evaluable population (n = 121) | |
|---|---|---|
| Age, y | Median (range) | 57.0 (21–88) |
| Sex, n (%) | Female/male | 62 (51.2)/59 (48.8) |
| Race, n (%) | White/Asian/Black or African American/other or not reported | 73 (60.3)/29 (24.0)/3 (2.5)/16 (13.2) |
| History of smoking (n = 118), n (%) | No/yes | 72 (61.0)/46 (39.0) |
| ECOG PS, n (%) | 0/1/2 | 53 (43.8)/57 (47.1)/11 (9.1) |
| Prior lines of systemic therapya, n (%) | 0/1/2/3/≥4 | 37 (30.6)/35 (28.9)/26 (21.5)/12 (9.9)/11 (9.1) |
| Any previous therapyb, n (%) | Chemotherapy/targeted therapy/hormonal therapy/immunotherapy | 88 (72.7)/24 (19.8)/10 (8.3)/13 (10.7) |
| CNS metastases at baselinec, n (%) | Present/measurable/absent | 20 (16.5)/6 (5.0)/95 (78.5) |
| Prior radiotherapy of the braind (n = 26), n (%) | Yes/no | 17 (65.4)/9 (34.6) |
| Time from end of prior radiotherapy of the brain to first dosee, n (%) | <2 mo/2 to <6 mo/≥6 mo | 7 (41.2)/5 (29.4)/5 (29.4) |
| NTRK fusion, n (%) | NTRK1/NTRK2/NTRK3 | 48 (39.7)/6 (5.0)/67 (55.4) |
| Tumor categoryf, n (%) | Sarcoma | 26 (21.5) |
| Salivary (MASC) | 24 (19.8) | |
| NSCLC | 22 (18.2) | |
| Thyroid | 13 (10.7) | |
| Colorectal | 10 (8.3) | |
| Breast | 7 (5.8) | |
| Neuroendocrine | 5 (4.1) | |
| Pancreatic | 4 (3.3) | |
| Cancer of unknown primary | 3 (2.5) | |
| Gynecologic | 2 (1.7) | |
| Head and neck (other) | 2 (1.7) | |
| Cholangiocarcinoma | 1 (0.8) | |
| Adenocarcinoma of upper GI tract | 1 (0.8) | |
| Neuroblastoma | 1 (0.8) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; GI, gastrointestinal; MASC, mammary analog secretory carcinoma.
aLines of therapy determined from the time of metastatic disease diagnosis.
bPrevious therapy in any setting.
cCNS metastases status as per investigator assessment.
dAmong patients with baseline CNS metastases per investigator assessment.
eAmong patients with baseline CNS metastases and prior radiotherapy of the brain.
fPatients may have had multiple sites of metastases at baseline.
Efficacy
After a median survival follow-up of 25.8 months (range, 0.0–48.8), the median duration of treatment was 11.0 months [interquartile range (IQR), 4.6–23.0]. In the efficacy-evaluable population, 74 of 121 patients (61.2%) had an OR: 19 CRs (15.7%), and 55 PRs (45.5%; Table 2). Responses were seen in all tumor types except neuroblastoma (n = 1; Table 3; Fig. 1A). Entrectinib led to a higher response rate in patients who had not received any prior systemic therapy for metastatic disease (n = 30/37; ORR 81.1%) versus those who had received ≥1 line of prior systemic therapy (n = 44/84; ORR 52.4%).
Table 2.
Overall efficacy (BICR assessed) of entrectinib in patients with NTRK fusion-positive solid tumors, by baseline investigator-assessed CNS metastases status.
| Efficacy-evaluable population | Baseline CNS metastasesa | No baseline CNS metastasesa | |
|---|---|---|---|
| Efficacy parameter | (n = 121) | (n = 26) | (n = 95) |
| ORR, n (%) | 74 (61.2) | 15 (57.7) | 59 (62.1) |
| (95% CI) | (51.9–69.9) | (36.9–76.7) | (51.6–71.9) |
| CR | 19 (15.7) | 2 (7.7) | 17 (17.9) |
| PR | 55 (45.5) | 13 (50.0) | 42 (44.2) |
| Stable disease | 13 (10.7) | 4 (15.4) | 9 (9.5) |
| Progressive disease | 13 (10.7) | 2 (7.7) | 11 (11.6) |
| Non-CR/non-PDb | 6 (5.0) | 0 | 6 (6.3) |
| Missing or unevaluablec | 15 (12.4) | 5 (19.2) | 10 (10.5) |
| DoR | n = 74 | n = 15 | n = 59 |
| Median, mo (95% CI) | 20.0 (13.0–38.2) | 17.2 (6.0–29.4) | 29.0 (12.9–NE) |
| PFS | |||
| Median, mo (95% CI) | 13.8 (10.1–19.9) | 11.7 (4.7–30.2) | 13.8 (10.2–20.8) |
| OS | |||
| Median, mo (95% CI) | 33.8 (23.4–46.4) | 19.9 (7.9–NE) | 37.1 (23.9–NE) |
Note: Data cut-off August 31, 2020.
aCNS metastases status determined by investigator.
bPatients with nonmeasurable lesions.
cMissing or unevaluable included patients with unevaluable on-study scans or who discontinued prior to obtaining adequate scans to evaluate or confirm response.
Table 3.
Overall efficacy (BICR assessed) of entrectinib in patients with NTRK fusion-positive solid tumors, by tumor type.
| Baseline CNS metastasesa | ORR, n (%) | Median DoR, mo | Median PFS, mo | Median OS, mo | ||
|---|---|---|---|---|---|---|
| Tumor category | n | n (%) | (95% CI) | (95% CI) | (95% CI) | (95% CI) |
| Sarcoma | 26 | 2 (7.7) | 15 (57.7) | 15.0 | 10.1 | 18.7 |
| (36.9–76.7) | (4.6–NE) | (6.3–13.7) | (14.5–NE) | |||
| Salivary (MASC) | 24 | 1 (4.2) | 20 (83.3) | NE | NE | NE |
| (62.6–95.3) | (NE–NE) | (13.8–NE) | (NE–NE) | |||
| NSCLC | 22 | 13 (59.1) | 14 (63.6) | 19.9 | 14.9 | NE |
| (40.7–82.8) | (10.4–29.4) | (6.5–30.4) | (20.8–NE) | |||
| Thyroid cancer | 13 | 7 (53.8) | 7 (53.8) | 13.2 | 19.9 | 19.9 |
| (25.1–80.8) | (7.9–NE) | (6.5–33.8) | (14.5–NE) | |||
| Colorectal carcinoma | 10 | 0 (0) | 2 (20.0) | 17.6 | 2.8 | 16.0 |
| (2.5–55.6) | (15.1–20.0) | (1.9–16.0) | (10.8–37.1) | |||
| Breast cancer | 7 | 2 (28.6) | 5 (71.4) | 12.9 | 10.1 | 19.2 |
| (29.0–96.3) | (4.2–NE) | (5.1–NE) | (5.1–NE) | |||
| Neuroendocrine tumors | 5 | 0 (0) | 2 (40.0) | NE | 15.6 | 40.5 |
| (5.3–85.3) | (11.1–NE) | (0.9–NE) | (28.6–40.5) | |||
| Pancreatic cancer | 4 | 0 (0) | 3 (75.0) | 12.9 | 12.8 | 22.0 |
| (19.4–99.4) | (7.1–12.9) | (6.2–17.5) | (11.2–30.7) | |||
| Cancer of unknown primary | 3 | 0 (0) | 1 (33.3) | 9.1 | 7.2 | 14.3 |
| (0.8–90.6) | (NE–NE) | (4.4–10.0) | (NE–NE) | |||
| Gynecologic | 2 | 0 (0) | 1 (50.0) | 38.2 | 27.4 | 39.3 |
| (1.3–98.7) | (NE–NE) | (13.7–41.2) | (32.1–46.4) | |||
| Head and neck | 2 | 0 (0) | 2 (100.0) | NE | NE | NE |
| (15.8–100.0) | (16.9–NE) | (17.6–NE) | (NE–NE) | |||
| Cholangiocarcinoma | 1 | 0 (0) | 1 (100.0) | 9.3 | 12.0 | 23.4 |
| (2.5–100.0) | (NE–NE) | (NE–NE) | (NE–NE) | |||
| Adenocarcinoma of upper GI tract | 1 | 0 (0) | 1 (100.0) | 29.0 | 30.0 | NE |
| (2.5–100.0) | (NE–NE) | (NE–NE) | (NE–NE) | |||
| Neuroblastoma | 1 | 1 (100.0) | 0 (0) | — | 0.1 | 0.1 |
| (NA) | (NE–NE) | (NE–NE) |
Note: Data cut-off August 31, 2020.
Abbreviations: GI, gastrointestinal; MASC, mammary analog secretory carcinoma; NA, not applicable; NSCLC, non–small cell lung cancer.
aCNS metastases status determined by investigator.
Figure 1.
Responses and time on entrectinib treatment in patients with NTRK fusion-positive solid tumors, by tumor type (BICR assessed). A, Best individual patient responses [n = 103; 18 patients with missing sum of the longest diameter (SLD) change were excluded from the plot]. B, Time on entrectinib treatment. Data cut-off August 31, 2020. The minimum shrinkage in the SLD of target lesions that defined an OR was 30%. Gastrointestinal (GI)-other, adenocarcinoma of upper GI tract; CRC, colorectal carcinoma; CUP, cancer of unknown primary; Inv, investigator; ND, not determined; SD, stable disease.
Entrectinib yielded similar response rates in patients with NTRK1 (26/48; 54.2%) or NTRK3 (47/67; 70.1%) gene fusions. Tumor reduction was observed in one of six (16.7%) patients with an NTRK2 gene fusion (Appendix Table A2 and Fig. A2). Overall, there was no observed relationship between response to entrectinib and fusion partner (Appendix Fig. A3).
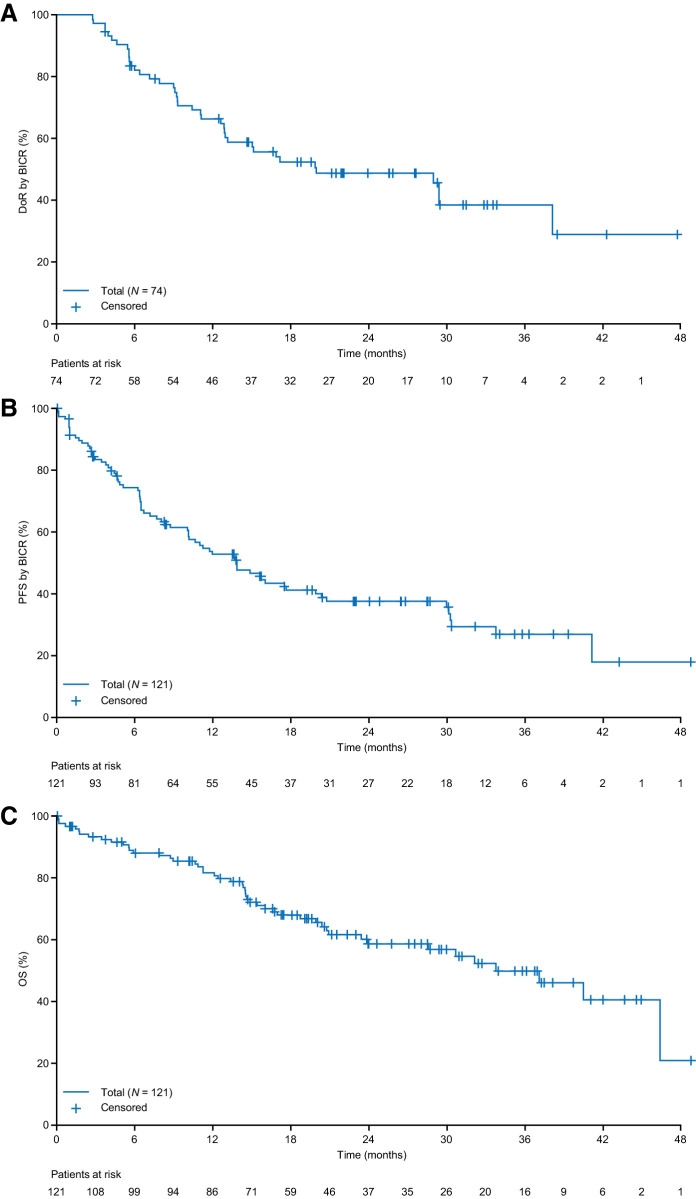
Responses to entrectinib usually occurred early (median time to first response, 0.95 months), with most responding patients achieving their first response by the end of the first treatment cycle, week 4, when the first postbaseline tumor assessment was performed (Fig. 1B). Median DoR per BICR in the 74 patients with an OR was 20.0 months (95% CI, 13.0–38.2) (Table 2; Fig. 2A). Five nonresponders with SD also remained on therapy for ≥8 months (Fig. 1B). At data cut-off, 72 of 121 (59.5%) efficacy-evaluable patients had experienced disease progression or died; median PFS was 13.8 months (95% CI, 10.1–19.9) and median OS was 33.8 months (95% CI, 23.4–46.4; Table 2; Fig. 2B and C). The event-free probability of PFS was 0.74 (95% CI, 0.66–0.82) at 6 months, 0.53 (95% CI, 0.43–0.62) at 12 months, and 0.41 (95% CI, 0.32–0.51) at 18 months.
Figure 2.
Time-to-event analyses for A, DoR in responding patients; B, PFS per BICR; C, OS in patients with NTRK fusion-positive solid tumors (N=121). Data cut-off August 31, 2020.
BICR-assessed ORR was similar in patients with investigator-assessed baseline CNS metastases (15/26; 57.7%) and in patients without investigator-assessed baseline CNS metastases (59/95; 62.1%; Table 2). In these two populations, median DoR was 17.2 months (95% CI, 6.0–29.4) and 29.0 months [95% CI, 12.9–not estimable (NE)], respectively; median PFS was 11.7 months (95% CI, 4.7–30.2) and 13.8 months (95% CI, 10.2–20.8), respectively (Table 2). Equivalent data for BICR-assessed baseline CNS metastases are presented in Appendix Table A3. In the overall efficacy-evaluable population, six of 121 patients (5.0%; all with baseline CNS metastases) experienced CNS progression (Appendix Fig. A4). Median time to CNS progression (exploratory endpoint; only scan-confirmed CNS progression counted as an event) was not estimable; the 12-month event-free probability was 100% in patients without investigator-assessed baseline CNS disease (no patient had experienced symptomatic, scan-confirmed CNS progression by data cut-off) and 81% in those with baseline CNS disease. Data for CNS PFS in these populations are described in the Appendix.
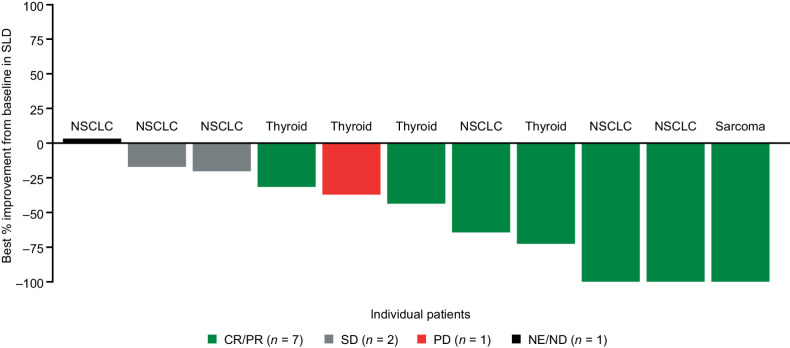
Among 11 patients with measurable CNS metastases at baseline per BICR, intracranial ORR was 63.6% [n = 7 (three CRs; four PRs); 95% CI, 30.8–89.1], median intracranial DoR was 22.1 (95% CI, 7.4–NE) months, and median intracranial PFS was 19.9 months (95% CI, 5.9–NE); six patients experienced an event (three CNS disease progression; three deaths; Appendix Table A4). One patient with thyroid cancer had PD as best intracranial response and overall PR (Fig. 3). This patient had received two prior lines of systemic therapy and, at data cut-off, was still under treatment after 38.1 months. Among patients with nonmeasurable CNS metastases at baseline per BICR, three had CR and five had non-CR/PD. Overall, patients with measurable or nonmeasurable baseline CNS disease per BICR (n = 19) showed an intracranial ORR of 52.6% (95% CI, 28.9–75.6) (Appendix Table A4). Median time to first intracranial response was 2.7 months (IQR, 0.9–4.6). Intracranial response according to prior brain radiotherapy status is described in Appendix Table A5.
Figure 3.
Best intracranial responses to entrectinib in patients with NTRK fusion-positive solid tumors and measurable CNS metastases (BICR assessed) at baseline. Data cut-off August 31, 2020. Response assessed by BICR. Patients with new CNS lesions or unequivocal progression of nontarget lesions had overall response classified as PD, even if the SLD of all lesions was reduced. ND, not determined; SD, stable disease.
Safety
Safety analyses included two different populations (Appendix Table A6): the overall entrectinib safety-evaluable population [n = 626 patients; 583 adults, 43 pediatric (pediatric study entrectinib dose, 250–750 mg)] comprising patients from STARTRK-1, STARTRK-2, ALKA-372-001, and STARTRK-NG and the subgroup of NTRK fusion-positive safety-evaluable adult patients (n = 193). At data cut-off, the median treatment duration was 8.3 months (IQR, 2.7–17.3) in the NTRK fusion-positive safety-evaluable population and 6.4 months (IQR, 1.9–18.4) in the overall safety population.
Almost all patients from the NTRK fusion-positive (99.5%) and overall safety populations (99.2%) experienced ≥1 AE of any grade (Appendix Table A7). Most patients from the NTRK (90.7%) and overall (92.0%) safety populations experienced ≥1 TRAE of any grade (Appendix Table A8). TRAEs reported in these populations were mostly grade 1 to 3 and nonserious; the most common were dysgeusia [35.2% (NTRK); 35.9% (overall)], diarrhea (31.1%; 25.9%), fatigue (27.5%; 28.8%), and weight increase (27.5%; 27.3%).
Grade ≥3 TRAEs were reported in 41.5% and 38.0% of patients in the NTRK fusion-positive and overall safety populations, respectively. In the NTRK safety population, the most common grade 3 or 4 TRAEs were weight gain (8.3%), anemia (5.2%), and fatigue (4.7%; Appendix Table A8).
Serious TRAEs were reported by 24 (12.4%) patients from the NTRK fusion-positive safety population and 72 (11.5%) patients from the overall safety population (Appendix Table A9). The most common were nervous system disorders (including dizziness and cognitive disorder), reported in nine patients (4.7%) from the NTRK safety population. At data cut-off, five deaths (all n = 1: atrioventricular block in MASC; cardiac arrest and ventricular fibrillation in papillary thyroid cancer; sudden death in neuroblastoma; cerebrovascular accident in head and neck cancer) were reported to have a potential relationship with entrectinib. They all occurred within 1 week of treatment initiation; the role of entrectinib in these deaths was thus unclear.
Most TRAEs were reversible and resolved following dose reductions or modifications. In the NTRK fusion-positive safety population, TRAEs that led to dose reduction occurred in 25.4% of patients, and median (IQR) dose intensity over the full treatment duration was 91.3% (65.9–99.6); patients' age had no influence on dose reductions. Drug interruptions due to TRAEs were seen in 65 (33.7%) patients. The most common all-cause TRAEs leading to dose reductions were dizziness (4.1%), anemia (n = 5; 2.6%), fatigue, and blood creatinine increase (both n = 4; 2.1%). Sixteen patients (8.3%) from the NTRK fusion-positive safety population discontinued entrectinib due to TRAEs.
In the overall safety population, TRAEs led to dose reductions in 25.6% of patients, drug interruptions in 31.2% of patients, and discontinuation in 6.5% of patients. Median dose intensity was 94.2% (IQR, 67.8–100.0).
Data from an earlier clinical data cut-off (October 31, 2018) were used to support the European approval of entrectinib. The key efficacy data from this cutoff (N = 74) are included in Appendix Tables A10, A11, A12, and Fig. A5, and the key safety data from this cut-off (n = 113 NTRK fusion-positive adult patients) are presented in Appendix Tables A13 and A14.
Discussion
We report data from an updated integrated efficacy and safety analysis of patients with NTRK fusion-positive solid tumors, including more patients (n = 121 vs. 54) and a longer follow-up (25.8 vs. 12.9 months) than the previous report (8). With additional clinical experience, entrectinib treatment continues to achieve high ORR (61.2%) and sustained responses (median DoR 20.0 months) in patients with NTRK fusion-positive solid tumors, confirming and improving upon our previously reported data (ORR, 57.4%; DoR, 10.4 months); median PFS was similar to previously reported, at 13.8 months versus 11.2 months previously.
Notably, although responses were observed across tumor types, ORR in NTRK fusion-positive colorectal carcinoma seemed markedly lower than in the other most common tumors examined. Local and large-scale analyses of molecular features of tyrosine kinase fusion-positive colorectal carcinoma identified it as a unique tumor type associated with high tumor mutational burden (TMB) and spontaneous high microsatellite instability (MSI) (12, 13). Importantly, median TMB was not increased in the other NTRK fusion-positive tumors, suggesting that NTRK fusion-positive colorectal carcinoma may have a different pathophysiologic profile to the other tumor types. This may explain the lower ORR observed in this population. However, the long DoR in those who responded to entrectinib indicates that some patients with this tumor type can derive benefit from TRK-targeted therapies. All these observations are very important for the clinical field of NTRK fusions because they suggest that treatment decisions for patients with NTRK fusion-positive colorectal carcinoma should also take TMB/MSI status into consideration. Overall, gathering further evidence on NTRK fusion-positive colorectal carcinoma would help shed light on the physiology of this unique tumor type and enable us to identify the patients who would benefit from TRK-targeted therapies.
Along with the confirmed overall efficacy in patients with and without CNS metastases at baseline per investigator assessment (overall ORR 57.7% and 62.1%, respectively), entrectinib also had intracranial activity in seven of 11 patients (63.6%; median intracranial DoR 22.1 months) with BICR-assessed measurable CNS metastases at baseline; this is consistent with its previously described mechanism of action and pharmacologic profile (7). Entrectinib was rationally designed to penetrate and remain in the CNS. Contrary to P-glycoprotein (P-gp) substrates such as larotrectinib, entrectinib is not exported out of the brain by P-gp and can therefore exert strong intracranial activity (7). Importantly, none of the 95 patients without investigator-assessed baseline CNS metastases experienced scan-confirmed symptomatic CNS progression, and median time to CNS progression (deaths censored) in patients with CNS metastases at baseline was 30.2 months (95% CI, 26.7–NE). CNS follow-up was only comprehensive for patients with baseline CNS metastases: regular CNS scans of those without baseline CNS metastases would have been performed as clinically indicated (e.g., if relevant neurologic symptoms were detected). It would have perhaps been preferable to mandate repeat CNS scans for patients with tumor types with a known high incidence of CNS progression (e.g., NSCLC or breast cancer; ref. 14) to provide more insights into the hypothetical CNS-protective effect of entrectinib. Indeed, The European Society for Medical Oncology (ESMO) Clinical Practice Guidelines for managing brain metastases from solid tumors recommend CNS screening at diagnosis in subgroups of patients at high risk of developing brain metastases (e.g., NSCLC, HER2-positive breast cancer, and grade IV melanoma; ref. 15). Additionally, in the entrectinib trials, CNS metastases were more frequent than expected in some tumor types such as NTRK fusion-positive thyroid cancer and sarcoma; extended life expectancy of patients may increase the likelihood of CNS metastases developing in histologies where they are normally uncommon. This, however, remains to be confirmed. Entrectinib can address the unmet need of a CNS-active treatment for patients with NTRK fusion-positive solid tumors with CNS metastases.
Larotrectinib is a TRK inhibitor also approved in the United States and Europe for the treatment of NTRK fusion-positive solid tumors. In updated results from the phase I/II integrated analysis of larotrectinib in 116 adult patients with NTRK fusion-positive solid tumors (16, 17), ORR per investigator was 71%, median DoR 35.2 months, and median PFS 25.8 months. Overall efficacy was reported for the 14 (12%) patients with baseline CNS metastases, but intracranial efficacy was not a predefined study endpoint. NTRK gene fusions occur in less than 1% of tumors, therefore the larotrectinib and entrectinib trials used single-arm study designs and integrated analyses to evaluate efficacy and safety. The study designs and patient populations differ substantially between the entrectinib and larotrectinib trials. For example, the entrectinib studies included a lower proportion of pediatric malignancies and a higher proportion of patients with CNS metastases at baseline (21% vs. 12% for larotrectinib). The utility of cross-study comparisons is therefore limited and further complicated by the fact that reporting methods differ between studies. For example, entrectinib studies primarily used BICR, whereas larotrectinib studies primarily used investigator-assessed response. This leads to slight discrepancies for ORR: in the larotrectinib studies, ORR was 71% by investigator and 66% by BICR (18).
Within the entrectinib studies, variation in response probabilities was observed between tumor types, likely due to differences in biology and prognoses across the multiple histologies; other patient parameters may also influence and confound clinical outcomes. As patient numbers were low and confidence intervals were accordingly large, further recruitment should give a clearer picture of the efficacy of entrectinib across NTRK fusion-positive tumor types. Additional molecular analyses, such as the identification of currently nontargetable alterations that may affect disease dynamics or be linked to resistance mechanisms are currently being performed but have so far not shown any correlation between the presence of coalterations and response or resistance to treatment.
In this study, entrectinib was well tolerated with low discontinuation rates and dose-reduction rates consistent with previous reports. The high median dose intensity (>91%) also indicates that any dose reductions and/or interruptions had a minor impact on overall dose exposure, with the majority of patients receiving most of the full planned dose. The safety profile was similar between the NTRK fusion-positive and overall safety populations and aligned with the safety profile of other TRK-targeted agents such as larotrectinib and with the previously reported safety profile (19, 20).
Conclusions
This updated integrated analysis of entrectinib phase I/II clinical trials included more patients and longer follow-up than the previously reported data. Entrectinib continued to demonstrate clinically meaningful, durable systemic responses in patients with NTRK fusion-positive solid tumors and was associated with intracranial responses in the small cohort of patients with baseline CNS metastases at baseline, suggesting it could address the unmet need of a CNS-active treatment for these patients. Although NTRK fusions are rare, our results should encourage broader screening for these fusions in patients with solid tumors as they may benefit from entrectinib (21).
Authors' Disclosures
G.D. Demetri reports grants, personal fees, and nonfinancial support to Dana-Farber Cancer Institute from Roche/Genentech and Bayer during the conduct of the study; personal fees and other support from Blueprint Medicines, Caris Life Sciences, G1 Therapeutics, Relay Therapeutics, CellCarta, Ikena Oncology, Kojin Therapeutics, Acrivon Therapeutics, EMD-Serono, WCG/Arsenal Capital and McCann Health; other support from Erasca Pharmaceuticals; personal fees, nonfinancial support, and other support from Pfizer and Epizyme; personal fees and nonfinancial support from Novartis; grants, personal fees, nonfinancial support, and other support from GlaxoSmithKline, PharmaMar, and Daiichi-Sankyo; grants from Janssen and Adaptimmune; and personal fees from Mirati, C4 Therapeutics, Synlogic, and RAIN Therapeutics outside the submitted work; in addition, G.D. Demetri has a patent for Imatinib for GIST issued, licensed, and with royalties paid from Novartis. F. De Braud reports personal fees from Roche, EMD Serono, NMS Nerviano Medical Science, Sanofi, Merck, Sharp & Dohme, Novartis, Incyte, Bristol Myers Squibb, Menarini, Merck Group, Pfizer, Servier, Amgen, Merck, Sharp & Dohme Serono, and Pharmadoc outside the submitted work. A. Drilon reports personal fees from Ignyta/Genentech/Roche during the conduct of the study; personal fees from Loxo/Bayer HealthCare/Eli Lilly and Company, Takeda/Ariad/Millenium, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Helsinn, BeiGene, BergenBio, Hengrui Therapeutics, Exelixis, Tyra Biosciences, Verastem, MORE Health, AbbVie, 14ner/Elevation Oncology, ArcherDX, Monopteros, Novartis, EMD Serono, Medendi, Repare RX, Nuvalent, Merus, Chugai Pharmaceutical Co., Ltd., Remedica Ltd., mBrace, AXIS, EPG Health, Harborside, Liberum Nexus, RV More Ology, Amgen, TouchIME, Janssen, Entos Pharmaceuticals, Treeline Biosciences, Prelude Therapeutics, and Applied Pharmaceutical Science, Inc. outside the submitted work; in addition, A. Drilon has a patent for Osimertinib-Selpercatinib pending and associated research paid to Memorial Sloan Kettering Cancer Center from Pfizer, Exelixis, GlaxoSmithKlein, Teva, Taiho Pharmaceutical Co., Ltd., PharmaMar; research from Foundation Medicine; royalties from Wolters Kluwer; other (food/beverage) from Boehringer Ingelheim, Merck, Puma, Merus; CME honoraria from Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice, Axis, Peerview Institute, Paradigm Medical Communications, WebMD, MJH Life Sciences, Med Learning, Imedex, Answers in CME, Clinical Care Options, EPG Health, JNCC/Harborside, Liberum, and Remedica Ltd. S. Siena reports advisory board member relationships with Agenus, AstraZeneca, Bayer, BMS, CheckmAb, Daiichi-Sankyo, Guardant Health, Menarini, Merck, Novartis, Roche-Genentech, and Seattle Genetics. M.R. Patel reports other support (advisory board) from Sanofi outside the submitted work. B.C. Cho reports grants from Novartis, Bayer HealthCare, AstraZeneca, MOGAM Institute, Dong-A ST, Champions Oncology, Janssen, Yuhan, Ono Pharmaceutical Co., Ltd., Dizal Pharma, Merck, Sharp & Dohme, AbbVie, Medpacto, GIInnovation, Eli Lilly and Company, Blueprint Medicines, and Interpark Bio Convergence Corp.; personal fees from Novartis, AstraZeneca, Boehringer-Ingelheim, Roche, Bristol Myers Squibb, Ono Pharmaceutical Co., Ltd., Yuhan, Pfizer, Eli Lilly and Company, Janssen, Takeda, Merck, Sharp & Dohme, Medpacto, Blueprint Medicines, TheraCanVac Inc, Gencurix Inc, Bridgebio Therapeutics, KANAPH Therapeutic Inc., Cyrus Therapeutics, Interpark Bio Convergence Corp., Guardant Health, Joseah BIO, and Champions Oncology; and was the founder of DAAN Biotherapeutics outside the submitted work. S.V. Liu reports grants from Alkermes, Merus, Nuvalent, Pfizer, Rain Therapeutics, and RAPT; personal fees from Amgen, AstraZeneca, BeiGene, Daiichi Sankyo, Eisai, Gilead, Guardant Health, Janssen, Jazz Pharmaceuticals, Eli Lilly and Company, Novartis, Regeneron, Sanofi, and Takeda; grants and personal fees from Bayer HealthCare, Blueprint Medicines, Bristol Myers Squibb, Elevation Oncology, Genentech, and Merck; and grants and Turning Point Therapeutics outside the submitted work. M.-J. Ahn reports personal fees from AstraZeneca, Alpha Pharmaceuticals, Merck, Takeda, Eli Lilly and Company, Yuhan, Merck, Sharp & Dohme, and Ono Pharmaceutical Co., Ltd. outside the submitted work. C.-H. Chiu reports personal fees from AstraZeneca, Boehringer-Ingelheim, Bristol Myers Squibb, Chugai Pharmaceutical Co., Ltd., Eli Lilly and Company, Janssen, Merck KGaA, Merck Sharp & Dohme, Novartis, Ono Pharmaceutical Co., Ltd., Pfizer, Roche, and Takeda outside the submitted work. J.J. Lin reports personal fees and other support from Roche/Genentech, Nuvalent, Turning Point Therapeutics, Novartis, Elevation Oncology, and Bayer HealthCare; personal fees from C4 Therapeutics, Blueprint Medicines, Pfizer, and Mirati Therapeutics; and other support from Hengrui Therapeutics, Neon Therapeutics, Linnaeus Therapeutics, Relay Therapeutics, OncLive, MedStar Health, and Northwell Health outside the submitted work. K. Goto reports grants and personal fees from Chugai Pharmaceutical Co., Ltd. and grants from Ignyta during the conduct of the study; grants and personal fees from Amgen K.K., AstraZeneca K.K., Boehringer Ingelheim Japan, Inc., Bristol Myers Squibb K.K., Daiichi Sankyo, Eisai, Eli Lilly and Company Japan K.K., Janssen Pharmaceutical K.K., Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda; grants from Kissei Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Loxo Oncology, Inc., Medical & Biological Laboratories Co., Ltd., Merck Biopharma Co., Ltd., Merus N.V., Merck, Sharp & Dohme K.K., NEC Corporation., Pfizer Japan Inc., Sumitomo Dainippon Pharma Co., Ltd., Spectrum Pharmaceuticals, Inc., Sysmex Corporation., Haihe Biopharma Co., Ltd., and Turning Point Therapeutics, Inc.; and personal fees from Amoy Diagnosties Co., Ltd., Bayer Yakuhin, Ltd., Guardant Health Inc., and Otsuka Pharmaceutical Co., Ltd. outside the submitted work. J. Lee reports advisory/consultancy relationships with Oncologie and Seattle Genetics and research grant/funding (self) from Astra Zeneca, Eli Lilly & Company, and Merck Sharp & Dohme. L. Bazhenova reports personal fees from ORIC, Turning Point Therapeutics, Inc., Neuvogen, Daichi Sankyo, Janssen, Merck, BeyondSpring, Regeneron, Bayer HealthCare, Takeda, Boehringer-Ingelheim, Novartis, Genentech, and Sanofi outside the submitted work. T. John reports personal fees from Roche, Merck, Merck, Sharp & Dohme, Puma, AstraZeneca, Bristol Myers Squibb, Novartis, Amgen, Gilead, PharmaMar, and Specialised Thrapeutics outside the submitted work. M. Fakih reports grants and personal fees from Amgen; personal fees from Array, Bayer HealthCare, GlaxoSmithKline, Guardant360, HalioDx, Mirati, Nouscom, Pfizer, Seattle Genetics, Taiho Pharmaceutical Co., Ltd., and Zhuhai Yufan Biotech; grants from AstraZeneca, Novartis, Bristol Myers Squibb, and Verstem outside the submitted work. S.P. Chawla reports grants or contracts from Amgen, Roche, GlaxoSmithKline, Threshold Pharmaceuticals, CytRx Corporation, Ignyta, Immune Design, TRACON Pharma, Karyopharm Therapeutics, SARC: Sarcoma Alliance for Research though Collaboration, Janssen, Advenchen Laboratories, Bayer HealthCare, InhibRx, and NKMax; consulting fees from Amgen, Roche, GlaxoSmithKline, Threshold Pharmaceuticals, CytRx Corporation, Ignyta, Immune Design, TRACON Pharma, Karyopharm Therapeutics, SARC: Sarcoma Alliance for Research though Collaboration, Janssen, Advenchen Laboratories, Bayer HealthCare, NKMax, and InhibRx; payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Amgen, Roche, GlaxoSmithKline, Threshold Pharmaceuticals, CytRx Corporation, Ignyta, Immune Design, TRACON Pharma, Karyopharm Therapeutics, SARC: Sarcoma Alliance for Research though Collaboration, Janssen, Advenchen Laboratories, Bayer HealthCare, InhibRx, NKMax, and Tyme; and stock or stock options of AADi, Cellestia Biotech, and Immix BioPharma. R. Dziadziuszko reports personal fees from Roche, AstraZeneca, FoundationMedicine, Merck, Sharp & Dohme, Takeda, Pfizer, Novartis, Karyopharm Therapeutics, and Boehringer-Ingelheim, and Bristol Myers Squibb during the conduct of the study. T. Seto reports personal fees from AstraZeneca, Bristol Myers Squibb, Kyowa Hakko Kirin, Mochida Pharmaceutical, Nippon Boehringer-Ingelheim, Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Towa Pharmaceutical, Covidien Japan; grants and personal fees from Chugai Pharmaceutical Co., Ltd., Daiichi Sankyo, Eli Lilly and Company Japan, Merck, Sharp & Dohme, Novartis Pharma, Pfizer Japan, Takeda; grants from AbbVie and Kissei Pharmaceutical; and employment from Precision Medicine Asia outside the submitted work. S. Heinzmann reports employment and shares from F. Hoffmann-La Roche Ltd. B. Pitcher reports employment from F. Hoffmann-La Roche Ltd. during the conduct of the study and outside the submitted work. D. Chen reports employment from Genentech/Roche during the conduct of the study and outside the submitted work. T.R. Wilson reports employment from Genentech during the conduct of the study and stocks from Roche outside the submitted work. C. Rolfo reports personal fees from ARCHER, Inivata, Bristol Myers Squibb, Novartis, Boston Pharmaceuticals, AstraZeneca, Roche, GuardantHealth, Merck, Sharp & Dohme, Mirati, Eisai, Daiichi Snakyo, and Sanofy Genzyme-Regeneron; personal fees and other support from EMD Serono; grants and personal fees from Pfizer; and personal fees from COR2RED outside the submitted work; in addition, C. Rolfo reports leadership roles as President of ISLB; Chair Educational Committee for IASLC; scientific board member of ESO; ESMO Advanced Lung Cancer Faculty member; Editor in Chief of CROH; and Associate Editor for ESMO Open. No disclosures were reported by the other authors.
Supplementary Material
Acknowledgments
This work was funded by F. Hoffmann-La Roche Ltd.
The authors would like to thank the patients, their families, and the participating study centers. We also thank Apurva Javery for her support with curating the data. Third-party medical writing assistance, under the direction of the authors, was provided by Laura Vergoz, PhD, of Ashfield MedComms, an Ashfield Health company, and was funded by F. Hoffmann-La Roche Ltd.
In memory of our dear colleague and friend Todd Riehl, whose contributions to the work in this article we would like to honor.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This article is featured in Highlights of This Issue, p. 1241
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Contributions
G.D. Demetri: Conceptualization, data curation, formal analysis, funding acquisition, investigation, writing–original draft, writing–review and editing. F. De Braud: Investigation, writing–original draft, writing–review and editing. A. Drilon: Conceptualization, data curation, supervision, investigation, methodology, writing–original draft, project administration, writing–review and editing. S. Siena: Conceptualization, validation, investigation, methodology, writing–original draft, writing–review and editing. M.R. Patel: Investigation, writing–original draft, writing–review and editing. B.C. Cho: Investigation, writing–original draft, writing–review and editing. S.V. Liu: Investigation, writing–original draft, writing–review and editing. M.-J. Ahn: Investigation, writing–original draft, writing–review and editing. C.-H. Chiu: Investigation, writing–original draft, writing–review and editing. J.J. Lin: Investigation, writing–original draft, writing–review and editing. K. Goto: Investigation, writing–original draft, writing–review and editing. J. Lee: Investigation, writing–original draft, writing–review and editing. L. Bazhenova: Investigation, writing–original draft, writing–review and editing. T. John: Investigation, writing–original draft, writing–review and editing. M. Fakih: Investigation, writing–original draft, writing–review and editing. S.P. Chawla: Investigation, writing–original draft, writing–review and editing. R. Dziadziuszko: Investigation, writing–original draft, writing–review and editing. T. Seto: Investigation, writing–original draft, writing–review and editing. S. Heinzmann: Writing–original draft, writing–review and editing. B. Pitcher: Data curation, formal analysis, investigation, writing–original draft, writing–review and editing. D. Chen: Data curation, writing–original draft, writing–review and editing. T.R. Wilson: Data curation, writing–original draft, writing–review and editing. C. Rolfo: Conceptualization, formal analysis, investigation, writing–original draft, writing–review and editing.
References
- 1. Vaishnavi A, Capelletti M, Le AT, Kako S, Butaney M, Ercan D, et al. Oncogenic and drug-sensitive NTRK1 rearrangements in lung cancer. Nat Med 2013;19:1469–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amatu A, Sartore-Bianchi A, Bencardino K, Pizzutilo EG, Tosi F, Siena S. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann Oncol 2019;30:viii5–viii15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol 2018;15:731–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hsiao SJ, Zehir A, Sireci AN, Aisner DL. Detection of tumor NTRK gene fusions to identify patients who may benefit from tyrosine kinase (TRK) inhibitor therapy. J Mol Diagn 2019;21:553–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rolfo C. NTRK gene fusions: a rough diamond ready to sparkle. Lancet Oncol 2020;21:472–4. [DOI] [PubMed] [Google Scholar]
- 6. Menichincheri M, Ardini E, Magnaghi P, Avanzi N, Banfi P, Bossi R, et al. Discovery of entrectinib: a new 3-aminoindazole as a potent anaplastic lymphoma kinase (ALK), c-ros oncogene 1 kinase (ROS1), and pan-tropomyosin receptor kinases (Pan-TRKs) inhibitor. J Med Chem 2016;59:3392–408. [DOI] [PubMed] [Google Scholar]
- 7. Fischer H, Ullah M, de la Cruz CC, Hunsaker T, Senn C, Wirz T, et al. Entrectinib, a TRK/ROS1 inhibitor with anti-CNS tumor activity: differentiation from other inhibitors in its class due to weak interaction with P-glycoprotein. Neuro Oncol 2020;22:819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol 2020;21:271–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Drilon A, Siena S, Ou S-HI, Patel M, Ahn MJ, Lee J, et al. Safety and antitumor activity of the multitargeted pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two Phase I trials (ALKA-372–001 and STARTRK-1). Cancer Discov 2017;7:400–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Desai AV, Brodeur GM, Foster J, Berg SL, Basu EM, Shusterman S, et al. Phase 1 study of entrectinib (RXDX-101), a TRK, ROS1, and ALK inhibitor, in children, adolescents, and young adults with recurrent or refractory solid tumors. J Clin Oncol 2018;36:10536. [Google Scholar]
- 11. Robinson GW, Gajjar AJ, Gauvain KM, Basu EM, Macy ME, Maese LD, et al. Phase 1/1B trial to assess the activity of entrectinib in children and adolescents with recurrent or refractory solid tumors including central nervous system (CNS) tumors. J Clin Oncol 2019;37:10009. [Google Scholar]
- 12. Singh H, Li YY, Spurr LF, Shinagare AB, Abhyankar R, Reilly E, et al. Molecular characterization and therapeutic targeting of colorectal cancers harboring receptor tyrosine kinase fusions. Clin Cancer Res 2021;27:1695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Westphalen CB, Krebs MG, Le Tourneau C, Sokol ES, Maund SL, Wilson TR, et al. Genomic context of NTRK1/2/3 fusion-positive tumours from a large real-world population. NPJ Precis Oncol 2021;5:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rick JW, Shahin M, Chandra A, Dalle Ore C, Yue JK, Nguyen A, et al. Systemic therapy for brain metastases. Crit Rev Oncol Hematol 2019;142:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le Rhun E, Guckenberger M, Smits M, Dummer R, Bachelot T, Sahm F, et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol 2021;32:1332–47. [DOI] [PubMed] [Google Scholar]
- 16. Drilon AE, Farago AF, Tan DS-W, Kummar S, McDermott RS, Berlin J, et al. Activity and safety of larotrectinib in adult patients with TRK fusion cancer: an expanded data set. J Clin Oncol 2020;38:3610. [Google Scholar]
- 17. McDermott R, van Tilburg CM, Farago AF, Kummar S, Tan DSW, Albert CM, et al. 1955P Survival benefits of larotrectinib in an integrated dataset of patients with TRK fusion cancer. Ann Oncol 2020;31:S1101–S2. [Google Scholar]
- 18. European Meadicines Agency. Larotrectinib (VITRAKVI) EU Summary of Product Characteristics. EMA; 2019. Available from: https://www.ema.europa.eu/en/documents/product-information/vitrakvi-epar-product-information_ en.pdf. [Google Scholar]
- 19. Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 2018;378:731–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol 2020;21:531–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cardona AF, Arrieta O, Ruiz-Patino A, Sotelo C, Zamudio-Molano N, Zatarain-Barron ZL, et al. Precision medicine and its implementation in patients with NTRK fusion genes: perspective from developing countries. Ther Adv Respir Dis 2020;14:1753466620938553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data were generated and analyzed under the auspices of Roche, which is a member of the Vivli Center for global clinical research data (https://vivli.org/ourmember/roche/). Roche will share/allow access to individual patient-level data from the clinical trials via Vivli, providing certain criteria are met. Please see the criteria and exceptions on the Roche member section of the Vivli homepage at https://vivli.org/ourmember/roche/. Please see also the Roche Global Data Sharing Policy (https://www.roche.com/dam/jcr:1c46aa73-cea0-4b9b-8eaa-e9a788ed021b/roche_global_policy_on_sharing_of_clinical_study_informationV2.1%20April2020%20(1).pdf) for more details. To request access to individual patient-level data from the clinical trials, first locate the clinical trial in Vivli (https://search.vivli.org/ requires sign up and log in) using the trial registration number (given above), then click the “Request Study” button and follow the instructions. In the event that you cannot see a specific study in the Roche list, an Enquiry Form can be submitted to confirm the availability of the specific study.
To request access to related clinical study documents (eg: protocols, clinical study reports, safety reports), please use Roche's Clinical study documents request form: https://www.roche.com/research_and_development/who_we_are_how_we_work/research_and_clinical_trials/our_commitment_to_data_sharing/clinical_study_documents_request_form.htm.



![Figure 1. Responses and time on entrectinib treatment in patients with NTRK fusion-positive solid tumors, by tumor type (BICR assessed). A, Best individual patient responses [n = 103; 18 patients with missing sum of the longest diameter (SLD) change were excluded from the plot]. B, Time on entrectinib treatment. Data cut-off August 31, 2020. The minimum shrinkage in the SLD of target lesions that defined an OR was 30%. Gastrointestinal (GI)-other, adenocarcinoma of upper GI tract; CRC, colorectal carcinoma; CUP, cancer of unknown primary; Inv, investigator; ND, not determined; SD, stable disease.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/75dd/9365368/39f0f639579d/1302fig1.jpg)