Abstract
Purpose:
Mesenchymal stem/stromal cell therapy may reduce radiation-induced xerostomia. We investigated the long-term safety of autologous adipose tissue-derived mesenchymal stem/stromal cell (ASC) injections into the submandibular glands.
Experimental Design:
An investigator-initiated, randomized, single-center, placebo-controlled trial. Previous patients with oropharyngeal squamous cell carcinoma with radiation-induced xerostomia were randomly (1:1) allocated to receive a 2.8 million ASCs/cm3 injection or placebo in both submandibular glands and followed for a minimum of 2 years. The primary endpoint was number of serious adverse events (SAE). Secondary endpoints included whole saliva flow rates and xerostomia-related symptoms. Data analysis was based on the intention-to-treat population using repeated measures mixed-effects linear models.
Results:
Thirty-three patients were randomized; 30 patients were treated (ASC group, n = 15; placebo group, n = 15). Long-term safety data were collected from all 30 patients. During follow-up, 6 of 15 (40%) of the ASC-treated patients versus 5 of 15 (33%) of the placebo patients experienced an SAE; no SAEs appeared to be treatment related. Unstimulated whole saliva flow rate increased to 0.20 and 0.16 mL/minute in the ASC and placebo group, respectively, yielding a 0.05 mL/minute (95% confidence interval: 0.00–0.10; P = 0.051) difference between groups. Patient-reported xerostomia symptoms diminished according to a decreased xerostomia questionnaire summary score of 35.0 and 45.1, respectively [−10.1 (−18.1 to −2.2); P = 0.013]. Three of the visual analog scale xerostomia measures indicated clinical benefit following use of ASC.
Conclusions:
Our data show that ASC therapy is safe with a clinically relevant effect on xerostomia-related symptoms. Confirmation in larger randomized controlled trials is warranted.
Translational Relevance.
Damage to the major salivary glands is a leading permanent side effect of radiotherapy for head and neck cancers, and adequate treatment is missing. Mesenchymal stem/stromal cell therapy may reduce radiation-induced xerostomia. We previously published the short-term results of intraglandular injections of autologous adipose tissue-derived mesenchymal stem/stromal cells (ASC), versus placebo, indicating a benefit of the intervention in previous patients with human papillomavirus + oropharyngeal squamous cell carcinoma. This study, an investigator-initiated, randomized, placebo-controlled trial, investigated the long-term safety of ASC injections into the submandibular glands.
This study demonstrates that an autologous ASC therapy is safe and that it significantly reduced xerostomia-related symptoms. This study also suggests that ASC therapy has a restorative effect on unstimulated whole saliva flow rate. Confirmation in larger randomized controlled trials is warranted.
Introduction
Damage to the major salivary glands is a leading permanent side effect of radiotherapy for head and neck cancers, and adequate treatment is missing (1). Around 800,000 patients are diagnosed worldwide with head and neck cancer annually, and in approximately 70%–80% of these cases, radiotherapy is a key treatment modality either alone, with chemotherapy or surgery, or both (2, 3). The salivary glands are highly sensitive to irradiation, which leads to chronic inflammation, loss of acinar cells, local intrinsic stem/progenitor cells and blood vessels, and progressive fibrosis. Reduced salivary flow rate may result in xerostomia; chewing, swallowing, and speech difficulties; dental decay; sleep deprivation due to oral dryness; and impaired quality of life.
Mesenchymal stromal/stem cells (MSC) are adult multipotent cells residing in abundance in the human body's vascularized tissues. Expanded MSCs can promote immunomodulation, antifibrosis, regeneration, angiogenesis, and restore tissue homeostasis (4, 5). Animal studies indicate that MSCs can potentially restore radiation-damaged tissues (6–9). However, although several trials with various clinical applications have been conducted only few studies have reported on the long-term safety and efficacy of MSCs in humans (10–14).
The MESRIX trial was the first randomized, double-blind, placebo-controlled trial in humans to investigate the safety and efficacy of autologous adipose tissue–derived MSC (ASC) injections into the submandibular glands in previous patients with oropharyngeal squamous cell carcinoma (OPSCC) with radiation-induced salivary gland hypofunction and xerostomia. The early effects after 4 months showed a tendency toward increased production of unstimulated whole saliva and reduced xerostomia-related symptoms (15). The objectives of the current study according to the trial registration were to report the long-term safety [serious adverse events (SAE)] and the effectiveness of intraglandular injection of ASCs into the submandibular glands of previous patients with human papillomavirus–associated oropharyngeal squamous cell carcinoma (HPV+ OPSCC), relative to placebo, after at least 2 years from baseline.
Materials and Methods
Study design and participants
The MESRIX trial was an investigator-initiated, single-center, randomized, placebo-controlled, phase I/II trial with a minimum of 2 years follow-up performed at the Department of Otolaryngology, Head and Neck Surgery & Audiology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark. The patients had undergone irradiation with volumetric-arc therapy to a total dose of 60–68 Gy, given in 2-Gy fractions. This treatment almost invariably delivers virtually the full dose to the ipsilateral submandibular gland and includes some dose to at least the lower part of the ipsilateral parotid gland. Patients were randomly assigned to either ASC or placebo. Outcomes were assessed at three consecutive timepoints following baseline for the primary efficacy outcome (i.e., at 0, 1, 4 months, and +2 years from baseline). The study protocol complied with the Declaration of Helsinki and was approved by The Danish National Committee on Health Research Ethics (1406653), the Danish Medicines Agency (2014-004349-29), and the Danish Data Protection Agency (30-1452). The trial was registered at clinicaltrials.gov before enrolling patients (NCT02513238) and monitored according to Good Clinical Practice principles. All patients provided written informed consent. The study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines for randomized clinical trials. The long-term follow-up was performed from May 2019 to April 2020.
The criteria for participation were described previously (15). The inclusion criteria were patients of both sexes over 18 years of age with radiation-induced salivary gland hypofunction and xerostomia after radiotherapy for HPV+OPSCC stage 1–2 (HPV+OPSCC, UICC 7), a minimum of 2 years after radiotherapy without relapse, no prior stem cell treatments, no pregnancy, and no transmitted infectious diseases. No use of supportive care of xerostomia was allowed during the study period. Patients were strictly monitored regarding intake or use of any treatment that may impact saliva flow rate or symptom relief.
Patients with cancer within the prior 2 years (not including OPSCC), xerogenic medications, previous stem cell therapy, previous surgery of the submandibular glands, known salivary gland disease, pregnancy, or breastfeeding were ineligible. Initially, we planned to include only patients with T1-T2 and N0-N2a but expanded it to N2b and T3 due to the low inclusion rate.
Randomization and concealed allocation
Patients were randomized 1:1 at inclusion to either ASC treatment or placebo by a computer-generated allocation sequence via http://www.randomization.com. The allocation sequence was concealed from the researcher(s) enrolling participants in sequentially numbered opaque, sealed, and stabled envelopes and only available to specified personnel at the Tissue Center, Department of Clinical Immunology, Rigshospitalet. Assessors and patients were blinded to treatment allocation for the first 4 months. The study was then unblinded to report 4-month results to the patients and academia. At long-term follow-up, the assessor was blinded to the baseline data, including data on the salivary flow rates, and radiologists were blinded as to which intervention group the patients belonged.
Treatment and outcomes
The ASCs were manufactured, characterized, and released as described previously (15), according to Good Manufacturing Practice and in compliance with the manufacturer's authorization of human investigational medicinal products issued by the Danish Medicines Agency. In brief, from the stromal vascular fraction of the lipoaspirate, the ASCs were culture expanded for approximately 2 weeks suspended in isotonic NaCl (0.9 mg/mL) and human albumin (HA) 1% to a volume of 1 mL. Placebo consisted of isotonic NaCl with 1% HA. The patients received ultrasound-guided injections of 1 mL blinded suspension of either ASCs or placebo into each submandibular gland. The dose of ASCs was 2.8 million ASCs/cm3 gland according to the volume of the glands determined by MRI.
The primary endpoint of this study (NCT02513238) was the safety assessed as SAEs. We also collected data on safety, including structural changes to the submandibular glands (assessed by MRI), recurrence of previous cancer, new primary cancer, and other SAEs with a minimum of grade 3 severity according to Common Terminology Criteria for Adverse Events v5.0 and treatment-related adverse events (infection, oral discomfort, and pain longer than 1 week after intervention). Secondary outcome measures were unstimulated and stimulated whole saliva flow rate assessed by sialometry, patient-reported outcomes measured on xerostomia symptoms, level of thirst, and amount of saliva. Finally, submandibular gland volume was evaluated by MRI.
Assessments were performed at baseline (preintervention), 1 and 4 months, and after a minimum of 2 years. At the long-term follow-up, the patients completed xerostomia questionnaires (XQ) and underwent sialometry; a complete ear, nose, and throat examination including fiberoptic laryngoscopy; and an MRI scan.
Data on safety were obtained from electronic patient records and the Danish National Pathological Register and Data Bank. Data on xerostomia symptoms were collected using the XQ and the visual analog scale (VAS) XQ, with higher scores indicating higher symptom burden (Supplementary Data S1; refs. 16, 17). The VAS scores cover eight subdomains related to xerostomia (difficulties speaking and eating, sensations of dryness) and the XQ results in a summary score. Sialometry was rigorously performed in an undisturbed room between 1 pm and 4 pm. Patients were asked to drink a minimum of 2 L of water the day before saliva collection and refrain from eating, drinking, and performing oral hygiene procedures 2 hours before the measurement. Unstimulated whole saliva (drooling method) was collected in a preweighed plastic cup over a 15-minute period (15). Stimulated whole saliva flow was collected over a 5-minute period (18, 19). First, the patient chewed on 1 g of paraffin wax for 1 minute and swallowed the newly produced whole saliva. The patient continued chewing, and the stimulated whole saliva was collected for the following 5 minutes. Saliva flow rates were calculated, whereby 1 g of whole saliva was equivalent to 1 mL (19).
The MRI examinations included axial T2-weighted turbo spin-echo sequences on which the glands were outlined. Gland delineation was performed using a semiautomatic 3D segmentation tool (AW server, GE Healthcare) by two radiologists (T.T. Andersen and U.M. Ciochon) under the supervision of a board-certified neuroradiologist (G.S. Rathje).
Statistical analysis
The sample size calculation was described previously (15). The prespecified statistical analysis plan (SAP) was developed while data collection was incomplete and the trial data inaccessible (Supplementary Data S2). The primary analyses were performed on the intention-to-treat (ITT) population, that is, independent of attrition during the trial period. The ITT principle asserts the effect of a planned treatment regimen rather than the actual treatment given. Accordingly, participants who were allocated to a treatment group (ASC and placebo, respectively) and had the injection as prescribed following the randomization were followed-up, assessed, and analyzed as members of that group, irrespective of their adherence to the planned course of treatment (i.e., independent of withdrawals and cross-over phenomena). Thus, as described in the SAP, we used a modified ITT (mITT) population, defined as randomized patients who received the invasive intervention (either placebo or ASC).
We analyzed continuous outcomes using repeated measures linear mixed-effects models, including participants as a random effects factor, with fixed-effect factors for Group (two levels) and Time [baseline + three levels (baseline, day 30, 120, and 730+)], and the interaction between them (Group × Time) while also adjusting for the level at baseline.
Missing data for the continuous data were handled indirectly and statistically modeled via the linear mixed-effects models (above; refs. 20, 21). The results for the continuous outcomes are reported on the basis of the least squares means with SEs for each group, and the corresponding difference between groups is reported with 95% confidence intervals (CI). Exact logistic regression was used to model the binary (safety) outcomes; exact logistic regression is appropriate because the safety outcomes are binary and the sample size was small with rare events. These models are preferable to a regular logistic regression; these estimates and corresponding P values given by exact logistic regression do not depend on asymptotic results. A two-sided P < 0.05 was taken to indicate statistical significance. Data analysis was conducted using SAS version 9.4 software (SAS Studio).
Data availability
Full dataset and software code to replicate the main analysis is available from the corresponding author on reasonable request.
Results
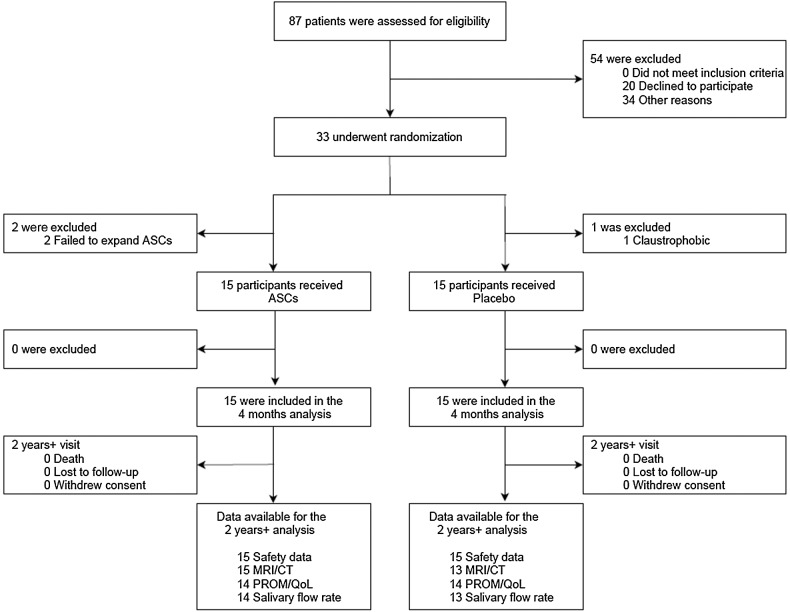
A diagram of the progress through the phases of the trial is presented in Fig. 1. Thirty of the 33 randomized patients received the intended intervention [ASC (15) or placebo (15)] between May 2015 and April 2017 (Table 1). The groups were balanced at baseline; baseline characteristics for the 30 participants included in the ITT population are presented in Table 1. The ASC group was followed for a median of 3.6 [interquartile range (IQR): 3.3–3.9] years and the placebo group for 3.4 (IQR: 2.9–3.8) years.
Figure 1.
CONSORT flow diagram of recruitment and study flow.
Table 1.
Baseline characteristics in the mITT population.
| ASCs (n = 15) | Placebo (n = 15) | Total (n = 30) | |
|---|---|---|---|
| Characteristica | |||
| Age (years) | 58.3 (±7.1) | 60.3 (±7.9) | 59.3 (±7.4) |
| Males, n (%) | 9 (60) | 10 (67) | 19 (63) |
| Ethnicity, Caucasian, n (%) | 15 (100) | 15 (100) | 30 (100) |
| Smoking status, n (%) | |||
| Current | 0 (0) | 1 (7) | 1 (3) |
| Prior | 6 (40) | 4 (27) | 10 (33) |
| Never | 9 (60) | 10 (67) | 19 (63) |
| Primary tumor site, n (%) | |||
| Tonsil | 13 (87) | 13 (87) | 26 (87) |
| Base of tongue | 2 (13) | 2 (13) | 4 (13) |
| T stage, n (%) | |||
| T1 | 7 (47) | 5 (33) | 12 (40) |
| T2 | 6 (40) | 10 (67) | 16 (53) |
| T3 | 2 (13) | 0 (0) | 2 (7) |
| N stage, n (%) | |||
| N0 | 2 (13) | 1 (7) | 3 (10) |
| N1 | 4 (27) | 2 (13) | 6 (20) |
| N2a | 2 (13) | 2 (13) | 4 (13) |
| N2b | 7 (47) | 9 (60) | 16 (53) |
| N2c | 0 (0) | 1 (7) | 1 (3) |
| Treatment, n (%) | |||
| Radiotherapy only | 2 (13) | 1 (7) | 3 (10) |
| Radiotherapy with cisplatin | 13 (87) | 14 (93) | 27 (90) |
| Duration from radiotherapy to intervention, years | 4.0 (±1.2) | 3.5 (±1.2) | 3.7 (±1.2) |
| Radiation of SMG (Gy): | |||
| Mean RT dose ipsilateral SMG | 65.7 (±3.9) | 65.4 (±2.8) | 65.5 (±3.2) |
| Mean RT dose contralateral SMG | 41.0 (±19.3) | 37.8 (±13.9) | 39.4 (±16.8) |
| Mean RT dose SMG, base of tongue | 59.9 (±6.6) | 63.5 (±3.9) | 61.7 (±5.7) |
| Whole saliva production (mL/minute)b | |||
| Unstimulated whole saliva flow rate | 0.12 (0.06–0.15) | 0.13 (0.09–0.21) | 0.12 (0.09–0.21) |
| Stimulated whole saliva flow rate | 0.74 (0.63–1.33) | 0.90 (0.79–1.18) | 0.85 (0.72–1.18) |
| Patient-reported complaints of xerostomiab | |||
| XQ, summary score (scale: 0–100) | 51 (39–65) | 49 (31–71) | 51 (33–71) |
| VAS, VAS score (scale: 0–100) | |||
| Difficulty speaking (#1) | 20 (7–41) | 22 (8–28) | 21 (8–28) |
| Difficulty swallowing (#2) | 45 (29–74) | 36 (23–60) | 44 (25–70) |
| Amount of saliva (#3) | 45 (20–77) | 48 (26–71) | 47 (26–75) |
| Dry mouth (#4) | 44 (19–86) | 47 (35–66) | 46 (26–77) |
| Dry throat (#5) | 46 (20–87) | 48 (40–67) | 48 (35–73) |
| Dry lips (#6) | 42 (23–74) | 47 (4–74) | 46 (9–74) |
| Dry tongue (#7) | 36 (17–47) | 39 (12–58) | 38 (17–51) |
| Level of thirst (#8) | 52 (22–92) | 41 (6–67) | 43 (16–67) |
| MRI-evaluated volume of SMG (cm3)b | |||
| MRI volume of left SMG | 5.3 (3.8–6.0) | 5.7 (4.6–7.9) | 5.5 (4.2–6.9) |
| MRI volume of right SMG | 5.5 (3.8–6.3) | 6.2 (4.0–6.5) | 5.8 (4.0–6.5) |
Abbreviations: Gy, gray; mITT, modified intention-to-treat; MRI, magnetic resonance imaging; RT, radiation therapy; SMG, submandibular gland; VAS, visual analog scale; XQ, Xerostomia Questionnaire.
aValues are reported as means (SD) unless otherwise stated.
bValues are reported as medians with IQRs.
Safety
Safety data were obtained from all 30 patients in the mITT population (Table 2). No patients experienced treatment-related or unexpected SAEs and no patients died. Between baseline and follow-up, an SAE was experienced by 6 (40%) patients in the ASC group and 5 of 15 patients (33%) in the placebo group. These figures correspond to an exact OR of 1.32 (95% CI: 0.20–7.67, P > 0.71). One patient (∼7%) with a previous OPSCC T1N2bM0 from the ASC group was diagnosed with a local recurrence—a squamous cell carcinoma in the ipsilateral mandible—during surgery for osteoradionecrosis (ORN) 7 months after the ASC treatment. The patient was diagnosed with persistent advanced ORN of the mandible before enrollment in the trial. The same patient received intravenous treatment for mucosal candidiasis months later. One patient with previous OPSCC T1N1M0 was treated with bevacizumab 7 years after radiotherapy for severe treatment juvenile-onset recurrent respiratory papillomatosis and was diagnosed with osteonecrosis of the hyoid bone. One patient (∼7%) in the placebo group was diagnosed with severe periodontal disease requiring surgical intervention. Two patients (∼13%) from the ASC group and one (∼7%) from the placebo group were diagnosed with a new non–head and neck cancer. No treatment-related adverse events comprising infection, oral discomfort, or prolonged pain were found.
Table 2.
Primary and secondary outcomes at 2 years or more after randomization in the mITT population.
| ASCs (n = 15) | Placebo (n = 15) | Difference | (95% CI) | P | |
|---|---|---|---|---|---|
| Variablea | |||||
| Primary outcome (composite outcome): | |||||
| Serious adverse events, patients, n (%) | 6 (40%) | 5 (33%) | OR = 1.32 | (0.24–7.67) | >0.70b |
| SAE components, n (%) | |||||
| Treatment-related SAEs | 0 (-) | 0 (-) | — | ||
| Death | 0 (-) | 0 (-) | — | ||
| Relapse | 1 (7%) | 0 (-) | — | ||
| New primary head and neck cancer | 0 (-) | 0 (-) | — | ||
| New primary cancer (not head and neck) | 2 (13%) | 1 (7%) | — | ||
| New osteonecrosis | 1 (7%) | 0 (-) | — | ||
| Oral infection (IV treated) | 1 (7%) | 0 (-) | — | ||
| Neurologic disorder | 1 (7%) | 1 (7%) | — | ||
| Cardiac disease | 0 (-) | 0 (-) | — | ||
| Other SAEs | 3 (20%) | 4 (27%) | — | ||
| Other SAEs | |||||
| Urinary disorder (cystocele) | 1 (7%) | 0 (-) | — | ||
| Fracture | 0 (-) | 2 (13%) | — | ||
| Recurrent laryngeal nerve palsy | 1 (7%) | 0 (-) | — | ||
| Fibroma cervicalis | 0 (-) | 1 (7%) | — | ||
| Pancreatitis | 1 (7%) | 0 (-) | — | ||
| Periodontal disease (severe) | 0 (-) | 1 (7%) | — | ||
| Disease in the submandibular glands, n (%) | 0 (-) | 0 (-) | |||
| Secondary outcomes | |||||
| Whole saliva production (mL/minute): | |||||
| Unstimulated whole saliva flow rate | 0.20 (±0.02) | 0.16 (±0.02) | 0.05 | (0.00 to 0.10) | 0.051 |
| Stimulated whole saliva flow rate | 1.13 (±0.09) | 1.16 (±0.09) | −0.03 | (−0.29 to 0.24) | 0.85 |
| Patient-reported complaints of xerostomia: | |||||
| XQ, summary score (scale: 0–100) | 35.0 (±2.79) | 45.1 (±2.87) | −10.1 | (−18.1 to −2.2) | 0.013 |
| VAS, VAS score (scale: 0–100) | |||||
| Difficulty speaking (#1) | 23 (±4.4) | 25 (±4.4) | −2.2 | (−14.7 to 10.3) | 0.72 |
| Difficulty swallowing (#2) | 35 (±5.2) | 49 (±5.2) | −15.0 | (−29.2 to 0.2) | 0.053 |
| Amount of saliva (#3) | 42 (±5.2) | 49 (±5.2) | −6.0 | (−20.6 to 8.5) | 0.41 |
| Dry mouth (#4) | 43 (±4.7) | 48 (±4.7) | −5.1 | (−18.3 to 8.2) | 0.45 |
| Dry throat (#5) | 44 (±4.3) | 48 (±4.3) | −4.0 | (−15.8 to 8.3) | 0.54 |
| Dry lips (#6) | 32 (±5.0) | 47 (±5.0) | −12.5 | (−26.5 to 1.4) | 0.077 |
| Dry tongue (#7) | 30 (±3.6) | 38 (±3.6) | −7.3 | (−17.5 to 2.9) | 0.16 |
| Level of thirst (#8) | 29 (±4.8) | 54 (±4.8) | −24.9 | (−38.4 to −11.3) | <0.001 |
| MRI-evaluated volume of SMG (cm3) | |||||
| MRI volume of left SMG | 6.3 (±0.17) | 6.8 (±0.17) | −0.45 | (−0.93 to 0.03) | 0.066 |
| MRI volume of right SMG | 5.7 (±0.15) | 5.5 (±0.16) | 0.19 | (−0.25 to 0.63) | 0.40 |
| Median time from baseline, days | 1,247 (1,064–1,399) | 1,307 (1,186–1,429) | −61 | (−212 to 90) | 0.43 |
Abbreviations: CI, confidence interval; IV, intravenously; MRI, magnetic resonance imaging; SAE, serious adverse event; SMG, submandibular gland; VAS, visual analog scale; XQ, Xerostomia Questionnaire.
aValues are reported as least squares means ± (SE) and differences presented with 95% CIs unless otherwise stated. Medians are reported with lower and upper quartile.
bCalculated on the basis of a χ2 test compared with a Fisher exact (two-sided) test, P = 1.00.
Key efficacy trajectories
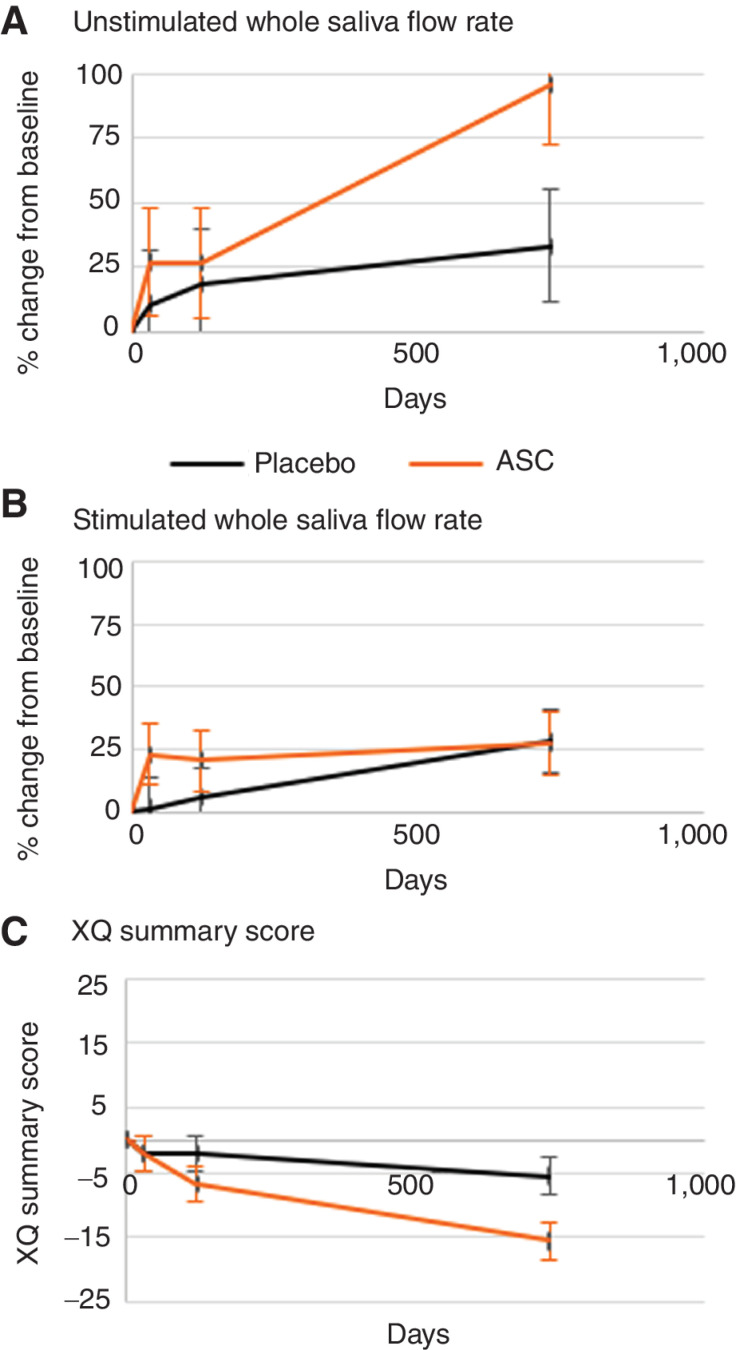
Of the 30 participants, we obtained all measures from 27 patients: 14 from the ASC group (93%) and 13 from the placebo group (87%). The trajectories for the key secondary outcomes are illustrated in Fig. 2, with the long-term effectiveness data after at least 2 years presented in Table 2. At long-term follow-up, unstimulated whole saliva flow rate in the ASC group was 0.20 compared with 0.16 mL/minute in the placebo group, corresponding to a difference between the least squares means of 0.05 mL/minute (95% CI: 0.00–0.10; P = 0.051). This is also illustrated in Fig. 2, where the trajectory of the relative increase was 95% (SE ± 21.7) in unstimulated whole saliva flow rate in the ASC group from baseline compared with an increase of 33% (SE ± 22.5) in the placebo group (Fig. 2A). The stimulated whole saliva flow rate was 1.13 (±0.09) mL/minute in the ASC group and 1.16 (±0.09) mL/minute in the placebo group at long-term follow-up with a difference of −0.03 mL/minute (95% CI: −0.29 to 0.24; P = 0.85) between the groups. Figure 2B illustrates the trajectory of the relative increase in stimulated whole saliva flow rate of 28% (SE ± 12.7) in the placebo group and 27% (SE ± 12.7) in the ASC group compared with baseline (Fig. 2B).
Figure 2.
Key secondary efficacy endpoints. A, Relative change in unstimulated whole saliva flow rate from baseline in percent. B, Relative change in stimulated whole saliva flow rate from baseline in percent. C, Change in Xerostomia Questionnaire (XQ) summary score from baseline.
Patient-reported outcomes
Of the 30 participants, 28 patients (14 from each group) had complete patient-reported outcomes. Patients in the ASC group reported an XQ summary score of 35.0 (±2.79) and the placebo group, a score of 45.1 (± 2.87), corresponding to a significant difference between the groups of −10.1 (95% CI: −18.1 to −2.2; P = 0.013; Fig. 2C). When side effects were evaluated according to the VAS scores, only the item covering the level of thirst (item 8) showed a statistically significant difference between the groups at long-term follow-up. In the ASC group, the level of thirst was 29 (± 4.8) compared with 54 (± 4.8) in the placebo group, corresponding to a significant difference of 24.9 units (95% CI: −38.4 to −11.3; P < 0.001) between the groups in favor of ASC (Table 2).
MRI assessments
In the ASC group, all 15 patients had scans performed: 14 had MRI scans of the neck and 1 had a CT scan of the neck. Thirteen patients from the placebo group underwent MRI scans. No structural changes or signs of salivary gland disease were observed in either group, nor did salivary glands volume differ between the groups (Table 2).
Discussion
This randomized, placebo-controlled trial reveals the first-in-human long-term safety and efficacy evidence of intraglandular injections into the submandibular glands with autologous culture–expanded ASCs in an attempt to restore and alleviate radiation-induced xerostomia and salivary gland hypofunction. Our results demonstrate that intraglandular injections with autologous ASCs in previous patients with HPV+OPSCC are safe as no treatment-related SAEs were identified, no patients died, and no MRI-detected signs of changes (size, structure, fat distribution) within the submandibular glands occurred. One patient in the ASC group was diagnosed with a local recurrence in the ipsilateral mandible during surgery 7 months after the ASC treatment for an advanced ORN known prior to the trial, and presumably the patient had developed the cancer before entering the trial. Six patients (6/15, 40%) experienced an SAE during the follow-up compared with 5 patients in the placebo group (5/15, 33%), corresponding to an exact OR of 1.32 (95% CI: 0.20–7.67; P > 0.71). The number of SAEs in both groups may seem high, but the patients were older (mean age, 59.3 at enrollment), predominately male (63%), 37% were previous smokers, and all had a previous history of cancer, all of which increases the risk of SAEs. In addition, Denmark has a public health care system, and all treatments are documented in electronic medical records, which may also have contributed to the high number of recorded SAEs.
On the basis of preclinical findings, we do not expect ASCs to differentiate or survive for a longer time (4–8). We do, however, expect the ASCs to support the innate regeneration processes within the submandibular glands. We find it highly unlikely that the ASC treatment is related to the onset of a new squamous cell carcinoma outside the submandibular glands.
A noteworthy finding was the statistically significant decrease in patient-reported symptoms of xerostomia in the ASC group as compared with the placebo group for the XQ summary score. For the subdomains collected with the VAS patient-reported outcomes, level of thirst differed significantly between the groups at long-term follow-up. The decreased xerostomia symptoms observed in the placebo arm are notable, because further substantial reduction in xerostomia-related symptoms or improvement in salivary gland function would not be expected exceeding a time span of 2 years after radiotherapy. However, this observation may suggest that a basic level of normal recovery from radiation damage might have been partly in progress in both groups and enhanced in the intervention arm. Symptoms of xerostomia and objective signs of reduced salivary flow rate are often weakly correlated (16, 22).
In this trial, however, the improvement in xerostomia at the long-term follow-up was paralleled by a clinically relevant increase in unstimulated whole saliva flow rate of 95% in the ASC group (increasing to 0.20 mL/minute) compared with a 33% increase (to 0.16 mL/minute) in the placebo group. Normal flow rates for unstimulated whole saliva are between 0.3 and 0.5 mL/minute; salivary gland hypofunction is defined as ≤0.2 mL/minute and hyposalivation as ≤0.10 mL/minute (1, 23). Thus, while an arbitrary cut-off value for the diagnosis of hyposalivation has been agreed upon, a clinically meaningful increase in unstimulated whole saliva flow rate has yet to be defined and would depend on a variety of objective and subjective parameters, here among the increase in salivary flow rate relative to the baseline flow rate and the variation in each individual patients’ threshold to the symptoms of xerostomia. Likewise, a clinically significant difference in the patient-reported outcome measure XQ summary score has not been defined.
At long-term follow-up, the unstimulated whole saliva flow rate for the ASC group was just at the limit of salivary gland hypofunction and was lower for the placebo group. The 33% increase of unstimulated whole saliva flow rate in the placebo group illustrates that the patients in this study experienced an ongoing natural repair of salivary gland function by endogenous stem cells years after undergoing radiotherapy, the capacity of which depends on the number of functional residual salivary stem cells surviving the total radiation dose to the salivary glands. This is consistent with results from studies following the first two years after irradiation (24, 25). Consequently, our results support that ASCs potentially assist innate tissue repair mechanisms. No difference within the stimulated whole saliva flow rate was seen between the two groups at long-term follow-up. This result was not surprising given that the intervention (ASC/placebo) was not provided to the parotid glands, which are responsible for approximately 50% of stimulated whole saliva secretion (1). We measured only whole saliva flow rates because submandibular gland flow rate is technically challenging to evaluate and is associated with the risk of several confounders, especially because more invasive measurements are not recommended for irradiated patients.
As our study is the first to present long-term safety and efficacy results of intraglandular ASC treatment, direct comparisons cannot be made with evidence from other trials.
This study has potential limitations. In accordance with the initial protocol, the study was unblinded after 4 months, which is a major limitation for the long-term follow-up especially for the reporting of xerostomia-related symptoms (i.e., potential performance bias). However, the results from the xerostomia questionnaires resemble both the objective changes in unstimulated whole saliva flow rate found at the long-term follow-up and the patient-reported outcomes reported before unmasking at 4 months. The radiologists and the assessor performing the sialometry were blinded to baseline values. Another limitation is the inclusion of only previous patients with HPV+ OPSCC. We observed a small but significant difference in the T and N categorization between the intervention and placebo arm that favored the intervention arm. We deem this unlikely to have impacted the results of this trial as there is no difference between the groups when comparing the radiation of the submandibular glands (measured in Gy). As the study only included 30 patients, no subanalysis was planned. In future larger trials, it would be interesting to investigate whether factors such as age, radiation dose to the glands, and time from radiotherapy to the ASC treatment affect the efficacy. Finally, no tissue biopsies were performed as we found it unethical to perform histologic biopsies from the placebo group again. However, histologic and proteomic analysis of the salivary gland tissue may provide information of changes induced by the ASC treatment in future studies.
In conclusion, our study is the first to provide a prospective long-term follow-up on autologous ASC therapy for radiation-induced salivary gland damage in humans. The study demonstrates that intraglandular injections with autologous ASCs are safe and have the potential to offer a clinically relevant restoring effect of increasing unstimulated whole saliva flow rate and decreasing xerostomia. Additional studies of this intervention in all types of head and neck cancer patients with radiation-induced salivary gland hypofunction and xerostomia are warranted.
Supplementary Material
Acknowledgments
The authors thank the patients who have participated in the study and the laboratory staff at the Cell Therapy Facility at the Blood Bank, Rigshospitalet.
This study was supported by the non-profit organization Candys Foundation (C.D. Lynggaard and C. Grønhøj). Section for Biostatistics and Evidence-Based Research, the Parker Institute is supported by a core grant from the Oak Foundation, a non-profit foundation (grant OCAY-18-774-OFIL). The funders of the study had no role in the design of the study, data collection, analysis, interpretation of the data, or any part in the article. The content of this article is solely the responsibility of the authors.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This article is featured in Highlights of This Issue, p. 2717
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
C.D. Lynggaard reports grants from Candys Foundation during the conduct of the study. C.D. Lynggaard, C. Grønhøj, and C. von Buchwald are co-inventors on patent PCT/EP2020/053878; the patent is owned by Rigshospitalet, Copenhagen University Hospital and the University of Copenhagen. A. Fischer-Nielsen is employee and shareholder of StemMedical A/S, a biotech company working with cell-enriched fat grafting. C. Grønhøj reports a patent for 5281-0127/18-7000 pending. L. Specht reports personal fees from Takeda, Kyowa Kirin, and MSD; grants from Varian, ViewRay, and Danish Cancer Society; and other support from Springer Verlag and Munksgaard Publishing outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
C.D. Lynggaard: Conceptualization, data curation, software, formal analysis, funding acquisition, validation, investigation, methodology, writing–original draft, project administration. C. Grønhøj: Conceptualization, resources, data curation, funding acquisition, investigation, methodology, project administration, writing–review and editing. S.B. Jensen: Conceptualization, resources, supervision, validation, investigation, methodology, writing–original draft, writing–review and editing. R. Christensen: Data curation, software, formal analysis, supervision, methodology, writing–review and editing. L. Specht: Conceptualization, supervision, methodology, writing–review and editing. E. Andersen: Conceptualization, resources, methodology, writing–review and editing. T.T. Andersen: Data curation, software, formal analysis, methodology, writing–review and editing. U.M. Ciochon: Data curation, software, formal analysis, methodology, writing–review and editing. G.S. Rathje: Conceptualization, software, supervision, writing–review and editing. A.E. Hansen: Conceptualization, resources, data curation, software, methodology, writing–review and editing. H. Stampe: Resources, visualization, project administration, writing–review and editing. A. Fischer-Nielsen: Conceptualization, resources, data curation, supervision, validation, investigation, methodology, project administration, writing–review and editing. C. von Buchwald: Conceptualization, resources, data curation, software, supervision, funding acquisition, validation, investigation, methodology, writing–original draft, project administration, writing–review and editing.
References
- 1. Mercadante V, Jensen SB, Smith DK, Bohlke K, Bauman J, Brennan MT, et al. Salivary gland hypofunction and/or xerostomia induced by nonsurgical cancer therapies: ISOO/MASCC/ASCO guideline. J Clin Oncol 2021;39:2825–43. [DOI] [PubMed] [Google Scholar]
- 2. Borras JM, Barton M, Grau C, Corral J, Verhoeven R, Lemmens V, et al. The impact of cancer incidence and stage on optimal utilization of radiotherapy: methodology of a population based analysis by the ESTRO-HERO project. Radiother Oncol 2015;116:45–50. [DOI] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 4. Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med 2019;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol 2011;6:457–78. [DOI] [PubMed] [Google Scholar]
- 6. Kojima T, Kanemaru S-I, Hirano S, Tateya I, Ohno S, Nakamura T, et al. Regeneration of radiation damaged salivary glands with adipose-derived stromal cells. Laryngoscope 2011;121:1864–9. [DOI] [PubMed] [Google Scholar]
- 7. Lim J-Y, Yi T, Choi J-S, Jang YH, Lee S, Kim HJ, et al. Intraglandular transplantation of bone marrow-derived clonal mesenchymal stem cells for amelioration of post-irradiation salivary gland damage. Oral Oncol 2013;49:136–43. [DOI] [PubMed] [Google Scholar]
- 8. Choi J-S, An H-Y, Shin H-S, Kim Y-M, Lim J-Y. Enhanced tissue remodelling efficacy of adipose-derived mesenchymal stem cells using injectable matrices in radiation-damaged salivary gland model. J Tissue Eng Regen Med 2018;12:e695–706. [DOI] [PubMed] [Google Scholar]
- 9. Rezvani M. Therapeutic potential of mesenchymal stromal cells and extracellular vesicles in the treatment of radiation lesions-a review. Cells 2021;10:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double-blind controlled trial. Lancet 2016;388:1281–90. [DOI] [PubMed] [Google Scholar]
- 11. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008;371:1579–86. [DOI] [PubMed] [Google Scholar]
- 12. Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (Prochymal) after acute myocardial infarction. J Am Coll Cardiol 2009;54:2277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Karantalis V, Difede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: the prospective randomized study of mesenchymal stem cell therapy in patients undergoing cardiac surgery (PROMETHEUS) trial. Circ Res 2014;114:1302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hare JM, Difede DL, Rieger AC, Florea V, Landin AM, El-Khorazaty J, et al. Randomized comparison of allogeneic vs. autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. J Am Coll Cardiolol 2017;69:526–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grønhøj C, Jensen DH, Vester-Glowinski P, Jensen SB, Bardow A, Oliveri RS, et al. Safety and efficacy of mesenchymal stem cells for radiation-induced xerostomia: a randomized, placebo-controlled phase 1/2 trial (MESRIX). Int J Radiat Oncol Biol Phys 2018;101:581–92. [DOI] [PubMed] [Google Scholar]
- 16. Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys 2001;50:695–704. [DOI] [PubMed] [Google Scholar]
- 17. Pai S, Ghezzi EM, Ship JA. Development of a visual analogue scale questionnaire for subjective assessment of salivary dysfunction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001;91:311–6. [DOI] [PubMed] [Google Scholar]
- 18. Navazesh M, Kumar SKS. Measuring salivary flow. J Am Dent Assoc 2014;139:35S–40S. [DOI] [PubMed] [Google Scholar]
- 19. Navazesh M, Christensen CM. A comparison of whole mouth resting and stimulated salivary measurement procedures. J Dent Res 1982;61:1158–62. [DOI] [PubMed] [Google Scholar]
- 20. Detry MA, Lewis RJ. The intention-to-treat principle: how to assess the true effect of choosing a medical treatment. JAMA 2014;312:85–6. [DOI] [PubMed] [Google Scholar]
- 21. Detry MA, Ma Y. Analyzing repeated measurements using mixed models. JAMA 2016;315:407–8. [DOI] [PubMed] [Google Scholar]
- 22. Dawes C. How much saliva is enough for avoidance of xerostomia? Caries Res 2004;38:236–40. [DOI] [PubMed] [Google Scholar]
- 23. Villa A, Connell C, Abati S. Diagnosis and management of xerostomia and hyposalivation. Ther Clin Risk Manag 2014;11:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jensen SB, Vissink A, Limesand KH, Reyland ME. Salivary gland hypofunction and xerostomia in head and neck radiation patients. J Natl Cancer Inst Monogr 2019;2019:lgz016. [DOI] [PubMed] [Google Scholar]
- 25. Burlage FR, Coppes RP, Meertens H, Stokman MA, Vissink A. Parotid and submandibular/sublingual salivary flow during high dose radiotherapy. Radiother Oncol 2001;61:271–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Full dataset and software code to replicate the main analysis is available from the corresponding author on reasonable request.