Abstract
Purpose:
Targeting CD79B using antibody–drug conjugates (ADC) is an effective therapeutic strategy in B-cell non-Hodgkin lymphoma (B-NHL). We investigated DCDS0780A, an anti-CD79B ADC with THIOMAB technology (TDC) that consistently conjugates two anti-neoplastic molecules per antibody, in contrast with ADCs with heterogeneous loads.
Patients and Methods:
This phase 1 study enrolled 60 patients with histologically confirmed B-NHL that had relapsed/failed to respond following ≥1 prior treatment regimens; 41 (68%) had diffuse large B-cell lymphoma (DLBCL). Fifty-one patients received DCDS0780A monotherapy once every 3 weeks (0.3–4.8 mg/kg); 9 received combination therapy (3.6–4.8 mg/kg) with rituximab.
Results:
Fifty-four (90%) patients experienced adverse events related to study drug, the most common of which were blurred vision, fatigue, corneal deposits, neutropenia, nausea, and peripheral neuropathy. 4.8 mg/kg was the highest dose tested and the recommended phase II dose. The pharmacokinetic profile was linear at doses ≥1.2 mg/kg. Response rate in all-treated patients (N = 60) was 47% (n = 28), including 17 complete responses (28%) and 11 partial responses (18%). The median duration of response (15.2 months) was the same for all responders (n = 28) and patients with DLBCL (n = 20).
Conclusions:
DCDS0780A as the TDC format for CD79B was tested at higher doses than its ADC counterpart investigated earlier, leading to deep responses. However, dose intensity was limited by ocular toxicities seen at the higher doses indicating that the TDC format was unable, in the current study, to expand the therapeutic index for the CD79B target. The encouraging antitumor activity advocates continuation of investigations into novel ADC technologies.
Translational Relevance.
Studies with the antibody–drug conjugate (ADC) polatuzumab vedotin have clinically validated CD79B as a useful target for the delivery of microtubule-disrupting agents, such as monomethyl auristatin E (MMAE), to the malignant cell. This study investigated DCDS0780A, an ADC containing humanized IgG1 anti-human CD79B monoclonal antibody (MCDS0593A) conjugated to MMAE via a protease labile linker. DCDS0780A uses novel THIOMAB technology (TDC) to consistently conjugate two MMAE molecules per antibody using engineered cysteine residues, in contrast with polatuzumab vedotin and other ADCs with heterogeneous numbers of drug molecules per antibody. TDC technology enabled dose escalation that led to improved efficacy in B-cell non-Hodgkin lymphoma. However, cumulative ocular toxicity occurred beyond the dose-limiting toxicity evaluation period. This study highlights the promise of novel ADC linker technology but also the need for further refinements to ensure clinical tolerability.
Introduction
CD79B is a cell-surface antigen expressed on mature B cells but absent on plasma cells (1). A majority of mature malignancies of B-cell origin, including non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia, express CD79B and demonstrate rapid internalization of anti-CD79B antibodies (2–4). Studies with polatuzumab vedotin, an antibody–drug conjugate (ADC) targeting CD79B for delivery of the microtubule-disrupting agent—monomethyl auristatin E (MMAE)—have clinically validated CD79B as a useful target for transporting cytotoxic agents to the malignant cell (5–7). Polatuzumab vedotin has been approved for the treatment of relapsed/refractory diffuse large B-cell lymphoma (DLBCL; ref. 8). DCDS0780A is another ADC that uses humanized IgG1 anti-human CD79B monoclonal antibody (MCDS0593A) conjugated to MMAE via a protease labile linker [maleimidocaproyl valine citrulline p aminobenzoyloxycarbonyl (MC-vc-PAB; ref. 9)]. DCDS0780A uses novel THIOMAB technology (TDC) to consistently conjugate two MMAE molecules per antibody using engineered cysteine residues—in contrast with other ADCs (including polatuzumab vedotin) that have a heterogeneous number of drug molecules per antibody (9, 10). Following internalization, TDC like other ADCs, is cleaved by lysosomal enzymes to release MMAE, which binds to tubulin and disrupts the microtubule network, resulting in inhibition of cell division and cell growth (11). With polatuzumab vedotin already demonstrating antitumor activity in B-cell NHL (B-NHL; ref. 8), the objective of the current study was to determine whether the therapeutic index of CD79B could be improved with a consistent payload of drug:antibody in a 2:1 ratio using the TDC conjugation platform. We undertook a phase I dose-escalation study of DCDS0780A as monotherapy and in combination with rituximab in patients with relapsed or refractory B-NHL.
Patients and Methods
Study design
This open label, multicenter, phase Ia/Ib study of DCDS0780A was designed to evaluate the safety, tolerability, pharmacokinetics (PK), and recommended phase II dose (RP2D) according to a 3+3 dose-escalation scheme. The phase Ia portion evaluated DCDS0780A monotherapy in escalation cohorts with a starting dose of 0.3 mg/kg administered intravenously once every 3 weeks (Q3W; 1 cycle = 21 days). The phase Ib portion evaluated DCDS0780A in dose-escalation cohorts starting at one dose level below that tolerated by completed monotherapy cohorts, and in combination with a fixed dose of rituximab (375 mg/m2). In phase Ib, rituximab was dosed on day 1 of every cycle; DCDS0780A was dosed on day 2 in the first two cycles and on day 1 thereafter in the absence of infusion-related reactions. Both monotherapy and combination treatment expansion cohorts were enrolled to further evaluate safety. Patients exhibiting acceptable safety and evidence of clinical benefit were permitted to receive DCDS0780A every 3 weeks for up to approximately 1 year or until disease progression or unacceptable toxicity.
The study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. Approval from the institutional review boards and ethics committees was obtained before study start. Written informed consent was obtained for all patients before performing study-related procedures in accordance with federal and institutional guidelines. The study was registered on ClinicalTrials.gov (NCT02453087).
Patients
Patients with histologically confirmed B-NHL that had relapsed after or failed to respond to at least one prior treatment regimen and for which no suitable therapy of curative intent or higher priority existed were eligible to enroll. Other inclusion criteria were: Age ≥18 years; ≥1 lesion measurable by computerized tomography scan (>1.5 cm); Eastern Cooperative Oncology Group Performance Status of 0 or 1; fasting (≥8 hours) glucose ≤160 mg/dL and stable regimen of diabetes medication if necessary; and adequate hematologic function without growth factor or transfusion support unless due to underlying disease (platelet count ≥75×109/L; absolute neutrophil count ≥1.5×109/L; hemoglobin >9 g/dL). Exclusion criteria were any one of the following: Prior use of monoclonal antibody therapy within 4 weeks to study start; treatment with radiotherapy, chemotherapy, systemic steroids used as an anti-neoplastic agent, or any investigational anticancer agent within 2 weeks before study start; allogeneic stem cell transplant; autologous stem cell transplant within 100 days; CNS lymphoma; clinically significant pulmonary disease or history of liver disease; peripheral neuropathy of grade >1; or prior lung radiation.
Safety
Adverse event (AE) severity grading scale defined by the National Cancer Institute Common Terminology Criteria for Adverse Events (v4.0) was used for categorizing AE grades in this study. Dose-limiting toxicity (DLT) was defined as an occurrence of any of the following AEs during the first cycle (unless attributed by the investigator to another clearly identifiable cause such as documented disease progression, concomitant medication, or pre-existing medical condition): Grade ≥3 non-hematologic and/or non-hepatic AE that required medical intervention (excluding hyperglycemia that resolved to grade 2 within 7 days after therapeutic intervention; alopecia of any grade; nausea, vomiting, or diarrhea that responded to supportive therapy within 72 hours; reversible non-allergic infusion toxicities; and asymptomatic laboratory abnormalities considered not clinically significant); grade 4 neutropenia that did not improve within a week and was accompanied by fever; grade ≥3 febrile neutropenia; grade 4 anemia; grade 4 thrombocytopenia accompanied by clinically significant bleeding that did not improve within 1 week; grade ≥3 elevation of serum hepatic transaminase lasting >7 days; or new onset of clinically significant respiratory symptoms.
Dose delays and reductions were permitted to mitigate AEs. Dosing was delayed for up to 2 weeks and could resume if all toxicities had resolved to grade ≤2 (or ≥80% of the baseline value in the case of hematologic toxicities, whichever was lower) within 2 weeks or to grade ≤1 for peripheral neuropathy. The dose of subsequent infusions of DCDS0780A could be reduced to that of the previous cohort or by approximately 30%−50%. Any patient in whom similar toxicity recurred at the reduced dose was discontinued from treatment with DCDS0780A.
PKs and immunogenicity
Serum concentrations of total antibody (conjugated and unconjugated antibody) were determined using a validated ELISA. The minimum quantifiable concentration of the assay was 75 ng/mL. Conjugate (antibody-conjugated MMAE, acMMAE) was measured in plasma samples using a validated immunoaffinity LC-MS/MS assay with a lower limit of quantitation (LLOQ) of 0.350 ng/mL. Unconjugated MMAE was measured in plasma samples using a validated LC-MS/MS assay with an LLOQ of 0.0359 ng/mL.
PK parameters for the total antibody, conjugate, and the unconjugated MMAE after the first dose of DCDS0780A in cycle 1 (once every 3-week schedule) were estimated by standard non-compartmental analysis (NCA) using WinNonlin 5.2.1 software (Pharsight). At subsequent cycles, the peak and trough (pre-infusion) concentrations were summarized using descriptive statistics.
Serum anti-therapeutic antibody (ATA) samples were collected at pre-dose from cycles 1 to 5 and at study completion/early termination from all treated patients and were analyzed using a validated bridging antibody ELISA. Positive antibody responses were further characterized by competitive binding to determine whether the response was primarily directed against the antibody or the linker-drug portion of the ADC.
Biomarkers
Tumor samples were retrospectively analyzed for expression of CD79B by IHC using the antibody clone AT107–2 (Serotec MCA2209). Baseline patient tumor samples were scored for CD79B in >50% of tumor cells with IHC = 3+ for strong staining intensity, IHC = 2+ for moderate staining intensity, IHC = 1+ for weak staining intensity; and IHC = 0 for samples with very weak or no staining.
Cell of origin (COO) was determined by targeted gene expression of archival formalin-fixed paraffin-embedded tissue specimens by quantitative RT-PCR using the Luminex Fluidigm platform and linear predictor score described previously (12).
Antitumor activity outcome measures
CT scans used for response assessment were performed at screening, at 6–8 weeks following C1D1, every 3 months thereafter, and at treatment completion/end of treatment (ET) visit. Patients who discontinued treatment for reasons other than disease progression were additionally followed for response for up to 1 year (2, 4, 6, 9, and 12 months after ET visit) or until progressive disease or initiation of another therapy. CT or combined PET/CT scans without contrast or MRI scans were permitted in patients for whom contrast was contraindicated. For patients with DLBCL, PET scans in conjunction with CT scans were required at screening, at the second post-screening assessment, and to confirm a complete response (CR). Objective response and disease progression were determined by the investigator using the 2014 Lugano Classification (13). Patients were evaluated for objective responses, duration of objective responses, progression-free survival (PFS), and relative dose intensity. Medians were based on the assessment date of the response data records, and ongoing duration of response (DoR) was assessed at the clinical data cutoff date.
Statistical analyses
The planned enrollment for phase Ia was approximately 24–72 patients, including expansion of certain dose levels to further evaluate safety, tolerability, and preliminary estimate of response rate as part of the process for selecting RP2D. The planned enrollment for phase Ib was approximately 9–36 patients. The sample size was based on numbers needed to obtain sufficient safety, activity, and PK data for subsequent clinical testing of DCDS0780A, and not on explicit assumptions about power and type I error. Patients who received any amount of DCDS0780A were evaluable for safety analyses. NCA for PK parameters was done with WinNonlin version 5.2.1. PFS and DoR were analyzed by the Kaplan–Meier method. Statistical analyses and creation of graphs were done with SAS version 9.4.
Data availability statement
For eligible studies, qualified researchers may request access to individual patient level clinical data through a data request platform. At the time of writing, this request platform is Vivli: https://vivli.org/ourmember/roche/.
For up to date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing.
Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.
Results
Patient population
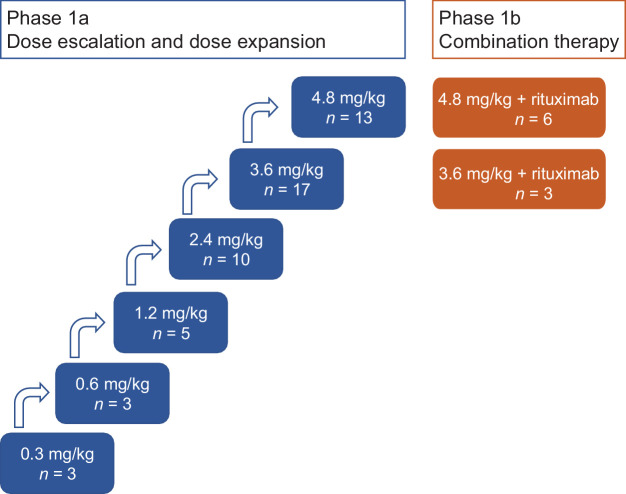
Between August 2015 and July 2019, 51 patients were enrolled in dose-escalation and expansion cohorts for DCDS0780A monotherapy (0.3–4.8 mg/kg), and 9 patients in combination therapy cohorts (DCDS0780A at 3.6 and 4.8 mg/kg with rituximab at 375 mg/m2; Fig. 1), at 7 clinical sites throughout the US. Patient demographic and baseline characteristics are summarized in Table 1. Two patients, both in the 3.6 mg/kg cohort, had received prior chimeric antigen receptor (CAR) T-cell therapy. None of the patients had prior CD79B-targeted therapy (including polatuzumab vedotin), although it was not an exclusion criterion. Patient sub-types for NHL included DLBCL (n = 41), follicular lymphoma (FL, n = 12), marginal zone lymphoma (MZL, n = 5), and mantle cell lymphoma (MCL, n = 2). At the data cutoff date of October 2019 for safety analysis, all patients had discontinued the study (Supplementary Fig. S1) due to lack of efficacy/disease progression (n = 30), other reasons (n = 14), death (n = 4), patient withdrawal (n = 4), physician decision (n = 4), AE (n = 3), and lost to follow-up (n = 1). Of the 14 patients who discontinued the study for “other reasons,” 10 patients completed the 12-month follow-up after completing/stopping treatment and 4 patients started other treatments. The data cutoff date for immunogenicity assessment was January 2018.
Figure 1.
Study scheme for the investigation of DCDS0780A monotherapy (phase 1a) and combination therapy (phase 1b) with rituximab (375 mg/m2).
Table 1.
Patient demographics and baseline characteristics.
| DCDS0780A <2.4 mg/kg monotherapy (n = 11) | DCDS0780A ≥2.4 mg/kg monotherapy (n = 40) | DCDS0780A ≥3.6 mg/kg + rituximab (n = 9) | Total N = 60 | |
|---|---|---|---|---|
| Age, y | ||||
| Median | 73 | 66 | 71 | 68 |
| Range | 60–86 | 32–85 | 44–82 | 32–86 |
| Sex, n (%) | ||||
| Female | 6 (55) | 16 (40) | 6 (67) | 28 (47) |
| Male | 5 (45) | 24 (60) | 3 (33) | 32 (53) |
| Race, n (%) | ||||
| Asian | 1 (9) | 4 (10) | 0 | 5 (8) |
| Black | 2 (18) | 0 | 1 (11) | 3 (5) |
| White | 8 (73) | 33 (83) | 7 (78) | 48 (80) |
| Other | 0 | 3 (8) | 1 (11) | 4 (7) |
| ECOG performance Status, n (%) | ||||
| 0 | 1 (9) | 9 (23) | 0 | 10 (17) |
| 1 | 10 (91) | 31 (78) | 9 (100) | 50 (83) |
| Disease type, n (%) | ||||
| DLBCL | 6 (55) | 27 (68) | 8 (89) | 41 (68) |
| FL | 3 (27) | 9 (23) | 0 | 12 (20) |
| MZL | 2 (18) | 2 (5) | 1 (11) | 5 (8) |
| MCL | 0 | 2 (5) | 0 | 2 (3) |
| No. prior systemic therapies | ||||
| Median | 3 | 3 | 3 | 3 |
| Range | 1–5 | 1–13 | 1–11 | 1–13 |
| Prior rituximab therapy | ||||
| Patients n (%) | 11 (100) | 39 (98) | 9 (100) | 59 (98) |
| Prior stem cell transplant | ||||
| Patients n (%) | 2 (18) | 1 (3) | 3 (33) | 6 (10) |
| Refractory to last therapy | ||||
| Patients n (%) | 8 (73) | 28 (70) | 6 (67) | 42 (70) |
| Prior radiotherapy | ||||
| Patients n (%) | 3 (27) | 8 (20) | 2 (22) | 13 (22) |
Study drug exposure
Across the cohorts, the median number of DCDS0780A doses received was 4 (range, 1–21) cycles in the monotherapy arm, and 5 (range, 3–13) cycles of DCDS0780A and rituximab in the combination therapy arm.
Safety
Sixty patients who received at least one dose of study drug were safety evaluable. Overall, all 60 (100%) patients experienced at least one AE regardless of relationship to study drug (Supplementary Table S1). AE profiles were similar between DCDS0780A monotherapy at ≥2.4 mg/kg and DCDS0780A + rituximab. Fifty-four (90%) patients experienced AEs of any grade related to study drug, the most common of which were blurred vision (35%), fatigue (28%), corneal deposits (28%), neutropenia (27%), nausea, and peripheral neuropathy (20% each; Table 2). Grade ≥3 AEs in ≥3 (5%) patients regardless of relationship to study drug included neutropenia (23%), hypercalcemia (5%), thrombocytopenia (5%), and white blood cell count decreased (5%).There was one death due to disease progression. Three deaths not due to disease progression were due to grade 5 events of hypoxic ischemic encephalopathy secondary to systemic inflammatory response syndrome (SIRS; 2.4 mg/kg, phase Ia), pneumonitis (4.8 mg/kg, phase Ib), and pulmonary embolism (3.4 mg/kg, phase Ia); the first two were considered related to treatment.
Table 2.
Adverse events related to study drug occurring in ≥6 (10%) of patients overall.
| MedDRA preferred term | DCDS0780A <2.4 mg/kg monotherapy (n = 11) | DCDS0780A ≥2.4 mg/kg monotherapy (n = 40) | DCDS0780A ≥3.6 mg/kg + rituximab (n = 9) | Total (N = 60) |
|---|---|---|---|---|
| Patients with ≥1 AE, n (%) | 9 | 37 | 8 (89) | 54 (90) |
| Total number of events | 38 | 193 | 45 | 276 |
| Vision blurred | 0 | 16 (40) | 5 (56) | 21 (35) |
| Corneal deposits | 1 (9) | 14 (35) | 2 (22) | 17 (28) |
| Fatigue | 2 (18) | 14 (35) | 1 (11) | 17 (28) |
| Neutropenia | 2 (18) | 14 (35) | 0 | 16 (27) |
| Nausea | 2 (18) | 9 (23) | 1 (11) | 12 (20) |
| Neuropathy peripheral | 1 (9) | 8 (20) | 3 (33) | 12 (20) |
| Diarrhea | 0 | 9 (23) | 2 (22) | 11 (18) |
| Peripheral sensory neuropathy | 2 (18) | 5 (13) | 1 (11) | 8 (13) |
| Decreased appetite | 1 (9) | 4 (10) | 1 (11) | 6 (10) |
| Keratitis | 0 | 3 (8) | 3 (33) | 6 (10) |
There was particular interest in AEs attributable to ADC with the MMAE payload. Using basket terms (standardized MedDRA queries) for peripheral neuropathy, pulmonary toxicities, and hepatic toxicities, there were 28 (47%), 23 (38%), and 5 (8%) patients, respectively, with AEs that fell under the basket terms (Supplementary Table S2). Protocol-defined AEs of special interest (AESI) included 3 patients with pneumonitis and 1 patient with lung infiltration, all considered related to study drug. There were 66 related ocular events that occurred in 28 (47%) patients consisting mainly of grades 1–2 and six grade 3 events in 5 (8%) patients; these ocular events were concentrated in dose groups ≥2.4 mg/kg ± rituximab (27 patients, 62 events; Supplementary Table S3). Time to onset for the 66 related ocular AEs from first study drug exposure ranged between 1 and 439 days with a median of 66 days. Ocular toxicity events peaked in cycle 3 (19/66, 29%), in contrast with AEs in general occurring early in the course of treatment with decreasing incidents in later cycles (Supplementary Fig. S2). Thirteen patients with blurred vision also had corneal deposits where both events were considered related to the study drug. Ocular findings and symptoms improved or resolved with dose delay or drug discontinuation, although study drug-related recurrence after the initial event was evident in 18 patients with continued drug exposure. Nineteen of 66 ocular AEs led to interruption or withdrawal of the study drug, among which 17 (89%) recovered with a median time to resolution of 43 days. Forty-seven of the 66 ocular events (71%) resolved with a median time to recovery of 47 days (range, 3–393 days). By the time of database lock, 4 additional events (6%) were in the process of resolving, with 15 events (23%) remaining unresolved.
Dose reductions occurred for 11 (18%) patients overall, including 5 of 20 (25%) patients dosed at 3.6 mg/kg ± rituximab and 6 of 19 (32%) patients at 4.8 mg/kg DCDS0780A ± rituximab. Dose reductions only occurred in patients who received 3 or more treatments, with 8 and 3 patients undergoing 1 and 2 dose reductions, respectively. Twenty-one (35%) patients discontinued DCDS0780A treatment due to AEs (ocular, n = 9; neuropathy, n = 5; pulmonary events, n = 4; one each for hypoxic ischemic encephalopathy, hypercalcemia, and muscular weakness).
One DLT of grade 4 SIRS occurred on study. The patient reporting the DLT was dosed at 2.4 mg/kg (monotherapy). The highest dose tested was 4.8 mg/kg; the protocol-specified MTD was not formally reached. The dose of 4.8 mg/kg DCDS0780A was determined to be the RP2D based on the overall safety and tolerability profile at that dose.
PKs and immunogenicity
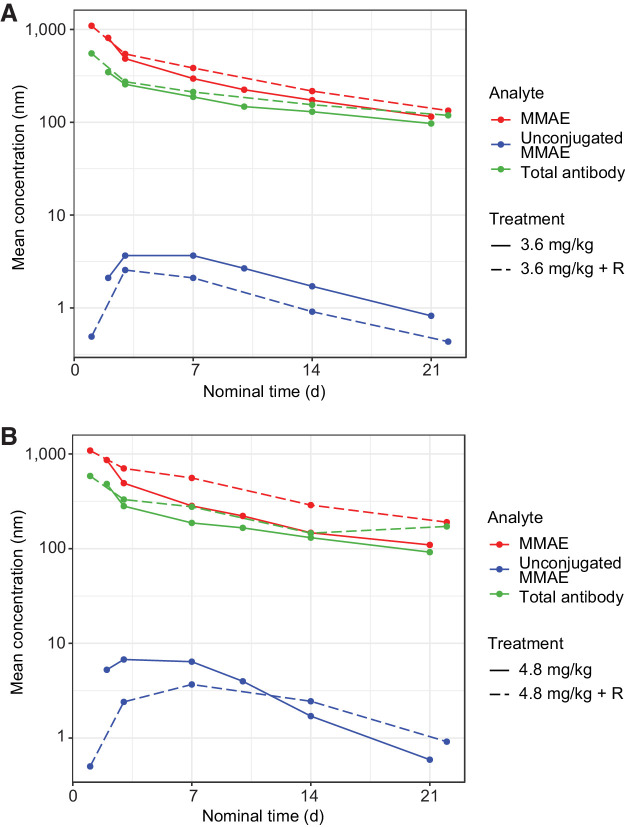
Preliminary summary statistics (mean, %CV) of key PK parameters for acMMAE, total antibody, and unconjugated MMAE by dose levels following the first doses of DCDS0780A given as a 90-minute infusion for the Q3W dosing regimen (cycle 1) are provided in Supplementary Table S4. The systemic exposures [maximum concentration (Cmax) and area under the plasma concentration−time curve from time 0 to infinity (AUC0−inf)] of acMMAE, total antibody, and unconjugated MMAE analytes appeared to increase with an increasing dose from 0.3 to 4.8 mg/kg (Fig. 2). The increases in Cmax and AUC0−inf were approximately dose-proportional at the clinically tested doses of ≥2.4 mg/kg for both the acMMAE and total antibody analytes, thereby demonstrating linear PKs. The clearance of total antibody and acMMAE analytes for TDC was similar, suggesting that the disposition of the MMAE component of the TDC appears to be primarily driven by the mAb component of the ADC. The volume of distribution of the acMMAE and total antibody analytes approximated physiological serum volume, with mean values ranging from 59 to 98 mL/kg for the acMMAE analyte and 66 to 114 mL/kg for the total antibody analyte across the dose levels tested, and did not appear to change substantially with dose. Across the doses, unconjugated MMAE exposure was consistently lower than the acMMAE with mean Cmax approximately 500-fold lower than that of the acMMAE, suggesting formation rate-limited kinetics. The Cmax of unconjugated MMAE ranged from 0.19 to 6.1 ng/mL across the clinical dose range. Furthermore, the PK profiles and parameters of DCDS0780A when administered in combination with rituximab remained unchanged at the 3.6 and 4.8 mg/kg doses.
Figure 2.
PKs of DCDS0780A at concentrations ≥2.4 mg/kg ± rituximab (R) for patients who received (A) 3.6 mg/kg and (B) 4.8 mg/kg.
Anti-drug antibodies (ADA) toward DCDS0780A were evaluable for 51 patients at baseline; one (2%) patient tested positive for baseline ADA. One (2%) patient tested positive for treatment-induced post-baseline ADA among 57 evaluable patients at post-treatment.
Efficacy
The best overall response rate (ORR) for patients on study (N = 60) was 47% (n = 28) and included 17 patients with CR (28%) and 11 patients with partial response (PR, 18%; Table 3 and Fig. 3). Among patients who received monotherapy, ORR was 9% at doses <2.4 mg/kg and 50% at doses ≥2.4 mg/kg. The ORR was 78% among all patients (n = 9) who received DCDS0780A at doses ≥3.6 mg/kg in combination with rituximab. For patients with DLBCL who received ≥2.4 mg/kg ± rituximab (n = 32), the ORR was 59%. The best overall response by investigators for the two patients in the 3.6 mg/kg cohort who had received prior CAR T-cell therapy was progressive disease; both patients discontinued treatment and from study due to progressive disease around 6-week after starting treatment.
Table 3.
Best overall responses.
| All lymphoma subtypes | |||||
|---|---|---|---|---|---|
| DCDS0780A <2.4 mg/kg monotherapy | DCDS0780A ≥2.4 mg/kg monotherapy | DCDS0780A ≥3.6 mg/kg + rituximab | All patients | DLBCL DCDS0780A ≥2.4 mg/kg ± rituximab | |
| (n = 11) | (n = 40) | (n = 9) | (N = 60) | (n = 32) | |
| Responders | 1 (9) | 20 (50) | 7 (78) | 28 (47) | 19 (59) |
| Complete response | 1 (9) | 12 (30) | 4 (44) | 17 (28) | 13 (41) |
| Partial response | 0 | 8 (20) | 3 (33) | 11 (18) | 6 (19) |
| Non-responders | 10 (91) | 18 (45) | 1 (11) | 29 (48) | 13 (41) |
| Stable disease | 3 (27) | 3 (8) | 0 | 6 (10) | 1 (3) |
| Progressive disease | 7 (64) | 15 (38) | 1 (11) | 23 (38) | 12 (38) |
| Missing data | 0 | 2 (5) | 1 (11) | 3 (5) | 0 |
Note: Percentages of each response category in one column may not add up to 100% due to rounding.
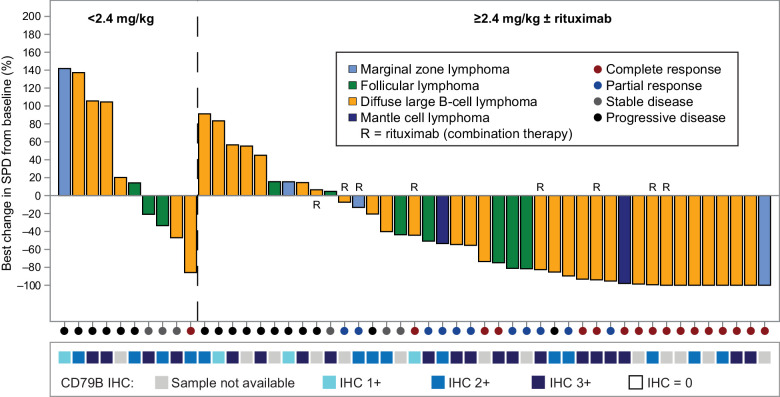
Figure 3.
Best percentage of change from baseline in sum of product of diameters (SPD) for all patients with assessable radiographic data (n = 51).
Patients who discontinued treatment for reasons other than disease progression were followed for response for up to 1 year or until progression of disease or initiation of another therapy. The median PFS for all patients on study (N = 60) was 4.4 months [95% confidence interval (CI), 2.6–13.2], and 3.9 months (95% CI, 2.4–9.5) for patients with DLBCL (n = 41); PFS for pooled subgroups are indicated (Supplementary Fig. S3). The median DoR for the 28 responders among all patients was 15.2 months (95% CI, 8.4–N.E.); DoR for other pooled groups are indicated (Supplementary Fig. S4).
Among the confirmed responders, 57% of patients who received monotherapy (phase Ia) and 71% of patients who received combination therapy (phase Ib) had ongoing responses at the clinical data cutoff date.
Biomarkers
Biomarker IHC samples were available for 38 of 51 patients who had sum of product of diameters response data available (Fig. 3). All 38 available patient samples stained positive for CD79B and a majority (90%) exhibited strong (2+/3+) CD79b expression. For these 38 patients, there was no clear relationship between CD79B expression and reduction in tumor size (Supplementary Fig. S5).
Tissue samples were available for 27 patients for the analysis of COO subtype for B-cell lymphoma. COO data indicated B-cell lymphoma to be of the activated B-cell subtype (ABC; n = 6, 22%), germinal center B-cell subtype (GCB; n = 17, 63%), and unclassifiable (UNC; n = 4, 15%). Tumor responses were seen in 5 (83%) patients with ABC subtype, 8 (47%) patients with GCB subtype, and 3 (75%) patients with UNC subtype (Supplementary Fig. S5).
Discussion
Targeting CD79B is an effective strategy in the treatment of B-NHL. Prior studies have demonstrated that polatuzumab vedotin, an anti-CD79B ADC with an MMAE payload, was well tolerated and produced antitumor responses as monotherapy as well as in combination with anti-CD20 monoclonal antibodies or chemotherapy (5–7, 14). Here, we evaluated the safety and antitumor activity of DCDS0780A, a TDC that has a consistent drug-to-antibody conjugation ratio of 2:1 as opposed to the heterogeneous conjugation ratio of polatuzumab vedotin, to determine whether the TDC format could improve the therapeutic index of an anti-CD79B ADC. Using the TDC, we were able to escalate beyond the approved dose of polatuzumab vedotin (1.8 mg/kg; ref. 8) and beyond the maximum administered dose of polatuzumab vedotin (2.4 mg/kg; ref. 5) investigated in a phase I study. Although the protocol-specified MTD was not formally reached, 4.8 mg/kg DCDS0780A was the maximum administered dose based upon the overall safety and tolerability profile at that dose. The single agent activity of DCDS0780A resulted in a response rate of 59% and CR rate of 41% at TDC doses of 2.4 mg/kg or higher for patients with DLBCL. Overall, the median DoR was 11.5 months with monotherapy and 15.7 months after DCDS0780A + rituximab, and a majority of patients who achieved CR continued to be in remission at the time of analysis. The depth and DoRs observed with DCDS0780A in patients with relapsed/refractory DLBCL compare favorably with the efficacy of other novel therapies in use for relapsed/refractory DLBCL (15–17).
Although only one DLT was observed in the entire phase Ia stage, other AEs were observed outside of the DLT window at higher DCDS0780A doses that limited duration of therapy in a sizable proportion of patients. Peripheral neuropathy and neutropenia, commonly seen with MMAE payloads, were observed with DCDS0780A but were manageable with dose delays and rarely caused drug discontinuations. However, unlike polatuzumab vedotin, ocular toxicities, including blurry vision, dry eyes, corneal deposits, and keratitis, were observed at a high frequency in patients treated with DCDS0780A. Although the ocular toxicities improved with dose delays, and some patients resumed therapy, the toxicities recurred with DCDS0780A re-challenge and a majority of patients subsequently discontinued therapy. Ocular AEs have been observed with payloads other than MMAE (18–21) and are generally associated with ADCs that have a stable linker (22–24). Similar to ocular AEs seen with other ADCs, the ocular toxicities were delayed in onset and mostly reversible. Mitigation strategies for ocular toxicities, such as prophylactic topical corticosteroids, have been used in other studies but have generally been unsuccessful.
In conclusion, our study demonstrated the proof-of-concept for TDC, which ensured a consistent ratio of drug conjugation and generated a TDC that could be escalated beyond the tolerable doses of ADCs with heterogeneous conjugation. The higher administered doses of the anti-CD79B TDC, DCDS0780A, produced higher response rates. However, increasing the dose of anti-CD79B TDC was also accompanied by a different toxicity profile that limited the ability to tolerate the study drug, and resulted in frequent dose delays and discontinuations. Our study demonstrated the challenges of expanding the therapeutic index of ADCs as incremental improvements in activity at higher doses were also accompanied by toxicities that limited tolerability. Despite the toxicities observed, the durable responses to DCDS0780A in patients with relapsed/refractory DLBCL suggest that novel ADC linker technologies may have future utility with further refinement needed to ensure the tolerability of therapy.
Supplementary Material
Acknowledgments
This study was funded by Genentech. We thank the patients, study investigators, and staff who participated in this study. Editing and writing support was provided by A. Daisy Goodrich (Genentech, Inc.) and was funded by Genentech. A.F. Herrera was supported by the National Cancer Institute of the National Institutes of Health under award numbers NIH 2K12CA001727‐21 and P50CA107399, the Emmet and Toni Stephenson Leukemia and Lymphoma Society Scholar Award, and the Lymphoma Research Foundation Larry and Denise Mason Clinical Investigator Career Development Award.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
A.F. Herrera reports grants and personal fees from Genentech, BMS, Merck, SeaGen, AstraZeneca, Kite Pharma, and ADC Therapeutics, grants from Gilead Sciences, and personal fees from KaryoPharm, Takeda, Tubulis, Genmab, and Regeneron outside the submitted work. M.R. Patel reports other support from Genentech during the conduct of the study; other support from Genentech advisory boards outside the submitted work. J.M. Burke reports personal fees from Adaptive Biotechnologies, Roche/Genentech, Epizyme, Kura, Abbvie, Morphosys, Beigene, Seagen, Kymera, Bristol Myers Squibb, X4 Pharmaceuticals, AstraZeneca, TG Therapeutics, and Lilly during the conduct of the study. R. Advani reports grants from Genentech during the conduct of the study; and personal fees from Genentech outside the submitted work. B.D. Cheson reports personal fees from Abbvie, Pharmacyclics, Morphosys, Epizyme, Karyopharm, Genmab, ADC Therapeutics, TG Therapeutics, AstraZeneca, Bristol Myers Squibb, Incyte, Lilly, Merck, and Symbio outside the submitted work. J.P. Sharman reports personal fees from Genentech during the conduct of the study; and personal fees from Abbvie, Beigene, BMS, TG Therapeutics, and AstraZeneca outside the submitted work. E. Penuel reports other support from Genentech/Roche and Genentech/Roche outside the submitted work. C.D. Liao reports employment and stockholder of Roche. C. Li reports other support from Genentech Inc. outside the submitted work; and reports being an employee and stock owner of Genentech/Roche. Genentech owns the published work. R. Elstrom reports personal fees from Genentech during the conduct of the study; personal fees from Fate Therapeutics outside the submitted work. J. Cooper reports employment of Genentech and owns stock in the parent company Roche. C.M. Diefenbach reports grants, personal fees, and non-financial support from Roche/Genentech during the conduct of the study; grants and personal fees from Roche/Genentech outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
A.F. Herrera: Resources, investigation, writing–review and editing, final approval of the article. M.R. Patel: Resources, investigation, writing–review and editing, final approval of the article. J.M. Burke: Resources, investigation, writing–review and editing, final approval of the article. R. Advani: Resources, investigation, writing–review and editing, final approval of the article. B.D. Cheson: Resources, investigation, writing–review and editing, final approval of the article. J.P. Sharman: Conceptualization, resources, investigation, writing–review and editing, final approval of the article. E. Penuel: Resources, investigation, writing–review and editing, final approval of the article. A.G. Polson: Investigation, writing–review and editing, final approval of the article. C.D. Liao: Formal analysis, investigation, final approval of the article. C. Li: Investigation, writing–review and editing, final approval of the article. E. Schuth: Investigation, writing–review and editing, final approval of article. A. Vaze: Investigation, writing–review and editing, approval of the final article. D. Samineni: Investigation, writing–review and editing, approval of the final article. R. Elstrom: Conceptualization, resources, supervision, investigation, approval of the final article. J. Cooper: Resources, supervision, investigation, writing–review and editing, approval of final article. C. Diefenbach: Resources, investigation, writing–review and editing, approval of final article.
References
- 1. Huang X, Takata K, Sato Y, Tanaka T, Ichimura K, Tamura M, et al. Downregulation of the B-cell receptor signaling component CD79b in plasma cell myeloma: a possible post transcriptional regulation. Pathol Int 2011;61:122–9. [DOI] [PubMed] [Google Scholar]
- 2. Polson AG, Calemine-Fenaux J, Chan P, Chang W, Christensen E, Clark S, et al. Antibody–drug conjugates for the treatment of non-Hodgkin's lymphoma: target and linker-drug selection. Cancer Res 2009;69:2358–64. [DOI] [PubMed] [Google Scholar]
- 3. Zheng B, Fuji RN, Elkins K, Yu SF, Fuh FK, Chuh J, et al. In vivo effects of targeting CD79b with antibodies and antibody–drug conjugates. Mol Cancer Ther 2009;8:2937–46. [DOI] [PubMed] [Google Scholar]
- 4. Polson AG, Yu SF, Elkins K, Zheng B, Clark S, Ingle GS, et al. Antibody–drug conjugates targeted to CD79 for the treatment of non-Hodgkin lymphoma. Blood 2007;110:616–23. [DOI] [PubMed] [Google Scholar]
- 5. Palanca-Wessels MC, Czuczman M, Salles G, Assouline S, Sehn LH, Flinn I, et al. Safety and activity of the anti-CD79B antibody–drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol 2015;16:704–15. [DOI] [PubMed] [Google Scholar]
- 6. Morschhauser F, Flinn IW, Advani R, Sehn LH, Diefenbach C, Kolibaba K, et al. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS). Lancet Haematol 2019;6:e254–e65. [DOI] [PubMed] [Google Scholar]
- 7. Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 2020;38:155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deeks ED. Polatuzumab vedotin: first global approval. Drugs 2019;79:1467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dornan D, Bennett F, Chen Y, Dennis M, Eaton D, Elkins K, et al. Therapeutic potential of an anti-CD79b antibody–drug conjugate, anti-CD79b-vc-MMAE, for the treatment of non-Hodgkin lymphoma. Blood 2009;114:2721–9. [DOI] [PubMed] [Google Scholar]
- 10. Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol 2008;26:925–32. [DOI] [PubMed] [Google Scholar]
- 11. Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 2003;21:778–84. [DOI] [PubMed] [Google Scholar]
- 12. Pfeifer M, Zheng B, Erdmann T, Koeppen H, McCord R, Grau M, et al. Anti-CD22 and anti-CD79B antibody–drug conjugates are active in different molecular diffuse large B-cell lymphoma subtypes. Leukemia 2015;29:1578–86. [DOI] [PubMed] [Google Scholar]
- 13. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tilly H, Morschhauser F, Bartlett NL, Mehta A, Salles G, Haioun C, et al. Polatuzumab vedotin in combination with immunochemotherapy in patients with previously untreated diffuse large B-cell lymphoma: an open-label, non-randomised, phase 1b-2 study. Lancet Oncol 2019;20:998–1010. [DOI] [PubMed] [Google Scholar]
- 15. Caimi PF, Ai W, Alderuccio JP, Ardeshna KM, Hamadani M, Hess B, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 2021;22:790–800. [DOI] [PubMed] [Google Scholar]
- 16. Kalakonda N, Maerevoet M, Cavallo F, Follows G, Goy A, Vermaat JSP, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol 2020;7:e511–e22. [DOI] [PubMed] [Google Scholar]
- 17. Jurczak W, Zinzani PL, Gaidano G, Goy A, Provencio M, Nagy Z, et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non-Hodgkin's lymphoma. Ann Oncol 2018;29:1266–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tannir NM, Forero-Torres A, Ramchandren R, Pal SK, Ansell SM, Infante JR, et al. Phase I dose-escalation study of SGN-75 in patients with CD70-positive relapsed/refractory non-Hodgkin lymphoma or metastatic renal cell carcinoma. Invest New Drugs 2014;32:1246–57. [DOI] [PubMed] [Google Scholar]
- 19. Shapiro GI, Vaishampayan UN, LoRusso P, Barton J, Hua S, Reich SD, et al. First-in-human trial of an anti-5T4 antibody-monomethylauristatin conjugate, PF-06263507, in patients with advanced solid tumors. Invest New Drugs 2017;35:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. van den Bent M, Gan HK, Lassman AB, Kumthekar P, Merrell R, Butowski N, et al. Efficacy of depatuxizumab mafodotin (ABT-414) monotherapy in patients with EGFR-amplified, recurrent glioblastoma: results from a multi-center, international study. Cancer Chemother Pharmacol 2017;80:1209–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gan HK, Reardon DA, Lassman AB, Merrell R, van den Bent M, Butowski N, et al. Safety, pharmacokinetics, and antitumor response of depatuxizumab mafodotin as monotherapy or in combination with temozolomide in patients with glioblastoma. Neuro Oncol 2018;20:838–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Donaghy H. Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody–drug conjugates. MAbs 2016;8:659–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Masters JC, Nickens DJ, Xuan D, Shazer RL, Amantea M. Clinical toxicity of antibody–drug conjugates: a meta-analysis of payloads. Invest New Drugs 2018;36:121–35. [DOI] [PubMed] [Google Scholar]
- 24. Campos MP, Konecny GE. The target invites a foe: antibody–drug conjugates in gynecologic oncology. Curr Opin Obstet Gynecol 2018;30:44–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For eligible studies, qualified researchers may request access to individual patient level clinical data through a data request platform. At the time of writing, this request platform is Vivli: https://vivli.org/ourmember/roche/.
For up to date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing.
Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.