Abstract
Purpose:
Preoperative chemoradiotherapy (CRT) and surgical resection are the standard treatment for locally advanced rectal cancer (LARC). Combining immune checkpoint inhibitors with radiation suggests a promising approach for enhancing efficacy. We investigated the efficacy of CRT followed by nivolumab and surgery in patients with LARC.
Patients and Methods:
In phase I, we investigated the feasibility of sequentially combined CRT, 5 cycles of nivolumab, and radical surgery. In phase II, patients with microsatellite stable (MSS) and microsatellite instability-high (MSI-H) LARC were evaluated.
Results:
Three patients in phase I received full courses of CRT and nivolumab without dose modification; the schedule was recommended for phase II. A pathologic complete response (pCR) was centrally confirmed in 30% [11/37; 90% confidence interval (CI), 18%–44%] and 60% (3/5) of the MSS and exploratory MSI-H cohorts, respectively. While immune-related severe adverse events were observed in 3 patients, no treatment-related deaths were observed. In 38 patients with MSS who underwent surgery, pCR rates of 75% (6/8) and 17% (5/30; P = 0.004, Fisher exact test) were observed in those with programmed cell death ligand 1 (PD-L1) tumor proportion score ≥1% and <1%, respectively; IHC staining was performed using pre-CRT samples. In 24 patients with MSS, pre-CRT samples were analyzed by flow cytometry; pCR rates of 78% (7/9) and 13% (2/15; P = 0.003, Fisher exact test) were observed for CD8+ T cell/effector regulatory T cell (CD8/eTreg) ratios of ≥2.5 and <2.5, respectively, in tumor-infiltrating lymphocytes.
Conclusions:
CRT followed by consolidation nivolumab could increase pCR. PD-L1 expression and an elevated CD8/eTreg ratio were positive predictors in patients with MSS LARC.
Translational Relevance.
In our clinical trial, preoperative chemoradiotherapy (CRT) followed by nivolumab and surgery indicated 30% [11/37; 90% confidence interval (CI), 18%–44%] and 60% (3/5) of pathologic complete response (pCR) rate with mild toxicities in patients with microsatellite stable (MSS) and microsatellite instability–high locally advanced rectal cancer (LARC), respectively. In the exploratory translational research for the MSS cohort, pCR rates of 75% (6/8) and 78% (7/9) were, respectively, observed in patients with PD-L1 ≥1% staining and elevated CD8+/effector regulatory T-cell ratio in tumor-infiltrating lymphocytes (TIL). Ki-67, CTLA-4, and PD-1 expression by CD8+ T cells in pre-CRT samples of TILs was higher in the good responders. High estimates of efficacy were also observed in patients with consensus molecular subtype 1 and higher tumor mutational burden in pre-CRT samples. Those can be the potential efficacy predictors of CRT plus immune-checkpoint inhibitor.
Introduction
Globally, CRC was the third and second most common cancer in men and women, respectively, in 2020 (1). For patients with locally advanced rectal cancer (LARC) of clinical T3 or T4 without distant metastasis, preoperative chemoradiotherapy (CRT) using radiation plus concurrent fluoropyrimidine-based chemotherapy followed by surgical resection with total mesorectal excision (TME; refs. 2, 3) has been performed as the standard treatment to reduce the risk of local recurrence (4). Adjuvant chemotherapy is generally administered to patients with stage II/III rectal cancer who did not receive neoadjuvant chemotherapy.
Immune checkpoint inhibitors (ICI) that block CTL-associated protein 4 (CTLA-4), programmed cell death 1 (PD-1), and programmed cell death ligand 1 (PD-L1) have become the standard therapy for various solid tumor types (5–7). In microsatellite instability–high (MSI-H) or mismatch-repair–deficient (dMMR) colorectal cancer, a strong association between the benefit of PD-1 blockade and the clinical benefit has been reported; although these are rare subtypes (8). Indeed, an anti–PD-1 antibody, pembrolizumab, was approved as first-line therapy both in the United States and Japan for metastatic disease. While neoadjuvant ICIs led to pathologic responses in microsatellite-stable (MSS) early-stage colon cancers in the Dutch NICHE study (NCT03026140; ref. 9), slight efficacies of ICIs have been reported in metastatic disease (10). Therefore, various combination treatments are being investigated to improve the efficacy of immunotherapies for this subtype (11–14).
Among the investigations above, combinations of ICIs with ionizing radiation are a promising approach because radiation may enhance the action of ICIs by several mechanisms, including tumor antigen release from direct tumor cell death and presentation of tumor-derived antigens, and activate the innate immune pathway through type I IFN and the stimulator of interferon genes (STING) pathway. Radiotherapy also increased T-cell infiltration and modulated immunosuppressive cells (15–17). Indeed, in preclinical melanoma and breast carcinoma xenografts models, the immune-stimulating effects of radiation were significantly increased when radiation was sequentially combined with an anti–PD-1 antibody. These increased the proportion of the tumor–antigen complexes and MHC molecules, and enhanced cross-presentation in lymph nodes and T-cell infiltration into tumors (18). The polyclonal T-cell response also mediated out-of-field (abscopal) effects following local radiotherapy in renal cell, breast, and colorectal carcinoma mouse models (19, 20). Clinical trials for various types of metastatic cancer have shown that high-dose stereotactic body radiotherapy combined with ICIs increased responses in nonirradiated metastatic lesions, leading to better progression-free survival (PFS) and overall survival (OS) outcomes, than with ICI monotherapies (21–23). Furthermore, in patients with locally advanced unresectable non–small cell lung cancer, the sequential combination of conventional platinum-based CRT plus consolidation therapy with an anti–PD-L1 antibody showed significant improvement in both PFS (24) and OS (25). However, the ability of CRT followed by ICIs to lead to better PFS and OS outcomes in LARC has not been investigated.
Patients and Methods
Study design
We initiated the proof-of-concept EPOC 1504 VOLTAGE study, which is an ongoing, nonrandomized, single-arm phase I/II trial across three academic hospitals (26). Phase I was designed to investigate the feasibility of PD-1 antibody nivolumab monotherapy and radical surgery after CRT, and to determine the recommended phase II dosing schedule in a “3+3” cohort-based design. As a presetting decision rule, if no dose-limited toxicities (DLT) were observed in the first 3 patients, we would move forward to phase II. If ≥1 DLT was observed, 3 more patients would be included in phase I. Phase II was designed to evaluate the efficacy and safety of nivolumab and radical surgery in patients with primary LARC (cohort A). Although primary analysis was conducted in patients with MSS LARC (cohort A1), those with MSI-H LARC were also included in the exploratory analyses (cohort A2).
The trial protocol was approved by the institutional review board of each participating site before study initiation. The study was conducted in accordance with the tenets of the Declaration of Helsinki and Good Clinical Practice Guidelines, after approval by the ethics board of each institution, and was registered at ClinicalTrials.gov (NCT02948348). Written informed consent was obtained from all patients. The full list of inclusion and exclusion criteria is provided in the trial protocol.
Patient eligibility
The major eligibility criteria were as follows: treatment-naïve with rectal cancer located 12 cm from the anal verge; clinical stage T3–4 N0–2 M0; age ≥20 to <80 years; Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1; treated using capecitabine (1,650 mg/m2 daily)-based concurrent CRT to a dose of 50.4 Gy (45 Gy/25 fractions to the pelvic cavity and 5.4 Gy/3 fractions boost to the primary lesion); and presence of sufficient organ function. Lymph node positivity was diagnosed on the basis of a short-axis diameter of >10 mm on MRI.
Procedures
The study treatments included five cycles of nivolumab monotherapy (240 mg every 2 weeks) and subsequent radical surgery using a sphincter-sparing procedure or abdominoperineal resection with TME, after CRT with capecitabine and radiation to a dose of 50.4 Gy, in 28 fractions. We planned to start nivolumab within 14 days of completion of preoperative CRT; the period between the end of CRT and radical surgery was planned for 12 weeks (Supplementary Fig. S1). For patients with favorable postoperative conditions, a maximum of 6 months on adjuvant treatment with mFOLFOX6 or CAPOX was recommended, at the investigator's discretion.
An exploratory biomarker study was also conducted using samples obtained from patients with MSS or MSI-H LARC. Serial tumor and blood samples were collected at four instances: pre-CRT, post-CRT, post-three cycles of nivolumab, and post-five cycles of nivolumab. The PD-L1 staining in pre-CRT tumor formalin-fixed paraffin-embedded samples was performed in vitro by diagnostic IHC staining (PD-L1 IHC 28–8 pharmDx, Agilent Technologies) according to the manufacturer's instructions. The PD-L1 status was evaluated using both the tumor proportion score (TPS) and the combined positive score (CPS). TPS is defined as the percentage of PD-L1 expression in tumor cells only, and CPS is defined as PD-L1 expression in tumor cells, lymphocytes, and macrophages/tumor cells × 100. Tumor-infiltrating lymphocytes (TIL), DNA, and RNA were extracted from tumor samples while peripheral blood mononuclear cells (PBMC) were isolated from the blood, as normal samples. Immune status was analyzed by flow cytometry using the collected TILs and PBMCs (27). Whole-exome and RNA-sequencing analyses were conducted using the extracted DNA and RNA, respectively. All analyses were centrally and independently performed, blinded to the clinical outcomes. The methodology of the exploratory biomarker study is presented in the Supplementary Materials and Methods.
Assessment
The primary endpoint for patients with MSS LARC was the pathologic complete response (pCR) rate, as determined by an independent central pathology assessment committee. Histopathologic assessment of tumor regression was performed in accordance with the assessment criteria of the American Joint Committee on Cancer (AJCC) 7th edition. Tumor regression grade (TRG) 0 indicates no remaining viable cancer cells (complete response); TRG 1 indicates a single cell or small groups of cancer cells (moderate response); TRG 2 indicates residual cancer outgrown by fibrosis (minimal response); and TRG 3 indicates minimal, or no tumor death, with extensive residual cancer (poor response). We also calculated the neoadjuvant rectal score, using the clinical T, and pathologic T and N stages as surrogates for long-term results (28). Data on recurrent and vital status were collected and reported. However, relapse-free survival (RFS), which is defined as the time between surgery and the date of relapse or death, and overall survival (OS) were calculated but were not evaluated using the Kaplan–Meier method in this short-term study.
Safety analyses were performed using the Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0, and the Clavien–Dindo classification (ver. 2.0) was added for postoperative complications. We established both radiation and central surgical assessment committees to ensure the quality and safety of the study treatments.
Statistical analysis
Assuming null and alternative hypotheses, pCR rates of 10% and 30%, the required sample size for patients with MSS LARC was 37, with a one-sided alpha of 5% and power of 90%. Five patients with MSI-H LARC were also included in the exploratory analyses.
The primary endpoint was set to be analyzed in the first 37 patients who were eligible and received protocol treatment at the recommended phase II dosing schedule at least once. The confidence intervals (CI) of the pCR rate were calculated using the Clopper and Pearson method. The efficacy endpoints for all eligible patients who received protocol treatment at least once were analyzed. Safety endpoints were analyzed in patients who received protocol treatment at least once. All statistical analyses were performed using SAS Release version 9.4 (SAS Institute).
Data availability
The RNA- and DNA-sequencing data will be deposited into the Japanese Genotype-Phenotype Archive and will be made available on reasonable request for academic use and within the limitations of the provided informed consent. Accession process is ongoing. Every request will be reviewed by the institutional review board of the National Cancer Center (NCC); the researcher will need to sign a data access agreement with the NCC after approval.
Results
Patient characteristics
Three patients with primary MSS LARC were enrolled in phase I. All 3 received both full courses of CRT and nivolumab (5 cycles) without dose modification and underwent radical surgical resection. No DLTs were observed (Supplementary Table S1). Therefore, five cycles of nivolumab (240 mg every 2 weeks) monotherapy prior to radical surgery was determined as the recommended phase II dosing schedule.
From January 2017 to October 2019, a total of 39 patients with MSS LARC and 5 patients with MSI-H LARC were enrolled. The first 37 consecutive patients with MSS LARC were included in the primary endpoint analysis, while the efficacy and safety analyses were performed using data from all 39 patients with MSS LARC. Patient characteristics of both MSS and MSI-H patients are shown in Table 1. Quality-assured CRT was performed for all enrolled patients according to the assessments of the radiation assessment committee.
Table 1.
Patient characteristics.
| MSS | MSI-H | |
|---|---|---|
| (n = 39) | (n = 5) | |
| Age [median (range)] | 61 (33–79) | 58 (42–67) |
| Sex (male/female) | 26 (67%)/13 (33%) | 3 (67%)/2 (33%) |
| ECOG PS (0/1) | 33 (85%)/6 (15%) | 4 (80%)/1 (20%) |
| T category (T3/T4) | 34 (87%)/5 (13%) | 5 (100%)/0 (0%) |
| N category (N0/N1–2) | 30 (77%)/9 (23%) | 3 (67%)/2 (33%) |
| Stage (II/III) | 30 (77%)/9 (23%) | 3 (67%)/2 (33%) |
| Location of primary site from anal verge [median (range)] | 7 cm (0–11.5) | 6 cm (3–8) |
| Time from the end of CRT to radical surgery [median (range)] | 12.7 weeks (10.9–15.7) | 12.7 weeks (11.9–13.4) |
| Surgery (sphincter sparing/not sphincter sparing/other) | 34 (87%)/4 (10%)/1 (3%)a | 5 (100%)/0 (0%)/0 (0%) |
| Adjuvant chemotherapy (performed/not performed) | 30 (77%)/9 (23%) | 1 (20%)/4 (80%) |
aSurgical resection was not performed in one patient who achieved near-complete clinical response and refused treatment.
Efficacy
According to the pathologic assessment by independent central pathologists, 11 patients [30% (11/37); 90% confidence interval (CI), 18%–44%] achieved TRG 0, resulting in a pCR rate of 30% in patients with MSS LARC. Thus, the primary endpoint of this study was met. When the 3 patients (8%) who achieved TRG 1 were included, 14 (38%) achieved TRG 0 or 1, which has been associated with a favorable prognosis (29). In T4- and/or N-positive cases, a pCR rate of 33% (3/9) was observed (Supplementary Table S2). In patients with MSI-H LARC, 3 patients (60%) achieved TRG 0 (pCR), and the remaining 2 patients achieved TRG 2. The median neoadjuvant rectal (NAR) scores of patients with MSS and MSI-H LARC were 8.4 (0.0–50.4) and 0.9 (0.9–20.4), respectively (Table 2).
Table 2.
Pathologic assessment of resected specimens by the independent central assessment committee.
| MSSa | MSI-H | |
|---|---|---|
| AJCC tumor regression grade | (N = 37) | (N = 5) |
| 0 (pCR) | 11 (30%) | 3 (60%) |
| 1 | 3 (8%) | 0 (0%) |
| 2 | 15 (41%) | 2 (40%) |
| 3 | 7 (19%) | 0 (0%) |
| Not evaluatedb | 1 (3%) | 0 (0%) |
| Neoadjuvant rectal score | 8.4 (0.0–50.4) | 0.9 (0.9–20.4) |
| Macroscopic evaluation of resection specimen (complete/nearly complete/incomplete/other) | 26/5/5/1b | 4/0/1/0 |
aIn total, 39 patients were included in cohort A1. The primary endpoint was analyzed in the first 37 consecutive patients. The AJCC tumor regression grades of the remaining two patients were grade 1 and grade 3.
bSurgical resection was not performed in one patient who achieved near-complete clinical response and refused treatment.
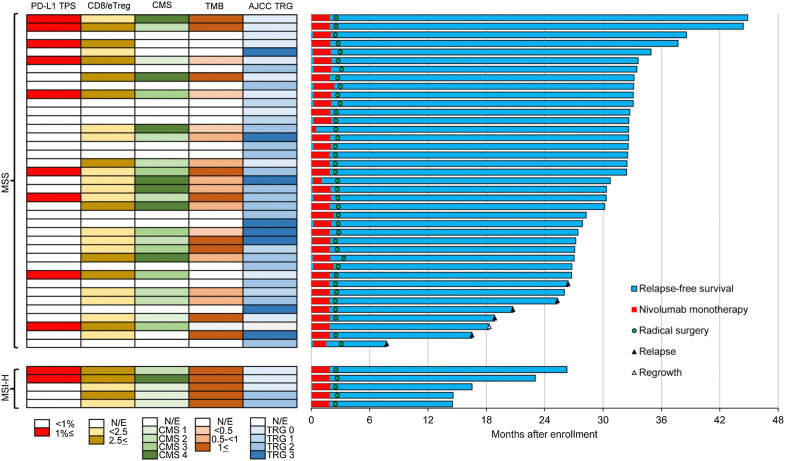
As of December 2020, 6 patients with MSS LARC experienced recurrence (two local and four distant), and no patients with MSI-H LARC experienced recurrence after a median follow-up of 32.9 and 17.2 months, respectively (Fig. 1).
Figure 1.
Representative effects of preoperative chemoradiation plus consolidation nivolumab monotherapy. Swimmer's plot of overall survival. From January 2017 to October 2019, a total of 39 patients with microsatellite stable (MSS) locally advanced rectal cancer (LARC) and 5 patients with microsatellite instability-high (MSI-H) LARC were enrolled. Radical surgery was performed for all patients, excluding one patient who declined surgery after achieving a near-complete clinical response with nivolumab. As of December 2020, six patients with MSS LARC experienced recurrence (two local and four distant), and no patients with MSI-H LARC experienced recurrence after a median follow-up of 32.9 and 17.2 months, respectively. One patient with MSS LARC died. The patient who achieved a near-complete clinical response and declined surgery experienced local regrowth and distant metastasis after 18.3 months of follow-up; there was no option for curative resection due to the multiple distant metastases.
Safety
In patients with MSS LARC, the major adverse events evaluated by the Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.0, were as follows: nivolumab-related adverse events were pruritus (20.5%), hyperthyroidism (12.8%), and AST (12.8%) and ALT elevation (12.8%); and surgery-related adverse events were ileus (10.5%), pelvic abscess (10.5%), and urinary retention (10.5%; Supplementary Table S3). Serious adverse events related to nivolumab or surgery were reported in 8 patients, and immune-related severe adverse events were observed in 3 patients (grade 3 myasthenia, grade 3 interstitial nephritis, and grade 2 peripheral motor neuropathy); all patients fully recovered, and radical surgical resection was performed. During the follow-up period, one additional patient developed grade 2 colitis. No treatment-related deaths were recorded.
PD-L1 positivity and elevated CD8/eTreg ratio as significant predictors of pCR in patients with MSS LARC
In the 38 patients with MSS LARC who had surgery, 8 (21%) had a positive PD-L1 TPS (PD-L1 expression in ≥1% of tumor cells) in pre-CRT samples according to in vitro diagnostic IHC staining (PD-L1 28–8 pharmDx assay, Agilent Technologies). Furthermore, pCR rates of 75% (6/8) and 17% (5/29) (P = 0.004, Fisher exact test) were observed in patients with positive and negative PD-L1 TPS, respectively (Table 3).
Table 3.
Correlation between the pCR rate and biomarkers of enhanced immune activation in both tumor and immune cells.
| Correlation between the pCR rate and PD-L1 status of tumor samples using the TPS before chemoradiotherapy | |||
|---|---|---|---|
| pCR rate in MSS patients | pCR rate in MSI-H patients | ||
| (N = 38) | (N = 5) | ||
| PD-L1 (TPS)a ≥1% | 75% (6/8)b | — | |
| PD-L1 (TPS) <1% | 17% (5/30)b | 60% (3/5) | |
| Correlation between the pCR rate and the CD8/eTreg ratio in tumor-infiltrating lymphocytes (TILs) before chemoradiotherapy | |||
|---|---|---|---|
| pCR rate in MSS patients | pCR rate in MSI-H patients | ||
| (N = 24) | (N = 5) | ||
| CD8/eTreg ratio before CRT ≥2.5 | 78% (7/9)c | 67% (2/3) | |
| CD8/eTreg ratio before CRT <2.5 | 13% (2/15)c | 50% (1/2) | |
| pCR rate by combined analysis of both PD-L1 expression using the TPS and the CD8/eTreg ratio before chemoradiotherapy in patients with MSS LARC (N = 24) | |||
|---|---|---|---|
| CD8/eTreg ≥ 2.5 | CD8/eTreg < 2.5 | Total | |
| PD-L1 (TPS) ≥1% | 100% (5/5) | 33% (1/3) | 75% (6/8) |
| PD-L1 (TPS) <1% | 50% (2/4) | 8% (1/12) | 19% (3/16) |
Abbreviations: pCR, pathologic complete response; TPS, tumor proportion score; CD8/eTreg, CD8+ T cell/effector regulatory T cell; TILs, tumor-infiltrating lymphocytes; MSS, microsatellite-stable; MSI-H, microsatellite-instability–high; LARC, locally advanced rectal cancer.
aTPS = (PD-L1 positive tumor cells/all tumor cells) × 100.
b P = 0.004, Fisher's exact test.
c P = 0.003, Fisher's exact test.
On the contrary, 28 patients (74%) had a positive PD-L1 CPS in pre-CRT samples and no significant differences in pCR were observed between positive (32%) and negative (20%) PD-L1 CPS (Supplementary Table S4). Remarkably, all 6 patients with MSS with recurrence tested negative for PD-L1/TPS (Fig. 1).
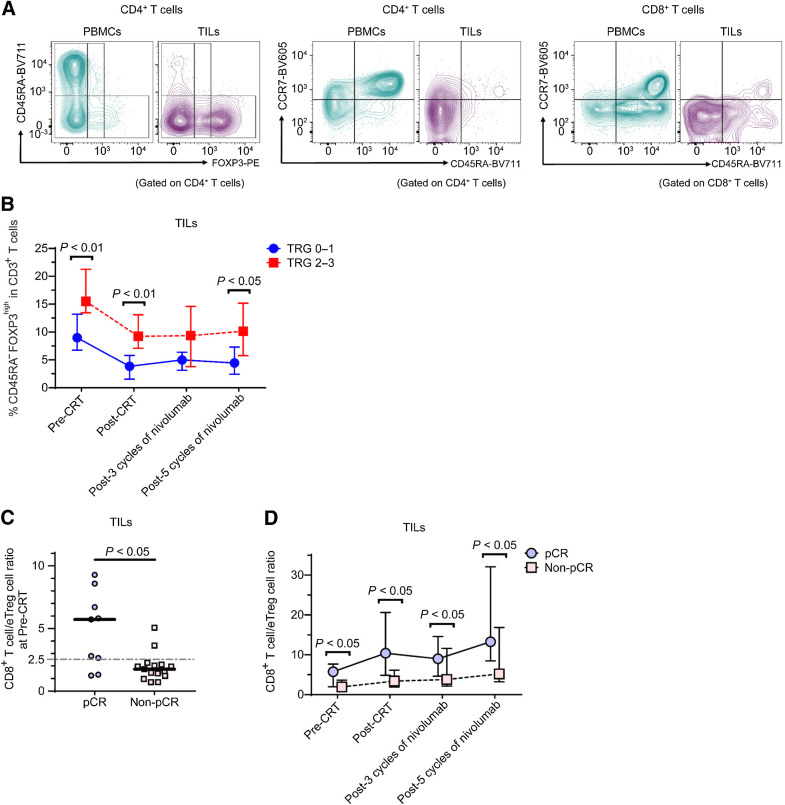
To investigate the correlation between T-cell activation and the efficacies of our sequential therapy, serial tumor and blood samples (pre-CRT, post-CRT, post three cycles of nivolumab, post five cycles of nivolumab) were collected from 24 patients and T-cell subsets evaluated by flow cytometry. We classified human Treg cells based on the expression levels of the naïve T-cell markers CD45RA and FOXP3 (27, 30, 31) to avoid interference with effector CD4+ T cells (32), as follows: naïve Treg cells (CD45RA+FoxP3loCD4+; I); effector Treg (eTreg) cells (CD45RA−FoxP3hiCD4+; II); and non-Treg cells (CD45RA−FoxP3loCD4+; III; Fig. 2A).
Figure 2.
Comparison of effector regulatory T (eTreg) cells and CD8+ T cells in tumor infiltrating lymphocytes (TILs) between responders and non-responders. A, Peripheral blood mononuclear cells (PBMCs) and TILs were collected from 24 patients with microsatellite stable (MSS) locally advanced rectal cancer (LARC) who were enrolled in the VOLTAGE and subjected to flow cytometry. Representative flow cytometry plots are shown. Based on the expression levels of the naive T-cell markers CD45RA and FOXP3, we defined naive Treg cells (CD45RA+FoxP3loCD4+) (I), effector Treg (eTreg) cells (CD45RA−FoxP3hiCD4+) (II), and non-Treg cells (CD45RA−FoxP3loCD4+) (III). B, Percentage of eTreg cells in CD3+ T cells of TILs in patients who achieved tumor regression grade (TRG) 2–3 and in patients who achieved TRG 0–1 at four time points (pre-CRT, post-CRT, post-3 cycles of nivolumab, and post-5 cycles of nivolumab) is shown. The median and interquartile range of TRG 0–1 and TRG 2–3 patients per group, respectively, are shown. The differences between the two groups of patients were compared using a Mann-Whitney U test. C, The CD8+ T cell/eTreg cell (CD8/eTreg) ratios in TILs in patients who achieved pathologic complete response (pCR) and in patients who achieved non-pCR at the pre-CRT time point are shown. The differences between the two groups of patients were compared using a Mann-Whitney U test. D, The CD8/eTreg ratios in TILs in patients who achieved pCR and in patients who achieved non-pCR at four time points (pre-CRT, post-CRT, post-3 cycles of nivolumab, and post-5 cycles of nivolumab) are shown. The median and interquartile range of pCR and non-pCR patients per group, respectively, are shown. The differences between the two groups of patients were compared using a Mann-Whitney U test. Naive (CCR7+CD45RA+); CM, central memory (CCR7+CD45RA−); EM, effector memory (CCR7−CD45RA−); TEMRA, terminally differentiated effector memory (CCR7−CD45RA+).
The frequency of eTreg cells was higher in TILs than in PBMCs in pre-CRT samples (P < 0.0001, Mann–Whitney U test, Supplementary Fig. S2A). The frequency of eTreg cells in TILs was consistently higher at all four time points in patients who achieved TRG 2 or 3 than in those with TRG 0 or 1 (Fig. 2B). As the percentage of CD3+ T cells in live TILs significantly decreased after CRT (Supplementary Fig. S2B) and the ratio of immunostimulant and immunosuppressive cells is expected to be an optimal measure for evaluating immune status (33), we investigated the CD8+ T cells/eTreg cells (CD8/eTreg) ratio in TILs for 24 patients with analyzable serial samples. The CD8/eTreg ratio in TILs pre-CRT in patients who achieved pCR was significantly higher than that in patients who did not achieve pCR (P = 0.012, Mann-Whitney U test; Fig. 2C). When we divided patients based on the ROC curve analysis (cut-off value: 2.5, Supplementary Fig. S2C), pCR rates of 78% (7/9) and 13% (2/15) were observed according to TIL CD8/eTreg ratios ≥2.5 and <2.5, respectively (P = 0.003, Fisher exact test, Table 3). Moreover, CD8/eTreg ratios in TILs were consistently higher in patients who achieved pCR during study treatments than in those who did not (non-pCR; Fig. 2D), and the same trends were also observed in patients with TRG 0–1 (Supplementary Fig. S2D and S2E).
When we evaluated PD-L1 expression and the CD8/eTreg ratio in TILs jointly in the 24 patients with serial specimen collection, a pCR rate of 100% (5/5) was observed in patients with both PD-L1/TPS ≥1% and a CD8/eTreg ratio in TILs ≥ 2.5. On the other hand, a pCR rate of 8% (1/12) was observed in patients with both PD-L1/TPS <1% and a CD8/eTreg ratio in TILs < 2.5 (Table 3), and all patients with TRG 3 were included in this subpopulation.
Five patients with MSI-H LARC were evaluated, and neither the PD-L1/TPS status nor the CD8/eTreg ratio in TILs from pre-CRT samples was associated with the efficacy of study treatment in this exploratory analysis (Table 3).
Taken together, these results suggest that both PD-L1 expression in pre-CRT tumor cells and the CD8/eTreg ratio in TILs were strong predictive markers of pCR in patients with MSS LARC treated with our sequential therapy. Thus, a combination of these biomarkers potentially identifies the patients who exhibit response to this sequential therapy with the highest efficacy.
Ki-67, CTLA-4, and PD-1 expression by T cells as significant predictors of the efficacy of chemoradiotherapy plus nivolumab in patients with MSS LARC
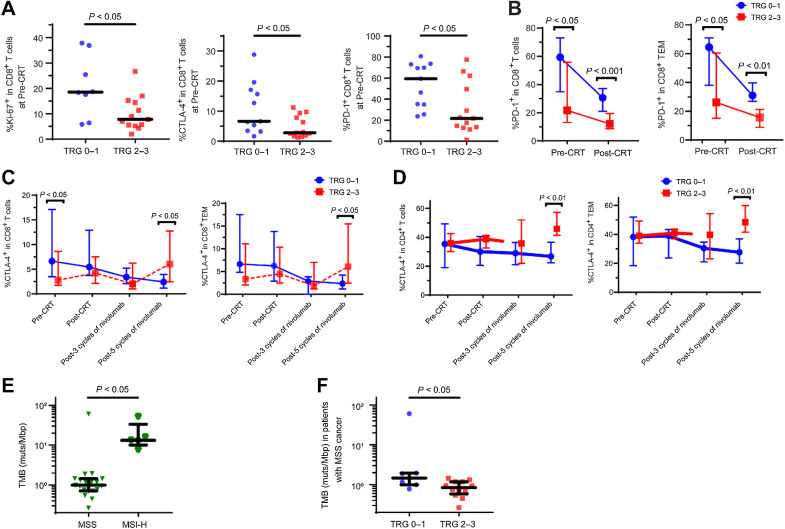
As the correlations between Ki-67, CTLA-4, and PD-1 expression levels in T cells and the efficacy of ICIs have been reported (9, 34, 35), we investigated the correlation between these factors and the efficacy of our sequential therapies. Ki-67, CTLA-4, and PD-1 expression levels by CD8+ T cells in TILs was significantly higher in the pre-CRT samples of patients who achieved TRG 0–1 than in those who achieved TRG 2–3 (P = 0.020, P = 0.030, P = 0.015, respectively, Mann–Whitney U test, Fig. 3A).
Figure 3.
Ki-67, PD-1, and CTLA-4 expression by CD8+ and CD4+ T cells in TILs in MSS LARC group and TMB in both the MSS LARC and MSI-H LARC groups. A, Ki-67, PD-1, and CTLA-4 expression by CD8+ T cells in tumor infiltrating lymphocytes (TILs) in patients who achieved tumor regression grade (TRG) 0–1 and in patients who achieved TRG 2–3 at pre-CRT are shown. The differences between the two groups of patients were compared using a Mann-Whitney U test. B, PD-1 expression by both CD8+ T cells and effector memory cells (TEM) in TILs in patients who achieved TRG 0–1 and in patients who achieved TRG 2–3 at both the pre-CRT and post-CRT time are shown. The differences between the two groups of patients were compared using a Mann-Whitney U test. Because PD-1 expression could not be correctly evaluated during nivolumab therapy due to crossing with the PD-1 antibody, the data obtained after nivolumab therapy are not shown. C, CTLA-4 expression by both CD8+ T cells and the TEM subset in TILs in patients who achieved TRG 0–1 and in patients who achieved TRG 2–3 at four time points (pre-CRT, post-CRT, post-3 cycles of nivolumab, and post-5 cycles of nivolumab) are shown. The differences between the two groups of patients were compared using a Mann-Whitney U test. D, CTLA-4 expression by both CD4+ T cells and the TEM subtype in TILs in patients who achieved TRG 0–1 and in patients who achieved TRG 2–3 at four time points (pre-CRT, post-CRT, post-3 cycles of nivolumab, and post-5 cycles of nivolumab) are shown. The differences between the two groups of patients were compared using a Mann-Whitney U test. E, The tumor mutational burden (TMB) in pre-CRT samples in patients with microsatellite instability-high (MSI-H) locally advanced rectal cancer and in patients with microsatellite stable (MSS) LARC is shown (median 13.2/Mbp vs. median 0.99/Mbp). The differences between the two groups of patients were compared using a Mann-Whitney U test. F, The TMB in pre-CRT samples in patients with MSS LARC who achieved TRG 0–1 (median 1.45 mutations/Mbp) and in patients who achieved TRG 2–3 (median 0.84 mutations/Mbp) is shown. The differences between the two groups of patients were compared using a Mann-Whitney U test. TEM, effector memory (CCR7−CD45RA−).
Although Ki-67 expression by CD8+ T cells was increased after CRT in both PBMCs and TILs, there were no significant differences between those who achieved TRG 0–1 and those who achieved TRG 2–3 (Supplementary Fig. S3B). In contrast, PD-1 expression by CD8+ T cells in TILs, especially in CD45RA−CCR7− effector memory (TEM) cell subset, was significantly higher in patients who achieved TRG 0–1, both before and after CRT, than in those who achieved TRG 2–3 (pre-CRT P = 0.015, post-CRT P = 0.0009, Mann–Whitney U test, Fig. 3B).
Conversely, CTLA-4 expression by both CD4+ and CD8+ T cells in TILs, especially in the TEM cell subset, was decreased in patients who achieved TRG 0–1 and tended to increase in patients who achieved TRG 2–3 during nivolumab treatment (Supplementary Fig. S3C and S3D). Furthermore, CTLA-4 expression by both CD4+ and CD8+ T cells, especially by TEM, was significantly higher after 5 cycles of nivolumab in patients who achieved TRG 2–3 than in those who achieved TRG 0–1 (CD4+ T cells and the TEM, P = 0.006 and P = 0.008; CD8+ T cells and the TEM, P = 0.024 and P = 0.024, Mann–Whitney U test, Fig. 3C and D). Taken together, Ki-67, CTLA-4, and PD-1 expression by CD8+ T cells in TILs from pre-CRT samples are positive predictive markers of pCR with our sequential therapies. The elevated CTLA-4 expressions by both CD4+ and CD8+ T cells after nivolumab might be associated with the reduced efficacy of nivolumab: a potential resistance mechanism of nivolumab monotherapy.
Consensus molecular subtype and tumor mutational burden as significant predictors of chemoradiotherapy plus nivolumab efficacy
To investigate the correlations between the status of gene mutations/expressions and the efficacy of our sequential therapy, whole-exome sequencing (WES) and RNA sequencing were performed in pre-CRT samples from 24 patients with MSS LARC and 5 patients with MSI-H LARC. Of 21 patients with MSS LARC whose consensus molecular subtype (CMS) was analyzable, those with CMS1 (MSI-immune) subtype achieved pCR rates of 100% (2/2). In contrast, patients with CMS2 (canonical), CMS3 (metabolic), and CMS4 (mesenchymal) achieved pCR rates of 29% (2/7), 40% (2/5), and 29% (2/7), respectively (Supplementary Table S5), indicating that CMS1 which is associated with gene expression of diffuse immune infiltrate is correlated with the efficacy of our sequential therapy. The tumor mutational burden (TMB) in the pre-CRT samples from the patients with MSI-H LARC (n = 5) was significantly higher than that in the samples from patients with MSS LARC (n = 19; median 13.2 mutations/Mbp vs. median 0.99 mutations/Mbp, P < 0.0001, Mann–Whitney U test, Fig. 3E). In patients with MSS LARC, the TMB in the pre-CRT samples was significantly higher in patients with TRG 0–1 (median 1.45 mutations/Mbp) than in those with TRG 2–3 (median 0.84 mutations/Mbp; P = 0.016, Mann–Whitney U test, Fig. 3F). However, no similar trends between those who achieved pCR and those who did not (non-pCR) were observed in the MSI-H group, although a small number of samples was investigated. Thus, higher TMB in patients with MSS LARC might be a good predictor of efficacy of our sequential therapy.
Microbiome analysis
To comprehensively investigate the correlations between fecal microbiomes and the efficacies of our sequential therapy, 16S ribosomal RNA (rRNA) sequencing was performed in the post-CRT stool samples from 32 patients with MSS LARC. The diversity of the fecal microbiomes was not significantly different between patients with pCR and those with non-pCR when evaluated using either the abundance of operational taxonomic units (OTU) or the Shannon index. The same was also true when comparing the diversity of the fecal microbiomes between patients who achieved TRG 0–1 and patients who achieved TRG 2–3 (Supplementary Fig. S4A). Beta diversities visualized using principal coordinate analysis (PCoA) were also not significantly different (Supplementary Fig. S4B). Considering the relative abundance of bacterial genera, no significant differences were found between patients with pCR and those with non-pCR (Supplementary Fig. S4C), indicating the diversity of fecal microbiome and the relative abundance of bacterial genera had no strong correlations to the efficacy of our sequential therapy in this limited sample.
Discussion
CRT followed by radical surgery is the standard therapy for patients with LARC. However, further developments are expected to improve both local and distant control. While ICIs have shown promise in solid tumors, they have been largely ineffective in patients with MSS CRC. To evaluate whether combining CRT and ICIs could improve efficacy in patients with MSS LARC, we performed this proof-of-concept study. Although several results of the combination of CRT and ICI in patients with LARC have been reported, this study is the first to investigate the efficacy and safety of preoperative CRT followed by ICIs in patients with LARC (26). We report that CRT followed by five cycles of consolidation nivolumab and radical surgery showed a promising pCR rate of 30% and acceptable mild toxicities in patients with MSS LARC, although it is a nonrandomized, single-arm, phase II trial. Our sequential combination also achieved a 60% pCR rate in patients with MSI-H LARC, suggesting the increased efficacy of nivolumab in this subgroup. Although the number of patients with MSI-H LARC was small due to the low incidence of left-sided MSI-H CRC (36), promising pCR rates suggested that CRT followed by nivolumab has potential to become the favorable treatment option for that population.
A meta-analysis that included previous prospective and retrospective studies suggested an association between longer intervals from CRT completion to surgery and improved pCR rates (37). However, the randomized GRECCAR6 trial demonstrated that an interval of 11 weeks yielded no significant differences in pCR rates as compared to 7 weeks (7 weeks, 15.0% vs. 11 weeks, 17.4%; P = 0.598; ref. 38). Another single-arm phase II trial demonstrated a 30% pCR rate with CRT followed by four cycles of consolidation FOLFOX at 12-week intervals (39). We observed a 30% pCR rate at a median 12.7-week interval in our MSS LARC cases, without consolidation FOLFOX, which suggested additional efficacy of nivolumab.
The recent NRG-GI002 study reported that preoperative FOLFOX followed by CRT with concurrent pembrolizumab did not improve pCR rates as compared with FOLFOX followed only by CRT (31.9% vs. 29.4%, P = 0.75; ref. 40). While there is no evidence to compare that concurrent or sequential combination of ICIs is a better strategy, our study suggests that CRT followed by consolidation nivolumab may enhance efficacy in certain subgroups of patients with LARC.
While a pCR rate of 30% in patients with MSS LARC was similar to that of previous single-arm phase II trials combining CRT and targeted therapies, our study differs in that we also performed various biomarker analyses to select the optimal beneficiaries of our study treatment. According to analyses using pre-CRT biopsy samples from our limited cohort, a 75% pCR rate was observed in patients with PD-L1/TPS-positive tumor samples. A promising pCR rate of 78% was also observed in patients with high (≥ 2.5) CD8/eTreg ratio in TILs. Given that pCR rates of 13%–17% in patients with negative PD-L1 expression or low (< 2.5) CD8/eTreg ratios in TILs are consistent with those of previous phase II/III clinical trials that investigated CRT followed by radical surgery, the synergistic effect of CRT plus nivolumab might be more evident in patients with positive PD-L1 expression or high CD8/eTreg ratios in TILs. In patients with both positive PD-L1 expression and high CD8/eTreg ratios, the highest pCR rate (100%; 5/5) was observed. As PD-L1 expression is related to the immunogenicity of tumor cells and elevated CD8/eTreg ratio in TILs is related to the potentiality of immune cells (33), those might be independent predictive factors and potential biomarkers to identify patients in whom our sequential therapy may be most effective. Because these findings came from small sample size analyses, future investigations with larger sample sizes are warranted.
Estimates of high efficacy were observed in the pre-CRT samples of patients with MSS LARC with a higher TMB. Because TMB is associated with a high frequency of neoantigens (41), those patients might be expected to have a higher efficacy similar to that observed in MSI-H LARC. In addition, both cases with CMS1 indicated pCR. While CMS1 is associated with gene expression of diffuse immune infiltrate (39), predicting efficacies of ICI, patients with other CMS levels had pCR rates of 29%–40%. Analyses with larger samples are needed to confirm the higher efficacy of ICIs in patients with CMS1. Higher Ki-67, CTLA-4, and PD-1 expression by CD8+ T cells in TILs was also higher in the pre-CRT samples of patients with TRG 0–1 than in those with TRG 2–3. These findings were consistent with those of previous reports on ICI treatments in various types of solid tumors, including MSS colon cancer responders treated with preoperative nivolumab plus ipilimumab in the Dutch NICHE study. (9, 35)
In contrast, CTLA-4 expression by both CD8+ and CD4+ T cells in TILs was high after the administration of nivolumab in poor responders; this was particularly evident in the TEM cell subset of the TILs. In addition, tumor-infiltrating eTreg cells, which can be decreased with ipilimumab, were highly elevated in poor responders (42). These findings suggest that the addition of ipilimumab might overcome resistance to the sequential combination of CRT plus ICIs. We are currently investigating the efficacy of this approach using the sequential combination of CRT plus nivolumab and ipilimumab in an ongoing study of patients with MSS LARC (VOLTAGE Cohort D). Further clinical and translational analyses will elucidate the situations in which CRT plus anti–PD-1 monotherapy, or the anti–PD-1 plus anti–CTLA-4 doublet should be used based on predictive biomarkers, leading to precision immunotherapy with higher efficacy rates and milder toxicities in the future.
Our study had several limitations. This was a nonrandomized, phase I/II study with a relatively small sample size. Although promising pCR rates were reported, long-term survival data has not been reported. To confirm the survival benefits of the sequential combination of CRT with ICIs, confirmatory, international, randomized phase III trials comparing standard CRT to our sequential combination, especially in patients with MSS LARC with biomarker selected populations, (PD-L1/TPS positive and/or high CD8/eTreg ratios) are needed.
In conclusion, preoperative CRT followed by nivolumab and radical surgery, which was associated with only mild toxicities, may also be associated with increased efficacy in patients with MSS LARC, as indicated by the promising pCR results observed. PD-L1 TPS expression and an elevated CD8/eTreg ratio in TILs appear to be positive predictors of nivolumab efficacy, warranting further study in a larger cohort.
Supplementary Material
Acknowledgments
This investigator-initiated trial was funded by Ono Pharmaceuticals Co., Ltd. We would like to express special thanks to all participating patients, their families, all participating investigators, the members of the data and safety monitoring board [Atsushi Sato (Hirosaki University), Keiji Koda (Teikyo University Chiba Medical Centre), and Koji Oba (Tokyo University)], the members of the radiation assessment committee [Hidenobu Tachibana, Naoki Nakamura, and Tetsuo Akimoto (National Cancer Centre Hospital East)], the members of the central surgical assessment committee [Ichiro Takemasa (Sapporo Medical University), Koki Otsuka (Iwate Medical University), and Masayoshi Nakanishi (Kyoto Prefectural University)], technical assistants (Miyuki Nakai, Chie Haijima, Megumi Hoshino, Kumiko Yoshida, Tomoka Takaku, Konomi Onagawa, Megumi Takemura, Yumi Osada, Miho Ozawa, and Yuka Nakamura), and Ayako Suzuki and Erina Ishikawa (University of Tokyo) for gene analysis. The VOLTAGE study is an investigator-initiated investigational new drug (IND) clinical trial. A research grant and nivolumab were provided by Ono Pharmaceutical Co., Ltd. (Osaka, Japan).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
This article is featured in Highlights of This Issue, p. 1053
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Authors' Disclosures
H. Bando reports grants from Ono Pharmaceutical during the conduct of the study as well as grants from Sysmex and AstraZeneca and other support from Taiho Pharmaceutical and Eli Lilly outside the submitted work. Y. Tsukada reports grants from Ono Pharmaceutical Co., Ltd. during the conduct of the study. K. Inamori reports grants from Ono Pharmaceutical Co., Ltd. during the conduct of the study. Y. Togashi reports grants and personal fees from Ono Pharmaceutical, BMS, and Chugai Pharmaceutical; personal fees from MSD; and grants from Kotai Biotechnologies, Daiichi Sankyo, and Kortuc outside the submitted work. D. Kotani reports grants and personal fees from Ono and MSD; personal fees from Pfizer, Taiho, Daiichi-Sankyo, Takeda, Chugai, Eli Lilly, Bristol Myers Squibb, and Merck Biopharma; and grants from Novartis, Janssen, IQVIA, Syneos, and CMIC Shift Zero outside the submitted work. S. Yuki reports personal fees from Ono Pharmaceutical Co., Ltd. and Bristol-Myers Squibb Co., Ltd. outside the submitted work. Y. Komatsu reports grants and personal fees from Ono Pharmaceutical Co., Ltd., Chugai, BMS, Taiho, Lilly, Nipro, and Sanofi outside the submitted work. T. Kato reports personal fees from Chugai Pharmaceutical Co., Ltd and Ono Pharmaceutical Co., Ltd. outside the submitted work. A. Sato reports grants from Ono Pharmaceutical Co., Ltd. during the conduct of the study as well as grants from MSD, Eisai Pharma, Taiho Pharma, Takeda Pharma, Bayer Pharma, Rakuten Medical Pharma, Oncolys Bio Pharma, and AstraZeneca Pharma outside the submitted work. H. Nishikawa reports grants and other support from Ono Pharmaceutical Co., Ltd., Chugai Pharmaceuticals, Bristol Myers Squibb, and MSD Pharmaceutical and grants from Taiho Pharmaceutical, Daiichi-Sankyo, Kyowa Kirin, Zenyaku Kogyo, Oncolys BioPharma, Debiopharma, Asahi-Kasei, Sysmex, Fujifilm, SRL, Astellas Pharmaceuticals, Sumitomo Dainippon Pharma, and Beckton Dickinson Japan outside the submitted work. M. Ito reports grants from Ono Pharmaceutical Co., Ltd. during the conduct of the study as well as grants from Nippon Covidien Ltd., Akita Sumitomo Bakelite Co., Ltd., Indivumed GmbH, Muranaka Medical Instruments Co., Ltd., Fujifilm Corporation, EP-CRSU Co., Ltd., Fujita Medical Instruments Co., Ltd., and Kawasumi Laboratories, Incorporated; grants and personal fees from Johnson & Johnson K.K. Medical Company; and personal fees from Olympus Corporation, Taiho Pharmaceutical Co., Ltd., Applied Medical Japan, Covidien Japan Inc., Daiichi Sankyo Company, Limited, Tsumura & Co., Sanofi K.K., Miyarisan Pharmaceutical Co., Ltd., Rakuten Medical, Kotobuki Medical Inc., and Chugai Pharmaceutical Co., Ltd. outside the submitted work. T. Yoshino reports grants from Ono Pharmaceutical Co., Ltd. during the conduct of the study as well as grants from Taiho Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Chugai Pharmaceuticals Co., Ltd., Amgen K.K., MSD K.K., Daiichi Sankyo Co., Ltd., Sanofi K.K., and Parexel International Corp. outside the submitted work. No disclosures were reported by the other authors.
Authors' Contributions
H. Bando: Conceptualization, resources, investigation, writing–original draft. Y. Tsukada: Conceptualization, resources, investigation, writing–original draft. K. Inamori: Resources, investigation. Y. Togashi: Investigation, writing–review and editing. S. Koyama: Resources, writing–review and editing. D. Kotani: Resources, writing–review and editing. S. Fukuoka: Resources, investigation, writing–review and editing. S. Yuki: Resources, investigation, writing–review and editing. Y. Komatsu: Resources, investigation, writing–review and editing. S. Homma: Resources, investigation, writing–review and editing. A. Taketomi: Resources, investigation, writing–review and editing. M. Uemura: Resources, investigation, writing–review and editing. T. Kato: Resources, investigation, writing–review and editing. M. Fukui: Validation, investigation, writing–review and editing. M. Wakabayashi: Formal analysis, writing–review and editing. N. Nakamura: Resources, investigation, writing–review and editing. M. Kojima: Resources, investigation, writing–review and editing. H. Kawachi: Validation, investigation, writing–review and editing. R. Kirsch: Validation, investigation, writing–review and editing. T. Yoshida: Validation, investigation, writing–review and editing. Y. Suzuki: Formal analysis, supervision, investigation, writing–review and editing. A. Sato: Data curation, formal analysis, project administration, writing–review and editing. H. Nishikawa: Formal analysis, supervision, writing–review and editing. M. Ito: Conceptualization, supervision, investigation, writing–review and editing. T. Yoshino: Conceptualization, supervision, funding acquisition, writing–original draft.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 2. Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery–the clue to pelvic recurrence? Br J Surg 1982;69:613–6. [DOI] [PubMed] [Google Scholar]
- 3. Lowry AC, Simmang CL, Boulos P, Farmer KC, Finan PJ, Hyman N, et al. Consensus statement of definitions for anorectal physiology and rectal cancer: report of the tripartite consensus conference on definitions for anorectal physiology and rectal cancer, Washington, D.C., May 1, 1999. Dis Colon Rectum 2001;44:915–9. [DOI] [PubMed] [Google Scholar]
- 4. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40. [DOI] [PubMed] [Google Scholar]
- 5. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461–71. [DOI] [PubMed] [Google Scholar]
- 7. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:1506–17. [DOI] [PubMed] [Google Scholar]
- 8. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 2020;26:566–76. [DOI] [PubMed] [Google Scholar]
- 10. Chen EX, Jonker DJ, Loree JM, Kennecke HF, Berry SR, Couture F, et al. Effect of combined immune checkpoint inhibition vs best supportive care alone in patients with advanced colorectal cancer: The Canadian Cancer Trials Group CO.26 study. JAMA Oncol 2020;6:831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawazoe A, Kuboki Y, Shinozaki E, Hara H, Nishina T, Komatsu Y, et al. Multicenter phase 1/2 Trial of napabucasin and pembrolizumab in patients with metastatic colorectal cancer (EPOC1503/SCOOP Trial). Clin Cancer Res 2020;26:5887–94. [DOI] [PubMed] [Google Scholar]
- 12. Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase Ib trial (REGONIVO, EPOC1603). J Clin Oncol 2020;38:2053–61. [DOI] [PubMed] [Google Scholar]
- 13. Cousin S, Cantarel C, Guegan JP, Gomez-Roca C, Metges JP, Adenis A, et al. Regorafenib-avelumab combination in patients with microsatellite stable colorectal cancer (REGOMUNE): a single-arm, open-label, phase II trial. Clin Cancer Res 2021;27:2139–47. [DOI] [PubMed] [Google Scholar]
- 14. Segal NH, Cercek A, Ku G, Wu AJ, Rimner A, Khalil DN, et al. Phase II single-arm study of durvalumab and tremelimumab with concurrent radiotherapy in patients with mismatch repair-proficient metastatic colorectal cancer. Clin Cancer Res 2021;27:2200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dovedi SJ, Adlard AL, Lipowska-Bhalla G, McKenna C, Jones S, Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014;74:5458–68. [DOI] [PubMed] [Google Scholar]
- 17. Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol 2015;1:1325–32. [DOI] [PubMed] [Google Scholar]
- 18. Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res 2015;3:345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park SS, Dong H, Liu X, Harrington SM, Krco CJ, Grams MP, et al. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol Res 2015;3:610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wei J, Montalvo-Ortiz W, Yu L, Krasco A, Ebstein S, Cortez C, et al. Sequence of alphaPD-1 relative to local tumor irradiation determines the induction of abscopal antitumor immune responses. Sci Immunol 2021;6:eabg0117. [DOI] [PubMed] [Google Scholar]
- 21. Sundahl N, Seremet T, Van Dorpe J, Neyns B, Ferdinande L, Meireson A, et al. Phase 2 trial of nivolumab combined with stereotactic body radiation therapy in patients with metastatic or locally advanced inoperable melanoma. Int J Radiat Oncol Biol Phys 2019;104:828–35. [DOI] [PubMed] [Google Scholar]
- 22. Sundahl N, Vandekerkhove G, Decaestecker K, Meireson A, De Visschere P, Fonteyne V, et al. Randomized phase 1 trial of pembrolizumab with sequential versus concomitant stereotactic body radiotherapy in metastatic urothelial carcinoma. Eur Urol 2019;75:707–11. [DOI] [PubMed] [Google Scholar]
- 23. Theelen W, Peulen HMU, Lalezari F, van der Noort V, de Vries JF, Aerts J, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol 2019;5:1276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 2017;377:1919–29. [DOI] [PubMed] [Google Scholar]
- 25. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342–50. [DOI] [PubMed] [Google Scholar]
- 26. Bando H, Tsukada Y, Ito M, Yoshino T. Novel immunological approaches in the treatment of locally advanced rectal cancer. Clin Colorectal Cancer 2021. [DOI] [PubMed] [Google Scholar]
- 27. Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, et al. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med 2016;22:679–84. [DOI] [PubMed] [Google Scholar]
- 28. George TJ, Allegra CJ, Yothers G. Neoadjuvant Rectal (NAR) score: a new surrogate endpoint in rectal cancer clinical trials. Current Colorectal Cancer Reports 2015;11:275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trakarnsanga A, Gonen M, Shia J, Nash GM, Temple LK, Guillem JG, et al. Comparison of tumor regression grade systems for locally advanced rectal cancer after multimodality treatment. J Natl Cancer Inst 2014;106:dju248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009;30:899–911. [DOI] [PubMed] [Google Scholar]
- 31. Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol 2019;16:356–71. [DOI] [PubMed] [Google Scholar]
- 32. Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood 2007;110:2983–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol 2020;21:1346–58. [DOI] [PubMed] [Google Scholar]
- 34. Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017;545:60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A 2017;114:4993–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Rosa N, Rodriguez-Bigas MA, Chang GJ, Veerapong J, Borras E, Krishnan S, et al. DNA mismatch repair deficiency in rectal cancer: benchmarking its impact on prognosis, neoadjuvant response prediction, and clinical cancer genetics. J Clin Oncol 2016;34:3039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Petrelli F, Sgroi G, Sarti E, Barni S. Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer: a meta-analysis of published studies. Ann Surg 2016;263:458–64. [DOI] [PubMed] [Google Scholar]
- 38. Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C, et al. Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6). J Clin Oncol 2016;34:3773–80. [DOI] [PubMed] [Google Scholar]
- 39. Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 2015;16:957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rahma OE, Yothers G, Hong TS, Russell MM, You YN, Parker W, et al. Use of total neoadjuvant therapy for locally advanced rectal cancer: Initial Results From the Pembrolizumab Arm of a phase 2 randomized clinical trial. JAMA Oncol 2021;7:1225–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 2017;377:2500–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Romano E, Kusio-Kobialka M, Foukas PG, Baumgaertner P, Meyer C, Ballabeni P, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A 2015;112:6140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA- and DNA-sequencing data will be deposited into the Japanese Genotype-Phenotype Archive and will be made available on reasonable request for academic use and within the limitations of the provided informed consent. Accession process is ongoing. Every request will be reviewed by the institutional review board of the National Cancer Center (NCC); the researcher will need to sign a data access agreement with the NCC after approval.