Summary
Background
China implemented strict non-pharmaceutical interventions to contain COVID-19 at the early stage. We aimed to evaluate the impact of COVID-19 on HIV care continuum in China.
Methods
Aggregated data on HIV care continuum between 1 January 2017 and 31 December 2020 were collected from centers for disease control and prevention at different levels and major infectious disease hospitals in various regions in China. We used interrupted time series analysis to characterize temporal trend in weekly numbers of HIV post-exposure prophylaxis (PEP) prescriptions, HIV tests, HIV diagnoses, median time intervals between HIV diagnosis and antiretroviral therapy (ART) initiation (time intervals, days), ART initiations, mean CD4+ T cell counts at ART initiation (CD4 counts, cells/μL), ART collections, and missed visits for ART collection, before and after the implementation of massive NPIs (23 January to 7 April 2020). We used Poisson segmented regression models to estimate the immediate and long-term impact of NPIs on these outcomes.
Findings
A total of 16,780 PEP prescriptions, 1,101,686 HIV tests, 69,659 HIV diagnoses, 63,409 time intervals and ART initiations, 61,518 CD4 counts, 1,528,802 ART collections, and 6656 missed visits were recorded during the study period. The majority of outcomes occurred in males (55·3-87·4%), 21-50 year olds (51·7-90·5%), Southwestern China (38·2-82·0%) and heterosexual transmission (47·9-66·1%). NPIs was associated with 71·5% decrease in PEP prescriptions (IRR 0·285; 95% CI 0·192-0·423), 36·1% decrease in HIV tests (0·639, 0·497-0·822), 32·0% decrease in HIV diagnoses (0·680, 0·511-0·904), 59·3% increase in time intervals (1·593, 1·270-1·997) and 17·4% decrease in CD4 counts (0·826, 0·746-0·915) in the first week during NPIs. There was no marked change in the number of ART initiations, ART collections and missed visits during the NPIs. By the end of 2020, the number of HIV tests, HIV diagnoses, time intervals, ART initiations, and CD4 counts reached expected levels, but the number of PEP prescriptions (0·523, 0·394-0·696), ART collections (0·720, 0·595-0·872), and missed visits (0·137, 0·086-0·220) were still below expected levels. With the ease of restrictions, PEP prescriptions (slope change 1·024/week, 1·012-1·037), HIV tests (1·016/week, 1·008-1·026), and CD4 counts (1·005/week, 1·001-1·009) showed a significant increasing trend.
Interpretation
HIV care continuum in China was affected by the COVID-19 NPIs at various levels. Preparedness and efforts to maintain the HIV care continuum during public health emergencies should leverage collaborations between stakeholders.
Funding
Natural Science Foundation of China.
Keywords: COVID-19, Non-pharmaceutical interventions (NPIs), HIV, Interrupted time series analysis, China
Research in context.
Evidence before this study
The COVID-19 pandemic and subsequent implementation of non-pharmaceutical interventions (NPIs) have had a significant impact on HIV prevention and care services. From March 7, 2021, to March 27, 2022, we searched all relevant literature on PubMed using the term (“COVID-19” [tiab] OR “SARS-CoV-2” [tiab] OR coronavirus[tiab] OR “2019-nCoV”[tiab] OR nCoV[tiab]) AND (HIV[tiab] OR AIDS[tiab] OR HIV/AIDS[tiab] OR “human immunodeficiency virus”[tiab] OR “Acquired Immune Deficiency Syndrome”[tiab]). Data from many large sexual health clinics and facilities around the world show significant decreases in HIV post-exposure prophylaxis (PEP) prescriptions, HIV tests, HIV diagnoses, HIV antiretroviral therapy (ART), and AIDS-related deaths following COVID-19 restrictions. However, most of the studies are from developed countries. Although indicators of HIV care continuum such as HIV pre-exposure prophylaxis (PrEP) initiations, PrEP adherence, condom uses, PEP prescriptions, health service provisions, HIV tests, HIV diagnoses, ART initiations, ART collections, viral load (VL) tests, VL suppressions, missed visits for ART collection, and AIDS-related deaths were reported in different studies, existing studies were mostly either from smaller sites or only reported part of the key indicators, over a short period. No nationally representative data that report on a complete spectrum of HIV care continuum have been published.
Added value of this study
Our study systematically analysed the impact of COVID-19 on a complete spectrum of HIV care continuum in China, using long-term data collected from various regions in the country. Parameters were adjusted for long-term trends and seasonality. At the first week of the COVID-19 related NPIs, PEP prescriptions, HIV tests, HIV diagnoses, and mean CD4 counts at ART initiation showed significant declines, while median time intervals between HIV diagnosis and ART initiation increase significantly. No significant changes in the number of ART initiations, ART collections, and missed visits for ART collection were observed in the first week. By the end of 2020, the number of HIV tests, HIV diagnoses, median time intervals between HIV diagnosis and ART initiation, ART initiations, and mean CD4 counts at ART initiation reached expected levels, but the number of PEP prescriptions, ART collections, and missed visits for ART collection were below expected levels.
Implications of all the available evidence
HIV care continuum in China was affected by the COVID-19 NPIs at various levels. Preparedness and efforts to maintain the HIV care continuum during public health emergencies should leverage collaborations between stakeholders. Future studies are needed to evaluate the long-term changes of PEP prescriptions, ART collections, and missed visits for ART collection in China after COVID-19.
Alt-text: Unlabelled box
Introduction
The ongoing coronavirus disease 2019 (COVID-19) global pandemic has been creating significant challenges to the health-care system and disrupting the delivery of routine medical services. COVID-19 and its impacts on the HIV/AIDS response might severely affect vulnerable populations such as men who have sex with men (MSM), migrant workers, sex workers, people who inject drugs (PWID) and sexually active adolescents and young people.1, 2, 3
Reductions in access to health services—such as post-exposure prophylaxis (PEP) for individuals at high risk of HIV infection, routine HIV testing, harm reduction services for PWID, initiation or continuation of antiretroviral therapy (ART) for people living with HIV (PLHIV)—due to the COVID-19 pandemic may prompt increases in HIV transmission, further challenging previous efforts toward the goals of Ending HIV. For example, London of the UK4 and Melbourne of Australia5 saw 80% and 66% decreases in PEP prescriptions comparing 4 weeks before and after restrictions. 47·6% decrease in HIV testing was reported in KwaZulu-Natal of South Africa during the first month of lockdown.6 Although less sexual contact translates into less transmission of HIV, this benefit may be offset by the suspension of HIV testing, interruption to ART and condom supply, poorer clinical care due to overstretched health facilities, and interruptions of supply of other drugs. For example, a modeling study found that in sub-Saharan Africa, a 6-month interruption of ART supply across 50% of PLHIV who are on treatment would lead to a median excess of 296,000 HIV deaths.7
China implemented strict nonpharmaceutical interventions (NPIs) such as city lockdowns and traffic controls, wearing a face mask, as well as mandating people to stay home, preventing mass gatherings, canceling or postponing large public events, and closing schools, universities, government offices, libraries, museums, and factories in the early stages of the outbreak to contain the spread of the epidemic.8, 9, 10, 11, 12, 13, 14, 15, 16 China has large populations who are at potential risk for HIV transmission and a large population of PLHIV, interruption of HIV care services exacerbated the HIV burden in the country. By the end of 2020, a total of 1·05 million PLHIV had been reported, with a population prevalence of 0·075%.17 PLHIV in China access HIV care services, including CD4 and viral load monitoring, ART collection, etc., at designated hospitals that are specialized in infectious disease treatment. HIV treatment and CD4 and viral load monitoring are publicly covered. In eastern China's Jiangsu Province, the HIV testing rate among the general population decreased by 49·0% in the first three months of implementing COVID-19 measures, compared to the expected proportion.18 HIV PEP prescriptions in Guangzhou halved between January and April 2020, when strict COVID-19 containing measures were in place. AIDS Concern Hong Kong closed its HIV testing center, stopped all physical outreach, venue-based testing, education programs and testing at saunas, and encouraged people to self-test during the COVID-19 pandemic.19 Collateral damage from the COVID-19 outbreak affected the PLHIV community in the country. The COVID-19 outbreak in China coincided with the Lunar New Year holiday when hundreds of millions of people traveled back to their hometowns. Transport suspension resulted in many PLHIV getting stranded in their hometowns, often far away from their designated clinics. On rare occasions patients may arrange to have the drugs shipped to them but this is done on an ad-hoc basis and at the discretion of the provider. Over one-third of PLHIV in China reported any risk of ATR interruption (ATI) during the early stage of COVID-19 outbreak, including 2·7% who experienced ATI, 18·0% at risk of imminent ATI and 14·4% at threatened but resolved risk.20
The latest study found that HIV testing and new ART initiations were inversely associated with stringency measures.21 Existing studies in China were either from smaller sites and only reported part of the key parameters. For example, a survey of 703 PLHIV found that 22·8% reported disruptions in their medication uptake and 67·5% reported concerns about disruption in their medication use and future clinical care.22 A study in Guangxi, China found that 22·0% of healthcare providers classified as working in HIV clinics with complete disruption, 15·4% classified as working in HIV clinics with moderate disruption, 21·9% and 40·8% classified as working in HIV clinics with minor or almost no disruption during the COVID-19 pandemic.23 Another study in Guangxi found that the prominent impacts of COVID-19 on patient care outcomes included “not being able to make follow-up visits on time”, “not being able to get antiretroviral drugs refills timely”, and “compromised ART adherence”.24
According to the World Health Organization (WHO), COVID-19 has affected more than 200 countries and territories, with more than 380 million confirmed cases and more than 5·70 million deaths as of 4 February 2022.25 Omicron and other new variants are emerging and vaccines have limited protection against infection.26 NPIs such as city lockdown are likely to remain in many places in the foreseeable future,27 and its impact on HIV care is likely to continue. To better understand the impact of COVID-19 NPIs on the HIV care continuum at the country-level and provide implications for potential measures to maintain HIV care during future large-scale public health emergencies, we conducted a systematic analysis on the changes in PEP prescriptions, HIV tests, HIV diagnoses, median time intervals between HIV diagnosis and ART initiation (median time intervals, days), ART initiations, mean CD4+ T cell counts at ART initiation (CD4 counts, cells/μL), ART collections, and missed visits for ART collection, based on data from various regions in China.
Methods
Study design
Aggregated data on HIV care continuum, including PEP prescriptions, HIV tests, HIV diagnoses, median time intervals, ART initiations, CD4 counts, ART collections, and missed visits were collected from seven provincial or municipal centers for disease control and prevention (CDC) in Jiangsu, Chongqing, Guangzhou, Shenzhen, Shenyang, Shijiazhuang, and Dehong, and ten major infectious disease hospitals specializing in HIV care in Guangzhou, Hohhot, Tianjin, Kunming, Shenyang, Nanjing, Beijing, and Dalian, between 1 January 2017 and 31 December 2020 (study period). These regions collectively represent 16·2% of the total population and 15·9% of newly reported HIV/AIDS cases in China in 2020. These regions do not overlap, except for Nanjing which is part of Jiangsu province. In the data used for each indicator calculation, we do not use data from Nanjing unless there is no data from Jiangsu. The intensity and timing of NPIs in each study region are shown in the Table S1.
After accounting for seasonality, interrupted time series analysis was used to assess whether exposure to the COVID-19 NPIs affected weekly PEP prescriptions, HIV tests, HIV diagnoses, median time intervals, ART initiations, CD4 counts, ART collections and missed visits. In China, centers for disease control and prevention at various levels are a public health system to implement public health technical management and services, and is responsible for HIV surveillance. Designated infectious disease hospitals specializing in HIV care are responsible for the treatment and care of PLHIV.
Outcomes
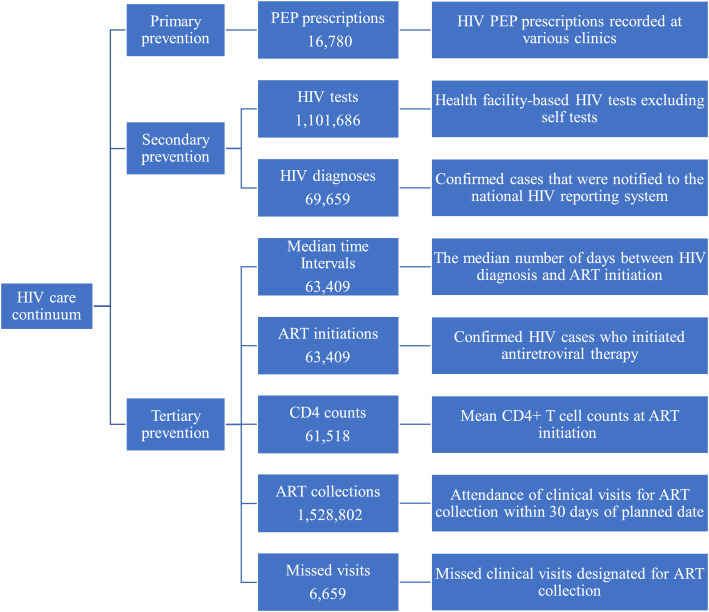
The primary outcome was the daily number of PEP prescriptions, HIV tests, HIV diagnoses, median time intervals, ART initiations, CD4 counts, ART collections and missed visits for ART collection before, during, and after the implementation of NPIs (23 January to 7 April, 2020; Wuhan lockdown, signaling the start of the nationwide NPIs). We stratified outcome data by gender, age, region and route of transmission. PEP prescriptions were based on HIV PEP visits recorded at all clinics. HIV tests refer to health facility-based HIV tests excluding self-tests. HIV diagnoses refer to confirmed cases that were notified to the National Comprehensive Response Information Management System-the real tie case reporting system for HIV care services in China. Median time intervals refer to the median number of days between HIV diagnosis and ART initiation. ART initiations refer to confirmed HIV cases who initiated ART. CD4 counts refer to mean CD4+ T cell counts at ART initiation. ART collections refer to attendance of clinical visits for ART collection within 30 days of the planned date. Missed visits for ART collection refer to missed clinical visits designated for ART collection (Figure 1).
Figure 1.
Definition of indicators of HIV care continuum.
PEP= post-exposure prophylaxis. ART= antiretroviral therapy. Median time intervals= median time intervals between HIV diagnosis and ART initiation (days). CD4 counts= mean CD4 counts at ART initiation (cells/μL). Missed visits=Missed visits for ART collection.
Statistical analysis
We used descriptive statistics to summarise demographic and clinical data before, during, and after the implementation of NPIs on a daily basis, and used an interrupted time series analysis to describe trends in changes in primary outcomes before and after the implementation of NPIs on a weekly basis. We used Poisson segmented regression models to estimate the immediate and long-term effect of NPIs on these outcomes, as well as post-NPIs trends. Due to the aggregation of data on a calendar weekly basis, the timepoint of the implementation of NPIs was adjusted to 20 January 2020 in the model to approximate the time of Wuhan lockdown and the start of the nationwide NPIs. The Poisson model included a time variable, a dummy NPIs variable indicating pre- and post-NPIs, and an interaction term between the time and the dummy variables.28,29 This approach allowed us to center time at different timepoints to assess the immediate and long-term effects of the NPIs.6,29 Since the impact of the COVID-19 NPIs may not be immediate, we show the changes in indicators of HIV care for each of the three weeks during NPIs.
To control for potential confounders of the COVID-19 NPIs effect, we adjusted for seasonality and long-term disease trends. We adjusted for seasonal variation in clinic activity by including a Fourier term consisting of two sine/cosine pairs in the model,30 and performed a sensitivity analysis that did not take into account seasonality. We then used the model before NPIs to predict what the data for the primary outcome would have looked like if COVID-19 had not occurred, starting on 20 January 2020, which represents the counterfactual. We calculated the incidence rate ratio (IRR) by comparing the fitted figures of the seasonally adjusted model with the expected figures of the counterfactual for the same period. To estimate the trend for each outcome in post-NPIs period, we summed the coefficients of the time and time-NPIs interaction terms. To eliminate autocorrelation and heteroskedasticity, we adjusted the standard errors of the parameters of the model by the Newey-west method to calculate the 95% CI of the IRR,30,31 with the lag taking the optimal value calculated.
To present more details about the regional heterogeneity, we divided the provinces and cities included in our study into five regions based on geographical locations and built separate models. We also built separate models according to gender, age, and route of transmission. Some regions did not report the number of missed visits. Route of transmission was available for median time intervals, ART initiations, CD4 counts, ART collections and missed visits. We performed all analyses in R 4·0·4 (R Foundation for Statistical Computing, Vienna, Austria; appendix).
Role of the funding source
This study was supported by the Natural Science Foundation of China Excellent Young Scientists Fund [82022064], the Special Support Plan for High-Level Talents of Guangdong Province [2019TQ05Y230] and the High Level Project of Medicine in Longhua, Shenzhen [HLPM201907020105]. All funding parties did not have any role in the design of the study or in the explanation of the data. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
A total of 16,780 PEP prescriptions, 1,101,686 HIV tests, 69,659 HIV diagnoses, 63,409 median time intervals and ART initiations, 61,518 CD4 counts, 1,528,802 ART collections, and 6656 missed visits for ART collection were recorded between 1 January, 2017 and 31 December, 2020. Detailed descriptions of indicators are provided in Figure 1. The majority of outcomes occurred in males (55·3-87·4%), 21-50 year olds (51·7-90·5%), Southwestern China (38·2-82·0%) and heterosexual transmission (47·9-66·1%). The age recorded in HIV tests and PEP prescriptions was the youngest, with median ages of 28 (interquartile range [IQR] 23-38) and 29 (24-34) years, respectively. The oldest, with median age of 49 (35-63) years, was recorded at missed visits. Detailed characteristics of people included in individual outcomes and data sources of indicators are provided in Table 1 and Table S2.
Table 1.
Demographics of people included in key indicators of HIV care continuum in China 1 January 2017 to 31 December 2020.
| PEP prescriptions | HIV tests | HIV diagnoses | Median time intervals | ART initiations | CD4 counts | ART collections | Missed visits | |
|---|---|---|---|---|---|---|---|---|
| Total | 16780 | 1101686 | 69659 | 63409 | 63409 | 61518 | 1528802 | 6659 |
| Gender | ||||||||
| Male | 14664 (87·4%) | 608992 (55·3%) | 57057 (81·9%) | 52788 (83·3%) | 52788 (83·3%) | 51161 (83·2%) | 1233567 (80·7%) | 5124 (76·9%) |
| Female | 2116 (12·6%) | 492694 (44·7%) | 12602 (18·1%) | 10621 (16·7%) | 10621 (16·7%) | 10357 (16·8%) | 295235 (19·3%) | 1535 (23·1%) |
| Age, years | ||||||||
| Median (IQR) | 29 (24-34) | 28 (23-38) | 43 (29-56) | 41 (29-55) | 41 (29-55) | 42 (29-55) | 41 (31-53) | 49 (35-63) |
| 0-20 | 1077 (6·4%) | 168586 (15·3%) | 3148 (4·5%) | 2516 (4·0%) | 2516 (4·0%) | 2429 (3·9%) | 21773 (1·4%) | 81 (1·2%) |
| 21-30 | 8969 (53·5%) | 467299 (42·4%) | 17351 (24·9%) | 16781 (26·5%) | 16781 (26·5%) | 16088 (26·2%) | 327156 (21·4%) | 1041 (15·6%) |
| 31-50 | 6203 (37%) | 372000 (33·8%) | 24023 (34·5%) | 22933 (36·2%) | 22933 (36·2%) | 22148 (36·0%) | 715340 (46·8%) | 2403 (36·1%) |
| >50 | 531 (3·2%) | 93801 (8·5%) | 25137 (36·1%) | 21179 (33·4%) | 21179 (33·4%) | 20853 (33·9%) | 464533 (30·4%) | 3134 (47·1%) |
| Region | ||||||||
| Eastern China | 2347 (14·0%) | 350544 (31·8%) | 16560 (23·8%) | 17129 (27·0%) | 17129 (27·0%) | 14935 (24·3%) | 178686 (11·7%) | 0 |
| South-Central China | 6983 (41·6%) | 155992 (14·2%) | 8842 (12·7%) | 2043 (3·2%) | 2043 (3·2%) | 2122 (3·4%) | 64870 (4·2%) | 0 |
| Southwestern China | 6402 (38·2%) | 420417 (38·2%) | 36012 (51·7%) | 28788 (45·4%) | 28788 (45·4%) | 29086 (47·3%) | 822004 (53·8%) | 5464 (82·0%) |
| Northeastern China | 124 (0·7%) | 91664 (8·3%) | 2609 (3·7%) | 6554 (10·3%) | 6554 (10·3%) | 6449 (10·5%) | 201398 (13·2%) | 0 |
| Northern China | 924 (5·5%) | 83069 (7·5%) | 5636 (8·1%) | 8895 (14·0%) | 8895 (14·0%) | 8926 (14·5%) | 261844 (17·1%) | 1195 (18·0%) |
| Route of transmission | ||||||||
| Heterosexual | NA | NA | NA | 31178 (49·2%) | 31178 (49·2%) | 30459 (49·5%) | 732498 (47·9%) | 4400 (66·1%) |
| Homosexual | NA | NA | NA | 27499 (43·4%) | 27499 (43·4%) | 26491 (43·1%) | 636031 (41·6%) | 1108 (16·6%) |
| Others | NA | NA | NA | 4732 (7·5%) | 4732 (7·5%) | 4568 (7·4%) | 160273 (10·5%) | 1151 (17·3%) |
PEP= post-exposure prophylaxis. ART= antiretroviral therapy. Median time intervals= median time intervals between HIV diagnosis and ART initiation (days). CD4 counts= mean CD4 counts at ART initiation (cells/μL). Missed visits=Missed visits for ART collection. Eastern China: Jiangsu and Nanjing. South-Central China: Guangzhou and Shenzhen. Southwestern China: Kunming, Dehong and Chongqing. Northeastern China: Hohhot, Shenyang and Dalian. Northern China: Tianjin, Shijiazhuang and Beijing. Route of transmission was available for median time intervals, ART initiations, CD4 counts, ART collections and missed visits.
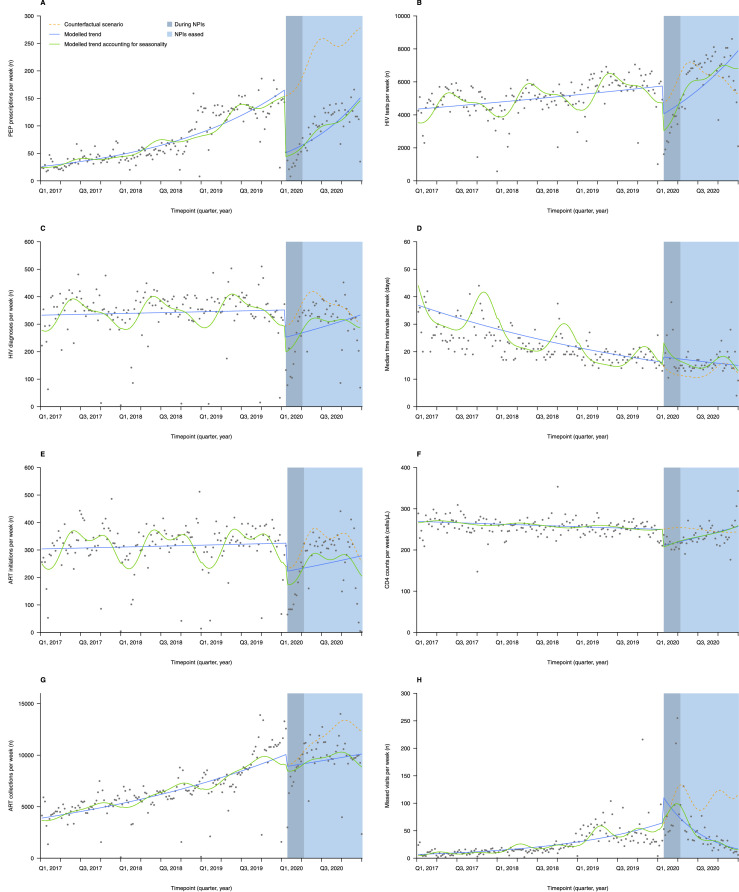
The median number of PEP prescriptions recorded per day was 8 (4-15) during the 1,117 days before NPIs (23 January, 2020), compared with 4 (2-7) during the 76 days of NPIs (23 January, 2020 to 7 April, 2020, the NPIs period), and 14 (9-20) during the 268 days post-NPIs (8 April, 2020 to 31 December, 2020, post-NPIs period). Poisson segmented regression analysis showed that the number of PEP prescriptions decreased by 71·5% in the first week of the NPIs period (20-26 January, 2020) (incidence rate ratio [IRR] 0·285; 95% confidence interval [CI] 0·192-0·423; Table 2 and Figure 2A; compared with counterfactual), then 71·2% (0·288, 0·197-0·423) and 70·8% (0·292, 0·201-0·424) in the second (27 January-2 February, 2020) and third week (3-9 February, 2020), respectively; the number of prescriptions showed an increasing trend of 2·4% per week in the post-NPIs period (1·024,1·012-1·037), and by the end of 2020 (28-31 December, 2020) the number of prescriptions still had not reached expected level (0·523,0·394-0·696). The results were similar for males and females (Table S3), and in a sensitivity analysis of PEP prescriptions (Table 2 and Table S4). The number of PEP prescriptions in each region decreased in the first, second and third weeks of the NPIs period, and the gap between the number of prescriptions and the expected level by the end of the study period was greater in Northeastern China (0·071,0·028-0·176) than that in South-Central China (0·546, 0·411-0·725) and Southwestern China (0·551,0·408-0·744).
Table 2.
Poisson segmented regression models of the impact of COVID-19 NPIs on HIV care continuum in China 1 January 2017 to 31 December 2020, accounting and not accounting for seasonality.
| Incidence rate ratio at the phases with massive NPIs |
Incidence rate ratio at the end of 2020 | Pre-NPIs trend | Post-NPIs trend | |||
|---|---|---|---|---|---|---|
| First week | Second week | Third week | ||||
| Seasonality unadjusted | ||||||
| PEP prescriptions | 0·306 (0·197-0·476) | 0·309 (0·202-0·475) | 0·313 (0·206-0·475) | 0·514 (0·367-0·721) | 1·012 (1·010-1·013) | 1·022 (1·009-1·036) |
| HIV tests | 0·705 (0·482-1·032) | 0·714 (0·493-1·033) | 0·722 (0·504-1·034) | 1·258 (0·917-1·726) | 1·002 (1·001-1·003) | 1·014 (1·001-1·026) |
| HIV diagnoses | 0·718 (0·484-1·066) | 0·722 (0·492-1·059) | 0·726 (0·501-1·053) | 0·931 (0·674-1·286) | 1·000 (0·999-1·001) | 1·006 (0·993-1·018) |
| Median time intervals | 1·122 (0·887-1·419) | 1·124 (0·894-1·412) | 1·125 (0·901-1·405) | 1·199 (0·921-1·561) | 0·995 (0·993-0·997) | 0·996 (0·988-1·004) |
| ART initiations | 0·686 (0·396-1·186) | 0·688 (0·405-1·169) | 0·691 (0·414-1·153) | 0·841 (0·477-1·481) | 1·000 (0·999-1·002) | 1·005 (0·985-1·025) |
| CD4 counts | 0·844 (0·784-0·909) | 0·848 (0·790-0·911) | 0·852 (0·795-0·913) | 1·052 (0·933-1·186) | 1·000 (0·999-1·000) | 1·004 (1·001-1·008) |
| ART collections | 0·881 (0·685-1·134) | 0·878 (0·687-1·124) | 0·875 (0·688-1·113) | 0·749 (0·612-0·915) | 1·006 (1·005-1·007) | 1·003 (0·995-1·010) |
| Missed visits | 1·692 (0·912-3·137) | 1·605 (0·878-2·934) | 1·524 (0·846-2·745) | 0·130 (0·069-0·245) | 1·014 (1·011-1·017) | 0·962 (0·945-0·980) |
| Seasonality adjusted | ||||||
| PEP prescriptions | 0·285 (0·192-0·423) | 0·288 (0·197-0·423) | 0·292 (0·201-0·424) | 0·523 (0·394-0·696) | 1·012 (1·011-1·013) | 1·024 (1·012-1·037) |
| HIV tests | 0·639 (0·497-0·822) | 0·649 (0·509-0·828) | 0·658 (0·520-0·833) | 1·300 (1·030-1·641) | 1·002 (1·001-1·003) | 1·016 (1·008-1·026) |
| HIV diagnoses | 0·680 (0·511-0·904) | 0·684 (0·519-0·902) | 0·689 (0·527-0·901) | 0·945 (0·748-1·192) | 1·000 (1·000-1·001) | 1·007 (0·998-1·017) |
| Median time intervals | 1·593 (1·270-1·997) | 1·580 (1·265-1·973) | 1·567 (1·259-1·950) | 1·072 (0·860-1·335) | 0·994 (0·992-0·996) | 0·986 (0·980-0·992) |
| ART initiations | 0·740 (0·474-1·154) | 0·741 (0·483-1·138) | 0·742 (0·491-1·122) | 0·798 (0·496-1·284) | 1·000 (0·999-1·001) | 1·002 (0·984-1·019) |
| CD4 counts | 0·826 (0·746-0·915) | 0·830 (0·752-0·917) | 0·835 (0·759-0·918) | 1·061 (0·940-1·197) | 1·000 (0·999-1·000) | 1·005 (1·001-1·009) |
| ART collections | 0·936 (0·737-1·189) | 0·931 (0·738-1·176) | 0·926 (0·738-1·163) | 0·720 (0·595-0·872) | 1·006 (1·005-1·007) | 1·000 (0·993-1·007) |
| Missed visits | 1·148 (0·784-1·683) | 1·100 (0·757-1·598) | 1·053 (0·731-1·518) | 0·137 (0·086-0·220) | 1·016 (1·013-1·018) | 0·972 (0·961-0·984) |
PEP= post-exposure prophylaxis. ART= antiretroviral therapy. Median time intervals= median time intervals between HIV diagnosis and ART initiation (days). CD4 counts= mean CD4 counts at ART initiation (cells/μL). Missed visits=Missed visits for ART collection. First week= 20-26 January, 2020. Second week= 27 January-2 February, 2020. Third week= 3-9 February, 2020.
Figure 2.
Weekly numbers, trends and fitted Poisson segmented regression models for eight indicators of HIV care continuum in China from 1 January 2017 to 31 December 2020, accounting and not accounting for seasonality.
PEP prescriptions (A), HIV tests (B), HIV diagnoses (C), median time intervals (D), ART initiations (E), CD4 counts (F), ART collections (G) and missed visits (H).
PEP= post-exposure prophylaxis. ART= antiretroviral therapy. Median time intervals= median time intervals between HIV diagnosis and ART initiation (days). CD4 counts= mean CD4 counts at ART initiation (cells/μL). Missed visits=Missed visits for ART collection. The legend is located on subfigure A.
The median number of HIV tests recorded per day was 789 (367-975) during the 1117 days before NPIs, compared with 410 (278-626) in the NPIs period and 1045 (524-1262) in post-NPIs period. Poisson segmented regression analysis showed a 36·1% (0·639, 0·497-0·822; Table 2 and Figure 2B) decrease in HIV tests in the first week of the NPIs period, then 35·1% (0·649, 0·509-0·828) and 34·2% (0·658,0·520-0·833) in the second and third week, respectively; in the NPIs period HIV tests showed a 1·6% weekly increase (1·016,1·008-1·026). By the end of 2020 it exceeded the expected level by 30·0% (1·300,1·030-1·641). The results were similar for males and females (Table S3), and in a sensitivity analysis of HIV tests (Table 2 and Table S4). The number of HIV tests in regions except for Southwestern China decreased in the first, second and third weeks of the NPIs period, and the recovery in the number of HIV tests by the end of 2020 was more significant in Northeastern China (2·137,1·442-3·167) and Eastern China (1·483,1·221-1·801) than that in South-Central China (0·862, 0·649-1·146).
The median number of HIV diagnoses recorded per day before NPIs was 49 (6-75), compared with 20 (6-46) in the NPIs period and 48 (9-73) in the post-NPIs period. Poisson segmented regression analysis showed a 32·0% decline in the number of HIV diagnoses in the first week of the NPIs period (0·680, 0·511-0·904; Table 2 and Figure 2C), then 31·6% (0·684, 0·519-0·902) and 31·1% (0·689, 0·527-0·901) in the second and third week, respectively; the number remained roughly constant in the post-NPIs period (1·007, 0·998-1·017); and had almost returned to expected level by the end of 2020 (0·945, 0·748-1·192). The results were similar across gender and age groups (Table S3), and in a sensitivity analysis of HIV diagnoses (Table 2 and Table S4). The decline in the number of HIV diagnoses in the first week of NPIs occurred mainly in Northeastern China (0·263,0·175-0·396). South-Central China (1·015, 1·008-1·023) and Northeastern China (1·034, 1·022-1·047) showed an increasing trend per week in the number of HIV diagnoses after NPIs, and significant differences compared to expected level by the end of 2020 were found in South-Central China (1·256, 1·006-1·567), Northeastern China (1·366, 1·006-1·855) and Northern China (0·415, 0·297-0·579).
The median number of median time intervals recorded per day was 21 (16-30) during the 1117 days pre-NPIs, compared with 18 (14-33) in the NPIs period, and 15 (13-19) in the post-NPIs period. Poisson segmented regression analysis showed that the median time intervals increased by 59·3% (1·593,1·270-1·997; Table 2 and Figure 2D), 58·0% (1·580,1·265-1·973) and 56·7% (1·567,1·259-1·950) during the first, second and third weeks of the NPIs period, respectively; the median time intervals showed a decreasing trend of 1·4% (0·986,0·980-0·992) per week in the post-NPIs period, and largely returned to the expected level (1·072,0·860-1·335) by the end of 2020. Results were similar for age groups (Table S3). The rise in the median time intervals in Eastern China (1·196,0·952-1·502), South-Central China (1·095,0·851-1·410) and Northern China (1·385,0·784-2·448) in the NPIs period was not statistically significant. The median time intervals in Northeastern China remained higher than expected level at the end of 2020 (1·750,1·202-2·548).Sensitivity analysis suggested that the decline in the NPIs period was not statistically significant (Table 2 and Table S4).
The median number of ART initiations recorded per day before NPIs was 54 (10-67), compared with 18 (4-30) in the NPIs period and 50 (8-64) in the post-NPIs period. Poisson segmented regression analysis showed no significant change in the number of ART initiations in the first (0·740, 0·474-1·154; Table 2 and Figure 2E), second (0·741, 0·483-1·138) and third weeks (0·742, 0·491-1·122) of the NPIs period; the number remained largely the same in the post-NPIs period (1·002, 0·984-1·019); and no significant difference from expected level by the end of 2020 (0·798, 0·496-1·284). The results were similar across gender, age and route of transmission groups (Table S3), and in a sensitivity analysis of ART initiations (Table 2 and Table S4).Northeastern China (0·479, 0·288-0·798) and Northern China (0·629, 0·437-0·904) showed significant decreases in the number of ART initiations in the first week of the NPIs period, as well as the second and third week. There were no significant differences from expected levels in the number in each region by the end of 2020.
The median number of CD4 counts recorded per day before NPIs was 256 (228-286), compared with 206 (162-242) in the NPIs period and 230 (205-262) in the post-NPIs period. Poisson segmented regression analysis showed that CD4 counts decreased by 17·4% (0·826, 0·746-0·915; Table 2 and Figure 2F), 17·0% (0·830, 0·752-0·917) and 16·5% (0·835, 0·759-0·918) during the first, second and third weeks of the NPIs period, respectively; in the post-NPIs period, CD4 counts showed an upward trend of 0·5% (1·005,1·001-1·009) per week in the post-NPIs period; then largely recovered to the expected level (1·061, 0·940-1·197) by the end of 2020. CD4 counts in Southwestern China and Northeastern China showed significant decreases in the first three weeks of the NPIs period. There were no significant differences from expected levels in the number in regions except for South-Central China and Northeastern China by the end of 2020. The results were similar in a sensitivity analysis of CD4 counts (Table 2 and Table S4).
The median number of ART collections recorded daily before NPIs was 949 (342-1295), compared with 1258 (368-1638) in NPIs period and 1728 (490-2054) in the post-NPIs period. Poisson segmented regression analysis showed no significant decrease in the number of ART collections in the first (0·936,0·737-1·189; Table 2 and Figure 2G), second (0·931, 0·738-1·176) and third week (0·926, 0·738-1·163) of the NPIs period; the number remained approximately the same in the post-NPIs period (1·000,0·993-1·007); however, by the end of 2020 there was a significant decrease compared to expected level (0·720,0·595-0·872). The results were similar across gender, age and route of transmission groups (Table S3), and in a sensitivity analysis of ART collections (Table 2 and Table S4). The number of ART collections in the first week of NPIs in the Eastern China showed a 19·3% (0·807,0·693-0·940) decrease. South-Central China (0·555,0·308-1·001) and Northern China (0·985,0·892-1·087) had recovered to the expected level by the end of 2020.
The median number of missed visits for ART collection recorded per day before the NPIs was 2 (1-5), compared with 9 (5-15) in the NPIs period and 4 (2-7) in the post-NPIs period. Poisson segmented regression analysis showed no significant increase in the number of missed visits for ART collection in the first week of the NPIs period (1·148,0·784-1·683; Table 2 and Figure 2H), as well as in the second (1·100, 0·757-1·598) and third week (1·053, 0·731-1·518); the number of missed visits showed a decreasing trend of 2·8% (0·972,0·961-0·984) in the post-NPIs period, and by the end of 2020 the number decreased significantly compared to expected level (0·137,0·086-0·220). The results of the study were similar across gender, age, region and route of transmission groups (Table S3), and in a sensitivity analysis of missed visits for ART collection (Table 2 and Table S4).
Discussion
Our study systematically analysed the impact of COVID-19 on the full spectrum of HIV care continuum in China. Compared with counterfactuals, significant declines in the number of PEP prescriptions (-71·5%), HIV tests (-36·1%), HIV diagnoses (-32·0%), and mean CD4 counts at ART initiation (-17·4%) in the first week of COVID-19 NPIs, and significant increase in median time intervals between HIV diagnosis and ART initiation (+59·3%) were found. No significant changes in the number of ART initiations, ART collections, and missed visits for ART collection were observed in the first week of COVID-19 NPIs. By the end of 2020, the number of HIV tests, HIV diagnoses, median time intervals between HIV diagnosis and ART initiation, ART initiations, and mean CD4 counts at ART initiation reached expected levels, but the number of PEP prescriptions, ART collections, and missed visits for ART collection were still well below expected levels.
From primary to tertiary prevention, HIV care services in China mostly take place at medical facilities and community-based organisations. Barriers to facility-based HIV care services during the COVID-19 pandemic, including the closure of health facilities, shortage of HIV specialists as many of them were shifted to do COVID work, cumbersome administrative procedures, COVID-related stigma and fear of being exposed to SARS-COV-2, complicated the management of HIV care services.32 Many provinces, autonomous regions and municipalities across China instituted a series of NPI measures to contain COVID under which all non-essential shops were closed, public transportation suspended, and residents confined to their homes.20 While deemed necessary to contain COVID-19, these measures profoundly affected the people at risk of HIV as well as PLHIV,1, 2, 3 increasing the likelihood of HIV transmission, drug resistance, late ART initiation, and opportunistic infections, particularly because HIV care and antiretroviral therapy (ART) in China are nearly exclusively provided through government-designated clinics and hospitals of their household registration. Collaborations among stakeholders, including people at high risk of HIV transmission, PLHIV, community based organisations (CBOs), HIV specialists and policy makers, were leveraged to mitigate these disruptions.32 The Chinese Center for AIDS/STD Control and Prevention released a nationwide directive on 26 January 2020 under which PLHIV could obtain one month of ART from any local HIV care clinic or hospital.33 This information was released to the public through official websites and social media official accounts. Subsequently, the address and telephone numbers of more than 5000 clinics were published to assist PLHIV to seeking help nearby. In addition, NCAIDS developed a special app to collect PLHIV's requests. CBOs played an important role in ART maintenance with innovative solutions such as peer navigation to refill ART and CBO-coordinated provision of ART through mail or home delivery services. Drug vendors contributed to ART maintenance by selling out-of-pocket ART. At designated hospitals and clinics, doctors and nurses worked with CBO members to post ART medications to PLHIV in need.32 These efforts put together alleviated ART interruption to the largest possible extent. However, services other than ART were affected to a greater extent for a longer period. When members of the community are recommended to reduce the number of non-urgent face-to-face interactions with healthcare, telemedicine system that enables remote consultation, prescription, and drug delivery, might be necessary. For example, during the COVID-19 pandemic, the Adelaide Sexual Health Centre provided telemedicine that included phone and video appointments, which facilitated timely uptake of PEP.34
As an indicator of primary prevention, declines in PEP prescriptions may be due to the changes in sexual behavior during the COVID-19 pandemic, such as the decreased sexual activity, the reduced number of casual partners and fewer condomless sex. A study of 177 MSM in Rhode Island, USA found that men reported an average of 2·60 fewer sexual partners (95% CI 4·04 -1·40) during the pandemic compared to pre-COVID-19.35 We observed rapid growth in PEP prescriptions from April through December 2020. In addition, we found that the decline in PEP prescriptions during the COVID-19 pandemic occurred primarily in people aged 50 years and younger, which may be because older adults are less adaptive to this unconventional and underutilized HIV prevention measure in China. A study in ten Chinese cities36 found people aged 50-64 years were less likely to use non-occupational postexposure prophylaxis (nPEP) (OR 0·179, 95% CI 0·036-0·894) compared to those aged 18-34 years, although it was not statistically significant for those aged 65 years and older (OR 0·232, 95% CI 0·014-3·975). As travel restrictions were attenuated and social life gradually returned to normal, people may resume their previous sexual frequency and risk. However, because of virus mutations and uncertainty of the effectiveness of COVID-19 vaccines, the extent and duration of containing measures in the long run, are still unclear. The number of PEP prescriptions at the end of 2020 is far from the expected level in most regions, with the gap between the expected level in Northeastern China being of concern. People's access to PEP may continue to be affected in the foreseeable future, especially in regions where the epidemic is ongoing or where economic levels are low. Public health authorities, healthcare facilities and community-based organizations are encouraged to work together to raise the awareness, knowledge and uptake of PEP among key populations.
As indicators of secondary prevention, the decrease in HIV tests was likely due to clinic closures, decreased willingness to seek medical care amidst the pandemic, and travel restrictions for residents.37 A study of routine data from 65 public sector primary care clinics in Kwazulu–Natal province, South Africa,6 found that the number of HIV tests declined by nearly 50% at the start of the lockdown, with gradual improvement over the next 3 months to pre-lockdown levels. A study of 11 sub-Saharan African countries21 found a small decline in number of HIV tests early in COVID-19. The decrease in HIV diagnoses may be due to NPIs reducing the number of newly-infected cases by affecting people's sexual activity. A study of MSM in the United States38 found that fewer sexual partners during COVID-19-related lockdown, which may temporarily reduce HIV transmission. But the decrease in HIV diagnoses may also simply be due to fewer HIV tests. A study of notifiable infectious disease incidence in China39 found no significant decrease in the number of HIV incidences (0·93, 0·78-1·11 Southern China; 0·89, 0·75-1·05 Northern China) in January 2020 after adjusting for changes in the volume of outpatient visits. In addition, we found that the decline in HIV tests during the COVID-19 pandemic occurred primarily in people aged 50 years and younger, which may be a result of older adults being more likely to have fewer clinic visits because of COVID-19-related NPIs. A study in Yinchuan, China40 found a decline in outpatient visits for most conditions in the population after week 3 of 2020, and the decline was greater for those 45 years and older than for younger people. More efforts should be made to ensure timely testing during a pandemic, especially for those who are already infected with HIV but not diagnosed, because of NPIs such as city lockdown.
As indicators of tertiary prevention, the increase in median time intervals between HIV diagnosis and ART initiation may be due to city lockdown and hospital focused on treating COVID-19 patients, limiting access to PLHIV. A study in China22 found that many hospitals specialising in HIV treatment were designated to treat COVID-19 and postponed or even suspended HIV services. The absence of significant decreases in ART initiation and ART collection during the COVID-19 may be related to China's efforts to prevent ART disruptions. A study in Shenzhen, China41 reported on measures taken by hospitals during the COVID-19 pandemic, such as increased medicine supply at single visits, express delivery of the medicine for PLHIV who were not able to attend the hospital, and provide counseling and consultation for patients affected by the lockdown outside the jurisdiction. Although unlike studies in some other countries or regions (11 sub-Saharan African countries,21 USA,42 Italy43 et al.) that have found a decrease in ART initiation and ART collection, there are still some problems with ART efforts in China that cannot be ignored. First, there was a significant decline in ART initiation among females after the first week, which may be related to more concern about being infected with COVID-19 in females.44 During the NPIs, the increase in median time intervals between HIV diagnosis and ART initiation occurred mainly in Southwestern China and Northeastern China, while the changes in Eastern China, South-Central China and Northern China were not statistically significant. Given the almost simultaneous adoption of public health emergency response level 1 everywhere in China (Table S1), this difference may indicate the suboptimal ART medicine delivery system in less economically developed regions. During NPIs, the increase in median time intervals between HIV diagnosis and ART initiation occurred mainly in heterosexually and homosexually transmitted populations and the decrease in mean CD4 counts at ART initiation occurred mainly in heterosexual transmitted populations, which may reflect that early ART initiation in these populations was affected by NPIs. Second, although the actual value of ART initiation did not differ significantly from the counterfactual in the first three weeks of NPIs and at the end of 2020, the point estimate suggested a decline of approximately 20%. The decrease in CD4 counts also reflects a possible delay in ART initiation in PLHIV. The lack of growth in ART collection to the expected level at the end of 2020 may be associated with declines in ART initiations and increased medicine supply at single visits. Moreover, the point estimate of the number of missed visits for ART collection also suggests a 14·8% rise in the first week of NPIs implementation.
Seasonality represents more of an intensive period of work in the health sector. When summer is approaching, national health administrative agencies issue annual assessment targets, and local health agencies begin to plan for the promotion and implementation of various HIV care efforts, so our study presents results with a cyclical peak in May-June. Data for all indicators except median time intervals and ART initiation showed a long-term increasing trend until 2020, which may be related to the rigorous efforts in massive HIV screening and testing strategies such as rapid expansion of voluntary counseling and testing clinics scale in the past decade.17 The decrease in median time intervals may be due to the implementation of the “treat-all” in China starting in June 2016, while the steady trend in ART initiation may be due to a lack of momentum in the effects of this revision.
To our knowledge, this is the first nationwide study on the impact of COVID-19 on HIV care continuum in Asia. The strengths of our study include data on a complete spectrum of HIV care continuum collected from various regions in China and parameters adjusted for long-term trends and seasonality. In the meantime, our study has some limitations. First, it is an ecological study and cannot demonstrate a causal relationship between COVID-19 NPIs and HIV care continuum. Second, different regions may reflect varied impact from COVID-19 as they have different HIV epidemic, COVID-19 epidemic and intensity of NPI measures. Nonetheless, during January 23 to April 8 and a period after that, different regions tended to take the upper level of NPI measures for COVID control purposes. Third, data from regions such as Wuhan, where NPI measures were set at the highest level and the impact of COVID-19 on HIV care continuum may be more pronounced, were not available in our analysis. The patients or service utilized in some regions were overrepresented in the sample. Future data that include patients or service utilized that were more representative may provide more precise estimates of parameters. Other than the Chinese CDC who has full access to the national HIV surveillance system, it is really unlikely that anyone could obtain data from all across the country. On top of this, the national HIV surveillance system does not routinely collect data on PEP. Albeit our data were only from a subset of the country, these data were from various regions with different levels of HIV/AIDS epidemic, representing a large population in China. Our data may be the best data available to clarify the changes in key HIV care continuum parameters due to COVID-19. Fourth, our study did not include data denoting the impact of COVID-19 on mental health and death among PLHIV as these parameters may reflect long-term impact from COVID-19 and will need future data to clarify. HIV Pre-Exposure Prophylaxis (PrEP) data were not available as currently there is a lack of national guideline on PrEP and PrEP data are not routinely collected.45
In conclusion, our findings suggest that HIV care continuum in China was affected by the COVID-19 NPIs at various levels. Preparedness and efforts to maintain the HIV care continuum during public health emergencies should leverage collaborations between stakeholders. Future studies are needed to evaluate the long-term changes of PEP prescriptions, ART collections, and missed visits for ART collection in China after COVID-19.
Contributors
HZ conceived and designed the study in consultation with the other authors. JX, XH, GF, GW, YM, XH, YY, YCai, JZ, LL, TZ, GL, PM, YQ, YChen, YFL, YG, YZ, WS, YW, RW, XY, LS, HW, QL, XX, LW, XW, RX, LY and XM contributed to data collection, data analysis and presentation. All authors contributed to interpretation of data and study findings. XW and HZ drafted the report with all authors critically reviewing the paper. All authors saw and approved the final report.
Data sharing statement
The data that support the findings of this study are available on request from the corresponding author, HZ. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanwpc.2022.100569.
Contributor Information
Jin Zhao, Email: szhaojin@foxmail.com.
Linghua Li, Email: llheliza@126.com.
Tong Zhang, Email: zt_doc@ccmu.edu.cn.
Junjie Xu, Email: xjjcmu@163.com.
Gengfeng Fu, Email: fugf@jscdc.cn.
Huachun Zou, Email: zouhuachun@mail.sysu.edu.cn.
Appendix. Supplementary materials
References
- 1.Simbayi LC, Moyo S, van Heerden A, et al. Global HIV efforts need to focus on key populations in LMICs. Lancet. 2021;398(10318):2213–2215. doi: 10.1016/S0140-6736(21)02692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enane LA, Apondi E, Aluoch J, et al. Social, economic, and health effects of the COVID-19 pandemic on adolescents retained in or recently disengaged from HIV care in Kenya. PLoS One. 2021;16(9) doi: 10.1371/journal.pone.0257210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vrazo AC, Golin R, Fernando NB, et al. Adapting HIV services for pregnant and breastfeeding women, infants, children, adolescents and families in resource-constrained settings during the COVID-19 pandemic. J Int AIDS Soc. 2020;23(9):e25622. doi: 10.1002/jia2.25622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Junejo M, Girometti N, McOwan A, Whitlock G. HIV postexposure prophylaxis during COVID-19. Lancet HIV. 2020;7(7):e460. doi: 10.1016/S2352-3018(20)30146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow EPF, Hocking JS, Ong JJ, Phillips TR, Fairley CK. Postexposure prophylaxis during COVID-19 lockdown in Melbourne, Australia. Lancet HIV. 2020;7(8):e528–e529. doi: 10.1016/S2352-3018(20)30204-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorward J, Khubone T, Gate K, et al. The impact of the COVID-19 lockdown on HIV care in 65 South African primary care clinics: an interrupted time series analysis. Lancet HIV. 2021;8(3):e158–e165. doi: 10.1016/S2352-3018(20)30359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jewell BL, Mudimu E, Stover J, et al. Potential effects of disruption to HIV programmes in sub-Saharan Africa caused by COVID-19: results from multiple mathematical models. Lancet HIV. 2020;7(9):e629–e640. doi: 10.1016/S2352-3018(20)30211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The People's Government of Beijing Municipality The announcement of cancellations of major events including temple fairs in Beijing. Jan 23, 2020. http://www.beijing.gov.cn/ywdt/gzdt/t1614497.htm.
- 9.The State Council of the People's Republic of China The announcement from Wuhan's headquarter on the novel coronavirus prevention and control. Jan 23, 2020. http://www.gov.cn/xinwen/2020-01/23/content_5471751.htm.
- 10.National Health Commission of the People's Republic of China The press conference on Jan 26, 2020. http://www.nhc.gov.cn/xcs/fkdt/202001/12ec9062d5d041f38e210e8b69b6d7ef.shtml. [DOI] [PMC free article] [PubMed]
- 11.The State Council of the People's Republic of China The State Council's announcement on extending the Lunar New Year Holiday in 2020. Jan 27, 2020. http://www.gov.cn/zhengce/content/2020-01/27/content_5472352.htm
- 12.The State Council of the People's Republic of China The State Council's announcement on the arrangement of public holidays in 2020. Nov 21, 2019. http://www.gov.cn/zhengce/content/2019-11/21/content_5454164.htm.
- 13.Liu N-N, Tan J-C, Li J, Li S, Cai Y, Wang H. COVID-19 pandemic: experiences in China and implications for its prevention and treatment worldwide. Curr Cancer Drug Targets. 2020;20(6):410–416. doi: 10.2174/1568009620666200414151419. [DOI] [PubMed] [Google Scholar]
- 14.Shen J, Duan H, Zhang B, et al. Prevention and control of COVID-19 in public transportation: experience from China. Environ Pollut. 2020;266 doi: 10.1016/j.envpol.2020.115291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao H, Dai X, Wagenaar BH, et al. The impact of the COVID-19 pandemic on health services utilization in China: time-series analyses for 2016-2020. Lancet Reg Health West Pac. 2021;9 doi: 10.1016/j.lanwpc.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao H, Liu F, He Y, et al. Unequal impact of the COVID-19 pandemic on paediatric cancer care: a population-based cohort study in China. Lancet Reg Health West Pac. 2022;19 doi: 10.1016/j.lanwpc.2021.100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He N. Research progress in the epidemiology of HIV/AIDS in China. China CDC Wkly. 2021;3(48):1022–1030. doi: 10.46234/ccdcw2021.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi L, Tang W, Hu H, et al. The impact of COVID-19 pandemic on HIV care continuum in Jiangsu, China. BMC Infect Dis. 2021;21(1):768. doi: 10.1186/s12879-021-06490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suen YT, Chidgey A. Disruption of HIV service provision and response in Hong Kong during COVID-19: issues of privacy and space. J Int Assoc Provid AIDS Care. 2021;20 doi: 10.1177/23259582211059588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Y, Li H, Luo G, et al. Antiretroviral treatment interruption among people living with HIV during COVID-19 outbreak in China: a nationwide cross-sectional study. J Int AIDS Soc. 2020;23(11):e25637. doi: 10.1002/jia2.25637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris TG, Jaszi E, Lamb MR, et al. Effects of the COVID-19 pandemic on HIV services: findings from 11 Sub-Saharan African countries. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun S, Hou J, Chen Y, Lu Y, Brown L, Operario D. Challenges to HIV care and psychological health during the COVID-19 pandemic among people living with HIV in China. AIDS Behav. 2020;24(10):2764–2765. doi: 10.1007/s10461-020-02903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X, Zeng C, Tam CC, et al. HIV service interruptions during the COVID-19 pandemic in China: the role of COVID-19 challenges and institutional response from healthcare professional’s perspective. AIDS Behav. 2021;26:1270–1278. doi: 10.1007/s10461-021-03484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao S, Yang X, Sun S, et al. Challenges to HIV service delivery and the impacts on patient care during COVID-19: perspective of HIV care providers in Guangxi, China. AIDS Care. 2021;33(5):559–565. doi: 10.1080/09540121.2020.1849532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO Coronavirus (COVID-19) Dashboard. 2021. https://covid19.who.int/.
- 26.Callaway E. Omicron likely to weaken COVID vaccine protection. Nature. 2021;600(7889):367–368. doi: 10.1038/d41586-021-03672-3. [DOI] [PubMed] [Google Scholar]
- 27.Omicron: the global response is making it worse. Nature. 2021;600(7888):190. doi: 10.1038/d41586-021-03616-x. [DOI] [PubMed] [Google Scholar]
- 28.Lopez Bernal J, Soumerai S, Gasparrini A. A methodological framework for model selection in interrupted time series studies. J Clin Epidemiol. 2018;103:82–91. doi: 10.1016/j.jclinepi.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Xiao H, Augusto O, Wagenaar BH. Reflection on modern methods: a common error in the segmented regression parameterization of interrupted time-series analyses. Int J Epidemiol. 2021;50(3):1011–1015. doi: 10.1093/ije/dyaa148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017;46(1):348–355. doi: 10.1093/ije/dyw098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bottomley C, Scott JAG, Isham V. Analysing interrupted time series with a control. Epidemiol Methods. 2019;8(1) [Google Scholar]
- 32.Sun Y, Zhan Y, Li H, et al. Stakeholder efforts to mitigate antiretroviral therapy interruption among people living with HIV during the COVID-19 pandemic in China: a qualitative study. J Int AIDS Soc. 2021;24(9):e25781. doi: 10.1002/jia2.25781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pagaoa M, Grey J, Torrone E, Kreisel K, Stenger M, Weinstock H. Trends in nationally notifiable sexually transmitted disease case reports during the US COVID-19 pandemic, January to December 2020. Sex Transm Dis. 2021;48(10):798–804. doi: 10.1097/OLQ.0000000000001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sentís A, Prats-Uribe A, López-Corbeto E, et al. The impact of the COVID-19 pandemic on sexually transmitted infections surveillance data: incidence drop or artefact? BMC Public Health. 2021;21(1):1637. doi: 10.1186/s12889-021-11630-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogers BG, Tao J, Darveau SC, et al. The impact of COVID-19 on sexual behavior and psychosocial functioning in a clinical sample of men who have sex with men using HIV pre-exposure prophylaxis. AIDS Behav. 2021;26:69–75. doi: 10.1007/s10461-021-03334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li H, Wei R, Piqueiras E, et al. HIV non-occupational postexposure prophylaxis (nPEP) usage among five key populations in China. Sex Transm Infect. 2021;97(6):411–413. doi: 10.1136/sextrans-2020-054791. [DOI] [PubMed] [Google Scholar]
- 37.Chang W-H. The influences of the COVID-19 pandemic on medical service behaviors. Taiwan J Obstet Gynecol. 2020;59(6):821–827. doi: 10.1016/j.tjog.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez TH, Zlotorzynska M, Rai M, Baral SD. Characterizing the Impact of COVID-19 on men who have sex with men across the United States in April, 2020. AIDS Behav. 2020;24(7):2024–2032. doi: 10.1007/s10461-020-02894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geng M-J, Zhang H-Y, Yu L-J, et al. Changes in notifiable infectious disease incidence in China during the COVID-19 pandemic. Nat Commun. 2021;12(1):6923. doi: 10.1038/s41467-021-27292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu L, Zhuo L, Zhang J, et al. Impact of the COVID-19 pandemic on outpatient service in primary healthcare institutions: an inspiration from Yinchuan of China. Int J Health Policy Manag. 2021 doi: 10.34172/ijhpm.2021.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H. HIV care during the coronavirus disease-2019 pandemic in Shenzhen, China. Curr Opin HIV AIDS. 2020;15(6):341–344. doi: 10.1097/COH.0000000000000653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell KM, Dimitrov D, Silhol R, et al. The potential effect of COVID-19-related disruptions on HIV incidence and HIV-related mortality among men who have sex with men in the USA: a modelling study. Lancet HIV. 2021;8(4):e206–e215. doi: 10.1016/S2352-3018(21)00022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quiros-Roldan E, Magro P, Carriero C, et al. Consequences of the COVID-19 pandemic on the continuum of care in a cohort of people living with HIV followed in a single center of Northern Italy. AIDS Res Ther. 2020;17(1):59. doi: 10.1186/s12981-020-00314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah SMA, Mohammad D, Qureshi MFH, Abbas MZ, Prevalence Aleem S. Psychological responses and associated correlates of depression, anxiety and stress in a global population, during the coronavirus disease (COVID-19) pandemic. Community Ment Health J. 2021;57(1):101–110. doi: 10.1007/s10597-020-00728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu J, Tang W, Zhang F, Shang H. PrEP in China: choices are ahead. Lancet HIV. 2020;7(3):e155–e157. doi: 10.1016/S2352-3018(19)30293-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.