Abstract
Context
Subacute thyroiditis is an inflammatory thyroid disease, which is treated by nonsteroidal anti-inflammatory drugs (NSAIDs) or steroids.
Objective
Defining characteristics of patients with subacute thyroiditis at diagnosis and during follow-up. Investigating the efficacies of NSAID and different doses of steroids and their effects on rates of relapse, recurrence, development of hypothyroidism and on quality of life and sleep parameters.
Design
A 3-year observational study in a tertiary referral center.
Subjects and Methods
A total of 63 patients with subacute thyroiditis were included. Clinical outcomes of patients treated with NSAIDs and NSAID unresponsive patients treated with prednisolone with initial doses of 0.5 mg/kg/day and 15 mg/day were evaluated.
Results
White blood cell count at diagnosis was an independent predictor of NSAID unresponsiveness. No relapse or recurrence was observed in patients receiving low dose of steroids. Long symptom duration until diagnosis and treatment with NSAIDs were associated with development of hypothyroidism. Subacute thyroiditis caused significant deterioration in quality of life and sleep of patients and low dose of steroid was as effective as higher doses in improving these parameters.
Conclusions
For patients with no response to NSAID therapy, an initial low dose of prednisolone (15 mg/day) is determined as a safe treatment method when dose reduction is performed with appropriate timing.
Keywords: subacute thyroiditis, steroid, non-steroidal anti-inflammatory drugs, relapse, life quality, sleep quality
Introduction
Subacute thyroiditis is a generally self-limiting inflammatory thyroid disease, which occurs in response to viral infections in genetically predisposed patients. Pain or feeling of discomfort in neck and thyrotoxicosis generally accompany to this disease (1, 2). Female predominance is observed and this disease tend to occur generally at ages of 30-50 (3-5). The incidence of subacute thyroiditis was reported to be 4.9 cases /100.000/year and subacute thyroiditis lie behind 0.5-22% of thyrotoxicosis cases (3, 6, 7).
The most important aim of treatment is to relieve the pain and tenderness as well as relieving thyrotoxicosis symptoms if any. Also, patients should be closely followed-up for occurrence of relapse or recurrence and development of hypothyroidism. Most of the patients are treated by either nonsteroidal anti-inflammatory drugs (NSAIDs) or steroids. The generally accepted approach is initiating NSAIDs primarily and deciding continuation of NSAIDs or replacing with steroids according to response to therapy. Cessation of the therapy by tapering is recommended for both treatment modalities (6). Although the standard recommended steroid dose for initial treatment is 40 mg prednisolone or 0.5 mg/kg prednisolone, there are multiple studies concerning initiating different doses of steroid treatment (3, 6, 8-16). Recent studies showed that lower initial steroid doses were equally effective in clinical improvement and recovery. Studies generally emphasize the importance of slow reduction of steroid dosing and adequate treatment time rather than initiating high doses of steroids (13).
The aim of this study is to describe clinical, biochemical and ultrasonographic features of the patients diagnosed with subacute thyroiditis in a tertiary center and evaluate the efficacies of NSAID therapies and different doses of steroid therapies and investigate the effects of these treatment modalities on relapse and recurrence rates, development of hypothyroidism and on quality of life and sleep.
Materials and Methods
Study protocol
This observational retrospective study was conducted between 2017 and 2019 in the outpatient unit of Endocrinology and Metabolism Clinic in Dokuz Eylul University Hospital. The study was approved by Clinical Research Ethical Committee of Dokuz Eylul University in 21.12.2017, 2017/29-21. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institution and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All patients were given detailed information and informed consent was obtained from all participants involved in this study. The diagnosis and treatment of subacute thyroiditis was made according to guidelines of American Thyroid Association issued in 2016 (6). During 2017 and 2019, 67 patients were referred to our department. Patients being treated with glucocorticoids for other indications (n=3) or with additional co-morbidities which could affect quality of life (n=1) were excluded from the study. At the final analysis, data of 63 patients were assessed. Clinical assessments of the patients were carried out by two endocrinologists with 15-years of clinical practice. All patients without prior history of treatment and without contraindications were initiated NSAIDs at presentation. Naproxen sodium 1000 mg/day was initiated unless contraindicated. Decisions of NSAID unresponsiveness, switching to prednisolone and its dose and duration were made by the endocrinologists monitoring and treating the patients.
As a routine part of treatment, patients were evaluated at approximately 7-14th day, 4th -6th week, 10th-12th week and 24th week. The decision to continue NSAID or switching to steroid treatment was made at 7-14th day following the initiation of treatment according to the absence or presence of neck pain. Patients without apparent relief of pain were defined to be unresponsive to NSAID treatment. Relapse was defined as the repeat of symptoms during tapering while recurrence was defined as the repeat of symptoms after cessation of therapy. Recurrence occurring in first 6-12 months period was defined as early recurrence. Glucocorticoid treatment was planned to be ceased with tapering in approximately 6 weeks in both groups. For the patients receiving an initial dose of 15 mg prednisolone, the tapering regimen included tapering by 5 mg every 2 weeks, and for the patients receiving 0.5 mg/kg initial dose of prednisolone, dose reduction was made weekly. In case of exacerbation, patients were recommended to increase the dose and tapered with longer intervals.
Laboratory Investigations
Biochemical tests including complete blood count (CBC), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), thyroid-stimulating hormone (TSH), free thyroxine (fT4), free triiodothyronine (fT3), glomerular filtration rate (GFR), alanine aminotransferase (ALT), thyroglobulin (Tg), were applied at each visit. Thyroid autoantibodies including anti-thyroid peroxidase antibody (Anti-TPO), anti-thyroglobulin (Anti-Tg), and TSH receptor antibody (TRAB) were analyzed at the time of diagnosis. CBC was performed with the impedance method (Beckman Coulter LH-780 Hematology Analyzer, USA). ALT and creatinine values were measured by the colorimetric method. CRP was measured by the immunoturbidimetric method. Thyroid function tests were measured by chemiluminescent immunoassay (Beckman Coulter DXI 800, USA). Anti-TPO and Anti-Tg measurements (Siemens Advia Contur XP, Germany) and TRAB and thyroglobulin measurements (Siemens Immulite 2000 XPI, Germany) were made by chemiluminescent immunoassays. ESR was measured with the photometric capillary flow (Alifax, Italy).
Ultrasonography
Ultrasonographic evaluation was made with GE Logic 5 ultrasonography. Ultrasonographic evaluation included thyroid volume measurements and evaluation of echo pattern, blood flow pattern and existence of thyroid nodules. In order to prevent interobserver variation, all ultrasonographic evaluations were made by one investigator. Ellipsoid formula was used for thyroid volume calculation and total volume was calculated by adding total volumes of the both lobes (17). Isthmus volume was not included in volume calculation. Echogenicity description was made as, isoechoic, hypoechoic, apparently hypoechoic and hyperechoic by comparing echogenicity with thyroid gland and infrahyoid muscle (18). Thyroid fine needle aspiration biopsy (FNAB) was only performed to the patients who had persistent nodules after complete amelioration of subacute thyroiditis and having nodules with high risk of malignancy according to TI-RADS (Thyroid Imaging, Reporting and Data System, TIRADS ≥4) in 6th month control ultrasonography (19).
Questionnaires
Pain scale was used for subjective evaluation of the patients’ degrees of pain at the time of diagnosis and during follow-up at control visits. Pain severity was measured with visual analog scale (0, no pain and 10, severe pain) (20). Short form 36 (SF-36) was applied for evaluation of life qualities of the patients and summary component calculations were done (21-24). Sleep qualities of the patients were evaluated with Pittsburgh Sleep Quality Index (PSQI) and a PSQI grade >5 is defined as bad sleep quality while a PSQI grade ≤5 is defined as good sleep quality (25-27). Patients were requested to fill SF-36 and PSQI questionnaires at the time of diagnosis and during follow up visits at 6th week, 12th week and 24th weeks.
Statistical Analysis
Statistical analyses were performed with IBM SPSS for Mac Version 20 (Statistical Package for Social Sciences for Mac). Numeric variables were summarized as median (minimum-maximum) or mean (±SD) according to distribution patterns. Categorical variables were evaluated with cross table analysis and shown numerically with a percentage. For pairwise comparisons student-T test was used for normally distributed variables, while Mann Whitney-U test was used for non-normally distributed variables. For dependent variables, normally distributed variables were compared using sample T-test and non-normally distributed variables were compared with Wilcoxon signed-ranked test. Categorical variables were compared using the chi-square test. Logistic regression analysis was used for evaluation of the correlation between laboratory values and pain severity with NSAID responsiveness. Also, the effects of laboratory values and symptom duration on development of hypothyroidism was evaluated by logistic regression analysis. Factors affecting prediction of NSAID unresponsiveness were evaluated with “Receiver Operating Characteristic” (ROC) curve analysis. p<0.05 was considered as statistically significant.
Results
Study participants
Demographic features and anthropometric measurements as well as number of patients receiving antibiotics, anti-thyroid drugs and NSAIDs before admission to our clinic are summarized in Table 1. Mean age of the patients was 45.2 (±11.1) years and female/male ratio was 1.86. Patients were mostly diagnosed in autumn and spring seasons (33.3%, 27%, respectively). Seven patients were positive for anti-TPO (11.1%) and 14 patients (22.2%) were positive for anti-Tg at the time of diagnosis. One patient had a history of use of levothyroxine due to autoimmune thyroiditis.
Table 1.
Demographic and anthropometric data, pain scale values and thyroid volumes of the patients at the time of diagnosis
| Parameter | Value |
|---|---|
| Age (year) | 45.2 (11.1) |
| Gender (F/M) | 41 (65.1%) / 22 (34.9%) |
| Smoker/Nonsmoker* | 10 (16.6%) / 50 (83.4%) |
| Education (primary school/high school/university)** | 17 (27.4%) /16 (25.8%) / 29 (46.8%) |
| Marital status (married/not-married) | 54 (85.7%) / 9 (14.3%) |
| Weight (kg) | 69.19 (14.1) |
| BMI (kg/m2) | 24.73 (3.68) |
| Use of antibiotics before admission | 28 (44.4%) |
| Use of anti-thyroid drugs before admission | 11 (17.4%) |
| Use of NSAIDs before admission | 11 (17.4%) |
| CRP (0.2-5 mg/L) | 59.1 (8.1-239.4) |
| ESR (0-20 mm/hour) | 61.3 (30.5) |
| Hemoglobin (12-16 g/dL) | 12.3 (8.5-15) |
| WBC (4-10.3 103/uL) | 8.23 (1.89) |
| Platelets (156-373 103/uL) | 348 (223-621) |
| TSH (0.38-5.33 mUI/L) | 0.0015 (0-1.45) |
| fT4 (0.5-1.51 ng/dL) | 2.34 (1.06) |
| fT3 (2.5-3.9 ng/dL) | 4.72 (3.19-15.93) |
| Tg (ng/mL) | 115 (3.3-1284) |
| ALT (0-35 U/L) | 20.5 (7-109) |
| Creatinine (0.51-0.95 mg/dL) | 0.62 (0.43-1.05) |
| GFR (>90 CKD-EPI) | 112 (76-130) |
| FPG (70-100 mg/dL) | 99 (75-224) |
| Pain scale | 7 (0-10) |
| Thyroid volume (cm3) | 19.5 (9.53-57.75) |
ALT; alanine aminotransferase, BMI: body mass index, CRP; C-reactive protein, ESR; erythrocyte sedimentation rate, F: female, FPG; fasting plasma glucose, GFR; glomerular filtration rate M: male, NSAID: nonsteroidal anti-inflammatory drug, fT3; free triiodothyronine, sT4; free thyroxine, Tg; thyroglobulin, TSH; thyroid stimulating hormone, WBC; white blood cell. *n=60. **n=62. Normally distributed values are presented as mean (±SD), non-normally distributed values are presented as median (min-max).
All 63 patients received NSAIDs as initial treatment. All but one patients had complaints of neck pain. Thyrotoxicosis were seen in 60 (95.2%) patients while 3 patients (4.8%) had normal thyroid function tests. Thyrotoxicosis symptoms were observed in 42 (66.7%) patients. Gastrointestinal side effects of NSAIDs leading to cessation of therapy was observed in 4 patients. Among these patients, two patients were followed-up without treatment due to relief of symptoms and two were treated with glucocorticoids.
NSAID unresponsiveness and indications of glucocorticoid treatment
Twenty-six (41.3%) patients were responsive to NSAID treatment while, 37 patients’ NSAID treatment were replaced by glucocorticoids. Unresponsiveness to NSAID treatment was observed in 35 patients. Two additional patients also received steroid treatment due to significant side effects of NSAIDs. We observed that among 37 patients treated with prednisolone, 18 (48.7%) were initiated 0.5 mg/kg/day, while 19 (51.3%) received a dose of 15 mg/day.
Data regarding patients who are responsive and unresponsive to NSAID treatment are summarized in Table 2. Age, duration of symptoms and CRP and ESR levels at initial presentation did not differ between the groups. However, NSAID unresponsive patients had significantly greater pain grades and WBC values than responsive patients (Table 2). Patients responsive to NSAIDs had significantly ameliorated pain and biochemical values at 1st week control (Table 2).
Table 2.
Comparison of patients responsive and unresponsive to NSAID therapy
| Parameters | Patients responsive to NSAID (n=26) | Patients unresponsive to NSAID (n=35)* | p |
|---|---|---|---|
| Age (year) | 46.85 (11.25) | 44.3 (11.3) | 0.384 |
| Symptom duration (day) | 30 (4-100) | 30 (4-90) | 0.534 |
| Pain scale diagnosis | 6.5 (0-10) | 8 (2-10) | 0.035 |
| Pain scale 1st week | 1 (0-7) | 6.9 (0-10) | p<0.001 |
| CRP diagnosis (mg/L) | 51.7 (8.1-239) | 64.4 (15.9-176) | 0.503 |
| CRP 1st week (mg/L) | 16.2 (1.5-95.8) | 72.6 (20.5-194) | p<0.001 |
| ESR diagnosis (mm/hour) | 59.3( 27.9) | 57 (23.4) | 0.753 |
| ESR 1st week (mm/hour) | 46.1 (23.5) | 65.6 (24) | 0.003 |
| WBC diagnosis (103/uL) | 7.43 (1.77) | 9 (1.66) | 0.002 |
| WBC 1st week (103/uL) | 6.82 (1.50) | 8.66 (2.17) | p<0.001 |
| TSH diagnosis (mUI/L) | 0.00 (0-1.06) | 0.01 (0-1.45) | 0.533 |
| TSH 1st week (mUI/L) | 0.00 (0-1.89) | 0 (0-2) | 0.401 |
| fT4 diagnosis (ng/dL) | 2.44 (1.14) | 2.24 (1) | 0.527 |
| fT4 1st week (ng/dL) | 1.85 (1.04) | 2.92 (1.32) | 0.001 |
| Tg (ng/mL) diagnosis | 147.5 (7.3-1084) | 85.65 (3.3-1284) | 0.190 |
| Tg (ng/mL) 1st week | 66 (5.3-948) | 126 (1.2-1061) | 0.510 |
CRP; C-reactive protein, ESR; erythrocyte sedimentation rate, , fT4; free thyroxine, NSAID: nonsteroidal anti-inflammatory drug, Tg; thyroglobulin TSH; thyroid stimulating hormone, WBC; white blood cell. Normally distributed values are presented as mean (±SD), non-normally distributed values are presented as median (min-max). *Two patients who were treated with steroids due to NSAID adverse effects were excluded from this analysis.
Direct logistic regression was performed to assess the impact of a number of factors on NSAID unresponsiveness. The model contained five independent variables (gender, age, pain scale, WBC and CRP levels at initial presentation). WBC at diagnosis was the sole independent predictor of NSAID unresponsiveness (B: 0.468, p=0.014, OR: 1.59; CI% 1.1-2.3).
Characteristics of patients treated with prednisolone
Biochemical and clinical parameters of patients receiving different doses of steroids are summarized in Table 3. Although age and baseline measures of pain scale, ESR, WBC and thyroid function test values of the patients were not significantly different between two groups, baseline CRP levels were significantly higher in patients receiving 0.5 mg/kg prednisolone treatment (p=0.044). Also, the duration of treatment was longer for patients receiving 0.5 mg/kg prednisolone treatment than patients receiving 15 mg prednisolone treatment (48 (42-126) days vs. 42 (42-59) days, p<0.001). None of the patients had clinical and biochemical signs of adrenal insufficiency after cessation of steroid therapy with tapering. All patients receiving steroid treatment had significant biochemical and clinical improvement at their 1st week control and their values are summarized in Table 3.
Table 3.
Biochemical parameters and pain scale grades of the patients receiving different doses of steroids before and after 1st week of treatment
| Prednisolone 0.5 mg/kg | Prednisolone 15 mg | |||||
|---|---|---|---|---|---|---|
| Parameter | Before | After | p | Before | After | p |
| Pain scale | 9 (1-10) | 0 (0-3) | p<0.001 | 7 (0-10) | 0 (0-5) | 0.001 |
| CRP (mg/L) | 105.3 (21.5-191) | 1.7 (0.6-12.2) | p<0.001 | 54 (20.5-194) | 2.4 (0.7-78) | 0.001 |
| ESR (mm/hour) | 68 (26.62) | 38.4 (23.17) | p<0.001 | 63.12 (21.41) | 28.65 (21.15) | p<0.001 |
| WBC (103/uL) | 8,69 (2,15) | 12.9 (2.45) | p<0.001 | 8.74 (2.24) | 10.68 (3.51) | 0.001 |
| TSH (mUI/L) | 0 (0-0.04) | 0 (0-0.13) | 0.324 | 0 (0-2) | 0 (0-18) | 0.345 |
| fT4 (ng/dL) | 3.26 (1,20) | 2.29 (1.16) | 0.001 | 2.57 (1.39) | 1.42 (0.92) | 0.001 |
| fT3 (ng/dL) | 6 (3.9-10.45) | 3.82 (2.40-6.64) | p<0.001 | 4.9 (3.2-12.1) | 3.41 (2.12-8.12) | 0.002 |
| Tg (ng/mL) | 153 (17-1061) | 9.24 (1-103) | p<0.001 | 55.90 (1.2-1011) | 22.65 (0-516) | 0.028 |
CRP; C-reactive protein, ESR; erythrocyte sedimentation rate, fT3; free T3, fT4; free T4, NSAID, nonsteroidal anti-inflammatory drug Tg; thyroglobulin, TSH; thyroid stimulating hormone, WBC; white blood cell. Normally distributed values are presented as mean (±SD), non-normally distributed values are presented as median (min-max).
Development of hypothyroidism
Twelve patients (19%) had increased TSH levels (TSH≥10 mUI/L) at the 6th week. At the 6th month, 11 patients (17.4%) had hypothyroidism requiring levothyroxine replacement. Among these patients 8 were in NSAID group while none of the patients in 0.5 mg/kg prednisolone group developed hypothyroidism. Thyroid volumes of patients who developed hypothyroidism at 6th month was significantly reduced when compared to their euthyroid counterparts (4.70 cm3 (1.48-11.34) vs. 9.87 cm3 (4-29.73), p=0.004).
Direct logistic regression was performed to assess the impact of a number of factors on development of hypothyroidism at the 6th month. The model contained five independent variables (NSAID use, age, symptom duration, anti-TPO and Tg levels at initial presentation). Duration of symptom until diagnosis (B: 0.035, p=0.048, OR: 1.03; CI% 1-1.07) and treatment with NSAIDs (B: 2.175, p=0.033, OR: 8.77; CI% 1.2-6.67) were found to be independent factors for development of hypothyroidism at 6th month.
Development of relapse and recurrence
Among 63 patients included in this study, relapse was observed in 3 patients and early recurrence was observed in 1 patient. Total relapse rate in our study was 4.8% and early recurrence rate was 1.6%. Total thyroidectomy was recommended to one patient who experienced relapse since it was not possible to cease the steroid treatment by tapering and due to side effects of long-term steroid treatment. None of the patients in NSAID group and 15 mg prednisolone group experienced a relapse or recurrence.
Ultrasonographic features
Ultrasonographic evaluation revealed decreased echogenicity and vascularization in affected areas in patients with subacute thyroiditis. Decreased echogenicity and vascularization was bilateral diffuse in 85.7%, unilateral diffuse in 7.7%, unilateral focal in 4.8% and bilateral focal in 1.6% of the patients. At the 3rd month evaluation, 33% of the patients had totally normal ultrasonographic appearance and at the 6th month evaluation 52.4% had normal appearance of the thyroid. Median thyroid volumes at the time of diagnosis were significantly higher (19.5 cm3 (9.53-57.76)) when compared to median thyroid volumes at 6th month (8.9 cm3 (1.48-29.73)) ,(p<0.001).
Thyroid nodules and thyroid malignancies
Thyroid nodules were detected in 14 patients at the time of diagnosis with thyroid ultrasound however, at the 6th month control, 21 patients were found to have thyroid nodules. Among 11 patients who received FNAB; 8 patients (72.7%) had benign results and 2 patients (18.2%) had atypia of undetermined significance with repeated biopsies after 3-6 months resulting in benign cytology. One patient’s (9.1%) FNAB was suspicious of papillary thyroid carcinoma. Among all patients in this study, two patients (3.17%) had thyroid malignancy including one patient with suspicious FNAB and one patient with incidental multifocal millimetric papillary carcinoma who had total thyroidectomy due to relapse.
Life quality evaluation
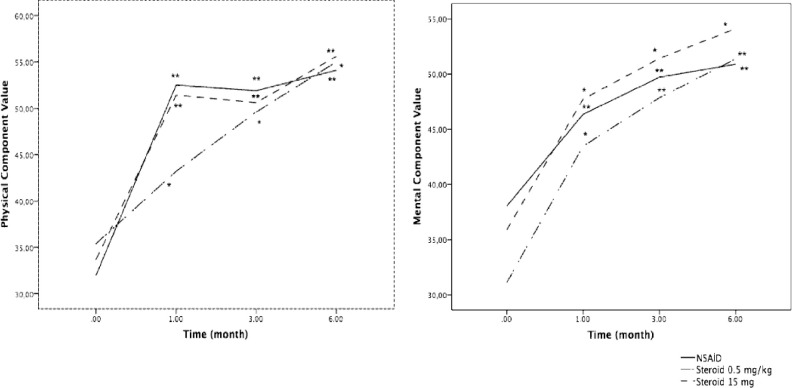
Obvious amelioration in terms of quality of life was observed during follow-up of the patients in all treatment groups. Median physical component summary (PCS) and mental component summary (MCS) values were obtained at the time of diagnosis and during follow-up. Median PCS value was 33 (20-58) at the time of diagnosis and were 48 (24-59), 50 (23-60) and 55 (35-62) during follow-up (at 6th week, 3rd month and 6th month respectively). On the other hand, median MCS value was 36 (17-70) at the time of diagnosis and were 46 (22-63), 49 (17-64) and 52 (19-62) during follow-up (at 6th week, 3rd month and 6th month respectively). Improvement curves according to treatment choices are shown in Figure 1.
Figure 1.
Short Form-36 physical component summary (median)-time curve and Short Form-36 mental component summary (median)-time curve according to treatment groups. *p<0.05; **p<0.01 vs. baseline values.
Sleep quality evaluation
Sleep quality was bad (PSQI>5) in 54 (85.7%) patients while sleep quality was good (PSQI≤5) in 9 (14.3%) patients at the time of diagnosis. At 6th month evaluation the number of patients with good sleep quality was 38 (60.3%), while the number of patients with bad sleep quality was 17 (27%). Median PSQI values and improvement curves according to treatment choices and are shown in Figure 2. No statistically significant differences were detected between treatment groups in terms of sleep quality.
Figure 2.
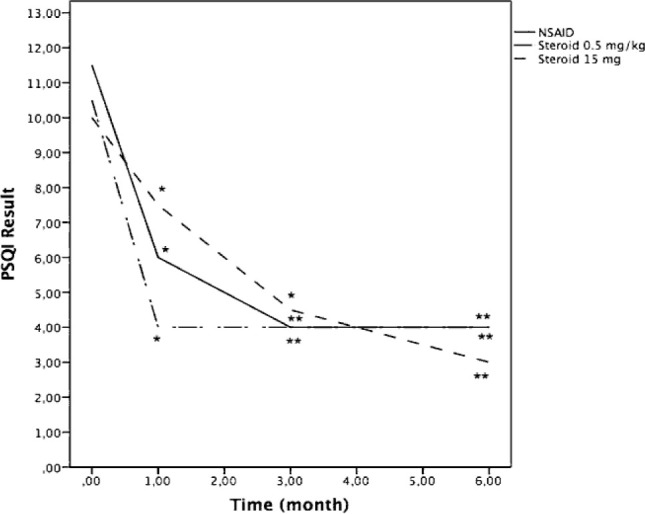
Pittsburgh Sleep Quality Index result (Median) -time curve according to treatment choices. *p<0.05; **p<0.01 vs. baseline values.
Discussion
In our study, we have investigated the short- and long-term effects of NSAID and different doses of steroid treatments on patients diagnosed with subacute thyroiditis. In addition, we have discussed the relationship of these treatments with pain, quality of life and sleep parameters of the patients. We have shown that for patients with no response to NSAID therapy, an initial low dose of prednisolone (15 mg/day) is a safe treatment method when dose reduction is performed with appropriate timing. WBC count at diagnosis was an independent predictor of NSAID unresponsiveness, whereas symptom duration until diagnosis and treatment with NSAIDs were found to be predictors of development of hypothyroidism at the 6th month. We have also showed that subacute thyroiditis significantly affected life quality and sleep quality of the patients and all treatment modalities improved these parameters.
Our results regarding the gender predominance (65.1% female), mean age at diagnosis (45.2y) and frequency of pain at diagnosis (98.4%) were in accordance with the studies on the epidemiology of subacute thyroiditis (3-5). It was reported in previous studies that almost all patients had biochemical findings of thyrotoxicosis, but the frequency of symptoms associated with thyrotoxicosis was 62.1% (28). In our study, 93.7% of the patients had biochemical findings compatible with thyrotoxicosis, and clinical findings of thyrotoxicosis were observed in 66.7% of the patients.
The median symptom duration of the patients was 30 days and 68.3% of the patients were diagnosed in more than 2 weeks. The length of symptom duration, which can be interpreted as the delay in diagnosis, was also associated with hypothyroidism at the 6th month. In addition, 44% of the patients had antibiotic use before application and 28% had a history of anti-thyroid drug use suggesting an incorrect use of antibiotic and anti-thyroid medications in different centers. Stasiak et al. (29) evaluated 64 patients with subacute thyroiditis in their study and found the mean time between the onset of symptoms and diagnosis to be 39.1 days. Only 27% of these patients were diagnosed before 2 weeks and in the remaining, the time from complaint to diagnosis was prolonged from 2 weeks to 6 months (29). In addition, similar to the rate of unnecessary antibiotic use in our study, their rate of unnecessary recommendation of antibiotics was 46.7% (29).
We have preferred the ultrasonography as the imaging method and while typical ultrasonographic findings of subacute thyroiditis were detected in all patients, the most common finding was bilateral diffuse thyroiditis, similar to the literature (30). In the follow-up, the ultrasonography image of 52.4% of the patients returned to normal at the 6th month. Moreover; the increased thyroid volume seen at the time of diagnosis decreased in the follow-ups and was within the normal range but close to the lower limit compared to the normal population values at the 6th month (17, 31, 32). Bennedbaek et al. (8), evaluated 23 patients in their study and they recorded a 68% reduction in thyroid volume at the 6th month when compared to baseline thyroid volumes at the time of diagnosis. They also found that 60% of the patients had a permanent abnormality in the function thyroid (8).
In our study, we have observed that NSAID treatment was applied primarily to all patients. The treatment of patients with clinical and biochemical responses to NSAID treatment was continued as NSAIDs and patients who did not benefit from NSAIDs were treated with steroids. Pain level and WBC values at initial diagnosis were significantly higher in those who were unresponsive to NSAIDs. WBC count at diagnosis was the sole independent predictor of NSAID unresponsiveness. To the best of our knowledge, this is the first study to show a predictor for NSAID unresponsiveness.
In patients with no response to NSAID treatment, initial glucocorticoid dose preferences were seen to be clustered in two groups as 0.5 mg/kg and 15 mg prednisolone. The mean initial steroid dose of the patient group receiving 0.5 mg/kg prednisolone was 33.92 (± 7.18) mg. CRP values were found to be higher in the 0.5 mg/kg prednisolone group when compared to 15 mg prednisolone group. CRP levels of all patients significantly decreased in the 1st week control after steroid treatment and CRP values were completely normalized in 84.4% of the patients. In addition, the patients receiving 0.5 mg/kg prednisolone had a significantly longer treatment time than the patients receiving 15 mg prednisolone.
In our study, the frequency of hypothyroidism was 17.4% at the 6th month and the majority of the patients who developed hypothyroidism were in the NSAID group (72.2%) while none of the patients in the 0.5 mg/kg prednisolone group developed hypothyroidism. On the other hand, long duration of symptoms until diagnosis and application of NSAID treatment were associated with the development of hypothyroidism at the 6th month. In studies, the frequency of hypothyroidism requiring long-term levothyroxine replacement therapy after subacute thyroiditis is approximately 10-15% (3, 33, 34). In a 2-year prospective follow-up study, maximum TSH value (7.83 mIU/L) in the first 3 months was determined as the predictor for the development of hypothyroidism (35). Studies regarding effects of treatment selection on development of hypothyroidism in long-term follow-up showed different results. In addition to studies showing that there is no significant relationship between treatment selection and the development of permanent hypothyroidism (34, 36, 37), there are also studies showing that the frequency of hypothyroidism is higher in patients receiving steroids (3) or hypothyroidism is less common in patients taking steroids (4). Fatourechi et al. (3), reported in their 28-year follow-up study that steroid treatment did not prevent the development of permanent hypothyroidism and they explained the reason that steroid treated group had more serious disease when compared with NSAID treated group (3). However, Nishihara et al. (4) found that the incidence of permanent hypothyroidism was lower in the patient group receiving steroids in their study and emphasized that the choice of treatment should be used in favor of steroid therapy in patients at risk for thyroid dysfunction. Although the mechanism of hypothyroidism development after subacute thyroiditis unclear, it is predicted that the damage to thyroid gland may be the cause (4). Although it is thought that steroid therapy may reduce thyroid parenchymal damage by preventing the secondary inflammatory response induced by cytokines, the mechanism is unclear (4, 38). In our study, the result that hypothyroidism was observed more frequently in the group treated with NSAIDs and hypothyroidism was not observed in the group receiving high-dose steroids can be interpreted as creating a more effective anti-inflammatory effect with high dose steroids and preventing thyroid parenchymal damage, but more studies are needed to confirm. Moreover, while the 6th month follow-up values in our study provide predictions in terms of development of permanent hypothyroidism, it is very important to evaluate at least after one year (3). In addition, similar to our study, other studies also emphasized that patients’ thyroid volumes increase during diagnosis and decrease during follow-up, and this decrease is more obvious in patients who develop hypothyroidism (34, 35).
Our findings show that the relapse rate is 4.8% and the early recurrence rate is 1.6%. No relapse or early recurrence was observed in the patient groups receiving NSAID and 15 mg steroid treatments. Different results have been obtained in many studies on this subject. Generally, it is seen that the frequency of relapse in subacute thyroiditis is 10-20% (9, 13, 16), the frequency of early recurrence is 10% (3) and the frequency of late recurrence is 1.6-4% (3, 13, 28, 39).
When steroid treatment doses in the literature are evaluated; the prednisolone starting dose appears to range from 25 to 60 mg (3, 8-12). Studies in the recent years showed that improvement can be achieved with lower steroid doses. In a study conducted in 122 patients, 20 mg of prednisolone/day tapered in 4 weeks was effective in relieving pain and decreasing ESR with a recurrence rate of 7% (14). In another study, the initial steroid dose of 15 mg/day tapered in 6 weeks resulted in recovery in 51.6% of the patients, while 27.9% required treatment for 8 weeks and 20% needed treatment longer than 8 weeks (12). Some studies emphasize that, for the prevention of recurrence of subacute thyroiditis, time to arrive at a prednisolone dose of 5 mg/day is more important than the prednisolone starting dose and this period should be longer than 6 weeks (13). In our study, when the appropriate period of treatment was given with low dose steroid therapy, no relapse or recurrence was observed in the early period.
Thyroid malignancy rate in subacute thyroiditis was found to be 3.17% in our study. None of the patients in the study of Fatourechi et al. (3) developed thyroid cancer, while according to Nishihara et al. (40) the rate of papillary thyroid cancer was 3.1%. When suspicious appearance is detected in patients diagnosed with subacute thyroiditis, biopsy should not be rushed; however, ultrasonography follow-ups should be continued at regular intervals due to possibility of hiding thyroid cancer.
Both hypothyroidism and hyperthyroidism were shown to be associated with impaired life and sleep quality both at the initial untreated phase as well as during follow-up despite adequate treatment (41). To the best of our knowledge, our study is the first to examine the effects of subacute thyroiditis on the quality of life and sleep of patients. We have found that subacute thyroiditis had significant negative effects on patients’ life and sleep quality parameters. According to the study of Demiral et al. (23), normal median PCS and MCS values were 54 and 52 in an urban Turkish population. Decreased median PCS (33) and MCS (36) values were observed at the time of diagnosis as well as during follow-up of the patients with subacute thyroiditis when compared to healthy individuals living in the same area. On the other hand, these values returned to normal at the 6th month (PCS, 55 and MCS, 52). Furthermore, both NSAID treatment and different initial steroid doses were found to be effective for the improvement of these parameters. Since subacute thyroiditis affects the life and sleep qualities of the patients in many ways, it is important to pay attention to these parameters at the time of diagnosis and during follow-up. All the treatment modalities were effective in not only fixing the biochemical parameters, but also were effective in improving life and sleep quality. On the other hand, low dose steroid treatment was non-inferior to higher dose of steroids in terms of life and sleep quality in suitable patients.
Limitations of our study include the retrospective pattern of the study and relatively small sample size with a relatively short duration of follow-up. Moreover, baseline CRP values of the patients in 0.5 mg/kg prednisolone group were higher than the 15 mg prednisolone group. This could indicate a bias in the selection of treatment with worse clinical and biochemical findings towards higher steroid administration.
In conclusion, initiating NSAIDs and then switching to steroid therapy when necessary according to clinical and laboratory response was found to be an appropriate method in treatment of subacute thyroiditis. No relapse or recurrence occurred when low-dose steroid therapy was given for an appropriate time. Life and sleep quality were impaired in patients with subacute thyroiditis and low initial dose of steroid was as effective as high dose steroids in improving these parameters.
Acknowledgements
The authors would like to thank to Prof. Dr. Yucel Demiral for his help in interpreting SF-36 data.
Conflict of interest
The authors declare that they have no conflict of interest.
Availability of data and material
The data that support the findings of this study are available on request from the corresponding author.
References
- 1.Davies TFLP, Bahn RS. Hyperthyroid Disorders. In: Melmed S PKS, Reed L. P, kronenberg H. M, editors. Vol. 13. Philadelphia: William’s Textbook of Endocrinology; 2015. pp. 406–408. [Google Scholar]
- 2.Martino E, Buratti L, Bartalena L, Mariotti S, Cupini C, Aghini-Lombardi F, Pinchera A. High prevalence of subacute thyroiditis during summer season in Italy. J Endocrinol Invest. 1987;10(3):321–323. doi: 10.1007/BF03348138. [DOI] [PubMed] [Google Scholar]
- 3.Fatourechi V, Aniszewski JP, Fatourechi GZ, Atkinson EJ, Jacobsen SJ. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab. 2003;88(5):2100–2105. doi: 10.1210/jc.2002-021799. [DOI] [PubMed] [Google Scholar]
- 4.Nishihara E, Amino N, Ohye H, Ota H, Ito M, Kubota S, Fukata S, Miyauchi A. Extent of hypoechogenic area in the thyroid is related with thyroid dysfunction after subacute thyroiditis. J Endocrinol Invest. 2009;32(1):33–36. doi: 10.1007/BF03345675. [DOI] [PubMed] [Google Scholar]
- 5.Erdem N, Erdogan M, Ozbek M, Karadeniz M, Cetinkalp S, Ozgen AG, Saygili F, Yilmaz C, Tuzun M, Kabalak T. Demographic and clinical features of patients with subacute thyroiditis: results of 169 patients from a single university center in Turkey. J Endocrinol Invest. 2007;30(7):546–550. doi: 10.1007/BF03346347. [DOI] [PubMed] [Google Scholar]
- 6.Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, Walter MA. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. 2016;26(10):1343–1421. doi: 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 7.Golden SH, Robinson KA, Saldanha I, Anton B, Ladenson PW. Clinical review: Prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94(6):1853–1878. doi: 10.1210/jc.2008-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennedbaek FN, Hegedus L. The value of ultrasonography in the diagnosis and follow-up of subacute thyroiditis. Thyroid. 1997;7(1):45–50. doi: 10.1089/thy.1997.7.45. [DOI] [PubMed] [Google Scholar]
- 9.Mizukoshi T, Noguchi S, Murakami T, Futata T, Yamashita H. Evaluation of recurrence in 36 subacute thyroiditis patients managed with prednisolone. Intern Med. 2001;40(4):292–295. doi: 10.2169/internalmedicine.40.292. [DOI] [PubMed] [Google Scholar]
- 10.Topuzovic N, Smoje J, Karner I. The therapeutic approach in subacute (de Quervain’s) thyroiditis. J Nucl Med. 1997;38(10):1665. [PubMed] [Google Scholar]
- 11.Vagenakis AG, Abreau CM, Braverman LE. Prevention of recurrence in acute thyoiditis following corticosteroid withdrawal. J Clin Endocrinol Metab. 1970;31(6):705–708. doi: 10.1210/jcem-31-6-705. [DOI] [PubMed] [Google Scholar]
- 12.Kubota S, Nishihara E, Kudo T, Ito M, Amino N, Miyauchi A. Initial treatment with 15 mg of prednisolone daily is sufficient for most patients with subacute thyroiditis in Japan. Thyroid. 2013;23(3):269–272. doi: 10.1089/thy.2012.0459. [DOI] [PubMed] [Google Scholar]
- 13.Arao T, Okada Y, Torimoto K, Kurozumi A, Narisawa M, Yamamoto S, Tanaka Y. Prednisolone Dosing Regimen for Treatment of Subacute Thyroiditis. J UOEH. 2015;37(2):103–110. doi: 10.7888/juoeh.37.103. [DOI] [PubMed] [Google Scholar]
- 14.Koirala KP, Sharma V. Treatment of Acute Painful Thyroiditis with Low Dose Prednisolone: A Study on Patients from Western Nepal. J Clin Diagn Res. 2015;9(9):MC01–03. doi: 10.7860/JCDR/2015/14893.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato J, Uchida T, Komiya K, Goto H, Takeno K, Suzuki R, Honda A, Himuro M, Watada H. Comparison of the therapeutic effects of prednisolone and nonsteroidal anti-inflammatory drugs in patients with subacute thyroiditis. Endocrine. 2017;55(1):209–214. doi: 10.1007/s12020-016-1122-3. [DOI] [PubMed] [Google Scholar]
- 16.Volpe R. The management of subacute (DeQuervain’s) thyroiditis. Thyroid. 1993;3(3):253–255. doi: 10.1089/thy.1993.3.253. [DOI] [PubMed] [Google Scholar]
- 17.Aydiner O, Karakoc Aydiner E, Akpinar I, Turan S, Bereket A. Normative Data of Thyroid Volume-Ultrasonographic Evaluation of 422 Subjects Aged 0-55 Years. J Clin Res Pediatr Endocrinol. 2015;7(2):98–101. doi: 10.4274/jcrpe.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YJ, Kim DW. Sonographic Characteristics and Interval Changes of Subacute Thyroiditis. J Ultrasound Med. 2016;35(8):1653–1659. doi: 10.7863/ultra.15.09049. [DOI] [PubMed] [Google Scholar]
- 19.Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, Cronan JJ, Beland MD, Desser TS, Frates MC, Hammers LW, Hamper UM, Langer JE, Reading CC, Scoutt LM, Stavros AT. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J Am Coll Radiol. 2017;14(5):587–595. doi: 10.1016/j.jacr.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 20.Langley GB, Sheppeard H. The visual analogue scale: its use in pain measurement. Rheumatol Int. 1985;5(4):145–148. doi: 10.1007/BF00541514. [DOI] [PubMed] [Google Scholar]
- 21.Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016;4:2050312116671725. doi: 10.1177/2050312116671725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware JE, Jr, Gandek B. Overview of the SF-36 Health Survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol. 1998;51(11):903–912. doi: 10.1016/s0895-4356(98)00081-x. [DOI] [PubMed] [Google Scholar]
- 23.Demiral Y, Ergor G, Unal B, Semin S, Akvardar Y, Kivircik B, Alptekin K. Normative data and discriminative properties of short form 36 (SF-36) in Turkish urban population. BMC Public Health. 2006;6:247. doi: 10.1186/1471-2458-6-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kocyigit H AO, Fisek G, Olmez N, Memis A. Kısa Form-36 (KF-36)’nın Türkçe versiyonunun güvenililiği ve geçerlilği. İlaç Tedavi Dergisi. 1999;12(2):102–106. [Google Scholar]
- 25.Agargun MY KH, Anlar O. Pittsburgh Uyku Kaltesi İndeksinin Geçerliği ve Güvenilirliği. Türk Psikiyatri Dergisi. 1996;7:107–115. [Google Scholar]
- 26.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 27.Mollayeva T, Thurairajah P, Burton K, Mollayeva S, Shapiro CM, Colantonio A. The Pittsburgh sleep quality index as a screening tool for sleep dysfunction in clinical and non-clinical samples: A systematic review and meta-analysis. Sleep Med Rev. 2016;25:52–73. doi: 10.1016/j.smrv.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Nishihara E, Ohye H, Amino N, Takata K, Arishima T, Kudo T, Ito M, Kubota S, Fukata S, Miyauchi A. Clinical characteristics of 852 patients with subacute thyroiditis before treatment. Intern Med. 2008;47(8):725–729. doi: 10.2169/internalmedicine.47.0740. [DOI] [PubMed] [Google Scholar]
- 29.Stasiak M, Michalak R, Stasiak B, Lewinski A. Time-Lag Between Symptom Onset and Diagnosis of Subacute Thyroiditis-How to Avoid the Delay of Diagnosis and Unnecessary Overuse of Antibiotics. Horm Metab Res. 2020;52(1):32–38. doi: 10.1055/a-1033-7524. [DOI] [PubMed] [Google Scholar]
- 30.Nordyke RA, Gilbert FI, Jr, Lew C. Painful subacute thyroiditis in Hawaii. West J Med. 1991;155(1):61–63. [PMC free article] [PubMed] [Google Scholar]
- 31.Maravall FJ, Gomez-Arnaiz N, Guma A, Abos R, Soler J, Gomez JM. Reference values of thyroid volume in a healthy, non-iodine-deficient Spanish population. Horm Metab Res. 2004;36(9):645–649. doi: 10.1055/s-2004-825901. [DOI] [PubMed] [Google Scholar]
- 32.Berghout A, Wiersinga WM, Smits NJ, Touber JL. Determinants of thyroid volume as measured by ultrasonography in healthy adults in a non-iodine deficient area. Clin Endocrinol (Oxf) 1987;26(3):273–280. doi: 10.1111/j.1365-2265.1987.tb00784.x. [DOI] [PubMed] [Google Scholar]
- 33.Gorges J, Ulrich J, Keck C, Muller-Wieland D, Diederich S, Janssen O. Long-term Outcome of Subacute Thyroiditis. Exp Clin Endocrinol Diabetes. 2020;128(11):703–708. doi: 10.1055/a-0998-8035. [DOI] [PubMed] [Google Scholar]
- 34.Schenke S, Klett R, Braun S, Zimny M. Thyroiditis de Quervain. Are there predictive factors for long-term hormone-replacement? Nuklearmedizin. 2013;52(4):137–140. doi: 10.3413/Nukmed-0536-12-10. [DOI] [PubMed] [Google Scholar]
- 35.Zhao N, Wang S, Cui XJ, Huang MS, Wang SW, Li YG, Zhao L, Wan WN, Li YS, Shan ZY, Teng WP. Two-Years Prospective Follow-Up Study of Subacute Thyroiditis. Front Endocrinol (Lausanne) 2020;11:47. doi: 10.3389/fendo.2020.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saklamaz A. Is There a Drug Effect on the Development of Permanent Hypothyroidism in Subacute Thyroiditis? Acta Endocrinol (Buchar) 2017;13(1):119–123. doi: 10.4183/aeb.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benbassat CA, Olchovsky D, Tsvetov G, Shimon I. Subacute thyroiditis: clinical characteristics and treatment outcome in fifty-six consecutive patients diagnosed between 1999 and 2005. J Endocrinol Invest. 2007;30(8):631–635. doi: 10.1007/BF03347442. [DOI] [PubMed] [Google Scholar]
- 38.Yamada T, Sato A, Aizawa T. Dissociation between serum interleukin-6 rise and other parameters of disease activity in subacute thyroiditis during treatment with corticosteroid. J Clin Endocrinol Metab. 1996;81(2):577–579. doi: 10.1210/jcem.81.2.8636270. [DOI] [PubMed] [Google Scholar]
- 39.Iitaka M, Momotani N, Ishii J, Ito K. Incidence of subacute thyroiditis recurrences after a prolonged latency: 24-year survey. J Clin Endocrinol Metab. 1996;81(2):466–469. doi: 10.1210/jcem.81.2.8636251. [DOI] [PubMed] [Google Scholar]
- 40.Nishihara E, Kudo T, Ito M, Fukata S, Nishikawa M, Nakamura H, Amino N, Miyauchi A. Papillary thyroid carcinomas are highly obscured by inflammatory hypoechoic regions caused by subacute thyroiditis: a longitudinal evaluation of 710 patients using ultrasonography. Endocr J. 2020;67(5):569–574. doi: 10.1507/endocrj.EJ19-0597. [DOI] [PubMed] [Google Scholar]
- 41.Watt T, Groenvold M, Rasmussen AK, Bonnema SJ, Hegedus L, Bjorner JB, Feldt-Rasmussen U. Quality of life in patients with benign thyroid disorders. A review. Eur J Endocrinol. 2006;154(4):501–510. doi: 10.1530/eje.1.02124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.