Abstract
Objective
The aim of this study is to compare the effect of photobiomodulation with low-level laser therapy (LLLT) and nimesulide on inflammatory parameters, biomarkers of oxidative stress and inflammation, and quality of life after lower third molar (L3M) surgery.
Material and methods
A randomized, two-factor, triple-blind, controlled, split-mouth clinical trial was performed with 40 volunteers who required bilateral L3M removal. Patients were allocated depending on the use or not of 100 mg nimesulide 1 hbefore surgery, as well as the use or not of LLLT in the preoperative period.
Results
Pain peaks occurred after 6 h (nimesulide-placebo [N-P] group) and 8 h (nimesulide group). In the N-P group, LLLT resulted in significantly lower mean pain scores than the subgroup without LLLT after 4 h (p = 0.009) and 6 h (p = 0.048). As for edema, a shorter distance between the mandibular angle and the outer canthus of the eyes after 7 days (p = 0.037) and a smaller cumulative effect (p = 0.036) were observed in the N-P group associated with LLLT. A direct effect between LLLT (p = 0.047) and a reduction in the mean scores of overall dissatisfaction with quality of life was detected.
Conclusions
Preemptive use of nimesulide only delayed peak pain. LLLT reduced edema, trismus, and contributed to a better perception of quality of life. Nimesulide inhibits peroxidation by increasing GSH and stopping neutrophil migration. The benefit of the association of both strategies was not superior to the use of LLLT alone.
Clinical relevance
Translational study with impact on clinical-surgical protocols involving L3M surgery related to pharmacological and non-pharmacological methods.
Keywords: Third molar; Analgesia; Anti-inflammatory agents, Non-steroidal; Low-level light therapy; Oxidative stress; Quality of life
Introduction
Mandibular third molar surgery can significantly affect the quality of life of patients, particularly during the first postoperative days because of the pain intensity as well as other inflammatory events, prompting many patients to resort to analgesic drugs after this surgical procedure [1–5]. In this context, clinical trials involving lower third molar removal have been considered important clinical models to identify adequate preemptive and non-preemptive pharmacological strategies to control postoperative inflammatory pain [6–8].
In addition to the use of non-steroidal anti-inflammatory drugs (NSAIDs) [1–3, 9], low-level laser (Light Amplification by Stimulated Emission of Radiation) therapy (LLLT) has been advocated in the modulation of inflammatory events resulting from the surgical removal of third molars. Photobiomodulation with LLLT promotes photochemical, photophysical, and photobiological effects through the transformation of photon energy into adenosine triphosphate (ATP) [10, 11]. The cell stimulation resulting from this therapy and other interrelated processes increases not only mitochondrial ATP production but also cell proliferation, which alleviates oxidative stress and preserves cellular components, exerting an important anti-inflammatory effect [12, 13]. Areas irradiated with LLLT increase O2 consumption, resulting in high rates of electron transport, which enhances ATP synthesis by the photosensitized cells. Thus, there is an increase in relative Cytochrome C oxidase (Cox) activity in mitochondrial channels [14]. The light-mitochondria interaction also increases the production of reactive oxygen species (ROS) in the mitochondria. ROS are a result of the univalent reduction of molecular oxygen, generating free radicals with high reactive potential, such as superoxide anion and hydrogen peroxide, which can be damaging to cells [15]. In addition to the formation of hydroxyl ions, the nitrogen-free radical may react with the nitric oxide free radical, generating a potentially reactive nitrogen species, known as peroxynitrite [10].
Clinical and experimental research on the pathogenesis and mediators involved in the pain process has been widely developed in the last 20 years. Although some mechanisms involved in this process have already been thoroughly described, others remain to be elucidated [16–19]. In this context, despite being considered of great relevance in the modulation of inflammatory events and studied in a considerable variety of human diseases [20], oxidative stress has not yet been investigated in clinical models of preemptive analgesia related to third molars surgery. ROS cause tissue changes induced by redox signaling, as well as modulate protein metabolism within complex networks of kinases, phosphatases, ion channels, and apoptotic cascades [21]. Oxidative stress arises from an imbalance between oxidants and antioxidants in favor of oxidant molecules, which leads to the interruption of redox signaling and physiological functions as well as irreversible chemical changes [22] through the deregulated production of various oxidizing agents [20], such as myeloperoxidase (MPO), Nitrite/Nitrate, glutathione (GSH), oxidized glutathione (GSSG), and malondialdehyde (MDA).
Petrini et al. investigated LLLT as a preemptive analgesic/anti-inflammatory strategy in lower third molar surgeries [23]. Patients were divided into two groups according to the laser therapy protocol (pre-or postoperatively), and it was found that preoperative LLLT resulted in better postoperative pain control, as well as lower consumption of rescue analgesic medication within the first 24 h after mandibular third molar surgery. However, no statistically significant difference regarding the type of laser therapy protocol was noted for edema and trismus occurrence. Landucci et al. reported that preemptive LLLT alone was effective in reducing postoperative discomfort (pain, edema, and trismus) associated with third molar extraction [24]. A possible explanation for the analgesic effect of LLLT is its ability to modulate various signaling pathways and physiological mechanisms involved in analgesia, such as increasing β-endorphin levels and modulating pain-related biochemicals, including substance P, TNF-alpha, and cyclooxygenase-2 [25–27]. Animal studies indicate that preoperative LLLT may locally act on the oxidative stress pathway to prevent ischemic muscle damage, decreasing the re-release of ROS, while increasing the levels of antioxidants and heat shock proteins [28].
These data reinforce the importance of carrying out further investigations into possible clinical benefits of LLLT only or in combination with NSAIDs (such as nimesulide, which is commonly studied in third molar clinical trials) [1–3, 9] in preemptive strategies for the relief of postoperative inflammatory events (notably pain, edema, and trismus) in mandibular third molar surgery. Moreover, it also constitutes a fertile field for translational studies involving the evaluation of certain biomarkers of oxidative stress that may be involved in the modulation of the inflammatory process that follows the local trauma resulting from the removal of mandibular third molars, while also allowing for the assessment of the quality of life of patients in the postoperative period.
Thus, the aim of this randomized clinical trial was to evaluate clinical parameters (pain, edema, and trismus), biomarkers of oxidative stress (myeloperoxidase, malonaldehyde, and glutathione) and inflammation (total protein concentration), and quality of life of patients submitted to preemptive analgesic protocols with LLLT and 100 mg nimesulide, either used isolated or in combination, after mandibular third molar surgery.
Material and methods
Ethical considerations
This work was carried out in accordance with the Health Research Standards of the Federal University of Ceará and the Brazilian National Health Council (Resolution n° 466, 2012). The research protocol was submitted and approved by the Human Research Ethics Committee of the Federal University of Ceará (Approval number 3,358,496). The volunteers of the present study received an informed consent form (ICF), in which the objectives, methodology, as well as related risks and benefits were explained, and patient confidentiality was guaranteed. Furthermore, the statements and guidelines of the Declaration of Helsinki were followed and respected.
Study design
A randomized, split-mouth, prospective, triple-blind, controlled, 2 × 2 factorial, analytical clinical trial was performed. This study was registered in the Brazilian Clinical Trials Registry (ReBEC) platform (https://ensaiosclinicos.gov.br). The present clinical trial followed the recommendations described in the latest version of the Consolidated Standards of Reporting Trials (CONSORT, http://www.consort-statement.org/extensions/checklists).
Participants
Healthy individuals (American Society of Anesthesiologists – ASA I) of both sexes, aged between 18 and 35 years, with an indication for bilateral third mandibular molar removal, whose consent was obtained through an IFC were recruited at the Oral Surgery Service of the Dentistry Course of the Faculty of Pharmacy, Dentistry, and Nursing of the Federal University of Ceará. Further inclusion criteria were (1) unerupted mandibular third molars; (2) third molars with similar patterns of root formation in panoramic radiographs, similar position, and degree of impaction between the right and left sides within the same individual.
Volunteers who met at least one of the following criteria were excluded: (1) history of photosensitivity; (2) smokers, pregnant, or lactating women; (3) patients using medication with drug-drug interaction with drugs used in this study; (4) patients with orthodontic bands on mandibular second molars; (5) confirmed history of NSAID allergy; (6) signs of any preoperative inflammatory or infectious condition; (7) systemic diseases; (8) neurological disorders; and (9) volunteers who had used any NSAID within a period of up to 21 days before the surgical procedure due to the possibility of a residual anti-inflammatory effect, which could affect the outcomes of the present study [3].
An appropriate clinical record was completed at the initial consultation and during the postoperative periods. In addition, biosecurity measures were taken regarding clinical signs or symptoms associated with the coronavirus disease 2019 (SARS-COV-2) [29].
Further exclusion criteria that were considered after inclusion in the study were (1) patients who did not follow the postoperative recommendations; (2) whose surgical procedure exceeded 2 h; or (3) who did not return for the postoperative evaluation consultations.
Interventions
In the consultation before the surgical procedure, data were collected regarding sex, age, general health status, blood and platelet count, international normalized ratio (INR), and fasting blood glucose concentration. A panoramic radiograph was also requested, and the following data were collected: degree of tooth development (Nolla stages), degree of impaction of the third molars, and their position according to the Pell & Gregory and Winter classifications. In addition, the Pernambuco index was used to assess the degree of surgical difficulty [30]. This index is based on scores assigned to variables related to the Pell & Gregory and Winter classifications; root curvature; number of roots; and relationship to the second molar, in addition to age and body mass index. The surgical difficulty is classified as low (8–12 scores), moderate (13–17 scores), and high (18–22 scores) [30].
Surgical procedures were scheduled to be performed in two separate clinical sessions (one side at a time), with an interval of at least 28 days between them [3]. All patients underwent a standardized surgical technique supported by scientific evidence performed in an outpatient setting under local anesthesia. A single oral and maxillofacial surgeon, with 3 years of experience in the respective specialty, performed all surgical procedures.
Initially, individuals considered eligible to participate in the study were randomly allocated through a list of random numbers generated in Microsoft Excel using the randbetween function [3] into four groups depending on the use of 100 mg nimesulide (N) or not (N-P, placebo) and the association with LLLT (N + LT or N-P + LT) or not (N + LT-P or N-P + LT-P), 1 h before the surgical procedure. Considering that each patient served as her/his control (split-mouth methodological design) and to ensure a 2 × 2 factorial design, the side of the face was also randomized according to the LLLT protocol (1 h before the surgical procedure), in which, with the patient blindfolded, one side received preemptive LLLT, while on the other side the device was turned off. Both the nimesulide and the placebo drug were similarly manipulated and dispensed in capsules to preserve the study blinding.
In the postoperative period, all volunteers in all study groups were assisted with supportive medication. Dipyrone (metamizole) at a dose of 500 mg was prescribed at 6-h intervals as it has been recommended as a rescue medication in similar study designs [31]. All patients were instructed to take the rescue medication when pain or local discomfort symptoms occurred. Postoperative instructions were also carefully read and explained to each patient, including following a liquid and cold diet for the first 24 h, performing strict oral hygiene, and avoiding vigorous mouthwash to prevent the occurrence of postsurgical hemorrhage. Patients were informed that they should initially contact the surgeon by telephone in case of persistent bleeding or if they deemed it necessary. In addition, patients were also asked to report any physical symptoms experienced during the postoperative periods of the study, such as nausea, vomiting, dizziness, headache, insomnia, and signs of infection. In cases of an infectious process, 500 mg amoxicillin would be prescribed at 8-h intervals for 7 days, or another more appropriate pharmacological regimen in case of allergy to this drug. In case of a dental abscess, a surgical drainage procedure would be performed in association with the drug prescription above.
The LLLT protocol was performed with a low-level gallium-aluminum-arsenic (Ga-Al-As) diode laser device (Therapy XT®, DMC, São Carlos, São Paulo, Brazil) using continuous emission at 100 mW (0.1 W) through a 600-μm-wide fiber in direct contact with the mucosa or the skin [32]. Patients allocated to the LLLT group received a total energy of 24 J (3 J per point), an irradiance of 3537 mW/cm2, and radiant exposure of 106 J/cm2. Four intraoral points were irradiated for 30 s: (I) suture region (center of the surgical cavity); (II) lingual face (cervical third); (III) lingual face (middle third); and (IV) lingual face (apical third). Extraorally, four points (30 s each) related to the masseter muscle were irradiated: (I) the most inferior region of the masseter muscle (near the insertion in the mandible); (II) lower intermediate region; (III) upper intermediate region; and (IV) the superior region (near the insertion in the zygomatic arch) [33, 34].
The same surgical protocol was adopted for both sides of the mouth to reduce differences in the level of intraoperative trauma. Removal of the lower third molars was performed under local anesthesia with 2% mepivacaine and 1:200,000 epinephrine (Mepivalem AD; Dentsply, USA), using a total of two or three cartridges containing 1.8 mL of the anesthetic solution. A full-thickness triangular flap was performed, followed by peripheral ostectomy using a high-speed bur under irrigation with 0.9% saline. Following a methodology similar to that of Albuquerque et al. (2017), two samples of soft tissue from the distal region of the third molar were collected, one obtained after local anesthesia and before performing the surgical flap (time 0'), and the other 30 min (time 30') after the surgical procedure for laboratory analysis. The surgical wound was strictly closed with 4–0 silk sutures. The biological material collected was properly packaged in sterile Eppendorf® tubes and stored in a freezer at − 80 °C until complete analysis.
Outcomes
The primary outcome of the present study was the occurrence of changes in clinical parameters related to inflammatory events (pain, swelling, and trismus). Secondary outcomes were the occurrence of quantitative changes in biomarkers of oxidative stress and inflammation and the impact on the quality of life of patients in the postoperative period. To measure the outcomes of the present study, the following methodologies were used:
Postoperative pain assessment
The occurrence of postoperative pain was evaluated through the assessment of pain intensity and the need for rescue medication. The volunteers were instructed to use a 10 cm visual analog scale (VAS), in which values range from 0 (no pain/discomfort) to 10 (maximum pain/discomfort) [1–3]. Before the surgical procedure, each patient received explanations on how to indicate pain intensity through the VAS. After the surgical procedure, each patient received a form to write down the postoperative pain values at home, and this document was returned to the researcher on the day of suture removal (seventh postoperative day). In this sense, the volunteers of this study were asked to inform the pain intensity scores in the following postoperative evaluation periods: 0 (end of the surgical procedure), 2, 4, 6, 8, 10, 24, 48, and 72 h, as well as at 7 days. Additionally, the form that each patient received contained a dedicated section to register the time required to take the first rescue medication (if necessary) as performed by Costa et al. [1], as well as the daily consumption of rescue medication during the postoperative period.
Assessment of postoperative edema and trismus
Lines between certain facial points on the operated side were drawn to measure edema [1, 3]. The distances were measured in millimeters from the mandibular angle (MA) to the tragus (MA-Tr distance); outer canthus of the eye (MA-OCE distance); ala of the nose (MA-AN distance); labial commissure (MA-LC distance); soft pogonion (MA-SP distance). The differences between the preoperative and postoperative values (24, 72 h, and 7 days) were compared between the groups.
The assessment of mouth opening limitation (trismus) was measured through the comparison of maximum mouth opening in predetermined periods (24, 72 h, and 7 days) among the groups using a digital caliper interposed between the incisal edges of the maxillary and mandibular central incisors.
Concentration of biomarkers of oxidative stress and inflammation
The samples collected at 0 and 30 min after the beginning of the surgical procedures were stored in a – 80 °C freezer and later processed to evaluate the following biomarkers: myeloperoxidase, malonaldehyde, glutathione, and total protein concentration.
Myeloperoxidase
Samples were macerated in a homogenizer with 250 μL of 0.2 M NaPO4 solution (pH = 4.7). After maceration, the samples were centrifuged at 3000 rpm for 15 min, and the supernatant was separated and immediately stored at – 80 °C. A lysing solution (1 mL 0.2% NaCl) was added to the pellets and vortexed for 30 s. After vortexing, the samples were centrifuged again (3000 rpm, 15 min), and the supernatant was discarded.
Then, 250 μL of hexadecyltrimethylammonium bromide (HTBA) was added to the pellets and the homogenate was macerated for 30 s at 4 °C. After the last maceration, the suspension was centrifuged at 10,000 rpm for 15 min before plating.
Ninety-six-well plates were incubated in duplicate with 25 μL of the homogenate and 25 μL of diluent (3,3′,5,5′-tetramethylbenzidine, H2O2, PBS). The reaction was halted after 5 min by adding sulfuric acid, and the plates were then read in a spectrophotometer (450 nm). Changes in absorbance were plotted on a neutrophil standard curve and expressed as neutrophils/mg of tissue [35].
Malonaldehyde
The samples were vigorously homogenized and subjected to centrifugation processes, and the supernatant was immediately frozen in liquid nitrogen at – 80 °C. Subsequently, the samples were brought to room temperature, 100 µL of the supernatant was removed, and 4 mL of ice-cold 1.15% potassium chloride (KCl) was added so that the sample was homogeneous, resulting in a concentrated acid solution.
Aqueous TBA solution (0.6%, 1 mL) and 3 mL 1% phosphoric acid (H3PO4) were added to a 0.5-mL aliquot of the sample, and the mixture was placed in a boiling bath, yielding a pink-colored product. Then, 4 mL of n-butanol was added, and the mixture was stirred for 2 min followed by centrifugation for 10 min at 3000 rpm.
The absorbance of the supernatant was measured in a Beckman spectrophotometer (520 nm to 535 nm), measuring the plasma concentration of thiobarbituric acid reactive substances (TBARS) in nmol malondialdehyde (MDA)/mL [35].
Glutathione dosage (oxidized and reduced forms)
The same supernatant obtained above was brought to room temperature, 100 µL was removed, and homogenization was carried out in 5 mL of 0.02 M EDTA. Four milliliters of the homogenate was mixed with 3.2 mL of distilled water and 0.8 mL of 50% trichloroacetic acid. The tube was shaken and centrifuged at 3000 rpm for 25 min. Then, 4 mL of 0.4 M TRIS (pH 8.9) and 0.1 mL of 0.01 M DTNB were added to 2 mL of the supernatant. The mixture was shaken and homogenized, followed by absorbance reading in a spectrophotometer at 412 nm. The final concentration of glutathione (µmol/mL) was obtained by comparing the absorbance value with a standard curve [35].
Total protein concentration
The samples obtained were vigorously homogenized and subjected to centrifugation processes, and the supernatant was immediately frozen in liquid nitrogen at − 80 °C. Subsequently, 300 µL of 0.85% sodium chloride was added to the samples and homogenized in a TissueLyser® for 5 min at 50,000 rpm. Then, 200 µL of the supernatant was mixed with the reagent, mixed, and kept in a water bath at 37 °C for 10 min. The absorbance of the supernatant phase was measured in a Beckman spectrophotometer (530 nm to 550 nm) [36].
Quality of life
To assess quality of life, each patient received two questionnaires: (1) one with a more general approach, namely the Oral Health Impact Profile (OHIP-14), which focuses mainly on negative repercussions; (2) a condition-specific instrument entitled Health-Related Quality of Life (HRQOL) [37]. Each patient was required to answer the questionnaires at the initial consultation, 24 h after the surgical procedure, and 7 days after surgery.
The OHIP-14 questionnaire consists of 14 questions distributed in seven domains. Two questions were applied to each of the seven domains (functional limitation, physical pain, psychological discomfort, physical disability, psychological disability, social disability, and handicap). Questions refer to how often individuals had trouble chewing, pronouncing words, felt discomfort while eating, felt tense, worse sense of taste, had to interrupt meals, felt upset or embarrassed, avoided going out or solving problems, or were unable to work because of dental problems.
Delayed clinical recovery was attributed to patients under the following conditions: prescription of antibiotics or other analgesics, surgical wound dehiscence, surgical wound debridement, or placement of a dressing [38].
Prevalence, recurrence, and severity of scores were calculated preoperatively and postoperatively according to Shugars et al. [37]. The percentage of volunteers reporting one or more items as “occasionally” or “very often” represented a measurement of prevalence. An item reported as “fairly often” or “very often” was considered clinically important and detrimental to the quality of life. In addition, the recurrence score of each patient was computed as the number of items reported “fairly often” or “very often,” while severity was computed as the sum of the OHIP-14 scores, coded as never? (0 points), almost never? (1 point), occasionally? (2 points), fairly often? (3 points), and very often? (4 points).
The HRQOL instrument was used to assess the patients’ perception of their recovery in the domains: pain, lifestyle, oral function, and other symptoms related to third molar extraction [37, 38]. Apart from pain, the results obtained were evaluated with a 5-point Likert scale, referring “no problems” (1 point) to “a lot of problems” (5 points). The means of worst pain levels in the last 24 h were evaluated with a 7-point Likert-type scale anchored in the descriptors “no pain” (1 point) to “worst imaginable pain” (7 points). The patient’s report of the sensory perception of pain and the affective impact or displeasure of pain being experienced at that time were recorded daily on Gracely scales, in which the patient could select 1 of 13 verbal descriptors [39]. Patients also recorded whether analgesics or other medications were needed for pain relief on each postoperative day.
The HRQOL instrument and the Gracely scale were used to determine the percentage of individuals who daily reported symptoms with high and low thresholds of severity. The percentage of patients with Likert-type responses being “a little” or “a lot” (4–5 out of 5) for lifestyle, oral function, or other symptoms; (5–7 out of 7) for pain; and on the Gracely scale affective words such as “very distressing,” “intolerable,” “very intolerable” and sensory words “intense,” “very intense,” and “extremely intense” were considered clinically important and harmful to the quality of life.
At first, the questionnaires were applied by a collaborator who did not know the group to which the patient belonged, as well as who did not participate in the performance of the surgical procedures or the measurement of any other outcome, thus ensuring the blinding of the study. In addition, the collaborator filled in the data obtained in an electronic spreadsheet with the groups coded by letters A and B to guarantee the blinding of the statistician.
Sample size
Based on the study by Sierra et al. who observed a reduction in edema measurements 7 days after the removal of impacted third molars using intraoral LLLT compared to a control group without LLLT (0.6 ± 0.3 cm versus 1.0 ± 0.4 cm), a sample of 17 patients per study group was estimated as necessary to achieve an evaluation with 90% power and 95% confidence [40]. Considering the possibility of sample loss, 10% was added to the sample, totaling 20 patients per study group.
Randomization
The randbetween function of the Microsoft Excel® version 2010 was used to generate the random allocation sequence through simple randomization without any restriction. Opaque envelopes containing randomization numbers on the outside were used to implement the random allocation sequence. Once opened, these envelopes showed the surgeon the group to which the study volunteer would be allocated. A collaborator who did not participate in any other stage of the study was responsible for generating the random allocation sequence, as well as for properly organizing and distributing the participants in the groups.
Blinding
To ensure triple blinding, the patient, researcher, and statistician were unaware of the group allocation of the volunteers [41]. Before the surgical procedures, a list containing the random distribution of all surgical sites (right and left sides) and respective drugs to be administered were kept in a sealed envelope by an external collaborator who was unaware of the study protocol until the final analysis of the data. Statistical analysis was performed initially with groups coded with the letters “A” (group 1) and “B” (group 2), subgroups coded with the letters “c” (with LLLT) and “d” (without LLLT). The coding system was revealed only after the results were computed to guarantee triple blinding.
Statistical methods
The data were tabulated in a spreadsheet on Microsoft Excel® (2010) and submitted to statistical analysis using the Statistical Package for the Social Sciences (SPSS) software, version 20.0 for Windows®.
To verify the normality of the quantitative data, the Kolmogorov–Smirnov test was used. This test considers the adherence and agreement between the distribution of a set of sample values and a specific theoretical reference distribution; in this case, the normal distribution. Data such as pain scales, quality of life, mouth opening limit, and edema measurements were expressed as mean and standard deviation values and analyzed using the Mann–Whitney and Kruskal Wallis/Dunn tests (for nonparametric data) or Student’s t test or ANOVA/Bonferroni (for parametric data). For intragroup analysis, Wilcoxon and Friedman/Dunn tests (nonparametric data) or repeated-measures ANOVA/Bonferroni (parametric data) were used. Correlation between study variables was performed using Pearson’s (normal distribution) or Spearman’s correlation test (non-normal distribution). The Kaplan-Meyer method was used to generate an analysis of rescue medication consumption. Categorical data were expressed as absolute and percentage frequencies and analyzed using the chi-square test, or Fisher’s exact test in case there was any variable with less than 5 measurements.
Finally, the association between the multiple variables of the study was evaluated by multiple linear regression analysis. The level of statistical significance adopted for all tests was 5% (p < 0.05) (Fig. 1).
Fig. 1.
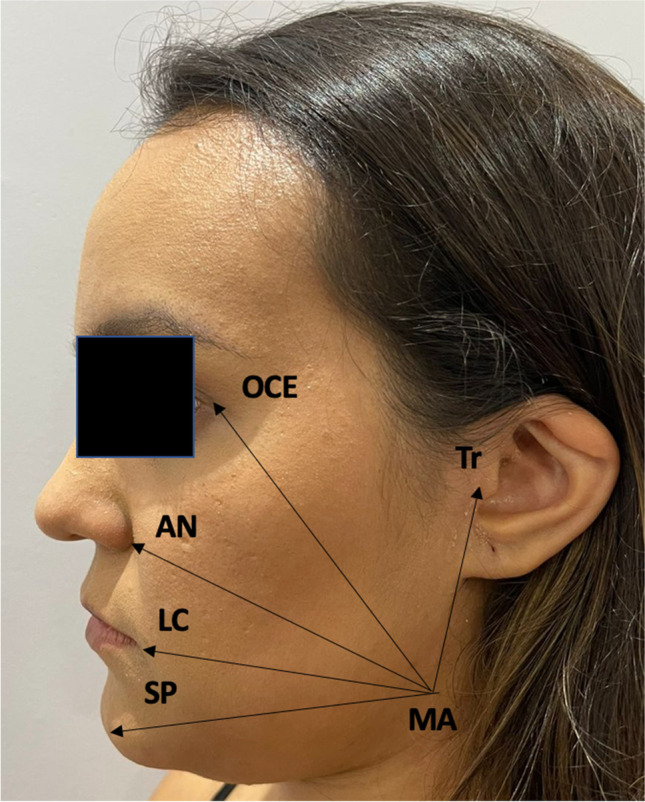
Linear measurements used to assess postoperative edema, from the mandibular angle (MA) to (1) tragus (MA-Tr distance), (2) outer canthus of the eye (MA-OCE distance), (3) ala of the nose (MA-AN distance), 4) labial commissure (MA-LC distance), and (5) soft pogonion (MA-SP distance)
Results
Sociodemographic profile and sample characterization
Five hundred patients were initially screened for the eligibility criteria. Of these, 460 were removed, as detailed in Fig. 2. The final sample consisted of 40 volunteers (8 males and 32 females), totaling 80 surgical sites divided into 4 subgroups of 20 surgical procedures. There was no sample loss in any of the groups. Most patients were young adults with a similar mean age between the study groups (80%) (p > 0.05). There were no statistically significant differences regarding the sociodemographic profile (Table 1), radiographic position of the third molars, mean number of anesthetic cartridges used, time, frequency of bone removal, surgical difficulty (Pernambuco index), and tooth development (Nolla stages) (Table 2).
Fig. 2.
Flow diagram of patients recruited according to the CONSORT guidelines
Table 1.
Demographic and socioeconomic profile of the sample
| Study groups | p value | ||
|---|---|---|---|
| Placebo (w/or w/o LLLT) | Nimesulide (w/or w/o LLLT) | ||
| Age (years) | 21.8 ± 2.6 | 23.8 ± 3.5 | 0.063a |
| Sex | |||
| Male | 4 (20.0%) | 4 (20.0%) | 1.000b |
| Female | 16 (80.0%) | 16 (80.0%) | |
| Marital status | |||
| Married | 1 (5.0%) | 2 (10.0%) | 0.548b |
| Single | 19 (95.0%) | 18 (90.0%) | |
| Race | |||
| White | 8 (40.0%) | 8 (40.0%) | 1.000b |
| Black | 1 (5.0%) | 1 (5.0%) | |
| Mixed-race | 11 (55.0%) | 11 (55.0%) | |
| Educational level (school years) | |||
| ≤ 8 | 1 (5.0%) | 1 (5.0%) | 0.626b |
| 9–11 | 7 (35.0%) | 10 (50.0%) | |
| ≥ 12 | 12 (60.0%) | 9 (45.0%) | |
| Family income* | |||
| < 1 | 1 (5.0%) | 2 (10.0%) | 0.701b |
| 2 | 10 (50.0%) | 7 (35.0%) | |
| 3 | 2 (10.0%) | 1 (5.0%) | |
| 4 | 3 (15.0%) | 3 (15.0%) | |
| 5 | 3 (15.0%) | 3 (15.0%) | |
| > 5 | 1 (5.0%) | 4 (20.0%) | |
*Number of minimum wages (R$1039.00 or equivalent to USD$186.47 considering the exchange rate variation for the year 2021)
aMann-Whitney test (mean ± SD)
bFisher’s exact test or Pearson's chi-square test (n, %)
Table 2.
Characterization of the third molars extracted and surgical aspects among the study groups
| Placebo | p value | Nimesulide | p value | |||
|---|---|---|---|---|---|---|
| w/o LLLT | w/ LLLT | w/o LLLT | w/ LLLT | |||
| Eruption status | ||||||
| Fully erupted | 0 (0.0%) | 0 (0.0%) | 1.000a | 1 (5.0%) | 1 (5.0%) | 0.762a |
| Impacted | 10 (100.0%) | 20 (100.0%) | 19 (95.0%) | 19 (95.0%) | ||
| Ramus relationship | ||||||
| Class I | 0 (0.0%) | 0 (0.0%) | 1.000a | 2 (10.0%) | 2 (10.0%) | 1.000a |
| Class II | 20 (100.0%) | 20 (100.0%) | 18 (90.0%) | 18 (90.0%) | ||
| Relationship to the second molar | ||||||
| A | 0 (0.0%) | 0 (0.0%) | 1.000a | 2 (10.0%) | 2 (10.0%) | 1.000a |
| B | 20 (100.0%) | 20 (100.0%) | 17 (85.0%) | 17 (85.0%) | ||
| C | 0 (0.0%) | 0 (0.0%) | 1 (5.0%) | 1 (5.0%) | ||
| Angulation | ||||||
| Horizontal | 0 (0.0%) | 1 (5.0%) | 1.000a | 6 (30.0%) | 8 (40.0%) | 0.766a |
| Vertical | 20 (100.0%) | 19 (95.0%) | 11 (55.0%) | 10 (50.0%) | ||
| Mesioangular | 0 (0.0%) | 0 (0.0%) | 3 (15.0%) | 2 (10.0%) | ||
| Proximity to the inferior alveolar nerve | ||||||
| Absent | 20 (100.0%) | 20 (100.0%) | 1.000a | 14 (70.0%) | 15 (75.0%) | 1.000a |
| Root darkening | 0 (0.0%) | 0 (0.0%) | 6 (30.0%) | 5 (25.0%) | ||
| Surgical aspects | ||||||
| Number of anesthetic cartridges used | 2.0 ± 0.0 | 2.0 ± 0.0 | 1.000b | 2.1 ± 0.2 | 2.0 ± 0.0 | 0.317b |
| Surgical time (minutes)* | 5.9 ± 3.8 | 5.33 ± 3.79 | 0.081b | 10.0 ± 6.2 | 11.6 ± 7.2 | 0.235b |
| Bone sectioning | 5 (25.0%) | 3 (15.0%) | 0.695a | 12 (60.0%) | 14 (70.0%) | 0.741a |
| Pernambuco index | ||||||
| Values (scores) | 12.33 ± 0.5 | 13.26 ± 2.6 | 0.628a | 12.33 ± 0.5 | 13.26 ± 2.6 | 0.628a |
| Nolla stages | ||||||
| 10 | 13 (65.0%) | 18 (90.0%) | 0.149 a | 13 (65.0%) | 18 (90.0%) | 0.149 a |
| 9 | 6 (30.0%) | 2 (10.0%) | 0.149 a | 6 (30.0%) | 2 (10.0%) | |
| 8 | 1 (5.0%) | 0 (0.0%) | 0.149 a | 1 (5.0%) | 0 (0.0%) | |
*Corresponding time from incision to suture. Captions: Pell & Gregory Class I: the crown of the third molar, in its mesiodistal diameter, is completely in front of the anterior border of the mandibular ramus; Pell & Gregory Class II: the third molar crown is partially within the mandibular ramus; Pell & Gregory position A: the occlusal surface of the third molar is in the same occlusal plane as the adjacent second molar; Pell & Gregory position B: the occlusal surface of the third molar is between the occlusal plane and the cervical line of the adjacent second molar; Pell & Gregory position C: the occlusal surface of the third molar is below the cervical line of the adjacent second molar
ap < 0.05, Fisher’s exact test or Pearson's chi-square test (n, %)
bWilcoxon test (mean ± SD)
Pain scores and rescue medication consumption
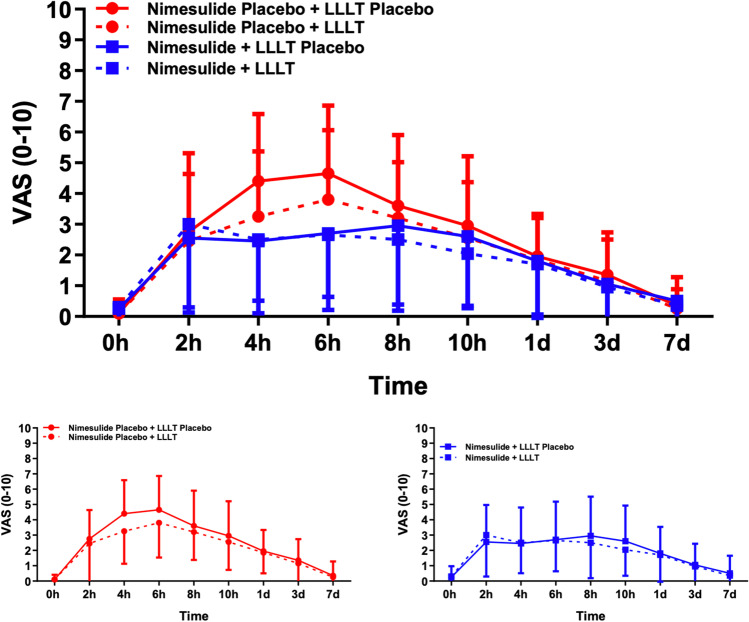
Postoperative scores were significantly high 2 h after the surgical procedure, with intensity peaks after 6 h in the N-P group, and 8 h in the N group. Lower mean VAS scores were observed when LLLT was used both in the N-P (3.80 ± 2.26) and N (2.75 ± 2.71) groups. For N-P patients, a significant reduction in pain intensity was observed after 10 h in the subgroups with and without LLLT (p < 0.001). In the N group, the reduction in pain intensity occurred after seven days both in the subgroup without (p = 0.025) and with (p = 0.031) LLLT. In the N-P group, mean pain scores were significantly lower in the subgroup with LLLT 4 h (p = 0.09) and 6 h = 0.048) after tooth extraction compared to the subgroup without LLLT, as well as in the cumulative effect throughout the study (p = 0.036). Multifactorial analysis revealed that, for patients who received nimesulide, pain scores were significantly lower regardless of LLLT (p = 0,065) and 4 h after surgery (p = 0.045) (Table 3). There was no statistically significant difference between the study groups regarding the mean consumption of rescue medication (p = 0.858) (Fig. 3).
Table 3.
Mean ± SD of VAS scores related to pain and rescue medication consumption among the study groups
| Placebo | p value | Nimesulide | p value | Multifactorial analysis | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| w/o LLLT | w/ LLLT | w/o LLLT | w/ LLLT | LLLT | Nimesulide | ||||
| VAS | |||||||||
| 0 hα | 0.10 ± 0.31 | 0.10 ± 0.45 | 1.000b | 0.25 ± 0.72 | 0.45 ± 1.39 | 0.541b | 0.547e | 0.457f | 0.671 h |
| 2 h | 2.75 ± 1.89* | 2.45 ± 2.86* | 0.584b | 2.55 ± 2.42* | 3.00 ± 2.70* | 0.513b | 0.863e | 0.390f | 0.754 h |
| 4 h | 4.40 ± 2.19* | 3.25 ± 2.12* | 0.009b | 2.45 ± 2.35* | 2.50 ± 1.99* | 0.908b | 0.065e | 0.045f | 0.021 h |
| 6 h | 4.65 ± 2.21* | 3.80 ± 2.26* | 0.048b | 2.70 ± 2.49* | 2.65 ± 2.01* | 0.909b | 0.137e | 0.185f | 0.017 h |
| 8 h | 3.60 ± 2.30* | 3.20 ± 1.82* | 0.504b | 3.00 ± 2.60* | 2.75 ± 2.71* | 0.480b | 0.346e | 0.827f | 0.370 h |
| 10 h | 3.10 ± 2.25*† | 2.60 ± 1.82*† | 0.242b | 2.90 ± 2.49* | 2.35 ± 2.11* | 0.242b | 0.096e | 0.936f | 0.795 h |
| 1 day | 2.30 ± 1.66*† | 1.90 ± 1.45*† | 0.163b | 2.30 ± 2.58* | 2.20 ± 2.53 | 0.881b | 0.487e | 0.676f | 0.855 h |
| 3 days | 1.40 ± 1.43*† | 1.20 ± 1.47† | 0.577b | 1.15 ± 1.53 | 1.25 ± 1.92 | 0.858b | 0.879e | 0.650f | 0.651 h |
| 7 days | 0.35 ± 0.93† | 0.25 ± 0.64† | 0.705b | 0.70 ± 1.66† | 0.65 ± 1.84† | 0.897b | 0.747e | 0.914f | 0.951 h |
| p value | < 0.001d | 0.001d | 0.025d | 0.031d | |||||
| Cumulative effect | 2.52 ± 1.28 | 2.08 ± 1.28 | 0.036a | 2.00 ± 1.67 | 1.98 ± 1.49 | 0.943a | 0.214e | 0.289f | 0.441 h |
| Rescue medication consupmtion** | 0.85 ± 0.99 | 1.00 ± 1.30 | 0.625a | 1.40 ± 1.54 | 1.10 ± 1.33 | 0.445a | 0.761e | 0.363f | 0.340 h |
Entries in bold refer to data that obtained a statistically significant p value
*p < 0.05 versus baseline; †p < 0.05 versus peak; **mean number of pills taken over the study evaluation periods
aPaired t test
bWilcoxon test
cRepeated-measures-1-way-ANOVA/Bonferroni test
dFriedman/Dunn test
eLLLT factor of the repeated-measures-2-way-ANOVA/Bonferroni test
fNimesulide factor of the repeated-measures-1-way-ANOVA/Bonferroni test
gANOVA-1-way/Bonferroni test
hKruskal-Wallis/Dunn test
αImmediately before anesthesia
Fig. 3.
Pain scale measurements throughout the postoperative period. Data expressed as Mean ± SD
Trismus and facial edema assessment
Both groups showed a significant reduction in the maximum mouth opening 24 h after the surgical procedure; however, maximum opening values were significantly increased in the 7-day postoperative period (p < 0.001; Table 4).
Table 4.
Characterization of variables related to inflammatory events in each study group
| Placebo | p value | Nimesulide | p value | Multifactorial analysis | ||||
|---|---|---|---|---|---|---|---|---|
| w/o LLLTA | w/ LLLTB | w/o LLLTC | w/ LLLTD | LLLT | Nimesulide | |||
| Mouth Opening (mm) | ||||||||
| Initialα | 49.05 ± 8.04 | 48.25 ± 6.84 | 0.389a | 48.39 ± 8.49 | 49.55 ± 7.27 | 0.142a | 0.762e | 0.106f |
| 24 h | 34.35 ± 12.38* | 33.90 ± 10.89* | 0.758a | 30.20 ± 13.82* | 29.65 ± 11.52* | 0.795a | 0.696e | 0.969f |
| 72 h | 35.70 ± 10.75* | 35.55 ± 10.16* | 0.921a | 34.55 ± 13.95*† | 33.00 ± 11.67* | 0.390a | 0.465e | 0.547f |
| 7 days | 43.25 ± 9.74*† | 42.45 ± 9.94*† | 0.655a | 41.60 ± 10.47*† | 40.60 ± 9.49*† | 0.594a | 0.485e | 0.938f |
| p value | < 0.001c | < 0.001c | < 0.001c | < 0.001c | ||||
| Cumulative effect | 40.68 ± 9.31 | 40.03 ± 8.35 | 0.615a | 38.68 ± 11.06 | 38.20 ± 8.81 | 0.693a | 0.526e | 0.698f |
| MA-Tr (mm) | ||||||||
| Initialα | 5.76 ± 0.55 | 5.58 ± 0.65 | 0.262a | 5.61 ± 0.78 | 5.65 ± 0.87 | 0.848a | 0.621c | 0.418d |
| 24 h | 6.67 ± 0.66* | 6.41 ± 0.72* | 0.105a | 6.20 ± 0.99* | 6.01 ± 0.78* | 0.452a | 0.133c | 0.828d |
| 72 h | 6.75 ± 0.73* | 6.43 ± 0.73* | 0.115a | 6.24 ± 1.11 | 6.16 ± 1.01 | 0.783a | 0.255c | 0.492d |
| 7 days | 6.02 ± 0.59*† | 5.77 ± 0.68*† | 0.151a | 5.78 ± 0.70† | 5.78 ± 0.86† | 0.982a | 0.369c | 0.388d |
| p value | < 0.001b | < 0.001b | 0.002b | 0.018b | ||||
| Cumulative effect | 6.30 ± 0.58 | 6.05 ± 0.63 | 0.098a | 5.96 ± 0.81 | 5.90 ± 0.75 | 0.784a | 0.232c | 0.455d |
| MA-OCE (mm) | ||||||||
| Initialα | 9.18 ± 0.72 | 8.91 ± 0.53 | 0.113a | 9.36 ± 0.88 | 9.16 ± 0.75 | 0.312a | 0.071c | 0.796d |
| 24 h | 10.03 ± 1.00* | 9.77 ± 0.83* | 0.130a | 9.75 ± 0.83* | 9.93 ± 0.83* | 0.335a | 0.752c | 0.081d |
| 72 h | 10.22 ± 0.93* | 9.99 ± 0.92* | 0.197a | 10.10 ± 0.93* | 10.12 ± 0.92* | 0.936a | 0.490c | 0.412d |
| 7 days | 9.41 ± 0.69*† | 9.11 ± 0.54*† | *0.037a | 9.45 ± 0.68† | 9.34 ± 0.74† | 0.590a | 0.091c | 0.409d |
| p value | < 0.001b | < 0.001b | 0.03b6 | < 0.001b | ||||
| Cumulative effect | 9.71 ± 0.79 | 9.44 ± 0.66 | 0.036a | 9.66 ± 0.65 | 9.64 ± 0.70 | 0.877a | 0.179c | 0.264d |
| MA-AN (mm) | ||||||||
| Initialα | 9.53 ± 0.53 | 9.35 ± 0.62 | 0.365a | 9.83 ± 0.74 | 9.75 ± 0.69 | 0.710a | 0.374c | 0.740d |
| 24 h | 10.75 ± 0.86* | 10.18 ± 0.87* | 0.007a | 10.77 ± 0.82* | 10.96 ± 0.92* | 0.527a | 0.301c | 0.039d |
| 72 h | 10.79 ± 0.77* | 10.42 ± 0.90* | 0.131a | 11.17 ± 0.86* | 10.90 ± 0.89* | 0.282a | 0.066c | 0.766d |
| 7 days | 9.83 ± 0.58*† | 9.53 ± 0.67*† | 0.120a | 9.99 ± 0.71† | 9.88 ± 0.67*† | 0.587a | 0.143c | 0.509d |
| p value | < 0.001b | < 0.001b | < 0.001b | 0.001b | ||||
| Cumulative effect | 10.22 ± 0.60 | 9.87 ± 0.71 | 0.034a | 10.44 ± 0.65 | 10.37 ± 0.62 | 0.759a | 0.143c | 0.314d |
| MA-LC (mm) | ||||||||
| Initialα | 7.44 ± 0.73 | 7.44 ± 0.75 | 1.000a | 8.07 ± 0.99 | 7.90 ± 0.86 | 0.566a | 0.634c | 0.634d |
| 24 h | 8.73 ± 0.87* | 8.42 ± 0.95* | 0.143a | 9.10 ± 0.92* | 9.17 ± 0.94* | 0.752a | 0.459c | 0.224d |
| 72 h | 8.87 ± 0.82* | 8.75 ± 0.96* | 0.597a | 9.08 ± 0.85* | 9.10 ± 0.82* | 0.943a | 0.772c | 0.689d |
| 7 days | 7.70 ± 0.85*† | 7.64 ± 0.79*† | 0.793a | 8.19 ± 0.93*† | 8.15 ± 0.77*† | 0.879a | 0.776c | 0.964d |
| p value | < 0.001b | < 0.001b | 0.001b | 0.001b | ||||
| Cumulative effect | 8.18 ± 0.67 | 8.06 ± 0.78 | 0.502a | 8.61 ± 0.78 | 8.58 ± 0.66 | 0.904a | 0.614c | 0.756d |
| MA-SP (mm) | ||||||||
| Initialα | 9.59 ± 0.76 | 9.43 ± 0.81 | 0.328a | 9.92 ± 0.97 | 9.77 ± 0.77 | 0.541a | 0.283c | 0.994d |
| 24 h | 10.68 ± 1.05* | 10.38 ± 0.88* | 0.153a | 11.19 ± 1.07* | 11.15 ± 1.29* | 0.917a | 0.387c | 0.492d |
| 72 h | 10.83 ± 1.02* | 10.57 ± 0.93* | 0.284a | 11.29 ± 1.12* | 11.09 ± 1.21* | 0.513a | 0.239c | 0.897d |
| 7 days | 9.86 ± 0.83*† | 9.59 ± 0.80*† | 0.100a | 9.68 ± 2.27*† | 10.00 ± 0.80*† | 0.591a | 0.940c | 0.334d |
| p value | < 0.001b | < 0.001b | < 0.001b | < 0.001b | ||||
| Cumulative effect | 10.24 ± 0.78 | 9.99 ± 0.80 | 0.124a | 10.52 ± 1.04 | 10.50 ± 0.76 | 0.950a | 0.407c | 0.471d |
| Total cumulative effect | 8.93 ± 0.55 | 8.68 ± 0.54 | 0.034a | 9.04 ± 0.56 | 9.00 ± 0.56 | 0.782a | 0.113c | 0.246d |
Entries in bold refer to data that obtained a statistically significant p value
*p < 0.05 versus baseline; †p < 0.05 versus peak; Mean ± SD
aPaired t test
bRepetead-measures-1-way ANOVA/Bonferroni test
cLLLT factor of the repeated-measures-2-way-ANOVA/Bonferroni test
dNimesulide factor of the repeated-measures-1-way -ANOVA/Bonferroni test
eOne-way ANOVA/Bonferroni Test
fKruskal-Wallis/Dunn Test
αimmediately before anesthesia
As depicted in Table 4, the MA-Tr and MA-OCE measurements showed higher statistically significant values 24 h after tooth extraction in all study groups. In both the placebo and nimesulide groups, a significant reduction in facial edema occurred within seven days of the surgical procedure. The MA-OCE measurement showed lower values after 7 days (p = 0.037) and a lower cumulative effect (p = 0.036) in the placebo group associated with LLLT.
In both study groups, the MA-AN measurement exhibited a statistically significant peak 24 h after surgery and up to 7 days after the surgical procedure (Table 4). There was no significant reduction in this measurement when LLLT was associated with nimesulide; however, a reduction was observed in the placebo group when LLLT was used (24-h postoperative period, p = 0.007; cumulative effect of the evaluation periods, p = 0.034).
The MA-LC and MA-SP measurements showed statistically significant higher values 24 h after tooth extraction in all study groups, with a significant reduction in these measurements occurring 7 days after the surgical procedure. The isolated or combined use of LLLT and nimesulide did not result in a statistically significant reduction of these measurements (Table 4).
Concentration of biomarkers of oxidative stress and inflammation
The N-P + LT-P (from 182.50 ± 128.60 to 192.50 ± 89.75 mg/dL, p = 0.841), N-P + LT (from 114.20 ± 50.54 to 104.70 ± 48.81 mg/dL, p = 0.674) and N + LT groups (from 182.30 ± 145.60 to 145.80 ± 33.64 mg/dL, p = 0.450) showed no significant variation in GSH concentration from T0 to T30, respectively. However, the N + LT-P group exhibited a significant increase in this biomarker in the evaluated period (from 131.70 ± 54.97 to 369.80 ± 195.70 mg/dL, p = 0.009). No difference was observed between the four study groups at T0 (p = 0.395), whereas at T30 the N + LT-P group had significantly higher GSH mean values than the other groups (p < 0.001) (Table 5) (Fig. 4).
Table 5.
Concentration of oxidative stress biomarkers
| N-P + LT-P 0' | N-P + LT-P 30’ | N-P + LT 0' | N-P + LT 30' | N + LT-P 0' | N + LT-P 30' | N + LT 0' | N + LT 30' | T0 | T30 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GSH | 182.50 ± 128.60 | 192.50 ± 89.75 | 0.841 | 114.20 ± 50.54 | 104.70 ± 48.81 | 0.674 | 131.70 ± 54.97 | 369.80 ± 195.70 | 0.009 | 182.30 ± 145.60 | 145.80 ± 33.64 | 0.450 | 0.395 | < 0.001 |
| MPO | 1456 ± 1106 | 10,340 ± 1563 | < 0.001 | 753.3 ± 263.3 | 1639 ± 235.3 | < 0.001 | 888.4 ± 264.3 | 1152 ± 478.8 | 0.145 | 653.3 ± 330.4 | 3014 ± 1173 | < 0.001 | 0.052 | < 0.001 |
| MDA | 1.13 ± 0.35 | 5.73 ± 1.01 | < 0.001 | 1.56 ± 0.67 | 2.57 ± 0.81 | 0.007 | 2.45 ± 1.30 | 1.92 ± 0.48 | 0.295 | 3.45 ± 1.96 | 3.98 ± 1.82 | 0.555 | 0.001 | < 0.001 |
Fig. 4.
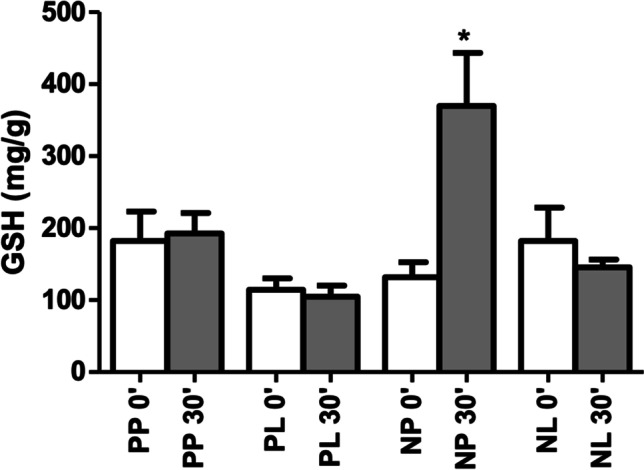
GSH concentration by group and surgical time
The myeloperoxidase (MPO) concentration showed a significant increase from T0 to T3 in the N-P + LT-P (1456 ± 1106 to 10,340 ± 1563 mg/dL, p < 0.001), N-P + LT (753.3 ± 263.3 to 1639 ± 235.3 mg/dL, p < 0.001) and N + LT (653.3 ± 330.4 to 3014 ± 1173 mg/dL, p < 0.001) groups, respectively. No significant variation in MPO was detected in the N + LT-P group (888.4 ± 264.3 to 1152 ± 478.8 mg/dL, p = 0.145). At T0, the four groups did not differ significantly (p = 0.052), but at T30 the N-P + LT-P group presented mean MPO values significantly higher than the other groups (p < 0.001) (Table 5; Fig. 5).
Fig. 5.
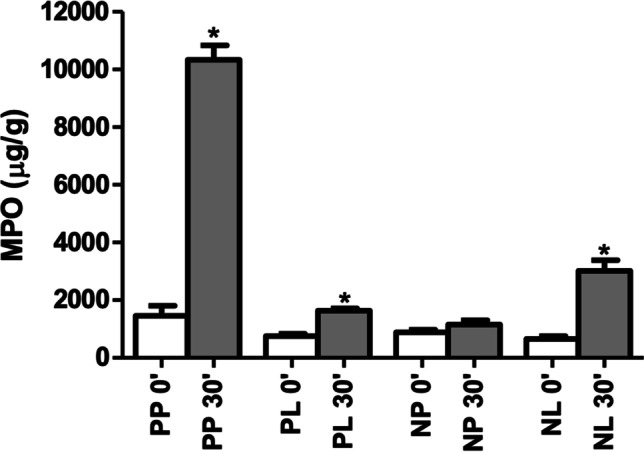
MPO concentration by group and surgical time
Malonaldehyde (MDA) levels showed a significant increase from T0 to T30 in the N-P + LT-P (1.13 ± 0.35 to 5.73 ± 1.01 mg/dL, p < 0.001) and PL (1.56 ± 0.67 to 2.57 ± 0.81 mg/dL, p = 0.007) groups, with no statistically significant difference in the N + LT-P (2.45 ± 1.30 to 1.92 ± 0.48 mg/dL, p = 0.295) and N + LT (3.45 ± 1.96 to 3.98 ± 1.82 mg/dL, p = 0.555) groups. At T0, the N + LT-P and N + LT groups exhibited higher values than the other groups (p = 0.001), whereas at T30 the N-P + LT-P group showed significantly higher concentration levels than the other groups (p < 0.00) (Table 5; Fig. 6).
Fig. 6.
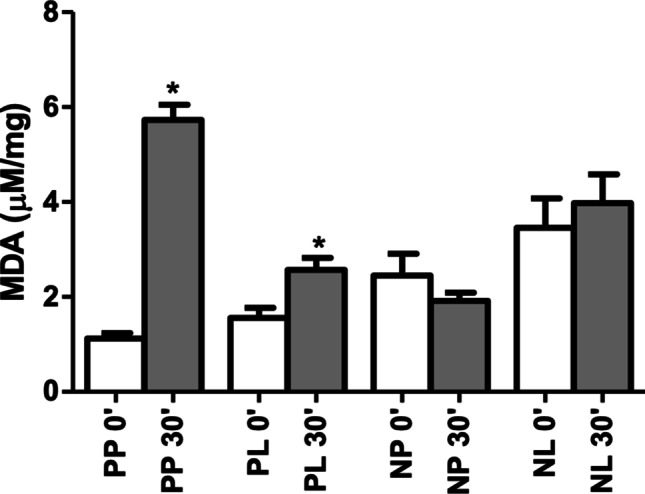
MDA concentration by group and surgical time
As for total protein concentration, in the N-P + LT-P (p = 0.040), N + LT-P (p < 0.001), and N + LT (p < 0.001) groups, there was a significant increase in protein leakage from T0 (0.199 ± 0.034, 0.256 ± 0.020, and 0.308 ± 0.028, respectively) to T30 (0.473 ± 0.106, 0.493 ± 0.007, and 0.505 ± 0.017, respectively). In the N + LT-P group, there was no significant variation from T0 (0.390 ± 0.102) to T30 (0.429 ± 0.05) (p = 0.722). No significant difference between the groups at T0 (p = 0.096) and T30 (p = 0.812) was detected (Fig. 7).
Fig. 7.
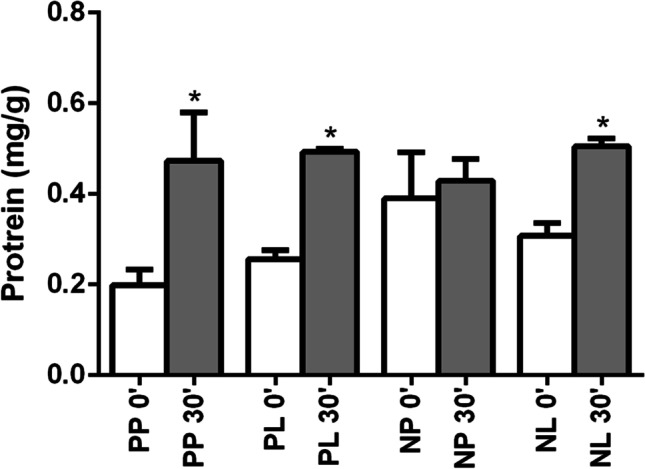
Total protein concentration by group and surgical time
Impact on oral health-related quality of life
Table 6 describes the findings related to the scores of the OHIP-14 domains according to the evaluation periods of the present study. In all study groups, there was a significant increase in the mean scores of functional limitation and physical pain after 24 h and a reduction after seven days. In the N-P group, the use of LLLT was associated with lower mean values of functional limitation (p = 0.027), physical pain (p = 0.033), and the cumulative effect of these domains (p = 0.020 and p = 0.034, respectively). A significant reduction in psychological discomfort was also observed seven days after the surgical procedure in this group. In the N group, the domain psychological discomfort did not show significant variation in the mean scores over seven days, regardless of the association (p = 0.054) or not (p = 0.071) with LLLT.
Table 6.
Quality of life characterization in each study group
| Placebo | p value | Nimesulide | p value | Multifactorial analysis | ||||
|---|---|---|---|---|---|---|---|---|
| w/o LLLTA | w/ LLLTB | w/o LLLC | w/ LLLTD | LLLT | Nimesulide | |||
| Functional limitation | ||||||||
| Initialα | 0.65 ± 1.27 | 0.50 ± 0.95 | 0.625a | 1.05 ± 1.76 | 0.80 ± 1.15 | 0.566a | 0.450c | 0.850d |
| 24 h | 3.90 ± 2.47* | 3.05 ± 2.46* | 0.235a | 3.55 ± 2.06* | 3.25 ± 1.86* | 0.594a | 0.202c | 0.539d |
| 7 dys | 1.60 ± 2.54† | 0.60 ± 0.94† | 0.027a | 2.35 ± 2.08† | 2.45 ± 2.31† | 0.881a | 0.280c | 0.188d |
| p value | < 0.001b | 0.001b | < .001b | < 0.001b | ||||
| Cumulative effect | 2.05 ± 1.61 | 1.38 ± 1.13 | *0.020a | 2.32 ± 1.52 | 2.17 ± 1.33 | 0.716a | 0.099c | 0.291d |
| Physical pain | ||||||||
| Initialα | 2.55 ± 2.52 | 2.15 ± 2.01 | 0.553a | 2.65 ± 2.46 | 3.30 ± 2.11 | 0.288a | 0.780c | 0.246d |
| 24 h | 4.95 ± 2.19* | 4.65 ± 2.54* | 0.611a | 5.10 ± 1.65* | 4.95 ± 2.01* | 0.780a | 0.570c | 0.850d |
| 7 days | 2.95 ± 2.70† | 1.60 ± 1.50† | 0.033a | 3.95 ± 2.01† | 2.85 ± 2.30† | 0.122a | 0.010c | 0.782d |
| p value | 0.001b | < 0.001b | 0.001b | 0.001b | ||||
| Cumulative effect | 3.48 ± 1.80 | 2.80 ± 1.62 | *0.034a | 3.90 ± 1.62 | 3.70 ± 1.47 | 0.669a | 0.166c | 0.385d |
| Psychological discomfort | ||||||||
| Initialα | 2.80 ± 1.91 | 2.75 ± 2.12 | 0.932a | 3.20 ± 2.48 | 3.40 ± 2.28 | 0.727a | 0.854c | 0.760d |
| 24 h | 3.35 ± 2.18* | 3.35 ± 2.56* | 1.000a | 2.30 ± 2.58 | 2.00 ± 1.78 | 0.619a | 0.736c | 0.736d |
| 7 days | 1.55 ± 2.09† | 1.50 ± 2.12† | 0.893a | 2.25 ± 2.53 | 2.05 ± 1.64 | 0.782a | 0.757c | 0.853d |
| p value | 0.004b | 0.003b | 0.071b | 0.054b | ||||
| Cumulative effect | 2.57 ± 1.30 | 2.53 ± 1.85 | 0.903a | 2.58 ± 2.16 | 2.48 ± 1.41 | 0.833a | 0.806c | 0.902d |
| Physical disability | ||||||||
| Initialα | 1.70 ± 2.27 | 0.70 ± 1.59 | 0.094a | 1.80 ± 1.85 | 1.65 ± 2.06 | 0.774a | 0.141c | 0..274d |
| 24 h | 4.20 ± 2.46* | 3.90 ± 2.13* | 0.600a | 4.35 ± 2.16* | 4.25 ± 2.38* | 0.853a | 0.609c | 0.798d |
| 7 days | 1.65 ± 2.30† | 1.15 ± 1.98† | 0.180a | 2.70 ± 2.45† | 2.25 ± 1.86† | 0.487a | 0.200c | 0.946d |
| pvalue | 0.001b | < 0.001b | 0.003b | < 0.001b | ||||
| Cumulative effect | 2.52 ± 1.44 | 1.92 ± 1.69 | *0.036a | 2.95 ± 1.63 | 2.72 ± 1.49 | 0.570a | 0.093c | 0.452d |
| Psychological disability | ||||||||
| Initialα | 1.30 ± 1.75 | 1.65 ± 1.66 | 0.467a | 1.50 ± 1.57 | 1.80 ± 1.64 | 0.566a | 0.357c | 0.943d |
| 24 h | 3.25 ± 2.51* | 2.65 ± 1.73* | 0.319a | 2.50 ± 1.99 | 2.45 ± 1.64* | 0.928a | 0.422c | 0.496d |
| 7 days | 1.65 ± 2.11† | 0.85 ± 1.27† | 0.084a | 1.90 ± 1.89 | 1.60 ± 1.60† | 0.570a | 0.114c | 0.466d |
| p value | 0.012b | < 0.001b | 0.092b | 0.037b | ||||
| Cumulative effect | 2.07 ± 1.47 | 1.72 ± 1.18 | 0.190a | 1.97 ± 1.49 | 1.95 ± 1.34 | 0.966a | 0.437c | 0.480d |
| Social disability | ||||||||
| Initialα | 0.65 ± 0.93 | 1.10 ± 1.71 | 0.225a | 1.80 ± 2.24 | 1.20 ± 1.64 | 0.254a | 0.811c | 0.100d |
| 24 h | 3.50 ± 2.04* | 2.75 ± 1.80* | 0.218a | 2.50 ± 1.76 | 2.90 ± 1.52* | 0.330a | 0.626c | 0.114d |
| 7 dys | 1.65 ± 2.18† | 0.70 ± 1.08† | *0.048a | 2.10 ± 2.22 | 1.75 ± 1.59*† | 0.557a | 0.044c | 0.422d |
| p value | < 0.001b | 0.001b | 0.561b | < 0.001b | ||||
| Cumulative effect | 1.93 ± 1.33 | 1.52 ± 1.03 | 0.118a | 2.13 ± 1.58 | 1.95 ± 1.20 | 0.600a | 0.169c | 0.589d |
| Handicap | ||||||||
| Initialα | 0.70 ± 1.34 | 0.75 ± 1.16 | 0.847a | 1.10 ± 1.59 | 0.90 ± 1.45 | 0.703a | 0.796c | 0.667d |
| 24 h | 3.00 ± 2.10* | 2.90 ± 1.80* | 0.850a | 2.35 ± 1.90 | 1.80 ± 1.67 | 0.270a | 0.367c | 0.531d |
| 7 days | 1.40 ± 2.26† | 0.70 ± 1.17† | 0.100a | 1.55 ± 2.11 | 1.45 ± 1.70 | 0.857a | 0.247c | 0.384d |
| p value | 0.001b | < 0.001b | 0.061b | 0.082b | ||||
| Cumulative effect | 1.70 ± 1.56 | 1.45 ± 1.18 | 0.345a | 1.67 ± 1.43 | 1.38 ± 1.26 | 0.459a | 0.249c | 0.942d |
| OHIP-14 | ||||||||
| Initialα | 10.35 ± 9.62 | 9.60 ± 8.86 | 0.772a | 13.10 ± 11.13 | 13.05 ± 9.49 | 0.986a | 0.834c | 0.855d |
| 24 h | 26.15 ± 13.73* | 23.25 ± 12.72* | 0.407a | 22.65 ± 10.50* | 21.60 ± 9.86* | 0.689a | 0.363c | 0.669d |
| 7 days | 12.45 ± 14.89† | 7.10 ± 8.20† | *0.039a | 16.80 ± 13.39† | 14.40 ± 10.82† | 0.539a | 0.047c | 0.519d |
| p value | < 0.001b | < 0.001b | 0.018b | < 0.001b | ||||
| Cumulative effect | 16.32 ± 9.13 | 13.32 ± 8.38 | *0.024a | 17.51 ± 9.68 | 16.35 ± 7.93 | 0.639a | 0.136c | 0.507d |
Entries in bold refer to data that obtained a statistically significant p value
*p < 0.05 versus baseline; †p < 0.05 versus peak; Mean ± SD
aWilcoxon test
bFriedman/Dunn test
cLLLT factor of the repeated-measures-2-way-ANOVA/Bonferroni test
dNimesulide factor of the repeated-measures-1-way-ANOVA/Bonferroni test
eANOVA-1-way/Bonferroni Test
fKruskal-Wallis/Dunn test
αBefore the surgical procedure
The mean scores regarding physical disability exhibited a peak in the evaluated period of 24 h, being significantly reduced 7 days after the surgical procedure in all groups. No statistically significant difference was observed in the comparison between the placebo and nimesulide subgroups.
In both N and N-P groups, the psychological and social disability domains showed a significant increase in mean scores 24 h after the surgical procedure, followed by a statistically significant reduction after 7 days. The cumulative effect of the scores in both domains exhibited lower values when associated with LLLT both in the placebo (p < 0.001) and nimesulide (p = 0.037) groups. In the 7-day evaluation period, there was a significant reduction in the mean scores related to the psychological domain when LLLT was used, both in the placebo (p < 0.001) and in the nimesulide group (p = 0.037). Regarding the social disability domain, both placebo and nimesulide groups, when associated with LLLT, showed lower mean scores in the evaluation on the 7th day compared to the peak observed 24 h after the surgical procedure (p < 0.001 in both groups).
The handicap scores exhibited a significant increase after 24 h and a decrease after 7 days in the N-P subgroups (p < 0.05), with no significant variation in the nimesulide subgroups in either evaluation period.
Total quality of life scores evidenced a significant increase after 24 h and a decrease after 7 days in all groups in the present study. The use of LLLT in the N-P group resulted in lower overall mean scores in the 7-day evaluation compared to the peak observed in the 24-h postoperative period (p = 0.039), as well as a lower mean of the cumulative effect of these scores (p = 0.024). In addition, multifactorial statistical analysis demonstrated a direct effect of LLLT (p = 0.047) in reducing the overall mean scores for quality of life, regardless of the association with nimesulide (p = 0.519), on the evaluation performed seven days after third molar surgery.
Discussion
Third molar surgery is generally associated with moderate to severe postoperative pain [42]. The quest to reduce the negative impacts on quality of life, with fewer side effects in patients undergoing these procedures, has been a field of continuous research in Oral and Maxillofacial Surgery. In this context, the present study evaluated different preemptive analgesia strategies, including the use of LLLT associated or not with nimesulide. The interest in this therapeutic protocol is based on the hypothesis that the preoperative administration of an anti-inflammatory drug can reduce the severity of pain resulting from the surgical procedure for the removal of third molars or even prevent the establishment of postoperative pain [1]. To the best of our knowledge, this therapeutic protocol has not been explored in other clinical studies on third molar surgery, which reinforces its importance in bringing new results to the scientific literature.
As noted in a recent systematic review with meta-analysis published by the present research group [9], preemptively administered NSAIDs are important interventions in the management of inflammatory events after lower third molar extraction, and a wide variety of drugs that affect the COX pathway could be used for this purpose. For the present study, the efficacy of nimesulide administered orally 1 h before the surgical procedure, alone or associated with LLLT, was evaluated, as this drug has already demonstrated modulation of postoperative inflammatory events related to the extraction of third molars [2, 43, 44]. Our methodological design has an unprecedented approach, being directly related to the need for well-established clinical-surgical protocols regarding the central theme of this study.
From a methodological perspective, this surgical procedure was chosen because it is one of the best clinical models for studying inflammatory pain, as observed in the randomized clinical trial by Albuquerque et al. [3]. This model generally allows for a more homogeneous sample by including young and healthy individuals while using a standardized surgical technique [42]. Another methodological aspect that supports these results is the fact that its split-mouth design allows the evaluation of radiographically similar surgical sites in terms of root formation and position/degree of tooth impaction in the same individual, in addition to being a methodological approach in which the individual is his control for the perception of postoperative pain [45]. Added to these aspects, the degree of surgical difficulty using the Pernambuco index showed that the operative trauma was similar among the groups, with the same degree of difficulty for both sides, which reduces the occurrence of bias in the results observed.
The findings of the present study demonstrated that the use of nimesulide delayed the peak of pain compared to the placebo group (8 and 6 h, respectively). In part, this finding can be explained by the pharmacological properties of nimesulide, which has a short half-life but displays a rapid onset of action (15 min on average) [31]. This mechanism leads to a faster reduction in peripheral and central nociception, secondary to a reduction in the sensory influx of nociceptive input from the peripheral to the central nervous system, which is a characteristic of NSAIDs. In the present study, mean pain scores were significantly increased 2 h after the surgical procedure in both N and N-P groups. The peak of pain after third molar surgery usually occurs within a period of 12 h [46], with some authors reporting specific peaks 5 h [47] and 6 h after the surgical intervention [48, 49].
In the nimesulide group, multifactorial statistical analysis demonstrated that this NSAID, regardless of whether LLLT was used or not, significantly reduced mean pain scores after 4 h. However, it is necessary to underline that the use of LLLT proved to be clinically beneficial, as the mean VAS scores were statistically lower in the subgroup treated with LLLT. These findings are supported by the study by Kahraman et al., who demonstrated that LLLT preemptively used in third molar surgeries, intraorally and near the surgical sites, caused a reduction in pain scores compared to an extraoral protocol (transcutaneous), promoting a reduction in the use of medication by patients [33].
The mean VAS scores in the nimesulide group treated with LLLT were lower than those in the placebo group with LLLT, and when the subgroups of the nimesulide group were compared, the association with LLLT did not significantly reduce the mean pain scores. This fact may reflect a competition for site of action between the LLLT and the NSAID. In this context, it can be speculated that the preemptive administration of nimesulide (1 h before the surgical procedure) has modulated the action of the LLLT, which would partially justify the results observed. In a systematic review, findings from four animal studies showed that there were no superior results when NSAIDs and LLLT were combined. This evidence derives from preclinical studies and suggests that LLLT can modulate inflammatory biochemical markers and produce local anti-inflammatory effects in cells and soft tissues, acting on COX-2 pathways similarly to what occurs with the use of NSAIDs. [50]. However, the scientific literature still lacks well-designed clinical trials that provide better explanations regarding the action of LLLT concomitantly with the administration of NSAIDs and possible competition for the site of action between these therapeutic approaches.
Petrini et al. [23] and Koszowski et al. [51] reported that the use of preemptive and preventive LLLT was effective in reducing the perception of pain and edema by patients in the LLLT group in the first 24-postoperative hours. The effect of LLLT in reducing postoperative inflammatory events has been considered a direct consequence of the activation of lymphatic flow and blood supply, characterized by the rapid increase in the number and diameter of capillaries in the initial hours until the peak in the twelfth hour and subsequent decrease to baseline values [52]. In addition, the analgesic effect of LLLT can also be explained by its ability to modulate various signaling pathways and physiological mechanisms involved in analgesia, such as increasing β-endorphin levels and influencing the production of substance P, tumor necrosis factor- α, and COX-2 [27]. Another aspect related to pain assessment was the consumption of rescue medication, which did not show a statistically significant difference when the study groups were evaluated.
As varying degrees of pain, edema, and trismus can be found in virtually all patients undergoing third molar surgery recovery, this surgical procedure has been chosen to assess the effects of LLLT on the inflammatory process related to bone and connective tissues as well as mastication muscles [32]. The irradiation protocol used in the present study was developed based on intraoral and extraoral points similarly used in the studies by Kahraman et al. [33] and Raiesian et al. [34], who reported favorable postsurgical clinical outcomes (pain, edema, and trismus).
The total cumulative effect of linear facial measurements showed that LLLT in the placebo group significantly reduced edema; however, this finding was not observed in the nimesulide group. Previous studies demonstrated that nimesulide did not present favorable clinical results regarding the reduction of edema and trismus [31]. Thus, the isolated action of LLLT was the main responsible for this clinical outcome. A study by Eshghpour et al. corroborates this hypothesis, demonstrating that the isolated use of LLLT with varying frequencies of red and infrared light generated better clinical results regarding pain and edema compared to the placebo group [53]. In addition, both study groups showed a significant reduction in the maximum mouth opening 24 h after the surgical procedure, with a significant increase in this parameter in the 7-day postoperative evaluation period. The studies by De Menezes and Cury [54] and Pouchain et al. [2] corroborate our findings, which evidenced a significant improvement in mouth opening values only 72 h after surgery.
Wang et al. reported an increase in GSH levels when NSAIDs were used [55], and the increase in GSH observed in the N + LT-P group of this study corroborates this finding. The increased GSH levels caused by nimesulide might affect the migration of neutrophils to the inflammation site, reducing the amount of MPO. MPO levels were reduced in all groups in this study, corroborating the findings by Pravalika et al. [56], who demonstrated the reduction of GSH levels through the expression of MPO, revealing an inversely proportional effect between GSH and MPO levels.
MDA levels increased in all groups, except for the N + LT-P group, which might be related to the fact that MDA is a result of peroxidation and oxidative stress [57]. As an NSAID, nimesulide inhibits peroxidation by increasing GSH levels while also reducing neutrophil migration (MPO), which could be responsible for the decrease in peroxidation seen in the N-P + LT-P and N-P + LT groups. Moreover, nimesulide also plays a role in the concentration of inflammatory biomarkers, which may help explain the increase in GSH concentration observed in the N + LT group. On the other hand, LLLT was not capable of blocking the peroxidation pathway nor contributed to the nimesulide effect, reinforcing the clinical finding that the overlap of these two treatments does not bring significant clinical benefit.
As for total protein concentration, in the N-P + LT-P, N + LT-P, and N + LT groups, a significant increase in protein leakage between T0 and T30 was detected. In the N + LT-P group, there was no significant variation from T0 to T30, that is, nimesulide alone was able to reduce the degree of inflammation (edema), resulting in less pain from a clinical point of view.
The overall quality of life scores was significantly high after 24 h, decreasing 7 days after the surgical procedure in both groups of the present study. The use of LLLT in the N-P group resulted in lower overall dissatisfaction at the 7th-day evaluation compared to the peak observed in the 24-h postoperative period, being statistically significant, as well as lower mean cumulative effect scores. In addition, multifactorial statistical analysis showed a direct effect of LLLT in reducing overall dissatisfaction with their quality of life regardless of the association with nimesulide 7 days after third molar removal. Thus, it was demonstrated that the reduction of pain perception with LLLT promoted a better quality of life through the modulation of various signaling pathways and physiological mechanisms involved in analgesia [44]. The study by Kazancioglu et al. corroborated these findings, as it showed that analgesic therapies positively affected the results of the OHIP-14 questionnaire, with similar scores during the postoperative period compared to the preoperative state [58]. An increase in the mean overall OHIP-14 score was observed on the first and third postoperative days. On the seventh postoperative day, the mean scores approached preoperative values.
None of the patients evaluated in the present clinical trial had adverse effects related to the use of nimesulide during the study period, similarly to the studies by Barbalho et al. [31], De Menezes and Cury [54], and Da Costa Araújo et al. [59]. Several side effects related to nimesulide have been reported, such as drowsiness, dizziness, headache, nausea, vomiting, allergy, syncope, and dyspnea. In a review of hepatic adverse effects, a greater number and severity of hepatotoxic events were observed in patients who used nimesulide compared to other NSAIDs [60]. These authors reported that the patients most at risk for hepatotoxicity with nimesulide were the elderly, females, and those who had used the drug for a mean period of 62 days. Factors that may explain the absence of self-reported adverse effects related to nimesulide intake could have been the age of the volunteers recruited for the present study (mean age of approximately 22 years) and the short period of use of this drug, as similarly reported by Barbalho et al. [31], De Menezes and Cury [54], and Da Costa Araújo et al. [59]. Furthermore, there were no adverse reactions when using LLLT alone or in combination with nimesulide, corroborating previous reports in the scientific literature [61].
In addition to the fact that a triple-blind randomized clinical trial was carried out, another relevant aspect of this work was its methodological design, which used a two-factor statistical model to better investigate the outcomes of two study groups. According to Richard [62], this approach involves testing two or more different interventions in the same group simultaneously. For the present work, the chosen factor was LLLT. Another benefit of bifactorial designs is the possibility of minimizing the number of patients needed for a given study group. Furthermore, factorial studies can not only address more than one question efficiently, as observed in the present study, but also increase the power of the sample [62].
In conclusion, the preemptive use of nimesulide delayed the peak of pain. LLLT reduced edema and trismus and contributed to a better perception of quality of life. Nimesulide inhibits peroxidation by increasing GSH levels and stopping neutrophil migration (MPO). LLLT did not block the peroxidation pathway and did not contribute to the nimesulide overall effect, reinforcing the clinical finding that the overlap of the two treatments does not bring significant clinical benefit, that is, the benefit of the association of both strategies was not superior to the isolated use of LLLT.
Acknowledgements
To the National Council for Scientific and Technological Development (CNPq) for granting Dr. Edson Cetira a scholarship and Dr. Fábio Costa a PQ scholarship Category 2.
Author contribution
All authors conducted the study read the articles, interpreted, and analyzed the data. All authors reviewed and approved the final manuscript.
Funding
This study received financial support from the National Council for Scientific and Technological Development (CNPq), Call MCTIC/CNPq No. 28/2018 – Universal/Track A, protocol number: 427620/2018–0.
Declarations
Ethics approval
Approval by the Ethics Committee, under protocol number 3,358,496.
Informed consent
All patients signed the consent form and agreed to participate in this study.
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
References
- 1.Costa FWG, Soares ECS, Esses DFS, Silva PGB, Bezerra TP, Scarparo HC, Ribeiro TR, Fonteles CSR. A split-mouth, randomized, triple-blind, placebo-controlled study to analyze the pre-emptive effect of etoricoxib 120 mg on inflammatory events following removal of unerupted mandibular third molars. Int J Oral Maxillofac Surg. 2015;44:1166–1174. doi: 10.1016/j.ijom.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Pouchain EC, Costa FWG, Bezerra TP, Soares ECS. Comparative efficacy of nimesulide and ketoprofen on inflammatory events in third molar surgery: a split-mouth, prospective, randomized, double-blind study. Int J Oral Maxillofac Surg. 2015;44:876–884. doi: 10.1016/j.ijom.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Albuquerque AFM, Fonteles CSR, Do Val DR, Chaves HV, Bezerra MM, Pereira KMA, Silva PGB, De Lima BB, Soares ECS, Ribeiro TR, Costa FWG. Effect of pre-emptive analgesia on clinical parameters and tissue levels of TNF-a and IL-1b in third molar surgery: a triple-blind, randomized, placebo-controlled study. Int J Oral Maxillofac Surg. 2017;46:1615–1625. doi: 10.1016/j.ijom.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Colorado-Bonnin M, Valmaseda-Castellón E, Berini-Aytés L, Gay-Escoda C. Quality of life following lower third molar removal. Int J Oral Maxillofac Surg. 2006;35:343–347. doi: 10.1016/j.ijom.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Zhao H, Weng Y, Zhao H, Ma C. Oral Bromelain for the control of facial swelling, trismus, and pain after mandibular third molar surgery: a systematic review and meta-analysis. J Oral Maxillofac Surg. 2019;77:1566–1574. doi: 10.1016/j.joms.2019.02.044. [DOI] [PubMed] [Google Scholar]
- 6.Forbes JA (1991) Oral surgery. In: M.E. Max, R.K. Portenoy, E.M. Laska, eds. Advances in pain research and therapy. The design of analgesic clinical trials. New York: Raven Press cap. 18, p. 347
- 7.Averbuch M, Katzper M. Severity of baseline pain and degree of analgesia in the third molar post-extraction dental pain model. Anesth Analg. 2003;97:163–167. doi: 10.1213/01.ane.0000063827.97392.5e. [DOI] [PubMed] [Google Scholar]
- 8.Saito K, Kaneko A, Machii K, Ohta H, Ohkura M, Suzuki M. Efficacy and safety of additional 200-mg dose of celecoxib in adult patients with postoperative pain following extraction of impacted third mandibular molar: a multicenter, randomized, double-blind, placebo-controlled, phase II study in Japan. Clin Ther. 2012;34:314–328. doi: 10.1016/j.clinthera.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Cetira Filho EL, Carvalho FSR, Barros Silva PG, Barbosa DAF, Pereira KMA, Ribeiro TR, Costa FWG. Preemptive use of oral nonsteroidal anti-inflammatory drugs for the relief of inflammatory events after surgical removal of lower third molars: A systematic review with meta-analysis of placebo-controlled randomized clinical trials. J Craniomaxillofac Surg. 2020;48:293–307. doi: 10.1016/j.jcms.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Barbosa KBF, Costa NMB, Alfenas RCC, de Paula SO, Minim VPR, Bressan J. Estresse oxidativo: conceito, implicações e fatores modulatórios. Ver Nutr. 2010;23:629–643. doi: 10.1590/S1415-52732010000400013. [DOI] [Google Scholar]
- 11.Koparal M, Kucuk AO, Alan H, Asutay F, Avci M. Effects of low-level laser therapy following surgical extraction of the lower third molar with objective measurement of swelling using a three-dimensional system. Exp Ther Med. 2018;15:3820–3826. doi: 10.3892/etm.2018.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lim W, Kim J, Lim C, Kim S, Jeon S, Karna S, Cho M, Choi H, Kim O. Effect of 635 nm light-emitting diode irradiation on intracellular superoxide anion scavenging independent of the cellular enzymatic antioxidant system. Photomed Laser Surg. 2012;30:451–459. doi: 10.1089/pho.2011.3199. [DOI] [PubMed] [Google Scholar]
- 13.Asutay F, Ozcan-Kucuk A, Alan H, Koparal M. Three-dimensional evaluation of the effect of low-level laser therapy on facial swelling after lower third molar surgery: a randomized, placebo-controlled study. Niger J Clin Pract. 2018;21:1107–1113. doi: 10.4103/njcp.njcp_38_18. [DOI] [PubMed] [Google Scholar]
- 14.Amaroli A, Ravera S, Baldini F, Benedicenti S, Panfoli I, Vergani L. Photobiomodulation with 808-nm diode laser light promotes wound healing of human endothelial cells through increased reactive oxygen species production stimulating mitochondrial oxidative phosphorylation. Lasers Med Sci 34: 495-504 10.1007/s10103-018-2623-5 [DOI] [PubMed]
- 15.Baratto L, Calzà L, Capra R, Gallamini M, Giardino L, Giuliani A, Lorenzini L, Traverso S. Ultra-low-level laser therapy. Lasers Med Sci. 2011;26:103–112. doi: 10.1007/s10103-010-0837-2. [DOI] [PubMed] [Google Scholar]
- 16.Kyrkanides S, Fiorentino PM, Miller JH, Gan Y, Lai Y, Shaftel SS, Puzas JE, Piancino MG, O'Banion MK, Tallents RH. Amelioration of pain and histopathologic joint abnormalities in the Col1-IL-1beta (XAT) mouse model of arthritis by intraarticular induction of mu-opioid receptor into the temporomandibular joint. Arthritis Rheum. 2007;56:2038–2048. doi: 10.1002/art.22635. [DOI] [PubMed] [Google Scholar]
- 17.Vernal R, Velásquez E, Gamonal J, Garcia-Sanz JA, Silva A, Sanz M. Expression of proinflammatory cytokines in osteoarthritis of the temporomandibular joint. Arch Oral Biol. 2008;53:910–915. doi: 10.1016/j.archoralbio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Chicre-Alcântara TC, Torres-Chávez KE, Fischer L, Clemente-Napimoga JT, Melo V, Parada CA, Tambeli CH. Local kappa opioid receptor activation decreases temporomandibular joint inflammation. Inflammation. 2012;35:371–376. doi: 10.1007/s10753-011-9329-1. [DOI] [PubMed] [Google Scholar]
- 19.Quinteiro MS, Napimoga MH, Mesquita KP, Clemente-Napimoga JT. The indirect antinociceptive mechanism of 15d-PGJ2 on rheumatoid arthritis-induced TMJ inflammatory pain in rats. Eur J Pain. 2012;16:1106–1115. doi: 10.1002/j.1532-2149.2012.00114.x. [DOI] [PubMed] [Google Scholar]
- 20.Frijhoff J, Winyard PG, Zarkovic N, Davies SS, Stocker R, Cheng D, Knight AR, Taylor EL, Oettrich J, Ruskovska T, Gasparovic AC, Cuadrado A, Weber D, Poulsen HE, Grune T, Schmidt HHHW, Ghezzi P. Clinical relevance of biomarkers of oxidative stress. Antioxid Redox Signal. 2015;23:1144–1170. doi: 10.1089/ars.2015.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frijhoff J, Dagnell M, Godfrey R, Östman A. Regulation of protein tyrosine phosphatase oxidation in cell adhesion and migration. Antioxid Redox Signal. 2014;20:1994–2010. doi: 10.1089/ars.2013.5643. [DOI] [PubMed] [Google Scholar]
- 22.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrini M, Ferrante M, Trentini P, Perfetti G, Spoto G. Effect of pre-operatory low-level laser therapy on pain, swelling, and trismus associated with third-molar surgery. Med Oral Patol Oral Cir Bucal. 2017;22:e467–e472. doi: 10.4317/medoral.21398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landucci A, Wosny AC, Uetanabaro LC, Moro A, Araujo MR. Efficacy of a single dose of low-level laser therapy in reducing pain, swelling, and trismus following third molar extraction surgery. Int J Oral Maxillofac Surg. 2016;45:392–398. doi: 10.1016/j.ijom.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Gür A, Karakoç M, Nas K, Cevik R, Saraç J, Demir E. Efficacy of low power laser therapy in fibromyalgia: a single-blind, placebo-controlled trial. Lasers Med Sci. 2002;17:57–61. doi: 10.1007/s10103-002-8267-4. [DOI] [PubMed] [Google Scholar]
- 26.De Carvalho PTC, Leal Junior ECP, Alves ACA, Rambo CSM, Sampaio LMM, Oliveira CS, Albertini R, Oliveira LVF. Effect of low-level laser therapy on pain, quality of life and sleep in patients with fibromyalgia: study protocol for a double-blinded randomized controlled trial. Trials. 2012;13:221. doi: 10.1186/1745-6215-13-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsieh YL, Hong CZ, Chou LW, Yang SA, Yang CC. Fluence-dependent effects of low-level laser therapy in myofascial trigger spots on modulation of biochemicals associated with pain in a rabbit model. Lasers Med Sci. 2015;30:209–216. doi: 10.1007/s10103-014-1654-9. [DOI] [PubMed] [Google Scholar]
- 28.Rizzi CF, Mauriz JL, Corrêa DSF, Moreira AJ, Zettler CG, Filippin LI, Marroni NP, González-Gallego J. Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle. Lasers Surg Med. 2006;38:704–713. doi: 10.1002/lsm.20371. [DOI] [PubMed] [Google Scholar]
- 29.Fiorillo L, Meto A, Cicciù F, De Stefano R. An eventual SARS-CoV-2 infection prevention protocol in the medical setting and dental office. Int J Environ Res Public Health. 2021;18:2593. doi: 10.3390/ijerph18052593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Carvalho RWF, Vasconcelos BC. Pernambuco index: predictability of the complexity of surgery for impacted lower third molars. Int J Oral Maxillofac Surg. 2018;47:234–240. doi: 10.1016/j.ijom.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Barbalho JC, Vasconcellos RJH, De Morais HH, Santos LAM, Almeida RAC, Rêbelo HL, Lucena EE, De Araújo SQ. Effects of co-administered dexamethasone and nimesulide on pain, swelling, and trismus following third molar surgery: a randomized, triple-blind, controlled clinical trial. Int J Oral Maxillofac Surg. 2017;46:236–242. doi: 10.1016/j.ijom.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Sierra SO, Deana AM, Ferrari RAM, Albarello PM, Bussadori SK, Fernandes KPS. Effect of low-level laser therapy on the post-surgical inflammatory process after third molar removal: study protocol for a double-blind randomized controlled trial. Trials. 2013;14:373. doi: 10.1186/1745-6215-14-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kahraman SA, Cetiner S, Strauss RA. The effects of transcutaneous and intraoral low-level laser therapy after extraction of lower third molars: a randomized single blind, placebo controlled dual-center study. Photomed Laser Surg. 2017;35:401–407. doi: 10.1089/pho.2016.4252. [DOI] [PubMed] [Google Scholar]
- 34.Raiesian S, Khani M, Khiabani K, Hemmati E, Pouretezad M. Assessment of low-level laser therapy effects after extraction of impacted lower third molar surgery. J Lasers Med Sci. 2017;8:42–45 . doi: 10.15171/jlms.2017.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melo RB, Guimarães SB, Silva PGB, Oriá RB, Melo JUS, Vasconcelos PRL. Antiperoxidative properties of oil mixes of high ratio Omega-9:Omega-6 and low ratio Omega-6:Omega-3 after molar extraction in rats. Acta Cir Bras. 2014;29:371–375. doi: 10.1590/s0102-86502014000600004. [DOI] [PubMed] [Google Scholar]
- 36.Burtis CA, Ashwood ER. Tiezt Textbook of Clinical Chemistry. 4. Philadelphia: WB Saunders Company; 1986. pp. 695–700. [Google Scholar]
- 37.Shugars DA, Gentile MA, Ahmad N, Stravropoulos MF, Slade GD, Phillpis C, Conrad SM, Fleuchaus PT, White Junior RP. Assessment of oral health-related quality of life before and after third molar surgery. J Oral Maxillofac Surg. 2006;64:1721–1730. doi: 10.1016/j.joms.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 38.White RP, Shugars DA, Shafer DM, Laskin DM, Buckley MJ, Phillips C. Recovery after third molar surgery: clinical and health-related quality of life outcomes. J Oral Maxillofac Surg. 2003;61:535–544. doi: 10.1053/joms.2003.50106. [DOI] [PubMed] [Google Scholar]
- 39.Gracely RH, Lota L, Walter DJ, Dubner R. A multiple random staircase method of psychophysical pain assessment. Pain. 1988;32:55–63. doi: 10.1016/0304-3959(88)90023-1. [DOI] [PubMed] [Google Scholar]
- 40.Sierra SO, Deana AM, Bussadori SK, Mota ACC, Ferrari RAM, Vale KL, Fernandes KPS. Choosing between intraoral or extraoral, red or infrared laser irradiation after impacted third molar extraction. Lasers Surg Med. 2016;48:511–518. doi: 10.1002/lsm.22488. [DOI] [PubMed] [Google Scholar]
- 41.Hochman B, Nahas FX, De Oliveira Filho RS, Ferreira LM. Research designs. Acta Cir Bras. 2005;20:2–9. doi: 10.1590/s0102-86502005000800002. [DOI] [PubMed] [Google Scholar]
- 42.Lustenberger FD, Grätz KW, Mutzbauer TS. Efficacy of ibuprofen versus lornoxicam after third molar surgery: a randomized, double-blind, crossover pilot study. Oral Maxillofac Surg. 2011;15:57–62. doi: 10.1007/s10006-010-0255-4. [DOI] [PubMed] [Google Scholar]
- 43.Avelar RL, Primo BT, Vogt BF, Silva EDO, Antunes AA, Magalhães MTC, Rocha A. Effect of partially selective cyclooxygenase-2 inhibitor in the removal of third molars. J Craniofac Surg. 2012;23:e108–e112. doi: 10.1097/SCS.0b013e318231e1d5. [DOI] [PubMed] [Google Scholar]
- 44.Neychev D, Chenchev I, Simitchiev K. Analysis of postoperative pain after extraction of impacted mandibular third molars and administration of preemptive analgesia. J of IMAB. 2017;23:1697–1701. doi: 10.5272/jimab.2017233.1697. [DOI] [Google Scholar]
- 45.Lokken P, Olsen I, Bruaset I, Norman-Pedersen K. Bilateral surgical removal of impacted lower third molar teeth as a model for drug evaluation: a test with ibuprofen. Eur J Clin Pharmacol. 1975;8:209–216. doi: 10.1007/BF00567117. [DOI] [PubMed] [Google Scholar]
- 46.Kaczmarzyk T, Wichlinski J, Stypulkowska J, Zaleska M, Woron J. Preemptive effect of ketoprofen on postoperative pain following third molar surgery. A prospective, randomized, double-blinded clinical trial. Int J Oral Maxillofac Surg. 2010;39:647–652. doi: 10.1016/j.ijom.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 47.Marković AB, Todorović L. Postoperative analgesia after lower third molar surgery: contribution of the use of long-acting local anesthetics, low-power laser, and diclofenac. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:e4–8. doi: 10.1016/j.tripleo.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 48.Bjørnsson GA, Haanaes HR, Skoglund LA. A randomized, double-blind crossover trial of paracetamol 1000 mg four times daily vs ibuprofen 600 mg: effect on swelling and other postoperative events after third molar surgery. Br J Clin Pharmacol. 2003;55:405–412. doi: 10.1046/j.1365-2125.2003.01779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seymour RA, Walton JG. Pain control after third molar surgery. Int J Oral Surg. 1984;13:457–485. doi: 10.1016/s0300-9785(84)80017-4. [DOI] [PubMed] [Google Scholar]
- 50.Bjordal JM, Johnson MI, Iversen V, Aimbire F, Lopes-Martins RAB. Low-level laser therapy in acute pain: a systematic review of possible mechanisms of action and clinical effects in randomized placebo-controlled trials. Photomed Laser Surg. 2006;24:158–168. doi: 10.1089/pho.2006.24.158. [DOI] [PubMed] [Google Scholar]
- 51.Koszowski R, Smieszek-Wilczewska J, Wiec G. Comparison of analgetic effect of magnetic and laser stimulation before oral surgery procedures. Wiad Lek. 2006;59:630–633. [PubMed] [Google Scholar]
- 52.Ihsan FR. Low-level laser therapy accelerates collateral circulation and enhances microcirculation. Photomed Laser Surg. 2005;23:289–294. doi: 10.1089/pho.2005.23.289. [DOI] [PubMed] [Google Scholar]
- 53.Eshghpour M, Ahrari F, Takallu M. Is low-level laser therapy effective in the management of pain and swelling after mandibular third molar surgery? J Oral Maxillofac Surg. 2016;74:1322. doi: 10.1016/j.joms.2016.02.030. [DOI] [PubMed] [Google Scholar]
- 54.De Menezes SA, Cury PR. Efficacy of nimesulide versus meloxicam in the control of pain, swelling and trismus following extraction of impacted lower third molar. Int J Oral Maxillofac Surg. 2010;39:580–584. doi: 10.1016/j.ijom.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z, Fang Y, Rock D, Ma J. Rapid screening and characterization of glutathione-trapped reactive metabolites using a polarity switch-based approach on a high-resolution quadrupole orbitrap mass spectrometer, Analytical and Bioanalytical. Chemistry. 2018;410:1595–1606. doi: 10.1007/s00216-017-0814-8. [DOI] [PubMed] [Google Scholar]
- 56.Pravalika K, Sarmah D, Kaur H, Vats K, Saraf J, Wanve M, Kalia K, Borah A, Yavagal DR, Dave KR, Bhattacharya P. Trigonelline therapy confers neuroprotection by reduced glutathione mediated myeloperoxidase expression in animal model of ischemic stroke. Life Sci. 2019;1:49–58. doi: 10.1016/j.lfs.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 57.Blair IA. DNA adducts with lipid peroxidation products. J Biol Chem. 2008;283:15545–15549. doi: 10.1074/jbc.R700051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kazancioglu HO, Kurklu E, Ezirganli S. Effects of ozone therapy on pain, swelling, and trismus following third molar surgery. Int J Oral Maxillofac Surg. 2014;43:644–648. doi: 10.1016/j.ijom.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 59.Da Costa Araújo FA, Santos TS, De Morais HHA, Laureano Filho JR, Silva EDO, Vasconcellos RJH. Comparative analysis of preemptive analgesic effect of tramadol chlorhydrate and nimesulide following third molar surgery. J Craniomaxillofac Surg. 2012;40:e346–e349. doi: 10.1016/j.jcms.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 60.Mació MA, Carvajal A, Vera E. Hepatotoxicity associated with nimesulide: data from the Spanish pharmacovigilance system. Clin Pharmacol Ther. 2002;72:596–597. [PubMed] [Google Scholar]
- 61.Eroglu CN, Tunc SK. Effectiveness of single session of low-level laser therapy with a 940 nm wavelength diode laser on pain, swelling, and trismus after impacted third molar surgery. Photomed Laser Surg. 2016;34:406–410. doi: 10.1089/pho.2016.4101. [DOI] [PubMed] [Google Scholar]
- 62.Richard C. Principles and practice of clinical trial medicine. Periods, Sequences, and Trial Design. 2008;95:118. [Google Scholar]