Abstract
Small-cell lung cancer (SCLC) is a recalcitrant cancer characterized by high metastasis. However, the exact cell type contributing to metastasis remains elusive. Using a Rb1L/L/Trp53L/L mouse model, we identify the NCAMhiCD44lo/– subpopulation as the SCLC metastasizing cell (SMC), which is progressively transitioned from the non-metastasizing NCAMloCD44hi cell (non-SMC). Integrative chromatin accessibility and gene expression profiling studies reveal the important role of the SWI/SNF complex, and knockout of its central component, Brg1, significantly inhibits such phenotypic transition and metastasis. Mechanistically, TAZ is silenced by the SWI/SNF complex during SCLC malignant progression, and its knockdown promotes SMC transition and metastasis. Importantly, ectopic TAZ expression reversely drives SMC-to-non-SMC transition and alleviates metastasis. Single-cell RNA-sequencing analyses identify SMC as the dominant subpopulation in human SCLC metastasis, and immunostaining data show a positive correlation between TAZ and patient prognosis. These data uncover high SCLC plasticity and identify TAZ as the key molecular switch in orchestrating SCLC phenotypic transition and metastasis.
Keywords: small cell lung cancer, SWI/SNF complex, TAZ, phenotypic transition, metastasis
INTRODUCTION
Small-cell lung cancer (SCLC) is characterized by very poor prognosis, with ∼15% global lung cancer incidence and a five-year survival lower than 7% [1]. This can be largely attributed to the highly metastatic capability of SCLC. Most SCLC patients are initially diagnosed at extensive stage, characterized by nearby lung and/or distant metastases. Therefore, exploration of the mechanisms involved in SCLC metastasis is urgently needed so as to provide helpful insights into clinical management.
Previous studies have shown that ∼90% of human SCLC harbors concurrent inactivating mutations or deletions of Rb1 and Trp53 [2]. Homozygous deletion of these two alleles in mouse lung epithelia promotes SCLC development and dramatic metastasis, which closely recapitulates human SCLC in the clinic [3]. Mouse SCLC in the Rb1L/L/Trp53L/L (RP) model typically expresses neuroendocrine markers including neuronal cell adhesion molecule (NCAM) and achaete-scute complex homolog 1 (ASCL1), and frequently metastasizes into distant organs [3]. Concurrent deletion of P130, an Rb-related gene, or Pten in the RP model, significantly accelerates malignant progression and SCLC metastasis [4,5]. Moreover, upregulated Nuclear Factor I B (NFIB) expression is found to promote SCLC metastasis through increasing the accessibility of global chromatin [6–8].
SCLCs are characterized by high heterogeneity [9–15]. It is proposed that human SCLC is composed of four different subtypes based on lineage-related transcription factors including ASCL1, NEUROD1, YAP and POU2F3 [14]. More recently, an inflamed SCLC subtype has been identified with a good response to immunotherapy [16]. Similar heterogeneity has also been found in mouse SCLC, e.g. the CD24hiCD44loEpCAMhi subpopulation from the RP model is identified as harboring a strong capability to form tumors in allograft assay [11]. Moreover, mouse SCLC is found to contain the neuroendocrine (NE) and non-neuroendocrine (non-NE) subpopulations according to distinct growth patterns in culture, with the NE subtype growing as suspension and the non-NE as adhesion [9]. The NE cells frequently express neuroendocrine markers including NCAM, synaptophysin (SYP) and ASCL1. In contrast, the non-NE cells tend to express mesenchymal markers such as VIMENTIN and CD44 [9]. It has been reported that the synergetic cooperation between NE and non-NE subpopulations is necessary for SCLC metastasis whereas neither subtype could metastasize on its own [9]. Therefore, the exact population responsible for SCLC metastasis still remains unknown.
The switch/sucrose-non-fermentable (mSWI/SNF) complexes, including canonical BRG1/BRM-associated factor (BAF), polybromo-associated BAF (PBAF) and non-canonical BAF (ncBAF), are essential for chromatin remodeling [17,18]. All three complexes contain a core ATPase subunit, e.g. BRG1 (Brahma/SWI2-related gene 1, also called SMARCA4), which catalyzes the hydrolysis of ATP [18]. Previous studies reveal that the SWI/SNF complexes tend to function as tumor suppressors during cancer development. Consistently, a high incidence of BRG1 inactivating mutation is detected in multiple cancer types including lung cancer [19]. Previous studies show that BRG1 promotes cell cycle arrest and senescence through the retinoblastoma pathway in cancer cells [20,21]. Interestingly, recent studies have also indicated an oncogenic role of BRG1. For example, BRG1 promotes pancreatic intraepithelial neoplasia (PanIN) development and gastric cancer metastasis [22–24]. In SCLC, BRG1 is preferentially required for cancer progression when MAX (Myc-associated factor) is inactivated [25]. These findings indicate that BRG1 might function as tumor suppressor or oncogenic driver in a cell-type- or genetic-context-dependent manner.
The Hippo pathway is initially defined as an important pathway during organ size control, and functions mainly via the synergetic interaction between the transcription factor TEAD1-4 and transcriptional co-activator YAP/TAZ (WWTR1) [26]. The oncogenic activities of YAP/TAZ have been well documented in multiple epithelial cancers [26–31]. It is well known that YAP/TAZ sustains self-renewal and tumor-initiating capability, and promotes cancer malignant progression and metastasis through epithelial-to-mesenchymal transition (EMT) [30]. The latest studies also reveal that YAP/TAZ might function as a tumor suppressor [32–34]. For instance, YAP expression is downregulated in breast cancer and knockdown of YAP promotes cancer cell migration and invasiveness [35]. Moreover, we have previously found that YAP acts as the barrier for adenocarcinoma-to-squamous-carcinoma transdifferentiation (AST) as well as for lung squamous-cell carcinoma progression [33,36]. Nonetheless, the exact role of YAP/TAZ during SCLC metastasis has not been characterized yet.
We here identify NCAMhiCD44lo/– cells as the major subpopulation responsible for SCLC metastasis. Moreover, this subpopulation is progressively transitioned from the non-metastatic NCAMloCD44hi cells via the SWI/SNF-complex-mediated TAZ silencing. Our data highlight the important link between epigenetically regulated TAZ and SCLC plasticity and metastasis.
RESULTS
Identification of the NCAMhiCD44lo/– subpopulation as SCLC metastasizing cells
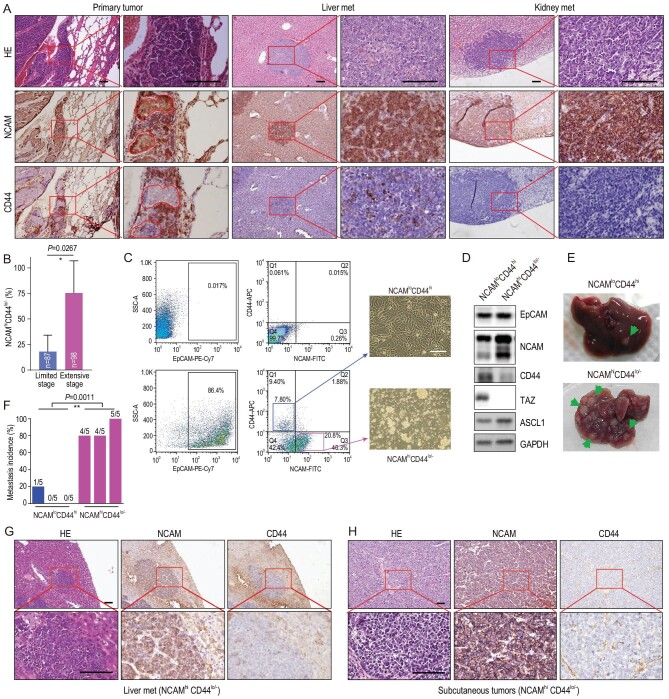
To study SCLC heterogeneity during cancer malignant progression and metastasis, we first performed immunohistochemistry (IHC) staining in RP tumors using NE marker NCAM and mesenchymal marker CD44. In primary RP tumors, we indeed observed the heterogeneous expression pattern of these two markers intra-tumorally and inter-tumorally (Fig. 1A and Table S1). We found that the percentage of NCAMhiCD44lo/– tumors, defined with over 50% of cancer cells highly expressing NCAM and with low or no CD44 expression [37], increased with malignant progression and metastasis (Fig. 1B, Fig. S1A and Table S1). Consistently, we found that distant organ metastases such as liver and kidney metastases uniformly exhibited the NCAMhiCD44lo/– expression pattern (Fig. 1A). These data indicate that the NCAMhiCD44lo/– subpopulation might be responsible for SCLC metastasis.
Figure 1.
Identification of the NCAMhiCD44lo/– cells as SCLC metastasizing cells in the RP mouse model. (A) Representative photos of Hematoxylin-Eosin (HE) staining, NCAM and CD44 IHC staining of primary tumors, liver and kidney metastases (met) from the RP mouse model. The intra-tumor heterogeneity of primary tumors is shown in high magnification. The marked areas in high magnification panels indicate the NCAMhiCD44lo/– expression pattern. Scale bars, 100 μm. (B) Statistic analyses of the NCAMhiCD44lo/– tumors at limited stage (no overt distant organ metastasis) and extensive stage (overt metastasis) in the RP model. The NCAMhiCD44lo/– tumors were defined when the lesions contained >50% of cells showing NCAMhi and CD44lo/– expression. Limited stage: 87 tumors from 4 mice were analyzed; extensive stage: 98 tumors from 4 mice were analyzed. Data are shown as mean ± S.E.M. P value was calculated by unpaired two-tailed t test. (C) Flow cytometry (FACS) analyses of primary tumors from the RP mouse model using antibodies towards EpCAM, NCAM and CD44. The tumor cells without primary antibody incubation are shown as negative control (top panels). The NCAMloCD44hi and NCAMhiCD44lo/– cells were sorted and cultured in vitro and the representative cell growth photos are shown on the right. Scale bar, 100 μm. (D) Western blot detection of EpCAM, NCAM, CD44, ASCL1 and TAZ expression in established NCAMloCD44hi and NCAMhiCD44lo/– SCLC primary cell lines. (E and F) Representative photos (E) and the incidence (F) of liver metastasis in nude mice subcutaneously transplanted with primary NCAMloCD44hi or NCAMhiCD44lo/– cells derived from the RP mouse model. Data are shown from three independent experiments (n = 5 mice for each experiment). The ratio of mice with liver metastasis was also indicated. P value was calculated by unpaired two-tailed t test. (G and H) Representative photos of HE staining, NCAM and CD44 IHC staining of (G) liver metastases, and (H) subcutaneous tumors in nude mice transplanted with NCAMhiCD44lo/– cell lines. Scale bars, 100 μm.
To test this, we then used Fluorescence Activated Cell Sorting (FACS) to isolate the NCAMhiCD44lo/– and NCAMloCD44hi subpopulations from primary RP tumors (Fig. 1C). Genotyping analyses confirmed the concurrent deletion of Rb1 and Trp53 in both subpopulations (Fig. S1B). We found that the NCAMhiCD44lo/– cells grew in culture as oncospheres with a suspension growth pattern (Fig. 1C), similar to classical human SCLC cell lines. In contrast, the NCAMloCD44hi cells grew as adhesion (Fig. 1C). Moreover, a higher ASCL1 level was detected in the NCAMhiCD44lo/– subpopulation (Fig. 1D). We then subcutaneously transplanted 5 × 106 cells from either the NCAMhiCD44lo/– or NCAMloCD44hi subpopulation into nude mice and waited for up to 10 weeks for distant organ metastasis analyses. Both subpopulations formed subcutaneous tumors at 100% in allograft assay, with comparable tumor growth (Fig. S1C and D). In contrast, the metastasis analyses revealed a huge difference. Most mice (13 out of 15) from the NCAMhiCD44lo/– group had spontaneous metastases in the liver whereas only 1 out of 15 mice from the NCAMloCD44hi group displayed distant metastasis (Fig. 1E and F). The liver metastases from the NCAMhiCD44lo/– group exhibited a characteristic marker expression pattern, similar to subcutaneous tumors (Fig. 1G and H). These data demonstrate that the NCAMhiCD44lo/– cells are mainly responsible for SCLC metastasis. We hereafter refer to the NCAMhiCD44lo/– and NCAMloCD44hi subpopulations as SCLC metastasizing cell (SMC) and non-SCLC metastasizing cell (non-SMC), respectively.
Phenotypic transition from non-SMC to SMC contributes to SCLC metastasis
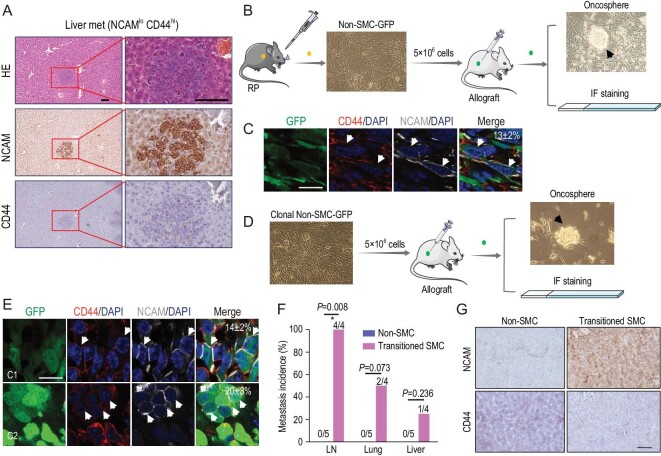
Consistent with SMC metastatic tumors, the liver metastasis lesion from the non-SMC allograft assay also exhibited the NCAMhiCD44lo/– expression pattern (Fig. 2A and Fig. S2). We speculated that there might exist phenotypic transition from non-SMC to SMC during SCLC malignant progression. To test this, we established a non-SMC cell line stably expressing GFP, termed non-SMC-GFP, and performed a subcutaneous allograft assay (Fig. 2B). Immunofluorescence (IF) staining in allograft tumors revealed that ∼13 ± 2% GFP-positive cancer cells displayed the NCAMhiCD44lo/– pattern whereas the rest remained as a non-SMC expression pattern (Fig. 2C and Table S2). Consistently, both suspension and adhesion growth patterns were observed when these allograft tumors were cultured in vitro (Fig. 2B). To further confirm such transition, we picked single-cell clones from non-SMC-GFP cells and performed allograft assay with the clonal non-SMC-GFP cell lines (Fig. 2D). Similarly, we found that these subcutaneous tumors also displayed the NCAMhiCD44lo/– pattern, ranging from 14 ± 2% to 20 ± 3% (Fig. 2E and Table S2). A mixed growth pattern was also observed in culture (Fig. 2D). We further isolated the transitioned SMC with NCAMhiCD44lo/– pattern and tested its metastasis capability using allograft assay. In contrast to no overt metastases in the non-SMC group, multiple distant organ metastases, e.g. lymph node, lung and liver metastases, were detectable in the transitioned SMC group (Fig. 2F and G). We found that the liver metastases also displayed the NCAMhiCD44lo/– pattern (Fig. 2G). These data together convincingly proved the transition from non-SMC to SMC and highlighted the important role of such phenotypic transition in SCLC metastasis.
Figure 2.
Phenotypic transition from non-SMC to SMC contributes to SCLC metastasis. (A) Representative photos for HE staining, NCAM and CD44 IHC staining in the only liver metastasis from nude mice subcutaneously transplanted with the NCAMloCD44hi cell lines (non-SMC) derived from the RP mouse model. Scale bars, 100 μm. (B) Experimental scheme to test phenotypic transition from non-SMC to SMC. Primary non-SMC was derived from the RP mouse model and ectopically expressed GFP, and then used for subcutaneous transplantation in nude mice. The subcutaneous tumors were analyzed through oncosphere formation, and NCAM and CD44 IF staining. Oncospheres in cell culture were indicated. (C) Representative photos of NCAM and CD44 IF staining in subcutaneous tumors from nude mice transplanted with non-SMC-GFP cells. The NCAMhiCD44lo/– subpopulation indicated by white arrows were microscopically counted and the mean ratio of NCAMhiCD44lo/– cells is indicated in the top right corner. Scale bar, 25 μm. Data are shown as mean ± S.E.M. (D) Experimental scheme to test the potential phenotypic transition using single-cell-derived clonal non-SMC-GFP. The subcutaneous tumors were then analyzed through oncosphere formation and NCAM and CD44 IF staining. Oncospheres in cell culture were indicated. (E) Representative photos of NCAM and CD44 IF staining in clonal non-SMC-GFP subcutaneous tumors. C1: clone #1; C2: clone #2. The NCAMhiCD44lo/– subpopulation indicated by white arrows was microscopically counted and the ratio of NCAMhiCD44lo/– cells is indicated in the top right corner. Scale bar, 25 μm. Data are shown as mean ± S.E.M. (F) Statistical analyses of the incidence of lymph node (LN), lung and liver metastases in nude mice subcutaneously transplanted with transitioned SMC or non-SMC, which were derived from the clonal non-SMC-GFP subcutaneous tumors. n = 4 mice for transitioned SMC group and n = 5 mice for paired non-SMC group. P values were calculated by Pearson chi-square test. (G) Representative photos of NCAM and CD44 IHC staining of mouse livers in (F). The livers from paired non-SMC showed no metastasis. Scale bar, 100 μm.
Brg1 knockout inhibits SMC phenotypic transition and SCLC metastasis
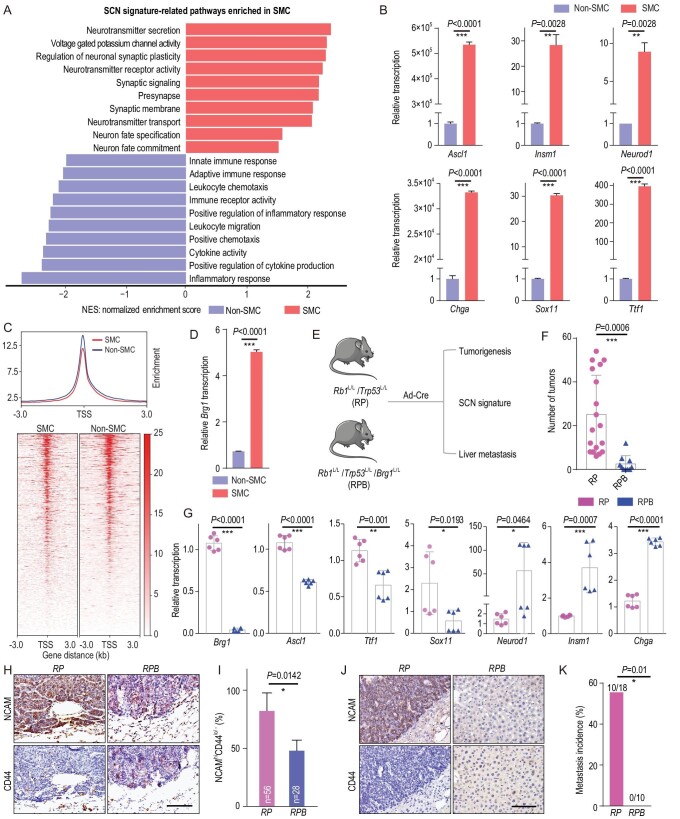
To further explore the molecular mechanisms underlying non-SMC-to-SMC transition, we performed RNA sequencing and comparatively analyzed the gene expression profiling of SMC and non-SMC. The small-cell neuroendocrine (SCN) signature has been recently established as an important index for SCLC metastasis [38]. Interestingly, we found a significant enrichment of SCN-signature-related pathways in SMC whereas non-SCN-related pathways (immune-related pathways) were enriched in non-SMC (Fig. 3A and Tables S3 and S4). Real-time PCR data further confirmed the increased expression of SCN signature genes, including Ascl1, Insm1, Neurod1, Chga, Sox11 and Ttf1, in SMC (Fig. 3B). These data might partially explain the high metastasis capability of SMC.
Figure 3.
Knockout of Brg1 in the RP mouse model significantly abrogates SMC phenotypic transition and SCLC metastasis. (A) The enrichment of small-cell neuroendocrine (SCN) signature-related pathways in SMC and the enrichment of immune-related pathways in non-SMC. NES, normalized enrichment score. (B) Real-time PCR detection of SCN-signature-related genes including Ascl1, Insm1, Neurod1, Chga, Sox11 and Ttf1 in SMC vs. non-SMC. Data are shown as mean ± S.E.M. P values were calculated by unpaired two-tailed t test. (C) Trend plot (top) and heat map (bottom) showing ATAC-seq signal over 6 kb regions centered at the transcription start sites (TSS) in SMC and non-SMC. (D) Real-time PCR detection of Brg1 expression in SMC vs. non-SMC. (E) Schematic illustration of the comparative analyses of Rb1L/L/Trp53L/L (RP) and Rb1L/L/Trp53L/L/Brg1L/L (RPB) mice. (F) Statistical analyses of primary tumor numbers in RP and RPB mice at 32 weeks after Ad-Cre treatment. n = 18 mice for the RP group, n = 10 mice for the RPB group. Data are shown as mean ± S.E.M. P value was calculated by unpaired two-tailed t test. (G) Real-time PCR detection of Brg1 and the SCN-signature-related genes in primary tumors from RP and RPB mice. n = 2 mice for each group. Data are shown as mean ± S.E.M. P values were calculated by unpaired two-tailed t test. (H) Representative photos of NCAM and CD44 IHC staining in primary tumors from RP and RPB mice at 32 weeks after Ad-Cre treatment. Scale bar, 100 μm. (I) Statistical analyses of the percentage of primary tumors with an NCAMhiCD44lo/– expression pattern in RP and RPB mice. The NCAMhiCD44lo/– tumors were defined when the lesions contained >50% of cells showing NCAMhi and CD44lo/– expression. A total of 56 tumors from 3 RP mice and 28 tumors from 4 RPB mice were analyzed. Data are shown as mean ± S.E.M. P value was calculated by unpaired two-tailed t test. (J) Representative photos of NCAM and CD44 IHC staining in livers of RP and RPB mice. The livers from RPB mice contained no metastasis. Scale bar, 100 μm. (K) Liver metastasis incidence in RP and RPB mice at 32 weeks after Ad-Cre treatment. n = 18 mice for the RP group, n = 10 mice for the RPB group. P value was calculated by Pearson chi-square test.
Epigenetic alterations have been implicated in cancer plasticity [39,40]. We performed the assay for transposase-accessible chromatin with next-generation sequencing (ATAC-seq) to determine the global chromatin accessibility of SMC and non-SMC. Our analyses on transcription start sites also revealed an overall reduced signal in the active promoter regions of SMC (Fig. 3C). The total reads of ATAC-seq for SMC and non-SMC were ∼36 million and 27 million, respectively. Chromatin remodelers, such as SWI/SNF complex, are critical for regulating chromatin architecture and accessibility [18]. We found that multiple members of the SWI/SNF complex, including the central catalytic ATPase Brg1, were markedly dysregulated between these two subpopulations (Fig. S3A). Real-time PCR quantification further confirmed the significant upregulation of Brg1 in SMC (Fig. 3D).
To test whether Brg1 is involved in the phenotypic transition and SCLC metastasis, we generated the Rb1L/L/Trp53L/L/Brg1L/L (RPB) mouse cohort and performed comparative analyses of tumorigenesis, SCN signature enrichment and metastasis in parallel with the RP model (Fig. 3E). We found that Brg1 knockout significantly reduced the tumor number (Fig. 3F, and Fig. S3B and C). Moreover, several SCN-signature-related genes, including Ascl1, Ttf1 and Sox11, were significantly downregulated in RPB tumors (Fig. 3G and Fig. S3B). IHC staining of the NCAM and CD44 showed that the percentage of primary tumors with an SMC expression pattern was also decreased in the RPB group (Fig. 3H and I and Table S5). Notably, no liver metastasis was detected in the RPB group in contrast to ∼50% incidence in the RP model (Fig. 3J and K). These data support the conclusion that the SWI/SNF complex is important for SMC phenotypic transition and SCLC metastasis.
Epigenetic silencing of TAZ by SWI/SNF complex in SMC
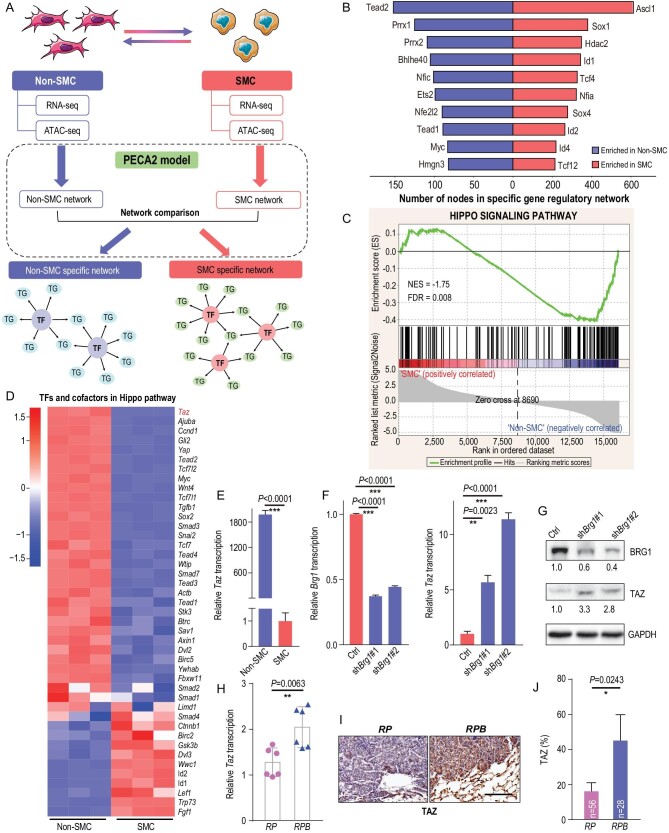
To identify the downstream mediator of the SWI/SNF complex in contribution to SCLC phenotypic transition and metastasis, we first constructed the dysregulated transcriptional factor (TF) network through the integrative analyses of RNA-seq and ATAC-seq data as previously described [41]. We found that Ascl1 and Tead2 were top-ranked TFs with the highest number of dysregulated target genes in SMC and non-SMC respectively (Fig. 4A and B, Figs S4 and S5, and Table S6). ASCL1 is known as the pioneering TF that initializes neuronal reprogramming and is also included in the SCN biomarker genes [42]. TEAD family members are important TFs that function with cofactor YAP/TAZ in cancer malignant progression [30,43,44]. Gene set enrichment analysis revealed that the Hippo pathway was significantly enriched in non-SMC (Fig. 4C). Moreover, Taz/Yap stood out as top hits among the dysregulated components of the Hippo pathway (Fig. 4D). Using real-time PCR, we further confirmed the decreased expression of Taz/Yap in SMC vs. non-SMC cells (Fig. 4E and Fig. S3D).
Figure 4.
TAZ is epigenetically silenced by the SWI/SNF complex in SMC. (A) Schematic illustration of the integrative analyses of RNA-seq and ATAC-seq in SMC and non-SMC. Specific TF networks in SMC and non-SMC were constructed according to the PECA2 model (see details in Materials and Methods). TG, target genes. (B) Enriched TFs in SMC and non-SMC through integrative analyses of ATAC-seq and RNA-seq data ranked according to the numbers of dysregulated target genes. (C) Gene set enrichment analysis (GSEA) plot of the Hippo signaling pathway in SMC vs. non-SMC. (D) Heat map of RNA-seq data showing the relative expression of TFs and cofactors in the Hippo pathway in SMC vs. non-SMC. (E) Real-time PCR detection of Taz in SMC and non-SMC. Data are shown as mean ± S.E.M. P value was calculated by unpaired two-tailed t test. (F) Real-time PCR detection of Brg1 and Taz in SMC with or without Brg1 knockdown. Gapdh served as the internal control. Data are shown as mean ± S.E.M. P values were calculated by unpaired two-tailed t test. (G) Western blot detection of BRG1 and TAZ levels in SMC with or without Brg1 knockdown. GAPDH served as the internal control. (H) Real-time PCR detection of Taz in primary tumors from RP and RPB mice at 32 weeks after Ad-Cre treatment. Gapdh served as the internal control. n = 2 for each group. Data are shown as mean ± S.E.M. P value was calculated by unpaired two-tailed t test. (I) Representative photos of TAZ IHC staining in primary tumors from RP and RPB mice at 32 weeks after Ad-Cre treatment. Scale bar, 100 μm. (J) Percentage of TAZ positive tumors in RP vs. RPB mice at 32 weeks after Ad-Cre treatment; 56 tumors from 3 RP mice and 28 tumors from 4 RPB mice were analyzed. Data are shown as mean ± S.E.M. P value was calculated by unpaired two-tailed t test.
We further asked whether Taz/Yap expression was regulated by Brg1. We found that Brg1 knockdown in SMC cells resulted in a significant upregulation of TAZ expression whereas the expression of YAP was downregulated (Fig. 4F and G and Fig. S3E). Such upregulation of TAZ was also detectable in RPB tumors in comparison to RP tumors (Fig. 4H and J and Table S5). Moreover, we observed an obvious decreased chromatin accessibility at the promoter region of Taz in SMC (Fig. S3F), which might explain the reduced TAZ expression (Fig. 1D). A similar but lesser degree of chromatin accessibility change was also observed at the Yap promoter region in SMC (Fig. S3F). In support of this, TAZ level was obviously downregulated in primary tumors at extensive stage (Fig. S1A). Moreover, knockdown of Arid1a or Arid2, another two important components of the SWI/SNF complex, obviously upregulated TAZ expression in SMC cells (Fig. S3G and H). However, YAP expression was only slightly upregulated with Arid2 knockdown, or even downregulated after Arid1a knockdown in SMC (Fig. S3G and H). Moreover, we performed BRG1 Chromatin Immunoprecipitation real-time quantitative PCR (ChIP-qPCR) analysis and found that BRG1 could bind to the promoter region of Taz (Fig. S3I). These results together demonstrate that TAZ is silenced during non-SMC-to-SMC transition through SWI/SNF-complex-mediated epigenetic reprogramming.
TAZ knockdown promotes non-SMC-to-SMC transition and accelerates SCLC metastasis
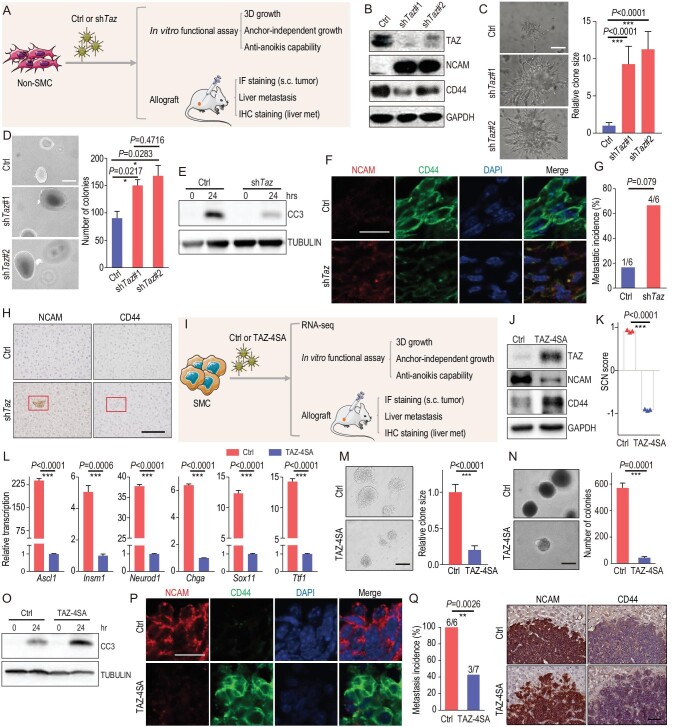
To explore the function of TAZ in phenotype transition and SCLC metastasis, we performed Taz knockdown in non-SMC for allograft assay (Fig. 5A). We found that Taz knockdown in non-SMC upregulated NCAM and SCN-related genes, whereas it downregulated CD44, without a dramatic effect upon Yap expression (Fig. 5B and Fig. S6A–C). Moreover, Taz knockdown also promoted the invasiveness in matrigel, colony formation in soft agar and anti-anoikis capability of non-SMC (Fig. 5C and E). IF staining of allograft tumors showed that Taz knockdown promoted the appearance of the NCAMhiCD44lo/– pattern, resembling the SMC-derived tumors (Fig. 5F and Fig. S6D). Importantly, knockdown of Taz in non-SMC promoted distant organ metastasis (Fig. 5G). IHC staining further confirmed that these metastases displayed the SMC expression pattern (Fig. 5H). These data together demonstrate that TAZ downregulation promotes phenotypic transition from non-SMC to SMC and SCLC metastasis.
Figure 5.
TAZ functions as a critical molecular switch in regulating the phenotypic transition and SCLC metastasis. (A) Schematic illustration of the comparative analyses of non-SMC with or without Taz knockdown. (B) Western blot detection of TAZ, NCAM and CD44 levels in non-SMC with or without Taz knockdown. GAPDH served as the internal control. (C) Representative photos of the matrigel invasiveness of non-SMC with or without Taz knockdown (left). The statistical analyses of the clone sizes were performed using Image J software. Scale bars, 100 μm. P values were calculated by unpaired two-tailed t test. (D) Representative photos (left) and number (right) of the soft-agar colonies of non-SMC with or without Taz knockdown. Scale bars, 100 μm. Data are shown as mean ± S.E.M. P value was calculated by unpaired two-tailed t test. (E) Western blot detection of cleaved caspase3 (CC3) in anti-anoikis assay of non-SMC with or without Taz knockdown. TUBULIN served as the internal control. (F) Representative photos of NCAM and CD44 IF staining in subcutaneous tumors from nude mice transplanted with non-SMC with or without Taz knockdown. Scale bar, 25 μm. (G) Metastasis incidence and (H) representative photos of NCAM and CD44 IHC staining in livers from nude mice transplanted by non-SMC with or without Taz knockdown. n = 6 for each group. P value was calculated by Pearson chi-square test. Scale bar, 100 μm. (I) Schematic illustration of the comparative analyses of SMC with or without ectopic TAZ-4SA expression. (J) Western blot detection of TAZ, NCAM and CD44 levels in SMC with or without ectopic TAZ-4SA expression. GAPDH served as the internal control. (K) SCN score of SMC with or without ectopic TAZ-4SA expression. Data are shown as mean ± S.E.M. P value was calculated by unpaired two-tailed t test. (L) Real-time PCR detection of the SCN-signature-related genes in SMC with or without ectopic TAZ-4SA expression. Gapdh served as the internal control. Data are shown as mean ± S.E.M. P values were calculated by unpaired two-tailed t test. (M) Representative photos of the matrigel invasiveness of SMC with or without ectopic TAZ-4SA expression (left). The statistical analyses of the clone sizes were performed using Image J software. Scale bar, 100 μm. P values were calculated by unpaired two-tailed t test. (N) Representative photos (left) and statistical analyses (right) of soft-agar colonies of SMC with or without ectopic TAZ-4SA expression. Scale bar, 100 μm. Data are shown as mean ± S.E.M. P value was calculated by unpaired two-tailed t test. (O) Western blot detection of CC3 in an anti-anoikis assay of SMC with or without ectopic TAZ-4SA expression. TUBULIN served as the internal control. (P) Representative photos of NCAM and CD44 IF staining in subcutaneous tumors from nude mice transplanted with SMC with or without ectopic TAZ-4SA expression. Scale bar, 25 μm. (Q) Metastasis incidence (left) and representative photos of NCAM and CD44 IHC staining of liver metastasis (right) in nude mice transplanted with SMC with or without ectopic TAZ-4SA expression. n = 6 mice for the control group, n = 7 mice for the TAZ-4SA group. Scale bar, 100 μm. P value was calculated by Pearson chi-square test.
Ectopic TAZ expression reversely promotes the transition from SMC to non-SMC and alleviates SCLC metastasis
To test if the phenotypic transition from non-SMC to SMC is reversible, we ectopically expressed a constitutive activated TAZ mutant (TAZ-4SA) [30] in SMC (Fig. 5I). We found that the downstream targets of TAZ, including Cyr61, Ctgf, Areg, Vim and Axl, were significantly upregulated after ectopic TAZ-4SA expression in SMC (Fig. S6E). Ectopic TAZ-4SA but not YAP-5SA [33] expression in SMC dramatically downregulated NCAM and upregulated CD44 expression in vitro, indicative of the potential reversible transition from SMC to non-SMC (Fig. 5J, Fig. S6F, and Tables S7 and S8). Moreover, the SCN score and related gene expression also decreased after ectopic TAZ-4SA expression (Fig. 5K and L and Tables S7 and S8). Functional assays showed that TAZ-4SA expression markedly suppressed the matrigel invasiveness, colony formation in soft agar and anti-anoikis capability of SMC (Fig. 5M and O). IF staining also showed that ectopic TAZ-4SA expression promoted the non-SMC expression pattern in comparison to SMC-derived subcutaneous tumors (Fig. 5P and Fig. S6G). More importantly, ectopic TAZ-4SA expression significantly suppressed the liver metastases of SMC (Fig. 5Q). Furthermore, all the liver metastases consistently showed no TAZ expression (Fig. S6H). These findings support the conclusion that ectopic TAZ expression promotes reverse transition from SMC to non-SMC and alleviates SCLC metastasis.
Low TAZ level is associated with SCN signature enrichment and predicts poor prognosis of SCLC patients
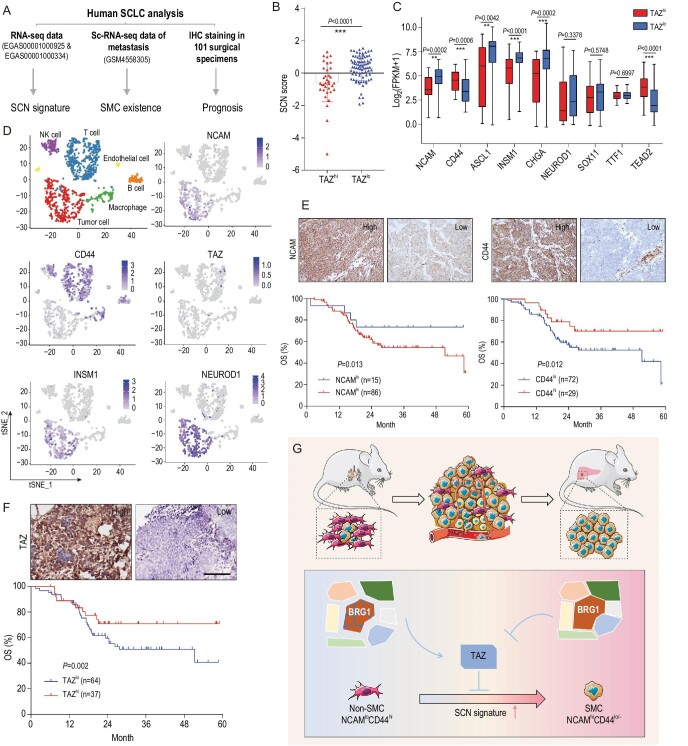
To evaluate whether our findings are clinically relevant, we downloaded a public RNA-sequencing dataset of 112 human SCLCs [2,45] and analyzed the correlation between TAZ and SCN signature, and single-cell sequencing data of liver metastasis [15], to detect whether SMC exists in metastatic lesion, and collected 101 Chinese surgical specimens for prognosis analyses (Fig. 6A). Bioinformatic analyses showed that human SCLC with low TAZ expression (TAZlo) displays a significantly higher SCN score (Fig. 6B and Table S9), indicative of strong metastasis capability. The SCN-signature-related pathways, including positive regulation of neurotransmitter transport, neurotransmitter secretion and synaptic vesicle membrane, were significantly enriched in TAZlo SCLC (Fig. S7A). Consistently, most SCN-signature-related genes, including ASCL1, INSM1 and CHGA, were significantly increased in TAZlo SCLC samples (Fig. 6C). Moreover, NCAM was increased, and CD44 was decreased in TAZlo SCLC specimens (Fig. 6C), indicative of the SMC pattern of these samples. TEAD also decreased in these TAZlo samples (Fig. 6C). Also, we observed a negative correlation between the SCN-signature-related genes and TAZ, and a positive correlation between CD44, TEAD2 and TAZ (Fig. S7B and Table S9).
Figure 6.
Low TAZ level is correlated with SCN signature enrichment and predicts poor prognosis of SCLC patients. (A) Schematic illustration of the analyses of human SCLC specimens. (B) SCN score of human SCLC specimens with high or low TAZ mRNA level. The RNA-seq data were downloaded from a public database (GSE69091 and EGAS00001000334). Data are shown as mean ± S.E.M. P value was calculated by unpaired two-tailed t test. (C) Correlation between individual SCN-signature-related genes, CD44 or TEAD2 expression with high or low TAZ level in human SCLC (GSE69091 and EGAS00001000334). Data are shown as mean ± S.E.M. P values were calculated by unpaired two-tailed t test. (D) Clustering and the NCAM, CD44, TAZ, NEUROD1 and INSM1 expression of the single cell sequencing data (GSM4558305) of a liver biopsy from an SCLC patient. (E and F) Representative photos of (E) NCAM, CD44 and (F) TAZ IHC staining in Chinese SCLC specimens (top) and survival curves of high or low expression of NCAM, CD44 or TAZ with overall survival (OS) (bottom). Scale bar, 100 μm. P values were calculated by Kaplan-Meier analysis with log-rank test. (G) Working model illustrating the essential role of SWI/SNF-complex-mediated TAZ expression in controlling the phenotypic transition from non-SMC to SMC and SCLC metastasis. TAZ, which is epigenetically silenced by the SWI/SNF complex, functions as a critical molecular switch during the phenotypic transition from non-SMC to SMC and SCLC metastasis. Disruption of the SWI/SNF complex through BRG1 knockout promotes TAZ upregulation and thus inhibits the phenotypic transition and cancer metastasis.
We further took advantage of the Ireland et al. single-cell RNA-sequencing data derived from SCLC liver metastasis [15]. Interestingly, we found that most SCLC metastatic cells showed high expression of NCAM with concurrent low expression of CD44, resembling the SMC pattern (Fig. 6D). Moreover, these cells showed high expression of the SCN signature markers INSM1 and NEUROD1, similar to the SMC in the RP model (Fig. 6D). Importantly, low or no TAZ expression was detected in these metastasis cells (Fig. 6D), confirming the silence of TAZ in metastasis.
Lastly, we put together a patient cohort containing 101 Chinese SCLC surgical specimens for immunostaining analyses of NCAM, CD44 and TAZ. Most of these patients were at limited stage without distant metastases. We found that high NCAM or low CD44 levels were significantly associated with worse patient overall survival (OS) (Fig. 6E and Table S10). Moreover, TAZlo patients also showed a worse overall survival (Fig. 6F). These data together provide strong clinical evidence in support of our findings of SMC in the RP model.
DISCUSSION
SCLC is the most lethal form of lung cancer, characterized by highly metastatic capacity. A growing body of evidence based on mouse models has demonstrated that SCLC is highly heterogeneous with distinct subpopulations playing different roles during malignant progression and metastasis [5–15]. In this study, we identify the NCAMhiCD44lo/– cells in the RP model as the SCLC metastasizing cells. We further reveal that the SMCs are progressively transitioned from non-SMCs during SCLC malignant progression and metastasis. Our data further show that the SWI/SNF-complex-mediated epigenetic downregulation of TAZ is essential for driving such a phenotype transition. Moreover, TAZ activation is sufficient to drive the reverse transition from SMC to non-SMC and thus alleviate SCLC metastasis. With the support of clinical specimen analyses, our data demonstrate that the NCAMhiCD44lo/– cells are mainly responsible for SCLC metastasis and the SWI/SNF-TAZ axis importantly orchestrates SCLC plasticity and metastasis (Fig. 6G).
To assess SCLC heterogeneity in the RP model, we use both NE marker NCAM and mesenchymal marker CD44 to do the immunostaining and FACS analyses, and identify the NCAMhiCD44lo/– cells as the SCLC metastasizing cells. A previous study shows that mouse SCLC cells contain both NE and non-NE subpopulations [9]. However, neither subpopulation alone can metastasize and a synergetic cooperation is necessary for distant organ metastasis [9]. In contrast, our data show that the NCAMhiCD44lo/– cells harbor strong metastasis capability in allograft assay, and the tumors metastasize into multiple distant organs including the lymph node, lung and liver. Since the SMC defined here also expresses classical NE biomarkers, we reason that the NCAMhiCD44lo/– cells might belong to the NE subpopulation, but with higher metastasis potential. In other words, the NCAMhiCD44lo/– cells might represent the highly metastatic subpopulation of the NE subtype. In addition, we used primary NCAMhiCD44lo/- cells and demonstrated their robust metastatic capability, whereas Calbo et al. [9] employed tumor-derived cell lines for metastasis assays. Future efforts looking into the heterogeneity of the NE subtype will hopefully uncover more subpopulations linked to SCLC malignant progression and metastasis.
We also find that phenotypic transition from non-SMC to SMC contributes to SCLC metastasis, which closely links cancer plasticity and malignant progression. Indeed, recent data also show, during SCLC drug resistance acquisition, that Notch signaling promotes the transition from an NE to non-NE subtype and thus provides a niche for resisting drug treatment [10]. A similar transition from NE to non-NE subtypes has also been found in another recent study [15]. Metastasis and drug resistance are two major hurdles in clinical SCLC management. Understanding molecular mechanisms involved in the phenotypic transition in these two important events will hopefully provide a solid base for the development of a novel therapeutic strategy to treat SCLC in the clinic.
Through integrative analyses of gene expression profiling and chromatin accessibility, we find that SWI/SNF complexes play an important role during non-SMC-to-SMC transition. Although the SWI/SNF complex is generally considered to be tumor suppressive [46], our results indicate that this complex has a different role in SCLC progression. Knockout of its ATPase BRG1 inhibits such phenotypic transition and cancer metastasis, indicating the oncogenic function of the SWI/SNF complex as well as BRG1 in SCLC. In agreement with our observation, a previous study reported that BRG1 is important for the activation of NE transcriptional programs to upregulate MYC targets, and depletion of BRG1 strongly hinders cell growth, specifically in MAX-deficient SCLC tumors [25]. Consistently, we find that BRG1 knockdown suppresses neuronal gene expression and several SCN-signature-related genes in the SMC subpopulation. Likewise, the dual roles of ARID1A have also been revealed in cancer [47]. Thus, the exact function of the SWI/SNF complex and its subunit as tumor suppressor or oncogenic driver might be cell-type or genetic-context dependent and vary with the type of malignancy.
We further find that TAZ is an important downstream mediator of the SWI/SNF complex during SCLC phenotypic transition. Although both YAP and TAZ are significantly upregulated in non-SMC, only TAZ is significantly upregulated when Brg1 is knocked down in SMC. Similar findings are also observed when Arid1a or Arid2 is knocked down. Consistently, Brg1 knockout in an RP mouse upregulates TAZ and significantly inhibits SMC appearance and SCLC metastasis. Moreover, we find that low TAZ expression is associated with SCN signature enrichment. In agreement with these observations, previous studies have shown that high YAP/TAZ expression correlates with decreased NE markers [48], and YAP loss defines NE differentiation [49]. Meanwhile, NE lineage markers are dominant in the SCN signature, which is significantly associated with SCLC malignant progression and metastasis [38,50–52]. Of course, considering the redundant function and concurrent decrease of YAP and TAZ, it remains possible that YAP may also contribute to SCLC phenotypic transition and metastasis albeit independent of the SWI/SNF complex. Future efforts will be necessary to clarify the detailed regulatory mechanisms underlying YAP expression during SCLC phenotypic transition and metastasis.
Our findings from loss-of-function and gain-of-function experiments support the tumor-suppressive role of TAZ in SCLC. YAP/TAZ is well established as oncogenic driver. Nonetheless, accumulated evidence has recently revealed the tumor-suppressive function of YAP/TAZ in multiple cancer types [53]. For instance, YAP restricts Wnt signals during intestinal regeneration, which results in rapid loss of intestinal crypts, and YAP loss promotes hyperplasia and microadenoma development [54]. In hematological cancer, low YAP level prevents nuclear ABL1-induced apoptosis and rescued YAP expression triggers cell death [55]. Another study shows that the growth inhibitory effect caused by LATS1/2 deletion is due to uncontrolled activation of YAP in colon cancer [56]. A recent report demonstrates that LATS1/2 promotes breast cancer cell growth through inhibition of YAP/TAZ [34]. Our findings with regard to the tumor-suppressive function of TAZ are also supported by clinical specimen analyses. Single-cell RNA-sequencing data support that the cancer cells from SCLC liver metastasis mainly display the SMC expression pattern and these metastatic cells show low or no expression of TAZ. Moreover, low TAZ level is significantly associated with poor patient survival. These data together support that TAZ works as a tumor suppressor in controlling SCLC plasticity and metastasis.
MATERIALS AND METHODS
RP and RPB mouse cohort generation, maintenance and analyses
Mice were housed in a specific pathogen-free environment at the Shanghai Institute of Biochemistry and Cell Biology, and treated in accordance with protocols conforming to the ARRIVE guidelines and approved by the Institutional Animal Care and Use Committee of the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (approval number: IBCB0011). Conditional knockout mice including Trp53L/L, Rb1L/L [3] and Brg1L/L [57] alleles were generously provided by Drs. Tyler Jacks, Ronald A. DePinho and Pierre Chambon. Mice were crossed to obtain Rb1L/L/Trp53L/L (RP) and Rb1L/L/Trp53L/L/Brg1L/L (RPB) cohorts. All experimental mice were maintained on a mixed genetic background as previously described [58]. Mice at 6–8 weeks old were treated with Adenovirus-CMV-Cre recombinase (Ad-Cre, 2 × 106 p.f.u.) by intratracheal intubation [59] to allow for Cre-lox mediated recombination of floxed alleles. Mouse tumors were used for immunostaining, FACS analyses, genomic DNA extraction and genotyping as previously described [3,57]. The primer sequences are shown in the supplementary data.
Statistical analysis
Statistical analyses were carried out using SPSS 16.0 or GraphPad Prism 5/7 software (San Diego, CA). The significance of differences was determined using a two-tailed Student's t test or chi-square test. Kaplan-Meier analysis with log-rank test was used to assess patients’ survival between subgroups. P value <0.05 was considered to be statistically significant.
DATA AVAILABILITY
Sequence data have been deposited in Gene Expression Omnibus (GEO) with the primary accession codes GSE158091 (ATAC-seq of SMC and non-SMC), GSE158290 (RNA-seq of SMC and non-SMC) and GSE158293 (RNA-seq of SMC-Ctrl and SMC-TAZ-4SA).
Supplementary Material
Acknowledgements
We thank Drs. Tyler Jacks and Ronald A. DePinho for the RP mouse model and Dr. Pierre Chambon for the Brg1L/L mouse. We are grateful to Drs. Fuming Li and Xiangkun Han for technical assistance and Drs. Carla F. Kim, Dangsheng Li, Cheng Li, Yujiang Geno Shi, Rui Fang and Nella Dost for constructive comments.
Contributor Information
Yujuan Jin, State Key Laboratory of Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China.
Qiqi Zhao, State Key Laboratory of Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China.
Weikang Zhu, Center for Excellence in Mathematical Sciences, National Center for Mathematics and Interdisciplinary Sciences, Key Laboratory of Management, Decision and Information System, Hua Loo-Keng Center for Mathematical Sciences, Academy of Mathematics and Systems Science, Chinese Academy of Sciences, Beijing 100190, China.
Yan Feng, State Key Laboratory of Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China.
Tian Xiao, Shenzhen Key Laboratory of Translational Medicine of Tumor, Department of Cell Biology and Genetics, Shenzhen University Health Sciences Center, Shenzhen 518060, China.
Peng Zhang, Shanghai Pulmonary Hospital, Tongji University, Shanghai 200092, China.
Liyan Jiang, Shanghai Chest Hospital, Shanghai Jiaotong University, Shanghai 200030, China.
Yingyong Hou, Zhongshan Hospital, Fudan University, Shanghai 200032, China.
Chenchen Guo, State Key Laboratory of Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China.
Hsinyi Huang, State Key Laboratory of Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China.
Yabin Chen, State Key Laboratory of Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China.
Xinyuan Tong, State Key Laboratory of Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China.
Jiayu Cao, State Key Laboratory of Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China.
Fei Li, Department of Pathology, School of Basic Medical Sciences, Fudan University, Shanghai 200032, China.
Xueliang Zhu, State Key Laboratory of Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China; School of Life Science and Technology, Shanghai Tech University, Shanghai 200120, China.
Jun Qin, CAS Key Laboratory of Tissue Microenvironment and Tumor, CAS Center for Excellence in Molecular Cell Science, Shanghai Institute of Nutrition and Health Sciences, Chinese Academy of Sciences, Shanghai 200031, China.
Dong Gao, State Key Laboratory of Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China.
Xin-Yuan Liu, State Key Laboratory of Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China.
Hua Zhang, Laura and Isaac Perlmutter Cancer Center, New York University Langone Medical Center, New York, NY 10016, USA.
Luonan Chen, State Key Laboratory of Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China; School of Life Science, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Hangzhou 310024, China.
Roman K Thomas, Department of Translational Genomics, Center of Integrated Oncology Cologne-Bonn, Medical Faculty, University of Cologne, Cologne 50931, Germany; Department of Pathology, University Hospital Cologne, Cologne 50937, Germany.
Kwok-Kin Wong, Laura and Isaac Perlmutter Cancer Center, New York University Langone Medical Center, New York, NY 10016, USA.
Lei Zhang, State Key Laboratory of Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China; School of Life Science, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Hangzhou 310024, China.
Yong Wang, Center for Excellence in Mathematical Sciences, National Center for Mathematics and Interdisciplinary Sciences, Key Laboratory of Management, Decision and Information System, Hua Loo-Keng Center for Mathematical Sciences, Academy of Mathematics and Systems Science, Chinese Academy of Sciences, Beijing 100190, China; School of Life Science, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Hangzhou 310024, China.
Liang Hu, State Key Laboratory of Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China.
Hongbin Ji, State Key Laboratory of Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Shanghai Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences, Shanghai 200031, China; Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences, Shanghai 200031, China; School of Life Science, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Hangzhou 310024, China.
FUNDING
This work was supported by the National Natural Science Foundation of China (81871875 and 82173340 to L.H., 82030083, 81872312, 82011540007 and 31621003 to H.J., 81402371 to Y.J., 12025107 to Y.W.), the National Basic Research Program of China (2017YFA0505501 and 2020YFA0803300 to H.J.), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB19020201 to H.J.), the Basic Frontier Scientific Research Program of the Chinese Academy of Sciences (ZDBS-LY-SM006 to H.J.), the International Cooperation Project of the Chinese Academy of Sciences (153D31KYSB20190035 to H.J.), the Innovative Research Team of High-Level Local Universities in Shanghai (SSMU-ZLCX20180500 to H.J.) and the Science and Technology Commission of Shanghai Municipality (21ZR1470300 to L.H.).
AUTHOR CONTRIBUTIONS
H.J. and Y.J. conceived the idea and designed the experiments. Y.J., Q.Z., Y.F., T.X. and H.H. performed all experiments and analyzed the data. W.Z., Y.W., J.C., Y.C. and L.C. performed the bioinformatics analyses. P.Z., L.J. and Y.H. provided human SCLC specimens. C.G., L.Z., K.K.W., R.K.T., H.Z., X.Z., D.G., J.Q., F.L. and X.Y.L. provided technical assistance and helpful comments. H.J., Y.J. and L.H. wrote the manuscript.
Conflict of interest statement. None declared.
REFERENCES
- 1. Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer 2017; 17: 725–37. 10.1038/nrc.2017.87 [DOI] [PubMed] [Google Scholar]
- 2. George J, Lim JS, Jang SJet al. . Comprehensive genomic profiles of small cell lung cancer. Nature 2015; 524: 47–53. 10.1038/nature14664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meuwissen R, Linn SC, Linnoila RIet al. . Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell 2003; 4: 181–9. 10.1016/S1535-6108(03)00220-4 [DOI] [PubMed] [Google Scholar]
- 4. Schaffer BE, Park KS, Yiu Get al. . Loss of p130 accelerates tumor development in a mouse model for human small-cell lung carcinoma. Cancer Res 2010; 70: 3877–83. 10.1158/0008-5472.CAN-09-4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McFadden DG, Papagiannakopoulos T, Taylor-Weiner Aet al. . Genetic and clonal dissection of murine small cell lung carcinoma progression by genome sequencing. Cell 2014; 156: 1298–311. 10.1016/j.cell.2014.02.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Semenova EA, Kwon MC, Monkhorst Ket al. . Transcription factor NFIB is a driver of small cell lung cancer progression in mice and marks metastatic disease in patients. Cell Rep 2016; 16: 631–43. 10.1016/j.celrep.2016.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Denny SK, Yang D, Chuang CHet al. . Nfib promotes metastasis through a widespread increase in chromatin accessibility. Cell 2016; 166: 328–42. 10.1016/j.cell.2016.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dooley AL, Winslow MM, Chiang DYet al. . Nuclear factor I/B is an oncogene in small cell lung cancer. Genes Dev 2011; 25: 1470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calbo J, van Montfort E, Proost Net al. . A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell 2011; 19: 244–56. 10.1016/j.ccr.2010.12.021 [DOI] [PubMed] [Google Scholar]
- 10. Lim JS, Ibaseta A, Fischer MMet al. . Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature 2017; 545: 360–4. 10.1038/nature22323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jahchan NS, Lim JS, Bola Bet al. . Identification and targeting of long-term tumor-propagating cells in small cell lung cancer. Cell Rep 2016; 16: 644–56. 10.1016/j.celrep.2016.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kwon MC, Proost N, Song J-Yet al. . Paracrine signaling between tumor subclones of mouse SCLC: a critical role of ETS transcription factor Pea3 in facilitating metastasis. Genes Dev 2015; 29: 1587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu N, Jia D, Ibrahim AHet al. . NFIB overexpression cooperates with Rb/p53 deletion to promote small cell lung cancer. Oncotarget 2016; 7: 57514–24. 10.18632/oncotarget.11583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rudin CM, Poirier JT, Byers LAet al. . Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer 2019; 19: 289–97. 10.1038/s41568-019-0133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ireland AS, Micinski AM, Kastner DWet al. . MYC drives temporal evolution of small cell lung cancer subtypes by reprogramming neuroendocrine fate. Cancer Cell 2020; 38: 60–78e12. 10.1016/j.ccell.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gay CM, Stewart CA, Park EMet al. . Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 2021; 39: 346–60. 10.1016/j.ccell.2020.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peterson CL, Herskowitz I. Characterization of the yeast SWI1, SWI2, and SWI3 genes, which encode a global activator of transcription. Cell 1992; 68: 573–83. 10.1016/0092-8674(92)90192-F [DOI] [PubMed] [Google Scholar]
- 18. Mashtalir N, D’Avino AR, Michel BCet al. . Modular organization and assembly of SWI/SNF family chromatin remodeling complexes. Cell 2018; 175: 1272–88. 10.1016/j.cell.2018.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mittal P, Roberts CWM. The SWI/SNF complex in cancer - biology, biomarkers and therapy. Nat Rev Clin Oncol 2020; 17: 435–48. 10.1038/s41571-020-0357-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wong AK, Shanahan F, Chen Yet al. . BRG1, a component of the SWI-SNF complex, is mutated in multiple human tumor cell lines. Cancer Res 2000; 60: 6171–7. [PubMed] [Google Scholar]
- 21. Strobeck MW, Knudsen KE, Fribourg AFet al. . BRG-1 is required for RB-mediated cell cycle arrest. Proc Natl Acad Sci USA 2000; 97: 7748–53. 10.1073/pnas.97.14.7748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wu Q, Lian JB, Stein JLet al. . The BRG1 ATPase of human SWI/SNF chromatin remodeling enzymes as a driver of cancer. Epigenomics 2017; 9: 919–31. 10.2217/epi-2017-0034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tsuda M, Fukuda A, Roy Net al. . The BRG1/SOX9 axis is critical for acinar cell-derived pancreatic tumorigenesis. J Clin Invest 2018; 128: 3475–89. 10.1172/JCI94287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang LY, Zhao J, Chen Het al. . SCF(FBW7)-mediated degradation of Brg1 suppresses gastric cancer metastasis. Nat Commun 2018; 9: 3569. 10.1038/s41467-018-06038-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Romero OA, Torres-Diz M, Pros Eet al. . MAX inactivation in small cell lung cancer disrupts MYC-SWI/SNF programs and is synthetic lethal with BRG1. Cancer Discov 2014; 4: 292–303. 10.1158/2159-8290.CD-13-0799 [DOI] [PubMed] [Google Scholar]
- 26. Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 2015; 163: 811–28. 10.1016/j.cell.2015.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zanconato F, Forcato M, Battilana Get al. . Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol 2015; 17: 1218–27. 10.1038/ncb3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benham-Pyle BW, Pruitt BL, Nelson WJ. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry. Science 2015; 348: 1024–7. 10.1126/science.aaa4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao B, Li L, Wang Let al. . Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev 2012; 26: 54–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lei QY, Zhang H, Zhao Bet al. . TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol 2008; 28: 2426–36. 10.1128/MCB.01874-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lau AN, Curtis SJ, Fillmore CMet al. . Tumor-propagating cells and yap/taz activity contribute to lung tumor progression and metastasis. EMBO J 2014; 33: 468–81. 10.1002/embj.201386082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Strano S, Monti O, Pediconi Net al. . The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA damage. Mol Cell 2005; 18: 447–59. 10.1016/j.molcel.2005.04.008 [DOI] [PubMed] [Google Scholar]
- 33. Huang H, Zhang W, Pan Yet al. . YAP suppresses lung squamous cell carcinoma progression via deregulation of the DNp63-GPX2 axis and ROS accumulation. Cancer Res 2017; 77: 5769–81. 10.1158/0008-5472.CAN-17-0449 [DOI] [PubMed] [Google Scholar]
- 34. Ma S, Wu Z, Yang Fet al. . Hippo signalling maintains ER expression and ER(+) breast cancer growth. Nature 2021; 591: E1–10. 10.1038/s41586-020-03131-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yuan M, Tomlinson V, Lara Ret al. . Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ 2008; 15: 1752–9. 10.1038/cdd.2008.108 [DOI] [PubMed] [Google Scholar]
- 36. Gao Y, Zhang W, Han Xet al. . YAP inhibits squamous transdifferentiation of Lkb1-deficient lung adenocarcinoma through ZEB2-dependent DNp63 repression. Nat Commun 2014; 5: 4629. 10.1038/ncomms5629 [DOI] [PubMed] [Google Scholar]
- 37. Maues De Paula A, Vasiljevic A, Giorgi Ret al. . A diagnosis of giant cell-rich tumour of bone is supported by p63 immunohistochemistry, when more than 50% of cells is stained. Virchows Arch 2014; 465: 487–94. 10.1007/s00428-014-1637-z [DOI] [PubMed] [Google Scholar]
- 38. Balanis NG, Sheu KM, Esedebe FNet al. . Pan-cancer convergence to a small-cell neuroendocrine phenotype that shares susceptibilities with hematological malignancies. Cancer Cell 2019; 36: 17–34. 10.1016/j.ccell.2019.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Flavahan WA, Gaskell E, Bernstein BE. Epigenetic plasticity and the hallmarks of cancer. Science 2017; 357: eaal2380. 10.1126/science.aal2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yuan S, Norgard RJ, Stanger BZ. Cellular plasticity in cancer. Cancer Discov 2019; 9: 837–51. 10.1158/2159-8290.CD-19-0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Duren Z, Chen X, Jiang Ret al. . Modeling gene regulation from paired expression and chromatin accessibility data. Proc Natl Acad Sci USA 2017; 114: E4914–23. 10.1073/pnas.1704553114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pang ZP, Yang N, Vierbuchen Tet al. . Induction of human neuronal cells by defined transcription factors. Nature 2011; 476: 220–3. 10.1038/nature10202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhao B, Li L, Lei Qet al. . The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev 2010; 24: 862–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell 2016; 29: 783–803. 10.1016/j.ccell.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rudin CM, Durinck S, Stawiski EWet al. . Comprehensive genomic analysis identifies SOX2 as a frequently amplified gene in small-cell lung cancer. Nat Genet 2012; 44: 1111–6. 10.1038/ng.2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wanior M, Kramer A, Knapp Set al. . Exploiting vulnerabilities of SWI/SNF chromatin remodelling complexes for cancer therapy. Oncogene 2021; 40: 3637–54. 10.1038/s41388-021-01781-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sun X, Wang SC, Wei Yet al. . Arid1a has context-dependent oncogenic and tumor suppressor functions in liver cancer. Cancer Cell 2017; 32: 574–89. 10.1016/j.ccell.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Horie M, Saito A, Ohshima Met al. . YAP and TAZ modulate cell phenotype in a subset of small cell lung cancer. Cancer Sci 2016; 107: 1755–66. 10.1111/cas.13078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ito T, Matsubara D, Tanaka Iet al. . Loss of YAP1 defines neuroendocrine differentiation of lung tumors. Cancer Sci 2016; 107: 1527–38. 10.1111/cas.13013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Borromeo MD, Savage TK, Kollipara RKet al. . ASCL1 and NEUROD1 reveal heterogeneity in pulmonary neuroendocrine tumors and regulate distinct genetic programs. Cell Rep 2016; 16: 1259–72. 10.1016/j.celrep.2016.06.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Osada H, Tatematsu Y, Yatabe Yet al. . ASH1 gene is a specific therapeutic target for lung cancers with neuroendocrine features. Cancer Res 2005; 65: 10680–5. 10.1158/0008-5472.CAN-05-1404 [DOI] [PubMed] [Google Scholar]
- 52. Osborne JK, Larsen JE, Shields MDet al. . NeuroD1 regulates survival and migration of neuroendocrine lung carcinomas via signaling molecules TrkB and NCAM. Proc Natl Acad Sci USA 2013; 110: 6524–9. 10.1073/pnas.1303932110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer 2015; 15: 73–9. 10.1038/nrc3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Barry ER, Morikawa T, Butler BLet al. . Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature 2013; 493: 106–10. 10.1038/nature11693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cottini F, Hideshima T, Xu Cet al. . Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nat Med 2014; 20: 599–606. 10.1038/nm.3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pan WW, Moroishi T, Koo JHet al. . Cell type-dependent function of LATS1/2 in cancer cell growth. Oncogene 2019; 38: 2595–610. 10.1038/s41388-018-0610-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ding Y, Li N, Dong Bet al. . Chromatin remodeling ATPase BRG1 and PTEN are synthetic lethal in prostate cancer. J Clin Invest 2019; 129: 759–73. 10.1172/JCI123557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Christensen CL, Kwiatkowski N, Abraham BJet al. . Targeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitor. Cancer Cell 2014; 26: 909–22. 10.1016/j.ccell.2014.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc 2009; 4: 1064–72. 10.1038/nprot.2009.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data have been deposited in Gene Expression Omnibus (GEO) with the primary accession codes GSE158091 (ATAC-seq of SMC and non-SMC), GSE158290 (RNA-seq of SMC and non-SMC) and GSE158293 (RNA-seq of SMC-Ctrl and SMC-TAZ-4SA).