Abstract
Background:
The risk of cardiovascular disease in type 1 diabetes remains extremely high, despite marked advances in blood glucose control and even the widespread use of cholesterol synthesis inhibitors. Thus, a deeper understanding of insulin regulation of cholesterol metabolism, and its disruption in type 1 diabetes, could reveal better treatment strategies.
Methods:
To define the mechanisms by which insulin controls plasma cholesterol levels, we knocked down the insulin receptor, FoxO1, and the key bile acid synthesis enzyme, CYP8B1. We measured bile acid composition, cholesterol absorption, and plasma cholesterol. In parallel, we measured markers of cholesterol absorption and synthesis in humans with type 1 diabetes treated with ezetimibe and simvastatin in a double-blind crossover study.
Results:
Mice with hepatic deletion of the insulin receptor showed marked increases in 12α-hydroxylated bile acids (12HBAs), cholesterol absorption, and plasma cholesterol. This phenotype was entirely reversed by hepatic deletion of FoxO1. FoxO1 is inhibited by insulin, and required for the production of 12HBAs, which promote intestinal cholesterol absorption and suppress hepatic cholesterol synthesis. Knockdown of Cyp8b1 normalized 12HBA levels and completely prevented hypercholesterolemia in mice with hepatic deletion of the insulin receptor (n=5–30) as well as mouse models of type 1 diabetes (n=5–22). In parallel, the cholesterol absorption inhibitor, ezetimibe, normalized cholesterol absorption and LDL-cholesterol in patients with type 1 diabetes as well as, or better than, the cholesterol synthesis inhibitor, simvastatin (n=20).
Conclusions:
Insulin, by inhibiting FoxO1 in the liver, reduces 12HBAs, cholesterol absorption, and plasma cholesterol levels. Thus, type 1 diabetes leads to a unique set of derangements in cholesterol metabolism, with increased absorption rather than synthesis. These derangements are reversed by ezetimibe, but not statins, which are currently the first line of lipid-lowering treatment in type 1 diabetes. Taken together, these data suggest that a personalized approach to lipid lowering in type 1 diabetes may be more effective and highlight the need for further studies specifically in this group of patients.
Keywords: bile acids and salts, cholesterol, diabetes mellitus, type 1, forkhead box protein O1, precision medicine
Introduction
The risk of cardiovascular disease in individuals with type 1 diabetes remains exceedingly high, up to 30-fold in some populations1, highlighting the urgent need for better therapies. The landmark Diabetes Control and Complications Trial/ Epidemiology of Diabetes Interventions and Complications study shows that the normalization of blood glucose levels dramatically lowers the risk of cardiovascular disease (CVD)2. One interpretation of these findings is that diabetes promotes CVD by increasing glucose levels. However, insulin was used to lower glucose levels, and insulin has many effects beyond maintaining glucose homeostasis. Given that glucose levels do not predict CVD risk in individuals with type 1 diabetes3 and the reduction of glucose levels per se does not consistently reduce CVD risk in type 2 diabetic individuals4, 5,6, it is possible that insulin acts via some other mechanism to lower CVD risk. Indeed, insulin also reduces plasma cholesterol levels7–9, a key driver of CVD10.
Cholesterol lowering medications play a central role in reducing the risk of CVD, and there are an increasing number of medications, with different mechanisms of action, that are now available11–13. At present, how exactly insulin reduces plasma cholesterol levels, and how this becomes deranged with diabetes is not clear. It is conceivable that defining these mechanistic links could provide a rational basis for selecting which cholesterol lowering drug to use and thereby enable us to personalize lipid lowering strategies for type 1 diabetes patients.
At the molecular level, insulin binds its receptor, activating the insulin receptor substrate (IRS) proteins, and, in turn, the kinase AKT. AKT phosphorylates and inhibits the transcription factor, Forkhead Box O1 (FoxO1). In the liver, FoxO1 has previously been shown to inhibit the master regulator of cholesterol synthesis, Sterol Regulatory Element Binding Protein (SREBP)-2, and its targets, the cholesterologenic genes14–16. Thus, these studies suggest that insulin, by inhibiting FoxO1, could induce SREBP-2 and cholesterol synthesis17. Consistent with this, mice with hepatocyte-specific deletion of the insulin receptor show reduced levels of activated SREBP-2 protein, reduced expression of the cholesterol synthesis genes and reduced cholesterol synthesis17. Similarly, mouse models of type 1 diabetes18 and people with type 1 diabetes show reduced cholesterol synthesis19.
In the intestine, insulin appears to reduce cholesterol absorption, but some discrepancies exist in the literature20–26. At the whole-body level, insulin treatment suppresses the absorption of cholesterol and its secretion as ApoB-containing lipoprotein particles—in both rodents and humans20–22, 26. In parallel, individuals with type 1 diabetes show an increase in cholesterol absorption20, 27, 28. However, insulin increases cholesterol uptake into intestinal cells in vitro; moreover, mice lacking insulin receptor in the intestine show reduced plasma cholesterol levels, suggesting that insulin may act on the intestine to increase absorption and plasma cholesterol levels23, 24.
Here, we show that insulin regulates plasma cholesterol levels by suppressing FoxO1 in the liver, which reduces 12α-hydroxylated bile acids (12HBAs). 12HBAs, which are more hydrophobic than non-12HBAs, increase cholesterol absorption and plasma cholesterol, while producing a compensatory decrease in SREBP-2 and the cholesterologenic genes29, 30. In parallel, we show that the defects in cholesterol metabolism observed in humans with type 1 diabetes are reversed by treatment with cholesterol absorption inhibitors, but not cholesterol synthesis inhibitors.
Experimental Methods and Materials
The data and analytic methods that support the mouse findings of this study will be available from the corresponding author upon reasonable request. Because of the sensitive nature of the data collected from the human studies, we cannot make these data available for public use.
An expanded and detailed materials and methods section is provided in the Expanded Methods of the Supplemental Materials.
Animal Studies
Generation of mice with floxed (flanked by loxP) insulin receptor and FoxO1 alleles has been described previously31, 32. To generate liver-specific knockout mice, floxed mice were crossed with Alb-Cre transgenic mice that express Cre recombinase under the albumin promoter. All mice were maintained on a C57BL/6Tac background. C57BL/6-Ins2AKITA/J and B6.129P2-Apoetm1Unc/J (ApoE) null mice were purchased from Jackson laboratories; both lines were crossed with C57BL/6 mice. Mice were randomized to treatments at the start of the experiment. Except where otherwise specified, we used male mice, fed a standard chow diet ad libitum, and sacrificed in the non-fasted state at 2pm. All animal experiments were performed with the approval of the Institutional Animal Care and Research Advisory Committee at Children’s Hospital Boston.
ASO and insulin experiments
A second-generation control antisense oligonucleotide (ASO, ION 141923–73) or antisense oligonucleotide against Cyp8b1 (ION 431981) was diluted in normal saline, and injected intraperitoneally (50 mg/kg each week). For insulin treatment, LinBit insulin pellets (LinShin, Canada, Inc.; 0.1U/day/mouse) were inserted aseptically in the dorsum. Mice were sacrificed one day after their final dose of antisense oligonucleotide.
Streptozotocin (STZ) treatment
For STZ treatment in the insulin or ASO studies, 8–9 week-old mice fed Western diet (Envigo TD.88137) for 3–4 weeks were injected with vehicle (0.1M citrate buffer, pH 4.2) or STZ (200 mg/kg body weight) and sacrificed 5 days later.
Phenotyping
Gene expression was performed using real-time PCR33; cholesterol absorption was assayed using the dual isotope method34; bile salt and lipoprotein profile were determined by HPLC35, 36; plasma lipids were determined using colorimetric assays17; physiological, biochemical and molecular characterizations were performed as described previously17, 33.
Human Studies
Cross-sectional study of individuals with type 1 diabetes and their controls: Male and female individuals with type 1 diabetes (n= 33) and non-diabetic controls (n=43) were recruited at Boston Children’s Hospital (see Supplemental Figure 5A for demographic information).
Simvastatin/ezetimibe treatment cross-over study: The selection criteria and simvastatin/ezetimibe treatment of people with type 1 diabetes have been previously reported (see Supplemental Figure 6 for demographic information)37.
Baseline fasting plasma was collected and subjected to measurements of HbA1c, lipid panel, and sterol markers. Individuals were then randomly assigned to therapy with either ezetimibe (n=11 individuals; 10 mg/day) or simvastatin (n=9 individuals; 40 mg/day) for 6 weeks, after which fasting plasma was collected. After a 4-week wash out period, the alternate treatment was administered to individuals for 6 weeks. Fasting plasma was again collected at the end of the therapy. LDL cholesterol was calculated using the Friedwald equation as previously reported37.
For cholesterol absorption measurements, individuals were asked to consume 8 ounces of Carnation ® Instant breakfast with a cholesterol tracer (cholesterol-d5) 24 hours prior each fasting plasma collection.
All the study procedures were approved by the Medical College of Wisconsin’s Institutional Review Board and/or the Institutional Review Board of Boston Children’s Hospital, and all individuals provided an informed consent.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS)
Sterols were extracted from plasma and subjected to liquid chromatography-tandem mass spectrometry as previously described38, 39.
Quantification and statistical analysis
Mouse Studies: Data are represented by the mean ± SEM, unless otherwise indicated. Significance was assessed by one-way, two-way, or repeated measures ANOVA, followed by Tukey post-hoc test to determine significant changes between individual groups. The sample size of individual groups was pre-determined based on prior experimental results.
Human Studies: For the cross-sectional study, group comparison was performed using 2-sided Student’s t-tests for continuous variables and Fisher’s exact tests for categorical variables. A linear model adjusted for age was used to determine a significant group effect. For the cross-over study, significance between treatments was assessed using mixed effects ANOVA to account for direct treatment, period, and carry-over effects. If the carry-over effects were not significant, additional paired t-tests for comparisons with baseline were performed. The secondary analyses were not adjusted for multiple comparisons as these results are hypothesis-generating. For triglyceride measurements, carry-over and period effects were reported as they were significant; the direct effect was not significant.
Results
The liver plays a central role in cholesterol metabolism and is a key site of insulin action. To dissect the roles of insulin and FoxO1 in cholesterol homeostasis, we used the Cre-LoxP system, with Cre driven by the albumin promoter, to generate mice with hepatocyte-specific deletion of the insulin receptor (Insulin Receptor Liver-Knockout, or IR L-KO), FoxO1 (FoxO1 Liver-Knockout, or FoxO1 L-KO), or both (Insulin Receptor and FoxO1 Liver-Double Knockout, or IR/FoxO1 L-DKO).
As expected from previous studies, IR L-KO mice showed a reduction in the expression of the cholesterologenic genes: Fdps and Cyp51 were reduced and Srebp-2 and Hmgcr trended downwards (Figure 1A–D). On the other hand, hepatic cholesterol, which suppresses SREBP-2 processing40, was slightly, but significantly increased (Figure 1E). FoxO1 deletion, though it had little effect on its own, increased cholesterologenic gene expression and lowered hepatic cholesterol in the context of insulin receptor deficiency. Thus, the effects of insulin receptor deletion were entirely lost in the IR/FoxO1 L-DKO mice (Figure 1A–E). Similarly, the deletion of FoxO1 in the livers of mice lacking IRS1 and IRS2 rescued the cholesterologenic genes (Supplemental Figure 1A, B).
Figure 1. Insulin regulates cholesterol homeostasis in a FoxO1-dependent manner.
(A-K) Mice with hepatic deletion of the insulin receptor (Insulin Receptor Liver-Knockout or IR L-KO), FoxO1 (FoxO1 Liver-Knockout, or FoxO1 L-KO), or both (Insulin Receptor and FoxO1 Liver-Double Knockout, or IR/FoxO1 L-DKO) as well as their littermate controls (FLOX) were sacrificed at 8–10 weeks of age (n=5–30). Livers were subjected to real-time PCR analysis (A-D,H-K) or cholesterol measurement (E). (F) Cholesterol absorption was measured at 13 weeks of age as the percentage of an oral bolus of [14C]-cholesterol absorbed using the dual isotope method41. (G) Gallbladder bile was subjected to HPLC analysis, and the percentage of 12HBAs was calculated as described in methods. Error bars represent SEM; *p<0.05. A.U., arbitrary units.
Cholesterol absorption from the intestine, in parallel with hepatic cholesterol, was increased by hepatic insulin receptor deletion in the presence but not absence of FoxO1 (Figure 1F). The fact that insulin receptor deletion solely in the hepatocytes could increase intestinal cholesterol absorption suggested a potential role for bile acids. Bile acids are synthesized in the liver and secreted into the gut, where they can regulate cholesterol absorption. More than 30 different bile acids can be detected in the plasma of humans and mice. These differ in their physiochemical and signaling properties. 12HBAs were of particular interest: first, mice with hepatic deletion of the insulin receptor as well as insulin-deficient mice show an increase in 12HBAs41–44, whereas mice with hepatic deletion of FoxO1 show a reduction in 12HBAs45; second, 12HBAs increase cholesterol absorption29, 46; and, finally, mice that lack 12HBAs show decreased hepatic cholesterol and an induction of SREBP-2 and its targets29. We therefore hypothesized that insulin and FoxO1 might regulate cholesterol absorption via 12HBAs. Consistent with this, hepatic deletion of the insulin receptor increased the proportion of 12HBAs in the bile from 60% to 78% (Figure 1G). Moreover, the ability of insulin receptor deletion to increase the proportion of 12HBAs was lost in the absence of FoxO1.
Two pathways for bile acid synthesis exist. The neutral pathway of bile acid synthesis, which is initiated by the enzyme CYP7A1, produces both 12HBAs and non-12HBAs47. The sole enzyme capable of catalyzing the 12α-hydroxylation reaction is CYP8B1, which is also known as sterol 12α-hydroxylase. The acidic pathway of bile acid synthesis, which is initiated by the enzymes CYP27A1 and CYP7B1, produces primarily non-12HBAs47–50. At the gene expression level, insulin receptor and FoxO1 in the liver showed complex interactions on the bile acid synthesis genes: IR L-KO mice showed a reduction in Cyp27a1 and Cyp7b1 which was reversed by the concomitant deletion of FoxO1, whereas FoxO1 L-KO mice showed a reduction in Cyp8b1, as well as an increase in Cyp7a1 upon deletion of the insulin receptor (Figure 1H–K).
To dissect the role of 12HBAs in mediating the effects of insulin and FoxO1 on cholesterol metabolism, we chose to manipulate CYP8B1, as the manipulation of CYP8B1 alone is sufficient to alter 12HBA levels29, 30, 51. Therefore, to reconstitute 12HBAs into IR/FoxO1 L-DKO mice, we treated them with an adenovirus encoding Cyp8b1. The adenovirus increased Cyp8b1 mRNA approximately 17-fold, and increased the proportion of 12HBAs in the bile from 30% to 56% (Supplemental Figure 2A, B). In parallel, hepatic cholesterol levels were increased two-fold, and Srebp-2 was reduced by 20% (Supplemental Figure 2C, D). The SREBP-2 targets, Hmgcr, Fdps, and Cyp51 were reduced by 20–70%, though the decrease in Hmgcr did not reach significance (Supplemental Figure 2E–G).
Conversely, we reduced 12HBA levels in IR L-KO mice using antisense oligonucleotides (ASOs) against Cyp8b1. ASO treatment reduced Cyp8b1 mRNA by 72% and the proportion of 12HBAs from 82% to 57% (Supplemental Figure 2H, I). In parallel, hepatic cholesterol was reduced, and mRNA levels of Srebp-2, Hmgcr, Fdps, and Cyp51 trended upwards (Supplemental Figure 2J–N). Protein levels of HMGCR mirrored mRNA levels (Supplemental Figure 2O).
Despite an increase in 12HBAs, IR L-KO mice on a chow diet failed to develop hyperlipidemia (Supplemental Figure 1C, D, Supplemental Figure 2P), consistent with prior studies52. Therefore, the mice were placed on a Western diet (42% kcal from fat, 43% from carbohydrate, 15% from protein). On this diet, IR L-KO mice developed severe hypercholesterolemia in parallel with an increase in 12HBAs. The excess cholesterol was associated with the atherogenic ApoB-containing lipoproteins, VLDL and LDL. Knockout of FoxO1 reduced Cyp8b1 mRNA and normalized 12HBA and plasma cholesterol levels (Figure 2A–D).
Figure 2. Hepatic insulin deficiency drives 12HBAs and hypercholesterolemia on a Western diet.
(A-E) 3–6 week-old mice with hepatic deletion of the insulin receptor (Insulin Receptor Liver-Knockout or IR L-KO), FoxO1 (FoxO1 Liver-Knockout, or FoxO1 L-KO), or both (Insulin Receptor and FoxO1 Liver-Double Knockout, or IR/FoxO1 L-DKO) and their littermate controls (FLOX) were placed on a Western diet for 6 weeks (n=5–25). (F-O) 5–9 week-old mice with hepatic deletion of the insulin receptor (IR L-KO) mice and their littermate controls (IR-FLOX) were placed on a Western diet and treated with control (CON) or Cyp8b1 ASO (50 mg/kg body weight per week, IP) for 5 weeks (n=6–9). Livers were subjected to real-time PCR (A, F, L-O) or cholesterol measurements (K). Gallbladder bile from individual mice (B) or pooled in equal amounts from 4–5 mice per group (G) were subjected to HPLC analysis, and the percentage of 12HBAs was calculated as described in methods. Plasma collected at sacrifice was used for measurements of cholesterol (C,H) and triglycerides (E,J). (D,I) Equal amounts of plasma from 4–5 mice per genotype were subjected to lipoprotein fractionation. Error bars represent SEM; *p<0.05.
To specifically test the role of 12HBAs, we treated IR L-KO mice and their littermate IR-FLOX controls with the Cyp8b1 ASO. Without affecting expression of the other bile acid synthesis genes, the Cyp8b1 ASO reduced Cyp8b1 by 70–90%, and lowered 12HBAs to approximately 30% in mice of both genotypes (Figure 2F, G, and data not shown). Importantly, the increase in plasma cholesterol and shift towards VLDL and LDL observed in IR L-KO mice were lost upon Cyp8b1 knockdown (Figure 2H, I). In parallel, the excess hepatic cholesterol observed in IR L-KO mice, which was much more prominent on the Western diet, was reduced by 33%. However, this reduction in hepatic cholesterol was not associated with a de-repression of the cholesterologenic genes (Figure 2K–O, Supplemental Figure 2Z). In control mice, Cyp8b1 knockdown induced Fdps expression, but did not affect plasma or hepatic lipids, or otherwise affect cholesterologenic gene expression. Plasma triglycerides were not significantly altered by manipulation of the insulin receptor, FoxO1, or Cyp8b1 on either diet (Figure 2E, J, Supplemental Figure 1E, 2Q).
These data reveal an important link between hepatic insulin signaling, bile acids, and plasma cholesterol levels. To determine the extent to which these effects of insulin are also observed in the context of general insulin deficiency, which is the pathophysiological lesion in type 1 diabetes, we treated Western diet-fed mice with the β-cell toxin, streptozotocin (STZ).
STZ-treated mice displayed the hallmarks of insulin deficiency, including severe hyperglycemia, weight loss, increased gluconeogenic gene expression, reduced lipogenic gene expression, and dyslipidemia with increased VLDL- and HDL-cholesterol and a marked increase in triglycerides (Figure 3A–F, Supplemental Figure 3A–E). They also showed changes in bile acid gene expression, with a two-fold increase in Cyp8b1, a 50% decrease in Cyp27a1, and no change in Cyp7a1 and Cyp7b1 (Figure 3G–J). Though bile acid gene expression differed somewhat between IR L-KO and STZ-treated mice, both IR L-KO and STZ-treated mice showed an increase in 12HBAs and suppression of Srebp-2, Hmgcr, Fdps, and Cyp51 (Figure 3K–O). Finally, almost all of the effects of streptozotocin, including those on plasma lipids and 12HBAs, were entirely reversed by the administration of insulin.
Figure 3. STZ-treated mice show increased 12HBA and plasma cholesterol levels that are reversed by insulin treatment.
(A-O) 6–9 week-old C57BL/6J mice were placed on Western diet for 4 weeks (n=6–10). Mice were injected with vehicle (VEH, 0.1 M citrate buffer, pH 4.2) or streptozotocin (STZ, 200 mg/kg body weight, IP) 5 days before sacrifice and insulin pellets (0.1 U/day/mouse) were inserted 2 days before sacrifice (STZ + INS). (A) Blood glucose was measured at the time of sacrifice. (B) Body weights were monitored every day after STZ injection and presented as % BW measured at the day of STZ injection. (C-F) Plasma collected at sacrifice was used for measurements of cholesterol (C), lipoprotein fractionation (D), and triglycerides (E) or was photographed (F). For lipoprotein fractionation, equal amounts of plasma from 4–5 mice per genotype were pooled. (G-J, L-O) Livers were subjected to real-time PCR analysis. (K) Gallbladder bile from individual mice were subjected to HPLC analysis, and the percentage of 12HBAs was calculated as described in methods. Error bars represent SEM; *p<0.05 for all analyses, except for body weight measurements where *p<0.05 for VEH vs STZ, #p<0.05 for STZ vs STZ + INS, &p<0.05 VEH vs STZ + INS; A.U., arbitrary units.
These data suggest that insulin’s ability to regulate 12HBA levels is necessary for maintaining normal plasma cholesterol levels. We therefore tested this further by knocking down Cyp8b1 in STZ-treated mice and their vehicle-injected controls (VEH). In STZ-treated mice, the Cyp8b1 ASO reduced Cyp8b1 by 64%, without significantly altering Cyp7a1, Cyp7b1 or Cyp27a1 (Figure 4A, and data not shown). In parallel, it lowered 12HBA levels; plasma cholesterol levels, including VLDL-cholesterol; and plasma triglycerides (Figure 4B–E). In contrast to insulin, however, Cyp8b1 knockdown in STZ-treated mice failed to significantly increase Srebp-2, Hmgcr, Fdps, or Cyp51, (Figure 4F–I). Cyp8b1 knockdown in STZ-treated mice also reduced gluconeogenic gene expression, but did not alter plasma glucose, body weight or lipogenic gene expression (Supplemental Figure 4A–G).
Figure 4. 12HBAs are required for hypercholesterolemia in mouse models of type 1 diabetes.
(A-I) 4–5 week-old C57BL/6J mice were placed on Western diet and treated with CON or Cyp8b1 ASO (50 mg/kg body weight per week, IP) for 4–6 weeks (n=5–16). Mice were injected with vehicle (VEH, 0.1M citrate buffer, pH 4.2) or streptozotocin (STZ, 200 mg/kg body weight, IP) five days prior to sacrifice. (J-R) 3–6 week-old C57BL/6-Ins2Akita/J mice (Ins2AKITA) and their wildtype littermates were placed on Western diet and treated with control (CON) or Cyp8b1 ASO (50 mg/kg body weight per week, IP) for 4–5 weeks (n=6–22). Livers were subjected to real-time PCR (A,F-I,J,O-R). (B,K) Gallbladder bile from individual mice was subjected to HPLC analysis, and the percentage of 12HBAs was calculated as described in methods. Plasma collected at sacrifice was subjected to measurements of cholesterol (C,L) and triglycerides (E,N) or lipoprotein fractionation (D,M). Error bars represent SEM; *p<0.05. A.U., arbitrary units.
Akita mice harbor a mutation in the Ins2 gene and represent a genetic model of type 1 diabetes. Akita mice, like STZ-treated mice, manifested hyperglycemia, increased gluconeogenic gene and Cyp8b1 expression, hypercholesterolemia and a suppression of the cholesterologenic genes; however, Akita body weights and plasma triglycerides were normal, suggestive of a milder degree of diabetes, and their excess plasma cholesterol was primarily associated with LDL (Figure 4J–R, Supplemental Figure 4N–Q). Nonetheless, Cyp8b1 ASO treatment of Akita mice, which lowered Cyp8b1 by 70% and normalized 12HBA levels, produced a marked reduction in plasma cholesterol, primarily LDL-cholesterol. Cyp8b1 knockdown did not significantly affect blood glucose, body weight, gluconeogenic and cholesterologenic genes, or plasma triglycerides in either Akita mice or their controls (Figure 4J–R, Supplemental Figure 4N–Q).
These data suggest that a key defect in cholesterol metabolism produced by type 1 diabetes in mice is an increase in cholesterol absorption. To assess cholesterol metabolism in humans, we measured plasma markers of cholesterol synthesis and absorption53–57 in a cohort of young adults with type 1 diabetes and non-diabetic controls (demographics in Supplemental Figure 5A). We found that the markers of cholesterol absorption, campesterol and β-sitosterol, were significantly higher in individuals with type 1 diabetes (Figure 5A, B). On the other hand, lathosterol, a marker of cholesterol synthesis, trended lower in individuals with type 1 diabetes (Figure 5C).
Figure 5. Type 1 diabetes is associated with increased cholesterol absorption but not increased cholesterol synthesis.
(A-C) Plasma from individuals with type 1 diabetes (T1D) and non-diabetic controls was taken after an overnight fast and subjected to LC-MS/MS analysis for measurement of cholesterol absorption (campesterol, β-sitosterol) and synthesis (lathosterol) markers (n=43 control and 33 T1D individuals). Error bars represent SEM; *p<0.05; adjusted for age. A.U., arbitrary units.
The fact that cholesterol absorption is increased in type 1 diabetes20, 27, 28 suggests that cholesterol absorption inhibitors might be effective in lipid lowering and CVD risk reduction. Indeed, we found that the ability of type 1 diabetes to induce hypercholesterolemia in an atherogenic mouse model was abolished by treatment with the cholesterol absorption inhibitor, ezetimibe (Supplemental Figure 5B, C). Moreover, we previously found that ezetimibe and simvastatin (a cholesterol synthesis inhibitor) had similar LDL-lowering effects in individuals with type 1 diabetes37. In an extension of this study, we assessed cholesterol metabolism by directly measuring cholesterol absorption using a stable isotope tracer, cholesterol-d5, and by measuring plasma sterol markers (demographics in Supplemental Figure 6).
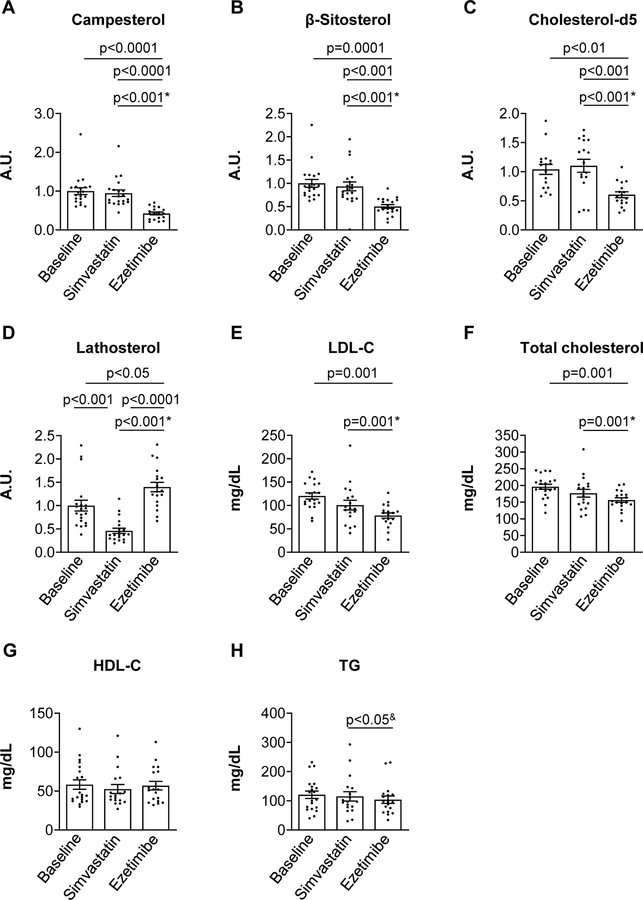
Ezetimibe significantly reduced cholesterol absorption, assayed by plasma campesterol and β-sitosterol, or cholesterol-d5. At the same time, ezetimibe increased cholesterol synthesis, assayed by plasma lathosterol (Figure 6A–D). On the other hand, simvastatin had no effect on campesterol, β-sitosterol, or cholesterol-d5, but reduced lathosterol levels. Importantly, both drugs reduced LDL and total cholesterol, though the reduction was slightly greater (33% versus 20%) and reached significance only with ezetimibe (Figure 6E, F). Neither drug affected HDL cholesterol or triglyceride levels (Figure 6G, H). Thus, ezetimibe was at least as effective, if not more effective, than simvastatin in this cohort.
Figure 6. Cholesterol absorption inhibitor restores cholesterol homeostasis in individuals with type 1 diabetes.
In this open-label, cross-over nonrandomized clinical trial37, individuals with type 1 diabetes were asked to withhold their lipid-lowering medications for four weeks prior to the collection of baseline samples (n=20). The individuals were then randomly assigned to treatment with either simvastatin (40 mg/day) or ezetimibe (10 mg/day) for 6 weeks. After a four-week wash out period, the second drug was administered. Plasma sterols (A-D) as well as total, LDL, HDL cholesterol (E-G), and triglycerides (H) were measured in plasma collected at baseline or after each intervention. (C) Cholesterol absorption was also measured at baseline and after each intervention as the amount of cholesterol-d5 tracer in the plasma 24 hours after an oral bolus. Error bars represent SEM; p-value between treatments represents a significant drug effect on the changes in measured lipid parameters. For the cross-over design analysis, the significant direct treatment* or carry-over and period effect& measurements are indicated. The other comparisons were based on paired t-tests since there was no evidence of carry-over effects for these variables (p>0.05). A.U., arbitrary units.
Discussion
We find that insulin prevents hypercholesterolemia by lowering 12HBAs. Mechanistically, insulin acts in the hepatocyte to suppress FoxO1, leading to a reduction in 12HBA levels, cholesterol absorption, and plasma cholesterol. In mouse models of type 1 diabetes, insulin treatment or the knockdown of Cyp8b1 prevents hypercholesterolemia; in patients, ezetimibe effectively reverses the abnormalities in cholesterol metabolism associated with type 1 diabetes.
There are multiple mechanisms by which insulin regulates cholesterol metabolism, which likely contribute in a context-dependent manner58–61. For example, insulin increases LDL receptor expression58, 62, 63, and we noted a reduction in LDL receptor protein in mice with hepatic deletion of the insulin receptor, but not STZ-treated mice (Supplemental Figure 2X, 3F, 4M). However, in IR L-KO, STZ-treated and Akita mice, we found an increase in 12HBAs. In parallel, we found an increase in plasma cholesterol, particularly VLDL/LDL-cholesterol, and suppression of the cholesterologenic genes, primarily Fdps and Cyp51. Importantly, the reduction of 12HBAs, which increase cholesterol absorption and decrease cholesterol synthesis29, 30, was able to normalize plasma cholesterol in all of these models, suggesting that the suppression of 12HBAs is critical to insulin’s regulation of cholesterol metabolism.
12HBAs promote cholesterol absorption because of their physiochemical properties64. Thus, the ability of insulin to suppress cholesterol absorption and the intestinal secretion of ApoB-containing lipoproteins in vivo could be mediated by 12HBAs20–22, 26. However, in vitro studies as well as studies using mice with intestinal knockout of the insulin receptor suggest that insulin increases cholesterol absorption23, 24. Interestingly, we found that Npc1l1, which promotes cholesterol absorption65, was reduced in the intestines of STZ-treated mice, with whole body insulin deficiency but not IR L-KO mice, with intact insulin signaling in the intestine (Supplemental Figure 2S–W, 4H–L). Taken together, these data suggest that insulin has two opposing effects on cholesterol absorption: while its major effect is to suppress cholesterol absorption by inhibiting 12HBA production in the liver, it may also increase cholesterol absorption by modulating cholesterol transporter expression in the intestine.
12HBAs appear to suppress cholesterologenic gene expression and cholesterol synthesis indirectly, by increasing hepatic cholesterol29, 30, 40. Consistent with this, increasing 12HBAs in IR/FoxO1 L-DKO mice by Cyp8b1 overexpression increased hepatic cholesterol and suppressed the cholesterologenic genes whereas lowering 12HBA levels in IR L-KO mice by Cyp8b1 knockdown lowered hepatic cholesterol, particularly on the Western diet (Figure 2K, Supplemental Figure 2C, J). However, lowering 12HBAs had modest effects on cholesterologenic gene expression, and cholesterologenic gene expression in IR L-KO, STZ-treated and Akita mice remained lower than their controls with normal insulin signaling, even after Cyp8b1 knockdown (Figure 2L–O, 4F–I, 4O–R, Supplemental Figure 2K–N). 12HBAs may also have additional effects on cholesterol metabolism by altering signaling via nuclear and G protein-coupled receptors, such as farnesoid X receptor and G protein-coupled bile acid receptor 166.
Insulin regulates 12HBA levels in large part via FoxO1. FoxO1 induces Cyp8b1, and insulin suppresses FoxO145. Thus, in STZ-treated and Akita mice, FoxO1 is dis-inhibited, leading to a two- to three-fold increase in Cyp8b1, and increased 12HBAs. FoxO1 is also central for 12HBA regulation in IR L-KO mice, as FoxO1 deletion normalizes 12HBA levels. However, the mechanisms by which 12HBAs become increased are more complex. First, Cyp8b1 levels are normal in IR L-KO livers despite dis-inhibition of FoxO1, potentially due to other transcriptional regulators of Cyp8b1. For example, LncLSTR is a long non-coding RNA that promotes Cyp8b1 expression67 and was decreased almost 80% in IR L-KO livers (Supplemental Figure 1F). Second, the fact that 12HBA, but not Cyp8b1, levels are increased in IR L-KO mice suggest that insulin may also suppress CYP8B1 at the post-transcriptional level or that insulin induces flux through the acidic pathway of bile acid synthesis, which produces primarily non-12HBAs47–50. The latter possibility is suggested by the fact that IR L-KO mice show lower hepatic Cyp7b1 and Cyp27a1 levels that are reversed by FoxO1 deletion in parallel with 12HBA levels (Figure 1G, J, K)41, 68, 69. Nonetheless, the fact that the three models studied, IR L-KO, STZ-treated and Akita mice, all show an increase in 12HBAs despite differences in bile acid synthesis gene expression, indicates that there are multiple redundant mechanisms by which insulin and FoxO1 can regulate 12HBAs.
The physiological effects of insulin and FoxO1 on cholesterol metabolism may be best understood in the context of energy utilization in the fed versus fasted state. Cholesterol biosynthesis is an intensively energy-consuming process, requiring over 36 ATP and 16 NADPH per molecule. Cholesterol absorption from the gut requires much less energy; however, the uptake of cholesterol from the gut is not entirely specific, and may result in the simultaneous uptake of toxins such as plant sterols. In the fed state, when nutrients are abundant, insulin licenses the synthesis of cholesterol, but inhibits the absorption of cholesterol. In the fasted state, insulin levels fall, and cholesterol synthesis is suppressed. In this case, the inhibition of cholesterol synthesis in the liver would allow the diversion of ATP and reducing equivalents towards other processes such as gluconeogenesis.
The disruption of insulin and FoxO1 control of cholesterol metabolism in our mouse models of type 1 diabetes resulted in an increase in plasma cholesterol, which was entirely dependent upon 12HBAs. In considering the translational implications of these findings, two notes should be made. First, bile acid metabolism differs between humans and mice, and these differences could theoretically impact the ability of 12HBAs versus non-12HBAs to drive cholesterol absorption. Nonetheless, the increase in cholesterol absorption markers and decrease in cholesterol synthesis markers observed in patients with type 1 diabetes20, 27, 28 suggest that, in this regard, 12HBAs may function similarly in humans. Second, in contrast to mice, patients with type 1 diabetes are treated with insulin, which lowers plasma cholesterol levels (Figure 3C). Consequently, only a subset of patients with type 1 diabetes, those that show elevated hemoglobin A1c levels, indicating poor glycemic control and insufficient insulin administration, develop hypercholesterolemia. Nonetheless, this subset is very important to consider: not only are individuals with elevated HbA1c common70, they have the highest cholesterol absorption39, and carry a disproportionate burden of the increased CVD risk associated with type 1 diabetes7, 71.
While it is clear that reducing cholesterol is critical for lowering CVD risk, which of the multiple drugs available is most effective at reducing cholesterol in a particular individual is less clear 72, 73. Statin drugs inhibit cholesterol synthesis; ezetimibe inhibits cholesterol absorption74; and bile acid sequestrants promote the excretion of bile acids, leading to an increase in cholesterol conversion to bile acids as well as a reduction in cholesterol absorption75. Because statins are much more effective than ezetimibe and bile acid sequestrants in the general population76, 77, they are the first line of lipid lowering treatment in type 1 diabetes78, 79. Consistent with this, meta-analyses and observational studies suggest an association between statin use and lower CVD risk in type 1 diabetes13, 80. However, the statin response, especially outside of the context of a clinical trial, is highly variable72, 73. Here, we show that insulin is an important modifier of cholesterol metabolism, and that the loss of insulin action in the liver promotes cholesterol absorption rather than cholesterol synthesis. Thus, the hypoinsulinemic condition of type 1 diabetes can produce changes in cholesterol metabolism that are distinct from those found in the hyperinsulinemic condition of type 2 diabetes and the general population.
In theory, personalizing the choice of lipid lowering drug to the specific defects in an individual’s metabolism of cholesterol could represent a more effective approach to CVD prevention. In individuals with type 1 diabetes in good control, we would expect hepatic insulin action, cholesterol synthesis levels, and the response to statin to be largely preserved. In individuals with poor control, insulin action in the liver as well as the statin response may be compromised17. In this case, other approaches, such as ezetimibe, might be more effective.
Another potential strategy for lipid lowering in type 1 diabetes may be CYP8B1 inhibition. Indeed, Cyp8b1 knockdown has beneficial effects beyond cholesterol lowering. Cyp8b1 deletion improves glucose metabolism81; in these studies, Cyp8b1 knockdown reduced gluconeogenic gene expression and/or glucose levels in STZ-treated and IR L-KO mice, though not Akita mice (Supplemental Figure 2R, 4A, C, D, N, P, Q). Cyp8b1 knockdown also reduced plasma triglycerides in STZ-treated mice, potentially due to the ability of 12HBAs to increase triglyceride absorption from the gut82, 83. However, this was not observed in other models, which were not hypertriglyceridemic at baseline. Finally, Cyp8b1 deletion reduces hepatic triglycerides82 though this was not observed in these studies (Supplemental Figure 2Y).
The studies here have some limitations. First, 12HBA levels were manipulated indirectly, by altering expression of Cyp8b1, raising the possibility that knockdown of Cyp8b1 reduced plasma cholesterol independently of 12HBAs. However, we think this is unlikely because (1) bile acids are the only known products of CYP8B184; (2) feeding rodents with 12HBAs is sufficient to increase cholesterol absorption46 and plasma cholesterol85, 86. Nonetheless, it remains possible that 12HBAs have effects on cholesterol metabolism beyond increasing cholesterol absorption, such as reducing ApoB clearance, another factor that contributes to dyslipidemia in diabetes. Second, the human studies here were performed on small cohorts, making it difficult to generalize conclusions. Finally, cholesterol absorption and synthesis were assessed with sterol markers, which are closely correlated with physiological measurements 84, but not direct measurements.
Nonetheless, these data in mice and humans show that insulin decreases plasma cholesterol, at least in part, by suppressing 12HBAs and cholesterol absorption. They further suggest that strategies to lower cholesterol absorption rather than synthesis may have a substantial impact on CVD risk reduction, particularly in the subset of patients who are under-insulinized with poor glycemic control. Testing the relative effects of cholesterol absorption and cholesterol synthesis inhibitors on lipid levels and cardiovascular outcomes specifically in type 1 diabetes is an important next step.
Supplementary Material
Clinical Perspective.
1). What’s new?
Insulin prevents hypercholesterolemia by lowering 12α-hydroxylated bile acids levels (12HBAs) and cholesterol absorption.
Restoring insulin signaling or reducing 12HBA levels normalizes plasma total and ApoB-associated cholesterol levels in mouse models of type 1 diabetes.
In individuals with type 1 diabetes, cholesterol absorption, rather than cholesterol synthesis is increased, and this is reversed by ezetimibe (cholesterol absorption inhibitor) but not simvastatin (cholesterol synthesis inhibitor).
2). What are the clinical implications?
Larger clinical studies to directly compare the efficacy of cholesterol absorption inhibitors versus cholesterol synthesis inhibitors for lipid lowering in type 1 diabetes are needed.
Acknowledgements
We thank Dr. William Pandak (VCU Health System) for the Cyp8b1 adenovirus and Matthew Davis (Wake Forest University School of Medicine) for performing the lipoprotein fractionation analysis.
Funding Sources
This work was funded by the American Diabetes Association Grant 9-18-CVD1-003 (IS), an American Heart Association Pre-doctoral Fellowship (AVL), NIH training grant No. T32 DK007260 (JK), John S. Ladue Memorial Fellowship (SSA), DK125898 and HL161092 (SBB), and a SPARC grant from the Broad Institute.
Non-standard Abbreviations and Acronyms
- 12HBA
12α-hydroxylated bile acid
- ApoB
Apolipoprotein B
- ASO
anti-sense oligonucleotide
- CYP8B1
Cytochrome P450 Family 8 Subfamily B Member 1
- FDPS
Farnesyl Diphosphate Synthase
- FOXO1
Forkhead box protein O1
- HbA1c
Hemoglobin A1c
- HMGCR
3-Hydroxy-3-Methylglutaryl-CoA Reductase
- HPLC
High-performance liquid chromatography
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- LncLSTR
long non-coding RNA, liver-specific triglyceride regulator
- SREBP-2
Sterol-regulatory element-binding protein-2
- STZ
Streptozotocin
Footnotes
Conflict of Interest Disclosures
None.
Supplemental Materials
Supplemental Figure legends 1 – 6
References 87–90
References
- 1.Rawshani A, Rawshani A, Franzen S, Eliasson B, Svensson AM, Miftaraj M, McGuire DK, Sattar N, Rosengren A, Gudbjornsdottir S. Mortality and cardiovascular disease in type 1 and type 2 diabetes. The New England journal of medicine. 2017;376:1407–1418. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B, Diabetes C, Complications Trial/Epidemiology of Diabetes I, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. The New England journal of medicine. 2005;353:2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, Ellis D, Becker DJ. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the pittsburgh epidemiology of diabetes complications study. Diabetes care. 2003;26:1374–1379. [DOI] [PubMed] [Google Scholar]
- 4.Gerstein HC, Miller ME, Byington RP, Goff DC Jr., Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr., et al. Effects of intensive glucose lowering in type 2 diabetes. The New England journal of medicine. 2008;358:2545–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reaven PD, Emanuele NV, Wiitala WL, Bahn GD, Reda DJ, McCarren M, Duckworth WC, Hayward RA, Investigators V. Intensive glucose control in patients with type 2 diabetes - 15-year follow-up. The New England journal of medicine. 2019;380:2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bornfeldt KE. Does elevated glucose promote atherosclerosis? Pros and cons. Circulation research. 2016;119:190–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bebu I, Braffett BH, Pop-Busui R, Orchard TJ, Nathan DM, Lachin JM, Group DER. The relationship of blood glucose with cardiovascular disease is mediated over time by traditional risk factors in type 1 diabetes: The dcct/edic study. Diabetologia. 2017;60:2084–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Effect of intensive diabetes management on macrovascular events and risk factors in the diabetes control and complications trial. Am.J Cardiol. 1995;75:894–903. [DOI] [PubMed] [Google Scholar]
- 9.Chance GW, Albutt EC, Edkins SM. Serum lipids and lipoproteins in untreated diabetic children. Lancet. 1969;1:1126–1128. [DOI] [PubMed] [Google Scholar]
- 10.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: The framingham heart study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 11.Singh AK, Singh R. Triglyceride and cardiovascular risk: A critical appraisal. Indian J Endocrinol Metab. 2016;20:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. Journal of the American College of Cardiology. 2006;48:438–445. [DOI] [PubMed] [Google Scholar]
- 13.Cholesterol Treatment Trialists C, Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, et al. Efficacy and safety of ldl-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. [DOI] [PubMed] [Google Scholar]
- 14.Haeusler RA, Han S, Accili D. Hepatic foxo1 ablation exacerbates lipid abnormalities during hyperglycemia. Journal of Biological Chemistry. 2010;285:26861–26868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang K, Li L, Qi Y, Zhu X, Gan B, DePinho RA, Averitt T, Guo S. Hepatic suppression of foxo1 and foxo3 causes hypoglycemia and hyperlipidemia in mice. Endocrinology. 2012;153:631–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tao R, Xiong X, DePinho RA, Deng CX, Dong XC. Hepatic srebp-2 and cholesterol biosynthesis are regulated by foxo3 and sirt6. Journal of lipid research. 2013;54:2745–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miao J, Haas JT, Manthena P, Wang Y, Zhao E, Vaitheesvaran B, Kurland IJ, Biddinger SB. Hepatic insulin receptor deficiency impairs the srebp-2 response to feeding and statins. Journal of lipid research. 2014;55:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uchida K, Takase H, Kadowaki M, Nomura Y, Matsubara T, Takeuchi N. Altered bile acid metabolism in alloxan diabetic rats. Jpn.J Pharmacol. 1979;29:553–562. [DOI] [PubMed] [Google Scholar]
- 19.Gylling H, Tuominen JA, Koivisto VA, Miettinen TA. Cholesterol metabolism in type 1 diabetes. Diabetes. 2004;53:2217–2222. [DOI] [PubMed] [Google Scholar]
- 20.Kojima H, Hidaka H, Matsumura K, Fujita Y, Yamada S, Haneda M, Yasuda H, Kikkawa R, Kashiwagi A. Effect of glycemic control on plasma plant sterol levels and post-heparin diamine oxidase activity in type 1 diabetic patients. Atherosclerosis. 1999;145:389–397. [DOI] [PubMed] [Google Scholar]
- 21.Pavlic M, Xiao C, Szeto L, Patterson BW, Lewis GF. Insulin acutely inhibits intestinal lipoprotein secretion in humans in part by suppressing plasma free fatty acids. Diabetes. 2010;59:580–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lally S, Owens D, Tomkin GH. The different effect of pioglitazone as compared to insulin on expression of hepatic and intestinal genes regulating post-prandial lipoproteins in diabetes. Atherosclerosis. 2007;193:343–351. [DOI] [PubMed] [Google Scholar]
- 23.Fuentes M, Santander N, Cortes V. Insulin increases cholesterol uptake, lipid droplet content, and apolipoprotein b secretion in caco-2 cells by upregulating sr-bi via a pi3k, akt, and mtor-dependent pathway. Journal of cellular biochemistry. 2018;120:1550–1559. [DOI] [PubMed] [Google Scholar]
- 24.Andres SF, Santoro MA, Mah AT, Keku JA, Bortvedt AE, Blue RE, Lund PK. Deletion of intestinal epithelial insulin receptor attenuates high-fat diet-induced elevations in cholesterol and stem, enteroendocrine, and paneth cell mrnas. Am J Physiol Gastrointest Liver Physiol. 2015;308:G100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy E, Sinnett D, Thibault L, Nguyen TD, Delvin E, Menard D. Insulin modulation of newly synthesized apolipoproteins b-100 and b-48 in human fetal intestine: Gene expression and mrna editing are not involved. FEBS letters. 1996;393:253–258. [DOI] [PubMed] [Google Scholar]
- 26.Bloks VW, Bakker-Van Waarde WM, Verkade HJ, Kema IP, Wolters H, Vink E, Groen AK, Kuipers F. Down-regulation of hepatic and intestinal abcg5 and abcg8 expression associated with altered sterol fluxes in rats with streptozotocin-induced diabetes. Diabetologia. 2004;47:104–112. [DOI] [PubMed] [Google Scholar]
- 27.Miettinen TA, Gylling H, Tuominen J, Simonen P, Koivisto V. Low synthesis and high absorption of cholesterol characterize type 1 diabetes. Diabetes care. 2004;27:53–58. [DOI] [PubMed] [Google Scholar]
- 28.Gylling H, Laaksonen DE, Atalay M, Hallikainen M, Niskanen L, Miettinen TA. Markers of absorption and synthesis of cholesterol in men with type 1 diabetes. Diabetes Metab Res Rev. 2007;23:372–377. [DOI] [PubMed] [Google Scholar]
- 29.Murphy C, Parini P, Wang J, Bjorkhem I, Eggertsen G, Gafvels M. Cholic acid as key regulator of cholesterol synthesis, intestinal absorption and hepatic storage in mice. Biochimica et biophysica acta. 2005;1735:167–175. [DOI] [PubMed] [Google Scholar]
- 30.Li-Hawkins J, Gafvels M, Olin M, Lund EG, Andersson U, Schuster G, Bjorkhem I, Russell DW, Eggertsen G. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. The Journal of clinical investigation. 2002;110:1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, et al. Foxos are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michael MD, Kulkarni RN, Postic C, Previs SF, Shulman GI, Magnuson MA, Kahn CR. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Molecular cell. 2000;6:87–97. [PubMed] [Google Scholar]
- 33.Ling AV, Gearing ME, Semova I, Shin DJ, Clements R, Lai ZW, Biddinger SB. Foxo1 is required for most of the metabolic and hormonal perturbations produced by hepatic insulin receptor deletion in male mice. Endocrinology. 2018;159:1253–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Temel RE, Lee RG, Kelley KL, Davis MA, Shah R, Sawyer JK, Wilson MD, Rudel LL. Intestinal cholesterol absorption is substantially reduced in mice deficient in both abca1 and acat2. Journal of lipid research. 2005;46:2423–2431. [DOI] [PubMed] [Google Scholar]
- 35.Hagey LR, Schteingart CD, Rossi SS, Ton-Nu HT, Hofmann AF. An n-acyl glycyltaurine conjugate of deoxycholic acid in the biliary bile acids of the rabbit. Journal of lipid research. 1998;39:2119–2124. [PubMed] [Google Scholar]
- 36.Haas ME, Levenson AE, Sun X, Liao WH, Rutkowski JM, de Ferranti SD, Schumacher VA, Scherer PE, Salant DJ, Biddinger SB. The role of proprotein convertase subtilisin/kexin type 9 in nephrotic syndrome-associated hypercholesterolemia. Circulation. 2016;134:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ciriacks K, Coly G, Krishnaswami S, Patel SB, Kidambi S. Effects of simvastatin and ezetimibe in lowering low-density lipoprotein cholesterol in subjects with type 1 and type 2 diabetes mellitus. Metab Syndr Relat Disord. 2015;13:84–90. [DOI] [PubMed] [Google Scholar]
- 38.Honda A, Yamashita K, Miyazaki H, Shirai M, Ikegami T, Xu G, Numazawa M, Hara T, Matsuzaki Y. Highly sensitive analysis of sterol profiles in human serum by lc-esi-ms/ms. Journal of lipid research. 2008;49:2063–2073. [DOI] [PubMed] [Google Scholar]
- 39.Semova I, Levenson AE, Krawczyk J, Bullock K, Williams KA, Wadwa RP, Shah AS, Khoury PR, Kimball TR, Urbina EM, et al. Type 1 diabetes is associated with an increase in cholesterol absorption markers but a decrease in cholesterol synthesis markers in a young adult population. J Clin Lipidol. 2019;13:940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radhakrishnan A, Goldstein JL, McDonald JG, Brown MS. Switch-like control of srebp-2 transport triggered by small changes in er cholesterol: A delicate balance. Cell metabolism. 2008;8:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biddinger SB, Haas JT, Yu BB, Bezy O, Jing E, Zhang W, Unterman TG, Carey MC, Kahn CR. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nature medicine. 2008;14:778–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogura M, Noda Y, Suzukui T. [Influence of nicotinamide administration on the bile-acid pattern in rats given streptozotocin]. Biol Chem Hoppe Seyler. 1990;371:637–640. [PubMed] [Google Scholar]
- 43.Li T, Francl JM, Boehme S, Ochoa A, Zhang Y, Klaassen CD, Erickson SK, Chiang JY. Glucose and insulin induction of bile acid synthesis: Mechanisms and implication in diabetes and obesity. The Journal of biological chemistry. 2012;287:1861–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uchida K, Makino S, Akiyoshi T. Altered bile acid metabolism in nonobese, spontaneously diabetic (nod) mice. Diabetes. 1985;34:79–83. [DOI] [PubMed] [Google Scholar]
- 45.Haeusler RA, Pratt-Hyatt M, Welch CL, Klaassen CD, Accili D. Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia. Cell metabolism. 2012;15:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang DQ, Tazuma S, Cohen DE, Carey MC. Feeding natural hydrophilic bile acids inhibits intestinal cholesterol absorption: Studies in the gallstone-susceptible mouse. Am J Physiol Gastrointest Liver Physiol. 2003;285:G494–502. [DOI] [PubMed] [Google Scholar]
- 47.Swell L, Gustafsson J, Schwartz CC, Halloran LG, Danielsson H, Vlahcevic ZR. An in vivo evaluation of the quantitative significance of several potential pathways to cholic and chenodeoxycholic acids from cholesterol in man. J Lipid.Res. 1980;21:455–466. [PubMed] [Google Scholar]
- 48.Duane WC, Javitt NB. 27-hydroxycholesterol: Production rates in normal human subjects. Journal of lipid research. 1999;40:1194–1199. [PubMed] [Google Scholar]
- 49.Anderson KE, Kok E, Javitt NB. Bile acid synthesis in man: Metabolism of 7 -hydroxycholesterol- 14 c and 26-hydroxycholesterol- 3 h. The Journal of clinical investigation. 1972;51:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosen H, Reshef A, Maeda N, Lippoldt A, Shpizen S, Triger L, Eggertsen G, Bjorkhem I, Leitersdorf E. Markedly reduced bile acid synthesis but maintained levels of cholesterol and vitamin d metabolites in mice with disrupted sterol 27-hydroxylase gene. The Journal of biological chemistry. 1998;273:14805–14812. [DOI] [PubMed] [Google Scholar]
- 51.Pandak WM, Bohdan P, Franklund C, Mallonee DH, Eggertsen G, Bjorkhem I, Gil G, Vlahcevic ZR, Hylemon PB. Expression of sterol 12alpha-hydroxylase alters bile acid pool composition in primary rat hepatocytes and in vivo. Gastroenterology. 2001;120:1801–1809. [DOI] [PubMed] [Google Scholar]
- 52.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Aleman JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, et al. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell metabolism. 2008;7:125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miettinen TA, Tilvis RS, Kesaniemi YA. Serum cholestanol and plant sterol levels in relation to cholesterol metabolism in middle-aged men. Metabolism: clinical and experimental. 1989;38:136–140. [DOI] [PubMed] [Google Scholar]
- 54.Miettinen TA, Tilvis RS, Kesaniemi YA. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol. 1990;131:20–31. [DOI] [PubMed] [Google Scholar]
- 55.Tilvis RS, Miettinen TA. Serum plant sterols and their relation to cholesterol absorption. The American journal of clinical nutrition. 1986;43:92–97. [DOI] [PubMed] [Google Scholar]
- 56.Simonen P, Gylling H, Miettinen TA. The validity of serum squalene and non-cholesterol sterols as surrogate markers of cholesterol synthesis and absorption in type 2 diabetes. Atherosclerosis. 2008;197:883–888. [DOI] [PubMed] [Google Scholar]
- 57.Mashnafi S, Plat J, Mensink RP, Baumgartner S. Non-cholesterol sterol concentrations as biomarkers for cholesterol absorption and synthesis in different metabolic disorders: A systematic review. Nutrients. 2019;11:1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kissebah AH, Alfarsi S, Evans DJ, Adams PW. Plasma low density lipoprotein transport kinetics in noninsulin-dependent diabetes mellitus. The Journal of clinical investigation. 1983;71:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldberg IJ, Hu Y, Noh HL, Wei J, Huggins LA, Rackmill MG, Hamai H, Reid BN, Blaner WS, Huang LS. Decreased lipoprotein clearance is responsible for increased cholesterol in ldl receptor knockout mice with streptozotocin-induced diabetes. Diabetes. 2008;57:1674–1682. [DOI] [PubMed] [Google Scholar]
- 60.Subramanian S, Han CY, Chiba T, McMillen TS, Wang SA, Haw A, 3rd, Kirk EA, O’Brien KD, Chait A. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese ldl receptor-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jun JY, Ma Z, Segar L. Spontaneously diabetic ins2(+/akita):Apoe-deficient mice exhibit exaggerated hypercholesterolemia and atherosclerosis. American journal of physiology. Endocrinology and metabolism. 2011;301:E145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mazzone T, Foster D, Chait A. In vivo stimulation of low-density lipoprotein degradation by insulin. Diabetes. 1984;33:333–338. [DOI] [PubMed] [Google Scholar]
- 63.Chait A, Bierman EL, Albers JJ. Low density lipoprotein receptor activity in fibroblasts cultured from diabetic donors. Diabetes. 1979;28:914–918. [DOI] [PubMed] [Google Scholar]
- 64.Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid.Res. 1989;30:719–730. [PubMed] [Google Scholar]
- 65.Garcia-Calvo M, Lisnock J, Bull HG, Hawes BE, Burnett DA, Braun MP, Crona JH, Davis HR Jr., Dean DC, Detmers PA, et al. The target of ezetimibe is niemann-pick c1-like 1 (npc1l1). Proceedings of the National Academy of Sciences of the United States of America. 2005;102:8132–8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Aguiar Vallim TQ, Tarling EJ, Edwards PA. Pleiotropic roles of bile acids in metabolism. Cell metabolism. 2013;17:657–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li P, Ruan X, Yang L, Kiesewetter K, Zhao Y, Luo H, Chen Y, Gucek M, Zhu J, Cao H. A liver-enriched long non-coding rna, lnclstr, regulates systemic lipid metabolism in mice. Cell metabolism. 2015;21:455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen C, Hu B, Wu T, Zhang Y, Xu Y, Feng Y, Jiang H. Bile acid profiles in diabetic (db/db) mice and their wild type littermates. J Pharm Biomed Anal. 2016;131:473–481. [DOI] [PubMed] [Google Scholar]
- 69.Al-Sharea A, Murphy AJ, Huggins LA, Hu Y, Goldberg IJ, Nagareddy PR. Sglt2 inhibition reduces atherosclerosis by enhancing lipoprotein clearance in ldlr(−/−) type 1 diabetic mice. Atherosclerosis. 2018;271:166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller RG, Mahajan HD, Costacou T, Sekikawa A, Anderson SJ, Orchard TJ. A contemporary estimate of total mortality and cardiovascular disease risk in young adults with type 1 diabetes: The pittsburgh epidemiology of diabetes complications study. Diabetes care. 2016;39:2296–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maahs DM, Wadwa RP, McFann K, Nadeau K, Williams MR, Eckel RH, Klingensmith GJ. Longitudinal lipid screening and use of lipid-lowering medications in pediatric type 1 diabetes. The Journal of pediatrics. 2007;150:146–150. [DOI] [PubMed] [Google Scholar]
- 72.Karlson BW, Wiklund O, Palmer MK, Nicholls SJ, Lundman P, Barter PJ. Variability of low-density lipoprotein cholesterol response with different doses of atorvastatin, rosuvastatin, and simvastatin: Results from voyager. Eur Heart J Cardiovasc Pharmacother. 2016;2:212–217. [DOI] [PubMed] [Google Scholar]
- 73.Bacquer D, Smedt D, Reiner Ž, Tokgözoğlu L, Clays E, Kotseva K, Rydén L, Wood D, Backer G. Percentage low-density lipoprotein-cholesterol response to a given statin dose is not fixed across the pre-treatment range: Real world evidence from clinical practice: Data from the esc-eorp euroaspire v study. Eur J Prev Cardiol. 2020;27:1630–1636. [DOI] [PubMed] [Google Scholar]
- 74.Sudhop T, Lutjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. [DOI] [PubMed] [Google Scholar]
- 75.Briones ER, Steiger D, Palumbo PJ, Kottke BA. Primary hypercholesterolemia: Effect of treatment on serum lipids, lipoprotein fractions, cholesterol absorption, sterol balance, and platelet aggregation. Mayo Clin Proc. 1984;59:251–257. [DOI] [PubMed] [Google Scholar]
- 76.Winkler K, Jacob S, Muller-Schewe T, Hoffmann MM, Konrad T. Ezetimibe alone and in combination lowers the concentration of small, dense low-density lipoproteins in type 2 diabetes mellitus. Atherosclerosis. 2012;220:189–193. [DOI] [PubMed] [Google Scholar]
- 77.Farnier M, Guyton JR, Jensen E, Polis AB, Johnson-Levonas AO, Brudi P. Effects of ezetimibe, simvastatin and ezetimibe/simvastatin on correlations between apolipoprotein b, ldl cholesterol and non-hdl cholesterol in patients with primary hypercholesterolemia. Atherosclerosis. 2013;229:415–422. [DOI] [PubMed] [Google Scholar]
- 78.Eliasson B, Svensson AM, Miftaraj M, Jonasson JM, Eeg-Olofsson K, Sundell KA, Gudbjornsdottir S. Clinical use and effectiveness of lipid lowering therapies in diabetes mellitus--an observational study from the swedish national diabetes register. PloS one. 2011;6:e18744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wadwa RP, Kinney GL, Maahs DM, Snell-Bergeon J, Hokanson JE, Garg SK, Eckel RH, Rewers M. Awareness and treatment of dyslipidemia in young adults with type 1 diabetes. Diabetes care. 2005;28:1051–1056. [DOI] [PubMed] [Google Scholar]
- 80.Hero C, Rawshani A, Svensson AM, Franzen S, Eliasson B, Eeg-Olofsson K, Gudbjornsdottir S. Association between use of lipid-lowering therapy and cardiovascular diseases and death in individuals with type 1 diabetes. Diabetes care. 2016;39:996–1003. [DOI] [PubMed] [Google Scholar]
- 81.Kaur A, Patankar JV, de Haan W, Ruddle P, Wijesekara N, Groen AK, Verchere CB, Singaraja RR, Hayden MR. Loss of cyp8b1 improves glucose homeostasis by increasing glp-1. Diabetes. 2015;64:1168–1179. [DOI] [PubMed] [Google Scholar]
- 82.Bertaggia E, Jensen KK, Castro-Perez J, Xu Y, Di Paolo G, Chan RB, Wang L, Haeusler RA. Cyp8b1 ablation prevents western diet-induced weight gain and hepatic steatosis because of impaired fat absorption. American journal of physiology. Endocrinology and metabolism. 2017;313:E121–E133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li R, Palmiotti A, de Vries HD, Hovingh MV, Koehorst M, Mulder NL, Zhang Y, Kats K, Bloks VW, Fu J, et al. Low production of 12α-hydroxylated bile acids prevents hepatic steatosis in cyp2c70(−/−) mice by reducing fat absorption. Journal of lipid research. 2021:100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Malmlof K, Zaragoza F, Golozoubova V, Refsgaard HH, Cremers T, Raun K, Wulff BS, Johansen PB, Westerink B, Rimvall K. Influence of a selective histamine h3 receptor antagonist on hypothalamic neural activity, food intake and body weight. Int.J Obes.(Lond.). 2005;29:1402–1412. [DOI] [PubMed] [Google Scholar]
- 85.Zurkinden L, Sviridov D, Vogt B, Escher G. Downregulation of cyp7a1 by cholic acid and chenodeoxycholic acid in cyp27a1/apoe double knockout mice: Differential cardiovascular outcome. Front Endocrinol (Lausanne). 2020;11:586980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chiang MT, Chen YC, Huang AL. Plasma lipoprotein cholesterol levels in rats fed a diet enriched in cholesterol and cholic acid. Int J Vitam Nutr Res. 1998;68:328–334. [PubMed] [Google Scholar]
- 87.Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell metabolism. 2008;8:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu M, Wan M, Leavens KF, Chu Q, Monks BR, Fernandez S, Ahima RS, Ueki K, Kahn CR, Birnbaum MJ. Insulin regulates liver metabolism in vivo in the absence of hepatic akt and foxo1. Nature medicine. 2012;18:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. Limma powers differential expression analyses for rna-sequencing and microarray studies. Nucleic acids research. 2015;43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reich M, Liefeld T, Gould J, Lerner J, Tamayo P, Mesirov JP. Genepattern 2.0. Nature genetics. 2006;38:500–501. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.