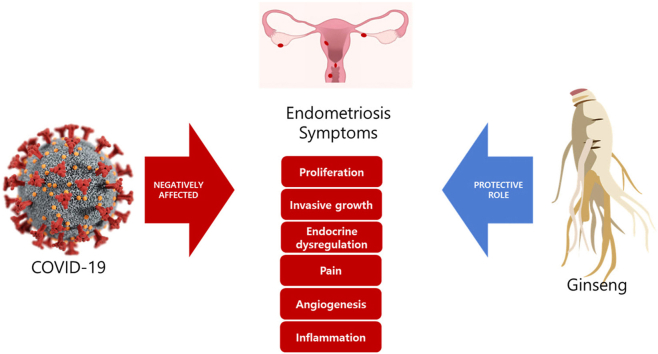
Abstract
The coronavirus disease 2019 (COVID) pandemic began in December 2019. Many countries have implemented restrictions such as mandatory mask wearing and social distancing. These measures have caused diverse and complex health problems, particularly in women's health, anxiety, and depression. This review examines an alternative approach to the treatment of endometriosis during the COVID pandemic. The efficacy of ginseng with anti-inflammatory activity and ability to relieve or prevent symptoms of endometriosis is discussed and reviewed.
Keywords: endometriosis, ginseng, korea red ginseng, COVID-19
Graphical abstract
1. Introduction
Around the end of 2019, a series of cases of pneumonia of unknown cause occurred in Wuhan, Hubei, China [1]. The clinical manifestations were like those of viral pneumonia. On January 7, the China Centers for Disease Control and Prevention found a novel coronavirus in these patients and reported that it had caused the cluster of pneumonia cases. The World Health Organization estimated that 527,842,668 patients caught COVID and 6,300,942 died between December 30, 2019, and January 3, 2022 [2]. COVID causes symptoms such as chest and sore throat, muscle pain, fever, cough, and respiratory insufficiency. It affects the heart, liver, kidney, and nervous system [3,4]. COVID is also responsible for multi-organ syndrome [5,6]. Neurological symptoms including depression, musculoskeletal, and digestive such as diarrhea are frequently observed in patients with post-COVID syndrome. Causes of post-COVID syndrome are under study. Hypotheses including autoimmune problem, persisting chronic inflammation, and hormonal imbalance as a consequence of a change in the hypothalamic-pituitary-adrenal axis have been proposed [7]. To prevent the spread of the virus by contact, a stringent approach has been taken. Measures include implementation of social distancing, mandatory use of face masks, events cancellation (e.g., meetings, exhibitions, and sports competitions), strict travel restrictions, and closure of most nonessential workplaces. Most countries have adopted these precautions to limit the spread of COVID. However, these measures themselves have caused a health crisis, worsening mental health and increasing numbers of suicides [8,9]. Endometriosis is one of the most common benign gynecological conditions in premenopausal women. An estimated 10–15% of women of reproductive age have pelvic endometriosis [10]. Endometriosis is a pelvic inflammatory disease triggered by inflammatory reactions caused by evasion of the local immune system [11]. It can lead to abrupt abdominal pelvic pain and reproductive problems such as infertility [12]. Local pre-inflammatory mediators such as tumor necrosis factor alpha (TNF-α) and interleukin (IL)-1β can activate nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and hypoxia-inducible factor 1-alpha (HIF-1α) signaling pathways, leading to cyclooxygenase-2 (COX-2) induction and angiogenesis [11]. A positive feed-forward loop is created by COX-2 to enhance local estrogen synthesis, aggravating endometriotic lesions [13]. These data suggest that suppression of COX-2 is a promising therapeutic strategy for endometriosis [14]. The COVID pandemic has had many mental and physical adverse effects on women's health, anxiety, and depression [15,16]. An internet-based survey for assess the influence of COVID was carried out on the care of people with endometriosis worldwide, to determine their priorities in relation to their clinical care during and after COVID, and whether they believed that endometriosis made them more vulnerable to COVID. Issues reported by 80.7% out of 6729 eligible respondents with endometriosis included difficulties obtaining medication (20.3%), cancelled/postponed gynaecology appointments (50.0%), and cancelled/postponed procedures (37.2%). More than half of respondents (54.2%; 95% confidence interval 53.0, 55.4) worried that their endometriosis made them more vulnerable to COVID [17]. In combination with or as an alternative to medical therapies, patients were encouraged to consider self-management strategies to combat endometriosis symptoms during the COVID pandemic [18].
2. Endometriosis and herb
Curcumin, genistein, ginsenoside, resveratrol, and puerarin have been assessed clinically in endometrial carcinoma, endothelial functions, and diseases such as breast cancer [19]. Genistein can inhibit the maintenance of endometriosis controlled by vascular endothelial growth factor (VEGF) and suppress neo-angiogenesis by inhibiting HIF-1α and mitogenic activity [20]. Curcumin can attenuate TNF-α-stimulated expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1, MCP-1, IL-8, and IL-6 by inhibiting activation of NF-κB, a key regulator of inflammation and inflammation gene activation, in human endometriotic stromal cells [21]. Regulated on activation normal T cell-expressed and secreted (RANTES) is a potent chemotactic factor for monocytes and activated T lymphocyte [22]. It is produced by peritoneal macrophages and endometriotic stromal cells. Its concentrations and levels are elevated in endometriotic patients, paralleling with disease severity. It is increased by the synthesis of cytokines (such as IL-1β) by activated macrophages and of NF-κB by endometriotic stromal cells [23]. Resveratrol can significantly reduce the expression of RANTES in ectopic endometrial stromal cells [23]. Several Chinese medicine formula containing various Chinese herbs were reviewed which can relieve various symptoms such as dysmenorreal relief, reduction of CA-125, normalization of prolactin serum levels, treatment of uterine fibroids and even infertility [19]. Table 1 lists active herbal alternative and complementary compounds reported to be effective for endometriosis. A number of studies have been reported that KRG and ginsenosides are effective against endometriosis associated symptoms by regulating angiogenic suppression, increasing apoptosis, immune system regulation, and anti- inflammation [[24], [25], [26], [27], [28], [29], [30]]. The following section focused on KRG of its effectiveness for endometriosis.
Table 1.
Active Herbal Compounds Effective for Endometriosis
| Bioactive compound | Source | Model | Action/mechanism | Reference |
|---|---|---|---|---|
| Resveratrol | Mulberry, peanuts, grapes, raspberry, cranberry, etc. | Ishikawa epithelial endometrial cells | Lowered IGF-1 and HGF levels | [65] |
| Curcumin | Turmeric. | Endometriotic stromal cells | Angiogenesis suppression and inflammation, | [66] |
| Female Sprague Dawley rats | Anti-inflammation, antioxidant. | [67] | ||
| Quercetin | Onions, curry plaques, apple peels, lettuce, peppers. | VK2/E6E7 and End1/E6E7 cells, and Endometriotic stromal cells | Anti-proliferation | [68] |
| Female C57BL/6 mice | Anti-proliferation, anti-inflammation. | [68] | ||
| Apigenin | Apple, beans, broccoli, celery, cherry, grape, onion, parsley, tomato, tea, wine, etc. | VK2/E6E7 and End1/E6E7 cells | Anti-proliferation. | [69] |
| Rosmarinic acid and carnosic acid | Lamiaceae hub | Endometriotic stromal cells | Anti-proliferation | [70] |
| Balb/c mice | Reduction of the lesion sized | [70] | ||
| Wogonin | Scutellaria baicalensis | Immortalized endometrial cell (T-HESC, ATCC CRL-4003) | Anti-proliferation | [70] |
| Female BALB/c mice | Reduction of lesion size | [70] | ||
| Delta-9- tetrahydrocannabinol | Hemp | Female C57Bl/6J mice | Reduction of lesion size and pain. | [71] |
3. Ginseng and endometriosis
Korea red ginseng (KRG) has immunomodulatory [28,31,32], anti-inflammatory [[32], [33], [34], [35], [36]], and anti-proliferative effects [37,38]. It can maintain immune system homeostasis and enhance resistance to microbial attack by regulating the immune system. The production of TNF-α, IL-18, IL-12, IL-6, IL-1β, and interferon-gamma (IFN-γ) is controlled by KRG [34,[39], [40], [41], [42]]. KRG attenuates not only the production of pro-inflammatory cytokines, but also the production of chemokines such as MCP-1 and MIP-2β thereby reducing leukocyte infiltration and the inflammatory response [43]. Anti-inflammatory effects of KRG are associated with cytokine regulation and phagocytosis in innate immunity as well as the activation of B and T lymphocytes [[44], [45], [46]]. Ginsenosides such as ginsenoside-Rh2 (Rh2) and ginsenoside-Rg3 (Rg3) and their metabolites protopanaxatriol (PPT) and protopanaxadiol (PPD) have antioxidant, antitumor, anti-inflammatory and immunomodulatory activities [[47], [48], [49], [50], [51]]. For instance, Rg3 can significantly reduce the activity of NF-kB, elevate caspase-3 expression, and inhibit VEGF expression [[52], [53], [54], [55], [56]]. These effects of KRG may be beneficial to symptoms of post-COVID as well autoimmune problems, persistent chronic inflammation, and hormonal imbalance as a consequence of changes in the hypothalamic-pituitary-adrenal axis [7,57].
We have reported that KRG can attenuate phthalate-induced endometriosis in a mouse model as indicated by a reduction in the expression of CD10, a sensitive marker of endometrial stromal cells [13]. COX-2 is overexpressed in endometriosis. COX-2 can induce or promote proliferation and inflammation [14]. KRG can decrease COX-2, NF-κB, and ERK1/2 levels in Ishikawa cells. It can inhibit COX-2 through diverse mechanisms, including the suppression of NF-kB [13]. Therefore, KRG can alleviate or prevent endometriotic symptoms. PPT and Rh2 can inhibit the viability and growth of ectopic endometrial stromal cells [24]. In endometriosis, PPD can reduce ectopic foci, promote endometrial receptivity and decidualization, suppress the inflammatory response of peritoneal macrophages, and increase the proportion, tolerance, and pro-angiogenetic phenotypes of natural killer cells. It can downregulate estrogen receptor α (ERα) and induce the expression of progesterone receptor in ectopic and normal endometrial stromal cells. ERα suppression mediated by PPD can induce autophagy of ectopic endometrial stromal cells, leading to increased NK cell cytotoxicity. These phenomena can enhance the immune surveillance of ectopic lesions thus inhibiting the development of endometriosis [24,29]. In a rat model, Rg3 can inhibit the development of endometriotic lesions induced by endometrial tissue allotransplantation by inhibiting angiogenesis [27]. Other studies have shown that Rg3 can inhibit the proliferation of ectopic endometriotic cells and significantly diminish the level of NF-κB p65 subunit as well as TNF-α induced nuclear translocation of NF-κB p65 subunit in ectopic endometriotic cells [25]. In addition, it can suppress endometriosis by regulating apoptosis and angiogenesis via NF-κB signaling in human ectopic endometrial stromal cells, suggesting that it can inhibit the growth of ectopic endometrium by blocking VEGF receptor-2-mediated PI3K/Akt/mTOR signaling pathway, thus promoting the halting angiogenesis and apoptosis of ectopic endometrial cells [27]. In an endometriosis mouse model, red ginsengs extract can significantly reduce the size of endometrial implant. Functional analyses have indicated that miRNAs with altered expression are involved in the immune system and multiple pathways, for example, PI3K/Akt/mTOR and Ras/Raf/MAPK pathways [30]. Through this regulation route, ginseng and its components can alleviate symptoms of endometriosis and inhibit the progression of endometriosis. Table 2 lists effects of KRG and its associated ginsenosides on endometriosis. The COVID-19 pandemic causes difficulties in endometriosis treatment and surgery [17]. Moreover, Covid-19 induced cytokine storms causing imbalances in inflammatory factors such as IL-6, IL-10 is believed to have an adverse effect on the health of endometriosis patients [58,59]. Therefore, it would be valuable to study the effectiveness of KRG on endometriosis under pandemic situation.
Table 2.
Effects of Ginsenosides and Ginseng in Endometriosis
| Source | Model | Action | Target | Reference |
|---|---|---|---|---|
| Rg3 | Female Sprague-Dawley (SD) | Angiogenesis suppression and increasing apoptosis | VEGFR-2-mediated PI3K/Akt/mTOR | [27] |
| Rg3 | Endometriotic stromal and Ishikawa cells | Inhibition of endometriosis-related fibrotic and invasion potential | miR-27b-3p | [26] |
| Rg3 | Female C57bl6 mice | Reduction lesion size, fibrotic and invasion potential | MMP9, MMP2, fibronectin, CTGF, Col-1, TGF-β1 | [26] |
| Rg3 | Endometriotic stromal cells | Suppression of cell proliferation, angiogenesis, and inflammation. | NF-κB p65 subunit, VEGF | [25] |
| PPD | Endometriotic stromal cells | Activation of the cytotoxicity of NK, autophagy induction, growth of lesions suppression, enhancing immune surveillance | ERα, PRα | [24] |
| PPD | Female BALB/C mice | Reduction of lesion size, inflammation, and the risk of abortion | IL-12, IFN-γ, CD16, NKp30, Ki67, VEGF, TGF-β | [29] |
| Red ginseng | FemaleC57b/6 mice | Reduction of lesion size, immune system regulation | miRNA | [30] |
| Red ginseng | DEHP-treated Ishikawa cells | Anti- inflammation | MMP-9, COX-2 | [13] |
4. Conclusion
Medicinal plants can relieve fever and cough in patients with COVID [60]. KRG may modulate acquired and natural immunity during COVID infection, indicating its potential as a preventive and supportive therapy [37,[60], [61], [62]]. It can promote health and prevent diseases by having immunomodulatory [63,64]. This review summarized a therapeutic potential of KRG as an adjunct for treating and preventing endometriosis [13,[25], [26], [27],29,30]. Further focused research is needed to reveal the precise functional effect and the mechanism of action of KRG in endometriosis in conjunction with viral spread of pandemic situation.
Acknowledgments
This research was supported by 2020 grant from The Korean Society of Ginseng.
References
- 1.Huang C., Wang y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO COVID-19 dashboard. World Health Organization; Geneva: 2020. https://covid19.who.int/(05.23.2022).2022 Available online. [Google Scholar]
- 3.Pascarella G., Strumia A., Piliego C., Bruno F., Del Buono R., Costa F., et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.da Rosa Mesquita R., Francelino Silva Junior L.C., Santos Santana F.M., Farias de Oliveira T., Campos Alcântara R., Monteiro Arnozo G., et al. Clinical manifestations of COVID-19 in the general population: systematic review. Wien Klin Wochenschr. 2021;133(7–8):377–382. doi: 10.1007/s00508-020-01760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zolotovskaia I.A., Shatskaia P.R., Davydkin I.L., Shavlovskaya O.A. [Post-COVID-19 asthenic syndrome] Zh Nevrol Psikhiatr Im S S Korsakova. 2021;121(4):25–30. doi: 10.17116/jnevro202112104125. [DOI] [PubMed] [Google Scholar]
- 6.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anaya J.-M., Rojas M., Salinas M.L., Rodríguez Y., Roa G., Lozano M., et al. Post-COVID study group Post-COVID syndrome. A case series and comprehensive review. Autoimmunity Rev. 2021;20(11) doi: 10.1016/j.autrev.2021.102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruinen de Bruin Y., Lequarre A.S., McCourt J., Clevestig P., Pigazzani F., Zare Jeddi M., et al. Initial impacts of global risk mitigation measures taken during the combatting of the COVID-19 pandemic. Saf Sci. 2020;128 doi: 10.1016/j.ssci.2020.104773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Both L.M., Zoratto G., Calegaro V.C., Ramos-Lima L.F., Negretto B.L., Hauck S., et al. COVID-19 pandemic and social distancing: economic, psychological, family, and technological effects. Trends Psychiatry Psychother. 2021;43(2):85–91. doi: 10.47626/2237-6089-2020-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asghari S., Valizadeh A., Aghebati-Maleki L., Nouri M., Yousefi M. Endometriosis: perspective, lights, and shadows of etiology. Biomed Pharmac. 2018;106:163–174. doi: 10.1016/j.biopha.2018.06.109. [DOI] [PubMed] [Google Scholar]
- 11.Han S.J., Wu S.P., Hawkins S.M., Park M.J., Kyo S., Qin J., et al. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell. 2015;163(4):960–974. doi: 10.1016/j.cell.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloski T., Pierson R. Endometriosis and chronic pelvic pain: unraveling the mystery behind this complex condition. Nursing Women's Health. 2008;12(5):382. doi: 10.1111/j.1751-486X.2008.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song H., Won J.E., Lee J., Han H.D., Lee Y.J. Korean red ginseng attenuates Di-(2-ethylhexyl) phthalate-induced inflammatory response in endometrial cancer cells and an endometriosis mouse model. J Ginseng Res. 2021 doi: 10.1016/j.jgr.2021.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y., Liu X., Guo S.-W. Therapeutic potential of andrographolide for treating endometriosis. Human Reprod. 2012;27(5):1300–1313. doi: 10.1093/humrep/des063. [DOI] [PubMed] [Google Scholar]
- 15.Almeida M., Shrestha A.D., Stojanac D., Miller L.J. The impact of the COVID-19 pandemic on women’s mental health. Archives Women’s Mental Health. 2020;23(6):741–748. doi: 10.1007/s00737-020-01092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connor J., Madhavan S., Mokashi M., Amanuel H., Johnson N.R., Pace L.E., et al. Health risks and outcomes that disproportionately affect women during the Covid-19 pandemic: a review. Soc Sci Med. 2020;266 doi: 10.1016/j.socscimed.2020.113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demetriou L., Cox E., Lunde C.E., Becker C.M., Inxitti A.L., Martínez-Burgo B., et al. The global impact of COVID-19 on the care of people with endometriosis. Front Glob Womens Health. 2021;2 doi: 10.3389/fgwh.2021.662732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leonardi M., Horne A.W., Vincent K., Sinclair J., Sherman K.A., Ciccia D., et al. Self-management strategies to consider to combat endometriosis symptoms during the COVID-19 pandemic. Hum Reprod Open. 2020;2020(2):hoaa028. doi: 10.1093/hropen/hoaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meresman G.F., Götte M., Laschke M.W. Plants as source of new therapies for endometriosis: a review of preclinical and clinical studies. Hum Reprod Update. 2021;27(2):367–392. doi: 10.1093/humupd/dmaa039. [DOI] [PubMed] [Google Scholar]
- 20.Sutrisno S., Aprina H., Simanungkalit H.M., Andriyani A., Barlianto W., Sujuti H., et al. Genistein modulates the estrogen receptor and suppresses angiogenesis and inflammation in the murine model of peritoneal endometriosis. J Tradit Complement Med. 2018;8(2):278–281. doi: 10.1016/j.jtcme.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim K.H., Lee E.N., Park J.K., Lee J.R., Kim J.H., Choi H.J., et al. Curcumin attenuates TNF-alpha-induced expression of intercellular adhesion molecule-1, vascular cell adhesion molecule-1 and proinflammatory cytokines in human endometriotic stromal cells. Phytother Res. 2012;26(7):1037–1047. doi: 10.1002/ptr.3694. [DOI] [PubMed] [Google Scholar]
- 22.Schall T.J., Bacon K., Toy K.J., Goeddel D.V. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347(6294):669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 23.Kolahdouz-Mohammadi R., Shidfar F., Khodaverdi S., Arablou T., Heidari S., Rashidi N., et al. Resveratrol treatment reduces expression of MCP-1, IL-6, IL-8 and RANTES in endometriotic stromal cells. J Cell Mol Med. 2021;25(2):1116–1127. doi: 10.1111/jcmm.16178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang B., Zhou W.J., Gu C.J., Wu K., Yang H.L., Mei J., et al. The ginsenoside PPD exerts anti-endometriosis effects by suppressing estrogen receptor-mediated inhibition of endometrial stromal cell autophagy and NK cell cytotoxicity. Cell Death Disease. 2018;9(5) doi: 10.1038/s41419-018-0581-2. 574-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang R., Chen S., Zhao M., Li Z., Zhu L. Ginsenoside Rg3 attenuates endometriosis by inhibiting the viability of human ectopic endometrial stromal cells through the nuclear factor-kappaB signaling pathway. J Gynecol Obstetrics Human Reprod. 2020;49(1) doi: 10.1016/j.jogoh.2019.101642. [DOI] [PubMed] [Google Scholar]
- 26.Kim M.K., Lee S.K., Park J.H., Lee J.H., Yun B.H., Park J.H., et al. Ginsenoside Rg3 decreases fibrotic and invasive nature of endometriosis by modulating miRNA-27b: in vitro and in vivo studies. Scientific Rep. 2017;7(1) doi: 10.1038/s41598-017-17956-0. 17670-17670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Y., Ye Q., Xie S., Zhong R., Cui J., Zhou J., et al. Ginsenoside Rg3 inhibits angiogenesis in a rat model of endometriosis through the VEGFR-2-mediated PI3K/Akt/mTOR signaling pathway. PLoS One. 2017;12(11):e0186520. doi: 10.1371/journal.pone.0186520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi J.H., Lee M.J., Park K.S., Kin S.H., In J.G., Kwak Y.S., et al. Korean Red Ginseng alleviates dehydroepiandrosterone-induced polycystic ovarian syndrome in rats via its antiinflammatory and antioxidant activities. J Ginseng Res. 2020;44(6):790–798. doi: 10.1016/j.jgr.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai Z.-Z., Yang H.-L., Shi J.-W., Shen H.-H., Wang Y., Chang K.-K., et al. Protopanaxadiol improves endometriosis associated infertility and miscarriage in sex hormones receptors-dependent and independent manners. Int J Biol Sci. 2021;17(8):1878–1894. doi: 10.7150/ijbs.58657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee J.H., Park J.H., Won B.H., Im W., Cho S.H. Administration of red ginseng regulates microRNA expression in a mouse model of endometriosis. Clin Exp Reprod Med. 2021;48(4):337–346. doi: 10.5653/cerm.2021.04392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim M., Sur B., Villa T., Yun J., Nah S.Y., Oh S. Gintonin regulates inflammation in human IL-1β-stimulated fibroblast-like synoviocytes and carrageenan/kaolin-induced arthritis in rats through LPAR2. J Ginseng Res. 2021;45(5):575–582. doi: 10.1016/j.jgr.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Min J.-H., Cho H.-J., Yi Y.-S. A novel mechanism of Korean red ginseng-mediated anti-inflammatory action via targeting caspase-11 non-canonical inflammasome in macrophages. J Ginseng Res. 2021 doi: 10.1016/j.jgr.2021.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X., Mo N., Li Z. Ginsenosides: potential therapeutic source for fibrosis-associated human diseases. J Ginseng Res. 2020;44(3):386–398. doi: 10.1016/j.jgr.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee J.-O., Yang Y., Tao Y., Yi Y.-S., Cho J.Y. Korean red ginseng saponin fraction exerts anti-inflammatory effects by targeting the NF-κB and AP-1 pathways. J Ginseng Res. 2022 doi: 10.1016/j.jgr.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heo H., Kim Y., Cha B., Brito S., Kim H., Kim H., et al. A systematic exploration of ginsenoside Rg5 reveals anti-inflammatory functions in airway mucosa cells. J Ginseng Res. 2022 doi: 10.1016/j.jgr.2022.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park J., Kim J., Ko E.-S., Jeong J.H., Park C.-O., Seo J.H., et al. Enzymatic bioconversion of ginseng powder increases the content of minor ginsenosides and potentiates immunostimulatory activity. J Ginseng Res. 2022;46(2):304–314. doi: 10.1016/j.jgr.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ratan Z.A., Youn S.H., Kwak Y.-S., Han C.-K., Haidere M.F., Kim J.K., et al. Adaptogenic effects of Panax ginseng on modulation of immune functions. J Ginseng Res. 2021;45(1):32–40. doi: 10.1016/j.jgr.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Y., He Y., Niu Z., Shen T., Zhang J., Wang X., et al. A review of the immunomodulatory activities of polysaccharides isolated from Panax species. J Ginseng Res. 2022;46(1):23–32. doi: 10.1016/j.jgr.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song S.B., Tung N.H., Quang T.H., Ngan N.T., Kim K.E., Kim Y.H. Inhibition of TNF-α-mediated NF-κB transcriptional activity in HepG2 cells by dammarane-type saponins from panax ginseng leaves. J Ginseng Res. 2012;36(2):146–152. doi: 10.5142/jgr.2012.36.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu H.L., Chen G.-H., Wu Y.-T., Xie L.-P., Tan Z.-B., Liu B., et al. Ginsenoside Ro, an oleanolic saponin of Panax ginseng, exerts anti-inflammatory effect by direct inhibiting toll like receptor 4 signaling pathway. J Ginseng Res. 2022;46(1):156–166. doi: 10.1016/j.jgr.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee S.Y., Kim M.-H., Kim S.-H., Ahn T., Kim S.-W., Kwak Y.-S., et al. Korean Red Ginseng affects ovalbumin-induced asthma by modulating IL-12, IL-4, and IL-6 levels and the NF-κB/COX-2 and PGE(2) pathways. J Ginseng Res. 2021;45(4):482–489. doi: 10.1016/j.jgr.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim M., Sur B., Villa S., Nah S.Y., Oh S. Inhibitory activity of gintonin on inflammation in human IL-1β-stimulated fibroblast-like synoviocytes and collagen-induced arthritis in mice. J Ginseng Res. 2021;45(4):510–518. doi: 10.1016/j.jgr.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shim J.-Y., Kim M.-H., Kim H.-D., Ahn J.-Y., Yun Y.-S., Song J.-Y. Protective action of the immunomodulator ginsan against carbon tetrachloride-induced liver injury via control of oxidative stress and the inflammatory response. Toxicol Appl Pharmacol. 2010;242(3):318–325. doi: 10.1016/j.taap.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Saba E., Lee Y.Y., Kim M.K., Kim S.-H., Hong S.-B., Rhee M.H. A comparative study on immune-stimulatory and antioxidant activities of various types of ginseng extracts in murine and rodent models. J Ginseng Res. 2018;42(4):577–584. doi: 10.1016/j.jgr.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim I.-K., Lee K.Y., Kang J., Park J.S., Jeong J. Immune-modulating effect of Korean red ginseng by balancing the ratio of peripheral T lymphocytes in bile duct or pancreatic cancer patients with adjuvant chemotherapy. In Vivo (Athens, Greece) 2021;35(3):1895–1900. doi: 10.21873/invivo.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hyun S.H., Ahn H.-Y., Kim H.-J., Kim S.W., So S.-H., In G., et al. Immuno-enhancement effects of Korean Red Ginseng in healthy adults: a randomized, double-blind, placebo-controlled trial. J Ginseng Res. 2021;45(1):191–198. doi: 10.1016/j.jgr.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ratan Z.A., Haidere M.F., Hong Y.H., Park S.H., Lee J.-O., Lee J., et al. Pharmacological potential of ginseng and its major component ginsenosides. J Ginseng Res. 2021;45(2):199–210. doi: 10.1016/j.jgr.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang W.-C., Huang T.-H., Yeh K.-W., Chen Y.-L., Shen S.-C., Liou C.-J. Ginsenoside Rg3 ameliorates allergic airway inflammation and oxidative stress in mice. J Ginseng Res. 2021;45(6):654–664. doi: 10.1016/j.jgr.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.You L., Cha S., Kim M.-Y., Cho J.Y. Ginsenosides are active ingredients in Panax ginseng with immunomodulatory properties from cellular to organismal levels. J Ginseng Res. 2021 doi: 10.1016/j.jgr.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wan Y., Wang J., Xu J.-F., Tang F., Chen L., Rao C.-L., et al. Panax ginseng and its ginsenosides: potential candidates for the prevention and treatment of chemotherapy-induced side effects. J Ginseng Res. 2021;45(6):617–630. doi: 10.1016/j.jgr.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jo S., Na H.G., Choi Y.S., Bae C.H., Song S.-Y., Kim Y.-D. Saponin attenuates diesel exhaust particle (DEP)-induced MUC5AC expression and pro-inflammatory cytokine upregulation via TLR4/TRIF/NF-κB signaling pathway in airway epithelium and ovalbumin (OVA)-sensitized mice. J Ginseng Res. 2022 doi: 10.1016/j.jgr.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hyun S.H., Ahn H.-Y., Kim H.-J., Kim S.W., So S.-H., In G., et al. Immuno-enhancement effects of Korean Red Ginseng in healthy adults: a randomized, double-blind, placebo-controlled trial. J Ginseng Res. 2021;45(1):191–198. doi: 10.1016/j.jgr.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee Y.Y., Irfan M., Quah Y., Saba E., Kim S.-D., Park S.-C., et al. The increasing hematopoietic effect of the combined treatment of Korean Red Ginseng and Colla corii asini on cyclophosphamide-induced immunosuppression in mice. J Ginseng Res. 2021;45(5):591–598. doi: 10.1016/j.jgr.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li W., Wang Y., Zhou X., Lü J., Sun H., Xie Z., et al. The anti-tumor efficacy of 20(S)-protopanaxadiol, an active metabolite of ginseng, according to fasting on hepatocellular carcinoma. J Ginseng Res. 2022;46(1):167–174. doi: 10.1016/j.jgr.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakhjavani M., Smith E., Yeo K., Tomita Y., Price T.J., Yool A., et al. Differential antiangiogenic and anticancer activities of the active metabolites of ginsenoside Rg3. J Ginseng Res. 2021 doi: 10.1016/j.jgr.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park E.-H., Kim Y.-J., Yamabe N., Park S.-H., Kim H.-K., Jang H.-.J., et al. Stereospecific anticancer effects of ginsenoside Rg3 epimers isolated from heat-processed American ginseng on human gastric cancer cell. J Ginseng Res. 2014;38(1):22–27. doi: 10.1016/j.jgr.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song J.-H., Kim K.-J., Choi S.-Y., Koh E.-J., Park J.D., Lee B.-Y. Korean ginseng extract ameliorates abnormal immune response through the regulation of inflammatory constituents in Sprague Dawley rat subjected to environmental heat stress. J Ginseng Res. 2019;43(2):252–260. doi: 10.1016/j.jgr.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mulchandani R., Lyngdoh T., Kakkar A.K. Deciphering the COVID-19 cytokine storm: systematic review and meta-analysis. Eur J Clin Invest. 2021;51(1) doi: 10.1111/eci.13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shuwa H.A., Knight S.B., Wemyss K., McClure F.A., Pearmain L., Prise I., et al. Alterations in T and B cell function persist in convalescent COVID-19 patients. Med (N Y) 2021;2(6):720–735.e4. doi: 10.1016/j.medj.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jalali A., Dabaghian F., Akbrialiabad H., Foroughinia F., Zarshenas M.M. A pharmacology-based comprehensive review on medicinal plants and phytoactive constituents possibly effective in the management of COVID-19. Phytother Res. 2021;35(4):1925–1938. doi: 10.1002/ptr.6936. [DOI] [PubMed] [Google Scholar]
- 61.Lee Y.Y., Quah Y., Shin J.-H., Kwon H.-W., Lee D.-H., Han J.E., et al. COVID-19 and Panax ginseng: targeting platelet aggregation, thrombosis and the coagulation pathway. J Ginseng Res. 2022;46(2):175–182. doi: 10.1016/j.jgr.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee W.S., Rhee D.-K. Corona-Cov-2 (COVID-19) and ginseng: comparison of possible use in COVID-19 and influenza. J Ginseng Res. 2021;45(4):535–537. doi: 10.1016/j.jgr.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.So S.-H., Lee J.W., Kim Y.S., Hyun S.H., Han C.K. Red ginseng monograph. J Ginseng Res. 2018;42(4):549–561. doi: 10.1016/j.jgr.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xiong Y., Chen L., Man J., Hu Y., Cui X. Chemical and bioactive comparison of Panax notoginseng root and rhizome in raw and steamed forms. J Ginseng Res. 2019;43(3):385–393. doi: 10.1016/j.jgr.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arablou T., Delbandi A.-A., Khodaverdi S., Arefi S., Kolahdouz-Mohammadi R., Heidari S., et al. Resveratrol reduces the expression of insulin-like growth factor-1 and hepatocyte growth factor in stromal cells of women with endometriosis compared with nonendometriotic women. Phytother Res. 2019;33(4):1044–1054. doi: 10.1002/ptr.6298. [DOI] [PubMed] [Google Scholar]
- 66.Chowdhury I., Banerjee S., Driss A., Xu W., Mehrabi S., Nezhat C., et al. Curcumin attenuates proangiogenic and proinflammatory factors in human eutopic endometrial stromal cells through the NF-κB signaling pathway. J Cell Physiol. 2019;234(5):6298–6312. doi: 10.1002/jcp.27360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jelodar G., Azimifar A. Evaluation of serum cancer antigen 125, resistin, leptin, homocysteine, and total antioxidant capacity in rat model of endometriosis treated with Curcumin. Physiol Rep. 2019;7(4) doi: 10.14814/phy2.14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park S., Lim W., Bazer F.W., Whang K.-Y., Song G. Quercetin inhibits proliferation of endometriosis regulating cyclin D1 and its target microRNAs in vitro and in vivo. J Nutr Biochem. 2019;63:87–100. doi: 10.1016/j.jnutbio.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 69.Park S., Lim W., Bazer F.W., Song G. Apigenin induces ROS-dependent apoptosis and ER stress in human endometriosis cells. J Cell Physiol. 2018;233(4):3055–3065. doi: 10.1002/jcp.26054. [DOI] [PubMed] [Google Scholar]
- 70.Ferella L., Bastón J.I., Bilotas M.A., Singla J.J., González A.M., Olivares C.M., et al. Active compounds present inRosmarinus officinalis leaves andScutellaria baicalensis root evaluated as new therapeutic agents for endometriosis. Reprod Biomed Online. 2018;37(6):769–782. doi: 10.1016/j.rbmo.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 71.Escudero-Lara A., Argerich j., Cabañero D., Maldonado R. Disease-modifying effects of natural Δ9-tetrahydrocannabinol in endometriosis-associated pain. Elife. 2020;9 doi: 10.7554/eLife.50356. [DOI] [PMC free article] [PubMed] [Google Scholar]