Abstract
Context
COVID-19 is a new viral infection affecting mainly the respiratory system with involvement of many other organs. Thyroid dysfunction has been described in COVID-19 but data are still unclear and conflicting on its frequency, severity and relationship with the outcome.
Patients and methods
We assessed thyroid function tests (TFT) in 50 patients admitted to our institution with confirmed COVID-19 infection. We excluded patients known to have thyroid diseases or taking drugs that may affect thyroid function. Serum free thyroxine (FT4), thyrotropin (TSH) and triiodothyronine (T3) were measured once or more during the first 10 days after admission. In about 50 % of the cases, a follow up TFT was obtained during the first year after discharge (at a median follow up of 6 months).
Results
We included 50 patients, 29 males (58 %) and 21 females (42 %). The median age was 47 years (range 25–89). Overall, TFTs were completely normal in all patients except for minor transient abnormalities in 5 patients (10 %) as follows: three patients had a mild transient elevated TSH, one had a mild transient suppressed TSH and one patient had a mildly low FT4 with normal TSH. There were no differences between the follow up TFTs obtained after discharge and TFTs obtained during admission in the acute phase.
Conclusion
In this study, thyroid dysfunction during acute COVID-19 infection was rare, mild and transient. However, the study might not be powered enough to detect an association between thyroid dysfunction and the severity of illness and further studies are needed to assess this issue. Late-onset thyroid dysfunction does not seem to occur in COVID-19 infection during the next year after discharge.
Keywords: COVID-19, Thyroid dysfunction, Thyroid function tests
1. Introduction
SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), named also as COVID-19 is a new coronavirus infection with many gaps in our knowledge about its pathogenesis and clinical spectrum. This new pandemic started in the late 2019 in Wuhan, China and spread across the globe causing COVID-19 infection in >534 million of people and >6.3 million deaths (https://www.worldometers.info/coronavirus/, accessed on 03 June 2022). While the respiratory system reveals the major manifestations of COVID-19 infection, it has become clear over the last 2.5 years that COVID-19 is a systemic infection that involves many body organs. The thyroid gland is rich in angiotensin converting enzyme ACE2 receptor, the main route of entry of the virus to the cells. Various types of involvement of the thyroid gland in COVID-19 have been reported in a number of recent studies (Gorini et al., 2020; Brancatella et al., 2020a; Wang et al., 2020; Campi et al., 2021; Khatri et al., 2021; Laurino et al., 2021). However, in a recent large study which prospectively screened 334 COVID-19 patients for thyroid function test abnormalities, the majority (86.6 %) were euthyroid (Khoo et al., 2021). In those with previous TFT in the year before admission, TSH and FT4 were found to be significantly lower during COVID-19 admission but in those patients who had follow up TFTs (55 patients), TSH and FT4 recovered to baseline levels (Khoo et al., 2021).
To assess the impact of COVID-19 infection on thyroid function in our patients, we performed thyroid function tests (TFT) during the acute phase of COVID-19 infection in 50 patients admitted to King Faisal Specialist Hospital & Research Centre (KFSHRC), Riyadh, Saudi Arabia during May 2020. In this report, we present our findings and briefly review the literature on COVID-19 and thyroid dysfunction.
2. Patients and methods
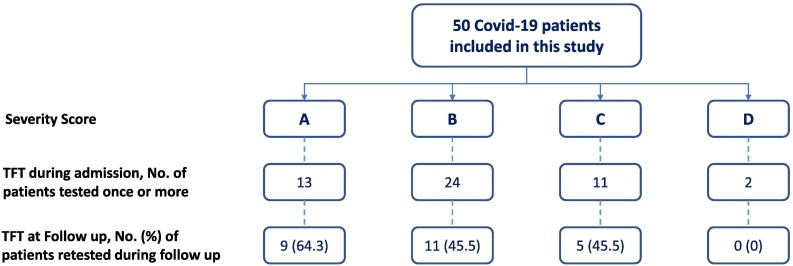
We included 50 consecutive adult patients (≥18 years of age) admitted for confirmed acute COVID-19 infection during 1–20 May 2020 (Fig. 1 ). All patients tested positive for COVID-19. We excluded patients who were known to have thyroid dysfunction or were on thyroid hormones, antithyroid drugs or drugs that may affect thyroid function such as glucocorticoids, amiodarone, anticonvulsants or biotin. The study was approved by the Office of Research Affairs and the Institutional Review Board of the KFSHRC (RAC # 2201062). After obtaining an informed consent, we measured TFT including free T4 (FT4), total T3 (T3) and thyrotropin (TSH) on one or more days during admission (days 1, 2, 3, 4, 8 and/or 10). We used the KFSHRC COVID-19 scoring system to assess the illness severity. This severity score was based on guidelines from the WHO (WHO, n.d.) and Centers for Disease Control of North America (CDC) (Prevention CfDCCa, n.d.) as follows:
-
1.
Asymptomatic (Stage A): Patients with positive COVID-19 test but no signs or symptoms of infection.
-
2.
Mild Infection (Stage B): Patients with upper respiratory tract infection symptoms and other mild symptoms (including fever and gastrointestinal symptoms) without evidence of pneumonia.
-
3.
Moderate Infection (Stage C): Patients with hypoxia with oxygen saturation <93 % at rest or presence of pneumonia on chest X-rays not requiring ICU admission.
-
4.Severe Infection (Stage D): Patients with pneumonia requiring ICU admission or any of the following:
-
a)Respiratory rate of >30 breaths/min
-
b)Arterial oxygen partial pressure to fractional inspiratory oxygen ratio (PaO2/FiO2) <300
-
c)More than 50 % lung involvement on imaging within 24–48 h
-
d)Critical respiratory failure requiring mechanical ventilation, septic shock or multiorgan dysfunction.
-
a)
Fig. 1.
A flow chart showing the total number of patients included and their distribution based on COVID-19 severity score and the number of those who had thyroid function testing during admission and those during the follow up.
2.1. Laboratory testing
COVID-19 testing: For RNA extraction, we used Qiagen EZ1 Virus mini kit extraction (Qiagen, Hilden, Germany). For real-time polymerase chain reaction (RT PCR) amplification, we used Roche cobas® SARS-CoV-2 Qualitative assay on 6800 System (Roche Diagnostics Nederland B.V., Netherland). Serum TSH, FT4, and T3 were all measured by electrochemiluminescence assays on Cobas e 801 immunoassay analyzer (Roche Diagnostics GmbH, Mannheim, Germany).
2.2. Statistical analysis
Numerical values are expressed in median and range or interquartile range or mean ± SD and categorical values in rates and percentages. One-way analysis of variance (ANOVA) was used to compare hormonal levels between different COVID-19 severity of illness groups. Paired t-test was used to compare TFTs during admission with follow up TFTs after discharge. The Statistical Package for the Social Sciences (SPSS) version 21 (IBM, Chicago) was used for the analysis. A two-tailed P value < 0.05 was considered significant.
3. Results
3.1. Patients
The initial characteristics, TFTs and outcome for the whole group and by severity score are summarized in Table 1 . We included 50 patients, 29 males (58 %) and 21 females (42 %). The median age was 47 years (range 25–89). The severity of illness was as follows: 13 (26 %) were in grade A, 24 (48 %) in grade B, 11 (22 %) in grade C and 2 (4 %) in grade D (Table 1). Thirty-seven patients (74 %) received Azithromycin and 31 (62 %) received Hydroxylcholoroquine, 7 (14 %) received Tocilizumab and 2 (4 %) were in the WHO Solidarity trial. Six patients (12 %) needed ICU admission.
Table 1.
Demographic, thyroid function tests and outcome of 50 COVID-19 patients with variable severity of illness.
| Characteristic | Covid-19 severity score |
||||
|---|---|---|---|---|---|
| All patients | A | B | C | D | |
| Median age (range) years | 47 (25–89) | 45 (26–67) | 49 (25–88) | 49 (25–89) | 57 |
| Sex (M:F) | 29:21 | 8:5 | 13:11 | 7:4 | 1:1 |
| Severity, no. (%) | 50 (100) | 13 (26) | 24 (48) | 11 (22) | 2 (4) |
| TFT at admission⁎ | |||||
| TSH, median (range) mU/l | 2.29 (1.1–3.1) | 2.65 (1.17–5.0) | 2.66 (0.0–7.36) | 1.32 (0.45–3.5) | 1.5 (0.35–2.69) |
| FT4, median (range) pmol/l | 14.6 (11.9–16.1) | 14.4 (10–18) | 14.3 (10.9–17.70) | 12.9 (12.3–16.0) | 17.25 (15–19.5) |
| T3, median (range) nmol/l | 1.5 (1.2–1.8) | 1.65 (1.4–2.4) | 1.7 (1.0–2.6) | 1.5 (1.10–1.80) | 1.35 (1.0–1.70) |
| Outcome, no (%) | |||||
| Discharged home, No ICU admission | 44 (88) | 13 (100) | 23 (95.8) | 7 (63.6) | 1 (50) |
| Discharged home, ICU admission | 4 (8) | 0 | 1 (4.2) | 3 (27.3) | 0 |
| Died | 2 (4) | 0 | 0 | 1 (9.1) | 1 (50) |
| Total | 50 (100) | 13 (100) | 24 (100) | 11 (100) | 2 (100) |
Normal ranges: TSH (0.4–4.2 Mu/l), FT4 (12–22 pmol/l), T3 (1.3–3.1 nmol/l).
3.2. Thyroid function assessment
TFTs were done 78 times in these 50 patients during their hospital stay. The mean TSH was 2.29 mU/l (IQ range 1.1–3.1) (normal range 0.4–4.2 mU/l), median FT4 14.6 pmol/l (IQ range 11.6–16.1) (normal range 12–22) and the median T3 1.5 nmol/l (IQ range 1.2–1.8) (normal range 1.3–3.1 nmol/l) (Table 1). Overall, TFTs were completely normal in all patients except for minor transient abnormalities in 5 patients (10 %) summarized in Table 2 . These include three patients who had mild transient elevated TSH, one had mild transient suppressed TSH and one patient had a mildly low FT4 with normal TSH (Table 2).
Table 2.
Demographic, clinical and laboratory data of five COVID-19 patients with abnormal thyroid function tests.
| Patient | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age in years/sex | 37/F | 48/M | 27/M | 58/M | 43/M |
| Severity score | A | A | B | B | C |
| WBC (×103/cc) | 6.93 | 4.13 | 7.50 | 4.9 | 4.68 |
| Neutrophils (%) | 65 | 56.7 | 58.6 | 62.3 | 41.3 |
| Lymphocytes (%) | 21.6 | 23.5 | 31.6 | 24.5 | 35.7 |
| CRP mg/l (<1) | 3.1 | 4.9 | 9.3 | 3.8 | >300 |
| Ferritin μg/l (13–150) | 32.5 | 330 | 106 | 124 | 277 |
| D-dimer μg/ml (0–50) | 0.87 | 0.29 | 0.59 | 0.27 | 1.91 |
| Baseline TFT | |||||
| Day of admission | 1 | 1 | 1 | 1 | 2 |
| TSH (0.4–4.2 mU/l) | 4.67 | 3.36 | 7.36 | 6.98 | 0.041 |
| FT4 (12–22 pmol/l) | 15.1 | 10.5 | 14.6 | 13.0 | 23.1 |
| T3 (1.3–3.1 nmol/l) | 1.8 | 1.5 | 2.1 | 2.9 | 1.2 |
| Follow up TFT | |||||
| Day of admission | 5 | 2 | 2 | ||
| TSH (2) | 3.84 | 4.65 | 5.5 | ||
| FT4 (2) | 16.7 | 14.7 | 13 | ||
| T3 (2) | 1.7 | 2.2 | 2.4 | ||
| Outcome | Recovery | Recovery | Recovery | Recovery | Recovery |
| Length of stay (days) | 13 | 8 | 8 | 7 | 28 |
3.3. Thyroid function tests and the severity of illness
Using ANOVA, there were no significant differences in the TSH (F 1.15, P 0.34), FT4 (F 1.72, P 0.18) or T3 (F 1.93, P 0.14) levels among patients with different severity of illnesses using the above mentioned severity score (A, B, C, D) (Table 1). There were also no differences in TSH (F 1.2, P 0.27), FT4 (F 2.04, P 0.13), and T3 (F 2.87, P 0.099) between those patients that were treated in the general ward and those who needed ICU admission.
3.4. Outcome and follow up thyroid function tests after discharge
Of the 50 patients included in this study, 44 patients (88 %) were treated in the general ward and did not need ICU admission. They all recovered and were discharged home. Six patients (12 %) needed ICU admission; 4 (8 %) recovered and were finally discharged home while 2 (4 %) died due to acute respiratory distress syndrome (ARDS) and multiorgan failure.
Of the initial 50 patients, 25 (50 %) had follow up TFTs at a mean duration of 6.0 ± 2.6 months after discharge (Fig. 1). There were no significant differences between admission and the follow up levels of TSH (2.5 ± 1.8 mU/l vs. 3.3 ± 4.3 mU/l, P 0.48), FT4 (14.8 ± 2.2 mU/l vs. 14.13 ± 14.13 ± 4.3 mU/l, P 0.71) and T3 (1.85 ± 0.13 nmol/l vs. 1.80 ± 1.02 nmol/l, P 0.92).
4. Discussion
There are many reports of thyroid dysfunction in patients with acute COVID-19 infection. Our study showed rare and mild changes in TFT. Only 10 % of cases had some minor abnormalities which frequently returned to normal over the next few days during admission. Our findings might be related to the selection of patients without known thyroid dysfunction prior to COVID-19 infection or might be due to the relatively small sample size. More likely is that thyroid dysfunction in COVID-19 is not common and frequently mild as this study and others (Khoo et al., 2021) have shown. One additional possibility is that thyroid dysfunction occurs later in the course of COVID-19 infection and evaluation for thyroid dysfunction was carried out early in this study. However, in our patients, follow up TFT in 50 % of patients were obtained at a median duration of 6 ± 2.6 months after discharge and were normal in all of them. Finally, the majority of our patients had mild to moderate severity COVID-19 infection and that may have been less likely associated with thyroid involvement.
Similar to our study, a study from China in which screening of 191 COVID-19 patients for abnormalities in thyroid function and thyroid autoimmunity at admission showed 13.1 % of them having mild abnormalities (Lui et al., 2020). In that study, lower TSH and free T3 were associated with more severe disease and low free T3 was associated with worse prognosis (Lui et al., 2020). In another study in which thyroid function abnormalities of 50 patients with recent COVID-19 infection were retrospectively reviewed and compared with normal control and patients with non-COVID19 pneumonia, 28 (56 %) of them had low TSH (Thyroid, 2021). TSH and total T3 were significantly lower in patients with COVID-19 infection than in control and patients with non-COVID-19 pneumonia. Low TSH and low total T3 correlated with the severity of the COVID-19 illness (Thyroid, 2021). In a study from England, all COVID-19 patients admitted to the hospital during a 6-week period were screened for thyroid function and compared with those admitted due to reasons other than COVID-19 (Khoo et al., 2021). They also compared thyroid function tests with previous results when available and with TFTs after recovery. The majority (86.6 %) of patients were euthyroid but TSH and FT4 were significantly lower in patients with COVID-19 than in non-COVID-19 patients and were also significantly lower than baseline levels (Khoo et al., 2021). At follow up, TSH returned to its baseline values. These changes are most likely due to non-thyroid sick syndrome (Khoo et al., 2021).
The pathophysiological mechanisms by which COVID-19 may affect thyroid gland (reviewed in Croce et al., 2021) include humoral effects related to the cytokine storm syndrome that leads to several effects on the hypothalamic pituitary thyroid axis, the thyroid hormone binding and the deiodinase function culminating in the well described non-thyroidal illness syndrome (Croce et al., 2021). However, there is no evidence of direct cytotoxic effect of cytokines on thyroid cells. On the other hand, COVID-19 may directly infect the thyroid and cause painful or painless thyroiditis. Finally, patients with COVID-19 infection frequently receive drugs that may alter TFT and confound their interpretation (Croce et al., 2021).
The main mechanism of entry of COVID-19 virus into cells is the attachment to the ACE2 and the transmembrane serine 2 (TMPRSS2) (Lam et al., 2020). These molecules are widely expressed in different tissues (Han et al., 2020; Lazartigues et al., 2020). Thyroid cells are rich in ACE2 (Rotondi et al., 2021) and this facilitates the entry of the virus into these cells with consequent direct injury or viral-induced immune-mediated injury (Gorini et al., 2020). The immune-mediated injury is triggered by several cells and cytokines including CD4+ and CD8+ T cells. T-helper 17 cells increase and T-regulatory 17:T-helper 17 ratio decrease. Several cytokines are increased including IL-6, IL2, IL-8, IL-17, IL-22, tumor necrosis factor, Interferon Gamma and alpha and colony stimulating factor. These cellular and humoral responses contribute to the severe inflammatory response called “cytokine storm” that is frequently associated with severe disease and acute respiratory distress syndrome (Swadling and Maini, 2020; Muyayalo et al., 2020; Moore and June, 2020; Zhang et al., 2020).
COVID-19 infection has been associated with different forms of thyroid dysfunction. Several case reports of subacute thyroiditis have been reported, occurring usually 2–7 weeks after the onset of COVID-19 infection and showing an excellent response to glucocorticoid therapy (Brancatella et al., 2020b; Campos-Barrera et al., 2020; Mattar et al., 2020; Asfuroglu Kalkan and Ates, 2020; Brancatella et al., 2020c; Chakraborty et al., 2020; Ippolito et al., 2020; Ruggeri et al., 2021). We have not seen any case of subacute thyroiditis in this study but that might be related to the early presentation of those patients after the onset of symptoms. We have follow up data beyond the duration of admission in 50 % of patients and we did not see evidence of thyroiditis in any of them. However, we cannot exclude this possibility between the time of discharge and time of obtaining follow up TFTs with complete recovery of overt or silent thyroiditis. However, this possibility seems unlikely given the normal TFTs in all patients tested during follow up.
In addition to subacute thyroiditis, which is likely due to direct viral damage of the thyroid cells, chronic autoimmune (Hashimoto's type) thyroiditis with hypothyroidism has been described in patients infected recently with COVID-19 (Lui et al., 2020; Tee et al., 2021; Caron, 2020). Most of these cases had pre existing autoimmune markers and it is thought that COVID-19 triggered or reactivated autoimmune responses leading to further autoimmune-mediated damage and clinically overt hypothyroidism.
The euthyroid sick syndrome is not specific for COVID-19 infection but has been frequently reported in patients with this infection (Somasundaram et al., 2020; Henry et al., 2020). Patients with COVID-19 have high IL-6 levels (Henry et al., 2020) and this is implicated in the pathogenesis of euthyroid sick syndrome, also called, low T3 syndrome and nonthyroidal illness syndrome (Davies et al., 1996; Yamazaki et al., 1996).
Thyrotoxicosis was also reported to be common in COVID-19 infection. In a retrospective study of 287 patients admitted at a single center in Italy, 58 patients (20.2 %) had thyrotoxicosis, 15 patients (5.2 %) had hypothyroidism and 214 patients (74.6 %) had normal thyroid function tests. Thyrotoxicosis was associated with elevated levels of IL-6 (Lania et al., 2020).
All of these studies indicate inconsistent data and widely variable rates of thyroid dysfunction indicating the need for further studies to define the rates, type and course and outcome of thyroid dysfunction in COVID-19 infection and its impact the disease.
Although this study adds to the evolving knowledge of thyroid function in COVID-19, it has several pitfalls including the small sample size, heterogeneity in frequency and timing of TFT measurements, the small number of patients with severe disease, lack of data on ultrasound, thyroid scans, thyroid autoantibodies and lack of disease severity markers in the majority of patients. Although this was a prospective study with a specific protocol, it was undertaken at the early time of the COVID-19 pandemic (May 2020) and the study protocol could not be applied fully due to some logistic issues related to the anxiety and concern about safety of the staff and refusal of some patients of repeated testing. The majority of patients in this study had mild to moderate illness (disease severity score A and B) and only about a quarter of this cohort had severe disease. Therefore, it is not surprising that TFT abnormalities were rare. However, in general, the vast majority of patients with COVID-19 infection have relatively mild to moderate illness and the spectrum of patients included in our cohort is probably representative of the usual spectrum of COVID-19 infection in general. Considering all of these shortcomings, this study may not be adequately powered to detect an association between severity of COVID-19 and thyroid dysfunction and further studies are needed to assess this issue.
In summary, in this study TFTs were only rarely, transiently and mildly abnormal in COVID-19 infection. Whether these mild abnormalities are direct consequences of COVID-19 itself, manifestations of sick euthyroid syndrome or unrelated to the illness is unclear. There is no suggestion of correlation between the severity of the illness or outcome and thyroid function. There is also no suggestion of late-onset sequel of COVID-19 on thyroid function.
CRediT authorship contribution statement
Noha Mukhtar: Obtained consent, enrolled patients, obtained blood tests, collected data and supervised the study undertaking
Abdulmohsin Bakhsh: Obtained consent, enrolled patients, obtained blood tests, collected data
Nahlah Alreshidi: Obtained consent, enrolled patients, obtained blood tests and collected data
Abeer Aljomaiah: Obtained consent, enrolled patients, obtained blood tests and collected data
Hadeel Aljamei: Obtained consent, enrolled patients, obtained blood tests and collected data
Nada Alsudani: Obtained consent, enrolled patients, obtained blood tests and collected data
Tarek Elsayed: Obtained consent, enrolled patients, obtained blood tests and collected data
Roqayh Fadel: Obtained consent, enrolled patients, obtained blood tests and collected data
Eman Alqahtani: Obtained consent, enrolled patients, obtained blood tests and collected data
Ali S. Alzahrani: Conceived the research idea, designed the study, supervised the study conduct, analyzed the data and wrote the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We would like to thank the patients and their relatives for agreeing to enrol in this study and our colleagues who looked after these patients during their admission and helped us obtain consents and blood testing.
Funding
No specific funding was allocated for this study.
Footnotes
Peer Review Summary
Peer Review Summary and Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.endmts.2022.100122.
Appendix Peer Review Summary
Supplementary material 1: Data collection sheet
Supplementary material 2: Proposal for studying the impact of COVID-19 on the Endocrine System
Supplementary material 3
Data availability
Data will be made available on request.
References
- Asfuroglu Kalkan E., Ates I. A case of subacute thyroiditis associated with COVID-19 infection. J. Endocrinol. Investig. 2020;43:1173–1174. doi: 10.1007/s40618-020-01316-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancatella A., Ricci D., Viola N., Sgrò D., Santini F., Latrofa F. Subacute thyroiditis after sars-COV-2 infection. J. Clin. Endocrinol. Metab. 2020;105 doi: 10.1210/clinem/dgaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancatella A., Ricci D., Viola N., Sgrò D., Santini F., Latrofa F. Subacute thyroiditis after SARS-CoV-2 infection. J. Clin. Endocrinol. Metab. 2020;105:2367–2370. doi: 10.1210/clinem/dgaa276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancatella A., Ricci D., Cappellani D., Viola N., Sgrò D., Santini F., Latrofa F. Is subacute thyroiditis an underestimated manifestation of SARS-CoV-2 infection? Insights from a case series. J. Clin. Endocrinol. Metab. 2020;105 doi: 10.1210/clinem/dgaa537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi I., Bulgarelli I., Dubini A., Perego G.B., Tortorici E., Torlasco C., Torresani E., Rocco L., Persani L., Fugazzola L. The spectrum of thyroid function tests during hospitalization for SARS COV-2 infection. Eur. J. Endocrinol. 2021;184(5):699–709. doi: 10.1530/EJE-20-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos-Barrera E., Alvarez-Cisneros T., Davalos-Fuentes M. Subacute thyroiditis associated with COVID-19. Case Rep. Endocrinol. 2020;2020 doi: 10.1155/2020/8891539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron P. Thyroid disorders and SARS-CoV-2 infection: from pathophysiological mechanism to patient management. Ann. Endocrinol. 2020;81:507–510. doi: 10.1016/j.ando.2020.09.001. (Paris) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty U., Ghosh S., Chandra A., Ray A.K. Subacute thyroiditis as a presenting manifestation of COVID-19: a report of an exceedingly rare clinical entity. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-239953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce L., Gangemi D., Ancona G., Liboà F., Bendotti G., Minelli L., Chiovato L. The cytokine storm and thyroid hormone changes in COVID-19. J. Endocrinol. Investig. 2021;44:891–904. doi: 10.1007/s40618-021-01506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P.H., Black E.G., Sheppard M.C., Franklyn J.A. Relation between serum interleukin-6 and thyroid hormone concentrations in 270 hospital in-patients with non-thyroidal illness. Clin. Endocrinol. 1996;44:199–205. doi: 10.1046/j.1365-2265.1996.668489.x. [DOI] [PubMed] [Google Scholar]
- Gorini F., Bianchi F., Iervasi G. COVID-19 and thyroid: progress and prospects. Int. J. Environ. Res. Public Health. 2020;17 doi: 10.3390/ijerph17186630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T., Kang J., Li G., Ge J., Gu J. Analysis of 2019-nCoV receptor ACE2 expression in different tissues and its significance study. Ann. Transl. Med. 2020;8:1077. doi: 10.21037/atm-20-4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- Ippolito S., Dentali F., Tanda M.L. SARS-CoV-2: a potential trigger for subacute thyroiditis? Insights from a case report. J. Endocrinol. Investig. 2020;43:1171–1172. doi: 10.1007/s40618-020-01312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri A., Charlap E., Kim A. Subacute thyroiditis from COVID-19 infection: a case report and review of literature. Eur. Thyroid J. 2021;9:324–328. doi: 10.1159/000511872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo B., Tan T., Clarke S.A., Mills E.G., Patel B., Modi M., Phylactou M., Eng P.C., Thurston L., Alexander E.C., Meeran K., Comninos A.N., Abbara A., Dhillo W.S. Thyroid function before, during, and after COVID-19. J. Clin. Endocrinol. Metab. 2021;106:e803–e811. doi: 10.1210/clinem/dgaa830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam S.D., Bordin N., Waman V.P., Scholes H.M., Ashford P., Sen N., van Dorp L., Rauer C., Dawson N.L., Pang C.S.M., Abbasian M., Sillitoe I., Edwards S.J.L., Fraternali F., Lees J.G., Santini J.M., Orengo C.A. SARS-CoV-2 spike protein predicted to form complexes with host receptor protein orthologues from a broad range of mammals. Sci. Rep. 2020;10:16471. doi: 10.1038/s41598-020-71936-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lania A., Sandri M.T., Cellini M., Mirani M., Lavezzi E., Mazziotti G. Thyrotoxicosis in patients with COVID-19: the THYRCOV study. Eur. J. Endocrinol. 2020;183:381–387. doi: 10.1530/EJE-20-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurino A., Gencarelli M., Buci L., Raimondi L. Commentary: euthyroid sick syndrome in patients with COVID-19. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.633097. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazartigues E., Qadir M.M.F., Mauvais-Jarvis F. Endocrine significance of SARS-CoV-2's reliance on ACE2. Endocrinology. 2020;161 doi: 10.1210/endocr/bqaa108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui D.T.W., Lee C.H., Chow W.S., Lee A.C.H., Tam A.R., Fong C.H.Y., Law C.Y., Leung E.K.H., To K.K.W., Tan K.C.B., Woo Y.C., Lam C.W., Hung I.F.N., Lam K.S.L. Thyroid dysfunction in relation to immune profile, disease status, and outcome in 191 patients with COVID-19. J. Clin. Endocrinol. Metab. 2020;106:e926–e935. doi: 10.1210/clinem/dgaa813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar S.A.M., Koh S.J.Q., Rama Chandran S., Cherng B.P.Z. Subacute thyroiditis associated with COVID-19. BMJ Case Rep. 2020;13 doi: 10.1136/bcr-2020-237336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science. 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- Muyayalo K.P., Huang D.-H., Zhao S.-J., Xie T., Mor G., Liao A.-H. COVID-19 and Treg/Th17 imbalance: potential relationship to pregnancy outcomes. Am. J. Reprod. Immunol. 2020;84 doi: 10.1111/aji.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevention CfDCCa Clinical care considerations: clinical considerations for care of children and adults with confirmed COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/clinical-considerations-index.html
- Rotondi M., Coperchini F., Ricci G., Denegri M., Croce L., Ngnitejeu S.T., Villani L., Magri F., Latrofa F., Chiovato L. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. J. Endocrinol. Investig. 2021;44:1085–1090. doi: 10.1007/s40618-020-01436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri R.M., Campennì A., Siracusa M., Frazzetto G., Gullo D. Subacute thyroiditis in a patient infected with SARS-COV-2: an endocrine complication linked to the COVID-19 pandemic. Hormones. 2021;20:219–221. doi: 10.1007/s42000-020-00230-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasundaram N.P., Ranathunga I., Ratnasamy V., Wijewickrama P.S.A., Dissanayake H.A., Yogendranathan N., Gamage K.K.K., de Silva N.L., Sumanatilleke M., Katulanda P., Grossman A.B. The impact of SARS-cov-2 virus infection on the endocrine system. J. Endocr. Soc. 2020;4 doi: 10.1210/jendso/bvaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadling L., Maini M.K. T cells in COVID-19 — united in diversity. Nat. Immunol. 2020;21:1307–1308. doi: 10.1038/s41590-020-0798-y. [DOI] [PubMed] [Google Scholar]
- Tee L.Y., Harjanto S., Rosario B.H. COVID-19 complicated by Hashimoto's thyroiditis. Singap. Med. J. 2021;62(5):265. doi: 10.11622/smedj.2020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Su X., Ding Y., Fan W., Zhou W., Su J., Chen Z., Zhao H., Xu K., Ni Q., Xu X., Qiu Y., Teng L. Thyroid function abnormalities in COVID-19 patients. Front. Endocrinol. 2020;11 doi: 10.3389/fendo.2020.623792. (Lausanne) [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO WHO. COVID-19 clinical care pathway. https://www.who.int/tools/covid-19-clinical-care-pathway
- Yamazaki K., Yamada E., Kanaji Y., Shizume K., Wang D.S., Maruo N., Obara T., Sato K. Interleukin-6 (IL-6) inhibits thyroid function in the presence of soluble IL-6 receptor in cultured human thyroid follicles. Endocrinology. 1996;137:4857–4863. doi: 10.1210/endo.137.11.8895357. [DOI] [PubMed] [Google Scholar]
- Zhang X., Tan Y., Ling Y., Lu G., Liu F., Yi Z., Jia X., Wu M., Shi B., Xu S., Chen J., Wang W., Chen B., Jiang L., Yu S., Lu J., Wang J., Xu M., Yuan Z., Zhang Q., Zhang X., Zhao G., Wang S., Chen S., Lu H. Viral and host factors related to the clinical outcome of COVID-19. Nature. 2020;583:437–440. doi: 10.1038/s41586-020-2355-0. [DOI] [PubMed] [Google Scholar]
- Thyroid function analysis in 50 patients with COVID-19: a retrospective studyThyroid. 2021;31:8–11. doi: 10.1089/thy.2020.0363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1: Data collection sheet
Supplementary material 2: Proposal for studying the impact of COVID-19 on the Endocrine System
Supplementary material 3
Data Availability Statement
Data will be made available on request.