Abstract
Thromboembolism is a major cause of death in patients who suffer from COVID-19. Studies examining the effects of aspirin (ASA) on mortality relating to this phenomenon have showed conflicting results with varying degrees and certainties of evidence. We performed an aggregate data meta-analysis of fourteen studies encompassing 164,539 COVID-19 patients, which showed a reduced risk of in-hospital mortality associated with ASA use in eight studies that reported risk ratios (RR 0.90; 95 % CI 0.82–0.98; I2 = 27.33 %, P = 0.01), six studies that reported hazard ratios (HR 0.56; 95 % CI 0.41–0.76, P ≤ 0.01; I2 = 85.92 %) and pooled effect size (0.71; 95 % CI 0.59–0.85, P = 0.00, I2 = 91.51 %). The objective of this study is to report the association between low dose ASA and a reduced risk of in-hospital mortality in patients with COVID-19.
Keywords: Aspirin, COVID-19, Mortality, Anti-thrombotic therapy, Thromboembolism
1. Introduction
Coronavirus disease 2019 (COVID-19) is associated with an increased risk of arterial and venous thrombosis through a proposed mechanism involving endothelial inflammation, thrombin and platelet recruitment, and complement activation [1]. Pulmonary microvascular thrombosis and venous thromboembolism have been associated with increased mortality in patients with COVID-19, although optimal inpatient therapy for these complications has yet to be well-defined [2]. Acetylsalicylic acid (ASA), or aspirin – an affordable, widely available anti-inflammatory, anticoagulant, and antithrombotic agent – has been proposed to be potentially protective among hospitalized COVID-19 patients given these thrombotic complications [3]. Prior data addressing this therapy has been derived from a small number of studies with conflicting results which makes it hard to draw robust conclusions. We performed aggregated data to study the association between pre-hospital and in-hospital administration of ASA among COVID-19 patients and mortality.
2. Materials and methods
Using PubMed, Google Scholar, OVID Medline, and the Cochrane Library, we performed a literature search for all peer-reviewed and published studies until March 30th, 2022 pertaining to the use of ASA in COVID-19 patients. The terms used in the search criteria were: Aspirin AND COVID-19 OR Aspirin AND SARS-CoV-2 OR Acetylsalicylic acid AND COVID-19 OR Acetylsalicylic acid AND SARS-CoV-2. Studies were assessed using the PRISMA guideline (Fig. S1). Two separate researchers (AS and JB) conducted the search and screening of the articles. The detailed search strategy is outlined in the supplemental materials. Inclusion criteria included clinical trials or observational studies, hospitalized adults (age ≥18) positive for SARS-CoV-2 with confirmed reverse transcriptase-polymerase chain reaction test, and use of ASA prior to hospitalization or during admission. Our outcome of interest was all-cause mortality among ASA users which could be calculated and compared with that among non-ASA users.
The quality of studies was assessed by calculating the Newcastle-Ottawa scale (NOS) for observational studies and JADAD score for RCTs (Table S1). Studies with NOS ≥ 7 and JADAD score ≥ 4 were included (Table S1). Discrepancies in the score were resolved by discussion. Table S1 shows the extracted data outlining the characteristics of included studies. Fourteen studies were included in the meta-analysis with a total of 164,539 COVID-19 patients. 30,438 patients were in the ASA cohort, and 134,101 patients were in non-ASA cohort. Twelve studies included in this meta-analysis were retrospective cohort studies, one study had a cross-sectional design, and one was a randomized controlled trial (RCT). The mean age of patients ranged from 18 to 81 years old.
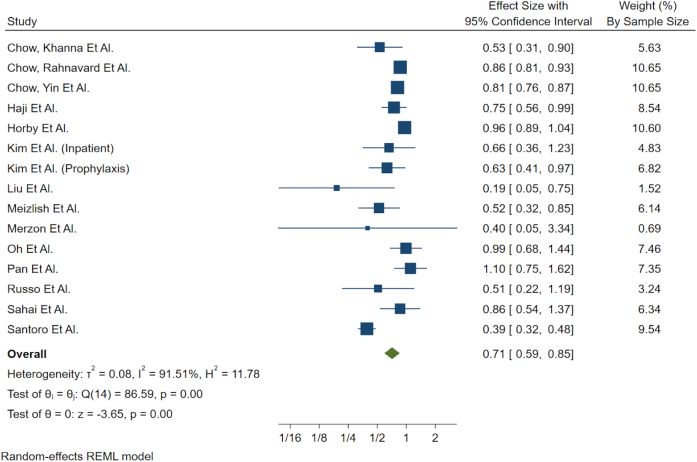
The effect of ASA on mortality was assessed by pooling the odds ratio (OR), risk ratio (RR), and hazard ratio (HR) between the ASA and non-ASA group. Studies were analyzed using a Random Effects Model, specifically Restricted Maximum Likelihood as the heterogeneity estimation method within Stata 17.0, which allows for significant heterogeneity between studies while still pooling. Studies that reported OR were converted to RR, and heterogeneity between these two groups was tested for conformity before grouping into a single stratum. This stratum was compared to studies utilizing a HR for effect size. Heterogeneity between the strata was present and was expected (Fig. 1). Statistical heterogeneity between studies was evaluated using the I2 statistic as a measure of variability. In addition, variables of clinical suspicion such as age and sex ratio were investigated for the source of heterogeneity using mixed effect meta-regression (Table S4). Heterogeneity testing was conducted to compare daily prophylactic dose and inpatient ASA administration. We also conducted heterogeneity testing to compare studies included and not included in prior meta-analyses (Table 1).
Fig. 1.
Forest plot showing pooled two way measure of effect using random effects model.
Table 1.
Heterogeneity test for timing of ASA administration (inpatient versus prophylaxis) showed no difference in mortality. Heterogeneity test for studies included and not included in prior meta-analyses showed no difference in mortality.
| P-Value | |
|---|---|
| Heterogeneity Test for timing (inpatient vs prophylactic) | |
| Risk ratio | 0.16 |
| Hazard ratio | 0.19 |
| Heterogeneity test for previous inclusion (yes vs no) | |
| Risk ratio | 0.14 |
| Hazard ratio | 0.44 |
3. Results
4. Discussion
Based on six studies which measured HR, the association of ASA use with reduced mortality was statistically significant (HR 0.56; 95 % CI 0.41–0.76; I2 = 85.92 %, P ≤ 0.01). Based on eight studies that measured RR, the association of ASA use with reduced mortality was also statistically significant (RR 0.90; 95 % CI 0.82–0.98; I2 = 27.33 %, P = 0.01). The pooled effect size showed reduced mortality was both statistically and clinically significant (0.71; 95 % CI 0.59–0.85, P = 0.00, I2 = 91.51 %). Heterogeneity testing for inpatient versus prophylactic ASA use showed no difference (P = 0.16 for RR; P = 0.19 for HR; Table 1). Heterogeneity testing showed no difference between studies included or not included in prior meta-analyses (P = 0.14 for RR; P = 0.44 for HR; Table 1). ASA use was relatively homogeneous, with most studies reporting ASA doses between 80 and 150 mg. The duration of ASA use was unspecified in several studies, ranging from a minimum of 7 days to several months. ASA users had higher rates of hypertension, diabetes mellitus, coronary artery disease and renal disease.
Each study was relatively equally weighted (Fig. 1) with the exception of studies from Horby et al. [4] (weight 10.60 %), Chow, Rahnavard et al. [5] (weight 10.65 %) and Chow, Yin et al. [6] (weight 10.65 %). The two largest cohort studies from Chow et al. reported decreased mortality associated with inpatient ASA use, with subgroup analysis demonstrating a greater benefit among elderly patients and those with additional comorbidities [5], [7]. The decreased mortality associated with ASA can be explained by its mechanism of action. ASA acts by inhibiting platelet function through irreversible inhibition of COX activity. It additionally reduces levels of C-Reactive Protein and interleukin-6, which helps mitigate cytokine storm [8], [9]. ASA also lowers serum fibrinogen levels through fibrinogen acetylation and fibrinolysis, which consequently decreases the risk of thrombotic events [7]. Observational studies in the literature show conflicting clinical outcomes, with the majority demonstrating an association between ASA use and decreased mortality.
Included in this meta-analysis, the RECOVERY RCT examined almost 15,000 patients with COVID-19 and found no significant association between ASA use and mortality [4]. However, this open-label RCT's sample was limited to patients who received ASA after hospitalization, whereas our meta-analysis also included patients who received ASA therapy prior to hospitalization. Notably, our meta-analysis found that ASA therapy significantly decreases mortality among hospitalized COVID-19 patients and found no difference in outcomes regardless of whether a patient received ASA inpatient or pre-hospital prophylactic use. Even within its limited sample, RCT reported a small increase in the rate (4 %) of being discharged alive within 28 days among patients who were given ASA after hospitalization.
In regards to hemorrhagic complications, four studies reported no difference in major bleeding between ASA and non-ASA users [5], [6], [7], [10]. The RECOVERY trial reported a 0.6 % absolute increase in major bleeding events in ASA group. Patients on ASA for cardiovascular disease were excluded. 150 mg of ASA was administered in the trial, whereas the median ASA dose was 81 mg in the four studies that reported no difference in major bleeding [5], [6], [7], [10]. Additionally, six studies reported thromboembolic events and the weighted mean in ASA users was 2.95 % [95 % CI 2.95–2.96] compared to 1.62 % [1.59–1.65] in non-ASA group (Table 2). Patients were either initiated on ASA within 24 h of admission, or were on chronic therapy for secondary prevention. This suggests that patients on long-term ASA for COVID-19 may be at a higher baseline risk for thromboembolic events. However, it is important to note that the raw average percentages can be misleading as these are not regression adjusted numbers. More studies are needed to confirm the role of thromboembolic events in mortality among COVID-19 patients.
Table 2.
Mean thromboembolic events in ASA and non-ASA users.
| Mean | 95 % confidence interval | ||
|---|---|---|---|
| Average percent of aspirin patients with thrombotic events | 2.95 | 2.95 | 2.96 |
| Average percent of non-aspirin patients with thrombotic events | 1.62 | 1.59 | 1.65 |
Our meta-analysis could not determine the optimal duration of antiplatelet therapy for COVID-19 due to limited data. There was difficulty in capturing ASA in electronic medical records prior to admission as ASA is an over-the-counter medication requiring no prescription. There was also insufficient data available to be able to compare the use of dual antiplatelet therapy to ASA monotherapy alone. The findings in our meta-analysis are not generalizable to the outpatient setting, as the data available addressed inpatient mortality only. Limitations stemming from the observational nature of included studies were addressed through qualitative assessment using the NOS scale. Asymmetry in the funnel plot was indicative of potential publication bias (Fig. 3S). In addition, variations in the baseline risk of death among studies could not be accounted for, therefore potentially leading to a difference in treatment effect.
In the HR studies, intra-strata heterogeneity was present, which was expected given the small number of studies within the HR group. Leave-one-out analysis (Fig. S1) was done on the HR group to help determine the source of heterogeneity, as well as sensitivity analysis for both strata across two factors considered for possible sources of differences. No source of heterogeneity was discovered, and with the use of a random effects model, and small number of studies used, and the I2 statistic bias, the confidence interval for the pooled effect size should be the most important metric. Regardless of which study was removed from the leave-one-out analysis (Fig. S2), the effect size confidence interval remained significant.
5. Conclusion
Overall, the use of ASA was associated with a protective effect, showing decreased inpatient mortality in patients hospitalized with COVID-19 infection. This is likely due to anti-inflammatory and anti-thrombotic effects. Further RCTs can help elucidate the benefits and risks of using ASA in conjunction with anticoagulant and antiplatelet medications for COVID-19 patients. Additional data can also explore the optimal length and time to initiation of ASA for these patients.
Funding
None.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ahjo.2022.100191.
Appendix A. Supplementary data
Supplementary material
References
- 1.McFadyen J.D., Stevens H., Peter K. The emerging threat of (Micro)Thrombosis in COVID-19 and its therapeutic implications. Circ. Res. 2020;127(4):571–587. doi: 10.1161/CIRCRESAHA.120.317447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sayed Ahmed H.A., Merrell E., Ismail M., et al. Rationales and uncertainties for aspirin use in COVID-19: a narrative review. Fam. Med. Community Health. 2021;9(2) doi: 10.1136/fmch-2020-000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.RECOVERY Collaborative Group Aspirin in patients admitted to hospital with COVID-19 (RECOVERY): randomized, controlled, open-label, platform trial. Lancet. 2022 Jan 8;399(10320):143–151. doi: 10.1016/S0140-6736(21)01825-0. Epub 2021 Nov 17. PMID: 34800427; PMCID: PMC8598213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow J.H., Rahnavard A., Gomberg-Maitland M., et al. N3C consortium and ANCHOR investigators. Association of Early Aspirin use with in-hospital mortality in patients with moderate COVID-19. JAMA Netw. Open. 2022 Mar 1;5(3) doi: 10.1001/jamanetworkopen.2022.3890. PMID: 35323950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow J.H., Yin Y., Yamane D.P., et al. Association of prehospital antiplatelet therapy with survival in patients hospitalized with COVID-19: a propensity score-matched analysis. J. Thromb. Haemost. 2021;19(11):2814–2824. doi: 10.1111/jth.15517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow J.H., Khanna A.K., Kethirddy S., et al. Aspirin use is associated with decreased mechanical ventilation, intensive care unit admission, and in-hospital mortality in hospitalized patients with coronavirus disease 2019. Anesth. Analg. 2021;132:930–941. doi: 10.1213/ANE.0000000000005292. [DOI] [PubMed] [Google Scholar]
- 8.Bianconi V., Violi F., Fallarino F., et al. Is acetylsalicylic acid a safe and potentially useful choice for adult patients with COVID-19? Drugs. 2020;80:1383–1396. doi: 10.1007/s40265-020-01365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santoro F., Nuñez-Gil I.J., Vitale E., et al. Antiplatelet therapy and outcome in COVID-19: the health outcome predictive evaluation registry. Heart. 2022;108(2):130–136. doi: 10.1136/heartjnl-2021-319552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material