Abstract
Objectives
To investigate the prevalence of post coronavirus disease (COVID-19) condition of the Omicron variant in comparison to other strains.
Study design
A single-center cross-sectional study.
Methods
Patients who recovered from Omicron COVID-19 infection (Omicron group) were interviewed via telephone, and patients infected with other strains (control group) were surveyed via a self-reporting questionnaire. Data on patients’ characteristics, information regarding the acute-phase COVID-19, as well as presence and duration of COVID-19-related symptoms were obtained. Post COVID-19 condition in this study was defined as a symptom that lasted for at least 2 months, within 3 months of COVID-19 onset. We investigated and compared the prevalence of post COVID-19 condition in both groups after performing propensity score matching.
Results
We conducted interviews for 53 out of 128 patients with Omicron and obtained 502 responses in the control group. After matching cases with controls, 18 patients from both groups had improved covariate balance of the factors: older adult, female sex, obesity, and vaccination status. There were no significant differences in the prevalence of each post COVID-19 condition between the two groups. The number of patients with at least one post COVID-19 condition in the Omicron and control groups were 1 (5.6%) and 10 (55.6%) (p = 0.003), respectively.
Conclusions
The prevalence of post Omicron COVID-19 conditions was less than that of the other strains. Further research with a larger sample size is needed to investigate the precise epidemiology of post COVID-19 condition of Omicron, and its impact on health-related quality of life and social productivity.
Keywords: Post COVID-19 conditions, Long COVID, Omicron, WHO definition
Abbreviations
- COVID-19
coronavirus disease
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- NCGM
National Center for Global Health and Medicine;
- BMI
body mass index
- IQR
interquartile range
- PSM
propensity score matching
- SMD
standardized mean difference
1. Introduction
Coronavirus disease (COVID-19) has become a global pandemic with 513 million cumulative cases and 6.2 million deaths worldwide as of May 4, 2022 [1]. Following the onset of COVID-19, 49% of patients had at least one prolonged symptom, lasting more than 12 months [2]. These post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are known as the post COVID-19 condition or long COVID [3,4]. Common symptoms of the post COVID-19 condition include fatigue, shortness of breath, dysosmia, hair loss, and cognitive impairment, with overlap and persistence of multiple symptoms [2,5,6].
A prospective longitudinal, observational study revealed the difference in the prevalence of acute-phase symptoms in patients with Omicron and Delta variants of SARS-CoV-2 [7]. In particular, sore throat was more common in Omicron than in Delta, while loss of smell was infrequent in Omicron compared to Delta. However, no epidemiological data on post COVID-19 condition of Omicron has been reported yet. Considering that many patients were infected with Omicron variant worldwide [8,9], several patients may suffer from post COVID-19 condition due to Omicron. In this study, we aimed to investigate and compare the prevalence of the post COVID-19 condition of Omicron with that of other strains.
2. Materials and methods
This study was a single-center, cross-sectional study, using telephone interviews and a self-reporting questionnaire survey for the Omicron and control group, respectively. The patients in the telephone and self-reported survey provided oral and written informed consent, respectively. This study was reviewed and approved by the ethics committee of the Center Hospital of the National Center for Global Health and Medicine (NCGM) (NCGM-G-004406-00, NCGM-G-004439-00).
2.1. Omicron group
Patients who were admitted due to COVID-19 at the NCGM between December 1, 2021, and February 9, 2022, were invited for telephone interviews. The patients were positive for SARS-CoV-2 E484A mutation, and negative for L452R mutation, as confirmed by the reverse transcription-polymerase chain reaction. Patients who died during admission were excluded from the study. The telephone interviews were conducted from April 14, 2022, to April 26, 2022, after confirming that 3 months had passed since the onset of COVID-19 symptoms for each patient. The investigators (M Suzuki, M Sanada, MA, YO, MT, KT, CK, TM) administered one-on-one structured telephone interviews with the patients using an interview guide (Supplement 1). The interviews lasted for 5–10 min, and each patient was interviewed only once. The investigators were nurses, researchers, and physicians working in the NCGM. In the individual telephone interviews, the participants were questioned regarding their symptoms and their duration, and were recorded by checking the electronic files. If the participants were unable to recall the symptom duration, the answers were regarded as missing values.
2.2. Control group
The participants recruited in the control group were patients who recovered from COVID-19, and who visited the outpatient service of the Disease Control and Prevention Center in the NCGM between February 2020 and November 2021 to undergo a pre-donation screening test for COVID-19 convalescent plasmapheresis [6]. All participants in this study were Japanese since the screening test was designed only for Japanese patients. Most of the participants had received acute-phase treatment for COVID-19 in other hospitals. A self-reporting, online or paper-based questionnaire was sent to eligible patients in February 2022, without any reminders (Supplement 2). Participation in this survey was voluntary, but not anonymous. The participants were requested to complete and return the questionnaire.
We developed the questionnaire based on previous similar studies [2,5,6,[10], [11], [12], [13], [14], [15]], including findings from our previous work on prolonged and late-onset symptoms of COVID-19 [16]. We attempted to minimize the number of questions required to maximize the response rate. Six non-medical NCGM employees were included in the pilot testing. They provided feedback on the content, clarity, and format of the items, as well as whether the survey questions were self-explanatory. Revisions were made on the questionnaire according to their feedback.
2.3. Items investigated
Information obtained included age, sex, ethnicity, smoking history, body mass index (BMI), underlying medical conditions, COVID-19 vaccination status, details on acute phase of COVID-19, as well as presence and duration of symptoms of post COVID-19 condition. Disease severity based on previous literature [2,12] was categorized as follows: 1) mild, no oxygen therapy; 2) moderate, oxygen therapy without mechanical ventilation; and 3) severe, mechanical ventilation with or without extracorporeal membrane oxygenation.
2.4. Definition of post COVID-19 condition
The definition of post COVID-19 condition in this study was a symptom that persisted for at least 2 months within 3 months of COVID-19 onset. The post COVID-19 condition included fatigue, shortness of breath, cough, dysosmia (including anosmia), dysgeusia (including ageusia), hair loss, depressed mood, brain fog, loss of concentration, and memory disturbance. Since this study did not include patient consultations, it could not be ascertained whether the symptoms were due to COVID-19 or an alternative diagnosis [3].
2.5. Statistical analysis
The patient characteristics, presence of pneumonia, disease severity, and treatment in the acute phase of COVID-19 were expressed as the median and interquartile range (IQR) for continuous variables, and as the absolute value (n) with percentage (%) for categorical variables.
A multivariable logistic regression model was developed to estimate a propensity score for being in the Omicron group. Age (older adult), sex (female), BMI ≥25, and vaccination status (vaccinated at least twice at the time of the survey) were included in the model, as the four factors were associated with increased risk of post COVID-19 condition [15,17]. Propensity score matching (PSM) was performed using the nearest neighbor matching with a caliper width of 0.2 [18]. The standardized difference was used to measure covariate balance, and an absolute standardized difference above 10% was interpreted as a meaningful imbalance. Subsequently, the proportion of patients with prolonged symptoms, and those with symptoms lasting at least 2 months within 3 months of onset have been described among the matched population. In addition, we estimated the average treatment effect (ATE) of Omicron infection using inverse-probability weighting (IPW) method. We calculated the weight of each case as the inverse of propensity score we used in the matching method. To avoid excessive weighting, the maximum weight for each case was set to 10. The ATE was estimated by linear regression analysis using presenting post COVID-19 condition as the outcome and infection of Omicron as the explanatory variable.
The level of significance in all statistical tests was set at α = 0.05. Data were analyzed using SPSS® Statistics version 25.0 software (IBM®, Armonk, NY, USA) and R, version 4.1.3 (R Foundation for Statistical Computing; 2018, Vienna, Austria).
3. Results
3.1. Omicron group
A total of 128 patients were potentially eligible for telephone interviews, one of whom died after discharge. Four opted not to participate, while interviews could not be conducted for 22 patients due to medical conditions such as dementia, and in one patient due to language barrier. Meanwhile, 47 patients could not be contacted via telephone. Overall, 53 patients were included and completed the interviews.
3.2. Control group
A self-reporting questionnaire was sent to 1148 patients (958 online; 190 paper-based) who had recovered from COVID-19. Of these, 502 responses (413 online; 89 paper-based) were obtained. The overall response rate was 43.7% (43.1% online; 46.8% paper-based). Among the 502 patients, 133 (31.5%), 205 (48.6%), and 84 (19.9%) (80 missing) got infected with COVID-19 between February 2020 and October 2020, November 2020 and June 2021 (Alpha strains predominant), and July 2021 and October 2021 (Delta strains predominant), respectively [8,19].
3.3. Characteristics of the participants in the Omicron and the control group before and after PSM
The characteristics of the participants in the Omicron and control groups before and after PSM are summarized in Table 1 and Table 2 , respectively. Before matching, the mean ages (years, IQR) and mean BMIs (IQR) in the Omicron and control groups were 56.0 (35.0, 69.5) and 48.0 (42.0, 55.0), and 24.4 (22.2, 26.8) and 23.1 (20.7, 25.9), respectively. Fourteen patients (26.4%) in the Omicron group and 300 patients (59.8%) in the control group were women. Forty-five patients (84.9%) in the Omicron group and 11 patients (2.2%) in the control group tested positive for SARS-CoV-2 at least seven days after their second COVID-19 vaccination when immunity had developed [20]. Forty-two patients (79.2%) in the Omicron group and 393 patients (78.3%) in the control group had mild disease. Missing values ranged from 0% to 15.5%.
Table 1.
Characteristics of the participants in the Omicron and control groups before propensity score matching.
| Characteristics | Omicron group |
Control group |
SMD | p-valuea |
|---|---|---|---|---|
| (n = 53) | (n = 502) | |||
| Age (years), median (IQR) | 56.0 (35.0, 69.5) | 48.0 (42.0, 55.0) | 0.011 | |
| Older adultb, No. (%) | 17 (32.1)c | 24 (4.8) | 0.767 | <0.001 |
| Female sex, No. (%) | 14 (26.4) | 300 (59.8) | 0.714 | <0.001 |
| BMId, median (IQR) | 24.4 (22.2, 26.8) | 23.1 (20.7, 25.9) | 0.045 | |
| Obesitye, No. (%) | 23 (43.4) | 150 (30.0) | 0.281 | 0.045 |
| Ethnicity, No. (%) | ||||
| Japanese | 44 (83.0) | 502 (100) | ||
| Asian | 4 (7.5) | 0 (0.0) | ||
| Caribbean | 3 (5.7) | 0 (0.0) | ||
| African | 2 (3.8) | 0 (0.0) | ||
| Smoking history, No. (%) | ||||
| Yes | 25 (47.2) | 206 (41.0) | 0.225 | |
| Individual comorbidities, No. (%) | ||||
| No medical conditions | 27 (50.9) | 234 (46.6) | 0.885 | |
| Hypertension | 10 (18.9) | 70 (13.9) | 0.450 | |
| Dyslipidemia | 10 (18.9) | 59 (11.8) | 0.200 | |
| Diabetes | 4 (7.5) | 19 (3.8) | 0.277 | |
| COPD | 1 (1.9) | 2 (0.4) | 0.275 | |
| Bronchial asthma | 3 (5.7) | 69 (13.7) | 0.700 | |
| Myocardial infarction | 1 (1.9) | 0 (0.0) | 0.102 | |
| Malignancy | 8 (15.1) | 11 (2.2) | <0.001 | |
| Immunodeficiency | 1 (1.9) | 2 (0.4) | 0.275 | |
| Chronic kidney disease | 2 (3.8) | 2 (0.4) | 0.053 | |
| Vaccinationf, No. (%) | 45 (84.9) | 11 (2.2) | 2.994 | <0.001 |
| Acute COVID-19 characteristics, No. (%) | ||||
| Pneumonia diagnosed | 12 (22.6) | 141 (28.1) | 0.214 | |
| Severityg | 0.173 | |||
| Mild | 42 (79.2) | 393 (78.3) | ||
| Moderate | 11 (20.8) | 58 (12.7) | ||
| Severe | 0 (0.0) | 4 (0.9) | ||
| Pharmacological treatments | ||||
| Antiviral | 13 (24.5) | 56 (11.2) | 0.020 | |
| Corticosteroids | 12 (22.6) | 58 (11.6) | 0.067 | |
| mAbh | 13 (24.5) | 4 (0.8) | <0.001 | |
Abbreviations: SMD, standardized mean difference; BMI, body mass index; IQR, interquartile range; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; IMV, invasive mechanical ventilation.
Mann–Whitney U test for continuous variables, Fisher's exact test or Kruskal−Wallis test for categorical variables.
Older adult is defined as aged 65 or more.
The denominator in each category depends on the number of missing values.
Calculated as weight in kilograms divided by height in meters squared.
Obesity is defined as BMI of 25 or more.
Patients tested positive for SARS-CoV-2 at least 7 days after their second vaccination when immunity had developed.
Highest severity during clinical course of COVID-19.
Monoclonal antibodies were either casirivimab/imdevimab or sotrovimab.
Table 2.
Characteristics of the participants in the Omicron and control groups after propensity score matching.
| Characteristics | Omicron group |
Control group |
SMD | p-valuea |
|---|---|---|---|---|
| (n = 18) | (n = 18) | |||
| Age, median (IQR), years | 37.5 (30.8, 66.5) | 52.0 (46.5, 61.3) | 0.239 | |
| Older adultb, No. (%) | 4 (22.2)c | 4 (22.2) | <0.001 | 1.000 |
| Female sex, No. (%) | 10 (55.6) | 10 (55.6) | <0.001 | 1.000 |
| BMId, median (IQR) | 23.3 (21.0, 24.9) | 23.3 (21.8, 25.1) | 0.696 | |
| Obesitye, No. (%) | 5 (27.8) | 5 (27.8) | <0.001 | 1.000 |
| Ethnicity, No. (%) | ||||
| Japanese | 12 (66.7) | 18 (100) | ||
| Asian | 2 (11.1) | 0 (0.0) | ||
| Caribbean | 2 (11.1) | 0 (0.0) | ||
| African | 2 (11.1) | 0 (0.0) | ||
| Smoking history, No. (%) | ||||
| Yes | 7 (38.9) | 10 (55.6) | 0.395 | |
| Individual comorbidities, No. (%) | ||||
| No medical conditions | 12 (66.7) | 9 (50.0) | 0.310 | |
| Hypertension | 1 (5.6) | 3 (16.7) | 0.603 | |
| Dyslipidemia | 2 (11.1) | 3 (16.7) | 1.0 | |
| Diabetes | 1 (5.6) | 2 (11.1) | 1.0 | |
| COPD | 0 (0.0) | 1 (5.6) | 1.0 | |
| Bronchial asthma | 1 (5.6) | 5 (27.8) | 0.177 | |
| Myocardial infarction | 0 (0.0) | 0 (0.0) | 1.00 | |
| Malignancy | 3 (16.7) | 0 (0.0) | 0.229 | |
| Immunodeficiency | 0 (0.0) | 0 (0.0) | 1.0 | |
| Chronic kidney disease | 0 (0.0) | 0 (0.0) | 1.0 | |
| Vaccinationf, No. (%) | 10 (55.6) | 10 (55.6) | <0.001 | 1.0 |
| Acute COVID-19 characteristics, No. (%) | ||||
| Pneumonia diagnosed | 4 (22.2) | 4 (22.2) | 1.0 | |
| Severityg | 0.635 | |||
| Mild | 16 (88.9) | 15 (83.3) | ||
| Moderate | 2 (11.1) | 3 (16.7) | ||
| Severe | 0 (0.0) | 0 (0.0) | ||
| Pharmacological treatments | ||||
| Antiviral | 3 (16.7) | 1 (5.6) | 0.603 | |
| Corticosteroids | 2 (11.1) | 4 (22.2) | 0.658 | |
| mAbh | 3 (16.7) | 1 (5.6) | 0.603 | |
Abbreviations: SMD, standardized mean difference; BMI, body mass index; IQR, interquartile range; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; IMV, invasive mechanical ventilation.
Mann–Whitney U test for continuous variables, Fisher's exact test or Kruskal−Wallis test for categorical variables.
Older adult is defined as aged 65 or more.
The denominator in each category depends on the number of missing values.
Calculated as weight in kilograms divided by height in meters squared.
Obesity is defined as BMI of 25 or more.
Patients tested positive for SARS-CoV-2 at least 7 days after their second vaccination when immunity had developed.
Highest severity during clinical course of COVID-19.
Monoclonal antibodies were either casirivimab/imdevimab or sotrovimab.
After matching, 18 patients each in the Omicron and control groups had improved covariate balance. The number of older adult (defined as age 65 and above), female, obese (defined as BMI ≥25), and vaccinated patients (defined as patients who tested positive for SARS-CoV-2 at least seven days after their second vaccination) was 4 (22.2%) (standardized mean difference: SMD <0.001, p = 1.000), 10 (55.6%) (SMD <0.001, p = 1.000), 5 (27.8%) (SMD <0.001, p = 1.000), and 10 (55.6%) (SMD <0.001, p = 1.000), respectively for both the Omicron and control groups.
3.4. Prevalence of post COVID-19 condition in the Omicron group and the control group
The prevalence of post COVID-19 condition in the Omicron and control groups before matching is summarized in Table 3 and Supplementary Table 3, respectively. In the Omicron group, 3 (5.7%) patients suffered from fatigue, 2 (3.8%) from shortness of breath, 2 (3.8%) from cough, 2 (3.8%) from depressed mood, and 3 (5.7%) from loss of concentration that lasted more than 2 months within 3 months of disease onset. As for the other symptoms of the Omicron group, 45 (84.9%) patients had fever, 30 (56.6%) had sore throat, and 15 (28.3%) had runny nose in the acute phase. However, none of the symptoms lasted more than 2 months within 3 months of disease onset. In addition, 3 patients (5.7%) had dysosmia, and 7 (13.2%) had dysgeusia.
Table 3.
The number of participants with post COVID-19 condition in the Omicron group (n = 53).
| Symptoms | Numbera (%b) | Lasting at least 2 months within 3 months since the onset (%b) |
|---|---|---|
| Fatigue | 22 (41.5) | 3 (5.7) |
| SoB | 10 (18.9) | 2 (3.8) |
| Cough | 23 (43.4) | 2 (3.8) |
| Dysosmia | 3 (5.7) | 0 (0.0) |
| Dysgeusia | 7 (13.2) | 0 (0.0) |
| Hair loss | 2 (3.8) | 0 (0.0) |
| Depressed mood | 5 (9.4) | 2 (3.8) |
| Brain fog | 0 (0.0) | 0 (0.0) |
| LoC | 8 (15.1) | 3 (5.7) |
| MD | 3 (5.7) | 1 (1.9) |
| At least one symptomc | 41 (77.4) | 7 (13.2) |
| Other symptoms | ||
| Fever | 45 (84.9) | 0 (0.0) |
| Sore throat | 30 (56.6) | 0 (0.0) |
| Sputum | 19 (35.8) | 2 (3.8) |
| Runny nose | 15 (28.3) | 0 (0.0) |
| Headache | 10 (18.9) | 1 (1.9) |
| Muscle pain | 1 (1.9) | 0 (0.0) |
| Joint pain | 7 (13.2) | 0 (0.0) |
| Chest pain | 3 (5.7) | 1 (1.9) |
| Palpitation | 1 (1.9) | 1 (1.9) |
| Abdominal pain | 4 (7.5) | 0 (0.0) |
| Diarrhea | 6 (11.3) | 0 (0.0) |
| Appetite loss | 3 (5.7) | 0 (0.0) |
Abbreviations: SoB, shortness of breath; LoC, loss of concentration; MD, memory disturbance.
Number of patients experiencing each symptom within 3 months since the onset.
Calculated by dividing the number of patients by the total number of participants (n = 53).
At least one symptom of fatigue, shortness of breath, cough, dysosmia, dysgeusia, hair loss, depressed mood, brain fog, loss of concentration, and memory disturbance (not including other symptoms).
The prevalence of post COVID-19 condition in the Omicron and control groups after matching is summarized in Table 4 . There were no significant differences in the prevalence of each post COVID-19 condition between the two groups. The number of patients with at least one post COVID-19 condition in the Omicron and control groups were 1 (5.6%) and 10 (55.6%) (p = 0.003), respectively. The IPW method showed similar results. The relative risk reduction of infection caused by Omicron was 0.33 (95%CI: 0.23–0.43).
Table 4.
Prevalence of post COVID-19 condition in the Omicron group and the control group.
| Symptomsa | Omicron group (n = 18) | Control group (n = 18) | p-valueb |
|---|---|---|---|
| Fatigue | 1 (5.6) | 1 (5.6) | 1.0 |
| SoB | 0 (0.0) | 1 (5.6) | 1.0 |
| Cough | 0 (0.0) | 3 (16.7) | 0.229 |
| Dysosmia | 0 (0.0) | 3 (16.7) | 0.229 |
| Dysgeusia | 0 (0.0) | 1 (5.6) | 1.000 |
| Hair loss | 0 (0.0) | 3 (16.7) | 0.229 |
| Depressed mood | 0 (0.0) | 0 (0.0) | 1.0 |
| Brain fog | 0 (0.0) | 1 (5.6) | 1.0 |
| LoC | 0 (0.0) | 1 (5.6) | 1.0 |
| MD | 0 (0.0) | 2 (11.1) | 0.486 |
| At least one post COVID-19 condition | 1 (5.6) | 10 (55.6) | 0.003 |
Abbreviations: SoB, shortness of breath; LoC, loss of concentration; MD, memory disturbance.
Definition of post COVID-19 condition is a symptom that last at least 2 months within 3 months since the onset of COVID-19.
Results of Fisher's exact test.
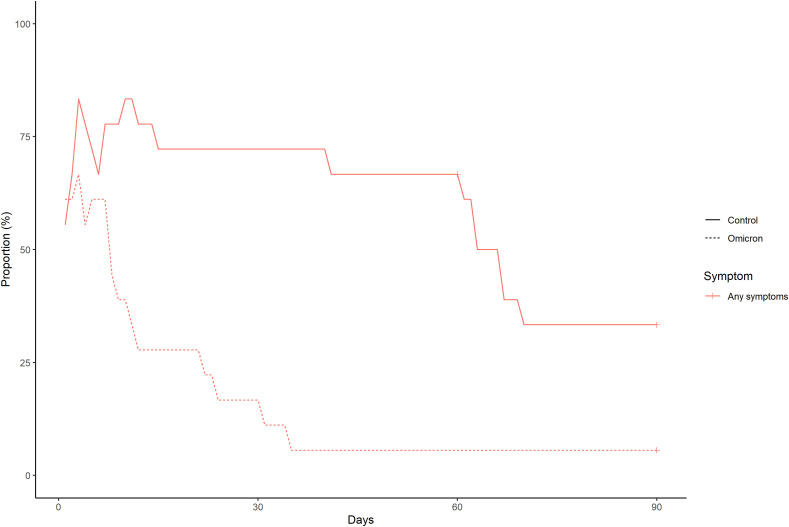
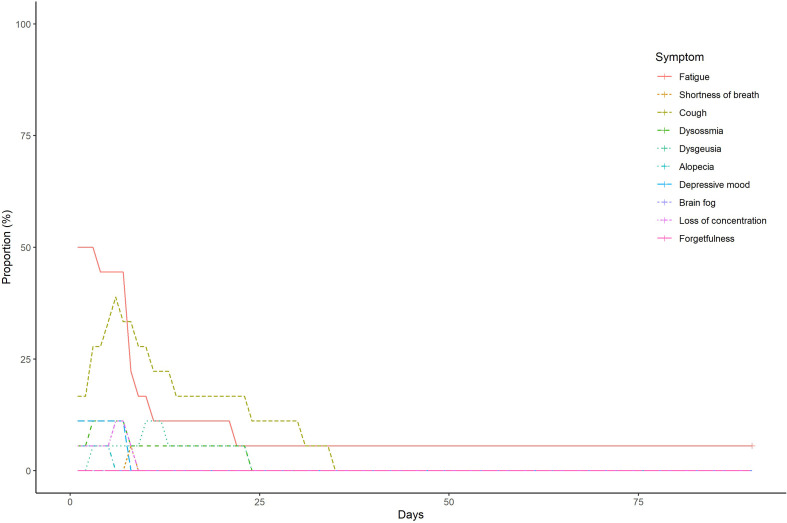
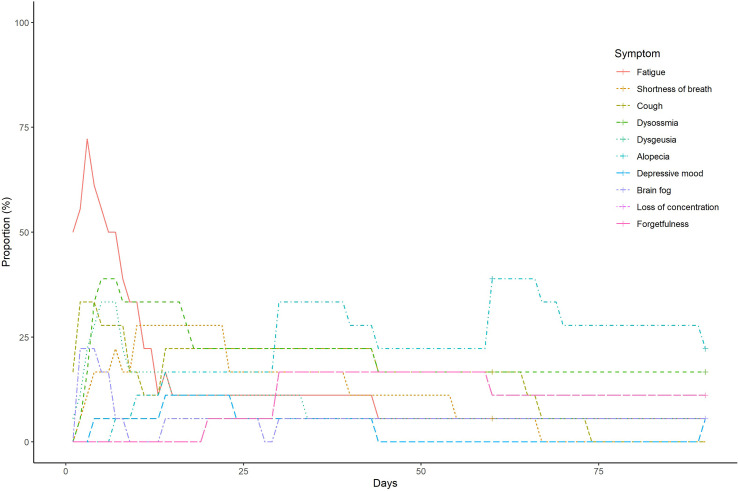
The frequency and duration of each symptom in the Omicron and control groups are shown in Supplementary Fig. 4 and Supplementary Fig. 5, respectively. The frequency and duration of at least one symptom in both groups are shown in Fig. 1 . The number of patients with at least one symptom at 60 and 90 days after symptom onset or diagnosis of COVID-19 in the Omicron group were 1 (5.6%) and 1 (5.6%), respectively. Meanwhile, 12 (66.7%) and 7 (38.9%) patients in the control group had at least one symptom that lasted at 60 and 90 days, respectively.
Fig. 1.
The frequency and duration of at least one symptom in the Omicron and control groups after propensity score matching.
4. Discussion
In this study, we investigated and compared the prevalence of post COVID-19 condition in patients with Omicron variant and the previous strains after performing PSM. Since we matched on age, sex, BMI and vaccination status, these factors are unlikely to confound our observation on the prevalence of post COVID-19 condition [15,17]. The main finding of this study was that there were no significant differences in the prevalence of post COVID-19 conditions between the Omicron and control groups, although the prevalence in the Omicron group tended to be less than that in the control group. In addition, the number of patients with at least one post COVID-19 condition in the Omicron group was significantly less than that in the control group. However, several patients were infected with Omicron variants worldwide. In particular, the World Health Organization reported that among the 432,470 specimens collected from 13 January to February 11, 2022, 98.3% were Omicron variant [9]. Thus, while the prevalence rate may be lower in Omicron group, the actual number of patients suffering from post COVID-19 conditions can be high. Further research with a larger sample size is needed to investigate the precise epidemiology of post COVID-19 conditions due to Omicron and assess its impact on health-related quality of life and social productivity.
We found that 84.9%, 56.6%, and 28.3% of patients with Omicron had fever, sore throat, and runny nose, respectively, but none of which lasted more than 2 months within 3 months of disease onset. Menni et al. reported that sore throat was more common with Omicron than with Delta variant (70.5% vs 60.8%, odds ratio 1.55; 95% CI [1.43–1.69], p < 0.001) [7], indicating that patients with Omicron tended to have upper respiratory symptoms. This is also consistent with an in vitro study stating that Omicron replicates faster than all other SARS-CoV-2 variants in the bronchus but less efficiently in the lung parenchyma, and appears to enter human cells by a different route [21]. Although we could not compare the prevalence and duration of these symptoms of Omicron with those of previous strains, characterizing the clinical course of infection by Omicron is not only of direct public health relevance providing public and clinicians awareness on what symptoms to look out for, but will also to assist in understanding the potential effects of future variants of concern [7].
We also found that 5.7%, 13.2% and 3.8% of the patients with Omicron had dysosmia, dysgeusia, and hair loss, respectively. This finding was consistent with a previous report stating that loss of smell was less common in participants infected with Omicron than with Delta variant (16.7% vs 52.7%, odds ratio 0.17; 95% CI [0.16–0.19], p < 0.001) [7]. These findings can also help raise awareness on what symptoms are more common with the Omicron variant to aide in rapid diagnosis, minimizing the spread of COVID-19.
Our study had several limitations. Firstly, we compared data obtained from two surveys with different methodologies: data of Omicron group from telephone interviews, and those of control group from a self-reported questionnaire-based online/paper-based survey, which may have influenced the results. Secondly, those with more severe symptoms might be less likely to respond to telephone calls if they were subsequently hospitalized, making data on the critically ill more likely to be scarce. Thirdly, the self-reported questionnaire-based online/paper-based survey was subject to various biases, such as selection, volunteer, and recall biases. In particular, the survey was limited to COVID-19 convalescent plasmapheresis patients who underwent the pre-donation screening test. It is unclear whether the results of this survey can be applied to all patients recovering from COVID-19. Fourthly, this was a single-center study with a small sample size. Fifthly, we regarded the results brought by PS matching as the main findings, because the IPW method sometimes distorts the results due to extremely large value of IPW. However, we believe that the result brought by the IPW method will support our conclusion although the small sample size will be an important limitation. Sixthly, the definition of post COVID-19 condition in this study was similar to a previous study [3]. However, whether the symptoms could be explained by an alternative diagnosis remains unclear in our study. Therefore, our findings may have overestimated the prevalence of post COVID-19 condition. Seventhly, some patients had persistent symptoms at the time of the survey. In these cases, the actual duration of the symptoms was unclear. Long-term observation is needed to better understand the duration of post-COVID conditions of Omicron. Eighthly, it was impossible to determine the type of variant in the control group. Considering that 31.5%, 48.6%, and 19.9% in the control group got infected with COVID-19 between February 2020 and October 2020, November 2020 and June 2021, and July 2021 and October 2021, respectively, it was likely that alpha (B.1.1.7) strains were most predominant [19,22,23]. The prevalence of the SARS-CoV-2 variants may have influenced the frequency of post-COVID conditions in the control group. Ninthly, we could not identify each Omicron variant (B.1.1.529, BA.1, BA.1.1, BA.2, BA.3, BA.4 and BA.5 lineages). Lastly, the immunity after vaccination would generally decrease over time. However, the time period between most recent vaccination and infection was not considered in this study.
5. Conclusions
We investigated the prevalence of post COVID-19 conditions in patients with Omicron and other strains of SARS-CoV-2 after matching on age, sex, BMI, and vaccination status. There were no significant differences in the prevalence of each post COVID-19 condition between the Omicron and control groups. However, the number of patients with at least one post COVID-19 condition in the Omicron group was significantly less than that in the control group. As many patients worldwide were infected with Omicron, several patients may suffer from post COVID-19 conditions. Further research with more participants is needed to investigate more precise epidemiology of post COVID-19 condition due to Omicron, and its impact on health-related quality of life and social productivity.
Funding
This work was supported by the Health, Labor and Welfare Policy Research Grants, Research on Emerging and Reemerging Infectious Diseases and Immunization [grant number 20HA1006].
Data statement
Due to the sensitive nature of the questions asked in this study, survey respondents were assured raw data would remain confidential and would not be shared.
Authors' contributions
MA, YO, CK, M Sanada KT, and TM conducted the research and investigation. SM was responsible for the data curation. SM and ST conducted the statistical analyses. SM and ST were major contributors to writing the original draft of the manuscript. SM acquired funds for the study and supervised the project. All authors read and approved the final manuscript.
Declaration of competing interest
None.
Acknowledgments
We would like to thank all the patients who participated in our study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jiac.2022.08.007.
Appendix A. Supplementary data
The following are the supplementary data to this article:
Supplementary Fig. 4.
The frequency and duration of each symptom in the Omicron group
Supplementary Fig. 5.
The frequency and duration of each symptom in the control group
References
- 1.World Health Organization COVID-19 Dashboard. https://www.gavi.org/covid19/dashboard?gclid=CjwKCAjwjtOTBhAvEiwASG4bCJqe6lbmoeoTy14wqJZsAW52-Jqd7HdYU4UUK7-IeVE8zEtoA3hmYxoCkG8QAvD_BwE
- 2.Huang L., Yao Q., Gu X., Wang Q., Ren L., Wang Y., et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398:747–758. doi: 10.1016/s0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Coronavirus disease (COVID-19): post COVID-19 condition. https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-post-covid-19-condition
- 4.Centers for Disease Control and Prevention Long COVID or post-COVID conditions. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html#:∼:text=Health%20conditions&text=Some%20people%2C%20especially%20those%20who,kidney%2C%20skin%2C%20and%20brain
- 5.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/s0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menni C., Valdes A.M., Polidori L., Antonelli M., Penamakuri S., Nogal A., et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399:1618–1624. doi: 10.1016/s0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization WHO coronavirus Dashboard. https://covid19.who.int/
- 9.World Health Organization Weekly epidemiological update on COVID-19. 15 February 2022. https://reliefweb.int/sites/reliefweb.int/files/resources/20220215_Weekly_Epi_Update_79.pdf
- 10.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Rio C., Collins L.F., Malani P. Long-term health consequences of COVID-19. JAMA. 2020;324:172–174. doi: 10.1001/jama.2020.19719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrigues E., Janvier P., Kherabi Y., Le Bot A., Hamon A., Gouze H., et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tenforde M.W., Kim S.S., Lindsell C.J., Rose E.B., Shapiro N.I., Files D.C., et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, March-June 2020. MMWR (Morb Mortal Wkly Rep) 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmad I., Rathore F.A. Neurological manifestations and complications of COVID-19: a literature review. J Clin Neurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonelli M., Penfold R.S., Merino J., Sudre C.H., Molteni E., Berry S., et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: a prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022;22:43–55. doi: 10.1016/s1473-3099(21)00460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyazato Y., Morioka S., Tsuzuki S., Akashi M., Osanai Y., Tanaka K., et al. Prolonged and late-onset symptoms of coronavirus disease 2019. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa507. ofaa507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C., et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho D., Imai K., King G., Stuart E.A. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Software. 2011;42:1–28. doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
- 19.Centers for Disease Control and Prevention SARS-CoV-2 variant classifications and definitions. https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html
- 20.Shrotri M., Navaratnam A.M.D., Nguyen V., Byrne T., Geismar C., Fragaszy E., et al. Spike-antibody waning after second dose of BNT162b2 or ChAdOx1. Lancet. 2021;398:385–387. doi: 10.1016/s0140-6736(21)01642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui K.P.Y., Ho J.C.W., Cheung M.-C., Ng K.-C., Ching R.H.H., Lai K.-L., et al. SARS-CoV-2 omicron variant replication in human bronchus and lung ex vivo. Nature. 2022;603:715–720. doi: 10.1038/s41586-022-04479-6. [DOI] [PubMed] [Google Scholar]
- 22.Ministry of Health, Labour and Welfare Number of domestic cases of variants of SARS-CoV-2 of concern by prefecture (genomic analysis) https://www.mhlw.go.jp/stf/seisakunitsuite/newpage_00054.html
- 23.Our World in data. Coronavirus pandemic (COVID-19) https://ourworldindata.org/coronavirus
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.