Abstract
Background Little is known about the prevalence of chronic postsurgical pain (CPSP) among patients receiving single-port video-assisted thoracic surgery (SP-VATS) compared with those undergoing multi-port VATS (MP-VATS). This study aimed to compare the incidence of CPSP between SP-VATS and MP-VATS lung resection and assess how the pain affected the daily living activities of this patient population.
Methods We prospectively collected data regarding the demographic features, clinical factors during surgery, the intensity of acute postoperative pain, and complications after surgery among patients receiving elective SP-VATS or MP-VATS lung resection at our institution between June 2015 and August 2018. At 3-, 6-, and 12-months following surgery, the patients were followed up through a telephonic interview. The Brief Pain Inventory-Short Form was adopted to assess the incidence, severity and location of the CPSP, analgesic consumption, as well as the interference of pain with daily activities.
Results In total, 476 patients were screened for eligibility, 411 patients were followed up for 12 months and included in final analysis. Among these, 190 patients had undergone an SP-VATS pulmonary resection and 221 had an MP-VATS pulmonary resection. At both 3 and 6 months, the incidence of CPSP in the SP-VATS group was lower than that in the MP-VATS group (25.3 vs. 38.0%, p = 0.006; 11.1 vs. 19.0%, p = 0.026, respectively). At 12 months after surgery, the prevalence of CPSP was comparable between groups (4.7 vs, 9.0%, p = 0.089). In addition, the SP-VATS technique alleviated pain interference with the daily living activities of patients with CPSP in several domains, including sleep, mood, and enjoyment of life compared with the MP-VATS technique. The two predictive factors for CPSP at postoperative 3 months were the MP-VATS technique (odds ratio [OR] = 1.792, p = 0.019) and postoperative chemotherapy (OR = 1.718, p = 0.033).
Conclusions This study indicated that the SP-VATS technique reduced the prevalence of CPSP at 3- and 6-months post-pulmonary resection compared with the MP-VATS technique. The SP-VATS technique also significantly alleviated pain interference with the daily living activities of the patients.
Keywords: Chronic postsurgical pain, single-port video-assisted thoracic surgery (SP-VATS), multi-port VATS (MP-VATS), pulmonary resection
Introduction
Chronic postsurgical pain (CPSP) is defined by the International Association for the Study of Pain (IASP) as pain that arises postoperatively and lasts for at least 3 months, which is longer than the normal healing time. 1 CPSP represents a frequent and important complication in thoracic surgery. It can occur in 25 to 60% of post-thoracic surgical patients, decrease the patients' quality of life, and hamper their daily activities. 2 3 4 5
Video-assisted thoracic surgery (VATS) has been extensively identified as an option to replace traditional thoracotomy with minimal invasion. The reported benefits of VATS include reduced risk of postoperative complication, shorter postoperative length of stay, and faster return to normal life. 6 7 However, as the conventional multi-port VATS (MP-VATS) using three or four ports is conducted through trocars inserted in intercostal spaces, intercostal nerves at trocar insertion sites are possibly impaired with this approach. Although MP-VATS is a minimally invasive surgical procedure, the rate of persistent pain following MP-VATS is nearly comparable to that following thoracotomy. 8 9
As surgical techniques and instruments have evolved, single-port VATS (SP-VATS) has recently emerged as an approach involving a lower level of invasion compared with the conventional multi-port approach. 10 SP-VATS can further reduce surgical incisions, decrease surgery-related trauma, and promote patient recovery. 11 12 Although SP-VATS has been suggested to decrease acute postoperative wound pain, only a few retrospective studies have examined its role in the development of CPSP, compared with that after MP-VATS. 13 14 15
This prospective study focused on comparing the incidence and features of CPSP after SP-VATS versus MP-VATS lung resection and assessing how the pain affected the daily activities of the patients. It was hypothesized that SP-VATS had a lower rate of CPSP when compared with MP-VATS.
We present the following article in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting checklist.
Methods
Study Design and Subjects
The present prospective observational research was approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University. Each patient provided written informed consent before enrolment. This work was performed in compliance with the revised Declaration of Helsinki (2013).
The patients who scheduled for elective VATS pulmonary resection, including lobectomy, segmentectomy, and wedge resection for pulmonary nodules at our institution, between June 2015 and August 2018 were enrolled. The inclusion criteria included aged over 18 years, American Society of Anesthesiologists (ASA) physical status between I and III, and ability to communicate in Mandarin. The exclusion criteria were patients who did not offer informed consent, history of thoracic surgery, history of chronic pain in the chest area, drug abuse, preoperative administration of chemotherapy and/or radiation, distant metastasis at the time of diagnosis, bilateral surgery, psychiatric illness, and inability to cooperate to personal or telephonic interviews.
Preoperative Interview
Each patient received an interview performed by a trained investigator after surgical admission. The questionnaire covered items including age, gender, marital status, employment status, education level, smoking history, preoperative comorbidities (chronic obstructive pulmonary disease, hypertension, diabetic mellitus, and coronary heart disease), as well as ASA physical status. Body weight and height were measured on admission to calculate body mass index (BMI).
Surgical Procedure
The operation was performed by three groups of surgeons in the thoracic department using the standardized surgical types of SP-VATS or MP-VATS. The adoption of single-port or multi-port approach was at the discretion of the attending surgeon. In each procedure, the patients were administered general anesthesia in the lateral decubitus position. Depending on the scheduled types of surgeries, the cases were categorized into the SP-VATS group or the MP-VATS group.
SP-VATS pneumonectomy was performed according to the following procedure. A 3- to 4-cm incision was made in the fourth or fifth intercostal space between anterior and middle axillary lines, and a wound retractor was connected. Thereafter, a 10-mm thoracoscope (30°) was fixed to the edge of the wound on the skin. MP-VATS was performed via three ports. A 3-cm anterior incision was made around the fourth or fifth interspace between anterior and middle axillary lines, following which the 1.0-cm camera port was inserted into the seventh interspace of the middle axillary line. After inserting the trocar into the camera port, a 1.5-cm incision was made within the eighth interspace in the posterior axillary line. Based on preoperative image data and results of the intraoperative frozen sections, lobectomy, segmentectomy, and wedge resection were selected as the surgical methods. Systemic lymph node dissection was performed when the malignant disease was evident from the frozen sections. Lymph nodes were grouped and then resected or sampled according to the American Joint Committee on Cancer criteria 16 and the Chinese Guidelines for the Diagnosis and Treatment of Primary Lung Cancer 17 . At the end of the operation, a protective specimen bag was used to retrieve the specimen, and then, a 28-Fr chest drain was inserted into the port site.
The surgical variables, including the location and size of the lesion, type of resection, histological type, pathological stage, operation time, and blood loss volume, were collected. Patients who were converted from VATS to thoracotomy were removed from the study.
Anesthesia and Analgesia
General anesthesia was induced with propofol, sufentanil, and rocuronium, and then, a double-lumen endotracheal tube was placed under bronchoscopic guidance. Subsequently, anesthesia was maintained through sevoflurane inhalation and intermittent sufentanil/rocuronium injection if necessary. Each case received intercostal blocks and wound infiltration with 0.375% ropivacaine. The management of postoperative pain was standardized at our division. Specifically, a patient-controlled intravenous analgesic system with sufentanil was routinely used for 1- or 2-days after operation. After the diet was reinstituted, oral celecoxib (200 mg) was administered twice daily until discharge from the hospital, unless otherwise indicated. Postoperative pain at rest and during movement was assessed every 12 hours with the numerical rating scale (NRS) (Scale D-10, indicating none to the most serious pain imaginable). The mean pain scores both at rest and during movement within postoperative 24 hours were calculated. When the pain control was insufficient (pain score of 4 or higher during rest), IV tramadol was administered as rescue medication.
Postoperative Management
Each patient was returned to the thoracic surgery ward after the operation. The chest drain tube was removed when no air leak was detected, and drainage volume was less than 200 mL/d. Patients who could mobilize independently and showed normal on the chest X-ray were allowed to discharge after chest tube removal. Information regarding chest drainage time, postoperative complications (pneumonia, atelectasis, arrhythmia, and surgical site infection), and postoperative length of stay were collected.
Evaluation of Chronic Postsurgical Pain
Follow-ups were performed at 3, 6, and 12 months after surgery by two trained investigators who were blinded to the patients' perioperative care to prevent any possibility of bias. After confirmation of no signs of recurrent or disseminated of disease, the patients were asked whether they experienced any pain at or near the surgical area over the previous 24 hours that they considered related to the thoracic surgery and whether the pain occurred after the surgery. Patients who replied with “no” to any of the above questions were required not to respond further questions, whereas the remaining patients were invited to finish the questionnaires.
The Chinese validated version of the Brief Pain Inventory-Short Form (BPI-SF) 18 was adopted to assess both pain intensity and pain interference of CPSP. BPI-SF has been demonstrated as a pain assessment tool that has adequate reliability and validity for patients with persistent pain and is sensitive to change over time. 19 20 The patients were instructed to score their worst, least, average, and current pain severity by NRS from the preceding 24 hours. Then, the patients were inquired about the pain location and their current analgesic use. The interference of pain with daily living activities of seven domains (general activity, mood, walking ability, normal work, relations with other people, sleep, and enjoyment of life) was also measured using the 0 to 10 NRS, wherein 0 and 10 points indicated none and complete interference, respectively.
In addition, data on the use of chemotherapy or radiation after surgery were also collected.
The primary outcome was the percentage of patients with CPSP 3 months after surgery. The CPSP was diagnosed as the pain that occurred at or near the operated area following surgery and persisted for at least 3 months, with the NRS score of > 0. Secondary outcomes included the incidence of CPSP at 6/12 months after surgery, the intensity of CPSP, and interference of pain with daily living activities, all measured with the BPI-SF questionnaire. Other secondary outcomes included the intensity of acute postoperative pain, duration of chest tube, occurrence of postoperative complications, and postoperative length of stay.
Statistical Analysis
As CPSP has been previously suggested to be present in around 30% of patients undergoing VATS at 3 months after surgery, 2 21 we determined that 121 cases were needed to enroll in each group for the detection of the 15% inter-group difference at the α and power values of 0.05 and 0.80, respectively. Therefore, 152 cases should be enrolled in each group when the predicted dropout rate was 20%.
Before the analysis, the distribution of the data was analyzed. The normally distributed continuous variables were displayed as mean (standard deviation) and analyzed using an independent samples t -test. The abnormally distributed data were displayed as medians (range) and tested using the Mann–Whitney U -test. The categorical data were displayed as case numbers and proportions. Chi-square tests were conducted to compare the categorical data between groups. To identify the possible predictive factors for CPSP at 3 months, univariate analysis was conducted between patients with and without CPSP. To find the independent factor for CPSP at 3 months after surgery, variables with p < 0.20 from the univariate analysis were taken into the multivariate analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) for each variable were determined to assess their impact on CPSP prediction. SPSS v. 22.0 (IBM, Armonk, NY, United States) was used for statistical analysis. p -Values of < 0.05 indicated statistical significance.
Results
Demographic and Clinical Features
Between June 2015 and August 2018, 476 patients were screened for eligibility, of which 14 declined participation, six canceled surgical procedures, and six were excluded according to the criteria. Thus, 450 patients (203 scheduled for SP-VATS and 247 scheduled for MP-VATS) completed preoperative assessment. Among these, eight cases quit during follow-up at the hospital, and 11, 12, and 8 cases dropped out at 3, 6, 12 months after surgery, respectively. As a result, 411 patients completed the study, among which, 190 had undergone SP-VATS and 221 had undergone MP-VATS ( Fig. 1 ).
Fig. 1.
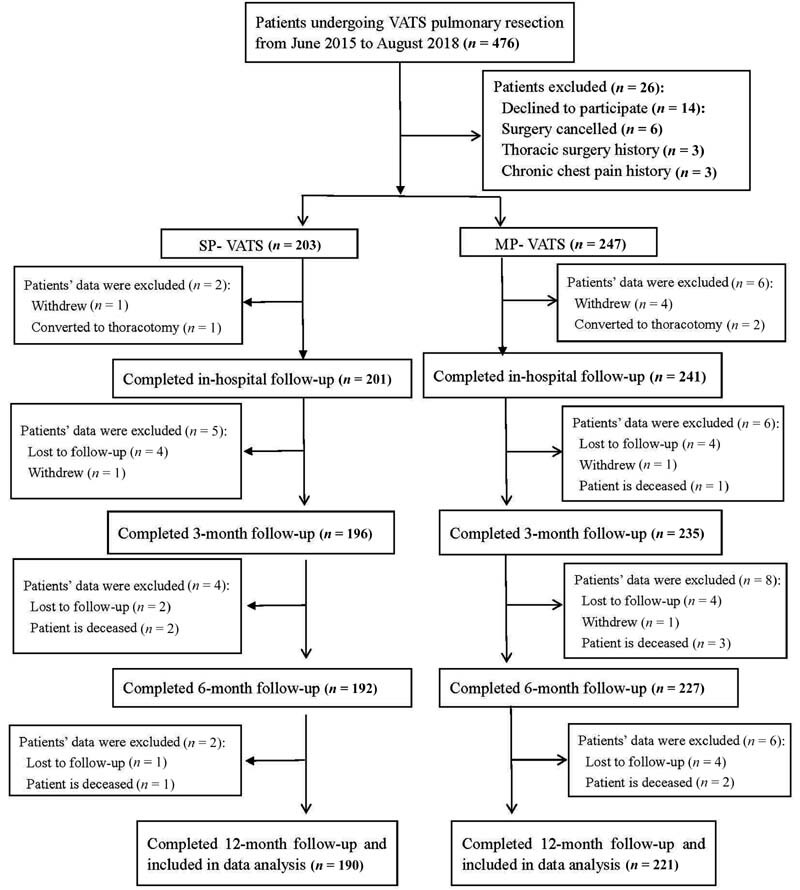
Flowchart of patients.
The demographic and clinical features of the patients, along with data on CPSP prevalence, are presented in Table 1 . Most of the demographic and clinical variables were comparable in the two groups. The SP-VATS group reported a significantly lower intensity of pain both at rest and during movement within 24 hours after surgery, less drainage duration, and shorter length of postoperative hospital stay compared with the MP-VATS group.
Table 1. Demographic, clinical features, and CPSP incidence of patients who underwent SP-VATS or MP-VATS pulmonary resection.
| SP-VATS ( n = 190) | MP-VATS ( n = 221) | p- Value | |
|---|---|---|---|
| Age (y) | 60 ± 10 | 60 ± 10 | 0.862 |
| Gender | 0.277 | ||
| Male | 106 (55.8) | 135 (61.1) | |
| Female | 84 (44.2) | 86 (38.9) | |
| Height (cm) | 163 ± 7 | 164 ± 6 | 0.346 |
| Weight (kg) | 62 ± 7 | 63 ± 7 | 0.202 |
| Body mass index (kg/m 2 ) | 23.3 ± 2.0 | 23.5 ± 2.1 | 0.365 |
| Marital status | 0.869 | ||
| Married | 150 (78.9) | 173 (78.3) | |
| Not married | 40 (21.1) | 48 (21.7) | |
| Employment status | 0.459 | ||
| Employed | 102 (53.7) | 105 (47.5) | |
| Unemployed | 21 (11.1) | 28 (12.7) | |
| Retired | 67 (35.3) | 88 (39.8) | |
| Education level | 0.667 | ||
| ≤ High school diploma | 81 (42.6) | 99 (44.8) | |
| Professional school diploma | 81 (42.6) | 85 (38.5) | |
| University/postgraduate degree | 28 (14.7) | 37 (16.7) | |
| Smoking | 0.954 | ||
| Never smoker | 91 (47.9) | 109 (49.3) | |
| Ex-smoker | 74 (38.9) | 83 (37.6) | |
| Current smoker | 25 (13.2) | 29 (13.1) | |
| Preoperative comorbidities | |||
| COPD | 24 (12.6) | 35 (15.8) | 0.355 |
| Hypertension | 64 (33.7) | 75 (33.9) | 0.957 |
| Diabetes mellitus | 39 (20.5) | 54 (24.4) | 0.345 |
| Coronary heart disease | 24 (12.6) | 20 (9.0) | 0.242 |
| ASA physical status | 0.894 | ||
| I | 37 (19.5) | 42 (19.0) | |
| II | 131 (68.9) | 150 (67.9) | |
| III | 22 (11.6) | 29 (13.1) | |
| Lesion location | 0.638 | ||
| Right | 110 (57.9) | 133 (60.2) | |
| Left | 80 (42.1) | 88 (39.8) | |
| Lesion size (cm) | 2.1 ± 0.8 | 2.2 ± 0.9 | 0.631 |
| Type of resection | 0.717 | ||
| Lobectomy | 123 (64.7) | 151 (68.3) | |
| Segmentectomy | 31 (16.3) | 34 (15.4) | |
| Wedge resection | 36 (18.9) | 36 (16.3) | |
| Histological type | 0.598 | ||
| Adenocarcinoma | 137 (72.1) | 164 (74.2) | |
| Squamous cell carcinoma | 36 (18.9) | 36 (16.3) | |
| Other malignant tumors | 9 (4.7) | 15 (6.8) | |
| Benign disease | 8 (4.2) | 6 (2.7) | |
| Pathologic stage | 0.796 | ||
| 0 | 10 (5.5) | 7 (3.3) | |
| IA | 90 (49.5) | 108 (50.2) | |
| IB | 16 (8.8) | 26 (12.1) | |
| IIA | 49 (26.9) | 58 (27.0) | |
| IIB | 12 (6.6) | 11 (5.1) | |
| IIIA | 4 (2.2) | 3 (1.4) | |
| IIIB | 1 (0.5) | 2 (0.9) | |
| Duration of surgery (min) | 122 ± 46 | 130 ± 49 | 0.115 |
| Blood loss (mL) | 75 (30–480) | 80 (30–500) | 0.267 |
| Average pain severity at rest within 24 h Postoperatively |
2.3 ± 0.7 | 3.0 ± 0.7 | <0.001 |
| Average pain severity during movement within 24 h postoperatively | 4.3 ± 0.9 | 5.1 ± 1.0 | <0.001 |
| Drainage duration (d) | 1.9 ± 0.7 | 2.2 ± 0.8 | 0.003 |
| Postoperative complications | |||
| Pneumonia | 11 (5.8) | 14 (6.3) | 0.818 |
| Atelectasis | 5 (2.6) | 9 (4.1) | 0.422 |
| Arrhythmia | 9 (4.7) | 5 (2.3) | 0.168 |
| Surgical site infection | 8 (4.2) | 17 (7.7) | 0.141 |
| Postoperative length of stay (d) | 4.7 ± 2.2 | 5.4 ± 1.9 | 0.001 |
| Postoperative chemotherapy | 44 (23.2) | 57 (25.8) | 0.536 |
| Postoperative radiation | 6 (3.2) | 9 (4.1) | 0.622 |
| Pain at 3 mo | 48 (25.3) | 84 (38.0) | 0.006 |
| Pain at 6 mo | 21 (11.1) | 42 (19.0) | 0.026 |
| Pain at 12 mo | 9 (4.7) | 20 (9.0) | 0.089 |
Abbreviations: ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; CPSP, chronic postsurgical pain; MP-VATS, multi-port video-assisted thoracoscopic surgery; SP-VATS, single-port video-assisted thoracoscopic surgery.
Note: Data are presented as mean ± standard deviation or medians (range) or numbers (%).
Incidence and Characteristics of CPSP
As Table 1 shows, at 3-months following surgery, the SP-VATS group showed lower CPSP incidence compared with the MP-VATS group (25.3 vs. 38.0%, p = 0.006). At 6 and 12 months, the percentage of patients with CPSP gradually decreased in both groups. However, compared with the MP-VATS group, the SP-VATS group continued to show a lower incidence of CPSP at 6 months (11.1 vs. 19.0%, p = 0.026). At 12 months after operation, the difference in the prevalence of CPSP between both groups was not statistically significant (4.7 vs. 9.0%, p = 0.089).
Table 2 presents the severity of CPSP, the location of pain, and pain interference as assessed using BPI-SF. At the 3-month follow-up, the SP-VATS group reported less pain intensity with lower NRS scores for all the pain measures compared with the MP-VATS group. However, the differences between both groups in worst, least, average, and current pain severity at 6 and 12 months were not statistically significant. In addition, the percentage of patients requiring pain medication was comparable in both groups at 3, 6, and 12 months. Regarding the location of CPSP, most of the patients reported pain along the surgical scar, although it was also reported in more than one site: the scapula, breast, shoulder, and sternal areas.
Table 2. Pain severity, analgesic use, pain location, and pain interference at postoperative 3, 6, and 12 months for patients who underwent SP-VATS or MP-VATS pulmonary resection.
| Pain at 3 mo | Pain at 6 mo | Pain at 12 mo | |||||||
|---|---|---|---|---|---|---|---|---|---|
| SP-VATS ( n = 48) |
MP-VATS ( n = 84) | p -Value | SP-VATS ( n = 21) |
MP-VATS ( n = 42) | p -Value | SP-VATS ( n = 9) |
MP-VATS ( n = 20) | p -Value | |
| Pain severity | |||||||||
| Worst pain | 4.3 ± 1.4 | 4.9 ± 1.4 | 0.037 | 3.5 ± 1.0 | 3.7 ± 1.3 | 0.602 | 3.2 ± 1.0 | 3.6 ± 1.2 | 0.476 |
| Least pain | 0.8 ± 0.9 | 1.1 ± 0.8 | 0.039 | 0.4 ± 0.7 | 0.7 ± 0.7 | 0.111 | 0.4 ± 0.7 | 0.6 ± 0.8 | 0.744 |
| Average pain | 2.5 ± 1.1 | 2.9 ± 0.9 | 0.024 | 2.0 ± 0.8 | 2.2 ± 0.9 | 0.307 | 1.7 ± 1.0 | 2.1 ± 0.8 | 0.288 |
| Current pain | 2.2 ± 1.5 | 2.7 ± 1.6 | 0.044 | 2.1 ± 1.5 | 2.3 ± 1.4 | 0.501 | 1.7 ± 1.7 | 1.8 ± 1.4 | 0.892 |
| Analgesic use | 17 (35.4) | 41 (48.8) | 0.136 | 6 (28.6) | 15 (35.7) | 0.571 | 1 (11.1) | 3 (15.0) | 0.779 |
| Pain location | |||||||||
| Surgical scar area | 33 (68.8) | 58 (69.0) | 15 (71.4) | 31 (73.8) | 6 (66.7) | 16 (80.0) | |||
| Breast area | 8 (16.7) | 18 (21.4) | 3 (14.3) | 9 (21.4) | 0 | 4 (20.0) | |||
| Sternal area | 2 (4.2) | 6 (7.1) | 1 (4.8) | 2 (4.8) | 0 | 1 (5.0) | |||
| Shoulder area | 5 (10.4) | 10 (11.9) | 1 (4.8) | 6 (14.3) | 1 (11.1) | 2 (10.0) | |||
| Scapula area | 11 (22.9) | 17 (20.2) | 4 (19.0) | 4 (9.5) | 3 (33.3) | 2 (10.0) | |||
| Pain interference | |||||||||
| General activity | 2.8 ± 1.7 | 3.4 ± 1.9 | 0.052 | 2.7 ± 1.8 | 3.3 ± 1.3 | 0.112 | 1.8 ± 1.3 | 2.4 ± 1.0 | 0.215 |
| Mood | 2.8 ± 1.8 | 3.9 ± 1.7 | 0.001 | 2.9 ± 1.8 | 3.7 ± 1.8 | 0.107 | 2.1 ± 1.3 | 3.1 ± 1.8 | 0.163 |
| Walking ability | 2.9 ± 1.5 | 3.0 ± 1.5 | 0.666 | 2.5 ± 1.5 | 2.6 ± 1.5 | 0.769 | 1.6 ± 1.2 | 2.0 ± 1.1 | 0.383 |
| Normal work | 3.3 ± 1.6 | 3.3 ± 1.6 | 0.826 | 2.9 ± 1.8 | 2.7 ± 1.4 | 0.730 | 1.9 ± 0.9 | 2.0 ± 0.9 | 0.872 |
| Relations with other people | 3.1 ± 1.7 | 3.4 ± 1.5 | 0.288 | 2.6 ± 1.2 | 3.1 ± 1.5 | 0.239 | 1.8 ± 1.4 | 2.2 ± 1.0 | 0.362 |
| Sleep | 2.7 ± 1.6 | 3.3 ± 1.4 | 0.018 | 2.5 ± 1.4 | 3.1 ± 1.4 | 0.144 | 2.1 ± 2.0 | 2.6 ± 1.2 | 0.476 |
| Enjoyment of life | 3.4 ± 1.7 | 4.1 ± 1.8 | 0.038 | 2.7 ± 1.6 | 3.9 ± 1.4 | 0.003 | 2.6 ± 1.3 | 3.7 ± 1.3 | 0.039 |
Abbreviations: MP-VATS, multi-port video-assisted thoracoscopic surgery; SP-VATS, single-port video-assisted thoracoscopic surgery.
Note: Data are presented as mean ± standard deviation or numbers (proportions).
At 3 months following the operation, no difference in pain interference was detected between the two groups in terms of four daily activity domains (general activity, walking ability, normal work, and relations with others), whereas the MP-VATS group showed greater interference of CPSP with the other three daily activity domains (mood, sleep, and enjoyment of life) compared with the SP-VATS group. At 6 and 12 months, the interference of CPSP with daily activities was comparable between both groups, except for a greater interference with the enjoyment of life in the MP-VATS group ( Table 2 ).
Predictors of CPSP at 3-Month Follow-Up
Based on the univariate analysis of patients with and without CPSP, factors including MP-VATS procedure, longer surgical duration, higher pain severities at rest and during movement within postoperative 24 hours, longer duration of drainage, surgical site infection, postoperative chemotherapy, and radiation were indicated as the potential risk factors ( p < 0.20) for 3-month CPSP following the thoracoscopic procedure. According to the multivariate regression analysis ( Table 3 ), only MP-VATS procedure (OR = 1.792, 95% CI = 1.101–2.915, p = 0.019) and postoperative chemotherapy (OR = 1.718, 95% CI = 1.043–2.830, p = 0.033) were shown to be the independent risk factors of chronic pain at 3 months following surgery.
Table 3. Multivariate logistic regression analysis of predictors of CPSP at 3 months after VATS pulmonary resection.
| β-coefficient | Odds ratio | 95% CI | p -Value | |
|---|---|---|---|---|
| Surgical approach (MP-VATS) | 0.583 | 1.792 | 1.101–2.915 | 0.019 |
| Duration of surgery (min) | 0.001 | 1.001 | 0.996–1.007 | 0.575 |
| Average pain intensity at rest within 24 h postoperatively | 0.101 | 1.106 | 0.754–1.623 | 0.605 |
| Average pain intensity on movement within 24 h postoperatively | −0.130 | 0.878 | 0.652–1.183 | 0.393 |
| Duration of drainage (d) | 0.089 | 1.093 | 0.793–1.507 | 0.587 |
| Surgical site infection | 0.373 | 1.452 | 0.614–3.436 | 0.396 |
| Postoperative chemotherapy | 0.541 | 1.718 | 1.043–2.830 | 0.033 |
| Postoperative radiation | 1.005 | 2.733 | 0.905–8.248 | 0.074 |
Abbreviations: CPSP, chronic postsurgical pain; MP-VATS, multi-port video-assisted thoracoscopic surgery; VATS, video-assisted thoracoscopic surgery.
Discussion
In this prospective cohort study, we observed that the SP-VATS technique reduced the 3- and 6-month chronic pain prevalence following pulmonary resection compared with the MP-VATS approach. Moreover, CPSP cases of the SP-VATS group showed markedly lower pain intensity than those of the MP-VATS group at 3-months after surgery. The multivariate analysis revealed that the MP-VATS technique accounted for one of the two significant predictive factors for CPSP at 3 months following the thoracoscopic procedure.
Comparisons of CPSP between SP-VATS and MP-VATS pulmonary resection have rarely been reported. Hirai et al conducted a retrospective study and showed that the SP-VATS technique dramatically decreased the frequency of CPSP compared with the MP-VATS technique at 1 month after surgery. Moreover, the SP-VATS group reported a lower NRS score on postoperative days 30, as well as a shorter duration of analgesics use within a month following surgery, when compared with the MP-VATS group. 13 In another retrospective study, the same authors compared the incidences of chronic pain following SP-VATS-based (142 cases) and MP-VATS-based (70 cases) lobectomy for lung cancer at 2 postoperative months. They revealed that SP-VATS predicted the markedly lower CPSP frequency compared with MP-VATS (2.8 vs. 11.4%, p = 0.025). 14 As previous studies of the incidence of CPSP after SP-VATS and MP-VATS procedure were retrospective, which were of lower quality and potential biases to the results, this prospective study has an advantage over them.
The prevalence of CPSP among patients undergoing thoracoscopic procedures is reported between 5 and 47%. 2 5 8 9 21 22 23 In this study, the overall incidence of CPSP following thoracoscopic surgery was 32, 15, and 7% at 3-, 6-, and 12-months after the operation, respectively. The difference in the prevalence of CPSP across different studies is possibly associated with the heterogeneities in study design, diverse definitions of chronic pain, diverse timing of evaluation, and differences in perioperative intervention. 2 21 23 Our study demonstrated that the incidence of chronic pain decreased with time in both groups, which was in line with other studies. 2 5 8 23 It may be speculated that spontaneous remission of chronic pain accounts is a natural process, as only less than 10% of the patients in each group continued to suffer from CPSP at 12 months after surgery.
CPSP after thoracic surgery involves the following mechanism. The damage to intercostal nerves during operation induces responses of the inflammatory system and nociceptor, along with remodeling of the nerve cells, which leads to central sensitization. 24 A possible reason for the lower incidence of CPSP in the SP-VATS group in our study is that the incision is simply made within the anterior axillary line that has a wider intercostal space, whereas the absence of ports within the posterior and middle axillary lines possibly avoids intercostal nerve stress and disorder in the intercostal space on the narrow dorsal side, resulting in less central sensitization and decreased risk of chronic pain. Moreover, Benedetti et al discovered that anterior thoracotomy was less likely to induce nerve impairment and the subsequent chronic pain compared with the posterolateral approach. 25
In this study, postoperative chemotherapy was shown to be a risk factor of CPSP, which is in line with previous research findings. 26 27 Neuropathic component significantly accounts for CPSP in thoracic procedures. 28 As chemotherapy-induced neuropathy is a well-known complication among cancer patients, 29 it may contribute to nerve damage associated with CPSP.
Several studies have demonstrated that the intensity of acute postoperative pain is closely related to CPSP after thoracic surgery. 2 5 21 However, in this study, we were unable to find that association for the VATS procedure alone. Further investigations are needed to address the role of acute postoperative pain in the development of CPSP.
The BPI-SF questionnaire allows the assessment of the interference of pain with the life dimensions of patients. In line with previous studies, 3 5 30 our results indicated that CPSP exerted a negative impact on the daily living activities of patients of both groups. Compared with the MP-VATS approach, the SP-VATS approach exerted less pain interference with daily activities for several domains, like mood, sleep, and enjoyment of life, which indicated that the SP-VATS technique might improve the quality of life of the surgical patients and, subsequently, decrease costs toward health care and social support systems of our societies.
This study has some limitations. First, in this observational study, the type of surgery was based on surgical preference. Second, both surgical procedures were not performed by the same surgeon, leading to possible result bias. Third, the pain intensity was assessed based on self-reports of the patients, and physical examination or quantitative sensory test was not applied in this study. Finally, all our results were from cases at a single institute, which limits the generalization of the conclusion. Therefore, randomized controlled trials with multicenter data should be conducted to confirm the role of the SP-VATS technique in the prevention of CPSP after pulmonary resection.
Conclusions
Our findings suggest that compared with the MP-VATS approach, SP-VATS lung resection might decrease the 3- and 6-month CPSP prevalence postoperatively and result in less interference of pain with daily living activities of the patients. There was a tendency toward a decrease in CPSP over time in patients undergoing either the SP-VATS or MP-VATS procedure.
Funding Statement
Funding This study was funded by Chongqing Municipal Bureau of Health Foundation Projects (No. 2013-2-002).Chongqing Municipal Bureau of Health Foundation Projects
Conflicts of Interest None declared.
Note
The authors have completed the STROBE reporting checklist.
Ethical Statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The First Affiliated Hospital of Chongqing Medical University, Chongqing (No.2013-61) and informed consent was taken from all individual participants.
Author Contributions
J.J., X.D., and S.M. contributed to conception and design. J.J. and S.M. contributed to administrative support. L.L. contributed to data analysis and interpretation. All authors contributed to the provision of study materials or patients, collection and assembly of data, the writing of this manuscript, and final approval.
References
- 1.Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1–S226. [PubMed] [Google Scholar]
- 2.Bayman E O, Parekh K R, Keech J, Selte A, Brennan T J. A prospective study of chronic pain after thoracic surgery. Anesthesiology. 2017;126(05):938–951. doi: 10.1097/ALN.0000000000001576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grosen K, Laue Petersen G, Pfeiffer-Jensen M, Hoejsgaard A, Pilegaard H K. Persistent post-surgical pain following anterior thoracotomy for lung cancer: a cross-sectional study of prevalence, characteristics and interference with functioning. Eur J Cardiothorac Surg. 2013;43(01):95–103. doi: 10.1093/ejcts/ezs159. [DOI] [PubMed] [Google Scholar]
- 4.Mongardon N, Pinton-Gonnet C, Szekely B, Michel-Cherqui M, Dreyfus J F, Fischler M. Assessment of chronic pain after thoracotomy: a 1-year prevalence study. Clin J Pain. 2011;27(08):677–681. doi: 10.1097/AJP.0b013e31821981a3. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Li S, Liang N, Liu W, Liu H, Liu H.Postoperative pain experiences in Chinese adult patients after thoracotomy and video-assisted thoracic surgery J Clin Nurs 201726(17-18):2744–2754. [DOI] [PubMed] [Google Scholar]
- 6.McKenna R J, Jr, Houck W, Fuller C B.Video-assisted thoracic surgery lobectomy: experience with 1,100 cases Ann Thorac Surg 20068102421–425., discussion 425–426 [DOI] [PubMed] [Google Scholar]
- 7.Paul S, Altorki N K, Sheng S. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139(02):366–378. doi: 10.1016/j.jtcvs.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Rizk N P, Ghanie A, Hsu M. A prospective trial comparing pain and quality of life measures after anatomic lung resection using thoracoscopy or thoracotomy. Ann Thorac Surg. 2014;98(04):1160–1166. doi: 10.1016/j.athoracsur.2014.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wildgaard K, Ravn J, Nikolajsen L, Jakobsen E, Jensen T S, Kehlet H. Consequences of persistent pain after lung cancer surgery: a nationwide questionnaire study. Acta Anaesthesiol Scand. 2011;55(01):60–68. doi: 10.1111/j.1399-6576.2010.02357.x. [DOI] [PubMed] [Google Scholar]
- 10.Migliore M, Deodato G. A single-trocar technique for minimally-invasive surgery of the chest. Surg Endosc. 2001;15(08):899–901. doi: 10.1007/s004640090033. [DOI] [PubMed] [Google Scholar]
- 11.Mizukami Y, Takahashi Y, Adachi H. Single-port vs conventional three-port video-assisted thoracoscopic pulmonary wedge resection: comparison of postoperative pain and surgical costs. Ann Thorac Cardiovasc Surg. 2021;27(02):91–96. doi: 10.5761/atcs.oa.20-00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamura M, Shimizu Y, Hashizume Y. Pain following thoracoscopic surgery: retrospective analysis between single-incision and three-port video-assisted thoracoscopic surgery. J Cardiothorac Surg. 2013;8:153. doi: 10.1186/1749-8090-8-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirai K, Takeuchi S, Usuda J. Single-incision thoracoscopic surgery and conventional video-assisted thoracoscopic surgery: a retrospective comparative study of perioperative clinical outcomes. Eur J Cardiothorac Surg. 2016;49 01:i37–i41. doi: 10.1093/ejcts/ezv320. [DOI] [PubMed] [Google Scholar]
- 14.Hirai K, Usuda J. Uniportal video-assisted thoracic surgery reduced the occurrence of post-thoracotomy pain syndrome after lobectomy for lung cancer. J Thorac Dis. 2019;11(09):3896–3902. doi: 10.21037/jtd.2019.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Liu D, Lu J, Zhang S, Yang X. The feasibility and advantage of uniportal video-assisted thoracoscopic surgery (VATS) in pulmonary lobectomy. BMC Cancer. 2017;17(01):75. doi: 10.1186/s12885-017-3069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edge S BBD, Byrd D R, Compton C C, Fritz A G, Greene F L. 7th ed. New York, NY: Springer-Verlag; 2010. AJCC Cancer Staging Handbook; pp. 299–323. [Google Scholar]
- 17.Zhi X Y, Yu J M, Shi Y K. Chinese guidelines on the diagnosis and treatment of primary lung cancer (2015 version) Cancer. 2015;121 17:3165–3181. doi: 10.1002/cncr.29550. [DOI] [PubMed] [Google Scholar]
- 18.Ger L P, Ho S T, Sun W Z, Wang M S, Cleeland C S. Validation of the Brief Pain Inventory in a Taiwanese population. J Pain Symptom Manage. 1999;18(05):316–322. doi: 10.1016/s0885-3924(99)00087-1. [DOI] [PubMed] [Google Scholar]
- 19.Gjeilo K H, Stenseth R, Wahba A, Lydersen S, Klepstad P. Validation of the brief pain inventory in patients six months after cardiac surgery. J Pain Symptom Manage. 2007;34(06):648–656. doi: 10.1016/j.jpainsymman.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Keller S, Bann C M, Dodd S L, Schein J, Mendoza T R, Cleeland C S. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(05):309–318. doi: 10.1097/00002508-200409000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Wildgaard K, Ringsted T K, Hansen H J, Petersen R H, Kehlet H. Persistent postsurgical pain after video-assisted thoracic surgery—an observational study. Acta Anaesthesiol Scand. 2016;60(05):650–658. doi: 10.1111/aas.12681. [DOI] [PubMed] [Google Scholar]
- 22.Steegers M A, Snik D M, Verhagen A F, van der Drift M A, Wilder-Smith O H. Only half of the chronic pain after thoracic surgery shows a neuropathic component. J Pain. 2008;9(10):955–961. doi: 10.1016/j.jpain.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Takenaka S, Saeki A, Sukenaga N. Acute and chronic neuropathic pain profiles after video-assisted thoracic surgery: a prospective study. Medicine (Baltimore) 2020;99(13):e19629. doi: 10.1097/MD.0000000000019629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kehlet H, Jensen T S, Woolf C J.Persistent postsurgical pain: risk factors and prevention Lancet 2006367(9522):1618–1625. [DOI] [PubMed] [Google Scholar]
- 25.Benedetti F, Vighetti S, Ricco C. Neurophysiologic assessment of nerve impairment in posterolateral and muscle-sparing thoracotomy. J Thorac Cardiovasc Surg. 1998;115(04):841–847. doi: 10.1016/S0022-5223(98)70365-4. [DOI] [PubMed] [Google Scholar]
- 26.Yoon S, Hong W P, Joo H. Long-term incidence of chronic postsurgical pain after thoracic surgery for lung cancer: a 10-year single-center retrospective study. Reg Anesth Pain Med. 2020;45(05):331–336. doi: 10.1136/rapm-2020-101292. [DOI] [PubMed] [Google Scholar]
- 27.Yoon S, Hong W P, Joo H, Jang D, Park S, Lee H J. Adjuvant chemotherapy as a risk factor for chronic postoperative pain after video-assisted thoracoscopic surgery: a 10-year single-centre retrospective study. Interact Cardiovasc Thorac Surg. 2021;32(02):276–283. doi: 10.1093/icvts/ivaa250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haroutiunian S, Nikolajsen L, Finnerup N B, Jensen T S. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain. 2013;154(01):95–102. doi: 10.1016/j.pain.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Brown M R, Ramirez J D, Farquhar-Smith P. Pain in cancer survivors. Br J Pain. 2014;8(04):139–153. doi: 10.1177/2049463714542605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin H H, Tse M M, Wong F K.Postoperative pain experience and barriers to pain management in Chinese adult patients undergoing thoracic surgery J Clin Nurs 201221(9-10):1232–1243. [DOI] [PubMed] [Google Scholar]


