Abstract
Objective
This study aimed to analyze the effect of traditional Chinese medicine (TCM) on the risk of readmission for rheumatoid arthritis (RA) patients with anemia.
Methods
In this study, 893 hospitalized RA patients were followed up by telephone. A retrospective cohort study was conducted using propensity score matching (PSM). The Cox proportional hazards model was used to assess the influence of various factors on the risk of readmission for RA patients with anemia. The Kaplan–Meier survival curve was utilized to analyze the effect of TCM intervention time on readmission.
Results
The incidence of anemia was 58.08% (471/811) in RA patients. After 1 : 1 PSM, 328 RA patients with anemia and 328 RA patients without anemia were finally included in our study. The readmission rate of anemia patients was higher than that of patients without anemia (P < 0.01). The readmission rate of RA patients with anemia was obviously lower in the TCM group than in the non-TCM group (P < 0.01). The Cox proportional hazards model showed TCM as an independent protective factor as it decreased the risk of readmission by 50% (HR = 0.50, 95% CI = 0.27–0.94, P=0.03) in RA patients with anemia. In addition, the risk of readmission was dramatically diminished in the high-exposure subgroup (TCM > 12 months) compared with the low-exposure subgroup (TCM ≤ 12 months) (log-rank P=0.016).
Conclusion
TCM, as a protective factor, is associated with a reduced risk of readmission in RA patients with anemia.
1. Introduction
Rheumatoid arthritis (RA) is an autoimmune disease involving numerous systems, with symmetric joint pain and swelling as the main clinical manifestations, which can lead to joint deformity and loss of function [1]. RA is accompanied by multifaceted extra-articular manifestations, including rheumatoid nodules, vasculitis, pleural and pulmonary lesions, hematologic changes, cardiac lesions, renal lesions, neurological lesions, ocular lesions, and Sjogren's syndrome [2, 3]. Involvement of the blood system is common in multisystem damage caused by RA. Anemia is one of the most frequent extra-articular manifestations of RA with an incidence rate of approximately 30%–70% [4, 5]. In fact, anemia is considered a predictor of radiographic progression of RA and an indicator of inflammatory status during active disease [6, 7]. In addition, anemia can lead to a range of adverse manifestations, such as fatigue, weakened muscle strength, decreased physical activity, augmented hospitalizations, and reduced quality of life [8–10]. Therefore, anemia is an important factor affecting local joint injury and systemic symptoms in RA patients.
Currently, there is no radical cure for RA. The main drugs for the treatment of RA comprise immunosuppressants, nonsteroidal anti-inflammatory drugs (NSAIDs), and glucocorticoids, which, however, often contribute to adverse reactions, such as liver and kidney dysfunction and gastrointestinal injury [11]. In addition to anemia, interstitial lung disease and Sjogren's syndrome are also prevalent secondary lesions of RA [12]. Studies have shown that interstitial lung disease is associated with poor prognosis of RA and is considered a critical factor in the occurrence of death in RA [13, 14]. 8.7–30% of RA patients can be complicated with Sjogren's syndrome, which is an essential factor affecting the disease activity, treatment, and prognosis of RA [15–17]. Therefore, it is urgent to develop drugs for the treatment of RA and secondary lesions.
In recent years, traditional Chinese medicine (TCM) has achieved some accomplishments in treating RA by alleviating clinical symptoms and improving prognosis in RA [18, 19]. The application of meta-analysis, network pharmacology, and systems biology has tremendously facilitated research on TCM-related therapeutics, pharmacy, and molecules [20–22]. Therefore, we performed a retrospective and controlled cohort study combined with data mining technology to ascertain the impact of TCM on the incidence of adverse events in RA patients with anemia, thus providing a reference for the application of TCM in the treatment of RA.
2. Materials and Methods
2.1. Data Sources and Study Subjects
In this telephone-based follow-up cohort analysis, we reviewed the clinical data of 893 RA patients admitted to the Department of Rheumatology in the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine from June 2013 to June 2021. These patients met the 2010 American College of Rheumatology/European Alliance of Associations for Rheumatology criteria for RA. The study was conducted in compliance with the principles of the Declaration of Helsinki. Our follow-up process fully protects the privacy of patients and did not interfere with the treatment option. The Ethics Committee of the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine exempted patients from the right of informed consent (Review no. 2022MCZQ01).
A flowchart describes the selection process for patients with RA (Figure 1). The follow-up started on December 10, 2021, and ended on February 10, 2022. Subsequent to follow-up and screening, 811 RA patients were followed up successfully, among which 471 patients suffered from anemia, accounting for 58.08%. In order to balance the bias caused by the baseline data, RA patients were arranged into an anemia group (n = 328) and an RA without anemia group (n = 328) after propensity score matching (PSM). Subsequently, RA patients with anemia were clarified into a TCM group (n = 243) and a non-TCM group (n = 85) according to whether TCM was used or not. Finally, patients in the TCM group were assigned into a high-exposure subgroup (>12 months) and a low-exposure subgroup (≤12 months) based on the time of TCM intervention.
Figure 1.
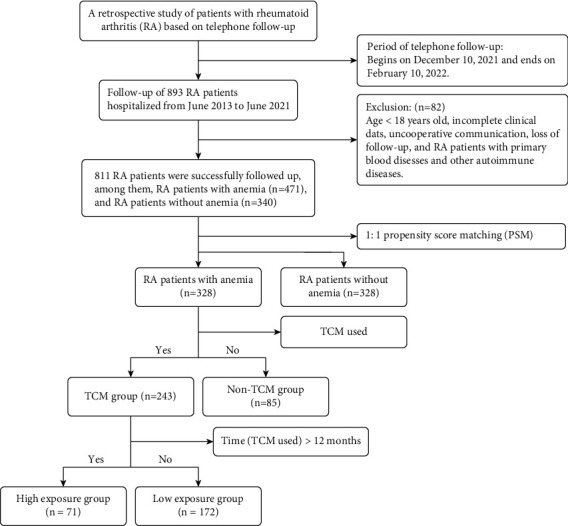
Flowchart of the study population.
2.2. Contents of Telephone Follow-Up
Basic data of patients, such as name, age, gender, telephone number, and diagnostic information, were obtained through the hospital information system (HIS) and verified through phone tracking. The follow-up included basic medication and time, TCM use and time, basic diseases, and end point events.
Basic medication consisted of disease-modifying antirheumatic drugs (DMARDs; including methotrexate, leflunomide, and hydroxychloroquine), NSAIDs (including celecoxib, meloxicam, and lornoxicam), glucocorticoids (including methylprednisolone and prednisone acetate), and folic acid. Combinations of basic diseases were listed as follows: hypertension, diabetes, and hyperlipidemia. The main end points were as follows: the exacerbation of RA leads to readmission, interstitial lung disease caused by RA, Sjogren's syndrome secondary to RA, surgical treatment of joints, and death event. All follow-up personnel were professional doctors in the Department of Rheumatology. Each follow-up was inquired by one doctor and supervised and verified by two doctors at the same time.
2.3. Anemia Evaluation and Laboratory Examinations
According to the criteria of anemia defined by the World Health Organization, hemoglobin (Hb) < 130 g/L for men and <120 g/L for women. It is divided into 4 grades according to the degree of anemia: mild anemia is Hb < 130 g/L (male) or Hb < 120 g/L (female) and Hb > 90 g/L; moderate anemia is 60 g/L ≤ Hb ≤ 90 g/L; severe anemia is 30 g/L ≤ Hb < 60 g/L; and extremely severe anemia is Hb < 30 g/L. Laboratory examination indicators were detected for the enrolled patients: red blood cell (RBC) count, Hb, serum Ferrum (Fe), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), rheumatoid factor (RF), α1-acid glycoprotein (α1-AGP), anticyclic citrullinated peptide (anti-CCP) antibody, immunoglobulin A (IgA), immunoglobulin G (IgG), and immunoglobulin M (IgM), complement component 3 (C3), complement component 4 (C4), serum creatinine (CREA), blood urea nitrogen (BUN), uric acid (UA), alanine aminotransferase (ALT), and aspartate transferase (AST).
2.4. Cox Proportional Hazards Model Analysis
In this study, the readmission of RA patients with anemia was used as the dependent variable, while age, gender, combined diseases, basic drugs, and TCM were utilized as independent variables. Combined with the time of readmission and follow-up, the Cox proportional hazards model analysis was carried out. A univariate analysis was performed to obtain preliminary results. A further multivariate analysis was conducted to study the independent influencing factors for readmission.
2.5. Association Rule Analysis
The specific TCM was defined as T and the unused TCM as F. After TCM treatment, the decrease of ESR, CRP, RF, anti-CCP, IgA, IgG, IgM, C3, and C4 was recorded as T, whereas their stability and increase was F. Hb, RBC, and Fe index values were regarded as T when they increased and F when they remained unchanged or decreased. Patient readmission was defined as F and no readmission was defined as T. This assisted in discovering the correlation between TCM and the observed indicator or with readmission. The specific calculation formula of association rule analysis refers to the research of Huang et al. [23].
2.6. Statistical Analysis
Count data as numbers or percentages and chi-squared test was used for comparison between groups. Continuous data were represented by median [quartile difference (IQR)] and comparisons were made using the rank-sum test. SPSS 24.0 was used for data statistics, Cox proportional risk model analysis, and Kaplan–Meier (K-M) survival curve analysis. There was a statistically significant difference when P < 0.05.
3. Results
3.1. Demographic Characteristics of RA Patients with or without Anemia
A total of 811 RA patients were included in this study, including 471 (58.08%) patients with anemia and 340 (41.92%) patients without anemia. There were significant differences in hypertension, hyperlipidemia, and folic acid use between the two groups (P < 0.05).
In order to avoid the bias caused by baseline, the method of 1 : 1 PSM was utilized to balance the bias between the two groups in terms of combined diseases and basic medication. Subsequent to matching, 656 RA patients were included, including 328 RA patients with anemia and 328 RA patients without anemia. No obvious difference was observed between the two groups regarding age, gender, combined diseases, and basic medication (P > 0.05). In terms of end point events, the incidence of readmission, interstitial lung disease, and surgical treatment was noticeably higher in RA patients with anemia than in RA patients without anemia (P < 0.05). In addition, Sjogren's syndrome and death were not statistically different between the two groups (P > 0.05; Table 1).
Table 1.
Comparison of demographic characteristics between RA patients with and without anemia.
| Characteristic | Before PSM matched | P value | After PSM matched | P value | ||||
|---|---|---|---|---|---|---|---|---|
| Total (n = 811) | RA with anemia (n = 471) | RA without anemia (n = 340) | Total (n = 656) | RA with anemia (n = 328) | RA without anemia (n = 328) | |||
| Age (years), n (%) | ||||||||
| 20–60 | 470 (57.95) | 261 (55.41) | 209 (61.47) | 0.09 | 384 (58.54) | 185 (56.40) | 199 (60.67) | 0.27 |
| >60 | 341 (42.05) | 210 (44.59) | 131 (38.53) | 272 (41.46) | 143 (43.60) | 129 (39.33) | ||
| Median (IQR) | 56 (49, 66) | 56 (49, 67) | 56 (49, 65) | 0.29 | 56 (49, 66) | 56 (48, 67) | 56 (49, 65) | 0.79 |
|
| ||||||||
| Gender, n (%) | ||||||||
| Female | 673 (82.98) | 392 (83.23) | 281 (82.65) | 0.83 | 539 (82.16) | 270 (82.32) | 269 (82.01) | 0.92 |
| Male | 138 (17.02) | 79 (16.77) | 59 (17.35) | 117 (17.84) | 58 (17.68) | 59 (17.99) | ||
|
| ||||||||
| Combined diseases, n (%) | ||||||||
| Hypertension | 176 (21.70) | 116 (24.63) | 60 (17.65) | 0.02 | 129 (19.66) | 69 (21.04) | 60 (18.29) | 0.38 |
| Diabetes | 34 (4.19) | 21 (4.46) | 13 (3.82) | 0.66 | 25 (3.81) | 13 (3.96) | 12 (3.66) | 0.84 |
| Hyperlipidemia | 91 (11.22) | 41 (8.70) | 50 (14.71) | 0.01 | 76 (11.59) | 37 (11.28) | 39 (11.89) | 0.81 |
|
| ||||||||
| Basic medicine, n (%) | ||||||||
| DMARDs | 767 (94.57) | 448 (95.12) | 319 (93.82) | 0.42 | 617 (94.05) | 309 (94.21) | 308 (93.90) | 0.87 |
| NSAIDs | 424 (52.28) | 253 (53.72) | 171 (50.29) | 0.34 | 328 (50.00) | 158 (48.17) | 170 (51.83) | 0.35 |
| Glucocorticoids | 202 (24.91) | 118 (25.05) | 84 (24.71) | 0.91 | 174 (26.52) | 90 (27.44) | 84 (25.61) | 0.60 |
| Folic acid | 241 (29.72) | 160 (33.97) | 81 (23.82) | <0.01 | 148 (22.56) | 67 (20.43) | 81 (24.70) | 0.19 |
|
| ||||||||
| End point events, n (%) | ||||||||
| Readmission | 107 (13.19) | 75 (15.92) | 32 (9.41) | 0.01 | 87 (13.26) | 59 (17.99) | 28 (8.54) | <0.01 |
| Interstitial lung disease | 94 (11.59) | 66 (14.01) | 28 (8.24) | 0.01 | 78 (11.89) | 50 (15.24) | 28 (8.54) | 0.01 |
| Sjogren's syndrome | 80 (10.11) | 55 (11.68) | 25 (7.35) | 0.04 | 56 (8.54) | 32 (9.76) | 24 (7.32) | 0.26 |
| Surgical treatment | 60 (7.40) | 46 (9.77) | 14 (4.12) | <0.01 | 45 (6.86) | 31 (9.45) | 14 (4.27) | 0.01 |
| Death | 21 (2.59) | 15 (3.18) | 6 (1.76) | 0.21 | 15 (2.29) | 9 (2.74) | 6 (1.83) | 0.43 |
Note. PSM: propensity score matching; DMARDs: disease-modifying antirheumatic drugs; NSAIDs: nonsteroidal anti-inflammatory drugs.
3.2. Demographic Characteristics of RA Patients with Anemia in the TCM Group and the Non-TCM Group
The 328 patients with RA anemia were allocated into the TCM group (n = 243) and the non-TCM group (n = 85). There existed no considerable difference in gender, age, combined diseases, anemia classification, and the incidence of interstitial lung disease, Sjogren's syndrome, surgical treatment, and death between the two groups (P > 0.05). In the TCM group, TCM was used in 80 cases for 0–6 months, 92 cases for 6–12 months, and 71 cases for more than 12 months. Compared with the non-TCM group, the incidence of readmission in the TCM group was evidently decreased (P < 0.01; Table 2).
Table 2.
Comparison of demographic characteristics of RA patients with anemia between the TCM group and the non-TCM group.
| Characteristic | TCM group (n = 243) | Non-TCM group (n = 85) | P value |
|---|---|---|---|
| Age (years), n (%) | |||
| 20–60 | 130 (53.50) | 55 (64.71) | 0.07 |
| >60 | 113 (46.50) | 30 (35.29) | |
|
| |||
| Gender, n (%) | |||
| Female | 200 (82.30) | 70 (82.35) | 0.99 |
| Male | 43 (17.70) | 15 (17.65) | |
|
| |||
| TCM (months), n (%) | |||
| 0 | 0 (0) | 85 (100) | — |
| >0, ≤6 | 80 (32.92) | NA | |
| >6, ≤12 | 92 (37.86) | NA | |
| >12 | 71 (29.22) | NA | |
|
| |||
| Anemia classification, n (%) | |||
| Mild anemia | 201 (82.72) | 69 (81.18) | 0.75 |
| Moderate anemia | 38 (15.64) | 16 (18.82) | 0.50 |
| Severe anemia | 4 (1.65) | 0 (0) | — |
|
| |||
| Combined diseases, n (%) | |||
| Hypertension | 54 (22.23) | 15 (17.65) | 0.37 |
| Hyperlipidemia | 30 (12.35) | 7 (8.24) | 0.30 |
| Diabetes | 8 (3.29) | 5 (5.88) | 0.29 |
|
| |||
| End event, n (%) | |||
| Readmission | 34 (13.99) | 25 (29.41) | <0.01 |
| Interstitial lung disease | 39 (16.05) | 11 (12.94) | 0.42 |
| Sjogren's syndrome | 20 (8.23) | 12 (14.12) | 0.12 |
| Surgical treatment | 22 (9.05) | 9 (10.59) | 0.68 |
| Death | 7 (2.88) | 2 (2.35) | 1.00 |
Note. TCM: traditional Chinese medicine.
3.3. Influencing Factors for the Readmission of RA Patients with Anemia
The Cox proportional risk model was used to determine the risk factors for readmission in RA patients with anemia (Table 3). The univariate analysis manifested that the TCM group had a substantially lower readmission than in the TCM group [Hazards Ratio (HR) = 0.50, 95% CI = 0.30–0.84, P=0.01]. Patients administering DMARDs had a prominently lower readmission risk (HR = 0.35, 95% CI = 0.16–0.77, P=0.01). Likewise, the readmission risk also was lower in patients receiving NSAID treatment (HR = 0.59, 95% CI = 0.35–0.99, P=0.05). Furthermore, the multivariate analysis was implemented to screen the independent factors influencing the readmission of RA patients with anemia. The results exhibited that the risk of readmission in patients of the TCM group was reduced by 50% (HR = 0.50, 95% CI = 0.27–0.94, P=0.03) compared with the patients of the non-TCM group. These results illustrated that the use of TCM was a protective factor to diminish the readmission rate of RA patients with anemia.
Table 3.
The Cox proportional hazards model for the readmission of RA patients with anemia.
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (year) | ||||||
| 20–60 | 1 | 1 | ||||
| ≥60 | 1.13 | 0.64–1.89 | 0.65 | 1.26 | 0.69–2.27 | 0.45 |
|
| ||||||
| Gender | ||||||
| Male | 1 | 1 | ||||
| Female | 1.05 | 0.53–2.07 | 0.90 | 1.03 | 0.52–2.06 | 0.93 |
|
| ||||||
| TCM used | ||||||
| No | 1 | 1 | ||||
| Yes | 0.50 | 0.30–0.84 | 0.01 | 0.50 | 0.27–0.94 | 0.03 |
| Hypertension | 1.23 | 0.68–2.22 | 0.50 | 1.42 | 0.72–2.78 | 0.31 |
| Diabetes | 0.72 | 1.18–2.98 | 0.65 | 0.52 | 0.11–2.33 | 0.39 |
| Hyperlipidemia | 1.07 | 0.46–2.50 | 0.88 | 1.00 | 0.41–2.39 | 0.09 |
| DMARDs | 0.35 | 0.16–0.77 | 0.01 | 0.48 | 0.19–1.25 | 0.13 |
| NSAIDs | 0.59 | 0.35–0.99 | 0.05 | 0.58 | 0.32–1.05 | 0.07 |
| Glucocorticoids | 0.96 | 0.54–1.70 | 0.88 | 0.53 | 0.27–1.04 | 0.07 |
| Folic acid | 1.39 | 0.75–2.57 | 0.30 | 1.69 | 0.88–3.25 | 0.11 |
Note. HR: hazards ratio; 95% CI: 95% confidence interval; TCM: traditional Chinese medicine; DMARDs: disease-modifying antirheumatic drugs; NSAIDs: nonsteroidal anti-inflammatory drugs.
3.4. The K-M Survival Curve Analysis of the Effects of TCM on the Readmission of RA Patients with Anemia
The K-M survival curve was adopted to compare the risk of readmission between the TCM group and the non-TCM group and further delve into the impact of TCM intervention time on the risk of readmission. Specifically, the risk of readmission in the TCM group was remarkably lower than that in the non-TCM group (log-rank P=0.004; Figure 2(a)). In the TCM group, the TCM intervention time of ≤12 months was defined as low exposure and the TCM intervention time of >12 months as high exposure. The readmission rate was significantly lower in the high-exposure group compared with the low-exposure group (P=0.02; Figure 2(b)). In addition, in contrast to the low-exposure group, the high-exposure subgroup had a significantly lower risk of readmission (log-rank P=0.016; Figure 2(c)).
Figure 2.
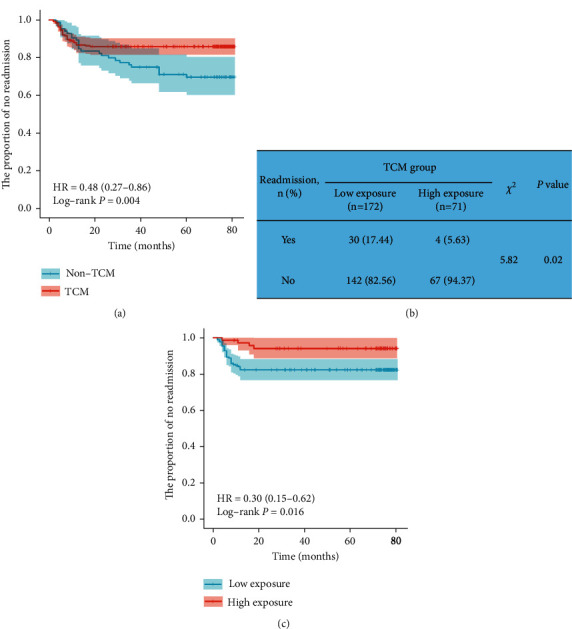
The K-M survival curve of readmission in RA patients with anemia. (a) The K-M survival curve was used to analyze the effect of TCM on the risk of readmission. (b) Incidence of readmission in low-exposure group and high-exposure group. (c) The K-M survival curve was utilized to assess the effect of TCM intervention time on the risk of readmission.
3.5. Characteristics of TCM in RA Patients with Anemia
The top 20 TCMs used by RA patients with anemia can be classified into five categories, including spleen-strengthening and dampness-removing drugs, heat-clearing and dampness-removing drugs, wind-dispelling and dehumidification drugs, blood-activating and stasis-removing drugs, and deficiency-tonifying drugs. The top 2 used Chinese patent medicines were Xinfeng capsules (XFC) and Huangqin Qingre Chubi capsules (HQC) (Supplementary Table S1).
3.6. Effects of TCM on Immune Inflammatory Indexes and Erythrocyte Parameters in RA Patients with Anemia
In the non-TCM group, compared with before treatment, Hb and RBC levels increased (P < 0.01), ESR, CRP, α1-AGP, IgG, C3, and C4 levels decreased (P < 0.01) after treatment. In TCM group, compared with before treatment, RBC and Hb levels were elevated (P < 0.01), and ESR, CRP, RF, α1-AGP, anti-CCP, IgA, IgG, C3, and C4 levels were reduced (P < 0.05) after treatment. In addition, TCM group was significantly better than non-TCM group in reducing RF level (P < 0.05; Table 4).
Table 4.
Effects of TCM on immune inflammatory indexes and erythrocyte parameters in RA patients with anemia.
| Index | Non-TCM group (n = 85) | TCM group (n = 243) | ||
|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | |
| Hb (g/L) | 99.00 (93.50, 108.00) | 103.00 (95.00, 110.50)∗∗ | 105.00 (95.00, 111.00) | 108.00 (99.00, 115.00)## |
| RBC (1012/L) | 3.67 (3.41, 4.00) | 3.87 (3.67, 4.23)∗∗ | 3.72 (3.45, 4.04) | 3.85 (3.58, 4.22)## |
| Fe (μmol/L) | 7.79 (5.47, 11.49) | 8.50 (6.05, 12.28) | 9.70 (6.31, 13.05) | 9.96 (6.70, 13.30) |
| ESR (mm/h) | 41.00 (26.00, 57.50) | 28.00 (18.50, 42.50)∗∗ | 41.00 (21.00, 68.00) | 24.00 (14.00, 44.00)## |
| CRP (mg/L) | 14.79 (3.77, 47.14) | 2.23 (0.58, 8.93)∗∗ | 9.99 (1.90, 36.84) | 1.45 (0.48, 225.50)## |
| RF (U/mL) | 92.20 (34.55, 235.50) | 87.90 (32.65, 238.25) | 113.10 (32.90, 238.30) | 98.60 (29.00, 201.80)##△ |
| α1-AGP (mg/dL) | 127.00 (96.00, 153.50) | 106.00 (84.00, 137.50)∗∗ | 118.00 (91.00, 154.00) | 101.00 (81.00, 130.00)## |
| Anti-CCP (U/mL) | 235.92 (27.75, 559.00) | 134.09 (27.23, 551.64) | 245.00 (64.17, 525.27) | 220.38 (49.17, 500.41)# |
| IgA (g/L) | 2.58 (1.78, 3.12) | 2.34 (1.77, 3.12) | 2.29 (1.65, 3.18) | 2.18 (1.64, 3.08)## |
| IgG (g/L) | 12.40 (9.67, 15.05) | 12.40 (9.72, 14.95)∗∗ | 12.30 (9.50, 15.49) | 11.75 (9.20, 14.80)## |
| IgM (g/L) | 1.27 (0.94, 1.68) | 1.25 (0.92, 1.71) | 1.20 (0.78, 1.63) | 1.19 (0.81, 1.64) |
| C3 (g/L) | 103.40 (83.20, 120.25) | 99.40 (83.70, 114.85)∗∗ | 104.20 (87.70, 122.30) | 100.80 (89.70, 115.10)## |
| C4 (g/L) | 21.80 (16.85, 28.30) | 20.90 (15.35, 25.15)∗∗ | 22.30 (17.20, 28.70) | 21.50 (15.60, 25.90)## |
Note. Compared with the non-TCM group before treatment, ∗∗P < 0.01. Compared with the TCM group before treatment, #P < 0.05 and ##P < 0.01. Compared with the non-TCM group difference (after treatment − before treatment), ΔP < 0.05. TCM: traditional Chinese medicine; Hb: hemoglobin; RBC: red blood cell count; Fe: serum Ferrum; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; RF: rheumatoid factor; α1-AGP: α1-acid glycoprotein; anti-CCP: anticyclic citrullinate peptide antibody; IgA: immunoglobulin A; IgG: immunoglobulin G; IgM: immunoglobulin M; C3: complement component 3; C4: complement component 4.
3.7. Effects of TCM on Kidney and Liver Function in RA Patients with Anemia
In non-TCM group, compared with before treatment, the level of UA decreased significantly after treatment (P < 0.01; Table 5). In TCM group, compared with before treatment, the level of UA decreased significantly after treatment (P < 0.01), and there was no statistical significance in the levels of CREA, BUN, ALT, and AST (P > 0.05; Table 5). There was no significant difference in the level of reduced UA between the two groups (P > 0.05; Table 5).
Table 5.
Effects of TCM on kidney and liver function in RA patients with anemia.
| Index | Non-TCM group (n = 85) | TCM group (n = 243) | ||
|---|---|---|---|---|
| Before treatment | After treatment | Before treatment | After treatment | |
| CREA (μmol/L) | 46.50 (39.10, 51.50) | 43.80 (39.65, 55.50) | 49.20 (43.90, 57.30) | 49.80 (43.10, 55.80) |
| BUN (mm/h) | 4.23 (3.29, 5.18) | 4.58 (3.61, 5.63) | 4.91 (3.62, 5.80) | 4.99 (3.90, 6.19) |
| UA (mg/L) | 211.00 (170.00, 263.50) | 196.00 (145.00, 236.50)∗∗ | 254.00 (202.00, 306.00) | 226.00 (187.00, 273.00)## |
| ALT (g/L) | 11.00 (8.00, 15.50) | 13.00 (10.00, 19.00) | 12.00 (9.00, 18.00) | 13.00 (9.00, 20.00) |
| AST (g/L) | 15.00 (13.00, 19.50) | 16.00 (13.00, 21.00) | 16.00 (13.00, 21.00) | 15.00 (13.00, 20.00) |
Note. Compared with the non-TCM group before treatment, ∗∗P < 0.01. Compared with the TCM group before treatment, ##P < 0.01. CREA: serum creatinine; BUN: blood urea nitrogen; UA: uric acid; ALT: alanine aminotransferase; AST: aspartate transferase.
3.8. Association Rules Analysis of the Correlation between TCM Treatment and Laboratory Indicators in RA Patients with Anemia
The association rule analysis was carried out with the TCM used as the antecedent and the improved laboratory indicators as the consequent. The results showed that Salvia, Poria, Angelicae Sinensis, Cyathulae radix, XFC, HQC, and other drugs were strongly correlated with the improvement of ESR, CRP, RF, α1-AGP, anti-CCP, RBC, and Hb. In the above results, the support is greater than 20%, the confidence is greater than 60%, and the lift is greater than 1 (Table 6).
Table 6.
Analysis of association rules between TCM treatment and laboratory indicators in RA patients with anemia.
| Number | The aforesaid | The consequent | Support (%) | Confidence (%) | Lift |
|---|---|---|---|---|---|
| 1 | Salvia | ESR↓ | 83.54 | 63.55 | 1.02 |
| 2 | Poria | ESR↓ | 85.60 | 65.38 | 1.05 |
| 3 | Salvia and poria | ESR↓ | 75.31 | 66.67 | 1.07 |
| 4 | HQC | ESR↓ | 24.28 | 62.71 | 1.01 |
| 5 | Dandelion and salvia | CRP↓ | 64.20 | 73.72 | 1.01 |
| 6 | XFC | CRP↓ | 31.28 | 73.68 | 1.01 |
| 7 | Spatholobi caulis | RF↓ | 44.03 | 64.49 | 1.03 |
| 8 | Spatholobi caulis and salvia | RF↓ | 39.09 | 64.21 | 1.03 |
| 9 | XFC | RF↓ | 31.28 | 64.47 | 1.03 |
| 10 | XFC | α1-AGP↓ | 31.28 | 60.53 | 1.14 |
| 11 | XFC and salvia | α1-AGP↓ | 29.22 | 60.56 | 1.14 |
| 12 | Hordei fructus germinatus | anti-CCP↓ | 24.28 | 61.02 | 1.24 |
| 13 | Hordei fructus germinatus and poria | anti-CCP↓ | 23.87 | 60.34 | 1.22 |
| 14 | Poria | RBC↑ | 85.60 | 60.10 | 1.03 |
| 15 | Coicis semen and poria | RBC↑ | 70.78 | 61.05 | 1.04 |
| 16 | Cyathulae radix | RBC↑ | 25.93 | 60.32 | 1.03 |
| 17 | Angelicae sinensis | RBC↑ | 28.81 | 64.29 | 1.10 |
| 18 | Salvia and poria | Hb↑ | 75.31 | 61.20 | 1.01 |
| 19 | Persicae semen and salvia | Hb↑ | 65.02 | 62.03 | 1.03 |
| 20 | Angelicae sinensis | Hb↑ | 28.81 | 64.29 | 1.06 |
Note. XFC: Xinfeng capsules; HQC: Huangqin Qingre Chubi capsules; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; RF: rheumatoid factor; α1-AGP: α1-acid glycoprotein; anti-CCP: anti-cyclic citrullinate peptide antibody; RBC: red blood cell count; Hb: hemoglobin.
3.9. Association Rule Analysis between TCM and Readmission or Not in RA Patients with Anemia
To investigate whether TCM is associated with readmission in RA patients with anemia, we defined readmission as F and no readmission as T. The results of association rule analysis showed that Spatholobi Caulis, XFC, Pinellia Ternata, Lonice Raejaponicae Caulis, Curcumae Radix, and Spatholobi Caulis were strongly associated with no readmission. The associated support is greater than 30%, the confidence is greater than 85%, and the lift is greater than 1 (Table 7).
Table 7.
The category of TCM associated with no readmission in RA patients with anemia.
| Number | The aforesaid | The consequent | Support (%) | Confidence (%) | Lift |
|---|---|---|---|---|---|
| 1 | Spatholobi caulis | No readmission | 44.03 | 89.72 | 1.04 |
| 2 | XFC | No readmission | 31.28 | 88.16 | 1.02 |
| 3 | Pinellia ternata | No readmission | 43.21 | 87.62 | 1.02 |
| 4 | Lonice raejaponicae caulis | No readmission | 41.15 | 87.00 | 1.01 |
| 5 | Curcumae radix | No readmission | 34.16 | 86.75 | 1.01 |
Note. XFC: Xinfeng capsules.
4. Discussion
Anemia induced by RA is majorly manifested as anemia of chronic disease (ACD) or inflammatory anemia, and the degree of anemia is mostly mild and moderate [24]. ACD in RA is attributed to a number of pathogenic mechanisms, such as the increased release of inflammatory cytokines, the disorder of iron metabolism, and the obstacle of erythropoiesis [25]. Our previous studies elucidated that the high-level immune inflammatory response in women was a risk factor for the increase of sharp scores in RA patients with anemia [26]. Similarly, increasing studies evidenced that inflammatory anemia might be an indicator of disease activity and structural damage in RA patients [7] and that RA patients with anemia have a longer hospital stay, more surgery times, and higher hospitalization costs [27]. Our study suggested that the incidence of anemia was 58.08% (471/811), consistent with the previous relevant literature [28]. The incidence of readmission was significantly higher in RA patients with anemia than in RA patients without anemia. Furthermore, TCM treatment contributed to declines in the risk of readmission in RA patients with anemia, and the longer the TCM use, the lower the risk of readmission.
TCM recently has attracted elevating attention from clinicians and relevant researchers for its role in the treatment of RA [29]. Former research has uncovered that the risk of fracture has been reduced by more than 50% in RA patients undergoing over 2 years of TCM treatment [30]. Chiu et al. noted that compared with the non-TCM group, the prognosis of the circulatory system of inpatients was favorable in the auxiliary TCM group, accompanied by two-thirds of reductions in the readmission rate of circulatory system events [31]. Our data unveiled that TCM effectively augmented the levels of RBC and Hb and reduced the levels of ESR, hs-CRP, RF, α1-AGP, anti-CCP, IgA, IgG, and UA in RA patients with anemia. In addition, it is worth noting that TCM intervention has no significant effect on liver and kidney function. The Cox proportional hazards regression analysis elaborated TCM intervention as the protective factor for readmission. The subsequent K-M survival curve analysis disclosed that not only did TCM reduce the risk of readmission of RA patients with anemia, but also the longer the use of TCM (>12 months), the lower the risk of readmission. In China, the combination of TCM and antirheumatism has been affirmed in the evaluation of clinical efficacy and safety [32], especially in repressing gastrointestinal diseases, abnormal liver function, leucopenia, skin allergy and rash, headache and dizziness, hair loss, and other adverse reactions.
The combined use of TCM is a common way to treat diseases, which often exerts the effect of increasing efficiency and reducing toxicity [33]. Multicomponents, multitargets, and multichannels are the characteristics of TCM in the treatment of RA and anemia [34]. The present study unraveled that the top 20 TCMs used by RA patients with anemia could be categorized into spleen-strengthening and dampness-removing drugs, heat-clearing and dampness-removing drugs, wind-dispelling and dehumidification drugs, blood-activating and stasis-removing drugs, and deficiency-tonifying drugs. Wang et al. [35] analyzed 302 RA patients related to “Zheng” in TCM, which indicated that the specific Zheng was associated with the characteristics of RA and that eliminating dampness, cooling patients, and promoting blood circulation might assist in treating severe RA. The association rules analysis in the present research manifested that Salvia, Poria, Angelicae Sinensis, Cyathulae radix, and Chinese patent medicine XFC and HQC were tightly correlated with the improvement of immune inflammatory indicators and erythrocyte parameters. In addition, further analysis showed that Spatholobi Caulis, XFC, Pinellia Ternata, Lonice Raejaponicae Caulis, Curcumae Radix, and Spatholobi Caulis were strongly associated with no readmission. Modern pharmacological research suggests that Poria has an excellent regulatory function in anti-inflammation and immune function [36]. Salvia miltiorrhiza exhibits definite curative effects in facilitating anti-inflammation, improving circulation, and reducing cardiovascular events [37]. Chen et al. [38] have observed that TCM has an obvious curative effect on improving anemia, among which Astragalus membranaceus and cinnamon decoction are the most frequently utilized single herbs and prescriptions in TCM for the treatment of anemia. The randomized controlled trial of XFC and leflunomide in the treatment of RA elucidated that XFC not only had superior efficacy and safety but also could improve the patient perception score [39]. XFC has been validated to be effective in improving extra-articular manifestations, such as cardiopulmonary function, anemia, and lipid metabolism disorders [40, 41]. In addition, HQC also assumes an established role in diminishing immune inflammatory responses and suppressing oxidative damage in RA [42].
Several limitations are present in this study. Only a single research institution is involved, and female patients account for more than 80% of the research population. Therefore, the research results may not be fully applicable to male RA patients. TCM formula is composed of many components, the specific active components are not clear, and the quality control is faced with some challenges. In addition, the combination of various herbs may cause gastrointestinal reactions in patients, increase the metabolic burden of liver and kidney, and potential adverse reactions.
In conclusion, TCM as a protective factor is associated with a reduced risk of readmission in RA patients with anemia. Furthermore, the longer the TCM use, the lower the risk of readmission. Although it is difficult to define whether this result is only caused by TCM, this study provides a reference for more comprehensive and extensive research in the future.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology National Key Research and Development Program Chinese Medicine Modernization Research Key Project (2018YFC1705204), National Nature Fund Program (82074373), the University Synergy Innovation Program of Anhui Province (GXXT-2020-025), Anhui Provincial Laboratory of Applied Basis and Development of Internal Medicine of Modern Traditional Chinese Medicine (2016080503B041), Anhui Famous Traditional Chinese Medicine Liu Jian Studio Construction Project (Traditional Chinese Medicine Development Secret [2018] no. 11), the Key Laboratory of Xin'an Ministry of Medical Education (no. 2020xayx10), the 2021 Open Fund of Anhui Key Laboratory of Applied Basic and Development Research of Modern Internal Medicine of Traditional Chinese Medicine (2021AKLMCM005), and the 2021 Anhui Province Major Difficult Diseases Collaborative Project of Traditional Chinese and Western Medicine (Anhui Traditional Chinese Medicine Development Secret [2021] no. 70).
Data Availability
The data used in this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this paper.
Authors' Contributions
YQS, JL, LX, and JTW participated in study design. SYQ, JTW, QZ, XLC, XD, and XHZ participated in telephone follow-up and recording. SYQ, QZ, and XLC collected clinical medical records and performed data analysis. SYQ performed manuscript writing. All authors reviewed the content of the final manuscript.
Supplementary Materials
Supplementary Table S1. TCM characteristics in treating rheumatoid arthritis patients with anemia.
References
- 1.Mcinnes I. B., Schett G. The pathogenesis of rheumatoid arthritis. New England Journal of Medicine . 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 2.Figus F. A., Piga M., Azzolin I., McConnell R., Iagnocco A. Rheumatoid arthritis: extra-articular manifestations and comorbidities. Autoimmunity Reviews . 2021;20(4) doi: 10.1016/j.autrev.2021.102776.102776 [DOI] [PubMed] [Google Scholar]
- 3.Marcucci E., Bartoloni E., Alunno A., et al. Extra-articular rheumatoid arthritis. Reumatismo . 2018;70(4):212–224. doi: 10.4081/reumatismo.2018.1106. [DOI] [PubMed] [Google Scholar]
- 4.Peeters H. R., Jongen-Lavrencic M., Raja A. N., et al. Course and characteristics of anaemia in patients with rheumatoid arthritis of recent onset. Annals of the Rheumatic Diseases . 1996;55(3):162–168. doi: 10.1136/ard.55.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song S. N. J., Iwahashi M., Tomosugi N., et al. Comparative evaluation of the effects of treatment with tocilizumab and TNF-α inhibitors on serum hepcidin, anemia response and disease activity in rheumatoid arthritis patients. Arthritis Research and Therapy . 2013;15(5):p. R141. doi: 10.1186/ar4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y., Xu W., Yang H., et al. Serum levels of hepcidin in rheumatoid arthritis and its correlation with disease activity and anemia: a meta-analysis. Immunological Investigations . 2021;50(2-3):243–258. doi: 10.1080/08820139.2020.1742731. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y. F., Xu S. Q., Xu Y. C., et al. Inflammatory anemia may be an indicator for predicting disease activity and structural damage in Chinese patients with rheumatoid arthritis. Clinical Rheumatology . 2020;39(6):1737–1745. doi: 10.1007/s10067-019-04873-y. [DOI] [PubMed] [Google Scholar]
- 8.Neidlein S., Wirth R., Pourhassan M. Iron deficiency, fatigue and muscle strength and function in older hospitalized patients. European Journal of Clinical Nutrition . 2021;75(3):456–463. doi: 10.1038/s41430-020-00742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikolaus S., Bode C., Taal E., van de Laar M. A. F. J. Fatigue and factors related to fatigue in rheumatoid arthritis: a systematic review. Arthritis Care & Research . 2013;65(7):1128–1146. doi: 10.1002/acr.21949. [DOI] [PubMed] [Google Scholar]
- 10.Yohannes A. M., Ershler W. B. Anemia in COPD: a systematic review of the prevalence, quality of life, and mortality. Respiratory Care . 2011;56(5):644–652. doi: 10.4187/respcare.01002. [DOI] [PubMed] [Google Scholar]
- 11.Conigliaro P., Triggianese P., De Martino E., et al. Challenges in the treatment of rheumatoid arthritis. Autoimmunity Reviews . 2019;18(7):706–713. doi: 10.1016/j.autrev.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 12.Conforti A., Di Cola I., Pavlych V., et al. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmunity Reviews . 2021;20(2) doi: 10.1016/j.autrev.2020.102735.102735 [DOI] [PubMed] [Google Scholar]
- 13.Dai Y., Wang W., Yu Y., Hu S. Rheumatoid arthritis-associated interstitial lung disease: an overview of epidemiology, pathogenesis and management. Clinical Rheumatology . 2021;40(4):1211–1220. doi: 10.1007/s10067-020-05320-z. [DOI] [PubMed] [Google Scholar]
- 14.Kadura S., Raghu G. Rheumatoid arthritis-interstitial lung disease: manifestations and current concepts in pathogenesis and management. European Respiratory Review . 2021;30(160) doi: 10.1183/16000617.0011-2021.210011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H., Cho S. K., Kim H. W., et al. The prevalence of sjögren’s syndrome in rheumatoid arthritis patients and their clinical features. Journal of Korean Medical Science . 2020;35(45):p. e369. doi: 10.3346/jkms.2020.35.e369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrold L. R., Shan Y., Rebello S., et al. Prevalence of Sjögren’s syndrome associated with rheumatoid arthritis in the USA: an observational study from the Corrona registry. Clinical Rheumatology . 2020;39(6):1899–1905. doi: 10.1007/s10067-020-05004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H., Zhang H., Gao D., Xie W., Geng Y., Zhang Z. Overlapping Sjogren’s syndrome reduces the probability of reaching target in rheumatoid arthritis patients: a propensity score matched real-world cohort from 2009 to 2019. Arthritis Research and Therapy . 2020;22(1):p. 100. doi: 10.1186/s13075-020-02189-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Chen S., Du K., et al. Traditional herbal medicine: therapeutic potential in rheumatoid arthritis. Journal of Ethnopharmacology . 2021;279 doi: 10.1016/j.jep.2021.114368.114368 [DOI] [PubMed] [Google Scholar]
- 19.Zhang P., Li J., Han Y., Wei Yu X., Qin L. Traditional Chinese medicine in the treatment of rheumatoid arthritis: a general review. Rheumatology International . 2010;30(6):713–718. doi: 10.1007/s00296-010-1370-0. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L., Cao Z., Yang Y., Tan X., Mao J., Su L. Traditional Chinese medicine on treating active rheumatoid arthritis: a protocol for systematic review and meta-analysis. Medicine (Baltimore) . 2020;99(24) doi: 10.1097/md.0000000000020642.e20642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang T., Kong B., Yan W., et al. Network pharmacology to identify the pharmacological mechanisms of a traditional Chinese medicine derived from trachelospermum jasminoides in patients with rheumatoid arthritis. Medical Science Monitor . 2020;26 doi: 10.12659/msm.922639.e922639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai N. S., Livneh H., Fan Y. H., Lu M. C., Liao H. H., Tsai T. Y. Use of Chinese herbal medicines by rheumatoid arthritis patients was associated with lower risk of stroke: a retrospective cohort study. Complementary Therapies in Medicine . 2019;45:124–129. doi: 10.1016/j.ctim.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 23.Huang D., Liu J., Xin L., et al. Data mining study on prescription patterns of different dosage forms of Chinese herbal medicines for treating and improving immune-inflammatory indices in patients with rheumatoid arthritis. Chinese Journal of Integrative Medicine . 2022;28(3):215–222. doi: 10.1007/s11655-020-3480-1. [DOI] [PubMed] [Google Scholar]
- 24.Nita E., Bairaktari E., Kolios G., et al. Role of hepcidin in anemia of chronic disease in rheumatoid arthritis. Journal of Laboratory Physicians . 2021;13(04):317–322. doi: 10.1055/s-0041-1732827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardang I. M., Lilleholt K., Hagve T. A. Anemia of chronic disease. Tidsskrift for den Norske laegeforening . 2017;137(17) doi: 10.4045/tidsskr.16.1128. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y., Liu J., Xin L., et al. Factors influencing the Sharp score of 1057 patients with rheumatoid arthritis and anemia: a retrospective study. Journal of International Medical Research . 2022;50(3) doi: 10.1177/03000605221088560.030006052210885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zlateva G., Diazaraque R., Viala-Danten M., Niculescu L. Burden of anemia in patients with osteoarthritis and rheumatoid arthritis in French secondary care. BMC Geriatrics . 2010;10(1):p. 59. doi: 10.1186/1471-2318-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson A., Yu H. T., Goodnough L. T., Nissenson A. R. Prevalence and outcomes of anemia in rheumatoid arthritis: a systematic review of the literature. The American Journal of Medicine . 2004;116(7):50S–57S. doi: 10.1016/j.amjmed.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 29.Guo B., Zhao C., Zhang C., et al. Elucidation of the anti-inflammatory mechanism of Er Miao San by integrative approach of network pharmacology and experimental verification. Pharmacological Research . 2022;175 doi: 10.1016/j.phrs.2021.106000.106000 [DOI] [PubMed] [Google Scholar]
- 30.Liao H. H., Livneh H., Chung Y. J., et al. A comparison of the risk of fracture in rheumatoid arthritis patients with and without receiving Chinese herbal medicine. Journal of Multidisciplinary Healthcare . 2021;14:3399–3409. doi: 10.2147/jmdh.s334134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiu H. E., Hong Y. C., Chang K. C., et al. Favorable circulatory system outcomes as adjuvant traditional Chinese medicine (TCM) treatment for cerebrovascular diseases in Taiwan. PLoS One . 2014;9(1) doi: 10.1371/journal.pone.0086351.e86351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xing Q., Fu L., Yu Z., Zhou X. Efficacy and safety of integrated traditional Chinese medicine and western medicine on the treatment of rheumatoid arthritis: a meta-analysis. Evidence-based Complementary and Alternative Medicine . 2020;2020:1–15. doi: 10.1155/2020/4348709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fang Y., Liu J., Xin L., et al. Identifying compound effect of drugs on rheumatoid arthritis treatment based on the association rule and a random walking-based model. BioMed Research International . 2020;2020:10. doi: 10.1155/2020/4031015.4031015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aihaiti Y., Song Cai Y., Tuerhong X., et al. Therapeutic effects of naringin in rheumatoid arthritis: network pharmacology and experimental validation. Frontiers in Pharmacology . 2021;12 doi: 10.3389/fphar.2021.672054.672054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang W., Wang X., Tang X., Jiang Q., Fan Y. Classifying rheumatoid arthritis by Traditional Chinese Medicine Zheng: a multi-center cross-sectional study. Journal of Traditional Chinese Medicine . 2019;39(3):425–432. [PubMed] [Google Scholar]
- 36.Ríos J. L. Chemical constituents and pharmacological properties of Poria cocos. Planta Medica . 2011;77(07):681–691. doi: 10.1055/s-0030-1270823. [DOI] [PubMed] [Google Scholar]
- 37.Lei W., Li X., Li L., et al. Compound Danshen Dripping Pill ameliorates post ischemic myocardial inflammation through synergistically regulating MAPK, PI3K/AKT and PPAR signaling pathways. Journal of Ethnopharmacology . 2021;281 doi: 10.1016/j.jep.2021.114438.114438 [DOI] [PubMed] [Google Scholar]
- 38.Chen W. D., Huang H. S., Su Y. C., et al. The characteristics and prescription patterns of Chinese herbal medicine in clinical practice for the treatment of anemia. Taiwanese Journal of Obstetrics & Gynecology . 2018;57(4):570–577. doi: 10.1016/j.tjog.2018.06.030. [DOI] [PubMed] [Google Scholar]
- 39.Liu J., Huang C. B., Wang Y., et al. Chinese herbal medicine Xinfeng Capsule in treatment of rheumatoid arthritis: study protocol of a multicenter randomized controlled trial. Journal of Integrative Medicine . 2013;11(6):428–434. doi: 10.3736/jintegrmed2013059. [DOI] [PubMed] [Google Scholar]
- 40.Liu J., Cao Y., Huang C., et al. Use of xinfeng capsule to treat abarticular pathologic changes in patients with rheumatoid arthritis. Journal of Traditional Chinese Medicine . 2014;34(5):532–538. doi: 10.1016/s0254-6272(15)30058-3. [DOI] [PubMed] [Google Scholar]
- 41.Wan L., Liu J., Huang C. B., et al. Xinfeng capsule for the treatment of rheumatoid arthritis patients with decreased pulmonary function--a randomized controlled clinical trial. Chinese Journal of Integrative Medicine . 2016;22(3):168–176. doi: 10.1007/s11655-015-2093-6. [DOI] [PubMed] [Google Scholar]
- 42.Wang X., Chang J., Zhou G., et al. The traditional Chinese medicine compound Huangqin Qingre Chubi capsule inhibits the pathogenesis of rheumatoid arthritis through the CUL4B/wnt pathway. Frontiers in Pharmacology . 2021;12 doi: 10.3389/fphar.2021.750233.750233 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. TCM characteristics in treating rheumatoid arthritis patients with anemia.
Data Availability Statement
The data used in this study are available from the corresponding author upon reasonable request.


